ACI211
混凝土外文参考文献

混凝土外文参考文献参考文献1. ACI Committee 211. (2011). Standard Practice for Selecting Proportions for Normal, Heavyweight, and Mass Concrete (ACI 211.1-91) (Reapproved 2009). American Concrete Institute.2. ASTM International. (2018). Standard Specification for Ready-Mixed Concrete (ASTM C94/C94M-18a). ASTM International.3. Neville, A.M., & Brooks, J.J. (2010). Concrete Technology (2nd ed.). Pearson Education Limited.4. Mehta, P.K., & Monteiro, P.J.M. (2013). Concrete: Microstructure, Properties, and Materials (4th ed.). McGraw-Hill Education.5. Mindess, S., Young, J.F., & Darwin D. (2003). Concrete (2nd ed.). Prentice Hall.6. Kosmatka, S.H., Kerkhoff B., & Panarese W.C. (2002). Designand Control of Concrete Mixtures (14th ed.). Portland Cement Association.7. ACI Committee 318M-14 Building Code Requirements for Structural Concrete and Commentary Metric Version. American Concrete Institute.8. ACI Committee 301-16 Specifications for Structural Concrete with Commentary Metric Version.American Concrete Institute.9. ACI Committee 308R-16 Guide to External Curing of Concrete.American Concrete Institute.10.Wang Lijiu,Wang Qiaoling,Yang Xiaoyong,et al.Research on the Properties of High Performance Steel Fiber Reinforced Self-compacting Lightweight AggregateConcrete[J].Journal of Wuhan University of Technology (Materials Science Edition),2017(4):821-826.11.Li Junjie,Li Xiaojun,Liu Yanjun.Experimental Study on the Mechanical Properties of High-strength Concrete Reinforced with Carbon Fiber[J].Journal of Building Materials,2017(4):501-508.12.Zhang Ying,Liu Haiqing,Zhang Jian.Experimental Studyon the Properties of Steel Fiber ReinforcedConcrete[J].Building Science Research of Sichuan,2017(1):103-106.13.Zhang Xiaojun,Wang Zhiqiang,Liu Xuejun.Experimental Study on the Mechanical Properties of Recycled Aggregate Concrete[J].Journal of Building Materials,2017(2):177-183.14.Wang Lijuan,Li Junjie,Li Xiaojun.Experimental Study on the Mechanical Properties of High-strength Lightweight Aggregate Concrete[J].Journal of Building Materials,2017(3):395-401.15.Wang Lijuan, Li Junjie, & Li Xiaojun. (2018). Experimental study on the mechanical properties and durability of high-performance concrete reinforced with polypropylene fiber. Construction and Building Materials, 190, 29-36.16.Li Junjie, Wang Lijuan, & Li Xiaojun. (2018). Experimental study on the mechanical properties and durability of high-performance concrete reinforced with basalt fiber. Journal of Wuhan University of Technology-Materials Science Edition, 33(2), 337-343.17.Wang Lijuan, Li Junjie, & Li Xiaojun. (2019). Experimental study on the mechanical properties and durability of high-performance concrete reinforced with steel fiber and polypropylene fiber. Construction and Building Materials, 225, 1128-1135.18.Li Junjie, Wang Lijuan, & Li Xiaojun. (2020). Experimental study on the mechanical properties and durability of high-performance concrete reinforced with hybrid fibers. Journal of Wuhan University of Technology-Materials Science Edition, 35(4), 739-746.19.Wang Lijuan, Li Junjie, & Li Xiaojun. (2021). Experimental study on the mechanical properties and durability of high-performance concrete reinforced with recycled steel fiber. Construction and Building Materials, 291, 123267.。
美国标准ACI中文版汇总

