WHO International Standard 1st WHO International Standard for Human Papillomavirus (HPV) Type 16 DNA
WHO_验证指南

世界卫生组织 ORGANISATION MONDIALE DE LA SANTE优良制造规(GMP) 辅助指南:验证有关对本文件的第一份草案的建议在第一轮的商讨中接受了审核,根据这些建议,南非比勒陀利亚卫生部医药控制委员会的Ms Joey Gouws 又对此文件进行了重重新起草。
如果对此文件有任何建议和/或发现需要改正之处,请与Dr S. Kopp 联系, 地址是Quality Assurance and Safety: Medicines, Essential Drugs and Medicines Policy,©世界卫生组织 2003保留所有权.本文件的提供是有限制的,例如,接受了此文件的个体或组织。
如果没有WHO的许可,这些个体或组织(包括组织成员和成员组织)之外的任何文件的形式或方法可能部分或全部没有被审核、提炼、引用、复制、传播、分发、翻译或可能不适用,本文件不能发布到上。
.如有任何许可的要求,请向Dr Sabine Kopp 提出,联系方式是Dr Sabine Kopp, Quality Assurance & Safety: Medicines (QSM), Department of Essential Drugs and Medicines Policy (EDM), WorldHealth Organization, CH-1211 Geneva 27, Switzerland.Fax: (41-22) 791 4730; s: koppswho.int; bonnywwho.int本文件中所指定的以及述的材料并不意味着WHO的任何表述都代表了其辖区的任何国家、区域、城市、或地区的界线的法定状况。
地图上的界线是一个大概,可能与实际的不完全一致。
文件中提到的特殊公司或产品并不意味着它们会比其它没有提及的具有类似条件的公司或产品被WHO优先推荐。
除去错误和遗漏,所有权产品的名字都由开头的大写字母区别。
欧洲低分子肝素钠标准说明书

WHO International Standard2nd International Standard Low Molecular Weight Heparin forMolecular Weight CalibrationNIBSC code: 05/112 Instructions for use(Version 3.0, Dated 14/05/2008)1. INTENDED USEThe 2nd International Standard Low Molecular Weight Heparin for Molecular Weight Calibration consists of ampoules, coded 05/112, containing aliquots of a freeze-dried material prepared from porcine mucosa. This preparation was established as the 2nd International Standard Low Molecular Weight Heparin for Molecular Weight Calibration by the Expert Committee on Biological Standardisation of the World Health Organisation in 20072. CAUTIONThis preparation is not for administration to humans .The material is not of human or bovine origin. As with all materials of biological origin, this preparation should be regarded as potentially hazardous to health. It should be used and discarded according to your own laboratory's safety procedures. Such safety procedures should include the wearing of protective gloves and avoiding the generation of aerosols. Care should be exercised in opening ampoules or vials, to avoid cuts.3. UNITAGEThere is no assigned unitage associated with this standard. The standard was calibrated by 15 laboratories in 10 countries, against the 1st International Reference Reagent Low Molecular Weight Heparin for Molecular Weight Calibration (1). It is characterised by the Table in Appendix 1.4. CONTENTSCountry of origin of biological material: Denmark.In June 2005 , 251.3 mg bulk material was dissolved in 10 litres water for injection. The solution was distributed at 4°C into 10000 ampoules (CV for volume of fill 0.15% (n=136)), coded 05/112. The contents of the ampoules were then freeze-dried under the conditions normally used for international biological standards. The mean dry weight (n=6) of the freeze-dried plug was 23.5 mg, with a water content of 0.29%.5. STORAGEUnopened ampoules should be stored in the dark at or below –20°C.6. DIRECTIONS FOR OPENINGDIN ampoules have an …easy -open‟ coloured stress point, where the narrow ampoule stem joins the wider ampoule body.Tap the ampoule gently to collect the material at the bottom (labeled) end. Ensure that the disposable ampoule safety breaker provided is pushed down on the stem of the ampoule and against the shoulder of the ampoule body. Hold the body of the ampoule in one hand and the disposable ampoule breaker covering the ampoule stem between the thumb and first finger of the other hand. Apply a bending force to open the ampoule at the coloured stress point, primarily using the hand holding the plastic collar.Care should be taken to avoid cuts and projectile glass fragments that might enter the eyes, for example, by the use of suitable gloves and an eye shield. Take care that no material is lost from the ampoule and no glass falls into the ampoule. Within the ampoule is dry nitrogen gas at slightly less than atmospheric pressure. A new disposable ampoule breaker is provided with each DIN ampoule.7. USE OF MATERIALNo attempt should be made to weigh out any portion of the freeze-dried material prior to reconstitutionThe calibrant is intended for use in the determination of the molecular weight distribution of low molecular weight heparins by size exclusion chromatography (SEC, also sometimes known as gel permeation chromatography (GPC)). It may be used to calibrate a chromatography system by broad standard calibration (as has been described for the previous calibrant (2)), using the molecular weight distribution information as listed in the table in Appendix 1. For each molecular weight (M) in the Table, the percent of sample above M (%>M) and the percent of sample below M (%<M) are given. The use of specialised SEC computer software for calibration of the chromatography system and for calculation of the molecular weights of low molecular weight heparin samples is strongly recommended. It should be noted that the 2nd International Standard Low Molecular Weight Heparin for Molecular Weight Calibration is not suitable for use in the method of Nielsen (3).8. STABILITYReference materials are held at NIBSC within assured, temperature-controlled storage facilities. Reference Materials should be stored on receipt as indicated on the label.Accelerated degradation studies have shown that the 2nd International Standard is very stable in unopened ampoules stored at –20°C. No change in molecular weight characteristics was observed even when the material was stored at +45°C for 6 months.9. REFERENCES1. Mulloy, B., Heath, A., Behr-Gross, M.-E. (2007) Pharmeuropa Bio 2007-1, 29-48.2. Mulloy, B., Gee, C., Wheeler, S. F., Wait, R., Gray, E., Barrowcliffe, T. W. (1997) Thrombosis and Haemostasis 77, 668-674.3. Nielsen, J.-I. (1992) Thrombosis and Haemostasis 68, 478-480.10. ACKNOWLEDGEMENTSAcknowledgements are made to the joint organisers of the collaborative study at the European Directorate for the Quality of Medicines, in particular to the study co-ordinator Dr M.-E. Behr-Gross, as well as to the participants in the study. We also thank the donors of the bulk material for this standard, Leo Pharma A/S, Industriparken 55, DK-2750 Ballerup, Denmark.11. FURTHER INFORMATIONFurther information can be obtained as follows; This material:enquiries@ WHO Biological Standards:Http://www.who.int/biologicals/en/JCTLM Higher order reference materials: /en/committees/jc/jctlm/ Derivation of International Units:http://www.who.int/biologicals/reference_preparations/en/ Ordering standards from NIBSC:/products/ordering_information/frequently_asked_questions.aspxNIBSC Terms & Conditions:/terms_and_conditions.aspx12. CUSTOMER FEEDBACKCustomers are encouraged to provide feedback on the suitability or use of the material provided or other aspects of our service. Please send any comments to enquiries@13. CITATIONIn all publications, including data sheets, in which this material is referenced, it is important that the preparation's title, its status, the NIBSCcode number, and the name and address of NIBSC are cited and cited correctly.15. LIABILITY AND LOSSInformation provided by the Institute is given after the exercise of all reasonable care and skill in its compilation, preparation and issue, but it is provided without liability to the Recipient in its application and use. It is the responsibility of the Recipient to determine the appropriateness of the standards or reference materials supplied by the Institute to the Recipient (“the Goods”) for the proposed application and ensure that it has the necessary technical skills to determine thatthey are appropriate. Results obtained from the Goods are likely to be dependant on conditions of use by the Recipient and the variability of materials beyond the control of the Institute.All warranties are excluded to the fullest extent permitted by law, including without limitation that the Goods are free from infectious agents or that the supply of Goods will not infringe any rights of any third party.The Institute shall not be liable to the Recipient for any economic loss whether direct or indirect, which arise in connection with this agreement.The total liability of the Institute in connection with this agreement, whether for negligence or breach of contract or otherwise, shall in no event exceed 120% of any price paid or payable by the Recipient for the supply of the Goods.If any of the Goods supplied by the Institute should prove not to meettheir specification when stored and used correctly (and provided that the Recipient has returned the Goods to the Institute together with written notification of such alleged defect within seven days of the time when the Recipient discovers or ought to have discovered the defect), the Institute shall either replace the Goods or, at its sole option, refund the handling charge provided that performance of either one of the above options shall constitute an entire discharge of the Institute‟s liability under this Condition.APPENDIX 1: BROAD STANDARD TABLE FOR 05/112 (LMW Heparin for Molecular Weight Calibration Proposed 2nd International Reference Reagent)Point Log 10(M) M % >M % <M1 2.78 600 0.40 99.602 3.08 1200 3.87 96.13 3 3.26 1800 8.94 91.06 4 3.38 2400 14.49 85.515 3.48 3000 20.68 79.326 3.56 3600 27.20 72.807 3.62 4200 33.89 66.118 3.68 4800 40.49 59.51 9 3.73 5400 46.83 53.17 10 3.78 6000 52.92 47.08 11 3.82 6600 58.59 41.41 12 3.86 7200 63.89 36.11 13 3.92 8400 72.96 27.04 14 3.98 9600 80.09 19.91 15 4.08 12000 89.21 10.79 16 4.13 13600 92.96 7.04 17 4.19 15600 95.95 4.05 184.261800097.772.23。
who标准品

