1-s2.0-0045794995002316-main
ASCII码表

ASCII码表信息在计算机上是用二进制表示的,这种表示法让人理解就很困难。
因此计算机上都配有输入和输出设备,这些设备的主要目的就是,以一种人类可阅读的形式将信息在这些设备上显示出来供人阅读理解。
为保证人类和设备,设备和计算机之间能进行正确的信息交换,人们编制的统一的信息交换代码,这就是ASCII码表,它的全称是“美国信息交换标准代码”。
ASCII码对照表在Web开发时,如下的ASCII码只要加上&#和;就可以变成Web可以辨认的字符了在处理特殊字符的时候特别有用,如:' 单引号在数据库查询的时候是杀手,但是如果转换成'(注意:转换后的机构有:&# +字符的ASCII码值+; 三个部分组成)再来存数据库,就没有什么影响了。
其他的字符与ASCII码的对照如下表ASCII表键盘常用ASCII码ESC键 VK_ESCAPE (27)回车键: VK_RETURN (13)TAB键: VK_TAB (9)Caps Lock键: VK_CAPITAL (20) Shift键: VK_SHIFT ($10)Ctrl键: VK_CONTROL (17)Alt键: VK_MENU (18)空格键: VK_SPACE ($20/32)退格键: VK_BACK (8)左徽标键: VK_LWIN (91)右徽标键: VK_LWIN (92)鼠标右键快捷键:VK_APPS (93)Insert键: VK_INSERT (45) Home键: VK_HOME (36)Page Up: VK_PRIOR (33) PageDown: VK_NEXT (34)End键: VK_END (35)Delete键: VK_DELETE (46)方向键(←): VK_LEFT (37)方向键(↑): VK_UP (38)方向键(→): VK_RIGHT (39)方向键(↓): VK_DOWN (40)F1键: VK_F1 (112)F2键: VK_F2 (113)F3键: VK_F3 (114)F4键: VK_F4 (115)F5键: VK_F5 (116)F6键: VK_F6 (117)F7键: VK_F7 (118)F8键: VK_F8 (119)F9键: VK_F9 (120)F10键: VK_F10 (121)F11键: VK_F11 (122)F12键: VK_F12 (123)Num Lock键: VK_NUMLOCK (144) 小键盘0: VK_NUMPAD0 (96)小键盘1: VK_NUMPAD0 (97)小键盘2: VK_NUMPAD0 (98)小键盘3: VK_NUMPAD0 (99)小键盘4: VK_NUMPAD0 (100)小键盘5: VK_NUMPAD0 (101)小键盘6: VK_NUMPAD0 (102)小键盘7: VK_NUMPAD0 (103)小键盘8: VK_NUMPAD0 (104)小键盘9: VK_NUMPAD0 (105)小键盘.: VK_DECIMAL (110)小键盘*: VK_MULTIPLY (106)小键盘+: VK_MULTIPLY (107)小键盘-: VK_SUBTRACT (109)小键盘/: VK_DIVIDE (111)Pause Break键: VK_PAUSE (19) Scroll Lock键: VK_SCROLL (145)。
1-s2.0-S1467803909000218-main

The larval alimentary canal of the Antarctic insect,Belgica antarcticaJames B.Nardi a ,*,Lou Ann Miller b ,Charles Mark Bee c ,Richard E.Lee,Jr.d ,David L.Denlinger eaDepartment of Entomology,University of Illinois,320Morrill Hall,505S.Goodwin Avenue,Urbana,IL 61801,USAbCenter for Microscopy and Imaging,College of Veterinary Medicine,University of Illinois,2001S.Lincoln Avenue,Urbana,IL 61801,USA cImaging Technology Group,Beckman Institute for Advanced Science and Technology,University of Illinois,405N Mathews Avenue,Urbana,IL 61801,USA dDepartment of Zoology,Miami University,Oxford,OH 45056,USA eDepartment of Entomology,Ohio State University,400Aronoff Laboratory,318West 12th Avenue,Columbus,OH 43210,USAa r t i c l e i n f oArticle history:Received 26October 2008Accepted 17April 2009Keywords:Alimentary canal Midge AntarcticaStomodeal valve MicrovilliRegenerative cellsa b s t r a c tOn the Antarctica continent the wingless midge,Belgica antarctica (Diptera,Chironomidae)occurs further south than any other insect.The digestive tract of the larval stage of Belgica that inhabits this extreme environment and feeds in detritus of penguin rookeries has been described for the first time.Ingested food passes through a foregut lumen and into a stomodeal valve representing an intussus-ception of the foregut into the midgut.A sharp discontinuity in microvillar length occurs at an interface separating relatively long microvilli of the stomodeal midgut region,the site where peritrophic membrane originates,from the midgut epithelium lying posterior to this stomodeal region.Although shapes of cells along the length of this non-stomodeal midgut epithelium are similar,the lengths of their microvilli increase over two orders of magnitude from anterior midgut to posterior ldings of the basal membranes also account for a greatly expanded interface between midgut cells and the hemocoel.The epithelial cells of the hindgut seem to be specialized for exchange of water with their environment,with the anterior two-thirds of the hindgut showing highly convoluted luminal membranes and the posterior third having a highly convoluted basal surface.The lumen of the middle third of the hindgut has a dense population of resident bacteria.Regenerative cells are scattered throughout the larval midgut epithelium.These presumably represent stem cells for the adult midgut,while a ring of cells,marked by a discontinuity in nuclear size at the midgut-hindgut interface,presumably represents stem cells for the adult hindgut.Ó2009Elsevier Ltd.All rights reserved.1.IntroductionDespite the dominance of insects and other terrestrial arthro-pods throughout the world,only a few species are found in Antarctica.Most are collembolans and mites,with the Class Insecta represented by only two endemic species of midges (Diptera:Chironomidae)(Convey and Block,1996).Of these,the range of the more abundant midge Belgica antarctica extends further south on the Antarctic Peninsula than that of any other insect.B.antarctica has a patchy distribution on the Peninsula,but it is particularly abundant near penguin rookeries on the small,off-shore islands near Palmer Station.In this habitat,midge larvae are subjected to a range of environmental stresses including desicca-tion,freezing,anoxia,pH fluctuation from the nitrogenous run-off,and inundation from seawater as well as fresh water fromprecipitation and ice melt.Numerous physiological adaptations are apparent in the larvae:heat shock proteins are continuously up-regulated (Rinehart et al.,2006),agents to counter oxidative stress are present in abundance (Lopez-Martinez et al.,2008),loss of a high percentage of body water is tolerated and in fact contributes to enhanced freeze tolerance (Hayward et al.,2007;Elnitsky et al.,2008).Dramatic changes in metabolites (Michaud et al.,2008)and gene expression (Lopez-Martinez et al.,2009)accompany these physiological responses to environmental stress.In addition to these distinctive biochemical features of cells of B.antarctica ,it is quite possible that structural features of this insect may also deviate somewhat from the patterns observed in insects at lower latitudes.The harsh environment of Antarctica obviously places great demands on the resiliency of cells that are exposed to periodic desiccation and freezing.To promote formation of ice crystals within extracellular spaces rather than within cells,insect cells must rapidly exchange water with their extracellular environments.Expansion of luminal and/or basal surface areas of epithelial cells would facilitate this rapid exchange.*Corresponding author.Tel.:þ12173336590;fax:þ12172443499E-mail address:j-nardi@ (J.B.Nardi).Contents lists available at ScienceDirectArthropod Structure &Developmentjournal homepage :www.else /locate/asd1467-8039/$–see front matter Ó2009Elsevier Ltd.All rights reserved.doi:10.1016/j.asd.2009.04.003Arthropod Structure &Development 38(2009)377–389Special emphasis in this manuscript has been placed on the cellular architecture of the three well-delineated epithelial regionsof the insect alimentary canal–foregut,midgut,and hindgut. Secretion from the midge’s large salivary gland cells aggregates detritus particles and facilitates uptake of detritus into the gut (Oliver,1971).After passage of ingested food through the midge larva’s short and narrow foregut,digestion and absorption of ingested material occurs across the peritrophic membrane and microvilli that line the lumen of the midgut.Subsequently digested food passes into the hindgut where ions,small molecules and water are absorbed across a cuticular lining.Comparisons of internal gut morphology of arthropods complement existing studies of external morphological features and molecular characters on which traditional phylogenetic relationships,representing genetic differences among organisms, are based.Although the alimentary canals of other species in the family Chironomidae have not been examined at the cellular level of organization examined in this manuscript,a compre-hensive comparison of internal morphological characters among related species would reveal differences that presumably are based on environmental adaptations.In this study we explore the possibility that the extreme environment in which these midge larvae live shapes the architecture of their gut epithelial cells.2.Materials and methodsLarvae of B.antarctica were collected from sites near penguin rookeries on Cormorant and Humble Islands,near Palmer Station, Antarctica(64 460S,64 040W)in January and February2007.The high nitrogen run-off from penguin rookeries provides the nutrient base for the microbes,algae(Prasiola crispa),lichens and moss with which midge larvae are usually rval development is restricted to the brief austral summer,from late December until mid-February;and larvae remain immobilized in the frozen substrate for the remainder of the year.Two years are required for the larva to complete its development(Usher and Edwards,1984),and adult life is compressed into a1–2week period in late December/early January(Sugg et al.,1983).Larvae collected in Antarctica were shipped to the laboratory at Ohio State University where they were maintained with their substrate at4 C.Digestive tracts and salivary glands from32individuals were dissected withfine watchmaker’s forceps in Grace’s insect culture medium.Tissues were immediatelyfixed at4 C in a primary fixative of2.5%glutaraldehyde(v/v)and0.5%paraformaldehyde (w/v)dissolved in a rinse buffer of0.1M cacodylate(pH7.4) containing0.18mM CaCl2and0.58mM sucrose.After3h in this fixative,tissues were washed three times with rinse buffer before being transferred to secondaryfixative(2%osmium tetroxide dissolved in rinse buffer,w/v)for4h.After thoroughly washing with rinse buffer,tissues were gradually dehydrated in a graded ethanol series(10–100%,v/v).From absolute ethanol,tissues were transferred to propylene oxide and infiltrated with mixtures of propylene oxide and resin before being embedded in pure LX112 resin.Semithin sections for light microscopy were mounted on glass slides and stained with0.5%toluidine blue in1%borax(w/v). Ultrathin sections were mounted on grids and stained briefly with saturated aqueous uranyl acetate and Luft’s lead citrate to enhance contrast.Images were taken with a Hitachi600transmission electron microscope operating at75kV.Whole mounts were prepared byfixing tissues at room temperature for30min in a4%solution of paraformaldehyde (w/v)dissolved in phosphate-buffered saline(PBS).After Fig.1.(a)Whole mount of larval gut with the three major divisions of the gut demarcated with arrows:fm¼foregut–midgut boundary;mh¼midgut–hindgut boundary.The stomodeal valve lies immediately posterior to fm(see Fig.4).The sclerotized head capsule is attached at the anterior end and four Malpighian tubules attach at the midgut–hindgut boundary.(b)Lateral view of the larva. Dorsal is to the right.Beneath the relatively translucent integument of the three thoracic segments(arrows point to prothoracic and metathoracic segments),lie imaginal discs for the three pairs of legs.Dorsal to these leg discs in the meso-thoracic and metathoracic segments are wing and haltere discs,respectively.Scale bar¼1.0mm.J.B.Nardi et al./Arthropod Structure&Development38(2009)377–389 378several rinses in PBS,nuclei of cells were labeled with1:1000 dilution of40,6-diamidino-2-phenylindole(DAPI,1mg/ml water)afterfirst permeabilizing cells for30min in a solution of 0.1%Triton X-100in PBS(v/v).Tissues were mounted under cover glasses in a solution of70%glycerin in0.1M Tris at pH 9.0(v/v).3.Results3.1.Global organization of the Belgica larval gutThe larval gut is a straight alimentary canal that is associated with a pair of salivary glands at its anterior end and four Malpighian tubules that converge with the alimentary canal at the junction of midgut and hindgut(Fig.1,mh).A prominent stomodeal valve occupies the interface between foregut and midgut(Fig.1,fm).The foregut occupies only about5%of the total length of the alimentary canal,while the endodermal midgut that occupies the region between fm and mh in Fig.1 clearly represents more than half the length of the alimentary canal.3.2.Salivary glands and foregutConspicuous salivary glands occupy the anterior end of the larval midge.These glands are both polyploid and polytene(Fig.2).Fig.3.Cuticles of foregut and epidermal epithelia have different stratification.At higher magnification,the internal folded foregut cuticle(a)is compared with the cuticle of the external epidermis(b).A bracket extends across the foregut cuticle.The gut lumen(*)is surrounded by relatively thin cuticle compared to the thicker epidermal cuticle in(b) epithelial cells(e),pigmented fat body(f).In(c)the convoluted integument of the foregut(arrowhead)is surrounded by muscles(arrows),tracheoles(t),neural tissue(n)and salivary gland(g).Scale bars:(a)1.0m m,(b)5m m,and(c)20mm.Fig.2.Whole mount of Belgica salivary gland as viewed with Nomarski optics(a)and after labeling DNA with DAPI(b).The salivary duct is located at the arrow.Scale bar¼50m m.J.B.Nardi et al./Arthropod Structure&Development38(2009)377–389379Like glands found in other larval members of the Chironomidae,these salivary glands secrete silken threads that entrap food particles.On the foregut’s apical surface,the cuticle lining the narrow foregut lumen is about 0.3–0.5m m thick (Fig.3a).Unlike the thicker,contiguous cuticle of the larva’s exoskeleton with its inner electron-dense layer (Fig.3b),this foregut cuticle is highly convoluted and its outermost layer is electron-dense.Conspicuous muscle layers surround the foregut.The large salivary glands and larval brain in turn surround these muscles on the basal surface of the larval foregut (Fig.3c).3.3.Stomodeal valve at foregut–midgut interfaceAfter being channeled through a foregut lumen lined by a convoluted cuticle,the contents of the gut pass through a conspicuous stomodeal valve into the endoperitrophic space of the midgut epithelium.The stomodeal valve represents anintussusception of the foregut epithelium into the midgut (Figs.4a–d and 5a–c).This folding of the foregut epithelium and its cuticle creates a caecum that is lined centrally by foregut cuticle and peripherally by midgut microvilli.The interface of foregut and midgut lies at the anterior end of the caecum.At this junction of foregut and midgut,a peritrophic membrane origi-nates and lines the lumen of the more posterior midgut epithelia.3.4.Spatial differentiation of the midgut epitheliumAn abrupt epithelial discontinuity marked by disparity in midgut microvillar length occurs at the interface between the stomodeal region and the more posterior midgut epithelium (Fig.4c and 5d).Certain cells at this interface are specialized for secretion (Fig.5d–f)and possibly are endocrine cells.These cells at the posterior edge of the stomodeal valve were the only cells of the midgut observed to contain conspicuous secretorygranulesFig.4.The stomodeal valve represents an intussusception of posterior foregut epithelium (fe)into anterior midgut epithelium (me).(a)The folded foregut epithelium is surrounded by the anterior midgut.Anterior is to the right.(b,c)Longitudinal sections show inner lumen cuticle (ic)and outer lumen cuticle (oc)of the foregut.Between these two cuticles lie two foregut epithelial layers and an enteric muscle layer (m).Midgut epithelium is the outermost layer of the valve.(c)Represents the region delimited by the rectangle in (b).The morphological discontinuity is indicated by the arrow.The peritrophic membrane (p)lies in the lumen separating foregut and midgut.(d)The concentric arrangement of three epithelia,two lumina and one muscle layer is shown in this transverse section of the valve.From periphery to center:(1)midgut epithelium with microvilli lining the lumen in which the peritrophic membrane forms;(2)foregut epithelium faces this lumen and (3)enteric muscles (m)occupy the space between this foregut epithelium and the foregut epithelium facing the innermost lumen.Scale bars:(a,b,d)50m m;(c)20m m.J.B.Nardi et al./Arthropod Structure &Development 38(2009)377–389380Fig.5.At higher resolution,longitudinal sections of the stomodeal valve reveal details of peritrophic membrane formation and the presence of special secretory cells.(a)At the interface between the foregut epithelium and midgut epithelium lies a confluence of muscle (m),foregut epithelium covered by cuticle (fg)and secretory microvilli (arrows)of midgut epithelium.Anterior is at the bottom.(b,c)Copious secretion of peritrophic membrane material (*)occurs from the microvilli of midgut epithelial cells at the anterior end of the stomodeal valve.Note the high density of mitochondria in the adjacent midgut cells.In (b)the newly formed peritrophic membrane (arrow)lies between the foregut (fg)cuticle and the tips of the microvilli.(d)Distinctive secretory cells (arrow)lie within the midgut epithelium of the stomodeal valve at the border between midgut cells with microvilli and midgut cells without obvious microvilli (See Fig.4c).The gut lumen is at upper left.(e,f)Close-ups of the secretory cell showing the nucleus (n),rough enoplasmic reticulum (arrowhead)and the high density of secretory granules (*).Scale bars:(a)10m m;(b,c)2m m;(d)5m m;(e)1m m;and (f)0.2m m.J.B.Nardi et al./Arthropod Structure &Development 38(2009)377–389381(Fig.5e and f).Posterior to the stomodeal valve,a striking gradient of microvillar length occurs along the antero-posterior (AP)axis of the midgut epithelium (Fig.6),with short (w 0.1to 0.2m m)microvilli occupying anterior regions of the midgut and extremely long,straight and densely packed (w 10m m,Figs.7and 9)microvilli occupying posterior regions of the midgut.This morphological gradient is evident in longitudinal sections of the midgut (Fig.6a–c)as well as the series of transverse sections from different locations along the AP axis of the Belgica gut (Figs.6d–f and 7–9).Underlying the antero-posterior topography revealed by the microvilli of the midgut is a parallel topography,at the base of the microvilli,reflected by contours of the apical surfaces of the midgut cells.These surfaces are most convoluted in regions of the midgut with the shortest microvilli and are least convoluted in regions of the midgut with the longest microvilli (Fig.6d–f).Rough endoplasmic reticulum (RER)is present throughout all regions of the midgut (Figs.7d,8d,and 9d).Stacks of large flattened sacs of endoplasmic reticulum are especially evident in the posterior region of the midgut epithelium.Some smooth endoplasmic reticulum (SER)is interspersed among the RER of the anterior third of the midgut,with little if any SER is observed in the middle third or the posterior third of the midgut.However,clearly defined Golgi complexes are not evident in any of the midgut cells.The lumen of the middle third of the Belgica midgut is lined with microvilli of intermediate length (w 1m m)that are associ-ated with electron-dense particles of uniform size (w 0.05m m).These particles lie within the ectoperitrophic space and show a strong affinity for the microvilli (Fig.8a–c).Within the cyto-plasm of the underlying midgut cells,electron-dense particles of identical size are concentrated in autophagic vacuoles,each delimited by a plasma membrane,and are presumed to enter these epithelial cells by endocytosis.Numerous coated vesicles atthe base of microvilli on the luminal surfaces of these midgut cells (Fig.8c)offer an obvious route for the cellular uptake of these electron-dense particles from the ectoperitrophic space of the gut lumen.Infoldings of the basal membranes of epithelial cells are most prominent in the anterior and the posterior regions of the midgut (Figs.7c,8e,and 9c);mitochondria are most conspicuous along the basal surface of the anterior midgut (Fig.7a,c);and infoldings of the basal plasma membrane contain numerous mitochondria and electron-lucent vacuoles (Figs.7c and 9c).Regenerative cells are scattered throughout the larval midgut epithelia and presumably represent imaginal stem cells that replace the larval epithelium at metamorphosis.These cells are located basally in the larval epithelium and are densely packed with ribosomes and endoplasmic reticulum (Fig.10).3.5.Contents of the midgut lumen3.5.1.Endoperitrophic spaceWithin the gut lumen,the peritrophic membrane separates an inner endoperitrophic space from an outer ectoperitrophic space (Terra and Ferreira,1994;Fig.6).The anterior endoperitrophic space of the midgut is packed with relatively intact multicellular microbial organisms.Gradual digestion of microbes is reflected in the disappearance of pigmentation from the gut lumen in the posterior third of the midgut (Fig.1a).Within the confines of the midgut’s peritrophic membrane,microbial cells arranged singly or in various aggregates can be readily discerned.These cells can be identified as predominantly nonbacterial microbes –i.e.,algae,fungi,lichens,protists (Fig.11a–c).3.5.2.Ectoperitrophic spaceThe surrounding ectoperitrophic space is lined by midgut epithelium with microvilli whose lengths are graded alongtheFig.6.Longitudinal (a–c)and transverse (d–f)sections of midgut epithelium posterior to the stomodeal valve show regional differences along the antero-posterior axis of the gut.Three equally spaced regions along this axis are illustrated:(a,d)anterior region;(b,e)middle region;(c,f)posterior region.In each image the microvillar surface and gut lumen are at top.The peritrophic membrane (arrow)is visible in (a,b,d,and e).Scale bars ¼20m m.J.B.Nardi et al./Arthropod Structure &Development 38(2009)377–389382antero-posterior axis.Within one of the 10whole mounts of larval guts prepared,gregarines with distinctive appendages were found in this midgut zone (between the peritrophic matrix and midgut epithelial surface)(Fig.11d and e).Considering the isolation of these gregarines from other related host species,these protists most likely represent a distinct species of dipteran parasite.3.6.Spatial differentiation of the hindgut3.6.1.Ring of undifferentiated,presumptive adult hindgut epitheliumIn the images of the Belgica gut labeled with DAPI (Fig.12a),a discontinuity in nuclear labeling is observed at the midgut–hindgut boundary.At the anterior-most region of the larval hindgut,an imaginal ring of undifferentiated presumptive adult hindgut cells appears in whole mounts as a zone of cells with small nuclei among the polyploid nuclei in cells of the larval hindgut epithelium.Immediately posterior to the imaginal ring of epithelial cells,the anterior third of the hindgut is lined by a smooth cuticle approximately 0.25m m thick.This hindgut cuticle,like the foregut cuticle,is contiguous with the exoskeleton.Also,like the foregut cuticle,the hindgut cuticle secretes a well-delineated,electron-dense outermost cuticular layer.The arrangement of these different layers secreted by hindgut epithelial cells is distinct from the arrangement of the cuticular layers secreted by epidermal epithelia (Fig.3b).The anterior hindgut cuticle is secreted by attenuated,highly folded extensions of the apical surfaces of the hindgut epithelium containing conspicuous mitochondria and separated by large vacuoles that lack electron-density (Fig.12b andc).Fig.7.These are ultrastructural images of the anterior region of the midgut epithelium.Note the high concentration of mitochondria along the basal surface of the epithelium in (a)as well as the dark cell (arrow)at the basal surface of this epithelium.Lumen is marked with an asterisk (*).(b)The epithelial surface facing the lumen (*)is covered by short microvilli.(c)Mitochondria (arrows)are localized among membrane infoldings that lie adjacent to the basal lamina and enteric muscles (arrowhead).(d)Perinuclear region of midgut cell.Rough endoplasmic reticulum (ER)marked with arrowhead.Smooth ER marked with double arrowhead.Arrows point to mitochondria.n ¼nucleus.Scale bars:(a)5m m;(b,c)2m m;and (d)1m m.J.B.Nardi et al./Arthropod Structure &Development 38(2009)377–389383While apical ends of epithelial cells in the anterior third of the hindgut have conspicuous large vacuoles,the central region of the hindgut is occupied by epithelial cells that lack vacuoles but that have extremely convoluted apical membranes characteristic of epithelial cells involved in active transport of ions and water (Fig.12d and e).Also in the central region of the hindgut,the presence of the electron-dense bacteria observed in high-resolu-tion images is reflected in the pigmentation of the central portion of the hindgut as viewed in whole mounts of larval guts (Fig.1a).In the posterior third of the hindgut or rectum,the highly convoluted cuticle lining the lumen mirrors the structure of the foregut.The apical surfaces of the epithelial cells of the rectum lack infolded membranes associated with mitochondria and are apparently not specialized for transport of ions and water.The basal surface of this region,by contrast,is highly infolded.Association of this basal surface with numerous muscles suggeststhis posterior-most hindgut epithelium serves a mechanical function.In this most posterior portion of the hindgut,the lumen is devoid of both resident microbes as well as ingested microbes (Fig.12f).3.7.Muscles associated with the gut epitheliaThe basal surface of the hindgut epithelium,like the foregut epithelium,is closely apposed to a uniformly thick layer of circum-ferential muscles (w 10m m)(Figs.3c,12e,f).By contrast,muscles associated with the midgut epithelium are sparsely but regularly distributed over the midgut’s basal surface (Figs.6–9).Relatively widely dispersed muscle cells lie on the basal surface of the midgut epithelium and are embedded in the matrix of the epithelial basal lamina.These represent the longitudinal muscles of the gut.Sparsely distributed circumferential muscles are alsopresent.Fig.8.Different magnifications of the middle region of the midgut are illustrated in (a–e).In images a and c,the midgut lumen is at the top;in e,it is down.Electron-dense particles occupy the space between the peritrophic membrane (arrow in a)and the microvillar parable particles are observed in autophagic vacuoles of midgut cells (arrowheads in b).(c)Higher resolution images suggest that these particles are taken up by endocytosis (arrows)at the microvillar surface.(d)Perinuclear region of midgut cell.Arrowheads indicate rough endoplasmic reticulum;arrows point to mitochondria;n ¼nucleus;me ¼membrane between two cells.(e)Basal lamina (arrowhead)and muscles (m)cover the basal surface of this ldings of the basal surface of plasma membrane are not conspicuous.Scale bars:(a)5m m;(b,c)1.0m m;(d)1m m;and (e)2m m.J.B.Nardi et al./Arthropod Structure &Development 38(2009)377–3893844.DiscussionThe study of insect diversity and evolution has been advanced by extensive surveys of molecular phylogeny and morphology of external integuments;however,knowledge and appreciation of insect diversity remain incomplete without adequate knowledge of the diversity of internal anatomy/physi-ology of insects and how this diversity is influenced by environment.With the paucity of information on the cellular architecture of insect guts,however,associating particular gut epithelial struc-tures with adaptation to particular diets and/or environments remains in a rudimentary state.Conventional descriptions of gut epithelial diversity (Lehane,1998;Noble-Nesbitt,1998;Terra et al.,1988)clearly do not consider the marked regional differ-entiation of microvilli on midgut cells of B.antarctica to be a common feature of epithelial cells of insect guts.Establishinghow general or how unique internal features are among insects,however,awaits additional structural studies on other related insect species.4.1.Differentiation of foregut–midgut boundary:structure of the peritrophic membrane and stomodeal valveA specialized luminal region at the foregut–midgut boundary can be visualized even in whole mounts of the alimentary canal (Fig.1a).In cross-sections and longitudinal sections of the alimentary canal at this boundary region,an inner concentric ring of folded foregut epithelium and associated muscle layers lies within the anterior midgut epithelium (Figs.4,5).Among the Diptera,the degree of specialization of foregut and midgut epithelial cells varies among the suborders.The most complex specialization of the foregut–midgut interface is found among the muscoid flies,in which specialized anteriormidgutFig.9.In a–c,the midgut lumen is to the left.Different magnifications of the posterior region of the midgut are illustrated.(a)Note numerous clear vacuoles (arrows)that lie between the luminal microvilli and the basal lamina (lower right).(b)The long microvilli are densely packed and extend approximately 10m m into the lumen.(c)The basal membranes of cells in this posterior region are highly folded.Basal lamina is indicated with arrowheads.Muscle ¼m.(d)Perinuclear region of midgut cell.Arrowheads indicate rough endoplasmic reticulum;arrows point to mitochondria.Scale bars:(a)10m m;(b)5m m;(c)2m m;and (d)1m m.J.B.Nardi et al./Arthropod Structure &Development 38(2009)377–389385epithelium is closely apposed to the stomodeal valve to form the distinctive cardia (Eisemann et al.,2001;Binnington,1988);but the simplest specialization of the foregut–midgut interface is observed in the suborder Nematocera,of which Belgica is a member.In these flies,the foregut has been described as forming a short intussusception into the anterior midgut referred to as the stomodeal valve (King,1991;Wigglesworth,1930).For Belgica ,however,this intussusception of foregut as a percentage of total foregut surrounded by midgut is higher than that reported by Volf et al.(2004)for four other nem-atoceran Diptera in the families Culicidae (Culex pipiens )and Psychodidae (Lutzomyia longipalpis ,Phlebotomus duboscqi ,Phle-botomus papatasi ).Peritrophic structures for many insect species have often been described as chitinous,reticulated membranes with chitin micro-fibrils in a hexagonal or orthogonal arrangement (Lehane,1998).These peritrophic structures consist of chitin networks embedded in protein–carbohydrate matrices (Wang and Granados,2001;Tellam et al.,1999).The origin and consistency of peritrophic membranes,gels and matrices differ among the insects (Terra,2001;Binnington et al.,1998).The peritrophic structures of some insects,such as lepi-dopteran larvae,have traditionally been described as arising from cells along the length of the midgut epithelium (type I peritrophic matrix).Recent studies involving labeling of lepidopteran peri-trophic proteins have indicated that while one peritrophic protein (i.e.,invertebrate intestinal mucin)is secreted by epithelial cells throughout the length of the midgut (Harper and Granados,1999),certain peritrophic proteins recognized by an antibody raised against the peritrophic membrane of Heliothis virescens are produced by specialized cells near the foregut–midgut interface (Ryerse et al.,1992).At the junction of foregut and midgut epithelia in Diptera,the peritrophic structure (type II)arises from microvilli of midgut epithelia (Eisemann et al.,2001)and lines the lumen of the more posterior midgut epithelia.In Belgica ,the midgut epithelial cells of the stomodeal valve are the only cells observed to produce a copious secretion associated with a newly formed structure that represents a type II peritrophic membrane.4.2.Regional differentiation of midgut epitheliumAt least in some insects,the midgut is differentiated both structurally and functionally along its length (Lehane,1998;Marana et al.,1997;Ferreira et al.,1990;Terra et al.,1988;Dow,1981).The marked and graded differences in microvillar lengths observed for Belgica midgut epithelial cells,however,represent an extreme example of such regional differentiation (Fig.6).Extensive infolding of the basal epithelial surface of midgut and hindgut cells,however,as frequently observed in other insects,is only evident in certain regions (anterior third and posterior third)of the Belgica midgut and the posterior third (rectum)of its hindgut (Villaro et al.,1999;Lehane,1998;Marana et al.,1997).See Figs.7c,8e,9c and 12f.The high concentrations of electron-dense particles that occupy the ectoperitrophic space of the central region of the midgut are not observed elsewhere in the alimentary canal.The presence of comparable particles in autophagic vacuoles of these midgut cells suggests that these particles are taken up by cells rather than secreted by gut cells.The high concentration of particles in the gut lumen also implies that their movement proceeds toward the low concentration of particles observed within the midgut epithelial cells.Although regenerative cells have not been observed in midgut epithelia of certain immature arthropods such aslarvalFig.10.Regenerative cells (asterisks)of midgut epithelium are scattered throughout the larval epithelium.Cells from different regions along the antero-posterior axis are shown.(a)Anterior third.(b)Middle third.(c)Posterior third.Arrowheads point to basal laminae;m ¼muscles;n ¼nuclei of regenerative cells.Scale bars ¼2.0m m.J.B.Nardi et al./Arthropod Structure &Development 38(2009)377–389386。
出货检验履历表

