14 group VIIA
Periodic Table 元素周期表(相对分子量)
Pu
Plutonium
Am
Americium
Cm
Curium
Bk
Berkelium
Cf
Californium
Es
Einsteinium
Fm
Fermium
Md
Mendelevium
No
Nobelium
Lr
Lawrencium
Chemical symbol Ac Ag Al Am Ar As At Au B Ba Be Bh Bi Bk Br C Ca Cd Ce Cf Cl Cm Co Cr Cs Cu Db Ds Dy Er Es Eu F Fe Fm Fr Ga Gd Ge H He Hf Hg Ho Hs I In Ir K Kr La Li Lr Lu Md Mg
Be
Beryllium 12 24.31
H
Hydrogen Atomic Name
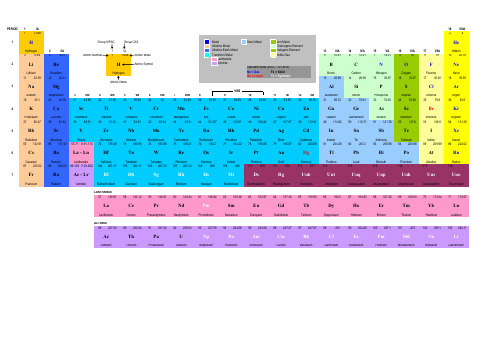
Metal Alkaline Metal Alkaline Earth Metal Transition Metal Lanthanide Actinide
Semi Metal
Non Metal Chalcogens Element Halogens Element Noble Gas
He
13 5 IIIA 10.81 14 6 IVA 12.01 15 7 VA 14.01 16 8 VIA 16 17 9 VIIA 19 10 Helium 20.18
Standard State (25oC - 101 kPA) Ne = Gas Fe = Solid Ga = Liquid Tc = Synthetic
91
231.04
92
periodic-table
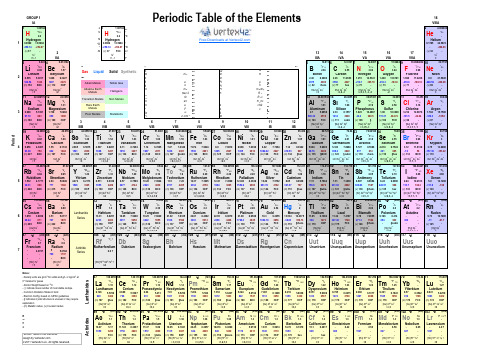
Atomic Number†Atomic WeightSymbol†Ground-State Level*Electronegativity (Pauling)Name*Density [Note]†Ionization Energy (eV)*Melting Point (°C)*Boiling Point (°C)Atomic radius (pm)[Note]Crystal Structure [Note]†Electron ConfigurationPossible Oxidation States [Note]Phase at STP†Common ConstantsSource: Absolute Zero -273.15 °CGravitation Constant 6.67428x10-11 m 3 kg -1 s -2Atomic Mass Unit 1.660539x10-27kg Molar Gas Constant8.314472 J mol -1 K -1Categories Avogadro Constant6.022142x1023 mol -1Molar Volume (Ideal Gas)0.02241410 m 3/mol Base of Natural Logarithms 2.718281828PI3.14159265358979Boltzmann constant 1.380650x10-23 J/K Planck Constant6.626069x10-34 J s Electron Mass9.10938215x10-31 kg Proton-Electron Mass Ratio 1836.152672470.5110 MeV Rydberg Constant10 973 732 m -1Electron Radius (Classical) 2.8179403x10-15 m 3.289842x1015 Hz Electron Volt 1.602176x10-19 J 13.6057 eV Elementry Charge 1.602176x10-19 C Second Radiation Constant 0.01438769 m K Faraday Constant 96 485.3399 C/mol Speed of Light in a Vacuum 299 792 458 m/s fine-structure constant 0.0072973525Speed of sound in air at STP 343.2 m/s [42]First Radiation Constant3.7417749x10-16 W m2Standard Pressure101 325 Pa{42}References:†, * (Mathematic),CRC Handbook of Chemistry and Physics 81st Edition, 2000-2001, and others18VIIIA Notes:- Density units are g/cm 3 for solids and g/L or kg/cm 3 at 0° Celsius for gases- Atomic Weight based on 12C- ( ) indicate mass number of most stable isotope - Common Oxidation States in bold- Electron Config. based on IUPAC guidelines- § indicates crystal structure is unusual or may require explanation- (m) Metallic radius, (v) Covalent radius © 2011 Vertex42 LLC. All rights reserved.Design by CnPeriodic Table of the Elements DbSg(v) 37-+1,-1LiPeriodic Table of the ElementsGROUP 1IA 1 1.0079424.00260216VIAFree Downloads at -1s 11s 11s 217VIIA0.178524.5874-259.14P e r i o d111.007940.089913.5984He1S 02.22.2-H2S 1/2H2S 1/2HydrogenHydrogenHelium2IIA 0.089913.5984(v) 37FCC +1,-1-268.93-259.14-252.87-(v) 3213IIIA 14IVA15VA-252.8709.012182510.8116236.941415.9994918.99840321012.0107714.0067820.17972S 1/2Be1S 0BG 2P°1/2C3P 0N3P 2F3.043.442.042.554S°3/2O2P°3/2Ne1S 00.981.57Gas Liquid Solid Synthetic3.98-LithiumBerylliumm u R BoronCarbonNitrogenOxygenFluorineNeon9.3227 2.468.2980 2.26p 207513.6181 1.69617.42280.911.2603 1.25114.5341 1.4294027-210.121.5645180.54134212872470Alkali Metals Noble Gas e -246.08(m) 152BCC(m) 112HCPk h (v) 82-195.79-218.3rhom.(v) 77hex(v) 75-188.12-248.59-182.9-219.640003550 -0.535 5.3917 1.848[He] 2s 1[He] 2s 2Alkaline EarthMetalsHalogens m e m e /m p [He] 2s 2 2p 1-(v) 73[He] 2s 2 2p 2[He] 2s 2 2p 3[He] 2s 2 2p 4[He] 2s 2 2p 5-(v) 69-(v) 71[He] 2s 2 2p 6+1+2me c 2R ∞+3+2,4,-4+2,3,4,5,-2,-3-2-1031122.9897701224.3050Transition Metals Non Metalsr0R ∞c 1839.9481530.973611632.065Na2S 1/2Mg1S 01735.4531326.9815381428.0855Si3P 0P4S°3/2eV R ∞hc Al2P°3/22.583.16Ar1S 00.93 1.31Rare Earth Metalse ch/k 1.611.902.19-SodiumMagnesiumF cAluminumSilicon PhosphorusSulfurChlorineArgon5.1391 1.7387.6462Poor Metals Metalloidsa2.710.4867 1.9610.36003.2145.9858 2.338.1517 1.8232P°1/2S3P 2Cl11IB 12IIB (m) 1436VIB 7VIIB 8VIII 9VIII §(v) 102FCO12.9676 1.78415.759697.7288365010902p hc 2660.32280.5115.21444.72-101.525191414290044.2-34.04-189.3-185.80.968(v) 99FCC(v) 111cubic(v) 106-(v) 97-[Ne] 3s 1[Ne] 3s 2[Ne] 3s 2 3p 1[Ne] 3s 2 3p 2[Ne] 3s 2 3p 3[Ne] 3s 2 3p 4[Ne] 3s 2 3p 5[Ne] 3s 2 3p 6+1+2+3+2,4,-4+3,4,5,-3+2,4,6,-2+1,3,5,7,-1010VIII (m) 186BCC(m) 160HCP3IIIB 4IVB 5VB 41939.09832063.3875984240.078K2S 1/22350.94152451.99612144.9559102247.8674.507 6.82810.856 4.3407 1.55 6.1132 2.985 6.5615 6.11 6.74627.14 6.7665+2,3,6Ni3F 4Cu1.881.912S 1/2Zn1S 02758.9332002858.69342554.9380492655.845312963.5463065.409Ti3F 2V4F 3/2Ca1S 0Sc2D 3/2Fe5D 4Co1.83Cr7S 3Mn6S 5/24F 9/2Ge3P 0As1.812.014S°3/2Se3P 2Br2.182.552P°3/2Kr79.9043683.7983374.921603478.9669.7233272.64351S 00.82 1.00 1.361.54 1.631.66 1.552.963PotassiumCalciumScandiumTitaniumVanadiumChromiumManganeseIronGalliumGermaniumArsenicSeleniumCobaltNickelCopperZincBromineKryptonGa1.901.652P°1/28.97.88108.9087.63987.477.43407.8747.9024 5.904 5.9993 5.3237.89948.927.72647.149.3942 3.1211.8138 3.7513.99965.7279.7886 4.8199.7524328719103407190714841541283016682861149529271455267112462061153890729.762204938.329131084.622927419.53685-7.359-157.362820817614221-153.22(m) 227BCC(m) 197FCC(m) 162HCP(m) 147HCP(m) 134§cubic(m) 126BCC(m) 125BCC(m) 128BCC(m) 127FCC(m) 134§hex(m) 135HCP(m) 124FCC(m) 128rhom.