文件编号DocumentNumber Date R/E/S Date Language 1ACI 1041971.01.01(R 1997) English2ACI 1172010.06.01(R 2015) English3ACI 117.1R2014.08.01English4ACI 117M ERTA2011.09.23English5ACI 117M2010.07.01(R 2015) English6ACI 121R2008.07.01English7ACI 122R2014.12.01English8ACI 131.1R2015.01.01English9ACI 132R2014.12.01English10ACI 201.1R2008.07.01English11ACI 201.2R2008.06.01English12ACI 207.1R2005.01.01(R 2012) English13ACI 207.2R2007.09.01English14ACI 207.3R1994.01.01(R 2008) English15ACI 207.4R2005.01.01(R 2012) English16ACI 207.5R2011.07.01English17ACI 209.1R2005.01.01English18ACI 209.2R ERTA2008.05.01English19ACI 209.2R ERTA2008.05.28English20ACI 209.2R2008.05.01English21ACI 209R1992.01.01(R 2008) English22ACI 210R1993.01.01(R 2008) English23ACI 211.11991.01.01(R 2009) English24ACI 211.21998.01.01(R 2004) English25ACI 211.3R2002.01.01(R 2009) English26ACI 211.4R2008.12.01English27ACI 211.5R2014.04.01English28ACI 211.6T2014.12.01English29ACI 211.7R2015.05.01English30ACI 211.8R2015.06.01English31ACI 212.3R2010.11.01English32ACI 213R2014.06.01English33ACI 214.4R2010.06.01English 34ACI 214R2011.04.01English 35ACI 215R1992.01.01(R 1997) English36ACI 216.12014.11.01English37ACI 216.1M2007.01.01English 38ACI 221.1R1998.01.01(R 2008) English 39ACI 221R1996.01.01(R 2001) English 40ACI 222.2R2014.10.01English 41ACI 222.3R2011.04.01English 42ACI 222R2001.01.01(R 2010) English 43ACI 223R2010.12.01English 44ACI 224.1R2007.03.01English 45ACI 224.2R1992.01.01(R 2004) English 46ACI 224.3R ERTA2008.01.31English 47ACI 224.3R1995.01.01(R 2013) English 48ACI 224.4R2013.12.01English 49ACI 224R ERTA2008.03.24English 50ACI 224R2001.01.01English 51ACI 225R1999.01.01(R 2009) English 52ACI 228.1R2003.11.01English 53ACI 228.2R2013.06.01English 54ACI 229R2013.06.01English 55ACI 230.1R2009.07.01English 56ACI 231R2010.01.01English57ACI 232.1R2012.07.01English 58ACI 232.2R2003.01.01English 59ACI 232.3R2014.10.01English 60ACI 233R2003.01.01(R 2011) English 61ACI 234R2006.01.01English 62ACI 237R2007.04.01English 63ACI 238.1R2008.02.01English 64ACI 238.2T2014.06.01English 65ACI 3012010.01.01English 66ACI 301 SPANISH2010.01.01Spanish 67ACI 301M2010.01.01English 68ACI 302.1R2015.06.01English 69ACI 302.2R2006.01.01English 70ACI 303.11997.01.01English71ACI 303R2012.06.01English 72ACI 304.2R1996.01.01(R 2008) English 73ACI 304.3R1996.01.01(R 2004) English 74ACI 304.4R1995.01.01(R 2008) English 75ACI 304.6R2009.03.01English76ACI 304R2000.01.01(R 2009) English 77ACI 305.12014.08.01English 78ACI 305R2010.10.01English 79ACI 306.11990.01.01(R 2002) English 80ACI 306R2010.10.01English81ACI 307 ERTA2013.01.21English 82ACI 307 ERTA2010.06.04English 83ACI 3072008.11.01English 84ACI 308.12011.07.01English 85ACI 308.1M2011.07.01English 86ACI 308-213R2013.06.01English 87ACI 308R2001.01.01(R 2008) English 88ACI 309.1R2008.08.01English 89ACI 309.2R2015.02.01English 90ACI 309.5R2000.01.01(R 2006) English 91ACI 309R2005.01.01English 92ACI 310R2013.12.01English 93ACI 311.4R2005.01.01English 94ACI 311.52004.01.01English 95ACI 311.62009.11.01English 96ACI 311.6M2009.12.01English 97ACI 311.72014.06.01English98ACI 3131997.01.07English 99ACI 314R2011.01.01English 100ACI 3182014.09.01English 101ACI 318 CD2011.01.01English 102ACI 318M2014.01.01English103ACI 318SUS2014.01.01Spanish 104ACI 325.9R2015.08.01English 105ACI 325.11R2001.01.01English 106ACI 325.12R2002.01.01(R 2013) English107ACI 325.13R2006.01.01English 108ACI 327R2014.01.01English 109ACI 329R2014.12.01English 110ACI 330.12014.11.01English111ACI 330.1M2014.01.01English112ACI 330R2008.06.01English113ACI 3322014.02.01English 114ACI 332.1R ERTA2006.12.19English 115ACI 332.1R2006.12.01English 116ACI 332M2010.08.01English 117ACI 334.1R1992.01.01(R 2002) English 118ACI 334.3R2005.01.01English 119ACI 336.12001.01.01English 120ACI 336.2R1988.01.01(R 2002) English 121ACI 336.3R2014.08.01English 122ACI 341.2R2014.06.01English123ACI 341.3R2007.03.01English124ACI 343.1R2012.11.01English125ACI 343R1995.01.01(R 2004) English 126ACI 345.1R2006.01.01English 127ACI 345.2R2013.07.01English 128ACI 345R2011.09.01English 129ACI 3462009.08.01English 130ACI 346M2009.08.01English131ACI 347.2R2005.01.01English 132ACI 347.3R2013.01.01English 133ACI 347R2014.07.01English134ACI 3492013.01.01English 135ACI 349.1R2007.06.01English 136ACI 349.2R ERTA2007.11.12English 137ACI 349.2R2007.11.01(R 2014) English 138ACI 349.3R2002.01.01(R 2010) English 139ACI 349M2013.02.27English 140ACI 3502006.01.01English141ACI 350.12010.01.01English 142ACI 350.1M2010.01.01English 143ACI 350.2R ERTA2009.07.23English 144ACI 350.2R2004.01.01English 145ACI 350.3 ERTA2008.11.10English 146ACI 350.32006.01.01English 147ACI 350.4R2004.01.01English 148ACI 350.52012.01.01English 149ACI 350.5M2012.01.01English 150ACI 350M2006.01.01English 151ACI 351.1R2012.03.01English 152ACI 351.2R2010.04.01English 153ACI 351.3R2004.01.01(R 2011) English 154ACI 351.42014.01.01English 155ACI 351.52015.05.01English 156ACI 351.5M2015.09.01English 157ACI 352.1R2011.01.01English 158ACI 352R2002.01.01(R 2010) English 159ACI 352RS2002.01.01Spanish 160ACI 355.22007.06.01English 161ACI 355.3R2011.05.01English 162ACI 355.42011.08.01English 163ACI 355.4M2011.09.01English 164ACI 357.2R2010.07.01English 165ACI 357.3R2014.10.01English 166ACI 357R1984.01.01(R 1997) English 167ACI 360R2010.04.01English 168ACI 362.1R2012.09.01English 169ACI 362.2R2000.06.01(R 2013) English 170ACI 363.2R2011.07.01English171ACI 363R2010.03.01English 172ACI 364.1R2007.05.01English 173ACI 364.3R2009.04.01English 174ACI 364.2T2008.01.01English 175ACI 364.3T2015.01.01English 176ACI 364.4T2010.01.01English 177ACI 364.5T2010.01.01English 178ACI 364.6T2002.07.01(R 2011) English 179ACI 364.7T2002.04.01(R 2011) English 180ACI 364.8T2002.05.01(R 2011) English 181ACI 364.9T2003.07.01(R 2011) English 182ACI 364.10T2014.07.01English 183ACI 364.11T2015.08.01English 184ACI 364.12T2015.10.01English 185ACI 364.13T2015.10.01English 186ACI 365.1R2000.01.01English 187ACI 369R2011.02.01English 188ACI 370R2014.07.01English 189ACI 371R2008.08.01English 190ACI 372R2013.12.01English 191ACI 374.12005.01.01(R 2014) English 192ACI 374.2R2013.08.01English 193ACI 3762011.01.01English194ACI 376M2011.01.01English 195ACI 408.2R2012.09.01English 196ACI 408.3R2009.10.01English 197ACI 408R2003.11.01English 198ACI 421.1R2008.06.01English199ACI 421.2R2010.04.01English 200ACI 421.3R2015.10.01English 201ACI 423.3R2005.01.01English 202ACI 423.4R2014.11.01English 203ACI 423.72014.11.01English 204ACI 423.8R2010.10.01English205ACI 423.9M2010.06.01English206ACI 435.8R1985.01.01(R 1997) English207ACI 435R1995.01.01(R 2000) English208ACI 4372012.08.01English209ACI 437.1R2007.03.01English210ACI 437.22013.01.01English211ACI 437.2M2013.01.01English 212ACI 437R2003.01.01English 213ACI 439.3R2007.03.01English 214ACI 439.4R2009.10.01English215ACI 440.1R2015.03.01English 216ACI 440.2R2008.07.01English 217ACI 440.3R2012.08.01English218ACI 440.4R2004.12.01(R 2011) English 219ACI 440.52008.07.01English 220ACI 440.5M2008.07.01English 221ACI 440.62008.07.01English 222ACI 440.6M2008.07.01English 223ACI 440.7R2010.04.01English 224ACI 440.82013.01.01English 225ACI 440.8M2013.01.01English226ACI 440.9R2015.05.01English227ACI 440R2007.09.01English 228ACI 441R1996.01.01English 229ACI 445.1R2012.01.01English 230ACI 445R1999.01.01(R 2009) English 231ACI 446.1R1991.01.01(R 1999) English232ACI 446.3R1997.01.01English 233ACI 446.4R2004.01.01English 234ACI 503.2-503.4English 235ACI 503.32010.10.01English 236ACI 503.3M2010.10.01English 237ACI 503.5R1992.01.01(R 1997) (R 2003) English 238ACI 503.72007.09.01English 239ACI 506.1R2008.11.01English 240ACI 506.22013.01.01English 241ACI 506.2M2013.01.01English 242ACI 506.4R1994.01.01(R 2004) English 243ACI 506.5R2009.08.01English 244ACI 506R2005.01.01English 245ACI 515.2R2013.07.01English 246ACI 522.12013.06.01English 247ACI 522.1M2013.10.01English 248ACI 522R2010.03.01English 249ACI 523.1R2006.01.01English 250ACI 523.2R1996.01.01English 251ACI 523.3R2014.04.01English 252ACI 523.4R2009.06.01English 253ACI 524R2008.08.01English 254ACI 530/530.12013.09.13English255ACI 533.1R2002.09.01English 256ACI 533R2011.01.01English 257ACI 543R2012.03.01English 258ACI 544.1R1996.01.01(R 2009) English 259ACI 544.2R1989.01.01(R 2009) English 260ACI 544.3R2008.11.01English 261ACI 544.4R1988.01.01(R 2009) English 262ACI 544.5R2010.03.01English 263ACI 544.6R2015.09.01English264ACI 546.2R2010.06.01English265ACI 546.3R2014.06.01English266ACI 546R2014.09.01English 267ACI 548.1R2009.03.01English 268ACI 548.3R2009.04.01English 269ACI 548.42011.01.01English 270ACI 548.4M2011.01.01English 271ACI 548.5R1994.01.01(R 1998) English272ACI 548.6R1996.01.01English 273ACI 548.82007.10.01English 274ACI 548.8M2007.10.01English 275ACI 548.92008.08.01English 276ACI 548.9M2008.08.01English 277ACI 548.102010.08.01English 278ACI 548.10M2010.09.01English 279ACI 548.11R2012.09.01English 280ACI 548.122012.01.01English 281ACI 548.132014.10.01English 282ACI 548.142014.01.01English 283ACI 548.14M2014.01.01English 284ACI 549.1R1993.01.01(R 2009) English 285ACI 549.2R2004.05.01(R 2013) English 286ACI 549.3R2009.12.01English 287ACI 549.4R2013.12.01English 288ACI 549R1997.01.24(R 2009) English 289ACI 550.1R2009.02.01English 290ACI 550.2R2013.04.01English 291ACI 550.32013.11.01English292ACI 550.3M2013.01.01English 293ACI 551.1R2014.11.01English 294ACI 551.2R2015.08.01English 295ACI 555R2001.01.01English296ACI 5622013.03.01English297ACI 562M2013.05.01English 298ACI ASCC-12005.01.01English 299ACI ASCC-1 SPANISH2005.01.01Spanish 300ACI C-071986.01.01English 301ACI C-081987.01.01English 302ACI CCS-01993.11.01English 303ACI CCS-12010.01.01English 304ACI CCS-31989.01.01English 305ACI CCS-42008.01.01English 306ACI CFPR1998.01.01English 307ACI CP-602009.01.01English308ACI CR MANUAL2013.01.01English 309ACI CT01 CD1998.01.01English 310ACI DCCM2011.01.01English 311ACI E12007.01.01English 312ACI E22000.01.01English 313ACI E42012.01.01English 314ACI EB0711991.01.01English 315ACI IJBRC2006.01.01English 316ACI IPS-12002.01.01English317ACI ITG-32004.01.01English 318ACI ITG-4.12007.03.01English 319ACI ITG-4.2R2006.10.01English 320ACI ITG-4.3R2007.09.01English321ACI ITG-5.12007.01.01English 322ACI ITG-5.1M2007.01.01English 323ACI ITG-5.22009.08.01English324ACI ITG-6R2010.08.01English 325ACI ITG-72009.11.01English 326ACI ITG-7M2009.11.01English 327ACI ITG-8R2010.12.01English 328ACI LICC2004.01.01English329ACI MCP-12014.01.01English330ACI MCP-22014.01.01English331ACI MCP-32014.01.01English332ACI MCP-42014.01.01English333ACI MCP-52014.01.01English334ACI MCP-62014.01.01English335ACI MCP-72014.01.01English 336ACI MCP COMBO2014.01.01English 337ACI MCP SET2015.01.01English 338ACI MCP SET CD2014.01.01English 339ACI MDG2013.01.01English 340ACI MSP2009.01.01English 341ACI NGBSPACK2008.01.01English 342ACI PCP2004.01.01English 343ACI PRCD.EM2014.01.01English 344ACI PTM2006.01.01English 345ACI RPMN13PACK2013.01.01English346ACI SCG12010.01.01EnglishTitle StatusPreparation of Notation for Concrete Active Specification for Tolerances for Concrete Construction andActive Materials (ACI 117-10) and Commentary (ACI 117R-10)Guide for Tolerance Compatibility in Concrete Construction ActiveSpecification for Tolerances for Concrete Construction andActive Materials (ACI 117M-10) and CommentarySpecification for Tolerances for Concrete Construction andActive Materials (ACI 117M-10) and Commentary (ACI 117RM-10)Guide for Concrete Construction Quality Systems inActive Conformance with ISO 9001Guide to Thermal Properties of Concrete and MasonryActive SystemsInformation Delivery Manual (IDM) for Cast-in-PlaceActive ConcreteGuide for Responsibility in Concrete Construction ActiveGuide for Conducting a Visual Inspection of Concrete inActive ServiceGuide to Durable Concrete Active Guide to Mass Concrete ActiveReport on Thermal and Volume Change Effects on Cracking ofActive Mass ConcretePractices for Evaluation of Concrete in Existing MassiveActive Structures for Service ConditionsCooling and Insulating Systems for Mass Concrete Active Report on Roller-Compacted Mass Concrete ActiveReport on Factors Affecting Shrinkage and Creep ofActive Hardened ConcreteGuide for Modeling and Calculating Shrinkage and Creep inActive Hardened ConcreteGuide for Modeling and Calculating Shrinkage and Creep inActive Hardened ConcreteGuide for Modeling and Calculating Shrinkage and Creep inActive Hardened ConcretePrediction of Creep, Shrinkage, and Temperature Effects inActive Concrete StructuresErosion of Concrete in Hydraulic Structures ActiveStandard Practice for Selecting Proportions for Normal,Active Heavyweight, and Mass ConcreteStandard Practice for Selecting Proportions for StructuralActive Lightweight ConcreteGuide for Selecting Proportions for No-Slump Concrete ActiveGuide for Selecting Proportions for High-Strength ConcreteActive Using Portland Cement and Other Cementitious MaterialsGuide for Submittal of Concrete Proportions Active Aggregate Suspension Mixture Proportioning Method ActiveGuide for Proportioning Concrete Mixtures with GroundActive Limestone and Other Mineral FillersGuide to Troubleshooting Concrete Mixture Issues asActive Influenced by Constitutive Materials, Jobsite Conditions,or Testing PracticesReport on Chemical Admixtures for Concrete Active Guide for Structural Lightweight-Aggregate Concrete ActiveGuide for Obtaining Cores and Interpreting CompressiveActive Strength ResultsGuide to Evaluation of Strength Test Results of Concrete ActiveConsiderations for Design of Concrete Structures SubjectedActive to Fatigue LoadingCode Requirements for Determining Fire Resistance ofActive Concrete and Masonry Construction AssembliesCode Requirements for Determining Fire Resistance ofActive Concrete and Masonry Construction AssembliesReport on Alkali-Aggregate Reactivity ActiveGuide for Use of Normal Weight and Heavyweight AggregatesActive in ConcreteReport on Corrosion of Prestressing Steels ActiveGuide to Design and Construction Practices to MitigateActive Corrosion of Reinforcement in Concrete StructuresProtection of Metals in Concrete Against Corrosion Active Guide for the Use of Shrinkage-Compensating Concrete ActiveCauses, Evaluation, and Repair of Cracks in ConcreteActive StructuresCracking of Concrete Members in Direct Tension Active Joints in Concrete Construction Active Joints in Concrete Construction Active Guide to Design Detailing to Mitigate Cracking Active Control of Cracking in Concrete Structures Active Control of Cracking in Concrete Structures Active Guide to the Selection and Use of Hydraulic Cements Active In-Place Methods to Estimate Concrete Strength ActiveReport on Nondestructive Test Methods for Evaluation ofActive Concrete in StructuresReport on Controlled Low-Strength Materials Active Report on Soil Cement ActiveReport on Early-Age Cracking: Causes, Measurement, andActive MitigationReport on the Use of Raw or Processed Natural Pozzolans inActive ConcreteUse of Fly Ash in Concrete ActiveReport on High-Volume Fly Ash Concrete for StructuralActive ApplicationsSlag Cement in Concrete and Mortar Active Guide for the Use of Silica Fume in Concrete Active Self-Consolidating Concrete ActiveReport on Measurements of Workability and Rheology ofActive Fresh ConcreteConcrete Thixotropy ActiveSpecifications for Structural Concrete - IncorporatingActive Errata: 23, February 2015Especificaciones para Concreto Estructural ActiveSpecifications for Structural Concrete - IncorporatingActive Errata: 23, February 2015Guide to Concrete Floor and Slab Construction ActiveGuide for Concrete Slabs that Receive Moisture-SensitiveActive Flooring MaterialsStandard Specification for Cast-In-Place ArchitecturalActive ConcreteGuide to Cast-in-Place Architectural Concrete Practice ActivePlacing Concrete by Pumping Methods Active Heavyweight Concrete: Measuring, Mixing, Transporting, andActive PlacingPlacing Concrete with Belt Conveyors ActiveGuide for Use of Volumetric-Measuring and Continuous-Active Mixing Concrete EquipmentGuide for Measuring, Mixing, Transporting, and PlacingActive ConcreteSpecification for Hot Weather Concreting Active Guide to Hot Weather Concreting Active Standard Specification for Cold Weather Concreting Active Guide to Cold Weather Concreting ActiveCode Requirements for Reinforced Concrete Chimneys (ACIActive 307-08) and CommentaryCode Requirements for Reinforced Concrete Chimneys andActive CommentaryCode Requirements for Reinforced Concrete Chimneys (ACIActive 307-08) and CommentarySpecification for Curing Concrete Active Specification for Curing Concrete ActiveReport on Internally Cured Concrete Using PrewettedActive Absorptive Lightweight AggregateGuide to Curing Concrete Active Report on Behavior of Fresh Concrete During Vibration ActiveGuide to Identification and Control of Visible SurfaceActive Effects of Consolidation on Formed Concrete SurfacesCompaction of Roller-Compacted Concrete Active Guide for Consolidation of Concrete Active Guide to Decorative Concrete Active Guide for Concrete Inspection ActiveGuide for Concrete Plant Inspection and Testing of Ready-Active Mixed ConcreteSpecification for Ready Mixed Concrete Testing Services Active Specification for Ready Mixed Concrete Testing Services ActiveInspection Services Specification for Cast-in-PlaceActive Concrete ConstructionStandard Practice for Design and Construction of ConcreteActive Silos and Stacking Tubes for Storing Granular MaterialsGuide to Simplified Design for Reinforced ConcreteActive BuildingsBuilding Code Requirements for Structural Concrete andActive Commentary - Incorporated Errata: October 9, 2015Building Code Requirements for Structural Concrete andActive Commentary - CD ROM: To Purchase Call 1-800-854-7179USA/Canada or 303-397-7956 WorldwideBuilding Code Requirements for Structural Concrete (ACIActive 318M-14) and Commentary (ACI 318RM-14)Building Code Requirements for Structural Concrete andActive Commentary-Spanish Inch-PoundGuide for Construction of Concrete Pavements Active Accelerated Techniques for Concrete Paving ActiveGuide for Design of Jointed Concrete Pavements for StreetsActive and Local RoadsConcrete Overlays for Pavement Rehabilitation ActiveGuide to Roller- Compacted Concrete Pavements Active Report on Performance-Based Requirements for Concrete Active Specification for Unreinforced Concrete Parking Lots andActive Site PavingSpecification for Unreinforced Concrete Parking Lots andActive Site PavingGuide for the Design and Construction of Concrete ParkingActive LotsResidential Code Requirements for Structural Concrete (ACIActive 332-10) and CommentaryGuide to Residential Concrete Construction Active Guide to Residential Concrete Construction ActiveResidential Code Requirements for Structural Concrete (ACIActive 332M-10) and CommentaryConcrete Shell Structures Practice and Commentary Active Construction of Concrete Shells Using Inflated Forms Active Specification for the Construction of Drilled Piers ActiveSuggested Analysis and Design Procedures for CombinedActive Footings and MatsReport on Design and Construction of Drilled Piers ActiveReport on Analysis and Design of Seismic-ResistantActive Concrete Bridge SystemsSeismic Evaluation and Retrofit Techniques for ConcreteActive BridgesGuide for the Analysis and Design of Reinforced andActive Prestressed Concrete Guideway StructuresAnalysis and Design of Reinforced Concrete BridgeActive StructuresGuide for Maintenance of Concrete Bridge Members Active Guide for Widening Highway Bridges Active Guide for Concrete Highway Bridge Deck Construction Active Specification for Cast-in-Place Concrete Pipe Active Specification for Cast-in-Place Concrete Pipe ActiveGuide for Shoring/Reshoring of Concrete MultisoryActive BuildingsGuide to Formed Concrete Surfaces Active Guide to Formwork for Concrete Active Code Requirements for Nuclear Safety-Related ConcreteActive Structures (ACI 349-13) and Commentary - IncorporatingErrata: 23, February 2015Reinforced Concrete Design for Thermal Effects on NuclearActive Power Plant StructuresConcrete Capacity Design (CCD) Method-Embedment DesignActive ExamplesGuide to the Concrete Capacity Design (CCD) Method -Active Embedment Design ExamplesEvaluation of Existing Nuclear Safety-Related ConcreteActive StructuresCode Requirements for Nuclear Safety-Related ConcreteActive Structures (ACI 349M-06) and Commentary - IncorporatesErrata: 2/24/2015Code Requirements for Environmental Engineering ConcreteActive Structures and Commentary - Incorporates Errata: 10/09/15Specification for Tightness Testing of EnvironmentalActive Engineering Concrete Containment Structures (ACI 350.1-10)and CommentarySpecification for Tightness Testing of EnvironmentalEngineering Concrete Containment Structures (ACI 350.1M-Active 10) and CommentaryConcrete Structures for Containment of Hazardous Materials Active Concrete Structures for Containment of Hazardous Materials ActiveSeismic Design of Liquid-Containing Concrete StructuresActive and CommentarySeismic Design of Liquid-Containing Concrete StructuresActive and CommentaryDesign Considerations for Environmental EngineeringActive Concrete StructuresSpecifications for Environmental Concrete Structures Active Specifications for Environmental Concrete Structures Active CODE REQUIREMENTS FOR ENVIRONMENTAL ENGINEERING CONCRETE STRUCTURES (ACI 350M-06) AND COMMENTARY - IncorporatingActive Errata: 4/29/2015Report on Grouting between Foundations and Bases forActive Support of Equipment and MachineryReport on Foundations for Static Equipment Active Foundations for Dynamic Equipment ActiveSpecification for Installation of Cementitious GroutingActive between Foundations and Equipment BasesSpecification for Installation of Epoxy Grout betweenActive Foundations and Equipment BasesSpecification for Installation of Epoxy Grout betweenActive Foundations and Equipment BasesGuide for Design of Slab-Column Connections in MonolithicActive Concrete StructuresRecommendations for Design of Beam-Column Connections inActive Monolithic Reinforced Concrete StructuresRecomendaciones para el Dise?o de Conexiones Viga-ColumnaActive en Estructuras Monolíticas de Concreto ReforzadoQualification of Post-Installed Mechanical Anchors inActive Concrete and CommentaryGuide for Design of Anchorage to Concrete: Examples UsingActive ACI 318 Appendix DQualification of Post-Installed Adhesive Anchors inActive Concrete (ACI 355.4) and CommentaryQualification of Post-Installed Adhesive Anchors inActive Concrete (ACI 355.4M-11) and CommentaryReport on Barge-Like Concrete Structures ActiveGuide for Design and Construction of Waterfront andActive Coastal Concrete Marine StructuresGuide for the Design and Construction of Fixed OffshoreActive Concrete StructuresGuide to Design of Slabs-on-Ground ActiveGuide for the Design and Construction of Durable ConcreteActive Parking StructuresGuide for Structural Maintenance of Parking Structures ActiveGuide to Quality Control and Assurance of High-StrengthActive ConcreteReport on High-Strength Concrete ActiveGuide for Evaluation of Concrete Structures Prior toActive RehabilitationGuide for Cementitious Repair Material Data Sheet ActiveIncreasing Shear Capacity Within Existing ReinforcedActive Concrete StructuresTreatment of Exposed Epoxy-Coated Reinforcement in Repair ActiveDetermining the Load Capacity of a Structure When As-BuiltActive Drawings are UnavailableImportance of Modulus of Elasticity in Surface RepairActive MaterialsConcrete Removal in Repairs Involving Corroded ReinforcingActive SteelEvaluation and Minimization of Bruising (Microcracking) inActive Concrete RepairUse of Hydrodemolition for Concrete Removal in UnbondedActive Post-Tensioned SystemsCracks in a Repair ActiveRehabilitation of Structure with Reinforcement SectionActive LossManaging Alkali-Aggregate Reaction Expansion in MassActive ConcreteRepair of Leaking Cracks in Walls of Liquid ContainmentActive StructuresRepairs for Reinforcement with Shallow Cover Active Service-Life Prediction - State-of-the-Art Report ActiveGuide for Seismic Rehabilitation of Existing ConcreteActive Frame Buildings and CommentaryReport for the Design of Concrete Structures for BlastActive EffectsGuide for the Analysis, Design, and Construction ofElevated Concrete and Composite Steel-Concrete WaterActive Storage TanksGuide to Design and Construction of Circular Wire- andActive Strand-Wrapped Prestressed Concrete StructuresAcceptance Criteria for Moment Frames Based on StructuralActive Testing and CommentaryGuide for Testing Reinforced Concrete Structural ElementsActive under Slowly Applied Simulated Seismic LoadsCode Requirements for Design and Construction of ConcreteActive Structures for the Containment of Refrigerated LiquefiedGases and CommentaryCode Requirements for Design and Construction of ConcreteActive Structures for the Containment of Refrigerated LiquefiedGases and CommentaryReport on Bond of Steel Reinforcing Bars Under CyclicActive LoadsGuide for Lap Splice and Development Length of HighActive Relative Rib Area Reinforcing Bars in Tension andCommentaryBond and Development of Straight Reinforcing Bars inActive TensionGuide to Shear Reinforcement for Slabs - IncorporatingActive Errata : 02/23/2015Guide to Seismic Design of Punching Shear Reinforcement inActive Flat PlatesGuide to Design of Reinforced Two-Way Slab Systems ActiveRecommendations for Concrete Members Prestressed withActive Unbonded TendonsCorrosion and Repair of Unbonded Single Strand Tendons Active Specification for Unbonded Single-Strand Tendon Materials ActiveReport on Corrosion and Repair of Grouted Multistrand andActive Bar Tendon SystemsTest Method for Bleed Stability of Cementitious Post-Active Tensioning Tendon GroutObserved Deflections of Reinforced Concrete Slab Systems,Active and Causes of Large DeflectionsControl of Deflection in Concrete Structures -Active incorporates Appendix B: 2003Code Requirements For Load Testing Of Existing ConcreteActive Structures And CommentaryLoad Tests of Concrete Structures: Methods, Magnitude,Active Protocols, and Acceptance CriteriaCode Requirements for Load Testing of Existing ConcreteActive Structures (ACI 437.2-13) and CommentaryCode Requirements for Load Testing of Existing ConcreteActive Structures (ACI 437.2M-13) and CommentaryStrength Evaluation of Existing Concrete Buildings Active Types of Mechanical Splices for Reinforcing Bars ActiveReport on Steel Reinforcement-Material Properties and U.S.Active AvailabilityGuide for the Design and Construction of StructuralActive Concrete Reinforced with Fiber-Reinforced Polymer (FRP)BarsGuide for the Design and Construction of Externally BondedFRP Systems for Strengthening Concrete Structures -Active Incorporating Errata : 02/27/2015Guide Test Methods for Fiber-Reinforced Polymer (FRP) Composites for Reinforcing or Strengthening Concrete andActive Masonry StructuresPrestressing Concrete Structures with FRP Tendons ActiveSpecification for Construction with Fiber-ReinforcedActive Polymer Reinforcing BarsSpecification for Construction with Fiber-ReinforcedActive Polymer Reinforcing BarsSpecification for Carbon and Glass Fiber-ReinforcedActive Polymer Bar Materials for Concrete ReinforcementSpecification for Carbon and Glass Fiber-ReinforcedActive Polymer Bar Materials for Concrete ReinforcementGuide for the Design and Construction of Externally BondedActive Fiber-Reinforced Polymer Systems for StrengtheningUnreinforced Masonry StructuresSpecification for Carbon and Glass Fiber-ReinforcedActive Polymer (FRP) Materials Made by Wet Layup for External Strengthening of Concrete and Masonry StructuresSpecification for Carbon and Glass Fiber-ReinforcedPolymer (FRP) Materials Made by Wet Layup for ExternalActive Strengthening of Concrete and Masonry Structures。
ACI发展心理学习题

