Laurolitsine_hydrochloride_SDS_MedChemExpress
洋甘菊提取液MSDS英文版

1. IDENTIFICATION OF THE SUBSTANCE/TREPARATION AND THE COMPANY/UNDERTAKING3.HAZARDS IDENTIFICATION4. FIRST AID MEASURESMATERIAL SAFETY DATA SHEETProduct name:Supplier:Tel:EMERGENCY OVERVIEW: May cause skin irritation and/or dermatitisPrinciple routes of exposure: Inhalation: Ingestion: Skin contact: Eye contact:SkinMay cause irritation of respiratory tract May be harmful if swallowed May cause allergic skin reaction Avoid contact with eyesStatements of hazard MAY CAUSE ALLERGIC SKIN REACTION.Statements of Spill of Leak Label Eliminate all ignition sources. Absorb and/or contain spill with inert materials (e.g., sand, vermiculite). Then place in appropriate container. For large spills, use water spray to disperse vapors, flush spill area. Prevent runoff from entering waterways or sewers.General advice:POSITION/INFORMATION ON INGREDIENTSInhalation:Skin contact:Ingestion:Eye contact:Protection of first – aiders:Medical conditions aggravated by exposure: In the case of accident or if you fell unwell, seek medical advice immediately (show the label where possible).Move to fresh air, call a physician immediately.Rinse immediately with plenty of water and seek medical adviceDo not induce vomiting without medical advice.In the case of contact with eyes, rinse immediately with plenty of water and seek medical advice.No information availableNone knownSuitable extinguishing media:Specific hazards:Special protective equipment for firefighters:Flash point:Autoignition temperature:NFPA rating Use dry chemical, CO2, water spray or “alcohol” foam Burning produces irritant fumes.As in any fire, wear self-contained breathing apparatus pressure-demand, MSHA/NIOSH (approved or equivalent) and full protective gearNot determinedNot determinedNFPA Health: 1 NFPA Flammability: 1 NFPA Reactivity: 0Personal precautions: Environmental precautions: Methods for cleaning up: Use personal protective equipment.Prevent product from entering drains.Sweep up and shovel into suitable containers for disposalStorage:7. HANDLING AND STORAGE5.FIRE-FIGHTING MEASURES6. ACCIDENTAL RELEASE MEASURESRoom temperature Handling:Safe handling advice: Incompatible products:Use only in area provided with appropriate exhaust ventilation.Wear personal protective equipment.Oxidising and spontaneously flammable productsEngineering measures: Respiratory protection: Skin and body protection:Eye protection: Hand protection: Hygiene measures:Ensure adequate ventilation.Breathing apparatus only if aerosol or dust is formed. Usual safety precautions while handling the product will provide adequate protection against this potential effect. Safety glasses with side-shieldsPVC or other plastic material glovesHandle in accordance with good industrial hygiene and safety practice.Melting point/range: Boiling point/range: Density: Vapor pressure: Evaporation rate: Vapor density: Solubility (in water): Flash point:Autoignition temperature:No Data available at this time. No Data available at this time. No data available No data available No data available No data available No data available Not determined Not determinedStability: Stable under recommended storage conditions. Polymerization: None under normal processing.Hazardous decomposition products: Thermal decomposition can lead to release of irritating gases and vapours such as carbon oxides.Materials to avoid: Strong oxidising agents.10. STABILITY AND REACTIVITY9. PHYSICAL AND CHEMICAL PROPERTIES8. EXPOSURE CONTROLS/PERSONAL PROTECTION11. TOXICOLOGICAL INFORMATIONConditions to avoid: Exposure to air or moisture over prolonged periods.Product information Acute toxicityChronic toxicity:Local effects: Chronic exposure may cause nausea and vomiting, higher exposure causes unconsciousness.Symptoms of overexposure may be headache, dizziness, tiredness, nausea and vomiting.Specific effects:May include moderate to severe erythema (redness) and moderate edema (raised skin), nausea, vomiting,headache.Primary irritation: Carcingenic effects: Mutagenic effects: Reproductive toxicity:No data is available on the product itself. No data is available on the product itself. No data is available on the product itself. No data is available on the product itself.Mobility:Bioaccumulation: Ecotoxicity effects: Aquatic toxicity:No data available No data available No data availableMay cause long-term adverse effects in the aquatic environment.12. ECOLOGICAL INFORMATION13. DISPOSAL CONSIDERATIONSWaste from residues/unused products:Contaminated packaging:Waste disposal must be in accordance with appropriate Federal, State and local regulations. This product, if unaltered by use, may be disposed of treatment at a permitted facility or as advised by your local hazardous waste regulatory authority. Residue from fires extinguished with this material may be hazardous.Do not re-use empty containers.UN/Id No:Not regulated14. TRANSPORT INFFORMATIONDOTProper shipping name: Not regulatedTGD(Canada)WHMIS hazard class: Non - controlledIMDG/IMOIMDG – Hazard Classifications Not ApplicableIMO – labels:15. REGULATORY INFOTMATION International Inventories16. OTHER INFORMATIONPrepared by: Health & SafetyDisclaimer: The information and recommendations contained herein are based upon tests believed to be reliable.However, XABC does not guarantee the accuracy or completeness NOR SHALL ANY OF THIS INFORMATION CONSTITUTE A WARRANTY, WHETHER EXPRESSED OR IMPLIED, AS TO THE SAFETY OF THE GOOD, THE MERCHANTABILITY OF THE GOODS, OR THE FITNESS OF THE FITNESS OF THE GOODS FOR A PARTICULAR PURPOSE. Adjustment to conform to actual conditions of usage maybe required. XABC assumes no responsibility for results obtained or for incidental or consequential damages, including lost profits arising from the use of these data. No warranty against infringement of any patent, copyright or trademark is made or implied.End of safety data sheet。
依达拉奉右莰醇联合阿替普酶治疗急性缺血性脑卒中的疗效观察

J Apoplexy and Nervous Diseases, October 2023, Vol 40,No. 10依达拉奉右莰醇联合阿替普酶治疗急性缺血性脑卒中的疗效观察李春颖1, 鞠东升1, 潘澍潇1, 朱辉2, 靳颖1摘要: 目的 观察依达拉奉右莰醇联合阿替普酶治疗急性缺血性脑卒中(AIS )的疗效性和安全性。
方法 收集2020年11月―2022年4月松原吉林油田医院收治的AIS 患者共计124例,随机分为实验组(阿替普酶静脉溶栓+依达拉奉右莰醇组)和对照组(阿替普酶静脉溶栓组),对比治疗效果。
结果 实验组治疗总有效率为82.3%,高于对照组的64.5%,差异有统计学意义(P < 0.05)。
其溶栓后不同阶段NIHSS 评分结果(5.40 ± 3.82)分、(4.14 ± 3.44)分、(0.57 ± 0.99)分均低于对照组,差异有统计学意义(P < 0.05)。
两组患者治疗期间均未发生药物不良反应。
结论 依达拉奉右莰醇联合阿替普酶治疗AIS 患者临床疗效确切。
关键词: 依达拉奉右莰醇; 阿替普酶; 急性缺血性脑卒中; 疗效中图分类号:R743.3 文献标识码:AEfficacy of edaravone dexborneol combined with alteplase in treatment of acute ischemic stroke LI Chunying ,JU Dongsheng , PAN Shuxiao , et al. (Songyuan Jilin Oilfield Hospital , Songyuan 138000, China )Abstract : Objective To investigate the efficacy and safety of edaravone dexborneol combined with alteplase in the treatment of acute ischemic stroke (AIS ).Methods The data were collected from 124 patients with AIS who were admitted to our hospital from November 2020 to April 2022. The patients were randomly divided into experimental group (intravenous thrombolysis with alteplase + treatment with edaravone dexborneol ) and control group (intravenous thrombolysis with al‑teplase ), and the two groups were compared for efficacy.Results The overall response rate in the experimental group was sig‑nificantly higher than that in the control group (82.3% vs 64.5%, P < 0.05). The National Institutes of Health Stroke Scale scores at different stages after thrombolysis were significantly lower in the experimental group (5.40 ± 3.82, 4.14 ± 3.44, and 0.57 ± 0.99) than in the control group (P < 0.05). No adverse drug reactions were observed in the two groups during the treat‑ment.Conclusion Edaravone dexborneol combined with alteplase has definite clinical efficacy in the treatment of AIS.Key words : Edaravone dexborneol ; Alteplase ; Acute ischemic stroke ; Efficacy 脑卒中是全球致残的主要原因和第二大死亡原因[1],至少50%幸存者将遗留残疾[2]。
