药品常用英语缩写
制药行业常用英语词汇(缩写、中英文对照)

制药行业常用英语词汇(缩写、中英文对照)制药行业常用英语词汇(缩写、中英文对照)序号中文英文及缩写 1 药品生产质量管理规范 GMP:GoodManufacturingPractice 2 国家食品与药品监督管理局 State Food and Drug Administration 3 总则 GeneralProvisions 4 《中华人民共和国药品管理法》 the DrugAdministration Law of the People"s Republic of China 5 制剂Preparation 6 原料药 API: Active PharmaceuticalIngredient 7 成品finished goods 8 工序 process 9 机构与人员 organization and personnel 10 专业知识 professional knowledge 11 生产经验 production e_perience 12 组织能力 organizational skill 13 技术人员 technical staff 14 实施implementation 15 药品生产 pharmaceutical manufacturing 16 质量管理quality management 17 质量检验 quality inspection 18 专业技术培训professional and technicaltraining 19 基础理论知识 basic theoreticalknowledge 20 实际操作技能 practical operationskills 21 高生物活性 highly potent 22 高毒性 high to_icity 23 污染 contamination 24 考核评估 assessment25 厂房与设施 buildings and facilities 26 生产环境 production environment 27 空气洁净级别 clean air level 28 昆虫 insect 29 洁净室(区)clean room(area) 30 光滑 smooth 31 无裂缝 no cracks 32 无颗粒物脱落no particle shedding 33 耐受 endure 34 消毒 disinfection 35 无菌 sterile 36 交界处 junction, joint 37 弧形 arc 38 灰尘积聚 dues accumulation 39 储存区 store area 40 生产规模 production scale 41 设备 equipment 42 物料material 43 中间产品 intermediate product 44 待验品 quarantined material 45 交叉污染 cross-contamination 46 管道 pipeline, ductwork 47 风口 tuber 48 公用设施, 公用工程 utilities of publicservice 49 照明 lighting 50 照度 illumination 51 应急紧急情况 emergency 52 净化 purification, clean 53 微生物, 微生物学, 微生物 micro-organism,microbiology,microbiologic的 54 监测 monitoring 55 记录 record 56 天棚天花板 ceiling, roof 57 密封 seal 58 静压差 Static DifferentialPressure 59 温度 temperature 60 相对湿度 RH: Relative Humidity 61 低漏地漏 floor drainer 62 青霉素penicillin 63 分装室 separating room, fillingroom 64 相对负压 relative negativepressure 65 废气 waste gas,e_hausted air 66 β-内酰胺结构类药品 β-Lactasestructure drug, drugs of β-Lactic group 67 避孕药品 contraceptives 68 激素类 hormone 69 抗肿瘤类 anti-tumor, oncology 70 放射性药品 Radiopharmaceuticals 71 包装 packing, package 72 循环使用recycling 73 微粒 particles 74 辐射 radiation, irradiation 75 细菌bacteria 76 病毒 virus 77 细胞 cell 78 脱毒前后 pre and postdeto_ification 79 活疫苗与灭活疫苗 activevaccine/inactivatedvaccine 80 人血液制品 blood products 81 预防制品prevention products82 灌装 filling 83 中药 Chinesetraditional medicines 84 前处理pretreatment 85 提取 e_traction 86 浓缩 concentration 87 动物脏器viscera of animal,organ ofanimal 88 蒸、炒、炙、煅 ing, frying,sunburn, testing 89 炮制concocted 90 通风 ventilation 91 除烟 smoke removal 92 除尘 dust removal 93 降温设施 temperature-reducingestablishment,cooling 94 筛选 screening, sift 95 切片 slicing 96 粉碎 grinding 97 压缩空气 pressed air 98 惰性气体 noble gas 99 取样 Sling 100 称量室weighing room, dispensingroom 中药标本 Chinese herbalsle,e_emplar of TCM 102 检定鉴定 verification, identification 103 同位素 Isoe 104 设备 equipment 105 选型 model/type selection 106 耐腐蚀anticorrosion 107 吸附 adsorption, absorption 108 润滑剂, 润滑 lubricant, lubricate 109 冷却剂 coolant 110 流向 flow direction111 纯化水 PW: Purified Water 112 注射用水 WFI: Water for Injection 113 滋生 breeding 114 储罐 tank 115 死角 neglected portion 116 盲管blind pipe 117 纤维 fiber 118 疏水性 hydrophobicity 119 仪表instrumentation 120 量具 measuring tool 121 衡器 weighing instrument 122 精密度 precision 123 维修 maintenance 124 不合格 disqualified reject 125 物料 material 126 购买 purchasing 127 发放 releasing 128 产地 origin 129 入库 loading 130 固体 solid 131 液体 liquid 132 挥发性 volatile 133 净药材 medicine, TCM 134 麻醉药品 narcotics 135 精神药品 psychotropic drug 136 易燃 bustible 137 易爆 e_plosive 138 验收 acceptance 139 使用说明书instruction140 标签 label 141 卫生, 清洁/消毒 sanitation 142 车间, 辅房workshop 143 间隔时间 time interval 144 清洁剂 detergent 145 消毒剂disinfectant 146 废弃物 wastes 147 更衣室 changing room 148 工作服, work clothes 149 颗粒性物质, 颗粒剂 granules 150 耐药菌株 drug-resistantstrain 151 传染病 infectiousdisease 152 皮肤病 dermatitis 153 验证verification, validation 154 确认 qualification 155 安装 installation156 运行 running operation 157 性能 performance 158 原辅料 raw material and incipient 159 文件 document 160 投诉 plaint 161 报废 reject 162 品名product name 163 处方 preion, formula 164 技术参数 technicalparameter165 容器 container 166 半成品 semi-finished product,intermediate 167 申请 lication 168 稳定性 stability169 起草 draft 170 生产管理 production management,manufacturing control.171 事故 accident 172 混淆 mi_-up 173 喷雾 spray 174 合格证certificate 175 清场 clearance 176 质量管理 quality management 177 内控internal control,on-line test 178 滴定液 tartan 179 培养基 medium 180 有效期 validity, e_piry date,shelf life 181 产品销售与收回 product sales andrecovery/recall 182 投诉与不良反应报告 plaints and adversereaction 自检self-inspection 184 附则 schedule endi_ 185 平衡 balance 186 饮用水drinking water, potablewater 187 蒸馏法 distillation 188 离子交换法 ion e_change 189 反渗透法 RO: Reverse Osmosis 190 附加剂添加剂 additives 191 滞留 stranded resort 192 批 batch, lot 193 组分, 组成 ponent 194 无纤维脱落的过滤器 non-fiber-releasingfilter 195 活性成份 Active Ingredient 196 非活性成份 Inactive ingredient 197 中间产品 in-processproduct,intermediate product198 批号 batch number 199 药用物料 medicated feed 20__药用预混合料 medicated premi_ 201 质量控制部门 Quality control department 202 理论产量 Theoretical yield 203 实际产量 Actual yield 204 比率 Percentage, rate 205 验收标准可接受标准 Acceptance criteria 206 代表性样品 Representative sle 207 微粒状的 particulate 208 污染物contaminant 209 石棉 asbestos 210 诊断 diagnosis 211 缓解 mitigation 212 化学变化 chemical change 213 组分 ingredient, ponent 214 制备 fabricate preparation 215 复合 pound 216 混合 blend 217 加工 processing 218 浓度concentration 219 单位剂量 unit dose 220 药品包装容器 drug product containers 221 密封件, 封盖 closure 222 效价 Titer 223 纯度 purity 224 规格 strength 225 监督 supervise, monitor 226 实验室 laboratory 227 无菌操作 aseptic operation,sterileoperation 228 层流 laminar flow 229 湍流 turbulent air flow 230 空气过滤 air filtration 231 空气加热 air heating 232 预过滤器 profiler 233 排气系统 e_haust system 234 管件 plumbing 235 虹吸倒流 back-siphon age 236 污水 sewage 237 废料 refuse 238 盥洗设备 toilet facilities 239 空气干燥器 air drier 240 垃圾 trash 241 有机废料 organic waste 242 杀鼠剂rodenticides 243 杀昆虫剂 insecticides 244 杀真菌剂 fungicides 245 熏蒸剂 fumigating reagents 246 去垢剂 cleaning agents 247 消毒剂 sanitizing agents 248 滂沱剂 lubricant 249 自动化设备 automatic, mechanical,or electronic equipment 250 微型胶卷 microfilm 251 注射剂 injection 252 灭菌设备 sterilization equipment 253 无菌取样技术 aseptic sling techniques 254 显微镜 microscope 255 热, 内毒素 pyrogen, endoto_in 256 偏差 deviation 257 变更 change control 258 进料 charge-in 259 项目代码 item code 260 鉴别 identify 261 片剂 tablet 262 胶囊 capsule 263 颗粒剂 granule 264 溶解时间溶出时间 dissolution time 265 澄明度clarity 266 隔离系统 quarantinesystem, isolation system 267 返工reprocessing 268 发放 issuance, release 269 非处方药 OTC:over-the-counter 270 处方药 preed medicine 271 皮肤科药、牙粉、胰岛素、喉片dermatological,dentifrice,insulin, or throat lozenge product 272 保险包装 ter-resistant package 273 明胶硬胶囊 hard gelatin capsule 274 顺势治疗homeopathic 275 入库 warehousing 276 变质 deteriorate 277 准确性accuracy 278 灵敏性 sensitivity 279 特异性 specificity 280 重复性reproducibility, repeatability 281 变应原提取物 allergenic e_tracts 282 眼膏 ophthalmic ointment 283 粗糙或磨蚀物质 harsh or abrasivesubstances 284 控释制剂 controlled-releasedosage form 285 实验动物 laboratory animals 286 供应商 Supplier 287 光谱 spectrum 288 测量单位 units of measure 289 换算系数 conversion factors 290 试剂 reagent 291 安慰剂placebo 292 明确地 e_plicitly 293 取代 supersede 294 溶液 solution 295 批准 roval 296 (美国)食品药品监督管理局 FDA: Food and DrugAdministration 297 标准操作程序 SOP: StandardOperatingProcedure 298 质量保证 QA: Quality Assurance 299 质量控制QC:Quality Control 300 批生产记录 BPR: Batch ProductionRecord 301 批检验记录 BAR: Batch AnalysisRecord 302 工艺规程 PP: Process Procedure 303 健康,安全,环保 EHS: Environment,Health andSafe 304 美国联邦法规 CFR: Code of FederalRegulation 305 美国药典USP: The UnitedStatesPharmacopeia 306 欧洲药典 EP: European pharmacopeia 307 英国药典 BP: British pharmacopeia 308 药物主文件 DMF: Drug Master File 309 验证主计划 VMP: Validation MasterPlan 310 验证方案 VP: Validation Protocol 311 验证报告 : Validation Report 312 安装确认 IQ: Installation Qualification313 运行确认 OQ: Operation Qualification 314 性能确认 PQ: Performance Qualification 315 超出标准(限度) OOS: Out of Specification 316 冻干产品 freeze-dry product,lyophilizated product 317 工厂主述文件SMF: Site Master File。
