Y染色体微缺失诊断与治疗手册2015年第2版
Y 染色体微缺失快速检测系统 说明书

Y染色体微缺失快速检测系统说明书 25人份/盒z概述本试剂盒用于快速检测人(男性)Y染色体AZF座位(Azoospermia Factor)的缺失。
多项研究发现,AZF基因家族共有AZFa、AZFb、AZFc三个座位(在AZFb与AZFc之间还存在AZFd座位,中等程度的少精和精子数目正常却形态异常多会与该区域的缺失有关),其中任何一个座位的微缺失都可导致生精障碍。
本试剂盒共设计15对引物和一对内控(内部质量控制)引物,利用PCR(Polymerase Chain Reaction)技术可从Y染色体上特异性扩增出15条非多态性短片段的序列标签位点(Sequence Tagged Sites,STS)和一条内控基因片段。
这些引物对分别组合成4套多重PCR系统,在相同条件下,进行4管PCR扩增反应,通过电泳结果判定15个序列标签位点(STS)的存在与否。
主要特点:1.可对15个STS序列标签位点进行检测,这15个位点覆盖了所有的AZF区域,其中AZFa有3个位点,AZFd有2个,AZFb有6个,AZFc 有4个位点;2.高特异引物确保结果的可靠性,并具有内控(内部质量控制)基因片断的检测;3.独特的防污染设计,有效避免假阳性的产生;4.符合并超过欧洲生殖学会推荐的检测标准。
应用:胞质内单精子注射(ICSI)技术辅助生育治疗前的检测、少精症与无精症男性患者遗传学检测、精子库的入库前筛查等。
z试剂盒组成组成成分数量保存条件PCR反应液Ⅰ(紫色) 25管(每管14μL ) -20℃PCR反应液Ⅱ(无色) 25管(每管14μL) -20℃PCR反应液Ⅲ(蓝色) 25管(每管14μL) -20℃PCR反应液Ⅳ(黄色) 25管(每管14μL) -20℃纯水z有效期六个月z需自备的仪器及试剂PCR仪(推荐使用PTC-100或PTC-200, M J Research Co.或其它国际知名品牌PCR仪)DNA电泳系统z操作步骤1.基因组DNA的获取使用本公司全血DNA快速提取试剂盒从抗凝全血中抽提人(男性)基因组DNA,按操作说明进行。
Y染色体微缺失检测

Y染色体微缺失检测试剂盒背景介绍根据国际卫生组织调查数据,约15%育龄夫妇存在生育障碍,其中男性因素引起的生育障碍约占一半。
在已知的导致男性不育的遗传学因素中,发病率最高的两种是Y染色体的微缺失和克氏综合症。
Y染色体具有大量重复基因序列及回文结构,这些结构在维持Y染色体进化稳定性的同时也使回文结构内部基因易于丢失,进而导致不育。
缺失率最高的三个影响精子发生的区域被命名为AZFa,AZFb和AZFc,它们之中任何一个出现缺失都有可能导致育性下降或不育。
Y染色体微缺失在无精症或少精症患者中发病率在10-15%,已成为男性不育患者的常规检查项目。
图1,Y染色体AZF区结构示意图(AZFa区两端有两段原病毒序列、AZFb/c区内部有5个序列高度一致的大的回文结构,这些序列和结构导致相应染色体片段易发生同源重组,造成缺失或复制。
)欧洲男科学协会和欧洲分子遗传实验质控协作网(EAA/EMQN)2004年发表“Y染色体微缺失分子诊断指导意见”建议通过对各AZF区的共6个STS位点和两个对照位点检测Y染色体微缺失。
产品简介本剂盒通过“多重定量荧光PCR技术”(Multiplex Quantitative Fluorescent PCR),以一个高度复合的扩增体系中扩增至少15个Y染色体微缺失检测相关的位点,并根据各位点有无扩增产物及扩增产物剂量判断缺失类型。
对于Y染色体微缺失,由于各AZF区片段相对较小,常规核型分析等方法难以发现其缺失。
本项目采用的检测方法是:在各AZF区上分别选择具有序列特异性的多个位点,通过多重定量荧光PCR方法检测各位点。
根据有无扩增产物判定样本染色体是否包含所检测的序列特异性位点,进而推断样本染色体是否在位点所在AZF区域发生缺失;通过部分位点扩增产物相对剂量确定相关位点拷贝数比例,根据拷贝数比例推断相关位点对应区域是否发生缺失或复制,并对于AZFb和/或AZFc区部分缺失或复制,实现了首次检测。
染色体缺失综合征的诊断及产前诊断

临床表现
❖ 在出生前,PWS胎儿即可表现出胎动减少;PWS新生儿可出现张力减退, 反射减弱,吸吮反应弱,吞咽困难,及外生殖器发育不全,1岁内手脚发 育在正常;患者在1岁到1岁半后出现无法控制的过量饮食、向心性肥胖, 但同时伴有生长发育迟缓和智力发育迟缓、特征性面容(窄长脸,杏仁 眼,斜眼,大下巴)和肌肉张力减弱引起的模仿能力降低;6岁后,患者 可出现体痒,抓后留痕,腹部出现嗅纹,嘴角含稠的唾液,对疼痛不敏 感;青春期后因糖摄入过多引发饮食性糖尿病,青春期发育差,大多数 患者25-30岁以后死于糖尿病和心肌衰竭。
临床表现
❖ 心血管畸形主要表现为弹力蛋白动脉病,主要累及大动脉如主动脉弓、 升主动脉、降主动脉、腹主动脉等,最常见且最有临床意义的为主动脉 瓣上狭窄,见于75%的病人;结缔组织异常表现为声音嘶哑,腹股沟疝、 脐疝、肠/膀胱憩室瘤、直肠脱垂、关节活动受限、皮肤松弛等;内分泌 异常表现为婴儿特发性高钙血症、尿钙过多、甲状腺功能减退、青春期 提早,成人可有糖尿病和亚临床甲状腺机能减退;大多数病人可出现轻 度到重度的精神发育迟缓,智商均值为56;行为异常主要表现为(对陌 生人)过分热情、注意力缺陷、广泛性焦虑和缺乏社会判断能力等;临 床表现的严重程度与患者缺失染色体片断的大小呈正相关。
诊断与产前诊断
❖ 临床诊断主要依据其临床表现如特征性的面容,生长发育、 智力发育迟缓、癫痫发作以及全身各组织器官的畸形等,可 行脑电图、X线(胸廓、脊柱)、心脏B超等检查明确。 FISH技术可有效检出WHSCR1和WHSCR2微缺失的患者。对 患者的父母应行FISH检查明确是否易位携带者,如再次生育 应行产前诊断。
治疗与预后
❖ 本病无根治手段,主要采取对症治疗;散发型患者 平均年龄34岁,遗传型患者平均年龄18岁,约21% 的患儿在出生两年内死亡,死因主要为先天性心脏 病、下呼吸道感染等。
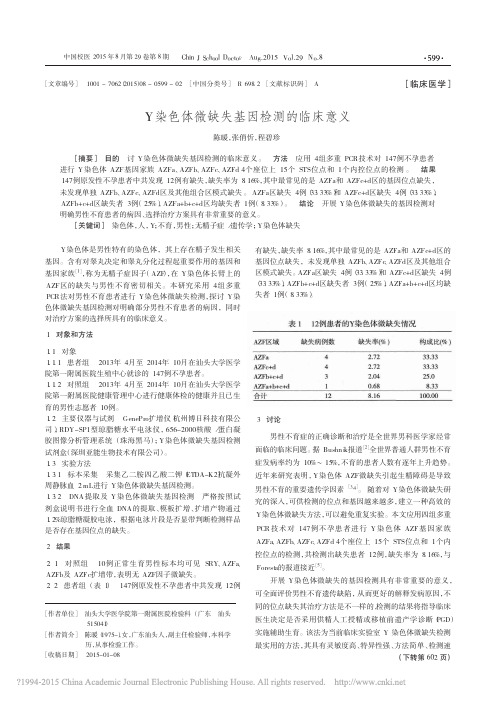
y染色体无精子症因子微缺失的筛查与临床探讨

·论 著·Y染色体无精子症因子微缺失的筛查与临床探讨欧妙玲,陈志华,刘丽雅,朱晓丹(广东省佛山市妇幼保健院检验科 528000) 【摘要】 目的 筛查男性不育患者Y染色体无精子症因子(AZF)微缺失发生率,并分析缺失类型与临床表型的关系。
方法 选取2013年1~12月于佛山市妇幼保健院男科就诊的男性不育症患者200例,其中非梗阻性无精子症141例,重度少精子症59例;另选取100例正常孕育男性作为阳性对照;100例正常孕育女性作为阴性对照。
