药物合成反应 (第三版 闻韧) 课后答案Chapter 1 Halogenation Reaction
药物合成反应习题及答案

药物合成反应习题及答案一、举例解释下列概念:1,官能团保护;为什么保护?当分子中有多个官能团,想在某一官能团进行转换反应,为了不使其他官能团影响反应,需对这些官能团进行衍生化,这就是官能团的保护。
达到反应目的后再还原这些官能团。
理想保护基:试剂易得、无毒,保护基稳定,引入和脱去反应选择性好,收率高。
2,相转移催化剂; 一种与水相中负离子结合的两性物质,可以把亲核试剂转移到有机相进行亲核反应。
相转移催化剂优点:克服溶剂化作用;不需无水操作;可用无机碱代替有机金属碱;降低反应温度。
3,重排反应;重排反应是指在同一分子内,某一原子或基团从一个原子迁移至另一原子而形成新分子的反应。
按反应机理可分为亲电重排、亲核重排、自由基重排和协同重排。
4,合成子;合成子:组成靶分子或中间体骨架的各个单元结构的活性形式.包括:离子合成子、自由基或周环反应所需的中性分子。
离子合成子:包括 d 合成子和a合成子d 合成子: 亲核性的离子合成子d---donor of electrond 合成子等价试剂a合成子:氧化性或亲电性的离子合成子a合成子:等价试剂5,协同反应协同反应:在反应过程中,若有两个或两个以上的化学键破裂和形成时,都必须相互协调地在同一步骤中完成。
6, 非均相催化氢化: 催化剂、反应物、试剂和氢供体在两项或多项中反应,催化剂自成一相,称为非均相催化氢化。
催化剂自成一相称为非均相催化剂如Pd/C为催化剂,氢气为氢供体,在反应液中还原双键的反应。
1) DMAP2) DMF3) DCC4) TBAF1) Aromatic Electrophilic Substitution; 芳香亲电取代2) Phase-transfer catalyst; 相转移催化剂3) Carbocations; 碳负离子4) trifluoroacetic anhydride.三氟乙酸酐Ryoji Noyori was awarded the Nobel Prize in 2001, What did he discover?Ryoji Noyori日本名古屋大学的野伊良治因在手性催化氢化反应方面做出了突出贡献而被授予2001年Nobel化学奖。
药物合成反应(闻韧_第三版)课后翻译

1、stream over a period of 20–30 minutes、自由流动得催化剂边搅拌边用滴液漏斗缓慢滴加81g苯乙酰。
Considerable heat is evolved, and, if the drops of ketone are not dispersed, darkening or charring occurs、放热反应,假如滴加得酮不能被分散,就会变黑bees a viscous ball-like mass that is difficult to stir、当三分之一得乙酰苯被滴加,反应混合物变成一个很难搅拌得粘性得球状团块。
Turning of the stirrer by hand or more rapid addition of ketone is necessary at this point、在这时,改用手动搅拌或快速滴加酮就是非常必要得。
The addition of ketone, however, should not be so rapid as to produce a temperature above 180°、然而,速度不能太快,当反应温度超过180℃时。
Near the end of the addition, the mass bees molten and can be stirred easily without being either明苯乙酰已经与三氯化铝混合完全,颜色也逐渐从黄褐色变为棕色。
Bromine (128 g、, 0、80 mole) is added dropwise to the well-stirred mixture over a period of 40 minutes (Note 4)、在40分钟内在搅拌下把溴缓慢滴加到混合物中。
After all the bromine has been added, the molten mixture is stirred at 80–85° on a steam bath for 1 hour、溴滴加完后,熔融混合物在80-85℃蒸气浴下搅拌1小时。
(完整word版)药物合成反应(闻韧_第三版)课后翻译(word文档良心出品)

1、About 216–224 g. (1.62–1.68 moles) of powdered anhydrous aluminum chloride is added to a 1Lthree-necked flask.在1L的三口烧瓶中加入大约216-224g(1.62–1.68 moles)的无水三氯化铝。
While the free-flowing catalyst is stirred (Note 3), 81 g. (0.67 mole) of acetophenone is added from the dropping funnel in a slow stream over a period of 20–30 minutes. 自由流动的催化剂边搅拌边用滴液漏斗缓慢滴加81g苯乙酰。
Considerable heat is evolved, and, if the drops of ketone are not dispersed, darkening or charring occurs. 放热反应,假如滴加的酮不能被分散,就会变黑或是碳化。
When about one-third of the acetophenone has been added, the mixture becomes a viscous ball-like mass that is difficult to stir.当三分之一的乙酰苯被滴加,反应混合物变成一个很难搅拌的粘性的球状团块。
Turning of the stirrer by hand or more rapid addition of ketone is necessary at this point. 在这时,改用手动搅拌或快速滴加酮是非常必要的。
The addition of ketone, however, should not be so rapid as to produce a temperature above 180°. 然而,速度不能太快,当反应温度超过180℃时。
药物合成反应(第三版_闻韧)第一章卤化反应

Organic Reactions for Drug Synthesis
例1.
