肾缺血再灌注模型细胞凋亡与吡咯烷二硫代氨基甲酸的干预
米诺环素对大鼠脑缺血再灌注后细胞凋亡的影响

米诺环素对大鼠脑缺血再灌注后细胞凋亡的影响摘要】目的:建立大鼠局灶性脑缺血再灌注模型,观察米诺环素对大鼠脑缺血再灌注后细胞凋亡的影响,为临床应用米诺环素提供实验室数据。
方法:采用大脑中动脉线栓闭塞法(线栓法)制备大鼠局灶性脑缺血再灌注模型,缺血120min后恢复再灌注24h。
36只健康成年雄性SD大鼠(250±10)g,随机分为假手术组(n=6)、缺血再灌注组(n=12)、米诺环素治疗组(n=12)。
采用TUNEL方法对缺血损伤的神经细胞DNA损伤情况进行观察,评价米诺环素的神经保护作用。
结论: 1、大鼠局灶性脑缺血再灌注模型(MCAO)能使脑组织细胞凋亡增加。
2、缺血再灌注组与米诺环素干预组细胞DNA损伤明显。
3、米诺环素对缺血半暗带、海马CA1区神经元凋亡有抑制作用,具有神经保护作用。
【关键词】米诺环素,缺血再灌注,凋亡急性脑梗死的发病率占脑卒中患者的50%~80%,其致残、致死率高,严重危害人民的身心健康。
脑缺血再灌注损伤是指脑组织经历一定时间的缺血后恢复血流供应,使缺血性损伤进一步加重的现象。
研究表明脑缺血-再灌注损伤后能启动脑细胞调亡,造成脑细胞不可逆的死亡,主动的细胞凋亡在缺血性尤其在缺血-再灌注损伤中发挥重要作用[1],直接针对凋亡过程的治疗方法,有助于保护半暗带区的神经细胞,对于脑缺血的治疗有着十分重要的意义。
米诺环素为第二代人工半合成四环素类抗生素,其抗菌谱与四环素相似。
米诺环素口服后迅速被吸收,脂溶性较高,组织穿透性好,可以通过血脑屏障作用于中枢神经系统。
近年表明,米诺环素不仅具有广谱的抗菌作用,且在脑缺血、脑外伤[2]、阿尔茨海默氏病、肌萎缩性(脊髓)侧索硬化和帕金森病模型上,均有显著的神经保护作用。
其中,在全脑和局灶性脑缺血模型上,发现米诺环素具有减少梗死面积等脑保护作用,治疗时间窗较传统的药物更长[3],所以该药既能抗感染又能保护神经,显示出具有很好的临床应用前途,但其抗凋亡作用机制尚没有完全阐明。
视网膜缺血再灌注损伤后凋亡相关基因表达的变化

视网膜缺血再灌注损伤后凋亡相关基因表达的变化作者:袁春燕牛膺筠张瑞丁玉枝【关键词】视网膜;再灌注损伤;基因,WTp53;基因,bax;基因,bcl-2;大鼠,Wistar[摘要]目的研究视网膜缺血再灌注损伤(RIRI)中凋亡相关基因野生型p53(WTp53)、bax和bcl-2表达的变化,探讨其作用机制。
方法将28只Wistar大鼠随机分为正常组、缺血组,其中缺血组又分为再灌注后1、6、12、24、48和72 h等6个组。
建立RIRI 动物模型,用免疫组织化学链霉亲和素-生物素-过氧化物酶复合物(SABC)法检测再灌注后不同时段视网膜组织中WTp53、bax和bcl-2基因表达的变化。
结果缺血组视网膜再灌注后WTp53、Bax蛋白表达增加,与正常组比较,再灌注6~72 h差异有显著意义( F=487.19、281.97,q=11.526~ 52.401 ,P <0.05)。
缺血组视网膜再灌注后Bcl-2蛋白表达无明显变化,与正常组比较,差异无显著性( P >0.05)。
结论缺血再灌注损伤引起WTp53、bax基因在视网膜神经节细胞层、神经纤维层与内核层表达增高;WTp53和bax可能通过诱导或促进神经节细胞凋亡参与了视网膜缺血再灌注损伤的发生。
[关键词]视网膜;再灌注损伤;基因,WTp53;基因,bax;基因,bcl-2;大鼠,Wistar[ABSTRACT]ObjectiveTo investigate the expression of WTp53,bax and bcl-2 in retina ischemia/reperfusion injury and explore the mechanism. MethodsTwenty-eight rats were randomly divided into normal (4 rats) and ischemia group, and the latter was subdivided into 1,6,12,24,48,and 72h after reperfusion,respectively. The rat model of experimental retina ischemia/reperfusion injury was made by increasing the intraocular pressure. The expression of WTp53, bax and bcl-2 was determined by streptavidin-biotin complex (SABC)immunohistochemistry. Results In ischemia group,WTp53 expression and bax expression increased significantly ( F=487.19,281.97;q=11.526-52.401; P <0.05),while the bcl-2 expression had little change. Conclu-sionWTp53 and bax expression increase in RIRI. WTp53 and bax may play a part in RIRI by promoting apoptosis.[KEY WORDS]retina; reperfusion injury; genes,WTp53; genes, bax; genes, bcl-2; rats, Wistar视网膜缺血再灌注损伤是眼科常见的病理过程,严重影响病人视功能。
缺氧-复氧肾小管上皮细胞损伤的信号传导机制

缺氧-复氧肾小管上皮细胞损伤的信号传导机制胡语航;王兴勇【摘要】目的探讨肾小管上皮细胞在缺氧-复氧(H-R)损伤后的信号传导机制.方法将不同方法处理的人近曲肾小管上皮细胞(HK-2)分为对照组、模型组和PDTC组,模型组和PDTC组再分别分成缺氧6、12、24 h组、缺氧24 h后分别复氧6、12、24 h组;检测细胞成活率、NF-κB p65蛋白表达、细胞间黏附分子-1(ICAM-1)mRNA及蛋白表达情况.结果与对照组比较,模型组随缺氧时间延长细胞数量逐渐减少,24 h达高峰(P<0.05),复氧后细胞数量略有增加;随缺氧时间延长NF-κB p65阳性蛋白表达增多,复氧6h组达高峰(P<0.05);ICAM-1 mRNA及蛋白表达随H-R时间延长渐增高(P<0.05).PDTC组中NF-κBp65蛋白和ICAM-1 mRNA及蛋白表达较模型组有明显下降(P<0.05).结论 H-R诱导HK-2细胞中NF-κcB p65的活化,从而上调ICAM-1 mRNA及蛋白表达.【期刊名称】《重庆医学》【年(卷),期】2016(045)028【总页数】3页(P3962-3964)【关键词】HK-2;缺氧/复氧;核因子-κB;细胞间黏附分子-1【作者】胡语航;王兴勇【作者单位】四川省妇幼保健院重症医学科,成都610045;重庆医科大学附属儿童医院 400014【正文语种】中文【中图分类】R459.7[摘要] 目的探讨肾小管上皮细胞在缺氧-复氧(H-R)损伤后的信号传导机制。
方法将不同方法处理的人近曲肾小管上皮细胞(HK-2)分为对照组、模型组和PDTC组,模型组和PDTC组再分别分成缺氧6、12、24 h组、缺氧24 h后分别复氧6、12、24 h组;检测细胞成活率、NF-κB p65蛋白表达、细胞间黏附分子-1(ICAM-1)mRNA及蛋白表达情况。
结果与对照组比较,模型组随缺氧时间延长细胞数量逐渐减少,24 h达高峰(P<0.05),复氧后细胞数量略有增加;随缺氧时间延长NF-κB p65阳性蛋白表达增多,复氧6 h组达高峰(P<0.05); ICAM-1 mRNA及蛋白表达随H-R时间延长渐增高(P<0.05)。
中药抗肾缺血再灌注损伤的研究进展_倪文娟

之弊;广木香行气止痛、健脾和胃,枳壳行气和中、散瘀消积,二药配伍以固护中焦胃气,使气血化生有源;三七活血止血、化瘀止痛,以防君臣活血之力太过;桂枝温阳通脉、鼓舞气血生长,使瘀滞去而气血能得以速生,上四药共为佐药;甘草健脾益气,缓急止痛以为使。
正与原发性痛经的病机相符〔8〕。
同时配合按摩疗法对痛经有巯通经络、行气活血、祛邪通阳、镇静止痛的作用。
现代药理研究证明:活血祛瘀药有明显的抑制前列腺素PGF2a的活性及解痉作用,能增加血液灌流量,改善微循环;当归含挥发油,其挥发油能抑制子宫肌收缩而使子宫松驰〔9〕。
故诸药配合从而达到治愈本病的目的。
临床实践表明,综合疗法治疗原发性痛经疗效显著,值得临床推广和应用。
参考文献〔1〕张惜阴.实用妇产科学〔M〕.第二版.北京:人民卫生出版社,2004,833-834.〔2〕马宝璋.中医妇科学〔M〕.北京:中国中医药出版社,2004,112.〔3〕安雅婷.按摩治疗痛经126疗效观察〔J〕.按摩与导引,2005,21(11):38-39.〔4〕欧阳智鸿.手足反射区推拿治疗痛经临床观察〔J〕.按摩与导引,1998,14(6):37.〔5〕国家中医药管理局.中医病证诊断疗效标准〔S〕.南京:南京大学卫生出版社,1994,61-62.〔6〕丰有吉.妇产科学〔M〕.第二版.北京:人民卫生出版社,2011,249.〔7〕马宝璋.中医妇科学〔M〕.上海:上海科学技术出版社社,1997,79-84.〔8〕齐律丽.辩证治疗原发性痛经60例〔J〕.辽宁中医杂志,2006,14(03):1621.〔9〕杜嫦燕.失笑散加减治疗原发性痛经68例临床观察〔J〕.中国民族民间·医药杂志,2003,42(2):99.中药抗肾缺血再灌注损伤的研究进展倪文娟(华东医药宁波有限公司宁波315041)摘要:根据造成肾缺血再灌注的两个主要因素—活性氧氧化损伤和炎症级联反应,综述了近年来中药在防治肾缺血再灌注损伤方面的研究进展。
甘草酸可改善肾缺血再灌注后HMGB1介导的细胞死亡和炎症

