纠正预防措施 中英文版
8D中英文纠正及预防--报告

Number/单号:8
QA/SQEconfirm:0
问题点发生阶段
Non-conformationFoundDuring客
户抽检发现不良
ቤተ መጻሕፍቲ ባይዱ进料IQC
制程检验IPQC 出货检验OQC/QA
客户抱怨CustomerComplain 客户退货ReturnGoodsbyCustomer
Discipline 3:Describe the problem's rootcause(Describe why conditionchange,or"hole"inthesystem,
Human\Machine\Material\Method\Environment)问题发生的根本原因(描述状况变化或系统出现“漏洞”的原因,人之因素、机器因素、物料因素、方法因素、环境因素)
8D报告示例
Corrective/PreventiveAtionReport(8DFormat)
改善措施报告(8D--报告)
Initiator/开立者:0
Customer/客户名称:候门行
IssueDate/开立日期:0
Issuedto/责任者:0
Cust.P/N/客户料号:*
DueDate/完成日期:0
Discipline 4:Problem containment plan(Describe who,what,when and how problem will becaptured orcontaind.)短期措施(描述此问题将由“谁”,“什么”,“何时”,“怎样”解决)
Discipline 5:Permanent corrective action plan(Describe who,what,when,and how changes will beimplemented.)长期措施(描述此问题将由“:谁”,“什么”,“何时”,“怎样”解决)
纠正和预防措施标准管理规程SMP for Corrective and Preventive Actions中英文双译
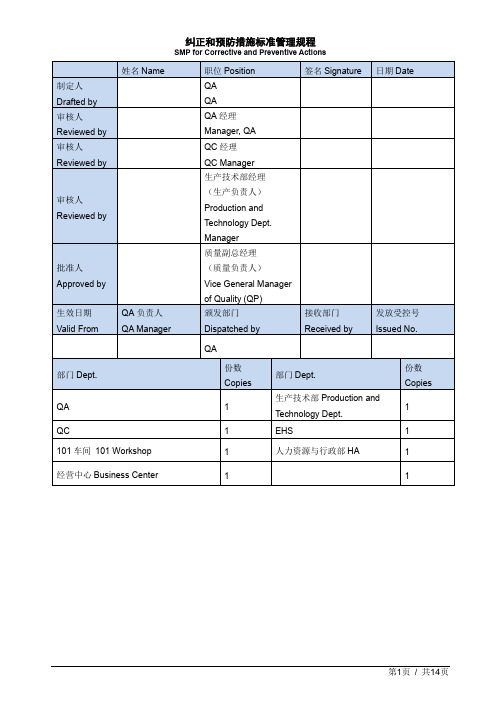
1.目的Purpose本文件规定企业纠正预防措施系统(CAPA)如何建立,通过对纠正措施的制订和实施进行有效的控制,消除产生问题的实际原因,防止再次发生不合格/不符合,以达到持续改进的目的。
This document defines how the company creates CAPA system and then effectively controls it by making and implementing corrective and active actions to eliminate the real reasons of problem, prevent against disqualification and non-compliance and reach the goal of continuous improvement.2.适用范围Scope本文件适用于全公司所有部门和场所对有关产品、过程及质量管理体系纠正和预防措施的控制。
This document applies to the control of corrective and preventive actions regarding products, process and quality management system .3.职责Responsibility3.1.责任部门为CAPA的发起人。
发起人负责发起CAPA,填写CAPA来源,描述事件,识别根本原因,写出采取的纠正与预防措施,预计的时限,CAPA人员的姓名并获得责任部门负责人的批准。
Responsible department is the initiator of CAPA. Initiator is responsible to raise CAPA, write the source of CAPA, description of incident, Identify the root cause, write CAPA to be taken, write estimated timeline, write the responsible person’s name for CAPA completion and take approval from the head of the responsible department.3.2.CAPA责任人负责CAPA的延期申请并在措施完成后通知QA。
不合格纠正及预防措施程序(中英文)

