Indapamide_26807-65-8_DataSheet_MedChemExpress
可提取性核抗原(ENA)自身抗体谱检测试剂盒(免疫印迹法) 产品技术要求万孚

1.性能指标
1.1外观
a)试剂盒应完整、牢固,标志应清晰;
b)膜条应洁净、无杂物;
c)试剂盒中各组份应配置齐备,且含有标准图谱、说明书;
d)各试剂应清亮无悬浮物、沉淀和颗粒(显色液 A 允许有颗粒状物悬浮)。
1.2试剂盒质控线的判断
阴性参考品、阳性参考品与膜条反应后,在膜条上距零线下约 2mm 处应出现一条棕色线。
在以下特异性、分析灵敏度、稳定性、精密度试验中,阴性、阳性膜条上均应出现。
试剂质控线是判断试剂有没有失效的标准。
1.3标准图谱精密性
棕色的线条,应与膜条零线平行,能与标准谱中相应区带对应,其偏差应不超过±1mm,边缘应清晰,肉眼应能辨认。
1.4分析特异性
a)与七种阳性参考品反应的膜条应出现标准谱中对应的阳性区带,无假阴性;
b)与十份阴性参考品反应的膜条不应出现阳性区带,无假阳性。
1.5分析灵敏度
阳性参考品 1:100 稀释后,按操作说明书标准程序操作,阳性区带应出现。
1.6精密度
1.6.1批内精密度
同批试剂盒中取足量的成品检测抗 Sm、抗 U 1RNP、抗 rRNP、抗 SSA、抗SSB、抗 Scl-70 及抗Jo-1 阳性参考品和 10 份阴性参考品,检测结果应一致。
1.6.2批间精密度
取连续三个批次的试剂盒检测抗 Sm、抗U 1RNP、抗rRNP、抗SSA、抗SSB、
抗 Scl-70 及抗 Jo-1 阳性参考品和 10 份阴性参考品,检测结果应一致。
1.7装量最大允许负偏差
各试剂的装量最大允许负偏差应≤5%。
Healthy Blue SC会员手册说明书
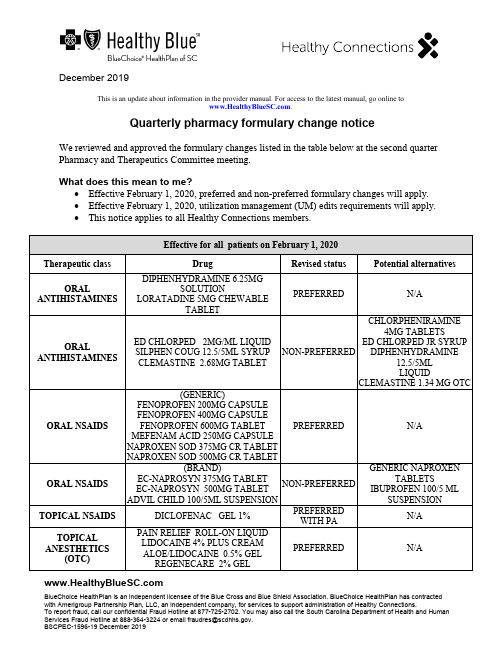
December 2019BlueChoice HealthPlan is an independent licensee of the Blue Cross and Blue Shield Association. BlueChoice HealthPlan has contracted with Amerigroup Partnership Plan, LLC, an independent company, for services to support administration of Healthy Connections.To report fraud, call our confidential Fraud Hotline at 877-725-2702. You may also call the South Carolina Department of Health and Human ************************************************************.BSCPEC-1596-19 December 2019This is an update about information in the provider manual. For access to the latest manual, go online to .Quarterly pharmacy formulary change noticeWe reviewed and approved the formulary changes listed in the table below at the second quarter Pharmacy and Therapeutics Committee meeting.What does this mean to me?• Effective February 1, 2020, preferred and non-preferred formulary changes will apply. • Effective February 1, 2020, utilization management (UM) edits requirements will apply. • This notice applies to all Healthy Connections members.Effective for all patients on February 1, 2020Therapeutic class DrugRevised status Potential alternativesORALANTIHISTAMINESDIPHENHYDRAMINE 6.25MGSOLUTIONLORATADINE 5MG CHEWABLETABLETPREFERREDN/AORALANTIHISTAMINESED CHLORPED 2MG/ML LIQUID SILPHEN COUG 12.5/5ML SYRUP CLEMASTINE 2.68MG TABLET NON-PREFERRED CHLORPHENIRAMINE4MG TABLETSED CHLORPED JR SYRUPDIPHENHYDRAMINE12.5/5MLLIQUIDCLEMASTINE 1.34 MG OTCORAL NSAIDS(GENERIC)FENOPROFEN 200MG CAPSULE FENOPROFEN 400MG CAPSULE FENOPROFEN 600MG TABLET MEFENAM ACID 250MG CAPSULE NAPROXEN SOD 375MG CR TABLET NAPROXEN SOD 500MG CR TABLETPREFERRED N/A ORAL NSAIDS(BRAND) EC-NAPROSYN 375MG TABLET EC-NAPROSYN 500MG TABLET ADVIL CHILD 100/5ML SUSPENSION NON-PREFERRED GENERIC NAPROXENTABLETSIBUPROFEN 100/5 ML SUSPENSIONTOPICAL NSAIDSDICLOFENAC GEL 1% PREFERREDWITH PAN/A TOPICALANESTHETICS(OTC)PAIN RELIEF ROLL-ON LIQUIDLIDOCAINE 4% PLUS CREAMALOE/LIDOCAINE 0.5% GELREGENECARE 2% GELPREFERRED N/ALIDODOSE 3% GELREGENECARE SPRAYALOCANE 4% GELAFTERBURN 2.5% GELXOLIDO 2% CREAM BURN RELIEF 0.5% AEROSAL ASPERCREME 4% SPRAYLIDOCAINE 3% CREAMLIDOCAINE 4% CREAMLIDOCAINE 5% CREAMAFTERSUN 0.5% GELLIDOCAINE 4% PADTOPICAL ANESTHETICS(RX)LIDOCAINE 3% CREAMLIDOCAINE 5% OINTMENT NON-PREFERREDOTC LIDOCAINEPRODUCTSRX LIDOCAINE5% PATCH(PA REQUIRED)MISCELLANEOUS ANTICONVULSANTSPREGABALIN 25MG CAPSULEPREGABALIN 50MG CAPSULEPREGABALIN 75MG CAPSULEPREGABALIN 100MG CAPSULEPREGABALIN 150MG CAPSULEPREGABALIN 200MG CAPSULEPREGABALIN 225MG CAPSULEPREGABALIN 300MG CAPSULEPREGABALIN SOL 20MG/MLPREFERREDWITH NO PRIORAUTHORIZATION(PA)N/AATOPICDERMATITIS PIMECROLIMUS 1% CREAMPREFERREDWITH STEPTHERAPY (ST)N/AFIBRATESFENOFIBRATE 130MG CAPSULEFENOFIBRATE 145MG TABLETFENOFIBRIC 35MG TABLETFENOFIBRIC 105MG TABLETFENOFIBRIC 135MG DR CAPSULENON-PREFERREDWITH STFENOFIBRATE134MG, 160MG, 200MG,43MG, 48MG,54 MG,67 MGFENOFIBRIC ACID 45 MGALCOHOL SWABS (MANUFACTURERS) GLOBAL DIABETICRITE AID NON-PREFERREDMANUFACTURERSBD DIABETESDYNAREXHEALTH MARTULTIMEDALCOHOL SWABS (MANUFACTURERS) BD DIABETESDYNAREXHEALTH MARTULTIMEDPREFERRED N/AIRON SUPPLEMENTS (GENERIC OTC)IRON 45MG TABLETSLOW-RELEASE FE 45MG TABLETHEMAX TABLETGENTLE IRON 28MG CAPSULEHIGH POTENCY FE 27MG TABLETNU-IRON 150 150MG CAPSULEABATRON AF TABLETSLOW IRON 50MG TABLETPREFERRED N/AFERGON 27MG TABLETIRON SUPPLEMENTS(BRAND OTC)FOLITAB 500 TABLET IRON 28MG TABLETFERROUS GLUC 324MG TABLETEZFE 200MG CAPSULEFERROUS GLUC TAB 324MGFERROUS SULF 324MG EC TABLETFERRETTS 325MG TABLETFERREX 150MG CAPSULEFERREX 28 MIS FERREX 150 PLUS CAPSULE FERREX 150 FORTE PL CAPSULECHEWABLE IRONPEDIATRIC IRON CHEWABLEFERROUS SUL 220/5ML LIQUIDFERROUS SULF 300/5ML SYRUPFEOSOL 200MG TABLETSLOW RELEASE FE 143MG CRTABLETNON- PREFERRED OTC GENERIC IRONSUPPLEMENTSRX PRODUCTS:HEMATOGEN FA CAPSULEHEMETAB TABLETMULTIGEN TABLETMULTIGEN PLS TABLETMULTIGEN FOLICTABLETFERRAPLUS 90 TABLETTARON FORTE CAPSULEFOLIVANE-F CAPSULEFOLIVANE-PLS CAPSULECENTRATEX CAPSULEIRON SUPPLEMENTS(PRESCRIPTIONSTRENGTH)IFEREX 150 FORTE CAPSULE HEMATOGEN CAPSULE HEMATOGEN FORTE CAPSULE TRICON CAPSULE MYFERON 150 FORTE CAPSULE FERROCITE PLUS TABLET FEROCON CAPSULE PUREVIT DUA FE PLUS CAPSULE HEMATINIC PL VIT/MIN TABLET HEMATINIC/FA TABLET POLY-IRON 150 FORT CAPSULE CORVITA 150 TABLET TRIGELS-F FORTE CAPSULE TL ICON CAPSULE SE-TAN PLUS CAPSULE NON- PREFERRED OTC GENERIC IRON SUPPLEMENTSRX PRODUCTS:HEMATOGEN FA CAPSULE HEMETAB TABLET MULTIGEN TABLET MULTIGEN PLS TABLET MULTIGEN FOLIC TABLET FERRAPLUS 90 TABLETTARON FORTE CAPSULE FOLIVANE-F CAPSULEFOLIVANE-PLS CAPSULE CENTRATEX CAPSULEUM edits — effective for all members no later than February 1, 2020 No changes in preferred/non-preferred status revision or addition to UM edit onlyANDROGENS*JATENZO CAPSULE ADD ST WITH QUANTITY LIMITS (QL)58 MG AND 198 MG QL: 4 PER DAY 237 MG QL: 2 PER DAY ANTICONVULSANTSNAYZILAM SPRAY 5MG ADD PA WITH QLQL: 50 MG PER 30 DAYS ANTICONVULSANTSOXTELLAR XR 150 MGOXTELLAR XR 600 MGREVISED QL LIMIT:150 MG: 3 TABLETS PER DAY 600 MG: 4 TABLETS PER DAYANTINEOPLASTICAGENTSPIQRAY 200 MG TABLETSPIQRAY 250 MG TABLETSPIQRAY 300 MG TABLETSADD PA WITH QL QL: 1 CARTON PER 28 DAYS ANTINEOPLASTICAGENTSXPOVIO PAK 60MGXPOVIO PAK 80MGXPOVIO PAK 100MGADD QL 1 CARTON PER 28 DAYSANTINEOPLASTICAGENTSNUBEQA 300MG TABLET ADD QL 4 TABLETS PER DAY ANTINEOPLASTICAGENTS TURALIO CAP 200MG ADD QL 4 TABLETS PER DAY ANTINEOPLASTICAGENTS PIQRAY 200MG TAB DOSE PIQRAY 300MG TAB DOSE PIQRAY 250MG TAB DOSE REVISE QL1 CARTON