Mosapride_112885-41-3_DataSheet_MedChemExpress
逆转录试剂盒( transcriptor first strand cdna synthesis kit ) 说明书

3
04 379 012 001
Transcriptor First Strand cDNA Synthesis Kit y Version 6.0
1. What this Product Does, continued
Vial/ Cap
Label
Store the kit at ؊15 to ؊25°C N Store Control RNA (vial 7 in
Cat. No 04 379 012 001) at —70°C or below.
y Version 6.0
Content version: September 2010
9 (7 for b,c) colorless
Water, PCR-grade
Content
a) Cat. No. 04 379 012 001 b) Cat. No. 04 896 866 001 c) Cat. No. 04 897 030 001
a) 1 vial, 1 ml b) 2 vials, each 1ml c) 3 vials, each 1 ml
L In Cat. No. 04 896 866 001 and Cat. No. 04 897 030 001 the control reagents (vial 7 and 8) are not included. Therefore, in these kits vial 7 is Water, PCR Grade.
Random Hexamer Primer
a) 1 vial, 100 l (600 M) b) 1 vial, 200 l (600 M) c) 2 vials, each 200 l (600 M)
Trigonox B(滴苷但羊水)产品数据表单说明书

Product Data SheetTrigonox BDi-tert-butyl peroxideTrigonox® B is a pure peroxide in liquid form.CAS number110-05-4EINECS/ELINCS No. 203-733-6TSCA statuslisted on inventory Molecular weight 146.2Active oxygen contentperoxide10.94%SpecificationsAppearance Clear liquidAssay≥ 99.0 %ApplicationsTrigonox® B (Di-tert-butyl peroxide) can be used for the market segments: polymer production, polymer crosslinking and acrylics production with their different applications/functions. For more information please check our website and/or contact us.Half-life dataThe reactivity of an organic peroxide is usually given by its half-life (t½) at various temperatures. For Trigonox® B in chlorobenzene half-life at other temperatures can be calculated by using the equations and constants mentioned below:0.1 hr at 164°C (327°F)1 hr at 141°C (286°F)10 hr at 121°C (250°F)Formula 1kd = A·e-Ea/RTFormula 2t½ = (ln2)/kdEa153.46 kJ/moleA 4.20E+15 s-1R8.3142 J/mole·KT(273.15+°C) KThermal stabilityOrganic peroxides are thermally unstable substances which may undergo self-accelerating decomposition. The lowest temperature at which self-accelerating decomposition may occur with a substance in the packaging as used for transport is the Self-Accelerating Decomposition Temperature (SADT). The SADT is determined on the basis of the Heat Accumulation Storage Test.SADT80°C (176°F)Method The Heat Accumulation Storage Test is a recognized test method for thedetermination of the SADT of organic peroxides (see Recommendations on theTransport of Dangerous Goods, Manual of Tests and Criteria - United Nations, NewYork and Geneva).StorageDue to the relatively unstable nature of organic peroxides, a loss of quality will occur over a period of time. To minimize the loss of quality, Nouryon recommends a maximum storage temperature (Ts max. ) for each organic peroxide product.Ts Max.40°C (104°F) andTs Min.-30°C (-22°F) to prevent crystallizationNote When stored according to these recommended storage conditions, Trigonox® Bwill remain within the Nouryon specifications for a period of at least 6 months afterdelivery.Packaging and transportIn North America Trigonox® B is packed in non-returnable, five gallon polyethylene containers of 30 lb net weight and steel drums of 100 or 340 lb net weight. In other regions the standard packaging is a 30-liter HDPE can (Nourytainer®) for 20 kg peroxide. Delivery in a 200 l steel drum for 150 kg peroxide is also possible in a number of countries. Both packaging and transport meet the international regulations. For the availability of other packed quantities consult your Nouryon representative. Trigonox® B is classified as Organic peroxide type E; liquid, Division 5. 2; UN 3107.Safety and handlingKeep containers tightly closed. Store and handle Trigonox® B in a dry well-ventilated place away from sources of heat or ignition and direct sunlight. Never weigh out in the storage room. Avoid contact with reducing agents (e. g. amines), acids, alkalis and heavy metal compounds (e. g. accelerators, driers and metal soaps). Please refer to the Safety Data Sheet (SDS) for detailed information on the safe storage, use and handling of Trigonox® B. This information should be thoroughly reviewed prior to acceptance of this product. The SDS is available at /sds-search.Major decomposition productsAcetone, Methane, tert-ButanolAll information concerning this product and/or suggestions for handling and use contained herein are offered in good faith and are believed to be reliable.Nouryon, however, makes no warranty as to accuracy and/or sufficiency of such information and/or suggestions, as to the product's merchantability or fitness for any particular purpose, or that any suggested use will not infringe any patent. Nouryon does not accept any liability whatsoever arising out of the use of or reliance on this information, or out of the use or the performance of the product. Nothing contained herein shall be construed as granting or extending any license under any patent. Customer must determine for himself, by preliminary tests or otherwise, the suitability of this product for his purposes.The information contained herein supersedes all previously issued information on the subject matter covered. The customer may forward, distribute, and/or photocopy this document only if unaltered and complete, including all of its headers and footers, and should refrain from any unauthorized use. Don’t copythis document to a website.Trigonox® and Nourytainer are registered trademarks of Nouryon Functional Chemicals B.V. or affiliates in one or more territories.Contact UsPolymer Specialties Americas************************Polymer Specialties Europe, Middle East, India and Africa*************************Polymer Specialties Asia Pacific************************2022-6-30© 2022Polymer crosslinking Trigonox B。
富马酸替诺福韦酯--------印度药典

NOTE - Prepare the solutions immediately before use. Test solution. Dissolve 100 mg of the substance under examination in 50 ml of methanol. Reference solution (a). A 0.2 per cent w/v solution of tenofovir disoproxil jitmarate RS in methanol. Reference solution (b). Dilute 1.0 ml ofreference solution (a) to 100.0 ml with methanol. Reference solution (c). Dissolve 10 mg ofthejUmaric acid in 50 ml of methanol.
TELMISARTAN TABLETS
IP 2010
Solvent mixture. 80 volumes of buffer solution prepared by diluting 5.0 ml of triethylamine to 2000ml with water and 20 volumes of methanol. Test solution. Weigh and powder 20 tablets. Disperse a quantity ofpowder containing about 100 mg ofTelmisartan in 100.0 ml ofsolvent mixture, sonicate for 45 minutes and filter. Reference solution. A 0.0005 per cent w/v solution of telmisartan RS in the solvent mixture.
_15国超说明书用药政策的循证评价

Evidence-Based Evaluation on Off-Label Drug Use Policies in 15 Countries
ZHANG Ling-li1,2, LI You-ping1*, ZENG Li-nan2, LIANG Yi2,3, HU Die2,3, LIU Yi2,3, LV Juan2,3
CJEBM • 426 •
© 2012 Editorial Board of Chin J Evid-based Med
中国循证医学杂志 2012, 12(4): 426~435
论 著 • 二次研究
tries. The right to prescribe off-label drug was defined in Britain and Ireland; b) Medical staff had to take the responsibility of off-label drug use in the country where the duty regulations were formulated; and c) Ten countries published guidelines or statements related to off label drug use by their official departments and academic organizations. And the regulation included the following procedures: firstly, to obtain the relative information and evidence; secondly, to get the informed consent; thirdly, to be approved by the ethics committee and/or pharmacy administration committee; fourthly, to record the reasons and effectiveness of off-label use; fifthly, to monitor the adverse reactions of off-label drug use. Besides monitoring the medical institutes, the pharmaceutical companies had also be monitored which included the following 3 aspects: a) to require companies to train specialized staffs to answer the questions related to off-label drug use; b) to open the contact information of medical departments of companies; and c) to prohibit preaching and advertising the off-label drug use. Conclusion Off-label drug use has its rationality and necessity. To protect the safety of patients, avoid the risk for hospitals and medical staffs, it requires formulating relative regulations soon in order to manage the off-label drug use in China. As a developing country, China is different from the developed countries in health care system. Therefore, when formulating the regulations, it is necessary to perform evidence-based evaluation on each country’s laws, regulations and guidelines about off-label drug use, with Chinese national conditions and experts’ opinions in combination. After a regulation is preliminarily drawn up, it needs to be put into pilot practice, and then revised and spread to the whole country.