发行日期 (Issuing date)
生效日期 (Effective date)
页 次 (Page) 3/6页 (3 of 6 pages)
负责人 Responsible
person 2.必要时,品
品质部主管人员 Chief person in Quality Dept.
Corrective defect, the Quality Dept. shall send 《Nonconformance Improvement Report》to responsible Dept. action 2. 相关部门或相关责任人在3个工作日内将采取的纠正行动回复给发单部门。
Responsible Dept. or responsilbe person should send corrective action which is taken back to the
审否会则议,前应7在个管
理W评it审hi会n 7议上
wothrekicnogrrdeacytsive
acAtioctniotnaken by
参考文件
Reference document 6.2 预防措
S《h管eet理" s评ho审ul程d 序》
Management Review
施6.2.1 各单
位应根现据或本分
document 6.1.3 成品
"Nonconforma 《检验与试验控制程序》、《生产过程控制程序》 《Inspection and Test Control Procedure》 《Production Process Control Procedure》
检验不合格 1.送检的批量
纠正和预防措施控制程序(英文版)

4.2.QA Department is the direct responsible department of this procedure.
5.Procedure
5.1Corrective actions as follows:
5.1.1Rejected raw material should be dealt according toControl of InspectionandControl of Abnormal Events.
5.1.3.1First piece quality confirmation.
5.1.3.2Quality confirmation after corrective actions taken.
5.1.3.3In process quality confirmation.
5.1.3.4Quality confirmation for finished goods after actions taken.
5.1.3.5Others.
5.1.4 In order to prevent the recurrence of similar quality issue, design, spec, work instruction etc should be revised to solve the quality issues thoroughly.
纠正预防措施报告模版(中英文对照)

纠正预防措施报告 Correction and Precaution Report
编号Serial Number 发起人Organizer (Print) 发起人签名 Organizer (Sign): 发行日期 Issued Date 报告回复期限 Reply Date 报告批准人 Approved By :
效果评审Effect Assessment
评审人签字: Auditor Sign: 备注Remark: 评审日期: Date: 处置结果: Result:
Pass Fail
Form-CPR-0001 Rev 00
Form-CPR-0001 Rev 00
Байду номын сангаас
Form-CPR-0001 Rev 00
问题描述 Description
根本原因分析 Root Cause Analysis
项目经理意见: Project Manager:
纠正措施 Corrective Action 措施项目 Action 责任人 (组) Owner 到期日 Action Date 完成日期 Complete Date
项目经理意见: Project Manager:
预防措施 Precaution 措施项目 Action 责任人 (组) Owner 到期日 Action Date 完成日期 Complete Date
Form-CPR-0001 Rev 00
其它 Other
跟踪结果/关闭 Follow-up Results / Completed
纠正措施与预防措施处理规程-翻译

1 目的为规范GMP运行过程产生的(潜在)不符合的处理行为,使药品生产符合法规、行业标准规定,降低产品缺陷率及偏差发生的机率,实现质量管理体系的持续改进,特制定纠正措施与预防措施(Corrective Action & Preventive Action,以下简称CAPA)的管理程序。
To regulate the incongruent processing behavior generated in the GMP, and meet the drug production in line with regulations and industry standards, and reduce product defect rate and the probability of the deviation occurrence, and realize the continuous improvement of the quality management system, we specially formulated corrective measures and prevention measures (Corrective Action & Preventive Action, hereinafter referred to as CAPA) management program.2 适用范围2.1 适用于药品GMP运行过程产生的各类(潜在)不符合的CAPA制定、实施及闭环确认。
Applies to all kinds of (potential) formulation, implementation and closed-loop confirmation which do not meet the CAPA in the GMP2.2在生产质量活动中,能够立即采取应急措施解决问题且相关批次产品质量无影响,可以不执行该程序。
纠正与预防措施控制程序(中英文)

