7 PCR143F120-0606S Final Inspection Report2010.4.6
pcr仪合格证书

pcr仪合格证书
PCR 仪合格证书是用于确认一台聚合酶链式反应(PCR)仪符合特定标准和要求的文件。
以下是对PCR 仪合格证书的介绍:
1. 证明仪器性能:PCR 仪合格证书是由相关权威机构或实验室颁发的,用于证明该仪器在一系列性能测试中达到了规定的标准。
这些测试通常包括温度控制准确性、升温速率、循环时间等关键参数的评估。
2. 确保实验结果可靠:合格证书的存在意味着该PCR 仪已经通过了严格的测试和验证程序,能够提供稳定和可靠的实验结果。
它为用户提供了一种信任和保证,即该仪器在进行基因扩增等实验时能够准确地执行预期的反应条件。
3. 合规性要求:在某些地区或行业,使用PCR 仪进行实验可能需要符合特定的法规或标准。
PCR 仪合格证书可以作为仪器符合这些要求的证据,帮助用户满足合规性要求。
4. 质量保证:合格证书是PCR 仪制造商对其产品质量的承诺。
它表示该仪器在设计、制造和测试过程中遵循了严格的质量控制标准,以确保其性能和可靠性。
PCR 仪合格证书对于确保仪器的性能、实验结果的可靠性以及合规性
要求的满足至关重要。
它为用户提供了一种信任和保证,使他们能够放心地使用该仪器进行基因扩增等实验。
Odyssey CLX 操作指南1

导入图片 ........................................................................................................................... 18 泳道设置 ........................................................................................................................... 19 设定 Marker....................................................................................................................... 20 创建新 marker ................................................................................................................... 20 自动识别条带 ................................................................................................................... 21 手动编辑条带 ................................................................................................................... 21 单通道信号归一化 ..............................................................................................................21 查看表格 ........................................................................................................................... 22
东洋纺快速Taq HS染料混合物使用手册 101盒说明书

manual Quick Taq HS Dye Mix 1306 F1138K Quick Taq HS DyeMixDTM-101 100 reactionsStore at -20°C Contents[1] Introduction[2] Components[3] Primer design[4] Analysis and Cloning of PCR products[5] Protocol1. Standard reaction setup2. Cycling conditions[6] Examples[7] TroubleshootingCAUTIONAll reagents in this kit are intended for research purposes. Do not use for diagnosis or clinical purposes. Please observe general laboratory precaution and utilize safety while using this kit.JAPAN CHINATOYOBO CO., LTD. TOYOBO Bio-Technology, CO., LTD.Tel(81)-6-6348-3888 Tel(86)-21-58794900.4140www.toyobo.co.jp/e/bio1JAPAN CHINA TOYOBO CO., LTD. TOYOBO Bio-Technology, CO., LTD.Tel(81)-6-6348-3888 Tel(86)-21-58794900.4140 www.toyobo.co.jp/bio/********************[ 1 ] Introduction[ 2 ] Components[ 3 ] Primer Design[ 4 ] Analysis andCloning of PCR productsDescriptionQuick Taq HS DyeMix is a Taq-based 2× master mix PCR reagent that contains anelectrophoresis dye (BPB; bromophenol blue) and anti-Taq antibodies for hot start PCR. This reagent contains all components for PCR except primers and template DNA. This reagent shows specific and efficient amplification. The amplified products can be directly loaded in the wells of agarose or acrylamide gels.Features-As this reagent contains bromophenol blue (BPB) as an electrophoresis dye; the PCR products can be analyzed directly with an agarose or acrylamide gel.-This reagent exhibits greater PCR performance than conventional rTaq DNA polymerase.-This reagent contains anti-Taq antibodies for hot start PCR. Hot start technology realizes highly specific and sensitive PCR.-This reagent is stable for at least three months at 4°C. No decrease in reaction efficiency is observed following 30 freeze-thaw cycles.-This reagent is suitable for a colony-direct PCR (see [6], Example 2).This reagent includes the following components for 100 reactions, 50 μl total reaction volume:2× Quick Taq HS DyeMix1.25ml× 2*In the case of the long-term storage (>3 months), this reagent should be stored at-20°C.Primers should be 22–35 bases long, with a melting temperature (Tm) > 60°C.-As this reagent contains bromophenol blue (BPB) as an electrophoresis dye and by adjusting its relative density, the PCR products can be applied directly to an agarose or acrylamide gel.-The PCR products can be cloned using general TA cloning technology.-The PCR products can be used as templates for sequencing after an appropriate treatment.JAPAN CHINA TOYOBO CO., LTD. TOYOBO Bio-Technology, CO., LTD.Tel(81)-6-6348-3888 Tel(86)-21-58794900.4140 www.toyobo.co.jp/bio/********************[ 5 ] Protocol[ 6 ] Examples1. Standard reactionBefore preparing the reaction mixture, the master mix solution should be completely thawed. *For the PCR reaction, thin-wall tubes are recommended. A total reaction volume of 50 μl is also recommended.2. PCR cycle conditionsExample 1. Amplification of the human p53 gene (2.9 kb)The human p53 gene (2.9 kb) was amplified using 50 ng of human genomic DNA.Quick Taq HS DyeMix successfully amplified the targets.Reaction volume 50 μl 20 μl Final concentrationAutoclaved, distilled water X μl X μl 2x Quick Taq HS DyeMix 25 μl 10 μl 1 × 10 pmol /μl Primer #1 1.0 μl 0.4 μl 0.2 μM 10 pmol /μl Primer #2 1.0 μl 0.4 μl 0.2 μM Template DNAY μlY μlGenomic DNA -200 ng / 50 μl Plasmid DNA -50 ng / 50 μl E. coli colonyTotal 50 μl 20 μl3-step cyclePredenaturation: 94°C , 2min. Denaturation: 94°C , 30sec. °C , 30sec. Extension: 68°C , 1min. /kb 2-step cyclePredenaturation: 94°C , 2min. Denaturation: 94°C , 30sec. Extension: 68°C , 1min. /kbJAPAN CHINA TOYOBO CO., LTD. TOYOBO Bio-Technology, CO., LTD.Tel(81)-6-6348-3888 Tel(86)-21-58794900.4140 www.toyobo.co.jp/bio/********************[ 7 ] Trouble shootingExample 2. Insert amplification by a colony-direct PCRThe inserts were amplified using Quick Taq HS DyeMix with universal primers from E. coli DH5α colonies bearing pTA2 plasmid (insert size: 500 bp). Quick Taq HS DyeMix successfully and efficiently amplified all targets.SymptomCauseSolutionNo PCR product / low yieldCycling conditions are not suitable.Increase the number of cycles by 2-5 cycles. Primer is not good.Check the quality of the primers. Redesign the primers.Template DNA is ofinsufficient quality and/or quantity.Check the quality of the template DNA. Increase the amount of the template DNA Too much sample Excessive amounts of bacterial cells may inhibit amplification. Decrease the sample volume. Smearing / Extra bandCycling conditions are not suitable.Decrease the number of cycles by 2-5 cycles.Primer concentration is not appropriate Optimize the primer concentration to around 0.1-0.2 μM.Annealing temperature is too low. Optimize the annealing temperature to around 55°C -65°C . Primer is not good. Check the quality of the primers. Redesign the primers. Too much template DNA Reduce the amount of template DNA。
