Progression of Aortic Valve Calcification Association With Coronary Atherosclerosis and Car
Method and system for aortic valve calcification e

专利名称:Method and system for aortic valvecalcification evaluation发明人:Sasa Grbic,Razvan Ioan Ionasec,FernandoVega-Higuera,Dominik Bernhardt,DorinComaniciu申请号:US13713603申请日:20121213公开号:US09730609B2公开日:20170815专利内容由知识产权出版社提供专利附图:摘要:A method and system for automatic aortic valve calcification evaluation isdisclosed. A patient-specific aortic valve model in a 3D medical image volume, such as a 3D computed tomography (CT) volume. Calcifications in a region of the 3D medical image volume defined based on the aortic valve model. A 2D calcification plot is generated that shows locations of the segmented calcifications relative to aortic valve leaflets of the patient-specific aortic valve model. The 2D calcification plot can be used for assessing the suitability of a patient for a Transcatheter Aortic Valve Replacement (TAVI) procedure, as well as risk assessment, positioning of an aortic valve implant, and selection of a type of aortic valve implant.申请人:Sasa Grbic,Razvan Ioan Ionasec,Fernando Vega-Higuera,Dominik Bernhardt,Dorin Comaniciu地址:Erlangen DE,Lawrenceville NJ US,Erlangen DE,Hausen DE,Princeton Junction NJ US国籍:DE,US,DE,DE,US更多信息请下载全文后查看。
Aortic Stenosis

INTRODUCTION
ETIOLOGY
When considering the etiology of aortic stenosis, it is important to consider the age and the demographics of the patient (Fig. 1). Rheumatic valvular disease, once the most common form of aortic stenosis, is less common today in developed countries. Commissural fusion is a hallmark of this disease and often presents with concomitant mitral stenosis (Fig. 2). Less commonly, the commissures may fuse eccentrically,producing a de facto bicuspid valve. In this case, differentiation from a congenitally bicuspid valve can be difficult.
Aortic Stenosis
Transthoracic echocardiography has largely replaced cardiac catheterization as the primary modality for the hemodynamic assessment of valvular heart disease. A comprehensive evaluation of valve structure, function,and hemodynamics is possible through a carefully performed transthoracic study. In the case of aortic stenosis,echocardiography is used to define the initial severity of disease, etiology, and monitor its progression through serial follow up studies. Evaluation of aortic stenosis would be incomplete without a comprehensive examination of overall left ventricular function and estimation of pulmonary artery pressures. Transthoracic echocardiography, therefore, can provide important information about the initial diagnosis, management, and follow-up of adult patients with native aortic valve stenosis.
心脏超声常用英文缩写及对照

心脏超声常用英文缩写及对照A-Mode Amplitude mode A型(超声)AMVL Anterior mitral valve leaflet 二尖瓣前叶AAO Aorta ascendens 升主动脉Ao Aorta 主动脉AI Aortic insufficiency 主动脉关闭不全AML Anterior mitral leaflet 二尖瓣前叶AR Aortic regurgitation 主动脉瓣返流AS Aortic stenosis 主动脉狭窄ASD Atrial septal defect 房间隔缺损ASH Asymmetrical septal hypertrophy 非对称性室间隔肥厚ATVL Anterior tricuspid valve leaflet 三尖瓣前叶AV Aortic valve 主动脉瓣AVA Aortic valve atresia 主动脉瓣闭锁AVR Aortic valve replacement 主动脉瓣置换术BAV bicuspid aortic valve 二叶式主动脉瓣B mode Brightness mode B型超声CDFI Color Doppler Flow Imaging 彩色多普勒血流显像CHD Congenital heart disease 先天性心脏病CPV common pulmonary vein 共同肺静脉干CW chest wall 胸壁CW-Doppler Continuous-wave Dopper 连续频谱多普勒CO Cardiac output 心输出量DA Descending aorta 降主动脉DCM dilated cardiomyopathy 扩张性心肌病DTI doppler tissue imaging 多普勒组织显像DORV double outlet right ventricle 右心室双出口DOLV double outlet left ventricle 左心室双出口ED End-diastole 舒张末期的EDV End-diastolic volume 舒张末期容积EF Ejection fraction 射血分数EPSS E-point of septal separation 舒张早期二尖瓣前叶与室间隔之间的距离ESD End-systolic dimension 收缩末直径ESV End-systolic volume 收缩末容量F3 trilogy of Fallot 法洛三联症F4 tetralogy of Fallot 法洛四联症FO fossa ovalis 卵圆窝FS fractional shortening 缩短分数HCM hypertrophic cardiomyopathy 肥厚性心肌病HOCM hypertrophic obstructive cardiomyopathy肥厚性梗阻性心肌病HPRF High pulse repetition frequency 高脉冲重复频率HR Heart rate 心率IAS Interatrial septum 房间隔IVC Inferior vena cava 下腔静脉IVCT Isovolumic contraction time 等容收缩期IVRT Isovolumic relaxation time 等容舒张期IVS interventricular septum 室间隔LA Left atrium 左心房LAA Left atrial appendage 左心耳LAD Left anterior descending cor 前降支LAE Left atrial enlargement 左心房扩大LAO Left anterior oblique 左前斜位LCC Left coronary cusp 左冠状动脉瓣LMCA Left main coronary artery 左主干LPA Left pulmonary artery 左肺动脉LSPV left superior pulmonary vein 左上肺静脉LSA left subclavian artery 左锁骨下动脉LV Left ventricle 左心室LVAN left ventricular aneurysm 左心室室壁瘤LVED Left ventricular end diastolic 左心室舒张未期内径LVH Left ventricular hypertrophy 左心室肥大LVDd Left ventricular end diastolic dimension左心室舒张末期内径LVDs left ventricular end-systolic dimension 左心室收缩末期内径LVPW left ventricular posterior wall 左心室后壁LVOT Left ventricular outflow tract 左室流出道LVIT Left ventricular inflow tract 左室流入道LVIW left ventricular inferior wall 左室下壁M-mode Motion mode M-型超声MAC Mitral annular calcification 二尖瓣环钙化MB Moderator band 隔缘肉柱MI mitral insufficiency 二尖瓣关闭不全MS Mitral stenosis 二尖瓣狭窄MR Mitral regurgitation 二尖瓣返流MVO Mitral valve orifice 二尖瓣口MVP mitral valve prolapse 二尖瓣脱垂NCC Noncoronary cusp 无冠瓣OMI old myocardial infarction 陈旧性心肌梗塞PA Pulmonary artery 肺动脉PAP