(v) 116§hex(v) 114§BCO(v) 122§cubic(v) 119BCO(v) 110-[Ar] 4s 1[Ar] 4s 2[Ar] 3d 1 4s 2[Ar] 3d 2 4s 2[Ar] 3d 3 4s 2[Ar] 3d 5 4s 1[Ar] 3d 5 4s 2+2,3,4,6,7[Ar] 3d 10 4s 2[Ar] 3d 10 4s 2 4p 1[Ar] 3d 10 4s 2 4p 2[Ar] 3d 10 4s 2 4p 3[Ar] 3d 6 4s 2[Ar] 3d 7 4s 2[Ar] 3d 8 4s 2[Ar] 3d 10 4s 1+2,3+1,2[Ar] 3d 10 4s 2 4p 4[Ar] 3d 10 4s 2 4p 5[Ar] 3d 10 4s 2 4p 6+1+2+3+2,3,4+2,3,4,5053785.46783887.623988.90585+2+34091.2244192.90638+2,4,6,-2+1,5,-1+2,4+3,5,-3+2,3+2,344101.0745102.905504295.9443(98)48112.41149114.8184654131.293Rb2S 1/2Sr1S 0Y2D 3/2Zr3F 2Nb6D 1/2Mo7S 31.602.16Tc6S 5/2Ru5F 51.91S 01.931.69In2P°1/2Sn3P 01.781.96106.4247107.868252127.6053126.9044750118.71051121.760Sb4S°3/2Te3P 22.052.10I2P°3/2Xe1S 02.662.60RubidiumStrontiumYttriumZirconium0.820.95 1.221.33IodineXenon2.20Rh4F 9/2Pd1S 02.282.20Ag2S 1/2CdIndiumTinAntimonyTelluriumRhodiumPalladiumSilverCadmium1.532 4.17712.63 5.6949 4.472 6.21738.57 6.758910.287.0924NiobiumMolybdenumTechnetiumRuthenium12.457.458912.0238.336911.57.2812.377.36057.31 5.78647.317.343910.497.57628.658.9938 4.9410.4513 5.912.12986.6978.6084 6.249.00961526334518554409-111.8-108630.631587449.519886.511 6.633939.31688777138221574265233441502477474426234639961.782162321.07767196436951554.92963113.7184.3156.62072231.932602(m) 146BCC(m) 139BCC(m) 180HCP(m) 160HCP(m) 134FCC(m) 137FCC(m) 136HCP(m) 134HCP(m) 167§tetra.(v) 141§tetra.(m) 144FCC(m) 151§hex(v) 133BCO(v) 130-(v) 138§rhom.(v) 135hex[Kr] 4d 4 5s 1[Kr] 4d 5 5s 1[Kr] 4d 5 5s 2[Kr] 4d 7 5s 1[Kr] 5s 1[Kr] 5s 2[Kr] 4d 1 5s 2[Kr] 4d 2 5s 2[Kr] 4d 10 5s 2 5p 1[Kr] 4d 10 5s 2 5p 2[Kr] 4d 10 5s 2 5p 3[Kr] 4d 10 5s 2 5p 4[Kr] 4d 8 5s 1[Kr] 4d 10[Kr] 4d 10 5s 1[Kr] 4d 10 5s 2[Kr] 4d 10 5s 2 5p 5[Kr] 4d 10 5s 2 5p 6(m) 248BCC(m) 215FCC+1+2+3+4+3,5+2,3,4,5,6+4,7+2,3,4,6,872178.49+3+2,4+3,5,-3+2,4,6,-2+2,3,4+2,4+1+283208.9803884(209)655132.9054556137.327Lanthanide Series73180.947974183.84+1,5,7,-1077192.21778195.07875186.20776190.2381204.383382207.279196.9665580200.5985(210)86(222)2.33 2.02Hf3F 2Ta4F 3/2Cs2S 1/2Ba1S 0Os5D 4Ir4F 9/2W5D 0Re6S 5/2PtPo3P 2At2P°3/22.0 2.2Rn1S 00.790.89 1.3 1.52.36 1.92.2 2.2-3D 3Au2S 1/22.28 2.54Hg1S 0Tl2P°1/221.62Pb3P 0Bi4S°3/2CesiumBariumHafniumTantalumTungstenRheniumOsmiumIridiumPlatinumBismuthPoloniumAstatineRadonGoldMercuryThalliumLead13.31 6.825116.657.54961.879 3.8939 3.51 5.211722.618.438222.658.967019.257.864021.027.833513.53410.437511.85 6.108221.098.958819.39.22559.1968.414--11.347.41679.787.28559.7310.748528.446717271870223346033017545830335012246644283422555531865596-38.83356.7330414731768.338251064.182856254962302-327.461749271.31564-71-61.7(m) 265BCC(m) 222BCC(m) 159HCP(m) 146BCC(m) 135HCP(m) 136FCC(m) 139BCC(m) 137HCP(m) 151§rhom.(m) 170HCP(m) 139FCC(m) 144FCC-§cubic--(m) 175FCC(v) 146§rhom.(v) 145-[Xe] 6s 1[Xe] 6s 2[Xe] 4f 14 5d 2 6s 2[Xe] 4f 14 5d 3 6s 2[Xe] 4f 14 5d 4 6s 2[Xe] 4f 14 5d 5 6s 2[Xe] 4f 14 5d 6 6s 2[Xe] 4f 14 5d 7 6s 2[Hg] 6p 2[Hg] 6p 3[Hg] 6p 4[Hg] 6p 5[Xe] 4f 14 5d 9 6s 1[Xe] 4f 14 5d 10 6s 1[Xe] 4f 14 5d 10 6s 2[Hg] 6p 1[Hg] 6p 6+1+2+4+5+2,3,4,5,6+2,4,6,7,-1+2,3,4,6,8+2,3,4,6+2,4+3,5+2,4+1,3,5,7,-1+1,3+1,2+1,3+2,4787(223)88Fr2S 1/2Francium(226)Actinide Series104(261)Ra1S 0Rf3F 2 ?RadiumRutherfordium- 4.07275 5.2784 6.0 ?+1+2+4107(264)108(277)105(262)106(266)111(272)112(285)109(268)110(281)118115116(292)HsMt117113114(289)UuhUusUutUuq UupDsRg DubniumSeaborgiumBohriumHassiumUuo0.70.9Ununtrium Ununquadium UnunpentiumUnunhexiumMeitnerium Darmstadtium RoentgeniumCoperniciumUnunseptiumUnunoctiumBh--7001737---BCC[Rn] 7s 1[Rn] 7s 2[Rn] 5f 14 6d 2 7s 2 ?59140.9076560144.2461(145)162.50067164.9303270173.0471174.96768167.25969168.9342157138.9055La2D 3/21.10Lanthanum62150.3663151.964A c t i n i d e s8958140.116Cerium L a n t h a n i d e s6664157.2565158.92534Ce1G°4Pr4I°9/21.121.13Nd 5I 4Pm6H°5/21.14-Sm7F 0Eu8S°7/21.17-Gd9D°2Tb6H°15/21.20-Dy5I 8Ho4I°15/21.221.23Er3H 6Tm2F°7/21.241.25Yb1S 0Lu2D 3/2-1.27ThuliumYtterbiumEuropiumGadoliniumTerbiumDysprosiumHolmiumErbiumPraseodymium NeodymiumPromethiumSamarium5.5827.353 5.6437 5.244Lutetium6.146 5.5769 6.689 5.5387 6.64 5.86388.551 5.93898.7955.67047.901 6.14988.219 6.1843 6.57 6.25429.8416.02159.066 6.10779.321 5.425992034647983360931329010213100110015271313325013563000107218038221196166327001497286815455.4737.01 5.52507.264§hex (m) 181§hex (m) 18319508193230141225671474HCP (m) 180§hex (m) 1803402(m) 187§hex (m) 182FCC (m) 182HCP (m) 178HCP (m) 176BCC (m) 180HCP (m) 177HCP (m) 176FCC (m) 174HCP (m) 176HCP (m) 176HCP [Xe] 5d 1 6s 2[Xe] 4f 1 5d 1 6s 2[Xe] 4f 3 6s 2[Xe] 4f 4 6s 2[Xe] 4f 5 6s 2[Xe] 4f 6 6s 2[Xe] 4f 7 6s 2[Xe] 4f 7 5d 1 6s 2[Xe] 4f 9 6s 2+2,3+3[Xe] 4f 10 6s 2[Xe] 4f 11 6s 2[Xe] 4f 12 6s 2[Xe] 4f 13 6s 2+3+3,4+3,4+3+3+2,3+3,4+3+3+3[Xe] 4f 14 6s 2[Xe] 4f 14 5d 1 6s 2+2,3+2,3+3(227)90232.038191231.035992238.028995(243)96(247)93(237)94(244)103(262)U5L°6101(258)102(259)99(252)100(257)Ac2D 3/2Th3F 2Pa4K 