QPCR及QRT-PCR系列产品

Invitrogen的ICFC系列产品促销1.QPCR及QRT-PCR系列产品Invitrogen公司专门为中国客户提供的定量PCR试剂盒,结合了 UDG 防止残余污染技术和SYBR® Green I 荧光染料(存在于SYBR® Green I荧光定量PCR试剂盒中),在美国接受了严格的质量监控,可提供极高灵敏度的目的序列定量检测,线性剂量低,反应浓度范围很大。
qPCR Supermix-- 即用型反应剂,专为高特异性、实时定量DNA扩增设计UDG-- 防止携带污染物,减少克隆片段假阳性结果ROX参考染料-- 适用ABI仪器的校正染料产品信息活动时间:即日起至2009年4月30日2.Gibco南美胎牛血清即日起凡优惠价¥1780购买Gibco胎牛血清500ml(目录号:C2027050)即可获赠送价值¥250现金抵用券。
您可以凭现金抵用券在英韦创津公司购买任何商品,此券有效期至2009年5月31日。
产品信息活动时间:即日起至2009年4月30日独特的采集方式:GIBCO采用无菌心脏穿刺的方式采血原装直送,避免污染:原产地采集、加工、检测、包装。
完善的质控:采集、处理、检测、运输等环节都有文件和证书。
3.Invitrogen TA Cloning克隆产品专门用于克隆Taq聚合酶扩增的PCR产物。
采用pCR载体,能产生80%以上的重组产物,90%以上重组产物都包含插入片段。
产品信息活动时间:即日起至2009年5月31日附:pCR载体优点及图谱:3’-T突出端可直接连接Taq扩增的PCR产物可选择T7或T7和Sp6启动子进行体外RNA转录和测序侧向EcoRⅠ位点的通用多接头位点方便了插入片段的切离可以选择卡那霉素或氨苄青霉素进行筛选非常简便的蓝/白克隆筛选具有M13正向和反向引物位点,方便测序4.GIBCO液体培养基系列产品创立近50年的历史,品质优秀,产品种类丰富;为了中国用户利益,特建立国内生产线;所有产品,从原材料到生产全部按照GIBCO质量标准进行,每批均送抵美国公司总部质检合格后,才在国内销售。
ASM 142系列蜜芬泄漏检测器说明书

G B 00207 - E d i t i o n 05 - F e b r u a r y 121/1A 100Introduction to the ASM 142 seriesA new generation of adixen helium leakdetectorModel photographed: ASM 142The ASM 142/142 D/142 S/ASM Graph/ASM Graph D/ASM Graph D+ are universal helium leak detectors which set new performance standards for multi-purpose unit.These detectors are the end-result of an innovative engineering approach utilizing the latest electronics technologies and vacuum concepts, whichmake them a truly universal unit:142I high performances, such as, a roughing capacity of 7 CFM (60 l/mn) with a usable helium sensitivity in the 10-11 /sec range.x x xI high performances, such as, a dry roughing capacity of 0.9 CFM (1.5 m 3/h) with a usable helium sensitivity in the 10-11 /sec range.x x xI a usable helium sensitivity in the10-7 /sec range (with auto-zero function).xI comprehensive control panel with two distinct areas (one for the operation of the unit, the other for entering the test parameters).x x x x x xI evolved features to assist the operator in his daily operation (auto-calibration, auto-zero, helium signal direct readout, ...).x x x x x xI very rugged design, based on field-proven components, which makes it ideal for any industrial environment.x x x x x xI various accessories to reinforce the versatility of the product (remote control, sniffer probe).x x x x x xI totally dry leak detector.x xxI specific to sniffing test mode applications.xI graphic interface.xxxWe suggest that you read this manual before you start to use your detector to obtain optimum levels of performance and complete satisfaction.adixen Vacuum Products - ASM 142 S Operating instructions。
艾本德pcr故障代码手册

艾本德pcr故障代码手册(实用版)目录1.概述2.艾本德 PCR 故障代码手册的主要内容3.艾本德 PCR 故障代码手册的使用方法4.艾本德 PCR 故障代码手册的优点5.总结正文1.概述艾本德 PCR 故障代码手册是一本针对艾本德 PCR 仪器故障排除的专业指南。
PCR(聚合酶链式反应)是一种生物技术方法,常用于扩增 DNA 分子。
艾本德作为一家知名的生物技术企业,其生产的 PCR 仪器在实验中可能会遇到各种故障。
为了帮助用户快速准确地解决这些问题,艾本德推出了这本故障代码手册。
2.艾本德 PCR 故障代码手册的主要内容艾本德 PCR 故障代码手册包含了众多故障代码及其对应的解决方案。
这些代码涵盖了从仪器启动、样本准备到数据分析等各个环节可能出现的问题。
每个故障代码都对应一个唯一的数字,方便用户快速查询。
同时,手册中还提供了详细的故障现象描述和故障排除步骤,以便用户根据实际情况进行操作。
3.艾本德 PCR 故障代码手册的使用方法在使用艾本德 PCR 故障代码手册时,用户应首先确定仪器出现的故障现象,然后根据现象在手册中查找对应的故障代码。
接着,根据代码所提供的解决方案,进行相应的操作,以排除故障。
若无法解决,可尝试联系艾本德技术支持获取进一步的帮助。
4.艾本德 PCR 故障代码手册的优点艾本德 PCR 故障代码手册具有以下优点:(1)针对性强:该手册专门针对艾本德 PCR 仪器设计,内容紧密贴合实际应用场景。
(2)易用性高:手册采用数字编码和分步骤解决方案,方便用户快速查询和操作。
(3)专业性强:手册中的内容由艾本德公司的专业技术团队编写,具有较高的专业水平和权威性。
5.总结艾本德 PCR 故障代码手册是一本实用的专业工具书,对于使用艾本德 PCR 仪器的用户来说,具有很高的参考价值。
SureSelect Target Enrichment RNA Reagent Kit - HSQ

SureSelect Target Enrichment RNA Reagent Kit - HSQ *************(24小时)化学品安全技术说明书GHS化学品标识应急咨询电话(带值班时间)::供应商/ 制造商:安捷伦科技(上海)有限公司中国(上海)外高桥自由贸易试验区英伦路412号(邮编:200131)电话号码: 800-820-3278传真号码: 0086 (21) 5048 2818SureSelect Target Enrichment RNA Reagent Kit - HSQ化学品的推荐用途和限制用途5190-4396 / 5190-4404 / 5190-44125190-4397 / 5190-4405 / 5190-4413SureSelect Hyb 45190-4398 / 5190-4406 / 5190-4414SureSelect Binding Buffer 5190-4399 / 5190-4407 / 5190-4415SureSelect Wash Buffer 15190-4400 / 5190-4408 / 5190-4416SureSelect Wash Buffer 25190-4401 / 5190-4409 / 5190-4417SureSelect Elution Buffer 5190-4402 / 5190-4410 / 5190-4418SureSelect Neutralization Buffer 5190-4403 / 5190-4411 / 5190-4419SureSelect RNase Block 5190-4383 / 5190-4386SureSelect Hyb 35190-4382 / 5190-4385SureSelect Block 25190-4381 / 5190-4384SureSelect Indexing Block 15190-4427 / 5190-4428SureSelect Indexing Block 35190-4445 / 5190-4446SureSelect ILM Indexing Post Capture Forward PCR Primer5190-4441 / 5190-4442SureSelect ILM Index Pre Capture PCR Reverse Primer5190-4443 / 5190-4444SureSelect Adaptor Oligo Mix 5190-4932 / 5190-3619SureSelect Primer 5190-4933 / 5190-3620PCR Primer Index 1-16各种各样的*部件号:物质用途:0.4 ml(毫升) - 12 ml(毫升)0.096 ml(毫升) - 1.25 ml(毫升)SureSelect Hyb 40.208 ml(毫升) - 6.25 ml(毫升)SureSelect Binding Buffer 13.2 ml(毫升) - 400 ml(毫升)SureSelect Wash Buffer 18 ml(毫升) - 240 ml(毫升)SureSelect Wash Buffer 224 ml(毫升) - 720 ml(毫升)SureSelect Elution Buffer0.96 ml(毫升) - 29 ml(毫升)SureSelect Neutralization Buffer 0.96 ml(毫升) - 29 ml(毫升)SureSelect RNase Block 0.016 ml(毫升) - 0.096 ml(毫升)SureSelect Hyb 30.16 ml(毫升) - 0.96 ml(毫升)SureSelect Block 20.045 ml(毫升) - 0.24 ml(毫升)SureSelect Indexing Block 10.045 ml(毫升) - 0.24 ml(毫升)SureSelect Indexing Block 30.012 ml(毫升) - 0.058 ml(毫升)SureSelect ILM Indexing Post Capture Forward PCR Primer0.045 ml(毫升) - 0.27 ml(毫升)SureSelect ILM Index Pre Capture PCR Reverse Primer0.045 ml(毫升) - 0.27 ml(毫升)SureSelect Adaptor Oligo Mix 1.2 ml(毫升) (16 反应)SureSelect Primer0.3 ml(毫升) (16 反应)PCR Primer Index 1-160.0125 ml(毫升) - 0.025 ml(毫升)部件号(化学品试剂盒):G9601A, G9601B, G9601C 注解 *:* PCR Primer Index 1-16: 5190-3021, 5190-3037, 5190-3022, 5190-3038, 5190-3023,5190-3039, 5190-3024, 5190-3040, 5190-3025, 5190-3041, 5190-3026, 5190-3042,5190-3027, 5190-3043, 5190-3028, 5190-3044, 5190-3029, 5190-3045, 5190-3030,5190-3046, 5190-3031, 5190-3047, 5190-3032, 5190-3048, 5190-4423, 5190-4468,5190-4424, 5190-4469, 5190-4425, 5190-4470, 5190-4426, 5190-4471安全技术说明书根据 GB/ T 16483-2008 和 GB/ T 17519-2013物质或混合物的分类根据 GB13690-2009 和 GB30000-2013紧急情况概述SureSelect Hyb 1液体。
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
HP 打印机说明书