Pulmonary artery pressure 肺动脉压PBMV percutaeous balloon mitral valvuloplasty经皮二尖瓣球囊扩张成形术PDA Patent ductus arteriosis 动脉导管未闭PE Pericardial effusion 心包积液PISA Proximal isovelocity surface area 最近等速线表面积PM papillary muscle 乳头肌PML Posterior mitral leaflet 二尖瓣后叶PTL posterior tricuspid leaflet 三尖瓣后叶PI Pulmonary insufficiency 肺动脉关闭不全PPM posterior papillary muscle 后乳头肌PS Pulmonary stenosis 肺动脉狭窄PV Pulmonic valve 肺动脉瓣PV Pulmonary vein 肺静脉PW-Doppler Pulsed-wave Doppler 脉冲频谱多普勒PWT Posterior wall thickness 舒张期后壁厚度RA Right atrium 右心房RAE Right atrial enlargement 右心房增大RAO Right anterior oblique 右前斜RCA Right coronary artery 右冠状动脉RCC Right coronary cusp 右冠瓣RPA Right pulmonary artery 右肺动脉RSPV Right superior pulmonary vein 右上肺静脉RV Right ventricle 右室RVAW Right ventricular anterior wall 右心室前壁RVE Right ventricular enlargement 右心室扩大RVH Right ventricular hypertrophy 右心室肥大RVI Right ventricular inflow 右室流入道RVOT Right ventricular outflow tract 右心室流出道SAM Systolic anterior motion 二尖瓣收缩期前向运动SBE subacute bacterial endocarditis 亚急性细菌心内膜炎SSN Suprasternal notch 胸骨上切迹ST Septal thickness 间隔厚度STP sinus transversus pericardii 心包横窦SOP sinus obliquus pericardium 心包斜窦SV Stroke volume 每搏输出量SVC Superior vena cava 上腔静脉SWMA Segmental wall motion abnormality节断性室壁运动异常TEE Transesophageal echocardiography经食管超声心动图TGA Transposition of the grea 大动脉移位TGC Time gain compensation 时间增益补偿TI tricuspid incompetence 三尖瓣关闭不全TL True lumen 真腔TOF Tetralogy of Fallot 法乐(氏)四联症TVP tricuspid valve prolapse 三尖瓣脱垂TS Tricuspid stenosis 三尖辨狭窄TTE Transthoracic echocardiography 经胸壁超声心动图TV Tricuspid valve 三尖瓣Veg Vegetation 赘生物Vmax Maximum velocity 最大速度VSD Ventricular septal defect 室间隔缺损VTI Velocity time integral 速度时间积分英文缩写英文全称中文名称AFI ATRIAL FIBRILLATION 心房纤维颤动AFL ATRIAL FLUTTER 心房扑动APC ATRIAL PREMATURE CONTRACTIONS 心房早期收缩APV ABSENCE OF PULMONARY VALVE 肺动脉瓣缺失APVC ANOMALOUS PULMONARY VENOUS CONNECTION 肺静脉连接异常APVR ANOMALOUS PULMONARY VENOUS RETURN 肺静脉回流异常AR AORTIC REGURGITATION 主动脉瓣关闭不全AS AORTIC STENOSIS 主动脉瓣狭窄ASD ATRIAL SEPTAL DEFECT 房间隔缺损AVB AV BLOCK, ATRIO-VENTRICULAR BLOCK 房室传导阻滞AVSD ATRIOVENTRICULAR SEPTAL DEFECT 房室间隔缺损BAV BICUSPID AORTIC VALVE 双叶性动脉瓣BT BLALOCK-TAUSSIG SHUNT BT分流术CAD CORONARY ARTERIAL DISEASE 冠状动脉疾病COA COARCTATION OF THE AORTA 主动脉窄缩DAA DOUBLE AORTIC ARCH 双主动脉弓DILV DOUBLE INLET LEFT VENTRICLE 左心室双入口DIRV DOUBLE INLET RIGHT VENTRICLE 右心室双入口DCM DILATED CARDIOMYOPATHY 扩张性心肌病变DOLV DOUBLE OUTLET LEFT VENTRICLE 左心室双出口DORV DOUBLE OUTLET RIGHT VENTRICLE 右心室双出口DYS DYSRHYTHMIA 节律异常、心律不整EBS EBSTEIN' S ANOMALY EBSTEIN氏畸形ECD ENDOCARDIAL CUSHION DEFECT 心内膜垫缺损HCM HYPERTROPHIC CARDIOMYOPATHY 肥厚型心肌病变HLHS HYPOPLASTIC LEFT HEART SYNDROME 左心发育不良综合征HLV HYPOPLASTIC LEFT VENTRICLE 左心室发育不全HRV HYPOPLASTIC RIGHT VENTRICLE 右心室发育不全IAA INTERRUPTION OF AORTIC ARCH 主动脉弓中断IE INFECTIVE ENDOCARDITIS 感染性心内膜炎IHSS IDIOPATHIC HYPERTROPHIC SUBAORTIC STENOSIS 特发性肥厚性主动脉瓣下狭窄JPC JUNCTIONAL PREMATURE CONTRACTION 房室节早期收缩KD KAWASAKI' S DISEASE 川崎病LAA LEFT AORTIC ARCH 左主动脉弓LAI LEFT ATRIAL ISOMERISM 两侧左心房LAR LEFT ATRIAL RHYTHM 左心房节律LCA LEFT CORONARY ARTERY DISEASE 左冠状动脉疾病LSVC LEFT SUPERIOR VENA CAVA 左上腔静脉MA MITRAL ATRESIA, MITRAL VALVE ATRESIA 二尖瓣闭锁MAPCA MAIN AORTO-PULMONARY COLLATERAL ARTERIES 联接主动脉与肺间的侧枝血管MR MITRAL REGURGITATION 二尖瓣关闭不全MS MITRAL STENOSIS 二尖瓣狭窄MVP MITRAL VALVE PROLAPSE 二尖瓣脱垂PA PULMONARY ATRESIA 肺动脉瓣闭锁PAB PULMONARY ARTERY BANDING ( PA BANDING ) 肺动脉绷扎PAPVC PARTIAL ANOMALOUS PULMONARY VENOUS CONNECTION 部份肺静脉连接异常PAPVR PARTIAL ANOMALOUS PULMONARY VENOUS RETURN 部份肺静脉回流异常PDA PATENT DUCTUS ARTERIOSUS 动脉导管未闭PE PERICARDIAL EFFUSION 心包膜积水PFO PATENT FORAMEN 开放性卵圆孔PHT PULMONARY ARTERY HYPERTENSION 肺动脉高压PR PULMONIC REGURGITATION 肺动脉瓣关闭不全PSVT PAROXYMAL SUPRAVENTRICULAR TACHYCARDIA 阵发性室上性心搏过速PS PULMONIC STENOSIS 肺动脉瓣狭窄PVO PULMONARY VENOUS OBSTRUCTION 肺静脉阻塞RAA RIGHT AORTIC ARCH 右主动脉弓RAI RIGHT ATRIAL ISOMERISM 两侧右心房RCA RIGHT CORONARY ARTERY DISEASE 右冠状动脉疾病RCC RIGHT CORONARY CUSP PROLAPSE, RCC PROLAPS 右冠状动脉瓣脱垂RDS RESPIRATORT DISTRESS DISEASE SYNDROME 呼吸窘迫症候群RF RHEUMATIC FEVER 风湿热RHD RHEUMATIC HEART DISEASE 风湿性心脏病SA SINGLE ATRIUM 单心房SBE SUBACUTE BACTERIAL ENDOCARDITIS 亚急性细菌性心内膜炎SSS SICK SINUS SYNDROME 病窦症候群SV SINGLE VENTRICLE 单心室TA TRICUSPID ATRESIA, TRICUSPID VALVE ATRESIA 三尖瓣闭锁TAPVC TOTAL ANOMALOUS PULMONARY VENOUS CONNECTION 全部肺静脉连接异常TAPVR TOTAL ANOMALOUS PULMONARY VENOUS RETURN 全部肺静脉回流异常TGA TRANSPOSITION OF THE GREAT ARTERIES 大动脉转位TOF TETRALOGY OF FALLOT 法洛氏四联症TR TRICUSPID REGURGITATION 三尖瓣关闭不全TS TRICUSPID STENOSIS 三尖瓣狭窄VPC VENTRICULAR PREMATURE CONTRACTION 心室早期收缩VSD VENTRICULAR SEPTAL DEFECT 室间隔缺损WPW WOLFF-PARKINSON-WHITE SYNDROME WPW症候群。
大梳理:经导管主动脉瓣置入术(TAVI)入路选择

大梳理:经导管主动脉瓣置入术(TAVI)入路选择继Anderson等在1992年首先报道了经导管主动脉瓣置入(TAVI)的动物实验后,2002年Cribier首次完成人体TAVI,到2015年全球共完成了289000例TAVI,2015年全年完成了71000例TAVI手术,到2025年这一数字将达到289000例。
TAVI使用导管将人工主动脉瓣通过合适入路置入于主动脉根部,替换原有的病变瓣膜的功能,在TAVI术中,选择合适的入路非常重要,临床研究表明,合适的血管入路可以提高手术成功率,减少并发症发生,对术后的康复和预后起到重要作用。
目前TAVI使用的入路有顺行入路和逆行入路,后者包括股动脉入路、锁骨下动脉入路、主动脉入路、颈动脉入路等,经心尖入路属顺行入路范畴。
一、顺行法(经股静脉、房间隔穿刺路径)TAVI早期多使用该方法,通过顺行穿刺房间隔,经二尖瓣、左心室到达主动脉根部,将人工主动脉瓣置入主动脉根部。
该方法的优点在于:外周静脉直径大、扩展性好,受到血管直径、疾病等限制小,血管并发症少。
其缺点在于:手术操作复杂、并发症较多,需经过房间隔穿刺,输送导管在经过二尖瓣口时,会损伤二尖瓣叶及瓣下结构。
目前该入路已经少用。
二、经股动脉入路图:经股动脉入路随着瓣膜设计理念更新,材料技术提高,瓣膜使用的导管直径逐渐变细,目前14F、16F和18F导管的应用使得逆行经股动脉途径成为可能。
Webb等对股动脉入路TAVI 临床资料的研究表明:由于存在学习曲线,最初25例手术的成功率为78%,后来提高到96%,术后30天的死亡率为l2%,中期随访未见瓣膜支架移位,瓣膜扭曲,关闭不全等, 3例在术后1个月出现瓣周漏。
