Montelukast sodium_151767-02-1_CoA_MedChemExpress
孟鲁司特钠杂质-(最新结构)列表

中文名称英文名称CAS规格图谱用途结构式孟鲁司特Montelukast 151767-02-110mg25mg50mg100mg更大规格请咨询随货COA,HNMR,MS,HPLC等确证谱图新药研发、项目报批纯度高于98%孟鲁司特杂质1(孟鲁司特EP杂质A)Montelukast Impurity 1(Montelukast EPImpurity A)190078-45-610mg25mg50mg100mg更大规格请咨询随货COA,HNMR,MS,HPLC等确证谱图新药研发、项目报批纯度高于98%孟鲁司特杂质2(孟鲁司特EP杂质B)Montelukast Impurity 2(Montelukast EPImpurity B)918972-54-10mg25mg50mg100mg更大规格请咨询随货COA,HNMR,MS,HPLC等确证谱图新药研发、项目报批纯度高于98%孟鲁司特杂质3(孟鲁司特EP杂质C)Montelukast Impurity 3(Montelukast EPImpurity C)909849-96-310mg25mg50mg100mg更大规格请咨询随货COA,HNMR,MS,HPLC等确证谱图新药研发、项目报批纯度高于98%孟鲁司特杂质4(孟鲁司特EP杂质D)Montelukast Impurity 4(Montelukast EPImpurity D)1187586-61-310mg25mg50mg100mg更大规格请咨询随货COA,HNMR,MS,HPLC等确证谱图新药研发、项目报批纯度高于98%扬信医药代理各品种杂质对照品:泊沙康唑杂质、替卡格雷杂质、依折麦布杂质、索拉菲尼相关杂质、索非布韦杂质、氨氯地平杂质、马来酸氯苯那敏杂质、头孢克肟杂质、瑞舒伐他汀杂质、瑞格列奈杂质、恩替卡韦杂质,替诺福韦杂质等;并提供COA、NMR、HPLC、MS等结构确证图谱。
更多杂质详情,点击头像咨询。
A级无机剧毒物品
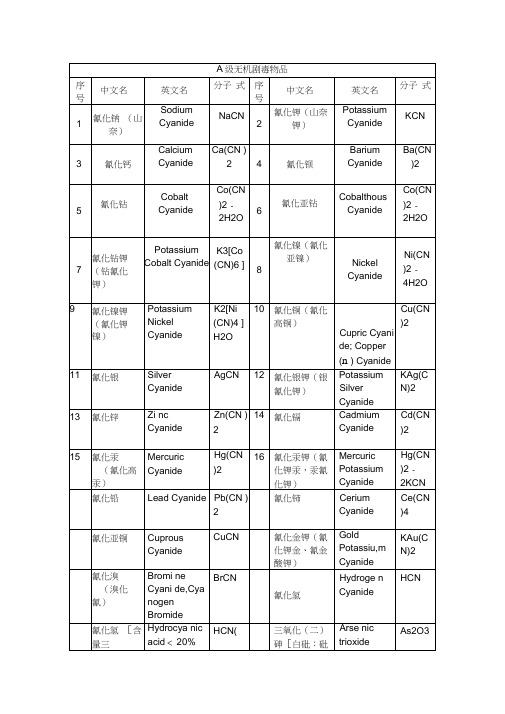
Strontium orthoarenite
Sr3(AsO3)
2•4H2O
亚砷酸 钡
Barium Arsenite
Ba(AsO2)2
亚砷酸 铁
Ferric
Arsenite
2FeAsO3
Fe2O3
•5H2O
亚砷酸 铜(亚砷 酸氢铜)
Cupric Arsenite;
Copper Arsenite
A级无机剧毒物品
序 号
中文名
英文名
分子 式
序 号
中文名
英文名
分子 式
1
氰化钠 (山奈)
Sodium Cyanide
NaCN
2
氰化钾(山奈 钾)
Potassium
Cyanide
KCN
3
氰化钙
Calcium Cyanide
Ca(CN )2
4
氰化钡
Barium Cyanide
Ba(CN
)2
5
氰化钻
Cobalt
Cyanide
Co(CN
)2-
2H2O
6
氰化亚钻
Cobalthous
Cyanide
Co(CN
)2-
2H2O
7
氰化钻钾 (钻氰化 钾)
Potassium Cobalt Cyanide
K3[Co
(CN)6 ]
8
氰化镍(氰化 亚镍)
Nickel Cyanide
Ni(CN )2-4H2O
9
氰化镍钾 (氰化钾 镍)
砷酸铜
Cupric Arsenate; Copper Arsenate
Cu3(AsO4
索马鲁肽 质量标准

索马鲁肽(Semaglutide)是一种新型长效胰高血糖素样肽-1(GLP-1)受体激动剂,主要用于治疗2型糖尿病。
其通过促进胰岛素分泌和抑制胰高血糖素分泌来降低血糖,同时具有减少食物摄入和降低体重的效果。
索马鲁肽的质量标准通常包括以下几个方面:
1. 纯度:索马鲁肽的纯度要求通常非常高,常见的要求为95%或99%以上。
这保证了药品中活性成分的浓度和一致性,从而确保疗效的稳定。
2. 分子式与分子量:索马鲁肽的分子式为C187H291N45O59,分子量约为411
3.57754。
这些参数是化学表征的重要指标,用于确保产品的正确性。
3. 外观:索马鲁肽通常为白色粉末状,这与其化学性质和纯度有关。
4. 溶解性:索马鲁肽在水中的溶解性良好,通常可溶解至1 mg/ml。
这与其作为注射剂的给药形式相关。
5. 储存条件:索马鲁肽应在-20°C的条件下储存,以保持其稳定性和活性。
6. 质量检验:在生产过程中,索马鲁肽会经过严格的质量检验,包括使用高效液相色谱法(HPLC)等分析技术来确保其纯度和含量。
7. 生物活性:索马鲁肽的生物活性是通过与GLP-1受体结合的能力来评估的。
这一点对于确保其治疗效果至关重要。
8. 稳定性:索马鲁肽在储存和使用过程中的稳定性也是质量标准的一部分,这包括对温度、pH 值和光照等条件的稳定性评估。
总的来说,索马鲁肽的质量标准旨在确保其作为药物的安全性和有效性,这些标准通常由药品制造商和监管机构设定并执行。
巯基吡啶氧化钠说明书

巯基吡啶氧化钠
中文名称巯基吡啶氧化钠
其他名称吡啶硫酮钠
英文名称Mercaptopyridine-N-oxide sodium salt
CAS NO.3811-73-2
规格25%w/W solution
说明
生物防腐剂、抗生素;能有效抑制霉菌和细菌生长;对膜蛋白有较强的相互作
用;对细菌膜离子通道有干扰作用
产品线生物工程、生物分析
级别BioThechnika
货号155092
性状浅黄色或琥珀色透明溶液
主要成分Mercaptopyridine-N-oxide sodium salt
使用说明高品质原料配制而成;推荐使用浓度0.5ml~5ml
注意事项本品有一定刺激性,只能体外使用不能用于体内
贮存密封,避光,在阴凉干燥处保存。
有效期2年
包装规格500g;25kg
深圳市美凯特科技有限公司曾老师I86846O449O
本公司提供产品符合本企业内部规格与质量标准;所有使用该产品的用户直接使用本产品或将本产品作为其产品的组成部分时应对本产品对于其本身的适用性进行确认。
本公司不对使用本产品产生的任何后果负有任何直接或间接连带责任。
Sigma-Aldrich是全世界领先的生命科学与高科技集团公司。

Sigma-Aldrich 是全世界领先的生命科学与高科技集团公司。
我们的生物化学与有机化学产品与试剂盒被广泛地应用研究于生命科学研究、有机化学研究、材料科学研究、分析化学研究与其他高科技生产中。
我们的用户遍及生命科学研究公司、大学与政府研究机构与企业,超过一百万科学家与科研人员使用到我们的产品。
SIGMA 原装促销欢迎垂询!sigma 51370 Harmane 1G 现货sigma 222984 dl-Propranolol HCl 5G 现货sigma 284785 S-6-Methoxy-a-Methyl-2-Naphthaleneacetic acid 25G 现货sigma 857416 3-(3,4-Dihydroxyphenyl)-2-methyl-L-Alanine 5g 现货sigma 857440 Chloramphenicol 25G 现货sigma A4669 Acycloguanosine 500mg 现货sigma A5882 Antipyrine 500G 现货sigma A7655 Atenolol 25G 现货sigma A8523 Amoxicillin 25g 现货sigma B7283 Benserazide HCl 5G 现货sigma C2505 Corticosterone 500MG 现货sigma C4024 Carbamazepine 25G 现货sigma C4216 Coumarin 500G 现货sigma C4255 Creatinine 1KG 现货sigma C4522 Cimetidine 25G 现货sigma C5793 Ceftriaxone Na 1G 现货sigma C6022 Cyproheptadin HCl 1G 现货sigma D1756 Dexamethasone 500MG 现货sigma D2521 Diltiazem 10G 现货sigma D4007 Phenytoin 500G 现货sigma D6270 1-(2,6-dichlorobenzyl-ideneamino)guanidine 100MG 现货sigma D9628 l-3,4-Dihydroxyphenyl-alanine 100G 现货sigma E6888 Enalapril Maleat 5G 现货sigma F4381 Furosemide 25G 现货sigma F8514 Flurbiprofen 25G 现货sigma F9677 Felodipine 25MG 现货sigma H0888 Hydrocortisone 10G 现货sigma H4759 Hydrochlorothiazide 100G 现货sigma I0899 Imipramine HCl 25G 现货sigma K1751 Ketoprofen 