九年级下册:第9单元测试卷
九年级化学下册《常见的酸、碱、盐》单元测试卷(含答案)
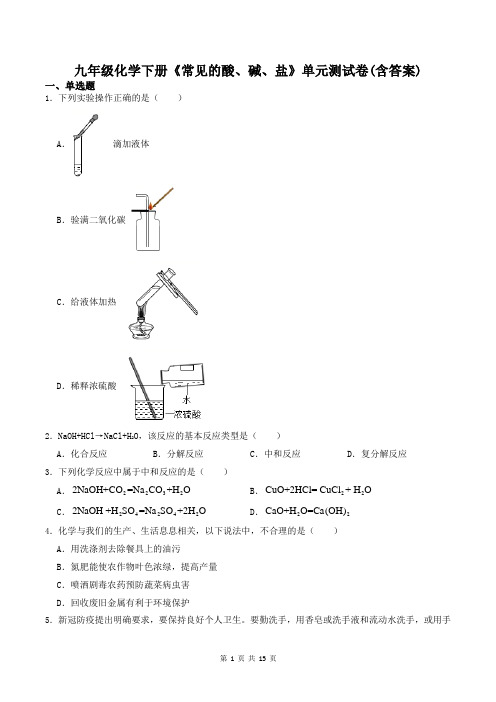
九年级化学下册《常见的酸、碱、盐》单元测试卷(含答案)一、单选题1.下列实验操作正确的是( )A . 滴加液体B .验满二氧化碳C .给液体加热D .稀释浓硫酸2.NaOH+HCl →NaCl+H 2O ,该反应的基本反应类型是( )A .化合反应B .分解反应C .中和反应D .复分解反应3.下列化学反应中属于中和反应的是( )A .22322NaOH+CO =Na CO +H OB .22CuO+2HCl= CuCl + H OC .242422NaOH +H SO =Na SO +2H OD .22CaO+H O=Ca(OH)4.化学与我们的生产、生活息息相关,以下说法中,不合理的是( )A .用洗涤剂去除餐具上的油污B .氮肥能使农作物叶色浓绿,提高产量C .喷洒剧毒农药预防蔬菜病虫害D .回收废旧金属有利于环境保护5.新冠防疫提出明确要求,要保持良好个人卫生。
要勤洗手,用香皂或洗手液和流动水洗手,或用手消毒剂消毒。
不同品牌的洗手液pH一般不同,25℃时四种洗手液的pH如图所示。
下列说法错误的是()A.洗手液a用蒸馏水稀释后pH减小B.洗手液b的酸性比a弱C.洗手液c能使石蕊试液变蓝色D.洗手液d和a混合液的pH可能等于76.科学的归纳推理可以由点到面得到普遍存在的规律。
以下推理所得规律正确的是()A.某碳原子的原子核里有6个质子和6个中子,则所有原子中质子数与中子数都相等B.碳酸钙与盐酸反应生成二氧化碳,则可溶性碳酸盐与盐酸反应都可能生成二氧化碳C.稀盐酸和氢氧化钠溶液中和时无明显现象,则所有酸碱中和反应都没有明显现象D.镁、锌可以置换出硫酸铜溶液中的铜离子,则所有金属都可以置换出盐溶液中的铜离子7.分析推理是一种重要的思维方法,以下推理不正确的是()A.物体振动一定产生声音,所以有声音产生则一定有物体发生了振动B.氯化钠、硫酸铜等盐中都含有金属元素,但是盐中不一定都含有金属元素C.惯性的大小只与物体的质量有关,所以物体的质量越大,惯性越大D.碳酸盐与盐酸反应放出气体,所以与盐酸反应放出气体的物质都是碳酸盐8.下列关于物质用途的描述中,不正确的是()A.石墨用作电极材料,活性炭用作吸附家装产生的有害气体B.聚乙烯塑料用作食品包装袋,可以大量使用C.磷酸二氢铵(NH4H2PO4)用作复合肥料D.氧气用作气焊9.某果农发现其种植的桔树生长迟缓,叶色淡黄。
人教版九年级英语Unit 9单元测试卷及答案

人教版九年级英语Unit 9单元测试卷第一部分(听力共30分)I.听对话,选答案(共15小题,计20分)第一节:听下面10段对话,每段对话后有一个问题,读两遍。
请根据每段对话的内容和后面的问题,从所给的三个选项中选出最恰当的一项。
(共10小题,计10分)( ) 1.A. Soft music. B. Loud music. C. Energetic music.( ) 2. A. America. B. Canada. C. Australia.( ) 3. A. Movies that are funny. B. Movies that give him something to think about.C. Movies that have great stories.( ) 4. A. A doctor. B. A driver. C. A teacher.( ) 5. A. Movies. B. Books. C. Music.( ) 6. A. On April 30th. B. On May 13th. C. On May 30th.( ) 7. A. Jazz music. B. Country music. C. Dance music.( ) 8.A. The bus station. B. The supermarket. C. The post office.( ) 9.A. The girl’s photos. B. The girl’s clothes. C. The girl’s collection.( ) 10. A. She ate a hamburger. B. She went swimming. C. She skated.第二节:听下面两段对话,每段对话后有几道小题,请根据每段对话的内容和后面的问题,从所给的三个选项中选出最恰当的一项。
每段对话读两遍。
(共5小题,计10分)听第11段对话,回答第11、12小题。
(人教版)九年级化学下册-第九单元 溶液-单元测试卷(含答案)

第九单元 溶液 单元测试题可能用到的相对原子质量:H 1- O 16- S 32- Cu 64-一、选择题(36分)1.下列物质加入水中不能形成溶液的是( ) A .食盐B .碳酸钠C .蔗糖D .食用油2.)下列有关溶液的说法正确的是( ) A .面粉与水混合可形成溶液 B .溶液中的溶质都是固体 C .溶液中可以含有多种溶质D .无色透明的液体都是溶液3.下列有关溶液的叙述错误的是( ) A .长期放置后不会分层的液体一定是溶液 B .衣服上的油污用汽油或用加了洗涤剂的水可除去 C .一瓶合格的生理盐水密封一段时间,不会出现浑浊D .实验室常将固体药品配制成溶液进行化学反应,以提高反应速率4.某“天气瓶”通过樟脑在酒精溶液中的结晶情况反映气温变化。
如图为该“天气瓶”在三种不同气温下的状况,则瓶内溶液一定为相应气温下樟脑饱和溶液的是( )A .甲、乙B .甲、丙C .乙、丙D .甲、乙、丙5.规范的实验操作是实验成功的关键。
下列配制20 g10%的氯化钠溶液的操作不规范的是( )A .称氯化钠B .量水C .溶解D .装瓶6.生活中的下列现象,不能说明气体溶解度随温度升高而减小的是( ) A .烧开水时,沸腾前有气泡逸出B .喝下汽水时,感到有气体冲击鼻腔C .打开啤酒瓶盖时,有大量气泡逸出D .天气闷热时,池塘里的鱼浮出水面7.小军同学需要快速配制一杯可口的白糖溶液,下列措施不能达到目的的是( ) A .用冰水溶解B .用热水溶解C .把白糖碾成粉末后溶解D .溶解时用筷子搅拌8.将100 g98%的浓硫酸注入900 g 水中,所得稀硫酸中溶质的质量分数为( ) A .9.8%B .10.0%C . 10.9%10.9%D .11.1%9.如图所示,向小试管中加入一些固体NaOH ,完全溶解后,玻璃管中液面变化情况是( ) A .左高右低B .左低右高C .几乎无变化D .无法判断10.硝酸钾的溶解度随温度升高而增大,如图是有关硝酸钾溶液的实验操作及变化情况。
第9单元溶液(B卷真题通关卷)(原卷版)

