ch15-intermediate acct
高锰酸钾滴定法测定钙片中钙的含量

高锰酸钾滴定法测定钙片中钙的含量第38卷第4期辽宁化工Vo.l38,No.4 2009年4月L iaoning Chem ical Industry Apr i,l2009高锰酸钾滴定法测定钙片中钙的含量陈妙兰(广东省江门市质量技量监督检测所,广东江门529000)摘要:为了准确测定钙片中钙的含量,建立了高锰酸钾容量滴定法测定钙片中钙含量的方法。
实验样品经湿法消化后,在酸性溶液中,钙与草酸生成草酸钙沉淀。
沉淀洗涤后,加入硫酸溶解,将草酸游离出来,在70~80e条件下,用高锰酸钾标准溶液滴定与钙等当量结合的钙含量。
测定结果的回收率(R)在98.40%~109.74%。
用高锰酸钾滴定法测定钙片中的钙含量,简单、快捷、准确、干扰小。
关键词:高锰酸钾滴定法;测定;钙含量中图分类号:O655.2文献标识码:A文章编号:1004 0935(2009)04028603钙是人体健康不可缺少的重要元素。
钙不仅是构成人体骨骼和牙齿的主要成分,而且在神经冲动的传递、血液凝固、细胞粘着、酶促反应的激活等一系列的生理过程中,发挥极其重要的作用[1]。
根据1992年全国营养调查结果,全国平均每标准每日摄入钙405mg,仅达到RDA的50%左右(我国成人钙的RDA值为800mg),全国尤其是儿童青少年和老年人缺钙比例很高[2]。
为了补充钙,补钙类保健食品及补钙制品在国内外发展很快。
因此,钙是保健食品、钙制品及乳品中常规营养分析必须检测的质量指标,其中钙片也是如此,而准确提供钙片中钙的含量,也是衡量钙片制品质量的主要依据。
食品中钙含量的测定通常采用火焰原子吸收光谱法或EDTA滴定法测定[3-5]。
火焰原子吸收光谱法适宜测定钙含量较低(以mg/kg计)的含钙食品。
该法虽干扰小,速度快,效果好,但因仪器昂贵、操作技术难掌握,普通实验室难以普及应用。
对于含量较高的(以g/100g计)食品,国家标准GB/T5009.92-2003第二方法为EDTA容量滴定法,该法虽操作简单,但存在着干扰现象严重、终点变化不明显、指示剂水溶液不稳定(固体指示剂用量不易掌握)且易封闭等问题,使得测定结果的准确度不高[6];并且,使用剧毒的KCN 易导致环境污染。
美国特色产品说明:0215型设备中文版说明书

verviewWell-suited for low traffic areas, this unit is similar to the 0210 but reduced capacity to dispense 200 C-fold or 275 multi-fold paper towels without adjustment or adapters and is fitted with tumbler lock and heavy-duty stainless steel piano hinge.American Specialties, I nc. | 441 Saw Mill River Road, Yonkers, NY 10701 | (914) 476-9000 | Model:#0215Issued:11/1/2021Revised:3/10/2023Page:1 of 2Revision :R1uStainability /lEEDPaper Towel Dispenser - Petite (Multi - C-Fold) -Surface MountedToilet Accessories CSI:10 28 13MR 6 (1)EQ 4.1 (1)EQ 4.2 (1)EQ 4.4 (1)661_1O peratiOn & M aintenancet echnical i nfOrMatiOn / p rOduct p rOpertiesi nstallatiOnW arrantySurface mount unit on wall or partition using five #10 self-threading screws (by others) through concealed mounting holes provided. Four mounting holes through back are keyhole slots for ease in hanging unit on pre-installed screws. Center top mounting hole is horizontal slot for ease of adjustment and vandal resistant locking. For compliance with 2010 ADA Accessibility Standars install unit so that centerline of towel dispenser slot is 48" [1219] maximum above finished floor (AFF).Manufacturer reserves the right to make changes to the design, dimensions or functionality of the product without formal notice.Towels are self-feeding as withdrawn by hand until supply is depleted. Unit may be reloaded with a partial load in-place and will continue to feed properly. Low level of towel supply is indicated from 35% capacity to empty. Unit is a reduced-size model and has less capacity than larger models, therefore it is recommended to use this unit in space-restricted or low traffic areas. Locking door prevents unauthorized access or removal.One (1) Y ear from date of InvoiceAmerican Specialties, I nc. | 441 Saw Mill River Road, Yonkers, NY 10701 | (914) 476-9000 | Model:CSI:#021510 28 13Toilet Accessories Issued:11/1/2021Revised:3/10/2023Page:Revision :Type 304 Stainless Steel - Matte Black Powder Coated Concealed multi-staked hinge - 3/16" Dia. [Ø5]200 C-fold or 275 multi-fold paper towelsPropertyValueDimensions Construction 11" [279] W x 8" [203] H x 4" [102] DManual - Pull T ype Surface MountedN/A2 of 2R1Power Operation Mounting Capacity。
PROMEGA逆转录试剂盒说明书

Reverse Transcription System
INSTRUCTIONS FOR USE OF PRODUCT A3500.
1. Description..........................................................................................................1 2. Product Components and Storage Conditions ............................................2 3. Reverse Transcription Protocol.......................................................................2
Printed in USA. Revised 3/09 Part# TB099 Page 1
2.
Product Components and Storage Conditions
Size 100 reactions Cat. # A3500
Product Reverse Transcription System
3.A. Reverse Transcri magnesium concentration may be optimized for any given sequence to achieve better yields. **Final concentration of reaction components: 5mM MgCl2; 1X Reverse Transcription Buffer (10mM Tris-HCl [pH 9.0 at 25°C]; 50mM KCl; 0.1% Triton® X-100); 1mM each dNTP; 1u/μl Recombinant RNasin® Ribonuclease Inhibitor; 15u/μg AMV Reverse Transcriptase (High Conc.); 0.5μg Oligo(dT)15 Primer or Random Primers per microgram RNA; 50ng/μl 1.2kb Kanamycin Positive Control RNA, poly(A)+ mRNA or total RNA.
Extech PRC15 电流 电压计ibrador用户指南说明书

GUÍA DEL USUARIOCalibrador de Corriente / VoltajeModelo PRC15IntroducciónGracias por seleccionar el Modelo PRC15 de Extech. Este instrumento se embarca completamente probado y calibrado y con uso apropiado le proveerá muchos años de servicio confiable. Por favor visite el sitio web de Extech Instruments () para descargar la versión más reciente de esta Guía del Usuario. Extech Instruments es una compañía certificada ISO-9001. SeguridadSeñales internacionales de seguridadEsta señal adyacente a otra señal o terminal, indica que el usuario debe referirse al manualpara mayor información.Esta señal, adyacente a una terminal, indica que, bajo uso normal, pueden existir voltajespeligrososDoble aislanteNotas de seguridad∙No exceda la escala de entrada máxima permisible.∙Apague la unidad cuando el dispositivo no esté en uso.∙Quite las baterías del dispositivo si lo va a guardar más de 60 días.∙Nunca deseche las baterías en el fuego. Las baterías pueden explotar o derramar.∙Nunca mezcle tipos distintos de baterías. Siempre instale baterías nuevas del mismo tipo. Precauciones∙El uso inapropiado de este medidor puede causar daños, choque, lesiones o la muerte. Lea y comprenda este manual del usuario antes de operar este medidor.∙Quite siempre los cables de prueba antes del reemplazar la batería.∙Inspeccione la condición de los cables de prueba y el medidor mismo por daños antes de su operación. Repare o reemplace cualquier daño antes de usar.∙Si el equipo es usado en una manera no especificada por el fabricante, la protección suministrada por el equipo puede ser afectada.Descripción del medidor1. Enchufe de entrada adaptador CA2. Pantallayencendido3. Apagado4. Botones de flechas de ajuste de la fuente de salidaSET5. Botón6. Botón UNIT (mA ó %)7. Botón MODO I/O8. MEM (botón memoria STEP "paso")Retroiluminación/CERO9. Botón10. Enchufes de entrada de cables de prueba Disposición de pantalla1. Icono modo FUENTE2. Icono de estado función CERO3. Icono modo MEDICIÓN4. Icono de estado de apagado automático5. Icono de estado de la batería6. Valor modo de medición7. Icono unidades modo de medición8. Ubicación en memoria del registrador de datos9. Valor modo fuente10. Icono unidades modo FuenteTeclado Descripciones y operaciónBOTÓN DE ENCENDIDO Y FUNCIÓN DE APAGADO AUTOMÁTICO1. Use el botón POWER para encender y apagar la unidad, Cuando enciende la unidad, seejecuta una prueba autónoma corta y luego se estabilizará la pantalla.2. Cuando el símbolo de batería parpadea en la pantalla, reemplace la batería tan pronto seapráctico. La batería débil puede causar lecturas imprecisas y operación errática delmedidor.3. Este instrumento está equipado con apagado automático para apagar el medidor despuésde 10 minutos de inactividad. Para desactivar esta función; presione y sostenga el botón(POWER) de encendido hasta que se apague el icono “ATP”.Botón UNITPresione el botón UNIT en la función corriente para seleccionar las unidades mA o % o V o mV en la función voltaje. En el modo MEDICIÓN el voltaje es de escala automática.Botón I/OPresione momentáneamente el botón I/O para seleccionar ya sea la fuente (SOURCE) o medición (MEASURE) (entrada).Botón MODOEn modo MEDICIÓN, presione y sostenga el botón MODE (I/O) durante 1 segundo paraseleccionar la función corriente (mA/%) o voltaje (mV/V). Suelte el botón cuando aparezca la función deseada.BOTÓN (Retroiluminación)Presione el botón retroiluminación para encender o apagar la retroiluminación.CERO () botónEn el modo MEDICIÓN o GENERACIÓN, presione y sostenga el ZERO () botón durante 1 segundo para ajustar el medidor a cero.►◄▼ y ▲BotonesLos botones de flecha se usan para ajustar el valor de salida en modo fuente (SOURCE).1. Seleccione el modo fuente (SOURCE)2. Presione el botón ► o ◄ para seleccionar un digito para ajuste. El cursor subrayadodestella identifica el digito seleccionado.3. Presione el botón ▼ o ▲ para ajustar el valor del digito. Presione y sostenga el botón ▼ o▲ para ajustar rápidamente el valor.Botón SETEl botón SET se usa para pasar entre los 5 valores de salida guardados.1. Seleccione el modo fuente (SOURCE)2. Presione el botón SET y el valor guardado en el sitio 01 de la memoria se tomará comofuente. En la pantalla aparece "MEM.01”3. Cada vez que presione el botón SET pasa por los 5 sitios de memoria.4. Puede usar los botones de flecha para ajustar el valor en cada sitio de memoria.BOTÓN STEP/MEMEl botón "paso" STEP/MEM se usa para pasar automáticamente por los 5 valores de salidaguardados. El medidor se puede ajustar para un ciclo único de los valores guardados o para unciclo continuo.1. Seleccione el modo fuente (SOURCE)2. Presione y SOSTENGA el botón STEP/MEM. En pantalla aparecerá de manera alterna"STEPSS" (ciclo único) "STEPSC" (ciclo continuo). Suelte el botón al ver el modo deseado.3. En modo de ciclo único el medidor producirá (Fuente) la corriente o el voltaje indicado enMEM01 durante 5 segundos. Enseguida el medidor avanzará a MEM02 durante 5segundos. Esto continuará hasta MEM05 y luego regresará pos los sitios de memoria. Elciclo terminará al llegar a MEM01.4. En modo continuo el ciclo continuará hasta que sea detenido a mano.5. Presione momentáneamente el botón MEM para detener el ciclo. En pantalla aparecerábrevemente el indicador “END”.GUARDAR VALORES EN LA MEMORIALos valores predeterminados guardados en los sitios de memoria son:mA % mV V MemoriaLocalizaciónM1 4.00mA 0.0% 0mV 001V M2 8.00mA 25% 500mV 5V1001V M3 12.00mA 50% 1000mV1501V M4 16.00mA 75% 1500mVM5 20.00mA 100% 2000mV 20VPara cambiar los valores en memoria:1. Seleccione el modo fuente (SOURCE)2. Presione el botón SET para seleccionar en la memoria el sitio a cambiar.3. Presione los botones de flecha para ajustar el valor nuevo4. Presione momentáneamente el botón MEM para guardar el valor. El icono del sito enmemoria destella mientras se evalúa el valor.Modos de operaciónModo de operación (entrada) MEDIREn este modo, la unidad medirá hasta 50mADC o 20VCD.1. Encienda el medidor.2. En pantalla aparecerá "MEASURE".3. Presione y sostenga el botón MODE durante 1 segundo para seleccionar mA ó % ó mV4. Conecte el cable de calibración al medidor.5. Conecte el cable de calibración al dispositivo o circuito a prueba.6. Lea la medida en la pantalla LCD.Modo de Operación SOURCE (Fuente)En este modo la unidad puede suministrar corriente hasta 24mACD a 1000 ohmios o voltaje hasta 20.00V La corriente o voltaje se puede suministrar manualmente o en pasos desde la memoria como se explicó previamente.1. Encienda el medidor2. En pantalla aparecerá "MEASURE".3. Presione y sostenga el botón MODE durante 1 segundo para seleccionar mA ó % ó mV4. presione momentáneamente el botón “I/O” para seleccionar fuente (SOURCE).5. Presione el botón UNIT para seleccionar %/ mA o mV / V.6. Conecte el cable de calibración al medidor7. Conecte el cable de calibración al dispositivo o circuito a prueba8. Use los botones de flecha para ajustar el valor de salida deseado en el indicador inferior.El indicador superior indica el valor real de la corriente o voltaje que se suministra. Si lapantalla superior no es igual al valor establecido, ya sea las baterías necesitan serreemplazadas de la impedancia de carga está fuera del rango especificado.Soporte inclinado / ColgadorEl soporte trasero proporciona dos métodos para comodidad en la visualización.1. Tire de la parte inferior del soporte hacia fuera para colocar la unidad sobre una superficieplana para su visualización.2. Tire hacia fuera de las partes inferior y superior del soporte, y luego gire el soporte paracolgar la unidad.Reemplazo de la bateríaCuando el icono de la batería aparece en la pantalla, debe reemplazar las seis pilas AA.El compartimiento de la batería se localiza en la parte posterior del medidor.1. Abra el soporte inclinado, afloje el tornillo cabeza Philips y quite la tapa de la batería.2. Quite y reemplace las baterías, observando la polaridad.3. Reemplace y asegure la tapa de la batería.Usted, como usuario final, está legalmente obligado (Reglamento de baterías) aregresar todas las baterías usadas; ¡el desecho en el desperdicio o basura de lacasa está prohibido! Usted puede entregar sus baterías en los centros de recolecciónde su comunidad o donde sea que se venden las baterías.Desecho: Cumpla las estipulaciones legales vigentes respecto al desecho del dispositivoal final de su vida útil.Recordatorios de seguridad de baterías∙Por favor deseche las baterías responsablemente; siempre observe las normas locales, estatales y federales referentes al desecho de baterías.∙Nunca deseche las baterías en el fuego. Las baterías pueden explotar o derramar.∙Nunca mezcle diferentes tipos de baterías o nuevas y usadas. Siempre instale baterías nuevas del mismo tipo.EspecificacionesEspecificaciones generalesPantallaLCD matriz de puntos Carga Máxima1000 ohmios @ 24mA Tensión del medidor6 baterías AA o adaptador CA Apagado automático El medidor automáticamente se apaga después de 10 minutos deinactividadCapacidad de suministro de corriente 24mACD a 1000 ohmiosImpedancia voltaje de entrada 10k ohmios mínimoTemperatura de operación 5ºC a 40ºC (41ºF a 104ºF)Temperatura de almacenamiento -20o C a 60o C (-4o F a 140o F)Humedad de operación 80% máx. hasta 31°C (87°F) con disminución linear hasta 50% a40°C (104°F)Humedad de almacenamiento < 80%Altitud de operación 2000 metros (7000ft.) máximaDimensiones 159 x 80 x 44mm (6.3 x 3.2 x 1.7")Peso 234 g (8.3 oz.) no bateríasEspecificaciones de escalaModo Función Escala (resolución) Precisión (% de la lectura) Corriente 0 a 50mA (0.01mA) Porcentaje(%)-25 a 230%0.1%) 0 a 1999mV (1mV) MediciónCD Voltaje(escalaautomática)2 a 20V (0.01v) Corriente 0 a 24mA (0.01mA) Porcentaje(%)-25 a 125%0.1%) 0 a 2000mV (1mV)Suministro(fuente) CD Voltaje 0 a 20V (0.01v)± (0.01% + 1 dígito)Copyright © 2013 Extech Instruments Corporación (una empresa FLIR)Reservados todos los derechos, incluyendo el derecho de reproducción total o parcial en cualquier medio.ISO-9001 certified。
《中级会计学》Kieso_IFRS_TestBank_Ch02