100G 现货sigma M5391 Metoprolol x 1/2 Tartrate 10G 现货sigma N144 Nitrendipine 500MG 现货sigma N7758 Naloxone 1G 现货sigma N8280 Naproxen 5G 现货sigma N9890 Norfloxacin 25G 现货sigma P0847 Piroxicam 10G 现货sigma P7412 Pirenzepine 1G 现货sigma Q3625 Quinidine 25G 现货sigma R101 Ranitidine 5G 现货sigma S0883 Sulfasalazine 100G 现货sigma S8010 Sulpiride 100G 现货sigma T1500 Testosterone 25G 现货sigma T2528 Terbutaline x 1/2 Tartrate 5G 现货sigma T69809 Trimethoxybenzamide HCl 100G 现货sigma V4629 Verapamil 10G 现货sigma E3763 Ethoxyresorufin 5MG 现货sigma A2500 Phenacetin 100G 现货sigma UC175 S-(+)Mephenytoin 5MG 现货sigma T1912 Paclitaxel 25MG 现货sigma UC214 S-Warfarin 10MG 现货sigma D6899 Diclofenac sodium salt 10G 现货sigma O104 Omeprazole 500MG 现货sigma D9684 Dextromethorphan hydrobromide 5G 现货sigma UC168 (±)-Bufuralol hydrochloride 10MG 现货sigma C4397 Chlorzoxazone 25G 现货sigma H24003 Umbelliferone 10G 现货sigma A7085 Acetaminophen 100G 现货sigma UC126 4-hydroxymephenytoin 5MG 现货sigma T2573 Troglitazone 5MG 现货sigma UC211 7-hydroxywarfarin 5MG 现货sigma H3661 4'-hydroxydiclofenac 1VL 现货sigma UC179 (R)-(?)-Nirvanol 5MG 现货sigma H147 Hydroxybufuralol maleate 2MG 现货sigma C188 6-Hydroxychlorzoxazone 5MG 现货sigma H2898 6β-Hydroxytestosterone 5MG 现货sigma M3501 8-Methoxypsoralen 1G 现货sigma S0758 Sulfaphenazole 1G 现货sigma C1240 Clomethiazole 10MG 现货sigma K1003 Ketoconazole 100MG 现货sigma T6514 Oleandomycin triacetate 1G 现货sigma D127 Dextrorphan tartrate 500MG 现货sigma Merck 580559 6alpha-OH-paclitaxel 5 mg 现货sigma N6505 NADPH (reduced form) 100 mg 现货sigma o3639 Ondansetron (7, 8) 50 mg 现货sigma UC448 Paracetamol 5 mg 现货sigma T5648 Tamoxifen (N-demethylation) 1g 现货sigma M4267 Mefenamic acid (3-methyl) 50g 现货sigma R2625 Retinoic acid (Tretinoin) 1g 现货sigma T0909 Tenoxicam (5') 1g 现货sigma T0781 Trimethadione (N-demethylation) 1g 现货sigma A8676 Alprenolol (Ar hydrox) 5g 现货sigma A8404 Amitryptyline 10 g 现货sigma C1290 Chlorpropamide 25 g 现货sigma C5270 Cinnarizine (Ar hydrox) 10g 现货sigma C7291 Clomipramine 5 g 现货sigma C6305 Clozapine 100 mg 现货sigma C5901 Codeine 1g 现货sigma D1306 Debrisoquine (4) 100 mg 现货sigma F6777 Flecainide 25 mg 现货sigma F8257 Flunarizine (Ar hydrox) 1 g 现货sigma i2909 Indoramin 10 mg 现货sigma M9651 Maprotiline 1g 现货sigma M1641 Methoxyphenamine 5g 现货sigma M2727 Mexiletine 25 g 现货sigma M3157 Minaprine 1g 现货sigma N7261 Nortriptyline 10g 现货sigma p3028 Perhexiline 5g 现货sigma p6402 Perphenazine 5g 现货sigma p7045 Phenformin 10g 现货sigma p4670 Propafenone 5g 现货sigma T9025 Thioridazine 5g 现货sigma A8423 Amiodarone (N-deethylation) 1 g 现货sigma B7777 Budesonide (6?) 250mg 现货sigma 46158 Dapsone (N) 250mg 现货sigma D5878 Digitoxin (oxidation) 1 g 现货sigma L8533 Lansoprazole (sulfoxyde-oxidation) 250 mg 现货sigma L7757 Lidocaine (N-deethylation) 25 g 现货sigma M2147 Lovastatin 25 mg 7 现货sigma N7634 Nifedipine (dihydropyridine) 5g 现货sigma S7507 Sulfamethoxazole (N) 10g 现货sigma T9772 Triazolam 10 mg 现货sigma N6505 Nicotinamide adenine dinucleotide phosphate 100 mg 现货sigma F6889 Famotidine 现货sigma C0378 Chloramphenicol 现货sigma T1633 Theophylline 现货sigma T1500 testosterone 现货sigma B9300 benzoicacid 现货sigma L1011 Labetalol hydrochloride 现货sigma S7507 Sulfamethoxazole 现货sigma B4144 Bromazepam 现货sigma D0899 Diazepam 现货sigma T7883 Trimethoprim 现货sigma Q1125 Quinine HCl 现货sigma D6899 Diclofenac sodium salt 现货sigma S0883 Sulfasalazine 现货sigma N144 Nitrendipine 现货sigma T9652 Terfenadine 现货sigma F9677 Felodipine 现货sigma N3889 Nitrazepam 现货sigma C4024 Carbamazepine 现货sigma C0750 Caffeine 现货sigma F4381 Furosemide 现货sigma K1751 Ketoprofen 现货sigma N9890 Norfloxacin 现货sigma C3045 Cholesterol 现货sigma G2878 Glycocholic acid hydrate 现货sigma P3556 L-α-Phosphatidylcholine 现货sigma D221104 Dodecane 现货sigma M8389 MOPSO 现货sigma FOR P450 inhibition 现货sigma N0505 NADP+ 现货sigma G7250 Glucose-6-phosphate 现货sigma G6378 G6PDH 现货sigma F-124 furafylline 现货sigma p8511 tranylcypromine 现货sigma UC455 CEC (3-cyano-7-ethoxycoumarin) 