欧盟OMCL仪器确认附件1-HPLC确认
仪器设备的4Q验证

仪器设备的4Q验证0、仪器验证的重要性仪器验证是国际上多个国家和权威组织对药品生产企业在仪器设备管理上的法规强制要求。
经济合作与发展组织(Organization for Economic Cooperation andDevelopment, OECD)良好实验室规范原则(GLP)明确要求GLP试验机构应对计算机化的实验仪器进行验证。
欧洲官方药品控制实验室(official medicine controllaboratory, OMCL)制定了仪器验证(确认)的核心文件。
美国药典(United StatesPharmacopoeia,USP)有专门应用于仪器分析的文件USP1058《分析仪器验证指导原则》,USP的标准在美国由药品与食品管理局 (FDA) 强制实施。
目前国内外药品行业对仪器的质量管理普遍实行的是“4Q验证”,“4Q验证”可分为4个连续阶段,依次是设计确认(DesignQualification,DQ)、安装确认(Installationqualification,IQ)、运行确认(OperationalQualification,OQ)和性能确认(PerformanceQualification,PQ)。
1、设计确认(DQ)设计确认(DQ)是确认仪器的功能性和操作指标满足仪器的预定用途,以此作为选择仪器供应商的标准。
DQ包括的内容有:实验室根据使用要求,提出实验室需求说明(Userrequirementsspecification,URS);仪器供应商有针对性地回复URS。
对仪器硬件/软件的各项指标进行设计确认;实验室对供应商的DQ进行确认,以保证满足仪器的预定用途;实验室选择供应商。
DQ由实验室负责,实验室应确保仪器适用于预期用途。
供应商只能作为仪器DQ的一部分,负责提供技术指标和其他有关信息。
URS是由实验室提出的,但不能完全依赖供应商。
DQ在仪器购买前完成。
2、安装确认(IQ)IQ是确认收到的仪器与设计和指定的仪器相符,仪器在选定的环境中正确安装,并且该仪器在这种环境中运行和使用是合适的。
欧盟:计算机化系统的验证-核心文件(中英文)

OMCL Network of the Council of EuropeQUALITY ASSURANCE DOCUMENTPA/PH/OMCL (08) 69 3R VALIDATION OF COMPUTERISED SYSTEMS计算机化系统的验证CORE DOCUMENT核心文件VALIDATION OF COMPUTERISED SYSTEMS计算机化系统验证CORE DOCUMENT核心文件SCOPE 范围This guideline defines basic principles for the validation of computerised systems used within Official Medicines Control Laboratories (OMCLs) with impact on quality of results. The purpose of this validation is to guarantee the confidence in scientific results obtained with each computerised system. A validated system ensures accurate results and reduces the risk of failure of the system.本指南给出了在OMCL化验室使用的计算机化系统验证的基本原则。
本验证的目的是保证由每个计算机化系统所得到的科学结果的可信性。
一个验证体系会保证准确的结果,降低系统失败的风险。
This document covers in-house and commercial software for calculation, database computerised systems, Laboratory Information Management Systems (LIMS), Electronic Laboratory Notebooks (ELN) and computers as part of test equipment.本文件包括了内控和商业计算软件,数据库计算机化系统,化验室信息管理系统(LIMS),化验室电子笔记本(ELN)和计算机作为检测仪器的一部分。
HPLC设备的确认(中英) Qualification of HPLC Equipment解读

OMCL Network of the Council of Europe QUALITY MANAGEMENT DOCUMENT欧洲委员会OMCL网络质量管理文件PA/PH/OMCL (11) 04QUALIFICATION OF EQUIPMENT设备的确认ANNEX 1: QUALIFICATION OF HPLC EQUIPMENT附件1:HPLC (高效液相色谱仪) 设备的确认ANNEX 1 OF THE OMCL NETWORK GUIDELINE“QUALIFICATION OF EQUIPMENT”OMCL网络指南“设备的确认”之附件1QUALIFICATION OF HPLC EQUIPMENTHPLC设备的确认Introduction 概述The present document is the first Annex of the core document “Qualification of Equipment”, and it should be used in combination with it when planning, performing and documenting the HPLC equipment qualification process.本文件是核心文件“设备的确认”第1个附件,在计划、实施和记录HPLC仪器的确认过程时,应将本文件与核心文件一起使用。
The core document contains the general introduction and the Level I and II of qualification, common to all type of instruments, and the present annex contains HPLC instrument-related recommendations on parameters to be checked and the corresponding typical acceptance Limits, as well as practical examples on the Methodology that can be used to carry out these checks.核心文件包括了简介和第一阶段和第二阶段的确认,它们适用于所有类型的仪器。
跟我一起做学习笔记之三—电子天平