药品常用英语缩写1

《GMP英语词汇及缩写》第一部分PIC/S的全称为:Pharmaceutical Inspection Convention/Pharmaceutical Inspection Cooperation Scheme, PIC/S(制药检查草案), 药品检查协会(PIC/S) ,也有人称PIC/S为医药审查会议/合作计划(PIC/S)PIC的权威翻译:药品生产检查相互承认公约API(Active Pharmaceutical Ingrediet) 原料药又称:活性药物组分AirLock 气闸Authorized Person 授权人Batch/Lot 批次Batch Number/Lot-Number 批号;Batch Numbering System 批次编码系统;Batch Records 批记录;Bulk Product 待包装品;Calibration 校正;Clean area洁净区;Consignmecnt(Delivery)托销药品。
FDA(FOOD AND DRUG ADMINISTRA TION):(美国)食品药品管理局IND(INVESTIGA TIONAL NEW DRUG):临床研究申请(指申报阶段,相对于NDA而言);研究中的新药(指新药开发阶段,相对于新药而言,即临床前研究结束)NDA(NEW DRUG APPLICA TION):新药申请ANDA(ABBREVIATED NEW DRUG APPLICATION):简化新药申请TREATMENT IND:研究中的新药用于治疗ABBREVIATED(NEW)DRUG:简化申请的新药DMF(DRUG MASTER FILE):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品。
只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND、NDA、ANDA时才能参考其内容)HOLDER:DMF持有者CFR(CODE OF FEDERAL REGULATION):(美国)联邦法规PANEL:专家小组BA TCH PRODUCTION:批量生产;分批生产BA TCH PRODUCTION RECORDS:生产批号记录POST-OR PRE- MARKET SURVEILLANCE:销售前或销售后监督INFORMED CONSENT:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)PRESCRIPTION DRUG:处方药OTC DRUG(OVER—THE—COUNTER DRUG):非处方药第二部分GMP文件常见缩写ABPI Association of the British Pharmaceutical IndustryADR Adverse Drug ReactionAE Adverse EventAIM Active Ingredient ManufacturerANDA Abbreviated New Drug ApplicationANOV A Analysis of VarianceASM: Active Substance ManufacturerATC Anatomical Therapeutic ChemicalATX Animal Test Exemption CertificateBAN British Approved NameBIRA British Institute of Regulatory AffairsBNF British National FormularyBP British PharmacopoeiaC of A Certificate of AnalysisC of S Certificate of SuitabilityCENTRE FOR DRUG EV ALUATION (CDE)Centre for Pharmaceutical Administration (CPA)CMS Concerned Member StateCMS每个成员国COS Certificate of SuitabilityCPMP Committee for Proprietary Medicinal ProductsCRA Clinical Research AssociateCRF Case Report FormCRO Contract Research OrganisationCTA Clinical Trial ApplicationCTC Clinical Trial CertificateCTD Common Technical DocumentCTX Clinical Trials ExemptionDDD Defined Daily DoseDGC Daily Global ComparisonDIA Drug Information AssociationDMF Drug Master FileDrug Registration Branch (DR, Product Evaluation & Registration Division, CPAEDQM (European Directorate for the Quality of Medicines) 欧洲联盟药品质量指导委员会EEA 欧洲经济地区EGMA European Generics Medicine AssociationELA Established Licence ApplicationEMEA European Medicines Evaluation AgencyEMEA (European Agency for the Evaluation of Medicinal Products)欧洲联盟药品评价机构EP European PharmacopoeiaEPAR European Public Assessment ReportsESRA European Society of Regulatory AffairsEuropean Pharmacopoeia Commission 欧洲药典委员会FDAFDA Food and Drug Administrationfinal evaluation report (FER)free sale certificates (FSCs)GCP Good Clinical PracticeGCP药品临床研究管理规范GLP Good Laboratory PracticeGLP 药品临床前安全性研究质量管理规范GMP Good Manufacturing PracticeGMP 药品生产质量管理规范GSP药品销售管理规范Health Sciences Authority (HSA)HSA’s Medicines Advisory Committee (MAC)IB Investigators BrochureICH International Conference for HarmonisationIDMC Independent Data-Monitoring CommitteeIEC Independent Ethics CommitteeIND Investigational New DrugINN International Non-proprietary NameInternational Conference on Harmonisation (ICH)IPC In Process ControlIRB Institutional Review BoardLICENCE HOLDERMA Marketing AuthorisationMAA Marketing Authorisation ApplicationMAA上市申请MAH Marketing Authorisation HolderMAH 销售许可持有者MCA Medicines Control AgencyMHW Ministry of Health and Welfare (Japan)MR Mutual RecognitionMRA 美国与欧盟的互认协议MRAs (Mutual Recognition Agreements) 互相認證同意MRFG Mutual Recognition Facilitation GroupMRP Mutual Recognition ProcedureNAS New Active SubstanceNCE New Chemical EntityNDA New Drug Applicationnew chemical entities (NCEs)new drug applications (NDAs)NSAID Non Steroidal Anti Inflammatory DrugNTA Notice To ApplicantsOOS Out of SpecificationOTC Over The CounterPAGB Proprietary Association of Great BritainPh Eur European PharmacopoeiaPIL Patient Information LeafletPL Product LicencePOM Prescription Only MedicinePRODUCT OWNERPSU Periodic Safety UpdatesQA Quality AssuranceQC Quality ControlRAJ Regulatory Affairs JournalRMS Reference Member StateRMS相互认可另一成员国RSD Relative Standard DeviationRx Prescription OnlySAE Serious Adverse EventSMF Site Master FileSOP Standard Operating ProcedureSOP (STANDARD OPERATION PROCEDURE)标准运作程序SPC/SmPC Summary of Product Characteristicssummary of product characteristics(SPC)Therapeutic Goods Administration (TGA)USP US PharmacopoeiaVMF Veterinary Master FileVPC Veterinary Products Committee第三部分常用缩略语A.A.A Addition and Amendments 增补和修订AC Air Conditioner 空调器ADR Adverse Drug Reaction 药物不良反应AFDO Association of Food and Drug Officials 食品与药品官员协会(美国)ACC Accept 接受AQL Acceptable Quality Level 合格质量标准ADNA Abbreviated New Drug Application 简化的新药申请BOM Bill of Material 物料清单BPC Bulk pharmaceutical Chemiclls 原料药CBER Center for Biologics Evaluation Research 生物制品评价与研究中心CFU Colony Forming Unet 菌落形成单位DMF Drug Master File 药品管理档案CDER Cemter for Drug Evaluation amd Research 药物评价与研究中心CI Corporate Identity (Image) 企业识别(形象)CIP Cleaning in Place 在线清洗CSI Consumer Safety Insepctor 消费者安全调查员CLP Cleaning Line Procedure 在线清洗程序DAL Defect Action Level 缺陷作用水平DEA Drug Enforcement Adminestration 管制药品管理DS Documentation Systim 文件系统FDA Food and Drug Administration 食品与药品管理局(美国)GA TT General Agreemernt on Tariffs and Trade 关贸总协会GMP Good Manufacturing Practice Gvp 药品生质量管理规范GCP Good Clinical Practice 药品临床实验管理规范GLP Good Laboratory Practice 实验室管理规范GSP Good Supply Practice 药品商业质量规范GRP Gook RaTAIL Practice 药品零业质量管理规范GAP Good Agriculture Practice 药材生产管理规范GVP Gook Validation Prctice 验证管理规范GUP Gook Use Practice 药品重用规范HV AC Heating Ventilation Air Conditioning 空调净化系统ISO Intematonal Organization for Standardization 车际标准化组织MOU Memorandum of Understanding 谅解备忘录PF Porduction File 生产记录用表格OTC Over the Counter (Drug) 非处方药品PLA Product License Application 产品许可申请QA Quality Assurance 质量保证QC Quality Control 质量控制QMP Quality Management Procedure 质量管理程序SDA State Drug Administration 国家药品监督管理局SMP Standard Managmert Procedure 标准管理程序SOP Standard Operating Procedure 标准操作程序TQC Tatal Quality Control 全面质量管理USA Uneted States Pharmacopeia 美国药典。
药品注册用英语,常见药品注册用英语缩写,FDA药品审评相关术语

药品注册用英语CEP:欧洲药典适应性证书certificate of suitability to monograph of European Pharmacopoeia。
是欧洲药典所收载的原料药的一种认证程序,用以确定原料药的质量可以用欧洲药典的方法加以控制。
这一程序适用于生产的和提取的有机或无机物质以及发酵生产的非直接基因产品。
DMF:Drug master File美国药物主文件档案。
是指提交给FDA的用于提供关于人用药品的生产设备、工艺或生产、工艺处理、包装和储存中使用的物料的详细的和保密的信息。
分为五种类型:I:生产地点、设备、操作程序和人员II:原料药、原料药中间体、生产原料药和中间体使用的物料和药品III:包装材料IV:赋形剂、色素、调味剂、香料或生产这些物质所用的物料V:FDA接受的参考信息EDMF:European Drug Master File欧洲药物主文件档案。
是指欧洲制剂申请中有关原料药信息的文件,又称原料药主文件档案(ASMF)。
EDMF 只有在制剂申请的支持下才能提交。
EDMF分为两部分:1.申请人部分(AP):供制剂申请人使用的非保密信息;2. 限制部分(RP):EDMF持有人认为是保密的信息。
EDMF的使用范围:1. 新原料药2. 已知的但欧洲药典或其成员国药典没有收载的原料药3. 欧洲药典或成员国药典已收载的原料药ANDA:Abbreviated New Drug Application 美国简略新药申请。
是FDA规定的仿制药申请程序。
Generic:仿制的,非特殊的API:Active Pharmaceutical Ingredient 原料药Dossier:文档,档案。
TSE:Transmitting animal Spongiform Encephalopathy agent 传播性动物海绵状脑病体Q7A:ICH(国际协调会议)原料药GMP 指南。
阐述了原料药生产商应遵循的GMP 指导原则。
药品常用英语缩写

《GMP英语词汇及缩写》第一部分PIC/S的全称为:Pharmaceutical Inspection Convention/Pharmaceutical Inspection Cooperation Scheme, PIC/S(制药检查草案), 药品检查协会(PIC/S) ,也有人称PIC/S为医药审查会议/合作计划(PIC/S)PIC的权威翻译:药品生产检查相互承认公约API(Active Pharmaceutical Ingrediet) 原料药又称:活性药物组分AirLock 气闸Authorized Person 授权人Batch/Lot 批次Batch Number/Lot-Number 批号;Batch Numbering System 批次编码系统;Batch Records 批记录;Bulk Product 待包装品;Calibration 校正;Clean area洁净区;Consignmecnt(Delivery)托销药品。
