A mild and convenient ‘dry’ hydrolysis of amides to carboxylic acids
氮化铝抗水化性能及其在浇注料中的应用
变化。
数与热处理温度呈线性关系( a= 3. 1152+ 1. 4249 10- 5T , c= 4. 9785+ 2. 2736 10- 5T ) , 因此他
们认为在热处理过程中有一部分氧元素固溶进入
加水量
5. 5 5. 5 5. 5 5. 5
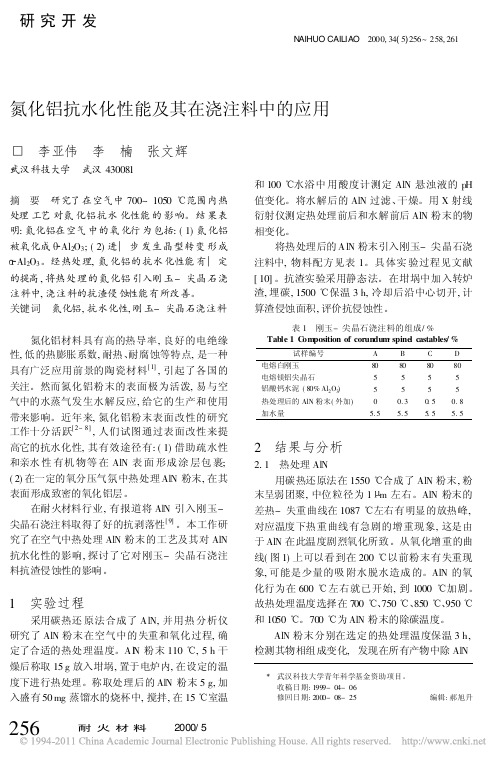
2 结果与分析
2. 1 热处理 AlN 用碳热还原法在 1550 合成了 AlN 粉末, 粉
末呈弱团聚, 中位粒径为 1 m 左右。AlN 粉末的 差热- 失重曲线在 1087 左右有明显的放热峰, 对应温度下热重曲线有急剧的增重现象, 这是由 于 AlN 在此温度剧烈氧化所致。从氧化增重的曲 线( 图 1) 上可以看到在 200 以前粉末有失重现 象, 可能是少量的吸 附水脱水造成 的。AlN 的氧 化行为在 600 左右就已开始, 到 1000 加剧。 故热处理温度选择在 700 、750 、850 、950 和 1050 。700 为 AlN 粉末的除碳温度。
于在高温下 AlN 与镁铝尖晶石形成含 Mg 的氮氧 化铝尖晶石固溶体, 这种带有空位缺陷的尖晶石 能吸收转炉渣中的 FeO。同时 CaO、MgO 等组分也 与 Al2O3 反应形成 CaO 6Al2O3 和 MgAl2O4, 改变了 熔渣的组成, 使渣粘度升高, 阻止熔渣在浇注料体 内进一步渗透, 从而提高了浇注料的抗渣侵蚀性。 但也发现在空气中, 引入 AlN 的浇注料抗渣性反 而下降, 这是因为 AlN 被氧化引起体积膨胀, 使浇 注料变松散, 熔渣侵入内部的结果。
AlN 晶格内形成 Al- O- N 相, 而使 AlN 晶格常数 增大。这种属 AlN 型的氮氧化铝相也有报道[ 13] 。 2. 2 AlN 粉末在水溶液中的稳定性
Chapter-3活性包装

barrier packaging.
Chemical forms of in-pack oxygen scavenging have been introduced to reduce…
These include amines formed rapidly in fish or rancid odors in oil-containing foods. Such
compounds can be present in trace amounts that are significant organoleptically but may not constitute a health hazard.
•Chemical deterioration : food components hydrolysis, industrial chemical oxidation Industrial chemical such as amines , and particularly some printing inks, are oxidized on storage, fried snacks’ oxidation and so on.
dioxide to the beverage.
The presence of an oxygen scavenger is required.(beer and White Wine)
Chemical deterioration:
The flavor of some foods changes on storage because of effects other than oxidation. Tainting is a recurrent problem.
汽车座席安全带维护指南说明书

InspectionAll parts of the seat belts, including the belt fabric, should be regularly inspected for fraying, loosening, wear and other damage. Keep the belts in good condition at all times to reduce the chance of being injured in an accident, and to minimize any in-juries that do occur. Make sure the buckles, retractors, tongue plates, guides and anchors all work properly.Don't let anything get inside the buckle or the retractor; it could cause latch or retractor failure.CleaningThe belts should always be kept clean and dry; wet or damp belts can cause rewinding problems. To clean the belts, pull them all the way out of their retractors and scrub them with warm water and a mild soap; then let them air-dry fully extended, in the shade, with the car windows open.Never bleach, dye or clean the belts with chemical solvents;it will weaken the fabric.Do not remove the seat belts from the car to wash them.ReplacementReplace the seat belt if:1. The belting is cut, punctured, burned, etc.2. The buckle or retractor does not work properly.3. It was being worn at the time of a collision (also check fordamage at the seat belt anchor points).4. Its condition is questionable.Anti Lock Brakes (GS)ALB (Anti Lock Brakes) help to maintain the road holding and trac-tability of your car during severe braking, and under slippery road conditions. The ALB system prevents the wheels from Socking (thus reducing the chance of skidding) to ensure controllable deceleration. When sudden braking might otherwise lock one or more wheels, the ALB system temporarily reduces the braking pressure to the wheel or wheels about to lock to ensure continued braking efficiency.When the ALB is regulating the braking pressure, the brake pedal pulsates slightly to make the driver aware that the system is com-pensating for critical braking conditions. The pulsating brake pedal can be an indication of hazardous road conditions, and a reminder for you to take extra care.Don't mix different diameter tires; it will confuse the ALB com-puter which monitors the road speed of each wheel. For exam-ple, if one or more tires are larger than the others, the computer will think they are rolling more slowly (as if they are about to lock-up) and reduce brake pressure to those wheels.On loose or uneven surfaces (gravel, ruts etc.) where all fourwheels lose traction intermittently, the ALB system may requirea longer stopping distance than an equivalent car with a con-ventional braking system.The ALB system cannot make up for extreme road conditions ordriver misjudgement. It is still the driver's responsibility to drive at a suitable speed and provide a margin of safety for the road, weather and traffic conditions at hand.CAUTION: Be careful not to damage the wiring or the speed sen-sors at the back of each wheel when removing mud or snow from the wheel housings.NOTE: You may hear a sound like a small motor running, coming from the engine while driving or after the ALB is applied. This in-dicates the ALB pump is in service and the system is working properly.Instrument Panel HAZARD WARNING SWITCHDIGITAL CLOCK REARWINDOWDEFOGGERSWITCH PANEL BRIGHTNESS CONTROL DIAL FOG LIGHTSWITCHCIGARETTELIGHTERASHTRAYWIPER/WASHERSWITCH TILT STEERING ADJUSTMENT LEVERPOWER MIRROR ADJUSTMENT SWITCH IGNITIONSWITCH LIGHT SWITCH FUSE BOX HOOD RELEASE HANDLE SUNROOF SWITCH COOLANT TEMPERATURE GAUGE TRIP METER TRIP METERRESET BUTTONFUEL GAUGE SPEEDOMETER ODOMETER SHIFT LEVERPOSITION INDICATOR(Automatic Trans. only)TACHOMETER GLOVE BOX HEATING/COOLING CONTROL PANEL CRUISE CONTROL MASTERSWITCHSpeedometerThe speed is indicated in miles per hour (outside scale) and kilo-meters per hour (inside).OdometerThe numbers on the odometer indicate miles.The odometer registers total distance traveled, and serves as your guide for determining when periodic maintenance is due. Federal law makes it illegal to alter the odometer of any motor vehicle with the intent to change the number of miles indicated.Trip MeterThe numbers on the trip meter indicate miles.The trip meter can be returned to zero by pushing in the reset but-ton. Use it for checking fuel consumption or distance traveled per trip.TachometerThe tachometer indicates engine speed in revolutions per minute.The beginning of the RED ZONE indicates the maximum allowa-ble engine R.P.M. Do not run the engine with the tachometer indicator needle in the RED ZONE.Fuel GaugeFUEL TANK CAPACITY: Approx. 50 (13.2 US gal)As a convenience, the gauge continues to show the same fuel level as when the ignition was last on. After refueling, the gauge will slowly change to the new fuel level when the ignition is swit-ched on.When the needle indicates E (empty), a usable reserve of about 4(1.1 US gal) remains in the tank.Coolant Temperature GaugeCAUTION: The needle should stay within the white range. If the needle reaches the red line at "H " (Hot), stop the engine and check the coolant level in the plastic tank on the left side of the radiator under the hood.Do not remove the radiator cap when the engine ishot. The coolant is under pressure and may blow out and scald you.Gauges。
艾拉莫德的合成工艺改进

广东药科大学学报Journal of Guangdong Pharmaceutical University Jan.2021,37(1)艾拉莫德的合成工艺改进王栋1,2,陆俊2,钟健2,龙玺国2,徐琳寓2,王崇益2,马玉恒1,马文静1(1.滁州学院材料与化学工程学院,安徽滁州239000;2.江苏润安制药有限公司生产技术部,江苏淮安223001)摘要:目的改进艾拉莫德合成中的胺基甲酰化反应工艺。
方法通过活性酯法,甲酸(2)与N,N‐羰基二咪唑(CDI)反应得到中间态甲酰基咪唑(3),再与起始原料2‐氨基‐1‐(2‐甲氧基‐4‐甲磺酰胺基‐5‐苯氧基苯基)乙酮盐酸盐(4)缩合得到甲酰胺基甲基‐2‐甲氧基‐4‐甲磺酰胺基‐5‐苯氧基苯基酮(5),收率由67.3%提高至91.7%;(5)经过甲氧基水解得到甲酰胺基甲基‐2‐羟基‐4‐甲磺酰胺基‐5‐苯氧基苯基酮(6);(6)经过环合反应得到艾拉莫德(1)。
结果与结论目标产物结构经1H‐NMR、13C‐NMR和MS确证,氨基甲酰化、甲氧基水解和环合3步反应总收率为68.1%。
改进后的甲酰化工艺反应进程快,条件温和,反应选择性高,具有较高的商业化价值。
关键词:艾拉莫德;环氧化酶‐2抑制剂;N,N'‐羰基二咪唑;合成中图分类号:R914.5文献标识码:A文章编号:2096‐3653(2021)01‐0051‐04DOI:10.16809/j/cnki.2096‐3653.2020091801Improvement of synthesis process of iguratimodW ANG Dong1,2,LU Jun2,ZHONG Jian2,LONG Xiguo2,XU Linyu2,WANG Chongyi2,MA Yuheng1*,MA Wenjing1(1.College of Material and Chemical Engineering,Chuzhou University,Chuzhou239000;2.ProductionTechnology Department,Jiangsu Runan Pharmaceutical CO.,LTD.Huai’an223001,China)*Corresponding author Email:****************Abstract Objective:The synthetic process of aminoformylation in the synthesis of iguratimod was improved.Methods Intermediate formyl imidazole(3)was synthesized by the reaction of formic acid(2)with N,N'‐carbonyldiimidazole(CDI),N‐(2‐(2‐methoxy‐4‐(methylsulfonamido)‐5‐phenoxyphen‐yl)‐2‐oxoe‐thyl)formamide(5)was synthesized by the reaction of N‐(4‐(2‐aminoacetyl)‐5‐methoxy‐2‐phenoxyphenyl) methanesulfonamide hydrochloride(4)and(3),and the yield increased from67.3%to91.7%.N‐(2‐(2‐hydroxy‐4‐(methylsulfonamido)‐5‐phenoxyphenyl)‐2‐oxoethyl)formamide(6)was obtained by methoxy hydrolysis reaction of(5),and iguratimod(1)was synthesized from(6)by cyclization.Results and Conclusion The structure of the target product has been verified by1H‐NMR/13C‐NMR and MS and thetotal yield of carbamoylation,methoxy hydrolysis and cyclization was68.1%.The improved formylationprocess has the advantages of fast reaction process,mild reaction condition and less side reaction andhigh commercial value.Key words iguratimod;COX‐2inhibitors;N,N'‐carbonyldiimidazole;synthesis收稿日期:2020‐09‐18基金项目:滁州学院校启动基金(2020qd09,2018qd06);滁州学院国家级大学生创新创业训练计划项目(2020CXXL119,202010377027)作者简介:王栋(1986-),男,硕士,高级工程师,从事药物合成研究,电话:*************,Email:****************通信作者:马玉恒(1983-),男,博士,讲师,主要从事药物化学及药物晶体学研究,Email:****************。
The floating egg problem(漂浮的鸡蛋)

