NMR Studies of Cathode Materials for Lithium-Ion Rechargeable Batteries
锂离子电池中重要正极材料体系的磁共振研究进展

第8卷 第6期2019年11月储能科学与技术Energy Storage Science and Technology V ol.8 No.6Nov. 2019收稿日期:2019-08-22; 修改稿日期:2019-08-24。
基金项目:国家自然科学基金项目(21872055)。
第一作者:耿福山(1992—),男,博士研究生,研究方向为磁共振与锂电池失效分析与测试技术专刊锂离子电池中重要正极材料体系的磁共振研究进展耿福山,胡炳文(华东师范大学物理与电子科学学院 & 上海市磁共振重点实验室,上海 200062)摘 要:锂离子电池得到了快速发展,并改变了我们的生活。
锂离子电池正极材料的研究是提高电池性能的关键;而理解正极材料的性能与结构之间的关系、阐释正极材料的电化学反应机理(尤其是性能衰减与失效机理)有助于提高材料的能量密度和功率密度。
磁共振技术(含核磁共振和顺磁共振)在过去三十多年的研究中不断进步,逐渐成为研究正极材料构效关系的关键技术之一。
本文总结了几个重要的已经商业化的正极材料(LiCoO 2、NCA 、NMC 和LiFePO 4)的磁共振研究进展,展示了核磁共振、顺磁共振在正极材料构效关系研究中的重要作用;尤其值得一提的是原位技术的发展在电化学反应机理中逐渐显示出其重要性。
本文有助于了解磁共振技术在电池材料研究中的重要价值,并进一步推动磁共振技术的发展。
关键词:正极材料;核磁共振;顺磁共振doi :10.19799/ki.2095-4239.2019.0186中图分类号:O 641 文献标志码:A 文章编号:2095-4239(2019)06-1017-07Progress in magnetic resonance research of important cathode materials in lithium ion batteriesGENG Fushan , HU Bingwen(School of Physics and Electronic Science & Shanghai Key Laboratory of Magnetic Resonance, Shanghai 200062, China )Abstract: Lithium-ion batteries have grown rapidly and have changed our lives. The research on the cathode materials of lithium ion battery is the key to improve the performance of the battery. Therefore, understanding the relationship between the structure-performance relationship and explaining the electrochemical reaction mechanism (especially the performance degradation and failure mechanism) of the cathode materials can help to improve the energy density and power density of the materials. Magnetic Resonance techniques, including NMR (nuclear magnetic resonance) and EPR (electron paramagnetic resonance), has been continuously improved during the past three decades of material research, and has gradually become one of the key technologies for studying the structure-performance relationship of cathode materials. NMR could be used to study light elements commonly found in battery materials such as Li, Na, F, P, C, H and O, while EPR can be employed to study transition metals such as Co, Ni, Mn, Fe and V. This paper summarizes the progress of magnetic resonance research on several important commercial cathode materials (LiCoO 2, NCA, NMC and LiFePO 4), and demonstrates the important role of NMR and EPR in the study of structure-performance relationship of cathode materials. It is emphasized here that the development of in-situ technology has gradually shown its importance to investigate the electrochemical reaction mechanism. This article will help to understand the important value of magnetic resonance technology in battery 电池,E-mail :545205908@ ;联系人:胡炳文,研究员,从事磁共振与电池研究,E-mail :bwhu@ 。
磷酸铁锂离子电池正极材料

Abstract Research progress in recent years on the preparation, modification, how to control crystal size, relationship between structure and performance, and prospect of olivine-type lithium iron phosphate LiFePO4 cathode materials for the lithium-ion batteries was reviewed. Particle size and its distribution, ionic and electronic conductivity, and the content of Fe( ) have much effect on the performance of the samples. The use of inert gas, the addition of conductive dope, and the control of crystal size to gain nano-powder are the useful way to improve the electrochemical performance of LiFePO4.
关键词 锂离子电池 正极材料 磷酸铁锂 改性
LiFePO4 Cathode Materials for Lithium-ion Batteries
Lü Zhengzhong, Zhou Zhentao
(College of Material Science and Engineering, South China University of Technology, Guangzhou 510641)
Na_xM_yFe_CN_6_M_省略_i_一类新颖的钠离子电池正极材料_钱江锋

近年来, 大容量锂离子电池被视为未来电动 汽车、 储能电站等大规模储能电池的主要选 择
[12 ]
, 其应用研究不断增加. 然而, 地球上的锂资
[3 ]
目前学术界大多持怀 源储量能否支持这类应用, 疑态度 . 即使在一定时期内, 锂的储量尚可维持 其高昂的材料成本也不利大规模应用. 应用需求, 因此, 发展资源丰富、 成本低廉的先进电池体系, 是解决未来大规模储电应用的必然出路 . 钠元素与锂同属一主族 , 化学性质相似 , 电极 电势也比较接近 , 且资源储量十分丰富 . 若能构建 性能良好的钠离子二次电池 , 从资源和环境方面 将比锂离子电池具有更大的应用潜力 . 但是 , 钠 离子的离子半径 ( 0. 102 nm) 较锂离子半径 ( 0. 076 nm) 大 30% , 其于固体晶格中通常较锂离子更稳 定, 因此使得寻找合适的钠离子嵌入电极材料十 分困难. 以嵌储钠正极材料为例, 迄今报道的含钠 过 渡 金 属 氧 化 物 ( 如 Na x CoO NaNi0. 5 Mn0. 5 O NaVPO4 F
· 109·
水溶液中, 即刻产生不溶性沉淀. 继续搅拌反应 6 h 后, 将沉淀产物离心分离, 洗涤烘干待用. 所得 普 鲁 士 蓝 类 产 物 分 别 标 记 为 NaFeFe ( CN ) 6 、 Na2 CoFe( CN) 6 和 Na2 NiFe( CN) 6 . 样品的晶体结构由 X射线粉末衍射法测定, 6000 型粉末衍射仪. 形貌表征 仪器为岛津 XRD使用 Sirion 2000 扫描电子显微镜测试. 样品中 Na、 Fe、 Co、 Ni 的元素含量由 X 射线荧光光谱定量标定 ( Bruker S4 PIONEER) . 正极膜电极制备 : 将 试 样 、 乙炔黑 ( 导电剂) 和 PTFE ( 粘结剂 ) 按质量比 70 ʒ 20 ʒ 10 称取, 混合 均匀后碾压成薄膜. 将此薄膜真空干燥后压在铝 网集流体上作为工作电极. 材料的充放电容量使 用 2032 型扣式电池测试, 扣式电池以上述膜片为 Celgard2400 型聚丙烯微孔 正极、 金属钠片为负极,
论文英文写作

论著(original articles)
综述(review)
会议摘要(meeting abstract) 评述类论文(comments) 读者来信(letters) 假说和观点类论文(hypothesis) 病例报道(case report)
SCI论文写作原则
试比较一下题名: a) Study of the solubility of polymers (聚合物的溶解性研 究) b) Study on the thermodynamic problem of polychlorotrifluoroethylene dissolution (聚氯三氟乙烯 的溶解热力学问题)
③ 陈述句式题名
由完整的句子组成,往往具有判断式的语意,即:使用一般 现在时在题名中提出结论,正文中却探讨性地论证。
④ 疑问句式题名
多用于评论性论文,使用探讨性的疑问句型显得比较生动, 激发读者兴趣。
例:
Dynamic capabilities: what are they? (动态能力:它们是 什么?)
SCI论文写作技巧
• 用词
熟悉、具体、简单、短句式
• 时态
过去时 现在时或现在完成时
• 词性
代词: is, this, these, those, that, which 冠词:a, an, the 动词:词性变化-ing, ed, en, d, t
• 标点
句号. 逗号, 括号(插入/附加) 所有格’ 连字符省略号…… 冒号:引号
Isolation of antigens from monkeys using complementfixation techniques (猴子使用补体固定技术分离的抗原) • 介词问题 ① “of”,“ for” 和 “in” 的使用 of——所有关系, for——目的、用途 例如: A design method of sliding mode robust controller with feed forward compensator is presented (提出了一种具有前馈补 偿的滑模鲁棒控制器设计方法)
层状Ni_Mn基锂离子电池正极材料进展

层状N-i Mn基锂离子电池正极材料进展¹叶尚云1*,张平伟2,乔芝郁1(1.北京科技大学物理化学系,北京100083; 2.个旧圣比和实业有限公司,云南个旧661000)摘要:层状N-i Mn基锂离子电池正极材料具有层状结构镍酸锂(LiNiO2)的高比容量以及尖晶石型结构锰酸锂(Li Mn2O4)的高安全性、低价格等特点,是最有可能代替或部分代替LiCoO2的新型正极材料用于小型锂离子电池,同时也可望用作低成本、高安全性和大容量动力型锂离子电池的正极材料。
本文综述了层状L-i N-i Mn-O系化合物和Li Ni1/3Mn1/3Co1/3O2的合成工艺、结构特点和电化学性能,阐述了层状N-i Mn基锂离子电池正极材料的发展、研究开发现状和应用前景。
关键词:层状结构N-i Mn基化合物;正极材料;锂离子电池中图分类号:TQ15文献标识码:A文章编号:0258-7076(2005)03-0328-11锂离子电池自1990年商业化应用以来,取得了飞速发展,已经垄断了便携式电子产品电源市场,并逐步向其它应用领域延伸,市场规模不断扩大,其应用已经超过N-i Cd电池和Ni M H电池。
随着锂离子电池的功率密度和安全性的改进和提高,未来将广泛应用于电动工具、储备电源、电动车等新兴领域,其市场前景广阔[1,2]。
尽管以LiCoO2为正极、炭材料为负极的锂离子电池相对以金属锂为负极的二次锂电池安全性有了很大程度的提高,小型锂离子电池的安全性得到了保障,但对于大容量和高功率动力型锂离子电池来说,成本和安全性仍是首要解决的核心问题。
由于LiCoO2成本高,耐过充性差,不适于用作动力型锂离子电池正极材料。
因此,开发具有低成本、安全性好并且环保的正极材料是高性能小型锂离子电池和大容量动力型锂离子电池发展的迫切要求。
尖晶石LiMn2O4、橄榄石结构的LiFePO4和新型层状N-i Mn基化合物Li N i1/3Mn1/3Co1/3O2由于热稳定性好、成本低而用于动力型锂离子电池目的,是近年来研究得最多的新型锂离子电池正极材料。
第八届全国大学生化学实验竞赛笔试题
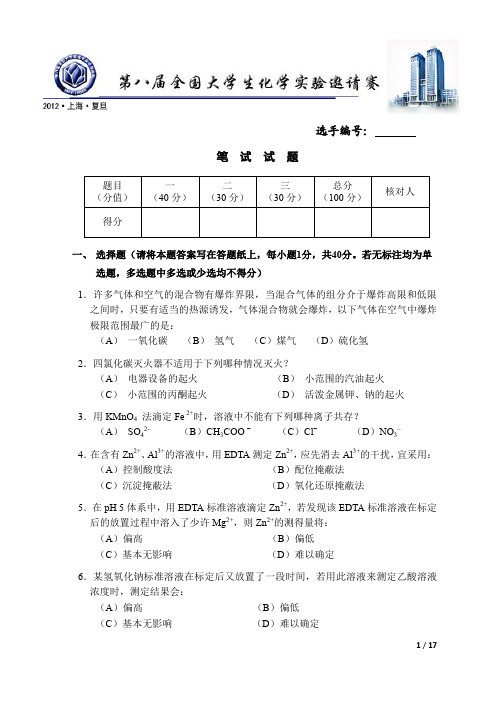
5.在 pH 5 体系中,用 EDTA 标准溶液滴定 Zn2+,若发现该 EDTA 标准溶液在标定 后的放置过程中溶入了少许 Mg2+,则 Zn2+的测得量将: (A)偏高 (C)基本无影响 (B)偏低 (D)难以确定
6.某氢氧化钠标准溶液在标定后又放置了一段时间,若用此溶液来测定乙酸溶液 浓度时,测定结果会: (A)偏高 (C)基本无影响 (B)偏低 (D)难以确定
图 1
a
b
c
25. 用 K2Cr2O7 溶液滴定 Fe2+溶液时,可选用的指示电极为: (A)硫酸根电极 (C)氟电极 (B)铂电极 (D)铅电极
26. 在电位滴定中,以2E/2V~V 绘制滴定曲线,滴定终点为: (A)2E/2V 为最正值时的点; (C)2E/2V 为零时的点; (B)2E/2V 为最负值时的点; (D)曲线的斜率为零时的点;
30.分析实验室对水质有较高要求,按照水的电阻率和总有机碳(TOC)指标,下 列水质最好的是: (A)电阻率 18 MΩ,TOC < 5 ppb (B)电阻率 18 MΩ,TOC > 5 ppb (C)电阻率 0.8 MΩ,TOC < 5 ppb (D)电阻率 0.8 MΩ,TOC > 5 ppb 31. 现需测定190-360 nm区域的分子吸收光谱图,可选用的光源为: (A)钨灯 (B)氘灯 (C)空心阴极灯 (D)低压汞灯
5 / 17 图 2
第八届全国大学生化学实验邀请赛
笔试试题
+
(C)C1极的电极反应式为 C2H5OH+3H2O-12e- 2CO2+12H
(D)该生物燃料电池的总反应式为 C2H5OH+3O2 2CO2+3H2O 36.1956年Van Deemter导出了著名的范氏方程,其简单形式为:
核磁共振波谱课程教学探索

