表面与界面化学试卷(a)2007
界面化学第一章

界面化学第一章————————————————————————————————作者:————————————————————————————————日期:ﻩ界面化学期末考试卷一、单项选择题1、关于表面张力和表面能有以下几种说法,试判断哪一个正确?(B)A、它们是体系的两个热力学变量B、它们描述体系的同一性质C、他们是指向体系内部的向量D、他们是完全相同的一个量2、纯水的表面张力是指恒温恒压组成时水与哪类相接触时的界面张力:(B)A、饱和水蒸气B、饱和了水蒸气的空气C、空气D、含有水蒸气的空气3、弯曲液面下的附加压力与表面张力的联系与区别在于(C)A、产生的原因与方向相同,而大小不同B作用点相同,而方向和大小不同C、产生的原因相同,而方向不同D作用点相同,而产生的原因不同4、在一个密闭的容器中,有大小不同的两个水珠,长期放置以后,会发生(A)A、大水珠变大,小水珠变小B、大水珠变大,小水珠变大C、大水珠变小,小水珠变大D、大水珠、小水珠均变小5、固体微粒在粘滞性液体中流动时,对其阻力无直接影响的是(C)A、介质粘度B、粒子大小C、粒子质量D、温度二、是非判断1、液体在毛细管内上升或下降决定于该液体的表面张力的大小。
( 错) 2、由于溶质在溶液的表面产生吸附,所以溶质在溶液表面的浓度大于他在溶液内部的浓度。
(错)3、表面活性物质是指那些加入到溶液中,可以降低溶液表面张力的物质。
(错)4、作布朗运动的粒子的平均位移与其质量无关。
(对)5、在等温等压下且组成不变时以可逆方式增加表面积做的功,量值等于表面能。
(对)ﻬ三、填空题1、丁达尔效应的本质溶胶对光的散射2、表面活性剂的结构特征两亲性3、润湿与不润湿的区分在于润湿角度小于九十度或大于九十度4、从吸附的角度考虑催化剂的活性取决于化学吸附强度,一个良好的催化剂应是中等强度的化学吸附5、植物的叶子一般都是憎水性的,所以配制农药时常常要加润湿剂,药对植物表面的润湿程度,使药物能在叶子表面上铺展。
《表面活性剂化学》题集

《表面活性剂化学》题集第一章绪论一、选择题1. 关于界面与表面的定义,下列哪项是正确的?()A. 界面是指不同物质相接触的线,表面是指液体与气体接触的面B. 界面是指不同物质相接触的面,表面是指液体与气体接触的线C. 界面是指不同物质相接触的面,表面是指液体与气体接触的面D. 界面和表面都是指液体与气体接触的面2. 表面张力是以下哪个现象的表现?()A. 液体表面层的分子受到向内的吸引力大于向外的吸引力B. 液体表面层的分子受到向外的吸引力大于向内的吸引力C. 液体表面层的分子受到均匀的吸引力D. 液体表面层的分子受到均匀的排斥力3. 下列哪种物质不属于表面活性剂?()A. 肥皂B. 洗发水中的活性成分C. 食盐D. 洗洁精4. 表面活性剂的HLB值代表的是:()A. 氢键长度B. 氢键能量C. 亲水亲油平衡D. 氢键数量5. 关于表面活性剂的活性,以下哪项描述是正确的?()A. 表面活性剂的活性与其分子量成正比B. 表面活性剂的活性与其分子量成反比C. 表面活性剂的活性与其分子结构无关D. 表面活性剂的活性取决于其在界面上的吸附能力二、填空题1. 界面是指两种不同______相互接触的区域,表面是指液体与气体接触时在液体表面形成的一个______薄层。
2. 表面张力是液体表面层的分子间作用力______液体内部,使液体表面具有______收缩的趋势。
3. 表面活性剂是一类能够显著降低液体表面张力的物质,其分子结构通常具有一个或多个______和一个或多个______。
4. 表面活性剂的HLB值反映了其分子的______程度,HLB值越高,亲水性越______,HLB值越低,亲油性越______。
5. 表面活性剂在生活和工业中有广泛的应用,如______、______、______等。
三、简答题1. 请简述界面张力与表面张力的区别。
2. 为什么液体表面层的分子会表现出比内部分子更大的相互作用力?3. 简述表面活性剂如何通过改变分子结构来降低液体表面张力。
物理化学试题卷A2007年1月11日

物理化学试题卷(A)考试时间:2007年1月11日8:00-10:00学号姓名一.填空(20分,每空1分)(可做在试题卷上,也可做在答题纸上)1、当液体饱和蒸汽压与外界压力相等时,出现_________的现象,此时相应的温度称为液体的___________。
2、每种气体都存在一个特殊的温度,在该温度以上,无论加多大压力,都不可能使气体液化。
这个温度称为___________;这个温度下的饱和蒸汽压称为__________。
3、焦耳实验证明理想气体的热力学能____________有关。
焦耳-汤姆逊实验证明,真实气体的焓H不只是___________的函数。
4、在高温、低温两热源间工作的所有可逆热机,其热效率必然______,与工质及其变化的类型________。
5、稀溶液中溶剂的蒸汽压下降、___________降低、_________升高和渗透压的数值,仅与一定量溶液中溶质的质点数有关,而与溶质的本性无关,故称这些性质为稀溶液的依数性。
6、理想气体混合物中组分B的化学势与其分压p B=y B p之间的关系式为B(pg)=Ө+RT ln(p B/pӨ)。
若真实气体混合物中组分B的逸度为f B,则其化学B(g)势可表示为_______________________,逸度因子定义为________________。
7、相律表示式F=C-P+2中,F为自由度数,C为_____________,P为_____________。
8、在稀溶液中,离子强度I定义为I=½bB z B2摩尔浓度为b的FeCl3溶液的离子强度为________。
9、胶体系统透明或不透明,但均可发生_________,胶体粒子扩散速率______,不能透过半透膜,有________的渗透压。
10、如下图所示,管中液体对毛细管壁完全浸润,当加热管中水柱的右端时,右端液面的表面张力,水柱将向移动。
二.简要回答下列问题(20分,每小题4分)1、热带海洋中,表面上的热水与表面之下的冷水间有一定的温差,能否利用这一现象造成一部热机?为什么没有看到使用这样的热机?加热2、已知25℃时,m(NaAc) = 91.0×10-4S·m2·mol-1,m(HCl)=426.2×10-4S·m2·mol-1,m (NaCl)=126.5×10-4S·m2·mol-1,请问25℃时m(HAc)是多少?3、什么是催化剂?为什么加入催化剂,化学反应速率会加快?催化剂能否改变化学反应当平衡状态?4、已知95C时,纯液态物质A(l)和纯液态物质B(l)的饱和蒸汽压分别为p A *=76.0kPa,pB*=120.0kPa二者形成理想液态混合物。
材料表面与界面知识复习资料题库

