ASTM F1580-18外科植入物覆层用钛和钛-6铝-4钒合金粉末的标准规格
(完整版)钛标准大全-国标-美标-日标-德标-俄标
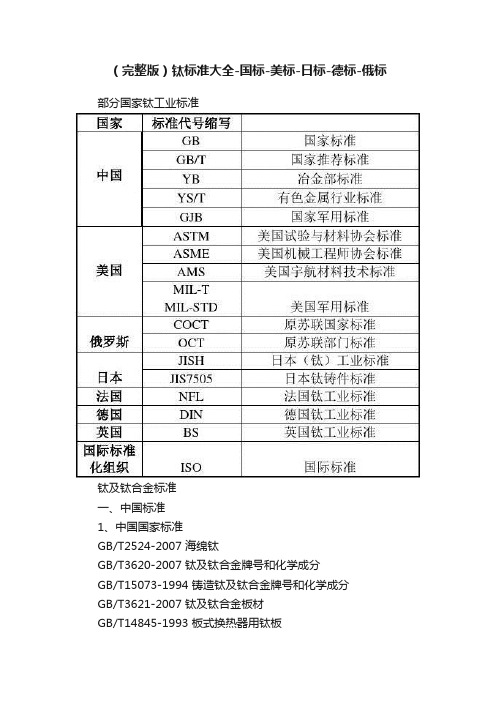
(完整版)钛标准大全-国标-美标-日标-德标-俄标部分国家钛工业标准钛及钛合金标准一、中国标准1、中国国家标准GB/T2524-2007 海绵钛GB/T3620-2007 钛及钛合金牌号和化学成分GB/T15073-1994 铸造钛及钛合金牌号和化学成分GB/T3621-2007 钛及钛合金板材GB/T14845-1993 板式换热器用钛板GB/T3622-1999 钛及钛合金带、箔材GB/T3623-2007 钛及钛合金丝材GB/T3624-2007 钛及钛合金管材GB/T3625-2007 换热器及冷凝器用钛及钛合金管GB/T2965-2007 钛及钛合金棒材GB/T16598-1996 钛及钛合金饼和环GB/T8546-1987 钛-不锈钢复合板GB/T8547-1987 钛-钢复合板GB/T6614-1994 钛及钛合金铸件GB/T5168-1985 两相钛合金高低倍组织检验方法GB/T6611-2008 钛及钛合金术语GB/T8755-2008 钛及钛合金术语金相图谱GB/T12769-2003 钛-铜复合棒GB/T13810-2007 外科植入物用钛及钛合金加工材GB/T12417-1990 外科金属植入物通用技术条件GB/T4698.1-4698.25-1996 海绵钛、钛及钛合金化学分析方法GB/T5193-2007 钛及钛合金加工产品超声波探伤方法GB/T12969.1-1991钛及钛合金管材超声波检验方法GB/T12969.2-1991 钛及钛合金管材涡流检验方法GB/T13149-1991 钛及钛合金符合钢板焊接技术条件GB/T6887-1986 烧结钛金属过滤元件和材料GB/T8180-2007 钛及钛合金加工产品的包装、标志、运输和贮存GB/T6612-1986 重要用途的TA7钛合金板材GB/T6613-1986 重要用途的TC4钛合金板材GB/T1216-1992 TA5钛合金焊接技术条件2、中国国家军用标准GJB2218-1994 航空用钛及钛合金棒材和锻坯规范GJB2219-1994 紧固件用钛及钛合金棒(线)规范GJB2220-1994 航空发动机用钛合金饼、环坯规范GJB2505-1995 航空用钛及钛合金板、带材规范GJB2744-1996 航空用钛及钛合金棒材和自由锻件和模锻件规范GJB2896-1996 钛及钛合金熔模精密铸件规范GJB2921-1997 超塑成形用TC4钛合金板材规范GJB3763A-2004 钛及钛合金热处理GJB391-1987 航天工业用TC4钛合金锻制饼材GJB493-1988 航空发动机叶片用TC4钛合金棒材GJB494-1988 航空发动机叶片用TC11钛合金棒材GJB495-1988 超低温用TA7-D钛合金棒材GJB943-1900 潜艇用TA5-A钛合金锻件GJB944-1900 TA5-A钛合金板材GJB1169-1991 航天用钛合金环材规范GJB1205-1991 TB2-1钛合金铆钉技术条件GJB1538-1992 飞机结构件用TC4钛合金棒材规范二、美国标准1、美国试验与材料协会标准ASTM B229-2001 海绵钛ASTM B265-2005 钛及钛合金带、薄板及板ASTM B337-1995 钛及钛合金无缝管和焊接管(已被B861-2002 钛及钛合金无缝管、B862-2002钛及钛合金焊接管代替)ASTM B338-2005a 钛及钛合金冷凝器和热交换器用无缝管和焊接管ASTM B348-2005 钛及钛合金棒和坯料ASTM B363-2004 非合金钛及钛合金无缝和焊接管件ASTM B367-2004 钛及钛合金铸件ASTM B861-2002 钛及钛合金无缝管ASTM B862-2002 钛及钛合金焊接管ASTM B381-2005 钛及钛合金锻件ASTM F67-2000 外科植入物用纯钛材ASTM F136-2002a 外科植入物用Ti-6Al-4V ELI加工材ASTM F620-2002 外科植入物用α+β相钛合金锻件ASTM F1108-2002 外科植入物用Ti-6Al-4V铸件ASTM F1295-2001 外科植入物用Ti-6Al-7Nb加工材ASTM F1341-1999 纯钛丝材ASTM F1472-2002a 外科植入物用Ti-6Al-4V加工材ASTM F1713-1996 外科植入物用Ti-13Nb-13Zr加工材ASTM F1813-2001 外科植入物用Ti-12Mo-6Zr-2Fe加工材ASTM F2063-2000 医疗器械和外科植入物用形状记忆合金加工材2、美国机械工程师协会标准ASME 第八部分:第一章压力容器(基本规则)美国宇航材料技术标准AMS 4900-2001 钛薄板、带和板材(退火状态)(380Mpa)AMS4901-2002 钛薄板、带和板材(退火状态)(485Mpa)AMS4902-2001 钛薄板、带和板材(退火状态)(275Mpa)AMS4907-2001 超低间隙元素级Ti-6Al-4V合金薄板、带和板材(退火状态)AMS4910-2003 Ti-5Al-2.5Sn合金薄板、带和中厚板(退火状态)AMS4911-2003 Ti-6Al-4V薄板、带和中厚板(退火状态)AMS4921-2004 钛的棒材、锻件和环件(退火状态)(485Mpa)AMS4924-2002 超低间隙元素级Ti-5Al-2.5Sn合金棒、锻件和环件(退火状态)AMS4926-2001 Ti-5Al-2.5Sn棒和环形件(退火状态)(760Mpa)AMS4928-2001 Ti-6Al-4V合金棒、锻件和环件(退火状态)(825Mpa)AMS4941-2003 钛焊管AMS4942-2001 无缝钛管(退火状态)(275Mpa)AMS4930-2001 超低间隙元素级Ti-6Al-4V合金棒材、锻件和环件(退火状态)AMS4951-2003 工业纯钛焊丝AMS4954-2003 Ti-6Al-4V合金焊丝AMS4965-2002 Ti-6Al-4V合金棒、锻件和环件(固溶和稳定化处理)AMS4966-2003 Ti-5Al-2.5Sn锻件AMS4967-2001 可热处理的Ti-6Al-4V合金棒、锻件和环件(退火状态)ASM4972-2003 Ti-8Al-1Mo-1V合金棒和环件(固溶和稳定化处理)ASM4973-2002 Ti-8Al-1Mo-1V钛合金锻件(固溶和稳定化处理)ASM4975-2003 Ti-6Al-2Sn-4Zr-2Mo合金棒和环件(固溶和稳定化处理)ASM4983-2002 Ti-10V-2F-3Al锻件(固溶处理和时效)ASM4985-2003 石蜡或石墨捣实法铸造的Ti-6Al-4V合金锻件ASM4991-2002 Ti-6Al-4V合金精锻件(退火状态)ASM2380-2003 优质钛合金认可和控制3、美国军用标准MIL-T-9046-1999 钛及钛合金薄板、带材和板材MIL-T-9047-2005 钛及钛合金棒材和锻坯MIL-R-81588-1986 钛及钛合金圆棒和丝MIL-F-83142-2000 钛及钛合金锻件(优质级)MIL-T-46077 钛合金可焊的装甲厚板MIL-T-13405 钛粉末MIL-T-46035-1989 高强度钛合金、变形材料MIL-T-81556-1996 钛及钛合金的圆棒、棒材、特殊形状面的挤压件MIL-T-81200 钛及钛合金的热处理三、英国标准BS2TA1:1974 工业纯钛的薄板和带(抗拉强度290-420Mpa)BS2TA2:1973 工业纯钛的薄板和带(抗拉强度390-540Mpa)BS2TA3:1973 机加工用的工业纯钛棒材和型材(抗拉强度390-540Mpa)BS2TA4:1973 工业纯钛的锻坯(抗拉强度390-540Mpa)BS2TA5:1973 工业纯钛的锻坯(抗拉强度390-540Mpa)BS2TA6:1973 工业纯钛的薄板和带(抗拉强度570-730Mpa)BS2TA7:1973 机加工用的工业纯钛棒材和型材(抗拉强度540-740Mpa)BS2TA8:1973 工业纯钛的锻坯(抗拉强度540-740Mpa)BS2TA9:1973 工业纯钛的锻件(抗拉强度540-740Mpa)BS2TA10:1974 钛-铝-钒合金的薄板和带材(抗拉强度960-1270Mpa)BS2TA11:1974 机加工用钛-铝-钒合金棒材和型材(抗拉强度900-1160Mpa)BS2TA12:1974 钛-铝-钒合金锻坯(抗拉强度900-1160Mpa)BS2TA13:1974 钛-铝-钒合金锻件(抗拉强度900-1160Mpa)BS2TA21:1973 钛-铜合金的薄板和带材(抗拉强度540-770Mpa)BS2TA22:1973 机加工用的钛-铜合金棒材和型材(抗拉强度540-770Mpa)BS2TA23:1973 钛-铜合金的锻坯(抗拉强度540-770Mpa)BS2TA24:1973 钛-铜合金的锻件(抗拉强度540-770Mpa)BS2TA28:1974 钛-铝-钒合金锻坯和丝材(抗拉强度1100-1300Mpa)BSTA38:1993 机加工用的钛-铝-钼-锡-硅-碳合金的棒材(抗拉强度1250-1420Mpa)BSTA39:1993 钛-铝-钼-锡-硅-碳合金的锻坯(抗拉强度1250-1420Mpa)BSTA40:1993 机加工用的钛-铝-钼-锡-硅-碳合金的棒材(抗拉强度1250-1375Mpa)BSTA41:1993 钛-铝-钼-锡-硅-碳合金的锻坯(抗拉强度1250-1375Mpa)BSTA42:1993 钛-铝-钼-锡-硅-碳合金的锻件(抗拉强度1250-1375Mpa)BSTA45:1993 机加工用的钛-铝-钼-锡-硅合金的棒材和型材(抗拉强度1100-1280Mpa)BSTA46:1993 机加工用的钛-铝-钼-锡-硅合金的棒材和型材(抗拉强度1050-1220Mpa)BSTA47:1993 钛-铝-钼-锡-硅合金的锻坯(抗拉强度1050-1220Mpa)BSTA48:1993 钛-铝-钼-锡-硅合金的锻坯(抗拉强度1050-1220Mpa)BSTA49:1993 机加工用的钛-铝-钼-锡-硅合金的棒材和型材(抗拉强度1000-1200Mpa)BSTA50:1993 钛-铝-钼-锡-硅合金的锻坯(抗拉强度1000-1200Mpa)BSTA51:1993 钛-铝-钼-锡-硅合金的锻件(抗拉强度1000-1200Mpa)BSTA52:1993 钛-铜合金的薄板和带材(抗拉强度690-920Mpa)BSTA56:1993 钛-铝-钒合金的厚板(抗拉强度895-1150Mpa)BSTA57:1993 钛-铝-钼-锡-硅的厚板(抗拉强度1030-1220Mpa)BSTA58:1993 钛-铜合金的厚板(抗拉强度520-640Mpa)BSTA100:1973 变形钛及钛合金的检验和实验方法BS5500:1997 无焰熔化焊压力容器CP3003 压力容器的衬里和化工用设备四、俄罗斯标准ΓOCT17746-79 海绵钛ΓOCT19807-91 变形钛及钛合金牌号ΓOCT22178-90 钛及钛合金薄板ΓOCT23755-87 钛及钛合金厚板ΓOCT21945-82 热轧无缝钛管ΓOCT22897-86 冷轧无缝钛管ΓOCT24890-81 焊接钛管ΓOCT26492-85 钛及钛合金轧棒ΓOCT27265-87 钛及钛合金填充丝说明书五、日本标准JISH2151-1983 海绵钛JISH4600-1993 钛及钛合金板和带JISH4630-1994 钛及钛合金无缝管JISH4631-1994 钛及钛合金热交换器用管JISH4635-1994 钛及钛合金焊接管JISH4650-2000 钛及钛合金棒JISH4657-1998 钛及钛合金锻件JISH4670-1993 钛及钛合金丝JIS7505 钛铸件六、德国标准DIN17850-1990 工业纯钛压力加工材的化学成分DIN17851-1990 钛合金压力加工材的化学成分DIN17860-1990 钛及钛合金板和带DIN17861-1990 钛及钛合金无缝管DIN17862-1990 钛及钛合金棒DIN17863-1973 钛及钛合金丝材DIN17864-1993 钛及钛合金锻件DIN17865-1990 铸钛DIN17866-1990 钛及钛合金焊接管DIN1737T1-1984 钛及钛钯合金填充材料的化学成分、技术条件DIN1737T2-1988 钛及钛钯合金填充材料全焊金属的试块、试样、力学与工艺性能DIN931 外六角螺栓半螺纹DIN933 外六角螺栓全螺纹DIN931 外六角螺母DIN125 普通垫片DIN127 弹簧垫片七、法国标准NFL21-110 1975 纯钛T40锻造用棒坯NFL21-270 1981 TA6V铆钉丝用杆材NFL14-601 1984 TA6V锻造用棒材NFL14-602 1984 TA6V锻件NFL14-603 1984 TA6V锻造用棒坯NFL14-604 1984 TA6V锻件NFL14-611 1984 TA6VZr5D棒坯NFL14-612 1984 TA6VZr5D锻件八、ISO国际标准(外科植入物用钛的标准)ISO5832-2-1999 纯钛ISO5832-3-1996 Ti-6Al-4V加工材ISO5832-11-1994 Ti-6Al-7Nb加工材。
2018年最新ASTM钛及钛合金中英对照标准汇编

