DDR1-IN-1_HNMR_12463_MedChemExpress
3_种常用碳青霉烯类抗生素血药浓度UPLC-MS

3种常用碳青霉烯类抗生素血药浓度UPLC-MS/MS检测方法的建立Δ秦怡1*,张瑞霞2,吕雅瑶2,翁莉莉1,张弋2 #(1.天津医科大学一中心临床学院,天津 300192;2.天津市第一中心医院药学部,天津 300192)中图分类号 R917;R978.1文献标志码 A 文章编号 1001-0408(2024)03-0343-05DOI 10.6039/j.issn.1001-0408.2024.03.14摘要目的建立3种临床常用碳青霉烯类抗生素——厄他培南(ETP)、亚胺培南(IPM)、美罗培南(MEM)血药浓度检测的超高效液相色谱-质谱联用(UPLC-MS/MS)法。
方法血浆样品经甲醇沉淀蛋白后,以3种抗生素的稳定性同位素(ETP-D4、IPM-D4、MEM-D6)为内标,采用ACQUITY UPLC BEH C18(2.1 mm×50 mm,1.7μm)色谱柱分离;流动相为98%乙腈+2%水+0.1%甲酸和98%水+2%乙腈+0.1%甲酸,梯度洗脱;流速为0.3 mL/min;柱温为40 ℃;采用正离子、多反应监测模式进行扫描分析。
结果该方法专属性良好,在ETP、IPM、MEM 0.2~200、0.1~100、0.1~100μg/mL范围内线性良好(r2≥0.993),批内、批间精密度和准确度良好(RE均≤5.14%,RSD均≤11.15%),基质效应、提取回收率较一致(RSD≤12.99%)。
结论本实验建立了一种可以同时定量ETP、IPM、MEM血药浓度的UPLC-MS/MS法,该方法样品前处理简单、检测时间短、所需样品量少,可满足临床需求。
关键词碳青霉烯类抗生素;超高效液相色谱-质谱联用;血药浓度;厄他培南;亚胺培南;美罗培南Establishment of UPLC-MS/MS method for the determination of plasma concentration of three common carbapenem antibioticsQIN Yi1,ZHANG Ruixia2,LYU Yayao2,WENG Lili1,ZHANG Yi2(1. First Central Clinical College of Tianjin Medical University,Tianjin 300192,China;2. Dept. of Pharmacy,Tianjin First Central Clinical Hospital,Tianjin 300192, China)ABSTRACT OBJECTIVE To establish a UPLC-MS/MS method for the determination of plasma concentration of three carbapenem antibiotics,i.e. ertapenem (ETP),imipenem (IPM)and meropenem (MEM).METHODS After protein precipitation with methanol,the plasma samples were separated by ACQUITY UPLC BEH C18column (2.1mm×50mm,1.7μm)using stable isotopes of three antibiotics (ETP-D4,IPM-D4,MEM-D6)as the internal standard. The mobile phases were 98%acetonitrile +2% water +0.1%formic acid and 98%water +2%acetonitrile +0.1%formic acid,by gradient elution. The flow rate was 0.3mL/min and the column temperature was 40 ℃. Scanning analysis was performed in the positive ion and multiple reaction monitoring mode. RESULTS The method had good specificity,good linearity (r2≥0.993)in the range of 0.2-200,0.1-100and 0.1-100μg/mL of ETP,IPM and MEM,and good intra-batch and inter-batch precision and accuracy (all RE≤5.14%,all RSD≤11.15%),the matrix effect and extraction recovery were consistent (RSD≤12.99%). CONCLUSIONS This study establishes the UPLC-MS/MS method to simultaneously quantify the plasma concentration of ETP,IPM and MEM. The method has the advantages of simple pretreatment, short detection time and small sample quantity to meet clinical requirement.KEYWORDS carbapenem antibiotics; UPLC-MS/MS; plasma concentration; ertapenem; imipenem; meropenem碳青霉烯类抗生素具有抗菌谱广、抗菌活性强、耐药率低的特点,已成为治疗重症感染的主要选择。
大动脉炎患者外周血单个核细胞RT-qPCR内参基因的选择

January 2021Vol.41 No.12021 年 1 月 第 41 卷 第 1 期基础医学与临床Basic & Clinical Medicine文章编号:1001-6325 ( 2021 ) 01-0087-06研究论文大动脉炎患者外周血单个核细胞RT-qPCR 内参基因的选择田苡箫,李菁*收稿日期:2019-11-18 修回日期:2020-04-30*通信作者(corresponding author ) :lijing6515@ (中国医学科学院北京协和医学院北京协和医院风湿免疫科风湿免疫病学教育部重点实验室国家皮肤与免疫疾病临床医学研究中心,北京100032)扌摘要:目的筛选适于在大动脉炎(TAK )患者和健康人群(HC )之间比较外周血单个核细胞(PBMC )中mRNA 表达水平的内参基因。
方法提取PBMC 中的总RNA,应用RT-qPCR ,分别采用geNorm 、NormFinder 、BestKeeper 3种软 件程序,分析 3-glucuronidase ,GAPDH ,ACTB ,SDHA ,HPRT1,RPL13A ,B2M , YWHAZ 和 PKG1 9 个基因的 mRNA 表达稳定性。
以T-bet 、GATA3和RORC 作为目的基因,比较不同稳定性的内参基因对mRNA 相对丰度的影响。
结果geNorm 筛选得到的基因组合为B 2M-SDHA , Nor^nFinder 和BestKeeper 筛选出最稳定的内参基因均为HPRT1 ; 3种方法均显示GAPDH 的稳定性较差。
结论自身免疫病患者在接受免疫抑制药物治疗时,原本稳定表达的基因可能会 上调或下调;在样本量较小时,稳定性更好的内参基因可能更有助于检测组间差异。
关键词:大动脉炎;实时定量聚合酶链式反应;RNA 稳定性;内参基因选择中图分类号:R593.2 文献标志码:AValidation of reference genes for the normalization of the RT-qPCRin peripheral blood mononuclear cells of patients with Takayasu arteritisTIAN Yi-xiao , LI Jing *(Department of Rheumatology and Immunology , Key Laboratory of Rheumatology and Clinical Immunology , Ministry of Education ,National Clinical Research Center for Dermatologic and Immunologic Diseases ( NCRC-DID ),Peking Union Medical College Hospital , CAMS & PUMC , Beijing 100032, China)Abstract : Objective To validate proper reference genes for quantitative real-time polymerase chain reaction ( RT-qPCR) used for comparing mRNA expression levels in Takayasu arteritis" (TAK) and healthy controls' ( HC ) pe ripheral blood mononuclear cells ( PBMC ). Methods Total RNA in PBMCs was extracted and used RT-qPCR to determine the profiles of 9 candidate genes , including 0-glucuronidase, GAPDH , ACTB , SDHA , HPRT1, RPL13A , B2M , YWHAZ and PKG1. Then compared their transcription stability by geNorm , NormFinder , and Best Keeper. Afterwards , with T-bet , GATA3 and RORC as the targeted genes , explored the influence of reference genes with different stability on mRNA relative abundance. Results The gene combination of B2M-SDHA was selected bygeNorm , and HPRT1 was the most stable one in analysis results of NormFinder and BestKeeper , while GAPDH was less stable. Conclusions Genes that have been expressed stably may be upregulated or downregulated whenpatients with autoimmune diseases received immunosuppressive drugs. When the sample size is small , the more sta ble internal reference may facilitate the identification of inter-groups difference.Key words : Takayasu arteritis ; real-time polymerase chain reaction ; RNA stability ; selection of reference gene88基础医学与临床Basic&Clinical Medicine2021.41(1)反转录实时荧光定量聚合酶链式反应(reverse quantitative real-time polymerase chain reaction,RT-qPCR)是目前分析基因表达水平的黄金标准,却经常表现出重复性欠佳的问题,选取合适的内参基因有助于改善这一情况[1]。
FDA批准的精准医疗诊断体外器械一览表List of Cleared or Approved Companion Diagnostic Devices
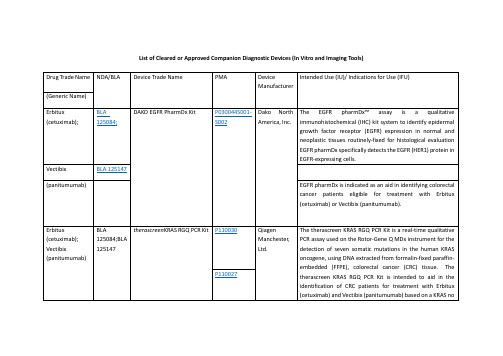
Drug Trade Name
NDA/BLA
Device Trade Name
PMA
Device Manufacturer
Intended Use (IU)/ Indications for Use (IFU)
(imatinibmesylate)
NDA 021588
The c-KitpharmDxis indicated as an aid in the differential diagnosis of gastrointestinal stromal tumors (GIST). After diagnosis of GIST, results from c-KitpharmDxmay be used as an aid in identifying those patients eligible for treatment withGleevec/Glivec(imatinibmesylate).
(deferasirox)
Gilotrif
NDA 201292
therascreenEGFR RGQ PCR Kit
P120022
QiagenManchester, Ltd.
ThetherascreenEGFR RGQ PCR Kit is a real-time PCR test for the qualitative detection of exon 19 deletions and exon 21 (L858R) substitution mutations of the epidermal growth factor receptor (EGFR) gene in DNA derived from formalin-fixed paraffin-embedded (FFPE) non-small cell lung cancer (NSCLC) tumor tissue. The test is intended to be used to select patients with NSCLC for whom GILOTRIF (afatinib), an EGFR tyrosine kinase inhibitor (TKI), is indicated. Safety and efficacy of GILOTRIF (afatinib) have not been established in patients whose tumors have L861Q, G719X, S768I, exon 20 insertions, and T790M mutations, which are also detected by thetherascreenEGFR RGQ PCR Kit.