FDA(FOOD AND DRUG ADMINISTRA TION):(美国)食品药品管理局IND(INVESTIGA TIONAL NEW DRUG):临床研究申请(指申报阶段,相对于NDA而言);研究中的新药(指新药开发阶段,相对于新药而言,即临床前研究结束)NDA(NEW DRUG APPLICA TION):新药申请ANDA(ABBREVIATED NEW DRUG APPLICATION):简化新药申请TREATMENT IND:研究中的新药用于治疗ABBREVIATED(NEW)DRUG:简化申请的新药DMF(DRUG MASTER FILE):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品。
只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND、NDA、ANDA时才能参考其内容)HOLDER:DMF持有者CFR(CODE OF FEDERAL REGULATION):(美国)联邦法规PANEL:专家小组BA TCH PRODUCTION:批量生产;分批生产BA TCH PRODUCTION RECORDS:生产批号记录POST-OR PRE- MARKET SURVEILLANCE:销售前或销售后监督INFORMED CONSENT:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)PRESCRIPTION DRUG:处方药OTC DRUG(OVER—THE—COUNTER DRUG):非处方药第二部分GMP文件常见缩写ABPI Association of the British Pharmaceutical IndustryADR Adverse Drug ReactionAE Adverse EventAIM Active Ingredient ManufacturerANDA Abbreviated New Drug ApplicationANOV A Analysis of VarianceASM: Active Substance ManufacturerATC Anatomical Therapeutic ChemicalATX Animal Test Exemption CertificateBAN British Approved NameBIRA British Institute of Regulatory AffairsBNF British National FormularyBP British PharmacopoeiaC of A Certificate of AnalysisC of S Certificate of SuitabilityCENTRE FOR DRUG EV ALUATION (CDE)Centre for Pharmaceutical Administration (CPA)CMS Concerned Member StateCMS每个成员国COS Certificate of SuitabilityCPMP Committee for Proprietary Medicinal ProductsCRA Clinical Research AssociateCRF Case Report FormCRO Contract Research OrganisationCTA Clinical Trial ApplicationCTC Clinical Trial CertificateCTD Common Technical DocumentCTX Clinical Trials ExemptionDDD Defined Daily DoseDGC Daily Global ComparisonDIA Drug Information AssociationDMF Drug Master FileDrug Registration Branch (DR, Product Evaluation & Registration Division, CPAEDQM (European Directorate for the Quality of Medicines) 欧洲联盟药品质量指导委员会EEA 欧洲经济地区EGMA European Generics Medicine AssociationELA Established Licence ApplicationEMEA European Medicines Evaluation AgencyEMEA (European Agency for the Evaluation of Medicinal Products)欧洲联盟药品评价机构EP European PharmacopoeiaEPAR European Public Assessment ReportsESRA European Society of Regulatory AffairsEuropean Pharmacopoeia Commission 欧洲药典委员会FDAFDA Food and Drug Administrationfinal evaluation report (FER)free sale certificates (FSCs)GCP Good Clinical PracticeGCP药品临床研究管理规范GLP Good Laboratory PracticeGLP 药品临床前安全性研究质量管理规范GMP Good Manufacturing PracticeGMP 药品生产质量管理规范GSP药品销售管理规范Health Sciences Authority (HSA)HSA’s Medicines Advisory Committee (MAC)IB Investigators BrochureICH International Conference for HarmonisationIDMC Independent Data-Monitoring CommitteeIEC Independent Ethics CommitteeIND Investigational New DrugINN International Non-proprietary NameInternational Conference on Harmonisation (ICH)IPC In Process ControlIRB Institutional Review BoardLICENCE HOLDERMA Marketing AuthorisationMAA Marketing Authorisation ApplicationMAA上市申请MAH Marketing Authorisation HolderMAH 销售许可持有者MCA Medicines Control AgencyMHW Ministry of Health and Welfare (Japan)MR Mutual RecognitionMRA 美国与欧盟的互认协议MRAs (Mutual Recognition Agreements) 互相認證同意MRFG Mutual Recognition Facilitation GroupMRP Mutual Recognition ProcedureNAS New Active SubstanceNCE New Chemical EntityNDA New Drug Applicationnew chemical entities (NCEs)new drug applications (NDAs)NSAID Non Steroidal Anti Inflammatory DrugNTA Notice To ApplicantsOOS Out of SpecificationOTC Over The CounterPAGB Proprietary Association of Great BritainPh Eur European PharmacopoeiaPIL Patient Information LeafletPL Product LicencePOM Prescription Only MedicinePRODUCT OWNERPSU Periodic Safety UpdatesQA Quality AssuranceQC Quality ControlRAJ Regulatory Affairs JournalRMS Reference Member StateRMS相互认可另一成员国RSD Relative Standard DeviationRx Prescription OnlySAE Serious Adverse EventSMF Site Master FileSOP Standard Operating ProcedureSOP (STANDARD OPERATION PROCEDURE)标准运作程序SPC/SmPC Summary of Product Characteristicssummary of product characteristics(SPC)Therapeutic Goods Administration (TGA)USP US PharmacopoeiaVMF Veterinary Master FileVPC Veterinary Products Committee第三部分常用缩略语A.A.A Addition and Amendments 增补和修订AC Air Conditioner 空调器ADR Adverse Drug Reaction 药物不良反应AFDO Association of Food and Drug Officials 食品与药品官员协会(美国)ACC Accept 接受AQL Acceptable Quality Level 合格质量标准ADNA Abbreviated New Drug Application 简化的新药申请BOM Bill of Material 物料清单BPC Bulk pharmaceutical Chemiclls 原料药CBER Center for Biologics Evaluation Research 生物制品评价与研究中心CFU Colony Forming Unet 菌落形成单位DMF Drug Master File 药品管理档案CDER Cemter for Drug Evaluation amd Research 药物评价与研究中心CI Corporate Identity (Image) 企业识别(形象)CIP Cleaning in Place 在线清洗CSI Consumer Safety Insepctor 消费者安全调查员CLP Cleaning Line Procedure 在线清洗程序DAL Defect Action Level 缺陷作用水平DEA Drug Enforcement Adminestration 管制药品管理DS Documentation Systim 文件系统FDA Food and Drug Administration 食品与药品管理局(美国)GA TT General Agreemernt on Tariffs and Trade 关贸总协会GMP Good Manufacturing Practice Gvp 药品生质量管理规范GCP Good Clinical Practice 药品临床实验管理规范GLP Good Laboratory Practice 实验室管理规范GSP Good Supply Practice 药品商业质量规范GRP Gook RaTAIL Practice 药品零业质量管理规范GAP Good Agriculture Practice 药材生产管理规范GVP Gook Validation Prctice 验证管理规范GUP Gook Use Practice 药品重用规范HV AC Heating Ventilation Air Conditioning 空调净化系统ISO Intematonal Organization for Standardization 车际标准化组织MOU Memorandum of Understanding 谅解备忘录PF Porduction File 生产记录用表格OTC Over the Counter (Drug) 非处方药品PLA Product License Application 产品许可申请QA Quality Assurance 质量保证QC Quality Control 质量控制QMP Quality Management Procedure 质量管理程序SDA State Drug Administration 国家药品监督管理局SMP Standard Managmert Procedure 标准管理程序SOP Standard Operating Procedure 标准操作程序TQC Tatal Quality Control 全面质量管理USA Uneted States Pharmacopeia 美国药典。
医院处方里常用的英语缩写

医院处方里常用的英语缩写aa 各a.c. 饭前ad 至.ext. 外用a.m. 上午A.s.t.!皮试aq.dest. 蒸馏水alt.2h. 每隔2小时一次b.I.d. 每日二次Cito! 急速地!D.S. 给予标记g. 克h.s. 睡时I.d 皮内注射I.h 皮下注射I.m 肌肉注射I.v 静脉注射I.v.derp 静脉滴注I.v.drip 静脉滴注I.v.gtt 静脉滴注I.u 国际单位Lent! 慢慢地!m.d. 用法口授,遵照医嘱M.D.S. 混合,给予,标记M.f.pulv. 混合制成散剂mg. 毫克ml. 毫升m.s. 用法口授,遵照医嘱p.a.a. 用于患处p 单位p.c 饭后pg. 微克p.m 下午p.o. 口服pr.aur. 耳用prim.vic.No2 首剂倍量p.r.n 必要时pr.nar. 鼻用pr.nar. 鼻用pr.ocul. 眼用p.t.c. 皮试后q.6h. 每6小时q.2d. 每二天一次q.d. 每天一次q.h. 每小时q.I.d. 每日四次q.m. 每晨q.n. 每晚q.o.d. 隔日q.s. 适量q.w.d. 每周Rp. 取S. 标记,用法Sig. 标记,用法s.I.d. 每日一次s.o.s. 需要时St! 立即! Staim! 立即!stat.! 立即!T! 皮试t.I.d. 每天三次t.c.s. 皮试u. 单位常用药品名称缩写简表:英文缩写药品名称5Fu 5-氟脲嘧啶6MP 6-巯基嘌呤ACV 无环鸟苷ADR 阿霉素APC 复方阿斯匹林Aza 硫唑嘌呤BTX-A A型肉毒毒素CBZ 卡马西平Cef 头孢呋辛钠CEL 赛利洛尔CIP 环丙沙星CLX 头孢氨苄CO SMZ 复方磺胺甲基异恶唑CO VB 复方维生素B CPZ 头孢哌酮CsA 环孢素ACZP 氯硝西泮DMPPC 甲氧西林DOC 多西紫杉醇Dox 阿霉素DRL 屈洛昔芬EE 炔雌醇EM 红霉素EPO 促红细胞生长素FOM 磷霉素G-CSF 重组人粒细胞集落刺激因子GL 格列齐特Gli 格列吡嗪GM 庆大霉素GM1 单唾液酸四己糖神经节苷脂GNS 葡萄糖钠盐GS 葡萄糖GTW 雷公藤多苷HCL 盐酸黄酮哌酯Ils 白细胞介素IN 肌醇烟酸酯LD 左旋多巴LFX 洛非西定LM 盐酸左旋咪唑LMWH 低分子肝素LNG 左炔诺孕酮LTB4 白三烯B4LTG 拉莫三嗪LVFX 左氧氟沙星MEBO 美宝湿润烧伤膏MINO 米诺环素MMC 丝裂霉素MMF 霉酚酸酯MP 甲泼尼龙MZR 咪唑立宾NK-104 伊它伐他汀NM 硫酸新霉素NS 生理盐水NTL 奈替米星OFLX 氧氟沙星OTC 盐酸土霉素PASNa 对氨基水杨酸钠PB 苯巴比妥PPA 盐酸苯丙醇胺Pred 泼尼松PSS 藻酸双酯钠rhEGF 重组人表皮生长因子rhG-CSF 重组人粒细胞集落刺激因子RSG 罗格列酮Ru486 米非司酮SB 碳酸氢钠SBT 舒巴坦SD 磺胺嘧啶SDM 磺胺邻二甲氧嘧啶SFZSIZ 磺胺二甲异恶唑SG 磺胺脒SM2 磺胺二甲嘧啶SMD 磺胺对甲氧嘧啶SMM 磺胺间甲氧嘧啶SMZ 磺胺甲基异恶唑TAM 三苯氧胺TC 盐酸四环素TMP 甲氧苄氨嘧啶TNZ 替硝唑TOB 妥布霉素TPM 托吡酯VA 维生素AVAD 维生素AD VB1 维生素B1VB12 维生素B12 VB2 维生素B2VB6 维生素B6 VC 维生素CVD 维生素D VE 维生素EVPA 丙戊酸钠 VPA 丙戊酸钠ivgtt。
制药工程专业英语--药品说明

去氧胆酸); 化学名为3a,7p dihydroxy-5p-
Cholanoic acid(3a,7p二羟基5p胆烷 酸)。
药品名称翻译可采用音译、意译、音意合译及谐音译意等方法:
1、音译:按英文药品名歌的读音译成相同或相近的汉语。如: Tamoxitn它莫西芬,Ritalin利他林,Amcacin 阿米卡星。音译较为 方便,但不能表意。
Insert原意为“插入物,插页”。
药品说明书即为附在每种药品包装盒中的一 份用药说明。经过注册的进口药品一般是国家 承认的有效药物,其说明书是指导医生与患者 合理用药的重要依据,具有一定的法律效力。
①药品名称(Drug Names), ②性状(Description), ③药理作用(Pharmacological Actions), ④适应症(Indications), ⑤禁忌症(Contraindications), ⑥用量与用法(Dosage and Administration). ⑦不良反应(Adverse Reactions)。 ⑧注意事项(Precautions), ⑨包装(Package), ⑩贮存(Storage), ⑾其他项目(Others)。