文献解读报告(2014-10-31)题目Glycyrrhizic acid ameliorates HMGB1-mediated cell death and inflammation after renal ischemia reperfusion injury甘草酸可改善肾缺血再灌注后HMGB1介导的细胞死亡和炎症出处Lau A, et al. Am J Nephrol. 2014;40(1):84-95. doi: 10.1159/000364908. Epub 2014 Jul 18.重点内容:肾脏缺血再灌注损伤(IRI)会导致急性肾损伤(AKI)和肾小管上皮细胞(TEC)坏死。
死亡细胞中释放出的高迁移率族1蛋白(HMGB1)可能诱发器官功能障碍和炎症。
甘草酸(GZA)具有抑制HMGB1功能的作用,但它减弱HMGB1介导TEC 损伤的能力尚未经过实验测试。
在体外,缺氧环境和细胞因子治疗可以杀死TEC并导致HMGB1逐步释放至上清液中,GZA可减少缺氧条件导致的TEC死亡,也可剂量依赖性的降低缺氧条件下TEC中MCP-1和CXCL1的表达量。
同样,GZA可抑制NK细胞相关的HMGB1活化。
肾脏的HMGB1蛋白表达量于肾缺血后4-24h逐渐增加,通过组织学分析和二聚体乙锭染色可检测到GZA可保护IRI后的肾功能并减少肾小管坏死和嗜中性粒细胞浸润。
结论:这些数据第一次证实AKI伴随缺氧和肾脏IRI可促进释放HMGB1,降低TEC幸存和炎症增强。
而通过GZA抑制HMGB1和TEC的相互作用可能作为一个新的治疗策略用于减轻肾脏IRI和移植后的肾损伤。
E-Mail karger@O riginal Report: Laboratory Investigation A m J Nephrol 2014;40:84–95D OI:10.1159/000364908 Glycyrrhizic Acid Ameliorates HMGB1-Mediated Cell Death and Inflammation after Renal Ischemia Reperfusion InjuryA rthur Lau a, c Shuang Wang b, d Weihua Liu a Aaron Haig c Zhu-Xu Zhang a–d Anthony M. Jevnikar a–daM atthew Mailing Centre for Translational Transplant Studies, London Health Sciences Centre, Departments of b M edicine and c P athology, University of Western Ontario, and d L awson Health Research Institute, L ondon,Ont.,Canadaafter IRI and reduced tubular necrosis and neutrophil infiltra-tion by histological analyses and ethidium homodimer stain-ing. C onclusions: Importantly, these data demonstrate for the first time that AKI following hypoxia and renal IRI may be promoted by HMGB1 release, which can reduce the survival of TEC and augment inflammation. Inhibition of the interac-tion of HMGB1 with TEC through GZA may represent a ther-apeutic strategy for the attenuation of renal injury following IRI and transplantation.© 2014 S. Karger AG, Basel I ntroducti on Ischemia reperfusion injury (IRI) occurs invariably in kidney transplantation and contributes to graft dysfunc-tion and rejection in recipients [1–3] . The initial ischemic insult induces widespread death of kidney parenchymal cells and in particular tubular epithelial cells (TEC) [4–8],which results in organ dysfunction and the release of damage-associated molecular pattern (DAMP) proteinsinto the extracellular space[9–13] . High-mobility group K ey WordsG lycyrrhizic acid · HMGB1 · Inflammation · Renal ischemia reperfusion injuryA bstractB ackground: Renal ischemia reperfusion injury (IRI) leads to acute kidney injury (AKI) and the death of tubular epithelial cells (TEC). The release of high-mobility group box-1 (HMGB1) and other damage-associated molecular pattern moieties from dying cells may promote organ dysfunction and in-flammation by effects on TEC. Glycyrrhizic acid (GZA) is a functional inhibitor of HMGB1, but its ability to attenuate the HMGB1-mediated injury of TEC has not been tested. M eth-ods/Results: In vitro, hypoxia and cytokine treatment killed TEC and resulted in the progressive release of HMGB1 into the supernatant. GZA reduced the hypoxia-induced TEC death as measured by annexin-V and propidium iodide. Hy-poxia increased the expression of MCP-1 and CXCL1 in TEC, which was reduced by GZA in a dose-dependent manner. Similarly, the HMGB1 activation of effector NK cells was in-hibited by GZA. To test the effect of HMGB1 neutralization by GZA in vivo, mice were subjected to renal IRI. HMGB1 pro-tein expression increased progressively in kidneys from 4 to 24 h after ischemia and was detected in tubular cells by 4 h using immunohistochemistry. GZA preserved renal functionReceived: February 5, 2014A ccepted: May 24, 2014 P ublished online: J uly 18, 2014NephrologyAmerican Journal ofAnthony M. JevnikarD epartment of Medicine, University of Western OntarioA 10-112, 339 Windermere RoadL ondon, ON N6A 5A5 (Canada) ©2014 S. Karger AG, Basel 0250–8095 /14/0401–0084$39.50/0Zhu-Xu Zhang and Anthony M. Jevnikar contributed equally to this report.d e d b y : T o r o n t o L i b r .190.39 - 10/9/2014 1:30:45 P MG ZA Blocks HMGB1 Effects during Renal IRI Am J Nephrol 2014;40:84–95DOI: 10.1159/00036490885box-1 (HMGB1) and other DAMP moieties may further contribute to proinflammatory injury [13–16].However, their effects on the survival or proinflammatory functions of TEC remain unknown and could alter allograft sur-vival.H MGB1 is a ubiquitous nuclear protein that is highly