目录1ASTM B265-15钛及钛合金带材、薄板和中厚板标准规范∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙1Standard Specification for Titanium and Titanium Alloy Strip,Sheet,and Plate∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙11 2ASTM B299-13海绵钛标准规范∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙23Standard Specification for Titanium Sponge∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙25 3ASTM B338-17冷凝器和热交换器用无缝和焊接钛及钛合金管标准规范∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙29Standard Specification for Seamless and Welded Titanium andTitanium Alloy Tubes for Condensers and HeatExchangers∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙39 4ASTM B348-13钛及钛合金棒材和坯锭标准规范∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙49Standard Specification for Titanium and Titanium Alloy Barsand Billets∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙57 5ASTM B363-14无缝和焊接的纯钛及钛合金焊接配件标准规范∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙65Standard Specification for Seamless and Welded UnalloyedTitanium and Titanium Alloy Welding Fittings∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙69 6ASTM B367-13(2017)钛及钛合金铸件标准规范∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙73Standard Specification for Titanium and Titanium Alloy Castings81 7ASTM B381-13钛及钛合金锻件标准规范∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙87Standard Specification for Titanium and Titanium Alloy Forgings95 8ASTM B481-68(2013)电镀用钛及钛合金制备标准∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙103Standard Practice for Preparation of Titanium and TitaniumAlloys for Electroplating∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙105 9ASTM B600-11(2017)钛及钛合金表面除鳞和清洁标准∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙109Standard Guide for Descaling and Cleaning Titanium andTitanium Alloy Surfaces∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙113 10ASTM B861-14钛及钛合金无缝管标准规范∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙117Standard Specification for Titanium and Titanium Alloy127Seamless Pipe∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙11ASTM B862-14钛及钛合金焊接管标准规范∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙137Standard Specification for Titanium and Titanium Alloy WeldedPipe∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙147 12ASTM B863-14钛及钛合金丝材标准规范∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙159Standard Specification for Titanium and Titanium Alloy Wire∙∙∙∙∙∙167 13ASTM B891-12具有强化表面以提高换热效果的冷凝器和换热器用钛及钛合金无缝管和焊管标准规范∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙175Standard Specification for Seamless and Welded Titanium andTitanium Alloy Condenser and Heat Exchanger Tubes WithEnhanced Surface for Improved Heat Transfer∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙183 14ASTM B977-13钛及钛合金铸锭标准规范∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙191Standard Specification for Titanium and Titanium Ingots∙∙∙∙∙∙∙∙∙∙∙∙∙197 15ASTM B988-13粉末冶金(PM)钛及钛合金结构件标准规范∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙205Standard Specification for Powder Metallurgy(PM)Titanium andTitanium Alloy Structural Components∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙209 16ASTM F67-13(2017)外科植入物用纯钛材标准规范(UNS R50250,UNS R50400,UNS R50550,UNS R50700)∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙213Standard Specification for Unalloyed Titanium,for SurgicalImplant Applications(UNS R50250,UNS R50400,UNSR50550,UNS R50700)∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙219 17ASTM F136-13外科植入物用锻制Ti-6Al-4V ELI(超低间隙)合金的标准规范(UNS R56401)∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙225Standard Specification for Wrought Titanium-6Aluminum-4Vanadium ELI(Extra Low Interstitial)Alloy for SurgicalImplant Applications(UNS R56401)∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙229 18ASTM F620-11(2015)外科植入物用α+β钛合金锻件标准规范∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙235Standard Specification for Titanium Alloy Forgings for SurgicalImplant in the Alpha Plus Beta Condition∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙239 19ASTM F1108-14外科植入物用铸造Ti-6Al-4V合金的标准规范(UNS R56406)∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙243Standard Specification for Titanium-6Aluminum-4VanadiumAlloy Castings for Surgical Implants(UNS R56406)∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙247 20ASTM F1295-16外科植入物用锻制Ti-6Al-7Nb合金的标准规范(UNS R56700)∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙253Standard Specification for Wrought Titanium-6Aluminum-7Niobium Alloy for Surgical Implant Applications(UNS R56700)∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙259 21ASTM F1472-14外科植入物用锻制Ti-6Al-4V合金的标准规范(UNS R56400)∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙265Standard Specification for Wrought Titanium-6Aluminum-4Vanadium Alloy for Surgical Implant Applications(UNSR56400)∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙269 22ASTM F1580-12外科植入物涂层用纯钛及Ti-6Al-4V合金粉末的标准规范∙∙∙∙∙∙275Standard Specification for Titanium and Titanium-6Aluminum-4Vanadium Alloy Powders for Coatings of Surgical Implants∙∙279 23ASTM F1709-97(2016)电子薄膜用高纯度钛溅射靶的标准规范∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙283Standard Specification for High Purity Titanium SputteringTargets for Electronic Thin Film Applications∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙285 24ASTM F1713-08(2013)外科植入物用锻制Ti-13Nb-13Zr合金的标准规范(UNS R58130)∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙289Standard Specification for Wrought Titanium-13Niobium-13Zirconium Alloy for Surgical Implant Applications(UNS R58130)∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙293 25ASTM F1813-13外科植入物用锻制Ti-12Mo-6Zr-2Fe合金的标准规范(UNS R58120)∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙299Standard Specification for Wrought Titanium-12Molybdenum-6Zirconium-2Iron Alloy for Surgical Implant(UNS R58120)∙∙303 26ASTM F2066-13ε1外科植入物用锻制Ti-15Mo合金的标准规范(UNS R58150)∙∙∙∙∙309Standard Specification for Wrought Titanium-15MolybdenumAlloy for Surgical Implant Applications(UNS R58150)∙∙∙∙∙∙∙∙∙∙∙∙315 27ASTM F2146-13外科植入物用锻制Ti-3Al-2.5V合金无缝管的标准规范(UNS R56320)∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙321Standard Specification for Wrought Titanium-3Aluminum-2.5Vanadium Alloy Seamless Tubing for Surgical ImplantApplications(UNS R56320)∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙∙325。
关节置换植入物材料、设计与临床应用相关标准综述

ISO 14630 中规定了临床评价的途径,即:a)与植入物 相似,或可证明相似的植入物的安全性、性能、设计特性和 预期使用有关的科学和临床文献的鉴定回顾;或 b)临床 研究的结果;或c)由以上 a)和 b)提供的临床数据的结合; d)分析从临床研究中获得的信息资料。与临床研究相关 的 ISO 标准为 ISO 14155 。 [31] 该标准的第 1、2 部分就制定 研究计划、临床研究等做出了详细规定。我国行标 YY/T 0297[32]等同采用该标准 1996 版。 1.4.3 上市后的监督
