Lersivirine_LCMS_08850_MedChemExpress
Study_on_the_pharmacological_activities_and_chemic

ReviewStudy on the pharmacological activities and chemicalstructures of Viburnum dilatatumZhiheng Gao, Yufei Xi, Man Wang, Xiaoxiao Huang*, Shaojiang Song*Key Laboratory of Computational Chemistry-Based Natural Antitumor Drug Research &Development, Liaoning Province, School of Traditional Chinese Materia Medica, ShenyangPharmaceutical University, Shenyang 110016, ChinaAbstractViburnum dilatatum (jiami in Chinese), belonging to the Caprifollaceae family, is widely distributed in Japan and China. Phytochemical investigations of Viburnum dilatatum (V. dilatatum) have resulted in the isolation of triterpenoids, phenolic glycosides essential oil, norisoprenoids, etc. Research results have shown that the chemical constituents of V. dilatatum possess various pharmacological activities, including antihyperglycemic, antioxidant activity and antiulcer effects. This study reviewed the chemical constituents and pharmacological activities of V. dilatatum to provide practical and useful information for further research and development of this plant.Keywords: Viburnum dilatatum; pharmacological activity; chemical structures1 IntroductionViburnum dilatatum (called jiami in Chinese, gamazumi in Japanese and snowball tree in English), beloinging to family Caprifoliaceae, is a deciduous low tree distributed widely in the hills of northern China and Japan [1]. There are many types of chemical constituents in Viburnum dilatatum (V. dilatatum), including triterpenoids, * Author to whom correspondence should be addressed. Address:School of Traditional Chinese Materia Medica, Shenyang Pharmaceutical University, 103 Wenhua Rd., Shenyang 110016, China; Tel.: +86-24-43520793 (Xiaoxiao Huang); +86-24-43520707 (ShaojiangSong);E-mail:*******************(XiaoxiaoHuang); ****************(ShaojiangSong).Received: 2021-04-16 Accepted: 2022-08-28phenolic glycosides and norisoprenoids [2-4]. The leaves have been utilized as a traditional Chinese medicine, and phenolic compounds have been reported as the main active chemical component of the leaves. Many researchers have analyzed the functions of these medicinal components and found that these components have good antioxidant antihyperglycemic and antiulcer effects. For example, the gamazumi crude extract obtained from the squeezed juice of the fruit prevented oxidative injury in rats [5]. This review described the chemical structures and pharmacological activities of V. dilatatum, so as to help readers understand comprehensively the research progress of V. dilatatum and provide help for the development of V. dilatatum.2 Chemical constituents and structuresPrevious reports have indicated that the main chemical constituents of V. dilatatum are phenolic glycosides and triterpenoids.2.1 Phenolic glycosidesThirteen phenolic glycosides were isolated and identified from V. dilatatum by extensive spectroscopic methods, namely p -hydroxyphenyl-6-O -trans-caffeoyl-β-D -glucoside (1) [6], p -hydroxyphenyl-6-O -trans-caffeoyl-β-D -alloside (2) [6], 4-allyl-2-methoxyphenyl-6-O -β-D -apiosyl(1→6)-β-D -glucoside (3) [6], 1-(4’-hydroxy-3’-methoxypheny1)-2-[2’’-hydroxy-4’’-(3’’’-hydroxypropyl)]-1,3-propanediol-l-O -β-D -glucopyranoside (erythro isomer) (4-7) [7], neochlorogenic acid methyl ester (8-9) [7], cryptochlorogenic acid methyl ester (10-11) [7], cyanidin-3-sambubioside (Cy-3-sam) (12) [8], cyanidin-3-glucoside (Cy-3-glc) (13) [8], 5-O -caffeoyl-4-methoxyl quinic acid (4-MeO-5-CQA) (14) [8], chlorogenic acid (5-CQA) (15) [8], quercetin (16) [8], 2-(glucopyranosyloxy)-benzyl-3-(glucopyranosyloxy)-benzoate (17) [9] and jiamizioside E (18) [10]. These structures are shown in Fig. 1.Fig. 1 Phenolic glycosides isolated from V . dilatatumContinued fig. 12.2 TriterpenoidsThere were about seventeen triterpenoids isolated and characterized from V. dilatatum , such as viburnols A (19) [11], viburnols B (20) [11], viburnols C (21) [11], viburnols D (22) [11], viburnols E (23) [11], viburnols F (24) [12], viburnols G (25) [12], viburnols H (26) [12], viburnols I (27) [12], viburnols J (28) [12],viburnols K (29) [12], viburnudienone B 2methyl ester (30) [13], viburnenone H 2 (31) [13],v i b u r n e n o n e B 2 m e t h y l e s t e r (32) [13], viburnudienone B 1 methyl ester (33) [13], viburnenone H 1 (34) [13], and viburnenone B 2 methyl ester (35) [13]. The structures are shown in Fig. 2.Continued fig. 23 Pharmacological activities3.1 Antioxidant activityOxidative stress caused by free radicals and their derivatives leads to disturbances in redox homeostasis. Reactive oxygen species (ROS) are not only endogenously produced during intracellular metabolic processes but also generated by exogenous stimuli such as UV radiation, pollutants, smoke and drugs. The cell triggers its defense systems or undergoes apoptosis when intracellular oxidative status increases. It influences numerous