conserved throughout many species. Physiologically, it binds to DNA within the nucleus and is involved in es-sential processes such as DNA replication and transcrip-tion [17] . HMGB1 has previously been identified as a DAMP molecule in different injury models in the liver [18] , lungs [19] , and heart [20] . Furthermore, previous studies in acute injury models have suggested that HMGB1 is not only released passively following cell death [21–23] but may be actively secreted [24, 25] by some cell types even while viable. The proinflammatory nature of HMGB1 is related to an increased expression of chemo-kines and cytokines that attract and activate diverse im-mune cells. This inflammatory response is typically medi-ated through Toll-like receptors (TLR), which have been implicated in having a major role in propagating tissue injury and inflammation [26–30] . TLR signaling follow-ing the binding of DAMP ligands such as HMGB1 [31] results in the recruitment of various adapter proteins (MyD88), leading to the activation of proinflammatory mediators such as TNF-α, IL-6, CCL2, CXCL8, and C X3CL1[32, 33] .A lthough targeting of HMGB1 has been suggested to limit acute renal injury, studies have been limited by the lack of a clear understanding of effects on renal parenchy-mal cells as well as clinically feasible reagents other than neutralizing antibodies [34, 35] . Glycyrrhizic acid (GZA), a functional inhibitor of HMGB1, has been tested clini-cally in patients with hepatitis C [36] and appears to ame-liorate both liver and kidney injury [37–40] . It is plausible that GZA may have the potential to reduce organ storage injury and IRI following kidney transplantation as well as diminishing inflammation due to immune reaction.I n the present study, we tested the effect of HMGB1 released from injured cells on TEC survival and function and whether GZA altered the effects of HMGB1 on kid-ney cells with IRI in vitro and in vivo. We have demon-strated that GZA can inhibit TEC death by blocking HMGB1, which may directly contribute to kidney injury in vivo as well as indirectly by the production of proin-flammatory molecules such as monocyte chemotactic protein-1 (MCP-1), CXCL1, and IL-6. Importantly, GZA neutralization blocks deleterious effects of HMGB1 on kidney cells, suggesting it may be useful to attenuate IRI and other forms of inflammatory kidney injury.M ateri als and MethodsA nimalsC57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, Maine, USA) and maintained in the animal facility at the University of Western Ontario using approved protocols and pro-cedures.C ell CulturesT he TEC line NG1.1 was developed from proximal TEC ofC57BL/6J mice by SV40 transformation as previously described[4] . NK cells were purified from C57BL/6J mouse spleens usinganti-CD49a MACS bead selection (Miltenyi Biotec) and were grown in the presence of IL-2 (1,000 IU/ml) in RPMI-1640. Thepurity of the NK cells was confirmed by flow cytometry, and >90%of the cells were CD3–/CD49b+ for each experiment. The NK cellswere treated with endotoxin-free recombinant HMGB1 (R&D Systems).K idney IRIR enal IRI was induced as previously described [8] . Briefly, arenal clamp was applied to the right kidney pedicle and removedafter 45 min while the left kidney was nephrectomized. Serum was collected at 48 h after IRI for creatinine detection by the Jaffe reac-tion method using an automated CX5 clinic analyzer (Beckman).G ZA (Sigma) was reconstituted in a minimum volume of DMSO, diluted with saline, and injected 2 h preoperatively and postoperatively at 8 h and 24 h into mice with IRI (1 mg GZA, <1% DMSO). Control mice were subjected to IRI and equivalent dosesof DMSO as a vehicle control.H ypoxia TreatmentT EC were made hypoxic with deoxygenated serum and glu-cose-free media in a hypoxia chamber for 20 min. The oxygen inthe chamber was displaced by a gas mixture of 3% H 2, 5% CO 2,anda balance of N 2(Praxair) at a rate of 0.1 l/min. The cells were col-lected at various time points for fluorescence-activated cell sorter analysis of apoptosis and necrosis by annexin-V and propidium iodide (PI; BD Bioscience), respectively.I mmunoblotting and Real-Time PCRP rotein was isolated from tissue and cells using a nonnuclear protein lysis buffer that excluded nuclear