Effective esterification of carboxylic acids using
三氟化硼甲醇酯化法

三氟化硼甲醇酯化法【标题】三氟化硼甲醇酯化法:一种高效、环保的化学反应方法【导言】在化学领域,合成新化合物的方法是科研和工业生产的重要基础。
其中,酯化反应是一项常见的重要化学反应,对于合成有机分子和药物具有广泛的应用。
而传统的酯化方法往往需要使用相对较多的反应试剂,并伴随着环境污染问题。
然而,近年来,全球科学家们发展出了一种高效、环保的酯化反应方法——三氟化硼甲醇酯化法,该方法在有机合成领域引起了广泛的关注。
【正文】一、三氟化硼甲醇酯化法的基本原理三氟化硼甲醇酯化法是一种新兴的酯化反应方法,其基本原理是利用三氟化硼和甲醇作为反应物,在适宜的条件下,通过酯化反应将酸和醇结合,生成酯化产物。
与传统酯化反应相比,三氟化硼甲醇酯化法具有以下特点:1. 反应条件温和:三氟化硼甲醇酯化法在常温下即可进行,大大降低了反应所需的能量消耗。
2. 催化效果显著:三氟化硼作为催化剂,能够有效促进酯化反应的进行,提高反应速率和产率。
三氟化硼可被循环使用,具有良好的催化寿命。
3. 反应副产物少:在传统酯化反应中,反应副产物通常较多,需要经过复杂的分离和纯化过程。
而三氟化硼甲醇酯化法生成的产物纯度较高,减少了后续处理的工作量,提高了反应的经济性。
二、三氟化硼甲醇酯化法的应用领域三氟化硼甲醇酯化法在有机化学合成领域涉及广泛。
其主要应用领域如下:1. 药物合成:在药物研发和合成中,酯化反应是常用的工具。
三氟化硼甲醇酯化法能够高效地合成多种药物酯化产物,如麻醉剂、抗癌药物等,对药物的活性和稳定性起到了重要的影响。
2. 化学品合成:三氟化硼甲醇酯化法也广泛应用于其他化学品的合成过程,如合成香料、染料、香精等领域。
其高效、低能耗的特点受到了工业界的青睐。
三、个人观点与思考作为一种新兴的合成方法,三氟化硼甲醇酯化法在有机合成领域具有巨大的潜力。
它不仅提高了反应速率和产率,还减少了反应副产物和能源消耗,从而达到了环境友好的目的。
我认为,随着对环境保护和可持续发展的要求越来越高,三氟化硼甲醇酯化法在未来将有更广泛的应用前景。
盐酸普鲁卡因的合成

盐酸普鲁卡因的合成张星(西北民族大学化工学院甘肃省兰州 730030)摘要目的合成局部麻醉药盐酸普鲁卡因。
方法利用水和二甲苯共沸脱水的原理进行羧酸的酯化,用盐析法对大分子物质进行分离及精制。
结果得盐酸普鲁卡因粗品2.5g,精制后称得0.4g,最终得盐酸普鲁卡因的产率是16%。
结论通过实验学习盐酸普鲁卡因的合成步骤,学习酯化,还原等单元反应。
由于对实验的操作方法的不熟练,导致了实验的误差。
关键词盐酸普鲁卡因的合成;酯化;盐析;硝基卡因The Synthesis Process of Procaine HydrochlorideZHANG Xing(College of Chemcial Engineering ,Northwest University for Nationalities , Lanzhou730030 ,China)Abstract Purpose Synthesis process of local anesthetic procaine hydrochloride. Method To get on the esterification of carboxylic acids by principle of water and xylene azeotropic dehydration,via salting out of macromolecules can be isolated and purified. Result Get Procaine hydrochloride crude 2.5g,after refined it get 0.4g.Finally the yield of Procaine Hyrochloride is 16%. Conclusion Through the experimental can study the synthesis step of Procaine hydrochloride.To learn the process of esterification, reduction, etc. Because of the unskilled of experimental procedure,leading the experimental error.Key words synthesis process of Procaine hydrochloride ; esterificario ; reduction ; the nitro paid盐酸普鲁卡因(chloroprocaine hyrochloride)作为一种酯类局麻药,在体内的代谢、麻醉效能与盐酸普鲁卡因相似,临床上主要用于浸润麻醉、产科阻滞麻醉和硬膜外麻醉等【3】。
有机化学中常见名词中英文对照