(ECL)NA931-GEHealthcareLifeSciences

Product Specification SheetCode: NA931WarningFor research use only.Not recommended or intended for diagnosis of disease in humans or animals.Do not use internally or externally in humans or animals. Before using this product, please read the instructions for safe handling, storage and disposalStorageStore at 2–8°C. Do not freeze. Under these conditions, the product is stable for at least 6 months from the date of despatch.ExpirySee outer packaging.Safety warnings and precautionsAll chemicals should be considered as potentially hazardous. We therefore recommend that this product is handled only by those persons who have been trained in laboratory techniques and that it is used in accordance with the principles of good laboratory practice. Wear suitable protective clothing such as laboratory overalls, safety glasses and gloves. Care should be taken to avoid contact with skin or eyes. In the case of contact with skin or eyes wash immediately with water. See material safety data sheet(s) and/or safety statement(s) for specific advice. ComponentsHorseradish Peroxidase conjugated antibody is supplied in Phosphate Buffered Saline (Sodium Phosphate 0.1 M, NaCl 0.1 M) pH 7.5, containing 1% (w/v) Bovine Serum Albumin and an anti-microbial agent.DescriptionPurification to ensure species-specificityThe antibody is prepared by hyper-immunizing sheep with purified immunoglobulin fractions from normal mouse serum to produce high affinity antibodies. The pooled antiserum is used to produce an immunoglobulin preparation which is then affinity adsorbed to remove cross-reacting antibodies towards rat, human and rabbit immunoglobulins. These activities are thoroughly depleted to ensure species-specificity.Finally, to select for specific binding to mouse IgG, the antibodies are purified using an affinity column of mouse IgG. After washingto remove non-specific serum components and low affinity antibodies, the species-specific antibodies are eluted using carefully selected, mild conditions which minimize aggregation and preserve immunological activity, yet which will elute high affinity antibodies.Preparation of labelled antibodyThe enzyme Horseradish Peroxidase is attached to the immunoglobulin molecules using an adaptation of the periodate oxidation technique (1). This method has been found not to affect the effective binding of the antibody to the antigen or the activity of the enzyme.Quality controlFor every batch of enzyme-linked antibody that is produced the antibody titre is determined in an ELISA. The substrate used forthe peroxidase is 2,2’-Azinobis[3-Ethylbenzothiazoline Sulphonate, diammonium salt], ABTS™.Every batch is also QC tested in a Western blotting system. Thisis performed using Amersham Protran™ Premium membrane containing serially-diluted tubulin protein and is immunodetected with: primary monoclonal anti-tubulin antibody and anti-mouse IgG HRP secondary antibody (NA931). Blots are detected using Amersham ECL and Amersham ECL Prime™ detection systems. Applications1. Protein blottinga) Detection with Amersham ECL Western blotting reagents (2) This reagent has been shown to be suitable for use in ECL Western blotting applications.The control system used was the detection of monoclonal anti-tubulin.We have found in our laboratories that dilutions of: 1:2000 for monoclonal anti-tubulin; and 1:5000 for anti-mouse IgG, HRP are suitable for the detection of 3 ng of tubulin on Amersham Protran Premium membrane, exposed to Hyperfilm™ ECL for 5 minutes.To achieve the same sensitivity level on Amersham Hybond™, concentrations would typically be: anti-tubulin - 1:2000; andNA931 - 1:10000.b) Detection with Amersham ECL Prime Western blotting reagents (3, 4)Amersham ECL Prime Western blotting reagent is highly sensitive, giving an increase, for this antibody, of 4-20 fold over ECL detection. This property can be utilized in 2 ways:• Preservation of antibodies that are rare or costly• Increase in detectable sensitivity levelsThe control system used was the same as for Amersham ECL.The suitable antibody dilutions, to detect 3 ng of tubulin on Amersham Protran Premium membrane are: anti-tubulin - 1:5000; and NA931 - 1:50 000.For Amersham Hybond membrane antibody dilutions are typically: anti-tubulin - 1:10 000; and NA931-1:100 000.c) Colorimetric detectionA dilution of 1:300 is recommended.2. ELISAIf this reagent is to be used to detect mouse immunoglobulins, we have found in our laboratories that a dilution of 1:6000 is suitable for the detection of 1 µg of IgG.For greater sensitivity (for example down to 300 pg) the reagent should be diluted rather less (for example 1:500). A suitable diluent is Phosphate-Buffered Saline containing 0.05% (v/v) Tween™ 20.Amersham™ECL™ Anti-Mouse IgG, Horseradish Peroxidase-Linked Species-Specific Whole Antibody (from sheep)imagination at work3. ImmunocytochemistryWhen using the reagent as a second antibody inimmunocytochemistry on sections of formalin-fixed wax-embedded tissue the antibody can typically be diluted 1:100 in Phosphate-Buffered Saline. The user may wish to adjust this to obtain the required sensitivity for the tissue under investigation. If frozensections are used, acceptable staining may be obtained using even higher dilutions of the reagent.Protocol recommendations MembranesNitrocellulose and PVDF membranes are suitable for use with both detection systems. PVDF membrane is highly recommended for use with Amersham ECL Prime detection reagents.For high quality results the following guidelines should be followed: Blocking: Use enough blocking agent to block all non-specific sites. A typical block 5% non-fat dried milk in PBS Tween or TBS Tween (order RPN2125).Washing: The volume of wash buffer (eg PBS-T or TBS-T) must be sufficient to cover the membrane completely.Optimization of primary and secondary antibodies ECL detectionAmersham ECL Western blotting is a very sensitive technique. As such it is essential to optimize the system under study for high signal and low background for both primary and secondary antibodies.Dot blots are a quick and effective method of determining the optimum dilutions required for primary and secondary antibodies. Optimization details are set out in the RPN2106/2108/2109/2209/ 2134 booklets.Amersham ECL Prime detectionDue to the improved sensitivity of Amersham ECL Prime compared to ECL, optimization details as set out in the RPN2132/2133 booklets.Typical anti-mouse secondary antibody dilution ranges:Amersham ECL for nitrocellulose membrane 1:1000 to 1:5000 Amersham ECL Prime for nitrocellulose membrane 1:2000 to 1:10 000For PVDF membrane the use of higher dilutions may be necessary. The exact concentration of the secondary antibody will always be dependent upon the primary antibody used and the sensitivity and exposure times required.Detection: Ensure any excess Amersham ECL or Amersham ECL Prime detection reagents are sufficiently drained prior to exposure.Exposure times:Amersham ECL - exposure times of 1 to 15 minutes are suggested. Amersham ECL Prime - initial exposure times of 1 to 5 minutes are suggested.Signal can still be obtained up to 24 hours after the application of Amersham ECL Prime reagents, and for this exposure times of 1 to 2 hours may be required.Related productsAmersham ECL Western blottingdetection reagentsRPN2106/2108/2109/ 2209/2134Amersham ECL Prime Western blotting detection systemRPN2232 & RPN2236Amersham Protran Premium 10600048 RPN2020D Amersham Hybond membrane 10600058 RPN2020FAmersham Hyperfilm ECL film28906835/28906836/ 28906837/28906838/ 28906839Amersham ECL protein molecular weight markersRPN2107/2124/2125References1) NAKANE, P.K. and KAWAOI, A., Journal of Histochemistry andCytochemistry , 22, pp.1084-1091, 1974.2) WHITEHEAD, T.P. et al., Clin. Chem., 25, pp.1532-1546, 1979.3) AKHAVEN-TAFTI, H. et al., Clin. Chem., 41, pp.1368-1369, 1995.4) AKHAVEN-TAFTI, H. et al., Biolum. And Chemilum. Fundamentals and Applied Aspects, pp.199-202, Chichester, 1994.LegalGE, imagination at work and GE Monogram are trademarks of General Electric Company.Amersham, Hybond, ECL, ECL Prime and Hyperfilm are trademarks of GE Healthcare companies.ABTS is a trademark of Boehringer Mannheim GmBH Tween is a trademark of ICI Americas Inc.Amersham ECL Prime and Amersham ECL Select is manufactured and sold under license from Cyanagen Srl and is subject of US patent application number 2008241868 and 2008176251, and Italian application number TO2010A000580, together with other equivalent granted patents and patent applications in other countries.© 2006 - 2014 General Electric Company – All rights reserved First published 2006All goods and services are sold subject to the terms and conditions of sale of the company within GE Healthcare which supplies them. A copy of these terms and conditions is available on request. Contact your local GE Healthcare representative for the most current information.For your local office contact information, visit /contact GE Healthcare UK Limited Amersham PlaceLittle Chalfont, Buckinghamshire, HP7 9NA, UKGE Healthcare offices:GE Healthcare Bio-Sciences AB Björkgatan 30, 751 84 Uppsala, SwedenGE Healthcare Europe GmbHMunzinger Strasse 5, D-79111 Freiburg, GermanyGE Healthcare Bio-Sciences Corp.800 Centennial Avenue, P.O. Box 1327, Piscataway, NJ 08855-1327, USAGE Healthcare Japan Corporation Sanken Bldg. 3-25-1, Hyakunincho, Shinjuku-ku, Tokyo 169-0073, Japan29093068 Rev AA 01-2014。
QPCR及QRT-PCR系列产品

Invitrogen的ICFC系列产品促销1.QPCR及QRT-PCR系列产品Invitrogen公司专门为中国客户提供的定量PCR试剂盒,结合了 UDG 防止残余污染技术和SYBR® Green I 荧光染料(存在于SYBR® Green I荧光定量PCR试剂盒中),在美国接受了严格的质量监控,可提供极高灵敏度的目的序列定量检测,线性剂量低,反应浓度范围很大。
qPCR Supermix-- 即用型反应剂,专为高特异性、实时定量DNA扩增设计UDG-- 防止携带污染物,减少克隆片段假阳性结果ROX参考染料-- 适用ABI仪器的校正染料产品信息活动时间:即日起至2009年4月30日2.Gibco南美胎牛血清即日起凡优惠价¥1780购买Gibco胎牛血清500ml(目录号:C2027050)即可获赠送价值¥250现金抵用券。
您可以凭现金抵用券在英韦创津公司购买任何商品,此券有效期至2009年5月31日。
产品信息活动时间:即日起至2009年4月30日独特的采集方式:GIBCO采用无菌心脏穿刺的方式采血原装直送,避免污染:原产地采集、加工、检测、包装。
完善的质控:采集、处理、检测、运输等环节都有文件和证书。
3.Invitrogen TA Cloning克隆产品专门用于克隆Taq聚合酶扩增的PCR产物。
采用pCR载体,能产生80%以上的重组产物,90%以上重组产物都包含插入片段。
产品信息活动时间:即日起至2009年5月31日附:pCR载体优点及图谱:3’-T突出端可直接连接Taq扩增的PCR产物可选择T7或T7和Sp6启动子进行体外RNA转录和测序侧向EcoRⅠ位点的通用多接头位点方便了插入片段的切离可以选择卡那霉素或氨苄青霉素进行筛选非常简便的蓝/白克隆筛选具有M13正向和反向引物位点,方便测序4.GIBCO液体培养基系列产品创立近50年的历史,品质优秀,产品种类丰富;为了中国用户利益,特建立国内生产线;所有产品,从原材料到生产全部按照GIBCO质量标准进行,每批均送抵美国公司总部质检合格后,才在国内销售。
SIGMA-ALDRICH MES hydrate M8250说明书

SIGMA-ALDRICH SAFETY DATA SHEETVersion 4.8Revision Date 08/02/2016Print Date 11/14/2016 1. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifiersProduct name : MES hydrateProduct Number : M8250Brand : SigmaCAS-No. : 1266615-59-11.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses : Laboratory chemicals, Synthesis of substances1.3 Details of the supplier of the safety data sheetCompany : Sigma-Aldrich3050 Spruce StreetSAINT LOUIS MO 63103USATelephone : +1 800-325-5832Fax : +1 800-325-50521.4 Emergency telephone numberEmergency Phone # : +1-703-527-3887 (CHEMTREC)2. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Hazards not otherwise classified (HNOC) or not covered by GHS -none3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms : 4-Morpholineethanesulfonic acid2-(N-Morpholino)ethanesulfonic acidFormula : C6H13NO4S · xH2OMolecular weight : 195.24 g/molCAS-No. : 1266615-59-1No components need to be disclosed according to the applicable regulations.4. FIRST AID MEASURES4.1 Description of first aid measuresIf inhaledIf breathed in, move person into fresh air. If not breathing, give artificial respiration.In case of skin contactWash off with soap and plenty of water.In case of eye contactFlush eyes with water as a precaution.If swallowedNever give anything by mouth to an unconscious person. Rinse mouth with water.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2) and/or in section 11 4.3 Indication of any immediate medical attention and special treatment neededNo data available5. FIREFIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, alcohol-resistant foam, dry chemical or carbon dioxide.5.2 Special hazards arising from the substance or mixtureNo data available5.3 Advice for firefightersWear self-contained breathing apparatus for firefighting if necessary.5.4 Further informationNo data available6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresAvoid dust formation. Avoid breathing vapours, mist or gas.For personal protection see section 8.6.2 Environmental precautionsDo not let product enter drains.6.3 Methods and materials for containment and cleaning upSweep up and shovel. Keep in suitable, closed containers for disposal.6.4 Reference to other sectionsFor disposal see section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingProvide appropriate exhaust ventilation at places where dust is formed.For precautions see section 2.2.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly closed in a dry and well-ventilated place.Keep in a dry place.7.3 Specific end use(s)Apart from the uses mentioned in section 1.2 no other specific uses are stipulated8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersContains no substances with occupational exposure limit values.8.2 Exposure controlsAppropriate engineering controlsGeneral industrial hygiene practice.Personal protective equipmentEye/face protectionUse equipment for eye protection tested and approved under appropriate government standards such asNIOSH (US) or EN 166(EU).Skin protectionHandle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (withouttouching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves afteruse in accordance with applicable laws and good laboratory practices. Wash and dry hands.Full contactMaterial: Nitrile rubberMinimum layer thickness: 0.11 mmBreak through time: 480 minMaterial tested:Dermatril® (KCL 740 / Aldrich Z677272, Size M)Splash contactMaterial: Nitrile rubberMinimum layer thickness: 0.11 mmBreak through time: 480 minMaterial tested:Dermatril® (KCL 740 / Aldrich Z677272, Size