与设计相关的 ISO 标准包括:ISO 7206[20]的第 1、2 部分 和 ISO 7207[21]的第 1、2 部分。
在 ISO 7206-1 和 ISO 7207-1 中,对髋、膝关节假体以及 其部件给出了定义,详细叙述了分类方法,对每一类型给 出了示意图和尺寸标注要求。为降低人体运动过程中关 节面部件之间的磨损,在 ISO 7206-2 中规定了髋关节假体 关节面的尺寸公差、表面粗糙度以及球形径向偏差等;在 ISO 7207-2 中,规定了膝关节假体关节面的表面粗糙度。 这些要求在行标 YY 0118 和 YY 0502 中采用。 1.4 设计评价
IDT
羟基磷灰石涂层
ISO 13779-2[15]
GB 23101.2[16]
IDT
最新ASTM中文版标准目录

ASTM A105/A105M-2011a中文版管道部件用碳钢锻件ASTM A123/A123M-2012中文版钢铁产品镀锌层(热浸镀)标准规范ASTM A148/A148M-2008中文版结构用高强度钢铸件标准ASTM A181/A181M-2012中文版一般管道用碳钢锻件标准规范ASTM A217/A217M-2011中文版高温承压件用马氏体不锈钢和合金钢铸件标准规范ASTM A240/A240M-2012中文版压力容器和一般用途用铬及铬-镍不锈钢钢板、薄板和钢带标准技术条件ASTM A312/A312M-2009中文版无缝和焊接的以及重度冷加工奥氏体不锈钢公称管标准技术条件ASTM A351/A351M-2012中文版承压件用奥氏体铸钢件标准规范ASTM A370-2012中文版钢制品力学性能试验的标准试验方法和定义ASTM A388/A388M-2011中文版大型钢锻件超声检验标准操作方法ASTM A473-2001(R2009)中文版不锈钢锻件标准ASTM A48/A48M-2003(R2012)中文版灰铸铁铸件标准规范ASTM A494/A494M-2009e1中文版镍和镍合金铸件ASTM A510/A510M-2011中文版碳素钢盘条、粗圆钢丝和合金钢的一般要求的标准规范ASTM A579/A579M-2004a(R2009)中文版超高强度合金钢锻件的标准规范ASTM A615/A615M-2012中文版混凝土配筋用变形及光面碳素钢棒材的标准规范ASTM A706/A706M-2009b中文版混凝土加固用低合金钢变形及光面钢筋规范ASTM A743/A743M-2006(R2010)中文版一般用途的铁-铬、铁-铬-镍耐蚀钢铸件标准ASTM A745/A745M-2012中文版奥氏体钢锻件超声波检验ASTM A781/A781M-2012b中文版一般工业用钢和合金铸件通用要求标准规范ASTM A82/A82M-2007中文版混凝土钢筋用普通钢丝的标准规范ASTM A820/A820M-2004中文版钢钎维混凝土用钢纤维ASTM B111/B111M-2011中文版铜和铜合金无缝冷凝器管子和压盖坯料ASTM B117-2011中文版盐雾试验仪的标准操作规程ASTM B456-2011e1中文版铜镍铬和镍铬电镀涂层的标准规范ASTM B564-2011中文版镍合金锻件标准规范ASTM B575-2010中文版低碳镍-铬-钼、低碳镍-铬-钼-铜、低碳镍-铬-钼-钽、低碳镍-铬-钼-钨和低碳镍-钼-铬的合金板材、薄板和带材的标准规范ASTM B841-1999(R2010)中文版锌镍合金沉积物电镀层的标准规范ASTM C825-2006(R2011)中文版预制混凝土删栏标准规范ASTM D1153-2006中文版甲基异丁基酮的标准规范ASTM D1193-2006(R2011)中文版试剂级纯水的标准规范ASTM D130-2010中文版石油产品铜片腐蚀标准试验方法ASTM D1319-2010中文版荧光指示剂吸附法测定液体石油产品中烃类标准方法ASTM D1321-2010中文版石油蜡针入度测定的标准方法ASTM D1364-2002(R2012)中文版挥发性溶剂中水分的试验方法(费歇尔试剂滴定法)ASTM D1401-2012中文版石油和合成液的水分离性测定的标准方法ASTM D1403-2010中文版全尺寸锥体的1/2或1/4比例锥体刺入润滑脂来测定稠度的标准方法ASTM D1465-2010中文版石油蜡粘点和结点标准试验方法ASTM D1533-2012中文版卡尔•费休电量滴定法测定绝缘液中水含量的标准方法ASTM D1603-2012中文版烯烃塑料中炭黑含量测定的标准方法ASTM D1796-2011中文版离心法测定燃油中水和沉淀物的标准方法ASTM D1840-2007中文版紫外分光光度法测定航空涡轮燃料中萘系烃含量的标准方法ASTM D217-2010中文版润滑脂锥入度测定的标准方法ASTM D2269-2010中文版紫外吸光度评定白油的标准方法ASTM D2270-2010e1中文版根据40℃和100℃下运动粘度计算粘度指数的标准规程ASTM D2272-2011中文版润滑油氧化安定性的测定——旋转氧弹法标准试验方法ASTM D2274-2010中文版馏分燃料油氧化安定性测定的标准方法(加速法)ASTM D240-2009中文版用弹式量热计测定液烃燃料燃烧热的试验方法ASTM D2425-2004(2009)中文版质谱法测量中间馏分烃类组成的标准方法ASTM D2638-2010中文版用氦气体密度仪测定煅烧石油焦真密度的标准方法ASTM D2887-2008中文版用气相色谱分析法测定石油馏分沸程分布的标准试验方法ASTM D2896-2011中文版用高氯酸电位滴定法测定石油产品碱值的试验方法ASTM D3237-2012中文版原子吸收光谱法测定汽油中铅含量的试验方法ASTM D3242-2011中文版航空涡轮燃料酸度标准试验方法ASTM D3329-2003(2009)中文版用气相色谱法测定甲基异丁基酮纯度的试验方法ASTM D3606-2010中文版气相色谱法测定车用汽油和航空汽油中苯和甲苯含量的标准方法ASTM D381-2012中文版喷射蒸汽法测定燃料胶质含量的标准方法ASTM D3829-2002(2007)中文版预测发动机油边界泵送温度的标准方法ASTM D4292-2010中文版煅烧石油焦振动松密度的标准测试方法ASTM D4422-2003(2008)中文版石油焦分析中灰分的测试标准方法ASTM D4530-2011中文版微量法测定残炭的标准方法ASTM D473-2007 中文版抽提法测定燃油和原油中沉淀物的标准方法ASTM D4815-2009中文版气相色谱法测定汽油中甲基叔丁基醚、乙基叔丁基醚、叔戊基甲基醚、二异丙基醚、叔戊醇及C1-C4醇类的标准方法ASTM D4931-2006(2011)中文版生石油焦中总水分的标准测试方法ASTM D4952-2012中文版定性分析燃料和溶剂中活性硫的标准试验方法(Doctor试验)ASTM D5116-2010中文版通过小型环境室测定室内材料/产品的有机排放物的标准指南ASTM D524-2010中文版石油产品兰氏法测定残炭标准方法ASTM D5293-2010e1中文版用冷起动模拟装置测量-5至-30℃发动机油表观粘度的标准方法ASTM D5709-2009中文版石油焦筛析的标准测试方法ASTM D5762-2012中文版石油和石油产品中氮含量的测定舟进样化学发光法ASTM D6376-2010中文版通过波长色散X射线荧光谱测定石油焦中痕量金属的试验方法ASTM D6584-2010ae1中文版气相色谱法测定B-100生物柴油脂肪酸甲酯中游离甘油和总甘油含量的试验方法ASTM D892-2011a中文版润滑油泡末特性标准试验方法ASTM D893-2012中文版用过的润滑油不溶物测定法ASTM E10-2012中文版金属材料布氏硬度的标准试验方法ASTM E1316-2011b中文版无损检测的标准术语ASTM E140-2007中文版金属的标准硬度转换表布氏硬度、维氏硬度、洛氏硬度、表面硬度、努氏硬度和肖氏硬度之间的关系ASTM E165/E165M-2012中文版通用工业液体渗透检验的标准操作方法ASTM E1742/E1742M-2011中文版射线照相检测标准规程ASTM E18-2011中文版金属材料洛氏硬度标准测试方法ASTM E190-1992(R2008)中文版焊缝塑性的导向弯曲试验的标准试验方法ASTM E290-2009中文版金属材料延性弯曲试验的标准试验方法ASTM E317-2011中文版不采用电子测量仪器评价脉冲回波式超声检测系统工作性能的方法ASTM E384-2011e1中文版材料的努氏和维氏硬度标准试验方法ASTM E4-2010中文版测试仪力验正标准规程ASTM E428-2008中文版超声检测用钢质参考试块的制作与质量控制方法ASTM E587-2010中文版接触式超声斜射检测方法ASTM E8/E8M-2011中文版金属材料拉伸试验方法ASTM F136-2012中文版外科植入用Ti-6Al-4V ELI(超低间隙)锻造合金标准(UNS R56401) ASTM F138-2008中文版外科植入物用锻造18铬-14镍-2.5 钼不锈钢棒材和线材标准(UNS S31673)ASTM F1472-2008e1中文版外科植入物用锻造Ti-6Al-4V钛合金标准ASTM F1537-2011中文版外科植入物用锻造Co-28Cr-6Mo合金标准(UNS R31537,UNS R31538和UNS R31539)ASTM F1580-2012中文版外科植入物涂层用钛和Ti-6AL-4V合金粉末标准ASTM F620-2011中文版外科植入物用α+β态钛合金锻件标准ASTM F648-2010a中文版外科植入物用超高分子量聚乙烯粉末和制成物的标准规范ASTM F75-2012中文版外科植入物用Co-28Cr-6Mo合金铸件和铸造合金标准(UNS R30075) ASTM F799-2011中文版外科植入物用Co-28Cr-6Mo合金锻件标准(UNS R31537,R31538,R31539)。
ti6al4v标准

ti6al4v标准
Ti-6Al-4V钛合金是一种常用的钛合金材料,其标准如下:
1. 化学成分:Ti-6Al-4V钛合金的化学成分要求符合GB/T 3620.1的规定。
其中,钛(Ti)的含量为余量,铁(Fe)的含量不超过0.30%,碳(C)的含量不超过0.08%,氮(N)的含量不超过0.05%,氢(H)的含量不超过0.015%,氧(O)的含量不超过0.2%。
另外,铝(Al)的含量在5.5%~6.75%之间,钒(V)的含量在3.5%~4.5%之间。
其他元素的含量也有一定的限制,单个元素含量不超过0.1%,总和不超过0.4%。
2. 力学性能:Ti-6Al-4V钛合金的力学性能要求符合相关标准。
其中,屈服强度(σs/MPa)应不小于825,拉伸强度(σb/MPa)应不小于895,断后伸长率(δ5/%)应不大于10,断面收缩率(ψ/%)应不小于25。
3. 热处理工艺:Ti-6Al-4V钛合金的热处理工艺包括加热温度、保温时间和冷却方式等。
通常,加热温度为700℃~800℃,保温时间为1小时~3小时,冷却方式为空冷。
总的来说,Ti-6Al-4V钛合金具有优良的耐蚀性、较小的密度、高的比强度以及良好的韧性和焊接性等优点,被广泛应用于航空航天、石油化工、医疗器械等领域。
国内外医用钛及钛合金标准及性能

国内外医用钛及钛合金标准及性能发布时间:2010-4-17 10:20:42 中国废旧物资网一、钛在医学中的应用1、钛作为一种新兴的材料在我国及世界制药工业、手术器械、人体植入物等领域使用已有几十年的历史,并已取得了极大地成功。
2、人体内应外伤、肿瘤造成的骨、关节损伤,采用钛及钛合金可制造人工关节、接骨板和螺钉现已广泛用于临床。
还用于髋关节(包括股骨头)、膝关节、肘关节、掌指关节、指间关节、下頜骨、人造椎体(脊柱矫形器)、心脏起搏器外壳、人工心脏(心脏瓣膜)、人工种植牙、以及钛网在头盖骨整形等方面。
3、对于植入物材料的要求可以归为三个方面:材料与人体的生物相容性、材料在人体环境中的耐腐蚀性和材料的力学性能,作为长期植入材料有下列七项具体要求:①、耐蚀性;②、生物相容性;③、优越的力学性能和疲劳性能;④、韧性;⑤、低的弹性模量;⑥、在组合体中有好的耐磨性;⑦、令人满意的价格;4、外科植入物材料主要有:金属、聚合物、陶瓷等,金属材料又包括不锈钢、鈷基合金和钛基合金。
材料性能与骨性能的比较和植入物材料的特性比较见表一和表二。
从表二可以看出,不锈钢价格低廉,易于加工,但耐蚀性和生物相容性不如钛合金;鈷鉻合金的耐磨性比钛合金好,但密度较大,太重;钛及钛合金由于比强度高,生物相容性好及耐体液腐蚀性好等特点正日益受到重视。
钛合金的不足之处识是耐磨性差、难于铸造,加工性能也差。
二、国内外外科植入物用钛及钛合金加工材标准情况1、国外外科植入物用加工材标准纯钛:国际标准化组织 ISO 5832/2 1999E《外科植入物-纯钛加工材》美国标准:ASTM F67 2006a 《外科植入物用纯钛》TC4: 国际标准化组织 ISO 5832/3 1996Z 《外科植入物-金属材料-Ti-6Al-4V加工材》ASTM F1472 2002 《外科植入物用Ti-6Al-4V合金加工材》TC4ELI: ASTM F136 2002a 《外科植入物用Ti-6Al-4VELI(超低间隙)加工材规范》TC20: ISO 5832/11 I994(E) 《外科植入物-金属材料-Ti-6Al-7Nb合金加工材》ASTM F1295:2005《外科植入物用Ti-6Al-7Nb合金加工材》2、中国国家标准①、《外科植入物用钛及钛合金加工材》中国国家标准为GB/T13810-2007,牌号有:TA 1ELI、TA1、TA2、TA3、TA4、TC4、TC4ELI、TC20.品种有:板材0.8~25mm;棒材7.0~90mm;丝材1.0~7.0mm;GB\T13810-2007标准中规定的各项性能指标:②、GB/T13810-2007标准中,为了保证外科植入物用钛及钛合金加工材的综合性能(强度、塑性、韧性、硬度、抗疲劳等性能的合理匹配),对两相钛合金的高倍金相组织和氢含量及其它间隙元素含量都有非常严格的要求和控制。