黄芩素通过调节HIF-1α

黄芩素通过调节HIF -1α/VEGF 信号通路抑制类风湿关节炎大鼠的炎症反应和病理性血管生成*杜红丽1,张晨宇1,赵清2△[1河南中医药大学第五临床医学院(郑州人民医院)风湿免疫科,河南郑州450053;2河南大学淮河医院风湿免疫科,河南开封475099][摘要]目的:探讨黄芩素(BA )调节缺氧诱导因子1α(HIF -1α)/血管内皮生长因子(VEGF )信号通路对类风湿关节炎(RA )大鼠炎症反应和病理性血管生成的影响。
方法:按照随机数字表法将SD 大鼠分为对照(control )组、模型(model )组、低剂量(10mg/kg )BA (BA -L )组、高剂量(30mg/kg )BA (BA -H )组、雷公藤多苷片(TWP ;6.25mg/kg )组和BA -H+HIF -1α激动剂二甲基草酰甘氨酸(DMOG ;40mg/kg )组,每组12只。
除control 组外,其它组大鼠均采用II 型胶原蛋白-完全弗氏佐剂法诱导RA 大鼠模型。
第2次免疫24h 后开始给药处理,每天给药一次,持续4周。
检测大鼠在给药第0、7、14和28天时的足趾肿胀度,计算关节炎指数;计算大鼠胸腺和脾脏指数;HE 染色检测大鼠踝关节滑膜组织病理损伤;ELISA 法检测大鼠踝关节滑膜组织中肿瘤坏死因子α(TNF -α)和白细胞介素6(IL -6)水平;免疫组化检测大鼠踝关节滑膜组织中VEGF 和VEGF 受体2(又称激酶插入域受体,KDR )表达;Western blot 检测各组大鼠踝关节滑膜组织中HIF -1α和VEGF 蛋白表达。
结果:与control 组比较,model 组大鼠踝关节滑膜组织病理损伤严重,足趾肿胀度、关节炎指数、胸腺和脾脏指数,以及滑膜组织TNF -α、IL -6、VEGF 、KDR 、HIF -1α和VEGF 水平均显著升高(P <0.05);与model 组比较,BA -L 组、BA -H 组和TWP 组对应指标变化趋势与上述相反(P <0.05);BA -H 组与TWP 组比较,上述指标变化差异无统计学意义(P >0.05);DMOG 减弱了BA -H 对RA 大鼠炎症反应和病理性血管生成的抑制作用。
QPCR及QRT-PCR系列产品

Invitrogen的ICFC系列产品促销1.QPCR及QRT-PCR系列产品Invitrogen公司专门为中国客户提供的定量PCR试剂盒,结合了 UDG 防止残余污染技术和SYBR® Green I 荧光染料(存在于SYBR® Green I荧光定量PCR试剂盒中),在美国接受了严格的质量监控,可提供极高灵敏度的目的序列定量检测,线性剂量低,反应浓度范围很大。
qPCR Supermix-- 即用型反应剂,专为高特异性、实时定量DNA扩增设计UDG-- 防止携带污染物,减少克隆片段假阳性结果ROX参考染料-- 适用ABI仪器的校正染料产品信息活动时间:即日起至2009年4月30日2.Gibco南美胎牛血清即日起凡优惠价¥1780购买Gibco胎牛血清500ml(目录号:C2027050)即可获赠送价值¥250现金抵用券。
您可以凭现金抵用券在英韦创津公司购买任何商品,此券有效期至2009年5月31日。
产品信息活动时间:即日起至2009年4月30日独特的采集方式:GIBCO采用无菌心脏穿刺的方式采血原装直送,避免污染:原产地采集、加工、检测、包装。
完善的质控:采集、处理、检测、运输等环节都有文件和证书。
3.Invitrogen TA Cloning克隆产品专门用于克隆Taq聚合酶扩增的PCR产物。
采用pCR载体,能产生80%以上的重组产物,90%以上重组产物都包含插入片段。
产品信息活动时间:即日起至2009年5月31日附:pCR载体优点及图谱:3’-T突出端可直接连接Taq扩增的PCR产物可选择T7或T7和Sp6启动子进行体外RNA转录和测序侧向EcoRⅠ位点的通用多接头位点方便了插入片段的切离可以选择卡那霉素或氨苄青霉素进行筛选非常简便的蓝/白克隆筛选具有M13正向和反向引物位点,方便测序4.GIBCO液体培养基系列产品创立近50年的历史,品质优秀,产品种类丰富;为了中国用户利益,特建立国内生产线;所有产品,从原材料到生产全部按照GIBCO质量标准进行,每批均送抵美国公司总部质检合格后,才在国内销售。
BP2015英国药典索引
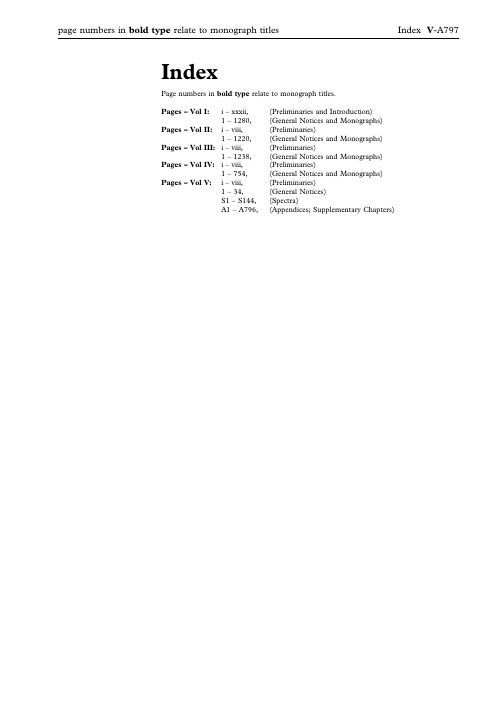
page numbers in bold type relate to monograph titles Index V-A797IndexPage numbers in bold type relate to monograph titles.Pages–Vol I:i–xxxii,(Preliminaries and Introduction)1–1280,(General Notices and Monographs)Pages–Vol II:i–viii,(Preliminaries)1–1220,(General Notices and Monographs)Pages–Vol III:i–viii,(Preliminaries)1–1238,(General Notices and Monographs)Pages–Vol IV:i–viii,(Preliminaries)1–754,(General Notices and Monographs)Pages–Vol V:i–viii,(Preliminaries)1–34,(General Notices)S1–S144,(Spectra)A1–A796,(Appendices;Supplementary Chapters)AAbacavir,V-S4Abacavir Oral Solution,III-85 Abacavir Sulfate,I-39Abacavir Tablets,III-86 Abbreviated,V-598Adjectives,V-598Anions,V-598Cations,V-598Preparations,V-598Titles of Monographs,V-598 Abbreviated Titles,Status of,I-7,II-7, III-7,IV-7,V-7Abbreviations and symbols,I-30,II-30, III-30,IV-30,V-30Abnormal Toxicity,Test for,V-409 About,definition of,I-5,II-5,III-5,IV-5,V-5Absence of Mycoplasmas,Test forV-487Absolute Ethanol,V-A61Absolute Ethanol R1,V-A62 Absorbent Cotton,IV-743Absorbent Viscose Wadding,IV-744 Absorption spectrophotometry,infrared, V-162Absorption Spectrophotometry, Ultraviolet and Visible,V-169 Acacia,I-41,V-A19Acacia Solution,V-A19Acacia Spray-dried,I-42 Acamprosate Calcium,I-43 Acanthopanax Bark,IV-49 Acarbose,I-44Accuracy,V-674Acebutolol Capsules,III-87 Acebutolol Hydrochloride,I-46,V-S5, V-A19Acebutolol Tablets,III-88 Aceclofenac,I-48Acemetacin,I-50Acenocoumarol,I-52,V-S5 Acenocoumarol Tablets,III-88 Acesulfame Potassium,I-52Acetal,V-A19Acetaldehyde,V-A19Acetaldehyde Ammonia Trimer Trihydrate,V-A20Acetaldehyde Standard Solution(100ppm C2H4O),V-A148 Acetaldehyde Standard Solution(100ppm C2H4O)R1,V-A148 Acetamide,V-A20Acetate Buffer pH2.8,V-A152 Acetate Buffer pH2.45,V-A152 Acetate Buffer pH3.4,V-A152 Acetate Buffer pH3.5,V-A152 Acetate Buffer pH3.7,V-A152 Acetate Buffer pH4.4,V-A152 Acetate Buffer pH4.6,V-A152 Acetate Buffer pH5.0,V-A152 Acetate Buffer pH6.0,V-A152 Acetate Buffer Solution pH4.7R1,V-A153Acetate Buffer Solution pH4.4,see Acetate Buffer pH4.4Acetate Buffer Solution pH4.6,see Acetate Buffer pH4.6Acetate Buffer Solution pH6.0,seeAcetate Buffer pH6.0Acetate Buffer Solution pH4.4,V-A152Acetate Buffer Solution pH4.5,V-A152Acetate Buffer Solution pH4.7,V-A152Acetate Buffer Solution pH5.0,V-A153Acetate Buffer Solution pH6.0,V-A153Acetate–edetate Buffer Solution pH5.5,V-A153Acetates,Reactions of,V-266Acetazolamide,I-54,V-S5Acetazolamide Oral Suspension,III-89Acetazolamide Tablets,III-90Acetic Acid,V-A20Acetic Acid(6per cent),I-56Acetic Acid(33per cent),I-56Acetic Acid,Anhydrous,V-A20Acetic Acid,Deuterated,V-A50Acetic Acid,Dilute,V-A20Acetic Acid,Dilute,see Acetic Acid(6per cent)Acetic Acid,Glacial,I-55,V-A20Acetic Acid in Synthetic Peptides,Determination of,V-299Acetic Acid VS,V-A142Acetic Acid,see Acetic Acid(33per cent)Acetic Anhydride,V-A20Acetic Anhydride Solution R1,V-A20Acetic Anhydride–Dioxan Solution,V-A20Acetic Anhydride–Sulfuric Acid Solution,V-A20Acetic Anhydride–Sulphuric AcidSolution,see Acetic Anhydride–SulfuricAcid SolutionAcetic Bromine Solution,V-A34Acetone,I-57,V-A20Acetone,Deuterated,V-A50Acetone Solution,Buffered,V-A153Acetone-dried Ox Brain,V-A98Acetonitrile,V-A20Acetonitrile for Chromatography,V-A20Acetonitrile R1,V-A20Acetoxyvalerenic Acid,V-A20Acetyl Chloride,V-A20Acetyl Groups,Reactions of,V-266Acetyl Salicylic Acid see AspirinAcetyl Value,Determination of,V-317Acetylacetamide,V-A20Acetylacetone,V-A20Acetylacetone Reagent R1,V-A20Acetylacetone Reagent R2,V-A204-Acetylbiphenyl,V-A20O-Acetyl Groups in PolysaccharideVaccines,V-467N-Acetyl-e-caprolactam,V-A20Acetylcholine Chloride,I-58,V-A20Acetylcysteine,I-59,V-S6Acetylcysteine Eye Drops,III-90Acetylcysteine Injection,III-91Acetyldigoxin,I-61b-Acetyldigoxin see AcetyldigoxinAcetyleugenol,V-A20N-Acetylglucosamine,V-A21Acetyl-11-keto-b-boswellic Acid,V-A21N-Acetyl-L-cysteine,V-A20N-Acetylneuraminic Acid,V-A21Acetylsalicylic Acid Tablets,see AspirinTabletsN-Acetyltryptophan,V-A21N-Acetyltryptophan see AcetyltryptophanAcetyltryptophan,I-63Acetyltyrosine,I-65N-Acetyltyrosine see AcetyltyrosineAcetyltyrosine Ethyl Ester,V-A21Acetyltyrosine Ethyl Ester,0.2M,V-A21Aciclovir,I-67Aciclovir Cream,III-93Aciclovir Eye Ointment,III-94Aciclovir Infusion,III-95Aciclovir Intravenous Infusion,seeAciclovir Infusion,Aciclovir Oral Suspension,III-97Aciclovir Sodium for Infusion,III-95Aciclovir Sodium for IntravenousInfusion,see Aciclovir Sodium forInfusion,Aciclovir Tablets,III-98Aciclovir Tablets,Dispersible,III-99Acid Blue92,V-A21Acid Blue92Solution,V-A21Acid Blue83,V-A21Acid Blue93Solution,V-A21Acid Blue90,V-A21Acid Gentian Mixture,IV-197Acid Gentian Oral Solution,IV-197Acid Potassium IodobismuthateSolution,V-A108Acid Value,V-317Acid/base Indicators,V-789Acid-base titrations,V-788Acidified Chloroform,V-A41Acidified Dichloromethane,V-A52Acidified Methanol,V-A85Acidified