Isoniazid_54-85-3_DataSheet_MedChemExpress
hss-p-5.75.09 - hyaluronic acid derivatives说明书

5.75.09Section:Prescription DrugsEffective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject:Hyaluronic Acid DerivativesPage:1 of 7Last Review Date:March 13, 2020Hyaluronic Acid DerivativesDescriptionDurolane, Euflexxa, GelSyn-3, GenVisc 850, Hyalgan , SodiumHyaluronate, Supartz , Synojoynt*, Triluron, TriVisc, Visco-3 (sodium hyaluronate)Gel-ONE , Hymovis, Monovisc, Orthovisc (hyaluronan)Synvisc, Synvisc-One (hylan G-F 20)Bolded medications are the preferred products*These medications are included in this policy but are not available in the market as of yetBackgroundOsteoarthritis of the knee is a disease in which the elastoviscous property of the synovial fluid in the knee joint becomes diminished, resulting in less protection and shock absorption. Durolane, Euflexxa, Gel-One, GelSyn-3, GenVisc 850, Hyalgan, Hymovis, Monovisc, Orthovisc, Sodium Hyaluronate, Synvisc, Synvisc-One, Supartz, Synojoynt, Triluron, TriVisc, Visco-3 are hyaluronan derivatives that are injected into the knee joints to increase the elastoviscous properties of arthritic joint fluid and slow its outflow from the joint . The goal of therapy is torestore the viscoelasticity in the affected joints, thereby decreasing pain, improving mobility, and restoring the natural protective functions (1).The American College of Rheumatology (ACR) updated its guidelines for the treatment of osteoarthritis (OA) of the knee in 2012. In mild symptomatic OA, treatment may be limited toFederal Employee Program® 1310 G Street, N.W.Washington, D.C. 20005 202.942.1000Fax 202.942.1125Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 2 of 7patient education, physical and occupational therapy and other non-pharmacologic modalities. Nonpharmacologic modalities strongly recommended for the management of knee OA were aerobic, aquatic, and/or resistance exercises as well as weight loss for overweight patients. Nonpharmacologic modalities conditionally recommended for knee OA included medial wedge insoles for valgus knee OA, subtalar strapped lateral insoles for varus knee OA, medially directed patellar taping, manual therapy, walking aids, thermal agents, tai chi, self-management programs, and psychosocial interventions. Pharmacologic modalities conditionally recommended for the initial management of patients with knee OA included acetaminophen, oral and topical NSAIDs, tramadol, and intraarticular corticosteroid injections (1).Regulatory StatusFDA-approved indication: Hyaluronic acid derivatives are indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative non-pharmacologic therapy, simple analgesics (e.g., acetaminophen), NSAIDs, tramadol, or intra-articular steroid injections (2-18).The hyaluronic acid derivatives are contraindicated for use in patients with known hypersensitivity to hyaluronan (sodium hyaluronate) preparations. Orthovisc lists hypersensitivity to gram positive bacterial proteins as an additional contraindication (4). Caution should be exercised when Gel-One, Hyalgan, Visco-3, Synvisc, Synvisc-One, Supartz, and Triluron are administered to patients with allergies to avian proteins, feathers, and egg products (3-8, 18).Hyaluronic acid derivatives are contraindicated to treat patients with knee joint infections, infections or skin diseases in the area of the injection site (2-17).A treatment cycle for most of the hyaluronan derivatives typically involves multiple weekly injections. Euflexxa, GelSyn-3, Sodium Hyaluronate, Synvisc, Triluron, TriVisc, and Visco-3 are given for a total of three injections. Orthovisc is given for three or four injections. GenVisc 850, Supartz and Hyalgan are given for a total of three or five injections. Durolane, Gel-One, Synojoynt, and Synvisc-One differ from the other hyaluronan derivatives in that it only requires one injection. Repeat courses of hyaluronan derivatives may be administered if symptoms return (2-18).Upon the basis of high quality supporting evidence, the American Academy of Orthopedic Surgeons cannot recommend using hyaluronic acid for patients with symptomatic osteoarthritis of the knee (19).Related policiesSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 3 of 7Hyaluronate PowderPolicyThis policy statement applies to clinical review performed for pre-service (Prior Approval, Precertification, Advanced Benefit Determination, etc.) and/or post-service claims.Hyaluronic acid derivatives may be considered medically necessary for the treatment of osteoarthritis of the knee and if the conditions indicated below are met.Hyaluronic acid derivatives may be considered investigational for all other indications.Prior-Approval RequirementsAge18 years or older (22 or older for Synvisc, Synvisc-One, and TriVisc)DiagnosisPatient must have the following:Osteoarthritis of the kneeAND ALL of the following:1. Inadequate response to TWO or more of the following conservative non-pharmacologic therapy:a. Cardiovascular (aerobic) activity, such as: walking, biking, stationarybike, aquatic exerciseb. Resistance exercisec. Weight reduction (for persons who are overweight)d. Participation in self-management programse. Wear of medially directed patellar tapingf. Wear of wedged insolesg. Thermal agentsh. Walking aidsi. Physical therapyj. Occupational therapy2. Inadequate response, intolerance, or contraindication to TWO or more of thefollowing:Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 4 of 7a. Acetaminophenb. Oral NSAIDsc. Topical NSAIDs3. Inadequate response, intolerance, or contraindication to intra-articularsteroid injections in which efficacy lasted less than 8 weeks4. Radiologic confirmation of Kellgren-Lawrence Scale score of grade 2 orgreater5. NO dual therapy with another hyaluronic acid injectable6. Non-preferred medications only: Patient MUST have tried at least TWO ofthe preferred products unless the patient has a valid medical exception (e.g.inadequate treatment response, intolerance, contraindication)Prior – Approval Renewal RequirementsAge18 years or older (22 or older for Synvisc, Synvisc-One, and TriVisc)DiagnosisPatient must have the following:Osteoarthritis of the kneeAND ALL of the following:1. Documentation of improvement in pain with previous course of treatment2. At least 12 months has elapsed since last injection of the prior treatmentcycle3. Documentation of reduction of dosing of NSAIDs or other analgesicsduring the 12 month period following the last injection of the prior treatmentcycle4. NO dual therapy with another hyaluronic acid injectable5. Non-preferred medications only: Patient MUST have tried at least TWOof the preferred products unless the patient has a valid medical exception(e.g. inadequate treatment response, intolerance, contraindication) Policy GuidelinesPre - PA AllowanceNoneSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 5 of 7Prior - Approval LimitsDuration12 monthsQuantity One course of therapy for each kneePrior – Approval Renewal LimitsSame as aboveRationaleSummaryOsteoarthritis of the knee is a disease in which the elastoviscous property of the synovial fluid in the knee joint becomes diminished, resulting in less protection and shock absorption. Durolane, Euflexxa, Gel-One, GelSyn-3, GenVisc 850, Hyalgan, Hymovis, Monovisc, Orthovisc, Sodium Hyaluronate, Synvisc, Synvisc-One, Supartz, Synojoynt, Triluron, TriVisc, Visco-3 are hyaluronan derivatives that are injected into the knee joints to increase the elastoviscous properties of arthritic joint fluid and slow its outflow from the joint. The goal of therapy is to restore the viscoelasticity in the affected joints, thereby decreasing pain, improving mobility, and restoring the natural protective functions (1-18).Prior approval is required to ensure the safe, clinically appropriate and cost effective use of the hyaluronic acid derivatives while maintaining optimal therapeutic outcomes.References1. American College of Rheumatology, Subcommittee on Osteoarthritis Guidelines.Recommendations for the medical management of osteoarthritis of the hip and knee:2012 update. Arthritis Care & Research 2012; 64(4):465-474.2. Euflexxa [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc.; July 2016.3. Hyalgan [package insert]. Parsippany, NJ: Fidia Pharma USA Inc.; May 2014.4. Orthovisc [package insert]. Woburn, MA: Anika Therapeutics; June 2005.5. Supartz [package insert]. Durham, NC: Bioventus LLC; April 2015.6. Synvisc [package insert]. Ridgefield, NJ: Genzyme Corp.; December 2014.7. Synvisc-One [package insert]. Ridgefield, NJ: Genzyme Corp.; September 2014;8. Gel-One [package insert]. Warsaw, IN: Zimmer Inc.; May 2011.9. Monovisc [package insert]. Bedford, MA: Anika Therapeutics; December 2013.10. Hymovis [package insert]. Parsippany, NJ: O Fidia Pharma USA Inc.; October 2015.Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 6 of 711. GenVisc 850 [package insert]. Doylestown, PA: OrthogenRx Inc.; January 2015.12. GelSyn-3 [package insert]. Durham, NC: Bioventus LLC; January 2016.13. Durolane [package insert]. Durham, NC: Bioventus LLC; November 2017.14. Visco-3 [package insert]. Warsaw, IN: Zimmer, Inc.; May 2017.15. Sodium Hyaluronate [package insert]. North Wales, PA: Teva Pharmaceuticals USA,Inc.; March 2019.16. Synojoynt [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc.;September 2019.17. TriVisc [package insert]. Doylestown, PA: OrthogenRx, Inc.; September 2018.18. Triluron [package insert]. Florham Park, NJ: Fidia Pharma USA Inc.; March 2019.19. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee.Evidence-based guideline 2nd edition. May 2013.Policy HistoryDate Action ReasonJanuary 2012 Added minimum age - only approved for adultsDecember 2012 Annual editorial review and reference updateDecember 2013 Annual editorial review and reference updateMarch 2014 Annual editorial reviewAddition of examples of non-pharmacological agents and agents of priorfailure medications.April 2014 Line-Addition of Monovisc to PAMarch 2015 Annual criteria review and reference updateMarch 2016 Change from one tried and failed to two tried and failed non-pharmacologic and pharmacologic therapies and addition of the tried and failed of intra-articular steroid and radiologic confirmation of Kellgren-Lawrence Scalescore of grade 2 or greaterAddition of HymovisPolicy # change from 5.11.04 to 5.75.09May 2016 Addition of GelSyn-3 and GenVisc 850December 2016 Annual editorial review and reference updateAdded: no dual therapy with another hyaluronic acid injectableMarch 2017 Bolded preferred products in the title pageJuly 2017 GelSyn-3 has been changed to preferredSeptember 2017 Annual reviewDecember 2017 Addition of Durolane and Visco-3March 2018 Annual editorial reviewRemoval of Tramadol from the T/F listSeptember 2019 Annual review and reference update. Addition of Sodium Hyaluronate,Synojoynt, and TriViscSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 7 of 7December 2019 Annual review. Addition of requirement to trial preferred products January 2020 Addition of TriluronMarch 2020 Annual reviewKeywordsThis policy was approved by the FEP® Pharmacy and Medical Policy Committee on March 13, 2020 and is effective on April 1, 2020.。
EDU说明书
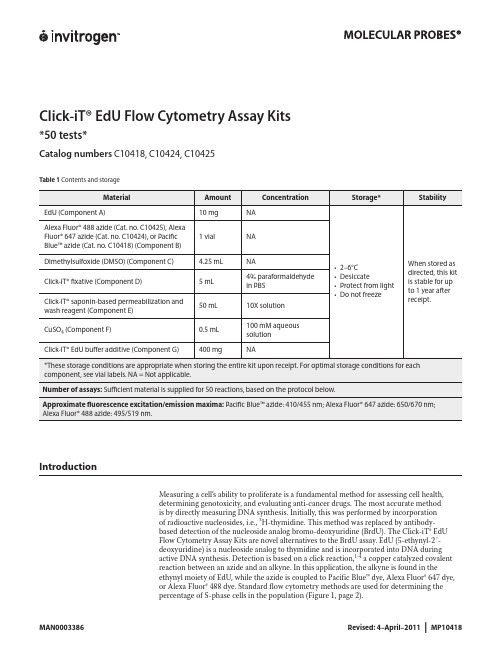
Click-iT® EdU Flow Cytometry Assay Kits*50 tests*Catalog numbers C10418, C10424, C10425Contents and storageTable 1IntroductionMeasuring a cell’s ability to proliferate is a fundamental method for assessing cell health,determining genotoxicity, and evaluating anti-cancer drugs. The most accurate methodis by directly measuring DNA synthesis. Initially, this was performed by incorporationof radioactive nucleosides, i.e., 3H-thymidine. This method was replaced by antibody-based detection of the nucleoside analog bromo-deoxyuridine (BrdU). The Click-iT® EdUFlow Cytometry Assay Kits are novel alternatives to the BrdU assay. EdU (5-ethynyl-2´-deoxyuridine) is a nucleoside analog to thymidine and is incorporated into DNA duringactive DNA synthesis. Detection is based on a click reaction,1-4 a copper catalyzed covalentreaction between an azide and an alkyne. In this application, the alkyne is found in theethynyl moiety of EdU, while the azide is coupled to Pacific Blue™ dye, Alexa Fluor® 647 dye,or Alexa Fluor® 488 dye. Standard flow cytometry methods are used for determining thepercentage of S-phase cells in the population (Figure 1, page 2).Figure 1 Fluorescence signal from Pacific Blue™, Alexa Fluor® 488, and Alexa Fluor® 647 Click-iT® EdU Flow Cytometry Assay Kits. Jurkat (human T-cell leukemia) cells were treated with 10 µM EdU for 2 hours and detected according to the recommended staining protocol. The figures show a clear separation of proliferating cells which have incorporated EdU and nonproliferating cells which have not. Panel A shows data from cells labeled with Pacific Blue™ azide analyzed on an Attune® Acoustic Focusing Cytometer using 405 nm excitation with a 450/40 nm bandpass emission filter; Panel B shows data from cells labeled with Alexa Fluor® 488 azide analyzed on an Attune® Acoustic Focusing Cytometer using 488 nm excitation and a 530/30 nm bandpass emission filter; Panel C shows data from cells labeled with Alexa Fluor® 647 azide analyzed on a flow cytometer using 633 nm excitation and a 660/20 nm bandpass emission filter.The advantage of Click-iT® EdU labeling is that the small size of the dye azide allows for efficient detection of the incorporated EdU using mild conditions. Standard aldehyde-based fixation and detergent permeabilization are sufficient for the Click-iT® detection reagent to gain access to the DNA. This is in contrast to BrdU assays that require DNA denaturation (using acid, heat, or digestion with DNase) to expose the BrdU so that it may be detected with an anti-BrdU antibody. Sample processing for the BrdU assay can result in signal alteration of the cell cycle distribution as well as the destruction of antigen recognition sites when using the acid denaturation method. In contrast, the EdU cell proliferation kit is compatible with cell cycle dyes (Figure 2). The EdU assay can also be multiplexed with antibodies againstsurface and intracellular markers. However, some reagents or antibody conjugates may not be compatible with the Click-iT® EdU detection reaction and may need some additional steps toensure compatibility (see Table 2, page 3 for details).Figure 2 Dual parameter plot of Click-iT® EdU Alexa Fluor® 488 and FxCycle™ Violet. Jurkat (human T-cell leukemia) cells were treated with 10 µM EdU for 2 hours and detected according to the recommended staining protocol. Data was collected and analyzed using an Attune® Acoustic Cytometer using 488 nm excitation and a 530/30 bandpass for detection of the EdU Alexa Fluor® 488 azide and 405 nm excitation and a 450/40 bandpass for detection of the FxCycle™ Violet fluorescence. This figure combines DNA content with EdU; cells that are positive for both labels are in S-phase of the cell cycle.CClick-iT ® EdU Alexa Fluor ® 647 fluorescenceC o u n t5010015020010210103104105A BTable 2 Click-iT® EdU detection reagent compatibilityBefore StartingMaterials Required butNot Provided• 1% bovine serum albumin (BSA) in phosphate buffered saline (PBS), pH 7.1–pH 7.4• Buffered saline solution, such as PBS, D-PBS, or TBS• Deionized water or 18 MΩ purified water• 12 × 75-mm tubes, or other flow cytometry tubesCautions• DMSO (Component C), provided as a solvent in this kit, is known to facilitate the entry oforganic molecules into tissues. Handle reagents containing DMSO using equipment andpractices appropriate for the hazards posed by such materials. Dispose of the reagents incompliance with all pertaining local regulations.• Click-iT® fixative (Component D) contains paraformaldehyde, which is harmful. Use withappropriate precautions.• Click-iT® saponin-based permeabilization and wash reagent (Component E) containssodium azide, which yields highly toxic hydrazoic acid under acidic conditions. Diluteazide compounds in running water before discarding to avoid accumulation of potentiallyexplosive deposits in plumbing.Preparing Reagents1.1 Allow vials to warm to room temperature before opening.1.2 To prepare a 10 mM solution of EdU, add 4 mL of DMSO (Component C) or aqueoussolution (PBS) to Component A and mix well. After use, store any remaining stock solution at≤–20°C. When stored as directed, the stock solution is stable for up to 1 year.1.3 To prepare a working solution of Pacific Blue™ azide (Cat. no. C10418), Alexa Fluor® 647azide (Cat. no. C10424), or Alexa Fluor® 488 azide (Cat. no. C10425), add 130 µL of DMSOto Component B and mix well. After use, store any remaining working solution at ≤–20°C.When stored as directed, this working solution is stable for up to 1 year.1.4 To prepare 500 mL of 1X Click-iT® saponin-based permeabilization and wash reagent, add50 mL of Component E to 450 mL of 1% BSA in PBS. Smaller amounts can be prepared bydiluting a volume of Component E 1:10 with 1% BSA in PBS. After use, store any remainingsolutions at 2–6˚C. When stored as directed, the 1X solution is stable for 6 months and the10X solution is stable for 12 months after receipt.Note: Component E contains sodium azide (see Cautions, page 3).1.5 To make a 10X stock solution of the Click-iT® EdU buffer additive (Component G), add2 mL of deionized water to the vial and mix until the Click-iT® EdU buffer additive is fullydissolved. After use, store any remaining stock solution at ≤–20˚C. When stored as directed,the stock solution is stable for up to 1 year.Experimental ProtocolsThe following protocol was developed with Jurkat cells, a human T cell line, and using anEdU concentration of 10 µM, and can be adapted for any cell type. Growth medium, celldensity, cell type variations, and other factors may influence labeling. In initial experiments,we recommend testing a range of EdU concentrations to determine the optimal concentrationfor your cell type and experimental conditions. If currently using a BrdU based assay for cellproliferation, a similar concentration to BrdU is a good starting concentration for EdU. If usingwhole blood as the sample, we recommend heparin as the anticoagulant for collection.Figure 3 Workflow diagram for the Click-iT® EdU Flow Cytometry Assay Kits.Incubate sample with Click-iT® EdUHarvest cells(Optional) Treat cells with antibodies to cell surface