采用聚合酶链反应分析男性Y染色体AZF微缺失。
结果 特发性不育组AZF微缺失率显著高于非特发性不育组,差异有统计学意义(P<0.05)。
在所有AZF微缺失类型中以AZFc最常见,占55.6%;非梗阻性无精子症组与重度少精子症组微缺失率及微缺失类型分布情况比较,差异无统计学意义(P>0.05)。
结论 Y染色体AZFa、AZFb、AZFc区微缺失与男性不育具有密切的相关性。
【关键词】 男性不育症; Y染色体; 无精子症因子DOI:10.3969/j.issn.1672‐9455.2015.03.031文献标志码:A文章编号:1672‐9455(2015)03‐0365‐03Screening and clinical phenotype explore of Y chromosome microdeletions of azoospermia factor in male infertility pa‐tients OU M iao‐lin g,C H EN Zhi‐hua,L IU L i‐y a,ZH U X iao‐dan(De p artment o f Clinical L aborator y,M aternaland Children H ealth Hos p ital o f Foshan Cit y,Foshan,Guan g dong528000,China)【Abstract】 Objective To investigate incidence of Y chromosome microdeletions of azoospermia factor(AZF)in male infertility patients,and to analyze the relationship between types of gene deletion and clinical phenotype.Methods A total of200cases of patients with male infertility were recruited in this study from January to December2013,including141cases with non‐obstructive azoospermia and59cases with severe oligozoospermia.Another100healthy males were enrolled as positive control group,and100healthy females were enrolled as negative controlg roup.M ale Y chromosome AZF microdeletions were analyzed by using polymerase chain reaction method.Results The incidence of AZF microdeletions of idiopathic infertility group was significantly higher than that of non‐idiopathic infertility group(P<0.05).In all types of AZF microdeletions,AZFc was the most common type,accounting for55.6%.No significant differences of AZF microdeletions incidence and distribution of types of AZF microdeletions were found between non‐obstructive azoospermia group and severe oligozoospermia group(P>0.05).Conclusion Y chromosome AZFa,AZFb and AZFc microdeletion could be closely correlated to male infertility.【Key words】 male infertility; Y chromosome; azoospermia factor 大约有10%~15%育龄夫妇存在不育情况,其中男性因素大约占了50%,在男性不育中30%是因为遗传因素所导致。
Y染色体微缺失与男性不育简介-机理和技术-2015年第2版

肉眼判读,条带大小相近,图片模糊 操作复杂,人工实验较繁琐 电泳检测,接触EB等致癌物 电泳时间长,需8小时
适用医疗机构及科室
男科(不育)
医院生殖遗传室
Y染色体微缺失检测
生殖医学中心
计划生育研究所
样本要求
推荐样品类型顺序:新鲜外周血>冷冻外周血 采血管选用非肝素抗凝管,推荐使用枸橼酸钠抗凝管和EDTA抗凝管
检验结果演示
同一荧光通路检测同一AZF区域位点 彩色S曲线结果,简单直观
组别 Group A 荧光 染料 FAM VIC ROX Cy5 检测 位点 SRY sY84 sY127 sY255 ZFX/ZF Y sY86 sY134 备注 对照 AZFa AZFb AZFc 对照 AZFa AZFb
检测位点 检测方法 内参基因 对照组设置
区域 STS
AZFa sY84 sY86
AZFb sY127 sY134
AZFc sY254 sY255
多重荧光PCR法,两管反应 ZFX/ZFY基因:检验样本是否正确提取,质量可靠 SRY基因:检验是否为男性样本
设置阳性对照(正常男性样本)、阴性对照(女性 样本)与空白对照(水)
基因检测,指导生育
检测对患者来说只要抽少量血液 即可,非常方便。
不同的位点缺失或是否存在缺失的治疗方法是不 一样的,检测结果指导医生是否采用ICSI辅助生 育; 同时为是否选择性移植女性胚胎提供依据,因为 男性后代将遗传父亲的不育缺陷。
Y染色体微缺失分子诊断
染色体显带方法-G显带
染色体显带 :经不同的方法 处理染色体,经染色后使染 色体在纵轴上显示明、暗或 着色深、浅相间的横纹即显 带(Banding)。
这种带对每一条染色体来说 都是独特的,可以区分和确 认每一条染色体。
11 Y染色体微缺失检测_8.15

Y染色体微缺失检测张磊博士深圳亚能医学检验所目录•背景知识•临床意义及适应症•亚能医学检验服务介绍•临床案例分享•其它服务简介背景知识1★据WHO数据统计,全世界有10~15%的夫妇患有不孕不育症;★其中男性因素占50%;★遗传因素占男性不育的30%;★在精子发生障碍引起的男性不育患者中,Y染色体微缺失的发生率仅次于克氏综合征,是居于第二位的遗传因素。