M)datasource:KCLGmbH,D-36124Eichenzell,phone+49(0)665987300,******************,testmethod:EN374If used in solution, or mixed with other substances, and under conditions which differ from EN 374, contact thesupplier of the CE approved gloves. This recommendation is advisory only and must be evaluated by anindustrial hygienist and safety officer familiar with the specific situation of anticipated use by our customers. Itshould not be construed as offering an approval for any specific use scenario.Body ProtectionChoose body protection in relation to its type, to the concentration and amount of dangerous substances, andto the specific work-place., The type of protective equipment must be selected according to the concentrationand amount of the dangerous substance at the specific workplace.Respiratory protectionRespiratory protection is not required. Where protection from nuisance levels of dusts are desired, use typeN95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and approved underappropriate government standards such as NIOSH (US) or CEN (EU).Control of environmental exposureDo not let product enter drains.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesa) Appearance Form: powderColour: whiteb) Odour No data availablec) Odour Threshold No data availabled) pH No data availableNo data availablee) Melting point/freezingpointf) Initial boiling point andNo data availableboiling rangeg) Flash point No data availableh) Evaporation rate No data availablei) Flammability (solid, gas) No data availableNo data availablej) Upper/lowerflammability orexplosive limitsk) Vapour pressure No data availablel) Vapour density No data availablem) Relative density No data availablen) Water solubility No data availableNo data availableo) Partition coefficient: n-octanol/waterp) Auto-ignitionNo data availabletemperatureNo data availableq) Decompositiontemperaturer) Viscosity No data availables) Explosive properties No data availablet) Oxidizing properties No data available9.2 Other safety informationNo data available10. STABILITY AND REACTIVITY10.1 ReactivityNo data available10.2Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available10.4 Conditions to avoidNo data available10.5 Incompatible materialsStrong oxidizing agents10.6 Hazardous decomposition productsHazardous decomposition products formed under fire conditions. - Carbon oxides, Nitrogen oxides (NOx), Sulphur oxidesOther decomposition products - No data availableIn the event of fire: see section 511. TOXICOLOGICAL INFORMATION11.1Information on toxicological effectsAcute toxicityNo data availableInhalation: No data availableDermal: No data availableNo data availableSkin corrosion/irritationNo data availableSerious eye damage/eye irritationNo data availableRespiratory or skin sensitisationNo data availableGerm cell mutagenicityNo data availableCarcinogenicityIARC: No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at levels greater than or equal to 0.1% is identified as a carcinogen or potential carcinogen by ACGIH.NTP: No component of this product present at levels greater than or equal to 0.1% is identified as a known or anticipated carcinogen by NTP.OSHA: No component of this product present at levels greater than or equal to 0.1% is identified as a carcinogen or potential carcinogen by OSHA.Reproductive toxicityNo data availableNo data availableSpecific target organ toxicity - single exposureNo data availableSpecific target organ toxicity - repeated exposureNo data availableAspiration hazardNo data availableAdditional InformationRTECS: Not availableTo the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly investigated.12. ECOLOGICAL INFORMATION12.1ToxicityNo data available12.2Persistence and degradabilityNo data available12.3Bioaccumulative potentialNo data available12.4 Mobility in soilNo data available12.5Results of PBT and vPvB assessmentPBT/vPvB assessment not available as chemical safety assessment not required/not conducted12.6Other adverse effectsNo data available13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductOffer surplus and non-recyclable solutions to a licensed disposal company.Contaminated packagingDispose of as unused product.14. TRANSPORT INFORMATIONDOT (US)Not dangerous goodsIMDGNot dangerous goodsIATANot dangerous goods15. REGULATORY INFORMATIONSARA 302 ComponentsNo chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 ComponentsThis material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 HazardsNo SARA HazardsMassachusetts Right To Know ComponentsNo components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components2-(N-Morpholino)ethanesulfonic acid hydrate CAS-No.1266615-59-1Revision DateNew Jersey Right To Know Components2-(N-Morpholino)ethanesulfonic acid hydrate CAS-No.1266615-59-1Revision DateCalifornia Prop. 65 ComponentsThis product does not contain any chemicals known to State of California to cause cancer, birth defects, or any other reproductive harm.16. OTHER INFORMATIONHMIS RatingHealth hazard: 0Chronic Health Hazard:Flammability: 0Physical Hazard 0NFPA RatingHealth hazard: 0Fire Hazard: 0Reactivity Hazard: 0Further informationCopyright 2016 Sigma-Aldrich Co. LLC. License granted to make unlimited paper copies for internal use only.The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to theproduct with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. Sigma-Aldrich Corporation and its Affiliates shall not be held liable for any damage resulting from handling or from contact with the above product. See and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.Preparation InformationSigma-Aldrich CorporationProduct Safety – Americas Region1-800-521-8956Version: 4.8Revision Date: 08/02/2016Print Date: 11/14/2016。
Luc-Pair

Luc-Pair™Duo-Luciferase HS Assay Kit-高灵敏性双荧光素酶检测试剂盒Cat.No.LF004(100reactions)Cat.No.LF005(300reactions)Cat.No.LF006(1000reactions)使用说明书GeneCopoeia,Inc.广州易锦生物技术有限公司9620Medical Center Drive,#101地址:广州科学城揽月路3号F区F801(510663)Rockville,MD20850电话:4006-020-200、************、************ USA网站:301-762-0888866-360-9531***********************©2016GeneCopoeia,Inc.使用说明书Luc-Pair™Duo-Luciferase HS Assay KitI.产品概述II.产品信息及储存条件III.细胞裂解IV.FLuc和RLuc工作液的配制V.荧光素酶检测流程VI.有限使用许可及质保声明I.产品概述对报告基因表达的转录调控的研究常被应用于生物学研究和药物发现。
荧光素酶在基因表达研究中应用最为广泛,其主要包含以下几个优点:1)在广泛动态范围内具有高灵敏度2)在哺乳动物细胞内无荧光素酶、背景极低3)实验重复性好4)成本低5)操作简单萤火虫和海肾荧光素酶都具有快捷、简便、灵敏度高的检测特点,被公认为是理想的报告基因,因为它们具有完全不同的进化起源、酶学结构和底物要求。
萤光素酶报告基因的测定需要用光度计或多功能微孔板检测仪,且发光强度与荧光素酶的数量成正比。
萤火虫荧光素酶(Photinus pyralis)已被证实是检测启动子活性和监测基因转录后调控状态的理想的报告基因。
它是在细胞质中作用的酶,分子量为61kDa并催化下列反应:海肾(Renilla reniformis)荧光素酶是一个36kDa单亚基蛋白质,酶活性不需要翻译后修饰,因此它可以作为一个实时转录报告基因,催化下面的生物发光反应:此体系可以在目的基因的附近监控顺式作用元件的转录激活。
达格列净中特定杂质分析及稳定性研究

检测认证达格列净中特定杂质分析及稳定性研究■ 范祥元1 杜陈侠2*〔1. 安徽省食品药品检验研究院;2. 中国科学技术大学附属第一医院(安徽省立医院)〕摘 要:为建立达格列净中的特定杂质DGL301分析方法,采用十四酸丙酰胺基团键合硅胶为填充剂(Poroshell 120 Bonus-RP,100 mm×4.6 mm,2.7 μm或效能相当的色谱柱);流动相A:0.085%的磷酸水溶液;流动相B:乙腈;控制体积流量为1.0 mL/min进行梯度洗脱;检测波长为225 nm;柱温为25℃;进样体积10 μL。
在优化的色谱条件下,主峰、特定杂质DGL301及其他杂质分离度符合要求;特定杂质DGL301在0.16~0.79 μg/mL的浓度范围内,呈现良好的线性关系,回归方程为A=24.869 C+0.5787,r=0.9992,检测限为17.56 ng·mL-1,定量限为59.85 ng·mL-1,RSD=0.9%(n=6),平均回收率为98.37%(n=9)。