CHAPTER 2CONCEPTUAL FRAMEWORK UNDERLYINGFINANCIAL ACCOUNTINGCHAPTER LEARNING OBJECTIVES1. Describe the usefulness of a conceptual framework.2. Describe efforts to construct a conceptual framework.3. Understand the objective of financial reporting.4. Identify the qualitative characteristics of accounting information.5. Define the basic elements of financial statements.6. Describe the basic assumptions of accounting.7. Explain the application of the basic principles of accounting.8. Describe the impact that constraints have on reporting accounting information.Test Bank for Intermediate Accounting: IFRS Edition2 - 2TRUE-FALSE—Conceptual1. The conceptual framework for accounting has been discovered through empirical research.2. A conceptual framework is a coherent system of interrelated objectives and fundamentalsthat can lead to consistent standards.3. The International Accounting Standards Board (IASB) uses a conceptual framework basedon individual concepts developed by each member of the standard-setting body.4. A soundly developed conceptual framework enables the International Accounting StandardsBoard (IASB) to issue more useful and consistent pronouncements over time.5. A soundly developed conceptual framework enables the International Accounting StandardsBoard (IASB) to quickly solve new and emerging practical problems by referencing basic theory.6. The IASB has issued a conceptual framework that is broadly consistent with that of theUnited States.7. The International Accounting Standards Board’s (IASB’s) Conceptual Framework includessupplementary information.8. The International Accounting Standards Board’s (IASB’s) Conceptual Framework includesthe elements of financial statements.9. The 2nd level of the IASB’s conceptual framework provides the qualitative characteristicsthat make accounting information useful and the elements of financial statements.10. One of the challenges in developing a common conceptual framework will be to agree onhow the framework should be organized since the FASB and IASB conceptual frameworks are organized in very different ways.11. The first level of the conceptual framework identifies the recognition and measurementconcepts used in establishing accounting standards.12. Decision usefulness is the underlying theme of the conceptual framework.13. Users of financial statements are assumed to have no knowledge of business and financialaccounting matters by financial statement preparers.14. The foundation of the International Accounting Standards Board’s (IASB’s) ConceptualFramework is found on the third level of the Framework and includes assumptions, principles, and constraints.15. An implicit assumption of the International Accounting Standards Board’s (IASB’s)Conceptual Framework is that users need to be experts in business and financial accounting matters to understand the information contained in financial statements.16. Relevance and reliability are the two primary qualities that make accounting informationuseful for decision making.Conceptual Framework Underlying Financial Accounting 2 - 3 17. The idea of consistency does not mean that companies cannot switch from one accountingmethod to another.18. Timeliness and neutrality are two ingredients of relevance.19. Verifiability and predictive value are two ingredients of reliability.20. The second level of the International Accounting Standards Board’s (IASB’s) ConceptualFramework serves as a bridge between the “why” of accounting and the “how” of accounting.21. In the International Accounting Standards Board’s (IASB’s) Conceptual Framew ork,qualitative characteristics are considered either relevant or prudent.22. In the International Accounting Standards Board’s (IASB’s) Conceptual Framework,qualitative characteristics distinguish better information from inferior information for decision-making purposes.23. In the International Accounting Standards Board’s (IASB’s) Conceptual Framework, anenhancing qualitative characteristic is predictive value.24. In the International Accounting Standards Board’s (IASB’s) Conceptual Framework,aningredient of a fundamental qualitative characteristic is understandability.25. To be a faithful representation as described by the International Accounting StandardsBoard’s (IASB’s) Conceptual Framework, information must be confirmatory.26. An enhancing quality as described by the International Accounting Standards Board’s(IASB’s) Conceptual Framework is comparability.27. Moon, Inc. applies different accounting treatments to similar events from period to period.Moon, Inc. is violating verifiability as described by the International Accounting Standards Board’s (IASB’s) Conceptual Framework.28. The International Accounting Standards Board’s (IASB) definition of retained earnings is“the residual interest in the assets of the entity after deducting all its liabilities.”29. The historical cost principle would be of limited usefulness if not for the going concernassumption.30. The economic entity assumption means that economic activity can be identified with aparticular legal entity.31. Materiality is one of the basic assumptions of accounting used by the InternationalAccounting Standards Board (IASB).32. Periodicity is one of the basic assumptions of accounting used by the InternationalAccounting Standards Board (IASB).33. Timeliness is one of the basic assumptions of accounting used by the InternationalAccounting Standards Board (IASB).Test Bank for Intermediate Accounting: IFRS Edition2 - 434. The periodicity basic assumptions of accounting (used by the International AccountingStandards Board) makes depreciation and amortization policies justifiable and appropriate.35. The IASB conceptual framework specifically identifies accrual basis accounting as one of itsfundamental assumptions.36. One of two assumptions made by the IASB conceptual framework is that the reporting entityis a going concern.37. The expense recognition principle states that debits must equal credits in each transaction.38. Revenues are realizable when assets received or held are readily convertible into cash orclaims to cash.39. Supplementary information may include details or amounts that present a differentperspective from that adopted in the financial statements.40. Companies consider only quantitative factors in determining whether an item is material.41. The International Accounting Standards Board has given companies the option of using fairvalue to report financial liabilities.42. Under International Financial Reporting Standards (IFRS) product costs are charged off inthe immediate period and period costs may be carried into future periods.43. Under International Financial Reporting Standards (IFRS) notes to the financial statementsmust qualify as an element.44. Under International Financial Reporting Standards (IFRS) supplementary information maybe information that is high in relevance but low in reliability.45. The cost-benefit constraint included in the International Accounting Standards Board’sconceptual framework states that financial information should be free from cost to users of the information.46. Th e International Accounting Standards Board’s (IASB) rule for materiality is any item under5% of net income is considered immaterial.47. The International Accounting Standards Board’s (IASB) conceptual framework includes theconcept of prudence or conservatism which means when in doubt, choose the solution that will be least likely to overstate assets or income and/or understate liabilities or expenses.48. Under International Financial Reporting Standards (IFRS) companies must consider bothquantitative and qualitative factors in determining whether an item is material.49. Under International Financial Reporting Standards (IFRS) companies need not reportimmaterial items within the body of the financial statements, but must disclose them in the notes or supplementary information that accompany the financial statements.50. The conceptual framework underlying U.S. GAAP is similar to that underlying IFRS.Conceptual Framework Underlying Financial Accounting 2 - 5MULTIPLE CHOICE—Conceptual51. A soundly developed conceptual framework of concepts and objectives shoulda. increase financial statement users' understanding of and confidence in financialreporting.b. enhance comparability among companies' financial statements.c. allow new and emerging practical problems to be more quickly solved.d. all of these.52. Which of the following (a-c) are not true concerning a conceptual framework in account-ing?a. It should be a basis for standard-setting.b. It should allow practical problems to be solved more quickly by reference to it.c. It should be based on fundamental truths that are derived from the laws of nature.d. All of the above (a-c) are true.53. What is a purpose of having a conceptual framework?a. To enable the profession to more quickly solve emerging practical problems.b. To provide a foundation from which to build more useful standards.c. Neither a nor b.d. Both a and b.S54. Which of the following is not a benefit associated with the FASB Conceptual Framework Project?a. A conceptual framework should increase financial statement users' understanding ofand confidence in financial reporting.b. Practical problems should be more quickly solvable by reference to an existingconceptual framework.c. A coherent set of accounting standards and rules should result.d. Business entities will need far less assistance from accountants because the financialreporting process will be quite easy to apply.Test Bank for Intermediate Accounting: IFRS Edition2 - 655. A soundly developed conceptual framework enables the International AccountingStandards Board (IASB) toI. Issue more useful and consistent pronouncements over time.II. More quickly solve new and emerging practical problems by referencing basic theory.a. I only.b. II only.c. Both I and II.d. Neither I nor II.56. In the conceptual framework for financial reporting, what provides "the why"--the goalsand purposes of accounting?a. Measurement and recognition concepts such as assumptions, principles, andconstraintsb. Qualitative characteristics of accounting informationc. Elements of financial statementsd. Objective of financial reporting57. The underlying theme of the conceptual framework isa. decision usefulness.b. understandability.c. reliability.d. comparability.58. What is the objective of financial reporting as indicated in the conceptual framework?a. provide information that is useful to those making investing and credit decisions.b. provide information that is useful to management.c. provide information about those investing in the entity.d. All of the above.59. The International Accounting Standards Board’s (IASB’s) Conceptual Framework includesall of the following except:a. Objective of financial reporting.b. Supplementary informationc. Elements of financial statements.d. Qualitative characteristics of accounting information.60. The second level in the International Accounting Standards Board’s (IASB’s) ConceptualFrameworka. Identifies the objective of financial reporting.b. Identifies recognition, measurement, and disclosure concepts used in establishing andapplying accounting standards.c. Provides the elements of financial statements.d. Includes assumptions, principles, and constraints.Conceptual Framework Underlying Financial Accounting 2 - 7 61. The objective of financial reporting in the Internatio nal Accounting Standards Board’s(IASB’s) Conceptual Frameworka. Is the foundation for the Framework.b. Includes the qualitative characteristics that make accounting information useful.c. Is found on the third level of the Framework.d. All of the choices are correct regarding the objective of financial reporting.62. An implicit assumption of the International Accounting Standards Board’s (IASB’s)Conceptual Framework is thata. Information must be decision-useful to all potential users of financial reporting.b. General-purpose financial reporting is the primary source of information for users offinancial reporting.c. Users need reasonable knowledge of business and financial accounting matters tounderstand the information contained in financial statements.d. All of the choices are correct.63. The overriding criterion by which accounting information can be judged is that ofa. usefulness for decision making.b. freedom from bias.c. timeliness.d. comparability.64. Which of the following is a fundamental quality of useful accounting information?a. Comparability.b. Relevance.c. Consistency.d. Materiality.65. Which of the following is a fundamental quality of useful accounting information?a. Conservatism.b. Comparability.c. Faithful representation.d. Consistency.66. What is meant by comparability when discussing financial accounting information?a. Information has predictive or feedback value.b. Information is reasonably free from error.c. Information that is measured and reported in a similar fashion across companies.d. Information is timely.67. What is meant by consistency when discussing financial accounting information?a. Information that is measured and reported in a similar fashion across points in time.b. Information is timely.c. Information is measured similarly across the industry.d. Information is verifiable.Test Bank for Intermediate Accounting: IFRS Edition2 - 868. Which of the following is an ingredient of relevance?a. Completeness.b. Representational faithfulness.c. Neutrality.d. Predictive value.69. Which of the following is an ingredient of faithful representation?a. Predictive value.b. Timeliness.c. Neutrality.d. Feedback value.70. Changing the method of inventory valuation should be reported in the financial statementsunder what qualitative characteristic of accounting information?a. Understandability.b. Verifiability.c. Timeliness.d. Comparability.71. Company A issuing its annual financial reports within one month of the end of the year isan example of which enhancing quality of accounting information?a. Neutrality.b. Timeliness.c. Predictive value.d. Representational faithfulness.72. What is the quality of information that enables users to better forecast future operations?a. Reliability.b. Materiality.c. Comparability.d. Relevance.73. Which of the following ingredients of fundamental qualities is part of faithful representation?a. Neutrality.b. Productive value.c. Confirmatory value.d. Timeliness.74. Decision makers vary widely in the types of decisions they make, the methods of decisionmaking they employ, the information they already possess or can obtain from other sources, and their ability to process information. Consequently, for information to be useful there must be a linkage between these users and the decisions they make. This link isa. relevance.b. reliability.c. understandability.d. materiality.Conceptual Framework Underlying Financial Accounting 2 - 9 75. The two fundamental qualities that make accounting information useful for decisionmaking area. comparability and consistency.b. materiality and timeliness.c. relevance and faithful representation.d. reliability and comparability.76. Accounting information is considered to be relevant when ita. can be depended on to represent the economic conditions and events that it isintended to represent.b. is capable of making a difference in a decision.c. is understandable by reasonably informed users of accounting information.d. is verifiable and neutral.77. The quality of information that gives assurance that it is reasonably free of error and biasa. relevance.b. faithful representation.c. verifiability.d. neutrality.78. Financial information does not demonstrate consistency whena. firms in the same industry use different accounting methods to account for the sametype of transaction.b. a company changes its estimate of the salvage value of a fixed asset.c. a company fails to adjust its financial statements for changes in the value of themeasuring unit.d. none of these.79. When information about two different enterprises has been prepared and presented in asimilar manner, the information exhibits the characteristic ofa. relevance.b. reliability.c. consistency.d. none of these.80. The second level of the International Accounting Standards Board’s (IASB’s) ConceptualFrameworka. provides conceptual building blocks that explain the qualitative characteristics ofaccounting information.b. defines the elements of financial statements.c. serves as a bridge between the “why” of accounting and the “how” of accounting.d. all of the choices are correct.81. In the Intern ational Accounting Standards Board’s (IASB’s) Conceptual Framework,qualitative characteristicsa. Are considered either fundamental or enhancing.b. Contribute to the decision-usefulness of financial reporting information.c. Distinguish better information from inferior information for decision-making purposes.d. All of the choices are correct.Test Bank for Intermediate Accounting: IFRS Edition2 - 1082. In the International Accounting Standards Board’s (IASB’s) Conceptual Framework, anenhancing qualitative characteristic isa. Predictive value.b. Free from error.c. Timeliness.d. Confirmatory value.83. In the International Accounting Standards Board’s (IASB’s) Conceptual Framework, aningredient of a fundamental qualitative characteristic isa. Neutrality.b. Verifiability.c. Timeliness.d. Understandability.84. In the International A ccounting Standards Board’s (IASB’s) Conceptual Framework, afundamental qualitative characteristic isa. Materiality.b. Faithful representation.c. Decision usefulness.d. Neutrality.85. To be a faithful representation as described by the International Accounting StandardsBoard’s (IASB’s) Conceptual Framework, information must be all of the following except:a. Complete.b. Free from error.c. Confirmatory.d. Neutral.86. Enhancing qualities as described by the International Accounting Standards Board’s(IASB’s) Conceptua l Framework, include all of the following except:a. Comparability.b. Neutrality.c. Understandability.d. Verifiability.87. Erin Company applies the same accounting treatment to similar events from period toperiod. Erin Company is exhibiting which of the following qualities as described by the International Accounting Standards Board’s (IASB’s) Conceptual Framework?a. Verifiability.b. Consistency.c. Predictive value.d. All of the choices are correct.S88. According to the IASB Conceptual Framework, the elements−assets, liabilities, and equity−describe amounts of resources and claims to resources at/during aMoment in Time Period of Timea. Yes Nob. Yes Yesc. No Yesd. No No89. Which of the following is not a basic element of financial statements?a. Assets.b. Statement of financial position.c. Equity.d. Income.90. Which of the following basic elements of financial statements is not associated with thestatement of financial position?a. Income.b. Equity.c. Liability.d. Asset.91. Issuance of common stock for cash affects which basic element of financial statements?a. Revenues.b. Losses.c. Liabilities.d. Equity.92. The International Accounting Standards Board (IASB) defines five interrelated elements offinancial statements. Which of the following is not one of those elements?a. Asset.b. Income.c. Equity.d. All of the choices are elements defined by the IASB.93. The International Accounting Standards Board (IASB) defines one of the 5 elements asfollows: “the residual interest in the assets of the entity after deducting all its liabilities”Which element matches this description?a. Retained earnings.b. Income.c. Equity.d. All of the choices match this definition.94. Which of the following is not a basic assumption underlying the financial accountingstructure?a. Economic entity assumption.b. Going concern assumption.c. Periodicity assumption.d. Historical cost assumption.95. Which basic assumption is illustrated when a firm reports financial results on an annualbasis?a. Economic entity assumption.b. Going concern assumption.c. Periodicity assumption.d. Monetary unit assumption.96. Which basic assumption may not be followed when a firm in bankruptcy reports financialresults?a. Economic entity assumption.b. Going concern assumption.c. Periodicity assumption.d. Monetary unit assumption.97. Which accounting assumption or principle is being violated if a company provides financialreports in connection with a new product introduction?a. Economic entity.b. Periodicity.c. Revenue recognition.d. Full disclosure.S98. Which of the following basic accounting assumptions is threatened by the existence of severe inflation in the economy?a. Monetary unit assumption.b. Periodicity assumption.c. Going-concern assumption.d. Economic entity assumption.S99. During the lifetime of an entity accountants produce financial statements at artificial points in time in accordance with the concept ofObjectivity Periodicitya. No Nob. Yes Noc. No Yesd. Yes Yes100. Under current IFRS, inflation is ignored in accounting due to thea. economic entity assumption.b. going concern assumption.c. monetary unit assumption.d. periodicity assumption.101. The economic entity assumptiona. is inapplicable to unincorporated businesses.b. recognizes the legal aspects of business organizations.c. requires periodic income measurement.d. is applicable to all forms of business organizations.102. Preparation of consolidated financial statements when a parent-subsidiary relationship exists is an example of thea. economic entity assumption.b. relevance characteristic.c. comparability characteristic.d. neutrality characteristic.103. During the lifetime of an entity, accountants produce financial statements at arbitrary points in time in accordance with which basic accounting concept?a. Cost/benefit constraintb. Periodicity assumptionc. Materiality constraintd. Expense recognition principle104. The assumption that a business enterprise will not be sold or liquidated in the near future is known as thea. economic entity assumption.b. monetary unit assumption.c. materiality assumption.d. none of these.105. Which of the following is an implication of the going concern assumption?a. The historical cost principle is credible.b. Depreciation and amortization policies are justifiable and appropriate.c. The current-noncurrent classification of assets and liabilities is justifiable and signify-cant.d. All of these.106. The basic assumptions of accounting used by the International Accounting Standards Board (IASB) include all of the following except:a. Going concern.b. Periodicity.c. Accrual basis.d. Materiality.107. The basic assumptions of accounting used by the International Accounting Standards Board (IASB) includea. Neutrality.b. Periodicity.c. Understandability.d. Materiality.108. The basic assumptions of accounting used by the International Accounting Standards Board (IASB) includea. Monetary unit.b. Decision usefulnessc. Timeliness.d. All of the choices are basic assumptions of accounting.109. Which of the following basic assumptions of accounting (used by the International Accounting Standards Board) makes depreciation and amortization policies justifiable and appropriate?a. Periodicity.b. Decision usefulnessc. Monetary unit.d. Going concern.110. Proponents of historical cost ordinarily maintain that in comparison with all other valuation alternatives for general purpose financial reporting, statements prepared using historical costs are morea. verifiable.b. relevant.c. indicative of the entity's purchasing power.d. conservative.111. Valuing assets at their liquidation values rather than their cost is inconsistent with thea. periodicity assumption.b. matching principle.c. materiality constraint.d. historical cost principle.112. Revenue is generally recognized when a sale occurs. This statement describes thea. consistency characteristic.b. matching principle.c. revenue recognition principle.d. relevance characteristic.113. Generally, revenue from sales should be recognized at a point whena. management decides it is appropriate to do so.b. the product is available for sale to the ultimate consumer.c. the entire amount receivable has been collected from the customer and there remainsno further warranty liability.d. none of these.114. Revenue generally should be recognizeda. at the end of production.b. at the time of cash collection.c. when realized.d. when a sale occurs.115. Which of the following is not a time when revenue may be recognized?a. At time of saleb. At receipt of cashc. During productiond. All of these are possible times of revenue recognition.116. The Allowance for Doubtful Accounts, which appears as a deduction from Accounts Receivable on a statement of financial position and which is based on an estimate of bad debts, is an application of thea. consistency characteristic.b. expense recognition principle.c. materiality constraint.d. revenue recognition principle.117. The accounting principle of expense recognition is best demonstrated bya. not recognizing any expense unless some revenue is realized.b. associating effort (expense) with accomplishment (revenue).c. recognizing prepaid rent received as revenue.d. establishing an Appropriation for Contingencies account.118. Application of the full disclosure principlea. is theoretically desirable but not practical because the costs of complete disclosureexceed the benefits.b. is violated when important financial information is buried in the notes to the financialstatements.c. is demonstrated by the use of supplementary information presenting the effects ofchanging prices.d. requires that the financial statements be consistent and comparable.119. Which of the following is an argument against using historical cost in accounting?a. Fair values are more relevant.b. Historical costs are based on an exchange transaction.c. Historical costs are reliable.d. Fair values are subjective.120. When is revenue generally recognized?a. When cash is received.b. When the warranty expires.c. When production is completed.d. When the sale occurs.121. Which of the following are the two components of the revenue recognition principle?a. Cash is received and the amount is material.b. It is probable that future economic benefits will flow to the company and it is possibleto reliably measure the amount.c. Production is complete and there is an active market for the product.d. Cash is realized or realizable and production is complete.122. Which of the following practices may not be an acceptable deviation from recognizing revenue at the point of sale?a. Upon receipt of cash.b. During production.c. Upon receipt of order.d. End of production.。
中级财务会计英文ch07

