A-317491_sodium_salt_hydrate_SDS_MedChemExpress
Roche MagNA Lyser 产品说明书

Ordering Information Cat. No. Product ***********MagNA Lyser Instrument (230 Volt)***********MagNA Lyser Instrument (110 Volt)(Instruments supplied with rotor and rotor cooling block)***********MagNA Lyser Green Beads (100 tubes)Related Products Cat. No. Product***********MagNA Pure LC DNA Isolation Kit II (Tissue)***********MagNA Pure LC mRNA Isolation Kit II (Tissue)03 330 591 001MagNA Pure LC RNA Isolation Kit III (Tissue)***********MagNA Pure LC DNA Isolation Kit III (Bacteria, Fungi)***********MagNA Pure LC RNA Isolation Tissue Lysis Buffer – Refill (70 ml)System DescriptionHomogenize up to 16 samples in just a few seconds.Save valuable lab space with a small benchtop instrument.Reduce hands-on time by replacing the mortar and pestle and other manual methods.Integrate your workflow with the automated nucleic acid isolation of the MagNA Pure LC Instrument.Perform consistent and reproducible sample disruption.Process many different sample types.Prevent nucleic acid degradation with the benchtop cooling unit.Ease your setup with a removable rotor and prefilled disposable vials.Automate with an easy-to-use instrumentVersatile, efficient, and rapid pre-preparationFigure 71. Add your sample and lysis buffer to the MagNA Lyser Green Beads.2. Homogenize with the MagNA Lyser Instrument.3. Centrifuge to pellet the debris.4. Proceeed with the supernatant to prepare nucleic acids or proteins.For detailed information,visit or contact your local representative.Trademarks:MagNA Pure, MagNA Lyser, LightCycler, and the MagNA Pure Logo are trademarks of a member of the Roche Group.The technology used for the LightCycler System is licensed from Idaho Technology Inc., Salt Lake City, UT, USA.Fully automated sample preparationon the PCR Workflow SystemRoche Diagnostics GmbH Roche Applied Science Nonnenwald 282372 Penzberg Germany0000Roche Applied Science Part of Roche DiagnosticsMagNA Lyser InstrumentStart the Ball Rollingwith Automated Tissue HomogenizationᕤᕣᕢᕡFigure 6Components of the system.The MagNA Lyser InstrumentAutomated tissue homogenizationProcessing conditionsRefer to the following tables for guidelines on setting up your homogenizationSample material(10 mg)*Time settings(seconds)Cooling(between the runs)Speed Average yield(µg)***Average purity(OD 280/260 nm)***Spleen 2 x 25 906,00030–40 1.9Liver 25-6,00016–18 1.8Lung 2 x 25906,00025 1.8Kidney25-6,000201.8Maize leaves **20-5,00010n.d.Maize polenta **20-5,0008n.d.Tortilla chips **20-5,0001n.d.*Aliqout containing 10 mg sample material (here mouse and food samples) was taken for the DNA purificationusing the MagNA Pure LC DNA Isolation Kit II (Tissue), (see pack insert)**Centrifugation after the homogenization for 5 minutes at 2,200 x g*** Yield and purity strongly depend on the condition of the sample material n.d.not determinedData kindly provided by Dr. Peterhänsel, RWTH Aachen, GermanyFigure 1Gel electrophoresis from genomic DNA isolated from tissue homogenized with the MagNA LyserInstrument, using the MagNA Pure LC DNA Kit II (Tissue).Marker: DNA Marker III*Aliquot containing 10 mg sample material (here mouse and human research samples) was taken to purify RNAeither with the MagNA Pure LC RNA Isolation Kit III (Tissue) or the MagNA Pure LC mRNA Isolation Kit II (Tissue) homogenized with the MagNA Lyser Instrument.** Yield and purity strongly depend on the condition of the sample material. The yield for mRNA was not determined.Sample material(10 mg)*Time settings(cycles/seconds)Cooling(between/afterthe runs in seconds)SpeedAverage yield (mg)(total RNA)**Average purity(OD 280/260 nm)**RNA/mRNARarely expressed targets in small numbers of target cells,as seen in experiments about minimalresidual diseases,are difficult to detect.Increasing the cell number can improve sensitivity and lead to accurate results.Without the MagNA Lyser pre-processing,the MagNA Pure mRNA HS Kit can efficiently obtain mRNA from a maximum of 1 x 107white blood cells (WBCs),as shown in research studies with human samples.However,using greater cell numbers results in a saturation effect with quantitative assays (Figure 3).Homogenization of the lysate with the MagNA Lyser Instrument prior to the purification eliminatesthe amplification saturation at 1 x 107cells and allows the use of up to 2.5 x 107WBCs (Figure 4 and 5),enhancing the analytical sensitivity of the assay.Eliminate sensitivity barriers with increased sample inputFigure 3mRNA was purified from different amounts of human white blood cells with the MagNA Pure mRNA HS Kit. G6PDH was amplified using the LightCycler t(9;22) Quantification Kit (see text beside).Figure 4mRNA was purified from different amounts of human white blood cells with the MagNA Pure mRNA HS Kit. The lysates from 2.5 x 107cells and 5 x 107cells were homogenized with the MagNA Lyser Instrument (2x50 seconds with 90 seconds cooling in between) prior to the mRNA purification. G6PDH was amplified using the LightCycler t(9;22) Quantification Kit (see text beside).Figure 5Scalability from 1 x 106cells to 2.5 x 107cells is represented in the graph and the table of the relationship between crossing points and cell numbers. The limitation of cell input is indicated by no change in crossing point with increased cell number (see text beside).Cell number 5 x 1072.5 x 1071 x 1075 x 1061 x 106Log (cell number)7.77.47.06.76.0Crossing point 20.320.321.822.424.4crossingpointLog(cell number)252423222120195.86.36.87.37.8Figure 2Gel electrophoresis from total RNA isolated from tissue homogenized with the MagNA Lyser Instrument, using the MagNA Pure LC RNA Kit III (Tissue).Ma r k e rS p l e e nL i v e rL u n gK i d n e yM a r k e rMa i z e l e a v e sMa i z e l e a v e sS p l e e nL i v e r11 kb5 kb5 kb28 S rRNA 18 S rRNASpleen 2 x 50 90 6,500–7,000 30–40 1.9Liver 50 - 6,500–7,000 13–17 2.0Thymoid tissue60906,500n.d.n.d.Heart 60 90 6,500 n.d. n.d.Abdominal fat 60 90 6,500 n.d. n.d.Aorta 60 90 6,500 n.d. n.d. Other samples1+n x 50 90 6,500–7,000- -1 x 105 x 101 x 10- 5 x 101 x 105 x 105 x 10- 5 x 102.5 x 10 5 x 10。
Bialaphos (sodium salt)_天然非选择性植物毒素_71048-99-2_Apexbio

Soluble in DMSO
Store at -20°C
For obtaining a higher solubility , please warm the tube at 37°C and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months.
ApexBio Technology
Evaluation sample solution : ship with blue ice All other available size: ship with RT , or blue ice upon request
生物活性
靶点 :
Others
信号通路:
Others
产品描述:
The antibiotic bialaphos (or SF1293) is a natural non-selective phytotoxin produced by Streptomyces hygroscopicus. Bialaphos is a tripeptide consisting of two L-alanine molecules and an L-glutamic acid analogue called phosphinothricin. Bialaphos is converted to the active agent phosphinothricin by peptidases. The phosphinothricin moiety is a potent inhibitor of glutamine synthetase [1].
SDSE所有详细试剂配方

SDSE所有详细试剂配方SDSE(Sodium dodecyl sulfate-electrolyte system)是一种常用的聚丙烯酰胺凝胶电泳缓冲液,主要用于蛋白质电泳分离。
SDSE的制备是根据一定的配方进行的,下面是SDSE的详细试剂配方:1.氯化钠(NaCl):18.3g溶解在1L去离子水中2.磷酸二氢钠(NaH2PO4):3.5g溶解在1L去离子水中3. 溴酚蓝(Bromophenol Blue):0.25g溶解在10mL水中4. SDS(Sodium Dodecyl Sulfate):10g溶解在1L去离子水中5. 甘油(Glycerol):10mL溶解在100mL去离子水中以上是SDSE的基础配方,下面是其制备步骤:1.将NaCl和NaH2PO4溶解在分别容量为1L的去离子水中,用磁力搅拌器搅拌均匀。
2.分别将溶解好的NaCl和NaH2PO4溶液倒入大容量烧杯中,混合均匀。
3.在pH计的帮助下调节缓冲液的pH值,使其在6.8-7.0之间。
4.加入溴酚蓝溶液,此时溴酚蓝的浓度为0.0025%,用磁力搅拌器搅拌均匀。
5.加入SDS溶液,用磁力搅拌器搅拌均匀。
注意,加入SDS时要小心,避免溅出。
6.最后加入甘油溶液,搅拌均匀。
甘油的添加主要是为了增加样品的密度,使其在凝胶中下沉更快。
SDSE的配方比较简单,主要成分包括NaCl、NaH2PO4、溴酚蓝、SDS和甘油。
其中,NaCl和NaH2PO4是缓冲液的主要组成部分,用于维持适当的离子强度和pH值;溴酚蓝用于着色样品,方便观察电泳结果;SDS主要起到断开蛋白质的二级结构和线性化作用;甘油则用于增加样品密度,使其在凝胶中下沉更快。
通过恰当的配方和制备步骤,可以获得高质量的SDSE缓冲液,用于蛋白质电泳分离和分析。
但需要注意的是,不同实验目的和要求可能需要微调配方中一些成分的浓度,因此在具体实验过程中,可以根据需要进行相应的调整。
生物纯度氨酸钠二氧化钙(氯酸氯酸钠)说明书

71188 Sodium acetate trihydrate (Acetic acid sodium salt)CAS number: 6131-90-4Product Description:Appearance: Clear colorless to very faint yellow liquidMolecular formula: CH3COONa • 3 H2OFormula weight: 136.08 g/molSolubility: 3 M in H2O, 20°C, complete, colorlesspH: 8.5-10.0 (3 M in H2O, 25°C)This product designated as BioUltra grade is suitable for different applications like purification, precipitation, crystallisation and other applications which require tight control of elemental content. Trace elemental analyses have been performed. The Certificate of Analysis provides lot-specific results.Applications:Sodium acetate is a widely used reagent in molecular biology applications. It is used as a buffer in conjunction with acetic acid, in the buffering range of pH 3.6 - 5.6. Sodium acetate is used in the purification and precipitation of nucleic acids,1,2,3 protein crystallization,4 staining of gels in protein gel electrophoresis,5 and HPLC.6 Large scale applications of sodium acetate include its use as a retardantin plastics manufacturing, as a mordant in dyeing, and in the tanning of leather.7 A DNA microarray study of E. coli response to different levels of sodium acetate has been reported.8 Protein unfolding during reversed phase chromatography in the presence of varying salts, including sodium acetate, at different ionic strengths has been investigated.9 Sodium acetate has been used in conjunction with sodium carbonate to enhance the activation of freeze-dried subtilisin Carlsberg in organic solvents.10 Sodium acetate may be used as a substrate for acetokinase (acetate kinase; EC 2.7.2.1).11Preparation InstructionsSodium acetate is soluble in water (3 mol/l), yielding a clear, colorless solution. The pH of a 0.1 M aqueous sodium acetate solution at 25°C is 8.9.8References:1.Evans, J. K., et al., Simultaneous purification of RNA and DNA from liver using sodium acetateprecipitation. BioTechniques, 24, 416-418 (1998).2.Molecular Cloning: A Laboratory Manual, 3rd ed., Sambrook, J. F., et al., Cold Spring HarborLaboratory Press (Cold Spring Harbor, NY: 2001), pp. 6.26-6.27, A8.12-A8.16.3.Wallace, D. M., Large- and Small-Scale Phenol Extractions, Meth. Enzymol., 152, 33-41 (1987).4.Baniecki, M. L., et al., Adenovirus proteinase: crystallization and preliminary X-ray diffractionstudies to atomic resolution. Acta Crystallogr. D Biol. Crystallogr., 58 (Pt 9), 1462-1464 (2002).5.Bjellqvist, B., et al., A nonlinear wide-range immobilized pH gradient for two-dimensionalelectrophoresis and its definition in a relevant pH scale. Electrophoresis, 14, 1357-1365 (1993).6.Clark, T. N., et al., Determination of 3'-azido-2',3'-dideoxyuridine in maternal plasma, amnioticfluid, fetal and placental tissues by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl., 755(1-2), 165-172 (2001).7.The Merck Index, 12th ed., Entry# 8711.8.Polen, T., et al., DNA microarray analyses of the long-term adaptive response of Escherichia colito acetate and propionate. Appl. Environ. Microbiol., 69(3), 1759-1774 (2003).The vibrant M and Sigma-Aldrich are trademarks of Merck KGaA, Darmstadt, Germany or its affiliates. Detailed information on trademarks is available via publicly accessible resources. © 2018 Merck KGaA, Darmstadt, Germany and/or its affiliates. All Rights Reserved. 9. McNay, J. L., et al., Protein unfolding during reversed-phase chromatography: II. Role of salt type and ionic strength. Biotechnol. Bioeng., 76(3), 233-240 (2001). 10. Ru, M. T., et al., Towards more active biocatalysts in organic media: increasing the activity of saltactivated enzymes. Biotechnol. Bioeng., 75(2), 187-196 (2001). 11. Rose, I., Acetate Kinase of Bacteria (Acetokinase), Meth. Enzymol., 1, 591-595 (1955)Precautions and Disclaimer This product is for R&D use only, not for drug, household, or other uses. Please consult the Material Safety Data Sheet for information regarding hazards and safe handling practices.The vibrant M and Sigma-Aldrich are trademarks of Merck KGaA, Darmstadt, Germany or its affiliates. Detailed information on trademarks is available via publicly accessible resources.© 2018 Merck KGaA, Darmstadt, Germany and/or its affiliates. All Rights Reserved.。
J. Heterocyclic Chem., 47, 1269 (2010).