ASTM F1580-18外科植入物覆层用钛和钛-6铝-4钒合金粉末的标准规格

Designation:F1580−18Standard Specification forTitanium and Titanium-6Aluminum-4Vanadium Alloy Powders for Coatings of Surgical Implants 1This standard is issued under the fixed designation F1580;the number immediately following the designation indicates the year of original adoption or,in the case of revision,the year of last revision.A number in parentheses indicates the year of last reapproval.A superscript epsilon (´)indicates an editorial change since the last revision or reapproval.1.Scope*1.1This specification covers the requirements for unalloyed titanium and Ti-6Al-4V alloy powders for use in fabricating coatings on titanium alloy implants.1.2Powders covered under this specification may be used to form coatings by sintering or thermal spraying techniques.1.3This specification covers powder requirements only.It does not address properties of the coatings formed from them.1.4Finely divided titanium powder may be considered pyrophoric and should be handled in accordance with the appropriate guidelines.1.5Units—The values stated in either SI units or inch-pound units are to be regarded separately as standard.The values stated in each system may not be exact equivalents;therefore,each system shall be used independently of the bining values from the two systems may result in non-conformance with the standard.1.6This international standard was developed in accor-dance with internationally recognized principles on standard-ization established in the Decision on Principles for the Development of International Standards,Guides and Recom-mendations issued by the World Trade Organization Technical Barriers to Trade (TBT)Committee.2.Referenced Documents2.1ASTM Standards:2B214Test Method for Sieve Analysis of Metal Powders B215Practices for Sampling Metal Powders B299Specification for Titanium SpongeE11Specification for Woven Wire Test Sieve Cloth and Test SievesE29Practice for Using Significant Digits in Test Data to Determine Conformance with SpecificationsE2371Test Method for Analysis of Titanium and Titanium Alloys by Direct Current Plasma and Inductively Coupled Plasma Atomic Emission Spectrometry (Performance-Based Test Methodology)F67Specification for Unalloyed Titanium,for Surgical Im-plant Applications (UNS R50250,UNS R50400,UNS R50550,UNS R50700)F981Practice for Assessment of Compatibility of Biomate-rials for Surgical Implants with Respect to Effect of Materials on Muscle and Insertion into BoneF1472Specification for Wrought Titanium-6Aluminum-4Vanadium Alloy for Surgical Implant Applications (UNS R56400)IEEE/ASTM SI 10American National Standard for Metric Practice2.2ISO Standards:3ISO 9001Quality Management System Requirements 2.3Aerospace Material Specifications:4AMS 2249Chemical Check Analysis Limits,Titanium and Titanium AlloysAMS 4998Powder,6Al-4V 3.Significance and Use3.1Coatings formed from metallic powders have become widely used as a means of improving tissue attachment to implants.Such coatings have also been demonstrated to improve bonding of acrylic cement to prostheses.This specification addresses the special requirements of the metal powders used to form these coatings.4.Ordering Information4.1Include with inquiries and orders for material under this specification the following information:4.1.1Quantity (weight),4.1.2ASTM specification and date of issue,1This specification is under the jurisdiction of ASTM Committee F04on Medical and Surgical Materials and Devices and is under the direct responsibility of Subcommittee F04.12on Metallurgical Materials.Current edition approved Nov.15,2018.Published December 2018.Originally approved in st previous edition approved in 2012as F1580-12.DOI:10.1520/F1580-18.2For referenced ASTM standards,visit the ASTM website,,or contact ASTM Customer Service at service@.For Annual Book of ASTM Standards volume information,refer to the standard’s Document Summary page on the ASTM website.3Available from American National Standards Institute (ANSI),25W.43rd St.,4th Floor,New York,NY 10036,.4Available from Society of Automotive Engineers (SAE),400Commonwealth Dr.,Warrendale,PA 15096-0001,.*A Summary of Changes section appears at the end of this standardCopyright ©ASTM International,100Barr Harbor Drive,PO Box C700,West Conshohocken,PA 19428-2959.United StatesThis international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for theDevelopment of International Standards,Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT)Committee.4.1.3Method of Manufacture—Type of powder(un-alloyed, sponge or Ti-6Al-4V),4.1.4Units to be Certified—SI or inch-pound,4.1.5Sieve analysis per7.1,4.1.6Powder cleanliness per8.2,4.1.7Special tests,if any,and4.1.8Other requirements.5.Methods of Manufacture5.1Powders may be manufactured by the plasma rotating electrode process,inert gas atomization,hydride-dehydride,or other method capable of producing powder meeting the re-quirements of this specification.6.Chemical Requirements6.1The chemical analysis of the powder shall conform to the requirements specified in Table1.6.1.1Requirements for the major and minor elemental constituents for unalloyed titanium and Ti-6Al-4V alloy pow-ders are listed in Table1.Also listed are all important residual elements.Analysis for elements not listed in Table1is not required to verify compliance with this specification.6.1.2All commercial metals contain small amounts of elements other than those which are specified.It is neither practical nor necessary to specify limits for unspecified elements,whether residual elements or trace elements.The producer is permitted to analyze for unspecified elements and is permitted to report such analyses.The presence of an unspecified element and the reporting of an analysis for that element shall not be a basis for rejection.6.1.3Intentional elemental additions other than those speci-fied in Table1are not permitted.6.2Product Analysis:6.2.1The product analysis tolerance shall conform to the requirements set forth in Table2.6.3For referee purposes,Test Method E2371shall be used.6.4Intentional elemental additions