跟我一起做学习笔记之三—电子天平电子天平是我们化验室日常检验必须用到的,也是非常关键的一种仪器,因为其很常见,很多人都认为它的使用很简单,但其合理应用与否可能直接影响到产品检测结果,它的操作可能在实际工作中却存在着巨大差异,这里我们来梳理一下,把握好天平的各种要求,保证称量的结果的准确性。
这次的学习笔记主要针对实验室最常用的万分之一和十万分之一天平进行的总结。
其中:万分之一天平(分析天平)常用于精密称量100mg以上的物质、炽灼残渣(坩埚)、干燥失重(称量瓶)十万分之一天平(半微量天平)常用于精密称量100mg-10mg物质,其中最小称量值要根据USP41进行确定允许误差:精密称定系指称重量应准确到所取重量的千分之一天平的法规要求GMP要求:质量管理,指明方向,但没有具体操作说明。
药典药求:药典关注天平称量结果的准确性,控制称量结果的相对误差,例如:称量区分:“精密称定”和“称定”。
USP(美国药典)USP WEIGHINGON AN ANALYTICAL BALANCE使用分析天平称量USPWEIGHTS ANDBALANCES砝码与天平USP ANALYTICALINSTRUMENT QUALIFICATION 分析仪器确认OMCL网络质量管理文件:很好的操作性。
OMCLNetwork of theCouncil of Europe QUALITY MANAGEMENT DOCUMENT:QUALIFICATION OF EQUIPMENT ANNEX 8: QUALIFICATION OF BALANCESOMCL网络质量管理文件仪器确认附件8:天平的确认注:编码为PA/PH/OMCL (12) 77 7R计量检定规程要求:关注天平本身,对天平的各项指标有具体的阐述,最后给一个检定结论:合格与否。
eg. 天平区分等级:“I级”、“II级”、“III级”、“IIII级”国标GB/T 26497-2011 《电子天平》注:适用于检定分度值不小于1mg 电子天平的设计与制造JJG 1036-2008 《电子天平》GB/T 4167-2011 《砝码》GB/T 26797-2011 《E1、E2、F1、F2、M1、M1-2、M2、M2-3、M3等级砝码》JJG 99-2006 《砝码》检定规程JJF 1139-2005 《计量器具检定周期确定原则和方法》OIML R 76-1: 2006天平的安装要求电源电压应在220V±10%之间,电源频率在50HZ±2%之间天平房间的选择:防风、防晒、温度恒定(温度的变化不应该超过5℃/小时)、湿度:(通常应在40%~60%之间)天平台:要先用防震、水平的周围应无影响校正的强电场、强磁场、气流(考虑到空调出风口的影响)天平的最小称量值由于测量的不确定度影响,当天平在低量程段称量时,绝对误差较小的情况下,相对误差会相当大,甚至超出了允许误差范围,这时为了保证天平的可靠性,必须知道天平的最小称量。
OMCL设备确认—— HPLC
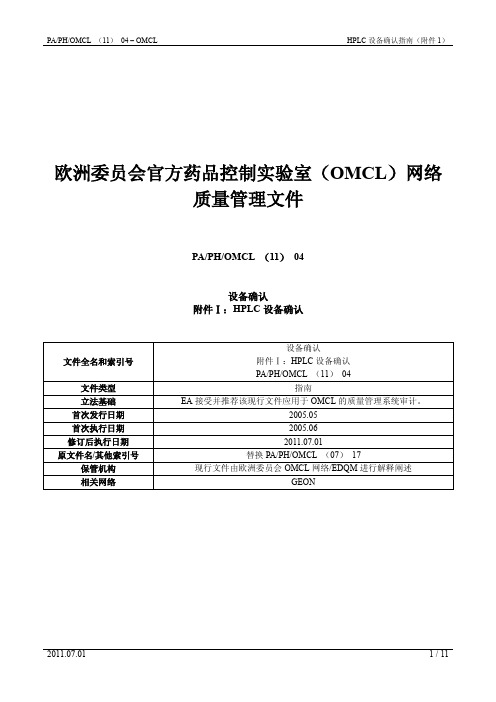
V t
f
V 60 t
f::测得的流速,ml/min.。
t:充满至刻度所用的时间,s。
V:容量瓶的体积,ml。
D
100
f
F F
D:偏差,%。
F:调节后的流速,ml/min.。
f::测得的流速,ml/min.。
限度:±5%
梯度配比和波动
设置: 不锈钢毛细管,如以 2000×0.12mm 的柱子代替色谱柱进行安装。 检测器:紫外检测器调节至 265nm。 流动相 A:脱气水。 流动相 B:含 0.5%的脱气水。 流速:1.0ml/min。
论或 MA 文件中的特定要求。)
保留时间精密度
RSD±5%
残留
(通过比较待进行定量检测物质的
≤0.2%
连续标准和空白进样)
信噪比(用于有关物质检测)
根据欧洲药典
2011.07.01
3 / 11
PA/PH/OMCL (11) 04 – OMCL
HPLC 设备确认指南(附件 1)
附件Ⅰ:
第Ⅲ级 周期性主动仪器检查
表 IV:
第 IV 级 在用仪器的检查
配备 UV 或 DAD 检测器的 HPLC 仪器的要求举例
需检查的参数
典型允差限度
方法的系统适用性检查
根据欧洲药典、MAH 文件或公司内部经过验证的方法
峰面积准确度 (适用于供试液中主峰面积)
RSD≤1.5% (除非另有方法在系统适用性里有描述,例如:EP2.2.46,原料药各
温度。 限度:38~42℃。
HPLC UV/DAD 检测器
对 HPLC UV/DAD 检测器的定期性主动检查可以采用测定线性和波长准确度的方法进行。
线性
OMCL指导文件 设备的确认