antigensFix and permeabilize cells(Optional) Treat cells with antibodies to intracellular antigensDetection of Click-iT® EdU(Optional) Treat cells with cell cycle stainAnalyze cells by flow cytometryLabeling Cells with EdU2.1 Suspend the cells in an appropriate tissue culture medium to obtain optimal conditions forcell growth. Disturbing the cells by temperature changes or washing prior to incubation withEdU slows the growth of the cells during incorporation.2.2 Add EdU to the culture medium at the desired final concentration and mix well. Werecommend a starting concentration of 10 μM for 1–2 hours. For longer incubations, uselower concentrations. For shorter incubations, higher concentrations may be required. For anegative staining control, include cells from the same population that have not been treatedwith EdU.2.3 Incubate under conditions optimal for cell type for the desired length of time. Altering theamount of time the cells are exposed to EdU or subjecting the cells to pulse labeling with EdUallows the evaluation of various DNA synthesis and proliferation parameters. Effective timeintervals for pulse labeling and the length of each pulse depend on the cell growth rate.2.4 Harvest cells and proceed immediately to step3.1 if performing antibody surface labeling;otherwise continue to step 4.1.Staining Cell-Surface Antigenswith Antibodies (Optional)3.1 Wash cells once with 3 mL of 1% BSA in PBS, pellet cells by centrifugation, and removesupernatant.3.2 Dislodge the pellet and resuspend cells at 1 × 107 cells/mL in 1% BSA in PBS.3.3 Add 100 µL of cell suspension or whole blood sample to flow tubes.3.4 Add surface antibodies and mix well (Table 2, page 3).Note: Do not use PE, PE-tandem, or Qdot® antibody conjugates before performing the clickreaction; wait until step 6.1 for labeling with these fluorophores.3.5 Incubate for the recommended time and temperature, protected from light.3.6 Proceed to step4.1 for cell fixation.Fixation and Permeabilization The Click-iT® saponin-based permeabilization and wash reagent can be used with wholeblood or cell suspensions containing red blood cells, as well as with cell suspensionscontaining more than one cell type. This permeabilization and wash reagent maintains themorphological light scatter characteristics of leukocytes while lysing red blood cells.4.1 Wash the cells once with 3 mL of 1% BSA in PBS, pellet the cells, and remove thesupernatant.4.2 Dislodge the pellet, add 100 µL of Click-iT® fixative (Component D), and mix well.4.3 Incubate the cells for 15 minutes at room temperature, protected from light.4.4 Wash the cells with 3 mL of 1% BSA in PBS, pellet the cells, and remove the supernatant.Repeat the wash step if red blood cells or hemoglobin are present in the sample. Remove allresidual red blood cell debris and hemoglobin before proceeding.4.5 Dislodge the cell pellet and resuspend the cells in 100 μL of 1X Click-iT® saponin-basedpermeabilization and wash reagent (prepared in step 1.4), and mix well. Incubate the cells for15 minutes or proceed directly to step 5.1 for click labeling.Click-iT® Reaction5.1 Prepare 1X Click-iT® EdU buffer additive by diluting the 10X stock solution (prepared instep 1.5) 1:10 in deionized water.5.2 Prepare the Click-iT® reaction cocktail according to Table 3.Table 3 Click-iT® reaction cocktailsNote: Use the Click-iT® reaction cocktail within 15 minutes of preparation.5.3 Add 0.5 mL of Click-iT® reaction cocktail to each tube and mix well.5.4 Incubate the reaction mixture for 30 minutes at room temperature, protected from light.5.5 Wash the cells once with 3 mL of 1X Click-iT® saponin-based permeabilization and washreagent (prepared in step 1.4), pellet the cells, and remove the supernatant. Dislodge the cellpellet and resuspend the cells in 100 μL of 1X Click-iT® saponin-based permeabilization andwash reagent, if proceeding with intracellular antibody labeleing in step 6.1. Otherwise, add500 μL of 1X Click-iT® saponin-based permeabilization and wash reagent and proceed withstep 7.1 for staining the cells for DNA content, or with step 8.1 for analyzing the cells on aflow cytometer.Staining Intracellular or SurfaceAntigens (Optional)6.1 Add antibodies against intracellular antigens or against surface antigens that use RPE,PE-tandem, or Qdot® antibody conjugates. Mix well.6.2 Incubate the tubes for the time and temperature required for antibody staining, protectedfrom light.6.3 Wash each tube with 3 mL of 1X Click-iT® saponin-based permeabilization and wash reagent(prepared in step 1.4), pellet the cells, and remove the supernatant. Dislodge the cell pelletand resuspend the cells in 500 μL of 1X Click-iT® saponin-based permeabilization and washreagent, and proceed with step 7.1 for staining the cells for DNA content, or with step 8.1 foranalyzing the cells on a flow cytometer.Staining Cells for DNA Content(Optional)7.1 If necessary, add Ribonuclease A to each tube and mix (Table 4).Table 4 Click-iT® EdU compatibility with DNA content stains7.2 Add the appropriate DNA stain to each tube, mix well, and incubate as recommended foreach DNA stain.Analysis by Flow Cytometry If measuring total DNA content on a traditional flow cytometer using hydrodynamicfocusing, use a low flow rate during acquisition. If using the Attune® Acoustic FocusingCytometer, all collection rates may be used without loss of signal integrity if the event rate iskept below 10,000 events per second. However, for each sample within an experiment, thesame collection rate and cell concentration should be used. The fluorescent signal generatedby DNA content stains is best detected with linear amplification. The fluorescent signalgenerated by Click-iT® EdU labeling is best detected with logarithmic amplification.8.1 Analyze the cells using a flow cytometer.• For the detection of EdU with Pacific Blue™ azide, use 405 nm excitation with a violetemission filter (450/50 nm or similar).• For the detection of EdU with Alexa Fluor® 647 azide use 633/635 nm excitation with a redemission filter (660/20 nm or similar).• For the detection of EdU with Alexa Fluor® 488 azide, use 488 nm excitation with a greenemission filter (530/30 nm or similar).EdU–Alexa Fluor ® 647 azideB r d U –A l e x a F l u o r ® 488101102103104105102103104105-45-58BrdU –EdU +BrdU –EdU –BrdU +EdU +BrdU +EdU –Figure 4 Dual pulse labeling with EdU and BrdU. Jurkat cells were first treated with 20 mM EdU for 1 hour. Without washing or removal of the EdU, BrdU was added at a 10 μM concentration, and the cells were incubated for 1 hour. The cells were harvested, washed, fixed with 70% ice-cold ethanol and stored at 4°C for 96 hours, followed by an HCl-based denaturation procedure. EdU was detected with Alexa Fluor® 488 azide using the Click-iT® EdU Flow Cytometry Kit (Cat. no. C10420). BrdU was then detected with anti-BrdU, Alexa Fluor® 647 conjugate (Cat. no. A21305). SYTOX® Blue nucleic acid stain (Cat. no. S11348) with RNase was used to detect DNA content. The labeled cells were analyzed by flow cytometry using 488 nm excitation with a 530/30 nm bandpass to detect EdU, 633 nm excitation with a 660/20 nm bandpass to detect BrdU, and 405 nm excitation with a 450/50 nm bandpass to detect DNA content. Four populations of cells are distinguished in the EdU vs BrdU plot: cells which are positive for both (Q2, upper right), cells which are negative for both (Q3, lower left), EdU positive but BrdU negative (Q4, lower right), and cells which are positive for BrdU but negative for EdU (Q1, upper left).Dual Pulse Labeling using EdU and BrdUFollow these guidelines to perform dual labeling of cultured cells by combining EdU with BrdU labeling.• Use EdU for the first pulse and BrdU for the second pulse.• Removal of EdU from the cell culture media is not required when BrdU is added as the second label.• Addition of BrdU to culture media containing EdU results in preferential incorporation of BrdU into the DNA with the exclusion of EdU, while simultaneous addition of EdU with equimolar or half equimolar BrdU to the media results in only BrdU incorporation. This simplifies the dual labeling protocol by eliminating the wash step normally required to remove the first label from the culture media prior to addition of the second label.• Process the cells after dual pulse labeling using a proven BrdU protocol.• After the DNA denaturation step in the BrdU protocol, click label the cells first for the detection of EdU, and then follow with an antibody labeling protocol for the detection of BrdU.• Select a BrdU antibody which does not have cross-reactivity to EdU, such as clone MoBu-1 (Cat. nos. B35129, B35139, B35140, B35141).References1. Chembiochem 4, 1147 (2003);2. J Am Chem Soc 125, 3192 (2003);3. Angew Chem Int Ed Engl 41, 2596 (2002);4. Angew Chem Int Ed Engl 40, 2004 (2001);5. BioTechniques 44, 927 (2008);6. Curr Protoc Cytom 55,7.38.1 (2011).Product List Current prices may be obtained at or from our Customer Service Department.Cat no. Product Name Unit SizeC10418 Click-iT® EdU Pacific Blue™ Flow Cytometry Assay Kit *50 assays*.................................................................. 