The floating egg problem----Can you make an egg float?Background:“For many years soap was made at homefrom a variety of recipes. Animal fat, usually fromcattle (also called tallow) was cooked with a lyesolution. Lye, though it was mostly simple sodiumhydroxide, could not be made from purifiedchemicals, as we do now. Instead, the solution wasobtained from ashes and water. The ashes weretreated with hot water, and then the mixture wasfiltered to obtain a solution.But before this could be used in soap making,one had to check the concentration of the lyesolution. One simple test was to try to "just float" a raw egg in the solution. If the egg sank, the concentration of the lye in the solution was too low.If the egg floated too high, the concentration of the solution was too great, and water was added before adding the fat. To “just float” in this case means to make the top of the egg just touch the top of the solution, without any significant amount of the egg protruding above the surface of the solution.” Because lye is caustic and corrosive, sodium chloride will be substituted in this lab.In this investigation, you will practice with volumetric glassware to improve your technique and to solve an important historical question in practical chemistry by answering "What is the density of an egg?"Firstly, a series of salt solutions are prepared by a variety of volumetric glasses to determine the concentration range which an egg can float. Then, prepare a salt solution with exact concentration in which an egg just floats. You will obtain mass and volume measurements of this solution using a balance to measure the mass and volumetric glasses to measure the volume. The values that you obtain for the mass and volume will be used to calculate the density of the salt solution and, by extension, the density of the egg itself. Hydrometer is also used to measurement the density of this solution. You will compare the density of a fresh egg with that of an egg that is past its prime.Safety note: Sodium chloride solution is not harmful unless it gets into the eyes. If this occurs, flush the eyes with large amount of water for 15 min. All solution may be poured down the drain with water after use.Note: Be sure to record all measurements using the numbers of significant figures allowed by that instrument.The density of a solution affects whether an object floats in that solution.Purpose:Practice skills of measuring masses on balance, measuring volumes with graduated cylinder, volumetric flask and pipets, and making solutions;To investigate the error associated with the measurements of volume in the chemistry labConsider the conceptions of accuracy, precision, and data analysisTo use significant figures correctlyMaterial available:electric Balances; beaker (50 mL, 100 mL); pipettes(10 mL, 25mL, 50mL) ; pipet bulb; graduated cylinders (10 mL, 50mL); V olumetric flasks(50 mL);Deionized water; Eggs ; Sodium chloride (salt)Pre-lab Questions1. Why is density a physical property useful for identifying an unknown substance?2. As the density of a given liquid increases, will the hydrometer used to determine it float higher or lower in the liquid? Explain.3. If a hydrometer touches bottom in a liquid, is its range too high or low for the liquid? Why?4. In what way dose each of the following affect the calculated density or the precision of the calculated density:(a) the type of the volume measuring device used: 10 mL graduated cylinder, 50 mL graduated cylinder, 100 mL beaker, or pipet?(b) the degree of freshness of the raw egg?5. A student, who performed this experiment, consistently viewed the meniscus of the liquid from a high angle (not at eye level) when reading the graduated cylinder. Explain how this error would affect both the accuracy and precision of the density result that he obtained when using this device.6. Determine the number of significant digit of figures below:0.0063 23.7003060 9.80×10-37. Calculate these equations using the appropriate number of significant figures.146.03 g + 175.1 g =21,500 g2÷58 g =Related reading1. Egg component:To determine the density of an egg, we need to described the components of an egg. It is these components, after all, that give an egg its mass and volume. According to the American Egg Board at , the main parts of an egg are:* Shell, which is mostly calcium carbonate and makes up 9 to 12 percent of the total weight of the egg (and which is actually very porous so that air can pass through)* Yolk (the yellow part consisting of fats, proteins, minerals and vitamins), which makes up about 34 percent of the liquid weight of the egg* Albumen (the egg white consisting of proteins, among other things), which makes up about 66 percent of the liquid weight of the egg* Air cell, which is a pocket of air found at the large end of the eggThere can be some variation of these parts.2. Density and density measurement:Density is a physical property of a substance that can be determined by scientific experiment. You might have learned that density is mass divided by volume, which means that if you can measure both the mass and the volume of an object, you can calculate its density. A substance will always have the same density no matter the size of the sample, so that density can be used to help identify a substance. Since an egg is an object that has a mass and a volume, you can calculate its density. The mass of the sample can be easily be measured with balance. While the measurement of the volume will be more complicated. The volume of an object can be measured in different ways. One way is to measure length with a ruler, and calculate the volume mathematically. This is easy to do if the shape of an object is something like a cube or a sphere. For objects that have irregular shapes, a common method is to use the water displacement method. Measure the volume of a certain amount of water (say, for example, 70 ml of water), then place the object into the water and see how much water it displaces (if the new volume is 100 ml, then 30 ml of water were displaced and that is the volume of the object). For smallerobjects, volume is typically measured in milliliters or cubiccentimeters.The hydrometer can measure the density of the liquiddirectly. An object that is less dense than a liquid will float inthat liquid density to a depth such that the mass of the objectsubmerged equals the mass of the of the liquid displaced(Archimedes' Principle). A hydrometer is a tube of constantmass that has been calibrated to measure density by floatingthe hydrometer in liquids of known densities and recording on a scale the fraction of the hydrometer submerged. Any hydrometer can be used over a limited range of densities because the hydrometer must float in the liquid being studied and the hydrometer level must be sufficiently submerged to obtain an on scale reading. Hydrometers may be calibrated in g/ml or some other unit of density. Figure above show how to read when using hydrometers.3. Precision and Accuracy:Chemists use special volumetric glassware in the chemistry laboratory (rather than the ordinary beakers an d flasks) when they need high-precision measurements of the volumes of liquids. Understanding the proper use and limitations of such glassware is important in achieving reliable results. Experimental technique and the inherent accuracy of the glassware both affect experimental results. One of your personal goals for this course is to develop sound experimental techniques.In this experiment you will practice using volumetric glassware and balance to improve your technique and to solve an important historical problem in practical chemistry.In any quantitative operation, one must always be concerned with both the precision and accuracy of the measurements. The precision is determined by how closely two or more measurements of the same quantity agree with each other. Random errors, unpredictable variations in measurements that result from fluctuations such as mechanical vibrations or differences in reading scales, are reflected in the precision of the data. The best way to evaluate the overall precision of an experiment is to repeat the entire experiment several times, varying the amounts of the substances used, but otherwise performing each operation the same way, and then to check the reproducibility of the data. The accuracy of a measurement refers to its degree of correspondence with a true or known value. Systematic errors, which result from such things as defective instrumentation or faulty lab technique, lead to inaccurate measurements. High precision might seem to imply high accuracy in a result, but this is not necessarily the case. If an instrument is improperly calibrated, for example, it may consistently give an inaccurate result with a high precision. Precision is not the same as accuracy.Every measuring device has a degree of precision, or uncertainty, associated with it. The general rule in using such a device is to estimate between the smallest calibrations of the measuring scale in order to obtain a reading with one more digit. The pieces of equipment you will use in the experiment have the following uncertainties:top-loading electric balance: ±0.01 g10 mL graduated cylinder: ±0.02 mL50 mL graduated cylinder: ±0.1 mL25 mL volumetric pipet: ±0.01 mL50 mL buret: ±0.01 mL4. Significant figures:The sensitivity of the instrument and the size of the sample govern the number of significant figures that can be reported. Th e significant figures in a number consist of all the digits known with certainty to be correct, plus one estimated digit. In other words, the last figure on the right should be the only approximate figure in the number. Always be sure your measurements include the last estimated digit, even if it is zero. It is important that a number which results from a calculation that incorporates more than one type of measurement does not claim a higher precision than was possible with the least precise measurement. In other words, the number of significant figures contained in the result cannot exceed the number of significant figures that are included in the least precise measurement. Keep this in mind when you read the ten-digit display on your calculator! Using the proper number of significant figures is a convenient way to estimate the uncertainty of a calculated result. The uncertainty will always be contained in the last digit of the result.The following are guidelines for using significant figures.1. All nonzero digits are significant, and zeroes between nonzero digits are significant. Zeroes to the left of the first nonzero digit are not significant.2. Trailing zeroes after a decimal point are always significant.3. Trailing zeroes before an implied decimal point are ambiguous and should be avoided by using scientific notation.4. In addition and subtraction calculations, the result carries the same number of decimal places as the quantity with the fewest decimal places.5. In multiplication and division calculations, the result carries the same number of significant figures as the factor with the fewest number of significant figures.6. When rounding to the correct number of significant figures, round down if the last (or leftmost) digit dropped is four or less; round up if the last (or leftmost) digit is five or more.7. To avoid rounding errors in multistep calculations, round only the final answer—do not round intermediate steps. If you write down intermediate steps, keep track of significant figures by underlining the least significant digit.Note: When a problem involves both addition or subtraction and multiplication or division, the answer may have a different number of significant figures than the initial quantities.5. Preparation of general-purpose solution:Solutions are usually prepared with respect to their molar concentrations (e.g. mol.L-1, mol.dm-3, or mol.m-3) or mass concentration (e.g. g.L-1, or kg.m-3); both can be regarded as an amount per unit volume, in accordance with the relationship:amount=concentrationvolumeIn general there are two levels of accuracy required for the preparation of solution: General-purpose solutions and Analytical solutions. The General-purpose solutions is the solution for chemicals used in qualitative and preparation procedure when the concentration of the chemical need not to be known more than one or two decimal places. For example:1) solution used in extraction and washing procedures, e.g. hydrochloric acid (0.1 mol.L-1),sodium carbonate (5% w/v)2) solutions for chemicals used in preparative experiments where the techniques of purification - distillation, recrystallization, filtration, etc - introduce intrinsic losses of substance that make accuracy to any greater level meaningless.Box below shows the steps involved in making up general-purpose aqueous solution. After weighting out the required mass or volume of chemical use distilled or deionized water or other solvent to make up solutions and stir with a clean glass rod or magnetic stirrers bar until all the chemical is dissolved. 'Obstinate' solutions may require heating, but only do this if you know that the chemical will not be damaged at the temperature used. Use a stirrer-heater to keep the solution mixed as you heat it. Allow the solution to cool to room temperature before you finalize its volume.6. Balances and weighing:Electronic single-pan balances with digital readouts are now favored over mechanical types and are common in most laboratories, There are essentially two types of balance:1) General purpose balances which weigh to the nearest 0.01 g with a capacity of about 200 g,Chemicals may be dispensed for weighing, into a suitable weighing container, directly onto these balances;2) Analytical four-figure balances for quantitative work, whichweigh to the nearest 0.0001 g (0.1 mg) and have a maximumcapacity of about 200 g, Chemicals must not be transferredonto the balance at any time and analytical balances usuallybe used for weighing by difference.Both types are illustrated in Figure below and you shouldfamiliarize yourself with their operation before use.Weighing - never weigh anything directly onto a balance's pan: you maycontaminate it for others. Use an appropriate weighing container such as aweighing boat, sample tube, weighing paper, conical flask, beaker.'Weighing paper' - It is common practice to put a piece of paper onto the pan ofgeneral-purpose balances, The mass of the paper is then 'tared off before theweighing container is placed on the balance pan. The paper protects the balancepan from corrosion by spillages and also allows you to discard easily any materialspilt without affecting the weighing.General-purpose balancesThe most useful feature of this type of balance is the electronic zero facility (self-taring), which means the mass of the weighing container can be subtracted automatically before weighing chemicals, To operate a standard self-taring balance:1. Check that it is level, using the adjustable feet to centre the bubble in the spirit level (usuallyat the back of the machine). For relatively accurate work or when using in a fume cupboard, make sure that the draught shield is in place.2. Ensure that the balance is switched on: the display should be lit.3. Place an empty weighing container centrally on the balance pan and allow the reading tostabilize, If the object is larger than the pan, take care that no part rests on the body of the balance or the draught shield as this will invalidate the reading. Press the tare bar to bring the reading to zero.4. Place the chemical or object carefully in the weighing vessel:(a) Solid chemicals should be dispensed with a suitably sized clean spatula.(b) Non-volatile liquids should be dispensed using a Pasteur pipette but take the weighingcontainer off the balance pan before dispensing; then reweigh the liquid plus container.Repeat until the desired weight is obtained.5. Allow the reading to stabilize and make a note of the reading.6. If you have added excess chemical. take great care when removing it. Remove the containerfrom the balance, remove the solid (with a spatula) or liquid (with a Pasteur pipette) andreweigh.7. If you need to clean any deposit accidentally left on or around the balance, switch off thebalance. Take care not to exceed the limits for the balance: while most have devices to protect against overloading, you may damage the mechanism.7. working with liquid:! Reading any volumetric scale - make sure your eye is level with the bottom of the liquid's meniscus and take the reading from this point.Measuring and dispensing liquidsThe equipment you should choose to measure out liquids depends upon the volume to be dispensed, the accuracy required and the number of times the job must be repeated.*If calibrated correctly and used properly;**Accuracy depends on width of barrel: large volumes less accurate;Conical flasks, beakers, measuring cylinders and volumetric flasks measure the volume of liquid contained in them, while burettes, pipettes, pipettors, syringes and micro syringes mostly measure the volume delivered from them: think about the requirements of the experiment.Certain liquids may cause problems:. High-viscosity liquids are difficult to dispense: allow time for the liquid to transfer.. Organic solvents may evaporate rapidly, making measurements inac-curate: work quickly; seal containers quickly.. Solutions prone to frothing (e.g. surfactant solutions) are difficult to measure and dispense: avoid forming bubbles; do not transfer quickly.Volumetric glassware is designed for two quite different purposes.⏹To deliver (TD) an accurate volume of liquid.⏹To contain (TC) an accurate volume of liquid.A container meant to contain an accurately known volume could of course be used to deliver the liquid to another vessel. However, the amount actually delivered would never exactly be equal to the amount originally in the container. Some liquid would inevitably remain behind, clinging to the interior walls. Some liquid also remains behind on the wallsof a piece of glassware that is TD designed, but the calibration of the TD glassware takes this into account.Pasteur pipettesHold correctly during use - keep the pipette vertical,with the middle finger gripping the barrel to support thepipette while the thumb and index finger provide controlledpressure on the bulb, and squeeze gently to provide individualdrops.To prevent liquid being sucked into the bulb and hencecross-contamination:. Ensure that the capacity of the bulb does not exceed that ofthe barrel.. Do not remove the tip of the pipette from the liquid whiledrawing up the liquid; the inrush of air may splash the liquidinto the bulb, This is particularly true when you lose patience trying to draw up viscous liquids, . Do not lie the pipette on its side during use.Conversely, if volatile liquids such as dichloromethane (DCM), ethanol, propanone (acetone) or diethylether (ether), for example, are to be dispensed, the warmth of the glass pipette will cause the liquid to squirt from the pipette without any pressure on the bulb. To prevent this, suck up the liquid several times into the pipette so as to cool the glass and then dispense as normal.Conical flasks and beakersThese have approximate graduations and should only be used for measuring volumes of solutions/liquids where accuracy is unimportant.Measuring cylinders and volumetric flasksThese must be used on a level surface (the laboratory bench) so that the scale is horizontal; you should first fill with solution until just below the desired mark, then fill slowly (e,g, using a Pasteur pipette) until the bottom of the meniscus is level with the mark, Remember to allow time for the solution to run down the walls of the vessel and tobend down so that your eyes are level with the graduationmark(s) and the meniscus.BurettesThese must be mounted vertically in a clamp - don'tover-tighten the clamp - or in a burette holder, on a stand, Firstensure that the tap is closed and, using a funnel, add a little ofthe solution to be dispensed, rinse the burette and discard thewashings through the tap: this is vital in titrations where a littlewater in the burette will alter the concentration of the solution,Refill the burette with solution, open the tap and allow the liquidto fill the barrel below the tap, then take a meniscus reading,noting the value in your notebook, Dispense the solution via the tap and measure the new meniscus reading, The volume dispensed is the difference between the two readings. PipettesThere are various designs, including graduated and bulb (volumetric) pipettes, Take care to look at the volume scale before use: some graduated pipettes empty from full volume to zero, others from zero to full volume; some scales refer to the shoulder of the tip, others to the tip by gravity, Never blowout volumetric (bulb) pipettes, just touch the tip against the inside wall of the vessel.Rinse out pipettes with a little of the solution to be delivered before commencing the accurate measurement. To prevent cross-contamination, never draw the solution into the pipette filler.KEY POINT For safety reasons, it is no longer permissible to mouth pipette - various aids (pipette fillers) are available, such as the rubber-bulb and Pi-Pump'".Pipettors (autopipettors)There are two basic types:1. Air displacement pipettors. For routine work with diluteaqueous solutions, One of the most widely used is the GilsonPipetman".2. Positive displacement pipettors, For non-standardapplications, including dispensing viscous, dense or volatileliquids where an air displacement pipettors might createaerosols leading to errors.Air displacement and positive displacement pipettors may be:●Fixed volume: capable of delivering a single factory-setvolume,●Adjustable: where the volume delivered is determined bythe operator across a particular range of values,●Pre-set: movable between a limited number of values.●Multichannel: able to deliver several replicate volumes at the same time.Whichever type of these routine but expensive devices you use, you must ensure that you understand the operating principles of the volume scale and the method for changing the volume delivered - some pipettors are easily misread.A pipettor must be fitted with the correct disposable tip before use and each manufacturer produces different tips to fit particular models, Specialized tips are available for particular applications.If you accidentally draw liquid into the barrel, seek assistance from your demonstrator/ supervisor since the barrel will need to be cleaned before further use (to prevent cross- contamination) and unskilled dismantling of the device will cause irreparable damage.Using syringes - take great care when handling syringe needles. They are very sharp and may be contaminated by Chemicals.SyringesSyringes should be used by placing the tip of the needle into the solution and slowly drawing the plunger up to the required point on the scale. Check the barrel to make sure no air bubbles have been drawn up, and expel the solution slowly, touching the needle tip on the side of the vessel to remove any adhering solution, If there is air in the barrel fill past the mark, invert the syringe and push the plunger to the mark so that the air and a little of the solution are expelled into a waste collection vessel. Then dispense the solution, The use of syringes for dispensing air-sensitive reagents is described latterly.Microsyringes should always be cleaned before and after use by repeatedly drawing up and expelling pure solvent. The dead space in the needle can occupy up to 4% of the nominal syringe volume, Some microsyringes have a fine wire attached to the plunger, which fills the dead space. Never pull the plunger out of the barrel.BalancesThese can be used to weigh accurately how much liquid you have dispensed. Convert mass to volume using the equation:Mass/density = volumee,g, a liquid (9.0g) of density (L2gmL- 1 ) = 7.5mL. Densities of common solvents and common chemicals can be found in Lide (2000), You will also need to know the liquid's temperature, since density is temperature dependent.。
有机化学专业词汇