本方法可用于达格列净中特定杂质DGL301的检测。
关键词:达格列净,特定杂质,高效液相色谱法DOI编码:10.3969/j.issn.1002-5944.2024.05.036Analysis and Stability Study of Specifi c Impurities in Dapaglifl ozinFAN Xiang-yuan1 DU Chen-xia2*(1. Anhui Institute for Food and Drug Control; 2. The First Affi liated Hospital of University of Science and Technology ofChina/Anhui Provincial Hospital)Abstract:To establish an analysis method for specific impurity DGL301 in dapagliflozin, the tetradecanoic acid propionamide group bonded silica gel was used as the fi ller (Poroshell 120 Bonus-RP, 100mm×4.6mm, 2.7 μm or equivalent chromatographic column); mobile phase A: 0.085% phosphoric acid aqueous solution, mobile phase B: acetonitrile; the volume flow rate was controlled to 1.0 mL/min for gradient elution; the detection wavelength was 225 nm; the column temperature was 25℃; the injection volume was 10 μL. Under optimized chromatographic conditions, the separation of the main peak, specific impurity DGL301, and other impurities met the requirement; specific impurity DGL301 was in the concentration range of 0.16 to 0.79 μg/mL, showing a good linear relationship. The regression equation was A=24.869C+0.5787, r=0.9992; the detection limit was 17.56 ng·mL-1; the quantifi cation limit was 59.85 ng·mL-1, RSD=0.9% (n=6); and the average recovery rate was 98.37% (n=9). This method can be used for the detection of specific impurity DGL301 in dapaglifl ozin.Keywords: dapaglifl ozin, specifi c impurity, high performance liquid chromatography基金项目:本文受安徽省自然基金项目“基于植物分类学与分子网络技术的贝母属药材止咳、化痰‘属效标志物’研究”(项目编号:2108085MB63)资助。
Etomoxir_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Etomoxir is a potent inhibitor of carnitine palmitoyltransferase–I (CPT–1).IC50 & Target: CPT–1[1]In Vitro: Etomoxir binds irreversibly to the catalytic site of CPT–1 inhibiting its activity, but also upregulates fatty acid oxidation enzymes. Etomoxir is developed as an inhibitor of the mitochondrial carnitine palmitoyltransferase–1 (CPT–1) located on the outer mitochondrial membrane. Etomoxir, in the liver can act as peroxisomal proliferator, increasing DNA synthesis and liver growth.Thus, etomoxir, in addition of being a CPT1 inhibitor could be considered as a PPARalpha agonist [1]. Etomoxir is a member of the oxirane carboxylic acid carnitine palmitoyl transferase I inhibitors and has been suggested as a therapeutic agent for the treatment of heart failure. Acute Etomoxir treatment irreversibly inhibits the activity of carnitine palmitoyltransferase I. As a result, fatty acid import into the mitochondria and β–oxidation is reduced, whereas cytosolic fatty acid accumulates and glucose oxidation is elevated. Prolonged incubation (24 h) with Etomoxir produces diverse effects on the expression of several metabolic enzyme [2].In Vivo: Etomoxir is an inhibitor of free fatty acid (FFA) oxidation–related key enzyme CPT1. P53 interacts directly with Bax, which is inhibited by Etomoxir, further confirming the direct interaction of P53 and Bax, and the involvement of FAO–mediatedmitochondrial ROS generation in db/db mice [3]. Rats are injected daily with Etomoxir, a specific CPT–I inhibitor, for 8 days at 20mg/kg of body mass. Etomoxir–treated rats display a 44% reduced cardiac CPT–I activity. The treatment of Lewis rats for 8 days with 20 mg/kg Etomoxir does not alter blood glucose, which is in line with comparable etomoxir–feeding studies. Similarly, Etomoxir feeding does not affect general growth characteristics such as gain in body mass, nor does it affect hindlimb muscle mass.However, heart mass and liver mass are both significantly increased by 11% in Etomoxir–treated rats [4].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay: Etomoxir is dissolved in DMSO and stored, and then diluted with appropriate medium before use [2]. [2]Rat heart H9c2 myoblastic cells are incubated in DMEM containing 10% fetal bovine serum until near confluence. In some experiments,cells are preincubated for 2 h with DMEM (serum–free) in the absence or presence of 1–80 μM Etomoxir and then incubated for 2 h with 0.1 mM [1–14C]oleic acid (10 μCi/dish, binds to BSA in a 1:1 molar ratio). In other experiments, cells arepreincubated for 2 h plus or minus 40 μM Etomoxir and then incubated for 2 h with 0.1 μM or 0.1 mM [1,3–3H]glycerol (10μCi/dish), 0.1 mM [1–14C]oleic acid (2 μCi/dish, binds to BSA in a 1:1 molar ratio), 0.1 mM [1–14C]palmitic acid (2μCi/dish, binds to BSA in a 1:1 molar ratio), 28 μM [3H]ethanolamine (2 μCi/dish), 28 μM [methyl–3H]choline (2 μCi/dish), 0.4mM [3H]serine (20 μCi/dish), or 40 μM myo–[3H]inositol (10 μCi/dish). The medium is removed and the cells washed twice withice–cold saline and then harvested from the dish with 2 mL methanol–water (1:1, v/v) for lipid extraction. An aliquot of thehomogenate is taken for the determination of total uptake of radioactivity into cells. Phospholipids are then isolated andradioactivity in these determined [2].Animal Administration: Etomoxir is dissolved in 0.9% (w/v) NaCl (Rat)[4].[3][4]Mice [3]Product Name:Etomoxir Cat. No.:HY-50202CAS No.:124083-20-1Molecular Formula:C 17H 23ClO 4Molecular Weight:326.82Target:Others Pathway:Others Solubility:10 mM in DMSO80 male C57BLKS/J lar–Lepr db/db mice and 20 wild type littermates (8 week) are used. db/db mice are randomly divided into four groups: db/db group, Etomoxir group, MitoQ group, and PFT–α group. In the Etomoxir group, mice are intraperitoneally injected with 1 mg/kg Etomoxir twice every week. In the MitoQ group, 50 μM MitoQ is given to the mice in water. Water bottles, containing either MitoQ, are covered with aluminum foil, and all bottles are refilled every 3 days. In the PFT–α group, mice are intraperitoneally injected with 1 mg/kg PFT–α twice every week. WT mice are administrated with vehicle instead. The experimental period is 8 weeks. At the end, peripheral blood samples and bone marrow cells are harvested for the assays.Rat[4]Male Lewis rats, weighing 150–200 g, are used in the present study. Animals are kept on a 12 h:12 h light/dark cycle and fed a Purina Chow diet and water ad libitum. The rats are divided into two groups: (1) control and (2) Etomoxir. Etomoxir (20 mg/kg of body weight) is dissolved in 0.9% (w/v) NaCl and administered intraperitoneally for 8 days. Control rats receive saline. The last injection is given 24 h before the experiment. Animals are anaesthetized with an intraperitoneal injection of a nembutal and heparin (3:1) mixture. Subsequently, the heart is removed for LCFA uptake studies and for analyses of transporter protein contents.References:[1]. Rupp H, et al. The use of partial fatty acid oxidation inhibitors for metabolic therapy of angina pectoris and heart failure. Herz. 2002 Nov;27(7):621–36.[2]. Xu FY, et al. Etomoxir mediates differential metabolic channeling of fatty acid and glycerol precursors into cardiolipin in H9c2 cells. J Lipid Res. 2003 Feb; 44(2):415–23.[3]. Li J, et al. FFA–ROS–P53–mediated mitochondrial apoptosis contributes to reduction of osteoblastogenesis and bone mass in type 2 diabetes mellitus. Sci Rep. 2015 Jul 31;5:12724.[4]. Luiken JJ, et al. Etomoxir–induced partial carnitine palmitoyltransferase–I (CPT–I) inhibition in vivo does not alter cardiac long–chain fatty acid uptake and oxidation rates. Biochem J. 2009 Apr 15;419(2):447–55.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