现货sigma F6377 Fluorescein 现货sigma 28605 CHC (3-cyano-7-hydroxycoumarin) 现货sigma L8533 Lansoprazole 现货sigma C4740 Cisapride 现货sigma N5757 alpha-naphthoflavone 现货sigma S6319 sertraline hydrochloride 现货sigma T6654 Ticlopidine hydrochloride 现货sigma 74437 Nootkatone 现货sigma 30024 Cyclosporin A 现货sigma 22530 Quinacrine 现货sigma B5016 Bepridil 现货sigma N149 Nimodipine 现货sigma F132 Fluoxetine 现货sigma M8046 Mifepristone 现货sigma 10798 Apigenin 现货sigma 0 2644 7-ethoxycoumarin 现货sigma M3512 Miconazole 现货sigma I4883 Ibuprofen 现货sigma F8929 Fluconazole 现货sigma 62630 Lobeline 现货sigma H1512 Haloperidol 现货sigma M2727 Mexiletine 现货sigma C6628 chloroquine 现货sigma D1306 Debrisoquine sulfate 现货sigma Q0125 Quercetin 现货sigma T0891 Tolbutamide 现货sigma 67580 4-Methylimidazole 现货sigma 4-methylpyrazole 现货sigma C6019 Clotrimazole 现货sigma A6424 Astemizole 现货sigma N7510 Nicardipine hydrochloride 现货sigma M5441 Mibefradil dihydrochloride hydrate 现货sigma I6657 Itraconazole 现货sigma B5774 Benzbromarone 现货sigma Fluvoxamine 现货sigma E4876 17α-Ethynylestradiol 现货sigma 01338 Bergamottin 现货sigma UC431 4-OH-midazolam 现货sigma n4505 500mg 现货sigma n1511 1g 现货sigma c5275-5mg concanavalin a form canavalia ensiformis 5mg 现货sigma a0937-1g norepinephrine bitartrate salt 1g 现货sigma c7527-100g Choline chloride cell culture tested 100g 现货sigma I6504-500mg Isoproterenol hydrochloride 500mg 现货sigma M5379-250mg L Methionine sulfoximine 250mg 现货sigma L9634-1g Laminarin from Laminaria digitata 1g 现货sigma F7131-100mg N 3 2 Furyl acryloyl]-Phe-Gly-Gly 100mg 现货sigma L8754-5mg Lectin from Phaseolus vulgaris (red kidney bean) Phytohemaggluti 5mg 现货sigma T5910-5g 2,3,5-Triiodobenzoic acid 5g 现货sigma E1253-100mg E1253 Estriol 100mg 现货sigma D9891-1G Doxycycline hyclate 1g 现货sigma F2878-100g Ficoll PM70 100g 现货sigma D5879-100ml D5879 DMSO 100ml 现货sigma I5879-100mg Isobutyl 1 methylxanthine 100mg 现货sigma D1756-25mg Dexamethasone 25mg 现货sigma I5500-50mg Insulin from bovine pancreas 50mg 现货sigma N5764-5g Nisin from Lactococcus lactis 2.5% 5g 现货sigma H1384-1g Hypotaurine 1g 现货sigma C7352-25g L-Cysteine 25g 现货sigma H3506-1g Hyaluronidase from bovine testes Type I-S, lyophilized 1g 现货sigma L4263-100ml Sodium DL lactate solution syrup 100ml 现货sigma B2261-25mg bisBenzimide H 33342 trihydrochloride 25mg 现货sigma D4263-5VL Deoxyribonuclease I from bovine pancreas Standardized vial 5VL 现货sigma a3307-5mg Sialyllactose sodium salt 5mg 现货sigma C9210-5g Cyanogen bromide activated Agarose lyophilized powder 5g 现货sigma S1782-0.2mg 3 Sialyl Lewis X tetrasaccharide 0.2mg 现货sigma c4805-5g b Cyclodextrin powder 5g 现货sigma D0627-25mg Dibutyryl cAMP 25mg 现货sigma S5265-25KU streptolysin 25KU 现货sigma R7256-100ml R7256 RPMI1640 100ml 现货sigma c8518-10g Cesium hydroxide hydrate 10g 现货sigma d95204-100g 100g 现货sigma c9253 Cholesteryl oleate ≥98%1g 现货sigma e6005-5g 现货sigma 15139 Bisphenol F bis(3-chloro-2-hydroxypropyl) ether 250mg 现货sigma 299537 Trifluoroacetic acid 100g 现货sigma 545201 Cucurbit[7]uril 100mg 现货sigma 545228 Cucurbit[8]uril 100mg 现货sigma T8949 Telmisartan 10mg 现货sigma D141 DigitoninSigma BSA 牛血清白蛋白A3912-10gSigma Cholesterol 胆固醇C8667-5g Sigma Indomethacin 吲哚美辛I7378-5gSigma Dexamethasone 地塞米松D1756-100mgSigma L-Ascorbic acid维生素C A4544-25GSigma insulin 牛胰岛素I5500-100mg Sigma Oil Red O 油红O O0625-25g Sigma LPS 脂多糖L2880-25mgSigma MTT 噻唑兰M2128-100mgSigma BrDu 溴脱氧尿嘧啶核苷B5002-500mg Sigma Proteinase K蛋白酶K P6556-25mg/100mgSigma Trypan Blue台盼蓝T6146-5gSigma Uracil 尿嘧啶U0750-5gSigma Thymidine 胸苷T9250-1gSigma L-Cysteine hydrochloride 半胱氨酸盐酸盐C1276-10G Sigma Ethambutol dihydrochloride E4630-25GSigma Phenol Red 酚红P3532-5GSigma Ethambutol dihydrochloride 盐酸乙胺丁醇E4630-25G Sigma Sodium tripolyphosphate 三聚磷酸钠238503-25G sigma Palmitic acid棕榈酸P5585-10Gsigma Levofloxacin左氧氟沙星28266-10G-Fsigma L-Tryptophan L-色氨酸T0254-1G。
商品名-圣马尔定医院
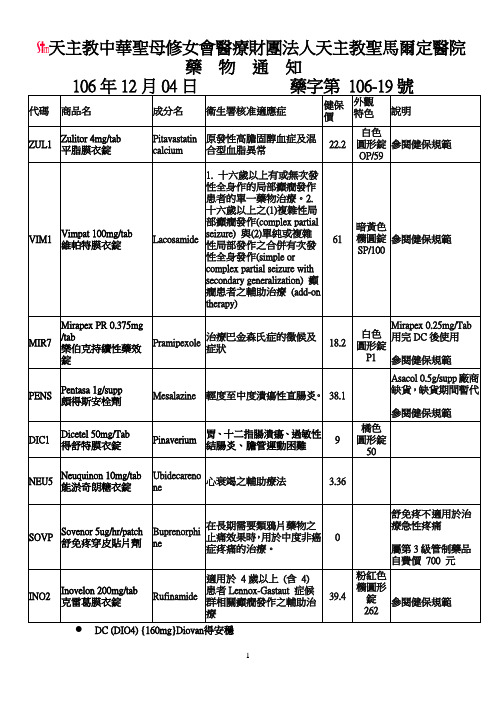
Vimpat 100mg/tab
維帕特膜衣錠
Lacosamide