cellular processes including core signaling pathways, which are associated with development of systematic and chronic disorders, such as aging and cancer. Therefore, it is critical to remove cellular oxidants and restore redox balance.solution of V. dilatatum (GSS) had strong antioxidant activity in vivo and prevent stress-induced oxidative damage by the XYZ-dish method and the澳electron spin resonance (ESR) method [14]. The experimental result showed that the concentrations of lipid peroxide in plasma, liver and stomach in the GSS group were reduced. Furthermore, the activities of plasma lactic dehydrogenase, amylase and creatine phosphokinase are ordinarily increased by stress. However, these activities in the GSS group decreased to that in the control group. It was concluded that gastric ulcer formation, increase of lipid peroxidation in plasma and tissues and elevation of plasma enzymatic activities were confirmed in rats with water immersion restraint stress. It was also found that intake of GSS could protect the stomach and other tissues from oxidative damage.Kim et al. identified and isolated two major anthocyanins by NMR and LC-ESI-MS/MS, namely, cyanidin 3-sambubioside (I) and kuromanin (II) [15]. By the electron spin resonance method, the superoxide anion radical scavenging activities of I and II were evaluated with the IC 50 values of 17.3 and 69.6 µM, and their activities on hydroxyl radicals were evaluated with the IC 50 values of 4.3 and 53.2 mM. As the positive control, the IC 50 values of ascorbic acid were 74.2 µM on superoxide anion radicals and 3.0 mM on hydroxyl radicals, respectively. The above results suggested that these anthocyanins with radical scavenging properties might be the key compounds contributing to the antioxidant activity and physiological effects of V . dilatatum fruits.Woo et al. determined the free radical scavenging capacity of VD (the leaves of V. dilatatum ) [16]. Anti-oxidant activity of the extracts was assessed by the ability to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH) or 3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radicals. Butylated hydroxytoluene (BHT), a synthetic antioxidant, or α-tocopherol, was used as the positive control in these assays. The experimental result showed that VD inducedincrease in radical scavenging activity. In addition, lipid peroxidation inhibitory activity was determined via measurement of MDA (Malondialdehyde) levels using mouse liver tissue homogenate treated with various concentrations of the extracts. The concentration-dependent decrease in MDA levels observed was consistent with radical scavenging activities of the extracts. To examine whether VD extracts could protect mam-malian cells from oxidative stress, cultures of a human mammary gland-derived epithelial cell line MCF-7 were treated with each extract prior to challenging them with tBHP. The intracellular ROS (Reactive oxygen species) production was determined with the relative intensity of dichlorofluorescein fluorescence. While intracellular ROS formation was significantly promoted by tBHP treatment, the augmented ROS level was significantly reduced after the treatment with VD extracts.3.2 Antihyperglycemic effectIwai et al. used an oral glucose tolerance test on the diabetic rats [17]. They found that the elevation of plasma glucose level after oral administration of 2 g/kg glucose was suppressed by the repeated administration of the freeze-dried powder of V. dilatatum fruit juice (CEV). The α-glucosidase inhibitory activities of isolated compounds from CEV were also measured. Cyanidin 3-sambubioside and 5-caffeoyl quinic acid A showed inhibitory activity. These results suggested that V. dilatatum fruit had the antihyperglycemic effects.4 ConclusionV. dilatatum is distributed widely in the hills of northern China and Japan. Currently, the studies on V. dilatatum have been conducted at home and abroad, but few studies focus on its chemical components and pharmacological activities. Previousphytochemical investigations showed that the constituents of V. dilatatum included triterpenoids, phenolic glycosides, norisoprenoids and other compounds. This study describes thirteen phenolic glycosides and seventeen triterpenoids and their different degrees of antihyperglycemic, antioxidant activity and antiulcer effects, aiming to provide a reference for further studies on V. dilatatum and pharmaceutical development.References[1] Jeffrey B, Harborne A. Colour atlas of medicinal plantsof Japan. Phytochemistry, 1981, 20: 1467.[2] Miyazawa M, Hashidume S, Takahashi T, et al. Aromaevaluation of gamazumi (Viburnum dilatatum) by aroma extract dilution analysis and odour activity value.Phytochem Anal, 2012, 23: 208-213.[3] Kurihara T, Kikuchi M. Studies on the constituentsof flowers. IV. On the components of the flower of Viburnum dilatatum Thunb. J Health Sci, 1975, 95: 1098-1102.