Route [`raut]【路线】Strategy ['strætidʒi]【策略】Rearrangement [ˏri:ә'reindʒmәnt]【重排】Asymmetric ['eisi'metrik]【不对称】Migration [mai'greiʃәn]【迁移】Mechanism ['mekәnizәm]【机理】Substrate ['sʌbstreit]【底物、培养基】Synthesis ['sinθisis]【合成】Labile['leibail]【不稳定的】(累白了)Azeotropic[әˏzi:ә'trɔpik]【共沸的】(一个零就热供沸)Scheme [ski:m]【图示】Derivative [di'rivәtiv]【衍生物】Ana logous [ә'nælәgәs]【类似物】(相同话的人)Pri mary ['praimәri]【伯-】最前Secondary ['sekәndәri]【仲-】Tertiary ['tә:ʃәri]【叔-】Functional ['fʌŋkʃәnl] group【官能团】Catalyst [`kætәlist] 【催化剂】Catalysis [kә`tælisis]【催化(作用)】Catalytical [kætә`litikәl] 【催化的】Hydrogen o lysis [ˏhaidrәudʒә'nɔlәsis]【氢化】Hydrogenous [hai'drɔdʒinәs] 【含氢的】peri cyclic [peri'saiklik] reaction【周环反应】(围绕)transition [træn'ziʒәn] state 【过渡态】homo lysis [hɔ'mɔlisis] 【均裂】(同)hetero lysis [ˏhetә'rɔlisis] 【异裂】(杂,异)homo geneous [ˏhɔmәu'dʒi:njәs]【均相的】hetero geneous [ˏhetәrәu'dʒi:niәs] 【非均相的】Ste ric ['sterik] hindrance[`hindrәns] 【立体位阻】(死呆)Lipophilic [ˏlipә`filik]【亲脂性的】(力婆非礼亲子)chiral [`kai rәl] 【手性的】(开弱-手不好)electro phile [i`lektrәfai l] 【亲电试剂】nucleo philic [ˏnju:kliәu'filik]【亲核的】hydro lysis [hai`drɔlisis] 【水解(作用)】acido lysis [ˏæsi`dɔlisis] 【酸解(作用)】displacement [dis'pleismәnt] 【取代】formalin ['fɔ:mәlin] 【甲醛溶液】di lute [dai'lju:t] 【稀释】(洗两次)de hydrate [di:'haidreit] 【脱水】dilat ion [dai'leiʃәn]【膨胀】lat-吹saponi fication [sәpɔnifi'keiʃәn]【皂化】sapo=soapemulsi fication [iˏmʌlsifi'keiʃәn] 【乳化】姨妈是如花reactant [ri:'æktәnt]【反应物】add uct [ә'dʌkt]【加合物】dis solve [di'zɔlv]【溶解】开,分散de grad ation [ˏdegrә'deiʃәn]【降解】mild [maild]【(反应)温和的】harsh [hɑ:ʃ] 【(反应)苛刻的】吓死race mic [rә'si:mik]【消旋的】弱市米小,酸substit uent [sʌb'stitjuәnt]【取代基】con juga tion [ˏkɔndʒu'geiʃәn]【偶和】连接在一起,共轭equi valent [i'kwivәlәnt]【当量的】vola tile ['vɔlәtail]【易挥发的】找着钛会发suscept ible [sә'septәbl]【易受影响的】想法不坚定in large scale【大量】prote ase ['prәutieis]【蛋白酶】不如踢死担百媚side chain【侧链】Im puritiy [im'pjuәriti]【杂质】Component [kәm'pәunәnt]【组分】Tare[tєә]【去皮】太恶去皮Syringe ['sirindʒ]【注射器】湿润着注射器Con cent rate ['kɔnsentreit]【浓缩】Aqueous ['eikwiәs]【水相(的)】一个哭儿跟水似的Method ology [meθә'dɔlәdʒi]【方法学】Pre cur sor [pri(:)'kә:sә] 【前体】走Separation [sepә'reiʃәn]【分离】Purification [ˏpjuәrifi'keiʃәn]【纯化】Re crystal lization ['rikristәlai'zeiʃәn]【重结晶】Crystal line ['kristәlain]【晶体】Di still ation [ˏdisti'leiʃәn]【蒸馏】(强调-保持(温度)Sublim ation [ˏsʌbli'meiʃәn] 【升华】低-极限Microwave ['maikrәuweiv]【微波】Infra red ['infrә'red]【红外】ultra violet ['ʌltrә'vaiәlit] active【紫外显色】iso tope ['aisәutәup] 【同位素】(不同类型)iso mer ['aisɔmә] 【同分异构体】Isomerization [aiˏsɔmәrai'zeiʃәn]【异构化】Trans formation [ˏtrænsfә'meiʃәn]【转化】Stability [stә'biliti]【稳定性】Poison ed ['pɔiznd]【中毒的】Filter ['filtә]【过滤器】Filtration [fil'treiʃәn]【过滤】Filtrate ['filtreit]【滤液】Filter Cake【滤饼】Eluent['eljuәnt]【淋洗液】流出Thin-layer-chromato graphy [ˏkrәumә'tɔgrәfi] TLC【薄层色谱】可柔摸偷-色Preparative [pri'pærәtiv] TLC【制备薄层色谱】HPLC【高效液相色谱】LC-MS【液质联用仪】Prot ic ['prәutik]【质子的】Apro tic[ә'prәutik]【非质子的】De compose [ˏdi:kәm'pәuz]【分解】Removal [ri'mu:vәl]【除去】Re duce d [ri'dju:st] pressure 【减压】Vac uum ['vækjuәm] 【真空】E limination [iˏlimi'neiʃәn]【消除】(限制出)Addition [ә'diʃәn]【添加、加成】Ligand ['laigәnd]【配体】(来真的,不要配体)Stop per ['stɔpә]【塞子】(阻止外物进入)Separatory/ addition/ Büchner funnel ['fʌnәl]【分液/加料/布什】发闹-露头(警察)column chromatography【柱层析】Run column【用柱层析分离】Stopcock ['stɔpkɔk] 【旋塞阀】Condenser[kәn'densә]【冷凝管】(气体-液体,浓缩)Cham ber ['tʃeimbә]【(薄层用)展缸】cham-小室Bench [bentʃ] 【实验台】Hood [hud] 【通风橱】呼呼的Rotavapor[ˏrәutә'veipә]【旋蒸】Evapor ation [iˏvæpә'reiʃәn] 【蒸发】off,气体vac uum hose [hәuz]【真空管】吼诗-管sili con ['silikәn] oil bath 【(硅)油浴】失利肯跪sink [siŋk]【水槽】深刻-水槽中下降neutral ize ['nju:trәlaiz]【中和】stir bar 【搅拌子】Dry ice trap【干冰冷阱】Glove box [glʌvә'bɔks]【手套箱】vacuum pump [pʌmp]【真空泵】spatula ['spætjulә] 【刮刀】失败丢了刮刀misci ble ['misibl]【混溶的】迷死薄毁容Quench [kwentʃ]【淬灭】Purging ['pә:dʒiŋ]【鼓气】破阵-鼓气Titration [tai'treiʃәn]【滴定】太脆-一滴定Tritu ration [tritju'reiʃәn]【研磨】锤丢不能研磨Dessi cator ['desikeitә:]【干燥剂】逮湿给他自己Hygroscopic[ˏhaigrәu's kɔpik] 【吸湿的】水+进自己的范围Lachrymator['lækriˏmeitә]【催泪剂】来客追模特用催泪弹DI (de-ion) water【去离子水】Stoichio metric [ˏstɔikiәuˋmєtrik]【化学量的】石洞跟偶滑雪Volum etric [vɔlju'metrik] 【测体积的】Weighing ['weiiŋ] paper【称量纸】Mechanical [mi'kænikl] stirring apparatus [ˏæpә'reitәs]【机械搅拌装置】heating mental ['mentl] 【加热套】Eclipse [i'klips] 【使失色】一客离谱失色Anomeric ['ænәˏmerik] Effect 【端基异构效应】Equatorial [ˏekwә'tɔ:riәl]【平伏(键)的】Axial['æksiәl]【直立(键)的】埃克斯直立Gauche [gәuʃ] 【构象】构思-笨-像Thermodynamic['θә:mәudai'næmik]【热力学的】舍帽逮奶热Kinetic [kai'netik]【动力学的】开奶-动力Enantio selective [eˏnæntiәsi'lektiv]【对映选择性的】爱奶提鹅腿硬Diastereo topic [ˏdaiәstiәriә'tɔpik] 【非对映的】Chemoselectivity 【化学选择性】Regio selective ['ri:dʒәu si'lektiv] 【区域选择性的】遇贼区域Stereo selectivity [ˏstiәriәˏsilek'tiviti]【立体选择性】Synthon['sinθɔn]【合成子】Nitro sation [naitrә'seiʃә:n] 【亚硝化】Tauto mer ['tɔ:tәmә]【(互变)异构体】湖边逃特魔Con firm ation [ˏkɔnfɔ:'meiʃәn] 【构型】Equi librium [ˏi:kwi'libriәm]【平衡】pyro lysis [ˏpaiә'rɔlisis] 【热解】派鹅肉-热ozono lysis [ ,әuzәu'nɔlisis ] 【臭氧分解】藕肉臭conversion [kәn'vә:ʃәn] ratio ['reiʃiәu] 【转化率】Deprotection【脱保护】Introduction【引入】Cleavage【分离,脱除】。
利用纤维素酶水解碱处理稻草生产葡萄糖