11/2Np6L 11/2Pu7F 097(247)98(251)FmBk6H°15/2Cf5I 8Am8S°7/2Cm9D°21S 0Lr1.31.33H 6Md1.31.281.31.31.32F°7/2No1.3Es1.34I°15/2- 5.973813.51 5.991420.45 6.265719.816 6.026011353927PlutoniumAmericiumCuriumBerkelium2P°1/2 ?1.11.31.51.381.36CaliforniumEinsteiniumFermiumMendelevium-ActiniumThorium ProtactiniumUraniumNeptuniumNobelium Lawrencium1050320017504820157240006444000640323010.07 5.1711.724 6.306715.37 5.8919.05 6.1941- 4.9 ?14.78 6.197915.1 6.28171050-900-1176201113453110827-827-860-1527-1627-- 6.58- 6.65- 6.42- 6.50--[Rn] 6d 1 7s 2[Rn] 6d 2 7s 2[Rn] 5f 2 6d 1 7s 2[Rn] 5f 3 6d 1 7s 2[Rn] 5f 4 6d 1 7s 2[Rn] 5f 6 7s 2[Rn] 5f 9 7s 2[Rn] 5f 10 7s 2[Rn] 5f 11 7s 2[Rn] 5f 12 7s 2-FCC(m) 179FCC (m) 163§tetra (m) 156BCP (m) 173HCP (m) 174HCP (m) 155SO (m) 159§mono.[Rn] 5f 7 7s 2[Rn] 5f 7 6d 7s 2--(m) 170hex -hex [Rn] 5f 13 7s 2[Rn] 5f 14 7s 2[Rn] 5f 14 7s 2 7p ?+3+4+4,5+3,4,5,6+3,4,5,6+3,4,5,6+3,4,5,6+3+2,3+2,3+3+3,4+3+3+3------。
化学专业英语 马永祥 兰州大学--翻译

1. The Elements and The Periodic Table元素和周期表The number of protons in the nucleus of an atom is referred to as the atomic number, or proton number, Z. The numbers of electrons in an electrically neutral atom is also equal to the atomic number, Z. The total mass of an atom is determined very nearly by the total number of protons and neutrons in its nucleus. This total is called the mass of the number, A. The number of neutrons in an atom, the neutron number, is given by the quantity A-Z.refer to sb. [sth.] as 称某人(物)为be determined by 由…确定原子核中质子的数目称为原子序数,或者质子数,以Z表示。
电中性原子中电子的数目也等于原子序数Z。
经测定,原子的总质量与原子核中质子与中子的总数差不多。
(几乎相同)(或者说原子的总质量几乎可以由原子核中质子与中子的总数确定。
)这个总数叫质量数,以A表示。
因此,原子中的质子的数目,质子数,可以定量地由A-Z给出。
即原子中质子数=A-ZThe term element refers to a pure substance with atoms all kinds of a single kind. To the chemist the “kind” of an atom is specified by its atomic number, since this is the property that determines its chemical behavior. At present all the atoms from Z=1 to Z=107 are known; there are 107 chemical elements. Each chemical element has been given a name and a distinctive symbol. For most elements the symbol is simply the abbreviated form of the English name consisting of one or two letters,for example:元素这个术语指的是仅仅由同一种类的原子组成的物质。
卤素规范SGS

Restricted Substance Testing Service國際無鹵(Halogen Free)規範及趨勢介紹2007/11/28ShinJyh,Chen陳新智RSTS E&E Technical CenterMulti-chemical Lab,UTIS Lab,Taipei,Taiwan綜合化學實驗室,超微量工業安全實驗室簡報大綱⏹⏹⏹1886⏹氟是一種極具腐蝕性的淡黃色雙原子氣體素,也是很強的氧化劑。
在常溫下,它幾乎能和所有的元素化合,並產生大量的熱能。
⏹1774⏹具有強氧化性,所以可以作為一種廉價的消毒劑,一般的自來水就採用它來消毒,同時氯氣也可用於紙漿和棉布的漂白⏹1824年法國⏹溴是唯一在室溫下為液體的非金屬元素,紅棕色蒸氣⏹常溫下為紅色發煙液體,化學性質較碘活潑,但不比氯活潑,能與許⏹1811⏹碘在常溫下是紫色的固體。
⏹碘的用途⏹砈像金屬,活性較碘低,固態,具放射性⏹1940年初次被合成的,可用α粒子轟擊鉍地衰變成⏹prEN14582Oxygenmethod⏹IEC61189-2Test methodsinterconnection鹵素含量測試方法比較法規編號prEN14582Method.ABS EN14582prEN14582Method.BASTM E442-91鹵素測試方法⏹IEC61189-2法主要針對⏹BS EN樣品燃燒/鹼液吸收HBr、HCl鹼液吸收IC儀器分析數據審核正式報告Optional產品應用及規範要求⏹IECReinforced and unclad woven laminated sheetsIPCadopted halogen-freeOther⏹acceptable⏹A testPBDEOther⏹All partsrequirements.⏹Homogenous揮發性有機化合物∙溶劑/助劑∙表面清洗劑∙冷煤–半揮發性有機化合物∙含溴化合物,作為––⏹含溴/氯化合物電子廢料、塑膠產品主要添加物質⏹燃燒時亦會產生酸性氣體HeatFuelAirFireH e a t t r a n s f e rMixing of fuel and airH ea t t r an sf e rF l am eR e t a r d a n tF l a m eR e t a rd a n t阻燃劑分類⏹無機類⏹含溴有機類常見耐燃劑種類DecaBDE十溴聯苯醚HBCD六溴環十二烷TPP磷酸三苯酯TribromoPhenol三溴酚無機類地區阻燃劑阻燃劑產品及應用Reactive Bisphenol ATypeBFR productgroup含鹵化合物使用範圍鹵素項目阻燃劑產品及應用Substance阻燃劑產品及應用Substance四溴雙酚Tetrabromobisphenol-A⏹TBBPA阻燃劑主要用於電路板⏹主要為反應型阻燃劑,因低成本、與電路板相關零件相容性佳,因而電子電機類產品禁用No.EU directive176/769/EEC電子電機類產品禁用No.1無鹵素材料對產業影響⏹傳統PCB⏹銅箔基板廠商主打完全無鹵素的環保材料,主要將鹵素系難燃無鹵替代性材料使用⏹FR4(epoxyresin/glass∙製程參數改變Information阻燃劑種類⏹不含溴(Non-Brominated ⏹絕大部分的對無鹵規範之因應建立規範•了解買家無鹵規範•訂定產品規範/因應時程Halogen Free 管控系統替代材料•功能性考量•成本考量產品了解•了解鹵素存在範圍•測試計劃Halogen Free無鹵產品管控系統。
材料工程专业英语2原子结构Atomic Structure and Interatomic Bonding

range) below the micrograph.
Why study atomic structure and interatomic
bonding?
An important reason to have an understanding of interatomic bonding in solids is that, in some instances, the type of bond allows us to explain a material’s properties. For example, consider carbon, which may exist as both graphite and diamond. Whereas graphite is relatively soft and has a ‘‘greasy’’ feel to it, diamond is the hardest known material. This dramatic ( significant 显著的) disparity (difference差异) in properties is directly attributable to (归因于) a type of interatomic bonding found in graphite that does not exist in diamond (see Section 3.9).
What should you be able to do after studying this chapter?
Name and explain the primary or chemical bond found in solids.
化学专业英语元素周期表

P-block Element
IIIA B Al Ga In Tl IV A Boron C Carbon Aluminium Si Silicon Gallium Ge Germanium Indium Sn Tin Thallium Pb Lead VIIA Oxygen Sulfur Selenium Tellurium Polonium F Fluorine Cl Chlorine Br Bromine I Iodine At Astatine VA N P As Sb Bi Nitrogen Phosphorus Arsenic Antimony Bismuth
Element Groups (Families) Alkali metal Rare Earth Alkaline Earth Transition Metals Other Metals Metalloids类金属 Noble Gases [′metəlɔid]
6
Non-Metals
Halogens卤素
16