美国阿格迪agdia公司转基因检测产品
STX74000/0050
50条
2450
阳性质控物
LPC73000
220
8Байду номын сангаас
Bt-Cry1F, ELISA DAS ELISA
内毒素蛋白检测试剂盒
过氧化物酶标记物
PSP10301/0096
96反应孔
2660
PSP10301/0288
288反应孔
4600
PSP10301/0480
480反应孔
6.Bt-Cry1F and Bt-Cry34Ab1货号:STX10900/0050规格:50条报价:2240元
7.Bt-Cry2A货号:STX05801/0050规格:50条报价:1540元
8.Bt-Cry34Ab1货号:STX04500/0050规格:50条报价:1540元
9.Bt-Cry3Bb1货号:STX06100/0050规格:50条报价:1540元
多克隆抗体/多克隆抗体,碱性磷酸酶标记物
PSA05900/0096
96反应孔
2660
PSA05900/0288
288反应孔
4600
PSA05900/0480
480反应孔
7210
PSA05900/4800
4800反应孔
38920
Bt-Cry3AImmunoStrip检测试纸条,必须与SEB4样品提取缓冲液结合使用,请另外购买SEB4缓冲液。
10.mBt-Cry3A货号:STX06700/0050规格:50条报价:1540元
11.neomycin phosphotransferase II货号:STX73000/0050规格:50条报价:2450元
Sigma-Aldrich实验室常用生化试剂大促销

缓冲液
产品货号 英文品名 中文品名 优惠价 (R M B ) 目录价 (RMB)
A1542-2.5KG A1542-250G A1542-500G B7901-1KG B7901-500G C3041-100CAP C3041-50CAP C3674-100G C3674-1KG C3674-500G E9508-100ML E9508-10UL E9508-1L E9508-2.5L E9508-500ML E6758-100G E6758-500G H3375-100G H3375-1KG H3375-250G H3375-25G H3375-500G H3375-5KG I0125-100G I0125-10G I0125-1KG I0125-25G I0125-500G I0125-5KG M2933-100G M2933-1KG M2933-25G M2933-500G M1254-100G M1254-1KG M1254-250G M1254-25G M1254-50KG M1254-5KG P5493-1L P5493-4L P4809-100TAB P4809-50TAB
Agarose Agarose Agarose Agarose Agarose Agarose Agarose Agarose Agarose Agarose Agarose Agarose
低熔点琼脂糖 低熔点琼脂糖 低熔点琼脂糖 低熔点琼脂糖 低熔点琼脂糖 低熔点琼脂糖 琼脂糖 琼脂糖 琼脂糖 琼脂糖 琼脂糖 琼脂糖
Ammonium acetate ~98% Ammonium acetate ~98% Ammonium acetate ~98% Boric acid Boric acid Carbonate-Bicarbonate Buffer Carbonate-Bicarbonate Buffer Citric acid trisodium salt Citric acid trisodium salt Citric acid trisodium salt Ethanolamine >=98% Ethanolamine >=98% Ethanolamine >=98% Ethanolamine >=98% Ethanolamine >=98% Ethylenediaminetetraacetic acid >=98.5% Ethylenediaminetetraacetic acid >=98.5% HEPES >=99.5% (titration) HEPES >=99.5% (titration) HEPES >=99.5% (titration) HEPES >=99.5% (titration) HEPES >=99.5% (titration) HEPES >=99.5% (titration) Imidazole >=98.5% (titration) Imidazole >=98.5% (titration) Imidazole >=98.5% (titration) Imidazole >=98.5% (titration) Imidazole >=98.5% (titration) Imidazole >=98.5% (titration) MES hydrate >=99.5% MES hydrate >=99.5% MES hydrate >=99.5% MES hydrate >=99.5% MOPS >=99.5% (titration) MOPS >=99.5% (titration) MOPS >=99.5% (titration) MOPS >=99.5% (titration) MOPS >=99.5% (titration) MOPS >=99.5% (titration) Phosphate buffered saline Phosphate buffered saline Phosphate-Citrate Buffer Phosphate-Citrate Buffer
Alda-1_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Alda–1 is a potent ALDH2 agonist, which significantly improves ALDH2 activity.In Vivo: Alda–1 treatment results in a significant decrease of 4–HNE–protein content in the plasma of apoE -/- mice. Alda–1administration leads to a slight increase in gene expression related to neurogenesis (Nog ), mitochondrial biogenesis (CYTB , ND1),and apoptosis (Bax , Gsk3b ) in the Hp of apoE -/- mice. Alda–1 administration leads to 2 and 10 differentially expressed proteins in theFCx and Hp of apoE -/- mice, respectively [1]. Alda–1 (1.5 mg/kg, b.w., i.p.) administration significantly increases the climbing time,tends to reduce the immobility time and increases the swimming time of the prenatally stressed rats in the forced swim test.Moreover, treatment of prenatally stressed rats with Alda–1 significantly increases number of entries into the open arms of the maze and the time spent therein, as assessed by elevated plus–maze test [2]. Alda–1 (8.5 mg/kg, i.p.) with glucose significantly lowers 4–HNE and FJB–positive cells in the cerebral cortex of Alda–1–treated rats than in DMSO–treated rats 24 h after glucose administration [3]. Alda–1 (10 mg/kg per day) treatment prevents aldehydic overload, mitochondrial dysfunction and improves ventricular function in post–MI cardiomyopathy rats [4].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay:[2]Spleen cells (4×106 cells/mL) are stimulated by optimal concentrations of concanavalin A (Con A; 2.5μg/mL and 0.6 μg/mL) and lipopolysaccharide (LPS, 5 μg/mL) and are incubated in 96–well plates at final volume of 0.2mL for 72 h. Cell proliferation is determined by adding 0.5 μCi of [3H]–thymidine per well at 16 h before the end of the incubation.The cultures are harvested with an automatic cell harvester, and [3H] thymidine incorporation is assessed using a liquid scintillationcounter.Animal Administration: Alda–1 is dissolved in 1 mL/kg b.w. DMSO/water 50/50.[2]After behavioral verification at three months of age,the animals are divided into the following four groups: control, control + Alda–1, prenatally stressed and prenatally stressed + Alda–1(6 animals per group). Alda–1 injections are given intraperitoneally (i.p.) once daily at a dose of 1.5 mg/kg b.w. (dissolved in 1 mL/kg b.w. DMSO/water 50/50) for 14 days. At the same time, the control and prenatally stressed rats receive 1 mL/kg b.w. DMSO/water 50/50. The injections of Alda–1 and vehicle are given between 10 a.m and 11 a.m. In the last five days of Alda–1 treatment the behavioral parameters in the elevated plus maze test and then in the forced swim test are measured.References:[1]. Stachowicz A, et al. Proteomic Analysis of Mitochondria–Enriched Fraction Isolated from the Frontal Cortex and Hippocampus of Apolipoprotein E Knockout Mice Treated with Alda–1, an Activator of Mitochondrial Aldehyde Dehydrogenase (ALDH2). Int J Mol Sci.[2]. Stachowicz A, et al. The impact of mitochondrial aldehyde dehydrogenase (ALDH2) activation by Alda–1 on the behavioral and biochemical disturbances in animal model of depression. Brain Behav Immun. 2016 Jan;51:144–53.Product Name:Alda–1Cat. No.:HY-18936CAS No.:349438-38-6Molecular Formula:C 15H 11Cl 2NO 3Molecular Weight:324.16Target:Aldehyde Dehydrogenase (ALDH)Pathway:Metabolic Enzyme/Protease Solubility:DMSO: ≥ 51 mg/mL[3]. Ikeda T, et al. Effects of Alda–1, an Aldehyde Dehydrogenase–2 Agonist, on Hypoglycemic Neuronal Death. PLoS One. 2015 Jun 17;10(6):e0128844.[4]. Gomes KM, et al. Aldehydic load and aldehyde dehydrogenase 2 profile during the progression of post–myocardial infarction cardiomyopathy: benefits of Alda–1. Int J Cardiol. 2015 Jan 20;179:129–138.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
欧洲药典7.5版

INDEX
To aid users the index includes a reference to the supplement in which the latest version of a text can be found. For example : Amikacin sulfate...............................................7.5-4579 means the monograph Amikacin sulfate can be found on page 4579 of Supplement 7.5. Note that where no reference to a supplement is made, the text can be found in the principal volume.