纠正与预防措施控制程序Corrective and Preventive Action Control Procedure1.目的Purpose:及时发现不符合,并采取有效的纠正和预防措施,以消除存在或潜在的不符合原因,实现管理体系的不断完善和持续改进。
To found non-conformity in time, and to take effective corrective and preventive action, that remove the actual or potential non-conformity reasons, to realize the management system is constantly improved and continually improved.2.范围Scope:适用于管理体系运行过程中的改进、纠正和预防措施的制定、实施和验证。
This document applies to the improvement in the process of management system, formulation, implementation and verification of corrective and preventive action.3.术语Terms:纠正—为消除已发现的不符合所采取的措施。
Corrective - the action taken to remove the non-conformity founded.纠正措施—为消除已发现的不合格或其他不期望情况的原因所采取的措施。
Corrective action - the action taken to remove the reason of the non-conformity founded or other not expected situation.预防措施—为消除潜在的不合格或其他潜在不期望情况的原因所采取的措施。
(完整版)纠正和预防措施表(中英文)

纠正和预防措施表corrective and preclude action table
编号NO.:QR-8.5-02序号Serial NO.:
不合格/体系(现在或潜在)缺陷描述Nonconformance/system (existing or potential) defect description:
日期Date:日期Date:
纠正和预防措施实施情况报告Progress report of corrective and preclude actions:
报告人Reporter: 部门经理Manager:
日期Date: 日期Date:
纠正和预防措施有效性验证Testing of Validity of corrective and preclude actions:
验证人Approved by: 日期Date:
调查人/日期Inspector/Date:
计划的纠正和预防措施: 预计完成/日期:
Planned corrective and preclude actions Planned action date
责任人:
Responsible person:
计划完成时间:
Actual action date:
编制D符合类型:
描述人Described by:日期Date:
责 任Responsibility
整改计划递交日期确认Date of corrective action confirmation
部门Department:
经理Manager:
完成日期Action date:
签 名Signature:
不合格原因调查Reasons of nonconformance:
纠正和预防措施程序中英文版本

5.1.1 Inthe incoming material inspection process, if it is inspected to be defective material by MRB, IQC should issueDefective Material Improvement Notification.
5.1.4生产过程中巡检检验不合格,不合格率超出5%时,由PQC发出《品质异常反馈报告》给责任单位。
5.1.5 If batch reject are detected in final quality control inspection, FQC issuesQuality Abnormity Feedback Report.
3.1各职能部门负责纠正预防措施中问题的提出,责任部门制订相应的纠正措施、预防措施的评估。
3.2 Management personnel affirm the effectiveness of corrective and preventive action.
3.2管理层对纠正预防措施有效性进行肯定。
4.4不合格:未满足明示的,通常隐含的或必须履行的需求或期望,即未满足要求。本程序中的“不合格”为不符合、不合格品、不合格项的统称。
4.5 Major reject: defect more than 5%.
4.5严重不合格:超过5%的不良。
5、Procedure Content /程序内容
File name文件名称
5.1.3制程生产中出现技术或工艺导致严重或批量不合格产生,由生产部门发出《品质异常反馈报告》给中央研究院、工程部。
纠正和预防措施程序中英文版

1.0 P URPOSE 目的1.1 The procedure defines the process for Corrective &Preventive Action and ContinuousImprovement and makes sure quality and environment management system is on continuingimprovement and continuously meets customer requirements.确定纠正预防和持续改进的流程,确保公司的质量管理体系和环境管理体系是处于持续改进的,不断满足客户需要。
2.0 S COPE 范围2.1 Apply to the products and processes which are related with quality management system andenvironment management system, such as internal products or processes required correctiveaction or improvement, customer complaint, internal/external audit and environmentnonconformity, KPI over target, and etc.适用于公司内质量管理体系和环境管理体系所涉及的产品和过程。
比如要求纠正或改进的产品、过程,客户投诉,内/外部审核和环境不符合,目标未达成,数据分析等。
3.0 D EFINITIONS定义3.1 Correction: action to eliminate a detected nonconformity. A correction can be, for example,rework or re-grade.纠正:为消除已发现的不合格所采取的措施,如返工,降级等。
纠正预防措施状态报告中英文版