C6H5 H
CC
H
COOC2H5
Br2 / CCl4
Br
C6H5
C
H C
H Br COOC2H5
C6H5 H CC
H Br COOC2H5
1. 卤素与烯烃的亲电加成反应
(1)反应历程: 第一步:卤正离子向π 键进攻,形成三员环卤正离子 或开放式碳正离子的过渡态。
R1 R3
R2
R4
δ +δ XX
Organic Reactions for Drug Synthesis
R1 R3 CC
R2 X R4
(1)
R1 R3
CC
R2
X R4
(2)
第二步:
反应类型
亲电加成 亲电取代 亲核取代 自由基反应
Organic Reactions for Drug Synthesis
常用的卤化剂 卤素(X2):Cl2、Br2
次卤酸(HOX):HOCl、HOBr
N-卤代酰胺:
如 N-溴(氯)代乙酰胺( NBA,NCA) N-溴(氯)代丁二酰亚胺(NBS,NCS)
Ph H CC
H CH3
NBS / DMSO / H2O
OH
Ph
H
CC
H
Br CH3
NBS / 干燥的DMSO
O H
Ph C C Br CH3
Organic Reactions for Drug Synthesis
五、卤化氢与烯烃的加成
药物合成第一章答案
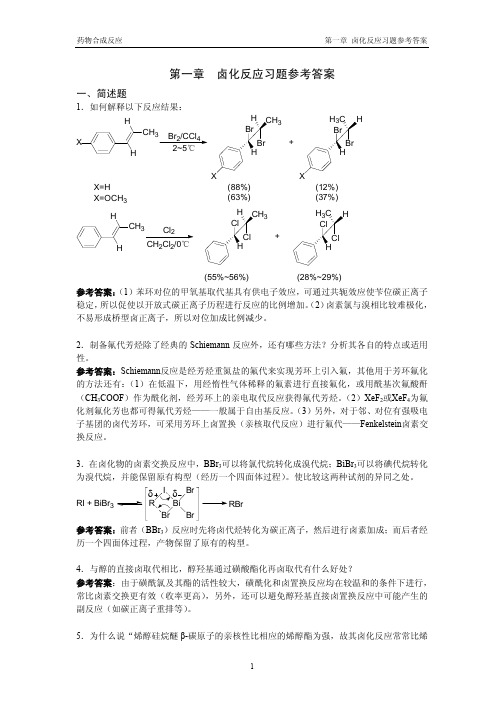
反应相对比较困难。对于碱催化情况下,α-位吸电子基有利于 α-氢脱去而促进反应。
7.酚和醚的卤置换反应各自有什么特点? 参考答案:酚羟基活性较小,一般需要用五卤化磷强卤化试剂,并在较高的反应温度下进行。 醚在氢卤酸作用下,生成一分子卤代烷和一分子醇。对于烷基芳基醚,则一般生成一分子卤 代烷和一分子酚。
8.简述卤化反应在药物合成中的作用和意义。
参考答案:(1)卤素原子的引入可以使有机分子的理化性质发生一定的变化,可以用于制备
具不同生理活性的含卤药物;(2)可以用以转化成其他官能团,卤化物常常是一类重要的中
间体;(3)卤素原子还可以作为保护基、阻断基等,用于提高反应的选择性等。
二、完成反应
1.完成反应式:
5
如下:
H
H
(H3C)3C C CH3 + (H3C)2C CH(CH3)2 + (H3C)3C C CH3
Cl
Cl
OAc
5.完成以下反应: Cl NO2 NaI/DMF ? , 15min
NO2
3
药物合成反应
参考答案:属于 Fenkelstein 卤素交换反应。 I NO2
第一章 卤化反应习题参考答案
1
药物合成反应
第一章 卤化反应习题参考答案
醇酯容易”(见教材 p26)? 参考答案:因为烯醇式的卤化是亲电性的,比较烯醇硅烷醚结构中的硅氧键极性情况与烯醇 酯结构中的碳氧键极性。用电子转移式表示如下:
O SiMe3
O OC
Me
烯醇硅醚中碳碳双键上的电子云密度要高于烯醇酯的双键碳。