有机化学中常见名词中英文对照英中对照abietic acid (松香酸)acetal(缩醛)acid anhydride(酸酐)A.Couper(古柏尔)acridine(吖啶)acronycine (山油柑碱)acidylating reaction(酰化反应)acyl group(酰基)acyl halide (酰卤)adenine(腺嘌呤)adrenal cortex hormone (肾上腺皮质激素) A.Kekule(开库勒)alanine(丙氨酸)alcoholysis(醇解)aldehyde(醛)alicyclic hydrocarbon(脂环烃)alizarin(茜草素)alizarin-type(茜素型)alkane(烷烃)alkene(烯烃)alkylation(傅-克烷基化反应)alkyne(炔烃)aloeemodin (芦荟大黄素)amines(胺类)amide(酰胺)amidino(脒基)amino acid(氨基酸)β-aminobutyric acid(β-氨基丁酸)ammonolysis(氨解)andiron formula(锯架式)andrographolide (穿心莲内酯)anisodine (樟柳碱)annulene(轮烯)anomer(异头物)anomeric effect(异头效应)anthocyanidin (花色素)anthraquinone (蒽醌)anthrol (蒽酚)anthrone (蒽酮)anthracene(蒽)antiaromaticity or antiaromatic compound(反芳香性化合物)apigenin (芹菜素)apple polyphenols (苹果多酚) aromatic compound(芳香性化合物)aromatic hydrocarbon(芳香烃)aromaticity(芳香性)aromatization(芳构化)arecoline (槟榔碱)arginine(精氨酸)aspartic acid(天冬氨酸)asymmetric carbon atom (手性碳原子) atomic orbital(原子轨道)A.Wurtz reaction(武兹反应)axial bond(直立键,a键)azulene)Baeyer(拜耳)baicalein (黄芩素)baicalin (黄芩苷)barbital(巴比妥)barbituric acid(巴比妥酸) base complementary(碱基配对) benzoimidazole(苯并咪唑)benzothiazole(苯并噻唑)benzene(苯)berberine (小檗碱)Berzelius(伯察留史)beta-pleated sheet( -折叠)bi-anthracene nucleus (双蒽核) biological methylate(生物甲基化)biuret reaction(缩二脲反应)σbond (σ键)πbond (π键)borneol (龙脑)Braun reaction (布朗反应)bridged hydrocarbon(桥环烃)bytrepob(布特力洛夫)camphor(樟脑)cardiac glycosides(强心苷) camptothecine (喜树碱)carotene (胡萝卜素)carthamin (红花苷)carbene(卡宾;碳烯)carbohydrate(碳水化合物)carbonyl (羰基)carboxyl(羧基)carboxylic acid(羧酸)cassiamine (山扁豆双醌)catechin (儿茶素)cellobiose(纤维二糖)cellulose(纤维素)cephalin(脑磷脂)chain carbon constitution(链状碳架)chain initiation step(链引发阶段)chain propagation step(链增长阶段)chain termination step(链终止阶段)chalcone (查尔酮)charge-transfer complex(电荷转移络合物)chemical bond(化学键)chemocholic acid(鹅去氧胆酸)chirality(手性)chitin(甲壳质)chitosamine(壳糖胺)chlorophyll(叶绿素)cholalic acid (胆甾酸)cholestane (胆甾烷)cholesterol (胆甾醇)chromatography(色谱法)chrysophanol 9-anthrone (9-蒽酮大黄酚)chrysarobin (柯桠素)cinchonine(金鸡宁)cis-trans isomer(顺反异构体)cistrans isomerism(顺反异构)citral(柠檬醛)Claisen rearrangement(克莱森重排)Claisen-Schmidt reaction(克莱森-斯密特反应)cocaine (古柯碱)codonopsine (党参碱)concerted reaction(协同反应)condensed nuclei hydrocarbon(稠环烃)conformation(构象)conformational isomerism(构象异构)coniine (毒芹碱)conjugated diene(共轭二烯烃)conjugation system (共轭体系)conjugative effect(共轭效应)conservation of orbital symmetry(分子轨道对称性守恒理论)constitutional isomerism (构造异构)coprostane (粪甾烷)cortisone (可的松)crown ether(冠醚)cumulative diene(聚集二烯烃)curcumenol (莪术醇)cyanidin (矢车菊素)cyclic carbon constitution(环状碳架)cycloaddition recation(环加成反应)cycloalkane (脂环烃)cyclodextrin(环糊精)cysteine(半胱氨酸)daidzein (大豆黄素)Darzens reaction(达尔森反应) decarboxylation(脱羧反应) delocaization(离域)delocalization energy(离域能) delocalized electron(离域电子)delocalized energy(离域能)delphinidin(飞燕草素)denature(变性)deoxyribonucleic acid(脱氧核糖核酸) derivative of carboxylic acid (羧酸衍生物) diastereoisomer(非对映体)diazotization reaction(重氮化反应)diazonium salt(重氮盐)diborane(乙硼烷)dichlorocarbene(二氯卡宾)β-dichroine (β-常山碱)Diels—Alder reaction(狄尔斯—阿尔德反应)diene(双烯体,二烯烃)dienophile(亲双烯体)dihydrochalcone (二氢查尔酮) β—dihydrotheelin (β—雌二醇)distillation(蒸馏法)diterpenoids (二萜类)effective atomic number(有效原子序数)E.J.Cory—H.House reaction(科瑞—郝思反应)electric field scan(电场扫描)18-electron rule(18电子规则)electromeric effect(电性效应)electrophilic addition(亲电加成反应)electrophilic substitution(亲电取代)electrophile(亲电性试剂)elimination reaction(消除反应)Emde degradation (埃姆德降解)1-emetine(1-吐根碱)emodin-type (大黄素型)enantiomerism(对映异构)enantiomer(对映体)end-group effect(端基效应)entgegen(E,相反之意)energy of activation(活化能)enzyme(酶)ephedrine (麻黄碱)epicatechin (表儿茶素)epicatechin gallate(表儿茶素没食子酸酯) epigallocatechin (表没食子儿茶素)epigallocatechin gallate (表没食子儿茶素,没食子酸酯) epimer(差向异构体)epoxidation(环氧化反应)equatorial bond(平伏键,e键)ergometrine(麦角新碱)ergostenol (麦角甾醇)essential amino acid(必需氨基酸)essential fatty acid(必需脂肪酸)ester(酯)esterification(酯化反应)extraction(萃取法)farnesol (金合欢醇)fatty acid(脂肪酸)Fischer projection formula(费歇尔投影式)flavanol (黄烷醇)flavanone (二氢黄酮)flavanonol (二氢黄酮醇)flavonoid (黄酮)flavonol (黄酮醇)formalin(福尔马林)free radical (自由基)free radical chain reaction (自由基链反应) Freon (氟利昂)Friedel-Crafts reaction (傅瑞德尔-克拉夫兹反应) reagent ( 试剂 )frontier orbital (前线轨道理论)fructose (果糖)fucose (海藻糖)furan (呋喃)fused ring carbon constitution (稠环碳架) F.Wohler (武勒)Gabreil reaction (盖布瑞尔合成法)galactose (半乳糖)..Frohde ..Frohdegallocatechin (没食子儿茶素)gallocatechin gallate (没食子儿茶素没食子酸酯) Gattermann-Koch reaction(盖特曼-科希反应)geometricalisomer(几何异构体)germacrone (杜鹃酮)glucose(葡萄糖)glutamic acid(谷氨酸)glutamine(谷酰胺)glycerol(甘油)glycocholic acid (甘氨胆酸)glycogen(糖原)glycoside(糖苷)glycyrrhizic acid (甘草酸)glycyrrhetinicacid (甘草次酸)Gmelin(哥美林)green tea polyphenols(绿茶多酚)Grignard Reaction(格氏反应)Grignard Reagent(格林那试剂,格氏试剂)G.Schiemann reaction(希曼反应)guaiazulene (愈创木奥)guanidine(胍)guanidino (胍基)guanine(鸟嘌呤)guanyl (脒基)haloform(卤仿)halogenation(卤代反应)halogenation reaction(卤化反应)Haworth(哈沃斯)heat of hydrogenation(氢化热)heat of reaction(反应热)hemiacetal(半缩醛)hesperetin (橙皮素)Hinsberg reaction(兴斯堡反应)histidine(组氨酸)H.Kolbe(科尔贝)Hoffmann degradation(霍夫曼降解反应)Hoffmann elimination(霍夫曼消除)Hoffmann exhaustive methylation(霍夫曼彻底甲基化反应)HOMO(Highest Occupied Molecnlar Orbital,最高被占用分子轨道) homolog(同系物)homologous series(同系列)hormone (激素)Hückel rule(休克尔规则)hybrid orbital(杂化轨道)hydroboration(硼氢化反应)hydrocortisone (氢化可的松) hydrogen bond(氢键)hydrolysis(水解)hyoscyamine (莨菪碱) hyperconjugation effect(超共轭效应)imidazole(咪唑)inclusion compound(包含物)indole(吲哚)inductive effect(诱导效应)infrared spectroscopy(红外光谱)insulin(胰岛素)invert sugar(转化糖)iodine number(碘值)tectoridin (鸢尾苷)isoelectric point(PI,等电点)isoflavanone (二氢异黄酮)isoflavone (异黄酮)isolated diene(隔离二烯烃)isoleucine(异亮氨酸)isoliquiritigenin (异甘草素)isomer(同分异构体)isoquinoline(异喹啉)isorhamnetin (异鼠李素) isorhynchophylline (异钩藤碱)isothiazole(异噻唑)isoxazole(异噁唑)Jones reagent(琼斯试剂)ketal(缩酮)ketone(酮)K.fries rearrangement(傅瑞斯重排)K.Fukui (福井谦一)Knoevenagel reaction(克脑文盖尔反应)Kolbe-Schmidt reaction(柯尔柏-施密特反应)Kutchcrov reaction(库切洛夫反应)lactose(乳糖)lecithin(卵磷脂)leptosidin (莱普西汀)leucine(亮氨酸)leucocyanidin (无色矢车菊素)limonene (苧烯)Lindlar(林德拉)liquiritin (甘草苷)lithium methide(甲基锂)lobeline (山梗菜碱)Lucas reagent(卢卡斯试剂)LUMO(Lowest Unoccupied Molecular Orbital)最低空余分子轨道lupinine (羽扇豆碱)lycopene (番茄红素)lycopodine (石松碱)lysine(赖氨酸)maackiain (高丽槐素)Macquis reagent (Macquis试剂)macrophylline (大叶千里光碱)magnesium acetate reaction (醋酸镁反应)magnetic field scan(磁场扫描)malonyl urea(丙二酰脲)maltose(麦芽糖)Mannich reaction(满尼希反应)mannose(甘露糖)mass spectroscopy(质谱)matrine (苦参碱)M. Besthelot(佰赛儒)Mclafferty(麦可拉费蒂重排)(-)-melacacidin [(-)黑金合欢素]menthol(薄荷醇)(±)-menthol [(±)-薄荷醇] menthone (薄荷酮)mesomer (内消旋体)methionine(蛋氨酸)methylporgestin (甲孕酮)provera(甲孕酮)methyltestosterone (甲基睾丸素) molecular orbital(分子轨道)molecular orbital theory(分子轨道理论)monoanthracene nucleus (单蒽核) monocrotaline (一野百合碱)monomer(单体)monoterpenoids (单萜) monosaccharide(单糖)morphine (吗啡)mutarotation(变旋光现象)naphthalene(萘)narcotine (那可汀)natrium amalgam reaction (钠汞齐反应) (±)-neomenthol [(±)-新薄荷醇] nerol (橙花醇)Newman projection(纽曼投影式)Newman projection formula(纽曼投影式)Nicol prism(尼科尔棱镜)nicotine (烟碱)ninhydrin(茚三酮)nitration(硝化反应)nitro compound(硝基化合物)nonaromatic compound(非芳香性化合物)nonbenzenoid hydrocarbon(非苯芳烃)nuclear magnetic resonance spectroscopy(核磁共振谱)nucleic acid(核酸)nucleophilic addition(亲核加成反应)nucleophilic reagent(亲核试剂)nucleoside(核苷)nucleotide(核苷酸)ocimene (罗勒烯)oligosaccharide(寡糖)frontier orbital (前线轨道理论)Oppenaner oxidizing reaction(欧芬脑尔氧化)optical isomer (旋光异构体)optical rotation instrument(旋光仪)organometallic compound(有机金属化合物)organometallics (金属有机化合物)orientation rule (定位规则)oxanthranol (氧化蒽酚)oxazole (噁唑)oxidation number (氧化值)oxidation state (氧化态)盐)oxonium salt oxymatrine (氧化苦参碱)palmatine (巴马汀)papaverine (罂粟碱)paraffin (烷烃)pelargonidin(天竺葵素)peptide (肽)peptide bond (肽键)peptide linkage(肽键)pericyclic reaction (周环反应)Perkin reaction (柏金反应)permeation (透析法)peroxide (过氧化物)peroxide effect (过氧化物效应)phellamurin (黄柏素-7-O-葡萄糖苷)phenanthrene (菲)phenylalanine (苯丙氨酸)phosphorus ylide(膦叶立德)phylloxanthin (叶黄素)physostigmine (毒扁豆碱)pinacol(频哪醇)pinene (蒎烯)piperine (胡椒碱)plane polarized light (平面偏振光) polycyclic aromatic hydrocarbon(多环芳烃)polymer(聚合物)polynuclear aromatic compound(稠环芳烃)polypeptide(多肽)polyreaction(聚合反应)polysaccharide(多糖)polytetrafluroethyleney(泰氟隆)unsaturated fatty acid(不饱和脂肪酸) precipitation(沉淀法)primary structure(一级结构)proanthocyanidin (原花色素) progesterone (黄体酮)protein(蛋白质)pseudoephedrine (伪麻黄碱)pteridine(蝶啶)purine(嘌呤)pyran(吡喃)pyrazine(吡嗪)pyrazole(吡唑)pyridazine(哒嗪)pyridine(吡啶)pyrimidine(嘧啶)pyrrole(吡咯)quaternary structure(四级结构)quercetin (槲皮素)quinine (奎宁)quinoline(喹啉)quinones(醌)racemic mixture(外消旋体)racemization(外消旋化)rancidity(酸败)Raney Ni(兰尼镍)reaction mechanism(反应历程)Reimer-Tiemann reaction(瑞穆尔-蒂曼反应)reserpine (利血平)residue(残基)resonance energy(共振能)resonance hybrid(共振杂化体)resonance theory(共振论)resonating structure(共振结构式)resveratrol (白藜芦醇)R.B.Woodward(伍德沃德)rhein (大黄酸)R.Hoffmann (霍夫曼)rhynchophylline (钩藤碱)ribonucleic acid(核糖核酸)ribose(核糖)rotation (旋光度)rutin (芦丁)saccharide(糖类)Sandmeyer-Gattermann reaction(桑得迈尔—盖特曼反应)Sandmeyer reaction(桑得迈尔反应)saponification(皂化)saponification number(皂化值)Sarrett reagent(沙瑞特试剂)Sawhares projection(萨哈斯投影式)sawhorse projection formula(锯架式)Schiff’s base(西佛碱)secondary structure(二级结构)securinine (一叶萩碱)sennoside A、B、C、D (番泻苷A、B、C、D) serine(丝氨酸)sesquiterpenoids (倍半萜)sigmatropic reaction(σ键迁移反应)silane(硅烷)single bond(单键)sinoacutine (清风藤碱)β—sitosterol (β—谷甾醇)skyrin (天精,醌茜素)S N(Nucleophilic substitution)(亲核取代)S N1(单分子亲核取代反应)S N2 (双分子亲核取代反sodium borohydride reaction (四氢硼钠反应) sparteine (金雀花碱)specific rotation (比旋光度)sphingomyelin(鞘磷脂)spiro hydrocarbon(螺环烃)squalene (鲨烯)stachydrine (水苏碱)starch(淀粉)stereochemistry (立体化学)stereoisomer(立体异构)stereocpecificity (立体专一性)steroidal compound (甾体化合物)Stevens rearrangement(史蒂文斯重排)stigmastane (豆甾烷)strychnine (士的宁)sucrose(蔗糖)sulfonation(磺化反应)systematic nomenclature(系统命名法)taurocholic acid (牛磺胆酸)tautomer(互变异构体)tautomerism(互变异构现象)taxifolin (黄杉素)tea polyphenols(茶多酚)Teflon (泰氟隆)terpenoid (萜类化合物)tertiary structure(三级结构)testosterone (睾丸素)tetrahydropalmatine (延胡索乙素, 四氢巴马汀) tetramethyl silane(四甲基硅烷)tetraterpenoid(四萜类)thiazole(噻唑)thiophene(噻吩)threonine(苏氨酸)torsional energy(扭转能)torsional strain(扭转张力)transition sate(过渡态)triglyceride(甘油三酯)trimethyl aluminium(三甲基铝)triptolide (雷公藤甲素)triterpenoid(三萜类)tryptophan(色氨酸)tylophorinine (娃儿藤定碱)tyrosine(酪氨酸)uridine(尿嘧啶)urea(脲)urotropine(乌洛托品)valence bond method(价键学说)valine(缬氨酸)visible-ultraviolet spectroscopy(可见-紫外光谱)vitamin A(维生素A)vitamin B12(维生素B12)Wilkinson (威尔克森)Williamson synthesis(威廉森合成法)Wittig reaction(维蒂希反应)zingiberene (姜烯)Zusammen(Z,德文,在一起之意)中英对照1 A1A 反应历程(1A1Areaction mechanism )吖啶(acridine)埃姆德降解(Emde degradation)安息香缩合反应(benzoic condensation reaction)氨基酸(amino acid)β-氨基丁酸(β-aminobutyric acid)氨解(ammonolysis)胺(amines)azulene)2 BAC 反应历程(2BACreaction mechanism )巴比妥(barbital)巴马汀(palmatine)白藜芦醇(resveratrol)拜耳(Baeyer)佰赛儒(M. Besthelot)半缩醛(semiacetal )半胱氨酸(cysteine)半乳糖(galactose)包含物(inclusion compound)苯丙氨酸(phenylalanine)苯(benzene)苯甲酸(benzoic acid)苯二甲酸(benzene dicarboxylic acid)苯并咪唑(benzimidazole)苯并噻唑(benzothiazole)倍半萜(sesquiterpenoid)比旋光度(specific rotation)必需氨基酸(essential amino acid)变性(denature)变旋光现象(mutarotation)表儿茶素(epicatechin)表儿茶素没食子酸酯(epicatechin gallate)表没食子儿茶素(epigallocatechin)表没食子儿茶素没食子酸酯(epigallocatechin gallate) 表面活性剂(surface active agent )槟榔碱(arecoline)丙氨酸(alanine)丙氨酸乙硫酯(ethyl alanine sulfide)丙二酰脲 (malonyl urea)伯察留史(Berzelius)柏金(Perkin)反应薄荷醇(menthol)(±)—薄荷醇((±)—menthol)薄荷酮(menthone)布特力洛夫(Bytrepob)布朗反应(Braun reaction)残基(residue)草酸(oxalic acid)超共轭效应(hyperconjugation effect)差向异构体(epimer)查尔酮(chalcone)茶多酚(tea polyphenols)β-常山碱(β-dichroine)沉淀法(precipitation)橙皮素(hesperetin)橙花醇(nerol)稠环芳烃(polynuclear aromatic compound)稠环碳架(fused ring carbon constitution)醇钠(sodium alcohols)醇解(alcoholysis)穿心莲内酯(andrographolide)磁场扫描(magnetic field scan)β—雌二醇(β—dihydrotheelin)醋酸镁反应(magnesium acetate reaction )萃取法(extraction)DDQ(2,3-二氯-5,6-氰基-1,4-苯醌)大黄素型(emodin-type)大黄酸(rhein)大豆黄素(daidzein)大叶千里光碱(macrophylline)达尔森(Darzen)反应哒嗪(pyridazine)单键(single bond)单体(monomer)单蒽核(monoanthracene nucleus)单萜(monoterpenoids)单糖(monosaccharide)单线态(singlet)蛋氨酸(methionine)蛋白质(protein)胆甾烷(cholestane)胆甾醇(cholesterol)胆甾酸(cholalic acid)胆甾烷(cholestane)胆甾醇(cholesterol)党参碱(codonopsine)等电点(isoelectric point ,PI)迪克曼反应(Dieckmann reaction )狄尔斯-阿尔德(Diels-Alher)电场扫描(electric field scan)电性效应(electromeric effect)电荷转移络合物(charge-transfer complex)碘仿试验(iodoform test)碘值( iodine number )淀粉(starch)敌敌畏(dichlorovos)蝶啶(pteridine)丁烯二酸(butene dioic acid)定位规则(orientationg rule)动力学概念(dynamical concept)豆甾烷(stigmastane)毒芹碱(coniine)毒扁豆碱(physostigmine)杜鹃酮 (germacrone)端基效应(end-group effect)对映异构(enantiomerism)对映体(enantiomers)对氨基苯磺酰胺(sulfanilamide)多环芳烃(polycyclic aromatic hydrocarbon)多糖(polysaccharide)多肽(polypeptide)多磷酸酯(polyphosphate ester)E(entgegen,德文,相反之意)EAN规则(EAN rule)莪术醇(curcumenol)鹅去氧胆酸(chemocholic acid)噁唑(oxazole)蒽(anthracene)蒽酚(anthrol)蒽醌(anthraquinones)蒽酮(anthrone)9-蒽酮大黄酚(chrysophanol 9-anthrone)儿茶素(catechin)二烯烃(diene)二氯卡宾(dichlorocarbene)二甲亚砜(dimethyl sulfoxide)二巯基丙醇(dimercaptopropanol )二级结构(secondary structure)二氢黄酮(flavanone)二氢黄酮醇(flavanonol)二氢异黄酮(isoflavanone)二氢查尔酮(dihydrochalcone)二萜类(diterpenoids)番泻苷A、B、C、D(sennoside A、B、C、D) 番茄红素(lycopene)反芳香性化合物(antiaromatic compound)反应历程(reaction mechanism)反应热(heat of reaction)芳香烃(aromatic hydrocarbon)芳香性(aromaticity)芳香性化合物(aromatic compound)芳构化(aromatization)放氮反应(denitrification)飞燕草素(delphinidin)非芳香性化合物(nonaromatic compound)非离子表面活性剂(nonionic)非对映体(diasteroisomer)非质子性溶剂(nonprotonic solvent )非苯芳烃(nonbenzenoid hydrocarbon)菲(phenanthrene)斐林溶液( Fehting solution )费歇尔(E.Fischer)费歇尔投影式(Fischer projection formula)分子轨道(molecular orbital)分子轨道对称性守恒理论(conservation of orbital symmetry theory)分子轨道理论(molecular orbital theory)酚苄明(Phenoxybenzamine)芬克尔斯坦(Finkelstein)芬氟拉明(fenfluramine)粪甾烷(coprostane)氟芬那酸(flufenamic Acid)福井谦一(K.Fukui)傅-克反应(Friedel-Crafts alkylation reaction)傅瑞斯重排(K.fries rearrangement)傅瑞德-克拉天茨反应(Friedel-Crafts reaction)辅酶Q10(coenzyme Q10)呋喃(furan)盖布瑞尔合成法(Gabreil reaction)盖特曼-科希(Gattermann-Koch)反应甘露糖(mannose)甘氨酸(glycine)甘油(glycerol)甘氨胆酸(glycocholic acid)甘草酸(glycyrrhizic acid)甘草次酸(glycyrrhetinic acid) 甘草苷(liquiritin)高丽槐素(maackiain)睾丸素(testosterone)隔离二烯烃(isolated diene)格林那试剂(Grignard reagent)哥美林(Gmelin)共振能(resonance energy)共振论(resonance theory)共振杂化体(resonance hybrid)共振结构式(resonating structure)共轭效应(conjugative effect)共轭体系(conjugation system)共轭二烯烃(conjugated diene)构象(conformation)构象异构(conformational isomerism)构造异构(constitutional isomerism)构型保持(configuration conservation)构型转化(configuration inversion)钩藤碱(rhynchophylline)β—谷甾醇(β—sitosterol)谷氨酸(glutamic acid)谷酰胺(glutamine)古柏尔(A. Couper)古柯碱(cocaine)胍(guanidine )寡糖(oligasaccharide)冠醚(crown ether)光学异构体(optical isomer)硅烷(silane)硅油(silicon oil)过氧化物(peroxide)过氧化物效应(peroxide effect)过渡态(transition state)果糖(fructose)哈沃斯(Haworth)海藻糖(fucose)核磁共振谱(nuclear magnetic resonance spectroscopy)核苷酸(nucleotide)核苷(nucleoside)核酸(nucleic acid)核糖(ribose)(-)-黑金合欢素[(-)melacacidin)]红古豆碱(cuskohygrine)红花苷(carthamin)红外光谱(infrared spectroscopy)互变异构现象(tautomerism)互变异构体(tautomer)胡萝卜素(carotene)胡椒碱(piperine)槲皮素(quercetin)化学键(chemical bond)花色素(anthocyanidin)环烃(cyclic hydrocarbon)环氧化反应(epoxidation)环加成反应(cycloaddition recation)环状碳架(cyclic carbon constitution)环己二酮(cyclic hexanedione)环糊精(cyclodextrin)α,β-环氧酸酯(α,β-cycloxacid ester)黄柏素-7-O-葡萄糖苷(phellamurin)黄芩素(baicalein)黄芩苷(baicalin)黄杉素(taxifolin)黄酮(flavonoid)黄酮醇(flavonol)黄烷醇(flavanol)黄体酮(progesterone)磺胺(sulfanilamide)磺化反应(sulfonation)活化能(energy of activation)霍夫曼(R.Hofmann)霍夫曼降解反应(Hoffmann degradation)霍夫曼消除(Hoffmann elimination)霍夫曼彻底甲基化反应(Hoffmann exhaustive methylation)几何异构体(geometricalisomer)己二胺(hexanediamine)季铵盐(quaternary ammonium salt)季铵碱(quaternnary ammonium hydrate )季膦盐( quaternary phosphonium salt )激素(hormone)甲孕酮(methporgestin)甲基睾丸素(methyl testosterone)甲基锂(lithium methlde)甲壳质(chitin)价键学说(valence bond method)假酸式(pseudo-acid form )σ键(σbond)σ键迁移反应(sigmatropic reaction)π键(πbond)碱基配对规律(base pairing rule)姜烯(zingiberene)胶束(micelle)交叉醇醛缩合反应(crossed aldol reaction)金合欢醇(farnesol)金属有机化合物(metalloorganic compound)金雀花碱(sparteine)金鸡宁(cinchonine)金刚烷胺(symmetrel)紧密离子对(tightness ionpair )精氨酸(arginine)竞争反应(competing reaction )聚合物(polymer)聚合反应(polyreaction)聚集二烯烃(cumulative diene)锯架式(andiron formula ; sawhorse projection formula) 卡宾(碳烯)(Carbene)开息纳尔-武尔夫(Kishner-Wolff)-黄鸣龙法凯库勒(A. Kekule)康尼查罗(Cannizzaro ) 反应科尔贝(H. Kolbe)科瑞—郝思反应(E.J.Cory—H.House reaction)可的松(cortisone)可见-紫外光谱(visible-ultraviolet spectroscopy)克莱森-斯密特(Claisen-Schmidt)反应克脑文盖尔(Knoevenagel)反应克莱门森(Clemmensen)还原反应克莱森重排(Claisen rearrangement)克莱森缩合反应(Claisen condensation reaction ) 柯尔柏-施密特反应(Kolbe-schmidt reaction)柯亚素(chrysarobin)壳糖胺(chitosamine)苦参碱(matrine)库切洛夫反应(Kutchcrov reaction)奎宁(quinine)喹啉(quinoline)醌(quinones)醌氢醌(quinhydrone)莱普西汀(leptosidin)赖氨酸(lysine)兰尼镍(Raney Ni)莨菪碱(hyoscyamine)雷公藤甲素(triptolide)利血平(reserpine)立体选择性(stereoselective)立体专一性(stereospecific)立体异构(stereoisomer)离域(delocalization)离域能(delocalization energy ;delocalized energy)离域电子(delocalized electron)离去基团(leaving group )链引发阶段(chain initiation step)链增长阶段(chain propagation step)链终止阶段(chain termination step)链状碳架(chain carbon constitution)亮氨酸(leucine)林德拉(Lindlar)膦叶立德(phosphorus ylide)膦(phosphureted hydrogen)鏻盐(phosphorate)膦酸(phosphonic acid )硫脲(thiourea )硫醇(thioalcohol )硫醚(thioether )硫酚(phenylsulfhydryl )留氮反应(reaction of nitrogen retention) 龙脑(borneol)卤代反应(halogenation)卤仿(haloform)卤仿反应(haloform reaction )卢卡斯试剂(Lucas reagent)芦荟大黄素(aloe-emodin)芦丁(rutin)α-卵磷脂(α-lecithine )轮烯(annulene)酪氨酸(tyrosine)罗勒烯(ocimene)罗森孟德(Rosenmund)还原法螺环烃(spiro hydrocarbon)氯乙酸甲酯(methyl chloroacetate) (chloro acetyl formate) 绿茶多酚(green tea polyphenols)马尔可夫尼可夫(Markovnikov)规则麻黄碱(ephedrine)吗啡(morphine)麦可拉费蒂(Mclafferty)麦芽糖(maltose)麦角甾醇(ergostenol)麦角新碱(ergometrine)麦克尔加成(Michael addition)满尼希(Mannich)反应梅尔外英-彭多夫(Meerwein-Poundorf)还原反应酶(enzyme)没食子儿茶素(gallocatechin)没食子儿茶素没食子酸酯(gallocatechin gallate)脒基(amidino)嘧啶(pyrimidine)咪唑(imidazole)那可汀(narcotine)钠汞齐反应(natrium amalgam reaction)萘(naphthalene)脑磷脂(cephalin)内消旋体(meso-form)尼科尔棱镜(Nicol prism)脲(尿素) (urea)鸟嘌呤(guanine)尿嘧啶(uracil)柠檬醛(citral)苧烯(limonene)牛磺胆酸(taurocholic acid)纽曼投影式(Newmans projection formular)扭转能(torsional energy)扭转张力(torsional strain)欧芬脑尔氧化反应(Oppenaner oxidizing reaction)偶氮基(azo)偶合(偶联)反应(coupling reaction)偶合组分(或偶合剂)(coupling agent )偶极-离子键(dipolar-ionic bond )蒎烯(pinene)硼氢化反应(hydroboration))硼氢化钠(NaBH4吡哆醛(pyridoxal)吡咯(pyrrole)吡喃(pyran)吡嗪(pyrazine)吡唑(pyrazole)吡啶(pyridine)嘌呤(purine)频哪醇(pinacol)平面偏振光(plane polarized light)平伏键(e键,equatorial bonds)苹果多酚(apple polyphenols)普鲁卡因(procaine )葡萄糖(glucose)歧化反应(disproportionation reaction )前列腺素( prostaglandin)前线轨道(frontier orbital)理论茜草素(alizarin)茜素型(alizarin-type)强心苷(cardiac glycosides)羟肟酸(hydroximic acid )β-羟基醛(β-hydroxy aldehyde )桥环烃(bridged hydrocarbon)鞘磷脂(sphingomyelinicacid)亲电性试剂(electrophile)亲电加成反应(electrophilic addition)亲电取代(electrophilic substitution)亲核试剂(nucleophilic reagent)亲核加成反应(nucleophilic addition)亲核取代(nucleophilic substitution)亲核取代反应历程(nucleophilic substitution reavtion mechanism) 亲双烯体(dienophile)芹菜素(apigenin)氢解( hydrogenolysis)氢化苯基锡(hydrogenation benztin)氢化正丁基锡( hydrogenation butyltin )氢化油(hydrogenated oil )氢化可的松(hydrocortisone)氢化热(heat of hydrogenation)氢甲酰化法(hydroformylation)氢键(hydrogen bond)清风藤碱(sinoacutine)琼斯试剂(Jones reagent)炔烃(alkyne)热力学概念(thermodynamic conception )溶剂化效应(solvating effect)乳糖(lactose)瑞穆尔-蒂曼反应(Reamer-Timann reaction)萨哈斯投影式(Sawhares projection)噻吩(thiophene)噻唑(thiazole)三萜(triterpenoids)三甲基铝(trimethyl aluminium)三级结构(tertiary structure)三线态(triplet state)三苯基膦(triphenyl phosphine)三磷酸腺苷(adenosine triphosphate)桑得迈尔反应(Sandmeyer reaction)桑得迈尔—盖特曼反应(Sandmeyer-Gattermann reaction)色氨酸(tryptophan)色谱法(chromatography)沙瑞特试剂(Sarrett reagent)鲨烯(squalene)山油柑碱(acronycine)山扁豆双醌(cassiamine)山梗菜碱(lobeline)肾上腺皮质激素(adrenal cortex hormone)生物甲基化(biological methylate)18电子规则(18- electron rule)史蒂文斯重排(Stevens rearrangement)石松碱(lycopodine)士的宁(strychnine)矢车菊素(cyanidin)手性(chirality)水苏碱(stachydrine)双蒽核(bi-anthracene nucleus)双烯体(diene)顺反异构(cistrans isomerism)顺反异构体(cis-trans isomer)四氢巴马汀(tetrahydropalmatine)四级结构(quaternary structure)四氢硼钠反应(sodium borohydride reaction) 四萜(quadruterpene)四甲基硅烷(tetramethylsilane)丝氨酸(serine)松香酸(abietic acid)苏氨酸(threonine)酸败(rancidity )缩醛(acetal)缩二脲反应(biuret reaction)肽(peptide)肽键(peptide bond)碳水化合物(carbohydrate)碳酰氯(carbonyl chloride ;phosgene )糖类(saccharide)糖苷(glycoside)糖原(glycogen)天冬氨酸(aspartic acid)天精(skyrin)天竺葵素(pelargonidin)萜类化合物(terpenoids)同系列(homologous series)同系物(homolog)同分异构体(isomer)酮(ketone)透析法(permeation)1-吐根碱(1-emetine)脱羧反应(decarboxylic reaction)娃儿藤定碱(tylophorinine)瓦尔登转化(Walden inversion)外消旋体(racemic mixture)外消旋化(racemization)烷烃(alkane)维生素A(vitamin A)维生素B12(vitamin B12)维生素K1、K2(vitamin K1、K2)维蒂希反应(Wittig reaction)伪麻黄碱(pseudoephedrine)威尔克森(wilkinson)威廉森合成法(Williamson synthesis)武勒(F. Wohler)无色矢车菊素(leucocyanidin)伍德沃德(R.B.Woodward)武兹反应(A.Wurtz reaction)烯烃(alkene)系统命名法(systematic nomenclature)西佛碱(Schiff ’s base)希夫(schiff)试剂吸电子共轭效应(electronwithdrawing conjugative effect) 喜树碱(camptothecine)西佛碱(schiff base)希曼反应(G.Schiemann reaction)纤维二糖(cellobiose)纤维素(cellulose)腺嘌呤(adenine)硝化反应(nitration)消除反应(elimination reaction)小檗碱(berberine)硝基化合物(nitro compounds)协同反应(concerted reaction)缬氨酸(valine)(±)—新薄荷醇[(±)—neomenthol]兴斯堡反应(Hinsberg reaction)胸腺嘧啶(thymine)12-15休克尔规则(Hückel rule)比旋光度(specific rotatory power)旋光仪(optical rotation instrument)旋光异构体(optical isomer)血红素(haemachrome)亚硝酸(nitrous acid)烟碱(nicotine)延胡索乙素(tetrahydropalmatine)盐酸-镁粉反应(HCl-Mg powder reaction)盐酸-锌粉反应(HCl-Zn powder reaction)盐(oxonium salt)氧化苦参碱(oxymatrine)氧化蒽酚(oxanthranol)氧化态(oxidation state)氧化值(oxidation number)阳离子表面活性剂(cationic surface active agent)叶黄素(phylloxanthin)叶绿素(chlorophyll)一叶萩碱(securinine)一野百合碱(monocrotaline)一级结构(primary structure)乙硼烷(diborane)1-乙炔基环戊醇(1-ethynyl cyclopentanol )乙二胺(ethylene diamine)乙烯酮(ethenone ;ketene )乙酸(acetic acid)乙二酸(ethanedioic acid )异甘草素(isoliquiritigenin)异黄酮(isoflavone)异鼠李素(isorhamnetin)异钩藤碱(isorhynchophylline)异亮氨酸(isoleucine)异头物(anomer)异头效应(anomeric effect)异噁唑(isoxazole)异噻唑(isothiazole)异腈(胩)(isonitrile)异喹啉(isoquinoline)胰岛素(insulin)阴离子表面活性剂(anionic surface active agent)茚三酮(ninhydrin )吲哚(indole)罂粟碱(papaverine)油脂(axunge;grease;lipin;)有效原子序数(effective atomic number)有机金属化合物(organometallic compound)有机锂(organic-Li) (organic lithium ) (organolithium compound) 诱导效应(inductive effect)羽扇豆碱(lupinine)愈创木奥(guaiazulene)原花色素(proanthocyanidin)鸢尾苷(iridin)原子轨道(atomic orbital)Z(Zusammen,德文,在一起之意)杂化轨道(hybrid orbital)杂环碳架(heterocycle carbon constitution)甾体化合物(steroidal compound)皂化反应(saponification reaction)皂化值(saponification value)扎依采夫(Saytzeff)规则樟柳碱(anisodine)樟脑(camphor)蔗糖(sucrose)-折叠(beta-pleated sheet)蒸馏法(distillation)质谱(mass spectroscopy)质子性溶剂(protonic solvent)脂环烃(alicyclic hydrocarbon;cycloalkane)直立键(a键,axial bond)重氮化反应(diazotization reaction)重氮盐(diazonium salt)重氮组分(diazocomponent)重氮甲烷(diazomethane)周环反应(pericyclic reaction)转化糖(invert sugar)自由基(free radical)自由基链反应(free radical chain reaction)β-紫罗兰酮(β-ionone )组氨酸(histidine)。
酯化反应机理 氢离子转移,脱去一分子水和氢离子得到酯。