DN QP220-RE-02 Effective Date:2020-2-9 Revision 1 Page 1 of 1纠正预防措施状态报告CAPA Status ReportSection A 纠正预防措施通知(Notification of CAPA due)纠正预防措施编号CAPA Ref: ___________ 预计完成时间Due Date: _______________调查人/制定人Investigator/Owner: _________________以上涉及的纠正预防措施必须在预定的时间内完成、关闭。
如制定的纠正预防措施不能按要求在预定时间内实施、关闭,请在期限当天填写下面相关内容,同时提交给QA。
(The CAPA referenced above is due on the date indicated. The referenced CAPA must be completed, closed and submitted to QA by the date listed. If the CAPA will not be closed by the due date, this form must be completed and submitted to QA by the due date listed.)QA Signature: ____________________________ Date: _________________Section B纠正预防措施状态CAPA Status(to be completed by the investigator and/or owner – attach additional sheets as needed)1. 哪些调查/措施已经按时完成?(What part(s) of the investigation/actions is/are completed?)_____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 2.哪些调查/措施没有按时?(What part(s) of the investigation/actions is/are pending?)_____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 3. 为什么调查/措施没有按时完成?(Why is/are the investigation/actions not completed?)__ ___________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 4. 何时调查/措施能够完成?(When is the investigation/actions expected to be finished?)_____________________________________________________________________________________调查人/制定人(Investigator/Owner): ________________________________ Date: _____________部门主管(Depart.Supervisor): Date:。
中英文纠正预防措施程序文件

Procedure 程序Issue版本:1TITLE:Corrective & Preventive Action Control 标题:糾正和預防措施控制DOC No.文件編號:FQP-CAR01 Pages: 1 of 6Revision History 更改记录Issue 版本Page#页码Rev版次CHANGE更改內容WRITTEN BY编写DATE日期Review and Approval審查和批准Review [ x ] [ x ] [ x ] [ x ] [ x ] [ x ] [ x ] [ x ] [ x ] [ x ] [ x ] Signature / DateISO Officer:________________________QC Sup:_____________________________QE Sup: ____________________________QA Mgr&MR:__________________________ProgramDirector: __________________________PM: ________________________________Store Mgr:_________________________Purchasing Mgr:______________________PMC Sup:_____________________________Shipping Sup:________________________Facility Mgr: ______________________Review[ x ][ x ][ x ][ x ][ x ][ x ][ x ][ x ][ x ]Signature / DateFacility Engineering Mgr:___________________________________ME Mgr:____________________________1st process Mgr:______________________2nd Process Mgr:______________________PPC Mgr: ____________________________Tooling Repair & Maintenance Mgr:___________________________________HR Mgr: ________________________ADM Mgr:_____________________________MIS Mgr:_____________________________APP[ x ]Signature / DatePlant. Manager:____________________Checked by DCC / Date: 文控審核/日期:TITLE:Corrective & Preventive Action Control 标题:糾正和預防措施控制DOC No. Rev文件編號: FQP-CAR01 版次: A Pages: 2 of 6Effective date Expiry date文件生效日期:____________________ 文件失效日期:_________________1.0目的Purpose:消除實際或潛在的不合格因素,及時采取有效的糾正和預防措施,并確保類似問題不再重复發生,以達到自我完善﹑自我提高的目的。
纠正和预防措施 英文范文

纠正和预防措施英文范文Here are some examples of corrective and preventive measures written in informal and conversational English:1. Spotting a Mistake and Fixing It.When I noticed that error in the report, I had to jump right in and correct it. No time to waste! I double-checked the data, made the necessary changes, and then verified everything again to ensure it was accurate. I know, it's a pain, but it's gotta be done.2. Preventing a Recurring Issue.To prevent this issue from happening again, we're going to set up a regular check-in system. Everyone will be assigned a task to review their part of the project every week and flag any potential problems. That way, we can catch things early and avoid bigger headaches later.3. Taking Action to Improve.I realized that our team communication wasn't as effective as it should be, so I suggested we have a weekly team meeting. It's a simple but effective way to keep everyone on the same page and address any issues that might arise. Plus, it's a great opportunity for everyone to share their ideas and collaborate.4. Identifying the Root Cause.To really understand why that problem occurred, we need to dig deeper. I'm going to.。
纠正及预防措施报告范文