other than those speci-fied in Table1are not permitted.6.5For powder that includes particle size fractionsfiner than74µm(200mesh),the oxygen content limits shall be agreed upon between buyer and seller.7.Particle Size7.1Powder shall be sieved to the customer’s requirements with stainless steel screens conforming to Specification E11. Analysis of sieved powder for conformance to the customer’s particle size range requirements shall be in accordance with Test Method B214.8.Cleanliness8.1Powder shall be handled at all times so as to ensure freedom from contamination with nonmetallic materials or other metal alloy powders or both.8.2Powder cleanliness shall be determined by examining a representative sample,per Practices B215or as agreed upon between buyer and seller,comprising at least6.45cm2(1in.2) of a closely packed mono-layer of powder per lot at20×magnification.No foreign material shall be visible under these conditions.9.Dimensions and Permissible Variation9.1Units of Measure:9.1.1Selection—This specification requires that the pur-chaser selects the units(SI or inch-pound)to be used for product certification.In the absence of a stated selection of units on the purchase order,this selection may be expressed by the purchaser in several alternate forms listed in order of precedence.9.1.2If the purchaser and supplier have a history of using specific units,these units shall continue to be certified until expressly changed by the purchaser.TABLE1Chemical RequirementsElementUnalloyedTi Powder A%(mass/mass)Ti SpongePowder B%(mass/mass)Ti-6Al-4VPowder C%(mass/mass) Min Max Min Max Min MaxAl0.05 5.50 6.75 V 3.50 4.50 O0.400.40D0.20 Fe0.500.150.30 C0.080.030.08 H0.050.030.015 N0.050.020.05Cu0.10Sn0.10Si0.04Cl0.20ENa FY0.005C Ti balance G balance G balance GA Chemistry per Specification F67except hydrogen.B Chemistry per Specification B299,general purpose grade.C Chemistry per Specification F1472.D Oxygen per Specification B299is0.15%.This level is reasonable for sponge product but not for powder because of the increased surface area of small particle powder product.E Lower maximum chlorine content may be agreed upon between buyer purchaser and seller supplier.F Sodium or magnesium,0.50maximum.G Approximately equal to the difference of100%and the sum percentage of the other specified elements.The percentage of the titanium difference is not required to be reported.TABLE2Product Analysis Tolerances A Element Element Variation Under Min or OverMaxAluminum0.04Vanadium0.015Oxygen0.03BOxygen0.02CHydrogen0.002Iron0.10Carbon0.02Nitrogen0.02Copper0.05Tin0.15Silicon0.02Yttrium0.0005CA Refer to AMS2249.B Forunalloyed Ti powder.C For Ti-6Al-4V alloy powder.9.1.3In the absence of historic precedence,if the units used to define the product on the purchaser’s purchase order, specification,and engineering drawing are consistent,these units shall be used by the supplier for product certification.9.1.4If the purchaser’s selection of units is unclear,the units of measure shall be agreed upon between the purchaser and supplier.9.1.5Conversion of Units—If the supplier’s test equipment does not report in the selected units,the test equipment units may be converted to the selected units for certification pur-poses.Accurate arithmetic conversion and proper use of significant digits should be observed when performing this conversion.IEEE/ASTM SI10provides guidelines for the use of SI units.Annex A of IEEE/ASTM SI10provides conversion tables and Annex B of IEEE/ASTM SI10provides rules for conversion and significance.10.Significance of Numerical Limits10.1The following applies to all specified numerical limits in this specification.To determine conformance to these limits,an observed or calculated value shall be rounded to the nearest unit in the last right hand digit used in expressing the specification limit,in accordance with the rounding method of Practice E29.11.Certification11.1The supplier shall provide a certification that the material was tested in accordance with this specification and met all requirements.A report of the test results shall be furnished to the purchaser at the time of shipment.12.Quality Program Requirements12.1The supplier shall maintain a quality program,such as that defined in ISO9001or similar quality program.13.Keywords13.1coatings;metallic;metals(for surgical implants tita-nium alloys);orthopaedic medical devices(titanium/titanium alloys);powder;porous coatings;titanium/titanium alloys(for surgical implants)APPENDIXES (Nonmandatory Information) X1.RATIONALEX1.1Coatings formed from metallic powders have become widely used as a means of improving tissue attachment to uncemented orthopedic joint prosthesis.Such coatings have also been demonstrated to improve bonding of acrylic cement to prostheses.X1.2The biocompatibility of metallic implants is a direct function of their composition.The compositions of titanium and titanium alloy powders allowed by this specification have been used in wrought form for surgical implants and are in widespread commercial use for fabrication of porous coatings. X1.3Chemical composition limits for oxygen,iron,carbon, and nitrogen in the unalloyed grade are taken from Specifica-tion F67,Grade4.Limits for silicon,chlorine,hydrogen,and sodium are taken from Specification B299,Grade SL.X1.4Chemical composition limits for aluminum, vanadium,oxygen,iron,carbon,hydrogen,and nitrogen in the Ti-6Al-4V grade are taken from Specification F1472.Limits for copper and tin are taken from AMS4998.X1.5Product analysis tolerances are taken directly from AMS2249.No recognized product analysis tolerances cur-rently exist specifically for chlorine or sodium in titanium alloys.X1.6Processing aids are frequently used to facilitate pow-der processing and application of porous coatings to implant surfaces.It is beyond the scope of this specification to identify suitable processing aids or define their use.It is the responsi-bility of the implant manufacturer to ensure that any processing aid or residue of a processing aid has no detrimental effect on biocompatibility or coating properties.X1.7It should be recognized that the