1.液体原子结构的主要特征。
(1)液体结构中近邻原子数一般为5~11个(呈统计分布),平均为6个,与固态晶体密排结构的12个最近邻原子数相比差别很大;(2)在液体原子的自由密堆结构中存在五种间隙,四面体间隙占了主要地位。
(3)液体原子结构在几个原子直径范围内是短程有序的,而长程是无序的。
2.液体表面能的产生原因。
液体表面层的分子,一方面受到液体内层的邻近分子的吸引,另一方面受到液面外部气体分子的吸引,而且前者的作用要比后者大。
因此在液体表面层中,每个分子都受到一个垂直于液面并指向液体内部的不平衡力。
这种吸引力使表面上的分子趋向于挤入液体内部,促成液体的最小表面积。
要使液体的表面积增大就必须要反抗液体内部分子的吸引力而做功,从而增加分子的位能,这种位能就是液体的表面能。
3.液体表面张力的概念和影响因素。
液体表面层的原子或分子受到内部原子或分子的吸引,趋向于挤入液体内部,使液体表面积缩小,因此在液体表面的切线方向始终存在一种使液体表面积缩小的力,其合力指向液体内部的作用力,这种力称为液体表面张力。
液体的表面张力大小受很多因素的影响。
如果不考虑液体内部其它组元向液体表面的偏聚和液体外部组元在液体表面的吸附,液体表面张力大小主要受物质本身结构、所接触的介质和温度的影响。
(1)液体的表面张力来源于液体内部原子或分子间的吸引力,因此液体内部原子或分子间的结合能的大小直接影响到液体的表面张力的大小。
一般来说,液体中原子或分子间的结合能越大,表面张力越大。
具有金属键原子结合的物质的表面张力最大;其次由大到小依次为:离子键结合的物质、极性共价键结合的物质、非极性共价键结合的物质。
(2)液体的表面张力的产生是由于处于表面层的原子或分子一方面受到液体内部原子或分子的吸引,另一方面受到液体外部原子或分子的吸引。
当液体处在不同介质环境时,液体表面的原子或分子与不同物质接触所受的作用力不同,因此导致液体表面张力的不同。
一般来说,介质物质的原子或分子与液体表面的原子或分子结合能越高,液体的表面张力越小;反之,介质物质的原子或分子与液体表面的原子或分子结合能越低,液体的表面张力越大。
材料表面与界面考试资料整理

材料表⾯与界⾯考试资料整理题型⼀、名词解释(8个,24分)⼆、简答(8个,48分,有画图题)三、简述(2个,28分)考试范围⼀、名词解释1.表界⾯:由⼀个相过渡到另⼀相的过渡区域。
2.表⾯:习惯上把固-⽓、液-⽓的过渡区域称为表⾯。
界⾯:把固-液、液-液、固-固的过渡区域称为界⾯。
物体与物体之间的接触⾯。
界⾯-两种物质(同种或不同种)之间的接触⾯、连接层和分界层。
3.理想表⾯:理论上结构完整的⼆维点阵平⾯。
4.清洁表⾯:不存在任何吸附、催化反应、杂质扩散等物理-化学效应的表⾯。
(表⾯的化学组成与体内相同,但结构可以不同于体内)5.驰豫表⾯:指表⾯层之间以及表⾯和体内原⼦层之间的垂直间距d s和体内原⼦层间距d0相⽐有所膨胀和压缩的现象。
6.驰豫:表⾯区原⼦(或离⼦)间的距离偏离体内的晶格常数,⽽晶胞结构基本不变,这种情况称为弛豫。
7.重构表⾯:指表⾯原⼦层在⽔平⽅向上的周期性不同于体内,但在垂直⽅向上的层间间距d0与体内相同。
8.台阶表⾯:表⾯不是平⾯,由规则或不规则台阶组成。
9.表⾯偏析:杂质由体内偏析到表⾯,使多组分材料体系的表⾯组成与体内不同。
10.吸附表⾯:在清洁表⾯上有来⾃体内扩散到表⾯的杂质和来⾃表⾯周围空间吸附在表⾯上的质点所构成的表⾯。
11.平移界⾯:在结构相同的晶体中,⼀部分相对于另⼀部分平滑移动⼀个位移⽮量。
其间的界⾯称为平移界⾯。
12.反演界⾯:当晶体结构由中⼼对称向⾮中⼼对称转变时,由反演操作联系起来的两个畴之间形成反演界⾯IB。
13.表⾯能:可以理解为系统增加单位⾯积时所需做的可逆功,单位是J/m2。
14.表⾯张⼒:是单位长度上的作⽤⼒,单位是N/m。
15.晶界:同质材料形成的固体/固体界⾯为晶界。
16.相界:异质材料形成的固体/固体界⾯为相界。
⼆、简答、简述1、表界⾯通常可以分为哪5类?固-⽓;液-⽓;固-液;液-液;固-固。
2、获得理想表⾯的理论前提?(1)、不考虑晶体内部周期性势场在晶体表⾯中断的影响;(2)、不考虑表⾯原⼦的热运动、热扩散、热缺陷等;(3)、不考虑外界对表⾯的物理-化学作⽤等;(4)、认为体内原⼦的位置与结构是⽆限周期性的,则表⾯原⼦的位置与结构是半⽆限的,与体内完全⼀样。
材料表面与界面复习题

