1,3丙二醇MSDS(GHS版本)
三羟甲基氨基甲烷77-86-1

q) 分解温度
无数据资料
r) 粘度
无数据资料
10.2 化学稳定性
强氧化剂
10.3 敏感性(危险反应的可能性)
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
吸入可能有害。引起呼吸道刺激。
10.6 危险的分解产物
如服入是有害的。
11 毒理学资料
5.2 源于此物质或混合物的特别的危害
使用个人防护用品。避免粉尘生成。避免吸入蒸气、烟雾或气体。保证充分的通风。人员疏散到安全区 域。避免吸入粉尘。
5.3 救火人员的预防
无数据资料
5.4 进一步的信息
无数据资料
6 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
收集和处置时不要产生粉尘。扫掉和铲掉。放入合适的封闭的容器中待处理。
欧洲陆运危规 : 无数据资料 国际海运危规 : 无数据资料 国际空运危规 : 无数据资料
14.2 联合国(UN)规定的名称
欧洲陆运危规:非危险货物 国际海运危规:非危险货物 国际空运危规:非危险货物
14.3 运输危险类别
欧洲陆运危规 : - 国际海运危规 : -
国际空运危规 : -
14.4 包裹组
欧洲陆运危规 : -
化学品安全技术说明书
https://
1 化学品及企业标识
1.1 产品标识符
化学品俗名或商品名: 三羟甲基氨基甲烷 CAS No.: 77-86-1 别名: 2-氨基-2-(羟甲基)-1,3-丙二醇;缓血酸铵;2-氨基-2-羟甲基-1,3-丙二醇;缓血酸胺;氨丁三醇;三(羟甲 基)氨基甲烷;
2.3 其它危害物
-无
3 成分/组成信息
1,3-丙二醇的工业应用和生产

1,3-丙二醇的工业应用和生产前言1,3-丙二醇(即1,3-PDO,化学式可表示为:CH2OHCH2CH2OH)是-种重要的化工原料,1990年代初,由于1,3-PDO的工业用途相对较少,全球的l,3-PDO的工业产量很低,价格却较高(30美元/kg),1991年的产量仅为 100t,市场占有率远不及乙二醇、l,2-丙二醇、l,4-丁二醇、2,3-丁二醇等其它二醇。
1990年代中期工业上成功地开发出以1,3-丙二醇为原料的新型聚酯材料—聚对苯二甲酸丙二醇酯,简称PTT。
1998年在美国PTT被评为六大石化新产品之-,世界很多跨国化学工业公司如壳牌、杜邦等也都对它产生了浓厚的兴趣。
1995年壳牌公司率先实现将PTT 商品化,商品名Colterra,2000年壳牌公司PTT的生产能力为100 kt/a,并且已于1999年底将在美国路易斯安那州2kt/a的 1,3-PDO生产装置扩产为72 kt/a。
2000年美国杜邦公司开发出PTT并且注册了商品名为Sorona。
另外德国Degussa公司也是世界上生产PTT和1,3-PDO的大公司,具有50kt/a的 1,3-PDO生产能力。
目前由于合成PTT的原料—1,3-PDO的合成方法的改进,成本大大降低,使工业生产风呼纤维的前景非常看好。
与壳牌公司和Degussa公司的化学法生产不同,杜邦公司拟采用生物转化法生产1,3-PDO,并且正与Tate& Lyle Citric Acid公司联合开发大规模工业化生产技术,中试实验正在进行当中,预计在2003年生物转化法生产1,3—PDO也将实现大规模生产。
1 1,3-PDO的性质及工业应用1,3-PDO为无色透明粘稠液体,无臭,有吸湿性,与水、醇混溶,对多种有机溶剂有较好的溶解度。
主要的物性参数如下:沸点为 213.5℃;熔点为-27℃;密度(20℃)为1.053;折光率l.4398。
l,3-PDO不仅是良好的溶剂、抗冻剂、保护剂,由于它含有双功能基还可以参与多个化学合成反应,如二氧六环的合成,以它为单体可以生产出特殊场合使用的聚酯、聚醚、聚氨酯等缩聚物。
丙二醇安全技术说明书
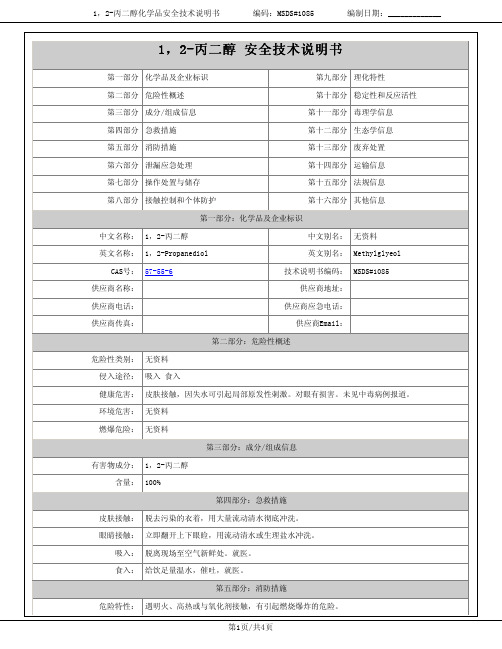
1,2-丙二醇化学品安全技术说明书编码:MSDS#1085编制日期:_____________建规火险分级:丙有害燃烧产物:一氧化碳、二氧化碳。
灭火方法:雾状水、泡沫、二氧化碳、干粉、砂土。
第六部分:泄漏应急处理应急处理:切断火源。
戴好防毒面具,穿一般消防防护服。
用大量水冲洗,经稀释的洗液放入废水系统。
如大量泄漏,利用围堤收容,然后收集、转移、回收或无害处理后废弃。
第七部分:操作处置与储存操作注意事项:无资料储存注意事项:储存于阴凉、通风仓间内。
远离火种、热源。
防止阳光直射。
保持容器密封。
应与氧化剂、酸类、碱类分开存放。
搬运时要轻装轻卸,防止包装及容器损坏。
第八部分:接触控制/个体防护中国MAC(mg/m3):未制订标准前苏联MAC(mg/m3):7TLVTN:无资料TLVWN:无资料接触限值:美国TLV-TWA:未制订标准美国TLV-STEL:未制订标准监测方法:无资料工程控制:生产过程密闭,全面通风。
呼吸系统防护:高浓度接触时,应该佩戴防毒面具。
眼睛防护:戴化学安全防护眼镜。
身体防护:穿工作服。
手防护:必要时戴防化学品手套。
其他防护:无资料第九部分:理化特性pH:无资料熔点(℃):-59沸点(℃):187.2分子式:C3H8O2主要成分:无资料饱和蒸气压(kPa):0.02(25℃)辛醇/水分配系数的对数值:无资料临界温度(℃):无资料闪点(℃):99引燃温度(℃):无资料自燃温度:引燃温度(℃):371燃烧性:可燃溶解性:与水混溶,可混溶于乙醇、乙醚、多数有机溶剂。
相对密度(水=1): 1.04(25℃)相对蒸气密度(空气=1): 2.62分子量:76.1燃烧热(kJ/mol):无资料临界压力(MPa):无资料爆炸上限%(V/V):12.6爆炸下限%(V/V): 2.6外观与性状:无色、有苦味、略粘稠吸湿的液体。
主要用途:用于生产防冻剂、热交换剂树脂和二醇衍生物,还用作溶剂、增塑剂和湿润剂等。
1,3-丙二醇结构式

