Gatifloxacin_DataSheet_MedChemExpress
Gatifloxacin用于呼吸道感染的新喹诺酮

Gatifloxacin用于呼吸道感染的新喹诺酮
佚名
【期刊名称】《德国临床用药》
【年(卷),期】2000(3)3
【摘要】@@ Gatifloxacin是一种新的氟喹诺酮,目前处于III期临床Ⅲ试验.本品可口服给药和胃肠外给药.与喹诺酮类老药(如环丙沙星)相比,本品作用谱广,对G+菌和厌氧菌也有效.根据Paul-Ehrlich化疗协会(注册)的分类,本品属于第IV类氟喹诺酮.根据迄今的临床研究结果,人们估计本品极宜用于治疗呼吸道感染.但是,它对淋病、尿道感染以及皮肤和软组织感染也有效.
【总页数】2页(P19-20)
【正文语种】中文
【中图分类】R5
【相关文献】
1.喹诺酮用于结核病治疗 [J], 韩咏霞
2.发现大肠埃希菌喹诺酮耐药株gyrA基因新亚型和aac(6')-Ⅰ6-Cr、mdfA基因[J], 文怡;糜祖煌;刘根焰;顾兵;陈友华;赵旺胜
3.新喹诺酮药物序贯疗法简化重度社区获得性肺炎治疗的临床观察 [J], 张杰明
4.7-溴-6-氯-4(3H)-喹诺啉酮和5-溴-6-氯-4(3H)-喹诺啉酮的合成 [J], 张越;牛玉环;董博芳;王银华;邸晓涛;杜会茹
5.蛋白质用于毛细管电泳-电导法快速检测喹诺酮的研究 [J], 刘青春;樊永霞;秦卫东
因版权原因,仅展示原文概要,查看原文内容请购买。
Teriflunomide_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Teriflunomide is the active metabolite of leflunomide, which inhibits pyrimidine de novo synthesis by blocking the enzyme dihydroorotate dehydrogenase, used as an immunomodulatory agent.In Vitro: Teriflunomide primarily acts as an inhibitor of dihydroorotate dehydrogenase (DHODH), a key mitochondrial enzymeinvolved in the de novo synthesis of pyrimidines in rapidly proliferating cells. By reducing the activity of high–avidity proliferating T lymphocytes and B lymphocytes, teriflunomide likely attenuates the inflammatory response to autoantigens in MS. Thus,teriflunomide can be considered a cytostatic rather than a cytotoxic drug to leukocytes [1].In Vivo: Teriflunomide has demonstrated beneficial effects in two independent animal models of demyelinating disease. In the dark agouti rat model of experimental autoimmune encephalitis (EAE), teriflunomide administration results in clinical, histopathological,and electrophysiological evidence of efficacy both as a prophylactic and therapeutic agent. Similarly, in the female Lewis rat model of EAE, teriflunomide administration results in beneficial prophylactic and therapeutic clinical effects, with a delay in disease onset and symptom severity [1].References:[1]. Oh J, et al. An update of teriflunomide for treatment of multiple sclerosis. Ther Clin Risk Manag. 2013;9:177–90.Product Name:Teriflunomide Cat. No.:HY-15405CAS No.:163451-81-8Molecular Formula:C 12H 9F 3N 2O 2Molecular Weight:270.21Target:Others Pathway:Others Solubility:DMSO: 26 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Gelucire-14-44-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Nov.-23-2018Print Date:Nov.-23-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Gelucire 14/44Catalog No. :HY-Y1892CAS No. :121548-04-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:N/AMolecular Weight:N/ACAS No. :121548-04-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Pure form-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Oil)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Gatifloxacin mesylate_316819-28-0_DataSheet_MedChemExpress

Product Name:Gatifloxacin mesylate CAS No.:316819-28-0Cat. No.:HY-10581BProduct Data SheetMWt:471.50Formula:C20H26FN3O7S Purity :>98%Solubility:DMSO 7 mg/Ml; Water <1 mg/mLMechanisms:Biological Activity:Gatifloxacin (mesylate)is an antibiotic of the fourth-generation fluoroquinolone family it inhibits the Pathways:Anti-infection; Target:Antibacterial Gatifloxacin (mesylate) is an antibiotic of the fourth generation fluoroquinolone family, it inhibits thebacterial enzymes DNA gyrase and topoisomerase IV.Target: Antibacterial Gatifloxacin (mesylate) is the mesylate salt of Gatifloxacin which is an antibiotic of the fourth-generation fluoroquinolone family, that like other members of that family, inhibits the bacterialenzymes DNA gyrase and topoisomerase IV. Gatifloxacin had activity equal to that of tosufloxacin and activity more potent than those