1.十六歲以上有或無次發性全身作的局部癲癇發作患者的單一藥物治療。2.十六歲以上之(1)複雜性局部癲癇發作(complex partial seizure)與(2)單純或複雜性局部發作之合併有次發性全身發作(simple or complex partial seizure with secondary generalization)癲癇患者之輔助治療(add-on therapy)
適用於4歲以上(含4)患者Lennox-Gastaut症候群相關癲癇發作之輔助治療
39.4
粉紅色橢圓形錠
262
參閱健保規範
DC(DIO4){160mg}Diovan得安穩
輕度至中度潰瘍性直腸炎。
38.1
Asacol 0.5g/supp廠商缺貨,缺貨期間暫代
參閱健保規範
DIC1
Dicetel 50mg/Tab
得舒特膜衣錠
Pinaverium
胃、十二指腸潰瘍、過敏性結腸炎、膽管運動困難
9
橘色
圓形錠50
NEU5
Neuquinon 10mg/tab
能淤奇朗糖衣錠
Ubidecarenone
心衰竭之輔助療法
3.36
SOVP
Sovenor 5ug/hr/patch
舒免疼穿皮貼片劑
Buprenorphine
在長期需要類鴉片藥物之止痛效果時,用於中度非癌症疼痛的治療。
0
舒免疼不適用於治療急性疼痛
屬第3級管制藥品
自費價700元
INO2
Inovelon 200mg/tab
克雷葛膜衣錠
Rufinamide
索马鲁肽

索马鲁肽
【产品名称】索马鲁肽
【英文名】Semaglutide,Ozempic
【序列】
H-His-Aib-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly-Gln-Ala-Ala-Lys(A EEAc-AEEAc-γ-Glu-17-carboxyheptadecanoyl)-Glu-Phe-Ile-Ala-Trp-Leu-Val-Arg-Gly-Arg-Gly-OH
【CAS号】910463-68-2
【分子式】C187H291N45O59
【分子量】4,113.64
【产品外观】白色结晶粉末
【纯度】99.38%
【鉴定】红外吸收
【干燥损失】≤0.2%
【氯化物】≤0.05%
【重金属】≤0.0015%
【硫酸盐】≤0.03%
【应用】
1.用作注射型或口服型药物。
3.改善心血管功能
4. II型糖尿病药物
【详细描述】
索马鲁肽是一种新的每周一次的胰高血糖素样肽-1(GLP-1)受体激动剂,对2型糖尿病(T2DM)的血糖控制和体重减轻有显着的作用。
索马鲁肽已证明有可能改善2型糖尿病,咨询委员会的积极建议标志着在美国为2型糖尿病患者提供索马鲁肽的重要一步。
在人类中,索马鲁肽在化学上类似于胰高血糖素样肽-1(GLP-1)。
索马鲁肽是胰高血糖素样肽-1(GLP-1)类似物,并且起GLP-1激动剂的作用。
脑血管葡萄糖还能改善心血管功能,将卒中风险降低39%,并降低心肌梗塞的风险。
此外,索马鲁肽对心血管结局和类似于其他GLP-1受体激动剂的安全性表现出显着的有益作用。
异鼠李素对照品

异鼠李素_CAS:480-19-3
异鼠李素是一种黄酮类化合物,属于甲基化代谢产物,具有抗食管癌、健胃消食的功效,它可以从银杏叶、白果、绞股蓝、黄花蒿当中分离取得。
【名称】异鼠李素
【别名】槲皮素3'-甲基醚
【英文名】Isorhamnetin
【英文别名】Quercetin 3'-methyl ether;EINECS 207-545-5
【IUPAC名称】
-3,5,7-三羟基-2-(4-羟基-3-甲氧基苯基)苯并吡喃-4-酮
【Isomeric SMILES】
COC1=C(C=CC(=C1)C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O
【分子式】C16H12O7
【分子量】316.265
【CAS号】480-19-3
【品牌】格利普生物科技
【检测方式】高效液相色谱法HPLC≥98%
【鉴才、定方法】Ms;NMR
【密度】1.662
【沸点】601.2°C
【稳定性】本产品在正常的温度和环境下比较稳定
【性状】本品为黄色颗粒结晶
【功效】抗食管癌、健胃消食
【提取来源】本品来源于银杏叶、白果、绞股蓝、黄花蒿。
山茱萸提取物

【颜色性状】山茱萸提取物为红棕色精细粉末
【产品目数】100%通过80目筛
【功能主治】补益肝肾,涩精固脱。用于眩晕耳鸣,腰膝酸痛,阳痿遗精,遗尿尿频,崩漏带下,大汗虚脱。内热消渴。
【包装方式】25公斤/纸板桶或铝箔袋
【存储条件】山茱萸提取物应密封遮光,贮存在干燥、阴凉、通风良好的地方,有效期为两年。
【产品名称】山茱萸提取物
【英文名称】Fructus Corni Extract
【提取来源】为茱萸科植物山茱萸的果肉
【化学成分】含莫罗忍冬甙(morroniside)、7-0-甲基莫罗忍冬甙(7-0-methylmorroniside)、獐牙菜甙(sweroside)、番木鳖甙(Ioganin)、山茱萸鞣质1、2、3、(cornus-tannin 1,2,3)等。
北京曼思特益母草苷结构式

北京曼思特益母草苷结构式
益母草苷:
分子式:C15H24O9
分子量:348.35
储存条件:-20℃,有效期2年,溶入溶剂后-20℃请尽量在一个月内使用
益母草苷是一种化学物质。
纯度98%以上,检测方法HPLC,用于医药研究及含量测定。
益母草苷是一种环烯醚萜苷类化合物,主要来源于地黄、益母草等。
益母草苷具有抗癌、神经保护、抗炎、利尿等功效。
一种预防原发性高血压的药物组合物,包含下列原料按照重量份数配制而成:无羁萜-3β-醇1~100份、黄芪多糖组分a310~100份、连翘苷3~100份,白藜芦醇10~100份和益母草苷a7~100份。
研究发现,无羁萜-3β-醇、黄芪多糖组分a3、连翘苷,白藜芦醇和益母草苷a 组成的复方可以有效降低高血压模型大鼠的收缩压和舒张压,降低血浆血管紧张素ii(angii)水平,升高血浆血管舒张因子一氧化氮(no)水平,增加血管组织一氧化氮合酶(enos)蛋白表达以及基因表达。
益母草苷或其前药的用途,尤其是益母草苷或其前药用于制备降血压的药物的用途。
顶空气相色谱法测定索玛鲁肽原料药的残留溶剂

顶空气相色谱法测定索玛鲁肽原料药的残留溶剂摘要:目的:建立索玛鲁肽原料药中甲醇、异丙醇、乙腈、甲基叔丁基醚、三乙胺5种溶剂的检测方法。
方法:采用Agilent DB-624(30m×0.53mm,3μm)毛细管柱;起始温度40℃,维持10min,以每分钟20℃的速率升温至200℃,维持7min;进样口温度200℃,检测器温度250℃,流速2ml/min;顶空平衡温度100℃,平衡时间20min。
结果:5种溶剂均达到完全分离,方法灵敏度高,在LOQ~200%限度范围内浓度和峰面积之间线性关系良好,精密度和回收率良好。
结论:本方法能简便、灵敏及准确地检测索玛鲁肽原料药溶剂残留量。
关键词:索玛鲁肽;气相色谱法;顶空进样;残留溶剂1仪器与设备Agilent 6890 Plus气相色谱仪、Agilent 7694E顶空进样器2试剂试药二甲基亚砜、甲醇、异丙醇、乙腈、三乙胺、甲基叔丁基醚和索玛鲁肽原料药3试验方法与结果3.1色谱条件以6%氰丙基苯-94%二甲基硅氧烷为固定液的毛细管柱为色谱柱[DB-624,30m×0.53mm,3μm]。
起始温度40℃,维持10min,以20℃/min的速率升温至200℃,维持6min;进样口温度200℃,检测器温度250℃,流速2ml/min,顶空平衡温度100℃,平衡时间20min。
3.2溶液配制空白溶液:二甲基亚砜(DMSO)。
定位溶液:分别取甲醇、异丙醇、乙腈和甲基叔丁基醚各10mg,精密称定,置不同50ml量瓶中,加空白溶液溶解并稀释至刻度,摇匀,即得。
对照品溶液:取甲醇240mg、异丙醇400mg、乙腈32.8mg、甲基叔丁基醚400mg、三乙胺25.6mg,精密称定,置同一50ml量瓶中,加空白溶液溶解并稀释至刻度,摇匀;精密量取0.5ml,置20ml量瓶中,用稀释剂稀释至刻度,摇匀;精密量取2ml,置20ml顶空瓶中,压盖密封,作为对照品溶液。
2009-2019专利到期原料药品种

2009年-2019年专利到期原料药品种2009.06 阿托伐他汀钙Atorvastatin calcium 134523-03-8据悉,去年立普妥为辉瑞创造了107亿美元的营收。
该药将于2011年11月30日丧失专利保护权。
辉瑞此前与兰伯西制药达成和解,给予后者6个月期限的独家销售立普妥仿制药权利。
立普妥将在2011年11月30日到期的专利,是指其化合物专利,其还拥有药物制剂、制备工艺、配方、用途等专利,来延长立普妥的专利保护期,阻碍潜在的仿制药竞争者进入市场。
目前,围绕立普妥的专利纠纷正在全球范围内愈演愈烈。
立普妥的专利将在2011年到期,而辉瑞的立普妥是1晶型,专利到2016年到期。
而且辉瑞还申请了其他20多种晶型。
开发其他晶型,要考虑样品的稳定性和生物等效性。
申请其他晶型,需要提供相当多的数据来证明你申报的晶型与上市晶型在毒性,临床的安全有效性的差异以及药学研究方面的差异如稳定性,不同晶型的溶解性等等。