酯化反应机理氢离子转移,脱去一分子水和氢离子得到酯。
In the process of esterification, a reaction mechanism called hydrogen ion transfer occurs. This involves the removal of one molecule of water and one hydrogen ion to form an ester.酯化反应是一种常见的有机化学反应,通常涉及酸催化。
在这个过程中,酯和水通过酸催化被转化为羧酸和醇。
The reaction mechanism begins with the protonation of the carboxylic acid by the catalyst, which is usually a strong acid such as sulfuric acid or hydrochloric acid. This protonation increases the electrophilicity of the carbonyl carbon in the carboxylic acid, making it more susceptible to nucleophilic attack.此反应机理从催化剂对羧酸进行质子化开始,催化剂通常是硫酸或盐酸等强酸。
质子化增加了羧基碳上亲电性,使其更容易受到亲核攻击。
Next, the nucleophile, which can be either an alcohol or an alkoxide ion, attacks the carbonyl carbon. The nucleophile donates a pair of electrons to form a new bond with the carbonyl carbon, simultaneously breaking the π bond between the carbon and oxygen and leading to tetrahedral intermediate formation.接下来,亲核试剂(可以是醇或烷氧根离子)将攻击羧基碳。
生物合成青蒿酸课件

7
生物合成青蒿酸
菌株改造:
Y337 (原始菌株, PMET3-ERG9 )
导入表达CYP71AV1 和CPR1 基因的高 拷贝质粒
14
结果
添加10%的IPM,使得所有菌株的生存能力显著加强。同时,对Y285 、Y301、Y657和 Y692菌株(缺乏ALDH1和ADH1基因)而言,添加IPM会导致中间产物的析出。而对有 ALDH1和ADH1基因的菌株,添加IPM会提高青蒿酸的产量
15
化学途径
1.the reduction of the D11(13) double bond. 2. the esterification of the carboxylic acid. 3. an ‘ene-type’ reaction of the C4 –C5 double bond with singlet oxygen. 4. the allylic hydroperoxide undergoes an acid-catalysed Hock fragmentation and rearrangement to afford a ringopened ketoaldehyde enol
12
添加IMP
表达ALDH1基因的菌株以胞外晶体沉淀的形式生产青蒿 酸,沉淀在初期发酵时便能观察到,这对使得多相发酵样 品中产品的精确测量变得复杂。为了克服由青蒿酸结晶沉 淀带来的困难,我们利用萃取发酵溶解沉淀的现象,在十 四酸异丙酯(IMP)环境中培养菌体。
羧酸的酯化反应

羧酸的酯化反应一、引言羧酸的酯化反应是一种重要的有机合成反应。
在这个反应中,羧酸与醇反应生成酯。
酯化反应在有机合成中广泛应用,可用于合成酯类化合物,具有重要的理论和实际意义。
本文将对羧酸的酯化反应进行全面、详细、完整且深入地探讨。
二、酯化反应的机理酯化反应的机理主要包括酸催化和酸碱催化两种方式。
以下将分别对两种机理进行介绍。
2.1 酸催化机理酸催化机理是指在酸性条件下进行的酯化反应。
在这种情况下,羧酸与醇在酸的催化下发生酯交换反应。
酸催化机理的反应步骤如下:1.酸性条件下,羧酸中的羧基质子化,形成羧离子;2.醇中的羟基质子化,形成醇离子;3.离子交换,羧离子与醇离子发生亲核取代反应,生成酯;4.生成的酯在酸催化下脱离羧基质子化,得到最终产物。
2.2 酸碱催化机理酸碱催化机理是指在碱性条件下进行的酯化反应。
在这种情况下,羧酸通过碱性催化剂转化为酸酐,再与醇反应生成酯。
酸碱催化机理的反应步骤如下:1.羧酸先与碱反应生成酸酐;2.酸酐与醇发生亲核取代反应,生成酯;3.反应结束后,酸酐通过水解还原为羧酸。
三、酯化反应的影响因素酯化反应的速率和产率受到多种因素的影响。
以下将对影响因素进行详细介绍。
3.1 底物结构底物结构对酯化反应的速率和产率有重要影响。
酯化反应中,存在两个底物:羧酸和醇。
它们的结构特点将直接影响反应的进行。
一般来说,较短的羧酸链和较长的醇链有利于酯化反应的进行。
3.2 催化剂种类酯化反应中常用的催化剂种类有强酸和碱。
强酸催化剂可以加速羧酸和醇之间的酯交换反应,而碱催化剂主要用于将羧酸转化为酸酐。
选择适当的催化剂对于提高反应速率和产率非常重要。
3.3 反应条件反应温度和反应时间是酯化反应中重要的反应条件。
适当的反应温度和反应时间可以提高反应速率和产率。
一般来说,较高的反应温度和较长的反应时间有利于反应的进行。
3.4 溶剂选择溶剂选择对酯化反应也有一定的影响。
常用的溶剂有水、乙醇、丙酮等。
不同的溶剂对反应速率和产率有不同的影响,适当选择溶剂可以改善反应效果。
重氮化合物在化学生物学中的运用