欧洲纳入61例患者数据的多中心研究显示:手术成功率96.4%,30天死亡率为8.2%,急诊主动脉瓣膜置换率均为l.6%,瓣膜栓塞均率为3.3%,脑血管意外发生率为3.3%,出血并发症为23.5%,血管并发症为28.4%,患者术后6个月及1年生存率为90.2%和78.7%。
钙化性主动脉瓣疾病的研究进展

钙化性主动脉瓣疾病的研究进展师瑞【摘要】大量研究显示,随着人口老龄化的加剧,钙化性主动脉瓣疾病发病率逐年上升.目前研究发现其是一类涉及内皮损伤、基质重塑、血管生成、钙化、骨形成及多种血管活性肽等参与的主动过程,其中血管活性肽较受关注.而且,目前尚无有效的药物治疗方案.近年来,他汀类药物及肾素-血管紧张素抑制剂的疗效备受关注,但结论尚不一致.基于此,现就钙化性主动脉瓣疾病的研究进展及治疗做一综述.【期刊名称】《心血管病学进展》【年(卷),期】2018(039)005【总页数】5页(P742-746)【关键词】钙化性主动脉瓣疾病;主动脉瓣狭窄;血管活性肽;他汀类药物【作者】师瑞【作者单位】重庆医科大学附属第一医院心血管内科,重庆 400016【正文语种】中文【中图分类】R543.1钙化性主动脉瓣疾病(calcific aortic valve disease,CAVD)是临床常见的一类心脏瓣膜疾病,根据疾病演变进程可分为主动脉瓣钙化与钙化性主动脉瓣狭窄。
随着风湿性心脏病发病率的降低以及人口老龄化,其发病率逐年上升,已成为继冠心病及高血压之后的第三大心血管疾病[1]。
既往CAVD被认为是“自然磨损”过程;但近年来研究发现内皮损伤、炎症、基质重塑、血管生成、钙化及骨形成及多种血管活性肽等多种途径均参与其中[2]。
此外,CAVD尚无有效的药物治疗方案,他汀类药物及肾素-血管紧张素系统(renin-angiotensin system,RAS)阻滞剂是研究最多的两类药物,但仍存在较多争议。
而近年来,在治疗方面主动脉瓣置换手术及经导管主动脉瓣置换术(transcatheter aortic valve replacement,TAVR)成为治疗CAVD的有效方案,但对于不能耐受手术的患者,治疗则受限。
现就CAVD的发病机制及治疗进展做一综述。
1 流行病学在美国,85岁以上人群中,CAVD的患病率为48%~57%。
经导管主动脉瓣植入术后的起搏器植入

经导管主动脉瓣植入术后的起搏器植入詹智;管丽华(综述)【摘要】经导管主动脉瓣植入术通过股动脉途径,将人工生物瓣膜支架送至狭窄的主动脉瓣区,以自膨胀或球囊膨胀的方式打开,主要用于治疗不能耐受外科换瓣手术的重度钙化性的主动脉瓣狭窄。
经导管主动脉瓣植入术后最主要的并发症之一包括心脏传导阻滞,部分患者需要永久起搏器的植入来改善预后。
然而,在经导管主动脉瓣植入术后起搏器植入的预测因素、植入指征、植入时机等多个方面仍存在很多争议。
现主要归纳总结经导管主动脉瓣植入术后起搏器植入的研究进展,以促进人们对此领域的认识。
%The transcatheter aortic valve implantation( TAVI) delivers a artificial valve to the aortic valve’ s place,which provides a treatment for the inoperable patients with severe,calcific,symptomatic aortic stenosis.Heart block was considered to be the most important complication associated with TAVI.Some of the patients who suffered from the heart block after TAVI had the requirement for the permanent pacemaker implantation.However,there were conversely opinions in the predictors ,timing and indication of permanent pacemaker implanta-tion.This review briefly summarizes recent advances in the research on permanent pacemaker implantation after TAVI.【期刊名称】《心血管病学进展》【年(卷),期】2016(037)005【总页数】5页(P455-459)【关键词】经导管主动脉瓣植入术;起搏器;植入【作者】詹智;管丽华(综述)【作者单位】复旦大学附属中山医院心血管内科,上海 200032;复旦大学附属中山医院心血管内科,上海 200032【正文语种】中文【中图分类】R318.11;R815从2002年经导管主动脉瓣植入术(transcatheter aortic valveimplantation/replacement,TAVI/TAVR)第一次被运用到临床[1],由于其显著的收益/风险比,TAVI每年的手术数量在全球各大心脏中心呈井喷式增长。
经导管主动脉瓣置换术的并发症

经导管主动脉瓣置换术的并发症邓秀琼;张晓刚【摘要】目前经导管主动脉瓣置换术(TAVR)的运用已经从外科主动脉瓣置换术风险极高危或高危患者发展至中低危患者.随着TAVR运用的拓展,其相关并发症引起越来越广泛的关注.本文将对TAVR的并发症做一综述.%At present,the application of transcatheter aortic valve replacement (TAVR)has been developed from the patients with extremely high or high-risk for surgical aortic valve replacement to moderate or low risk patients.With the development of TAVR,its related complications have attracted more and more attention.This article will review the complications of TAVR.【期刊名称】《中国心血管杂志》【年(卷),期】2018(023)002【总页数】4页(P180-183)【关键词】经导管主动脉瓣置换术;并发症【作者】邓秀琼;张晓刚【作者单位】400016 重庆医科大学附属第一医院心内科;400016 重庆医科大学附属第一医院心内科【正文语种】中文主动脉瓣狭窄是一种常见的瓣膜性心脏病,最常见的病因是与年龄相关的瓣膜退行性改变。
据估计,约有2%的65岁以上的老年人患有此病,超过85岁者达4%[1]。
随着全球人口老龄化的加剧,主动脉瓣狭窄发病率正逐年升高。
主动脉瓣狭窄患者早期可无明显症状,一旦出现症状即需要手术治疗。
既往外科主动脉瓣置换术(surgical aortic valve replacement,SAVR)为主动脉瓣狭窄的标准治疗,但自2002年Cribier等[2]第1例经导管人主动脉瓣置换术的开展,SAVR因其创伤大、风险高、恢复慢等缺点逐渐被经导管主动脉瓣置换术(transcatheter aortic valve replacement,TAVR)取代。
Aortic Valve
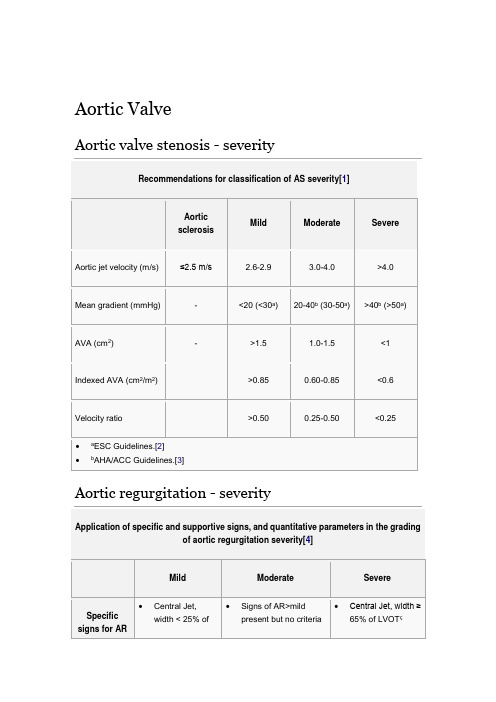
Aortic ValveAortic valve stenosis - severityRecommendations for classification of AS severity[1]Aortic sclerosisMild Moderate SevereAortic jet velocity (m/s) ≤2.5 m/s 2.6-2.9 3.0-4.0 >4.0Mean gradient (mmHg) - <20 (<30a)20-40b (30-50a)>40b (>50a)AVA (cm 2)- >1.5 1.0-1.5 <1Indexed AVA (cm 2/m 2)>0.85 0.60-0.85 <0.6Velocity ratio >0.50 0.25-0.50 <0.25∙ a ESC Guidelines.[2] ∙bAHA/ACC Guidelines.[3]Mitral ValveMitral regurgitation - severityApplication of specific and supportive signs, and quantitative parameters in the gradingof mitral regurgitation severity[4]Mild Moderate SevereSpecific signs of severity ∙Small centraljet <4 cm2 or<20% of LAareaψ∙Venacontractawidth <0.3cm∙No orminimal flowconvergence∙Signs of MR>mildpresent, but nocriteria for severe MR∙Vena contracta width ≥0.7cm with large centralMR jet (area < 40% of LA)or with a wall-impingingjet of any size, swirling inLAψ∙Large flow convergenceς∙Systolic reversal inpulmonary veins∙Prominent flail MV leafletor ruptured papillarymuscleSupportive signs ∙Systolicdominantflow inpulmonaryveins∙A-wavedominantmitral inflowΦ∙Soft density,parabolicCW DopplerMR signal∙Normal LVsize∗∙Intermediatesigns/findings∙Dense, triangular CWDoppler MR jet∙E-wave dominant mitralinflow (E >1.2m/s)Φ Enlarged LV andLA size∗∗, (particularlywhen normal LV functionis present).Quantitative parametersφR Vol(ml/beat)< 30 30-44 45-59 ≥ 60RF (%) < 30 30-39 40-49 ≥ 50EROA (cm2) < 0.20 0.20-0.29 0.30-0.39 ≥ 0.40∙CW, Continuous wave; EROA, effective regurgitant orifice area; LA, left atrium; LV, left ventricle; MV, mitral valve; MR, mitral regurgitation; R Vol, regurgitant volume; RF,regurgitant fraction.∙∗ LV size applied only to chronic lesions. Normal 2D measurements: LV minor axis ≤ 2.8 cm/m2, LV end-diastolic volume ≤ 82 ml/m2, maximal LA antero-posterior diameter ≤ 2.8 cm/m2, maximal LA volume ≤ 36 ml/m2 (2;33;35).