NBD-556_333353-44-9_DataSheet_MedChemExpress
NBD-557_333352-59-3_DataSheet_MedChemExpress
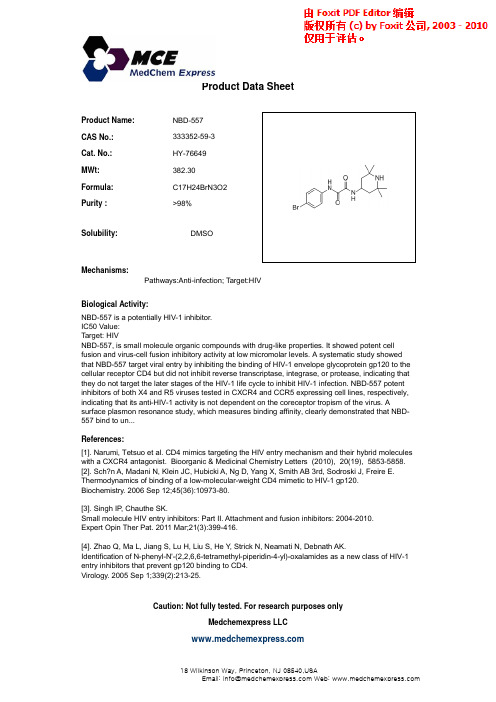
Product Name:NBD-557CAS No.:333352-59-3Cat. No.:HY-76649Product Data SheetMWt:382.30Formula:C17H24BrN3O2Purity :>98%Solubility:DMSOy Mechanisms:Biological Activity:NBD 557is a potentially HIV 1inhibitor Pathways:Anti-infection; Target:HIVNBD-557 is a potentially HIV-1 inhibitor.IC50 Value:Target: HIV NBD-557, is small molecule organic compounds with drug-like properties. It showed potent cell fusion and virus-cell fusion inhibitory activity at low micromolar levels. A systematic study showed that NBD-557 target viral entry by inhibiting the binding of HIV-1 envelope glycoprotein gp120 to the cellular receptor CD4 but did not inhibit reverse transcriptase, integrase, or protease, indicating that they do not target the later stages of the HIV-1 life cycle to inhibit HIV-1 infection. NBD-557 potent inhibitors of both X4 and R5 viruses tested in CXCR4 and CCR5 expressing cell lines, respectively,References:[1]. Narumi, Tetsuo et al. CD4 mimics targeting the HIV entry mechanism and their hybrid moleculeswith a CXCR4 antagonist. Bioorganic & Medicinal Chemistry Letters (2010), 20(19), 5853-5858. [2]. Sch?n A, Madani N, Klein JC, Hubicki A, Ng D, Yang X, Smith AB 3rd, Sodroski J, Freire E.p g ,p y,indicating that its anti-HIV-1 activity is not dependent on the coreceptor tropism of the virus. Asurface plasmon resonance study, which measures binding affinity, clearly demonstrated that NBD-557 bind to un...Thermodynamics of binding of a low-molecular-weight CD4 mimetic to HIV-1 gp120.Biochemistry. 2006 Sep 12;45(36):10973-80.[3]. Singh IP , Chauthe SK. Small molecule HIV entry inhibitors: Part II. Attachment and fusion inhibitors: 2004-2010.Expert Opin Ther Pat. 2011 Mar;21(3):399-416.[4]. Zhao Q, Ma L, Jiang S, Lu H, Liu S, He Y , Strick N, Neamati N, Debnath AK.Id tifi ti f N h l N'(2266t t th l i idi 4l)l id l f HIV 1Caution: Not fully tested. For research purposes onlyMedchemexpress LLCIdentification of N-phenyl-N'-(2,2,6,6-tetramethyl-piperidin-4-yl)-oxalamides as a new class of HIV-1entry inhibitors that prevent gp120 binding to CD4.Virology. 2005 Sep 1;339(2):213-25.18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c om。
艾森生物 实验室耗材和试剂 目录t
21127-030
P0018 31458
20W 500ml 500ml 100ml PL009 PL017 353002 3516
11644807001
2227S/100ul 3175S/100ul 7002s/100ul 7004s/100ul 9803s/15ml G7126-1KG
胎牛血清FBS MEM
FX 384 30ul 枪头
Horse serum马血清
DMSO
PMSF
25ml移液管
DMEM 细胞筛网 70um
细胞培养皿 65×15mm
384孔板
真空抽气泵
BSA牛血清蛋白
EGF Receptor (D38B1) XP® Rabbit mAb
western 一抗二抗去除液(弱碱性)
96孔细胞培养板 细胞网筛
25ml移液管 96孔板
无酚红1640 0.1-20ul排枪枪头 10-250ul排枪枪头 1ml盒装蓝色枪头 Trypsin EDTA,0.05%
DMSO 医用酒精棉球
医用酒精棉 麻面无粉手套 1ml灭菌盒装蓝枪头
DMEM L-15 384 HT assay plates
P3563-10PAK
PL017
SM0671/2x250ul
PL009 4685s/100ul 9271s/100ul
9101s/200ul
9102s/200ul 7002s/100ul 7004s/100ul 9803s/15ml
3777s/100ul
21127-022
11644807001 11966-025 6570 SD6031 华东 CU50
Gibco 华东 华东 Roche Gibco CORNING 上海生工 M号 中新 迪申 sigma AXYGEN Gibco
NBD-556_SDS_MedChemExpress
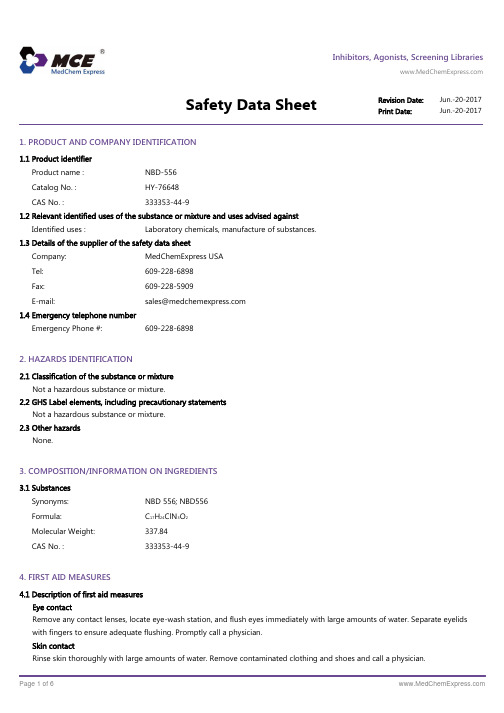
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jun.-20-2017Print Date:Jun.-20-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :NBD-556Catalog No. :HY-76648CAS No. :333353-44-91.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NBD 556; NBD556Formula:C17H24ClN3O2Molecular Weight:337.84CAS No. :333353-44-94. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
YM-155_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:YM155 (Sepantronium bromide) is a novel small molecule survivin suppressant with an IC50 of 0.54 nM for the inhibition of survivin promoter activity.IC50 Value: 0.54 nMTarget: SurvivinIn vitro: YM155 is not sensitive to survivn gene promoter–driven luciferase reporter activity even at 30 μM. YM155 significantly inhibits endogenous survivin expression in PC–3 and PPC–1 human HRPC cells with deficient p53 through transcriptional inhibition of the survivin gene promoter. On the contrary YM155 shows no sufficient effect on protein expression of c–IAP2, XIAP, Bcl–2, Bcl–xL, Bad,α–actin, and β–tubulin at 100 nM. YM155 indicates great apoptosis in human cancer cell lines including PC–3 and PPC–1 with a concomitant increase in caspase–3 activity. YM155 potently inhibits human cancer cell lines (mutated or truncated p53) including PC–3, PPC–1, DU145, TSU–Pr1, 22Rv1, SK–MEL–5 and A375 with IC50 from 2.3 to 11 nM, respectively [1]. YM155 increases thesensitivity of NSCLC cells to γ–radiation. The combination of YM155 and γ–radiation increases both the number of apoptotic cells and the activity of caspase–3. YM155 delays the repair of radiation–induced double–strand breaks in nuclear DNA [2].In vivo: YM155 completely inhibits the tumor growth of PC–3 s.c. xenografted prostate tumors at doses of 3 and 10 mg/kg, without body weight loss and blood cell count decrease. Pharmacokinetic analysis shows that YM155 is highly distributed to tumor tissue.Moreover, YM155 shows 80% TGI at a dose of 5 mg/kg in PC–3 orthotopic xenografts [1]. The combination therapy with YM155 and γ–radiation shows great antitumor activity against H460 or Calu6 xenografts in nude mice [2].PROTOCOL (Extracted from published papers and Only for reference)Enzyme assay [1]:The caspase–3 activity was measured with a CPP32/Caspase–3 Fluometric Protease Assay Kit (MBL) according to the manufacturer's instructions. After incubation with YM155 for 48 h, PC–3 and PPC–1 cells were lysed in 100 μL of a cell lysis buffer (provided with the kit) and equal volumes (50 μL) of cell lysate were obtained (100 μg of protein). After addition of 2× reaction buffer, the mixture was added to a black 96–well plate. The DEVD–AFC substrate (appended with the kit) was then added at 5 mL/well and the mixture incubated at 37°C for 30 min. Fluorescence emissions were quantified with a spectrofluorometer at an excitation wavelength of 390nm and an emission wavelength of 460 nm.Cell assay(growth inhibition) [1]:The antiproliferative activity of YM155 was measured by the method used at the National Cancer Institute. After treatment with YM155for 48 h, the cell count was determined by sulforhodamine B assay. The GI50 value was calculated by logistic analysis, which is thedrug concentration resulting in a 50% reduction in the net protein increase (as measured by sulforhodamine B staining) in control cells during the drug incubation. The assay was done in triplicate, and the mean GI50 value was obtained from the results of fourProduct Name:YM–155Cat. No.:HY-10194CAS No.:781661-94-7Molecular Formula:C 20H 19BrN 4O 3Molecular Weight:443.29Target:Survivin; Autophagy Pathway:Apoptosis; Autophagy Solubility:DMSO: 10.66 mg/mLindependent assays.Animal administration [1]:Five–week–old male nude mice (BALB/c nu/nu) were purchased from Charles River Japan, Inc. PC–3 cells (2 × 106–3 × 106) were injected into the flanks of the mice and allowed to reach a tumor volume of >100 mm3 in tumor volume (length × width2 × 0.5). YM155 was s.c. administered as a 3–day continuous infusion per week for 2 weeks using an implanted micro–osmotic pump (Alzet model 1003D, Durect) or i.v. administered five times a week for 2 weeks. The percentage of tumor growth inhibition 14 days after initial YM155 administration was calculated for each group using the following formula: MTV = 100 × {1–[(MTV of the treated group on day 14)–(MTV of the treated group on day 0)] / [(MTV of the control group on day 14)–(MTV of the control group on day 0)]}, where MTV is mean tumor volume. For both the frozen tumors and plasma samples, survivin expression levels were analyzed by Western blotting and YM155 drug concentration by high–performance liquid chromatography/triple quadrupole mass spectrometry (LC/MS/MS) using validated methods.References:[1]. Nakahara T, et al. YM155, a novel small–molecule survivin suppressant, induces regression of established human hormone–refractory prostate tumor xenografts. Cancer Res. 2007 Sep 1;67(17):8014–21.[2]. Iisa T, et al. Radiosensitizing effect of YM155, a novel small–molecule survivin suppressant, in non–small cell lung cancer cell lines. Clin Cancer Res. 2008 Oct 15;14(20):6496–504.[3]. Guo K, et al. A combination of YM–155, a small molecule survivin inhibitor, and IL–2 potently suppresses renal cell carcinoma in murine model. Oncotarget. 2015 Aug 28;6(25):21137–47.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
美国贝克曼库尔特流式细胞分析仪

美国贝克曼库尔特流式细胞分析仪(Beckman coulter cell)产品型号:Cell Lab Quanta SC当前价格:0.00元产品数量:0新旧程度:全新有效期至:0000-00-00所在地:产品简介:仪器简介:T细胞亚群检测的CD45/CD4/CD8/CD3、CD45/CD56/CD19/CD3;阵发性血红蛋白尿(PNH)检测的CD55、CD59;血小板无力症(GT)检测的CD41、CD61等等详细信息仪器简介:T细胞亚群检测的CD45/CD4/CD8/CD3、CD45/CD56/CD19/CD3;阵发性血红蛋白尿(PNH)检测的CD55、CD59;血小板无力症(GT)检测的CD41、CD61等等。
但对于白血病/淋巴瘤免疫分型,国际上迄今为止也没有统一的抗体组合。
在2000年国际细胞分析学会(ISAC)大会上,临床血细胞计数协会组织了一次国际专家会议,以期对检测血液淋巴系统肿瘤所需最少、最有效的单抗数达成共识。
75%与会者一致认为,对于慢性淋巴系统增殖性疾病(CLD)有9种单抗:CD5,CD19,κ,λ,CD3,CD20,CD23,CD10,CD45对初诊来说是最基本的。
淋巴瘤和CLD相似,需要至少12-16种单抗。
对于急性白血病(AL),75%的与会者认为大约13-15种单抗是最基本的:CD10,CD19,CD79a,CD13,CD33,CD34,CD45,CD2,MPO,CD7,CD14,CD3,HLA-DR等,对初步鉴别白血病系列是必需的。
其他一些(CD16,CD56,CDw65,TdT,cyCD3)可能对某些病例有用。
几乎所有的投票者都认为,要对急性白血病完善分类所需单抗的恰当数量平均为20-24种。
但这些抗体之间组合也是一大难题,目前也无统一规定(如表二)。
大会多数发言者(11/13)指出,对已确诊病人的监护和分期来说,仅需较少单抗。
抗体的质量控制是实验的关键环节。
抗体的质量包括其特异性、灵敏度、精密度。
封闭添加试剂(按字母顺序排列)

询价
4、BMPA
改变巯基基团为羧基基团,用于制备肽段-蛋白质交联物。
产品特点:
•反应基团:马来酰亚胺和羧基
•反应指向:巯基基团和胺(当与EDC结合使用时)
参考文献
Hermanson, G.T. (2008). Bioconjugate Techniques, 2nd ed., Elsevier Inc., pp.111-113. (Product # 20036)
25g
473
相关产品:
产品货号
产品名称
价格¥
24510
Sulfo-NHS
1848
22980
EDC
1005
20320
DCC
488
14、STAT,SATP和巯基添加试剂盒
添加保护性巯基防止二肽形成。
产品特点:
•与伯胺反应添加保护性巯基
•简单的脱保护步骤即可产生游离的巯基
•Thermo Scientific巯基添加试剂盒包含硫醇化作用,脱保护,纯化和定量所有需要的组分
订购信息:
产品货号
产品名称
包装
价格¥
23031
二盐酸乙二胺
10g
399
8、碘乙酰胺(Iodoacetamide),一次性使用
质谱前可靠的烷基化试剂。产品ຫໍສະໝຸດ 点:•与巯基基团形成共价键
•质谱级别(高纯度)
•方便,一次性使用形式(3个微型管,每管含有9.4mg)
•当用132μl碳酸氢铵(ammonium bicarbonate)溶解时,每管产生375mM的溶液
500mg
620
10、MMTS
可与巯基反应的可逆试剂。
产品特点:
•将-SH基团转化为-S-S-CH3
抗体公司

赛信通(上海)生物试剂有限公司
上海市浦东南路1101号远东大厦514室,200120 info@cst www.cst 2158356288 公司总部: 美国
Established in Beverly, MA in 1999, Cell Signaling Technology (CST) is a privatelyowned company with over 400 employees worldwide. We are dedicated to providing innovative research tools that are used to help define mechanisms underlying cell function and disease. Since its inception, CST has become the world leader in the production of the highest quality activationstate and total protein antibodies utilized to expand knowledge of cell signaling pathways. Our mission is to deliver the world's highest quality research tools that accelerate progress in biological research and personalized medicine. 总引用数为4670,来自于1966篇文章。最常引用的试剂包括: Akt, ERK2, ERK1, p38, Akt1。
AbD Serotec (BioRad)
MEDICA EasyRA

REF 10213-4 4 x 24mL总蛋白 (TP)楔形瓶,每瓶含试剂可用量 24mL。
预期用途EasyRA® TP 试剂目的是用于通过 MEDICA EasyRA 临床化学分析仪进行人血清或血浆(采用肝素锂作为抗凝血剂)中总蛋白的定量测定。
仅用于体外诊断用途。
仅供专业人员使用。
摘要和说明1, 2, 3在人血清中,血清蛋白主要包括白蛋白(占总蛋白的50-60%),其余部分包括α1-、α2-、β- 和γ-球蛋白。
总蛋白的浓度对维持血液和组织之间水的正常平衡与交换是很重要的。
血清蛋白浓度较低,可能是由于吸收不良、合成受损引起的,或是由于出血或过度分解代谢导致蛋白损失而引起的,如肾病和肝病中观察到的情况,这种情况可能导致低蛋白血症。
在高免疫球蛋白血症(如多发性骨髓瘤和感染)或脱水情况下,可能发生高蛋白血症。
方法的原理本分析法采用双缩脲反应测定血清蛋白,其中碱性溶液中的二价铜离子与化合物(含 2 个或多个、与碳原子结合的酰胺基或肽基)反应,形成一种有色络合物4。