For most elements the symbol is simply, the abbreviated form of the English name consisting of one or two letters。 对大部分元素来说,这个含有一个或两个字母的符 号仅仅是英文名字的缩写形式。 Beginning in the late seventeenth century with the work of Robert Boyle, who proposed the presently accepted concept of an element, numerous investigations produced a considerable knowledge of the properties of elements and their compounds. 早在17世纪末期,罗伯特·波义耳就开始了这项工 作,他提出了现在公认的元素概念,大量的研究使 我们对元素及其化合物的性质有了相当的了解。 17
完整版)化学专业英语

完整版)化学专业英语Teaching Material for Scientific EnglishI。
Naming of XXX1.Naming of XXXThe English word for both "元素" and "单质" is "element"。
To distinguish een the two。
"free element" may be used when emphasizing "单质"。
Therefore。
the English names for XXX are the same。
The following are the names of elements that are also names of free elements:Group IA:XXXXXXSodiumGroup IIA:XXXMagnesiumGroup IIIA: Boron AluminumGroup IVA: Carbon Silicon GermaniumGroup VA: Nitrogen PhosphorusGroup VIA: Oxygen Sulfur XXXXXXPoloniumGroup VIIA: Fluorine Chlorine Bromine IodineXXXGroup 0: XXXNeon ArgonXXX Xenon RadonGroup IA: Potassium CalciumGroup IIA:RubidiumCesiumFranciumGroup IIIA:GalliumIndiumXXXGroup IVA:ArsenicXXXXXXXXXLead2.Naming of CompoundsCompounds are named from left to right according to their chemical formula。
第11章 第VIIA族428

第十一章第VIIA族,卤素 Group 7, the halogens第一节卤素 The halogens学习目标 Learning objectives:∙元素周期表中第VIIA族元素的原子半径有何变化趋势,存在这种趋势的原因?∙元素周期表中第VIIA族元素的电负性有何变化趋势,存在这种趋势的原因?大纲参考:3.2.5第VIIA族元素位于元素周期表的右手边,由非金属元素组成。
第VIIA族单质是双原子分子,包括 F2、Cl2、Br2、I2和At2,这些单质称为卤素。
物理性质 Physical properties气态卤素外观差别很大,如图1所示。
室温条件下,氟气是淡黄色气体,氯气是黄绿色气体,溴气是红棕色液体,而碘是黑色固体。
随着卤素在元素周期表内排列位置越靠下,卤素颜色越来越深、越来越浓。
卤素都有一种“室内游泳池”的气味。
氟气的物理性质没有代表性,原因在于与其他卤素单质内部化学键力量强度相比,F-F单键之间的力量非常弱。
氟原子很小,造成非成键的电子之间存在排斥力,因为这些电子之间靠得太近:Repulsion 排斥力氟气、氯气、溴气和碘的物理性质如表2所示。
提示Hint卤素这个词指的是形成盐的物质。
卤素能与多种金属发生反应,形成氟化物、氯化物、溴化物和碘盐。
图1气体卤素,依次排列为:氟气、氯气、溴气和碘。
表1氟气、氯气、溴气和碘的键能表中的红色箭头指示了这些变化趋势很清晰,可解释如下:原子的大小Size of atoms随着卤素在元素周期表内排列位置越靠下,卤素原子越大,原因在于与上一种元素相比,每种元素都多一层电子,如表2所示。
表2 第VIIA族元素氟气-碘的物理性质Chlorine 氯 bromine 溴 iodine碘图2 随着卤素在周期表内的位置越靠下,外壳距离原子核越远。
电负性 Electronegativity电负性可衡量原子吸引电子的能力,也可以称为电子密度,是指在一个共价键内吸引电子朝向自身原子的能力(如章节3.3所示)。
初中全英文授课资料 bonds

Molar masses with empirical formulas --> chemical formula
Expressing chemical equations
Stoichiometric calculations
Limiting Reactant : determines amount of product formed Theoretical yields vs actual yields
Chemical Bonding
THE PERIODIC TABLE By the late 1800’s it was realized that elements could be grouped by similar chemical properties and that the chemical and physical properties of elements are periodic functions of their atomic numbers – PERIODIC LAW. The arrangements of the elements in order of increasing atomic number, with elements having similar properties placed in a vertical column, is called the PERIODIC TABLE.
Columns are called GROUPS (FAMILIES) and rows are called PERIODS.
Use as a chemical compound and those ion conductor

专利名称:Use as a chemical compound and those ion conductors which were induced fromLa2Mo2O9发明人:フランソワ グーテノワール,フィリップ ラコル申请号:JP特願2001-575504(P2001-575504)申请日:20010405公开号:JP特表2003-530291(P2003-530291A)A公开日:20031014专利内容由知识产权出版社提供摘要:The invention concerns novel compounds derived from La2Mo2O9 and their use as ion conductors. The compounds of the invention have formula (1): A2-x A'x B2-y B'y O9-z+delta X z in which A is at least one trivalent element selected from trivalent rare earths, trivalent bismuth, trivalent antimony and trivalent arsenic; A' is at least one monovalent element selected from alkalis; or a divalent element selected from alkaline-earths, tin, lead, samarium, europium, erbium, thulium and ytterbium; or a quadrivalent element selected from thorium, uranium, group IVA elements, cerium, praseodymium and terbium; B is at least one hexavalent element from groups VIA, VIIA, VIII and group VIB with the exception of oxygen; B' is at least one element selected from lithium, sodium, magnesium, calcium, scandium, yttrium, rare earths with atomic numbers 63 to 71, elements from groups IVA to IIB with an oxidation number of less than 6, aluminium III, silicon IV, gallium III, germanium IV, indium III, tin IV, phosphorus V, antimony V and bismuth V; X is selected from sulphur, fluorine and chlorine; where 0<=x<2; 0<=y<2;0<=z<=3; and with the condition that when A is lanthanum and B is tungsten or molybdenum, at least one of x, y or z is different from 0; the compounds having a cationiccubic or pseudo-cubic beta-SnWO4 type lattice.申请人:ロディア テレ ラレ,サントル ナスィオナル ド ラ ルシェルシュ スィアンティフィク地址:フランス国 エフ17041 ラ ロシェル、リュ シェフ ド ベ、26、ゾヌ アンデュストリエル,フランス国 エフ75016 パリ、リュ ミシェル アンジェ、3国籍:FR,FR代理人:倉内 基弘 (外1名)更多信息请下载全文后查看。
QuantStudio