DimensionsW x D x H . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .660 mm x 650 mm x 725 mm ( 26 in x 26 in x 29 in)Weight . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .103 kg (227 lb)Operating EnvironmentTemperature . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15 – 35 °C Humidity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .20 – 80%, non-condensing Altitude . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3048 m (10,000 ft) max Heat Generation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4092 BTU/h Power Consumption . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1200 VA max Power Input Requirements . . . . . . . . . . . . . . . . . . . .100 – 240 V ~, 50 – 60 Hz User Interface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Integrated LCD touchscreen Adapter/Ports■■Ethernet on RJ45 port ■■RS232 on DB9 port■■External DVI-I connectors (with VGA)■■ 6 External USB portsLiquid Waste Handling■■ 2 external waste bottles (5 L each)■■Can be connected to a laboratory drain Printing Options■■On-screen result review ■■Electronic printing to PDF ■■External printer (available)Required Maintenance■■Empty waste bottles (as needed)■■Clean surfaces, touchscreen, and racks (as needed)■■Perform the Clean System procedure (2 minute automated process, every 30,000 tests)LIS Specifi cations■■Bi-directional capability with broadcast download of test orders ■■Option to add patient demographics to printed results ■■LIS result output with option to include comment record ■■Chromatogram PDF can be sent automatically to a network folderD-100™ HbA 1c Elution Buffer A Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2600 mL Number of Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . > 1100 tests per bottle Storage Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15 – 35°C Onboard Capacity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 bottles D-100™ HbA 1c Elution Buffer B Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1400 mL Number of Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . > 3000 tests per bottle Storage Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15 – 35°C Onboard Capacity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 bottles D-100™ Wash Solution Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3300 mL Number of Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . > 1100 tests per bottle Storage Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15 – 35°C Onboard Capacity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 bottles D-100™ HbA 1c Analytical CartridgeNumber of Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10,000Storage Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 – 8°C Onboard Capacity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 cartridge D-100™ HbA 1c Calibrator Pack Calibration Frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .once per cartridge Storage Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 – 8°C D-100™ Prefi ltersNumber of Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,000Storage Requirements . . . . . . . . . . . . . . . . . . . . . .2 – 8°C, 120 days at 15 – 35°C Onboard Capacity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 prefi lterGeneral Specifi cationsConsumable SpecificationsHbA1c Testing for DiabetesD-100™ Hemoglobin Testing SystemFor further information, please contact the Bio-Rad office nearest you or visit our website at /diagnosticsClinical Diagnostics Group Web site /diagnostics USA 180****6723Australia 61 2 9914 2800 Austria 43 1 877 8901 Belgium 32 03 710 53 00Brazil 55 31 3689 6600 Canada 151****4372China 86 21 61698500 Czech Republic 420 241 430 532 Denmark 45 4452 1000 Finland 358 9 804 22 00 France 33 1 47 95 60 00 Germany 49 0 89 318 840 Greece 30 210 7774396 Hong Kong 852 2789 3300 Hungary 36 1 459 6100 India 180****1224 Israel 972 3 9636050 Italy 39 02 216091 Japan 81 3 6361 7070 Korea 82 2 3473 4460 Mexico 52 55 5488 7670 The Netherlands 31 318 540666 New Zealand 64 9 415 2280 Norway 47 23 38 41 30 Poland 48 22 3319999 Portugal 351 21 472-7700 Russia 7 495 721 1404 Singapore 65 6415 3170 South Africa 27 11 442 85 08 Spain 34 91 590 5200 Sweden 46 8 555 127 00 Switzerland 41 0 26 674 55 05 06 Taiwan 886 2 2578 7189Thailand 662 651 8311 United Kingdom 44 0 20 8328 2000Bio-RadLaboratories, Inc.© 2016 Bio-Rad Laboratories, Inc. Printed in USA DG16-0012 A-318 Rev. 07/2016Printed on recycled paperwith soy-based inksD-100™ Hemoglobin Testing SystemSample Handling SpecificationsSample Throughput . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .80 tests/hour Time to First Result . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 minutes, 15 seconds Sample Analysis Time . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .45 seconds Sample Preparation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .None required Sample Capacity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .100 samples, continuous loading Stat Area Sample Capacity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3 samples (primary tube or microvial) Minimum Sample Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1 mL (13 x 75 mm primary tube) Specimen Type . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Whole blood, capillary** Whole Blood Sample Aspiration Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9 μL Prediluted Sample Aspiration Volume . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .495 μL Acceptable Sample Tube Types■■K2-EDTA■■K3-EDTA■■Potassium Oxalate/Sodium Fluoride■■Sodium Citrate ■■Sodium Heparin ■■Lithium HeparinSupported Barcode Symbologies■■Code 39■■Code 128■■Codabar■■Interleaved 2 of 5■■EAN■■UPCPerformance SpecificationsTotal Precision* . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .≤1 .7% CV (NGSP units) Accuracy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Total Error <6% Linear Range . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3 .5% – 20% HbA1c Minimum QC Frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Once per day Sample Concentration Range . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .50,000 – 350,000 mAU HbA1c Units Reported . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .IFCC and NGSP Hemoglobin Interferences■■No interference from hemoglobins S, C, D, or E ■■No interference from fetal hemoglobin up to 30%■■No interference from labile HbA1c■■No interference from carbamylated hemoglobin* Total precision is based on a 3-instrument, 60-day precision study using 3 lots of reagents to generate a total of 720 data points per sample . It includes the following sources of variation: within-run (repeatability), between-run, between-day, between-instrument, and between-lot .** Not available in US for the capillary collection kit .。
