Ambrisentan_177036-94-1_CoA_MedChemExpress
胆汁酸谱在肺炎和肺癌鉴别诊断中的应用价值

文章编号:1673-8640(2021)01-001-07 中图分类号:R446.1 文献标志码:A DOI:10.3969/j.issn.1673-8640.2021.01.001胆汁酸谱在肺炎和肺癌鉴别诊断中的应用价值徐润灏1,邹 琛1,张 洁2,李 敏2,张舒林1(1.上海交通大学医学院,上海 200025;2.上海交通大学医学院附属仁济医院检验科,上海 200001)摘要:目的探讨肺癌患者血清胆汁酸谱的变化及其在肺炎与肺癌鉴别诊断中的价值。
方法 采用液相色谱-串联质谱法(LC-MS/MS)检测80例肺炎患者(肺炎组)、108例肺癌患者(肺癌组)和106名体检健康者(正常对照组)血清胆汁酸谱[5种游离胆汁酸,包括胆酸(CA)、鹅脱氧胆酸(CDCA)、脱氧胆酸(DCA)、石胆酸(LCA)、熊脱氧胆酸(UDCA);10种结合胆汁酸,包括甘氨胆酸(GCA)、甘氨鹅脱氧胆酸(GCDCA)、甘氨脱氧胆酸(GDCA)、甘氨石胆酸(GLCA)、甘氨熊脱氧胆酸(GUDCA)、牛磺胆酸(TCA)、牛磺鹅脱氧胆酸(TCDCA)、牛磺脱氧胆酸(TDCA)、牛磺石胆酸(TLCA)、牛磺熊脱氧胆酸(TUDCA)],同时检测血清总胆汁酸(TBA)及肿瘤标志物[糖类抗原(CA)125、CA19-9、癌胚抗原(CEA)、细胞角蛋白19片段(CYFRA 21-1)、神经元特异性烯醇化酶(NSE)]。
采用二元Logistic回归分析筛选指标并建立诊断模型。
采用受试者工作特性(ROC)曲线分析各项指标及诊断模型鉴别诊断肺炎与肺癌的效能。
结果与正常对照组比较,肺炎组血清游离胆汁酸DCA、LCA、UDCA水平及结合胆汁酸GDCA、GLCA、TDCA、TLCA水平降低(P<0.01),血清结合胆汁酸GCDCA、TCDCA水平升高(P<0.01);肺癌组血清游离胆汁酸CA、CDCA水平及结合胆汁酸GCDCA、TCDCA水平升高(P<0.01),血清结合胆汁酸TDCA、TLCA水平降低(P<0.01)。
梅思安(MSA)面罩呼吸管

梅思安(MSA)面罩呼吸管
品牌:梅思安(MSA) 型号:10027724
功能:
防毒气型式:头戴式
规格:详见说明重量:详见说明(kg)
材质:硅胶
供应梅思安(MSA)面罩呼吸管产品信息:和赛富
①、MSA德国产品,配单滤罐使用;
②、内置口鼻罩,热塑弹性体材料,可有效减少雾气产生;
③、四点式橡胶头带,带有预调功能,调节更加简便;
④、聚碳酸酯材料的透视,视野宽阔,无视觉扭曲,且机械性能好,坚固稳定;
⑤、透镜带防刮渡层,有效减少刮蹭对透镜的损坏;
⑥、设计优良的呼气及吸气阀,在有效过滤外界毒气体的同时,又降低了呼吸阻力,使用舒适;
⑦、配套使用滤毒片,可选配配件如透镜保护膜片、内置眼镜架。
产品编号:。
稳定性英文版

HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationFLUOXETINE HClC17H18F3NO•HClM.W. = 345.79CAS — 59333-67-4STABILITY INDICATINGA S S A Y V A L I D A T I O NMethod is suitable for:ýIn-process controlþProduct ReleaseþStability indicating analysis (Suitability - US/EU Product) CAUTIONFLUOXETINE HYDROCHLORIDE IS A HAZARDOUS CHEMICAL AND SHOULD BE HANDLED ONLY UNDER CONDITIONS SUITABLE FOR HAZARDOUS WORK.IT IS HIGHLY PRESSURE SENSITIVE AND ADEQUATE PRECAUTIONS SHOULD BE TAKEN TO AVOID ANY MECHANICAL FORCE (SUCH AS GRINDING, CRUSHING, ETC.) ON THE POWDER.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationTABLE OF CONTENTS INTRODUCTION........................................................................................................................ PRECISION............................................................................................................................... System Repeatability ................................................................................................................ Method Repeatability................................................................................................................. Intermediate Precision .............................................................................................................. LINEARITY................................................................................................................................ RANGE...................................................................................................................................... ACCURACY............................................................................................................................... Accuracy of Standard Injections................................................................................................ Accuracy of the Drug Product.................................................................................................... VALIDATION OF FLUOXETINE HCl AT LOW CONCENTRATION........................................... Linearity at Low Concentrations................................................................................................. Accuracy of Fluoxetine HCl at Low Concentration..................................................................... System Repeatability................................................................................................................. Quantitation Limit....................................................................................................................... Detection Limit........................................................................................................................... VALIDATION FOR META-FLUOXETINE HCl (POSSIBLE IMPURITIES).................................. Meta-Fluoxetine HCl linearity at 0.05% - 1.0%........................................................................... Detection Limit for Fluoxetine HCl.............................................................................................. Quantitation Limit for Meta Fluoxetine HCl................................................................................ Accuracy for Meta-Fluoxetine HCl ............................................................................................ Method Repeatability for Meta-Fluoxetine HCl........................................................................... Intermediate Precision for Meta-Fluoxetine HCl......................................................................... SPECIFICITY - STABILITY INDICATING EVALUATION OF THE METHOD............................. FORCED DEGRADATION OF FINISHED PRODUCT AND STANDARD..................................1. Unstressed analysis...............................................................................................................2. Acid Hydrolysis stressed analysis..........................................................................................3. Base hydrolysis stressed analysis.........................................................................................4. Oxidation stressed analysis...................................................................................................5. Sunlight stressed analysis.....................................................................................................6. Heat of solution stressed analysis.........................................................................................7. Heat of powder stressed analysis.......................................................................................... System Suitability stressed analysis.......................................................................................... Placebo...................................................................................................................................... STABILITY OF STANDARD AND SAMPLE SOLUTIONS......................................................... Standard Solution...................................................................................................................... Sample Solutions....................................................................................................................... ROBUSTNESS.......................................................................................................................... Extraction................................................................................................................................... Factorial Design......................................................................................................................... CONCLUSION...........................................................................................................................ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationBACKGROUNDTherapeutically, Fluoxetine hydrochloride is a classified as a selective serotonin-reuptake inhibitor. Effectively used for the treatment of various depressions. Fluoxetine hydrochloride has been shown to have comparable efficacy to tricyclic antidepressants but with fewer anticholinergic side effects. The patent expiry becomes effective in 2001 (US). INTRODUCTIONFluoxetine capsules were prepared in two dosage strengths: 10mg and 20mg dosage strengths with the same capsule weight. The formulas are essentially similar and geometrically equivalent with the same ingredients and proportions. Minor changes in non-active proportions account for the change in active ingredient amounts from the 10 and 20 mg strength.The following validation, for the method SI-IAG-206-02 , includes assay and determination of Meta-Fluoxetine by HPLC, is based on the analytical method validation SI-IAG-209-06. Currently the method is the in-house method performed for Stability Studies. The Validation was performed on the 20mg dosage samples, IAG-21-001 and IAG-21-002.In the forced degradation studies, the two placebo samples were also used. PRECISIONSYSTEM REPEATABILITYFive replicate injections of the standard solution at the concentration of 0.4242mg/mL as described in method SI-IAG-206-02 were made and the relative standard deviation (RSD) of the peak areas was calculated.SAMPLE PEAK AREA#15390#25406#35405#45405#55406Average5402.7SD 6.1% RSD0.1ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::PRECISION - Method RepeatabilityThe full HPLC method as described in SI-IAG-206-02 was carried-out on the finished product IAG-21-001 for the 20mg dosage form. The method repeated six times and the relative standard deviation (RSD) was calculated.SAMPLENumber%ASSAYof labeled amountI 96.9II 97.8III 98.2IV 97.4V 97.7VI 98.5(%) Average97.7SD 0.6(%) RSD0.6PRECISION - Intermediate PrecisionThe full method as described in SI-IAG-206-02 was carried-out on the finished product IAG-21-001 for the 20mg dosage form. The method was repeated six times by a second analyst on a different day using a different HPLC instrument. The average assay and the relative standard deviation (RSD) were calculated.SAMPLENumber% ASSAYof labeled amountI 98.3II 96.3III 94.6IV 96.3V 97.8VI 93.3Average (%)96.1SD 2.0RSD (%)2.1The difference between the average results of method repeatability and the intermediate precision is 1.7%.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationLINEARITYStandard solutions were prepared at 50% to 200% of the nominal concentration required by the assay procedure. Linear regression analysis demonstrated acceptability of the method for quantitative analysis over the concentration range required. Y-Intercept was found to be insignificant.RANGEDifferent concentrations of the sample (IAG-21-001) for the 20mg dosage form were prepared, covering between 50% - 200% of the nominal weight of the sample.Conc. (%)Conc. (mg/mL)Peak Area% Assayof labeled amount500.20116235096.7700.27935334099.21000.39734463296.61500.64480757797.52000.79448939497.9(%) Average97.6SD 1.0(%) RSD 1.0ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::RANGE (cont.)The results demonstrate linearity as well over the specified range.Correlation coefficient (RSQ)0.99981 Slope11808.3Y -Interceptresponse at 100%* 100 (%) 0.3%ACCURACYACCURACY OF STANDARD INJECTIONSFive (5) replicate injections of the working standard solution at concentration of 0.4242mg/mL, as described in method SI-IAG-206-02 were made.INJECTIONNO.PEAK AREA%ACCURACYI 539299.7II 540599.9III 540499.9IV 5406100.0V 5407100.0Average 5402.899.9%SD 6.10.1RSD, (%)0.10.1The percent deviation from the true value wasdetermined from the linear regression lineHPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::ACCURACY OF THE DRUG PRODUCTAdmixtures of non-actives (placebo, batch IAG-21-001 ) with Fluoxetine HCl were prepared at the same proportion as in a capsule (70%-180% of the nominal concentration).Three preparations were made for each concentration and the recovery was calculated.Conc.(%)Placebo Wt.(mg)Fluoxetine HCl Wt.(mg)Peak Area%Accuracy Average (%)70%7079.477.843465102.27079.687.873427100.77079.618.013465100.0101.0100%10079.6211.25476397.910080.8011.42491799.610079.6011.42485498.398.6130%13079.7214.90640599.413080.3114.75632899.213081.3314.766402100.399.618079.9920.10863699.318079.3820.45879499.418080.0820.32874899.599.4Placebo, Batch Lot IAG-21-001HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::VALIDATION OF FLUOXETINE HClAT LOW CONCENTRATIONLINEARITY AT LOW CONCENTRATIONSStandard solution of Fluoxetine were prepared at approximately 0.02%-1.0% of the working concentration required by the method SI-IAG-206-02. Linear regression analysis demonstrated acceptability of the method for quantitative analysis over this range.ACCURACY OF FLUOXETINE HCl AT LOW CONCENTRATIONThe peak areas of the standard solution at the working concentration were measured and the percent deviation from the true value, as determined from the linear regression was calculated.SAMPLECONC.µg/100mLAREA FOUND%ACCURACYI 470.56258499.7II 470.56359098.1III 470.561585101.3IV 470.561940100.7V 470.56252599.8VI 470.56271599.5(%) AverageSlope = 132.7395299.9SD Y-Intercept = -65.872371.1(%) RSD1.1HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSystem RepeatabilitySix replicate injections of standard solution at 0.02% and 0.05% of working concentration as described in method SI-IAG-206-02 were made and the relative standard deviation was calculated.SAMPLE FLUOXETINE HCl AREA0.02%0.05%I10173623II11503731III10103475IV10623390V10393315VI10953235Average10623462RSD, (%) 5.0 5.4Quantitation Limit - QLThe quantitation limit ( QL) was established by determining the minimum level at which the analyte was quantified. The quantitation limit for Fluoxetine HCl is 0.02% of the working standard concentration with resulting RSD (for six injections) of 5.0%. Detection Limit - DLThe detection limit (DL) was established by determining the minimum level at which the analyte was reliably detected. The detection limit of Fluoxetine HCl is about 0.01% of the working standard concentration.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::VALIDATION FOR META-FLUOXETINE HCl(EVALUATING POSSIBLE IMPURITIES)Meta-Fluoxetine HCl linearity at 0.05% - 1.0%Relative Response Factor (F)Relative response factor for Meta-Fluoxetine HCl was determined as slope of Fluoxetine HCl divided by the slope of Meta-Fluoxetine HCl from the linearity graphs (analysed at the same time).F =132.7395274.859534= 1.8Detection Limit (DL) for Fluoxetine HClThe detection limit (DL) was established by determining the minimum level at which the analyte was reliably detected.Detection limit for Meta Fluoxetine HCl is about 0.02%.Quantitation Limit (QL) for Meta-Fluoxetine HClThe QL is determined by the analysis of samples with known concentration of Meta-Fluoxetine HCl and by establishing the minimum level at which the Meta-Fluoxetine HCl can be quantified with acceptable accuracy and precision.Six individual preparations of standard and placebo spiked with Meta-Fluoxetine HCl solution to give solution with 0.05% of Meta Fluoxetine HCl, were injected into the HPLC and the recovery was calculated.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::META-FLUOXETINE HCl[RECOVERY IN SPIKED SAMPLES].Approx.Conc.(%)Known Conc.(µg/100ml)Area in SpikedSampleFound Conc.(µg/100mL)Recovery (%)0.0521.783326125.735118.10.0521.783326825.821118.50.0521.783292021.55799.00.0521.783324125.490117.00.0521.783287220.96996.30.0521.783328526.030119.5(%) AVERAGE111.4SD The recovery result of 6 samples is between 80%-120%.10.7(%) RSDQL for Meta Fluoxetine HCl is 0.05%.9.6Accuracy for Meta Fluoxetine HClDetermination of Accuracy for Meta-Fluoxetine HCl impurity was assessed using triplicate samples (of the drug product) spiked with known quantities of Meta Fluoxetine HCl impurity at three concentrations levels (namely 80%, 100% and 120% of the specified limit - 0.05%).The results are within specifications:For 0.4% and 0.5% recovery of 85% -115%For 0.6% recovery of 90%-110%HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::META-FLUOXETINE HCl[RECOVERY IN SPIKED SAMPLES]Approx.Conc.