KDM5-IN-1_DataSheet_MedChemExpress
KDM5A-IN-1_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :KDM5A-IN-1Catalog No. :HY-100014CAS No. :1905481-36-81.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:C15H22N4O2Molecular Weight:290.36CAS No. :1905481-36-84. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
KW-2449_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:KW–2449 is a multiple–targeted inhibitor, mostly for Flt3 with IC50 of 6.6 nM, modestly potent to FGFR1, Bcr–Abl and Aurora A; little effect on PDGFRβ, IGF–1R, EGFR.IC50 value: 6.6 nM [1]Target: Flt3in vitro: Phosphorylation levels of FLT3 and STAT5 are decreased by KW–2449 in a dose–dependent manner. In addition, it potently inhibits ABL–T315I, which is associated with IM resistance, with IC50 of 4 nM. On the other hand, KW–2449 has little effect on PDGFRβ,IGF–1R, EGFR, and various serine/threonine kinases even at a concentration of 1 μM. KW–2449 has the potent growth inhibitory activities against not only FLT3/ITD–expressing leukemia cells but also FLT3/KDM–activated and wild–type FLT3–overexpressing leukemia cells [1]. KW–2449 is rapidly absorbed and converted to a major metabolite M1.Preclinical studies reveal that KW–2449 is converted by monoamine oxidase–B (MAO–B) and aldehyde oxidase into its major metabolite M1.KW–2449 mediates cytotoxicity thru inhibition of FLT3/ITD.KW–2449 is a direct inhibitor of FLT3 and induces inhibition of its downstream target STAT5 [2]. KW2449interacts synergistically with HDACIs to induce apoptosis in Ph+ CML cells in a time– and concentration–dependent manner. KW2449synergistically enhances the lethality of vorinostat/SNDX275 in CML cells.KW–2449 regimens are active against additional IM–resistant Bcr/Abl+ leukemia cells. KW2449 moderately reduces phosphorylation of histone H3, an indicator of Aurora B activity, innocodozole–treated K562 cells [3].in vivo: In the MOLM–13 tumor xenograft model, oral administration of KW–2449 for 14 days shows a potent and significant antitumor effect in a dose–dependent manner [1].PROTOCOL (Extracted from published papers and Only for reference)Cell assay [1] Cell viability was determined by the sodium 3′–[1–(phenylaminocarbonyl)–3, 4–tetrazolium]–bis (4–methoxy–6–nitro)benzene sulfonic acid hydrate assay after incubation with or without KW–2449 for 72 hours at 37°C. The number of viable cells was determined using the Cell Proliferation Kit II (Roche Diagnostics). For cell–cycle analysis, MOLM–13 and RS4;11 cells were treated with KW–2449. After 24, 48, and 72 hours of incubation at 37°C, DNA contents were analyzed as previously described.28 Cell cycledistribution of K562, TCC–Y, and TCC/Ysr was analyzed 24 hours after treatment with KW–2449 or imatinib. Animal administration [3]Animal studies were performed in CBySmn.CB17–Prkds scid/J (BALB/C) mice (The Jackson Laboratory). A total of 2 × 106BV173/E255K/Luc cl4 cells in 100uL phosphate–buffered saline (PBS) were injected into tail vein. Tumor infiltration was monitored by bioluminescence imaging one or twice a week. These animals were noninvasively imaged using the In Vivo Imaging System (IVIS–200;Xenogen, Hopkinton, MA) after injection with the luciferase substrate (D–Luciferin, Research Products International). For in vivostudies, KW2449 was dissolved in 0.5% methylcellulose 400 solution (Wako). SNDX–275 and vorinostat were first dissolved in DMSO and stored in –80°C in small aliquots. SNDX–275 was further diluted in sterile water before use. Vorinostat was further diluted in 1:1poethylene glycol 400 (Fluka analytical) and sterile water to a final composition of 10% DMSO, 45% PEG400, 45% water before use.Product Name:KW–2449Cat. No.:HY-10339CAS No.:1000669-72-6Molecular Formula:C 20H 20N 4O Molecular Weight:332.40Target:FLT3Pathway:Protein Tyrosine Kinase/RTK Solubility:10 mM in DMSOBoth SNDX–275 and KW2449 was orally administered at 15mg/kg/day and 32mg/kg/day respectively. Vorinostat 70 mg/kg/day was administered intraperitoneally (IP). All drugs were given 5 days/week. The weight of each mouse was monitored once or twice a week.References:[1]. Shiotsu Y, et al. KW–2449, a novel multikinase inhibitor, suppresses the growth of leukemia cells with FLT3 mutations or T315I–mutated BCR/ABL translocation. Blood, 2009, 114(8), 1607–17.[2]. Pratz KW, et al. A pharmacodynamic study of the FLT3 inhibitor KW–2449 yields insight into the basis for clinical response. Blood, 2009, 113(17), 3938–46.[3]. Nguyen T, et al. HDAC inhibitors potentiate the activity of the BCR/ABL kinase inhibitor KW–2449 in imatinib–sensitive or –resistant BCR/ABL+ leukemia cells in vitro and in vivo. Clin Cancer Res, 2011, 17(10), 3219–32.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
黄芩素通过调节HIF-1α

黄芩素通过调节HIF -1α/VEGF 信号通路抑制类风湿关节炎大鼠的炎症反应和病理性血管生成*杜红丽1,张晨宇1,赵清2△[1河南中医药大学第五临床医学院(郑州人民医院)风湿免疫科,河南郑州450053;2河南大学淮河医院风湿免疫科,河南开封475099][摘要]目的:探讨黄芩素(BA )调节缺氧诱导因子1α(HIF -1α)/血管内皮生长因子(VEGF )信号通路对类风湿关节炎(RA )大鼠炎症反应和病理性血管生成的影响。
方法:按照随机数字表法将SD 大鼠分为对照(control )组、模型(model )组、低剂量(10mg/kg )BA (BA -L )组、高剂量(30mg/kg )BA (BA -H )组、雷公藤多苷片(TWP ;6.25mg/kg )组和BA -H+HIF -1α激动剂二甲基草酰甘氨酸(DMOG ;40mg/kg )组,每组12只。
除control 组外,其它组大鼠均采用II 型胶原蛋白-完全弗氏佐剂法诱导RA 大鼠模型。
第2次免疫24h 后开始给药处理,每天给药一次,持续4周。
检测大鼠在给药第0、7、14和28天时的足趾肿胀度,计算关节炎指数;计算大鼠胸腺和脾脏指数;HE 染色检测大鼠踝关节滑膜组织病理损伤;ELISA 法检测大鼠踝关节滑膜组织中肿瘤坏死因子α(TNF -α)和白细胞介素6(IL -6)水平;免疫组化检测大鼠踝关节滑膜组织中VEGF 和VEGF 受体2(又称激酶插入域受体,KDR )表达;Western blot 检测各组大鼠踝关节滑膜组织中HIF -1α和VEGF 蛋白表达。
结果:与control 组比较,model 组大鼠踝关节滑膜组织病理损伤严重,足趾肿胀度、关节炎指数、胸腺和脾脏指数,以及滑膜组织TNF -α、IL -6、VEGF 、KDR 、HIF -1α和VEGF 水平均显著升高(P <0.05);与model 组比较,BA -L 组、BA -H 组和TWP 组对应指标变化趋势与上述相反(P <0.05);BA -H 组与TWP 组比较,上述指标变化差异无统计学意义(P >0.05);DMOG 减弱了BA -H 对RA 大鼠炎症反应和病理性血管生成的抑制作用。
QPCR及QRT-PCR系列产品

Invitrogen的ICFC系列产品促销1.QPCR及QRT-PCR系列产品Invitrogen公司专门为中国客户提供的定量PCR试剂盒,结合了 UDG 防止残余污染技术和SYBR® Green I 荧光染料(存在于SYBR® Green I荧光定量PCR试剂盒中),在美国接受了严格的质量监控,可提供极高灵敏度的目的序列定量检测,线性剂量低,反应浓度范围很大。