不良率
检验日期
受检方
检验方式
#DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0!
#DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0!
#DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0!
462 463 464 465 466 467 468 469 470 471 472 473 474 475 476 477 478 479 480 481 482
483 484 485 486 487 488 489 490 491 492 493 494 495 496 497 498 499 500 501 502 503
#DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0! #DIV/0!
ASCII码中英文对照表

关键字:最全ASCII码对照表ASCII码值对照表 ASCI I码值A SCII码中英文对照表Bi n DecH ex 缩写/字符解释0000 0000 0 00 NUL(null) 空字符0000 0001 1 01 SOH (start of h andin g) 标题开始0000 0010202 S TX (s tartof te xt) 正文开始00000011 303 ET X (en d oftext)正文结束0000 0100 4 04 EOT(endof tr ansmi ssion) 传输结束0000 0101 5 05 ENQ (enq uiry)请求00000110 606 AC K (ac knowl edge)收到通知0000 0111 7 07 BEL (bel l) 响铃0000 1000 8 08 BS(back space) 退格0000 1001 9 09 HT(hori zonta l tab) 水平制表符0000 1010 10 0A LF (N L lin e fee d, ne w lin e) 换行键00001011 11 0B VT(vert icaltab)垂直制表符00001100 12 0C FF(NP f orm f eed,new p age)换页键0000 1101 13 0D C R (ca rriag e ret urn)回车键0000 111014 0E SO (shi ft ou t) 不用切换0000 111115 0F SI (shi ft in) 启用切换0001 0000 16 10 DLE(data link esca pe) 数据链路转义0001 0001 17 11 DC1(devi ce co ntrol 1) 设备控制10001 0010 18 12 DC2 (devic e con trol2) 设备控制20001 0011 19 13 D C3 (d evice cont rol 3) 设备控制30001 010020 14 DC4 (de vicecontr ol 4)设备控制4 00010101 21 15 NAK (neg ative ackn owled ge) 拒绝接收0001 0110 22 16 S YN (s ynchr onous idle) 同步空闲0001 0111 23 17 ETB(endof tr ans.block) 传输块结束0001 1000 24 18 C AN (c ancel) 取消0001 1001 25 19 EM (e nd of medi um) 介质中断0001 101026 1A SU B (su bstit ute)替补0001 101127 1B ES C (es cape)溢出0001 110028 1C FS (fil e sep arato r) 文件分割符0001 1101 29 1D GS (group sepa rator) 分组符0001 111030 1E RS (rec ord s epara tor)记录分离符00011111 31 1F US(unit sepa rator) 单元分隔符0010 0000 32 20 空格0010 0001 33 21 !0010 0010 34 22 "0010 0011 35 23 #0010 0100 36 24 $0010 0110 38 26 & 0010 0111 39 27 ' 0010 1000 40 28 ( 0010 1001 41 29 ) 0010 1010 42 2A * 0010 1011 43 2B + 0010 1100 44 2C , 0010 1101 45 2D -0010 1110 46 2E . 0010 1111 47 2F / 0011 0000 48 30 0 0011 0001 49 31 1 0011 0010 50 32 2 0011 0011 51 33 3 0011 0100 52 34 4 0011 0101 53 35 5 0011 0110 54 36 6 0011 0111 55 37 7 0011 1000 56 38 8 0011 1001 57 39 9 0011 1010 58 3A : 0011 1011 59 3B ; 0011 1100 60 3C < 0011 110161 3D =0011 111062 3E >0011 111163 3F ?0100 000064 40 @0100 000165 41 A0100 001066 42 B0100 001167 43 C0100 010068 44 D0100 010169 45 E0100 011070 46 F0100 011171 47 G0100 100072 48 H0100 100173 49 I0100 101074 4A J0100 101175 4B K0100 110076 4C L0100 110177 4D M0100 111078 4E N0100 111179 4F O0101 000080 50 P0101 000181 51 Q0101 001183 53 S0101 010084 54 T0101 010185 55 U0101 011086 56 V0101 011187 57 W0101 100088 58 X0101 100189 59 Y0101 101090 5A Z0101 101191 5B [0101 110092 5C \0101 110193 5D ]0101 111094 5E ^0101 111195 5F _0110 000096 60 `0110 000197 61 a0110 001098 62 b0110 001199 63 c0110 0100100 64 d 01100101 101 65 e 0110 0110 102 66 f 0110 0111 10367 g 0110 1000 104 68 h0110 1001105 69 i 01101010 106 6A j 0110 1011 107 6B k 0110 1100 1086C l 0110 1101 109 6D m0110 1110110 6E n 01101111 111 6F o 0111 0000 112 70 p 0111 0001 11371 q 0111 0010 114 72 r0111 0011115 73 s 01110100 116 74 t 0111 0101 117 75 u 0111 0110 11876 v 0111 0111 119 77 w0111 1000120 78 x 01111001 121 79 y 0111 1010 122 7A z 0111 1011 1237B { 0111 1100 124 7C |0111 1101125 7D } 01111110 126 7E ~01111111 127 7F DEL(dele te) 删除E SC键V K_ESC APE (27)回车键:VK_RE TURN(13)TAB键: VK_TAB(9)C aps L ock键: VK_C APITA L (20)Shi ft键: VK_SHIFT ()C trl键:V K_CON TROL(17)Alt键: VK_M ENU (18)空格键: VK_S PACE(/32)退格键: VK_BACK (8)左徽标键:VK_LW IN (91)右徽标键: VK_LWIN (92)鼠标右键快捷键:V K_APP S (93)In sert键: VK_INSE RT (45)Ho me键: VK_H OME (36)P age U p: VK_PRIO R (33)Pag eDown: VK_NEXT(34)End键:VK_EN D (35)Del ete键: VK_D ELETE (46)方向键(←): VK_L EFT (37)方向键(↑):VK_UP (38)方向键(→): VK_RIGHT (39)方向键(↓): VK_DOWN(40)F1键: VK_F1 (112)F2键: VK_F2 (113)F3键:VK_F3 (114)F4键:V K_F4(115)F5键: VK_F5 (116)F6键: VK_F6 (117)F7键: VK_F7 (118)F8键:VK_F8 (119)F9键:V K_F9(120)F10键: VK_F10 (121)F11键: VK_F11 (122)F12键:VK_F12 (123)Nu m Loc k键:V K_NUM LOCK(144)小键盘0:V K_NUM PAD0(96)小键盘1: VK_NUMP AD0 (97)小键盘2: VK_NUMPA D0 (98)小键盘3: VK_N UMPAD0 (99)小键盘4:VK_NU MPAD0 (100)小键盘5:VK_NU MPAD0 (101)小键盘6:VK_NU MPAD0 (102)小键盘7:VK_NU MPAD0 (103)小键盘8:VK_NU MPAD0 (104)小键盘9:VK_NU MPAD0 (105)小键盘.:VK_DE CIMAL (110)小键盘*:VK_MU LTIPL Y (106)小键盘+: VK_M ULTIP LY (107)小键盘-: VK_SUBTR ACT (109)小键盘/: VK_DIVI DE (111)P auseBreak键: VK_PAUS E (19)Scr oll L ock键: VK_S CROLL (145)关键字:最全AS CII码对照表 AS CII码值对照表A SCII码值 ASC II码中英文对照表作者:f lydoo飞度欢迎加QQ编程群:26438718飞度编程学社博客:htt p://f lydoo s.blo g.16网盘软件下载:htt p://f lydoo.ys168.com。
ASCII码值对照表