图1: 原发性不孕全球发病率统计(WHO, 2010年数据)图2: 继发性不孕全球发病率统计(WHO, 2010年数据)EAA/EMQN发布2013版指南12背景知识1Y染色体微缺失Y染色体长臂存在精子发生相关的基因,被称为无精子因子(AZF);缺失率最高的三个影响精子发生的区域被命名为AZFa,AZFb和AZFc,它们之中任何一个出现缺失都有可能导致不育症。
3据WHO统计,男性原发性无精子症与少精子症患者中约10%~15%存在Y染色体微缺失。
背景知识1在中国,约有11.5%的男性不育是由AZF 区缺失引起的。
资料来源:全球不育男性中AZF缺失所占比例(Simoni et al., 2008 发行)Y染色体微缺失4背景知识1Y染色体AZF区结构示意图资料来源:Y染色体结构及微缺失模型(Repping et al., 2002)Ratio ~80%0.5-4%1-5%1-3%背景知识1AZFa区缺失AZFc区缺失AZFb区缺失AZFa、AZFb和AZFc缺失患者主要表现为唯支持细胞综合征(SCO综合征)伴睾丸体积缩小,无精子生成,占整个AZF缺失0.5~4%患者主要表现为生精过程阻滞在精母细胞阶段,无精子生成,占整个AZF缺失1~5%患者表现多样化,会出现无精子症和少精子症的不同临床表现,临床上最常见,约80%患者表现为100%无精子症临床表现Y Y染色体AZF区微缺失资料来源:2013年EAA/EMQN 指南(C. Krausz, et al., 2013)1资料来源:2013年EAA/EMQN 指南(C. Krausz, et al., 2013)2013年EAA/EMQN 指南AZF 区基础分析指南建议进行AZF区筛查流程背景知识AZF区筛查流程1资料来源:2013年EAA/EMQN 指南(C. Krausz, et al., 2013)2013年EAA/EMQN 指南AZF 区扩展分析背景知识临床意义及适应症2Y染色体微缺失检测临床意义3减少病人痛苦,提高辅助生殖成功几率1确定无精、少精患者病因,避免不必要的药物及手术治疗2尽量避免将有缺陷基因传递给下一代4为未来的基因治疗提供理论依据资料来源:2013年EAA/EMQN指南(C. Krausz, et al., 2013)2临床意义及适应症Y染色体微缺失检测适应症常规适应症☆男性不育症IVF、ICSI治疗前☆非梗阻性无精子症患者☆严重少精症者(少于5×106/mL)☆精子库入库前质量筛选☆无精症患者术前☆原因不明的男性不育症用药前资料来源:2004年EAA/EMQN指南(M. SIMONI, et al., 2004)2临床意义及适应症Y染色体微缺失检测适应症推荐适应症☆少精子症患者(精子数目少于20×106/mL)☆精子密度正常,但原因不明的男性不育症患者☆男性不育伴隐睾和精索静脉曲张的患者☆妻子有不明原因习惯性流产的患者资料来源:2004年EAA/EMQN指南(M. SIMONI, et al., 2004)二、STS-多重定性PCR法(EAA 和EMQN 推荐使用)一、实时荧光PCR法(简便、快速)三、基因芯片法四、荧光原位杂交技术Y染色体微缺失检测方法亚能医学检验服务介绍3服务介绍—PCR-荧光探针法3采用多重PCR结合多色Taqman荧光探针技术,选择与男性不育高度相关的Y染色体AZF区域6个序列标签位点(EAA/EMQN指南推荐)进行检测,以判断AZF区域是否存在微缺失。
Y染色体微缺失结果判读表

I管(紫色) 条带 STS SRY 产物 座位 472bp / 条带
II管(白色) STS SRY 产物 座位 条带
III管(蓝色) STS SRY 产物 座位 条带
IV管(黄色) STS SRY 产物 472bp / 座位
472bp /
Y染色体微缺失电泳检测结果判读表
产物座标 (bp) 450 400 350 300 250 200 150 100 50 0 1、正常男性(阳性对照)Y染色体微缺失电泳结果显示15个STS位点无缺失(即:以上的所有条带都应该有。) 2、SRY是内控,4个管中都有,都应该显带,没有显带说明结果存在问题,操作或试剂质量产生问题的可能性都有。 结果解读: 3、如果某管中的某个STS位点未出现(如sY143和sY242),说明该位点缺失,结果报相应的座位缺失: 如:AZFb+AZFc联合缺失 sY255 126p AZFc sY152 125bp AZFc
472bp /
sY254 400bp AZFc
sY143 310bp AZFb
sY840bp AZFa
sY134 301bp AZFb sY82 264bp /
sY127 274bp AZFb sY242 233bp AZFc sY293 200bp AZFc
sY128 228bp AZFb sY133 177bp AZFb
Y染色体微缺失基因检测的临床意义
[7] 何斌, 王洪源. 2008- 2010 年北京市海淀区手足口病流行病学分 析[J].首都公共卫生, 2012, 6(3): 110-112.
[11] Onozuka D, Hashizume M. The influence of temperature and humidity பைடு நூலகம்n theincidenceofhand,foot,and mouth diseasein Japan[J]. Sci Total Environ, 2011, 410-411: 119-125.
[17] Ooi EE, Phoon MC, Ishak B, et al. Seroepidemiology of human enterovirus 71, Singapore[J].Emerg Infect Dis, 2002, 8(9): 995-997.
[18] 李佳萌, 张颖, 高璐, 等. 天津市 2009-2010 年健康人群肠道病毒 71 型中和抗体血清流行病学调查[J].中华流行病学杂志, 2011, 32(6): 568-570. [编辑] 何洪江
[2] Bushnik T,Cook JL,Yuzpe AA, et al. Estimating the prevalence of infertility in Canada[J].Hum Reprod,2012,27⑶:738-746.
(上接第 602 页)
度快且经济的特点,适合临床推广应用。 [参 考 文 献]
[1] Kent-First M.The Y chromosome and its role in testis differentiation and spermatogenesis[J].Semin Report Med, 2000,18(1):67-80.