1.液体原子构造的主要特征。
〔1〕液体构造中近邻原子数一般为5~11个〔呈统计分布〕,平均为6个,与固态晶体密排构造的12个最近邻原子数相比差异很大;〔2〕在液体原子的自由密堆构造中存在五种间隙,四面体间隙占了主要地位。
〔3〕液体原子构造在几个原子直径范围内是短程有序的,而长程是无序的。
2.液体外表能的产生原因。
液体外表层的分子,一方面受到液体内层的邻近分子的吸引,另一方面受到液面外部气体分子的吸引,而且前者的作用要比后者大。
因此在液体外表层中,每个分子都受到一个垂直于液面并指向液体内部的不平衡力。
这种吸引力使外表上的分子趋向于挤入液体内部,促成液体的最小外表积。
要使液体的外表积增大就必须要对抗液体内局部子的吸引力而做功,从而增加分子的位能,这种位能就是液体的外表能。
3.液体外表张力的概念与影响因素。
液体外表层的原子或分子受到内部原子或分子的吸引,趋向于挤入液体内部,使液体外表积缩小,因此在液体外表的切线方向始终存在一种使液体外表积缩小的力,其合力指向液体内部的作用力,这种力称为液体外表张力。
液体的外表张力大小受很多因素的影响。
如果不考虑液体内部其它组元向液体外表的偏聚与液体外部组元在液体外表的吸附,液体外表张力大小主要受物质本身构造、所接触的介质与温度的影响。
〔1〕液体的外表张力来源于液体内部原子或分子间的吸引力,因此液体内部原子或分子间的结合能的大小直接影响到液体的外表张力的大小。
一般来说,液体中原子或分子间的结合能越大,外表张力越大。
具有金属键原子结合的物质的外表张力最大;其次由大到小依次为:离子键结合的物质、极性共价键结合的物质、非极性共价键结合的物质。
(2)液体的外表张力的产生是由于处于外表层的原子或分子一方面受到液体内部原子或分子的吸引,另一方面受到液体外部原子或分子的吸引。
当液体处在不同介质环境时,液体外表的原子或分子与不同物质接触所受的作用力不同,因此导致液体外表张力的不同。
一般来说,介质物质的原子或分子与液体外表的原子或分子结合能越高,液体的外表张力越小;反之,介质物质的原子或分子与液体外表的原子或分子结合能越低,液体的外表张力越大。
胶体与界面化学习题
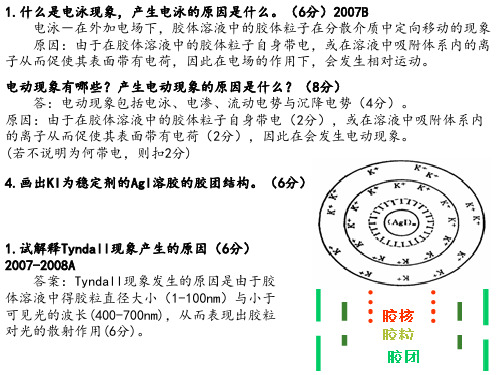
1.对于胶团[(AgBr)m•n Ag+•(n-x)NO3-]x+•xNO3-,下列说法中不正确的是( B )
A. 胶核是(AgBr)m
B. m=n+x
C. 胶粒是[(AgBr)m•nAg+•(n-x)NO3-]x+ D. 在电场中胶粒向负极移动
2.对于As2S3(负溶胶),聚沉能力最大的是( C )
3.试解释毛细凝结现象发生的原因。(6分) 2007-2008A 答:根据Kelvin公式(2分),凹面上的蒸汽压比平面上小(2分),所以在小于
饱和蒸汽压时,气体凹面上已达饱和而发生凝聚,这就是毛细凝聚现象(2分), 即蒸汽变成液体在毛细管中凝聚,吸附量迅速增加。
4. 试解释过饱和蒸汽产生的原因。(6分) 答:过饱和蒸汽现象的产生是由于如果蒸汽凝结成为液滴,那么需要先形成
2. 表面张力又称为表面(吉布斯)自由能 ,液体的表面张力的方向是垂直于表 面的边界指向液体方向并与表面相切。
3. 水能润湿洁净玻璃,而水银则不能。现将一根毛细玻璃管插入水中,管内液面 将 上升 ;如在管内液面处加热,则液面将 下降 ;当毛细管插入水银 时,管内液面将 下降 ;若在管内液面处加热,则液面将 上升 。
答:当泡沫表面由于扩展而变薄,从而产生新的表面时(2分),由于在新的 表面上溶质的吸附尚未达到平衡(2分),使其表面张力增加,产生了表面张力梯 度(2分),这种表面张力的梯度就会引起液体从低表面张力的周围向高表面张力 区域流动,直到此张力差消失为止(1分)。
7. 根据DLVO理论,试解释外加电解质对带电溶胶稳定性的影响。(7分) 答:根据DLVO理论,在胶团之间, 既存在着斥力势能, 又存在着吸力势能(1
答:硅胶自四氯化碳中吸附苯甲酸的量大于在水中吸附的量(3分),因为硅 胶是极性吸附剂(1分),水的极性比苯甲酸强(1分),硅胶对水有强烈的吸引力, 所以减少了硅胶对苯甲酸的吸附(1分),而硅胶对四氯化碳的吸引力弱,所以硅 胶易于吸附苯甲酸(1分)。
2007A卷答案

