Tedizolid_856866-72-3_MSDS_MedChemExpress
吲哚英文版MSDS

MSDS29.10.2015 SECTION 1:Identification of the substance/mixture and of the company/undertaking1.1 Product identifiersProduct name :IndoleProduct Number : I3408Brand : AldrichREACH No. : A registration number is not available for this substance as the substanceor its uses are exempted from registration, the annual tonnage does notrequire a registration or the registration is envisaged for a laterregistration deadline.CAS-No. : 120-72-91.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses : Laboratory chemicals, Manufacture of substances1.3 Details of the supplier of the safety data sheetCompany : HuBei SiBo Technology Co.,Ltd.FanHu Industrial parkPanJiaWan Town437200 XianNing Hubei,CHINA湖北斯柏生物科技有限公司湖北省咸宁市嘉鱼县潘家湾镇畈湖工业园邮政编码:437200Telephone : +86 0715-*******Fax : +86 0715-*******1.4 Emergency telephone numberEmergency Phone # : +86 0715-*******SECTION 2: Hazards identification2.1 Classification of the substance or mixtureClassification according to Regulation (EC) No 1272/2008Acute toxicity, Oral (Category 4), H302Acute toxicity, Dermal (Category 3), H311Skin irritation (Category 2), H315Serious eye damage (Category 1), H318Specific target organ toxicity - single exposure (Category 3), H335Acute aquatic toxicity (Category 1), H400For the full text of the H-Statements mentioned in this Section, see Section 16. Classification according to EU Directives 67/548/EEC or 1999/45/ECXn, N Harmful, Dangerous for theenvironmentR21/22, R37/38, R41, R50/53For the full text of the R-phrases mentioned in this Section, see Section 16.2.2 Label elementsLabelling according Regulation (EC) No 1272/2008Signal word DangerHazard statement(s)H302 Harmful if swallowed.H311 Toxic in contact with skin.H315 Causes skin irritation.H318 Causes serious eye damage.H335 May cause respiratory irritation.H400 Very toxic to aquatic life.Precautionary statement(s)P261 Avoid breathing dust.P273 Avoid release to the environment.P280 Wear protective gloves/ eye protection/ face protection.P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.P312 Call a POISON CENTER or doctor/ physician if you feel unwell.Supplemental HazardStatementsnone2.3 Other hazardsStench.SECTION 3: Composition/information on ingredients3.1 SubstancesSynonyms : 1H-Benzo[b]pyrroleFormula : C8H7NMolecular Weight : 117,15 g/molCAS-No. : 120-72-9EC-No. : 204-420-7Hazardous ingredients according to Regulation (EC) No 1272/2008Component Classification ConcentrationIndoleAcute Tox. 4; Acute Tox. 3;Skin Irrit. 2; Eye Dam. 1;STOT SE 3; Aquatic Acute 1;H302, H311, H315, H318,H335, H400-Hazardous ingredients according to Directive 1999/45/ECComponent Classification ConcentrationIndoleXn, N, R21/22 - R37/38 - R41- R50/53-For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16SECTION 4: First aid measures4.1 Description of first aid measuresGeneral adviceConsult a physician. Show this safety data sheet to the doctor in attendance.If inhaledIf breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician. In case of skin contactWash off with soap and plenty of water. Take victim immediately to hospital. Consult a physician.In case of eye contactRinse thoroughly with plenty of water for at least 15 minutes and consult a physician.If swallowedNever give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2) and/or insection 114.3 Indication of any immediate medical attention and special treatment neededno data availableSECTION 5: Firefighting measures5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, alcohol-resistant foam, dry chemical or carbon dioxide.5.2 Special hazards arising from the substance or mixtureCarbon oxides, nitrogen oxides (NOx)5.3 Advice for firefightersWear self contained breathing apparatus for fire fighting if necessary.5.4 Further informationno data availableSECTION 6: Accidental release measures6.1 Personal precautions, protective equipment and emergency proceduresWear respiratory protection. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust.For personal protection see section 8.6.2 Environmental precautionsPrevent further leakage or spillage if safe to do so. Do not let product enter drains. Discharge into the environment must be avoided.6.3 Methods and materials for containment and cleaning upPick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed containers for disposal.6.4 Reference to other sectionsFor disposal see section 13.SECTION 7: Handling and storage7.1 Precautions for safe handlingAvoid contact with skin and eyes. Avoid formation of dust and aerosols.Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire protection.For precautions see section 2.2.7.2 Conditions for safe storage, including any incompatibilitiesStore in cool place. Keep container tightly closed in a dry and well-ventilated place.Air and light sensitive.7.3 Specific end use(s)A part from the uses mentioned in section 1.2 no other specific uses are stipulatedSECTION 8: Exposure controls/personal protection8.1 Control parametersComponents with workplace control parameters8.2 Exposure controlsAppropriate engineering controlsAvoid contact with skin, eyes and clothing. Wash hands before breaks and immediately after handling the product.Personal protective equipmentEye/face protectionFace shield and safety glasses Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).Skin protectionHandle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands.The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC andthe standard EN 374 derived from it.Full contactMaterial: Nitrile rubberMinimum layer thickness: 0,11 mmBreak through time: 480 minMaterial tested:Dermatril® (KCL 740 / Aldrich Z677272, Size M)Splash contactMaterial: Nitrile rubberMinimum layer thickness: 0,11 mmBreak through time: 480 minMaterial tested:Dermatril® (KCL 740 / Aldrich Z677272, Size M)datasource:KCLGmbH,D-36124Eichenzell,phone+49(0)665987300,******************,test method: EN374If used in solution, or mixed with other substances, and under conditions which differ from EN 374, contact the supplier of the CE approved gloves. This recommendation is advisory only and mustbe evaluated by an industrial hygienist and safety officer familiar with the specific situation of anticipated use by our customers. It should not be construed as offering an approval for any specific use scenario.Body ProtectionComplete suit protecting against chemicals, The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Respiratory protectionWhere risk assessment shows air-purifying respirators are appropriate use a full-face particle respirator type N100 (US) or type P3 (EN 143) respirator cartridges as a backup to engineering controls. If the respirator is the sole means of protection, use a full-face supplied air respirator. Use respirators and components tested and approved under appropriate government standards suchas NIOSH (US) or CEN (EU).Control of environmental exposurePrevent further leakage or spillage if safe to do so. Do not let product enter drains. Discharge into the environment must be avoided.SECTION 9: Physical and chemical properties9.1 Information on basic physical and chemical propertiesa) Appearance Form: flakesColour: light brownb) Odour no data availablec) Odour Threshold no data availabled) pH no data availablee) Melting point/freezingpointMelting point/range: 51 - 54 °C - lit.f) Initial boiling point andboiling range253 - 254 °C - lit.g) Flash point 121 °C - closed cuph) Evapouration rate no data availablei) Flammability (solid, gas) no data availablej) Upper/lowerflammability orexplosive limitsno data availablek) Vapour pressure no data availablel) Vapour density no data availablem) Relative density no data availablen) Water solubility no data availableo) Partition coefficient: noctanol/waterno data availablep) Auto-ignitiontemperatureno data availableq) Decompositiontemperatureno data availabler) Viscosity no data availables) Explosive properties no data availablet) Oxidizing properties no data available9.2 Other safety informationno data availableSECTION 10: Stability and reactivity 10.1 Reactivityno data available10.2 Chemical stabilityStable under recommended storage conditions. 