7.Kanendomycin is a very stable antibiotic, and its activity do es not decrease when the powder is placed in an airtight conta iner and kept at room temperatures for more than 2 years. 卡内多霉素是一种很稳定的抗生素,其粉沫置于密封容器中,在室温 下保存二年以上,活性不减。
处方里常用的英语缩写

处方里常用的英语缩写aa 各a.c.饭前ad 至.ext.外用a.m.上午A.s.t.!皮试aq.dest.蒸馏水alt.2h.每隔2小时一次b.I.d.每日二次Cito!急速地!D.S.给予标记g.xxh.s.睡时I.d 皮内注射I.h 皮下注射I.m 肌肉注射I.v 静脉注射I.v.derp 静脉滴注I.v.drip 静脉滴注I.v.gtt 静脉滴注I.u 国际单位Lent!慢慢地!m.d.用法口授,遵照医嘱M.D.S.混合,给予,标记M.f.pulv.混合制成散剂mg.毫克ml.毫升m.s.用法口授,遵照医嘱p.a.a.用于患处p 单位p.c 饭后pg.微克p.m 下午p.o.口服pr.aur.耳用prim.vic.No2首剂倍量p.r.n 必要时pr.nar.鼻用pr.nar.鼻用pr.ocul.眼用p.t.c.皮试后q.6h.每6小时q.2d.每二天一次q.d.每天一次q.h.每小时q.I.d.每日四次q.m.每晨q.n.每晚q.o.d.隔日q.s.适量q.w.d.每周Rp.取S.标记,用法Sig.标记,用法s.I.d.每日一次s.o.s.需要时St!立即! Staim!立即!stat.!立即!T!皮试t.I.d.每天三次t.c.s.皮试u.单位常用药品名称缩写简表:英文缩写药品名称5Fu 5-氟脲嘧啶6MP 6-巯基嘌呤ACV 无环鸟苷ADR 阿霉素APC 复方阿斯匹林Aza 硫唑嘌呤BTX-A A型肉毒毒素CBZ 卡马西平Cef 头孢呋辛钠CEL 赛利xxCIP 环丙沙星CLX 头孢氨苄CO SMZ 复方磺胺甲基异恶唑CO VB 复方维生素B CPZ 头孢哌酮CsA 环孢素ACZP 氯硝西泮DMPPC 甲氧xxDOC 多西xxDox 阿霉素DRL xxEE 炔雌醇EM 红霉素EPO 促红细胞生长素FOM 磷霉素G-CSF 重组人粒细胞集落刺激因子GL xxxxGli xx吡嗪GM 庆大霉素GM1单唾液酸四己糖神经节苷脂GNS 葡萄糖钠盐GS 葡萄糖GTW xx多苷HCL 盐酸黄酮哌酯Ils 白细胞介素IN 肌醇烟酸酯LD xxLFX xxxxLM 盐酸xx咪唑LMWH 低分子肝素LNG 左炔诺孕酮LTB4xxB4LTG 拉莫三嗪LVFX 左氧氟沙星MEBO 美宝湿润烧伤膏MINO 米诺环素MMC 丝裂霉素MMF 霉酚酸酯MP 甲泼xxMZR 咪唑xxNK-104伊它伐他汀NM 硫酸新霉素NS 生理盐水NTL 奈替米星OFLX 氧氟沙星OTC 盐酸土霉素PASNa 对氨基水杨酸钠PB 苯巴比妥PPA 盐酸苯丙醇胺Pred 泼尼松PSS 藻酸双酯钠rhEGF 重组人表皮生长因子rhG-CSF 重组人粒细胞集落刺激因子RSG 罗格列酮Ru486米非司酮SB 碳酸氢钠SBT xxSD 磺胺嘧啶SDM 磺胺邻二甲氧嘧啶SFZSIZ 磺胺二甲异恶唑SG 磺胺脒SM2磺胺二甲嘧啶SMD 磺胺对甲氧嘧啶SMM 磺胺间甲氧嘧啶SMZ 磺胺甲基异恶唑TAM 三苯氧胺TC 盐酸四环素TMP 甲氧苄氨嘧啶TNZ 替硝唑TOB 妥布霉素TPM 托吡酯VA xxAVAD xxAD VB1xxB1VB12xxB12 VB2xxB2VB6xxB6 VC xxCVD xxD VE xxEVPA 丙戊酸钠Rp.请取:Tab.Aminophyllini 氨茶碱片Sig.0.2g.t.i.d.p.c.用法:0.2克每次每日3次饭后服用Rp.Tab.Terramycini 土霉素片剂D.S.0.5g,q,6h.p.o.用法:0.5克每次,每6个小时一次,口服Rp.Inj.Adrenalini 肾上腺素注射液S.1mI.i.m.用法:肌肉注射,每次1毫升Rp.Inj.Lobelini xx注射液S.1 amp.q.2h.i.m.用法:肌肉注射,2小时一次,每次1毫升Rp.Inj.Atropini xx注射液S.0. 5mg.b.i.d.i.m.用法:0.5毫克,肌注2次每日Inj.Penicillini 注射用青霉素钠40万u×12支S.80万i.u.b.i.d.i.m.t.c.用法:80万u 肌注2次/日皮试Rp.Inj.Penicillini 注射用青霉素钠Inj.Streptomycini 链霉素注射液M.D.S.b.i.d.i.m.p.t.c.给与混合肌注2次/日皮试Rp.Inj.Erythromycini 红霉素注射液Inj.Glucosi 葡萄糖注射液M.D.S.i.v./gtt.q.d.给与混合静脉注射每天一次Rp.Mist.Pepsini 胃蛋白酶合剂S.10ml.t.i.d.p.c.用法10ml 3次/日饭前Rp.Lot.Calaminae 甘油洗剂S.m.d.用法:遵照医嘱Rp.Ocust.Chloramphenicoli 氯霉素滴眼液S.pr.ocul.用法:Rp.Naristill.Ephedrini 鼻病及呋嘛滴鼻剂S.2 gtt.p.r.n.用法:需要时按情而定请取2滴p.r.n.缩写词= pro re nata (= as the situation demands )【拉】【医】处方常用拉丁词缩写与中文对照表[原]缩写字拉丁文中文aa. Ana 各a.c. Ante cibos 饭前a.d. Ante decubitum 睡前a.h. Alternis horis 每2小时,隔1小时a.j. Ante jentaculum 早饭前a.m. Ante meridiem 上午,午前a.p. Ante parndium 午饭前a.u.agit Ante usum agitetur 使用前振荡Abs.febr. Absente febri 不发烧时Ac.;acid. Acidum 酸Ad.;add Ad 到、为、加至Ad lid Ad libitum 随意、任意量Ad us Ad usum 应用Ad us.ext Ad usum externum 外用Ad us.int. Ad usum internum 内服Alt.die.(a.d.)Alternis diebus 隔日(alterno die)Amp. Ampulla xx(瓿)en. Ante coenam 晚饭前Aq. Aqua 水Aq.bull Aqua bulliens 开水,沸水Aq.cal. Aqua calida 热水Ap.dest. Aqua destillata 蒸馏水Ap.ferv. Aqua fervens 热水Ap.font. Aqua fontana 泉水Ap.steril. Aqua sterilisata 无菌水b.i.d. Bis in die 1日2次Cap Cape,capiat 应服用Caps.amyl. Capsula amylacea 淀粉囊Caps.gelat.Capsula gelatinosa 胶囊Caps.dur. Capsula dura 硬胶囊Caps.moll. Capsula mollis 软胶囊Catapl. Cataplasma 泥济c.c Centimetrum cubicum 西西,公撮,立方公分c.g. Centigramma 厘克,百分之一公分Cit. Cito 快Collum. Collunarium 洗鼻剂Collut. Collutorium 漱口济Collyr. Collyrium 洗眼剂Co. Compcitus 复方的Ccen. Coena 晚饭Cons Consperus 撒布剂Cort. Cortex 皮Crem. Cremor 乳剂c.t. Cutis testis 皮试d. Da,dentur 给与,须给与d.d De die 每日d.i.d Dies in dies 每日,日日d.in amp. Da in ampullis 给安瓿d.in caps. Da in capsulis 给胶囊Dec. Decoctum 煎剂Deg. Deglutio 吞服Dest. Destillatus 蒸馏的Dg. Decigramma 分克Dieb.alt Diebus alternis 间日,每隔一日Dil. Dilue,dilutus 稀释,稀的Dim. Dimidius 一半Div. Divide 分开,分成Div.in p. Divide in partes 分……次服用Div.inpar.aeg Divide inpartis aegualis 分成等分d.t.d Da tales doses 给与此量Em.;emuls Emulsum,emulsio 乳剂Emp. Emplastrum 硬膏(剂)Ext Externus 外部的Extr. Extractum 浸膏Feb.urg Febri urgente 发烧时Fl. Flos,flose 花Fol. Folium folia xxFort. Fortis 强的,浓的Fr. Fructus 果实Garg. Gargarisma 含漱剂g.;gm. Gramma,grammata 克h. Hora 小时Hb. Herba 草h.d. Hora decubitus 睡觉时,就寝时h.s. Hora somni 睡觉时h.s.s Hora somni sumendus 睡觉服用Hod. Hodie 今日In.d In die 每日Inf. Inrfsum 浸剂Inj. Injectio 注射剂i.h. Injectio hypodermatica 皮下注射i.m. Injectio musculosa 肌肉注射i.v. Injectio venosa 静脉注射Lin. Inimentum 擦剂Liq. Liquor,liquidus 溶液,液体的Lit. Litrum 升Lot Lotio 洗剂Mist. Mistura 合剂Ml. Millitrum 毫升Mg. Milligramma 毫克Muc. Mucilago 胶浆剂N Nocte 夜晚n.et.m Nocte et mane 在早晚Neb. Nebula 喷雾剂o.d. Omni die 每日O.D. Oculus dexter 右眼O.L. Oculus laevus 左眼O.S. Oculus sinister 左眼O.U. Oculi utrigue 双眼Ol. Oleum 油Om.bid. Omni biduo 每2日Om.d.(o.d.) Omni die 每日Om.hor.(o.h.) Omni hora 每小时Om.man. Omni mane 每日早晨Om.noc.(o.n.)Omni nocte 每日晚上p.c. Post cibos 饭后p.o. Per os 口服Pil. Pilula 丸剂p.j. Post jentaculum 早饭后p.m. Post meridiem 午后p.prand. Post prandium 午饭后Pcoen. Post coenam 晚饭后Pro us.ext Pro usu externo 外用.int. Pro usu interno 内用,内服Pro us.med. Pro usu medicinali 药用Pro us.vet. Prousu veterinario 兽医用Pulv. Pulvis 粉剂、散剂Pt. Partes 部分p.r.n. Pro kre nata 必要时q.d. Quaque die 每日q.i.d. Quarter in die 每日4次q.h. Quaque hora 每1小时q.4.h. Quaque 4 hora 每4小时q.l. Quantum libet 任意量q.n. Quante nocte 每日晚上q.s. Quantum sufficit 足够量Quantum satis 足够量,适量q.semih. Quaque semihora 每半小时r.;rad Radix 根Rec Recens xx的Rp. Recipe 取Rhiz. Rhizoma 根茎s.i.d Semel in die 每日1次s.l Saccharum lactis 乳糖s.o.s Si opus(est)sit 需要时Sem. Semen 种子Ser.;syr. Sirupu,ssyrupus 糖浆Solut. Solutio 溶液Semih. Semihora 半小时Sp. Spiritus 醵剂Stat.;st Statim 立刻,立即Supp. Suppositouium 栓剂t.i.d. Ter in die 每日3次t.;tr. Tinctura 酊剂Troch. Trochscus 锭剂,糖锭Tab. Tabella 片剂Us. Usus 应用,用途Ug.;ung. Unguentum 软膏Us.ext. Usus externus 外用Us.int. Usus internus 内服Ut dict Ut dictum 依照嘱咐Vesp. Vespere 晚上。
药理中英文缩写
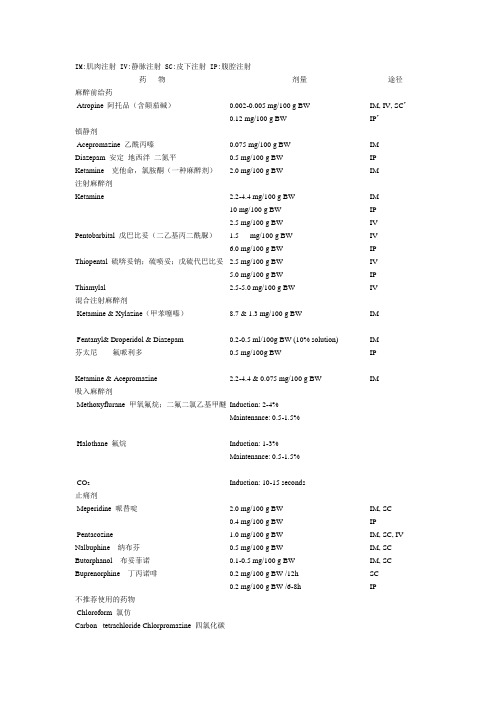
IM:肌肉注射 IV:静脉注射 SC:皮下注射 IP:腹腔注射药物剂量途径麻醉前给药Atropine 阿托品(含颠茄碱)0.002-0.005 mg/100 g BW0.12 mg/100 g BW IM, IV, SC* IP*镇静剂Acepromazine 乙酰丙嗪Diazepam 安定地西泮二氮平Ketamine 克他命,氯胺酮(一种麻醉剂)0.075 mg/100 g BW0.5 mg/100 g BW2.0 mg/100 g BWIMIPIM注射麻醉剂KetaminePentobarbital 戊巴比妥(二乙基丙二酰脲)Thiopental 硫喯妥钠;硫喷妥;戊硫代巴比妥Thiamylal 2.2-4.4 mg/100 g BW10 mg/100 g BW2.5 mg/100 g BW1.5 mg/100 g BW6.0 mg/100 g BW2.5 mg/100 g BW5.0 mg/100 g BW2.5-5.0 mg/100 g BWIMIPIVIVIPIVIPIV混合注射麻醉剂Ketamine & Xylazine(甲苯噻嗪)Fentanyl& Droperidol & Diazepam 芬太尼氟哌利多Ketamine & Acepromazine 8.7 & 1.3 mg/100 g BW0.2-0.5 ml/100g BW (10% solution)0.5 mg/100g BW2.2-4.4 & 0.075 mg/100 g BWIMIMIPIM吸入麻醉剂Methoxyflurane 甲氧氟烷;二氟二氯乙基甲醚 Halothane 氟烷CO2Induction: 2-4% Maintenance: 0.5-1.5%Induction: 1-3% Maintenance: 0.5-1.5%Induction: 10-15 seconds止痛剂Meperidine 哌替啶Pentacozine Nalbuphine 纳布芬Butorphanol 布妥菲诺Buprenorphine 丁丙诺啡2.0 mg/100 g BW0.4 mg/100 g BW1.0 mg/100 g BW0.5 mg/100 g BW0.1-0.5 mg/100 g BW0.2 mg/100 g BW /12h0.2 mg/100 g BW /6-8hIM, SCIPIM, SC, IVIM, SCIM, SCSCIP不推荐使用的药物Chloroform 氯仿Carbon tetrachloride Chlorpromazine 四氯化碳Ether 乙醚Trichloroethylene 三氯乙烯Tribromoethanol 三溴乙醇药物剂量途径麻醉前给药Atropine 阿托品0.002-0.005 mg/100 g BW IM, IV, SC 镇静剂Acepromazine Diazepam Xylazine Ketamine 0.5 mg/100 g BW0.25 mg/100 g BW1.3 mg/100 g BW2.2 mg/100 g BW2.0 mg/100 g BWIM, SCIPIMIMIP注射麻醉剂Fentanyl & Droperidol KetaminePentobarbital 0.2-0.4 ml/kg (10% solution)4.4 mg/100 g BW4-16 mg/100 g BW5 mg/100 g BW3-4 mg/100 g BW3-5 mg/100 g BWIPIMIPIVIVIP混合注射麻醉剂Ketamine & XylazineKetamine & AcepromazineKetamine & PentobarbitalInnovar-Vet & PentobarbitalInactinAcepromazine & Pentobarbital 8.7 & 1.3 mg/100 g BW2-4 & 0.075 mg/100 g BW4.4 & 2.5 mg/100 g BW6.0 & 2.1 mg/100 g BW(I)0.13-0.3 ml/kg (10% solution) & (P)1-2mg/100 g BW100 mg/kg0.5 & 2.5-3 mg/100 g BWIMIM(K)IM, (P)IP(K)IM, (P)IV(I)IM, (P)IPIP(A)IM, SC, (P)IP吸入麻醉剂MethoxyfluraneHalothaneEnflurane Ether Induction: 2-4%Maintenance: 0.5-1.5%Induction: 1-3%Maintenance: 0.5-1.5%Induction: 3-4%Maintenance: 1-3%棉花上沾1-2ml放到chamber中作用止痛剂Meperidine Pentacozine Butorphanol Nalbuphine Buprenorphine 0.3-0.5 mg/100 g BW0.2 mg/100 g BW0.05-2 ml/kg0.1-0.2 mg/100 g BW0.01-0.05 mg/100g every 12 hrIM, IV,IPIM, IV, SCIM, SCIM, SCSC不推荐使用的药物Chlorpromazine Chloral hydrate Chloroform Tribromoethanol。