最全ASCII码对照表ASCII码值对照表ASCII码值ASCII码中英文对照表0010 0000 32 20 空格0010 0001 33 21 !0010 0010 34 22 "0010 0011 35 23 #0010 0100 36 24 $0010 0101 37 25 %0010 0110 38 26 &0010 0111 39 27 '0010 1000 40 28 (0010 1001 41 29 )0010 1010 42 2A *0010 1011 43 2B +0010 1100 44 2C ,0010 1101 45 2D -0010 1110 46 2E .0010 1111 47 2F /0011 0000 48 30 00011 0001 49 31 10011 0010 50 32 20011 0011 51 33 30011 0100 52 34 40011 0101 53 35 50011 0110 54 36 60011 0111 55 37 70011 1000 56 38 80011 1001 57 39 90011 1010 58 3A :0011 1011 59 3B ;0011 1100 60 3C <0011 1101 61 3D =0011 1110 62 3E >0011 1111 63 3F ?0100 0000 64 40 @0100 0001 65 41 A0100 0010 66 42 B0100 0011 67 43 C0100 0100 68 44 D0100 0101 69 45 E0100 0110 70 46 F0100 0111 71 47 G0100 1000 72 48 H0100 1001 73 49 I0100 1010 74 4A J0100 1011 75 4B K0100 1100 76 4C L0100 1101 77 4D M0100 1110 78 4E N0100 1111 79 4F O0101 0000 80 50 P0101 0001 81 51 Q0101 0010 82 52 R0101 0011 83 53 S0101 0100 84 54 T0101 0101 85 55 U0101 0110 86 56 V0101 0111 87 57 W0101 1000 88 58 X0101 1001 89 59 Y0101 1010 90 5A Z 0101 1011 91 5B [ 0101 1100 92 5C \ 0101 1101 93 5D ] 0101 1110 94 5E ^ 0101 1111 95 5F _ 0110 0000 96 60 ` 0110 0001 97 61 a 0110 0010 98 62 b 0110 0011 99 63 c 0110 0100 100 64 d 0110 0101 101 65 e 0110 0110 102 66 f 0110 0111 103 67 g 0110 1000 104 68 h 0110 1001 105 69 i 0110 1010 106 6A j 0110 1011 107 6B k 0110 1100 108 6C l 0110 1101 109 6D m 0110 1110 110 6E n 0110 1111 111 6F o 0111 0000 112 70 p 0111 0001 113 71 q 0111 0010 114 72 r 0111 0011 115 73 s 0111 0100 116 74 t 0111 0101 117 75 u 0111 0110 118 76 v 0111 0111 119 77 w 0111 1000 120 78 x 0111 1001 121 79 y 0111 1010 122 7A z 0111 1011 123 7B { 0111 1100 124 7C | 0111 1101 125 7D } 0111 1110 126 7E ~ 0111 1111 127 7F DEL (delete)删除ESC键 VK_ESCA PE (27)回车键: VK_RETU RN (13) TAB键: VK_TAB(9)Caps Lock键: VK_CAPI TAL (20) Shift键: VK_SHIF T ()Ctrl键: VK_CONT ROL (17) Alt键:VK_MENU (18)空格键: VK_SPAC E (/32)退格键: VK_BACK (8)左徽标键: VK_LWIN (91)右徽标键: VK_LWIN (92)鼠标右键快捷键:VK_APPS (93) Insert键: VK_INSE RT (45) Home键: VK_HOME (36) Page Up: VK_PRIO R (33) PageDow n: VK_NEXT (34)End键: VK_END(35) Delete键: VK_DELE TE (46)方向键(←): VK_LEFT (37)方向键(↑):VK_UP (38)方向键(→): VK_RIGH T (39)方向键(↓): VK_DOWN (40)F1键:VK_F1 (112)F2键:VK_F2 (113)F3键:VK_F3 (114)F4键:VK_F4 (115)F5键:VK_F5 (116)F6键:VK_F6 (117)F7键:VK_F7 (118)F8键:VK_F8 (119)F9键:VK_F9 (120)F10键: VK_F10(121)F11键: VK_F11(122)F12键: VK_F12(123)Num Lock键:VK_NUML OCK (144)小键盘0: VK_NUMP AD0 (96)小键盘1: VK_NUMP AD0 (97)小键盘2: VK_NUMP AD0 (98)小键盘3: VK_NUMP AD0 (99)小键盘4: VK_NUMP AD0 (100)小键盘5: VK_NUMP AD0 (101)小键盘6: VK_NUMP AD0 (102)小键盘7: VK_NUMP AD0 (103)小键盘8: VK_NUMP AD0 (104)小键盘9: VK_NUMP AD0 (105)小键盘.: VK_DECI MAL (110)小键盘*: VK_MULT IPLY (106)小键盘+: VK_MULT IPLY (107)小键盘-: VK_SUBT RACT (109)小键盘/: VK_DIVI DE (111)Pause Break键: VK_PAUS E (19)ScrollLock键: VK_SCRO LL (145)注意:1.在ASCII码中,有4组字符:一组是控制字符,如LF,CR等,其对应ASCI I码值最小;第2组是数字0~9,第3组是大写字母A~Z,第4组是小写字母a~z。
1-s2.0-S0146638012000599-main

A first step towards identification of tannin-derived black carbon:Conventional pyrolysis (Py–GC–MS)and thermally assisted hydrolysis and methylation (THM–GC–MS)of charred condensed tanninsJoeri Kaal a ,⇑,Klaas G.J.Nierop b ,Peter Kraal c ,Caroline M.Preston daInstituto de Ciencias del Patrimonio (Incipit),Consejo Superior de Investigaciones Científicas (CSIC),San Roque 2,15704Santiago de Compostela,Spain bDepartment of Earth Sciences –Organic Geochemistry,Faculty of Geosciences,Utrecht University,P.O.Box 80021,3508TA Utrecht,The Netherlands cSouthern Cross GeoScience,Southern Cross University,P.O.Box 157,Lismore,2480New South Wales,Australia dPacific Forestry Centre,Natural Resources Canada,506West Burnside Rd.,Victoria,BC,Canada V8Z 1M5a r t i c l e i n f o Article history:Received 5October 2011Received in revised form 13March 2012Accepted 26March 2012Available online 5April 2012a b s t r a c tTannins account for a significant proportion of plant biomass and are likely to contribute to the residues formed by incomplete biomass combustion (black carbon,BC).Nonetheless,the molecular properties of thermally modified tannins have not been investigated in laboratory charring experiments.We applied conventional analytical pyrolysis–gas chromatography–mass spectrometry (Py–GC–MS)and thermally assisted hydrolysis and methylation (THM–GC–MS)to investigate the effects of heat treatment with a muffle furnace on the properties of condensed tannins (CT)from Corsican pine (Pinus nigra )needles.Py–GC–MS showed a decrease in the relative abundance of the 1,2,3-trihydroxybenzenes (pyrogallols)at P 300°C and of the dihydroxybenzenes (mainly catechols)at P 350°C due to dehydroxylation of the CT B ring.Further dehydroxylation led to formation of monohydroxybenzenes (phenols),which showed a strong enrichment between 350and 400°C and,at higher temperatures,to a series of mono-cyclic and polycyclic aromatics [benzene,alkyl benzenes and polycondensed aromatic hydrocarbons (PAHs)].Degradation of the A ring could not be recognized via Py–GC–MS,probably because of the poor chromatographic behavior of 1,3,5-trihydroxybenzenes (phloroglucinols).The progressive dehydroxyla-tion and eventual polycondensation of the CT B ring was corroborated using THM–GC–MS.In addition,with THM–GC–MS the thermal rearrangement of CT A rings at 300°C and higher was inferred from the relative abundance of 1,3,5-trimethoxybenzenes (methylated phloroglucinol derivatives).These com-pounds were observed at moderate/high temperature (up to 450°C)and can not be produced from THM of lignin,suggesting that they may be markers of CT in natural BC samples.Ó2012Elsevier Ltd.All rights reserved.1.IntroductionTannins are among the most abundant plant biopolymers,typ-ically comprising 10–25%of foliar mass (Kraus et al.,2003).In leaves,needles and bark,tannin content often exceeds that of lig-nin (Hernes and Hedges,2004)and it is also present in woody tis-sue (Rogge et al.,1998).Tannins are strong antioxidants with multiple ecosystem functions,such as defense against herbivores,metal mobilization,radical scavenging and regulation of nutrient dynamics by protein precipitation and suppression of microbial activity (Zucker,1983;Kennedy et al.,1996;Fierer et al.,2001).Tannins from terrestrial plants can be divided into two main groups:condensed tannins (CT)and hydrolyzable tannins.Con-densed tannins are oligomers and polymers based on flavan-3-ol monomers linked through covalent bonds (Fig.1).Within thegroup of CT,there is variation in the distribution of OH groups on the aromatic B ring,forming procyanidin and prodelphinidin CT (e.g.Khanbabaee and van Ree,2001).Each CT monomer con-tains up to six OH functionalities concentrated on the aromatic A and B rings (Fig.1).These aromatic OH groups,especially those in adjacent positions on the B ring,give rise to the exceptional reactivity of CT in the environment (Slabbert,1992).Despite the fact that tannins form a major component of plant biomass,they have often been ignored as a possible source of poly-phenolic substances in soil organic matter;these have commonly been ascribed to lignin (Filley et al.,2006).This is also the case for phenolic moieties in biomass burning residue (black carbon,BC)(Baldock and Smernik,2002;Krull et al.,2003),which are abundant in BC formed at low/moderate temperature (e.g.Knicker et al.,2005,2007;Rumpel et al.,2006).In the light of growing interest in BC or ‘biochar’,amendment programs for soil ameliora-tion and C sequestration (Lehmann et al.,2006;Jeffery et al.,2011),the possible effects of charred tannins on soil microbial and nutri-ent dynamics must be understood (Warnock et al.,2010),as they0146-6380/$-see front matter Ó2012Elsevier Ltd.All rights reserved./10.1016/geochem.2012.03.009Corresponding author.Tel.:+34881813588;fax:+34881813601.E-mail address:joeri.kaal@incipit.csic.es (J.Kaal).may be anticipated to be vastly different from that of charred lig-nin.This is not possible,however,as methodologies for identifying charred tannins are not available and the thermal degradation pathways of tannins are largely unknown.The thermal alteration of plant tissue has been investigated in numerous studies,as reviewed by e.g.González-Pérez et al.(2004)and Preston and Schmidt (2006).Pyrolysis–gas chromatog-raphy–mass spectrometry (Py–GC–MS)is one method that can provide information on the molecular properties of BC (De la Rosa et al.,2008;Kaal and Rumpel,2009;Kaal et al.,2009;Fabbri et al.,2012),despite the fact that pyrolysis itself is a heat-induced scission reaction and that secondary rearrangements generate structures that may resemble the pyrolysis products of BC (Saiz-Jiménez,1994;Wampler,1999).Pyrolysis is a relatively inex-pensive and rapid technique that has also proven of value for tan-nin characterization (Galletti et al.,1995).Flash heating in the presence of tetramethylammonium hydroxide (TMAH)is referred to as thermally assisted hydrolysis and methylation (THM)or ther-mochemolysis.With THM,hydrolyzable bonds are cleaved and the resulting CO 2H and OH groups are transformed in situ to the corre-sponding methyl esters and methyl ethers,respectively (Challinor,2001;Hatcher et al.,2001;Shadkami and Helleur,2010),which are more amenable to GC than their underivatized counterparts.As such,THM–GC–MS provides additional information on tannin structure through detection of derivatized polyfunctionalized A and B rings (Nierop et al.,2005).In the present study the thermal degradation of CT was studied using laboratory charring experiments followed by characteriza-tion with Py–GC–MS and THM–GC–MS.The aim was to provide guidelines for the identification of CT-derived BC and identify the molecular changes as a function of charring temperature.2.Material and methodsCondensed tannins were isolated from Corsican pine (Pinus ni-gra var.maritima )needles from the coastal dunes in The Nether-lands (52°2004500N,4°3105700E)using the scheme proposed by Preston (1999)and described in detail by Nierop et al.(2005,2006).The CT were completely isolated from other components and had a prodelphinidin:procyanidin ratio of 2:1and average chain length of 6.6(Nierop et al.,2005).It has been used in various studies (Kaal et al.,2005;Nierop et al.,2006;Kraal et al.,2009).For the charring experiments,ca.200mg of CT were double wrapped in Al foil to simulate limited O 2availability during wild-fires.The samples were placed (30min)in a preheated muffle fur-nace at temperatures (T CHAR )from 200°C to 600°C.Similar experiments have been performed by Turney et al.(2006),Hall et al.(2008)and Wiesenberg et al.(2009).Weight loss was deter-mined gravimetrically before and after charring.C and H contents were determined by way of combustion using a LECO carbon ana-lyzer (model CHN-1000).Uncharred CT was used as a control.Py–GC–MS was performed in duplicate using a Pt filament coil probe Pyroprobe 5000pyrolyzer (CDS Analytical,Oxford,USA).Approximately 1–1.5mg sample was embedded in quartz tubes using glass wool.Pyrolysis was applied at 750°C for 10s (heating rate 10°C/ms).The method produces limited artificial charring during pyrolysis and a relatively high proportion of pyrolyzable biomass in comparison with pyrolysis at lower temperatures (Pastorova et al.,1994;Kaal et al.,2009;Song and Peng,2010).The pyrolysis interface was coupled to a 6890N GC instrument and 5975MSD (Agilent Technologies,Palo Alto,USA).The pyrolysis interface and GC inlet (split ratio 1:20)were set at 325°C.The GC instrument was equipped with a (non-polar)HP-5MS 5%phenyl,95%dimethylpolysiloxane column (30m Â0.25mm i.d.;film thickness 0.25l m)and He was the carrier gas (constant flow 1ml/min).The GC oven was heated from 50to 325°C (held 10min)at 20°C/min.The GC–MS transfer line was held at 270°C,the ion source (electron impact mode,70eV)at 230°C and the quadrupole detector at 150°C scanning a range between m /z 50and 500.Peak areas of the pyrolysis products were obtained from one or two characteristic or dominant fragment ions,the sum of which (total quantified peak area;TQPA)was set as 100%.Relative contributions of the pyrolysis products were calculated as %of TQPA.This is a semi-quantitative exercise that allows better comparison between samples than visual inspection of pyrolysis chromatograms alone.Benzofuran and styrene could not be quantified because of co-elution with contaminants.For THM–GC–MS,samples were pressed onto Curie-Point wires,after which a droplet of a 25%solution of TMAH in water was added,prior to drying under a 100W halogen lamp.THM was car-ried out using a Horizon Instruments Curie-Point pyrolyzer.Sam-ples were heated for 5s at 600°C.The pyrolysis unit was connected to a Carlo Erba GC8060furnished with a fused silica col-umn (Varian,25m Â0.25mm i.d.)coated with CP-Sil 5(film thick-ness 0.40l m).He was the carrier gas.The oven temperature program was:40°C (1min)to 200°C at 7°C/min and then to 320°C (held 5min)at 20°C/min.The column was coupled to a Fi-sons MD800MS instrument (m /z 45–650,ionization energy 70eV,cycle time 0.7s).Like Py–GC–MS,the relative contributions of the THM products were calculated as relative contributions to TQPA using 1–2dominant fragment ions.Benzene and toluene were not detected because they co-eluted with trimethylamine,the main side product of TMAH-based THM (Challinor,2001),i.e.with-in the solvent delay period (3min).Py–GC–MS and THM–GC–MS results were analyzed via princi-pal component analysis (PCA)to illustrate the major effects of heating on the pyrolysis and THM fingerprints,respectively,using SPSS 13.0.3.Results and discussion3.1.Weight loss and elemental compositionWeight loss from CT increased from 17%at T CHAR 200°C towards 56%at T CHAR 600°C (Table 1).The CT C content increased from 51%to 81%with increasing T CHAR .The atomic H/C ratio of the samples declined from 1.2to 0.6with increasing T CHAR ,reflecting loss of functional groups and formation of fused aromatic clusters through condensation (Braadbaart et al.,2004).Model structure of a condensed tannin oligomer;procyanidin,prodelphinidin,R =OH.47(2012)99–108chromatograms of uncharred(control)and charred(200–600°C)CT,from Py–GC–MS.Relative peak intensity vs.retention time3.2.Charred condensed tannin composition:Py–GC–MSPy–GC–MS total ion chromatograms are depicted in Fig.2.Total quantified peak area (Table 1),a rough measure of signal intensity,decreased with increasing T CHAR .This can be explained by the for-mation of non-pyrolyzable structures upon charring,probably through the formation of polycondensed aromatic clusters stable under pyrolysis conditions.However,this does not imply that the results from the high temperature chars should be dismissed for representing only a small and relatively volatilefraction:a more appropriate interpretation is that the samples consist largely of non-pyrolyzable fused aromatic clusters,corroborated by the dom-inant pyrolysis products of such samples (benzene and PAHs;see below)and lack of pyrolysis products from less intensely charred structures.The major pyrolysis products are listed in sponding retention times,fragment ions used and relative proportions.Products were structure,in particular the hydroxylation between benzenes (benzene and alkyl (with one OH),dihydroxybenzenes (DHB),(THB)and other compounds.The DHB are while the THB are based on pyrogallol moieties exclusively from prodelphinidin B rings.The uncharred CT isolate produced mainly pyrolysis (Table 2;Fig.3),which originate from prodelphinidin B rings,respectively.In contrast delphinidin:procyanidin ratio determined et al.,2005),the DHB were more abundant may be explained by way of the poor ‘‘visibility’’a non-polar GC column.The high proportion of and 4-methylpyrogallol points to scission of the the heterocyclic pyran C ring (Fig.1),which has sociation energy than the aromatic A and B acetone and acetic acid may represent the after pyrolysis.Products from the A ring were may be explained by the poor chromatographic rivatized 1,3,5-trihydroxybenzene principal pyrolysis product from the A ring (The lack of unambiguous A ring markers implies that Py–GC–MS cannot be used to study the degradation of the predominantly C-4/C-8and C-4/C-6intermonomeric linkages (Fig.1),and thus to investigate CT depolymerization.Some methoxyphenols (guaiacol and 4-vinylguaiacol)and catechol carbonate were detected.The guaiacols are commonly attributed to lignin and its derivatives (e.g.Kögel-Knabner,2002),but here they might alternatively orig-inate from C ring fission in CT (Galletti et al.,1995).The possibility of tannins as a source of the guaiacols is supported by the absence of resonances from methoxyphenols in liquid-state 13C NMR tra (Nierop et al.,2005)and guaiacols bearing a C 3side chain pyrolyzate,which should be detectable if residual lignin was ent in the fresh needle isolate (Saiz-Jiménez and de Leeuw,The results for uncharred CT were in good agreement with earlier pyrolysis experiments with tannin,catechin and gallocate-Relative proportion (TQPA)of pyrolysis product groups from CT vs.charring temperature (200–600°C);0°C,control (uncharred CT);THB,trihydroxybenzene;dihydroxybenzene;PAH,polycyclic aromatic hydrocarbon.Error bars reflect standard error of mean of two replicates.Note differences in y -axis scaling.Pyrolysis products plotted in PC1–PC2space (PCA).THB,trihydroxybenzene;dihydroxybenzene;PAH,polycyclic aromatic hydrocarbon.Arrow indicates trend in pyrolysis patterns with increasing charring temperature.The sample charred at200°C gave a pyrogram similar to that of uncharred CT,indicating limited thermal rearrangement at this temperature(Fig.2).At T CHAR300°C,the proportion of THB de-creased from ca.20%to ca.5%of the TQPA,while the proportion of DHB increased towards ca.70%(Fig.3).This reflects elimination of one OH from the prodelphinidin B ring during charring,causing a relative increase in the contribution of DHB to the pyrolyzate. Thus,the presence of DHB in pyrolyzates does not necessarily indi-cate the presence of uncharred CT.This sheds new light on results from previous studies(Quénéa et al.,2005a,b)in which the pres-ence of DHB in the pyrolyzate of BC-containing forest soil was interpreted as being from uncharred CT,whereas it may alterna-tively originate from CT-derived BC.A more drastic shift in pyro-lyzate composition occurred at T CHAR350°C:the relative abundance of DHB diminished,with a concomitant increase in phenols(from ca.10%to50%),as well as benzenes,PAH and other compounds(Fig.3).Also,the relative contribution of THB de-creased further.The results are indicative of strong B ring dehydr-oxylation at350°C.At T CHAR400°C,a further decrease in DHB contribution and increased relative abundance of benzenes and PAH were observed.The high biphenyl/naphthalene ratio may be specific for the pyrolyzate of CT-derived BC,as it is usually much lower in the pyrolyzate of char obtained from lignocellulose(Kaal et al.,2009).At T CHAR450°C,phenols decreased while the relative abundance of benzenes increased towards60%and that of PAHs to-wards10%,suggesting the loss of most of the OH groups from theB Fig.5.Total ion chromatograms of uncharred(control)and charred(200–600°C)CT,from THM–GC–MS.ring.The relatively weak signal for this sample (Table 1;Fig.2)sug-gested that a significant proportion of the CT was converted to non-pyrolyzable polycondensed aromatics.After charring at 600°C the phenolic pyrolysis products and the possible products of the C ring (acetylacetone,acetone and acetic acid)were absent,while the benzenes and PAH had increased to 75%and 20%,respec-tively (Fig.3).This combination constitutes a typical set of pyroly-sis products from strongly charred biomass (Kaal et al.,2009;Fabbri et al.,2012).The general trends for experimental charring of CT as deter-mined with Py–GC–MS became apparent with PCA.In Fig.4,the pyrolysis products are plotted in PC1–PC2space.PC1explained 62%of the total variance and PC222%.PC1and PC2reflect the same process however,namely thermally-induced dehydroxylation:benzenes and PAH had positive loadings on PC1(recording increas-ing abundance with increasing T CHAR )while DHB and THB had neg-ative loadings (compounds showing an opposite trend of decreasing abundance with increasing T CHAR ).PC2separated the phenols from the other pyrolysis products:the phenols had a small contribution to the pyrolyzate at the lower and highest tempera-tures,while they dominated the pyrolyzates of the samples charred between 350and 400°C.The arrow in Fig.4represents the dehydroxylation pathway of CT with increasing T CHAR .The pro-cess is reflected in the THB/DHB,DHB/phenol and phenol/benzene ratios (not shown),which decreased significantly with increasing T CHAR (P <0.001for all ratios).Under the experimental conditions of the present study,the thermal modification of the CT B ring oc-curred predominantly between 300and 400°C.3.3.Charred condensed tannin composition:THM–GC–MSTHM–GC–MS total ion chromatograms are depicted in Fig.5.Similar to TQPA from Py–GC–MS,TQPA decreased with increasing T CHAR (Table 1).The THM products are listed in Table 3,with corresponding relative contributions to TQPA.The likely origin of the THM products was identified on the basis of the substitution pattern of the functional groups.As such,the THM products were grouped according to the number of O-containing functional groups (OFG).Furthermore,trimethoxybenzenes with the methoxyl groups in the m positions (methylated phloroglucinol derivatives)were assumed to originate from A ring moieties,while the trimethoxybenzenes with the methoxyl groups in the o positions (methylated pyrogallol derivatives)were assumed to originate from prodelphinidin B ring moieties.For the uncharred CT,major products from the A ring were 1,3,5-trimethoxybenzene and 2-methyl-1,3,5-trimethoxybenzene,while procyanidin and prodelphinidin B ring products were pres-ent mainly as methyl esters of 3,4-dimethoxybenzoic acid and 3,4,5-trimethoxybenzoic acid,respectively.These compounds have been found to be the dominant THM products of CT isolated from various plant species (Nierop et al.,2005).The presence of A ring products and absence of CH 2-bridged diaromatic (i.e.diphenylme-thane-based)products suggests that CT was readily depolymerized during THM.The exact location of depolymerization is unknown because it cannot be elucidated whether the Me group in 2-methyl-1,3,5-trimethoxybenzene originated from the C-4carbon in the same monomer or from a C-4carbon in the C ring of an adja-cent monomer.The fact that uncharred CT produced no detectable intermonomeric THM products implies that charring-induced depolymerization cannot be studied either.Parameters used by Nierop et al.(2005)to indicate the %of procyanidin B rings of CT were ‘‘PC-acid’’(dimethoxybenzoic acid,methyl ester/sum di-and trimethoxybenzoic acids,methyl esters;26.9%)and ‘‘PC-THM’’(based on all compounds related to di-and trihydroxy B rings;36.2%),are 49.4%and 34.2%,respectively,for the (uncharred)CT used isolated from Corsican pine used here.The cause of the large difference in the ‘‘PC-acid’’parameter may be of an analytical nature.The fact that the ‘‘PC-THM’’values,which were often closer to those determined with NMR (Nierop et al.,2005),were similar suggests that this parameter to estimate the %procyanidin B rings from THM–GC–MS is reproducible.Analogous to Py–GC–MS,the THM–GC–MS pattern from the CT charred at 200°C was similar to the uncharred CT (Fig.5).At T CHAR 300°C,a major decline was observed for 1,3,5-trimethoxybenzene and 2-methyl-1,3,5-trimethoxybenzene from the A ring (Fig.6),which coincided with an increase in relative contribution of most of the products with two or three adjacent methoxyl groups de-rived from CT B-rings.This is suggestive of a greater thermalstabil-Relative proportion (%of TQPA)of THM product groups from CT vs.charring temperature (200–600°C).0°C,control (uncharred CT);OFG refers to number containing functional groups;PAH,polycyclic aromatic hydrocarbon.Note differences in y -axis scaling.ity of B rings than A rings.Between T CHAR300and400°C,the rela-tive contribution of compounds with three and four OFG de-creased,that of two OFG maximized,while that of compounds with only one OFG increased.The trend was especially strong for the compounds with three o methoxyl groups,suggesting thor-ough thermal rearrangement of prodelphinidin B rings in this tem-perature range.At higher charring temperatures,the proportions of benzene,alkyl benzenes and PAH increased strongly,while the proportions of the other compounds decreased.Like the benzenes, benzoic acid methyl ester increased progressively with increasing T CHAR,but it is not clear whether the carboxylic group was formed upon oxidation during the charring experiment or upon Cannizz-aro reactions during THM(Hatcher and Minard,1995;Tanczos et al.,1997).Relatively intact CT A ring products were still recog-nized at T CHAR450°C(R1,3,5-trimethoxybenzenes>10%of TQPA), suggesting that THM–GC–MS might allow unequivocal identifica-tion of CT markers in weak/moderately charred BC.McKinney et al.(1996)identified these THM products from cutan isolated from Agave americana.Apart from its presence in CAM plants only (Boom et al.,2005),a possible interference from cutan-derived 1,3,5-trimethoxybenzenes would be recognized by the presence of methylated aliphatic compounds including fatty acid methyl es-ters.More importantly,these1,3,5-trimethoxybenzenes are not formed upon THM of lignin(e.g.Chefetz et al.,2002;Nierop and Filley,2008;Shadkami and Helleur,2010),a more likely interfering component in plant-derived BC.No O-substituted PAH such as methoxynaphthalenes were de-tected,suggesting that thorough elimination of functional groups preceded the polycondensation reactions.Finally,methylated ben-zene polycarboxylic acids(with three or more carboxyl groups) found among the THM products of aged charcoal(Kaal et al., 2008)were not detected,probably because the necessary oxidation reactions occur during aging in soil and not during heat treatment under limited O2availability.The PC1(58%)–PC2(22%)plot of the THM products showed a similar distribution according to hydrox-ylation pattern(Fig.7).The arrow indicates the progressive loss of OFG with increasing T CHAR.Unsurprisingly,the main difference between Py–GC–MS and THM–GC–MS was the higher abundance of OFG among THM prod-ucts,independent of charring intensity,confirming the protection of functional groups resulting from TMAH derivatization and the loss and/or poor detection of polar compounds using conventional Py–GC–MS.4.ConclusionsThe charring of CT caused progressive dehydroxylation at T CHAR6400°C(under the conditions of the present study)and polyaromatization in the higher temperature range at T CHAR400–600°C.Based on Py–GC–MS,it is suggested that pyrogallol and, more tentatively,catechol derivatives may act as indicators of CT-derived BC formed at low temperature,while a high relative abundance of biphenyl might be indicative of a significant CT con-tribution in more severely charred material.Py–GC–MS is not suit-able for detection of A ring products.From THM–GC–MS,initial A ring degradation occurred at lower temperatures than B ring deg-radation.Nonetheless,the significant contribution of1,3,5-tri-methoxybenzenes from the phloroglucinol A ring up to T CHAR 450°C suggested that these compounds can be used to distinguish between lignin and CT-derived BC in weakly/moderately charred BC samples.The results show that CT is a possible source of pheno-lic moieties in BC and provide a framework for estimating the de-gree of thermal degradation of CT based on the functional group distribution of Py–GC–MS and THM–GC–MS products.Incubation experiments using this CT are currently being developed,aimed at determining the effects of CT charred at different temperatures on organic matter mineralization.AcknowledgmentsWe thank Carmen Pérez Llaguno(Universidade de Santiago de Compostela)for elemental analysis and two anonymous reviewers for their time and comments.Associate Editor—S.DerenneReferencesBaldock,J.A.,Smernik,R.J.,2002.Chemical composition and bioavailability of thermally altered Pinus resinosa(red pine)anic Geochemistry33, 1093–1109.Boom,A.,Sinninghe Damsté,J.S.,De Leeuw,J.W.,2005.Cutan,a common aliphatic biopolymer in cuticles of drought-adapted anic Geochemistry36, 595–601.Braadbaart,F.,Boon,J.J.,Veld,H.,David,P.,Van Bergen,P.F.,boratory simulations of the transformation of peas as a result of heat treatment:changes of the physical and chemical properties.Journal of Archaeological Science31, 821–833.Challinor,J.M.,2001.Review:the development and applications of thermally assisted hydrolysis and methylation reactions.Journal of Analytical and Applied Pyrolysis61,3–34.Chefetz, B.,Salloum,M.J.,Deshmukh, A.P.,Hatcher,P.G.,2002.Structural components of humic acids as determined by chemical modifications and13C NMR,pyrolysis-,and thermochemolysis–gas chromatography/mass spectrometry.Soil Science Society of America Journal66,1159–1171.De la Rosa,J.M.,Knicker,H.,López-Capel,E.,Manning,D.A.C.,González-Pérez,J.A., González-Vila,F.J.,2008.Direct detection of black carbon in soils by py–GC–MS, 13C NMR spectroscopy and thermogravimetric techniques.Soil Science Society of America Journal72,258–267.Fabbri, D.,Torri, C.,Spokas,K.A.,2012.Analytical pyrolysis of synthetic chars derived from biomass with potential agronomic application(biochar).Relationships with impacts on microbial carbon dioxide production.Journal of Analytical and Applied Pyrolysis93,77–84.Fierer,N.,Schimel,J.P.,Cates,R.G.,Zou,J.,2001.Influence of balsam poplar tannin fractions on carbon and nitrogen dynamics in Alaskan taigafloodplain soils.Soil Biology and Biochemistry33,1827–1839.Filley,T.R.,Nierop,K.G.J.,Wang,Y.,2006.The contribution of polyhydroxyl aromatic compounds to tetramethylammonium hydroxide lignin-based anic Geochemistry37,711–727.Galletti,G.C.,Reeves,J.B.,1992.Pyrolysis/gas chromatography/ion-trap detection of polyphenols(vegetable tannins):preliminary anic Mass Spectrometry27,226–230.Galletti,G.C.,Modafferi,V.,Poiana,M.,Bocchini,P.,1995.Analytical pyrolysis and thermally assisted hydrolysis–methylation of wine tannin.Journal of Agricultural and Food Chemistry43,1859–1863.González-Pérez,J.A.,González-Vila,F.J.,Almendros,G.,Knicker,H.,2004.The effect offire on soil organic matter–a review.Environment International30,855–870.THM products plotted in PC1–PC2space(from PCA).OFG refers to numberO-containing functional groups;PAH,polycyclic aromatic hydrocarbon.Theindicates the major trend in dominant THM products with increasing charringtemperature.47(2012)99–108107。
常用ASCII码对照表