碱性 pH血清蛋白质类 + Cu++———— 紫色络合物紫色络合物以分光光度法在 550nm 处测定,以 700nm 作为空白波长。
在较宽的线性范围内,该络合物的密度与蛋白质浓度成正比5。
试剂五水合硫酸铜0.3g/dL氢氧化钠0.8g/dL碘化钾、酒石酸钾钠和叠氮化钠作为防腐剂。
注意事项1.当处理任何实验室试剂时,应遵守良好实验室安全规范(CLSI, GP17-A2)。
2.氢氧化钠具有腐蚀性,能引起烧伤。
不要用嘴吸取试剂。
如果吞服,应立即就医处理。
避免眼部和皮肤接触。
如发生眼部接触,立即用大量水清洗眼睛,并就医处理。
如果发生皮肤接触,用水清洗皮肤至少 15 分钟。
3.本试剂含叠氮化钠 <0.1%;叠氮化钠可与铅和铜水管反应,形成高爆炸性金属叠氮化物。
请参考化学品安全说明书中的危险、危害和安全信息。
4.就任何诊断试验方法而言,其结果应根据所有其它试验结果和患者的临床状况加以解释。
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
植物病害诊断试剂盒

植物病害诊断试剂盒美国阿格迪agdia 公司是全球最大的植物病害诊断试剂生产商,产品品种最多,可检测项目多达200多个。
包装规格最全,不同的包装规格适合不同规模的实验室。
从中您一定能发现适合您使用的产品。
选购试剂说明,请仔细阅读。
1,kit, 订货号PSAxxxxx/xxxx 或PSPxxxxx/xxxx 为完整的试剂盒包装,包括样品提取缓冲液、包被好抗体的微孔板(可拆分)、酶标记物、稀释液、缓冲液、底物发色剂、阳性质控(如果应该供应)。
特别注明Indirect ELISA 方法的kit 包括未包被的微孔板及联接用的抗体,所含有的其他组分同上。
2, Reagent Set, 订货号SRAxxxxx/xxxx ,XRAxxxxx/xxxx或SRPxxxxx/xxxx 只含有未包被的微孔板、包被需要的抗体或联接用的抗体、酶标记物。
其他试剂如样品提取缓冲液、稀释液、缓冲液、底物发色剂、质控物需另外订购或自己配制。
我公司销售原厂的上述试剂,详见目录。
3, Bacterial Reagent Set, 订货号BRAxxxxx/xxxx 只含有未包被的微孔板、包被需要的抗体或联接用的抗体、酶标记物。
其他试剂如样品提取缓冲液、稀释液、缓冲液、底物发色剂、质控物需另外订购或自己配制。
我公司销售原厂的上述试剂,详见目录。
4, Bacterial ID订货号BIDxxxxx/xxxx 为完整的试剂盒包装,包括样品提取缓冲液、包被好抗体的微孔板(可拆分)、酶标记物、稀释液、缓冲液、底物发色剂、质控(如果应该供应)。
用于鉴定培养基中或有病症植物提取液中的细菌。
操作简便快速。
5, PS A或SR A中的A代表碱性磷酸酶标记;PS P或SR P中的P代表过氧化物酶标记。
6, Immunostrip test, 为检试纸条,操作简单,几分钟内得到结果,非常适合于现场检测。
该试条必须与相应的样品提取缓冲液配套使用。
实验室需要单独购买该样品提取缓冲液,详见目录。
重组贻贝粘蛋白的表征及功效评价

生物技术进展 2023 年 第 13 卷 第 4 期 596 ~ 603Current Biotechnology ISSN 2095‑2341研究论文Articles重组贻贝粘蛋白的表征及功效评价李敏 , 魏文培 , 乔莎 , 郝东 , 周浩 , 赵硕文 , 张立峰 , 侯增淼 *西安德诺海思医疗科技有限公司,西安 710000摘要:为了推进重组贻贝粘蛋白在医疗、化妆品领域的应用,对大肠杆菌规模化发酵及纯化生产获得的重组贻贝粘蛋白进行了表征及功效评价。
经Edman 降解法、基质辅助激光解吸电离飞行时间质谱、PITC 法、非还原型SDS -聚丙烯酰胺凝胶电泳法、凝胶法、改良的Arnow 法对重组贻贝粘蛋白进行氨基酸N 端测序、相对分子量分析、氨基酸组成分析、蛋白纯度分析、内毒素含量测定、多巴含量测定;通过细胞迁移、斑马鱼尾鳍修复效果对重组贻贝粘蛋白进行功效评价。
结果显示,获得的重组贻贝粘蛋白与理论的一级结构一致,蛋白纯度达95%以上,内毒素<10 EU ·mg -1,多巴含量大于5%;重组贻贝粘蛋白浓度为60 μg ·mL -1时能够显著促进细胞增殖的活性(P <0.01);斑马鱼尾鳍面积样品组与模型对照组相比极显著增加(P <0.001)。
研究结果表明,重组贻贝粘蛋白具有显著的促细胞迁移和修复愈合的功效,具备作为生物医学材料的潜质。
关键词:贻贝粘蛋白;基因重组;生物材料;表征;功效评价DOI :10.19586/j.20952341.2023.0021 中图分类号:S985.3+1 文献标志码:ACharacterization and Efficacy Evaluation of Recombinant Mussel Adhesive ProteinLI Min , WEI Wenpei , QIAO Sha , HAO Dong , ZHOU Hao , ZHAO Shuowen , ZHANG Lifeng ,HOU Zengmiao *Xi'an DeNovo Hith Medical Technology Co., Ltd , Xi'an 710000, ChinaAbstract :In order to promote the application of recombinant mussel adhesive protein in the medical and cosmetics field , the recombi⁃nant mussel adhesive protein obtained from scale fermentation and purification of Escherichia coli was characterized and its efficacy was evaluated. Amino acid N -terminal sequencing , relative molecular weight analysis , amino acid composition analysis , protein purityanalysis , endotoxin content , dihydroxyphenylalanine (DOPA ) content of recombinant mussel adhesive protein were determined by the following methods : Edman degradation , matrix -assisted laser desorption ionization time -of -flight mass spectrometry (MALDI -TOF -MS ), phenyl -isothiocyanate (PITC ), nonreductive SDS -polyacrylamide gel electrophoresis (SDS -PAGE ), gel method , modified Ar⁃now. The efficacy of recombinant mussel adhesive protein was evaluated by cell migration and repairing effect of zebrafish tail fin. Re⁃sults showed that the obtained recombinant mussel adhesive protein was confirmed to be consistent with the theoretical primary structure , protein purity of more than 95%, endotoxin <10 EU ·mg -1, DOPA content above 5%. When the recombinant mussel adhesive protein concentration was 60 μg ·mL -1, the effect of promoting cell proliferation was the most obvious , and it had very significant activity (P <0.01). The caudal fin area of zebrafish in sample group was significantly increased compared with model control group (P <0.001). The results indicated that recombinant mussel adhesive protein can promote cell migration and repair healing and has the potential to be used as biomedical materials.Key words :mussel adhesive protein ; gene recombination ; biological materials ; representation ; efficacy evaluation贻贝粘蛋白(mussel adhesive protein , MAP )也称作贻贝足丝蛋白(mussel foot protein ,Mfps ),收稿日期:2023⁃02⁃24; 接受日期:2023⁃03⁃31联系方式:李敏 E -mail:*******************;*通信作者 侯增淼 E -mail:***********************.cn李敏,等:重组贻贝粘蛋白的表征及功效评价是海洋贝类——紫贻贝(Mytilus galloprovincalis)、厚壳贻贝(Mytilus coruscus)、翡翠贻贝(Perna viri⁃dis)等分泌的一种特殊的蛋白质,贻贝中含有多种贻贝粘蛋白,包括贻贝粘蛋白(Mfp 1~6)、前胶原蛋白(precollagens)和基质蛋白(matrix proteins)等[1]。
ADC药物研发现状

Title Originator Highest Dev Status 111In-capromab pendetide Cytogen Corp Launched111In-imciromab pentetate Janssen Biotech Inc Launched131I-chTNT-1/B Peregrine Pharmaceuticals Inc Launched131I-metuximab Fourth Military Medical University PLA Launchedbrentuximab vedotin Seattle Genetics Inc Launchedgemtuzumab Wyeth Research Launchedibritumomab tiuxetan IDEC Pharmaceuticals Corp Launchedtrastuzumab emtansine Genentech Inc LaunchedATL-101, ATLAB Cornell University Phase 3 Clinical inotuzumab ozogamicin Wyeth Research Phase 3 Clinical oportuzumab monatox (intratumoral, head and neck cancer), Viventia University of Zurich Phase 3 ClinicalRIGS CC49Navidea Biopharmaceuticals Inc Phase 3 ClinicalABT-414Abbott Laboratories Phase 2 ClinicalCDX-1401Celldex Therapeutics Inc (pre-merger)Phase 2 Clinical glembatumumab vedotin CuraGen Corp Phase 2 ClinicalLMB-2National Cancer Institute Phase 2 Clinical lorvotuzumab mertansine ImmunoGen Inc Phase 2 Clinical moxetumomab pasudotox National Cancer Institute Phase 2 Clinical oportuzumab monatox (intravesicular, bladder cancer), Viventia University of Zurich Phase 2 ClinicalPSMA-ADC Cytogen Corp Phase 2 ClinicalRG-7593Genentech Inc Phase 2 ClinicalRG-7596Genentech Inc Phase 2 ClinicalSAR-3419ImmunoGen Inc Phase 2 Clinical212-Pb-TCMC-trastuzumab National Cancer Institute Phase 1 ClinicalActimab-A PDL BioPharma Inc Phase 1 ClinicalAGS-16M8F Agensys Inc Phase 1 Clinicalanti-CD3/anti-CD20 bispecific antibody-armed activated T-cells (non-Hodgkin's lymphoma), Wayne State University/Barbara Ann KarmanosCancer Institute Barbara Ann Karmanos Cancer Institute Phase 1 ClinicalASG-5ME Agensys Inc Phase 1 ClinicalBAY-79-4620MorphoSys AG Phase 1 Clinical citatuzumab bogatox Viventia Biotech Inc Phase 1 Clinical doxorubicin-loaded anti-EGFR immunoliposomes (solid tumors), UniversityHospital Basel University Hospital of Basel Phase 1 ClinicalHuM195/rGel (intravenous infusion, AML/CML/meylodisplastic syndrome),Targa Therapeutics Memorial Sloan-Kettering Cancer Center Phase 1 ClinicalIMGN-529ImmunoGen Inc Phase 1 ClinicalIMGN-853ImmunoGen Inc Phase 1 ClinicalIMMU-132Immunomedics Inc Phase 1 Clinical labetuzumab-SN-38Immunomedics Inc Phase 1 ClinicalNHS-IL-12National Cancer Institute Phase 1 ClinicalRG-7450Genentech Inc Phase 1 ClinicalRG-7458Genentech Inc Phase 1 Clinical RG-7598Genentech Inc Phase 1 Clinical RG-7599Genentech Inc Phase 1 Clinical RG-7600Genentech Inc Phase 1 Clinical RG-7636Genentech Inc Phase 1 Clinical SAR-566658ImmunoGen Inc Phase 1 Clinical T-Guard University Medical Center St Radboud Phase 1 Clinical vorsetuzumab mafodotin Seattle Genetics Inc Phase 1 Clinical 131I-catuximab (colorectal cancer), Pacific Meinuoke Fourth Military Medical University PLA Discovery177Lu-tetraxetan-tetulomab (non-Hodgkin's lymphoma), Nordic Nanovector Nordic Nanovector AS Discovery227Th-epratuzumab (hematological cancer), Algeta Algeta ASA Discovery227Th-rituximab (cancer), Algeta Algeta ASA Discovery227Th-trastuzumab (cancer), Algeta Algeta ASA Discovery4s3-0014s3 Bioscience Inc Discovery4s3-0024s3 Bioscience Inc Discovery64Cu-NOTA-ALT-836Altor BioScience Corp DiscoveryAA-A225Actinium Pharmaceuticals Inc Discovery AbGn-107AbGenomics Corp Discovery Actimab-B Fred Hutchinson Cancer Research Center Discovery Actimab-C Actinium Pharmaceuticals Inc Discovery Actimab-P Actinium Pharmaceuticals Inc Discovery adalimumab + anti-Ang2 Zybody (rheumatoid arthritis/inflammatory boweldisease), Zyngenia Zyngenia Inc DiscoveryAGS-15E ADC Agensys Inc DiscoveryAGT-160ArmaGen Technologies Inc DiscoveryAGT-185ArmaGen Technologies Inc DiscoveryAGT-190ArmaGen Technologies Inc Discovery amanitin-trastuzumab conjugate (cancer), Heidelberg Pharma Heidelberg Pharma Holding Ltd Discoveryanti-CD133-immunotoxin conjugates (photochemical internalization, cancer),PCI Biotech PCI Biotech Holding ASA Discoveryanti-ET8R-MC-vc-PAB-MMAE Genentech Inc Discoveryanti-NaPi3b antibody-drug conjugate (cancer), Genentech/Roche Genentech Inc Discovery antibody drug conjugates (cancer), Sanofi Sanofi Discovery antibody-drug conjugates (cancer), ADC Therapeutics ADC Therapeutics Sarl Discovery antibody-drug conjugates (cancer), Seattle Genetics/Oxford BioTherapeutics Seattle Genetics Inc Discovery antibody-drug conjugates (TAP, cancer), Lilly Eli Lilly & Co Discovery antibody-IFN lambda conjugates (cancer), Immunomedics Immunomedics Inc Discovery anticancer therapy (TAP technology), Amgen Amgen Inc DiscoveryAPH-0912Aphios Corp Discovery Aurixin BioIntegrator DiscoveryAZ-05Allozyne Inc Discovery BIOO-1BIOO Therapeutics DiscoveryBIOO-2BIOO Therapeutics Discovery BIOO-3BIOO Therapeutics Discovery BIOO-4BIOO Therapeutics Discovery BIOO-5BIOO Therapeutics Discovery BIOO-6BIOO Therapeutics Discovery BIOO-7BIOO Therapeutics Discovery botulinum toxin B inhibitor (injectable, heteropolymer mAbs, botulism),Immunome Immunome Inc Discovery BT-2111biOasis Technologies Inc Discovery C2-2b-2b Immunomedics Inc Discovery CDX-014CuraGen Corp Discovery chiHEA-125-Ama Heidelberg Pharma Holding Ltd Discovery CK-22-(20)-(20)Immunomedics Inc Discovery complement factor H-derived short consensus repeat-antibody constructs(infection), LysoVac University of Innsbruck Discovery Cymac-001Cytoguide ApS Discovery CYP-Ab Cytune Pharma Discovery D2C7-based immunotoxins (glioma), Duke University Duke University Discovery EGFR modulators (antibody conjugates, PIT, cancer), Aspyrian Aspyrian Therapeutics Inc Discovery engineered cysteine drug conjugates mAbs (cancer), Seattle Genetics Seattle Genetics Inc Discovery epratuzumab-SN-38Immunomedics Inc Discovery ETBs (cancer), Molecular Templates/ Imclone Molecular Templates Inc Discovery Fluorescent-labeled bevacizumab (imaging, ocular disease), Mivenion mivenion Gmbh Discovery gemcitabine + paclitaxel (prodrug, nanomAb, cancer), ImmunePharmaceuticals Immune Pharmaceuticals Corp Discovery Herceptin:Endostatin-P125A University of Miami Discovery hLL1-CL2A-SN-38Immunomedics Inc Discovery hPAM4-CL2A-SN-38Immunomedics Inc Discovery human monoclonal antibody-toxin conjugates (myocardial infarction), Celdara Celdara Medical LLC Discovery HuMax-TF-ADC Genmab A/S Discovery IFNalpha-fused mAbs (HBV infection), Roche Roche Holding AG Discovery IL-13 receptor alpha 2 inhibitors (iv, cancer), Pfizer Pfizer Inc Discovery IMGN-289ImmunoGen Inc Discovery intracellular antibodies (Intraphilin, inflammatory diseases/infectiousdiseases/ophthalmic diseases), Permeon Biologics Permeon Biologics Inc Discovery mapp-66Mapp Biopharmaceutical Inc Discovery MB-2003Mapp Biopharmaceutical Inc Discovery monoclonal antibody-drug conjugates, Chirogenix/ImmunoGen/Celltrion Chirogenix Co Ltd Discovery MP-Ter-ADC MediaPharma Srl Discovery N01-OX2Intellect Neurosciences Inc Discovery PC-91ProCell Therapeutics Inc Discovery ProstaLite PhotoBiotics Ltd Discoveryrecombinant mAb-biocide fusion proteins (oral/Directed