沙眼衣原体核酸检测试剂盒[2]
![沙眼衣原体核酸检测试剂盒[2]](https://img.taocdn.com/s3/m/2509adfbaeaad1f346933ff5.png)
沙眼衣原体核酸检测试剂盒(PCR-荧光探针法)【名称】通用名称:沙眼衣原体核酸检测(PCR-荧光探针法)英文名称:Diagnostic for Chlamydia Trachomatis DNA (PCR—Fluoresecence Probing)【包装规格】20人份/盒【预期用途】本产品用于尿道或女性阴道分泌物标本中的沙眼衣原体核酸定性检测,适用于沙眼衣原体(CT)感染的辅助诊断,不能用于沙眼衣原体感染的筛查,仅限用于经卫生行政机构认可的医疗单位的试验室。
【检验原理】本产品用一对沙眼衣原体特异性引物和沙眼衣原体特异性荧光探针,配以PCR反应液、耐热dna聚合酶(Taq酶)、核苷酸单体(dNTPs)等成分,用PCR体外扩增法检测沙眼衣原体DNA。
【主要组成成份】【储存条件及有效期】于–20℃,避免反复冻融;有效期6个月。
【适用仪器】DA—620荧光检测仪,ABI Prism 7000,ABI GeneAmp 5700,Roche LightCycler、Mx3000P荧光定量PCR仪等。
【样本要求】男性:取尿道分泌物或细小棉拭子伸入尿道约2~4厘米,略捻动拭子取出分泌物(应略带黏膜)。
将分泌物或棉拭子置入无菌玻璃管,用无菌棉球将试管塞紧后,密闭送检。
女性:阴道-用无菌生理氯化钠溶液棉球洗去宫颈外分泌物,再用无菌棉拭子插入宫颈内,停5秒钟后旋动棉拭子采集宫颈分泌物,将棉拭子置入无菌玻璃管,用无菌棉球将试管塞紧后,密闭送检。
尿道—用无菌生理氯化钠溶液棉球洗净尿道口,再用无菌棉拭子插入尿道约2厘米,略捻拭子取出分泌物,将棉拭子置入无菌玻璃管(应略带黏膜)。
用无菌棉球将试管塞紧后,密闭送检。
标本可立即用于测试,也可保存于-20℃待测,保存期为6个月。
标本运送应采用0℃冰壶。
并于48小时内送达。
【检验方法】标本及对照品的处理标本试管中加入1ml无菌生理氯化钠溶液1ml打匀,12,000转/分离心5分钟,再重复洗涤一次。
QIAquick_PCR_Purification_Kit

QIAquick® PCR Purification KitThe QIAquick PCR Purification Kit (cat. nos. 28104 and 28106) can be stored at room temperature (15–25°C) for up to 12 months.For more information, please refer to the QIAquick Spin Handbook, March 2008, which can be found at: /handbooks.For technical assistance, please call toll-free 00800-22-44-6000, or find regional phone numbers at /contact.Notes before startingAdd ethanol (96–100%) to Buffer PE before use (see bottle label for volume).All centrifugation steps are carried out at 17,900 x g (13,000 rpm) in a conventional table-top microcentrifuge at room temperature.Add 1:250 volume pH indicator I to Buffer PB. The yellow color of Buffer PB with pH indicator I indicates a pH of ≤7.5. If the purified PCR product is to be used in sensitive microarray applications, it may be beneficial to use Buffer PB withoutthe addition of pH indicator I. Do not add pH indicator I to buffer aliquots.1.Add 5 volumes Buffer PB to 1 volume of the PCR reaction and mix. If the color ofthe mixture is orange or violet, add 10 μl 3 M sodium acetate, pH 5.0, and mix.The color of the mixture will turn yellow.2.Place a QIAquick column in S a provided 2 ml collection tube or intoz a vacuum manifold. For details on how to set up a vacuum manifold, refer to the QIAquick Spin Handbook.3.To bind DNA, apply the sample to the QIAquick column and S centrifuge for30–60 s or z apply vacuum to the manifold until all the samples have passedthrough the column. S Discard flow-through and place the QIAquick columnback in the same tube.October 20104.To wash, add 0.75 ml Buffer PE to the QIAquick column S centrifuge for30–60 s or z apply vacuum. S Discard flow-through and place the QIAquick column back in the same tube.5.Centrifuge the QIAquick column once more in the provided 2 ml collection tubefor 1 min to remove residual wash buffer.6.Place each QIAquick column in a clean 1.5 ml microcentrifuge tube.7.To elute DNA, add 50 μl Buffer EB (10 mM Tris·Cl, pH 8.5) or water (pH 7.0–8.5) to the center of the QIAquick membrane and centrifuge the column for1 min. For increased DNA concentration, add 30 μl elution buffer to the centerof the QIAquick membrane, let the column stand for 1 min, and then centrifuge.8.If the purified DNA is to be analyzed on a gel, add 1 volume of Loading Dye to5 volumes of purified DNA. Mix the solution by pipetting up and down beforeloading the gel.For up-to-date licensing information and product-specific Array disclaimers, see the respective QIAGEN kit handbook or usermanual.Trademarks: QIAGEN®, QIAquick® (QIAGEN Group). 1063920 10/2010© 2010 QIAGEN, all rights reserved.。
科威特医学实验室——微生物检测试试瓶设备及其代码快速指南(2017年10月12日修订)说明书

Should also be used for: Group B Streptococcus by PCR
Tube Type:
MRSA by PCR Rapid Trichomonas assay
ESWAB
Rapid Strep Throat assay (Group A Streptococcus)
Tube Type: URGRAY
Stool Collection Devices
BLACK TOP—Total-Fix medium for parasitological evaluation. YELLOW TOP - Cary-Blair for the Comprehensive GI Panel by PCR or Targeted Culturing (CGIPCR/TOCURE)
University of Kentucky Hospital—Microbiology Specimen Collection Devices and Their Codes Quick Guide
Revised 10/12/2017
Urine Vacutainer with Boric Acid Preservative This is the main collection/transport device used for Urine Cultures. Urine Culture (URNC) Urine Culture Catheter (URCATH)
Abbott Multi-Collect Vaginal/Urethral or Urine Collection System
This collection devise is strictly for the use on the Abbott M2000 instrument for the detection of Neisseria gonorrhoeae and Chlamydia trachomatis by PCR
GoTaq Probe qPCR Master Mix 说明书

Revised 6/14TM378GoTaq ® Probe qPCRMaster MixInstruc Ɵ ons for Use of ProductsA6101 and A6102T E C H N I C A L M A N U A LPromega Corpora Ɵ on · 2800 Woods Hollow Road · Madison, WI 53711-5399 USA · Toll Free in USA 800-356-9526 · 608-274-4330 · Fax 608-277-2516 1 TM378 · Revised 6/14All technical literature is available at: /protocols/Visit the web site to verify that you are using the most current version of this Technical Manual.E-mailPromegaTechnicalServicesifyouhavequestionsonuseofthissystem:********************GoTaq ® Probe qPCR Master Mix1. DescriptionThe GoTaq ® Probe qPCR Master Mix (a,b) is optimized for quantitative PCR assays in the hydrolysis probe detection format. It is provided as a ready-to-use, stabilized 2X formulation that includes all components for qPCR (except template, primers and probe). This master mix does not contain a reference dye; however, a separate tube of carboxy-X-rhodamine (CXR) reference dye is included with this system, allowing users to add reference dye to amplifi cation reactions if desired.The GoTaq ® Probe qPCR Master Mix is designed to provide resistance to a wide range of PCR inhibitors. This formulation uses antibody-mediated hot-start chemistry, allowing reaction setup to be performed at room temperature. The master mix also employs rapid hot-start activation and processive enzymes, making it compatible with both