20232024学年九年级化学下册单元测试卷(人教版)第九单元溶液(B卷•真题通关卷)1、训练范围:人教版九年级第9单元2、可能用到的相对原子质量:H1 C12 N14 O16 F19 Na23 S32 C135.5 K39Ca40 Fe56 Cu64 Zn65 I127第Ⅰ卷选择题一、选择题(本题共20小题,每小题2分,共40分)1.(2023•济南)下列说法中,合理的是()A.催化剂一定都能加快化学反应速率B.含有氧元素的化合物一定是氧化物C.溶液一定是均一、稳定的混合物D.生成单质和化合物的反应一定是置换反应2.(2023•宁夏)推理是重要的科学思维方法。
下列推理正确的是()A.单质是由同种元素组成的,所以由同种元素组成的物质一定是单质B.一定条件下,溶液是均一的、稳定的,所以均一的、稳定的液体一定是溶液C.某物质在氧气中燃烧生成二氧化碳和水,则该物质一定含有碳、氢、氧三种元素D.元素是质子数相同的一类原子的总称,因此质子数相同的原子一定属于同种元素3.(2022•重庆)王亚平在太空做了油和水“难分难舍”的神奇实验:她用力摇晃装有油和水的瓶子,让油水充分混合、静置,发现油水不分层。
下列说法不正确的是()A.太空中水不能作溶剂B.在地面油水混合物会产生分层现象C.在不同环境中同一实验可能现象不同D.在地面向油水混合物中加入洗涤剂会产生乳化现象4.(2023•娄底)如图是甲、乙、丙三种固体物质在水中的溶解度曲线。
下列说法正确的是()A.20℃时,甲、乙两种物质的溶解度相同B.可用降温结晶的方法将丙从它的饱和溶液中结晶出来C.20℃时,将12.5g乙物质溶于50g水中,充分溶解后溶质的质量分数小于20%D.10℃时,将乙、丙两种饱和溶液升温到20℃,此时两种溶液都变成了不饱和溶液5.(2023•永州)下列溶液中,溶剂不是水的是()A.稀硫酸B.生理盐水C.蔗糖溶液D.碘的酒精溶液6.(2022•盘锦)下列说法中错误的是()A.水是由H、O两种元素组成的B.生铁的硬度小于纯铁C.氧气约占空气体积的五分之一D.洗涤剂可以乳化油污7.(2022•呼伦贝尔)溶液的知识广泛应用于生产生活。
人教版九年级英语全一册《Unit 9 单元综合测试卷》测试题及参考答案

人教版九年级英语全一册Unit9单元综合测试卷(分数:100分时间:90分钟)第一卷听力部分(20分)Ⅰ.听句子,选择与所听句子相符合的图片(5分)1.2.3.4.5.Ⅱ.听对话和对话后面的问题,选择最佳答案(5分)6.A.Pop music. B.Classical music. C.Country music.7.A.Yes,she does.B.No,she doesn’t. C.We don’t know.8.A.Pirates(海盗)of Caribbean. B.Harry Potter. C.Titanic.9.A.Yes,she does.B.No,she doesn’t. C.We don’t know.10.A.She went to the cinema. B.She went to a concert.C.She went swimming.Ⅲ.听短文和短文后面的问题,选择最佳答案(5分)11.A.He was a famous musician.B.He was an owner of a restaurant.C.He was a poet.12.A.Yes,he did. B.No,he didn’t. C.We don’t know.13.A.To write a poem for his Berceuse.B.To have dinner together with his friend.C.To see if he could find some friends who could lend him some money for a meal.14.A.He was also a musician.B.He was very kind.C.He didn’t understand music very much.15.A.The passage didn’t tell us.B.At the age of thirty.C.Soon after he wrote his famous Berceuse.Ⅳ.听短文,完成表格信息。
人教版九年级全一册英语单元测试卷:Unit 9 I like music that I can dance to.(含答案与解析)

Unit9 I like music that I can dance to.单元测试卷一.阅读理解(共两节,满分40分)第一节(共15小题,每小题2分,满分30分)阅读下列短文,从每题所给的四个选项(A、B、C和D)中选出最佳选项。
A1.You probably eat Chinese, Italian and Indian food at .A.City HallB.Central ParkC.the City TheatreD.City College2.The Zoo here is a .A.music group concertC.popular parkD.museum theatre3.The passage is mainly about .A.city newsB.weekend activitiesC.music weekD.food festivalBLittle Women is a novel by an American writer, which was published in 1868 and 1869. The novel follows the lives of four sisters—Meg, Jo, Beth, and Amy March from childhood to womanhood.These four sisters live with their mother. Meg and Jo March, the elder sisters, both work outside the home for money to support the family. Meg teaches four children in a nearby family, while Jo helps her grandaunt March, who is very rich. Beth helps with the housework, and Amy attends school. Their nearest neighbor is a rich man whose orphaned grandson lives with him. The sisters introduce themselves to the handsome shy boy, Laurie, who is the age of Jo.Meg is the beautiful sister;Jo is the tomboy(爱打闹的女孩);Beth is the musician;and Amy is the charming artist with blond curls. Jo is easy to get angry. One of her challenges(挑战) in growing up is to control her anger. Laurie enjoys his neighbors, joining the family often in play. His grandfather, Mr. Laurence, is charmed(吸引) by Beth, and gives her the piano used by Laurie's late sister.1.Who wrote the novel Little Women?A.Louisa May Alcott.B.Jo March.urence.urie.2.What does Little Women tell about?A.About the lives of four sisters when they are young.B.About the lives of four sisters when they are old.C.About the lives of four sisters after they get married.D.About the lives of four sisters when they grow up.3.What is Amy and how does she look like?A.She is an artist and has golden curly hair.B.She is a musician and charming.C.She is a teacher and has blond curls.D.She is an artist and easy to get angry.4.What do we know about Laurie from the passage?A.He lives with the four sisters.B.He is an orphan.C.He is easy to get angry.D.He doesn't like his late sister.CFor the first time in Hong Kong's history, an artist has been written into middle school textbooks. He is considered one of the best actors in Hong Kong. Yes, he is Chow Yun Fat!The example of his long hard struggle for success has been used in a lesson in a Hong Kong middle school textbook. Using his experiences, students can learn to make the most of their time and catch every chance to succeed.Indeed, Chow has travelled a long and difficult way to reach his success. In 1955, he was born into a poor family in Hong Kong. His father was a seaman and died when he was little. He had to get up very early to feed the chickens and the pigs and worked in the fields. He described that period of life as “happy though difficult”.When he was 17, he had to leave school. He worked in a number of jobs—as a postman, camera salesman and taxi driver. These experiences gave him the way to play all kinds of roles later in life.Chow broke into the film career in the late 1970s. He was one of the hardest-working actors and starred in a number of popular TV plays and films including Shanghai Bund and A Better Tomorrow. Since 1985, he has won many awards including Taiwan's Golden Horse Awards and Hong Kong Film Awards. In 1995, he went to Hollywood. Although he was already in his 40s, he had to learn English. He even put chopsticks in his mouth to practice pronunciation.His efforts paid off. He became worldwide famous for the film Crouching Tiger,Hidden Dragon. In 1998, the mayor of Chicago set January 12 as “Chow Yun Fat Day”.1.Why was Chow's story chosen into the middle school textbook?A.All the students will understand they are living a better life.B.Young people will know they must travel a lot to reach their success.C.Chow's experiences will encourage young people to fight for success.D.Students will find something interesting from Chow's experiences.2.What did Chow Yun Fat do when he was young?A.He travelled a long journey.B.He became the best actor in Hong Kong.C.He worked as a seaman.D.He raised animals and worked in the fields.3.What's the correct order of the following events?a.Chow won Taiwan's Golden Horse Awards.b.Chow learned to speak English for films.c.Chow lived a happy and difficult life.d.“Chow Y un Fat Day”was set.e.Chow worked as a taxi driver.A.c-a-e-d-bB.c-e-a-b-dC.e-c-b-a-dD.e-a-c-b-d4.What is the best title of this passage?A.A famous actorB.A bitter childhoodC.Famous films in Hong KongD.An example of successDOnce Louis the Twelfth, the King of France, happened to walk into the kitchen of his palace. There he found a small boy busy at work and singing happily.The boy had bright eyes, and a happy, sunny face, and his looks and manners pleased the king. With a smile Louis asked him his name. He replied that his name was Simon. In answer to further questions, the king learnt that the boy was an orphan;both his father and mother were dead.“Are you content(满足的)to do this kind of work?”the king asked, pointing at the bowls which the boy was washing. “Are you willing to spend your time in the kitchen when so many of the others work upstairs?”“Why not?”answered the boy, with a twinkle in his eye.“I am doing as well as the best of them.The king himself can do no better.”“Indeed!”said the king, no little surprised at the boy’s words.“How do you know that?”“Well, sir, the king lives, and so do I. I am content with my life. Can the king say that he is content with his life?”Louis walked away, his mind full of strange, sad thoughts. No one knew better than he did that kings seldomfeel content.The next day, much to Simon’s surprise, he was called before the king. He was still more surprised when he found that his visitor of the day before was Louis himself. The king talked with him about many things,and found him to be a very quick and clever boy.After that Simon was made to serve the king directly.Step by step, he rose from one important task to another, until he became one of the best soldiers of that time. He is known in history as General La Roche, and considered as one of the greatest men of France.If Simon had not, early in life, learnt to be content with his life, he might never have been given those chances. The king did not want people who were always unhappy because they did not have as good a place as some others. You cannot too soon learn that only those who are content are happy.1.What did Simon do before he met the king?A.He sang i n the king’s palace.B.He served in the king’s army.C.He worked in the king’s kitchen.D.He guarded the king’s palace.2.Simon was made to serve the king directly because he .A.knew what the king thoughtB.was content with his lifeC.did as well as the best of othersD.had bright eyes, and a happy, sunny face3.Which of the following is the correct order about Simon?①He served the king directly.②He found the visitor was the king.③He became one of the best soldiers.④He had a chat with a visitor in the kitchen.⑤He is a well-known man in French history.A.①②⑤③④B.④②⑤①③C.③④②①⑤D.④②①③⑤4.We can learn from the story that.A.being content makes us happyB.working hard can lead to successC.it’s important to be kind to othersD.children are always more honest第二节(共5小题,每小题2分,满分10分)下面文章中有五处(第1-5题)需要添加小标题。
牛津译林版九年级英语下册9B Unit4 单元综合测试卷(含答案)