1 kitC10424 Click-iT® EdU Alexa Fluor® 647 Flow Cytometry Assay Kit *50 assays* .............................................................. 1 kitC10425 Click-iT® EdU Alexa Fluor® 488 Flow Cytometry Assay Kit *50 assays* .............................................................. 1 kit Related productsC10419 Click-iT® EdU Alexa Fluor® 647 Flow Cytometry Assay Kit *100 assays*............................................................. 1 kitC10420 Click-iT® EdU Alexa Fluor® 488 Flow Cytometry Assay Kit *100 assays*............................................................. 1 kitA10027 Click-iT® EdU Alexa Fluor® 488 High-Throughput Imaging (HCS) Assay *2-plate size*............................................... 1 kitA10028 Click-iT® EdU Alexa Fluor® 488 High-Throughput Imaging (HCS) Assay *10-plate size*.............................................. 1 kitA10044 EdU (5-ethynyl-2´-deoxyuridine)................................................................................................. 50 mg A10208 Click-iT® EdU Alexa Fluor® 647 High-Throughput Imaging (HCS) Assay *2-plate size*............................................... 1 kitA10209 Click-iT® EdU Alexa Fluor® 594 High-Throughput Imaging (HCS) Assay *2-plate size*............................................... 1 kitB35129 BrdU mouse monoclonal antibody (Clone MoBU-1) - Pacific Blue™ *for flow cytometry* *100 tests*................................ 1 eachB35139 BrdU mouse monoclonal antibody (Clone MoBU-1) Alexa Fluor® 488 *for flow cytometry* *100 tests*.............................. 1 eachB35140 BrdU mouse monoclonal antibody (Clone MoBU-1) Alexa Fluor® 647 *for flow cytometry* *100 tests*.............................. 1 eachB35141 BrdU mouse monoclonal antibody (Clone MoBU-1) unconjugated *for flow cytometry* *100 tests*................................ 1 eachF10347 FxCycle™ Violet Stain *for flow cytometry* *500 assays* .......................................................................... 1 kitF10348 FxCycle™ Far Red Stain *for flow cytometry* *500 assays* ........................................................................ 1 kitH3570 Hoechst 33342, trihydrochloride, trihydrate *10 mg/mL solution in water* ........................................................ 10 mLP3566 Propidium Iodide – 1.0mg/ml solution in water .................................................................................. 10 mLS10349 SYTOX® AADvanced™ dead cell stain *for 488 excitation* *for flow cytometry* *100 tests* ........................................ 1 kitV35003 Vybrant® DyeCycle™ Violet stain *5 mM in water* *200 assays*.................................................................... 200 µL12091-021 RNase A (20 mg/mL)............................................................................................................. 10 mL14190-144 Dulbecco’s Phosphate Buffered Saline 1X without Calcium Chloride without Magnesium Chloride................................. 500 mL14190-250 Dulbecco’s Phosphate Buffered Saline 1X without Calcium Chloride without Magnesium Chloride.............................10 x 500 mLContact InformationCorporate Headquarters5791 Van Allen WayCarlsbad, CA 92008USAPhone: +1 760 603 7200Fax: +1 760 602 6500Email: techsupport@ European HeadquartersInchinnan Business Park3 Fountain DrivePaisley PA4 9RFUKPhone: +44 141 814 6100Toll-Free Phone: 0800 269 210Toll-Free Tech: 0800 838 380Fax: +44 141 814 6260Tech Fax: +44 141 814 6117Email: euroinfo@Email Tech: eurotech@ Japanese HeadquartersLOOP-X Bldg. 6F3-9-15, KaiganMinato-ku, Tokyo 108-0022JapanPhone: +81 3 5730 6509Fax: +81 3 5730 6519Email: jpinfo@ Additional international offices are listed at These high-quality reagents and materials must be used by, or directl y under the super v ision of, a tech n ically qualified individual experienced in handling potentially hazardous chemicals. Read the Safety Data Sheet provided for each product; other regulatory considerations may apply.Web ResourcesVisit the Invitrogen website at for:• Technical resources, including manuals, vector maps and sequences, application notes, Meds, FAQs, formulations, citations, handbooks, etc.• Complete technical support contact information• Access to the Invitrogen Online Catalog• Additional product information and special offersSDSSafety Data Sheets (SDSs) are available at /sds.Certificate of AnalysisThe Certificate of Analysis provides detailed quality control and product qualification information for each product. Certificates of Analysis are available on our website. Go to /support and search for the Certificate of Analysis by product lot number, which is printed on the product packaging (tube, pouch, or box).Limited WarrantyInvitrogen (a part of Life Technologies Corporation) is committed to providing our customers with high-quality goods and services. Our goal is to ensure that every customer is 100% satisfied with our products and our service. If you should have any questions or concerns about an Invitrogen product or service, contact our Technical Support Representatives.All Invitrogen products are warranted to perform according to specifications stated on the certificate of analysis. The Company will replace, free of charge, any product that does not meet those specifications. This warranty limits the Company’s liability to only the price of the product. No warranty is granted for products beyond their listed expiration date. No warranty is applicable unless all product components are stored in accordance with instructions. The Company reserves the right to select the method(s) used to analyze a product unless the Company agrees to a specified method in writing prior to acceptance of the order.Invitrogen makes every effort to ensure the accuracy of its publications, but realizes that the occasional typographical or other error is inevitable. Therefore the Company makes no warranty of any kind regarding the contents of any publications or documentation. If you discover an error in any of our publications, please report it to our Technical Support Representatives.Life Technologies Corporation shall have no responsibility or liability for any special, incidental, indirect or consequential loss or damage whatsoever. The above limited warranty is sole and exclusive. No other warranty is made, whether expressed or implied, including any warranty of merchantability or fitness for a particular purpose.Limited Use Label License No. 358: Research Use OnlyThe purchase of this product conveys to the purchaser the limited, non-transferable right to use the purchased amount of the product only to perform internal research for the sole benefit of the purchaser. No right to resell this product or any of its components is conveyed expressly, by implication, or by estoppel. This product is for internal research purposes only and is not for use in commercial services of any kind, including, without limitation, reporting the results of purchaser’s activities for a fee or other form of consideration. For information on obtaining additional rights, please contact outlicensing@ or Out Licensing, Life Technologies Corporation, 5791 Van Allen Way, Carlsbad, California 92008.The trademarks mentioned herein are the property of Life Technologies Corporation or their respective owners.©2011 Life Technologies Corporation. All rights reserved.For research use only. Not intended for any animal or human therapeutic or diagnostic use.。
硝苯地平缓释片Ⅰ释放度影响因素研究

作者简介:刘景宜,本科,主管药师。
研究方向:药品检验。
E-mail :***************硝苯地平缓释片Ⅰ释放度影响因素研究刘景宜洛阳市食品药品检验所,河南 洛阳 471023[摘要]目的:研究硝苯地平缓释片Ⅰ释放度的影响因素。
方法:参照国家食品药品监督管理局国家药品标准WS1-(X-056)-2004Z ,采用紫外可见分光光度法在237 nm 测定吸光度,计算硝苯地平缓释片Ⅰ释放度。
结果:对照品性质稳定,多次测量RSD 为0.65%;滤膜对2 h 释放度结果影响误差为5.1%~9.4%;温度在相差1 ℃时,吸光度的平均差值为2.3%。
结论:对照品在正常检验时不用考虑;温度仅在检验结果为边缘时需要考虑;滤膜是影响释放度结果的主要因素,在检验时必须考虑滤膜的影响。
[关键词]硝苯地平缓释片Ⅰ;药物释放;滤膜;温度;溶出仪;紫外可见分光光度法DOI: 10.19939/ki.1672-2809.2021.08.07Study on the Influence Factors of Nifedipine Sustained Release Tablet I Release DegreeLIU JingyiLuoyang Food and Drug Inspection Institute, Luoyang Henan 471023, China.[Abstract] Objective: To study the influence factors of Nifedipine sustained release tablet I release degree. Methods: By reference to the National Drug Standard WS1-(X-056)-2004Z of the State Food and Drug Administration, the absorbance was measured at 237 nm using ultraviolet visible method to calculate the degree of release. Results: The standard substance was stable with RSD of 0.65%; the influence error of membrane on 2 h release rate was 5.1%~9.4%; when the temperature difference is 1 ℃, the average difference of absorbance is 2.3%. Conclusion: The standard substance should not be considered in normal test; temperature only needs to be considered when the inspection result is edge; filter membrane is the main factor affecting the results of release, so the influence of filter membrane must be considered in the test.[Key Words] Nifedipine sustained release tablet I; Drug liberation; Filter membrane; Temperature; Dissolution apparatus; Ultraviolet visible spectrodiometer确保检验方法的准确性[12]。
Get清风SDS聚丙烯酰胺凝胶电泳筛选霉变烟叶特异性标志蛋白

SDS-聚丙烯酰胺凝胶电泳筛选霉变烟叶特异性标志蛋白目录摘要 (3)前言 (4)l 材料与方法 (6)1.1 样品采集 (6)仪器与试剂 (6)1.3 试剂的配制 (6)电泳缓冲液的配制 (6)样品缓冲液制备 (6)考马斯亮蓝R-250固定液的配制 (6)考马斯亮蓝R-250染液的配制 (6)考马斯亮蓝脱色液的配置 (6)烟叶蛋白的提取 (6)聚丙烯酰胺凝胶电泳 (6)考马斯亮蓝染色 (7)2 结果与讨论 (7)3 展望 (8)致谢 (8)参考文献 (9)SDS-聚丙烯酰胺凝胶电泳筛选霉变烟叶特异性标志蛋白摘要:为了找出霉变烟叶里的特异性标记,我们采集了75个霉变烟叶样品,以及11个正常烟叶样品作为对照。