heat treatments used to form porous coatings can create microstructures that are substantially different from wrought titanium alloys.Porous coated implants also exhibit much greater surface area than monolithic implants.For these reasons,the biocompatibility and corrosion behavior must be characterized onfinished coatings.X1.8Likewise,these heat treatments can create microstruc-tures that give substantially different corrosion fatigue behavior from that of typical wrought titanium alloys.Corrosion fatigue behavior must be evaluated onfinished coated substrates.X1.9Pore size and morphology are important factors influ-encing tissue ingrowth and acrylic penetration of porous coatings.Particle size,size distribution,and shape are critical to controlling the pore size and morphology in thefinal coating.Particle size and size distribution are conventionally controlled by screening.The referenced ASTM International standards allow comparison of powder to a manufacturer’s specifications for a given coating process.A number of methods to characterize particle shape exists.The coating manufacturer should select a means of particle shape charac-terization suitable for this process.X1.10This specification requires sampling for particle size and powder cleanliness on each powder lot.In some cases, sampling on each shipping container of powder may beappropriate.X1.11Other process parameters are also critical to deter-mining final pore size and morphology in the final coating.Because these parameters are not directly related to the chemical and physical characteristics of the starting powder,they are not addressed in this specification.X1.12The requirements for powder cleanliness ensure free-dom from contaminants that might adversely affect either the biocompatibility or the finished coatings or the ability to bondthe coating properly during manufacturing.The method in 8.2(Practices B215)is commonly used for relatively coarse spherical powders used to fabricate sintered porous coatings.Other types of powders may require different methods for cleanliness characterization.The development and implemen-tation of such methods are the responsibility of the implant manufacturer.X2.BIOCOMPATIBILITYX2.1The alloy composition covered by this specification has a long history of successful clinical application in soft tissue and bone implants in humans,with a well-characterized level of biological response.X2.2No known surgical implant material has ever been shown to be completely free from adverse reactions in thehuman body.Long-term clinical experience of the use of the material referred to in this specification,however,has shown that an acceptable level of biological response can be expected,if the material is used in appropriate applications.SUMMARY OF CHANGESCommittee F04has identified the location of selected changes to this standard since the last issue (F1580–12)that may impact the use of this standard.(Approved Nov.15,2018.)(1)Updated to agree with the ASTM Subcommittee F04.12template language.(2)Added Section 4.(3)Revised Footnote G of Table 1with new template wording.(4)Revised Section 11due to addition of Section 4.ASTM International takes no position respecting the validity of any patent rights asserted in connection with any item mentioned in this ers of this standard are expressly advised that determination of the validity of any such patent rights,and the risk of infringement of such rights,are entirely their own responsibility.This standard is subject to revision at any time by the responsible technical committee and must be reviewed every five years and if not revised,either reapproved or withdrawn.Your comments are invited either for revision of this standard or for additional standards and should be addressed to ASTM International Headquarters.Your comments will receive careful consideration at a meeting of the responsible technical committee,which you may attend.If you feel that your comments have not received a fair hearing you should make your views known to the ASTM Committee on Standards,at the address shown below.This standard is copyrighted by ASTM International,100Barr Harbor Drive,PO Box C700,West Conshohocken,PA 19428-2959,United States.Individual reprints (single or multiple copies)of this standard may be obtained by contacting ASTM at the above address or at 610-832-9585(phone),610-832-9555(fax),or service@ (e-mail);or through the ASTM website ().Permission rights to photocopy the standard may also be secured from the Copyright Clearance Center,222Rosewood Drive,Danvers,MA 01923,Tel:(978)646-2600;/。
外科植入物 金属材料 第 11 部分:锻造钛-6 铝-7 铌合金-2023最新国标
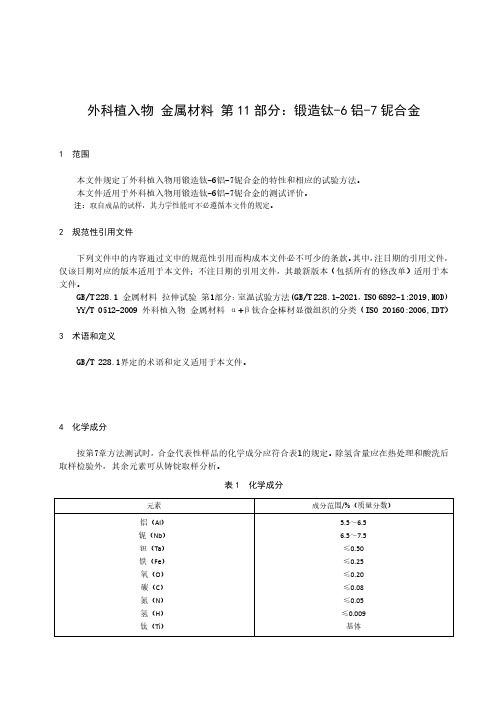
外科植入物金属材料第11部分:锻造钛-6铝-7铌合金1范围本文件规定了外科植入物用锻造钛-6铝-7铌合金的特性和相应的试验方法。
本文件适用于外科植入物用锻造钛-6铝-7铌合金的测试评价。
注:取自成品的试样,其力学性能可不必遵循本文件的规定。
2规范性引用文件下列文件中的内容通过文中的规范性引用而构成本文件必不可少的条款。
其中,注日期的引用文件,仅该日期对应的版本适用于本文件;不注日期的引用文件,其最新版本(包括所有的修改单)适用于本文件。
GB/T228.1金属材料拉伸试验第1部分:室温试验方法(GB/T228.1-2021,ISO6892-1:2019,MOD) YY/T0512-2009外科植入物金属材料α+β钛合金棒材显微组织的分类(ISO20160:2006,IDT)3术语和定义GB/T228.1界定的术语和定义适用于本文件。
4化学成分按第7章方法测试时,合金代表性样品的化学成分应符合表l的规定。
除氢含量应在热处理和酸洗后取样检验外,其余元素可从铸锭取样分析。
表1化学成分元素成分范围/%(质量分数)铝(Al)铌(Nb)钽(Ta)铁(Fe)氧(O)碳(C)氮(N)氢(H)钛(Ti)5.5~6.56.5~7.5≤0.50≤0.25≤0.20≤0.08≤0.05≤0.009基体5显微组织按表3的试验方法测试时,显微组织应为等轴α或拉长α组成的β转变组织,并未在原始β相晶界处析出连续网状α,退火态棒材横截面显微组织评级应符合附录A中图A1~图A9。
6力学性能按第7章的方法测试时,合金的力学性能应符合表2的规定。
表2退火态力学性能状态抗拉强度R m/MPa规定非比例延伸强度RP0.2/MPa伸长率A/%断面收缩率Z/%棒材a≥900≥800≥10≥25a最大直径或厚度=100mm。
如果有任一试验样品在标距范围内断裂并且不符合规定的性能要求,对于每件失效样品,应从同一批次中另取出两件试样,用同样的方法进行试验。
植入钛涂层标准

植入钛涂层标准全文共四篇示例,供读者参考第一篇示例:植入钛涂层标准是指对植入医疗器械进行表面处理的一种技术标准,它通过在器械表面涂覆一层钛材料,以增加器械在人体内的耐腐蚀性和生物相容性,提高器械的使用寿命和安全性。
植入钛涂层标准的制定和执行对于医疗器械行业具有重要意义,不仅可以保证植入器械的质量和性能,也可以保障患者的健康和安全。
植入钛涂层标准包括材料选择、涂层制备、涂层性能测试和产品质量控制等内容,具体的标准和要求可以根据不同类型的植入器械和具体的临床需求来制定。
在制定植入钛涂层标准时,需要考虑以下几个方面:首先是材料选择。
钛材料是一种优良的生物医用金属材料,具有良好的生物相容性和耐腐蚀性,适合用于植入器械的表面涂层。
在选择钛材料时,需要考虑其纯度、晶格结构和力学性能等因素,以确保其具有良好的性能和稳定性。
其次是涂层制备。
植入钛涂层标准需要明确涂层的制备方法和工艺流程,包括表面处理、涂覆技术、烧结工艺等环节。
涂层制备工艺的稳定性和重复性对于最终涂层的质量和性能至关重要,因此需要严格控制每一道工艺环节,确保涂层的厚度均匀、结合强度良好。
另外是涂层性能测试。
植入钛涂层标准需要规定涂层的性能测试方法和指标,包括耐腐蚀性、生物相容性、机械性能等方面。
通过对涂层的各项性能进行测试,可以评估其是否符合要求,并及时发现和解决潜在的质量问题。
最后是产品质量控制。
植入钛涂层标准需要规定产品的质量控制要求和检验方法,包括对成品植入器械和涂层质量的检测和审核。