第61卷 第3期吉林大学学报(理学版)V o l .61 N o .32023年5月J o u r n a l o f J i l i nU n i v e r s i t y (S c i e n c eE d i t i o n )M a y2023d o i :10.13413/j .c n k i .jd x b l x b .2022291利用纤维素酶水解碱处理稻草生产葡萄糖顾天华1,曹亚群2,李烜琦1,任晓冬1(1.吉林大学生命科学学院,长春130012;2.内蒙古自治区城乡人居环境发展促进中心,呼和浩特010020)摘要:针对木质纤维素结构复杂㊁难以降解利用,焚烧时导致环境污染的问题,利用纤维素酶将木质纤维素中的纤维素水解为葡萄糖.首先,使用N a O H 溶液对稻草进行预处理;其次,对预处理前后的稻草进行扫描电镜㊁F o u r i e r 变换红外光谱和X 射线衍射分析;最后,使用A s p e r g i l l u s n i ge r Q 7生产的纤维素酶水解预处理稻草.结果表明:预处理后的稻草纤维结构被打开,表面积增大,结晶度上升;预处理稻草纤维素含量上升,半纤维素和木质素含量下降;预处理后比预处理前的稻草产生的葡萄糖量高.关键词:稻草;预处理;纤维素;纤维素酶;酶解中图分类号:Q 93 文献标志码:A 文章编号:1671-5489(2023)03-0702-05P r o d u c t i o no fG l u c o s eU s i n g C e l l u l a s eH y d r o l yz e dA l k a l i T r e a t m e n t f o rR i c e S t r a wG U T i a n h u a 1,C A O Y a q u n 2,L IX u a n q i 1,R E N X i a o d o n g1(1.S c h o o l o f L i f eS c i e n c e s ,J i l i nU n i v e r s i t y ,C h a n gc h u n 130012,C h i n a ;2.I n n e rM o n g o l i aA u t o n o m o u sR e gi o nU r b a na n dR u r a lH u m a n S e t t l e m e n t E n v i r o n m e n tD e v e l o p me n tP r o m o t i o nC e n t e r ,H o h h o t 010020,C h i n a )收稿日期:2022-06-27.第一作者简介:顾天华(1998 ),女,满族,硕士研究生,从事生物与医药的研究,E -m a i l :t h g u 20@m a i l s .jl u .e d u .c n .通信作者简介:任晓冬(1970 ),男,汉族,博士,副教授,从事生物与医药的研究,E -m a i l :r e n x i a o d o n g @m a i l s .jl u .e d u .c n .基金项目:内蒙古自治区科技计划项目(批准号:2019G G 032).A b s t r a c t :A m i n g a t t h e p r o b l e mt h a t t h e l i g n o c e l l u l o s eh a dac o m pl e xs t r u c t u r ea n dw a sd i f f i c u l t t o d e g r a d ea n d u t i l i z e ,r e s u l t i n g in e n v i r o n m e n t a l p o l l u t i o n w h e ni t b u r n e d ,w e u s e d c e l l u l a s et o h y d r o l y z ec e l l u l o s ef r o m l i g n o c e l l u l o s ei n t o g l u c o s e .F i r s t l y ,t h er i c es t r a w w a s p r e t r e a t e d u s i n g s o d i u mh y d r o x i d e s o l u t i o n .S e c o n d l y ,t h e r i c e s t r a wb e f o r ea n da f t e r t h e p r e t r e a t m e n tw a sa n a l yz e d b y s c a n n i n g e l e c t r o n m i c r o s c o p y ,F o u r i e rt r a n s f o r m i n f r a r e ds p e c t r o s c o p y ,a n d X -r a y di f f r a c t i o n .F i n a l l y ,t h ec e l l u l a s e p r o d u c e db y A s p e r g i l l u sn i ge r Q 7w a su s e dt oh y d r o l y z ea n d p r e t r e a tr i c e s t r a w.T h er e s u l t ss h o wt h a taf t e r p r e t r e a t m e n t ,t h ef i b e rs t r u c t u r eo fr i c es t r a wi so p e n e d ,t h e s u r f a c e a r e a i n c r e a s e s ,a n dt h ec r y s t a l l i n i t y in c r e a s e s .T h ec e l l u l o s ec o n t e n t i nr i c es t r a wi n c r e a s e s a f t e r p r e t r e a t m e n t ,w h i l e t h ec o n t e n to fh e m i c e l l u l o s ea n d l i g n i nd e c r e a s e s .T h e g l u c o s ec o n t e n to f p r e t r e a t e d r i c e s t r a wi s h i g h e r t h a n t h a t o f u n pr e t r e a t e d r i c e s t r a w.K e yw o r d s :r i c e s t r a w ;p r e t r e a t m e n t ;c e l l u l o s e ;c e l l u l a s e ;e n z y m o l y s i s 木质纤维素是一种丰富的自然资源,全球每年产量超过两千亿吨,但其结构复杂,难以降解利用.目前,大部分的木质纤维素以焚烧和堆积等方式处理,不但浪费资源,而且污染环境[1].木质纤维素Copyright ©博看网. All Rights Reserved.主要由纤维素㊁半纤维素和木质素3种成分组成,其中半纤维素和木质素相互缠绕,将纤维素包裹,形成结晶结构,这种复杂的空间结构使木质纤维素不易降解.为利用木质纤维素,需先破坏木质纤维素的晶体结构,再用纤维素酶降解[2].目前,对木质纤维素预处理方法主要有物理法㊁化学法和生物法等[3].稻草中的主要成分为木质纤维素.本文采用N a O H 对稻草进行预处理,分别用扫描电镜(S E M )㊁F o u r i e r 变换红外光谱(F T I R )和X 射线衍射(X R D )对预处理的稻草进行分析,研究N a O H预处理对秸秆结构的影响,并利用纤维素酶水解预处理稻草生产葡萄糖,为利用稻草生产生物化工产品提供了理论基础.1 实 验1.1 仪器和试剂Z Q L Y -180V 型立式全温震荡培养箱(上海知楚仪器有限公司);S P -722E 型可见分光光度计(上海光谱仪器有限公司);HH -6A 型恒温水浴锅(上海皓庄仪器有限公司);K Y K Y -E M 3900型扫描电子显微镜(北京中科科仪股份有限公司);N i c o l e t 6700型F o u r i e r 红外光谱(美国,T h e r m oF i s h e r 公司);E m p y r e a n 型X 射线晶体衍射仪(荷兰,P A N a l yt i c a l B V 公司);T G A P T 1000型热重分析仪(上海林赛斯科学仪器有限公司).P D L 培养基:土豆洗净去皮,切成小块,取200~300g 熬汁,用8层纱布过滤出汁液,加入葡萄糖20g ,用去离子水定容至1000m L .产酶培养基:配制含蛋白胨3g /L ,酵母提取物0.5g /L ,K H 2P O 43g /L ,(N H 4)2S O 42g /L ,尿素0.3g /L ,M g S O 40.3g /L ,C a C O 35g/L ,气爆秸秆30g /L 的溶液,调节溶液p H=5.5.1.2 纤维素酶制备从土壤中分离纯化一株产纤维素酶的菌株A s p e r g i l l u s n i g e r Q 7.将A .n i ge r Q 7接种到40m L P D L 培养基中,于30ħ,130r /m i n 条件下培养3d .再按5%接种量接种到产酶培养基中,于30ħ,130r /m i n 条件下培养5d .将发酵液于8000r /m i n 离心15m i n ,弃沉淀,收集上清液为粗酶液.1.3 纤维素酶纯化利用70%相对饱和度的(NH 4)2S O 4进行沉淀,于4ħ盐析2h 后,于4ħ,8000r /m i n 离心30m i n ,弃上清液.使用少量缓冲液(pH=5.0)溶解沉淀,于4ħ保存.将获得的酶转移至透析袋中(相对分子质量截留10000),透析除盐,4ħ保存备用[4].1.4 酶活力测定采用滤纸酶活测定法[5]测酶活力.滤纸酶活力定义为:在温度50ħ,p H=4.8条件下,每分钟水解底物产生1μg 还原糖所需的酶量,定义为一个酶活力单位(U ).1.5 稻草预处理将30g 稻草粉末加入500m L 蓝盖瓶中,加入质量分数为1%的N a O H 溶液300m L ,用双层牛皮纸包扎,放入高压灭菌器中,于121ħ反应60m i n .待反应液降至室温后取出,用8层纱布过滤预处理液,用蒸馏水反复洗涤预处理后的稻草,室温下晾干,于-20ħ保存备用.1.6 预处理稻草的S E M 分析用S E M 对预处理稻草表面形貌进行分析.取少量预处理后的稻草,在样品台上固定双面导电胶带,于真空条件下进行镀金处理.待样品表面均匀镀上一层导电膜后,将样品置于扫描电子显微镜下,在10k V 和15m A 下,放大400倍和2000倍进行观察.1.7 预处理稻草的F T I R 分析取预处理后的稻草,与溴化钾以1ʒ100的质量比混匀,用玛瑙研钵研磨至粉末状,取少量样本在压片机上制成薄片.利用F o u r i e r 红外光谱仪进行分析,扫描波长范围为400~4000c m -1,光谱分辨率为4c m -1,单一样品进行50次扫描.1.8 预处理稻草的X R D 分析取预处理样品用X 射线衍射仪进行结晶度分析.对预处理稻草进行扫描,工作电压和电流分别为307 第3期 顾天华,等:利用纤维素酶水解碱处理稻草生产葡萄糖Copyright ©博看网. All Rights Reserved.407吉林大学学报(理学版)第61卷40k V和40m A,扫描范围(2θ)为5ʎ~40ʎ,扫描速度2ʎ/m i n.样品结晶度(I C r)通过(1)I C r=I002-I a mI002ˑ100%计算,其中I002为纤维素结晶区衍射角的峰值,I a m为无定形区衍射角的峰值.1.9稻草成分分析按照美国国家可再生能源实验室(N R E L)的L A P002,003,004方法进行分析[6].1.10利用纤维素酶降解预处理前后的稻草向20m L的磷酸氢二钠-柠檬酸缓冲液(p H=5)中分别加入50m g预处理前后的稻草,按20U/g纤维素加入纤维素酶,于50ħ水浴振荡,分别在12,18,24,30,36h取样,利用葡萄糖试剂盒法测量葡萄糖的质量浓度.2结果与讨论2.1预处理前后稻草的成分分析按照N R E L方法对预处理前后的稻草进行成分分析,结果列于表1.由表1可见,经质量分数为1%N a O H预处理的稻草,木质素的质量分数降低,有助于纤维素酶吸附到纤维素上,从而促进纤维素降解成葡萄糖.表1预处理前后稻草的成分分析T a b l e1C o m p o n e n t a n a l y s i s o f r i c e s t r a wb e f o r e a n da f t e r p r e t r e a t m e n t %处理方式w(纤维素)w(半纤维素)w(木质素)w(灰分)未处理37.4030.4418.471.00 w(N a OH)=1%58.8724.3915.300.702.2S E M分析结果用S E M观察预处理前后稻草的结构,结果如图1所示.由图1(A),(B)可见,未处理的稻草表面结构平整光滑.经N a O H预处理后,稻草表面结构被破坏,出现孔洞,纤维断裂弯曲,表明天然木质纤维素结构被破坏.可见碱预处理可有效降解植物细胞壁中的木质素[7].稻草中半纤维素㊁纤维素和木质素缠绕在一起,形成紧凑结构,去除木质素可大幅度提高纤维素的降解率[8].图1预处理前后稻草的S E M照片F i g.1S E Mi m a g e s o f r i c e s t r a wb e f o r e a n da f t e r p r e t r e a t m e n t2.3F T I R结果分析利用F T I R分析预处理前后的稻草,结果如图2所示.由于稻草为混合物,本文以羟基的O HCopyright©博看网. All Rights Reserved.伸缩振动3410c m -1为强度标度归一化比较其他吸收带.由图2可见:3300~3500c m -1处振动来自羟基的O H 伸缩振动模式,其强吸收和较宽的吸收带表明稻草分子内或分子间可能产生了氢键[9];2848,2916c m -1处的吸收带为亚甲基的对称伸缩和不对称伸缩振动吸收峰,相比于羟基峰,预处理后明显少于预处理前稻草的亚甲基含量,这可能是因为部分纤维素的糖环在处理后被打开;1728c m -1处的吸收带为羰基碳氧双键的伸缩振动[10],与羟基相比,预处理后明显比预处理前稻草羰基的含量增多,预处理前稻草在1251c m -1(羧基或酯羰基的碳氧单键伸缩振动)处未出现吸收峰,但处理后的稻草在该处出现了吸收峰,推断羰基可能来自纤维素糖环打开后,亚甲基氧化为羧基,部分亚甲基被氧化成了羧酸;1621,1531c m -1处的峰是苯环的碳碳双键伸缩振动峰,是木质素特征峰,由于处理后仅失去部分木质素,因此该峰仍存在于F T I R 中.2.4 X R D 分析结果木质纤维素中有结晶区和非结晶区.结晶度是木质纤维素聚集形成结晶程度的指标[11].对预处理前后的稻草进行X 射线晶体衍射分析,并计算结晶度,结果如图3所示.经N a O H 预处理的稻草结晶度为43.36%,而未预处理的稻草结晶度为18.51%.这是由于预处理后,木质纤维素的非结晶成分溶出,导致稻草整体的结晶度升高[12].图2 预处理前后稻草的F T I RF i g.2 F T I Ro f r i c e s t r a wb e f o r e a n da f t e r p r e t r e a t m e nt 图3 预处理前后稻草的X R D 谱F i g.3 X R D p a t t e r n s o f r i c e s t r a wb e f o r e a n da f t e r p r e t r e a t m e n t2.5 利用纤维素酶酶解预处理前后稻草的结果利用A .n i g e r Q 7纤维素酶分别水解预处理前后稻草,水解时间分别为12,18,24,30,36h .预处图4 A .n i ge r Q 7纤维素酶水解预处理前后稻草生产葡萄糖的时间进程曲线F i g.4 T i m e c o u r s e c u r v e s o f g l u c o s e p r o d u c t i o n f r o m r i c e s t r a wb e f o r e a n da f t e r p r e t r e a t m e n t w i t h A .n i g e r Q 7c e l l u l a s e h y d r o l ys i s 理前后稻草水解产生葡萄糖的质量浓度如图4所示.由图4可见,水解预处理后稻草的葡糖糖产量始终高于水解预处理前稻草的葡萄糖产量[13].综上所述,本文用质量分数为1%的N a O H 对稻草进行预处理,稻草中纤维素质量分数从37.40%提高至58.87%,半纤维素质量分数为24.39%,木质素质量分数为15.30%.对预处理前后的稻草进行分析,S E M 结果表明,预处理后的稻草表面出现塌陷,纤维断裂弯曲,木质纤维素原有的结构被破坏;X R D 结果表明,预处理后稻草的结晶度大幅度提高.用从土壤中分离纯化的A .n i ge r Q 7产生的纤维素酶水解稻草,预处理稻草比未预处理稻草产生葡萄糖的质量比提高了47.5%.结果表明,预处理稻草中的木质素被降解,稻草结构发生显著改变,水解效率显著提高.因此,先用N a O H 预处理稻草,再用纤维素酶水解稻草生产葡萄糖是稻草利用的有效方式.507 第3期 顾天华,等:利用纤维素酶水解碱处理稻草生产葡萄糖Copyright ©博看网. All Rights Reserved.607吉林大学学报(理学版)第61卷参考文献[1]刘亦陶,魏佳,李军.废弃生物质水热炭化技术及其产物在废水处理中的应用进展[J].化学与生物工程,2019,36(1):1-10.(L I U YT,W E I J,L I J.P r o g r e s s i nH y d r o t h e r m a l C a r b o n i z a t i o n o fW a s t e B i o m a s s a n dA p p l i c a t i o n o fB i o c h a r i n W a s t e w a t e rT r e a t m e n t[J].C h e m i s t r y&B i o e n g i n e e r i n g,2019,36(1):1-10.)[2] P E D E R S E N M,V I K SØ-N I E L S E N A,M E Y E R A S.M o n o s a c c h a r i d eY i e l d sa n dL i g n i n R e m o v a l f r o m W h e a tS t r a wi nR e s p o n s e t oC a t a l y s tT y p e a n d p Hd u r i n g M i l dT h e r m a l P r e t r e a t m e n t[J].P r o c e s sB i o c h e m i s t r y,2010, 45(7):1181-1186.[3]张鑫,刘岩.木质纤维原料预处理技术的研究进展[J].纤维素科学与技术,2005,13(2):54-58.(Z HA N G X,L I U Y.A d v a n c e o fL i g n o c e l l u l o s e sP r e t r e a t m e n tT e c h n o l o g y[J].J o u r n a l o fC e l l u l o s eS c i e n c ea n dT e c h n o l o g y, 2005,13(2):54-58.)[4] F A R J A N AI,N A R A Y A N R.S c r e e n i n g,P u r i f i c a t i o na n dC h a r a c t e r i z a t i o no fC e l l u l a s e f r o m C e l l u l a s eP r o d u c i n gB a c t e r i a i n M o l a s s e s[J].B M CR e s e a r c hN o t e s,2018,11(1):445-1-445-6.[5]张瑞萍.纤维素酶的滤纸酶活和C M C酶活的测定[J].印染助剂,2002,19(5):51-53.(Z HA N G R P.D e t e r m i n a t i o no f F i l t e r P a p e rE n z y m a t i c A c t i v i t y a n d C M C E n z y m a t i c A c t i v i t y o f C e l l u l a s e[J].T e x t i l eA u x i l i a r i e s,2002,19(5):51-53.)[6]任广跃,毛志怀,李栋.秸秆饲用处理及其有效利用的研究进展[J].粮食与饲料工业,2004,1(7):29-30.(R E N G Y,MA O Z H,L ID.R e s e a r c hP r o g r e s s i nt h eF e e d O r i e n t e d T r e a t m e n to fS t r a w a n dI t sE f f e c t i v e U t i l i z a t i o n[J].C e r e a l&F e e d I n d u s t r y,2004,1(7):29-30.)[7]李雅丽,高锦红,王丽.碱处理对农作物秸秆中木质素含量的影响[J].化学与生物工程,2017,34(10):58-60.(L IYL,G A OJH,WA N GL.E f f e c t s o fA l k a l i T r e a t m e n t o nL i g n i nC o n t e n t i nC r o p S t r a w[J].C h e m i s t r y&B i o e n g i n e e r i n g,2017,34(10):58-60.)[8] HA Q U E M A,B A R MA N D N,K A N G T H,e t a l.E f f e c t o fD i l u t eA l k a l i P r e t r e a t m e n t o nS t r u c t u r a lF e a t u r e sa n dE n h a n c e dE n z y m a t i cH y d r o l y s i s o f M i s c a n t h u s s i n e n s i s a t B o i l i n g T e m p e r a t u r ew i t hL o wR e s i d e n c eT i m e[J].B i l o s y s t e m sE n g i n e e r i n g,2013,114(3):294-305.[9]郑明霞,李来庆,郑明月,等.碱处理对玉米秸秆纤维素结构的影响[J].环境科学与技术,2012,35(6):27-31.(Z H E N G M X,L I LQ,Z H E N G M Y,e t a l.E f f e c t s o fA l k a l i P r e-t r e a t m e n t o nC e l l u l o s i c S t r u c t u r a l C h a n g e s o fC o r nS t o v e r[J].E n v i r o n m e n t a l S c i e n c e&T e c h n o l o g y,2012,35(6):27-31.)[10] G A S T A L D IG,C A P R E T T IG,F O C H E RB,e t a l.C h a r a c t e r i z a t i o n a n dP r o p r i e t i e s o f C e l l u l o s e I s o l a t e d f r o mt h eC r a m b e a b b y s s i n i c a h u l l[J].I n d u s t r i a l C r o p s a n dP r o d u c t s,1998,8(3):205-218.[11]刘书钗.制浆造纸分析与检测[M].北京:化学工业出版社,2004:13-17.(L I U SC.A n a l y s i s a n dT e s t i n g o fP u l p i n g P a p e r[M].B e i j i n g:C h e m i c a l I n d u s t r y P r e s s,2004:13-17.)[12]唐洪涛,王锋,李伟明,等.γ射线辐照与N a OH溶液协同预处理对玉米秸秆酶解产糖率及微观结构的影响[J].核农学报,2021,26(3):535-542.(T A N G H T,WA N GF,L IW M,e t a l.E f f e c t s o fγ-R a y I r r a d i a t i o n a n d N a OH S o l u t i o nS y n e r g i s t i cP r e t r e a t m e n t o nS u g a rY i e l d a n dM i c r o s t r u c t u r e o f C o r nS t o v e rE n z y m a t i cH y d r o l y s i s [J].J o u r n a l o fN u c l e a rA g r i c u l t u r a l S c i e n c e s,2021,26(3):535-542.)[13]张晓君,赵志海,刘爽,等.稻草半纤维素预提取对浆料性能的影响[J].吉林大学学报(理学版),2014,52(2):360-363.(Z HA N G XJ,Z HA O Z H,L I U S,e ta l.I m p a c to fH e m i c e l l u l o s eP r e-e x t r a c t i o no fR i c eS t r a wo nC o o k i n g P u l p P r o p e r t i e s[J].J o u r n a l o f J i l i nU n i v e r s i t y(S c i e n c eE d i t i o n),2014,52(2):360-363.)(责任编辑:单凝)Copyright©博看网. All Rights Reserved.。
酶专业外语学习