4-(Coumarin-3-yl)thiazol-2-ylhydrazone DerivativesFranco Chimenti,a Bruna Bizzarri,a *Adriana Bolasco,a Daniela Secci,aPaola Chimenti,a Arianna Granese,a Simone Carradori,a Melissa D’Ascenzio,aM.Maddalena Scaltrito,b and Francesca Sisto baDipartimento di Chimica e Tecnologie del Farmaco,University ‘‘La Sapienza,’’P.le A.Moro 5,00185Rome,ItalybDipartimento di Sanita`Pubblica-Microbiologia-Virologia,Universita `degli Studi di Milano,via Pascal 36,20133Milan,Italy *E-mail:zarri@uniroma1.itReceived January 13,2010DOI 10.1002/jhet.464Published online 20August 2010in Wiley Online Library().A novel class of coumarin-thiazole conjugated systems (1–31)were synthesized by Hantzsch conden-sation between a -bromo-3-acetyl coumarin and several thiosemicarbazone intermediates.This scaffold was also evaluated for selective antibacterial activity against 20isolates of H.pylori clinical strains,including four metronidazole resistant ones.J.Heterocyclic Chem.,47,1269(2010).INTRODUCTIONHelicobacter pylori are spiral-shaped Gram-negative bacteria with polar flagella that live near the surface of the human gastric mucosa.They have evolved specific mechanisms to avoid the bactericidal acid environment in the gastric lumen to survive near,to attach to,and to communicate with the human gastric epithelium and host immune system.This interaction sometimes results in severe gastric pathology.In fact,H.pylori infection is indeed the most known risk factor for the development of gastroduodenal ulcers,gastric adenocarcinoma,and gastric mucosa-associated lymphoid tissue lymphoma.H.pylori infections are difficult to cure and success-ful treatment generally requires the simultaneous som-ministration of several antibacterial agents.Antibiotic resistance has resulted in unsatisfactory eradication with dual and now triple therapy in many countries.Newer antibiotics and changes in dosing and duration of therapy may overcome resistant strains but may only provide limited improvement in eradication rates [1–3].In our previous works [4,5]and from the analysis of the structure of natural coumarins reported as potentanti-H .pylori agents [6],we have pointed out that the coumarin ring might play an important role in determin-ing activity and seemed to be crucial for the selective antimicrobial activity of such compounds.Recently,we have synthesized and chemically and biologically char-acterized some new conjugated coumarin-thiazole sys-tems,which were endowed with interesting industrial properties and especially antimicrobial activity on H.pylori clinical strains [7].Furthermore,interest in these structures has renewed due to the recent discovery of their promising antibacte-rial,antifungal,and antimycobacterial activity [8–11].Moving from these indications,in this report we described the synthesis and selective antimicrobial evalu-ation of a new series of 4-(coumarin-3-yl)thiazol-2-ylhy-drazone derivatives which differ for the electronic and steric characteristics on the hydrazone nitrogen (aliphatic chains,cycloaliphatic moiety,and heterocyclic rings).RESULTS AND DISCUSSIONThe coumarin-thiazole derivatives (1–30)were pre-pared in high yields (69–99%)according to a protocolused in our laboratory(Table1).Different carbonyl compounds reacted directly with thiosemicarbazide in ethanol with catalytic amounts of acetic acid,and the obtained thiosemicarbazones were subsequently con-verted into4-(coumarin-3-yl)-2-thiazolylhydrazones by reaction with a-bromo-3-acetyl coumarin in the same solvent at room temperature(Hantzsch condensation).a-Bromo-3-acetyl coumarin has been synthesized by direct halogenation of3-acetyl coumarin with bromine in chlo-roform.Moreover,knowing that all reported structures possess an imine bond,which could be hydrolyzed in the acidic environment of the stomach(reproduced in the biological assay),we also synthesized and assayed their common intermediate(31)by direct reaction between thiosemicabazide and a-bromo-3-acetyl couma-rin in ethanol at room temperature.All synthesized products were purified with petroleum ether and diethyl ether and,if requested,by chromatog-raphy before characterization by spectroscopic methods (IR and1H NMR)and elemental analysis.The com-pounds,correctly analyzed for their molecular formula, showed in the IR spectrum strong bands at1710and 1600cmÀ1due to the presence of a d-lactone C¼¼O and C¼¼N group,respectively.Moreover,the presence of a C¼¼N double bond can give rise to isomeric geometry E/Z.The1H NMR(in CDCl3)spectra analysis revealed that the E isomer was more favored and stable than the Z-configuration.The amounts of both conformers were measured by area integration of the signal relative to the CH3(R1)protons (area ratio of proton signals E:Z was generally6:1).The low-field signal was assigned to the E isomer,as it is widely accepted in thiosemicarbazone derivatives[17]. Our choice,as reaction medium,of a polar alcoholic solvent appeared to be preferred to obtain the E-configu-ration and limit the interconversion according to the results of our previous theoretical and chromatographic study for similar compounds[18].Then,all compounds were evaluated,as mixture of E/ Z conformers,against20clinical strains of H.pylori, which are more resistant to conventional therapy.Metro-nidazole was used as standard antibacterial drug(Table 2).Most of the assayed compounds showed no anti-H. pylori activity or comparable activity with respect to Metronidazole(MIC!16l g/mL).Only some com-pounds(14,21,and26),bearing a specific heterocyclic ring(furan,pyridine,and naphthalene)on the hydrazone nitrogen,possessed MIC values slightly inferior to the reference drug(MIC¼8l g/mL)against some clinical H.pylori strains.Unfortunately,it was not possible to correlate this biological activity with lipophilicity (Clog P).EXPERIMENTALThe chemicals,solvents for synthesis and spectral grade sol-vents were purchased from Aldrich(Italy)and used without further purification.Melting points are uncorrected and were determined automatically on an FP62apparatus(Mettler-Tol-edo).1H NMR spectra were recorded at400MHz on a Bruker spectrometer.Chemical shifts are expressed as d units(parts per millions)relative to the solvent peak.Coupling constants J are valued in Hertz(Hz).IR spectra were registered on a Per-kin Elmer FTIR Spectrometer Spectrum1000in KBr.Elemen-tal analysis for C,H,and N were recorded on a Perkin-Elmer 240B microanalyzer and the analytical results were within 60.4%of the theoretical values for all compounds.All reac-tions were monitored by TLC performed on0.2-mm-thick silica gel plates(60F254Merck).Lipophilicity parameter, Clog P,has been calculated for each molecule by using Chem-Draw ultra8.0.The synthesis of some compounds has been described in previous references(Table1)and was performed with slight changes.Their analytical and spectral data were in full agreement with those reported in the literature.Typical procedure for the thiosemicarbazones synthesis.The appropriate carbonylic compound(50mmol) was dissolved in100mL of ethanol and stirred vigorously atTable1Structure of derivatives1–31.Comp R R11[ref.12]CH3CH32CH2CH3CH33CH(CH3)2CH34(CH2)2CH3CH35CH2CH3CH2CH36(CH2)2CH¼CH2CH37(CH2)4CH3CH38(CH2)3CH3CH2CH39(CH2)5CH3CH3102-CH3-Cyclopentyliden113-CH3-Cyclopentyliden12Cyclooctyliden13Cyclohexyl CH314[ref.11]Fur-2-yl H15Fur-2-yl CH316Tiophen-2-yl H17Tiophen-2-yl CH318[ref.13]Phenyl CH319Pyridin-2-yl CH320Pyridin-3-yl H21Pyridin-3-yl CH322Pyridin-4-yl H23Pyridin-4-yl CH3241H-indol-3-yl H25[ref.14]3,4-Methylendioxophenyl H26Naphtalen-1-yl H27Naphtalen-2-yl CH328[ref.15]Coumarin-3-yl CH3292-COOH-9H-fluoren-5-yliden30Thiazol-2-yl CH331[ref.16]H HS.Carradori,M.D’Ascenzio,M.Maddalena Scaltrito,and F.Sistoroom temperature with an equimolar amount of thiosemicarba-zide for24h with catalytic amount of acetic acid.The desired thiosemicarbazone precipitated from reaction mixture wasfil-tered and crystallized from suitable solvent and dried. Typical procedure for the Hantzsch protocol for the preparation of derivatives1–30.Equimolar amounts of theprepared thiosemicarbazones(50mmol)and freshly synthe-sized3-a-bromo-acetyl coumarin(50mmol),both dissolved in ethanol,were reacted at room temperature under magnetic stir-ring for4h.The precipitate wasfiltered and dried to give compounds1–30in69–99%yield.3-(2-(2-Butylidenehydrazynyl)thiazol-4-yl)-2H-chromen-2-one(2).Light brown crystals,96%yield,mp205–210 C;1H NMR(CDCl3):d1.15–1.18(t,3H,J¼7.2,CH3),2.20(s,3H, CH3),2.42–2.47(q,2H,J¼7.2,CH2),7.35–7.39(m,1H,J7-6¼J7-8¼7.8Hz,J7-5¼2.3Hz,C7H-chrom),7.41–7.43(dd, 1H,J5-6¼7.9,J5-7¼2.4Hz,C5H-chrom),7.62–7.65(m,1H, J6-5¼J6-7¼7.8Hz,J6-8¼2.3Hz,C6H-chrom),7.68(s,1H, C5H-thiaz.),7.77–7.83(dd,1H,J8-7¼7.8Hz,J8-6¼2.2Hz, C8H-chrom.),10.75(bs,1H,NH,D2O exch.);Anal.Calcd.for C15H13N3O2S:C,60.18;H,4.38;N,14.04.Found:C,60.13; H,4.37;N,14.06.3-(2-(2-(3-Methyl-2-butylidene)hydrazynyl)thiazol-4-yl)-2H-chromen-2-one(3).Yellow crystals,99%yield,mp170–173 C;1H NMR(CDCl3):d0.95–0.97(d,J¼6.6Hz,6H,2ÂCH3),1.98–2.11(m,J¼6.6Hz,1H,CH),2.17(s,3H, CH3),7.35–7.38(m,J7-6¼J7-8¼7.3Hz,J7-5¼1.8Hz,1H, C7H-chrom.),7.39–7.41(dd,J5-6¼7.3Hz,J5-7¼1.8Hz,1H, C5H-chrom.),7.61–7.65(m,J6-5¼J6-7¼7.3Hz,J6-8¼1.8 Hz,1H,C6H-chrom.),7.79–7.82(dd,J8-7¼7.3Hz,J8-6¼1.9 Hz,1H,C8H-chrom.),7.84(s,1H,C5H-thiaz.),8.54(s,1H, C4H-chrom.),12.00(br s,1H,NH,D2O exch.);Anal.Calcd. for C17H17N3O2S:C,62.36;H, 5.23;N,12.83.Found:C, 62.41;H,5.24;N,12.82.3-(2-(2-(2-Pentanylidene)hydrazynyl)thiazol-4-yl)-2H-chro-men-2-one(4).Orange crystals,82%yield,mp186–187 C; 1H NMR(CDCl3):d0.97–1.03(t,J¼7.4Hz,3H,CH3), 1.59–1.65(m,J¼7.4Hz,J¼5.6Hz,2H,CH2),2.18(s,3H, CH3),2.33–2.38(t,J¼5.6Hz,2H,CH2),7.34–7.37(m,J7-6¼J7-8¼7.6Hz,J7-5¼2.1Hz,1H,C7H-chrom.),7.62–7.65 (dd,J5-6¼7.6Hz,J5-7¼2.2Hz,1H,C5H-chrom.),7.68–7.75 (m,J6-5¼J6-7¼7.7Hz,J6-8¼2.1Hz,1H,C6H-chrom.), 7.77–7.81(dd,J8-7¼7.8Hz,J8-6¼2.1Hz,1H,C8H-chrom.), 7.85(s,1H,C5H-thiaz.),8.62(s,1H,C4H-chrom.),11.90(br s,1H,NH,D2O exch.);Anal.Calcd.for C17H17N3O2S:C, 62.36;H,5.23;N,12.83.Found:C,62.39;H,5.22;N,12.83. 3-(2-(2-(3-Pentanylidene)hydrazynyl)thiazol-4-yl)-2H-chro-men-2-one(5).Yellow crystals,82%yield,mp180–183 C; 1H NMR(CDCl3):d1.16–1.19(t,J¼7.3Hz,6H,2ÂCH3), 2.40–2.46(m,4H,2ÂCH2),7.35–7.37(m,J7-6¼J7-8¼6.8 Hz,J7-5¼1.4Hz,1H,C7H-chrom.),7.38–7.41(dd,J5-6¼6.8 Hz,J5-7¼1.4Hz,1H,C5H-chrom.),7.59–7.63(m,J6-5¼J6-7¼6.8Hz,J6-8¼1.4Hz,1H,C6H-chrom.),7.78–7.80(dd,J8-7¼6.8Hz,J8-6¼1.4Hz,1H,C8H-chrom.),7.84(s,1H, C5H-thiaz.),8.61(s,1H,C4H-chrom.),12.01(br s,1H,NH, D2O exch.);Anal.Calcd.for C17H17N3O2S:C,62.36;H,5.23; N,12.83.Found:C,62.38;H,5.24;N,12.82.3-(2-(2-(5-Hexen-2-ylidene)hydrazynyl)thiazol-4-yl)-2H-chromen-2-one(6).Light yellow crystals,73%yield,mp 195–197 C;1H NMR(CDCl3):d2.19(s,3H,CH3),2.38–2.45 (t,J¼6.5Hz,2H,CH2),2.48–2.53(m,J¼6.5Hz,J¼7.2 Hz,2H,CH2),5.05–5.08(dd,J cis¼8.8Hz,J gem¼1.7Hz, 1H,CH¼¼),5.09–5.13(dd,J trans¼17.7Hz,J gem¼1.7Hz, 1H,CH¼¼),5.78–5.85(m,J cis¼8.8Hz,J trans¼17.8Hz,J¼7.2Hz1H,CH¼¼),7.36–7.40(m,J7-6¼J7-8¼7.5,J7-5¼1.5,1H,C7H-chrom.),7.41–7.43(dd,J5-6¼7.5,J5-7¼1.5, 1H,C5H-chrom.),7.62–7.64(m,J6-5¼J6-7¼7.5,J6-8¼1.4, 1H,C6H-chrom.),7.79–7.81(dd,J8-7¼7.6,J8-6¼1.4,1H, C8H-chrom.),7.86(s,1H,C5H-thiaz.),8.61(s,1H,C4H-chrom.),12.00(br s,1H,NH,D2O exch.);Anal.Calcd.for C18H17N3O2S:C,63.70;H,5.05;N,12.38.Found:C,63.75; H,5.04;N,12.38.3-(2-(2-(2-Heptanylidene)hydrazynyl)thiazol-4-yl)-2H-chro-men-2-one(7).Yellow crystals,99%yield,mp198–201 C; 1H NMR(DMSO-d6):d0.93–0.95(m,3H,CH3),1.22–1.30 (m,2H,CH2), 1.32–1.38(m,2H,CH2),1.55–1.61(m,2H, CH2),2.18(s,3H,CH3),2.36–2.40(m,2H,CH2),7.37–7.39 (m,J7-6¼J7-8¼7.1Hz,J7-5¼3.7Hz,1H,C7H-chrom.), 7.40–7.42(dd,J5-6¼7.16,J5-7¼3.8,1H,C5H-chrom.), 7.61–7.66(m,J6-5¼J6-7¼7.2Hz,J6-8¼3.8Hz,1H,C6H-chrom.),7.79–7.81(dd,J8-7¼7.1,J8-6¼3.7,1H,C8H-chrom.),7.84(s,1H,C5H-thiaz.),8.61(s,1H,C4H-chrom.), 12.06(br s,1H,NH,D2O exch.);Anal.Calcd.for C19H21N3O2S:C,64.20;H,5.95;N,11.82.Found:C,64.15; H,5.93;N,11.84.Table2MIC values(l g/mL)of derivatives1–31and M(metronidazole)against20H.pylori strains.Compound Metronidazole sensitivestrains(16strains)Metronidazole resistantstrains(4strains)1!16>162!16>163!16>164!16>165!16>166!16!167!16!168!16!169!16!1610!16!1611!16!1612!16!1613!16>16148–!168–!1615!16!1616>16!1617!16!1618!16>1619!16!1620!16!16218–!16!1622!16!1623!16!1624>16!1625!16!16268–!16!1627!16!1628>16>1629!16>1630>16>1631!16>16M0.5–16>164-(Coumarin-3-yl)thiazol-2-ylhydrazone Derivatives3-(2-(2-(3-Heptanylidene)hydrazynyl)thiazol-4-yl)-2H-chro-men-2-one (8).Yellow crystals,77%yield,mp 175–180 C;1H NMR (CDCl 3):d 0.95–0.98(m,3H,CH 3),1.13–1.19(m,2H,CH 2),1.34–1.40(m,2H,CH 2),1.55–1.62(m,3H,CH 3),2.39–2.42(m,2H,CH 2),2.45–2.51(m,2H,CH 2),7.36–7.38(m,1H,C 7H-chrom.),7.39–7.41(m,1H,C 5H-chrom.),7.60–7.64(m,1H,C 6H-chrom.),7.79–7.82(m,1H,C 8H-chrom.),7.83(s,1H,C 5H-thiaz.),8.61(s,1H,C 4H-chrom.),12.14(br s,1H,NH,D 2O exch.);Anal.Calcd.for C 19H 21N 3O 2S:C,64.20;H, 5.95;N,11.82.Found:C,64.25;H, 5.95;N,11.81.3-(2-(2-(2-Octanylidene)hydrazynyl)thiazol-4-yl)-2H-chro-men-2-one (9).Yellow crystals,74%yield,mp 149–150 C;1H NMR (DMSO-d 6):d 0.84–0.88(m,3H,CH 3),1.25–1.32(m,6H,3ÂCH 2),1.47–1.51(m,2H,CH 2),1.88–1.91(m,3H,CH 3),2.19–2.23(m,2H,CH 2),7.37–7.39(m,1H,C 7H-chrom.),7.42–7.44(m 1H,C 5H-chrom.),7.60–7.64(m,C 6H-chrom.),7.68(s,1H,C 5H-thiaz.),7.77–7.80(m,1H,C 8H-chrom.),8.53(s,1H,C 4H-chrom.),10.71(br s,1H,NH,D 2O exch.);Anal.Calcd.for C 20H 23N 3O 2S:C,65.01;H,6.27;N,11.37.Found:C,65.06;H,6.28;N,11.35.3-(2-(2-(2-Methylcyclopentylidene)hydrazynyl)thiazol-4-yl)-2H-chromen-2-one (10).Yellow crystals,79%yield,mp 143–145 C;1H NMR (CDCl 3):d 1.21–1.23(m,3H,CH 3),1.31–1.39(m,1H,cyclopentyl), 1.71–1.77(m,1H,cyclopentyl),1.95–2.02(m,1H,cyclopentyl),2.05–2.12(m,1H,cyclopen-tyl),2.25–2.33(m,1H,cyclopentyl),2.37–2.46(m,1H,cyclo-pentyl),2.58–2.64(m,1H,cyclopentyl),7.27–7.33(m,J 7-6¼J 7-8¼7.7Hz,J 7-5¼3.63Hz,1H,C 7H-chrom.),7.34–7.37(dd,J 5-6¼7.8,J 5-7¼3.6,1H,C 5H-chrom.),7.50–7.54(m,J 6-5¼J 6-7¼7.7Hz,J 6-8¼3.7Hz,1H,C 6H-chrom.),7.57–7.60(dd,J 8-7¼7.7,J 8-6¼3.6,1H,C 8H-chrom.),7.88(s,1H,C 5H-thiaz.),8.49(s,1H,C 4H-chrom.),12.00(br s,1H,NH,D 2O exch.);Anal.Calcd.for C 18H 17N 3O 2S:C,63.70;H,5.05;N,12.38.Found:C,63.75;H,5.04;N,12.39.3-(2-(2-(3-Methylcyclopentylidene)hydrazynyl)thiazol-4-yl)-2H-chromen-2-one (11).Light yellow crystals,99%yield,mp 214–216 C;1H NMR (CDCl 3):d 1.10–1.12(m,3H,CH 3),1.49–1.56(m,1H,cyclopentyl),2.10–2.12(m,1H,cyclopen-tyl),2.14–2.17(m,1H,cyclopentyl),2.19–2.22(m,1H,cyclo-pentyl), 2.54–2.62(m,1H,cyclopentyl), 2.64–2.73(m,1H,cyclopentyl), 2.75–2.81(m,1H,cyclopentyl),7.35–7.38(m,J 7-6¼J 7-8¼7.9Hz,J 7-5¼3.3Hz,1H,C 7H-chrom.),7.38–7.40(dd,J 5-6¼8.0,J 5-7¼3.2,1H,C 5H-chrom.),7.63–7.67(m,J 6-5¼J 6-7¼7.9Hz,J 6-8¼3.3Hz,1H,C 6H-chrom.),7.77–7.79(dd,J 8-7¼7.9,J 8-6¼3.4,1H,C 8H-chrom.),7.84(s,1H,C 5H-thiaz.),8.59(s,1H,C 4H-chrom.),11.80(br s,1H,NH,D 2O exch.);Anal.Calcd.for C 18H 17N 3O 2S:C,63.70;H,5.05;N,12.38.Found:C,63.65;H,5.06;N,12.38.3-(2-(2-(Cyclooctylidene)hydrazynyl)thiazol-4-yl)-2H-chro-men-2-one (12).Yellow crystals,69%yield,mp 143–145 C;1H NMR (CDCl 3):d 1.47–1.50(m,2H,cyclooctyl),1.52–1.58(m,4H,cyclooctyl),1.79–1.84(m,4H,cyclooctyl),2.43–2.46(m,4H,cyclooctyl),7.27–7.30(m,J 7-6¼J 7-8¼7.5Hz,J 7-5¼1.6Hz,1H,C 7H-chrom.),7.38–7.40(dd,J 5-6¼7.4,J 5-7¼1.7,1H,C 5H-chrom.),7.50–7.54(m,J 6-5¼J 6-7¼7.4Hz,J 6-8¼1.7Hz,1H,C 6H-chrom.),7.69–7.71(dd,J 8-7¼7.4,J 8-6¼1.7,1H,C 8H-chrom.),7.87(s,1H,C 5H-thiaz.),8.51(s,1H,C 4H-chrom.),11.97(br s,1H,NH,D 2O exch.);Anal.Calcd.for C 20H 21N 3O 2S:C,65.37;H, 5.76;N,11.44.Found:C,65.33;H,5.76;N,11.45.3-(2-(2-(1-(Cyclohexyl)ethyliden)hydrazynyl)thiazol-4-yl)-2H-chromen-2-one (13).Yellow crystals,69%yield,mp 195–200 C;1H NMR (DMSO-d 6):d 1.48–1.52(m,2H,cyclo-hexyl), 1.75–1.79(m,4H,cyclohexyl), 2.41–2.45(m,4H,cyclohexyl),7.37–7.41(m,J 7-6¼J 7-8¼7.5Hz,J 7-5¼1.8Hz,1H,C 7H-chrom.),7.41–7.43(dd,J 5-6¼7.6,J 5-7¼1.9,1H,C 5H-chrom.),7.53–7.57(m,J 6-5¼J 6-7¼7.5Hz,J 6-8¼1.8Hz,1H,C 6H-chrom.),7.68–7.70(dd,J 8-7¼7.9,J 8-6¼1.7,1H,C 8H-chrom.),7.85(s,1H,C 5H-thiaz.),8.54(s,1H,C 4H-chrom.),11.75(br s,1H,NH,D 2O exch.);Anal.Calcd.for C 20H 21N 3O 2S:C,65.37;H, 5.76;N,11.44.Found:C,65.33;H,5.77;N,11.45.3-(2-(2-(1-(Furan-2-yl)ethyliden)hydrazynyl)thiazol-4-yl)-2H-chromen-2-one (15).Light green crystals,77%yield,mp 218–220 C;1H NMR (DMSO-d 6):d 2.25(s,3H,CH 3),6.57–6.58(d,J 3-4¼1.7Hz,1H,C 3H-furan),6.84–6.86(dd,J 4-5¼3.3Hz,J 4-3¼1.7Hz,1H,C 4H-furan),7.37–7.41(m,J 7-6¼J 7-8¼7.6Hz,J 7-5¼2.9Hz,1H,C 7H-chrom.),7.44–7.47(dd,J 5-6¼7.6Hz,J 5-7¼2.6Hz,1H,C 5H-chrom.),7.61–7.63(m,J 6-5¼J 6-7¼7.2Hz,J 6-8¼2.9Hz,1H,C 6H-chrom.),7.73–7.75(d,J 5-4¼3.3Hz,1H,C 5H-furan),7.76(s,1H,C 5H-thiaz.),7.80–7.83(dd,J 8-7¼7.6Hz,J 8-6¼2.9Hz,1H,C 8H-chrom.),8.56(s,1H,C 4H-chrom.),11.25(br s,1H,NH,D 2O exch.);Anal.Calcd.for C 18H 13N 3O 3S:C,61.53;H,3.73;N,11.96.Found:C,61.56;H,3.72;N,11.98.3-(2-(2-(Thiophen-2-ylmethylen)hydrazynyl)thiazol-4-yl)-2H-chromen-2-one (16).Yellow crystals,99%yield,mp 230–235 C;1H NMR (DMSO-d 6):d 7.08–7.12(m,1H,thio-phene),7.37–7.40(m,1H,thiophene),7.41(s,1H,C 5H-thiaz.),7.43–7.48(m,J 7-6¼J 7-8¼6.8Hz,J 7-5¼3.4Hz,1H,C 7H-chrom.),7.58–7.62(dd,J 5-6¼6.3Hz,J 5-7¼3.3Hz,1H,C 5H-chrom.),7.63–7.68(m,1H,thiophene),7.75–7.78(m,1H,C 8H-chrom.),7.82–7.87(m,J 6-5¼J 6-7¼6.3Hz,J 6-8¼3.4Hz,1H,C 6H-chrom.),8.24(s,1H,CH ¼¼N),8.53(s,1H,C 4H-chrom.),12.10(br s,1H,NH,D 2O exch.);Anal.Calcd.for C 17H 11N 3O 2S 2:C,57.77;H,3.14;N,11.89.Found:C,57.72;H,3.15;N,11.90.3-(2-(2-(1-(Thiophen-2-yl)ethyliden)hydrazynyl)thiazol-4-yl)-2H-chromen-2-one (17).Yellow crystals,92%yield,mp 221–223 C;1H NMR (DMSO-d 6):d 2.31(s,3H,CH 3),7.03–7.06(m,1H,thiophene),7.37–7.40(m,1H,C 7H-chrom.),7.45–7.47(dd,J 5-6¼7.4Hz,J 5-7¼2.1Hz,1H,C 5H-chrom.),7.52–7.55(m,1H,thiophene),7.57–7.60(m,1H,thiophene),7.61–7.64(m,1H,C 6H-chrom.),7.77(s,1H,C 5H-thiaz.),7.81–7.84(dd,J 8-7¼7.4,J 8-6¼2.5,1H,C 8H-chrom.),8.57(s,1H,C 4H-chrom.),11.20(br s,1H,NH,D 2O exch.);Anal.Calcd.for C 17H 11N 3O 2S 2:C,57.61;H,3.41;N,11.86.Found:C,57.60;H,3.42;N,11.86.3-(2-(2-(1-(Pyridin-2-yl)ethyliden)hydrazynyl)thiazol-4-yl)-2H-chromen-2-one (19).Orange crystals,99%yield,mp 258–262 C;1H NMR (DMSO-d 6):d 2.41(s,3H,CH 3),7.37–7.40(m,1H,C 7H-chrom.),7.42–7.47(dd,J 5-6¼7.5Hz,J 5-7¼1.9Hz,1H,C 5H-chrom.),7.50–7.54(m,J 6-5¼J 6-7¼7.3Hz,J 6-8¼1.2Hz,1H,C 6H-chrom.),7.55–7.61(m,1H,C 5H-pyridine),7.81(s,1H,C 5H-thiaz.),7.82–7.84(dd,J 8-7¼7.3Hz,J 8-6¼1.3Hz,1H,C 8H-chrom.),8.05–8.10(m,1H,C 4H-pyridine),8.11–8.13(m,1H,C 3H-pyri-dine),8.58(s,1H,C 4H-chrom.),8.63–8.65(m,1H,C 6H-pyri-dine),11.77(br s,1H,NH,D 2O exch.);Anal.Calcd.for C 19H 14N 4O 2S:C,62.97;H,3.89;N,15.46.Found:C,62.95;H,3.88;N,15.45.S.Carradori,M.D’Ascenzio,M.Maddalena Scaltrito,and F.Sisto3-(2-(2-(1-(Pyridin-3-yl)methylen)hydrazynyl)thiazol-4-yl)-2H-chromen-2-one(20).Yellow crystals,99%yield,mp 257–258 C;1H NMR(DMSO-d6):7.32–7.35(m,1H,C7H-chrom.),7.38–7.41(dd,J5-6¼7.5Hz,J5-7¼1.3Hz,1H, C5H-chrom.),7.60–7.64(m,1H,C6H-chrom.),7.80–7.83(dd, J8-7¼7.6Hz,J8-6¼1.4Hz,1H,C8H-chrom.),7.84(s,1H, C5H-thiaz.),7.85–7.88(m,1H,C5H-pyridine),8.17(s,1H, CH¼¼N),8.48–8.52(m,1H,C4H-pyridine),8.56(s,1H,C4H-chrom.),8.73–8.75(m,1H,C6H-pyridine),9.01(s,1H,C2H-pyridine),12.75(br s,1H,NH,D2O exch.);Anal.Calcd.for C18H12N4O2S:C,62.06;H,3.47;N,16.08.Found:C,62.07; H,3.48;N,16.10.3-(2-(2-(1-(Pyridin-3-yl)ethyliden)hydrazynyl)thiazol-4-yl)-2H-chromen-2-one(21).Yellow crystals,99%yield,mp 269–270 C;1H NMR(DMSO-d6):d2.41(s,3H,CH3),7.40–7.44(m,1H,C7H-chrom.),7.48–7.50(dd,J5-6¼7.8Hz,J5-7¼1.7Hz,1H,C5H-chrom.),7.58–7.62(m,1H,C6H-chrom.), 7.80–7.83(dd,J8-7¼7.7Hz,J8-6¼1.7Hz,1H,C8H-chrom.),7.84(s,1H,C5H-thiaz.),7.87–7.90(m,1H,C5H-pyridine),8.52(s,1H,C4H-chrom.),8.55–8.58(m,1H,C4H-pyridine), 8.72–8.74(m,1H,C6H-pyridine),9.07(s,1H,C2H-pyridine), 11.75(br s,1H,NH,D2O exch.);Anal.Calcd.for C19H14N4O2S:C,62.97;H,3.89;N,15.46.Found:C,62.98; H,3.90;N,15.46.3-(2-(2-(1-(Pyridin-4-yl)methylen)hydrazynyl)thiazol-4-yl)-2H-chromen-2-one(22).Orange crystals,99%yield,mp> 300 C;1H NMR(DMSO-d6):7.40–7.43(m,1H,C7H-chrom.), 7.48–7.50(dd,J5-6¼7.9Hz,J5-7¼1.4Hz,1H,C5H-chrom.), 7.59–7.63(m,1H,C6H-chrom.),7.85–7.88(dd,J8-7¼7.6Hz, J8-6¼1.5Hz,1H,C8H-chrom.),7.91(s,1H,C5H-thiaz.), 8.07–8.10(d,J¼4.1Hz,2H,pyridine),8.16(s,1H,CH¼¼N), 8.55(s,1H,C4H-chrom.),8.81–8.83(d,J¼4.5Hz,2H,pyri-dine),13.00(br s,1H,NH,D2O exch.);Anal.Calcd.for C18H12N4O2S:C,62.06;H,3.47;N,16.08.Found:C,62.05; H,3.46;N,16.08.3-(2-(2-(1-(Pyridin-4-yl)ethyliden)hydrazynyl)thiazol-4-yl)-2H-chromen-2-one(23).Yellow crystals,98%yield,mp 215–220 C;1H NMR(DMSO-d6):d2.41(s,3H,CH3),7.42–7.46(m,1H,C7H-chrom.),7.48–7.50(dd,J5-6¼7.4Hz,J5-7¼1.6Hz,1H,C5H-chrom.),7.64–7.68(m,1H,C6H-chrom.), 7.76–7.78(dd,J8-7¼7.5Hz,J8-6¼1.9Hz,1H,C8H-chrom.), 7.85(s,1H,C5H-thiaz.),8.45–8.48(d,J¼5.8Hz,2H,pyri-dine),8.67(s,1H,C4H-chrom.),8.78–8.81(d,J¼5.8Hz, 2H,pyridine),10.75(br s,1H,NH,D2O exch.);Anal.Calcd. for C19H14N4O2S:C,62.97;H, 3.89;N,15.46.Found:C, 62.95;H,3.98;N,15.45.3-(2-(2-(1-(1H-indol-4-yl)methylen)hydrazynyl)thiazol-4-yl)-2H-chromen-2-one(24).Yellow crystals,90%yield,mp248–250 C;1H NMR(DMSO-d6):6.95–6.98(t,J¼3.5,1H,C5H-indole),7.12–7.15(t,J¼3.7,1H,C6H-indole),7.32(s,1H,C2H-indole),7.39–7.43(m,J7-6¼J7-8¼7.3Hz,J7-5¼1.7Hz,1H, C7H-chrom.),7.44–7.46(d,J¼3.7,1H,C7H-indole),7.48–7.50 (dd,J5-6¼7.3Hz,J5-7¼1.7Hz,1H,C5H-chrom.),7.49–7.53 (m,1H,C6H-chrom.),7.55(s,1H,C5H-thiaz.),7.58–7.60(dd,J8-7¼7.3Hz,J8-6¼1.3Hz,1H,C8H-chrom.),7.62–7.64(d,J¼3.5,1H,C4H-indole),8.16(s,1H,CH¼¼N),8.57(s,1H,C4H-chrom.),10.79(br s,1H,NH,D2O exch.),11.51(br s,1H,NH, D2O exch.);Anal.Calcd.for C21H14N4O2S:C,65.27;H,3.65;N, 14.50.Found:C,65.25;H,3.64;N,14.51.3-(2-(2-(1-(Naphthalen-1-yl)methylen)hydrazynyl)thiazol-4-yl)-2H-chromen-2-one(26).Yellow crystals,70%yield,mp 240–242 C;1H NMR(DMSO-d6):7.40–7.44(m,1H,C7H-chrom.),7.49–7.51(dd,J5-6¼7.8Hz,J5-7¼1.5Hz,1H, C5H-chrom.),7.53–7.55(m,1H,C6H-chrom.),7.56(s,1H, C5H-thiaz.),7.57–7.60(dd,J8-7¼7.8Hz,J8-6¼1.3Hz,1H, C8H-chrom.),7.77–7.81(m,2H,naphtalene),7.82–7.85(m, 1H,naphtalene),7.97–8.02(m,2H,naphtalene),8.08–8.10(m, 1H,naphtalene),8.12(s,1H,CH¼¼N),8.22–8.24(m,1H, naphtalene),8.60(s,1H,C4H-chrom.),11.54(br s,1H,NH, D2O exch.);Anal.Calcd.for C24H17N3O2S:C,70.05;H,4.16; N,10.21.Found:C,70.00;H,4.15;N,10.22.3-(2-(2-(1-(Naphthalen-2-yl)ethyliden)hydrazynyl)thiazol-4-yl)-2H-chromen-2-one(27).Yellow crystals,91%yield,mp 244–245 C;1H NMR(DMSO-d6):7.38–7.42(m,1H,C7H-chrom.),7.46–7.48(dd,J5-6¼7.4Hz,J5-7¼1.8Hz,1H, C5H-chrom.),7.49–7.53(m,1H,C6H-chrom.),7.54(s,1H, C5H-thiaz.),7.58–7.60(dd,J8-7¼7.3Hz,J8-6¼1.4Hz,1H, C8H-chrom.),7.77–7.82(m,2H,naphtalene),7.90–7.94(m, 2H,naphtalene),7.98–8.01(m,1H,naphtalene),8.08–8.10(m, 1H,naphtalene),8.21–8.23(m,1H,naphtalene),8.59(s,1H, C4H-chrom.),11.50(br s,1H,NH,D2O exch.);Anal.Calcd. for C24H17N3O2S:C,70.05;H, 4.16;N,10.21.Found:C, 70.00;H,4.15;N,10.22.9-(2-(4-(2H-2-oxo-chromen-3-yl)thiazol-2-yl)hydrazono)-9H-fluorene-2-carboxylic acid(29).Yellow crystals,99%yield, mp190–192 C;1H NMR(DMSO-d6):7.28–7.30(m,1H,fluo-rene),7.38–7.42(m,1H,C7H-chrom.),7.45–7.47(dd,J5-6¼7.7Hz,J5-7¼1.3Hz,1H,C5H-chrom.),7.49–7.53(m,1H, C6H-chrom.),7.56(s,1H,C5H-thiaz.),7.57–7.61(m,2H,fluo-rene),7.62–7.64(dd,J8-7¼7.4Hz,J8-6¼1.4Hz,1H,C8H-chrom.),7.78–7.82(m,2H,fluorene),8.33–8.38(m,2H,fluo-rene),8.57(s,1H,C4H-chrom.),11.77(br s,1H,COOH,D2O exch.),12.50(br s,1H,NH,D2O exch.);Anal.Calcd.for C26H15N3O4S:C,67.09;H,3.25;N,9.03.Found:C,67.13;H, 3.25;N,9.04.3-(2-(2-(1-(Thiazol-2-yl)ethyliden)hydrazynyl)thiazol-4-yl)-2H-chromen-2-one(30).Light brown crystals,99%yield,mp 256–260 C;1H NMR(DMSO-d6):d2.43(s,3H,CH3),7.39–7.43(m,1H,C7H-chrom.),7.46–7.48(dd,J5-6¼8.0Hz,J5-7¼1.6Hz,1H,C5H-chrom.),7.63–7.67(m,1H,C6H-chrom.), 7.79(s,1H,C5H-thiaz.),7.83–7.85(dd,J8-7¼7.9Hz,J8-6¼1.8Hz,1H,C8H-chrom.),7.86–7.89(m,2H,thiazole),8.72(s, 1H,C4H-chrom.),11.75(br s,1H,NH,D2O exch.);Anal. Calcd.for C17H12N4O2S2:C,55.42;H,3.28;N,15.21.Found: C,55.47;H,3.28;N,15.24.Procedure for the synthesis of derivative31.3-a-Bromo-acetyl coumarin(50mmol)was dissolved in2-propanol and reacted with an equimolar amount of thiosemicarbazide at room temperature under magnetic stirring for4h.The precipi-tate wasfiltered and dried to give intermediate31.H.pylori culture.The H.pylori strains used in this study were maintained atÀ80 C in Wilkins Chalgren broth with 10%(v/v)horse serum(Seromed)and20%(v/v)glycerol (Merck)until required for the experiments.Before being used the bacteria were subcultured twice on Columbia agar base (Difco Laboratories)supplemented with10%horse serum and 0.25%Bacto yeast extract(Difco).Plates were incubated for 72h at37 C in an atmosphere of10%CO2in a gas incubator. Anti-Helicobacter pylori activity.Antimicrobial activity against H.pylori was determined by the agar dilution standard method[19].The strains were inoculated onto Columbia agar base(Difco)supplemented with10%horse serum and0.25%4-(Coumarin-3-yl)thiazol-2-ylhydrazone Derivativesbacto yeast extract(Difco)and were incubated for72h at 37 C in an atmosphere of10%CO2in a gas incubator.Colo-nies were suspended in Wilkins Chalgren broth to achieve a turbidity equivalent to0.5Mc Farland.Columbia agar plates with10%horse serum were prepared by using twofold dilu-tions of the antimicrobial agents(128–0.0039l g/mL).The inoculum was delivered to the surface of the agar plates with a Steer’s replicator to obtain$5Â105CFU per spot.Growth control plates without antibiotics were inoculated in each se-ries of tests.All plates were incubated at37 C for72h under conditions(10%CO2in a gas incubator).The minimal inhibi-tory concentration was defined as the lowest concentration of drug inhibiting visible bacterial growth.REFERENCES AND NOTES[1]Hunt,R.H.Scand J Gastroenterol1996,220,3.[2]Bardhan,P.K.Clin Infect Dis1997,25,973.[3]IARC.IARC monographs on the evaluation of carcinogenic risks to humans,Vol.61;IARC:Lyon,1994;pp177–240.[4]Chimenti,F.;Bizzarri,B.;Bolasco,A.;Secci,D.;Chimenti, P.;Carradori,S.;Granese,A.;Rivanera,D.;Lilli,D.;Scaltrito,M.M.; Brenciaglia,M.I.Eur J Med Chem2006,41,208.[5]Chimenti,F.;Bizzarri,B.;Bolasco,A.;Secci,D.;Chimenti, P.;Carradori,S.;Granese,A.;Rivanera,D.;Lilli,D.;Zicari,A.;Scal-trito,M.M.;Sisto,F.Bioorg Med Chem Lett2007,17,3065.[6]Kawase,M.;Motohashi,N.Curr Med Chem Anti-Infect Agents2004,3,89.[7]Chimenti, F.;Carradori,S.;Secci, D.;Bolasco, A.;Chi-menti,P.;Granese,A.;Bizzarri,B.J Heterocycl Chem2009,46,575.[8]Raghu,M.;Nagaraj,A.;Reddy,Ch.S.J Heterocycl Chem 2009,46,261.[9]Rao,V.R.;Reddy,M.M.M.Indian J Heterocycl Chem 2003,13,69.[10]Kalluraya,B.;Isloor,A.M.;Shenoy,S.Indian J Heterocycl Chem2001,11,159.[11]Kalluraya, B.;Vishwanatha,P.;Isloor, A.M.;Rai,G.; Kotian,M.Bollettino Chimico Farmaceutico2000,139,263.[12]Rao,V.R.;Kumar,V.R.;Vardhan,V.A.Phosphorus Sul-fur1999,152,257.[13]Srimanth,K.;Rao,V.R.Indian J Chem B1999,38B,473.[14]Gursoy,A.J Fac Pharm Istanbul U1974,10,57.[15]Gursoy,A.;Eczacilik F.J Fac Pharm Istanbul U1973,9, 51.[16]Rao,V.R.;Srimanth,K.J Chem Res S2002,9,420.[17]Benassi,R.;Benedetti, A.;Taddei, F.;Cappelletti,R.; Nardi,D.;Tajana, Magn Reson1982,20,26.[18]Cirilli,R.;Ferretti,R.;La Torre,F.;Secci,D.;Bolasco,A.; Carradori,S.;Pierini,M.J Chromatogr A2007,1172,160.[19]National Committee for Clinical Laboratory Standards. Methods for Antimicrobial Susceptibility Testing of Anaerobic bacte-ria.Approved standard M11-A6,6th ed.;National Committee for Clin-ical Laboratory Standards:Villanova,PA,2004.S.Carradori,M.D’Ascenzio,M.Maddalena Scaltrito,and F.Sisto。
Extract-N-Amp Tissue PCR Kit 产品说明书