of norfloxacin, ofloxacin, ciprofloxacin, and sparfloxacin against the second-step mutants (grlA gyrA; gatifloxacin MIC range, 1.56 to 3.13 microg/ml) and had the most potent activity against the third-step mutants (grlA gyrA grlA; gatifloxacin MIC range, 1.56 toReferences:[1]. Fukuda, H., S. Hori, and K. Hiramatsu, Antibacterial activity of gatifloxacin (AM-1155, CG5501,BMS 206584)l d l d fl i l i t ti ll i d i l i t t 6.25 microg/ml), suggesting that gatifloxacin possesses the most potent inhibitory activity against singly m...BMS-206584), a newly developed fluoroquinolone, against sequentially acquired quinolone-resistant mutants and the norA transformant of Staphylococcus aureus. Antimicrob Agents Chemother, 1998.42(8): p. 1917-22.[2]. Jensen, H., et al., Comparison of ophthalmic gatifloxacin 0.3% and ciprofloxacin 0.3% in healing of corneal ulcers associated with Pseudomonas aeruginosa-induced ulcerative keratitis in rabbits. JOcul Pharmacol Ther, 2005. 21(1): p. 36-43.[3]. Cheung, O., et al., Gatifloxacin-induced hepatotoxicity and acute pancreatitis. Ann Intern Med,2004. 140(1): p. 73-4.[4]Fteha A et al Gatifloxacin induced torsades de pointes Pacing Clin Electrophysiol 2004Caution: Not fully tested. For research purposes onlyMedchemexpress LLC[4]. Fteha, A., et al., Gatifloxacin induced torsades de pointes. Pacing Clin Electrophysiol, 2004.27(10): p. 1449-50....18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
CAS25812-30-0_Gemfibrozil_MedBio合成路线
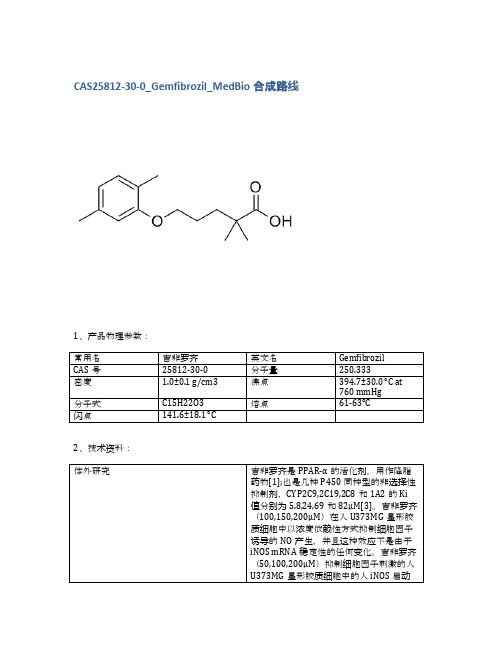
1、产品物理参数:
常用名
吉非罗齐
英文名
Gemfibrozil
CAS号
25812-30-0
分子量
250.333
密度
1.0±0.1 g/cm3
沸点
394.7±30.0 °C at 760 mmHg
分子式
C15H22O3
熔点
61-63°C
闪点
141.6±18.1 °C
2、技术资料:
体外研究
吉非罗齐是PPAR-α的活化剂,用作降脂药物[1];也是几种P450同种型的非选择性抑制剂,CYP2C9,2C19,2C8和1A2的Ki值分别为5.8,24,69和82μM[3]。吉非罗齐(100,150,200μM)在人U373MG星形胶质细胞中以浓度依赖性方式抑制细胞因子诱导的NO产生,并且这种效应不是由于iNOS mRNA稳定性的任何变化。吉非罗齐(50,100,200μM)抑制细胞因子刺激的人U373MG星形胶质细胞中的人iNOS启动子衍生的荧光素酶活性。此外,吉非罗齐(50,100,150和200μM)对细胞活力没有影响[1]。吉非罗齐显着抑制M-23和M-1的形成(由CYP2C8和CYP3A4催化),在人肝微粒体中Ki(IC50)值分别为69μM(95μM)和273μM(>250μM)。吉非罗齐(0-250μM)剂量依赖性地抑制重组CYP2C8中M-23(IC50,68μM)和M-1(IC50,78μM)的形成,但对重组CYP3A4中这些代谢物的形成没有显着影响。[3]。
10mM (in 1mL DMSO)
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15072
Calcipotriol_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Sep.-13-2017Print Date:Sep.-13-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :CalcipotriolCatalog No. :HY-10001CAS No. :112965-21-61.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:MC 903; CalcipotrieneFormula:C27H40O3Molecular Weight:412.60CAS No. :112965-21-64. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature: 4°C, protect from light, stored under nitrogenShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Cilengitide_DataSheet_MedChemExpress
Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Cilengitide is a potent and selective integrin inhibitor for αv β3 and αv β5 receptor, inhibits binding of isolated αv β3 and αv β5 to vitronectin with an IC 50 value of 4 and 79 nM, respectively.IC50 & Target: IC50: 4 and 79 nM (αv β3 and αv β5)[1]In Vitro: Cilengitide (EMD 121974) is the αv β3 and αv β5 integrin receptor antagonist. In cell adhesion studies assessing the human melanoma M21 or UCLA–P3 human lung carcinoma cell lines, Cilengitide inhibits integrin–mediated binding to vitronectin with IC 50s of 0.4 and 0.4 μM [1]. In vitro treatment of Cilengitide, at a concentration greater than 1 μM, shows concentration– andtime–dependent cytotoxic effects. However, lower doses of Cilengitide monotherapy (0.1 and 0.5 μM) does not elicit the effective death of the both U87MG and U251MG cells. Significant cytotoxic effects are