10.3 Possibility of hazardous reactions no data available10.4 Conditions to avoidAir Light.10.5 Incompatible materialsStrong oxidizing agents, Iron and iron salts.10.6 Hazardous decomposition products Other decomposition products - no data available In the event of fire: see section 5SECTION 11: Toxicological information11.1 Information on toxicological effectsAcute toxicityLD50 Oral - rat - 1.000 mg/kgLD50 Dermal - rabbit - 790 mg/kgSkin corrosion/irritationSerious eye damage/eye irritationEyes - rabbitResult: Severe eye irritation - 24 hRespiratory or skin sensitisationno data availableGerm cell mutagenicityno data availableCarcinogenicityCarcinogenicity - mouse - SubcutaneousTumorigenic:Equivocal tumorigenic agent by RTECS criteria. Lungs, Thorax, or Respiration:Tumors. LeukaemiaCarcinogenicity - mouse - SubcutaneousTumorigenic:Equivocal tumorigenic agent by RTECS criteria. Tumorigenic:Tumor types after systemic administration not seen spontaneously.IARC: No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.Reproductive toxicityno data availableSpecific target organ toxicity - single exposureInhalation - May cause respiratory irritation.Specific target organ toxicity - repeated exposureno data availableAspiration hazardno data availableAdditional InformationRTECS: NL2450000Nausea, Headache, V omiting, To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly investigated.SECTION 12: Ecological information12.1 ToxicityToxicity to daphnia andother aquaticinvertebratesLC50 - Daphnia magna (Water flea) - 1 mg/l - 48 hToxicity to algae Growth inhibition EC100 - Scenedesmus acuminatus - > 10 mg/l - 96 h12.2 Persistence and degradabilityno data available12.3 Bioaccumulative potentialno data available12.4 Mobility in soilno data available12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment not available as chemical safety assessment not required/not conducted 12.6 Other adverse effectsVery toxic to aquatic life.SECTION 13: Disposal considerations13.1 Waste treatment methodsProductOffer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. Contaminated packagingDispose of as unused product.SECTION 14: Transport information14.1 UN numberADR/RID: 2811 IMDG: 2811 IATA: 281114.2 UN proper shipping nameADR/RID: TOXIC SOLID, ORGANIC, N.O.S. (Indole)IMDG: TOXIC SOLID, ORGANIC, N.O.S. (Indole)IATA: Toxic solid, organic, n.o.s. (Indole)14.3 Transport hazard class(es)ADR/RID: 6.1 IMDG: 6.1 IATA: 6.114.4 Packaging groupADR/RID: III IMDG: III IATA: III14.5 Environmental hazardsADR/RID: yes IMDG Marine pollutant: yes IATA: no14.6 Special precautions for userno data availableSECTION 15: Regulatory informationThis safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.15.1 Safety, health and environmental regulations/legislation specific for the substance or mixtureno data available15.2 Chemical Safety AssessmentFor this product a chemical safety assessment was not carried outSECTION 16: Other informationFull text of H-Statements referred to under sections 2 and 3.Acute Tox. Acute toxicityAquatic Acute Acute aquatic toxicityEye Dam. Serious eye damageH302 Harmful if swallowed.H311 Toxic in contact with skin.H315 Causes skin irritation.H318 Causes serious eye damage.H335 May cause respiratory irritation.H400 Very toxic to aquatic life.Skin Irrit. Skin irritationSTOT SE Specific target organ toxicity - single exposureFull text of R-phrases referred to under sections 2 and 3N Dangerous for the environmentXn HarmfulR21/22 Harmful in contact with skin and if swallowed.R37/38 Irritating to respiratory system and skin.R41 Risk of serious damage to eyes.R50/53 Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.。
洋甘菊提取液MSDS英文版

1. IDENTIFICATION OF THE SUBSTANCE/TREPARATION AND THE COMPANY/UNDERTAKING3.HAZARDS IDENTIFICATION4. FIRST AID MEASURESMATERIAL SAFETY DATA SHEETProduct name:Supplier:Tel:EMERGENCY OVERVIEW: May cause skin irritation and/or dermatitisPrinciple routes of exposure: Inhalation: Ingestion: Skin contact: Eye contact:SkinMay cause irritation of respiratory tract May be harmful if swallowed May cause allergic skin reaction Avoid contact with eyesStatements of hazard MAY CAUSE ALLERGIC SKIN REACTION.Statements of Spill of Leak Label Eliminate all ignition sources. Absorb and/or contain spill with inert materials (e.g., sand, vermiculite). Then place in appropriate container. For large spills, use water spray to disperse vapors, flush spill area. Prevent runoff from entering waterways or sewers.General advice:POSITION/INFORMATION ON INGREDIENTSInhalation:Skin contact:Ingestion:Eye contact:Protection of first – aiders:Medical conditions aggravated by exposure: In the case of accident or if you fell unwell, seek medical advice immediately (show the label where possible).Move to fresh air, call a physician immediately.Rinse immediately with plenty of water and seek medical adviceDo not induce vomiting without medical advice.In the case of contact with eyes, rinse immediately with plenty of water and seek medical advice.No information availableNone knownSuitable extinguishing media:Specific hazards:Special protective equipment for firefighters:Flash point:Autoignition temperature:NFPA rating Use dry chemical, CO2, water spray or “alcohol” foam Burning produces irritant fumes.As in any fire, wear self-contained breathing apparatus pressure-demand, MSHA/NIOSH (approved or equivalent) and full protective gearNot determinedNot determinedNFPA Health: 1 NFPA Flammability: 1 NFPA Reactivity: 0Personal precautions: Environmental precautions: Methods for cleaning up: Use personal protective equipment.Prevent product from entering drains.Sweep up and shovel into suitable containers for disposalStorage:7. HANDLING AND STORAGE5.FIRE-FIGHTING MEASURES6. ACCIDENTAL RELEASE MEASURESRoom temperature Handling:Safe handling advice: Incompatible products:Use only in area provided with appropriate exhaust ventilation.Wear personal protective equipment.Oxidising and spontaneously flammable productsEngineering measures: Respiratory protection: Skin and body protection:Eye protection: Hand protection: Hygiene measures:Ensure adequate ventilation.Breathing apparatus only if aerosol or dust is formed. Usual safety precautions while handling the product will provide adequate protection against this potential effect. Safety glasses with side-shieldsPVC or other plastic material glovesHandle in accordance with good industrial hygiene and safety practice.Melting point/range: Boiling point/range: Density: Vapor pressure: Evaporation rate: Vapor density: Solubility (in water): Flash point:Autoignition temperature:No Data available at this time. No Data available at this time. No data available No data available No data available No data available No data available Not determined Not determinedStability: Stable under recommended storage conditions. Polymerization: None under normal processing.Hazardous decomposition products: Thermal decomposition can lead to release of irritating gases and vapours such as carbon oxides.Materials to avoid: Strong oxidising agents.10. STABILITY AND REACTIVITY9. PHYSICAL AND