单元综合测试Test for Unit 4 of 9B一、单项填空(20分)( )1. There is a special part the main body of the machine.A. connects withB. connect withC. connected toD. is connecting to( )2. This is a big class, and of the students boys.A. two-thirds; isB. second-three; isC. two-thirds; areD. two-three; is( )3. I don't doubt she can do it well, but I doubt she is willing to do it.A. that; thatB. that;ifC. if; ifD. if; that( )4. Between 1950 and 1990, the world population doubled to 5.3 . Nearly eighty of these people live in developing, or poorer nations.A. billion; percentB. billion; percentageC. billions; percentsD. billions; percent( )5. Do you believe food Mars will be the form pills?A. on; on; toB. on; in; ofC. in; in; ofD. in; of ; in( )6. So you are still in Shanghai now. I you to Beijing.A. think: wentB. thought; have goneC. thought; had goneD. think; has been( )7. The surface of Mars is like the surface of the Earth than that of planet in our solar system(太阳系).A. much; the otherB. more; any otherC. more; the otherD. much; any other( )8. ―It's surp rising that John came out of the plane alive.―Yes, only a few people the crash.A. survivedB. surviveC. survivesD. survived in ( )9. ―I didn't know you took a bus to school.―Oh, I take a bus, but it is snowing today.A. hardlyB. neverC. sometimesD. usually( )10. The teenager plays the piano , if not better than, Mike.A. as wellB. as well asC. so wellD. so well as ( )11. Do you feel like to the cinema or would you like at home?A. to go; stayB. going; stayingC. going; stayD. going; to stay( )12. The way he did was different we were used to.A. in whichB. in whatC. from whatD. from that( )13. Don't always your daughter others. It's not good for her growth.A. compare; fromB. compare; withC. connect; toD. prevent; from ( )14. Eddie can't get his food his helmet.A. to; becauseB. /; becauseC. with; forD. to; because of ( )15. Mr Hu spoke loudly he could be heard clearly.A. for exampleB. as a resultC. so thatD. in order to( )16. We're not sure there'll be or not tomorrow.A, if; rains B. if; rainy C. whether; rain D. whether; raining ( )17. There was a big stone on the road, but it until a boy ran into it.A. didn't seeB. didn't seenC. isn’ t seenD. wasn't seen ( )18. Li Ling's mother wanted to know .A. how did she study at schoolB. what she has studied at schoolC. whether did she study hard at schoolD. if she studied hard at school( )19. ―of volunteers will be needed for 2018 International Robot Competition in Shanghai.―Let's go and them.A. Thousands; joinB. Thousand; be a member ofC. Three thousand; take part inD. Thousands; join in( )20. ―Can I get you a drink?.―. I have already got one.A.That's very nice of youB. No, thanksC. Yes, pleaseD. with pleasure二、完形填空(10分)Today some people call Amsterdam the "City of Bicycles" because it is a city which is flat and convenient(方便的)for bicycles.In the 1960s, a group of cycling fans 1 an idea. They believed that it would be better for everybody if only bicycles were allowed in the city center. They were 2 that this would help to save energy, reduce pollution and provide free public transport. The group painted hundreds of bicycles 3 and placed them in public places around Amsterdam for people to use. 4 was allowed to take them and use them for short journeys, whether he was a local or a foreigner. Wherever someonef inished a journey, thev would 5 the bike there for someone else to use. The problem was that it didn't work―all the bicycles were 6 within weeks!7 , more than thirty years later, the "white bike" is back in town―this time with a computer chip(芯片)to 8 its every move! To take a bicycle, you have to put a special card inside. The new "white bike" is not white any more but is an unusual 9 with bright colours. The bikes are parked at special parking places and people who want to use them have to take them to another place that has enough room.There is already less traffic in central Amsterdam, 10 both locals and tourists have been using the white bikes. Thanks to the good ideas of lots of people, like the cycling fans in the 1960s, many people around the world have been enjoying the city centre streets without cars for many years.( )1. A. stole B. had C. refused D. dropped( )2. A. thoughtful B. helpful C. hopeful D. thankful( )3. A. black B. brown C. blue D. white( )4. A. Anyone B. Any one C. No one D. Someone( )5. A. take B. leave C. carry D. send( )6. A. produced B. kept C. bought D. stolen( )7. A. However B. Instead C. Therefore D. Though( )8. A. make B. mark C. record D. describe( )9. A. design B. idea C. size D. experiment( )10. A. so B. because C. but D. while三、阅读理解(10分)The weather is getting hotter and you'll be getting thirstier playing basketball or riding home from school. A cold drink may be just the thing. But be careful with what you pour down your throat. Something thar looks cool may not be good for your health.There are plenty of so-called energy drinks on the market. Most of them have an attractive colour and a cool name. Their nutrition (营养)list also has various things from vitamins to ginseng(人参). Sounds greatBut after a careful check you may find that most energy drinks have high levels of caffeine(咖啡因). These drinks are aimed at young people, students, busy people and sports players.Makers sometimes say their drinks make you better at sports and can keep you awake. But be careful not to drink too much.Caffeine raises your heartbeat(心跳). Because of this, the International Olympic Committee has limited (限制) its use. The amount of caffeine in most energy drinks is at least as high as in a cup of strong coffee or strong tea.There are potential (潜在的)health dangers connected with energy drinks. Just one can of energy drink can make you nervous, have difficulty sleeping and can cause heart attacks."Teenagers should be discouraged from taking drinks with a lot of caffeine in them," an expert from the Australia Nutrition Foundation said.( )1. The teenagers like drinking energy drinks because of the following EXCEPT that .A. they have an attractive colour and a cool nameB. they have high levels of caffeineC. they can keep them awake and better at sportsD. they are said to have various nutrtrion( )2. The teenagers should not drink too many energy drinks. Which of the following should be the best reason for it?A. The drinks can cause heart attacks.B. The drinks make them nervous.C. The drinks make them have difficulty in sleeping.D. The drinks have potential health dangers.( )3. The underlined word "discouraged" in the last paragraph can be replaced(替换) by .A. droppedB. stoppedC. helpedD. asked( )4. From the passage we can infer(推断) that .A. advertisements play an important part in getting people to buy the goodsB. the amount of caffeine in most energy drinks is lower than that in a cup of strongcoffeeC. Australian teenagers drink more energy drinks than those in other countriesD. the energy drinks are aimed at young people( )5. Which of the following can be the best title of the passage?A. What's the Use of Energy Drinks?B. Who Can Drink Energy Drinks?C. What's That in Energy Drinks?D. Why Can't We Buy Energy Drinks?Rock art is the name given to pictures drawn on rock by ancient peoples. In the American Southwest, rock art can be seen on the walls of caves and mountains. Many of these places are in the Four Comers area, where the states of Arizona, Colorado, Utah, and New Mexico meet. More than 7,000 rock art places have been found just in Utah, where the area’s dry climate (气候)has helped keep the art.There are three forms of rock art. The first is petroglyphs,which are pictures carved (雕刻)into the surface of rock. A sharp (尖利的)stone may have been used as a carving tool. A heavy stone may have been used to hit the sharp stone into the surface. The second form of rock art is pictographs, which are pictures painted on the rock. The paint was made from plants and trees. The artists painted with fingers, brushes made from hair, or bird bones. The third kind of rock art is geoglyphs, which are designs made in the ground by taking away stones.Some rock art shows faces, hands, animals, and trees. Other pictures are symbols such as lines, circles, and squares. Some scientists think these symbols marked the location of water or good hunting grounds. Some think the symbols were put there during special celebrations. Others think the symbols showed the movement of planets and stars. Still others believe that they are just doodles.Some rock art may be a form of writing. Large scenes are shown on mountains. Some scenes seem to tell a story. A hunting scene may include animals and people with hunting tools. A scene with many people holding hands could mean friendship. Rock art in caves may have been a way to decorate the artis t’s home.Some rock art in the Southwest is about 200 years old. Other rock art may be 10,000 years old. Scientists think an ancient people called the Anasazi created the older works. They were farmers and lived in caves.( )6.The writer mainly wantsA. to introduce an ancient art formB. to describe pictures drawn on rockC. to tell the history of a certain areaD. to solve the mystery of old symbols( )7.According to the passage, what are "doodles" probably like?A. They are carelessly written or drawn.B. They show different symbols.C. They are carefully chosen or designed.D. They have special meanings.( )8.What can we learn about rock art?A.The artists were usually farmers and lived in caves.B.It requires special paint made from plants and trees.C.Most of it describes the daily life of ancient peoples.D.Utah has the largest collection of it in the Southwest.( )9.What can we infer (推断)from the passage?A.Petroglyphs are the easiest to be washed away by rain.B.Artists of modem times can copy rock art rather easily.C.The works created by the Anasazi must be pictographs.D.Places like rainforests are not ideal for keeping rock art.四、单词拼写(13分)1. The train stopped suddenly and all the (乘客)were worried.2. The cookies are so (味道好的)that I can hardly stop eating.3. When speaking to a (陌生人), you'd better be careful.4. We have to explore the (可能性)of working with them.5. I will not pay the (增加)in my bills unless you make all this clear.6. She changed her clothes (迅速地)and went outside.7. Look! Some fish are dead. The water in the river must be (被污染的).8. The bus is old and dirty.It makes my journey (不舒服的).9. The boots are designed (专门地)for small children.10. The car is moving at a of 120 km/h. Too fast!11.―Have you guys decided who will be chosen as the new chairperson―Yes. We have reached the that Andy is the best choice.12.―Mum says we are going to stay outside for the whole night, aren't we?―Yes. You'd better take your bag if you need some sleep.13.―I will never forgive him for lying to me.―Don't say that. All, he has plenty of reasons.五、句子翻译(14分)1.我们有望在火星上重新开始。
(人教版)2020-2021学年九年级下册化学单元测试卷 第九单元 溶液基础卷(Word版含答案)