[4] Machida K, Kikuchi M. Norisoprenoids from Viburnumdilatatum. Phytochemistry, 1996, 41: 1333-1336. [5] Iwai K, Onodera A, Matsue H. Mechanism of preventiveaction of Viburnum dilatatum Thunb (gamazumi) crude extract on oxidative damage in rats subjected to stress. J Sci Food Agric, 2010, 83: 1593-1599.[6] Machida K, Nakano Y, Kikuchi M. Phenolic glycosidesfrom Viburnum dilatatum. Phytochemistry, 1991, 30: 2013-2014.[7] Machida K, Kikuchi M. Phenolic compounds fromViburnum dilatatum. Phytochemistry, 1992, 31: 3654-3656.[8] Kim MY, Iwai K, Matsue H. Phenolic compositions ofViburnum dilatatum Thunb. fruits and their antiradical properties. J Food Compos Anal, 2005, 18: 789-802. [9] Lu D, Yao S. Phenolic glycoside from the roots ofViburnum dilatatum. Nat Prod Commun, 2009, 4: 945-946.[10] Wu B, Zeng X, Zhang Y. New metabolite fromViburnum dilatatum. Nat Prod Commun, 2010, 5: 1097-1098.[11] Machida K, Kikuchi M. Viburnols: Novel triterpenoidswith a rearranged dammarane skeleton from Viburnum dilatatum. Tetrahedron Lett, 1996, 37: 4157-4160. [12] Machida K, Kikuchi M. Viburnols: Six noveltriterpenoids from Viburnum dilatatum. Tetrahedron Lett, 1997, 38: 571-574.[13] Machida K, Kikuchi M. Studies on the Constituents ofViburnum Species. XIX. Six New Triterpenoids from Viburnum dilatatum Thunb. Chem Pharm Bull, 1999, 47: 692-694.[14] Iwai K, Onodera A, Matsue H, et al. Antioxidant activityand inhibitory effect of Gamazumi (Viburnum dilatatum THUNB.) on oxidative damage induced by water immersion restraint stress in rats. Int J. Food Sci Nutr, 2001, 52: 443-451.[15] Kim MY, Iwai K, Onodera A, et al. Identification andAntiradical Properties of Anthocyanins in Fruits of Viburnum dilatatum Thunb. J Agric Food Chem, 2003, 51: 6173-6177.[16] Woo YJ, Lee HJ, Jeong YS, et al. Antioxidant Potentialof Selected Korean Edible Plant Extracts. Bio Med Res Int, 2017, 2017: 1-9.[17] Iwai K, Kim MY, Akio O, et al. Alpha-glucosidaseinhibitory and antihyperglycemic effects of polyphenols in the fruit of Viburnum dilatatum Thunb. J Agric Food Chem, 2006, 54: 4588-4592.。
西红花提取物调控免疫细胞,提高程序性死亡受体-1抑制剂治疗肺腺癌效果的实验研究

西红花提取物调控免疫细胞,提高程序性死亡受体-1抑制剂治疗肺腺癌效果的实验研究作者:李诗颖李存雅张雪钟薏来源:《上海医药》2024年第01期摘要目的:多项研究提示,西红花提取物能影响肿瘤的发展进程。
本实验探究西红花提取物在肺腺癌小鼠模型中对肿瘤免疫微环境和免疫治疗的影响,为西红花提取物抗肿瘤研究提供更多基础性数据。
方法:构建Lewis肺癌细胞和萤光素酶稳定结合的小鼠皮下瘤模型,观察西红花提取物对小鼠皮下瘤和肿瘤免疫微环境的影响:运用活体成像技术跟踪肿瘤生长情况;运用流式细胞技术检测小鼠CD4+、CD8+ T细胞的数量及占比;运用反转录-聚合酶链式反应技术检测程序性死亡受体配体1、含有T细胞免疫球蛋白和黏蛋白结构域的蛋白3(T cell immunoglobulin and mucin domaincontaining protein 3, TIM3)、淋巴细胞活化基因-3(lymphocyte-activation gene-3, LAG3)、具有免疫球蛋白和ITIM结构域的T细胞免疫受体(T cell immunoreceptor with immunoglobulin and ITIM domain, TIGIT)、胸腺细胞选择相关的高迁移率族蛋白(thymocyte selection-associated high mobility group box, TOX)1、TOX2、TOX3基因的mRNA表达情况。
结果:与对照组相比,给予西红花提取物能一定程度地抑制小鼠皮下瘤的生长(P关键词西红花免疫微环境肺腺癌免疫治疗中图分类号:R965; R282.71 文献标志码:A 文章编号:1006-1533(2024)01-0003-09引用本文李诗颖,李存雅,张雪,等. 西红花提取物调控免疫细胞,提高程序性死亡受体-1抑制剂治疗肺腺癌效果的实验研究[J]. 上海医药, 2024, 45(1): 3-11; 28.基金项目:上海市2022年度“科技创新行动计划”医学创新研究专项项目(22Y31920104);上海市虹口区第二轮“国医强优”三年行动计划(2022—2024年)中西医结合重点专科、薄弱专科建设项目(HKGYQYXM-2022-10);上海市2021年度“科技创新行动计划”扬帆计划项目(21YF444400);上海市2022年度“科技创新行动计划”启明星培育(扬帆专项)项目(22YF1444900);山东省乡村振兴基金会张秀兰慈善基金项目Experimental study of saffron extracts to modulate immune cells to improve the efficacy of a programmed death-1 inhibitor in the treatment of lung adenocarcinomaLI Shiying1, LI Cunya1, ZHANG Xue2, ZHONG Yi1(1. Department of Oncology, Shanghai TCM-Integrated Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200082, China; 2. Shanghai Traditional Chinese Medicine Co., Ltd., Shanghai 200082, China)ABSTRACT Objective: A number of studies have shown that saffron extracts can affect the development of tumor. This study explored the effect of saffron extract on tumor immune microenvironment and immunotherapy in a mouse model of lung adenocarcinoma so as to provide more basic data for the anti-tumor research of saffron extracts. Methods: The transplanted tumor model of Lewis lung carcinoma-luciferase in mice was established to detect the effect of saffron extracts on the transplanted tumor in vivo. At the same time, the tumor growth was tracked by in vivo imaging technique. The number and proportion of CD4+ and CD8+ T cells were determined by flow cytometry. The mRNA levels of programmed death-ligand 1, T cell immunoglobulin and mucin domain-containing protein 3 (TIM3), lymphocyte-activation gene-3 (LAG3), T cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT), thymocyte selection-associated high mobility group box (TOX) 1, TOX2 and TOX3 were detected by reverse transcription-polymerase chain reaction (RT-PCR) and immunohistochemical techniques to verify the effect of saffron extracts on the regulation of tumor immune microenvironment. Results:Compared with the control group, the administration of saffron extracts could inhibit the growth of subcutaneous tumor in mice to a certain extent, and the number and proportion of CD4+ and CD8+ T cells were increased (PKEY WORDS saffron; immune microenvironment; lung adenocarcinoma; immunotherapy肿瘤是一类恶性疾病,2018年全球肿瘤死亡病例数达约960万人,较2008年增加26.3%,其中男性肿瘤死亡病例数增加最多的是肺癌,增加了23.4万人[1-2]。