General European OMCL Network (GEON) QUALITY MANAGEMENT DOCUMENTPA/PH/OMCL (08) 73 R3QUALIFICATION OF EQUIPMENTCORE DOCUMENTFull document titleand reference Qualification of Equipment – Core documentPA/PH/OMCL (08) 73 R3Document typeGuidelineLegislative basisCouncil Directive 2001/83/EC and 2001/82/EC, as amended The present document was also accepted by EA asrecommendation document to be used in the context of Quality Management System audits of OMCLs Date of first adoption1st October 1999 Date of original entry into force1st February 2000 Date of entry into force of revised document1st May 2017Previous titles/other references / last valid version This document replaces document PA/PH/OMCL (08) 73 2R Custodian Organisation The present document was elaborated by the OMCL Network / EDQM of the Council of Europe Concerned Network GEONQUALIFICATION OF EQUIPMENTCORE DOCUMENTIntroductionThe standard ISO/IEC 17025 requires an appropriate choice and qualification of equipment to be used for testing purposes. Particularly, checks and calibrations before and during use and, if needed, intermediate tests (see ISO/IEC 17025 chapter 5.5.10) are necessary.In order to guarantee a harmonized interpretation and application within the OMCL Network, the guideline « Qualification of Equipment » has been elaborated.From experience, the terms DQ, IQ, OQ and PQ (not explicitly mentioned by ISO/IEC 17025) have been used in a non-harmonized way amongst the different OMCLs. Therefore their mention has been avoided in this document. This does not exclude their use in OMCL’s quality systems where already approved and in application, or a reference to literature using this nomenclature.In order to simplify the management of the guideline, the present document contains only the general introduction and the first two levels of qualification, which are common to all type of equipment. The third and fourth levels of qualification can be found in separate instrument-related annexes. When considered appropriate, additional requirements and/or examples related to Level I and/or Level II have also been included in the annexes, which are to be used in combination with the general recommendations given in the core document.The list of annexes, included in this document, will be updated as soon as new annexes are issued. This document should be considered as a guide to OMCLs for planning, performing and documenting the equipment qualification process. It should not be taken as an exhaustive list of compulsory tests. It is left to the professional judgement and background experience of each OMCL to decide on the most relevant tests and the most appropriate tolerance limits for each of the parameters, in order to give evidence that the instrument is working properly and is appropriate for its intended use.If the qualification of equipment is done by the manufacturer itself or an external service, it is under the responsibility of the OMCL to make sure that the checks performed are in line with the minimum requirements set in this guideline.To facilitate the implementation of a documented qualification process for the various analytical instruments, specific recommendations on minimum requirements are given in the corresponding annexes.For the more technique-related aspects of equipment qualification checks, practical examples of possible approaches are also presented in the annexes.The following four levels of Equipment Qualification should be considered by the OMCLs:Level I. Selection of instruments and suppliersThe selection and purchase of new instruments shall follow a conscious decision process, based on the needs related to the intended use of the instrument.An example for setting and documenting such specifications and decisions taken is given in Table I.Level II. Installation and release for useWhen receiving an instrument, the OMCL should check that it is received in good conditions, as ordered, and should monitor and document the installation process of the instrument in the selected environment. This includes the start up checks done by the supplier, followed by a full periodic check as described in Level III.The release for use shall be documented and authorised by the person responsible for the instrument.An example for documenting the instrument installation and release for use and decisions taken is given in Table II.Level III. Periodic and motivated instrument checksWhen instruments are installed or moved into a new environment a series of checks have to be carried out to verify the key performance parameters of the instrument avoiding additional contributory effects from the analytical method. Depending on the frequency of use and the experienced stability of the instrument this shall be repeated periodically.The same verifications (or a relevant part of them) shall be carried out following events like significant repair or maintenance operations.Examples of parameters to be checked on instruments and their typical acceptance limits can be found in the Table III of the corresponding instrument-related annex.The specifications from the manufacturer of the instrument should be taken into account when setting the tolerance limits.Some examples on how these checks may be performed on each type of instrument are also provided in the corresponding Annexes.Level IV. In-use instrument checksDuring the day-to-day use of the instruments, checks are necessary to demonstrate continued evidence of satisfactory performance by the instrument itself and compliance with the system suitability criteria as defined in the applied analytical procedure for each product or group of products tested at this occasion.Examples of parameters to be checked on instruments and their typical acceptance limits can be found in the Table IV of the corresponding instrument-related annex.In the case of OMCLs performing routine testing (batch release of vaccines and blood products), the use of control charts provides supplementary information on equipment performance, which can also be used in this context.List of instrument-related annexesThe qualification levels dealt with in each annex are indicated in brackets.∙Annex 1: Qualification of HPLC equipment (Levels III and IV)∙Annex 2: Qualification of GC equipment (Levels III and IV)∙Annex 3: Qualification of UV-Visible spectrophotometers (Levels I, III and IV)∙Annex 4: Qualification of IR spectrophotometers (Levels I, III and IV)∙Annex 5: Qualification of automatic titrators (Levels III and IV)∙Annex 6: Qualification of piston pipettes (Levels III and IV)∙Annex 7: Qualification of mass spectrometers (Levels III and IV)∙Annex 8 : Qualification of balances (Levels I to IV)∙Annex 9: Calibration/qualification of pH meters (Levels I to IV)∙Annex 10: Qualification of Atomic Absorption / Atomic Emission Spectrometers (Levels III and IV)TABLE I Level I. Selection of instruments and suppliersExample of check-list (non-exhaustive)Manufacturer:Provider/Distributor:Name of instrument and type:Attribute(This list may be adapted ifnecessary) SpecificationsBenefits(Instrument/supplier)AssessmentPass FailsTechniqueCommunication and datahandlingInterface RS232Data transfer to spreadsheetsCompatible with other hard-and software such as LIMS…SafetyIrradiation Explosion protectionDocumentationManual (paper copy)HandlingUser languageService and maintenanceServices offeredWarranty SupportDelivery (duration etc.)Installation(Service / Laboratory)Training (in-house / externalcourses)TABLE I (cont.) Requirements for media and environmentCost / Benefit AnalysisComments / DecisionsDate / Signature:Date / Signature for approval:TABLE IILevel II. Installation and release for useExample of check-list (non-exhaustive)Name of instrument and type:Identification code:Conformity with order (instrument / material / documentation)Pass Fails (description of deficiencies)Check of damagesPass Fails (description of deficiencies)Check of required media supply (connections / environmental conditions)Pass Fails (description of deficiencies)Installation of instrument(s) including possible control modulesPass Fails (description of deficiencies)Performance of start-up checks and diagnosis functions 1Pass Fails (description of deficiencies)1CommentsWhen appropriate, raw data are attached to the instrument documentationDate / Signature:Release and authorisation for use: Date / Signature:Disclaimer:The present Core Document of the OMCL Guideline “Qualification of Equipment”, as well as all its Annexes, have been drafted by ad-hoc working groups of technical experts, mainly coming from Official Medicines Control Laboratories (OMCLs) and only occasionally from other public institutions. These working groups do not include any representative from any commercial organisation.This Core Document and its Annexes may contain trade names of laboratory instruments, materials and/or reagents. These are exclusively given as example in order to make these guidelines easier to understand and implement, and were found to be suitable when the guideline was being developed. These references do not imply in any way that the mentioned instruments, materials or reagents or their suppliers are especially endorsed, recommended or certified by the EDQM, the OMCL Network or the Council of Europe, in preference to others of a similar nature which are not mentioned. It is therefore acceptable to use instruments, materials and reagents from another source, provided that they fulfil the necessary criteria laid down in these documents and appropriately satisfy the needs of the concerned laboratories in the frame of their specific activities.。
OMCL设备确认——附件3_UV

需检查的参数
典型允许限度
1. 方法的系统适用性检查
根据欧洲药典、MAH 文件或经验证内部方法。
‐ 如,重复性;
‐ 如,溶液(若定量分析要求的话)
2. 吸收池
吸光度差异≤0.005
2007.12
4 / 10
PA/PH/OMCL (07) 11 DEF CORR– OMCL
紫外-可见分光光度计确认指南(附件 3)
Gain = (100) see 4 below
增益=(100)见下述第 4 项
3. 选择“Option”(选择)项,设定以下参数:
原文
翻译
SWB = 4 nm
SWB=4nm
Beam mode = Single front
光束模式=单光束在前
Lamps on = Deuterium
开灯=氘
Source change = 700.0 nm
486.0nm(Dβ)
302.25nm(Hg)
486.1nm(Hβ)
313.16nm(Hg)
536.3nm(Ho)
334.15nm(Hg)
546.07nm(Hg)
361.5nm(Ho)
576.96nm(Hg)
365.48nm(Hg)
579.07nm(Hg)
如果使用的是商品滤光片,应当将测得的吸收值与供应商提供的值进行比较,测量应扣除空气空白。
限度: 重复性:最大吸收处的相对标准偏差应满足供应商提供的标准。 6 个单独峰之间的差值应符合供应商提供的标准(例如<0.5nm)。
如果仪器较陈旧,波长可能采用转动转轮以达到选定波长的方式进行设定。这种情况下,机械磨损可 能会导致实际波长有差异,这取决于是从较高波长还是从较低波长开始向目标波长调节。如果存在差异, 可以采用从高波长和低波长进行设置的方式对该变化进行检查。
EDQM对实验结果的评估及报告(核心文件)

欧洲委员会的OMCL网络质量控制文件PA/PH/OMCL (13) 113 2R Evaluation and Reporting of ResultsCore document Full document title and reference Evaluation and Reporting of Results – Core DocumentPA/PH/OMCL (13) 113 2RDocument type Guideline Legislative basis /Date of first adoption October 1999 Date of original entry into force February 2000 Date of entry into force of revised document October 2014Previous titles / other references / last valid version This document replaces documentPA/PH/OMCL (07) 28 DEF CORR Former titles / references:Evaluation and Reporting of Results from Assays, PA/PH/OMCL (02) 52 DEF Evaluation and Reporting of Results,PA/PH/OMCL (99) 38 DEFCustodian Organisation The present document was elaborated by theOMCL Network / EDQM of the Council ofEuropeConcerned Network GEON对实验结果的评估及报告EVALUATION AND REPORTING OF RESULTS核心文件CORE DOCUMENT对于政府药品质控实验室(OMCL)的指导原则GUIDELINE FOR OMCLs1. 范围1. SCOPE本指导原则针对政府药品质控实验室(OMCL)在对工业化生产的药品1、中间产品及API进行的检测,定义了在报告结果时的一些基本原则。
OMCL设备确认——核心文件