Product InformationExtract-N-Amp™ Tissue PCR KitXNAT2, XNAT2RProduct DescriptionThe Extract-N-Amp™ Tissue PCR Kit for direct PCR contains the reagents needed to rapidly extract and amplify genomic DNA from mouse tails and other animal tissues, buccal swabs, hair shafts, and saliva. Briefly, the DNA is released from the starting material by incubating the sample with a mixture of the Extraction Solution and the Tissue Preparation Solution at room temperature for 10 minutes. There is no need for mechanical disruption, organic extraction, column purification, or precipitation of the DNA.After adding Neutralization Solution B, the extract is ready for PCR. An aliquot of the neutralized extract is then combined with the Extract-N-Amp™ PCR Reaction Mix and user-provided PCR primers to amplify target DNA. The Extract-N-Amp™ PCR Reaction Mix is a 2X ready mix containing buffer, salts, dNTPs, and Taq polymerase. It is optimized specifically for use with the extraction reagents. It also contains the JumpStart Taq antibody for hot start PCR to enhance specificity but does not contain the inert red dye found in the REDExtract-N-Amp™ PCR Reaction Mix.Reagents Provided Cat. No. XNAT2 100 Preps,100 PCRsXNAT2R 1000 Preps, 1000 PCRsExtraction SolutionE7526 24 mL 240 mL Tissue Preparation Solution T3073 3 mL 30 mL Neutralization Solution BN391024 mL240 mLExtract-N-Amp™ PCR Reaction Mix This is a 2X PCR reaction mix containing buffer, salts, dNTPs, Taq polymerase, and JumpStart™ Taq antibody.E30041.2 mL12 mLReagents and Equipment Required(Not Provided)•Microcentrifuge tubes (1.5 or 2 mL) or multi-well plate for extractions (200 μL minimal well volume) • Small dissecting scissors• Forceps (small to medium in size)• Buccal swab - Sterile foam tipped applicator (Cat. No. WHAWB100032)•Sample collection card - Bloodstain card (Cat. No. WHAWB100014)• Tubes or plate for PCR• Heat block or thermal cycler at 95 °C • PCR Primers (Cat. No. OLIGO) • Thermal cycler•Water, PCR Reagent (Cat. No. W1754)Precautions and DisclaimerThis product is for R&D use only. Not for drug, household, or other uses. Please consult the Safety Data Sheet for information regarding hazards and safe handling practices.StorageThe Extract-N-Amp™ Tissue PCR Kit can be stored at 2 to 8 °C for up to 3 weeks. For long-term storage, greater than 3 weeks, -20 °C is recommended. Do not store in a "frost-free" freezer.ProcedureAll steps are carried out at room temperature unless otherwise noted.DNA Extraction from Mouse Tails, Animal Tissues, Hair, or Saliva1.Pipette 100 μL of Extraction Solution into amicrocentrifuge tube or well of a multi-well plate.Add 25 μL of Tissue Preparation Solution to thetube or well and pipette up and down to mix.Note: If several extractions will be performed,sufficient volumes of Extraction and TissuePreparation Solutions may be pre-mixed in a ratio of 4:1 up to 2 hours before use.2.For fresh or frozen mouse tails: Rinse thescissors and forceps in 70% ethanol prior to useand between different samples. Place a 0.5–1 cm piece of mouse tail tip (cut end down) into thesolution. Mix thoroughly by vortexing or pipetting.Ensure the mouse tail is in solution.Note: For fresh mouse tails, perform extractions within 30 minutes of snipping the tail.For animal tissues: Rinse the scissors or scalpel and forceps in 70% ethanol prior to use andbetween different samples. Place a 2–10 mgpiece of tissue into the solution. Mix thoroughlyby vortexing or pipetting. Ensure the tissue is inthe solution.For hair shafts: Rinse the scissors and forceps in 70% ethanol prior to use and between differentsamples. Trim excess off of the hair shaft leaving the root and place sample (root end down) intosolution. Only one hair shaft, with root, isrequired per extraction.For Saliva: Pipette 10 μL of saliva into thesolution. Mix thoroughly by vortexing or pipetting.For saliva dried on card: Pipette 50 μL of saliva onto collection card and allow the card to dry.Rinse the punch in 70% ethanol prior to use andbetween different samples. Punch a disk(preferably 1/8 inch or 3 mm) out of the cardfrom the area with the dried saliva sample. Place disk into the solution. Tap tube or plate on hardsurface to ensure disk is in solution forincubation period.3.Incubate sample at room temperature for10 minutes.4.Incubate sample at 95 °C for 3 minutes.Note: Tissues will not be completely digested atthe end of the incubations. This is normal and will not affect performance.5.Add 100 μL of Neutralization Solution B to sampleand mix by vortexing.6.Store the neutralized tissue extract at 4 °C oruse immediately in PCR amplification.Note: For long term storage, remove theundigested tissue or transfer the extracts tonew tubes or wells. Extracts may now be storedat 4 °C for at least 6 months without notable loss in most cases.DNA Extraction for Buccal Swabs1.Collect buccal cells on swab and allow theswab to dry. Drying time is approximately10 to 15 minutes.Note: Due to the low volume of solution used for DNA extraction, a foam tipped swab should beused. Swabs with fibrous tips, such as cotton orDacron®, should be avoided because the solution cannot be recovered efficiently.2.Pipette 200 μL of Extraction Solution into amicrocentrifuge tube. Add 25 μL of TissuePreparation Solution to the tube and pipette upand down to mix.Note: If several extractions will be performed,sufficient volumes of Extraction and TissuePreparation Solutions may be pre-mixed ina ratio of 8:1 up to 2 hours before use.3.Place dried buccal swab into solution and incubateat room temperature for 1 minute.4.Twirl swab in solution 10 times and then removeexcess solution from the swab into the tube bytwirling swab firmly against the side of the tube.Discard the swab. Close the tube andvortex briefly.5.Incubate sample at room temperature for10 minutes.6.Incubate sample at 95 °C for 3 minutes.7.Add 200 μL of Neutralization Solution B to sampleand mix by vortexing.8.Store the neutralized extract at 4 °C or useimmediately in PCR. Continue to PCRamplification.Note: Extracts may be stored at 4 °C for at least6 months without notable loss in most cases. PCR AmplificationThe Extract-N-Amp™ PCR Reaction Mix contains JumpStart™ Taq antibody for specific hot start amplification. Therefore, PCR mixtures can be assembled at room temperature without premature Taq DNA polymerase activity.Typical final primer concentrations are approximately 0.4 μM each. The optimal primer concentration and cycling parameters will depend on the system being used.1.Add the following reagents to a thin-walled PCRmicrocentrifuge tube or plate:Reagent VolumeWater, PCR grade VariableExtract-N-Amp™ PCRreaction mix 10 μLForward primer VariableReverse primer VariableTissue extract 4 μL*Total volume 20 μL*The Extract-N-Amp™ PCR Reaction Mix isformulated to compensate for components in the Extraction, Tissue Preparation, and Neutralization Solutions. If less than 4 µL of tissue extract isadded to the PCR reaction volume, use a 50:50mixture of Extraction and Neutralization BSolutions to bring the volume of tissue extract upto 4 μL.2.Mix gently.3.For thermal cyclers without a heated lid, add20 μL of mineral oil on top of the mixture in eachtube to prevent evaporation.4.Perform thermal cycling. The amplificationparameters should be optimized for individualprimers, template, and thermal cycler.Common cycling parameters:Step Temperature Time Cycles InitialDenaturation 94 °C 3 minutes 1 Denaturation 94 °C 30 seconds Annealing 45 to 68 °C 30 seconds 30-35 Extension 72 °C 1-2 minutes(1 min/kb)FinalExtension 72 °C 10 minutes 1 Hold 4 °C Indefinitely5.The amplified DNA can be loaded onto an agarosegel after the PCR is completed with the addition ofa separate loading buffer/tracking dye such as GelLoading Solution, Cat. No. G2526.Note: PCR products can be purified, if desired, fordownstream applications such as sequencing withthe GenElute PCR Clean-Up Kit, Cat. No.NA1020.Troubleshooting GuideProblem Cause SolutionLittle or no PCR product is detected. PCR reaction may beinhibited due tocontaminants in thetissue extract.Dilute the tissue extract with a 50:50 mix of Extractionand Neutralization Solutions. To test for inhibition, includea DNA control and/or spike a known amount of template(100-500 copies) into the PCR along with the tissue extract. Extraction isinsufficient.Incubate samples at 55 °C for 10 minutes instead ofroom temperature.A PCR component maybe missing or degraded.Run a positive control to ensure that componentsare functioning. A checklist is also recommendedwhen assembling reactions.There may be too fewcycles performed. Increase the number of cycles (5-10 additional cycles at a time). The annealingtemperature maybe too high.Decrease the annealing temperature in 2-4 °C increments.The primers may notbe designed optimally.Confirm the accuracy of the sequence information. If theprimers are less than 22 nucleotides long, try to lengthen theprimer to 25-30 nucleotides. If the primer has a GC contentof less than 45%, try to redesign the primer with a GCcontent of 45-60%.The extension timemay be too short.Increase the extension time in 1-minute increments, especiallyfor long templates.Target templateis difficult.In most cases, inherently difficult targets are due to unusuallyhigh GC content and/or secondary structure. Betaine, Cat. No.B0300, has been reported to help amplification of high GCcontent templates at a concentration of 1.0-1.7 M.Multiple products JumpStart™ Taqantibody is notworking correctly.Do not use DMSO or formamide with Extract-N-Amp™ PCRReaction Mix. It can interfere with the enzyme-antibodycomplex. Other cosolvents, solutes (e.g., salts), and extremesin pH or other reaction conditions may reduce the affinity ofthe JumpStart™ Taq antibody for Taq polymerase and therebycompromise its effectiveness.TouchdownPCR maybe needed.“Touchdown” PCR significantly improves the specificity of manyPCR reactions in various applications. Touchdown PCR involvesusing an annealing/extension temperature that is higher thanthe TM of the primers during the initial PCR cycles. Theannealing/extension temperature is then reduced to the primerTM for the remaining PCR cycles. The change can be performedin a single step or in increments over several cycles.Negative control shows a PCR product or “false positive” result. Reagents arecontaminated.Include a reagent blank without DNA template be included asa control in every PCR run to determine if the reagents used inextraction or PCR are contaminated with a template froma previous reaction.Tissue is not digested after incubations. Tissue is not expectedto be completelydigested.The REDExtract-N-Amp™ Tissue PCR Kit does not require thetissue to be completely digested. Sufficient DNA is released forPCR without completely digesting the tissue.Buccal swab absorbed all the solution. The recommended typeof swab was not used.Due to the low volume of solution used for DNA extraction, afoam tipped swab should be used. Swabs with fibrous tips, suchas cotton or Dacron®, should be avoided because the solutioncannot be recovered efficiently.References1.Dieffenbach, C.W., and Dveksler, G.S. (Eds.), PCRPrimer: A Laboratory Manual, 2nd ed., Cold Spring Harbor Laboratory Press, New York (1995).2.Don, R.H. et al., ‘Touchdown' PCR to circumventspurious priming during gene amplification.Nucleic Acids Res., 19, 4008 (1991).3.Erlich, H.A. (Ed.), PCR Technology: Principles andApplications for DNA Amplification, StocktonPress, New York (1989).4.Griffin, H.G., and Griffin, A.M. (Eds.), PCRTechnology: Current Innovations, CRC Press,Boca Raton, FL (1994).5.Innis, M.A., et al., (Eds.), PCR Strategies,Academic Press, New York (1995).6.Innis, M., et al., (Eds.), PCR Protocols: A Guide toMethods and Applications, Academic Press, SanDiego, California (1990).7.McPherson, M.J. et al., (Eds.), PCR 2: A PracticalApproach, IRL Press, New York (1995).8.Newton, C.R. (Ed.), PCR: Essential Data, JohnWiley & Sons, New York (1995).9.Roux, K.H. Optimization and troubleshooting inPCR. PCR Methods Appl., 4, 5185-5194 (1995).10.Saiki, R., PCR Technology: Principles andApplications for DNA Amplification, Stockton, New York (1989). Product OrderingOrder products online at Related Products Cat. No.Ethanol E7148; E7023; 459836 Forceps,micro-dissecting F4267PCR Marker P9577PCR microtubes Z374873; Z374962;Z374881PCR multi-well plates Z374903Precast Agarose Gels P6097Sealing mats & tapes Z374938; A2350TBE Buffer T4415, T6400, T9525The life science business of Merck operatesas MilliporeSigma in the U.S. and Canada.Merck, Extract-N-Amp, REDExtract-N-Amp, JumpStart, GenElute and Sigma-Aldrich are trademarks of Merck KGaA, Darmstadt, Germany or its affiliates. All other trademarks are theproperty of their respective owners. Detailed information on trademarks is available via publicly accessible resources.NoticeWe provide information and advice to our customers on application technologies and regulatory matters to the best of our knowledge and ability, but without obligation or liability. Existing laws and regulations are to be observed in all cases by our customers. This also applies in respect to any rights of third parties. Our information and advice do not relieve our customers of their own responsibility for checking the suitability of our products for the envisaged purpose. The information in this document is subject to change without notice and should not be construed as a commitment by the manufacturing or selling entity, or an affiliate. We assume no responsibility for any errors that may appear in this document. Technical AssistanceVisit the tech service page at/techservice.Terms and Conditions of SaleWarranty, use restrictions, and other conditions of sale may be found at /terms. Contact InformationFor the location of the office nearest you, go to /offices.。
L 012 sodium salt_化学发光探针_143556-24-5_Apexbio