Diva Decloaker 10X Pretreatment Reagent 说明书

Intended Use:For In Vitro Diagnostic UseHeat induced antigen retrieval of formalin-fixed paraffin-embedded (FFPE) tissues for immunohistochemistry (IHC) procedures. The clinical interpretation of any staining or its absence should be complimented by morphological studies using proper controls and should be evaluated within the context of the patient's clinical history and other diagnostic tests by a qualified pathologist.Summary & Explanation:Diva Decloaker is a heat retrieval solution that is compatible with virtually all antibodies and eliminates the need for multiple buffers including citrate buffer, EDTA or high pH tris buffers. Antibody titers are doubled and tripled when compared to citrate buffer, pH 6.0. Diva Decloaker incorporates Assure™ tech nology, a color-coded high temperatures pH indicator solution. The end-user is assured by visual inspection that the solution is at the correct dilution and pH. This product is specially formulated for superior pH stability at high temperatures and will help prevent the possibility of losing pH sensitive antigens. Diva Decloaker is non-toxic, non-flammable, odorless and sodium azide and thimerosal free.Known Applications:Immunohistochemistry (formalin-fixed paraffin-embedded tissues) Supplied As:100mlDiva Decloaker, 10X concentrate (DV2004LX)500mlDiva Decloaker, 10X concentrate (DV2004MX)Materials and Reagents (Needed But Not Provided): Microscope slides, positively chargedDesert Chamber* (Drying oven)Positive and negative tissue controlsXylene (Could be substituted with xylene substitute*)Ethanol or reagent alcoholDecloaking Chamber* (Pressure cooker)Deionized or distilled waterWash buffer*(TBS/PBS)Enzyme digestion*Avidin-Biotin Blocking Kit*(Labeled Streptavidin Kits Only) Peroxidase block*Protein block*Primary antibody*Negative control reagents*Detection kits*Detection components*Chromogens*Hematoxylin*Bluing reagent*Mounting medium** Biocare Medical Products: Refer to a Biocare Medical catalog for further information regarding catalog number and ordering information. Certain reagents listed above are based on specific application and detection system used. Storage and Stability:Store at room temperature. Do not use after expiration date printed on vial. If reagents are stored under conditions other than those specified in the package insert, they must be verified by the user. Diluted reagents should be used promptly; any remaining reagent should be stored at room temperature.Protocol Recommendations:1. Deparaffinize tissues and hydrate to water. If necessary, block for endogenous peroxidase and wash in DI water.2. Dilute concentrated Diva Decloaker at a ratio of 1:10 (1 ml Diva to 9 ml of deionized water).3. Place slides into 1X retrieval solution in a slide container (e.g. Coplin Jar, Tissue -Tek™ staining dish or metal slide canister).4. Retrieve sections under pressure using Biocare's Decloaking Chamber. Follow the recommendations on the antibody data sheet and Decloaking Chamber User Manual.5. Check solution for appropriate color change. (See Technical Note #1)6. Gently rinse by gradually adding DI water to the solution, then remove slides and rinse with DI water.Technical Notes:1. Concentrated Diva Decloaker is a bright yellow color. RTU or 1X solution is a pale yellow color. When the solution reaches 80-125°C, the solution turns yellow and indicates that the high temperature solution is at correct pH. Should the pH rise above 7.0, the solution turns a fuschia red color. Should the pH drop too low, thesolution turns a pink color.2. If using Biocare’s Desert Chamber Pro (a programmable turbo-action drying oven), dry sections at 25ºC overnight or at 37ºC for 30-60 minutes and then dry slides at 60ºC for 30 minutes.3. Use positive char ged slides (use Biocare’s Kling-On HIER Slides) and cut tissues at 4-5 microns. Do not use any adhesives in the water bath. Poor fixation and processing of tissues will cause tissue sections to fall off the slides, especially fatty tissues such as breast. Tissues should be fixed a minimum of 6-12 hours.4. Protocol time and temperatures for HIER can vary depending on the Decloaking Chamber model used. Please refer to the relevant Decloaking Chamber manual for appropriate protocol times and temperatures.Limitations:The protocols for a specific application can vary. These include, but are not limited to: fixation, heat-retrieval method, incubation times, tissue section thickness and detection kit used. Due to the superior sensitivity of these unique reagents, the recommended incubation times and titers listed are not applicable to other detection systems, asresults may vary. The data sheet recommendations and protocols are based on exclusive use of Biocare products. Ultimately, it is the responsibility of the investigator to determine optimal conditions. The clinical interpretation of any positive or negative staining should be evaluated within the context of clinical presentation, morphology and other histopathological criteria by a qualified pathologist. The clinical interpretation of any positive or negative staining should be complemented by morphological studies using proper positive and negative internal and external controls as well as other diagnostic tests.Catalog Number: DV2004 LX, MX Description: 100, 500 ml, concentrateQuality Control:Refer to CLSI Quality Standards for Design and Implementation of Immunohistochemistry Assays; Approved Guideline-Second edition (I/LA28-A2). CLSI Wayne, PA, USA (). 