利用聚丙烯酰胺凝胶电泳,可以检测和分析出不同的蛋白带型。
正常烟叶的特异性标志蛋白已经被鉴定出来〔相对分子质量大约为20Da〕。
SDS-聚丙烯酰胺凝胶电泳筛选霉变烟叶里的特异性标志蛋白,找出了霉变烟叶和正常烟叶里的蛋白质指纹差异,为建立快速、精确的烟叶霉变检测方法提供了先决条件。
关键词:霉变烟叶;蛋白指纹;SDS-聚丙烯酰胺凝胶电泳Screen for Specific Biomarker in Molded Flue-Cured Tobacco Leaves by SDS - Polyacrylamide Gel ElectrophoresisAbstract: To screen relatively specifically markers in molded flue-cured tobacco leaves (FCTL), 75 samples of molded FCTL were collected and an additional 11 samples of unmolded FCTL were used as controls. The protein profiles were detected and analyzed bySDS - polyacrylamide gel electrophoresis. A FCTL-specific biomarker (relative molecular weight is 20Da) was identified. The pattern of the marker provides a powerful and reliable diagnostic method for molded FCTL with high sensitivity and specificity.Key words: Molded flue-cured tobacco leaves; Fingerprints; SDS-Polyacrylamide Gel Electrophoresis前言烟草是以收获叶片为主的重要经济作物,烟叶的自然醇化或人工发酵是提高烟叶燃吸品质和可用性的重要环节之一。
黄芪化学成分

1 黄芪的化学成分黄芪的化学成分众多, 主要含皂苷类、黄酮类、多糖类及氨基酸类等, 另外还含有蔗糖、黏液质、苦味素、胆碱、甜菜碱、叶酸等。
屠鹏飞等[ 2]对黄芪进行了较系统的化学成分研究, 到目前为止已分离得到32种化合物, 鉴定了其中的14种化合物, 包括4种异黄酮[芒柄花素( X-64)、芒柄花素-7-O--D葡萄糖苷( K-12-1)、毛蕊异黄酮( X-100-21)和毛蕊异黄酮-7-O-?-D葡萄糖苷], 2种异黄烷[ ( 3R) -8, 2"-二羟基- 7, 4*-二甲氧基异黄烷( X-66)、2", 3" 4"-三甲氧基异黄烷-7-O-?-D-葡萄糖苷], 1种紫檀烷[ ( 6aR, 11aR) 9, 10- 二甲氧基紫檀烷-3-O-?-D-葡萄糖苷( X-186) ], 1种皂苷[黄芪甲苷( h-29) ], 6种其它类成分[ ?-谷甾醇( E-191)、5-甲基- 呋喃甲醛( X-30)、5?甲氧基?2?吡咯甲醛( X-67)、1, 3, 5-三烯-1, 6-己二醇( E-32-10-17)、2, 4-二烯- 己二酸( E-32-19-14)和尿嘧啶核苷( h-33-7) ]。
其他化合物正在鉴定中。
图1 黄芪的HPLC-APCI-MS(A)和HPLC-DAD(B)色谱指纹图谱Fig. 1 Chromatographic fingerprints of Astragali Radix by HPLC-APCI-MS (A) and byHPLC-DAD (B)表1 黄芪HPLC-DAD-MS 指纹图谱中各色谱峰的保留时间、质谱和紫外光谱数据峰号t/min λmax/nm 质谱(m/z) 化合物1 17.336 258, 288 447, 285 calycosin-7-O-β-D-glycoside2 20.730 280, 325 2173 22.752 280, 325 2244 26.642 280, 325 489, 2855 30.390 250, 300 431, 269 ononin6 34.898 248, 288 285 calycosin7 40.733 230, 282 3018 44.394 250, 300 269 formononetin9 44.394 280 303 (3R)-7,2′-dihydroxy,4-dimethoxyisoflavone10 53.372 230 295, 293, 34811 55.662 –295, 227, 31112 61.692 –24513 62.250 –313, 295, 27714 64.555 –27915 65.001 –297, 279, 31516 65.483 –27917 65.884 –297, 279, 31518 66.585 –437, 455, 419 astragaloside IV19 67.312 –29320 67.562 280 361, 27721 68.126 230 27722 69.266 –35323 70.505 280 295, 29624 71.616 280 29525 74.030 230 2791 黄芪多糖蒙古黄芪的多糖研究已取得一定进展, 其多糖含量处于中上水平。
Mettler Toledo 试验用品说明书
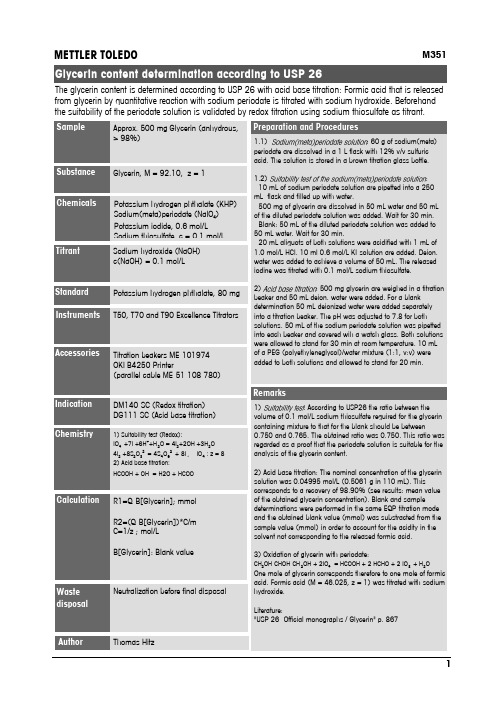
M351Glycerin, M = 92.10, z = 1Potassium hydrogen phthalate (KHP)Sodium(meta)periodate (NaIO 4)Potassium iodide, 0.6 mol/LSodium thiosulfate c =01mol/L Sodium hydroxide (NaOH)c(NaOH) = 0.1 mol/LT50, T70 and T90 Excellence Titrators1) Suitability test (Redox):IO 4-+7I -+6H ++H 2O = 4I 2+2OH -+3H 2O 4I 2+8S 2O 32-= 4S 4O 62-+ 8I -, IO 4-: z = 82) Acid base titration:HCOOH + OH -= H2O + HCOO-Titration beakers ME-101974OKI B4250 Printer(parallel cable ME-51 108 780)METTLER TOLEDONeutralization before final disposalApprox. 500 mg Glycerin (anhydrous,> 98%)DM140-SC (Redox titration)DG111-SC (Acid base titration)1.1): 60 g of sodium(meta)periodate are dissolved in a 1 L flask with 12% v/v sulfuric acid. The solution is stored in a brown titration glass bottle.1.2):- 10 mL of sodium periodate solution are pipetted into a 250mL flask and filled up with water.- 500 mg of glycerin are dissolved in 50 mL water and 50 mL of the diluted periodate solution was added. Wait for 30 min.- Blank: 50 mL of the diluted periodate solution was added to 50 mL water. Wait for 30 min.- 20 mL aliquots of both solutions were acidified with 1 mL of 1.0 mol/L HCl. 10 ml 0.6 mol/L KI-solution are added. Deion.water was added to achieve a volume of 50 mL. The released iodine was titrated with 0.1 mol/L sodium thiosulfate.2): 500 mg glycerin are weighed in a titration beaker and 50 mL deion. water were added. For a blank determination 50 mL deionized water were added separately into a titration beaker. The pH was adjusted to 7.8 for both solutions. 50 mL of the sodium periodate solution was pipetted into each beaker and covered wih a watch glass. Both solutions were allowed to stand for 30 min at room temperature. 10 mL of a PEG (polyethyleneglycol)/water mixture (1:1, v:v) were added to both solutions and allowed to stand for 20 min.1): According to USP26 the ratio between the volume of 0.1 mol/L sodium thiosulfate required for the glycerin containing mixture to that for the blank should be between0.750 and 0.765. The obtained ratio was 0.750. This ratio was regarded as a proof that the periodate solution is suitable for the analysis of the glycerin content.2) Acid base titration: The nominal concentration of the glycerin solution was 0.04995 mol/L (0.5061 g in 110 mL). This corresponds to a recovery of 98.90% (see results: mean value of the obtained glycerin concentration). Blank and sample determinations were performed in the same EQP-titration mode and the obtained blank value (mmol) was substracted from the sample value (mmol) in order to account for the acidity in the solvent not corresponding to the released formic acid.3) Oxidation of glycerin with periodate:CH 2OH-CHOH-CH 2OH + 2IO 4-= HCOOH + 2 HCHO + 2 IO 3-+ H 2OOne mole of glycerin corresponds therefore to one mole of formicacid. Formic acid (M = 46.025, z = 1) was titrated with sodium hydroxide.Literature:"USP 26 -Official monographs / Glycerin" p. 867R1=Q-B[Glycerin]; mmol R2=(Q-B[Glycerin])*C/m C=1/z ; mol/LB[Glycerin]: Blank valueThe glycerin content is determined according to USP 26 with acid base titration: Formic acid that is released from glycerin by quantitative reaction with sodium periodate is titrated with sodium hydroxide. Beforehand the suitability of the periodate solution is validated by redox titration using sodium thiosulfate as titrant.Potassium hydrogen phthalate, 80 mg Thomas HitzName: Thomas Hitz, ID Glycerin ContentRx Result Unit Name1/5 -- 27.03.2007 11:30:27R1 =0.98657 mmol ContentR2 =0.04933 mol/L Concentration 2/5 -- 27.03.2007 11:34:16R1 =0.98932 mmol ContentR2 =0.04947 mol/L Concentration 3/5 -- 27.03.2007 11:41:09R1 =0.98976 mmol ContentR2 =0.04949 mol/L Concentration 4/5 -- 27.03.2007 11:47:34R1 =0.98991 mmol ContentR2 =0.04950 mol/L Concentration 5/5 -- 27.03.2007 11:53:14R1 =0.98406 mmol ContentR2 =0.04920 mol/L Concentration StatisticsRx Name n Mean Unit s srel [%]R1 Cont.50.98792 mmol0.00255 0.258R2 Conc.50.04940 mol/L0.00013 0.264。
7. Endotoxin LALTests

Charles River Endosafe
2
Woo Jung BSC Inc.
August 25, 2003
LAL Discoveries by Bang and Levin
Described role of endotoxin in coagulation of Limulus blood
Prepared Endotoxin - responsive lysate from Amoebocytes
Endotoxicity
ENDOTOXIN CAUSES HUMAN TISSUE TO RELEASE INFLAMMATORY MEDIATORS INFLAMMATION INDUCES A VARIETY OF TISSUE DAMAGE SHOCK and MULTIPLE ORGAN DYSFUNCTION MAY OCCUR
Endotoxins and Pyrogens
Pyrogens are fever-inducing agents in humans and animals
include endotoxin, gram + cell debris, fungi
Endotoxins are components from the outer membrane of gram-negative bacteria
Clotting Enzyme
Liquid Coagulogen
M++ pH=7.2
Clotted Coagulin Gel
Summary of Gel Clot Test
Endpoint sought by 180 inversion of sample tube
糖尿病患者诊断应用血清C肽及糖化血红蛋白联合检测的价值分析

DOI:10.16658/ki.1672-4062.2023.14.085糖尿病患者诊断应用血清C肽及糖化血红蛋白联合检测的价值分析倪胜南,陈少,陈一鸣泗阳康达医院检验科,江苏宿迁223700[摘要]目的探讨糖尿病患者诊断应用血清C肽联合糖化血红蛋白检测的价值。
方法将2022年1月—2023年1月泗阳康达医院收治的74例疑似糖尿病患者作为研究对象,检测入组患者糖化血红蛋白(glycosylated hemoglobin, HbA1c)以及血清C肽水平,以口服葡萄糖耐量试验(glucose tolerance test check, OGTT)为金标准,统计血清C肽联合糖化血红蛋白检测与单一项目检测的敏感性、特异度和诊断符合率。
结果74例疑似糖尿病患者根据葡萄糖耐量试验结果,确诊患者67例,确诊率为90.54%(67/74);与血清C肽、HbA1c单一检测相比,血清C肽+HbA1c联合检测敏感度更高,差异有统计学意义(P<0.05);血清C肽+HbA1c联合检测的特异度略高于血清C肽、HbA1c单一检测,但差异无统计学意义(P>0.05);联合检测诊断符合率明显高于血清C 肽、HbA1c单项检测,差异有统计学意义(P<0.05)。
结论血清C肽与糖化血红蛋白是临床诊断糖尿病的重要参考指标,二者表达水平的变化有助于检测患者胰岛素分泌功能,评估疾病严重程度,两者联合检验灵敏性与特异度良好,有助于早期明确诊断,临床参考价值较高。
[关键词] 糖尿病;血清C肽;糖化血红蛋白;诊断价值[中图分类号] R446.1 [文献标识码] A [文章编号] 1672-4062(2023)07(b)-0085-04Analysis of the Value of the Diagnostic Application of Combined Serum C-peptide and Glycosylated Hemoglobin Testing in Patients with Diabetes MellitusNI Shengnan, CHEN Shao, CHEN YimingDepartment of Laboratory Medicine, Siyang Kangda Hospital, Suqian, Jiangsu Province, 223700 China[Abstract] Objective To explore the value of applying serum C-peptide combined with glycated hemoglobin test for the diagnosis of diabetic patients. Methods A total of 74 patients with suspected diabetes admitted to Siyang Kangda Hospital from January 2022 to January 2023 were selected as the research objects. The levels of glycosylated hemoglo‐bin (HbA1c) and serum C-peptide were detected. Oral glucose tolerance test (OGTT) was used as the gold standard. The sensitivity, specificity and diagnostic coincidence rate of serum C-peptide combined with glycosylated hemoglo‐bin detection and single item detection were statistically analyzed. Results According to the results of glucose toler‐ance test, 67 patients were diagnosed in 74 patients with suspected diabetes, and the diagnosis rate was 90.54% (67/ 74). Compared with the single detection of serum C-peptide and HbA1c, the sensitivity of combined detection of se‐rum C peptide and HbA1c was higher, and the difference was statistically significant (P<0.05). The specificity of com‐bined detection of serum C-peptide and HbA1c was slightly higher than that of single detection of serum C-peptide and HbA1c, but the difference was no statistically significant (P>0.05). The diagnostic coincidence rate of combined detection was significantly higher than that of single detection of serum C-peptide and HbA1c, and the difference was statistically significant (P<0.05). Conclusion Serum C-peptide and glycosylated hemoglobin are important reference indexes for clinical diagnosis of diabetes mellitus, and changes in the expression levels of the two can help to detect the insulin secretion function of patients and assess the severity of the disease. The sensitivity and specificity of the [作者简介]倪胜南(1991-),女,本科,主管检验师,研究方向为免疫学、分子生物学检验。