6.试从反应机理出发解释酮羰基的 α-卤代在酸催化下一般只能进行一卤代,而在碱催化时
由于磺酰氯及其酯的活性较大磺酰化和卤置换反应均在较温和的条件下进行常比卤素交换更有效收率更高另外还可以避免醇羟基直接卤置换反应中可能产生的副反应如碳正离子重排等
药物合成反应 (第三版 闻韧) 课后答案Chapter 1 Halogenation Reaction
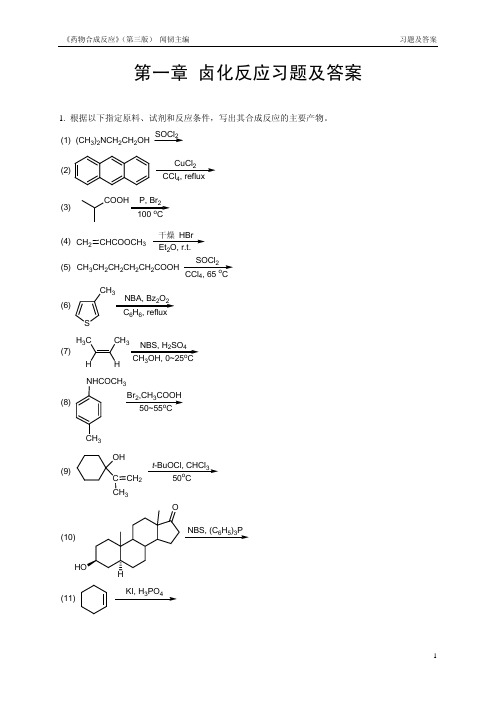
第一章 卤化反应习题及答案1. 根据以下指定原料、试剂和反应条件,写出其合成反应的主要产物。
(1)(CH 3)2NCH 2CH 2OHSOCl 2(2)CuCl 24(3)P, Br 2o(4)CH 2CHCOOCH 3干燥 HBr 2 (5)CH 3CH 2CH 2CH 2CH 2COOHSOCl 24o(6)S CH 32266(7)H 3C CH 3NBS, H 2SO 43o(8)NHCOCH 3CH 3Br 2,CH 3COOH o(9)OHC CH 3CH 2t -BuOCl, CHCl 3o(10)NBS, (C 6H 5)3P(11)KI, H 3PO 4(12)C 6H 5H 3Br 2,Cl 4o (13)CH 3CH CH CH 3232(14)(CH 3)3CCH 2OHHBr(15)OOP2(16)NBS, Et 3N ·3HF 22o(17)OHBr 24o(18)O23o2. 在下列指定原料和产物的反应式中分别填入必需的化学试剂(或反应物)和反应条件。
(1)CH 3CH 2CH 2CH 2CH CHCH 3CH 3CH 2CH 2CHCHCHCH 3Br(2)COOHBr(3)(4)OHBr(5)2CH 2BrBr(6)(7)(CH 3)3CCH 2OH(CH 3)3CCH 2Br(8)OOBocHNO OBocHNBr(9)OOBr OO BrBr2. 在下列指定原料和产物的反应式中分别填入必需的化学试剂(或反应物)和反应条件。
(参考答案)题号答案注释(1) NBS/(PhCO)2O, CCl4, △(2) Br2/HgO/tetrachloroethaneNaNO2, HCl, H2O; 2. HPF6; 3. △ (168℃)(3) 1.(4) Ph3P, Br2, CH3CN, △ (200-340℃)(5) NBS/(PhCO)2O, CCl4refluxing (10min. )acetone,(6) NaI,(7) Bu3P, Br2, DMF(8) NBS/hv, CCl4(9) NBS/(PhCO)2O, CCl4, reflux3. 阅读(翻译)以下有关反应操作的原文,请在理解基础上写出:(1)此反应的完整反应式(原料、试剂和主要反应条件);(2)此反应的反应机理(历程)。