CHEMICAL PROPERTIES8. EXPOSURE CONTROLS/PERSONAL PROTECTION11. TOXICOLOGICAL INFORMATIONConditions to avoid: Exposure to air or moisture over prolonged periods.Product information Acute toxicityChronic toxicity:Local effects: Chronic exposure may cause nausea and vomiting, higher exposure causes unconsciousness.Symptoms of overexposure may be headache, dizziness, tiredness, nausea and vomiting.Specific effects:May include moderate to severe erythema (redness) and moderate edema (raised skin), nausea, vomiting,headache.Primary irritation: Carcingenic effects: Mutagenic effects: Reproductive toxicity:No data is available on the product itself. No data is available on the product itself. No data is available on the product itself. No data is available on the product itself.Mobility:Bioaccumulation: Ecotoxicity effects: Aquatic toxicity:No data available No data available No data availableMay cause long-term adverse effects in the aquatic environment.12. ECOLOGICAL INFORMATION13. DISPOSAL CONSIDERATIONSWaste from residues/unused products:Contaminated packaging:Waste disposal must be in accordance with appropriate Federal, State and local regulations. This product, if unaltered by use, may be disposed of treatment at a permitted facility or as advised by your local hazardous waste regulatory authority. Residue from fires extinguished with this material may be hazardous.Do not re-use empty containers.UN/Id No:Not regulated14. TRANSPORT INFFORMATIONDOTProper shipping name: Not regulatedTGD(Canada)WHMIS hazard class: Non - controlledIMDG/IMOIMDG – Hazard Classifications Not ApplicableIMO – labels:15. REGULATORY INFOTMATION International Inventories16. OTHER INFORMATIONPrepared by: Health & SafetyDisclaimer: The information and recommendations contained herein are based upon tests believed to be reliable.However, XABC does not guarantee the accuracy or completeness NOR SHALL ANY OF THIS INFORMATION CONSTITUTE A WARRANTY, WHETHER EXPRESSED OR IMPLIED, AS TO THE SAFETY OF THE GOOD, THE MERCHANTABILITY OF THE GOODS, OR THE FITNESS OF THE FITNESS OF THE GOODS FOR A PARTICULAR PURPOSE. Adjustment to conform to actual conditions of usage maybe required. XABC assumes no responsibility for results obtained or for incidental or consequential damages, including lost profits arising from the use of these data. No warranty against infringement of any patent, copyright or trademark is made or implied.End of safety data sheet。
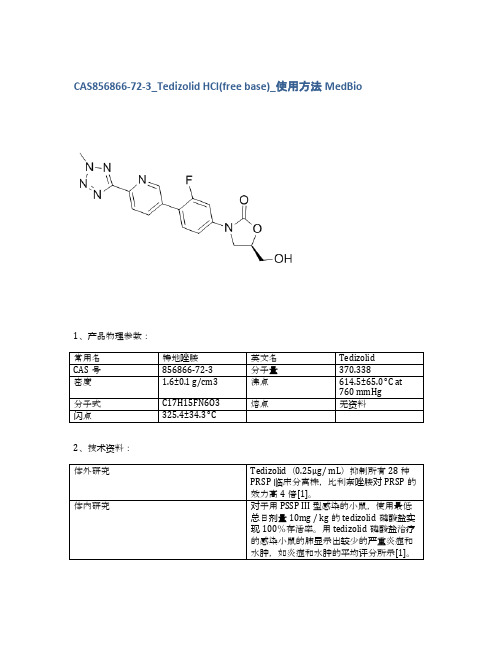
CAS856866-72-3_Tedizolid HCl(free base)_使用方法MedBio
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBห้องสมุดไป่ตู้o
MED15401
Indolmycin
Indolmycin
21200-24-8
1mg
≥98%
Colistin Methanesulfonate (sodium salt)
Colistin Methanesulfonate (sodium salt)
8068-28-8
1g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15145
Tenofovir Disoproxil Fumarate
MED15316
Tedizolid HCl(free base)
Tedizolid HCl(free base)
856866-72-3
5mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15250
Famciclovir
Famciclovir
104227-87-4
100mg
≥98%
品牌
体内研究
对于用PSSP III型感染的小鼠,使用最低总日剂量10mg / kg的tedizolid磷酸盐实现100%存活率。用tedizolid磷酸盐治疗的感染小鼠的肺显示出较少的严重炎症和水肿,如炎症和水肿的平均评分所示[1]。
3、同类产品列表:
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
甲磺酸倍他司汀片治疗耳石症复位后残余头晕的疗效研讨

DOI:10.19368/ki.2096-1782.2023.24.086甲磺酸倍他司汀片治疗耳石症复位后残余头晕的疗效研讨谢书华上海市杨浦区控江医院耳鼻喉科,上海200090[摘要]目的分析在耳石症复位后残余头晕患者中甲磺酸倍他司汀片的治疗效果。
方法选择2021年5月—2023年6月上海市杨浦区控江医院治疗的62例耳石症复位后残余头晕患者作为研究对象,按照治疗方式分为控制组和研究组,各31例。
控制组进行手法复位治疗,研究组在控制组治疗方案基础上给予患者甲磺酸倍他司汀片治疗,对比两组患者的康复指标、脑血流速度、治疗有效率。
结果治疗后,研究组患者的眩晕障碍量表评分、前庭症状指数评分低于控制组,Berg平衡量表评分高于控制组,差异有统计学意义(P均< 0.05)。
研究组患者右椎动脉、左椎动脉、基底动脉的平均血流速度均高于控制组,差异有统计学意义(P均< 0.05)。
研究组患者的总有效率(96.77%)高于控制组(77.42%),差异有统计学意义(χ2=5.166,P=0.023)。
结论在治疗耳石症复位后残余头晕的过程中,使用甲磺酸倍他司汀片价值发挥显著,临床意义深远。
[关键词]耳石症复位后残余头晕;甲磺酸倍他司汀片;疗效[中图分类号]R764 [文献标识码]A [文章编号]2096-1782(2023)12(b)-0086-04Study on the Efficacy of Betahistine Mesylate Tablets in the Treatment of Residual Dizziness after Repositioning of OtolithiasisXIE ShuhuaDepartment of Otorhinolaryngology, Shanghai Yangpu District Kongjiang Hospital, Shanghai, 200090 China[Abstract] Objective To analyze the therapeutic effect of betahistine mesylate tablets in patients with residual dizzi⁃ness after repositioning of otolithiasis. Methods A total of 62 patients with residual dizziness after repositioning of oto⁃lithiasis who were treated at Shanghai Yangpu District Kongjiang Hospital from May 2021 to June 2023 were selected as the study subjects. They were divided into a control group and a study group according to the treatment method, with 31 cases in each group. The control group was treated with manipulative repositioning, and the study group was given betahistine mesylate tablets on the basis of the treatment program of the control group. The rehabilitation indica⁃tors, cerebral blood flow rates, and treatment effectiveness of the two groups of patients were compared. Results After treatment, the vertigo disorder scale score of the study group and vestibular symptom index score were lower than those of the control group, while the Berg balance scale score was higher than that of the control group, and the differ⁃ences were statistically significant (all P<0.05). The mean blood flow velocity of right vertebral artery, left vertebral artery, and basilar artery in the study group were higher than that in the control group, and the differences were sta⁃tistically significant (all P<0.05). The total treatment effectiveness rate of patients in the study group (96.77%) was higher than that in the control group (77.42%), and the difference was statistically significant (χ2=5.166, P=0.023).Conclusion In the treatment of residual dizziness after repositioning of otolithiasis, the use of betahistine mesylate tablets has significant value and profound clinical significance.[Key words] Residual dizziness after repositioning of otolithiasis; Betahistine mesylate tablets; Therapeutic effect近几年,耳石症发病率呈上升趋势,最常见的表现是有强烈的眩晕感,一般持续1 min以内,严重[作者简介] 谢书华(1982-),女,本科,主治医师,研究方向为耳源性眩晕疾病。
STERIS Vaporized Hydrogen Peroxide (VHP) 生物消毒系统及服务

STERIS provides a wide range of vaporized hydrogen peroxide (VHP®) bio-decontamination systems and services, utilizing Vaprox® Sterilant for broad-spectrum efficacy against viruses, bacteria, yeasts, and bacterial spores. Vaporized hydrogenperoxide bio-decontamination is crucial, not only for pharmaceutical and biotechnology production, but also for agricultural industries and healthcare facilities. The STERIS bio-decontamination systems use a “dry process” hydrogenperoxide vapor distribution, which eliminates the risk of condensation on surfaces.The advantages ofdecontamination with vaporized H 2O 2 include:• Easy to use• Effective against biological contaminants• Ideal for low-temperature processes• Processes can be validated • Compatible with a wide variety of materials• Environmentally friendly and safe for operators• Leaves no toxic residue, only water vapor and oxygenA trusted partnerFor several years, STERIS has used a trusted sensing technology from Vaisala. In 2018, STERIS became interested in a new solutionfrom Vaisala: the HPP270-series hydrogen peroxide vapor probe. The probes feature PEROXCAP® sensing technology. The sensors provide accurate measurements for hydrogen peroxideconcentration or ppm (parts per million) and several other parameters, most importantly: relative humidity, temperature, and a new parameter: relativesaturation — which indicates when condensation will occur.Validating bio-decontaminationIn DSVA (surface disinfection by airways) the aim is to prove that bacteria and microorganisms have been eradicated and the results must demonstrate maximumeffectiveness throughout the bio-decontaminated area. To validate a cleanroom bio-decontamination, it is essential that STERIS use a highly accurate sensor that can provide stable, repeatable data on the concentration of hydrogen peroxide vapor ppm. Vaisala’s unique technology met STERIS’s requirements for measurement reliability and repeatability. Thanks to Vaisala, STERIS has been ableSTERIS provides efficient, effective bio-decontamination with accurate vaporized H 2O 2 sensorsSTERIS is a world leader in bio-decontamination and equipmentsterilization for pharmaceutical production. The company has advanced the science of sterilization, cleaning and infection control with solutions that meet the high standards and requirements of its customers. In particular, cleanroom applications used for pharmaceuticals andbiotechnology require a high degree of environmental control. In thesehighly regulated environments, bio-decontamination is crucial and must be controlled, validated, and documented. Case Studyto prove maximum effectiveness of bio-decontamination in clean rooms.