We now come to the most remarkable and highly specialized proteins, the enzymes. Enzymes are the reaction catalysts of biological systems. They have extraordinary catalytic power, often far greater than that of synthetic catalysts. They have a high degree of specificity for their substrates, they accelerate specific chemical reactions, and they function in aqueous solutions under very mild conditions of temperature and pH. Few nonbiological catalysts show all these properties.我们现在来最显着的和高度专业化的蛋白质,酶。
酶是生物系统的反应的催化剂。
他们具有非凡的催化能力,往往远远大于合成催化剂。
它们具有高度的专一性,它们的底物,它们加速具体的化学反应,并在非常温和的条件下的温度和pH值下,它们在水溶液中的功能。
很少有非生物催化剂显示所有这些属性。
Enzymes are one of the keys to understanding how cells survive and proliferate. Acting in organized sequences, they catalyze the hundreds of stepwise reactions in metabolic pathways by which nutrient molecules are degraded, chemical energy is conserved and transformed, and biological macromolecules are made from simple precursors. Some of the many enzymes participating in metabolism are regulatory enzymes, which can respond to various metabolic signals by changing their catalytic activity accordingly. Through the action of regulatory enzymes, enzyme systems are highly coordinated to yield a harmonious interplay among the many different metabolic activities necessary to sustain life.酶是了解细胞存活和增殖的关键之一。
芳烃论文:芳烃胺甲基化酰胺甲基化一锅法