proteins. Protein from supernatant was concentrated by centrifugation (Millipore). Membranes were probed with anti-HMGB1 (Abcam) or mouseanti-β-actin (Sigma).T otal RNA was extracted from tissue and cells by TRIzol (Invi-trogen) as described by the manufacturer. cDNA was generatedfrom RNA using Superscript II (Invitrogen) as described by the manufacturer. It was quantified by real-time PCR using SYBR Green (Bio-Rad) as described by the manufacturer. The primers (Invitrogen) used for Q-PCR include the following: HMGB15 ′-TAAAAAGCCGAGAGGCAAAA-3′,5′-GCAGACATGGTC TTCCACGT-3 ′; MCP-1 5 ′-AGCACCAGCCAACTCTCACT-3′,5 ′-CGTTAACTGCATCTGGCTGA-3′; RANTES 5 ′-ATATGGC TCGGACACCACTC-3 ′,5′-TCCTTCGAGTGACAAACACG-3′;CXCL1 5 ′-AGACTGCTCTGATGGCACCT-3′,5′-TGCACTTC TTTTCGCACAAC-3 ′; IL-6 5 ′-GAGGATACCACTCCCAACA GACC-3 ′,5′-AAGTGCATCATCGTTGTTCATACA-3′;IFN-γ5 ′-CATTGAAAGCCTAGAAAGTCTGA-3′,5′-TAGCGATGCAdedby:TorontoLibr.19.39-1/9/2141:3:45PML au/Wang/Liu/Haig/Zhang/Jevnikar Am J Nephrol 2014;40:84–95DOI: 10.1159/00036490886AATGCTTGATATC-3 ′ ; perforin 5 ′-GAAGACCTATCAG GAC CAGTACAACTT-3 ′,5′-CAAGGTGGAGTGGAGGTTTTTG-3′,and granzyme B 5 ′-CGATCAAGGATCAGCAGCC-3′,5′-CTG GGTCTTCTCCTGTTCT-3 ′ . β-Actin was used as the endogenous control. The normalized delta threshold cycle value and relativeexpression levels (2 –ΔΔCt) were calculated according to the manu-facturer’s protocol.H istology and Immunoh istoch emistryT issue sections were H&E stained and scored by a pathologist in a blinded fashion using an injury scoring method as previously described [7] . The criteria for kidney injury include tubular necro-sis, immune cell infiltration, lumen casts, and glomerular cell ne-crosis. Immunohistochemistry was performed using anti-HMGB1 (Abcam). To visualize and quantify kidney tissue necrosis in vivo,frozen tissue sections were scored from mice undergoing renal ar-tery infusion of ethidium homodimer (Invitrogen) as previouslydescribed [41] . Briefly, 5 μmol/l ethidium homodimer was injected at 1 ml/min for 10 min into the renal artery through the aorta and then flushed with perfusion buffer at 1 ml/min for 5 min. The sec-tions were analyzed and quantified using a fluorescent microscope and an automated image analysis program (Nikon) measuring the area and fluorescent intensity.S tatistical AnalysisD ata were compared using Student’s t test for unpaired values and one-way ANOVA for multiple comparisons. The data are pre-sented as means ± SEM, and p < 0.05 was considered to be signifi-cantly different.R esults HMGB1 Protein Expression Is Upregulated during Renal IRIR enal IRI causes severe tissue injury and various forms of cell death[4–8] including apoptosis, necrosis, autophagy, and other nonclassical forms of cell death[42] . D uring necrotic cell death, cells invariably lose membrane integrity and eventually lyse, resulting in the release of intracellular contents and various DAMP such as HMGB1.T o first demonstrate the kinetics of HMGB1 release in renal IRI, we tested mRNA and nonnuclear protein levels in kidneys for up to 24 h following IRI. As shown in f igure 1 a and b, nonnuclear HMGB1 protein increased in the kidney progressively for up to 24 h of reperfusion after ischemia (control density ratio: 0.953 ± 0.707; 24 h after IRI: 5.368 ± 0.239). In contrast, mRNA expression of HMGB1 was decreased after ischemia as compared with controls (sham: 1 ± 0; 0 h after ischemia: 0.17 ± 0.04) and remained at low levels for up to 24 h of reperfusion, as shown in f igure 1 c . Together, these results indicate that the presence of HMGB1 protein outside the nucleus in-creased over the course of renal IRI but was not due to increased transcription.A s expected, mice with IRI demonstrated a decreased kidney function, as indicated by an increase in serum cre-atinine 24 h following reperfusion ( f ig. 1 d ). HMGB1 ex-pression analyses by immunohistochemistry showed that HMGB1 expression was detected in tubules as early as4 h after IRI ( f ig. 1 e ). While TEC expressing HMGB1 ap-pear to be located in cortical areas, these data do not dis-tinguish their identity as proximal or distal tubules. How-ever, in vitro results using previously characterized NG TEC suggest that proximal tubular cells are likely to be a prominent source of HMGB1 in vivo.G ZA Neutralization of