常用ASCII码对照表1. ASCII码在计算机内部,所有的信息最终都表示为一个二进制的字符串。
每一个二进制位(bit)有0和1两种状态,因此八个二进制位就可以组合出256种状态,这被称为一个字节(byte)。
也就是说,一个字节一共可以用来表示256种不同的状态,每一个状态对应一个符号,就是256个符号,从0000000到。
上个世纪60年代,美国制定了一套字符编码,对英语字符与二进制位之间的关系,做了统一规定。
这被称为ASCII码,一直沿用至今。
ASCII码一共规定了128个字符的编码,比如空格“SPACE”是32(十进制的32,用二进制表示就是00100000),大写的字母A是65(二进制01000001)。
这128个符号(包括32个不能打印出来的控制符号),只占用了一个字节的后面7位,最前面的1位统一规定为0。
2、非ASCII编码英语用128个符号编码就够了,但是用来表示其他语言,128个符号是不够的。
比如,在法语中,字母上方有注音符号,它就无法用ASCII码表示。
于是,一些欧洲国家就决定,利用字节中闲置的最高位编入新的符号。
比如,法语中的é的编码为130(二进制)。
这样一来,这些欧洲国家使用的编码体系,可以表示最多256个符号。
但是,这里又出现了新的问题。
不同的国家有不同的字母,因此,哪怕它们都使用256个符号的编码方式,代表的字母却不一样。
比如,130在法语编码中代表了é,在希伯来语编码中却代表了字母Gimel (),在俄语编码中又会代表另一个符号。
但是不管怎样,所有这些编码方式中,0—127表示的符号是一样的,不一样的只是128—255的这一段。
至于亚洲国家的文字,使用的符号就更多了,汉字就多达10万左右。
一个字节只能表示256种符号,肯定是不够的,就必须使用多个字节表达一个符号。
比如,简体中文常见的编码方式是GB2312,使用两个字节表示一个汉字,所以理论上最多可以表示256x256=65536个符号。
1-s2.0-S0306261907001742-main