欧洲委员会官方药品控制实验室(OMCL)网络质量管理文件PA/PH/OMCL(08)732R设备确认核心文件文件全名和索引号设备确认附件4:核心文件PA/PH/OMCL(08)732R文件类型指南立法基础EA接受并推荐该现行文件应用于OMCL的质量管理系统审计。
首次发行日期1999.10.01首次执行日期2000.12.01修订后执行日期2011.07.01原文件名/其他索引号替换PA/PH/OMCL(08)73R 保管机构现行文件由欧洲委员会OMCL网络/EDQM进行解释阐述相关网络GEON设备确认核心文件介绍ISO/IEC17025标准要求用于检测的仪器应进行适当的选择和确认,特别是在使用前和使用中应进行检查和校正,必要时应进行中间测试(见ISO/ICE17025第5.5.10章节)。
为了保证在OMCL网络内的统一解释和应用,特制订此指南《仪器确认》。
根据经验,术语DQ,IQ,OQ和PQ(在ISO/IEC17025中未明确提出)已由非协调方式在不同OMCL 中采用,因此在本文中避免对这些术语的使用。
但这并不排除OMCL的质量体系在已批准和实际中采用这些术语,或者引用一些使用了这些术语的文献。
为了简化指南管理,本文件仅包括通用介绍,以及前两个级别的确认,可以适用于所有类型的仪器。
第Ⅲ级和第Ⅳ级的确认可以在单独的仪器相关的附件中找到。
考虑到适用性,附加要求和/或第Ⅰ级和/或第Ⅱ级的相关举例也列在了附件中,这些内容应与该核心文件中的通用推荐结合使用。
如有新附件增加,包括在本文件中的附件清单将同步更新。
本文件应被作为OMCL的指南,用于计划、实施和记录仪器确认过程。
它不应该作为一个必检内容的完全列举清单。
各OMCL应根据自己的专业判断和背景经验来决定最相关的测试,每个参数的最合适允许限度,以证明仪器能正常工作,并适合其使用目的。
如果仪器的确认由供应商提供,或委托外部服务机构进行,那么OMCL有责任保证所进行的检查符合本指南的最低要求。
欧盟GMP附录15确认和验证中英文新版

欧盟GMP附录15确认和验证欧盟GMP附录15确认和验证ANNEX 15 附件15Qualification and Validation确认和验证Table of Contents 目录1. Qualification and Validation 确认和验证2. Planning for Validation 验证计划3. Documentation 文件4. Qualification 确认5. Process Validation 工艺验证6. Cleaning Validation 清洁验证7. Change Control 变更控制8. Revalidation 再验证9. Glossary 术语表Qualification and Validation 确认和验证Principle 原理1.This Annex describes the principles of qualification and validation which are applicable to the manufacture of medicinal products. It is a requirement of GMP that manufacturers identify what validation work is needed to prove control of the critical aspects of their particular operations. Significant changes to the facilities, the equipment and the processes, which may affect the quality of the product, should be validated. A risk assessment approach should be used to determine the scope and extent of validation.1.本附件描述了确认和验证的原理,适用于医药产品的生产者。
欧盟OMCL仪器确认附件7-质谱

OMCL Network of the Council of EuropeQUALITY MANAGEMENT DOCUMENT质量管理文件PA/PH/OMCL (10) 86 2RQUALIFICATION OF EQUIPMENT仪器确认ANNEX 7: QUALIFICATION OF MASS SPECTROMETERS附件7:质谱仪的确认ANNEX 7 OF THE OMCL NETWORK GUIDELINE“QUALIFICATION OF EQUIPMENT”OMCL网络指南“仪器确认”附件77.1: QUALIFICATION OF GAS CHROMATOGRAPHY-MASS SPECTROMETERS WITH ELECTRON IMPACT IONIZATION (GC-EI-MS) 装备电子碰撞电离的气相色谱-质谱仪的确认Introduction 介绍The present document is the first part of the 7th Annex of the core document “Qualification of Equipment”, and it should be used in combination with it when planning, performing and documenting the qualification process of GC-EI-MS.本文件是核心文件“仪器确认”的第7个附件,在计实施和记录GC-EI-MS的确认过程中,应与核心文件结合使用。
The core document contains the Introduction and general forms for Level I and II of qualification, which are common to all type of instruments.核心文件包括了介绍和适用于所有类型仪器的第一级和第二级确认用通用表格。
OMCL设备确认——附件2_GC
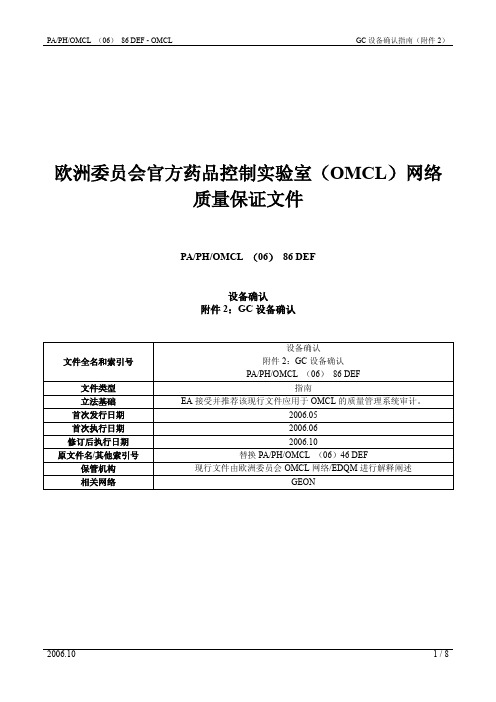
如果仪器有一个内置的自动系统进行噪音和漂移确认,应根据供应商的指示进行,并采用供应商的可 接受标准。如无,则采用以下检查: 设置:
所安装的色谱柱; 合适的流速,取决于柱长度/直径; 无进样; 柱温:40℃ 打开检测器,加热至工作温度(270~300℃)。 方法: 系统平衡后,记录 15min 信号; 噪音:计算 10 次 1min 内的噪音,计算平均值; 漂移:计算 15min 内基线漂移的斜率。 限度:
见附件 1
3.4 漂移
见附件 2
表 IV:
第 IV 级 在用仪器的检查
采用 FID 检测器的 GC 仪器要求举例
需检查的参数 方法系统适用性检查
峰面积精密度 保留时间重复性 灵敏度(如果相关,例如对于有关物质)
典型可接受限度
根据欧洲药典、MAH 相关文件或经验证的内部方法 除另有规定外,RSD≤3.0%* RSD≤2.0%
2006.10
1/8
PA/PH/OMCL (06) 86 DEF - OMCL
GC 设备确认指南(附件 2)
OMCL 网络指南附件 2 “设备确认”
GC 设备确认
概述 本文件是核心文件“仪器的确认”的第 2 个附件,在计划、实施和记录 HPLC 仪器的确认过程时,应
将本文件与核心文件一起使用。 核心文件包括了一般介绍和第Ⅰ级、第Ⅱ级确认,适用于所有类型的仪器,本附件包括了 GC 仪器相
全面检查 2:
全面检查 2 包括以下参数: 进样器、柱温箱和检测器温度准确性和稳定性:保留时间重复性。
建议采用两个可相互替代的方法之一。
全面检查 2A
供试液:
‐ 0.035ml(1-辛醇);
‐ 0.035ml(2-辛酮);
PA PH OMCL (07) 11 DEF CORR 欧盟质量保证文件 仪器验证——紫外可见分光光度计验证