Biocide,cryptosporidium infection), ioGenetics ioGenetics Inc Discovery SGN-CD33A Seattle Genetics Inc Discovery SGN-LIV1A Seattle Genetics Inc Discovery SL-101Stemline Therapeutics Inc Discovery SYD-983Synthon Biopharmaceuticals Discovery T01-OX2Intellect Neurosciences Inc Discovery TBL-0306L Transgene Biotek Ltd Discovery TBL-0306M Transgene Biotek Ltd Discovery TBL-0805E Transgene Biotek Ltd Discovery thio-trastuzumab-mpeo-DM1Genentech Inc Discovery trastuzumab-PNU-159682 antibody-drug conjugate (cancer), Genentech Genentech Inc Discovery veltuzumab-IFN alpha 2b conjugate (cancer), IBC/Immunomedics IBC Pharmaceuticals Inc Discovery BIIB-015Biogen Inc Suspended Pretarget technology (gastrointestinal adenocarcinoma), NeoRx Poniard Pharmaceuticals Inc Suspended125I-AnnA1 IgG Sidney Kimmel Cancer Center No Development Reported131I-CC49-SCA Enzon Labs Inc No Development Reported177Lu-capromab pendetide Cytogen Corp No Development Reported90Y-capromab pendetide Cytogen Corp No Development Reported99mTC-BERH2Medac GmbH No Development Reportedanti-CD133-vcMMAF Seattle Genetics Inc No Development Reportedanti-CD22 antibody drug-conjugates, Medarex/BMS Medarex Inc No Development Reportedanti-PSMA antibody-drug conjugates (cancer), Medarex Medarex Inc No Development Reportedantibody-drug conjugates (solid tumors), Daiichi Sankyo Seattle Genetics Inc No Development ReportedAVE-9633ImmunoGen Inc No Development Reportedbectumomab Immunomedics Inc No Development ReportedCA125/MUC16-targeting antibody-drug conjugate (ovarian cancer),Genentech Genentech Inc No Development Reportedcathepsin B-sensitive prodrugs, BMS Bristol-Myers Squibb Pharmaceutical ResearchInstituteNo DevelopmentReportedCC49 humanized radioimmunoconjugates, National Cancer Institute,University of Alabama at Birmingham National Cancer Institute No Development ReportedCD4-BFFI Roche Holding AG No Development ReportedCHB-111ViRexx Medical Corp No Development ReportedCHT-25University College London No Development ReportedCMD-193Wyeth No Development ReportedCNTO-95 immunoconjugates (cancer), Centocor Janssen Biotech Inc No Development Reportedconjugated PEI/anti-CD133 mAb plasmid-based gene therapy (brain tumor),Discovery genomics Discovery Genomics Inc No Development ReportedcT84.66City of Hope No Development Reporteddelta 9-cadherin targeting antibody (gastric cancer), Actinium Helmholtz Zentrum München No Development Reporteddiphtheria toxin, RCT Research Corporation Technologies No Development Reporteddoxorubicin-C225 conjugate (STEALTH), SEQUUS SEQUUS Pharmaceuticals Inc No Development ReportedDTPA-BrE-3University of Colorado System No Development ReportedDXL-625InNexus Biotechnology Inc No Development ReportedG3.519-PAP-S Tanox Inc No Development ReportedhuHMFG1-caspase Antisoma plc No Development ReportedIMGN-007ImmunoGen Inc No Development ReportedIMGN-009ImmunoGen Inc No Development Reportedimmunoconjugate (cancer MN), Bayer Bayer AG No Development ReportedImmuRAID-AFP-99mTc, Immunomedics Immunomedics Inc No Development ReportedIMTOX 22-97A University of Texas Southwestern MedicalCenterNo DevelopmentReportedKSB-201KS Biomedix Holdings plc No Development ReportedLA22-radioimmunoconjugates (cancer), Welson/Peking University Welson Pharmaceuticals Inc No Development Reportedlabetuzumab Immunomedics Inc No Development ReportedLMB-1, NIH National Institutes of Health No Development ReportedLu-177-trastuzumab Tarbiat Modares University No Development ReportedLymphoScan Immunomedics Inc No Development ReportedMDX-11Medarex Inc No Development ReportedMDX-1203Medarex Inc No Development ReportedMDX-1206Medarex Inc No Development Reportedmonoclonal-porphyrins, Quadra Logic QLT Inc No Development Reportedmonoclonals, Quest Quest Biotechnology Inc No Development ReportedNogo receptor modulators, Biogen Idec Yale University No Development ReportedONS-1210Oncobiologics Inc No Development ReportedOP-06 program (cancer), Onco-Pharmakon Onco-Pharmakon Inc No Development Reportedpaclitaxel analogs and immunoconjugates (cancer), Bioxel Bioxel Pharma Inc No Development ReportedPE38-conjugated anti-CD30 immunotoxin, NCI National Cancer Institute No Development Reportedprostate-specific MAb, NIH National Institutes of Health No Development ReportedR-1549The UK Imperial Cancer Research Fund No Development Reportedradiolabeled Tx3.833Beth Israel Deaconess Medical Center No Development Reportedscu-PA-59D8Bristol-Myers Squibb Co No Development ReportedSGN-17/19Seattle Genetics Inc No Development Reportedtaxane-monoclonal antibody conjugates, ImClone ImClone Systems Inc No Development Reportedtranscobalamin (vitamin B12) receptor-targeting mAbTCR23-saporinconjugate (cancer), Kyto Kyto Biopharma Inc No Development Reportedtrastuzumab-autophilic peptide conjugate (breast cancer), InNexusBiotechnology InNexus Biotechnology Inc No Development Reportedtrastuzumab-MC-vc-PAB-MMAF Genentech Inc No Development ReportedTRP-targeted antibody conjugate (Yttrium 90/MX-DTPA), SomantaPharmaceuticals Immunodex Inc No Development Reportedtucotuzumab celmoleukin EMD Lexigen Research Center Corp No Development ReportedVB4-011Viventia Biotech Inc No Development ReportedVB6-011Viventia Biotech Inc No Development ReportedVB6-050Viventia Biotech Inc No Development ReportedXomaZyme-791XOMA Corp No Development Reportednofetumomab Poniard Pharmaceuticals Inc Withdrawn 131I-81C6Duke University Discontinued 131I-ImmuRAIT-HCG, Immunomedics Immunomedics Inc Discontinued B-B4-DC1ImmunoGen Inc Discontinued CC49 radioimmunoconjugates, University of Alabama at Birmingham University of Alabama at Birmingham Discontinued CD5 monoclonals/RIPs, Italfarmaco Italfarmaco SpA Discontinued CVX-045CovX Pharmaceuticals Inc Discontinued CVX-060CovX Pharmaceuticals Inc Discontinued CVX-241CovX Pharmaceuticals Inc Discontinued CVX-343CovX Pharmaceuticals Inc Discontinued doxorubicin-BR96 conjugate, BMS Bristol-Myers Squibb Co Discontinued doxorubicin-CEA conjugate, Immunomedics Immunomedics Inc Discontinued doxorubicin-LL2 conjugate, Immunomedics Immunomedics Inc Discontinued FAP5-DM1Boehringer Ingelheim Corp Discontinued FGFR4-CovX-Body CovX Pharmaceuticals Inc Discontinued HuM-195-Bi-213PDL BioPharma Inc Discontinued human placental growth factor 1-CVX-2000 monoclonal antibody conjugatedtherapeutic (CovX-body, cancer), CovX CovX Pharmaceuticals Inc Discontinued huN901-CC-1065ImmunoGen Inc Discontinued huN901-DC1ImmunoGen Inc Discontinued ImmuRAID-HCG-99mTc, Immunomedics Immunomedics Inc Discontinued ImmuRAIT-CEA-rhenium-188, Immunomedics Immunomedics Inc Discontinued MDX-214Medarex Inc Discontinued MEDI-547MedImmune LLC Discontinued MLN-2704Cornell University Discontinued Oncolym Peregrine Pharmaceuticals Inc Discontinued Oncolysin B Dana-Farber Cancer Institute Inc DiscontinuedOncolysin CD6Dana-Farber Cancer Institute Inc Discontinued Oncolysin M Dana-Farber Cancer Institute Inc Discontinued Oncolysin S Dana-Farber Cancer Institute Inc Discontinued Oncopurge Poniard Pharmaceuticals Inc Discontinued rhenium-188-LL2, Immunomedics Immunomedics Inc Discontinued SMART ABL-364Novartis AG Discontinued targeted ranpirnase conjugates (cancer), Alfacell Tamir Biotechnology Inc DiscontinuedHighest Dev Status。
VX-745_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:VX–745 is a potent and selective inhibitor of p38α, and possesses anti–inflammatory activity.In Vitro: VX–745 exhibits PBMC IL–1β and TNFα IC 50 values of 45 and 51 nM, respectively. VX–745 is also effective in whole blood,blocking IL–1β and TNFα release with IC 50 values of 150 and 180 nM, respectively. VX–745 shows a promising selectivity profile,with 20–fold selectivity for p38α over p38β (K i =220 nM)[1]. VX–745 solutions in DMSO/DMEM inhibits the IL–6 production with IC 50of 15±9 nM [2]. VX–745 (5.0 nM) displays potent activity and 1000–fold selectivity over closely related kinases, including ERK1,JNK1–3 and MK2. VX–745 (10 nM–50 μM) increasingly inhibits the anisomycin–induced activity of p38α[3]. VX–745 (0.06 μM–20 μM)inhibits IL–6 and VEGF secretion in BMSCs. VX–745 can inhibit cytokine (TNF–α, IL–6, VEGF)–induced paracrine MM cell growth,survival, and drug resistance in the BM microenvironment. VX–745 induces modest growth inhibition of MM.1S, RPMI8226, and U266 cell lines in a dose–dependent fashion, with inhibitory concentration of 50% (IC 50) of 10 μM [4].In Vivo: VX–745 (2.5, 5, and 10 mg/kg) improves the inflammatory scores in mice by 27%, 31%, and 44%, respectively [1]. VX–745(1.06 mg/kg) significantly decreases the inflammation score from 2.07±0.29 for the control group to 1.42±0.06[2].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]VX–745 inhibits p38α and p38β. The IC 50 for the inhibition of these two p38 homologs are obtained by aspectrophotometric coupled–enzyme assay. A fixed concentration of enzyme (15 nM of p38α or p38β) is incubated with various concentrations of VX–745 in DMSO for 10 min. at 30°C in 0.1 M HEPES buffer, pH 7.5, containing 10% glycerol, 10 mM MgCl 2, 2.5mM phosphoenolpyruvate, 200 μM NADH, 150 μg/mL pyruvate kinase, 50 μg/mL lactate dehydrogenase, and 200 μM EGF receptor peptide. The reaction is initiated with 100 μM and 70 μM ATP for p38α and p38β assays, respectively. The decrease of absorbance at 340 nm is monitored to follow the rate of the reaction. IC 50 is evaluated from the rate data as a function of the inhibitor concentration.Cell Assay: VX–745 is dissolved in DMSO.[2]For these experiments, cells are plated at a density of 60,000 cells/well in 96–well plates.Each condition is tested in triplicate or more. The following day, all particle suspensions, active substances or control solutions are freshly prepared, distributed and incubated for 24 h at 37°C. Then, supernatants are carefully discarded. To perform the MTT test, 50μL of 0.1% MTT solution is added to each well for 3 h. Each well is then incubated for 1 h with 200 μL of dimethyl sulfoxide.Absorbance is measured at 595 nm. Reported results are expressed as the means±SD.Animal Administration: VX–745 is prepared in 100% propylene glycol.[1]Type II collagen–induced arthritis is established in male DBA/1mice with a minor modification. 10–Week old male DBA/1 mice are immunized by two intradermal injections within a 3 week interval using 100 μL of an emulsion consisting of a 1:1 (v/v) mixture of chick type II collagen (200 μg in 10 mM acetic acid) and complete Freund's adjuvant. Following the booster immunization, the mice are left untreated for 2–3 weeks, and are randomized into five treatment groups after they exhibit focal carpal (wrist) swelling (level 2 arthritic severity score) in both front paws. The five treatment groups are: 1: water control, 10 mL/kg, p.o., bid, (n=14); 2: 100% propylene glycol (PG) vehicle control, 10 mL/kg, p.o., bid, (n=8); 3:VX–745 in PG, at 10 mg/kg, p.o., bid, (n=7); 4: VX–745 in PG, at 5 mg/kg, p.o., bid, (n=10); and 5: VX–745 in PG, at 2.5 mg/kg, p.o., bid,Product Name:VX–745Cat. No.:HY-10328CAS No.:209410-46-8Molecular Formula:C 19H 9Cl 2F 2N 3OS Molecular Weight:436.26Target:p38 MAPK Pathway:MAPK/ERK Pathway Solubility:DMSO: 13.08 mg/mL (Need ultrasonic)(n=11). Arthritic symptoms are scored every other day using a level 1 to level 5 scoring system. Paw inflammation begins with erythema at the wrist (level 1), progressing to focal swelling of the wrist (level 2), to complete swelling of the wrist (level 3), to complete swelling of wrist and palm (level 4), and finally to complete swelling of wrist, palm and fingers (level 5). The sums of the scores from both front paw scores are used for plotting disease progression curves. Mice are sacrificed on day 20 and paws are removed, sectioned sagitally, stained with hemotoxylin & eosin, and scored for inflammation. Histologically, wrist joint inflammation begins with an infiltration of the synovium into the joint space (level 1), progressing to joint cartilage erosion (level 2), to joint cartilage and bone erosion (level 3), and finally to erosion of cartilage and bone accompanied by pannus formation (level 4). References:[1]. Duffy JP, et al. The Discovery of VX–745: A Novel and Selective p38α Kinase Inhibitor. ACS Med Chem Lett. 2011 Jul 28;2(10):758–63.[2]. Pradal J, et al. Intra–articular bioactivity of a p38 MAPK inhibitor and development of an extended–release system. Eur J Pharm Biopharm. 2015 Jun; 93:110–7.[3]. Bagley MC, et al. Rapid synthesis of VX–745: p38 MAP kinase inhibition in Werner syndrome cells. Bioorg Med Chem Lett. 2007 Sep 15;17(18):5107–10. Epub 2007 Jul 13.[4]. Hideshima T, et al. Targeting p38 MAPK inhibits multiple myeloma cell growth in the bone marrow milieu. Blood, 2003, 101(2), 703–705.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