READMEQuantStudio™ Real-Time PCR Software v1.3CONTENTSOVERVIEWFEATURESUPDATESFIXED DEFECTSLICENSECOMPATIBILITYONLINE HELPKNOWN ISSUESSYSTEM REQUIREMENTSINSTALL SOFTWAREUNINSTALL SOFTWAREOVERVIEWThe QuantStudio Real-Time PCR Software v1.3 supports the ViiA™ 7 Real-Time PCR System, QuantStudio™ 6 Flex and QuantStudio™ 7 Flex Real-Time PCR Systems. The QuantStudio Software allows the user to open and analyze experiments generated with the ViiA7 System, QuantStudio 6 Flex, and 7 Flex Real-Time PCR Systems. The software also enables the user to set up experiments, send experiments to the instrument, control the thermal cycling process, collect data and analyze the collected data from these platforms, all in an integrated and streamlined fashion.FEATURES∙Ability to connect to three different instrument types in the instrument console: ViiA7 Real-Time PCR System, QuantStudio 6 Flex and QuantStudio 7 Flex Real-Time PCRInstrument Systems∙Setting up experiments for both novice and advanced users∙Customizable optical filter selection for more complex applications, such as multiplexingUPDATESVersion 1.3∙Added more options to customize export in ViiA7 format (control number of rows and columns).∙Added sorting feature in well table and persist the sorting in export table and exported file.∙Allow the user re-order the column in Export table and persist the column order in exported file.∙Allow the software connect to the instrument that has firmware v1.0.3 (QS7) and v1.2.3 (ViiA7) or later.Version 1.2∙User may toggle manual analysis with F5 key or Analyze button∙Improved editing of SNP assay workflow for Genotyping experiments∙User may enable or disable the “new export set” message when opening an old experiment file∙User may open the example files and templates directly from the menu bar∙User may save the template file to a .eds file with the "Save As" button∙Allow user default the Data/Import/Export folder to the last used location∙Increased tooltip duration on plots for better user experience∙Enabled zoom in and zoom out function on Right click in Analysis Plots∙Standardized the label and format of “Quality” in Report, Well Table, and Export for Genotyping experiment∙Enabled Clipped Data in 7900 export format∙Enabled "Auto Adjust" and "Fixed Range" options for Y-axis of Amplification plot∙Enabled "Auto" and "Manual" analysis feature for Amplification plot∙The C rt (relative threshold) analysis method algorithm updated to harmonize processing workflow between our online C rt analysis module and the desktop software.Version 1.1∙Removal of BASE license requirement. SAE and HRM still require licensing∙Auto baseline will start at cycle 3 or earlier∙Improved reliability of saving a run file to a network drive∙Improved error logs for network issues∙Improved handling of temporary files and memory that may cause software to crash∙Improved file management within Windows® such that only the login user’s home directory will require read/write access∙Added Target option as filter in Amplification Curve view∙When Touchscreen is in secure mode, remove access to change time, date, network information or instrument name, firmware upgrade/downgrade as well as restore settings ∙Print report layout is improvedFIXED DEFECTSVersion 1.3∙Fixed sample name not fully displayed in report.∙Fixed Passive Reference not persists in edt file.∙Fixed open file permission.∙Fixed import of SDS/SDT file.Version 1.2Compatibility with Remote Viewing Application∙Improved WebEx and Remote Desktop display qualityInstrument Run∙Allow runs to proceed after click Start Run from template fileExperiments∙In a Genotyping experiment, the edited information in the SNP Assay will persist after reopening the fileAnalysis∙Allow user to view RQ value for all the Biological group∙Standardize the sorting method for target option and well table∙Displays correct curve and threshold line in Amplification plot for the selected target ∙Displays correct SNP Assay when moves the slider to different cycles∙Allow user opens the experiment file when the cycle number is more than 40∙Displays correct tooltip for the Amplification plot∙Persist number format for the Y-axis of Amplification plot∙Display Crt values correctly for all the targetExport∙Enable "Use Last File Location" function for the Export in Study∙Display correct calibration status in export fileE-signature∙Allow user save the e-signature and retract or signs the signature for second time∙Fixed the software hung issue when user sign the meaning of “Review and Approve Results”Version 1.1Compatibility with Automation Controller software∙User selected password can be used∙Prevent user from closing the software when Automation Controller software is runningSetup∙For Standard Curves, under Define and Set Up Standards, allow setup for multiplex targets Import∙Experiments that import 7900 setup files can be ran on instrument without errorAnalysis∙Plot settings will persist even after selecting different wellsExport∙Unselected export tabs will not auto-export∙Fixed incorrect default file name of export∙Fixed Quick Start function when SAE is enabled∙Ct values are exported as number instead of text∙Undetermined Ct results will not be empty∙Melt Curve Raw tab is consistently available∙Fixed reports for results using Crt∙Allow export of Comparative Ct (ddCt) in 7900 Format∙Slope, Y-Intercept and R2 values are available in 7900 Format for Standard Curves∙Multicomponent files are exported in 7900 FormatReport∙Print preview is available immediately after run∙Fixed incorrect timestampsCalibration∙Verification run can be opened when