吲哚菁绿注射剂说明书(英语)

IC-GREEN™(Indocyanine Green for Injection, USP)SterileDescription: IC-GREEN™ is a sterile, lyophilized green powder containing 25 mg of indocyanine green with no more than 5 % sodium iodide. It is packaged with an Aqueous Solvent consisting of Sterile Water for Injection used to dissolve the indocyanine green. IC-GREEN™ is to be administered intravenously.Idocyanine green is a water soluble, tricarbocyanine dye with peak spectral absorption at 800 nm. The chemical name for Indocyanine Green is 1 H-Benz[e]indolium,2-[7[1,3-dihydro-1,1-dimethyl-3-(-4-sulfobutyl)-2H-benz[e]indo-2-ylidene]-1,3,5-heptatrienyl]-1,1-dimethyl-3-(4-sulfobutyl)-,hydroxide, innersalt, sodium. 2-[7-[1,1-Dimethyl-3-(4-sulfobuttyl)benz[e]indolin-2-ylidene]-1,3,5-heptatrienyl]-1,1-dimethyl-3-(4-sulfobutyl)-1Hbenz[e]indolium hydroxide , inner salt, sodium salt. IC-GREEN™ has a pH pf approximately 6.5 when reconstituted. Each vial of IC-GREEN™ contains 25 mg of indocyanine green as a sterile lyophilized powder.Clinical Pharmacology: Following intravenous injection, IC-GREEN™ is rapidly bound to plasma protein, of which albumin is the principle carrier (95%). IC-GREEN™ undergoes no significant extrahepatic or enterohepatic circulation; simultaneous arterial and venous blood estimations have shown negligible renal, peripheral, lung or cerebro-spinal uptake of the dye. IC-GREEN™ is taken up from the plasma almost exclusively by the hepatic parenchymal cells and is secreted entirely into the bile. After biliary obstruction, the dye appears in the hepatic lymph, independently of the bile, suggesting that the biliary mucosa is sufficiently intact to prevent diffusion of the dye, though allowing diffusion of bilirubin. These characteristics make IC-GREEN™ a helpful index of hepatic function. The plasma fractional disappearance rate at the recommended 0.5 mg/kg dose has been reported to be significantly greater in women than in men, although there was no significant difference in the calculated value for clearance.Indications and Usage: For determining cardiac output, hepatic function and liver blood flow, and for ophthalmic angiography.Contraindications: IC-GREEN™ contains sodium iodide and should be used with caution in patients who have a history of allergy to iodides.Warnings: Anaphylactic deaths have been reported following IC-GREEN™ administration during cardiac catheterization.Precautions: General: IC-GREEN™ Powder and Solution: IC-GREEN™ is unstable in aqueous solution and must be used within 6 hours. However, the dye is stable in plasma and whole blood so that samples obtained in discontinuous sampling techniques may be read hours later. Sterile techniques should be used in handling the dye solution as well as in the performance of the dilution curves.IC-GREEN™ (indocyanine green for injection) powder may cling to the vial or lump together because it is freeze-dried in the vials.Drug Interactions: Heparin preparations containing sodium bisulfate reduce the absorption peak of IC-GREEN™ in blood and, therefore, should not be used as an anticoagulant for the collection of samples for analysis.Drug/Laboratory Test Interactions: Radioactive iodine uptake studies should not be performed for at least a week following the use of IC-GREEN™.Carcinogenesis, Mutagenesis, Impairment of Fertility: No studies have been performed to evaluate the carcinogenicity, mutagenicity, or impairment of fertility.Pregnancy: Teratogenic Effects: Pregnancy Category C: Animal Reproduction studies have not been conducted with IC-GREEN™. It is also not known whether IC-GREEN™ can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. IC-GREEN™ should be given to a pregnant woman only if clearly indicated.Nursing Mothers: It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when IC-GREEN™ is administered to a nursing woman.Pediatric Use: Safety and effectiveness in pediatric patients have been established.Adverse Reactions: Anaphylactic or urticarial reactions have been reported in patients with or without history of allergy to iodides. If such reactions occur, treatment with the appropriate agents, e.g., epinephrine, antihistamines, and corticosteroids should be administered.Overdosage: There are no data available describing the signs, symptoms, or laboratory findings accompanying overdosage. The LD 50 after I.V. administration ranges between 60 and 80 mg/kg in mice, 50 and 70 mg/kg in rats and 50 and 80 mg/kg in rabbits.Dosage and Administration: INDICATOR-DILUTION STUDIES: IC-GREEN™ permits recording of the indicator-dilution curves for both diagnostic and research purpose independently of fluctuations in oxygen saturation. In the performance of dye dilution curves, a known amount of dye is usually injected as a single bolus as rapidly as possible via a cardiac catheter into selected sites in the vascular system. A recording instrument (oximeter or densitometer) is attached to a needle or catheter for sampling of the dye-blood mixture from a systemic arterial sampling site.Under sterile conditions, the IC-GREEN™ powder should be dissolved with the Aqueous Solvent provided for this product, and the solution used within 6 hours after it is prepared. If a precipitate is present, discard the solution. The amount of solvent to be used can be calculated from the dosage form which follows. It is recommended that the syringe used for injection of the dye be rinsed with this diluent. Saline is used in all other parts of the catheterization procedure.This matter of rinsing the dye syringe with distilled water may not be critical, since it is known that an amount of sodium chloride sufficient to make an isotonic solution may be added to dye that has first been dissolved in distilled water. This procedure has been used for constant-rate injection techniques without precipitation of the dye.The usual doses of IC-GREEN™ which have been used for dilution curves are as follows:Adults -5.0 mgChildren -2.5 mgInfants -1.25 mgThese doses of the dye are usually injected in a mL volume. An average of five dilution curves are required in the performance of a diagnostic cardiac catheterization. The total dose of dye injected should be kept below 2 mg/kg.Calibrating Dye Curves: To quantitate the dilution curves, standard dilutions of IC-GREEN™ in whole blood are made as follows. It is strongly recommended that the same dye that was used for the injections be used in the preparation of these standard dilutions. The most concentrated dye solution is made by accurately diluting 1 mL of the 5 mg/ mL dye with 7 mL of distilled water. This concentration is then successively halved by diluting 4 mL of the previous concentration with 4 mL of distilled water. (If a 2.5 mg/ mL concentration was used for the dilution curves, 1 mL of the 2.5 mg/ mL dye is added to 3 mL of distilled water to make the most concentrated "standard" solution. This concentration is then successively halved by diluting 2 mL of the previous concentration with 2 mL of distilled water.) Then 0.2 mL