1,3-丙二醇结构式
1,3-丙二醇是一种有机化合物,其结构式如下:
1,3-丙二醇的结构式解析
1,3-丙二醇的化学式为C3H8O2,分子量为76.10。
从结构式中我们可以看到,它由三个碳原子、八个氢原子和两个氧原子组成。
碳原子之间通过单键连接,形成主链。
在主链上,第一个碳原子和第三个碳原子各连接了一个羟基(-OH),形成了两个醇基。
而第二个碳原子则连接了一个甲基(-CH3)。
这种结构使得1,3-丙二醇具有一些独特的性质。
首先,由于两个醇基的存在,它具有很好的水溶性,可以与水以任意比例互溶。
其次,由于甲基的存在,它具有一定的脂溶性,可以与脂肪和其他有机溶剂混合。
此外,由于其分子结构中含有多个官能团,因此它还具有一些化学反应活性,可以参与多种化学反应。
在自然界中,1,3-丙二醇并不存在,而是通过化学合成方法得到的。
它被广泛应用于化工、医药、食品、化妆品等领域。
在化工领域,它可以作为溶剂、增塑剂、润滑剂等使用。
在医药领域,它可以作为药物中间体、合成原料等使用。
在食品领域,它可以作为食品添加剂、甜味剂等使用。
在化妆品领域,它可以作为保湿剂、柔润剂等使用。
总之,1,3-丙二醇是一种重要的有机化合物,其结构式揭示了它的化学组成和分子结构特点。
这些特点使得它在多个领域都有广泛的应用前景。
新戊二醇化学品安全技术说明书
将剩余的和未回收的溶液交给处理公司。联系专业的拥有废弃物处理执照的机构来处理此物质。与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
废弃注意事项:
危险货物编号:
UN编号:
包装标志:
包装类别:
包装方法:
塑料袋
运输注意事项:
铁路运输时应严格按照铁道部《危险货物运输规则》中的危险货物配装表进行配装。运输时运输车辆应配备相应品种和数量的消防器材及泄漏应急处理设备。夏季最好早晚运输。运输时所用的槽(罐)车应有接地链,槽内可设孔隔板以减少震荡产生静电。严禁与氧化剂、酸类、食用化学品等混装混运。运输途中应防曝晒、雨淋,防高温。中途停留时应远离火种、热源、高温区。装运该物品的车辆排气管必须配备阻火装置,禁止使用易产生火花的机械设备和工具装卸。公路运输时要按规定路线行驶,勿在居民区和人口稠密区停留。铁路运输时要禁止溜放。严禁用木船、水泥船散装运输。
操作注意事项:
防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。 一般性的防火保护措施。
储存注意事项:
容器保持紧闭,储存在干燥通风处。贮存在阴凉处。
中国MAC(mg/m3):
前苏联MAC(mg/m3):
TLVTN:
TLVWN:
监测方法:
工程控制:
密闭操作,局部排风。提供安全淋浴和洗眼设备。
其它理化性质:
稳定性:
禁配物:
强氧化剂,酰氯,酸酐
避免接触的条件:
防潮。
聚合危害:
分解产物:
在着火情况下,会生成有害分解产物。-碳氧化物
急性毒性:
LD50:≥6400mg/kg大鼠经口;小鼠经口LD50为3200~6400mg/kg
新版MSDS——1,2-丙二醇
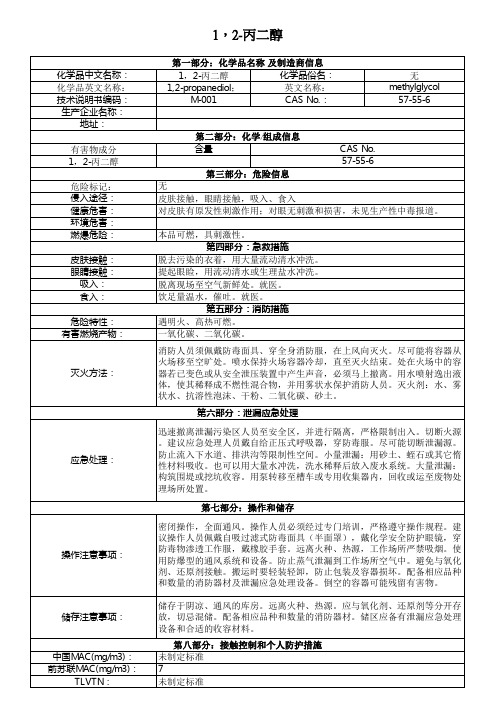
1,2-丙二醇
化学品中文名称: 化学品英文名称: 技术说明书编码: 生产企业名称:
地址: 有害物成分 1,2-丙二醇 危险标记: 侵入途径: 健康危害: 环境危害: 燃爆危险: 皮肤接触: 眼睛接触:
吸入: 食入: 危险特性: 有害燃烧产物:
灭火方法:
应急处理:
操作注意事项:
储存注意事项:
中国MAC(mg/m3): 前苏联MAC(mg/m3):
储存于阴凉、通风的库房。远离火种、热源。应与氧化剂、还原剂等分开存 放,切忌混储。配备相应品种和数量的消防器材。储区应备有泄漏应急处理 设备和合适的收容材料。
第八部分:接触控制和个人防护措施 未制定标准 7 未制定标准
1,2-丙二醇
TLVWN: 监测方法: 工程控制: 呼吸系统防护: 眼睛防护: 身体防护: 手防护: 其他防护:
运输注意事项:
法规信息
1,2-丙二醇
处置前应参阅国家和地方有关法规。建议用焚烧法处置。
无资料 无资料
第十四部分:运输信息
Z01 无资料。
运输前应先检查包装容器是否完整、密封,运输过程中要确保容器不泄漏、 不倒塌、不坠落、不损坏。严禁与氧化剂、还原剂、食用化学品等混装混运 。运输车船必须彻底清洗、消毒,否则不得装运其它物品。船运时,配装位 置应远离卧室、厨房,并与机舱、电源、火源等部位隔离。公路运输时要按 规定路线行驶。
2-氨基-2-甲基-1,3-丙二醇115-69-5

14.5 环境危害
欧洲陆运危规 :否 国际海运危规 海运污染物 :否 国际空运危规 : 否
https:// 4/5
化学品安全技术说明书
14.6 对使用者的特别预防
无数据资料
15 法规信息
15.1 专门对此物质或混合物的安全,健康和环境的规章 / 法规
法规信息 无数据资料
https://
12 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
13 废弃处置
13.1 废物处理方法
产品 无数据资料 污染了的包装物 无数据资料 进一步的说明: 无数据资料
Powered by TCPDF ()
5/5
P302+P352
如沾染皮肤:用大量肥皂和水清洗。
P304+P340
如果吸入:将受害人移到空气新鲜处,在呼吸舒适的地方休息。
P312
如果你感到不适,呼叫解毒中心/医生。
P321
特殊明确的治疗见本标签上的...。
P332+P313
如发生皮肤刺激:求医/就诊。
P337+P313
如果发生眼刺激:求医/就诊。?
4.2 最重要的症状和影响,急性的和滞后的
最重要的症状和健康影响据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
如有必要,佩戴自给式呼吸器进行消防作业。
2-氨基-1,3-丙二醇534-03-2

14.1 UN编号
欧洲陆运危规 : 3263
国际海运危规 : 3263
国际空运危规 : 3263
14.2 联合国(UN)规定的名称
欧洲陆运危规:CORROSIVESOLID,BASIC,ORGANIC,N.O.S.(2-Aminopropane-1,3-diol) 国际海运危规:CORROSIVESOLID,BASIC,ORGANIC,N.O.S.(2-Aminopropane-1,3-diol) 国际空运危规:CORROSIVESOLID,BASIC,ORGANIC,N.O.S.(2-Aminopropane-1,3-diol)
5 消防措施
5.1 灭火介质
火灾特征 无数据资料 灭火方法及灭火剂 碳氧化物,氮氧化物
5.2 源于此物质或混合物的特别的危害
使用个人防护用品。避免粉尘生成。避免吸入蒸气、烟雾或气体。保证充分的通风。人员疏散到安全区 域。避免吸入粉尘。
5.3 救火人员的预防
无数据资料
5.4 进一步的信息
无数据资料
6 泄露应急处理
警告申明
P280
戴防护手套/防护服/护眼/防护面具。
P305+P351+P338
如进入眼睛:用水小心清洗几分钟。如果可以做到,摘掉隐形眼镜,继续冲洗。P3源自0立即呼叫解毒中心/医生。
RS
Hazard symbol(s)
C
R-phrase(s)
R34
S-phrase(s)
S26;S;S39/;S45;S
f) 起始沸点和沸程
277 °C (531 °F) - lit.
g) 闪点
113 °C (235 °F) - closed cup
丙二醇安全技术说明书

1,2丙二醇物质安全技术说明书MSDS第一部分:化学品名称化学品中文名称: 12-丙二醇化学品英文名称: 12-propanediol中文名称2:英文名称2: methylglycol技术说明书编码: 1673CAS No.: 57—55-6分子式: C3H8O2分子量: 76。
10第二部分:成分/组成信息有害物成分含量 CAS No。
1,2—丙二醇 57-55-6第三部分:危险性概述危险性类别:侵入途径:健康危害:对皮肤有原发性刺激作用;对眼无刺激和损害,未见生产性中毒报道。
环境危害:燃爆危险:本品可燃,具刺激性.第四部分:急救措施皮肤接触:脱去污染的衣着,用大量流动清水冲洗。
眼睛接触:提起眼睑,用流动清水或生理盐水冲洗。
吸入:脱离现场至空气新鲜处。
就医。
食入:饮足量温水,催吐。
就医。
第五部分:消防措施危险特性: 遇明火、高热可燃.有害燃烧产物:一氧化碳、二氧化碳.灭火方法:消防人员须佩戴防毒面具、穿全身消防服,在上风向灭火。
尽可能将容器从火场移至空旷处。
喷水保持火场容器冷却,直至灭火结束。
处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。
用水喷射逸出液体,使其稀释成不燃性混合物,并用雾状水保护消防人员。
灭火剂:水、雾状水、抗溶性泡沫、干粉、二氧化碳、砂土.第六部分:泄漏应急处理应急处理:迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。
切断火源。
建议应急处理人员戴自给正压式呼吸器,穿防毒服.尽可能切断泄漏源。
防止流入下水道、排洪沟等限制性空间.小量泄漏:用砂土、蛭石或其它惰性材料吸收。
也可以用大量水冲洗,洗水稀释后放入废水系统.大量泄漏:构筑围堤或挖坑收容。
用泵转移至槽车或专用收集器内,回收或运至废物处理场所处置.第七部分:操作处置与储存操作注意事项:密闭操作,全面通风。
操作人员必须经过专门培训,严格遵守操作规程。
建议操作人员佩戴自吸过滤式防毒面具(半面罩),戴化学安全防护眼镜,穿防毒物渗透工作服,戴橡胶手套。
丙二醇MSDS