HMGB1 Released from Hypoxic TEC Can Inhibit Cell DeathI t has previously been reported that cells release HMGB1 during necrotic cell death[13, 22] . As hypoxia similarly results in TEC death, we tested their capacity to release HMGB1 after hypoxia. As shown in f igure 2 a , TEC cultures underwent increasingly higher levels of cell death and were primarily and maximally annexin-V/PI positive 24 h after hypoxia (7.5 vs. 30.6%). In addition, the percentage of viable cells (annexin-V/PI negative) had decreased by 24 h after hypoxia (75.7 vs. 29.7%).N ext, we tested whether HMGB1 was released from TEC after hypoxic cell death. The analysis of supernatant from TEC following hypoxia over a 24-hour period ( f ig. 2 b , c) clearly demonstrated that HMGB1 was re-leased from killed and remaining TEC at detectable levels immediately after hypoxia treatment (0.574 with no treat-ment vs. 8.876 24 h after hypoxia). In addition, it was also observed that the lysate fraction containing protein from both the nuclear and the cytoplasmic compartment of re-maining adherent TEC did not similarly show increased levels of HMGB1.A s supernatant from hypoxia-treated cells contains a complex number of mediators that could affect cell death or viability, we attempted to clarify the role of HMGB1 by the addition of GZA, which specifically in-hibits HMGB1 [40] . As shown in f igure 2 d and e, the vi-ability of TEC was reduced from 81.00 ± 1.87% (annex-in-V/PI negative) to 41.73 ± 7.26% with hypoxia (p = 0.003). The addition of 1,000 ng/ml of GZA to TEC dur-ing hypoxia modestly increased the cell viability from 41.73 ± 7.26 to 58.03 ± 7.39% (p = 0.04). Although many TEC-expressed mediators in the conditioned media have an effect on cell viability, these data suggest HMGB1 has a role in cell death that might be inhibited by GZA in vivo.d e d b y : T o r o n t o L i b r .190.39 - 10/9/2014 1:30:45 P MG ZA Blocks HMGB1 Effects during Renal IRIAm J Nephrol 2014;40:84–95DOI: 10.1159/00036490887F ig. 1. Characterization of HMGB1 expression in the kidney after renal IRI. C57BL/6 mice were subjected to acute ischemia for 45 min using a renal clamp at 32 ° C . The sham mice did not have a renal clamp applied. Reperfusion injury occurred over a 24-hour period, during which the mice were sacrificed at various timepoints.a ,b Nonnuclear kidney protein was isolated and analyzed by immunoblotting using anti-HMGB1 (representative of 3 inde-pendent experiments). The relative protein concentration was de-termined by semiquantitative densitometry and normalized byβ-actin ( * p < 0.01; n = 3/group).c mRNA expression of HMGB1 after renal IRI was measured by real-time PCR. The fold change in mRNA expression was normalized by β-actin (n = 3/group). d Kidney function was determined by serum creatinine in naive and IRI mice at 24 h ( * * * p < 0.001; n = 4–5/group). e Kidney sec-tions were analyzed for HMGB1 by immunohistochemistry (n = 3/group). Arrows: tubules positive for HMGB1. ×200.Sham0 148+RXUV RI UHSHUIXVLRQ DIWHU LVFKHPLDd e d b y : T o r o n t o L i b r .190.39 - 10/9/2014 1:30:45 P ML au/Wang/Liu/Haig/Zhang/Jevnikar Am J Nephrol 2014;40:84–95DOI: 10.1159/00036490888101102103100100101102103A3A4Annexin-VA10.2%7.5%75.7%16.6%A2P INo treatment101102103100100101102103A3A4Annexin-VA10.7%11.6%73.3%14.5%A2P I0 h after hypoxia101102103100100101102103A3A4Annexin-VA10.3%12.5%66.4%20.8%A2P I3 h after hypoxia101102103100100101102103A3A4Annexin-VA11.4%15.3%67.4%15.9%A2P I6 h after hypoxia101102103100100101102103A3A4Annexin-VA12.4%14.2%79.2% 4.3%A2P I12 h after hypoxia101102103100100101102103D3D4Annexin-VD136.4%30.6%29.7%3.3%D2P I24 h after hypoxiaaC o l o r v e r s i o n a v a i l a b l e o nF ig. 2. GZA neutralization of HMGB1 re-leased from hypoxic TEC can inhibit celldeath.a TEC were subjected to hypoxia, and cell death was measured by annexin-V/PI for apoptosis and necrosis, respectively, at various time points (representative of 3independent experiments).b ,c Total cell lysate and supernatants were collected from TEC, and HMGB1 was detected by immu-noblotting. The relative protein concentra-tions were determined by semiquantitative densitometry and normalized by β-actin ( * p < 0.05; n = 3/group). CL = Cell lysate; S = supernatant.