Application of the Miller cycle to reduce NO x emissionsfrom petrol enginesYaodong Wang a,b,*,Lin Lin c ,Shengchuo Zeng b ,Jincheng Huang b ,Anthony P.Roskilly a ,Yunxin He b ,Xiaodong Huang b ,Shanping Li daThe Sir Joseph Swan Institute for Energy Research,Newcastle University,Newcastle upon Tyne,NE17RU,United KingdombMechanical Engineering College,Guangxi University,Nanning,Guangxi 530004,China cNanning College for Vocational Technology,Nanning,Guangxi 530003,ChinadGuangxi University of Technology,Liuzhou,545006,ChinaAccepted 26October 2007Available online 7February 2008AbstractA conceptual analysis of the mechanism of the Miller cycle for reducing NO x emissions is presented.Two versions of selected Miller cycle (1and 2)were designed and realized on a Rover ‘‘K ”series 16-valve twin-camshaft petrol engine.The test results showed that the application of the Miller cycle could reduce the NO x emissions from the petrol engine.For Miller cycle 1,the least reduction rate of NO x emission was 8%with an engine-power-loss of 1%at the engine’s full-load,compared with that of standard Otto cycle.For Miller cycle 2,the least reduction rate of NO x emission was 46%with an engine-power-loss of 13%at the engine’s full-load,compared with that of standard Otto cycle.Ó2007Elsevier Ltd.All rights reserved.Keywords:Petrol engine;Miller cycle;NO x emission1.IntroductionIt has been more than a century since petrol engines were first and widely used as primary movers for human activities,such as transportation and stand-by power generation.The technologies to design and to make petrol engines are well developed.But environmental concerns since the 1970s have made the control of engine emissions a challenge for the engine industry.Engineers and researchers have taken numerous mea-sures to reduce engine emissions and to comply with restrictions on the quality and quantity of emissions allowed in different applications.The need to meet the emissions legislation means that it is appropriate con-tinuously to investigate the ways of reducing emissions without compromising engine-efficiency or increasing the cost of manufacturing engines.0306-2619/$-see front matter Ó2007Elsevier Ltd.All rights reserved.doi:10.1016/j.apenergy.2007.10.009*Corresponding author.Address:Newcastle University,The Sir Joseph Swan Institute,Newcastle upon Tyne NE17RU,United Kingdom.Tel.:+4401912464934;fax:+4401912464961.E-mail address:y.d.wang@ (Y.Wang).Available online at Applied Energy 85(2008)463–474/locate/apenergyAPPLIED ENERGYThe main gaseous emissions from petrol engines are hydrocarbon (HC),carbon monoxide (CO),carbon dioxide (CO 2)and nitrogen oxides (NO x ,i.e.NO and NO 2).Among them,NO x is the most harmful gas that needs to be minimized.Currently there are two ways to reduce NO x emissions:one way is reducing NO x at source,such as exhaust-gas recirculation or homogenous combustion.This method is preferred from the view point of cost.Another way is after-treatment.This is an effective but expensive way to reduce NO x emissions.In order to reduce the NO x emissions at source,it is necessary to know the mechanism of NO x formation in the engine cylinder.The factors that influence the formation of NO x in engines are:(a)the peak flame-tem-perature during the combustion process,(b)the duration of the heat-release process,and (c)the air–fuel ratio.Among these factors,the peak flame-temperature in the cylinder is the key factor.If the highest temperature of the flame is reduced,the amount of NO x formed in the cylinder will be less.Consequently,the NO x emissions will be reduced.Thus,searching for a way to lower or to control the peak flame-temperature in the engine’s cylinder is one of the main aim for engine engineers and scientists.The Miller cycle was first proposed by ler in 1947.The proposal was for the use of early intake valve closing (EIVC)to provide internal cooling before compression so as to reduce the compression work [1].Miller further proposed increasing the boost of the inlet charge to compensate for the reduced inlet duration [2].The cycle that Miller proposed is a cold cycle which has allowed an increase in engine performance with an upraise of the knocking threshold.At that time,the Miller cycle was focused on improving the thermal efficiency of engine [3–10].This is still the aim [11–15].Since the Miller cycle is a cold cycle,there is the possibility to apply it to reduce the combustion temperatures in engines thus reducing the NO x formation and emissions.The objective of this study is to investigate experimentally the feasibility of the application of the Miller cycle in order to reduce NO x emissions from petrol engines.2.The concept of Miller cycle 2.1.Description of Miller cycleFor the Miller cycle,the expansion-ratio exceeds its compression-ratio [15],that is,the effective expansion stroke of the engine is longer than the compression stroke.A comparison of the standard Otto cycle with the Miller cycle is shown in Fig.1.Assuming the cylinder pressure at the starting point 0is P 0,the volume is V 0,the swept volume of cylinder for Otto cycle is V c and for Miller cycle is V 0c .As shown in Fig.1a,the work processes of Otto cycle are:intake process 0?1,compression process 1?2,combustion and expansion process 2?3?4,and exhaust process 4?1?0.For the cycle,theNotation M 1Miller cycle 1M 2Miller cycle 2n engine speed (r/min)P pressure in the cylinder (kPa)P 0ambient pressure (kPa)V volume of cylinder (m 3)V 0clearance volume (m 3)V c swept volume of Otto cycle (m 3)V 0c swept volume of Miller cycle (m 3)D Pe power difference between the Otto cycle and the Miller cycle (kW)D Tr exhaust-temperature difference between the Otto cycle and the Miller cycle (°C)e D NO x relative NO x emission difference between the Otto cycle and the Miller cycle e D Pe relative power difference of Otto cycle from that of the Miller cyclee D Trrelative exhaust-temperature difference between the Otto cycle and the Miller cycle464Y.Wang et al./Applied Energy 85(2008)463–474Y.Wang et al./Applied Energy85(2008)463–474465compression-ratio is identical to the expansion-ratio;a higher expansion-ratio causes a higher compression-ratio.However,the Miller cycle allows the compression-and expansion-ratios to be preset independently,as shown in Fig.1b.The work processes are:intake process0?1a?1;then an additional‘‘intake blow-back”process1?1a,which is the main difference between the Miller cycle and the Otto cycle;compression process 1a?2;combustion and expansion process2?3?4?4a;and exhaust process4a?1?1a?0.From the P–V diagram of the Miller cycle,it can be seen that a higher engine-efficiency is expected with an increased expansion-ratio because more heat is changed to mechanical power.This was the original idea behind the Miller cycle.466Y.Wang et al./Applied Energy85(2008)463–4742.2.Basic idea of the Miller cycle to reduce NO x emissionsAs mentioned above,NO x is one of the most harmful gases emitted from engines and the main cause of NO x formation is the peakflame-temperature in the engine cylinder during the combustion.The Miller cycle is a‘‘cold cycle”.The application of this‘‘cold”characteristic may reduce the temperature at the end of the compression process(at point2in the P–V diagram).Thus it reduces the temperature at the end of the com-bustion process(point3in the P–V diagram).Therefore,it reduces the NO x emissions.This is the basic idea of the application of the Miller cycle to reduce the NO x emission from petrol engines.Fig.2presents the P–V diagram for this concept.Cycle0?1?2?3?4?1?0is the standard Otto cycle.Cycle0?1?1a?2a?3a?4a?1?0is the Miller cycle.The intake valve is kept open during a portion of the compression stroke.Some of intake air into the cylinder is rejected.Thus the amount of intake air into the cylinder is relatively less than for the Otto cycle and this reduces the effective compression-ratio.At the end of the compression stroke,the pressure and temperature in the cylinder are lower than those of stan-dard Otto cycle.The combustion temperature is then lower;this may result in less NO x formation in the cyl-inder of engine.2.3.Main methods to realize the Miller cycleThere are three main methods to realize a Miller cycle in practice[5–8]:(a)installing a rotating valve between intake manifold and intake valve(on the cylinder head)to control the intake air quantity–early rotary-valve closing(ERVC);(b)closing the intake valve before the termination of the intake stroke–early intake valve closing(EIVC);and(c)keeping the intake valve open during a portion of the compression stroke, thus rejecting part of the charge and reducing the net compression-ratio–late intake valve closing(LIVC–as shown in Fig.2).For this experimental study,the LIVC version of the Miller cycle was selected.A schematic valve timing diagram of the LIVC is shown in Fig.3.Two versions of the LIVC Miller cycle were designed and tested; the detail parameters are presented in Section3.6.3.Experimental rig,instrumentation and test plan3.1.The engineA Rover‘‘K”series16-valve twin-camshaft petrol engine,type K-161400TBI,made by the Rover Group Ltd.in1991,shown Fig.4,was used for the experimental investigation.It has a1397cm3displacement,max-imum power70.8kW/6250r/min(torque106.7Nm),maximum torque124Nm/4000r/min,equipped for Rover200&400series cars.Y.Wang et al./Applied Energy85(2008)463–4744673.2.The dynamometer(see Fig.5)A Heenan Dynamatic Dynamometer MK1,made by Froude Consine Ltd.,was used to measure the engine performance:i.e.its torque,power and fuel consumption.3.3.Emission analyzersFour exhaust-gas analyzers,as shown in Fig.6,made by Analytical Development Company Ltd.(Hoddes-don,Hertfordshire,EN110DB,England),were used to analyze the exhaust emissions(carbon monoxide,car-bon dioxide,hydrocarbon and nitrogen oxides)from the engine.Prior to testing,the analyzers were calibrated separately by using the special sample gases supplied by BOC Ltd.3.4.Pressure and temperature measurementPressures were measured at the air-inlet manifold,for the engine oil at the outlet of the oilfilter,and the ambient-air pressure was measured by a barometer.Thermocouples type K (which have a temperature range from À200°C to 1200°C)were used to measure the temperature at the following positions on the engine:air-inlet,exhaust-gas,engine oil,and the engine’s cooling-water inlet andoutlet.Fig.5.Dynamometer.Fig.6.Emission analyzers.468Y.Wang et al./Applied Energy 85(2008)463–474Y.Wang et al./Applied Energy85(2008)463–474469 3.5.The test rigFig.7presents the schematic design of the test rig for the experimental study.Fig.8shows the completed test rig in the laboratory.470Y.Wang et al./Applied Energy85(2008)463–4743.6.Experimental planA test plan was designed to carry out the engine tests on the original Otto cycle and two Miller cycles.For comparison,the intake throttle wasfixed at the maximum open position for all the tests.There were no changes for the other engine systems,except for the intake valve timing.The running range of the engine was from2000r/min to6250r/min.Two versions of the Miller cycle were designed and tested as follows:ler1:the intake valve closed15°later than that of original Otto cycle;ler2:the intake valve closed30°later than that of original Otto cycle.The whole experimental plan was realized in two stages:(i)running engine on standard Otto cycle;and(ii) running engine on the two Miller cycles.Each test was repeated3times to make sure the data were reliable. The detailed test plan is listed in Table1.4.Test results and discussionThe test results of the engine-power output,brake specific fuel-consumption(BSFC),exhaust-gas temper-ature and the NO x emissions for the original Otto cycle and the two Miller cycles are shown in Figs.9–16.The engine’s brake engine-power outputs at different engine speeds from the three cycles are presented in Fig.9.The engine’s power outputs of Miller cycle1were almost the same as those of the Otto cycle;the Table1The experimental planEngine speed(r/min)Cycle testedOtto cycle Miller cycle1Miller cycle2Time tested2000Three Three Three3000Three Three Three3500Three Three Three4000Three Three Three4500Three Three Three5000Three Three Three5500Three Three Three6250Three Three ThreeY.Wang et al./Applied Energy85(2008)463–474471472Y.Wang et al./Applied Energy85(2008)463–474Y.Wang et al./Applied Energy85(2008)463–474473differences were from0.0to1.2kW.The differences between Miller cycle1and Otto cycle were from0%to 2%,as shown in Fig.10.For Miller cycle2,the engine-power outputs at different engine speeds were much less than those of original Otto cycle.The differences of power outputs were between4.7and10.8kW,as shown in Fig.9.The relative differences were from13%to22%for the Miller cycle2compared with those of Otto cycle.The results are also presented in Fig.10.The engine’s brake specific fuel-consumption related to the power outputs at different engine speeds for the three cycles are shown in Fig.11.For the Miller cycle1,the BSFCs were from2.5to28.2g/kWh,higher than those of the Otto cycle.The relative differences were under8%in all the cases.The results are shown in Fig.12.For the Miller cycle2,the BSFCs were also higher than those of the Otto cycle,i.e.from57.5to146.6g/ kWh,which are also presented in Fig.11.The relative differences were from17%to44%,as shown in Fig.12.The exhaust-gas temperatures at the outlet of the engine’s exhaust-manifold related to the power outputs at different engine speeds for the Otto cycle and the two Miller cycles are shown in Fig.13.The exhaust-gas tem-peratures for the Miller cycles at different engine speeds were all lower than those of Otto cycle.For the Miller cycle1,as shown in Fig.13,the differences of exhaust-gas temperatures were from20°C to 62°C,compared with those of Otto cycle.The relative differences were from2%to11%–see Fig.14.For the Miller cycle2,compared with that of the Otto cycle,the differences of exhaust-gas temperatures were between45°C and112°C.The relative differences were from6%to19%–see Fig.14.The results of NO x emissions from the three cycles at different engine speeds are presented in Fig.15.For the cycles tested,the NO x emissions from the Otto cycle were the highest;those from the Miller cycle1came second;and those from the Miller cycle2were the lowest.For the Miller cycle1,compared with the Otto cycle,the difference of NO x emissions ranged from130to 665ppm.The relative differences were from8%to51%.The results are shown in Figs.15and16.For the Miller cycle2,compared with the Otto cycle,the differences of NO x emissions were from360to 850ppm.The relative differences were from44%to69%.The results are also shown in Figs.15and16.From these results,it can be seen that the engine-power outputs of the Miller cycle1(M1)were nearly the same as those of the original Otto cycle;the exhaust-gas temperatures of M1were lower than those of the Otto cycle;and the NO x emissions were also lower than those of the Otto cycle.For the Miller cycle2(M2),the exhaust-gas temperatures were lower than those of M1and the Otto cycle; and the NO x emissions were much lower than those of the Otto cycle.The effect of the Miller cycle in reducing the NO x emission is obvious,although the engine power outputs were much lower than those of the Otto cycle.The reason for the power-loss is because the late intake valve closure during the compression stroke led to some of the mixture of air and fuel being pushed out of the cylinder;this resulted in the charge being less than that of original Otto cycle.As a result,the engine-power outputs were reduced.474Y.Wang et al./Applied Energy85(2008)463–4745.Conclusions and recommendationThe investigation of the feasibility of applying the Miller cycle to petrol engines to reduce NO x emissions was completed.The results showed that it was feasible to apply the Miller cycle to petrol engines in order to reduce NO x emissions.For the two versions of the Miller cycles tested,the NO x emissions were less than those of the original Otto cycle.Of the two versions of the Miller cycle tested,Miller2is the better,in terms of the reductions of NO x emis-sion only.Of the two versions of the Miller cycle tested,Miller1is the better,in terms of both the reductions of NO x emissions and the engine-power outputs.For the two Miller cycles tested,the engine-power outputs were all less than those of the Otto cycle.This is due to there being less charge in the engine cylinder,which is a characteristic of Miller cycle.In order to make up for the charge losses as well as to make up for the power-losses,it is necessary to carry out an investigation on the application of a supercharger with an inter-cooler added to the above Miller cycles.A better engine performance with NO x reduction may then be able to be achieved. AcknowledgementsThe authors wish to thank Mr.Ian Pinks who helped set up the test rig for the experiments.The support of the Faculty of Computing,Engineering and Technology of Staffordshire University,UK is greatly appreciated.References[1]Miller RH.Supercharging and internal cooling cycle for high output.Trans ASME1947;69:453–7.[2]Miller RH,Lieberherr HU.The Miller supercharging system for diesel and gas engines operating characteristics,CIMAC,1957.In:Proceedings of the4th international congress on combustion engines,Zurich.June15–22;1957.p.787–803.[3]Okamoto K,Zhang FR,Shimogata S,Shoji F,Kanesaka H,Sakai H.Study of a Miller-cycle gas-engine for co-generation systems–effect of a Miller cycle on the performance of a gas engine,vol.1171.1996:SAE Special Publications;1996,p.125–36.[4]Thring RH.Theflexible diesel engine.In:Proceedings of the international congress and exposition,Detroit,USA,1990.SAE PaperNo.900175.SAE Special Publications;1990,p.484–92.[5]Clarke D,Smith WJ.Simulation,implementation and analysis of the miller cycle using an inlet control rotary-valve,variable valveactuation and power boost,vol.1258(SAE,No.970336).SAE Special Publications;1997.p.61–70.[6]Shimogata S,Homma R,Zhang FR,Okamoto K,Shoji F.Study on Miller cycle gas engine for co-generation systems-numericalanalysis for improvement of efficiency and power.SAE Paper No.971709.SAE Special Publications;1997.p.61–67.[7]Franca ler cycle–outline and general considerations,Diesel Ricerche S.P.A.Technical report;1996.[8]Okamoto K,Zhang FR,Morimoto S,Shoji F.Development of a high-performance gas engine operating at a stoichiometric condition–effect of Miller cycle and EGR.In:Proceedings of CIMAC congress1998Copenhagen.1998.p.1345–60.[9]Stebler H,Weisser G,Horler H,Boulouchos K.Reduction of NO x emissions of D.I.diesel engines by application of the Millersystem:an experimental and numerical investigation.SAE Paper No.960844.SAE Special Publications;1996.p.1238–48.[10]Ueda N,Sakai H,Iso N,Sasaki J.A naturally aspirated Miller cycle gasoline engine–its capability of emission,power and fueleconomy.SAE Paper No.960589.SAE Special Publications;1996.p.696–703.[11]Hatamura Koichi,Hayakawa Motoo,Goto Tsuyoshi,Hitomi Mitsuo.A study of the improvement effect of the Miller-cycle on meaneffective pressure limit for high-pressure supercharged gasoline engines.JSAE Rev1997;18:101–6.[12]Hiroyuki Endo,Kengo Tanaka,Yoshitaka Kakuhama,Yasunori Goda,Takao Fujiwaka,Masashi Nishigaki.Development of thelean-burn Miller cycle gas engine(3-04).In:Proceedings of thefifth international symposium on diagnostics and modeling of combustion in internal combustion engines(COMODIA2001).Nagoya,Japan:July1–4;2001.p.374–81.[13]Fukuzawa Yorihiro,Shimoda Hiromi,Kakuhama Yoshitaka,Endo Hiroyuki,Tanaka Kengo.Development of a high efficiencyMiller cycle gas engine,Mitsubishi Heavy Industries Ltd..Tech Rev2001;38(3):146–50.[14]Wu Chih,Puzinauskas Paul V,Tsai Jung S.Performance analysis and optimization of a supercharged Miller cycle otto engine.ApplTherm Eng2003;23:511–21.[15]Al-Sarkhi A,Jaber JO,Probert SD.Efficiency of a Miller engine.Appl Energy2006;83:343–51.。
IBM错误代码解释以及解决方法

IBM错误代码解释以及解决方法在使用IBM产品或服务的过程中,有时可能会遇到各种错误代码。
这些错误代码可能涉及到不同的问题,需要我们仔细检查和解决。
在本文中,我们将解释一些常见的IBM错误代码,并提供相应的解决方法。
1. 500 - Internal Server Error(内部服务器错误)这个错误代码表示服务器遇到了一个无法处理的问题。
可能的原因包括服务器配置错误、网络连接问题或应用程序错误。
解决方法包括:-检查服务器配置文件,确保配置正确。
-检查网络连接是否正常,尝试重启网络设备。
-使用日志文件来查找潜在的应用程序错误,并修复相应的代码。
2. 404 - Not Found(未找到)这个错误代码表示请求的资源在服务器上不存在。
可能的原因包括文件被误删除、链接错误或服务器配置问题。
解决方法包括:-检查请求的资源是否存在于服务器上。
如果不存在,可以尝试恢复文件或重新上传文件。
-检查链接是否正确。
如果链接错误,可以尝试修复链接或更改链接地址。
-检查服务器配置文件,确保资源的路径和文件名称正确。
3. 403 - Forbidden(禁止访问)这个错误代码表示服务器拒绝了对请求资源的访问。
可能的原因包括权限不足、IP地址被拦截或访问规则被禁止。
解决方法包括:-检查访问权限,确保用户有足够的权限来访问资源。
-检查IP地址是否被服务器拦截。
如果是,则可以尝试解除拦截或添加到允许访问列表。
-检查访问规则,确保没有禁止访问请求资源的规则。
4. 502 - Bad Gateway(网关错误)这个错误代码表示作为代理或网关的服务器从上游服务器接收到了一条无效的响应。
可能的原因包括上游服务器故障、网络连接问题或配置错误。
解决方法包括:-检查网络连接是否正常。
如果网络连接有问题,可以尝试重启网络设备或使用其他网络连接。
-检查代理或网关服务器的配置文件,确保配置正确。
5. 503 - Service Unavailable(服务不可用)这个错误代码表示服务器当前无法处理请求,可能是因为过载或维护。
第四版人民币冠号大全

第四版人民币冠号大全四版纸币冠号表(转)壹角券〔517种〕8001已见发行517种冠号〔其中补号30种〕第一大组〔90种JY为样票〕其中补号5种JT JU JW JX JZAP AQ AR AS AT ——AX AY AZBP BQ BR BS BT BU BW BX BY BZCP CQ CR CS CT CU CW CX CY CZDP DQ DR DS DT DU DW DX ——EP EQ ER ES ET EU EW EX EY EZ———FS FT FU FW FX FY FZGP GQ GR GS GT GU GW GX GY GZHP HQ HR HS HT HU —HX HY HZIP IQ IR IS IT IU IW IX IY IZJP JQ JR —JT JU JW JX —JZ第二大组〔99种〕其中补号3种ZH ZI ZJPA PB PC PD PE PF PG PH PI PJQA QB QC QD QE QF QG QH QI QJRA RB RC RD RE RF RG RH RI RJSA SB SC SD SE SF SG SH SI SJTA TB —TD TE TF TG TH TI TJUA UB UC UD UE UF UG UH UI UJWA WB WC WD WE WF WG WH WI WJ XA XB XC XD XE XF XG XH XI XJYA YB YC YD YE YF YG YH YI YJZA ZB ZC ZD ZE ZF ZG ZH ZI ZJ第三大组〔50种〕其中补号3种ZM ZN ZOPK PL PM PN POQK QL QM QN QORK RL RM RN ROSK SL SM SN SOTK TL TM TN TOUK UL UM UN UOWK WL WM WN WOXK XL XM XN XOYK YL YM YN YOZK ZL ZM ZN ZO第四大组〔50种〕其中补号2种JN JOAK AL AM AN AOBK BL BM BN BOCK CL CM CN CODK DL DM DN DOEK EL EM EN EOFK FL FM FN FOGK GL GM GN GOHK HL HM HN HOIK IL IM IN IOJK JL JM JN JO第五大组〔50种〕其中补号2种NY NZKP KQ KR KS KT KU KW KX KY KZLP LQ LR LS LT LU LW LX LY LZMP MQ MR MS MT MU MW MX MY MZ NP NQ NR NS NT NU NW NX NY NZOP OQ OR OS OT OU OW OX OY OZ第六大组〔95种〕其中补号7种JA JB JC JG JH JI JJAA AB AC AD AE AF AG AH AI AJBA BB BC BD BE BF BG BH BI —CA CB CC CD CE CF CG CH CI CJDA —DC DD DE DF DG DH DI DJEA EB EC ED ——EG EH EI EJFA FB FC FD FE FF FG FH FI FJGA GB GC GD GE GF GG GH GI GJHA HB HC HD HE HF HG HH HI HJIA IB IC ID IE IF IG IH II IJJA JB JC —JE JF JG JH JI JJ第七大组〔12种〕其中补号1种NJKA KB KC KD KE KF KG KH KI KJ——MC ————————————————NJ第八大组〔69种〕其中补号6种ZT ZU ZW ZX ZY ZZPP PQ PR PS PT PU PW PX PY PZQP QQ QR ————QX QY QZRP RQ RR RS RT RU RW RX RY RZSP ——SS —SU SW SX SY —TP TQ TR TS TT TU TW TX TY TZUS UUWP WQ —WS WT WU WW WX WY ———XR XS XZYP —YR YS ———YX —YZZP ZQ ZR —ZT ZU ZW ZX ZY ZZ第九大组〔2种〕其中补号1种NNMKNN贰角券〔242种,其中补号6种〕第一大组〔99种JY为样票〕其中补号2种JX JZAP AQ AR AS AT AU AW AX AY AZ BP BQ BR BS BT BU BW BX BY BZ CP CQ CR CS CT CU CW CX CY CZ DP DQ DR DS DT DU DW DX DY DZ EP EQ ER ES ET EU EW EX EY EZFP FQ FR FS FT FU FW FX FY FZGP GQ GR GS GT GU GW GX GY GZ HP HQ HR HS HT HU HW HX HY HZ IP IQ IR IS IT IU IW IX IY IZJP JQ JR JS JT JU JW JX —JZ第二大组〔100种〕其中补号2种ZI ZJPA PB PC PD PE PF PG PH PI PJQA QB QC QD QE QF QG QH QI QJ RA RB RC RD RE RF RG RH RI RJ SA SB SC SD SE SF SG SH SI SJTA TB TC TD TE TF TG TH TI TJUA UB UC UD UE UF UG UH UI UJWA WB WC WD WE WF WG WH WI WJXA XB XC XD XE XF XG XH XI XJYA YB YC YD YE YF YG YH YI YJZA ZB ZC ZD ZE ZF ZG ZH ZI ZJ第三大组〔43种〕其中补号2种ZN ZOPK PL PM PN POQK QL QM QN QORK RL RM RN ROSK SL SM SN SOTK TL TM TN TOUK —UM UN UOWK WL WM WN WOXK XL ———YK YL YM YN YO———ZN ZO伍角券〔387种〕第一大组〔92种JY为样票〕其中补号4种JU JW JX JZAP AQ AR AS AT AU AW AX AY AZBP BQ BR —————BY BZ CP CQ CR CS CT CU CW CX CY CZDP DQ DR ——DU DW DX DY DZ EP EQ ER ES ET EU EW EX EY EZFP FQ FR FS FT FU FW FX FY FZGP GQ GR GS GT GU GW GX GY GZHP HQ HR HS HT HU HW HX HY HZIP IQ IR IS IT IU IW IX IY IZJP JQ JR JS JT JU JW JX —JZ第二大组〔100种〕其中补号2种ZI ZJPA PB PC PD PE PF PG PH PI PJQA QB QC QD QE QF QG QH QI QJRA RB RC RD RE RF RG RH RI RJSA SB SC SD SE SF SG SH SI SJTA TB TC TD TE TF TG TH TI TJUA UB UC UD UE UF UG UH UI UJWA WB WC WD WE WF WG WH WI WJ XA XB XC XD XE XF XG XH XI XJYA YB YC YD YE YF YG YH YI YJZA ZB ZC ZD ZE ZF ZG ZH ZI ZJ第三大组〔50种〕其中补号2种ZN ZOPK PL PM PN POQK QL QM QN QORK RL RM RN ROSK SL SM SN SOTK TL TM TN TOUK UL UM UN UOWK WL WM WN WOXK XL XM XN XOYK YL YM YN YOZK ZL ZM ZN ZO第六大组〔45种〕其中补号3种JH JI JJAA AB AC AD AE AF AG AH AI AJ CA CB CC CD CE CF CG CH CI CJEA EB EC ———EG EH EI EJ GA GB GC GD GE GF GG GH GI GJ IA IB IC ID IE ————————————JH JI JJ第八大组〔93种〕其中补号4种ZW ZX ZY ZZPP PQ PR PS PT PU PW PX PY PZ QP QQ QR QS QT QU QW QX QY QZSP SQ SR SS ST SU SW SX SY SZTP TQ TR TS TT TU TW TX TY TZUP UQ UR US UT UU UW UX UY UZWP WQ WR WS WT WU WW WX WY WZXP ------ XT XU XW XX XY --YP YQ YR YS YT YU YW YX YY YZZP ZQ -- ZS ---- ZW ZX ZY ZZ第九大组(7种)其中补号1种NOKK ---- KN KOMK ML -------------- NO------ ON --注:此冠号明细不含四联体冠号。
ASCII码表_全_完整版
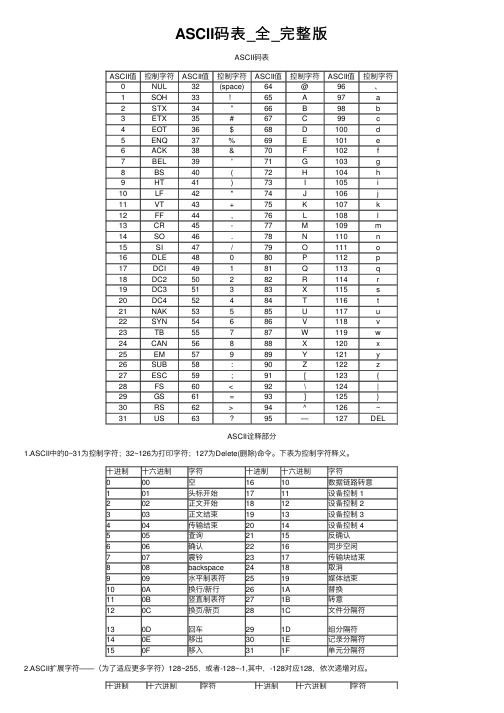
ASCII码表_全_完整版ASCII码表ASCII值控制字符ASCII值控制字符ASCII值控制字符ASCII值控制字符0NUL32(space)64@96、1SOH33!65A97a2STX34”66B98b3ETX35#67C99c4EOT36$68D100d5ENQ37%69E101e6ACK38&70F102f7BEL39'71G103g8BS40(72H104h9HT41)73I105i10LF42*74J106j11VT43+75K107k12FF44,76L108l13CR45-77M109m14SO46.78N110n15SI47/79O111o16DLE48080P112p17DCI49181Q113q18DC250282R114r19DC351383X115s20DC452484T116t21NAK53585U117u22SYN54686V118v23TB55787W119w24CAN56888X120x25EM57989Y121y26SUB58:90Z122z27ESC59;91[123{28FS60< 92\124|29GS61=93]125}30RS62> 94^126~31US63?95—127DELASCII诠释部分1.ASCII中的0~31为控制字符;32~126为打印字符;127为Delete(删除)命令。
下表为控制字符释义。
⼗进制⼗六进制字符⼗进制⼗六进制字符000空1610数据链路转意101头标开始1711设备控制 1202正⽂开始1812设备控制 2303正⽂结束1913设备控制 3404传输结束2014设备控制 4505查询2115反确认606确认2216同步空闲707震铃2317传输块结束808backspace2418取消909⽔平制表符2519媒体结束100A换⾏/新⾏261A替换110B竖直制表符271B转意120C换页/新页281C⽂件分隔符130D回车291D组分隔符140E移出301E记录分隔符150F移⼊311F单元分隔符2.ASCII扩展字符——(为了适应更多字符)128~255,或者-128~-1,其中,-128对应128,依次递增对应。
ASCII码表