who标准品
WHO标准品。
WHO标准品(World Health Organization International Standard)是世界卫生组织制定的一系列国际标准品,用于确保医疗产品的质量和安全性。
这些标准品包括药品、疫苗、诊断试剂和生物制品等,对于保障全球公共卫生具有重要意义。
首先,WHO标准品在医疗产品质量控制中起着至关重要的作用。
在医疗产品的生产过程中,确保产品的质量和安全性是至关重要的。
而WHO标准品作为国际认可的标准,可以帮助生产商确保其产品的质量符合国际标准,从而提高产品的质量和安全性。
其次,WHO标准品对于全球疫苗接种计划具有重要意义。
在全球范围内,疫苗的质量和安全性对于预防和控制传染病至关重要。
而WHO标准品作为国际认可的标准,可以帮助各国政府和组织确保其采购的疫苗符合国际标准,从而保障疫苗接种计划的有效性和安全性。
此外,WHO标准品还对于全球药品质量控制具有重要意义。
在全球范围内,药品的质量和安全性对于治疗疾病和保障患者健康至关重要。
而WHO标准品作为国际认可的标准,可以帮助各国政府和组织确保其采购的药品符合国际标准,从而提高药品的质量和安全性。
总的来说,WHO标准品在医疗产品质量控制、全球疫苗接种计划和全球药品质量控制中起着至关重要的作用。
通过采用国际认可的标准,可以帮助各国政府和组织确保医疗产品的质量和安全性,从而保障全球公共卫生的健康和安全。
因此,WHO标准品的制定和执行对于全球公共卫生具有重要意义,值得各国政府和组织的重视和支持。
世界卫生组织致癌物评级

世界卫生组织致癌物评级摘要:1.世界卫生组织致癌物评级的背景和意义2.世界卫生组织如何进行致癌物评级3.致癌物的分类和级别4.对人类健康的影响和建议正文:【世界卫生组织致癌物评级的背景和意义】世界卫生组织(World Health Organization,简称WHO)是联合国下属的一个专门机构,负责国际公共卫生问题。
其中一个重要任务就是对可能对人体健康产生危害的物质进行致癌物评级。
这一评级旨在为全球公众提供科学依据,以便人们了解和预防癌症等疾病。
【世界卫生组织如何进行致癌物评级】世界卫生组织下属的国际癌症研究机构(International Agency for Research on Cancer,简称IARC)负责进行致癌物评级。
该机构成立于1965 年,是一个独立的科学研究组织,其专家来自全球各地。
IARC 通过对大量科学文献和研究进行评估,对可能具有致癌风险的物质进行分类和评级。
【致癌物的分类和级别】根据IARC 的评估结果,致癌物分为五级,从低到高风险依次为:1.类别1:对人体有明确致癌作用,如烟草、石棉、砒霜等。
2.类别2A:对人体很可能有致癌作用,如丙烯酰胺、红肉等。
3.类别2B:对人体可能有致癌作用,如手机辐射、氯仿等。
4.类别3:对人类致癌性尚无法分类,如咖啡、茶、糖精等。
5.类别4:对人体不可能致癌,如醋、柠檬汁等。
【对人类健康的影响和建议】了解致癌物的级别对人类健康具有重要意义。
人们可以根据致癌物评级来调整生活方式,减少接触可能致癌的物质,从而降低患病风险。
此外,政府部门和企业也可以根据IARC 的评级结果,制定相应政策和措施,加强对致癌物的监管和管理,保障公众健康。
总之,世界卫生组织致癌物评级是一项重要的公共卫生工作,它为人们提供了科学依据,有助于预防癌症等疾病。
WHO International Standard 1st WHO International Standard for Human Papillomavirus (HPV) Type 16 DNA