丙二醇M S D S -CAL-FENGHAI-(2020YEAR-YICAI)_JINGBIAN1,2-丙二醇安全技术说明书第一部分:化学品名称化学品中文名称:1,2-丙二醇化学品俗名:化学品英文名称:methylglycol 英文名称:技术说明书编码:1673 CAS No.:57-55-6生产企业名称:地址:?生效日期:第二部分:成分/组成信息有害物成分含量CAS No.1,2-丙二醇57-55-6第三部分:危险性概述危险性类别:侵入途径:健康危害:对皮肤有原发性刺激作用;对眼无刺激和损害,未见生产性中毒报道。
环境危害:燃爆危险:本品可燃,具刺激性。
第四部分:急救措施皮肤接触:脱去污染的衣着,用大量流动清水冲洗。
眼睛接触:提起眼睑,用流动清水或生理盐水冲洗。
吸入:脱离现场至空气新鲜处。
就医。
食入:饮足量温水,催吐。
就医。
第五部分:消防措施危险特性:遇明火、高热可燃。
有害燃烧产物:一氧化碳、二氧化碳。
灭火方法:第六部分:泄漏应急处理应急处理:迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。
切断火源。
建议应急处理人员戴自给正压式呼吸器,穿防毒服。
尽可能切断泄漏源。
防止流入下水道、排洪沟等限制性空间。
小量泄漏:用砂土、蛭石或其它惰性材料吸收。
也可以用大量水冲洗,洗水稀释后放入废水系统。
大量泄漏:构筑围堤或挖坑收容。
用泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。
第七部分:操作处置与储存操作注意事项:密闭操作,全面通风。
操作人员必须经过专门培训,严格遵守操作规程。
建议操作人员佩戴自吸过滤式防毒面具(半面罩),戴化学安全防护眼镜,穿防毒物渗透工作服,戴橡胶手套。
远离火种、热源,工作场所严禁吸烟。
使用防爆型的通风系统和设备。
防止蒸气泄漏到工作场所空气中。
避免与氧化剂、还原剂接触。
搬运时要轻装轻卸,防止包装及容器损坏。
配备相应品种和数量的消防器材及泄漏应急处理设备。
倒空的容器可能残留有害物。
1,3-丙二醇报告书

1 总论1.1 项目由来1,3-丙二醇(PDO)是一种重要的化工原料,可作为有机溶剂应用于油墨、涂料、润滑剂、抗冻剂等行业,还可用作药物合成中间体。
其最主要的用途是作为聚合体单体合成性能优异的高分子材料。
1,3-丙二醇可以替代乙二醇,1,4丁二醇和新戊二醇等中间体用于生产多醇聚酯及作为碳链延伸剂。
其与苯二甲酸合成的聚对苯二甲酸丙二酯(PTT),显示了比乙二醇、丁二醇为单体合成的聚对苯二甲酸乙二酯(PET)、聚对苯二甲酸丁二酯(PBP)等更优良的性能,被认为是一种兼具PET的高性能和PBT 的易加工性的新型聚酯材料。
目前,世界范围内对聚酯的需求十分旺盛,生产及消费量逐年递增,使得对原料二元醇的需求量也持续增长。
黑龙江省辰能生物工程有限公司从2002年8月开始至2003年9月止对已基本完成1,3-丙二醇项目的工业试验,验证了清华大学的二步发酵法工艺技术。
专家认为,生物发酵法生产1,3-丙二醇,与化学合成法(环氧乙烷法、丙烯醛法)相比,具有利用可再生资源、设备装备简便、操作条件温和、环境友好、大大降低成本等先进性。
本项目的建设将会大大推进我国的发酵法生产1,3-丙二醇这一领域的竞争能力,有利地促进我国发酵行业、合成纤维行业以及纺织业地发展。
国家发展计划委员会于2001年6月30日作了关于黑龙江省电力开发公司发酵法生产1,3-丙二醇高技术产业化示范工程项目可行性研究报告的批复,文号为计高技[2001]1912号。
拟建项目选址于黑龙江肇东市西面高新技术开发区内。
生产能力2500t/a。
受黑龙江省辰能生物工程有限公司的委托,黑龙江省环境保护科学研究院承担了该项目的环境影响评价工作,在现场调查及资料调研的基础上,编制了该项目的环境影响报告书,现提交主管部门及专家审查。
1.2 编制依据1.2.1 相关法律、法规⑴中华人民共和国环境保护法⑵中华人民共和国环境影响评价法⑶中华人民共和国大气污染防治法⑷中华人民共和国水污染防治法⑸中华人民共和国噪声污染防治法⑹中华人民共和国固体废物污染防治法⑺中华人民共和国清洁生产促进法⑻中华人民共和国国务院令第253号《建设项目环境保护管理条例》⑼黑龙江省人民政府令第23号《黑龙江省建设项目环境保护管理办法》⑽《建设项目环境保护分类管理名录》1.2.2 相关技术规范⑴《环境影响评价技术导则》(HJ/T2.1~2.3-93)⑵《环境影响评价技术导则》(HJ/T2.4-1995)⑶《环境影响评价技术导则非污染生态影响》(HJ/T19-1997)1.2.3 相关文件国家发展计划委员会文件《关于黑龙江省电力开发公司发酵法生产1,3丙二醇产业化示范工程项目可行性研究报告的批复》1.3 编制目的按照环评工作的要求,充分分析该建设项目的基本情况(组成、功能、规模等),详细调查项目所在区域的环境概况和环境质量现状,确认项目建设可能产生的环境问题和环境保护目标,确定本次环评的技术路线和实施方案。
3-二甲胺基-1-2-丙二醇-安全技术说明书MSDS

第一部分化学品及企业标识化学品中文名:3-二甲胺基-1,2-丙二醇化学品英文名:3-dimethylaminopropane-1,2-diolCAS No.:623-57-4分子式:C5H13NO2产品推荐及限制用途:工业及科研用途。
第二部分危险性概述紧急情况概述造成严重皮肤灼伤和眼损伤。
GHS危险性类别皮肤腐蚀 / 刺激类别 1B严重眼损伤 / 眼刺激类别 1标签要素:象形图:警示词:警告危险性说明:H314 造成严重皮肤灼伤和眼损伤●预防措施:—— P260 不要吸入粉尘/烟/气体/烟雾/蒸气/喷雾。
—— P264 作业后彻底清洗。
—— P280 戴防护手套/穿防护服/戴防护眼罩/戴防护面具。
●事故响应:—— P301+P330+P331 如误吞咽:漱口。
不要诱导呕吐。
—— P303+P361+P353 如皮肤(或头发)沾染:立即脱掉所有沾染的衣服。
用水清洗皮肤/淋浴。
—— P363 沾染的衣服清洗后方可重新使用。
—— P304+P340 如误吸入:将人转移到空气新鲜处,保持呼吸舒适体位。
—— P310 立即呼叫解毒中心/医生—— P305+P351+P338 如进入眼睛:用水小心冲洗几分钟。
如戴隐形眼镜并可方便地取出,取出隐形眼镜。
继续冲洗。
●安全储存:—— P403+P233 存放在通风良好的地方。
保持容器密闭。
—— P405 存放处须加锁。
●废弃处置:—— P501 按当地法规处置内装物/容器。
物理和化学危险:无资料。
健康危害:造成严重皮肤灼伤和眼损伤。
环境危害:无资料。
第三部分成分/组成信息√物质混合物第四部分急救措施急救:吸入:如果吸入,请将患者移到新鲜空气处。
皮肤接触:脱去污染的衣着,用肥皂水和清水彻底冲洗皮肤。
如有不适感,就医。
眼晴接触:分开眼睑,用流动清水或生理盐水冲洗。
如有不适感,就医。
食入:饮水,禁止催吐。
如有不适感,就医。
对保护施救者的忠告:将患者转移到安全的场所。
咨询医生。
精品(环境管理)1,3丙二醇环境影响报告书