∙∗∗ In the absence of other etiologies of LV and LA dilatation and acute MR.1 Highly mobile valve with only leaflet tips restrictedLeaflets near normal in thickness (4-5 mm)A single area of increased echobrightnessMinimal thickening just below the mitral leaflets2 Leaflet mid and base portions have normal mobilityMidleaflets normal, considerable thickening of margins (5-8 mm)Scattered areas of brightness confined to leaflet marginsThickening of chordal structures extending to one-third of the chordallength3 Valve continues to move forward in diastole, mainly from the baseThickening extending through the entire leaflet (5-8mm)Brightness extending into the mid-portions of the leafletsThickening extended to distal third of thechords4 No or minimal forward movement of the leaflets in diastoleConsiderable thickening of all leaflet tissue(>8-10mm)Extensive brightness throughout muchof the leaflet tissue Extensive thickening and shortening of all chordal structures extending down to thepapillary musclesThe total score is the sum of the four items and ranges between 4 and 16.Tricuspid ValveTricuspid regurgitation - severityEchocardiographic and Doppler parameters used in grading tricuspid regurgitationseverity[4]Parameter Mild Moderate SevereTricuspid valve Usually normal Normal orabnormalAbnormal/Flailleaflet/Poor coaptationRV/RA/IVC size Normal∗Normal or dilated Usually dilated∗∗Jet area-central jets(cm2)§< 5 5-10 > 10VC width (cm)ΦNot defined Not defined, but <0.7> 0.7PISA radius (cm)ψ≤ 0.50.6-0.9 > 0.9Jet density and Soft and parabolicDense, variable Dense, triangular withPulmonary ValvePulmonary regurgitaion - severityEchocardiographic and Doppler parameters used in grading pulmonary regurgitationseverity[4]Parameter Mild Moderate SeverePulmonic valve Normal Normal orabnormalAbnormalRV size Normal∗Normal or dilated DilatedJet size by color Doppler§Thin (usually < 10 mmin length) with anarrow originIntermediateUsually large, with awide origin; May be briefin durationJet density and deceleration rate –CW†Soft; SlowdecelerationDense; variabledecelerationDense; steepdeceleration, earlytermination of diastolicflowPulmonic systolic flowcompared to systemicflow –PWφSlightly increased Intermediate Greatly increased。
胎羊体外循环与胎盘功能保护研究进展

胎羊体外循环与胎盘功能保护研究进展心血管病学进展 2000年第4期第21卷综述作者:钟慧陈张根单位:上海医科大学儿科医院心血管中心,上海200032 中图分类号:R654.1 文献标识码:A文章编号:1004-3934(2000)04-0214-03Advances in Research of Fetal CardiacBypass and Prevention of Placental FunctionZHONG Hui,CHEN Zhang-gen(Cardiovascular Center,Children's Hospital of Shanghai Medical University, Shanghai 200032) Abstract: Fetal cardiac operation has been done in sheep so a method to protect the fetal and placenta after cessation of bypass is important.This is to review the progress in research of fetal cardiac bypass and prevention of placental dysfunction.The placental dysfunction results from different factors such as distribution of blood flow,stress response,placental perfusion,hypothermia and higher pressureoxygen.Observations were made in fetal Cardiac Bypass.Administration of high dose of sodium nitropusside,the indomethacin,total spinal anesthetic to block the fetal stress response and the improvement the equipment of the cardiopulmonary bypass devices and the normotherimia with high flow can maintain the placental function.Currently information indicates that intrauterine correction of selected congenital cardiac defects will be available in human fetuses in not remote future.Key Words: Cardiac bypass fetal;Placental function 随着诊断技术的不断提高,许多胎儿先天性心脏病在宫内即能通过超声心动图早期诊断[1]。
钙化性主动脉瓣疾病基础和临床研究进展

持对无症状的极重度主动脉瓣狭窄患者进行换瓣手术mi。
主动脉瓣狭窄若患者及时行换瓣手术,经年龄校正的10年 生存率接近正常人群。 AVC及CAS的确诊需要依靠病理检查。但由于心脏超 声简便、准确性较高,AVC及CAS通常由心脏超声即可诊 断。心脏超声显示瓣膜局限性或弥漫回声增强且瓣膜增厚 (≥1 mm),瓣叶活动不受限制,瓣口面积≥3 cm2,跨瓣血流 速率<2.5 m/s为AVC;跨瓣血流速率>2.5 m/s、瓣口面 积<3 on2为CAS¨1。除此之外,AVC也可以通过普通x片 发现,但敏感性较差;而多排CT特别是电子束CT(EBCT)亦 能检测AVC,并能对钙化进行定量积分分析。 五、治疗进展 对于AVC,目前主要治疗时控制合并的危险因素,尚无 直接针对AVC的有效治疗手段。而针对无症状者CAS,尚 无特殊治疗方法。对于症状性CAS或极重度的无症状 CAS,外科换瓣仍是目前最主要的治疗手段。近年来,TAVI 在治疗CAS上获得重大进展,药物治疗CAVD也正在研究 探讨中。 TAVI所用的带瓣膜支架发展已经历3代,目前第3代 的代表性产品主要有两种:一种为Cribier—Edwards生物瓣; 另一种为CoreValve生物瓣。TAVI途径包括经静脉顺行法 (经静脉穿刺房间隔,再进入左心房一二尖瓣-左心室.主动 脉)、经动脉逆行法(股动脉-主动脉路径)及经心尖法。目 前,TAVI主要的适应证为:(1)有症状的严重主动脉瓣狭窄 (瓣膜El面积<1 cm2);(2)欧洲心脏手术风险评分 (EuroSCORE)I>20%或美国胸外科学会危险(STS)评分≥ 10%;(3)解剖上适合TAVI(主要为主动脉瓣环内径、外周 动脉内径在合适的范围内)。截至目前,全球已有35 000多 例患者接受了TAVI治疗。新近研究报道的病例数越来多, 手术效果也越来越令人鼓舞。近2年来发表的大型TAVI研 究的结果显示,TAVI成功率很高(93.3%~98.4%),30 d病 死率8.5%~12.7%L23j。由于入选的是高危患者,这样的病 死率还是可以接受的。常见并发症包括需要置入起搏器的 房室传导阻滞、脑卒中及局部血管并发症。虽然国外TAVI 研究较多、进展迅速,我国在这方面的研究起步较晚。欣喜 的是,近期葛均波教授完成国内首例TAVI术,开创了我国 TAVI的先河ⅢJ。 动物实验、回顾性研究及RAAVE研究‘25。结果显示,他 汀类药物能减缓CAVD的进展速度。人们因此对他汀治疗 CAVD寄予厚望。但是,新近的大规模前瞻性SALTIRE-2刮
二叶式主动脉瓣疾病发病机理和治疗的新感悟英文
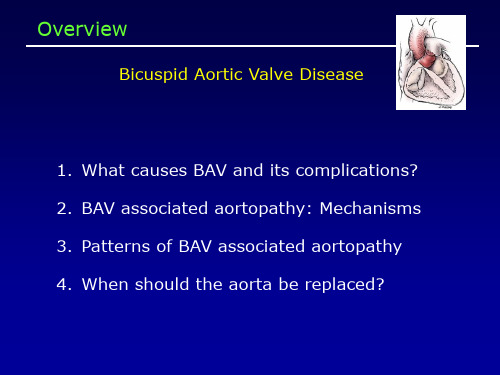
Patterns of Aortic Dilation in Bicuspid Aortic Valves
• A: aortoventricular jxn • B: Sinuses of Valsalva • C: Sinotubular jxn • D: Tubular ascending aorta • E: Proximal innominate • F: Distal innominate • G: Proximal left subclavian • H: Distal left subclavian • I: Proximal descending • J: Descending at diaphragm
Molecular Mechanisms of Aortic Dilatation in Bicuspid Aortic Valve Disease: Role of Matrix Remodeling
Fedak, Verma, et al. JTCVS 2003; 126(3): 797-806
Aortopathy in Bicuspid Valve
When Should the Ascending Aorta Be Replaced in Patients with Bicuspid Aortic Valve Disease?