WHO International Standard1st WHO International Standard for Human Papillomavirus (HPV)Type 16 DNA NIBSC code: 06/202 Instructions for use(Version 2.0, Dated 10/11/2010)1. INTENDED USEThe 1st International Standard for HPV Type 16 (HPV-16) DNA Nucleic Acid Amplification Techniques consists of a freeze-dried preparation of recombinant plasmid containing full-length HPV-16 DNA cloned via its unique BamH1 site (Quint et al., 2006). The standard has been formulated in a background of purified human genomic DNA, lyophilized in 0.5 ml aliquots and stored at -20 °C. The material was calibrated in an international collaborative study involving 19 laboratories (Wilkinson et al., 2008). The International Standard contains material that is proprietory to third parties and should be used for the sole purpose of calibrating in-house or working standards for the amplification and detection of HPV-16 DNA. The International Standard should not be used for any other purpose and should be discarded after use. 2. CAUTIONThis preparation is not for administration to humans .This material contains DNA derived from C33A cells. As with all materials of biological origin, this preparation should be regarded as potentially hazardous to health. It should be used and discarded according to your own laboratory's safety procedures. Such safety procedures should include the wearing of protective gloves and avoiding the generation of aerosols. Care should be exercised in opening ampoules or vials, to avoid cuts.3. UNITAGEThe 1st International Standard for HPV-16 DNA Nucleic Acid Amplification Techniques has been assigned a unitage of 5 x 106 International Units (IU) per ampoule.Traceability statement:It was proposed at a WHO meeting in January 2008 (WHO Meeting Report, 2008) that the instructions for use of the International Standard for HPV-16 DNA include the calculations and assumptions used in determining the theoretical HPV-16 qenome equivalents (GEq) of the bulk material used in formulating the International Standard, thus demonstrating that 1 IU is equivalent to 1 GEq for HPV-16 DNA . The definitive unitage of the 1st WHO International Standard for HPV-16 DNA therefore remains as IU while the traceability statement would allow users to equate IU with GEq.Assays for DNA concentration of the recombinant HPV-16 plasmid stock preparation were performed in Dr Cosette Wheeler‟s laboratory, University of New Mexico (UNM). DNA concentrations were determined by absorbance at 260 nm as well as spectrofluorometrically using the Picogreen assay (Invitrogen Corporation, USA). A correlation coefficient of 0.95 or higher was obtained between the two DNA measurements. 10 ng HPV-16 plasmid DNA/μl was supplied to NIBSC for formulating the bulk material for subsequent freeze-drying. The UNM laboratory also provided NIBSC with a statement indicating that 1.0 x 1011 GEq/ml for HPV-16 is equal to 1.17 ng/μl. 10 ng HPV-16 plasmid DNA/μl plasmid stock preparation is therefore equivalent to 8.547 x 1011 HPV-16 GEq/ml. NIBSC used this data in formulating the 1st International Standard for HPV Type 16 DNA.Formulation of bulk material for the 1st International Standard for HPV Type 16 DNA (NIBSC code 06/202):At NIBSC, the bulk HPV-16 plasmid DNA material was prepared according to the formula:HPV GEq/ml of bulk material = (HPV GEq/ml of plasmid stock x volume plasmid stock) / volume bulk material.Therefore,HPV-16 GEq/ml of bulk material = (8.547 x 1011 HPV-16 GEq/ml plasmid stock) x (0.02223 ml HPV-16 plasmid stock) / 1900 ml HPV-16 bulk material = 1.0 x 107 HPV-16 GEq/ml bulk materialThe HPV-16 DNA bulk material was subsequently freeze-dried in 0.5 ml aliquots.Certain assumptions are required for equating IU to GEq for the 1st International Standard for HPV-16 DNA: 1) 1.0 x 1011 GEq/ml for HPV-16 is equal to 1.17 ng/μl. 2) There is no loss in activity of the HPV-16 DNA upon lyophilization. 3) The recombinant HPV-16 plasmid DNA accurately mimics the activity of HPV-16 viral DNA in biological samples.Independent calculation of GEq/ml for recombinant HPV-16 plasmid DNA.NIBSC also independently calculated the genome equivalence of the HPV-16 plasmid stock preparation and bulk preparation in which the molecular weights of the full-length HPV-16 genome and pBR322 DNA were based on sequence content using BioEdit Sequence Alignment Editor v7.0.5.3 (Tom Hall, Isis Pharmaceuticals Inc., USA). The sequences used for determining the molecular weights are GenBank Accession number J01749.1 for pBR322 and the reference sequence for HPV16 (Accession K02718).BioEdit dataDNA molecule: HPV16 Accession K02718 Length = 7904 base pairsMW= 4786756.00 Daltons, double strandedDNA molecule: cloning vector pBR322 Length = 4361 base pairsMW= 2653867.00 Daltons, double strandedFormulaeGEq/ml of the HPV plasmid stock was calculated according to the formula: GEq/ml of the HPV plasmid stock = (DNA concentration of HPV plasmid stock) x (MW of HPV DNA + MW of pBR322)-1 x (Avogadro‟s Number) where Avogadro‟s Number = 6.022x1023 molecules/molGEq/ml of the bulk HPV DNA materials was calculated according to the formula:HPV GEq/ml of bulk material = (HPV GEq/ml of plasmid stock x volume plasmid stock) / volume bulk material.CalculationThe recombinant HPV-16 plasmid stock preparation was supplied to NIBSC at a concentration of 10 ng/μl. Using the MW determinations shown above, the GEq/ml of the HPV-16 plasmid stock is:= (10 x 10-9 g/μl) x (mol/(7440623 g) x (6.022x1023 molecules/mol) = 8.093 x 108 molecules/μl = 8.093 x 1011molecules/ml = 8.093 x 1011 HPV-16 GEq/ml22.23μl of the recombinant HPV-16 plasmid stock was diluted to a final volume of 1900ml, therefore,HPV-16 GEq/ml of bulk material = (8.093 x 1011 HPV-16 GEq/ml plasmid stock) x (0.02223 ml HPV-16 plasmid stock) / 1900 ml HPV-16 bulk material = 0.947 x 107 HPV-16 GEq/ml bulk material4. CONTENTSCountry of origin of biological material: United Kingdom.Each ampoule contains the lyophilized equivalent of 0.5 ml HPV-16 plasmid DNA in 10mM Tris buffer pH7.4 containing 1mM EDTA, 5 mg/ml trehalose and ~1 x 106 human GEq/ml derived from C33a cells.5. STORAGEThe ampoule should be stored at -20 °C or below on receipt.Please note: because of the inherent stability of lyophilized material, NIBSC may ship these materials at ambient temperature.6. DIRECTIONS FOR OPENINGDIN ampoules have an …easy -open‟ coloured stress point, where the narrow ampoule stem joins the wider ampoule body.Tap the ampoule gently to collect the material at the bottom (labeled) end. Ensure that the disposable ampoule safety breaker provided is pushed down on the stem of the ampoule and against the shoulder of the ampoule body. Hold the body of the ampoule in one hand and the disposable ampoule breaker covering the ampoule stem between the thumb and first finger of the other hand. Apply a bending force to open the ampoule at the coloured stress point, primarily using the hand holding the plastic collar.Care should be taken to avoid cuts and projectile glass fragments that might enter the eyes, for example, by the use of suitable gloves and an eye shield. Take care that no material is lost from the ampoule and no glass falls into the ampoule. Within the ampoule is dry nitrogen gas at slightly less than atmospheric pressure. A new disposable ampoule breaker is provided with each DIN ampoule.7. USE OF MATERIALNo attempt should be made to weigh out any portion of the freeze-dried material prior to reconstitution.The 1st International Standard for HPV-16 DNA contains high copy number template. There is a high risk of HPV-16 plasmid DNA contamination via aerosolization upon opening of the glass ampoule. The material must be opened and handled in a separate laboratory environment, away from other pre-amplification components such as reagents, labware and samples.The material is supplied lyophilized and, before use, should be reconstituted in 0.5 ml sterile nuclease-free water. Ensure that the inside surface of the ampoule is wetted with the added water so that any particles of freeze-dried material adhering to the glass are reconstituted. The reconstituted material has a final concentration of 1 X 107 IU/ml. The reconstituted material is suitable for calibration of in-house or working standards for the amplification and detection of HPV-16 DNA.. The material is not suitable for calibrating or assessing extraction, precipitation or centrifugation procedures. The material has NOT been calibrated for human DNA nucleic acid amplification techniques.8. STABILITYReference materials are held at NIBSC within assured, temperature-controlled storage facilities. The 1st International Standard for HPV-16 DNA should be stored at -20 °C or below on receipt.Studies on the stability of reconstituted standard are underway. Users should determine the stability of the reconstituted material according to their own method of preparation, storage and use.NIBSC follows the policy of WHO with respect to its reference materials.9. REFERENCESQuint, W. G. V., Pagliusi, S. R., Lelie, N., de Villiers, E. M., Wheeler, C. M. and the World Health Organization Human Papillomavirus DNA International Collaborative Study Group. (2006). Results of the First WorldHealth Organization International Collaborative Study of Detection of Human Papillomavirus DNA. J. Clin. Microbiol. 44: 571-579.Wilkinson, D.E., Baylis, S.A., Padley, D., Heath, A.B., Ferguson, M., Pagliusi, S.R., et al. Establishment of the 1st World Health Organization international standards for human papillomavirus type 16 DNA and type 18 DNA. Int J Cancer 2010 Jun 15;126(12):2969-83.WHO meeting report, on “Standardization of HPV assays and the role of HPV LabNet in supporting vaccine introduction” Geneva, Switzerland, 23-25 January 2008, in preparation.10. ACKNOWLEDGEMENTS11. FURTHER INFORMATIONFurther information can be obtained as follows; This material: enquiries@ WHO Biological Standards:http://www.who.int/biologicals/en/JCTLM Higher order reference materials: /en/committees/jc/jctlm/ Derivation of International Units:/products/biological_reference_materials/frequently _asked_questions/how_are_international_units.aspx Ordering standards from NIBSC:/products/ordering_information/frequently_asked_q uestions.aspxNIBSC Terms & Conditions:/terms_and_conditions.aspx12. CUSTOMER FEEDBACKCustomers are encouraged to provide feedback on the suitability or use of the material provided or other aspects of our service. Please send any comments to enquiries@13. CITATIONIn all publications, including data sheets, in which this material is referenced, it is important that the preparation's title, its status, the NIBSC code number, and the name and address of NIBSC are cited and cited correctly.15. LIABILITY AND LOSSInformation provided by the Institute is given after the exercise of all reasonable care and skill in its compilation, preparation and issue, but it is provided without liability to the Recipient in its application and use. It is the responsibility of the Recipient to determine the appropriateness of the standards or reference materials supplied by the Institute to the Recip ient (“the Goods”) for the proposed application and ensure that it has the necessary technical skills to determine that they are appropriate. Results obtained from the Goods are likely to be dependant on conditions of use by the Recipient and the variability of materials beyond the control of the Institute.All warranties are excluded to the fullest extent permitted by law, including without limitation that the Goods are free from infectious agents or that the supply of Goods will not infringe any rights of any third party.The Institute shall not be liable to the Recipient for any economic loss whether direct or indirect, which arise in connection with this agreement.The total liability of the Institute in connection with this agreement, whether for negligence or breach of contract or otherwise, shall in no event exceed 120% of any price paid or payable by the Recipient for the supply of the Goods.If any of the Goods supplied by the Institute should prove not to meet their specification when stored and used correctly (and provided that the Recipient has returned the Goods to the Institute together with written notification of such alleged defect within seven days of the time when the Recipient discovers or ought to have discovered the defect), the Institute shall either replace the Goods or, at its sole option, refund the handling charge provided that performance of either one of the above options shall constitute an entire discharge of the Institute‟s liability under this Condition.。
世界卫生组织儿童标准处方集