化学性质
产品名: Cas No.: 分子量: 分子式:
L 012 sodium salt 143556-24-5 310.67 C13H8CIN4NaO2
产品名: L 012 sodium salt 修订日期: 6/30/2016
化学名: SMILES: 溶解性: 储存条件: 一般建议:
运输条件:
特别声明产品仅用于研ຫໍສະໝຸດ ,不针对患者销售,望谅解。
每个产品具体的储存和使用信息显示在产品说明书中。ApexBio 产品在推荐的条件下是稳定 的。产品会根据不同的推荐温度进行运输。许多产品短期运输是稳定的,运输温度不同于长 期储存的温度。我们确保我们的产品是在保持试剂质量的条件下运输的。收到产品后,按照 产品说明书上的要求进行储存。
生物活性
sodium 8-amino-5-chloro-4-hydroxy-7-phenylpyrido[3,4-d]pyridazin-1-olate
ClC1=NC(C2=CC=CC=C2)=C(N)C3=C1C(O)=NN=C3[O-].[Na+]
Soluble in DMSO > 10 mM
Store at -20°C
参考文献: [1]. Nishinaka Y, Aramaki Y, Yoshida H, et al. A new sensitive chemiluminescence probe, L-012, for measuring the production of superoxide anion by cells. Biochem Biophys Res Commun, 1993, 193(2): 554-559. [2]. Sohn HY, Gloe T, Keller M, et al. Sensitive superoxide detection in vascular cells by the new chemiluminescence dye L-012. J Vasc Res, 1999, 36(6): 456-464. [3]. Imada I, Sato EF, Miyamoto M, et al. Analysis of reactive oxygen species generated by neutrophils using a chemiluminescence probe L-012. Anal Biochem. 1999, 271(1): 53-58. [4]. Asghar MN, Emani R, Alam C, et al. In vivo imaging of reactive oxygen and nitrogen species in murine colitis. Inflamm Bowel Dis, 2014, 20(8): 1435-1447.
Pimecrolimus_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :PimecrolimusCatalog No. :HY-13723CAS No. :137071-32-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:SDZ⁻ASM 981Formula:C43H68ClNO11Molecular Weight:810.45CAS No. :137071-32-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Mettler Toledo 试验用品说明书
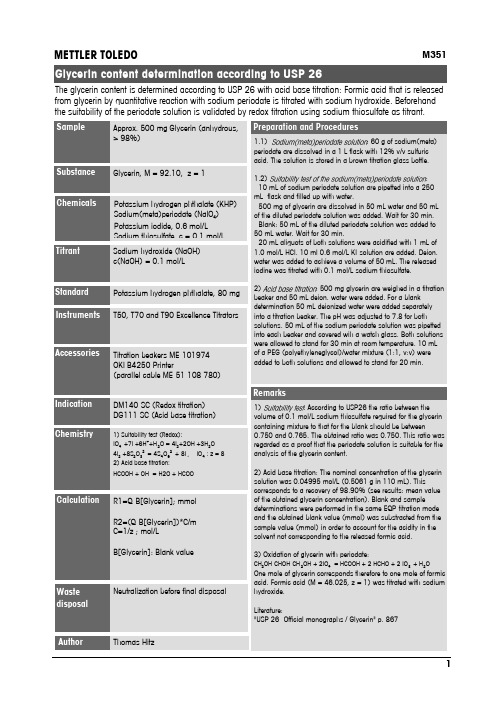
M351Glycerin, M = 92.10, z = 1Potassium hydrogen phthalate (KHP)Sodium(meta)periodate (NaIO 4)Potassium iodide, 0.6 mol/LSodium thiosulfate c =01mol/L Sodium hydroxide (NaOH)c(NaOH) = 0.1 mol/LT50, T70 and T90 Excellence Titrators1) Suitability test (Redox):IO 4-+7I -+6H ++H 2O = 4I 2+2OH -+3H 2O 4I 2+8S 2O 32-= 4S 4O 62-+ 8I -, IO 4-: z = 82) Acid base titration:HCOOH + OH -= H2O + HCOO-Titration beakers ME-101974OKI B4250 Printer(parallel cable ME-51 108 780)METTLER TOLEDONeutralization before final disposalApprox. 500 mg Glycerin (anhydrous,> 98%)DM140-SC (Redox titration)DG111-SC (Acid base titration)1.1): 60 g of sodium(meta)periodate are dissolved in a 1 L flask with 12% v/v sulfuric acid. The solution is stored in a brown titration glass bottle.1.2):- 10 mL of sodium periodate solution are pipetted into a 250mL flask and filled up with water.- 500 mg of glycerin are dissolved in 50 mL water and 50 mL of the diluted periodate solution was added. Wait for 30 min.- Blank: 50 mL of the diluted periodate solution was added to 50 mL water. Wait for 30 min.- 20 mL aliquots of both solutions were acidified with 1 mL of 1.0 mol/L HCl. 10 ml 0.6 mol/L KI-solution are added. Deion.water was added to achieve a volume of 50 mL. The released iodine was titrated with 0.1 mol/L sodium thiosulfate.2): 500 mg glycerin are weighed in a titration beaker and 50 mL deion. water were added. For a blank determination 50 mL deionized water were added separately into a titration beaker. The pH was adjusted to 7.8 for both solutions. 50 mL of the sodium periodate solution was pipetted into each beaker and covered wih a watch glass. Both solutions were allowed to stand for 30 min at room temperature. 10 mL of a PEG (polyethyleneglycol)/water mixture (1:1, v:v) were added to both solutions and allowed to stand for 20 min.1): According to USP26 the ratio between the volume of 0.1 mol/L sodium thiosulfate required for the glycerin containing mixture to that for the blank should be between0.750 and 0.765. The obtained ratio was 0.750. This ratio was regarded as a proof that the periodate solution is suitable for the analysis of the glycerin content.2) Acid base titration: The nominal concentration of the glycerin solution was 0.04995 mol/L (0.5061 g in 110 mL). This corresponds to a recovery of 98.90% (see results: mean value of the obtained glycerin concentration). Blank and sample determinations were performed in the same EQP-titration mode and the obtained blank value (mmol) was substracted from the sample value (mmol) in order to account for the acidity in the solvent not corresponding to the released formic acid.3) Oxidation of glycerin with periodate:CH 2OH-CHOH-CH 2OH + 2IO 4-= HCOOH + 2 HCHO + 2 IO 3-+ H 2OOne mole of glycerin corresponds therefore to one mole of formicacid. Formic acid (M = 46.025, z = 1) was titrated with sodium hydroxide.Literature:"USP 26 -Official monographs / Glycerin" p. 867R1=Q-B[Glycerin]; mmol R2=(Q-B[Glycerin])*C/m C=1/z ; mol/LB[Glycerin]: Blank valueThe glycerin content is determined according to USP 26 with acid base titration: Formic acid that is released from glycerin by quantitative reaction with sodium periodate is titrated with sodium hydroxide. Beforehand the suitability of the periodate solution is validated by redox titration using sodium thiosulfate as titrant.Potassium hydrogen phthalate, 80 mg Thomas HitzName: Thomas Hitz, ID Glycerin ContentRx Result Unit Name1/5 -- 27.03.2007 11:30:27R1 =0.98657 mmol ContentR2 =0.04933 mol/L Concentration 2/5 -- 27.03.2007 11:34:16R1 =0.98932 mmol ContentR2 =0.04947 mol/L Concentration 3/5 -- 27.03.2007 11:41:09R1 =0.98976 mmol ContentR2 =0.04949 mol/L Concentration 4/5 -- 27.03.2007 11:47:34R1 =0.98991 mmol ContentR2 =0.04950 mol/L Concentration 5/5 -- 27.03.2007 11:53:14R1 =0.98406 mmol ContentR2 =0.04920 mol/L Concentration StatisticsRx Name n Mean Unit s srel [%]R1 Cont.50.98792 mmol0.00255 0.258R2 Conc.50.04940 mol/L0.00013 0.264。
A-317491 sodium salt hydrate__DataSheet_MedChemExpress