碧云天线粒体膜电位检测试剂盒(Rhodamine 123)说明书

线粒体膜电位检测试剂盒(Rhodamine 123)产品简介:碧云天的线粒体膜电位检测试剂盒(Rhodamine 123) (Mitochondrial Membrane Potential Assay Kit with Rhodamine 123)是一种以Rhodamine 123为荧光探针对线粒体进行染色并进行膜电位检测的试剂盒。
本试剂盒可用于活细胞线粒体膜电位的检测,也用于细胞凋亡的检测。
本试剂盒可使用荧光显微镜、流式细胞仪、荧光酶标仪等荧光检测设备进行检测。
Rhodamine 123也称2-(6-Amino-3-imino-3H-xanthen-9-yl)benzoic acid methyl ester ,中文名为罗丹明123,分子式为C 21H 17ClN 2O 3,分子量为380.82,CAS 号为62669-70-9。
Rhodamine 123是一种通透细胞膜的黄绿色阳离子荧光探针,最大激发光波长为507nm ,最大发射光波长为529nm 。
Rhodamine 123的化学结构式和激发、发射光谱图参考图1。
图1. Rhodamine 123的化学结构式和激发、发射光谱图。
本试剂盒检测线粒体膜电位的原理如下。
在正常细胞中,Rhodamine 123能够依赖线粒体跨膜电位(mitochondrial transmembrane potential, ΔΨm)选择性进入线粒体基质,可发出明亮的黄绿色荧光;当细胞发生凋亡或坏死时,线粒体膜电位丢失,线粒体通透性转换孔(mitochondrial permeability transition pore, MPTP)持续开放,引起线粒体跨膜电位(ΔΨm )的崩溃,Rhodamine 123从线粒体中释放出来,从而导致线粒体内黄绿色荧光强度的明显降低。
此外有报道,在个别特定情况下,Rhodamine 123探针在线粒体内过度聚集后可能会出现自发淬灭(self-quenching)现象,线粒体内黄绿色荧光强度降低,而在凋亡发生时,线粒体中黄绿色荧光增强。
进口器械耗材试剂目录表1

1.乳酸脱氢酶试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2002.肌酸激酶试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004第3400291号(更3.碱性磷酸酶试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004第3400293号(4.丙氨酸氨基转移酶试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004第34005.淀粉酶试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004第3400294号(更))6.CD45RO-PE荧光单克隆抗体试剂 (法国 IMMUNOTECH S.A.S (美国贝克曼库尔特有限公司分公司) 国7.CD3-FITC/CD(16+56)-PE荧光单克隆抗体试剂 (法国 IMMUNOTECH S.A.S (美国贝克曼库尔特有限公司8.CD45RA-FITC荧光单克隆抗体试剂 (法国 IMMUNOTECH S.A.S (美国贝克曼库尔特有限公司分公司)9.ISE-缓冲液 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004第3400162号(更))10.稀释液 ( 国食药监械(进)字2007第3400992号)11.质控品 (美国SYSMEX CORPORATION希森美康株式会社 国食药监械(进)字2007第3400993号)12.质控品 (美国 SYSMEX CORPORATION希森美康株式会社 国食药监械(进)字2007第3400994号)13.皮质醇试剂包 ( 国食药监械(进)字2007第3401009号)14.三碘甲状腺原氨酸摄取试剂包 (Ortho-Clinical Diagnostics Inc., 国食药监械(进)字2007第3415.肌酸激酶MB亚单位质量试剂包 ( 国食药监械(进)字2007第3401011号)16.采血针 (德国 罗氏诊断有限公司 国食药监械(进)字2007第3410922号)17.苯妥英试剂盒 ( 国食药监械(进)字2005第3401316号(更))18.凝血酶原时间测试试剂 (德国 Dade Behring Marburg公司 国食药监械(进)字2007第3400990号)19.GPA-PE荧光单克隆抗体试剂 (法国 IMMUNOTECH S.A.S (美国贝克曼库尔特有限公司分公司) 国食20.腔镜手助器(商品名:兰碟斯) (Hakko Co., Ltd. 国食药监械(进)字2007第3660631号)21.高压注射连接管 (美国 Argon Medical Devices, Inc 国食药监械(进)字2007第3660652号)22.球囊扩张导管(商品名:Extensor) ( 国食药监械(进)字2007第3770536号)23.弹簧圈(商品名:GDC) (Boston Scientific Corporation 国食药监械(进)字2007第3770569号)24.穿刺导引套装 英文名称:Exacta Percutaneous Sheath Introducer Kits (Becton Dickinson Cri25.7.382缓冲液 ( 国食药监械(进)字2004第3401401号(更))26.尿液总蛋白试剂盒 ( 国食药监械(进)字2005第3401707号(更))27.网织红细胞稀释液·染色液 (日本 SYSMEX CORPORATION希森美康株式会社 国食药监械(进)字20028.肺通气功能测定仪(商品名:肺功能仪) (日本福田产业株式会社 国食药监械(进)字2007第2400718号29.高密度脂质胆固醇诊断试剂盒 (日本 协和医药株式会社 国食药监械(进)字2007第2400722号)30.白蛋白检测试剂盒 (西班牙博士泰公司 国食药监械(进)字2007第2400723号)31.镁检测试剂盒 (西班牙博士泰公司 国食药监械(进)字2007第2400724号)32.葡萄糖检测试剂盒 (西班牙博士泰公司 国食药监械(进)字2007第2400725号)33.HL-9000/IONEA型高电位治疗器 (株式会社日本医疗科学 国药管械(进)2002第2210693号(更))34.BIO 2001 生殖泌尿系统生物反馈电刺激治疗仪 (METRASOL公司 国食药监械(进)字2004第3260453号35.CD3-FITC荧光单克隆抗体试剂 (法国 IMMUNOTECH S.A.S (美国贝克曼库尔特有限公司分公司) 国36.CD38-FITC荧光单克隆抗体试剂 (法国 IMMUNOTECH S.A.S (美国贝克曼库尔特有限公司分公司) 国37.胰淀粉酶测定试剂盒 (关东化学株式会社 国食药监械(进)字2007第2400711号)38.类风湿因子诊断试剂盒 ( 国食药监械(进)字2005第3401715号(更))39.前白蛋白诊断试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2005第240145140.组织脱水机(商品名:珊顿组织脱水机) (美国 Thermo Electron Corporation 国食药监械(进)字2041.切片机(商品名:珊顿冷冻切片机) (美国 Thermo Electron Corporation 国食药监械(进)字2007第42.组织脱水机(商品名:珊顿全密封组织脱水机) (美国 Thermo Electron Corporation 国食药监械(进43.呼吸球及附件 (VBM Medizintechnik GmbH 国食药监械(进)字2007第1540638号)44.麻醉面罩 (Mallinckrodt Dar S.r.l. 国食药监械(进)字2007第1540643号)45.牙科种植体手术工具 (MegaGen Co.,Ltd. 国食药监械(进)字2007第1550546号)46.酒精诊断试剂盒 (德国 罗氏诊断有限公司 国食药监械(进)字2007第3400693号)47.转铁蛋白诊断试剂盒 ( 国食药监械(进)字2005第3401771号(更))48.全段甲状旁腺激素试剂盒 ( 国食药监械(进)字2006第3401974号(更))49.全自动荧光磁微粒酶免分析仪 (日本东曹株式会社日本东曹株式会社 国食药监械(进)字2007第240050.化学发光分析仪 (德国 DiaSorin Deutschland GmbH 国食药监械(进)字2007第2400559号)51.丙氨酸氨基转移酶试剂盒 (Dade Behring Inc. 国食药监械(进)字2007第2400705号)52.低密度脂质胆固醇诊断试剂盒 (协和医药株式会社 国食药监械(进)字2007第2400708号)53.甘油三酯诊断试剂盒 (协和医药株式会社 国食药监械(进)字2007第2400709号)54.手术膜 (美国 3M Company3M Company 国食药监械(进)字2007第2640542号)55.高密度/低密度胆固醇校准液 ( 国食药监械(进)字2005第3402329号(更))56.通用稀释液8 ( 国食药监械(进)字2005第3401703号(更))57.植入式心脏起搏器 (美国 Guidant Corporation Cardiac Pacemakers Inc. 国食药监械(进)字200658.通用稀释液1 ( 国食药监械(进)字2005第3401697号(更))59.中空螺钉(商品名:Magana-Fx内固定中空螺钉) (美国 Zimmer Inc. 捷迈公司 国食药60.带锁髓内钉(商品名:M/DN) (美国 Zimmer Inc. 捷迈公司 国食药监械(进)字2007第3461044号)61.全髋关节系统(商品名:Elite Plus) (英国 DePuy International Ltd 国食药监械(进)字2007第62.内固定线缆系统(商品名:ATLAS) (美国 Medtronic Sofamor Danek USA Inc. 国食药监械(进)字63.髋关节假体(商品名:VerSys推荐型髋关节假体) (美国 Zimmer Inc. 捷迈公司 国食药监械(进)字64.鼻塞(商品名:Raucocel 鼻塞) ((Deutschland)Lohmann & Rauscher 国际股份有限公司 国食药监65.腹腔镜及附件(商品名:腹腔镜及附件) (奥林巴斯苇音特和意北公司 国食药监械(进)字2007第322066.Acculan电池动力系统 (Aesculap AG & Co. KG 国食药监械(进)字2004第2211422号(更))67.透析用碳酸氢钠干粉 (B. Braun Medizintechnologie GmbH 国食药监械(进)字2004第3451444号(更68.二氧化碳校准液/稀释液 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2005第340269.乙烯基聚硅氧烷咬合检测印模材料(商品名:而至精确咬合记录II型) (日本株式会社而至 国食药监械70.用于口腔粘膜的亲水乙烯基聚硅氧烷印模材料(商品名:而至精确义齿记录) (日本株式会社而至 国食71.而至快速自凝基托树脂 (株式会社而至 国食药监械(进)字2005第3632051号(更))72.而至义齿贴合点指示剂 (而至株式会社 国食药监械(进)字2005第2633109号(更))73.胰岛素校准液 ( 国食药监械(进)字2005第3402318号(更))74.通用稀释液10 ( 国食药监械(进)字2005第3401704号(更))75.铜蓝蛋白试剂盒 (英国 THE BINDING SITE公司 国食药监械(进)字2007第3400649号)76.补体C4试剂盒 (The Binding Site Limited 国食药监械(进)字2007第3400650号)77.气动动力系统 (Aesculap AG & CO.KG 国食药监械(进)字2005第2541844号(更))78.避孕套 (马来西亚康乐工业有限公司 国食药监械(进)字2006第3661816号(更))79.导引导管 (Boston Scientific Corporation 国食药监械(进)字2005第3773129号(更))80.Cardio MD(单光子发射计算机断层)伽玛相机系统 (ADAC Laboratories A Philips Medical Syst81.全自动组织脱水机 (德国徕卡仪器公司Leica Microsystems Nussloch GmbH 国食药监械(进)字200382.耐甲氧西林金黄色葡萄球菌鉴定培养基 (bioMerieux,sa 国食药监械(进)字2006第3400205号(更))83.洗脱缓冲液 (日本 TOSOH CORPORATION 国食药监械(进)字2007第3400983号)84.C反应蛋白校准品 ( 国食药监械(进)字2007第3400985号)85.胆红素定标品 ( 国食药监械(进)字2007第3400986号)86.脂类定标品 (SYSMEX CORPORATION 国食药监械(进)字2007第3400987号)87.质控品 ( 国食药监械(进)字2007第3400988号)88.糖化血清蛋白测定试剂盒(生化酶法) (英国 Genzyme Diagnostic 国食药监械(进)字2007第34089.高频电烧装置 (日本 奥林巴斯医疗株式会社 OLYMPUS MEDICAL SYSTEMS CORP. 国食药监械(进)字290.正电子发射及计算机断层扫描系统 (美国 Siemens Medical Solutions USA,Inc 国食药监械(进)字91.荧光探针脱氧核糖核酸检测系统 (美国 Becton,Dickinsonand Company公司 国食药监械(进)字200792.促肾上腺皮质激素定标液 (Roche Diagnostics GmbH 国食药监械(进)字2007第3400501号)93.水杨酸盐试剂盒 (Dade Behring, Inc. 国食药监械(进)字2007第3400646号)94.叶酸诊断试剂盒 (德国 罗氏诊断有限公司Roche Diagnostics GmbH 国食药监械(进)字2007第3400695.免疫球蛋白M试剂盒 (The Binding Site Limited 国食药监械(进)字2007第3400648号)96.一次性胰岛素注射器带针头 ( 国食药监械(进)字2004第3152002号(更))97.带恒速调节器的输液管路 (Baxter S.A. 国食药监械(进)字2004第3661717号(更))98.百耐凝胶(Bionect gel) (意大利 Fidia制药厂 国食药监械(进)字2005第2640099号(更))99.自粘性硅胶片(商品名:仙卡) (Smith & Nephew Medical Ltd. 国食药监械(进)字2007第2660635号100.一次性使用真空采血管 (Greiner Bio-One Gmbh 国食药监械(进)字2007第2660642号)101.喉罩导气管 (塞舌尔 The Laryngeal Mask Company Limited 国食药监械(进)字2007第2660719号) 102.甲状腺过氧化酶自身抗体试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字20 103.通用稀释液7 ( 国食药监械(进)字2005第3401702号(更))104.X射线摄影暗匣(商品名:柯达 X-OMAT 暗盒) (美国 EASTMAN KODAK COMPANYEASTMAN KODAK COMPA 105.电解质参比液 (德国 罗氏诊断有限公司 国食药监械(进)字2007第1400716号)106.诱导剂 (芬兰 PerkinElmer Life and Analytical Sciences,Wallac Oy 国食药监械(进)字2007第107.甲状腺球蛋白试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004第340054108.甲状腺过氧化物酶抗体试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004 109.四碘甲状腺原氨酸试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004第34 110.植入式心脏起搏器 (美国 Guidant Corporation Cardiac Pacemakers Inc. 国食药监械(进)字200 111.超声诊断系统和探头 (Philips Ultrasound, INC. 国食药监械(进)字2004第3230502号(更))112.血糖监测仪 (Infopia CO.,Ltd. 国食药监械(进)字2005第2402628号(更))113.血糖试条 (Infopia Co.,Ltd 国食药监械(进)字2005第2402611号(更))114.低温等离子体灭菌器 ( 国食药监械(进)字2005第2571415号(更))115.地高辛试剂盒 ( 国食药监械(进)字2005第3401304号(更))116.万古霉素试剂盒 ( 国食药监械(进)字2005第3401383号(更))117.卡马西平试剂盒 ( 国食药监械(进)字2005第3401379号(更))118.高频电外手科术和电凝设备(商品名:VIO系列高频电外科系统) (德国 爱尔博电子医疗仪器公司 国119.肺炎链球菌抗生素敏感实验用抗生素 (美国 biomerieux,Inc. 国食药监械(进)字2007第3400685号120.维生素B12试剂盒 ( 国食药监械(进)字2005第3401380号(更)121.白蛋白试剂盒Albumin (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004第34001 122.甘油三酯试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004第3400140号( 123.齿科烤瓷合金(商品名:Ceradelta 2) (Metalor Technologies SA 国食药监械(进)字2007第26306124.排龈膏 (法国 Produits Dentaires Pierre Rolland SAS,Acteon Pharma Division 国食药监械(进125.烤瓷瓷粉(商品名:烤瓷瓷粉) (德国 Hager & Werken GmbH & Co.,KG 国食药监械(进)字2007第2 126.医疗压力带(商品名:医疗压力带) (LABORATORI PIAZZA S.r.l. 国食药监械(进)字2007第2640509 127.藻酸钙止血贴 (美国 TZ Medical Inc.TZ Medical Inc. 国食药监械(进)字2007第2640512号) 128.抗核抗体谱(IgG)检测试剂盒(欧蒙印迹法) (德国 EUROIMMUN Medizinische Labordiagnostika AG 129.医用诊断X射线管组件 (美国 Varian Medical Systems 国食药监械(进)字2007第2310622号)130.血糖仪 (韩国 i-SENS.Inc 国食药监械(进)字2007第2400442号)131.尿液分析仪 (盈东电子株式会社 国食药监械(进)字2007第2400447号)132.碳13红外光谱仪 (日本 大塚电子株式会社 国食药监械(进)字2007第2400451号)133.血糖检测系统(商品名:罗康全优越型) (德国 罗氏诊断有限公司 国食药监械(进)字2007第240045 134.半自动血凝分析仪 (德国 MERLIN medical GmbH 国食药监械(进)字2007第2400476号)135.疝环充填补片(商品名:巴德) (美国 Davol Inc.,Subsidiary of C.R. Bard,Inc. 国食药监械(进 136.疝修补平片和预裁补片(商品名:巴德) (美国 Davol Inc.,Subsidiary of C.R. Bard,Inc. 国食137.切口疝补片(商品名:巴德) (美国 Davol Inc.,Subsidiary of C.R. Bard,Inc. 国食药监械(进)字138.348 Hct Slope 试剂盒 ( 国食药监械(进)字2004第3401406号(更))139.6.838缓冲液 ( 国食药监械(进)字2004第3401410号(更))140.病人固定系统(商品名:UON-DUON) (比利时 ORFIT INDUSTRIES N.V 国食药监械(进)字2007第1100 141.病人固定系统(商品名:EFFICAST) (比利时 ORFIT Industries N.V 国食药监械(进)字2007第1100 142.病人固定系统(商品名:AIO Solution) (比利时 ORFIT INDUSTRIES N.V 国食药监械(进)字2007第143.矫形外科(骨科)手术器械 (奥地利 I.T.S Implantat-Technologie Systeme GmbH 国食药监械(进144.椎间融合器安装工具(商品名:椎间融合器安装工具) (俄罗斯 《KIMPF》股份有限公司 Closedjoi 145.组件式短柄假体工具(商品名:Metha) (AESCULAP AG & CO.KG 国食药监械(进)字2007第1100568号146.矫形外科手术器械 (韩国GS医疗公司GS Medical 国食药监械(进)字2007第1100706号)147.医用干式胶片(商品名:富士) (日本 Fuji Photo Film Co.,Ltd 国食药监械(进)字2007第1240484 148.接骨板和接骨螺钉(商品名:LINK 接骨板和接骨螺钉系统) ( 国食药监械(进)字2007第3461012号149.机械心脏瓣膜(商品名:Regent) (美国 圣犹达医疗用品有限公司 国食药监械(进)字2007第3461 150.血气分析仪 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004第2401337号(更)) 151.血气分析仪 (英国 Siemens Medical Solutions Diagnostics Manufacturing Limited 国食药监152.