PER 28 DAYS CHOLESTEROLAGENTS EZALLOR SPRINKLE 5 MG CAP EZALLOR SPRINKLE 10 MG CAP EZALLOR SPRINKLE 20 MG CAP EZALLOR SPRINKLE 40 MG CAP ADD PA AND QLQL: 1 TABLET PER DAY COPD AGENTS DUAKLIR 400/12 INHALER ADD ST AND QLQL: 1 INHALER PER 30 DAYSCYSTIC FIBROSISAGENTSKALYDECO PAK 25MG ADD QL2 PACKETS PER DAYCYSTIC FIBROSISAGENTSORKAMBI GRANULES ADD QL2 PACKETS PER DAY HIVDOVATO TABLET EDURANT 25 MG TABLET DELSTRIGO TABLET COMPLERA TABLET ODEFSEY TABLET JULUCA TABLET ADD PA FOR NEW STARTS AND ADD QLQL: 1 PER DAY HIVINTELENCE TABLET ADD PA FOR NEW STARTS AND ADD QLQL:200 MG- 2 TABLETS PER DAY 400 MG- 4 TABLETS PER DAY 25 MG – 16 TABLETS PER DAYHIVATRIPLA TABLET BIKTARVY TABLET CIMDUO TABLET DESCOVY TABLETEMTRIVA 200 MG CAPSULE EPIVIR 300 MG TABLET EPZICOM TABLET EVOTAZ TABLET GENVOYA TABLET PIFELTRO 100 MG TABLET PREZCOBIX TABLET PREZISTA 800 MG TABLET REYATAZ 300 MG CAPSULESTRIBILD TABLET SUSTIVA 600 MG TABLETSYMFI TABLET SYMFI LO TABLET SYMTUZA TABLET TRIUMEQ TABLET TRUVADA TABLET TYBOST 150 MG TABLET VIDEX EC 400 MG CAPSULE VIDEX EC 250 MG CAPSULE VIRAMUNE XR 400 MG TABLETADD QL 1 PER DAYTEMIXYS TABLETHIVREYATAZ 200 MG CAPSULE REYATAZ 150 MG CAPSULE VIDEX EC 200 MG CAPSULE ZERIT 40 MG CAPSULE ZERIT 30 MG CAPSULE COMBIVIR TABLET DUTREBIS TABLET EPIVIR 150 MG TABLET ISENTRESS HD 600 MG TABLET PREZISTA 600 MG TABLET RETROVIR 300 MG TABLET SELZENTRY 75 MG TABLET TIVICAY 10 MG, 25 MG AND 50 MGTABLETTRIZIVIR TABLETVIRAMUNE 200 MG TABLET ZIAGEN 300 MG TABLET ADD QL 2 PER DAYHIV ISENTRESS 100 MG GRANULE PACKET FOR SUSPENSION ADD QL2 PACKETS PER DAYHIVVIDEX EC 125 MG CAPSULE VIRAMUNE XR 100MG TABLET ADD QL 3 PER DAYHIVAPTIVUS 250 MG CAPSULE INVIRASE 500 MG TABLET ISENTRESS 400 MG TABLET KALETRA 200 MG-50 MG TABLETLEXIVA 700 MG TABLET SELZENTRY 300 MG TABLET SELZENTRY 150 MG TABLET SUSTIVA 200 MG CAPSULE VIRACEPT 625 MG TABLET ZERIT 20 MG CAPSULE ZERIT 15 MG CAPSULEADD QL 4 PER DAYHIVREYATAZ 50 MG POWDER FORSUSPENSIONADD QL5 PACKETS PER DAYHIVCRIXIVAN 400 MG CAPSULE PREZISTA 150 MG TABLET RESCRIPTOR 200 MG TABLET RETROVIR 100 MG CAPSULE ISENTRESS 100 MG CHEWABLEADD QL 6 PER DAY HIV SELZENTRY 25 MG TABLET ADD QL 8 PER DAY HIV TROGARZO 150MG/ML VIAL ADD QL8 VIALS PER 28 DAYSHIVINVIRASE 200 MG CAPSULE KALETRA 100 MG-25 MG TABLETPREZISTA 75 MG TABLET VIRACEPT 250 MG TABLET ADD QL 10 PER DAY HIVCRIXIVAN 200 MG CAPSULE NORVIR 100 MG TABLET NORVIR 100 MG CAPSULEADD QL 12 PER DAYNORVIR 100 MG ORAL POWDERPACKETRESCRIPTOR 100 MG TABLET SUSTIVA 50 MG CAPSULEHIV APTIVUS 100 MG/ML SOLUTION ADD QL 13 ML PER DAYHIV PREZISTA 100 MG/ML SUSPENSION ADD QL 14 ML PER DAY HIV KALETRA 400 MG-100 MG/5 MLORAL SOLUTIONNORVIR 80 MG/ML ORAL SOLUTION ADD QL 16 ML PER DAY HIV ISENTRESS 25 MG CHEWABLE ADD QL24 TABLETS PER DAYHIV EMTRIVA 10 MG/ML SOLUTION ADD QL 29 ML PER DAYHIVEPIVIR 10 MG/ML ORAL SOLUTION ZIAGEN 20 MG/ML SOLUTION ADD QL 32 ML PER DAY HIVVIDEX 4 GM PEDIATRIC ORALSOLUTIONVIDEX 2 GM PEDIATRIC ORALSOLUTIONVIRAMUNE 50 MG/5 MLSUSPENSION ADD QL 40 ML PER DAY HIV VIRACEPT 50 MG/G POWDERADD QL 53 GM PER DAYHIV FUZEON 90 MG VIAL ADD QL60 VIALS PER 30 DAYSHIV LEXIVA 50 MG/ML SUSPENSION ADD QL 60 ML PER DAYHIV SELZENTRY 20 MG/ML ORALSOLUTION ADD QL 62 ML PER DAYHIV RETROVIR 10 MG/ML SYRUP ADD QL 64 ML PER DAYHIVZERIT 1 MG/ML SOLUTION ADD QL 80 ML PER DAY IRRITABLE BOWEL SYNDROME (IBS)AGENTSZELNORM 6MG TABLET ADD PA AND QL QL 2 TABLETS PER DAY LAMBERT-EATON MYASTHENIC SYNDROME AGENTSRUZURGI 10MG TABLET ADD PA AND QL QL 10 TABLETS PER DAYNARCOTIC ANTAGONISTS SUBLOCADE 100/0.5 INJECTION SUBLOCADE 300/1.5 INJECTION REMOVE PANARCOTIC ANTAGONISTS VIVITROL 380MG INEJCTION REMOVE PA AND ADD QL QL 1 VIAL PER 28 DAYSNARCOTIC ANTAGONISTS ZUBSOLV 2.9-0.71 SUB REVISE QL QL 5 PER DAY ORAL DIABETICAGENTS*QTERNMET XR TABLETADD ST AND QLQL:5 MG/5 MG/1000 MG, 10 MG/5 MG/1000 MG:1 TABLET PER DAY2.5 MG/2.5 MG/1000 MG, 5 MG/2.5 MG/10000MG: 2 TABLETS PER DAYORAL DIABETICAGENTS QTERN 5-5MG TABLET ADD QL1 TABLET 28 DAYSINJECTABLE DIABETIC AGENTSOZEMPIC 2/1.5ML INJECTION ADD QL 1 PER 28 DAYSPRENATAL VITAMINS DUET DHADUET DHA BALANCEDNESTABS ABC NESTABS DHA OBTREX DHA SELECT-OB+DHATHERANATAL COMPLETEVITAFOL FE+ VITAFOL-OB+DHABAL-CARE DHA ESSENTIAL ADD QL 2 PER DAYPRENATAL VITAMINS CITRANATAL B-CALMADD QL 3 PER DAYTOPICAL ANTIPRURITICS DOXEPIN HCL 5% CREAM,ZONALON 5% CREAM, PRUDOXIN5% CREAM ADD PA AND QLQL 1 TUBE PER FILL; 1 FILL PER 3 MONTHSTOPICAL ANESTHETIC COMBINATIONSLIDOCAINE/PRILOCAINE CREAMREVISE QL30 GM PER 30 DAYS* Clinical edits will be put in place as these new drugs to come market.What action do I need to take?Please review these changes and work with your Healthy Connections members to transition them to formulary alternatives. If you determine preferred formulary alternatives are notclinically appropriate for specific members, you will need to obtain prior authorization (PA) to continue coverage beyond the applicable effective date.What if I need assistance?We recognize the unique aspects of member cases. If your Healthy Connections member cannot be converted to a formulary alternative for medical reasons, call our Pharmacy department at 866-902-1689 and follow the voice prompts for pharmacy PA.You can find the Preferred Drug List on our website at > Providers > Pharmacy Information. If you need assistance with any other item, contact the Customer Care Center at 866-757-8286.。
2015-2021年FDA批准的505b2药物