发展心理学第三章第一节单选题1.皮亚杰把儿童的心理发展划分为()个阶段。
A. 3B. 5C. 4D. 6【答案】C【解析】《心理咨询师三级基础知识》P190 第三章发展心理学第一节概述皮亚杰把认知(智慧)发展视为认知结构的发展过程,以认知结构为依据区分心理发展阶段。
他把认知发展分为四个阶段:1)感知运动阶段;2)前运算阶段;3)具体运算阶段;4)形式运算阶段。
2.皮亚杰认为心理起源于()。
A. 成熟B. 经验C. 模仿D. 动作【答案】D【解析】《心理咨询师三级基础知识》P188 第三章发展心理学第一节概述皮亚杰认为,心理既不是起源于先天的成熟,也不是起源于后天的经验,而是起源于动作,即动作是认识的源泉,是主客体相互作用的中介。
3.给我一打健全儿童,我可以用特殊的方法将他们任意改变,或者使他们成为医生,或者使他们成为艺术家和富商,或者使他们成为乞丐和强盗,这种心理发展动因属于()。
A. 遗传因素决定心理发展B. 环境因素决定心理发展C. 社会文化因素决定心理发展D. 通过社会学习获得心理发展【答案】B【解析】《心理咨询师三级基础知识》P184 第三章发展心理学第一节概述环境因素决定心理发展的理论被称为环境决定论。
行为主义心理学派创始人华生是这种观点的代表人物。
华生的名言:“给我一打健全的儿童,我可以用特殊的方法任意地将他们加以改变,或者使他们成为医生、律师、艺术家和富商,或者使他们成为乞丐和强盗”,完全否定了儿童的素质、年龄特征和内部状态的作用。
4.为了观察个体随时间的变化,科学家进行()。
A. 智力测验B. 纵向研究C. 横断面研究D. 个案研究【答案】B【解析】《ACI 心理咨询师上册》P179 第三章第一节纵向研究设计是对相同的研究对象在不同的年龄或阶段进行的长期反复观测的设计方式,也称为纵向跟踪研究。
多选题5.处于前运算阶段的儿童具有的特征包括()。
A. 泛灵论B. 自我中心C. 思维的可逆性D. 掌握守恒【答案】AB【解析】《心理咨询师三级基础知识》P190 第三章发展心理学第一节概述皮亚杰指出前运算阶段儿童思维的特点有:1)泛灵论;2)自我中心主义;3)不能理顺整体和部分的关系;4)思维的不可逆性;5)缺乏守恒。
ACI目录

ACI 规范总目录S/NTitleACI 104-71Preparation of Notation for ConcreteACI 116R-00Cement and Concrete TerminologyACI 117-90Standard Specifications for Tolerances for Concrete Construction and MaterialsACI 117R-90Commentary on Standard Specifications for Tolerances for Concrete Construction and MaterialsACI 121R-98Quality Management System for Concrete ConstructionACI 122R-02Guide to thermal properties of concrete and masonry systemACI 209R-92Prediction of creep, shrinkage and temperature effects in concrete structuresACI 210R-93Erosion of concrete in hydraulic structuresACI 213R-87Guide for structural lightweight aggregate concreteACI 214R-02Evaluation of strength test results of concreteACI 251R-74Considerations for design of concrete structures subjected to fatigue loadingACI 216R-89Guide for determining fire endurance of concrete elementsGuide for use of normal weight and heavyweight aggregates in concreteACI 222R-01Protection of metals in concrete against corrosionACI 223-98Standard practice for the use of shrinkage -compensating concreteACI 224R-01Control of cracking in concrete structuresACI 225R-99Guide to selection and use of hydraulic elementsACI 229R-99Controlled low-strength materialACI 233R-95Ground granulated blast-furnace slag as cementitious constituent in concreteACI 234R-96Guide for the use of silica fume in concreteACI 301-99Specifications for structural concreteACI 301M-99Specifications for structural concreteACI 304R-00Guide for measuring, mixing, transporting and placing concreteACI 305R-99Hot weather concretingACI 306R-88Cold weather concretingACI 307-98Design and construction of reinforced concrete chimneysCommentary on design and construction of reinforced concrete chimneysACI 308R-01Guide to curing concreteACI 309R-96Guide for consolidation of concreteACI 313-97Standard practice for design and construction of concrete silos and stacking tubes for storing granular materialsACI 313R-97Commentary on standard practice for design and construction of concrete silos and stacking tubes for storing granular materialsACI 315-99Details and detailing of concrete reinforcementACI 318-02Building code requirements for structural concrete (ACI 318-02) and commentary (ACI 318R-02)ACI 318M-02Building code requirements for structural concrete (ACI 318M-02) and commentary (ACI 318RM-02)ACI 330R-01Guide for design and construction of concrete parking lotsACI 332R-84Guide to residential cast-in-place concrete constructionACI 343R-95Analysis and design of reinforced concrete bridge structuresACI 345R-91Guide for concrete highway bridge deck constructionACI 346-01Specification for cast-in-place concrete pipeGuide to formwork for concreteACI 349-01Code requirements for nuclear safety related concrete structuresACI 350-01Code requirements for environmental engineering concrete structures (ACI 350-01) and commentary (ACI 350R-01)ACI 352R-02Recommendations for design of beam-column connections in monolithic reinforced concrete structuresACI 357R-84Guide for the design and construction of fixed offshore concrete structuresACI 359-01Code for concrete containmentsACI 360R-92Design of slabs on gradeACI 363R-92State-of-the-art report on high strength concreteACI 371R-98Guide for the analysis, design and construction of concrete-pedestal water towersACI 372R-00Design and construction of circular wire- and strand- wrapped prestressed- concrete structuresACI 373R-97Design and construction of circular prestressed concrete structures with circumferential tendonACI 435R-95Control of deflection in concrete structuresACI 437R-91Strength evaluation of existing concrete buildingsState-of-the-art report on fiber reinforced plastic reinforcement for concrete structuresACI 441R-96High strength concrete-columns- state of the artACI 445R-99Recent approaches to shear design of structural concreteACI 503R-93Use of epoxy compounds with concreteACI 504R-90Guide to sealing joints in concrete structuresACI 506R-90Guide to shotcreteACI 524R-93Guide to Portland cement plasteringACI 530-02Building code requirements for masonry structuresACI 530R-02Commentary on building code requirements for masonry structuresACI 533R-93Guide for precast concrete wall panelsACI 543R-00Design, manufacture and installation of concrete pilesACI 546R-96Concrete repair guideACI 549R-97State-of-the-art report on ferrocementACI 550R-96Design recommendations for precast concrete structuresTilt-up concrete structuresACI 555R-01Removal and reuse of hardened concreteACI 124.2R-94The mercer mile buildingsACI 126.3R-99Guide to recommended format for concrete in material property databaseACI 201.1R-92Guide for making a condition survey of concrete in serviceACI 201.2R-01Guide to durable concreteACI 207.1R-96Mass concreteACI 207.2R-95Effect of restraint, volume change, and reinforcement on cracking of mass concreteACI 207.3R-94Practices for evaluation of concrete in existing massive structures for service conditionsACI 207.4R-93Cooling and insulating system for mass concreteACI 207.5R-99Roller-compacted mass concreteACI 210.1R-94Compendium of case histories on repair of erosion-damaged concrete in hydraulic structuresACI 211.1-91Standard practice for selecting proportions for normal, heavyweight and mass concreteStandard practice for selecting proportions for structural lightweight concreteACI 211.3R-02Guide for selecting proportions for no-slump concreteACI 211.4R-93Guide for selecting proportions for high-strength concrete with Portland cement and fly ashACI 211.5R-01Guide for submittal of concrete portionsACI 212.3R-91Chemical admixtures for concreteACI 212.4R-93Guide for the use of high-range water-reducing admixtures (superplasticizers) in concreteACI 216.1-97Standard method for determining fire resistance of concrete and masonry construction assembliesACI 221.1R-98State-of-the-art report on alkali-aggregate reactivityACI 222.1-96Provisional standard test method for water-soluable chloride available for corrosion of embedded steel in mortar and concrete using the soxhlet extractorACI 222.2R-01Corrosion of prestressing steelsACI 224.1R-93Causes, evaluation and repair of cracks in concrete structuresACI 224.2R-92Cracking of concrete members in direct tensionACI 224.3R-95Joints in concrete constructionIn-place methods to estimate concrete strengthACI 228.2R-98Nondestructive test methods for evaluation of concrete in structuresACI 230.1R-90State-of-the-art report on soil cementACI 232.1R-00Use of raw or processed natural pozzlans in concreteACI 232.2R-96Use of fly ash in concreteACI 302.1R-96Guide for concrete floor and slab constructionACI 303.1-97Standard specifications for cast-in-place architectural concreteACI 304.1R-92Guide for the use of replaced aggregate concrete for structural and mass concrete applicationsACI 304.2R-96Placing concrete by pumping methodsACI 304.3R-96Heavyweight concrete: measuring, mixing, transporting and placingACI 304.5R-91Batching, mixing and job control of lightweight concreteACI 304.6R-91Guide for the use of volumetric-measuring and continuous-mixing concrete equipmentACI 306.1-90Standard specifications for cold weather concretingStandard specification for curing concreteACI 309,1R-93Behavior of flesh concrete during vibrationACI 309.2R-98Identification and control of visible effects of consolidation on formed concrete surfacesACI 309.3R-92Guide to consolidation of concrete in congested areasACI 309.5R-00Compaction of roller-compacted concreteACI 311.1R-99ACI manual of concrete inspectionACI 311.4R-00Guide for concrete inspectionACI 311.5R-02Guide for concrete plant inspection and testing of ready-mixed concreteACI 325.3R-85Guide for foundations and shoulders for concrete pavementsACI 325.6R-88Texturing concrete pavementsACI 325.9R-91Guide for construction of concrete pavements and concrete basesACI 330.1-94Standard specification for plain concrete parking lotsACI 334.1R-92Concrete shell structures practice and commentaryACI 336.1-01Specifications for the construction of drilled piersSuggested analysis and design procedures for combined footings and matsACI 336.3R-93Design and construction of drilled piersACI 341.2R-97Seismic analysis and design of concrete bridge systemACI 345.1R-92Routine maintenance of concrete bridgesACI 345.2R-98Guide for widening highway bridgesACI 349.1R-91Reinforced concrete design for thermal effects on nuclear power plant structuresACI 349.2R-97Embedment design examplesACI 349.3R-02Evaluation of existing nuclear safety-related concrete structuresACI 350.1R-01Tightness testing of environmental engineering concrete structures (ACI 350,1-01) and commentary (350.1R-01)ACI 350.2R-97Concrete structures for containment of hazardous materialsACI 350.3-01Seismic design of liquid-containing concrete structures (ACI 350.3-01) and commentary (350.3R-01)ACI 351.1R-99Grouting between foundations and bases for support of equipment and machineryACI 351.2R-94Foundations for static equipmentRecommendations for design of slab-column connections in monolithic reinforced concrete structuresACI 355.1R-91State-of-the-art report on anchorage to concreteACI 355.2-01Evaluating the performance of post-installed mechanical anchors in concreteACI 357.1R-91State-of-the-art report on offshore concrete structures for the ArcticACI 357.2R-88State-of-the-art report on barge-like concrete structuresACI 358.1R-92Analysis and design of reinforced and prestressed-concrete guideway structuresACI 362.1R-97Guide for the design of durable parking structuresACI 362.2R-00Guide for structural maintenance of parking structuresACI 363.2R-98Guide to quality control and testing of high-strength concreteACI 364.1R-94Guide for evaluation of concrete structures prior to rehabilitationACI 365.1R-00Service-lift prediction – state-of-the-art reportACI 408.2R-92State-of-the-art report on bond under cyclic loadsACI 408.3-01/408.3R-01Splice and development length of high relative rib area reinforcing bars in tension (408.3-01) and commentary (408.3R-01)ACI 421.1R-99Shear reinforcement for slabsRecommendations for concrete members prestressed with unbonded tendonsACI 423.4R-98Corrosion and repair of unbonded single strand tendonsACI 423.5R-99State-of-the-art report on partially stressed tendonsACI 423.6-01/423.6R-01Specification for unbonded single-strand tendons and commentaryACI 435.7R-85State-of-the-art report on temperature-induced deflections of reinforced concrete membersACI 435.8R-85Observed deflections of reinforced concrete slab system, and causes of large deflectionsACI 439.3R-91Mechanical connections of reinforcing barsACI 440.1R-01Guide for design and construction of concrete reinforced with FRP barsACI 440.2R-02Guide for design and construction of externally bonded FRP systems for strengthening concrete structuresACI 446.1R-91Fracture mechanics of concrete: concepts, models and determination of material propertiesACI 446.3R-97Finite element analysis of fracture in concrete structures: state -of -the -artACI 503.1-92Standard specification for bonding hardened concrete, steel, wood, brick and other materials to hardened concrete with a multi-component epoxy adhesiveStandard specification for bonding plastic concrete to hardened concrete with a multi-component epoxy adhesiveACI 503.3-92Standard specification for producing a skid-resistant surface on concrete by the use of a multi-component epoxy systemACI 503.4-92Standard specification for repairing concrete with epoxy mortarsACI 503.5R-92Guide for selection of polymer adhesives with concreteACI 503.6R-97Guide for the application of epoxy and latex adhesives for bonding freshly mixed and hardened concretesACI 506.1R-98Committee report on fiber reinforced shotcreteACI 506.2-95Specification for shotcreteACI 506.3R-91Guide to certification of shotcrete nozzlemenACI 506.4R-94Guide for evaluation of shotcreteACI 532.2R-96Guide for precast cellular concrete floor, roof and wall unitsACI 530.1-02Specifications for masonry structuresACI 530.1R-02Commentary on specifications for masonry structuresACI 533.1R-02Design responsibility for architectural precast concrete projectsState-of-the-art report on fiber reinforced concreteACI 544.2R-89Measurement of properties of fiber reinforced concreteACI 544.3R-93Guide for specifying, proportioning, mixng, placing and finishing steel fiber reinforced concreteACI 544.4R-88Design considerations for steel fiber reinforced concreteACI 546.2R-98Guide to underwater repair of concreteACI 548.1R-97Guide for the use of polymers in concreteACI 548.2R-93Guide for mixing and placing sulfur concrete in constructionACI 548.4-93Standard specification for latex-modified concrete (LMC) overlaysACI 548.5R-94Guide for polymer concrete overlaysACI 549.1R-93Guide for design, construction and repair of ferrocementACI 550.1R-01Emulating cast-in-place detailing in precast concrete structuresACI 325.10R-95Report on roller-compacted concrete pavementsACI 325.11R-01Accelerated techniques for concrete pavingACI 325.12R-02Guide for design of jointed concrete pavements for streets and local roadsSP-4Formwork for concreteSP-15(99)Field reference manualACI 340R-97ACI design handbook – design of structural reinforced concrete elements in accordance with the strength design method of ACI 318-95SP-66(94)ACI detailing manualSP-71(02)ASTM standards in ACI 318ACI T1.1-01Acceptance criteria for moment frames based on structural testing。
紧密堆积混凝土配合比设计方法研究