山东化工SHANDONGCHEMICALINDUSTRY-158-2020年第49卷核磁共振波谱课程教学探索李晓虹(苏州大学材料与化学化工学部,江苏苏州215123)摘要:核磁共振波谱作为鉴定化合物结构、组分含量、动力学参数等信息的重要手段,在化学、医药、材料等领域科研生产中起着关键作用。
其课程教学长期以来受到理论内容难、仪器开放难等因素困扰’结合苏州大学核磁共振波谱课程的双语教学实践提出了相应的对策与改进举措,探讨通过更新改进教学方法和内容,突破传统教学模式,使学生从理论联系实践,从“会用”到“用好”核磁技术’关键词:核磁共振波谱;远程虚拟终端%网络课堂中图分类号:G642O文献标识码:B文章编号:1008-021X(2020)23-0158-02Exploration of Teaching in Nuclear Magnetic Resonance Spectroscopy CourseLi Xiaohong(Colleae of Chemist—,Chemicai Enginee/ng and Materials Science of Soochow University,Suzhou215123,China) Abstract:Nuclear magnetic resonance spectroscopy(NMR),as an Onportant method of studying compound structures, component contents and kinetic parameters,plays a key rolo in the fields of chemist—,pharmaceutical indust—and materials science.For a long time,its course teaching has been troubled by the dOficulta of theo—tical content and the lack of instmmentai peacicce.Based on ihebcocnguaoieachcngpeacicceooNMR couesecn Soochow Unceeesciy,ihcspapeedcscu s eshow iobeeak iheough iheieadciconaoieachcngmodebycmpeoecngiheieachcngmeihodsand conienis,soihaisiudeniscan combcneiheoeywcih peacicceand makegood useooNMRiechnooogy.Key wordt:NMR%VNC%online coa s es核磁共振波谱作为鉴定化合物结构的重要手段,对样品无损,分辨率高,较灵敏,可获得准确的定性定量信息。
高容量锂离子电池电极材料研究的新进展

250
200
150
100
50
0 0 2 4 6 8 10 12 14 16 18 20
Cycle number
Current density: 10mA/g (~C/16), Temperature:30 oC
Capacity (mAh/g)
The changes in Valence of Mn ions
Li2MnxFe1-xSiO4 332
235 (x=0.5)
Two lithium ions should be reversible extracted or inserted
in principle without distinctively changes of the crystal
structure.
Cycle Number
Voltage/V vs. Li+/Li
Li2Mn1xFexSiO4/C
5.0
4.5
4.0
3.5
3.0
2.5 a:x=0.9
b:x=0.7 2.0 c:x=0.5
1.5 d:x=0.2 e:x=0
1.0
0
50
ae b dc 100 150 200 250
Specific capacity/mAh/g
Gas evolution,
e.g. O2, CO2
~ 270mAh/g or even higher,
High capacity
cathode materials Charging to high voltage
In-situ Electrochemical Mass spectroscopic techniques and Its use in
原位固体NMR用于研究分子筛表面酸性

第20卷第3期催 化 学 报1999年5月Vol.20No.3Chinese Journal of Catalysis May1999原位固体NMR用于研究分子筛表面酸性刘宪春赵 琦刘秀梅王 毅李文丽韩秀文包信和(中国科学院大连化学物理研究所催化基础国家重点实验室,大连116023)提 要 描述了固体NMR样品原位处理、装样和密封的一体化装置,可用于催化剂样品预处理.利用该装置可进行样品的脱气、脱水、吸附探针分子及氧化还原等操作,还可以原位将处理后的样品转移到样品管中封存.通过两个实例展示了该装置的实际效果.(1)研究MgO改性的HY分子筛的1H MAS NMR谱发现,随着MgO担载量的变化分子筛表面的SiOH数量也相应改变,显示MgO与分子筛之间存在较强的相互作用.(2)利用较大的碱性有机胺分子吸附在分子筛外表面,研究HY分子筛外表面的酸性,发现其酸性主要来源于分子筛表面的SiOH.关键词 固体核磁共振,表面酸性,HY分子筛,吸附,原位试验分类号 O643实现固体核磁共振(NMR)的原位研究,从而将固体NMR技术应用到多相催化反应过程的研究是催化研究者梦寐以求的愿望,国内外科学家对此进行了富有成效的探索.Haw[1]和Hunger[2,3]等分别发展了自己的原位NMR技术,对很多催化反应进行了研究,取得了很好的结果.然而,原位NMR在固体粉末样品上的应用从技术角度较难实现.主要原因一是高分辨NMR仪器在很强的磁场下工作,对在其中工作的仪器部件要求极高;二是为了消除固体样品中特有的强相互作用,在进行固体NMR检测时,样品必须绕与磁场方向成54°44′角的轴作高速旋转(即MAS技术).以上两点极大地限制了原位NMR方法的应用.用固体NMR方法研究催化剂时,经常需要对样品进行脱水和脱气处理,处理后的样品应在不暴露大气的情况下转移到固体NMR转子中.该过程通常是采用玻璃封管的方法实现,即将样品放于小玻璃管中,进行必要的处理后将小安瓿密封,再将小安瓿装入转子进行NMR 检测.由于转子要在1~15kHz转速下高速旋转,所以需要对称性很高的安瓿及高超的封管技术,并且无法再次进行吸附,限制了其在催化中的应用.为克服上述缺点我们试制了一种装置[4],可以很方便地对样品进行各种处理,包括抽空、焙烧、氧化还原、吸附以及样品的密封等.本文详细介绍了该装置,并结合纳米分子筛的粒度大小对分子筛结构和酸性的影响以及改性HY分子筛上酸性变化等进行了研究,给出了该装置的实际应用效果.1 实验部分1.1 反应器结构 图1为样品处理、装样和密封的一体化实验装置示意图.该装置的主体包括石英反应器和样品捣杆与波纹管连接形成的机械手.机械手通过O圈与石英反应器之间密封.机械手的作用是模仿固体核磁实验的装样方法,将经过处理的样品在保证与原处理条件一致的环境下装于转子中,密封之后再将转子转移到探头中完成NMR实验.实验结果显示该反应器具有良好的密封效果,样品的脱水、脱气,在线的氧化、还原处理或保护气氛下的处理等操作均可在该装置上完成.当样品吸附各种碱性探针分子则可用于分子筛等的酸性表征,常用的碱有吡啶、全氟丁胺和三甲基膦等.将该反应器连接在真空线上,系统的真空度可以达到10-3Pa.密封塞可根据不同的要求选择不同的材料,一般可以选择橡收稿日期:1998211225.第一作者:刘宪春,男,1961年生,助理研究员.联系人:包信和.Tel:(0411)46719912551;Fax:(0411)4694447;E2mail:xhbao@.图1 固体样品处理装样和密封装置示意图Fig 1 Schematic diagram of the treating ,loading and sealing apparatus for solid samples 1O 2ring ,2Bellows ,3Sample cell ,4Tamper ,5Ro 2tor rack ,6Plug rack ,7,9,11,12Vacuum cock ,8Quartz tube ,10Adsorbate胶、四氟化物等材料.对于有些实验甚至可以不加密封塞,但转子的盖子要塞紧,同时样品的转移需要在惰性气体保护下进行.实验表明在不用密封胶塞的情况下,测试HY 分子筛样品的1H MAS NMR 谱,一天以后没有变化.用硅橡胶塞密封的样品,在测试SAPO 244分子筛的样品时,NMR 信号四天以后仍然没有变化.用该方法装填的样品可以达到理想的旋转效果.在实验中用了12kHz 的转速,可以达到探头的设计转速.1.2 样品处理 (1)将改性的HY 分子筛样品装入石英反应器中,在真空中缓慢加热到400℃,在10-2Pa 下抽空20h ,脱水后的样品原位装入转子并密封,用于1H MAS NMR 检测.(2)HY 分子筛样品在真空中缓慢加热到400℃,在10-2Pa 下抽空20h ,然后将样品温度降至室温,在全氟丁胺(C 4F 9)3N 的饱和蒸气压下吸附40min ,在线装样后进行1H MAS NMR 检测.全氟丁胺为分析纯试剂.1.3 NMR 实验 NMR 实验是在Bruker DRX 2400型核磁共振谱仪上完成.采用固体DB MAS 探头,4mm ZrO 2转子.13C 核共振频率为10016MHz ,转子转速为4kHz ;1H 核共振频率为400MHz ,转子转速为8kHz.化学位移参考四甲基硅(TMS ).1H →13C CP/MAS (交叉极化和魔角旋转)NMR 脉冲序列中接的触时间和弛豫延迟分别为2ms 和2s.2 结果与讨论2.1 改性H Y 分子筛的1H MAS NMR 研究 HY 分子筛经MgO 改性后表面酸性和骨架结构都有很大的变化.图2示出了经MgO 改性的HY 分子筛的1H MAS NMR 谱,对其进行分峰拟合求得各个羟基的数量,结果显示:随着MgO 含量的增加,HY 分子筛超笼中的B 酸量(δ=317)和方钠石笼中的B 酸量(δ=414)明显减少.当HY 担载1%MgO 时,分子筛中SiOH (δ=1167)的量比未担载MgO 的HY 分子筛的SiOH 的量减少许多,这是由于担载的MgO 覆盖在分子筛表面,使得分子筛表面的SiOH 数量减少.而随着MgO 担载量的逐渐增大,分子筛表面的SiOH 数量反而逐渐增多,这表明MgO 与分子筛骨架存在相互作用,使得Si —O —Si 键或Si —O —Al 键断裂,在分子筛骨架中造成了缺陷位,从而形成了SiOH.2.2 碱吸附实验研究H Y 分子筛外表面的酸性 由于全氟丁胺具有较大的分子直径(0194nm ),无法进入HY 分子筛的笼中(孔口最大直径为0174nm )[5],用全氟丁胺吸附在HY 分子筛的外表面上可以研究HY 分子筛外表面的酸中心.由于(C 4F 9)3N 碱性较弱,通常它与羟基上的质子只形成氢键.图3所示为吸附(C 4F 9)3N 前后的HY 分子筛的1H MAS NMR 谱.吸附(C 4F 9)3N 后,代表硅羟基(δ=1167)的峰强度比吸附前略有降低,这可能是因为位于HY 分子筛外表面的SiOH 与(C 4F 9)3N 相互作用,氟原子与质子产生偶极相互作用,以至于峰展宽而不可见所引起的.吸附(C 4F 9)3N 后,在低场出现两个较尖锐的小峰,在高场接近δ=0处也002催 化 学 报20卷图2 MgO/H Y 分子筛脱水后的1H MAS NMR 谱Fig 2 1H MAS NMR spectra of dehydratedMgO/HY zeolites with differentMgOloading 图3 脱水H Y 吸附全氟丁胺前后的1H MAS NMR 谱Fig 3 1H MAS NMR spectra of dehydrated HY zeolite (1)and after perfluorobutylamine adsorption (2)发现了尖锐的小峰,这几个小峰的归属有待进一步考察.参考文献1 Haw J F ,Richardson B R ,Oshiro I S et al.J A m Chem Soc ,1989,111(6):20522 Hunger M ,Horvath T ,Weitkamp J.S tud S urf Sci Catal ,1996,105:8533 Hunger M ,Horvath T.J Catal ,1997,167:1874 刘宪春,赵琦,包信和.CN98238330.4,19985 徐如人,庞文琴,屠昆岗.沸石分子筛的结构与合成.吉林:吉林大学出版社,1987.7IN 2SITU SOL ID 2STATE NMR STU DY ON SURFACE ACIDIT Y OF ZEOL ITESLiu Xianchun ,Zhao Qi ,Liu Xiumei ,Wang Y i ,Li Wenli ,Han Xiuwen ,Bao Xinhe(S tate Key L aboratory of Catalysis ,Dalian Institute of Chemical Physics ,The Chinese Academy of Science ,Dalian 116023)Abstract An integrative apparatus made on our own employed in treating ,loading and sealing of samples that can be measured in solid 2state NMR is described.A series of pre 2treatment can be performed in the apparatus ,such as degasification ,dehydra 2tion ,adsorption and redox.Then ,the treated sample can be transferred i n sit u into the rotor and sealed.The following two experiments were carried out in it.(1)Acid 2ity on the surface of H Y zeolites modified by MgO was investigated by 1H MAS NMR.It is found that the amount of SiOH on the surface changes with that of MgO loading in H Y zeolites ,which shows that there is a strong interaction between MgO and H Y zeolites.(2)The observation of 1H MAS NMR spectra of H Y with adsorbed (C 4F 9)3N on the external surface shows that the acidity on external surface comes mainly from SiOH on the external surface of H Y zeolites.K ey w ords solid 2state NMR ,surface acidity ,H Y zeolite ,adsorption ,in situ test(Ed L YX )1023期刘宪春等:原位固体NMR 用于研究分子筛表面酸性。
Raman and NMR studies of aged LiFePO4 cathode