2011 Precautions:1. This product is not classified as hazardous. The preservative used in this reagent is Proclin 300 and the concentration is less than 0.25%. Overexposure to Proclin 300 can cause skin and eye irritation and irritation to mucous membranes and upper respiratory tract. The concentration of Proclin 300 in this product does not meet the OSHA criteria for a hazardous substance. Wear disposable gloves when handling reagents.2. Specimens, before and after fixation, and all materials exposed to them should be handled as if capable of transmitting infection and disposed of with proper precautions. Never pipette reagents by mouth and avoid contacting the skin and mucous membranes with reagents and specimens. If reagents or specimens come in contact with sensitive areas, wash with copious amounts of water.3. Microbial contamination of reagents may result in an increase in nonspecific staining.4. Incubation times or temperatures other than those specified may give erroneous results. The user must validate any such change.5. Do not use reagent after the expiration date printed on the vial.6. The SDS is available upon request and is located at /.7. Consult OSHA, federal, state or local regulations for disposal of any toxic substances. Proclin is a trademark of Rohm and Haas Company, or of its subsidiaries or affiliates.Troubleshooting:Follow the antibody specific protocol recommendations according to data sheet provided. If atypical results occur, contact Biocare's Technical Support at 1-800-542-2002.。
HPLC法测定紫果西番莲叶中异牡荆素的含量

HPLC法测定紫果西番莲叶中异牡荆素的含量孔秋玲;赵瑞瑞;邹江冰;蒋琳兰【期刊名称】《西南国防医药》【年(卷),期】2012(22)11【摘要】Objective To detect the content of isovitexin in the leaves of Passiflora edulis by high performance liquid chromatography ( HPLC ). Methods Firstly, the extraction and purification were carried out by silica gel column chromatography. HPLC separation was carried out in Hypersil ODS C_18 column ( 4. 6 × 250 mm, 5μm ) with the mobile phase of acetonitrile - 0. 1% H_3PO_4 solution (18: 82 ), and the flow rate was 1. 0 m l/min. The sample size was 10 μl, and the detection wavelength was 350 nm. Results Within the range of 0.012 - 0. 120 mg/ml, isovitexin had a good linear relationship ( R =0. 997 ). The average recovery was 98. 26% , and RSD was 1. 4% . The average content of isovitexin was 0. 0873% . Conclusion The method is accurate, reliable, and repeatable, and can be used to detect the content of isovitexin in the leaves of passiflora edulis.%目的应用HPLC法,对紫果西番莲叶中异牡荆素的含量进行测定.方法首先采用硅胶柱色谱进行分离纯化;采用HPLC,Hypersil ODS C18色谱柱(4.6×250 mm,5μm);乙腈-0.1磷酸(18:82)为流动相;流速1.0 ml/min;进样量:10 μl,检测波长350 nm.结果异牡荆素在0.012~0.120 mg/ml范围内时,呈现良好线性关系(R2=0.997);平均回收率为98.26%,RSD 1.4%;紫果西番莲叶中异牡荆素的平均含量为0.0873%.结论该方法准确、可靠、重复性良好,可用于西番莲属植物中异牡荆素含量的测定.【总页数】3页(P1168-1170)【作者】孔秋玲;赵瑞瑞;邹江冰;蒋琳兰【作者单位】510010,广州,广州军区广州总医院药学部;510010,广州,广州军区广州总医院药学部;510010,广州,广州军区广州总医院药学部;510010,广州,广州军区广州总医院药学部【正文语种】中文【中图分类】R285.5【相关文献】1.HPLC法检测明绿萌动过程中牡荆素和异牡荆素含量变化规律 [J], 万娅琼;程江华;吴翔2.HPLC测定山楂叶中牡荆素-4"-O-葡萄糖苷、牡荆素鼠李糖苷、牡荆素、芦丁、金丝桃苷的含量 [J], 赵彩云;王强3.HPLC法测定地菍药材中牡荆素和异牡荆素的含量 [J], 曹丹;姜岩;林瑞超;马志强;王金凤;赵崇军4.HPLC 法测定竹叶提取物中荭草苷、异荭草素、牡荆素和异牡荆素的含量 [J], 王晖;陈梅荣;刘良玉5.RP-HPLC法测定山楂叶中牡荆素鼠李糖苷及牡荆素葡萄糖苷的含量 [J], 龚青;张叶萍;祝明因版权原因,仅展示原文概要,查看原文内容请购买。
Lersivirine_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :LersivirineCatalog No. :HY-14267CAS No. :473921-12-91.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:UK⁻453061; UK453061; UK 453061Formula:C17H18N4O2Molecular Weight:310.35CAS No. :473921-12-94. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance light yellow to khaki (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Gelucire-14-44-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Nov.-23-2018Print Date:Nov.-23-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Gelucire 14/44Catalog No. :HY-Y1892CAS No. :121548-04-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:N/AMolecular Weight:N/ACAS No. :121548-04-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Pure form-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Oil)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
LYVE1+巨噬细胞在RA_患者关节滑膜组织中表达变化及对RA-FLS_细胞迁移、侵袭、FMT_的抑

LYVE1+巨噬细胞在RA 患者关节滑膜组织中表达变化及对RA -FLS 细胞迁移、侵袭、FMT 的抑制作用李骁瀚,王洪星,王玺龙,赵娜,刘治璞,张义山东大学齐鲁医院检验科,济南250012摘要:目的 观察淋巴管内皮受体-1(LYVE1)+巨噬细胞在类风湿性关节炎(RA )患者关节滑膜组织中的表达变化及对RA 成纤维样滑膜细胞(RA -FLS )迁移、侵袭、向肌成纤维细胞转化(FMT )的抑制作用。