重氮化合物在化学生物学中的运用摘要: 重氮化合物的制备方法进行了简要介绍。
重叠化合成物在化学生物学中的应用:环加成反应、作为探针研究生物分子、蛋白质的烷基化、生物可逆蛋白质修饰、生成卡宾对肽和蛋白质的修饰和核酸的烷基化。
关键词:重氮化合物、化学生物学、蛋白质近日,来自威斯康星大学麦迪逊分校(University of Wisconsin–Madison)的Ronald T. Raines教授在ACS Chemical Biology杂志上发表综述文章介绍了重氮化合物在化学生物学中的应用[1](Diazo Compounds: Versatile Tools for Chemical Biology)。
相信学化学的同学们对重氮化合物肯定不陌生。
现在,我简要粗略的梳理一下这篇综述。
我们先仰望一下这位本科毕业于麻省理工,博士毕业于哈佛的通讯作者Ronald T. Raines教授。
综述开篇,作者先是简要介绍了一下什么是重氮化合物(R1R2C=N2),以及重氮化合物相对叠氮化合物(R1R2CH-N3)在生物学应用中的优势,比如体积更小和更广泛的反应活性。
与叠氮化合物另一个不同点是,自然界存在着含有重氮基团的天然产物,作者列举了含有重氮基团的氨基酸,以及两类活性显著的天然产物kinamycins和lomaiviticins(结构如下图)。
作者接着就重氮化合物的制备方法进行了简要介绍。
主要概括:(i) 重氮基转移[2,3];(ii) 胺类直接重氮化[4,5];(iii) 腙类分解或氧化[6,7];(iv) N-亚硝基化合物重排[8,9];(v) 1,3-二取代酰基三嗪分解[10,12];(vi) 来自其他重氮化合物[13-17]。
尽管在有机化学上有许多制备重氮化合物的方法,但是在化学生物学领域当中也受到很大限制,这主要受制于重氮化合物的多官能团兼容性以及水溶性。
当然作者也不忘推广一下自己开发的从叠氮化合物制备重氮化合物的方法,该方法一定程度上提高了反应的水溶性。
碳酸二甲酯在有机合成中的应用

| 1432 碳酸二甲酯应用于生物来源平台化学品的升级生物来源的化学品通常用于替代现有石化产品,有利于降低化工生产过程中的有毒性、减少温室气体排放、实现绿色可持续性发展。
然而目前生物来源的平台化学品清单中大部分仍为最初鉴定的化合物,例如乙醇、功能化的一元和二元羧酸(乳酸、乙酰丙酸、羟基丙酸和琥珀酸)、呋喃类产品(糠醛、羟甲基糠醛和呋喃二羧酸)、异戊二烯的生物碳氢衍生物、甘油及其衍生物、其他糖类(山梨糖醇和木糖醇)等。
DCM 作为甲基化试剂和甲氧羰基化试剂已经被应用于一些生物来源平台化学品的升级[12]。
甘油与DMC 通过酯交换反应制备碳酸甘油酯(GC)或甘油二碳酸酯(GDC)[13]。
在催化剂的作用下,通常在40-80℃下只需要几个小时会以非常高的选择性和90%以上的收率生成GC ,而GDC 产生需要过量的DMC 和更长的反应时间。
甘油缩醛也可与DMC 通过甲氧羰基化或甲基化反应制备其衍生物。
采用阳离子交换树脂吸附水相发酵液中的琥珀酸二钠盐,然后被吸附的琥珀酸盐在季铵离子催化下与DMC 发生O-甲基化反应生成琥珀酸二甲酯。
在碱催化下,乙酰丙酸与DMC 反应可以制备多种衍生产品,包括乙酰丙酸甲酯、4,4-二甲氧基戊酸甲酯和琥珀酸二甲酯[14]。
在固定化脂肪酶B 的催化下,羟甲基糠醛与DMC 反应生成甲氧羰基化衍生物。
使用大孔树脂吸附含2,5-呋喃二甲酸二钠的微生物发酵液,然后在100℃高压釜中与过量的DMC 进行甲基化制备2,5-呋喃二甲酸二甲酯[15]。
在碱性催化剂存在下,D-山梨糖醇与DMC 直接反应制备异山梨醇,也可一锅法进一步制备二甲基异山梨醇。
首先将反应混合物在90℃加热以允许定量D-山梨糖醇环化为异山梨醇,然后在200℃下进行甲基化反应得到二甲基异山梨醇[16]。
3 碳酸二甲酯在聚合物合成中的最新应用聚氨酯(PU)种类繁多,作为商业化应用广泛的塑料家族,仅2014年在欧洲的年产量就达到四百多万吨。
_苯乙醇对位硝化工艺研究

( 3) 以 - 苯乙醇为原料, 用乙酰氯保护醇羟基再用 发烟硝酸硝化后酸性水解, 收率为 49% [ 5] 。该法操作 简单, 易于工业化, 但尚有不足: 一方面乙酰氯、 发烟 硝酸极易挥发, 污染环境, 且乙酰氯易水解; 另一方面 在酸性条件下酯水解不完全 [ 6] 。本文参考方法 ( 3) , 以苯乙醇为原料, 先用乙酸酐酰化保护羟基, 然后用 m ( 硝酸) m ( 硫酸) = 1 1的混酸硝化 , 硝化产物以对 位为主, 再碱性水解, 最后分离出对硝基 - - 苯乙 醇。反应原理如下 : O O NO2 OH + NO2 NaOH 质量分数为 40% , 工业品, 配成含 NaOH 质量 分数为 20% 的水溶液; 浓硫酸、 浓硝酸、 乙酸酐、 苯、 石油醚均为工业品。 Nicolet, AVARTE 360 傅立叶变换红外光谱仪, 固体 KBr 压片 法; XT - 4 双目 体视显 微熔点 测定 仪 , 温度计未校正 ; Perkin- Elmer 240C 元素分析仪; 515 HPLC Pump 高效液相色谱分析仪。 1 2 工艺流程 对硝基 - - 苯乙醇的工艺流程示意图见图 1。 OH NO2
摘 要: - 苯乙 醇在浓硫酸催化下与乙酸酐发生酯化反应 , 然后与硝 酸和硫酸组 成的混酸发 生对位硝 化反应 , 再 - 苯乙醇 , 讨论了反应温度 、 反应时间 、 硝酸用量对硝化反应收 率的影响 , 在优化 条 - 苯乙醇的总收率和 纯度分 别达到 71 7% 、 98 3% 。 经 IR 、 元素分 析证实 了 - 苯乙醇 文章 编号 : 1671- 7643( 2004) 05- 0001- 04 1, 2 1 1 1
实验次序 1 2 3 4 5 6 平均值
产量 /g 125 8 124 2 124 9 125 4 123 9 124 4 124 8
果胶甲酯化反应及应用高甲氧基果胶制备纳米乳液

果胶甲酯化反应及应用高甲氧基果胶制备纳米乳液丁萍;汪明明;迟坤蕊;华霄;杨瑞金【摘要】以商业橘皮果胶为原料,在无水甲醇环境中采用盐酸催化甲酯化反应制备高甲氧基果胶.通过对反应时间、反应温度、料液比以及盐酸添加量的调节,可以制备得到酯化度在90%以上的高甲氧基果胶.分子质量分布结果表明,随着反应温度升高、反应时间延长和盐酸浓度增加,产物的酯化度逐渐提高,但数均分子质量逐渐降低.在料液比1:50,盐酸添加量0.1 mol/L,温度60℃下反应12h,产物酯化度达到91.20%,但数均分子质量降低为15.00 kDa.基于极高酯化度果胶所具有的双亲性(甲氧基为疏水基团而羟基为亲水基团)和分子链短的特点,进一步研究了极高酯化度果胶的乳化性质.用高能量法(高速剪切)分别制备了油滴体积分数为10%、20%和30%的纳米乳液,并考察了7d内乳状液的稳定性、粒径和Zeta-电位变化.结果显示采用极高酯化度果胶可制备得到粒径为3 500 nm的乳状液(油滴体积分数10%~30%),并具有较好的稳定性.【期刊名称】《食品与发酵工业》【年(卷),期】2018(044)008【总页数】8页(P188-195)【关键词】高甲氧基果胶:甲酯化反应;酯化度;乳化;粒径;乳液稳定性【作者】丁萍;汪明明;迟坤蕊;华霄;杨瑞金【作者单位】江南大学食品学院,江苏无锡,214122;江南大学食品学院,江苏无锡,214122;江南大学食品学院,江苏无锡,214122;江南大学食品学院,江苏无锡,214122;江南大学食品科学与技术国家重点实验室,江苏无锡,214122;江南大学食品学院,江苏无锡,214122;江南大学食品科学与技术国家重点实验室,江苏无锡,214122【正文语种】中文果胶是植物细胞间层和初生细胞壁的重要成分[1]。
通常认为其结构包含3个结构区域[2]:均一半乳糖醛酸区(HG)、鼠李半乳糖醛酸聚糖Ⅰ区(RG-Ⅰ)以及鼠李半乳糖醛酸聚糖Ⅱ区(RG-Ⅱ)[3]。
用石脑油生产溶剂油的工艺的研究

25.苗望春影响煤油型溶剂油气味的因素[期刊论文]-河南石油 2002(2)
26.何力.李发忠.李谦定陕北原油生产210#专用油墨溶剂油的研究 1994(03)
27.王云方.徐经茂.朱洪亮120#溶剂油吸附法脱芳烃精制技术的研究 1996(06)
28.王云芳.朱洪亮.徐经茂6#溶剂油脱芳精制吸附剂再生技术[期刊论文]-石油大学学报(自然科学版) 1996(4)
37.何雅仙.韩松.郁桂芝精制芳烃用颗粒白土的研制[期刊论文]-精细石油化工进展 2001(6)
38.邹德东.曲晓廉溶剂油装置的扩能技术改造.工业生产与技术进步 1999(02)
39.戴逸云沸石分子筛技术展望[期刊论文]-精细石油化工进展 2000(3)
40.杨学萍沸石催化剂在炼油和石油化工中的应用[期刊论文]-工业催化 2003(6)
45.Hu X C.Chuah G K.Jaenicke S Solid acid catalysts for the efficient synthesis of 1-(2,4-difluorophenyl) propane 2001
46.Hu X C.Foo M L.Chuah G K Pore size engineerin g on MCM- 41:Selectivity Tuning of Heterogenized AlCl3 for the synthesis of linear alkyl benzenes 2001
磷酸奥司他韦中相关杂质的合成