APPLICATIONNUMBERPROPRIETARY NAME ESTABLISHED NAME通用名APPLICANTNDA208746(1)PEMETREXED培美曲塞HOSPIRA INC NDA 206610ACETAMINOPHEN对乙酰氨基酚RISINGNDA 214313NOREPINEPHRINEBITARTRATE IN 5%DEXTROSENOREPINEPHRINEBITARTRATE酒石酸去甲肾上腺素BAXTER HEALTHCARECORPNDA 204803POSIMIR BUPIVACAINE布比卡因DURECT CORPNDA 204957ACETAMINOPHEN对乙酰氨基酚B BRAUN MEDICAL INCNDA 212994AZSTARYS SERDEXMETHYLPHENIDATE ANDDEXMETHYLPHENIDATE对甲苯磺酸盐和对甲苯磺酸盐COMMAVETHERAPEUTICS SANDA 211844MIDAZOLAM咪达唑仑INFORLIFE SANDA 213072ROSZET ROSUVASTATIN ANDEZETIMIBE瑞舒伐他汀和依折麦布ALTHERAPHARMACEUTICALSLLCNDA 214154NEXTSTELLIS DROSPIRENONE ANDESTETROL TABLETS屈螺酮和雌醇片MAYNE PHARMA LLCNDA 212045KLOXXADO NALOXONE HCL盐酸纳洛酮HIKMA PHARMACEUTICALS USA INCNDA214657(1)PEMETREXED SANDOZ INCNDA 211988ZYNRELEF BUPIVACAINE ANDMELOXICAM布比卡因和美洛昔康HERON THERAPEUTICSINCNDA 214253LEVOTHYROXINESODIUMCUSTOPHARM INCNDA 211488CAMCEVI LEUPROLIDE亮丙瑞林FORESEE PHARMACEUTICALS CO LTDNDA 214846MYFEMBREE RELUGOLIX 40 MG,ESTRADIOL 1 MG,AND NORETHINDRONEACETATE 0.5MGRELUGOLIX 40 mg、雌二醇 1 mg 和醋酸炔诺酮0.5 mgMYOVANT SCIENCESGMBHNDA 213378LYBALVI OLANZAPINE ANDSAMIDORPHAN奥氮平和沙美芬ALKERMES INCNDA 215025SODIUMPHENYLACETATE ANDSODIUM BENZOATE苯乙酸钠和苯甲酸钠MAIAPHARMACEUTICALSINCNDA 213218SOAANZ TORSEMIDE托拉塞米SARFEZ PHARMACEUTICALS INCNDA 213536REZIPRES EPHEDRINEHYDROCHLORIDE盐酸麻黄碱ETONPHARMACEUTICALSINCNDA 212156MICAFUNGIN米卡芬净PAR STERILE PRODUCTS LLCNDA 210282DAPTOMYCIN达托霉素HOSPIRA INC NDA 214965VERKAZIA CYCLOSPORINE环孢素SANTEN INCNDA 212303(1)DULUTEGRAVIR,LAMIVUDINE, ANDTENOFOVIRDISOPROXIL度替拉韦、拉米夫定和富马酸替诺福韦酯LUPIN LTDNDA 214902TWYNEO TRETINOIN ANDBENZOYL PEROXIDE维 A 酸和过氧化苯甲酰SOL-GELTECHNOLOGIES LTDNDA 215143SUCCINYLCHOLINECHLORIDE琥珀胆碱氯化物HIKMAPHARMACEUTICALSUSA INCNDA 210735CYCLOPHOSPHAMIDE环磷酰胺EUGIA PHARMA SPECIALITIES LTDNDA 213895VANCOMYCIN万古霉素XELLIA PHARMACEUTICALS APSNDA 214826LOREEV XR LORAZEPAM劳拉西泮ALMATICA PHARMA LLCNDA 211566(1)SITAGLIPTIN西他列汀ZYDUS WORLDWIDEDMCCNDA 213436TRUDHESA DIHYDROERGOTAMINEMESYLATE甲磺酸二氢麦角胺IMPEL NEUROPHARMANDA 215133SERTRALINEHYDROCHLORIDE盐酸舍曲林ALMATICA PHARMALLCNDA 212854ZIMHI NALOXONEHYDROCHLORIDE盐酸纳洛酮ADAMISPHARMACEUTICALSCORPNDA 213426SEGLENTIS CELECOXIB ANDTRAMADOLHYDROCHLORIDE塞来昔布和盐酸曲马多KOWAPHARMACEUTICALSAMERICA INCNDA 213978TYRVAYA VARENICLINESOLUTION伐尼克兰溶液OYSTER POINTPHARMA INCNDA 211950XIPERE TRIAMCINOLONEACETONIDE曲安奈德BAUSCH AND LOMBINCNDA 214028VUITY PILOCARPINEHYDROCHLORIDE盐酸匹洛卡品ABBVIE INCNDA 210526DYANAVEL XR AMPHETAMINE安非他明TRIS PHARMA INCNDA 213005(1)YUTREPIA TREPROSTINIL曲前列尼尔LIQUIDIATECHNOLOGIESNDA 214679EPRONTIA TOPIRAMATE托吡酯AZURITY PHARMACEUTICALS INCNDA 214869DHIVY CARBIDOPA ANDLEVODOPACARBIDOPA 和左旋多巴AVIONPHARMACEUTICALSLLCNDA 215668(1)BENDAMUSTINEHYDROCHLORIDE盐酸苯达莫司汀DR REDDYSLABORATORIES LTDNDA 213312FYARRO SIROLIMUS PROTEIN-BOUND PARTICLESSIROLIMUS 蛋白结合颗粒AADI BIOSCIENCEINCNDA 215422LYVISPAH BACLOFEN巴氯芬SAOL THERAPEUTICS RESEARCH LTDNDA 215650XACIATO CLINDAMYCINPHOSPHATE磷酸克林霉素DARE BIOSCIENCEINCNDA 215423ENTADFI TADALAFIL ANDFINASTERIDE他达拉非和非那雄胺VERU INCNDA 215935TARPEYO BUDESONIDE布地奈德CALLIDITAS THERAPEUTICS ABNDA 215019DARTISLA ODT GLYCOPYRROLATE甘氨酰吡咯酸EDENBRIDGE PHARMACEUTICALS LLCNDA 2I4032ILLUCCIX KIT FOR THEPREPARATION OF GA-68 PSMA-11GA-68 PSMA-11 制备试剂盒TELIXPHARMACEUTICALS USINCNDA 215395LANREOTIDE ACETATE醋酸来那度胺INVAGEN PHARMACEUTICALS INCNDA 214133RECORLEV LEVOKETOCONAZOLE左酮康唑STRONGBRIDGE DUBLIN LTDREVIEW CLASSIFI CATION 505(B)(2)APPROVALAPPROVAL DATE批准类型S Y1/8/2021Type 3 - New Dosage FormS Y1/15/2021Type 5 - New Formulation or New ManufacturerS Y1/15/2021Type 5 - New Formulation or New ManufacturerS Y2/1/2021Type 3 - New Dosage FormS Y2/18/2021Type 5 - New Formulation or New ManufacturerS Y3/2/2021Type 1 - New Molecular Entity and Type 4 - New CombinationS Y3/22/2021Type 5 - New Formulation or New ManufacturerS Y3/23/2021Type 4 - New CombinationS Y4/15/2021Type 1 - New Molecular EntityS Y4/29/2021Type 5 - New Formulation or New ManufacturerS Y5/6/2021Type 5 - New Formulation or New ManufacturerP Y5/12/2021Type 4 - New CombinationS Y5/17/2021Type 5 - New Formulation or New ManufacturerS Y5/25/2021Type 2 - New Active IngredientS Y5/26/2021Type 4 - New CombinationS Y5/28/2021Type 1 - New Molecular Entity and Type 4 - New CombinationS Y6/10/2021Type 5 - New Formulation or New ManufacturerS Y6/14/2021Type 5 - New Formulation or New ManufacturerS Y6/14/2021Type 5 - New Formulation or New ManufacturerNew ManufacturerS Y6/21/2021Type 5 - New Formulation or New ManufacturerS,O Y6/23/2021Type 5 - New Formulation or New ManufacturerS Y6/25/2021Type 4 - New Combination S Y7/26/2021Type 4 - New CombinationS Y8/20/2021Type 5 - New Formulation or New ManufacturerS Y8/25/2021Type 5 - New Formulation or New ManufacturerP Y8/26/2021Type 5 - New Formulation or New ManufacturerS Y8/27/2021Type 3 - New Dosage FormS Y9/2/2021Type 2 - New Active IngredientS Y9/2/2021Type 5 - New Formulation or New ManufacturerS Y10/4/2021Type 3 - New Dosage FormS Y10/15/2021Type 5 - New Formulation or New ManufacturerS Y10/15/2021Type 4 - New Combination S Y10/15/2021Type 3 - New Dosage Form S Y10/22/2021Type 3 - New Dosage FormS Y10/28/2021Type 5 - New Formulation or New ManufacturerS Y11/4/2021Type 3 - New Dosage Form S Y11/4/2021Type 3 - New Dosage FormS Y11/5/2021Type 3 - New Dosage FormS Y11/12/2021Type 5 - New Formulation or New ManufacturerNew ManufacturerP,O Y11/22/2021Type 5 - New Formulation or New ManufacturerS Y11/22/2021Type 3 - New Dosage FormP Y12/7/2021Type 5 - New Formulation or New ManufacturerS Y12/9/2021Type 4 - New CombinationP,O Y12/15/2021Type 5 - New Formulation or New ManufacturerS Y12/16/2021Type 3 - New Dosage FormS Y12/17/2021Type 3 - New Dosage Form and Type 4 - New CombinationS Y12/17/2021Type 5 - New Formulation or New ManufacturerS,O Y12/30/2021Type 2 - New Active Ingredient适应症NSCLC、Mesothelioma间皮瘤(注射剂)止痛、退烧(注射剂)升高低血压(注射剂)局部麻醉(注射剂)止痛、退烧CNS兴奋剂注射麻醉剂降低LDL-C预防妊娠阿片类拮抗剂,用于阿片类过量NSCLC、Mesothelioma间皮瘤术后止痛(注射剂)粘液性水肿昏迷myxedema coma晚期前列腺癌子宫平滑肌瘤月经量过多成人精神分裂症、成人双相 i 型障碍急性高血氨症成人心力衰竭、肾病相关水肿麻醉状态的低血压念珠菌血症、急性播散性念珠菌病、念珠菌腹膜炎和脓肿cSSSI、Bacteremia菌血症春季角结膜炎(Tentative Approval)痤疮局部治疗作为全身麻醉的辅助治疗、便于气管插管、在手术或机械通气期间提供骨骼肌松弛label not available败血症、感染性心内膜炎、皮肤和皮肤结构感染、骨感染、下呼吸道感染、艰难梭菌相关性腹泻、金黄色葡萄球菌引起的小肠结肠炎label not availablelabel not available偏头痛急性治疗MDD、OCD紧急治疗阿片类药物过量治疗需要阿片类镇痛剂且替代治疗效果不佳的成人急性疼痛。
接触碟产品目录

5
产品规格
90 mm 预灌装培养基平皿:用于空气中沉降菌的测定以及实验室 相关微生物指标的测定等。 75 mm 预灌装培养基平皿:用于 MILLIPORE 浮游生物检测仪配 套使用。 55 mm 预灌装培养基接触性平皿(Rodac Plate 接触碟) :用于设 备、车间、人员、包装材料等表面微生物的测定。
海南办事处 海口市南沙路 78 号南沙公寓 320 室 570206 电话: (0898)66703090 传真: (0898)66703090
8
2
产品用途
本品适用于物品表面(洁净厂房、机械设备、洁净服、包装材料等)表 面微生物的检测。广泛应用于制药、食品、医院以及化妆品等企事业单位 的环境微生物检测,比传统的棉签方法更简便、准确。可用于对洁净环境 中的微生物进行动态/静态监测,以及检验消毒效果等。
培养基种类(进口) :
∗ 胰蛋白大豆琼脂(TSA) ∗ 含卵磷脂及吐温 80 胰蛋白大豆琼脂(TSAWLP) ∗ 沙堡氏琼脂(SDA) ∗ 麦康凯琼脂(MCA) ∗ 营养琼脂 NA ∗ 玫瑰红纳琼脂 RBA、R2A 等 可在培养基中添加青霉素酶等催化酶,用于去除生产中产生的抗生素 粉尘的抗菌性。
江苏益玛生物科技有限公司(EM-BIO) ,成立于 2011 年 7 月,注册资
金 500 万元。公司创建于国内唯一的国家级医药产业园区——江苏泰州・中国医 药城,是国内首家与国外著名制药企业合作,按照欧盟 EMEA 标准,开发无菌环 境检测产品的民营企业;公司紧跟新版 GMP 要求,研发出高水平、高质量的预灌 装培养基平皿系列产品,产品的灌装及包装等主要环节,均在 B 级背景下的 A 级 半隔离器中完成,为国内首创。产品经与国际知名企业产品对照试验,性能优良, 反应灵敏,品质稳定,完全达到欧盟相关技术要求。目前,已有多家国内外知名 药企使用本公司产品。
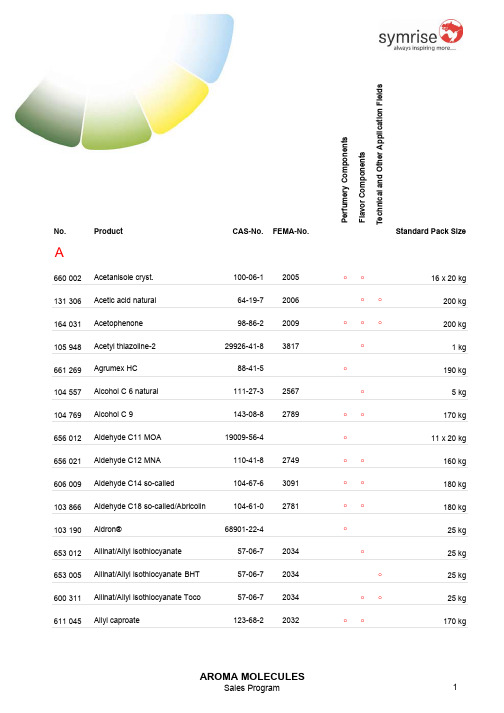
2007 德之馨产品目录(英文)
◦◦
180 kg
AROMA MOLECULES
Sales Program
5
Perfumery Components Flavor Components Technical and Other Application Fields
No.