Borger, Fedak, Verma et al. JTCVS 2005
Aortic Dilatation and BAV
Poor Correlation Between Degree of AS and Aortic Dilation
Magrad et al. JTCVS 2001
Adventitia Media Intima
主动脉瓣钙化生物学相关研究进展

Vol.46 No.4Aug. 2020第46卷第4期2020年8月兰州大学学报(医学版)Journal of Lanzhou University (Medical Sciences)文章编号:1000—2812(2020)04—0035-06主动脉瓣钙化生物学相关研究进展冯茹',李啥',李昕二宋兵1兰州大学第一临床医学院,甘肃兰州7300002兰州大学第一医院心外科,甘肃兰州730000摘要:主动脉瓣钙化(CAVD)是钙结节在主动脉瓣膜表面异位蓄积,导致主动脉瓣膜增厚、功能性狭窄,继而引起血流动力学紊乱,诱发心血管疾病及相关并发症,已成为心血管疾病的重要致死原因之一。
目前CAVD 的发病机制尚不明确,但最新研究证明其与很多分子生物学过程有关,包括转化生长因子-p(TGF-p),骨形成蛋白(BMP)、Wnt 、 Notch 、Sox9、胞外膜泡及机械压力和流量。
关键词:主动脉瓣钙化;发病机制;信号通路;研究进展中图分类号:R34文献标识码:A doi : 10.13885/j.issn. 1000-2812.2020.04.008Developmental biology perspective of calcific aortic valve diseaseFeng Ru 1, Li Han 1, Li Xin 12, Song Bing 1 21 The First School Clinical Medicine, Lanzhou University, Lanzhou 730000, China2 Deppartment of Cardial Surgery, The First Hospital of Lanzhou University, Lanzhou 730000, ChinaAbstract : Calcific aortic valve disease (CAVD) is an abnonnal accumulation of calcium nodules on the surface of the aorta, leading to thickening of aortic valve and functional stenosis, and thus to hemodynamic disorder,cardiovascular disease and related complications. It has become one of important fatal causes of cardiovascu ・lar disease. At present, the pathogenesis of CAVD is still unclear, but recent studies have proved that its occur rence is related to several molecular biological processes, including transforming growth factor-卩(TGF ■卩),bone morphogenetic protein (BMP), Wnt, Notch, Sox9, extracellular membrane vesicle, mechanical pressureand flow.Keywords: calcific aortic valve disease; pathogenesis; signal pathway; research development主动脉瓣钙化(calcific aortic valvular disease,CAVD)是常见的瓣膜病,中国50岁以上中老年人群中瓣膜钙化检出率为12.5%,其中94.4%为主 动脉瓣受累,极大影响患者的生活质量叫 传统意义上认为CAVD 是由于主动脉瓣老化而发生的退行性改变。
心脏主动脉瓣膜关闭不全手术流程

心脏主动脉瓣膜关闭不全手术流程Heart aortic valve regurgitation, also known as aortic valve insufficiency or aortic valve incompetence, is a condition where the aortic valve does not close properly, leading to the backflow of blood from the aorta into the left ventricle. This can result in various symptoms like shortness of breath, fatigue, chest pain, and even heart failure. In severe cases, surgical intervention may be required to repair or replace the faulty valve. Thesurgical procedure for aortic valve regurgitation aims to restore the normal functioning of the valve and improve the patient's overall quality of life.Before the surgery, a thorough evaluation is done to assess the severity of the valve regurgitation and determine the most appropriate treatment plan. This evaluation includes a physical examination, echocardiogram, and other diagnostic tests. Once the decision for surgeryis made, the patient is prepared for the procedure.The surgical procedure for aortic valve regurgitationis typically performed under general anesthesia. The surgeon makes an incision in the chest to gain access to the heart. The patient is connected to a heart-lung bypass machine, which takes over the function of the heart and lungs during the surgery.The next step involves removing the damaged aortic valve. This can be done through either a valve repair or a valve replacement. Valve repair involves techniques to reconstruct and restore the function of the existing valve, while valve replacement involves removing the damaged valve and replacing it with a prosthetic valve. The choice between repair and replacement depends on the individual patient's condition and the surgeon's expertise.If a valve repair is feasible, the surgeon will proceed with the necessary repairs, such as removing excess tissue, reattaching loose leaflets, or reinforcing the valve structure. In cases where a valve replacement is necessary, the surgeon will choose the most suitable type ofprosthetic valve, either mechanical or biological, based onfactors like the patient's age, lifestyle, and overall health.After the repair or replacement, the surgeon carefully checks the valve's functionality and ensures that there are no leaks or abnormalities. Once satisfied with the result, the surgeon closes the incision in the chest using suturesor staples. The patient is then taken off the heart-lung bypass machine, and the heart is allowed to resume its normal function.After the surgery, the patient is closely monitored in the intensive care unit (ICU) for a period of time toensure a smooth recovery. Medications may be prescribed to manage pain, prevent infection, and regulate blood pressure. Physical therapy and rehabilitation are also initiated to help the patient regain strength and mobility.In conclusion, the surgical procedure for heart aortic valve regurgitation aims to correct the faulty valve and restore normal blood flow. It involves a meticulous evaluation, a surgical intervention under generalanesthesia, and either a repair or replacement of the damaged valve. The choice between repair and replacement depends on the individual patient's condition. After the surgery, close monitoring and post-operative care are essential for a successful recovery. This procedure plays a crucial role in improving the patient's quality of life and preventing further complications associated with aortic valve regurgitation.。
钙化性主动脉瓣疾病药物治疗研究进展

钙化性主动脉瓣疾病药物治疗研究进展杜苗苗;马改改;施育平【期刊名称】《浙江大学学报(医学版)》【年(卷),期】2016(045)004【摘要】With the population aging and declining incidence of rheumatic heart disease, calcific aortic valve disease ( CAVD ) has become the most frequent valve disease and the common cause of aortic valve replacement.Patients with CAVD need to cope with a deteriorating quality of life and valve replacement is the only effective clinical option for the patients.Therefore, early pharmacotherapy is of great significance in prevention or slow-down of the progression of CAVD. For years CAVD was considered to be a passive wear and tear process of valves, but now it is recognized as an active and multi-factorial process. Histopathologic studies have revealed that inflammation, disorder of calcium and phosphorus metabolism and dyslipidemia are involved in the process of CAVD.Clinical trials of CAVD pharmacotherapy have been carried out based on those histopathologic studies.Statin, renin-angiotensin inhibitors and anti-osteoporosis drug are well studied in recent years.This article reviews the recent research progress of the pharmacotherapy for CAVD.%随着人口老龄化及风湿性心脏病发生率的下降,钙化性主动脉瓣疾病( CAVD)已经成为最常见的瓣膜病,并已成为主动脉瓣膜置换的首要原因。
钙化性主动脉瓣疾病的发病机制

[Jj.N Engl J Med,2005,352(23):2389—2397. [6]
Rossehop AB,Pedersen TR,Boman K.et a1.Intensive lipid
in aortic
lowering with simvastatin and ezetimibe N Engl J
aortic
valve stenosis and
function[J].Am J Cardiol,2010,105(6):862
Mills
DOI:10.3969/i.issn.1673—6583.2012.05.002
1
概述
胞一信号通路在其进展中发挥重要作用。此外遗传 因素亦参与其进展。CAVS是多种危险因素作用下 的主动渐进性疾病。 2脂代谢治疗对CAVS的影响 钙化性主动脉瓣疾病与动脉粥样硬化不仅在 危险因素方面,而且病理改变也有许多相似,因此, 人们尝试通过他汀类药物治疗来阻断或延缓CAVS 进展。早期动物模型研究显示高胆固醇血症可致 主动脉瓣硬化和血流动力学改变,回顾性研究亦提 示他汀可延缓瓣膜钙化形成和狭窄进展,但是大规 模的前瞻性临床对照试验却未能一致性证实他汀 能有效延缓或阻断CAVS的发生和进展。 SALTIRE研究入选155例严重CAVS患者, 随机给予阿托伐他汀80 mg/d或安慰剂治疗25个 月,结果两组在血流动力学进展变化和钙化程度均 上无统计学差异¨j。大规模的SEAS研究,人选 1873例CAVS患者,随机接受依折麦布/辛伐他汀 或安慰剂治疗4.4年,结果两组在主动脉瓣相关事 件(外科主动脉瓣置换术、心力衰竭、心血管死亡) 上无统计学差异¨J。ASTRON()MER研究,人选 269例主动脉瓣狭窄患者,随机给予瑞舒伐他汀
Fetuin A与主动脉瓣膜钙化

Fetuin A与主动脉瓣膜钙化刘亚; 刘艳【期刊名称】《《医学理论与实践》》【年(卷),期】2019(032)023【总页数】3页(P3787-3789)【关键词】Fetuin; A; 主动脉瓣膜钙化; 脂质代谢; 钙磷代谢; 胰岛素抵抗; 炎症反应【作者】刘亚; 刘艳【作者单位】上海交通大学临床医学院附属第九人民医院上海市 200011【正文语种】中文【中图分类】R543.1Fetuin A,也称Alpha2-Heremans-Schmid glycoprotein(AHSG),是一种由肝脏细胞合成和分泌的血浆糖蛋白,属于半胱氨酸蛋白酶抑制剂(Cystatin)超家族成员。
Fetuin A前体由一条含282个氨基酸残基的重链、一条含27个氨基酸残基的轻链和一条含40个氨基酸残基的连接链组成。
在翻译过程中,连接链被糜蛋白酶水解,重链与轻链在Cys-14和Cyst-340之间由二硫键连接,形成FetuinA[1] ,其分子量为55~59kD。
Fetuin A蛋白共有3个结构域: 2个半胱氨酸蛋白酶抑制剂样结构域,其含有1个磷酸钙结合位点和1个TGF-β细胞因子结合单元,通过与钙磷及TGF-β的结合,阻断了钙磷沉积及TGF-β介导的信号传导,在调节机体钙化、胰岛素抵抗、抑制肿瘤生长和血管生成,以及抗炎症反应中发挥了重要的作用;1个独特的羧基末端结构域,该结构域结构易变,是抗原决定簇所在的位置,目前关于其结构和功能尚不明确。
1 Fetuin A与主动脉瓣膜钙化主动脉瓣膜钙化疾病(Calcific aortic valve disease,CAVD)是一组以主动脉瓣及其周围组织纤维化、硬化、钙盐沉积为主要改变,主动脉瓣狭窄(Aortic stenosis,AS)为主要功能损害的疾病。
流行病学调查数据显示,CAVD发病成日益上升趋势,其与年龄明显呈正相关。
在一项关于AS发病率与预后的国外人群研究[2]中提示,在50~59 岁人群中,AS发病率为0.2%(95%CI:0%~0.4%),60~69 岁的发病率为1.3%(95%CI:0.9%~1.7%),70~79岁为3.9%(95%CI:3.2%~4.6%),80~89岁则为 9.8%(95%CI:7.8%~11.8%)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