observed in the U87MG cells with the addition of 1μM Cilengitide in combination with Belotecan monotherapy at concentration of 6.25 nM. Higher concentrations of Cilengitide (5and 25 μM) does not significantly increase cell death in the U87MG and U251MG compare to a lower concentration of Cilengitide (1 μM)[2].In Vivo: In nude mice bearing M21–L melanoma tumors, Cilengitide dose i.p. at 10, 50, and 250 μg three times per weekdemonstrated inhibition of tumor growth with a reduction in both tumor volume (55%, 75%, and 89%, respectively) and tumor weight (23%, 38%, and 61%, respectively), when compared to controls [2]. In the rat model studied, the systemic pharmacokineticsof i.p. Cilengitide are not affected by ILP with Cilengitide alone or ILP with Cilengitide plus Melphalan, TNF or both. Systemic Cilengitide levels reach around 20 μg/mL (approximately 35 μM) within 10 min of i.p. administration and continued to rise toapproximately 40 μg/mL (approximately 70 μM) in the first hour. Thereafter Cilengitide levels in serum dropped with an elimination half–life of 2.1 hr [3].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay: Cilengitide is supplied as an apyrogenic sterile infusion solution in physiological saline. Cilengitide is diluted in saline to a concentration of 1 mM [2]. [2]The cytotoxicity of the two drugs, Belotecan and Cilengitide, is measured by the Cell Counting Kit–8(CCK–8). U87MG and U251MG cells are seeded in 96 well plates at a density of 4×103 cells per well to allow for adhesion overnight.After this, the cells are treated with Cilengitide at a concentration of 0, 0.1, 0.5, 1, 5 and 25 μM and Belotecan at a concentration of 0,6.25, 12.5, 25, 50 and 100 nM. All possible combinations of concentrations are used to assess the combined therapeutic effect of Cilengitide and Belotecan. After 3 days, 10 μL of the CCK–8 solution is added to each well of the plate, and the plate is incubated for 3 hr in the incubator (37°C; 5% CO 2). The optical density (OD) of the sample plate is measured at 450 nm in a microplate reader.The viability of tumor cells is assessed by calculating the OD ratio of the specific OD in each sample to that of the control. Each experiment is conducted in quadruplicate. The values are averaged and normalized against the controls to generate dose–response curves [2].Animal Administration: Cilengitide is prepared in saline.[2][3]Mice [2]Product Name:Cilengitide Cat. No.:HY-16141CAS No.:188968-51-6Molecular Formula:C 27H 40N 8O 7Molecular Weight:588.66Target:Integrin; Autophagy Pathway:Cytoskeleton; Autophagy Solubility:DMSO: ≥ 44 mg/mL; H 2O: ≥ 32 mg/mLMale Balb/c–nu mice, at 8 weeks of age, are randomly assigned to four groups: control (n=10), Cilengitide (n=10), Belotecan (n=10) and combination (n=10). Cilengitide is administered intraperitoneally at a dose of 20 mg/kg daily and the Belotecan at a dose of 10 mg/kg every 4 days. The optimal dose is calculated. The control group of animals is injected with saline only. A single dose of the drugs comprised a 3–sec infusion in a volume of 3 mL/kg. The drug treatments began 7 days after the implantation of tumor cells for 16 days. Half of the animals are sacrificed 1 month after the implantation of the tumor cells for tumor volume analysis and the rest of the animals are observed for another 2 months to analyze survival. The death of the animals is defined as a weight reduction of over 25% of the initial weight or an unexpected sudden death beforehand.Rat[3]Male inbred Brown Norway rats (250 to 300 g) are injected i.p. with 50 mg/kg Cilengitide or saline 2 hr before and 3 hr after Isolated limb perfusion. The Rats