downloaded from Instrument ConsoleLICENSESee accompanying End User License Agreement for details. Users must agree to the terms of the license before installing or using the software.COMPATIBILITY∙This software can be installed as new installation or an upgrade from QuantStudio Real-Time PCR System Software v1.0 onwards∙This software can open template and run files created with the ViiA7 System Software, QuantStudio 6 and 7 Flex Real-Time PCR System Software or QuantStudio Real-TimePCR System Software∙The software can import setup files created by 7900 SDS v2.3ONLINE HELP∙Any changes not mentioned in the Online Help are covered in Release Notes and/or User BulletinKNOWN ISSUESCalibration∙The Close button in the Calibration workspace is not functional when the connection to the instrument is lost during a calibration run. Select the Cancel button to return to the Mainpage of the Calibration workspace∙Wells that failed or Caution QC are not highlighted to the user in the plate layout. Navigate to Well Table to view calibration results∙In Background calibration, wells that missed QC mark are not highlighted to user in the plate layout. Click the wells in the plate layout to view the QC markExport∙After run, auto exported files will not open automatically if the checkbox is selected∙Exported data in 7900 Format is not sorted the same way as 7900 Software export Instrument Run∙Changing the target dye during the run will cause Amp Curve to show incorrect data∙Due to high utilization of the instrument CPU resources during an instrument run, the Melt Curve Plot will not be able to copy properly during the last 15 minutes of the instrumentrun. It is advisable to copy the Melt Curve plot only after an instrument run has completed ∙If a run is terminated during an infinite hold, the system incorrectly labels experiment with status of “Run Terminated,” rather than “Run Completed”. The system is still able to open and analyze these files correctly∙For a High Resolution Melt test, it is recommended that users save the run file under analysis before concurrently opening another run fileConnecting to the Instrument∙The status of the instrument remains as "READY" in the Instrument Console after Auto Discovery has been disabled from the eGUI. Restart the software after changing AutoDiscovery settings from the eGUI to update the instrument status∙The status of the instrument remains as "Not Connected" in the Instrument Console after the IP address of the instrument has changed. Restart the software or refresh the MyInstrument list after changing the IP address of an instrumentPerformance∙Software can continue operation for at least 3.5 days. However, we recommend that you re-launch the software application after 3.5 days of continuous operation for optimalperformanceNotifications∙Email notification operation does not function correctly with SMTP encryption enabled.Uncheck the ‘Encryption Require d?’ option in Preferences for SMTP Settings to allownotifications∙User is required to contact their corporate IT for help if using corporate smtp server for email notificationGenotyping Experiments∙The initial view of the allelic discrimination plot does not show the different colors for each of the genotypes. Click on the empty region of the plot to see the genotype colorsHigh Resolution Melt Experiments∙For a High Resolution Melt experiment, manual calls cannot be made in the Variant Call column in the Well Table. To make a manual call, double-click a well in the Plate Layout tab and select the control from the Control drop-down list∙For a High Resolution Melt experiment, the Derivative Melt Curve plot in the Run area is empty. To view the derivative melt curve, click Analysis, then HRM Plots ∙Exporting with more than 65,536 rows is not supported by the Excel® 2003 format. To export such data use either the Text format or the Excel® 2007 format∙Recommended hard drive space available when performing an instrument run for a High Resolution Melt experiment is 50MB∙The software is not able to properly analyze an HRM experiment file when the target dye added to the plate is “SYBR”in the “Define” tab but the reagent selected is MeltDoctor™and the run protocol contains a PCR stage in the “Experiment Properties” tab. Please select “Other” as the reagent in the “Experiment Properties” tab and ensure you have done anHRM calibration for that dyeExperiments∙If the user opens more than 10 experiments at the same time, the bottom right arrow to move the tab becomes invisible. Close some experiments to access the hidden ones.