Applied Surface Science 259 (2012) 49–54Contents lists available at SciVerse ScienceDirectApplied SurfaceSciencej o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /a p s u scRaman and NMR studies of aged LiFePO 4cathodeShrikant C.Nagpure a ,Bharat Bhushan a ,∗,S.S.Babu ba Nanoprobe Laboratory for Bio-&Nanotechnology and Biomimetics (NLBB),Ohio State University,Columbus,OH 43210,USA bDepartment of Material Science and Engineering,Ohio State University,Columbus,OH 43210,USAa r t i c l ei n f oArticle history:Received 6April 2012Received in revised form 19June 2012Accepted 20June 2012Available online 28 June 2012Keywords:Lithium-ion batteries AgingCarbon-coatingRaman spectroscopy (RS)Magic angle spinning (MAS)nuclearmagnetic resonance (NMR)spectroscopya b s t r a c tThe carbon coated LiFePO 4nanoparticles are used in advanced lithium-ion batteries due to low cost,high energy and power density.In this paper Raman spectroscopy is used to analyze the degradation of carbon coating around these nanoparticles in several commercial cells aged with different C-rate.Magic angle spinning 7Li Nuclear magnetic resonance (NMR)spectroscopy is used to characterize these nanoparticles for the presence of Li.In Raman spectroscopy data,structural change in the carbon leading to low electrical conductivity is observed for the cells aged at higher C-rate.In NMR spectroscopy data,isotropic 7Li peak is observed in an unaged cell,while the similar peak is absent in the aged cells.© 2012 Elsevier B.V. All rights reserved.1.IntroductionSony commercialized the lithium-ion battery in 1991,and since this technology has been growing and gaining popularity at a rapid pace.The lithium-ion batteries were initially used in mobile phones,laptop computers,and digital cameras.But the advanced lithium-ion batteries have shown potential for application as an energy storage device for electric vehicles (EV),hybrid electric vehi-cles (HEV)and plug-in hybrid electric vehicles (PHEV).Lithium-ion batteries do not have memory effect and require minimal mainte-nance.Its energy density is in the range of 110–170Wh/kg,which is the highest among the other competing chemistries such as Lead-acid and nickel metal hydride.The cycling of these batter-ies causes an increase in the internal resistance (power fade)and a decrease of capacity (charge acceptance)[1].According to United States Advanced Battery Consortium (USABC),a 42V battery in a hybrid electric vehicle (HEV)should have a calendar life of 15years [2].Electric vehicles (EV)should have a battery system that can last for 10years [3].In terms of cycles,1000cycles at 80%depth-of-discharge are expected in EV [3],and 300,000cycles at 50Wh are expected in a plug-in HEV [4].Thus,to improve the calendar life and cycle life of the batteries,the research has been focused on understanding the aging mechanisms of these batteries.The degra-dation or “aging”is a result of several simultaneous physiochemical∗Corresponding author.E-mail address:bhushan.2@ (B.Bhushan).processes that occur within the electrode,electrode–electrolyte interface,and within the electrolyte [5].Among different cathode electrode material LiFePO 4have shown great potential because this cathode material has the highest capacity (160mAh/g)at moderate current densities [6].In addition,it is inexpensive and nontoxic,as compared to cobalt-oxide-based materials for large-scaled applications such as HEV.The perfor-mance of this material is improved when it is synthesized in form of nanoparticles.The nanosized particles provide better electronic contact,high surface area to volume ratio and better diffusion kinet-ics through decreased diffusion length [7–9,10,11].In our previous studies the degradation of these nanoparticle based LiFePO 4cathodes in commercial batteries has been studied through a systematic multi-scale characterization plan.The phys-ical/morphological changes in these cathodes have been studied at different length scales using thermography,scanning electron microscopy (SEM),atomic force microscopy (AFM)and high resolu-tion transmission electron microscopy (TEM)imaging [12,13].The change in functional properties such as surface resistance and sur-face potential has been studied with scanning spreading resistance microscopy and Kelvin probe microscopy [14,15].In this paper Raman spectroscopy (RS)is used to analyze the carbon coating on the LiFePO 4nanoparticles in several commer-cial cells aged with different C-rate.LiFePO 4particles have poor electronic conductivity.To increase the electronic conductivity,it is a common practice in the production of lithium-ion battery electrodes to add carbon,either by use of carbon additives to the LiFePO 4matrix or by surface coating of LiFePO 4particles with thin layers of carbon.The addition of carbon has then the advantage0169-4332/$–see front matter © 2012 Elsevier B.V. All rights reserved./10.1016/j.apsusc.2012.06.08050S.C.Nagpure et al./Applied Surface Science259 (2012) 49–54of improving the electronic conductivity of originally high capac-ity LiFePO4cathode material.In particular,a capacity of about 160mAh/g has been found for LiFePO4coated with1wt%carbon [11].Degradation in the quality of the carbon coating can lead to decreased electronic performance of the cathode.Also magic angle spinning(MAS)nuclear magnetic resonance(NMR)with7Li probe is used to probe the presence of lithium in the unaged and aged cells. As Li is vital in charging and discharging of the batteries it is neces-sary to understand its local environment within the host structure and any changes to the structure due to aging during cycling of the cells.Before we discuss our experiments and the results,a brief review of Raman and NMR studies related to lithium-ion batteries is provided in next section.2.Review of Raman and nuclear magnetic spectroscopy studies2.1.Raman spectroscopyRaman spectroscopy is a unique analytical tool for identifying and characterizing the elements within the sample.It is a non-destructive tool and requires very minimal sample preparation. The atmospheric CO2and H2O do not interfere with the Raman signal,so no special atmospheric conditions are necessary during the experiments.Several different researchers have used Raman spectroscopy to characterize different types of carbon in differ-ent forms such as crystalline,non-crystalline,amorphous carbons [16–19,20].Saito et al.[21]have used Raman spectroscopy to ana-lyze the single-wall carbon nanotubes.Recently Raman spectroscopy has been used to analyze the car-bon coating of LiFePO4nanoparticles used in lithium-ion batteries. The studies have been conducted to understand the effect of car-bon coating on the electrochemical performance of LiFePO4based lithium-ion batteries.In the studies done by Doeff et al.[22]they showed that the structure of the residual carbon present on the LiFePO4powder affects its electrochemical performance and the better performance is dependent on the quality of the carbon rather than the quantity of the carbon.Similarly,Cho et al.[23]have used RS for studying the effect of the coating thickness on the capac-ity of LiFePO4composite cathodes.Julien et al.[24]characterized the carbon-film coating on the LiFePO4nanoparticles and discussed the Li+diffusion through the carbon coating.Wilcox et al.[25]used RS to study the factors,in particular the synthetic additives,which can affect the quality of the carbon coatings.Also,the effects of the thermal treatments on the performance of the carbon coat-ing using RS have been studied by Maccario et al.[11].Raman spectroscopy is very useful in characterizing the carbon coating of LiFePO4nanoparticles for two main reasons.One,carbon is a strong scatterer with two E2g modes predicted to be Raman active [25]Second,the penetration depth of light inside the LiFePO4par-ticles is very small,thus the coating of carbon can be easily probed with Raman spectroscopy[24].2.2.Nuclear magnetic resonance spectroscopyIn lithium-ion batteries Li is the most important element as it is directly involved in the electrochemical process during the charg-ing and discharging cycles of batteries.As such understanding the Li concentration as well as the local crystallographic and electronic structure of Li within the host LiFePO4structure is critical to predict the performance of the lithium-ion batteries in terms of operating voltage,residual capacity,and rate capability.The common electron spectroscopy techniques fail to identify and characterize lithium within the sample.The energy dispersive spectroscopy(EDS)technique,which is commonly used to identify the atomic percent of the elements in the component,fails to detect Li.The EDS detectors are very sensi-tive to impurities.To avoid repeated exposure of the detectors to atmosphere,they are maintained under vacuum and are separated from the column vacuum in the electron microscope by beryllium window.The thin beryllium window on the EDS detector absorbs low energy X-rays and thus prevents the use of EDS in the detection of elements with an atomic number less thanfive.Electron energy loss spectroscopy(EELS)provides an indirect method to probe the lithium and its local environment within the sample.Nagpure et al.[26]have used electron energy loss spectroscopy(EELS)to probe the local electronic structure of the LiFePO4nanoparticles in unaged and aged samples.The EELS study showed the change in the density of states of O in the aged LiFePO4 cathode sample and the subsequent loss of Li from the host LiFePO4 structure.X-ray diffraction techniques are useful in identifying the phases of the LiFePO4material present in the cathode and long-range structural data.However,it lacks the ability to provide local crys-tallographic and electronic structure of lithium within the sample.As such neutron based techniques such as neutron imaging,neu-tron depth profiling(NDP)or NMR etc.prove very vital in studying lithium within the sample as neutrons have high penetration power and detectors do not require any special protection from environ-ment[27,28].Neutron depth profiling(NDP)has been used to study Li content in the host structure[13].NDP studies are limited to the concentration in bulk cathode material.Among all these neutron techniques,NMR can play a vital complementary role to study cath-ode materials in lithium-ion batteries as it directly probes the local Li environment[29].Solid state NMR is extremely useful for studying the local struc-ture in ordered and disordered materials.Solid state Li NMR in particular is very useful due to its high sensitivity towards the atomic and electronic environment at the lithium site within the host cathode structure[30].NMR can distinguish between the metallic and semiconductor behavior of the materials.While prob-ing the local and electronic environment of the nuclear probe it can also monitor the electronic structure of the surrounding cations [29].In NMR spectroscopy it is also possible to quantify the species taking part in battery charging and discharging and monitor the effect on the local structural changes of these species as the function of the aging of the battery.Due to its sensitivity towards the local electronic structure it can also distinguish between diamagnetic and paramagnetic behavior of materials.Most of the materials used as cathode in lithium-ion batteries show paramagnetic behavior in charged and discharged state[31].The natural abundant7Li isotope(93%)has larger quadrupolar and gyromagnetic moments as compared to the less abundant6Li (7%)isotope.The quadrupolar interactions result from the inter-actions between the quadrupolar nucleus and the electricfield gradient at the nucleus.The quadrupolar interactions of6Li are smaller compared with7Li but they result in higher resolution spec-trum that is easier to interpret[31].Thus the coupling between the lithium nucleus and the unpaired electrons can be exploited in magic angle spinning(MAS)NMR to study the changes in the local electronic structures as the function of the battery aging in the paramagnetic cathode materials.Given these advantages of NMR it has been used by several researchers to study different types of cathode yered oxides such as LiCoO2,LiNiO2have been studied by Dahn et al.[32],Marichal et al.[33],Levasseur et al.[34],and Carlier et al.[35], Carlier et al.[36].The spinel structured materials such as LiMn2O4 have been studied by Gee et al.[37],Lee et al.[38],Lee et al.[39], Lee and Grey[40],Morgan et al.[41],Tucker et al.[42],and Ver-hoeven et al.[43].The spinel structured materials such as LiFePO4S.C.Nagpure et al./Applied Surface Science259 (2012) 49–5451have also been studied by Gaubicher et al.[44],Arrabito et al.[45], and Tucker et al.[42],Tucker et al.[30],Tucker et al.[46].The goal in these studies has been to understand and predict the effect of local and electronic structure on lithium NMR shift in these battery materials.In situ NMR studies using toroid detector with limited resolution have also been conducted by Gerald et al.[47].Cheval-lier et al.[48]have also shown that NMR signals from plastic bag batteries can be successfully obtained for analysis.3.Experimental details3.1.Sample preparationCommercial lithium ion cells used in these experiments have a graphite anode and a cathode comprising of LiFePO4nanoparti-cles(40–50nm).Graphite is bonded onto a copper substrate,and layers of LiFePO4nanoparticles are bonded onto an aluminum sub-strate using a polyvinylidene difluoride(PVDF)binder.The anode and cathode strips,with a separator in between,are rolled and then packed into a can to form a cylindrical cell.The electrolyte used in this cell is a lithium hexafluorophosphate(LiPF6)salt in1:1 ethylene carbonate and dimethyl carbonate.The cell has an oper-ating voltage of3.3V and a nominal discharge capacity of2.3Ah. LiFePO4has poor electronic conductivity( =2×10−9S cm−1)[49]. To improve the conductivity,the nanoparticles are coated with car-bon[50].Table1describes the condition of the cells used in this study.The effect of charging or discharging current rate(C-rate) on the lithium concentration was studied on cells#C1,C2,and C4 cycled from0%to10%state of charge(SOC)with a C-rate of1C,2C and4C,respectively(1C-rate=2.3Ah).Two cells were cycled with higher C-rates and at a higher SOC to study the effect of the SOC on the lithium concentration profiles.Cell C6was cycled from60% to70%SOC with6C rate.A cell that underwent only one complete charge-discharge cycle with1C-rate was established as the base-line cell in our studies(C0).The cycling of the cells was terminated when the cells reached∼80%of their rated capacity.This protocol was found to be consistent with the automotive industry standard, which considers a cell to be dead when its capacity drops below 80%of the original rating[4].The cells are completely discharged after they have reached the ∼80%of their rated capacity.The cylindrical cell was then opened in a glove boxfilled with Argon atmosphere.The oxygen level was maintained at∼88ppm and the dew point was∼−34◦C.The cell was unrolled,and the long anode and cathode strips were sepa-rated.The samples for RS and NMR were taken from the part of the strip that was near the center of the roll when packed in the cylindrical cell.3.2.Raman spectroscopyLabram®,an integrated confocal Raman microscope system, made by ISA Group Horiba was used to analyze the carbon-coating. Since the positions of the Raman bands are dependent on the wave-length of the incident laser,a He Ne laser with512nm excitation wavelength was used in these experiments for comparison of ourTable1Different samples used to study the lithium concentration profile.Sample Aging condition Residual capacityC0New–no aging100%C11C,0–10%SOC,55◦C∼80%C22C,0–10%SOC,55◦C∼80%C44C,0–10%SOC,55◦C∼80%C6∼6C,60–70%,SOC,45◦C∼80%SOC:state of charge.data with the published literature.The laser power was adjusted to2.5mW and the laser spot size was at∼5m.RS experiments were conducted under ambient conditions at room temperature. The data acquisition time was set at10s for all the samples.The commercial software package included in the Labram®system was used for background subtraction and baseline correction.3.3.NMR spectroscopyBruker DSX300MHz NMR spectrometer was used to probe the lithium in LiFePO4nanoparticles.A7mm triple resonance MAS probe was tuned to7Li frequency of38.9MHz.The shifts in the7Li were referenced with1M LiCl(aq)solution.The Bloch Decay exper-iment method was used with relaxation delay of10s and spin rate of10kHz.4.Results and discussion4.1.Raman spectroscopyFig.1shows a experimental(dotted line)andfitted(solid dark line)Raman spectra obtained on sample C0.The Raman spec-troscopy of a disordered carbon shows two distinct peaks.The peak at∼1600cm−1is referred to as G(G for graphite)peak and the peak at∼1350cm−1is referred to as D(D for disordered)peak.The G peak is attributed to the optically allowed E2g zone-center mode of crys-talline graphite while D peak is attributed to the disorder allowed zone-edge modes of graphite[18,24].The Raman spectra is deconvoluted according to these two char-acteristic D and G peaks.As can be seen in Fig.1the Raman spectra was satisfactorily deconvoluted with Breit–Wigner–Fano(BWF) plus Lorentzian scheme.A BWF line is used for the G peak and a Lorentzian line is used for the D peak.The BWF line shape is given byI(ω)=I0[1+2(ω−ω0)/Q ]21+[2(ω−ω0)/ ]2(1)Fig.1.Deconvolution method used forfitting of experimental Raman spectra.The method is demonstrated with the experimental Raman spectra(dots)for C0battery. The data is deconvoluted in two peaks(dotted line)and thenfitted(solid line).The D peak isfitted with Lorentzian and G peak isfitted with Breit–Wigner–Fano(BWF) line shape.52S.C.Nagpure et al./Applied Surface Science 259 (2012) 49–54Fig.2.Raman spectra of all the cells.The details of the aging cycle for the batteries are given in Table 1.Each spectra was fitted with the deconvolution method shown in Fig.1.where I 0is the peak intensity,ω0is the peak position, is assumed as the full width at half maximum (FWHM)and Q −1is the BWF coupling coefficient.Due to the coupling of a discrete mode to the continuum BWF line has an asymmetric line shape [20,51].A Lorentzian line shape given by Eq.(2),which belongs to the same family as BWF is used for the D peak:L =I 0(ω−ω0)2+ 2(2)The deconvolution of the Raman spectra based on BWF +Lorentzian scheme is shown for the data obtained for sample C0in Fig.1.Fig.2shows the fitted Raman spectra for all the samples using the deconvolution method described above.The deconvolution procedure satisfactorily fits the experimental Raman spectra for all the samples.The two characteristic D and G peaks are observed in all the samples.Note that the positions of the Raman bands are dependent on the incident laser wavelength,so for quantitative comparison between spectra given here,we have maintained the excitation wavelength of 512nm.The data shows a uniform coating of carbon on the LiFePO4particles with highly disordered carbon.The relative intensities of the D and G band are associated with the disorder in carbon structure similar to disorder in microcrystalline graphite [22,52].According to Doeff et al.[22]the electrochemical performance of carbon coated LiFePO 4cathode is not only dependent on the quan-tity of the carbon but also on the quality of carbon.The quality of the carbon coating in the different samples is compared by com-paring the intensity ratios of D and G band (I D /I G ).Usually a lower I D /I G ratios are desired in a good quality LiFePO 4cathode.As can be seen in Fig.3,I D /I G ratio increases with increasing C-rate.Accord-ing to Tuinstra and Koenig [53]the in-plane correlation length L a is related to the I D /I G ratio,which quantifies the mean basal-plane diameter of graphite parallel to (001)[11].The following modified Tuinstra–Koenig relation gives the value of L a ,L a =C ( L )ID /I G(3)where C ( L )is a variable scaling coefficient depending on excitation wavelength L and given byC ( L )=C 0+ L C 1(4)Fig.3.Intensity ratio analyses (I D /I G ).The intensity is calculated as the area under the respective curve.The intensity increases with the C-rate indicating poor quality of carbon leading to loss of electrical conductivity in batteries cycled at higher C-rate.where C 0=−126˚Aand C 1=0.033[54].As the I D /I G ratio increases from 0.76to 0.81the in-plane correlation length L a calculated using Eqs.(3)and (4),drops from 5.64to 5.27nm for cells C0through C6.The increasing trend of the ratio and decreasing trend of the in-plane correlation length suggest the lower amounts of graphite clusters in very highly disordered carbon.Thus higher I D /I G ratio here indicates poor quality carbon in cells aged with higher C-rate [25].In case of sample C6the higher I D /I G ratio could be the effect of combination of higher SOC and higher C-rate.This leads to poor electronic properties of the carbon coating and contributes to the increased ohmic resistance of the cell.Thus the overall elec-trode performance is affected in cells aged with higher C-rate.The composite cathode cycled with higher C-rate have poor electronic conductivity due to degradation in the quality of the carbon.4.2.Nuclear magnetic resonanceFig.4shows the NMR spectra for C0and C6samples.In case of the C0sample a single isotropic 7Li peak is observed while this peak is absent in case of C6sample.The NMR spectra of C0shows a small chemical shift of ∼8ppm.There is also considerable broadening of the peak.The multiple spinning side bands accompanying the isotropic peak observed by Tucker et al.[30],Tucker et al.[46]are not observed in this case.The single isotropic band indicates one local environment for the lithium in case ofC0.Fig.4.NMR spectra of samples harvested from C0and C6batteries.An isotropic 7Li peak is observed in C0while similar speak is absent in C6.S.C.Nagpure et al./Applied Surface Science259 (2012) 49–5453Fig.5.Crystal structure of LiFePO4showing one unit cell constructed using Materials Studio.From[26].LiFePO4forms an orthorhombic olivine structure with a slightly distorted hexagonal close packed arrangement with Li and Fe in octahedral and P in tetrahedral oxygen array belonging to the sym-metry group Pnma[55–57],listed as no.62in the International Tables for Crystallography[58].Fig.5shows the crystal structure of this material with one unit cell constructed with Materials Stu-dio.The O atoms are located at the tetrahedral sites around each P atom.The divalent Fe ions form an octahedral arrangement with the O atoms.The Li atoms are located in channels along the b axis of the orthorhombic structure[26,57].LiFePO4shows a paramagnetic behavior and the single isotropic peak in C0is as expected for paramagnetic materials containing a single type of Li site.Lithium NMR spectra for paramagnetic materials are dominated by series of larger interactions such as quadrupole coupling(6Li,I=1;7Li,I=3/2)and hyperfine inter-actions between nucleus and the unpaired electrons[31].The possibility of a Knight shift in the battery materials is excluded due to their electronic insulating character[46].According to Tucker et al.[30,46]the shift in case of LiFePO4can be attributed to the hyperfine interactions through-bond transfer (specifically Li Fe O bond)of unpaired electron density via the oxygen p-orbitals to the Li s-orbitals.Since LiFePO4is electronically less conductive the Knight shift characteristic of metal conductors is ignored for this material.The broadening of the peak can be attributed to the considerable local disorder in the coordination sphere of Li in LiFePO4[30].The absence of peak in case of C6sam-ples indicates presence of FePO4instead of LiFePO4phase.This is consistent with observation by Nagpure et al.[26],using EELNS. The Li depletion in case of aged samples caused the changes in the local electronic structure of the sample.The Li depletion in aged sample caused the transitions from the1s core orbital of oxygen and strong hybridization between the Fe3d,O2p,and P3s and 3p states,giving rise to empty states(above the Fermi level)with some O2p states.The lithium starved FePO4phase indicates loss of cycling capability of the battery.5.SummaryRaman spectroscopy was used to analyze the carbon coating of aged LiFePO4and NMR was used to probe the local Li environment of the LiFePO4nanoparticles.According to the Raman studies the carbon coating degraded in quality as the batteries were aged at higher C-rates.The higher I D/I G ratio in case of batteries cycled at higher C-rate indicate poor quality of carbon leading to loss of elec-trical conductivity and subsequent decrease in the performance of the battery.The solid state7Li NMR is very critical in studying the local environment and electronic structure of the LiFePO4nanopar-ticles as it directly probes the Li within the sample.An isotropic peak with a small chemical shift,a characteristic of paramagnetic materials,is observed in unaged LiFePO4sample.The absence of such peak in the aged sample indicates the Li starved FePO4phase. The loss of active Li directly affects the loss of cycling capacity of the battery.References[1]Z.Chehab,L.Serrao,Y.Guezennec,G.Rizzoni,Aging characterization ofnickel–metal hydride batteries using electrochemical impedance spectroscopy, in:Proceedings of the2006ASME International Mechanical Engineering Congress and Exposition,2006.[2]Anonymous,FreedomCAR42V Energy Storage System End-of-Life Perfor-mance Goals,USABC,Southfield,MI,2002,Available from:http://www./guest/view team.php?teams id=12.[3]Anonymous,USABC Goals for Advanced Batteries for EVs,USABC,South-field,MI,2006,Available from:/guest/view team.php?teams id=12.[4]Anonymous,USABC Requirements of End of Life Energy Storage Sys-tems for PHEVs,USABC,Southfield,MI,2006,Available from:http://www./guest/view team.php?teams id=12.[5]J.Vetter,P.Novák,M.R.Wagner,C.Veit,K.C.Möller,J.O.Besenhard,M.Win-ter,M.Wohlfahrt-Mehrens,C.Vogler,A.Hammouche,Ageing mechanisms in lithium-ion batteries,Journal of Power Sources147(2005)269–281.[6]J.B.Goodenough,Cathode materials:a personal perspective,Journal of PowerSources174(2007)996–1000.[7]A.Yamada,S.C.Chung,K.Hinokuma,Optimized LiFePO4for lithium batterycathodes,Journal of the Electrochemical Society148(2001)A224–A229. [8]S.Franger,F.Le Cras,C.Bourbon,H.Rouault,Comparison between differentLiFePO4synthesis routes and their influence on its physico-chemical proper-ties,Journal of Power Sources119–121(2003)252–257.[9]G.Arnold,J.Garche,R.Hemmer,S.Strobele,C.Vogler,A.Wohlfahrt-Mehrens,Journal of Power Sources119–121(2003)247–251.[10]C.Delacourt,P.Poizot,S.Levasseur,C.Masquelier,Size effects on carbon-freeLiFePO4powders.The key to superior energy density,Electrochemical and Solid State Letters9(2006)A352–A355.[11]M.Maccario,L.Croguennec,B.Desbat,M.Couzi,F.Le Cras,L.Servant,Raman andFTIR spectroscopy investigations of carbon-coated Li x FePO4materials,Journal of the Electrochemical Society155(2008)A879–A886.[12]S.C.Nagpure,R.Dinwiddie,S.S.Babu,G.Rizzoni,B.Bhushan,T.Frech,Thermaldiffusivity study of aged Li-ion batteries usingflash method,Journal of Power Sources195(2010)872–876.[13]S.C.Nagpure,R.G.Downing,B.Bhushan,S.S.Babu,L.Cao,Neutron depth pro-filing technique for studying aging in Li-ion batteries,Electrochimica Acta56 (2011)4735–4743.[14]S.C.Nagpure,B.Bhushan,S.S.Babu,G.Rizzoni,Scanning spreading resistancecharacterization of aged Li-ion batteries using atomic force microscopy,Scripta Materialia60(2009)933–936.[15]S.C.Nagpure,S.S.Babu,B.Bhushan,Surface potential measurement of agedLi-ion batteries using Kelvin probe microscopy,Journal of Power Sources196 (2011)1508–1512.[16]N.-H.Cho,K.M.Krishnan,D.K.Veirs,M.D.Rubin,C.B.Hopper,B.Bhushan,Chem-ical structure and physical properties of diamond-like amorphous carbonfilms prepared by magnetron sputtering,Journal of Materials Research5(1990) 2543–2554.[17]M.A.Tamor,W.C.Vassell,Raman“fingerprinting”of amorphous carbonfilms,Journal of Applied Physics76(1994)3823–3830.[18]J.Schwan,S.Ulrich,V.Bathori,H.Erhardt,S.R.P.Silva,Raman spectroscopy onamorphous carbonfilms,Journal of Applied Physics80(1996)440–447. [19]B.Bhushan,Chemical,mechanical and tribilogical characterization of ultra-thinand hard amorphous carbon coatings as thin as3.5nm:recent developments, Diamond and Related Materials8(1999)1985–2015.。
张凯华文献综述