南开大学智慧树知到“药学”《药物合成反应》网课测试题答案1

南开大学智慧树知到“药学”《药物合成反应》网课测试题答案(图片大小可自由调整)第1卷一.综合考核(共15题)1.醛与氢氰酸反应生成α-羟基腈的反应机理是()。
A.亲核反应B.亲电反应C.亲核加成D.亲电加成2.羧酸衍生物的氨解比水解和醇解更容易进行。
()A.错误B.正确3.合成格式试剂常用的溶剂是()。
A.醇B.醚C.酯D.石油醚4.羧酸和下列哪些化合物直接作用可以制得活性酰胺类酰化剂?()A.CDIB.噻唑琳酮C.噁唑酮D.苯并三氮唑5.醇的烃化反应中,下面的卤代烃,哪个活性最高?()A.CH3IB.CH3BrC.CH3ClD.CH3F6.最易和间苯二酚发生反应的是()。
A.CH3CNB.PhCNC.ClCH2CND.Cl3CCN7.乙烯型卤代烃和苯型卤代烃中的卤原子的反应活性很大,容易被亲核试剂所取代。
()A.错误B.正确8.下列不能与硝酸银的氨溶液反应的是?()A.丙炔B.丙烯C.2-戊炔D.2-戊烯9.以下哪几个化合物能作为烃化剂?()A.硫酸二甲酯B.重氮甲烷C.氯仿D.盐酸10.在醇的消除反应和亲核取代反应中,羟基是离去基团。
()A.错误B.正确11.通过Hofmann重排,可以用来制备什么类型的化合物?()A.酰胺B.伯胺C.烯酮D.酯12.醛在稀碱溶液中都可发生羟醛缩合反应。
()A.错误B.正确13.利用卢卡斯试剂可以区别分子中含6个或6个以下碳原子的伯醇、仲醇和叔醇。
()A.错误B.正确14.有利于Diels-Alder反应的因素有哪些?()A.亲二烯连有吸电子基团B.共轭二烯烃具有给电子基团C.亲二烯连有给电子基团D.共轭二烯烃具有吸电子基团15.下列哪个反应可以得到四氢异喹啉?()A.Aldol反应B.Grignard反应C.Blanc反应D.Pictet-Spengler反应第2卷一.综合考核(共15题)1.阿司匹林(乙酰水杨酸)是水杨酸的衍生物,所以可与FeCl3溶液发生显色反应。
药物合成反应(第三版)第一章课后翻译

About 216–224 g. (1.62–1.68 moles) of powdered anhydrous aluminum chloride is added to a 1Lthree-necked flask.在1L的三口烧瓶中加入大约216-224g(1.62–1.68 moles)的无水三氯化铝。
While the free-flowing catalyst is stirred (Note 3), 81 g. (0.67 mole) of acetophenone is added from the dropping funnel in a slow stream over a period of 20–30 minutes.自由流动的催化剂边搅拌边用滴液漏斗缓慢滴加81g苯乙酰。