知识产权:本品(阿托伐他汀钙)在中国无化合物专利,国内2007年行保到期,二类新药保护也在2007年到期。
有原料晶形,工艺和制剂处方专利,申报中需要避开。
2009.07 盐酸氨基戊酮Aminolevulinic acid hydrochloride 5451-09-22009.11 拉米夫定Lamivudine 134678-17-42009.11 甲磺酸阿拉曲伐沙星Alatrofloxacin Mesylate 146961-77-52009.12 阿那曲唑Anastrozole 120511-73-12010年专利到期2010 盐酸加巴喷丁(盐酸卡巴潘定)Gabapentin hydrochloride 60142-96-32010 泛昔咯韦Famciclovir 104227-87-42010 多奈哌齐Donepezil 120014-06-42010 孟鲁司特钠Montelukast sodium 151767-02-12010.01 卡莫司汀Carmustin 154-93-82010.01 加巴喷丁盐酸盐Gabapentin hydrochloride 60142-96-32010.05 扎鲁司特Zafirlukast 107753-78-62010.05 阿达帕林Adapalene 106685-40-92010.07 盐酸罗哌卡因单水合物Ropivacaine hydrochloride monohydrate 132112-35-7 2010.08 布林佐胺Brinzolamide 138890-62-72010.09 右雷佐生Dexrazoxane 24584-09-62010.10 盐酸阿洛司琼Alosetron hydrochloride 122852-69-12011年专利到期2011 钆喷酸葡胺Gadopentetate dimeglumine 86050-77-32011 西地那非Sildenafil 139755-83-22011.01 氯吡格雷Clopidogrel 113665-84-22011.03 拉坦前列腺素Latanoprost 130209-82-42011.03 盐酸普拉克索Pramipexole dihydrochloride 104632-25-92011.05 盐酸地尔硫卓Diltiazem hydrochloride 33286-22-52011.10 盐酸氮卓斯汀Azelastine hydrochloride 79307-93-02011.11 奈韦拉平Nevirapine 129618-40-22011.11 硫酸茚地那韦Indinavir sulfate 157810-81-62011.11 醋酸戈舍瑞林Goserelin acetate 145781-92-62012年专利到期2012 盐酸司来吉兰Selegiline hydrochloride 14611-52-02012 舍曲林Sertraline 79617-96-22012 沙美特罗羟萘甲酸盐Salmeterol hydroxynaphthoate 94749-08-32012 酒石酸托特罗定Tolterodine tartrate 124937-52-62012 缬沙坦Valsartan 137862-53-42012 顺氯氨铂Cisplatin 15663-27-12012 多西他赛Docetaxel 114977-28-52012 佐米曲普坦Zolmitriptan 139264-17-82012.02 混合雌激素Conjugated Estrogens 12126-59-92012.07 氨磷汀Amifostine 20537-88-62012.08 盐酸舍曲林Sertraline hydrochloride 79559-97-02012.09 比马前列素Bimatoprost 155206-00-12012.12 阿仑膦酸钠Alendronate sodium 121268-17-52013年专利到期2013 盐酸安非他酮Bupropion hydrochloride 31677-93-72013 盐酸非索非那定Fexofenadine hydrochloride 153439-40-82013 琥珀酸舒马曲坦Sumatriptan succinate 103628-48-42013 硫酸沙丁胺醇Albuterol sulfate 51022-70-92013.05 依法韦仑Efavirenz 154598-52-42013.06 醋酸去氨加压素Desmopressin acetate 16789-98-32013.07 美沙拉嗪Mesalamine 89-57-62013.09 白消安Busulfan 55-98-12013.10 奈非那韦Nelfinavir 159989-64-72013.11 阿托喹酮Atovaquone 95233-18-42013.11 安普那韦Amprenavir 161814-49-92014年专利到期2014.01 格列吡嗪Glipizide 29094-61-92014.02 吉妥珠单抗奥唑米星Gemtuzumab ozogamicin 220578-59-6 2014.06 盐酸奥洛他定Olopatadine hydrochloride 140462-76-6 2014.07 醋酸亮丙瑞林Leuprolide acetate 74381-53-62014 糠酸莫美他松Mometasone furoate 83919-23-72014 奥氮平Olanzapine 132539-06-12014 阿伐他汀Atorvastatin 134523-00-52014 雷洛昔芬Raloxifene 84449-90-12014 利托那韦Ritonavir Norvir 155213-67-52014.01 阿扎那韦Atazanavir 198904-31-32014.01 埃索美拉唑Esomeprazole 119141-88-72014.10 莫达非尼Modafinil 68693-11-82014.06 盐酸倍他洛尔Betaxolol hydrochloride 63659-19-82014.05 伊曲康唑Itraconazole 84625-61-62015年专利到期2015 丙泊酚Propofol 2078-54-82015 妊马雌酮Almestrone 10448-96-12015 鲑鱼降钙素Calcitonin salmon 47931-85-12015 卡维地洛Carvedilol 72956-09-32015 法莫替丁Famotidine 76824-35-62015.07 二丙酸氯地米松Beclomethasone dipropionate 5534-09-8 2015.08 维替泊芬Verteporfin 129497-78-52016年专利到期2016 利巴韦林Ribavirin 36791-04-52016 盐酸替罗非班Tirofiban hydrochloride 142373-60-22016 曲格列酮Troglitazone 97322-87-72019年专利到期2019.03 马来酸噻吗洛尔Timolol maleate 26921-17-5。
日本药典-孟鲁司特钠-英文

001-1409-2(仮訳).pdfMontelukast SodiumモンテルカストナトリウムC35H35ClNNaO3S: 608.17Sodium (1-{[((1R)-1-{3-[(1E)-2-(7-chloroquinolin-2-yl)ethenyl] phenyl}-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl)sulfanyl] methyl}cyclopropyl)acetate[151767-02-1]Montelukast Sodium contains not less than 98.0% and not more than 102.0% of C35H35ClNNaO3S, calcu-lated on the anhydrous basis and corrected on the amount of the residual solvent.Description Montelukast Sodium occurs as a white to pale yellowish white powder.It is very soluble in methanol and in ethanol (99.5), and freely soluble in water.It is hygroscopic.It turns yellow on exposure to light.Identification(1)Place 0.1 g of Montelukast Sodium in a crucible, and heat until a white residue is formed. To the residue add 2 mL of water, and then filter. To the filtrate add 2 mL of potassium carbonate solution (3 in 20), and heat to boiling: no precipitate is observed. To this solution add 4 mL of potassium hexahydroxoantimonate (V) TS, heat to boiling, and cool immediately in ice water: a white precipitate is formed. Rub the inside wall of the test tube with a glass rod, if necessary.