Learning Objectives
1. Describe the characteristics of intangible assets. 2. Identify the costs to include in the initial valuation of intangible assets. 3. Explain the procedure for amortizing intangible assets. 4. Describe the types of intangible assets. 5. Explain the conceptual issues related to goodwill. 6. Describe the accounting procedures for recording goodwill. 7. Explain the accounting issues related to intangible-asset
5. Expected actions of competitors, regulatory bodies, and others.
Chapter 7-12
Intangible Assets Amortization of Cost
Intangible Assets With a Finite Life Are Amortized.
Chapter Patents
7-3
Copyrights
Franchises
Intangible Assets
Intangible assets are those noncurrent economic resources that are used in the operations of the business but have no physical existence.
CA153 Calset

肿瘤相关抗原15-3TMⅡ定标液说明书Elecsys CA 15-3 TM II CalSetElecsys® Systems 1010/2010/MODULAR ANALYTICS E170 03045856 4×1ml【名称】通用名:肿瘤相关抗原15-3TMⅡ定标液英文名: Elecsys CA 15-3 TM II CalSet汉语拼音:Zhong liu xiang guan kang yuan 15-3 TM II ding biao ye【用途】Elecsys CA15-3 Ⅱ定标液用于Elecsys1010/2010和MODULAR ANALYTICS E170免疫分析系统上Elecsys CA15-3 Ⅱ定量检测的定标。
【概述】Elecsys CA15-3 Ⅱ定标液由加入两种浓度的人CA15-3人血清基质组成。
标准化: Elecsys CA15-3 Ⅱ参照Elecsys CA15-3 Ⅱ标定1。
该定标液可以用于所有批号的试剂。
【试剂主要成份】Elecsys CA15-3 Ⅱ定标液,货号03045846CA15-3 Ⅱ Cal 1:2瓶,每瓶包含1.0ml定标液1CA15-3 Ⅱ Cal 2:2瓶,每瓶包含1.0ml定标液2含两种浓度的CA15-3 Ⅱ(人)(大约15U/ml和大约100U/ml)人血清基质。
准确的批特异性定标液的值包含在条形码中,同时亦打印在定标液条形码纸上。
【预防措施和警告】仅用于体外诊断。
采用使用实验室试剂所必须的标准预防措施。
所有废物的丢弃必须遵循当地的要求。
该产品使用捐献者血液,使用FDA建议方法检测乙肝表面抗原、丙型肝炎、HIV 1+2抗体检测均为阴性。
然而,任何实验方法都不能绝对地排除潜在感染的危险,所以该产品仍需作为病人标本一样仔细处理。
一旦泄漏,应遵循有关健康权威机构的指令。
2.3【定标液的使用】提供的定标液为即用型,试剂瓶与系统兼容。
高等有机化学 亲电加成反应

有时有重排产物出现
CH3 H3C C CH CH2 CH3 CH3 H3C CH3 rearrangement H3C CH3 C CH CH3
+
H+
+
C CH CH3
X-
CH3 H3C C CH CH3 X CH3
CH3
重排产物的出现可作为经碳正离子中间体历程的证据之一。
6
(2)鎓型离子历程
H Br H H CH3 CH3 Br H CH3 CH3
+
CH3 CH3
OH
CH3 CH3
H
CH3 C C H H
DCl CH3COOD
D
+C
D Cl C C CH3 H H
C CH3 H
H
5
通常不具有立体选择性
CH CH3 3 + CH3 + H2O CH3 H
+
CH3 CH3 顺式 OH HO + H CH3
H
OH
OH CH3 H CH3
反式
CH3 H
H+ F C C F F F HF FF F FF F
F C C F
F F
NC C NC C
CN CN
H C C+ F F F F F
H C C F F F F F F
C CF F
H+
C C H F F
亲核加成产物
19
2. 亲电试剂对加成速率的影响
对于特定烯烃,卤化氢的加成速率与酸性强弱一致 HI > HBr > HCl > HF 对于特定烯烃,混合卤素的加成速率与其异裂难易程度相符 ICl > IBr > I2
intermediate accounting 中级会计(wiley)ch02

First level
LO 2 Describe efforts to construct a conceptual framework.
First Level: Basic Objective
OBJECTIVE
“To provide financial information about the reporting entity that is useful to present and potential equity investors, lenders, and other creditors in making decisions in their capacity as capital providers.”
ELEMENTS 1. 2. 3. 4. 5. Assets Liabilities Equity Income Expenses
Second level
Chapter 2-8
OBJECTIVE Provide information about the reporting entity that is useful to present and potential equity investors, lenders, and other creditors in their capacity as capital Providers.
Chapter 2-6
LO 2 Describe efforts to construct a conceptual framework.
Conceptual Framework
Overview of the Conceptual Framework
Three levels:
First Level = Basic objective Second Level = Qualitative characteristics and elements of financial statements Third Level = Recognition, measurement, and disclosure concepts
xi第三章碳正与碳负离子