portions (accurately measured from a calibrated syringe) of these dye solutions are added to 5 mL aliquots of the subject's blood, giving final concentrations of the dye in blood beginning with 24.0 mg/liter, approximately (actual concentration depends on the exact volume of dye added). This concentration is, of course, successively halved in the succeeding aliquots of the subject's blood. These aliquots of blood containing known amounts of dye, as well as a blank sample of which 0.2 mL of saline containing no dye has been added, are then passed through the detecting instrument and a calibration curve is constructed from the deflections recorded.HEPATIC FUNCTION STUDIES: Due to its absorption spectrum, changing concentrations of IC-GREEN™ (indocyanine green for injection) in the blood can be monitored by ear densitometry or by obtaining blood specimens at timed intervals. The technique for both methods is as follows.The patient should be studied in a fasting, basal state. The patient should be weighed and the dosage calculated on the basis of 0.5 mg/kg of body weight.Under sterile conditions, the IC-GREEN™ powder should be dissolved with the Aqueous Solvent provided. Exactly 5 mL of aqueous solvent should be added to the 25 mg vial giving 5 mg of dye per mL of solution.Inject the correct amount of dye into the lumen of an arm vein as rapidly as possible, without allowing the dye to escape outside the vein. (If the photometric method is used, prior to injecting IC-GREEN™, withdraw 6 mL of venous blood from the patient's arm for serum blank and standard curve construction, and through the same needle, inject the correct amount of dye )Ear Densitometry: Ear oximetry has also been used and makes it possible to monitor the appearance and disappearance of IC-GREEN™ without the necessity of withdrawal and spectrophotometric analysis of blood samples for calibration. An ear densitometer which has a compensatory photo-electric cell to correct for changes in blood volume and hematocrit, and a detection photocell which registers levels has been described. This device permits simultaneous measurement of cardiac output, blood volume and hepatic clearance of IC-GREEN™∗ and was found to provide a reliable index of plasma removal kinetics after single injections or continuous intrusions of IC-GREEN™. This technique was employed in newborn infants, healthy adults and in children and adults with liver disease. The normal subject has a removal rate of 18-24% per minute. Due to the absence of extra-hepatic removal, IC-GREEN™ was found to be ideally suited for serial study of severe chronic liver disease and to provide a stable measurement of hepatic blood flow. In larger doses, IC-GREEN™ has proven to be particularly valuable in detecting drug-induced alterations of hepatic function and in the detection of mild liver injury.Using the ear densitometer, a dosage of 0.5 mg/kg in normal subjects gives the following clearance pattern.Photometric Method-Determination Using Percentage Retention of Dye:A typical curve obtained by plotting dye concentration versus optical density is shown below. Percent retention can be read from this plot.∗Dichromatic earpiece densitometer supplied by The Waters Company, Rochester, Minnesota.If more accurate results are desired, a curve using the patient's blood and the vial of IC-GREEN™ being used in the determination can be constructed as follows:1.Take 6 mL of non-dye-containing venous blood from the patient's arm. Place in a test tube andallow the blood to clot. The serum is separated by centrifugation.2.Pipette 1 mLof the serum into a microcuvette.3.Add 1 lambda ((lambda)) of the 5 mg/mL aqueous IC-GREEN™ (sterile indocyanine green)solution to the serum, giving a dilution of 5 mg/liter, the standard for 50% retention. (Theaddition of 2 lambda (lambda) of the 5 mg/mL IC-GREEN™ solution would give 100%retention; however, this concentration cannot be read on the spectrophotometer.)4.The optical density of this solution is read at 805 nm, using normal serum as the blank.5.Plot the 50% figure obtained in Step 4, and draw a line connecting this point with the zerocoordinates.Percentage Retention: A single 20-minute sample (withdrawn from a vein in the opposite arm to that injected) is allowed to clot, centrifuged and its optical density is determined at 805 nm using the patient's normal serum as the blank. Dye concentration is read from the curve above. A single 20-minute sample of serum in healthy subjects should contain no more than 4% of the initial concentration of the dye. The use of percentage retention is less accurate than percentage disappearance rate, but provides reproducible results. Hemolysis does not interfere with a reading.Determination Using Disappearance Rate of Dye: To calculate the percentage disappearance rate, obtain samples at 5, 10, 15 and 20 minutes after injecting the dye. Prepare the sample as in the previous section and measure the optical densities at 805 nm, using the patient's normal serum as the blank. The IC-GREEN™ concentration in each timed specimen can be determined by using the concentration curve illustrated. Plot values on semilogarithmic paper.Specimens containing IC-GREEN™ should be read at the same temperature since its optical density is influenced by temperature variations.Normal Values: Percentage disappearance rate in healthy subjects is 18-24% per minute. Normal biological half-time is 2.5-3.0 minutes.OPHTHALMIC ANGIOGRAPHY STUDIES: The excitation and emission spectra (Figure 1) and the absorption spectra (Figure 2) of IC-GREEN™ make it useful in ophthalmic angiography. The peak absorption and emission of IC-GREEN™ lie in a region (800-850 nm) where transmission of energy by the pigment epithelium is more efficient than in the region of visible light energy. IC-GREEN™ also has the property of being nearly 98% bound to blood protein, and therefore, excessive dye extravasation does not take place in the highly fenestrated choroidal vasculature It is, therefore, useful in both absorption and fluorescence infrared angiography of the choroidal vasculature when using appropriate filters and film in a fundus camera.Dosages up to 40 mg IC-GREEN™ dye in 2 mL of aqueous solvent have been found to give optimal angiograms, depending on the imaging equipment and technique used. The antecubital vein injected IC-GREEN™ dye bolus should immediately be followed by a 5 mL bolus of normal saline.Clinically, angiograms of uniformly good quality can be assured only after taking care to optimize the contributions of all possible factors such as, patient cooperation and dye injection. The foregoing injection regimen is designed to provide delivery of a spatially limited dye bolus of optimal concentration to the choroidal vasculature following intravenous injection.How Supplied: IC-GREEN™ is supplied in a kit (NDC 17478-701-02) containing six 25 mg IC-GREEN™ vials and six 10mL Aqueous Solvent ampules:NDC 17478-701-25 IC-Green vial. 25 mg fill in 50 mL vial.NDC 17478-701-10 Aqueous Solvent ampule. 10 mL fill in 10 mL ampule.Storage: Store at 15° to 25° C (59° to 77°).Rx OnlyManufactured for:Akorn, IncBuffalo Grove, IL 60089IGGON Rev 03/06Proposed Container Label for IC-GREEN (not actual size)Proposed Kit Carton Label for IC-GREEN (not actual size)。
常用蛋白酶切割位点
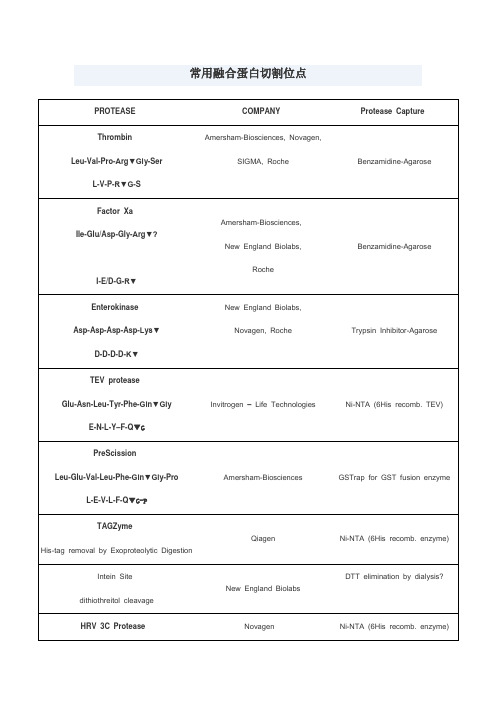
4.溴化氰处理,专一性的切割甲硫氨酸羧基端的肽键。
SIGMA, Roche
Benzamidine-Agarose
Factor Xa
Ile-Glu/Asp-Gly-Arg▼?