A卷答案1、锅炉成分分析的基准有收到基、空气干燥基、干燥基、干燥无灰基四种。
其换算关系为:由于燃料中水分和灰分的含量易受外界条件的影响而发生变化,水分或灰分的含量变化了,其他元素成分的含量也会随之而变化。
例如,水分含量增加时,其他成分的百分含量便相对地减小;反之,水分含量减少时,其他成分的百分含量便相对地增加。
所以不能仅用各成分的质量百分数来表示燃料的成分组成特性。
有时为了使用或研究工作的需要,在计算燃料的各成分百分含量时,可将某种成分(例如水分或灰分)不计算在内。
这样按不同的“成分组合”计算出来的各成分百分数就会有较大的差别。
这种根据燃料存在的条件或根据需要而规定的“成分组合”称为基准。
如果所用的基准不同,同一种煤的同一成分的百分含量结果便不一样。
2、由于燃料中水分和灰分的含量易受外界条件的影响而发生变化,水分或灰分的含量变化了,其他元素成分的含量也会随之而变化。
例如,水分含量增加时,其他成分的百分含量便相对地减小;反之,水分含量减少时,其他成分的百分含量便相对地增加。
所以不能仅用各成分的质量百分数来表示燃料的成分组成特性。
有时为了使用或研究工作的需要,在计算燃料的各成分百分含量时,可将某种成分(例如水分或灰分)不计算在内。
这样按不同的“成分组合”计算出来的各成分百分数就会有较大的差别。
这种根据燃料存在的条件或根据需要而规定的“成分组合”称为基准。
如果所用的基准不同,同一种煤的同一成分的百分含量结果便不一样。
3、链条炉中,习惯上将从炉排下送入的空气称为一次风,二次风是指从火床上方送入炉膛的一股强烈气流。
二次风的主要作用是扰乱炉内气流,使之自相混合,从而使气体不完全燃烧损失和炉膛内的过量空气系数都得以降低。
与炉拱相比,二次风的优点是布置灵活,调节方便,而且炉膛结构也不会因之复杂化。
但二次风要消耗一定能量。
一般情况下,二次风配合炉拱使用,以取得最佳效果。
除搅乱和混合烟气外,二次风若布置恰当,它还能起多种其它的良好作用,二次风的工质可以是空气,也可以是蒸汽,甚至是烟气。
界面化学

A2 A1
γdA
=
γ
( A2
−
A1 )
=
γ
(4 Nπr22
−
4πr12 )
[ ( ) ( ) ] = {4π × 0.07288 ×[109 × 1.0 ×10−6 2 − 1.0 ×10−3 2 ]}J
= 9.15 ×10−4 J
(3)
W f = −ΔGA = −9.15 ×10−4 J
2. 已知汞溶胶中胶粒(设为球形)的直径 22nm,在 1.0dm3的溶胶中含Hg为 8×10-5kg,试计算: ⑴在 1.0cm3的溶胶中的胶粒数. ⑵胶粒的总表面积. ⑶若把质量为 8×10-5kg的汞滴,分散成上述溶胶粒子时,表面Gibbs自由能增加多少?已知汞
( ) ΔGA = γΔA = γ A − 4πr02
{ [ ]} ( ) = 0.375 × 1.604 ×10−3 − 4π × 1.12 ×10−3 2 J
= 5.96 ×10−4 J
3 试证明: ⑴(δU/δAs)T.p=γ-T(δγ/δT)p.As –p(δγ/δp)T.As ⑵(δH/δAs)T.p=γ-T(δγ/δT)p.As
⎟⎟⎠⎞T , p
+
T
⎜⎜⎝⎛
∂S ∂AS
⎟⎟⎠⎞T , p
⎜⎜⎝⎛
∂G ∂AS
⎟⎟⎠⎞T , p
=γ
⎜⎜⎝⎛
∂S ∂AS
⎟⎟⎠⎞T , p
=
−⎜⎛ ∂γ ⎝ ∂T
⎟⎞ ⎠ p, AS
⎜⎜⎝⎛
∂U ∂AS
⎟⎟⎠⎞T , p
=γ
−
T
⎜⎛ ⎝
∂γ ∂T
⎟⎞ ⎠ p, AS
4. 已知水的表面张力与温度的关系式为:γ=(75.64-0.00495T/K)×10-3N/m 在 283K时,可逆地使一定量纯水的表面积增加 0.01m2(设体积不变),求系统的如下各个量: △U,△H,△S,△A,△G,Q和W。
材料表面与界面-习题含答案