CH+
2
CH3
13PPm
H3C 4.5PPm
2014-9-8
40
Ph
Ph
++
Ph Ph Br Br Ph Ph Ph Ph
SbF5-SO2 -60oC
Ph
Ph
+ +
Ph 8.64 7.87
显示3个峰 8.64(d), 2H, o8.26(t), 1H, p7.87(t), 2H, m-
8.26
2014-9-8
平面构型,如1-金刚烷碳正离子:
2014-9-8
28
溶剂效应:
1) 溶剂的诱导极化作用, 利于底物的解离。 2) 溶剂使碳正离子稳定:
C
3) 极性溶剂:溶剂化作用 强,利于底物的解离。
CH3
溶剂
2014-9-8
空的 p 轨道易于溶剂化 29
(3)碳正离子的生成:
① 直接离子化 通过化学键的异裂而产生。
>
2014-9-8
17
环丙甲基正离子的结构:
C
CH2 空的 p 轨道与弯曲轨道的交盖 随着环丙基的数目增多, 正碳离子稳定性提高。
CH2
CH2
中心碳原子上的空的 p轨道与环丙基中 的弯曲轨道进行侧面交盖,其结果是使 正电荷分散。
2014-9-8 18
直接与杂原子相连的正碳离子结构:
氧上未共有电子对所 占 p 轨道 与中心碳原子上的空的 p轨道 侧面交盖,未共有电子对离域, 正电荷分散。
2014-9-8
37
它们的稳定性可以用pKR(醇和碳正离子浓度相等的 pH值)来表示。这里,三苯甲醇的pKR,值为-6;三对甲 氧基苯甲醇为0.8,下图所示的醇为9.1;通过uv或nmr可 以测定离子的浓度,从而得到pKR值。
UL153

Table of Content ﹝內容﹞
- Scope of UL153 ﹝UL153的範圍﹞ - Part A: Mechanical Construction ﹝機械結構﹞ - Part B: Electrical Construction ﹝電子結構﹞ - Part C: Components ﹝零件﹞ - Part D: Particular Requirements For Specific Portable Luminaires
﹝UL153之最新版本為發行於2002年3月25日之12版本,最新修訂日期為2005 年12月28日﹞
• UL 153 covers portable luminaires whose primary function is task or ambient illumination and provided with a flexible cord and an attachment plug for connection to a nominal 120 volt branch circuit
Guide to Portable Luminaires Design on US and Canadian Market
﹝銷往美國及加拿大的可移動燈具設計指南﹞
UL 153 12th Edition and CSA C22.2 No. 12-1982
Presented by Ms. Shelley Liu (1 March, 2006)
之間的電線,如非於下列情況下,裝配時不可轉動多於360度﹞
- Internal diameter of tubing ≥ 12.7mm ﹝套管內徑大過或等於12.7毫米﹞
- Not more than 1 revolution for 3 inch ﹝於三寸內不會轉多於一圈﹞
G902 G902-15 Cherry Lightweight Nut-Plate Rivete

Original InstructionsG902Cherry Lightweight Nut-Plate Riveter1224 East Warner Ave, Santa Ana, Ca 92705THE G902 / G902-15T A B L E O F C O N T E N T SDescription (1)Specifications for G902 / G902-15 (1)Safety Warnings (2)How to use G902 / G902-15 (2)Maintenance and Repair (2)Troubleshooting (2)Overhaul (3)Air Valve (3)Handle Sub-Assembly (3)Pulling Heads (3)Installing Pulling Heads (3)Cross Section Drawing Of G902 (4)Parts List for G902/G902-15 Riveters (5)Exploded View of G902 / G902-15 (6)Declaration of Conformity ...................................................................................................................................................Back CoverD E S C R I P T I O NThe G902 feed-through/G902-15 side-eject all pneumatic lightweight tools are designed specifically for the most efficient installation of blind rivets. Its durable, all metal housing makes this extremely robust tool ideal for use in rugged shop environments. This tool utilizes a straight pulling head which can install 3/32", 1/8’’ pull through type Nut-Plate and 3/32" CherryLock “A” rivets. Extensions are available for the G902 to allow the pulling heads to reach limited access areas.See the section on pulling heads for the correctpulling head part number.S P E C I F I C A T I O N S F O RG902/G902-15Cherry® Aerospace (CHERRY) policy is one ofcontinuous development. Specifications shown inthis document may be subject to change whichmay be introduced after publication. For the latestinformation always consult CHERRY®.AIR PRESSURE: 90 PSI to 110 PSI(6.2 bar to 7.6 bar)STROKE: 3/4 Inch (19 mm)PULLING FORCE: 550 Pounds (2.45 kN)@ 90 PSI (6.2 bar)CYCLE TIME: One Second approx.WEIGHT 2 Pounds (.91 kg)NOISE LEVEL 60 dB (A)VIBRATION: less than 2.5 m/s2AIR CONSUMPTION:05 SCF/Cycle (1.42 L/cycle)SAFETY WARNINGS•Operating this tool with a damaged or missing stem deflector, or using the deflector as a handle, may result in severe personal injury. Rotate the pin deflector until the aperture is facing away from the operator and other persons working in the vicinity. •Approved eye protection should be worn when operating, repairing, or overhauling this tool.•Do not use beyond the design intent.•Do not use substitute components for repair. Any modification to the tool, pulling heads, accessories or any component supplied by CHERRY®, or their representatives, shall be at the customer’s entire responsibility.CHERRY® will be pleased to advise on any proposed modification.•The tool must be maintained in a safe working condition at all times and examined at regular intervals for damage.•Before disassembling the tool for repair, refer to the maintenance instructions. All repairs shall be undertaken only by personnel trained by CHERRY®. Contact us with your training requirement.•Always disconnect the air line from the tool inlet before attempting to service, adjust, fit or remove any accessory.•Do not operate the tool when it is directed at any person.•Make sure the vent holes do not become blocked or covered and that air line hoses are always in good condition.•Do not operate the tool without the pulling head in place.•All retaining rings, screwed end caps, air fittings, trigger valves and pulling heads should be attached securely and examined at the end of each working shift•Do not pull rivet in the air.•The precautions to be used when using this tool must be explained by the customer to all operators. Any questions regarding the correct operation of the tool and operator safety should be directed to CHERRY®.•Do not pound on the rear of the tool head to force rivets into holes as this will damage the tool.HOW TO USE THE G902 / G902-15After selecting the proper pulling head and attaching it securely to the G902 or G902-15, connect the air line to the tool.Insert the rivet stem into the pulling head until the head of the rivet is in contact with the pulling head nosepiece; this will ensure full engagement between the jaws and the rivet stem and will prevent slippage.MAINTENANCE AND REPAIRThe G902 or G902-15 has been manufactured to give maximum service with minimum care.In order that this may be accomplished, the following recommendations should be followed:1. Keep excessive moisture and dirt out of air supply to prevent wear of air valve, air cylinder and air piston.2. Tool should be routinely inspected for damage or air leaks.3. Periodically tighten the threaded connection between the rear piston sub-assembly (14) and the front piston (11)(see instructions on page 3). A loose connection will result in an air leak and a loss of pulling force. TROUBLESHOOTING (see Parts List, page 5)1. Check for excessive air leakage from the air valve: If an O-ring on the valve stem (24) or on the valve sleeve (22) is worn or dam-aged, air will leak through the bottom of the handle at the exhaust port or at the front of the trigger valve.•If an O-ring (2) on valve sleeve (22) is worn or damaged, replace.•If an O-ring (23) on valve stem (24) is worn or damaged, replace.2. Check movement of the head piston rod (11). If it does not move freely or it is slow in operation:•O-rings (19), (17), (16), (12), (9), (2), (6), (2) and O-ring (17) for G902-15 may be damaged and require replacement.•Front and rear piston sub-assemblies (15 and 8) may be seized due to damaged parts and need replacement.•The Ø.094" (2.388 mm) vent holes on the side of main cylinder may be blocked or damaged; clean as necessary.3. Rivet stem sticks in the pulling head:•Pulling head needs maintenance. Disassemble the pulling head; clean and replace worn parts as needed.•Spent rivet stems are wedged inside the pistons. Disassemble the pulling head and remove jammed stems.OVERHAULThe disassembly and reassembly procedures can be accomplished by following the instructions below and the drawings on pages 4 & 6. Use extreme care during disassembly and reassembly not to mar, nick or burr any smooth surface that comes in contact with O-rings. Before installing O-rings, be sure to apply an O-ring lubricant. It is recommended that a special assembly tool, which can be ordered under part number 902-027, be used to overhaul this tool.AIR VALVE• Remove spring pin (21) by tapping it gently with a punch.• Remove the valve sub-assembly by pressing on trigger (27) from behind with both thumbs using the handle (20) for leverage, orpull and wiggle the trigger from the front. • Remove the set screw (28) from the trigger (27). • Disassemble the trigger (27) from valve stem (24). • Remove retaining ring (26).• Remove washer (25) from the counter bore of valve sleeve (22). • Pull valve stem (24) out from valve sleeve (22).HANDLE SUB-ASSEMBLY (see parts list)• Always remove the complete pulling head from the tool before attempting to dissemble the head assembly. Remove front cap sub-assembly (3) by unscrewing counterclockwise. • Remove deflector pin (31) and deflector fitting (30) (G902-15 model does not use these components). Insert a 3/16 hex wrenchthrough the back of the tool into the rear piston sub-assembly (14). • With hex wrench in place, locate the flats on the front piston rod (11). Use a 7/16 wrench, (for G902-15 model insert a pin in thecrossed drill hole of front piston rod (11)), to unscrew the front piston rod (11) by turning it counterclockwise.Note: Do not use Loctite TM when reassembling the front piston rod (11) to the rear piston sub-assembly (14); it would block the air passage. Instead, torque to about 90 in-lbs. • With the front piston rod (11) and front piston sub-assembly (3) out of the main housing, remove retaining ring (7) then push offthe front air piston sub-assembly (3) off of piston rod (11). • Remove retaining ring (10) from the main handle bore.• Use a 3/16 hex wrench to push out the rear piston sub-assembly (14) and bulk head (13).• Use plug wrench 902-027 and locate the two holes on the rear plug (18) inside the main handle bore. Unscrew the rear plug byturning the plug wrench counterclockwise.G902 / G902-15 PULLING HEADSINSTALLING H902-3NPR PULLING HEAD ON RIVETER1. Place jaws inside collet and place jaw follower in the collet against jaws. Then screw drawbar adapter onto the collet and tighten securely using wrenches on flats provided.2. Insert spring into head piston. Screw collet sub-assembly onto head piston and tighten securely using wrenches on flats provided.3. Thread tightly the proper nosepiece onto sleeve.C R O S S S E C T I O N O F G902PARTS LIST FOR THE G902 (902-15) NUT-PLATE RIVETER ASSEMBLY** NOT SOLD SEPARATELY.NOTE: ITEMS 29 TO 32 (P-880, 703A13, 530A16 AND 670A20) ARE NOT USED IN G902-15 TOOL.E X P L O D E D V I E W OF G902/G902-151224 East Warner Ave, Santa Ana, Ca 92705 Tel: 1-714-545-5511Fax: 1-714-850-6093 © 2007 Cherry AerospaceLOCTITE ® is a registered trademark of Henkel Corporation DEXRON ® is a registered trademark of GM corporation.PARKER ®is a trademark of Parker Hannifin CorporationLUBRRIPLATE ® is a trademark of Fiske Brothers Refining Co.TM-G902。
欧洲药典索引版3