WHO Model Formulary for ChildrenBased on the Second Model List of Essential Medicines for Children 2009世界卫生组织儿童标准处方集基于2009年儿童基本用药的第二个标准目录WHO Library Cataloguing-in-Publication Data:WHO model formulary for children 2010.Based on the second model list of essential medicines for children 2009.1.Essential drugs.2.Formularies.3.Pharmaceutical preparations.4.Child.5.Drug utilization. I.World Health Organization.ISBN 978 92 4 159932 0 (NLM classification: QV 55)世界卫生组织实验室出版数据目录:世界卫生组织儿童标准处方集基于2009年儿童基本用药的第二个标准处方集1.基本药物 2.处方一览表 3.药品制备 4儿童 5.药物ISBN 978 92 4 159932 0 (美国国立医学图书馆分类:QV55)World Health Organization 2010All rights reserved. Publications of the World Health Organization can be obtained fromWHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: ******************). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the aboveaddress(fax:+41227914806;e-mail:*******************).世界卫生组织2010版权所有。
NIBSC 85-506+WHO CRP参考品C反应蛋白 标准物质 参考物质 量值溯源 WHO International Standard

WHO International StandardHUMAN C-REACTIVE PROTEIN 1st International StandardNIBSC code: 85/506 Instructions for use(Version 3.0, Dated 19/03/2008)1. INTENDED USECalibrant for C-reactive protein assays2. CAUTIONThis preparation is not for administration to humans or animals in the human food chain .The preparation contains material of human origin, and either the final product or the source materials, from which it is derived, have been tested and found negative for HBsAg, anti-HIV and HCV RNA. As with all materials of biological origin, this preparation should be regarded as potentially hazardous to health. It should be used and discarded according to your own laboratory's safety procedures. Such safety procedures should include the wearing of protective gloves and avoiding the generation of aerosols. Care should be exercised in opening ampoules or vials, to avoid cuts.3. UNITAGE49 milli-International Units (IU) per ampoule.4. CONTENTSCountry of origin of biological material: United Kingdom.Each ampoule contains the residue after freeze drying of 0.5ml of a solution that contained:50 µg Human C-reactive protein 0.5ml pooled normal human serumThe material has not been sterilized and the ampoules contain no bacteriostat.5. STORAGEStore at -20ºC or belowPlease note: because of the inherent stability of lyophilized material, NIBSC may ship these materials at ambient temperature.6. DIRECTIONS FOR OPENINGTap the ampoule gently to collect the material at the bottom (labelled) end. Ensure ampoule is scored all round at the narrow part of the neck, with a diamond or tungsten carbide tipped glass knife file or other suitable implement before attempting to open. Place the ampoule in the ampoule opener, positioning the score at position 'A'; shown in the diagram below. Surround the ampoule with cloth or layers of tissue paper. Grip the ampoule and holder in the hand and squeeze at point 'B'. The ampoule will snap open. Take care to avoid cuts and projectile glass fragments that enter eyes. Take care that no material is lost from the ampoule and that no glass falls into theampoule.Side view of ampoule opening device containing an ampoule positioned ready to open. 'A' is the score mark and 'B' the point of applied pressure.7. USE OF MATERIALNo attempt should be made to weigh out any portion of the freeze-dried material prior to reconstitution.For all practical purposes each ampoule contains the same amount of the same materials. Dissolve the total contents in a known amount of suitable buffer solution with carrier protein (free of peptidase), where extensive dilution is required, to minimise loss by surface adsorption.For economy of use it is recommended that the solution be subdivided into several small containers, which are frozen rapidly to below –40ºC and then stored below –40ºC in the dark. Repeated freezing and thawing should be avoided.8. STABILITYReference materials are held at NIBSC within assured, temperature-controlled storage facilities. Reference Materials should be stored on receipt as indicated on the label.Once reconstituted, diluted or aliquoted, users should determine the stability of the material according to their own method of preparation, storage and use.NIBSC follows the policy of WHO with respect to its reference materials.9. REFERENCESWHO Expert Committee on Biological Standardization (1992), 42nd Report. WHO Technical Report Series, 760, p21, 1987.10. ACKNOWLEDGEMENTS Not Applicable11. FURTHER INFORMATIONFurther information can be obtained as follows; This material: enquiries@ WHO Biological Standards:http://www.who.int/biologicals/en/JCTLM Higher order reference materials: /en/committees/jc/jctlm/ Derivation of International Units:/standardisation/international_standards.aspx Ordering standards from NIBSC:/products/ordering.aspx NIBSC Terms & Conditions:/terms_and_conditions.aspx12. CUSTOMER FEEDBACKCustomers are encouraged to provide feedback on the suitability or use of the material provided or other aspects of our service. Please send any comments to enquiries@13. CITATIONIn all publications, including data sheets, in which this material is referenced, it is important that the preparation's title, its status, the NIBSC code number, and the name and address of NIBSC are cited and cited correctly.14. MATERIAL SAFETY SHEETClassification in accordance with Directive 2000/54/EC, Regulation (EC)15. LIABILITY AND LOSSIn the event that this document is translated into another language, the English language version shall prevail in the event of any inconsistencies between the documents.Unless expressly stated otherwise by NIB SC, NIBSC’s Standard Terms and Conditions for the Supply of Materials (available at /About_Us/Terms_and_Conditions.aspx or upon request by the Recipient) (“Conditions”) apply to the exclusion of all other terms and are hereby incorporated into this document by reference. The Recipient's attention is drawn in particular to the provisions of clause 11 of the Conditions.17. CERTIFICATE OF ANALYSISNIBSC does not provide a Certificate of Analysis for WHO Biological Reference Materials because they are internationally recognised primary reference materials fully described in the instructions for use. The reference materials are established according to the WHO Recommendations for the preparation, characterization and establishment of international and other biological reference standards http://www.who.int/bloodproducts/publications/TRS932Annex2_Inter_bi olefstandardsrev2004.pdf (revised 2004). They are officially endorsed by the WHO Expert Committee on Biological Standardization (ECBS) based on the report of the international collaborative study which established their suitability for the intended use.。
生产制造英文缩写