摘要水泥混凝土广泛应用于基础建设各个领域,随着经济发展、科技进步,人们对其使用品质要求越来越高。
现行混凝土配合比设计方法设计的混凝土以悬浮密实型结构为主,易在集料与水泥石粘结处发生破坏,且尚未充分发挥粗集料的作用。
为此,本研究在体积法的基础上,提出了粗集料紧密堆积结构与紧密堆积型水泥混凝土概念,并对其工作性、强度特性及其设计方法开展了系统研究,以期节约成本,提高混凝土性能,具有重要工程实用价值。
粗集料紧密堆积结构是指骨架颗粒与填充颗粒之间充分嵌锁、紧密排列、不干涉或少干涉,使其达到合理密实状态时形成一个多级空间骨架结构;在此基础上,利用砂填充粗集料振实剩余空隙,粉煤灰作为填充砂振实剩余空隙,再用水泥净浆润滑和填充混合料的剩余空隙,形成紧密堆积型水泥混凝土。
综合研究成果,提出了紧密堆积型混凝土配合比设计方法,并与现行设计方法对比表明,同等强度、工作性要求下,紧密堆积型水泥混凝土比现行设计方法确定的混凝土的经济性更好,且设计方法可操作性强,简便实用,可以直接应用于工程实际。
关键词:水泥混凝土,工作性,强度特性,配合比设计方法ABSTRACTConcrete widely used in infrastructure construction in various fields, along with economic development, scientific and technological progress, people use their increasingly high-quality. The existing design of concrete mix designed to suspension-compacting concrete structure-oriented and easy to damage in bonding of aggregate and cement, and has yet to give full play to the role of coarse aggregate. For this reason, basing on the Volume and Interference theory, the study put forward a coarse aggregate embedded lock skeleton structure and embedded lock dense concrete concept, and research systematically on its working, strength and design, with a view save costs and improve the properties of concrete,important works have practical value.The coarse aggregate embedded lock skeleton structure is that skeleton particles embed fully, work closely, non-interference or less with peanuts, and to reach a state of reasonable density to form a multi-level space frame structure; on this basis, use sand to fill coarse aggregate remaining gap, use cement paste to lubricate and fill the remaining gap of coarse aggregate and sand mixture, forming dense embedded lock-cement concrete.Comprehensive research results, put forward the embedded lock-dense concrete mix design method, and compared the existing design methods show that the same intensity, working, embedded lock density cement concrete cement concrete mix design are better than the existing concrete on economy better, can be highly workable, simple and practical, can be directly applied to engineering practice.Key words: cement concrete, working, strength, mix design methods目录摘要 (I)ABSTRACT (II)第一章绪论 (1)1.1引言 (1)1.2研究背景 (1)1.3 国内外混凝土配合比设计方法研究概况 (3)1.4 主要研究内容与技术路线 (7)1.4.1 主要研究内容 (7)1.4.2 技术路线 (7)第二章普通混凝土、紧密堆积混凝土 (9)2.1普通混凝土配合比设计 (9)2.1.1普通水泥混凝土(ordinary cement concrete) (9)2.1.2普通水泥混凝土的组成设计 (9)2.2紧密堆积混凝土配合比设计 (14)第三章试验研究 (17)3.1试验设计思想 (17)3.2试验方法 (17)3.3试验仪器及设备 (18)3.4试验原材料选择 (18)3.4.1水泥 (18)3.4.2粗集料 (19)3.4.3细集料 (19)3.4.4水 (20)3.4.5原材料试验 (20)3.5初步紧密堆积混凝土试验 (25)3.5.1初步试验设计 (25)3.5.2初步试验结果及分析 (26)3.6对比试验 (28)3.6.1对比试验设计 (27)3.6.2对比试验结果及分析 (28)3.7 综合对比分析 (28)第四章经济技术分析 (29)4.1经济效益分析 (29)4.2环境效益分析 (30)第五章结论与建议 (31)5.1结论 (31)5.2建议 (32)参考文献 (33)致谢: (34)第一章绪论1.1引言建筑工程的质量问题是关系到国家人民生命财产安危的千年大计。
水泥混凝土方面的SCI期刊

水泥混凝土方面的SCI期刊Construction and Building MaterialsJournal of Engineering Materials and Technologyadvances in cement researchConcrete Science and Engineering以下提供与建筑材料相关SCI期刊的基本信息!希望对大家的投稿能起到一定的帮助作用。
ADVANCES IN CEMENT RESEARCH(1) Cement and concrete researchImpact factor: 0.727(2006)1.028 (2007)Full Journal Title: CEMENT AND CONCRETE RESEARCHISO Abbrev. Title: Cem. Concr. Res.JCR Abbrev. Title: CEMENT CONCRETE RESISSN: 0008-8846Issues/Year: 12Language: MULTI-LANGUAGEJournal Country/Territory: ENGLANDPublisher: PERGAMON-ELSEVIER SCIENCE LTDPublisher Address: THE BOULEVARD, LANGFORD LANE, KIDLINGTON, OXFORD OX5 1GB, ENGLANDSubject Categories: CONSTRUCTION & BUILDING TECHNOLOGYMATERIALS SCIENCE, MULTIDISCIPLINARY(2) Cement concrete and aggregate (Stop publication)Impact factor: 0.067 (2006)Full Journal Title: CEMENT CONCRETE AND AGGREGATESISO Abbrev. Title: Cem. Concr. Aggreg.JCR Abbrev. Title: CEMENT CONCRETE AGGRISSN: 0149-6123Issues/Year: 2Language: ENGLISHJournal Country/Territory: UNITED STATESPublisher: AMER SOC TESTING MATERIALSPublisher Address: 100 BARR HARBOR DR, W CONSHOHOCKEN, PA 19428-2959Subject Categories: CONSTRUCTION & BUILDING TECHNOLOGY MATERIALS SCIENCE, MULTIDISCIPLINARY(3) CEMENT & CONCRETE COMPOSITESImpact factor: 0.457(2006)0.962(2007)Full Journal Title: CEMENT & CONCRETE COMPOSITESISO Abbrev. Title: Cem. Concr. Compos.JCR Abbrev. Title: CEMENT CONCRETE COMPISSN: 0958-9465Issues/Year: 8Language: ENGLISHJournal Country/Territory: ENGLANDPublisher: ELSEVIER SCI LTDPublisher Address: THE BOULEVARD, LANGFORD LANE, KIDLINGTON, OXFORD OX5 1GB, OXON, ENGLANDSubject Categories: CONSTRUCTION & BUILDING TECHNOLOGY MATERIALS SCIENCE, COMPOSITES(4) ADVANCES IN CEMENT RESEARCHImpact factor: 0.5 (2006)0.4 (2007)Full Journal Title: ADVANCES IN CEMENT RESEARCHISO Abbrev. Title: Adv. Cem. Res.JCR Abbrev. Title: ADV CEM RESISSN: 0951-7197Issues/Year: 4Language: ENGLISHJournal Country/Territory: ENGLANDPublisher: THOMAS TELFORD PUBLISHINGPublisher Address: THOMAS TELFORD HOUSE, 1 HERON QUAY, LONDON E14 4JD, ENGLANDSubject Categories: CONSTRUCTION & BUILDING TECHNOLOGY MATERIALS SCIENCE, MULTIDISCIPLINARY(5) JOURNAL OF MATERIALS IN CIVIL ENGINEERINGImpact factor: 0.449 (2006)0.452 (2007)Full Journal Title: JOURNAL OF MATERIALS IN CIVIL ENGINEERINGISO Abbrev. Title: J. Mater. Civ. Eng.JCR Abbrev. Title: J MATER CIVIL ENGISSN: 0899-1561Issues/Year: 4Language: ENGLISHJournal Country/Territory: UNITED STATESPublisher: ASCE-AMER SOC CIVIL ENGINEERSPublisher Address: 1801 ALEXANDER BELL DR,RESTON, VA 20191-4400Subject Categories: CONSTRUCTION & BUILDING TECHNOLOGY ENGINEERING, CIVIL MATERIALS SCIENCE,MULTIDISCIPLINARY(6) ACI MATERIALS JOURNALImpact factor: 0.419 (2006)0.670 (2007)Full Journal Title: ACI MATERIALS JOURNALISO Abbrev. Title: ACI Mater. J.JCR Abbrev. Title: ACI MATER JISSN: 0889-325XIssues/Year: 6Language: ENGLISHJournal Country/Territory: UNITED STATESPublisher: AMER CONCRETE INSTPublisher Address: 38800 INTERNATIONAL WAY, COUNTRY CLUB DRIVE, PO BOX 9094, FARMINGTON HILLS, MI 48333-9094 Subject Categories: CONSTRUCTION & BUILDING TECHNOLOGY MATERIALS SCIENCE, MULTIDISCIPLINARY(7) CONSTRUCTION AND BUILDING MATERIALSImpact factor: 0.343 (2006)0.841 (2007)Full Journal Title: CONSTRUCTION AND BUILDING MATERIALSISO Abbrev. Title: Constr. Build. Mater.JCR Abbrev. Title: CONSTR BUILD MATERISSN: 0950-0618Issues/Year: 8Language: ENGLISHJournal Country/Territory: ENGLANDPublisher: ELSEVIER SCI LTDPublisher Address: THE BOULEVARD, LANGFORD LANE, KIDLINGTON, OXFORD OX5 1GB, OXON, ENGLANDSubject Categories: CONSTRUCTION & BUILDING TECHNOLOGY MATERIALS SCIENCE, MULTIDISCIPLINARY(8) MAGAZINE OF CONCRETE RESEARCHImpact factor: 0.379(2006)0.317 (2007)Full Journal Title: MAGAZINE OF CONCRETE RESEARCHISO Abbrev. Title: Mag. Concr. Res.JCR Abbrev. Title: MAG CONCRETE RESISSN: 0024-9831Issues/Year: 6Language: ENGLISHJournal Country/Territory: ENGLANDPublisher: THOMAS TELFORD PUBLISHINGPublisher Address: THOMAS TELFORD HOUSE, 1 HERON QUAY, LONDON E14 4JD, ENGLANDSubject Categories: CONSTRUCTION & BUILDING TECHNOLOGYMATERIALS SCIENCE, MULTIDISCIPLINARY(9) INTERNATIONAL JOURNAL OF IMPACT ENGINEERINGImpact factor: 0.824 (2007)Full Journal Title: INTERNATIONAL JOURNAL OF IMPACT ENGINEERINGISO Abbrev. Title: Int. J. Impact Eng.JCR Abbrev. Title: INT J IMPACT ENGISSN: 0734-743XIssues/Year: 10Language: ENGLISHJournal Country/Territory: ENGLANDPublisher: PERGAMON-ELSEVIER SCIENCE LTDPublisher Address: THE BOULEVARD, LANGFORD LANE, KIDLINGTON, OXFORD OX5 1GB, ENGLANDSubject Categories: ENGINEERING, MECHANICAL(10) ENGINEERING FRACTURE MECHANICSImpact factor: 1.227 (2007)Full Journal Title: ENGINEERING FRACTURE MECHANICSISO Abbrev. Title: Eng. Fract. Mech.JCR Abbrev. Title: ENG FRACT MECHISSN: 0013-7944Issues/Year: 18Language: MULTI-LANGUAGEJournal Country/Territory: ENGLANDPublisher: PERGAMON-ELSEVIER SCIENCE LTDPublisher Address: THE BOULEVARD, LANGFORD LANE, KIDLINGTON, OXFORD OX5 1GB, ENGLANDSubject Categories: MECHANICS(11) International Journal of FractureImpact factor: 1.003(2007)Full Journal Title: INTERNATIONAL JOURNAL OF FRACTURE ISO Abbrev. Title:Int. J. Fract.JCR Abbrev. Title: INT J FRACTUREISSN: 0376-9429Issues/Year: 18Language: ENGLISHJournal Country/Territory: NETHERLANDSPublisher: SPRINGERPublisher Address: VAN GODEWIJCKSTRAAT 30, 3311 GZ DORDRECHT, NETHERLANDSSubject Categories: MECHANICS(12) THEORETICAL AND APPLIED FRACTURE MECHANICSImpact factor: 0.781 (2007)Full Journal Title: THEORETICAL AND APPLIED FRACTURE MECHANICSISO Abbrev. Title: Theor. Appl. Fract. Mech.JCR Abbrev. Title: THEOR APPL FRACT MECISSN: 0167-8442Issues/Year: 6Language: ENGLISHJournal Country/Territory: NETHERLANDSPublisher: ELSEVIER SCIENCE BVPublisher Address: PO BOX 211, 1000 AE AMSTERDAM, NETHERLANDSSubject Categories: ENGINEERING, MECHANICALMECHANICS(13) FATIGUE & FRACTURE OF ENGINEERING MATERIALS & STRUCTURESImpact factor: 0.726 (2007)Full Journal Title: FATIGUE & FRACTURE OF ENGINEERING MATERIALS & STRUCTURESISO Abbrev. Title: Fatigue Fract. Eng. Mater. Struct.JCR Abbrev. Title: FATIGUE FRACT ENG MISSN: 8756-758XIssues/Year: 12Language: ENGLISHJournal Country/Territory: ENGLANDPublisher: BLACKWELL PUBLISHINGPublisher Address: 9600 GARSINGTON RD, OXFORD OX4 2ZG, OXON, ENGLANDSubject Categories: ENGINEERING, MECHANICALMATERIALS SCIENCE, MULTIDISCIPLINARY(14) StrainImpact factor: 0.642 (2007)Full Journal Title: STRAINISO Abbrev. Title: StrainJCR Abbrev. Title: STRAINISSN: 0039-2103Issues/Year: 4Language: ENGLISHJournal Country/Territory: ENGLANDPublisher: BLACKWELL PUBLISHINGPublisher Address: 9600 GARSINGTON RD, OXFORD OX4 2ZG, OXON, ENGLANDSubject Categories: MATERIALS SCIENCE, CHARACTERIZATION & TESTING(15) Computers and ConcreteImpact factor: 0.351 (2007)Full Journal Title: Computers and ConcreteISO Abbrev. Title: Comput. Concr.JCR Abbrev. Title: COMPUT CONCRETEISSN: 1598-8198Issues/Year: 6Language: ENGLISHJournal Country/Territory: SOUTH KOREAPublisher: TECHNO-PRESSPublisher Address: PO BOX 33, YUSEONG, DAEJEON 305-600, SOUTH KOREASubject Categories: COMPUTER SCIENCE, INTERDISCIPLINARY APPLICATIONSCONSTRUCTION & BUILDING TECHNOLOGYENGINEERING, CIVILMATERIALS SCIENCE, CHARACTERIZATION & TESTING(16) EXPERIMENTAL MECHANICSImpact factor: 0.985 (2007)Full Journal Title: EXPERIMENTAL MECHANICSISO Abbrev. Title: Exp. Mech.JCR Abbrev. Title: EXP MECHISSN: 0014-4851Issues/Year: 4Language: ENGLISHJournal Country/Territory: ENGLANDPublisher: SPRINGERPublisher Address: 233 SPRING STREET, NEW YORK, NY 10013Subject Categories: MATERIALS SCIENCE, MULTIDISCIPLINARYMECHANICSMATERIALS SCIENCE, CHARACTERIZATION & TESTING(17) ENGINEERING FAILURE ANALYSISImpact factor: 0.565Full Journal Title: ENGINEERING FAILURE ANALYSISISO Abbrev. Title: Eng. Fail. Anal.JCR Abbrev. Title: ENG FAIL ANALISSN: 1350-6307Issues/Year: 6Language: ENGLISHJournal Country/Territory: ENGLANDPublisher: PERGAMON-ELSEVIER SCIENCE LTDPublisher Address: THE BOULEVARD, LANGFORD LANE, KIDLINGTON, OXFORD OX5 1GB, ENGLANDSubject Categories: ENGINEERING, MECHANICALMATERIALS SCIENCE, CHARACTERIZATION & TESTING(18) Materials and StructuresImpact factor: 0.892 (2008)Full Journal Title: MATERIALS AND STRUCTURESISO Abbrev. Title: Mater. Struct.JCR Abbrev. Title: MATER STRUCTISSN: 1359-5997Issues/Year: 10Language: MULTI-LANGUAGEJournal Country/Territory: NETHERLANDSPublisher: SPRINGERPublisher Address: VAN GODEWIJCKSTRAAT 30, 3311 GZ DORDRECHT, NETHERLANDSSubject Categories:CONSTRUCTION & BUILDINGTECHNOLOGY ENGINEERING, CIVIL MATERIALS SCIENCE, MULTIDISCIPLINARY。
ASTRAL评分对急性前后循环脑梗死预后预测价值的比较_王大力

Role of ASTRAL score in predicting prognosis of anterior and posterior circulation acute cerebral infarction
WANG Da-li,Fment of Neurology,Affiliated Hospital of North China University of Science and Ttechnology,Tangshan063000,Hebei Province,china)
Abstract:Objective To study the role of ASTRAL score in predicting the prognosis of anterior and posterior circulation acute cerebral infarction (ACI).Methods Six hundred and ninety-two ACI patients were divided into anterior circulation ACI group (n=481)and posterior circulation ACI group(n=211)according to their head MRI.Patients were assessed with a ASTRAL score within 24hours after admission.The outcome of the 30day of onset was the end of the study. Good prognosis was defined when the mRS score was 0-2and poor prognosis was defined when the mRS score was 3-5.The role of ASTRAL score in predicting the prognosis of anterior and posterior circulation ACI was analyzed according to its ROC curve.Results There were 81anteri- or circulation patients with poor prognosis,and there were 44posterior circulation infarction pa- tients with poor prognosis.The ASTRAL score was lower in ACI patients with a good prognosis than in those with a poor prognosis(17.30±3.66 vs 26.81±7.90,17.25±4.11 vs 23.66±7.28, P<0.01).The rate of poor prognosis increased with the increasing ASTRAL score.The area un- der the ROC curve for ASTRAL was 0.902and 0.788respectively.The best cut-off points was 22 and 19respectively.The sensitivity of anterior circulation and posterior circulation infarction was 79.0% and 72.7% respectively,and the specificity was 88.2% and 68.9% respectively.Conclu- sion ASTRAL score can predict the poor prognosis of ACI patients. Key words:brain infarction;ROC curve;magnetic resonance imaging;forecasting;prognosis
21CFR PART211(FDA的cGMP)