qPCR Supermix-- 即用型反应剂,专为高特异性、实时定量DNA扩增设计UDG-- 防止携带污染物,减少克隆片段假阳性结果ROX参考染料-- 适用ABI仪器的校正染料产品信息活动时间:即日起至2009年4月30日2.Gibco南美胎牛血清即日起凡优惠价¥1780购买Gibco胎牛血清500ml(目录号:C2027050)即可获赠送价值¥250现金抵用券。
您可以凭现金抵用券在英韦创津公司购买任何商品,此券有效期至2009年5月31日。
产品信息活动时间:即日起至2009年4月30日独特的采集方式:GIBCO采用无菌心脏穿刺的方式采血原装直送,避免污染:原产地采集、加工、检测、包装。
完善的质控:采集、处理、检测、运输等环节都有文件和证书。
3.Invitrogen TA Cloning克隆产品专门用于克隆Taq聚合酶扩增的PCR产物。
采用pCR载体,能产生80%以上的重组产物,90%以上重组产物都包含插入片段。
产品信息活动时间:即日起至2009年5月31日附:pCR载体优点及图谱:3’-T突出端可直接连接Taq扩增的PCR产物可选择T7或T7和Sp6启动子进行体外RNA转录和测序侧向EcoRⅠ位点的通用多接头位点方便了插入片段的切离可以选择卡那霉素或氨苄青霉素进行筛选非常简便的蓝/白克隆筛选具有M13正向和反向引物位点,方便测序4.GIBCO液体培养基系列产品创立近50年的历史,品质优秀,产品种类丰富;为了中国用户利益,特建立国内生产线;所有产品,从原材料到生产全部按照GIBCO质量标准进行,每批均送抵美国公司总部质检合格后,才在国内销售。
D1000 Reagents, Part Number 5067-5583说明书

D1000 Reagents, Part Number 5067-5583*************(24小时)化学品安全技术说明书GHS product identifier 应急咨询电话(带值班时间)::供应商/ 制造商:安捷伦科技贸易(上海)有限公司中国(上海)外高桥自由贸易试验区英伦路412号(邮编:200131)电话号码: 800-820-3278传真号码: 0086 (21) 5048 2818D1000 Reagents, Part Number 5067-5583化学品的推荐用途和限制用途D1000 Sample Buffer 5190-6502D1000 Ladder5067-5586部件号:物质用途:仅限研究使用。
不可用于诊断程序 (RUO)。
5190-6502D1000 Sample Buffer 1 x 400 µl 样品瓶5067-5586D1000 Ladder 1 x 10 µl 样品瓶部件号(化学品试剂盒):5067-5583安全技术说明书根据 GB/ T 16483-2008 和 GB/ T 17519-2013GHS化学品标识:D1000 试剂, 部件号码5067-5583有关环境保护措施,请参阅第 12 节。
物质或混合物的分类根据 GB13690-2009 和 GB30000-2013紧急情况概述D1000 Sample Buffer 液体。
D1000 Ladder 液体。
D1000 Sample Buffer 无色。
D1000 Ladder 无色。
D1000 Sample Buffer 无气味的。
D1000 Ladder无气味的。
物理状态:颜色:气味:GHS危险性类别警示词:D1000 Sample Buffer 无信号词。
D1000 Ladder无信号词。
危险性说明:D1000 Sample Buffer 没有明显的已知作用或严重危险。
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
MK591_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:MK591 is a selective and specific 5–Lipoxygenase–activating protein (FLAP ) inhibitor.In Vitro: MK591 and SB203580 are able to block SEB–induced human PBMC cell proliferation. MK591 down regulates three genes [for cathepsin L, IL–17 and guanylate binding protein (GBP)–2] that are up regulated by SEB [1]. MK591 undergoes apoptosis withinhours of treatment. MK591 also induces rapid activation of the stress kinase, c–Jun N–terminal kinase (JNK), which plays animportant role in the apoptosis process. MK591 triggers apoptosis in prostate cancer cells without inhibition of PI3K–Akt, or ERK.Moreover, MK591 and LY294002 (an inhibitor of PI3K) exert synergistic effect in inducing apoptosis in prostate cancer cells [2].MK–591 influences cAMP response element–binding protein but not Sp1[4].In Vivo: Hyperoxia groups of mice treated with MK–0591 (20, 40 mg/kg) show alveolarization that resembles that of room aircontrols while untreated hyperoxia groups show definite evidence of aberrant alveolarization but no inflammation [3]. Comparison of the Aβ–immunopositive areas between the placebo and MK–591 (320 mg/kg)–treated group reveals a statistically significant reduction of the amyloid burden in the treated mice. MK–591 also has a significant reduction in brain levels of IL–1β. Mice