Y染色体微缺失检测指导

丫染色体微缺失是严重少精子或无精子症的重要原因,是导致男性不育的第二大遗传因素,其发生率仅次于Klinefelter综合征(克氏综合征)。
从1999年开始,欧洲男科学协会和欧洲分子遗传实验质控网(EAA/EMQN为提高诊断质量,出版了丫染色体微缺失分子诊断指南,并提供了客观的实验质量评价方法。
最新版的实验室指南是2013年9月EAA/EMQN根据12年的临床积累和专家共识,在1999版和2004版的基础上修订而成。
新指南重点阐明:在少精子症或无精子症男性中发现的丫染色体微缺失区域,主要是无精子因子(azoospermia factor,AZF)区域仅包含AZFa AZFb AZFbc AZFc和AZFabc区,独立的AZFd区并不存在;AZFc 区中gr/gr 缺失是影响精子生成的一个危险因素,但临床意义尚存争议,不作为常规检查指标;检测位点增加并不能提高检测灵敏度,反而可能使结果复杂化;基于两管多重PCR的检测方法仍适用于整个AZF缺失检测。
EAA/EMQN 12年国际质量评估计划(EQA计划)的实施表明,参与实验室通过规范实验操作,改善报告质量,可有效降低诊断错误率。
丫染色体微缺失在中国不育男性中的发生频率为11.5%,处于较高水平,我们建议将AZF检测作为男性严重少精子或无精子症的常规检测项目,呼吁国内AZF检测实验室加入EQA计划,完善中国丫染色体微缺失检测实验操作规范。
丫染色体微缺失分子检测在中国已开展多年,国内专家对AZF缺失模式、检测序列标签位点(sequenee- tagged site, STS的数量、检测方法和内部质量控制等临床问题未达成共识。
各实验室的诊断操作方法有很大不同,导致不准确或错误诊断时有发生,迫切需要建立丫染色体微缺失诊断标准和质量控制标准。
EAA/EMQN 更新的2013版指南对以上问题都给出了明确的专家共识,对我国建立自己的检测指南有重要的指导意义。
一、丫染色体微缺失发生频率丫染色体微缺失在健康人群中发生率约为1/4 000,但在不育男性中显着升高,微缺失发生频率为2%〜10%(甚至更高)。
中国人Y染色体微缺失分子诊断指南(草案)

中国人Y染色体微缺失分子诊断指南(草案)2005.4 上海前言在精子发生障碍引起的男性不育患者中,Y染色体微缺失的发生率仅次于Klinefelter’s syndrome(克氏综合征),是居于第二位的遗传因素。
Y染色体微缺失已成为男性不育患者的常规检查项目。
欧洲男科学协会和欧洲分子遗传实验质控协作网为提高诊断质量,在1999年和2004年先后发布了第一版和第二版Y染色体微缺失分子诊断指南。
经过多年的临床实践证明该指南准确、灵敏和易于操作。
2005年4月在上海召开了中国人Y 染色体微缺失分子诊断的研讨会,成立了Y染色体微缺失分子诊断协作网。
会议在欧洲指南的基础上起草了符合目前我国男性不育诊疗现状,并反应最新生物技术发展的中国人Y染色体微缺失分子诊断指南。
本指南重点讨论Y染色体微缺失分子诊断具体实施时的标准化和规范化,推荐的相关方法和设计是根据欧洲指南和我国已有的实验研究基础上综合而成。
对机理研究和背景知识介绍部分在本指南中不再详细讨论。
男性不育症患者Y染色体微缺失分子筛查适应症常规检测的适应症:1、男性不育症患者选择单精子卵泡浆内注射(ICSI)或体外受精(IVF)生育子代前;2、非梗阻性无精子症患者;3、严重少精子症患者(精子数目少于5×106/ml);4、无精子症患者进行睾丸活检术前;5、男性不育症患者(如精索静脉曲张)手术前;6、原因不明的男性不育症患者用药前。
推荐检测的适应症:1、少精子症患者(精子数目少于20×106/ml);2、精子密度正常,但原因不明的男性不育症患者;3、男性不育伴隐睾和精索静脉曲张的患者;4、妻子有不明原因习惯性流产的患者。
诊断实验指南Y染色体上存在影响精子发生的无精子因子(AZF)区域,进一步可分为AZFa,AZFb和AZFc三个区域。
Y染色体微缺失分子诊断实验利用多重聚合酶链反应(multiplex-PCR)特异性扩增Y染色体AZF区域的序列标签位点(STS),扩增产物用电泳或杂交等方法进行检测。
Y染色体微缺失2

Azoospermia is defined as complete absence of sperm from ejaculate and approximately occurs in 10-15 per cent of infertile men with abnormal semen analysis 1. Azoospermia is classified as obstructiveHigh prevalence of AZFb microdeletion in Iranian patients with idiopathic non-obstructive azoospermiaReza Mirfakhraie, Farzaneh Mirzajani *, Sayed Mahdi Kalantar **, Maryam Montazeri *, Nasser Salsabili +, Gholam Reza Pourmand ++ & Massoud Houshmand *Biology Department Islamic Azad University (IAU), Science & Research Branch, Tehran, *Medical Genetics Department National Institute of Genetic Engineering & Biotechnology, Tehran, **Genetics Department Research & Clinical Centre for Infertility, Yazd, +IVF Department, Mirza Kouchak Khan Hospital, Tehran University of Medical Sciences, Tehran & ++Research Center, Sina Hospital, Tehran University of Medical Sciences, Tehran, IranReceived October 9, 2009Background & objectives : Genetic factors contribute about 10 per cent of male infertility. Among these, genes in azoospermia factor (AZF) region including AZFa, AZFb, AZFc and AZFd on the long arm of Y chromosome are considered most important for spermatogenesis. Deletions in these regions are thought to be involved in some cases of male infertility associated with azoospermia or oligozoospermia. We studied the incidence of AZF deletions among Iranian infertile men with idiopathic non-obstructive azoospermia.Methods : A total of 100 Iranian azoospermic infertile men were selected for the molecular study of Y chromosome microdeletions. The presence of 13 sequence tagged site (STS) markers from AZF region was investigated using multiplex polymerase chain reaction (M-PCR). One hundred fertile men were also studied as control group.Results : Twelve (12%) patients showed Y chromosome microdeletions and among these, deletion in AZFb region was the most frequent (66.67%) followed by AZFc (41.67%), AZFd (33.33%) and AZFa (8.33%), respectively.Interpretation & conclusions : Because of relatively high incidence of Y chromosome microdeletions among Iranian azoospermic patients, molecular screening may be advised to infertile men before using assisted reproductive treatments.Key words AZF - azoospermia - Iran - microdeletion - Y chromosomeand non-obstructive, although in some clinical protocols aetiologies for azoospermia fall into pre-testicular, testicular and post-testicular categories 1-3. Microdeletions on the long arm of the Y chromosomeIndian J Med Res 132, September 2010, pp 265-270265are the most frequent genetic causes of azoospermia and have been reported in 5-10 per cent of infertile men and 6-16 per cent of azoospermic men4.Most of the candidate genes involved in spermatogenesis have been mapped to the proximal long arm of the Y chromosome (Yq11) and are arranged in azoospermia factor (AZF) region including AZFa, AZFb, and AZFc sub regions5. Further, a fourth AZF region has been suggested to exist in the area where AZFb and AZFc overlap, and is termed as AZFd6.There are two main genes in AZFa region including DFFRY and DBY. Protein-encoding gene families on the AZFb region include RBMY, PRY, and CDY2 which are expressed only in the testis. AZFc region contains 8 gene families including BPY2, CDY, DAZ, CSPG4LY, GOLGAZLY, TTY3.1, TTY4.1, and TTY7.1 among which the 5 former are expressed only in the testis4,5. Microdeletions in the more proximal regions (AZFa and AZFb) cause spermatogenic arrest and sertoli cell only (SCO) syndrome, whereas partial deletions in these regions and deletions in distal regions (AZFc and AZFd) cause variable phenotypes ranging from normal to oligozoospermia and azoospermia4,5,7.Using assisted reproductive technologies such as intracytoplasmic sperm injection (ICSI) and in vitro fertilization (IVF) for treatment of male infertility may result in the transmission of Y chromosome microdeletions to the male offspring, causing the persistence of infertility problem over the