[Code of Federal Regulations][Title 21, Volume 4][Revised as of April 1, 2010][CITE: 21CFR211]TITLE 21--FOOD AND DRUGSCHAPTER I--FOOD AND DRUG ADMINISTRATIONDEPARTMENT OF HEALTH AND HUMAN SERVICESSUBCHAPTER C--DRUGS: GENERALPART 211 CURRENT GOOD MANUFACTURING PRACTICE FOR FINISHED PHARMACEUTICALSSubpart A--General ProvisionsSec. 211.1 Scope.(a) The regulations in this part contain the minimum current good manufacturing practice for preparation of drug products for administration to humans or animals.(b) The current good manufacturing practice regulations in this chapter as they pertain to drug products; in parts 600 through 680 of this chapter, as they pertain to drugs that are also biological products for human use; and in part 1271 of this chapter, as they are applicable to drugs that are also human cells, tissues, and cellular and tissue-based products (HCT/Ps) and that are drugs (subject to review under an application submitted under section 505 of the act or under a biological product license application under section 351 of the Public Health Service Act); supplement and do not supersede the regulations in this part unless the regulations explicitly provide otherwise. In the event of a conflict between applicable regulations in this part and in other parts of this chapter, or in parts 600 through680 of this chapter, or in part 1271 of this chapter, the regulation specifically applicable to the drug product in question shall supersede the more general.(c) Pending consideration of a proposed exemption, published in theFederal Registerof September 29, 1978, the requirements in this part shall not be enforced for OTC drug products if the products and all their ingredients are ordinarily marketed and consumed as human foods, and which products may also fall within the legal definition of drugs by virtue of their intended use. Therefore, until further notice, regulations under part 110 of this chapter, and where applicable, parts 113 to 129 of this chapter, shall be applied in determining whether these OTC drug products that are also foods are manufactured, processed, packed, or held under current good manufacturing practice.Link to an amendment published at 74 FR 65431, Dec. 10, 2009.[43 FR 45077, Sept. 29, 1978, as amended at 62 FR 66522, Dec. 19, 1997; 69 FR 29828, May 25, 2004]Sec. 211.3 Definitions.The definitions set forth in 210.3 of this chapter apply in this part.Subpart B--Organization and PersonnelSec. 211.22 Responsibilities of quality control unit.(a) There shall be a quality control unit that shall have the responsibility and authority to approve or reject all components, drug product containers, closures, in-process materials, packaging material, labeling, and drug products, and the authority to review production records to assure that no errors have occurred or, if errors have occurred,that they have been fully investigated. The quality control unit shall be responsible for approving or rejecting drug products manufactured, processed, packed, or held under contract by another company.(b) Adequate laboratory facilities for the testing and approval (or rejection) of components, drug product containers, closures, packaging materials, in-process materials, and drug products shall be available to the quality control unit.(c) The quality control unit shall have the responsibility for approving or rejecting all procedures or specifications impacting on the identity, strength, quality, and purity of the drug product.(d) The responsibilities and procedures applicable to the quality control unit shall be in writing; such written procedures shall be followed.Sec. 211.25 Personnel qualifications.(a) Each person engaged in the manufacture, processing, packing, or holding of a drug product shall have education, training, and experience, or any combination thereof, to enable that person to perform the assigned functions. Training shall be in the particular operations that the employee performs and in current good manufacturing practice (including the current good manufacturing practice regulations in this chapter and written procedures required by these regulations) as they relate to the employee's functions. Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with sufficient frequency to assure that employees remain familiar with CGMP requirements applicable to them.(b) Each person responsible for supervising the manufacture, processing, packing, or holding of a drug product shall have the education, training, and experience, or any combination thereof, to perform assigned functions in such a manner as to provide assurance that the drug product has the safety, identity, strength, quality, and purity that it purportsor is represented to possess.(c) There shall be an adequate number of qualified personnel to perform and supervise the manufacture, processing, packing, or holding of each drug product.Sec. 211.28 Personnel responsibilities.(a) Personnel engaged in the manufacture, processing, packing, or holding of a drug product shall wear clean clothing appropriate for the duties they perform. Protective apparel, such as head, face, hand, and arm coverings, shall be worn as necessary to protect drug products from contamination.(b) Personnel shall practice good sanitation and health habits.(c) Only personnel authorized by supervisory personnel shall enter those areas of the buildings and facilities designated as limited-access areas.(d) Any person shown at any time (either by medical examination or supervisory observation) to have an apparent illness or open lesions that may adversely affect the safety or quality of drug products shall be excluded from direct contact with components, drug product containers, closures, in-process materials, and drug products until the condition is corrected or determined by competent medical personnel not to jeopardize the safety or quality of drug products. All personnel shall be instructed to report to supervisory personnel any health conditions that may have an adverse effect on drug products.Sec. 211.34 Consultants.Consultants advising on the manufacture, processing, packing, or holding of drug products shall have sufficient education, training, and experience, or any combination thereof, to advise on the subject for which they are retained. Records shall be maintained stating the name, address, and qualifications of any consultants and the type of service they provide.Subpart C--Buildings and FacilitiesSec. 211.42 Design and construction features.(a) Any building or buildings used in the manufacture, processing, packing, or holding of a drug product shall be of suitable size, construction and location to facilitate cleaning, maintenance, and proper operations.(b) Any such building shall have adequate space for the orderly placement of equipment and materials to prevent mixups between different components, drug product containers, closures, labeling, in-process materials, or drug products, and to prevent contamination. The flow of components, drug product containers, closures, labeling, in-process materials, and drug products through the building or buildings shall be designed to prevent contamination.(c) Operations shall be performed within specifically defined areas of adequate size. There shall be separate or defined areas or such other control systems for the firm's operations as are necessary to prevent contamination or mixups during the course of the following procedures:(1) Receipt, identification, storage, and withholding from use of components, drug product containers, closures, and labeling, pending the appropriate sampling, testing, or examination by the quality control unit before release for manufacturing or packaging;(2) Holding rejected components, drug product containers, closures, and labeling before disposition;(3) Storage of released components, drug product containers, closures, and labeling;(4) Storage of in-process materials;(5) Manufacturing and processing operations;(6) Packaging and labeling operations;(7) Quarantine storage before release of drug products;(8) Storage of drug products after release;(9) Control and laboratory operations;(10) Aseptic processing, which includes as appropriate:(i) Floors, walls, and ceilings of smooth, hard surfaces that are easily cleanable;(ii) Temperature and humidity controls;(iii) An air supply filtered through high-efficiency particulate air filters under positive pressure, regardless of whether flow is laminar or nonlaminar;(iv) A system for monitoring environmental conditions;(v) A system for cleaning and disinfecting the room and equipment to produce aseptic conditions;(vi) A system for maintaining any equipment used to control the aseptic conditions.(d) Operations relating to the manufacture, processing, and packing of penicillin shall be performed in facilities separate from those used for other drug products for human use.[43 FR 45077, Sept. 29, 1978, as amended at 60 FR 4091, Jan. 20, 1995]Sec. 211.44 Lighting.Adequate lighting shall be provided in all areas.Sec. 211.46 Ventilation, air filtration, air heating and cooling.(a) Adequate ventilation shall be provided.(b) Equipment for adequate control over air pressure, micro-organisms, dust, humidity, and temperature shall be provided when appropriate for the manufacture, processing, packing, or holding of a drug product.(c) Air filtration systems, including prefilters and particulate matter air filters, shall be used when appropriate on air supplies to production areas. If air is recirculated to production areas, measures shall be taken to controlrecirculation of dust from production. In areas where air contamination occurs during production, there shall be adequate exhaust systems or other systems adequate to control contaminants.(d) Air-handling systems for the manufacture, processing, and packing of penicillin shall be completely separate from those for other drug products for human use.Sec. 211.48 Plumbing.(a) Potable water shall be supplied under continuous positive pressure in a plumbing system free of defects that could contribute contamination to any drug product. Potable water shall meet the standards prescribed in the Environmental Protection Agency's Primary Drinking Water Regulations set forth in 40 CFR part 141. Water not meeting such standards shall not be permitted in the potable water system.(b) Drains shall be of adequate size and, where connected directly to a sewer, shall be provided with an air break or other mechanical device to prevent back-siphonage.[43 FR 45077, Sept. 29, 1978, as amended at 48 FR 11426, Mar. 18, 1983]Sec. 211.50 Sewage and refuse.Sewage, trash, and other refuse in and from the building and immediate premises shall be disposed of in a safe and sanitary manner.Sec. 211.52 Washing and toilet facilities.Adequate washing facilities shall be provided, including hot and cold water, soap or detergent, air driers or single-service towels, and clean toilet facilities easily accesible to working areas.Sec. 211.56 Sanitation.(a) Any building used in the manufacture, processing, packing, or holding of a drug product shall be maintained in a clean and sanitary condition, Any such building shall be free of infestation by rodents, birds, insects, and other vermin (other than laboratory animals). Trash and organic waste matter shall be held and disposed of in a timely and sanitary manner.(b) There shall be written procedures assigning responsibility for sanitation and describing in sufficient detail the cleaning schedules, methods, equipment, and materials to be used in cleaning the buildings and facilities; such written procedures shall be followed.(c) There shall be written procedures for use of suitable rodenticides, insecticides, fungicides, fumigating agents, and cleaning and sanitizing agents. Such written procedures shall be designed to prevent the contamination of equipment, components, drug product containers, closures, packaging, labeling materials, or drug products and shall be followed. Rodenticides, insecticides, and fungicides shall not be used unless registered and used in accordance with the Federal Insecticide, Fungicide, and Rodenticide Act (7 U.S.C. 135).(d) Sanitation procedures shall apply to work performed by contractors or temporary employees as well as work performed by full-time employees during the ordinary course of operations.Sec. 211.58 Maintenance.Any building used in the manufacture, processing, packing, or holding of a drug product shall be maintained in a good state of repair.Subpart D--EquipmentSec. 211.63 Equipment design, size, and location.Equipment used in the manufacture, processing, packing, or holding of a drug product shall be of appropriate design, adequate size, and suitably located to facilitate operations for its intended use and for its cleaning and maintenance.Sec. 211.65 Equipment construction.(a) Equipment shall be constructed so that surfaces that contact components, in-process materials, or drug products shall not be reactive, additive, or absorptive so as to alter the safety, identity, strength, quality, or purity of the drug product beyond the official or other established requirements.(b) Any substances required for operation, such as lubricants or coolants, shall not come into contact with components, drug product containers, closures, in-process materials, or drug products so as to alter the safety, identity, strength, quality, or purity of the drug product beyond the official or other established requirements.Sec. 211.67 Equipment cleaning and maintenance.(a) Equipment and utensils shall be cleaned, maintained, and, as appropriate for the nature of the drug, sanitized and/or sterilized at appropriate intervals to prevent malfunctions or contamination that would alter the safety, identity,strength, quality, or purity of the drug product beyond the official or other established requirements.(b) Written procedures shall be established and followed for cleaning and maintenance of equipment, including utensils, used in the manufacture, processing, packing, or holding of a drug product. These procedures shall include, but are not necessarily limited to, the following:(1) Assignment of responsibility for cleaning and maintaining equipment;(2) Maintenance and cleaning schedules, including, where appropriate, sanitizing schedules;(3) A description in sufficient detail of the methods, equipment, and materials used in cleaning and maintenance operations, and the methods of disassembling and reassembling equipment as necessary to assure proper cleaning and maintenance;(4) Removal or obliteration of previous batch identification;(5) Protection of clean equipment from contamination prior to use;(6) Inspection of equipment for cleanliness immediately before use.(c) Records shall be kept of maintenance, cleaning, sanitizing, and inspection as specified in 211.180 and 211.182.[43 FR 45077, Sept. 29, 1978, as amended at 73 FR 51931, Sept. 8, 2008]Sec. 211.68 Automatic, mechanical, and electronic equipment.(a) Automatic, mechanical, or electronic equipment or other types of equipment, including computers, or related systems that will perform a function satisfactorily, may be used in the manufacture, processing, packing, and holding of a drug product. If such equipment is so used, it shall be routinely calibrated, inspected, or checked according to a written program designed to assure proper performance. Written records of those calibration checks and inspections shall be maintained.(b) Appropriate controls shall be exercised over computer or related systems to assure that changes in master production and control records or other records are instituted only by authorized personnel. Input to and output from the computer or related system of formulas or other records or data shall be checked for accuracy. The degree and frequency of input/output verification shall be based on the complexity and reliability of the computer or related system. A backup file of data entered into the computer or related system shall be maintained except where certain data, such as calculations performed in connection with laboratory analysis, are eliminated by computerization or other automated processes. In such instances a written record of the program shall be maintained along with appropriate validation data. Hard copy or alternative systems, such as duplicates, tapes, or microfilm, designed to assure that backup data are exact and complete and that it is secure from alteration, inadvertent erasures, or loss shall be maintained.(c) Such automated equipment used for performance of operations addressed by 211.101(c) or (d), 211.103, 211.182, or 211.188(b)(11) can satisfy the requirements included in those sections relating to the performance of an operation by one person and checking by another person if such equipment is used in conformity with this section, and one person checks that the equipment properly performed the operation.[43 FR 45077, Sept. 29, 1978, as amended at 60 FR 4091, Jan. 20, 1995; 73 FR 51932, Sept. 8, 2008]Sec. 211.72 Filters.Filters for liquid filtration used in the manufacture, processing, or packing of injectable drug products intended for human use shall not release fibers into such products. Fiber-releasing filters may be used when it is not possible to manufacture such products without the use of these filters. If use of a fiber-releasing filter is necessary, an additional nonfiber-releasing filter having a maximum nominal pore size rating of 0.2 micron (0.45 micron if the manufacturing conditions so dictate) shall subsequently be used to reduce the content of particles in the injectable drug product. The use of an asbestos-containing filter is prohibited.[73 FR 51932, Sept. 8, 2008]Subpart E--Control of Components and Drug Product Containers and ClosuresSec. 211.80 General requirements.(a) There shall be written procedures describing in sufficient detail the receipt, identification, storage, handling, sampling, testing, and approval or rejection of components and drug product containers and closures; such written procedures shall be followed.(b) Components and drug product containers and closures shall at all times be handled and stored in a manner to prevent contamination.(c) Bagged or boxed components of drug product containers, or closures shall be stored off the floor and suitably spaced to permit cleaning and inspection.(d) Each container or grouping of containers for components or drug product containers, or closures shall be identified with a distinctive code for each lot in each shipment received. This code shall be used in recording the dispositionof each lot. Each lot shall be appropriately identified as to its status (i.e., quarantined, approved, or rejected). Sec. 211.82 Receipt and storage of untested components, drug product containers, and closures.(a) Upon receipt and before acceptance, each container or grouping of containers of components, drug product containers, and closures shall be examined visually for appropriate labeling as to contents, container damage or broken seals, and contamination.(b) Components, drug product containers, and closures shall be stored under quarantine until they have been tested or examined, whichever is appropriate, and released. Storage within the area shall conform to the requirements of 211.80.[43 FR 45077, Sept. 29, 1978, as amended at 73 FR 51932, Sept. 8, 2008]Sec. 211.84 Testing and approval or rejection of components, drug product containers, and closures.(a) Each lot of components, drug product containers, and closures shall be withheld from use until the lot has been sampled, tested, or examined, as appropriate, and released for use by the quality control unit.(b) Representative samples of each shipment of each lot shall be collected for testing or examination. The number of containers to be sampled, and the amount of material to be taken from each container, shall be based upon appropriate criteria such as statistical criteria for component variability, confidence levels, and degree of precision desired, the past quality history of the supplier, and the quantity needed for analysis and reserve where required by 211.170.(c) Samples shall be collected in accordance with the following procedures:(1) The containers of components selected shall be cleaned when necessary in a manner to prevent introduction of contaminants into the component.(2) The containers shall be opened, sampled, and resealed in a manner designed to prevent contamination of their contents and contamination of other components, drug product containers, or closures.(3) Sterile equipment and aseptic sampling techniques shall be used when necessary.(4) If it is necessary to sample a component from the top, middle, and bottom of its container, such sample subdivisions shall not be composited for testing.(5) Sample containers shall be identified so that the following information can be determined: name of the material sampled, the lot number, the container from which the sample was taken, the date on which the sample was taken, and the name of the person who collected the sample.(6) Containers from which samples have been taken shall be marked to show that samples have been removed from them.(d) Samples shall be examined and tested as follows:(1) At least one test shall be conducted to verify the identity of each component of a drug product. Specific identity tests, if they exist, shall be used.(2) Each component shall be tested for conformity with all appropriate written specifications for purity, strength, and quality. In lieu of such testing by the manufacturer, a report of analysis may be accepted from the supplier ofa component, provided that at least one specific identity test is conducted on such component by the manufacturer, and provided that the manufacturer establishes the reliability of the supplier's analyses through appropriate validation of the supplier's test results at appropriate intervals.(3) Containers and closures shall be tested for conformity with all appropriate written specifications. In lieu of such testing by the manufacturer, a certificate of testing may be accepted from the supplier, provided that at least a visual identification is conducted on such containers/closures by the manufacturer and provided that the manufacturer establishes the reliability of the supplier's test results through appropriate validation of the supplier's test results at appropriate intervals.(4) When appropriate, components shall be microscopically examined.(5) Each lot of a component, drug product container, or closure that is liable to contamination with filth, insect infestation, or other extraneous adulterant shall be examined against established specifications for such contamination.(6) Each lot of a component, drug product container, or closure with potential for microbiological contamination that is objectionable in view of its intended use shall be subjected to microbiological tests before use.(e) Any lot of components, drug product containers, or closures that meets the appropriate written specifications of identity, strength, quality, and purity and related tests under paragraph (d) of this section may be approved and released for use. Any lot of such material that does not meet such specifications shall be rejected.[43 FR 45077, Sept. 29, 1978, as amended at 63 FR 14356, Mar. 25, 1998; 73 FR 51932, Sept. 8, 2008]Sec. 211.86 Use of approved components, drug product containers, and closures.Components, drug product containers, and closures approved for use shall be rotated so that the oldest approved stock is used first. Deviation from this requirement is permitted if such deviation is temporary and appropriate.Sec. 211.87 Retesting of approved components, drug product containers, and closures.Components, drug product containers, and closures shall be retested or reexamined, as appropriate, for identity, strength, quality, and purity and approved or rejected by the quality control unit in accordance with 211.84 as necessary, e.g., after storage for long periods or after exposure to air, heat or other conditions that might adversely affect the component, drug product container, or closure.Sec. 211.89 Rejected components, drug product containers, and closures.Rejected components, drug product containers, and closures shall be identified and controlled under a quarantine system designed to prevent their use in manufacturing or processing operations for which they are unsuitable.Sec. 211.94 Drug product containers and closures.(a) Drug product containers and closures shall not be reactive, additive, or absorptive so as to alter the safety, identity, strength, quality, or purity of the drug beyond the official or established requirements.(b) Container closure systems shall provide adequate protection against foreseeable external factors in storage and use that can cause deterioration or contamination of the drug product.(c) Drug product containers and closures shall be clean and, where indicated by the nature of the drug, sterilizedand processed to remove pyrogenic properties to assure that they are suitable for their intended use. Such depyrogenation processes shall be validated.(d) Standards or specifications, methods of testing, and, where indicated, methods of cleaning, sterilizing, and processing to remove pyrogenic properties shall be written and followed for drug product containers and closures.[43 FR 45077, Sept. 29, 1978, as amended at 73 FR 51932, Sept. 8, 2008]Subpart F--Production and Process ControlsSec. 211.100 Written procedures; deviations.(a) There shall be written procedures for production and process control designed to assure that the drug products have the identity, strength, quality, and purity they purport or are represented to possess. Such procedures shall include all requirements in this subpart. These written procedures, including any changes, shall be drafted, reviewed, and approved by the appropriate organizational units and reviewed and approved by the quality control unit.(b) Written production and process control procedures shall be followed in the execution of the various production and process control functions and shall be documented at the time of performance. Any deviation from the written procedures shall be recorded and justified.Sec. 211.101 Charge-in of components.Written production and control procedures shall include the following, which are designed to assure that the drug productsproduced have the identity, strength, quality, and purity they purport or are represented to possess:(a) The batch shall be formulated with the intent to provide not less than 100 percent of the labeled or established amount of active ingredient.(b) Components for drug product manufacturing shall be weighed, measured, or subdivided as appropriate. If a component is removed from the original container to another, the new container shall be identified with the following information:(1) Component name or item code;(2) Receiving or control number;(3) Weight or measure in new container;(4) Batch for which component was dispensed, including its product name, strength, and lot number.(c) Weighing, measuring, or subdividing operations for components shall be adequately supervised. Each container of component dispensed to manufacturing shall be examined by a second person to assure that:(1) The component was released by the quality control unit;(2) The weight or measure is correct as stated in the batch production records;(3) The containers are properly identified. If the weighing, measuring, or subdividing operations are performed by automated equipment under 211.68, only one person is needed to assure paragraphs (c)(1), (c)(2), and (c)(3) of this。
ACI(美国混凝土协会)标准目录最新