磷酸奥司他韦中相关杂质的合成吴丕业;赖小燕;汤清华;戴鹏;查高峰【摘要】磷酸奥司他韦作为选择性的流感病毒神经氨酸酶抑制剂,是治疗禽流感最有效的药物.美国药典38版收载了磷酸奥司他韦中的两个杂质.杂质(3R ,4R ,5S )-3-(1-乙基丙氧基)-4-乙酰胺-5-氨基-1-环己烯-1-羧酸(A )是原料药在精制和存储过程中发生微量水解产生的.对合成条件进行了优化,采用了常规分离手段得到了杂质A ,合成收率达到86.6%.杂质3-羟基-4-乙酰氨基-苯甲酸乙酯(B )是美国药典38版所载磷酸奥司他韦中的另一个杂质,目前没有合成方面的报道.设计了杂质B的合成路线,以3-羟基-4-氨基苯甲酸为原料,用乙酸酐作为酰化试剂对原料进行乙酰化,然后加入氢氧化钠和盐酸,调节pH ,发生水解,最后用溴乙烷作为烃化剂得到了目标产物,合成收率达到52.1%.整条合成路线操作简单、反应条件温和、没有环境污染,对磷酸奥司他韦的生产检验和贮存均具有现实意义.%Oseltamivir phosphate as a potent selective neuraminidase inhibitor is the most effective treat-ment of avian influenza now. There are two related substances of Oseltamivir phosphate recorded in USP38. Impurity (3R, 4R, 5S )-4-acetamido-5-amino-3- (1-ethylpropoxy )-1-cyclohexene-1- carboxylic acid(A) is produced during the refining and storage of the raw material medicine. We optimized the synthesis process of A and got it with a 86. 6% yield by conventional separation methods. Impurity 4-Acetylamino-3-hydroxybenzoic acid ethyl ester(B)is the other related substance recorded, and there is no report about synthetic route of it so far. After exploring the references related to B, we proposed a synthesis process, using 3-hydroxy-4-amino benzoic acidas raw material and acetic anhydride as acyla-tion reagent, then adding sodium hydroxide and hydrochloric acid to regulate pH, sequently hydrolysis occurring. Finally we got B with a 52. 1% yield by bromoethane as alkylating agent. The entire syn-thetic route has the advantages of simple operation, mild reaction condition and no environmental pollu-tion, w hich has practical significance for the production, inspection and storage of Oseltamivir phos-phate.【期刊名称】《武汉工程大学学报》【年(卷),期】2014(000)010【总页数】4页(P13-16)【关键词】磷酸奥司他韦;杂质合成;结构表征【作者】吴丕业;赖小燕;汤清华;戴鹏;查高峰【作者单位】武汉工程大学材料科学与工程学院,湖北武汉430200;武汉理工大学化学化工与生命科学学院,湖北武汉430070;武汉理工大学化学化工与生命科学学院,湖北武汉430070;武汉理工大学化学化工与生命科学学院,湖北武汉430070;武汉理工大学化学化工与生命科学学院,湖北武汉430070【正文语种】中文【中图分类】TQ460.60 引言磷酸奥司他韦(Oseltamivir,phosphate) 化学名为(3R,4R,5S)- 3 -(1-乙基丙氧基)-4-乙酰胺-5-氨基-1-环己烯-1-羧酸乙酯磷酸盐(1),由瑞士罗氏制药公司研制,在临床上是用于预防和治疗甲型和乙型流感的选择性神经氨酸酶抑制剂,是治疗禽流感最为有效的药物[1-4].美国药典38 版提出了1 中的2 个有关物质A、B ( 图1)并对其进行了限度控制.图1 1和杂质A、B结构式Fig.1 The formula of 1 and impurities A and B其中有关物质A是1的水解物,化学名为(3R,4R,5S)-3-(1-乙基丙氧基)-4-乙酰胺-5-氨基-1-环己烯-1-羧酸,已有多种合成方法被报道.其中,根据Fujiko Konno[5]等人所采用的方法,尝试在100 ℃下进行水解反应,发现原料大量分解,不利于产品纯度及产率优化.而根据Bischofberger[6]的方法,利用四氢呋喃作溶剂,在室温下进行反应,能得到需要的反应物,但其使用制备液相法进行分离纯化,无法获得较大量的样品,也不利于大规模推广应用.因此对其优化后处理工艺进行了监控,使用常规分离手段得到了高纯度的杂质A,合成收率为86.6% ( 图2).图2 杂质A的合成路线Fig.2 The synthetic route of the impurity A有关物质B是美国药典38 版所载磷酸奥司他韦中的另一个杂质,化学名为3-羟基-4-乙酰氨基苯甲酸乙酯,为非叠氮合成工艺合成磷酸奥司他韦时产生的工艺杂质,目前没有相关的合成报道.因此制备高纯度的杂质B,对于该品种的生产、贮存和检验均具有现实意义.实验[7-9]设计了杂质B的合成路线:以3-羟基-4-氨基-苯甲酸为原料,在醋酸钠和盐酸存在下经乙酸酐双乙酰化反应得到3-乙酰氧基-4-乙酰胺基-苯甲酸2,再在碱性条件下选择性水解得到3-羟基-4-乙酰胺基-苯甲酸3,3 在碳酸氢钠催化下与溴乙烷进行乙酯化得到杂质B,总收率为52.1% ( 图3). 图3 杂质B的合成路线Fig.3 The synthetic route of the impurity B1 实验部分1.1 分析仪器与试剂Avance HD III 500NMR核磁共振仪(瑞士Bruker公司,TMS为内标);质谱仪(Thermo-LTQXL);磷酸奥司他韦((3R,4R,5S)- 3 -(1-乙基丙氧基)-4-乙酰胺-5-氨基--1-环己烯-1-羧酸乙酯磷酸盐)来自宜昌长江药业有限公司,纯度为99.9%;3-羟基-4-氨基-苯甲酸购自aladdin试剂,纯度98.0%;氢型732阳离子交换树脂(国药试剂);氢氧化钠、氢氧化钾、盐酸、碳酸氢钠、四氢呋喃、N,N-二甲基甲酰胺、乙酸乙酯、甲醇、醋酸钠、乙酸酐、溴乙烷等均为分析纯.1.2 磷酸奥司他韦杂质A的合成向100 mL反应瓶中投入磷酸奥司他韦(1.00 g)、乙酸乙酯(20 mL)、水(10 mL),室温下搅拌,滴加1 mol/L的碳酸钠溶液,待水层pH值至7~8时,停止滴加,分层,水层使用乙酸乙酯萃取2次(每次20 mL),合并有机层,用蒸馏水(5 mL)搅拌洗涤1次,有机层加入无水硫酸钠干燥8 h,浓缩得白色固体.向浓缩物中加入四氢呋喃(40 mL)、1 mol/L氢氧化钾溶液3.6 mL,控温15~20 ℃下搅拌反应2 h,TLC监测反应结束后,加入氢型732阳离子交换树脂约0.9 g,搅拌10 min后,检测pH值应为5~6,滤去树脂,并使用少量四氢呋喃淋洗,合并洗滤液浓缩至干,加入石油醚降温至0~5 ℃搅拌结晶2 h,过滤,减压干燥得白色固体0.60g(收率86.6%).1H-NMR (500 MHz, DMSO) 6.37 (s, 1H,2-CH), 4.11 (d, 1H,3-CH), 3.69 (dd, J = 19.8, 9.1 Hz, 1H,4-CH), 3.40~3.28 (m, 1H,6-CH), 3.15 (dd, J = 16.2, 10.8 Hz, 1H,6-CH), 2.84~2.73 (m, 1H,5-CH), 2.26~2.15 (m,1H,1'-CH), 1.84 (s, 3H,CO-CH3), 1.50~1.27 (m, 4H,—CH2-,—CH2-), 0.83 (t, J=7.3 Hz, 3H,—CH3), 0.78 (t, J = 7.3 Hz, 3H,—CH3). MS(ESI):m/z 286.1 (M+1).1.3 磷酸奥司他韦杂质B的合成1.3.1 3-乙酰氧基-4-乙酰氨基-苯甲酸(2)的合成向100 mL单口瓶中,加入2mol/L盐酸20 mL、再加入醋酸钠10.0 g搅拌下使之溶解,降温至0~5 ℃,加入3-羟基-4-氨基苯甲酸1.0 g,缓慢滴加乙酸酐10 mL,滴毕,保持0~5 ℃下搅拌30 min,升温至15~25 ℃下反应6 h,逐步析出大量棕色沉淀,TLC监测反应结束后,降温至0~5 ℃结晶1 h,过滤,水洗,减压干燥得淡棕色固体1.09 g,产率70.4%.1H-NMR (500 MHz, DMSO) δ 8.16 (d, J=8.6 Hz, 1H,5-H), 7.78 (dd, J=8.6, 1.6 Hz, 1H,6-H), 7.68 (d, J=1.6Hz, 1H,2-H), 2.34 (s,3H,CH3COO—), 2.13 (s, 3H,CH3CON—).1.3.2 3-羟基-4-乙酰氨基-苯甲酸(3)的合成在100 mL的单口瓶中,加入30 mL 水,再加入0.12 g氢氧化钠,溶解后加入3-乙酰氧基-4-乙酰氨基苯甲酸0.6 g,控温15~20 ℃搅拌反应30 min,TLC监测反应结束后,用1 mol/L盐酸调节pH 值为2,有大量固体析出,降温至0~5 ℃结晶1 h,过滤,水洗,减压干燥得淡棕色固体0.44 g,产率89.1%.1H-NMR (500 MHz, DMSO) δ 8.05 (d, J = 8.4 Hz, 1H,5-H), 7.44 (d, J = 1.6 Hz, 1H,2-H), 7.38 (dd, J = 8.4, 1.6 Hz, 1H,6-H),2.14 (s, 3H, CH3CON—).1.3.3 3-羟基-4-乙酰氨基苯甲酸乙酯(杂质B)的合成在25 mL单口瓶中,加入0.2g 3-羟基-4-乙酰氨基苯甲酸、1 mL DMF、0.1 g碳酸氢钠,在室温下搅拌20 min,再向反应中加入溴乙烷0.17 g,升温至45~50 ℃反应12 h,TLC监测反应结束后,降温至室温,加入20 mL水,加入乙酸乙酯萃取2次(每次20 mL),合并有机层水洗1次,再加入水10 mL,搅拌下用1 mol/L的NaOH溶液调pH=8~9,分层,弃去有机层,水层加1 mol/L盐酸调节pH=5~6,有大量固体析出,过滤,水洗,减压干燥得淡棕色固体0.21 g,产率83.0%.1H-NMR (500 MHz, DMSO) δ 8.09 (d, J =8.4 Hz, 1H, 5-H), 7.46 (d, J=1.7 Hz, 1H, 2-H), 7.40 (dd, J=8.4, 1.6 Hz, 1H, 6-H). 4.27 (q, J=7.1 Hz, 2H,—OCH2), 2.14 (s,3H,—CH3CON—), 1.30 (t, J=7.1 Hz, 3H,—CH2CH3). MS(ESI):m/z 224.1 (M+1).2 结语杂质的存在在药品中是不可避免的,而且对于杂质的控制是药品安全保证的关键,因此对于一种药品进行杂质研究是一项重要的工作.本研究对杂质A的合成工艺进行了改进,改进后工艺操作简单,高收率的得到了杂质A,验证了杂质A的产生机理,提示生产企业在贮存磷酸奥司他韦过程中保持干燥,其结果经1H-NMR确证其结构.杂质B经Scifinder检索目前没有其合成方法,本实验探索性的合成了杂质B,并对其进行了条件优化,整条路线的总收率为52.1%,虽然总的收率不高,但是三步操作较为简单,成本较低,没有引起环境污染,首次对该化合物进行了合成研究,并经1H-NMR确证其结构,对于企业在生产磷酸奥司他韦的过程中对杂质进行控制有重要意义.致谢感谢武汉理工大学化学化工与生命科学学院对本实验的帮助.参考文献:[1] HAYDEN F G,TREANOR J J,FRITZ R S . Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza randomized controlled trials for prevention and treatment[J]. JAMA,1999,282: 1240-1246.[2] TREANOR J J,HAYDEN F G,VROOMAN P S. Efficacy and safety of the oral neuraminidase inhibitor Oseltamivir in treating acute influenza[J]. JAMA,2000,283: 1016-1024.[3] 王孟昭,孙武装,王亚梅,等. 磷酸奥司他韦治疗流行性感冒的临床疗效和安全性研究[J].中华传染病杂志,2003,21:114-117.WANG Meng-zhao, SUN Wu-zhuang, WANG Ya-mei, et al. Clinical efficacy and safety study on Oseltamivir phosphate for influenza[J]. Chinese Journalof Infectious Diseases, 2003, 21:114-117.[4] 陈晓红,祝亚非,关山越,等. 抗流感药物奥司他韦的结构确证[J].广州化工,2006(6):34-35.CHEN Xiao-hong, ZHU Ya-fei, GUAN Shan-yue, et al. The structure confirmation of the anti flu drug - Oseltamivir[J]. Guangzhou Chemical Industry, 2006(6):34-35.[5] FUJIKO K, TAKUYA A, ZHANG M R. Radiosyntheses of two positron emission tomography probes:[11C]Oseltamivir and its active metabolite [11C]Ro 64-0802[J].Bioorganic & Medicinal Chemistry Letters,2008,18: 1260-1263.[6] BISCHOFBERGER, NORBERT W. Compounds containing six-membered rings processes for their preparation and their use asmedicaments:WO,9914185[P].1999-03-25.[7] LUO M, KONISHI W J. Inhibitors of influenza virus neuraminidase and methods of making and using the same:WO,5453533[P].1995-10-26. [8] LUO M, BIRMINGHAM A. Methods of inhibition bacterial sialidase:US, 5985859[P]. 1999-06-07.[9] GUO W, LI J F, FAN N J, et al. A simple and effective method for chemisetiove esterification of phenolicacids [J].Synthetic Conmunications,2005 , 35:145-152.。
cooh是什么基团

cooh是什么基团COOH是羧基(carboxyl group)的化学式缩写,它是一种常见的有机化合物功能基团。
在有机化学中,羧基是由一个碳原子与一个氧原子以及一个氢原子结合而成的。
羧基通常以-COOH的形式出现,代表了一个碳原子上连接着一个含氧功能基团和一个氢原子。
可以通过羧酸(carboxylic acid)来表示这种化学基团,例如乙酸(acetic acid)的化学式为CH3COOH。
羧基具有非常重要的化学性质和生物学功能,广泛存在于天然物质中。
在有机化学中,羧基是一个极性功能基团,它使得羧酸具有亲水性。
这种亲水性让羧基有很强的溶解性,能够在许多溶剂中溶解,并与水分子发生氢键作用。
羧基也是许多生物分子的重要组成部分,如脂肪酸、氨基酸和核苷酸。
在脂肪酸中,羧基与甘油结合形成三酯,而在氨基酸中,羧基与氨基结合形成肽键,从而构成蛋白质的多肽链。
羧基还具有酸性反应。
由于羧基中的氧原子带有部分负电荷,它能够释放出氢离子(H+),形成一个共振稳定的羧酸阴离子。
这使羧酸成为一类具有酸性的有机物质,可以与碱反应生成盐和水。
例如,乙酸可以与氢氧化钠反应生成乙酸钠和水。
此外,羧基还参与了许多重要的化学反应和反应机制。
其中最常见的就是通过酯化(esterification)反应,羧基与醇(酸醇反应)发生酯键的形成,生成酯化合物。
酯是一类重要的有机化合物,广泛存在于水果香味、香精、药物和塑料等许多生物和工业化合物中。
总结起来,COOH是羧基的化学式缩写,代表了一个碳原子上连接着一个含氧功能基团和一个氢原子的有机化合物。
羧基是有机化学中的常见功能基团,具有强烈的亲水性、酸性以及参与酯化等许多重要化学反应和反应机制。
在生物学中,羧基是脂肪酸、氨基酸和核苷酸等生物分子的重要组成部分。
2_2_二甲基环丙烷甲酸的合成与拆分[1]
![2_2_二甲基环丙烷甲酸的合成与拆分[1]](https://img.taocdn.com/s3/m/b6139871a26925c52cc5bfbc.png)
2005 年 6 月 Journal of Chemical Engineering of Chinese Universities June 2005文章编号:1003-9015(2005)03-0384-042,2-二甲基环丙烷甲酸的合成与拆分石晓华, 周舞阗, 陈新志(浙江大学化学工程与生物工程学系, 浙江杭州 310027)摘要:S-(+)-2,2-二甲基环丙烷甲酸是合成西司他丁(一种肾脱氢二肽酶抑制剂)的关键中间体,今设计了一条新的2,2-二甲基环丙烷甲酸合成路线并改进了拆分工艺,它是以异戊烯酸为原料,经酸的酯化、烯键的环丙烷化、酯水解制得2,2-二甲基环丙烷甲酸,收率为44.1%。
其中,环丙烷化反应用锌粉/氯化亚铜-乙酰氯作为催化剂,二溴甲烷作为环丙烷化试剂,这样可以在温和的条件下进行反应,降低成本。
此外用L-肉碱草酸盐作为手性拆分试剂,经酰化、成盐、部分结晶、水解得到S-(+)-2,2-二甲基环丙烷甲酸。
收率为16.7%,手性纯度大于95%。
此路线工艺简单、环境友好、成本较低,易于工业化。
关键词:S-(+)-2,2-二甲基环丙烷甲酸;手性拆分;异戊烯酸;环丙烷化中图分类号:R914.5 文献标识码:ASynthesis and Chiral Resolution of 2,2- dimethylcyclopropane Carboxylic AcidSHI Xiao-hua, ZHOU Wu-tian, CHEN Xin-zhi(Department of Chemical and Biochemical Engineering, Zhejiang University, Hangzhou 310027, China)Abstract: (S)-(+)-2,2-dimethylcyclopropane carboxylic acid is the key intermediate of Cilastatin (an excellent inhibitor of dehydropeptidase-I). Using 2-methylbutenoic acid as raw material, 2,2-dimethylcyclopropane carboxylic acid was achieved via esterification, cyclopropanantion and hydrolysis, the yield is 44.1%. Specially, using zinc powder/copper (I) chloride-acetyl chloride as catalysts, the cyclopropanation proceedes easily with dibromomethane. Then 2,2-dimethylcyclopropane carboxylic acid is converted to acid chloride and reacted with L-carnitine oxalate (the chiral reagent for resolution). After fractional crystallization and hydrolysis, (S)-(+)-2,2-dimethylcyclopropane carboxylic acid was prepared with 16.7% yield. Thus a new synthesis route of 2,2-dimethylcyclopropane carboxylic acid was designed and its chiral resolution procedure was improved, this new route and improved procedure are clean, convenient to operate and apt for industrialization with lower cost.Key words: (S)-(+)-2,2-dimethylcyclopropane carboxylic acid; chiral resolution; 2-methylbutenoic acid;cyclopropanation1 引言S-(+)-2,2-二甲基环丙烷甲酸是合成西司他丁(一种肾脱氢二肽酶抑制剂)的关键中间体,西司他丁与亚胺培南的复方制剂泰能特别适用于多种菌联合感染以及需氧菌和厌氧菌的混合感染[1]。
糠硫醇及其羧酸酯的合成与香气特征

C atalytic Synthesis ofα2Amyl Cinnamaldehyde by Potassium Fluoride/AluminaWang Hongqing(Depart ment of Chemical Engineering,Cent ral South Institute of Technology,Hengyang,Postcode421001)Xia Liming(The Cent re of A nalysis,Hu’nan Institute of Chemical Technology,Changsha,Postcode410007)Abstract:Benzaldehyde and heptaldehyde as materials,theα2amyl cinnamaldehyde is synthesized in the pres2 ence of potassium fluoride on alumina.The sequence of the effects on reaction is benzaldehyde>catalyst>re2 action temperature>the dropping speed of heptaldehyde>reaction time.The optimum production conditions are as follows:benzaldehyde∶heptaldehyde∶catalyst is1.2mol∶1.0mol∶0.06mol,the dropping speed of hep2 taldehyde is6h,reaction temperature is60℃,reaction time is2h.Under the optimum conditions,the yield of α2amyl cinnamaldehyde can reach92.1%.K eyw ords:potassium fluoride/alumina,catalysis,synthesis,α2amyl cinnamaldehydeα2糠硫醇及其羧酸酯的合成与香气特征姚立红 苏长安 徐 锐 齐立权 杨永珍(辽宁大学化学与工程学院,沈阳,邮编110036)摘要 等摩尔α2糠醇与硫脲在18%盐酸中于50~60℃反应,放置过夜后碱性水解4h,得α2糠硫醇,收率为60%。
羧酸叔丁酯的保护方法

羧酸叔丁酯的保护方法1) 质子酸(如H2SO4)催化羧酸与异丁烯反应得到叔丁酯。
To a solution of compound 8(21 g, 43.12 mmol) in DCM (420 mL) was added H2SO4 (2.1 mL) at -780C under N2. Then inlet the isobutene in DCM for 30 min, stirred overnight at RT. The mixture was poured into sat.aq. NaHCO3, filtered an dried the organic layer and purified by flash chromatography to get the compound 9(16.6 g, Yield: 70.9%) as a oily for the next step.2) 在DMAP-Boc2O条件下,催化羧酸形成叔丁酯。
( 示例反应用了1eq的Boc2O是为了保证两个羧基只上一个叔丁酯。
实际反应中会有少量上双叔丁酯的副产物生成)To a flame dried flask containing t-BuOH (500mL), was added compound 1 (50g,1.0 eq), DMAP (1.97g, 0.1eq), anhydrous pyridine (12.8mL, 1.0eq). Boc2O (34.7g, 1.0eq) was then added slowly, the resulting mixture was stirred for overnight at 30 oC. Reaction was monitored by LC-MS, 100mL H2O was added to the reaction mixture, and some insoluble material was formed, it was filtered. The solid was dried over vacuum, and it was purified by silica gel column with eluent of PE/ EtOAc (5:1) to obtain the desired product as a white powder. (20.5g, 35.2% yelid)3) DCC-DMAP催化羧酸与t-BuOH直接缩合形成叔丁酯。
酚 醛 羧酸 酯化学方程式