1 总论1.1 项目由来1,3-丙二醇(PDO)是一种重要的化工原料,可作为有机溶剂应用于油墨、涂料、润滑剂、抗冻剂等行业,还可用作药物合成中间体。
其最主要的用途是作为聚合体单体合成性能优异的高分子材料。
1,3-丙二醇可以替代乙二醇,1,4丁二醇和新戊二醇等中间体用于生产多醇聚酯及作为碳链延伸剂。
其与苯二甲酸合成的聚对苯二甲酸丙二酯(PTT),显示了比乙二醇、丁二醇为单体合成的聚对苯二甲酸乙二酯(PET)、聚对苯二甲酸丁二酯(PBP)等更优良的性能,被认为是一种兼具PET的高性能和PBT 的易加工性的新型聚酯材料。
目前,世界范围内对聚酯的需求十分旺盛,生产及消费量逐年递增,使得对原料二元醇的需求量也持续增长。
黑龙江省辰能生物工程有限公司从2002年8月开始至2003年9月止对已基本完成1,3-丙二醇项目的工业试验,验证了清华大学的二步发酵法工艺技术。
专家认为,生物发酵法生产1,3-丙二醇,与化学合成法(环氧乙烷法、丙烯醛法)相比,具有利用可再生资源、设备装备简便、操作条件温和、环境友好、大大降低成本等先进性。
本项目的建设将会大大推进我国的发酵法生产1,3-丙二醇这一领域的竞争能力,有利地促进我国发酵行业、合成纤维行业以及纺织业地发展。
国家发展计划委员会于2001年6月30日作了关于黑龙江省电力开发公司发酵法生产1,3-丙二醇高技术产业化示范工程项目可行性研究报告的批复,文号为计高技[2001]1912号。
拟建项目选址于黑龙江肇东市西面高新技术开发区内。
生产能力2500t/a。
受黑龙江省辰能生物工程有限公司的委托,黑龙江省环境保护科学研究院承担了该项目的环境影响评价工作,在现场调查及资料调研的基础上,编制了该项目的环境影响报告书,现提交主管部门及专家审查。
1.2 编制依据1.2.1 相关法律、法规⑴中华人民共和国环境保护法⑵中华人民共和国环境影响评价法⑶中华人民共和国大气污染防治法⑷中华人民共和国水污染防治法⑸中华人民共和国噪声污染防治法⑹中华人民共和国固体废物污染防治法⑺中华人民共和国清洁生产促进法⑻中华人民共和国国务院令第253号《建设项目环境保护管理条例》⑼黑龙江省人民政府令第23号《黑龙江省建设项目环境保护管理办法》⑽《建设项目环境保护分类管理名录》1.2.2 相关技术规范⑴《环境影响评价技术导则》(HJ/T2.1~2.3-93)⑵《环境影响评价技术导则》(HJ/T2.4-1995)⑶《环境影响评价技术导则非污染生态影响》(HJ/T19-1997)1.2.3 相关文件国家发展计划委员会文件《关于黑龙江省电力开发公司发酵法生产1,3丙二醇产业化示范工程项目可行性研究报告的批复》1.3 编制目的按照环评工作的要求,充分分析该建设项目的基本情况(组成、功能、规模等),详细调查项目所在区域的环境概况和环境质量现状,确认项目建设可能产生的环境问题和环境保护目标,确定本次环评的技术路线和实施方案。
丙二醇msds

Material Safety Data SheetPropylene glycol MSDSCheck for and remove any contact lenses. Immediately flush eyes with running water for at least 15 minutes, keeping eyelids open. Cold water may be used. Get medical attention.Skin Contact:In case of contact, immediately flush skin with plenty of water. Cover the irritated skin with an emollient. Remove contaminated clothing and shoes. Cold water may be used.Wash clothing before reuse. Thoroughly clean shoes before reuse. Get medical attention.Serious Skin Contact:Wash with a disinfectant soap and cover the contaminated skin with an anti-bacterial cream. Seek immediate medical attention.Inhalation:If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention.Serious Inhalation: Not available.Ingestion:Do NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. If large quantities of this material are swallowed, call a physician immediately. Loosen tight clothing such as a collar, tie, belt or waistband.Serious Ingestion: Not available.Precautions:Keep away from heat. Keep away from sources of ignition. Empty containers pose a fire risk, evaporate the residue under a fume hood. Ground all equipment containing material. Do not ingest. Do not breathe gas/fumes/ vapor/spray. Wear suitable protective clothing. In case of insufficient ventilation, wear suitable respiratory equipment. If ingested, seek medical advice immediately and show the container or the label. Avoid contact with skin and eyes. Keep away from incompatibles such as oxidizing agents, reducing agents, acids, alkalis, moisture.Storage:Hygroscopic. Keep container tightly closed. Keep container in a cool, well-ventilated area. Do not store above 23°C (73.4°F).Solubility: Soluble in cold water, hot water, acetone.Products of Biodegradation:Possibly hazardous short term degradation products are not likely. However, long term degradation products may arise. Toxicity of the Products of Biodegradation: The products of degradation are less toxic than the product itself. Special Remarks on the Products of Biodegradation: Not available.Other Special Considerations: Not available.Created: 10/10/2005 08:24 PMLast Updated: 06/09/2012 12:00 PMThe information above is believed to be accurate and represents the best information currently available to us. However, we make no warranty of merchantability or any other warranty, express or implied, with respect to such information, and we assume no liability resulting from its use. Users should make their own investigations to determine the suitability of the information for their particular purposes. In no event shall be liable for any claims, losses, or damages of any third party or for lost profits or any special, indirect, incidental, consequential or exemplary damages, howsoever arising, even if has been advised of the possibility of such damages.。
溴硝丙二醇52-51-7

H318
造成了严重的视力损害。
H335
可能引起呼吸道发炎。
H400
对水生生物非常有毒。
警告申明
P261
避免吸入粉尘/烟/气体/烟雾/蒸汽/喷雾。
P273
避免释放到环境中。
P280
戴防护手套/防护服/护眼/防护面具。
P305+P351+P338 如进入眼睛:用水小心清洗几分钟。如果可以做到,摘掉隐形眼
6.2 环境预防措施
在确保安全的条件下,采取措施防止进一步的泄漏或溢出。不要让产物进入下水道。防止排放到周围环境 中。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。用防电真空清洁器或湿的刷子将溢出物收集起来并放置到容器中去,根据当地规定处理(见第 13部分)。存放在合适的封闭的处理容器内。容器溢出,用电保护的真空吸尘器或者湿的刷子除去,然后装 入容器按照当地法规去处理(见第13部分)。
5.2 源于此物质或混合物的特别的危害
碳氧化物,氮氧化物,溴化氢气
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
水喷雾可用来冷却未打开的容器。
6 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
戴呼吸罩。防止粉尘的生成。防止吸入蒸汽、气雾或气体。保证充分的通风。将人员撤离到安全区 域。避免吸入粉尘。
https://
Powered by TCPDF ()
5/5
12 生态学资料
12.1 毒性
对鱼类的毒性半致死浓度(LC50)-Oncorhynchusmykiss(红鳟)-20mg/l-96.0h 对水蚤和其他水生无脊椎动物的毒性。 半致死有效浓度(EC50)-Daphniamagna(大型蚤)-1.6mg/l-48h
1,3-丙二醇