next generations8-10.The aim of this study was to evaluate the frequency of AZF deletions among Iranian infertile men with idiopathic non-obstructive azoospermia using multiplex polymerase chain reaction (M-PCR).Material & Methods Patients:In a case-control study, that was carried out between June 2008 and July 2009 in National Institute of Genetic Engineering and Biotechnology (NIGEB), 100 non-obstructive azoospermic patients with a normal karyotype aged between 21 and 60 (mean ± SD = 32.41 ± 6.43 yr) were screened for the presence of Y chromosome microdeletions. The patients were candidates for ICSI and referred to NIGEB from the whole country. Semen analysis was performed according to normal standard parameters using the World Health Organization (WHO) criteria11. Urological examination was performed in all of the patients for anatomical integrity of genital system. Horm one analysis including folloicle - stimulating hormone (FSH) and luteinizing hormone (LH) were also done. One hundred age-matched (mean ± SD = 32.1 ± 7.06 yr) fertile men who had at least one child with no history of requiring assisted reproduction technology were considered as control group; also a female sample was used as negative control. Informed written consent was obtained from each azoospermic and fertile control man. The study protocol was approved by the ethics committee of the NIGEB.Detection of Y chromosome microdeletions:Genomic DNA was extracted by a salting out method from peripheral blood leukocytes12. A series of 13 STS markers on Yq11 were used for the detection of submicroscopic deletions according to the European Academy of Andrology (EAA), the European Molecular Genetics Quality Network (EMQN), and previous protocols7,13,14. The STS markers included sY81, sY84 and sY86 for AZFa; sY121, sY124, sY127 and sY134 for AZFb; sY242, sY239, sY254 and sY255 for AZFc; and sY145 and sY153 for AZFd region. The primers’ sequences and the size of related PCR products are shown in Table I. In addition, two sets of primers were used to amplify SRY and ZFY regions as internal controls. Five set of multiplexes were designed as follows:Multiplex 1: SRY, sY84, sY134, sY255; Multiplex 2: ZFY, sY86, sY127, sY254; Multiplex 3: SRY, sY145, sY153; Multiplex 4: ZFY, sY81, sY121; and Multiplex 5: SRY, sY124, sY242, sY239.Genomic DNA (100 ng) was added to a mixture of 200 mmol/l Tris–HCl (pH 8.3), 100 mmol/l KCl, 3 mmol/l MgCl2, 5 mmol/l each dNTP, 10 per cent dimethyl sulfoxide (DMSO), 5 pmol/l of primer pairs, 2 U Taq DNA polymerase (Genfanavaran Co., Tehran, Iran), adjusted to a final volume of 25 μl. Amplifications were carried out on a Techne thermocycler (Techn Ltd., Cambridge, UK) with the following program: Initial denaturation at 94 °C for 5 min and a subsequent series of 35 cycles of 94 °C for 30 sec (denaturation), 57 °C for 45 sec (annealing), and 72°C for 1 min (extension). Final extension was carried out at 72°C for 5 min. All of the PCR amplification products were subjected to electrophoresis on 2.5 per cent agarose gel and/or 10 per cent acrylamide gel prepared in 0.5X TBE, stained with ethidium bromide and visualized by exposure to ultraviolet light. In order to confirm the absence of the unamplified STS markers, two additional PCRs were carried out.266 INDIAN J MED RES, SEPTEMBER 2010Statistical analyses:χ2 test was used to compare differences between the two studied groups. The statistical analyses were performed with SPSS 16 statistical software (SPSS Inc., Chicago, Illinois, USA). P<0.05 was considered significant.ResultsA total of 200 men, including 100 patients with non-obstructive azoospermia and 100 fertile controls, were analyzed for the presence of submicroscopic Y chromosome deletions. Of these, 12 patients (12%) showed Y chromosome microdeletions, while no microdeletion was detected in controls (P<0.001). Among the patients, deletion in AZFb region was the most common (66.67%) followed by AZFc (41.67%), AZFd (33.33%) and AZFa (8.33%) respectively (Fig. 1). Individual deletions in AZFb and AZFc regions were detected in 6 and 1 of the patients which accounted for 50 and 8.33 per cent of the total deletion respectively (Fig. 2). Combined deletions including AZFab, AZFcd, and AZFbcd were also detected in 1, 3, and 1 of the patients with microdeletion respectively (Fig. 2). Characteristics, hormonal analysis results, and treatment outcomes for the patients who showed Y chromosome microdeletion are mentioned in Table II.AZFaAZFb AZFc41.67%66.67%33.33%Fig. 1. Venn diagram illustrating frequencies of different types of observed Y chromosome microdeletions in AZF regions. AZFa, b, c and d deletions were involved in 8.33, 66.67, 41.67, and 33.33 per cent of total deletions respectively. Combined deletions including AZFab, AZFcd, and AZFbcd were also detected with the frequencies of 8.33, 25, and 8.33 per cent respectively. Individual deletions were also detected only in AZFb and AZFc regions with the frequencies of 50 and 8.33 per cent respectively.Table I. STS markers and primer sequences used for screening Y chromosome microdeletionsSTS Region Size(bp)Primer sequencesY81AZFa2095’AGG CAC TGG TCA GAA TGA AG3’5’AAT GGA AAA TAC AGC TCC CC3’sY84AZFa3265’AGA AGG GTC TGA AAG CAG GT3’5’GCC TAC TAC CTG GAG GCT TC3’sY86AZFa3205’GTG ACA CAC AGA CTA TGC TTC3’5’ACA CAC AGA GGG ACA ACC CT3’sY121AZFb1905’AGT TCA CAG AAT GGA GCC TG3’5’CCT GTG ACT CCA GTT TGG TC3’sY124AZFb1095’CAG GCA GGA CAG CTT AAA AG3’5’ACT GTG GCA AAG TTG CTT TC3’sY127AZFb2745’GGC TCA CAA ACG AAA AGA AA3’5’CTG CAG GCA GTA ATA AGG GA3’sY134AZFb3015’GTC TGC CTC ACC ATA AAA CG3’5’ACC ACT GCC AAA ACT TTC AA3’sY145AZFd1255’CAA CAC AAA AAC ACT CAT ATACTCG3’5’GGG CAT TGT ATG TTA ATA AGA