第一章1、什么是Young 方程?接触角的大小与液体对固体的润湿性好坏有怎样的关系?答:Young 方程:界面化学的基本方程之一。
它是描述固气、固液、液气界面自由能γsv ,γSL ,γLv 与接触角θ之间的关系式,亦称润湿方程,表达式为:γsv -γSL =γLv COSθ.该方程适用于均匀表面和固液间无特殊作用的平衡状态.关系:一般来讲,接触角θ的大小是判定润湿性好坏的依据,若θ=0.cosθ=1,液体完全润湿固体表面,液体在固体表面铺展;若0<θ<90°,液体可润湿固体,且θ越小,润湿性越好;90°<θ<180°,液体不润湿固体;θ=180°,完全不润湿固体,液体在固体表面凝集成小球。
2、水蒸气骤冷会发生过饱和现象,在夏天的乌云中,用飞机撒干冰微粒,试气温骤降至293K ,水气的过饱和度(P/Ps )达4,已知在293K 时,水的表面能力为0。
07288N/m,密度为997kg/m 3,试计算:(1)在此时开始形成雨滴的半径.(2)每一雨滴中所含水的分子数。
答:(1)根据Kelvin 公式有'2ln 0R RT M P P ργ=开始形成的雨滴半径为:0ln 2'p pRT MR ργ=将数据代入得:m R 101079.74ln 997293314.8018.007288.02'-⨯=⨯⨯⨯⨯⨯=(2)每一雨滴中所含水的分子数为N=N A n ,n=m/M=ρV/M ,得个661002.6018.03997)1079.7(14.34)(34233103'=⨯⨯⨯⨯⨯⨯⨯===-A A N M R N M V N ρπρ3、在293k 时,把半径为1。
0mm 的水滴分散成半径为1。
0μm 的小水滴,试计算(已知293K 时水的表面Gibbs 自由为0。
07288J .m —2)(1)表面积是原来的多少倍?(2)表面Gibbs 自由能增加了多少?(9分)答:(1)设大水滴的表面积为A 1,小水滴的总表面积为A 2,则小水滴数位N ,大水滴半径为r 1,小水滴半径为r 2。
(完整版)界面现象测试题答案

(完整版)界面现象测试题答案界面现象测试题(二)参考答案1. 液体的表面自由能γ可以表示为:( )参考答案: C(A) (H/A)T,p,n(B) (F/A)T,p,n(C) (U/A)S,V,n(D) (G/A)T,V,n2.下列叙述不正确的是:参考答案: D(A) 比表面自由能的物理意义是,在定温定压下,可逆地增加单位表面积引起系统吉布斯自由能的增量;(B)表面张力的物理意义是,在相表面的功面上,垂直作用于表面上任意单位长度功线的表面紧缩力;(C)比表面自由能与表面张力量纲相同,单位不同;(D)比表面自由能单位为J·m2,表面张力单位为N·m-1时,两者数值不同。
3. 对大多数纯液体其表面张力随温度的变化率是:( ) 参考答案: B(A) (γ/T)p> 0 (B) (γ/T)p< 0(C) (γ/T)p= 0 (D) 无一定变化规律4.同一体系,比表面自由能和表面张力都用σ表示,它们:参考答案: D(A) 物理意义相同,数值相同; (B) 量纲和单位完全相同;(C) 物理意义相同,单位不同;(D) 前者是标量,后者是矢量。
5.纯水的表面张力是指恒温恒压组成时水与哪类相接触时的界面张力:参考答案: B(A) 饱和水蒸气;(B) 饱和了水蒸气的空气;(C) 空气; (D) 含有水蒸气的空气。
6. 已知400 K 时,汞的饱和蒸气压为p0,密度为ρ,如果求在相同温度下,一个直径为10-7 m 的汞滴的蒸气压,应该用公式:( ) 参考答案: C(A)p =p0+ 2γ/R' (B) ln(p/p0) =ΔV ap Hm(1/T0- 1/T)/R(C) RTln(p/p0) = 2γM/ρR' (D) p = nRT/V7.某温度压力下,有大小相同的水滴、水泡和气泡,其气相部分组成相同,见图。
它们三者表面自由能大小为:参考答案: A(A) G a = G c < G b;(B) G a = G b > G c ;(C) G a < G b < G c ;(D) G a = G b = G c 。
材料表面与界面复习题

材料表面与界面复习题第一章1.试述表面张力(表面能)产生的原因。
怎样测试液体的表面张力?(1)原因液体表面层的分子所受的力不均匀而产生的。
液体表面层即气液界面中的分子受到指向液体内部的液体分子的吸引力,也受到指向气相的气体分子的吸引力,由于气相吸引力太小,这样,气液界面的分子净受到指向液体内部并垂直于表面的引力作用,即为表面张力。
这里的分子间作用力为范德华力。
(2)测试①毛细管上升法测定原理将一支毛细管插入液体中,液体将沿毛细管上升,升到一定高度后,毛细管内外液体将达到平衡状态,液体就不再上升了。
此时,液面对液体所施加的向上的拉力与液体总向下的力相等。
则丫=1 /2( p l- p g)ghrcos6 (1)(1)式中Y为表面张力,r为毛细管的半径,h为毛细管中液面上升的高度,p l为测量液体的密度,P g为气体的密度(空气和蒸气),g为当地的重力加速度,e为液体与管壁的接触角。
若毛细管管径很小,而且B =0时,则上式(1)可简化为Y =1/2 p ghr (2)②Wilhelmy盘法测定原理用铂片、云母片或显微镜盖玻片挂在扭力天平或链式天平上,测定当片的底边平行面刚好接触液面时的压力,由此得表面张力,公式为:W 总-W 片=2 丫lcos©式中,W总为薄片与液面拉脱时的最大拉力,W 片为薄片的重力,l为薄片的宽度,薄片与液体的接触的周长近似为2l,4为薄片与液体的接触角。
③悬滴法测定原理悬滴法是根据在水平面上自然形成的液滴形状计算表面张力。
在一定平面上,液滴形状与液体表面张力和密度有直接关系。
由Laplace公式,描述在任意的一点P曲面内外压差为式中R1, R2为液滴的主曲率半径;z为以液滴顶点0为原点,液滴表面上P的垂直坐标; P0为顶点O处的静压力。
定义S= ds/de式中de为悬滴的最大直径, ds为离顶点距离为de处悬滴截面的直径再定义H= 3 (de/b)2 则得丫= ( p l- p g)gde2/H 式中 b 为液滴顶点O处的曲率半径。
表面与界面化学试卷(a)2008