危重症检测分析仪系列 ( 国食药监械(进)字2006第2210279号(更))153.348 Buffer Pack 试剂盒 ( 国食药监械(进)字2004第3401443号(更))154.348/248冲洗液试剂盒 ( 国食药监械(进)字2004第3401531号(更))155.玻璃离子水门汀(商品名:FUJI PLUS) (日本 株式会社而至 国食药监械(进)字2006第3631801号(156.乙烯基聚硅氧烷印模材料(膏剂型)(商品名:而至精确硅橡胶 膏剂型) (株式会社而至 国食药监械157.球囊预装支架传送系统(商品名:TriMaxx 冠状动脉预装支架传送系统) (美国 Abbott Vascular D 158.导丝(商品名:HiWire) (美国 库克泌尿外科公司;Cook Urological Incorporated 国食药监械(进159.导引导管(商品名:Launcher) (Medtronic, Inc. 国食药监械(进)字2007第3770627号)160.护套介入系统(商品名:Brite Tip) (Cordis Corporation 国食药监械(进)字2007第3770629号) 161.革兰氏阴性细菌药敏实验用抗生素 (美国 biomerieux,Inc. 国食药监械(进)字2007第3400686号) 162.皮质醇试剂盒 (美国 Bayer HealthCare LLC 国食药监械(进)字2007第3400687号)163.系列冲洗液/废液试剂盒 (美国 Siemens Medical Solutions Diagnostics 国食药监械(进)字2007 164.测量试剂盒 (美国 Siemens Medical Solutions Diagnostics 国食药监械(进)字2007第3400689号165.便潜血测定试剂盒 (日本株式会社常光 国食药监械(进)字2007第3400690号)166.肌钙蛋白-I测定试剂盒 (日本株式会社常光 国食药监械(进)字2007第3400691号)167.肌红蛋白测定试剂盒 (日本株式会社常光 国食药监械(进)字2007第3400692号)168.穆法 MV 呼吸机 (G.LOHMEIER GmbH 国食药监械(进)字2005第3541326号(更))169.全自动蛋白印迹仪 (Genelabs Diagnostics Pte. Ltd. 国食药监械(进)字2005第3402673号(更)) 170.泌尿系统致病菌鉴定培养基 (bioMerieux,sa 国食药监械(进)字2005第3403472号(更))171.庆大霉素试剂盒 ( 国食药监械(进)字2005第3401378号(更))172.髋部螺钉系统(商品名:亚洲型) (美国 Smith & Nephew, Inc. Orthopaedic Division 国食药监械173.肌酸激酶MB同工酶校准液 ( 国食药监械(进)字2005第3401797号(更))174.移动式X射线诊断设备 (Siemens AG 国食药监械(进)字2007第2300704号)175.影像板扫描处理系统(商品名:柯达 Point-of-Care CR 系统) (美国 Eastman Kodak Company 国食176.X射线口内影像系统 (韩国 VATECH Co.Ltd. 国食药监械(进)字2007第2310517号)177.血液透析用管道Lines for hemodialysis (Gambro DASCO S.p.A 国食药监械(进)字2007第34507 178.管路及滤器H.E.L.P. Consumables (德国 B.Braun Medizintechnologie GmbH 国食药监械(进)字179.血浆分离器(商品名:Haemoselect) (德国 B. Braun Medizintechnologie GmbH 国食药监械(进)180.补体C3诊断试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2005第3401775号181.可吸收骨替代材料(商品名:固骼生) (美国诺邦生物制品有限公司 国食药监械(进)字2004第346 182.尿分析阳性和阴性质控试纸 ( 国食药监械(进)字2006第3401062号(更))183.尿液分析仪 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004第2401280号(更)) 184.尿液分析仪 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004第2402147号(更)) 185.校准液C ( 国食药监械(进)字2005第3401788号(更))186.校准液B ( 国食药监械(进)字2005第3401800号(更))187.校准液A ( 国食药监械(进)字2005第3401787号(更))188.C反应蛋白(Ⅱ)乳胶试剂盒X2 (日本 DENKA SEIKEN CO., LTD. 国食药监械(进)字2006第3400929 189.D-二聚体排除试验试剂盒 (bioMerieux,sa 国食药监械(进)字2005第3401130号(更))190.多功能医疗护理床系列 (捷克共和国Linet spol.sr.o. 国食药监械(进)字2004第2542365号(更)) 191.Multifiltrate Cassette 管路系统 (德国 Fresenius Medical Care AG & Co.KGaA 国食药监械(进192.透析液过滤器 (Fresenius Medical Care AG & Co.KGaA 国食药监械(进)字2005第3453077号(更)) 193.穿刺针 (Fresenius Medical Care AG & Co.KGaA 国食药监械(进)字2004第3150589号(更))194.游离三碘甲状腺原氨酸试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004 195.游离甲状腺素试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004第340060 196.超声诊断系统 (意大利 ESAOTE SpAESAOTE SpA 国食药监械(进)字2007第3230621号)197.MicroPlex 弹簧圈系统 (MicroVention,Inc 国食药监械(进)字2005第3770791号(更))198.运动负荷试验诊断系统 (Philips Medical Systems 国食药监械(进)字2005第2212430号(更)) 199.动态心电图系统 (Philips Medical Systems 国药管械(进)字2003第2210893号(更))200.心电图机 ( 国食药监械(进)字2004第2210828号(更))201.监护除颤器 (Philips Medical System 国食药监械(进)字2004第3211207号(更))202.正电子发射断层成像系统 (Philips Medical Systems (Cleveland),Inc. 国食药监械(进)字2003第203.免疫球蛋白G诊断试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2005第340204.Rapidpoint Coag 快速凝血仪PT-NC测试卡 (Siemens Medical Solutions Diagnostics 国食药监械205.髋关节假体(商品名:SDC and PLC) (德国 AESCULAP AG & Co.KGGermany AESCULAP AG & Co.KG 国206.膝关节系统(商品名:AGC DA) (英国 Biomet UK LTD.Biomet UK LTD. 国食药监械(进)字2007第34 207.疝修补网织片(商品名:巴德 Modified Kugel) ( 国食药监械(进)字2007第3460593号)208.角膜接触镜(商品名:强生彩镜) (美国 VISTAKON Johnson&Johnson Vision Care Inc. 国食药监械209.角膜接触镜(商品名:西武) (G&G CONTACT LENS CO. 国食药监械(进)字2007第3220572号)210.超声诊断仪 (GE Medical Systems Kretztechnik GmbH&Co.,OHG 国食药监械(进)字2007第3230494 211.超声诊断系统(商品名:SONOLINE G60 S) (美国 SIEMENS MEDICAL SOLUTIONS USA, INC. 国食药监212.Rapidpoint Coag 快速凝血aPTT测试卡 (Siemens Medical Solutions Diagnostics 国食药监械(进213.银粉玻璃离子水门汀(商品名:而至 Miracle Mix) (日本株式会社而至 国食药监械(进)字2007第3 214.氟化泡沫(商品名:氟化泡沫) (美国 Laclede, A Laclede, Inc. 国食药监械(进)字2007第215.去白细胞滤器 (Fresenius Kabi AG 国食药监械(进)字2006第3660078号(更))216.硬膜下电极 (AD-Tech Medical Instrument Corporation 国食药监械(进)字2005第3210648号(更) 217.一次性使用去白细胞滤器 ( 国食药监械(进)字2007第3451019号)218.股骨头及内称(商品名:股骨头及内称) (法国 GROUPE LEPINE 国食药监械(进)字2007第3460914 219.补体C4诊断试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2005第3401774号220.生化校准液 ( 国食药监械(进)字2006第3400191号(更))221.口腔科手术器械 (德国 Alfred Becht GmbHGermany Alfred Becht GmbH 国食药监械(进)字2007第222.泌尿肛肠外科手术器械 (德国 Aesculap AG & Co.KG 国食药监械(进)字2007第1090477号)223.游离四碘甲状腺原氨酸试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004 224.维生素B12稀释液 ( 国食药监械(进)字2005第3401705号(更))225.B型心钠素校准液 ( 国食药监械(进)字2005第3401785号(更))226.体外循环用插管--心室插管 (Maquet Cardioplumonary AG 国食药监械(进)字2004第3450692号(更227.体外循环用插管--静脉插管 (Maquet Cardioplumonary AG 国食药监械(进)字2004第3450694号(更228.脊椎动力平衡治疗系统 (Optima Health Solutions International Corporation 国食药监械(进) 229.手术室包 (Buckley Lamb Limited 国食药监械(进)字2005第2641933号(更))230.超声成像诊断仪 (Esaote Europe B.V. 国食药监械(进)字2006第3210425号(更))231.免疫球蛋白M诊断试剂盒 ( 国食药监械(进)字2005第3401709号(更))232.妥布霉素试剂盒 ( 国食药监械(进)字2005第3401695号(更))233.苯巴比妥试剂盒 ( 国食药监械(进)字2005第3401693号(更))234.氨测定试剂盒 (日本 关东化学株式会社 国食药监械(进)字2007第3400674号)235.肌红蛋白测定试剂盒 (日本 关东化学株式会社 国食药监械(进)字2007第3400675号)236.胃蛋白酶原Ⅰ测定试剂盒 (关东化学株式会社 国食药监械(进)字2007第3400676号)237.全瓷(商品名:维他全瓷系列) (德国 维他公司 VITA Zahnfabrik H.Rauter Gmbh & Co.KG 国食药238.齿科用铸造合金 (商品名:Solaro 3 ) (瑞士 Metalor Technologies SAMetalor Technologies S 239.齿科烤瓷合金(商品名:V-Gnathos plus ) ( 国食药监械(进)字2007第2630623号)240.齿科烤瓷合金(商品名:V-Deltaloy) (Metalor Technologies SA 国食药监械(进)字2007第263062 241.可吸收外科缝线(商品名:万福(Monosyn)) (德国 AESCULAP AG & CO.KG 国食药监械(进)字2007 242.静脉插管 (美国 Edwards Lifesciences LLC 国食药监械(进)字2007第3660520号)243.肾造瘘扩张器及套装(商品名:Amplatz) (Cook Urological Incorporated 国食药监械(进)字2007 244.连通板英文名称: Merit Manifolds(商品名:麦瑞连通板) (美国 麦瑞医疗设备有限公司麦瑞医疗245.铁蛋白试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004第3400273号(更246.高密度脂蛋白测定试剂盒 (日本 关东化学株式会社 国食药监械(进)字2007第3400673号)247.图像处理装置 (日本 オリンパスメディカルシステムズ株式会社 国食药监械(进)字2007第222044 248.内窥镜摄像系统 (Karl Storz GmbH & Co.KG 国食药监械(进)字2007第2220473号)249.接触式压电眼压计(商品名:动态轮廓眼压计) (SMT Swiss Microtechnology AG 国食药监械(进)字250.内窥镜下无源手术器械(商品名:蛇牌) (Aesculap AG&Co.KG 国食药监械(进)字2007第2220528号) 251.内窥镜摄像系统 (德国 Karl Storz GmbH & Co.KG 国食药监械(进)字2007第2220554号)252.医学影像存储和传输系统(商品名:医学影像存储和传输系统) (德国 Siemens AGSiemens AG 国食253.内窥镜冷光源 (德国 Richard Wolf GmbH 国食药监械(进)字2007第2220633号)254.牛心包生物瓣(商品名:SJM Biocor) (圣犹达医疗用品公司 国食药监械(进)字2007第3460701号) 255.呼吸机(商品名:纽邦呼吸机) (美国 Newport Medical Instruments,Inc.U.S.A.Newport Medical 256.ADVIA 70 血液分析仪 (Siemens Medical Solutions Diagnostics 国药管械(进)2003第2400740号257.ADVIA 血液分析仪 (Siemens Medical Solutions Diagnostics 国药管械(进)2003第2400657号(更258.Kodak Min-R EV乳房X线影像胶片 (Carestream Health,Inc 国食药监械(进)字2004第1310201号(更259.Kodak DVM胶片 ( 国食药监械(进)字2005第1311759号(更))260.超声诊断设备(商品名:超声诊断设备Aplio) (日本 TOSHIBA MEDICAL SYSTEMS CORPORATION 国食261.层析柱 (TOSOH CORPORATION 国食药监械(进)字2007第3460551号)262.自动体外除颤仪 (Medtronic Emergency Response Systems,Inc. 国药管械(进)2002第3211124号( 263.电动动力系统 ( 国食药监械(进)字2005第2211873号(更))264.显微外科手术器械 (德国 Aesculap AG & Co.KGAesculap AG & Co.KG 国食药监械(进)字2007第10 265.根管扩大器 (日本 Mani, Inc. 国食药监械(进)字2007第2550455号)266.喷粉洁牙手机(商品名:PROPHY-MATE) (日本株式会社 中西/Nakanishi Inc. 国食药监械(进)字20 267.脉动预真空压力蒸汽灭菌器 (Tuttnauer Co.Ltd. 国食药监械(进)字2007第2570703号)268.牙科复合树脂充填材料(商品名:SwissTec Composite) (Coltene/Whaledent AG 国食药监械(进)字269.ADVIA 70 鞘液 ( 国食药监械(进)字2003第3400427号(更))270.ADVIA 70 稀释液 ( 国食药监械(进)字2003第3400428号(更))271.ADVIA 70 溶血剂 ( 国食药监械(进)字2003第3400429号(更))272.ADVIA 60 TIMEPAC ( 国食药监械(进)字2005第2400926号(更))273.ADVIA 60 血液分析仪 ( 国食药监械(进)字2005第2401875号(更))274.万古霉素校准液 ( 国食药监械(进)字2005第3401795号(更))275.庆大霉素校准液 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2005第3401794号( 276.远程心电事件记录器 (德国 TMS Telemedizinische Systeme GmbH 国食药监械(进)字2007第22105 277.生物显微镜 (德国 Carl Zeiss AG,Werk Gottingen 国食药监械(进)字2007第2220441号)278.深部电极 (Ad-Tech Medical Instrument Corporation 国食药监械(进)字2005第3210459号(更)) 279.硬膜外麻醉导管 (美国 ARROW INTERNATIONAL INC 国食药监械(进)字2007第3660588号)280.膜型血浆分离器 (日本 旭化成医疗株式会社 国食药监械(进)字2007第3660590号)281.血栓抽吸导管(商品名:Rebirth ) (日本 株式会社Goodman 国食药监械(进)字2007第3660607号) 282.一次性使用输液器用输液帽、防回流阀 (德国 Fresenius Kabi AGFresenius Kabi AG 国食药监械283.Rapidpoint Coag 快速凝血仪 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2003284.胃蛋白酶原Ⅱ测定试剂盒 (日本 关东化学株式会社 国食药监械(进)字2007第3400677号)285.血脂正常值质控 (德国罗氏诊断有限公司 国食药监械(进)字2007第3400678号)286.血脂病理值质控 (德国罗氏诊断有限公司 国食药监械(进)字2007第3400679号)287.免疫球蛋白G2试剂 (德国 Dade Behring Marburg GmbH 国食药监械(进)字2007第3400680号)288.免疫球蛋白G1试剂 (德国 Dade Behring Marburg GmbH 国食药监械(进)字2007第3400681号)289.铁蛋白检测试剂盒 (西班牙博士泰公司 国食药监械(进)字2007第3400682号)290.脑脊液/尿液总蛋白检测试剂盒 (西班牙博士泰公司 国食药监械(进)字2007第3400683号)291.HydroCoil栓塞系统 (MicroVention,Inc 国食药监械(进)字2005第3770789号(更))292.泵用输液器 英 文 名 称:Original Infusomat Tubing(商品名:Original Infusomat) (B. Brau 293.麻醉系统(商品名:Zeus) ( 国食药监械(进)字2007第3540615号)294.呼吸加湿过滤系统 (马来西亚 Rusch Sdn Bhd. 国食药监械(进)字2007第3540736号)295.光固化玻璃离子水门汀(商品名:而至富士 II LC ) (日本 株式会社而至 国食药监械(进)字2007第296.C-肽试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004第3400619号(更)) 297.胰岛素试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004第3400287号(更298.胰岛素试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2004第3400637号(更299.校准液O ( 国食药监械(进)字2005第3401792号(更))300.茶碱试剂盒 ( 国食药监械(进)字2005第3401694号(更))301.动力系统(商品名:Colibri ) (瑞士 Synthes GmbH 国食药监械(进)字2007第2540714号)302.免疫球蛋白A诊断试剂盒 ( 国食药监械(进)字2005第3401710号(更))303.KODAK DirectView CR950 系统 (Carestream Health,Inc 国食药监械(进)字2003第2310504号(更) 304.通用稀释液3 ( 国食药监械(进)字2005第3401699号(更))305.校准液Z ( 国食药监械(进)字2005第3401786号(更))306.校准液E ( 国食药监械(进)字2005第3401799号(更))307.肌酸激酶MB同工酶液体试剂盒 (DiaSys Diagnostic Systems GmbH 国食药监械(进)字2005第24031 308.自体血连续回输机 (Fresenius Kabi AG 国食药监械(进)字2005第3452722号(更))309.S5L/C5L 血小板套件 (Fresenius Kabi AG 国食药监械(进)字2006第3450453号(更))310.PL1血浆置换组件 (Fresenius Kabi AG 国食药监械(进)字2006第3450452号(更))311.血细胞分离机 (Fresenius Kabi AG 国食药监械(进)字2005第3453128号(更))312.CATS自体血回输机耗材 (Fresenius Kabi AG 国食药监械(进)字2006第3450451号(更))313.去白细胞滤器 (Fresenius Kabi AG 国食药监械(进)字2005第3453185号(更))314.通用稀释液4 ( 国食药监械(进)字2005第3401700号(更))315.C反应蛋白诊断试剂盒 (Siemens Medical Solutions Diagnostics 国食药监械(进)字2005第34017316.腹腔镜用缝合材料及辅助器械 (Ethicon Endo-Surgery, Inc. 国食药监械(进)字20317.一次性使用冲洗装置(商品名:CritiFlo) (新加坡 Becton Dickinson Critical Care Systems P 318.一次性使用电刀笔 (TELEFLEX MEDICAL 国食药监械(进)字2005第2252933号(更))319.导电性粘合电极板 (TELEFLEX MEDICAL 国食药监械(进)字2005第2252946号(更))320.R-Stent Evolution 2 冠状动脉支架系统 (美国 Orbus Neich Medical,Inc. 国食药监械(进)字20 321.程控仪(商品名:ZOOMLATITUDE) (美国 Guidant Corporation Cardiac Pacemakers Inc. 国食药322.植入式心脏除颤电极导管 ( 国食药监械(进)字2006第3211955号(更))323.二氧化碳(CO2)液体试剂盒 ( 国食药监械(进)字2004第3400627号(更))324.铁蛋白测定试剂 ( 国食药监械(进)字2004第3400628号(更))325.超声治疗仪(商品名:骨科超声治疗仪) (赛特力公司 国食药监械(进)字2007第2230109号)326.中频电疗仪(商品名:Superkine SK-SERIES) ( 国食药监械(进)字2007第2260157号)327.影像板扫描处理系统(商品名:柯达 CR 7400 牙科计算机放射成像系统) (美国 Eastman Kodak C 328.凝血酶原时间测定试剂盒 ( 国食药监械(进)字2007第2400118号)329.凝血酶原时间测定试剂盒 ( 国食药监械(进)字2007第2400119号)330.谷氨酰转肽酶测定试剂盒 (和光纯药工业株式会社 国食药监械(进)字2007第2400121号)331.PTA 导管(Amphirion DEEP) (意大利 Invatec S.r.l. 国食药监械(进)字2006第2771389号)。
Bioanalytical Method ValidationGuidance for Indust