EUROPEAN PHARMACOPOEIA5.5INDEXTo aid users the index includes a reference to the supplement where the latest version of a text can be found.For example:Acetone...............................................5.1-2875means the monograph Acetone can be found on page2875of Supplement5.1.Note that where no reference for a supplement is made,the text can be found in the principal volume.Monographs deleted from the5th edition are not included in the index;the list of deleted texts is found in the Contents of this supplement,page xxx.EUROPEAN PHARMACOPOEIA5.5Numerics1.1.General statements (5)1.2.Other provisions applying to general chapters and monographs (5)1.3.General chapters (6)1.4.Monographs (7)1.5.Abbreviations and symbols (9)1.6.Units of the International System(SI)used in the Pharmacopoeia and equivalence with other units (10)1.General notices (5)2.1.1.Droppers (17)parative table of porosity of sintered-glass filters (17)2.1.3.Ultraviolet ray lamps for analytical purposes (17)2.1.4.Sieves (18)2.1.5.Tubes for comparative tests (19)2.1.6.Gas detector tubes (19)2.1.Apparatus (17)2.2.10.Viscosity-Rotating viscometer method.........5.3-3337 2.2.11.Distillation range (30)2.2.12.Boiling point (31)2.2.13.Determination of water by distillation (32)2.2.14.Melting point-capillary method (32)2.2.15.Melting point-open capillary method (33)2.2.16.Melting point-instantaneous method (33)2.2.17.Drop point (33)2.2.18.Freezing point (34)2.2.19.Amperometric titration (34)2.2.1.Clarity and degree of opalescence of liquids (23)2.2.20.Potentiometric titration (35)2.2.21.Fluorimetry (35)2.2.22.Atomic emission spectrometry (35)2.2.23.Atomic absorption spectrometry (36)2.2.24.Absorption spectrophotometry,infrared (37)2.2.25.Absorption spectrophotometry,ultraviolet and visible.................................................................................5.2-3089 2.2.26.Paper chromatography. (40)2.2.27.Thin-layer chromatography...............................5.2-3090 2.2.28.Gas chromatography.. (42)2.2.29.Liquid chromatography (43)2.2.2.Degree of coloration of liquids (24)2.2.30.Size-exclusion chromatography (45)2.2.31.Electrophoresis (45)2.2.32.Loss on drying (50)2.2.33.Nuclear magnetic resonance spectrometry (51)2.2.34.Thermal analysis (52)2.2.35.Osmolality (54)2.2.36.Potentiometric determination of ionic concentration using ion-selective electrodes (55)2.2.37.X-ray fluorescence spectrometry (56)2.2.38.Conductivity.........................................................5.1-2783 2.2.39.Molecular mass distribution in dextrans (57)2.2.3.Potentiometric determination of pH (26)2.2.40.Near-infrared spectrophotometry (59)2.2.41.Circular dichroism (63)2.2.42.Density of solids (64)2.2.43.Mass spectrometry (65)2.2.44.Total organic carbon in water for pharmaceutical use (68)2.2.45.Supercritical fluid chromatography (68)2.2.46.Chromatographic separation techniques (69)2.2.47.Capillary electrophoresis (74)2.2.48.Raman spectrometry (79)2.2.49.Falling ball viscometer method (80)2.2.4.Relationship between reaction of solution, approximate pH and colour of certain indicators (27)2.2.54.Isoelectric focusing (81)2.2.55.Peptide mapping (82)2.2.56.Amino acid analysis.......................................................862.2.5.Relative density.. (27)2.2.6.Refractive index (28)2.2.7.Optical rotation......................................................5.4-3695 2.2.8.Viscosity (29)2.2.9.Capillary viscometer method (29)2.2.Physical and physicochemical methods (23)2.3.1.Identification reactions of ions and functional groups...............................................................................5.5-4101 2.3.2.Identification of fatty oils by thin-layer chromatography. (98)2.3.3.Identification of phenothiazines by thin-layer chromatography (99)2.3.4.Odour (99)2.3.Identification (95)2.4.10.Lead in sugars (107)2.4.11.Phosphates (108)2.4.12.Potassium (108)2.4.13.Sulphates (108)2.4.14.Sulphated ash......................................................5.3-3341 2.4.15.Nickel in polyols.. (108)2.4.16.Total ash (108)2.4.17.Aluminium (108)2.4.18.Free formaldehyde (109)2.4.19.Alkaline impurities in fatty oils (109)2.4.1.Ammonium (103)2.4.21.Foreign oils in fatty oils by thin-layer chromatography (109)position of fatty acids by gas chroma-tography (110)2.4.23.Sterols in fatty oils..............................................5.1-2787 2.4.24.Identification and control of residual solvents (113)2.4.25.Ethylene oxide and dioxan (118)2.4.26.N,N-Dimethylaniline (119)2.4.27.Heavy metals in herbal drugs and fatty oils (119)2.4.28.2-Ethylhexanoic acid (120)position of fatty acids in oils rich inomega-3-acids...................................................................5.5-4107 2.4.2.Arsenic (103)2.4.30.Ethylene glycol and diethylene glycol in ethoxylated substances........................................................................5.2-3095 2.4.3.Calcium.. (103)2.4.4.Chlorides (104)2.4.5.Fluorides (104)2.4.6.Magnesium (104)2.4.7.Magnesium and alkaline-earth metals (104)2.4.8.Heavy metals (104)2.4.9.Iron (107)2.4.Limit tests (103)2.5.10.Oxygen-flask method (130)plexometric titrations (130)2.5.12.Water:semi-micro determination (130)2.5.13.Aluminium in adsorbed vaccines (131)2.5.14.Calcium in adsorbed vaccines (131)2.5.15.Phenol in immunosera and vaccines (131)2.5.16.Protein in polysaccharide vaccines (131)2.5.17.Nucleic acids in polysaccharide vaccines (132)2.5.18.Phosphorus in polysaccharide vaccines (132)2.5.19.O-Acetyl in polysaccharide vaccines (132)2.5.1.Acid value................................................................5.2-3099 2.5.20.Hexosamines in polysaccharide vaccines. (132)2.5.21.Methylpentoses in polysaccharide vaccines (133)2.5.22.Uronic acids in polysaccharide vaccines (133)2.5.23.Sialic acid in polysaccharide vaccines (133)2.5.24.Carbon dioxide in gases (134)2.5.25.Carbon monoxide in gases (134)2.5.26.Nitrogen monoxide and nitrogen dioxide in gases (135)2.5.27.Oxygen in gases (136)2.5.28.Water in gases (136)2.5.29.Sulphur dioxide (136)2.5.2.Ester value (127)2.5.30.Oxidising substances (137)2.5.31.Ribose in polysaccharide vaccines (137)2.5.32.Water:micro determination (137)2.5.33.Total protein (138)2.5.34.Acetic acid in synthetic peptides (141)2.5.35.Nitrous oxide in gases (141)2.5.36.Anisidine value (142)2.5.3.Hydroxyl value (127)2.5.4.Iodine value (127)2.5.5.Peroxide value (128)2.5.6.Saponification value (129)2.5.7.Unsaponifiable matter (129)2.5.8.Determination of primary aromaticamino-nitrogen (129)2.5.9.Determination of nitrogen by sulphuric acid digestion (129)2.5.Assays (127)2.6.10.Histamine (153)2.6.11.Depressor substances (153)2.6.12.Microbiological examination of non-sterile products (total viable aerobic count) (154)2.6.13.Microbiological examination of non-sterile products (test for specified micro-organisms) (156)2.6.14.Bacterial endotoxins (161)2.6.15.Prekallikrein activator........................................5.5-4111 2.6.16.Tests for extraneous agents in viral vaccines for human use (169)2.6.17.Test for anticomplementary activity of immunoglobulin (170)2.6.18.Test for neurovirulence of live virus vaccines (172)2.6.19.Test for neurovirulence of poliomyelitis vaccine (oral) (172)2.6.1.Sterility (145)2.6.20.Anti-A and anti-B haemagglutinins(indirect method) (174)2.6.21.Nucleic acid amplification techniques............5.5-4111 2.6.22.Activated coagulation factors...........................5.5-4115 2.6.24.Avian viral vaccines:tests for extraneous agents in seed lots............................................................................5.4-3699 2.6.25.Avian live virus vaccines:tests for extraneous agents in batches of finished product.....................................5.3-3345 2.6.26.Test for anti-D antibodies in human immunoglobulin for intravenous administration....................................5.3-3348 2.6.2.Mycobacteria. (149)2.6.7.Mycoplasmas (149)2.6.8.Pyrogens (152)2.6.9.Abnormal toxicity (153)2.6.Biological tests (145)2.7.10.Assay of human coagulation factor VII (203)2.7.11.Assay of human coagulation factor IX............5.5-4120 2.7.12.Assay of heparin in coagulation factors (204)2.7.13.Assay of human anti-D immunoglobulin (205)2.7.14.Assay of hepatitis A vaccine..............................5.1-2795 2.7.15.Assay of hepatitis B vaccine(rDNA). (207)2.7.16.Assay of pertussis vaccine(acellular) (208)2.7.17.Assay of human antithrombin III (209)2.7.18.Assay of human coagulation factor II (209)2.7.19.Assay of human coagulation factor X (210)2.7.1.Immunochemical methods (187)2.7.20.In vivo assay of poliomyelitis vaccine (inactivated) (210)2.7.21.Assay of human von Willebrand factor...........5.5-4120 2.7.22.Assay of human coagulation factor XI............5.5-4121 2.7.2.Microbiological assay of antibiotics. (188)2.7.4.Assay of human coagulation factor VIII...........5.5-4119 2.7.5.Assay of heparin.. (195)2.7.6.Assay of diphtheria vaccine(adsorbed).....................1962.7.7.Assay of pertussis vaccine (197)2.7.8.Assay of tetanus vaccine(adsorbed)..................5.1-2791 2.7.9.Test for Fc function of immunoglobulin. (202)2.7.Biological assays (187)2.8.10.Solubility in alcohol of essential oils (216)2.8.11.Assay of1,8-cineole in essential oils (216)2.8.12.Determination of essential oils in vegetable drugs (217)2.8.13.Pesticide residues (218)2.8.14.Determination of tannins in herbal drugs (221)2.8.15.Bitterness value (221)2.8.16.Dry residue of extracts (222)2.8.17.Loss on drying of extracts (222)2.8.1.Ash insoluble in hydrochloric acid (215)2.8.2.Foreign matter (215)2.8.3.Stomata and stomatal index (215)2.8.4.Swelling index (215)2.8.5.Water in essential oils (216)2.8.6.Foreign esters in essential oils (216)2.8.7.Fatty oils and resinified essential oils in essential oils (216)2.8.8.Odour and taste of essential oils (216)2.8.9.Residue on evaporation of essential oils (216)2.8.Methods in pharmacognosy (215)2.9.10.Ethanol content and alcoholimetric tables (237)2.9.11.Test for methanol and2-propanol...................5.3-3362 2.9.12.Sieve test (239)2.9.13.Limit test of particle size by microscopy (239)2.9.14.Specific surface area by air permeability (239)2.9.15.Apparent volume (241)2.9.16.Flowability (242)2.9.17.Test for extractable volume of parenteral preparations.....................................................................5.3-3363 2.9.18.Preparations for inhalation:aerodynamic assessment of fine particles...............................................................5.2-3103 2.9.19.Particulate contamination:sub-visible particles (253)2.9.1.Disintegration of tablets and capsules..............5.3-3351 2.9.20.Particulate contamination:visible particles. (255)2.9.22.Softening time determination of lipophilic suppositories (256)2.9.23.Pycnometric density of solids (257)2.9.24.Resistance to rupture of suppositories and pessaries (258)2.9.25.Dissolution test for medicated chewing gums..................................................................................5.2-3116 2.9.26.Specific surface area by gas adsorption.........5.1-2811 2.9.27.Uniformity of mass of delivered doses from multidose containers. (263)2.9.28.Test for deliverable mass or volume of liquid and semi-solid preparations (263)2.9.29.Intrinsic dissolution............................................5.4-3705 2.9.2.Disintegration of suppositories and pessaries (227)2.9.36.Powder flow..........................................................5.3-3363 2.9.37.Optical microscopy..............................................5.3-3366 2.9.38.Particle-size distribution estimation by analytical sieving...............................................................................5.3-3368 2.9.3.Dissolution test for solid dosage forms............5.3-3353 2.9.40.Uniformity of dosage units................................5.3-3370 2.9.42.Dissolution test for lipophilic solid dosage forms..................................................................................5.3-3373 2.9.4.Dissolution test for transdermal patches (231)2.9.5.Uniformity of mass of single-dose preparations (233)2.9.6.Uniformity of content of single-dose preparations..234 2.9.7.Friability of uncoated tablets..............................5.2-3103 2.9.8.Resistance to crushing of tablets.. (235)2.9.9.Measurement of consistency by penetrometry (235)2.9.Pharmaceutical technical procedures (225)3.1.10.Materials based on non-plasticised poly(vinyl chloride) for containers for non-injectable,aqueous solutions (289)3.1.11.Materials based on non-plasticised poly(vinyl chloride)for containers for dry dosage forms for oral administration..........................................................................2913.1.1.1.Materials based on plasticised poly(vinyl chloride)for containers for human blood and blood components. (269)3.1.1.2.Materials based on plasticised poly(vinyl chloride)for tubing used in sets for the transfusion of blood andblood components (272)3.1.13.Plastic additives (293)3.1.14.Materials based on plasticised poly(vinyl chloride)for containers for aqueous solutions for intravenous infusion......................................................................................2963.1.15.Polyethyleneterephthalatefor containers forpreparations not for parenteral use.....................................2983.1.1.Materials for containers for human blood and blood components. (269)3.1.3.Polyolefines (274)3.1.4.Polyethylene without additives for containers for parenteral preparations and for ophthalmic preparations..............................................................................2783.1.5.Polyethylene with additives for containers for parenteral preparations and for ophthalmicpreparations..............................................................................2793.1.6.Polypropylene for containers and closures for parenteral preparationsand ophthalmic preparations (282)3.1.7.Poly(ethylene -vinyl acetate)for containers and tubing for total parenteral nutrition preparations........................2853.1.8.Silicone oilused as a lubricant (287)3.1.9.Silicone elastomer for closures and tubing..............2883.1.Materials used for the manufacture of containers.....2693.2.1.Glass containers for pharmaceutical use..................3033.2.2.1.Plastic containers for aqueous solutions for parenteral infusion..................................................................3093.2.2.Plastic containers and closures for pharmaceuticaluse...............................................................................................3083.2.3.Sterile plastic containers for human blood and bloodcomponents...............................................................................3093.2.4.Empty sterile containers of plasticised poly(vinylchloride)forhuman blood and blood components...........3113.2.5.Sterile containers of plasticisedpoly (vinylchloride)for human blood containing anticoagulant solution.......3123.2.6.Sets for the transfusion of blood and blood components................................................................................3133.2.8.Sterile single-use plastic syringes................................3143.2.9.Rubber closures for containers for aqueous parenteral preparations,for powders and for freeze-dried powders..3163.2.Containers...........................................................................3034.1.1.Reagents..................................................................5.4-37094.1.1.Reagents..................................................................5.5-41254.1.2.Standard solutions for limit tests.......................5.4-38174.1.2.Standard solutions for limit tests.......................5.5-41264.1.3.Buffer solutions.....................................................5.4-38214.1.3.Buffer solutions.....................................................5.5-41264.1.Reagents,standard solutions,buffer solutions..5.4-37094.2.1.Primary standards for volumetric solutions....5.4-38274.2.2.Volumetric