I-E/D-G-R▼
Amersham-Biosciences,
New England Biolabs,
Roche
Benzamidine-Agarose
Enterokinase
Asp-Asp-Asp-Asp-Lys▼
羧肽酶
羧肽酶B可以切割C端的Lys或Arg;羧肽酶A可以切割C端除了Lys、Arg、Pro的氨基酸,但如果倒数第二个氨基酸为Pro两种羧肽酶均不能作用
1.胰蛋白酶属肽链内切酶,能把多肽链中Lys和Arg残基中的羧基侧切断。
2.胰凝乳蛋白酶(亦称糜蛋白酶)属肽链内切酶,主要切断多肽链中的芳香族氨基酸(Phe、Trp、Tyr)残基的羧基一侧。
LifeSensors
Ni-NTA (6His recomb. enzyme)
Kex-2
-Arg-X-Lys/Arg-Arg▼
Invitrogen – Life Technologies,
Ni-NTA (6His recomb. enzyme)
KEX2对arg的专一性高,要求最重要。
Arg前为lys效率最高,不切-Arg-lys,Pro影响KEX2切割
Ni-NTA (6His recomb. TEV)
PreScission
Leu-Glu-Val-Leu-Phe-Gln▼Gly-Pro
CAS1204313-51-8_Icotinib Hydrochloride_MedBio相关资料

在体外激酶测定中,将2.4 ng /μLEGFR蛋白与32 ng /μLCrk在含有1μM冷ATP和1μCi32P-γ-ATP的25μL激酶反应缓冲液中混合。将混合物与Icotinib在0,0.5,2.5,12.5或62.5nM下在冰上温育10分钟,然后在30℃温育20分钟。用SDS样品缓冲液在100℃猝灭4分钟后,通过在10%SDS-PAGE凝胶中电泳分离蛋白质混合物。然后暴露干燥的凝胶以检测放射性。量化由软件[1]执行。
CAS
1、产品物理参数:
常用名
凯美纳
英文名
Icotinib Hydrochloride
CAS号
1204313-51-8
分子量
427.881
密度
无资料
沸点
无资料
分子式
C22H22ClN3O4
熔点
无资料
闪点
无资料
2、技术资料:
体外研究
与Iconitib在0.5μM孵育导致激酶活性抑制分别为91%,99%,96%,61%和61%。 Iconitib抑制A431和BGC-823 A549,H460和KB细胞系的增殖,IC50分别为1,4.06,12.16,16.08,40.71μM。当用88种激酶进行分析时,Icotinib仅对EGFR及其突变体显示出有意义的抑制活性。 Icotinib阻断人表皮样癌A431细胞系中EGFR介导的细胞内酪氨酸磷酸化(IC50 = 45 nM)并抑制肿瘤细胞增殖[1]。
10mM (in 1mL DMSO)
≥98%
1172133-28-6
5mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
硫酸羟氯喹

药代动力学
羟氯喹具有和氯喹相似的药理作用、药代动力学和体内代谢过程。口服后,羟氯喹被快速和几乎全部吸收。 在一项研究中,给予健康志愿者400mg单剂量的羟氯喹后,其平均血浆峰浓度在53-208ng/ml范围,平均水平为 105ng/ml。血浆达峰浓度的平均时间为1.83小时。根据给药后的时间,平均血浆消除半衰期变化如下:血浆达峰 浓度后-10、10-48、48-504小时时分别为5.9个小时、26.1个小时和299个小时。母体化合物和代谢物广泛分布 于机体,消除主要通过尿液。在一项研究中,24小时可观察到3%的给药剂量。
贮藏
密封,25
本品活性成份为:硫酸羟氯喹 化学名称:(±)-2[[4-[(7-氯-4-喹啉基)氨基]苯基]-乙氨基]乙醇硫酸盐 化学结构式: 分子式:C18H26ClN3O·H2SO4 分子量:434.0
性状
本品为白色薄膜衣片,除去包衣后显白色。 一面刻有HCQ,另一面刻有200字样。
适应症
硫酸羟氯喹
介绍
目录
01 基本信息
02 药品说明书基本信息
基本信息
硫酸羟氯喹,临床用于类风湿关节炎,青少年慢性关节炎,盘状和系统性红斑狼疮,以及由阳光引发或加剧 的皮肤病变。
基本信息
1
物化性质
2
安全信息
3
毒理学数据
4
生态学数据
5
性质与稳定性
物化性质
外观与性状:无气味的固体 熔点:240 °C 沸点:516.7ºC at 760 mmHg 闪 点 : 2 6 6 . 3 ºC 水溶解性:易溶 储存条件:Store in a cool, dry place. Do not store in direct sunlight. Store in a tightly closed container.
注射用盐酸罗沙替丁醋酸酯项目简介及技术转让-20200728

盐酸罗沙替丁醋酸酯
项目分析及技术转让
桐晖药业提供技术转让
中文名称:注射用盐酸罗沙替丁醋酸酯
英文名称:Roxatidine Acetate Hydrochloride for Injection
规格:75mg
适应症:本品适用于上消化道出血(由消化性溃疡,急性应激性溃疡,出血性胃炎等引起)的低危患者。
注册分类:化药3类
项目简介
盐酸罗沙替丁乙酸酯是由日本Teikoku Hormone公司开发的第四代组胺H2-受体拮抗剂(H2RA)、一种长效H2RA,抑酸作用较雷尼替丁较强,而且具有黏膜保护作用,1986年首次在日本上市。
本品为脂溶性药物,它在小肠、血浆和肝脏内经去乙酰化作用后,迅速转变为有活性的代谢物-罗沙替丁,临床主要用于预防和治疗由于胃酸高分泌状态引起的消化系统疾病。
与传统的组胺H2-受体拮抗剂西咪替丁、雷尼替丁相比,其在疗效和安全性上有了较大改进,其不良反应发生率为1.7%。
研发进度:待申报
合作方式:委托开发、联合开发
(桐晖药业提供技术转让)。
52-89-1,L-半胱氨酸,盐酸盐,技术规格说明书(SDS)

产品技术规格说明书由上海创赛科技有限公司收集整理,仅做参考使用。
L-半胱氨酸,盐酸盐详细介绍:
中文名称:
L-半胱氨酸,盐酸盐
中文别名:
L-半胱氨酸,盐酸盐;L-半胱氨酸盐酸盐水合物;L-丰胱胺酸盐酸盐;半胱氨酸,盐酸盐水合物;L-半胱氨酸盐酸盐(无水物);Α-氨基-Β-巯基丙酸盐酸盐;盐酸L-半胱氨酸;L-无水巯基丙氨酸盐酸盐;L-半胱氨酸盐酸盐无水物;DL-半胱氨酸盐酸盐;L-半胱氨酸盐酸盐,BR;L-半胱氨酸盐酸盐,无水;L―半胱氨酸盐酸盐无水物;L-半胱氨酸盐无水物;L-半胱胺酸盐酸盐;L-半胱胺酸盐酸盐无水物;L-巯基丙氨酸盐酸盐;L-盐酸半胱氨酸无水物;盐酸半胱氨酸;L-半胺氨酸盐酸盐;L-2-氨基-3-巯基丙酸盐酸盐;L-半胱氨酸盐酸盐-水物;L-无水半胱盐酸盐;半胱氨酸,盐酸盐;孚氨基酸;盐酸半胱氨酸(无水);L-半胱氨酸,盐酸盐,医药级,纯度:>99%