ISSN: 1524-4539Copyright © 2001 American Heart Association. All rights reserved. Print ISSN: 0009-7322. OnlineCirculation is published by the American Heart Association. 7272 Greenville Avenue, Dallas, TX 72514DOI: 10.1161/hc4101.0975272001;104;1927-1932 Circulation Werner G. Daniel and Stephan AchenbachKarsten Pohle, Ralph Mäffert, Dieter Ropers, Werner Moshage, Nicolaos Stilianakis,Atherosclerosis and Cardiovascular Risk Factors Progression of Aortic Valve Calcification: Association With Coronary/cgi/content/full/104/16/1927located on the World Wide Web at:The online version of this article, along with updated information and services, is/reprints Reprints: Information about reprints can be found online atjournalpermissions@ 410-528-8550. E-mail:Kluwer Health, 351 West Camden Street, Baltimore, MD 21202-2436. Phone: 410-528-4050. Fax: Permissions: Permissions & Rights Desk, Lippincott Williams & Wilkins, a division of Wolters/subscriptions/Subscriptions: Information about subscribing to Circulation is online atProgression of Aortic Valve Calcification Association With Coronary Atherosclerosis and CardiovascularRisk FactorsKarsten Pohle,MD;Ralph Mäffert,MD;Dieter Ropers,MD;Werner Moshage,MD;Nicolaos Stilianakis,PhD;Werner G.Daniel,MD;Stephan Achenbach,MDBackground—Recent studies demonstrated an influence of atherosclerotic risk factors on the progression of aortic valve stenosis.The extent of aortic valve calcification(AVC)was also found to be a strong predictor of stenosis progression.We investigated the influence of the LDL cholesterol level(LDL),other standard cardiovascular risk factors,and the extent of coronary calcification(CC)on the progression of AVC quantified by electron beam tomography(EBT). Methods and Results—In104patients(64.7Ϯ8years,89male)with an EBT scan positive for AVC,CC and AVC were quantified using a volumetric score.EBT was repeated at a mean interval of15months(10to36months),and the progression of AVC and CC was determined.Patients were divided into2groups according to LDL:group1, LDLՅ3.36mmol/L(130mg/dL),57patients;group2,LDLϾ3.36mmol/L(130mg/dL),47patients.Mean values for CC were546Ϯ932mm3in scan1and665Ϯ1085mm3in scan2for AVC324Ϯ796mm3and404Ϯ1076mm3, respectively.The mean progression of CC was27Ϯ37%(group1,16Ϯ22%;group2,39Ϯ46%,PՅ0.001)and of AVC was25Ϯ38%(group1,9Ϯ22%;group2,43Ϯ44%,PՅ0.001).Conclusions—Quantification of AVC by EBT permits new insights into the progression of aortic valve sclerosis.We observed a strong influence of LDL cholesterol level on the progression of AVC and CC,suggesting that lipid-lowering therapy may decrease the progression of aortic valve calcification.(Circulation.2001;104:1927-1932.)Key Words:imagingⅢheart diseasesⅢlipidsⅢrisk factorsⅢatherosclerosisA ortic valve stenosis has a prevalence of2%to7%in thepopulation above65years of age.1In industrialized countries,aortic valve stenosis is most frequently caused by progressive calcification and degeneration of the aortic cusps.2,3The disease shows a progressive course,especially after the threshold to mild aortic stenosis has been crossed.2,4 Common pathomechanisms of aortic valve stenosis and atherosclerosis have been discussed,and several studies have demonstrated an influence of cardiovascular risk factors on the progression and outcome of aortic valve stenosis,but results have been inhomogeneous as to the relative impor-tance of specific risk factors.5–9Several studies have identi-fied the degree of aortic valve calcification as a strong predictor both for the progression and outcome of aortic stenosis.10,11Presently,there are no accurate methods to quantify the extent of aortic valve calcification;most studies rely on categorical scoring systems derived from echocardio-graphic valve morphology.1,12See p1881Electron beam tomography(EBT)is a cross-sectional imaging technique with high temporal resolution.13,14It has so far mainly been used to sensitively detect and quantify coronary calcifications15–17;several studies used EBT to follow the progression of coronary calcification.An influence of cardiovascular risk factors,especially the LDL cholesterol level,on the degree of coronary calcium progression could be demonstrated.18–22Some studies suggested the use of EBT or computed tomography to detect and quantify aortic valve calcification.23–25A weak correlation between the extent of calcification and the severity of aortic valve stenosis has been described.25We therefore used EBT in a group of104patients to quantify the extent of aortic valve calcification,to determine the rate of progression,and to analyze the influence of cardiovascular risk factors on the course of aortic valve calcification.In addition,we inves-tigated the relationship between the progression of aortic valve calcification and the extent and progression of coronary atherosclerosis,expressed through the amount of coronary calcification.We hypothesized that adults with aortic sclerosis and low levels of LDL cholesterol would have a lower increase in aortic valve and coronary calci-fication,as measured by EBT,compared with those with higher LDL cholesterol levels.Received May30,2001;revision received August6,2001;accepted August9,2001.From the Department of Internal Medicine II(K.P.,R.M.,D.R.W.M.,W.G.D.,S.A.)and Department of Medical Information,Biometry,and Epidemiology(N.S.),University of Erlangen,Germany.Correspondence to Dr Karsten Pohle,Medizinische Klinik II,Universität Erlangen-Nürnberg,Östliche Stadtmauerstr.29,D-91054Erlangen,Germany. E-mail Falk-Karsten.Pohle@rzmail.uni-erlangen.de©2001American Heart Association,Inc.Circulation is available at MethodsPatientsOne hundred four patients (89men and 15women,mean age 64.7Ϯ8years)with aortic valve calcification in EBT were included in the study in a retrospective fashion.The patients were recruited by reviewing 2124EBT studies that had been obtained for detection of coronary artery calcification on an outpatient basis in our centerbetween 1997and 2000.Two hundred seventy two individuals with aortic valve calcification in the EBT scan (volume score Ͼ10mm 3)were identified and invited for a follow-up EBT investigation to assess the progression of coronary and aortic valve calcification.One hundred sixty eight patients with aortic calcification had to be excluded because they refused return for follow-up or for other reasons that did not permit inclusion in the investigation (established diagnosis or symptoms suggestive of coronary artery disease or aortic valve stenosis at the baseline scan,arrhythmias,possible pregnancy,history of renal disease or renal failure [elevated serum creatinine concentrations],or change of lipid-lowering medications within the observation interval).All participating patients gave written informed consent to the investigation,and the study protocol was approved by the institutional ethics committee.Assessment of Cardiovascular Risk FactorsCardiovascular risk factors were determined by interviewing the patients at the time of the follow-up scan.The following risk factors were assessed:patient age (Ն55years),present smoking,hyperten-sion (antihypertensive medication or known and untreated hyperten-sion),and diabetes (use of insulin or oral hypoglycemic agents).In addition,fasting blood samples were taken from all patients,and the serum levels of LDL cholesterol and total cholesterol were measured.Image Acquisition and EvaluationImaging was performed with an Imatron C-150XP EBT scanner (Imatron Inc).Patients were scanned in supine position.After determination of the heart position,40axial cross-sections of the heart were acquired during inspiratory breathhold.Imaging was performed using the high-resolution single slice mode of the scanner with 100-ms exposure time,3-mm slice thickness,and 3-mm table feed between consecutive slices.Image acquisition was triggered to the patient ’s ECG at 40%of the cardiac cycle.Cross-sectional images were reconstructed with a 26-cm field of view using the scanner ’s sharp kernel.To assess the interscan variability of aortic valve calcification measurements by EBT,a second scan was performed in 50patients at the time of the follow-up investigation.After repositioning the patient,the EBT scan was repeated with identical parameters as the first scan,but only 12images were acquired to selectively cover the region of the aorticvalve.Figure 1.Cross-sectional EBT image of a calcified aortic valve (arrow).Arrowheads indicate coronary artery calcifications.Clinical Characteristics of the Patients and Results of Coronary and Aortic Valve Calcification MeasurementsAll PatientsGroup 1,LDL Cholesterol Յ3.36mmol/LGroup 2,LDL Cholesterol Ͼ3.36mmol/LPNo.of patients 1045747Men/women 89/1549/840/7Age,y64.7Ϯ865.8Ϯ763.4Ϯ9NS Present smoker,%44.240.448.9NS Hypertension,%51.050.951.1NS Diabetes,%11.512.310.6NS LDL cholesterol,mmol/L 3.4Ϯ1.12.6Ϯ0.64.4Ϯ0.8Ͻ0.001Coronary calcification Volume score,initial scan 546.8Ϯ932632.9Ϯ1180455.5Ϯ554NS Volume score,follow-up scan 665.1Ϯ1085705.5Ϯ1278620.2Ϯ834NS Percent annualized increase 27.3Ϯ3716.2Ϯ2339.7Ϯ46Ͻ0.001Aortic valve calcifications Volume score,initial scan 324.8Ϯ796239.9Ϯ356427.6Ϯ1116NS Volume score,follow-up scan 404.1Ϯ1076272.8Ϯ409563.5Ϯ1530NS Percent annualized increase24.5Ϯ389.1Ϯ2243.2Ϯ44Ͻ0.001Continuous variables are expressed as mean ϮSD.1928Circulation October 16,2001Acquired images were transferred to an offline workstation (NetraMD,ScImage).Coronary and aortic valve calcifications were defined as areas of at least2contiguous pixels(areaϾ0.51mm2) with a density of130HU or more(see Figure1).Using an interpolated volume score,26the total volumes of coronary and aortic valve calcification were determined.Calcifications of the aortic wall that were immediately connected to calcifications of aortic valve cusps were included in the aortic valve calcification score.To determine interscan variability,the absolute difference of the two aortic valve calcification scores was divided by the mean score and expressed as percent value.To determine the change of aortic valve and coronary artery calcification over time,the initial score was subtracted from the follow-up score and the difference was divided by the initial score and expressed as percent value.This value was divided by the actual number of days between the initial and follow-up scan and multiplied by365to obtain the annualized percent change in aortic valve and coronary calcification. Statistical AnalysisStatistical analysis was performed using a PC-based computer program(SPSS version10.0).To compare the influence of LDL cholesterol levels on the progression of coronary and aortic valve calcification,patients were divided into