医药常用英语单词及缩写

FDA(food and drug adminisration):(美国)食品药品监督管理局NDA(new drug application):新药申请ANDA(abbreviated new drug application):简化新药申请EP(export application):出口药申请(申请出口不被批准在美国销售的药品)treatment IND:研究中的新药用于治疗abbreviated(new)drug:简化申请的新药DMF(drug master file):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品。
只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND、NDA、ANDA时才能参考其内容)holder:DMF持有者CFR(code of federal regulation):(美国)联邦法规PANEL:专家小组batch production:批量生产;分批生产batch production records:生产批号记录post or pre-market surveillance:销售前或销售后监督informed consent:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)prescription drug:处方药OTC drug(over—the—counter drug):非处方药U.S. public health service:美国卫生福利部NIH(national institute of health):(美国)全国卫生研究所animal trail:动物试验accelerated approval:加速批准standard drug:标准药物investigator :研究人员;调研人员preparing and submitting:起草和申报submission:申报;递交benefit(s):受益risk(s):受害drug product:药物产品drug substance:原料药established name:确定的名称generic name:非专利名称proprietary name:专有名称;INN(international nonproprietary name):国际非专有名称narrative summary: 记叙体概要adverse effect:副作用adverse reaction:不良反应protocol:方案archival copy:存档用副本review copy:审查用副本official compendium:法定药典(主要指USP、NF).USP(the united state pharmacopeia):美国药典(现已和NF合并一起出版)NF(national formulary):(美国)国家药品集official=pharmacopeial = compendial:药典的;法定的;官方的agency:审理部门(指FDA)sponsor:主办者(指负责并着手临床研究者)identity:真伪;鉴别;特性strength:规格;规格含量(每一剂量单位所含有效成分的量)labeled amount:标示量regulatory specification:质量管理规格标准(NDA提供)regulatory methodology:质量管理方法(FDA用于考核原料药或药物产品是否符合批准了的质量管理规格标准的整套步骤)regulatory methods validation:管理用分析方法的验证(FDA对NDA提供的方法进行验证)Dietary supplement:食用补充品ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use)人用药物注册技术要求国际协调会议ICH:Quality-质量Q1A(R2): Stability Testing of New Drug Substances and Products (Second Revision)新原料药和制剂的稳定性试验(第二版)Q1B: Photostability Testing of New Drug Substances and Products新原料药和制剂的光稳定性试验Q1C: Stability Testing for New Dosage Forms新制剂的稳定性试验Q1D: Bracketing and Matrixing Designs for Stability Testing of DrugSubstances and Drug Products原料药和制剂稳定性试验的交叉和矩阵设计Q1E: Evaluation of Stability Data对稳定性数据的评估处理Q1F: Stability Data Package for Registration Applications in ClimaticZones III and IV在气候带III和IV,药物注册申请所提供的稳定性数据Q2A: Text on Validation of Analytical Procedures分析程序的验证Q2B: Validation of Analytical Procedures: Methodology分析程序的验证:方法学Q3A(R): Impurities in New Drug Substances (Revised Guideline)新原料药中的杂质(修订版)Q3B(R): Impurities in New Drug Products (Revised Guideline)新制剂中的杂质(修订版)Q3C: Impurities: Guideline for Residual Solvents杂质:残留溶剂指南Q3C(M): Impurities: Guideline for Residual Solvents (Maintenance)杂质:残留溶剂指南(修改内容)Q4: Pharmacopoeias药典Q4A: Pharmacopoeial Harmonisation 药典的协调Q4B: Regulatory Acceptance of Pharmacopoeial Interchangeability药典互替在法规上的可接受性Q5A: Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin来源于人或者动物细胞系的生物技术产品的病毒安全性评估Q5B: Quality of Biotechnological Products: Analysis of the ExpressionConstruct in Cells Used for Production of r-DNA Derived Protein Products 生物技术产品的质量:源于重组DNA的蛋白质产品的生产中所用的细胞中的表达构建分析Q5C: Quality of Biotechnological Products: Stability Testing ofBiotechnological/Biological Products生物技术产品的质量:生物技术/生物产品的稳定性试验Q5D: Derivation and Characterisation of Cell Substrates Used forProduction of Biotechnological/Biological Products用于生产生物技术/生物产品的细胞底物的起源和特征描述Q5E: Comparability of Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process基于不同生产工艺的生物技术产品/生物产品的可比较性Q6: Specifications for New Drug Substances and Products新原料药和制剂的质量规格Q6A: Specifications: Test Procedures and Acceptance Criteria for New DrugSubstances and New Drug Products: Chemical Substances质量规格:新原料药和新制剂的检验程序和可接收标准:化学物质Q6B: Specifications: Test Procedures and Acceptance Criteria forBiotechnological/Biological Products质量规格:生物技术/生物产品的检验程序和可接收标准Q7: Good Manufacturing Practices for Pharmaceutical Ingredients活性药物成份的GMPQ7A: Good Manufacturing Practice Guide for Active PharmaceuticalIngredients活性药物成份的GMP指南Q8: Pharmaceutical Development药物研发Q9: Quality Risk Management质量风险管理ICH:Safety-安全S1A: Guideline on the Need for Carcinogenicity Studies of Pharmaceuticals药物致癌性研究需要的指南S1B: Testing for Carcinogenicity of Pharmaceuticals药物致癌性的检验S1C: Dose Selection for Carcinogenicity Studies of Pharmaceuticals药物致癌性研究之剂量选择S1C(R): Addendum: Addition of a Limit Dose and Related Notes附录:极限剂量和有关注释的的补充S2A: Guidance on Specific Aspects of Regulatory Genotoxicity Tests for Pharmaceuticals受法规管辖的药物基因毒性检验的特定方面的指南S2B: Genotoxicity: A Standard Battery for Genotoxicity Testing forPharmaceuticals基因毒性:药物基因毒性检验的标准S3A: Note for Guidance on Toxicokinetics: The Assessment of Systemic Exposure in Toxicity Studies毒物代谢动力学指南的注释:毒性研究中的全身性暴露量的评估S3B: Pharmacokinetics: Guidance for Repeated Dose Tissue Distribution Studies药物代谢动力学:重复剂量的组织分布研究指南S4: Single Dose Toxicity Tests单剂量毒性检验S4A: Duration of Chronic Toxicity Testing in Animals (Rodent andNon-Rodent Toxicity Testing)动物体内慢性毒性持续时间的检验(啮齿动物和非啮齿动物毒性检验)S5A: Detection of Toxicity to Reproduction for Medicinal Products药物对生殖发育的毒性的检验S5B(M): Maintenance of the ICH Guideline on Toxicity to Male Fertility: An Addendum to the Guideline on Detection of Toxicity to Reproduction forMedicinal Products对男性生殖能力的毒性的指南的变动:药物对生殖发育的毒性的检验指南增加了一个附录S6: Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals生物技术生产的药物的临床前安全评价S7A: Safety Pharmacology Studies for Human Pharmaceuticals人用药的安全药理学研究S7B: The Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization(QT Interval Prolongation) By Human Pharmaceuticals药物延迟心室复极化(QT间期)潜在作用的非临床评价S8: Immunotoxicology Studies for Human Pharmaceuticals人用药免疫毒理学研究M3(M): Maintenance of the ICH Guideline on Non-Clinical Safety Studies forthe Conduct of Human Clinical Trials for Pharmaceuticals药物的对人临床试验的非临床安全研究指南的变动E-Efficacy(有效)E1: The Extent of Population Exposure to Assess Clinical Safety for Drugs Intended for Long-Term Treatment of Non-Life-Threatening Conditions 对用于无生命危险情况下长期治疗的药物进行临床安全评估的族群暴露量范围E2A: Clinical Safety Data Management: Definitions and Standards forExpedited Reporting临床安全数据管理:速报制度的定义和标准E2B(R): Revision of the E2B(M) ICH Guideline on Clinical Safety DataManagement Data Elements for Transmission of Individual Case Safety Reports个案安全报告送交的临床安全数据管理的数据要素指南(E2B(M))的修订版E2B (M): Maintenance of the Clinical Safety Data Management including: Data Elements for Transmission of Individual Case Safety Reports临床安全数据管理的变动包括:个案安全报告送交的数据要素E2B(M): Maintenance of the Clinical Safety Data Management including Questions and Answers临床安全数据管理的变动,包括问答E2C: Clinical Safety Data Management: Periodic Safety Update Reports forMarketed Drugs临床安全数据管理:已上市药品的周期性安全数据更新报告Addendum to E2C: Periodic Safety Update Reports for Marketed Drugs E2C的附录:已上市药品的周期性安全数据更新报告E2D: Post-Approval Safety Data Management: Definitions and Standards forExpedited Reporting批准后的安全数据管理:速报制度的定义和标准E2E: Pharmacovigilance Planning药物警戒计划E3: Structure and Content of Clinical Study Reports临床研究报告的结构和内容E4: Dose-Response Information to Support Drug Registration支持药品注册的剂量-效应资料E5: Ethnic Factors in the Acceptability of Foreign Clinical Data引入海外临床数据时要考虑的人种因素E6: Good Clinical Practice: Consolidated GuidelineGCP:良好的临床规范:统一的指南E7: Studies in Support of Special Populations: Geriatrics对特定族群的支持的研究:老人病学E8: General Considerations for Clinical Trials对临床试验的总的考虑E9: Statistical Principles for Clinical Trials临床试验的统计原则E10: Choice of Control Group and Related Issues in Clinical Trials临床试验中控制组和有关课题的选择E11: Clinical Investigation of Medicinal Products in the PediatricPopulation小儿科药物的临床调查E12A: Principles for Clinical Evaluation of New Antihypertensive Drugs 新抗高血压药物的临床评价原则E14: The Clinical Evaluation of QT/QTc Interval Prolongation