包装:
25, 100 g in poly bottle
生化/生理作用:
NMDA谷氨酸受体激动剂也是AMPA谷氨酸受体在高浓度时的激动剂。
查询关键词:“52-89-1,L-半胱氨酸,盐酸盐,L-Cysteine Hydrochloride,Sigma-Aldrich,上海现货”。
L-半胱氨酸,盐酸盐参考文献:
英文名称:
L-Cysteine hydrochloride
英文别名:
迷迭香酸对过氧化氢处理下的皮肤黑色素瘤的抗氧化作用(原文翻译)

迷迭香酸(罗丹酚酸)对H2O2处理过的皮肤黑色素瘤细胞的抗氧化作用Sun Mi Yoo1 and Jeong Ran Kang2*1.韩国光州500-741号东冈大学美容系2.韩国首尔143-701号建国大学生物工程系2009.2.6收到 2009.4.17接收本学科旨在检测迷迭香酸对人工孵育的皮肤黑色素瘤细胞在ROS下的抗氧化作用。
通过XTT比色法,以细胞毒性和抗氧化作用来分析细胞粘附活性,DPPH自由基清除活性以及H2O2处理1-10h和未经处理的两种情况下乳酸脱氢酶的活性。
用20-110 μM 的H2O2处理皮肤黑色素瘤细胞5-7h后,细胞活性的降低呈剂量和时间依赖性。
通过XTT比色法测得H2O2的半抑制浓度(IC50 )为90μM。
同时H2O2增强了LDH细胞的剂量依赖性。
用50-90μM的H2O2处理8h后测得LDH50为60 μM H2O2。
迷迭香酸能增强细胞活性和DPPH自由基清除活性,降低乳酸盐脱氢酶的活性。
细胞的H2O2处理证实了对人工孵育的皮肤黑色素瘤细胞的强抗氧化作用。
通过H2O2的处理,迷迭香酸能在细胞内能增强细胞活性和DPPH 自由基清除活性,降低乳酸盐脱氢酶的活性。
这被认为是迷迭香酸对ROS(ROS)如H2O2的抗氧化作用。
Key words:DPPH-radical scavenging, LDH, rosmarinic acid, XTT assay关键字:DPPH自由基清除活性,乳酸脱氢酶,迷迭香酸,XTT比色法据研究发现,ROS通过氧化应激对细胞的损伤和一些脑部疾病比如帕金森症或心脏疾病例如心肌梗塞之间有很大的关联[Difazio et al., 1992; Delanty and Dichter, 1998].尤其是研究人员认为ROS是皮肤老化的一个主要的因素后,一直试图从ROS方面研究衰老。
[Yokozawa et al., 1998].据研究表明,ROS的氧化应激会通过萎缩细胞引起各种疾病,例如超氧自由基、H2O2(H2O2)或羟基自由基的巯基蛋白反应中断酶的活性,破坏脱氧RMA(DNA)或RMA(RNA),诱导细胞膜脂质过氧化。
索莱宝 羟自由基清除能力检测试剂盒 说明书 可见分光光度法
羟自由基清除能力检测试剂盒说明书可见分光光度法注意:本产品试剂有所变动,请注意并严格按照该说明书操作。
货号:BC1320规格:50T/48S产品组成:使用前请认真核对试剂体积与瓶内体积是否一致,有疑问请及时联系索莱宝工作人员。
试剂名称规格保存条件提取液液体55 mL×1瓶4℃保存试剂一液体10 mL×1瓶4℃保存试剂二液体20 mL×1瓶4℃保存试剂三液体20 mL×1瓶4℃保存试剂四液体0.1 mL×1瓶4℃保存溶液的配制:1、试剂四:液体置于试剂瓶内EP 管中。
临用前加入9.9 mL 蒸馏水混匀,也可按试剂四体积(mL ):蒸馏水(mL )=1∶99的比例,现用现配,配好的试剂4℃保存一周。
产品说明:羟自由基是人体在新陈代谢过程中产生的对生物体毒性强、危害大的一种自由基。
它可以使组织中的糖类、氨基酸、蛋白质、核酸等物质发生氧化,遭受氧化性损伤和破坏,导致细胞坏死或突变。
羟自由基清除能力是样本抗氧化能力的重要指标之一,在抗氧化类保健品和药品研究中得到广泛应用。
H 2O 2/Fe 2+通过Fenton 反应产生羟自由基,将邻二氮菲-Fe 2+水溶液中Fe 2+氧化为Fe 3+,导致536 nm 的吸光度下降,样本536 nm 吸光度下降速率的抑制程度,反映了样本清除羟自由基的能力。
注意:实验之前建议选择2-3个预期差异大的样本做预实验。
如果样本吸光值不在测量范围内建议稀释或者增加样本量进行检测。
需自备的仪器和用品:可见分光光度计、恒温水浴锅、1 mL 玻璃比色皿、低温离心机、研钵/匀浆器和蒸馏水。
操作步骤:一、样本处理(可适当调整待测样本量,具体比例可以参考文献)1、组织样本的制备:称取约0.1 g 组织,加入1 mL 提取液进行冰浴匀浆,10000 g 4℃离心10 min ,取上清,置冰上待测。
2、血清、果汁等液体样本可直接测定。
3、提取物(或者药物)可配制成一定浓度,如5 mg/mL 。
罗氏生化仪用清洗液

罗氏生化仪用清洗液型号︰Modular、Cobas品牌︰百龙腾原产地︰中国电话︰************地址︰北京市海淀区二里庄35号万和大夏206适用于罗氏 Roche Modular系列生化分析仪适用模块品名货号规格P、D模块(生化模块)清洗液碱性清洗液B1812L/桶(10桶/箱)酸性清洗液B182500ml抗菌无磷清洁剂B183500ml,1L, 12×60mlISE模块(电解质模块)试剂内标液B2812L/桶(10桶/箱)稀释液B2822L/桶(10桶/箱)参比液B283500ml/桶离子清洗液(ISE-Cleaning Sol)B284110ml/瓶上十年数千台大型生化仪使用考验:百龙腾,生化清洗液、离子试剂的领跑者生化分析仪用电解质试剂(电极法)- 测量项目:K+、Na+、Cl-、(Ca2+、CO2)- 特点:专业·稳居行业领先地位;·获北京市自然科学基金资助项目;·在电解质(ISE)领域近20年的研发从业经验,对电极(ISE)及其工作过程有着深刻研究优质·直接装机,与原厂产品完全互换,不需调整仪器参数。
测量结果与原厂试剂相关系数≥0.998,结果无显著性差异·采用进口添加剂,长期使用更能延长电极寿命生化分析仪专用清洗液-居国内行业领先地位- 久经考验,产品齐全,几乎涵盖国内外所有生化仪,并为国内数个生化分析仪器厂家合作提供专用清洗液或解决方案- 每种仪器所需清洗液量体裁衣,专机专用,既清洗彻底,又保护机器- 进口分装或使用进口原料生产,产品外观(颜色、气味等)与原厂产品一致- 产品经市场多年考验,对测量结果及仪器均无不良影响。
仿制药参比制剂目录(第六十三批)
欧盟上市
63-27
匹伐他汀钙片
Pitavastatin Caical Europe GmbH
未进口原研药品
欧盟上市
63-28
匹伐他汀钙片
Pitavastatin Calcium Tablets
2mg
Kowa Pharmaceutical Europe GmbH
未进口原研药品
增加持证商Angelini Pharma Česká Republika s.r.o.