第九单元溶液基础卷1.生活中的下列事件,利用了溶解原理的是()A.将硬水变为软水B.用洗洁精洗去餐具上的油污C.用汽油洗去衣服上的油污D.海水晒盐2.下列说法正确的是( )A.植物油与水混合一定形成溶液B.将100 g 10%的氯化钠溶液倒掉一半,剩余溶液的质量分数变为5%C.饱和溶液一定是浓溶液D.生活中利用洗涤剂清洗油污属于乳化现象3.通常状况下,下列溶液的溶质为液体的是( )A.蔗糖溶液B.酒精溶液C.氯化钠溶液D.澄清石灰水4.下列有关溶液的说法正确的是()A.溶液均是无色的、透明的B.可溶性物质溶于水时一定会放出热量C.石灰水的溶质是石灰D.溶液的均一性是指同一溶液各部分的性质、组成相同5.下列过程吸收热量的是( )A.氢氧化钠固体溶于水B.硝酸铵固体溶于水C.把水加入生石灰中D.氢氧化钠溶液与稀硫酸反应6.下列洗涤或除污过程应用乳化原理的是( )A.用钢丝球刷除炒菜锅上的污垢B.用酒精除去衣服上的碘C.用汽油除去衣服上的油污D.用洗洁精洗去餐具上的油脂7.使某不饱和溶液变为饱和溶液,下列方法中最可靠的是()A.升高温度B.加入溶质C.降低温度D.倒掉一部分溶液8.向一瓶接近饱和的氯化铵溶液中,逐渐加人氯化铵晶体,下列图像符合溶液中溶质质量变化规律的是( )A. B.C. D.9.生活中的下列现象,能说明气体的溶解度随压强变化而变化的是()A.夏季,鱼塘中的鱼常常会浮在水面呼吸B.喝了汽水以后,常常会打嗝C.打开汽水瓶盖,有大量气泡冒出D.烧开水时,沸腾前水中有气泡产生10.若要增加汽水中的二氧化碳,下列操作可行的是()A.增加水量B.持续通二氧化碳C.升温D.加压11.现有一杯20C︒的某溶质的溶液.欲改变其溶质质量分数,一定可行的方法是()A.加入一定量的溶质 B.增大压强C.升温到60 C︒ D.加入一定量的水12.用溶质质量分数为98%的浓硫酸10 mL(密度为1.84 g. mL-1)配制溶质质量分数为10%的硫酸溶液,需要量取水的体积是()A.180 mLB. 170 mLC.162 mLD. 88 mL13.某同学模拟闽籍化学家侯德榜的“侯氏制碱法”制纯碱,需用50.0g水配制20℃的NaCl 饱和溶液(20℃时NaCl的溶解度为36.0g),应称取NaCl的质量为( )A.18.0gB.16.0gC.13.2gD.11.5g14.如图是甲、乙、丙三种物质的溶解度曲线,下列说法中正确的是()A.P点表示甲、丙两种物质的饱和溶液质量相等B.t1C︒时仍是饱和溶液︒时,乙物质的饱和溶液升温至t2CC.t1C︒时,甲物质的饱和溶液中溶质和溶剂的质量比为1 : 4 D.将三种物质的溶液从t2C︒,析出晶体最多的是甲物质︒降温至t1C15.已知氯化钾、硝酸钾在不同温度时的溶解度如下表:依据上表数据和溶解度曲线判断,下列说法错误的是( )A.能表示硝酸钾和氯化钾的溶解度曲线分别是甲和乙B.t1℃时,氯化钾和硝酸钾的溶解度相等,在34.0g 至35.0g 之间C.t2℃时,将接近饱和的丙物质的溶液升高温度,可使其变成饱和溶液D.氯化钾中混有少量的硝酸钾,可采用降温结晶的方法提纯16.指出下列溶液中的溶质和溶剂。
九年级数学下册《直角三角形的边角关系》单元测试卷(附答案)