(For figure 2d, e see next page.)3624+RXUV DIWHU K\SR[LDCL S*d e d b y : T o r o n t o L i b r .190.39 - 10/9/2014 1:30:45 P MG ZA Blocks HMGB1 Effects during Renal IRIAm J Nephrol 2014;40:84–95DOI: 10.1159/0003649088910010110210A3A4Annexin-VA10.0%0.1%89.1%10.8%A210010110210A3A4Annexin-VA10.7%17.7%34.2%47.3%A210010110210B3B4Annexin-VB12.8%8.0%59.2%30.1%B2(For figure see next page.)F ig. 3. The increased TEC expression of proinflammatory cyto-kines and NK cell activation are inhibited by GZA.a –c C57BL/6mice were subjected to renal IRI. Total renal mRNA was analyzedfor MCP-1 (a ), CXCL1 (b ), and RANTES (c ) expression by real-time PCR ( *p <0.05,* * * p < 0.001; n = 3/group).d ,e TEC were subjected to hypoxia and treated with various concentrations of GZA. Total mRNA was analyzed at 24 h for MCP-1 ( d ) and CXCL1 (e ) expression by real-time PCR ( *p <0.05,* *p <0.01,* * *p <0.001; n = 3–4/group).f ,g Total mRNA expression of IL-6 wasanalyzed in C57BL/6 mouse kidneys subjected to renal IRI (f ) or in treated TEC 24 h after hypoxia with 800 ng/ml of GZA (g ; ** p < 0.01; n = 3/group). h NK cells were treated with 1,000 ng/ml of rHMGB1 and 1,000 ng/ml of GZA for 24 h. Total mRNA from the NK cells was analyzed for IFN-γ, perforin, and granzyme B by real-time PCR (* p < 0.05; n = 3/group).F ig. 2. GZA neutralization of HMGB1 released from hypoxic TEC can inhibit cell death. d , e TEC were treated with hypoxia and various concentrations of GZA, and cell death was measured at 24 h using annexin-V/PI. Viable TEC were negative for annexin-V/PI labeling (* p <0.05,* * p < 0.01; n = 4/group).d e d b y : T o r o n t o L i b r .190.39 - 10/9/2014 1:30:45 P ML au/Wang/Liu/Haig/Zhang/Jevnikar Am J Nephrol 2014;40:84–95DOI: 10.1159/000364908903d e d b y : T o r o n t o L i b r .190.39 - 10/9/2014 1:30:45 P MG ZA Blocks HMGB1 Effects during Renal IRI Am J Nephrol 2014;40:84–95DOI: 10.1159/00036490891I ncreased TEC Expression of ProinflammatoryCytokines and NK Cell Activation Are Inhibited byGZAR enal IRI is associated with an upregulation of local proinflammatory mediators within the kidney, which promotes further injury from the influx of effector cells including neutrophils, T cells, and NK cells [43] . The re-lease of HMGB1 into the microenvironment with the subsequent activation of several key TLR pathways likely accounts for much of the upregulation of proinflamma-tory molecules. It has been demonstrated that GZA can induce an anti-inflammatory effect in an ischemic spinal cord injury model with downregulation of cytokines and chemokines [44] . Therefore, we tested whether inflam-mation could similarly be ameliorated by neutralizing HMGB1 through the use of GZA.W e first confirmed that inflammatory chemokine mRNA expression increased in the total kidney after IRI. As shown in f igure 3 a and b, there was an upregulation of MCP-1 (naive: 1 ± 0; 48 h after IRI: 5.13 ± 1.39; p = 0.03; n = 3) and CXCL1 (naive: 1 ± 0; 48 h after IRI: 5.22 ± 1.03; p = 0.04; n = 3) peaking by 8 h but persisting for up to 48 h after reperfusion. In addition, the proinflammatory cy-tokine IL-6 was upregulated in the kidney after renal IRI (naive: 1 ± 0; 48 h after IRI: 20.17 ± 5.48; p = 0.04; n = 3), as shown in f igure 3 f. In contrast, RANTES mRNA ex-pression ( f ig. 3 c) peaked by 24 h but was downregulated by 48 h after IRI (naive: 1 ± 0; 48 h after IRI: 0.30 ± 0.05; p = 0.0004; n = 3). Other chemokines tested included MIP-1α and CX3CL1, but they did not demonstrate any increased expression after renal IRI (data not shown). These data confirmed that there is an increase in the pro-duction of proinflammatory molecules during IRI.T o determine whether the increased chemokine ex-pression observed during renal IRI might involve TEC, total mRNA from TEC 24 h after hypoxia were tested for MCP-1, CXCL1, and IL-6 mRNA expression. As shown in f igure 3 d and e, there was an increase in both MCP-1 (no treatment: 1 ± 0; 24 h after hypoxia: 9.32 ± 2.85) and CXCL1 (no treatment: 1 ± 0; 24 h after hypoxia: 10.22 ± 0.52) 24 h after hypoxia. RANTES mRNA expression was unchanged after hypoxia treatment (data not shown). However, when GZA was added to TEC cultures during hypoxia, it had a dose-dependent inhibitory effect (as de-termined by one-way ANOVA) on MCP-1 [F(5, 11) = 4.13; p = 0.02] and