andProarrhythmic Potential for Non-Antiarrhythmic Drugs非抗心率失常药物的QT/QTc 间期和致心率失常潜在作用的临床评价Multidisciplinary Guidelines 多学科兼容的指南M1: Medical Terminology医学术语M2: Electronic Standards for Transmission of Regulatory Information (ESTRI)药政信息传递之电子标准M3: Timing of Pre-clinical Studies in Relation to Clinical Trials (SeeSafety Topics)有关临床试验的临床前研究的时间安排M4: The Common Technical Document (See CTD section for complete Status ofthe guidelines)通用技术文件(见有关CTD章节)M5: Data Elements and Standards for Drug Dictionaries药物词典的数据要素和标准临床试验常用的英文缩略语TTP:time-to-progression 疾病进展时间SAE:severity Adverse Event 严重不良事件AE:Adverse Event 不良事件SOP:Standard Operating Procedure 标准操作规程CRF:Case Report form 病例报告表DLT:剂量限制毒性MTD:最大耐受剂量KPS:Karnofsky Performance Status行为状态评分CR:complete response完全缓解PR:partial response部分缓解SD:病情稳定PD:progressive disease病情进展CTC:常用药物毒性标准IEC:independent ethics committee 独立伦理委员会IRB :institutional review board 伦理委员会CRA:临床研究助理CRO:Contract Research Organization 合同研究组织DFS:Disease Free Survival 无病生存期OS:(Overall Survival)总生存时间IC:Informed consent 知情同意ADR:Adverse Drug Reaction 不良反应GAP:Good Agricultural Practice 中药材种植管理规范GCP:Good Clinical Practice 药物临床试验质量管理规范GLP:Good Laboratory Practice 药品实验室管理规范GMP:Good Manufacturing Practice 药品生产质量管理规范GSP:Good Supply Practice 药品经营质量管理规范GUP:Good Use Practice 药品使用质量管理规范PI :Principal investigator 主要研究者CI:Co-inveatigator 合作研究者SI :Sub-investigator 助理研究者COI :Coordinating investigtor 协调研究者DGMP:医疗器械生产质量管理规范ICF:Informed consent form 知情同意书RCT :randomized controlled trial, 随机对照试验NRCCT:non-randomized concurrent controlled trial, 非随机同期对照试验EBM:evidence-based medicine 循证医学RCD:randomized cross-over disgn 随机交叉对照试验HCT:historial control trial, 历史对照研究RECIST:Response Evaluation Criteria In Solid Tumors. 实体瘤疗效反应的评价标准QC:Quality Control质量控制UADR:Unexpected Adverse Drug Reaction,非预期药物不良反应。
常用医用英语缩写

常用医用英语缩写RP表示请取药;P.O表示此药口服;INJ.表示注射剂;MIXT.表示合剂;TAD.表示片剂;SOL.表示溶液;CO.表示复方;PR.表示灌肠;I.D表示皮内注射;I.V表示静脉注射;I.V.GTT.表示静脉点滴; IH表示皮下注射;IM表示肌肉注射;O.M表示每晨;O.N表示每晚;HS.表示睡时用;AM.表示上午;PM.表示下午;A.C.表示饭前;P.C.表示饭后;SOS.表示需要时用一次; ST.表示立即;QD表示每日一次;BID表示每日两次;TID表示每日三次;QID表示隔日一次;QH表示每小时一次;Q2H表示每两小时一次;Q3H表示每三小时一次;依次类推;MCG表示微克;MG表示毫克;G表示克;ML表示毫升;sig表示用法;医学常见缩写与符号ACE 血管紧张素转换酶 Mg 镁 ACTH 促肾上腺皮质激素 mg 毫克 ADH 抗利尿激素 MI 心肌梗死 AIDS 获得性免疫缺陷综合征(艾滋病) MIC 最小抑制浓度ALT 丙氨酸转氨酶(以前称SGPT) min 分钟AST 天门冬氨酸转氨酶(以前称SGOT) mIU 毫国际单位ATP 三磷酸腺苷 ml 毫升 BCG 卡介苗 mm 毫米bid 每日2次 mmol 毫摩尔BMR 基础代谢率 molwt 分子量BP 血压 mOsm 毫渗摩尔BSA 体表面积 MRI 磁共振成像 BUN 血尿素氮 N 氮;正常(溶液浓度) C 摄氏温度;百分度;补体 Na 钠 Ca 钙 NaCl 氯化钠cAMP 环腺苷酸 ng 纳克(,毫微克) CBC 全血计数 nm 纳米(,毫微米) cGy 厘戈瑞 nmol 纳摩尔Ch 节 NSAID 非类固醇抗炎药Ci 居里 O2 氧CK 肌酸激酶 OTC 非处方用药Cl 氯化物;氯 P 磷;压力cm 厘米 PACO2 肺泡二氧化碳压CNS 中枢神经系统 PaCO2 动脉二氧化碳压 CO2 二氧化碳 PAO2 肺泡氧压COPD 慢性阻塞性肺部疾病 PaO2 动脉氧压 CPR 心肺复苏 PAS 过碘酸-希夫CSF 脑脊液 PCO2 二氧化碳压(或张力) cu 立方的 pg 皮克(,微微克) D&C 扩张和刮除术 pH 氢离子浓度 dl 分升(,100ml) PMN 多形核白细胞 DNA 脱氧核糖核酸 PO 口服DTP 白喉-破伤风-百日咳(类毒素/疫苗) PO2 氧压(或张力)D/W 葡萄糖水 PPD 精制白蛋白衍生物(结核菌素)ECF 细胞外液 ppm 百万分之几ECG 心电图 prn 必要时EEG 脑电图 q 每ENT 耳,鼻,喉 qid 每日4次ERCP 内镜逆行胰胆管造影 RA 类风湿性关节炎 ESR 红细胞沉降率 RBC 红细胞FDA 美国食物及药品管理局 RNA 核糖核酸 FUO 不明原因发热 SaO2 动脉氧饱和度 Gy 戈瑞 SBE 亚急性细菌性心内膜炎 g 克 sc 皮下GFR 肾小球滤过率 sec 秒GI 胃肠的,消化道的 SI 国际单位制 G6PD 葡萄糖-6-磷酸脱氢酶 SIDS 婴儿猝死综合征GU 泌尿生殖的 SLE 系统性红斑狼疮 h 小时 soln 溶液Hb 血红蛋白 sp gr 比重HCl 盐酸;氢氯化物 sq 平方HCO3 重碳酸盐 STS 梅毒血清试验 Hct 血细胞比容 TB 结核病 Hg 汞 tid 每日3次HIV 人类免疫缺陷病毒 TPN 全肠外营养 HLA 人类白细胞抗原 u 单位 Hz 赫(兹)(周/秒) URI 上呼吸道感染ICF 细胞内液 UTI 尿路感染 ICU 强化监护室 WBC 白血细胞 IgA,etc 免疫球蛋白A,等 WHO 世界卫生组织IM 肌内注射 wk 周INR 国际标准化率 wt 重量 IPPB 间歇性正压呼吸μ 微;微米 IU 国际单位μCi 微居里 IV 静脉注射μg 微克IVU 静脉尿路造影μl 微升 K 钾μm 微米kcal 千卡(食物热卡) μmol 微摩尔 kg 千克μOsm 微渗摩尔 L 升mμ 毫微米(,纳米) LDH 乳酸脱氢酶M 摩尔m 米m2 平方米MCH 平均红细胞血红蛋白量 MCHC 平均红细胞血红蛋白浓度 mCi 毫居里MCV 平均红细胞容量mEq 毫当量GNS。
医药常用英语单词及缩写

FDA(food and drug adminisration):(美国)食品药品监督管理局NDA(new drug application):新药申请ANDA(abbreviated new drug application):简化新药申请EP(export application):出口药申请(申请出口不被批准在美国销售的药品)treatment IND:研究中的新药用于治疗abbreviated(new)drug:简化申请的新药DMF(drug master file):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品。
只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND、NDA、ANDA时才能参考其内容)holder:DMF持有者CFR(code of federal regulation):(美国)联邦法规PANEL:专家小组batch production:批量生产;分批生产batch production records:生产批号记录post or pre-market surveillance:销售前或销售后监督informed consent:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)prescription drug:处方药OTC drug(over—the—counter drug):非处方药U.S. public health service:美国卫生福利部NIH(national institute of health):(美国)全国卫生研究所animal trail:动物试验accelerated approval:加速批准standard drug:标准药物investigator :研究人员;调研人员preparing and submitting:起草和申报submission:申报;递交benefit(s):受益risk(s):受害drug product:药物产品drug substance:原料药established name:确定的名称generic name:非专利名称proprietary name:专有名称;INN(international nonproprietary name):国际非专有名称narrative summary: 记叙体概要adverse effect:副作用adverse reaction:不良反应protocol:方案archival copy:存档用副本review copy:审查用副本official compendium:法定药典(主要指USP、NF).USP(the united state pharmacopeia):美国药典(现已和NF合并一起出版)NF(national formulary):(美国)国家药品集official=pharmacopeial = compendial:药典的;法定的;官方的agency:审理部门(指FDA)sponsor:主办者(指负责并着手临床研究者)identity:真伪;鉴别;特性strength:规格;规格含量(每一剂量单位所含有效成分的量)labeled amount:标示量regulatory specification:质量管理规格标准(NDA提供)regulatory methodology:质量管理方法(FDA用于考核原料药或药物产品是否符合批准了的质量管理规格标准的整套步骤)regulatory methods validation:管理用分析方法的验证(FDA对NDA提供的方法进行验证)Dietary supplement:食用补充品ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use)人用药物注册技术要求国际协调会议ICH:Quality-质量Q1A(R2): Stability Testing of New Drug Substances and Products (SecondRevision)新原料药和制剂的稳定性试验(第二版)Q1B: Photostability Testing of New Drug Substances and Products新原料药和制剂的光稳定性试验Q1C: Stability Testing for New Dosage Forms新制剂的稳定性试验Q1D: Bracketing and Matrixing Designs for Stability Testing of DrugSubstances and Drug Products原料药和制剂稳定性试验的交叉和矩阵设计Q1E: Evaluation of Stability Data对稳定性数据的评估处理Q1F: Stability Data Package for Registration Applications in ClimaticZones III and IV在气候带III和IV,药物注册申请所提供的稳定性数据Q2A: Text on Validation of Analytical Procedures分析程序的验证Q2B: Validation of Analytical Procedures: Methodology分析程序的验证:方法学Q3A(R): Impurities in New Drug Substances (Revised Guideline)新原料药中的杂质(修订版)Q3B(R): Impurities in New Drug Products (Revised Guideline)新制剂中的杂质(修订版)Q3C: Impurities: Guideline for Residual Solvents杂质:残留溶剂指南Q3C(M): Impurities: Guideline for Residual Solvents (Maintenance)杂质:残留溶剂指南(修改内容)Q4: Pharmacopoeias药典Q4A: PharmacopoeialHarmonisation药典的协调Q4B: Regulatory Acceptance of Pharmacopoeial Interchangeability药典互替在法规上的可接受性Q5A: Viral Safety Evaluation of Biotechnology Products Derived from CellLines of Human or Animal Origin来源于人或者动物细胞系的生物技术产品的病毒安全性评估Q5B: Quality of Biotechnological Products: Analysis of the ExpressionConstruct in Cells Used for Production of r-DNA Derived Protein Products生物技术产品的质量:源于重组DNA的蛋白质产品的生产中所用的细胞中的表达构建分析Q5C: Quality of Biotechnological Products: Stability Testing ofBiotechnological/Biological Products生物技术产品的质量:生物技术/生物产品的稳定性试验Q5D: Derivation and Characterisation of Cell Substrates Used forProduction of Biotechnological/Biological Products用于生产生物技术/生物产品的细胞底物的起源和特征描述Q5E: Comparability of Biotechnological/Biological Products Subject toChanges in Their Manufacturing Process基于不同生产工艺的生物技术产品/生物产品的可比较性Q6: Specifications for New Drug Substances and Products新原料药和制剂的质量规格Q6A: Specifications: Test Procedures and Acceptance Criteria for New DrugSubstances and New Drug Products: Chemical Substances质量规格:新原料药和新制剂的检验程序和可接收标准:化学物质Q6B: Specifications: Test Procedures and Acceptance Criteria forBiotechnological/Biological Products质量规格:生物技术/生物产品的检验程序和可接收标准Q7: Good Manufacturing Practices for Pharmaceutical Ingredients活性药物成份的GMPQ7A: Good Manufacturing Practice Guide for Active PharmaceuticalIngredients活性药物成份的GMP指南Q8: Pharmaceutical Development药物研发Q9: Quality Risk Management质量风险管理ICH:Safety-安全S1A: Guideline on the Need for Carcinogenicity Studies of Pharmaceuticals药物致癌性研究需要的指南S1B: Testing for Carcinogenicity of