Guidance for Industry Bioanalytical Method ValidationU.S. Department of Health and Human ServicesFood and Drug AdministrationCenter for Drug Evaluation and Research (CDER)Center for Veterinary Medicine (CVM)May 2001BPGuidance for Industry Bioanalytical Method ValidationAdditional copies are available from:Drug Information Branch (HFD-210)Center for Drug Evaluation and Research (CDER)5600 Fishers Lane, Rockville, MD 20857 (Tel) 301-827-4573Internet at /cder/guidance/index.htmorCommunications Staff (HFV-12)Center for Veterinary Medicine (CVM)7500 Standish Place, Rockville, MD 20855 (Tel) 301–594-1755Internet at /cvmU.S. Department of Health and Human ServicesFood and Drug AdministrationCenter for Drug Evaluation and Research (CDER)Center for Veterinary Medicine (CVM)May 2001BPTable of ContentsI.INTRODUCTION (1)II.BACKGROUND (1)A.F ULL V ALIDATION (2)B.P ARTIAL V ALIDATION (2)C.C ROSS-V ALIDATION (3)III.REFERENCE STANDARD (4)IV.METHOD DEVELOPMENT: CHEMICAL ASSAY (4)A.S ELECTIVITY (4)B.A CCURACY, P RECISION, AND R ECOVERY (5)C.C ALIBRATION/S TANDARD C URVE (5)D.S TABILITY (6)E.P RINCIPLES OF B IOANALYTICAL M ETHOD V ALIDATION AND E STABLISHMENT (8)F.S PECIFIC R ECOMMENDATIONS FOR M ETHOD V ALIDATION (10)V.METHOD DEVELOPMENT: MICROBIOLOGICAL AND LIGAND-BINDING ASSAYS (11)A.S ELECTIVITY I SSUES (11)B.Q UANTIFICATION I SSUES (12)VI.APPLICATION OF VALIDATED METHOD TO ROUTINE DRUG ANALYSIS (13)A CCEPTANCE C RITERIA FOR THE R UN (15)VII.DOCUMENTATION (16)A.S UMMARY I NFORMATION (16)B.D OCUMENTATION FOR M ETHOD E STABLISHMENT (17)C.A PPLICATION TO R OUTINE D RUG A NALYSIS (17)D.O THER I NFORMATION (19)GLOSSARY (20)GUIDANCE FOR INDUSTRY1Bioanalytical Method ValidationI.INTRODUCTIONThis guidance provides assistance to sponsors of investigational new drug applications (INDs), new drug applications (NDAs), abbreviated new drug applications (ANDAs), and supplements in developing bioanalytical method validation information used in human clinical pharmacology, bioavailability (BA), and bioequivalence (BE) studies requiring pharmacokinetic (PK) evaluation. This guidance also applies to bioanalytical methods used for non-human pharmacology/toxicology studies and preclinical studies. For studies related to the veterinary drug approval process, this guidance applies only to blood and urine BA, BE, and PK studies.The information in this guidance generally applies to bioanalytical procedures such as gas chromatography (GC), high-pressure liquid chromatography (LC), combined GC and LC mass spectrometric (MS) procedures such as LC-MS, LC-MS-MS, GC-MS, and GC-MS-MS performed for the quantitative determination of drugs and/or metabolites in biological matricessuch as blood, serum, plasma, or urine. This guidance also applies to other bioanalytical methods, such as immunological and microbiological procedures, and to other biological matrices, such as tissue and skin samples.This guidance provides general recommendations for bioanalytical method validation. The recommendations can be adjusted or modified depending on the specific type of analytical method used. II.BACKGROUND1 This guidance has been prepared by the Biopharmaceutics Coordinating Committee in the Center for Drug Evaluation and Research (CDER) in cooperation with the Center for Veterinary Medicine (CVM) at the Food and Drug Administration.This guidance has been developed based on the deliberations of two workshops: (1) Analytical Methods Validation: Bioavailability, Bioequivalence, and Pharmacokinetic Studies (held on December 3B5, 19902 ) and (2) Bioanalytical Methods Validation C A Revisit With a Decade of Progress (held on January 12B14, 20003).Selective and sensitive analytical methods for the quantitative evaluation of drugs and their metabolites (analytes) are critical for the successful conduct of preclinical and/or biopharmaceutics and clinical pharmacology studies. Bioanalytical method validation includes all of the procedures that demonstrate that a particular method used for quantitative measurement of analytes in a given biological matrix, such as blood, plasma, serum, or urine, is reliable and reproducible for the intended use. The fundamental parameters for this validation include (1) accuracy, (2) precision, (3) selectivity, (4) sensitivity, (5) reproducibility, and (6) stability. Validation involves documenting, through the use of specific laboratory investigations, that the performance characteristics of the method are suitable and reliable for the intended analytical applications. The acceptability of analytical data corresponds directly to the criteria used to validate the method.Published methods of analysis are often modified to suit the requirements of the laboratory performing the assay. These modifications should be validated to ensure suitable performance of the analytical method. When changes are made to a previously validated method, the analyst should exercise judgment as to how much additional validation is needed. During the course of a typical drug development program, a defined bioanalytical method undergoes many modifications. The evolutionary changes to support specific studies and different levels of validation demonstrate the validity of an assay’s performance. Different types and levels of validation are defined and characterized as follows:A.Full Validation•Full validation is important when developing and implementing a bioanalytical method for the first time.•Full validation is important for a new drug entity.• A full validation of the revised assay is important if metabolites are added to an existing assay for quantification.B.Partial ValidationPartial validations are modifications of already validated bioanalytical methods. Partial validation can range from as little as one intra-assay accuracy and precision determination to a nearly full2 Workshop Report: Shah, V.P. et al., Pharmaceutical Research: 1992; 9:588-592.3 Workshop Report: Shah, V.P. et al., Pharmaceutical Research: 2000; 17:in press.validation. Typical bioanalytical method changes that fall into this category include, but are not limited to:•Bioanalytical method transfers between laboratories or analysts•Change in analytical methodology (e.g., change in detection systems)•Change in anticoagulant in harvesting biological fluid•Change in matrix within species (e.g., human plasma to human urine)•Change in sample processing procedures•Change in species within matrix (e.g., rat plasma to mouse plasma)•Change in relevant concentration range•Changes in instruments and/or software platforms•Limited sample volume (e.g., pediatric study)•Rare matrices•Selectivity demonstration of an analyte in the presence of concomitant medications•Selectivity demonstration of an analyte in the presence of specific metabolitesC.Cross-ValidationCross-validation is a comparison of validation parameters when two or more bioanalytical methods are used to generate data within the same study or across different studies. An example of cross-validation would be a situation where an original validated bioanalytical method serves as thereference and the revised bioanalytical method is the comparator. The comparisons should be done both ways.When sample analyses within a single study are conducted at more than one site or more than one laboratory, cross-validation with spiked matrix standards and subject samples should be conducted at each site or laboratory to establish interlaboratory reliability. Cross-validation should also be considered when data generated using different analytical techniques (e.g., LC-MS-MS vs.ELISA4) in different studies are included in a regulatory submission.All modifications should be assessed to determine the recommended degree of validation. The analytical laboratory conducting pharmacology/toxicology and other preclinical studies for regulatory submissions should adhere to FDA=s Good Laboratory Practices (GLPs)5 (21 CFR part 58) and to sound principles of quality assurance throughout the testing process. The bioanalytical method for human BA, BE, PK, and drug interaction studies must meet the criteria in 21 CFR 320.29. The analytical laboratory should have a written set of standard operating procedures (SOPs) to ensure a complete system of quality control and assurance. The SOPs should cover all aspects of analysis from the time the sample is collected and reaches the laboratory until the results of the analysis are reported. The SOPs also should include record keeping, security and chain of sample custody4 Enzyme linked immune sorbent assay5 For the Center for Veterinary Medicine, all bioequivalence studies are subject to Good Laboratory Practices.(accountability systems that ensure integrity of test articles), sample preparation, and analytical tools such as methods, reagents, equipment, instrumentation, and procedures for quality control and verification of results.The process by which a specific bioanalytical method is developed, validated, and used in routine sample analysis can be divided into (1) reference standard preparation, (2) bioanalytical method development and establishment of assay procedure, and (3) application of validated bioanalytical method to routine drug analysis and acceptance criteria for the analytical run and/or batch. These three processes are described in the following sections of this guidance.III.REFERENCE STANDARDAnalysis of drugs and their metabolites in a biological matrix is carried out using samples spiked with calibration (reference) standards and using quality control (QC) samples. The purity of the reference standard used to prepare spiked samples can affect study data. For this reason, an authenticated analytical reference standard of known identity and purity should be used to prepare solutions of known concentrations. If possible, the reference standard should be identical to the analyte. When this is not possible, an established chemical form (free base or acid, salt or ester) of known purity can be used. Three types of reference standards are usually used: (1) certified reference standards (e.g., USP compendial standards); (2) commercially supplied reference standards obtained from a reputable commercial source; and/or (3) other materials of documented purity custom-synthesized by an analytical laboratory or other noncommercial establishment. The source and lot number, expiration date, certificates of analyses when available, and/or internally or externally generated evidence of identity and purity should be furnished for each reference standard.IV.METHOD DEVELOPMENT: CHEMICAL ASSAYThe method development and establishment phase defines the chemical assay. The fundamental parameters for a bioanalytical method validation are accuracy, precision, selectivity, sensitivity, reproducibility, and stability. Measurements for each analyte in the biological matrix should be validated. In addition, the stability of the analyte in spiked samples should be determined. Typical method development and establishment for a bioanalytical method include determination of (1) selectivity, (2) accuracy, precision, recovery, (3) calibration curve, and (4) stability of analyte in spiked samples.A.SelectivitySelectivity is the ability of an analytical method to differentiate and quantify the analyte in thepresence of other components in the sample. For selectivity, analyses of blank samples of theappropriate biological matrix (plasma, urine, or other matrix) should be obtained from at leastsix sources. Each blank sample should be tested for interference, and selectivity should be ensured at the lower limit