瑞舒伐他汀Rosuvastatin全套杂质结构列表
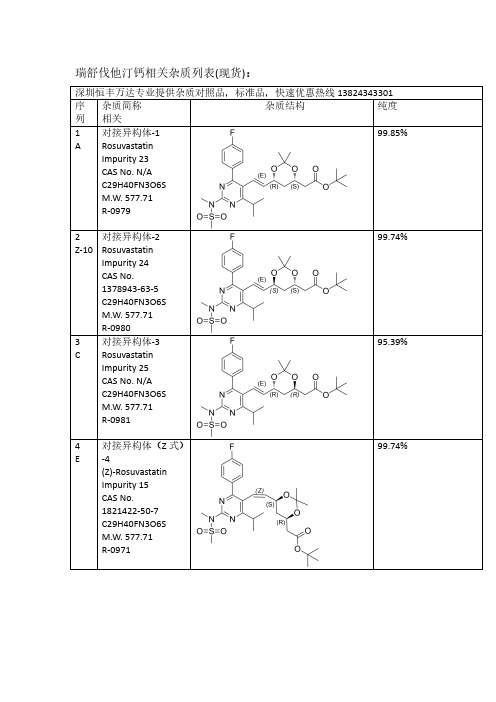
瑞舒伐他汀钙相关杂质列表(现货):深圳恒丰万达专业提供杂质对照品,标准品,快速优惠热线138****3301序列杂质简称相关杂质结构纯度1A 对接异构体-1RosuvastatinImpurity 23CAS No.N/A C29H40FN3O6S M.W.577.71R-097999.85%2Z-10对接异构体-2RosuvastatinImpurity 24CAS No.1378943-63-5C29H40FN3O6S M.W.577.71R-098099.74%3C 对接异构体-3RosuvastatinImpurity 25CAS No.N/A C29H40FN3O6S M.W.577.71R-098195.39%4E对接异构体(Z 式)-4(Z)-Rosuvastatin Impurity 15CAS No.1821422-50-7C29H40FN3O6S M.W.577.71R-097199.74%5Q对接光降解-1CAS No.N/A C29H40FN3O6S M.W.577.71R-099099.31%6P对接光降解-2CAS No.N/A C29H40FN3O6S M.W.577.71R-099299.69%7I丙酮加合物CAS No.N/A C32H46FN3O7S M.W.635.7998.23%8Z-12对接脱氟CAS No.N/AC29H41N3O6S M.W.559.72R-099399.46%9Z-16双键环氧脱丙酮叉CAS No.N/AC29H40FN3O7S M.W.593.7199.18%10B脱丙酮叉异构体-1ent-Rosuvastatin tert-Butyl Ester CAS No.615263-60-0C26H36FN3O6S M.W.537.65R-091899.90%11Z-11脱丙酮叉异构体-2(3S,5S)-Rosuvastatintert-Butyl Ester CAS No.2185805-16-5C26H36FN3O6S M.W.537.65R-097699.66%12D 脱丙酮叉异构体-3(3R,5R)-tert-ButylRosuvastatin (Rosuvastatin Impurity)CAS No.2162136-65-2C26H36FN3O6S M.W.537.65R-0911100%13F 脱丙酮叉异构体(Z 式)-4CAS No.1821422-51-8C26H36FN3O6S M.W.537.65R-099699.44%14S脱丙酮叉光降解-1CAS No.N/A C26H36FN3O6S M.W.537.65R-098999.61%15R脱丙酮叉光降解-2CAS No.N/A C26H36FN3O6S M.W.537.65R-099199.20%16J丙酮加合物脱丙酮叉Rosuvastatin EP Impurity A CAS No.1714147-49-5C29H42FN3O7S M.W.595.72R-099799.65%17Z-13无氟脱丙酮叉CAS No.N/AC26H37N3O6S M.W.519.65R-099599.17%18Z-255-甲氧基脱叉杂质RosuvastatinImpurity 42CAS No.N/A C27H38FN3O6S M.W.551.67R-0910098.80%19Z-263-甲氧基脱叉杂质RosuvastatinImpurity 47CAS No.N/A C27H38FN3O6S M.W.551.67R-0910598.65%203,5-二甲氧基脱叉杂质Rosuvastatin Impurity 44CAS No.N/A C28H40FN3O6S M.W.565.70R-0910698%21G钙盐异构体-1Rosuvastatin EP Impurity G Calcium Salt CAS No.1242184-42-4(free acid)C22H27FN3O6S.1/2Ca M.W.480.5420.0499.88%22Z-1钙盐异构体-2(3S,5S)Rosuvastatin Calcium Salt CAS No.1584149-34-7(free acid)C22H27FN3O6S.1/2Ca M.W.480.5420.04R-091598.15%23H钙盐异构体-3Rosuvastatin EP Impurity B Calcium Salt(3R,5R)-Rosuvastatin Calcium Salt CAS No.1422515-55-61094100-06-7(free acid)C22H27FN3O6S.1/2Ca M.W.480.5420.04R-091296.13%24V钙盐异构体(Z 式)-4Rosuvastatin Z-Isomer Calcium Salt CAS No.1444772-08-01445208-17-2(free acid)C22H27FN3O6S.1/2Ca M.W.480.5420.0499.51%25Z-245-甲氧基钠盐CAS No.N/AC23H29FN3NaO6S M.W.517.5599.50%26Z-273-甲氧基钠盐CAS No.N/AC23H29FN3NaO6S M.W.517.5596.83%27Z-293,5-二甲氧基钠盐CAS No.N/AC24H31FN3NaO6S M.W.531.5797.78%28U钙盐异构体光降解-5RosuvastatinImpurity 1Calcium Salt CAS No.854898-49-0854898-48-9(free acid)C22H27FN3O6S.1/2Ca M.W.480.5420.0499.75%29T 钙盐异构体光降解-6RosuvastatinImpurity 2Calcium Salt CAS No.854898-50-3854898-53-6(free base)C22H27FN3O6S.1/2Ca M.W.480.5420.0499.83%30Z-18钠盐异构体光降解-7(混合物)RosuvastatinImpurity 1Calcium SaltCAS No.N/A C22H27FN3O6S.1/2Ca M.W.480.5420.04R-093499.16%31Z-20钙盐异构体光降解-8Rosuvastatin Impurity CAS No.N/A C22H26FN3O6S M.W.479.5296.69%32Z-14钙盐无氟DesfluoroRosuvastatin Calcium Salt CAS No.N/A847849-66-5(acid)C22H28N3O6S.1/2Ca M.W.462.5420.04R-099499.78%33K 丙酮加合物钙盐Rosuvastatin EPImpurity A Calcium Salt CAS No.1714147-47-31715120-13-0(free acid)C25H33FN3O7S.1/2Ca M.W.538.6120.04R-092599.46%34M 内酯Rosuvastatin EPImpurity D CAS No.503610-43-3C22H26FN3O5S M.W.463.52R-09199.46%35N 内酯异构体CAS No.N/AC22H26FN3O5S M.W.463.5299.46%36内酯异构体CAS No.N/A C22H26FN3O5S M.W.463.5297%37Z-283-甲氧基内酯CAS No.N/AC23H28FN3O5S M.W.477.5598.97%38L5-氧代Rosuvastatin EP Impurity C freed acid CAS No.1620823-61-1(sodium salt)1422619-13-3(acid)C22H26FN3O6S M.W.479.5297.92%39Z-63-氧代3-Oxo Rosuvastatin Sodium Salt CAS No.1346606-28-71346747-49-6(acid)C22H25FN3O6S.Na M.W.478.5222.99R-091798.61%40O内酯脱水Rosuvastatin2,6-Diene Lactone Impurity CAS No.1246665-85-9C22H24FN3O4S M.W.445.5299.36%R-094141Z-44,6-二烯Rosuvastatin4,6-Diene Impurity CAS No.1422954-13-9C22H26FN3O5S M.W.463.53R-092795.44%42Z-152,6-二烯CAS NO.1422954-12-8(free acid)C22H26FN3O5S R-092695.70%43Z-8脱甲基二钠盐(体内代谢产物)N-Desmethyl Rosuvastatin Disodium Salt CAS No.371775-74-5(free base)C21H24FN3O6S.2Na M.W.465.50222.99R-09598.87%44W光降解内酯CAS No.854898-47-8C22H26FN3O5S M.W.463.5299.80%45X 光降解内酯CAS No.854898-46-7C22H26FN3O5S M.W.463.5297.84%46Z-2Z 式异构体内酯CAS No.N/A C22H26FN3O5S M.W.463.5296.52%47Z-9脱甲基内酯(体内代谢产物)N-Desmethyl Rosuvastatin Lactone CAS:1797419-58-9C21H24FN3O5S M.W.449.5097.42%48Z-7基因毒性杂质(体内代谢产物)Rosuvastatin Impurity 28CAS No.N/A C22H28FN3O7S M.W.497.54R-098498.94%49Z-5母核烯醛(工艺相关杂质)CAS No.890028-66-7C18H20FN3O3S M.W.377.4398.85%50Z-42母核无氟膦盐CAS No.N/A C34H35BrN3O2PS M.W.660.6099.14%51Y 本体脱丙酮叉CAS No.N/A C26H36FN3O6S M.W.537.6599.74%更多其他项目:雷西纳德,阿格列汀,依鲁替尼,唑吡坦,利奈唑胺,西他沙星,沙丁胺醇,罗替戈汀,丙戊酸钠,左乙拉西坦,西格列汀,特地唑胺,帕瑞昔布钠,帕布昔利布,赛乐西帕,伏硫西汀,曲格列汀,米拉贝隆,沙芬酰胺,达泊西汀,吡格列酮,达泊西汀,吉非替尼,去甲肾上素,阿昔洛韦,文拉法辛,普拉洛芬,普拉克索,曲唑酮,茚达特罗,阿扎胞苷,酮替芬,依折麦布,西那卡塞,氨曲南,替诺福韦,厄多司坦,孤法辛,雷贝拉唑,阿法替尼,依达拉奉,帕利哌酮,达比加群酯,鲁拉西酮,尼非卡兰,瑞巴派特,苯达莫司汀,利伐沙班,法舒地尔,普拉格雷,维格列汀,索非布韦,文拉法辛等。
l-鸟氨酸盐酸盐的标准

L-鸟氨酸盐酸盐(L-Ornithine Hydrochloride)是一种氨基酸盐酸盐,常用于生物研究和实验分析。
其标准品通常按照相关标准进行生产和质量控制。
以下是关于L-鸟氨酸盐酸盐的一些参考标准:
1. 英国药典(BP)标准:L-鸟氨酸盐酸盐的标准品应符合英国药典中关于L-鸟氨酸盐酸盐的质量要求,包括性状、旋光度、重金属、含水量、干燥失重、灼烧残渣等指标。
2. 中国标准品网:L-鸟氨酸盐酸盐标准品货号为O695550,英文名为L-Ornithine Hydrochloride。
其质量应符合中国标准品网关于L-鸟氨酸盐酸盐的质量要求。
3. 检测方法:对于L-鸟氨酸盐酸盐的检测,可以参考GB/T 14751-2008《食品中氨基酸的测定离子交换色谱法》或GB/T 18902-2002《食品中氨基酸的测定紫外分光光度法》等方法。
这些方法可以用于测定L-鸟氨酸盐酸盐的含量。
4. 存储条件:L-鸟氨酸盐酸盐标准品应避光冷藏,以保证其质量和稳定性。
高效液相色谱法测定3-氨基哌啶二盐酸盐中氨基吡啶类基因毒性杂质的含量

4理但检验-佗字分册PTCACPART B:CHEM.ANAL.)DOI:!0.ll973/lhjy-hx201912006高效液相色谱法测定3-氨基哌唳二盐酸盐中氨基毗唳类基因毒性杂质的含量董淑波",杨汉跃',徐亮S陈学民'(1.南京大学化学化工学院,南京210023; 2.江苏德源药业股份有限公司,连云港222000)摘要:在降血糖药物苯甲酸阿格列汀的合成过程中,有可能因原料的因素而引入氨基毗•呢类基因毒性杂质而对上述药物的用药安全性造成影响。
为此试验提出了用高效液相色谱法测定3-氨基哌味二盐酸盐中3种氨基毗•陀化合物(4-氨基毗•喘、3-氨基毗■啜和2-氨基毗•味)的方法。
称取试样0.3g,用磷酸盐缓冲溶液(pH7.0)-甲醇(90+10)混合溶液超声溶解并定容至10.0mL。
选择Shim-p ack Scepter Cg色谱柱作为分离柱,用pH7.0的磷酸盐缓冲溶液和甲醇(90+10)的混合溶液等度洗脱,流量为0.5mL•min-1,柱温为35°C,检测波长为280nm,进样量10“L。
在选定的条件下,上述3种化合物可达到很好的分离,4-氨基毗•喘、3-氨基毗•噪和2-氨基毗•啜间的分离度分别为30.3,&4,其检出限(3S/N)分别为0.0289,0.0711,0.0702mg•I^1o在3个浓度水平上对3种化合物进行回收试验,测得回收率依次为101%,98.4%,97.2%。
重复性和重现性试验所测得3种化合物测定值的相对标准偏差(”=6)分别依次为1.5%,0.90%,0.70%和6.2%,3.0%,2.2%。
样品加标溶液和对照話溶液在室温下放置24h内稳定性良好。
关键词:高效液相色谱法;苯甲酸阿格列汀;3-氨基哌喘二盐酸盐;氨基"比味;基因毒性杂质中图分类号:()652.63文献标志码:A文章编号:1001-4020(2019)12-1396-05苯甲酸阿格列汀是一种能高效、高度选择性抑制二肽基肽酶-IV的降血糖药物,用于D型糖尿病患者的血糖控制〔切。
板蓝根研究进展

板蓝根为十字花科植物菘蓝(Isatis indigotica Fort.)的干燥根,其性寒、味苦,具有清热解毒、凉血利咽的功效,常用于温毒发斑、大头瘟疫、烂喉丹痧、痄腮、丹毒等症的治疗。
菘蓝在国内栽培历史悠久,产地广泛,古籍记载的产地有河北、江苏、安徽、河南等省,其他省零星分布。
文献对板蓝根的道地产区地域记载并不明确,部分文献记载河北为道地产区。
但近几十年来,菘蓝种植区域发生了很大变化,文献记载的传统大产区种植量明显减少,山西和安徽以生产板蓝根种子为主,甘肃、黑龙江、内蒙古、新疆变成了新型种植区,产量较大。
1历史沿袭板蓝根作为我国传统中药材历史悠久,其栽培历史已有2000多年,首见于《尔雅·释草》,作为药材使用始载于《神农本草经》,被列为上品,谓之“蓝实”,其后历代本草均有记载。
但“蓝实”究竟来源于何种植物,从古至今说法不尽相同。
《新修本草》中记载“本经所用乃是蓼蓝实也”[1]。
《本草衍义》认为蓝实“即大蓝实也”。
关于板蓝根入药的记载,在唐代更是有增无减。
在《千金方》中就记录了几个含“蓝”中药材不同部位的成方。
在《新修本草》中,第一次记录了“蓝”有3种,即菘蓝、木蓝、蓼蓝。
这进一步表明,从唐代初期板蓝根研究进展付照羽冯治朋韩颜超姚振林陈文雪杨树林吴相周*(石家庄以岭药业股份有限公司,河北石家庄050035)摘要板蓝根是中药抗病毒的主要成分之一,板蓝根及其复方制剂在一些疾病治疗方面效果显著。
目前板蓝根供不应求,加大板蓝根研究力度对促进板蓝根产业发展具有重要意义。
通过阅览近年来全球关于板蓝根研究的文献了解到,其化学成分复杂,内在活性物质不明确,作用机制不清楚,阻碍了板蓝根产业现代化、国际化发展。
本文主要从板蓝根的历史沿袭、化学成分、药理作用、临床应用、栽培现状等方面加以综述,以期为板蓝根的开发利用提供参考。
关键词板蓝根;化学成分;药理作用;临床应用;栽培现状中图分类号R282文献标识码A文章编号1007-5739(2023)14-0041-05DOI:10.3969/j.issn.1007-5739.2023.14.013开放科学(资源服务)标识码(OSID):Research Progress on Radix isatidisFU Zhaoyu FENG Zhipeng HAN Yanchao YAO Zhenlin CHEN Wenxue YANG Shulin WU Xiangzhou* (Shijiazhuang Yiling Pharmaceutical Co.,Ltd.,Shijiazhuang Hebei050035) Abstract Radix isatidis is one of the main components of traditional Chinese medicine against virus,Radix isatidis and its polypill has remarkable effects in the treatment of some diseases.At present,the supply of Radix isatidis is in short supply,and increasing its research is of great significance for promoting the development of Radix isatidis industry.Based on the recent global literature of Radix isatidis,it has been found that its chemical composition is complex,its intrinsic active substances are unclear,and its mechanism of action is unclear,which hinders the modernization and internationalization of Radix isatidis industry.This paper mainly reviewed the history,chemical composition,pharmacological effects,clinical applications and cultivation status of Radix isatidis,so as to provide references for the development and utilization of Radix isatidis.Keywords Radix isatidis;chemical composition;pharmacological effect;clinical application;cultivation status基金项目国家重点研发计划项目(2017YFC1701700);石家庄市科技研发计划项目(161200343A);河北省重点研发计划资助项目(19226433D)。
世界卫生组织《结核分枝杆菌耐药相关基因突变目录(第2版)》解读

录-包含高质量$全面的表型耐药相关基因突变列表 行了更细致分层'其根据MN! 对不同药敏试验
及其置信度分级%%%&"但纳入菌株的地理区域代表性 方法的认可程度"将表型药敏试验数据分为7个层
较差"且有关新药和再利用药物耐药相关基因突变 级"前2个层级为使用MN!推荐试验方法得到的