27-423
左甲状腺素钠片
Levothyroxine Sodium Tablets/Euthyrox;Levothyrox
100μg(以左甲状腺素钠计)
Merck Serono GmbH/Merck Sante/Merck GesellschaftmbH/Merck Healthcare Germany GmbH
100ml:1g(10mg/ml)
B Braunmedical Inc
未进口原研药品
美国橙皮书
63-14
钆特醇注射液
Gadoteridol Injection
/ProHance
279.3mg/mL(1.3965 g/5mL)
Bracco Diagnostics Inc
未进口原研药品
美国橙皮书
63-15
63-241
依折麦布瑞舒伐他汀锌胶囊
Ezetimibe rosuvastatin zinc hard capsule/Cholecomb
20mg/10mg
Proterapia Hungary Ltd
未进口原研药品
欧盟上市
63-251
盐酸氨酮戊酸凝胶
aminolevulinic acid hydrochloride gel/AMELUZ
OECD Test No. 460 Fluorescein Leakage Test Method for Identifying Ocular Corrosives and Severe Irrit

OECD/OCDE460Adopted: 2 October 2012© OECD, (2012)You are free to use this material for personal, non-commercial purposes without seeking prior consent from the OECD, provided the source is duly mentioned. Any commercial use of this material is subject to written permission from the OECD.OECD GUIDELINE FOR THE TESTING OF CHEMICALS Fluorescein Leakage Test Method for Identifying Ocular Corrosives and Severe IrritantsINTRODUCTION1. The Fluorescein Leakage (FL) test method is an in vitro test method that can be used under certain circumstances and with specific limitations to classify chemicals (substances and mixtures) as ocular corrosives and severe irritants, as defined by the United Nations (UN) Globally Harmonized System of Classification and Labelling of Chemicals (GHS) (Category 1), the European Union (EU) Regulation on Classification, Labelling and Packaging of Substances and Mixtures (CLP) (Category 1), and the U.S. Environmental Protection Agency (EPA) (Category I) (1) (2) (3). For the purpose of this Test Guideline, severe irritants are defined as chemicals that cause tissue damage in the eye following test substance administration that is not reversible within 21 days or causes serious physical decay of vision, while ocular corrosives are chemicals that cause irreversible tissue damage to the eye. These chemicals are classified as UN GHS Category 1, EU CLP Category 1, or U.S. EPA Category I.2. While the FL test method is not considered valid as a complete replacement for the in vivo rabbit eye test, the FL is recommended for use as part of a tiered testing strategy for regulatory classification and labelling. Thus, the FL is recommended as an initial step within a Top-Down approach to identify ocular corrosives/severe irritants, specifically for limited types of chemicals (i.e. water soluble substances and mixtures) (4)(5).3. It is currently generally accepted that, in the foreseeable future, no single in vitro eye irritation test will be able to replace the in vivo eye test (TG 405 (6)) to predict across the full range of irritation for different chemical classes. However, strategic combinations of several alternative test methods within a (tiered) testing strategy may be able to replace the in vivo eye test (5). The Top-Down approach (5) is designed to be used when, based on existing information, a chemical is expected to have high irritancy potential.Based on the prediction model detailed in paragraph 35, the FL test method can identify Category 1; EU CLP Category 1; U.S. EPA Category I) without any further testing. The same is assumed for mixtures although mixtures were not used in the validation. Therefore, the FL test method may be used to determine the eye irritancy/corrosivity of chemicals, following the460OECD/OCDEsequential testing strategy of TG 405 (6). However, a chemical that is not predicted as ocular corrosive or severe irritant with the FL test method would need to be tested in one or more additional test methods (in vitro and/or in vivo) that are capable of accurately identifying i) chemicals that are in vitro false negative ocular corrosives/severe irritants in the FL (UN GHS Category 1; EU CLP Category 1; U.S. EPA Category I); ii) chemicals that are not classified for eye corrosion/irritation (UN GHS No Category; EU CLP No Category; U.S. EPA Category IV); and/or iii) chemicals that are moderate/mild eye irritants (UN GHS Categories 2A and 2B; EU CLP Category 2; U.S. EPA Categories II and III).5. The purpose of this Test Guideline is to describe the procedures used to evaluate the potential ocular corrosivity or severe irritancy of a test substance as measured by its ability to induce damage to an impermeable confluent epithelial monolayer. The integrity of trans-epithelial permeability is a major function of an epithelium such as that found in the conjunctiva and the cornea. Trans-epithelial permeability is controlled by various tight junctions. Increasing the permeability of the corneal epithelium in vivo has been shown to correlate with the level of inflammation and surface damage observed as eye irritation develops.6. In the FL test method, toxic effects after a short exposure time to the test substance are measured by an increase in permeability of sodium fluorescein through the epithelial monolayer of Madin-Darby Canine Kidney (MDCK) cells cultured on permeable inserts. The amount of fluorescein leakage that occurs is proportional to the chemical-induced damage to the tight junctions, desmosomal junctions and cell membranes, and can be used to estimate the ocular toxicity potential of a test substance. Annex I provides a diagram of MDCK cells grown on an insert membrane for the FL test method.7. Definitions are provided in Annex II.INITIAL CONSIDERATIONS AND LIMITATIONS8. This Test Guideline is based on the INVITTOX protocol No. 71 (7) that has been evaluated in an international validation study by the European Centre for the Validation of Alternative Methods (ECVAM) (8), in collaboration with the US Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) and the Japanese Center for the Validation of Alternative Methods (JaCVAM).9. The FL test method is not recommended for the identification of chemicals which should be classified as mild/moderate irritants or of chemicals which should not be classified for ocular irritation (substances and mixtures) (i.e. GHS Cat. 2A/2B, no category; EU CLP Cat. 2, no category; US EPA Cat. II/III/IV), as demonstrated by the validation study (4) (8).10. The test method is only applicable to water soluble chemicals (substances and mixtures). The ocular severe irritation potential of chemicals that are water soluble and/or where the toxic effect is not affected by dilution is generally predicted accurately using the FL test method (8). To categorise a chemical as water soluble, under experimental conditions, it should be soluble in sterile calcium-containing (at a concentration of 1.0-1.8 mM), phenol red-free, Hanks’ Buffered Salt Solution (HBSS) at a concentration ≥ 250 mg/mL (one dose above the cut-off of 100 mg/mL). However, if the test substance is soluble below the concentration 100 mg/mL,2© OECD, (2012)OECD/OCDE 460 but already induces a FL induction of 20 % at that concentration (meaning FL20 < 100 mg/mL), it can still be classified as GHS Cat. 1 or EPA Cat. 1.11. The identified limitations for this test method exclude strong acids and bases, cell fixatives and highly volatile chemicals from the applicability domain. These chemicals have mechanisms that are not measured by the FL test method, e.g. extensive coagulation, saponification or specific reactive chemistries. Other identified limitations for this method are based upon the results for the predictive capacity for coloured and viscous test substance (8). It is suggested that both types of chemicals are difficult to remove from the monolayer following the short exposure period and that predictivity of the test method could be improved if a higher number of washing steps was used. Solid chemicals suspended in liquid have the propensity to precipitate out and the final concentration to cells can be difficult to determine. When substances within these chemical and physical classes are excluded from the database, the accuracy of FL across the EU, EPA, and GHS classification systems is substantially improved (8).12. Based on the purpose of this test method (i.e. to identify ocular corrosives/severe irritants only), false negative rates (see Paragraph 13) are not critical since such substances would be subsequently tested with other adequately validated in vitro tests or in rabbits, depending on regulatory requirements, using a sequential testing strategy in a weight of evidence approach (6) (see also paragraphs 3 and 4).13. Other identified limitations of the FL test method are based on false negative and false positive rates. When used as an initial step within a Top-Down approach to identify water soluble ocular corrosive/severe irritant substances and mixtures (UN GHS Category 1; EU CLP Category 1; U.S. EPA Category I), the false positive rate for the FL test method ranged from 7% (7/103; UN GHS and EU CLP) to 9% (9/99; U.S. EPA) and the false negative rate ranged from 54% (15/28; U.S. EPA) to 56% (27/48; UN GHS and EU CLP) when compared to in vivo results. Chemical groups showing false positive and/or false negative results in the FL test method are not defined here.14. Certain technical limitations are specific to the MDCK cell culture. The tight junctions that block the passage of the sodium-fluorescein dye through the monolayer are increasingly compromised with increasing cell passage number. Incomplete formation of the tight junctions results in increased FL in the non-treated control. Therefore, a defined permissible maximal