treatedwith MK–591 show a statistically significant decrease in the steady–state levels of total CREB and its phosphorylated form at Ser133[4]. PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[2]LNCaP cells (appr 3×105) are plated and treated with inhibitors or solvent vehicle for varying periods of time. Then the cells are lysed in lysis buffer containing 0.2% CHAPS detergent plus protease and phosphatase inhibitors, and the enzymatic activity of Akt is measured by a kit following methods supplied by the manufacturer.Animal Administration:[4]The Tg2576 transgenic mice expressing human APP with the Swedish mutation (K670N/M671L) are used in these studies. They are genotyped by PCR analysis using tail DNA and kept in a pathogen–free environment, on a 12–hour light/dark cycle and have access to food and water ad libitum. All the experiments presented in this paper are performed with female mice.Starting at 7 months of age, mice are randomized to receive MK–591 (40 mg/kg weight) (n=11) or vehicle (n=9) in their chow diet for 8 months until they are 15 months old. Considering that each mouse eats on average 5 g/day of chow diet and the diet is formulated for 320 mg MK–591 per kg diet, the final dose of the active drug is approximately 40 mg/kg weight/day. During the study, mice in both groups gain weight regularly, and no significant difference in weight is detected between the two groups. No macroscopic effect on the overall general health is observed in the animals receiving the active treatment. Post–mortem examination shows no sign of macroscopic pathology in any of the organs considered (spleen, liver, thymus, ileum).References:[1]. Mendis C, et al. Effect of 5–lipoxygenase inhibitor MK591 on early molecular and signaling events induced by staphylococcal enterotoxin B in human peripheral blood mononuclear cells. FEBS J. 2008 Jun;275(12):3088–98.Product Name:MK591Cat. No.:HY-50714CAS No.:147030-01-1Molecular Formula:C 34H 34ClN 2NaO 3S Molecular Weight:609.15Target:FLAP Pathway:Immunology/Inflammation Solubility:10 mM in DMSO[2]. Sarveswaran S, et al. MK591, a leukotriene biosynthesis inhibitor, induces apoptosis in prostate cancer cells: synergistic action with LY294002, an inhibitor of phosphatidylinositol 3'–kinase. Cancer Lett. 2010 May 28;291(2):167–76.[3]. Park MS, et al. 5–Lipoxygenase–activating protein (FLAP) inhibitor MK–0591 prevents aberrant alveolarization in newborn mice exposed to 85% oxygen ina dose– and time–dependent manner. Lung. 2011 Feb;189(1):43–50.[4]. Chu J, et al. Involvement of 5–lipoxygenase activating protein in the amyloidotic phenotype of an Alzheimer's disease mouse model. J Neuroinflammation. 2012 Jun 14;9:127.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
世界卫生组织儿童标准处方集