Product Name:A-317491 sodium salt hydrateCAS No.:Product Data SheetCat. No.:HY-15568A MWt:606.57Formula:C33H29NNaO9+Purity :>98%Solubility:Mechanisms:Biological Activity:Pathways:Membrane Transporter/Ion Channel; Target:P2X Receptor water >50 mg/mlg y A-317491 is a non-nucleotide P2X3 and P2X2/3 receptor antagonist, which inhibits calcium fluxmediated by the receptors.IC50 value:Target: P2X2/3 receptor It is known that P2X3 and P2X2/3 receptors stimulate the pronociceptive effects of ATP upon activation. Studies indicate that the P2X3 receptor is implicated in both neuropathic andinflammatory pain. P2X3 receptor is a promising target for therapeutic intervention in cancer patients for pain management.References:[1]. Hansen RR, Nasser A, Falk S, et al. Chronic administration of the selective P2X3, P2X2/3receptor antagonist, A-317491, transiently attenuates cancer-induced bone pain in mice. Eur JPharmacol. 2012 Aug 5;688(1-3):27-34.[2]. Wu JX, Xu MY, Miao XR, et al. Functional up-regulation of P2X3 receptors in dorsal root p g[]p g pganglion in a rat model of bone cancer pain. Eur J Pain. 2012;16(10):1378-88.[3]. Xu J, Chu KL, Brederson JD, et al. Spontaneous firing and evoked responses of spinalnociceptive neurons are attenuated by blockade of P2X3 and P2X2/3 receptors in inflamed rats. JNeurosci Res. 2012;90(8):1597-606.[4]. Yasuda M, Shinoda M, Kiyomoto M, et al. P2X3 receptor mediates ectopic mechanical allodyniawith inflamed lower lip in mice. Neurosci Lett. 2012 18;528(1):67-72.[5]. Maria Cláudia G. Oliveiraa, Adriana Pelegrini-da-Silvaa, et al. Peripheral m...Caution: Not fully tested. For research purposes onlyMedchemexpress LLC。
waters质谱masslynx软件使用说明

Copyright Notice
Micromass UK Limited believes that the information in this publication is accurate. However the information is subject to change without notice and should not be construed as a contractual undertaking by Micromass UK Limited. Despite the care that has been given to the preparation of this publication, Micromass UK Limited accepts no responsibility for any loss or any other matter that may arise from any error or inaccuracy that may inadvertently have been included. Copyright 1993-2002 Micromass Ltd. All Rights Reserved. No part of this publication may be copied without the express written permission of Micromass UK Limited.
Page ii
MassLynx NT Users Guide
Contents
MassLynx NT User’s Guide............................................................................
高效液相色谱-串联质谱法检测肉类原料中的药物多残留

肉,菇业MEAT INDUSTRY 2020年第12期总第476期❖由醃妥全与检测❖高效液相色谱-串联质谱法检测肉类原料中的药物多残留沈春华厦门古龙食品有限公司技术中心实验室福建厦门361000摘要建立了高效液相色谱-串联质谱技术,同时检测肉类原料中磺胺类(磺胺二甲囉味、磺胺间二甲氧疇啜、磺胺间甲氧嗨呢、磺胺甲基异噁哇、礦胺囉睫、礦胺二甲异噁哇、磺胺甲氧哒嗪)和喳诺飼类(恩诺沙星)8种药物残留量的方法。
前处理采用1%甲酸-乙睛提取,浓缩后用0.1%甲酸-乙睛定容,正己烷去脂,用高效液相-串联质谱进行定性及定量分析。
所涉8种药物在1~100|xg/L范围内线性良好,相关系数为0.9956~0.9998,方法最低定量限为1jig/kg。
在3个添加水平下,加标回收率为67.8%-115%,相对标准偏差为1.2%~9.1%。
$匕方法可满足肉类原料中,磺胺类和喳•诺飼类多种药物残留的检测与验证。
关键词液相色谱-串联质谱药物残留肉类检测Detection of drugs residue in meat raw materials by high performance liquidchromatography-tandem mass spectrometry methodSHEN ChunhuaAbstract A high performance liquid chromatography-tandem mass spectrometry method was established,and8kinds of drugs residue including sulfonamides(sulfamethazine,sulfadimethoxine, sulfamo nomethoxine,sulfamethoxazole,sulfadiazine,sulfisoxazole and sulfamethoxypyridazine)and quinolones(enrofloxacin)in meat raw materials could be detected simultaneously.1%acidic acetonitrile was adopted to extract in pretreatment.After concentration,the volume was fixed with0.1%formic acid acetonitrile,and the fat was removed with n一Hexane,high performance liquid chromatography-tandem mass spectrometry method was used for qualitative and quantitation analysis・The8kinds of drugs involved had good linearity in the range of1~100|xg/L,and the correlation coefficient was0.9956〜0.9998,and the minimum limit of quantitation was1|xg/kg・Under the three addition levels,the recovery of standard addition was67.8%~115%,and the relative standard deviations was1.2%to9.1%.This method could meet the needs of detection and validation of sulfonamides and quinolones residues in meat raw materials.Key words high performance liquid chromatography一tandem mass spectrometry method;drugs residue;meat;detection肉类作为食品加工行业的一大类原料,随着食品安全问题备受社会日益关注的同时,肉类原料是否安全可靠,已引起了国内食品安全监管机构甚至国际食品法典委员会(CAC)的高度重视⑷。
巴斯夫的清洁剂资料