GTT3’sY153AZFd1355’GCA TCC TCA TTT TAT GTC CA3’5’ATG AGT CAC GAA AAC CCA AC3’sY242AZFc2335’ACA CAG TAG CAG CGG GAG TT3’5’TCT GCC ACT AAA CTG TAA GCT CC3’sY239AZFc2015’CAT TCA TCT TCC CTT TTG AAG G3’5’ATG CAA GTC GCA GGA AAT CT3’sY254AZFc4005’GGG TGT TAC CAG AAG GCA AA3’5’GAA CCG TAT CTA CCA AAG CAG C3’sY255AZFc1265’GTT ACA GGA TTC GGC GTG AT3’5’CTC GTC ATG TGC AGC CAC3’STS, sequence tagged siteTable II. Clinical features of 12 azoospermia patients with Y chromosome microdeletionsPatientNo.FSH(mIU/ml)LH(mIU/ml)TestisbiopsyTreatment Sperm A16012NA NA NAA84510SCO NA NA Inf47612MA TESE-Inf67010.5MA TESE-Inf136017MA TESE-Inf243019MA TESE-Inf382512MA TESE-Inf522018MA TESE-Inf1044020SCO TESE-Inf121107.5SCO TESE-Y238.57NA TESE-Y303514NA TESE-FSH, follicule-stimulating hormone; LH, leuterizing hormone; NA, not available; SCO, sertoli cell only; MA, maturation arrest; TESE, testicular sperm extraction; -, no sperm.MIRFAKHRAIE et al: AZOOSPERMIA & Y CHROMOSOME MICRODELETIONS IN IRANIAN INFERTILE MALE 267The mean value for FSH concentration in 12 patients with Y chromosome microdeletions was 44.90 mIU/ ml compared with 35.50 mIU/ml in patients with no microdeletions (P<0.001). These values were 13.91 and 13.69 mIU/ml for LH concentration respectively (P = 0.178) (Table II). Nine patients who showed microdeletions underwent testicular biopsy. In six patients, maturation arrest was observed at different steps of spermatogenesis and three patients showed SCO syndrome. In all of these patients sperm retrieval failed using testicular sperm extraction (TESE) (Table II).DiscussionPrevious reports have revealed that Y chromosome microdeletions vary from 1 to 55 per cent among infertile men all over the world, but most studies have reported an incidence below 15 per cent7. In the present study the estimated frequency was 12 per cent among azoospermic patients that is within the range of the published data. Omrani et al15 showed that the incidence of microdeletions among azoospermic patients of the Kurd and Azari ethnic groups in North West of Iran was about 30 per cent (18 of 60). Malekasgar et al 14also indicated that the incidence of Yq microdeletions in South of Iran was higher than international frequency. They found microdeletions in 51.6 per cent (16 of 31) of patients with azoospermia14. These variations in deletion frequencies may be due to geographic and ethnic origins of the studied population and differences in the study design including the composition of the study population and sample size. However, Krausz et al16,17suggested that the major factor influencing deletion frequency was the composition of study population and ethnic or geographical differences apparently had no influence on it. They found that the highest deletion frequency was in the group defined as idiopathic azoospermic/cryptozoospermic with the incidence of 17 per cent and the incidence decreases progressively with the inclusion of less severe phenotypes17. It seems that the discrepancy in reported deletion frequencies in Iranian population could be explained by (i) differences in the sample size, and (ii) ethnic variations since our samples were collected from the whole country irrespective of ethnic origin. Deletions of the AZF region occur with different frequency. It has been suggested that AZFc is the most frequently deleted region (60%), followed by deletions of the AZFb and combined deletions involving different AZF regions (35%). AZFa deletions are extremely rare (5%) and isolated deletions have been reported in this region16.None of the patients in the present study showed individual deletion in AZFa region using sY81, sY84 and sY86 sequence tagged sites, although deletion in this region was observed in combination with deletions in other AZF regions in one patient. In our study, AZFa region was involved in a total of 8.33 per cent of the observed Y chromosome microdeletions. This region contains single copies of DFFRY (USP9Y) and DBY (DDX3Y) genes. It is suggested that complete deletion of AZFa region may result in complete SCO syndrome and azoospermia4,7,18,19. Identification of deletions in this region is very important since it is impossible to retrieve testicular sperm for ICSI4,7.AZFb deletion was involved in 66.67 per cent of the total deletions of which AZFb alone was involved in 50 per cent. Selected STS markers for detecting deletions in AZFb region in the present study were located in the median and distal part of AZFb and in the most cases the deletion of sY127 and sY134 markers indicated a complete deletion of the AZFb region which may cause SCO syndrome or spermatogenetic arrest resulting in azoospermia7. Five patients showed complete deletionPatientd F ZAa F ZA AZFcAZFbFig. 2. Schematic diagram illustrating different deletion patterns ofthe sequence-tagged site markers in the studied patients. +: PCRproduct was present; –: PCR product was not detected.268 INDIAN J MED RES, SEPTEMBER 2010of AZFb whereas two patients showed partial deletion in AZFb region. RBMY genes, including RBM1 and RBM2, located in AZFb region, are specifically expressed in testis and germ cells. The gene encodes a RNA binding protein that localizes to the nucleus of all spermatogenic cell types4,20. Since several copies of these genes are located in the AZFb region, it is not clear whether the loss of the RBMY genes in men may result in male infertility or not, therefore, the role of deletion in these genes is not clear in the process of spermatogenesis21. Several reports have shown that complete deletions in AZFb region will have the same results as deletions in AZFa region for testicular sperm extraction (TESE)4,7. Patients with complete or partial AZFb deletion in our study