北京理工大学硕士学位论文开题文献综述报告硕士学位论文开题文献综述报告报告题目纳米硅橡胶与含能材料之间的相互作用学号____2220100317__姓名____张凯华___导师____董晓_____研究方向____计算化学______二级学科____化学工程______一级学科___化学工程与技术_学院___化工与环境学院_2011年 11月 5日纳米硅橡胶与含能材料之间的相互作用摘要:本课题以纳米硅橡胶和含能材料之间的相互作用为主要研究对象。
在DFT水平上,通过量子化学计算,获得hydroxy-terminated siloxane 晶体及含有C、H、O、N、Si原子的小分子的势能曲线图及状态方程。
在这些数据的基础上优化适于HMX、RDX、TNT以及硅橡胶的ReaxFF 参数,并通过ReaxFF MD 研究含有hydroxy-terminated siloxane 及HMX/RDX/TNT的复合材料对热与冲击的反应。
关键词:纳米硅橡胶;含能材料;相互作用;Gaussian;分子动力学模拟;1纳米硅橡胶的研究现状硅橡胶是一种直链状的高分子量的聚有机硅氧烷,其结构通式如下:通式中,n代表链段数,R’是烷基或羟基,R通常是甲基。
构成硅橡胶骨架的化学键主要是Si-O和Si-C。
Si-O键是组成硅氧链的骨架,其键能为451kJ/mol,使聚硅氧烷的热稳定性很好;键长较长(0.164nm),使得键对侧基转动的位阻小;Si和O的电负性差值为1.7,Si-O键有50%的离子性,这个键在通常情况下是稳定的,但在强酸强碱作用下仍然会被打断;Si-O-Si的键角很大(1430),使得Si-O之间容易旋转,链非常柔软。
Si-C键是组成有机硅化合物的特征键,也是有机硅聚合物侧基的键型[1]。
硅橡胶具有优异的耐高低温、耐候、耐臭氧、抗电弧、电气绝缘性、耐化学品、高透气性及生理惰性等特点,在航空、宇航、电气电子、化工仪表、汽车、机械等工业以及医疗卫生、日常生活的各个领域得到了广泛的应用。
CATHODE MATERIAL