English index ........................................................................ 4707
Latin index ................................................................................. 4739
EUROPEAN PHARMACOPபைடு நூலகம்EIA 7.5
Index
Numerics 1. General notices ................................................................... 7.5-4453 2.1.1. Droppers...................
HOE-S 785026_132869-83-1_DataSheet_MedChemExpress

Product Name:HOE-S 785026CAS No.:132869-83-1Cat. No.:HY-15561Product Data SheetMWt:424.50Formula:C25H24N6O Purity :>98%Solubility:DMSO or water Protect from lightMechanisms:Biological Activity:Hoechst stains are part of a family of blue fluorescent dyes used to stain DNA HOE-S 785026is a Pathways:Others; Target:DNA Stain Hoechst stains are part of a family of blue fluorescent dyes used to stain DNA. HOE S 785026 is acell dye for DNA.IC50 Value:These Bis-benzimides were originally developed by Hoechst AG, which numbered all theircompounds so that the dye Hoechst 33342 is the 33342nd compound made by the company. There are three related Hoechst stains: Hoechst 33258, Hoechst 33342, and Hoechst 34580. The dyes Hoechst 33258 and Hoechst 33342 are the ones most commonly used and they havesimilarexcitation/emission spectra. Both dyes are excited by ultraviolet light at around 350 nm, and both emit blue/cyan fluorescent light around anemission maximum at 461 nm. Unbound dye has its References:[1]. Latt SA, Stetten G, Juergens LA, Recent developments in the detection of deoxyribonucleic acid synthesis by 33258 Hoechst fluorescence. The journal of histochemistry and cytochemistry : officialjournal of the Histochemistry Society 23 (7): 493-505.maximum fluorescence emission in the 510-540 nm range. Hoechst dyes are soluble in water and in organic solvents such as dimethyl formamide or dimethyl sulfoxide. Concentrations can be achieved of up to 10 mg/...j y y ()[2]. a b c "Hoechst Stains". Invitrogren (Molecular Probes).[3]. Portugal J, Waring MJ. Assignment of DNA binding sites for 4',6-diamidine-2-phenylindole and bisbenzimide (Hoechst 33258). A comparative footprinting study. Biochimica et Biophysica Acta 949(2): 158-68.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
M5 Hiper 高难阳离子转染试剂 使用说明书

M5 Hiper“转必得”高难阳离子转染试剂使用说明书产品名称单位货号M5 Hiper“转必得”高难阳离子转染试剂1ml MF979-01【储存条件】2-8℃储存,保质期两年【产品简介】“转必得”高难阳离子转染试剂是一种新型高效的阳离子聚合物。
它可以与核酸(包括质粒、siRNA、寡聚核苷酸)相互作用形成一种复合物将核酸转运到真核细胞内,适用于大部分真核细胞的细胞转染。
已经成功转染过的细胞系:HeLa、HEK293T、293T、HUVEC、HepG2、HEK293、CNE2、SW480、HEK293FT、HT29、Raw264.7、THP-1、HCT116【产品特性】大量试验测试表明,与常规转染试剂产品相比,“转必得”高难阳离子转染试剂具有以下优势:(1)转染效率较高。
(2)批次稳定,实验重复性好。
(3)对于贴壁细胞和悬浮细胞都适用。
(4)无明显细胞毒性。
(5)适用于siRNA细胞转染。
(6)“转必得”高难阳离子转染试剂适用于质粒共转染,慢病毒包装及感染实验。
【注意事项】1、转染前细胞应处于良好的生长状态,以对数生长期为佳,推荐细胞传代后12-24小时内、细胞密度为70-90%时进行转染;2、使用高质量的质粒有利于获得较高的转染效率,推荐使用无内毒素质粒抽提试剂盒抽提质粒,A260/A280比值为1.8~2.0,质粒浓度在300ng/μL以上;3、在配制转染试剂与质粒复合物时,需使用无血清、无抗生素的基础培养基作为溶剂,细胞培养孔中的完全培养基不影响细胞转染效率;4、在细胞转染实验中,根据细胞转染效率可适当调整质粒与转染试剂的用量比例,一般质粒的量(μg)与“转必得”高难阳离子转染试剂剂量(μL)使用比例在1:1~1:4之间,推荐比例为1:2;5、对于毒性比较敏感的细胞,建议转染4-6小时后,半量更换新鲜完全培养基;6、转染试剂如遇沉淀,轻轻摇动管身,至沉淀溶解即可使用;7、本产品仅限用于科学研究,不得用于临床诊断和治疗。
SYTOXOrangeNucleicAcidStainSYTOX橙核酸染色
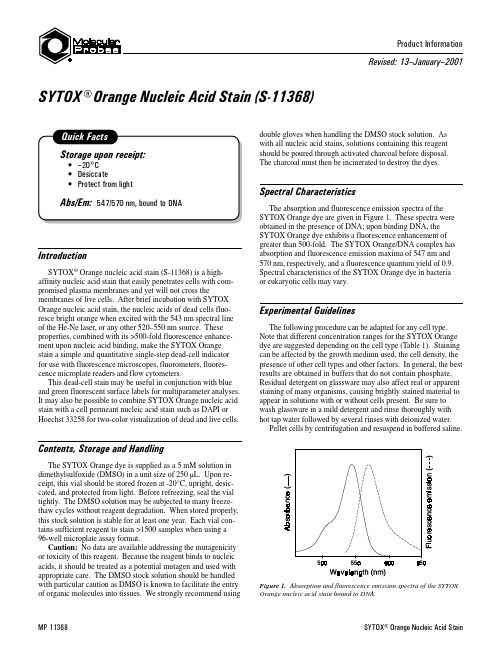
SYTOX® Orange Nucleic Acid StainMP 11368Revised: 13 January 2001Product Information IntroductionSYTOX ® Orange nucleic acid stain (S-11368) is a high-affinity nucleic acid stain that easily penetrates cells with com-promised plasma membranes and yet will not cross themembranes of live cells. After brief incubation with SYTOX Orange nucleic acid stain, the nucleic acids of dead cells fluo-resce bright orange when excited with the 543 nm spectral line of the He-Ne laser, or any other 520–550 nm source. These properties, combined with its >500-fold fluorescence enhance-ment upon nucleic acid binding, make the SYTOX Orange stain a simple and quantitative single-step dead-cell indicator for use with fluorescence microscopes, fluorometers, fluores-cence microplate readers and flow cytometers.This dead-cell stain may be useful in conjunction with blue and green fluorescent surface labels for multiparameter analyses.It may also be possible to combine SYTOX Orange nucleic acid stain with a cell permeant nucleic acid stain such as DAPI or Hoechst 33258 for two-color visualization of dead and live cells.Contents, Storage and HandlingThe SYTOX Orange dye is supplied as a 5 mM solution in dimethylsulfoxide (DMSO) in a unit size of 250 µL. Upon re-ceipt, this vial should be stored frozen at -20°C, upright, desic-cated, and protected from light. Before refreezing, seal the vial tightly. The DMSO solution may be subjected to many freeze-thaw cycles without reagent degradation. When stored properly,this stock solution is stable for at least one year. Each vial con-tains sufficient reagent to stain >1500 samples when using a 96-well microplate assay format.Caution: No data are available addressing the mutagenicity or toxicity of this reagent. Because the reagent binds to nucleic acids, it should be treated as a potential mutagen and used with appropriate care. The DMSO stock solution should be handled with particular caution as DMSO is known to facilitate the entry of organic molecules into tissues. We strongly recommend usingdouble gloves when handling the DMSO stock solution. As with all nucleic acid stains, solutions containing this reagent should be poured through activated charcoal before disposal.The charcoal must then be incinerated to destroy the dyes.Spectral CharacteristicsThe absorption and fluorescence emission spectra of the SYTOX Orange dye are given in Figure 1. These spectra were obtained in the presence of DNA; upon binding DNA, the SYTOX Orange dye exhibits a fluorescence enhancement of greater than 500-fold. The SYTOX Orange/DNA complex has absorption and fluorescence emission maxima of 547 nm and 570 nm, respectively, and a fluorescence quantum yield of 0.9.Spectral characteristics of the SYTOX Orange dye in bacteria or eukaryotic cells may vary.Experimental GuidelinesThe following procedure can be adapted for any cell type.Note that different concentration ranges for the