Nonionic surfactantsLutensol®A 4 N C12C14-Fatty alcohol+ 4 EO ca. 62/ELutensol®A 7 N+7 EO ca. 56/A Lutensol®A 79 N+7 EO90 %,ca. 56/A Lutensol®A 8+8 EO90 %,ca. 52/ALutensol®AT 11C16C18-Fatty alcohol+11 EO ca. 87/ALutensol®AT 18+18 EO ca. 92/B Lutensol®AT 18 Solution+18 EO20 %,ca. 92/B Lutensol®AT 25 Powder+25 EO ca. 95/B Lutensol®AT 25 E+25 EO ca. 95/B Lutensol®AT 25 Flakes+25 EO ca. 95/B Lutensol®AT 50 Powder+50 EO ca. 92/B Lutensol®AT 50 Flakes+50 EO ca. 92/B Lutensol®AT 80 Powder+80 EO ca. 87/B Lutensol®AT 80 E+80 EO ca. 87/B Lutensol®AT 80 Flakes+80 EO ca. 87/BLutensol®AO 3C13C15-Oxo alcohol+ 3 EO ca. 45/ELutensol®AO 4+ 4 EO ca. 57/E Lutensol®AO 5+ 5 EO ca. 62/E Lutensol®AO 7+7 EO ca. 43/A Lutensol®AO 79+7 EO90 %,ca. 43/A Lutensol®AO 8+8 EO ca. 52/A Lutensol®AO 89+8 EO90 %,ca. 52/A Lutensol®AO 109+10 EO90 %,ca. 80/A Lutensol®AO 11+11 EO ca. 86/A Lutensol®AO 30+30 EO ca. 91/B Lutensol®AO 3109+3/+10EO 90 %,ca. 73/ELutensol®TO 2C13-Oxo alcohol+ 2 EO ca. 37/DLutensol®TO 3+ 3 EO ca. 40/E Lutensol®TO 5+ 5 EO ca. 62/E Lutensol®TO 6+ 6 EO ca. 67/E Lutensol®TO 65+6,5 EO ca. 68/D Lutensol®TO 7+7 EO ca. 70/E Lutensol®TO 79+7 EO90 %,ca. 70/E Lutensol®TO 8+8 EO ca. 60/A Lutensol®TO 89+8 EO90 %,ca. 60/A Lutensol®TO 10+10 EO ca. 70/A Lutensol®TO 109+10 EO85 %,ca. 70/A Lutensol®TO 11+11 EO ca. 70/B Lutensol®TO 12+12 EO ca. 75/B Lutensol®TO 129+12 EO85 %,ca. 75/B Lutensol®TO 15+15 EO ca. 80/B Lutensol®TO 20+20 EO ca. 86/B Lutensol®TO 389+3 /+8EO90 %,ca. 70/E Unless otherwise indicated, the product concentration is 100% Cloud point in °C according to EN 1890Method A: 1 g of surfactant + 100 g of dist. WaterMethod B: 1 g of surfactant + 100 g of NaCl solution (c = 50 g/l) Method C: 1 g of surfactant + 100 g of NaCl solution (c = 100 g/l) Method D: 5 g of surfactant + 45 g of butyldiglycol solution(c = 250 g/l)Method E: 5 g of surfactant + 25 g of butyldiglycol solution(c = 250 g/l)Lutensol®XP 30C10-Guerbet alcohol+ 3 EO ca. 31/ELutensol®XP 40+ 4 EO ca. 44/E Lutensol®XP 50+ 5 EO ca. 56/E Lutensol®XP 60+ 6 EO ca. 62/E Lutensol®XP 69+ 6 EO85 %,ca. 62/E Lutensol®XP 70+7 EO ca. 68/E Lutensol®XP 79+7 EO85 %,ca. 68/E Lutensol®XP 80+8 EO ca. 56/A Lutensol®XP 89+8 EO85 %,ca. 56/A Lutensol®XP 90+9 EO ca. 69/A Lutensol®XP 99+9 EO85 %,ca. 69/A Lutensol®XP 100+10 EO ca. 80/A Lutensol®XP 140+14 EO ca. 78/BLutensol®XL 40C10-Guerbet alcohol+ 4 EO ca. 43/ELutensol®XL 50alkoxylate+ 5 EO ca. 58/E Lutensol®XL 60+ 6 EO ca. 65/E Lutensol®XL 70+7 EO ca. 68/E Lutensol®XL 79+7 EO85 %,ca. 68/E Lutensol®XL 80+8 EO ca. 56/A Lutensol®XL 89+8 EO85 %,ca. 56/A Lutensol®XL 90+9 EO ca. 69/A Lutensol®XL 99+9 EO ca. 69/A Lutensol®XL 100+10 EO ca. 80/A Lutensol®XL 140+14 EO ca. 78/BLutensol®ON 30C10-Oxo alcohol+ 3 EO ca. 53/ELutensol®ON 50+ 5 EO ca. 67/E Lutensol®ON 60+ 6 EO ca. 36/A Lutensol®ON 66+6,5 EO ca. 53/A Lutensol®ON 70+7 EO ca. 60/A Lutensol®ON 80+8 EO ca. 80/A Lutensol®ON 110+11 EO ca. 78/B Lutensol®AP 6Alkylphenol+ 6 EO ca. 61/E Lutensol®AP 7+7 EO ca. 62/E Lutensol®AP 8+8 EO ca. 34/A Lutensol®AP 9+9 EO ca. 51/A Lutensol®AP 10+10 EO ca. 60/A Lutensol®AP 14+14 EO ca. 76/B Lutensol®AP 20+20 EO ca. 85/B Lutensol®FA 12Oleyl amine+12 EO ca. 86/B Lutensol®FA 12 K Coco amine+12 EO ca. 92/B Lutensol®FA 15 T Tallow amine+15 EO ca. 97/B Lutensol®FSA 10Oleic acid amine+10 EO ca. 85/E Lutensol®GD 70Alkyl polyglucoside70 %,>100/BLow-foaming nonionic surfactantsPlurafac®LF 120Fatty alcohol alkoxylate ca. 28/A Plurafac®LF 22095 %,ca. 42/A Plurafac®LF 22195 %,ca. 33/A Plurafac®LF 22398 %,ca. 33/E Plurafac®LF 224ca. 27/E Plurafac®LF 226ca. 28/A Plurafac®LF 300ca. 22/A Plurafac®LF 301ca. 32/E Plurafac®LF 303ca. 29/E Plurafac®LF 305ca. 38/E Plurafac®LF 400ca. 33/A Plurafac®LF 401ca. 74/A Plurafac®LF 403ca. 41/E Plurafac®LF 404ca. 45/E Plurafac®LF 40595 %,ca. 55/E Plurafac®LF 500ca. 32/E Plurafac®LF 600ca. 55/A Plurafac®LF 711ca. 45/E Plurafac®LF 1300ca. 21/E Plurafac®LF 1430Amine alkoxylate ca. 35/A Plurafac®SLF-18B4590 % Fatty alcohol alkoxylate 90 %,ca. 21/E Plurafac®LF 131Fatty alcohol alkoxylate, end-capped ca. 35/E Plurafac®LF 132ca. 30/E Plurafac®LF 231ca. 28/E Plurafac®LF 431ca. 39/E Pluronic®PE 3100PO–EO-block polymer10 % EO ca. 41/E Pluronic®PE 350050 % EO ca. 68/A Pluronic®PE 430030 % EO ca. 61/E Pluronic®PE 610010 % EO ca. 23/A Pluronic®PE 612012 % EO ca. 41/E Pluronic®PE 620020 % EO ca. 33/A Pluronic®PE 640040 % EO ca. 60/A Pluronic®PE 680080 % EO ca. 88/B Pluronic®PE 740040 % EO ca. 60/A Pluronic®PE 810010 % EO ca. 36/E Pluronic®PE 920020 % EO ca. 49/E Pluronic®PE 940040 % EO ca. 80/E Pluronic®PE 1010010 % EO ca. 35/E Pluronic®PE 1030030 % EO ca. 71/E Pluronic®PE 1040040 % EO ca. 81/A Pluronic®PE 1050050 % EO ca. 75/B Pluronic®PE 10500 Solution50 % EO,18 %,ca. 75/B Pluronic®RPE 1720EO-PO-block polymer20 % EO ca. 37/E Pluronic®RPE 174040 % EO ca. 51/E Pluronic®RPE 203535 % EO ca. 41/E Pluronic®RPE 252020 % EO ca. 31/E Pluronic®RPE 252525 % EO ca. 38/E Pluronic®RPE 311010 % EO ca. 25/E Tetronic®RED 9040Ethylene diamineEO- PO- block polymer40 % EO ca. 48/A AminopolyolQuadrol®L Ethylene diamine + 4 POPolyalkylene glykolsPluriol®A 010 R Allyl alcohol ethoxylatePluriol®A 11 RE Allyl alcohol alkoxylatePluriol®A 13 RPluriol®A 22 RPluriol®A 23 RPluriol®A 308 R Butyne-1,4-diol ethoxylatePluriol®A 350 E Methylpolyethylene glycol M 350 Pluriol®A 500 E M 500 Pluriol®A 750 E1)M 750 Pluriol®A 760 E M 750 Pluriol®A 1000 E M 1000 Pluriol®A 1020 E1)M 1000 Pluriol®A 2000 E M 2000 Pluriol®A 3010 E1)M 3000 Pluriol®A 5010 E1)M 5000 Pluriol®A 1000 PE Alkyl polyalkylene glykol M 1000 Pluriol®A 1320 PE1)M 1400 Pluriol®A 2000 PE M 2000 Pluriol®A 2020 PE1)M 2000 Pluriol®A 1350 P Alkyl polypropylene glykol M 1350 Pluriol®A 2000 P M 2000 Pluriol®A 3 TE Modified polyglycol ether M 275 Pluriol®A 15 TE M 800 Pluriol®A 15 TERC M 800 Pluriol®A 18 TERC M 900 1)non filtratedPluriol®E 200Polyethylene glycol M 200 Pluriol®E 2052)M 200 Pluriol®E 300M 300 Pluriol®E 3052)M 300 Pluriol®E 400M 400 Pluriol®E 4052)M 400 Pluriol®E 600M 600 Pluriol®E 6052)M 600 Pluriol®E 1000M 1000 Pluriol®E 1500 E M 1500 Pluriol®E 1500 Powder M 1500 Pluriol®E 1500 Flakes M 1500 Pluriol®E 1505 Flakes2)M 1500 Pluriol®E 3400 E M 3400 Pluriol®E 3400 Powder M 3400 Pluriol®E 3400 Flakes M 3400 Pluriol®E 3405 E2)M 3400 Pluriol®E 3405 Flakes2)M 3400 Pluriol®E 4000 E M 4000 Pluriol®E 4000 Powder M 4000 Pluriol®E 4000 Flakes M 4000 Pluriol®E 4005 E2)M 4000 Pluriol®E 4005 Flakes2)M 4000 Pluriol®E 6000 E M 6000 Pluriol®E 6000 Powder M 6000 Pluriol®E 6000 Flakes M 6000 Pluriol®E 6005 E2)M 6000 Pluriol®E 6005 Flakes2)M 6000 Pluriol®E 8000 E M 8000 Pluriol®E 8000 Flakes M 8000Pluriol®E 8005 E2)M 8000 Pluriol®E 8005 Flakes2)M 8000 Pluriol®E 9000 Powder M 9000 Pluriol®E 9000 Fine Powder M 9000 Pluriol®E 9000 Flakes M 9000 Pluriol®P 400Polypropylene glycol M 430 Pluriol®P 600M 600 Pluriol®P 900M 900 Pluriol®P 2000M 2000 Pluriol®P 4000M 4000 2)Care - QualityReactive SolventsPluriol®BP 30 E Bisphenol A+ 3 EOPluriol®BP 40 E+ 4 EOPluriol®BP 60 E+ 6 EOPluriol®BP 100 E+ 10 EOEmulsifiersEmulan®A Oleic acid ethoxylate ca. 52/E Emulan®AF Fatty alcohol ethoxylate ca. 65/E Emulan®AT 9ca. 68/A Emulan®EL Castor oil ethoxylate97 %,ca. 71/B Emulan®EL 40ca. 72/E Emulan®ELH 6090 %,ca. 85/B Emulan®EL 200 Powder> 100/A Emulan®ELP ca. 51/E Emulan®LVA Oxo alcohol ethoxylate85 %,ca. 56/A Emulan®NP 3070Alkylphenol ethoxylate70 %,ca. 90/B Emulan®OC Fatty alcohol ethoxylate ca. 90/B Emulan®OC Solution30 %,ca. 90/B Emulan®OG ca. 92/B Emulan®OK 5ca. 62/E Emulan®OP 25Alkylphenol ethoxylate ca. 88/B Emulan®OU Fatty alcohol ethoxylate ca. 90/B Emulan®P ca. 52/E Emulan®PO Alkylphenol ethoxylate ca. 46/EEmulan®TO 2080C13-Oxo alcohol ethoxylate80 %,ca. 93/BEmulan®TO 307070 %,ca. 91/B Emulan®TO 407070 %,ca. 91/B Emulan®XCA 23Polyisobutene derivative70 % Emulphor®OPS 25Alkylphenol ether sulfate, sodium salt34 % Emulphor®NPS 2531 % Emulphor®FAS 30Fatty alcohol ether sulfate, sodium salt30 % SolubilizerEmulan®HE 50Alcohol ethoxylate ca. 72/B Emulan®EL S104Fatty alcohol alkoxylate ca. 56/E Ionic surfactantsLutensit®A-BO Dioctylsulphosuccinate, sodium salt60 % Lutensit®A-EP Acid phosphoric esterLutensit®A-ES Alkylphenol ether sulphate, 40 %sodium saltLutensit®A-FK Fatty acid condensation product,sodium salt55 % Lutensit®A-LBA Dodecylbenzenesulphonate, amine salt55 % Lutensit®A-LBS Dodecylbenzenesulphonic acid98 % Lutensit®A-PS Alkylsulphonate, sodium salt60 %Lutensit®AN 10Anionic/nonionic surfactant combinationbased on an alkylphenol ethoxylateLutensit®AN 30Anionic/nonionic surfactant combinationbased on a fatty alcohol ethoxylateLutensit®AN 40Mixture of nonionic surfactants andalkylcarboxylic acids70 % Lutensit®AN 45Mixture of nonionic surfactants andalkylcarboxylic acids80 % Lutensit®AN 50Anionic/nonionic surfactant combinationbased on a fatty alcohol ethoxylateNekal®BX Dry Alkylnaphthalenesulfonate, sodium salt68 %Nekal®BX Dry Paste60 %Nekal®BX Conc. Paste 40 %34 %Nekal®BX 30%22 %Nekal®BX Conc. Paste60 %Nekal®SBC Alkylnaphthalenesulphonic acid72 %Foam suppressorsDegressal®SD 20Fatty alcohol alkoxylateDegressal®SD 21Degressal®SD 22Degressal®SD 23Alkohol alkoxylateDegressal®SD 30Carbonylic esterDegressal®SD 40Phosphoric esterDispersing agentsPolycarboxylateSokalan®CP 5Maleic acid/Acrylic acid copolymer,sodium salt M 70 00040 % Sokalan®CP 5 Granules M 70 00092 % Sokalan®CP 5 Powder G M 70 00092 % Sokalan®CP 453)M 70 00045 % Sokalan®CP 45 Granules3)M 70 00092 % Sokalan®CP 7M 50 00040 % Sokalan®CP 7 Granules NL M 50 00092 % Sokalan®CP 9Maleic acid/olefin copolymer,sodium salt M 12 00025 % Sokalan®CP 9 Granules87 % Sokalan®CP 10Modified Polyacrylic acid,sodium salt M 4 00045 % Sokalan®CP 10 S Modified Polyacrylic acid M 4 00050 % Sokalan®CP 12 S M 3 00050 % Sokalan®CP 13 S M 20 00025 % Sokalan®PA 15Polyacrylic acid, M 1 20045 %sodium saltSokalan®PA 15 CL M 1 20045 % Sokalan®PA 20M 2 50045 % Sokalan®PA 20 PN3)M 2 50054 % Sokalan®PA 25 CL M 4 00045 % Sokalan®PA 25 CL Granules M 4 00092 % Sokalan®PA 25 CL PN3)M 4 00049 % Sokalan®PA 30M 8 00045 % Sokalan®PA 30 CL M 8 00045 % Sokalan®PA 30 CL Granules M 8 00092 % Sokalan®PA 30 CL PN48 % Sokalan®PA 30 CL PN Granules93 %please turn overSokalan®PA 40M 15 00035 % Sokalan®PA 40 Powder M 15 00092 % Sokalan®PA 70 PN3)M 70 00030 % Sokalan®PA 80 S Polyacrylic acid M 100 00035 % Sokalan®PA 110 S M 250 00035 % Special polymersSokalan®HP 22 G Nonionic copolymer M 24 00020 % Sokalan®HP 25Modified polycarboxylate M 3 00045 % Sokalan®HP 165Polyvinylpyrrolidone M 9 00030 % Sokalan®HP 50M 40 00095 % Sokalan®HP 53M 40 00030 % Sokalan®HP 53 K M 40 00030 % Sokalan®HP 56Vinylpyrrolidon/Vinylimidazolcopolymer M 70 00030 % Sokalan®HP 56 Granules M 70 00095 % Sokalan®HP 59Polyvinylpyrrolidon M 500 00045 % Sokalan®HP 60M 1 000 00020 % Sokalan®HP 66Vinylpyrrolidon/VinylimidazolCopolymer modified41 % Sokalan®AF PolyetherSokalan®DCS Mixture of dicarboxylic acidsSokalan®PM 10 I Maleic acid copolymer, M 4 00044 %sodium saltSokalan®PM 15 I Modified polycarboxylate, 40 %sodium saltSokalan®PM 70M 4 00040 % Sokalan®SR 100Esterified polyether3)= partly neutralizedSulphonic acid condensation product/Sulfonates Tamol®NH 7519Naphthaline sulphonic acid condensation 95 % Tamol®NN 2406product, sodium salt21 % Tamol®NN 290131 % Tamol®NN 450145 % Tamol®NN 771895 % Tamol®NN 890695 % Tamol®NN 910495 % Tamol®NN 940195 % Tamol®PP Phenol sulphonic acid condensation,95 % Tamol®DN product, sodium salt95 % PolyethyleneiminesLupasol®FG Polyethylenimine M 80099 % Lupasol®FC M 80050 % Lupasol®G 20 waterfree M 1 30099 % Lupasol®G 20M 1 30050 % Lupasol®PR 8515M 2 00099 % Lupasol®G 35M 2 00050 % Lupasol®G 100M 5 00050 % Lupasol®WF M 25 00099 % Lupasol®HF M 25 00056 % Lupasol®G 500M 25 00040 % Lupasol®P M 750 00050 % Lupasol®PS M 750 00033 % Lupasol®PO 100M 5 00050 % Lupasol®HEO 1M 13 00080 % Lupasol®PN 50M 1 000 00050 % Lupasol®SK M 2 000 00024 %Chelating agentsTrilon®AS Nitrilotriacetic acid (NTA)99 %Trilon®A 92 R Trisodium salt of NTA92 %Trilon®A liquid40 %Trilon®BS Ethylenediaminetetraacetic acid (EDTA)99 %Trilon®B-A-T liquid Triammonium salt of EDTA50 %Trilon®B Powder Tetrasodium salt of EDTA87 %Trilon®BX Powder83 %Trilon®B liquid40 %Trilon®BX liquid40 %Trilon®BD Disodium salt of EDTA89 %Trilon®BVT Chelating agent with highspecification for iron (III)21 %Trilon®C liquid Pentasodium salt ofDiethylenetriaminepentaacetic acid (DTP)40 %Trilon®C liquid 50%50 %Trilon®D liquid Trisodium salt of Hydroxyethyl-ethylenediaminetriacetic acid (HEDTA)40 %Trilon®M liquid Trisodium salt of Methylglycinediacetic 40 %acid (MGDA)Trilon®M Powder83 %Trilon®M Granules73 %Trilon®P Liquiol Modified anionic Polyamine40 % Biocide% Protectol®BN2-Bromo-2-nitropropane-1,3-diol 99(Bronopol)Protectol®BN 3030 % Protectol®BN 1818 % Protectol®GA 50Glutaraldehyde50 % Protectol®GA 2424 % Protectol®PE Phenoxyethanol99 % Protectol®PE S99 % Protectol®PP Phenoxypropanol99 % Protectol®GL Glyoxal40 % Protectol®HT Hexahydrotriazine derivate76 % Protectol®TD Tetramethylolacetylene diurea47 % Protectol®DF Dimethoxytetrahydrofurane99 % Protectol®DZ Thiadiazin derivate99 % Protectol®DZ P99 % Protectol®DA2,4-Dichlorbenzyl alkohol98 % Protectol®DA S98 % Corrosion inhibitorsKorantin®BH solid2-Butine-1,4-diol> 98 % Korantin®BH 50> 50 % Korantin®LUB Acid phosphoric ester of a polyetherKorantin®MAT Alkanolamine salt of a nitrogenious, organic acidKorantin®PAT80 % Korantin®PM Propargylalcohol alkoxylate99,5 % Korantin®PP67 % Korantin®SMK Phosphoric monoesterWaxesPolyethylene waxesLuwax®A Ethylene homopolymer waxes withLuwax®AH 3different densities and molar massesLuwax®AH 6Luwax®AL 3Luwax®AL 61Luwax®AM 3Luwax®AM 6Luwax®AF 29Micronized polyethylene waxes withLuwax®AF 30different particle size distributionLuwax®AF 31Luwax®AF 32Luwax®PE 10 MLuwax®OA Oxidized polyethylene waxes, emulsifiableLuwax®OA 2Luwax®OA 3Luwax®OA 5Luwax®ES 9696Luwax®ES 9698Luwax®EVA 1Copolymer polyethylene waxesLuwax®EVA 3Luwax®EAS 3Luwax®EAS 4Luwax®EAS 5Polyether waxLuwax®V Polyvinyl ether waxMontanwaxesLuwax®S Montanic acid waxesLuwax®LSLuwax®E Montanic ester waxesLuwax®LGLuwax®LGE Montanic ester waxes, contains emulsifierLuwax®OP Partially saponified montanic ester waxWax emulsionsPoligen®WE 1Polyethylene wax emulsions35 % Poligen®WE 325 % Poligen®WE 421 % Poligen®WE 635 % Poligen®WE 739 % Poligen®WE 8 BW35 % Poligen®ES 9100235 % Poligen®ES 9100540 % Poligen®ES 9100920 %Specialty chemicals for the electroplatingand electronics industriesEspecially for formulators of the electroplating and printed circuit board industry the following products are offered:Golpanol®ALS Allyl sulfonate25 % Golpanol®ALS 3535 % Golpanol®ATPN Carboxyethylisothiuronium betaine> 98 % Golpanol®BEO Butyne diol ethoxylateGolpanol®BMP Butyne diol propoxylateGolpanol®BOZ Crystals Butyne diol> 98 % Golpanol®DEP Diethylaminopropyne97,5 % Golpanol®HD Hexyne diol80 % Golpanol®MPA Dimethylpropinylamine89 % Golpanol®PA Propargyl alcohol99,3 % Golpanol®PAP Propargyl alcohol propoxylate67 % Golpanol®PME Propargyl alcohol ethoxylateGolpanol®PS Propyne sulphonate20 % Golpanol®VS Vinyl sulphonate25,5 % Lugalvan®ANA Anisaldehyde> 98,5 % Lugalvan®BNO 12ß-Naphtol ethoxylate99 % Lugalvan®BNO 2475 % Lugalvan®BPC 48Benzylpyridinium carboxylate48 % Lugalvan®DC Aqueous dispersion of an ethylene copolymer21 % Lugalvan®EH 158Ethylhexanol ethoxylate80 % Lugalvan®G 15000Polyethyleneimine50 % Lugalvan®G 2050 % Lugalvan®G 3550 % Lugalvan®HS 1000Thiodiglykol ethoxylate> 98 % Lugalvan®IMZ Imidazole> 99,5 % Lugalvan®IZE Reaction product of imidazole45 %and epichlorohydrineLugalvan®NES Sodium salt of a sulphonatedalkylphenol ethoxylate40 % Basotronic®PVI Polyvinylimidazole, quarternized44 % Basotronic®PYR Pyrrole (electronic grade)> 99,9 % Lutron®HF 1Modified polyglykol etherLutron®HF 3Lutron®HF 8Mixture of varous alkoxylates, stabilizedLutron®KS 1Modified PolyglykoletherLutron®WF 20 DLutron®WF 21Lutron®WF 21 D Modified Polyglykolether, stabilizedLutropur®FEG 28Formaldehyde (electronic grade)28 % Lutropur®MSA Methanesulfonic acid (electronic grade)70 % Lutropur®Q 75Ethylendiamine + 4 PO75 % Lutropur®Q Ethylendiamine + 4 PO。
红色REDTaq DNA多合物说明书