Fig.1Figure 1 shows an adsorbed structure on an fcc surface. How might this adsorbate structure on the surface be described武汉理工大学教务处试题标准答案及评分标准用纸课程名称—表面与界面化学——(A 卷)1. chemisorption: a chemical bond, involving substantial rearrangement of electron density, is formed between the adsorbate and substrate.Physisorption : the only bonding is by weak Van der Waals - type forces. There is no significant redistribution of electron density in either the molecule or at the substrate surface.2. the surface energies determined are not equal. in air<under argon<and in a vacuum. When the mica is prepared in air, chemisorptions will occur on the mica surface and release heat. Under argon, there is only physisorption occur, the released energy is lower. In a vacuum, no adsorption occurs, the real surface energy is determined.3. The first raindrop sliding down the window glass, the contact angle of the water and glass is the advancing contact angle, which gives a large force to prevent the sliding of raindrop. The first raindrop leaves a water film on the glass. The later raindrop will show a spreading wetting on the window and give a quick sliding rate.4. (1) M=WRT/ A=(2) sodium dodecylsulfate is an ionic surfactant。
表面与界面习题讲解

Chapter 11、表面与界面的定义。
1)表面:固体与真空的界面;2)界面:相邻两个结晶空间的交界面称为“界面”。
2、叙述表面与本体的不同点。
表面与本体:结构、化学组成、性质都存在不同。
材料与外界的相互作用是通过表面来进行的。
因此,表面具有特殊性,它的性质将直接影响材料的整体性质。
材料的性质虽然与组成的本体有关,但其表面对性能的影响却占很大的比重。
因为,不少性能是通过表面来实现的,如表面硬度、表面电导,同时,材料某些性能将通过表面受到外界环境的影响。
3、什么叫相界面?有哪几类?1)相界面:相邻相之间的交界面成为相界面。
2)分为3类:固相与固相的界面,固相与气相的界面,固相与液相的界面。
4、材料表面与界面的表征手段有哪些?材料表面与界面的表征主要通过对比表面积、表面张力(表面能)等测定来实现1)比表面积a 静态吸附法(BET )(测量准确度和精度都很好,但达到吸附平衡慢,仪器装置较复杂,需要高真空系统,并且要使用大量的汞,逐步被动态吸附法所取代)b 动态吸附法:常压流动法,气相色谱法(操作简单而快速 )2)表面张力a 高聚物熔体表面张力外推法(γ∝T 成直线关系,测定不同温度下高聚物熔体的表面张力,外推到20℃时的表面张力)b Zisman 的浸润临界表面张力法(测定固体在已知表面张力的液体中的接触角 )C 还有几何平均方程求解法、状态方程测求法等等d 理论计算:等张比容法、内聚能密度法、Tg 参数计算法5、试述表面张力产生的原因。
材料的表面结构和性质与其本体有明显的差别,这是因为位于材料本体的原子受到周围原子的相互作用是相同的,处于对称力场之中,总的作用之和等于0;而处于表面的原子只有局部受到与本体相同的相互作用,而其余的部分则完全不同,表面由此产生表面张力。
6、单位体积的物体所具有的表面积称为比表面,请得 出下列结果:(1)半径为r 的球形颗粒,其比表面为:(2)质量为m ,密度为ρ的球形颗粒的比表面:(3) 边长为L 的立方体的比表面为:(4) 质量为m ,密度为ρ的立方体的比表面为:7.水蒸气迅速冷却至25℃会发生过饱和现象。
界面化学各题型试题汇总【自己整理】

界面化学各题型试题汇总一、选择题1.液体表面最基本的特性是( )A.倾向于收缩 B.倾向于铺展 C.形成单分子吸附层2.若将液体与毛细管壁间的接触角近似看作0°,则液体在毛细管中的液面可以看作( )A.凹型B.凸型C.球面3.下列方程均为计算液a/液b 界面张力γab 的经验公式,其中Fowkes 公式为( )A.γab =γa -γbB.γab =γa + γb -2(γa γb )1/2C . γab =γa + γb -2(γa d γb d )1/24.吊片法测定液体表面张力时,要求尽可能采用表面粗糙的吊片材料,其目的是( )A.改善液体对吊片的润湿使θ接近于0°B. 改善液体对吊片的润湿使θ接近于90°C.改善液体对吊片的润湿使θ接近于180°5.溶液中溶剂记为1、溶质记为2,则吸附量Γ2(1)的含义为( )A.单位面积表面相与含有相等总分子数的溶液相比较,溶质的过剩量B.单位面积表面相与含有等量溶质的溶液相比较,溶剂的过剩量C.单位面积表面相与含有等量溶剂的溶液相比较,溶质的过剩量8.下列说法中不正确的是( )A.任何液面都存在表面张力B.平面液体没有附加压力C.弯曲液面的表面张力方向合力指向曲率中心D.弯曲液面的附加压力指向曲率中心9.运用过滤手段进行溶胶净化的目的是( )A.除掉反应过程中过量的副产物B.除掉过量的电解质C.除掉溶胶体系中的粗离子10.溶液中胶体离子表面的热力学电位是指下列哪个电学参数( )A.φ0B. φsC. ξ11.造成接触角滞后的主要原因是( )A.测量方法B.实验者过失C.表面不均匀和表面不平整12.固体自稀溶液中吸附等温线可分为4类18种,对于具有L 型吸附等温线的吸附过程的特征,下列说法正确的是( )A.溶液有强烈的吸附竞争,且溶质分子以单一端基近似垂直吸附于固体表面B.溶质与固体表面具有高亲和力,有类似与化学吸附特征的强烈吸附行为ngmuir 型,溶质比溶剂易于吸附,吸附时溶质分子多以其长轴或平面平行于固体表面13.对于胶体体系下列说法正确的是( )A.电解质引发胶体体系聚沉的主要原因是使扩散层变厚B.低浓度的聚合物可以使胶体体系发生聚沉,而高浓度的聚合物却可以使胶体体系稳定C.胶体体系属于热力学多相体系,由于界面自由能显著,所以无论采取何种措施都不可能获得相对稳定的胶体溶液二、填空题1. 液-固润湿过程有________,_________,_________.2. 固体自溶液中吸附时,极性吸附剂易于从非极性溶液中吸附_______物质,而非极性吸附剂易于从极性溶液中吸附________物质。
界面化学部分复习题