九年级数学下册《直角三角形的边角关系》单元测试卷(附答案)一.选择题(共10小题,满分30分)1.已知在Rt△ABC中,∠C=90°,AC=3,BC=4,则tan A的值为()A.B.C.D.2.在Rt△ABC中,各边的长度都扩大2倍,那么锐角A的正切值()A.都扩大2倍B.都扩大4倍C.没有变化D.都缩小一半3.在直角坐标系中,P是第一象限内的点,OP与x轴正半轴的夹角α的正切值是,则cos α的值是()A.B.C.D.4.计算sin45°的值等于()A.B.C.D.5.在Rt△ABC中,∠C=90°,AB=5,BC=3,则tan A的值是()A.B.C.D.6.在Rt△ABC中,∠C=90°,若sin A=,则cos B的值是()A.B.C.D.7.已知tan A=0.85,用计算器求∠A的大小,下列按键顺序正确的是()A.B.C.D.8.若用我们数学课本上采用的科学计算器计算sin42°16′,按键顺序正确的是()A.B.C.D.9.在△ABC中,已知∠C=90°,AC=4,sin A=,那么BC边的长是()A.2B.8 C.4D.1210.α为锐角,若sinα+cosα=,则sinα﹣cosα的值为()A.B.±C.D.0二.填空题(共10小题,满分30分)11.如图,在平面直角坐标系内有一点P(5,12),那么OP与x轴正半轴的夹角α的余弦值.12.若α为锐角,且,则m的取值范围是.13.用科学计算器计算: tan16°15′≈(结果精确到0.01)14.如果3sinα=+1,则∠α=.(精确到0.1度)15.计算:sin225°+cos225°﹣tan60°=.16.在Rt△ABC中,∠C=90°,∠A、∠B、∠C的对边分别为a、b、c,且c=3a,则tan A 的值为.17.在Rt△ABC中,∠C=90°,如果AC=4,sin B=,那么AB=.18.已知∠A是锐角,且tan A=2,那么cos A=.19.已知∠A+∠B=90°,若,则cos B=.20.化简=.三.解答题(共7小题,满分60分)21.如图,在Rt△ABC中,∠C=90°,BC=6,tan A=.求AB的长和sin B的值.22.已知cos45°=,求cos21°+cos22°+…+cos289°的值.23.计算下列各题:(1);(2)sin60°•cos60°﹣tan30°tan60°+sin245°+cos245°.24.在△ABC中,∠C=90°,BC=3,AB=5,求sin A,cos B,tan A的值.25.如图,在所示的直角坐标系中,P是第一象限的点,其坐标是(6,y),且OP与x轴的正半轴的夹角α的正切值是,求角α的正弦值.26.如图,△ABC是等腰三角形,AB=AC,以AC为直径的⊙O与BC交于点D,DE⊥AB,垂足为E,ED的延长线与AC的延长线交于点F.(1)求证:DE是⊙O的切线;(2)若⊙O的半径为2,BE=1,求cos A的值.27.如图,已知∠ABC和射线BD上一点P(点P与点B不重合),且点P到BA、BC的距离为PE、PF.(1)若∠EBP=40°,∠FBP=20°,PB=m,试比较PE、PF的大小;(2)若∠EBP=α,∠FBP=β,α,β都是锐角,且α>β.试判断PE、PF的大小,并给出证明.参考答案与解析一.选择题1.解:如图所示:∵在Rt△ABC中,∠C=90°,AC=3,BC=4,∴tan A==.故选:B.2.解:根据锐角三角函数的定义,知各边的长度都扩大2倍,那么锐角A的大小不变,所以其正切值不变.故选:C.3.解:如图:过点P作PE⊥x轴于点E,∵tanα=,∴设PE=4x,OE=3x,在Rt△OPE中,由勾股定理得OP=,∴cosα=.故选:C.4.解:sin45°=故选:C.5.解:∵∠C=90°,AB=5,BC=3,∴AC===4,∴tan A==,故选:D.6.解:Rt△ABC中,∠C=90°,∴∠A+∠B=90°,∴cos B=sin A=,故选:C.7.解:根据计算器功能键,先按反三角2ndF,再按正切值.故选:A.8.解:若用我们数学课本上采用的科学计算器计算sin42°16′,按键顺序正确的是.故选:C.9.解:由sin A==,不妨设BC=2k,则AB=3k,由勾股定理得,AC2+BC2=AB2,即(4)2+(2k)2=(3k)2,解得k=4(取正值),所以BC=2k=8,故选:B.10.解:∵sinα+cosα=,∴(sinα+cosα)2=2,即sin2α+cos2α+2sinαcosα=2.又∵sin2α+cos2α=1,∴2sinαcosα=1.∴(sinα﹣cosα)2=sin2α+cos2α﹣2sinαcosα=1﹣2sinαcosα=1﹣1=0.∴sinα﹣cosα=0.故选:D.二.填空题(共10小题,满分30分)11.解:过P作PA⊥OA,∵P点坐标为(5,12),∴OA=5,PA=12,由勾股定理得,OP===13.∴cosα==.故答案为:.12.解:∵0<cosα<1,∴0<<1,解得,故答案为:.13.解: tan16°15′≈0.71,故答案为:0.71.14.解:∵3sinα=+1,∴sinα=,解得,∠α≈65.5°,故答案为:65.5°.15.解:∵sin225°+cos225°=1,tan60°=,∴sin225°+cos225°﹣tan60°=1﹣,故答案为:1﹣.16.解:在Rt△ABC中,∠C=90°,c=3a,∴b===2a,∴tan A===,故答案为:.17.解:∵sin B=,∴AB===6.故答案是:6.18.解:设∠A所在的直角三角形为△ABC,∠C=90°,∠A、∠B、∠C所得的边为a,b,c,∵tan A=2,即=2,设b=k,则a=2k,∴c==k,∴cos A==,故答案为:.19.解:由∠A+∠B=90°,若,得cos B=,故答案为:.20.解:∵tan30°=<1,∴原式=1﹣tan30°=1﹣=.三.解答题(共7小题,满分60分)21.解:∵在Rt△ABC中,∠C=90°,BC=6,tan A==,∴AC=12,∴AB===6,∴sin B===.22.解:原式=(cos21°+cos289°)+(cos22°+cos288°)+…+(cos244°+cos246°)+cos245 =(sin21°+cos21°)+(sin22°+cos22°)+…+(sin244°+cos244°)+cos245=44+()2=44.23.解:(1)=(2×﹣)+=2﹣+=2;(2)sin60°•cos60°﹣tan30°tan60°+sin245°+cos245°.=×﹣×+()2+()2=﹣1++=.24.解:∵在△ABC中,∠C=90°,BC=3,AB=5,根据勾股定理可得:AC=4,∴sin A=,cos B==,tan A==.25.解:作PC⊥x轴于C.∵tanα=,OC=6∴PC=8.则OP=10.则sinα=.26.(1)证明:法一、连接AD、OD,∵AC是直径,∴AD⊥BC,∵AB=AC,∴D是BC的中点,又∵O是AC的中点,∴OD∥AB,∵DE⊥AB,∴OD⊥DE,∴DE是⊙O的切线.法二、连接OD,∵OC=OD,∴∠OCD=∠ODC,∵AB=AC,∴∠OCD=∠B,∴∠B=∠ODC,∴OD∥AB,∵DE⊥AB,∴OD⊥DE,∴DE是⊙O的切线.(2)解:由(1)知OD∥AE,∴∠FOD=∠FAE,∠FDO=∠FEA,∴△FOD∽△FAE,∴,∴,∴,解得FC=2,∴AF=6,∴Rt△AEF中,cos∠FAE====.27.解:(1)在Rt△BPE中,sin∠EBP==sin40°在Rt△BPF中,sin∠FBP==sin20°又sin40°>sin20°∴PE>PF;(2)根据(1)得sin∠EBP==sinα,sin∠FBP==sinβ又∵α>β∴sinα>sinβ∴PE>PF.。
九年级化学下册第九单元《溶液》测试卷-人教版(含答案)

九年级化学下册第九单元《溶液》测试卷-人教版(含答案)附相对原子质量:H-1 C-12 N-14 O-16一、本大题包括12小题,每小题1分,共12分。
每小题的4个备选答案中只有一个答案符合题意。
1、下列物质与水混合,不可能形成溶液的是()A.胆矾B.纯碱C.汽油D.白醋2、下列去“污”的方法中,利用了乳化原理的是()A.用洗涤剂除去餐具上的油脂B.用酒精除去附着在试管内壁的碘C.用汽油除去衣物上的油污D.用热水除去附着在烧杯底部的硝酸钾3、2021年7月,新冠病毒的变异体德尔塔病毒在整个欧洲大规模传播。
75%的酒精可杀灭新冠病毒,酒精溶液中的溶剂是()A.乙醇B.白醋C.水D.食盐4、水是一种最常用的溶剂。
下列做法或说法正确的是()A.用絮凝剂明矾可降低水的硬度B.硝酸铵溶于水制成的冰袋可给高烧病人降温C.天然水经过自然沉降、过滤、吸附,即得纯水D.用150mL 酒精和50mL 蒸馏水混合配制成200mL 的75%的医用酒精5、下列物质加入水中,能形成溶液并且温度不会发生明显改变的是()A.硝酸铵B.牛奶C.氢氧化钠D.氯化钠6、配制一定溶质质量分数的溶液,下列仪器不需要的是()A.量筒B.胶头滴管C.烧杯D.漏斗7、啤酒内溶有一定量的二氧化碳气体,打开瓶盖时,你会发现啤酒会喷出来。
喝了啤酒后又会常常打嗝,这说明气体在水中的溶解度与压强和温度有关。
下列关于气体溶解度的说法正确的是()A.压强减小,气体溶解度增大B.压强增大,气体溶解度减小C.温度升高,气体溶解度减小D.温度降低,气体溶解度减小8、甲、乙的溶解度曲线如图所示(不考虑水的变化),下列说法正确的是()A.t2℃时,甲的饱和溶液中溶质质量分数为40%B.t1℃时,将甲、乙的饱和溶液分别升温到t2℃,两溶液中溶质质量分数相等C.依据溶解度曲线可判断,甲的溶解度比乙的大D.t2℃时,将甲、乙的饱和溶液分别降温到t1℃,甲析出的晶体质量大9、下列对课本中的图表、数据的使用,叙述不正确的是()A.根据金属活动性顺序表,判断金属能否与稀盐酸、稀硫酸反应产生氢气B.根据元素周期表,可以查找元素原子核内中子的数目C.根据“一些常见元素和根的化合价”表,可以确定铵根的化合价为+1价D.根据物质的溶解度曲线图,可确定该物质在某一温度时的溶解度10、下列关于“生活中的化学”的叙述中,不正确的是()A.冬天用炭火盆取暖要注意关闭门窗B.常用铁制品保持清洁干燥能防止生锈C.钻石项链不能放到火上灼烧D.菜汤太咸了可以适当加些水11、为了探究物质的溶解现象,设计了如下实验:实验现象固体溶解,形成紫色溶液固体几乎不溶解固体溶解,形成紫红色溶液根据上表得出下列说法错误的是()A.物质的溶解性受到溶质与溶剂性质影响B.②号试管所得液体是乳浊液C.不同溶质在同一种溶剂中的溶解特性不同D.同种溶质在不同溶剂中的溶解特性不同12、下列排序正确的是()A.溶解时放出的热量: B.氮元素的化合价:C.地壳中元素含量:D.金属活动性顺序:二、本大题包括5小题,共28分13、阅读下列短文,回答问题。
第九单元 金属 单元综合测验九年级下册化学单元测试卷鲁教版