Pharmaceuticals药物致癌性的检验S1C: Dose Selection for Carcinogenicity Studies of Pharmaceuticals药物致癌性研究之剂量选择S1C(R): Addendum: Addition of a Limit Dose and Related Notes附录:极限剂量和有关注释的的补充S2A: Guidance on Specific Aspects of Regulatory Genotoxicity Tests forPharmaceuticals受法规管辖的药物基因毒性检验的特定方面的指南S2B: Genotoxicity: A Standard Battery for Genotoxicity Testing forPharmaceuticals基因毒性:药物基因毒性检验的标准S3A: Note for Guidance on Toxicokinetics: The Assessment of SystemicExposure in Toxicity Studies毒物代谢动力学指南的注释:毒性研究中的全身性暴露量的评估S3B: Pharmacokinetics: Guidance for Repeated Dose Tissue DistributionStudies药物代谢动力学:重复剂量的组织分布研究指南S4: Single Dose Toxicity Tests单剂量毒性检验S4A: Duration of Chronic Toxicity Testing in Animals (Rodent andNon-Rodent Toxicity Testing)动物体内慢性毒性持续时间的检验(啮齿动物和非啮齿动物毒性检验)S5A: Detection of Toxicity to Reproduction for Medicinal Products药物对生殖发育的毒性的检验S5B(M): Maintenance of the ICH Guideline on Toxicity to Male Fertility:An Addendum to the Guideline on Detection of Toxicity to Reproduction forMedicinal Products对男性生殖能力的毒性的指南的变动:药物对生殖发育的毒性的检验指南增加了一个附录S6: Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals生物技术生产的药物的临床前安全评价S7A: Safety Pharmacology Studies for Human Pharmaceuticals人用药的安全药理学研究S7B: The Nonclinical Evaluation of the Potential for Delayed VentricularRepolarization(QT Interval Prolongation) By Human Pharmaceuticals药物延迟心室复极化(QT间期)潜在作用的非临床评价S8: Immunotoxicology Studies for Human Pharmaceuticals人用药免疫毒理学研究M3(M): Maintenance of the ICH Guideline on Non-Clinical Safety Studies for the Conduct of Human Clinical Trials for Pharmaceuticals药物的对人临床试验的非临床安全研究指南的变动E-Efficacy(有效)E1: The Extent of Population Exposure to Assess Clinical Safety for DrugsIntended for Long-Term Treatment of Non-Life-Threatening Conditions对用于无生命危险情况下长期治疗的药物进行临床安全评估的族群暴露量范围E2A: Clinical Safety Data Management: Definitions and Standards forExpedited Reporting临床安全数据管理:速报制度的定义和标准E2B(R): Revision of the E2B(M) ICH Guideline on Clinical Safety DataManagement Data Elements for Transmission of Individual Case SafetyReports个案安全报告送交的临床安全数据管理的数据要素指南(E2B(M))的修订版E2B (M): Maintenance of the Clinical Safety Data Management including:Data Elements for Transmission of Individual Case Safety Reports临床安全数据管理的变动包括:个案安全报告送交的数据要素E2B(M): Maintenance of the Clinical Safety Data Management includingQuestions and Answers临床安全数据管理的变动,包括问答E2C: Clinical Safety Data Management: Periodic Safety Update Reports forMarketed Drugs临床安全数据管理:已上市药品的周期性安全数据更新报告Addendum to E2C: Periodic Safety Update Reports for Marketed DrugsE2C的附录:已上市药品的周期性安全数据更新报告E2D: Post-Approval Safety Data Management: Definitions and Standards for Expedited Reporting批准后的安全数据管理:速报制度的定义和标准E2E: Pharmacovigilance Planning药物警戒计划E3: Structure and Content of Clinical Study Reports临床研究报告的结构和内容E4: Dose-Response Information to Support Drug Registration支持药品注册的剂量-效应资料E5: Ethnic Factors in the Acceptability of Foreign Clinical Data引入海外临床数据时要考虑的人种因素E6: Good Clinical Practice: Consolidated GuidelineGCP:良好的临床规范:统一的指南E7: Studies in Support of Special Populations: Geriatrics对特定族群的支持的研究:老人病学E8: General Considerations for Clinical Trials对临床试验的总的考虑E9: Statistical Principles for Clinical Trials临床试验的统计原则E10: Choice of Control Group and Related Issues in Clinical Trials临床试验中控制组和有关课题的选择E11: Clinical Investigation of Medicinal Products in the PediatricPopulation小儿科药物的临床调查E12A: Principles for Clinical Evaluation of New Antihypertensive Drugs新抗高血压药物的临床评价原则E14: The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs非抗心率失常药物的QT/QTc间期和致心率失常潜在作用的临床评价Multidisciplinary Guidelines 多学科兼容的指南M1: Medical Terminology医学术语M2: Electronic Standards for Transmission of Regulatory Information (ESTRI)药政信息传递之电子标准M3: Timing of Pre-clinical Studies in Relation to Clinical Trials (SeeSafety Topics)有关临床试验的临床前研究的时间安排M4: The Common Technical Document (See CTD section for complete Status of the guidelines)通用技术文件(见有关CTD章节)M5: Data Elements and Standards for Drug Dictionaries药物词典的数据要素和标准临床试验常用的英文缩略语TTP:time-to-progression 疾病进展时间SAE:severity Adverse Event 严重不良事件AE:Adverse Event 不良事件SOP:Standard Operating Procedure 标准操作规程CRF:Case Report form 病例报告表DLT:剂量限制毒性MTD:最大耐受剂量KPS:Karnofsky Performance Status行为状态评分CR:complete response完全缓解PR:partial response部分缓解SD:病情稳定PD:progressive disease病情进展CTC:常用药物毒性标准IEC:independent ethics committee 独立伦理委员会IRB :institutional review board 伦理委员会CRA:临床研究助理CRO:Contract Research Organization 合同研究组织DFS:Disease Free Survival 无病生存期OS:(Overall Survival)总生存时间IC:Informed consent 知情同意ADR:Adverse Drug Reaction 不良反应GAP:Good Agricultural Practice 中药材种植管理规范GCP:Good Clinical Practice 药物临床试验质量管理规范GLP:Good Laboratory Practice 药品实验室管理规范GMP:Good Manufacturing Practice 药品生产质量管理规范GSP:Good Supply Practice 药品经营质量管理规范GUP:Good Use Practice 药品使用质量管理规范PI :Principal investigator 主要研究者CI:Co-inveatigator合作研究者SI :Sub-investigator 助理研究者COI :Coordinating investigtor协调研究者DGMP:医疗器械生产质量管理规范ICF:Informed consent form 知情同意书RCT :randomized controlled trial, 随机对照试验NRCCT:non-randomized concurrent controlled trial, 非随机同期对照试验EBM:evidence-based medicine 循证医学RCD:randomized cross-over disgn随机交叉对照试验HCT:historial control trial, 历史对照研究RECIST:Response Evaluation Criteria In Solid Tumors. 实体瘤疗效反应的评价标准QC:Quality Control质量控制UADR:Unexpected Adverse Drug Reaction,非预期药物不良反应。
药店常用英语

药店常用英语药店 drugstore、pharmacy、dispensary非处方药 (Non-prescription Drug or Over-the-Counter, OTC) 处方药 (Prescription Drug, Ethical Drug,or Receptor X, Rx) 症状 symptom用法用量:usage and dosage感冒 catch(have)a cold发高/低烧 have a high/slight fever鼻塞 have a stuffy nose流涕 have a runny nose咽喉痛 have a sore throat咳嗽得很厉害 cough a lot咳嗽有痰 cough up some phlegm您需要什么,先生/女士? Can I help you Sir/Madam? 或What can I do for you?2shivery 发冷 sleepy 发困 syrup 糖浆 capsule 胶囊essence 口服液 tablet 药片 ache all over 浑身酸痛cough mixture 咳嗽药水My throat feels swollen. 我的喉咙肿了。
Golden throat 金嗓子喉宝What’s wrong with you? 您怎么啦。
I need some medicine for my cold. 我要一些感冒药。
Do you have a temperature? 您量过体温了吗?Do you cough up any phlegm? 咳嗽有痰吗?How long have you been like this? 像这样多长时间了?Take this medicine 3 tablets once after meals, and three times a day.一天三次,每次三片,饭后服用。
医药行业专业英语词汇(非常有用)

FDA和EDQM术语:CLINICAL TRIAL:临床试验ANIMAL TRIAL:动物试验ACCELERATED APPROVAL:加速批准STANDARD DRUG:标准药物INVESTIGATOR:研究人员;调研人员PREPARING AND SUBMITTING:起草和申报SUBMISSION:申报;递交BENIFIT(S):受益RISK(S):受害DRUG PRODUCT:药物产品DRUG SUBSTANCE:原料药ESTABLISHED NAME:确定的名称GENERIC NAME:非专利名称PROPRIETARY NAME:专有名称;INN(INTERNATIONAL NONPROPRIETARY NAME):国际非专有名称ADVERSE EFFECT:副作用ADVERSE REACTION:不良反应PROTOCOL:方案ARCHIVAL COPY:存档用副本REVIEW COPY:审查用副本OFFICIAL COMPENDIUM:法定药典(主要指USP、NF).USP(THE UNITED STATES PHARMACOPEIA):美国药典NF(NATIONAL FORMULARY):(美国)国家处方集OFFICIAL=PHARMACOPEIAL= COMPENDIAL:药典的;法定的;官方的AGENCY:审理部门(指FDA)IDENTITY:真伪;鉴别;特性STRENGTH:规格;规格含量(每一剂量单位所含有效成分的量)LABELED AMOUNT:标示量REGULATORY SPECIFICATION:质量管理规格标准(NDA提供)REGULATORY METHODOLOGY:质量管理方法REGULATORY METHODS VALIDATION:管理用分析方法的验证COS/CEP 欧洲药典符合性认证ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use)人用药物注册技术要求国际协调会议ICH文件分为质量、安全性、有效性和综合学科4类。
常用临床医学英文缩写中英文对照

常用临床医学英文缩写中英文对照AB 实际碳酸氢盐ACEI 血管紧张素转换酶抑制剂ACT 激活凝血时间AG 离子间隙AMI 急性心梗ANA 抗核抗体ARDS 急性呼吸窘迫综合征ASO 抗链球菌溶血素“0” ATP 三磷酸腺苷AVNRT 房室结折返性心动过速AVRT 房室折返性心动过速BB 缓冲碱BEE 基础能量消耗BT 出血时间BuN 尿素氮C3 补体C3 CBC 血常规CCU 心血管监护室CHE 胆碱酯酶CK 肌酸磷酸激酶CPAP 持续正压通气CPR 心肺复苏CT 凝血时间CVP 中心静脉压DBP 舒张压DCT 双氢克尿噻DIC 弥散性血管内凝血DM 舒张期杂音EF 射血分数ENT 耳鼻喉科(五官科) FDP 纤维蛋白原降解产物FUO 不明原因发热GNS 葡萄糖生理氯化钠溶液Hb 血红蛋白HCO3- 碳酸氢根HCT 红细胞比容HIV 人类免疫缺陷病毒Holter 24h动态心电图IABP 主动脉内气囊反搏术IHSS 特发性肥厚型主动脉瓣下狭窄INR 国际标准比率IU 国际单位KPTT 部分凝血活酶时间KUB 腹部平片LDH 乳酸脱氢酶NS 生理氯化钠溶液NTG 硝酸甘油OB 隐油P(A-a)O2 肺泡气-动脉血氧分压差P2 肺动脉第二心音PaCO2 动脉二氧化碳分压PAMPA 氨甲苯酸PaO2 动脉氧分压PCAP 肺小动脉压PCWP 肺毛细血管压PEEP 呼气末正压pH 酸碱度PPD 结核菌素纯蛋白衍生物Prn 必要时PT 凝血酶原时间qh 每小时1次qid 每天4次qn 每晚1次qod 隔日1次RF 类风湿因子RI 胰岛素RR 呼吸频率S3 第3心音S4 第4心音SaO2 血氧饱和度SB 标准碳酸氢盐SBE 亚急性细菌性心内膜炎SBP 收缩压SGOT 血清谷草转氨酶SGPT 血清谷丙转氨酶SK 链激酶SM 收缩期杂音T3 三碘甲状原氨酸T4 甲状腺素TAT 抗蛇毒血清TIL 短暂脑缺血发作tid 每天3次t-PA 组织型纤溶酶原激活物TPN 全肠道外营养TSH 促甲状腺激素UK 尿激酶V/Q 通气/灌注比Qd 一天一次Bid 两天一次Bid 两天一次Tid 三天一次im 肌肉注射ICU 重症监护bp 血压R 呼吸P 心率Ca 癌症Qid表示为一天4次,Tid表示为一天3次。