of quantification (LLOQ).Potential interfering substances in a biological matrix include endogenous matrix components, metabolites, decomposition products, and in the actual study, concomitant medication and other exogenous xenobiotics. If the method is intended to quantify more than one analyte, each analyte should be tested to ensure that there is no interference.B.Accuracy, Precision, and RecoveryThe accuracy of an analytical method describes the closeness of mean test results obtained by the method to the true value (concentration) of the analyte. Accuracy is determined by replicate analysis of samples containing known amounts of the analyte. Accuracy should be measured using a minimum of five determinations per concentration. A minimum of three concentrations in the range of expected concentrations is recommended. The mean value should be within 15% of the actual value except at LLOQ, where it should not deviate by more than 20%. The deviation of the mean from the true value serves as the measure of accuracy.The precision of an analytical method describes the closeness of individual measures of an analyte when the procedure is applied repeatedly to multiple aliquots of a single homogeneous volume of biological matrix. Precision should be measured using a minimum of five determinations per concentration. A minimum of three concentrations in the range of expected concentrations is recommended. The precision determined at each concentration level should not exceed 15% of the coefficient of variation (CV) except for the LLOQ, where it should not exceed 20% of the CV. Precision is further subdivided into within-run, intra-batch precision or repeatability, which assesses precision during a single analytical run, and between-run, inter-batch precision or repeatability, which measures precision with time, and may involve different analysts, equipment, reagents, and laboratories.The recovery of an analyte in an assay is the detector response obtained from an amount of the analyte added to and extracted from the biological matrix, compared to the detector response obtained for the true concentration of the pure authentic standard. Recovery pertains to the extraction efficiency of an analytical method within the limits of variability. Recovery of the analyte need not be 100%, but the extent of recovery of an analyte and of the internal standard should be consistent, precise, and reproducible. Recovery experiments should be performed by comparing the analytical results for extracted samples at three concentrations (low, medium, and high) with unextracted standards that represent 100% recovery.C.Calibration/Standard CurveA calibration (standard) curve is the relationship between instrument response and known concentrations of the analyte. A calibration curve should be generated for each analyte in thesample. A sufficient number of standards should be used to adequately define the relationship between concentration and response. A calibration curve should be prepared in the same biological matrix as the samples in the intended study by spiking the matrix with known concentrations of the analyte. The number of standards used in constructing a calibration curve will be a function of the anticipated range of analytical values and the nature of theanalyte/response relationship. Concentrations of standards should be chosen on the basis of the concentration range expected in a particular study. A calibration curve should consist of a blank sample (matrix sample processed without internal standard), a zero sample (matrix sample processed with internal standard), and six to eight non-zero samples covering the expected range, including LLOQ.1.Lower Limit of Quantification (LLOQ)The lowest standard on the calibration curve should be accepted as the limit ofquantification if the following conditions are met:C The analyte response at the LLOQ should be at least 5 times the responsecompared to blank response.C Analyte peak (response) should be identifiable, discrete, and reproducible witha precision of 20% and accuracy of 80-120%.2.Calibration Curve/Standard Curve/Concentration-ResponseThe simplest model that adequately describes the concentration-response relationshipshould be used. Selection of weighting and use of a complex regression equation should be justified. The following conditions should be met in developing a calibration curve:C#20% deviation of the LLOQ from nominal concentrationC#15% deviation of standards other than LLOQ from nominal concentrationAt least four out of six non-zero standards should meet the above criteria, including the LLOQ and the calibration standard at the highest concentration. Excluding thestandards should not change the model used.D.StabilityDrug stability in a biological fluid is a function of the storage conditions, the chemical properties of the drug, the matrix, and the container system. The stability of an analyte in a particular matrix and container system is relevant only to that matrix and container system and should not be extrapolated to other matrices and container systems. Stability procedures should evaluate the stability of the analytes during sample collection and handling, after long-term (frozen at theintended storage temperature) and short-term (bench top, room temperature) storage, and after going through freeze and thaw cycles and the analytical process. Conditions used in stability experiments should reflect situations likely to be encountered during actual sample handling and analysis. The procedure should also include an evaluation of analyte stability in stock solution.All stability determinations should use a set of samples prepared from a freshly made stock solution of the analyte in the appropriate analyte-free, interference-free biological matrix. Stock solutions of the analyte for stability evaluation should be prepared in an appropriate solvent at known concentrations.1.Freeze and Thaw StabilityAnalyte stability should be determined after three freeze and thaw cycles. At least three aliquots at each of the low and high concentrations should be stored at the intendedstorage temperature for 24 hours and thawed unassisted at room temperature. Whencompletely thawed, the samples should be refrozen for 12 to 24 hours under the sameconditions. The freeze–thaw cycle should be repeated two more times, then analyzedon the third cycle. If an analyte is unstable at the intended storage temperature, thestability sample should be frozen at -700C during the three freeze and thaw cycles.2.Short-Term Temperature StabilityThree aliquots of each of the low and high concentrations should be thawed at roomtemperature and kept at this temperature from 4 to 24 hours (based on the expectedduration that samples will be maintained at room temperature in the intended study) and analyzed.3.Long-Term StabilityThe storage time in a long-term stability evaluation should exceed the time between the date of first sample collection and the date of last sample analysis. Long-term stabilityshould be determined by storing at least three aliquots of each of the low and highconcentrations under the same conditions as the study samples. The volume of samples should be sufficient for analysis on three separate occasions. The concentrations of allthe stability samples should be compared to the mean of back-calculated values for the standards at the appropriate concentrations from the first day of long-term stabilitytesting.4.Stock Solution StabilityThe stability of stock solutions of drug and the internal standard should be evaluated at room temperature for at least 6 hours. If the stock solutions are refrigerated or frozenfor the relevant period, the stability should be documented. After completion of thedesired storage time, the stability should be tested by comparing the instrumentresponse with that of freshly prepared solutions.5.Post-Preparative StabilityThe stability of processed samples, including the resident time in the autosampler, should be determined. The stability of the drug and the internal standard should be assessedover the anticipated run time for the batch size in validation samples by determiningconcentrations on the basis of original calibration standards.Although the traditional approach of comparing analytical results for stored samples with those for freshly prepared samples has been referred to in this guidance, other statistical approaches based on confidence limits for evaluation of an analyte=s stability in abiological matrix can be used. SOPs should clearly describe the statistical method andrules used. Additional validation may include investigation of samples from dosedsubjects.E.Principles of Bioanalytical Method Validation and Establishment•The fundamental parameters to ensure the acceptability of the performance of a bioanalytical method validation are accuracy, precision, selectivity, sensitivity,reproducibility, and stability.• A specific, detailed description of the bioanalytical method should be written. This can be in the form of a protocol, study plan, report, and/or SOP.•Each step in the method should be investigated to determine the extent to which environmental, matrix, material, or procedural variables can affect the estimation of analyte in the matrix from the time of collection of the material up to and including the time ofanalysis.•It may be important to consider the variability of the matrix due to the physiological nature of the sample. In the case of LC-MS-MS-based procedures, appropriate steps should be taken to ensure the lack of matrix effects throughout the application of the method,especially if the nature of the matrix changes from the matrix used during method validation.• A bioanalytical method should be validated for the intended use or application. All experiments used to make claims or draw conclusions about the validity of the methodshould be presented in a report (method validation report).•Whenever possible, the same biological matrix as the matrix in the intended samples should be used for validation purposes. (For tissues of limited availability, such as bone marrow, physiologically appropriate proxy matrices can be substituted.)•The stability of the analyte (drug and/or metabolite) in the matrix during the collection process and the sample storage period should be assessed, preferably prior to sampleanalysis.•For compounds with potentially labile metabolites, the stability of analyte in matrix from dosed subjects (or species) should be confirmed.•The accuracy, precision, reproducibility, response function, and selectivity of the method for endogenous substances, metabolites, and known degradation products should beestablished for the biological matrix. For selectivity, there should be evidence that thesubstance being quantified is the intended analyte.•The concentration range over which the analyte will be determined should be defined in the bioanalytical method, based on evaluation of actual standard samples over the range,including their statistical variation. This defines the standard curve.