∙Software prompts user to save unchanged changes when user opens a ViiA7 file containing email notification settings even when no changes are made∙The software is not able to analyze the experiment file if the selected dye has not been calibrated on the instrument. Workaround: Override the calibration data in the experiment file with that of an experiment file containing calibration data for the missing dye. Thecalibration has to be from the same instrument and block typeSecurity and Auditing∙Disabling audit and e-signature settings in a secured environment does not hide / disable the Audit section in the Experiment MenuStudy∙Gene Expression study files created in this software will not be supported in legacy Data Collection SoftwareSYSTEM REQUIREMENTSThe computer hardware and operating system requirements for the QuantStudio Real-Time PCR System Software v1.3 are:∙Windows® 7 (32-bit or 64-bit) or Windows® XP with Service Pack 3∙Pentium® 4 processor or compatible, with minimum 4 GB of RAM and 500 GB of hard drive capacity∙Minimum monitor resolution of 1280x1024∙One open Ethernet port for connecting to the instrument directly∙Internet Explorer® 6.0 or higher∙Excel® softwareINSTALL SOFTWAREIMPORTANT - To help prevent data loss, it is strongly advised that all user data is backed up before upgrading the software.∙Log on to the Windows® system with Administrator privileges∙Obtain the software installation package. Double click on the installer application to start the installation∙A gree to the End User License Agreement (EULA) when prompted and complete the installation∙The software will be installed, by default, to “C:\Program Files\AppliedBiosystems\QuantStudio Real-Time PCR Software” (referred to as the home directory ofthe QuantStudio Software)∙ A program group, “QuantStudio Real-Time PCR Software” will be created during installation. In addition, a short cut to the application, “QuantStudio Real-Time PCRSoftware” will be installed on the computer desktop∙When a previous version of the software is detected on the system, the installer will perform an upgrade. The user will be prompted to proceed with the upgrade or to cancel the operationUNINSTALL SOFTWAREIMPORTANT: To prevent data loss, it is strongly advised that all user data is backed up before uninstalling the Software.∙From Windows® “Start” button, find the “QuantStudio Real-Time PCR Software”program group∙Click on “Uninstall QuantStudio Real-Time PCR Software”∙In the wizard, follow the instructions and complete the uninstall operation∙Optionally, rename or delete the home directory of the QuantStudio Real-Time PCR Software application. This ensures a clean environment for the next installationCopyright © 2016 Thermo Fisher Scientific Inc. All rights reserved.For Research Use Only. Not for use in diagnostic procedures.TO THE EXTENT ALLOWED BY LAW, LIFE TECHNOLOGIES AND/OR ITSAFFILIATE(S) WILL NOT BE LIABLE FOR SPECIAL, INCIDENTAL, INDIRECT, PUNITIVE, MULTIPLE OR CONSEQUENTIAL DAMAGES IN CONNECTION WITH OR ARISING FROM THIS DOCUMENT, INCLUDING YOUR USE OF IT.All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified. Windows, Excel, Internet Explorer and PowerPoint are registered trademarks of Microsoft Corporation in the United States and other countries.Pentium is a registered trademark of Intel Corporation.。
重组凝血因子VIIa在出血性患者中的应用解析

重组凝血因子VIIa在出血性患者中的应用中山大学附属第一医院麻醉科510080刘宽智黄文起重组凝血因子VIIa(rFVIIa)于1999年由美国FDA批准上市,用于治疗血友病及伴有抑制物患者的出血情况[1]。
此后rFVIIa被证明能增加已被激活的血小板上凝血酶的生成,可用于血小板减少症及血小板功能障碍患者出血时的治疗[2-4]。
rFVIIa也被证实可以成功地用于治疗围术期出现的急性出血情况[5-8],及在肝功能受损的患者上亦取得良好的疗效[9,10]。
故rFVIIa在外科止血方面的应用越来越受到人们的重视。
rFVIIa的结构rFVIIa是一种依赖维生素K的凝血因子,由406个氨基酸残基组成的糖蛋白。
Thim等[11]比较了重组样品与人血浆纯化样品的区别,发现两者的氨基酸序列完全相同,均与cDNA推测的结果一致,只是在翻译后修饰存在着较大的区别,但Hedner等[12]证明,两者生物学功能无差别。
rFVIIa的凝血机制目前提出了2种旁路作用的可能机制,而且很可能2种机制都在起作用。
一是经典的外源性凝血途径,又称作凝血的组织因子(TF)途径。
TF是一种跨膜糖蛋白,存在于大多数组织细胞中,与FVIIa有很高的亲和性。
TF通常不与血液接触,但当组织损伤时,TF暴露于血液中,与FVIIa形成复合物,激活一系列的凝血因子,最终产生大量的凝血酶,完成凝血过程[13]。
二是血小板表面依赖模型。
TF-FVIIa 复合物能够与活化的血小板直接结合,可致血小板表面上的Fva和FXa产生凝血酶爆发,这对形成稳定的纤维蛋白血栓非常重要[14,15]。
rFVIIa的作用机制与人体内正常凝血机制稍有不同。
体外实验证明,FVII会和rFVIIa竞争TF受体。
大剂量注射rFVIIa后,在启动凝血瀑布阶段,血浆高水平的rFVIIa克服了FVII的干扰,使TF与rFVIIa饱和,从而确保其发挥最大的生理作用,产生足够的活化血小板。
此外,rFVIIa与活化血小板的亲和力较低,并且不依赖TF产生FXa,因而比血浆中的FVII更加有效。
氮的族序数

氮的族序数
氮(Nitrogen)的族序数为7(Group 7, Period 2)。
氮元素的原子
序数为7,因此它属于第7族(即碱金属族)。
氮元素是非金属元素,位
于第2周期中,有7个电子在其外层。
氮是第7族(VIIA)中非金属元素
中最外层电子数最多的元素,目前已知其核外电子配置是2,5,其中2
个电子为第一能级,5个电子为第二能级。
氮是空气中最主要的组成成分,其性质属于暖性无色气体。
它是一种
无色无味的气体,不溶于水,能溶于有机溶剂,可以与氢,氧,碳,磷等
元素构成多种物质,如氨,氮氧化物,氨基酸,蛋白质等。
氮是植物生长
的重要物质,是一种必需的养分,可以帮助植物合成多种含氮的有机物质,而土壤中的氮是植物根系营养和发育所必须的物质。
氮是重要的工业原料,可以制造各种复杂的化合物,如胺、硝酸、酰胺、氨基酸等,广泛应用于医药、农业、化工等领域,被用于炸药、液体
燃料、农药、染料等的生产。
此外,氮也是生物细胞的重要组成部分,在
生命的演化、发展和进化中扮演着重要角色。
化学周期的名词解释

化学周期的名词解释化学周期是指元素周期表中元素的有序排列方式。
元素周期表将所有已知的化学元素按照一定的规律排列,以便更好地理解元素之间的相似性和周期性。
在这篇文章中,我们将对几个常见的化学周期名词进行解释。
1. 元素周期表元素周期表是化学中最重要的工具之一。
它将所有已知的化学元素按照一定的规律排列,并提供了每个元素的原子序数、原子量和化学符号。
通过元素周期表,我们可以更好地理解元素的性质和相互之间的联系。
2. 周期元素周期表中的每一行被称为一个周期。
目前,元素周期表共有7个周期。
周期性的存在使得我们能够观察到元素性质的重复出现。
每一个周期都开始于一个主族元素(具有相似的化学性质)并以一个惰性气体元素结束。
3. 周期性元素周期表中元素性质的重复出现被称为周期性。
这种周期性可以分为原子半径、离子半径、电负性、原子核电荷以及其他化学性质等方面。
4. 主族元素主族元素是指元素周期表中IA到VIIA族的元素。
这些元素具有相似的化学性质,比如金属元素通常具有很好的导电性和热导性,而非金属元素则通常是诸如卤素(Group VIIA)那样的不良导电体。
5. 过渡金属元素过渡金属元素是指元素周期表中III至IIB组的元素。
这些元素通常具有良好的导电性和热导性,并且在化学反应中具有多样化的电价(氧化态)。
6. 族周期表中的纵向列被称为族。
每个族都有特定的元素性质和一定的原子结构。
一些常见的族包括碱金属族、碱土金属族和卤族等。
7. 原子半径原子半径是指元素中心核电荷到其外层最外电子轨道的距离。
一般来说,原子半径随着周期增加而减小。
这是因为周期表中的元素原子核的正电荷增加了,吸引外层电子的能力也增强了。
8. 离子半径离子半径是指形成带电离子的元素或化合物中离子的半径。
正离子通常比原子半径小,因为失去了电子后形成更加稳定的结构。
而负离子则通常比原子半径大,因为获得了电子后需要更多的空间。
9. 电负性电负性是衡量元素吸引外层电子的能力的物理量。
AtomicTheoryHistoryoftheAtom-WVU:原子理论史的原子西弗吉尼..