two groups using an arbi-trary threshold.In group1,the LDL cholesterol level wasՅ3.36mmol/L(130mg/dL);in group2,the LDL cholesterol level wasϾ3.36mmol/L(130mg/dL).Comparisons between groups were performed using the t test for unpaired samples.The relationship between the progression of coronary and aortic valve calcification was analyzed by bivariate correlation using the Pearson coefficient. In addition,stepwise multiple regression analysis was performed to identify independent predictors of the progression of aortic valve and coronary calcification.PՅ0.05was considered to indicate a signif-icant difference.ResultsBaseline Patient CharacteristicsThe mean interval between the initial and follow-up EBT scan was15.3Ϯ5months(range,10to36months).There were no significant differences concerning age,sex,and cardiovascular risk factors between the two patient groups divided according to LDL cholesterol levels.Fifty four patients(39in group1and15in group2)were treated with HMG-CoA reductase inhibitors.The Table shows the patient characteristics in all patients as well as in the subgroups.The mean initial aortic valve calcification score in all104patients was324Ϯ796mm3.The baseline amount of aortic valve calcification was not significantly different in the2patient groups,and it was not associated with any of the other tested cardiovascular risk factors(age,Pϭ0.46;diabetes,Pϭ0.22; hypertension,Pϭ0.52;smoking,Pϭ0.33).All patients had coronary calcifications in the baseline scan(mean score, 541Ϯ929mm3).No significant correlation was found be-tween the amount of coronary and aortic valve calcification in the initial EBT investigation(rϭ0.04,Pϭ0.7). Variability of Aortic ValveCalcification MeasurementsIn50patients,measurement of aortic valve calcification was repeated at the follow-up investigation to determine interscan variability.The mean aortic valve calcification score was 410.5mm3for the first and386.6mm3for the second measurement(Pϭ0.9),resulting in a mean variability of 8.2Ϯ9%and a median variability of6.9%.There was a significant influence of the total amount of aortic valve calcification on interscan variability:Mean and median vari-abilities in the lowest tercile of aortic valve calcification (scoreϽ96.8mm3)were14.3Ϯ11%and11.9%,whereas the mean and median variabilities in the upper tercile of aortic valve calcification(scoreϾ1531.7mm3)were4.8Ϯ6%and 5.9Ϯ5%,respectively(Pϭ0.05).Progression of Aortic Valve andCoronary CalcificationThe mean aortic valve calcification score of all104patients increased from324Ϯ796mm3in the initial scan to 404Ϯ1076mm3in the follow-up scan,corresponding to a mean annualized progression of24.5Ϯ38%.Eighty five patients(82%)showed progression,whereas19patients (18%)showed regression in the amount of aortic valve calcification.There was no significant influence of the amount of aortic valve calcification in the initial scan on the rate of progression.In the lowest tercile(scoreϽ34mm3),the mean progression was39.2Ϯ57%(median18.8),whereas in the upper tercile(scoreϾ274mm3),the mean progression was14.7Ϯ11%(median14.7,Pϭ0.18).The mean coronary artery calcification score increased from546Ϯ932mm3to665Ϯ1085mm3during the study period.The mean annualized progression was27.3Ϯ37%. There was a significant correlation between the annualized progression of coronary and aortic valve calcification (rϭ0.42,PϽ0.001,Figure2).In the upper tercile of annual-ized coronary calcium progression(annual progression Ͼ33.8%),the mean annual increase of aortic valve calcifica-tion was47.3Ϯ33%(median38.6),whereas in the lowest tercile of coronary calcium progression(Ͻ8.3%),the mean annual increase of aortic valve calcium was only9.5Ϯ22% (median6.5,PϽ0.001).Influence of Cardiovascular Risk Factors on the Progression of Aortic Valve CalcificationThere was a significant influence of serum LDL cholesterol levels on the progression both of aortic valve andcoronary Figure2.Scatterplot of percent annualized progression of coro-nary calcifications(x-axis)and aortic valve calcifications(y-axis). Bold line indicates regression line;thin lines,95%CI.Corre-sponding correlation coefficient was rϭ0.42(PϽ0.001).Associ-ated estimate of the regression coefficient was bϭ0.44(95%CI 0.27to0.59,PϽ0.001).Pohle et al Progression of Aortic Valve Calcification1929calcifications (Table,Figures 3A and 3B).Patients were divided according to their LDL cholesterol level,using a predefined value of 3.36mmol/L (130mg/dL)as an arbi-trarily chosen cut point.In patients with a LDL cholesterol level Յ3.36mmol/L (group 1,n ϭ57),the mean annual progression of aortic valve calcification was 9.1Ϯ22%,whereas in patients with LDL cholesterol levels Ͼ3.36mmol/L (group 2,n ϭ47),the mean annual progression was 43.2Ϯ44%(P Ͻ0.001).Correspondingly,the mean an-nual coronary calcium progression was 16.1Ϯ22%(group 1)and 39.7Ϯ46%(group 2),respectively (P Ͻ0.001,Figure 4).We found no influence of smoking,hypertension,diabetes,or patient age on the rate of progression,possibly because of the small size of the respective subgroups.The use of cholesterol-lowering medication by itself had no significant influence on the progression of aortic valve calcification.Fifty four patients were treated with HMG-CoA reductase inhibitors during the follow-up interval;their mean LDL cholesterol level was 2.97Ϯ0.83mmol/L (114.7Ϯ32mg/dL).The mean annual progression of aortic valve calci-fication in these 54patients was 21.5Ϯ44%.The mean LDLcholesterol level in the 50patients not treated with HMG-CoA inhibitors was 3.96Ϯ1.16mmol/L (153.2Ϯ45mg/dL),and these patients displayed a mean annual progression of 27.8Ϯ31%(P ϭ0.4).If,however,the 54patients treated with statins were divided according to their LDL cholesterol level (Յ3.36mmol/L,39patients;Ͼ3.36mmol/L,15patients),a statistically significant difference of annualized aortic valve calcium progression was found (10.1Ϯ26%and 51.1Ϯ65%,P ϭ0.002).Multiple Regression AnalysisStepwise multiple regression analysis was performed for two reasons:to identify predictors of the progression of aortic valve and coronary calcifications and to verify an indepen-dent association between the degree of progression of coro-nary calcifications and the progression of aortic valve calci-fication.For entry into calculation,a univariate probability value of 0.10was set for all parameters.LDL cholesterol levels and age were entered as continuous variables,whereas hypertension,diabetes,and smoking were entered as categor-ical variables.The serum LDL level could be identified as a parameter with an independent influence both on the progression of aortic valve (slope 0.29;95%CI 0.13to 0.46;P ϭ0.001)and coronary calcifications (slope 0.22;95%CI 0.05to 0.39;P ϭ0.01).The overall data fit of the model was R 2ϭ0.11for the progression of aortic valve calcifications and R 2ϭ0.06for the influence on progression of coronary calcifications.When the annualized relative progression of coronary artery calcification was added to the regression analysis,it could be identified as an independent predictor of the pro-gression of aortic valve calcification (slope 0.37;95%CI 0.18to 0.57;P Ͻ0.001)along with the LDL cholesterol level (slope 0.19;95%CI 0.03to 0.36;P ϭ0.02).The overall data fit of this model was R 2ϭ0.22.DiscussionThe degree of aortic valve calcification is of high predictive value concerning the progression and clinical outcome of aortic valve stenosis 10and has also been identified as a predictor of cardiovascular mortality,echocardiographic ev-idence of aortic valve sclerosis being associated with a50%Figure 3.A,Scatterplot of LDL cholesterol level in mmol/L (x-axis)and the percent annualized progression of aortic valve calci fications (y-axis).Bold line indicates regression line;thin lines,95%CI.The corresponding correlation coef ficient was r ϭ0.35(P Ͻ0.001).Associated estimate of the regression coef fi-cient was b ϭ10.55(95%CI 5.09to 16.02,P Ͻ0.001).B,Scat-terplot of LDL cholesterol level in mmol/L (x-axis)and the per-cent annualized progression of coronary calci fications (y-axis).Bold line indicates regression line;thin lines,95%CI.Corre-sponding correlation coef ficient was r ϭ0.25(P ϭ0.01).Associ-ated estimate of the regression coef ficient was b ϭ8.49(95%CI 1.87to 15.12,P ϭ0.01)Figure 4.Box plots of the annualized progression of coronary calci fications and of aortic valve calci fications in the two patient groups divided according to their LDL cholesterol level.Box plots display median,25th and 75th percentile,and the extreme values.1930Circulation October 16,2001increase in the risk of death from cardiovascular causes. Clinical and histopathological data suggest that aortic valve sclerosis and stenosis represent different stages of the same disease.12In our study,we could demonstrate that electron beam tomography permits the quantification of aortic valve calci-fication with high interscan reproducibility.It was demon-strated that aortic valve calcification,even in asymptomatic patients,is progressive,with a mean increase of24.5%per year.In addition,we could show that the degree of progres-sion of aortic valve calcification is influenced by the LDL cholesterol level,and that,independent from risk factors,the progression of aortic valve calcification is more rapid in patients with a rapid progression of coronary artery calcifi-cation,a surrogate marker for the amount of coronary atherosclerotic plaque.27These results strongly add to the findings of previous