solutions.............................................5.4-38274.2.2.Volumetric solutions.............................................5.5-41274.2.Volumetric analysis...................................................5.4-38274.Reagents.........................................................................5.4-37095.10.Control of impurities in substances for pharmaceuticaluse......................................................................................5.5-41455.11.Characters section in monographs (565)5.1.1.Methods of preparation of sterile products..............4455.1.2.Biological indicators of sterilisation (447)5.1.3.Efficacy of antimicrobial preservation.......................4475.1.4.Microbiological quality of pharmaceuticalpreparations (449)5.1.5.Application of the F 0concept to steam sterilisation of aqueous preparations....................................................5.1-2821 5.1.6.Alternative methods for control of microbiological quality................................................................................5.5-41315.1.Generaltexts onsterility..................................................4455.2.1.Terminology used in monographs on vaccines (453)5.2.2.Chicken flocks free from specified pathogens for the production and quality control of vaccines...............5.1-28255.2.3.Cell substrates for the production of vaccines for human use.................................................................................4555.2.4.Cell cultures for the production of veterinaryvaccines (458)5.2.5.Substances of animal origin for the production ofveterinary vaccines (460)5.2.6.Evaluation of safety of veterinary vaccines andimmunosera ....................................................................5.1-28275.2.7.Evaluation of efficacy of veterinary vaccines and immunosera.....................................................................5.1-28295.2.8.Minimising the risk of transmitting animal spongiform encephalopathy agents via human and veterinary medicinal products (463)5.2.9.Evaluation of safety of each batch of veterinary vaccines and immunosera.............................................5.1-28305.2.General texts on vaccines (453)5.3.Statistical analysis of results of biological assays andtests (475)5.4.Residual solvents...............................................................5075.5.Alcoholimetric tables.........................................................5195.6.Assay of interferons..................................................5.3-33815.7.Table of physical characteristics of radionuclidesmentioned in the European Pharmacopoeia.....................5395.8.Pharmacopoeial harmonisation.....................................5515.9.Polymorphism (555)AAbbreviationsand symbols (1.5.) (9)Abnormal toxicity (2.6.9.) (153)Absinthiiherba ........................................................................2710Absorption spectrophotometry,infrared (2.2.24.). (37)Absorption spectrophotometry,ultraviolet and visible (2.2.25.).............................................................................5.2-3089Acacia (905)Acaciae gummi (905)Acaciae gummi dispersione desiccatum .............................905Acacia,spray-dried (905)Acamprosate calcium................................................................906Acamprosatum calcicum (906)Acarbose..............................................................................5.1-2873Acarbosum .........................................................................5.1-2873Acebutololhydrochloride................................................5.4-3889Acebutololi hydrochloridum .........................................5.4-3889Aceclofenac (909)Aceclofenacum (909)Acesulfame potassium.....................................................5.4-3890Acesulfamum kalicum ....................................................5.4-3890Acetazolamide (912)Acetazolamidum (912)Acetic acid,glacial (913)Acetic acid in synthetic peptides (2.5.34.) (141)Acetone................................................................................5.1-2875Acetonum ...........................................................................5.1-2875Acetylcholine chloride...............................................................914Acetylcholini chloridum .. (914)Acetylcysteine..............................................................................915Acetylcysteinum (915)β-Acetyldigoxin..................................................................5.5-4185β-Acetyldigoxinum ...........................................................5.5-4185Acetylsalicylic acid (917)Acetyltryptophan,N - (918)Acetyltyrosine,N - (920)Aciclovir..............................................................................5.3-3436Aciclovirum.......................................................................5.3-3436 Acidum4-aminobenzoicum (973)Acidum aceticum glaciale (913)Acidum acetylsalicylicum (917)Acidum adipicum (926)Acidum alginicum (942)Acidum amidotrizoicum dihydricum (967)Acidum aminocaproicum (974)Acidum ascorbicum (1025)Acidum asparticum (1029)Acidum benzoicum (1072)Acidum boricum (1117)Acidum caprylicum (1172)Acidum chenodeoxycholicum (1247)Acidum citricum anhydricum (1306)Acidum citricum monohydricum (1307)Acidum edeticum.............................................................5.4-3933 Acidum etacrynicum.. (1542)Acidum folicum (1630)Acidum fusidicum (1645)Acidum glutamicum (1670)Acidum hydrochloridum concentratum (1755)Acidum hydrochloridum dilutum (1756)Acidum iopanoicum (1824)Acidum iotalamicum (1825)Acidum ioxaglicum (1826)Acidum lacticum..............................................................5.2-3227 Acidum lactobionicum.. (1885)Acidum maleicum (1966)Acidum malicum (1966)Acidum mefenamicum (1984)Acidum methacrylicum et ethylis acrylas polymerisatum 1:1 (2005)Acidum methacrylicum et ethylis acrylas polymerisatum 1:1dispersio30per centum (2005)Acidum methacrylicum et methylis methacrylas polymerisatum1:1 (2006)Acidum methacrylicum et methylis methacrylas polymerisatum1:2 (2007)Acidum nalidixicum (2080)Acidum nicotinicum (2097)Acidum nitricum (2105)Acidum oleicum (2132)Acidum oxolinicum (2165)Acidum palmiticum (2179)Acidum phosphoricum concentratum (2237)Acidum phosphoricum dilutum (2238)Acidum pipemidicum trihydricum (2249)Acidum salicylicum.........................................................5.1-3007 Acidum(S)-lacticum........................................................5.2-3227 Acidum sorbicum.. (2467)Acidum stearicum (2490)Acidum sulfuricum (2520)Acidum tartaricum (2534)Acidum thiocticum...........................................................5.5-4312 Acidum tiaprofenicum.. (2578)Acidum tolfenamicum (2601)Acidum tranexamicum (2609)Acidum trichloraceticum (2620)Acidum undecylenicum (2658)Acidum ursodeoxycholicum (2662)Acidum valproicum (2669)Acid value(2.5.1.)..............................................................5.2-3099 Acitretin. (922)Acitretinum (922)Acriflavinii monochloridum (924)Acriflavinium monochloride (924)Actinobacillosis vaccine(inactivated),porcine (784)Activated charcoal....................................................................1246Activated coagulation factors(2.6.22.).........................5.5-4115 Additives,plastic(3.1.13.) (293)Adenine (924)Adeninum (924)Adenosine (925)Adenosinum (925)Adeps lanae.......................................................................5.2-3285 Adeps lanae cum aqua.. (2709)Adeps lanae hydrogenatus (2708)Adeps solidus (1711)Adipic acid (926)Adrenaline tartrate (927)Adrenalini tartras (927)Aer medicinalis (929)Aer medicinalis artificiosus (932)Aerodynamic assessment of fine particles in preparations for inhalation(2.9.18.).........................................................5.2-3103 Aether.. (1548)Aether anaestheticus (1549)Agar (928)Agni casti fructus.............................................................5.4-3892 Agnus castus fruit.............................................................5.4-3892 Agrimoniae herba (929)Agrimony (929)Air,medicinal (929)Air,synthetic medicinal (932)Alanine (933)Alaninum (933)Albendazole (934)Albendazolum (934)Albumini humani solutio...............................................5.3-3511 Albumin solution,human................................................5.3-3511 Alchemilla (935)Alchemillae herba (935)Alcohol benzylicus...........................................................5.5-4197 Alcohol cetylicus...............................................................5.3-3475 Alcohol cetylicus et stearylicus....................................5.3-3474 Alcohol cetylicus et stearylicus emulsificans A.. (1239)Alcohol cetylicus et stearylicus emulsificans B (1241)Alcoholes adipis lanae (2703)Alcoholimetric tables(2.9.10.) (237)Alcoholimetric tables(5.5.) (519)Alcohol isopropylicus (1841)Alcohol oleicus (2134)Alcohol stearylicus...........................................................5.3-3621 Alcuronii chloridum.. (935)Alcuronium chloride (935)Alexandrian senna pods (2404)Alfacalcidol (937)Alfacalcidolum (937)Alfadex (938)Alfadexum (938)Alfentanil hydrochloride (939)Alfentanili hydrochloridum (939)Alfuzosin hydrochloride (941)Alfuzosini hydrochloridum (941)Alginic acid (942)Alkaline-earth metals and magnesium(2.4.7.) (104)Alkaline impurities in fatty oils(2.4.19.) (109)Allantoin (942)Allantoinum (942)Allergen products (569)Allii sativi bulbi pulvis (1651)Allium sativum ad praeparationes homoeopathicas (897)Allopurinol (943)Allopurinolum (943)all-rac-α-Tocopherol..........................................................5.5-4313 all-rac-α-Tocopheryl acetate...........................................5.5-4314 Almagate.............................................................................5.2-3169Almagatum.........................................................................5.2-3169 Almond oil,refined...........................................................5.4-3893 Almond oil,virgin.............................................................5.3-3437 Aloe barbadensis.. (947)Aloe capensis (948)Aloes,barbados (947)Aloes,Cape (948)Aloes dry extract,standardised (949)Aloes extractum siccum normatum (949)Alphacyclodextrin (938)Alprazolam (950)Alprazolamum (950)Alprenolol hydrochloride (952)Alprenololi hydrochloridum (952)Alprostadil (953)Alprostadilum (953)Alteplase for injection (956)Alteplasum ad iniectabile (956)Alternative methods for control of microbiological quality (5.1.6.)................................................................................5.5-4131 Althaeae folium (1974)Althaeae radix...................................................................5.2-3232 Alum. (959)Alumen (959)Aluminii chloridum hexahydricum (960)Aluminii hydroxidum hydricum ad adsorptionem..5.5-4186 Aluminii magnesii silicas (961)Aluminii oxidum hydricum (962)Aluminii phosphas hydricus (963)Aluminii phosphatis liquamen.....................................5.3-3438 Aluminii sulfas (964)Aluminium(2.4.17.) (108)Aluminium chloride hexahydrate (960)Aluminium hydroxide,hydrated,for adsorption........5.5-4186 Aluminium in adsorbed vaccines(2.5.13.).. (131)Aluminium magnesium silicate (961)Aluminium oxide,hydrated (962)Aluminium phosphate gel...............................................5.3-3438 Aluminium phosphate,hydrated.. (963)Aluminium sulphate (964)Amantadine hydrochloride (964)Amantadini hydrochloridum (964)Ambroxol hydrochloride (965)Ambroxoli hydrochloridum (965)Amfetamine sulphate (966)Amfetamini sulfas (966)Amidotrizoic acid dihydrate (967)Amikacin (968)Amikacini sulfas...............................................................5.4-3894 Amikacin sulphate............................................................5.4-3894 Amikacinum. (968)Amiloride hydrochloride..................................................5.3-3439 Amiloridi hydrochloridum.............................................5.3-3439 Amino acid analysis(2.2.56.).. (86)Aminobenzoic acid,4- (973)Aminocaproic acid (974)Aminoglutethimide (975)Aminoglutethimidum (975)Amiodarone hydrochloride (977)Amiodaroni hydrochloridum (977)Amisulpride (978)Amisulpridum (978)Amitriptyline hydrochloride (980)Amitriptylini hydrochloridum (980)Amlodipine besilate (981)Amlodipini besilas (981)Ammonia(13N)injection (817)Ammoniae(13N)solutio iniectabilis (817)Ammoniae solutio concentrata.............................................983Ammonia solution,concentrated. (983)Ammonii bromidum (985)Ammonii chloridum (986)Ammonii glycyrrhizas....................................................5.1-2876 Ammonii hydrogenocarbonas.. (988)Ammonio methacrylate copolymer(type A) (983)Ammonio methacrylate copolymer(type B) (984)Ammonio methacrylatis copolymerum A (983)Ammonio methacrylatis copolymerum B (984)Ammonium(2.4.1.) (103)Ammonium bromide (985)Ammonium chloride (986)Ammonium glycyrrhizate................................................5.1-2876 Ammonium hydrogen carbonate.. (988)Amobarbital (988)Amobarbital sodium (989)Amobarbitalum (988)Amobarbitalum natricum (989)Amoxicillin sodium (990)Amoxicillin trihydrate......................................................5.3-3440 Amoxicillinum natricum (990)Amoxicillinum trihydricum...........................................5.3-3440 Amperometric titration(2.2.19.).. (34)Amphotericin B (995)Amphotericinum B (995)Ampicillin,anhydrous (996)Ampicillin sodium (998)Ampicillin trihydrate (1001)Ampicillinum anhydricum (996)Ampicillinum natricum (998)Ampicillinum trihydricum (1001)Amygdalae oleum raffinatum.......................................5.4-3893 Amygdalae oleum virginale..........................................5.3-3437 Amylum pregelificatum (2490)Anaesthetic ether (1549)Analysis,thermal(2.2.34.) (52)Analytical sieving,particle-size distribution estimation by (2.9.38.).............................................................................5.3-3368 Angelicae radix (1003)Angelica root (1003)Anhydrous silica,hydrophobic colloidal......................5.5-4297 Animal anti-T lymphocyte immunoglobulin for human use (1010)Animal spongiform encephalopathies,products with risk of transmitting agents of (577)Animal spongiform encephalopathy agents,minimising the risk of transmitting via human and veterinary medicinal products(5.2.8.) (463)Aniseed (1006)Anise oil (1004)Anisi aetheroleum (1004)Anisidine value(2.5.36.) (142)Anisi fructus (1006)Anisi stellati aetheroleum (2488)Anisi stellati fructus.........................................................5.5-4297 Antazoline hydrochloride. (1006)Antazolini hydrochloridum (1006)Anthrax spore vaccine(live)for veterinary use (715)Anti-A and anti-B haemagglutinins(indirect method)(2.6.20.) (174)Antibiotics,microbiological assay of(2.7.2.) (188)Anticoagulant and preservative solutions for human blood (1007)Anticomplementary activity of immunoglobulin(2.6.17.)..170 Anticorpora monoclonalia ad usum humanum......5.2-3127 Anti-D antibodies in human immunoglobulins for intravenous administration,test for(2.6.26.)..................................5.3-3348 Anti-D immunoglobulin,human. (1732)Anti-D immunoglobulin,human,assay of(2.7.13.) (205)。
荷尔登生物医学检测产品说明书
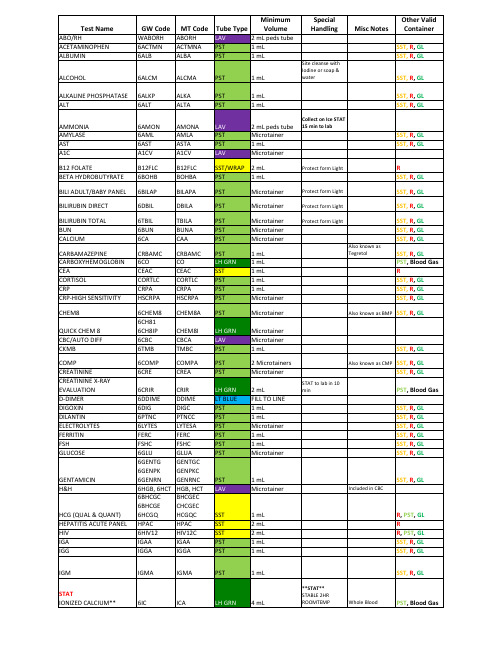
1mL
R
1mL
SST, R, GL
1mL
No capillary
1mL
collection
Order of Draw: 1. BLOOD CULTURES 2. LT BLUE 3. RED 4. SST 5. PST/LH GRN 6. LAV 7. GRAY
M2909 (1-19)
Microtainer Microtainer 1 mL
2 Microtainers Microtainer
2 mL FILL TO LINE 1 mL 1 mL Microtainer 1 mL 1 mL Microtainer
SST, R, GL
STAT to lab in 10 min
Also known as CMP SST, R, GL SST, R, GL
TOBRAMYCIN TOTAL PROTEIN TROPONIN TSH
TYPE AND CROSSMATCH
TYPE AND SCREEN URIC ACID
VANCOMYCIN VALPROIC ACID VITAMIN D
FEA
6LA LHC 6LIP
6LIVER 6LIPID LIA 6MG 6METX 6MONO PHNOC 6PFA 6PHOS 6PBNP 6PCAL 6KA 6PTIMEN PSAC 6PTT 6RETCT 6RENAL WHRIG 6SAL SYPHB TBSATA TT3C T4C TESTOC THEOC
CHEM8
QUICK CHEM 8 CBC/AUTO DIFF CKMB
COMP CREATININE CREATININE X-RAY EVALUATION D-DIMER DIGOXIN DILANTIN ELECTROLYTES FERRITIN FSH GLUCOSE
二氯二取代茂锆配合物的FTIR研究