EVT(Engineering Verification Test)工程验证测试阶段DVT(Design Verification Test)设计验证测试阶段DMT(Design Maturity Test)成熟度验证MVT(Mass-Production Verification Test)量产验证测试PVT(Production/Process Verification Test)生产/制程验证测试阶段MP(Mass Production)量产工程师类:PE: Product Engineer 产品工程师Process Engineer 制程工程师ME: Mechanical Engineer 机构工程师IE:Industrial Engineer 工业工程师QE: Quality Engineer 品质工程师SQESupplier Quality Engineer供货商质量工程师QC quality control 品质管理人员FQC final quality control 终点质量管理人员IPQC in process quality control 制程中的质量管理人员OQC output quality control 最终出货质量管理人员IQC incoming quality control 进料质量管理人员TQC total quality control 全面质量管理POC passage quality control 段检人员QA quality assurance 质量保证人员OQA output quality assurance 出货质量保证人员QE quality engineering 品质工程人员TE Test Engineer 测试工程师AE Automatic Engineer 自动化工程师研发类:R&D Research & Design 设计开发部ID (Industry Design)工业设计MD (Mechanical Design)结构设计HW(Hardware) 硬件设计SW(Software)软件设计PDM Product Data Management 产品数据管理PLM product lifecycle management 产品生命周期管理电子设计:ICT In Circuit Test 电路测试PCB Printed Circuit Board 印刷电路板PCBA Printed Circuit Board Assembly 印刷电路板装配FPC FlexiblePrintedCircui 挠性电路板EMI Electrical Magnetic Interference 电子干扰RFI adio Frequency Interference射频干扰。
罗氏电化学发光质控品溯源性与不确定度V201501
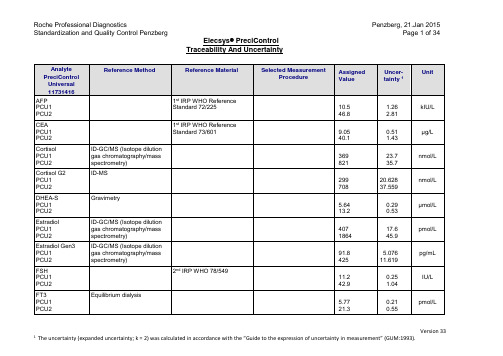
ACTH
PCMM 1
PCMM 2
Gravimetry
71.0
1640
3.075
58.8Байду номын сангаас3
pg/mL
C-Peptid
PCMM 1
PCMM 2
WHO Reference Reagent
IRR 84/510 (NIBSC)
1.95
9.71
0.009
0.072
ng/mL
Insulin
µg/L
S100
PCU1
PCU2
Fa. Affinity S100 antigen
lot. no. Z03833
0.21
2.7
0.008
0.10
µg/L
SHBG
PCU1
PCU2
NIBSC 1stInternational Standard 95/560
42.5
16.2
0.70
0.30
nmol/L
Testosterone II
Reference Sera Pools with value of 0 and 1
1.47
0.78
0.01
0.02
TBI
Tg
PCU1
PCU2
Certified Reference Material (CRM), 457 (Community Bureau of Reference)
17.3
70.4
./.
19.4
97.7
./.
0.221
2.17
µg/L
Reference Material
Selected Measurement Procedure
国际疾病分类标准

国际疾病分类标准国际疾病分类标准(International Classification of Diseases,简称ICD),是世界卫生组织(World Health Organization,简称WHO)制定的一套用于对各种疾病、伤害和死因进行分类和编码的标准。
ICD对于医疗机构、研究机构以及保险公司等单位在诊断、统计和数据分析上起着重要的作用。
该标准的最新版本是ICD-11,于2018年发布。
ICD的使用可以追溯到1850年代。
当时,各个国家都有自己的疾病分类系统,这导致了在不同国家之间进行病例比较和数据交流时的困难。
为了解决这一问题,国际卫生统计会议于1893年决定建立一个国际统一的疾病分类标准,即ICD。
这个标准首次于1893年正式发布,标志着世界各国开始使用同一套疾病分类系统。
ICD的编码体系十分详细。
每个疾病或伤害都有一个唯一的编码,该编码由字母和数字组成。
编码的实质是将疾病或伤害按病因、病理生理以及发病部位进行分类,以方便统计与分析。
ICD的编码系统十分灵活,可以根据需要进行修订和扩展,以适应不断变化的医学知识和临床实践。
ICD不仅仅是一个医学分类系统,它还提供了一种方法来记录和报告全球范围内的疾病和死因数据。
这种全球范围的数据收集和统计有助于监测疾病的传播、病患的治疗情况以及识别流行病的爆发。
同时,ICD的统一标准还为同一疾病在全球范围内的研究提供了便利,促进了不同国家之间的交流和合作。
随着时代的发展,ICD也在不断进化。
最新版本的ICD-11相比之前的版本有着一些重要的改变。
例如,ICD-11将精神疾病更加细化,对许多常见的精神障碍进行了全面的描述和定义。
此外,ICD-11还加入了一些新的疾病和伤害,比如游戏障碍等,以适应当前社会中出现的新型问题。
尽管ICD是一套世界通用的疾病分类标准,但它也存在一些局限性。
首先,ICD的分类系统在某些情况下可能存在主观判断的问题,不同的医生可能对同一个病例进行不同的分类。
安全管理国际信息安全技术标准发展英文版

Protect
Detect
React/ Response
Business Continuity Planning
Activate BCP
Prepare & Test
Plan
Plan
Prepare & Test
Activate DCRP
Disaster Contingency & Recovery Planning
27001
ISMS Requirements
27002
Code of Practice
27000
Fundamental & Vocabulary
27006
Accreditation Requirements
ISMS Family
27003
ISMS Implementation
Guidance
27005
Security breaches and compromises
安全管理国际信息安全技术标准发展 英文版
SC27 WG4 Roadmap
ICT Readiness for Business Continuity (27031)
Cybersecurity (27032)
Network Security (27033)
Disaster Events
IT Systems Failures
安全管理国际信息安全技术标准发展 英文版
ICT Readiness for Business Continuity
• What is ICT Readiness?
• Prepare organization ICT technology (infrastructure, operation, applications), process, and people against unforeseeable focusing events that could change the risk environment
国际标准化组织的英语简称

国际标准化组织的英语简称。
其全称是International Organization for Standardi zation。
ISO一来源于希腊语“ISOS”,即“EQUAL”——平等之意。
国际标准化组织(ISO)是由各国标准化团体(ISO成员团体)组成的世界性的联合会。
制定国际标准工作通常由ISO的技术委员会完成。
各成员团体若对某技术委员会确定的项目感兴趣,均有权参加该委员会的工作。
与ISO保持联系的各国际组织(官方的或非官方的)也可参加有关工作。
ISO与国际电工委员会(IEC)在电工技术标准化方面保持密切合作的关系。
由来国际标准化活动最早开始于电子领域,于1906年成立了世界上最早的国际标准化机构——国际电工委员会(IEC)。
其他技术领域的工作原先有成立于1926年的国家标准化协会的国际联盟(International Federation of the National Standardizing Associations,简称ISA)承担,重点在于机械工程方面。
ISA的工作由于二次大战在1942年终止。
1946年,来自25个国家的代表在伦敦召开会议,决定成立一个新的国际组织,其目的是促进国际间的合作和工业标准的统一。
于是,ISO这一新组织于1947年2月23日正式成立,总部设在瑞士的日内瓦。
ISO于1951年发布了第一个标准——工业长度测量用标准参考温度。
许多人注意到国际标准化组织(International Organization for Standardization)的全名与缩写之间存在差异,为什么不是“IOS”呢?其实,“ISO”并不是首字母缩写,而是一个词,它来源于希腊语,意为“相等”,现在有一系列用它作前缀的词,诸如“is ometric”(意为“尺寸相等”)、“isonomy”(意为“法律平等”)。
从“相等”到“标准”,内涵上的联系使“ISO”成为组织的名称。
如今ISO是一个国际标准化组织,由91个成员国和173个学术委员会组成。
疾病与健康问题的国际分类标准