的数据非常有限'因此"MN!于(&(2年修订并发 数据集!MN! 数据集#'第%层级包括根据最新
序的耐药结核病分子诊断技术%%&&'新一代靶向测 #:@5>+,?-5:"SIT##或欧洲核苷酸档案馆数据库
序技术可以通过对临床标本直接检测"实现对十几
的同步更新 !*E>5/A,:SE4.A5?-FAC>4G-PA"*SC#
"
种抗结核药物耐药性的快速判定"但基于目前对部 可以获取更多公开数据'因此"第(版,目录-纳入
9A45:FAF-?-5:5@?GAF")"/'@-*'<.-)"),'50,5 $%&'("&)*+,-.)-(*+&-/'0,0&'.#/*O"5I)3*,+"00'&,"),'5 P,)3I+-@ +*0,0)"5&*-:S5PA+ZA>(&(23JG-94,?,.5BEAFA94>-ZAF+5>A45+/>AGA:9-PA,:F,44E>,?AF>EB;>A9-9?,:4A;>A.,?AFBA:A +E?,?-5:9Z,9AF5:?GA.,>BA9?45..A4?-5:5@+E.?-:,?-5:,. $%&'("&)*+,-.)-(*+&-/'0,045+/.A0-95.,?A9"95,9?5 9E//5>??GAFAPA.5/+A:?,:F-+/>5PA+A:?5@?GA +5.A4E.,>F>EB9E94A/?-Z-.-?H?A9?-:B?A4G:5.5BH3JG-9,>?-4.A
超高效液相串联质谱法测定牛奶中8种氟喹诺酮类兽药残留

分析检测超高效液相串联质谱法测定牛奶中8种氟喹诺酮类兽药残留张瑞婷(北京市产品质量监督检验研究院,北京 101300)摘 要:建立了超高效液相串联质谱法同时测定牛奶中8种氟喹诺酮类兽药残留的分析方法。
牛奶样品用提取剂提取后,用固相萃取柱净化,选用C18色谱柱梯度洗脱分离。
通过串联质谱在电喷雾电离-正离子、多反应监测模式下进行定性和定量分析。
结果表明,8种兽药在0.5~500.0 μg·mL-1呈良好的线性关系,R2>0.996,在10 μg·kg-1、50 μg·kg-1、100 μg·kg-1 3个浓度添加水平下,回收率为81.3%~98.8%,RSD为2.3%~9.8%。
该方法灵敏、快速、可靠,适用于牛奶中多种兽药残留的快速筛查。
关键词:超高效液相串联质谱法;氟喹诺酮类;牛奶Determination of 8 Fluoroquinolones Veterinary Drug Residues in Milk by Ultra Performance Liquid ChromatographyTandem Mass SpectrometryZHANG Ruiting(Beijing Institute of Product Quality Supervision and Inspection, Beijing 101300, China) Abstract: A method for the simultaneous determination of eight fluoroquinolone veterinary drug residues in milk by ultra performance liquid chromatography tandem mass spectrometry was established. After the milk sample was extracted with the extractant, it was purified by a solid phase extraction column and separated by gradient elution on a C18 chromatographic column. Qualitative and quantitative analyzes were then performed by tandem mass spectrometry in electrospray ionization-positive ion, multiple reaction monitoring mode. The results showed that the eight veterinary drugs showed a good linear relationship in the range of 0.5~500.0 μg·mL-1, R2>0.996. The recoveries ranged from 81.3% to 98.8% at the spiked levels of 10 μg·kg-1, 50 μg·kg-1, 100 μg·kg-1 with the RSD of 2.3% to 9.8%. The method is sensitive, rapid and reliable, and is suitable for rapid screening of various veterinary drug residues in milk.Keywords: ultra performance liquid chromatography tandem mass spectrometry; fluoroquinolones; milk氟喹诺酮类药物为白色或淡黄色晶型粉末,属于广谱抗生素,常被用于预防和治疗奶牛疾病[1]。
顺铂通过增加HeLa人宫颈癌细胞中Nrf2入核促进细胞自噬和凋亡

[收稿日期]2020-09-08 [修回日期]2020-12-01[作者单位]江苏省无锡市妇幼保健院宫颈科,214000[作者简介]季 静(1985-),女,硕士,主治医师.[通信作者]詹惠英,主任医师.E⁃mail:wxzhy2013@[文章编号]1000⁃2200(2023)03⁃0301⁃06㊃基础医学㊃顺铂通过增加HeLa 人宫颈癌细胞中Nrf2入核促进细胞自噬和凋亡季 静,张 晔,陆晓红,徐 锋,詹惠英[摘要]目的:探讨顺铂对HeLa 人宫颈癌细胞中Nrf2蛋白表达及核转位的影响,并观察细胞自噬和凋亡的变化㊂方法:使用不同浓度的顺铂(0㊁2.5㊁5㊁10μmol /L)干预HeLa 人宫颈癌细胞,MTT 法检测细胞活性,Transwell 实验观察细胞迁移能力,mCherry⁃GFP⁃LC3B 融合蛋白腺病毒转染HeLa 细胞观察自噬水平的变化,流式细胞术检测细胞凋亡水平,免疫蛋白印迹检测Nrf2在细胞质和细胞核内的表达情况,免疫荧光法观察Nrf2的核转位情况㊂结果:与对照组相比,2.5㊁5㊁10μmol /L 的顺铂均可抑制HeLa 细胞的活性及细胞迁移能力(P <0.05),且随着药物浓度增高抑制作用增强(P <0.05)㊂与对照组相比,2.5㊁5㊁10μmol /L 的顺铂均可促进HeLa 细胞中LC3B 的表达㊁细胞凋亡及Nrf2的核转位(P <0.05),降低细胞质中Nrf2的蛋白表达水平(P <0.05),并升高细胞核中Nrf2的蛋白表达水平(P <0.05)㊂结论:顺铂通过增加HeLa 人宫颈癌细胞中Nrf2入核促进细胞自噬和细胞凋亡,并抑制细胞的活性和迁移能力㊂[关键词]HeLa 人宫颈癌细胞;顺铂;Nrf2;自噬;细胞凋亡[中图法分类号]R 737.33 [文献标志码]A DOI :10.13898/ki.issn.1000⁃2200.2023.03.005Cisplatin increases nuclear translocation of Nrf2and promotes autophagy and apoptosis in HeLa human cervical cancer cellsJI Jing,ZHANG Ye,LU Xiao⁃hong,XU Feng,ZHAN Hui⁃ying(Department of Cervical ,Wuxi Maternal and Child Health Care Hospital ,Jiangsu Wuxi 214000,China )[Abstract ]Objective :To investigate the effect of cisplatin on the protein expression and nuclear translocation of Nrf2,and on the changes of autophagy and apoptosis in HeLa human cervical cancer cells.Methods :After the HeLa human cervical cancer cells were treated with different concentrations of cisplatin(0,2.5,5and 10μmol /L),MTT assay was used to detect the cell activity,Transwell assay was used to observe the cell migration ability,mCherry⁃GFP⁃LC3B fusion protein adenovirus transfected HeLa cells were applied to observe the autophagy changes,flow cytometry was employed to detect the apoptosis,Western blotting was used to detect the expression of Nrf2in the cytoplasm and nucleus,and immunofluorescence staining was used to observe the nuclear translocation of Nrf2.Results :Compared with the control group,2.5,5and 10μmol /L cisplatin could inhibit the activity and migration ability of HeLa cells (P <0.05),and the inhibitory effect increased with the increase of drug concentration(P <0.05).Compared with the control group,2.5,5and 10μmol /L cisplatin could promote the expression of LC3B,apoptosis and nuclear translocation of Nrf2in HeLa cells(P <0.05),reduce the protein expression level of Nrf2in the cytoplasm(P <0.05),and increase the protein expression level of Nrf2in thenucleus(P <0.05).Conclusions :Cisplatin promotes autophagy and apoptosis by increasing nuclear translocation of Nrf2in HeLa human cervical cancer cells,and inhibits cell viability and migration ability.[Key words ]HeLa human cervical cancer cells;cisplatin;Nrf2;autophagy;apoptosis 虽然近年来我国宫颈癌的死亡率大幅下降,但作为女性常见的恶性肿瘤,我国每年宫颈癌新发病例仍高达13.15万,每年约5.3万女性死于宫颈癌[1]㊂顺铂作为宫颈癌经典的化疗药物,在单药化疗㊁联合其他药物化疗或同步放化疗的应用中都显示出对宫颈癌病人的有益作用[2]㊂自噬广泛参与宫颈癌的发生㊁发展㊁药物耐受等[3],有学者[4]报道顺铂对HeLa 人宫颈癌细胞自噬具有诱导作用,但顺铂对宫颈癌自噬的调节及其可能机制仍值得深入探索㊂本研究从顺铂对HeLa 人宫颈癌细胞中Nrf2入核影响的角度,探讨顺铂通过增加Nrf2的核转位促进细胞自噬相关的细胞凋亡抑制HeLa 细胞生长和迁移的可能机制㊂1 材料与方法1.1 药物与试剂 顺铂(Sigma⁃Aldrich 公司,货号:P4394);MEM 培养基(美国Thermo Fisher Scientific 公司,货号:11095072);胎牛血清(美国ThermoFisher Scientific公司,货号:10099);细胞消化液(美国Thermo Fisher Scientific公司,货号:12605010);青霉素-链霉素(美国Thermo Fisher Scientific公司,货号:15070063);噻唑蓝(MTT)(上海碧云天生物技术有限公司,货号:ST316);结晶紫染色液(上海碧云天生物技术有限公司,货号:C0121); mCherry⁃GFP⁃LC3B融合蛋白腺病毒(上海碧云天生物技术有限公司,货号:C3011);Annexin V⁃FITC细胞凋亡检测试剂盒(上海碧云天生物技术有限公司,货号:C1062M);DAPI染色液(上海碧云天生物技术有限公司,货号:C1006);细胞核蛋白与细胞质蛋白抽提试剂盒(上海碧云天生物技术有限公司,货号:P0027);GAPDH兔多克隆抗体(武汉三鹰生物技术有限公司,货号:10494⁃1⁃AP);Nrf2兔多克隆抗体(武汉三鹰生物技术有限公司,货号:16396⁃1⁃AP);LaminB1兔多克隆抗体(武汉三鹰生物技术有限公司,货号:12987⁃1⁃AP);HRP⁃标记的山羊抗兔IgG(武汉三鹰生物技术有限公司,货号:SA00001⁃2);Fluorescein(FITC)⁃标记的山羊抗兔IgG(武汉三鹰生物技术有限公司,货号:SA00003⁃2);其他试剂为分析纯,购自国药集团化学试剂有限公司㊂1.2 仪器 高速低温离心机(德国Sartorius公司,型号:Centrisart®D⁃16C);光学显微镜(日本Olympus公司,型号:IX83);荧光显微镜(德国Leica 公司,型号:AF6000);酶标仪(美国Thermo Fisher Scientific公司,型号:Multiskan FC);流式细胞仪(美国BD公司,型号:Accuri®C6Plus);电泳电源(美国Bio⁃Rad公司,型号:PowerPac Basic);垂直电泳/转膜槽(美国Bio⁃Rad公司,型号:Mini⁃PROTEAN Tetra);凝胶成像系统(美国Bio⁃Rad公司,型号: BIO⁃RAD Gel Doc XR+)㊂1.3 HeLa人宫颈癌细胞的培养及分组 HeLa人宫颈癌细胞株(产品目录号:TCHu187)购买于中国科学院典型培养物保藏委员会细胞库㊂使用MEM 培养基(90%)和胎牛血清(10%)配制完全培养基,在95%空气和5%CO2的37℃培养箱中培养HeLa人宫颈癌细胞㊂细胞生长至80%亚融合状态时,胰酶消化,分瓶继续培养㊂所有实验使用细胞复苏后4~6代的HeLa人宫颈癌细胞㊂实验分组:对照组㊁顺铂2.5μmol/L组㊁顺铂5μmol/L组及顺铂10μmol/L组㊂1.4 MTT法检测细胞的活性 取HeLa细胞,计数后用MEM完全培养基稀释,使细胞密度为1×105个/毫升,将细胞接种于96孔板,每孔5000个细胞,每组10个复孔,共4组,12h细胞贴壁后弃去原培养液,各组加入含有对应浓度顺铂的完全培养基200μL,对照组加入MEM完全培养基200μL,培养24h后,每孔加入20μL MTT(5mg/mL)试剂,培养4h后,吸弃培养液,加入150μL二甲基亚砜,摇床震荡10min后,使用酶标仪在490nm波长下测定吸光值(OD)㊂将对照组的细胞存活率定义为100%,细胞活性=给药组OD值/对照组OD值×l00%㊂1.5 Transwell实验检测细胞迁移 用含有不同浓度顺铂(0㊁2.5㊁5㊁10μmol/L)的单培养基将HeLa 细胞孵育24h后,收集各组细胞并计数,混匀于MEM单培养基中㊂Transwell小室上室中加入各组细胞5000个,每组6个重复,Transwell小室下室中加入含10%胎牛血清的MEM完全培养基,上室底部浸入下室完全培养基㊂培养24h后,4%多聚甲醛浸泡Transwell上室固定细胞30min,PBS缓冲液清洗2次,使用结晶紫染色液染色5min,再次清洗2次,使用棉签擦去Transwell上室内侧细胞,将小室置于载玻片上,使用倒置光学显微镜,对Transwell 上室外(下)侧的细胞进行拍照㊂1.6 mCherry⁃GFP⁃LC3B融合蛋白腺病毒转染细胞检测自噬 参考mCherry⁃GFP⁃LC3B融合蛋白腺病毒使用说明书,对HeLa细胞进行转染,6孔板中放入细胞爬片,并接种HeLa细胞,每组1块6孔板㊂细胞生长至50%融合时加入1.2mL单培养液,并加入腺病毒,培养24h㊂之后弃去所有培养液并更换为完全培养液后,继续培养24h㊂各组加入含有对应浓度顺铂的完全培养基2mL,对照组加入MEM完全培养基2mL,培养24h㊂4%多聚甲醛固定细胞30min,PBS缓冲液清洗2次后,取出细胞爬片,覆盖在载玻片上后,使用荧光显微镜观察mCherry⁃GFP⁃LC3B的荧光强度并拍照㊂1.7 流式细胞术检测细胞凋亡 用含有不同浓度顺铂(0㊁2.5㊁5㊁10μmol/L)的单培养基将HeLa细胞孵育24h后,收集各组细胞㊂参考Annexin V⁃FITC细胞凋亡检测试剂盒说明书检测HeLa细胞的凋亡率㊂将5~10万重悬细胞离心弃上清后,加入195μL Annexin V⁃FITC结合液,再加入5μL Annexin V⁃FITC,混匀后加入10μL碘化丙啶(PI)染色液,孵育20min,放入流式细胞仪样本架,设置仪器通道后检测并导出结果㊂1.8 免疫蛋白印迹法检测细胞Nrf2的蛋白表达 用含有不同浓度顺铂(0㊁2.5㊁5㊁10μmol/L)的完全培养基将HeLa细胞孵育24h后,收集各组细胞至新EP管内㊂使用细胞核蛋白与细胞浆蛋白提取试剂盒分别提取HeLa细胞的膜蛋白核核蛋白,BCA 法测定蛋白浓度㊂蛋白变性后,取等质量总蛋白(30μg)进行SDS⁃PAGE 电泳㊂之后将凝胶上的蛋白转移至PVDF 膜㊂PVDF 膜使用封闭液封闭2h㊂对于提取的细胞质内的蛋白,在封闭后孵育抗体GAPDH(1∶5000)和Nrf2(1∶500),对于提取的细胞核内的蛋白,在封闭后孵育抗体LaminB1(1∶5000)和Nrf2(1∶500),4℃过夜后洗膜3次㊂HRP 标记的山羊抗兔IgG(1∶10000)孵育2h 后,再次洗膜3次㊂使用凝胶成像系统化学发光成像并保存印迹图片㊂1.9 免疫荧光法检测细胞Nrf2的核转位 6孔板放入细胞爬片,并接种HeLa 细胞,每组1块6孔板㊂用含有不同浓度顺铂(0㊁2.5㊁5㊁10μmol /L)的完全培养基孵育24h 后,4%多聚甲醛固定细胞30min,PBS 缓冲液清洗2次,加入Nrf2(1∶100)抗体4℃孵育过夜㊂PBS 缓冲液清洗2次,加入FITC 标记的山羊抗兔IgG(1∶500)孵育2h 后,再次使用PBS 清洗2次,加入DPAI 染细胞核5min㊂使用PBS 清洗细胞3次,取出细胞爬片,覆盖在载玻片上,使用荧光显微镜观察Nrf2的荧光强度并拍照㊂1.10 统计学方法 采用方差分析和q 检验㊂2 结果2.1 顺铂抑制HeLa 人宫颈癌细胞的生长及迁移 与对照组相比,2.5㊁5㊁10μmol /L 的顺铂均可抑制HeLa 细胞的活性及细胞迁移能力(P <0.05),且随着药物浓度增高抑制作用增强(P <0.05)(见图1㊁表1)㊂2.2 顺铂促进HeLa 人宫颈癌细胞的自噬和细胞凋亡 使用mCherry⁃GFP⁃LC3B 融合蛋白腺病毒转染HeLa 细胞,采用荧光显微镜观察自噬相关蛋白LC3B 的表达情况,结果显示,与对照组相比,2.5㊁5㊁10μmol /L 的顺铂均可促进HeLa 细胞中LC3B 的表达(P <0.05)(见图2㊁表2)㊂使用流式细胞术检测各组HeLa 细胞的凋亡情况,结果显示,与对照组相比,2.5㊁5㊁10μmol /L 的顺铂均可促进HeLa 细胞的凋亡(P <0.05)(见图2㊁表2)㊂2.3 顺铂促进HeLa 人宫颈癌细胞中Nrf2的入核 免疫蛋白印迹结果显示,与对照组相比,2.5㊁5㊁10μmol /L 的顺铂均可降低HeLa 细胞的细胞质中Nrf2的蛋白表达水平(P <0.05),并升高细胞核中Nrf2的蛋白表达水平(P <0.05)(见图3A㊁表3)㊂使用免疫荧光法观察各组细胞中Nrf2的入核情况,结果显示,与对照组相比,2.5㊁5㊁10μmol /L 的顺铂均可促进HeLa 人宫颈癌细胞的中Nrf2的核转位(P <0.05)(见图3B㊁表3)㊂ 表1 顺铂对HeLa人宫颈癌细胞的细胞活性及迁移能力的影响(x±s)分组细胞存活率/%(n i=10)迁移细胞数/个(n i=6)对照组100.0±5.6213.0±14.5顺铂2.5μmol/L组75.5±4.1*107.0±9.9*顺铂5.0μmol/L组63.0±4.3*#83.7±6.1*#▲顺铂10.0μmol/L组40.6±4.6*#▲59.5±7.1*#▲F281.72279.04P<0.01<0.01MS组内21.84798.391 q检验:与对照组比较*P<0.05;与顺铂2.5μmol/L组比较#P <0.05;与顺铂5.0μmol/L组比较▲P<0.053 讨论 