are used to investigate the effects of perfusing various combinations of melphalan, TNF and cilengitide with or without the additional i.p. administration of cilengitide before and after the ILP procedure itself. The i.p. administration pre– and post–ILP is intended to optimally saturate available αVβ3 and αVβ5 integrins. Saline is used as a control in both the i.p. and perfusion settings.References:[1]. Hariharan S, et al. Assessment of the biological and pharmacological effects of the alpha nu beta3 and alpha nu beta5 integrinreceptor antagonist, Cilengitide (EMD 121974), in patients with advanced solid tumors. Ann Oncol. 2007 Aug;18(8):1400–7.[2]. Kim YH, et al. Combination therapy of cilengitide with belotecan against experimental glioblastoma. Int J Cancer. 2013 Aug 1;133(3):749–56.[3]. Ten Hagen TL, et al. The αVβ3/αVβ5 integrin inhibitor cilengitide augments tumor response to melphalan isolated limb perfusion in a sarcoma model. Int J Cancer. 2012 Nov 13. [Epub ahead of print]Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Alda-1_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Alda–1 is a potent ALDH2 agonist, which significantly improves ALDH2 activity.In Vivo: Alda–1 treatment results in a significant decrease of 4–HNE–protein content in the plasma of apoE -/- mice. Alda–1administration leads to a slight increase in gene expression related to neurogenesis (Nog ), mitochondrial biogenesis (CYTB , ND1),and apoptosis (Bax , Gsk3b ) in the Hp of apoE -/- mice. Alda–1 administration leads to 2 and 10 differentially expressed proteins in theFCx and Hp of apoE -/- mice, respectively [1]. Alda–1 (1.5 mg/kg, b.w., i.p.) administration significantly increases the climbing time,tends to reduce the immobility time and increases the swimming time of the prenatally stressed rats in the forced swim test.Moreover, treatment of prenatally stressed rats with Alda–1 significantly increases number of entries into the open arms of the maze and the time spent therein, as assessed by elevated plus–maze test [2]. Alda–1 (8.5 mg/kg, i.p.) with glucose significantly lowers 4–HNE and FJB–positive cells in the cerebral cortex of Alda–1–treated rats than in DMSO–treated rats 24 h after glucose administration [3]. Alda–1 (10 mg/kg per day) treatment prevents aldehydic overload, mitochondrial dysfunction and improves ventricular function in post–MI cardiomyopathy rats [4].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay:[2]Spleen cells (4×106 cells/mL) are stimulated by optimal concentrations of concanavalin A (Con A; 2.5μg/mL and 0.6 μg/mL) and lipopolysaccharide (LPS, 5 μg/mL) and are incubated in 96–well plates at final volume of 0.2mL for 72 h. Cell proliferation is determined by adding 0.5 μCi of [3H]–thymidine per well at 16 h before the end of the incubation.The cultures are harvested with an automatic cell harvester, and [3H] thymidine incorporation is assessed using a liquid scintillationcounter.Animal Administration: Alda–1 is dissolved in 1 mL/kg b.w. DMSO/water 50/50.[2]After behavioral verification at three months of age,the animals are divided into the following four groups: control, control + Alda–1, prenatally stressed and prenatally stressed + Alda–1(6 animals per group). Alda–1 injections are given intraperitoneally (i.p.) once daily at a dose of 1.5 mg/kg b.w. (dissolved in 1 mL/kg b.w. DMSO/water 50/50) for 14 days. At the same time, the control and prenatally stressed rats receive 1 mL/kg b.w. DMSO/water 50/50. The injections of Alda–1 and vehicle are given between 10 a.m and 11 a.m. In the last five days of Alda–1 treatment the behavioral parameters in the elevated plus maze test and then in the forced swim test are measured.References:[1]. Stachowicz A, et al. Proteomic Analysis of Mitochondria–Enriched Fraction Isolated from the Frontal Cortex and Hippocampus of Apolipoprotein E Knockout Mice Treated with Alda–1, an Activator of Mitochondrial Aldehyde Dehydrogenase (ALDH2). Int J Mol Sci.