Methylene Chloride,seeAcidified DichloromethaneAcid-insoluble Ash,Determination of,V-336Acid-washed Diatomaceous Support,V-A51Acitretin,I-69Acitretin Capsules,III-100Acknowledgements,I-xxviiAcrylamide,V-A21Acrylamide/bisacrylamide(29:1)Solution,30per cent,V-A21Acrylamide/bisacrylamide(36.5:1)Solution,30per cent,V-A21Acrylic Acid,V-A21Actein,V-A21Acteoside,V-A21Action and Use Statement,Status of,I-17,II-17,III-17,IV-17,V-17Activated Acid Aluminium Oxide,V-A23Activated Attapulgite,I-220Activated Charcoal,I-496,V-A40Activated Zinc,V-A140Active Moiety,V-651Adamantane,V-A21Adapalene,I-71Adapalene Cream,III-101Adapalene Gel,III-103Additions,List of,I-xxviiiAdditions,List of Monographs,I-xxiiAdditives,Plastic,V-592Adenine,I-72,V-A21Adenosine,I-73,V-A21Adipic Acid,I-75,V-A21Adrenaline,V-A21Adrenaline/Epinephrine,I-76page numbers in bold type relate to monograph titles Index V-A799Adrenaline Acid Tartrate,V-A21 Adrenaline Acid Tartrate/Epinephrine Acid Tartrate,I-77Adrenaline and Cocaine Intranasal Solution,III-107Adrenaline(Epinephrine),V-S6 Adrenaline Eye Drops,Epinephrine Eye Drops,Neutral,III-104Adrenaline Eye Drops/Epinephrine Eye Drops,III-104Adrenaline Injection,Bupivacaine and, III-220Adrenaline Injection,Dilute(1in10,000),III-106Adrenaline Injection,Lidocaine and,III-751Adrenaline Injection/Epinephrine Injection,III-105Adrenaline Solution/Epinephrine Solution,III-106Adrenaline Tartrate see Adrenaline Acid TartrateAdrenaline TartrateInjection/Epinephrine Tartrate Injection,III-105Adrenaline TartrateSolution/Epinephrine Tartrate Solution,III-106Adrenalone Hydrochloride,V-A21 Adsorbed Diphtheria and Tetanus Vaccine,IV-537Adsorbed Diphtheria and Tetanus Vaccine for Adults and Adolescents, see Adsorbed Diphtheria and Tetanus Vaccine(adsorbed,Reduced Antigen(s) Content)Adsorbed Diphtheria,Tetanus and Pertussis(Acellular Component) Vaccine,IV-541Adsorbed Diphtheria,Tetanus,Pertussis (Acellular Component)and Haemophilus Type b Conjugate Vaccine,IV-545Adsorbed Diphtheria,Tetanus,Pertussis (Acellular Component)and Hepatitis B(rDNA)Vaccine,IV-547 Adsorbed Diphtheria,Tetanus,Pertussis (Acellular Component)and Inactivated Poliomyelitis Vaccine,IV-548Adsorbed Diphtheria Vaccine,IV-534 Adsorbed Diphtheria Vaccine for Adults and Adolescents,see Diphtheria Vaccine (Adsorbed,Reduced Antigen Content) Adsorbed Pertussis Vaccine(Acellular Component),IV-604Adsorbed Pertussis Vaccine(Acellular, Co-purified),IV-605Adsorbed Tetanus Vaccine,IV-633 Adsorbed Vaccines,Aluminium in,V-463Adsorbed Vaccines,Calcium in,V-464 Adsorption,Gas,Specific Surface Area By(2.9.26.)(5.8.),V-701Aescin,V-A22Aflatoxin B1,V-A22Aflatoxin B,in Herbal Drugs, Determination of,V-341Agar,I-79,V-A22Agarose for Chromatography,V-A22Agarose for Chromatography,Cross-linked,V-A22Agarose for Chromatography R1,Cross-linked,V-A22Agarose for Electrophoresis,V-A22Agarose/Cross-linked Polyacrylamide,V-A22Agarose-DEAE for Ion ExchangeChromatography,V-A22Agnus Castus Fruit,IV-50Agrimony,IV-52Air,Medical,I-78Air,Medicinal see Medical AirAir Permeability,Specific Surface Areaby,V-505Air,Synthetic,I-81Air,Synthetic Medicinal see Synthetic AirAlanine,I-83,V-A22ß-Alanine,see3-Aminopropionic AcidAlbendazole,I-84Albumin,Bovine,V-A22Albumin,Bovine R1,V-A22Albumin,Human,V-A22Albumin Solution,IV-467Albumin Solution,Human,V-A22Albumin Solution R1,Human,V-A22Alchemilla,IV-53Alcohol(20per cent),I-900Alcohol(25per cent),I-900Alcohol(45per cent),I-900Alcohol(50per cent),I-900Alcohol(60per cent),I-900Alcohol(70per cent),I-900Alcohol(80per cent),I-900Alcohol(90per cent),I-900Alcohol,Aldehyde-free,see Ethanol(96%),Aldehyde-freeAlcoholic Calcium Standard Solution(100ppm Ca),V-A149Alcoholic DimethylaminobenzaldehydeSolution,V-A55Alcoholic Hydroxylamine Solution,V-A74Alcoholic Iodine Solution,III-696,V-A75Alcoholic Potassium Hydroxide,2M,V-A108Alcoholic Potassium Hydroxide,seePotassium Hydroxide VS,EthanolicAlcoholic Potassium Hydroxide Solution,V-A108Alcoholic Potassium Hydroxide SolutionR1,V-A108Alcoholic Solution of Sulfuric Acid,V-A128Alcoholic Sulfuric Acid,0.25M,V-A128Alcoholimetric Tables,V-687Alcohol,see Ethanol(96%)Alcuronium Chloride,I-85Aldehyde Dehydrogenase,V-A22Aldehyde Dehydrogenase Solution,V-A22Aldehyde-free alcohol,see Ethanol(96%),Aldehyde-freeAldehyde-free Ethanol(96%),V-A62Aldehyde-free Methanol,V-A85Aldehydes,Determination of,V-321Aldrin,V-A22Alendronate Sodium Tablets,seeAlendronic Acid TabletsAlendronic Acid Tablets,III-109Aleuritic Acid,V-A22Alexandrian Senna Fruit,IV-362Alfacalcidol,I-87Alfadex,I-88Alfentanil Hydrochloride,I-89Alfuzosin,V-S7Alfuzosin Hydrochloride,I-91Alfuzosin Tablets,III-111Alfuzosin Tablets,Prolonged-release,III-112Alginate Antacid Oral Suspension,Compound,III-113Alginate Oral Suspension,Raft-forming,III-114Alginate Raft-forming Oral Suspension,III-114Alginic Acid,I-92Alimemazine,V-S7Alimemazine Oral Solution,Paediatric,III-115Alimemazine Tablets,III-116Alimemazine Tartrate,I-93Alizarin S,V-A22Alizarin S Solution,V-A22Alkaline Corallin Solution,V-A46Alkaline Eye Drops,see Hypromellose EyeDropsAlkaline Gentian Mixture,IV-198Alkaline Gentian Oral Solution,IV-198Alkaline Hydroxylamine Solution,V-A74Alkaline Hydroxylamine Solution R1,V-A74Alkaline Potassium Mercuri-iodideSolution,V-A109Alkaline Potassium TetraiodomercurateSolution,V-A109Alkaline Pyrogallol Solution,V-A111Alkaline Sodium Picrate Solution,V-A124Alkaline Tetrazolium Blue Solution,V-A132Alkali-washed Diatomaceous Support,V-A51Alkaloids,Complete Extraction of,V-335Alkaloids,Reactions of,V-266all--Alpha-Tocopherol,II-1051Allantoin,I-94,V-A23Allergen Products,I-95Allium Sativum for HomoeopathicPreparations,IV-427Allopurinol,I-98Allopurinol Oral Suspension,III-117Allopurinol Tablets,III-118all-rac-Alpha-Tocopheryl Acetate,II-1054all-rac-a-Tocopheryl see all-rac-Alpha-Tocopherylall-rac-Tocopheryl Acetate see all-rac-Alpha-Tocopheryl AcetateAlmagate,I-100Almond Oil Ear Drops,III-119Almond Oil,Refined,I-100Almond Oil,Virgin,I-99Almond Oil see Virgin Almond OilAloes,Barbados,IV-53Aloes,Cape,IV-54Alovudine,V-A23Alovudine(18F)Injection,IV-669V-A800IndexAloxiprin,I-102Aloxiprin Tablets,III-119Alpha Tocopheryl Acetate Concentrate (Powder Form),II-1057Alpha Tocopheryl Hydrogen Succinate, II-1058Alpha Tocopheryl Succinate Tablets,III-1164Alphacyclodextrin see Alfadex Alprazolam,I-103Alprenolol Hydrochloride,I-105 Alprostadil,I-107Alteplase for Injection,I-109 Alternative methods,I-20,II-20,III-20, IV-20,V-20Alternative Methods for Control of Microbiological Quality,V-745 Altizide,I-113Alum,I-114Aluminium,V-A23Aluminium Acetate Ear Drops,III-120 Aluminium Chloride,V-A23 Aluminium Chloride Hexahydrate,I-114 Aluminium Chloride Reagent,V-A23 Aluminium Chloride Solution,III-121, V-A23Aluminium Glycinate,I-115 Aluminium Hydroxide