Development of a Ru/C catalyst for glycerol hydrogenolysis incombination with an ion-exchange resinTomohisa Miyazawa,Shuichi Koso,Kimio Kunimori,Keiichi Tomishige *Institute of Materials Science,University of Tsukuba,1-1-1Tennodai,Tsukuba,Ibaraki 305-8573,Japan Received 3August 2006;received in revised form 8November 2006;accepted 9November 2006Available online 12December 2006AbstractThe combination of Ru/C and Amberlyst ion-exchange resin is effective for the dehydration and hydrogenation (denoted as hydrogenolysis)of glycerol to 1,2-propanediol under mild reaction conditions (393K).A Ru/C catalyst prepared by using active carbon with a low surface area ($250m 2/g)showed better performance than that prepared by using active carbon with a high surface area.In addition,treatment of Ru/C catalysts prepared from Ru(NO)(NO 3)3with Ar flowing at the appropriate temperature enhanced the performance compared to that of the commercially available Ru/C catalysts.This temperature treatment can be influenced by the decomposition of Ru precursor salt and aggregation of Ru metal particles.In addition,the degradation reaction as a side-reaction to C1and C2compounds of glycerol hydrogenolysis was more structure-sensitive than the hydrogenolysis reaction,and the selectivity of hydrogenolysis was lower on smaller Ru particles.The combination of Ru/C with the Amberlyst resin enhanced the turnover frequency of 1,2-propanediol formation drastically,and this indicates that 1,2-propanediol can be formed mainly by dehydration of glycerol to acetol catalyzed by Amberlyst and subsequent hydrogenation of acetol to 1,2-propanediol catalyzed by Ru/C.#2006Elsevier B.V .All rights reserved.Keywords:Glycerol;Hydrogenolysis;Ruthenium;Ion-exchange resin;Propanediol1.IntroductionInterest in the catalytic conversion of renewable feedstocks and chemicals has been increasing.Such conversion to hydrogen can contribute to the utilization of renewable energy sources [1–5],and conversion to petrochemicals can facilitate the replacement of petroleum by renewable resources [6,7].Recently,it has been proposed that commodity chemicals that are used to produce pharmaceuticals,agricultural adjuvants,plastics and transportation fuel that are derived from fossil resources might be producible in future biorefineries from renewable resources,such as plant-derived sugar and other compounds [8].Glycerol is a building block that might serve as an important biorefinery feedstock [8].In addition,it is a byproduct from the production of biodiesel from vegetable oils [9].Glycerol can be converted to hydrogen and synthesis gas by a reforming reaction [10,11].One of the methods is conversion of glycerolto 1,2-propanediol and 1,3-propanediol,which have been produced from petroleum derivatives [12].Several routes to the formation of propanediol can be traced from renewable feedstocks.The commonest route is the conversion of sugar or sugar alcohols at high temperatures and pressures in the presence of a metal catalyst to produce propanediol and other lower polyols [13].It has been reported that propanediol is producible through catalytic conversion of polyols [14]and glycerol.Supported metal catalysts,hydrogen pressure of $6–10MPa and reaction temperatures of 453–513K have been applied [15–18].Recently,it was reported that the reaction of glycerol proceeded at a hydrogen pressure of 1.4MPa and a temperature of 473K [13].Judging from these various reports,it seems to be difficult to use milder reaction conditions,especially reduced reaction temperatures.Our group reported recently that the addition of solid acid catalysts to Ru/C enhanced conversion and selectivity in glycerol hydrogenolysis [7,19].Our results suggest that the conversion of glycerol to propanediols proceeds by the combination of dehydration over acid catalysts with subsequent hydrogenation over metal catalysts [7,19].In this study,it is found that Ru/C catalysts prepared by using Ru(NO)(NO 3)3and active carbon with a low/locate/apcataApplied Catalysis A:General 318(2007)244–251*Corresponding author.Tel.:+81298535030;fax:+81298535030.E-mail address:tomi@tulip.sannet.ne.jp (K.Tomishige).0926-860X/$–see front matter #2006Elsevier B.V .All rights reserved.doi:10.1016/j.apcata.2006.11.006surface area and treated under Arflowing at the optimum temperature exhibited much higher performance in the glycerol reaction under H2in combination with Amberlyst as a solid acid catalyst than the commercial Ru/C.In addition,from the comparison of data obtained in the optimization process of Ru/ C catalysts,the structure-sensitivity of the glycerol hydro-genolysis and degradation reaction is discussed,and the reaction route to various products is discussed on the basis of the turnover frequency.2.Experimental2.1.CatalystActive carbon-supported Ru catalysts were prepared by using various active carbon materials.Vulcun-XC72was supplied by Cabot Corporation Ltd.,Shirasagi DO-2,Shirasagi M and Carboraffin were supplied by Japan EnviroChemicals Ltd.Vulcun-XC72,Shirasagi DO-2,Shirasagi M and Carbor-affin are denoted as C(I),C(II),C(III)and C(IV),respectively. Ru was loaded by impregnating carbon supports with an aqueous solution of Ru(NO)(NO3)3and RuCl3,and an acetone solution of Ru(acac)3as a precursor.After impregnation and solvent removal by evaporation,the catalysts were dried for 12h at393K.The amount of Ru loaded was in the range of3–10wt%.The amount of Ru loaded is denoted as Ru5/C(I)in the case of5wt%Ru supported on active carbon(I).These catalysts were used also after treatment under Arflowing at a temperature of473–773K.In addition,5wt%Ru/C was purchased from Wako Pure Chemical Industries Ltd.,and this catalyst is denoted as Ru5/C(V).The cation-exchange resin Amberlyst15(4.7equiv./kg resin dried,particle size0.4–1.2mm,highest operating temperature393K;MP Biomedi-cals),which consists of highly cross-linked styrene–divinyl benzene copolymer beads that are functionalized with sulfonic groups,was used as the solid acid catalyst.All catalysts were in powder form,with less than0.1mm.2.2.Activity testGlycerol hydrogenolysis was carried out with a20ml aqueous solution of glycerol in a70ml stainless-steel autoclave.The standard reaction was conducted under the following conditions:393K reaction temperature,8.0MPa initial hydrogen pressure,10h reaction time,20wt%glycerol aqueous solution,150mg Ru/C catalysts,and300mg Amberlyst.Reaction conditions were varied for investigation of the effects of different conditions.Details of the reaction conditions are described for each result.In addition,to elucidate the mechanism of the glycerol reaction,we used an aqueous solution of2wt%.1,3-Propanediol(1,3-PD),and1,2-propanediol(1,2-PD)as a reactant.In all experiments,the aqueous solution of the reactant,the catalyst powder,and the spinner were put into the autoclave; then the reactor was purged with H2(99.99%;Takachiho Trading Co.Ltd.).After purging,the reactor was heated to the required temperature and the H2pressure was increased to 8.0MPa in standard experiments.The temperature was monitored with a thermocouple that was inserted into the autoclave and connected to the thermo-controller.The reaction consumed hydrogen,and the total pressure decreased.However,the decrease in hydrogen pressure was,at most,1/10of the initial pressure.After the reaction, the gas phase products were collected in a gasbag and the liquid phase products were separated from the used catalyst byfiltration.These products were analyzed using a gas chromatograph(GC-353;GL Sciences Inc.)equipped with a flame ionization detector(FID).A TC-WAX capillary column (diameter0.25mm,length20m)was used for separation and the column temperature was493K.Products were identified also using GC–MS(GCMS-QP5050,column Stabilwax; Shimadzu Corp.)and the products detected were:1,3-propanediol(1,3-PD),1,2-propanediol(1,2-PD),1-propanol (1-PO),and2-propanol(2-PO)are hydrogenolysis products, and ethylene glycol(EG),ethanol,methanol and methane are degradation products.Conversion of the reactants in all reaction tests were calculated on the basis of the following equation:conversion of reactantð%Þ¼sum of C-based mol of all productssum of C-based mol of reactant and all productsÂ100The conversion of a reactant is usually defined as:reactant beforeÀreactant afterwardsreactant beforebut we have to determine the conversion and the selectivity even when the level of conversion is very low.Considering the error of the analysis procedure,we applied the above method of calculation.It should be noted that the conversions calculated by our method and the method based on mass balance agreed well when the conversion was greater than5%.The selectivity of the products in all reaction tests was calculated with the following equation,considering that the degradation byproducts(ethylene glycol,ethanol,methanol, and methane)were always formed:selectivityð%Þ¼C-based mol of the productÂ100Here,we simply assume that the degradation products are formed directly from glycerol and other reactants in terms of carbon number in each molecule.For example,when one molecule of glycerol is converted to one molecule of ethylene glycol and one molecule of methane,the selectivity of ethylene glycol and methane is calculated to be66.7%and33.3%, respectively.Here,it is interpreted that two-thirds of a glycerol molecule is converted to one molecule of ethylene glycol molecule,and at the same time one-third of the glycerol molecule is converted to one molecule of methane.The yield is calculated as:conversionð%ÞÂselectivityð%Þ100:T.Miyazawa et al./Applied Catalysis A:General318(2007)244–2512452.3.CharacterizationThe surface area of the supported metal catalysts was measured using the BET method (N 2adsorption)with a Gemini apparatus (Micromeritics Instrument Corporation).The sizes of metal particles and the dispersion of the carbon-supported Ru catalyst were estimated from irreversible CO adsorption measurements performed at room temperature.The gas pressure at the adsorption equilibrium was about 1.1kPa and the sample weight was about 0.2g.The dead-volume of the apparatus was about 60cm 3.The results of the characterization of the fresh metal catalysts are given in Table 1.Before the CO adsorption,the catalyst was treated without evaporating to the atmosphere under Ar flowing at a temperature of 473–773K for 2h,and reduced under flowing hydrogen at 393K for 1h.X-ray diffraction (XRD)spectra recorded with a Philips X’pert diffractometer was used in order to confirm the graphite phase of the carbon supports.Transmission electron microscope (TEM)images were taken for determination of the particle size with a JEM 2010instrument (JEOL)operated at 200kV .The samples after the glycerol reaction were dispersed by supersonic waves in 2-propanol;they were placed on Cu grids under air atmosphere.Temperature-programmed desorption (TPD)of the carbon supports was done in a closed circulating vacuum system equipped with a variable leak valve,by which the gas was introduced into a differentially pumped quadrupole mass spectrometer (Balzers QMS 200F).Without any pretreatment,the sample support (10mg)was heated under vacuum from room temperature to 1273K at 10K/min.Desorbed CO 2and CO were analyzed by QMS.In order to characterize the effect of treatment with Ar,the thermal stability of impregnated Ru/C catalyst was evaluated using the TPD profile of Ru5/C(I).Fresh catalyst (25mg)was used without pretreatment.The sample was heated under vacuum from room temperature to 773K at 10K/min.3.Results and discussion3.1.Glycerol reaction on various active carbon-supported Ru catalystsResults of the activity test with various combinations of Ru/C and Amberlyst in the reaction of glycerol are described inFig.1and the characterization results are given in Table 1.Except for Ru5/C(V),all the carbon-supported Ru catalysts were pretreated under 30cm 3/min Ar flow at 573K.This treatment with Ar is the optimum activation condition,as shown later.It is found that Ru5/C(I)+Amberlyst exhibited a higher level of glycerol conversion and selectivity toward 1,2-propanediol than the commercially available Ru5/C(V)+Am-berlyst,which was shown to be an effective catalyst in our earlier studies [7,19].On the other hand,Ru5/C(II),Ru5/C(III)and Ru5/C(IV)catalysts showed a much lower level of activity than the commercial Ru5/(V).In addition,we tested Ru5/C(V)after treatment with Ar and found that the performance was almost the same as that without the treatment with Ar.From a comparison between the catalytic activity and characterization results,such as BET surface area and metal dispersion,it seems that a lower surface area is more suitable,and the metal dispersion seems to be unrelated.Fig.2shows the XRDTable 1Properties of various active carbon-supported Ru catalysts Catalysts Surface area (m 2/g)Pretreatment temperature Dispersion Ru5/C(I)a 254573K,Ar 0.60Ru5/C(II)a 521573K,Ar 0.88Ru5/C(in)a 629573K,Ar 0.90Ru5/C(IV)a 1046573K,Ar0.70Ru5/C(V)485None0.41aRu were impregnated from RuNO(NO 3)3and loading amount of Ru is 5wt%.C(I),Vulcun-XC72;C(II),Shirasagi DO-2;C(III),Shirasagi M;C(IV),Carboraffin;Ru/C(V),Ru/C(Wako).Fig.1.Glycerol reaction on various active carbon-supported Ru catalyst-s +Amberlyst.Reaction conditions:20mass%glycerol aqueous solution 20ml,393K reaction temperature,8.0MPa initial H 2pressure,10h reaction time,150mg of Ru catalyst +300mg of Amberlyst.PD,propanediol;PO,propanol;others,ethylene glycol +ethanol +methanol +methane.