在生产过程中,需要对关键环节和关键参数进行严格控制,确保产品的质量稳定和可靠。
植入钛涂层标准的制定和执行可以提高植入器械的品质和性能,保障患者的健康和安全。
医疗器械企业应该严格遵守相关标准和要求,加强质量管理和控制,不断提升自身技术水平和产品竞争力,为患者提供更加安全和可靠的医疗服务。
【2000字】第二篇示例:植入钛涂层是一种颇具前景和应用价值的表面技术,广泛应用于航空航天、汽车制造、医疗器械等领域。
钒系列产品标准
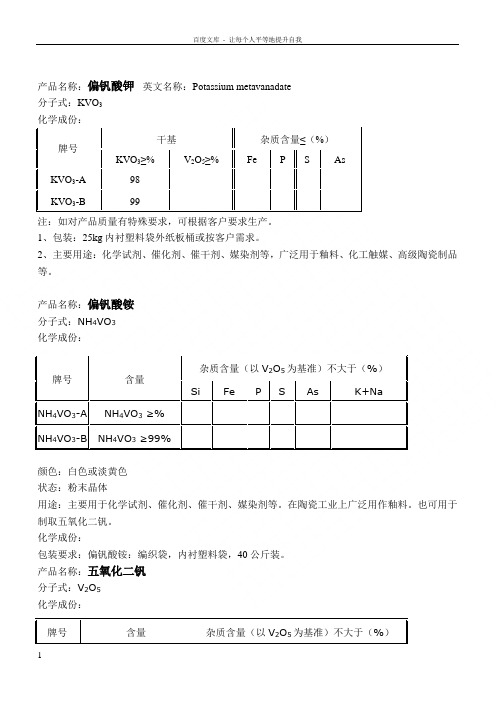
产品名称:偏钒酸钾 英文名称:Potassium metavanadate 分子式:KVO 3 牌号 干基杂质含量≤(%) KVO 3≥%V 2O 5≥%Fe P S As KVO 3-A 98 KVO 3-B99注:如对产品质量有特殊要求,可根据客户要求生产。
1、包装:25kg 内衬塑料袋外纸板桶或按客户需求。
2、主要用途:化学试剂、催化剂、催干剂、媒染剂等,广泛用于釉料、化工触媒、高级陶瓷制品 等。
产品名称:偏钒酸铵 分子式:NH 4VO 3 化学成份:颜色:白色或淡黄色 状态:粉末晶体用途:主要用于化学试剂、催化剂、催干剂、媒染剂等。
在陶瓷工业上广泛用作釉料。
也可用于制取五氧化二钒。
化学成份:包装要求:偏钒酸铵:编织袋,内衬塑料袋,40公斤装。
产品名称:五氧化二钒 分子式:V 2O 5 化学成份: 牌号 含量 杂质含量(以V 2O 5为基准)不大于(%) SiFe P S As K+Na NH 4VO 3-ANH 4VO 3 ≥%NH 4VO 3-B NH 4VO 3 ≥99%牌号含量杂质含量(以V 2O 5为基准)不大于(%)Si Fe P S As K+NaV2O5-A V2O5≥98%(片状)V2O5-B V2O5≥98%(粉状)V2O5-C V2O5≥99% (粉状)V2O5-C V2O5≥%(粉状)颜色:砖红色状态:粉状或片状用途:主要用于生产钒铁、钒铝合金、氮化钒、碳化钒等钒制品,并作为特种钢、合金钢和不锈钢的添加原料;在合成氨工业中用于脱碳、脱硫;用做印染、玻璃制造业及陶瓷工业中的着色剂;制备硫酸和石油化工生产中的催化剂、缓蚀剂;制备各种钒化合物,用于生产化肥、尼龙等产品还可以用于做钒电池等广大领域。
化学成份:包装要求:2)五氧化二钒:采用编织袋、纤维板桶和复合袋三种,内衬塑料袋。
编织袋净重40公斤或1000公斤装,纤维板桶和复合袋净重25公斤装。
3)需方如有特殊要求可以协商。
植入性医疗器械表面处理工艺规程

文件制修订记录1、目的:为消除内应力,提高表面性能,改善医用操作性。
2、范围:适用于III类植入性产品、人工关节(无菌)、及II类介入类产品手术入路器械(无菌和非无菌),采用外科植入物用不锈钢、外科植入物用钛合金加工材(TC4)金属材料所制造的零件的表面强化、钝化、洁净和除氢处理。
3、设备:3.1 CK900喷砂机。
3.2 非标清洗作业线。
3.3 101FAF-1型电热鼓风干燥箱。
3.4 ZLP78AK烘干消毒柜。
3.5 压缩空气净化系统W-1.47/7 空压机1-1.0MPa 储气罐HAD-1HTF 冷干机HF-C 过滤器3µHF-T 过滤器1µ。
3.6 0~300℃水银玻璃温度计。
3.7 1.10~1.20g/cm2密度计。
4、材料:4.1 硝酸。
分析纯GB626-89。
4.2 玻璃珠。
成球率〉80%。
4.3 纯化水。
5、工艺规程:5.1流程5.1.1钛合金零件表面处理流程:1.检查→2.强化→3.清洗→4.检验。
5.1.2不锈钢零件表面处理流程:1.检查→2.强化→3.清洗→4.检验→5.钝化→6.清洗→7.除氢处理→8.检验。
5.1.3不锈钢零件经抛光后的处理流程:1.检查→2.清洗→3.检验→4.钝化→5.清洗→6.除氢处理→7.检验。
5.2 工艺5.2.1检查:a) 零件外表面应光滑,不得有油污、锋棱、裂纹、毛刺、附着物等缺陷;b) 零件表面的粗糙度应符合相应的产品要求;c) 零件生产跟踪卡的上道工序有合格的检验结果和检验员签章。
5.2.2强化:在喷砂机中进行。
III类植入性产品:a)不锈钢零件表面强化:喷射材料:120#玻璃珠;喷射压力:4~5Kg/ cm2;喷射倾角:80~90º;喷射距离:100~150mm;喷射时间:一网筛零件不大于18min有效时间,符合6.a 要求为准。
b)钛合金零件表面强化:喷射材料:180#玻璃珠;喷射压力:5~6Kg/ cm2;喷射倾角:80~90º;喷射距离:100~150mm;喷射时间:一网筛零件不大于20min有效时间,符合6.a 要求为准。
ASTM_F136-02a(中文)

析方法。
7.3 为确保材料的化学分析样品在测试中具有代表性,化学分析取样时要特别小心,因为钛
与氧,氮和和氢等元素有很强的亲和力,因此,在切割化学分析样品时,应尽可能在没有灰
尘的环境中进行,切割工具也应洁净和锋利,用来测试的样品应放在合适的容其中。
8. 力学性能
8.1 按本规范供应的材料应符合表 3 中规定的力学性能要求。
生产商应该保持一质量程序,如 ASQ C1 中的定义的 12. 关键词 12.1 金属(外科植入物用):矫形医疗器械;钛合金;钛合金(外科植入物用)。
E8 金属材料拉伸试验方法 E120 钛及钛合金化学成分分析试验方法 E290 测定金属材料塑性的导向弯曲试验方法 E527 金属及合金代码的描述(UNS) E1409 根据惰性气体熔融技术,测定钛及钛合金中氧的试验方法 E1447 根据惰性气体熔融导热方法,测定钛及钛合金中氢的试验方法 E981 关于外科植入物材料对人体肌肉和骨头生物相容性的测定方法 2.2 ASO 标准 ASO C1 质量控制程序的通用技术要求 2.3 宇航材料技术规范: AMS 2249 钛及钛合金化学检验分析范围 AMS 4930 6Al-4V ELI 退火钛合金棒,锻件和环材 2.4 美国汽车工程师协会标准 SAE J1086 金属及合金代码描述(UNS) 3. 术语 3.1 本标准专用术语 3.1.1 批(lot),名词,由同一炉次熔炼,在同一条件下基本在同一时间生产的加工产品。 4. 产品分类 4.1 带材(strip)-厚度小于 0.1875 英寸(4.75mm),宽度小于 24 英寸(610mm)的产品。 4.2 薄板(sheet)-厚度小于 0.1875 英寸(4.75mm),宽度等于或大于 24 英寸(610mm) 的产品。 4.3 板(plate)厚度不小于 0.1875 英寸(4.75mm),宽度大于 10 英寸(254mm)的产品。 且宽度大于厚度的 5 倍,本规范适用于厚度不大于 4.00 英寸(101.60mm)的板材。 4.4 棒(bar)-直径或厚度从 0.1875 英寸(4.75mm)到 4.00 英寸(101.60mm)的圆棒和 扁棒(其他尺寸和形状为特殊订货)。 4.5 锻棒(forging bar)-按 4.4 所述的棒,用锻造方法生产,可以热轧加工态提供。 4.6 线(wire)-直径小于 0.1875 英寸(4.75mm)的圆形产品。 5. 订单说明 5.1 按本规范采购的材料订单应包括以下信息; 5.1.1 数量 5.1.2 ASTM 代号和发布日期; 5.1.3 形状(薄板,带材,板材,棒材,或线材); 5.1.4 状态(见 6.3) 5.1.5 力学性能(如果适用,对特殊状态);
18nicrmo6-4化学成分标准

18nicrmo6-4化学成分标准1. 前言18nicrmo6-4合金钢是一种常用的工程材料,具有较高的强度和韧性,在机械制造、航空航天、汽车制造等领域有着广泛的应用。
18nicrmo6-4合金钢的化学成分标准对其性能和用途具有重要意义,下面将对18nicrmo6-4合金钢的化学成分标准进行详细介绍。
2. 化学成分标准18nicrmo6-4合金钢的化学成分标准主要包括元素含量和相关标准。
2.1 元素含量根据国际标准,18nicrmo6-4合金钢的元素含量标准如下:- C:0.15-0.21%- Si:≤0.40%- Mn:1.30-1.60%- P:≤0.035%- S:≤0.035%- Cr:1.30-1.70%- Mo:0.15-0.25%- Ni:1.30-1.70%根据以上标准,18nicrmo6-4合金钢的化学成分主要含有碳、硅、锰、磷、硫、铬、钼和镍等元素,其中碳、硅、锰、磷和硫是常规杂质元素,而铬、钼和镍是主要合金元素。
2.2 相关标准除了元素含量之外,18nicrmo6-4合金钢的化学成分标准还包括相关的国际标准和行业标准。
国际标准主要包括ISO 683-1:2016(EN)和ISO 683-18:2014(EN)等,这些标准对18nicrmo6-4合金钢的化学成分、物理性能和机械性能等方面进行了详细的规定和说明。
行业标准主要包括汽车制造行业标准和航空航天行业标准等,这些标准是根据实际应用需求制定的,对18nicrmo6-4合金钢的化学成分标准有着具体的规定和要求。
3. 化学成分标准的意义18nicrmo6-4合金钢的化学成分标准对其性能和用途具有重要意义。
3.1 对性能的影响化学成分直接影响着合金钢的力学性能、物理性能和化学性能。
碳含量的增加可以提高合金钢的硬度和耐磨性,但会降低其韧性和塑性;铬、钼和镍等合金元素的含量则会提高合金钢的抗腐蚀性能和高温性能。
严格控制化学成分是保证18nicrmo6-4合金钢性能稳定和可靠的关键。
行业标准《金属注射成形钛及钛合金异形件》--编制说明(送审稿)

金属注射成形钛及钛合金异形件编制说明(送审稿)金属注射成形钛及钛合金异形件行业标准编制说明一、工作简况1.1 项目来源根据《工业和信息化部办公厅关于印发2019年第一批行业标准制修订和外文版项目计划的通知》(工信厅科函[2019]126号)文的要求,由广东省材料与加工研究所负责组织制定《金属注射成形钛及钛合金异形件》有色行业标准,项目计划编号为XX,计划完成年限:2020年。
1.2 本标准所涉及的产品简况钛材料具有比强度高、耐腐蚀性好、生物相容性优异等特点,广泛应用于航空航天、生物医用、石油化工等领域。
但是钛制品传统方法难以加工,导致生产成本一直居高不下,成为制约钛大规模应用的关键问题,如何发展低成本钛材料制品及其制备成形技术成为目前一个重要研究方向。
采用粉末冶金技术可以直接制备出具有或接近最终形状的零件,可避免或减少机加工,提高了材料产出,降低了制备成本。
其中金属粉末注射成形技术( Metal Injection Molding,简称MIM) 是将现代塑料注射成形技术引入粉末冶金领域,而形成的一种近终形成形技术。
它的基本工艺过程为: 将粉末与粘结剂均匀混合并制成粒状喂料,在注塑机上注射成形出所需零件形状,经脱除粘结剂后,高温烧结致密化而获得所需的零件。
粉末注射成形制备钛合金的优点在于:①可实现小型三维复杂形状零件的批量制备;②成分均匀,组织细小,力学性能优异;③易于添加合金元素和制备复合材料;④易于控制材料微观结构,可用于制备多孔钛合金材料。
因此,钛合金粉末注射成形技术是实现钛及其合金材料的低成本制备,推动合金实用化进程的有效途径。
1992 年,日本的Nippon Tungsten 公司制备出首件钛粉末注射成形产品。
随后美国、加拿大、新西兰、德国、中国等国家便相继大量开展了有关钛粉末注射成形技术的研究。
随着研究的深入,粉末注射成形钛制件的性能不断提高,有的甚至已与铸态、锻态合金相当。
采用粉末注射成形技术制备的钛产品涉及航空航天、兵器、医疗、汽车以及日常消费品领域。
用于骨科植入物涂层的钛粉和钛

用于骨科植入物涂层的钛粉和钛-6铝-4钒合金粉的标准规范1.范围:1.1 本标准规定了用于制备钛合金植入物涂层的非合金钛粉和Ti-6Al-4V合金粉的要求.1.2 所规定的粉末可采用烧结工艺或热喷涂工艺用来形成涂层.1.3 该标准规范仅仅规定了粉料的要求,未涉及用粉料制成的涂层的性能.2.引用文件2.1 ASTM标准B214 颗粒状金属粉料的筛分分析检测方法B215 多量金属粉末的取样方法B299 海绵钛规范E11 检测用金属丝网筛规范E120 钛和钛合金化学分析检测方法F67 用于外科植入物的非合金钛规范F981外科植入用生物材料与肌肉及骨骼材料效应相容性的评定F1472 外科植入物用煅制钛-6铝-4钒合金规范2.2 美国质量标准协会ASQ C1 质量程序通用要求2.3 空间材料规范AMS 2249 化学检测分析范围,钛和钛合金AMS 4998A 优质粉,6Al-4V,3.制造方法3.1 粉料可以采用等离子旋转电极法、惰性气体雾化法, 氢化-脱氢法或能生产满足本规范要求的粉料的其它方法.4.化学要求4.1 粉料的化学分析结果应符合表1所列规定的要求, 化学分析应在添加任何加工助剂之前进行.表1. 化学成分要求元素非合金Ti粉(wt%) Ti-6Al-4V粉(wt%)最小值最大值小值最大值Al 5.50 6.75V 3.50 4.50O 0.40 0.20Fe 0.50 0.30C 0.10 0.08H 0.05 0.015N 0.05 0.05Cu 0.10Sn 0.10Si 0.04Cl 0.20ANa 0.19ATi 基体B基体BA-更低的氯元素和钠元素的最大值含量可由买卖双方商定.B-钛含量由差值确定,不需要测定.4.1.1表1列出了对非合金化钛粉和Ti-6Al-4V合金粉的主要元素和次要元素的含量要求,也列元素小于最小值或大于最大值的偏差Al 0.04V 0.015O 0.03BO 0.02CFe 0.10H 0.002C 0.02N 0.02Cu 0.05Sn 0.15Si 0.02出了所有重要残余元素的要求.表1未列出的元素成分不要求与本规范相符.4.2 产品的分析误差应符合表2所列规定的要求表2. 产品分析误差AA-针对AMS 2249B-针对Ti粉C-针对Ti-6Al-4V粉4.3 作为参照,可采用检测方法E120.4.4 表1所列以外的有益元素不允许加入.4.5 对于粒度小于200目(74um)的粉料,氧含量极限可由供货商和客户商定.5.颗粒尺寸和形状5.1 颗粒按规范E11用不锈钢筛网筛分至符合顾客要求.按照顾客.筛分粉料的检验根据顾客粒度范围要求按试验方法B214进行.5.2 采用等离子旋转电极法、惰性气体雾化法制得的粉料多呈圆球形,采用氢化-脱氢法制得的粉料多呈棱角形,海绵状粉料多呈不规则形状.6.纯度6.1 粉料作任何处理时,应避免非金属材料或其它合金粉末或二者的污染.6.2 粉料纯度应依照实施细则B215通过检测典型试样确定,或由买卖双方商定.在这些条件下,无其它材料混入.粉料纯度在加入工艺添加剂之前确定.7.特殊要求7.1 被称作工艺添加剂的各种材料允许加入粉料中,以提高工艺性能.粉料供应商应在材料证明书中提供任何工艺添加剂的化学成分和重量百分含量.7.2 工艺添加剂应对最终涂层的耐腐蚀性和生物相容性没有不确定影响.注释1-8.说明书8.1 本规范涉及的粉料应在在说明书中附带以下内容:8.1.1 ASTM标准名称和发布日期8.1.2 质量(重量)8.1.3 制造方法8.1.4 化学成分,见4.18.1.5 粒度分布,见5.18.1.6 粉料纯度,见6.18.1.7 特殊要求,见7.18.1.8 其它要求9. 质量体系9.1 生产者应实施如ASQ C1所确定的质量体系9.2 粉料生产商的质量体系应确保外科植入物生产商与ASQ C1或其它通过认证的体系的要求相一致.10. 关键词10.1 涂层金属的金属(用于外科植入物的钛合金) 骨科医疗器械(钛/钛合金) 多孔涂层钛/钛合金(用于外科植入物)附录(非强制信息)X1. 一般说明X1.1。