REDTaq® DNA PolymeraseCatalog Number D4309Storage Temperature –20 ︒CTECHNICAL BULLETINProduct DescriptionREDTaq DNA Polymerase is a unique blend of our high quality Taq DNA Polymerase combined with an inert red dye. The dye enables quick visual recognition of reactions to which enzyme has been added, as well as confirmation of complete mixing. The formulation allows aliquots (5-10 μL) from the PCR to be directly loaded onto an agarose gel without addition of electrophoresis loading buffers. The red dye migrates at the same rate as a 125 bp fragment in a 1% agarose gel. Because gel loading buffer is not added to the reaction mix, a sample can be re-amplified, such as in nested PCR.The red dye has no effect on automated or manual DNA sequencing, ligase mediated ligations, or exonucleolytic PCR product digestion. Though exceptions may exist, the dye is generally inert in restriction enzyme digestions. If desired, the dye can be removed from the amplicon using any standard purification method.The enzyme is provided at one unit/μL for more accurate volume measurement and less waste. Reactions using REDTaq DNA Polymerase and its optimized 10⨯ PCR buffer are formulated as any PCR mixtures. There are no additional reaction preparation steps or protocol changes required.Unit Definition: One unit incorporates 10 nmol of total deoxyribonucleoside-triphosphates into acid precipitable DNA in 30 min at 74 ︒C.Reagents Provided∙REDTaq DNA Polymerase, Catalog Number D56841 unit/μL in 20 mM Tris-HCl, pH 8.0, 100 mM KCl,0.1 mM EDTA, 1 mM DTT, stabilizers, inert dye,50% glycerol. Provided as 50, 250, 1,000 or 2,500 units∙ 10⨯ REDTaq PCR Reaction Buffer, Catalog Number B5926, 100 mM Tris-HCl, pH 8.3, 500mM KCl, 11 mM MgCl2 and 0.1% gelatin. Provided as 1 ml package Reagents and equipment required but not provided∙Deoxynucleotide Mix, Catalog Number D729510 mM each dATP, dCTP, dGTP, and TTP∙Water, PCR Reagent, Catalog Number W1754∙Mineral Oil, Catalog Number M8662 (optional)∙ Primers∙DNA to be amplified∙ Dedicated pipettes∙PCR pipette tips∙0.5 ml or 0.2 ml thin-walled PCR tubes, Catalog Numbers P3114 and P3364∙ Thermal cyclerPrecautions and DisclaimerThis product is for R&D use only, not for drug, household, or other uses. Please consult the Material Safety Data Sheet for information regarding hazards and safe handling practices.StorageStore all components at –20 ︒C.ProcedureNote: REDTaq 10⨯ PCR Reaction Buffer has been formulated to be compatible with REDTaq DNA Polymerase. If other buffers are to be used, they must be formulated to account for 0.4 mM magnesium being added to the PCR from the enzyme. In this case the final enzyme concentration in the PCR is assumed to be 0.05 units/μL Other enzyme concentrations may require further magnesium concentration optimization. Optimal concentrations of template DNA, MgCl2, KCl, and PCR adjuncts, as well as pH, are often target specific. It may be necessary to determine the optimal concentration of each component.21. Add the following reagents to a thin-walled 200 μLor 500 μL PCR tube in the order listed below. Amount Component FinalConcentration5 μL 10⨯ REDTaq PCRReaction Buffer1⨯1 μL Deoxynucleotide Mix,D7295 200 μM (each dNTP)- μL Primer 0.1-0.5 μM- μL Primer 0.1-0.5 μM2.5 μL REDTaq DNAPolymerase0.05 unit/μL- μL Template DNA(typically 10 ng)200 pg/μL q.s. Water (Cat. # W1754)50 μL Total volumeNote 1: A master mix is highly recommended when setting up multiple reactions.Note 2:10x REDTaq PCR Reaction Buffer should be vortexed vigorously prior to use.2. Mix gently by vortex and briefly centrifuge tocollect all components to the bottom of the tube.3. Add 50 μL of mineral oil to the top of each tube toprevent evaporation if not using a thermal cyclerwith a heated lid.4. Optimum cycling parameters vary with PCRcomposition (i.e. primer sequences, template,MgCl2 concentration etc.) and thermal cycler. Itmay be necessary to optimize the cyclingparameters to achieve maximum product yieldand/or quality. Typical cycling parameters are:.For cycles 1-30Denaturation 94 ︒C 1 min Annealing 55 ︒C 2 min Extension 72 ︒C 3 min Note: 25-30 cycles of amplification are recommended.5. The amplified DNA can be evaluated by agarosegel electrophoresis by loading 5-10 μL of the PCR reaction onto the gel without the addition of gelloading buffers. Note: A minimum of 1.5 units of REDTaq DNA polymerase must be added per 50 μL reaction for unencumbered gel loading. The red dye migrates as a 125 bp fragment in a 1% agarose gel.If a more intense tracking dye is desired, an unused lane can be used to run any common tracking dye (i.e. as provided by a DNA marker). Alternatively, a tracking solution devoid of DNA can be added to a previously loaded REDTaq PCR product well. Amplification products can be visualized by standard methodologies (e.g. ethidium bromide staining). Mineral oil overlay may be removed by a single chloroform extraction (1:1), recovering the aqueous phase. Alternatively, an aliquot of the aqueous phase can be taken by withdrawing solution from below the aqueousphase/mineral oil interface.Related ProductsReagents and Kits∙Lambda Hind III DNA Marker, Catalog Number D9780∙Enhanced Avian HS RT-PCR kits, Catalog Number HSRT100 (100 reactions). The RT-PCR kit combines twopowerful techniques to convert mRNA into cDNA andsubsequently to amplify the cDNA. The enhanced avianreverse transcriptase has an enhanced ability to transcribe through difficult secondary structure at elevatedtemperatures (up to 65 ︒C). Includes JumpStart AccuTaq™LA for hot-start PCR.Equipment∙PCR Multiwell Plate, 96-well, Catalog No. Z374903∙PCR Multiwell Plate, 384-well, Catalog No. Z374911∙PCR Microtubes, 0.2 ml, attached caps, Catalog No.Z374873∙PCR Microtubes, 0.2 ml strip tubes with strip caps, Catalog No. Z374962∙Sealing accessory for PCR vessels, Micro Mats, Catalog No. Z374938∙PCR Workstation, 120V, Catalog No. Z376213∙PCR Workstation, 240V, Catalog No. Z376221NOTICE TO PURCHASER: LIMITED LICENSEUse of this product is covered by one or more of the following US patents and corresponding patent claims outside the US: US 8,404,464 and US 7,972,828. The purchase of this product includes a limited, non-transferable immunity from suit under the foregoing patent claims.REDTaq is a registered trademark of Sigma-Aldrich Co, LLCGAR,PHC,KNV 10/16-1©2016 Sigma-Aldrich Co. LLC. All rights reserved. SIGMA-ALDRICH is a trademark of Sigma-Aldrich Co. LLC, registered in the US and other countries. Sigma brand products are sold through Sigma-Aldrich, Inc. Purchaser must determine the suitability of the product(s) for their particular use. Additional terms and conditions may apply. Please see product information on the Sigma-Aldrich website at and/or on the reverse side of the invoice or packing slip.。
Sodium Bisulfite 1 percent 安全技术说明书