OMCL Network of the Council of Europe QUALITY ASSURANCE DOCUMENT PA/PH/OMCL (07) 11 DEF CORRQUALIFICATION OF EQUIPMENTANNEX 3: QUALIFICATION OF UV-VISIBLESPECTROPHOTOMETERSFull document title and reference Qualification of EquipmentAnnex 3: Qualification of UV-Visible spectrophotometers PA/PH/OMCL (07) 11 DEF CORRDocument type GuidelineLegislative basis The present document was also accepted by EA asrecommendation document to be used in the context of QualityManagement System audits of OMCLsDate of first adoption May 2007Date of original entryinto forceJuly 2007Date of entry into forceof revised documentDecember 2007Previous titles/otherreferencesThis document replaces document PA/PH/OMCL (07) 11 DEFCustodian Organisation The present document was elaborated by the OMCL Network/EDQM of the Council of EuropeConcerned Network GEONANNEX 3 OF THE OMCL NETWORK GUIDELINE“QUALIFICATION OF EQUIPMENT”QUALIFICATION OF UV-VISIBLE SPECTROPHOTOMETERSIntroductionThe present document is the third Annex of the core document “Qualification of Equipment”, and it should be used in combination with it when planning, performing and documenting the UV-Visible spectrophotometer qualification process.The core document contains the Introduction and general forms for Level I and II of qualification, which are common to all type of instruments.For UV-Visible spectrometers, an example has been added to give instrument-specific proposals that may be used in combination with the general requirements presented in the core document “Qualification of Equipment”, when drawing up a Level I checklist.The present annex contains instrument-related recommendations on parameters to be checked at Level III and IV of qualification and the corresponding typical acceptance limits, as well as practical examples on the methodology that can be used to carry out these checks.TABLE I Level I. Selection of instruments and suppliersExample of check-list (non-exhaustive)Manufacturer:Provider/Distributor:Name of instrument and type:Notes:-This check-list, containing examples of attributes that can be taken into account in the selection of an instrument and supplier, can be used in combination with the general check-list presented in Level I in the core document “Qualification of Equipment”.-For Table II (Level II of Equipment Qualification: Installation and release for use) please refer to the core document.TABLE III Level III. Periodic and motivated instrument checksExamples of requirements for UV-Visible spectrophotometers Parameter to be checked Typical tolerance limits1. Spectral slit-width (if applicable)± 10 %2. Wavelength accuracy± 1 nm for the UV range± 3 nm for the Visible range3. Wavelength precisionSee manufacturer’s specifications(for mechanically set wavelengths)4. Photometric accuracySee Annex I(Control of absorbance)5. Photometric linearityr2≥ 0.9996. Limit of stray lightA > 2.0 at 198 nm7. Baseline noise± 0.002 Absorbance units (500 nm)or± 0.01 Absorbance units (200, 300, 400 nm)8. Photometric drift± 0.001 Absorbance units/h (250 nm)or± 0.002 Absorbance units/h (500 nm)TABLE IV Level IV. In-use instrument checksExamples of requirements for UV-Visible spectrophotometers Parameter to be checked Typical tolerance limits1. System suitability check of the method- e.g. Repeatability- e.g. Resolution (if required forqualitative analysis)According to Ph. Eur.or MAH dossier or validated in-house method2. Absorption cells Absorbance difference ≤ 0.005ANNEX I Level III. Periodic and motivated instrument checksThis Annex contains practical examples of tests and their associated tolerance limits for several parameters related to the performance of a UV-Visible spectrophotometer.These examples can be considered by the OMCLs as possible approaches to perform the Level III of the equipment qualification process: “Periodic and motivated instrument checks”. GENERAL CONSIDERATIONS-Measurements made by comparing samples against external standards should be made under conditions during which temperature is held constant. This is particularly relevant where the carrier solvent is organic and measurements may be distorted by expansion or evaporation of the solvent.-It is recommended to perform the qualification within the spectral range corresponding to the region of analytical interest.-Ensure that the spectrophotometer has stabilized, according to the manufacturer’s recommendations, before starting the qualification tests.-When references are made to the European Pharmacopoeia, e.g. reagents R, then the reagent quality complies with the EP specifications.-When using commercial filters as alternative to the proposed tests, a set of filters covering the entire range of interest should be used. They should be calibrated with traceability to national/international standards, preferable through a national metrology laboratory or NIST.1.SPECTRAL SLIT WIDTH (if applicable)When using an instrument on which the slit-width is variable at the selected wavelength, the slit-width must be small compared with the half-width of the absorption band but it must be as large as possible to obtain a high value of I0. Therefore, a slit-width is chosen such that further reduction does not result in a change in absorbance reading.Method and Limits:1. Switch the system on and start the Scan module.2. Select SETUP and set the following parameters:X Mode = NanometresStart wavelength = 660.0 nmStop wavelength = 650.0 nmY Mode = % TScan rate = 10 nm/minGain = (100) see 4 below3. Select Options tab and set the following parameters:SWB = 4 nmBeam mode = Single frontLamps on = DeuteriumSource change = 700.0 nm4. Start a scan and examine the trace for a spectral peak around 656.1 nm.If no peak is seen or it is less than 50% T, increase the gain.If the signal exceeds 100% T, reduce the gain.5. Measure the width of the peak (in nanometres) at half the height of the peak.This represents the spectral bandwidth and should be within ± 10% of that selected via the computer.6. Check the calibration at a slit width of 0.2 nm. If the measured slits are too small then, for a selected width, the instrument will have more photometric noise than normal. If the slit widthis unacceptable, then reset the slit calibration.Effect of spectral slit width on absorbance fluctuation (performed with pure solvents).Slit width 5.0 nm Slit width2.0 nmSlit width1.0 nmSlit width0.05 nmDifferencein Abs.TheoreticalValuesTest statusCyclohexane0.016 0.015 0.015 0.015 < 0.001 < 0.010 PassedEthanol0.020 0.019 0.019 0.019 < 0.001 < 0.010 PassedMethanol0.005 0.005 0.005 0.005 0.00 < 0.010 Passed2.WAVELENGTH ACCURACYNote: if the determination is performed by using the specific absorbance (), the frequency of this check should be higher.Materials:Verify the wavelength accuracy using the absorption maxima of holmium perchlorate solution R prepared, for example, with a 40 g/l solution of holmium oxide R in a solution of perchloric acid R containing 141 g/l of HClO4 (Ph. Eur. Chapter 4.1.1, ref 1043101), or the line of a hydrogen or deuterium discharge lamp.If available, the built-in mercury lamp of the instrument may be used for this test.Alternatively, use suitable commercial certified filters1typically made incorporating rare earth metal oxides. Ideally the calibrant should demonstrate traceability to national or international standards and should carry a statement about the associated uncertainty.Method:Compare the measured absorbance maxima with the absorbance maxima shown in the table below:241.15 nm (Ho) 404.66 nm (Hg)253.7 nm (Hg) 435.83 nm (Hg)287.15 nm (Ho) 486.0 nm (D )302.25 nm (Hg) 486.1 nm (H )313.16 nm (Hg) 536.3 nm (Ho)334.15 nm (Hg) 546.07 nm (Hg)361.5 nm (Ho) 576.96 nm (Hg)365.48 nm (Hg) 579.07 nm (Hg)When using commercial filters, compare the measured absorbance with the certified values given by the manufacturer. The measurement is made against air.Limits:± 1 nm in the UV range± 3 nm in the Visible range1 Commercial certified filters, e.g. Hellma or NIST (National Institute of Standards and Technology).3.WAVELENGTH PRECISION (for mechanically set wavelengths)Materials:For this test, the same materials of the previous test can be used:Holmium perchlorate solution R prepared, for example, with a 40 g/l solution of holmium oxide R in a solution of perchloric acid R containing 141 g/l of HClO4(Ph. Eur. Chapter 4.1.1, ref 1043101).If available, the built-in mercury lamp of the instrument may be used for this test. Alternatively, suitable commercial certified filters may be used1.Method:Carry out 6 measurements of the absorbance maxima.Limits:Repeatability: the relative standard deviation of the absorbance maxima should satisfy the manufacturer’s specifications.The difference between the 6 individual peaks should comply with the manufacturer’s specifications (e.g. < 0.5 nm).Where old instruments are involved, the wavelength may be set by turning a wheel until the selected wavelength is reached. Where this is the case, mechanical wear may result in a different wavelength being selected depending on whether starting above or below the target wavelength. Examine the variation by setting a wavelength from above and below and seeing if there is a difference.4.PHOTOMETRIC ACCURACY (CONTROL OF ABSORBANCE)Note: if the determination is performed by using the specific absorbance (), the frequency of this check should be higher.Materials:Solution of potassium dichromate R, previously dried to constant mass at 130 °C.For the control of absorbance at 235 nm, 257 nm, 313 nm and 350 nm, dissolve 57.0-63.0 mg of potassium dichromate R in 0.005 M sulphuric acid and dilute to 1000.0 ml with the same acid. For the control of absorbance at 430 nm, dissolve 57.0-63.0 mg of potassium dichromate R in 0.005 M sulphuric acid and dilute to 100.0 ml with the same acid.Alternatively, suitable commercial certified filters may be used2.Note: If commercial filters are used, the wavelengths and the exact absorption values with the corresponding tolerance limits will depend on the type of filters. The selection of the filters should be adequate in order to cover the range used in routine work.Method:Check the absorbance using the solution of potassium dichromate R or the certified filters at the wavelengths indicated in the table below, which gives for each wavelength the exact values and the permitted limits of the specific absorbance.2 Commercial certified filters, e.g. Hellma or NIST (e.g. NIST Standard Reference Material 930e).Limits:Wavelength (nm)Specific absorbanceMaximum tolerance235 124.5 122.9 to 126.2 257 144.5 142.8 to 146.2 313 48.6 47.0 to 50.3 350 107.3 105.6 to 109.0 43015.915.7 to 16.1Example of certified commercial filters:Filter Wavelength (nm)Maximum tolerance235 0.750 ± 0.01 257 0.868 ± 0.01 313 0.292 ± 0.01 Potassium dichromate insulphuric acid 3, against sulphuric acid (blank)3500.644 ± 0.01FilterWavelength (nm)Maximum tolerance235 0.043 ± 0.01 257 0.040 ± 0.01 313 0.035 ± 0.01 Sulphuric acid (blank), against air350 0.034 ± 0.01FilterWavelength (nm)Maximum tolerancePotassium dichromate in sulphuric acid, against sulphuric acid (blank) 430 0.960 ± 0.01 Sulphuric acid (blank), against air430 0.033 ± 0.015. PHOTOMETRIC LINEARITYMaterials:A series of solutions of a suitable absorbing compound (e.g. potassium dichromate) spanning the concentration range of interest. The absorbing solutions need to be stable. The concentrations should be regularly spaced (1, 2, 3, 4, 5) rather than doubling (1, 2, 4, 8, 16) in order to reduce the leverage effects when fitting the line.3A potassium dichromate in perchloric acid cuvette is also available from Hellma.Method:Measure the net absorbance of the solutions against a blank at 235, 313, 257 and 350 nm. Make 3 measurements for each solution. Calculate the mean value and the linearity of the concentration of each solution against the measured absorbance at the control wavelengths. Limits:At each wavelength: r2≥ 0.999Note: It is recommended to use these results to regularly monitor the trend of the instrument (control chart of r2).6.LIMIT OF STRAY LIGHTMaterials:Stray light may be measured at a given wavelength with suitable certified filters or different solutions. For example, a 12 g/l solution of potassium chloride R in water.Method:The absorbance of a 12 g/l solution of potassium chloride R in a 1 cm cell increases steeply between 220 nm and 200 nm.When available, use the in-built software of the instrument.Limits:A > 2.0 at 198 nm, when compared with water as compensation liquid.Alternatively:Method: Check stray light at 220 nm by using a 20 g/l NaI solution.Limits: according to manufacture’s specifications (e.g. Transmittance ≤ 0.1 %).Note:A range of tests using other salts are described in the literature to cover different wavelength ranges4.7. BASELINE NOISEFor this test, 2 alternative methods are proposed.TEST 1Method:Make 61 absorbance measurements with an integration time of 1 second at a wavelength of 500 nm, with no sample in the sample chamber, and calculate the mean.Limits:Mean ± 0.002 Absorbance units4 e.g. ASTM E 387-2004: Standard test method for estimating stray radiant power ratio of dispersive spectrophotometers by the opaque filter method.TEST 2Method:Record the absorbance for 60 seconds at 200, 300 and 400 nm with a highly pure, synthetic Quartz block 5. The measurement is made against air.Note: If commercial filters are used, the wavelengths and the exact absorption values with the corresponding tolerance limits will depend on the type of filters.Example of limits:Filter Wavelength (nm) Maximum tolerance200 0.049 ± 0.01 3000.033 ± 0.01 Quartz block (against air) 4000.031 ± 0.018. PHOTOMETRIC DRIFTNotes:- Photometric drift should be checked at both the Visible and UV region, at appropriate wavelengths.- The limits are in accordance with the user’s requirements (as defined in Level I) and with manufacturer’s specifications.For this test, 2 alternative examples are proposed.TEST 1Method :As routine test, the drift is measured at 250 nm over a period of 2 hours by using the Time Scan mode of the instrument, with no sample in the sample chamber.Limits : ± 0.001 Absorbance units/hNote: In certain cases, for example when several samples are measured over a long period of time (or when using auto sampler) the drift can also be determined at the wavelength used for the analytical method, in the same way, before the measurement of the samples.TEST 2Method :Record the baseline for 60 minutes at 500 nm and compare the absorbance with the initial value.Limits: ± 0.002 Absorbance units/h5e.g. Hellma filter 667-UV 0.ANNEX IILevel IV. In-use instrument checksThis Annex contains practical examples of tests and their associated tolerance limits for several parameters related to the performance of an UV-Visible spectrophotometer.These examples can be considered by the OMCLs as possible approaches to perform the Level IV of the equipment qualification process: “In-use instrument checks”.1. SYSTEM SUITABILITY TEST OF THE METHOD- REPEATABILITY (for quantitative analysis)- RESOLUTIONMethod:Criteria such as repeatability or resolution are usually given when test methods are performed according to Ph. Eur., MAH dossier or a suitably validated in-house method.- RESOLUTION (if required for qualitative analysis)Materials:0.02 per cent V/V solution of toluene R in hexane R.Alternatively, suitable certified reference materials may be used.Method:When prescribed in a monograph, measure the resolution of the apparatus as follows: record the spectrum of a 0.02 per cent V/V solution of toluene R in hexane R.Alternatively, record the spectrum of the reference material.Limits:The minimum ratio of the absorbance at the maximum at 269 nm to that at the minimum at 266 nm is stated in the monograph or in the test method description.2. ABSORPTION CELLSMethod:The following method for checking cleanliness and gross differences in thickness or parallelism of the windows of optical cells is described in ASTM E275-01 (American society of Testing and Materials), “Standard Practice for Describing and Measuring Performance of UV, Visible, and Near Infrared Spectrophotometers”:Fill the cell with distilled water and measure its apparent absorbance against air at 240 nm for quartz cells and at 650 nm for glass cells. With recording instruments it is desirable to scan over the optical region of interest. The apparent absorbance should be not greater than 0.093 for 1 cm quartz cells (UV region) and 0.035 for 1 cm glass cells (Visible region). Rotate the cell in its holder (180°) and measure the apparent absorbance again.Limits:Rotating the cells should give an absorbance difference not greater than 0.005.REFERENCES(For all references, the latest version applies)1)Ph Eur. 2.2.25, Absorption spectrophotometry, Ultraviolet and Visible.2)Guidance on Equipment Qualification of Analytical Instruments: UV-VisibleSpectro(photo)meters (UV-Vis). Valid Analytical Measurement (VAM) Programme.。
OMCL设备确认附录7:质谱确认