*Ru was impregnated from RuNO(NO 3)3and treated with Ar at 573K.The loading amount of Ru is 5wt%.Fig.2.XRD patterns of various Ru/C catalysts.C(I),Vulcun-XC72;C(II),Shirasagi DO-2;C(III),Shirasagi M;C(IV),Carboraffin,All samples were used without treatment after impregnation.T.Miyazawa et al./Applied Catalysis A:General 318(2007)244–251246patterns of various Ru/C catalysts.The peak around 25.28is assigned to diffraction of the graphite phase (JCPDS File,no.41–1487).It is characteristic that the graphite phase was observed much more clearly for Ru/C(I)than for other catalysts.This suggests that Ru metal particles on the graphite phase have a high level of catalytic activity.Fig.3shows the TPD profiles of CO and CO 2on the various carbon supports.It is clear that the desorption of CO and CO 2on C(I)was much less than it was on other supports.The desorption of CO 2and CO is due to the decomposition of surface functional groups,such as carboxyl and carbonyl groups [20–22].This result indicates also that C(I)has a much fewer surface functional groups than other carbon supports.The order of the total amount of desorbed CO and CO 2(C(IV))C(III)>C(II))C(I))can be related to the order of the catalytic activity in the glycerol reaction (Ru/C(I))Ru/C(II)>Ru/C(III)>Ru/C(IV)).This suggests that the carbon surface with less oxygen-containing functional groups,such as carbonyl and carboxyl groups,is more suitable as a support for Ru metal particles.This can be related to the suitability of the graphite phase.At present,the reason for the suitability of a graphite phase with less surface carboxylic and carbonyl functional groups is not clear and further investigation is necessary.The Ru5/C(I)catalysts were used for subsequent experiments.The effect of the Ru precursor,the loading amount and the catalyst treatment over Ru/C(I)+Amberlyst in the glycerol reaction at 393K are given in Table 2.Except for Ru5/C(I)from Ru(acac)3,it is clear that pretreatment with Ar enhanced the catalyst activity.In the case of Ru5/C(I)from Ru(acac)3after treatment with Ar,the conversion of glycerol was very low (<0.2%)(data not shown).This can be related to the sublimation of Ru(acac)3during the treatment.On the other hand,regarding RuCl 3,the sublimation was negligible.This is based on the thermogravimetric analysis (TGA)of Ru5/C(I)from RuCl 3under Ar flow from room temperature to 573K.Except the vaporization of water in the catalysts at room temperature to 373K,the weight loss corresponds to almost 5%,and this could be due to the amount of Cl in the Ru precursor (RuCl 3),where the decomposition of HCl was detected by MS.This is supported by the fact that the treatment with Ar improved the catalytic activity of Ru5/C(I)from RuCl 3+Amberlyst,and it is interpreted that the precursor salt decomposed during the treatment.Ru5/C(I)from Ru(NO)(NO 3)3+Amberlyst exhibited thegreatestFig.3.TPD profiles of various carbon supports.(a)CO 2desorption;(b)CO desorption;C(I),Vulcun-XC72;C(II),Shirasagi DO-2;C(III),Shirasagi M;C(IV),Carboraffin.Sample weight 10mg,heating rate 10K/min.All samples were used without pretreatment.Table 2Effect of Ru precursor,loading amount and catalyst treatment over Ru/C +Amberlyst Ru catalystPretreatment temperatureConversion (%)Selectivity of each product (%)a 1,2-PD1,3-PD 1-PO 2-PO Others Ru3b /C(I)Ru(NO)(NO 3)3None 2.469.6 3.414.1 1.611.4Ru5/C(I)Ru(NO)(NO 3)3None 4.759.4 6.713.50.819.6RulO/C(I)Ru(NO)(NO 3)3None 3.665.5 3.110.5 1.319.6Ru5/C(I)Ru(acac)3None 3.732.911.817.6 1.136.5Ru5/C(I)c RuCl 3None 0.324.00.00.015.234.7Ru3/C(I)Ru(NO)(NO 3)3573K,Ar 9.575.8 4.68.1 1.310.2Ru5/C(I)Ru(NO)(NO 3)3573K,Ar 21.376.7 1.5 2.50.518.8RulO/C(I)Ru(NO)(NO 3)3573K,Ar 18.146.8 5.216.30.631.1Ru5/C(I)RuCl 3573K,Ar5.273.84.913.91.75.7Reaction conditions:20mass%glycerol aqueous solution 20ml,393K reaction temperature,8.0MPa initial H 2pressure,10h reaction time,150mg Ru catalyst +300mg Amberlyst,PD,propanediol;PO,propanol;others,ethylene glycol +ethanol +methanol +methane.aC-based selectivity.bloading amount of Ru(wt%).cRest 26%product is acetol.T.Miyazawa et al./Applied Catalysis A:General 318(2007)244–251247conversion of glycerol both with and without treatment with Ar.In addition,regarding the effect of the loading amount of Ru,the activity of Ru10/C(I)+Amberlyst with Ar treatment was comparable to the case of Ru5/C(I);however,it showed higher degradation selectivity.These results indicate that the amount of Ru on the carbon support must be optimized for high selectivity of hydrogenolysis reactions,and too much Ru promotes degradation reactions.This tendency is compatible with earlier results [7].On the basis of the results presented here,we focused on the Ru5/C(I)catalyst in subsequent studies.3.2.Effect of catalyst pretreatment temperature on glycerol reaction over Ru/C(I)The effect of catalyst pretreatment temperature on the glycerol reaction over Ru5/C(I)+Amberlyst is shown in Fig.4.The activity was maximum on the catalyst treated at 573K,and this showed the highest level of selectivity for 1,2-propanediol (1,2-PD).It should be noted that Ru5/C(I)+Amberlyst treated at 573K is highly active in glycerol hydrogenolysis compared to Ru5/(V)+Amberlyst.As a reference,the effect of pretreatment temperature on the glycerol reaction over Ru5/C(I)in the absence of Amberlyst is shown in Fig.5.Thepretreatment temperature-dependence is similar to that of Ru5/C(IV)+Amberlyst.From comparison of the results illustrated by Figs.4and 5,it is clear that the addition of Amberlyst enhanced the activity of glycerol hydrogenolysis remarkably,compared to the degradation activity.The selectivity for hydrogenolysis products was promoted drastically.In addition,without pretreatment with Ar,Ru5/C(I)and Ru5/C(I)+Am-berlyst exhibited a lower level of activity than the correspond-ing commercial Ru5/C(IV)catalysts.In order to investigate the structural change of the Ru catalyst during treatment with Ar,we measured the CO uptake and the results are given in Table 3.The CO uptake and dispersion decreased monotonously with increasing pretreatment temperature.This phenomenon is explained easily as the aggregation of Ru by the thermal treatment.Here,an important point is that Ru5/C(I)treated below 523K showed a lower level of activity in the glycerol reaction,in spite of the large amount of CO adsorption.In order to investigate what happens on Ru5/C(I)during the treatment,the TPD profile was obtained,and the result is described in Fig.6.A large amount of NO desorption was observed in the temperature range 400–600K.The total amount of desorbed NO corresponded to a molar ratio of NO/Ru of nearly 4.Since the precursor is Ru(NO)(NO 3)3,it is suggested thattheFig.4.Effect of temperature of pretreatment of catalysts on glycerol reaction over Ru5/C(I)+Amberlyst at 393K.The reaction conditions are the same as those given in the legend to Fig.1.*Ru5/C(V)+Amberlyst was used as areference.Fig.5.Effect of pretreatment temperature of Ru5/C(I)on the glycerol reaction at 393K.The reaction conditions are the same as those given in the legend to Fig.1,except the amount of Amberlyst.*Ru5/C(V)was used as a reference.Table 3Characterization results of Ru5/C CatalystsAr pretreatment temperature (K)CO uptake amount (mmol)Dispersion (CO/Ru)Acetol hydrogenation Conversion (%)TOF (1/100h À1)Ru5/C(I)4730.400.8122.7 2.15230.410.8229.6 2.65730.300.6041.3 5.06230.270.5536.5 4.86730.230.4730.6 4.77730.190.3822.1 4.3Ru5/C(V)None0.200.4124.64.3Acetol hydrogenation reaction conditions:2mass%acetol aqueous solution 20ml,393K reaction temperature,1.0MPa initial H 2pressure,1h reaction time,1.5mg Ru catalyst.T.Miyazawa et al./Applied Catalysis A:General 318(2007)244–251248precursor can be maintained during the drying process in the catalyst preparation procedure,and the decomposition of the precursor starts at 400K.At higher temperatures,NO and NO 3Àspecies can be desorbed as NO.In addition,the result means that NO molecules from the precursor exist even after treatment with Ar at 473–523K.On the other hand,considering the result of the large amount of CO adsorption,the precursor salt before the decomposition can also adsorb the CO molecule.However,the activity in the glycerol hydrogenolysis of this species is thought to be very low,probably because adsorbed NO can suppress the hydrogenolysis activity.In contrast,it is possible to interpret the behavior of the catalysts treated at temperatures higher than 573K as simply being influenced by the aggregation of Ru metal particles.Fig.7shows the TEM images of the Ru/C(I)and Ru/C(V)used in the glycerol reaction.The average particle size of Ru is calculated as:mean diameter ðd s Þ¼P n i d3i P n i d 2iwhere n i is the number of particles having a characteristic diameter d i (within a given diameter range)[23].The metalparticle size on Ru/C(I)is calculated as 1.7Æ0.3nm,and that on Ru/C(V)is 2.5Æ0.3nm.This indicates that Ru metal particles have greater dispersion on Ru/C(I).This tendency agreed with that from the CO adsorption measurement.On the basis of the relationship D =1.32/d between particle size d (in nm)and D (in %)[24],the dispersion is calculated to be 78Æ12%on Ru/C(I)and 53Æ7%on Ru/C(V).The disper-sion estimated from TEM is a little higher than that from CO adsorption (Table 3)for both catalysts.This is probably due to the error in the assumption of CO/Ru s =1,because a CO bridge can be present.This tendency in terms of metal dispersion can be related to the higher level of activity of Ru/C(I)compared to that of Ru/C(V).3.3.Reaction schemeOn the basis of CO uptake,we calculated the turnover frequency (TOF)of hydrogenolysis and degradationreactionsFig. 6.Temperature-programmed desorption profile of NO on Ru5/C(I).Sample weight 25mg,heating rate 10K/min.Fig.7.TEM image of Ru/C catalysts after the glycerol reaction at 393K for 10h.(a)Ru5/C(I);(b)Ru5/C(V).Fig.8.Effect of the pretreatment temperature on the TOF in the glycerol reaction.TOFs were calculated from the data shown in Figs.3and 4.T.Miyazawa et al./Applied Catalysis A:General 318(2007)244–251249in the glycerol reaction,and the results are shown in Fig.8.In particular,TOFs of hydrogenolysis are represented as 1,2-propanediol (1,2-PD)and 1,3-propanediol (1,3-PD)+1-pro-panpol (1-PO)+2-propanol (2-PO)separately.This is because 1-PO and 2-PO are formed mainly via 1,3-PD,as discussed later.The TOF of glycerol hydrogenolysis over Ru5(I)/C in the treatment temperature range 573–773K was not changed,although the dispersion was changed from 0.38to 0.60(Table 3).On the other hand,the TOF of the degradation reaction over the Ru5/C(I)catalysts was changed remarkably.This indicates that the reaction to degradation products (EG +C 2H 5OH +CH 3OH +CH 4)is more influenced than the hydrogenolysis reaction over Ru5/C(I)catalysts.This suggests that smaller metal particles can decrease the hydrogenolysis selectivity.From the comparison of Ru5/C(I)with and without Amberlyst,it was found that the TOF of 1,3-PD +1-PO +2-PO was not influenced by the presence of Amberlyst,and this suggests that the formation of 1,3-PD +1-PO +2-PO can be catalyzed by only Ru5/C(I).In contrast,it is interesting that the TOF of the 1,2-PD formation can be enhanced remarkably by the presence of Amberlyst.This result suggests that 1,2-PD formation can be catalyzed by Amberlyst and Ru5/C(I).From the combination of the present discussion and that reported previously [7],the reaction route from glycerol to 1,2-PD is thought to be as follows:dehydration of glycerol to acetol is catalyzed by Amberlyst and consecutive hydrogenation of acetol to 1,2-PD is catalyzed by Ru/C.The possible reaction route is shown in Fig.9.In order to confirm this expectation,we carried out an activity test of acetol hydrogenation,and the results are summarized in Table 3.The behavior of 1,2-PD formation in the glycerol reaction agrees well with that of acetol hydrogenation,and this supports the expected reaction route to 1,2-PD.In addition,the TOF of degradation was increased by the presence of Amberlyst.However,the effect is not so significant as that in the TOF of 1,2-PD formation.This can be explained by the degradation via 1,2-PD.In order to investigate the consecutive reactions,the reaction tests of 1,2-PD and 1,3-PD were carried out (Table 4).The conversion in the glycerol reaction was comparable to that of the 1,3-PD reaction;in contrast,the conversion of 1,2-PD was much lower than that of glycerol.The low reactivity of 1,2-PD is related to the high yield of 1,2-PD in the glycerol reaction,and the high reactivity of 1,3-PD causes the very low yield of 1,3-PD in the glycerol reaction.In addition,the selectivity ratio of 1-PO to 2-PO is characteristic.In the reaction of 1,3-PD,mainly 1-PO was formed;in contrast,the formation of 1-PO was comparable to that of 2-PO in the 1,2-PD reaction.The selectivity ratio of 1-PO to 2-PO in the glycerol reaction was more similar to that in the 1,3-PD reaction,and this suggests that 1-PO and 2-PO can be formed via 1,3-PD in the glycerol reaction.Furthermore,the degradation reaction proceeded in the reactions of both 1,2-PD and 1,3-PD,and this can be related to the tendency that the TOF of degradation was increased slightly by the presence of Amberlyst,as shown in Fig.8.Fig.9.Reaction scheme of glycerol hydrogenolysis and degradation.Table 4Results of the reaction test of 2mass%aqueous solution of various compounds over Ru5/C(I)+Amberlyst under H 2CatalystsReactantConversion (%)Selectivity of each product (%)a 1,2-PD1,3-PD 1-PO 2-PO EG C 2H 5OH CH 3OH CH 4Ru5/C(I)+AmberlystGlycerol 79.374.70.07.7 1.6 6.8 3.80.4 4.91,2-PD 20.1–0.035.730.90.321.90.011.11.3-PD81.00.0–23.50.10.050.90.025.4Reaction conditions:2mass%glycerol aqueous solution 20ml,393K reaction temperature,8.0MPa initial H 2pressure,10h reaction time,150mg Ru catalyst +300mg Amberlyst.PD,propanediol;PO,propanol;EG,ethylene glycol.aC-based selectivity.T.Miyazawa et al./Applied Catalysis A:General 318(2007)244–251250。
1,3-丙二醇 MSDS