芳烃论文:芳烃胺甲基化酰胺甲基化一锅法【中文摘要】胺甲基化反应是众多化学反应中非常重要的一类反应,尤其是曼尼希(Mannich)胺甲基化反应。
利用Mannich反应可以合成出许多在医药、涂料、香料、有机助剂、农药等行业有重大应用价值的医药中间体、精细有机化学品及一般方法难以合成的化合物。
本文选择廉价的多聚甲醛和乙酰胺或氯乙酰胺作为酰胺甲基化试剂,在酸催化下,采用一锅法,对芳香化合物的酰胺甲基化反应进行了研究,该反应也属于Mannich反应。
但一般Mannich反应都是以醛、酮或含活泼氢的芳烃作为酸性组分,二级胺为碱性组分。
而本文以芳烃为酸性组分,不仅包括活泼芳烃,还包括钝化芳烃、杂环芳烃,以一级酰胺为碱性组分,这种从二级胺到一级酰胺的变换是一种非常有用的改变,不仅阻止了一级胺的多次胺甲基化,也是一种一步合成酰胺甲基芳环化合物的有效方法。
该类酰胺甲基化合物非常稳定、易于保存,在酸性或碱性条件下可水解成伯胺甲基芳环化合物,从而对伯胺甲基芳环化合物起到了保护作用。
通过对芳香化合物的酰胺甲基化反应的研究,找到了部分芳环酰胺甲基化反应的最佳条件,同时也确立了一条经芳环酰胺甲基化反应,再水解制备伯胺甲基芳环的路线。
该方法操作简单,原料便宜,反应条件温和,后处理简单,产率适中。
本文合成了N-(2-甲基-5-硝基苄基)乙酰胺、N-(2,5-二甲基苄基)乙酰胺、2,5-二(乙酰胺甲基)对二甲苯、N-(2,4-二甲基苄基)乙酰胺、4,6-二(乙酰胺甲基)间二甲苯、N-(3,4-二甲基苄基)乙酰胺、N-(2,5-二氟苄基)乙酰胺、N-(4-甲基苄基)乙酰胺、N-(4-氯苄基)乙酰胺、N-(2-氯-5-三氟甲基苄基)乙酰胺、2-氯-5-乙酰胺甲基苯甲酸、4,4′-二氯乙酰胺甲基联苯、4-乙酰胺甲基联苯、α-乙酰胺甲基萘、2-乙酰胺甲基噻吩等。
其次,本文还考察了酰胺甲基芳烃的水解,合成了4,4′-二氨甲基联苯盐酸盐、2-甲基-5-硝基苄胺盐酸盐、2,5-二甲基苄胺盐酸盐、2,5-二氟苄胺盐酸盐、2,5-二胺甲基对二甲苯盐酸盐。
要保持电器干燥的英语作文

要保持电器干燥的英语作文Electricity and water don't mix well, so keeping your electronics dry is crucial. Think of it like this: your phone or laptop is like a delicate flower – it needs to be sheltered from the rain!Imagine you're at the beach, and suddenly a wave hits your phone. Oops! That's not good. The saltwater can corrode the metal parts inside, and the moisture can mess with the circuitry. So, always remember to stash your gadgets in a waterproof bag or case when you're near any wet stuff.And let's not forget about humidity. High humidity can be a sneaky enemy for electronics. It can cause condensation on the internal parts, leading to all sorts of issues. So, if you live in a humid area, consider investing in a dehumidifier to keep your home – and your electronics – dry as a bone.Now, let's talk about spills. Whether it's coffee, tea, or even soda, liquids and electronics just don't get along. If you accidentally spill something on your device, don't panic! Turn it off immediately and gently blot the liquid with a soft, dry cloth. Don't use any harsh chemicals or blowers, as they can further damage the electronics.In the end, it's all about being proactive. Keep your electronics away from any potential sources of moisture, and they。
瓶装水既有好处英语作文