Product
164 019 Ethyl acetoacetate
CAS-No. FEMA-No.
1646-26-0 93-51-6 2671
104-93-8 2681 68901-15-5
68039-69-0 706-14-9 2360
10519-11-6 431-03-8 2370
2222-33-5 1210-35-1
119-84-6 2381 75-18-3 2746
600 169 Dibenzosuberenone
613 135 Dibenzosuberone
690 616 Dihydrocoumarin
103 259 Dimethyl sulfide natural
130 273 Diphenyl oxide
E
131 341 Ethyl acetate natural
123-11-5 2670 104-21-2 2098 105-13-5 2099
Standard Pack Size
◦◦
180 kg
◦◦
170 kg
◦◦
200 kg
◦
170 kg
◦
180 kg
◦
25 kg
◦
20 kg
◦◦
20 kg
◦◦◦
200 kg
◦◦◦
220 kg
哥伦比亚CAN血琼脂平板产品说明书

化学品安全技术说明书第一部分化学品及企业标识产品中文名称:哥伦比亚CAN血琼脂平板产品英文名称:Columbia CNA Blood Agar Plate产品编号:CP0910企业名称:广东环凯微生物科技有限公司地址:广东省广州市黄埔区广州开发区科学城神舟路788号邮编:510663公司网址电子邮件地址:*********************传真号码:************销售热线:************-8602技术热线:************-8877/8876推荐用途和限制用途:生化研究/分析第二部分危险性概述GSH危害性类别非危险物质或混合物GSH标签要素非危险物质或混合物其它危害(健康危害、环境危害)未见报道第三部分成分/组成信息混合物化学品成分:参考培养基使用说明。
有害物质成分:无第四部分急救措施一般信息:无特殊的措施要求。
皮肤接触:立即用清水彻底清洗。
眼睛接触:立即提起眼睑,用大量流动清水冲洗15min。
如不适就医。
吸入:如果吸入,将人员移动到新鲜空气处,如果没有呼吸,进行人工呼吸操作,并联系医生。
食入:如误食,用水冲洗口腔,如不适就医。
就医信息:出示产品使用说明或者此SDS。
第六部分 泄露应急处理个人防护:穿个人实验服,佩戴手套和口罩。
环境保护措施:用湿布和地拖擦拭干净。
清洁/收集措施:保持干燥。
迅速清洗弄脏的区域。
第七部分 操作处置与储存安全操作注意事项:无特殊要求。
储存注意事项:贮存于2-8℃避光、干燥处。
第八部分 接触控制/个人防护职业接触限值没有已知的国家规定的暴露极限。
工程控制:提供安全淋浴和洗眼设备个人保护措施呼吸系统防护:在通风橱里称取产品,佩戴口罩。
眼睛防护:佩戴安全眼镜。
身体防护:穿实验室服。
手防护:戴防化学品手套。
其他防护:常规的工业卫生操作,工作后及时清洗双手。
第九部分 理化特性外观:凝胶 pH 值: 7.3±0.2 颜色:血红色 气味: 特征性 熔点:无数据资料 沸点: 无数据资料 燃点:无数据资料 闪点: 无数据资料 爆炸限度下限:无数据资料 上限: 无数据资料 热分解:无数据资料 溶解性:无数据资料。
肯定列表799项农业化学品汇总(1)

0.02 0.2 0.5 0.1 0.2 0.04
0.02 0.2 0.5 1 0.2 0.1
0.02
0.02 0.2 5 1 0.2 0.1 0.1
0.02 0.2 0.5 1 0.2 0.1
0.02
0.02 0.2 0.5 1 0.2 0.1 0.1
0.1
0.02 0.2 10
0.02 0.2 0.5
0.1
0.2
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
0.1
氨丙啉 AMPROLIUM 動物薬・合成抗菌剤・寄生虫駆除剤 VD 甲基碘 磺隆 IODOSULFURON METHYL 農薬・除草剤 P(H) 氯唑磷 ISAZOPHOS 農薬・殺虫剤・線虫駆除剤 P(I,N) 异恶隆 ISOURON 農薬・除草剤 P(H) 异丁子 香酚 動物薬・麻酔剤 VD 双苯恶 ISOEUGENOL 唑酸 恶唑磷 ISOXATHION 農薬・殺虫剤 P(I) 异恶氟 草 ISOXAFLUTOLE P(H) 農薬・除草剤 0.02 0.02 0.02 0.02 0.2 0.02 0.02 0.02 0.02 0.02 0.02 0.02 0.02 0.02
0.1 0.1 N.D 0.05
0.1
11 11‐1 現01 12 現02 13 現03 14 15 16 17 18 ★
4-クロ ルフェ ノキシ 酢酸 4-CPA 農薬・成長調整剤 0.02 0.02 0.02 0.02 0.02 P(GR) 5-プロピルスルホニル・1H・ベンズイミダゾール・2・アミン 5-(PROPYLSULPHONYL)-1-H-BENZIMIDAZOLE-2-AMINE 動物薬・寄生虫駆除剤 VD BHC 農薬/動物薬・殺虫剤 P(I), VD 0.2 0.2 DBED 六六六 BHC C 胺磺铜 DBEDC 農薬・殺菌剤・抗菌剤 P(F) 0.5 0.5 0.5 0.5 0.5 二氯乙 DCIP 丙醚 DCIP 農薬・殺虫剤 P(I) 1 1 1 1 1 DDT 滴滴涕 DDT 農薬・殺虫剤 P(I) 0.2 0.2 0.5 0.5 0.2 EPN 苯硫磷 EPN 農薬・殺虫剤 P(I) 0.1 EPTC 茵草敌 EPTC 農薬・除草剤 P(H) 0.1 0.04 0.04 0.04 0.1 MCPA 2甲4氯 MCPA 農薬・除草剤 P(H) 2甲4氯 MCPB 丁酸 MCPB 農薬・除草剤 P(H) 邻二氯 ODB 苯 ODB 動物薬・消毒薬 VD Sec-ブ チルア 仲丁胺 Sec-BUTYLAMINEP(H) 農薬・除草剤 0.1 0.1 0.1 0.1 0.1Biblioteka ★0.05 N.D
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
危险化学品目录表二

有机过氧化物,C型
单过氧马来酸叔丁酯[含量≤52%,糊状物]
tert-butyl monoperoxymaleate (not more than 52% as a paste)
107-08-4
易燃液体,类别3
185
2-碘丙烷
异丙基碘;碘代异丙烷
2-iodopropane;isopropyl iodide;iodo-iso-propane
75-30-9
易燃液体,类别3
186
1-碘丁烷
正丁基碘;碘代正丁烷
1-iodobutane;n-butyl iodide;iodo-n-butane
严重眼损伤/眼刺激,类别1
特异性靶器官毒性-一次接触,类别3(呼吸道刺激)
190
碘化亚汞
一碘化汞
mercurous iodide;mercurous monoiodide
15385-57-6
急性毒性-经口,类别2*
急性毒性-经皮,类别1
急性毒性-吸入,类别2*
特异性靶器官毒性-反复接触,类别2*
危害水生环境-急性危害,类别1
遇水放出易燃气体的物质和混合物,类别3
严重眼损伤/眼刺激,类别2
皮肤致敏物,类别1
生殖毒性,类别2
危害水生环境-急性危害,类别1
危害水生环境-长期危害,类别1
171
单过氧马来酸叔丁酯[含量>52%]
tert-butyl monoperoxymaleate (more than 52%)
仿制药参比制剂目录(第八批)
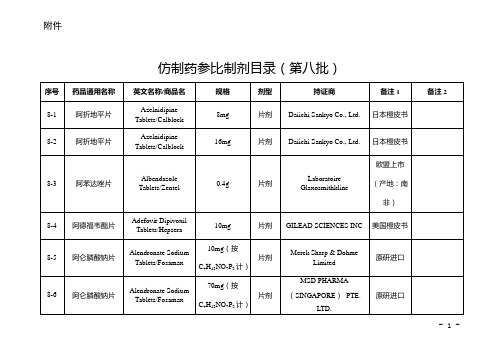
8-2
阿折地平片
16mg
片剂
Daiichi Sankyo Co., Ltd.
日本橙皮书 欧盟上市
8-3
阿苯达唑片
Albendazole Tablets/Zentel
0.4g
片剂
Laboratoire Glaxosmithkline
(产地:南 非)
8-4
阿德福韦酯片
Adefovir Dipivoxil Tablets/Hepsera Alendronate Sodium Tablets/Fosamax
原研进口 日本橙皮书收
8-17
氨酪酸片
Aminobutyric Acid Tablets/GAMMALO N
0.25g
片剂
Daiichi Sankyo Co., Ltd.
日本橙皮书
载名称: γ-Aminobutyri c Acid Tablets
- 3 -
序号 8-18
药品通用名称 奥氮平片
英文名称/商品名 Olanzapine Tablets/ Zyprexa Olanzapine Tablets/ Zyprexa Olanzapine Tablets/ Zyprexa Orlistat Capsules/Alli Orlistat Capsules/Xenical
片剂
Pfizer Inc.
原研进口
8-11
阿托伐他汀钙片
Atorvastatin Calcium Tablets/Lipitor
C33H35FN2O5 计)
片剂
Pfizer Inc.