but they do not themselves break apart. • Atoms of the same element are identical in mass and other properties. • Atoms of different elements differ in mass and other properties. • Chemical combination of elements to form compounds occurs. However, in
Example:
If the relative mass of Mo:12C is 7.995, what is the atomic mass of Mo on the 12C atomic mass scale?
Example:
If the relative mass of Fe:S is 1.74 and the relative mass of Fe:12C is 4.65, what is the atomic mass of S?
Atomic masses shown on periodic table are average atomic masses taking into account the different isotopes of each element and their percent abundances. Isotopes are atoms of the same element but with a different mass. These isotopes occur in different percentages in nature (percent abundances or isotopic abundances).
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
HI
1.40
μ/(10-30c· m)
b.p./℃
6.37
3.57
2.76
polarity
m.p./℃ *-83.57 -114.18 -86.87 -50.80 mp
* 19.52
-85.05 -66.71 -35.1 bp
-92.3
△ fHm /kJ· mol-1 -271.1
-36.4
-26.5
Polyhalogen cations
Polyhalogen cations, such as Br2+, I2+, Cl3+, Br3+, I3+, Br5+, I5+, and I42+ are well known, and prepared the dihalogens X2 using strong oxidants. In the X2 the removal of a p* electron shortens the bond (increases the bond order to 1.5) in Br2+ to 215 pm (vs 227 pm in Br2) and in I2+ to 258 pm (vs 272 pm in I2)
322 155 3.96
(kJ · mol–1)
Electronegativity
(Pauling)
The Elements
Physical Properties: State
Intermolecular Forces
F2 g
Small
Cl2 g
Br2 l
I2 s
Large
b.p./℃
-188
-34
Interhalogens
The interhalogens are a large group of compounds containing combinations of different halogen atoms, such as ClF3 or I2Cl6. They are all prepared by direct combinations of the elements, with the product distribution controlled by temperature and the relative amounts of the halogens used. In general lower tempertures give lower oxidation states of the central atom, while higher oxidation states are achieved by raising the temperature of the reaction. Other features are: • F is always in the oxidation state of -1 • the highest oxidation states for X reached are Cl < Br < I
• Reaction with H2O: Oxidation:
X2 2H2O 4HX O2
F2 Cl 2 Br2 X2 H2O HXO HX Proportionation: Cl 2 Br2 I 2
K (Cl2 ) 4.210
-4
K (Br2 ) 7.210-9
+ HF
2KMnO4+2KF+10HF+3H2O2
== 2K MnF +8H O+3O
2 6 2
2
SbCl5+5HF K2MnF6+2SbF5
423K
== SbF +5HCl
6
2KSbF5+MnF4 MnF3+1/2F2
Industrial:
electrolysis 2NaCl 2H2O H2 Cl2 2NaOH
Twenty electrons are found on the outer iodines (14 e-) or central iodine (6 e-) centered non-bonding MOs, which may be treated as lone pairs of electrons.
Lab:
metathesis
500OC
NaCl H2SO4 (concentrated ) HCl NaHSO4
2NaCl H2SO4 (c) 2HCl Na 2SO4
•HF metathesis
CaF 2 H2SO4 (c) CaSO 4 2HF
• Redox F2
-
Cl2
Br2
I2
E (X 2 /X ) /V: 2.889 1.360 1.0774 0.5345
X2 oxidant: S X reductant: W Conclusion: W S
Strongest Oxidant: F2,Strongest Reductant Iˉ。
F2+NaOH NaF+OF2↑+H2O
300
>1500 1000 Dec T/℃ 432 Bond E/kJ· mol-1 570 Acidity Weak
stability
366
298
Strong
HX polar,easily dissolvable in H2O. @ 273K,1m3 dissolve 500m3 HCl, HF miscible with H2O. forms azeotropic solution with water
+,
X
In the X3+, the cations are bent, with similar X-X distances as in X2.
The I42+ cation is a “rectangular” dimer of I2+, with I-I distances of 258 and 326 pm
-
12H
MnO2 4H 2I
-
-
Mn I 2 2H2 O
2-
2IO3 5HSO3 I2 2SO4 3HSO4 H2O
Hydrogen Halides
1.Properties
@ Ambient T,colorless, pungent HCl HBr HF
BrO3 5Br- 6H 3Br2 3H2 O
-
-
-
•I2 (s) From seaweed :
(Reverse Proportionation)
Cl2 2I- I2 2Cl2IO
3
6H 2 O 5Cl 2 ( excess ) I2
10Cl
2
Lab:
MnO2 4HCl MnCl 2 Cl 2 (g) 2H2 O
•Br2(l)
Br2 2Cl oxidant: Cl 2 2Br 23Br2 3CO3 5Br - BrO3 3CO2 (proportionation) Purification:
Polyhalides
I 2 KI KI3
I
3
linear
I
I
I
-
Molecular Orbital Theory – I3-
I
I ---- I
The linear I3- molecule, with D∞h symmetry, is an example of 3c-2e bonding. I3- has 22 valence electrons and all but one of the MOs are filled (only the su* orbital is empty). A partial MO diagram, with only the interactions of the Xe 5pz orbital and the LGOs derived from the F 2pz orbitals (su and sg) shown, may be constructed to illustrate the 3c-2e bonding.
Halogens
Fluorine, Chlorine, Bromine, Iodine, Astatine
General Properties
Symbol
Electronic Configuration
Major Oxidation Number Electron Affinity/ (kJ · mol–1 )
HF in solid state:
in dilute solution:
HF H++F- HF2- Kaθ = 6.6×10–4 Kaθ = 5
F–+HF
in not-so-dilute solution, HF exists as dimer (HF)2: H 2 F2 H++HF2-
HF reacts with SiO2 and silicates, etching glass
K (I2 ) 2.010-13
The majority exists as elements in aqueous solutions
Preparation of the Elements: (Oxidation)
•F2 (g) Electrolysis: •Cl2 (g)