(%)Known Conc.(µg/100mL)Area in spikedSample Found Conc.(µg/100mL)Recovery (%)[0.4%]0.4174.2614283182.66104.820.4174.2614606187.11107.370.4174.2614351183.59105.36[0.5%]0.5217.8317344224.85103.220.5217.8316713216.1599.230.5217.8317341224.81103.20[0.6%]0.6261.3918367238.9591.420.6261.3920606269.81103.220.6261.3920237264.73101.28RECOVERY DATA DETERMINED IN SPIKED SAMPLESHPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::REPEATABILITYMethod Repeatability - Meta Fluoxetine HClThe full method (as described in SI-IAG-206-02) was carried out on the finished drug product representing lot number IAG-21-001-(1). The HPLC method repeated serially, six times and the relative standard deviation (RSD) was calculated.IAG-21-001 20mg CAPSULES - FLUOXETINESample% Meta Fluoxetine % Meta-Fluoxetine 1 in Spiked Solution10.0260.09520.0270.08630.0320.07740.0300.07450.0240.09060.0280.063AVERAGE (%)0.0280.081SD 0.0030.012RSD, (%)10.314.51NOTE :All results are less than QL (0.05%) therefore spiked samples with 0.05% Meta Fluoxetine HCl were injected.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::Intermediate Precision - Meta-Fluoxetine HClThe full method as described in SI-IAG-206-02 was applied on the finished product IAG-21-001-(1) .It was repeated six times, with a different analyst on a different day using a different HPLC instrument.The difference between the average results obtained by the method repeatability and the intermediate precision was less than 30.0%, (11.4% for Meta-Fluoxetine HCl as is and 28.5% for spiked solution).IAG-21-001 20mg - CAPSULES FLUOXETINESample N o:Percentage Meta-fluoxetine% Meta-fluoxetine 1 in spiked solution10.0260.06920.0270.05730.0120.06140.0210.05850.0360.05560.0270.079(%) AVERAGE0.0250.063SD 0.0080.009(%) RSD31.514.51NOTE:All results obtained were well below the QL (0.05%) thus spiked samples slightly greater than 0.05% Meta-Fluoxetine HCl were injected. The RSD at the QL of the spiked solution was 14.5%HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSPECIFICITY - STABILITY INDICATING EVALUATIONDemonstration of the Stability Indicating parameters of the HPLC assay method [SI-IAG-206-02] for Fluoxetine 10 & 20mg capsules, a suitable photo-diode array detector was incorporated utilizing a commercial chromatography software managing system2, and applied to analyze a range of stressed samples of the finished drug product.GLOSSARY of PEAK PURITY RESULT NOTATION (as reported2):Purity Angle-is a measure of spectral non-homogeneity across a peak, i.e. the weighed average of all spectral contrast angles calculated by comparing all spectra in the integrated peak against the peak apex spectrum.Purity Threshold-is the sum of noise angle3 and solvent angle4. It is the limit of detection of shape differences between two spectra.Match Angle-is a comparison of the spectrum at the peak apex against a library spectrum.Match Threshold-is the sum of the match noise angle3 and match solvent angle4.3Noise Angle-is a measure of spectral non-homogeneity caused by system noise.4Solvent Angle-is a measure of spectral non-homogeneity caused by solvent composition.OVERVIEWT he assay of the main peak in each stressed solution is calculated according to the assay method SI-IAG-206-02, against the Standard Solution, injected on the same day.I f the Purity Angle is smaller than the Purity Threshold and the Match Angle is smaller than the Match Threshold, no significant differences between spectra can be detected. As a result no spectroscopic evidence for co-elution is evident and the peak is considered to be pure.T he stressed condition study indicated that the Fluoxetine peak is free from any appreciable degradation interference under the stressed conditions tested. Observed degradation products peaks were well separated from the main peak.1® PDA-996 Waters™ ; 2[Millennium 2010]ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationFORCED DEGRADATION OF FINISHED PRODUCT & STANDARD 1.UNSTRESSED SAMPLE1.1.Sample IAG-21-001 (2) (20mg/capsule) was prepared as stated in SI-IAG-206-02 and injected into the HPLC system. The calculated assay is 98.5%.SAMPLE - UNSTRESSEDFluoxetine:Purity Angle:0.075Match Angle:0.407Purity Threshold:0.142Match Threshold:0.4251.2.Standard solution was prepared as stated in method SI-IAG-206-02 and injected into the HPLC system. The calculated assay is 100.0%.Fluoxetine:Purity Angle:0.078Match Angle:0.379Purity Threshold:0.146Match Threshold:0.4272.ACID HYDROLYSIS2.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as in method SI-IAG-206-02 : An amount equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent was added and the solution sonicated for 10 minutes. 1mL of conc. HCl was added to this solution The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with NaOH 10N, made up to volume with Diluent and injected into the HPLC system after filtration.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 98.8%.SAMPLE- ACID HYDROLYSISFluoxetine peak:Purity Angle:0.055Match Angle:0.143Purity Threshold:0.096Match Threshold:0.3712.2.Standard solution was prepared as in method SI-IAG-206-02 : about 22mg Fluoxetine HCl were weighed into a 50mL volumetric flask. 20mL Diluent were added. 2mL of conc. HCl were added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with NaOH 10N, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 97.2%.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSTANDARD - ACID HYDROLYSISFluoxetine peak:Purity Angle:0.060Match Angle:0.060Purity Threshold:0.099Match Threshold:0.3713.BASE HYDROLYSIS3.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as per method SI-IAG-206-02 : An amount equivalent to 20mg Fluoxetine was weight into a 50mL volumetric flask. 20mL Diluent was added and the solution sonicated for 10 minutes. 1mL of 5N NaOH was added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with 5N HCl, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 99.3%.SAMPLE - BASE HYDROLYSISFluoxetine peak:Purity Angle:0.063Match Angle:0.065Purity Threshold:0.099Match Threshold:0.3623.2.Standard stock solution was prepared as per method SI-IAG-206-02 : About 22mg Fluoxetine HCl was weighed into a 50mL volumetric flask. 20mL Diluent was added. 2mL of 5N NaOH was added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH=5.5 with 5N HCl, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease - 99.5%.STANDARD - BASE HYDROLYSISFluoxetine peak:Purity Angle:0.081Match Angle:0.096Purity Threshold:0.103Match Threshold:0.3634.OXIDATION4.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as per method SI-IAG-206-02. An equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent added and the solution sonicated for 10 minutes.1.0mL of 30% H2O2 was added to the solution and allowed to stand for 5 hours, then made up to volume with Diluent, filtered and injected into HPLC system.Fluoxetine peak intensity decreased to 95.2%.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSAMPLE - OXIDATIONFluoxetine peak:Purity Angle:0.090Match Angle:0.400Purity Threshold:0.154Match Threshold:0.4294.2.Standard solution was prepared as in method SI-IAG-206-02 : about 22mg Fluoxetine HCl were weighed into a 50mL volumetric flask and 25mL Diluent were added. 