方法 采用免疫荧光染色法对45例RA 患者及45例骨关节炎(OA )患者滑膜组织LYVE1、CD68进行定性、定量检测。
取对数生长期人单核白血病细胞THP -1,在培养液中加入LYVE1过表达慢病毒,培养48 h 获得表达LYVE1的THP -1细胞,在表达LYVE1的THP -1细胞中加入100 ng /mL 的佛波酯(PMA )诱导培养48 h ,获得LYVE1+巨噬细胞;另取部分THP -1细胞,仅加入100 ng /mL 的PMA 诱导培养48 h 获得LYVE1-巨噬细胞。
取对数生长期人类风湿性关节炎成纤维细胞MH7A 分为LYVE1+巨噬细胞组、LYVE1-巨噬细胞组,分别加入LYVE1+巨噬细胞、LYVE1-巨噬细胞,另将仅含培养基的小室设为空白对照组,采用划痕实验观察各组细胞的迁移能力。
取MH7A 细胞分为A 组、B 组,分别加入LYVE1+巨噬细胞、LYVE1-巨噬细胞,将仅含培养基小室设为C 组,采用Transwell 侵袭实验观察各组细胞的侵袭能力。
取MH7A 细胞分为一组、二组,分别加入LYVE1+巨噬细胞、LYVE1-巨噬细胞,将仅含培养基小室设为空白组,培养48 h 时采用实时定量PCR 法检测各组MH7A 细胞FMT 相关基因(COL1A1 、fibronectin 、α-SMA )的mRNA 。
结果 RA 与OA 患者滑膜组织中LYVE1、CD68表达位置基本重叠;RA 与OA 患者滑膜组织LYVE1相对表达量分别为0.319 ± 0.033、1.000 ± 0.159,二者比较,P <0.05。
用于检测和定量分析极性细胞代谢物的一种新颖的HILIC LC-MS方法

用于检测和定量分析极性细胞代谢物的一种新颖的HILIC LC-MS方法来源:沃特世公司阅读数:61 时间:2011-04-20引言复杂生物样品通常包含大量可反映有机体代谢状态的内源性代谢物。
特别是骨骼肌可根据其是快收缩肌(白色肌肉,糖酵解型)还是慢收缩肌(红色肌肉,氧化型)而表现出特征性的代谢组“指纹图谱”。
例如,红色肌肉应显示一种富含线粒体代谢物(如三羧酸循环)的代谢组指纹图谱;而白色肌肉应显示富含糖酵解代谢物的代谢组指纹图谱,其线粒体代谢物的浓度大大低于红色肌肉。
虽然很多这类代谢物可具有较强的极性,但这些样本通常使用反相液相色谱-质谱(RP-LC-MS)进行分析,由此而来的多是较差的保留。
在本研究中,我们开发了一种用于检测和定量强极性细胞代谢物的新颖LC-MS方法。
本研究采用了基于亚2微米颗粒BEH酰胺柱的亲水作用液相色谱(HILIC),并联用了杂化四极杆飞行时间质谱仪和三重四极杆质谱。
方法这些试验借助联用了沃特世Synapt TM HDMS TM、沃特世Synapt G2 HDMS和Xevo TQ的一台UPLC系统而进行:液相色谱条件(1):使用碱性流动相液相色谱系统:沃特世ACQUITY UPLC®系统色谱柱:ACQUITY UPLC BEH Amide 酰胺基柱,2.1×100mm,1.7µm柱温:45℃流速:500µL/分钟碱性流动相A:乙腈/水,95/5(v/v),10mM醋酸铵,pH=9.0碱性流动相B:乙腈/水,50/50(v/v),10mM醋酸铵,pH=9.0梯度(采用碱性流动相进行分离时):线性梯度,先用2% B - 85% B洗脱8分钟,然后在98% B的水平下保持2分钟,接着返回到2% B的水平进行再平衡(5分钟)进样量:5-20µL液相色谱条件(2):使用酸性流动相液相色谱系统:沃特世®ACQUITY UPLC®系统色谱柱:ACQUITY UPLC BEH Amide 酰胺基柱,2.1×100mm,1.7µm柱温:40℃流速:600µL/分钟酸性流动相A:乙腈/水,95/5(v/v),10mM醋酸铵,pH=3.0酸性流动相B:水,10mM醋酸铵,pH=3.0-梯度:先用5% B - 30% B洗脱5分钟,在第5.5min时升至60% B并保持7分钟,接着返回到5% B 的水平进行再平衡(5分钟)进样量:20µL满定量环质谱分析Synapt HDMS和Synapt G2 HDMS该质谱仪在正离子MS E模式下运行。
VX-11e_LCMS_08581_MedChemExpress
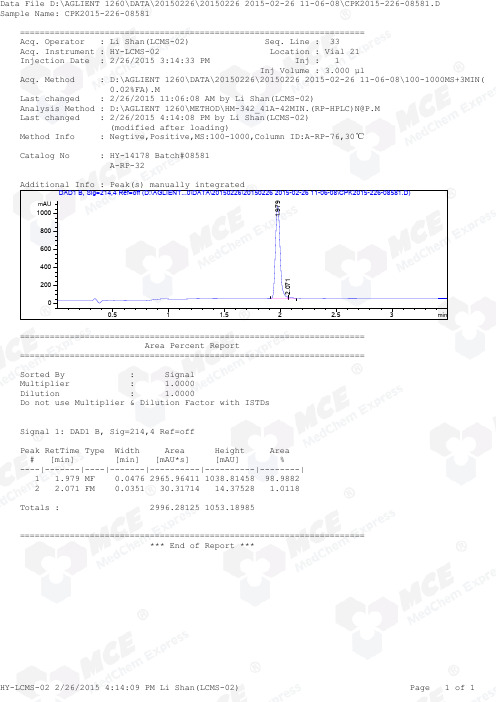
=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 33Acq. Instrument : HY-LCMS-02 Location : Vial 21Injection Date : 2/26/2015 3:14:33 PM Inj : 1Inj Volume : 3.000 µlAcq. Method : D:\AGLIENT 1260\DATA\20150226\20150226 2015-02-26 11-06-08\100-1000MS+3MIN( 0.02%FA).MLast changed : 2/26/2015 11:06:08 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\HM-342_41A-42MIN.(RP-HPLC)N@P.M Last changed : 2/26/2015 4:14:08 PM by Li Shan(LCMS-02) (modified after loading)Method Info : Negtive,Positive,MS:100-1000,Column ID:A-RP-76,30℃Catalog No : HY-14178 Batch#08581 A-RP-32Additional Info : Peak(s) manually integratedmin0.511.522.53mAU 02004006008001000 DAD1 B, Sig=214,4 Ref=off (D:\AGLIENT...0\DATA\20150226\20150226 2015-02-26 11-06-08\CPK2015-226-08581.D)1.9792.071===================================================================== Area Percent Report =====================================================================Sorted By : Signal Multiplier : 1.0000Dilution : 1.0000Do not use Multiplier & Dilution Factor with ISTDsSignal 1: DAD1 B, Sig=214,4 Ref=offPeak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------| 1 1.979 MF 0.0476 2965.96411 1038.81458 98.9882 2 2.071 FM 0.0351 30.31714 14.37528 1.0118Totals : 2996.28125 1053.18985===================================================================== *** End of Report ***=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 33Acq. Instrument : HY-LCMS-02 Location : Vial 21Injection Date : 2/26/2015 3:14:33 PM Inj : 1Inj Volume : 3.000 µlAcq. Method : D:\AGLIENT 1260\DATA\20150226\20150226 2015-02-26 11-06-08\100-1000MS+3MIN( 0.02%FA).MLast changed : 2/26/2015 11:06:08 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\HM-342_41A-42MIN.(RP-HPLC)N@P.M Last changed : 2/26/2015 4:15:29 PM by Li Shan(LCMS-02) (modified after loading)Method Info : Negtive,Positive,MS:100-1000,Column ID:A-RP-76,30℃Catalog No : HY-14178 Batch#08581 A-RP-32Additional Info : Peak(s) manually integratedmin0.511.522.5350000100000150000200000250000300000350000 MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20150226\20150226 2015-02-26 11-06-08\CPK2015-226-08581.D) ES-API, Pos, Sc1.984MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts.Reportable Ion Abundance: > 10%.Retention Mol. Weight Time (MS) MS Area or Ion1.984 1960436 503.80 I 502.75 I 501.80 I 500.80 I 499.75 Im/z10020030040050020406080100*MSD1 SPC, time=1.963:2.017 of D:\AGLIENT 1260\DATA\20150226\20150226 2015-02-26 11-06-08\CPK2015-226-08581.D ES-API Max: 137924503.8500.8501.8499.8*** End of Report ***。
高效液相色谱_电喷雾串联质谱法_LC_ESI_MS_MS_检测人血浆中罗红霉素浓

摘要 目的: 建立人血浆中罗红霉素的高效液相色谱 - 电 喷雾串联 质谱 ( LC- ES I- M S /M S) 检测方 法。方法: 血浆样 品经乙
腈沉淀蛋白后, 反相液相色谱分离后进行质谱分析, 采用选择反 应监测 模式 ( SRM ) 进行检 测, 罗红霉 素和内标 物克拉 霉素的
检测离子对分别为 m /z 837 5 679 5和 m /z 748 5 158 1。结果: 罗红霉 素在 10~ 1, 000 ng mL- 1浓度范围 内线性 关系良
GUO Be i- ning1, YU Ji- cheng1, ZHANG J ing1, CAO Guo- ying1, SH I Y ao- guo1, L IU Fe i2
( 1. Inst itu te of A nt ib iot ics, H uashan H osp ita,l Fudan U n iversity, Shangha i 200040, Ch in a; 2. Therm o F isher S cien tif ic, Shanghai 201206, Ch ina)