Considerable heat is evolved, and, if the drops of ketone are not dispersed, darkening or charring occurs. 放热反应,假如滴加的酮不能被分散,就会变黑或是碳化。
When about one-third of the acetophenone has been added, the mixture becomes a viscous ball-like mass that is difficult to stir.当三分之一的乙酰苯被滴加,反应混合物变成一个很难搅拌的粘性的球状团块。
Turning of the stirrer by hand or more rapid addition of ketone is necessary at this point. 在这时,改用手动搅拌或快速滴加酮是非常必要的。
The addition of ketone, however, should not be so rapid as to produce a temperature above 180°. 然而,速度不能太快,当反应温度超过180℃时。
药物合成习题答案
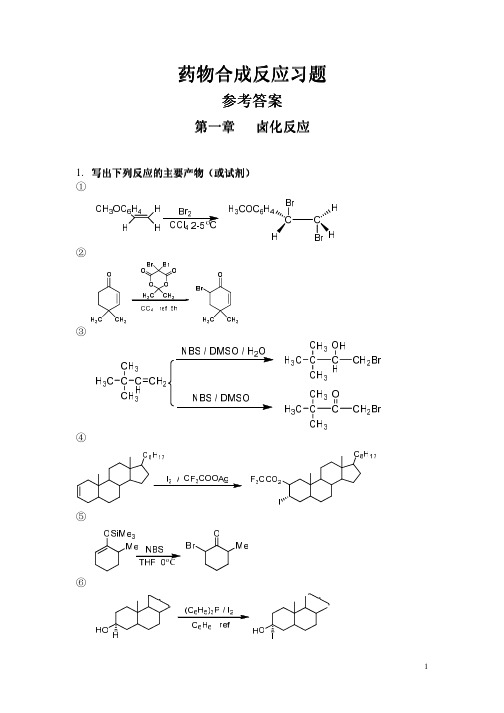
C2H5ONa
(COOC2H5)2CH(CH2)6CH(COOC2H5)2 Na, NH3 PhCH3 (CH2)2 HO O O (CH2)2
C2H5OOC(CH2)8COOC2H5
O
O
②试由
O
OCH3 合成
。
OH 0.25 NaBH4 OCH3 H2O H+ OCH3
OH H+ O O
H COOEt CH2CH 2COOH
HO
2.完成下列合成过程 (1)
O CHO CN PCC NaOAc O O O NaOH H3O O O COOH OH
O
(2)
CH3 O CH2OH + CH3 CHO O OC2H5 O O CH3 Claisen
Cope O
CH3 CHO
H2, Pd-C O
CHO CH3
(3)
C2H5 CH3 OCH3 OCH3 C2H5 O CO2CH3 + H3CO2C CH3 CH3 Claisen Rearrangement OH 2,4-O2NC6H5OH Tol C2H5 CH3 CO2CH3 O
药物合成反应习题
参考答案
第一章 卤化反应
1. 写出下列反应的主要产物( 写出下列反应的主要产物(或试剂) 或试剂) ①
②
③
④
⑤
⑥
1
⑦
⑧
⑨
⑩
2.写出下列反应的可能产物
①
②
2
③
④
3.
①
写出下列反应的主要试剂及条件
②
③ቤተ መጻሕፍቲ ባይዱ
④
第二章 1.