2mL of 30% H2O2 were added to this solution which was standing for 5 hours, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity decreased to 95.8%.STANDARD - OXIDATIONFluoxetine peak:Purity Angle:0.083Match Angle:0.416Purity Threshold:0.153Match Threshold:0.4295.SUNLIGHT5.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as in method SI-IAG-206-02 . The solution was exposed to 500w/hr. cell sunlight for 1hour. The BST was set to 35°C and the ACT was 45°C. The vials were placed in a horizontal position (4mm vials, National + Septum were used). A Dark control solution was tested. A 2%w/v quinine solution was used as the reference absorbance solution.Fluoxetine peak decreased to 91.2% and the dark control solution showed assay of 97.0%. The difference in the absorbance in the quinine solution is 0.4227AU.Additional peak was observed at RRT of 1.5 (2.7%).The total percent of Fluoxetine peak with the degradation peak is about 93.9%.SAMPLE - SUNLIGHTFluoxetine peak:Purity Angle:0.093Match Angle:0.583Purity Threshold:0.148Match Threshold:0.825 ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSUNLIGHT (Cont.)5.2.Working standard solution was prepared as in method SI-IAG-206-02 . The solution was exposed to 500w/hr. cell sunlight for 1.5 hour. The BST was set to 35°C and the ACT was 42°C. The vials were placed in a horizontal position (4mm vials, National + Septum were used). A Dark control solution was tested. A 2%w/v quinine solution was used as the reference absorbance solution.Fluoxetine peak was decreased to 95.2% and the dark control solution showed assay of 99.5%.The difference in the absorbance in the quinine solution is 0.4227AU.Additional peak were observed at RRT of 1.5 (2.3).The total percent of Fluoxetine peak with the degradation peak is about 97.5%. STANDARD - SUNLIGHTFluoxetine peak:Purity Angle:0.067Match Angle:0.389Purity Threshold:0.134Match Threshold:0.8196.HEAT OF SOLUTION6.1.Sample solution of IAG-21-001-(2) (20 mg/capsule) was prepared as in method SI-IAG-206-02 . Equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent was added and the solution was sonicated for 10 minutes and made up to volume with Diluent. 4mL solution was transferred into a suitable crucible, heated at 105°C in an oven for 2 hours. The sample was cooled to ambient temperature, filtered and injected into the HPLC system.Fluoxetine peak was decreased to 93.3%.SAMPLE - HEAT OF SOLUTION [105o C]Fluoxetine peak:Purity Angle:0.062Match Angle:0.460Purity Threshold:0.131Match Threshold:0.8186.2.Standard Working Solution (WS) was prepared under method SI-IAG-206-02 . 4mL of the working solution was transferred into a suitable crucible, placed in an oven at 105°C for 2 hours, cooled to ambient temperature and injected into the HPLC system.Fluoxetine peak intensity did not decrease - 100.5%.ED. N0: 04Effective Date:APPROVED::。
Enzyme immunoassay for antigen and solid phase use

专利名称:Enzyme immunoassay for antigen and solid phase used therefor发明人:Marui, Yoji,Hayashi, Chozo,Ito, Shigeki,Fujio, Mieko,Tanaka, Hiroki,Nagasaki,Toshihide,Soda, Yasuji,Kaneta, Hitoshi申请号:EP90112454.5申请日:19900629公开号:EP0405578A2公开日:19910102专利内容由知识产权出版社提供摘要:An enzyme immunoassay for an antigen using a solid phase, which employs (a) a biotinylated antibody in which a biotin derivative is bound to a thiol group of an Fab′fragment of an antibody, (b) an enzyme-labelled antibody, and (c) a solid phase immobilized with a substance capable of specifically reacting with the above biotin derivative selected from the group consisting of avidin, streptoavidin and a derivative thereof which is bound to the solid phase directly or via a linkage between another biotin derivative and a high molecular weight substance bound to the solid phase, or alternatively employs (a′) a solid phase immobilized with a biotinylated antibody via a substance selected from the group consisting of avidin, streptoavidin and a derivative thereof wherein said substance is bound to the biotin moiety of said biotinylated antibody and is also bound to the solid phase directly or via a linkage between another biotin derivative and a high molecular weight substance bound to the solid phase, and (b′) an enzyme-l abelled antibody, and a solid phase used therefor.申请人:NIPPON SHOJI KABUSHIKI KAISHA地址:No. 2-9, Kokumachi 2-chome Chuo-ku Osaka-shi Osaka-fu JP 国籍:JP代理机构:Hansen, Bernd, Dr. Dipl.-Chem.更多信息请下载全文后查看。
(完整word版)USP38通用章节目录中文

USP38-通用章节指导目录(附录)Guide to General Chapters 通用章节指导General Requirements for Test and Assays检查与含量分析的一般要求<1>INJECTIONS AND IMPLANTED DRUG PRODUCTS (PARENTERALS)—PRODUCT QUALITY TESTS 注射和植入药物产品(注射用) —产品质量测试<1>INJECTIONS注射剂<2>ORAL DRUG PRODUCTS—PRODUCT QUALITY TESTS 口服药物产品质量测试<3>TOPICAL AND TRANSDERMAL DRUG PRODUCTS—PRODUCT QUALITY TESTS 局部和透皮药物产品—产品质量测试<4>MUCOSAL DRUG PRODUCTS—PRODUCT QUALITY TESTS 粘膜药物产品质量测试<5>INHALATION AND NASAL DRUG PRODUCTS—GENERAL INFORMATION AND PRODUCT QUALITY TESTS 吸入剂产品—产品质量测试<7>LABELING 标签<11>USP REFERENCE STANDARDS USP标准品Apparatus for Test and Assays用于检查与含量分析的器具<17>PRESCRIPTION CONTAINER LABELING处方容器标签<21>THERMOMETERS温度计<31>VOLUMETRIC APPARATUS容量器具<41>BALANCES天平Microbiological Tests 微生物检查法<51>ANTIMICROBIAL EFFECTIVENESS TESTING抗菌剂有效性检查法<55>BIOLOGICAL INDICATORS—RESISTANCE PERFORMANCE TESTS生物指示剂-耐药性实验<61>MICROBIOLOGICAL EXAMINATION OF NONSTERILE PRODUCTS: MICROBIAL ENUMERATION TESTS非无菌产品的微生物限度检查:微生物列举检查法<62>MICROBIOLOGICAL EXAMINATION OF NONSTERILE PRODUCTS: TESTS FOR SPECIFIED MICROORGANISMS 非无菌产品的微生物限度检查:特定微生物检查法<63>MYCOPLASMA TESTS 支原体检查法<71>STERILITY TESTS无菌检查法Biological tests and assays生物检查法与测定法<81>ANTIBIOTICS—MICROBIAL ASSAYS抗生素-微生物测定<85>BACTERIAL ENDOTOXINS TEST细菌内毒素检查法<87>BIOLOGICAL REACTIVITY TESTS, IN VITRO体外的生物反应性检查法<88>BIOLOGICAL REACTIVITY TESTS, IN VIVO 体内的生物反应性检查法<89>ENZYMES USED AS ANCILLARY MATERIALS IN PHARMACEUTICAL MANUFACTURING 药品生产中酶作为辅料所使用<90>FETAL BOVINE SERUM—QUALITY ATTRIBUTES AND FUNCTIONALITY TESTS 牛胎儿血清-质量品质和功能检查法<91>CALCIUM PANTOTHENATE ASSAY泛酸钙测定法<92>GROWTH FACTORS AND CYTOKINES USED IN CELL THERAPY MANUFACTURING 在细胞疗法中使用生长因子和细胞因子<111>DESIGN AND ANALYSIS OF BIOLOGICAL ASSAYS 生物测定法的设计与分析<115>DEXPANTHENOL ASSAY右泛醇(拟胆碱药)测定法<121>INSULIN ASSAYS胰岛素测定法<121.1>PHYSICOCHEMICAL ANALYTICAL PROCEDURES FOR INSULINS胰岛素的物理化学分析程序<123>GLUCAGON BIOIDENTITY TESTS 高血糖素的生物鉴别检查法<124>ERYTHROPOIETIN BIOASSAYS 红细胞生成素的微生物测定<126>SOMATROPIN BIOIDENTITY TESTS 生长激素的生物鉴别检查法<130>PROTEIN A QUALITY ATTRIBUTES 蛋白质A的质量特征<151>PYROGEN TEST热原检查法<161>TRANSFUSION AND INFUSION ASSEMBLIES AND SIMILAR MEDICAL DEVICES 输血输液用具以及相类似的医疗器械<171>VITAMIN B12 ACTIVITY ASSAY……2548维生素B12活性测定法Chemical Tests and assays化学实验检查与测定法鉴别检查<181>IDENTIFICATION—ORGANIC NITROGENOUS BASES鉴别-有机氮碱化合物<191>IDENTIFICATION TESTS—GENERAL鉴别实验-通用<193>IDENTIFICATION—TETRACYCLINES鉴别-四环素类<197>SPECTROPHOTOMETRIC IDENTIFICATION TESTS分光光度计鉴别实验<201>THIN-LAYER CHROMATOGRAPHIC IDENTIFICATION TEST薄层色谱鉴别实验Limit Tests 限度检查法<206>ALUMINUM铝<207>TEST FOR 1,6-ANHYDRO DERIV ATIVE FOR ENOXAPARIN SODIUM依诺肝素钠的酐类衍生物实验<208>ANTI-FACTOR Xa AND ANTI-FACTOR IIa ASSAYS FOR UNFRACTIONATED AND LOW MOLECULAR WEIGHT HEPARINS普通肝素和低分子肝素产品中抗体Xa和抗体IIa测定<209>LOW MOLECULAR WEIGHT HEPARIN MOLECULAR WEIGHT DETERMINATIONS 低分子肝素钠分子量测定<211>ARSENIC砷<221>CHLORIDE AND SULFATE氯和硫<223>DIMETHYLANILINE二甲基苯胺<226>4-EPIANHYDRO-TETRACYCLINE4-?