酚醛羧酸酯化学方程式英文回答:Phenol, aldehyde, and carboxylic acid esterification is a chemical reaction that involves the conversion of phenol, aldehyde, and carboxylic acid into an ester. This reaction is commonly used in organic synthesis to produce a wide range of esters with various applications.The reaction mechanism starts with the formation of an intermediate called an acyloxyphosphonium salt. This intermediate is formed by the reaction between the carboxylic acid and a phosphonium salt, which acts as a catalyst. The phosphonium salt transfers its acyloxy group to the phenol, resulting in the formation of an ester and regenerating the catalyst.For example, let's consider the esterification of phenol with acetic acid. In this case, acetic acid (the carboxylic acid) reacts with a phosphonium salt catalyst toform an acyloxyphosphonium salt intermediate. This intermediate then reacts with phenol to produce phenyl acetate (the ester) and regenerate the catalyst.Another example is the esterification of benzaldehyde (the aldehyde) with butyric acid (the carboxylic acid). The reaction starts with the formation of an acyloxyphosphonium salt intermediate using a phosphonium salt catalyst. This intermediate then reacts with benzaldehyde to produce benzyl butyrate (the ester) and regenerate the catalyst.Overall, phenol, aldehyde, and carboxylic acid esterification is a versatile reaction that allows for the synthesis of various esters. It is widely used in the production of flavors, fragrances, and pharmaceuticals.中文回答:酚、醛和羧酸酯化反应是一种化学反应,涉及将酚、醛和羧酸转化为酯。
碳酸二甲酯甲基化应用综述
Jason_liu@frontierchem
8
Methylation of activated methylene groupMechanism
J. Chem. Soc. Perkin Trans.1 1994, 1323
Obviously, DMC has advantage over DMS, methyl halide and phosgene,
Pure and Applied Chemistry 2001,73, 1117–1124
2
Jason_liu@frontierchem
Two possible mechanism
3
Jason_liu@frontierchem
Methylation of indoles
Br + N H 1 O 2 3 O O Br 0.1 eq base 90 oC, 5 h N O O 4 + N Me Br
• •
DABCO is an extremely effective catalyst for the methylation of indoles in conjunction with dimethyl carbonate J. Org. hem, 2003, 68, 1984
4
Jason_liu@frontierchem
Methylation of indoles
The indole can be methylated in almost quantitative yields with DMC. J. Org. Chem, 2003, 68, 1984
EEDQ作为缩合剂的主要原理
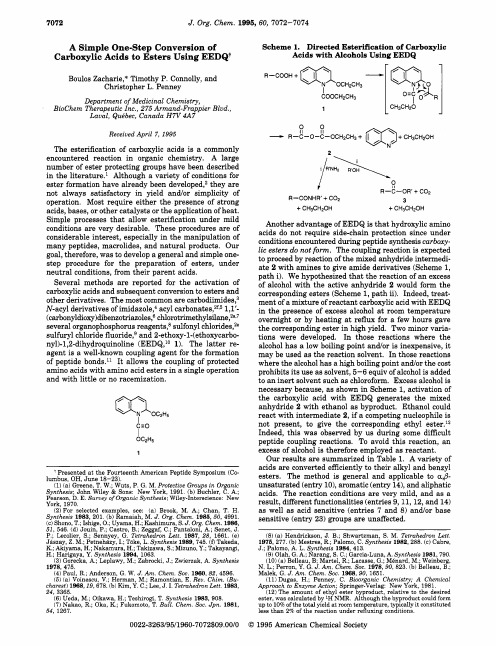
I
Indeed, this was observed by us during some difficult
OC2H5
peptide coupling reactions. To avoid thiLeabharlann reaction, an1
excess of alcohol is therefore employed as reactant.
(carbonyldioxy)dibenzotriazoles,6chlorotrimethyl~ilane,~~~~overnight or by heating at reflux for a few hours gave several organophosphorus reagents,Ssulfonyl chlorides,2e the corresponding ester in high yield. Two minor variasulfuryl chloride f l ~ o r i d ea,n~d 2-ethoxy-l-(ethoxycarbo- tions were developed. In those reactions where the nyl)-1,2-dihydroquinoline(EEDQ,1° 1). The latter re- alcohol has a low boiling point andor is inexpensive, it agent is a well-known coupling agent for the formation may be used as the reaction solvent. In those reactions of peptide bonds.ll It allows the coupling of protected where the alcohol has a high boiling point and/or the cost amino acids with amino acid esters in a single operation prohibits its use as solvent, 5-6 equiv of alcohol is added
系列环状螯合硼酸酯键合剂的合成及工艺优化