* 上海市重点学科基金 ( B119)资助项目 第一作者 Te:l ( 021) 52888198; E - ma i:l gu obein ing@ yahoo. com. cn
# 1538 #
药 物分析杂志 Ch in J Pharm Ana l 2010, 30( 8)
要用于敏感菌引起的呼吸道感染包括鼻窦炎、中耳 炎、支气管炎及肺炎、皮肤软组织感染以及衣原体所
致的泌尿生殖道感染。本文建立了血浆中罗红霉素
的高效液相色谱 - 串联质谱 ( LC- M S /M S) 测定法, 方法简单、灵敏, 血样需要量及进样量少, 分析时间 短, 实用性强, 可用于临床药代动力学等研究。 1 仪器、试剂和材料
医院制剂复方苯海拉明滴鼻液中硫酸卡那霉素的含量测定

医院制剂复方苯海拉明滴鼻液中硫酸卡那霉素的含量测定1. 引言1.1 背景介绍医院制剂复方苯海拉明滴鼻液是常用的治疗鼻塞、鼻炎等鼻腔疾病的药物。
其主要成分为苯海拉明和硫酸卡那霉素。
苯海拉明具有镇静、抗组织胺等作用,能有效缓解鼻塞症状;而硫酸卡那霉素则具有抗菌作用,可预防或治疗鼻腔感染。
本研究旨在对医院制剂复方苯海拉明滴鼻液中硫酸卡那霉素的含量进行准确测定,并对其质量进行控制,以确保药物的安全有效使用。
通过本实验的开展,将为医院制剂复方苯海拉明滴鼻液的质量控制提供一定的参考依据,进一步提高药物的治疗效果和安全性。
1.2 研究目的研究目的是对医院制剂复方苯海拉明滴鼻液中硫酸卡那霉素的含量进行测定,以确保药品质量符合标准要求。
通过本研究可以验证硫酸卡那霉素的含量测定方法的准确性和精准度,为今后的药品生产和质量控制提供参考依据。
本研究还旨在探讨药物质量控制的重要性,以确保患者在使用医院制剂复方苯海拉明滴鼻液时能够获得有效的治疗效果,并减少潜在的药物不良反应和风险。
通过本研究的结果分析和实验数据,可以为医院制剂的生产与质量管理提供科学依据,提高药品的安全性和有效性,从而更好地满足患者的临床需求。
2. 正文2.1 医院制剂复方苯海拉明滴鼻液的制备所要制备的医院制剂复方苯海拉明滴鼻液是一种常用于治疗鼻腔炎症的药物。
其主要成分包括苯海拉明和硫酸卡那霉素。
制备该复方药液的步骤如下:1.准备工作:准备所需原料苯海拉明、硫酸卡那霉素、辅料等,并确保工作台面的清洁和消毒。
2.称量和混合:按照处方要求,称取适量的苯海拉明和硫酸卡那霉素,并根据比例混合均匀。
3.加入辅料:在混合好的药物中加入适量的辅料,如蒸馏水、甘油等,以调整药液的浓度和稳定性。
4.搅拌和过滤:将药物溶液搅拌均匀,然后进行过滤,去除其中的杂质和固体颗粒。
5.灭菌和包装:经过过滤的药液进行灭菌处理,然后进行分装和包装,确保药液的无菌性和稳定性。
通过以上制备步骤,医院制剂复方苯海拉明滴鼻液可以制备出符合药典标准的高质量药液,用于临床治疗鼻腔炎症。
益生菌对阿尔茨海默病作用的研究进展

益生菌对阿尔茨海默病作用的研究进展发布时间:2021-12-14T06:08:15.523Z 来源:《中国结合医学杂志》2021年12期作者:宋鑫萍1,2,李盛钰2,金清1[导读] 阿尔茨海默病已成为威胁全球老年人生命健康的主要疾病之一,患者数量逐年攀升,其护理的经济成本高,给全球经济造成重大挑战。
近年来研究显示,益生菌在适量使用时作为有益于宿主健康的微生物,在防治阿尔茨海默病方面具有积极影响,其作用机制可能通过调节肠道菌群,影响神经免疫系统,调控神经活性物质以及代谢产物,通过肠-脑轴影响该病发生和发展。
宋鑫萍1,2,李盛钰2,金清11.延边大学农学院,吉林延吉 1330022.吉林省农业科学院农产品加工研究所,吉林长春 130033摘要:阿尔茨海默病已成为威胁全球老年人生命健康的主要疾病之一,患者数量逐年攀升,其护理的经济成本高,给全球经济造成重大挑战。
近年来研究显示,益生菌在适量使用时作为有益于宿主健康的微生物,在防治阿尔茨海默病方面具有积极影响,其作用机制可能通过调节肠道菌群,影响神经免疫系统,调控神经活性物质以及代谢产物,通过肠-脑轴影响该病发生和发展。
本文综述了近几年来国内外益生菌对阿尔茨海默病的作用进展,以及其预防和治疗阿尔茨海默病的潜在作用机制。
关键词:益生菌;阿尔茨海默病;肠道菌群;机制Recent Progress in Research on Probiotics Effect on Alzheimer’s DiseaseSONG Xinping1,2,LI Shengyu2,JI Qing1*(1.College of Agricultural, Yanbian University, Yanji 133002,China)(2.Institute of Agro-food Technology, Jilin Academy of Agricultural Sciences, Chanchun 130033, China)Abstract:Alzheimer’s disease has become one of the major diseases threatening the life and health of the global elderly. The number of patients is increasing year by year, and the economic cost of nursing is high, which poses a major challenge to the global economy. In recent years, studies have shown that probiotics, as microorganisms beneficial to the health of the host, have a positive impact on the prevention and treatment of Alzheimer’s disease. Its mechanism may be through regulating intestinal flora, affecting the nervous immune system, regulating the neuroactive substances and metabolites, and affecting the occurrence and development of the disease through thegut- brain axis. This paper reviews the progress of probiotics on Alzheimer’s disease at home and abroad in recent years, as well as its potential mechanism of prevention and treatment.Key words:probiotics; Alzheimer’s disease; gut microbiota; mechanism阿尔茨海默病(Alzheimer’s disease, AD),系中枢神经系统退行性疾病,属于老年期痴呆常见类型,临床特征主要包括:记忆力减退、认知功能障碍、行为改变、焦虑和抑郁等。
Alda-1_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Alda–1 is a potent ALDH2 agonist, which significantly improves ALDH2 activity.In Vivo: Alda–1 treatment results in a significant decrease of 4–HNE–protein content in the plasma of apoE -/- mice. Alda–1administration leads to a slight increase in gene expression related to neurogenesis (Nog ), mitochondrial biogenesis (CYTB , ND1),and apoptosis (Bax , Gsk3b ) in the Hp of apoE -/- mice. Alda–1 administration leads to 2 and 10 differentially expressed proteins in theFCx and Hp of apoE -/- mice, respectively [1]. Alda–1 (1.5 mg/kg, b.w., i.p.) administration significantly increases the climbing time,tends to reduce the immobility time and increases the swimming time of the prenatally stressed rats in the forced swim test.Moreover, treatment of prenatally stressed rats with Alda–1 significantly increases number of entries into the open arms of the maze and the time spent therein, as assessed by elevated plus–maze test [2]. Alda–1 (8.5 mg/kg, i.p.) with glucose significantly lowers 4–HNE and FJB–positive cells in the cerebral cortex of Alda–1–treated rats than in DMSO–treated rats 24 h after glucose administration [3]. Alda–1 (10 mg/kg per day) treatment prevents aldehydic overload, mitochondrial dysfunction and improves ventricular function in post–MI cardiomyopathy rats [4].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay:[2]Spleen cells (4×106 cells/mL) are stimulated by optimal concentrations of concanavalin A (Con A; 2.5μg/mL and 0.6 μg/mL) and lipopolysaccharide (LPS, 5 μg/mL) and are incubated in 96–well plates at final volume of 0.2mL for 72 h. Cell proliferation is determined by adding 0.5 μCi of [3H]–thymidine per well at 16 h before the end of the incubation.The cultures are harvested with an automatic cell harvester, and [3H] thymidine incorporation is assessed using a liquid scintillationcounter.Animal Administration: Alda–1 is dissolved in 1 mL/kg b.w. DMSO/water 50/50.