原研进口
- 2 -
序号
药品通用名称
英文名称/商品名
规格 40mg(按
药用辅料中英文对照

药用辅料中英文对照药用辅料中英文对照1 阿拉伯胶(Acacia)2 乙酰舒泛钾(Acesulfame Potassium)3 冰醋酸(Acetic Acid,Glacial)4 乙酰枸橼酸三丁酯(AcetyltributylCitrate)5 乙酰枸橼酸三乙酯(AcetyltriethylCitrate)6 人血白蛋白(Albumin)7 乙醇(Alcohol)8 海藻酸(Alginic Acid)9 脂肪族聚酯(Aliphatic Polyesters)10 阿力糖(Alitame)11 杏仁油(Almond Oil)12 维生素E(Alpha Tocopherol)13 氨溶液(Ammonia Solution)14 维生素C(Ascorbic Acid)15 棕榈酸维生素C酯(Ascorbyl Palmitate)16 阿司帕坦(Aspartame)17 绿坡缕石(Attapulgite)18 皂土(Bentonite)19 苯扎氯铵(Benzalkonium Chloride)20 苄索氯铵(Benzethonium Chloride)21 苯甲酸(Benzoic Acid)22 苯甲醇(Benzyl Alcohol)23 苯甲酸苄酯(Benzyl Benzoate)24 溴硝丙二醇(Bronopol)25 丁羟茴醚(Butylated Hydroxyanisole)26 丁羟甲苯(Butylated Hydroxytoluene)27 羟苯丁酯(Butylparaben)28 碳酸钙(Calcium Carbonate)29 无水磷酸氢钙(Calcium Phosphate,Dibasic Anhydrous)30 磷酸氢钙二水合物(Calcium Phosphate,Dibasic Dihydrate)31 磷酸钙(Calcium Phosphate,Tribasic)32 硬脂酸钙(Calcium Stearate)33 硫酸钙(Calcium Sulfate)34 低芥酸菜籽油(Canola Oil)35 卡波姆(Carbomer)36 二氧化碳(Carbon Dioxide)37 羧甲纤维素钙(Carboxymethylcellulose Calcium)38 羧甲纤维素钠(Carboxymethylcellulose Sodium)39 角叉菜胶(Carrageenan)40 蓖麻油(Castor Oil)41 氢化蓖麻油(Castor Oil,Hydro-genated)42 微晶纤维素(Cellulose,Microcr ystalline)43 粉状纤维素(Cellulose,Powdered)44 微粉硅胶微晶纤维素(Cellulose, Silicified Microcrystalline)45 醋酸纤维素(Cellulose Acetate)46 纤维醋法酯(Cellulose Acetate Phthalate)47 角豆胶(Ceratonia)48 十八十六醇(Cetostearyl Alcohol)49 西曲溴铵(Cetrimide)50 十六醇(Cetyl Alcohol)51 壳聚糖(Chitosan)52 氯己定(Chlorhexidine)53 三氯叔丁醇(Chlorobutanol)54 氯甲酚(Chlorocresol)55 一氯二氟乙烷(Chlorodifluoroe-thane)56 氟里昂(Chlorofluorocabons)57 对氯间二甲酚(Chloroxylenol)58 胆固醇(Cholesterol)59 枸橼酸(Citric Acid Monohydrate)60 胶态二氧化硅(微粉硅胶)(Colloidal Silicon Dioxide)61 着色剂(Coloring Agents)62 玉米油(Corn Oil)63 棉籽油(Cottonseed Oil)64 甲酚(Cresol)65 交联羧甲纤维素钠(Croscarmellose Sodium)66 交联聚维酮(Crospovidone)67 环糊精(Cyclodextrins)68 环甲基硅酮(Cyclomethicone)69 苯甲地那铵(Denatonium Benzoate)70 葡萄糖结合剂(Dextrates)71 糊精(Dextrin)72 葡萄糖(Dextrose)73 邻苯二甲酸二丁酯(Dibutyl Phthalate)74 癸二酸二丁酯(Dibutyl Sebacate)75 二乙醇胺(Diethanolamine)76 邻苯二甲酸二乙酯(Diethyl Phthalate)77 二氟乙烷(Difluoroethane)78 二甲硅油(Dimethicone)79 二甲醚(Dimethyl Ether)80 邻苯二甲酸二甲酯(Dimethyl Phthalate)81 二甲亚砜(Dimethyl Sulfoxide)82 多库酯钠(Docusate Sodium)83 依地酸(乙二胺四乙酸)(Edetic Acid)84 乙酸乙酯(Ethyl Acetate)85 乙基麦芽酚(Ethyl Maltol)86 油酸乙酯(Ethyl Oleate)87 乙基香草醛(Ethyl Vanillin)88 乙基纤维素(Ethylcellulose)89 硬脂酸棕榈酸乙二醇酯(Ethylene Glycol Palmitostearate)90 羟苯乙酯(Ethylparaben)91 果糖(Fructose)92 富马酸(Fumaric Acid)93 明胶(Gelatin)94 液体葡萄糖(Glucose,Liquid)95 甘油(Glycerin)96 山萮酸甘油酯(Glyceryl Behenate)97 单油酸甘油酯(Glyceryl Monooleate)98 单硬脂酸甘油酯(Glyceryl Monostearate)99 硬脂酸棕榈酸甘油酯(Glyceryl Palmitostearate)100 四氢呋喃聚乙二醇醚(Glycofurol)101 瓜耳胶(Guar Gum)102 七氟丙烷(HFC)(Heptafluoro-propane)103 海克西定(Hexetidine)104 烷烃类(HC) (Hydrocarbons)105 盐酸(Hydrochloric Acid)106 羟乙纤维素(Hydroxyethyl Cellulose)107 羟乙甲纤维素(Hydroxyethylmethyl Cellulose)108 羟丙纤维素(Hydroxypropyl Cellulose)109 低取代羟丙纤维素(Hydroxypropyl Cellulose,Low-substituted) 110 羟丙甲纤维素(Hypromellose)111 羟丙甲纤维素酞酸酯(Hypromellose Phthalate)112 咪唑烷脲(Imidurea)113 异丙醇(Isopropyl Alcohol)114 肉豆蔻酸异丙酯(Isopropyl Myristate)115 棕榈酸异丙酯(Isopropyl Palmitate)116 白陶土(Kaolin)117 乳酸(Lactic Acid)118 拉克替醇(Lactitol)119 乳糖(Lactose)120 羊毛脂(Lanolin)121 含水羊毛脂(Lanolin,Hydrous)122 羊毛醇(Lanolin Alcohols)123 卵磷脂(Lecithin)124 硅酸镁铝(Magnesium Aluminum Silicate)125 碳酸镁(Magnesium Carbonate)126 氧化镁(Magnesium Oxide)127 硅酸镁(Magnesium Silicate)128 硬脂酸镁(Magnesium Stearate)129 三硅酸镁(Magnesium Trisilicate)130 苹果酸(Malic Acid)131 麦芽糖醇(Maltitol)132 麦芽糖醇溶液(Maltitol Solution)133 麦芽糖糊精(Maltodextrin)134 麦芽酚(Maltol)135 麦芽糖(Maltose)136 甘露醇(Mannitol)137 中链脂肪酸甘油三酯(Medium-chain Triglycerides) 138 葡甲胺(Meglumine)139 薄荷脑(Menthol)140 甲基纤维素(Methylcellulose)141 羟苯甲酯(Methylparaben)142 液体石蜡(Mineral Oil)143 轻质液体石蜡(Mineral Oil,Light)144 液体石蜡羊毛醇(Mineral Oil and Lanolin Alcohols) 145 单乙醇胺(Monoethanolamine)146 谷氨酸一钠(Monosodium Glutamate)147 硫代甘油(Monothioglycerol)148 氮(Nitrogen)149 一氧化二氮(Nitrous Oxide)150 油酸(Oleic Acid)151 橄榄油(Olive Oil)152 石蜡(Paraffin)153 花生油(Peanut Oil)154 凡士林(Petrolatum)155 凡士林羊毛醇(Petrolatum and Lanolin Alcohols)156 苯酚(Phenol)157 苯氧乙醇(Phenoxyethanol)158 苯乙醇(Phenylethyl Alcohol)159 醋酸苯汞(Phenylmercuric Acetate)160 硼酸苯汞(Phenylmercuric Borate)161 硝酸苯汞(Phenylmercuric Nitrate)162 磷酸(Phosphoric Acid)163 波拉克林钾(Polacrilin Potassium)164 泊洛沙姆(Poloxamer)165 葡聚糖(Polydextrose)166 聚乙二醇(Polyethylene Glycol)167 聚氧乙烯(Polyethylene Oxide)168 聚(甲基)丙烯酸树脂(Polymethacr-ylates)169 聚氧乙烯烷基醚(Polyoxyethylene Alkyl Ethers)170 聚氧乙烯蓖麻油衍生物(Polyoxyeth-ylene Castor Oil Derivatives) 171 聚山梨酯(Polyoxyethylene Sorbitan Fatty Acid Esters)172 硬脂酸聚氧乙烯酯(Polyoxyethylene Stearates)173 聚醋酸乙烯酞酸酯(Polyvinyl Acetate Phthalate)174 聚乙烯醇(Polyvinyl Alcohol)175 苯甲酸钾(Potassium Benzoate)176 碳酸氢钾(Potassium Bicarbonate)177 氯化钾(Potassium Chloride)178 枸橼酸钾(Potassium Citrate)179 氢氧化钾(Potassium Hydroxide)180 焦亚硫酸钾(Potassium Metabisulfite)181 山梨酸钾(Potassium Sorbate)182 聚维酮(Povidone)183 丙酸(Propionic Acid)184 没食子酸丙酯(Propyl Gallate)185 碳酸丙烯酯(Propylene Carbonate)186 丙二醇(Propylene Glycol)187 海藻酸丙二醇酯(Propylene Glycol Alginate)188 羟苯丙酯(Propylparaben)189 糖精(Saccharin)190 糖精钠(Saccharin Sodium)191 芝麻油(Sesame Oil)192 虫胶(Shellac)193 二氧化硅二甲硅油(Simethicone)194 海藻酸钠(Sodium Alginate)195 抗坏血酸钠(Sodium Ascorbate)196 苯甲酸钠(Sodium Benzoate)197 碳酸氢钠(Sodium Bicarbonate)198 氯化钠(Sodium Chloride)199 枸橼酸钠二水合物(Sodium Citrate Dihydrate)200 环拉酸钠(Sodium Cyclamate)201 氢氧化钠(Sodium Hydroxide)202 月桂硫酸钠(十二烷基硫酸钠)(Sodium Lauryl Sulfate) 203 焦亚硫酸钠(偏亚硫酸钠)(Sodium Metabisulfite) 204 磷酸氢二钠(Sodium Phosphate,Dibasic)205 磷酸二氢钠(Sodium Phosphate ,Monobasic)206 丙酸钠(Sodium Propionate)207 羧甲淀粉钠(Sodium Starch Glycolate)208 硬脂富马酸钠(Sodium Stearyl Fumarate)209 山梨酸(Sorbic Acid)210 山梨坦酯Sorbitan Esters(Sorbitan Fatty Acid Esters)211 山梨醇(Sorbitol)212 大豆油(Soybean Oil)213 淀粉(Starch)214 预胶化淀粉(Starch,Pregelatinized)215 灭菌玉米淀粉(Starch,Sterilizable Maize)216 硬脂酸(Stearic Acid)217 硬脂醇(Stearyl Alcohol)218 羟糖氯(Sucralose)219 蔗糖(Sucrose)220 可压性蔗糖(Sugar,Compressible)221 蔗糖粉(Sugar,Confectioner’s)222 蔗糖球形颗粒(Sugar Spheres)223 硫酸(Sulfuric Acid)224 葵花籽油(Sunflower Oil)225 氢化植物油(硬脂)栓剂基质(Sup-pository Bases,Hard Fat) 226 滑石粉(Talc)227 酒石酸(Tartaric Acid)228 四氟乙烷(HFC)(Tetrafluoroe-thane)229 硫柳汞(Thimerosal)230 二氧化钛(Titanium Dioxide)231 西黄蓍胶(Tragacanth)232 海藻糖(Trehalose)233 三醋汀(Triacetin)234 枸橼酸三丁酯(Tributyl Citrate)235 三乙醇胺(Triethanolamine)236 枸橼酸三乙酯(Triethyl Citrate)237 香草醛(Vanillin)238 氢化植物油(Vegetable Oil,Hydrogenated)239 水(Water)240 阴离子乳化蜡(Wax,Anionic Emulsifying)241 巴西棕榈蜡(Wax,Carnauba)242 十六醇酯蜡(Wax,Cetyl Esters)243 微晶蜡(Wax,Microcrystalline)244 非离子乳化蜡(聚西托醇乳化蜡)(Wax,Nonionic Emulsifying) 245 白蜡(Wax,White)246 黄蜡(Wax,Yellow)247 黄原酸胶(Xanthan Gum)248 木糖醇(Xylitol)796249 玉米朊(玉米蛋白)(Zein)250 硬脂酸锌(Zinc Stearate)。
电磁炉系列产品说明书

Serie 8, Placa de inducción, 80 cm, NegroPIE875DC1EHEZ390090 Wok para radiantes e inducciónHEZ390210 Sartén antiadherente de 15 cm. de base. HEZ390220 Sartén antiadherente de 19 cm de base HEZ390230 Sartén antiadherente de 21 cm de base HEZ390250 Sartén antiadherente de 28 cm de base HEZ394301 Accesorio de uniónHEZ9ES100 Cafetera 4 tazasHEZ9FE280 Sartén de hierro Ø 18 / 28 cmHEZ9SE030 Set de 2 ollas y 1 sarténHEZ9SE040 set de 4 piezasHEZ9SE060 set de 6 piezas Encimera de inducción con PerfectFry: logra resultados de fritura perfectos gracias al control automático de temperatura.• DirectSelect Premium: selección directa y fácil de la zona decocción, potencia y funciones deseada.• PerfectFry: Un perfecto dorado al momento de freír gracias al sensor de control con 5 niveles de potencia.• PowerBoost: hasta un 50% más de potencia para una cocción más rápida.• QuickStart: start comience de inmediato a cocinar y seleccione el nivel de cocción deseado.• ReStart: si algo se derrama, la placa de cocción se apagaautomáticamente y guarda la última configuración seleccionada.Familia de Producto: ..........................Placa independie.vitrocerámica Tipo de construcción: .........................................................Integrable Entrada energética: ...............................................................Eléctrico Número de posiciones que se pueden usar al mismo tiempo: . (4)Medidas del nicho de encastre: ...............51 x 750-780 x 490-500 mm Anchura: .................................................................................816 mm Dimensiones aparato (alto, ancho, fondo): ...........51 x 816 x 527 mm Medidas del producto embalado: ........................126 x 953 x 603 mm Peso neto: ...............................................................................13.3 kg Peso bruto: ..............................................................................17.0 kg Indicador de calor residual: .................................................Separado Ubicación del panel de mandos: ...............Frontal de la vitrocerámica Material de la superficie básica: ....................................Vitrocerámica Color de superficie superior: ........................Negro, Aluminio gratado Longitud del cable de alimentación eléctrica: ......................110.0 cm Sealed Burners: ..............................................................................No Zonas con booster: ....................................................................Todas Potencia del elemento calentador: ...........................................2.2 kW Potencia del tercer elemento calentador: ................................1.4 kW Potencia del quinto elemento calentador: ...............................2.6 kW Power of heating element (kW in boost): ..............................[3.7] kW Power of 3rd heating element (kW in boost): ........................[2.1] kW Power of 5th heating element (kW in boost): ........................