leakage in the non-treated controls is important (see paragraph 38: 0% leakage). As with all in vitro assays there is the potential for the cells to become transformed over time, thus it is vital that passage number ranges for the assays are stated.15. The current applicability domain might be increased in some cases, but only after analyzing an expanded data set of studied test substances, preferably acquired through testing (4). This Test Guideline will be updated accordingly as new information and data are considered.16. For any laboratory initially establishing this assay, the proficiency chemicals provided in Annex III should be used. Laboratories can use these chemicals to demonstrate their technical competence in performing the FL test method prior to submitting FL assay data for regulatory hazard classification purposes.PRINCIPLE OF THE TEST3© OECD, (2012)460OECD/OCDE17. The FL test method is a cytotoxicity and cell-function based in vitro assay that is performed on a confluent monolayer of MDCK CB997 tubular epithelial cells that are grown on semi-permeable inserts and model the non-proliferating state of the in vivo corneal epithelium. The MDCK cell line is well established and forms tight junctions and desmosomal junctions similar to those found on the apical side of conjunctival and corneal epithelia. Tight and desmosomal junctions in vivo prevent solutes and foreign materials penetrating the corneal epithelium. Loss of trans-epithelial impermeability, due to damaged tight junctions and desmosomal junctions, is one of the early events in chemical-induced ocular irritation.18. The test substance is applied to the confluent layer of cells grown on the apical side of the insert. A short 1 min exposure is routinely used to reflect the normal clearance rate in human exposures. An advantage of the short exposure period is that water-based substances and mixtures can be tested neat, if they can be easily removed after the exposure period. This allows more direct comparisons of the results with the chemical effects in humans. The test substance is then removed and the non-toxic, highly fluorescent sodium-fluorescein dye is added to the apical side of the monolayer for 30 minutes. The damage caused by the test substance to the tight junctions is determined by the amount of fluorescein which leaks through the cell layer within a defined period of time.19. The amount of sodium-fluorescein dye that passes through the monolayer and the insert membrane into a set volume of solution present in the well (to which the sodium-fluorescein dye leaks in) is determined by measuring spectrofluorometrically the fluorescein concentration in the well. The amount of fluorescein leakage (FL) is calculated with reference to fluoresence intensity (FI) readings from two controls: a blank control, and a maximum leakage control. The percentage of leakage and therefore amount of damage to the tight junctions is expressed, relative to these controls, for each of the set concentrations of the test substance. Then the FL20 (i.e. concentration that causes 20% FL relative to the value recorded for the untreated confluent monolayer and inserts without cells), is calculated. The FL20 (mg/mL) value is used in the prediction model for identification of ocular corrosives and severe irritants (see paragraph 35).20. Recovery is an important part of a test substance’s toxicity profile that is also assessed by the in vivo ocular irritation test. Preliminary analyses indicated that recovery data (up to 72 h following the chemical exposure) could potentially increase the predictive capacity of INVITTOX Protocol 71 but further evaluation is needed and would benefit from additional data, preferably acquired by further testing (7). This Test Guideline will be updated accordingly as new information and data are considered.PROCEDUREPreparation of the cellular monolayer21. The monolayer of MDCK CB997 cells is prepared using sub-confluent cells growing in cell culture flasks in DMEM/Nutrient Mix F12 (1x concentrate with L-glutamine, 15 mM HEPES, calcium (at a concentration of 1.0-1.8 mM) and 10% heat-inactivated FCS/FBS). Importantly, all media/solutions used throughout the FL assay should contain calcium at a concentration between 1.8 mM (200 mg/L) and 1.0 mM (111 mg/L) to ensure tight junction formation and integrity. Cell passage number range should be controlled to ensure even and4© OECD, (2012)OECD/OCDE 460 reproducible tight junctions formation. Preferably, the cells should be within the passage range 3-30 from thawing because cells within this passage range have similar functionality, which aids assay results to be reproducible.22. Prior to performing the FL test method, the cells are detached from the flask by trypsinisation, centrifuged and an appropriate amount of cells is seeded into the inserts placed in 24-well plates (see Annex I). Twelve mm diameter inserts with membrane of mixed cellulose esters, a thickness of 80-150 µm and a pore size of 0.45 µm, should be used to seed the cells. In the validation study, Millicell-HA 12 mm inserts were used. The properties of the insert and membrane type are important as these may affect cell growth and chemical binding. Certain types of chemicals may bind to the Millicell-HA insert membrane, which could affect the interpretation of results. Proficiency chemicals (see Annex III) should be used to demonstrate equivalency if other membranes are used.23. Chemical binding to the insert membrane is more common for cationic chemicals, such as benzalkonium chloride, which are attracted to the positively charged membrane (8). Chemical binding to the insert membrane may increase the chemical exposure period, leading to an over-estimation of the toxic potential of the chemical, but can also physically reduce the leakage of fluorescein through the insert by binding of the dye to the cationic chemical bound to the insert membrane, leading to an under-estimation of the toxic potential of the chemical. This can be readily monitored by exposing the membrane alone to the top concentration of the chemical tested and then adding sodium-fluorescein dye at the normal concentration for the standard time (no cell control). If binding of the sodium-fluorescein dye occurs, the insert membrane appears yellow after the test material has been washed-off. Thus, it is essential to know the binding properties of the test substance in order to be able to interpret the effect of the chemical on the cells.24. Cell seeding on inserts should produce a confluent monolayer at the time of chemical exposure. 1.6 x 105 cells should be added per insert (400 µL of a cell suspension with a density of 4 x 105 cells / mL). Under these conditions, a confluent monolayer is usually obtained after 96 hours in culture. Inserts should be examined visually prior to seeding, so as to ensure that any damages recorded at the visual control described at paragraph 30 is due to handling.25. The MDCK cell cultures should be kept in incubators in a humidified atmosphere, at 5% ± 1% CO2and 37 ± 1 ºC. The cells should be free of contamination by bacteria, viruses, mycoplasma and fungi.Application of the Test and Control Chemicals26. A fresh stock solution of test substance should be prepared for each experimental run and used within 30 minutes of preparation. Test substances should be prepared in calcium-containing (at a concentration of 1.0-1.8 mM), phenol red-free, HBSS to avoid serum protein binding. Solubility of the chemical at 250 mg/mL in HBSS should be assessed prior to testing. If at this concentration the chemical forms a stable suspension or emulsion (i.e.maintains uniformity and does not settle or separate into more than one phase) over 30 minutes, HBSS can still be used as solvent. However, if the chemical is found to be insoluble in HBSS at this concentration, the use of other test methods instead of FL should be considered. The use of light mineral oil as a solvent, in cases where the chemical is found to be insoluble in HBSS, should be5© OECD, (2012)460OECD/OCDEconsidered with caution as there is not enough data available to conclude on the performance of the FL assay under such conditions.27. All chemicals to be tested are prepared in sterile calcium-containing (at a concentration of 1.0-1.8 mM), phenol red-free, HBSS from the stock solution, at five fixed concentrations diluted on a weight per volume basis: 1, 25, 100, 250 mg/mL and a neat or a saturated solution. When testing a solid chemical, a very high concentration of 750 mg/mL should be included. This concentration of chemical may have to be applied on the cells using a positive displacement pipette. If the toxicity is found to be between 25 and 100 mg/mL, the following additional concentrations should be tested twice: 1, 25, 50, 75, 100 mg/mL. The FL20value should be derived from these concentrations provided the acceptance criteria were met.28. The test substances are applied to the confluent cell monolayers after removal of the cell culture medium and washing twice with sterile, warm (37ºC), calcium-containing (at a concentration of 1.0-1.8 mM), phenol red-free, HBSS. Previously, the filters have been visually checked for any pre-existing damages that could be falsely attributed to potential incompatibilities with test chemicals. At least three replicates should be used for each concentration of the test substance and for the controls in each run. After 1 min of exposure at room temperature, the test substance should be carefully removed by aspiration, the monolayer should be washed twice with sterile, warm (37ºC), calcium-containing (at a concentration of 1.0-1.8 mM), phenol red-free, HBSS, and the fluorescein leakage should be immediately measured. 