170 ACI 332.1R 171 ACI 546.3R 172 ACI SP-238
173 ACI ITG-4.2R 2006.10.01 174 ACI 234R 175 ACI 350M 176 ACI 350 177 ACI 349M 178 ACI 345.1R 179 ACI 305.1 180 ACI 350.3 181 ACI 302.2R 182 ACI 325.13R 183 ACI 523.1R 184 ACI 440.1R 185 ACI SP-231 186 ACI SP-225 187 ACI 207.1R
100 ACI ITG-7M 101 ACI 311.6 102 ACI ITG-7 103 ACI 439.4R 104 ACI 408.3R 105 ACI 506.5R 106 ACI ITG-5.2
107 ACI 346M 108 ACI 346
ACI 440.2R ERTA ACI 350.2R 110 ERTA
ACI 530/530.1 2011.01.01
53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79
ACI 376 ACI 314R ACI 352.1R ACI 548.4 ACI 533R ACI 548.4M ACI 301 ACI 223R ACI ITG-8R ACI 212.3R ACI 423.8R ACI 503.5R ACI 503.3M ACI 306R ACI 305R ACI 503.3 ACI 548.10M ACI ITG-6R ACI 548.10 ACI 332M ACI 440.2R ERTA ACI 332 ACI 357.2R ACI 117M
ACI发展心理学习题

发展心理学第三章第一节单选题1.皮亚杰把儿童的心理发展划分为()个阶段。
A. 3B. 5C. 4D. 6【答案】C【解析】《心理咨询师三级基础知识》P190 第三章发展心理学第一节概述皮亚杰把认知(智慧)发展视为认知结构的发展过程,以认知结构为依据区分心理发展阶段。
他把认知发展分为四个阶段:1)感知运动阶段;2)前运算阶段;3)具体运算阶段;4)形式运算阶段。
2.皮亚杰认为心理起源于()。
A. 成熟B. 经验C. 模仿D. 动作【答案】D【解析】《心理咨询师三级基础知识》P188 第三章发展心理学第一节概述皮亚杰认为,心理既不是起源于先天的成熟,也不是起源于后天的经验,而是起源于动作,即动作是认识的源泉,是主客体相互作用的中介。
3.给我一打健全儿童,我可以用特殊的方法将他们任意改变,或者使他们成为医生,或者使他们成为艺术家和富商,或者使他们成为乞丐和强盗,这种心理发展动因属于()。
A. 遗传因素决定心理发展B. 环境因素决定心理发展C. 社会文化因素决定心理发展D. 通过社会学习获得心理发展【答案】B【解析】《心理咨询师三级基础知识》P184 第三章发展心理学第一节概述环境因素决定心理发展的理论被称为环境决定论。
行为主义心理学派创始人华生是这种观点的代表人物。
华生的名言:“给我一打健全的儿童,我可以用特殊的方法任意地将他们加以改变,或者使他们成为医生、律师、艺术家和富商,或者使他们成为乞丐和强盗”,完全否定了儿童的素质、年龄特征和内部状态的作用。
4.为了观察个体随时间的变化,科学家进行()。
A. 智力测验B. 纵向研究C. 横断面研究D. 个案研究【答案】B【解析】《ACI 心理咨询师上册》P179 第三章第一节纵向研究设计是对相同的研究对象在不同的年龄或阶段进行的长期反复观测的设计方式,也称为纵向跟踪研究。
多选题5.处于前运算阶段的儿童具有的特征包括()。
A. 泛灵论B. 自我中心C. 思维的可逆性D. 掌握守恒【答案】AB【解析】《心理咨询师三级基础知识》P190 第三章发展心理学第一节概述皮亚杰指出前运算阶段儿童思维的特点有:1)泛灵论;2)自我中心主义;3)不能理顺整体和部分的关系;4)思维的不可逆性;5)缺乏守恒。
ACI_INTERNATIONAL_(ACI)._ACI_211.1.--美国混凝土配合比规范

3.4 CLEANING
清理
3.5 SURFACE SEALING 表面密封
3.6 PROTECTION OF WORK 保护工作
3.7 DEFECTIVE WORK
针对缺陷的处理工作
-- End of Section Table of Contents -- 目录内容的结束部分
SECTION 03330 Page 1
2.1.6 Form Release Agents 模板隔离剂 2.1.7 Surface Sealer 表面密封剂
PART 3 EXECUTION
第3部分执行
3.1 FORMWORK ERECTION 模板安装
3.2 CONCRETE FINISHES 混凝土表面光洁度
3.3 JOINT SEALING 接头密封
**************************************************************************
USACE / NAVFAC / AFCESA 美国陆军工兵部队/海军设施工程控制/空军土建工程师支持机构
Preparing Activity: USACE
ACI SP-66 美国混凝土学会 ACI SP-66规范
(2003) Guide to Formwork for Concrete
混凝土模板工程的指导2003 (2004) ACI Detailing Manual
美国混凝土学会 ACI的详细手册2004
ASTM INTERNATIONAL (ASTM) 美国试验材料学会ASTM
ACI 301
(1999) Specifications for Structural
美国混凝土学会 ACI 301规范
21CFR211中英文对照版(SMART)

PART 211 211部分- CURRENT GOOD MANUFACTURING PRACTICE FOR—制剂药品的CGMPFINISHED PHARMACEUTICALSSubpart A--General Provisions A.总 则§211.1 - Scope.211∙1范围§211.3 - Definitions.211∙3定义Subpart B--Organization and Personnel B.组织与人员§211.22 - Responsibilities of quality control unit.211∙22质量控制部门的职责§211.25 - Personnel qualifications.211∙25人员资格§211.28 - Personnel responsibilities.211∙28人员职责§211.34 - Consultants.211∙34顾问Subpart C--Buildings and Facilities C.厂房和设施§211.42 - Design and construction features.211∙42设计与建造特征§211.44 - Lighting.211∙44照明§211.46 - Ventilation, air filtration, air heating and211∙46通风、空气过滤、空气加热与冷却§211.48 - Plumbing.211∙48管件§211.50 - Sewage and refuse.211∙50污水和废料§211.52 - Washing and toilet facilities.211∙52洗涤和盥洗设备§211.56 - Sanitation.211∙56卫生§211.58 - Maintenance.211∙58保养Subpart D--Equipment D.设备§211.63 - Equipment design, size, and location.211∙63设备的设计、尺寸及位置§211.65 - Equipment construction.211∙65设备构造§211.67 - Equipment cleaning and maintenance.211∙67设备清洁与保养§211.68 - Automatic, mechanical, and electronic211∙68自动化设备、机械化设备和电子设备equipment.§211.72 - Filters.211∙72过滤器Subpart E--Control of Components and DrugE.成分、药品容器和密封件控制Product Containers and Closures§211.80 - General requirements.211∙80总要求§211.82 - Receipt and storage of untested211∙82未检验的成份、药品容器和密封件的接收与贮存components, drug product containers, and closures.§211.84 - Testing and approval or rejection of211∙84成份、药品容器和密封件的试验、批准或拒收components, drug product containers, and closures.§211.86 - Use of approved components, drug product211∙86获准的成份、药品容器和密封件的使用containers, and closures.§211.87 - Retesting of approved components, drug211∙87获准的成份、药品容器和密封件的复检product containers, and closures.§ 211.89 - Rejected components, drug product211∙89拒收的成份、药品容器和密封件containers, and closures.§ 211.94 - Drug product containers and closures.211∙94药品密封容器和密封件Subpart F--Production and Process Controls F .生产和加工控制§211.100 - Written procedures; deviations.211∙100成文的规程、偏差§211.101 - Charge-in of components.211∙101成分的控制§211.103 - Calculation of yield.211∙103 产量计算§211.105 - Equipment identification.211∙105设备鉴别§211.110 - Sampling and testing of in-process211∙110中间体和药品的取样与检验materials and drug products.§211.111 - Time limitations on production.211∙111生产时间限制§211.113 - Control of microbiological contamination.211∙113微生物污染的控制§211.115 - Reprocessing.211∙115返工Subpart G--Packaging and Labeling Control G.包装和标签控制§211.122 - Materials examination and usage criteria.211∙122材料的检查和使用标准§211.125 - Labeling issuance.211∙125标签的发放§211.130 - Packaging and labeling operations.211∙130包装和贴签操作§211.132 - Tamper-evident packaging requirements211∙132人用非处方药(OTC)保险包装的要求for over-the-counter (OTC) human drug products.§211.134 - Drug product inspection.211∙134药品检查§ 211.137 - Expiration dating.211∙137有效期Subpart H--Holding and Distribution H.贮存和销售§ 211.142 - Warehousing procedures.211∙142入库程序§ 211.150 - Distribution procedures.211∙150销售程序Subpart I--Laboratory Controls I∙实验室控制§ 211.160 - General requirements.211∙160总要求§ 211.165 - Testing and release for distribution.211∙165销售前的检验与发放§ 211.166 - Stability testing.211∙166稳定性试验§211.167 - Special testing requirements.211∙167特别检验要求§ 211.170 - Reserve samples.211∙170留样§ 211.173 - Laboratory animals.211∙173实验动物§ 211.176 - Penicillin contamination.211∙176青霉素污染Subpart J--Records and Reports J.记录和报告§ 211.180 - General requirements.211∙180总要求§ 211.182 - Equipment cleaning and use log.211∙182设备清洁和使用记录§ 211.184 - Component, drug product container,211∙184成份、药品容器、密封件及标签记录closure, and labeling records.§ 211.186 - Master production and control records.211∙186主要生产和控制的记录§ 211.188 - Batch production and control records.211∙188批生产和控制记录§ 211.192 - Production record review.211∙192产品记录复查§ 211.194 - Laboratory records.211∙194实验室记录§ 211.196 - Distribution records.211∙196销售记录§ 211.198 - Complaint files.211∙198客户投诉档案Subpart K--Returned and Salvaged Drug Products K.退货的药品和回收处理§ 211.204 - Returned drug products.211∙204退货的药品§ 211.208 - Drug product salvaging.211∙208 药品的回收利用Subpart A-General Provisions A.总 则§ 211.1 Scope211∙1范围(a) The regulations in this part contain the minimum current good manufacturing practice for preparation of drug products for administration to humans or animals.(a)本部分的条例包含人用或兽用药品制备的现行最低限度的药品生产质量管理规范(GMP)(b) The current good manufacturing practice regulations in this chapter, as they pertain to drug products, and in parts 600 through 680 of thischapter, as they pertain to biological products for human use, shall be considered to supplement, not supersede, the regulations in this part unless the regulations explicitly provide otherwise. In the event it is impossible to comply with applicable regulations both in this part and in other parts of this chapter or in parts 600 through 680 of this chapter, the regulation specifically applicable to the drug product in question (b)在本章里的这些针对药品的现行GMP条例和本章600至800的所有部分针对人用生物制品的现行GMP条例,除非明确另有说明者外,应认为是对本部分条例的补充,而是不代替。
国外模具钢和工具钢材料对照表

ACERO INDEFORMABLE 12% Cr. U211
U212
ACERO INDEFORMABLE AL Cr Mn
U213
ACERO INDEFORMABLE BAJO AL Cr.
UA-2 U12 UA-3 U13
B-2
F-524
ACERO PARA BURILES U221
UB-1 U21
GRUPO F-330
GRUPO F-320
GRUPO F-310
ACEROS DE EMERGENCIA
ACEROS REFRACTARIOS
ACEROS PARA VÁ LVULAS
ACEROS INOXIDABLES
F-313
F-314 F-315
ACERO INOXIDABLE AL Cr - Ni 621
F-525
ACERO PARA BUTEROLAS U222
UB-2 U22
F-526
U231
ACEROS PARA TRABAJOS EN CALIENTE, ALTO AL W.
UC-2 U32
36.072 36.072 36.072 36.072 36.072
36.072
RUPO F-550 ROS RÁPIDOS
111320 111330
111440 111510 111520 111530 111540 111550
111410 111420 111430
GRUPO F-220 ACEROS DE FÁCIL SOLDADURA
GRUPO F210
GRUPO F-170
GRUPOS F-150 F-160 ACEROS DE CEMENTACI
关于《中国民航大学学报》改用彩色印刷的通告