standard and fast instrument cycling programs.1. Description (1)2. Product Components and Storage Conditions (3)3. General Considerations (3)3.A. Prevention of Contamination (3)3.B. qPCR Primers and Probes (4)3.C. Genomic DNA Template Quantity (4)3.D. CXR Reference Dye (4)3.E. Instruments for Low-Level (30nM) Reference Dye (4)3.F. Instruments for High-Level (500nM) Reference Dye (5)4. GoTaq ® Probe qPCR Protocol (5)4.A. Addition of CXR Reference Dye to the GoTaq ® Probe qPCR Master Mix (Optional) (5)4.B. Preparation of GoTaq ® Probe qPCR Amplifi cations (6)5. Thermal Cycling (7)6. General qPCR References (7)7. Summary of Changes (8)2 Promega Corpora Ɵ on · 2800 Woods Hollow Road · Madison, WI 53711-5399 USA · Toll Free in USA 800-356-9526 · 608-274-4330 · Fax 608-277-2516TM378 · Revised 6/14 1. Description (continued)11006TAPrepare gDNA, primers andprobe and GoTaq ® ProbeqPCR Master Mix.Perform qPCR using standardor FAST mode on a real-time PCRinstrument.Assemble reaction.Analyze amplificationand standard curve data.Figure 1. Flow diagram of the GoTaq ® Probe qPCR Master Mix protocol.Promega Corpora Ɵ on · 2800 Woods Hollow Road · Madison, WI 53711-5399 USA · Toll Free in USA 800-356-9526 · 608-274-4330 · Fax 608-277-2516 3 TM378 · Revised 6/142. Product Components and Storage ConditionsP R O D U C TS I Z E C AT.#GoTaq® Probe qPCR Master Mix 200 reac Ɵ ons A6101For Research Use Only. Not for use in diagnostic procedures. Contains suffi cient reagents for 200 × 20μl assays. Includes:• 2 × 1ml GoTaq ® Probe qPCR Master Mix, dTTP (2X)• 100µl CXR Reference Dye, 30µM• 2 × 1.25ml Nuclease-Free WaterP R O D U C T S I Z E C AT.#GoTaq® Probe qPCR Master Mix 1,000 reac Ɵ ons A6102For Research Use Only. Not for use in diagnostic procedures. Contains suffi cient reagents for 1,000 × 20μl assays. Includes:• 10 × 1ml GoTaq ® Probe qPCR Master Mix, dTTP (2X)• 2 × 200µl CXR Reference Dye, 30µM• 13ml Nuclease-Free WaterAvailable SeparatelyP R O D U C T S I Z E C AT.#GoTaq 1-Step RT-qPCR System*200 reac Ɵ ons A6120GoTaq 2-Step RT-qPCR System*200 reac Ɵ ons A6110Nuclease-Free Water** 50ml P1193*For Research Use Only. Not for use in diagnostic procedures.**For Laboratory Use.3. General Considerations3.A. Prevention of Contamination• Use designated work areas and pipettes for pre- and post-amplifi cation steps to minimize the potential for cross-contamination between samples and revent carryover of nucleic acid from one experiment to the next.• Wear gloves and change them often.• Do not open the reactions after amplifi cation is complete. Opening increases the risk of contaminating subsequent reactions with the amplifi ed product.• Prevent contamination by using aerosol-resistant pipette tips.4 Promega Corpora Ɵ on · 2800 Woods Hollow Road · Madison, WI 53711-5399 USA · Toll Free in USA 800-356-9526 · 608-274-4330 · Fax 608-277-2516TM378 · Revised 6/14 3.B. qPCR Primers and ProbesThe concentrations of primers and probes should be optimized for each primer/probe combination. For geneexpression assays, primer and probe concentration may need to be adjusted based on target abundance. As a general rule, a concentration of 900nM for PCR primers and 250nM for the hydrolysis probe is a recommended starting point. Concentrations of PCR primers may range from 200nM to 1µM, while probe concentration may range from 100nM to 300nM; titrations should be performed to ensure optimal results.We recommend preparing and storing 20X solutions of the PCR primers and hydrolysis probe.3.C. Genomic DNA Template QuantityUse ≤250ng of genomic DNA.3.D. CXR Reference DyeThe GoTaq ® Probe qPCR Master Mix formulation does not contain a reference dye; however, a separate tube ofcarboxy-X-rhodamine (CXR) reference dye is included with this system, allowing users to add reference dye if desired. Addition of the reference dye will help maximize eff ectiveness of the GoTaq ® Probe qPCR Master Mix when used on real-time PCR instruments that allow normalization. The CXR reference dye has the same spectral properties as ROX™dye. The dye is provided at a concentration of 30µM.Some instrumentation is designed to normalize with a low concentration of ROX™ reference dye. We recommend that the CXR reference dye be added to a fi nal reaction concentration of 30nM for instruments that recommend a “low” level of ROX™ dye. Other instruments require ROX™ at a high concentration for normalization. We recommend that the CXR reference dye be added to a fi nal reaction concentration of 500nM for instruments that recommend a “high” level of ROX™ dye.Examples of instrument recommendations are listed below. Directions for setting up qPCR amplifi cation reactions for both “low dye” and “high dye” instruments are included in Section 4.3.E.Instruments for Low-Level (30nM) Reference Dye •Applied Biosystems 7500 and 7500 FAST Real-Time PCR System •Bio-Rad CFX96 Real-Time PCR Detection System • Bio-Rad DNA Engine Opticon ® and Opticon ® 2 Real Time PCR Detection Systems• Bio-Rad/MJ Research Chromo4™ Real-Time Detector• Cepheid SmartCycler ® system• Corbett Rotor-Gene™ 3000 and 6000 Real-Time Rotary Analyzer• Eppendorf Mastercycler ® ep realplex Real-Time PCR System• Roche LightCycler ® 480 Real-Time PCR System• Stratagene Mx3000P ® and Mx3005P ® Real-Time PCR Systems• Stratagene Mx4000® Multiplex Quantitative PCR SystemPromega Corpora Ɵ on · 2800 Woods Hollow Road · Madison, WI 53711-5399 USA · Toll Free in USA 800-356-9526 · 608-274-4330 · Fax 608-277-2516 5 TM378 · Revised 6/143.F.Instruments for High-Level (500nM) Reference Dye •Applied Biosystems ABI PRISM ® 7000 and 7700 Sequence Detection System •Applied Biosystems 7300 and 7900HT Real-Time PCR System •Applied Biosystems GeneAmp ® 5700 Thermal Cycler • Applied Biosystems StepOne™ and StepOnePlus™ Real-Time PCR Systems4. GoTaq ® Probe qPCR ProtocolMaterials to Be Supplied by the User• real-time PCR instrument and related equipment (i.e., appropriate PCR plates and plate covers)• sterile, aerosol-resistant pipette tips• nuclease-free pipettors dedicated to pre-amplifi cation work• DNA template• qPCR primers and probe4.A. Addition of CXR Reference Dye to the GoTaq ® Probe qPCR Master Mix (Optional)For users who wish to include CXR Reference Dye in their amplifi cation reactions, we recommend adding an aliquot ofconcentrated CXR Reference