and Magnesium Trisilicate Tablets,Chewable,III-776 Aluminium Hydroxide,Dried,I-115 Aluminium Hydroxide Gel,V-A23 Aluminium Hydroxide,Hydrated for Adsorption,I-114Aluminium Hydroxide Oral Suspension, III-121Aluminium Hydroxide Oral Suspension, Magnesium Hydroxide and,III-401 Aluminium Hydroxide Tablets, Chewable,III-122Aluminium Hydroxide Tablets, Magnesium Hydroxide and,III-402 Aluminium Hydroxide Tablets see Chewable Aluminium Hydroxide Tablets,III-122Aluminium in Adsorbed Vaccines,V-463 Aluminium Magnesium Silicate,I-118 Aluminium Nitrate,V-A23Aluminium Oxide,Activated Acid,V-A23Aluminium Oxide,Anhydrous,V-A23 Aluminium Oxide,Basic,V-A23 Aluminium Oxide,Deactivated,V-A23 Aluminium Oxide G,V-A23 Aluminium Oxide,Neutral,V-A23 Aluminium Paste,Compound,III-120 Aluminium Phosphate Gel,I-120 Aluminium Phosphate,Hydrated see Dried Aluminium Phosphate Aluminium Potassium Sulfate,V-A23 Aluminium Potassium Sulphate,see Aluminium Potassium Sulfate Aluminium Powder,I-121Aluminium Salts,Reactions of,V-266 Aluminium Sodium Silicate,I-122 Aluminium Standard Solution(2ppm Al),V-A148Aluminium Standard Solution(10ppm Al),V-A148Aluminium Standard Solution(100ppm Al),V-A148Aluminium Standard Solution(200ppmAl),V-A148Aluminium Stearate,I-123Aluminium Sulfate,I-125,V-A23Aluminium Sulphate,see AluminiumSulfateAlverine Capsules,III-122Alverine Citrate,I-126,V-S7Amantadine Capsules,III-123Amantadine Hydrochloride,I-127Amantadine Oral Solution,III-124Amantidine,V-S8Amaranth S,V-A23Amaranth Solution,V-A23Ambroxol Hydrochloride,I-128Americium-243Spiking Solution,V-A23Amethocaine Eye Drops,see TetracaineEye DropsAmfetamine Sulfate,I-130Amfetamine Sulphote,see AmfetamineSulfate,I-130Amido Black10B Solution,V-A23Amidohexadecylsilyl Silica Gel forchromatography,V-A115Amidotrizoic Acid Dihydrate,I-130Amikacin,I-132Amikacin Injection,III-124Amikacin Sulfate,I-135Amiloride and Furosemide Tablets,seeCo-amilofruse TabletsAmiloride and Hydrochlorothiazide OralSolution,see Co-amilozide Oral SolutionAmiloride and HydrochlorothiazideTablets,see Co-amilozide TabletsAmiloride Hydrochloride,I-138Amiloride Tablets,III-125Amines,Primary Aromatic,Reactions of,V-266Amino Acid Analysis,V-221Amino Acid Analysis(2.2.56.)(5.8.),V-700Amino Acids,Use of Codes for,I-8,II-8,III-8,IV-8,V-8Aminoazobenzene,V-A23Aminobenzoic Acid,I-139,V-A23,V-A244-Aminobenzoic Acid Solution,V-A24(4-Aminobenzoyl)-L-glutamic Acid,V-A244-Aminobutanoic acid,see4-Amino-n-butyric Acid2-Aminobutan-1-ol,V-A24Aminocaproic Acid,I-1402-Amino-5-chlorobenzophenone,V-A24Aminochlorobenzophenone,see2-Amino-5-chlorbenzophenone4-Aminofolic Acid,V-A24Aminoglutethimide,I-141,V-S8Aminoglutethimide Tablets,III-126Aminohexadecylsilyl Silica Gel forChromatography,V-A1156-Aminohexanoic Acid,V-A24p-Aminohippuric Acid,V-A24Aminohippuric Acid Reagent,V-A244-Amino-3-hydroxynaphthalene-1-sulfonic Acid,V-A24Aminohydroxynaphthalenesulfonic AcidSolution,V-A24Aminohydroxynaphthalenesulfonic AcidSolution,Strong,V-A24AminohydroxynaphthalenesulphonicAcid Solution,Strong,seeAminohydroxynaphthalenesulfonic AcidSolution,StrongAminohydroxynaphthalenesulphonicAcid Solution,seeAminohydroxynaphthalenesulfonic AcidSolution4-Amino-3-hydroxynaphthalene-1-sulphonic Acid,see4-Amino-3-hydroxynaphthalene-1-sulfonic AcidAminohydroxynaphthalenesulphonic,Acid,see Aminonaphthalenesulfonic AcidSolution5-Aminoimidazole-4-carboxamideHydrochloride,V-A24cis-Aminoindanol,V-A24Aminomethylalizarindiacetic AcidReagent,V-A24Aminomethylalizarindiacetic AcidSolution,V-A253-Aminomethylalizarin-N,N-diaceticAcid,V-A244-Aminomethylbenzoic acid,V-A253-(Aminomethyl)pyridine,V-A258-Aminonaphthalene-2-sulfonic Acid,V-A25Aminonaphthalenesulfonic AcidSolution,V-A25Aminonaphthalenesulphonic AcidSolution,see AminonaphthalenesulfonicAcid Solution8-Aminonaphthalene-2-sulphonic Acid,see8-Aminonaphthalene-2-sulfonic Acid4-Amino-n-butyric Acid,V-A242-Amino-5-nitrobenzophenone,V-A25Aminonitrobenzophenone,see2-Amino-5-nitrobenzophenone4-Aminophenazone,V-A25Aminophenazone Solution,V-A253-Aminophenol,V-A254-Aminophenol-free Paracetamol,V-A99Aminophylline,I-143Aminophylline Hydrate,I-145Aminophylline Injection,III-128Aminophylline Tablets,III-128Aminophylline Tablets,Prolonged-release,III-129Aminopolyether,V-A253-Aminopropanol,V-A253-Aminopropionic Acid,V-A25Aminopropylmethylsilyl Silica Gel forChromatography,V-A115Aminopropylsilyl Silica Gel forChromatography,V-A115Aminopropylsilyl Silica Gel forChromatography R1,V-A115Aminopyrazolone,see4-AminophenazoneAminopyrazolone Solution,seeAminophenazone Solution3-Aminosalicylic Acid,V-A25Amiodarone,V-S8Amiodarone Concentrate,Sterile,III-130Amiodarone Hydrochloride,I-147Amiodarone Infusion,III-129Amiodarone Intravenous Infusion,seeAmiodarone Infusion,Amiodarone Oral Suspension,III-131Amiodarone Sterile Concentrate,III-130page numbers in bold type relate to monograph titles Index V-A801Amiodarone Tablets,III-132 Amisulpride,I-149,V-S9Amisulpride Oral Solution,III-133 Amisulpride Tablets,III-134 Amitriptyline Embonate,I-150 Amitriptyline Hydrochloride,I-151 Amitriptyline Tablets,III-135 Amlodipine Besilate,I-153 Ammonia,V-A25Ammonia(13N)Injection,IV-672 Ammonia Buffer pH9.5,see Ammonium Chloride Buffer Solution pH9.5 Ammonia Buffer pH10.9,V-A153 Ammonia Buffer pH10.9,Dilute,V-A153Ammonia Buffer pH10.0,V-A153 Ammonia,Chloride-free,V-A25 Ammonia,Concentrated,V-A25 Ammonia,Lead-free,V-A25 Ammonia,Methanolic,V-A25 Ammonia R1,Concentrated,V-A26 Ammonia R1,Dilute,V-A26 Ammonia R2,Dilute,V-A26 Ammonia R3,Dilute,V-A26 Ammonia Solution,Aromatic,III-136 Ammonia Solution,Concentrated see Strong Ammonia SolutionAmmonia Solution,Dilute,III-137 Ammonia Spirit,Aromatic,III-137 Ammoniacal Copper Oxide Solution,V-A46Ammoniacal Silver Nitrate Solution,V-A120Ammoniacal Solution of Copper Tetrammine,V-A46Ammonia-free Water,V-A139 Ammonio Methacrylate Copolymer (Type A),I-155Ammonio Methacrylate Copolymer (Type B),I-1560.5M Ammonium acetate buffer solution pH4.5,see Ammonium acetate buffer pH4.5,0.5M0.01M Ammonium and Cerium Nitrate, see Ammonium Cerium(IV)Nitrate VS 0.1M Ammonium and Cerium Sulfate, see Ammonium Cerium(IV)Sulfate VS Ammonium Acetate,V-A26 Ammonium acetate buffer pH4.5,0.5M, V-A153Ammonium Acetate Solution,V-A26 Ammonium Acetate Solution,Strong,III-137Ammonium and Cerium Nitrate,see Ammonium Cerium(IV)Nitrate Ammonium and Cerium Sulfate,see Ammonium Cerium(IV)Sulfate Ammonium and Cerium Sulphate,see Ammonium and Cerium Sulfate Ammonium Bicarbonate,I-157 Ammonium Bromide,I-158 Ammonium Carbamate,V-A26 Ammonium Carbonate,V-A26 Ammonium Carbonate Buffer Solution pH10.3,0.1M,V-A153Ammonium Carbonate Solution,V-A26 Ammonium Carbonate Solution,Dilute, V-A26Ammonium carbonate solution R1,V-A26Ammonium Cerium(IV)Nitrate,V-A26Ammonium Cerium(IV)Nitrate