“Today, Vaisala is the best technology on the market tomeasure the concentration of H 2O 2 reliably, accurately, and repeatably over time,” says Philippe Muylaert, Room Decontamination Service Specialist with STERIS SAS. “The Vaisala model HPP272 is the most effective sensor for bio-decontamination with hydrogen peroxide. Thus, we haveconfidence in the data, and we can prove to our customers the effectiveness of bio-decontamination cycles.”Muylaert appreciates that the HPP270 probes provide H 2O 2 concentration measurement curves throughout the bio-decontamination process in addition to real-time monitoring data.In-line data throughout a process is valuable for cycle development, especially to help determine pressure binary mixture, water concentration, temperature, etc.In-line measurement of vaporized H 2O 2To remain in a gaseous state,hydrogen peroxide vapor requires controlled parameters, including temperature, relative humidity, pressure and volume. Anydeparture from ideal conditions can cause hydrogen peroxide vapor to condense, essentially returning the H 2O 2 to its natural state: liquid. For STERIS’s dry method, it is necessary to avoid condensation that can lead to equipment deterioration.In the absence of hydrogen peroxide vapor, the relativehumidity of the air is equal to the relative saturation (1). When vaporized H 2O 2 is introduced, the relative saturation is greater than the relative humidity (2).During H 2O 2 vapor bio-decontamination processes, there is always water vapor in addition to hydrogen peroxide vapor. To control condensation, you need to know both the humidity of the air caused by water vapor and by hydrogen peroxide vapor.Relative saturation, which indicates the concentration of vaporizedhydrogen peroxide and water vapor in the air, is the only value thatrepresents both vapors. Monitoring the relative saturation value during a process is therefore crucial, because it indicates saturation point of the combined vapors: water and hydrogen peroxide.Reliable measurements mean reliable processesSTERIS systems required a probe capable of providing accurate measurements for hydrogen peroxide ppm, temperature, relative humidity and relative saturation. Using the unique Vaisala PEROXCAP ® hydrogen peroxide sensor technology, the HPP272 probe can also measure two other parameters: dewpoint and vapor pressure, which can also be useful parameters in bio-decontamination. The probe guarantees reliable and precise hydrogen peroxide measurements throughout thebio-decontamination cycle, even in high humidity.The reliable and reproducible measurements of Vaisala’s vaporized hydrogen peroxide probes allow STERIS to achieve a high degree of confidence in their bio-decontamination procedures, success during annual audits, and a high level of product quality.Please contact us at/contactusScan the code formore informationRef. B212075EN-A ©Vaisala 2020This material is subject to copyright protection, with all copyrights retained by Vaisala and its individual partners. All rights reserved. Any logos and/or product names are trademarks of Vaisala or its individual partners. The reproduction, transfer, distribution or storage of information contained in this brochure in any form without the prior written consent of Vaisala is strictly prohibited. All specifications — technical included — are subject to change without notice.。
抗体公司

赛信通(上海)生物试剂有限公司
上海市浦东南路1101号远东大厦514室,200120 info@cst www.cst 2158356288 公司总部: 美国
Established in Beverly, MA in 1999, Cell Signaling Technology (CST) is a privatelyowned company with over 400 employees worldwide. We are dedicated to providing innovative research tools that are used to help define mechanisms underlying cell function and disease. Since its inception, CST has become the world leader in the production of the highest quality activationstate and total protein antibodies utilized to expand knowledge of cell signaling pathways. Our mission is to deliver the world's highest quality research tools that accelerate progress in biological research and personalized medicine. 总引用数为4670,来自于1966篇文章。最常引用的试剂包括: Akt, ERK2, ERK1, p38, Akt1。
AbD Serotec (BioRad)
恩格列净联合西格列汀治疗老年2_型糖尿病患者的临床疗效分析

·药物与临床·糖尿病新世界 2023年3月DOI:10.16658/ki.1672-4062.2023.05.059恩格列净联合西格列汀治疗老年2型糖尿病患者的临床疗效分析臧道军,龚红燕江苏省常州市德安医院老年内科,江苏常州213000[摘要]目的探讨老年2型糖尿病患者使用恩格列净+西格列汀治疗的临床效果。
方法选取2020年1月—2021年12月常州市德安医院接诊的100例老年2型糖尿病患者作为研究对象,根据不同用药方式分为对照组与研究组,各50例,对照组接受西格列汀治疗,研究组接受恩格列净+西格列汀治疗,就两组患者血糖指标、炎性指标、胱抑素C(Cys-C)、血尿素氮(BUN)、血同型半胱氨酸(Hcy)指标进行比较。
结果治疗前两组血糖指标相比,差异无统计学意义(P>0.05),治疗后,研究组HbA1c、FPG及2 hPG明显低于对照组,差异有统计学意义(P<0.05);治疗前两组炎性指标比较,差异无统计学意义(P>0.05),治疗后,研究组IL-4、IL-6及TNF-α明显低于对照组,差异有统计学意义(P<0.05);治疗前两组Cys-C、BUN及Hcy相比,差异无统计学意义(P>0.05),治疗后,研究组患者Cys-C、BUN及Hcy明显低于对照组,差异有统计学意义(P<0.05)。
结论对于老年2型糖尿病患者开展恩格列净+西格列汀治疗能有效改善血糖指标,降低Hcy,提升肾功能,治疗效果显著。
[关键词] 老年人群;恩格列净;西格列汀;2型糖尿病[中图分类号] R4 [文献标识码] A [文章编号] 1672-4062(2023)03(a)-0059-04Clinical Efficacy Analysis of Empagliflozin Combined with Sitagliptin in the Treatment of Elderly Patients with Type 2 Diabetes MellitusZANG Daojun, GONG HongyanDepartment of Geriatric Medicine, Changzhou De'an Hospital, Changzhou, Jiangsu Province, 213000 China[Abstract] Objective To investigate the clinical effect of treatment with empagliflozin + sitagliptin in elderly patients with type 2 diabetes mellitus.Methods A total of 100 elderly patients with type 2 diabetes mellitus admitted to Chang⁃zhou De'an Hospital from January 2020 to December 2021 were selected as study subjects. The cases were divided into control group and study group according to different medication administration, fifty cases in each. The control group received sitagliptin treatment and the study group received empagliflozin + sitagliptin treatment. The blood glu⁃cose index, inflammatory index, cystatin C (Cys-C), blood urea nitrogen (BUN), and blood homocysteine (Hcy) index were compared between the two groups.Results There was no statistically significant difference in blood glucose in⁃dexes between the two groups before treatment (P>0.05). After treatment, HbA1c, FPG and 2 hPG of the study group were significantly lower than those in the control group, the difference was statistically significant (P<0.05). There was no statistically significant difference in inflammatory indexes between the two groups before treatment (P>0.05). After treatment, IL-4, IL-6 and TNF-α in the study group were significantly lower than those in the control group, the dif⁃ference was statistically significant (P<0.05). There was no statistically significant difference in the Cys-C, BUN and Hcy between the two groups before treatment (P>0.05). After treatment, the Cys-C, BUN and Hcy of the study group were significantly lower than those in the control group, the difference was statistically significant (P<0.05).Conclusion For elderly patients with type 2 diabetes mellitus, treatment with empagliflozin + sitagliptin can effec⁃tively improve blood glucose index, reduce Hcy and enhance renal function, with significant therapeutic effects.[作者简介]臧道军(1974-),男,本科,副主任医师,研究方向为老年内科。