[3.7] kW Potencia: .................................................................................7400 W Tensión: ..............................................................................220-240 V Frecuencia: ..........................................................................60; 50 Hz Entrada energética: ...............................................................Eléctrico Tipo de clavija: ..................................................................sin enchufe Appliance Dimensions (h x w x d) (in): ..........................................x x Dimensions of the packed product: ....................4.96 x 23.74 x 37.51 Net weight: .........................................................................29.000 lbs Gross weight: .....................................................................37.000 lbs Número de posiciones que se pueden usar al mismo tiempo: . (4)Longitud del cable de alimentación eléctrica: ......................110.0 cm Medidas del nicho de encastre: ...............51 x 750-780 x 490-500 mm Dimensiones aparato (alto, ancho, fondo): ...........51 x 816 x 527 mm Medidas del producto embalado: ........................126 x 953 x 603 mm Peso neto: ...............................................................................13.3 kg Peso bruto: ..............................................................................17.0 kgSerie 8, Placa de inducción, 80 cm, NegroPIE875DC1EEncimera de inducción con PerfectFry: logra resultados de fritura perfectos gracias al control automático de temperatura.Diseño:- Combinación perfecta con el resto de placas de cristal vitrocerámico y terminación PremiumConfort:- 4 zonas de inducción- Programación de tiempo de cocción para cada zona y avisador acústico- Avisador acústico- STFE- Regulación electrónica con 17 niveles de potencia- Detección de recipiente- Posibilidad de limitar la potencia total de la encimera- Desconexión de seguridad de la placa- Bloqueo de seguridad para niños automático o manual- Función Clean: bloqueo temporal del control- Display de consumo de energía- Función Sprint en todas las zonasSeguridad:- Indicador de calor residual dual (H/h)- Main Switch- Sensor PerfectFry con 5 ajustes de temperaturaSerie 8, Placa de inducción, 80 cm,NegroPIE875DC1E。
Pyxis Lab ST-588 PTSA Fluorescent Polymer Dual Inl

ST-588PTSA/Fluorescent Polymer DualInline SensorUser ManualOctober12,2020Rev.2.00Pyxis Lab,Inc.1729Majestic Dr.Suite5Lafayette,CO80026USA©2017Pyxis Lab,Inc.Pyxis Lab Proprietary and ConfidentialTable of Contents1Introduction21.1Main Features (2)2Specifications3 3Unpacking Instrument43.1Standard Accessories (4)3.2Optional Accessories (5)4Installation64.1ST-588Piping (6)4.2ST-588SS Piping (6)4.3Wiring (7)4.4Connecting via Bluetooth (8)4.5Connecting via USB (8)5Setup and Calibration with uPyxis®Mobile App95.1Download uPyxis®Mobile App (9)5.2Connecting to uPyxis®Mobile App (9)5.3Calibration Screen and Reading (10)5.4Diagnosis Screen (11)5.5Device Info Screen (12)6Setup and Calibration with uPyxis®Desktop App126.1Install uPyxis®Desktop App (12)6.2Connecting to uPyxis®Desktop App (13)6.3Information Screen (13)6.4Calibration Screen (14)6.5Diagnosis Screen (14)7Outputs157.14–20mA Output Setup (15)7.2Communication using Modbus RTU (15)8Sensor Maintenance and Precaution158.1Methods to Cleaning the ST-588 (16)8.2Storage (16)9Troubleshooting17 10Contact Us18Warranty InformationConfidentialityThe information contained in this manual may be confidential and proprietary and is the property of Pyxis Lab,rmation disclosed herein shall not be used to manufacture,construct,or otherwise reproduce the goods rmation disclosed herein shall not be disclosed to others or made public in any manner without the express written consent of Pyxis Lab,Inc.Standard Limited WarrantyPyxis Lab warrants its products for defects in materials and workmanship.Pyxis Lab will,at its option,repair or replace instrument components that prove to be defective with new or remanufactured components (i.e.,equivalent to new).The warranty set forth is exclusive and no other warranty,whether written or oral, is expressed or implied.Warranty TermThe Pyxis warranty term is thirteen(13)months ex-works.In no event shall the standard limited warranty coverage extend beyond thirteen(13)months from original shipment date.Warranty ServiceDamaged or dysfunctional instruments may be returned to Pyxis for repair or replacement.In some in-stances,replacement instruments may be available for short duration loan or lease.Pyxis warrants that any labor services provided shall conform to the reasonable standards of technical com-petency and performance effective at the time of delivery.All service interventions are to be reviewed and authorized as correct and complete at the completion of the service by a customer representative,or des-ignate.Pyxis warrants these services for30days after the authorization and will correct any qualifying deficiency in labor provided that the labor service deficiency is exactly related to the originating event.No other remedy,other than the provision of labor services,may be applicable.Repair components(parts and materials),but not consumables,provided during a repair,or purchased individually,are warranted for90days ex-works for materials and workmanship.In no event will the in-corporation of a warranted repair component into an instrument extend the whole instrument’s warranty beyond its original term.Warranty ShippingA Repair Authorization(RA)Number must be obtained from Pyxis Technical Support before any product can be returned to the factory.Pyxis will pay freight charges to ship replacement or repaired products to the customer.The customer shall pay freight charges for returning products to Pyxis.Any product returned to the factory without an RA number will be returned to the customer.To receive an RMA you can generate a request on our website at https:///request-tech-support/.Pyxis Technical SupportContact Pyxis Technical Support at+1(866)203-8397,*********************,or by filling out a request for support at https:///request-tech-support/.1IntroductionThe Pyxis ST-588inline fluorometer probe simultaneously measures the concentration of PTSA and Fluores-cent Polymer in water.It can be simply inserted to the compression fitting port of a custom-made tee.The standard ST-001installation tee provided with each ST-588sensor,has two¾inch female NPT ports and can be placed to an existing¾inch sample water line.Pyxis Lab also offers2”and3”Tee formats for larger flow installations.The4–20mA current output of the ST-588probe can be connected to any controller that accepts an isolated or non-isolated4–20mA input.The ST-588probe is a smart device.In addition to mea-suring PTSA and Fluorescent Polymer,the ST-588probe has extra photo-electric components that monitor the color and turbidity of the sample water.This extra feature allows automatic color and turbidity com-pensation to eliminate interference commonly associated with real-world waters.The Pyxis ST-588probe has a short fluidic channel and can be easily cleaned.The fluidic and optical ar-rangement of the ST-588probe is designed to overcome shortcomings associated with other fluorometers that have a distal sensor surface or a long,narrow fluidic cell.Traditional inline fluorometers are susceptible to color and turbidity interference and fouling and are difficult to properly clean.1.1Main FeaturesThe ST-588measures PTSA and Fluorescent Polymer in a water sample and includes the following features:•Easy calibration with using uPyxis®Mobile or Desktop App.•Automatic compensation for turbidity up to150NTU and color created by up to10ppm iron or equivalent to10ppm humic acid.•Diagnostic information(probe fouling,color or turbidity over range,failure modes)are available in uPyxis®App or via Modbus RTU.•Easy to remove from the system for cleaning and calibration without the need for any tools.2SpecificationsTable1.ST-588Specifications*With Pyxis’s continuous improvement policy,these specifications are subject to change without notice.†The fluorescent polymer concentration scale is based on the polymer containing0.25mole%fluorescent monomer.Typical polymer specifications are attached below but may vary by producer.‡See Figure4for ST-588SS dimensions.3Unpacking InstrumentRemove the instrument and accessories from the shipping container and inspect each item for any damage that may have occurred during shipping.Verify that all accessory items are included.If any item is missing or damaged,please contact Pyxis Lab Customer Service at*********************.3.1Standard Accessories•Tee Assembly3/4”NPT(1x Tee,O-ring,and Nut)P/N:ST-001*NOTE*ST-001is not included for ST-588SS•8-Pin Female Adapter/Flying Leads Cable(1.5ft)•User Manual available online at https:///support/3.2Optional AccessoriesFigure1.4Installation4.1ST-588PipingThe provided ST-001Tee Assembly can be connected to a pipe system through the3/4”female ports,either socket or NPT threaded.To properly install the ST-588probe into the ST-001Tee Assembly,follow the steps below:1.Insert the provided O-ring into the O-ring groove on the tee.2.Insert the ST-588probe into the tee.3.Tighten the tee nut onto the tee to form a water-tight,compression seal.Figure2.Dimension of the ST-588and the ST-001Tee Assembly(mm)4.2ST-588SS PipingThe ST-588SS probe has3/4”female NPT threaded ports on the probe itself and therefore does not require a custom tee assembly.It is recommended that two3/4”NPT to1/4”tubing adapters are used to connect the probe to the sampling system.Sample water entering the probe must be cooled down to below104°F (40°C).The probe can be held by a1.75-inch pipe clamp or mounted to a panel with four1/4-28bolts.See Figure4for ST-588SS dimensions.Figure3.Dimension of the ST-588SS(inch)4.3WiringIf the power ground terminal and the negative4–20mA terminal in the controller are internally connected (non-isolated4–20mA input),it is unnecessary to connect the4–20mA negative wire(gray)to the4–20mA negative terminal in the controller.If a separate DC power supply other than that from the controller is used,make sure that the output from the power supply is rated for22–26VDC@85mA.