VS,V-A142Ammonium Cerium(IV)Sulfate,V-A26Ammonium Cerium(IV)Sulfate VS,V-A142Ammonium Cerium(IV)Sulphate VS,seeAmmonium Cerium(IV)Sulfate VSAmmonium Cerium(IV)Sulphate,seeAmmonium Cerium(iv)SulfateAmmonium Chloride,I-159,V-A26Ammonium Chloride Buffer SolutionpH10.0,see Ammonia Buffer pH10.0Ammonium Chloride Buffer SolutionpH10.4,V-A153Ammonium Chloride Buffer SolutionpH10.7,V-A153Ammonium Chloride Buffer SolutionpH9.5,V-A153Ammonium Chloride Buffer SolutionpH10.0,V-A153Ammonium Chloride Mixture,III-137Ammonium Chloride Oral Solution,III-137Ammonium Chloride Solution,V-A26Ammonium Citrate,V-A26Ammonium Citrate Solution,V-A26Ammonium CobaltothiocyanateSolution,V-A26Ammonium DihydrogenOrthophosphate,V-A26Ammonium Formate,V-A26Ammonium Glycyrrhizinate,I-160Ammonium Hexafluorogermanate,V-A26Ammonium Hydrogen Carbonate,V-A26Ammonium Hydrogen Carbonate seeAmmonium BicarbonateAmmonium Ichthosulphonate seeIchthammolAmmonium Iron(II)Sulfate,V-A26Ammonium Iron(II)Sulfate VS,V-A142Ammonium Iron(II)Sulphate VS,seeAmmonium Iron(II)Sulfate VSAmmonium Iron(II)Sulphate,seeAmmonium Iron(ii)SulfateAmmonium Iron(III)Citrate,V-A26Ammonium Iron(III)Sulfate,V-A26Ammonium Iron(III)Sulfate Solution R1,V-A26Ammonium Iron(III)Sulfate Solution R2,V-A26Ammonium Iron(III)Sulfate Solution R5,V-A26Ammonium Iron(III)Sulfate Solution R6,V-A27Ammonium Iron(III)Sulfate VS,V-A142Ammonium Iron(III)Sulphate SolutionR1,see Ammonium Iron(iii)SulfateSolution R1Ammonium Iron(III)Sulphate SolutionR2,see Ammonium Iron(iii)SulfateSolution R2Ammonium Iron(III)Sulphate SolutionR5,see Ammonium Iron(iii)SulfateSolution R5Ammonium Iron(III)Sulphate VS,seeAmmonium Iron(III)Sulfate VSAmmonium Iron(III)Sulphate,seeAmmonium Iron(iii)SulfateAmmonium Mercaptoacetate Solution,V-A27Ammonium Mercurithiocyanate Reagent,V-A27Ammonium Metavanadate,V-A27Ammonium Metavanadate Solution,V-A27Ammonium Molybdate,V-A27Ammonium Molybdate Reagent,V-A27Ammonium Molybdate Reagent R1,V-A27Ammonium Molybdate Reagent R2,V-A27Ammonium Molybdate Solution,V-A27Ammonium Molybdate Solution R2,V-A27Ammonium Molybdate Solution R3,V-A27Ammonium Molybdate Solution R4,V-A27Ammonium Molybdate Solution R5,V-A27Ammonium Molybdate Solution R6,V-A27Ammonium Molybdate-Sulfuric AcidSolution,V-A27Ammonium Molybdate-Sulphuric AcidSolution,see Ammonium Molybdate-Sulfuric Acid SolutionAmmonium Muriaticum,V-609Ammonium Nitrate,V-A27Ammonium Nitrate R1,V-A27Ammonium Oxalate,V-A27Ammonium Oxalate Solution,V-A27Ammonium Persulfate,V-A27Ammonium Persulphate,see AmmoniumPersulfateAmmonium Phosphate,see DiammoniumHydrogen OrthophosphateAmmonium Polysulfide Solution,V-A27Ammonium Polysulphide Solution,seeAmmonium Polysulfide SolutionAmmonium Pyrrolidinedithiocarbamate,V-A27Ammonium PyrrolidinedithiocarbamateSolution,V-A27Ammonium Reineckate,V-A27Ammonium Reineckate Solution,V-A28Ammonium Salts and Salts of VolatileBases,Reactions of,V-267Ammonium Salts Reactions of,V-266Ammonium Standard Solution(1ppmNH4),V-A148Ammonium Standard Solution(2.5ppmNH4),V-A148Ammonium Standard Solution(3ppmNH4),V-A148Ammonium Standard Solution(100ppmNH4),V-A148Ammonium Sulfamate,V-A28Ammonium Sulfate,V-A28Ammonium Sulfide Solution,V-A28Ammonium Sulphamate,see AmmoniumSulfamateAmmonium Sulphate,see AmmoniumSulfateAmmonium Sulphide Solution,seeAmmonium Sulfide SolutionV-A802IndexAmmonium Thiocyanate,V-A28 Ammonium Thiocyanate Solution,V-A28Ammonium Thiocyanate VS,V-A142 Ammonium Vanadate Solution,V-A28 Ammonium Vanadate,see Ammonium MetavanadateAmobarbital,I-161Amobarbital Sodium,I-162Amomum fruit,IV-56Amorphous Organosilica Polymer, Octadecylsilyl,V-A98Amoxicillin and Potassium Clavulanate Injection,see Co-amoxiclav Injection Amoxicillin and Potassium Clavulanate Oral Suspension,see Co-amoxiclav Oral SuspensionAmoxicillin and Potassium Clavulanate Tablets,Dispersible,see Dispersible Co-amoxiclav TabletsAmoxicillin and Potassium Clavulanate Tablets,see Co-amoxiclav Tablets Amoxicillin Capsules,III-138 Amoxicillin Injection,III-139 Amoxicillin Oral Suspension,III-141 Amoxicillin Sodium,I-163,V-S9 Amoxicillin Sodium for Injection,III-139Amoxicillin Trihydrate,I-165,V-S9,V-A28Ampere,Definition of,I-32,II-32,III-32,IV-32,V-32 Amperometric,Potentiometric and Voltametric Titrations,V-280 Amperometric Titration,V-280 Amphotericin,I-168Amphotericin B see Amphotericin Amphotericin for Infusion,III-142 Amphotericin Lozenges,I-xxix Amphotericin Oral Suspension,I-xxix Ampicillin,I-170Ampicillin Capsules,III-143Ampicillin Capsules,Flucloxacillin and, see Co-fluampicil CapsulesAmpicillin Injection,III-144Ampicillin Oral Suspension,III-146 Ampicillin Oral Suspension, Flucloxacillin and,see Co-fluampicil Oral SuspensionAmpicillin Sodium,I-172,V-S10 Ampicillin Sodium for Injection,III-144 Ampicillin Trihydrate,I-175,V-S10 Amyl Acetate,V-A28Amyl Alcohol,see Isoamyl Alcohola-Amylase,V-A28a-Amylase Solution,V-A28 Amylmetacresol,I-178,V-S10Amylose-derivative Silica Gel for Chromatography,V-A115b-Amyrin,V-A28Anacardium for Homoeopathic Preparations,IV-428Anaesthetic Ether,I-902Analytical Procedures,Validation of,V-673Analytical Sieving,Particle-size Distribution Estimation By,V-503 Anastrozole,I-179cis-Anethole,V-A28Anethum Graveolens L.Sowa Group,seeAnethum Graveolens Sowa Fruit,Anethum Graveolens Sowa Fruit,IV-58Angelica Archangelica Root,IV-59Angelica Dahurica Root,IV-60Angelica Pubescens Root,IV-62Angelica Sinensis Root,IV-63Angelica Sinensis Root,see ProcessedAngelica Sinensis RootAnhydrous Acetic Acid,V-A20Anhydrous Aluminium Oxide,V-A23Anhydrous Ampicilin see AmpicillinAnhydrous Azapropazone,V-S12Anhydrous Beclometasone Dipropionate,I-239Anhydrous Caffeine,see CaffeineAnhydrous Calcipotriol,I-353Anhydrous Calcium Acetate,see CalciumAcetateAnhydrous Calcium Chloride,V-A37Anhydrous Calcium Gluconate,I-372Anhydrous Calcium HydrogenPhosphate,I-377Anhydrous Calcium Lactate,I-378Anhydrous Chlorobutanol,I-518Anhydrous Citric Acid,I-569,V-A45Anhydrous Copper Sulfate,I-647Anhydrous Disodium HydrogenOrthophosphate,V-A59Anhydrous Disodium HydrogenPhosphate,I-788Anhydrous Docetaxel,I-796Anhydrous Ephedrine,I-849Anhydrous Formic Acid,V-A67Anhydrous Glucose,I-1083Anhydrous Iron(III)Chloride,V-A77Anhydrous Lactose,II-66Anhydrous Lithium Metaborate,V-A81Anhydrous Magnesium Citrate,II-166Anhydrous Methanol,V-A85Anhydrous Morphine,V-A91Anhydrous Nevirapine,II-358Anhydrous Niclosamide,II-362Anhydrous Paroxetine Hydrochloride,II-504Anhydrous Phloroglucinol,II-566Anhydrous Pyridine,V-A110Anhydrous Silica Gel,V-A114Anhydrous Silica,HydrophobicColloidal,II-807Anhydrous Sodium Acetate,V-A120Anhydrous Sodium Carbonate,II-830,V-A121,V-A141Anhydrous Sodium DihydrogenOrthophosphate,V-A122Anhydrous Sodium DihydrogenPhosphate,II-839,V-A122Anhydrous Sodium Sulfate,II-872,V-A124Anhydrous Sodium Sulfite,II-873,V-A124Anhydrous