专利内容由知识产权出版社提供
专利名称:CATHODE MATERIAL 发明人:TRAXLER, Hannes,TRAXLER,
Hannes,WESEMANN, Ingmar,WESEMANN, Ingmar,KNABL, Wolfram,KNABL, Wol fr am,T AUT ERMANN, Alexander,TAUTERMANN, Alexander,NILIUS, Maria,NILIUS, Maria 申请号:AT 2018/000032 申请日:201804 26 公开号:WO2018/213858A3 公开日:20190131
专利附图:
摘要:The invention relates to a cathode material for use in a high intensity discharge lamp, said material containing at least the following elements: a matrix based on tungsten having a tungsten content of more than or equal to 95 wt.%; tungsten carbide; oxides and or predominantly oxidic phases of one or more emitter elements from the group consisting of (rare earth metals, Hf, Zr), wherein the cathode material contains predominantly carbide phases from one or more emitter elements from the group (rare earth metals, Hr, Zr). The invention relates to a high intensity discharge lamp which comprises a cathode made from the claimed cathode material and to a method for producing a cathode material.
nmr用途英文介绍

nmr用途英文介绍NMR Applications: An Introduction in EnglishNuclear Magnetic Resonance (NMR) spectroscopy is a powerful analytical technique that plays a crucial role in various scientific fields. Its ability to provide detailed information about molecular structure and dynamics has contributed significantly to advancements in chemistry, biology, medicine, and materials science. This article serves as an introductory guide to the applications of NMR, highlighting its versatility and importance.1. Chemical Structure ElucidationNMR spectroscopy is commonly used to determine the chemical structure of organic and inorganic compounds. By analyzing the unique magnetic properties of atoms within a molecule, NMR can identify functional groups, confirm molecular connectivity, and provide insights into the three-dimensional arrangement of atoms. It is an indispensable tool for chemists, enabling them to characterize and understand the properties of new compounds.2. Drug Development and Pharmaceutical AnalysisIn the pharmaceutical industry, NMR aids in the development and analysis of drugs. It allows researchers to determine the purity and identify impurities in drug substances, ensuring high-quality pharmaceutical products. NMR can also assess the stability of drug formulations and investigate drug-protein interactions, facilitating the design of more effective and safe medications.3. MetabolomicsMetabolomics, the study of small molecules involved in metabolic processes, heavily relies on NMR spectroscopy for metabolite identification and quantification. NMR can provide a comprehensive analysis of biological samples such as blood, urine, and tissues, allowing researchers to understand the metabolic changes associated with diseases, drug responses, and environmental factors. This information contributes to the development of personalized medicine and biomarker discovery.4. Structural BiologyIn structural biology, NMR spectroscopy plays a vital role in determining the three-dimensional structures of macromolecules, including proteins and nucleic acids. By studying the interactions and dynamics of these biological molecules, NMR provides insights into their functions and mechanisms. This information is essential for understanding diseases at a molecular level and designing targeted therapies.5. Materials ScienceNMR spectroscopy is instrumental in characterizing various materials, including polymers, catalysts, and nanoparticles. It can elucidate the molecular structure, composition, and physical properties of materials, aiding in their design and optimization. NMR techniques such as solid-state NMR and diffusion NMR provide valuable information about material properties at the atomic and molecular levels.6. Environmental AnalysisEnvironmental scientists employ NMR spectroscopy to investigate pollutants, monitor water quality, and study interactions between contaminants and natural substances. NMR can identify and quantify organic compounds in environmental samples, helping to assess their impact on ecosystems and human health. This knowledge supports environmental remediation efforts and the development of sustainable practices.7. Food ScienceNMR spectroscopy finds extensive applications in food science and quality control. It can analyze food composition, detect adulteration, and determine nutritional content. NMR also assists in identifying flavor compounds and understanding the mechanisms behind food spoilage. This information is vital for ensuring food safety and improving food processing techniques.In conclusion, NMR spectroscopy is an invaluable scientific tool with diverse applications across numerous disciplines. Its ability to provide detailed molecular insights has revolutionized research and contributed to significant advancements in various fields. From chemical structure elucidation to drug development, from structural biology to environmental analysis, NMR continues to shape our understanding of the world around us. As technology advances, NMR will undoubtedly uncover new applications, further expanding its impact in science and beyond.。
纳米铁酸铜的制备及对rdx热分解的催化作用

纳米铁酸铜的制备及对rdx热分解的催化作用下载提示:该文档是本店铺精心编制而成的,希望大家下载后,能够帮助大家解决实际问题。
文档下载后可定制修改,请根据实际需要进行调整和使用,谢谢!本店铺为大家提供各种类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by this editor. I hope that after you download it, it can help you solve practical problems. The document can be customized and modified after downloading, please adjust and use it according to actual needs, thank you! In addition, this shop provides you with various types of practical materials, such as educational essays, diary appreciation, sentence excerpts, ancient poems, classic articles, topic composition, work summary, word parsing, copy excerpts, other materials and so on, want to know different data formats and writing methods, please pay attention!纳米铁酸铜的制备及对RDX热分解的催化作用1. 简介在爆炸性能研究领域,对于提高炸药的热分解速率和能量释放效率具有重要意义。
第五章 纳米材料的表征