[2]. Stachowicz A, et al. The impact of mitochondrial aldehyde dehydrogenase (ALDH2) activation by Alda–1 on the behavioral and biochemical disturbances in animal model of depression. Brain Behav Immun. 2016 Jan;51:144–53.Product Name:Alda–1Cat. No.:HY-18936CAS No.:349438-38-6Molecular Formula:C 15H 11Cl 2NO 3Molecular Weight:324.16Target:Aldehyde Dehydrogenase (ALDH)Pathway:Metabolic Enzyme/Protease Solubility:DMSO: ≥ 51 mg/mL[3]. Ikeda T, et al. Effects of Alda–1, an Aldehyde Dehydrogenase–2 Agonist, on Hypoglycemic Neuronal Death. PLoS One. 2015 Jun 17;10(6):e0128844.[4]. Gomes KM, et al. Aldehydic load and aldehyde dehydrogenase 2 profile during the progression of post–myocardial infarction cardiomyopathy: benefits of Alda–1. Int J Cardiol. 2015 Jan 20;179:129–138.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries
Data Sheet
BIOLOGICAL ACTIVITY:
Gatifloxacin is an antibiotic of the fourth–generation fluoroquinolone family, it inhibits the bacterial enzymes DNA gyrase and topoisomerase IV.
Target: Antibacterial
Gatifloxacin is an antibiotic of the fourth–generation fluoroquinolone family, that like other members of that family, inhibits the
bacterial enzymes DNA gyrase and topoisomerase IV. Gatifloxacin had activity equal to that of tosufloxacin and activity more potent than those of norfloxacin, ofloxacin, ciprofloxacin, and sparfloxacin against the second–step mutants (grlA gyrA; gatifloxacin MIC
range, 1.56 to 3.13 microg/ml) and had the most potent activity against the third–step mutants (grlA gyrA grlA; gatifloxacin MIC range,
1.56 to 6.25 microg/ml), suggesting that gatifloxacin possesses the most potent inhibitory activity against singly mutated topo IV and singly mutated DNA gyrase among the quinolones tested [1].
Ophthalmic gatifloxacin 0.3% is at least as effective as ciprofloxacin at healing corneal ulcers infected with Pseudomonas aeruginosa when gatifloxacin is administered less frequently than ciprofloxacin. Trends favored gatifloxacin in fluorescein retention scores [2].Clinical indications: Bacterial infection
FDA Approved Date:
Toxicity: Hepatotoxicity; Acute pancreatitis [3]; Torsades de pointes [4]
References:
[1]. Fukuda, H., S. Hori, and K. Hiramatsu, Antibacterial activity of gatifloxacin (AM–1155, CG5501, BMS–206584), a newly developed fluoroquinolone, against sequentially acquired quinolone–resistant mutants and the norA transformant of Staphylococcus aureus. Antimicrob Agents Chemother, 1998. 42(8): p.1917–22.
[2]. Jensen, H., et al., Comparison of ophthalmic gatifloxacin 0.3% and ciprofloxacin 0.3% in healing of corneal ulcers associated with Pseudomonas aeruginosa–induced ulcerative keratitis in rabbits. J Ocul Pharmacol Ther, 2005. 21(1): p. 36–43.
[3]. Cheung, O., et al., Gatifloxacin–induced hepatotoxicity and acute pancreatitis. Ann Intern Med, 2004. 140(1): p. 73–4.
[4]. Fteha, A., et al., Gatifloxacin induced torsades de pointes. Pacing Clin Electrophysiol, 2004. 27(10): p. 1449–50.
Product Name:
Gatifloxacin Cat. No.:
HY-10581CAS No.:
112811-59-3Molecular Formula:
C 19H 22FN 3O 4Molecular Weight:
375.39Target:
Bacterial Pathway:
Anti–infection Solubility:
10 mM in DMSO
Caution: Product has not been fully validated for medical applications. For research use only.
Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