(2)Determine the absorption spectrum of a solution of Montelukast Sodium in a mixture of methanol and water (3:1) (1 in 100,000) as directed under Ultraviolet-visible Spectrophotometry <2.24>, and compare the spectrum with the Reference Spectrum or the spectrum of a solution of Montelukast Sodium RS prepared in the same manner as the sample solution: both spectra exhibit similar intensities of absorption at the same wavelengths.(3)Determine the infrared absorption spectrum of Mon-telukast Sodium as directed in the paste method under Infra-red Spectrophotometry <2.25>, and compare the spectrum with the Reference Spectrum or the spectrum of Montelukast Sodium RS: both spectra exhibit similar intensities of ab-sorption at the same wave numbers. Or, perform the test by the potassium bromide disk method or ATR method, and compare the spectrum with the spectrum of Montelukast So-dium RS: both spectra exhibit similar intensities of absorp-tion at the same wave numbers.Purity (1) Heavy metals-Dissolve 0.5 g of Montelukast Sodium in 20 mL of a mixture of acetone and water (4:1), and use this solution as the sample solution. Separately, take 0.5 mL of Standard Lead Solution, add 20 mL of the mixture of acetone and water (4:1), and use this solution as the stand-ard solution. To the sample solution and the standard solution add 2 mL of acetate buffer solution, pH 3.5, and shake. To these solutions add 1.2 mL of thioacetamide-alkaline glycerin TS, shake immediately, then allow to stand for 2 minutes, and filter through a membrane filter with pore size 0.45 µm (about 13 mm in diameter). Compare the color on the mem-brane filters through which each solution is filtered: the color obtained from the sample solution is not darker than that obtained from the standard solution (not more than 10 ppm).(2)Related substances-Conduct this procedure using light-resistant vessels. Dissolve 50 mg of Montelukast So-dium in 50 mL of a mixture of methanol and water (9:1), and use this solution as the sample solution. Perform the test with 10 µL of the sample solution as directed under Liquid Chromatography <2.01>according to the following condi-tions. Determine each peak area by the automatic integration method, and calculate the amount of them by the area per-centage method: the amount of the peak having the relative retention time of about 1.9 to montelukast (related substance F) is not more than 0.3%, the amount of the peak having the relative retention time of about 0.4 (related substance A) is not more than 0.2%, the amounts of the peaks having the relative retention times of about 0.8 (related substance B) and about 1.2 (related substance E) are not more than 0.15%, respectively, the total amount of the two peaks having the relative retention time about 0.9 (related substances C and D) is not more than 0.15%, and the amounts of the peaks other than montelukast and the peaks mentioned above are not more than 0.10%, respectively. The total amount of the peaks other than montelukast is not more than 0.6%.Operating conditions-Detector, column, column temperature, mobile phase, and flow rate: Proceed as directed in the operating conditions in the Assay.Time span of measurement: For 16 minutes after injection, beginning after the solvent peak.System suitability-System performance: Proceed as directed in the system suitability in the Assay.Test for required detectability: Pipet 1 mL of the sample solution, add the mixture of methanol and water (9:1) to make exactly 100 mL. Pipet 1 mL of this solution, add the mixture of methanol and water (9:1) to make exactly 20 mL, and use this solution as the solution for system suitability test.001-1409-2(仮訳).pdf When the procedure is run with 10 µL of the solution forsystem suitability test under the above operating conditions,the SN ratio of the peak of montelukast is not less than 10.For the calculations mentioned above, the peak areassmaller than that of montelukast, founded in the chromato-gram obtained with 10 µL of the solution for system suitabil-ity test, are excluded.