例一、热氧化法制备WO 2.9纳米棒利用SEM 知道了棒的集体形貌、大体尺寸、取向特性等。
利用XRD 分析获取了纳米棒的晶体结构以及取向分布等信息。
利用TEM 、SAD 、HRTEM 、EDS 等分析可以获取单根纳米棒的结构、直径、化学成分、生长方向等信息。
例一、热氧化法制备纳米棒材料的分析与表征概述Raman 光谱和光致发光谱给出了化学键合和光学性能信息。
JEOL-200CX 分析型透射电镜透射电镜观察法(TEM观察法).透射电镜观察法(TEM观察法).纳米TiO2晶粒尺寸分布•C 透射电镜观察法(TEM观察法).X射线法X射线法X射线法比表面积法比表面积法方程:吸附质分压P0: 吸附剂饱和蒸汽压V之间的数量关系,为比表面积测定提供了很好的理论基础。
轴,为方程做图进行线性拟合,得到直线的斜率和截距,从而求得值计算出被测样品比表(二)纳米材料的颗粒尺寸与粒度分析拉曼散射法拉曼原理:当一束波长为λ的光照射到物质上之后,一部分拉曼散射与粉体粒径的关系:拉曼散射法z 特点:灵敏度较低,一般只能测定样品中含量在1%以上的物相,同时,定量测定的准确度也不高,一般在1%的数量级。
z 所需样品量大(0.1g),才能得到比较准确的结果,对非晶样品不能分析。
z 样品的颗粒度对X射线的衍射强度及重现性有大的影响。
一般颗粒越大,则参与衍射的晶粒数就越少,且产生初级消光效应,使得强度的重现性较差。
z 要求粉体样品的颗粒度大小在0.1 ~10μm范围。
对吸收系数大的样品,参加衍射的晶粒数减少,也会使重现性变差。
因此在选择参比物质时,尽可能选择结晶完好,晶粒小于5μm,吸收系数小的样品。
z 可采用压片,胶带粘以及石蜡分散的方法进行制样。
由于X射线的吸收与其质量密度有关,因此要求样品制备均匀,否则会严重影响定量结果的重现性(三)纳米材料的结构形貌分析X射线衍射结构/物相分析:XRD 物相分析是基于多晶样品对X 射线的衍射效应,对样品中各组分的存在形态进行分析。
固体核磁在钠离子电池层状氧化物正极材料

固体核磁在钠离子电池层状氧化物正极材料一、引言固体核磁共振 (NMR) 技术是一种非常重要的应用于材料科学领域的分析技术。
它通过观察核自旋在外加磁场下的共振现象来获得材料的结构和性质信息。
在新能源材料领域中,固体核磁共振技术被广泛应用于研究钠离子电池正极材料的结构和性能,尤其是层状氧化物正极材料。
本文将围绕固体核磁共振在钠离子电池层状氧化物正极材料中的应用展开深度讨论。
二、固体核磁共振技术在钠离子电池层状氧化物正极材料中的应用1. 结构分析固体核磁共振技术能够通过检测不同核自旋的共振信号,获取材料的局域结构信息,包括化学环境、晶格构型等。
在钠离子电池层状氧化物正极材料中,固体核磁共振技术可以帮助研究人员确定钠离子在晶格中的分布情况,揭示材料的离子导电路径,为提高电池性能提供结构指导。
2. 锂钠动力学研究通过固体核磁共振技术,可以研究锂钠离子在正极材料中的扩散动力学行为,包括迁移率、扩散路径等。
这对于理解材料的离子输运机制,优化电池的循环性能具有重要意义。
3. 晶格缺陷和杂质探测固体核磁共振技术可以探测材料中的晶格缺陷和杂质情况,比如氧缺陷、金属离子掺杂等。
这些缺陷和杂质可能对材料的电化学性能产生重要影响,固体核磁共振技术能够帮助科研人员深入了解材料的结构-性能关系。
三、固体核磁共振技术在钠离子电池层状氧化物正极材料中的挑战与展望1. 挑战目前固体核磁共振技术在钠离子电池层状氧化物正极材料中还面临一些挑战,比如样品制备的难度、信号分辨率的提高、结构模拟和实验数据的匹配等。
这些挑战需要材料科学家和固体核磁共振专家们共同努力,才能更好地发挥固体核磁共振技术在新能源材料研究中的作用。
2. 展望随着固体核磁共振技术的不断发展和完善,相信在未来,固体核磁共振技术将更加广泛地应用于钠离子电池层状氧化物正极材料的研究中。
通过结合计算模拟、实验表征等多种手段,固体核磁共振技术将为钠离子电池正极材料的设计与优化提供更丰富的信息,推动新能源电池领域的进展。
锂离子电池纳米钒基正极材料的研究进展

锂离子电池纳米钒基正极材料的研究进展梁叔全;潘安强;刘军;钟杰;陈涛;周江【摘要】锂离子电池因为其较高的能量密度、优良的循环性能及较强的荷电保持能力被广泛应用于便捷式电子器件中.同时作为混合动力汽车(Hv)和电动汽车(Ev)潜在的电源设备也被广泛地研究,但是,目前其电化学性能还不能完全满足高能量密度、大功率的要求.主要是因为商品化和即将进入开发性研究的正极材料大多是嵌锂过渡金属氧化物,这些正极材料存在致命的本征制约——较低的比容量.钒基正极材料,如V2O5、LiV3O8和Li3V2(PO4)3等,由于可以嵌入多个Li+离子,从而具有较高的理论比容量,但受材料微结构的影响,这类材料的实际比容量远低于理论值.材料微结构纳米化,可以形成独特形貌,获得高比表面积,缩短Li+离子的扩散距离,使这类材料的实际比容量接近理论值,从而有可能在能量的高效率储存中扮演十分重要的角色.本文作者重点综述钒基正极材料的主要晶体结构特点和相关纳米材料合成方法、结构表征及其对应电化学性能的研究进展.%Lithium-ion batteries (LIBs) were widely used in portable electronic devices, mainly due to their high energy density, good cycle performance and charge retention ability. Moreover, as the potential power sources of the hybrid vehicles (HV) and electric vehicles (EV), LIBs were widely studied. But at present their electrochemical properties cannot fully meet the requirements of high energy density, high power for power sources of HV and EV. This is mainly because most commercial and studied cathode materials are lithium transition metal oxides, which have an intrinsic constraint, I.e. Low capacity. V-based cathode materials, such as V2O5, LiV3O8 and Li3V2(PO4)3, possess relatively high theoretical specific capacity because of their abilities tointercalate more Li+ ions per formula. However, due to the structure limitation of these materials, their actual capacity is much lower than the theoretical value. Synthesis of these materials with nanostructures can greatly enlarge their surface areas and reduce the Li+ ion diffusion distance significantly, resulting in the fact that the actual specific capacity is closer to the theoretical value. Such V-based nanomaterials may make LIBs play an important role in the high efficiency store of energy, especially for power sources of HV and EV. This review focuses on the research development of synthesis of V-based nanomaterials, characterization and their corresponding electrochemical properties.【期刊名称】《中国有色金属学报》【年(卷),期】2011(021)010【总页数】17页(P2448-2464)【关键词】纳米材料;锂离子电池;V2O5;LiV3O8;Li3V2(PO4)3;电化学性能【作者】梁叔全;潘安强;刘军;钟杰;陈涛;周江【作者单位】中南大学材料科学与工程学院,长沙410083;中南大学材料科学与工程学院,长沙410083;中南大学材料科学与工程学院,长沙410083;中南大学材料科学与工程学院,长沙410083;中南大学材料科学与工程学院,长沙410083;中南大学材料科学与工程学院,长沙410083【正文语种】中文【中图分类】TM912.9;O646.54随着石油、天然气等不可再生能源的快速消耗和生态环境的日益恶化,在可支撑经济和社会可持续发展的新清洁能源出现之前,人类只能在不断提高能量的使用效率上下功夫,因此,对能量的储存和释放将提出越来越高的要求。
电池的制备