ASCII码表信息在计算机上是用二进制表示的,这种表示法让人理解就很困难。
因此计算机上都配有输入和输出设备,这些设备的主要目的就是,以一种人类可阅读的形式将信息在这些设备上显示出来供人阅读理解。
为保证人类和设备,设备和计算机之间能进行正确的信息交换,人们编制的统一的信息交换代码,这就是ASCII码表,它的全称是“美国信息交换标准代码”。
ASCII码对照表在Web开发时,如下的ASCII码只要加上&#和;就可以变成Web可以辨认的字符了在处理特殊字符的时候特别有用,如:' 单引号在数据库查询的时候是杀手,但是如果转换成'(注意:转换后的机构有:&# +字符的ASCII码值+; 三个部分组成)再来存数据库,就没有什么影响了。
其他的字符与ASCII码的对照如下表ASCII表键盘常用ASCII码ESC键 VK_ESCAPE (27)回车键: VK_RETURN (13)TAB键: VK_TAB (9)Caps Lock键: VK_CAPITAL (20)Shift键: VK_SHIFT ($10)Ctrl键: VK_CONTROL (17)Alt键: VK_MENU (18)空格键: VK_SPACE ($20/32)退格键: VK_BACK (8)左徽标键: VK_LWIN (91)右徽标键: VK_LWIN (92)鼠标右键快捷键:VK_APPS (93)Insert键: VK_INSERT (45) Home键: VK_HOME (36)Page Up: VK_PRIOR (33) PageDown: VK_NEXT (34)End键: VK_END (35)Delete键: VK_DELETE (46)方向键(←): VK_LEFT (37)方向键(↑): VK_UP (38)方向键(→): VK_RIGHT (39)方向键(↓): VK_DOWN (40)F1键: VK_F1 (112)F2键: VK_F2 (113)F3键: VK_F3 (114)F4键: VK_F4 (115)F5键: VK_F5 (116)F6键: VK_F6 (117)F7键: VK_F7 (118)F8键: VK_F8 (119)F9键: VK_F9 (120)F10键: VK_F10 (121)F11键: VK_F11 (122)F12键: VK_F12 (123)Num Lock键: VK_NUMLOCK (144) 小键盘0: VK_NUMPAD0 (96)小键盘1: VK_NUMPAD0 (97)小键盘2: VK_NUMPAD0 (98)小键盘3: VK_NUMPAD0 (99)小键盘4: VK_NUMPAD0 (100)小键盘5: VK_NUMPAD0 (101)小键盘6: VK_NUMPAD0 (102)小键盘7: VK_NUMPAD0 (103)小键盘8: VK_NUMPAD0 (104)小键盘9: VK_NUMPAD0 (105)小键盘.: VK_DECIMAL (110)小键盘*: VK_MULTIPLY (106)小键盘+: VK_MULTIPLY (107)小键盘-: VK_SUBTRACT (109)小键盘/: VK_DIVIDE (111)Pause Break键: VK_PAUSE (19)Scroll Lock键: VK_SCROLL (145)025532CA . B9 90AB5502 mov ecx, 0255AB90 ; b07cgmrf2rff36jfj4n82k06545454545454545454545454b&r product licensing。
ASCII码表

ASCII码表信息在计算机上是用二进制表示的,这种表示法让人理解就很困难。
因此计算机上都配有输入和输出设备,这些设备的主要目的就是,以一种人类可阅读的形式将信息在这些设备上显示出来供人阅读理解。
为保证人类和设备,设备和计算机之间能进行正确的信息交换,人们编制的统一的信息交换代码,这就是ASCII码表,它的全称是“美国信息交换标准代码”。
八进制十六进制十进制字符八进制十六进制十进制字符00000nul1004064@ 01011soh1014165A 02022stx1024266B 03033etx1034367C 04044eot1044468D 05055enq1054569E 06066ack1064670F 07077bel1074771G 10088bs1104872H 11099ht1114973I 120a10nl1124a74J 130b11vt1134b75K 140c12ff1144c76L 150d13er1154d77M 160e14so1164e78N 170f15si1174f79O 201016dle1205080P 211117dc11215181Q 221218dc21225282R 231319dc31235383S 241420dc41245484T251521nak1255585U 261622syn1265686V 271723etb1275787W 301824can1305888X 311925em1315989Y 321a26sub1325a90Z 331b27esc1335b91[ 341c28fs1345c92\ 351d29gs1355d93] 361e30re1365e94^ 371f31us1375f95_ 402032sp1406096' 412133!1416197a 422234"1426298b 432335#1436399c 442436$14464100d 452537%14565101e 462638&14666102f 472739`14767103g 502840(15068104h 512941)15169105i 522a42*1526a106j 532b43+1536b107k 542c44,1546c108l 552d45-1556d109m 562e46.1566e110n 572f47/1576f111o 603048016070112p 613149116171113q623250216272114r633351316373115s643452416474116t653553516575117u663654616676118v673755716777119w703856817078120x713957917179121y723a58:1727a122z733b59;1737b123{743c60<1747c124|753d61=1757d125}763e62>1767e126~773f63?1777f127delASCII码对照表在Web开发时,如下的ASCII码只要加上&#和;就可以变成Web可以辨认的字符了在处理特殊字符的时候特别有用,如:' 单引号在数据库查询的时候是杀手,但是如果转换成'(注意:转换后的机构有:&# +字符的ASCII码值+; 三个部分组成)再来存数据库,就没有什么影响了。
最全ASCII对应码表-键值

最全ASCII对应码表-键值OCT(八进制)最全ASCII码对应表—与键盘按键对应值(二进)Bin (十进)Dec (十六进)Hex 缩写/字符解释0000 0000 0 00 NUL (null)空字符0000 0001 1 01 SOH (start o f handing) 标题开始0000 0010 2 02 STX (start o f text) 正文开始0000 0011 3 03 ETX (end of text) 正文结束0000 0100 4 04 EOT (end of transmission) 传输结束0000 0101 5 05 ENQ (enquir y) 请求0000 0110 6 06 ACK (acknowl edge) 收到通知0000 0111 7 07 BEL (bell)响铃0000 1000 8 08 BS (backspac e) 退格0000 1001 9 09 HT (horizont al tab) 水平制表符0000 1010 10 0A LF (NL line fee d, new line) 换行键0000 1011 11 0B VT (vertical ta b) 垂直制表符0000 1100 12 0C FF (NP form fee d, new page) 换页键0000 1101 13 0D CR (carriage re turn) 回车键0000 1110 14 0E SO (shift out) 不用切换0000 1111 15 0F SI (shift in) 启用切换0001 0000 16 10 DLE (data link escape) 数据链路转义0001 0001 17 11 DC1 (device con trol 1) 设备控制10001 0010 18 12 DC2 (device con trol 2) 设备控制2 0001 0011 19 13 DC3 (device con trol 3) 设备控制3 0001 0100 20 14 DC4 (device con trol 4) 设备控制4 0001 0101 21 15 NAK (negative a cknowledge) 拒绝接收0001 0110 22 16 SYN (synchronou s idle) 同步空闲0001 0111 23 17 ETB (end of tra ns. block) 传输块结束0001 1000 24 18 CAN (cancel) 取消0001 1001 25 19 EM (end of medi um) 介质中断0001 1010 26 1A SUB (substitut e) 替补0001 1011 27 1B ESC (escape) 溢出0001 1100 28 1C FS (file separa tor) 文件分割符0001 1101 29 1D GS (group separ ator) 分组符0001 1110 30 1E RS (record sepa rator) 记录分离符0001 1111 31 1F US (unit separa tor) 单元分隔符0010 0000 32 20 空格0010 0001 33 21 !0010 0010 34 22 "0010 0011 35 23 #0010 0100 36 24 $0010 0101 37 25 %0010 0110 38 26 &0010 0111 39 27 '0010 1000 40 28 (0010 1001 41 29 )0010 1010 42 2A *0010 1011 43 2B +0010 1100 44 2C ,0010 1101 45 2D -0010 1110 46 2E .0010 1111 47 2F /0011 0001 49 31 1 0011 0010 50 32 2 0011 0011 51 33 3 0011 0100 52 34 4 0011 0101 53 35 5 0011 0110 54 36 6 0011 0111 55 37 7 0011 1000 56 38 8 0011 1001 57 39 9 0011 1010 58 3A : 0011 1011 59 3B ; 0011 1100 60 3C < 0011 1101 61 3D = 0011 1110 62 3E > 0011 1111 63 3F ? 0100 0000 64 400100 0001 65 41 A 0100 0010 66 42 B 0100 0011 67 43 C 0100 0100 68 44 D0100 0110 70 46 F 0100 0111 71 47 G 0100 1000 72 48 H 0100 1001 73 49 I 0100 1010 74 4A J 0100 1011 75 4B K 0100 1100 76 4C L 0100 1101 77 4D M 0100 1110 78 4E N 0100 1111 79 4F O 0101 0000 80 50 P 0101 0001 81 51 Q 0101 0010 82 52 R 0101 0011 83 53 S 0101 0100 84 54 T 0101 0101 85 55 U 0101 0110 86 56 V 0101 0111 87 57 W 0101 1000 88 58 X 0101 1001 89 59 Y 0101 1010 90 5A Z。
ASCII码表

ASCII码表信息在计算机上是用二进制表示的,这种表示法让人理解就很困难。
因此计算机上都配有输入和输出设备,这些设备的主要目的就是,以一种人类可阅读的形式将信息在这些设备上显示出来供人阅读理解。
为保证人类和设备,设备和计算机之间能进行正确的信息交换,人们编制的统一的信息交换代码,这就是ASCII码表,它的全称是“美国信息交换标准代码”。
八进制十六进制十进制字符八进制十六进制十进制字符00 00 0 nul 100 40 64 @01 01 1 soh 101 41 65 A02 02 2 stx 102 42 66 B03 03 3 etx 103 43 67 C04 04 4 eot 104 44 68 D05 05 5 enq 105 45 69 E06 06 6 ack 106 46 70 F07 07 7 bel 107 47 71 G10 08 8 bs 110 48 72 H11 09 9 ht 111 49 73 I12 0a 10 nl 112 4a 74 J13 0b 11 vt 113 4b 75 K14 0c 12 ff 114 4c 76 L15 0d 13 er 115 4d 77 M16 0e 14 so 116 4e 78 N17 0f 15 si 117 4f 79 O20 10 16 dle 120 50 80 P21 11 17 dc1 121 51 81 Q22 12 18 dc2 122 52 82 R23 13 19 dc3 123 53 83 S24 14 20 dc4 124 54 84 T25 15 21 nak 125 55 85 U26 16 22 syn 126 56 86 V27 17 23 etb 127 57 87 W30 18 24 can 130 58 88 X31 19 25 em 131 59 89 Y32 1a 26 sub 132 5a 90 Z33 1b 27 esc 133 5b 91 [34 1c 28 fs 134 5c 92 \35 1d 29 gs 135 5d 93 ]36 1e 30 re 136 5e 94 ^37 1f 31 us 137 5f 95 _40 20 32 sp 140 60 96 '41 21 33 ! 141 61 97 a42 22 34 " 142 62 98 b43 23 35 # 143 63 99 c44 24 36 $ 144 64 100 d45 25 37 % 145 65 101 e46 26 38 & 146 66 102 f47 27 39 ` 147 67 103 g50 28 40 ( 150 68 104 h51 29 41 ) 151 69 105 i52 2a 42 * 152 6a 106 j53 2b 43 + 153 6b 107 k54 2c 44 , 154 6c 108 l55 2d 45 - 155 6d 109 m56 2e 46 . 156 6e 110 n57 2f 47 / 157 6f 111 o 60 30 48 0 160 70 112 p61 31 49 1 161 71 113 q62 32 50 2 162 72 114 r63 33 51 3 163 73 115 s64 34 52 4 164 74 116 t65 35 53 5 165 75 117 u66 36 54 6 166 76 118 v67 37 55 7 167 77 119 w70 38 56 8 170 78 120 x71 39 57 9 171 79 121 y72 3a 58 : 172 7a 122 z73 3b 59 ; 173 7b 123 {74 3c 60 < 174 7c 124 |75 3d 61 = 175 7d 125 }76 3e 62 > 176 7e 126 ~77 3f 63 ? 177 7f 127 delASCII码对照表在Web开发时,如下的ASCII码只要加上&#和;就可以变成Web可以辨认的字符了在处理特殊字符的时候特别有用,如:' 单引号在数据库查询的时候是杀手,但是如果转换成'(注意:转换后的机构有:&# +字符的ASCII码值+; 三个部分组成)再来存数据库,就没有什么影响了。
ascII码表

ASCII码表信息在计算机上是用二进制表示的,这种表示法让人理解就很困难。
因此计算机上都配有输入和输出设备,这些设备的主要目的就是,以一种人类可阅读的形式将信息在这些设备上显示出来供人阅读理解。
为保证人类和设备,设备和计算机之间能进行正确的信息交换,人们编制的统一的信息交换代码,这就是ASCII码表,它的全称是“美国信息交换标准代码”。
ASCII码对照表在Web开发时,如下的ASCII码只要加上&#和;就可以变成Web可以辨认的字符了在处理特殊字符的时候特别有用,如:' 单引号在数据库查询的时候是杀手,但是如果转换成'(注意:转换后的机构有:&# +字符的ASCII码值+; 三个部分组成)再来存数据库,就没有什么影响了。
其他的字符与ASCII码的对照如下表ASCII表键盘常用ASCII码SC键VK_ESCAPE (27)回车键:VK_RETURN (13)AB键:VK_TAB (9)aps Lock键:VK_CAPITAL (20) hift键:VK_SHIFT ($10)trl键:VK_CONTROL (17)lt键:VK_MENU (18)空格键:VK_SPACE ($20/32)退格键:VK_BACK (8)左徽标键:VK_LWIN (91)右徽标键:VK_LWIN (92)鼠标右键快捷键:VK_APPS (93) Insert键:VK_INSERT (45)Home键:VK_HOME (36)Page Up:VK_PRIOR (33) PageDown:VK_NEXT (34)End键:VK_END (35)Delete键:VK_DELETE (46)方向键(←):VK_LEFT (37)方向键(↑):VK_UP (38)方向键(→):VK_RIGHT (39)方向键(↓):VK_DOWN (40)F1键:VK_F1 (112)F2键:VK_F2 (113)F3键:VK_F3 (114)F4键:VK_F4 (115)F5键:VK_F5 (116)F6键:VK_F6 (117)F7键:VK_F7 (118)F8键:VK_F8 (119)F9键:VK_F9 (120)F10键:VK_F10 (121)F11键:VK_F11 (122)F12键:VK_F12 (123)Num Lock键:VK_NUMLOCK (144) 小键盘0:VK_NUMPAD0 (96)小键盘1:VK_NUMPAD0 (97)小键盘2:VK_NUMPAD0 (98)小键盘3:VK_NUMPAD0 (99)小键盘4:VK_NUMPAD0 (100)小键盘5:VK_NUMPAD0 (101)小键盘6:VK_NUMPAD0 (102)小键盘7:VK_NUMPAD0 (103)小键盘8:VK_NUMPAD0 (104)小键盘9:VK_NUMPAD0 (105)小键盘.:VK_DECIMAL (110)小键盘*:VK_MULTIPLY (106)小键盘+:VK_MULTIPLY (107)小键盘-:VK_SUBTRACT (109)小键盘/:VK_DIVIDE (111)Pause Break键:VK_PAUSE (19)Scroll Lock键:VK_SCROLL (145)。
姓名代码查询表