地特胰岛素联合门冬胰岛素治疗妊娠期糖尿病疗效与安全性及对母婴结局的影响研究

DOI:10.16658/ki.1672-4062.2023.18.113地特胰岛素联合门冬胰岛素治疗妊娠期糖尿病疗效与安全性及对母婴结局的影响研究王霞平遥县人民医院产科,山西晋中031100[摘要]目的探讨妊娠期糖尿病(gestational diabetes mellitus, GDM)产妇应用地特胰岛素联合门冬胰岛素治疗的效果。
方法选取2021年7月—2022年9月期间在平遥县人民医院进行分娩的GDM产妇66例为研究对象,按隐匿数字随机法分为单药组(33例,门冬胰岛素治疗),联合组(33例,门冬胰岛素+地特胰岛素治疗),观察记录两组血糖变化、胰岛素水平、母婴结局,进行比较分析。
结果治疗前,两组患者血糖控制水平比较,差异无统计学意义(P>0.05);治疗后,联合组的空腹血糖(fasting plasma glucose, FPG)、餐后2 h血糖(2-hourpostprandial blood glucose,2 hPG)、糖化血红蛋白(glycated hemoglobin, HbA1c)水平均低于单药组,差异有统计学意义(P<0.05);联合组的FPG达标、2 hFPG达标、FPG和2 hFPG均达标的时间均显著短于单药组,差异有统计学意义(P<0.05);联合组的自然分娩率为72.73%显著高于单药组的48.48%,差异有统计学意义(P< 0.05);单药组的不良妊娠结局发生率(24.24%)高于联合组(9.09%),差异无统计学意义(P>0.05)。
结论地特胰岛素联合门冬胰岛素治疗GDM患者,可以获得较为理想的血糖控制效果,能更快的使患者血糖达到理想的标准,自然分娩率更高。
[关键词] 妊娠期糖尿病;地特胰岛素;门冬胰岛素;母婴结局[中图分类号] R714 [文献标识码] A [文章编号] 1672-4062(2023)09(b)-0113-04Study on the Efficacy and Safety of Insulin Detemir Combined with Insu⁃lin Aspart in the Treatment of Gestational Diabetes and Its Impact on Ma⁃ternal and Fetal OutcomesWANG XiaDepartment of Obstetrics, Pingyao County People's Hospital, Jinzhong, Shanxi Province, 031100 China[Abstract] Objective To explore the effect of insulin detemir combined with insulin aspart in the treatment of gesta‐tional diabetes mellitus (GDM). Methods 66 GDM women who gave birth in Pingyao County People's Hospital from July 2021 to September 2022 were selected as research objects. According to the concealed number random method, 33 patients were divided into a single-drug group (treated with insulin aspart) and 33 patients were combination group (treated with insulin aspart+insulin detemir). Observed and recorded the data on blood sugar changes, insulin levels, and maternal and infant outcomes between the two groups for comparative analysis. Results Before treatment, there was no statistically significant difference in blood glucose control levels between the two groups (P>0.05). After treat‐ment, the levels of fasting plasma glucose (FPG), 2-hour postprandial blood glucose (2 hPG), and glycated hemoglobin (HbA1c) in the combination group were lower than those in the single-drug group, the difference was statistically sig‐nificant (P<0.05). The time for FPG to reach the target, 2 hPG to reach the target, and both FPG and 2 hPG to reach the target in the combination group were significantly shorter than those in the single-drug group, the difference were statistically significant (P<0.05). The natural delivery rate in the combination group was 72.73%, which was signifi‐cantly higher than the 48.48% in the single-drug group, the difference was statistically significant (P<0.05). The inci‐dence rate of adverse pregnancy outcomes in the single-drug group (24.24%) was higher than that in the combination group (9.09%), and the difference was statistically significant (P>0.05). Conclusion Insulin detemir combined with in‐sulin aspart can achieve ideal blood sugar control effects in patients with GDM, and can bring patients' blood sugar to the ideal standard faster, and the natural delivery rate is higher.[作者简介]王霞(1979-),女,本科,副主任医师,研究方向为产科及相关疾病诊治。
TEDA-L33E商品说明书

SAFETY DATA SHEET: TEDA – L33E – US-GHS VersionTEDA - L33E1. IDENTIFICATION OF THE SUBSTANCE OR MIXTURE AND OF THE SUPPLIERPRODUCT IDENTIFIER: TEDA – L33EMANUFACTURER / IMPORTER:TOSOH SPECIALTY CHEMICALS USA, Inc. ADDRESS: 1720 Windward Concourse, Suite 125Alpharetta, Georgia 30005 PHONE: 1-770-442-9501EMERGENCY PHONE :CHEMTREC 1-800-424-9300 OR 1-703-527-3887RECOMMENDED USE:General industrial products2. HAZARDS IDENTIFICATIONGHS CLASSIFICATIONAcute toxicityOral: Category 4 Skin corrosion/irritation Category 2 Serious eye damage/eye irritation Category 2A Specific target organ toxicity – single exposure Category 3 Specific target organ toxicity – repeat exposure Category 2HAZARD SYMBOL:SIGNAL WORD : WARNINGHAZARD STATEMENTS :Harmful if swallowed. Causes skin irritation.Causes serious eye irritation.May cause drowsiness or dizziness.May cause damage to kidneys through prolonged or repeated exposure.PREVENTION :Wash thoroughly after handling.Do not eat, drink or smoke when using thisproduct.Wear protective gloves/eye protection/faceprotection.Avoid breathingdust/fume/gas/mist/vapors/spray.Use only outdoors or in a well-ventilated area.distributed by:Request Quote or SamplesSAFETY DATA SHEET: TEDA – L33E – US-GHS Version2. HAZARDS IDENTIFICATION (continued)RESPONSE :If in eyes: Rinse cautiously with water for several minutes.Remove contact lenses, if present and easy to do.Continue rinsing.If eye irritation persists: Get medical advice/attention.If on skin (or hair): Wash with plenty of water. If skin irritation occurs: Get medical advice/attention.Take off contaminated clothing and wash it before reuse.If inhaled: Remove person to fresh air and keep comfortable for breathing.Call a poison control center/doctor if you feel unwell.If swallowed: Rinse mouth.Call a poison control center/doctor if you feel unwell.STORAGE: Store in a well-ventilated place. Keep container tightly closed.Store locked up.DISPOSAL :Dispose of contents/container in accordance with Federal and state regulations.3. COMPOSITION/INFORMATION ON INGREDIENTSOSHAChemical Name CAS # Hazardous(Y/N) Concentration (%) Triethylenediamine 280-57-9 Y 33 Ethylene glycol 107-21-1 Y 674. FIRST AID MEASURESEYE CONTACT:Hold eyelids open and flush with a steady, gentle stream of water for at least 15 minutes. Seek medical attention if eye irritation develops or persists.SKIN CONTACT:Remove contaminated clothing and shoes. Wash with plenty of water, for at least 15minutes. Seek medical attention if skin irritation develops or persists. Launder contaminated clothing and shoes before re-use.SAFETY DATA SHEET: TEDA – L33E – US-GHS Version4. FIRST AID MEASURES (continued)INGESTION:Do not induce vomiting. If victim is conscious and alert, give 1-2 glasses of water to drink. Do not give anything by mouth to an unconscious person. Seek immediate medical attention. Do not leave victim unattended.INHALATION:If respiratory irritation or distress occurs, remove victim to fresh air. Seek imedical attention if respiratory irritation or drowsiness develops or persists.NOTES TO PHYSICIAN :All treatments should be based on observed signs and symptoms of distress in the patient. Consideration should be given to the possibility that overexposure to materials other than this product may have occurred. Treatsymptomatically. No specific antidote available.5. FIRE FIGHTING MEASURESEXTINGUISHING MEDIA: Water spray, fog, dry chemical, foam, CO 2UNUSUAL FIRE AND EXPLOSION HAZARDS:Closed containers may rupture due to buildup of pressure when exposed to extreme heat. SPECIAL PROTECTIVE EQUIPMENT FOR FIRE FIGHTERS:Firefighters should wear NIOSH/MSHA-approved self-contained breathing apparatus and full protective clothing. Cool containers exposed to fire with water.HAZARDOUS DECOMPOSITIONMATERIALS UNDER FIRE CONDITIONS : Oxides of carbon, oxides of nitrogen, ammonia.6. ACCIDENTAL RELEASE MEASURESPERSONAL PRECAUTIONS: Wear appropriate protective gear for thesituation. (See Personal Protection Information in Section 8).ENVIROMENTAL PRECAUTIONS :Do not flush to drain. Spills