Dye to the 1ml tube of GoTaq ® Probe qPCR Master Mix. Depending on your instrumen-tation, the CXR Reference Dye should be added at either “low dye” concentration or “high dye” concentration, as follows (refer to the list in Section 3 to determine if your instrument requires low CXR or high CXR):1. Thaw the GoTaq ® Probe qPCR Master Mix and the Nuclease-Free Water. Do not thaw the Master Mix at elevatedtemperatures (i.e., above room temperature).2. Briefl y vortex the GoTaq ® Probe qPCR Master Mix for 3–5 seconds to mix.3.Add CXR Reference Dye to the 1ml tube of GoTaq ® Probe qPCR Master Mix:For “Low Dye” Instruments: Add 2µl of CXR Reference Dye (at 30µM) to the 1ml tube of GoTaq ® Probe qPCR Master Mix. For “High Dye” Instruments: Add 17µl of CXR Reference Dye (at 30µM) to the 1ml tube of GoTaq ® ProbeqPCR Master Mix.4. Briefl y vortex the GoTaq ® Probe qPCR Master Mix with CXR added for 3–5 seconds to mix.5. After adding the CXR to the GoTaq ® Probe qPCR Master Mix, mark the tube to indicate that you have performedthis step. The GoTaq ® Probe qPCR Master Mix with CXR added should be stored at –20°C.4.B. Preparation of GoTaq® Probe qPCR Amplifi cationsThe GoTaq® Probe qPCR Master Mix uses a hot-start chemistry, allowing reaction setup to be performed at room temperature.1. Thaw the GoTaq® Probe qPCR Master Mix and the Nuclease-Free Water. Do not thaw the Master Mix at el-evated temperatures (i.e., above room temperature).2. Briefl y vortex the GoTaq® Probe qPCR Master Mix for 3–5 seconds to mix.3. Determine the number of reactions to be set up. This should include negative control reactions. Add 1 or 2 reac-tions to this number to compensate for pipetting error. While this approach does require using a small amount of extra reagent, it ensures that you will have enough PCR master mix for all samples.Notes:• The reagent composition for a 20μl reaction volume is shown. Component volumes may be scaled for larger or smaller reaction volumes.• The reaction concentrations of primers and hydrolysis probe should be optimized for each primer/probe combi-nation.Nuclease-Free Water To a 20µl total reaction volume4. Prepare the reaction mix (without the template DNA) by combining the GoTaq® Probe qPCR Master Mix, thePCR primers, hydrolysis probe, and Nuclease-Free Water. Vortex briefl y to mix.5. Add the appropriate volume of reaction mix (without the template DNA) to each PCR tube or to each well of anoptical grade PCR plate.6. Add DNA template to the sample reactions.7. Seal the tubes or optical plates; centrifuge briefl y to collect the contents of the wells at the bottom. The samplesare ready for thermal cycling. Protect from extended light exposure or elevated temperatures before cycling.6Promega CorporaƟ on · 2800 Woods Hollow Road · Madison, WI 53711-5399 USA · Toll Free in USA 800-356-9526 · 608-274-4330 · Fax 608-277-2516 TM378 · Revised 6/14 Promega Corpora Ɵ on · 2800 Woods Hollow Road · Madison, WI 53711-5399 USA · Toll Free in USA 800-356-9526 · 608-274-4330 · Fax 608-277-2516 7 TM378 · Revised 6/145. Thermal CyclingThe cycling parameters below are off ered as a guideline and may be modifi ed as necessary for optimal results.Standard Cycling ConditionsFAST Cycling ConditionsDenaturation95°C 3 seconds 6. General qPCR References 1. Bustin, S.A. et al . (2009) The MIQE guidelines: Minimum information for publication of quantitative real-timePCR experiments. Clin. Chem . 55, 611–22.2.Dorak, M.T. (2009) Glossary of real-time PCR terms. This can be viewed online at: /genet-ics/glosrt.html 3. Fleige, S. and Pfaffl , M.W. (2006) RNA integrity and the eff ect on the real-time qRT-PCR performance. Mol.Aspects Med. 27, 126–39.4. Lefever, S. et al. (2009) RDML: Structured language and reporting guidelines for real-time quantitative PCRdata. Nucleic Acids Res . 37, 2065–9.5. Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real-time quantitativePCR and the 2(-delta delta C(T)) method. Methods 25, 402–8.7. Summary of ChangesThe following changes were made to the 6/14 revision of this document:1. Expired patent and license statements were removed.2. Document design was updated.(a)U.S. Pat. No. 6,242,235, Australian Pat. No. 761757, Canadian Pat. No. 2,335,153, Chinese Pat. No. ZL99808861.7, Hong Kong Pat. No. HK 1040262, Japanese Pat. No. 3673175, European Pat. No. 1088060 and other patents pending.(b)NOTICE TO PURCHASER: DISCLAIMER OF LICENSENo license is conveyed with the purchase of this product under any of US Pat. Nos. 5,210,015, 5,487,972, 5,804,375, 5,994,056, 6,171,785, 6,214,979,5,538,848, 5,723,591, 5,876,930, 6,030,787, and 6,258,569, and corresponding patents outside the United States, or any other patents or patent applicaƟ ons, relaƟ ng to the 5’ Nuclease and dsDNA-Binding Dye Processes. For further informaƟ on contact the Director of Licensing, Applied Biosystems, 850 Lincoln Centre Drive, Foster City, California 94404, USA.© 2012, 2014 Promega CorporaƟ on. All Rights Reserved.GoTaq and RNasin are registered trademarks of Promega CorporaƟ on. GoScript is a trademark of Promega CorporaƟ on.ABI PRISM is a registered trademark and ROX, StepOne and StepOnePlus are trademarks of Applera CorporaƟ on. GeneAmp is a registered trademark of Roche Molecular Systems, Inc. DNA Engine OpƟ con is a registered trademark and Chromo4 is a trademark of Bio-Rad Laboratories, Inc. LightCycler is a registered trademark of Roche DiagnosƟ cs, GmbH. Mastercycler is a registered trademark of Eppendorf-Netheler-Hinz GmbH. Mx3000P, Mx3005P and Mx4000 are registered trademarks of Stratagene. Rotor-Gene is a trademark of CorbeƩ Research Pty Ltd. SmartCycler is a registered trademark of Cepheid CorporaƟ on.Products may be covered by pending or issued patents or may have certain limitaƟ ons. Please visit our Web site for more informaƟ on.All prices and specifi caƟ ons are subject to change without prior noƟ ce.Product claims are subject to change. Please contact Promega Technical Services or access the Promega online catalog for the most up-to-dateinformaƟ on on Promega products.8Promega CorporaƟ on · 2800 Woods Hollow Road · Madison, WI 53711-5399 USA · Toll Free in USA 800-356-9526 · 608-274-4330 · Fax 608-277-2516 TM378 · Revised 6/14 。
PicoMaxx高保真PCR系统说明书