(3)Optical isomer-Conduct this procedure usinglight-resistant vessels. Dissolve 50 mg of Montelukast So-dium in 50 mL of a mixture of water and acetonitrile (1:1),and use this solution as the sample solution. Perform the testwith 10 µL of the sample solution as directed under LiquidChromatography <2.01>according to the following condi-tions. Determine each peak area by the automatic integra-tion method, and calculate the amounts of them by the areapercentage method: the amount of the peak having the rela-tive retention time of about 0.7 to montelukast is not morethan 0.2%.Operating conditions-Detector: An ultraviolet absorption photometer (wave-length: 280 nm).Column: A stainless steel column 4.0 mm in inside diame-ter and 15 cm in length, packed with α1-acid glycoproteinbinding silica gel for liquid chromatography (5 µm in particlediameter).Column temperature: A constant temperature of about30℃.Mobile phase A: Dissolve 2.3 g of ammonium acetate in1000 mL of water, and adjust to pH 5.7 with acetic acid(100).Mobile phase B: A mixture of methanol and acetonitrile(3:2).Flowing of mobile phase: Control the gradient by mixingthe mobile phases A and B as directed in the following table.Time after injection of sample (min) Mobile phase A(vol%)Mobile phase B(vol%)0 -30 70 →60 30 →4030 -35 60 40Flow rate: 0.9 mL per minute (the retention time of mon-telukast is about 25 minutes).System suitability-Test for required detectability: Pipet 1 mL of the sample solution, add the mixture of water and acetonitrile (1:1) to make exactly 100 mL. Pipet 1 mL of this solution, add the mixture of water and acetonitrile (1:1) to make exactly 10 mL. When the procedure is run with 10 µL of this solution under the above operating conditions, the SN ratio of the peak of montelukast is not less than 10.System performance: Dissolve about 5 mg of Montelukast Racemate RS in the mixture of water and acetonitrile (1:1) to make 50 mL. When the procedure is run with 10 µL of this solution under the above operating conditions, the resolution between the peak of montelukast and the peak having the relative retention time of about 0.7 to montelukast is not less than 2.9.(4)Residual solvent-Being specified separately when the drug is granted approval based on the Pharmaceutical Affairs Law.Water <2.48>Not more than 4.0% (0.3 g, volumetric titra-tion, direct titration).Assay Conduct this procedure using light-resistant vessels. Weigh accurately about 50 mg of Montelukast Sodium, and dissolve in a mixture of methanol and water (9:1) to make exactly 50 mL. Pipet 10 mL of this solution, add the mixture of methanol and water (9:1) to make exactly 100 mL, and use this solution as the sample solution. Separately, weigh accurately about 26 mg of Montelukast Dicyclohexylamine RS, dissolve in the mixture of methanol and water (9:1) to make exactly 50 mL. Pipet 5 mL of this solution, add the mixture of methanol and water (9:1) to make exactly 20 mL, and use this solution as the standard solution. Perform the test with exactly 10 µL each of the sample solution and standard solution as directed under Liquid Chromatography <2.01> according to the following conditions. Determine the peak areas, A T and A S, of montelukast in each solution.Amount (mg) of C35H35ClNNaO3S=M S×A T/A S×5/2 ×0.792M S: Amount (mg) of Montelukast Dicyclohexylamine RSOperating conditions-Detector: An ultraviolet absorption