*NOTE*The negative24V power terminal(power ground)and the negative4–20mA ter-minal on the ST-588probe are internally connected.Follow the wiring table below to connect the ST-588probe to a controller:Table2.*Internally connected to the power ground4.4Connecting via BluetoothA Bluetooth adapter(P/N:MA-WB)can be used to connect a ST-588probe to a smart phone with the uPyxis®Mobile App or a computer with the uPyxis®Desktop App.Figure4.Bluetooth connection to ST-588probe4.5Connecting via USBA USB-RS485adapter(P/N:MA-485)can be used to connect a ST-588probe to a computer with the uPyxis®Desktop App.*NOTE*Using non-Pyxis USB-RS485adapters may result in permanent damage of the ST-588probe communication hardware.B connection to ST-588probe5Setup and Calibration with uPyxis®Mobile App5.1Download uPyxis®Mobile AppDownload uPyxis®Mobile App from Apple App Store or Google Play.Figure6.5.2Connecting to uPyxis®Mobile AppTurn on Bluetooth on your mobile phone(Do not pair the phone Bluetooth to the ST-588probe).Open uPyxis®Mobile App.Once the app is open the app will start to search for the sensor.Once the uPyxis®Mobile App connects to the sensor,press the ST-588probe.Figure7.5.3Calibration Screen and ReadingWhen connected,the uPyxis®Mobile App will default to the Calibration screen.From the Calibration screen,you can perform calibrations by pressing on Zero Calibration,Slope Calibration,and4–20mA Span for either Fluorescent Polymer or PTSA,independently.Follow the screen instructions for each calibration step.Figure8.5.4Diagnosis ScreenFrom the Diagnosis screen,you can check the diagnosis condition.This feature may be used for technical support when communicating with*********************.To preform a probe cleaniness check,first select the Diagnosis Condition which defines the fluid type that the ST-588probe in currently measuring,then press Cleanliness Check.If the probe is clean,a Clean mes-sage will be shown.If the probe is severely fouled,a Dirty message will be shown.In this case,follow the procedure in the Methods to Cleaning the ST-588section of this manual.Figure9.5.5Device Info ScreenFrom the Device Info screen.You can name the Device or Product as well as set the Modbus address.Figure10.6Setup and Calibration with uPyxis®Desktop App6.1Install uPyxis®Desktop AppDownload the latest version of uPyxis®Desktop software package from:https:///upyxis/this setup package will download and install the Framework4.5(if not previously installed on the PC),the USB driver for the USB-Bluetooth adapter(MA-NEB),the USB-RS485adapter(MA-485),and the main uPyxis®Desktop application.Double click the uPyxis.Setup.exe file to install.Figure11.Click Install to start the installation process.Follow the screen instructions to complete the USB driver and uPyxis®installation.6.2Connecting to uPyxis®Desktop AppWhen the uPyxis®Desktop App opens,click on Device,then click either Connect via USB-Bluetooth or Connect via USB-RS485depending on the connection type.Figure12.6.3Information ScreenOnce connected to the device,a picture of the device will appear on the top left corner of the window and the uPyxis®Desktop App will default to the Information screen.On the Information screen you can set the information description for Device Name,Product Name,and Modbus Address,then click Apply Settings to save.Figure13.6.4Calibration ScreenTo calibrate the device,click on Calibration.On the Calibration screen there are six calibration options:•Fluorescent Polymer:Zero Calibration,Slope Calibration,and4-20mA Span•PTSA:Zero Calibration,Slope Calibration,and4-20mA SpanThe screen also displays the reading of the device.The reading refresh rate is every4seconds.Figure14.6.5Diagnosis ScreenAfter the device has been calibrated and installation has been completed,to check diagnosis,click on Di-agnosis.When in the Diagnosis screen you can view the Diagnosis Condition of the device.This feature may be used for technical support when communicating with*********************.To preform a probe Cleaniness Check,first select the Diagnosis Condition which defines the fluid type that the ST-588probe inCheck.If the probe is clean,a Clean message will be shown.message will be shown.In this case,follow the procedure in theof this manual.Figure15.7Outputs7.14–20mA Output SetupThe4–20mA output of the ST-588sensor is scaled as:•Fluorescent Polymer:–4mA=0ppm–20mA=20ppm•PTSA:–4mA=0ppb–20mA=200ppb7.2Communication using Modbus RTUThe ST-588probe is configured as a Modbus slave device.In addition to the ppm Fluorescent Polymer and ppb PTSA values,many operational parameters,including warning and error messages,are available via a Modbus RTU connection.Contact Pyxis Lab Customer Service(*********************)for more informa-tion.8Sensor Maintenance and PrecautionThe ST-588probe is designed to provide reliable and continuous Fluorescent Polymer and PTSA readings even when installed in moderately contaminated industrial cooling waters.Although the optics are com-pensated for the effects of moderate fouling,heavy fouling will prevent the light from reaching the sensor, resulting in low readings and the potential for product overfeed if the ST-588probe is used as part of an au-tomated control system.When used to control product dosing,it is suggested that the automation system be configured to provide backup to limit potential product overfeed,for example by limiting pump size or duration,or by alarming if the pumping rate exceeds a desired maximum limit.The ST-588probe is designed to be easily removed,inspected,and cleaned if required.It is suggested that the ST-588probe be checked for fouling and cleaned/calibrated on a monthly basis.Heavily contam-inated waters may require more frequent cleanings.Cleaner water sources with less contamination may not require cleaning for several months.The need to clean the ST-588probe can be determined by the Cleanliness Check using either the uPyxis®Mobile App(see the Mobile Diagnosis Screen section)or the uPyxis®Desktop App(see the Desktop Diagnosis Screen section).8.1Methods to Cleaning the ST-588Any equipment in contact with industrial cooling systems is subject to many potential foulants and con-taminants.Our inline probe cleaning solutions below have been shown to remove most common foulants and contaminants.A small,soft bristle brush,Q-Tips cotton swab,or soft cloth may be used to safely clean the probe housing and the quartz optical sensor channel.These components and more come with a Pyxis Lab Inline Probe Cleaning Solution Kit(P/N:SER-01)which can be purchased at our online Estore/Catalog https:///product/probe-cleaning-kit/Figure16.Inline Probe Cleaning Solution KitTo clean the ST-588probe,soak the lower half of the probe in100mL inline probe cleaning solution for 10minutes.Rinse the ST-588probe with distilled water and then check for the flashing blue light inside the ST-588probe quartz tube.If the surface is not entirely clean,continue to soak the ST-588probe for an e the small,soft bristle brush and Q-Tips cotton swabs as necessary to remove any remaining contaminants in the ST-588probe quartz tube.8.2StorageAvoid long term storage at temperature over100°F.In an outdoor installation,properly shield the ST-588 probe from direct sunlight and precipitation.9TroubleshootingIf the ST-588probe output signal is not stable and fluctuates significantly,make an additional ground con-nection––connect the clear(shield,earth ground)wire to a conductor that contacts the sample water electrically such as a metal pipe adjacent to the ST-588tee.Carry out routine calibration verification against a qualified Fluorescent Polymer and PTSA combined stan-dard.After properly cleaning the ST-588sensor,carry out the zero point calibration with distilled water and slope calibration using the qualified Fluorescent Polymer and PTSA combined standard.10Contact UsPyxis Lab,Inc1729Majestic Dr.Suite5Lafayette,CO80026USAPhone:+1(866)203-8397Email:*********************。
欧盟GMP指南第5章生产(中英文20150123)

欧盟GMP指南第5章生产(中英文20150123)EU GMP 第1部分第5章生产——增加基因毒性的评估生效时限2015-02-25 11:50:00(粉色字为2015年1月23日新增内容,红字斜体为2014年8月13日修订内容)Ref. Ares(2015)283689 - 23/01/2015 EUROPEAN COMMISSIONHEALTH AND CONSUMERS DIRECTORATE-GENERALPublic Health and Risk AssessmentMedicinal products – quality, safety and efficacyBrussels, 13 August 2014Ares(2014)2674301EudraLexThe Rules Governing Medicinal Products in the European UnionVolume 4EU Guidelines forGood Manufacturing Practice forMedicinal Products for Human and Veterinary UsePart 1Chapter 5: Production人兽药EU GMP指南第1部分第5章:生产Legal basis for publishing the detailed guidelines: Article 47 of Directive 2001/83/EC on the Community code relating to medicinal products for human use and Article 51 of Directive 2001/82/EC on the Community code relating to veterinarymedicinal products. This document provides guidance for the interpretation of the principles and guidelines of good manufacturing practice (GMP) for medicinal products as laid down in Directive 2003/94/EC for medicinal products for human use and Directive 91/412/EEC for veterinary use.Status of the document: Revision [a]Reasons for changes: Changes have been made to sections 17 to 20 to improve the guidance on prevention of cross-contamination and to refer to toxicological assessment guidance. Changes were also introduced in sections 26 to 28 on the qualification of suppliers in order to reflect the legal obligation of manufacturing authorisation holders to ensure that active substances are produced in accordance with GMP. The changes include supply chain traceability. Section (33) is inserted to clarify and harmonise expectations of manufacturers regarding the testing of starting materials while section (68) introduces guidance on notification of restrictions in supply.变更理由:对17-20条进行变更,以改进指南中防止交叉污染的部分,及引用毒理学评估指南。