-四环素<227>4-AMINOPHENOL IN ACETAMINOPHEN-CONTAINING DRUG PRODUCTS 对乙酰氨酚药物产品中氨基酚<228>ETHYLENE OXIDE AND DIOXANE 环氧乙烷和二氧六环<231>HEA VY METALS重金属(删除)<232>ELEMENTAL IMPURITIES—LIMITS 元素杂质-限度<233>ELEMENTAL IMPURITIES—PROCEDURES 元素杂质-规程<241>IRON铁<251>LEAD铅<261>MERCURY汞<267>POROSIMETRY BY MERCURY INTRUSION 水银孔隙仪<268>POROSITY BY NITROGEN ADSORPTION–DESORPTION 氮吸附-解吸测定孔隙率<271>READILY CARBONIZABLE SUBSTANCES TEST易碳化物检查法<281>RESIDUE ON IGNITION炽灼残渣<291>SELENIUM硒Other Tests and Assays 其它检查法与测定法<301>ACID-NEUTRALIZING CAPACITY酸中和容量<311>ALGINATES ASSAY藻酸盐测定法<341>ANTIMICROBIAL AGENTS—CONTENT 抗菌剂-含量<345>Assay for Citric Acid/Citrate and Phosphate 柠檬酸/柠檬酸盐和磷酸盐的测定<351>ASSAY FOR STEROIDS类固醇(甾类化合物)测定法<361> BARBITURATE ASSAY 巴比妥类药物测定法<371>COBALAMIN RADIOTRACER ASSAY钴铵素放射性跟踪剂测定法<381>ELASTOMERIC CLOSURES FOR INJECTIONS 注射剂的弹性密封件<391>EPINEPHRINE ASSAY肾上腺素测定法<401>FATS AND FIXED OILS脂肪与混合油<411>FOLIC ACID ASSAY叶酸测定法<413>IMPURITIES TESTING IN MEDICAL GASES 医用气体杂质检查<415>MEDICAL GASES ASSAY 医用气体含量检查<425>IODOMETRIC ASSAY—ANTIBIOTICS碘量检查法-抗生素<429>LIGHT DIFFRACTION MEASUREMENT OF PARTICLE SIZE粒径的光衍射测量法<431>METHOXY DETERMINATION甲氧基测定法<441>NIACIN OR NIACINAMIDE ASSAY 烟酰或烟酰胺测定法<451>NITRITE TITRATION亚硝酸盐滴定<461>NITROGEN DETERMINATION氮测定法<466>ORDINARY IMPURITIES一般杂质<467>RESIDUAL SOLVENTS残留溶剂<469>ETHYLENE GLYCOL, DIETHYLENE GLYCOL, AND TRIETHYLENE GLYCOL IN ETHOXYLATED SUBSTANCES乙氧基物质中乙二醇、二甘醇、三甘醇测定<471>OXYGEN FLASK COMBUSTION氧瓶燃烧法<481>RIBOFLAVIN ASSAY核黄素(维生素B2)测定法<501>SALTS OF ORGANIC NITROGENOUS BASES有机氮盐<503>ACETIC ACID IN PEPTIDES 多肽类中乙酸测定<511>SINGLE-STEROID ASSAY单一的类固醇测定法<525>SULFUR DIOXIDE 二氧化硫<531>THIAMINE ASSAY硫胺素测定法<541>TITRIMETRY滴定法<551>VITAMIN E ASSAY维生素E测定法<561>ARTICLES OF BOTANICAL ORIGIN植物起源的药品<563>IDENTIFICATION OF ARTICLES OF BOTANICAL ORIGIN植物药品的鉴别<565>BOTANICAL EXTRACTS植物提取<571>VITAMIN A ASSAY维生素A测定法<581>VITAMIN D ASSAY维生素D测定法<591>ZINC DETERMINATION锌的测定法Physical Test and Determinations物理检查与测定法<601>INHALATION AND NASAL DRUG PRODUCTS: AEROSOLS, SPRAYS, AND POWDERS—PERFORMANCE QUALITY TESTS吸入剂、鼻雾剂:气溶胶,喷雾,干粉-质量通则<602>PROPELLANTS 推进剂<603>TOPICAL AEROSOLS 局部喷雾剂<604>LEAK RATE 渗漏率<610>ALTERNATIVE MICROBIOLOGICAL SAMPLING METHODS FOR NONSTERILE INHALED AND NASAL PRODUCTS非无菌吸入和鼻雾剂可供选择的微生物取样方法<611>ALCOHOL DETERMINATION乙醇测定法<616>BULK DENSITY AND TAPPED DENSITY堆密度与振实密度<621>CHROMATOGRAPHY色谱法<631>COLOR AND ACHROMICITY呈色与消色<641>COMPLETENESS OF SOLUTION溶解度<643>TOTAL ORGANIC CARBON总有机碳<645>W ATER CONDUCTIVITY水电导率<651>CONGEALING TEMPERATURE凝点温度<659>PACKAGING AND STORAGE REQUIREMENTS 包装和储藏要求<660>CONTAINERS—GLASS 容器-玻璃<661>CONTAINERS—PLASTICS容器-塑料<670>AUXILIARY PACKAGING COMPONENTS 辅助包装部件<671>CONTAINERS—PERFORMANCE TESTING容器-性能测试<691>COTTON棉花<695>CRYSTALLINITY结晶度<696>CHARACTERIZATION OF CRYSTALLINE SOLIDS BY MICROCALORIMETRY AND SOLUTION CALORIMETRY 通过溶液量热学测定结晶性<697>CONTAINER CONTENT FOR INJECTIONS 注射剂容器容积<698>DELIVERABLE VOLUME抽取体积<699>DENSITY OF SOLIDS固体密度<701>DISINTEGRATION崩解时限<705>QUALITY ATTRIBUTES OF TABLETS LABELED AS HA VING A FUNCTIONAL SCORE ?<711>DISSOLUTION 溶出度<721>DISTILLING RANGE馏程<724>DRUG RELEASE药物释放度<729>GLOBULE SIZE DISTRIBUTION IN LIPID INJECTABLE EMULSIONS脂类可注射的乳剂的粒径分布<730>Plasma Spectrochemistry 血浆光谱化学?<731>LOSS ON DRYING4干燥失重<733>LOSS ON IGNITION灼烧失重<735>X-RAY FLUORESCENCE SPECTROMETRY X射线光谱<736>MASS SPECTROMETRY 质谱<741>MELTING RANGE OR TEMPERATURE熔距或熔点<751>METAL PARTICLES IN OPHTHALMIC OINTMENTS眼用软膏中的金属粒子<755>MINIMUM FILL最低装量<761>NUCLEAR MAGNETIC RESONANCE核磁共振<771>OPHTHALMIC OINTMENTS眼用软膏<776>OPTICAL MICROSCOPY光学显微镜<781>OPTICAL ROTATION旋光度<785>OSMOLALITY AND OSMOLARITY渗透压<786>PARTICLE SIZE DISTRIBUTION ESTIMATION BY ANALYTICAL SIEVING 筛分法估算粒径分布<787>SUBVISIBLE PARTICULATE MATTER IN THERAPEUTIC PROTEIN INJECTIONS显微计数法在治疗性蛋白注射剂中应用<788>PARTICULATE MATTER IN INJECTIONS注射剂中的不溶性微粒<789>PARTICULATE MATTER IN OPHTHALMIC SOLUTIONS眼用溶液中的不溶性微粒<790>VISIBLE PARTICULATES IN INJECTIONS 注射剂中可见异物<791>pH<795>PHARMACEUTICAL COMPOUNDING—NONSTERILE PREPARATIONS药物混合-非无菌制剂<797>PHARMACEUTICAL COMPOUNDING—STERILE PREPARATIONS药物混合-无菌制剂<801>POLAROGRAPHY极谱法<811>POWDER FINENESS粉剂细度<821>RADIOACTIVITY放射性<823>POSITRON EMISSION TOMOGRAPHY DRUGS FOR COMPOUNDING, INVESTIGATIONAL, AND RESEARCH USES用于正电子发射断层造影术的放射性药物<831>REFRACTIVE INDEX折光率<841>SPECIFIC GRAVITY比重<846>SPECIFIC SURFACE AREA 比表面积<851>SPECTROPHOTOMETRY AND LIGHT-SCATTERING分光光度计与光散射<852>ATOMIC ABSORPTION SPECTROSCOPY 原子吸收光谱<853>FLUORESCENCE SPECTROSCOPY 荧光光谱<854>MID-INFRARED SPECTROSCOPY 中红外光谱<857>ULTRAVIOLET-VISIBLE SPECTROSCOPY 紫外可见光谱<861>SUTURES—DIAMETER缝线-直径?<871>SUTURES—NEEDLE ATTACHMENT缝线-穿孔实验<881>TENSILE STRENGTH张力<891>THERMAL ANALYSIS热分析<905>UNIFORMITY OF DOSAGE UNITS制剂单位的含量均匀度<911>VISCOSITY—CAPILLARY METHODS黏度-毛细管法<912>VISCOSITY—ROTATIONAL METHODS 黏度-旋转法<913>VISCOSITY—ROLLING BALL METHOD 黏度-球法<921>W ATER DETERMINATION水分测定<941>CHARACTERIZATION OF CRYSTALLINE AND PARTIALLY CRYSTALLINE SOLIDS BY X-RAY POWDER DIFFRACTION (XRPD)X光衍射General Information通用信息<1005>ACOUSTIC EMISSION 声频发射<1010>ANALYTICAL DATA—INTERPRETATION AND TREATMENT分析数据-解释与处理<1015>AUTOMATED RADIOCHEMICAL SYNTHESIS APPARATUS放射性自动合成装置<1024>BOVINE SERUM 牛血清<1027>FLOW CYTOMETRY 流式细胞仪<1030>BIOLOGICAL ASSAY CHAPTERS—OVERVIEW AND GLOSSARY生物测定章节-综述和术语<1031>THE BIOCOMPATIBILITY OF MATERIALS USED IN DRUG CONTAINERS, MEDICAL DEVICES, AND IMPLANTS用于药物容器、医疗设施和植入剂的材料的生物相容性<1034>ANALYSIS OF BIOLOGICAL ASSAYS 生物测定分析<1035>BIOLOGICAL INDICATORS FOR STERILIZATION灭菌用生物指示剂<1041>BIOLOGICS生物制剂<1043>Ancillary Material for Cell, Gene, and Tissue-Engineered Products细胞,基因与组织设计产品的辅助材料<1044>CRYOPRESERV ATION OF CELLS 细胞低温保存<1045>BIOTECHNOLOGY-DERIVED ARTICLES生物技术提取产品<1046>CELLULAR AND TISSUE-BASED PRODUCTS细胞与组织产品<1047>GENE THERAPY PRODUCTS 基因治疗产品<1048>QUALITY OF BIOTECHNOLOGICAL PRODUCTS: ANALYSIS OF THE EXPRESSION CONSTRUCT IN CELLS USED FOR PRODUCTION OF r-DNA DERIVED PROTEIN PRODUCTS 生物技术产品的质量:从蛋白质产品中提取的r-DNA产品在细胞中表达结构的分析<1049>QUALITY OF BIOTECHNOLOGICAL PRODUCTS: STABILITY TESTING OF BIOTECHNOLOGICAL/BIOLOGICAL PRODUCTS生物技术产品的质量:生物技术/生物产品的稳定性实验<1050>VIRAL SAFETY EV ALUATION OF BIOTECHNOLOGY PRODUCTS DERIVED FROM CELL LINES OF HUMAN OR ANIMAL ORIGIN从人或动物细胞中提取的生物技术产品的病毒安全性评估<1051>CLEANING GLASS APPARATUS玻璃容器的清洗<1052>BIOTECHNOLOGY-DERIVED ARTICLES—AMINO ACID ANALYSIS生物技术提取法-氨基酸测定<1053>CAPILLARY ELECTROPHORESIS 毛细管电泳法<1054>BIOTECHNOLOGY-DERIVED ARTICLES—ISOELECTRIC FOCUSING生物技术提取法-等电点聚集<1055>BIOTECHNOLOGY-DERIVED ARTICLES—PEPTIDE MAPPING生物技术提取法-肽谱<1056>BIOTECHNOLOGY-DERIVED ARTICLES—POLYACRYLAMIDE GEL ELECTROPHORESIS 生物技术提取法-凝胶电泳<1057>BIOTECHNOLOGY-DERIVED ARTICLES—TOTAL PROTEIN ASSAY生物技术提取法-总蛋白测定<1058>ANALYTICAL INSTRUMENT QUALIFICATION 分析仪器要求<1059>EXCIPIENT PERFORMANCE 赋形剂<1061>COLOR—INSTRUMENTAL MEASUREMENT显色-仪器测量<1065>Ion Chromatography 离子色谱法<1066>PHYSICAL ENVIRONMENTS THAT PROMOTE SAFE MEDICATION USE物理环境促使安全使用药物<1072>DISINFECTANTS AND ANTISEPTICS 消毒剂和防腐剂<1074>EXCIPIENT BIOLOGICAL SAFETY EV ALUATION GUIDELINES赋形剂(辅料)生物安全性评估指导<1078>GOOD MANUFACTURING PRACTICES FOR BULK PHARMACEUTICAL EXCIPIENTS 批药品赋形剂的生产管理规范<1079>Good Storage and Shipping Practices 良好的贮存与运输规范<1080>BULK PHARMACEUTICAL EXCIPIENTS—CERTIFICATE OF ANALYSIS批药品赋形剂-COA<1084>GLYCOPROTEIN AND GLYCAN ANALYSIS—GENERAL CONSIDERATIONS 糖蛋白和多糖分析-一般通则<1086>IMPURITIES IN DRUG SUBSTANCES AND DRUG PRODUCTS药物和药物产品中的杂质<1087>APPARENT INTRINSIC DISSOLUTION—DISSOLUTION TESTING PROCEDURES FOR ROTATING DISK AND STATIONARY DISK内部的溶出度-旋转和静止溶出检测程序?<1088>IN VITRO AND IN VIVO EV ALUATION OF DOSAGE FORMS体内与体外的剂型的评估<1090>ASSESSMENT OF DRUG PRODUCT PERFORMANCE-BIOAV AILABILITY, BIOEQUIV ALENCE, AND DISSOLUTION药物产品性能评估:生物利用度、生物等效性和溶出<1091>LABELING OF INACTIVE INGREDIENTS非活性成分的标示<1092>THE DISSOLUTION PROCEDURE: DEVELOPMENT AND V ALIDATION溶出程序:开发与验证<1094>CAPSULES—DISSOLUTION TESTING AND RELATED QUALITY ATTRIBUTES 胶囊-关于产品质量的溶出测定<1097>BULK POWDER SAMPLING PROCEDURES:粉末样品取样程序<1102>IMMUNOLOGICAL TEST METHODS—GENERAL CONSIDERATIONS免疫测试方法-总则<1103>IMMUNOLOGICAL TEST METHODS—ENZYME-LINKED IMMUNOSORBENT ASSAY (ELISA) 免疫学测试方法-酶联免疫吸附测定<1104>IMMUNOLOGICAL TEST METHODS—IMMUNOBLOT ANALYSIS免疫测试方法-免疫印迹法<1105>IMMUNOLOGICAL TEST METHODS—SURFACE PLASMON RESONANCE 免疫测试方法-表面等离子体共振<1106>IMMUNOGENICITY ASSAYS—DESIGN AND VALIDATION OF IMMUNOASSAYS TO DETECT ANTI-DRUG ANTIBODIES?