① 完成下列反应
烃化反应
药物合成反应习题及答案

药物合成反应习题及答案药物合成反应习题及答案⼀、举例解释下列概念:1,官能团保护;为什么保护?当分⼦中有多个官能团,想在某⼀官能团进⾏转换反应,为了不使其他官能团影响反应,需对这些官能团进⾏衍⽣化,这就是官能团的保护。
达到反应⽬的后再还原这些官能团。
理想保护基:试剂易得、⽆毒,保护基稳定,引⼊和脱去反应选择性好,收率⾼。
2,相转移催化剂; ⼀种与⽔相中负离⼦结合的两性物质,可以把亲核试剂转移到有机相进⾏亲核反应。
相转移催化剂优点:克服溶剂化作⽤;不需⽆⽔操作;可⽤⽆机碱代替有机⾦属碱;降低反应温度。
3,重排反应;重排反应是指在同⼀分⼦内,某⼀原⼦或基团从⼀个原⼦迁移⾄另⼀原⼦⽽形成新分⼦的反应。
按反应机理可分为亲电重排、亲核重排、⾃由基重排和协同重排。
4,合成⼦;合成⼦:组成靶分⼦或中间体⾻架的各个单元结构的活性形式. 包括:离⼦合成⼦、⾃由基或周环反应所需的中性分⼦。
离⼦合成⼦:包括 d 合成⼦和 a 合成⼦d 合成⼦: 亲核性的离⼦合成⼦ d---donor of electrond 合成⼦CN KCNCH 2-CHO CH3CHOMeSHMeS RCH C RCH CHBu BuLi等价试剂a 合成⼦:氧化性或亲电性的离⼦合成⼦ a 合成⼦:Me 2C-OH Me 2C=OCH 2COCH 3BrCH 2COCH 3CH 2-CH-COOR CH 2=CH-COOR等价试剂5,协同反应协同反应:在反应过程中,若有两个或两个以上的化学键破裂和形成时,都必须相互协调地在同⼀步骤中完成。
6, ⾮均相催化氢化: 催化剂、反应物、试剂和氢供体在两项或多项中反应,催化剂⾃成⼀相,称为⾮均相催化氢化。
催化剂⾃成⼀相称为⾮均相催化剂如Pd/C 为催化剂,氢⽓为氢供体,在反应液中还原双键的反应。
1) DMAP 2) DMF 3) DCC 4) TBAF1) Aromatic Electrophilic Substitution; 芳⾹亲电取代2) Phase-transfer catalyst; 相转移催化剂4) trifluoroacetic anhydride.三氟⼄酸酐Ryoji Noyori was awarded the Nobel Prize in 2001, What did he discover?Ryoji Noyori⽇本名古屋⼤学的野伊良治因在⼿性催化氢化反应⽅⾯做出了突出贡献⽽被授予2001年Nobel化学奖。
药物合成反应与设计翻译部分(优.选)

药物合成反应与设计翻译部分(第三版闻韧主编)第一章翻译:About 216–224 g. (1.62–1.68 moles) of powdered anhydrous aluminum chloride is added to a 1Lthree-necked flask.在1L的三口烧瓶中加入大约216-224g(1.62–1.68 moles)的无水三氯化铝。
While the free-flowing catalyst is stirred (Note 3), 81 g. (0.67 mole) of acetophenone is added from the dropping funnel in a slow stream over a period of 20–30 minutes. 自由流动的催化剂边搅拌边用滴液漏斗缓慢滴加81g苯乙酰。
Considerable heat is evolved, and, if the drops of ketone are not dispersed, darkening or charring occurs. 放热反应,假如滴加的酮不能被分散,就会变黑或是碳化。
When about one-third of the acetophenone has been added, the mixture becomes a viscous ball-like mass that is difficult to stir.当三分之一的乙酰苯被滴加,反应混合物变成一个很难搅拌的粘性的球状团块。
Turning of the stirrer by hand or more rapid addition of ketone is necessary at this point. 在这时,改用手动搅拌或快速滴加酮是非常必要的。
The addition of ketone, however, should not be so rapid as to produce a temperature above 180°. 然而,速度不能太快,当反应温度超过180℃时。
《药物合成反应》第一章 卤化反应

精品课程
药物合成反应
材料与化学工程系
药物合成反应
医药工业与制药工程
• 医药行业是按国际标准划分的15类国际化 产业之一,被称为“永不衰落的朝阳产业”
• 包括医药工业和医药商业
• 医药工业按原材料来分,又可分为化学制 药业、中药业、生物制药业及医疗器械业
药物合成反应
医药工业发展前景
• 随着社会的的现代化发展要求,对药的品 种、质量、效果等相应地提出了越来越高 的要求
• 随着中国步入老龄化社会,对医药的需求 带来相当大的机会
• 医药产业已成为世界经济强国竞争的焦点, 世界上许多国家都把建立制药学科视为国 家强盛的现状
• 中国医药工业的现状
药物合成反应
一 不饱和烃卤加成反应 1.卤素对烯烃的加成
(加成)
药物合成反应
F2:加成反应激烈,副产物多,实用性小; I2:C-I键不稳定,易消除,不实用; Cl2和Br2常用,重要,资源丰富,且活泼