8.某水溶液发生负吸附后,在干净的毛吸管中的上升高度比 纯水在该毛吸管中上升的高度低。 ( ) 8. 答: × 9.通常物理吸附的速率较小,而化学吸附的速率较大。( ) 9. 答: ×
10.兰缪尔定温吸附理论只适用于单分子层吸附。( ) 10. 答:
二、选择题
选择正确答案的编号,填在各题后的括号内:
=5.45 ×10 - 4 Pa -1
82.5
dm 3 kg 1
Pa
( 2 ) V=
93.8dm 3 kg 1 5.45 10 4 Pa 1 6.67 103 Pa 1 5.45 10 4 Pa 1 6.67 103 Pa
= 73.5dm3· -1 kg
= 143.9×10 3 Pa = 1439 kPa ln
p r 2σ M p RTr
8.314
2 71.97 103
N m 1 18.02 10 3 K 0.9971 10 3
kg mol1 kg cm 3 1 10 6 m
r
所以 h 2cos
rg
=
2 483 10 3 5 10 4 m 13.55 103
N m 1 1 kg m 3 9.81 m s 2
= —0.0145 m 即汞面会降低0.0145 m
5 25 ℃时乙醇水溶液的表面张力随乙醇浓度c的变化关系为:
2
泡压法测定丁醇水溶液的表面张力。20℃实测最大泡压力为
0.4217 kPa,20℃时测的水的最大泡压力为0.5472 kPa,已知20℃时 水的表面张力为 72.75×10 -3 N· -1,请计算丁醇溶液的表面张力。 m 解:设p1,p2,1,2 分别为丁醇溶液及水的最大泡压力与表面 张力。 根据拉普拉斯公式及泡压法的原理可知:
材料物理化学 表面与界面 习题

球状较稳定,还是在境界上呈双球冠形较为稳定?
(b)如果 β 在晶界上呈薄膜状,情况又将如何?
解:(a)若设 γ αβ 为 α-β 界面上的表面张力; γ αα 为 α -α 界面上的表面张力。 当 β 相为球冠状存在于晶界上时,如图 5-12-1 示,表面能为:
(γ
)
A晶
界
=
2[
2
r
2 α
β
(1
3)真实表面:它是在清洁表面上有来自体内扩散到表面的杂质和来自表面周围空 间吸附在表面上的质点所构成的表面。根据原子在基底上的吸附位置,一般可分为四种 吸附情况,即顶吸附、桥吸附、填充吸附和中心吸附等。
4、固体表面的驰豫与无机超细粉体性能之间有何关系? 解:由于固相的三维周期性在固体表面处突然中断,表面上原子产生的相对于正常
位置的上、下位移,称为表面弛豫。
材料物理化学
湖南工学院
粉体:微细的固体微料集合体,原料加工成微细颗粒以利于成型和烧结。粉体制备:反 复粉碎形成一系列新表面。而离子极化变形重排畸变有序性降低,随粒子的微细化从表 面增大,无序性增大并向纵深发展,不断影响内部结构,最后使粉体表面结构趋于无定 形化。
一种认为粉体表面层是无定形结构。一种认为粉体表面层是粒度极小的微晶结构。 所以在无机超细粉体上可以发生表面驰豫现象。
解:每 1g 石英所占体积 1/2.65=0.3774cm3/g
一粒石英所占体积
4 / 3 r 3= 4 / 3 π (10 4 ) 3 = 4 .188 10 - 12 cm 3
每克石英含粒子数
0 .3774
= 9 10 10
4 .188 10 12
1 .02 = 0 .3849 cm 3 / g
表面与界面化学试卷(a)2007

Fig.2 An adsorbed structure on an fcc (100) surface Fig.3 adsorption isothermsAt 77K a 1.5g sample of a porous solid showed the following results for the adsorption of nitrogen:武汉理工大学教务处试题标准答案及评分标准用纸课程名称—表面与界面化学—— ( A 卷)1. ① surface tension: Surface tension is defined as the force acting at right angles to any line of unit length on the liquid surface or surface tension is the work required to increase the area of a surface isothermally and reversibly by unit amount. (3 points)②CMC: CMC is the concentration above which micelle formation becomes appreciable. (3 points)③solubilisation: Surfactant solutions above the CMC can solubilise otherwise insoluble organic material by incorporating it into the interior of the micelles. This is known as micellar solubilisation. (3 points)④ interface: The point, area, or surface along which two substances or other qualitatively different things meet. (3 points)⑤coverage: a measure of the extent of adsorption of a species onto a surface. Usually defined as , Where N ads is the number of adsorbate atoms per unit area and N m is the number of atoms adsorbated per unit area to produce one complete layer on the surface. (3 points)⑥unit mesh: the unit cell of a two-dimensional surface structure. (3 points) ⑦surface atom density: the number of atoms per unit surface area 。
界面试题