Sodium Bisulfite 1 percent *************(24小时)化学品安全技术说明书GHS product identifier 应急咨询电话(带值班时间)::供应商/ 制造商:供应商/ 制造商: Agilent Technologies, Inc.(美国安捷伦科技有限公司)住所:5301 Stevens Creek Boulevard, Santa Clara, CA, 95051, United States 联系电话:+1 800 227 9770 供应商/ 制造商: Agilent Technologies Singapore (International) Pte Ltd.(安捷伦科技新加坡(国际)私人有限公司)住所:No. 1 Yishun Avenue 7, Singapore, 768923 联系电话:(65) 6276 2622供应商/ 制造商: Agilent Technologies Denmark ApS (安捷伦科技丹麦私人有限公司)住所:Produktionsvej 42, DK-2600 Glostrup, Denmark 联系电话: +45 44859500 Sodium Bisulfite 1 percent化学品的推荐用途和限制用途AR176, AR376部件号:物质用途:实验室使用容器类型: 一次性包装AR176 // Sodium Bisulfite 1% // Artisan Grocott's Methenamine Silver Stain Kit // 65 mL & 115 mLAR376 // Sodium Bisulfite 1% // Artisan Grocott's Methenamine Silver Eosin Stain Kit // 65 mL 参考号码: SDS055安全技术说明书根据 GB/ T 16483-2008 和 GB/ T 17519-2013本安全技术说明书责任人的e-mail地址:***************GHS化学品标识:1%亚硫酸氢钠有关环境保护措施,请参阅第 12 节。
A-317491 sodium salt hydrate__MSDS_MedChemExpress

Version 2.1 Revision Date: 07/08/2013Print Date: 11/21/2013MSDS1 Composition7 Accident Release MeasureProduct Name:A-317491 sodium salt hydrateChemical Name:PROCEDURE(S) OF PERSONAL PRECAUTION(S)-Wear respirator, chemical safety goggles, rubber boots, and heavy rubber gloves.METHODS FOR CLEANING UP-Sweep up, place in a bag and hold for waste disposal. Avoid raising dust. Ventilate area andwash spill site after material pickup is complete.(S)-5-((3-phenoxybenzyl)(1,2,3,4-tetrahydronaphthalen-1-yl)carbamoyl)benzene-1,2,4-tricarboxylic acid, sodium salthydrateCAS No.:8 Accident Release MeasureAppearance:White to off-white(solid)Formula:C33H29NNaO9+9 Toxicological InformationSolubility:To the best of our knowledge, the chemical, physical, andtoxicological properties have not been thoroughly investigated.No data available.p p p p water >50 mg/ml2 Handling and Storage10 Regulary Information3 Stability and ReactivityDisposal CLASSIFICATION- Substance not yet fully tested.SAFETY PHASES- 26-36 (In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Wear suitable protective clothing.) 36/37/38 (Irritating to eyes,respiratory system and skin.)STABILITY- Stable under normal handling conditions.HANDLING- Do not breathe dust. Avoid contact with eyes,skin,and clothing.Avoid prolonged or repeated exposure.STORAGE- Please store the product under the recommended conditions in the Certificate of Analysis.11Considerations4 Hazards Identification12 Transport InformationFirst AidRID/ADR- Non-hazardous for road transport.IMDG- Non-hazardous for sea transport.IATA - Non-hazardous for air transport.As specific country, federal, state and local environmentalregulations vary and change frequently we suggest you contact a local, authorized waste disposal contractor for adequate disposal.Special indication of hazards to humans and the environment.Irritating to eyes, respiratory system and skin.MATERIALS TO AVOID- Strong oxidizing agents.REACTIVITY- May emit toxic gasses like Carbon monoxide,Carbon dioxide, Nitrogen oxides upon thermal decomposition.513 Other InformationThe above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. Medchemexpress LLC shall not be held liable for any damage resulting from h dli f t t ith th b d tINHALATION- If inhaled, remove to fresh air. If not breathing give, artificial respiration. If breathing is difficult, give oxygen.SKIN CONTACT- In case of contact, immediately wash skin with soap and copious amounts of water.EYE CONTACT- In case of contact, immediately flush eyes with copious amounts of water for at least 15 minutes.INGESTION- If swallowed, wash out mouth with water provided person is conscious. Call a physician.6 Fire Fighting Measureshandling or from contact with the above product.EXTINGUISHING MEDIAWater spray- Carbon dioxide, dry chemical powder, or appropriate foam.SPECIAL RISKSSpecific Hazard(s)- Emits toxic fumes under fire conditions.SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS Wear self-contained breathing apparatus and protective clothing Caution: Not fully tested. For research purposes onlyMedchemexpress LLCto prevent contact with skin and eyes.㪈㪏㪮㫀㫃㫂㫀㫅㫊㫆㫅㩷㪮㪸㫐㪃㩷㪧㫉㫀㫅㪺㪼㫋㫆㫅㪃㩷㪥㪡㩷㪇㪏㪌㪋㪇㪃㪬㪪㪘㪜㫄㪸㫀㫃㪑㩷㫀㫅㪽㫆㪗㫄㪼㪻㪺㪿㪼㫄㪼㫏㫇㫉㪼㫊㫊㪅㪺㫆㫄㩷㪮㪼㪹㪑㩷㫎㫎㫎㪅㫄㪼㪻㪺㪿㪼㫄㪼㫏㫇㫉㪼㫊㫊㪅㪺㫆㫄。
Phosphatidylcholine Assay Kit 产品说明书

Phosphatidylcholine Assay Kit Catalog Number MAK049Storage Temperature –20 °CTECHNICAL BULLETINProduct DescriptionPhosphatidylcholine (PC) accounts for more than 50% of the phospholipids composing mammalian plasma membranes and is particularly enriched in theextracellular leaflet. PC is synthesized in the liver and is required for lipoprotein assembly and secretion. PC is cleaved by phospholipase D, which hydrolyses the choline head, producing the signaling intermediate phosphatidic acid.In this assay, PC concentration is determined by acoupled enzyme reaction, which results in a colorimetric (570 nm)/fluorometric (λex = 535/λem = 587 nm) product, proportional to the PC present.ComponentsThe kit is sufficient for 100 assays in 96 well plates.PC Assay Buffer25 mL Catalog Number MAK049AFluorescent Peroxidase Substrate, in DMSO0.2 mL Catalog Number MAK049B PC Hydrolysis Enzyme1 vl Catalog Number MAK049C PC Development Mix1 vl Catalog Number MAK049D PC Standard, 10 µmole1 vlCatalog Number MAK049EReagents and Equipment Required but Not Provided.• 96 well flat-bottom plate –It is recommended to useblack plates with clear bottoms for fluorescence assays and clear plates for colorimetric assays.• Fluorescence or spectrophotometric multiwell platereaderPrecautions and DisclaimerThis product is for R&D use only, not for drug,household, or other uses. Please consult the Material Safety Data Sheet for information regarding hazards and safe handling practices.Preparation InstructionsBriefly centrifuge vials before opening. Use ultrapure water for the preparation of reagents. To maintain reagent integrity, avoid repeated freeze/thaw cycles.PC Assay Buffer –Allow buffer to come to roomtemperature before use.Fluorescent Peroxidase Substrate –Thaw at roomtemperature to melt the solution prior to use.Aliquot and store protected from light and moisture at –20 °C. Upon thawing, the Fluorescent Peroxidase Substrate is ready-to-use in the colorimetric assay.For the fluorescence assay, dilute an aliquot of the Fluorescent Peroxidase Substrate 5 to 10-fold with PC Assay Buffer, just prior to use. This will reduce the background of the fluorescence assay.PC Hydrolysis Enzyme and PC Development Mix –Reconstitute each with 220 µL of PC Assay Buffer. Mix well by pipetting, then aliquot and store,protected from light, at –20 °C. Use within 2 months of reconstitution and keep cold while in use.PC Standard –Reconstitute in 200 µL of water to0generate a 50 mM (50 nmole/µL) PC Standard solution. Mix well by pipetting until it forms a uniform suspension, then aliquot and store at –20 °C. Use within 2 months of reconstitution and keep cold while in use.Storage/StabilityThe kit is shipped on wet ice. Storage at –20 °C, protected from light, is recommended.2ProcedureAll samples and standards should be run in duplicate. PC Standards for Colorimetric DetectionDilute 10 µL of the 50 mM (50 nmole/µL) PC Standard Solution with 990 µL of water to prepare a 0.5 mM (0.5 nmole/µL) standard solution. Add 0, 2, 4, 6, 8,10 µL of the 0.5 mM PC standard solution into a 96 well plate, generating 0 (blank), 1, 2, 3, 4, and 5 nmole/well standards. Add PC Assay Buffer to each well to bring the volume to 50 µL.PC Standards for Fluorometric DetectionDilute 10 µL of the 50 mM (50 nmole/µL) PC Standard Solution with 990 µL of water to prepare a 0.5 mM (0.5 nmole/µL) standard solution.Dilute 10 µL of the 0.5 mM standard solution with 90 µL of water to generate a 0.05 mM (0.05 nmole/µL). Add 0, 2, 4, 6, 8, 10 µL of the 0.05 mM PC standard solution into a96 well plate, generating 0 (blank), 0.1, 0.2, 0.3, 0.4, and 0.5 nmole/well standards. Add PC Assay Buffer to each well to bring the volume to 50 µL.Sample PreparationTissue (10 mg) or cells (2 ×106) should be rapidly homogenized in 4 volumes of cold PC Assay buffer. Centrifuge at 13,000 ×g for 10 minutes at 4 °C to remove insoluble material.Serum samples may be assayed directly.Bring samples to a final volume of50 µL with PC Assay Buffer.For unknown samples, it is suggested to test several sample dilutions to ensure the readings are within the linear range of the standard curve.Choline present in the sample can generate background. To control for choline background, include a blank sample for each sample by omitting the PC Hydrolysis Enzyme in the Reaction Mix. Assay Reaction1.Set up the Master Reaction Mixes according to thescheme in Table 1. 50 µL of the appropriate Master Reaction Mix is required for each reaction (well). Table 1.Reaction Mixes2.Add 50 µL of the appropriate Reaction Mix to eachof the wells. Mix well using a horizontal shaker orby pipetting, and incubate the reaction for30 minutes at room temperature. Protect the platefrom light during the incubation.3.For colorimetric assays, measure the absorbanceat 570 nm (A570). For fluorometric assays, measure fluorescence intensity (λex= 535/λem= 587 nm).3 ResultsCalculationsThe background for the assays is the value obtained for the 0 (blank) PC Standard. Correct for the background by subtracting the 0 (blank) value from all readings. Background values can be significant and must be subtracted from all readings. Use the values obtained from the appropriate PC standards to plot a standard curve.Note: A new standard curve must be set up each time the assay is run.Subtract the blank sample value from the sample reading to obtain the corrected measurement. Using the corrected measurement, the amount of PC present in the sample may be determined from the standard curve Concentration of PCS a/S v= CS a= Amount of PC in unknown sample (nmole) from standard curveS v= Sample volume (µL) added into the wellsC = Concentration of PC in samplePC molecular weight: 768 g/moleSample CalculationAmount of PC (S a) = 5.84 nmole(from standard curve)Sample volume (S v) = 50 µLConcentration of PC in sample5.84 nmole/50 µL = 0.1168 nmole/µL0.1168 nmole/µL ×768 ng/nmole= 89.7 ng/µL4LS,MAM 09/12-1©2012 Sigma-Aldrich Co. LLC. All rights reserved. SIGMA-ALDRICH is a trademark of Sigma-Aldrich Co. LLC, registered in the US and other countries. Sigma brand products are sold through Sigma-Aldrich, Inc. Purchaser must determine the suitability of the product(s) for their particular use. Additional terms and conditions may apply. Please see product information on the Sigma-Aldrich website at and/or on the reverse side of the invoice or packing slip.。
卡达尔健康肾上腺素产品说明书
R e a g e n t sALT/GPT ReagentTHERMO SCIEnTIFIC - AlT is found primarily in the liver and kidneys . Increased levels of AlT are generally a result of liver disease associated with some degree of hepatic necrosis such as cirrhosis, carcinoma, viral or toxic hepatitis and obstructive jaundice . Slight to moderate elevations have been observed after administration of medications such as nSAIDS, antibiotics, antiepileptic drugs, statins or opiates .ūIntended for the in vitro quantitative determination of Alanine Aminotransferase in serumūReagent is suitable for manual use or on automated clinical chemistry analyzersūApplications for a wide range of automated analyzers are available on requestSpecifications:linearity . . . . . . . . . . . . . . . . . . . . . . .0 - 450 U/l (TR71121)Stability . . . . . . . . . . . . . . . . . . . . . . .12 Stable liquid (TR71121)Cat. No.Mfr. No.DescriptionQty.B5358-178TR71121Infinity AlT/GPT 2 x 125ml2/eaAmmonia ReagentTHERMO SCIEnTIFIC - Excess ammonia exerts toxic effects on the central nervous system . Elevated levels of ammonia may be either due to inborn errors of metabolism or secondary to other conditions . Elevated ammonia may be observed in advanced liver disease caused by Reye’s syndrome, viral hepatitis or cirrhosis .Cat. No.Mfr. No.DescriptionQty.B5358-170TR60101Ammonia 2 x 28ml2/eaAST/GOT ReagentTHERMO SCIEnTIFIC - AST is widely distributed with high concentrations in the heart, liver, skeletal muscle and kidney . Damage or disease to any of these tissues such as myocardial infarction, viral hepatitis, liver necrosis, cirrhosis and muscular dystrophy may result in raised serum levels of AST .ūIntended for the in vitro quantitative determination of aspartate aminotransferase in serum or plasmaūReagent is suitable for manual use or on automated clinical chemistry analyzersūApplications for a wide range of automated analyzers are available on requestSpecifications:linearity . . . . . . . . . . . . . . . . . . . . . . .0 - 450 U/lStability . . . . . . . . . . . . . . . . . . . . . . .Stable liquid (TR70121)Cat. No.Mfr. No.DescriptionQty.B5358-175TR70121Infinity AST/GOT 2 x 125ml2/eaCalcium ReagentTHERMO SCIEnTIFIC - Thermo Scientific Calcium Reagent is intended for the in vitro quantitative determination of calcium in serum or plasma .Cat. No.Mfr. No.DescriptionQty.–1265-250Infinity Calcium Arsenazo 2 x 125ml2/ea– To order, call your customer service representative and provide them with Mfr. No.。
3. 氘代试剂相关知识介绍

d5-Pyridine: 文献上一些天然产物用此试剂检测 (三萜皂苷, 甾体皂苷)
DCl: 中性化合物不溶, 改成盐酸盐后才溶解. (杀星)
价格
CDCl3 d6-DMSO D2O
(元/样品) 2
10 4
d6-Acetone
10
CD3OD
30
氘代试剂的污染防范?
因为样品量大, 细微杂质感觉不出.
平时可以不必太在意, 多半没感觉. 样品本身的杂质比溶剂多.
若检测极微量极纯品, 略注意污染细节. 绝大多数东西都会污染氘代氯仿: 出现 0.8 / 1.2 ppm 峰 -- 塑料的增塑剂. 常见的污染峰. -- 塑料滴管, 核磁帽, 手指, 棉花, 白纸, 橡皮管... 不污染: 玻璃, 四氟乙烯制品 -- 进行空白实验数据
结论: 对于含水量 0.01% 的氘代氯仿, 将氯仿峰积分定为 10, 则水峰的积分为 6.69. 若水峰的积分小于 6.69, 表示含水量 < 0.01%.
氘代试剂的氘代度? 99.8%
定义: 氘代氯仿氘代度 99.8%, 表 1000 个粒子, 有 998 个 CDCl3, 有 2 个CHCl3
若样品珍贵泼在大蒸发皿几天后得固体样品若仍未全干加石油醚曾有老师将样品收集在瓶中满数百ml蒸馏回收但费时建议不考虑
中仪标化
核磁共振培训
氘代试剂 相关知识介绍
林崇熙 2014.11
北京大学化学学院
常见的氘代试剂? 氘代试剂种类几十种, 常用的十几种 选择氘代试剂的考虑? 价格, 溶解度, 沸点, 特殊性质
60 MHz NMR: 1%
毛细管内标: 化学位移或需要校正, 不很鼓励.
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :A-317491 (sodium salt hydrate)Catalog No. :HY-15568ACAS No. :None1.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:A 317491; A317491;(S)⁻5⁻((3⁻phenoxybenzyl)(1,2,3,4⁻tetrahydronaphthalen⁻1⁻yl)carbamoyl)benzene⁻1,2,4⁻tricarboxylic acid, sodium salt hydrateFormula:C33H29NNaO9+Molecular Weight:606.57CAS No. :None4. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