[2]After behavioral verification at three months of age,the animals are divided into the following four groups: control, control + Alda–1, prenatally stressed and prenatally stressed + Alda–1(6 animals per group). Alda–1 injections are given intraperitoneally (i.p.) once daily at a dose of 1.5 mg/kg b.w. (dissolved in 1 mL/kg b.w. DMSO/water 50/50) for 14 days. At the same time, the control and prenatally stressed rats receive 1 mL/kg b.w. DMSO/water 50/50. The injections of Alda–1 and vehicle are given between 10 a.m and 11 a.m. In the last five days of Alda–1 treatment the behavioral parameters in the elevated plus maze test and then in the forced swim test are measured.References:[1]. Stachowicz A, et al. Proteomic Analysis of Mitochondria–Enriched Fraction Isolated from the Frontal Cortex and Hippocampus of Apolipoprotein E Knockout Mice Treated with Alda–1, an Activator of Mitochondrial Aldehyde Dehydrogenase (ALDH2). Int J Mol Sci.[2]. Stachowicz A, et al. The impact of mitochondrial aldehyde dehydrogenase (ALDH2) activation by Alda–1 on the behavioral and biochemical disturbances in animal model of depression. Brain Behav Immun. 2016 Jan;51:144–53.Product Name:Alda–1Cat. No.:HY-18936CAS No.:349438-38-6Molecular Formula:C 15H 11Cl 2NO 3Molecular Weight:324.16Target:Aldehyde Dehydrogenase (ALDH)Pathway:Metabolic Enzyme/Protease Solubility:DMSO: ≥ 51 mg/mL[3]. Ikeda T, et al. Effects of Alda–1, an Aldehyde Dehydrogenase–2 Agonist, on Hypoglycemic Neuronal Death. PLoS One. 2015 Jun 17;10(6):e0128844.[4]. Gomes KM, et al. Aldehydic load and aldehyde dehydrogenase 2 profile during the progression of post–myocardial infarction cardiomyopathy: benefits of Alda–1. Int J Cardiol. 2015 Jan 20;179:129–138.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 67
Acq. Instrument : HY-LCMS-02 Location : P1-D-03Injection Date : 3/24/2015 6:42:57 PM Inj : 1
Inj Volume : 3.000 µl
Acq. Method : D:\AGLIENT 1260\DATA\20150324\20150324 2015-03-24 13-23-05\100-1000MS+3MIN- 1.5_(0.02%FA).M
Last changed : 3/24/2015 1:23:05 PM by Li Shan(LCMS-02)
Analysis Method : D:\AGLIENT 1260\DATA\20150324\20150324 2015-03-24 13-23-05\100-1000MS+3MIN- 1.5_(0.02%FA).M (Sequence Method)
Last changed : 3/25/2015 10:16:55 AM by Li Shan(LCMS-02) (modified after loading)
Method Info : Postive,MS:100-1000,Column ID:A-RP-132,40℃
Catalog No : HY-14267 Batch#08850 A-RP-132
Additional Info : Peak(s) manually integrated
min
0.5
1
1.52
2.53mAU 0
2505007501000125015001750 DAD1 B, Sig=214,4 Ref=off (D:\AGLIENT...0\DATA\20150324\20150324 2015-03-24 13-23-05\CPK2015-324-08850.D)
1.435
1.717 1.802 1.882
1.962
2.135
2.249 2.335
2.442
===================================================================== Area Percent Report =====================================================================
Sorted By : Signal Multiplier : 1.0000Dilution : 1.0000
Do not use Multiplier & Dilution Factor with ISTDs
Signal 1: DAD1 B, Sig=214,4 Ref=off
Peak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %
----|-------|----|-------|----------|----------|--------| 1 1.435 MM 0.0429 15.38021 5.97674 0.2955 2 1.717 MM 0.0496 28.26757 9.50315 0.5432 3 1.802 MM 0.0439 2.89154 1.09685 0.0556 4 1.882 MM 0.0447 10.52832 3.92737 0.2023 5 1.962 MM 0.0454 4996.16406 1833.14172 96.0035 6 2.135 MM 0.0437 111.47768 42.47631 2.1421 7 2.249 MM 0.0490 25.69613 8.73846 0.4938 8 2.335 MM 0.0409 6.36347 2.59539 0.1223 9 2.442 MM 0.0575 7.38008 2.13974 0.1418
Totals : 5204.14906 1909.59573
===================================================================== *** End of Report ***
=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 67
Acq. Instrument : HY-LCMS-02 Location : P1-D-03Injection Date : 3/24/2015 6:42:57 PM Inj : 1
Inj Volume : 3.000 µl
Acq. Method : D:\AGLIENT 1260\DATA\20150324\20150324 2015-03-24 13-23-05\100-1000MS+3MIN- 1.5_(0.02%FA).M
Last changed : 3/24/2015 1:23:05 PM by Li Shan(LCMS-02)
Analysis Method : D:\AGLIENT 1260\DATA\20150324\20150324 2015-03-24 13-23-05\100-1000MS+3MIN- 1.5_(0.02%FA).M (Sequence Method)
Last changed : 3/25/2015 10:22:39 AM by Li Shan(LCMS-02) (modified after loading)
Method Info : Postive,MS:100-1000,Column ID:A-RP-132,40℃
Catalog No : HY-14267 Batch#08850 A-RP-132
Additional Info : Peak(s) manually integrated
min
0.5
1
1.5
2
2.5
3
20000
400006000080000100000120000140000 MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20150324\20150324 2015-03-24 13-23-05\CPK2015-324-08850.D) ES-API, Pos, Sc
1.960
MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts.
Reportable Ion Abundance: > 10%.
Retention Mol. Weight Time (MS) MS Area or Ion
1.960 532409 311.90 I 310.90 I
m/z
100
200
300
400
500
600
20406080100*MSD1 SPC, time=1.944:1.981 of D:\AGLIENT 1260\DATA\20150324\20150324 2015-03-24 13-23-05\CPK2015-324-08850.D ES-API Max: 80307
332.9
311.9
310.9
*** End of Report ***。