CXCL1 [F(4, 10) = 12.83; p = 0.001] expression, which resulted in a return to untreated con-trol levels. TEC expression of proinflammatory IL-6 mRNA was clearly inhibited by the addition of GZA fol-lowing hypoxia [12.61 ± 2.7 vs. 0.71 ± 0.22; F(2, 6) = 18.88; p = 0.002], as shown in f igure 3 g. Given that HMGB1 neutralization by GZA reduces proinflammatory chemo-kine and cytokine expression in TEC, GZA may poten-tially ameliorate inflammation during renal IRI.W e have recently demonstrated the significance of theNK cell contribution to TEC injury as a result of inflamma-tion during renal IRI [8] . As NK cells can be activated by HMGB1, we attempted to elucidate the effects of HMGB1 inhibition by GZA on NK cell activation. As shown in f ig-ure 3 h, the addition of HMGB1 activated the NK cells and resulted in an upregulation of IFN-γ, perforin, and gran-zyme B mRNA. In the presence of GZA, the NK cell activa-tion by HMGB1 was inhibited and led to the downregula-tion of IFN-γ (2.9 ± 0.5 vs. 0.8 ± 0.1; p = 0.03), perforin (2.2 ± 0.4 vs. 0.3 ± 0.1; p = 0.02), and granzyme B mRNA (2.1 ± 0.6 vs. 0.5 ± 0.4; p = 0.04). These data suggest thatGZA may prevent the HMGB1-mediated activation of NKcells and thereby reduce TEC injury during renal IRI.H MGB1 Inhibition by GZA Improves Renal Functionafter Kidney IRIB ased on the anti-inflammatory and survival-induc-ing effects of GZA on TEC in vitro, we tested the capacityof GZA to inhibit HMGB1 in vivo and its potential to at-tenuate renal dysfunction after IRI. Mice were subjectedto IRI with or without GZA pretreatment, and renal func-tion was assessed at 48 h. As shown in f igure 4 a, mice withrenal IRI had markedly elevated serum creatinine levelsas compared with mice with renal IRI plus GZA treat-ment (48 h after IRI: 120 ± 35 μmol/l; 48 h after IRI plus GZA: 31.4 ± 5; p = 0.03). Consistent with renal function( f ig. 4 c), the blinded injury scores were higher in the micewith renal IRI than in those additionally treated withGZA (48 h after IRI: 2.8 ± 0.45; 48 h after IRI plus GZA:1 ± 0; p = 0.0004). In particular, more neutrophil infiltra-tion was observed (indicated by arrows) in the controlmice than in the GZA-treated mice ( f ig. 4 b). Unlike the evaluation of apoptosis, which can be quantified in tissuesby TUNEL and other methods, the quantitative assess-ment of necrosis in tissue to date has been difficult to quantitate, relying on histological patterns and electron micrographs. To address this, we have modified a methodthat quantifies the release of an easily measured fluoro-chrome (ethidium homodimer) from intact cells follow-ing organ perfusion to measure tissue necrosis [41].Con-sistent with histology and functional data, we observed greater necrosis in the mice with renal IRI than in those additionally treated with GZA, as shown in f igure 4 dand e (necrosis score 48 h after IRI: 27.54 ± 5.94; 48 h af-ter IRI plus GZA: 12.04 ± 5.21; p = 0.04). Collectively,dedby:TorontoLibr.19.39-1/9/2141:3:45PML au/Wang/Liu/Haig/Zhang/Jevnikar Am J Nephrol 2014;40:84–95DOI: 10.1159/00036490892these in vitro and in vivo data demonstrate that GZA treatment can improve cell viability and reduce renal IRI. These findings suggest that the deleterious effects of IRI can be mediated by HMGB1 released from dying cells and that GZA may neutralize HMGB1 therapeutically.D iscussionD espite our considerable knowledge of the adaptive immune system and the effectiveness of current immu-nosuppressive therapies (directed against T and B cells), kidney allografts have a limited survival. It has been sug-gested that innate immunity by its ability to promote in-flammation through DAMP during IRI may have a large but perhaps underappreciated role in limiting allograft survival. TEC, which represent the majority cell type of the renal parenchyma, are particularly sensitive to isch-emia, inflammation, and acute kidney injury (AKI). Dur-ing renal IRI, cells undergo prominent forms of cell death,namely apoptosis and necrosis. As a result of cell death,DAMP are released to the extracellular compartment, al-lowing for interaction with TLR which can be found on a variety of cell types including renal parenchyma. The rec-510152025303540N e c r o s i s s c o r eeControl *GZAF ig. 4. GZA can improve renal function