姓名代码查询表1、下列汉字取自国标(GB 2312-80)中的分级与排列内容;包含所有的第一级汉字和第二级汉字中的常用部分。
2、第一级汉字(16—55区的汉字)以拼音字母为序进行排列,同音字以笔形顺序横、竖、撇、捺、折为序,起笔相同的按第二笔,依次类推;第二级汉字(56-87区的汉字)按部首为序进行排列。
3、对于多音字,仅在表中出现一次。
如:柏音(bai,bo),表中仅出现在bai中。
4、汉字区位码用阿拉伯数字表示,每个汉字对应4个数字。
5、本汉字代码表摘自《字符集和信息编码国家标准汇编》,(中国标准出版社,1998年编)a啊1601 阿1602 吖6325 嗄6436 腌7571 锕7925ai埃1603 挨1604 哎1605 唉1606 哀1607 皑1608 癌1609蔼1610 矮1611 艾1612 碍1613 爱1614 隘1615 捱6263嗳6440 嗌6441 嫒7040 瑷7208 暧7451 砹7733 锿7945霭8616an鞍1616 氨1617 安1618 俺1619 按1620 暗1621 岸1622胺1623 案1624 谙5847 埯5991 揞6278 犴6577 庵6654桉7281 铵7907 鹌8038 黯8786ang肮1625 昂1626 盎1627ao凹1628 敖1629 熬1630 翱1631 袄1632 傲1633 奥1634懊1635 澳1636 坳5974 拗6254 嗷6427 岙6514 廒6658遨6959 媪7033 骜7081 獒7365 聱8190 螯8292 鏊8643鳌8701 鏖8773ba芭1637 捌1638 扒1639 叭1640 吧1641 笆1642 八1643疤1644 巴1645 拔1646 跋1647 靶1648 把1649 耙1650坝1651 霸1652 罢1653 爸1654 茇6056 菝6135 岜6517灞6917 钯7857 粑8446 鲅8649 魃8741bai白1655 柏1656 百1657 摆1658 佰1659 败1660 拜1661稗1662 捭6267 呗6334 掰7494ban斑1663 班1664 搬1665 扳1666 般1667 颁1668 板1669版1670 扮1671 拌1672 伴1673 瓣1674 半1675 办1676绊1677 阪5870 坂5964 钣7851 瘢8103 癍8113 舨8418bang邦1678 帮1679 梆1680 榜1681 膀1682 绑1683 棒1684磅1685 蚌1686 镑1687 傍1688 谤1689 蒡6182 浜6826bao苞1690 胞1691 包1692 褒1693 剥1694 薄1701 雹1702保1703 堡1704 饱1705 宝1706 抱1707 报1708 暴1709豹1710 鲍1711 爆1712 葆6165 孢7063 煲7650 鸨8017褓8157 趵8532 龅8621bei杯1713 碑1714 悲1715 卑1716 北1717 辈1718 背1719贝1720 钡1721 倍1722 狈1723 备1724 惫1725 焙1726被1727 孛5635 陂5873 邶5893 蓓6177 悖6703 碚7753鹎8039 褙8156 鐾8645 鞴8725ben奔1728 苯1729 本1730 笨1731 畚5946 坌5948 贲7458锛7928beng崩1732 绷1733 甭1734 泵1735 蹦1736 迸1737 嘣6452甏7420bi逼1738 鼻1739 比1740 鄙1741 笔1742 彼1743 碧1744蓖1745 蔽1746 毕1747 毙1748 毖1749币1750 庇1751痹1752 闭1753 敝1754 弊1755 必1756 辟1757 壁1758臂1759 萆6141 避1760 陛1761 匕5616 俾5734 荜6074荸6109 薜6221 吡6333 哔6357 狴6589 庳6656 愎6725滗6868 濞6908 弼6986 妣6994 婢7030 嬖7052 璧7221畀7815 铋7873 秕7985 裨8152 筚8357 箅8375 篦8387舭8416 襞8437 跸8547 髀8734bian鞭1762 边1763 编1764 贬1765 扁1766 便1767 变1768卞1769 辨1770 辩1771 辫1772 遍1773 匾5650 弁5945苄6048 忭6677 汴6774 缏7134 煸7652 砭7730 碥7760窆8125 褊8159 蝙8289 笾8354 鳊8693biao标1774 彪1775 膘1776 表1777 婊7027 骠7084 杓7228飑7609 飙7613 飚7614 镖7958 镳7980 瘭8106 裱8149鳔8707 髟8752bie鳖1778 憋1779 别1780 瘪1781 蹩8531bin彬1782 斌1783 濒1784 滨1785 宾1786 摈1787 傧5747豳6557 缤7145 玢7167 槟7336 殡7375 膑7587 镔7957髌8738 鬓8762bing兵1788 冰1789 柄1790 丙1791 秉1792 饼1793 炳1794病1801 并1802 禀5787 邴5891 摒6280bo玻1803 菠1804 播1805 拨1806 钵1807 波1808 博1809勃1810 搏1811 铂1812 箔1813 伯1814 帛1815 舶1816脖1817 膊1818 渤1819 泊1820 驳1821 亳5781 啵6403饽6636 檗7362 擘7502 礴7771 钹7864 鹁8030 簸8404跛8543 踣8559bu捕1822 卜1823 哺1824 补1825 埠1826 不1827 布1828步1829 簿1830 部1831 怖1832 卟6318 逋6945 瓿7419晡7446 钚7848 钸7863 醭8519ca擦1833 嚓6474 礤7769cai猜1834 裁1835 材1836 才1837 财1838 睬1839 踩1840采1841 彩1842 菜1843 蔡1844can餐1845 参1846 蚕1847 残1848 惭1849 惨1850 灿1851孱6978 骖7078 璨7218 粲8451 黪8785cang苍1852 舱1853 仓1854 沧1855 藏1856cao操1857 糙1858 槽1859 曹1860 草1861 嘈6448 漕6878螬8309 艚8429ce厕1862 策1863 侧1864 册1865 测1866 恻6692cen岑6515 涔6825ceng层1867 蹭1868 噌6465cha插1869 叉1870 茬1871 茶1872 查1873 碴1874 搽1875察1876 岔1877 差1878 诧1879 猹6610 馇6639 汊6766姹7017 杈7230 楂7311 槎7322 檫7363 锸7942 镲7979衩8135chai拆1880 柴1881 豺1882 侪5713 钗7846 瘥8091 虿8218chan搀1883 掺1884 蝉1885 馋1886 谗1887 缠1888 铲1889产1890 阐1891 颤1892 冁5770 谄5838 蒇6159 廛6660忏6667 潺6893 澶6904 羼6981 婵7031 骣7086 觇7472禅7688 镡7966 蟾8324 躔8580chang昌1893 猖1894 场1901 尝1902 常1903 长1904 偿1905肠1906 厂1907 敞1908 畅1909 唱1910 倡1911 伥5686鬯5943 苌6041 菖6137 徜6568 怅6674 惝6714 阊6749娼7029 嫦7047 昶7438 氅7509 鲳8680chao超1912 抄1913 钞1914朝1915 嘲1916 潮1917 巢1918吵1919 炒1920 怊6687 晁7443 焯7644 耖8173che车1921 扯1922 撤1923 掣1924 彻1925 澈1926 坼5969砗7726chen郴1927 臣1928 辰1929 尘1930 晨1931 忱1932 沉1933陈1934 趁1935 衬1936 伧5687 谌5840 谶5863 抻6251嗔6433 宸6923 琛7201 榇7320 碜7755 龀8619cheng撑1937 称1938 城1939 橙1940 成1941 呈1942 乘1943程1944 惩1945 澄1946 诚1947 承1948 逞1949 骋1950秤1951 丞5609 埕5984 枨7239 柽7263 塍7583 瞠7810铖7881 铛7885 裎8146 蛏8241 酲8508chi吃1952 痴1953 持1954 匙1955 池1956 迟1957 弛1958驰1959 耻1960 齿1961 侈1962 尺1963 赤1964 翅1965斥1966 炽1967 傺5749 坻5970 墀6015 茌6061 叱6319哧6374 啻6420 嗤6445 彳6560 饬6633 媸7042 敕7523眙7784 眵7787 鸱8023 瘛8101 褫8161 蚩8231 螭8304笞8355 篪8388 豉8489 踟8556 魑8746chong充1968 冲1969 虫1970 崇1971 宠1972 茺6091 忡6671憧6731 铳7905 舂8409 艟8430chou抽1973 酬1974 畴1975 踌1976 稠1977 愁1978 筹1979仇1980 绸1981 瞅1982 丑1983 臭1984 俦5717 帱6492惆6716 瘳8112 雠8637chu初1985 出1986 橱1987 厨1988 躇1989 锄1990 雏1991滁1992 除1993 楚1994 础2001 储2002 矗2003 搐2004触2005 处2006 亍5601 刍5927 怵6680 憷6732 绌7109杵7238 楮7290 樗7343 褚8150 蜍8260 蹰8573 黜8777chuai揣2007 搋6285 啜6408 嘬6460 膪7590 踹8563chuan川2008 穿2009 椽2010 传2011 船2012 喘2013 串2014舛6622 遄6955 巛7161 氚7516 钏7843 舡8413chuang疮2015 窗2016 幢2017 床2018 闯2019 创2020 怆6675chui吹2021 炊2022 捶2023 锤2024 垂2025 陲5879 棰7302槌7319chun春2026 椿2027 醇2028 唇2029 淳2030 纯2031 蠢2032莼6127 鹑8040 蝽8277chuo戳2033 绰2034 辍7401 踔8554 龊8626ci疵2035 茨2036 磁2037 雌2038 辞2039 慈2040 瓷2041词2042 此2043 刺2044 赐2045 次2046 茈6075 呲6358祠7684 鹚8043 糍8457cong聪2047 葱2048 囱2049 匆2050 从2051 丛2052 苁6042淙6840 骢7085 琮7193 璁7214 枞7240cou凑2053 楱7308 辏7403 腠7577cu粗2054 醋2055 簇2056 促2057 蔟6193 徂6562 猝6607殂7367 酢8501 蹙8530 蹴8577cuan蹿2058 篡2059 窜2060 氽5764 撺6305 爨7664 镩7973cui摧2061 崔2062 催2063 脆2064 瘁2065 粹2066 淬2067翠2068 萃6145 啐6393 悴6718 璀7213 榱7333 毳7505隹8631cun村2069 存2070 寸2071 忖6666 皴8169cuo磋2072 撮2073 搓2074 措2075 挫2076 错2077 厝5640嵯6547 脞7566 锉7917 矬7983 痤8078 鹾8526 蹉8567da搭2078 达2079 答2080 瘩2081 打2082 大2083 耷6239哒6353 嗒6410 怛6682 妲7007 沓7719 疸8067 褡8155笪8346 靼8716 鞑8718dai呆2084 歹2085 傣2086 戴2087 带2088 殆2089 代2090贷2091 袋2092 待2093 逮2094 怠2101 埭6004 甙6316呔6330 岱6523 迨6942 骀7070 绐7110 玳7173 黛8776dan耽2102 担2103 丹2104 单2105 郸2106 掸2107 胆2108旦2109 氮2110 但2111 惮2112 淡2113 诞2114 弹2115蛋2116 儋5757 萏6144 啖6402 澹6903 殚7373 赕7470眈7781 瘅8087 聃8185 箪8376dang当2117 挡2118 党2119 荡2120 档2121 谠5852 凼5942菪6148 宕6920 砀7724 裆8141dao刀2122 捣2123 蹈2124 倒2125 岛2126 祷2127 导2128到2129 稻2130 悼2131 道2132 盗2133 叨6322 忉6665氘7514 焘7666 纛8478de德2134 得2135 的2136 锝7929deng蹬2137 灯2138 登2139 等2140 瞪2141 凳2142 邓2143噔6466 嶝6556 戥7413 磴7767 镫7975 簦8403di堤2144 低2145 滴2146 迪2147 敌2148 笛2149 狄2150涤2151 翟2152 嫡2153 抵2154 底2155 地2156 蒂2157第2158 帝2159 弟2160 递2161 缔2162 氐5621 籴5765诋5814 谛5848 邸5901 荻6122 嘀6454 娣7023 柢7260棣7306 觌7475 砥7738 碲7758 睇7791 镝7965 羝8438骶8730dia嗲6439dian颠2163 掂2164 滇2165 碘2166 点2167 典2168 靛2169垫2170 电2171 佃7172 甸2173 店2174 惦2175 奠2176淀2177 殿2178 阽5871 坫5967 巅6559 玷7172 钿7868癜8116 癫8118 簟8401 踮8558diao碉2179 叼2180 雕2181 凋2182 刁2183 掉2184 吊2185钓2186 调2187 铞7886 铫7902 貂8585 鲷8684die跌2188 爹2189 碟2190 蝶2191 迭2192 谍2193 叠2194垤5976 堞6006 揲6273 喋6409 牒7526 瓞8012 耋8183蹀8562 鲽8688ding丁2201 盯2202 叮2203 钉2204 顶2205 鼎2206 锭2207定2208 订2209 仃5674 啶6404 玎7164 腚7575 碇7754町7814 铤7890 疔8059 耵8184 酊8490diu丢2210 铥7891dong东2211 冬2212 董2213 懂2214 动2215 栋2216 侗2217恫2218 冻2219 洞2220 垌5977 咚6343 岽6520 峒6528氡7517 胨7543 胴7556 硐7747 鸫8020dou兜2221 抖2222 斗2223 陡2224 豆2225 逗2226 痘2227蔸6190 窦8128 蚪8229 篼8391du都2228 督2229 毒2230 犊2231 独2232 读2233 堵2234睹2235 赌2236 杜2237 镀2238 肚2239 度2240 渡2241妒2242 芏6022 嘟6429 渎6834 椟7292牍7525 蠹8328笃8338 髑8739 黩8782duan端2243 短2244 锻2245 段2246 断2247 缎2248 椴7318煅7649 簖8393dui堆2249 兑2250 队2251 对2252 怼7701 憝7713 碓7752镦7970dun墩2253 吨2254 蹲2255 敦2256 顿2257 囤2258 钝2259盾2260 遁2261 沌6771 炖7632 砘7727 礅7766 盹7779趸8527掇2262 哆2263 多2264 夺2265 垛2266 躲2267 朵2268跺2269 舵2270 剁2271 惰2272 堕2273 咄6345 哚6365沲6785 缍7122 催柁7262 铎7876 裰8154 踱8566e蛾2274 峨2275 鹅2276 俄2277 额2278 讹2279 娥2280恶2281 厄2282 扼2283 遏2284 鄂2285 饿2286 噩5612谔5844 垩5949 苊6035 莪6113 萼6164 呃6332 愕6721阏6753 屙6977 婀7025 轭7378 腭7581 锇7916 锷7941鹗8042 颚8206 鳄8689ei诶5832en恩2287 蒽6176 摁6284而2288 儿2289 耳2290 尔2291 饵2292 洱2293 二2294贰2301 佴5706 迩6939 珥7177 铒7879 鸸8025 鲕8660fa发2302 罚2303 筏2304 伐2305 乏2306 阀2307 法2308珐2309 垡5950 砝7732fan藩2310 帆2311 番2312 翻2313 樊2314 矾2315 钒2316繁2317 凡2318 烦2319 反2320 返2321 范2322 贩2323犯2324 饭2325 泛2326 蕃6212 蘩6232 幡6506 梵7283燔7660 畈7818 蹯8576fang坊2327 芳2328 方2329 肪2330 房2331 防2332 妨2333仿2334 访2335 纺2336 放2337 邡5890 彷6561 枋7242钫7853 舫8419 鲂8648fei菲2338 非2339 啡2340 飞2341 肥2342 匪2343 诽2344吠2345 肺2346 废2347 沸2348 费2349 芾6032 狒6584悱6713 淝6839 妃6990 绯7119 榧7328 腓7572 斐7619扉7673 镄7948 痱8082 蜚8267 篚8385 翡8468 霏8613鲱8678fen芬2350 酚2351 吩2352 氛2353 分2354 纷2355 坟2356焚2357 汾2358 粉2359 奋2360 份2361 忿2362 愤2363粪2364 偾5739 瀵6915 棼7291 鲼8687 鼢8787feng丰2365 封2366 枫2367 蜂2368 峰2369 锋2370 风2371疯2372 烽2373 逢2374 冯2375 缝2376 讽2377 奉2378凤2379 俸5726 酆5926 葑6155 唪6384 沣6767 砜7731fo佛2380fou否2381 缶8330fu夫2382 敷2383 肤2384 孵2385 扶2386 拂2387 辐2388幅2389 氟2390 符2391 伏2392 俘2393 服2394 浮2401涪2402 福2403 袱2404 弗2405 甫2406 抚2407 辅2408俯2409 釜2410 斧2411 脯2412 腑2413 府2414 腐2415赴2416 副2417 覆2418 赋2419 复2420 傅2421 付2422阜2423 父2424 腹2425 负2426 富2427 讣2428 附2429妇2430 缚2431 咐2432 匐5775 凫5776 郛5914 芙6029苻6062 茯6082 莩6119 菔6142 拊6252 呋6327 幞6505怫6686 滏6870 艴6985 孚7058 驸7066 绂7106 绋7108桴7285 赙7471 祓7680 砩7741 黻7774 黼7775 罘7823稃7991 馥8005 蚨8222 蜉8261 蝠8280 蝮8283 麸8479趺8535 跗8538 鲋8654 鳆8691ga噶2433 嘎2434 伽5704 尬6246 尕7056 尜7057 旮7424钆7837gai该2435 改2436概2437 钙2438 盖2439 溉2440 丐5604陔5875 垓5982 戤7414 赅7464gan干2441 甘2442 杆2443 柑2444 竿2445 肝2446 赶2447感2448 秆2449 敢2450 赣2451 坩5965 苷6053 尴6247擀6306 泔6779 淦6838 澉6887 绀7104 橄7347 旰7426矸7723 疳8065 酐8491gang冈2452 刚2453 钢2454 缸2455 肛2456 纲2457 岗2458港2459 杠2460 戆7716 罡7824 筻8364gao篙2461 皋2462 高2463 膏2464 羔2465 糕2466 搞2467镐2468 稿2469 告2470 睾5626 诰5830 郜5912 藁6227缟7141 槔7332 槁7334 杲7429 锆7915ge哥2471 歌2472 搁2473 戈2474 鸽2475 胳2476 疙2477割2478 革2479 葛2480 格2481 蛤2482 阁2483 隔2484铬2485 个2486 各2487 鬲5610 仡5678 哿5933 圪5957塥6010 嗝6435 纥7092 搿7501 膈7585 硌7749 镉7951袼8143 虼8220 舸8420 骼8732gei给2488gen根2489 跟2490 亘5608 茛6102 哏6371 艮8462geng耕2491 更2492 庚2493 羹2494 埂2501 耿2502 梗2503哽6376 赓6657 绠7114 鲠8665gong工2504 攻2505 功2506 恭2507 龚2508 供2509 躬2510公2511 宫2512 弓2513 巩2514 汞2515 拱2516 贡2517共2518 珙7178 肱7537 蚣8228 觥8601gou钩2519 勾2520 沟2521 苟2522 狗2523 垢2524 构2525购2526 够2527 佝5694 诟5824 岣6524 遘6960 媾7037缑7135 枸7259 觏7477 彀7616 笱8349 篝8384 鞲8724gu辜2528 菇2529 咕2530 箍2531 估2532 沽2533 孤2534姑2535 鼓2536 古2537 蛊2538 骨2539 谷2540 股2541故2542 顾2543 固2544 雇2545 嘏5637 诂5812 菰6152呱6341 崮6536 汩6773 梏7284 轱7379 牯7484 牿7486臌7591 毂7617 瞽7813 罟7825 钴7860 锢7932 鸪8019鹄8032 痼8083 蛄8233 酤8494 觚8593 鲴8681 鹘8729gua刮2546 瓜2547 剐2548 寡2549 挂2550 褂2551 卦5652诖5820 栝7273 胍7550 鸹8027guai乖2552 拐2553 怪2554 掴6266guan棺2555 关2556 官2557 冠2558 观2559 管2560 馆2561罐2562 惯2563 灌2564 贯2565 倌5736 莞6124 掼6272涫6842 盥7834 鹳8057 矜8170 鳏8704guang光2566 广2567 逛2568 咣6359 犷6578 桄7270 胱7555gui瑰2569 规2570 圭2571 硅2572 归2573 龟2574 闺2575轨2576 鬼2577 诡2578 癸2579 桂2580 柜2581 跪2582贵2583 刽2584 匦5648 刿5659 庋6649 宄6919 妫7003桧7277 炅7433 晷7448 皈8007 簋8394 鲑8657 鳜8712gun辊2585 滚2586 棍2587 衮5782 绲7121 磙7762 鲧8671guo锅2588 郭2589 国2590 果2591 裹2592 过2593 馘5769埚5986 呙6335 帼6494 崞6538 猓6603 椁7304 虢7529聒8188 蜾8268 蝈8269ha哈2594 铪7894hai骸2601 孩2602 海2603 氦2604 亥2605 害2606 骇2607嗨6443 胲7560 醢8516han酣2608 憨2609 邯2610 韩2611 含2612 涵2613 寒2614函2615 喊2616 罕2617 翰2618 撼2619 捍2620 旱2621憾2622 悍2623 焊2624 汗2625 汉2626 邗5885 菡6153撖6294 瀚6911 晗7447 焓7642 顸8192 颔8205 蚶8232鼾8793hang夯2627 杭2628 航2629 沆6776 绗7112 颃8194hao壕2630 嚎2631 豪2632 毫2633 郝2634 好2635 耗2636号2637 浩2638 蒿6179 薅6222 嗥6438 嚆6467 濠6909灏6916 昊7427 皓8009 颢8211 蚝8226he呵2639 喝2640 荷2641 菏2642 核2643 禾2644 和2645何2646 合2647 盒2648 貉2649 阂2650 河2651 涸2652赫2653 褐2654 鹤2655 贺2656 诃5813 劾5932 壑5954嗬6432 阖6756 曷7434 盍7833 颌8202 翮8471hei嘿2657 黑2658hen痕2659 很2660 狠2661 恨2662heng哼2663 亨2664 横2665 衡2666 恒2667 蘅6231 珩7181桁7276hong轰2668 哄2669 烘2670 虹2671 鸿2672 洪2673 宏2674弘2675 红2676 黉5768 訇5774 讧5807 荭6106 蕻6214薨6216 闳6740 泓6792hou喉2677 侯2678 猴2679 吼2680 厚2681 候2682 后2683堠6009 後6565 逅6943 瘊8090 篌8383 糇8455 鲎8655骺8731hu呼2684 乎2685 忽2686 瑚2687 壶2688 葫2689 胡2690蝴2691 狐2692 糊2693 湖2694 弧2701 虎2702 唬2703护2704 互2705 沪2706 户2707 冱5792 唿6392 囫6481岵6518 猢6609 怙6679 惚6717 浒6816 滹6879 琥7190槲7346 轷7385 觳7618 烀7635 煳7646 戽7670 扈7672祜7679 瓠8013 鹕8041 鹱8055 笏8343 醐8513 斛8590hua花2708 哗2709 华2710 猾2711 滑2712 画2713 划2714化2715 话2716 骅7072 桦7275 砉7725 铧7892huai槐2717 徊2718 怀2719 淮2720 坏2721 踝8555huan欢2722 环2723 桓2724 还2725 缓2726 换2727 患2728唤2729 痪2730 豢2731 焕2732 涣2733 宦2734 幻2735郇5908 奂5928 萑6140 擐6307 圜6487 獾6621 洹6801浣6829 漶6881 寰6930 逭6953 缳7157 锾7944 鲩8673鬟8763huang荒2736 慌2737 黄2738 磺2739 蝗2740 簧2741 皇2742凰2743 惶2744 煌2745 晃2746 幌2747 恍2748 谎2749隍5882 徨6569 湟6850 潢6874 遑6956 璜7211 肓7533癀8105 蟥8308 篁8382 鳇8692hui灰2750 挥2751 辉2752 徽2753 恢2754 蛔2755 回2756毁2757 悔2758 慧2759 卉2760 惠2761 晦2762 贿2763秽2764 会2765 烩2766 汇2767 讳2768 诲2769 绘2770诙5822 茴6078 荟6086 蕙6205 咴6352 哕6360 喙6425隳6736 洄6807 浍6811 彗6971 缋7132 珲7185 晖7445恚7703 虺8219 蟪8319 麾8766hun荤2771 昏2772 婚2773 魂2774 浑2775 混2776 诨5827馄6638 阍6752 溷6867huo豁2777活2778 伙2779 火2780 获2781 或2782 惑2783霍2784 货2785 祸2786 劐5669 藿6229 攉6311 嚯6475夥6623 钬7856 锪7933 镬7976 耠8175 蠖8322ji击2787 圾2788 基2789 机2790 畸2791 稽2792 积2793箕2794 肌2801 饥2802 迹2803 激2804 讥2805 鸡2806姬2807 绩2808 缉2809 吉2810 极2811 棘2812 辑2813籍2814 集2815 及2816 急2817 疾2818 汲2819 即2820嫉2821 级2822 挤2823 几2824 脊2825 己2826 蓟2827技2828 冀2829 季2830 伎2831 祭2832 剂2833 悸2834济2835 寄2836 寂2837 计2838 记2839 既2840 忌2841际2842 妓2843 继2844 纪2845 丌5602 亟5629 乩5632剞5662 佶5705 偈5742 诘5821 墼5952 芨6024 芰6033荠6089 蒺6180 蕺6210 掎6265 叽6320 咭6350 哜6366唧6383 岌6507 嵴6553 洎6809 屐6976 骥7087 畿7160玑7165 楫7314 殛7374 戟7410 戢7411 觊7473 犄7487齑7620 矶7722 羁7831 嵇7990 稷8002 瘠8104 虮8217笈8337 笄8339 暨8463 跻8550 跽8553 霁8611 鲚8661鲫8674 髻8757 麂8768jia嘉2846 枷2847 夹2848 佳2849 家2850 加2851 荚2852颊2853 贾2854 甲2855 钾2856 假2857 稼2858 价2859架2860 驾2861 嫁2862 郏5903 葭6171 岬6521 浃6804迦6940 珈7176 戛7409 胛7546 恝7702 铗7882 镓7956痂8072 瘕8093 袷8142 蛱8244 笳8353 袈8434 跏8542jian歼2863 监2864 坚2865 尖2866 笺2867 间2868 煎2869兼2870 肩2871 艰2872 奸2873 缄2874 茧2875 检2876柬2977 碱2878 硷2879 拣2880 捡2881 简2882 俭2883剪2884 减2885 荐2886 槛2887 鉴2888 践2889 贱2890见2891 键2892 箭2893 件2894 健2901 舰2902 剑2903饯2904 渐2905 溅2906 涧2907 建2908 僭5752 谏5841谫5857 菅6149 蒹6183 搛6286 囝6478 湔6853 蹇6931謇6932 缣7144 枧7237 楗7305 戋7407 戬7415 牮7480犍7489 毽7506 腱7576 睑7790 锏7921 鹣8047 裥8148笕8340 翦8469 趼8534 踺8561 鲣8668 鞯8721jiang僵2909 姜2910 将2911 浆2912 江2913 疆2914 蒋2915桨2916 奖2917 讲2918 匠2919 酱2920 降2921 茳6092洚6814 绛7113 缰7154 犟7481 礓7768 耩8180 糨8461豇8488jiao蕉2922 椒2923 礁2924 焦2925 胶2926 交2927 郊2928浇2929 骄2930 娇2931 嚼2932 搅2933 铰2934 矫2935侥2936 脚2937 狡2938 角2939 饺2940 缴2941 绞2942剿2943 教2944 酵2945 轿2946 较2947叫2948 窖2949佼5714 僬5753 艽6020 茭6090 挢6256 噍6461 峤6529徼6572 湫6848 姣7015 敫7524 皎8008 鹪8052 蛟8252醮8520 跤8551 鲛8662jie揭2950 接2951 皆2952 秸2953 街2954 阶2955 截2956劫2957 节2958 桔2959 杰2960 捷2961 睫2962 竭2963洁2964 结2965 解2966 姐2967 戒2968 藉2969 芥2970界2971 借2972 介2973 疥2974 诫2975届2976 拮6255喈6414 嗟6421 婕7028 孑7061 桀7278 碣7757 讦5806疖8060 颉8201 蚧8227 羯8441 鲒8658 骱8726jin巾2977 筋2978 斤2979 金2980 今2981 津2982 襟2983紧2984 锦2985 仅2986 谨2987 进2988 靳2989 晋2990禁2991 近2992 烬2993 浸2994 尽3001 劲3002 卺5865荩6103 堇6132 噤6468 馑6643 廑6659 妗7001 缙7138瑾7210 槿7340 赆7465 觐7478 衿8138jing荆3003 兢3004 茎3005 睛3006 晶3007 鲸3008 京3009惊3010 精3011 粳3012 经3013 井3014 警3015 景3016颈3017 静3018 境3019 敬3020 镜3021 径3022 痉3023靖3024 竟3025 竞3026 净3027 刭5657 儆5751 阱5869菁6128 獍6616 憬6729 泾6794 迳6941 弪6982 婧7026肼7534 胫7554 腈7570 旌7626 靓8606jiong炯3028 窘3029 迥6936 扃7671jiu揪3030 究3031 纠3032 玖3033 韭3034 久3035 灸3036九3037 酒3038 厩3039 救3040 旧3041 臼3042 舅3043咎3044 就3045 疚3046 僦5754 啾6417 阄6746 柩7249桕7274 鸠8015 鹫8053 赳8481 鬏8761ju鞠3047 拘3048 狙3049 疽3050 居3051 驹3052 菊3053局3054 咀3055 矩3056 举3057 沮3058 聚3059 拒3060据3061 巨3062 具3063 距3064 踞3065 锯3066 俱3067句3068 惧3069 炬3070 剧3071 倨5738 讵5810 苣6036苴6058 莒6076 掬6268 遽6965 屦6980 琚7202 椐7307榘7316 榉7323 橘7357 犋7488 飓7611 钜7850 锔7924窭8132 裾8153 趄8482 醵8522 踽8565 龃8620 雎8634瞿8636 鞫8722juan捐3072 鹃3073 娟3074 倦3075 眷3076 卷3077 绢3078鄄5918 狷6590 涓6824 桊7280 蠲7835 锩7935 镌7952 隽8633jue撅3079 攫3080 抉3081 掘3082 倔3083 爵3084 觉3085决3086 诀3087 绝3088 厥5642 劂5667 谲5860 矍5939蕨6207 噘6457 噱6469 崛6540 獗6617 孓7062 珏7169桷7286 橛7351 爝7663 镢7967 蹶8574 觖8591jun均3089 菌3090 钧3091 军3092 君3093 峻3094 俊3101竣3102 浚3103 郡3104 骏3105 捃6260 皲8168 筠8362麇8769ka喀3106 咖3107 卡3108 咯3109 佧5691 咔6339 胩7544kai开3110 揩3111 楷3112 凯3113 慨3114 剀5660 垲5978蒈6160 忾6673 恺6693 铠7888 锎7920 锴7939kan刊3115 堪3116 勘3117 坎3118 砍3119 看3120 侃5709莰6108 阚6759 戡7412龛7772 瞰7811kang康3121 慷3122 糠3123 扛3124 抗3125 亢3126 炕3127伉5688 闶6742 钪7854kao考3128 拷3129 烤3130 靠3131 尻6974 栲7264 犒7491铐7877ke坷3132 苛3133 柯3134 棵3135 磕3136 颗3137 科3138壳3139 咳3140 可3141 渴3142 克3143 刻3144 客3145课3146 嗑6430 岢6519 恪6701 溘6859 骒7076 缂7128珂7170 轲7380 氪7520 瞌7807 钶7861 锞7930 稞7993疴8066 窠8129 颏8204 蚵8234 蝌8282 髁8733肯3147 啃3148 垦3149 恳3150 裉8144keng坑3151 吭3152 铿7912kong空3153 恐3154 孔3155 控3156 倥5737 崆6539 箜8377kou抠3157 口3158 扣3159 寇3160 芤6050 蔻6202 叩6321眍7778 筘8356ku枯3161 哭3162 窟3163 苦3164 酷3165 库3166 裤3167刳5658 堀6005 喾6423 绔7111 骷8728夸3168 垮3169 挎3170 跨3171 胯3172 侉5708kuai块3173 筷3174 侩3175 快3176 蒯5665 郐5906 哙6364狯6586 脍7558kuan宽3177 款3178 髋8737kuang匡3179 筐3180 狂3181 框3182 矿3183 眶3184 旷3185况3186 诓5818 诳5831 邝5887 圹5959 夼6237 哐6349囗6477 纩7094 贶7460kui亏3187 盔3188 岿3189 窥3190 葵3191 奎3192 魁3193傀3194 馈3201 愧3202 溃3203 馗5624 匮5649 夔5771隗5883 蒉6162 揆6281 喹6413 喟6416 悝6706 愦6720逵6951 暌7450 睽7805 聩8189 蝰8281 篑8381 跬8545kun坤3204 昆3205 捆3206 困3207 悃6707 阃6745 琨7191锟7931 醌8511 鲲8679 髡8753kuo括3208 扩3209 廓3210 阔3211 蛞8250la垃3212 拉3213 喇3214 蜡3215 腊3216 辣3217 啦3218剌5661 邋6969 旯7425 砬7739 瘌8088lai莱3219 来3220 赖3221 崃6533 徕6566 涞6821 濑6894赉7467 赍7469 睐7789 铼7910 癞8114 籁8405lan蓝3222 婪3223 栏3224 拦3225 篮3226 阑3227 兰3228澜3229 谰3230 揽3231 览3232 懒3233 缆3234 烂3235滥3236 岚6516 漤6877 榄7313 斓7621 罱7829 镧7971褴8160lang琅3237 榔3238 狼3239 廊3240 郎3241 朗3242 浪3243莨6125 蒗6185 啷6405 阆6747 锒7922 稂7992 螂8275lao捞3244 劳3245 牢3246 老3247 佬3248 姥3249 酪3250烙3251 涝3252 唠6375 崂6532 栳7265 铑7878 铹7909痨8076 耢8176 醪8518le勒3253 乐3254 仂5676 叻6323 泐6778 鳓8706lei雷3255 镭3256 蕾3257 磊3258 累3259 儡3260 垒3261擂3262 肋3263 类3264 泪3265 羸5790 诔5819 嘞6447嫘7048 缧7148 檑7359 耒8171 酹8510leng棱3266 楞3267 冷3268 塄6008 愣6722li厘3269 梨3270 犁3271 黎3272篱3273 狸3274 离3275漓3276 理3277 李3278 里3279 鲤3280 礼3281 莉3282荔3283 吏3284 栗3285 丽3286 厉3287 励3288 砾3289历3290 利3291 傈3292 例3293 俐3294 痢3301 立3302粒3303 沥3304 隶3305 力3306 璃3307 哩3308 俪5719俚5721 郦5910 坜5962 苈6034 莅6116 蓠6181 藜6228呖6331 唳6406 喱6412 猁6591 溧6864 澧6902 逦6946娌7018 嫠7043 骊7074 缡7142 枥7232 栎7261 轹7386戾7669 砺7734 詈7826 罹7830 锂7914 鹂8031 疠806 1疬8063 蛎8235 蜊8259 蠡8327 笠8350 篥8386 粝8447醴8523 跞8540 雳8608 鲡8666 鳢8715 黧8783lia俩3309lian联3310 莲3311 连3312 镰3313 廉3314 怜3315 涟3316帘3317 敛3318 脸3319 链3320 恋3321 炼3322 练3323蔹6192 奁6238 潋6882 濂6905 琏7186 楝7312 殓7371臁7601 裢8145 裣8147 蠊8325 鲢8667liang粮3324 凉3325 梁3326 粱3327 良3328 两3329 辆3330量3331 晾3332 亮3333 谅3334 墚6014 椋7303 踉8552魉8743liao撩3335 聊3336 僚3337 疗3338 燎3339 寥3340 辽3341潦3342 了3343 撂3344 镣3345 廖3346 料3347 蓼6204尥6245 嘹6458 獠6618 寮6928 缭7152 钌7841 鹩8051lie列3348 裂3349 烈3350 劣3351 猎3352 冽5793 埒5988捩6270 咧6354 洌6803 趔8483 躐8581 鬣8764lin琳3353 林3354 磷3355 霖3356 临3357 邻3358 鳞3359淋3360 凛3361 赁3362 吝3363 蔺6194 啉6388 嶙6555廪6662 懔6733 遴6964 檩7361 辚7405 膦7602 瞵7812粼8452 躏8579 麟8775ling。
全国pos机代码查询