随着国内电动自行车的普及, 电动车电池得到了快速的发展, 大容量的电动车电池已成为电池企业的研发重点。
与传统的VRLA 蓄电池相比, 胶体蓄电池可以有效地防止硫酸电解液发生分层和干涸现象[1]。
天津蓝天公司生产的12 V 20 Ah 电动车用的高极板电池存在早期容量衰减(PCL)问题, 通过研究发现, 这种高极板电池极板上、下两部分的硫酸密度相差较大, 电解液出现了分层现象, 而这种分层现象会导致电池极板下部分活性物质的反应活性降低, 从而加剧了电池的早期容量衰减问题, 严重地影响了电池的使用性能。
为了解决硫酸电解液的分层现象, 本文通过在硫酸电解液中加入气相SiO2 粉末, 制成胶体铅酸蓄电池。
由于胶体电解质属于类固体电解质, 其流动性小, 可以有效地抑制硫酸分层现象, 防止高极板电池出现早期容量衰减现象。
1 实验1.1 实验电池的制备胶体电解液的配制: 将一定量的气相SiO2 粉末与1.25g/mL 的硫酸电解液混合并搅拌均匀后, 灌入阀控蓄电池中,电解液在阀控蓄电池内完成胶凝过程, 电池的其它生产工艺同普通VRLA 电池。
1.2 电池性能测试将制备好的胶体电池与没有添加SiO2 粉末的普通VRLA电池(简称空白电池)进行如下性能测试。
1.2.1 不同倍率放电性能测试室温下, 将经过10 次循环的单只胶体电池和空白电池分别以0.25 C、0.5 C、1 C 电流放电, 定时记录放电过程中电池电压的变化, 放电终止电压为10.5 V。
1.2.2 析气量测试把电池通气阀连接上胶皮管, 胶皮管的另一端插入充满水的倒立量筒中, 分别将胶体电池和空白电池在室温下进行充电, 记录各自气泡冒出的时间, 并随时记录量筒中液体下降的体积, 即析出的气体量。
1.2.3 低温性能测试把充好电的胶体电池与空白电池分别在低温箱中放置12 h, 设置温度为- 10 ℃, 然后以0.5 C 放电, 定时记录电池的放电电压, 直到放电完毕。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
NMR Studies of Cathode Materials for Lithium-Ion Rechargeable BatteriesClare P.Grey*and Nicolas Dupre´Department of Chemistry,State University of New York at Stony Brook,Stony Brook,New York11794-3400Received February24,2004Contents1.Introduction44932.NMR Background44942.1.Lithium NMR Spectra of Cathode Materials:Introduction44942.2.NMR Spectra of Paramagnetic Materials44942.2.1.Fermi-Contact Interaction44962.2.2.Dipolar Coupling44963.Extracting Chemical Information from the Spectraof Paramagnetic Materials44973.1.Fermi-Contact Interaction44973.2.Dipolar Interaction45004.Applications of NMR Spectroscopy to the Studyof Cathode Materials45014.1.Spinels45014.1.1.Cation-Doped Spinels45024.2.Cr3+-and Ni2+-Substituted Layered LithiumManganates45044.3.LiCoO2and Related Materials45054.4.Lithium Phosphates45064.5.Vanadates45074.5.1.51V NMR45074.5.2.NMR Studies of Vanadium Oxides45074.5.3.Vanadium Phosphates45095.Conclusions45106.Acknowledgments45107.References4510 1.IntroductionLithium intercalation or insertion materials have been widely investigated in the search for new electrode materials for use in high-voltage recharge-able batteries.1-6The first commercial Li-ion re-chargeable battery contains the layered materials LiCoO2(Figure1)and graphite as the cathode(or positive electrode)and anode(or negative electrode), respectively.7Although this battery is the current standard in many applications including cell phones and laptops,its slow charge and discharge rates and cost have prevented its use in applications that require cheap high power and capacity,such as hybrid electric vehicles and electric vehicles.The toxicity of Co is also an issue.A wide variety of materials have been studied,5,6which include doped LiCoO2phases,layered compounds based on the LiCoO2structure(e.g.,LiNiO28and LiNi0.5Mn0.5O29,10),*To whom correspondence should be addressed.E-mail:cgrey@.Clare P.Grey received her B.A.(1987)and D.Phil.(1990)degrees inChemistry from the University of Oxford.At Oxford she worked withProfessors Tony Cheetham and Christopher Dobson on the applicationof solid-state NMR to problems in solid-state chemistry.She then spenta year as a postdoctoral fellow at the University of Nijmegen in TheNetherlands with Professor Wiebren Veeman,where she developed newNMR methods for measuring internuclear distances in systems withquadrupolar nuclei.She was a visiting scientist at the DuPont ExperimentalStation in Wilmington(1992−1994),where she worked with Dr.AlexanderVega on NMR theory and on the application of NMR to molecular sievesand inorganic−organic composites.She joined the faculty at SUNY StonyBrook in1994as Assistant Professor and was promoted to Full Professorin2001.She uses solid-state NMR spectroscopy,in combination withother characterization techniques such as diffraction,to understand therole that local structure plays in controlling the physical properties of awide range of materials.Current studies include the investigation ofelectrode materials for lithium-ion rechargeable batteries,anionic conduc-tors,and ion-exchange and sorption properties of soil minerals,molecularsieves,and layeredmaterials.Nicolas Dupre´was born in Chatillon-sous-Bagneux,France,in1975.Hereceived his Ph.D.(2001)degree from Universite´Pierre et Marie Curie−Paris VI working under the direction of Professor Michel Quarton.Hewas appointed as a postdoctoral associate at SUNY Stony Brook in2002,where he works with Professor Clare P.Grey.His current researchinterests are focused on the study of the behavior of materials for lithiumbatteries using solid-state NMR.4493 Chem.Rev.2004,104,4493−451210.1021/cr020734p CCC:$48.50©2004American Chemical SocietyPublished on Web09/03/2004and a series of materials with two-and three-dimensional hosts for Li (e.g.,Li 1+x Mn 2-x O 411and LiFePO 412).While long-range structural information is typically available from diffraction methods,solid-state NMR is an extremely useful tool for character-izing local structure in these materials,even in highly disordered systems.The lithium nuclei (7Li and 6Li)are typically (but by no means exclusively)used as probes because it is the lithium ions that are directly involved in the electrochemical processes.The NMR spectra are strongly influenced by the electronic structure of the materials,and it is often possible to distinguish between insulators and conductors and between diamagnets and paramagnets.The method is quantitative and can be used to determine which species are removed on charging the battery and how the local structures change on extended cycling.NMR is also sensitive to dynamics that occur on the NMR time scale,and one-dimensional (1D)and two-dimensional (2D)NMR have,for example,been used to investigate Li-ion motion in vanadates (1D)13and between two different nanosized domains in anatase,Li y TiO 2.14This paper describes the approaches taken by us and other researchers over the past few years to interpret and extract chemical information from this class of materials.We focus on ex-situ analysis of electrode materials (i.e.,samples that have been extracted from cycled batteries)since these ap-proaches allow higher resolution spectra to be e of a toroid detector (to obtain the NMR signal)has allowed working batteries to be studied in situ by spectroscopy and imaging methods,15and more recent studies have shown that NMR signals may be obtained from plastic bag batteries.16In-situ methods have not,to date,been combined with the high-resolution method magic angle spinning (MAS)due to a number of experimental difficulties that still need to be surmounted and are,therefore,not discussed here.The theory required to interpret the NMR spectra is first presented (section 2);the use of this theory to extract chemical information is then described (section 3).This is followed by illustrative recent examples from the field.In so far as this is possible,section 4has been written so that it does not require a detailed understanding of NMR theory (beyond that discussed in section 2.1)and should be accessible to the non-NMR audience.A more comprehensive discussion of some of the techni-cal aspects associated with obtaining NMR spectra of cathode materials can be found in an earlier review article.172.NMR Background2.1.Lithium NMR Spectra of Cathode Materials:IntroductionThe chemical shift range for lithium NMR spectra is very small,and it is not always possible to resolve resonances due to different local environments in the NMR spectra of diamagnetic materials based solely on the chemical shift interaction.Sometimes im-proved resolution can be obtained at higher field strengths,allowing chemical information to be ex-tracted from the spectra.18,19Fortunately,the lithium NMR spectra of most battery samples are strongly affected by a series of larger interactions which include quadrupole coupling (6Li,I )1;7Li,I )3/2)and interactions with unpaired electrons for para-magnetic samples (hyperfine interactions)and with the conduction electrons in metals (the Knight shift).7Li has the much higher natural abundance (93%)and larger quadrupolar and gyromagnetic moments.In contrast,6Li is only 7%abundant,but its smaller quadrupole and gyromagnetic moments can result in higher resolution spectra that are often easier to interpret.The quadrupolar interaction,which results from the interaction of the quadrupolar nucleus with the electric field gradient (EFG)at the nucleus,is typically very small for 6Li but can result in charac-teristic broadening in static and a series of spinning sidebands in magic angle spinning (MAS)7Li NMR spectra due to the satellite (|+3/2〉-|1/2〉and |-1/2〉-|-3/2〉)transitions.This (anisotropic)interaction contains information concerning the local environ-ments at the lithium nucleus and can be used to distinguish between ions in distorted and more symmetric environments.Many battery materials are paramagnetic in the discharged or charged state.For example,the cathode material LiMn 2O 4is a mixed-valence compound containing Mn 3+(d 4)and Mn 4+(d 3)ions.Although the Co 3+d electrons in Li x CoO 2are paired in the fully discharged state,Li x CoO 2contains Co 4+d 5ions when charged.The NMR spectra of paramagnetic materials are dominated by the inter-actions between the nuclear and electronic spins (Figures 2and 3).These interactions may be much larger than any of the other interactions and can dominate the spectra of these materials,but they can also contain valuable information concerning both local crystallographic and electronic structure.Hence,we will now consider these interactions in some detail.2.2.NMR Spectra of Paramagnetic MaterialsParamagnetic ions with electronic spin,S (e.g.,S )3/2for d 3ions Mn 4+and Cr 3+),are associated with magnetic moments,µe ,that align in the presence of a static magnetic field,B 0(Figure 2),typically defined to be the z -direction.S z represents the component of the spin along this direction.Electron spin resonance (ESR)probes the transitions between the different spin (or Zeeman)states |m s 〉.Often the lifetime of an ion in a particular electronic state (T 1e )is very short on the relatively long time scale probed by NMR (from many seconds to 10-8s,depending on thesizeFigure 1.Structure of the cathode material LiCoO 2,showing the alternating layers formed by edge-sharing CoO 6octahedra and Li +.4494Chemical Reviews,2004,Vol.104,No.10Grey and Dupre ´of the electron -nuclear interaction).In this situation,the NMR spins cannot couple to S z (i.e.,the different spin states |m s 〉)and instead couple with the time average of the local field,〈S z 〉.This is nonzero due to the differences in populations of the different |m s 〉states in a magnetic field.The time average of S z is proportional to the net magnetic moment of an ensemble,which is the quantity measured in a magnetic susceptibility measurementwhere µ0is the permeability,g the electron g -factor,µB the Bohr magneton,N 0Avogadro’s number,and M the magnetic molar susceptibility in m 3mol -1.20The size of the electron -nuclear interaction can be quantified via a hyperfine coupling constant,A/h (in Hz)(see below),and NMR experiments are feasible for ions with short T 1e s,such that 21As the T 1e s lengthen and 1/T 1e (s -1)approaches the size of the electron -nuclear interaction,considerable NMR line broadening can occur,and it may not be possible to acquire high-resolution NMR spectra under these conditions.The effect of T 1e on the nuclear relaxation times is discussed in more detail in ref rge hyperfine constants are observed (of many MHz)when the nuclear and electronic spins are on the same atom.For example,a hyperfine constant A/h of -324MHz was measured by electronspin resonance (ESR)for the S )1/251V 4+vanadyl ions in VO(H 2O)52+,23and the T 1e ’s for these ions are 10-8-10-9s.221/T 1e is similar in magnitude to A /h,and high-resolution NMR spectra are not observed.When the nuclei under observation are further from the paramagnet,A is much smaller (the proton -electron hyperfine coupling constants are only 2.1-0.01MHz for water molecules in the same vanadyl complex 24-26)and the condition in eq 2is more readily achieved.This is the case for lithium spins,which are generally separated by two or more bonds from the paramagnet.In addition,bonding involving Li +is largely ionic,again reducing the size of A .Shorter T 1e s are generally observed for ions with S >1/2(S *5/2),such as Mn 3+,Ni 2+,and Co 2+,and higher resolution spectra are more readily achieved.22Broader 6Li/7Li resonances have been observed for local environments containing the paramagnetic S )1/2ion Ni 3+(e.g.,for LiNiO 227and LiCo 1-x Ni x O 228),but the resonances are not sufficiently broad to prevent detailed analysis of the local environments in these compounds.28,29The nuclear spins can interact with the time-averaged magnetic moments via either through-space (dipolar)or through-bond (Fermi-contact)interac-tions.The 6Li MAS NMR spectrum of LiMn 2O 4isFigure 2.(a,b)Effect of a static magnetic field on a paramagnet with magnetic moment µe and electronic spin S )1/2.Note that the lowest energy level for S )1/2in the magnetic field is the |-1/2〉state due to negative charge of the electron.(The magnetic moment of the electron µe associated with this state is still aligned along the direction of the static magnetic field;in many of the subsequent drawings,we will use arrows to represent directions of the net magnetic moments on the electrons/paramagnets.)(c)ESR probes the transitions between the spins states |+1/2〉T |-1/2〉.Spins in these states relax with a rate 1/T 1e ,which is typically much faster than the size of many of the interactions probed by NMR (i.e.,T 1e is shorter than the NMR time scale).Thus,NMR experiments are generally only sensitive to the time-averaged value of the magnetic moment,µj e (-g µB 〈S z 〉).〈S z 〉)-B 0µ0gN 0µBM(1)|A /h |,1/T 1e(2)Figure 3.(a)6Li MAS NMR spectrum of the spinel LiMn 2O 4synthesized at 850°C acquired with a spinning speed of 10kHz at a field strength of 4.7T.The spinning sidebands and the isotropic shift are marked with asterisks and its shift value (520ppm),respectively.(b)Li local environment in LiMn 2O 4showing a tetrahedrally coordi-nated Li ion and the 12nearest Mn ions (in the octahedral sites of the spinel structure).Schematics illustrating (c)the transfer of unpaired electron spin density from the un-paired electrons in the t 2g d electrons (on one of the 12nearby Mn ions)via the 2p orbitals on the intervening oxygen atom to the 2s orbital on Li,which causes the large shift seen for the LiMn 2O 4isotropic resonance and (d)the dipolar coupling between a 6Li nuclear spin and net magnetic moment due to a nearby Mn ion.The spinning sidebands shown in (a)primarily result from this interac-tion,which is not completely removed by MAS.34Materials for Lithium-Ion Rechargeable Batteries Chemical Reviews,2004,Vol.104,No.104495presented in Figure3a to illustrate the effect of the different interactions on the NMR spectrum of this paramagnetic material.2.2.1.Fermi-Contact InteractionThis is a measure of the unpaired electron spin density that is transferred from the paramagnet to the nucleus of the spin under observation.In the regime defined by eq2,the NMR shift(δ)(∆ω/ω0)) induced by the Fermi-contact interaction is directly proportional to〈S z〉30,31The sign(and size)of the hyperfine constant A c/h (Hz)determines the direction(and size)of the shift and depends on the electron spin(i.e.,unpaired electron)density at the nucleus,F(r)0).