第九单元金属单元综合测验一、单选题1.某同学为了探究甲、乙、丙三种金属的活动性强弱,做了如下实验。
则三种金属的活动性顺序是()D.丙>乙>甲2.为验证Zn、Cu、Ag三种金属的活动性顺序,下列试剂可以选用的是()A.ZnSO4溶液B.稀盐酸C.AgNO3溶液D.CuSO4溶液3.化学行业中把材料分为:金属材料、无机非金属材料、有机高分子材料等.金属材料在现实生活中有极为重要的作用.下列金属材料的运用与金属所具有的性质不一致的是()A.制造白炽灯灯丝﹣﹣熔点高B.制造飞机﹣﹣坚硬而质轻C.制造保险丝﹣﹣硬度大D.制造装化工原料的容器﹣﹣耐腐蚀4.如图所示,烧杯中盛有质量相等、质量分数相等的稀盐酸,天平调平后,同时向其中左右盘分别加入等质量的锌片和铁片,则从反应开始到金属完全反应的过程中,天平指针指向的变化是()A.向左偏B.向右偏C.先向左偏后向右偏D.先向右偏后向左偏5.医用热敷袋使用时要轻揉,袋内的反应可看作是铁粉、空气和水相互作用,产生氢氧化亚铁[Fe(OH)2],最终转化为氢氧化铁[Fe(OH)3]。
下列说法不正确的是()A .热敷袋放出的热量是由化学反应产生的B .该过程发生缓慢氧化反应C .总反应为2Fe+O 2+3H 2O=2Fe(OH)3D .上述含铁物质中铁元素的化合价有0、+2、+36.将一定量的锌粉投入到溶质为Mg (NO 3)2、Cu (NO 3)2、AgNO 3的溶液中,充分反应后过滤,滤液蓝色:向滤液中滴加稀盐酸,无明显现象。
下列说法正确的是( )A .滤渣中一定有Ag 、CuB .滤液中可能有AgNO 3C .滤渣中一定有Ag ,可能有Cu ,没有ZnD .滤液中含有两种溶质:Cu (NO 3)2、Mg (NO 3)27.下列观点正确的是( )A .酸和碱一定含有氢元素B .显碱性的物质一定属于碱C .有单质生成的反应一定是置换反应D .等质量的酸溶液和碱溶液混合后,溶液一定显中性8.芯片是电脑、智能家电的核心部件,它是以高纯度的单质硅(Si )为材料制成的。
九年级化学下册第九单元《溶液》综合测试卷-人教版(含答案)

九年级化学下册第九单元《溶液》综合测试卷-人教版(含答案)一、选择题。
1、分别将下列物质加入到足量水中,能得到无色、澄清溶液的是( ) A .NaCl B .CuSO 4 C .CaCO 3D .FeCl 32、小军同学需要快速配制一杯可口的白糖溶液,下列措施不能达到目的的是( )A .用冰水溶解B .用热水溶解C .把白糖碾成粉末后溶解D .溶解时用筷子搅拌3、化学知识在生产、生活中有着广泛的应用。
用下列物质除去油污时,利用乳化作用的是 (填字母)。
A.汽油B.洗洁精C.氢氧化钠溶液4、如图所示,一木块漂浮于X 中,向X 中缓缓加入(或通入)少量物质Y 后,最终木块上浮,则X 与Y 可能是( )(溶液的体积变化忽略不计)A ②④B ①③C ①②③D ②③④5、将热的硝酸钾不饱和溶液降温至如图所示的状态。
对该过程的描述错误的是( )序号 X Y ① 水食盐 ② 氢氧化钙溶液 二氧化碳 ③ 稀硫酸 镁粉 ④硫酸铜溶液铁粉A.降温过程中硝酸钾溶液逐渐达到饱和状态B.饱和的硝酸钾溶液继续降温,会析出硝酸钾晶体C.硝酸钾以晶体的形式析出的过程叫作结晶D.析出晶体后的硝酸钾溶液是不饱和溶液6、如图是甲、乙、丙三种物质的溶解度曲线,下列说法中正确的是()A.P点表示甲、丙两种物质的饱和溶液质量相等B.t1℃时,将乙物质的饱和溶液升温至t2℃,仍是饱和溶液C.t1℃时,甲物质的饱和溶液中溶质和溶剂的质量比为1∶4D.将三种物质的溶液从t2℃降温至t1℃,析出晶体最多的是甲物质的溶液7、以下说法正确的是()A.溶液一定是均一、无色、稳定的B.均一、稳定的液体一定是溶液C.溶液的溶质一定是固体D.溶液一定是混合物8、关于100克5%的氯化钠溶液,下列叙述正确的是()A. 100克水中溶有5克氯化钠B. 5克氯化钠溶于95克水中C. 溶液中氯化钠与水的质量比为1:20D. 溶液中氯化钠与水的质量比为19:19、实验室用硝酸钾固体配制100.0 g溶质质量分数为3.0%的硝酸钾溶液,下列说法正确的是()A.用50 mL量筒量取水B.将固体放于托盘天平的右盘称取C.将固体直接投入量筒中溶解D.将配好的溶液装入贴有标签(如图SY5-3)的试剂瓶中,盖好瓶塞10、把少量下列物质分别放入足量水中,充分搅拌,可以得到无色溶液的是()A 氯化铁B 氢氧化镁C 小苏打D 汽油11、有关溶液的说法正确的是()A.具有均匀性、稳定性的液体一定是溶液B.配制溶液时,搅拌可以增大固体溶质的溶解度C.饱和溶液的浓度一定比不饱和溶液的浓度大D.融雪剂的原理是利用某些物质水溶液的凝固点低于0℃12、将金属钠投入水中会发生反应(2Na+2H2O═2NaOH+H2↑),现将4.6g钠投入95.4g水中充分反应后,溶液中溶质质量分数()A. 等于4.6%B. 等于8.0%C. 大于8.0%D. 小于8.0%13、用溶质质量分数为98%的浓硫酸配制490 g溶质质量分数为20%的稀硫酸,下列说法不正确的是()A.实验中用到的玻璃仪器:量筒、胶头滴管、烧杯、玻璃棒B.配制该稀硫酸需要加水390 mLC.配制时需要溶质质量分数为98%的浓硫酸100 gD.配制的步骤是计算、称量、量取、溶解、装瓶贴标签二、填空题。
人教版数学九年级下册单元测试卷

人教版数学九年级下册单元测试卷一、选择题(每小题3分,共30分)1.下列关于圆的描述中,正确的是()A. 圆的切线垂直于半径B. 弦的中点与圆心的连线垂直于弦C. 垂直于弦的直线必过圆心D. 平分弦的直径垂直于弦2.下列二次根式中最简二次根式是()A. √(12)B. √(27)C. √(30)D. √(18)3.下列命题中,是真命题的是()A. 四个角相等的四边形是矩形B. 对角线相等的四边形是矩形C. 对角线互相垂直的四边形是菱形D. 邻边相等的四边形是菱形4.下列函数图像中,与x 轴有两个交点的是()A. y = x^2 + 1B. y = x^2 - 2x + 3C. y = x^2 - 4x + 4D. y = x^2 - 4x5.下列函数中,图像经过坐标原点的是()A. y = 2x + 1B. y = 3/xC. y = x^2 - 1D. y = -2x^2 + 16.下列关于概率的描述中,正确的是()A. 必然事件的概率为0B. 不可能事件的概率为1C. 随机事件的概率介于0 和1 之间D. 某事件的概率可能大于17.下列关于一元二次方程的根的判别式Δ = b^2 - 4ac 的说法中,错误的是()A. 当Δ > 0 时,方程有两个不相等的实数根B. 当Δ = 0 时,方程有两个相等的实数根C. 当Δ < 0 时,方程没有实数根D. Δ 的值越大,方程的根越大8.下列关于反比例函数的描述中,正确的是()A. 反比例函数的图像是一条直线B. 反比例函数的图像分布在第二、四象限C. 反比例函数的图像关于原点对称D. 反比例函数的值随着x 的增大而增大9.下列关于三角函数的说法中,正确的是()A. sinθ = cos(90° - θ)B. tanθ = sinθ/cosθ (θ ≠ 90°)C. cosθ = sin(90° + θ)D. tanθ = cosθ/sinθ (θ ≠ 0°)10.下列关于投影的说法中,正确的是()A. 投影线互相平行时,它们的投影是平行投影B. 投影线互相垂直时,它们的投影是中心投影C. 物体的正投影不改变物体的形状和大小D. 中心投影比平行投影更能真实地反映物体的形状和大小二、填空题(每小题2分,共20分)11.已知圆的半径为r,则圆的周长为_______。
人教版英语九年级全册单元unit 9 知识点+测试卷+思维导图

Unit 9 I like music that I can dance to.1.重点词汇:case, war, director, dialogue, pain, pity, total, master, wound, prefer, suppose, stick, shut, sense, reflect, perform, praise...2. 短语归纳:1. dance to (music) 随着(音乐)跳舞2. sing along with 随着……一起唱3. electronic music 电子音乐4. not much 没什么(事)5. suppose sb to do sth. 猜想某人做某事6. be supposed to do sth 应该做某事7. suppose sb (to be) +adj. 原以为……8. have spare time 有空闲时间9. in one’s spare time在某人的空闲时间10. spare the time to do sth 抽时间做……11. a film director 一名电影导演12. think too much 想太多13. in that case 既然那样14. World War II 第二次世界大战15. smooth music 悦耳的音乐16. prefer A to B 比起B来更喜欢A17. prefer doing A to doing B 比起做B更喜欢做A18. prefer to do A rather than do B 宁愿做A而不愿做B3. 必背典句:1. -What kind of music do you like? 你喜欢什么样的音乐?-I love music that/which I can sing along with. 我喜欢和我能跟着一起唱的音乐。
2. I prefer movies that/ which give me something to think about.我更喜欢能给我一些思考的东西的电影。
人教版英语九年级下册第九单元测试卷3份含答案