瓶装水既有好处英语作文Bottled water has several advantages. First of all, it is convenient and portable, making it easy to stay hydrated while on the go. Additionally, bottled water is usually purified and filtered, ensuring that it is safe to drink. Furthermore, many bottled water brands offer different sizes and varieties to cater to individual preferences. Lastly, bottled water is often readily available in stores, making it easily accessible in times of need.Despite these advantages, there are also some drawbacks to bottled water. One major issue is the environmental impact of plastic bottles. The production and disposal of plastic bottles contribute to pollution and harm wildlife. Additionally, bottled water can be expensive compared to tap water, which is a more sustainable and cost-effective option. Furthermore, the quality of bottled water can vary dependingon the brand, leading to concerns about consistency and transparency in labeling.In conclusion, while bottled water has its benefits in terms of convenience and safety, it is important to consider the environmental and financial consequences. By being mindful of these drawbacks and making informed choices, we can better balance the advantages and disadvantages of bottled water in our daily lives.。
解决缺水的办法英文作文

解决缺水的办法英文作文英文:As a global issue, water scarcity has become a serious problem for many countries. To solve this problem, there are several ways we can consider.Firstly, we can improve water conservation by using water-saving technologies. For example, low-flow showerheads, dual-flush toilets, and efficient irrigation systems can help reduce water usage. Additionally, we can also promote water conservation through education and awareness campaigns to change people's behavior and attitudes towards water usage.Secondly, we can increase water supply by exploring alternative sources of water. For instance, desalination, rainwater harvesting, and wastewater treatment can provide additional sources of water. Furthermore, we can also establish water storage facilities such as dams andreservoirs to store water during rainy seasons for use during dry seasons.Lastly, we can also reduce water demand by changing our consumption patterns. For example, we can reduce meat consumption as it takes a lot of water to produce meat. We can also reduce our use of bottled water and switch to reusable water bottles.Overall, solving water scarcity requires a combinationof approaches, including water conservation, alternative sources of water, and changing our consumption patterns.中文:作为全球性问题,水资源短缺已经成为许多国家面临的严峻问题。
油酸甘油酯水解条件

油酸甘油酯水解条件英文回答:Oil acid glycerol ester hydrolysis is a process in which the ester bond between the oil acid and glycerol is broken down by the addition of water. This hydrolysis reaction can be carried out under various conditions, depending on the desired outcome.One common condition for the hydrolysis of oil acid glycerol ester is the use of an acid catalyst. This can be achieved by adding a small amount of acid, such as sulfuric acid or hydrochloric acid, to the reaction mixture. The acid catalyst helps to speed up the hydrolysis reaction by providing protons that can attack the ester bond. The reaction can be further accelerated by heating the mixture, as higher temperatures increase the rate of chemical reactions.Another condition for the hydrolysis of oil acidglycerol ester is the use of an enzyme catalyst. Lipases, for example, are enzymes that can catalyze the hydrolysis of ester bonds. These enzymes are often used in biotechnological processes, as they are highly specific and can work under mild reaction conditions. The presence of water is essential for the enzyme to function, as it provides the necessary medium for the hydrolysis reaction to occur.In addition to the catalyst, the concentration of water in the reaction mixture also plays a crucial role in the hydrolysis of oil acid glycerol ester. A higher water concentration can increase the rate of hydrolysis, as more water molecules are available to attack the ester bond. However, excessive amounts of water can also lead to side reactions and decrease the overall yield of the desired product.Furthermore, the pH of the reaction mixture can affect the hydrolysis of oil acid glycerol ester. Different pH values can favor different reaction pathways and result in different products. For example, under acidic conditions,the hydrolysis reaction may lead to the formation of free oil acids and glycerol. On the other hand, under alkaline conditions, the hydrolysis reaction may result in the formation of soap and glycerol.Overall, the hydrolysis of oil acid glycerol ester can be achieved under various conditions, including the use of acid or enzyme catalysts, different water concentrations, and varying pH values. The choice of conditions depends on the specific requirements of the reaction and the desired outcome.中文回答:油酸甘油酯水解是指通过加水使油酸和甘油之间的酯键断裂的过程。
保持充足的水分英语作文

保持充足的水分英语作文英文回答:Staying hydrated is an essential aspect of maintaining overall health and well-being. Adequate hydration ensures that our bodies have the necessary water to perform various physiological functions, such as regulating body temperature, transporting nutrients, and eliminating waste products.Dehydration occurs when fluid intake is insufficient to meet the body's needs. It can result from several factors, including excessive sweating, inadequate fluid intake, or certain medical conditions. Dehydration can lead to a range of symptoms, including dizziness, fatigue, headaches, dry mouth, and decreased urine output.Maintaining sufficient hydration involves consuming fluids regularly throughout the day, even when not thirsty. Water is the ideal hydration source, but other beveragessuch as fruit juices, sports drinks, and tea can also contribute to fluid intake. It is recommended to drinkeight glasses of water per day, but individual fluid needs may vary depending on factors such as activity level, climate, and overall health.In addition to consuming fluids, certain foods can also contribute to hydration. Fruits and vegetables, such as watermelon, cucumber, and spinach, have high water content and can help replenish fluids. Electrolyte-rich foods, such as sports drinks and bananas, can also aid in maintaining electrolyte balance, which is important for hydration.Proper hydration is particularly crucial during physical activity. Sweating can lead to significant fluid loss, which must be replaced to maintain performance and prevent dehydration. Sports drinks or electrolyte-rich fluids are recommended for hydration during exercise.Maintaining adequate hydration is essential for good health. By consuming sufficient fluids and incorporating water-rich foods into our diets, we can ensure that ourbodies have the hydration they need to function optimally.中文回答:保持水分充足是维持整体健康和幸福的重要组成部分。
新型选择性雄激素调节剂Enobosarm的合成工艺改进

收稿日期:2020-07-29作者简介:邓玉晓,硕士,主管药师,从事新药研发工作,Tel**************,E-mail:***********************通讯作者:孙晋瑞,主任药师,从事新药研发工作,Tel**************,E-mail:**************新型选择性雄激素调节剂Enobosarm 的合成工艺改进邓玉晓,刘宜辉,樊志萍,任业明,段崇刚,孙晋瑞*(山东省药学科学院 山东省化学药物重点实验室,山东 济南 250101)摘 要:目的 改进Enobosarm 的合成工艺。
方法 以D-脯氨酸为起始物料,经缩合反应,溴内酯化反应,水解反应,缩合反应,取代反应生成Enobosarm 。
结果 总收率由21.0 %提高至51.4 %,产物纯度99.8 %,手性纯度99.8 %。
结论 优化后的反应条件温和,工艺操作简便,适合工业化生产。
关键词:Enobosarm ;选择性雄激素受体调节剂;工艺优化;合成中图分类号:R914.5 文献标识码:A 文章编号:1672-979X (2021)02-0138-04DOI :10.3969/j.issn.1672-979X.2021.02.010Improved Synthesis of New Selective Androgen Modulator EnobosarmDENG Yu-xiao, LIU Yi-hui, FAN Zhi-ping, REN Ye-ming, DUAN Chong-gang, SUN Jin-rui(Shandong Provincial Key Laboratory of Chemical Drugs , Shandong Academy of Pharmaceutical Sciences , Jinan250101, China )Abstract: Objective To improve the synthesis process of Enobosarm. Methods Starting from D-proline, Enobosarm was obtained by condensation, bromolactonization, hydrolysis, condensation and substitution reactions. Results Through the improvement of synthesis process, the total yield increased from 21.0 % to 51.4 %, the product purity was 99.8 %, and the chiral purity was 99.8 %. Conclusion The optimized reaction conditions are mild, the process is simple and convenient, and is suitable for industrial production.Key Words: Enobosarm; selective androgen receptor modulator; process optimization; synthesisEnobosarm (GTx-024),化学名(S )-N -(4-氰基-3-(三氟甲基)苯基)-3-(4-氰基苯氧基)-2-羟基-2-甲基丙酰胺(1),是由GTx 公司研发的一种口服非甾体选择性雄激素受体调节剂[1],具有合成代谢选择性[2],在II 期和III 期临床实验中表现出良好的耐受性[3],目前处于III 期临床试验阶段,对肌营养不良症、与癌症、慢性病等有关的肌肉萎缩症、性激素受体(AR )阳性转移性乳腺癌、晚期癌症患者的恶病质等[4]均表现出了良好的治疗效果,具有良好的临床应用前景。
不能开大的干燥剂课英语作文

不能开大的干燥剂课英语作文英文回答:Desiccants that cannot be opened have several potential hazards and should be handled with caution:Chemical hazard: Desiccants typically contain hygroscopic materials that absorb moisture from the air. If the desiccant is not properly sealed, these materials can react with oxygen and other chemicals in the air, releasing harmful gases or vapors.Physical hazard: Desiccants are often made of small, porous beads or granules. If the desiccant is not properly sealed, these beads or granules can spill out and create a slip-and-fall hazard.Environmental hazard: Desiccants can contain toxic chemicals that can harm the environment if they are not properly disposed of. If the desiccant is not properlysealed, these chemicals can leak out and contaminate soil, water, and air.To avoid these hazards, it is important to follow the manufacturer's instructions for handling and disposing of desiccants. Desiccants should always be stored in a cool, dry place away from sources of heat and moisture. When disposing of desiccants, they should be placed in a sealed container and disposed of according to local regulations.中文回答:不可以开启的干燥剂有着许多潜在的危害,因此在处理时应当谨慎:化学危害,干燥剂通常包含具有吸湿性的材料,可以吸收空气中的水分。
不能开大的干燥剂课英语作文

不能开大的干燥剂课英语作文【中英文版】Title: The English Essay We Can"t Miss: The Dangers of DesiccantsIn the realm of chemistry, there lies a substance that appears innocuous yet possesses a silent threat, the desiccant.It"s a小小的, often overlooked packet that keeps our surroundings dry, preventing moisture from ruining our precious goods.However, when the label reads "Do Not Eat" or "Keep Away from Children", it"s a sign that there"s more to this silent savior than meets the eye.在化学的世界里,有一种看似无害却暗藏风险的物质——干燥剂。
这些小小的包装常常被忽视,它们保护我们的物品不受潮湿侵害。
然而,当标签上写着“请勿食用”或“远离儿童”时,这就暗示了这个默默无闻的守护者并非看上去那么简单。
The unsuspecting desiccant, commonly found in packaging, is not to be taken lightly.Its primary function is to absorb excess moisture, a trait that can become dangerous if the packet is ingested.The chemicals inside, such as silica gel or calcium chloride, are not designed for human consumption and can lead to severe health issues if accidentally eaten.不起眼的干燥剂,通常存在于各种包装中,绝不能被轻视。
小儿腹泻(英文)

Non infective
Allergic Viruses Symptomatic Bacteria Inappropriate feeding Parasites Food intolerance Climate Fungi
11
Viral Enteropathogens
Viral enteropathogens cause most illnesses in pediatric population.
In China 836 million episodes of diarrhea every year 1/4-1/3 of all outdoor patients and a large amount of hospitalizations of children are due to diarrhea
Dietary Diarrhea
Inappropriate feeding:
Overfeeding Indigestible diet Sudden change of formula Inappropriate feeding for a milk-fed baby shifting into solid food (too much, too early, too rapid…)
Rotavirus (morn than 50% acute diarrhea) Astrovirus Norwalk virus Coronavirus Calicivirus Enteric adenovirus (serotypes 40 and 41)
12
Rotavirus
13
Bacterial Enteropathogens
关于医疗的英语作文