showed maturation arrest in their testis biopsy; therefore, attempts for retrieving sperm were not successful.Deletions in AZFc region are the most commonly reported deletions among AZF microdeletions and its complete deletion is one of the most frequent molecular genetics causes of severe male infertility. The prevalence of this type of deletion is 5-10 per cent in cases of azoospermia and severe oligozoospermia22. DAZ gene family is the most important candidate gene for male infertility in this region and consists of four functional copies including DAZ1, DAZ2, DAZ3 and DAZ4 arranged in two clusters18,23. DAZ encodes for a testis specific RNA binding protein containing 8-24 copies of 24 amino acid sequences, known as ‘DAZ repeat’24. Deletions in DAZ genes may have different effects. It has been suggested that only partial AZFc deletions removing DAZ1/DAZ2 are associated with spermatogenic impairment and male infertility, whereas those removing DAZ3/DAZ4 are found in both fertile and infertile men25.In our study, deletion in AZFc region accounted for 41.67 per cent of the total deletion detected in the patients, using sY239, sY242, sY254 and sY255 sequence tagged sites which are specific for DAZ gene. The absence of these markers indicates deletion of the entire AZFc region, which removes all copies of DAZ7. Partial or complete deletions in AZFc region may result in different phenotypes vary from normal to oligozoospermia and azoospermia, so there may be a chance for retrieving sperm from testis and TESE/ ICSI can be attempted in these patients. However, in our study patients with AZFc deletion showed SCO syndrome or maturation arrest in histological analysis. None of the patients showed individual deletion in AZFd region, however deletion in this region was observed in combination with deletions in other AZF regions. It is believed that AZFd STS markers are located within the AZFc region and the existence of this region separately is seriously questioned4. Our results were contrary to this idea since a patient (no.A1) who showed the complete deletion of AZFc region did not show deletion of AZFd STS markers. However, three patients with AZFc deletion (Inf6, Inf38, and Inf121) also showed deletion of sY145 marker within the AZFd region. In addition, one patient (no.A8) with complete deletion of AZFbc showed deletion of both STS markers for AZFd region. Hence, our results are similar to those who consider AZFd as a separate region between AZFb and AZFc regions6,26,27.Finally, several studies have shown that serum FSH levels were significantly above the mean value in azoospermic patients and in patients with microdeletions16,17,28. Sertoli cell function depends on adequate stimulation by FSH and the elevation of FSH may result in abnormal spermatogenesis28. In the present study, same results were obtained, further the FSH values were significantly higher in patients with microdeletions than patients without microdeletions (P<0.001). However, this may be due to small sample size of the patients with microdeletions (n = 12) and further studies are needed to confirm these results.Since the involvement of AZFb region was seen in 66.67 per cent of total deletions, we may conclude that genes located in the AZFb region were more involved in the fertility process in the studied patients and this is contrary to previous reports in which AZFc deletion was reported to be the most frequent deletion in azoospermic patients4,13-15,29-31.Due to relatively high incidence of Y chromosome microdeletions among Iranian candidates for ICSI, molecular screening for detection of these microdeletions may have diagnostic, prognostic and preventive value, and for genetic counselling in infertility clinics.AcknowledgmentThe authors are grateful to Yazd Clinical Centre for Infertility, Day Hospital IVF section, and Kowsar Infertility Treatment Center for their kind collaboration.ReferencesEzeh UIO. Beyond the clinical classification of azoospermia.1.Hum Reprod 2000; 15 : 2356-9.Sharif K. Reclassification of azoospermia: The time has come?2.Hum Reprod 2000; 15 : 237-8.MIRFAKHRAIE et al: AZOOSPERMIA & Y CHROMOSOME MICRODELETIONS IN IRANIAN INFERTILE MALE 269Ezeh UIO, Taub N, Moore HDM, Cooke ID. Establishment of 3.predictive variables associated with testicular sperm retrieval in men with non-obstructive azoospermia. Hum Reprod 1999;14 : 1005-12.Sadeghi-Nejad H, Farrokhi F. Genetics of azoospermia: Current 4.knowledge, clinical implications, and future directions. Part II. Urol J 2007; 4 : 192-206.Raicu F, Popa L, Apostol P, Cimponeriu D, Dan L, Ilinca E, 5.et al. Screening for microdeletions in human Y chromosome-AZF candidate genes and male infertility. J Cell Mol Med 2003; 7 : 43-8.Kent-First M, Muallem A, Shultz J, Pryor J, Roberts K, Nolten 6.W, et al. Defining regions of the Y-chromosome responsible for male infertility and identification of a fourth AZF region (AZFd) by Y-chromosome microdeletion detection.Mol Reprod Dev 1999; 53 : 27-41.Simoni M, Bakker E, Krausz C. EAA/EMQN best practice 7.guidelines for molecular diagnosis of y-chromosomal microdeletions. State of the art 2004. Int J Androl 2004; 27 : 240-9.Page DC, Silber S, Brown LG. Men with infertility caused by 8.AZFc deletion can produce sons by intracytoplasmic sperm injection, but are likely to transmit the deletion and infertility.Hum Reprod 1999; 14 : 1722-6.Kuhnert B, Gromoll J, Kostova E, Tschanter P, Luetjens CM, 9.Simoni M, et al. Case report: natural transmission of an AZFc Y-chromosomal microdeletion from father to his sons. Hum Reprod 2004; 19 : 886-8.Kamischke A, Gromoll J, Simoni M, Behre HM, Nieschlag10.E. Transmission of a Y chromosomal deletion involving thedeleted in