photometer (wave-length: 238 nm).Column: A stainless steel column 4.6 mm in inside diame-ter and 5 cm in length, packed with phenylsilanized silica gel for liquid chromatography (1.8 µm in particle diameter). Column temperature: A constant temperature of about 30℃.Mobile phase A: A mixture of water and trifluoroacetic acid (2000:3).Mobile phase B: A mixture of acetonitrile and trifluoroa-cetic acid (2000:3).Flowing of mobile phase: Control the gradient by mixing the mobile phases A and B as directed in the following table.Time after injectionof sample (min)Mobile phase A(vol%)Mobile phase B(vol%)0 - 3 60 403 -16 60 →49 40 →51 Flow rate: 1.2 mL per minute (the retention time of mon-telukast is about 7 minutes).001-1409-2(仮訳).pdfSystem suitability-System performance: Dissolve 10 mg of Montelukast for Peak Identification RS in the mixture of methanol and water (9:1) to make 10 mL, and use this solution as the solution A for peak identification. Perform the test with 10 µL of the solution A for peak identification under the above operating conditions, and identify the peaks having the relative reten-tion times to montelukast of about 0.4 (related substance A), about 0.9 (related substances C and D), about 1.2 (related substance E), and about 1.9 (related substance F). Place 1 mL of the solution A for peak identification in a clear glass con-tainer, allow to stand for about 20 minutes, and use this solu-tion as the solution B for peak identification. When the pro-cedure is run with 10 µL of the solution B for peak identifi-cation under the above operating conditions, and identify the peak having the relative retention time of about 0.8 to mon-telukast (related substance B), the resolution between the peaks of related substance B and montelukast is not less than 2.5, and between the peaks of montelukast and related sub-stance E is not less than 1.5.System repeatability: When the test is repeated 5 times with 10 µL of the standard solution under the above operat-ing conditions, the relative standard deviation of the peak area of montelukast is not more than 0.73%.Containers and storage Containers-Tight containers Storage-Light-resistant.Related substancesRelated substance A:(1-{[(1-{3-[(1E)-2-(7-Chloroquinolin-2-yl)ethenyl]phenyl} -3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl)sulfinyl] methyl}cyclopropyl)acetic acid Related substance B:(1-{[((1R)-1-{3-[(1Z)-2-(7-Chloroquinolin-2-yl)ethenyl] phenyl}-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl)sulfanyl]methyl}cyclopropyl)acetic acidRelated substance C:(1-{[((1R)-1-{3-[(1R)-1-({[1-(Carboxymethyl)cyclopropyl] methyl}sulfanyl)-2-(7-chloroquinolin-2-yl)ethyl]phenyl}-3- [2-(1-hydroxy-1-methylethyl)phenyl]propyl)sulfanyl]methyl}cyclopropyl)acetic acidRelated substance D:(1-{[((1R)-1-{3-[(1S)-1-({[1-(Carboxymethyl)cyclopropyl] methyl}sulfanyl)-2-(7-chloroquinolin-2-yl)ethyl]phenyl}-3- [2-(1-hydroxy-1-methylethyl)phenyl]propyl)sulfanyl]methyl}cyclopropyl)acetic acidRelated substance E:(1-{[((1R)-3-(2-Acetylphenyl)-1-{3-[(1E)-2-(7-chloroquinolin -2-yl)ethenyl]phenyl}propyl)sulfanyl]methyl}cyclopropyl)acetic acid001-1409-2(仮訳).pdfRelated substance F:(1-{[((1R)-1-{3-[(1E)-2-(7-Chloroquinolin-2-yl)ethenyl]phenyl}-3-[2-(1-methylethenyl)phenyl]propyl)sulfanyl]methyl}cyclopropyl)acetic acidAdd the following to 9.01 Reference Standards(1):Montelukast Sodium RSMontelukast Dicyclohexylamine RSMontelukast Racemate RSMontelukast for Peak Identification RS。
欧前胡素检测

迪信泰检测平台
欧前胡素检测
欧前胡素(Imperatorin),又称白茅苷、前胡内酯、白芷乙素。
具有抗菌、平喘及抗过敏等作用,是多种止痛药物中的质量控制标准成分之一。
极性小,不溶于水,易溶于极性小的有机溶剂。
存在于伞形科植物兴安白芷的根、芸香科植物芸香的全草中,属于呋喃香豆素类化合物。
迪信泰检测平台采用高效液相色谱(HPLC)和液质联用(LC-MS)法,可高效、精准的检测欧前胡素的含量变化。
此外,我们还提供其他苯丙素类检测服务,以满足您的不同需求。
HPLC和LC-MS测定欧前胡素样本要求:
1. 请确保样本量大于0.2g或者0.2mL。
周期:2~3周。
项目结束后迪信泰检测平台将会提供详细中英文双语技术报告,报告包括:
1. 实验步骤(中英文)。
2. 相关质谱参数(中英文)。
3. 质谱图片。
4. 原始数据。
5. 欧前胡素含量信息。
迪信泰检测平台可根据需求定制其他物质测定方案,具体可免费咨询技术支持。