研究[5-6]表明,癌细胞中Nef2的激活可促进癌症的进展和转移,并且会导致化疗和放疗敏感性的降低[7-8]㊂Nrf2在肿瘤中具有刺激肿瘤细胞增殖㊁抑制肿瘤细胞凋亡㊁增强肿瘤细胞复制潜力和肿瘤血管生产㊁增强肿瘤细胞对组织的侵袭和转移㊁促进炎症和增加基因突变等作用[9]㊂Nrf2在癌症中广泛的作用表明靶向Nrf2可能是治疗肿瘤的有效方法,如Nrf2的抑制剂可用于癌症治疗[9]㊂未来Nrf2可能会成为癌症的预后指标和治疗靶点㊂研究[10]显示Nrf2的高表达会增加肿瘤细胞的抗氧化能力,核内Nrf2高表达宫颈癌细胞具有增加的恶性潜能,Nrf2可能是宫颈癌病人预后不良的标志㊂有学者[11]报道,宫颈癌病变程度的升级增加Nrf2的核水平,并增强肿瘤细胞抗氧化反应中下游蛋白的表达㊂此外,有研究[12]证实辣椒素通过抑制宫颈癌细胞中Nrf2的表达抑制宫颈癌细胞的迁移与侵袭㊂本研究结果显示,顺铂作用于HeLa人宫颈癌细胞后,细胞核中的Nrf2蛋白水平升高,但降低了细胞质中的Nrf2水平㊂作为宫颈癌常用的化疗药物,顺铂对各类肿瘤的作用被广泛报道,包括其对宫颈癌细胞的抑制作用[13]㊂本研究结果还表明,顺铂对HeLa细胞的生长与迁移具有抑制作用,这些结果与前人的报道相一致㊂关于顺铂通过抑制Nrf2调节HeLa人宫颈癌细胞生长与迁移的潜在机制,本研究主要关注了顺铂对HeLa人宫颈癌细胞自噬依赖性的细胞凋亡的影响㊂据报道[14],自噬在化疗药物发挥作用中有重要的作用㊂有证据[15-16]显示,宫颈癌细胞中自噬作用的增强促进了宫颈癌细胞的凋亡,但也有研究[17-18]证实阻断自噬增加了耐药宫颈癌细胞对顺铂的敏感性㊂本研究结果显示,顺铂促进HeLa细胞的自噬并增加细胞凋亡㊂ 表2 顺铂对HeLa细胞自噬及凋亡的影响(n i=6;x±s)分组GFP⁃LC3B阳性面积/%mCherry⁃LC3B阳性面积/%细胞凋亡率/%对照组 5.4±1.5 3.2±1.87.3±0.2顺铂2.5μmol/L组22.0±1.4*20.4±1.5*20.6±0.2*顺铂5.0μmol/L组22.9±1.8*22.1±1.6*63.1±0.6*#顺铂10.0μmol/L组29.5±3.6*#▲28.1±3.2*#▲68.4±0.3*#▲F126.62150.2544835.04P<0.01<0.01<0.01MS组内 5.004 4.5520.124 q检验:与对照组比较*P<0.05;与顺铂2.5μmol/L组比较#P<0.05;与顺铂5.0μmol/L组比较▲P<0.05 表3 顺铂对HeLa细胞中Nrf2表达的影响(n i=6;x±s)分组细胞质Nrf2蛋白相对表达水平细胞质Nrf2蛋白相对表达水平细胞核内Nrf2荧光强度(总光密度)对照组 3.7±0.3 1.1±0.1411.0±30.3顺铂2.5μmol/L 2.2±0.1* 2.2±0.1*1438.7±69.0*顺铂5.0μmol/L 2.2±0.2* 3.9±0.2*#1730.5±37.5*#顺铂10.0μmol/L 1.1±0.1*#▲ 3.9±0.3*#2163.3±57.9*#▲F219.46351.791278.08P<0.01<0.01<0.01MS组内0.0310.0322607.613 q检验:与对照组比较*P<0.05;与顺铂2.5μmol/L组比较#P<0.05;与顺铂5.0μmol/L组比较▲P<0.05 综上,顺铂通过增加HeLa人宫颈癌细胞中Nrf2入核促进细胞自噬和细胞凋亡,并抑制细胞的活性和迁移能力㊂[参考文献][1] 许驰,何玉.宫颈癌筛查方法的现状及进展[J].蚌埠医学院学报,2018,43(11):1538.[2] 周莉,陆安伟.局部晚期宫颈癌治疗的争议与对策[J].中国实用妇科与产科杂志,2019,35(10):1116.[3] 何白云,王艳林,黄利鸣.细胞自噬与宫颈癌关系的研究进展[J].现代妇产科进展,2018,27(2):149.[4] 邹霞,郑建英,赖开发,等.RAC3激活Akt通路促进宫颈癌对顺铂耐药的实验研究[J].临床肿瘤学杂志,2020,25(6):516.[5] TAO S,ROJO DE LA VEGA M,CHAPMAN E,et al.The effects ofNRF2modulation on the initiation and progression of chemicallyand genetically induced lung cancer[J].Mol Carcinog,2018,57(2):182.[6] 周燕妮,章宏,张玉媛.Nrf2在镉诱导的氧化损伤和致癌中保护作用的研究进展[J].蚌埠医学院学报,2017,42(8):1149.[7] PADMANABHAN B,TONG KI,OHTA T,et al.Structural basis fordefects of Keap1activity provoked by its point mutations in lungcancer[J].Mol Cell,2006,21(5):689.[8] 邰宵辉,张玲芳,张旭霞,等.Nrf2/ARE信号通路及其在肿瘤发生发展中作用的研究进展[J].现代肿瘤医学,2021,29(17):3113.(下转第310页)Parkinson′s disease pathology[J].Neuroscientist,2020,27(4):340.[7] 史晓燕,李京涛.内质网应激及其对肝纤维化调控作用的研究进展[J].中华肝脏病杂志,2018,26(11):865. [8] PASQUALE ED,Condorelli G,PASQUALE,CHEVET E.Endoplasmic reticulum stress at the crossroads of progeria andatherosclerosis[J].Embo Molecular Medicine,2019,11(4):e10360.[9] LARA⁃GUZMAN OJ,GIL⁃IZQUIERDO A,MEDINA S,et al.Oxidized LDL triggers changes in oxidative stress andinflammatory biomarkers in human macrophages[J].Redox Biol,2018,15(1):11.[10] ZHOU AX,TABAS IRA.The UPR in atherosclerosis[J].SeminImmunopathol,2013,35(3):321.[11] YANG X,YIN M,YU L,et al.Simivastatin inhibited oxLDL⁃induced proatherogenic effects through calpain⁃1/PPARγ/CD36pathway[J].Can J Physiol Pharmacol,2016,94(12):1. [12] WEINSTOCK A,FISHER EA.Methods to study monocyte andmacrophage trafficking in atherosclerosis progression andresolution[J].Methods Mol Biol,2019,1951:153.[13] WANG D,YANG Y,LEI Y,et al.Targeting foam cell formation inatherosclerosis:therapeutic potential of natural products[J].Pharmacol Rev,2019,71(4):596.[14] HERNANDEZ⁃TRUJILLO Y,RODRIGUEZ⁃ESPARRAGON F,MACIAS⁃RERES A,et al.Rosiglitazone but not losartan preventsNrf⁃2dependent CD36gene expression up⁃regulation in an in vivoatherosclerosis model[J].Cardiovascular Diabetology,2008,7(1):3.[15] 胥亚.TLR4/NF⁃κB通路在oxLDL/β2GPI/anti⁃β2GPI复合物诱导小鼠巨噬细胞泡沫化中的作用探讨[D].镇江:江苏大学,2014.[16] ZHANG P,ZHOU H,HE C,et al.OxLDL/β2GPⅠ/β2GPⅠ⁃Abcomplex in regulating the phenotypic transformation of A7r5andthe expression of lipid transporters[J].Chin J Clin Lab Sci,2019,37(3):195.[17] 杨金伟,赵灿,刘秀,等.左归降糖舒心方含药血浆对ox⁃LDL诱导小鼠巨噬细胞泡沫化和凋亡的影响[J].南京中医药大学学报,2020,36(3):96.(本文编辑 赵素容)(上接第305页)[9] ROJO DE LA VEGA M,CHAPMAN E,ZHANG DD.NRF2andthe hallmarks of cancer[J].Cancer Cell,2018,34(1):21. [10] MA JQ,TUERSUN H,JIAO SJ,et al.Functional role of NRF2incervical carcinogenesis[J].PLoS One,2015,10(8):e0133876.[11] MA X,ZHANG J,LIU S,et al.Nrf2knockdown by shRNAinhibits tumor growth and increases efficacy of chemotherapy incervical cancer[J].Cancer Chemother Pharmacol,2012,69(2):485.[12] ZHANG Q,YANG D.Allicin suppresses the migration andinvasion in cervical cancer cells mainly by inhibiting NRF2[J].Exp Ther Med,2019,17(3):1523.[13] 毛万丽,李杰慧,冉立.宫颈癌顺铂耐药研究进展[J].现代肿瘤医学,2021,29(16):2927.[14] CHUDE CI,AMARAVADI RK.Targeting autophagy in cancer:update on clinical trials and novel inhibitors[J].Int J Mol Sci,2017,18(6):1279.[15] DAVIS MA,DELANEY JR,PATEL CB,et al.Nelfinavir iseffective against human cervical cancer cells in vivo:a potentialtreatment modality in resource⁃limited settings[J].Drug DesDevel Ther,2016,10:1837.[16] 阎臻,付晓瑞,李新敏,等.HRAS基因表达对宫颈癌细胞自噬及凋亡作用及机制[J].青岛大学学报(医学版),2021,57(2):240.[17] LI N,ZHANG W.Protein kinase C beta inhibits autophagy andsensitizes cervical cancer Hela cells to cisplatin[J].Biosci Rep,2017,37(2):BSR20160445.[18] LIN WM,LI ZG.Blockage of cisplatin⁃induced autophagysensitizes cervical cancer cells to cisplatin[J].Genet Mol Res,2015,14(4):16905.(本文编辑 赵素容)。
细胞角蛋白(广谱)抗体试剂(免疫组织化学)说明书

1. 供专业人员使用。 2. 该产品中含有叠氮钠 (NaN3),纯品具有高度的化学毒性。产品中叠氮钠的浓度虽然不能被认定为危险
性浓度,但是叠氮钠可以与铅、铜发生化学反应,形成具有爆炸性危险的叠氮化金属物质。处理时需 要用大量清水冲洗,防止管道中形成金属叠氮化物质。 3. 与所有生物来源的产品相同,必须遵循相关操作步骤。 4. 穿戴合适的个人防护装置,避免皮肤和眼睛接触。 5. 请按照当地、地区以及国家的相关法规,处理未使用的溶液。
【主要组成成份】
1. 提供的试剂 即用型单克隆小鼠抗体以液体形式提供,缓冲液中含有稳定蛋白和0.015 mol/L NaN3。 克隆:AE1/AE3 同型:IgG1,kappa 2.免疫原 人体表皮细胞愈伤组织 (1)。 3.特异性 AE1/AE3 包括两种单克隆抗体,采用人愈伤组织细胞角蛋白免疫小鼠的方法得到 (2)。研究显示, AE1/AE3 能够识别大部分人细胞角蛋白,因此可以作为 IHC 中单层和复层上皮来源的判定工具 (1,2,4)。 抗体 AE1 能够与大部分亚组 A 细胞角蛋白的抗原决定簇发生反应,包括 Moll 分型(4) 中的 10、13、14、 15、16 和 19(分子量分别为 56.5、54'、50、50'、48 和 40 kDa),但是不包括编号为 12、17 和 18(分 子量分别为 55、47 和 45 kDa)的细胞角蛋白(4)。抗体 AE3 能够与亚组 B 细胞角蛋白的抗原决定簇发生 反应,包括编号为 1 和 2、3、4、5、6、7 和 8(分子量分别为 65、67、64、59、58、56、54 和 52 kDa) 的细胞角蛋白(5)。
5. Eichner R, Bonitz P, Sun T-T. Classification of epidermal keratins according to their immunoreactivity, isoelectric point and mode of expression. J Cell Biol 1984; 98:1388
Isoniazid_Microbiology_CAS号54-85-3说明书_AbMole中国
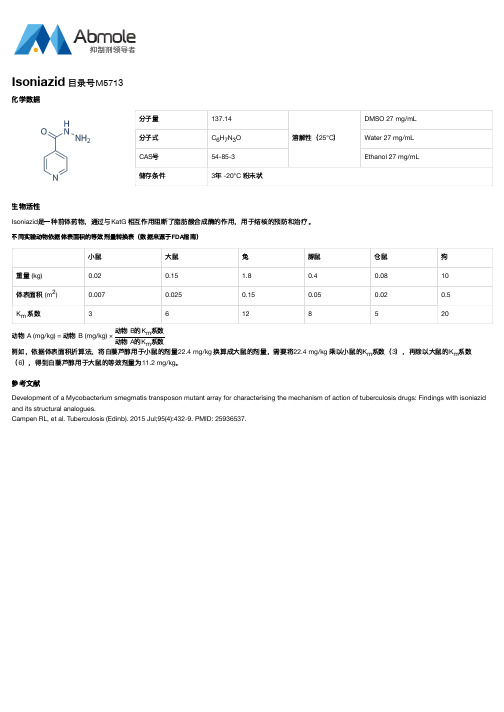
分子量137.14溶解性(25°C)DMSO 27 mg/mL分子式C H N O Water 27 mg/mLCAS号54-85-3Ethanol 27 mg/mL储存条件3年 -20°C 粉末状生物活性Isoniazid是一种前体药物,通过与KatG 相互作用阻断了脂肪酸合成酶的作用,用于结核的预防和治疗。
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)小鼠大鼠兔豚鼠仓鼠狗重量 (kg)0.020.15 1.80.40.0810体表面积 (m)0.0070.0250.150.050.020.5K系数36128520动物 A (mg/kg) = 动物 B (mg/kg) ×动物 B的K系数动物 A的K系数例如,依据体表面积折算法,将白藜芦醇用于小鼠的剂量22.4 mg/kg 换算成大鼠的剂量,需要将22.4 mg/kg 乘以小鼠的K系数(3),再除以大鼠的K系数(6),得到白藜芦醇用于大鼠的等效剂量为11.2 mg/kg。
参考文献Development of a Mycobacterium smegmatis transposon mutant array for characterising the mechanism of action of tuberculosis drugs: Findings with isoniazid and its structural analogues.Campen RL, et al. Tuberculosis (Edinb). 2015 Jul;95(4):432-9. PMID: 25936537.Isoniazid 目录号M5713化学数据6732mmmm m。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Product Name:
Isoniazid CAS No.:
54-85-3Cat No :HY-B0329
Product Data Sheet
Cat. No.:
HY B0329MWt:
137.14Formula:
C6H7N3O Purity :>98%
DMSO 282mg/mL;Water 28
2
Solubility:Mechanisms:
Biological Activity:
Pathways:Anti-infection; Target:Antibacterial DMSO 28.2 mg/mL; Water 28.2mg/mL
Isoniazid is an antibacterial agent used primarily as a tuberculostatic.
Target: Antibacterial Isoniazid is a prodrug and must be activated by a bacterial catalase-peroxidase enzyme that in M.tuberculosis is called KatG [1]. KatG couples the isonicotinic acyl with NADH to form isonicotinic acyl-NADH complex. This complex binds tightly to the enoyl-acyl carrier protein reductase known as InhA, thereby blocking the natural enoyl-AcpM substrate and the action of fatty acid synthase. This process inhibits the synthesis of mycolic acid, required for the mycobacterial cell wall. A range of radicals are produced by KatG activation of isoniazid, including nitric oxide, which has also been shown to be important in the action of another antimycobacterial prodrug PA 824[23]Isoniazid is References:
[1]. Suarez, J., et al., An oxyferrous heme/protein-based radical intermediate is catalytically
competent in the catalase reaction of Mycobacterium tuberculosis catalase-peroxidase (KatG). J Biol
Chem, 2009. 284(11): p. 7017-29.[2]Ti i G S t l Nit i id t d f i i id ti ti b K tG f it i shown to be important in the action of another antimycobacterial prodrug PA-824 [2, 3]. Isoniazid is bactericidal to rapidly dividing mycobacteria, but is bacteriostatic if the mycobacteria ...
[2]. Timmins, G.S., et al., Nitric oxide generated from isoniazid activation by KatG: source of nitric oxide and activity against Mycobacterium tuberculosis. Antimicrob Agents Chemother, 2004. 48(8):
p. 3006-9.[3]. Singh, R., et al., PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO
release. Science, 2008. 322(5906): p. 1392-5.[4]. Ahmad, Z., et al., Biphasic kill curve of isoniazid reveals the presence of drug-tolerant, not drug- resistant, Mycobacterium tuberculosis in the guinea pig. J Infect Dis, 2009. 200(7): p. 1136-43....
Caution: Not fully tested. For research purposes only
Medchemexpress LLC
18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