WHO Model Formulary for ChildrenBased on the Second Model List of Essential Medicines for Children 2009世界卫生组织儿童标准处方集基于2009年儿童基本用药的第二个标准目录WHO Library Cataloguing-in-Publication Data:WHO model formulary for children 2010.Based on the second model list of essential medicines for children 2009.1.Essential drugs.2.Formularies.3.Pharmaceutical preparations.4.Child.5.Drug utilization. I.World Health Organization.ISBN 978 92 4 159932 0 (NLM classification: QV 55)世界卫生组织实验室出版数据目录:世界卫生组织儿童标准处方集基于2009年儿童基本用药的第二个标准处方集1.基本药物 2.处方一览表 3.药品制备 4儿童 5.药物ISBN 978 92 4 159932 0 (美国国立医学图书馆分类:QV55)World Health Organization 2010All rights reserved. Publications of the World Health Organization can be obtained fromWHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: ******************). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the aboveaddress(fax:+41227914806;e-mail:*******************).世界卫生组织2010版权所有。
BD破膜剂试剂手册中文

破膜剂试剂手册BD Cytofix / Cytoperm 固定/渗透试剂盒手册(目录号554714)BD Cytofix / Cytoperm Plus固定/渗透试剂盒(BD GolgiStop含有莫能菌素的蛋白质转运抑制剂)(目录号554715)BD Cytofix / Cytoperm Plus固定/渗透试剂盒(含布雷菲德菌素A的BDGolgiPlug蛋白质转运抑制剂)(目录号555028)Ficoll是Amersham Biosciences AB的注册商标。
Hypaque是Amersham Health AS的注册商标。
BD流式细胞仪是1流激光产品。
仅供研究使用。
不用于诊断或治疗程序。
2016年Becton,Dickinson 和公司。
版权所有。
本出版物的任何部分不得以电子,机械,磁性,光学,化学,手册或其他方式以任何形式或通过任何方式复制,传播,转录,存储在检索系统中或翻译成任何语言或计算机语言,未经BD Biosciences事先书面许可。
购买不包括或携带任何权利转售或转让本产品作为独立产品或另一产品的组成部分。
未经Becton,Dickinson和Company明确书面许可,严禁使用本产品以外的许可使用。
2016BD,BD徽标和所有其他商标均为Becton,Dickinson和Company的产权。
目录BD Cytofix / Cytoperm固定/渗透试剂盒BD Cytofix / Cytoperm Plus固定/渗透试剂盒(BD GolgiStop蛋白质转运抑制剂)BD Cytofix / Cytoperm Plus固定/渗透试剂盒(BD GolgiPlug蛋白质运输抑制剂)警告和注意事项1.一般程序A.刺激细胞1.使用BD GolgiStop的程序蛋白质转运抑制剂(含有莫能菌素)2。
使用BD GolgiPlug 蛋白质转运抑制剂的程序(含布雷菲德菌素A)B.方案:细胞表面抗原和细胞内细胞因子的多色染色1收集细胞2.阻止Fc受体3.细胞表面抗原染色4.固定和渗透细胞5.备选固定和渗透方案6细胞内细胞因子染色C.流式细胞分析。
Mdivi-1_CAS号338967-87-6说明书_AbMole中国

分子量353.22溶解性(25°C )DMSO 71 mg/mL 分子式C H Cl N O S Water <1 mg/mL CAS 号338967-87-6Ethanol <1 mg/mL储存条件3年 -20°C 粉末状生物活性Mdivi-1是一个具有选择性和细胞穿膜性的线粒体分裂抑制剂,抑制了DRP1(发动蛋白相关GTP 酶)和Dynamin I (Dnm1)的IC50为1-10 μM 。
Mdivi-1是一个可以穿越细胞膜的喹唑酮类化合物,抑制了酵母的Dnm1和人源的Drp1分裂DRPs(发动蛋白相关GTP 酶),并且高效可逆的诱导线粒体融合进一个巢样的结构。
细胞外的研究表明mdivi-1通过变构调节阻断了Dnm1的ATP 酶活性(IC50<10μM)和自组装。
在HeLa 细胞和细胞外鼠源肝细胞线粒体配置液中,Mdivi-1有效地分别抑制了STS 和C8-Bid 诱导的MOMP (线粒体外膜通透性增加)。
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA 指南)小鼠大鼠兔豚鼠仓鼠狗重量 (kg)0.020.15 1.80.40.0810体表面积 (m )0.0070.0250.150.050.020.5K 系数36128520动物 A (mg/kg) = 动物 B (mg/kg) ×动物 B 的K 系数动物 A 的K 系数例如,依据体表面积折算法,将白藜芦醇用于小鼠的剂量22.4 mg/kg 换算成大鼠的剂量,需要将22.4 mg/kg 乘以小鼠的K 系数(3),再除以大鼠的K 系数(6),得到白藜芦醇用于大鼠的等效剂量为11.2 mg/kg 。
Mdivi-1 目录号M2830化学数据15102222m m m m m。
ICP-MS法测定五层共挤输液用袋中8种元素在碳酸氢钠林格注射液中的迁移量

·药物检验与分析·ICP MS法测定五层共挤输液用袋中8种元素在碳酸氢钠林格注射液中的迁移量杨文宇1 ,李 飞1,兰婉玲2,赵代国2 ,李 勇3,张加宇3,赖 朋1,李玉锋1(1 西华大学食品与生物工程学院,四川成都610039;2 四川省食品药品检验检测院,四川成都611731;3 四川太平洋药业有限责任公司,四川成都611731)摘要:目的 考察五层共挤输液用袋中锂、铝、铬、铜、砷、镉、锡、铅8种元素在碳酸氢钠林格注射液中的迁移情况。
方法 采用电感耦合等离子体质谱法,先对模拟试验溶液进行半定量分析以确定待测金属元素,再对样品溶液中各元素含量进行测定。
射频功率为1550W,载气流量为1 01L·min-1,雾化泵转数0 1r·s-1,雾化室温度为2℃,采样深度为8mm,采样锥类型为Ni,采样锥孔径为1mm,采样间隔为105s,重复次数为3次,积分时间为0 1s。
结果 样品在加速试验条件下〔温度:(40±2)℃、相对湿度:75%±5%〕储存0、3、6个月后样品溶液中锂、铝、铬、铜、砷、镉、锡、铅的最大迁移量分别为<0 02、3 62、1 88、<0 09、<0 05、<0 02、2 22、<0 03μg/袋。