[医学]药学英语常用缩写
![[医学]药学英语常用缩写](https://img.taocdn.com/s3/m/1a8f30544a7302768e9939d4.png)
等方式来做为新药申报,这些药物在国外已经通过临床试验或 者已经上市使用,安全性资料已经积累,用于国内进行临床试验 的参照.而国内的临床试验需要考虑到的就是不同种族之间的 差异这些人口学资料的影响。
FDA关于新药的IND门槛较低,但临床试验研究非常严格, 失败的例子比比皆是,通过率很低, 其原因是:
GDP 国民生产总值 Gross National Product
CPI 消费者物价指数 Consumer Price Index
新药注册相关的英文缩略语 药代动力学相关的英文缩略语 调剂相关的英文缩略语 制剂相关的英文缩略语 科研相关的英文缩略语
SFDA
State Food and Drug Admistraton ——(中国的)国家食品药品监督管理局
NDA
—— New Drug Application ——(美国的)新药上市申请
INDA
——Abbreviated New Drug Application ——(美国的)简略的新药上市申请(仿制药)
SIPO
State Intellectual Property Office 国家知识产权局
CDE
Center For Durg Evaluation ——国家食品药品监督管理局药品审评中心
FDA
药学英语常用缩写

CDER
—— Center for Drug Evaluation and Research ——药品评价和研究中心
IND
——Investigational New Drug ——(美国的)研究用的新药,即:新药(临床)研究 目前国内新药研制的现状是:IND的门槛比较高,一旦通过 IND后,几乎100%通过临床试验并上市销售。其原因是: 国内的新药大多是通过改变剂型或者改变药物侧链,取代酸盐 等方式来做为新药申报,这些药物在国外已经通过临床试验或 者已经上市使用,安全性资料已经积累,用于国内进行临床试验 的参照.而国内的临床试验需要考虑到的就是不同种族之间的 差异这些人口学资料的影响。
PDR
——Physician's Desk Reference ——医生案头手册
作为一本美国医生必备参考书,是美国定期把药厂的产品介绍和 说明书汇编成册,而形成PDR。每年综合汇编一次,介绍市场上的新 药,内容比较全面,并且还出补充本,用途较广。 PDR(医师药物参考书):收集2800多种FDA核准的处方药,以 及250多家药厂的相关报道。医师靠着它,能够更准确的开立处方, 以免一个病没治好,药物副作用制造另一个病。 该书内容按生产厂家的名称字母排列,名厂上市的许多药品再按 其字母排列。本书每年内容皆修订,次料较为新颖,是医师查阅最新 药品资讯的便捷工具。在各个药品条目下,包含了药品的处方规格、 基本辅料、使用说明书、药理、毒理、临床应用,甚至有的还有药品 的包装图片,十分详尽。本书特色是非处方药与饮食补品 PDR及眼科 用药皆有说明,包括最新的FDA批准的处方药、非处方药与特殊眼用 药物。 PDR的网站地址为:
一期临床试验主要测试药物的安全性,主要副作用、代谢机 理等,如果有严重的不良反应,立刻就被停掉。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
《GMP英语词汇及缩写》第一部分PIC/S的全称为:Pharmaceutical Inspection Convention/Pharmaceutical Inspection Cooperation Scheme, PIC/S(制药检查草案), 药品检查协会(PIC/S) ,也有人称PIC/S为医药审查会议/合作计划(PIC/S)PIC的权威翻译:药品生产检查相互承认公约API(Active Pharmaceutical Ingrediet) 原料药又称:活性药物组分AirLock 气闸 Authorized Person 授权人 Batch/Lot 批次Batch Number/Lot-Number 批号;Batch Numbering System 批次编码系统;Batch Records 批记录;Bulk Product 待包装品;Calibration 校正;Clean area洁净区;Consignmecnt(Delivery)托销药品。
FDA(FOOD AND DRUG ADMINISTRATION):(美国)食品药品管理局IND(INVESTIGATIONAL NEW DRUG):临床研究申请(指申报阶段,相对于NDA而言);研究中的新药(指新药开发阶段,相对于新药而言,即临床前研究结束)NDA(NEW DRUG APPLICATION):新药申请ANDA(ABBREVIATED NEW DRUG APPLICATION):简化新药申请TREATMENT IND:研究中的新药用于治疗ABBREVIATED(NEW)DRUG:简化申请的新药DMF(DRUG MASTER FILE):药物主文件(持有者为谨慎起见而准备的保密资料,可以包括一个或多个人用药物在制备、加工、包装和贮存过程中所涉及的设备、生产过程或物品。
只有在DMF持有者或授权代表以授权书的形式授权给FDA,FDA在审查IND、 NDA、ANDA时才能参考其内容)HOLDER:DMF持有者CFR(CODE OF FEDERAL REGULATION):(美国)联邦法规PANEL:专家小组BATCH PRODUCTION:批量生产;分批生产BATCH PRODUCTION RECORDS:生产批号记录POST-OR PRE- MARKET SURVEILLANCE:销售前或销售后监督INFORMED CONSENT:知情同意(患者对治疗或受试者对医疗试验了解后表示同意接受治疗或试验)PRESCRIPTION DRUG:处方药OTC DRUG(OVER—THE—COUNTER DRUG):非处方药第二部分 GMP文件常见缩写ABPI Association of the British Pharmaceutical Industry ADR Adverse Drug ReactionAE Adverse EventAIM Active Ingredient ManufacturerANDA Abbreviated New Drug ApplicationANOVA Analysis of VarianceASM: Active Substance ManufacturerATC Anatomical Therapeutic ChemicalATX Animal Test Exemption CertificateBAN British Approved NameBIRA British Institute of Regulatory AffairsBNF British National FormularyBP British PharmacopoeiaC of A Certificate of AnalysisC of S Certificate of SuitabilityCENTRE FOR DRUG EVALUATION (CDE)Centre for Pharmaceutical Administration (CPA)CMS Concerned Member StateCMS每个成员国COS Certificate of SuitabilityCPMP Committee for Proprietary Medicinal ProductsCRA Clinical Research AssociateCRF Case Report FormCRO Contract Research OrganisationCTA Clinical Trial ApplicationCTC Clinical Trial CertificateCTD Common Technical DocumentCTX Clinical Trials ExemptionDDD Defined Daily DoseDGC Daily Global ComparisonDIA Drug Information AssociationDMF Drug Master FileDrug Registration Branch (DR, Product Evaluation & Registration Division, CPA EDQM (European Directorate for the Quality of Medicines) 欧洲联盟药品质量指导委员会EEA 欧洲经济地区EGMA European Generics Medicine AssociationELA Established Licence ApplicationEMEA European Medicines Evaluation AgencyEMEA (European Agency for the Evaluation of Medicinal Products)欧洲联盟药品评价机构EP European PharmacopoeiaEPAR European Public Assessment ReportsESRA European Society of Regulatory AffairsEuropean Pharmacopoeia Commission 欧洲药典委员会FDAFDA Food and Drug Administrationfinal evaluation report (FER)free sale certificates (FSCs)GCP Good Clinical PracticeGCP药品临床研究管理规范GLP Good Laboratory PracticeGLP 药品临床前安全性研究质量管理规范GMP Good Manufacturing PracticeGMP 药品生产质量管理规范GSP药品销售管理规范Health Sciences Authority (HSA)HSA’s Medicines Advisory Committee (MAC)IB Investigators BrochureICH International Conference for HarmonisationIDMC Independent Data-Monitoring CommitteeIEC Independent Ethics CommitteeIND Investigational New DrugINN International Non-proprietary Name International Conference on Harmonisation (ICH) IPC In Process ControlIRB Institutional Review BoardLICENCE HOLDERMA Marketing AuthorisationMAA Marketing Authorisation ApplicationMAA上市申请MAH Marketing Authorisation HolderMAH 销售许可持有者MCA Medicines Control AgencyMHW Ministry of Health and Welfare (Japan)MR Mutual RecognitionMRA 美国与欧盟的互认协议MRAs (Mutual Recognition Agreements) 互相認證同意MRFG Mutual Recognition Facilitation Group MRP Mutual Recognition ProcedureNAS New Active SubstanceNCE New Chemical EntityNDA New Drug Applicationnew chemical entities (NCEs)new drug applications (NDAs)NSAID Non Steroidal Anti Inflammatory DrugNTA Notice To ApplicantsOOS Out of SpecificationOTC Over The CounterPAGB Proprietary Association of Great Britain Ph Eur European PharmacopoeiaPIL Patient Information LeafletPL Product LicencePOM Prescription Only MedicinePRODUCT OWNERPSU Periodic Safety UpdatesQA Quality AssuranceQC Quality ControlRAJ Regulatory Affairs JournalRMS Reference Member StateRMS相互认可另一成员国RSD Relative Standard DeviationRx Prescription OnlySAE Serious Adverse EventSMF Site Master FileSOP Standard Operating ProcedureSOP (STANDARD OPERATION PROCEDURE)标准运作程序SPC/SmPC Summary of Product Characteristicssummary of product characteristics(SPC)Therapeutic Goods Administration (TGA)USP US PharmacopoeiaVMF Veterinary Master FileVPC Veterinary Products Committee第三部分常用缩略语A.A.A Addition and Amendments 增补和修订AC Air Conditioner 空调器ADR Adverse Drug Reaction 药物不良反应AFDO Association of Food and Drug Officials 食品与药品官员协会(美国)ACC Accept 接受AQL Acceptable Quality Level 合格质量标准ADNA Abbreviated New Drug Application 简化的新药申请BOM Bill of Material 物料清单BPC Bulk pharmaceutical Chemiclls 原料药CBER Center for Biologics Evaluation Research 生物制品评价与研究中心CFU Colony Forming Unet 菌落形成单位DMF Drug Master File 药品管理档案CDER Cemter for Drug Evaluation amd Research 药物评价与研究中心CI Corporate Identity (Image) 企业识别(形象)CIP Cleaning in Place 在线清洗CSI Consumer Safety Insepctor 消费者安全调查员CLP Cleaning Line Procedure 在线清洗程序DAL Defect Action Level 缺陷作用水平DEA Drug Enforcement Adminestration 管制药品管理DS Documentation Systim 文件系统FDA Food and Drug Administration 食品与药品管理局(美国)GATT General Agreemernt on Tariffs and Trade 关贸总协会GMP Good Manufacturing Practice Gvp 药品生质量管理规范GCP Good Clinical Practice 药品临床实验管理规范GLP Good Laboratory Practice 实验室管理规范GSP Good Supply Practice 药品商业质量规范GRP Gook RaTAIL Practice 药品零业质量管理规范GAP Good Agriculture Practice 药材生产管理规范GVP Gook Validation Prctice 验证管理规范GUP Gook Use Practice 药品重用规范HVAC Heating Ventilation Air Conditioning 空调净化系统ISO Intematonal Organization for Standardization 车际标准化组织MOU Memorandum of Understanding 谅解备忘录PF Porduction File 生产记录用表格OTC Over the Counter (Drug) 非处方药品PLA Product License Application 产品许可申请QA Quality Assurance 质量保证QC Quality Control 质量控制QMP Quality Management Procedure 质量管理程序SDA State Drug Administration 国家药品监督管理局SMP Standard Managmert Procedure 标准管理程序SOP Standard Operating Procedure 标准操作程序TQC Tatal Quality Control 全面质量管理USA Uneted States Pharmacopeia 美国药典欢迎您的下载,资料仅供参考!致力为企业和个人提供合同协议,策划案计划书,学习资料等等打造全网一站式需求。