Sodium Sulphate seeAnhydrous Sodium SulfateAnhydrous Sodium Sulphite seeAnhydrous Sodium SulfiteAnhydrous Torasemide,II-1066Anhydrous Valaciclovir Hydrochloride,II-1134Aniline,V-A28Aniline Hydrochloride,V-A28Aniline Hydrochloride Solution,V-A28Animal Spongiform EncephalopathyAgents Via Human and VeterinaryMedicinal Products,Minimising theRisk of Transmitting,V-611Animals,Use of,I-15,II-15,III-15,IV-15,V-15Anion Exchange Resin,V-A28Anion Exchange Resin forChromatography,Strongly Basic,V-A28Anion Exchange Resin R1,V-A28Anion Exchange Resin R2,V-A28Anion exchange resin R3,V-A29Anion Exchange Resin,Strongly Basic,V-A28Anion Exchange Resin,Weak,V-A29Anion-exchange Resin forChromatography,Strongly Basic R1,V-A28Anionic Emulsifying Wax,see EmulsifyingWaxAnisaldehyde,V-A29Anisaldehyde Solution,V-A29Anisaldehyde Solution R1,V-A29Anise Ketone,V-A29Anise Oil,IV-71Anise Water,Concentrated,IV-73Aniseed,IV-66Aniseed Oil,see Anise Oilp-Anisidine,V-A29Anisidine Value,V-326Anolyte for Isoelectric Focusing pH3to5,V-A29Antazoline Hydrochloride,I-181Anthracene,V-A29Anthranilic Acid,see2-Aminobenzoic AcidAnthrax,see Anthrax Vaccine for HumanUse(Adsorbed,Prepared from CultureFiltrates)Anthrax Vaccine for Human Use(Adsorbed,Prepared from CultureFiltrates),IV-527Anthrone,V-A29Anthrone Reagent,V-A29Antibiotics,Microbiological Assay of,V-396,V-655Antibiotics,Potency of,I-14,II-14,III-14,IV-14,V-14Anticoagulant and Preservative Solutionsfor Blood,IV-461Anti-D Immunoglobulin for IntravenousUse,IV-497Anti-D(Rh0)Immunoglobulin,IV-496Antimicrobial Preservation,Efficacy of,V-494,V-653Antithrombin III ConcentrateAnticomplimentary activity ofimmunoglobulin,Test for V-427Anti-D immunoglobulin,human,Assayof V-429Anti-D antibodies in humanimmunoglobulin V-431Anti-A and anti-B haemogglutininsV-432Antimicrobial Preservatives,Definition ofSuitable,I-11,II-11,III-11,IV-11,V-11Antimony Compounds,Reactions of,V-267page numbers in bold type relate to monograph titles Index V-A803。
伯乐1708895系列说明书

Reaction ProtocolIncubate complete reaction mix in a real-time thermal detection system as follows:cDNA synthesis:10 min at 50°CiScript reverse transcriptase inactivation: 5 min at 95°CPCR cycling and detection (30 to 45 cycles):10 to 15 sec at 95°C30 sec at 55°C to 60°C (data collection step) Recommendations for optimal results using the iScript One-Step RT-PCR Kit for ProbesProbe and primers should be designed according to standard qPCR guidelines.Suggested input quantities of template are: 1 pg to 1 µg total RNA; 10 fg to 100 ng polyA(+) RNA.First strand synthesis can be performed between 40°C and 52°C. Optimal results are generally obtained with a 10-minute incubation at 50°C. Incubation at temperatures higher than 50°C can delay or eliminate the detection of some non-specific amplification artifacts. However, this may also delay the C t for detection of specific targets. We also recommend a 5-minute incubation at 95°C to fully inactive the reverse transcriptase prior to PCR cycling.Thaw all components, except the iScript reverse transcriptase, at room temperature. Mix gently, but thoroughly, and then centrifuge at 4°C to collect contents to the bottom of the tube. Chill on ice before using. Centrifuge again briefly at 4°C if needed. Preparation of a reaction cocktail is crucial in quantitative PCR applications to reduce pipetting errors and maximize assay precision and accuracy. Assemble the reaction cocktail with all required components except sample template (total RNA) and dispense equal aliquots into each reaction tube. Add target sample to each reaction as the final step. Addition of sample as 5–10 µl volumes will improve assay precision. Replicate samples should be assembled as a master mix with a single addition of sample template.Reagents and Materials Not SuppliedGene-specific primers and probePipet tips, aerosol barrier tips, such as:Xcluda®Style B, 211-2006Nuclease-free tubes or plates, such as:0.2 ml thin-wall tubes, 223-9473 or plates, 223-9441RNA purification kit, such as:Aurum™total RNA mini kit, 732-6820, orAurum total RNA kit, 2 x 96 well, 732-6800To learn more about Bio-Rad's complete solution for Amplification, visit our website:/genomicsNOTICE TO PURCHASER: LIMITED LICENSEPractice of the patented polymerase chain reaction (PCR) process requires a license. The Bio-Rad real-time detection systems include a licensed thermal cycler and may be used with PCR licenses available from Applied Biosystems. Its use with authorized reagents also provides a limited PCR license in accordance with the label rights accompanying such reagents. Some applications may require licenses from other parties. Aurum, iScript, iTaq, iQ, iCycler,Xcluda, and MyiQ are trademarks of Bio-Rad Laboratories. SYBR Green is a registered trademark of Molecular Probes, Inc. Bio-Rad Laboratories, Inc. is licensed by Molecular Probes, Inc. to sell reagents containing SYBR Green I for use in real-time PCR, for research purposes only.A license to perform the patented 5’ Nuclease Process for research is obtained by the purchase of (i) both Authorized 5’ Nuclease Core Kit and Licensed Probe, (ii) a Licensed5’ Nuclease Kit, or (iii) license rights from Applied Biosystems.This product is an Authorized 5’ Nuclease Core Kit. Use of this product is covered by one or more of the following US patents and corresponding patent claims outside the US: 5,079,352, 5,789,224, 5,618,711, 6,127,155, 5,677,152, (Claims 1-23 only), 5,773,258, (claims 1 and 6 only), 5,407,800, 5,322,770, 5,310,652, 5,210,015, 5,487,972, and claims outside the US corresponding to US Patent No. 4,889,818. The purchase of this product includes a limited, non-transferable immunity from suit under the foregoing patent claims for using only this amount of product for the purchaser’s own internal research. Separate purchase of a Licensed Probe would convey rights under the applicable claims on US Patents Nos. 5,538,848, 5,723,591, 5,876,930, 6,030,787, 6,258,569, 5,804,375 (claims 1-12 only), and 6,214,979, and corresponding claims outside the United States. No right under any other patent claim and no right to perform commercial services of any kind, including without limitation reporting the results of purchaser’s activities for a fee or other commercial consideration, is conveyed expressly, by implication, or by estoppel. This product is for research use only. Diagnostic uses under Roche patents require a separate license from Roche. Further information on purchasing licenses may be obtained from the Director of Licensing, Applied Biosystems, 850 Lincoln Centre Drive, Foster City, California 94404, USA.Bio-Rad Laboratories2000 Alfred Nobel Drive, Hercules, CA 94547510-741-10004106270 Rev C。
Thermo Scientific Rever id First Strand cDNA Synthesis Kit K 说明书 第一链cDNA合成试剂盒