生态毒理毒性:
生物降解性: 非生物降解性: 生物富集或生物积累性: 其它有害作用: 无资料。
第十三部分:废弃处置
废弃物性质: 废弃处置方法: 处置前应参阅国家和地方有关法规。建议用焚烧法处置。 废弃注意事项:
第十四部分:运输信息
危险货物编号: 无资料 UN 编号: 无资料 包装标志: 包装类别: Z01 包装方法: 无资料。 运输注意事项: 运输前应先检查包装容器是否完整、密封,运输过程中要确保容器不泄漏 、 不倒塌、不坠落、不损坏。严禁与氧化剂、还原剂、食用化学品等混装混运。运输车船必须 彻底清洗、消毒,否则不得装运其它物品。船运时,配装位置应远离卧室、厨房,并与机舱 、 电源、火源等部位隔离。公路运输时要按规定路线行驶。
第九部分:理化特性
主要成分: 纯品 外观与性状: 无色、无臭,具咸味、吸湿性的粘稠液体。
pH: 熔点(℃): -27 沸点(℃): 210-211 相对密度(水=1): 1.05(25℃) 相对蒸气密度(空气=1): 2.6 饱和蒸气压(kPa): 0.13(60℃) 燃烧热(kJ/mol): 无资料 临界温度(℃): 无资料 临界压力(MPa): 无资料 辛醇/水分配系数的对数值: 无资料 闪点(℃): 79 引燃温度(℃): 400 爆炸上限%(V/V): 无资料 爆炸下限%(V/V): 无资料 溶解性: 与水混溶,可混溶于乙醇、乙醚。 主要用途: 用作溶剂, 用于有机合成。 其它理化性质:
干粉、二氧化碳、砂土。
第六部分:泄漏应急处理
应急处理: 迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。 建议应急处理人员戴自给正压式呼吸器,穿一般作业工作服。尽可能切断泄漏源。防止流入 下水道、排洪沟等限制性空间。小量泄漏:用砂土、蛭石或其它惰性材料吸收。也可以用大 量水冲洗,洗水稀释后放入废水系统。大量泄漏:构筑围堤或挖坑收容。用泵转移至槽车或 专用收集器内,回收或运至废物处理场所处置。
丙二醇
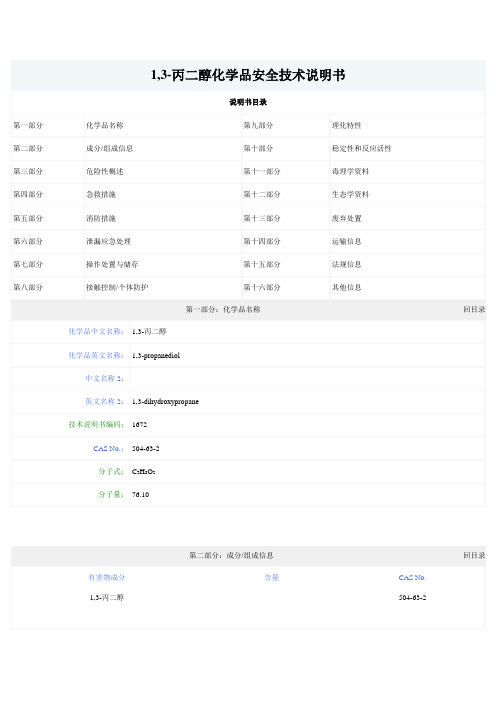
第二部分:成分/组成信息回目录有害物成分含量CAS No.
1,3-丙二醇504-63-2
第六部分:泄漏应急处理回目录
应急处理:迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。
切断火源。
建议应急处理人员戴自给正压式呼吸器,穿一般作业工作服。
尽可能切断泄漏源。
防止流入下水道、排洪沟等限制性空间。
小量泄漏:用
砂土、蛭石或其它惰性材料吸收。
也可以用大量水冲洗,洗水稀释后放入废水系统。
大量泄漏:构筑围堤或
挖坑收容。
用泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。
第十三部分:废弃处置回目录
第十五部分:法规信息回目录法规信息化学危险物品安全管理条例(1987年2月17日国务院发布),化学危险物品安全管理条例实施细则(化劳发[1992] 677号),工作场所安全使用化学品规定([1996]劳部发423号)等法规,针对化学危险品的安全使用、生产、储存、运输、装卸等方面均作了相应规定。
丙二醇理化性质及危险特性