may be reportable to the National Response Center (800-424-8802) and to state and/or local agencies.METHOD FOR CLEAN UP:Extinguish or remove all sources of ignition. Absorb with an inert absorbent, sweep up and place in an appropriate closed container. Clean up residual material by washing area with water. Collect washings for disposal. Spills may be reportable to the National Response Center (800-424-8802) and to state and/or local agencies.SAFETY DATA SHEET: TEDA – L33E – US-GHS Version7. HANDLING AND STORAGEPRECAUTIONS FOR SAFE HANDLING : Handle material with suitable protection (SeeSection 8). Handle with adequate ventilation. Avoid breathing vapors. Avoid contact with eyes, skin and clothing.VENTILATION:General area dilution/exhaust ventilation.CONDITIONS FOR SAFE STORAGE :Store upright in a cool, dry, well ventilated area out of direct sunlight. Keep away from heat,open flames and ignition sources. Keep container tightly closed. Do not reuse container.8. EXPOSURE CONTROLS/PERSONAL PROTECTIONENGINEERING MEASURES:Set up hand-wash station and eyewash station near work area.General area dilution/exhaust ventilation.EXPOSURE LIMITS:Ethylene glycol – 100 mg/M 3 - ACGIH ceilingPERSONAL PROTECTION MEASURES:Respiratory protection :When respirators are required, selectNIOSH/MSHA approved equipment based on actual or potential airborne concentrations and in accordance with regulatory standards and/or industrial recommendations. Self-contained or supplied-air respiratory equipmment is recommended.Eye protection : Safety glasses with side shields, goggles or face shield are recommended.Skin protection :Skin contact should be minimized through the use of chemical-resistant gloves and boots, and suitable protective clothing.The following general measures should be taken when working or handling this material:1) Do not store, use, and/or consume foods, beverages, tobacco products, or cosmetics in areas where this material is stored.2) Wash hands and face carefully before eating, drinking, using tobacco, applying cosmetics, or using the toilet.3) Wash exposed skin promptly to remove accidental splashes of contact with this material.9. PHYSICAL AND CHEMICAL PROPERTIESPHYSICAL STATE: Liquid COLOR: Pale yellow ODOR: Ammonia-like pH: 11.0 (@10% aqueous) MELTING POINT: No data availableSAFETY DATA SHEET: TEDA – L33E – US-GHS Version9. PHYSICAL AND CHEMICAL PROPERTIES (continued)BOILING POINT: 363-385F (184-196C) FLASH POINT: 219F (104C) AUTOIGNITION POINT: 608F (320C) EXPLOSIVE LIMITS(Lower): No data available EXPLOSIVE LIMITS(Upper): No data available VAPOR PRESSURE: < 13 Pa @ 20C (68F) VAPOR DENSITY: 2.52 (Air = 1) EVAPORATION RATE: No data available RELATIVE DENSITY: 1.10 SOLUBILITY IN WATER : Soluble PARTITION COEFFICIENT: No data available DECOMPOSITION TEMPERATURE: No data available10. STABILITY AND REACTIVITYCHEMICAL STABILITY:This material is stable under normal handlingand storage conditions described in Section 7.CONDITIONS TO AVOID: Heat, open flame, sparks, direct sunlight.INCOMPATIBLE MATERIALS:Strong oxidizing agents, strong acids, copper, zinc, aluminum and their alloys.HAZARDOUS DECOMPOSITION PRODUCTS: Oxides of carbon, oxides of nitrogen, ammonia.HAZARDOUS POLYMERIZATION: Not applicable11. TOXICOLOGICAL INFORMATIONEYE CORROSION/IRRITATION: Severely irritating, rabbit. (Data for Triethylenediamine)SKIN CORROSION/IRRITATION: Moderately irritating, rabbit. (Data for Triethylenediamine)ACUTE TOXICITY:ACUTE ORAL TOXICITY: LD 50 = 1700 mg/kg, rat. (Data for Triethylenediamine)ACUTE DERMAL TOXICITY : LD 50 > 2000 mg/kg, rat. (Data for Triethylenediamine)ACUTE INHALATION TOXICITY :LC 50 ≥ 20.2 mg/L/1 hour, rat (tested as a 20% solution). (Data for Triethylenediamine)SKIN SENSITIZATIONNot a sensitizer (guinea pig). (Data for Triethylenediamine)GENETIC TOXICITYNot mutagenic in the Ames test or in vivo mouse micronucleus test. (Data for Triethylenediamine)SAFETY DATA SHEET: TEDA – L33E – US-GHS Version11. TOXICOLOGICAL INFORMATION (continued)CARCINOGENICITY:This product does not contain any substances that are considered by OSHA, NTP, IARC or ACGIH to be “probable” or “suspected” human carcinogens.REPRODUCTIVE TOXICITY:In a combined repeat-dose/reproductive study (OECD 422) with Triethylenediamine, theNOAEL (no-observed-adverse-effect level) for F0 reproductive toxicity was considered to be 300 mg/kg/day. The NOAEL for Fl neonatal toxicity was considered to be 300 mg/kg/day. The NOAEL for F0 parental systemic toxicity was considered to be 100 mg/kg/day.Reproductive studies with ethylene glycol show that in repeated dose toxicity studies, noevidence of an adverse impact on reproductive organs was observed. In special studies, including a three generation study in rats and continuous breeding protocols in mice, evidence of reproductive effects have been restricted to mice (but not rabbits or rats) exposed to doses considerably higher than those associated with developmental effects in this species or renal effects in rats.STOT-SINGLE EXPOSURE : Ethylene glycol may cause central nervous system depression and drowsiness.STOT-REPEATED EXPOSURE:In a combined repeat-dose/reproductive study (OECD 422) with Triethylenediamine, reversible, treatment-related effects wereobserved in the kidneys and bladders of mid-to-high dose animals. The NOAEL for ethylene glycol was determined to be 150 mg/kg/day and appears to be a threshold dose below which no renal toxicity occurs.12. ECOLOGICAL INFORMATIONECOTOXICITY:96hr LC 50 > 100 mg/L (carp)48hr EC 50 > 92 mg/L (daphnia magna)72hr EC 50 > 110 mg/L (algae, biomass), > 180mg/L (algae, growth rate) (All data for Triethylenediamine)PERSISTENCE AND DEGRADABILITY: Not readily biodegradable (Data forTriethylenediamine)MOBILITY IN SOIL:No data availableSAFETY DATA SHEET: TEDA – L33E – US-GHS Version13. DISPOSAL CONSIDERATION (INCLUDING CONTAINER)RESIDUAL WASTE:Chemical additions, processing or otherwise altering this material may make the waste management information presented in this MSDS incomplete, inaccurate or otherwiseinappropriate. Please be advised that state and local requirements for waste disposal may be more restrictive or otherwise different fromFederal laws and regulations. Consult state and local regulations regarding the proper disposal of this material.CONTAMINATED VESSELS AND CONTAINERS :Rinse containers before disposal. Do not allow rinsate to enter the water systems.EPA Hazardous Waste = No14. TRANSPORTATION INFORMATIONPROPER SHIPPING NAME: NOT REGULATED UN NUMBER:None UN CLASS or DIVISION: None UN PACKING GROUP: None LABELS :None EMERGENCY GUIDE#:None15. REGULATORY INFORMATIONInventory Status:US (TSCA): YesCanada (DSL): Yes EU (REACH): Yes Australia (AICS): Yes Japan (METI): Yes Korea (KECL): YesWhere: Yes = all ingredients are listed on the inventory, Exempt = All ingredients are either on the inventory or exempt from the requirements of listing, No = Not determined, or one or more ingredients are not on the inventory and are not exempt from listingSARA Title III Hazard Classes: Fire Hazard: No Reactive Hazard: No Release of Pressure: No Acute Health Hazard: Yes Chronic Health Hazard: YesSARA Extremely Hazardous Substances/CERCLA Hazardous Substances: Ethylene glycol (107-21-1) (33%), TPQ=5000 pounds, 2270 kgCalifornia Proposition 65: This product does not contain any components that are regulated under Proposition 65.SAFETY DATA SHEET: TEDA – L33E – US-GHS Version16. OTHER INFORMATION INCLUDING INFORMATION ON PREPARATION AND REVISION OF THIS MSDSNational Fire Protection Association (“NFPA”) Hazard Ratings: Health: 2 (Moderate)Flammability: 1 (Slight)Reactivity: 