天津市 1200
山东省 3700
辽宁省 2100? ?
山西省 1400
河南省 4100
河北省 1300
吉林省 2200
黑龙江省 2300
陕西省 6100
甘肃省 6200
青海省 6300南省 4600
香港区 8100
澳门区 8200
上海市 2900
重庆市 5000
广东省 4400 广西区 4500 湖南省 4300 湖北省 4200 江苏省 3200 浙江省 3300 内蒙古区 1500 安徽省 3400 福建省 3500 江西省 3600 四川省 5100 贵州省 5200 云南省 5300 西藏区 5400 台湾省 7100
POS机消费单代码识别(商户代码)
每张POS单上都有一个编号,这个编号共有 15 位。 例如:69
-0569
3 位银行-4 位行政区划-4 位消费类型-4 位商户代码
69,代码含义: 102 银行收单,?1000 代表了北京,5812 公共饮食行业、餐馆,0569 商户代码全聚德
1、头三位代表收单银行
661
荷兰银行
662
荷兰安智银行股份有限公司
671
渣打银行
672
英国苏格兰皇家银行公众有限公司
691
法国(中国)有限公司
694
法国东方汇理银行股份有限公司
695
法国外贸银行股份有限公司
711
德国德累斯登银行股份公司
712
德意志银行(中国)有限公司
713
德国商业银行股份有限公司
714
德国西德银行股份有限公司
荷兰合作银行有限公司
781
厦门国际银行
782
18位身份证号码验证公式及标准

18位身份证号码验证工具
计算依据及说明
根据《中华人民共和国国家标准》(GB 11643-1999)规定:
公民身份号码是特征组合码,由十七位数字本体码和一位数字校验码组成。
排列顺序从左至右依次为:六位数字地址码,八位数字出生日期码,三位数字顺序码和一位数字校验码。
地址码(1-6位):表示编码对象常住户口所在县(市、旗、区)的行政区划代码
生日码(7-14位):表示编码对象出生的年、月、日,其中年份用四位数字表示,年、月、日之间不用分隔符。
例如:1981年05月11日就用19810511表示。
顺序码(15-17位):为同一地址码所标识的区域范围内,对同年、月、日出生的人员编定的顺序号。
其中奇数分给男性,偶数分给女性。
校验码(18位):是根据前面十七位数字码,按照ISO 7064:1983.MOD 11-2校验码计算出来的检验码。
第十八位数字的计算方法为:
1.将前面的身份证号码17位数分别乘以不同的权数。
从第一位到第十七位的权数分别为:7、9、10、5、8、4、2、1、6、3、7、9、10、5、8、4、2。
2.将这17位数字和其对应的权数分别相乘并加总。
3.用加总来和除以11,看余数是多少?
4余数只可能有0 1 2 3 4 5 6 7 8 9 10这11个数字。
其分别对应的最后一位身份证的号码为1 0 X 9 8 7 6 5 4 3 2。
5.通过上面得知,如果余数是2,就会在身份证的第18位数字上出现罗马数字的Ⅹ。
如果余数是10,身份证的最后一位号码就是2。
以上算法来源于网络,具体规范见国家相关标准。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
w
> Pergamon
0045-7949(95NO231-6
. ,
Compurm & Snucrurrr Vol. 59. No. I. pp. 131-140. 1996 CopyrIght Q.I 1996 Elsevier Science Ltd Printed in Great Bntain. All rights reserved 0045.7949/96 fl5.00 + 0.00
(Received 19 August 1994)
Abstract-An efficient method is developed for the analysis of scissor-link foldable structures. The stiffness matrix of a unit of such a structure, called a duplet, is derived and incorporated into a standard stiffness method. A computer program is developed and many examples are studied. The results are compared to the analysis using uniplet elements of Ref. [l]. Substantial improvement is obtained through the introduction of duplets in place of the use of uniplets.
1. INTRODUCTION
The need for mobile and reusable structures that are characterized by fast and easy erection procedures has existed for a long period. Such structures are often used in temporary construction industry, quick sheltering after natural disasters and in the aerospace industry. One of the most important foldable (deployable) structures is the scissor-link structure. The first such structure has been designed and constructed by Pinero [2]. Substantial contributions to the general understanding of geometric and kinematic behaviour of scissor-link structures is due to Escrig [3], and Escrig and Vaicarcel[4]. Further studies have been made by Zeigler [S], Derus [6], Nodskov [7], Gantes et al. [8], Rosenfeld et al. [9] and Shan [IO]. A pantograph is a foldable structure which consists of scissor-link units called duplets (see Fig. 1). Such a structure will be referred to as “p-structure” from now on. A duplet consists of two elements, called uniplets, which are capable of rotating about their intermediate pivot node, Fig. 2. Two uniplets which form a duplet are in fact beam elements with three nodes acting as pin-joints having only translational degrees of freedom. No torsion is produced in the members, however, axial forces and bending moments can be developed. A uniplet together with its degrees of freedom and the corresponding nodal forces are illustrated in Fig. 3. The stiffness matrix of a uniplet can be obtained by assembling first the stiffness matrix of two beam type elements, and then condensing and removing the rotational degrees of freedom of three nodes, Refs [I, lo]. The result is a 9 x 9 stiffness matrix for the uniplet element. This matrix is given in Appendix A. Once such a matrix is obtained, the analysis of p-structures follows a standard stiffness method. In this paper the stiffness matrix of a duplet is formulated using the stiffness matrices of its consti-
where d, and p2 are the force and displacement vectors of uniplet 2 in XJJZcoordinate system corresponding to uniplet 1. R and T are orthogonal transformation matrices. Using eqn (2) and eqn (5)
2. STIFFNESS MATRIX OF A DUPLET Consider a typical duplet element of a space pstructure, as shown in Fig. 4. The geometric properties of this element are illustrated in Fig. 4a and local and system coordinate are depicted in Fig 4b. The local coordinate system of uniplet l-S-2 is taken as xyz and that of uniplet 3-S-4 is considered as @.?. Both x and 2 axis are coincident with the axial longitudinal axes of the uniplets and the axes y-z and p-2 are parallel to the principal axes of the sections of uniplet 1-5-2 and 3-5-4, respectively. Obviously the plane x-y will coincide with plane a-j, and the angle between x and i in a counter clockwise direction is measured as 4. The nodal forces in local coordinate systems xyz and ,?j% are illustrated in Fig. 5, where the nodal forces in node 5 are eliminated in both uniplets for clarity. Uniplet (l-5-2) will be specified by index 1 and (3-5-4) will be denoted by index 2. The forcedisplacement relationships for these uniplets can be written as
tuting uniplets. For this purpose the stiffness matrices of uniplets with the angle (p between them are assembled in a 15 x 15 matrix. Then the translational degrees of the freedom of the connecting pivot node are condensed, in order to obtain a 12 x 12 stiffness matrix for a duplet element. A comparison of the efficiency of the analysis of p-structures using duplet and uniplet elements is presented in subsequent sections.
p-structure
system, and &, d2 are force and displacement vectors for uniplet (3-5-4) in system coordinate .@Z. These vectors are