investigations,which have suggested a similar nature of calcified aortic valve stenosis and coronary artery disease,for example by establishing an association between atherosclerotic risk factors or the presence of coro-nary artery disease and the progression of aortic stenosis5,28,29 or by demonstrating that aortic valves affected by degenera-tive stenosis contain higher amounts of oxidized LDL cho-lesterol and show increased expression of metalloproteinases compared with healthy valves,9,30–32observations that are also made in coronary atherosclerotic plaque.33,34Even though it therefore seems possible that risk factor modifica-tions that have proven to beneficially influence the progres-sion and outcome of coronary artery disease,such as the reduction of LDL cholesterol,may also be able to slow the progression of calcified aortic valve stenosis,this remains to be proven in intervention studies,especially because aortic valve stenosis may be a multifactorial disease28and nonath-erosclerotic risk factors for disease progression have also been identified.29Our study,intended as a first investigation to analyze a possible association between the progression of aortic valve and coronary artery calcification,has several limitations.It is a retrospective analysis in patients who had been referred for coronary calcification scanning and is therefore subjected to selection bias,because patients with cardiovascular risk factors are overrepresented.Even though a significant influ-ence of LDL cholesterol levels on the progression of aortic valve calcification could be demonstrated,the sample size was too small to reliably analyze the effect of other risk factors,such as diabetes,which was only present in a very small subgroup of our patients.Apart from the determination of reproducibility in our study and a small,previously published group of19patients,25no other validations of EBT for the quantification of aortic valve calcification have been performed.However,it is reasonable to assume that the close association that has been found for EBT and histological measurements of coronary artery calcium also should hold true for the aortic valve.35Most importantly,we only assessed aortic valve calcification,and no measurements concerning the functional status of the aortic valve were performed. Patients with symptoms suggestive of severe aortic valve stenosis were excluded from our investigation.However, echocardiographic data were not available in all subjects and,therefore,our patients may have been an inhomogeneous group,consisting of patients with aortic sclerosis without obstruction and patients with asymptomatic,calcified valvu-lar stenosis.Even though other authors have described a weak correlation between the extent of aortic valve calcification and the degree of aortic stenosis,25the data we obtained as to the progression of valve calcification cannot be directly extrapolated to the progression of aortic valve stenosis. Despite these limitations,our study demonstrates that electron beam tomography permits new insights into the progression of aortic valve disease by quantification of aortic valve calcification.We could show that the LDL cholesterol level influences the progression of aortic valve calcification and that there is a significant,independent correlation be-tween the progression of calcifications in the coronary arter-ies and the aortic valve,suggesting similar mechanisms of disease and possible benefits of risk factor modification on the clinical course of calcified aortic valve stenosis.References1.Stewart BF,Siscovick D,Lind BK,et al.Clinical factors associated withcalcific aortic valve disease.J Am Coll Cardiol.1997;29:630–634. 2.Otto CM,Burwash IG,Legget ME,et al.Prospective study of asymp-tomatic valvular aortic stenosis:clinical,echocardiographic,and exercise predictors of outcome.Circulation.1997;95:2262–2270.3.Lindroos M,Kupari M,Valvanne J,et al.Factors associated with calcificaortic valve degeneration in the elderly.Eur Heart J.1994;15:865–870.4.Iivanainen AM,Lindroos M,Tilvis R,et al.Calcific degeneration of theaortic valve in old age:is the development of flow obstruction pre-dictable?J Intern Med.1996;239:269–273.5.Roger VL,Tajik AJ,Bailey KR,et al.Progression of aortic stenosis inadults:new appraisal using Doppler echocardiography.Am Heart J.1990;119:331–338.6.Ngo MV,Gottdiener JS,Fletcher RD,et al.Smoking and obesity areassociated with the progression of aortic stenosis.Am J Geriatr Cardiol.2001;10:86–90.7.Faggiano P,Aurigemma GP,Rusconi C,et al.Progression of valvularaortic stenosis in adults:literature review and clinical implications.Am Heart J.1996;132:408–417.8.Peter M,Hoffmann A,Parker C,et al.Progression of aortic stenosis:roleof age and concomitant coronary artery disease.Chest.1993;103: 1715–1719.9.Otto CM,Kuusisto J,Reichenbach D,et al.Characterisation of the earlylesion of“degenerative”valvular aortic stenosis:histological and immu-nohistochemical studies.Circulation.1994;90:844–853.10.Rosenhek R,Binder T,Porenta G,et al.Predictors of outcome in severe,asymptomatic aortic stenosis.N Engl J Med.2000;343:611–617.11.Otto CM.Timing of aortic valve surgery.Heart.2000;84:211–218.12.Otto CM,Lind BK,Kitzman DW,et al.Association of aortic-valvesclerosis with cardiovascular mortality and morbidity in the elderly.N Engl J Med.1999;341:142–147.13.Gould RG.Principles of ultrafast computed tomography:historicalaspects,mechanism,and scanner characteristics.In:Stanford W,Rum-berger JA,eds.Ultrafast Computed Tomography in Cardiac Imaging: Principles and Practice.Mt Kisco,NY:Futura;1993:1–16.14.Boyd D,Gould RG,Quinn J.A proposed cardiac3-D densitometer foreasy detection and evaluation of heart disease.IEEE Trans Nucl Sci.1979;26:2724–2727.15.Rumberger JA,Simons DB,Fitzpatrick LA,et al.Coronary arterycalcium area by electron-beam computed tomography and coronary ath-erosclerotic plaque area:a histopathologic correlative study.Circulation.1995;92:2157–2162.16.Agatston AS,Janowitz WR,Hildner FJ,et al.Quantification of coronaryartery calcium using ultrafast computed tomography.J Am Coll Cardiol.1990;15:827–832.17.O’Rourke RA,Brundage BH,Froelicher VF,et al.American College ofCardiology/American Heart Association expert consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease.Circulation.2000;102:126–140.Pohle et al Progression of Aortic Valve Calcification193118.Callister TQ,Raggi P,Cooil B,et al.Effect of HMG-CoA reductaseinhibitors on coronary artery disease as assessed by electron-beam computed tomography.N Engl J Med.1998;339:1972–1978.19.Hecht HS,Superko HR,Smith LK,et al.Relation of coronary arterycalcium identified by electron beam tomography to serum lipoprotein: implications for treatment.Am J Cardiol.2001;87:406–412.20.Budoff MJ,Lane KL,Bakhsheshi H,et al.Rates of progression ofcoronary calcium by electron beam tomography.Am J Cardiol.2000;86:8–11.21.Bielak LF,Sheedy PF,Peyser PA.Coronary artery calcification measuredat electron-beam CT:agreement in dual scan runs and change over time.Radiology.2001;218:224–229.22.Schmermund A,Baumgart D,Erbel R.Coronary calcification by electronbeam tomography:comparison with coronary risk factors and angiography.J Cardiovasc Risk.2000;7:99–106.23.MacMillan RM,Rees MR,Lumia FJ,et al.Preliminary experience in theuse of ultrafast computed tomography to diagnose aortic valve stenosis.Am Heart J.1988;115:665–671.24.Boughner DR,Thornton M,Dunmore-Buyze J,et al.The radiographicquantitation of aortic valve calcification:implications for assessing bio-prosthetic valve calcification in vitro.Physiol Meas.2000;21:409–416.25.Kizer JR,Gefter WB,de Lemos AS,et al.Electron beam computedtomography for the quantification of aortic valve calcification.J Heart Valve Dis.2001;10:361–366.26.Callister TQ,Cooil B,Raya SP,et al.Coronary artery disease:improvedreproducibility of calcium scoring with an elctron beam CT volumetric method.Radiology.1998;208:807–814.27.Sangiorgi G,Rumberger JA,Severson A,et al.Arterial calcification andnot lumen stenosis is highly correlated with atherosclerotic plaque burden in humans:a histologic study of723coronary artery segments using nondecalcifying methodology.J Am Coll Cardiol.1998;31:126–133. 28.Mohler ER.Are atherosclerotic processes involved in aortic-valve calci-fication?Lancet.2000;356:524–525.29.Palta S,Pai AM,Gill KS,et al.New insights into the progression of aorticstenosis:implications for secondary prevention.Circulation.2000;101: 2497–2502.30.Olsson M,Thyberg J,Nilsson J.Presence of oxidized low densitylipoprotein in nonrheumatic stenotic aortic valves.Arterioscler Thromb Vasc Biol.1999;19:1218–1222.31.Edep ME,Shirani J,Wolf P,et al.Matrix metalloproteinase expression innonrheumatic aortic stenosis.Cardiovasc Pathol.2000;9:281–336. 32.Mehrabi MR,Sinzinger H,Ekmekcioglu C,et al.Accumulation ofoxidized LDL in human semilunar valves correlates with coronary ath-erosclerosis.Cardiovasc Res.2000;45:874–882.33.Fuster V,Badimon J,Chesebro JH,et al.Plaque rupture,thrombosis,andtherapeutic implications.Haemostasis.1996;26:269–284.34.Lee RT,Schoen FJ,Loree HM,et al.Circumferential stress and matrixmetalloproteinase1in human coronary atherosclerosis:implications for plaque rupture.Arterioscler Thromb Vasc Biol.1996;16:1070–1073. 35.Mautner GC,Mautner SL,Froehlich J,et al.Coronary artery calcifi-cation:assessment with electron beam CT and histomorphometric corre-lation.Radiology.1994;192:619–623.1932Circulation October16,2001。