0 (Minimal)National Paint and Coatings Hazardous Materials Identification System (“HMIS”) Hazard Ratings: Health: 2 (Moderate)Flammability: 1 (Slight) Physical Hazard: 0 (Minimal)HISTORY: Date previous SDS: April 7, 2015 Date of issue: November 13, 2015 Reasons for Revision: Revised Phone NumberDisclaimer: The information set forth herein has been gathered from standard reference materials and/or TOSOH SPECIALTY CHEMICALS USA, INC and its related, subsidiary and affiliated companies’ test data and is to the best knowledge and belief of TOSOH SPECIALTY CHEMICALS USA, INC and its related, subsidiary and affiliated companies, accurate andreliable. Such information is offered solely for your consideration, investigation, and verification, and is not suggested or guaranteed that the hazard precautions or procedures mentioned are the only ones that exist. TOSOH SPECIALTY CHEMICALS USA, INC and its related, subsidiary and affiliated companies make no warranties, express or implied, and expressly disclaim any and all such warranties with respect to the use of such information or the use of specific materialidentified herein in combination with any other material or process, and assume no responsibility therefor. TOSOH SPECIALTY CHEMICALS USA, INC and its related, subsidiary and affiliated companies make no representation or warranty, express or implied, and EXPRESSLY DISCLAIM ANY AND ALL SUCH WARRANTIES, as to the usefulness, sufficiency, MERCHANTABILITY or FITNESS FOR ANY PURPOSE whatsoever of the materials identified herein. The purchaser bears sole responsibility for testing, evaluating and determining the suitability of these materials for whatever use(s), manufacturing and refining processes, and any other such application(s) for which it intends or ultimately makes of these materials. Purchaser bears sole responsibility for obtaining any and all regulatory, legal and governmental approval necessary for such use(s).END OF SAFETY DATA SHEETdistributed by:Request Quote or Samples。
胰岛素抵抗的实验研究

本工作根据S自0rlien LH的配方制作高脂饲料(hi曲fat diet,m酗LT),分别喂饲正
常成年sD大鼠30天和60天,用K瑚gen Ew方法建立正糖钳技术评估瓜,以葡
萄糖输注率(西uc0∞in向sion Late,GIR)反映IR,观察m形成和发展过程中禁食期 血糖,血脂,血清胰岛素,脂肪细胞大小和相关脂肪细胞源性因子lep血(瘦素)及 肿瘤坏死因子a(tI蛐or necmsis factor。alpha,1NF d)的改变。对大鼠附睾脂肪垫脂 肪细胞进行体外培养。
论文题目
中国汕和医科大学硕士学位论文
胰岛素抵抗的实验研究
研究生:张荣 导 师:许荣煜研究员 学 科:生理学 研究方向:脂肪细胞源性因子与胰岛素抵抗 所在院所:北京协和医科大学
中国医学科学院基础医学研究所
2003年6月
中国协和医科大学硕士学位论文
论文:胰岛素抵抗的实验研究
英文缩略语表
缩语
英文原文
IR
glucose iIl】融sion rate Ⅱonesteri丘cd fatIy∞id fkefIatacid
TG CH0 RIA ELISA AT.1NF GLUT-4 LPL BAT
tri91yc嘶des
cholesterol
radioimmune嬲say
cn巧me-linked imm啪osorbent assay adipose tis锄e is sollre of耵吓
德谷门冬双胰岛素注射液治疗2_型糖尿病临床效果及安全性探讨

DOI:10.16658/ki.1672-4062.2023.17.098德谷门冬双胰岛素注射液治疗2型糖尿病临床效果及安全性探讨林生,谢平,陈予福州市长乐区人民医院内分泌科,福建福州350200[摘要]目的研究德谷门冬双胰岛素注射液治疗2型糖尿病的临床效果及安全性。
方法选取于2022年7月—2023年4月福州市长乐区人民医院收治的2型糖尿病患者98例为研究对象,采用随机抓阄法分为两组,每组49例。
两组均联用常规降糖药物治疗,对照组采用甘精胰岛素注射液治疗,观察组采用德谷门冬双胰岛素注射液治疗。
对比两组临床治疗效果、临床症状好转时间和胰岛素用量情况、糖代谢指标、胰岛素功能指标、不良反应发生情况、心血管不良事件发生情况。
结果观察组总有效率高于对照组,差异有统计学意义(P<0.05)。
观察组尿酮体转阴时间、血糖达标时间、胰岛素用量均优于对照组,差异有统计学意义(P< 0.05)。
观察组空腹血糖、餐后2 h血糖、糖化血红蛋白均低于对照组,差异有统计学意义(P<0.05)。
观察组胰岛β细胞功能指数高于对照组,胰岛素抵抗指数、空腹胰岛素低于对照组,差异有统计学意义(P<0.05)。
两组恶心呕吐、倦怠乏力、低血糖总发生率比较,差异无统计学意义(P>0.05)。
两组心绞痛、心力衰竭总发生率比较,差异无统计学意义(P>0.05)。
结论德谷门冬双胰岛素注射液治疗2型糖尿病临床效果显著优于甘精胰岛素注射液,但是治疗安全性无显著变化。
[关键词] 2型糖尿病;德谷门冬双胰岛素注射液;不良反应;心血管不良事件[中图分类号] R59 [文献标识码] A [文章编号] 1672-4062(2023)09(a)-0098-04Discussion on the Clinical Effect and Safety of Insulin Degludec and Insu⁃lin Aspart Injection in the Treatment of Type 2 Diabetes MellitusLIN Sheng, XIE Ping, CHEN YuDepartment of Endocrinology, Changle District People's Hospital, Fuzhou, Fujian Province, 350200 China[Abstract] Objective To study the clinical effect and safety of insulin degludec and insulin aspart injection in the treatment of type 2 diabetes mellitus. Methods A total of 98 patients with type 2 diabetes admitted to Fuzhou Changle District People's Hospital from July 2022 to April 2023 were selected as the study objects and divided into two groups with 49 cases in each group by random lottery method. Both groups were treated with conventional hypoglycemic drugs, the control group was treated with insulin glargine injection, and the observation group was treated with Degu asparton double insulin injection. The clinical therapeutic effect, time of improvement of clinical symptoms, insulin dosage, glucose metabolism index, insulin function index, occurrence of adverse reactions and cardiovascular adverse events were compared between the two groups. Results The total effective rate of the observation group was higher than that of the control group, and the difference was statistically significant (P<0.05). The time of urine ketone body turning negative, blood glucose reaching standard and insulin dosage in observation group were better than those in control group, and the differences were statistically significant (P<0.05). Fasting plasma glucose, 2-hour postprandial blood glucose and glycated hemoglobin in the observation group were lower than those in the control group, and the differences were statistically significant (P<0.05). The function index of islet β cells in observation group was higher than that in control group, the insulin resistance index and fasting insulin was lower than that in control group, the dif⁃ference was statistically significant (P<0.05). There was no statistically significant difference in the total incidence of [作者简介]林生(1981-),男,本科,副主任医师,研究方向为糖尿病及其并发症的相关临床研究。
依达拉奉右莰醇治疗缺血性脑卒中的研究进展

- 179 -①滨州医学院附属医院神经内科 山东 滨州 256600通信作者:鹿树军依达拉奉右莰醇治疗缺血性脑卒中的研究进展席娅琳① 汪临华① 鹿树军① 【摘要】 缺血性脑卒中是脑血管疾病中的常见病,严重可导致高级认知及运动障碍,甚至死亡。
缺血性脑卒中的治疗方法主要包括早期溶栓和保护神经细胞等治疗,然而目前神经保护剂的临床疗效有待考证,大多数神经保护剂仍未得出有益的证据。
新型双靶点复合型神经保护剂依达拉奉右莰醇(ED)可抑制诱导型一氧化氮合酶(iNOS)和肿瘤坏死因子-α(TNF-α)的表达,降低自由基过氧化亚硝基阴离子(ONOO -)水平,从而改善缺血性脑卒中所致的神经损伤症状、功能障碍及活动障碍,本文将对ED 的作用机制及其应用发展做一综述,并对ED 的临床应用进行展望,为后续的用药提供指导。
【关键词】 缺血性脑卒中 自由基清除剂 神经保护剂 依达拉奉右莰醇 Research Progress of Edaravone Dexborneol in the Treatment of Ischemic Stroke/XI Yalin, WANG Linhua, LU Shujun. //Medical Innovation of China, 2024, 21(10): 179-183 [Abstract] Ischemic stroke is a common type of cerebrovascular disease that can lead to advanced cognitive and motor deficits and even death. The treatment of ischemic stroke mainly includes early thrombolysis and neuroprotection. However, the clinical efficacy of neuroprotective agents remains to be verified, and most neuroprotective agents have not yet received useful evidence. Edaravone Dexborneol (ED), a new dual-target neuroprotective agent, can inhibit the expression of inducible nitric oxide synthase (iNOS) and tumor necrosis factor-α(TNF-α), reduce the level of peroxynitrite anion (ONOO -), and improve the symptoms of nerve injury, dysfunction, and activity disorder caused by ischemic stroke. This article will review the mechanism of ED and its application development, and prospect the clinical application of ED, so as to provide guidance for subsequent medication. [Key words] Ischemic stroke Free radical scavenger Neuroprotective agent Edaravone Dextrogenol First-author's address: Department of Neurology, Binzhou Medical University Hospital, Binzhou 256600, China doi:10.3969/j.issn.1674-4985.2024.10.041 脑卒中已成为我国居民寿命的“第一杀手”,其中,急性缺血性脑卒中(acute ischemic stroke,AIS)约占我国脑卒中的70%,为最常见的卒中类型[1-2]。
磷酸特地唑胺产品说明

450.32
Bacterial
Anti-infection
项目 Test
外观 Appearance
纯度 Purity
溶解性 Solubility
保存/复检期 Storage/Recommended Retest Period
检测指标 Specification White to off-white (Solid)
北京索莱宝科技有限公司磷酸特地唑胺产品说明产品编号tcatnumberit0410产品名称tproductname磷酸特地唑胺tedizolidphosphate产品类型tproducttype小分子抑制剂smallmoleculeinhibitorscas
磷酸特地唑胺产品说明
北京索莱宝科技有限公司
产品编号 Cat Number 产品名称 Product Name 产品类型 Product Type
CAS.