29. Concurrent negative (NC) and positive controls (PC) should be used in each run to demonstrate that monolayer integrity (NC) and sensitivity of the cells (PC) are within a defined historical acceptance range. The suggested PC chemical is Brij 35 (CAS No. 9002-92-0) at 100 mg/mL. This concentration should give approximately 30% fluorescein leakage (acceptable range 20-40% fluorescein leakage, i.e. damage to cell layer). The suggested NC chemical is calcium-containing (at a concentration of 1.0-1.8 mM), phenol red-free, HBSS (untreated, blank control).A maximum leakage control should also be included in each run to allow for the calculation of FL20 values. Maximum leakage is determined using a control insert without cells.Determination of fluorescein permeability30. Immediately after removal of the test and control substances, 400μL of 0.1 mg/mL sodium-fluorescein solution (0.01% (w/v) in calcium-containing [at a concentration of 1.0-1.8 mM], phenol red-free, HBSS) is added to the Millicell-HA inserts. The cultures are kept for 30 minutes at room temperature. At the end of the incubation with fluorescein, the inserts are carefully removed from each well. Visual check is performed on each filter and any damage which may have occurred during handling is recorded.31. The amount of fluorescein that leaked through the monolayer and the insert is quantified in the solution which remained in the wells after removal of the inserts. Measurements are done in a spectrofluorometer at excitation and emission wavelengths of 485 nm and 530 nm, respectively. The sensitivity of the spectrofluorometer should be set so that there is the highest numerical difference between the maximum FL (insert with no cells) and the minimum FL (insert with confluent monolayer treated with NC). Because of the differences in the used spectrofluorometer, it is suggested that a sensitivity is used which will give fluorescence intensity > 4000 at the maximum fluorescein leakage control. The maximum FL value should not be6© OECD, (2012)OECD/OCDE 460 greater than 9999. The maximum fluorescence leakage intensity should fall within the linear range of the spectrofluorometer used.Interpretation of results and Prediction model32. The amount of FL is proportional to the chemical-induced damage to the tight junctions. The percentage of FL for each tested concentration of chemical is calculated from the FL values obtained for the test substance with reference to FL values from the NC (reading from the confluent monolayer of cells treated with the NC) and a maximum leakage control (reading for the amount of FL through an insert without cells).The mean maximum leakage fluorescence intensity = xThe mean 0% leakage fluorescence intensity (NC) = yThe mean 100% leakage is obtained by subtracting the mean 0% leakage from the mean maximum leakage,i.e. x - y = z33. The percentage leakage for each fixed dose is obtained by subtracting the 0% leakage to the mean fluorescence intensity of the three replicate readings (m), and dividing this value by the 100% leakage, i.e. %FL = [(m-y) / z] x 100%, where:m = the mean fluorescence intensity of the three replicate measurements for the concentration involved% FL = the percent of the fluorescein which leaks through the cell layer34. The following equation for the calculation of the chemical concentration causing 20% FL should be applied:FL D = [(A-B) / (C-B)] x (M C –M B) + M BWhere:D = % of inhibitionA = % damage (20% fluorescein leakage)B = % fluorescein leakage < AC = % fluorescein leakage > AM C = Concentration (mg/mL) of CM B = Concentration (mg/mL) of B35. The cut-off value of FL20 for predicting chemicals as ocular corrosives/severe irritants is given below:7© OECD, (2012)460OECD/OCDE36. The FL test method is recommended only for the identification of water soluble ocular corrosives and severe irritants (UN GHS Category 1, EU CLP Category 1, U.S. EPA Category I) (see paragraphs 1 and 10).37. In order to identify water soluble chemicals (substances and mixtures) (4) (7) (8) as "inducing serious eye damage" (UN GHS/EU CLP Category 1) or as an "ocular corrosive or severe irritant" (U.S. EPA Category I), the test substance should induce an FL20 value of ≤ 100 mg/mL.Acceptance of results38. The mean maximum fluorescein leakage value (x) should be higher than 4000 (see paragraph 31), the mean 0% leakage (y) should be equal or lower than 300, and the mean 100% leakage (z) should fall between 3700 and 6000.39. A test is considered acceptable if the positive control produced 20% to 40% damage to the cell layer (measure as % fluorescein leakage).DATA AND REPORTINGData40. For each run, data from individual replicate wells (e.g. fluorescence intensity values and calculated percentage FL data for each test substance, including classification) should be reported in tabular form. In addition, means ± SD of individual replicate measurements in each run should be reported.Test Report41. The test report should include the following information:Test and Control Substances-Chemical name(s) such as the structural name used by the Chemical Abstracts Service (CAS), followed by other names, if known;-Chemical CAS number, if known;-Purity and composition of the substance or mixture (in percentage(s) by weight), to the extent this information is available;-Physical-chemical properties relevant to the conduct of the study (e.g. physical state, volatility, pH, stability, water solubility, chemical class);-Treatment of the test/control substance prior to testing, if applicable (e.g. warming, grinding);-Storage conditions;Justification of the Test Method and Protocol Used-Should include considerations regarding applicability domain and limitations of the test method;Test Conditions8© OECD, (2012)OECD/OCDE 460 -Description of cell system used, including certificate of authenticity and the mycoplasma status of the cell line;-Details of test procedure used;-Test substance concentration(s) used;-Duration of exposure to the test substance;-Duration of incubation with fluorescein;-Description of any modifications of the test procedure;-Description of evaluation criteria used;-Reference to historical data of the model (e.g. negative and positive controls, benchmark chemicals, if applicable);-Information on the technical proficiency demonstrated by the laboratory;Results-Tabulation of data from individual test substances and controls for each run and each replicate measurement (including individual results, means and SDs);-The derived classification(s) with reference to the prediction model and/or decision criteria used;-Description of other effects observed;Discussion of the Results-Should include considerations regarding a non-conclusive outcome (paragraph 35: FL20 > 100 mg/mL) and further testing;Conclusions9© OECD, (2012)460OECD/OCDELITERATURE1.UN (2009), United Nations Globally Harmonized System of Classification and Labelling ofChemicals (GHS), Third revised edition, New York & Geneva: United Nations Publications.ISBN: 978-92-1-117006-1. Available at:[/trans/danger/publi/ghs/ghs_rev03/03files_e.html]2.EC (2008), Regulation (EC) No 1272/2008 of the European Parliament and of the Council of16 December 2008 on classification, labelling and packaging of substances and mixtures,amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006, Official Journal of the European Union L353, 1-1355.3.U.S. EPA (1996), Label Review Manual: 2nd Edition, EPA737-B-96-001, Washington DC:U.S. Environmental Protection Agency.4.EC-ECVAM (2009), Statement on the scientific validity of cytotoxicity/cell-function based invitro assays for eye irritation testing. Available under Publications at: [http://ecvam.jrc.it/index.htm]5.Scott, L. et al. (2010), A proposed eye irritation testing strategy to reduce and replace in vivostudies using Bottom-Up and Top-Down approaches, Toxicol. In Vitro 24, 1-9.6.OECD (2002), Test No. 405: Acute Eye Irritation/Corrosion, OECD Guidelines for theTesting of Chemicals, Section 4, OECD Publishing. doi: 10.1787/9789264070646-en7.EC-ECVAM (1999), INVITOX Protocol 71: Fluorescein Leakage Test, Ispra, Italy:European Centre for the Validation of Alternative Methods (ECVAM). Available at: [http://ecvam-dbalm.jrc.ec.europa.eu]8.EC-ECVAM (2008), Fluorescein Leakage Assay Background Review Document as anAlternative Method for Eye Irritation Testing. Available under Validation Study Documents, Section Eye Irritation at: [http://ecvam.jrc.it/index.htm]9.OECD (2005), Guidance Document on the Validation and International Acceptance of Newor Updated Test Methods for Hazard Assessment, OECD Series on Testing and Assessment No. 34. OECD, Paris. Available at: [/env/testguidelines]10© OECD, (2012)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jul.-26-2017Print Date:Jul.-26-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Laurolitsine (hydrochloride)Catalog No. :HY-N2352ACAS No. :None1.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:(+)–Norboldine hydrochlorideFormula:C18H20ClNO4Molecular Weight:349.81CAS No. :None4. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