纠正及预防措施报告范文Addressing and preventing corrective measures are essential in creating a safe and secure environment for individuals. 纠正及预防措施是创造一个安全稳定环境的重要措施。
Whether it be in a professional setting or personal interactions, being proactive in addressing issues can help prevent future problems from arising. 无论是在专业环境还是个人交往中,积极解决问题有助于预防未来出现问题。
When faced with a situation that requires corrective action, it is important to handle it promptly and effectively. 面对需要纠正的情况,及时有效地处理是重要的。
By taking steps to correct and prevent issues, individuals can promote a sense of accountability and responsibility. 通过采取纠正和预防措施,个人可以促进责任感和责任心。
In this report, we will explore the importance of addressing and preventing corrective measures in various aspects of life. 在这份报告中,我们将探讨纠正及预防措施在生活的各个方面的重要性。
Taking accountability for one's actions is a key component of addressing and preventing corrective measures. 对自己的行为负责是纠正及预防措施的关键组成部分。
纠正和预防措施程序-中英文版本

Version: A0 Page: 1/7Status:PROCEDURE 程序文件Corrective and Preventive Actions ManagementProcedure 纠正和预防措施程序目的OBJECTIVE 2 范围SCOPE2 职责RESPONSIBILITY2 正文PROCEDURAL ELEMENTS2流程FLOW CHART 3 任务描述TASKS DESCRIPTION 4 参考文件REFERENCE DOCUMENTS 6 记录RECORDS7 定义与缩写DEFINITIONS/ ABBREVIATIONS 7___________________________________________________________________Version: A0 Page: 2/7Status:PROCEDURE 程序文件Corrective and Preventive Actions ManagementProcedure 纠正和预防措施程序Objective 目的To ensure continually improve the effectiveness of the quality management system through the use of corrective and preventive actions.利用纠正与预防措施,确保持续改进质量管理体系和环境管理体系的有效性。
__________________________________________________________Scope 范围Applied to the corrective , correction action and continual improvement. 适用于纠正、预防和持续改进。
__________________________________________________________Responsibility 职责It is the responsibility of the related departments to implement the corrective and preventive action in compliance with this procedure.相关部门负责按照此程序实施纠正与预防措施。
纠正和预防措施控制程序(中英文)

质量管理体系程序文件QUALITY SYSTEM PROCEDURE编制Initiated by:审核Reviewed by:批准Approved by:发放范围(applied for):生效日期(Effective Date):2022年05月10日文件修改履历Document History Summary目录Ta b l e o f C o n t e n t序号名称页码Item #Title Page1 目的(Purpose) (2)2 范围(Scopes) (2)3. 定义(Definitions) (2)4 职责(Responsibilities) (2)5 工作流程(Working procedures) (3)6 相关文件(Relevant Documents) (8)7 相关记录(Relevant Records) (8)8 附件(Appendixes) (8)Document History Summary (1)1 目的(Purpose)本程序规范了为消除实际或潜在的不合格而采取纠正预防措施的流程,以确保类似或潜在不合格不再发生,促进质量管理体系的持续改进。
The procedure clarifies the process to adopt corrective and preventive action to eliminate the existing or potential non-conforming and prevent similar non-conforming in the future and promote the sustained improvement of the quality management system.2 范围(Scopes)本程序适用于本公司质量管理体系范围内纠正措施和预防措施的制定、实施与有效性验证。
The procedure is applicable to the establishment, implementation and validation of corrective and preventive action within the quality management system.3. 定义(Definitions)3.1 纠正:为消除已发现的不合格所采取的措施;Correction: Action to eliminate a detected nonconformity.3.2 纠正措施:为消除已发现的不合格或其他不期望情况的原因所采取的措施;Corrective action: Action to eliminate the cause(s) of a detected nonconformity or other undesirable situation. 3.3 预防措施:为消除潜在不合格或其他潜在不期望情况的原因所采取的措施;Preventive action: Action to eliminate the cause of a potential nonconformity or other undesirable potential4 职责(Responsibilities)4.1 质量管理体系内各部门负责将质量管理体系持续改进的机会,包括不合格情况、潜在不合格情况、改进机会反馈至QA。