分子式 Molecular Formula 分子量 Molecular Wt
靶点 Target
通路 Pathway
IT0410 磷酸特地唑胺 Tedizolid phosphate 小分子抑制剂 Small molecule inhibitors 856867-55-5
Purityห้องสมุดไป่ตู้98%
Soluble in DMSO Powder 4℃ 2 years In solvent -20℃ 1 month
注意:我司生产的小分子抑制剂均为非无菌包装,若用于细胞实验,请提前做好预处理。
AZD7545_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :AZD7545Catalog No. :HY-16082CAS No. :252017-04-21.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:AZD 7545; AZD⁻7545Formula:C19H18ClF3N2O5SMolecular Weight:478.87CAS No. :252017-04-24. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutantIATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
德谷门冬双胰岛素注射液治疗2_型糖尿病的疗效及安全性研究

DOI:10.16658/ki.1672-4062.2023.19.084德谷门冬双胰岛素注射液治疗2型糖尿病的疗效及安全性研究戴卉,张开凤,朱凤丽江苏省镇江市丹徒区人民医院内分泌科,江苏镇江212000[摘要]目的探讨德谷门冬双胰岛素注射液在2型糖尿病中的效果以及安全性。
方法选取2022年1月—2023年7月江苏省镇江市丹徒区人民医院收治的62例2型糖尿病患者为研究对象,按随机数表法分为对照组(n=31)和观察组(n=31)。
对照组患者接受门冬胰岛素30注射液治疗,观察组患者接受德谷门冬双胰岛素注射治疗。
对比两组患者临床疗效、血糖变化和不良反应发生率。
结果观察组治疗有效为96.77%,高于对照组的77.42%,差异有统计学意义(χ2=5.167,P=0.023)。
治疗前,两组患者血糖水平比较,差异无统计学意义(P>0.05);治疗后,两组患者血糖水平均改善,且观察组血糖指标低于对照组,差异有统计学意义(P< 0.05)。
观察组不良反应发生率低与对照组,差异有统计学意义(P<0.05)。
结论德谷门冬双胰岛素的应用可以明显改善2型糖尿病患者血糖水平,疗效更为确切,且安全性更高,不会增加用药后不良反应。
[关键词] 2型糖尿病;德谷门冬双胰岛素;门冬胰岛素30注射液;安全性[中图分类号] R587 [文献标识码] A [文章编号] 1672-4062(2023)10(a)-0084-04Study on the Efficacy and Safety of Insulin Degludec and Insulin Aspart Injection in the Treatment of Type 2 Diabetes MellitusDAI Hui, ZHANG Kaifeng, ZHU FengliDepartment of Endocrinology, Zhenjiang Dantu District People's Hospital, Zhenjiang, Jiangsu Province, 212000 China [Abstract] Objective To explore the effect and safety of insulin degludec and insulin aspart injection in type 2 diabe⁃tes mellitus.Methods 62 patients of type 2 diabetes mellitus patients admitted to Zhenjiang Dantu District People's Hospital, Jiangsu Province from January 2022 to July 2023 were selected as study objects and divided into the control group (n=31) and the observation group (n=31) by taking the random number table method. The patients in the control group were treated with insulin aspart 30 injection and the patients in the observation group were treated with insulin degludec and insulin aspart injection. Compared the clinical efficacy, the changes in blood glucose and the incidence of adverse reactions between the two groups of patients.Results The treatment effectiveness of the observation group was 96.77%, which was higher than that of the control group, which was 77.42%, and the difference was statistically significant (χ2=5.167, P=0.023). There was no statistically significant difference in blood glucose levels between the two groups before treatment (P>0.05). After treatment, blood glucose levels improved in both groups, and the level of blood glucose in the observation group were lower than those in the control group, and the difference was statistically significant (P<0.05). The incidence of adverse reactions in the observation group was lower than that in the control group, and the difference was statistically significant (P<0.05).Conclusion The application of insulin degludec and in⁃sulin aspart can significantly improve the blood glucose level of patients with type 2 diabetes mellitus, the efficacy is more accurate, and the safety is higher, and it will not increase the occurrence of adverse reactions after the use of medication.[作者简介]戴卉(1985-),女,本科,主治医师,研究方向为内分泌科。
保健食品毒理学安全性评价

• 任何一个化学物在一定条件下都可能是 对机体产生任有害作用。
保健食品毒理学安全性评价
第5页
毒理学
• 描述毒理学、机制毒理学、管理毒理学 • (法规毒理学) • 毒作用谱: • 系统毒性;致突变;致畸;致癌
保健食品毒理学安全性评价
第6页
┫
○
┫
┫
50
┫ ┫
┫
*
┫
○
┫○
┗┳┳┳┳┳┳┳┳┳┳┳┳┳┳┳
15 30
60
NOAEL LOAEL
阈
mg/kg
保健食品毒理学安全性评价
第8页
描述毒理学试验基础标准
○化学物在试验动物产生作用, 能够外推于 人。
○试验动物必须暴露于高剂量, 这是发觉对 人潜在危害必需和可靠方法。
○成年健康(雄性和雌性未孕)试验动物 和人可能暴露路径是基础选择。
保健食品毒理学安全性评价
第30页
保健食品原料
药食同源(附件1) 可用于保健食品(附件2) ???? 禁用于保健食品(附件3)。
保健食品毒理学安全性评价
第31页
???
• 以普通食品、卫生部要求药食同源物质和允许用作 保健食品物质以外动植物或动植物提取物、微生物、 化学合成物为原料生产保健食品, 应对该原料和用该 原料生产保健食品分别进行安全性评价。
保健食品毒理学安全性评价
第28页
附件2 可用于保健食品物品名单
人参、人参叶、人参果、三七、土茯苓、大蓟、女贞子、山
茱萸、川牛膝、川贝母、川芎、马鹿胎、马鹿茸、马鹿骨、
丹参、五加皮、五味子、升麻、天门冬、天麻、太子参、巴
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
MSDS
1 Composition
7 Accident Release Measure
Product Name:Tedizolid
Chemical Name:
PROCEDURE(S) OF PERSONAL PRECAUTION(S)-Wear respirator, chemical safety goggles, rubber boots, and heavy
rubber gloves.METHODS FOR CLEANING UP-Sweep up, place in a bag and hold for waste disposal. Avoid raising dust. Ventilate area and
wash spill site after material pickup is complete.
2-Oxazolidinone, 3-[3-fluoro-4-[6-(2-methyl-2H-tetrazol-5-yl)-3-pyridinyl]phenyl]-5-(hydroxymethyl)-, (5R)-
CAS No.:856866-72-3
8 Accident Release Measure
Appearance:White to off-white(solid)Formula:C17H15FN6O39 Toxicological Information
Solubility:
To the best of our knowledge, the chemical, physical, and
toxicological properties have not been thoroughly investigated.
No data available.
p p p p DMSO
2 Handling and Storage
10 Regulary Information
3 Stability and Reactivity
11Disposal Considerations
CLASSIFICATION- Substance not yet fully tested.SAFETY PHASES- 26-36 (In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Wear suitable protective clothing.) 36/37/38 (Irritating to eyes,respiratory system and skin.)
STABILITY- Stable under normal handling conditions.HANDLING- Do not breathe dust. Avoid contact with eyes,
skin,and clothing.Avoid prolonged or repeated exposure.STORAGE- Store in a properly sealed container store at -20℃,shelflife is 2 years.
11 Disposal Considerations 4 Hazards Identification
12 Transport Information
5First Aid RID/ADR- Non-hazardous for road transport. IMDG- Non-hazardous for sea transport.IATA - Non-hazardous for air transport.
As specific country, federal, state and local environmental
regulations vary and change frequently we suggest you contact a local, authorized waste disposal contractor for adequate disposal.
Special indication of hazards to humans and the environment.Irritating to eyes, respiratory system and skin.
MATERIALS TO AVOID- Strong oxidizing agents.REACTIVITY- May emit toxic gasses like Carbon monoxide,Carbon dioxide, Nitrogen oxides upon thermal decomposition.
5 First Aid
13 Other Information
The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. Medchemexpress LLC shall not be held liable for any damage resulting from h dli f t t ith th b d t
INHALATION- If inhaled, remove to fresh air. If not breathing give, artificial respiration. If breathing is difficult, give oxygen.SKIN CONTACT- In case of contact, immediately wash skin with
soap and copious amounts of water.EYE CONTACT- In case of contact, immediately flush eyes with
copious amounts of water for at least 15 minutes.INGESTION- If swallowed, wash out mouth with water provided person is conscious. Call a physician.
6 Fire Fighting Measures
handling or from contact with the above product.
EXTINGUISHING MEDIA Water spray- Carbon dioxide, dry chemical powder, or appropriate foam.
SPECIAL RISKS Specific Hazard(s)- Emits toxic fumes under fire conditions. SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS Wear self-contained breathing apparatus and protective clothing Caution: Not fully tested. For research purposes only
Medchemexpress LLC
to prevent contact with skin and eyes.
18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