程度适中,反应相对易控制; Cl2来自于氯碱工业,Br2来自于海洋。
药物合成反应
• (2)反应机理(两种机理,形成两类产品) • 以anti为主,但比例影响因素较多
• 在1999年制药工程专业招生时,全国共有 34所高等院校设置制药工程专业,其中医 药类院校13所,理工类院校12所,综合性 大学9所,招生人数为1165人。
• 2004年5月,国内已有121所高校设置了制 药工程专业,
药物合成反应
分布状况
• 制药工程专业在各省市的分布不是十分均衡的
• 最多的江苏省有10所以上的高校设置了制药工程 专业,但是全国却有近1/5的省、自治区则没有设 置该专业。
药物合成反应(第三版)

Br
Br
Br
Br
(选 择 性 溴 化 试 剂 )
第三节 羰基化合物的卤代反应
一、醛酮α-位氢的卤代反应
选择性溴化剂
O CH3 O Br Br O O Br CH3 CH3 CH3 O CH-Ar Br O CH-Ar
第一节 不饱和烃的卤加成反应
• 加卤素
C C X2 C X C X
概述
X2=Cl2, Br2
• 加卤化氢
C C HX C H C X HX=HCl, HBr, HI
无过氧化物 CH3CH=CH2
CH3CHBrCH3
Markovnikov加 成 反Markovnikov加 成
过氧化物
CH3CH2CH2Br
一、醛酮α-位氢的卤代反应
1,3二羰基化合物
O O H3C C CHCCH3 Cl
O
O
H3C C CH2CCH3
CF3SO2Cl Et3N
第三节 羰基化合物的卤代反应
一、醛酮α-位氢的卤代反应
α-羰基自由基取代
O
游离基反应促进剂 选择性地对烷基取代较多的α -H进行溴代
O Br C R''' R''
E π-络 合 物
X+-X-
H X -H+
σ-络 合 物
CH3 CH3 Cl + CH3 2mol Cl CH3 Br2 /Fe Br + Br CH3 Cl Cl
X
H
CH3
Cl2 /Fe
例
+
第二节 烃类的卤代反应
二、芳烃卤代反应(亲电取代)
OH H2O 3Br2 OH H2O 2Br2 Br
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
第一章 卤化反应习题及答案1. 根据以下指定原料、试剂和反应条件,写出其合成反应的主要产物。
(1)(CH 3)2NCH 2CH 2OHSOCl 2(2)CuCl 24(3)P, Br 2o(4)CH 2CHCOOCH 3干燥 HBr 2 (5)CH 3CH 2CH 2CH 2CH 2COOHSOCl 24o(6)S CH 32266(7)H 3C CH 3NBS, H 2SO 43o(8)NHCOCH 3CH 3Br 2,CH 3COOH o(9)OHC CH 3CH 2t -BuOCl, CHCl 3o(10)NBS, (C 6H 5)3P(11)KI, H 3PO 4(12)C 6H 5H 3Br 2,Cl 4o (13)CH 3CH CH CH 3232(14)(CH 3)3CCH 2OHHBr(15)OOP2(16)NBS, Et 3N ·3HF 22o(17)OHBr 24o(18)O23o2. 在下列指定原料和产物的反应式中分别填入必需的化学试剂(或反应物)和反应条件。
(1)CH 3CH 2CH 2CH 2CH CHCH 3CH 3CH 2CH 2CHCHCHCH 3Br(2)COOHBr(3)(4)OHBr(5)2CH 2BrBr(6)(7)(CH 3)3CCH 2OH(CH 3)3CCH 2Br(8)OOBocHNO OBocHNBr(9)OOBr OO BrBr2. 在下列指定原料和产物的反应式中分别填入必需的化学试剂(或反应物)和反应条件。
(参考答案)题号答案注释(1) NBS/(PhCO)2O, CCl4, △(2) Br2/HgO/tetrachloroethaneNaNO2, HCl, H2O; 2. HPF6; 3. △ (168℃)(3) 1.(4) Ph3P, Br2, CH3CN, △ (200-340℃)(5) NBS/(PhCO)2O, CCl4refluxing (10min. )acetone,(6) NaI,(7) Bu3P, Br2, DMF(8) NBS/hv, CCl4(9) NBS/(PhCO)2O, CCl4, reflux3. 阅读(翻译)以下有关反应操作的原文,请在理解基础上写出:(1)此反应的完整反应式(原料、试剂和主要反应条件);(2)此反应的反应机理(历程)。
The apparatus consists of a 1-l. three-necked flask equipped with a condenser, a dropping funnel, anda stirrer terminating in a stiff, crescent-shaped Teflon polytetrafluoroethylene paddle. The stirrer motor must have good torque (Note 1). The assembled apparatus, which is protected from moisture by means of drying tubes in the condenser and funnel, is preferably predried. About 216–224 g. (1.62–1.68 moles) of powdered anhydrous aluminum chloride is added to the apparatus with as little exposure