0.02(25℃)
溶解性
与水混溶,可混溶于乙醇、乙醚、多数有机溶剂。
毒性及健康危害
职业接触限值
最高容许浓度(mg/m3)
-
时间加权平均容许浓度(mg/m3)
-
短时间接触容许浓度(PC-STEL)(mg/m3)
-
侵入途径
吸入、食入、经皮吸收。源自毒性LD50:21000~32200mg/kg(大鼠经口);22000mg/kg(小鼠经口)
包装方法
小开口钢桶;安瓿瓶外普通木箱;螺纹口玻璃瓶、铁盖压口玻璃瓶、塑料瓶或金属桶(罐)外普通木箱。
储存注意事项
储存于阴凉、通风仓间内。远离火种、热源。防止阳光直射。保持容器密封。应与氧化剂、酸类、碱类分开存放。搬运时要轻装轻卸,防止包装及容器损坏。
泄露处
理
切断火源。戴好防毒面具,穿一般消防防护服。用大量水冲洗,经稀释的洗液放入废水系统。如大量泄漏,利用围堤收容,然后收集、转移、回收或无害处理后废弃。
建规火险分级
稳定性
稳定
聚合危害
不聚合
禁忌物
酰基氯、酸酐、氧化剂、还原剂。
灭火方法
雾状水、泡沫、二氧化碳、干粉、砂土。
防护措施
呼吸系统防护
一般不需要特殊防护,高浓度接触时可佩带自给式呼吸器。
眼睛防护
可采用安全面罩。
身体防护
穿工作服。
手防护
必要时戴防化学品手套。
其他防护
工作现场严禁吸烟。注意个人清洁卫生。避免长期反复接触。
丙二醇理化性质及危险特性
标
识
中文名:丙二醇
危险化学品目录序号:
英文名:Propanediol
UN编号:
分子式:C3H8O2
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
化学品安全技术说明书(MSDS)
1,3-丙二醇
版本:20110901第一部分:化学品名称
化学品中文名称:1,3-丙二醇
化学品英文名称:1,3-propanediol
英文名称2:1,3-dihydroxypropane
技术说明书编码:1672
第二部分:危险性概述
危险性类别:无
侵入途径:无
健康危害:对眼和皮肤无刺激作用。
未见中毒报道。
环境危害:无
燃爆危险:本品可燃。
风险术语:无
安全术语:S24/25;
危险标志:
第三部分:成分/组成信息
有害物成分
1,3-丙二醇
CAS No.:504-63-2
分子式:C3H8O2
分子量:76.10
第四部分:急救措施
皮肤接触:脱去污染的衣着,用流动清水冲洗。
眼睛接触:提起眼睑,用流动清水或生理盐水冲洗。
吸入:脱离现场至空气新鲜处。
就医。
食入:饮足量温水,催吐。
就医。
第五部分:消防措施
危险特性:遇明火、高热可燃。
有害燃烧产物:一氧化碳、二氧化碳。
灭火方法:尽可能将容器从火场移至空旷处。
喷水保持火场容器冷却,直至灭火结束。
处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。
用水喷射逸出液体,使其稀释成不燃性混合物,并用雾状水保护消防人员。
灭火剂:水、雾状水、抗溶性泡沫、干粉、二氧化碳、砂土。
第六部分:泄漏应急处理
应急处理:迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。
切断火源。
建议应急处理人员戴自给正压式呼吸器,穿一般作业工作服。
尽可能切断泄漏源。
防止流入下水道、排洪沟等限制性空间。
小量泄漏:用砂土、蛭石或其它惰性材料吸收。
也可以用大量水冲洗,洗水稀释后放入废水系统。
大量泄漏:构筑围堤或挖坑收容。
用泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。
第七部分:操作处置与储存
操作注意事项:密闭操作,全面通风。
操作人员必须经过专门培训,严格遵守操作规程。
远离火种、热源,工作场所严禁吸烟。
使用防爆型的通风系统和设备。
防止蒸气泄漏到工作场所空气中。
避免与氧化剂、还原剂接触。
搬运时轻装轻卸,防止包装破损。
配备相应品种和数量的消防器材及泄漏应急处理设备。
倒空的容器可能残留有害物。
储存注意事项:储存于阴凉、通风的库房。
远离火种、热源。
应与氧化剂、还原剂等分开存放,切忌混储。
配备相应品种和数量的消防器材。
储区应备有泄漏应急处理设备和合适的收容材料。
第八部分:接触控制/个体防护
工程控制:生产过程密闭,全面通风。
呼吸系统防护:一般不需要特殊防护,但建议特殊情况下,建议佩戴自吸过滤式防毒面具(半面罩)。
眼睛防护:一般不需特殊防护。
身体防护:穿一般作业防护服。
手防护:戴一般作业防护手套。
其他防护:工作现场严禁吸烟。
避免长期反复接触。
定期体检。
第九部分:理化特性
主要成分:纯品
外观与性状:无色、无臭,具咸味、吸湿性的粘稠液体。
熔点(℃):-27
沸点(℃):210-211
相对密度(水=1):1.05(25℃)
相对蒸气密度(空气=1):2.6
饱和蒸气压(kPa):0.13(60℃)
闪点(℃):79
引燃温度(℃):400
爆炸上限%(V/V):无资料
爆炸下限%(V/V):无资料
溶解性:与水混溶,可混溶于乙醇、乙醚。
主要用途:用作溶剂, 用于有机合成。
其它理化性质:
第十部分:稳定性和反应活性
禁配物:酰基氯、酸酐、氧化剂、还原剂。
第十一部分:毒理学资料
急性毒性:LD50:16080 mg/kg(大鼠经口);6500 mg/kg(小鼠经口)
第十二部分:生态学资料
无资料。
第十三部分:废弃处置
废弃处置方法:处置前应参阅国家和地方有关法规。
建议用焚烧法处置。
第十四部分:运输信息
包装类别:Z01
运输注意事项:运输前应先检查包装容器是否完整、密封,运输过程中要确保容器不泄漏、不倒塌、不坠落、不损坏。
严禁与氧化剂、还原剂、食用化学品等混装混运。
运输车船必须彻底清洗、消毒,否则不得装运其它物品。
船运时,配装位置应远离卧室、厨房,并与机舱、电源、火源等部位隔离。
公路运输时要按规定路线行驶。
第十五部分:法规信息
法规信息化学危险物品安全管理条例(1987年2月17日国务院发布),化学危险物品安全管理条例实施细则(化劳发[1992] 677号),工作场所安全使用化学品规定([1996]劳部发423号)等法规,针对化学危险品的安全使用、生产、储存、运输、装卸等方面均作了相应规定。