篇一:《英语作文中国医疗教育》The Strength and Problems ofMedical Education in ChinaMedical science is one of the most absruse and lofty subjects. To be a graduate from ShanDong university school of stomatology and a dentist who have six years clinical experience, I think I have some advantages to talk about the strength and problems of medical education in China.Undoubtedly, the medical students in China are the students of fine qualities. They always nose to the grindstone and are the pride of their families. As far as we know that the medical students have almost the busiest college lives compare with other majors, and most of medical students keep the high strict standards to themselves.Expect hard working, Chinese medical students have another superiority which is envied by the prospective doctors in other countries. AS is well-known that in China the sample capacity of patients is the biggest in the world, so the Chinese medical students can come into contact with varied kinds of patients under the leadership of their teachers. It is a precious resources for a medical student in his special period when a student become transforms into a doctor.However, contrasted with some developed countries, we should discuss the issue about why we can not cultivate the outstanding doctors in the world even if we have hard working students and big patient sample capacity ? In my perspective, the two main factors maybe summerized as follows.First, Chinese medical students lack the attic faith to medical science and also tothe stantus quo of medical care service. Recently, so many incidents of violence were reported directed at medical personnels, and the outcomes often shocking by the sight. The grim medical care environment in China has a negative effect on these students who want to be a doctor. So how to establish the medical students’ faith should be a important content in the medical education.Second, the medical education in China overemphasis the konwledge on the book and lose sight of the important of practice. It results in a consequence that a medical student can take high score in the test but can not answer a simple question posed by a patient.Above all, medical education in China even has lots of deficiencies, but the tendency is still in the better direction step-by-step. I hope Chinese medical studends will have a perfecting medical professional training system in the near future.Step-by –step building of the Chinese medical professional training system in order to efficient health care providersStrengthen and reorgnize the educational systems for training better qualityThe purpose is that through analysis and discussion, we could explore different options as well as provide insightful and helpful tips for Chinese medical education and health care reform, which are currently being conducted in ChinaAs far as we know that the medico medics have almost the busiest college life compare with other majors. Room for individual studyChina充足准备,背景各异动力十足,作为一生职业费用巨大病人量不足不努力就滚蛋多种学习方式高中毕业,少经历少,对病人责任感不足犹豫是否应该学医分数够就行经验上有优势竞争少主要在课堂篇二:《医疗英文》dispense分配、分发、配药vi.免除、省掉 medication药物,药物处理,处方prescription命令,处方,惯例 ailment病痛,(轻微的)疾病,精神失调 hybrid 混血儿,混合物,混合的 bactericidal杀菌的,有杀菌性的spectrum谱,光谱,色谱 resistance抵抗力、抗药性beta-lactamase B-内酰胺酶 meningitis 脑(脊)膜炎affinity 亲和力,亲和性 intestinal 肠的,肠内的pneumonia 肺炎 bronchitis 支气管炎resistance 抗药性 tolerance 耐受性selectivity 选择性 dependence 依赖性gonorrhea 淋病 Metoprolol 美托洛尔Propranolol 普萘洛尔 Timolol 噻吗洛尔Pindolol ISA 吲哚洛尔 Atenolol 阿替洛尔Labetalol 拉贝洛尔 blocker 阻滞剂,阻断剂competitive 竞争性 hypertension 高血压angina pectoris 心绞痛 cardiac arrhythmias 心律失常myocardial infarction 心肌梗塞 heart failure 心力衰竭hyperthyroidism 甲亢 migraine 偏头痛Omeprazole 奥美拉唑 ulcer 溃疡esophagitis 食管炎 gastric 胃的duodenal 十二指肠的 pediatric 儿科的endoscopic 内窥镜检查的 hypersensitivity 过敏症Lansoprazole 兰索拉唑 Pantoprazole 泮托拉唑Rabeprazole 雷贝拉唑 diclofenac 双氯酚酸anti-inflammatory 抗炎药,抗炎的 analgesic 止痛的,止痛剂antipyretic 退热的,退热剂 prostaglandin 前列腺素absorption 吸收 metabolites 代谢产物airway 气道,导气管 hyper responsiveness 高反应性wheezing 喘鸣 breathlessness 气喘,呼吸急促chest tightness 胸部紧迫感 cough 咳嗽reversible 可逆的,可翻转的 spontaneously 自发地,自然地,本能的 antipyretic 解热 analgesic 镇痛osteoarthritis 骨关节炎 rheumatoid arthritis 类风湿性关节炎 ankylosing spondylitis 强制性脊柱炎 allergic reaction 过敏反应itching 瘙痒 hives 寻麻症swell 肿胀 tingling 麻刺感gastrointestinal(GI) effects 胃肠道反应 GI ulceration 胃肠道溃疡bleeding 出血 perforation 穿孔Pharmacokientics 药动学 absorption 吸收distribution 分布 metabolism 代谢excretion 排泄 elimination 消除It involves the interpretation of prescription orders; the compounding, labeling, and dispensing of drugs and devices.它包括解释处方,混合、标记、调配药物和装置。
毒死蜱-新《生活饮用水卫生标准》GB5749-项目解读

新《生活饮用水卫生标准》GB5749- 项目解读毒死蜱
英文通用名chlorpyrifos
其他名称乐斯本
毒性毒死蜱属中等杀虫剂,是一个传统的有机磷杀虫剂。
对眼睛有轻度刺激,对皮肤有明显刺激,长时间接触会产生灼伤。
在试验剂量下未见致畸、致突变、致癌作用。
对鱼和水生动物毒性较高,对蜜蜂有毒。
剂型乐斯本40.7%乳油,杀死虫蓝珠14%颗粒剂。
特点毒死蜱具有触杀、胃毒和熏蒸作用。
在叶片上残留期不长,但在土壤中残留期较长,因此对地下害虫防治效果较好,对烟草有药害。
适用范围适用于水稻、小麦、棉花、果树、蔬菜、茶树上多种咀嚼式和刺吸式口器害虫,也可用于防治卫生害虫。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Pergamon Tetrahedron Letters41(2000)3855–3857TETRAHEDRON LETTERSA mild and convenient‘dry’hydrolysis of amides tocarboxylic acidsFarid ChematLaboratoire de Chimie des Substances Naturelles et des Sciences des Aliments,Facultédes Sciences et Technologies, Universitéde la Réunion,15avenue RenéCassin,B.P.7151,F-97715Saint Denis messag cedex9,La Réunion,France DOMReceived8February2000;accepted22March2000AbstractA one-pot preparation of carboxylic acids is described that proceeds from their corresponding amides by a‘dry’hydrolysis with phthalic anhydride in the absence of water and solvent.The method affords carboxylic acids in good yields and is applicable to a variety of substrates.©2000Elsevier Science Ltd.All rights reserved. Keywords:hydrolysis;amides;carboxylic acids and derivatives.Hydrolysis is a fundamental process in organic chemistry.The introduction of new reagents and the modification of existing ones are a continuous challenge.1,2Reagents are now available for almost every conceivable type of hydrolysis,but in very many instances there are disadvantages associated with their use:high cost,drastic conditions,lack of sensitivity,toxicity,instability,etc.3,4The hydrolysis of amides by phthalic anhydride is roughly one century old5and during this period, there have been very few studies.6,7The need for high reaction temperature,expensive co-reagents (tetrachloro and tetrafluoro phthalic anhydrides)and long reaction periods(6days to obtain often only low to moderate yields)has overshadowed its potential advantages and applications in organic chemistry.7We have now found that using a moderate pressure of4to10atm in a closed reactor2under solvent and water-free conditions,the hydrolysis of amides with phthalic anhydride takes place in excellent yield and short reaction times.The reaction allows for the presence of some functional groups thanks to the rather mild acidic conditions.Identification with GLC–MS(gas chromatography with a mass spectrometer detector)of the com-ponents of the reaction mixtures,initially containing equimolar quantities of benzamide and phthalic anhydride,allowed the reaction pathway illustrated in Scheme1to be established.The expected com-pounds4and5,respectively the carboxylic acid and phthalimide corresponding to the starting amide1 and phthalic anhydride2,as well as the reaction intermediate3,have been identified.8The water equivalent needed for complete hydrolysis of the amide is provided by phthalic anhydride, which is converted to phthalimide.Because this hydrolysis reaction is run without the addition of water, we call it‘dry’hydrolysis.0040-4039/00/$-see front matter©2000Elsevier Science Ltd.All rights reserved.P I I:S0040-4039(00)00507-4tetl167863856Scheme1.The generally drastic conditions of amide hydrolysis lead to severe drawbacks with compounds containing sensitive functional groups.The method described here was tested as a possible alternative.9 The results obtained with a series of bifunctional compounds are displayed(see Table1).10Fairly good yields were obtained simply by using the mild reaction conditions even for pivalamide with its steric hindrance.No side product was detected,except for ethylamidoacetate.In this case,decarboxylation also occurs:malonic acid,acetic acid and acetamide have been identified as side products.Table1‘Dry’hydrolysis of amides1to carboxylic acids4(T=240–250°C;P=4atm)3857 In summary,an extremely simple method for the preparation of carboxylic acids and phthalimides has been developed that uses cheap co-reagents and a reactor with moderate pressure of a few atms, available in every organic laboratory.This method generally affords good yields of carboxylic acids in short reaction times(less than1h)using phthalic anhydride as a means of generating water in situ.AcknowledgementsThe author thanks Prof.Jacqueline Smadja for her valuable comments and helpful discussions. References1.McKillop,A.;Tarbin,J.A.Tetrahedron Lett.1983,24,1505–1508.2.(a)Chemat,F.;Poux,M.;Berlan J.J.Chem.Soc.,Perkin Trans.21994,2597–2602.(b)Chemat,F.;Poux,M.;Berlan J.J.Chem.Soc.,Perkin Trans.21996,1781–1784.3.Jacobson,A.;Sayre,L.M.Inorg.Chem.1992,31,935–937.4.Brown,R.S.;Bennet A.J.;Slebocka-Tilk H.Acc.Chem.Res.1992,25,481–488.5.Mathews,J.A.J.Am.Chem.Soc.1898,20,648–668.6.Strain,W.H.;Rochester,N.Y.;Dec, Patent2508418(Chem.Abstr.1950,44,9983i).7.Eaton,J.T.;Rounds,W.D.;Urbanowicz,J.H.;Gribble,G.W.Tetrahedron Lett.1988,29,6553–6556.8.The intermediate3(C15H9O3N;m/z251)was detected by GLC(HP3690)with a mass spectrometer detector(HP1090).All the carboxylic acids prepared were compared with authentic samples(GLC,HPLC,FTIR,NMR,mp).9.In a typical run,the amide(30mmol)and phthalic anhydride(30mmol)were introduced into the reactor,and heated understirring.The reactor was made of stainless steel.It was connected to a pressure gauge,andfitted with a thermocouple for temperature control.At the desired reaction time,the reactor was rapidly cooled down in a water–ice mixture,and chloroform(30mL)is added.The mixture was stirred for5min,and the solidfiltered off.The chloroform solution contained the unreacted amide and the carboxylic acid.The residual solid contained unchanged phthalic anhydride and phthalimide.The volume of the chloroform solution was adjusted to50mL and naphthalene was added as internal standard.The resulting solution was analysed by GLC(Hewlett–Packard3690;QC BP20capillary column,25m).10.The amides and their corresponding carboxylic acids were purchased from Janssen and Aldrich(99%purity)except forvaleramide,which was given by Sanofi.。