PicoMaxx High Fidelity PCR System, Part Number 600420*************(24小时)化学品安全技术说明书GHS product identifier 应急咨询电话(带值班时间)::供应商/ 制造商:安捷伦科技贸易(上海)有限公司中国(上海)外高桥自由贸易试验区英伦路412号(邮编:200131)电话号码: 800-820-3278传真号码: 0086 (21) 5048 2818PicoMaxx High Fidelity PCR System, Part Number 600420化学品的推荐用途和限制用途PicoMaxx High Fidelity PCR System 600420-5110X PicoMaxx Reaction Buffer 600420-52部件号:部件号(化学品试剂盒):600420安全技术说明书根据 GB/ T 16483-2008 和 GB/ T 17519-2013GHS化学品标识:PicoMaxx 高保真 PCR 系统,货号600420推荐用途分析试剂。
600420-51PicoMaxx High Fidelity PCR System0.04 ml (100 U 2.5 U/µl)600420-5210X PicoMaxx Reaction Buffer1 ml:有关环境保护措施,请参阅第 12 节。
物质或混合物的分类根据 GB13690-2009 和 GB30000-2013紧急情况概述PicoMaxx High Fidelity PCR System液体。
10X PicoMaxx Reaction Buffer 液体。
PicoMaxx High Fidelity PCR System无资料。
10X PicoMaxx Reaction Buffer 无资料。
PicoMaxx High Fidelity PCR System无资料。
维萨拉验证服务商品说明书

维萨拉验证服务/ 高效、合规的温湿度分布试验 卓越的质量管理体系为确保满足GM P 《药品生产质量管理规范》的要求,各机构必须证明其仓储和运输区域的环境条件都处于适当的控制下。
因此,质量计划的首要步骤应是通过分布研究深入了解这些区域的温度属性。
然而,分布研究需要的不仅仅是设备,还需要验证和校准方面的专业知识与技能。
维萨拉为您提供由娴熟的技术人员完成的世界级验证服务,服务包含用以进行分布研究,内容翔实的验证计划及分布试验协议。
深入透彻的验证后报告将会提升您对受控环境的了解。
这些文档和专业技能将有助于体现您的企业正在遵循受控环境验证与文档记录的当前最佳实践。
我们提供验证所需的专业技能和设备服务,所以您的质量和设施经理能专注于他们的日常工作。
降低生命科学环境的风险在过去20年间,我们已为生命科学行业的受控环境专门设计了创新型设备和软件,这些行业包括:• 制药• 生物技术• 医疗器械• 营养和保健品应用领域:• 冰箱/冷冻柜/冷藏室 • 培养箱• 稳定性试验箱• 仓库/存储区/批发中心翔实、精确的存档为进行分布研究,验证服务技术人员将会收集并分析您所在区域、房间或试验箱的数据,辨识工艺过程中的热点或冷点。
技术人员可对空载/满载试验箱,或仓储区的静态/动态过程进行挑战性实验。
测试可能会根据应用需要部署热电偶或配有传感器的数据记录仪,使用维萨拉验证软件下载分析测试数据。
所有分布研究文档均为安全、演示级品质。
报告包含最小值、最大值以及平均值统计和平均动力学温度(MKT) 数据,可轻松导出为供深入分析使用的通用.csv文件。
温度传感器工作范围:-90 °C至70 °C,精度高达±0.1 °C, 分辨率0.02 °C.相对湿度传感器工作范围:10%至90%,精度高达±1%,分辨率0.05%.合规性: 21 CFR Part 11, E U Annex 11 21随着全球范围的监管机构对制药、生物技术和医疗器械制造商和经销商的审查日趋严格,许多企业开始为其受控环境寻求验证解决方案。
PRISM用户手册说明书

Pharmaceutical Regulatory Information System(PRISM) Internet –CT Expedited Safety Report ModuleUser ManualVersion 3.1 (Aug 2018)HSA-NCS Confidential Page 1 of 19PRISMUser Manual For Internet – CT Expedited Safety Report Ver 3.1 REVISION HISTORYPRISMUser Manual For Internet – CT Expedited Safety Report Ver 3.1Table of Contents1INTRODUCTION (4)P URPOSE (4)S COPE (4)O VERVIEW (4)2FUNCTION (5)2.1T O A PPLY FOR S UBMISSION OF E XPEDITED S AFETY R EPORT (5)2.1.1Login (5)2.2C OMMON ICONS AND LINKS IN ALL SECTIONS: (7)2.3A PPLICATION F ORM OF C LINICAL T RIAL S UBMISSION OF E XPEDITED S AFETY R EPORT (8)2.3.1Introduction (8)2.3.2Particulars of Clinical Trial Application (9)2.3.3Applicant Particulars (10)2.3.4Safety Report Summary (11)2.3.5Supporting Attachments (15)2.3.6Confirmation (16)2.3.7Acknowledgment (19)1 IntroductionPurposeThe purpose of this user manual is to ensure that all nominated application users will be proficient in the use of the online application system.ScopeThe scope of the manual is to provide information on the use of the eService for the online application of the extended function –Submission of Expedited Safety ReportOverviewThis document provides brief details on the standards and guidelines that a user should adhere to in doing an online preparation and submission of an application. It divides the application procedure into sections and provides the brief guidelines for each of them.2 Function2.1 To Apply for Submission of Expedited Safety ReportSteps:1) CRIS administrator grants access to eService of CT - Submission of Expedited Safety Report2) Please access the following URL (DO NOT click on link, please copy the URL to address bar ofbrowser).sg/osc/portal/jsp/AA/process.jsp?eService=1902.1.1 Login1) Fill in your CorpPass ID and password and click the Submit button.2) The predefined roles of the users, comprising of drafter, submitter, CRIS administrator, counter staff, willbe verified against the CRIS authorisation.3) Upon successful authentication, a page will be shown for the applicant to select the company.4) Select the specific company and click the Submit button.5) The Terms and Conditions page will be shown. The application user is required to read the Terms andConditions before indicating accept or reject. If the Accept button is clicked, the user will be able to proceed with the eService. If the Cancel button is clicked, the page will be re-directed to the HSA homepage.2.2 Common icons and links in all sections:∙Attach icon. This will allow user to go to the Supporting Attachments page to attach relevant documents.∙Save icon. This will allow the user to save the form information at any desired point of time.∙Application form links. This will allow the user to toggle to different sections of the application form.∙This will allow the user to proceed to the next section of the application form.∙Previous button. This will allow the user to proceed to the previous section of the application form.∙Reset button. This will clear the information the user has input in the page.∙Fields with a red asterix * are mandatory input fields. Unless it is entered, the system validation will highlight error and application submission will be disabled.2.3 Application Form of Clinical Trial Submission of Expedited Safety ReportThe application form consists of 6 sections:It is recommended for users to fill in the application form details in a systematic serial manner as the later sections could reference information in the earlier sections.2.3.1 IntroductionThis section shows the list of Clinical Trial Application numbers. Select a Clinical Trial Application Number and click the Retrieve button to go to the Clinical Trial Application information section.2.3.2 Particulars of Clinical Trial ApplicationThis section shows the Clinical Trial Application information after a Clinical Trial Application Number is selected. The Clinical Trial Application information is auto-populated. Select a study drug and click the Next button to go to the Applicant Particulars section.2.3.3 Applicant ParticularsThis section allows the input of applicant particulars. Please note that drafter will not be able to see this page since they are not required to enter information for applicant.1) Fill in Name or NRIC.2) Click on the Retrieve button to populate the remaining fields.3) Fill in the other details if applicable.4) Click on the Next button to go to the Safety Report Summary section.2.3.4 Safety Report SummaryInitial Safety ReportFollow up Safety ReportSearch for Manufacturer Control Number (MCN)Safety Report AddedTo add a Safety ReportPlease note that more than 1 CIOMS report for the same drug can be