人教版英语九年级下册第九单元测试卷3份人教版英语九年级下册第九单元测试卷AⅠ. 根据句意及所给汉语提示,写出句中所缺单词。
1. Robert said this kind of paper wasn't _________ (平滑的) enough.2. What language do most _________ (澳大利亚人) speak?3. I couldn't get any replies when I called Ben, so I _________ (推断) that he's gone out.4. The music started and Anna walked out. To our surprise, she forgot the _________(歌词).5. The _________ (部门负责人) have a meeting every Friday afternoon.Ⅱ. 根据语境及所给汉语提示,完成下列句子或对话,每空一词。
1. Sandy loves to _________ _________ (随着……跳舞) fast music.2. Many senior high school students hardly _________ _________ _________ (有空) for sports.3. —I'm afraid Mr. Wood can't see you until 4 o'clock.—Oh, _________ _________ _________ (假使那样的话) I won't wait.4. The beautiful love story in this film takes place _________ _________ _________ _________ (第二次世界大战期间).5. Carmen likes _________ _________ (电子音乐) while her sister likes classical music.Ⅲ. 根据对话内容,从方框中选择恰当的选项补全对话,其中有两项多余。
九年级化学下册《初识酸、碱和盐》单元测试卷(附答案)

九年级化学下册《初识酸、碱和盐》单元测试卷(附答案)一、单选题1.有关物质对应的化学式,书写正确的是A .干冰:H 2OB .硫酸钠:Na 2SO 3C .火碱:NaOHD .熟石灰:CaCO 32.食醋中含有醋酸,下列使用食醋肯定不能达到目的是( )A .除去水壶壁上的水垢B .加入紫色石蕊溶液,溶液变红C .除去菜刀上的铁锈D .鉴别黄金饰品中是否含有铜3.下列实验操作正确的是A .稀释浓硫酸B .过滤C .测溶液的酸碱度D .证明CO 2能与H 2O 发生反应 4.下列对实验现象的分析合理的是( )A .某无色气体混入空气后点燃爆炸,可证明该气体一定是H 2B .氧化钙放入水中使液体温度升高,可证明氧化钙溶于水吸热C .某气体在空气中燃烧产生蓝色火焰,可证明该气体一定是一氧化碳D .煤燃烧产生二氧化硫等有害气体,可证明该物质中一定含有硫元素5.下列各组离子在pH=1的溶液中能大量共存的是( )A .K +、2Cu +、24SO -、Cl -B .K +、Na +、23CO -、Cl -C .4NH +、Na +、3NO -、OH -D .Na +、2Ba +、3NO -、24SO -6.向 20g 质量分数为 10%的稀硫酸中加入 20g 质量分数 10%的氢氧化钙溶液。
向反应后溶液中滴加紫色石蕊溶液,溶液的颜色是( )A .紫色B .红色C .蓝色D .不确定7.下列说法正确的是A.过氧化氢和水都是由氢元素和氧元素组成的B.加碘食盐中的“碘”是指碘单质C.二氧化碳和氧气都极易溶于水D.纯碱属于碱8.下列装置不能用于证明CO2和NaOH 溶液反应的(均做与水的对照实验)是A.B.C.D.9.A~E为初中化学常见的物质,其中A、B、D、E是不同类别的化合物,A的浓溶液具有吸水性,C是相对分子质量最小的氧化物,D广泛用于玻璃、造纸、纺织和洗涤剂的生产,它们的部分反应和转化关系如图所示(“一”表示两端的物质间能发生化学反应,“→”表示一种物质生成另一种物质,部分反应物、生成物和反应条件已略去)。
2021年九年级化学下册第九单元《溶液》经典测试卷(答案解析)(2)