第39卷第1期韩鹏,赵嶷飞,刘宏:无人机地面撞击风险评估体系构建及趋势展望-47 -national Conference on Unmanned Aircraft Systems (ICUAS), June 912, 2015, Denver, CO, USA . IEEE, 2015: 1301-1309.[7] AWAD A I. An analysis of the risk from UAS missions in the nationalairspace[D]. Washington: University of Washington, 2013.[N] BARR L C, NEWMAN R, ANCEL E, et al. Preliminary risk assessmentfor small unmanned aircraft systems[C]//17th AIAA Aviation Technolo gy, Integration, and Operations Conference, June5-9, 2017, Denver,Colorado . Reston, Virginia: AIAA, 2017.[9] FORD A, MCENTEE K. Assessment of the risk to ground populationdue to an unmanned aircraft in-flight failure[C]//10th AIAA AviationTechnology, Integration, and Operations Conference, September 13- 15,2010, Fort Worth, Texas . Reston, Virginia: AIAA, 2010.[10] LUM C, WAGGONER B. A risk based paradigm and model for un manned aerial systems in the national airspace[M]. Missouri: Aerospace,2011.[11] WEIBEL R E, HANSMAN R J. Safety considerations for operation ofunmanned aerial vehicles in the national airspace system[R]. Cam bridge: MIT International Center for Air Transportation, 2005.[12] WOLF S E. Modeling small unmanned aerial system mishaps using lo gistic regression and artificial neural networks[R]. Dayton: Air Force In stitute of Technology, 2012.[13] ANCEL E, CAPRISTAN F M, FOSTER J V, et al. Real-time risk as sessment framework for unmanned aircraft system (UAS) traffic manage - ment(UTM)[C]//17th AIAA Aviation Technology, Integration, and Ope rations Conference, June 5-9, 2017, Denver, Colorado . Reston, Virginia:AIAA, 2017.[14] COUR -HARBO A L .Ground impact probability distribution for smallunmanned aircraft in ballistic descent[C]//2020 International Confer ence on Unmanned Aircraft Systems(ICUAS), September 1-4, 2020,Athens, Greece . IEEE, 2020: 1442-1451.[15] HAARTSEN Y, AALMOES R, CHEUNG Y S. Simulation of unmannedaerial vehicles in the determination of accident locations[C]//2016 IEEE International Conference on Unmanned Aircraft Systems, Arlington VA,June 7-10, 2016: 993-1002.[16] KING D W, BERTAPELLE A, MOSES C. UAV failure rate criteria forequivalent level of safety [C]//International Helicopter Safety Sympo sium, Montreal, Quebec, September 26-29, 2005: 1-11.[17] MELNYK R V, SCHRAGE D P, VOLOVOI V, et al. A third-party ca sualty risk model for UAS operations[J]. Reliability Engineering and System Safety, 2014, 124: 105-116.[18] STEVENSON J D, YOUNG S, ROLLAND L. Estimated levels of safetyfor small unmanned aerial vehicles and risk mitigation strategies [J]. Journal of Unmanned Vehicle Systems, 2015, 3(4): 205-221.[19] WU P, CLOTHIER R A. The development of ground impact models forthe analysis of the risks associated with unmanned aircraft operationsover inhabited areas[C]//11th Probabilistic Safety Assessment and Mana gement Conference, Helsinki, Finland, June 25-29, 2012: 1-14.[20] ANDREW D, KNOTT M, BURKE D. Population density modeling tool[R]. Maryland: Department of the Navy, Naval Air Warfare Center Air craft Division, 2012.[21] BURKE D A, HALL C E, COOK S P. System-level airworthiness tool[J]. Journal of Aircraft, 2011,48(3): 777-785.[22] DALAMAGKIDIS K, VALAVANIS K P, PIEGL L A. On integrating un manned aircraft systems into the national airspace system: issues, chal lenges, operational restrictions, certification, and recommendations [M]. Berlin: Springer Science and Business Media, 2011.[23] GUGLIERI G, QUAGLIOTTI F, RISTORTO G. Operational issues andassessment of risk for light UAVs[J]. Journal of Unmanned Vehicle Sys tems, 2014, 2(4): 119-129.[24] WAGGONER B. Developing a risk assessment tool for unmanned air 一craft system operations[D]. Washington: University of Washington, 2010.[25] BALL J A, KNOTT M, BURKE D. Crash lethality model[R]. Maryland:Naval Air Warfare Centre Aircraft Division, 2012.[26] LARSON E, HABER J M. Final quantitative risk analysis for genericlifting entry vehicle landing at edwards air force base[R]. California: AirForce Flight Test Center, 2001.[27] 韩 鹏,赵嶷飞•基于飞行环境建模的UAV 地面撞击风险研究[J].中国安全科学学报,2020, 30(1): 142-147.(责任编辑:刘佩佩)关于《中国民航大学学报》改用彩色印刷的通告为更好地呈现论文图片信息,《中国民航大学学报》于2021年第1期开始采用彩色印刷。
国际会议级别
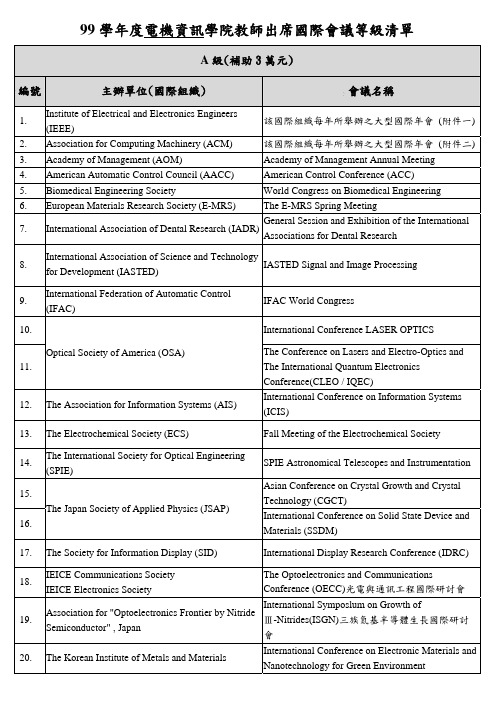
23. European Union Control Association (EUCA)
European Control Conference (ECC)
Innovative Computing, Information and Control 24.
(ICIC)
International Symposium on Intelligent Informatics (ISII)
24. Kobe Gakuin University
The 40th International Conference on Computers & Industrial Engineering(CIE40)
99 學年度電機資訊學院教師出席國際會議等級清單
B 級(補助 2 萬元)
編號
主辦單位(國際組織)
(IFAC)
IFAC World Congress
10.
International Conference LASER OPTICS
Optical Society of America (OSA) 11.
12. The Association for Information Systems (AIS)
Associations for Dental Research
International Association of Science and Technology
8.
IASTED Signal and Image Processing
for Development (IASTED)
International Federation of Automatic Control 9.
(IFAC)
211A_Manual说明书(1)

User’s Manual
Model 211
Temperature Monitor
Serial Numbers 21A0000 and Subsequent
西三一大学IMBA简介

西三一大学IMBA西三一大学坐落于加拿大不列颠哥伦比亚省的兰尼市(Langley),靠近国际港口温哥华,离美国华盛顿州的西雅图市仅125英里,是一所国际认可的综合性大学,也是加拿大最大的私立教会综合性大学。
创建于1962年,作为一所综合性高等学府,它得到国际同行的广泛承认,颁发的学位被国际认可。
前联合国主席Charles Malik博士曾评价TWU是一所不断提高教学质量并以培养高素质人才为已任的大学。
西三一大学的校园分别座落于兰尼市和温哥华市中心。
每年入学学生在4000人左右,其中77%的学生来自加拿大,10%来自美国,13%的学生来自世界其他国家。
学校学习、生活、医疗设施先进齐备,学生宿舍管理有序。
项目介绍西三一大学MBA项目,全日制12个月完成课程。
项目开始比较国际管理课程,这门课程主要是旅行教学的形式,让学生在不同国家不同文化的企业中学习管理实践。
入学前无需语言成绩和GMAT,项目为没有时间准备语言考试的人开设pre-masters program (PMP),学生可以在1月,5月或9月灵活入学MBA的学生要先学习ESLI Pre-Masters Program training(开课月份有1、5、9月三期)课程有:Marketing Management(市场管理)Human Resource Management(人力资源管理)Managing a Global Organization(国际组织管理)Managerial Economics(管理经济学)Managerial Accounting(管理会计学)Global Competitiveness(全球竞争力)International Business Law(国际商法)Comparative International Management (Travel Studies)(国际管理比较)(旅游研究)Managerial Finance(管理金融学)Information and Knowledge Management Systems(信息和知识管理体系)Operations Management(经营管理)International Finance and Accounting(国际金融与会计)Christian Leadership and Ethics(基督教领袖及道德规范)Strategic Management(战略管理)申请条件:1,学士学位不要求相关专业背景,无须工作经验。
老年ACI患者血清CYR61、氧化应激分子水平及其对ACI患者90_d预后的预测价值孙国才,赵旭锐,