系列环状螯合硼酸酯键合剂的合成及工艺优化王祝愿;张习龙;邓剑如【摘要】为解决硼酸酯键合剂易水解的问题,选择酯化和酯交换的方法合成出了2个系列共8种环状螯合硼酸酯键合剂,用FTIR、1 H NMR、硼含量和羟值测定表征产物结构。
采用单因素法和正交实验的方法,探究各系列硼酸酯键合剂的最佳合成条件。
结果表明,硼酸酯成环后耐水解性提高;CBA1系列采用酯化法,合成最优工艺为二醇与硼酸的摩尔比为2∶1.4,温度为105℃,收率为98.4%;CBA2系列采用酯交换法,合成最优条件为硼酸、二醇、连接单体的摩尔比为1∶1.05∶0.45,温度为105℃,收率为97.2%。
%To solve the limitation of the borate ester bonding agent’ s suscept ibility to hydrolysis,methods of esterification and transesterification were adopted to synthesize two series of eight cyclic borate chelate bonding agents,whose corresponding product structures were characterized by using FTIR,1H NMR and determinations of boron content and hydroxyl value.Besides,the optimal synthetic conditions of each series were discussed by adopting orthogonal and single-factor experiment.The results show that:the re-sistance to hydrolysis of boric acid ester is improved after cyclization,CBA1 is synthesized by esterification,under the optimal condi-tion that the molar ratio of boric acid to glycol was 2 ∶ 1.4,with a temperature of 105℃,the yield was98.4%;CBA2 is synthesized by transesterification, under the optimal condition that the m olar ratio of boric acid, glycol to link was 1 ∶ 1.05 ∶ 0.45,with a tem-perature of 105 ℃,the yield was 97.2%.【期刊名称】《固体火箭技术》【年(卷),期】2016(039)002【总页数】5页(P231-235)【关键词】环状硼酸酯键合剂;酯化法;酯交换法;水解稳定性【作者】王祝愿;张习龙;邓剑如【作者单位】湖南大学化学化工学院,长沙 410082;湖南大学化学化工学院,长沙 410082;湖南大学化学化工学院,长沙 410082【正文语种】中文【中图分类】V512高能丁羟四组元(AP/RDX/Al/HTPB)复合固体火箭推进剂中,填料黑索今(RDX)、奥克托今(HMX)在复合材料中的含量高,且表面惰性,造成粘合剂基体与硝铵颗粒的界面粘结性能差,容易引起“脱湿”现象,导致推进剂力学性能无法达到使用要求[1-3]。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Effective esterification of carboxylic acids using (6-oxo-6H -pyridazin-1-yl)phosphoric acid diethylester as novel coupling agentsJu-Eun Won,a Ho-Kyun Kim,a Jeum-Jong Kim,a Heong-Seup Yim,a Min-Jung Kim,a Seung-Beom Kang,a Hyun-A Chung,a Sang-Gyeong Lee b ,*and Yong-Jin Yoon a ,*aDepartment of Chemistry and Environmental Biotechnology National Core Research center,Research Institute of Natural Science,Graduate School for Molecular Materials and Nanochemistry,Gyeongsang National University,900Gazwa-dong,Jinju,Gyeongnam 660-701,Republic of KoreabDepartment of Chemistry,Research Institute of Natural Science,Graduate School for Molecular Materials and Nanochemistry,Gyeongsang National University,900Gazwa-dong,Jinju,Gyeongnam 660-701,Republic of KoreaReceived 24July 2007;revised 1October 2007;accepted 1October 2007Available online 6October 2007Abstract —(6-Oxo-6H -pyridazin-1-yl)phosphoric acid diethyl esters (3)are efficient and selective coupling agents for equimolar esterifica-tion of carboxylic acids and alcohols.Esterification of aliphatic and aromatic carboxylic acids with aliphatic and aromatic alcohols using 3afforded the corresponding esters chemoselectively in good to excellent yield.Ó2007Elsevier Ltd.All rights reserved.1.IntroductionEffective esterification of carboxylic acids with alcohols is the most fundamental reaction in organic synthesis.1It has long been known that the process of esterification is enor-mously accelerated by the addition of strong acid such as sulfuric acid.There are also many methods of esterification that use specific dehydrating reagents.2However,the classi-cal esterifications have some disadvantages of the corrosive-ness of strong acid,with accompanying side reactions such as carbonization and oxidation.Although many reagents for esterification of carboxylic acid have been developed,2–5the research in this field is still very active even now.6For di-rect esterification of carboxylic acid in the absence of strong acid,carboxylic acid must be activated to more reactive species by using an activator.In our previous paper,3,5we have reported on the synthesis of anhydrides and esters using 4,5-dichloro-2-[(4-nitrobenzen-sulfonyl)]pyridazin-3(2H )-one as an activator.Because this esterification proceeds via the corresponding anhydrides as the intermediate,52equiv of carboxylic acids are required in this reaction.Therefore,we attempted to develop more ef-fective coupling agents that contain pyridazinone derivativesfor high-yielding esterifications with equimolar reactions of carboxylic acids and alcohols.Pyridazin-3(2H )-one is a stable and good leaving group,and shows an electron withdrawing ability.3,5,7–9Also various organophosphorus compounds have been developed as carboxylic acid activa-tors.10Therefore,we designed and synthesized some (6-oxo-6H -pyridazin-1-yl)phosphoric acid diethyl esters as carboxylic acid activator.In this paper,we report on effec-tive and convenient esterification of carboxylic acids with al-cohols by using (6-oxo-6H -pyridazin-1-yl)phosphoric acid diethyl esters in one port.2.Results and discussionSome 4,5-disubstituted-pyridazin-3(2H )-ones were readily prepared by the reported methods.11From preliminary ex-periments (Table 1,entries 1–5),12we selected triethylamine and acetonitrile as a suitable base/solvent system for the syn-thesis of 3.The reaction of 4,5-disubstituted-pyridazin-3(2H )-ones (1)with diethyl chlorophosphate (2)in the presence of triethyl-amine in acetonitrile at room temperature afforded the corre-sponding 3a –3e in excellent yields (Table 1,entries 1and 6–9)(Scheme 1).Initially,direct esterification of 4-nitrobenzoic acid (4a )with methanol (5a )using 3a was studied in a variety ofKeywords :(6-Oxo-6H -pyridazin-1-yl)phosphoric acid diethyl ester;Coupling agent;Carboxylic acid;Pyridazinone;Esterification.*Corresponding authors.Tel.:+820557516019;fax:+820557610244;e-mail:yjyoon@gnu.ac.kr0040–4020/$-see front matter Ó2007Elsevier Ltd.All rights reserved.doi:10.1016/j.tet.2007.10.011Tetrahedron 63(2007)12720–12730representative organic solvents and bases (Table 2,entries 1–10).Exclusive esterification in excellent yields was obtained in triethylamine/THF (or toluene,acetone,acetonitrile,di-ethyl ether,and ethyl acetate),13potassium carbonate/ethyl acetate (or THF),and N ,N -dimethylaminopyridine/THF (or ethyl acetate).Among these systems,we selected the po-tassium carbonate/ethyl acetate system for direct esterifica-tion of carboxylic acid using 3a .The efficacy of 3b –3e for esterification was evaluated using the reaction of 4-nitroben-zoic acid (4a )with methanol (5a )in the presence ofpotassium carbonate in ethyl acetate at reflux temperature (Table 2,entries 13–16).Compounds 3a –3d showed similar efficacy for esterification under this condition.Therefore,we selected compound 3a as a novel coupling agent for the esterification of carboxylic acid because 1b –1d were prepared by the conversion ofTable 1.Synthesis of diethyl 6-oxo-6H -pyridazin-1-ylphosphonate 3aN H NY X O+EtO P ClOEtO Base 3123N NYX O P OEtO OEtEntry1Base Time (h)Yield b (%)XY 1a Cl Cl Et 3N 13a (96)2a Cl Cl DMAP c 283a (65)3a Cl Cl NaH 263a (58)4a Cl Cl K 2CO 3103a (89)5a Cl Cl Cs 2CO 3183a (78)6b Cl OMe Et 3N 63b (89)7c Cl N 3Et 3N 43c (86)8d Cl OPh Et 3N 83d (88)9e Br BrEt 3N 193e (79)a Reaction was carried out at room temperature.b Isolated yield.cDMAP ¼N ,N -dimethylaminopyridine.13Cl P OOEtOEt 233R C OHO R C OR'O +R'OH 3Base/Solvent456a RCOOH =R'OH =xY ClCl b ClOMe ClN 3d ClOPhe Br BrcN H NY X ON NY X O P OEtO OEt Scheme 1.Table 2.Esterification of compound 4a with methanol (5a )using 3at reflux temperature34a5a6aC O OH O 2NC OOMeO 2NEntry 3Base Solvent Time (h)6a Yield a (%)13a Et 3N THF 0.59523a Et 3N EtOAc 0.59733a Et 3N H 2O 209d 43a K 2CO 3THF 0.39853a K 2CO 3EtOAc 0.59963a K 2CO 3H 2O 617d 73a DMAP b THF 29883a DMAP b EtOAc 29593a DMAP b H 2O 4211d 103a Resin c THF 4317113a Resin c EtOAc 2066123a Resin c H 2O 5011d 133b K 2CO 3EtOAc 197143c K 2CO 3EtOAc 0.598153d K 2CO 3EtOAc 0.896163eK 2CO 3EtOAc0.589a Isolated yield.4,5-Dichloropyridazin-3(2H )-one was isolated quantita-tively.b DMAP ¼N ,N -dimethylaminopyridine.c Resin is Amberite-IRA66.dCompound 3was decomposed.12721J.-E.Won et al./Tetrahedron 63(2007)12720–127301a .Esterification of 4-nitrobenzoic acid (4a )with various ali-phatic and aromatic alcohols 5b –5i using 3a in the presence of potassium carbonate in refluxing ethyl acetate afforded the corresponding esters 6b –6i except for 6e in good to excellent yields (Table 3,entries 1–8).The reaction of 4-nitrobenzoic acid (4a )with benzenethiol (5j )under the same condition also afforded the correspond-ing thioester 6j in excellent yields,whereas reaction of 4-ni-trobenzoic acid (4a )with tert -butyl alcohol (5e )under the same condition obtained 4-nitrobenzoic anhydride instead of the corresponding ester 6e in 21%yield (Table 3,entry 4).This result may be attributed to the steric hindrance of tert -butanol.Treatment of aliphatic or aromatic carboxylic acids 4b –4g with various alcohols 5a ,5c ,5f ,and 5i in the presence of potassium carbonate in refluxing ethyl acetate easily afforded the corresponding ester 6k –6ag in good to excellent yields (Table 4).Selective esterification of primary alcohol in mixed alcohol such as 1 /2 alcohol and 1 /3 alcohol is also often required.Therefore we examined the selective esterification of a mix-ture of two alcohols such as 1 /2 alcohols,1 /3 alcohols,2 /3 alcohols,aromatic/aliphatic alcohols,or bifunctional alcohols such as 2-mercaptoethanol (5k )and 4-aminophenol (5l ).The esterification of octanoic acid (4d )with a mixtureof 1 /2 alcohols or 1 /3 alcohols afforded primary alkyl es-ter 6t selectively (Table 5,entries 1and 3).But we found the corresponding anhydrides as the main products in the com-petition reaction of 2 /3 alcohols with octanoic acid (4d )and benzoic acid (4h )(Table 5,entries 5and 6).This selec-tivity may be due to the steric hindrance of the carbonyl car-bon of acyl phosphate and alcohols (2 or 3 ).In the reaction of carboxylic acids with 2 or 3 alcohol under our reaction condition,the formation of anhydride may be more favor-able than esterification.Esterification of octanoic acid (4d )with a mixture of cyclohexanol (5d )/phenol (5f )afforded phenyl ester 6u selectively in good yield (Table 5,entry 7).We investigated the chemoselectivity in the esterification of a mixture of phenol (5f )/benzenethiol (5j )and bifunc-tional alcohols such as 2-mercaptoethanol (5k )and 4-amino-phenol (5l ).The esterification of a mixture of phenol (5f )/benzenethiol (5j )with octanoic acid (4d )afforded the cor-responding ester 6u chemoselectively as the main product instead of the thioester.Also diphenyldisulfide was obtained as the side product (Table 5,entry 11).The reaction of 2-mercaptoethanol (5k )with octanoic acid (4d )under the same condition also afforded the thioester 6ak selectively in 86%yield (Table 5,entry 13).However,the reaction of 4-aminophenol (5l )with octanoic acid (4d )under the same condition yielded the corresponding amide 6am chemo-selectively in 99%yield (Table 5,entry 15).Table 3.Esterification of compound 4a with alcohols 5using 3a at reflux temperature+ R'XH3a 4a56C OOH O 2NC OXR'O 2N23X = O or SEntry 5Time (h)Product 6Yield a (%)15bi -PrOH1.5C O 2NO O(i-Pr)6b (86)25c CH 3(CH 2)5OH 2C O 2NOO(CH 2)5CH 36c (80)35dOH5.5C O 2NOO 6d (77)45eOH3C O 2NOO 6e (—)b55fOH2C O 2NOO6f (98)65gOHO 2N0.5C O 2NOO NO 26g (99)75hOH Cl0.5C O 2NOO Cl6h (99)85i NOH0.5C O 2N OO N6i (97)95jSH1C O 2NOS6j (98)a Isolated yield.4,5-Dichloropyridazin-3(2H )-one was isolated quantitatively.b4-Nitrobenzoic anhydride instead of 6e was obtained in 21%yield.12722J.-E.Won et al./Tetrahedron 63(2007)12720–12730Table 4.Esterification of some carboxylic acids 4b –4g with 5a ,5c ,5f ,and 5i using 3a in the presence of potassium carbonate in refluxing ethyl acetateRCOOH + R'OH 3a 23RCOOR'4b-4g 56k-6agEntry 45Time (h)Product 6Yield a (%)14b MeCOOH5a MeOH 2MeC OOMe 6k (95)24b Me COOH5c CH 3(CH 2)5OH9MeC OO(CH 2)5CH 36l (71)34b Me COOH5f OH 3.5Me C O O 6m (86)44b Me COOH5i NOH4MeC O ON6n (89)54c COOH5a MeOH 1C OOMe 6o (81)64c COOH5c CH 3(CH 2)5OH4C OO(CH 2)5CH 36p (83)74cCOOH5fOH0.5C O O 6q (80)84c COOH 5i NOH0.5C O ON6r (96)94d 5a MeOH1.36s (91)104d 5c CH 3(CH 2)5OH52)5CH36t (76)114d5f OH36u (95)124d5iNOH1.5N6v (94)134ePhPh COOH 5a MeOH 0.5PhPhCOOMe 6w (92)144e PhPh COOH 5c CH 3(CH 2)5OH 0.5Ph Ph COO(CH 2)5CH 36x (93)154e PhPh COOH 5fOH2Ph Ph CO O6y (87)164e PhPh COOH 5i NOH0.5PhPh CO ON6z (95)174fC OHO 5c CH 3(CH 2)5OH 9CO(CH 2)5CH 3O 6aa (70)184fC OHO 5fOH1C OO 6ab (86)194f C OHO 5i NOH0.5C O O N6ac (97)204gOCOOH5aMeOH1.5OCOMeO 6ad (92)214gOCOOH5c CH 3(CH 2)5OH 1OCO(CH 2)5CH 3O 6ae (77)(continued )12723J.-E.Won et al./Tetrahedron 63(2007)12720–12730Table 4.(continued )Entry 45Time (h)Product 6Yield a (%)224g OCOOH5f OH 3OC O O 6af (95)234gOCOOH 5iNOH2.5OC OO N6ag (95)aIsolated yield.4,5-Dichloropyridazin-3(2H )-one was isolated quantitatively.Table petition reaction of octanoic acid (4d )or benzoic acid (4h )with mixed alcohols at room temperatureRCOOH + R'XH/R"XH3a23RCOXR'(or R")456Entry 4R 0XH/R 00XH (5)Time (h)Product 6Yield a (%)14d CH 3(CH 2)5OH (5c )/i -PrOH(5b )12)5CH 36t (87)24h COOH 5b C OO(CH 2)5CH 36ah (90)34d CH 3(CH 2)5OH (5c )/t -BuOH(5e )0.32)5CH 36t (82)44h COOH 11C OO(CH 2)5CH 36ah (22)c 54d i -PrOH(5b )/t -BuOH(5e )3.5——d 64h COOH 3.5——c 74d c -C 6H11OH(5d )/C 6H 5OH(5f )26u (80)84h COOH 2C OO 6ai (86)94d CH 3(CH 2)5OH(5c )/C 6H 5OH(5f )0.56u (88)104h COOH 4C OO 6ai (82)114d C 6H 5OH (5f )/C 6H 5SH (5j )66u (71)e 124h COOH 3.5C OS 6aj (50)e 134d HSCH 2CH 2OH (5k )1.52)2OH6ak (86)144h COOH 4CS(CH 2)2OH O6al (93)154dH 2NOH (5l )1.5CNHOH6am (99)164hCOOH2.5fCNHO OH6an (92)a Isolated yield.4,5-Dichloropyridazin-3(2H )-one was isolated quantitatively.b Reaction temperature ¼at reflux temperature.c Benzoic anhydride was obtained in 54%yield.d Octanoic anhydride was obtained in 75%yield.e Diphenyldisulfide was also obtained.fThe solvent was THF.12724J.