OMCL设备确认附录7:质谱确认作者:JULIA 来源:Julia法规翻译PA/PH/OMCL (10) 86 R6 –OMCL设备确认附录7:质谱确认 201804 生效日期20180801前版本:PA/PH/OMCL(10)86 2R 201107ANNEX 7 OF THE OMCL NETWORKGUIDELINE“QUALIFICATION OF EQUIPMENT”QUALIFICATION OF MASSSPECTROMETERS设备确认附录7:质谱仪的确认Note: Mandatory requirements in this guideline andits annexes are defined using the terms «shall» or«must».The use of «should» indicates are commendation. For these parts of the text other appropriately justified approaches are acceptable.The term«can» indicates a possibility or an example with non-binding character.注:本指南及其附录中的强制要求采用术语“应”或“必须”进行定义。
使用“应”表示是建议,其下所列要求可采用经过适当论证的其它方法来实施。
术语“可”表示一种可能性,或非强制特性举例。
Introduction 介绍The present document is the 7th Annex of the core document “Qualification of Equipment” and it shall be usedincombination with it when planning, performing and documenting the qualificationprocess of Mass Spectrometers coupled with Chromatographic equipment.本文件是核心文件“仪器确认”的第7个附件,在规划、实施和记录质谱确认过程中,应与核心文件结合使用。
仪器确认6活塞移液管确认中英文