Melinta制药厂宣布美国FDA批准德拉沙星用于治疗急性细菌性皮肤和皮肤结构感染

中国感染与化疗杂志2018年3月20日第18卷第2期 Chin J Infect Chemother, March 2018, Vol. 18, No. 2141参考文献[1] LOW CY,ROTSTEIN C. Emerging fungal infections inimmunocompromised patients[J]. F1000 Med Rep,2011,3:14.[2] HAHN-AST C,GLASMACHER A,MUCKTER S,etal. Overall survival and fungal infection-related mortality in patients with invasive fungal infection and neutropenia after myelosuppressive chemotherapy in a tertiary care centre from 1995 to 2006[J]. J Antimicrob Chemother,2010,65(4):761-768.[3] WALSH TJ, ANAISSIE EJ, DENNING DW, et al. Treatmentof aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America[J]. Clin Infect Dis,2008,46(3):327-360.[4] MAERTENS J, MAICHETTI O, HERBRECHT R, et al.European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3--2009 update[J]. Bone Marrow Transplant, 2011, 46(5):709-718.[5] BADEN LR, BENSINGER W, ANGARONE C, et al.Prevention and treatment of cancer-related infections[J]. J Natl Compr Canc Netw, 2012,10(11):1412-1415.[6] 中国侵袭性真菌感染工作组. 血液病/恶性肿瘤患者侵袭性真菌病的诊断标准与治疗原则(第四次修订版)[J].中华内科杂志,2013,52(8):704-709.[7] ALLINSON K,KOLVE H,GUMBINGER HG,et al.Secondary antifungal prophylaxis in paediatric allogeneic haematopoietic stem cell recipients[J].J Antimicrob Chemother,2008,61(3):734-742.[8] GUPTA V, GORDON-SMITH EC, COOK G, et al. A thirdcourse of anti-thymocyte globuhn in aplastic anemia is only beneficial in previous responders[J]. Bri J Haematol, 2005,129(1):110-117.[9] ROBENSHTOK E,GAFTER-GVILI A,GOLDBERG E,etal. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis[J]. J Clin Oncol,2007,25(34):5471-5489.[10] DÖRING M, BLUME O, HAUFE S, et al.Comparisonof itraconazole, voriconazole, and posaconazoleas oral antifungal prophylaxis in pediatric patients following allogeneic hematopoietic stem cell transplantation[J]. Eur J Clin Microbiol Infect Dis, 2014, 33(4):629-638.[11] 黄劲龙,肖世极,田丽红,等. 泊沙康唑预防侵袭性真菌感染的Mata分析[J].中国真菌学杂志,2015,10(6):357-364.收稿日期:2017-07-12 修回日期:2017-12-09Melinta 制药厂宣布美国FDA批准德拉沙星用于治疗急性细菌性皮肤和皮肤结构感染Melinta therapeutics announces US FDA approval of delafloxacin for acute bacterial skin and skin structure infections·信息交流·2017年6月19日,Melinta制药厂宣布美国FDA已批准其新药德拉沙星(Delafloxacin)用于治疗敏感菌引起的成人急性细菌皮肤和皮肤结构感染。
difene_英文说明书

Package: Blister Pack (10 tablets per blister)Ingredients:- Active Ingredient: Ibuprofen (200 mg)- Inactive Ingredients: Magnesium stearate, cellulose, lactose monohydrate, pregelatinized starch, croscarmellose sodium, hypromellose, titanium dioxide, and iron oxide.Indications:Difene Tablets are a nonsteroidal anti-inflammatory drug (NSAID) used to relieve symptoms of various conditions, including:- Pain: such as headache, dental pain, menstrual cramps, arthritis, backache, and muscular pain.- Fever: to reduce feverish symptoms.- Inflammation: to reduce inflammation in conditions such as arthritis, ankylosing spondylitis, and tendinitis.Contraindications:Do not use Difene Tablets if you have any of the following conditions:- Hypersensitivity to ibuprofen or any other NSAID.- Severe kidney disease.- Active peptic ulcer or gastrointestinal bleeding.- Third trimester of pregnancy.- Severe liver disease.- Hemorrhagic diathesis.- Children under 12 years of age (unless directed by a physician).Warnings and Precautions:- Consult a healthcare professional before taking Difene Tablets if you have a history of stomach ulcers, gastrointestinal bleeding, heart disease, high blood pressure, liver or kidney disease, asthma, or if you are taking any other medications.- Do not exceed the recommended dose to avoid potential side effects.- Do not use Difene Tablets for more than 10 days unless directed by a healthcare professional.- Avoid alcohol consumption while taking Difene Tablets.- Use caution when driving or operating machinery as Difene Tablets may cause drowsiness or dizziness in some individuals.- Difene Tablets may interact with certain medications, including anticoagulants, corticosteroids, and diuretics. Consult a healthcare professional if you are taking any of these medications.Dosage:The recommended dosage of Difene Tablets is as follows:- Adults and children over 12 years of age: 1 tablet every 4 to 6 hours as needed, not to exceed 6 tablets in 24 hours.- Elderly patients: Adjust dosage based on renal function and healthcare professional advice.How to Use:- Swallow the tablet whole with a glass of water.- Do not chew or crush the tablet.- Take Difene Tablets with or without food.Side Effects:The following side effects may occur while taking Difene Tablets:- Gastrointestinal: Nausea, vomiting, stomach pain, heartburn, indigestion, diarrhea, constipation, and ulcers.- Hematologic: Increased risk of bleeding and bruising.- Dermatologic: Skin rash, itching, and hives.- Cardiovascular: Increased blood pressure, heart failure, and myocardial infarction.- Central nervous system: Dizziness, headache, and drowsiness.Overdose:If an overdose is suspected, contact a healthcare professional immediately. Symptoms of an overdose may include severe stomach pain, vomiting, bleeding, and kidney damage.Storage:- Store Difene Tablets at room temperature (15°C to 30°C or 59°F to 86°F).- Keep the blister pack tightly closed when not in use.- Protect from light and moisture.- Do not use after the expiration date.Manufactured by:[Manufacturing Company Name][Address][City, State, ZIP Code]Please read this leaflet carefully before taking Difene Tablets. If you have any questions or concerns, consult your healthcare professional.---Note: This is a fictional product and the information provided here is for illustrative purposes only. The actual dosage, contraindications, warnings, and side effects may vary based on the specific product andits labeling. Always consult the product's official labeling and a healthcare professional before use.。
哌啶的产品包装说明和使用说明书

哌啶的产品包装说明和使用说明书编辑整理:尊敬的读者朋友们:这里是精品文档编辑中心,本文档内容是由我和我的同事精心编辑整理后发布的,发布之前我们对文中内容进行仔细校对,但是难免会有疏漏的地方,但是任然希望(哌啶的产品包装说明和使用说明书)的内容能够给您的工作和学习带来便利。
同时也真诚的希望收到您的建议和反馈,这将是我们进步的源泉,前进的动力。
本文可编辑可修改,如果觉得对您有帮助请收藏以便随时查阅,最后祝您生活愉快业绩进步,以下为哌啶的产品包装说明和使用说明书的全部内容。
哌啶的产品包装说明和使用说明书1。
化学品及企业标识中文名:哌啶英文名:Piperidine中文别名:哌啶;六氢吡啶;一氮六环;胡椒环;环五甲亚胺;哌哔定;氮杂环己烷;氮己环英文别名:Piperidine;Hexahydropyridine;Pentamethyleneimine推荐用途:实验室用化验、试验及科学实验。
限制用途:不可作为药品、食品、家庭或其它用途2。
危险性概述2.1紧急情况概述:高度易燃液体和蒸气。
吞咽有害。
皮肤接触或吸入可致中毒。
造成严重皮肤灼伤和眼损伤。
对水生生物有害并具有长期持续影响。
过量接触需采取特殊急救措施和进行医疗随访。
火灾时:使用二氧化碳、沙粒、灭火粉末灭火。
如必要的话,戴自给式呼吸器去救火。
2.2GHS危险性分类:易燃液体(类别2)急性毒性(经口)(类别4)急性毒性(吸入)(类别3)急性毒性(经皮)(类别3)皮肤腐蚀(类别1B)严重眼睛损伤(类别1)急性水生毒性(类别3)2.3GHS标记要素,包括预防性的陈述:象形图:警示词:危险危险信息:高度易燃液体和蒸气。
吞咽有害。
皮肤接触或吸入可致中毒。
造成严重皮肤灼伤和眼损伤。
对水生生物有害并具有长期持续影响。
预防措施:远离热源/火花/明火。
禁止吸烟.保持容器密闭.容器和装载设备接地/等势联接。
使用防爆的电气/通风/照明设备.只能使用不产生火花的工具。
采取防止静电放电的措施。
原料药国际注册

DMF:Drug Master File(药物主文件),美国对涉药类产品的资料管 理系统,分为5类
I:生产场地,设施,操作规程及人员(2000年1月12日已废止) II:药物物质,药物物质中间体,制备过程使用的原料,药品 III:包装材料 IV:药用辅料,色素,矫味剂,制备过程使用的原料 V:FDA接受的其他参考信息
Issue Date Issue Date 24/10/2012 21/02/2006 17/03/2015 23/01/2008
Status Status VALID WITHDRAWN BY HOLDER VALID WITHDRAWN BY HOLDER
End date End date
Type Type Chemistry
Substance Number 260 260 260 260
Amoxicillin trihydrate India, Process O DSM Anti-Infectives B.V. NL 2613 AX Delft Amoxicillin trihydrate Compacted Amoxicillin trihydrate India, Process 1
定义/概念
注册资料: 按照各国 药品注册法规要求编 写的申请药品市场准 入许可的文件资料
ICH(人用药物注册 技术要求国际协调会 议:美国、日本和欧 盟三方的政府药品注 册部门和制药行业在 1990年发起成立;
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Product Name:
Indapamide CAS No.:
26807-65-8Cat. No.:
HY-B0259
Product Data Sheet
MWt:
365.83Formula:
C16H16ClN3O3S Purity :>98%
Solubility:
DMSO 75 mg/mL; Water <1 mg/mL
Mechanisms:
Biological Activity:
Indapamide is a non-thiazide sulphonamide diuretic compound generally used in the treatment of
Pathways:Membrane Tranporter/Ion Channel; Target:Potassiun Channel Indapamide is a non thiazide sulphonamide diuretic compound, generally used in the treatment of
hypertension, as well as decompensated cardiac failure.
Target: Potassiun Channel Indapamide is a thiazide-like diuretic drug marketed by Servier, generally used in the treatment of hypertension, as well as decompensated cardiac failure. The US trade name for indapamide is
Lozol. It is described as a thiazide-like diuretic. From Wikipedia Indapamide evidently induces redistribution of the cardiac output, with enhanced muscle blood flow and reduced renal perfussion, and that AVP does not seem to be involved in blood pressure regulation in mild to moderate essential hypertension under basal conditions [1]. Indapamide SR References:
[1]. Pedersen, E.B., H. Danielsen, and E.S. Spencer, Effect of indapamide on renal plasma flow,glomerular filtration rate and arginine vasopressin in plasma in essential hypertension. Eur J Clin Pharmacol,1984.26(5):p.543-7.provides an effective option for initial antihypertensive monotherapy and a basis for multidrug
antihypertensive strategies[2] ....
Pharmacol, 1984. 26(5): p. 5437.[2]. Robinson, D.M. and K. Wellington, Indapamide sustained release: a review of its use in the
treatment of hypertension. Drugs, 2006. 66(2): p. 257-71.Caution: Not fully tested. For research purposes only
Medchemexpress LLC
18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