<1111>MICROBIOLOGICAL EXAMINATION OF NONSTERILE PRODUCTS: ACCEPTANCE CRITERIA FOR PHARMACEUTICAL PREPARATIONS AND SUBSTANCES FOR PHARMACEUTICAL USE非无菌产品的微生物学检查:药用制剂和制药过程使用的物质接受标准<1112>MICROBIAL CHARACTERIZATION, IDENTIFICATION, AND STRAIN TYPING 非无菌药物产品水活性测定应用<1113>MICROBIOLOGICAL ATTRIBUTES OF NONSTERILE PHARMACEUTICAL PRODUCTS 非无菌药品中的微生物分布<1115>BIOBURDEN CONTROL OF NONSTERILE DRUG SUBSTANCES AND PRODUCTS 非无菌药物和产品的生物负载控制<1116>MICROBIOLOGICAL CONTROL AND MONITORING OF ASEPTIC PROCESSING ENVIRONMENTS洁净的房间与其它可控环境的微生物评估<1117>MICROBIOLOGICAL BEST LABORATORY PRACTICES 微生物最优实验室规范<1118>MONITORING DEVICES—TIME, TEMPERATURE, AND HUMIDITY监控装置-时间、温度与湿度<1119>NEAR-INFRARED SPECTROPHOTOMETRY近红外分光光度测定法<1120>Raman Spectrophotometry 拉曼分光光度测定法<1121>NOMENCLATURE命名<1125>NUCLEIC ACID-BASED TECHNIQUES—GENERAL 核酸技术-通则<1126>NUCLEIC ACID-BASED TECHNIQUES—EXTRACTION, DETECTION, AND SEQUENCING 核酸技术-提取、检测、测序<1127>NUCLEIC ACID-BASED TECHNIQUES—AMPLIFICATION 核酸技术-扩增<1128>NUCLEIC ACID-BASED TECHNIQUES—MICROARRAY 核酸技术-微阵列<1129>NUCLEIC ACID-BASED TECHNIQUES—GENOTYPING 核酸技术-基因分型<1130>NUCLEIC ACID-BASED TECHNIQUES—APPROACHES FOR DETECTING TRACE NUCLEIC ACIDS (RESIDUAL DNA TESTING)核酸技术-探测微量核酸的应用(残留DNA测试)<1136>PACKAGING AND REPACKAGING—SINGLE-UNIT CONTAINERS包装和再包装-单一容器<1151>PHARMACEUTICAL DOSAGE FORMS药物剂型<1152>ANIMAL DRUGS FOR USE IN ANIMAL FEEDS兽药在动物饲料中的使用<1160>PHARMACEUTICAL CALCULATIONS IN PRESCRIPTION COMPOUNDING 按处方混合的药物的计算<1163>QUALITY ASSURANCE IN PHARMACEUTICAL COMPOUNDING按处方混合的药物的质量保证<1171>PHASE-SOLUBILITY ANALYSIS相溶解分析<1174>Powder Flow 粉末流动性<1176>PRESCRIPTION BALANCES AND VOLUMETRIC APPARATUS 处方天平与容量器具<1177>Good Packaging Practices 良好的包装操作<1178>Good Repackaging Practices 良好的再包装操作<1180>HUMAN PLASMA 人血浆<1181>SCANNING ELECTRON MICROSCOPY扫描电子显微镜<1184>SENSITIZATION TESTING 致敏测试<1191>STABILITY CONSIDERATIONS IN DISPENSING PRACTICE分装操作中稳定性考察<1195>SIGNIFICANT CHANGE GUIDE FOR BULK PHARMACEUTICAL EXCIPIENTS 散装药用辅料更换指导原则<1197>GOOD DISTRIBUTION PRACTICES FOR BULK PHARMACEUTICAL EXCIPIENTS 散装药用辅料良好的分装操作<1207>STERILE PRODUCT PACKAGING—INTEGRITY EV ALUATION无菌产品包装-完整性评估<1208>STERILITY TESTING—V ALIDATION OF ISOLATOR SYSTEMS无菌实验-隔离系统的验证<1209>STERILIZATION—CHEMICAL AND PHYSICOCHEMICAL INDICATORS AND INTEGRATORS灭菌-化学与物理化学的指示剂以及二者的综合<1211>STERILIZATION AND STERILITY ASSURANCE OF COMPENDIAL ARTICLES 药典物品中的灭菌与灭菌保证<1216>TABLET FRIABILITY片剂的脆碎度<1217>TABLET BREAKING FORCE 片剂断裂力<1222>TERMINALLY STERILIZED PHARMACEUTICAL PRODUCTS—PARAMETRIC RELEASE 药品终端灭菌-放行参数<1223>V ALIDATION OF ALTERNATIVE MICROBIOLOGICAL METHODS可供选择的微生物学方法的验证<1224>TRANSFER OF ANALYTICAL PROCEDURES 分析方法转移<1225>V ALIDATION OF COMPENDIAL PROCEDURES药典方法的验证<1226>VERIFICATION OF COMPENDIAL PROCEDURES 药典方法的确认<1227>V ALIDATION OF MICROBIAL RECOVERY FROM PHARMACOPEIAL ARTICLES从药物中回收微生物的验证<1229>STERILIZATION OF COMPENDIAL ARTICLES 药典灭菌过程<1229.1>STEAM STERILIZATION BY DIRECT CONTACT 直接蒸汽灭菌<1229.2>MOIST HEAT STERILIZATION OF AQUEOUS LIQUIDS 水溶液的湿热灭菌<1229.3>MONITORING OF BIOBURDEN 生物负载监控<1229.4>STERILIZING FILTRATION OF LIQUIDS 溶液的无菌过滤器<1229.6>LIQUID-PHASE STERILIZATION 液态灭菌<1229.7>GASEOUS STERILIZATION 气态灭菌<1229.8>DRY HEAT STERILIZATION 干热灭菌<1229.10>RADIATION STERILIZATION 辐射灭菌<1230>W ATER FOR HEMODIALYSIS APPLICATIONS 血液透析过程用水<1231>W ATER FOR PHARMACEUTICAL PURPOSES制药用水<1234>VACCINES FOR HUMAN USE—POLYSACCHARIDE AND GLYCOCONJUGATE VACCINES 人用疫苗-多糖和糖复合物疫苗<1235>V ACCINES FOR HUMAN USE—GENERAL CONSIDERATIONS 人用疫苗-通则<1237>VIROLOGY TEST METHODS 病毒测试方法<1238>V ACCINES FOR HUMAN USE—BACTERIAL V ACCINES 人用疫苗-细菌疫苗<1240>VIRUS TESTING OF HUMAN PLASMA FOR FURTHER MANUFACTURE下一步使用人血浆的病毒测试<1241>W ATER–SOLID INTERACTIONS IN PHARMACEUTICAL SYSTEMS在药物系统中水与固体的相互作用<1251>WEIGHING ON AN ANALYTICAL BALANCE关于分析天平的称重<1265>Written Prescription Drug Information-Guidelines 书面的处方药信息-指南<1285>PREPARATION OF BIOLOGICAL SPECIMENS FOR HISTOLOGIC AND IMMUNOHISTOCHEMICAL ANALYSIS为了组织和免疫组织分析的生物标本制备<1285.1>HEMATOXYLIN AND EOSIN STAINING OF SECTIONED TISSUE FOR MICROSCOPIC EXAMINATION显微镜观察用苏木精和伊红染色的切片<1601>PRODUCTS FOR NEBULIZATION—CHARACTERIZATION TESTS 产品雾化状态-性状描述<1644>THEORY AND PRACTICE OF ELECTRICAL CONDUCTIVITY MEASUREMENTS OF SOLUTIONS溶液电导值测量方法的理论与实践<1660>EV ALUATION OF THE INNER SURFACE DURABILITY OF GLASS CONTAINERS 玻璃容器内表面耐久性评估<1724>SEMISOLID DRUG PRODUCTS—PERFORMANCE TESTS 半固态药物产品-性能测试<1736>APPLICATIONS OF MASS SPECTROMETRY 质谱应用<1761>APPLICATIONS OF NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY核磁共振光谱应用<1787>MEASUREMENT OF SUBVISIBLE PARTICULATE MATTER IN THERAPEUTIC PROTEIN INJECTIONS用显微镜测量方法测量治疗性蛋白注射剂的不溶性微粒<1788>METHODS FOR THE DETERMINATION OF PARTICULATE MATTER IN INJECTIONS AND OPHTHALMIC SOLUTIONS注射剂和眼用溶液的不溶性微粒测定的方法选择<1852>ATOMIC ABSORPTION SPECTROSCOPY—THEORY AND PRACTICE原子吸收光谱-理论与实践<1853>FLUORESCENCE SPECTROSCOPY—THEORY AND PRACTICE荧光光谱-理论与实践<1854>MID-INFRARED SPECTROSCOPY—THEORY AND PRACTICE中红外光谱-理论与实践<1857>ULTRA VIOLET-VISIBLE SPECTROSCOPY—THEORY AND PRACTICE紫外可见光谱-理论与实践<1911>RHEOMETRY 流变测定Dietary Supplements营养补充剂General Tests and Assays 一般检查法与测定法<2021>MICROBIAL ENUMERATION TESTS—NUTRITIONAL AND DIETARY SUPPLEMENTS…3080微生物数量实验-营养与食品添加剂<2022>MICROBIOLOGICAL PROCEDURES FOR ABSENCE OF SPECIFIED MICROORGANISMS—NUTRITIONAL AND DIETARY SUPPLEMENTS (3083)不得检出特定微生物的程序-营养与营养补充剂<2023>MICROBIOLOGICAL ATTRIBUTES OF NONSTERILE NUTRITIONAL AND DIETARY SUPPLEMENTS……3087非无菌的营养与食品添加剂中的微生物分布<2040>DISINTEGRATION AND DISSOLUTION OF DIETARY SUPPLEMENTS (3089)食品添加剂的崩解与溶出<2091>WEIGHT V ARIATION OF DIETARY SUPPLEMENTS……3092食品添加剂的重量差异<2750>MANUFACTURING PRACTICES FOR DIETARY SUPPLEMENTS (3093)食品添加剂的生产操作。
苯环喹溴铵药理毒理研究进展

苯环喹溴铵药理毒理研究进展刘红1王宝辉1王宇1张俊毅1王祥艳1孙艾楠1程颜彬1赵李宏2(1白求恩医科大学制药厂,吉林长春130012;2吉林省成大方圆医药连锁有限公司,吉林长春130041)【摘要】变应性鼻炎和慢性阻塞性肺病是当前两种发病率高、发病人群广泛、病程缠绵且危害显著的呼吸系统疾病。
作为有针对性的新一代治疗药物,苯环喹溴铵是我国自主研发的具有自主知识产权的国家一类新药,具有广阔的应用前景。
【关键词】苯环喹溴铵;药理;毒理;研究进展Research progress of pharmacological effects and toxicological informationof bencycloquidium bromideLIU Hong1 WANG Bao-hui1WANG Y u1 ZHANG Jun-yi1 W ANG Xiang-yan1 SUN Ai-nan1CHENG Y an-bin1 ZHAO Li-hong2(1 Pharmaceutical Factory Norman Bethune University of Medical Science,Jilin Changchun 130012;2 Cheng DaFang Y uan Pharmaceutical Co,Ltd of Jilin Province,Jilin Changchun 130041)【Abtract】At present, allergic rhinitis and chronic obstructive pulmonary disease are the two kinds of respiratory diseases, with the features of high incidence、widespread population incidence、long course and remarkable harm. As a new generation of targeted therapeutic drug, bencycloquidium bromide is an independent intellectual property rights and national class of drug that would get broad application prospect. 【KeyWords】bencycloquidium bromide;pharmacological effects;toxicological information;research progress变应性鼻炎(allergic rhinitis,AR)又称为过敏性鼻炎,是特应性个体接触致敏源后导致的,包含IgE介导的炎症介质释放和多种免疫活性细胞因子、细胞因子参与的鼻黏膜慢性反应性疾病。
抗体公司

赛信通(上海)生物试剂有限公司
上海市浦东南路1101号远东大厦514室,200120 info@cst www.cst 2158356288 公司总部: 美国
Established in Beverly, MA in 1999, Cell Signaling Technology (CST) is a privatelyowned company with over 400 employees worldwide. We are dedicated to providing innovative research tools that are used to help define mechanisms underlying cell function and disease. Since its inception, CST has become the world leader in the production of the highest quality activationstate and total protein antibodies utilized to expand knowledge of cell signaling pathways. Our mission is to deliver the world's highest quality research tools that accelerate progress in biological research and personalized medicine. 总引用数为4670,来自于1966篇文章。最常引用的试剂包括: Akt, ERK2, ERK1, p38, Akt1。