azoospermia (DAZ) and chromodomain (CDY1) genes from father to son through intracytoplasmic sperm injection: case report. Hum Reprod 1999; 14 : 2320-2.World Health Organization.11. WHO laboratory manual forthe examination of human semen and semen-cervical mucus interaction, 4th ed. Cambridge: Cambridge University Press;1999.Miller SA, Dykes DD. A simple salting out procedure for 12.extracting DNA from human nucleated cells. Nucleic Acids Res 1988; 16 : 1215.Zamani AG, Kutlu R, Durakbasi-Dursan HG, Gorkemli H, 13.Acar A. Y chromosome microdeletions in Turkish infertile men. Indian J Hum Genet 2006; 12 : 66-71.Malekasgar AM, Mombaini H. Screening of Y chromosome 14.microdeletions in Iranian infertile males. J Hum Reprod Sci 2008; 1 : 2-9.Omrani MD, Samadzadae S, Bagheri M, Attar K. Y 15.chromosome microdeletions in idiopathic infertile men from West Azarbaijan. Urol J 2006; 3 : 38-43.Krausz C, Forti G, McElreavey K. The Y chromosome and 16.male fertility and infertility. Int J Androl 2003; 26 : 70-5.Krausz C, Rajpert-De Meyts E, Frydelund-Larsen L, 17.Quintana-Murci L, McElreavey K, Skakkebaek NE. Double-blind Y chromosome microdeletion analysis in men with known sperm parameters and reproductive hormone profiles: microdeletions are specific for spermatogenic failure. J Clin Endocrinol Metab 2001; 86 : 2638-42.V ogt PH, Edelmann A, Kirsch S, Henegariu O, Hirschmann 18.P, Kiesewetter F, et al. Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum Mol Genet 1996; 5 : 933-43.Krausz C, Quintana-Murci L, McElreavey K. Prognostic 19.value of Y deletion analysis: what is the clinical prognostic value of Y chromosome microdeletion analysis? Hum Reprod 2000; 15 : 1431-4.Elliot DJ,20. Millar M R, Oghene K, Ross A, Kiesewetter F, PryorJ, et al. Expression of RBM in the nuclei of human germ cells is dependent on a critical region of the Y chromosome long arm. Proc Natl Acad Sci USA 1997; 94 : 3848-53.Delbridge ML, Harry JL, Toder R, O’Neill21. RJW, Ma K,Chandley AC, et al. A human candidate spermatogenesis gene, RBM1, is conserved and amplified on the marsupial Y chromosome. Nat Genet 1997; 15 : 131-6.Foresta C, Moro E, Ferlin A. Y chromosome microdeletions 22.and alterations of spermatogenesis. Endocr Rev 2001; 22: 226-39.Saxena R, de Vries JW, Repping S, Alagappan RK, Skaletsky 23.H, Brown LG, et al. Four DAZ genes in two clusters found in the AZFc region of the human Y chromosome. Genomics 2000; 67 : 256-67.Reijo R, Lee TY, Salo P, Alagappan R, Brown LG, Rosenberg 24.M, et al. Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat Genet 1995; 10 : 383-93.Ferlin A, Tessari A, Ganz F, Marchina E, Barlati S, Garolla 25.A, et al. Association of partial AZFc region deletions with spermatogenic impairment and male infertility. J Med Genet 2005; 42 : 209-13.Muslumanoglu MH, Turgut M, Cilingir O, Can C, Ozyurek 26.Y, Artan S. Role of the AZFd locus in spermatogenesis. Fertil Steril 2005; 84 : 519-22.Cram DS, Ma K, Bhasin S, Arias J, Pandjaitan M, Chu B, 27.et al. Y chromosome analysis of infertile men and their sons conceived through intracytoplasmic sperm injection: vertical transmission of deletions and rarity of de novo deletions.Fertil Steril 2000; 74 : 909-15.Lammarrone E, Balet R, Lower AM, Gillott C, Grudzinskas 28.JG. Male infertility. Best Pract Res Clin Obstet Gynaecol 2003; 17 : 211-29.Simoni M. The EAA International Quality Control Programme 29.for Y-Chromosomal microdeletions. European Academy of Andrology. Int J Androl 1998; 21 : 315-6.Krausz C, Forti G, McElreavey K. The Y chromosome and 30.male fertility and infertility. Int J Androl 2003; 26 : 70-5.Thangaraj K, Gupta NJ, Pavani K, Reddy AG, Subramainan S, 31.Selvi Rani D, et al. Y chromosome deletions in azoospermic men in India. J Androl 2003; 24 : 588-97.Reprint requests: Dr Reza Mirfakhraie, Department of Medical Genetics, National Institute of Genetic Engineering & Biotechnology Pajoohesh Blv., 17 Km, Tehran-Karaj Highway, Tehran, Irane-mail: reza_mirfakhraie@yahoo.co m, rmirfakhraie@nigeb.ac.i r270 INDIAN J MED RES, SEPTEMBER 2010。
Y染色体微缺失检测指导

Y染色体微缺失是严重少精子或无精子症的重要原因,是导致男性不育的第二大遗传因素,其发生率仅次于Klinefelter综合征(克氏综合征)。
从1999年开始,欧洲男科学协会和欧洲分子遗传实验质控网(EAA/EMQN)为提高诊断质量,出版了Y 染色体微缺失分子诊断指南,并提供了客观的实验质量评价方法。
最新版的实验室指南是2013年9月EAA/EMQN根据12年的临床积累和专家共识,在1999版和2004版的基础上修订而成。
新指南重点阐明:在少精子症或无精子症男性中。
测指南有重要的指导意义。
一、Y染色体微缺失发生频率Y染色体微缺失在健康人群中发生率约为1/4 000,但在不育男性中显着升高,微缺失发生频率为2%~10%(甚至更高)。
2013版指南指出Y染色体微缺失在中国不育男性中发生频率为11.5%,处于较高水平。
2006年朱晓斌等[1]针对中国不育男性染色体的研究表明AZF微缺失在非梗阻性无精子症患者中的发生率为9.94%,严重少精子症患者中发生率为5.82%,与国内外其他学者的研究基本一致。
随着微缺失分子机制的阐明和Y染色体男性特异区域的结构(MSY)测序完成,结合十多年临床数据分析,EAA专家总结沿用Repping等[2]对Y染色体微缺失区域的定义模式,分为:AZFa区缺失、AZFb区缺失、AZFbc区缺失和AZFc区缺失,认为只有该微缺失模式有明确的临床表现。
国内外学者对AZFd区缺失是否存在一直存有争议。
尽管发现在AZFb与AZFc两区域之间存在新的缺失位点(一些学者认为的AZFd区缺失),但是该区域缺失没有明确的临床意义,也并非独立存在的缺失模式。
所以第四区域AZFd区缺失仍存在较多争议。
Müslümanolu等[3]在2005年的一项研究中指出AZFd缺失可能与精子形成有关,但仍缺乏有力的临床证据。
2013年,陈科等[4]在精子正常和轻度少精子症患者中都发现了假定的AZFd 区缺失。
Y染色体微缺失及其检测方法

Y染色体微缺失及其检测方法高云;陈嘉昌;彭焕玉;朱振宇【摘要】全世界大约有15%的夫妇不育,其中男性不育约占50%.Y染色体长臂上的AZF缺失是导致男性不育的重要因素.AZF进一步分为AZFa、AZFb和AZFc 3个区域,不同区域的微缺失引起不同程度的精子发生障碍.因此Y染色体微缺失的检测对男性不育的诊治有很重要的指导意义.目前Y染色体微缺失常用的检测方法有多重定性PCR法、实时荧光PCR法、基因芯片法和荧光原位杂交法.【期刊名称】《分子诊断与治疗杂志》【年(卷),期】2011(003)005【总页数】4页(P326-329)【关键词】Y染色体微缺失;男性不育;AZF【作者】高云;陈嘉昌;彭焕玉;朱振宇【作者单位】中山大学中山医学院,广东,广州510080;中山大学达安基因诊断中心,广东,广州510665;中山大学达安基因诊断中心,广东,广州510665;中山大学中山医学院,广东,广州510080【正文语种】中文目前全世界大约有15%的夫妇不育,其中男性不育约占50%[1]。
引起男性不育的因素有很多,其中由Y染色体微缺失引起的生精障碍是导致男性不育的重要原因,表现为原发性无精子症(Azoospermia)或少精子症(Oligospermia)。
研究表明男性原发无精和少精症患者中大约有10%~15%存在Y染色体微缺失[2,3]。
1 Y染色体微缺失与男性不育1976年,Tiepolo等在1170例男性不育患者中发现6例无精子症患者存在显微镜下可见的Y染色体长臂缺失,于是提出Y染色体长臂上可能存在着控制精子生成的基因,并将其称为无精子症因子(azoospermia factor,AZF)。
1996年Vogot将 AZF 划分为AZFa、AZFb、AZFc 3个相互独立的区域(见图1[4])。
各区域内包括若干AZF候选基因并主导精子形成过程中的不同阶段,它们的缺失或突变可能导致精子发生障碍,引起少精子症或无精子症。