-E.Won et al./Tetrahedron 63(2007)12720–12730The reaction of benzoic acid (4h )with a mixture of 1 /2 alcohols afforded primary alkyl ester 6ah in excellent selec-tivity and in high yield (Table 5,entry 2).The esterification of benzoic acid (4h )with a mixture of 1 /3 alcohols af-forded primary alkyl ester 6ah (22%)and benzoic anhy-dride (54%)(Table 5,entry 4).The reaction of a mixture of 2 /3 alcohols with benzoic acid (4h )isolated only ben-zoic anhydride instead of the corresponding ester (Table 5,entry 6).Aromatic alcohols were selectively esterified with benzoic acid (4h )in the competition reaction of aliphatic/ar-omatic alcohol under our condition (Table 5,entries 8and 10).The esterification of a mixture of phenol (5f )/benzene-thiol (5i )with benzoic acid (4h )using 3a afforded the corre-sponding thioester 6aj (50%)chemoselectively and diphenyldisulfide (Table 5,entry 12).The reaction of 2-mercaptoethanol (5k )with benzoic acid (4h )using 3a under same condition also afforded selectively thioester 6al in 93%yield (Table 5,entry 14).The structure of 6al was established by IR,NMR,and elemental analysis.However,the reaction of 4-aminophenol (5l )with benzoic acid (4h )using 3a under the same condition yielded the corresponding amide 6an chemoselectively in 92%yield (Table 5,entry 16).In the esterification of octanoic acid (4d )or benzoic acid (4h ),the different selectivity of cyclohexanol (5d )and phenol (5f )may be attributed to the steric hindrance and/or the difference of p K a for two alcohols.Also,the different chemoselectivity for esterification of octanoic acid (4d )or benzoic acid (4h )with phenol (5f )or benzenethiol (5j )may be due to the different nucleophilicity of two nucleo-philes (5f and 5j )and/or the different electrophilicity of the carbonyl carbons for aliphatic and aromatic carboxylic acids.In all the reactions described above,reusable 4,5-dichloro-pyridazin-3(2H )-one (1a )was also isolated quantitatively.On the other hand,acid anhydride was not detected during these reactions when monitored with TLC except for the use of 2 and 3 alcohols.Actually,only 1equiv of carboxy-lic acid was required for the esterification under these reac-tion conditions.This esterification mechanism is different from that of the esterification of carboxylic acid with alcohol by using 4,5-dichloro-2-[(4-nitrobenzenesulfonyl)]pyrida-zin-3(2H )-one.6The esterification of carboxylic acid using compound 3may be proceeded via two steps,the formation of acyl phosphate in the first step and then alcohol reacts with acyl phosphate to give the ester in the second step.Therefore,diethyl 6-oxo-6H -pyridazin-1-ylphosphonate is a more effective coupling agent than 4,5-dichloro-2-[(4-ni-trobenzenesulfonyl)]pyridazin-3(2H )-one 6for equimolar esterification of carboxylic acids and alcohols.The struc-tures of all the products were established by IR,NMR,and elemental analyses (Scheme 2).3a / BaseR'OH / Base2CO ORPOEtO OEtCO OH R CO O RR'CO O R CR O EsterAnhydride-Scheme 2.3.ConclusionCompound 3a is an efficient coupling agent for equimolar esterification of carboxylic acids with alcohols under the ba-sic condition.It also has some advantages:(i)the reaction condition is basic,(ii)this method shows excellent selecti-vity for primary or secondary alcohols,(iii)the coupling agent is easily prepared from commercially available com-pound 1,and (iv)compound 1can be recovered quantita-tively for reuse.We also believe that these coupling agents would be applicable particularly to solid-phase syntheses and amidation of carboxylic acid.4.Experimental4.1.GeneralColumn chromatography was carried out on silica gel 60(70–230mesh).Melting points were determined with a Thomas–Hoover capillary apparatus and are uncorrected.1H and 13C NMR spectra were recorded on a 300MHz spectrophotometer with chemical shift values reported in d units (part per million)relative to an internal standard (tetramethylsilane).IR spectra were obtained on a Hitachi 270-50or Mattson Genesis Series FT-IR spectrophotome-ter.Elemental analyses were performed with CHNS-932(Leco).4.2.4,5-Disubsituted-6-oxo-6H -pyridazin-1-ylphos-phonates (3a–3e)A solution of diethyl chlorophosphate (10.4mmol)in sol-vent such as CH 2Cl 2,THF,CH 3CN,acetone,or toluene was added slowly to a solution of 4,5-disubsituted-pyrid-azin-3(2H )-ones 10(9.4mmol)in solvent such as CH 2Cl 2,THF,CH 3CN,acetone,or toluene in the presence of a base (10.4mmol)at the appropriate temperature with stirring.The mixture was stirred at room temperature until compound 1disappeared.After filtering the mixture,water (150mL)was added to the filtrate.The product was extracted with methylene chloride (150mL).The methylene chloride solu-tion was dried over anhydrous magnesium sulfate.The resulting solution was evaporated under reduced pressure to give compound 3as liquid.4.2.1.Diethyl-4,5-dichloro-6-oxo-(6H )-pyridazin-1-yl-phosphonate (3a).Liquid.R f ¼0.33(EtOAc/n -hexane ¼1:1,v/v).IR (KBr)3020,2970,1700,1610,1560,1460,1430,1400,1380,1310,1180,1150,1120,1050,980,940cm À1.1H NMR (CDCl 3)d :9.04(s,1H),4.52–4.42(m,4H),1.48–1.43(m,6H).13C NMR (CDCl 3)d :157.6,149.6,138.3,127.2,65.9,16.0.Elemental analysis calcd for C 8H 11Cl 2N 2O 4P:C,31.92;H,3.68;N,9.30.Found:C,31.93;H,3.74;N,9.33.4.2.2.Diethyl-5-chloro-4-methoxy-6-oxo-(6H )-pyrid-azin-1-ylphosphonate (3b).Liquid.R f ¼0.33(EtOAc/n -hexane ¼1:2,v/v).IR (KBr)3010,2960,2940,1695,1620,1580,1480,1460,1400,1360,1290,1170,1120,1040,980cm À1.1H NMR (CDCl 3)d :8.91(s,1H),4.50–4.40(m,4H),4.15(s,3H),1.44(t,6H,J ¼7.1Hz).13C NMR (CDCl 3)d :157.8,156.9,137.3,113.8,65.5,57.7,12725J.-E.Won et al./Tetrahedron 63(2007)12720–1273016.0.Elemental analysis calcd for C9H14ClN2O5P:C,36.44; H,4.76;N,9.44.Found:C,36.46;H,4.77;N,9.52.4.2.3.Diethyl-4-azido5-chloro-6-oxo-(6H)-pyridazin-1-ylphosphonate(3c).Liquid.R f¼0.30(EtOAc/n-hexane¼1:1,v/v).IR(KBr)3010,2960,2260,1690,1580,1460, 1380,1360,1300,1250,1210,1180,1080,1050,1020, 860cmÀ1.1H NMR(CDCl3)d:8.90(s,1H),4.96–4.40 (m,4H),1.46–1.41(m,6H).13C NMR(CDCl3)d:157.7, 141.9,141.1,116.8,65.8,16.0.Elemental analysis calcd for C8H11ClN5O4P:C,31.23;H,3.60;N,22.77.Found:C, 31.34;H,3.69;N,22.78.4.2.4.Diethyl-5-chloro-4-phenoxy-6-oxo-(6H)-pyridazin-1-ylphosphonate(3d).Liquid.R f¼0.40(EtOAc/n-hexane¼1:1,v/v).IR(KBr)3010,1690,1580,1500,1380,1320, 1300,1230,1210,1050,980,900,820,780cmÀ1.1H NMR (CDCl3)d:8.51(s,1H),7.52–7.13(m,5H),4.53–4.44(m, 4H),1.48–1.43(m,6H).13C NMR(CDCl3)d:158.3,155.3, 153.2,140.3,130.7,126.6,120.1,115.5,65.7,16.1.Elemen-tal analysis calcd for C14H16ClN2O5P:C,46.88;H,4.50;N,7.81.Found:C,46.91;H,4.52;N,7.84.4.2.5.Diethyl-4,5-dibromo-6-oxo-(6H)-pyridazin-1-yl-phosphonate(3e).Liquid.R f¼0.30(EtOAc/n-hexane¼1:1, v/v).IR(KBr)3030,2980,2950,1700,1600,1540,1430, 1420,1380,1300,1180,1100,1050,970cmÀ1.1H NMR (CDCl3)d:9.04(s,1H),4.52–4.42(m,4H),1.47–1.42(m, 6H).13C NMR(CDCl3)d:158.5,151.5,132.4,121.4, 65.9,16.1.Elemental analysis calcd for C8H11Br2N2O4P: C,24.64;H,2.84;N,7.18.Found:C,24.66;H,2.95;N,7.21.4.3.Esterification of carboxylic acid derivatives with alcohol derivativesA solution of carboxylic acid(4.1mmol,1equiv),alcohol(4.5mmol,1.1equiv),base(4.5mmol,1.1equiv),coupling agent3(6.1mmol,1.5equiv),and solvent(30mL)was stirred at reflux temperature or at room temperature until carboxylic acid disappeared by TLC monitoring.After cool-ing to room temperature,the mixture wasfiltered.The sol-vent was evaporated under reduced pressure.The resulting residue was applied to the top of an open-bed silica gel column(2.5Â10cm).The column was eluted with methyl-ene chloride.Fractions containing the ester were combined, and evaporated under reduced pressure to give the ester.And fractions containing pyridazinone derivative were com-bined,and evaporated under reduced pressure to give pyrid-azinone derivative.4.3.1.Methyl4-nitrobenzoate(6a).Mp94–95 C.R f¼0.58 (EtOAc/n-hexane¼1:2,v/v).IR(KBr)3120,3080,2950, 2850,1720,1610,1530,1440,1350,1310,1280,1100, 960,880,820,720cmÀ1.1H NMR(CDCl3)d:8.29(d, 2H,J¼9.0Hz),8.21(d,2H,J¼9.0Hz),3.99(s,3H).13C NMR(CDCl3)d:165.2,150.6,136.5,130.7,123.6,52.9.El-emental analysis calcd for C8H7NO4:C,53.04;H,3.89;N,7.73.Found:C,49.96;H,3.97;N,7.80.4.3.2.Isopropyl4-nitrobenzoate(6b).Mp106–108 C. R f¼0.67(EtOAc/n-hexane¼1:2,v/v).IR(KBr)3110, 3070,3050,2990,2940,2870,1710,1610,1520,1460, 1340,1290,1090,1000,830,710cmÀ1.1H NMR(CDCl3)d:8.28(d,2H,J¼8.9Hz),8.20(d,2H,J¼8.8Hz),5.29 (m,1H),1.41(d,6H,J¼6.3Hz).13C NMR(CDCl3)d: 164.2,150.4,136.3,130.6,123.4,69.7,21.9.Elemental analysis calcd for C10H11NO4:C,57.41;H,5.30;N,6.70. Found:C,57.49;H,5.31;N,6.72.4.3.3.Hexyl4-nitrobenzoate(6c).Liquid.R f¼0.71 (EtOAc/n-hexane¼1:2,v/v).IR(KBr)3140,2950,2890, 1730,1610,1540,1470,1360,1320,1280,1110,1020, 880,720cmÀ1.1H NMR(CDCl3)d:8.29(d,2H, J¼9.0Hz),8.21(d,2H,J¼9.0Hz), 4.38(t,2H, J¼6.7Hz),1.85–1.75(m,2H),1.51–1.26(m,6H),0.91(t, 3H,J¼7.0Hz).13C NMR(CDCl3)d:165.7,151.4,136.9, 131.6,124.5,67.1,32.3,29.5,26.6,23.5,14.9.Elemental analysis calcd for C13H17NO4:C,62.14;H,6.82;N,5.57. Found:C,62.20;H,6.89;N,5.59.4.3.4.Cyclohexyl4-nitrobenzoate(6d).Liquid.R f¼0.73 (EtOAc/n-hexane¼1:2,v/v).IR(KBr)3150,2970,2900, 1730,1610,1530,1460,1410,1360,1340,1290,1180, 1110,1020,960,890cmÀ1.1H NMR(CDCl3)d:8.28(d, 2H,J¼9.0Hz),8.21(d,2H,J¼9.0Hz),5.11–5.03(m,1H), 2.00–1.95(m,2H),1.83–1.75(m,2H),1.68–1.57(m,3H), 1.53–1.33(m,3H).13C NMR(CDCl3)d:164.0,150.4, 136.4,130.6,123.4,74.4,31.5,25.3,23.6.Elemental analysis calcd for C13H15NO4:C,62.64;H,6.07;N,5.62.Found:C, 62.67;H,6.10;N,5.67.4.3.5.Phenyl4-nitrobenzoate(6f).Mp127–129 C. R f¼0.75(EtOAc/n-hexane¼1:2,v/v).IR(KBr)3130,1750, 1610,1530,1490,1360,1320,1280,1190,1080,1020, 870,850,760,720cmÀ1.1H NMR(CDCl3)d:8.40–8.33 (m,4H),7.46(t,2H,J¼8.1Hz),7.31(t,1H,J¼7.4Hz), 7.23(d,2H,J¼7.5Hz).13C NMR(CDCl3)d:163.3,150.9, 150.5,135.0,131.3,129.7,126.4,123.7,121.4.Elemental analysis calcd for C13H9NO4:C,64.20;H,3.73;N,5.76. Found:C,64.23;H,3.75;N,5.78.4.3.6.4-Nitrophenyl4-nitrobenzoate(6g).Mp158–159 C.R f¼0.77(CH2Cl2).IR(KBr)3150,1760,1600, 1560,1540,1500,1360,1340,1270,1220,1080,870, 720cmÀ1.1H NMR(CDCl3)d:8.40(s,4H),8.36(d,2H, J¼9.1Hz),7.96(d,2H,J¼9.1Hz).13C NMR(CDCl3)d: 162.5,155.1,151.2,145.8,133.9,131.5,125.5,123.9, 122.5.Elemental analysis calcd for C13H8N2O6:C,54.18; H,2.80;N,9.72.Found:C,54.22;H,2.88;N,9.81.4.3.7.2-Chlorophenyl4-nitrobenzoate(6h).Mp119–121 C.R f¼0.77(CH2Cl2).IR(KBr)3130,3100,1760, 1620,1540,1490,1370,1290,1270,1220,1090,900,860, 780,730cmÀ1.1H NMR(CDCl3)d:8.45–8.37(m,4H), 7.53(d,1H,J¼7.9Hz),7.39–7.28(m,4H).13C NMR (CDCl3)d:162.5,151.1,146.8,134.3,131.5,130.5,128.0, 127.6,126.8,123.8,123.6.Elemental analysis calcd for C13H8ClNO4:C,56.23;H,2.90;N,5.04.Found:C,56.31; H,2.97;N,5.09.4.3.8.Pyridin-3-yl4-nitrobenzoate(6i).Mp111–112 C. R f¼0.24(EtOAc/n-hexane¼1:2,v/v).IR(KBr)3120, 1740,1620,1600,1560,1520,1480,1420,1340,1320, 1260,1200,1060,1000,840,700cmÀ1.1H NMR(CDCl3) d:8.61–8.58(m,2H),8.39(s,4H),7.66(d,1H, J¼8.4Hz),7.46–7.41(m,1H).13C NMR(CDCl3)d:12726J.-E.Won et al./Tetrahedron63(2007)12720–12730162.9,151.1,147.5,143.2,137.2,134.1,131.4,129.2,124.1, 123.8.Elemental analysis calcd for C12H8N2O4:C,59.02;H,3.30;N,11.47.Found:C,59.05;H,3.38;N,11.49.4.3.9.S-Phenyl4-nitrobenzothioate(6j).Mp155–157 C. R f¼0.71(CH2Cl2).IR(KBr)3100,3070,1670,1600,1520, 1480,1440,1340,1320,1200,1100,920,850,750, 680cmÀ1.1H NMR(CDCl3)d:8.34(d,2H,J¼8.8Hz), 8.18(d,2H,J¼8.8Hz),7.53–7.48(m,5H).13C NMR (CDCl3)d:188.8,150.7,141.3,134.9,130.1,129.5,128.5, 125.2,124.0.Elemental analysis calcd for C13H9NO3S:C, 60.22;H,3.50;N,5.40.Found:C,60.24;H,3.53;N,5.48.4.3.10.4-Nitrobenzoic anhydride.Mp55–56 C.R f¼0.84 (EtOAc/n-hexane¼1:1,v/v).IR(KBr)3150,3000,1730, 1620,1540,1360,1280,1110,1030,880,810,720cmÀ1.1H NMR(CDCl3)d:8.12(d,2H,J¼9.0Hz),8.12(d,2H,J¼9.0Hz).13C NMR(CDCl3)d:164.7,150.5,135.8, 130.6,123.5.Elemental analysis calcd for C15H10N2O6:C, 57.33;H,3.21;N,8.91.Found:57.37;H,3.33;N,8.96. 4.3.11.Methyl4-methylbenzoate(6k).Liquid.R f¼0.77(EtOAc/n-hexane¼1:2,v/v).IR(KBr)3030,1740,1620, 1450,1290,1190,1120,1030,850,760cmÀ1.1H NMR (CDCl3)d:7.91(d,2H,J¼8.2Hz),7.20(d,2H,J¼8.2Hz), 3.87(s,3H),2.37(s,3H).13C NMR(CDCl3)d:167.1, 143.5,129.6,129.0,127.4,51.8,21.5.Elemental analysis calcd for C9H10O2:C,71.98;H,6.71.Found:C,72.03;H, 6.79.4.3.12.Hexyl4-methylbenzoate(6l).Liquid.R f¼0.81 (EtOAc/n-hexane¼1:2,v/v).IR(KBr)2940,2850,1720, 1620,1460,1380,1270,1180,1100,1020,760cmÀ1.1H NMR(CDCl3)d:7.93(d,2H,J¼8.2Hz),7.21(d,2H, J¼8.2Hz),4.29(t,2H,J¼6.7Hz),2.39(s,3H),1.79–1.70 (m,2H),1.36–1.30(m,6H),0.92–0.87(m,3H).13C NMR (CDCl3)d:166.7,143.3,129.5,129.0,127.8,64.8,31.5, 28.7,25.7,22.5,21.6,14.0.Elemental analysis calcd for C14H18O2:C,77.03;H,8.31.Found:C,77.11;H,8.36. 4.3.13.Phenyl4-methylbenzoate(6m).Mp75–76 C. R f¼0.72(EtOAc/n-hexane¼1:2,v/v).IR(KBr)3050, 2950,1720,1610,1590,1480,1460,1400,1270,1250, 1190,1170,1090,1020,840,750cmÀ1.1H NMR(CDCl3) d:8.09(d,2H,J¼8.2Hz),7.42(t,2H,J¼8.1Hz),7.30(d, 2H,J¼8.3Hz),7.25–7.19(m,3H),2.44(s,3H).13C NMR (CDCl3)d:165.3,151.1,144.1,130.2,129.5,129.3,126.9, 125.8,121.8,21.8.Elemental analysis calcd for C14H12O2: C,79.22;H,5.70.Found:C,79.18;H,5.82.4.3.14.Pyridin-3-yl4-methylbenzoate(6n).Mp75–76 C. R f¼0.45(EtOAc/CH2Cl2¼1:5,v/v).IR(KBr)3120,3050, 3000,2930,2850,1740,1650,1610,1580,1470,1430, 1280,1210,1180,1140,940cmÀ1.1H NMR(CDCl3)d: 8.56(d,1H,J¼2.1Hz),8.53(d,1H,J¼4.2Hz),8.09(d, 2H,J¼8.2Hz),7.61(d,1H,J¼8.3Hz),7.41–7.36(m,1H), 7.33(d,2H,J¼8.0Hz),2.46(s,3H).13C NMR(CDCl3)d: 164.8,147.4,146.9,145.0,143.6,130.3,129.4(2),126.0, 123.9,21.8.Elemental analysis calcd for C13H11NO2:C, 73.23;H,5.20;N,6.57.Found:C,73.25;H,5.25;N,6.64.4.3.15.Methyl cyclohexanecarboxylate(6o).Liquid. R f¼0.73(EtOAc/n-hexane¼1:2,v/v).IR(KBr)2970,2900,1760,1470,1270,1210,1190,1150,1050cmÀ1.1H NMR(CDCl3)d:3.55(s,3H),2.24–2.15(m,1H),1.18–1.76(m,2H), 1.66–1.59(m,2H), 1.55–1.52(m,1H), 1.40–1.27(m,2H),1.24–1.05(m,3H).13C NMR(CDCl3) d:176.2,51.2,42.9,28.9,25.6,25.3.Elemental analysis calcd for C8H14O2:C,67.57;H,9.92.Found:C,67.59;H, 10.01.4.3.16.Hexyl cyclohexanecarboxylate(6p).Liquid. R f¼0.85(EtOAc/n-hexane¼1:2,v/v).IR(KBr)2970, 2900,1750,1690,1470,1400,1330,1260,1180,1140, 1050cmÀ1.1H NMR(CDCl3)d:4.05(t,2H,J¼6.7Hz), 2.33–2.24(m,1H),1.19–1.86(m,2H),1.77–1.72(m,2H), 1.66–1.57(m,3H), 1.51–1.23(m,11H),0.89(t,3H, J¼6.9Hz).13C NMR(CDCl3)d:166.7,143.3,129.5, 129.0,127.8,64.8,31.5,28.7,25.7,22.5,21.6,14.0.Ele-mental analysis calcd for C13H22O2:C,74.24;H,10.54. Found:C,74.23;H,10.60.4.3.17.Phenyl cyclohexanecarboxylate(6q).Liquid. R f¼0.71(EtOAc/n-hexane¼1:2,v/v).IR(KBr)3100, 2960,2900,1770,1610,1500,1460,1390,1330,1260, 1210,1170,1140,1040,950cmÀ1.1H NMR(CDCl3)d: 7.30(t,2H,J¼8.2Hz),7.14(t,1H,J¼7.4Hz),7.03(d, 2H,J¼7.4Hz),2.56–2.46(m,1H),2.05–2.00(m,2H), 1.80–1.75(m,2H),1.66–1.50(m,3H),1.38–1.21(m,3H). 13C NMR(CDCl3)d:176.1,64.2,43.2,31.4,29.0,28.6, 25.7,25.6,25.4,22.5,13.4.Elemental analysis calcd for C13H16O2:C,76.44;H,7.90.Found:C,76.50;H,7.98. 4.3.18.Pyridin-3-yl cyclohexanecarboxylate(6r).Liquid. R f¼0.50(EtOAc/n-hexane¼1:2,v/v).IR(KBr)3100,2960, 2900,1720,1600,1500,1460,1280,1210,1190,1110,1050, 810cmÀ1.1H NMR(CDCl3)d:8.43(d,1H,J¼4.5Hz),8.40 (d,1H,J¼2.2Hz),7.44(d,1H,J¼8.3Hz),7.30–7.26(m, 1H),2.62–2.52(m,1H),2.07–2.02(m,2H),1.83–1.78(m, 2H),1.69–1.51(m,3H),1.42–1.23(m,3H).13C NMR (CDCl3)d:173.7,147.5,146.5,143.3,129.1,123.7,42.9, 28.7,25.6,25.1.Elemental analysis calcd for C12H15NO2: C,70.22;H,7.37;N,6.82.Found:C,70.24;H,7.41;N,6.85.4.3.19.Methyl octanoate(6s).Liquid.R f¼0.86(EtOAc/n-hexane¼1:1,v/v).IR(KBr)2960,2890,1760,1470,1450, 1380,1210,1180cmÀ1.1H NMR(CDCl3)d:3.66(s,3H), 2.31(t,2H,J¼7.4Hz),1.62(t,2H,J¼7.4Hz),1.33–1.29 (m,8H),0.88(t,3H,J¼6.6Hz).13C NMR(CDCl3)d: 174.1,51.2,34.0,31.6,29.0,28.8,24.9,22.5,13.9.Elemen-tal analysis calcd for C9H18O2:C,68.31;H,11.47.Found:C, 68.33;H,11.51.4.3.20.Hexyl octanoate(6t).Liquid.R f¼0.82(EtOAc/n-hexane¼1:2,v/v).IR(KBr)2960,2900,1760,1580,1400, 1380,1270,1190,1120cmÀ1.1H NMR(CDCl3)d:4.06 (t,2H,J¼6.7Hz),2.29(t,2H,J¼7.6Hz),1.66–1.57(m, 4H),1.40–1.23(m,14H),0.92–0.86(m,6H).13C NMR (CDCl3)d:173.9,64.3,34.4,31.6,31.4,29.1,28.9,28.6, 25.6,25.0,22.5,22.4,14.0,13.0.Elemental analysis calcd for C14H26O2:C,74.29;H,11.58.Found:C,74.33;H,11.61.4.3.21.Phenyl octanoate(6u).Liquid.R f¼0.66(CH2Cl2). IR(KBr)3090,2950,2880,1770,1600,1500,1470,1380, 1300,1200,1140,1100,1030,930cmÀ1.1H NMR (CDCl3)d:7.34(t,2H,J¼7.4Hz),7.18(t,1H,J¼7.4Hz),12727J.-E.Won et al./Tetrahedron63(2007)12720–12730。