一、选择题1.下列有关溶液的叙述正确的是()A.均一、稳定的液体一定是溶液B.一份溶液中可以含有多种溶质C.饱和溶液就是不能再溶解任何物质的溶液D.饱和溶液一定比不饱和溶液浓度大B解析:BA.溶液的特征是是均一、稳定、混合物,则均一、稳定的液体不一定是混合物,A错误;B.溶质是被溶解的物质,所以一份溶液中可以溶解多种物质,如碳酸饮料中就溶解多种物质,则一份溶液中可以含有多种溶质,B正确;C.在一定温度和一定量的溶剂里,不能溶解某种溶质的溶液,叫做这种溶质的饱和溶液,所以饱和溶液只是不能再溶解某种溶质,但可以溶解其他溶质,如氯化钠的饱和溶液可以溶解氯化钾等其他溶质,C错误;D.对于不同溶质的溶液,饱和溶液不一定比不饱和溶液的浓度大,而对于同种溶质的溶液,在同一温度下,饱和溶液比不饱和溶液的浓度大,D错误;故选:B。
2.化学概念之间在逻辑上存在并列、交叉和包含等关系。
下列各图中概念之间关系不正确的是( )A.B.C.D. A解析:AA、物质分为混合物和纯净物,混合物是由两种或两种以上的物质组成,因此混合物与纯净物属于并列关系,不是交叉关系,错误符合题意,故选项正确;B、由两种或两种以上的元素组成的纯净物叫化合物。
氧化物是指由两种元素组成的化合物中,其中一种元素是氧元素,因此它们之间属于包含关系,正确但不符合题意,故选项错误;C、化合反应是有两种或两种以上的物质生成一种物质的化学反应,氧化反应是物质与氧发生的化学反应,因此它们是交叉关系,正确但不符合题意,故选项错误;D、溶液分为饱和溶液与不饱和溶液,饱和溶液与不饱和溶液是并列关系,正确但不符合题意,故选项错误;故选:A 。
3.某同学在帮助实验员整理化学试剂时发现了一瓶标签残缺的无色溶液(如图所示),经实验员分析可知原瓶溶液中的溶质可能是233NaCl NaOH Na CO NaHCO 、、、中的一种。上述四种物质的溶解度如下,据此可知该溶液中的溶质一定不是( )物质NaCl NaOH Na 2CO 3 NaHCO 3 常温下的溶解度/g 36 109 21.5 9.6 A .NaClB .NaOHC .Na 2CO 3D .NaHCO 3D 解析:D【分析】要确认溶质一定不是哪种物质,需要根据溶质该温度下的溶解度计算出物质最高的溶质质量分数,低于标签上的溶质质量的分数的物质,一定不能配制。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
2019-2020年九年级下册:第9单元测试卷姓名班级学号一、选择题(每小题只有1个选项符合题意。
)1.下列化学仪器中,可用于溶解固体、配制溶液、加热较大量液体的是()A.集气瓶B.烧杯C.量筒D.试管2.溶液在我们日常生活中有很广泛的用途,下列属于溶液的是()A.蒸馏水 B.碘酒C.石灰浆 D.植物油3.已知50℃时氯化铵的溶解度为50g,则在50℃时的氯化铵饱和溶液中,溶质质量∶溶剂质量∶溶液质量等于()A.1∶2∶3 B.2∶3∶1 C.3∶1∶2 D.1∶3∶24.据《说文解字》记载,我们的祖先在神农氏时代就开始利用海水晒盐。
海水晒盐的原理是()A.日晒风吹使海水中的氯化钠蒸发B.日晒风吹使溶液由饱和变为不饱和C.日晒风吹使水分蒸发、晶体析出D.日晒风吹使氯化钠的溶解度变小5.夏日里想随时喝到凉爽的饮料,可以自制化学“冰箱”,即把一种化学试剂放入到一定量的水中,就可以形成低温小环境。
这种试剂可以是下列物质中的()A.食盐B.熟石灰C.蔗糖D.硝酸铵6.通常情况下,欲将一杯不饱和的蔗糖溶液变成饱和溶液,最适宜的方法是()A.加蔗糖B.加水C.升温D.增大压强7.下列加速溶解的措施中,能改变固体溶解度的是( )A.把固体研细 B.加热 C.搅拌 D.振荡 8.小欣拉开易拉罐汽水的拉环时,听到“啵”的一声,并有气体自汽水中冲出。
下列有关此现象的说明正确的是( )A.因压强减小使气体的溶解度变小 B.因压强增大使气体的溶解度变小 C.因温度升高使气体的溶解度变小 D.因温度降低使气体的溶解度变小9.右图是A 、B 两种固体物质的溶解度曲线,下列说法正确的是( ) A .A 的溶解度大于B 的溶解度B .t 1℃时,用等量水分别制成的A 、B 饱和溶液中,A 的质量比B 大C .t 2℃时,A 、B 两种饱和溶液中,溶质的质量分数相等D .A 、B 两种饱和溶液都从t 3℃降温到t 2℃,析出的晶体一定等量10.夏天,实验员在实验室里配制了一瓶饱和的硝酸钾溶液并密封放置,到了冬天发现瓶底有大量的晶体析出。
这是因为( )A.溶液中溶剂质量减小 B.变成了不饱和溶液 C.温度下降,硝酸钾溶解度减小 D.溶液中溶质质量增加二、选择题(每小题有1~2个选项符合题意。
)11.一个封闭的池塘受大量生活污水的污染出现富营养化,则该水体中溶解氧含量的变化曲线是( )12.下列有关溶液的认识中,不正确的是( )溶 解 度/gA.析出晶体后的溶液是该温度下的不饱和溶液B.在溶液里进行的化学反应,通常是比较快的C.同种溶质的饱和溶液一定比它的不饱和溶液的浓度大D.食物里的营养成分经消化变成溶液,容易被人体吸收13.在一定温度下,向一定量的氯化钠不饱和溶液中不断加氯化钠固体,并搅拌。
在此过程中,加入氯化钠的质量(n)与溶液中溶质的质量分数(m)的变化关系如图所示,其中正确的是()14.在25℃时,向饱和的澄清石灰水中加入少量氧化钙[已知CaO+H2O===Ca(OH)2],恢复到25℃时,关于该溶液的下列说法中正确的是()A.溶质质量不变B.溶质质量减少C.溶质的质量分数不变D.溶质的质量分数增大15.如右图所示,在盛冷水烧杯中放入甲、乙两支试管(试管中都有未溶解的该溶液的溶质),若使甲试管中晶体减少,乙试管中晶体增加,需向烧杯中加入的物质是()A.氯化钠B.氢氧化钠C.冰块D.硝酸铵三、填空题16.在①食盐、②花生油、③木炭粉、④蔗糖这4种物质中,与水混合能形成乳浊液的是_________(选填序号,下同),混合后该物质与水能采用过滤的方法分离的是_________。
17.化学源于生活,生活中蕴含着许多化学知识。
(1)医用葡萄糖注射液中的溶剂是_________。
(2)用加了洗涤剂的水清洗油腻的餐具,是利用洗涤剂的_________(选填“乳化”或“溶解”)功能。
(3)硬水洗涤衣物,既浪费肥皂也洗不净衣物,时间长了还会使衣物变硬。
日常生活中常用_________的方法来降低水的硬度。
18.实验室要配制100克溶质质量分数为12%的硝酸钾溶液。
(1)配制溶液需完成计算、称量、三个步骤。
(2)会导致所得溶液溶质质量分数偏小的选项有哪些?(选填字母)。
A.硝酸钾中有杂质 B.称量时使用了生锈的砝码C.称量时硝酸钾和砝码位置颠倒 D.称量时使用了缺损的砝码E.用量筒量取水时,仰视读数 F.配制完成后移取液体时有部分溅出19.化学中有许多概念是对立的,又是统一的。
右图反映了饱和溶液、不饱和溶液、浓溶液以及稀溶液的关系。
(1)从右图可以看出,饱和溶液与浓溶液、稀溶液的关系是_________________________________________________。
(2)0.9%的医用生理盐水可归于右图中_______区域(选填“Ⅰ”、“Ⅱ”、“Ⅲ”、“Ⅳ”);现有一瓶接近饱和的氯化钠溶液,若要使它变为饱和溶液,一般采用的方法是_______________________________或_______________________________。
20.某化学兴趣小组进行溶液的配制和粗盐的初步提纯实验。
(1)配制200g质量分数为8%的氢氧化钠溶液:①计算:需要氢氧化钠固体的质量为克,需要水的体积为mL(水的密度近似看作1g/mL)。
②称量:调节托盘天平平衡,将一个烧杯放在托盘天平的盘,称量其质量。
然后(按操作的先后顺序选填字母),直至天平平衡。
A.将氢氧化钠固体加入烧杯中 B.按需要添加砝码、移动游码该步骤中用烧杯而不用纸称量氢氧化钠的原因是。
③用量取所需要的水,倒入盛有氢氧化钠固体的烧杯中,用搅拌,使其溶解,并冷却到室温。
④将配制好的溶液放入试剂瓶,塞好橡胶塞并,放到指定的地方。
(2)粗盐的初步提纯:①称取5.0克粗盐,用药匙逐渐加入到10mL水中,直到粗盐不再溶解为止。
还需进行的实验操作步骤的顺序为:称量剩余粗盐、 (填编号)。
A.过滤 B.计算产率 C.蒸发 D.称量精盐②过滤操作如右图所示,指出其中的错误之处:;;;。
21.下图是固物质体甲的溶解度曲线。
22.在实验探究过程中,如果有多种因素同时影响某一实验结果,当我们要探究其中的一种因素时,就要保持其他因素保持不变,以探究此种因素对实验结果的影响。
完成实验探究:哪些因素影响固体物质在水中的溶解度?因素1:溶质的种类在实验中要改变的因素是:溶质的种类要保持不变的因素是:温度操作步骤:在常温下,将NaCl、KNO3、Ca(OH)2各2g,分别加入①、②、③三支试管中,用量筒各量取5mL蒸馏水,倒入①、②、③三支试管中,并不断(填操作步骤)一段时间,观察各物质的溶解情况。
实验现象:试管①中有少量NaCl未溶,试管②中KNO3全部溶解,试管③中有较多Ca(OH)2未溶。
实验结论:在___________保持不变的情况下,不同种类固体物质在水里的溶解度___________(填“相等”或“不相等”)。
因素2:温度(以KNO3为例)在实验中要改变的因素是:______________要保持不变的因素是:溶质的种类(KNO3)操作步骤:用量筒各量取用量筒各量取5mL蒸馏水,分别加入①、②、③三支试管中,再分别加入5gKNO3晶体,将试管①保持在常温下,将试管②加热并保持在40℃,将试管③加热并保持在60℃,振荡,观察各试管中KNO3的溶解情况。
实验现象:试管①中有多量KNO3未溶,试管②中有少量KNO3未溶,试管③中KNO3全部溶解。
实验结论:在不同温度下,固体物质(KNO3)在水里的溶解度__________(填“相等“或”不相等)。
四、计算题23.溶液与人类的生活息息相关,溶液的配制是日常生活和化学实验中的常见操作。
下表是硫酸溶液和氨水的密度与其溶质的质量分数对照表(20℃)。
请仔细分析后回答下列问题:(1)20℃时,随着溶液中溶质的质量分数逐渐增大,硫酸溶液的密度逐渐_________(填“增大”、“减小”或“不变”),氨水的密度逐渐___________(填“增大”、“减小”或“不变”)。
(2)取12%的硫酸溶液100g配制成6%的溶液,向100g12%的硫酸溶液中加水的质量应__________(填“大于”、“小于”或“等于”)100g。
(3)向100g24%的氨水中加入100g水,摇匀,溶液的体积是_______mL(计算结果保留到0.1mL)。
24.100g某硫酸恰好与13g锌完全起反应,试计算:(1)生成氢气的质量;(2)该硫酸中溶质的质量分数;(3)反应后所得溶液中溶质的质量分数(保留到0.1%)。
五、附加题25.下表是KNO3、NaCl在不同温度下的溶解度:0 10 20 30 40 50 60 70 80 90 100 温度(℃)KNO313.3 20.9 32 45.8 64 85.5 110 138 169 202 246 NaCl 35.7 35.8 36 36.3 36.6 37 37.3 37.8 38.4 39 39.8 (1)KNO3溶液中含有少量NaCl时,可通过________的方法提纯。
(2)对(1)析出的晶体和剩余溶液描述正确的是________(填写编号)。
A.剩余溶液一定是KNO3饱和溶液 B.剩余溶液一定是NaCl不饱和溶液C.上述方法可以将两者完全分离 D.析出的晶体中只含有KNO3(3)在一定温度下,将含69gKNO3、18gNaCl的混合物完全溶解在50g水中。
欲改变温度使KNO3析出,NaCl不析出,则温度T(℃)的范围是________℃(硝酸钾和氯化钠溶解度互不影响)。
26.如图所示,在室温下的饱和食盐水中放了一个塑料小球。
(1)现加入少量食盐晶体,充分搅拌和静置后,在温度不变的情况下,小球在液面沉浮情况有何变化,并简述原因:____________________________________________________。
(2)若要在不用外力的情况下使小球略上浮,你准备采取的具体方法是:______________________________________________________。
参考答案及评分标准一、选择题(若不完成附加题,每小题2分,共20分;若完成附加题,每小题1分,共10分。
)题号 1 2 3 4 5 6 7 8 9 10 答案 B B A C D A B A C C二、选择题(每小题2分,共10分。
)题号11 12 13 14 15答案 D AC A BC B三、填空题(每空1分,共36分。
)21.(1)不饱和(2)22.振荡温度不相等温度不相等四、计算题(共14分。
)23.(1)增大(1分)减小(1分)(2)等于(2分)(3)210.5(2分)24.(1)0.4g(2分)(2)19.6%(3分)(3)28.6%(3分)项目 1 2 3 4A→B不变不变饱和B→C变小变小饱和五、附加题(每空2分,共10分。