AbD Serotec (BioRad)
GC测定盐酸普拉克索中三乙胺残留量

高效液相色谱法测定注射用美罗培南的有关物质

第35卷第3期 长治医学院学报2021 年 6 月JOURNAL OF CHANGZHI MEDICAI COLLEGE167Vol. 35 No. 3Jun. 2021高效液相色谱法测定注射用美罗培南的有关物质李金格禹玉洪**作者单位山西医科大学药学院药剂教研室(030001)* 通信作者(E-mail :3024546064@ qq. com)摘要目的:探讨优化注射用美罗培南杂质A 、B 的测定方法。
方法:运用高效液相色谱法(HPLC) 进行检测,色谱柱以十八烷基硅烷键合硅胶为填充剂;流动相A :20. 0 mmol-L'1磷酸二氢钠-甲醇(89 :11, V/V),流动相B :甲醇,流速1.0 mL-min 1,检测波长220 nm,柱温30 P 。
结果:主成分峰与杂质峰可实现基线分离,杂质A 检测限和定量限分别为1. 62,5.15 ng,杂质B 检测限和定量限分别为0. 85,2. 51 ng ;1.2~24.0 ixg-mL -1的杂质A 具有良好的线性关系(r=0. 999 9) ,0.7-14.0 ixg-mL 1的杂质B 具有良好的线性 关系(r=0. 999 9);杂质A 平均加样回收率为101.2%(RSD= 1.38%,“ = 9),杂质B 平均加样回收率为100.2%(RSD=1.29%,n = 9)o 经破坏性试验,美罗培南可能的降解杂质A 、B 均不干扰美罗培南主峰的测定。
结论:检测限及定量限、精密度、稳定性、耐用性试验结果均符合HPLC 有关物质测定的方法学验证要求。
本HPLC 法专属性良好,可用于美罗培南的主要杂质A 、B 的定量控制。
关键词美罗培南;有关物质;高效液相色谱法中图分类号R97&1文献标识码 A 文章编号1006(2021)03-167-05Determination of Related Substances of Meropenem for Injection by High Performance Liquid ChromatographyLI Jinge , YU YuhongDepartment of Pharmacy , School of Pharmacy , Shanxi Medical UniversityAbstract Objective : To explore and optimize the determination method of impulity A andB of meropenem for injection. Meth ods :Using the high performance liquid chromatography ( HPLC ) to detection , Octadecylsilane-bonded silica gel was used as the fi ler ; The mobile phase A : 20. 0 mmol * L -1 sodium dihydrogen phosphate-methanol ( 89 : 11, V/V) . The mobile phase B : methanol ,the flow rate was 1. 0 mL *m in _1 and the detection wavelength was set at 220 nm. The column temperature was set at 30 % . Re sults :The principal component peak and impurity peak could achieve baseline separation. The detection limit and quantitative limit of impurity A were 1. 62 ng and 5. 15 ng respectively , and the detection limit and quantitative lim 让 of impurity B were 0. 85 ng and 2. 51 ng respectively. There was A good linear relationship between impurity A (r = 0. 999 9) and impurity B ( r= 0. 999 9 ) in therange of 1. 2-24. 0 |xg *m L _1 and 0. 7 ~ 14. 0 jig * mL -1. The average recovery of impurity A was 101. 2% ( RSD = 1. 38% , n = 9),and that of impurity B was 100. 2% ( RSD = 1. 29% , n= 9). After stressing test, both of impurities A and B of meropenem didn * tinterfere w 让h the determination of meropenem main peak. Conclusion : The test results of detection lim 让 and quant N ative lim 让,pre cision ,stabil 让y and durability all meet the methodological verification requirements of HPLC related substance determination. The HPLC method has good specificity and can be used for the quant N ative control of major impurities A and B.Key words meropenem ; related substances ; HPLC注射用美罗培南(Meropenem, C ”H25弘0申) 是由日本住友制药公司与英国ICI 制药公司共同 开发的第二代碳青霉烯抗生素,通过干扰细菌细胞壁的合成发挥杀菌作用,具有广谱耐酶的特 点[1_4]o 在美罗培南原料中常检测出杂质A 及杂质B,杂质A(C 17H 27N 3O 6S)为美罗培南四元内酰 胺环结构发生水解反应而形成,系美罗培南的降 解产物;杂质B(C 34H 50N 6O 10S 2)为美罗培南与杂质A 发生聚合反应而形成,系美罗培南的二聚体X 。
化学仿制药参比制剂目录(第四十四批)(征求意见稿)
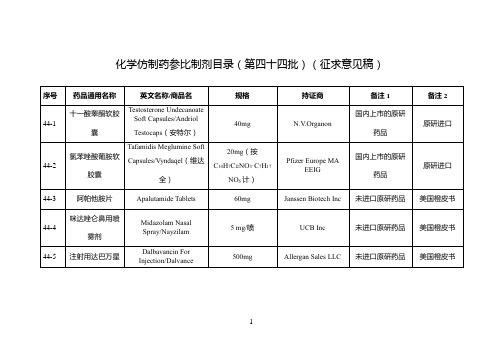
Chiesi Farmaceutici S.p.A.
未进口原研药品
欧盟上市
44-12
伊拉地平缓释胶囊
Isradipine Sustained-release Capsules/Vascal
2.5mg
Cheplapharm Arzneimittel GmbH
未进口原研药品
欧盟上市
44-13
伊拉地平缓释胶囊
未进口原研药品
美国橙皮书
44-33
西洛他唑片
Cilostazol Tablets / Pletal
50mg
Teva Pharmaceuticals USA Inc
国际公认的同种药品
美国橙皮书
44-34
格列吡嗪片
Glipizide Tablets/Mindiab
5mg
Pfizer Italia s.r.l.
20ml:0.5g
Hospira Australia Pty Ltd
国内上市的原研药品
原研进口
44-24
ω-3鱼油脂肪乳注射液
ω-3 Fish Oil Fat Emulsion Injection/Omegaven
100ml:10g(精制鱼油):1.2g(卵磷脂)
Fresenius Kabi Austria GmbH
2mg/ml(40ml、100ml)
Sanofi
未进口原研产品
欧盟上市
44-44
苯丁酸甘油酯口服液
Glycerol phenylbutyrate oral liquid/Ravicti
1.1g/ml
Immedica Pharma AB
未进口原研药品
欧盟上市
世界卫生组织儿童标准处方集

WHO Model Formulary for ChildrenBased on the Second Model List of Essential Medicines for Children 2009世界卫生组织儿童标准处方集基于2009年儿童基本用药的第二个标准目录WHO Library Cataloguing-in-Publication Data:WHO model formulary for children 2010.Based on the second model list of essential medicines for children 2009.1.Essential drugs.2.Formularies.3.Pharmaceutical preparations.4.Child.5.Drug utilization. I.World Health Organization.ISBN 978 92 4 159932 0 (NLM classification: QV 55)世界卫生组织实验室出版数据目录:世界卫生组织儿童标准处方集基于2009年儿童基本用药的第二个标准处方集1.基本药物 2.处方一览表 3.药品制备 4儿童 5.药物ISBN 978 92 4 159932 0 (美国国立医学图书馆分类:QV55)World Health Organization 2010All rights reserved. Publications of the World Health Organization can be obtained fromWHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: ******************). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the aboveaddress(fax:+41227914806;e-mail:*******************).世界卫生组织2010版权所有。
人血白蛋白_规格_解释说明以及概述

人血白蛋白规格解释说明以及概述1. 引言1.1 概述人血白蛋白是一种重要的血浆蛋白,具有多种生理功能和广泛的应用领域。
它是人体内含量最丰富的血浆蛋白,占据了总血浆蛋白含量的60-70%。
人血白蛋白的结构稳定、溶解性强,并且具有较长的半衰期,使其在医学领域具有广泛用途。
由于其重要性和特殊性质,人血白蛋白一直受到科学家和医护人员的高度关注。
1.2 文章结构本文首先会介绍人血白蛋白的定义和简介,包括其基本特征以及生理功能等方面。
接着会对目前已知的人血白蛋白规格和分类进行综述分析。
在解释说明部分,则会详细阐述人血白蛋白的来源与制备方法,以及其物理化学性质,并探讨其在应用领域和临床上的意义。
最后,在结论与展望部分将对主要观点进行总结,并展望未来研究方向。
1.3 目的本文的目的是全面介绍和解释人血白蛋白及其相关知识,让读者对人血白蛋白有更深入的了解。
通过对人血白蛋白生理功能、规格以及应用领域的系统概述,旨在提供一份权威和全面的参考资料,为科学家、医务人员和研究者进一步开展相关研究提供便利,并促进该领域的发展与进步。
2. 人血白蛋白2.1 定义和简介人血白蛋白是一种重要的生物分子,在人体中存在于血液中。
它是一种由肝脏合成的蛋白质,属于血浆蛋白家族。
人血白蛋白在维持人体内正常生理功能方面起着重要作用。
2.2 生理功能人血白蛋白具有多种生理功能。
首先,它在维持渗透压平衡方面发挥关键作用。
通过控制体内水分分布,人血白蛋白能够防止水分从循环系统渗出到周围组织中,同时还能保持细胞的正常结构和功能。
此外,人血白蛋白参与运输许多重要的物质和代谢产物,如药物、激素、矿物质等。
它还可以提供重要的营养源,并起到解毒和抗氧化的作用。
2.3 规格和分类根据不同的规格和分类方法,人血白蛋白可以被细分为不同类型。
常见的分类方法包括纯度、来源以及规格等方面。
纯度较高的人血白蛋白可以用于制备药物,而一般纯度的人血白蛋白则可供临床使用。
来源方面,主要有血浆来源和基因工程来源的人血白蛋白。
A new antibiotic kills pathogens without detectable resistance

factors through the chambers enables growth of uncultured bacteria in their natural environment. The growth recovery by this method approaches 50%, as compared to 1% of cells from soil that will grow on a nutrient Petri dish10. Once a colony is produced, a substantial number of uncultured isolates are able to grow in vitro14. Extracts from 10,000 isolates obtained by growth in iChips were screened for antimicrobial activity on plates overlaid with S. aureus. An extract from a new species of b-proteobacteria provisionally named Eleftheria terrae showed good activity. The genome of E. terrae was sequenced (Supplementary Discussion). Based on 16S rDNA and in silico DNA/DNA hybridization, this organism belongs to a new genus related to Aquabacteria (Extended Data Fig. 2, Supplementary Discussion). This group of Gram-negative organisms is not known to produce antibiotics. A partially purified active fraction contained a compound with a molecular mass of 1,242 Da determined by mass spectrometry, which was not reported in available databases. The compound was isolated and a complete stereochemical assignment has been made based on NMR and advanced Marfey’s analysis (Fig. 1, Extended Data Figs 3 and 4 and Supplementary Discussion). This molecule, which we named teixobactin, is an unusual depsipeptide which contains enduracididine, methylphenylalanine, and four D-amino acids. The biosynthetic gene cluster (GenBank accession number KP006601) was identified using a homology search (Supplementary Discussion). It consists of two large non-ribosomal peptide synthetase (NRPS)-coding genes, which we named txo1 and txo2, respectively (Fig. 1). In accordance with the co-linearity rule, 11 modules are encoded. The in silico predicted adenylation domain specificity perfectly matches the amino acid order of teixobactin (Fig. 1), and allowed us to predict the biosynthetic pathway (Extended Data Fig. 5).
仿制药参比制剂目录(第六十三批)
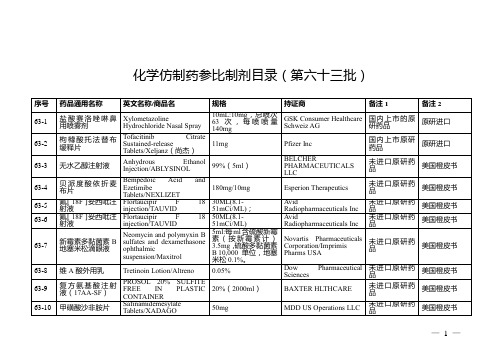
欧盟上市
63-27
匹伐他汀钙片
Pitavastatin Caical Europe GmbH
未进口原研药品
欧盟上市
63-28
匹伐他汀钙片
Pitavastatin Calcium Tablets
2mg
Kowa Pharmaceutical Europe GmbH
未进口原研药品
增加持证商Angelini Pharma Česká Republika s.r.o.