作者简介:孙国才,男,主治医师,主要从事老年病相关诊疗方面的研究㊂ ә 通信作者,E -m a i l :632273959@q q.c o m ㊂㊃论 著㊃D O I :10.3969/j.i s s n .1672-9455.2024.02.018老年A C I 患者血清C Y R 61㊁氧化应激分子水平及其对A C I 患者90d 预后的预测价值孙国才1,赵旭锐1ә,权泽威1,朱 娇2,王彩霞1,秦 燕1兵器工业五二一医院:1.老年病科;2.肿瘤血液病科,陕西西安710065摘 要:目的 探讨老年急性脑梗死(A C I )患者血清富半胱氨酸蛋白61(C Y R 61)㊁丙二醇(M D A )㊁超氧化物歧化酶(S O D )㊁髓磷脂碱性蛋白(M B P )水平及其对A C I 患者90d 预后的预测价值㊂方法 选取2019年6月至2022年1月该院收治的211例A C I 患者作为A C I 组,另选取同期200例健康体检者作为对照组,检测两组血清C Y R 61㊁M D A ㊁S O D ㊁M B P 水平㊂根据改良R a n k i n 量表(m R S )评分将随访90d 的A C I 患者分为预后良好组(m R S 评分ɤ2分)和预后不良组(m R S 评分>2分)㊂比较预后良好组和预后不良组临床资料,并进行多因素L o gi s t i c 回归分析,采用受试者工作特征(R O C )曲线评估A C I 患者血清C Y R 61水平对90d 预后的预测价值㊂结果 A C I 组血清C Y R 61㊁M D A ㊁M B P 水平均高于对照组,S O D 水平低于对照组,差异均有统计学意义(P <0.05)㊂P e a r s o n 相关分析结果显示,A C I 患者血清C Y R 61水平与M D A ㊁M B P 水平均呈正相关,与S O D 水平呈负相关(r =0.447㊁0.605㊁-0.551,P <0.05)㊂预后良好组和预后不良组年龄㊁入院当天美国国立卫生研究院卒中量表(N I H S S )评分㊁发病至治疗时间,以及C Y R 61㊁M D A ㊁M B P 和S O D 水平比较,差异均有统计学意义(P <0.05)㊂多因素L o gi s t i c 回归分析结果显示,入院当天N I H S S 评分和血清C Y R 61水平是A C I 患者90d 预后不良的影响因素(P <0.05)㊂R O C 曲线分析结果显示,血清C Y R 61预测A C I 患者90d 预后不良的曲线下面积(A U C )为0.798,灵敏度㊁特异度分别为0.857和0.652;入院当天N I H S S 评分预测A C I 患者90d 预后不良的A U C 为0.811,灵敏度㊁特异度分别为0.857和0.783㊂结论 A C I 患者血清C Y R 61水平升高,可能参与氧化应激分子水平的调控㊂C Y R 61水平是A C I 患者预后不良的影响因素,具有一定的早期预测价值㊂关键词:急性脑梗死; 富半胱氨酸蛋白61; 氧化应激分子; 预后; 老年中图法分类号:R 743.33文献标志码:A 文章编号:1672-9455(2024)02-0221-04S e r u m l e v e l s o f C Y R 61a n d o x i d a t i v e s t r e s s m o l e c u l e s i n e l d e r l y pa t i e n t s w i t h A C I a n d t h e i r p r e d i c t i v e v a l u e f o r 90d p r o gn o s i s i n p a t i e n t s w i t h A C I S U N G u o c a i 1,Z HA O X u r u i 1ә,Q U A N Z e w e i 1,Z HU J i a o 2,WA N G C a i x i a 1,Q I N Y a n11.D e p a r t m e n t o f g e r i a t r i c s ;2.D e p a r t m e n t o f O n c o l o g y a n d H e m a t o l o g y ,521H o s p i t a l o f O r d n a n c e I n d u s t r y ,X i 'a n ,S h a a n x i 710065,C h i n a A b s t r a c t :O b j e c t i v e T o i n v e s t i g a t e t h e l e v e l s o f s e r u m c y s t e i n e -r i c h p r o t e i n 61(C Y R 61),m a l o n d i a l d e h y d e (M D A ),s u p e r o x i d e d i s m u t a s e (S O D )a n d m y e l i n b a s i c p r o t e i n (M B P )i n e l d e r l y pa t i e n t s w i t h a c u t e c e r eb r a l i n f a rc t i o n (A C I )a nd t he i r p r e d i c t i v e v a l u ef o r 90-d a y p r o gn o s i s .M e t h o d s A t o t a l o f 211A C I p a t i e n t s a d m i t -t e d t o t h e h o s p i t a l f r o m J u n e 2019t o J a n u a r y 2022w e r e s e l e c t e d a s t h e A C I g r o u p ,a n d 200h e a l t h y p h y s i c a l e x a m i n a t i o n s u b j e c t s d u r i n g t h e s a m e p e r i o d w e r e s e l e c t e d a s t h e c o n t r o l g r o u p.T h e s e r u m C Y R 61,M D A ,S O D a n d M B P l e v e l s o f t h e t w o g r o u p s w e r e d e t e c t e d .A c c o r d i n g to t h e m o d i f i e d R a n k i n s c a l e (m R S )s c o r e ,A C I p a t i e n t s w h o w e r e f o l l o w e d u p f o r 90d w e r e d i v i d e d i n t o g o o d p r o g n o s i s g r o u p (m R S s c o r e ɤ2)a n d p o o r p r o g n o s i s g r o u p (m R S s c o r e >2).T h e c l i n i c a l d a t a o f t h e g o o d p r o g n o s i s g r o u p a n d t h e p o o r p r o g n o s i s g r o u p w e r e c o m p a r e d ,a n d m u l t i v a r i a t e L o g i s t i c r e g r e s s i o n a n a l y s i s w a s p e r f o r m e d .T h e r e c e i v e r o p e r a t i n g ch a r a c t e r -i s t i c (R O C )c u r v e w a s u s e d t o e v a l u a t e t h e p r e d i c t i v e v a l u e o f s e r u m C Y R 61l e v e l f o r t h e 90d p r o g n o s i s o f A C I p a t i e n t s .R e s u l t s T h e l e v e l s o f C Y R 61,M D A a n d M B P i n A C I g r o u p w e r e s i g n i f i c a n t l y h i gh e r t h a n t h o s e i n c o n t r o l g r o u p (P <0.05),a n d t h e l e v e l o f S O D w a s s i g n i f i c a n t l y l o w e r t h a n t h a t i n c o n t r o l g r o u p (P <0.05).P e a r s o n c o r r e l a t i o n a n a l y s i s s h o w e d t h a t s e r u m C Y R 61l e v e l i n A C I p a t i e n t s w a s p o s i t i v e l y co r r e l a t e d w i t h M D A a n d M B P l e v e l s ,a n d n e g a t i v e l y co r r e l a t e d w i t h S O D l e v e l (r =0.447,0.605,-0.551,P <0.05).T h e r e w e r e s i g n i f i c a n t d i f f e r e n c e s i n a g e ,N a t i o n a l I n s t i t u t e s o f H e a l t h S t r o k e S c a l e (N I H S S )s c o r e o n t h e d a yo f a d m i s s i o n ,t i m e f r o m o n s e t t o t r e a t m e n t a n d l e v e l s o f C Y R 61,M D A ,M B P a n d S O D b e t w e e n t h e g o o d p r o g-n o s i s g r o u p a n d t h e p o o r p r o g n o s i s g r o u p (P <0.05).M u l t i v a r i a t e L o g i s t i c r e g r e s s i o n a n a l ys i s s h o w e d t h a t ㊃122㊃检验医学与临床2024年1月第21卷第2期 L a b M e d C l i n ,J a n u a r y 2024,V o l .21,N o .2N I H S S s c o r e a n d s e r u m C Y R61l e v e l o n a d m i s s i o n w e r e t h e i n f l u e n c i n g f a c t o r s o f p o o r p r o g n o s i s o f A C I p a-t i e n t s a t90d(P<0.05).R O C c u r v e a n a l y s i s s h o w e d t h a t t h e a r e a u n d e r t h e c u r v e(A U C)o f s e r u m C Y R61 f o r p r e d i c t i n g p o o r p r o g n o s i s o f A C I p a t i e n t s a t90d w a s0.798,a n d t h e s e n s i t i v i t y a n d s p e c i f i c i t y w e r e0.857 a n d0.652r e s p e c t i v e l y.T h e A U C o f N I H S S s c o r e o n a d m i s s i o n t o p r e d i c t p o o r p r o g n o s i s a t90d w a s0.811, a n d t h e s e n s i t i v i t y a n d s p e c i f i c i t y w e r e0.857a n d0.783r e s p e c t i v e l y.C o n c l u s i o n T h e s e r u m C Y R61l e v e l i s i n c r e a s e d i n A C I p a t i e n t s,w h i c h m a y b e i n v o l v e d i n t h e r e g u l a t i o n o f o x i d a t i v e s t r e s s.T h e C Y R61l e v e l i s a i n-f l u e n c i n g f a c t o r f o r p o o r p r o g n o s i s i n p a t i e n t s w i t h A C I,a n d h a s a c e r t a i n e a r l y p r e d i c t i v e v a l u e.K e y w o r d s:a c u t e c e r e b r a l i n f a r c t i o n;c y s t e i n e-r i c h p r o t e i n61;o x i d a t i v e s t r e s s m o l e c u l e;p r o g n o s i s; t h e e l d e r l y急性脑梗死(A C I)是一种严重威胁中老年人群身体健康㊁生命安全的缺血性脑血管疾病㊂有研究表明,氧化应激在脑梗死发生㊁发展中具有促进作用,氧化应激指标水平变化可对A C I的转归进行判断[1-2]㊂血清富半胱氨酸蛋白61(C Y R61)在炎症反应㊁血管生成㊁纤维组织修复等方面发挥重要作用,是新型炎症调节因子[3-4]㊂炎症反应是导致A C I脑损伤进展的重要机制之一㊂基于此,本研究选取老年A C I患者作为研究对象,分析血清C Y R61水平与氧化应激指标的相关性及其对A C I患者90d预后的预测价值,以期为老年A C I的临床预防和诊疗提供参考,现报道如下㊂1资料与方法1.1一般资料选取2019年6月至2022年1月本院收治的211例A C I患者作为A C I组,其中男98例,女113例;年龄45~78岁,平均(61.73ʃ9.62)岁;体质量指数(B M I)21~27k g/m2,平均(23.84ʃ2.12)k g/m2㊂另选取同期200例健康体检者作为对照组,其中男108例,女92例;年龄43~79岁,平均(62.73ʃ9.95)岁;B M I21~26k g/m2,平均(23.76ʃ2.09)k g/m2㊂两组性别㊁年龄㊁B M I等一般资料比较,差异均无统计学意义(P>0.05),具有可比性㊂纳入标准:(1)A C I组患者符合中华医学会神经病学分会的‘中国急性缺血性脑卒中诊治指南2018“[5]中的相关标准,经M R I或头颅C T检查确诊;(2)首次发作的A C I,无既往病史;(3)发病至入院时间在48h内;(4)临床资料完整㊁愿意接受随访㊂排除标准:(1)合并恶性肿瘤㊁血液系统疾病者;(2)有神经系统疾病既往病史者;(3)妊娠期㊁哺乳期妇女;(4)既往有脑出血㊁脑外伤者㊂所有研究对象均知情同意并签署知情同意书㊂本研究经本院医学伦理委员会审核通过㊂1.2仪器与试剂血清C Y R61试剂盒(温州科淼生物科技有限公司);丙二醇(M D A)测定试剂盒(上海弘顺生物科技有限公司);超氧化物歧化酶(S O D)活性测定试剂盒(上海索宝生物科技有限公司);髓磷脂碱性蛋白(M B P)E L I S A试剂盒(上海拜力生物科技有限公司);A l l e g r a X-15R台式冷冻离心机(美国贝克曼库尔特有限公司);酶标仪[伯乐生命医学产品(上海)有限公司(b i o-r a d)]㊂1.3方法(1)血清C Y R61㊁M D A㊁S O D㊁M B P水平测定:于清晨采集两组空腹肘静脉血2m L,在4ħ环境以3000r/m i n离心10m i n,取上清液放于内含蛋白酶抑制剂㊁抗凝剂E D T A的E P试管内,并置于-80ħ冷冻环境中保存待检㊂采用E L I S A及试剂盒检测血清C Y R61㊁M D A㊁S O D㊁M B P水平,具体操作严格按照试剂盒说明书进行㊂(2)A C I预后相关因素收集:收集A C I预后的相关因素,包括平均年龄㊁性别㊁平均B M I㊁生活习惯(有无吸烟史㊁饮酒史)㊁慢性病史(有无高血压史㊁糖尿病史㊁冠心病史)㊁入院当天美国国立卫生研究院卒中量表(N I H S S)评分㊁发病至治疗时间及入院24h内的实验室检测指标(空腹血糖㊁血小板计数和血肌酐)㊂(3)A C I患者90d预后的随访:自入院后开始随访,第90天时采用改良R a n k i n 量表(m R S)进行评价,认定m R S评分ɤ2分为预后良好,归为预后良好组(154例),m R S评分>2分为预后不良,归为预后不良组(57例)㊂1.4统计学处理采用S P S S22.0统计软件进行数据分析处理㊂符合正态分布的计量资料以xʃs表示,两组间比较采用独立样本t检验;计数资料以例数或百分率表示,组间比较采用χ2检验;采用多因素L o g i s t i c回归分析A C I患者90d预后不良的影响因素;采用受试者工作特征(R O C)曲线分析血清C Y R61水平对A C I患者90d预后的预测价值;采用P e a r s o n相关分析血清C Y R61水平与M D A㊁S O D㊁M B P水平的相关性㊂以P<0.05为差异有统计学意义㊂2结果2.1两组血清C Y R61㊁M D A㊁S O D㊁M B P水平比较 A C I组血清C Y R61㊁M D A㊁M B P水平均高于对照组,S O D水平低于对照组,差异均有统计学意义(P< 0.05)㊂见表1㊂表1两组血清C Y R61㊁M D A㊁S O D㊁M B P水平比较(xʃs)组别nC Y R61(p g/m L)M D A(n m o l/L)M B P(μg/L)S O D(U/m L) A C I组2112403.75ʃ895.036.08ʃ1.1417.84ʃ3.2950.01ʃ6.23对照组2001067.77ʃ161.264.82ʃ1.0312.78ʃ2.6559.28ʃ7.66 t31.4711.8128.08-17.50P<0.01<0.01<0.01<0.01 2.2血清C Y R61水平与M D A㊁S O D㊁M B P水平的㊃222㊃检验医学与临床2024年1月第21卷第2期 L a b M e d C l i n,J a n u a r y2024,V o l.21,N o.2相关性 P e a r s o n相关分析结果显示,A C I患者血清C Y R61水平与M D A㊁M B P水平均呈正相关,与S O D 水平呈负相关(r=0.447㊁0.605㊁-0.551,P<0.05)㊂2.3预后良好组和预后不良组临床资料比较预后良好组和预后不良组年龄㊁入院当天N I H S S评分㊁发病至治疗时间,以及C Y R61㊁M D A㊁M B P和S O D水平比较,差异均有统计学意义(P<0.05);预后良好组和预后不良组男性占比㊁B M I㊁吸烟史㊁饮酒史㊁高血压史㊁糖尿病史㊁冠心病史㊁空腹血糖㊁血小板计数㊁血肌酐比较,差异均无统计学意义(P>0.05)㊂见表2㊂2.4 A C I患者90d预后影响因素的多因素L o g i s t i c 回归分析以A C I患者90d预后作为因变量(预后良好=0,预后不良=1),将表2中差异有统计学意义的年龄(<70岁=0,ȡ70岁=1)㊁入院当天N I H S S 评分(<15分=0,ȡ15分=1)㊁发病至治疗时间(< 4.5h=0,ȡ4.5h=1)㊁C Y R61(原值输入)㊁M D A(原值输入)㊁M B P(原值输入)㊁S O D(原值输入)作为自变量㊂多因素L o g i s t i c回归分析结果显示,入院当天N I H S S评分和血清C Y R61水平是A C I患者90d预后不良的影响因素(P<0.05)㊂见表3㊂2.5A C I患者90d预后不良的R O C曲线分析 R O C曲线分析结果显示,血清C Y R61预测A C I 患者90d预后不良的A U C为0.798,灵敏度㊁特异度分别为0.857和0.652;入院当天N I H S S评分预测A C I患者90d预后不良的A U C为0.811,灵敏度㊁特异度分别为0.857和0.783㊂见图1㊁表4㊂图1血清C Y R61和入院当天N I H S S评分对A C I患者90d预后不良的R O C曲线表2预后良好组和预后不良组临床资料比较[xʃs或n(%)]组别n年龄(岁)男性B M I(k g/m2)吸烟史饮酒史高血压史预后良好组15465.88ʃ9.7371(46.10)23.67ʃ2.8152(33.77)48(31.17)62(40.26)预后不良组5770.67ʃ8.9527(47.37)23.48ʃ3.1419(33.33)18(31.58)25(43.86) t/χ2-2.6110.5511.0120.3310.4791.231 P0.0030.8020.6570.9590.8370.562组别n糖尿病史冠心病史入院当天N I H S S评分(分)发病至治疗时间(h)空腹血糖(mm o l/L)血小板计数(ˑ109/L)预后良好组15449(31.82)38(24.68)10.87ʃ2.453.13ʃ1.015.51ʃ0.68183.98ʃ22.67预后不良组5718(31.58)15(26.32)15.35ʃ2.516.42ʃ1.225.48ʃ0.62183.70ʃ21.34 t/χ20.9870.690-2.934-3.2640.6790.342 P0.6710.737<0.001<0.0010.8000.943组别n血肌酐(μm o l/L)C Y R61(p g/m L)M D A(n m o l/L)M B P(μg/L)S O D(U/m L)预后良好组15468.84ʃ7.842403.75ʃ161.265.58ʃ0.5312.28ʃ2.7959.78ʃ8.16预后不良组5769.60ʃ9.022833.00ʃ404.776.55ʃ0.7834.72ʃ6.2650.51ʃ6.67 t/χ2-1.118-3.305-3.414-3.2193.394P0.578<0.001<0.001<0.001<0.001表3 A C I患者90d预后影响因素的多因素L o g i s t i c回归分析因素βS E W a l dχ2P O R(95%C I)年龄0.7710.5132.2650.1322.162(0.792~5.907)入院当天N I H S S评分0.9670.3975.9350.0152.630(1.208~5.722)发病至治疗时间0.5580.4781.3650.2431.747(0.685~4.461) C Y R611.1150.3848.4320.0043.050(1.437~6.478) M D A0.5260.4691.2580.2621.692(0.675~4.241) M B P0.6750.4901.8990.1681.964(0.752~5.129) S O D-0.1920.3970.2350.6280.825(0.379~1.796)㊃322㊃检验医学与临床2024年1月第21卷第2期 L a b M e d C l i n,J a n u a r y2024,V o l.21,N o.2表4血清C Y R61和入院当天N I H S S评分对A C I患者90d预后不良的预测价值指标S E P A U C(95%C I)灵敏度特异度入院当天N I H S S评分0.1100.0190.811(0.563~0.999)0.8570.783血清C Y R610.1050.0140.798(0.578~0.999)0.8570.6523讨论A C I是严重威胁人类生命健康的心脑血管疾病,其发病率逐年攀升㊂A C I可引起患者脑组织缺氧㊁缺血甚至坏死,进而引起不同程度的功能障碍,严重影响患者日常工作和生活㊂自由基在脑缺氧和缺血过程中发挥重要作用,自由基可通过与中风损伤级联反应中的钙超载㊁兴奋性氨基酸等发生相互作用,最终形成凋亡㊁炎症反应等,从而损害脑组织㊂M D A㊁S O D㊁MB P是常见的脑自由基,其水平可用于判断中枢神经系统髓鞘损伤和实质性损伤的严重程度[6-9]㊂C Y R61是重要的促炎因子,参与炎症微环境和自身免疫性疾病的调节㊂C Y R61既往主要在肿瘤血管生成中研究较多[10-11],近年来有研究显示其含有丰富的半胱氨酸,不仅能参与血管生成,而且在炎症反应㊁纤维组织修复等方面也发挥重要作用[12]㊂目前,血清C Y R61在A C I患者中的表达及其临床意义尚未阐明,基于此,本研究选取老年A C I患者作为研究对象,分析血清C Y R61水平与MD A㊁S O D㊁M B P水平的相关性及其对A C I患者90d预后的预测价值,以期为老年A C I的临床预防和诊疗提供参考㊂本研究结果显示,A C I组血清C Y R61㊁M D A㊁M B P水平均明显高于对照组,S O D水平低于对照组,差异均有统计学意义(P<0.05),表明C Y R61可能参与M D A㊁M B P㊁S O D水平的调控并发挥其促炎作用㊂S O D能清除机体的氧自由基,从而保护脑血管组织免受自由基及过氧化物的损伤,其活性水平降低与神经功能损伤程度有关[13-16]㊂P e a r s o n相关分析结果显示,A C I患者血清C Y R61水平与M D A㊁M B P水平均呈正相关,与S O D水平呈负相关(P<0.05)㊂A C I起病迅速,可在短时间内引发严重炎症反应㊁氧化应激及神经元凋亡,随着坏死病灶迅速扩大,会加剧神经功能损伤,最终表现为肢体功能㊁认知功能㊁语言功能等不同程度障碍㊂入院当天N I H S S评分可大概评估患者的脑损伤严重程度,A C I病情较重的患者治疗结局不理想的概率也更高㊂多因素L o-g i s t i c回归分析结果显示,入院当天N I H S S评分是A C I患者90d预后不良的影响因素,其A U C与血清C Y R61的A U C比较无差异,但血清C Y R61可重复性好,可动态监测,实用价值更高㊂而N I H S S评分项目较多,难以收集,限制其临床应用㊂综上所述,A C I患者血清C Y R61水平升高,可能参与M D A㊁S O D㊁M B P水平的调控㊂C Y R61水平是A C I患者预后不良的影响因素,具有一定的早期预测价值㊂参考文献[1]李万华,王绍谦,刘艳,等.苦碟子注射液联合吡拉西坦治疗急性脑梗死的临床研究[J].现代药物与临床,2023,38(5):1113-1117.[2]梁菊萍,杨旸,董继存.急性脑梗死患者流行病学调查及危险因素[J].中国老年学杂志,2021,41(12):2484-2487.[3]南晓强,杨浩峰,雷鹏.息痛散通过富含半胱氨酸蛋白61对痛风性关节炎大鼠炎症反应的干预机制[J].陕西中医,2022,43(12):1659-1663.[4]单洁,李晓梅.循环生物标志物在急性心肌梗死患者预后评估中的价值[J].医学综述,2021,27(14):2709-2714.[5]中华医学会神经病学分会,中华医学会神经病学分会脑血管病学组.中国急性缺血性脑卒中诊治指南2018[J].中华神经科杂志,2018,51(9):666-682.[6]何保明,喻良.丁苯酞治疗急性脑梗死的临床疗效及对M D A㊁S O D㊁M B P的影响[J].四川医学,2019,40(3): 258-261.[7]迟蕾,张倩,梅继林,等.醒脑阴阳透刺针法联合温针灸治疗急性脑梗死疗效及对血清M D A㊁S O D㊁M B P水平影响[J].四川中医,2022,40(6):208-210.[8]张雷,孔祥丽,李军朝,等.远隔缺血适应联合静脉溶栓对急性脑梗死患者临床预后及氧化应激表达的影响[J].中国脑血管病杂志,2022,19(9):611-617.[9]姜飞,王东玉.依达拉奉右莰醇联合尿激酶静脉溶栓治疗急性脑梗死合并糖尿病对患者血清氧化应激水平㊁颈总动脉内-中膜厚度㊁血清基质金属蛋白酶-12水平的影响[J].陕西医学杂志,2022,51(1):88-91.[10]李江佩,孔祥波,孙音,等.H E R2㊁C y r61㊁I G F B P3表达情况对老年子宫内膜癌病人预后的价值[J].实用老年医学,2022,36(7):697-701.[11]王怡,郭婉军,郭冬婕.C y r61在皮肤相关疾病中的研究进展[J].中国皮肤性病学杂志,2022,36(2):230-234.[12]庹虎,唐其柱,余诗倩,等.C y r61的生物学作用及其调控机制[J].武汉大学学报(医学版),2021,42(5):846-850.[13]张银仙,潘永康,王妙.血清超氧化物歧化酶和缺血修饰白蛋白在脑梗死患者中的水平变化及临床价值[J].数理医药学杂志,2022,35(7):995-997.[14]张静,魏抗洪.鼠神经生长因子对新生儿缺氧缺血性脑病合并心肌损伤患儿神经功能及S O D㊁V E G F㊁I L-10水平的影响[J].中国医学工程,2022,30(3):134-136. [15]杜双霞,张晓红,苑艳尊,等.血清氧化应激水平与血管性痴呆患者认知功能㊁神经损伤因子水平及预后的关系[J].山东医药,2022,62(5):67-70.[16]唐映红,黄小平,谭华,等.三七总皂苷对脑缺血再灌注后细胞外基质破坏及氧化应激的影响[J].中华中医药学刊,2010,28(11):2327-2330.(收稿日期:2023-05-10修回日期:2023-11-03)㊃422㊃检验医学与临床2024年1月第21卷第2期 L a b M e d C l i n,J a n u a r y2024,V o l.21,N o.2。