SYTOX Orange dye are suggested depending on the cell type (Table 1). Staining can be affected by the growth medium used, the cell density, the presence of other cell types and other factors. In general, the best results are obtained in buffers that do not contain phosphate.Residual detergent on glassware may also affect real or apparent staining of many organisms, causing brightly stained material to appear in solutions with or without cells present. Be sure to wash glassware in a mild detergent and rinse thoroughly with hot tap water followed by several rinses with deionized water.Pellet cells by centrifugation and resuspend in buffered saline.SYTOX® Orange Nucleic Acid Stain (S-11368)Figure 1. Absorption and fluorescence emission spectra of the SYTOXOrange nucleic acid stain bound to DNA.SYTOX® Orange Nucleic Acid Stain2The binding of SYTOX Orange stain may be reduced somewhat in solutions containing very high concentrations of monovalentor divalent cations. Adherent cells such as mammalian tissue cells may be stained in situ on coverslips. Add SYTOX Orange stain using the concentrations listed in Table 1 as a guide. In initial experiments, it may be best to try several dye concen-trations over the entire suggested range to determine the concen-tration that yields optimal staining.Cells stained with SYTOX Orange dye can be viewed with a fluorescence microscope equipped with a filter set appropriate for tetramethylrhodamine.Stained eukaryotic cells will generally have bright orange nuclei as well as some low-level cytoplasmic staining. Bacteria generally stain uniformly once the intracellular dye is at equilib-rium with the staining solution. Allow 5 minutes or more for staining of bacteria or eukaryotic cells to reach completion.Product List Current prices may be obtained from our Web site or from our Customer Service Department.Cat #Product NameUnit Size S-11368SYTOX ® Orange nucleic acid stain *5 mM solution in DMSO*.................................................................................................250 µLContact InformationFurther information on Molecular Probes' products, including product bibliographies, is available from your local distributor or directly from Molecular Probes. Customers in Europe, Africa and the Middle East should contact our office in Leiden, the Netherlands. All others should contact our Technical Assis-tance Department in Eugene, Oregon.Please visit our Web site for the most up-to-date informationMolecular Probes, Inc.PO Box 22010, Eugene, OR 97402-0469Phone: (541) 465-8300 Fax: (541) 344-6504Customer Service : 7:00 am to 5:00 pm (Pacific Time)Phone:(541)465-8338 Fax:(541)344-6504 ****************Toll-Free Ordering for USA and Canada:Order Phone: (800) 438-2209 Order Fax: (800) 438-0228Technical Assistance : 8:00 am to 4:00 pm (Pacific Time)Phone:(541)465-8353 Fax:(541)465-4593 ***************Molecular Probes Europe BV PoortGebouw, Rijnsburgerweg 102333 AA Leiden, The NetherlandsPhone: +31-71-5233378 Fax: +31-71-5233419Customer Service : 9:00 to 16:30 (Central European Time)Phone: +31-71-5236850 Fax: +31-71-5233419******************Technical Assistance : 9:00 to 16:30 (Central European Time)Phone: +31-71-5233431 Fax: +31-71-5241883******************Molecular Probes products are high-quality reagents and materials intended for research purposes only. These products must be used by, or directly under the supervision of, a technically qualified individual experienced in handling potentially hazardous chemicals. Please read the Material Safety Data Sheet provided for each product; other regulatory considerations may apply.Several of Molecular Probes products and product applications are covered by U.S. and foreign patents and patents pending. Our products are not available for resale or other commercial uses without a specific agreement from Molecular Probes, Inc. We welcome inquiries about licensing the use of our dyes,*************************************************************************.Allnamescontainingthedesignation®areregisteredwiththe U.S. Patent and Trademark Office.Copyright 2001, Molecular Probes, Inc. All rights reserved. This information is subject to change without notice.Table 1. Recommended conditions for staining cells with SYTOX。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Product Name:
Mosapride CAS No.:
112885-41-3Cat. No.:
HY-B0189Product Data Sheet
MWt:
421.89Formula:
C21H25ClFN3O3Purity :>98%
Solubility:
DMSO
y Mechanisms:Biological Activity:
Mosapride is a gastroprokinetic agent that acts as a selective 5HT4agonist
Pathways:GPCR/G protein; Target:5-HT Receptor Pathways:Neuronal Signaling; Target:5-HT Receptor
Mosapride is a gastroprokinetic agent that acts as a selective 5HT4 agonist.
Target: 5HT4 Mosapride is a gastroprokinetic agent that acts as a selective 5HT4 agonist. The major active metabolite of mosapride, known as M1, additionally acts as a 5HT3 antagonist, which accelerates gastric emptying throughout the whole of the gastrointestinal tract in humans, and is used for the treatment of gastritis, gastro-oesophageal reflux disease, functional dyspepsia and irritable bowel syndrome. It is recommended to be taken on an empty stomach (i.e. at least one hour before food or
two hours after food).In addition to its prokinetic properties, mosapride also exerts anti-inflammatory effects on the
References:
[1]. Tack J, et al. Systematic review: cardiovascular safety profile of 5-HT(4) agonists developed for
gastrointestinal disorders. Aliment Pharmacol Ther. 2012 Apr;35(7):745-67.[2]. Curran MP , et al. Mosapride in gastrointestinal disorders. Drugs. 2008;68(7):981-91.p p p p y gastrointestinal tract which may contribute to some of its therapeutic effects. Mosapride also promotes neurogenesis in the gastrointestinal tract which may prove useful in certain...
[]p g g ()[3]. Odaka T, et al. Serotonin 5- HT4 receptor agonist (mosapride citrate). Nihon Rinsho. 2006
Aug;64(8):1491-4.Caution: Not fully tested. For research purposes only
Medchemexpress LLC
18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