结论 五层共挤输液用袋中的锂、铝、铬、铜、砷、镉、锡、铅在碳酸氢钠林格注射液中的迁移量均符合有关规定。
关键词:电感耦合等离子体质谱法;碳酸氢钠林格注射液;五层共挤输液用袋;有害元素;迁移量中图分类号:R927 文献标识码:A 文章编号:1006 3765(2020) 11 0047 06作者简介:杨文宇,男(1973-)。
学历:博士。
职称:副教授。
研究方向:药物分析技术。
通讯作者:赵代国,男(1982-)。
学历:硕士。
职称:工程师。
研究方向:药品包装材料。
基金项目:四川省经信委创新能力提升 创新平台建设项目“负压式软袋输液组合袋”(No 2016NL015)DeterminationofMigrationofEightElementsfromFive layerCo extrusionBagsinBicarbonatedRinger′sInjectionbyICP MSYANGWen yu1 ,LIFei1,LANWan ling2,ZHAODai guo2 ,LIYong3,ZHANGJia yu3,LAIPeng1,LIYufeng1(1 SchoolofFoodandBioengineering,XihuaUniversity,Chengdu610039,China;2 SichuanInstituteforFoodandDrugControl,Chengdu611731,China;3 SichuanPacificPharmaceuticalCo ,Ltd,Chengdu611731,China)ABSTRACT:OBJECTIVE Toinvestigate8kindsofinorganicelements(Li,Al,Cr,Cu,As,Cd,SnandPb)inBicarbonatedRinger′sInjectionmigratedfromitspackagingmaterials,five layerco extrusioninfusionbags METHODS ICP MSmethodwasused Firstly,conductingsemi quantitativeanalysisofsimulationtestsolu tiontoclarifywhichelementstobequantitatived,thendeterminingthecontentofeachinorganicelementinthesam plesolution Parametersofexperimentalconditionswereasfollows:theRFpowerwas1550W,thecarriergasvelocitywas1 01L·min-1,theperistalticpumpratewas0 1r·s-1,theatomizingchambertemperaturewas2℃,thesam plingdepthwas8mm,thesamplingconetypewasNi,thesamplingconediameterwas1mm,thesamplingintervalwas105s,therepetitionwas3times,theintegrationtimewas0 1s RESULTS Undertheacceleratedtestconditions〔(temperature:(40±2)℃andrelativehumidity:75%±5%)〕,themaximummigrationofLi,Al,Cr,Cu,As,Cd,SnandPbinthesamplesolutionafterstoragefor0,3and6monthswere<0 02,3 62,1 88,<0 09,<0 05,<0 02,2 22and<0 03μgperbagrespectively CONCLUSION ThemigrationofLi,Al,Cr,Cu,As,Cd,SnandPbintheBicarbonatedRinger′sInjectionfromthefive layerco extrusioninfusionbagsareallinlinewithrelatedstandards KEYWORDS:ICP MS;Bicarbonatedringer′sinjection;Five layerco extrusioninfusionbag;Hazardouselements;Migration·74· 碳酸氢钠林格注射液为复方电解质输液,由氯化钠、氯化钾、氯化镁、枸橼酸钠、碳酸氢钠、枸橼酸、氯化钙和注射用水制成。
NDM-51NDM-5
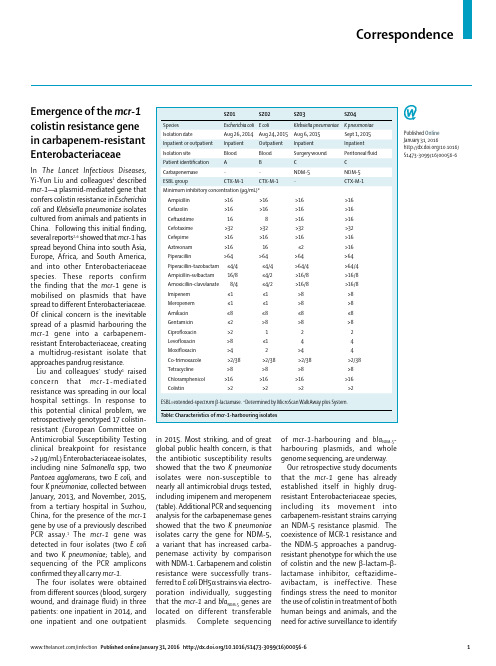
Correspondenceof mcr-1-harbouring and bla NDM-5-harbouring plasmids, and whole genome sequencing, are underway.Our retrospective study documents that the mcr-1 gene has already established itself in highly drug-resistant Enterobacteriaceae species, including its movement into carbapenem-resistant strains carrying an NDM-5 resistance plasmid. The coexistence of MCR-1 resistance and the NDM-5 approaches a pandrug-resistant phenotype for which the use of colistin and the new β-lactam–β-lactamase inhibitor, ceftazidime–avibactam, is ineffective. These findings stress the need to monitor the use of colistin in treatment of both human beings and animals, and the need for active surveillance to identifyEmergence of the mcr-1colistin resistance genein carbapenem-resistant Enterobacteriaceae In The Lancet Infectious Diseases, Yi-Yun Liu and colleagues 1 described mcr-1—a