◎ I6 ◎
石化公司研究院 高 级工 程师 . 主要从 事 大型 仪 器 的分析
工作 。
维普资讯
第 2期
潘玲 。 二氯二取代茂锫配台 物的 f I 等 T R研 究
甲基 的不 对称 伸缩 振动 c H和对 称 伸缩 振动 — ( — H) 29 8 m 和 28 0 m 分 别 为 亚 甲基 c ; 2 c 6 c
是 鉴定其 结 构 的重 要 途径 。 因此 , 者 对 中国 石 笔 油 吉林 石化公 司研究 院合 成橡 胶 研究 所 开发 的两 种 二 氯二取代 茂 错 配合物 的 中红 外光 谱及 远 红外 光谱 进行 了较 为详 细 的研 究 . 并对 其 特征 吸收 峰 进 行 了归属 , 确定 配合 物 的结 构提供 了依据 。 为
1157cm一l186cm归属为季碳的骨架振动吸收峰而1599em则归属为苯环的cc1074cm归属为苯环的面内ch8l8cm和1032cm分别归属为茂环的面外8ch和面内8ch与图1相比可看出1383cm和1366em中等强度的双峰消失了取而代之的是l378cm的弱吸收峰说明1378cm是烷基上的甲基对称弯曲振动而季碳上的甲基被苯基取代了由此证明了此配合物的配体结构为
为 2 分 束 器选 择 2 . 。
烯 烃 聚合 的立 体 选 择性 都 很 高 J 因 此 得 到 广 ,
泛 的 应 用 。对 于 此 类 配 合 物 的 红 外 光 谱 研 究 一 直
2 结 果 与讨 论
2 1 两 种 配 台 物 在 4 O一4O O mI 波 数 范 围 内 . 0 Oc 1 特 征 吸 收 峰 的 归 属
维普资讯
分析 测 试
弹 C22(E4 性,.52R 体l.2:C 20.)I H AM5 041 5 0ET2 N LO AS
同步检测10种转基因玉米品系的试剂盒、及其使用方法[发明专利]
![同步检测10种转基因玉米品系的试剂盒、及其使用方法[发明专利]](https://img.taocdn.com/s3/m/23aaaf3d50e2524de4187e9e.png)
专利名称:同步检测10种转基因玉米品系的试剂盒、及其使用方法
专利类型:发明专利
发明人:徐君怡,曹际娟,赵昕,曹冬梅
申请号:CN201210172121.5
申请日:20120530
公开号:CN102719533A
公开日:
20121010
专利内容由知识产权出版社提供
摘要:本发明公开一种实时荧光RCA技术同步检测10种转基因玉米品系的试剂盒、及其使用方法,属于转基因食品检测技术领域,其通过锁式探针RCA技术与实时荧光PCR技术相结合,建立了实时荧光PCR-锁式通用探针滚环扩增(RCA),针对10种转基因玉米品系分别设计锁式探针SEQ ID NO:1~10,通过滚环扩增技术与待检测样品中的靶标序列互补结合,在连接酶作用下使探针环化;进一步通过滚环扩增的通用引物区,实现环状锁式探针的级联扩增;本发明试剂盒具有高通量、特异性、准确性、高灵敏等特点,锁式探针独特的设计,满足了专化性检测的需要,而且可以结合多种扩增方式及检测标记物,适应多种检测平台,填补了国内外空白。
申请人:徐君怡,曹际娟,赵昕,曹冬梅
地址:116001 辽宁省大连市中山区长江东路60号
国籍:CN
代理机构:大连东方专利代理有限责任公司
代理人:贾汉生
更多信息请下载全文后查看。
核糖核酸定量测定——改良苔黑酚法