OMCL Network of the Council of Europe QUALITY ASSURANCE DOCUMENT质量保证文件PA/PH/OMCL (09) 64 2RQUALIFICATION OF EQUIPMENT仪器确认ANNEX 6: QUALIFICATION OF PISTON PIPETTES附件6:活塞移液器的确认ANNEX 6 OF THE OMCL NETWORK GUIDELINE “QUALIFICATION OF EQUIPMENT”QUALIFICATION OF PISTON PIPETTES活塞移液器的确认INTRODUCTION 介绍The present document is the 6th Annex of the core document “Qualification of Equipment”, and it should be used in combination with it when planning, performing and documenting the qualification process of piston pipettes.本文是核心文件“仪器确认”的第6个附件,在计划、实施和记录活塞移液器的确认过程中,应与核心文件结合使用。
The core document contains the Introduction and general forms for Level I and II of qualification, which are common to all type of instruments.核心文件包括介绍,和对所有类型仪器都适用的第一级和第二级确认通用表格。
The present annex contains instrument-related recommendations on parameters to be checked at Level III and IV of qualification and the corresponding typical acceptance limits, as well as practical examples on the methodology that can be used to carry out these checks.本附件包含与仪器相关参数及其相应的典型可接受限度的推荐,这些参数应在第三级和第四级确认时进行检查,以及在实施这些检查时可以使用的一些实用方法举例。
欧盟OMCL仪器确认附件1-HPLC确认

---------------------------------------------------------------最新资料推荐------------------------------------------------------ 欧盟OMCL仪器确认附件1-HPLC确认BEIJING GELEG SCI.-TECH.CO.LTDOMCL Network of the Council of Europe QUALITY MANAGEMENT DOCUMENT PA/PH/OMCL (11) 04 QUALIFICATION OF EQUIPMENT 仪器确认 ANNEX 1: QUALIFICATION OF HPLC EQUIPMENT 附件 1:HPLC 仪器的确认 Full document title and reference 文件全名和索引号 Qualification of Equipment Annex 1: Qualification of HPLC equipment PA/PH/OMCL (11) 04 Document type 文件类型 Legislative basis 立法基础 Guideline 指南 The present document was also accepted by EA as recommendation document to be used in the context of Quality Management System audits of OMCLs Date of first adoption 首次发行日期 Date of original entry into force 首次执行日期 Date of entry into force of revised document 修订后执行日期Previous titles/other references 原文件名/其它索引号Custodian Organisation 保管机构Concerned Network 相关网络This document replaces document PA/PH/OMCL (07) 17 DEF The present document was elaborated by the OMCL Network/ EDQM of the Council of Europe GEON 1st July 2011 June 2005 May 20051/ 32BEIJING GELEG SCI.-TECH.CO.LTDANNEX 1 OF THE OMCL NETWORK GUIDELIN E “QUALIFICATION OF EQUIPMENT” OMCL 网络指南“仪器的确认”之附件 1 QUALIFICATION OF HPLC EQUIPMENT HPLC 仪器的确认 Introduction 概述 The present document is the first Annex of the core document “Qualification of Equipment”, and it should be used in combination with it when planning, performing and documenting the HPLC equipment qualification process. 本文件是核心文件“仪器的确认”第 1 个附件,在计划、实施和记录HPLC 仪器的确认过程时,应将本文件与核心文件一起使用。
仪器确认--天平确认-中英文