submitted per application.1) Select a report type, initial or follow up report.2) For initial report, enter a Manufacturer Control Number (MCN) and click Add MCN button.For follow up report, select a Manufacturer Control Number (MCN) from dropdown list and click Add MCN button. To search for Manufacturer Control Number (MCN), click on the Search for MCN link to search for MCN.If the Manufacturer Control Number (MCN) is not found in the dropdown list, enter a Manufacturer Control Number (MCN) and click Add MCN button.3) After adding the safety report, the Manufacturer Control Number (MCN) will appear in the list atbottom. Click on the Manufacturer Control Number (MCN) hyperlink to enter report details.4) To add another CIOMS report, please return to steps 1-3.Safety Report DetailsSearch for SAE Description and SOC1SAE Description and SOC1 are populatedTo enter Safety Report DetailsPlease note that only events that meet the 3 criteria of serious, unexpected and drug-related are to be entered. MedDRA Preferred Terms are used for SAE Description.1) Enter all mandatory fields, date of onset (if available), indication, start and stop dates of drug.To enter SAE Description and SOC1, enter a keyword and click on search keyword. Select the appropriate SAE term from the search results. Both SAE Description and SOC1 fields will be populated.To delete SAE Description and SOC1 entry, click on Clear button.2) Attach at least one document of any required document type and click on Attach Files button to uploadthe document.3) After entering the details of safety report, click on Back to Summary button to save changes and goback to summary page.2.3.5 Supporting AttachmentsThis section allows the attachment of the supporting documents for the application.Add Attachment1) Click on the Browse button to select the required file for attachment.2) Select the required file.3) Click on the Ok button.4) Click on the Attach File button for the file to be attached to this application.5) Fill up remarks with regards to the attachment if required.Remove Attachment1) Click on the checkbox beside the attachment or attachments from the List of Attachments table.2) To delete all attachments, click on the checkbox beside S/n.3) Click on the Remove button.The file extensions, which are acceptable and supported, are:∙tif∙jpg∙pdf∙doc∙xls∙ppt∙avi (audio visual, if required)∙mpeg(audio visual, if required)2.3.6 ConfirmationThis section shows all the information the user has entered into the different sections of the application form. It allows the user to manually verify all the information fields.Confirmation Page for DrafterNotifyThe drafter would need to click on the Notify button to inform the applicant the application of Clinical Trial Submission of Expedited Safety Report. The notification email will be sent to the email of the latest submitter of Clinical Trial Extended eService. The applicant will fill in the applicant details and submit the application.Confirmation Page for SubmitterTo proceed with the system verification of the information on the application form,1) Click on the Validate button.2) If there is any missing mandatory information or details, which do not meet the application requirements,a pop up screen will appear with the details of the validation error. (Please disable any popup blockersto allow the notification to show.)3) If there is no validation error, click on the Submit button to submit the application.This will allow the user to take note of the relevant changes to be made and return back to the application form and amend accordingly.2.3.7 AcknowledgmentThis section acknowledges that the application has been submitted to HSA for processing. An application number will be generated for the application.Links1) Show Printer Friendly versionThis allows the applicant to print or view the application.2) Click here to start a new ESR applicationThis allows the applicant to start a new ESR application.3) Back to HSA HomeThis allows the applicant to go back to HSA home page.。
荷尔登生物医学检测产品说明书
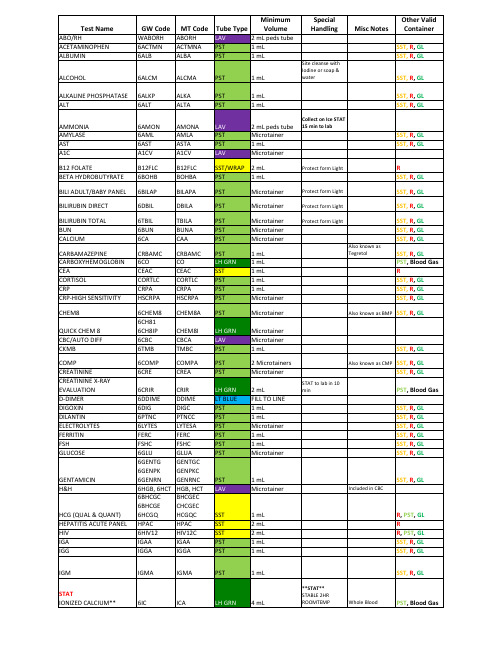
1mL
R
1mL
SST, R, GL
1mL
No capillary
1mL
collection
Order of Draw: 1. BLOOD CULTURES 2. LT BLUE 3. RED 4. SST 5. PST/LH GRN 6. LAV 7. GRAY
M2909 (1-19)
Microtainer Microtainer 1 mL
2 Microtainers Microtainer
2 mL FILL TO LINE 1 mL 1 mL Microtainer 1 mL 1 mL Microtainer
SST, R, GL
STAT to lab in 10 min
Also known as CMP SST, R, GL SST, R, GL
TOBRAMYCIN TOTAL PROTEIN TROPONIN TSH
TYPE AND CROSSMATCH
TYPE AND SCREEN URIC ACID
VANCOMYCIN VALPROIC ACID VITAMIN D
FEA
6LA LHC 6LIP
6LIVER 6LIPID LIA 6MG 6METX 6MONO PHNOC 6PFA 6PHOS 6PBNP 6PCAL 6KA 6PTIMEN PSAC 6PTT 6RETCT 6RENAL WHRIG 6SAL SYPHB TBSATA TT3C T4C TESTOC THEOC
CHEM8
QUICK CHEM 8 CBC/AUTO DIFF CKMB
COMP CREATININE CREATININE X-RAY EVALUATION D-DIMER DIGOXIN DILANTIN ELECTROLYTES FERRITIN FSH GLUCOSE
仪器设备期间核查记录(安捷伦)
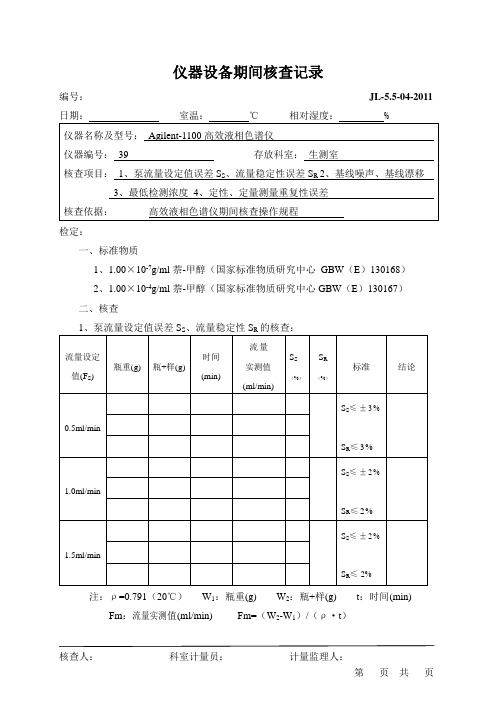
仪器设备期间核查记录编号:JL-5.5-04-2011检定:一、标准物质1、1.00×10-7g/ml萘-甲醇(国家标准物质研究中心GBW(E)130168)2、1.00×10-4g/ml萘-甲醇(国家标准物质研究中心GBW(E)130167)二、核查注:ρ=0.791(20℃)W1:瓶重(g) W2:瓶+样(g) t:时间(min) Fm:流量实测值(ml/min) Fm=(W2-W1)/(ρ·t)J L-5.5-04-2011S S=(Fm-Fs)/Fs×100% S R=(Fmax-Fmin)/F×100%2、基线漂移和基线噪声的核查:(图谱见第页)色谱柱:C18 流动相:甲醇流速:1 ml/min 波长:254nm①基线漂移= Au/h(规定:基线漂移≤5×10-3AU/h)规定②基线噪声= Au(规定:基线噪声≤5×10-4AU)规定3、最低检测浓度的核查:(图谱见第页)色谱条件同2项进样量:20ul(定量环)最低检测浓度:Cl=2·Nd·C/H== g/ml注:Cl:最小检测浓度Nd :基线噪声峰-峰高C:标准溶液浓度H:标准溶液色谱峰高(规定:Cl≤1×10-7g/ml)规定4、定性、定量测量重复性试验的核查:(图谱见第页)色谱条件同2项进样量:20ul(定量环)标准溶液的配制:精取标准物质萘-甲醇溶液(1.00×10-4g/ml)4ml,置10ml 量瓶中,加甲醇稀释至刻度,摇匀,即得4.00×10-5g/ml的标准溶液。
峰保留时间:RSD= %(规定:RSD≤1.5%)规定峰面积:RSD= %(规定:RSD≤1.5%)规定核查结论:按照《高效液相色谱仪期间核查操作规程》核查,结果规定。
采购技术参数要求须按顺序提供相应证明材料,未提供不得分

★7、检测性能:能够检测低生物量样本的微生物状态,并能够有效避免试剂耗材微生
物带来检测准确性干扰。
8、原始数据:可提供本检测所产生的原始数据(包括高通量原始下机数据Fastq文件)。
9、产品特性:单样本测序1,00,OOO条序列,InaPPingrate>70%;已知内膜炎致病菌条种,含量高于千分之一均可检出。
4、单细胞全基因组扩增方法具有授权专利,且应用成果在《Science》、《Ce11》、
(Ferti1SteriD等期刊;
★5>单细胞扩增技术性能:脱扣率低(V10%),覆盖度高(293%),均一性好(CV
≤0.25);
6、具有配套的胚胎植入前染色体非整倍体分析软件,II类医疗器械注册证;
7、检测实验室通过CAP认证、通过国家室间质评;
★5、对于新发型和家系样本不全的单基因病家系,有完善单体型预实验构建方案,需包括二代测序、芯片、三代测序技术平台;
6、检测实验室通过CAP认证、通过国家室间质评;
★7、能够提供PGT-M患者检测方案决策树;
8、提供遗传咨询服务
9、免费为受检者提供保险服务
10、对于检测后妊娠的患者,免费提供胎儿样本的验证方案。
7、平台适用性广:项目试剂盒可适用于i11umina、1ife、MG1和SNP芯片平台。
8、提供全程本地化服务方案:试剂、耗材、仪器、培训、生物信息学分析和遗传咨询
9、本地化生物信息学分析平台:对于该检测项目可提供配套的生物信息分析服务器,分析方法采用国家专利认可的拷贝数变异分析技术,自动化输入检测报告,无需专业生信人员即可完成整个检测项目;