γN is the gyromagnetic ratio for the isotope studied by NMR, andω0is its Larmor frequency(in radians).The 6Li/7Li hyperfine shift can be very large for many transition-metal oxides and shift the Li resonances well out of the typical range observed in the Li NMR of diamagnetic materials(0(5ppm)(Figure3a).F(0)depends on the connectivity between the orbitals on the paramagnet and the orbitals on the NMR-active atom(Figure3c).Only the electrons in s orbitals are associated with a finite probability of being found at the nucleus,and only the electron density transferred to the s orbitals needs to be considered.Electron density may be transferred either directly from the paramagnet or indirectly via a transferred hyperfine interaction to the s orbitals, and thus,the interaction contains chemical informa-tion about the bonding involving the paramagnets, Li,and the intervening oxygen atoms.For example, in LiMn2O4,the transferred hyperfine interaction will primarily involve the manganese t2g,oxygen2p,and Li2s orbitals(Figure3c).The Fermi contact shift is generally considered to be additive,so that the shift due to many magnetic ions may be obtained from a sum of the shifts induced by each magnetic ion, although exceptions occur in systems with very delocalized spin states.One challenge in this field has been to relate shift to local environment to extract chemical information from these often-complex systems.The correlation between shift and local environment will be explored in some detail in section3.The Knight shift,which dominates the spectra of metallic samples,is analogous to the Fermi-contact shift,except that now the shift is a measure of the density of states at the Fermi level,N(E f).This relationship arises because the Pauli paramagnetism p of a metal is proportional to N(E f),32and thus,since the electron-nucleus interaction is dependent on〈S z〉and hence (eqs1and3),the Knight shift is dependent on N(E f).Again,since hyperfine coupling requires that unpaired electron density is present at the nucleus under investigation,the Knight shift depends,to a first approximation,on the contribution of the s orbitals of the NMR-active atom(lithium)to the crystal orbitals with energies at or very close to E f(i.e.,N(E f)Li,2s the Li2s partial density of states at the Fermi level;this can be quite different than the value of N(E f)that is important in controlling the bulk conductivity of the sample).Often the d or p orbitals at the atom under investigation may have larger contributions to N(E f).This will be the case for some metals and the51V Knight shifts of vana-dates.Here,spin density can also be transferred to the s orbitals via a polarization mechanism and both positive and negative Knight shifts can result,de-pending on the nature,populations,and locations of the orbitals involved in the process.Polarization mechanisms are discussed in more detail in section 3.1.2.2.2.Dipolar CouplingThe dipolar interaction is the same interaction that occurs between two or more nuclear moments and is caused by the local magnetic fields of the nuclear or electronic(S)spins that are felt at the nearby nuclear (I)spin(Figure3d).When the dipolar interaction involves coupling to S)1/2nuclear spins,coupling to both the|1/2〉and|-1/2〉eigenstates will occur, resulting in the classic Pake doublet pattern for a powder.In contrast,when the second set of coupled spins are electrons(or paramagnetic ions),the nuclear spins can only couple to the time-averaged magnetic moment(again because the electronic relaxation time T1e is extremely fast on the NMR time scale).The line shape in this case resembles that of the chemical shift anisotropy(CSA),and like the CSA,it scales linearly with the field.33The Hamiltonian for this interaction,H en,can be represented by33,34whereµj e is the time(or thermally)averaged magnetic moment of the electrons(i.e.,-gµB〈S z〉)and D˜en is the dipolar coupling tensor between the unpaired elec-tron and nucleus,which is defined by its matrix elements as follows35,36and depends on both the distance between the nuclear and electronic spins,r(e.g.,the Li and Mn atoms in Figure3b),and the orientation of the interatomic(Li-Mn)vector with respect to the static magnetic field,B0.δij is the Kronecker delta(δij)1 for i)j and0for i*j),e i and e j are the x,y,z components of a unit vector pointing from the nuclear spin to the electron spin in a chosen coordinate system.These interactions(and the CSA)are second-rank tensors and therefore averaged by MAS.Since the size of the dipolar interaction is generally larger than the MAS frequency,a series of spinning side-bands result(Figure3a)that contains information concerning the size of the interaction and the relative orientation of the spins.37∆ωω0)-Acω0h〈S z〉(3)A c /h)gµBγN F(r)0)µo/3S(4)Hen)µ04πµj e D˜enµN(5)Dij)1r3(δij-3eiej)(6)4496Chemical Reviews,2004,Vol.104,No.10Grey and Dupre´For isotropic magnetic moments,µj e ,the interaction results in a line broadening of the resonance only but no overall shift.38This will not be the case for an anisotropic magnetic moment.Here,both line broad-ening and a “pseudo-contact”shift will occur.This is an important mechanism for “shift reagents”in solution NMR and has also been shown to be neces-sary to explain the 89Y shifts that arise due to lanthanide ions in the solid state.39The pseudo-contact shift arises from a similar mechanism as the broadening and line splitting due to a coupling to a quadrupolar nucleus (the interaction involves a fourth-rank tensor).Thus,the line broadening is no longer completely removed by MAS.In many of the batteries studied to date,the size of the shift and line broaden-ing are small and the interaction can often be ignored to a first approximation.More detailed studies are still,however,required to correlate this interaction with line broadening.The size of the dipolar interaction is often straight-forward to calculate as 〈S z 〉can be readily estimated from expressions for the susceptibility.For example,assuming spin-only paramagnetism,which is a good approximation for d 3ions such as Mn 4+and Cr 3+40,41and thus µj e is given by 42where k is the Boltzmann constant.In an extended solid,the nuclear spins are typically surrounded by more than one paramagnet and the total Hamilto-nian for the dipolar coupling,H en ,is obtained by summing the individual coupling tensors for coupling to each individual paramagnetic ions in successive coordination spheres.34,43Since µN ,the magnetic moment ()γN (h /2π)I z ),depends on the gyromagnetic ratio,γN ,larger dipolarcouplings are seen for nuclei with larger values of γN .Thus,7Li with its much larger gyromagnetic ratio than 6Li (γ7Li /γ6Li )2.6)results in MAS spectra with much larger spinning sideband manifolds.Further-more,unlike the dipolar coupling between nuclear spins,the size of the interaction depends on the magnetic field strength (eq 8).Consequently,the effect of this interaction can be minimized and spectra simplified by using low fields,fast MAS,and nuclei with low γN s.The effect of γN is illustrated in Figure 4,which compares the 6Li and 7Li spectra of the spinel LiMn 2O 4prepared at 600°C obtained at an identical spinning speed and magnetic field strength.The sidebands in this system result prima-rily from the dipolar interactions.Clearly,it is difficult to see all the resonances in the 7Li spectrum at this spinning speed,and some of the weaker peaks are obscured by the sidebands originating from the more intense resonance at ca.511ppm.3.Extracting Chemical Information from the Spectra of Paramagnetic Materials3.1.Fermi-Contact InteractionTo extract chemical information from the NMR spectra of paramagnetic battery samples (e.g.,Figure 3a),we need to be able to assign a particular value of the hyperfine shift to a specific local environment.To achieve this goal,we 44-47and other workers 28,48,49have now studied a series of lithium compounds containing transition metals with electronic configu-rations from d 1to d.8This has allowed us to rational-ize the differences in the shift mechanisms that result from the presence of unpaired electrons in the t 2g and e g orbitals of the transition-metal ions.Since the interaction is analogous to the J -coupling that occurs between nuclei and to magnetic interactions between paramagnets,approaches similar to those used in these fields may be used to predict the sign and magnitude of the hyperfine interaction.The correla-tions developed between the shift and local environ-ment in these lithium NMR studies will now be explored.To rationalize the large hyperfine shifts observed for lithium manganates such as the spinel LiMn 2O 4(Figure 3a),we synthesized a whole series of com-pounds within the MnO 2-LiMnO 2-Li 2MnO 3phase diagram.The NMR spectra of these compounds were then acquired to obtain typical hyperfine shift ranges for these materials (Figure 5).Even without a complete understanding of the causes of these large shifts,it then became possible to assign different shift ranges to different lithium local environments.For example,lithium spins in the octahedral site of a Mn 4+spinel typically give rise to a resonance at 1800-2300ppm,while lithium spins in the tetrahe-dral site of the spinel resonate at ca.800ppm.This is illustrated in Figure 5for the ordered spinel (Li 0.5-Zn 0.5)tet (Li 0.5Mn 1.5)O 4,which contains Li ions on both the octahedral and tetrahedral sites of the spinel structure.Two resonances are clearly visible,which can be assigned using our hyperfine shift scale to the two different sites in this structure.50Similar ap-proaches have been used by us and others todeter-Figure parison of the spectra of LiMn 2O 4synthe-sized at 600°C obtained with 6Li and 7Li MAS NMR at a MAS spinning speed of ∼10.5kHz and a magnetic field strength of 4.7T (200MHz for 1H).(Reprinted with permission from ref 17.Copyright 2003Elsevier.))(µ0/4π)N 0g 2µB 2S (S +1)/3kT(7)µj e )g 2µB 2S (S +1)B 0/3kT(8)Materials for Lithium-Ion Rechargeable Batteries Chemical Reviews,2004,Vol.104,No.104497mine the locations of lithium ions in a series of transition-metal-doped spinels,LiMn 2-x M x O 4(M )Cu,Co,Li,Ni,Cr).17,51We examined the local environments around lithium in a series of Mn(IV)compounds to explain the different hyperfine shifts in these materials.By breaking up the local environments according to the numbers of manganese ions in the first cation coor-dination sphere around lithium and the Li -O -Mn bond angles,we determined the contribution of each Li -O -Mn interaction to the overall lithium shift.17,44This analysis involves two assumptions:(1)that the major shift contribution arises from the hyperfine shift due to manganese ions in the first cation coordination sphere and (2)that there are no signifi-cant interactions between paramagnets at the tem-peratures at which the NMR spectra were acquired (close to room temperature).This latter assumption allows the shifts between different compounds to be compared.(The validity of this assumption can be tested by studying the temperature dependence of the shifts to determine whether there is any evidence for large deviations from Curie -Weiss behavior.45,46)For example,one local environment for Li in Li 2MnO 3contains 12sets of Li -O -Mn 4+bonds,each with a bond angle of close to 90°(Figure 6a).This environ-ment is associated with a hyperfine shift of 1500ppm or a shift of 125ppm per Li -O -Mn 4+interaction.More generally,Li +-O -Mn 4+bonds with bond angles of 90°were found to result in large positive shifts of ca.120-150ppm,while linear Li -O -Mn 4+bonds resulted in smaller negative shifts from -60to -125ppm,the exact value depending largely on the coordination number of oxygen (Table 1).47The size and direction of the shifts was then rationalized by using the Goodenough -Kanamoori rules,originally designed to predict the sign of the coupling between d electrons in transition-metal oxides.For example,overlap between a half-filled t 2g orbital of a manganese ion and an empty 2s lithium orbital in a Li -O -Mn 90°arrangement may occur either directly or via an intervening p orbital on an oxygen atom.In the case of the transferred hyperfine interaction,an interaction with the t 2g electron andthe filled oxygen p orbital can only occur via transfer of spin density of the opposite sign to that already present in the half-filled t 2g orbital since the t 2g orbital is half-filled (Figure 6b).This then results in transfer of positive spin density to the empty Li 2s orbital and shifts of ca.120-150ppm per Li -O -Mn interaction.In the case of a 180°interaction,no direct overlap mechanism is possible involving the t 2g orbitals.In this case,the interaction occurs via the empty e g orbitals (Figure 6c).Here,the exchange coupling between the e g and t 2g orbitals on the same atom favors the transfer of spin density with the same sign of spin polarization as that in the t 2g orbitals to the e g orbitals.This results in a net transfer of negative spin polarization from manganese to the empty2sFigure 5.6Li MAS NMR spectrum of the Mn(IV)spinel(Li 0.5Zn 0.5)tet (Li 0.5Mn 1.5)Oct O 4and the typical hyperfine shifts observed for lithium in a series of local environments.Hyperfine shifts are given next to the two isotropic resonances in the 6Li spectrum;all other peaks are spin-ning sidebands,which are predominantly caused by the electron -nuclear dipolarcoupling.Figure 6.6Li MAS NMR spectrum of the layered com-pound Li 2MnO 3acquired at a MAS frequency,νr ,of 35kHz.Spinning sidebands are marked with asterisks.The local environment in the Mn 4+/Li +layers that gives rise to the isotropic resonance at 1500ppm is shown.Spin density may be transferred to the 2s orbital of Li via the interaction with (b)a half-filled t 2g orbital and (c)an empty d z 2Mn orbital to produce the hyperfine shifts seen in the spectrum of Li 2MnO 3.The large arrows represent the magnetic moments of the electrons in the t 2g and p orbitals,while the smaller arrows indicate the sign of the spin density that is transferred to the Li 2s and transition-metal d orbitals.Table parison of the Hyperfine Shifts (in ppm)Observed for Lithium Coordinated to a Single Mn 4+Ion via an Intervening Oxygen Ion for a Series of Local Environments a Li -O -M bond angle (deg)O oct b O tet c O linear d9012216312276180-60-75-125aShifts are classified according to the coordination number or environment of the intervening oxygen atom in each Li -O -Mn 4+interaction.b Li 2MnO 3.47c Spinel compounds such as (Li 0.5Zn 0.5)(Li 0.5Mn 1.5)O 4.50d Perovskite and K 2NiF 4-related compounds such as La 2(LiMn)0.5O 4.534498Chemical Reviews,2004,Vol.104,No.10Grey and Dupre ´。