to the moisture of the air as possible (Note 2). While the free-flowing catalyst is stirred (Note 3), 81 g. (0.67 mole) of acetophenone is added from the dropping funnel in a slow stream over a period of 20–30 minutes. Considerable heat is evolved, and, if the drops of ketone are not dispersed, darkening or charring occurs. When about one-third of the acetophenone has been added, the mixture becomes a viscous ball-like mass that is difficult to stir. Turning of the stirrer by hand or more rapid addition of ketone is necessary at this point. The addition of ketone, however, should not be so rapid as to produce a temperature above 180°. Near the end of the addition, the mass becomes molten and can be stirred easily without being either heatedor cooled. The molten mass, in which the acetophenone is complexed with aluminum chloride, ranges in color from tan to brown.Bromine (128 g., 0.80 mole) is added dropwise to the well-stirred mixture over a period of 40 minutes (Note 4). After all the bromine has been added, the molten mixture is stirred at 80–85° on a steam bath for1 hour, or until it solidifies if that happens first (Note 5). The complex is added in portions to a well-stirred mixture of 1.3 l. of cracked ice and 100 ml. of concentrated hydrochloric acid in a 2-l. beaker (Note 6). Partof the cold aqueous layer is added to the reaction flask to decompose whatever part of the reaction mixture remains there, and the resulting mixture is added to the beaker. The dark oil that settles out is extracted from the mixture with four 150-ml. portions of ether. The extracts are combined, washed consecutively with 100 ml. of water and 100 ml. of 5% aqueous sodium bicarbonate solution, dried with anhydrous sodium sulfate, and transferred to a short-necked distillation flask. The ether is removed by distillation at atmospheric pressure, and crude 3-bromoacetophenone is stripped from a few grams of heavy dark residueby distillation at reduced pressure. The colorless distillate is carefully fractionated in a column 20 cm. long and 1.5 cm. in diameter that is filled with Carborundum or Heli-Pak filling. The combined middle fractionsof constant refractive index are taken as 3-bromoacetophenone; weight, 94–100 g. (70–75%); b.p. 75–76°/0.5 mm.; n D25 1.5738–1.5742; m.p. 7–8° (Note 7) and (Note 8).3. 阅读(翻译)以下有关反应操作的原文,请在理解基础上写出:(1)此反应的完整反应式(原料、试剂和主要反应条件);(2)此反应的反应机理(历程)。
(参考答案)(翻译略)反应式:反应机理:亲电取代(略)。