27-423
左甲状腺素钠片
Levothyroxine Sodium Tablets/Euthyrox;Levothyrox
100μg(以左甲状腺素钠计)
Merck Serono GmbH/Merck Sante/Merck GesellschaftmbH/Merck Healthcare Germany GmbH
100ml:1g(10mg/ml)
B Braunmedical Inc
未进口原研药品
美国橙皮书
63-14
钆特醇注射液
Gadoteridol Injection
/ProHance
279.3mg/mL(1.3965 g/5mL)
Bracco Diagnostics Inc
未进口原研药品
美国橙皮书
63-15
63-241
依折麦布瑞舒伐他汀锌胶囊
Ezetimibe rosuvastatin zinc hard capsule/Cholecomb
20mg/10mg
Proterapia Hungary Ltd
未进口原研药品
欧盟上市
63-251
盐酸氨酮戊酸凝胶
aminolevulinic acid hydrochloride gel/AMELUZ
高效液相色谱-质谱联用法检查止喘灵胶囊中非法添加的醋酸泼尼松和茶碱

高效液 相色谱 一质谱联用法检查 止喘灵胶囊中非法添加 的醋酸泼尼松和茶碱
王 戈, 赵培敬
4 7 3 0 6 1 ) ( 河 南省 南阳市食 品药 品检验 所 , 河南 南阳
摘要 : 目 的 采 用 高 效 液 相 色谱 一质 谱 联 用技 术 , 检 查止喘灵胶 囊中非法添加 的醋酸泼尼松和茶碱 。 方 法 色谱 柱 为 S h i m a d z u s h i m— p a c k V P— O D S柱 ( 2 5 0 mm x 4 . 6 m m, 5 m) , 0 . 0 2 m o l / L乙酸铵 一 0 . 1 % 冰醋 酸 水溶 液 : 乙腈 ( 7 1 : 2 9 ) 为 流动 相 , 流速 为 1 . 0 m L / mi n ; A g i l e n t 6 3 1 0型 离子 阱质 谱仪 , 电喷 雾 离子源 ( E S I ) , 雾化 气压 力为 5 0 p s i , 干燥 气温 度 为 3 8 0 ℃, 流速 为 9 . 5 mL / m i n , 毛 细管 电压 为 3 5 0 0 V, 扫 描 范 围为 1 0 0~ 5 0 0m/ z , 检 测方式为正、 负模 式 一 级 、 二 级 同时 扫 描 。 结 果 在 高 效 液 相 色谱 、 质谱 中, 样 品 出现 与 醋 酸 泼 尼松 、 茶 碱 成 分 一 致 的 色谱峰 、 质 谱峰 。 结 论 所 用方 法 简 单 可行 , 结 果 准确 可 靠 , 可 用于 止 喘 灵胶 囊 中非 法 添加 醋 酸 泼 尼松 、 茶碱 的定 性 鉴 别 。
A b s t r a c t : 0 b j e c t i v e T o e s t a b l i s h a H P L C— MS / MS m e t h o d f o r d e t e c t i n g p r e d n i s o n e a c e t a t e a n d t h e o p h y l l i n e i l l e g a l l y a d d e d i n
荷尔登生物医学检测产品说明书
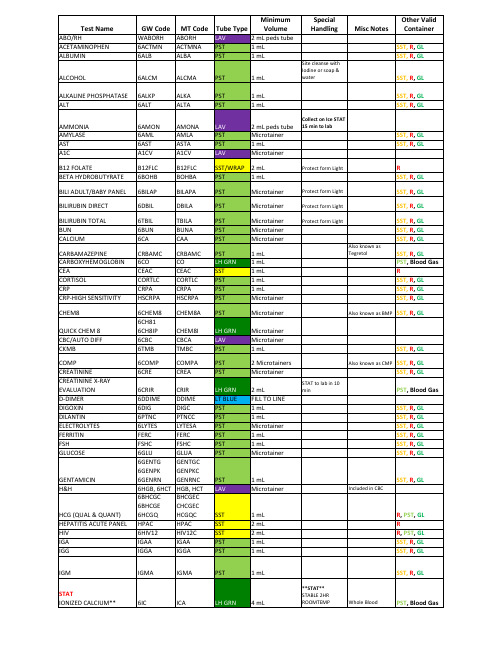
1mL
R
1mL
SST, R, GL
1mL
No capillary
1mL
collection
Order of Draw: 1. BLOOD CULTURES 2. LT BLUE 3. RED 4. SST 5. PST/LH GRN 6. LAV 7. GRAY
M2909 (1-19)
Microtainer Microtainer 1 mL
2 Microtainers Microtainer
2 mL FILL TO LINE 1 mL 1 mL Microtainer 1 mL 1 mL Microtainer
SST, R, GL
STAT to lab in 10 min
Also known as CMP SST, R, GL SST, R, GL
TOBRAMYCIN TOTAL PROTEIN TROPONIN TSH
TYPE AND CROSSMATCH
TYPE AND SCREEN URIC ACID
VANCOMYCIN VALPROIC ACID VITAMIN D
FEA
6LA LHC 6LIP
6LIVER 6LIPID LIA 6MG 6METX 6MONO PHNOC 6PFA 6PHOS 6PBNP 6PCAL 6KA 6PTIMEN PSAC 6PTT 6RETCT 6RENAL WHRIG 6SAL SYPHB TBSATA TT3C T4C TESTOC THEOC
CHEM8
QUICK CHEM 8 CBC/AUTO DIFF CKMB
COMP CREATININE CREATININE X-RAY EVALUATION D-DIMER DIGOXIN DILANTIN ELECTROLYTES FERRITIN FSH GLUCOSE
Mesh类目词表
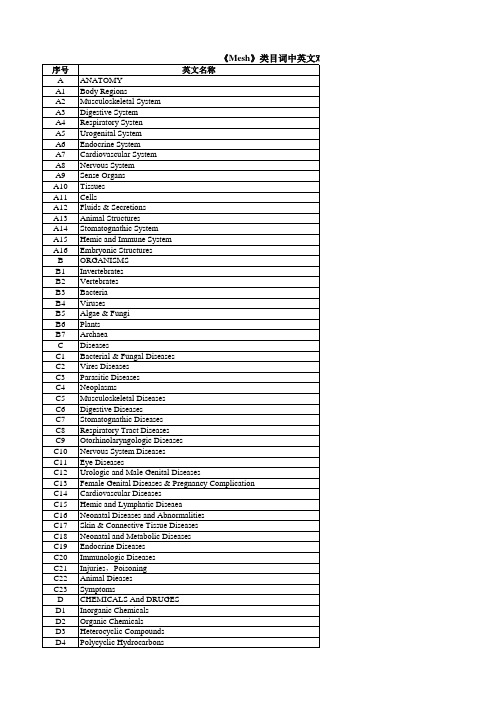
环境污染物,病原农药和杀虫剂 激素,激素代用品和激素拮抗剂 避孕药 酶、辅酶和酶抑制剂 碳水化合物和降糖剂 脂类和抗血脂类 促生长物质、色素和维生素 氨基酸、肽和蛋白质 核酸、核苷酸和核苷 神经递质和神经递质物 中枢神经系统药物 周围神经系统药物 抗炎药、抗风湿药、炎性介质 心血管系统药物 血液、胃和肾脏药 抗感染药物 变态反应和呼吸系统药物 抗肿瘤药和免疫抑制剂 皮肤病药物 免疫和生长因子 生物医学和牙科材料 其他药剂 分析、诊断、治疗技术和设备 诊断 治疗 麻醉和镇痛 外科操作,外科 包埋技术 牙科 设备和供应 精神病学和心理学 行为和行为机制 心理现象和过程 精神疾病 行为训练和活动 生物科学 生物科学 卫生职业 环境和公共卫生 生物现象、细胞现象和免疫 遗传学 生物化学现象、代谢和营养 生理学过程 生殖和泌尿生理 循环和呼吸生理 消化、口腔和皮肤生理 肌肉、骨骼和视生理 化学和药物现象 自然科学 人类学、教育、社会学和社会现象 社会科学 教育 人类活动 工艺学、工业、农业 工艺学、工业和农业
Environmental Pollutants,Noxae & Pesticides Hormones, Hormone Substitutes Reproductive Control Agents Enzymes, Conezymes Enzyme Inhibitore Carbohydrates & Hypoglycemic Lipids & Antilipemis Growth Substances, Pigments Amino Acids, Peptides & Vitamins Nucleic Acid, Nucletides Neurotransmitter & Neurotransmitter Agents Central Nervous System Agents Peripheral Nervous System Agents Anti-Inflammatory Agents, Antirheumatic,Agents,& Inflammation Mediators Cardiovascular Agents Hematologic, Gastrointestinal,& Renal Agents Anti-Infective Agents Anti-Allergic & Respiratory System Agents Anti-Neoplastic & Immunosuppressive Agents Dermatologic Agents Immunologic and Biologic Factors Biomedical and Dental Materials Miscellaneous Drugs and Agents ANALYTICAL, DIAGNOSTIC &THERAPEUTIC TECHNIGUES AND EQUIPMENT Diagnosis Therapeutics Anesthesia & Analgesia Surgical Procedures, Operative Investigative Techniques Dentistry Equipment & Supplies PSYCHIATRY AND PSYCHOLOGY Behavior & Behavior Mechanisms Psychological Phenomena & Processes Mental Disorders Behavioral Disciplines & Actives BIOLOGICAL SCIENCES Biological Sciences Health Occupations Environmental & Public Health Biological Phenomena, Cell Phenomena, & Immunity Genetics Biochemical Phenomena, Metabolism, & Nutrition Physiological Processes Reproductive & Urinary Physiology Cirulatory & Respiratory Physiology Digestive, Oral, & Skin Physiology Musculoskeletal, Neural, & Ocular Physiology Chemical & Pharmacologic Phyenomena PHYSICAL SCIENCES ANTHROPOLOGY, EDUCATION, SOCIOLOGY & SOCIAL PHENOMENA Social Sciences Education Human Activities TECHNOLOGY, INDUSTRY & AGRICULTURE Technology, Industry & Agriculture