疾病与健康问题的国际分类标准在医学领域,了解与研究疾病与健康问题是非常重要的。
为了方便医生、科研人员、保险公司等更好地理解和统计各种疾病与健康问题的情况,国际上制定了疾病与健康问题的分类标准。
本文将介绍国际上通用的疾病与健康问题的分类标准,以及其在世界范围内的应用。
1. 简介疾病与健康问题的国际分类标准是世界卫生组织(World Health Organization,简称WHO)制定的一套用于统计、研究和管理疾病与健康问题的工具。
该标准被称为国际疾病分类(International Classification of Diseases,简称ICD)。
2. ICD的历史与发展ICD最早的版本可以追溯到1893年,当时被用于统计死因。
经过多次修订和更新,ICD逐渐发展成为用于记录各种疾病与健康问题的统一标准。
目前,ICD的第十个版本(ICD-10)是最为广泛应用的版本。
3. ICD的分类结构ICD按照一定的层次结构对疾病与健康问题进行分类。
它包括主要类别(Chapter)、次要类别(Block)、类别组(Category)和类别(Code)。
主要类别根据病因、器官系统或疾病类型进行划分,如传染病、肿瘤、心脑血管疾病等。
次要类别用于进一步细分主要类别,类别组则进一步细分次要类别,最后类别则是具体的疾病或健康问题的代码。
4. ICD的应用领域ICD主要用于医疗机构、科研机构、保险公司等对疾病与健康问题进行统计与分析。
在医疗机构中,医生可以使用ICD来诊断和记录患者的疾病信息,进而为其制定合适的治疗方案。
在科研机构中,研究人员可以使用ICD对不同地区、不同年龄段、不同性别等人群中的疾病与健康问题进行比较与分析。
在保险业中,保险公司可以根据ICD对被保险人的疾病风险进行评估,制定相应的保险政策。
5. ICD的更新与版本转换为了跟上疾病与健康问题的发展,ICD每隔一段时间就会进行更新与修订。
各国根据自身情况与需求,选择合适的ICD版本进行使用。
重组肠道病毒71型疫苗(汉逊酵母)抗原冻干参考品的制备及稳定性研究

国际生物制品学杂志2020年丨2月第43卷第6期Im J BiologicaLs,December 2020, Vol. 43, No. 6• 267 ••论著•重组肠道病毒71型疫苗(汉逊酵母)抗原冻干参考品的制备及稳定性研究张改梅陈磊赵丽丽谢学超李国顺肖海峰郭林徐颖之朱芮波刘建凯顾美荣北京民海生物科技有限公司研发中心1〇26()()通信作者:顾美荣,Email: 6088gmr@【摘要】目的制备重组肠道病毒71型(enterovirus71,EV71)疫苗(汉逊酵母)抗原冻干参考品,用于重组EV71疫苗(汉逊酵母)的抗原含量测定。
方法选取检定合格的重组EV71疫苗(汉逊酵母)原液.加人冻干保护剂,冷冻干燥制备重组EV71疫苗(汉逊酵母)抗原冻干参考品,对其进行保护剂抗原含量标定,并对其进行反复冻融试验,37 t、2〜8 X;和-20X;稳定性研究。
结果制备的抗原冻干参考品鉴别试验、无菌检査结果均符合规定,水分含量为1. 4%。
经标定,抗原含量的几何均值为2 146 U/ml,几何变异系数为7.2%。
10次反复冻融,抗原含量仍无明显变化;37 t放置3个月,抗原含量无明显变化;分别于2〜8 T:和-20C放置3<)个月,抗原含量仍较为稳定。
结论制备了一批稳定的、均一性良好的抗原冻干参考品,可以用于重组EV71型疫苗(汉逊酵母)抗原含量测定。
【关键词】肠道病毒71型;疫苗,合成;抗原参考品;稳定性D0I:10. 3760/cma. j. cn311962-20200831-00085Preparation and stability of freeze-dried recombinant enterovirus 71 vaccine (Hansenula)antigen referenceZhang Gaimei,Chen L ei,Zhao L ili, Xie Xuechao,Li Guoshun ♦Xiao H a ifen g,Guo L in, XuYingzhi, Zhu Ruibo, Liu Jiankai, Gu MeirongCenter o f Research and Development,Beijing Minhai Biotechnology Co.,L td.,Beijing 102600,ChinaCorresponding author :Gu Meirong 9Email:****************【Abstract】Objective To prepare a lyophilized reference antigen of recombinant enterovirus 71(EV71) vaccine (Hansenula) for the determination of antigen content of recombinant EV71 vaccine{Hansenula).Methods Bulk of qualified recombinant EV71 vaccine (Hansenula)was selected as theraw material of reference, adding freeze-dried protective agent, then lyophilized to prepared freeze-driedrecombinant EV71 (Hansenula) vaccine antigen reference, after the calibration on the antigen content,the reference was carried on the freeze-thaw test, stored at 37 °C, 2-8 °C and _ 20 °C for the stabilitystudy. Results All the quality indexes of prepared reference met the relevant requirements, and themoisture content was 1. 4%. After calibration, geometry mean of reference antigen content was2 146 U/ml and geometric variation coefficient was 1.2%.There was no significant change in antigencontent after repeated freezing and thawing for 10 times. There was no significant change in antigencontent for 3 months at 37 °C. The antigen content remained stable at 2-8 and ~ 20 °C for 30 months,respectively. Conclusion A batch of stable and homogeneous reference antigens were prepared, whichcould be applied for the determination of antigen content in recombinant EV71 vaccine {Hansenula).【Keywords】Enterovirus 71; Vaccine,synthetic; Antigen reference; StabilityDOI :10. 3760/cma. j. cn311962-20200831 -00085手足 口病(hand,foot,and mouth disease, HFMD)是一种由多种肠道病毒引起的传染性疾病,常见于5岁以下婴幼儿[14]。
乳胶颗粒凝集法检测梅毒特异性抗体的性能评估

乳胶颗粒凝集法检测梅毒特异性抗体的性能评估胡亮;陈艺;陈长强;瞿晨芸;顾志冬;樊绮诗【摘要】目的:对乳胶颗粒凝集法梅毒螺旋体特异性抗体检测试剂( Mediace TPLA)进行性能评估。
方法参考美国临床实验室标准化协会(CLSI)EP系列文件,评估Mediace TPLA的分析性能。
用Mediace TPLA检测111例梅毒确诊患者和78例排除梅毒感染者的血清,并与化学发光微粒子免疫分析法( CMIA )进行比较。
结果Mediace TPLA检测结果在8.0、28.6及166.0 T.U.这3个水平的总变异系数( CV)分别为8.77%、3.94%和6.57%;检测线性范围为0~250 T.U.;总回收率为95.31%;一定程度的溶血(血红蛋白<4.8 g/L)、脂血(甘油三酯<8 mmol/L)、黄疸(胆红素<20μmol/L)及类风湿因子(<450 IU/mL)对检测结果无明显影响;排除梅毒感染的30例抗核抗体( ANA)阳性患者、10例肝炎患者及10例妊娠期妇女血清检测结果为阴性。
以CMIA结果为相对标准,Mediace TPLA Cut-off 值取10 T.U.时,其检测灵敏度为99%、特异性为100%。
结论Mediace TPLA具有抗干扰能力强、重复性好、准确性好、线性范围宽及特异度高等特点,可在全自动生化分析仪中使用。
%Objective To evaluate the analytic performance of a latex agglutination assay for treponemal specific antibody (MediaceTPLA).Methods The EP documents established by Clinical and Laboratory Standards Institute ( CLSI) were referred to evaluate the analytic performance .A total of 110 syphilitic positive and 78 syphilitic negative sera were detected by Mediace TPLA , and the results were compared with those of chemiluminescence microparticle immunoassay ( CMIA).Results The total coefficients of variation ( CV) were 8.77%, 3.94% and6.57%respectively at the concentration levels of 8.0, 28.6 and 166.0T.U..The linear range was from 0 to 250 T.U., and the total recovery rate was95.31%.The results were not significantly affected by hemolysis (hemoglobin<4.8 g/L), lipidemia ( triglyceride<8 mmol/L) , jaundice( bilirubin <20 μmol/L) and rheumatoid factor ( <450 IU/mL) .The results of 30 antinuclear antibody ( ANA ) positive patients , 10 hepatitis patients and 10 pregnant women were all negative.When the results of CMIA were defined as the reference standard ,the sensitivity of Mediace TPLA was 99%, the specificity was 100%, and the cut-off value was 10T.U..Conclusions Mediace TPLA has excellent anti-interference ability , repeatability , high accuracy , wide linear range and specificity .It can also be used in automated biochemical analyzers .【期刊名称】《检验医学》【年(卷),期】2015(000)003【总页数】4页(P250-253)【关键词】梅毒;乳胶颗粒凝集法;血清学检测【作者】胡亮;陈艺;陈长强;瞿晨芸;顾志冬;樊绮诗【作者单位】上海交通大学医学院附属瑞金医院北院检验科,上海201801;上海交通大学医学院附属瑞金医院北院检验科,上海201801;上海交通大学医学院附属瑞金医院北院检验科,上海201801;上海交通大学医学院附属瑞金医院北院检验科,上海201801;上海交通大学医学院附属瑞金医院检验科,上海200025;上海交通大学医学院附属瑞金医院北院检验科,上海201801【正文语种】中文【中图分类】R446.1梅毒(syphilis)是由梅毒螺旋体感染人体所引起的一种常见的性传播疾病。
生产制造英文缩写