钛合金Ti-6Al-4V材料参数

Titanium Ti-6Al-4V (Grade 5), Annealed Subcategory: Alpha/Beta Titanium Alloy; Metal; Nonferrous Metal; Titanium Alloy Close Analogs: 4 other heat treatments of this alloy are listed in MatWeb. Key Words: Ti-6-4; UNS R56400; ASTM Grade 5 titanium; UNS R56401 (ELI); Ti6Al4V, biomaterials, biomedical implants, biocompatibility
0.342 17 J 240 MPa 510 MPa 75 MPa-m� 44 GPa 550 MPa
0.342 12.5 ft-lb 34800 psi 74000 psi 68.3 ksi-in� 6380 ksi 79800 psi Ultimate shear strength V-notch at 1E+7 cycles. Kt (stress concentration factor) = 3.3 Unnotched 10,000,000 Cycles
Applications: Blades, discs, rings, airframes, fasteners, components. Vessels, cases, hubs, forgings. Biomedical implants. Biocompatibility: Excellent, especially when direct contact with tissue or bone is required. Ti-6Al-4V's poor shear strength makes it undesirable for bone screws or plates. It also has poor surface wear properties and tends to seize when in sliding contact with itself and other metals. Surface treatments such as nitriding and oxidizing can improve the surface wear properties.
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Designation:F1580−18Standard Specification forTitanium and Titanium-6Aluminum-4Vanadium Alloy Powders for Coatings of Surgical Implants 1This standard is issued under the fixed designation F1580;the number immediately following the designation indicates the year of original adoption or,in the case of revision,the year of last revision.A number in parentheses indicates the year of last reapproval.A superscript epsilon (´)indicates an editorial change since the last revision or reapproval.1.Scope*1.1This specification covers the requirements for unalloyed titanium and Ti-6Al-4V alloy powders for use in fabricating coatings on titanium alloy implants.1.2Powders covered under this specification may be used to form coatings by sintering or thermal spraying techniques.1.3This specification covers powder requirements only.It does not address properties of the coatings formed from them.1.4Finely divided titanium powder may be considered pyrophoric and should be handled in accordance with the appropriate guidelines.1.5Units—The values stated in either SI units or inch-pound units are to be regarded separately as standard.The values stated in each system may not be exact equivalents;therefore,each system shall be used independently of the bining values from the two systems may result in non-conformance with the standard.1.6This international standard was developed in accor-dance with internationally recognized principles on standard-ization established in the Decision on Principles for the Development of International Standards,Guides and Recom-mendations issued by the World Trade Organization Technical Barriers to Trade (TBT)Committee.2.Referenced Documents2.1ASTM Standards:2B214Test Method for Sieve Analysis of Metal Powders B215Practices for Sampling Metal Powders B299Specification for Titanium SpongeE11Specification for Woven Wire Test Sieve Cloth and Test SievesE29Practice for Using Significant Digits in Test Data to Determine Conformance with SpecificationsE2371Test Method for Analysis of Titanium and Titanium Alloys by Direct Current Plasma and Inductively Coupled Plasma Atomic Emission Spectrometry (Performance-Based Test Methodology)F67Specification for Unalloyed Titanium,for Surgical Im-plant Applications (UNS R50250,UNS R50400,UNS R50550,UNS R50700)F981Practice for Assessment of Compatibility of Biomate-rials for Surgical Implants with Respect to Effect of Materials on Muscle and Insertion into BoneF1472Specification for Wrought Titanium-6Aluminum-4Vanadium Alloy for Surgical Implant Applications (UNS R56400)IEEE/ASTM SI 10American National Standard for Metric Practice2.2ISO Standards:3ISO 9001Quality Management System Requirements 2.3Aerospace Material Specifications:4AMS 2249Chemical Check Analysis Limits,Titanium and Titanium AlloysAMS 4998Powder,6Al-4V 3.Significance and Use3.1Coatings formed from metallic powders have become widely used as a means of improving tissue attachment to implants.Such coatings have also been demonstrated to improve bonding of acrylic cement to prostheses.This specification addresses the special requirements of the metal powders used to form these coatings.4.Ordering Information4.1Include with inquiries and orders for material under this specification the following information:4.1.1Quantity (weight),4.1.2ASTM specification and date of issue,1This specification is under the jurisdiction of ASTM Committee F04on Medical and Surgical Materials and Devices and is under the direct responsibility of Subcommittee F04.12on Metallurgical Materials.Current edition approved Nov.15,2018.Published December 2018.Originally approved in st previous edition approved in 2012as F1580-12.DOI:10.1520/F1580-18.2For referenced ASTM standards,visit the ASTM website,,or contact ASTM Customer Service at service@.For Annual Book of ASTM Standards volume information,refer to the standard’s Document Summary page on the ASTM website.3Available from American National Standards Institute (ANSI),25W.43rd St.,4th Floor,New York,NY 10036,.4Available from Society of Automotive Engineers (SAE),400Commonwealth Dr.,Warrendale,PA 15096-0001,.*A Summary of Changes section appears at the end of this standardCopyright ©ASTM International,100Barr Harbor Drive,PO Box C700,West Conshohocken,PA 19428-2959.United StatesThis international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for theDevelopment of International Standards,Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT)Committee.1Copyright ASTM International。