plasmid-mediated gene that confers colistin resistance in Escherichia coli and Klebsiella pneumoniae isolates cultured from animals and patients in China. Following this initial fi nding, several reports 2–6 showed that mcr-1 has spread beyond China into south Asia, Europe, Africa, and South America, and into other Enterobacteriaceae species. These reports confirm the finding that the mcr-1 gene is mobilised on plasmids that have spread to diff erent Enterobacteriaceae. Of clinical concern is the inevitable spread of a plasmid harbouring the mcr-1 gene into a carbapenem-resistant Enterobacteriaceae, creating a multidrug-resistant isolate that approaches pandrug resistance.Liu and colleagues’ study 1 raised concern that mcr-1-mediated resistance was spreading in our local hospital settings. I n response to this potential clinical problem, weretrospectively genotyped 17 colistin-resistant (European Committee onAntimicrobial Susceptibility Testing clinical breakpoint for resistance >2 μg/mL) Enterobacteriaceae isolates, including nine Salmonella spp, two Pantoea agglomer ans , two E coli, and four K pneumoniae , collected between January, 2013, and November, 2015, from a tertiary hospital in Suzhou, China, for the presence of the mcr-1 gene by use of a previously described PCR assay.1 The mcr-1 gene was detected in four isolates (two E coli and two K pneumoniae ; table), and sequencing of the PCR amplicons confi rmed they all carry mcr-1. The four isolates were obtained from diff erent sources (blood, surgery wound, and drainage fl uid) in three patients: one inpatient in 2014, and one inpatient and one outpatient in 2015. Most striking, and of great global public health concern, is that the antibiotic susceptibility results showed that the two K pneumoniae isolates were non-susceptible to nearly all antimicrobial drugs tested, including imipenem and meropenem (table). Additional PCR and sequencing analysis for the carbapenemase genes showed that the two K pneumoniae isolates carry the gene for NDM-5, a variant that has increased carba-penemase activity by comparison with NDM-1. Carbapenem and colistin resistance were success f ully trans-ferred to E coli DH5α strains via electro-poration individually, suggesting that the mcr-1 and bla NDM-5 genes are located on different transferable plasmids. Complete sequencing Published Online January 31, 2016/10.1016/S1473-3099(16)00056-6Correspondence 3 Arcilla MS, van Hattem JM, Matamoros S, et al. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 2016; 16: 147–49. 4 Olaitan AO, Chabou S, Okdah L, Morand S, Rolain J-M. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 2016; 16: 147. 5 Webb HE, Granier SA, Marault M, et al. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 2016; 16: 144–45. 6 Tse H, Yuen K-Y. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect Dis 2016; 16: 144–45.Tuberculosis Center, New Jersey medical school, Rutgers University, Newark, NJ 07103, USA (LC, BNK); and Department of Laboratory Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA (Y-WT) 1 Liu Y-Y, Wang Y, Walsh TR, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 2016; 16: 161–68. 2 Hasman H, Hammerum AM, Hansen F, et al. Detection of mcr-1 encoding plasmid-mediated colistin-resistant Escherichia coli isolates from human bloodstream infection and imported chicken meat, Denmark 2015. Euro Surveill 2015; 20: 30085.colistin resistance and for heightened infection control practices to restrict its further dissemination.This study was supported by grants from the National Institutes of Health (grant R01AI090155) and National Natural Science Foundation of China (number 81572032). We declare no competing interests. LC, YWT, and BNK conceived and designed the experiments. HD collected data and did the experiments. HD and LC analysed the data. LC, YWT, and BNK wrote the report. All authors reviewed and approved the fi nal report. Hong Du, *Liang Chen, Yi-Wei Tang, Barry N Kreiswirth *******************.edu Department of Clinical Laboratory, The Second Affi liated Hospital of Soochow University, Suzhou, Jiangsu, China (HD); Public Health Research Institute。
毛细管PCR芯片电泳快速检测NDM-1耐药菌方法的建立

毛细管PCR芯片电泳快速检测NDM-1耐药菌方法的建立王秋平【摘要】目的建立一种毛细管聚合酶链反应(PCR)芯片电泳方法,实现快速、准确地检测新德里金属-β-内酰胺酶(NDM-1)耐药菌.方法根据NDM-1基因设计1对特异引物,对临床微生物检测的80株耐药肠道杆菌和60株鲍曼不动杆菌的细菌培养液进行毛细管振荡流PCR扩增,芯片电泳快速检测PCR产物.将NDM-1阳性标本进行测序验证,同时对NDM-1阳性菌细菌悬液定量梯度稀释进行PCR,考察免核酸提取PCR的敏感度.结果毛细管PCR芯片电泳方法检测出2例阳性标本,其产物经测序验证,确定携带NDM-1基因,阳性率为100%.该方法在40 min内实现产NDM-1耐药菌扩增产物的快速分离检测,细菌检测线为1.15×101 CFU/mL.结论毛细管PCR芯片电泳方法检测NDM-1基因准确性高、特异性强,具有快速、廉价等特点,适合NDM-1阳性菌的早期快速现场诊断.【期刊名称】《检验医学与临床》【年(卷),期】2018(015)024【总页数】5页(P3674-3677,3681)【关键词】新德里金属-β-内酰胺酶;芯片电泳;聚合酶链反应【作者】王秋平【作者单位】南华大学附属第一医院检验科,湖南衡阳421001【正文语种】中文【中图分类】R446滥用抗生素的问题由来已久,2000年出现了多重耐药假单胞菌和肺炎克雷伯菌。
2010年8月The Lancet Infectious Diseases杂志报道了抗生素抗新德里金属-β-内酰胺酶(NDM-1)超级细菌。
NDM-1是一种新鉴定的对β-内酰胺类抗生素具有广谱水解作用的酶,是编码碳青霉烯类酶基因家族成员之一。
NDM-l可以水解碳青霉烯类抗生素,以及头孢菌素和青霉素。
携带该基因的细菌对几乎所有一线治疗重症感染的广谱抗生素,如青霉素类、头孢菌素类、头霉素类等β-内酰胺和碳青霉烯类抗生素均耐药,仅对多黏菌素E及米诺环素衍生物——替加环素敏感,被称为“超级细菌”[1-2]。
M5 微量临床基因组 DNA 快速提取试剂盒使用说明书

M5微量临床基因组DNA快速提取试剂盒使用说明书产品名称单位货号M5微量临床基因组DNA快速提取试剂盒50T M669-01【储存条件】Poly Carrier和蛋白酶K粉(可选)20mg/ml-20˚C保存,其他组分室温储存。
【产品简介】本试剂盒采用特制的DNA吸附柱和独特的缓冲液系统,特别适合于从微量血液、法医材料、干血点、药签、口香糖、尿液等微量样品中分离纯化基因组DNA。
各种来源样品裂解消化处理后DNA在高离序盐状态下选择性吸附于离心柱内硅基质膜(特别配备了Poly Carrier可以从体系中轻松捕获微量核酸),再通过一系列快速的漂洗-离心的步骤,将盐、细胞代谢物、蛋白等杂质去除,最后低盐的洗脱缓冲液将纯净的基因组DNA从硅基质膜上洗脱。
纯化后的DNA无杂质和PCR抑制剂,可直接适用于PCR分析。
【产品特色】1.不需要使用有毒的苯酚等试剂,也不需要乙醇沉淀等步骤。
2.节省时间,简捷,单个样品操作一般可在20分钟内完成。
3.配备了Poly Carrier用于充分收集特别微量DNA。
4.多次柱漂洗确保高纯度,提取的DNA纯度高,质量稳定可靠,适用于各种常规操作,包括PCR、酶切、测序、Southern杂交等。
【产品组份】50T注意事项裂解液ML11ml室温密闭干燥保存结合液CB15ml室温密闭干燥保存抑制物去除液IR25ml室温密闭干燥保存漂洗液WB25ml第一次使用前按说明加指定量乙醇Poly Carrier200μl-20˚C保存。
洗脱缓冲液EB10ml室温密闭干燥保存蛋白酶K粉(20mg/ml)20mg-20˚C保存。
吸附柱AC50个室温密闭干燥保存收集管(2ml)50个室温密闭干燥保存1.结合液CB或者抑制物去除液IR低温时可能出现析出和沉淀,可以在37℃水浴几分钟帮助重新溶解,恢复澄清透明后冷却到室温即可使用。
2.为避免降低活性,方便运输,提供蛋白酶K为冻干粉状,收到后,可短暂离心后,加入1毫升灭菌水溶解。