EVT(Engineering Verification Test)工程验证测试阶段令狐采学DVT(Design Verification Test)设计验证测试阶段DMT(Design Maturity Test)成熟度验证MVT(Mass-Production Verification Test)量产验证测试PVT(Production/Process Verification Test)生产/制程验证测试阶段MP(Mass Production)量产工程师类:PE: Product Engineer 产品工程师Process Engineer 制程工程师ME: Mechanical Engineer 机构工程师IE:Industrial Engineer 工业工程师QE: Quality Engineer 品质工程师SQESupplier Quality Engineer供货商质量工程师QC quality control 品质管理人员FQC final quality control 终点质量管理人员IPQC in process quality control 制程中的质量管理人员OQC output quality control 最终出货质量管理人员IQC incoming quality control 进料质量管理人员TQC total quality control 全面质量管理POC passage quality control 段检人员QA quality assurance 质量保证人员OQA output quality assurance 出货质量保证人员QE quality engineering 品质工程人员TE Test Engineer 测试工程师AE Automatic Engineer 自动化工程师研发类:R&D Research & Design 设计开发部ID (Industry Design)工业设计MD (Mechanical Design)结构设计HW(Hardware) 硬件设计SW(Software)软件设计PDM Product Data Management 产品数据管理PLM product lifecycle management 产品生命周期管理电子设计:ICT In Circuit Test 电路测试PCB Printed Circuit Board 印刷电路板PCBA Printed Circuit Board Assembly 印刷电路板装配FPC FlexiblePrintedCircui 挠性电路板EMI Electrical Magnetic Interference 电子干扰RFI adio Frequency Interference射频干扰。
世界卫生组织生存质量测定简表 (WHOQOL) -BREF