#K1621, #K1622
分析证明书 #K1621 Lot
质量控制
采用100 fg对照GAPDH RNA和对照引物进行RT-PCR反应,通过在1%琼脂糖上进行凝胶电泳和溴 化乙锭染色显示得到足够量的496 bp的产物
质量认证人:Jurgita Zilinskiene
RT-PCR…………………………………………………………….6 合成cDNA用于克隆………..………………………………………7 实验对照……………………………………………………………….8 问题分析与解决……………………………………………………...10
Page 1
试剂盒成分
RevertAid™第一链cDNA合成试剂盒
2. 37°C 孵育 30 分钟。
3. 加入1 μl 50 mM EDTA,65°C 孵育10分钟。在含有二价阳离子(1)而缺乏螯合剂的环境中,RNA
产品说明
RevertAid™第一链cDNA合成试剂盒以mRNA或者总RNA为模板,高效合成第一链cDNA。本试剂 盒使用RevertAid™ M-MuLV反转录酶,它的RNA酶H的活性与AMV反转录酶相比较低。该反转录酶可 耐受42-50°C温度,合成的cDNA片段长度达13kb。
试剂盒中含有RiboLock™ 重组RNA酶抑制剂,防止RNA降解,可耐受55°C高温。 试剂盒同时含有oligo(dT)18和随机六聚体引物。随机六聚体引物与模板非特异性地结合,以总RNA 中任何RNA为模板合成cDNA。oligo(dT)18选择性和RNA 3’poly(A)配对结合,只以有poly(A)尾巴的 mRNA为模板合成cDNA。使用本试剂盒也可采用序列特异性引物。 合成的第一链cDNA能直接用作PCR或荧光定量PCR的模板,第二链cDNA的合成或线性RNA扩增, 也可用于需要用带有放射性或非放射性核苷酸标记第一链cDNA的实验,比如将标记好的第一链cDNA 作为杂交实验中的探针或者用于微阵列分析。
AR-A014418_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:AR–A014418 is a selective and effective GSK3β inhibitor with an IC 50 value of 104 nM, and has no significant inhibition on 26 other kinases.IC50 & Target: IC50: 104 nM (GSK3β)In Vitro: AR–A014418 inhibits tau phosphorylation at a GSK3–specific site (Ser–396) in 3T3 fibroblasts expressing human four–repeat tau protein with IC 50 of 2.7 μM, and protects cultured N2A cells from death induced by blocking PI3K/PKB pathway. In hippocampal slices, AR–A014418 inhibits neurodegeneration mediated by beta–amyloid peptide [1]. While in NGP and SH–5Y–SY cells,AR–A014418 reduces neuroendocrine markers and suppresses neuroblastoma cell growth [2].In Vivo: In ALS mouse model with the G93A mutant human SOD1, AR–A014418 (0–4 mg/kg, i.p.) delays the onset of symptoms,improves motor activity, slows down disease progression, and postpons the endpoint of the disease [3]. In addition, AR–A014418produces inhibition effect on acetic acid– and formalin–induced nociception in mice by modulating NMDA and metabotropic receptor signaling as well as TNF–α and IL–1β transmission in the spinal cord [4].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]The competition experiments are carried out in duplicate with 10 concentrations of the inhibitor inclear–bottomed microtiter plates. The biotinylated peptide substrate, biotin–AAEELDSRAGS(PO3H2)PQL, is added at a final concentration of 2 μM in an assay buffer containing 6 milliunits of recombinant human GSK3 (equal mix of both α and β), 12mM MOPS, pH 7.0, 0.3 mM EDTA, 0.01% β–mercaptoethanol, 0.004% Brij 35, 0.5% glycerol, and 0.5 μg of bovine serumalbumin/25 μL and preincubated for 10–15 min. The reaction is initiated by the addition of 0.04 μCi of [γ–33P]ATP and unlabeled ATP in 50 mM Mg(Ac)2 to a final concentration of 1 μM ATP and assay volume of 25 μL. Blank controls without peptide substrate are used.After incubation for 20 min at room temperature, each reaction is terminated by the addition of 25 μL of stop solution containing 5mM EDTA, 50 μM ATP, 0.1% Triton X–100, and 0.25 mg of streptavidin–coated SPA beads corresponding to appr 35 pmol of binding capacity. After 6 h the radioactivity is determined in a liquid scintillation counter. Inhibition curves are analyzed by non–linear regression using GraphPad Prism.Cell Assay: AR–A014418 is dissolved in DMSO.[1]Cell viability is assessed by calcein/propidium iodide uptake. Calcein AM is taken up and cleaved by esterases present within living cells, yielding yellowish–green fluorescence, whereas PI is only taken up by dead cells,which become orange–red fluorescent. In brief, N2A cells are cultured for 2 days in vitro and then treated with 50 μM LY–294002 in the presence of AR–A014418 or vehicle (DMSO) for 24 h. Subsequently, N2A cells are incubated for 30 min with 2 μM PI and 1 μM calcein–AM. The cultures are then rinsed three times with Hanks' buffered saline solution containing 2 mM CaCl 2, and the cells are visualized by fluorescence microscopy using a Zeiss Axiovert 135 microscope. Three fields (selected at random) are analyzed per well (appr 300 cells/field) in at least three different experiments. Cell death is expressed as percentage of PI–positive cells from the total number of cells. In every experiment, specific cell death is obtained after subtracting the number of dead cells present inProduct Name:AR–A014418Cat. No.:HY-10512CAS No.:487021-52-3Molecular Formula:C 12H 12N 4O 4S Molecular Weight:308.31Target:GSK–3; GSK–3Pathway:Stem Cell/Wnt; PI3K/Akt/mTOR Solubility:10 mM in DMSOvehicle–treated cultures.Animal Administration: AR–A014418 is formulated in normal saline.[3]First, to examine the effects of GSK–3 inhibition on the clinical symptoms, life span, and motor behavior function of ALS, 56 Tg mice are divided into four groups. In each group, 0.5 mL of normal saline is mixed with either 0 μg (control group), 1 μg (group A), 2 μg (group B) or 4 μg (group C) of AR–A014418 per gram of mouse, and injected intraperitoneally into 14 animals per group 5 days a week beginning 60 days after birth. The mice are sacrificed at the endpoint described below.References:[1]. Bhat R, Xue Y, Berg S, Structural insights and biological effects of glycogen synthase kinase 3–specific inhibitor AR–A014418. J Biol Chem. 2003 Nov 14; 278(46):45937–45.[2]. Carter YM, et al. Specific glycogen synthase kinase–3 inhibition reduces neuroendocrine markers and suppresses neuroblastoma cell growth. Cancer Biol Ther. 2014 May;15(5):510–5.[3]. Koh SH, et al. Inhibition of glycogen synthase kinase–3 suppresses the onset of symptoms and disease progression of G93A–SOD1 mouse model of ALS. Exp Neurol. 2007 Jun;205(2):336–46.[4]. Martins DF, et al. The antinociceptive effects of AR–A014418, a selective inhibitor of glycogen synthase kinase–3 beta, in mice. J Pain. 2011 Mar;12(3):315–22.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
MedDRA