• A sufficient number of standards should be used to adequately define the relationship between concentration and response. The relationship between response and concentration should be demonstrated to be continuous and reproducible. The number of standards used should be a function of the dynamic range and nature of the concentration-responserelationship. In many cases, six to eight concentrations (excluding blank values) can define the standard curve. More standard concentrations may be recommended for nonlinear than for linear relationships.•The ability to dilute samples originally above the upper limit of the standard curve should be demonstrated by accuracy and precision parameters in the validation.•In consideration of high throughput analyses, including but not limited to multiplexing, multicolumn, and parallel systems, sufficient QC samples should be used to ensure control of the assay. The number of QC samples to ensure proper control of the assay should be determined based on the run size. The placement of QC samples should be judiciously considered in the run.•For a bioanalytical method to be considered valid, specific acceptance criteria should be set in advance and achieved for accuracy and precision for the validation of QC samples over the range of the standards.F.Specific Recommendations for Method Validation•The matrix-based standard curve should consist of a minimum of six standard points, excluding blanks, using single or replicate samples. The standard curve should cover the entire range of expected concentrations.•Standard curve fitting is determined by applying the simplest model that adequately describes the concentration-response relationship using appropriate weighting and statistical tests for goodness of fit.•LLOQ is the lowest concentration of the standard curve that can be measured with acceptable accuracy and precision. The LLOQ should be established using at least five samples independent of standards and determining the coefficient of variation and/orappropriate confidence interval. The LLOQ should serve as the lowest concentration on the standard curve and should not be confused with the limit of detection and/or the low QC sample. The highest standard will define the upper limit of quantification (ULOQ) of an analytical method.•For validation of the bioanalytical method, accuracy and precision should be determined using a minimum of five determinations per concentration level (excluding blank samples).The mean value should be within ±15% of the theoretical value, except at LLOQ, where it should not deviate by more than ±20%. The precision around the mean value should not exceed 15% of the CV, except for LLOQ, where it should not exceed 20% of the CV.Other methods of assessing accuracy and precision that meet these limits may be equally acceptable.•The accuracy and precision with which known concentrations of analyte in biological matrix can be determined should be demonstrated. This can be accomplished by analysis ofreplicate sets of analyte samples of known concentrations C QC samples C from anequivalent biological matrix. At a minimum, three concentrations representing the entire range of the standard curve should be studied: one within 3x the lower limit of quantification (LLOQ) (low QC sample), one near the center (middle QC), and one near the upperboundary of the standard curve (high QC).•Reported method validation data and the determination of accuracy and precision should include all outliers; however, calculations of accuracy and precision excluding values that are statistically determined as outliers can also be reported.•The stability of the analyte in biological matrix at intended storage temperatures should be established. The influence of freeze-thaw cycles (a minimum of three cycles at twoconcentrations in triplicate) should be studied.•The stability of the analyte in matrix at ambient temperature should be evaluated over a time period equal to the typical sample preparation, sample handling, and analytical run times.•Reinjection reproducibility should be evaluated to determine if an analytical run could be reanalyzed in the case of instrument failure.•The specificity of the assay methodology should be established using a minimum of six independent sources of the same matrix. For hyphenated mass spectrometry-basedmethods, however, testing six independent matrices for interference may not be important.In the case of LC-MS and LC-MS-MS-based procedures, matrix effects should beinvestigated to ensure that precision, selectivity, and sensitivity will not be compromised.Method selectivity should be evaluated during method development and throughout methodvalidation and can continue throughout application of the method to actual study samples.•Acceptance/rejection criteria for spiked, matrix-based calibration standards and validation QC samples should be based on the nominal (theoretical) concentration of analytes.Specific criteria can be set up in advance and achieved for accuracy and precision over therange of the standards, if so desired.V.METHOD DEVELOPMENT: MICROBIOLOGICAL AND LIGAND-BINDING ASSAYSMany of the bioanalytical validation parameters and principles discussed above are also applicable to microbiological and ligand-binding assays. However, these assays possess some unique characteristics that should be considered during method validation.A.Selectivity IssuesAs with chromatographic methods, microbiological and ligand-binding assays should be shown to be selective for the analyte. The following recommendations for dealing with two selectivity issues should be considered:1.Interference From Substances Physiochemically Similar to the Analyte•Cross-reactivity of metabolites, concomitant medications, or endogenouscompounds should be evaluated individually and in combination with the analyteof interest.•When possible, the immunoassay should be compared with a validated reference method (such as LC-MS) using incurred samples and predetermined criteria foragreement of accuracy of immunoassay and reference method.。
试剂 耗材清单
反渗透膜清洗药片(密理博)
个
反渗透膜预处理组件(密理博)
个
反渗透膜清洗口(RIOS 100系统)(密理博)
19
化学试剂
日本Abbott Japan Co., Ltd.
100人份/包
人类免疫缺陷病毒(HIV1/2)抗体诊断试剂盒(胶体硒法)(Abbott)
20
化学试剂
日本Fujirebio Inc.
胃蛋白酶原I检测试剂盒(BIOHIT OYJ)
96人份/盒
胃蛋白酶原II检测试剂盒(BIOHIT OYJ)
9
化学试剂
韩国Standard Diagnostics Incorporation
30人份/包
人类免疫缺陷病毒抗体检测试剂盒(胶体金法)BIOLINE HIV-1/2 3.0(SD)
10
化学试剂
480人份/盒
丙肝病毒抗体诊断试剂盒:HCV(酶联免疫法)(强生)
13
化学试剂
美国Becton,Dickinson and Company
100支/盒
BD960分枝杆菌培养管(BD)
100人份/盒
BD960分枝杆菌培养添加剂试剂盒(BD)
盒
BD960联合药敏试剂盒SIRE(BD)
100人份/盒
BD960分枝杆菌PZA培养管(BD)
600人份/盒
GLU葡萄糖检测试剂盒(beckman)
600人份/盒
BUN尿素氮检测试剂盒(beckman)
600人份/盒
TG甘油三酯检测试剂盒(beckman)
800人份/盒
TBIL总胆红素检测试剂盒(beckman)
600人份/盒
CHOL胆固醇试剂盒(beckman)
百灵威核磁耗材产品

NMR Consumables and Accessories
客服热线:400-666-7788
全球 NMR 耗材 引领 60 年
百灵威
百灵威科技有限公司成立于 1992 年,始终以“为科研和生产提供世界一流的产品和服务”为宗旨,致力于超精细化学品的研发与 制造。经过近二十年的发展,百灵威已具备为化学、分析、生物、材料、物理及药物研发等领域提供近五十万种产品和专业服务 的能力。 百灵威拥有一支强大的具有丰富经验和创新能力的研发团队,在江苏、河北设立的两个研发中心可迅速研发出毫克至数百公斤级 的医药、生化、材料等中间体及特殊高端化学品,并可为客户定制合成各类产品,尤其擅长小分子药物中间体以及催化剂配体的 合成。 百灵威人坚信“发展民族科技”的理念,坚持依靠中国人自己的智慧和力量, 不断建设和发展位于潮白河畔的现代化工业生产基地,发挥百灵威在尖端技术研究、敏捷制造和系统性物流管理等方面的突出优 势,积极地将中国的各种高端化合物推荐给国际同行,为促进中国化学事业发展,推动世界文明与和谐进步而奋斗不息。 百灵威的使命 促进科技和工业发展,造福人类……
管壁厚度(mm) 平均凸度(µm)
包装
0.27
<60>
50只/塑料筒装
0.27
<60>
50只/塑料筒装
0.43
<60>
50只/塑料筒装
0.43
<60>
100只/纸盒装
0.43
<60>
50只/塑料筒装
0.43
<60>
100只/纸盒装
0.60
<60>
50只/塑料筒装
SampleJet®核磁管
细胞粘附的检测方法
包被: 1、 96 孔板每孔加入 100ul 包被液。 2、 将培养板置 2-8°C 过夜。 3、 移除包被液,用洗涤液洗涤 2-3 次。
细胞接种: 1、 待测定细胞处理好后,用胰酶消化,PBS 洗涤,然后用相应培养基重悬,制成细胞
悬液。 2、 按 5×104 细胞/孔接种 96 孔板,建议设 3-5 个复孔。同时设立对照组,即孵育后不
体损失导致试剂量不够用。 ● 细胞处理需要小心操作,尽量避免人为的损伤细胞。离心力在不损失细胞的前提下尽可能小,重悬
细胞是动作要轻柔,避免多次反复的激烈吹打。不用涡旋振荡器。 ● 染色培养时间根据细胞种类的不同和每孔内细胞数量的多少而异。一般情况下,白细胞较难染色,
因此需要较长的培养时间。培养时间一般为 1- 4 小时,但在培养 30 分钟左右即可取出肉眼观察 染色程度 (根据细胞种类而定,需要摸索一下条件)。当使用标准 96 孔板时,贴壁细胞的最小接 种量至少为 1,000 个/孔 (100 μl 培养基)。检测白细胞时的灵敏度相对较低,因此推荐接种量不 低于 2,500 个/孔 (100 μl 培养基)。如果要使用 24 孔板或 6 孔板实验,请先计算每孔相应的接 种量,并按照每孔培养基总体积的 10%加入染色液 B。 ● 有条件的情况下建议采用多通道移液器,可以减少平行孔间的差异。加染色液 B 试剂时,建议斜
产品说明书
细胞粘附检测试剂盒
货号:BB-48120
V2.16
试剂盒储存条件: 2-8℃保存。
开盖后组份按要求条件保存。 Nhomakorabea试剂盒组成:
产品组成
BB-48120-1
BB-48120-2
规格
100 T
250 T
试剂 A:包被液 A
BD破膜剂试剂手册中文

破膜剂试剂手册BD Cytofix / Cytoperm 固定/渗透试剂盒手册(目录号554714)BD Cytofix / Cytoperm Plus固定/渗透试剂盒(BD GolgiStop含有莫能菌素的蛋白质转运抑制剂)(目录号554715)BD Cytofix / Cytoperm Plus固定/渗透试剂盒(含布雷菲德菌素A的BDGolgiPlug蛋白质转运抑制剂)(目录号555028)Ficoll是Amersham Biosciences AB的注册商标。
Hypaque是Amersham Health AS的注册商标。
BD流式细胞仪是1流激光产品。
仅供研究使用。
不用于诊断或治疗程序。
2016年Becton,Dickinson 和公司。
版权所有。
本出版物的任何部分不得以电子,机械,磁性,光学,化学,手册或其他方式以任何形式或通过任何方式复制,传播,转录,存储在检索系统中或翻译成任何语言或计算机语言,未经BD Biosciences事先书面许可。
购买不包括或携带任何权利转售或转让本产品作为独立产品或另一产品的组成部分。
未经Becton,Dickinson和Company明确书面许可,严禁使用本产品以外的许可使用。
2016BD,BD徽标和所有其他商标均为Becton,Dickinson和Company的产权。
目录BD Cytofix / Cytoperm固定/渗透试剂盒BD Cytofix / Cytoperm Plus固定/渗透试剂盒(BD GolgiStop蛋白质转运抑制剂)BD Cytofix / Cytoperm Plus固定/渗透试剂盒(BD GolgiPlug蛋白质运输抑制剂)警告和注意事项1.一般程序A.刺激细胞1.使用BD GolgiStop的程序蛋白质转运抑制剂(含有莫能菌素)2。
使用BD GolgiPlug 蛋白质转运抑制剂的程序(含布雷菲德菌素A)B.方案:细胞表面抗原和细胞内细胞因子的多色染色1收集细胞2.阻止Fc受体3.细胞表面抗原染色4.固定和渗透细胞5.备选固定和渗透方案6细胞内细胞因子染色C.流式细胞分析。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Product Name:
NBD-556CAS No.:
333353-44-9Cat. No.:
HY-76648Product Data Sheet
MWt:
337.84Formula:
C17H24ClN3O2Purity :>98%
Solubility:
DMSO
y Mechanisms:
Biological Activity:
NBD 556is small molecule mimetic of CD4NBD 556recognizes the HIV 1envelope protein gp120 Pathways:Anti-infection; Target:HIV NBD-556 is small molecule mimetic of CD4, NBD-556 recognizes the HIV-1 envelope protein gp120
and induces restructuring of gp120 analogous to CD4 binding.
IC50 Value:
Target: HIV NBD-556 N-phenyl-N ′-(2,2,6,6-tetramethyl-piperidin-4-yl)-oxalamide analogs as a novel class of human immunodeficiency virus type 1 (HIV-1) entry inhibitors that block the gp120–CD4 interaction.Human immunodeficiency virus (HIV-1) interaction with the primary receptor, CD4, induces
conformational changes in the viral envelope glycoproteins that allow binding to the CCR5 second receptor and virus entry into the host cell. The small molecule NBD-556 mimics CD4 by binding the References:
[1]. Narumi, Tetsuo et al. CD4 mimics targeting the HIV entry mechanism and their hybrid molecules
with a CXCR4 antagonist. Bioorganic & Medicinal Chemistry Letters (2010), 20(19), 5853-5858. [2]. Sch?n A, Madani N, Klein JC, Hubicki A, Ng D, Yang X, Smith AB 3rd, Sodroski J, Freire E.receptor and virus entry into the host cell. The small molecule NBD 556 mimics CD4 by binding the gp120 exterior envelope glycoprotein, moderately inhibiting virus entry into CD4-expressing target cells and enhancing CCR5 binding and virus entry into CCR5-expressing cells lacking CD4....
Thermodynamics of binding of a low-molecular-weight CD4 mimetic to HIV-1 gp120.
Biochemistry. 2006 Sep 12;45(36):10973-80.
[3]. Singh IP , Chauthe SK. Small molecule HIV entry inhibitors: Part II. Attachment and fusion inhibitors: 2004-2010.
Expert Opin Ther Pat. 2011 Mar;21(3):399-416.
[4]. Zhao Q, Ma L, Jiang S, Lu H, Liu S, He Y , Strick N, Neamati N, Debnath AK.Identification of N phenyl N'(2266tetramethyl piperidin 4yl)oxalamides as a new class of HIV 1Caution: Not fully tested. For research purposes only
Medchemexpress LLC
Identification of N-phenyl-N'-(2,2,6,6-tetramethyl-piperidin-4-yl)-oxalamides as a new class of HIV-1
entry inhibitors that prevent gp120 binding to CD4.
Virology. 2005 Sep 1;339(2):213-25.
18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