差
一般
好
很好
15.
您行动的能力如何?
1
2
3
4
5
非常不满意
不满意
一般
满意
很满意
16. 17. 18. 19. 20. 21. 22. 23. 24. 25.
您对自己的睡眠情况满意吗? 您对自己做日常生活事情的 能力满意吗? 您对自己的工作能力满意吗? 您对自己满意吗? 您对自己的人际关系满意吗? 您对自己的性生活满意吗? 您对自己从朋友那里得到的 支持满意吗? 您对自己居住地的条件满意 吗? 您对您能享受到的卫生保健 服务满意吗? 您对自己的交通情况满意吗?
世界卫生组织生存质量测定简表
T HE W ORLD H EALTH O RGANIZATION Q UALITY OF L IFE (WHOQOL) -BREF
The World Health Organization Quality of Life (WHOQOL)-BREF © World Health Organization 2004 All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: bookorders@who.int). Requests for permission to reproduce or translate WHO publications—whether for sale or for noncommercial distribution— should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: permissions@who.int). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use.
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
WHO International Standard1st WHO International Standard for Human Papillomavirus (HPV)Type 16 DNA NIBSC code: 06/202 Instructions for use(Version 2.0, Dated 10/11/2010)1. INTENDED USEThe 1st International Standard for HPV Type 16 (HPV-16) DNA Nucleic Acid Amplification Techniques consists of a freeze-dried preparation of recombinant plasmid containing full-length HPV-16 DNA cloned via its unique BamH1 site (Quint et al., 2006). The standard has been formulated in a background of purified human genomic DNA, lyophilized in 0.5 ml aliquots and stored at -20 °C. The material was calibrated in an international collaborative study involving 19 laboratories (Wilkinson et al., 2008). The International Standard contains material that is proprietory to third parties and should be used for the sole purpose of calibrating in-house or working standards for the amplification and detection of HPV-16 DNA. The International Standard should not be used for any other purpose and should be discarded after use. 2. CAUTIONThis preparation is not for administration to humans .This material contains DNA derived from C33A cells. As with all materials of biological origin, this preparation should be regarded as potentially hazardous to health. It should be used and discarded according to your own laboratory's safety procedures. Such safety procedures should include the wearing of protective gloves and avoiding the generation of aerosols. Care should be exercised in opening ampoules or vials, to avoid cuts.3. UNITAGEThe 1st International Standard for HPV-16 DNA Nucleic Acid Amplification Techniques has been assigned a unitage of 5 x 106 International Units (IU) per ampoule.Traceability statement:It was proposed at a WHO meeting in January 2008 (WHO Meeting Report, 2008) that the instructions for use of the International Standard for HPV-16 DNA include the calculations and assumptions used in determining the theoretical HPV-16 qenome equivalents (GEq) of the bulk material used in formulating the International Standard, thus demonstrating that 1 IU is equivalent to 1 GEq for HPV-16 DNA . The definitive unitage of the 1st WHO International Standard for HPV-16 DNA therefore remains as IU while the traceability statement would allow users to equate IU with GEq.Assays for DNA concentration of the recombinant HPV-16 plasmid stock preparation were performed in Dr Cosette Wheeler‟s laboratory, University of New Mexico (UNM). DNA concentrations were determined by absorbance at 260 nm as well as spectrofluorometrically using the Picogreen assay (Invitrogen Corporation, USA). A correlation coefficient of 0.95 or higher was obtained between the two DNA measurements. 10 ng HPV-16 plasmid DNA/μl was supplied to NIBSC for formulating the bulk material for subsequent freeze-drying. The UNM laboratory also provided NIBSC with a statement indicating that 1.0 x 1011 GEq/ml for HPV-16 is equal to 1.17 ng/μl. 10 ng HPV-16 plasmid DNA/μl plasmid stock preparation is therefore equivalent to 8.547 x 1011 HPV-16 GEq/ml. NIBSC used this data in formulating the 1st International Standard for HPV Type 16 DNA.Formulation of bulk material for the 1st International Standard for HPV Type 16 DNA (NIBSC code 06/202):At NIBSC, the bulk HPV-16 plasmid DNA material was prepared according to the formula:HPV GEq/ml of bulk material = (HPV GEq/ml of plasmid stock x volume plasmid stock) / volume bulk material.Therefore,HPV-16 GEq/ml of bulk material = (8.547 x 1011 HPV-16 GEq/ml plasmid stock) x (0.02223 ml HPV-16 plasmid stock) / 1900 ml HPV-16 bulk material = 1.0 x 107 HPV-16 GEq/ml bulk materialThe HPV-16 DNA bulk material was subsequently freeze-dried in 0.5 ml aliquots.Certain assumptions are required for equating IU to GEq for the 1st International Standard for HPV-16 DNA: 1) 1.0 x 1011 GEq/ml for HPV-16 is equal to 1.17 ng/μl. 2) There is no loss in activity of the HPV-16 DNA upon lyophilization. 3) The recombinant HPV-16 plasmid DNA accurately mimics the activity of HPV-16 viral DNA in biological samples.Independent calculation of GEq/ml for recombinant HPV-16 plasmid DNA.NIBSC also independently calculated the genome equivalence of the HPV-16 plasmid stock preparation and bulk preparation in which the molecular weights of the full-length HPV-16 genome and pBR322 DNA were based on sequence content using BioEdit Sequence Alignment Editor v7.0.5.3 (Tom Hall, Isis Pharmaceuticals Inc., USA). The sequences used for determining the molecular weights are GenBank Accession number J01749.1 for pBR322 and the reference sequence for HPV16 (Accession K02718).BioEdit dataDNA molecule: HPV16 Accession K02718 Length = 7904 base pairsMW= 4786756.00 Daltons, double strandedDNA molecule: cloning vector pBR322 Length = 4361 base pairsMW= 2653867.00 Daltons, double strandedFormulaeGEq/ml of the HPV plasmid stock was calculated according to the formula: GEq/ml of the HPV plasmid stock = (DNA concentration of HPV plasmid stock) x (MW of HPV DNA + MW of pBR322)-1 x (Avogadro‟s Number) where Avogadro‟s Number = 6.022x1023 molecules/molGEq/ml of the bulk HPV DNA materials was calculated according to the formula:HPV GEq/ml of bulk material = (HPV GEq/ml of plasmid stock x volume plasmid stock) / volume bulk material.CalculationThe recombinant HPV-16 plasmid stock preparation was supplied to NIBSC at a concentration of 10 ng/μl. Using the MW determinations shown above, the GEq/ml of the HPV-16 plasmid stock is:= (10 x 10-9 g/μl) x (mol/(7440623 g) x (6.022x1023 molecules/mol) = 8.093 x 108 molecules/μl = 8.093 x 1011molecules/ml = 8.093 x 1011 HPV-16 GEq/ml22.23μl of the recombinant HPV-16 plasmid stock was diluted to a final volume of 1900ml, therefore,HPV-16 GEq/ml of bulk material = (8.093 x 1011 HPV-16 GEq/ml plasmid stock) x (0.02223 ml HPV-16 plasmid stock) / 1900 ml HPV-16 bulk material = 0.947 x 107 HPV-16 GEq/ml bulk material4. CONTENTSCountry of origin of biological material: United Kingdom.Each ampoule contains the lyophilized equivalent of 0.5 ml HPV-16 plasmid DNA in 10mM Tris buffer pH7.4 containing 1mM EDTA, 5 mg/ml trehalose and ~1 x 106 human GEq/ml derived from C33a cells.5. STORAGEThe ampoule should be stored at -20 °C or below on receipt.Please note: because of the inherent stability of lyophilized material, NIBSC may ship these materials at ambient temperature.6. DIRECTIONS FOR OPENINGDIN ampoules have an …easy -open‟ coloured stress point, where the narrow ampoule stem joins the wider ampoule body.Tap the ampoule gently to collect the material at the bottom (labeled) end. Ensure that the disposable ampoule safety breaker provided is pushed down on the stem of the ampoule and against the shoulder of the ampoule body. Hold the body of the ampoule in one hand and the disposable ampoule breaker covering the ampoule stem between the thumb and first finger of the other hand. Apply a bending force to open the ampoule at the coloured stress point, primarily using the hand holding the plastic collar.Care should be taken to avoid cuts and projectile glass fragments that might enter the eyes, for example, by the use of suitable gloves and an eye shield. Take care that no material is lost from the ampoule and no glass falls into the ampoule. Within the ampoule is dry nitrogen gas at slightly less than atmospheric pressure. A new disposable ampoule breaker is provided with each DIN ampoule.7. USE OF MATERIALNo attempt should be made to weigh out any portion of the freeze-dried material prior to reconstitution.The 1st International Standard for HPV-16 DNA contains high copy number template. There is a high risk of HPV-16 plasmid DNA contamination via aerosolization upon opening of the glass ampoule. The material must be opened and handled in a separate laboratory environment, away from other pre-amplification components such as reagents, labware and samples.The material is supplied lyophilized and, before use, should be reconstituted in 0.5 ml sterile nuclease-free water. Ensure that the inside surface of the ampoule is wetted with the added water so that any particles of freeze-dried material adhering to the glass are reconstituted. The reconstituted material has a final concentration of 1 X 107 IU/ml. The reconstituted material is suitable for calibration of in-house or working standards for the amplification and detection of HPV-16 DNA.. The material is not suitable for calibrating or assessing extraction, precipitation or centrifugation procedures. The material has NOT been calibrated for human DNA nucleic acid amplification techniques.8. STABILITYReference materials are held at NIBSC within assured, temperature-controlled storage facilities. The 1st International Standard for HPV-16 DNA should be stored at -20 °C or below on receipt.Studies on the stability of reconstituted standard are underway. Users should determine the stability of the reconstituted material according to their own method of preparation, storage and use.NIBSC follows the policy of WHO with respect to its reference materials.9. REFERENCESQuint, W. G. V., Pagliusi, S. R., Lelie, N., de Villiers, E. M., Wheeler, C. M. and the World Health Organization Human Papillomavirus DNA International Collaborative Study Group. (2006). Results of the First WorldHealth Organization International Collaborative Study of Detection of Human Papillomavirus DNA. J. Clin. Microbiol. 44: 571-579.Wilkinson, D.E., Baylis, S.A., Padley, D., Heath, A.B., Ferguson, M., Pagliusi, S.R., et al. Establishment of the 1st World Health Organization international standards for human papillomavirus type 16 DNA and type 18 DNA. Int J Cancer 2010 Jun 15;126(12):2969-83.WHO meeting report, on “Standardization of HPV assays and the role of HPV LabNet in supporting vaccine introduction” Geneva, Switzerland, 23-25 January 2008, in preparation.10. ACKNOWLEDGEMENTS11. FURTHER INFORMATIONFurther information can be obtained as follows; This material: enquiries@ WHO Biological Standards:http://www.who.int/biologicals/en/JCTLM Higher order reference materials: /en/committees/jc/jctlm/ Derivation of International Units:/products/biological_reference_materials/frequently _asked_questions/how_are_international_units.aspx Ordering standards from NIBSC:/products/ordering_information/frequently_asked_q uestions.aspxNIBSC Terms & Conditions:/terms_and_conditions.aspx12. CUSTOMER FEEDBACKCustomers are encouraged to provide feedback on the suitability or use of the material provided or other aspects of our service. Please send any comments to enquiries@13. CITATIONIn all publications, including data sheets, in which this material is referenced, it is important that the preparation's title, its status, the NIBSC code number, and the name and address of NIBSC are cited and cited correctly.15. LIABILITY AND LOSSInformation provided by the Institute is given after the exercise of all reasonable care and skill in its compilation, preparation and issue, but it is provided without liability to the Recipient in its application and use. It is the responsibility of the Recipient to determine the appropriateness of the standards or reference materials supplied by the Institute to the Recip ient (“the Goods”) for the proposed application and ensure that it has the necessary technical skills to determine that they are appropriate. Results obtained from the Goods are likely to be dependant on conditions of use by the Recipient and the variability of materials beyond the control of the Institute.All warranties are excluded to the fullest extent permitted by law, including without limitation that the Goods are free from infectious agents or that the supply of Goods will not infringe any rights of any third party.The Institute shall not be liable to the Recipient for any economic loss whether direct or indirect, which arise in connection with this agreement.The total liability of the Institute in connection with this agreement, whether for negligence or breach of contract or otherwise, shall in no event exceed 120% of any price paid or payable by the Recipient for the supply of the Goods.If any of the Goods supplied by the Institute should prove not to meet their specification when stored and used correctly (and provided that the Recipient has returned the Goods to the Institute together with written notification of such alleged defect within seven days of the time when the Recipient discovers or ought to have discovered the defect), the Institute shall either replace the Goods or, at its sole option, refund the handling charge provided that performance of either one of the above options shall constitute an entire discharge of the Institute‟s liability under this Condition.。