核糖核酸定量测定——改良苔黑酚法一、目的学习用定糖法测定核糖核酸(RNA)的含量。
二、原理RNA与浓盐酸共热酸解生成嘧啶核苷酸、嘌呤碱基及核糖。
核糖在浓酸中脱水环化成糠醛。
它与苔黑酚(3,5-二羟甲苯)作用显示蓝绿色,在670毫微米有最大吸收。
本测定用铜离子代替苔黑酚法中的铁离子进行催化,故称为改良苔黑酚法。
它使反应灵敏度提高一倍以上。
待测样品中若RNA在5~50微克/毫升之间,则光密度与RNA 的浓度成线性关系。
样品中少量脱氧核糖核酸(DNA)存在对测定无干扰,蛋白质、粘多糖则干扰测定。
由于测糖法只能测定RNA中与嘌呤连接的糖,而不同来源的RNA含的嘌呤、嘧啶的比例各不相同,因此用所测得的核糖量来换算各种RNA的含量是不正确的。
最好用与被测物相同来源的纯化RNA作RNA-核糖标准曲线,然后通过此标准曲线查出被测物中RNA的含量。
三、实验材料,仪器和试剂1、实验材料RNA 标准溶液:在分析天平上精确称取纯RNA,用0.001N NaOH 配成50微克/毫升的溶液。
待测RNA样品:用0.001N NaOH将其配成10~100微克/毫升溶液。
2、仪器移液管、试管、72型分光光度计、水浴锅3.试剂苔黑酚铜离子试剂:甲、苔黑酚贮备溶液:5克苔黑酚溶于10毫升95%乙醇中,溶液呈深红色。
乙、铜离子溶液:0.75克氯化铜(CuCl₂·2H₂O)溶于500毫升12N盐酸中,溶液呈深黄色。
使用前2 毫升苔黑酚贮备液加100毫升铜离子溶液混匀。
四、操作步骤1、标准曲线的制作按下表次序在7支试管内加入50微克/毫升的RNA 标准溶液与试剂,充分摇匀后在100₂水浴中保温35分钟,流动水冷却。
以1号管作空白,在分光光度计上于670毫微米下测光密度(OD)值。
2、样品中RNA 含量测定吸取2毫升含RNA 约10~100微克/毫升的待测样品溶液与2毫升苔黑酚铜离子溶液充分摇匀。
同制作标准曲线同样步骤操作。
670毫微米下测得OD值,在标准曲线上可找出相应的RNA 量。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
CHAPTER 15EQUITYCHAPTER LEARNING OBJECTIVES1. Discuss the characteristics of the corporate form of organization.2. Identify the key components of equity.3. Explain the accounting procedures for issuing shares.4. Describe the accounting for treasury shares.5. Explain the accounting for and reporting of preference shares.6. Describe the policies used in distributing dividends.7. Identify the various forms of dividend distributions.8. Explain the accounting for share dividends and share splits.9. Indicate how to present and analyze equity.*10. Explain the different types of preference share dividends and their effect on book value per share.Test Bank for Intermediate Accounting, IFRS Edition, 2e15 - 2TRUE-FALSE—Conceptual1. A corporation is incorporated in only one country regardless of the number of countries inwhich it operates.2. The preemptive right allows shareholders the right to vote for directors of the company.3. Ordinary shares is the residual corporate interest that bears the ultimate risks of loss.4. Earned capital consists of contributed capital and retained earnings.5. True no-par shares should be carried in the accounts at issue price without any sharepremium reported.6. Companies allocate the proceeds received from a lump-sum sale of securities based onthe securities’ par values.7. Companies should record shares issued for services or noncash property at either the fairvalue of the shares issued or the fair value of the consideration received.8. Treasury shares are a company’s own shares that have been reacquired and retired.9. The cost method records all transactions in treasury shares at their cost and reports thetreasury shares as a deduction from ordinary shares.10. When a corporation sells treasury shares below its cost, it usually debits the differencebetween cost and selling price to Share Premium—Treasury.11. Participating preference shares require that if a company fails to pay a dividend in anyyear, it must make it up in a later year before paying any ordinary dividends.12. Callable preference shares permit the corporation at its option to redeem the outstandingpreference shares at stipulated prices.13. The laws of some jurisdictions require that corporations restrict their contributed capitalfrom distribution to shareholders.14. Many companies pay dividends in amounts equal to their legally available retainedearnings.15. All dividends, except for liquidating dividends, reduce the total shareholders’equity of acorporation.16. Dividends payable in assets of the corporation other than cash are called propertydividends or dividends in kind.17. When a share dividend is declared on the ordinary shares outstanding, a company isrequired to transfer the par value of the shares issued from retained earnings.Equity 15 - 3 18. Share splits and share dividends have the same effect on a company’s retained earningsand total shareholders’ equity.19. The return on ordinary share equity is computed by dividing net income by the averageordinary equity.20. The payout ratio is determined by dividing cash dividends paid to ordinary shareholdersby net income available to ordinary shareholders.MULTIPLE CHOICE—Conceptual21. The residual interest in a corporation belongs to thea. management.b. creditors.c. ordinary shareholders.d. preference shareholders.22. The pre-emptive right of an ordinary shareholder is the right toa. share proportionately in corporate assets upon liquidation.b. share proportionately in any new issues of stock of the same class.c. receive cash dividends before they are distributed to preference shareholders.d. exclude preference shareholders from voting rights.23. The pre-emptive right enables a shareholder toa. share proportionately in any new issues of shares of the same class.b. receive cash dividends before other classes of stock without the pre-emptive right.c. sell ordinary shares back to the corporation at the option of the shareholder.d. receive the same amount of dividends on a percentage basis as the preferenceshareholders.S24. Special characteristics of the corporate form that affect accounting include thea. influence of corporate law.b. use of the share system.c. development of a variety of ownership interests.d. All of these answer choices are correct.Test Bank for Intermediate Accounting, IFRS Edition, 2e15 - 425. Hiro Corp. issues shares which bear the ultimate risks of loss and receive the benefit ofsuccess. These shares are not guaranteed dividends nor assets upon dissolution. These shares are consideredOrdinary Preferencea. Yes Yesb. Yes Noc. No Yesd. No No26. Categories of equity include all of the following excepta. Non-controlling interest.b. Accumulated other comprehensive income.c. Liquidating dividends.d. Treasury shares.S27. Shareholders of a business enterprise are said to be the residual owners. The term residual owner means that shareholdersa. are entitled to a dividend every year in which the business earns a profit.b. have the rights to specific assets of the business.c. bear the ultimate risks and uncertainties and receive the benefits of enterpriseownership.d. can negotiate individual contracts on behalf of the enterprise.28. Total shareholders’ equity representsa. a claim to specific assets contributed by the owners.b. the maximum amount that can be borrowed by the enterprise.c. a claim against a portion of the total assets of an enterprise.d. only the amount of earnings that have been retained in the business.29. A primary source of shareholders’ equity isa. income retained by the corporation.b. appropriated retained earnings.c. contributions by shareholders.d. both income retained by the corporation and contributions by holders.30. Equity is generally classified into two major categories:a. contributed capital and appropriated capital.b. appropriated capital and retained earnings.c. retained earnings and unearned capital.d. earned capital and contributed capital.31. The accounting problem in a lump sum issuance is the allocation of proceeds between theclasses of securities. An acceptable method of allocation is thea. pro forma method.b. proportional method.c. incremental method.d. either the proportional method or the incremental method.Equity 15 - 5 32. When a corporation issues its ordinary shares in payment for services, the leastappropriate basis for recording the transaction is thea. fair value of the services received.b. par value of the shares issued.c. fair value of the shares issued.d. Any of these provides an appropriate basis for recording the transaction.33. Direct costs incurred to sell shares such as underwriting costs should be accounted for as1. a reduction of share premium.2. an expense of the period in which the shares are issued.3. an intangible asset.a. 1b. 2c. 3d. 1 or 334. A “secret reserve” will be created ifa. inadequate depreciation is charged to income.b. a capital expenditure is charged to expense.c. liabilities are understated.d. shareholders’ equity is overstated.P35. Which of the following represents the total number of shares that a corporation may issue under the terms of its charter?a. authorized sharesb. issued sharesc. unissued sharesd. outstanding sharesS36. Shares that have a fixed per-share amount printed on each share certificate are calleda. stated value shares.b. fixed value shares.c. uniform value shares.d. par value shares.S37. Which of the following is not a legal restriction related to profit distributions by a corporation?a. The amount distributed to owners must be in compliance with the laws governingcorporations.b. The amount distributed in any one year can never exceed the net income reported forthat year.c. Profit distributions must be formally approved by the board of directors.d. Dividends must be in full agreement with the capital contracts as to preferences andparticipation.38. Ordinary no-par sharesa. Are considered illegal.b. May be subject to high taxes.c. May be sold at a premium.d. All of these answer choices are correct.Test Bank for Intermediate Accounting, IFRS Edition, 2e15 - 639. Dunn Trading Co. issued 2,500 ordinary shares, The shares have a ₤2 par value and soldfor ₤12 per share. Dunn incurre d ₤3,000 to sell the shares related to underwriting costs and legal fees. Dunn Trading Co. will record the ₤3,000 asa. A debit to Share Premium—Ordinary.b. A debit to Financing Expense.c. A credit to Share Premium—Ordinary.d. A credit to Share Capital—Ordinary.S40. In January 2015, Finley Corporation, a newly formed company, issued 10,000 shares of its $10 par ordinary shares for $15 per share. On July 1, 2015, Finley Corporation reacquired 1,000 shares of its outstanding shares for $12 per share. The acquisition of these treasury sharesa. decreased total shareholders’ equity.b. increased total shareholders’ equity.c. did not change total shareholders’ equity.d. decreased the number of issued shares.P41. Treasury shares area. shares held as an investment by the treasurer of the corporation.b. shares held as an investment of the corporation.c. issued and outstanding shares.d. issued but not outstanding shares.42. When treasury shares are purchased for more than the par value of the shares and thecost method is used to account for treasury shares, what account(s) should be debited?a. Treasury shares for the par value and share premium for the excess of the purchaseprice over the par value.b. share premium for the purchase price.c. Treasury shares for the purchase price.d. Treasury shares for the par value and retained earnings for the excess of thepurchase price over the par value.43. “Gains” on sales of treasury shares (using the cost method) should be credited toa. share premium—treasury.b. share capital.c. retained earnings.d. other income.44. Porter Corp. purchased its own par value shares on January 1, 2015 for $20,000 anddebited the treasury shares account for the purchase price. The shares were subsequently sold for $12,000. The $8,000 difference between the cost and sales price should be recorded as a deduction froma. share premium—treasury to the extent that previous net “gains”from sales of thesame class of stock are included therein; otherwise, from retained earnings.b. share premium—treasury without regard as to whether or not there have beenprevious net “gains” from sales of the same class of shares included therein.c. retained earnings.d. net income.Equity 15 - 7 45. How should a “gain” from the sale of treasury shares be reflected when using the costmethod of recording treasury shares transactions?a. As other income shown on the income statement.b. As share premium from treasury share transactions.c. As an increase in the amount shown for share capital.d. As an increase in the retained earnings amount.46. Which of the following best describes a possible result of treasury share transactions by acorporation?a. May increase but not decrease retained earnings.b. May increase net income if the cost method is used.c. May decrease but not increase retained earnings.d. May decrease but not increase net income.47. Which of the following features of preference shares makes the security more like debtthan an equity instrument?a. Participatingb. Votingc. Redeemabled. Noncumulative48. The cumulative feature of preference sharesa. limits the amount of cumulative dividends to the par value of the preference shares.b. requires that preference dividends not paid in any year must be made up in a lateryear before dividends are distributed to ordinary shareholders.c. means that the shareholder can accumulate preference shares until it is equal to thepar value of ordinary shares at which time it can be converted into ordinary shares.d. enables a preference shareholder to accumulate dividends until they equal the parvalue of the shares and receive the shares in place of the cash dividends.P49. According to IFRS, redeemable preference shares should bea. included with ordinary shares.b. included as a liability.c. excluded from the statement of financial position.d. included as a contra item in shareholders’ equity.S50. Cumulative preference dividends in arrears should be shown in a corporation’s statement of financial position asa. an increase in current liabilities.b. an increase in equity.c. a footnote.d. an increase in current liabilities for the current portion and non-current liabilities for thelong-term portion.51. The features most frequently associated with preference shares include all of the followingexcepta. Callable at the option of the shareholder.b. Convertible into ordinary shares.c. Non-voting.d. Preference as to assets in the event of liquidation.Test Bank for Intermediate Accounting, IFRS Edition, 2e15 - 852. When preference shares share ratably with the ordinary shareholders in any profitdistributions beyond the prescribed rate this is known as thea. Cumulative feature.b. Participating feature.c. Callable feature.d. Redeemable feature.53. Liquidating dividendsa. Are prohibited under IFRS.b. Require a credit to Share Capital—Ordinary.c. Reduce amounts paid-in by shareholders.d. All of these answer choices are correct.54. At the date of the financial statements, ordinary shares issued would exceed ordinaryshares outstanding as a result of thea. declaration of a share split.b. declaration of a share dividend.c. purchase of treasury shares.d. payment in full of subscribed shares.55. An entry is not made on thea. date of declaration.b. date of record.c. date of payment.d. An entry is made on all of these dates.56. Cash dividends are paid on the basis of the number of sharesa. authorized.b. issued.c. outstanding.d. outstanding less the number of treasury shares.57. Which of the following statements about property dividends is not true?a. A property dividend is usually in the form of securities of other companies.b. A property dividend is also called a dividend in kind.c. The accounting for a property dividend should be based on the carrying value (bookvalue) of the nonmonetary assets transferred.d. All of these statements are true.58. Houser Corporation owns 4,000,000 shares of Baha Corporation. On December 31, 2015,Houser distributed these shares as a dividend to its shareholders. This is an example of aa. property dividend.b. share dividend.c. liquidating dividend.d. cash dividend.59. A dividend which is a return to shareholders of a portion of their original investments is aa. liquidating dividend.b. property dividend.c. liability dividend.d. participating dividend.Equity 15 - 9 60. A mining company declared a liquidating dividend. The journal entry to record thedeclaration must include a debit toa. Retained Earnings.b. Share Premium.c. Accumulated Depletion.d. Accumulated Depreciation.61. If management wishes to “capitalize” part of the earnings, it may issue aa. cash dividend.b. share dividend.c. property dividend.d. liquidating dividend.62. Which dividends do not reduce equity?a. Cash dividendsb. Share dividendsc. Property dividendsd. Liquidating dividends63. The declaration and issuance of a share dividenda. increases ordinary shares outstanding and increases total equity.b. decreases retained earnings but does not change total equity.c. may increase share premium but does not change total equity.d. increases retained earnings and increases total equity.64. Quirk Corporation issued a 100% share dividend of its ordinary shares which had a parvalue of $10 before and after the dividend. At what amount should retained earnings be capitalized for the additional shares issued?a. There should be no capitalization of retained earnings.b. Par valuec. Fair value on the declaration dated. Fair value on the payment date65. The issuer of an ordinary share dividend to ordinary shareholders should transfer fromretained earnings to contributed capital an amount equal to thea. fair value of the shares issued.b. book value of the shares issued.c. minimum legal requirements.d. par or stated value of the shares issued.66. At the date of declaration of an ordinary share dividend, the entry should not includea. a credit to Share Capital—Ordinary.b. a credit to Ordinary Share Dividend Distributable.c. a debit to Retained Earnings.d. All of these are acceptable.Test Bank for Intermediate Accounting, IFRS Edition, 2e15 - 1067. The balance in Ordinary Share Dividend Distributable should be reported as a(n)a. deduction from share capital—ordinary.b. addition to share capital—ordinary.c. current liability.d. contra current asset.68. A feature common to both share splits and share dividends isa. a transfer to earned capital of a corporation.b. that there is no effect on total equity.c. an increase in total liabilities of a corporation.d. a reduction in the contributed capital of a corporation.69. What effect does the issuance of a 2-for-1 share split have on each of the following?Par Value per Share Retained Earningsa. No effect No effectb. Increase No effectc. Decrease No effectd. Decrease Decrease70. Which one of the following disclosures should be made in the equity section of thestatement of financial position, rather than in the notes to the financial statements?a. Dividend preferencesb. Liquidation preferencesc. Call pricesd. Conversion or exercise prices71. The return on ordinary share equity is calculated by dividinga. net income less preference dividends by average ordinary shareholders’ equity.b. net income by average ordinary shareholders’ equity.c. net income less preference dividends by ending ordinary shareholders’ equity.d. net income by ending ordinary shareholders’ equity.72. The payout ratio can be calculated by dividinga. dividends per share by earnings per share.b. cash dividends by net income less preference dividends.c. cash dividends by market price per share.d. dividends per share by earnings per share and dividing cash dividends by net incomeless preference dividends.73. Younger Company has outstanding both ordinary shares and nonparticipating, non-cumulative preference shares. The liquidation value of the preference shares is equal to its par value. The book value per share of the ordinary shares is unaffected bya. the declaration of a share dividend on preference payable in preference shares whenthe market price of the preference is equal to its par value.b. the declaration of a share dividend on ordinary shares payable in ordinary shareswhen the market price of the ordinary shares is equal to its par value.c. the payment of a previously declared cash dividend on the ordinary shares.d. a 2-for-1 split of the ordinary shares.P74. Ordinary shareholders’equity divided by the number of ordinary shares outstanding is calleda. book value per share.b. par value per share.c. stated value per share.d. market value per share.75. Which of the following is a required statement under IFRS?Statement of financial position Statement of changes in equitya. Yes Nob. Yes Yesc. No Nod. No Yes76. Trading on the equity is:a. The ratio of the company’s cash dividends to net income.b. A return on assets that is higher than the cost of financing these assets.c. The amount each share would receive if the company were liquidated.d. The “Revaluation Surplus” related to increases or decreases in items such as property,plant, and equipment.*77. Dividends are not paid ona. noncumulative preference shares.b. nonparticipating preference shares.c. treasury shares.d. Dividends are paid on all of these.*78. Noncumulative preferred dividends in arrearsa. are not paid or disclosed.b. must be paid before any other cash dividends can be distributed.c. are disclosed as a liability until paid.d. are paid to preference shareholders if sufficient funds remain after payment of thecurrent preference dividend.*79. How should cumulative preference dividends in arrears be shown in a corporation’s statement of financial position?a. Note disclosureb. Increase in shareholders’ equityc. Increase in current liabilitiesd. Increase in current liabilities for the amount expected to be declared within the year oroperating cycle, and increase in non-current liabilities for the balanceMULTIPLE CHOICE—ComputationalUse the following information for questions 80 and 81.Presented below is information related to Hale Corporation:Share Capital—Ordinary, $1 par $4,300,000Share premium—Ordinary 550,000Share Capital—Preference 8 1/2%, $50 par 2,000,000Share premium—Preference 400,000Retained Earnings 1,500,000Treasury Shares—Ordinary (at cost) 150,00080. The total equity of Hale Corporation isa. $8,600,000.b. $8,750,000.c. $7,100,000.d. $7,250,000.81. The total contributed capital related to the ordinary shares isa. $4,300,000.b. $4,850,000.c. $5,250,000.d. $4,700,000.82. Manning Company issued 10,000 shares of its $5 par value ordinary shares having a fairvalue of $25 per share and 15,000 shares of its $15 par value preference shares having a fair value of $20 per share for a lump sum of $480,000. How much of the proceeds would be allocated to the ordinary shares?a. $50,000b. $218,182c. $250,000d. $255,00083. Norton Company issues 4,000 shares of its $5 par value ordinary shares having a fairvalue of $25 per share and 6,000 shares of its $15 par value preference shares having a fair value of $20 per share for a lump sum of $192,000. What amount of the proceeds should be allocated to the preference shares?a. $172,000b. $120,000c. $104,727d. $90,00084. Berry Corporation has 50,000 shares of $10 par ordinary shares authorized. The followingtransactions took place during 2015, the first year of the corporation’s existence: Sold 5,000 ordinary shares for $18 per share.Issued 5,000 ordinary shares in exchange for a patent valued at $100,000.At the end of the Berry’s first year, total contributed capital amounted toa. $40,000.b. $90,000.c. $100,000.d. $190,000.85. Glavine Company issues 6,000 shares of its $5 par value ordinary shares having a marketvalue of $25 per share and 9,000 shares of its $15 par value preference shares having a fair value of $20 per share for a lump sum of $288,000. The proceeds allocated to the ordinary shares isa. $30,000b. $130,909c. $150,000d. $157,09186. Wheeler Company issued 5,000 shares of its $5 par value ordinary shares having amarket value of $25 per share and 7,500 shares of its $15 par value preference shares having a market value of $20 per share for a lump sum of $240,000. The proceeds allocated to the preference shares isa. $215,000b. $150,000c. $130,909d. $109,09187. Hiro Corp. issues 1,000 €5 par value ordinary shares and 1,000 €20 par value preferenceshares for a lump sum of €60,000. At the issue date, the ordinary shares were selling for €36 and the preference shares were selling for €28. The Share Premium—Ordinary account will be credited fora. €31,000b. €36,000c. €26,250d. €28,75088. Hiro Corp. issues 1,000 €5 par value ordinary shares and 1,000 €20 par value preferenceshares for a lump sum of €60,000. At the issue date, the ordinary shares were sell ing for €36 and the preference shares were selling for €28. How much is recorded in Hiro’s statement of financial position for the preference shares?a. €31,000b. €36,000c. €26,250d. €28,75089. On January 1 Hiro Corp. issues 1,000 no-par ordinary sha res for €15 per share. Theshares have a stated value of €5 per share. When Hiro prepares the journal entry to record the issuance of the shares which of the following will be recorded?a. Debit Share Capital—Ordinary €5,000.b. Credit Share Capital—Ordina ry €15,000.c. Debit Share Premium—Ordinary €15,000.d. Credit Share Premium—Ordinary €10,00090. Five years ago, Dunn Trading Co. issued 2,500 ordinary shares. The shares have a ₤2par value and sold at that time for ₤12 per share. On January 1, 2015, Dunn Trading Co.Purchased 1,000 of these shares for ₤24 per share. On September 30, 2015, Dunn reissued 500 of the shares for ₤28 per share. The journal entry to record the reissuance will includea. A debit to Treasury Shares ₤12,000.b. A credit to Share Premium—Treasury ₤2,000.c. A credit to Treasury Shares ₤14,000.d. A credit to cash ₤14,000.91. Pember Corporation started business in 2009 by issuing 200,000 shares of $20 parordinary shares for $36 each. In 2014, 20,000 of these shares were purchased for $52 per share by Pember Corporation and held as treasury shares. On June 15, 2015, these 20,000 shares were exchanged for a piece of property that had an assessed value of $810,000. Pember’s shares are actively traded and had a fair price of $60 on June 15, 2015. The cost method is used to account for treasury shares. The amount of share premium—treasury resulting from the above events would bea. $800,000.b. $480,000.c. $390,000.d. $160,000.92. On September 1, 2015, Valdez Company reacquired 12,000 shares of its $10 par valueordinary shares for $15 per share. Valdez uses the cost method to account for treasury shares. The journal entry to record the reacquisition of the shares should debita. Treasury Shares for $120,000.b. Share Capital—Ordinary for $120,000.c. Share Capital—Ordinary for $120,000 and Share Premium—Ordinary for $60,000.d. Treasury Shares for $180,000.93. Gannon Company acquired 6,000 shares of its own ordinary shares at $20 per share onFebruary 5, 2010, and sold 3,000 of these shares at $27 per share on August 9, 2015.The fair value of Gannon’s ordinary shares was $24 per share at December 31, 2014, and $25 per share at December 31, 2015. The cost method is used to record treasury shares transactions. What account(s) should Gannon credit in 2015 to record the sale of 3,000 shares?a. Treasury Shares for $81,000.b. Treasury Shares for $60,000 and Share Premium—Treasury for $21,000.c. Treasury Shares for $60,000 and Retained Earnings for $21,000.d. Treasury Shares for $72,000 and Retained Earnings for $9,000.94. Long Co. issued 100,000 shares of $10 par ordinary shares for $1,200,000. Longacquired 8,000 shares of its own shares at $15 per share. Three months later Long sold 4,000 of these shares at $19 per share. If the cost method is used to record treasury shares transactions, to record the sale of the 4,000 treasury shares, Long should credita. Treasury Shares for $76,000.b. Treasury Shares for $40,000 and Share Premium—Treasury for $36,000.c. Treasury Shares for $60,000 and Share Premium—Treasury Stock for $16,000.d. Treasury Shares for $60,000 and Share Premium—Ordinary for $16,000.95. An analysis of equity of Hahn Corporation as of January 1, 2015, is as follows:Share capital—ordinary, par value $20; authorized 100,000 shares;issued and outstanding 90,000 shares $1,800,000 Share premium—ordinary 900,000Retained earnings 760,000Total $3,460,000 Hahn uses the cost method of accounting for treasury shares and during 2015 entered into the following transactions:Acquired 2,500 of its shares for $75,000.Sold 2,000 treasury shares at $35 per share.Sold the remaining treasury shares at $20 per share.Assuming no other equity transactions occurred during 2015, what should Hahn report at December 31, 2015, as total share premium?a. $895,000b. $900,000c. $905,000d. $915,00096. Percy Corporation was organized on January 1, 2015, with an authorization of 1,200,000ordinary shares with a par value of $6 per share. During 2015, the corporation had the following capital transactions:January 5 issued 675,000 shares @ $10 per shareJuly 28 purchased 90,000 shares @ $11 per shareDecember 31 sold the 90,000 shares held in treasury @ $18 per share Percy used the cost method to record the purchase and reissuance of the treasury shares.What is the total amount of share premium as of December 31, 2015?a. $-0-.b. $2,070,000.c. $2,700,000.d. $3,330,000.。