MedDRA®术语选择:考虑要点ICH 认可的 MedDRA 用户指南2022年3月目录SECTION 1 –引言 (1)1.1 本文档的目的 (1)1.2 使用 MedDRA (1)1.3 如何使用本文档 (2)1.4 首选方案 (2)1.5 MedDRA 浏览工具 (2)SECTION 2 –术语选择一般原则 (3)2.1 源数据的质量 (3)2.2 质量保证 (3)2.3 不要改动 MedDRA (3)2.4 始终选择低位语 (3)2.5 只选择现行低位语 (5)2.6 何时就术语提出申请 (5)2.7 选择术语时采用医学判断 (5)2.8 选择多个术语 (5)2.9 查看层级结构 (6)2.10 编码所有报告信息,但不要添加信息 (6)SECTION 3 –术语选择要点 (7)3.1 在有或没有报告体征和症状情况下的确定诊断和临时诊断 (7)3.2 死亡和其他患者转归 (10)3.2.1 死亡且报告了 AR/AE (10)3.2.2 报告信息里只有死亡 (11)3.2.3 提供重要临床信息的死亡术语 (11)3.2.4 其他患者转归(非致命) (11)3.3 自杀和自我伤害 (12)3.3.1 报告了用药过量 (12)3.3.2 报告了自我伤害 (12)3.3.3 实施自杀行为致死 (12)3.4 矛盾/有歧义/含糊的信息 (13)3.4.1 矛盾的信息 (13)3.4.2 有歧义的信息 (13)3.4.3 含糊的信息 (13)3.5 组合术语 (14)3.5.1 诊断和体征/症状 (14)3.5.2 一个状况比另一个更具体 (14)3.5.3 可以找到 MedDRA 组合术语 (15)3.5.4 何时“拆分”成多个 MedDRA 术语 (15)3.5.5 事件伴随原有状况 (16)3.6 年龄与事件 (16)3.6.1 MedDRA 术语能同时包含年龄和事件 (16)3.6.2 MedDRA 术语不能同时包含年龄和事件 (16)3.7 身体部位与事件 (17)3.7.1 MedDRA 术语能同时包含身体部位和事件 (17)3.7.2 MedDRA 术语不能同时包含身体部位和事件 (17)3.7.3 发生在多处身体部位的事件 (18)3.8 具体部位与具体微生物感染 (18)3.8.1 MedDRA 术语能同时包含微生物和解剖学部位 (18)3.8.2 MedDRA 术语不能同时包含微生物和解剖学部位 (18)3.9 原有状况发生变化 (19)3.10 妊娠和哺乳期暴露 (20)3.10.1 事件发生在母亲身上 (20)3.10.2 事件发生在儿童或者胎儿身上 (20)3.11 先天性术语 (21)3.11.1 先天性状况 (21)3.11.2 获得性状况(出生时不存在) (21)3.11.3 不能明确是先天性还是获得性的状况 (22)3.12 肿瘤 (22)3.12.1 不推断恶性 (23)3.13 医疗和手术操作 (23)3.13.1 仅报告了操作 (23)3.13.2 同时报告了操作和诊断 (24)3.14 各类检查 (24)3.14.1 检查结果作为 AR/AE (24)3.14.2 检查结果与诊断一致 (25)3.14.3 检查结果与诊断不相关 (25)3.14.4 一组检查结果术语 (25)3.14.5 不带限定词的检查结果 (26)3.15 用药错误、意外暴露和职业暴露 (26)3.15.1 用药错误 (26)3.15.2 意外暴露和职业暴露 (31)3.16 误用、滥用和成瘾 (32)3.16.1 误用 (32)3.16.2 滥用 (32)3.16.3 成瘾 (33)3.16.4 药物流弊 (33)3.17 感染性病原体通过产品传播 (34)3.18 用药过量、毒性和中毒 (34)3.18.1 用药过量有临床后果 (35)3.18.2 用药过量没有临床后果 (35)3.19 器械相关术语 (36)3.19.1 器械相关事件有临床后果 (36)3.19.2 器械相关事件没有临床后果 (36)3.20 药物相互作用 (37)3.20.1 报告明确指出是相互作用 (37)3.20.2 报告没有明确指出是相互作用 (37)3.21 无不良作用和“正常”术语 (38)3.21.1 无不良作用 (38)3.21.2 “正常”术语的使用 (38)3.22 意外治疗效果 (38)3.23 治疗效果的改变 (38)3.23.1 缺乏治疗效果 (38)3.23.2 不推断缺乏治疗效果 (39)3.23.3 治疗效果增强、减弱、延长 (39)3.24 社会环境 (39)3.24.1 该 SOC 中术语的使用 (39)3.24.2 犯罪或虐待的非法行为 (40)3.25 病史和社会史 (41)3.26 产品使用的适应症 (41)3.26.1 医学状况 (42)3.26.2 复杂适应症 (42)3.26.3 带基因标记物或者异常病变的适应症 (43)3.26.4 防治与预防 (43)3.26.5 操作和诊断性检查作为适应症 (44)3.26.6 补充和替代治疗 (44)3.26.7 未报告适应症 (44)3.27 超说明书使用 (45)3.27.1 超说明书使用报告为适应症 (45)3.27.2 超说明书使用同时报告了 AR/AE (45)3.28 产品质量问题 (46)3.28.1 产品质量问题有临床后果 (46)3.28.2 产品质量问题没有临床后果 (47)3.28.3 产品质量问题与用药错误 (47)SECTION 4 –附录 (49)4.1 版本更新 (49)4.1.1 版本更新方法 (49)4.1.2 新版本采用时间 (50)4.2 链接及参考文献 (50)SECTION 1 –引言《监管活动医学词典》(MedDRA)术语集的设计旨在共享人用医疗产品的法规监管信息。
760-78-1_DL-正缬氨酸_MED11074技术资料_上海_Medbio脉铂
1g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11127
Fmoc-L-丝氨酸
Fmoc-Ser-OH
73724-45-5
100g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
CAS
包装
纯度
MedBio
MED11025
N-羟基琥珀酰亚胺
HOSu
6066-82-6
100g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11040
丝氨酸苄酯盐酸盐
H-Ser-OBzl.HCl
1738-72-3
100g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11086
5g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11059
N-Fmoc-N'-Boc-L-2,3-二氨基丙酸
Fmoc-Dap(Boc)-OH
162558-25-0
1g
≥98%
纯度
MedBio
MED11049
Fmoc-N-三苯甲基-L-天冬酰胺
Fmoc-Asn(Trt)-OH
132388-59-1
100g
≥98%
品牌
货号
中文名称
D8-MMAE_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Aug.-08-2017Print Date:Aug.-08-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :D8-MMAECatalog No. :HY-15162ACAS No. :2070009-72-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:D8–Monomethyl auristatin E; D8–Vedotin; D8 MMAEFormula:C39H59D8N5O7Molecular Weight:726.03CAS No. :2070009-72-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature: 4°C, stored under nitrogenShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
DDR1促进胰腺癌细胞AsPC-1的迁移及侵袭能力

DDR1促进胰腺癌细胞AsPC-1的迁移及侵袭能力杨佳纯;张毅;曹佳;徐雷鸣【摘要】Objective To investigate the effect of discoidin domain receptor 1 (DDR1) on the migration and in-vasion of human pancreatic cancer cell line AsPC-1. Methods The expressions of DDR1 mRNA in pancreatic cancer tissues and adjacent tissues were determined by real-time PCR. The expressions of DDR1, matrix metalloproteinase 2 (MMP2) and matrix metalloproteinase 2 (MMP9) were detected by Western blot after the DDR1 expression plasmid was transfected into AsPC-1 cell line. The ability of migration and invasion was determined by Wound Healing and Tran-swell. Results The expression of DDR1 mRNA in pancreatic cancer tissues was significantly higher than that in adja-cent tissues (P<0.05). After the DDR1 plasmid was transfected into AsPC-1, the protein expression of DDR1 was signifi-cantly up-regulated (P<0.05). Compared with cells transfected with empty vector (control), the ability of migration and invasion for cells transfected with DDR1 plasmid were increased by (51.11 ± 11.51)%and (77.25 ± 10.64)%, and the ex-pression levels of MMP2 and MMP9 after overexpression of DDR1 were also significantly up-regulated. The differences were all statistically significant (P<0.05). Conclusion DDR1 can promote the migration and invasion of human pancre-atic caner cell line AsPC-1 via up-regulating the expressions of MMP2 and MMP9. It could be considered as a potential target for gene therapy for pancreatic cancer.%目的探讨盘状结构域受体1 (DDR1)对胰腺癌细胞迁移及侵袭能力的影响.方法利用qRT-PCR检测DDR1在胰腺癌旁组织及癌组织中的表达水平.通过脂质体转染DDR1表达质粒至胰腺癌细胞AsPC-1中,应用Western blot验证其转染效果.通过划痕实验及Transwell法检测转染DDR1表达质粒后细胞迁移及侵袭能力的变化情况.Western blot检测转染后基质金属蛋白酶2 (MMP2)和基质金属蛋白酶9 (MMP9)的表达水平.结果与癌旁组织比较,胰腺癌组织的DDR1 mRNA 表达水平显著升高(P<0.05).转染质粒后,AsPC-1细胞DDR1蛋白表达量显著升高(P<0.05).应用划痕试验及Transwell试验结果显示,与对照组比较,AsPC-1/DDR1迁移力上调(51.11±11.51)%,侵袭力上调(77.25±10.64)%,差异均具有统计学意义(P<0.05).过表达DDR1后,AsPC-1细胞中MMP2和MMP9表达水平显著升高(P<0.05).结论 DDR1通过改变MMP2和MMP9的表达水平,促进胰腺癌细胞的迁移及侵袭,有望成为靶向治疗的新方向.【期刊名称】《海南医学》【年(卷),期】2016(027)002【总页数】4页(P173-176)【关键词】盘状结构域受体1;胰腺癌;迁移;侵袭;基质金属蛋白酶2;基质金属蛋白酶9【作者】杨佳纯;张毅;曹佳;徐雷鸣【作者单位】上海交通大学医学院附属新华医院消化内科,上海 200092;上海交通大学医学院附属新华医院消化内科,上海 200092;上海交通大学医学院附属新华医院消化内科,上海 200092;上海交通大学医学院附属新华医院消化内科,上海200092【正文语种】中文【中图分类】R735.9胰腺癌是消化系统恶性程度极高的肿瘤之一,其发病率和病死率逐年升高,五年生存率通常不超过5%。
Prdx1与肿瘤

Prdx1与肿瘤高军;朱虹;高国兰【摘要】Peroxiredoxin 1(Prdx1)belongs to peroxide oxidoreductase protein(peroxiredoxin, Prdx)family,which is over-expressed in multiple cancers and it play an important role in antagonizing reactive oxygen species (ROS) therefore as works as anti-oxidations. Its expression is closely related with tumor proliferation, differentiation and metastasis as well as with sensibility to radiotherapy and chemotherapy. Its structure and biological function in tumors and regulation mechanism were reviewed in this paper. It can provide evidence to screen new therapeutic targets in the treatment of tumors.%Prdx1(peroxiredoxin 1)属于过氧化物氧化还原酶蛋白(peroxiredoxin,Prdx)家族成员,是一类在对抗活性氧簇(ROS)、抗氧化过程中发挥关键作用的蛋白质,在多种肿瘤组织中高表达,其表达与肿瘤细胞增殖、分化、转移、复发及放化疗的敏感性密切相关。
本文就Prdx1的结构、在肿瘤中的生物学功能及其信号调控机制等进行综述,为寻找新的肿瘤生物治疗靶点提供依据。
【期刊名称】《天津医药》【年(卷),期】2015(000)012【总页数】3页(P1464-1466)【关键词】Prdx1;过氧化物酶;肿瘤【作者】高军;朱虹;高国兰【作者单位】江西省人民医院妇科邮编330006;江西省人民医院妇科邮编330006;中国医科大学航空总医院妇科【正文语种】中文【中图分类】R730.2;R73-3众所周知,生命的进化经历了无氧至有氧的发展过程。
【实验】转基因植物产品检测实验室一览

【关键字】实验转基因植物产品检测实验室一览其他设备:细胞融合仪、核酸提取仪、紫外分光光度计、核酸蛋白检测仪磁力搅拌机杂交仪、-30℃低温冰箱、超低温冰箱、漩涡混合器、超声波细胞粉碎仪、自动恒温酶标。
7 操作步骤7.1 抽样参照 NY/T672 转基因植物及其产品检测通用要求和NY/T673 转基因植物及其产品检测抽样。
7.2 制样参照 NY/T672 转基因植物及其产品检测通用要求和NY/T673 转基因植物及其产品检测抽样(按照GB 5491中四分法制备样品进行送检)。
7.3 DNA模板的制备a称取200-400 mg试样,在液氮中磨碎,装入已经用液氮预冷的1.5 ml离心管中。
b加入1ml预冷至4 ℃的抽提液,剧烈摇动混匀后,在冰上静置5分钟,用13 000 r/min离心机,4 ℃离心15 min,弃去上清液。
c加入600 μl 预热到65 ℃的抽提裂解液,用玻棒搅拌上下颠倒充分混匀,在65 ℃的水浴锅中裂解40 min。
d用13 000 r/min离心机室温离心10 min,将上清液转至另一离心管中,加入5 μl RNase A (10 mg/ml),37 ℃水浴30 min。
e分别用等体积苯酚:氯仿:异戊醇(25:24:1)和氯仿:异戊醇(24:1)各抽提一次。
f用13 000 r/min离心机室温离心10 min,将上清转至另一离心管中。
加入2/3体积异丙醇,1/10 体积3M乙酸钠(pH 5.6),-20 ℃放置2-3 h,充分沉淀DNA。
g13 000 r/min,4 ℃离心15 min,用70%乙醇洗沉淀一次,倒出乙醇,晾干DNA。
加入50 μl TE(pH8.0)溶解DNA。
h把DNA溶液浓度用重蒸馏水调制为100ng/μl,储存于-20 ℃备用。
注意:I 1 g试样(如棉花种子)提取的DNA量应不小于200 μg。
II DNA的OD260/OD280的比值应在1.8左右,且OD260的值应在曲线的最高峰。