界面(胶体)化学练习题1 选择题1.同一体系,比表面自由能和表面张力都用γ表示,它们()(a)物理意义相同,数值相同(b)量纲和单位完全相同(c)物理意义相同,单位不同(d)前者是标量,后者是矢量2.液体在毛细管中上升的高度与下列哪一个因素()无关?(a)温度(b)液体密度(c)重力加速度(d)大气压力3.水对玻璃润湿,汞对玻璃不润湿,将一玻璃毛细管分别插入水和汞中,下列叙述不正确的是()(a)水为凹球面(b)汞为凸球面(c)内水面高于水平面(d)内汞面与汞平面一致4.一根毛细管插入水中,液面上升的高度为h,当在水中加入少量氯化钠,这时毛细管中液面高度为()(a)等于h (b)大于h (c)小于h (d)无法确定5.下列说法中不正确的是()(a)平面液体没有附加压力(b)凸液面曲率半径为正,凹液面曲率半径为负(c)弯曲液面表面张力的方向指向曲率中心(d)弯曲液面的附加压力方向指向曲率中心6.有两根半径相同的玻璃毛细管插入水中,水面上升高度为h,其中一根在h/2处使其弯曲向下,试问水在此毛细管端的行为是()(a)水从毛细管端滴下(b)毛细管端水面呈凸形弯月面(c)毛细管端水面呈凹形弯月面(d)毛细管端水面呈水平面7.在298K时,已知A液面的表面张力是B液的一半,其密度是B液的2倍:如果A、B液分别用相同的毛细管产生大小相同的气泡时,A液的最大气泡压力差等于B液的()(a)1/2倍(b)1倍(c)2倍(d)4倍8.将装有润湿性液体的毛细管水平放置,在其右端加热,则管内液体将()(a)向右移动(b)向左移动(c)不移动(d)无法确定9.天空中的水滴大小不等,在运动中,这些水滴的变化趋势如何?()(a)大水滴分散成小水滴,半径趋于相等(b)大水滴变大,小水滴缩小(c)大小水滴的变化没有规律(d)不会产生变化10.一个U形管的两壁直径不同,一端为1×10-3m,另一端为3×10-3m,水的表面张力为0.072N·m-1。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Fig.2 An adsorbed structure on an fcc (100) surface Fig.3 adsorption isotherms
At 77K a 1.5g sample of a porous solid showed the following results for the adsorption of nitrogen:
武汉理工大学教务处
试题标准答案及评分标准用纸
课程名称—表面与界面化学—— ( A 卷)
1. ① surface tension: Surface tension is defined as the force acting at right angles to any line of unit length on the liquid surface or surface tension is the work required to increase the area of a surface isothermally and reversibly by unit amount. (3 points)
②CMC: CMC is the concentration above which micelle formation becomes appreciable. (3 points)
③solubilisation: Surfactant solutions above the CMC can solubilise otherwise insoluble organic material by incorporating it into the interior of the micelles. This is known as micellar solubilisation. (3 points)
④ interface: The point, area, or surface along which two substances or other qualitatively different things meet. (3 points)
⑤coverage: a measure of the extent of adsorption of a species onto a surface. Usually defined as , Where N ads is the number of adsorbate atoms per unit area and N m is the number of atoms adsorbated per unit area to produce one complete layer on the surface. (3 points)
⑥unit mesh: the unit cell of a two-dimensional surface structure. (3 points) ⑦surface atom density: the number of atoms per unit surface area 。
(3 points) ⑧ surface activity: The strong adsorption of such materials at surfaces or interfaces in m ads
N N /=θ
the form of an orientated monomolecular layer is termed surface activity. (3 points)
⑨Krafft point: Micelle-forming surfactants exhibit another unusual phenomenon in that their solubilities show a rapid increase above a certain temperature, known as the Krafft point.
⑩surface excess: The difference in concentration between surface and inner due to the adsorption is termed “surface excess”.
2.P/(V(P0-P))⨯104(cm-3) 4.17 16.67
P/P00.04 0.2
The slop and the intercept are 0.0078125, 0.0001045 respectively. (3 points) Vm=1/(0.0078125+0.0001045)=126.31 cm3 (3 points)
The specific sruface area
A=N0σV m/m22400=6.02⨯1023⨯16.2⨯10-20⨯126.31/1.5/22400
=367m2/g (4 points)
3.Read off a number of pairs of values of pressure (P) and temperature (T) which yield the
same surface coverage, (5 points) and then plot a line of ( ln P ) v's (1/T), the slope of which yields the adsorption heat. (5 points)
The unit cell is illustrated below - note, this cell contains only one repeat unit and is the primitve unit cell for the adsorbate.
It is rectangular in shape and shows no rotational misalignment with the substrate unit cell. Using the substrate vectors given on the diagram we have the following relations: b1 = a1; b2 = 3.a2
Hence, this structure is a M(110)(1x3) structure.
The ratio of the areas the unit cells of the adsorbate and substrate is 3:1 and the adsorbate cell contains only one adsorbed entity - consequently
Coverage = ( 1 / 3 ) x 1 = 0.33
4. The real space overlayer structures for c(2⨯4) and (1⨯2) are shown in the Fig.(a) and Fig.(b), respectively.
(a) (3points) (b) (3points)
The corresponding LEED patterns are shown as Fig (c) and Fig.(d)
(c) (3 points) (d) (3 points)
7. (1) the work of adhesion W a = γw +γO -γwO =72.8+21.8-50.8=43.8mN m -1 (4 points)
(2) the work of cohesion for n-octane W c =2γO =2⨯21.8=43.6 mN m -1(2 points)
the work of cohesion for water W c ’=2γw =2⨯72.8=145.6 mN m -1(2 points)
(3) the initial spreading coefficient of n-octane on water
S=γw -(γO +γwO )=72.8-(21.8+50.8)=0.2 mN m -1(4 points)
Pd atoms
O atoms
Pd spots
O spots。