ANNEX 8OF THE OMCL NETWORK GUIDELINE“QUALIFICATIONOF EQUIPMENT”仪器确认QUALIFICATION OF BALANCES天平的确认1.INTRODUCTION 介绍This document isthe 8th Annex to the core document “Qualification of Equipment”, which togethershould be used when planning, performing and documenting the qualification processof balances.本文件是核心文件“仪器的确认”第8个附件,在计划、实施和记录天平的确认过程时,应将本文件与核心文件一起使用。
The core documentcontains the introduction and general forms for Level I and II of qualification,which are common to all types of instruments.核心文件包括了第一级和第二级确认的介绍和通用表格,适用于所有类型的仪器。
Annex 8 containsinstrument-related recommendations on parameters to be checked at Level III andIV of qualification and the corresponding typical acceptance limits, as well aspractical examples on the methodology that can be used to carry out thesechecks.附件8包括了仪器相关的的推荐:在第三级和第四级确认中需要检查的参数和对应的典型的可接受标准,以及可用于进行这些检查的实用方法学举例。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
OMCL Network of the Council of EuropeQUALITY MANAGEMENT DOCUMENTPA/PH/OMCL (11) 04QUALIFICATION OF EQUIPMENT仪器确认ANNEX 1: QUALIFICATION OF HPLC EQUIPMENT 附件1:HPLC仪器的确认ANNEX 1 OF THE OMCL NETWORK GUIDELINE“QUALIFICATION OF EQUIPMENT”OMCL网络指南“仪器的确认”之附件1QUALIFICATION OF HPLC EQUIPMENTHPLC仪器的确认Introduction 概述The present document is the first Annex of the core document “Qualification of Equipment”, and it should be used in combination with it when planning, performing and documenting the HPLC equipment qualification process.本文件是核心文件“仪器的确认”第1个附件,在计划、实施和记录HPLC仪器的确认过程时,应将本文件与核心文件一起使用。
The core document contains the general introduction and the Level I and II of qualification, common to all type of instruments, and the present annex contains HPLC instrument-related recommendations on parameters to be checked and the corresponding typical acceptance Limits, as well as practical examples on the Methodology that can be used to carry out these checks.核心文件包括了第一级和第二级确认的通用介绍,适用于所有类型的仪器,本附件包括了HPLC仪器相关的需要检查的参数和相应典型的可接受标准的推荐,以及可用于进行这此检查的实用方法学举例。
When qualifying HPLC equipment, it should be noted that it is acceptable to check at Level III and IV several of the mentioned parameters at the same time in a combined test procedure (e.g. “overall” system performance test giving information on peak area precision, retention time precision, gradient reproducibility, etc).在进行HPLC仪器确认时,应注意也可以对第三级和第四级几个提到的参数采用联合检测程序同时进行检查(例如“全面”系统性能检查,同时给出峰面积精密度、保留时间精密度、梯度重复性等的信息)TABLE III 表三Level III. Periodic and motivated instrument checks第三级定期主动仪器检查Examples of requirements for HPLC instruments and detectorsHPLC仪器和检测器要求举例TABLE IV 表四Level IV. In-use instrument checks第四级在用仪器检查Examples of requirements for HPLC instruments with UV or DAD detectors配备UV或DAD检测器的HPLC仪器的要求举例ANNEX I 附件1Level III. Periodic and motivated instrument checks第三级定期性的主动仪器检查This Annex contains practical examples of tests and their associated tolerance Limits for several parameters related to the performance of the different modules of a HPLC.本附件包括一些实用几个与HPLC不同模块相关的参数检测举例及其相应的可接受限度。
These examples can be considered by the OMCLs as possible approaches to perform the Level III of the equipment qualification process: “Periodic and motivated instrument checks”.这些例子可以认为是OMCL实验室可能的进行第三级仪器确认的方法“周期主动仪器检查”HPLC SOLVENT DELIVERY SYSTEM 高压液相溶剂传输系统The following tests are proposed for the periodic and motivated check of the HPLC solvent delivery system: flow rate and gradient test.以下测试作为HPLC溶剂传输系统周期性主动检查:流速和梯度检查FLOW RATE 流速Materials: 材料Volumetric flask of 5 or 10 ml5ml或10ml的容量瓶Calibrated chronometer校正过的计时计Settings: 设置Mobile phase: degassed water流动相:脱过气的水No column (open end)*无柱(开放式)*Flow rate: adjusted between 0.5 and 3.0 ml/min流速:调节在0.5到3.0ml/minIf high-pressure mixing systems are installed, this test has to be done on each solvent channel.如果有安装高压混合系统,本测试应在每一溶剂通道进行。
For certain equipment, e.g. in the case of low flow rates, the check would be performed by using a column or a backpressure regulator.对于一定的仪器,例如,流速较低时,采用一根柱子或背压调整器进行检查Method方法:Set the flow rate at an appropriate level and measure the time needed to fill the volumetric flask up to the mark. Record the time needed.将流速设定在一个合适的水平,测量将容量瓶装满至刻度的时间。
记录所用的时间。
f=V /t f=(V×60)/tf ................. measured flow rate [ml/min] 测量的流速t ................. elapsed time to fill up to mark [s] 充满至刻度所花的时间V ............... volume of the volumetric flask [ml] 容量瓶的体积D=100×(f-F)/FD ............... deviation [%] 偏差F ............... adjusted flow rate [ml/min] 调节后的流速f ................. measured flow rate [ml/min] 测量的流速Limits限度: ±5%GRADIENT COMPOSITION AND RIPPLE 梯度组成和波动Settings: 设置Stainless steel capillary e.g. 2000 x 0.12 mm installed instead of a column不锈钢毛细管,例如2000X0.12mm,代替色谱柱安装于仪器Detection: UV-Detector adjusted to 265 nm检测器:紫外检测器调节至265nmMobile phase A: degassed water流动相A:脱气水Mobile phase B: degassed water containing 0.5% acetone流动相B:含0.5%丙酮的脱气水Flow rate: 1.0 ml/min流速:1.0ml/分钟Method方法:The test is carried out in the following way by using a gradient program depending on the number of solvent channels and the configuration of the system:按下列方法进行测试,根据通道数量不同使用下列不同系统参数和梯度进行洗脱A-BA-B and A-CA-C, A-B and B-D开始测试前,先用水平衡系统至少10分钟。
The zero % value at the start of the test is the baseline. All steps are measured at the beginning of the horizontal part of the line either by software or manually on the paper print using a liner. The height of the 100% water/acetone mixture is used as the 100% value in the following calculation.开始时的0%值是基线值。