and prevent tissue necro-sis during renal IRI. C57BL/6 mice were subjected to renal IRI. GZA was injected intraperitoneally before and after ischemia. Control mice were injected with only DMSO vehicle. a Renal func-tion was determined by serum creatinine at 48h (* p < 0.05; n = 5/group). b ,c Kidney tissue was collected 48 h after ischemia and stained by H&E. Arrows: areas of neutrophil infiltration. Sections were scored for injury by an unbiased blinded pathologist ( * * *p <0.001; n = 5/group). ×100. d ,e Kidneys were perfused with ethid-ium homodimer 48 h after IRI. Sections were analyzed by fluores-cent microscopy and scored by automated software analysis (* p < 0.05;n =5/group).×40.Renal IRIRenal IRI + GZAd e d b y : T o r o n t o L i b r .190.39 - 10/9/2014 1:30:45 P M。
大鼠肢体缺血再灌注后骨骼肌细胞凋亡及其BQ123的保护作用

大鼠肢体缺血再灌注后骨骼肌细胞凋亡及其BQ123的保护作用刘燕;张连元【摘要】目的观察大鼠肢体缺血再灌注(LIR)后骨骼肌细胞凋亡情况,探讨内皮素A(ETA)受体拮抗剂BQ123对其的保护机制.方法雄性Wistar大鼠随机分为3组(n=8):对照组、肢体缺血再灌注组和选择性ETA受体阻断剂BQ123处理组,缺血4 h再灌注4 h后检测血浆一氧化氮(NO)、内皮素(ET-1)、血栓素B2(TXB2)、前列环素(6-KetoPGF1.)的含量以及NO/ET-1比值、TXB2/6-Keto-PGF 1α比值的变化;采用免疫组织化学方法检测腓肠肌组织中凋亡相关基因Bcl-2、Bax和凋亡蛋白酶(Caspase-3)的蛋白表达情况,利用原位脱氧核糖核甘酸末端转移酶介导的缺口末端标记法(TUNEL)检测各组腓肠肌细胞的凋亡情况.结果再灌注组与对照组相比较,血浆NO、ET-1、TXB2、6-Keto-PGF1α含量均增加,但TXB2/6-Keto-PGF1α比值增大,NO/ET-1比值减小,凋亡指数(AI)明显增高,Bax、Bcl-2、Caspase-3蛋白表达明显较对照组增强,但Bcl-2/Bax比值减小;与再灌注组比较,BQ123处理组血浆NO、6-KetoPGF1α含量升高,ET-1、TXB2下降,NO/ET-1比值升高,TXB2/6-Keto-PGF1α比值下降;腓肠肌组织Bcl-2/Bax比值升高,Caspase-3表达水平明显减弱,AI下降.结论 ETA受体拮抗剂BQ123可减轻大鼠肢体缺血再灌注后骨骼肌细胞凋亡.【期刊名称】《西安交通大学学报(医学版)》【年(卷),期】2011(032)004【总页数】3页(P414-416)【关键词】再灌注损伤;骨骼肌;内皮缩血管肽1;凋亡【作者】刘燕;张连元【作者单位】河北联合大学基础医学院机能实验室,河北唐山063000;河北联合大学基础医学院机能实验室,河北唐山063000【正文语种】中文【中图分类】R363肢体缺血再灌注(limb ischemia reperfusion,LIR)损伤是临床上常见的病理过程。
丁苯酞通过下调NF-κB_信号通路抑制细胞焦亡减轻大鼠肾缺血-_再灌注损伤
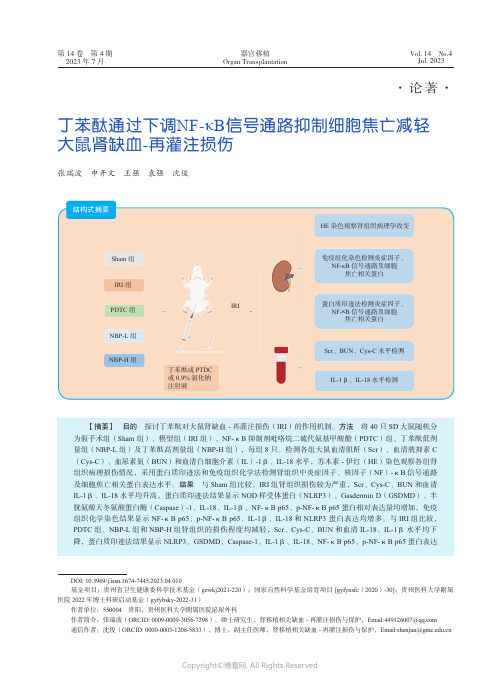
第14卷 第4期2023年7月Vol. 14 No.4Jul. 2023器官移植Organ Transplantation ·论著·丁苯酞通过下调NF-κB 信号通路抑制细胞焦亡减轻大鼠肾缺血-再灌注损伤张瑞波 申开文 王强 袁强 沈俊【摘要】 目的 探讨丁苯酞对大鼠肾缺血-再灌注损伤(IRI )的作用机制。
方法 将40只SD 大鼠随机分为假手术组(Sham 组)、模型组(IRI 组)、NF-κB 抑制剂吡咯烷二硫代氨基甲酸酯(PDTC )组、丁苯酞低剂量组(NBP-L 组)及丁苯酞高剂量组(NBP-H 组),每组8只。
检测各组大鼠血清肌酐(Scr )、血清胱抑素C (Cys-C )、血尿素氮(BUN )和血清白细胞介素(IL )-1β、IL-18水平,苏木素-伊红(HE )染色观察各组肾组织病理损伤情况,采用蛋白质印迹法和免疫组织化学法检测肾组织中炎症因子、核因子(NF )-κB 信号通路及细胞焦亡相关蛋白表达水平。
结果 与Sham 组比较,IRI 组肾组织损伤较为严重,Scr 、Cys-C 、BUN 和血清IL-1β、IL-18水平均升高,蛋白质印迹法结果显示NOD 样受体蛋白(NLRP3)、Gasdermin D (GSDMD )、半胱氨酸天冬氨酸蛋白酶(Caspase )-1、IL-18、IL-1β、NF-κB p65、p-NF-κB p65蛋白相对表达量均增加,免疫组织化学染色结果显示NF-κB p65、p-NF-κB p65、IL-1β、IL-18和NLRP3 蛋白表达均增多。
与IRI 组比较,PDTC 组、NBP-L 组和NBP-H 组肾组织的损伤程度均减轻,Scr 、Cys-C 、BUN 和血清IL-18、IL-1β水平均下降,蛋白质印迹法结果显示NLRP3、GSDMD 、Caspase-1、IL-1β、IL-18、NF-κB p65、p-NF-κB p65蛋白表达DOI: 10.3969/j.issn.1674-7445.2023.04.010基金项目:贵州省卫生健康委科学技术基金(gzwkj2021-220);国家自然科学基金培育项目 [gyfynsfc (2020)-30];贵州医科大学附属医院2022年博士科研启动基金(gyfybsky-2022-31)作者单位:550004 贵阳,贵州医科大学附属医院泌尿外科作者简介:张瑞波(ORCID: 0009-0009-3056-7298),硕士研究生,肾移植相关缺血-再灌注损伤与保护,Email:****************通信作者:沈俊(ORCID: 0000-0003-1208-5833),博士,副主任医师,肾移植相关缺血-再灌注损伤与保护,Email:***************.cn结构式摘要HE 染色观察肾组织病理学改变免疫组化染色检测炎症因子、 NF-κB 信号通路及细胞焦亡相关蛋白IRISham 组IRI 组PDTC 组NBP-H 组丁苯酞或PTDC 或0.9%氯化钠注射液NBP-L 组蛋白质印迹法检测炎症因子、 NF-κB 信号通路及细胞焦亡相关蛋白Scr 、BUN 、Cys-C 水平检测IL-1β、IL-18水平检测·540·第14卷器官移植肾脏缺血-再灌注损伤(ischemia-reperfusion injury ,IRI )通常由器官移植、肾部分切除手术、败血症等引起,如不及时采取有效措施加以预防,很容易发展为急性肾损伤,导致病死率上升[1-6]。
米诺环素对大鼠脑缺血再灌注后细胞凋亡的抑制作用

米诺环素对大鼠脑缺血再灌注后细胞凋亡的抑制作用熊建忠;易飞【期刊名称】《上海医学》【年(卷),期】2012(35)2【摘要】目的观察米诺环素对大鼠脑缺血再灌注后细胞凋亡的影响。
方法 30只雄性Sprague-Dawley(SD)大鼠随机分为假手术组、氯化钠溶液组、预处理组、大剂量组、小剂量组,每组6只。
采用线栓法制作大鼠脑缺血再灌注模型。
采用反转录聚合酶链反应(RT-PCR)、免疫组织化学、Hoechst染色、磁共振T2加权像等方法,了解米诺环素对缺血后细胞凋亡的影响。
结果假手术组可见少量半胱氨酸天冬氨酸蛋白酶(caspase)-3阳性细胞,氯化钠溶液组缺血灶周围可见大量caspase-3阳性细胞。
与假手术组相比,氯化钠溶液组的caspase-3光密度值显著升高(P<0.01);与氯化钠溶液组相比,预处理组、大剂量组、小剂量组的caspase-3光密度值均显著降低(P值分别<0.05、0.01)。
假手术组大鼠大脑皮层只有少量凋亡细胞,氯化钠溶液组大鼠脑缺血灶周围可见大量凋亡细胞。
与假手术组相比,氯化钠溶液组的细胞凋亡率显著降低(P<0.01);与氯化钠溶液组相比,预处理组、大剂量组、小剂量组的细胞凋亡率均显著升高(P值均<0.01)。
与氯化钠溶液组相比,预处理组、大剂量组、小剂量组的相对脑缺血体积均显著缩小(P值分别<0.05、0.01)。
与假手术组相比,氯化钠溶液组白细胞介素-1β转化酶(ICE)的表达显著升高(P<0.05);与氯化钠溶液组相比,预处理组、大剂量组、小剂量组ICE的表达均显著降低(P值均<0.05)。
结论米诺环素能抑制缺血后细胞凋亡。
【总页数】5页(P129-132)【关键词】脑缺血再灌注;米诺环素;凋亡【作者】熊建忠;易飞【作者单位】江西省萍乡市人民医院神经内科【正文语种】中文【中图分类】R277.733【相关文献】1.填髓益脑法对大鼠脑缺血再灌注损伤后细胞凋亡的影响 [J], 丁念;张觉人2.米诺环素对脑缺血再灌注后大鼠细胞凋亡的影响 [J], 熊建忠;易飞;揭文环3.缺血后处理对大鼠肢体缺血再灌注后肾细胞凋亡的抑制作用 [J], 赵利军;李开济;吴静;门秀丽4.脑源性神经营养因子预先给药对大鼠局灶性脑缺血再灌注后神经细胞凋亡的影响[J], 谭永星;庾俊雄;蒋奕红;林高翔5.脑脉泰对大鼠脑缺血再灌注后神经细胞凋亡和Akt,bcl-243,Bax和caspase3表达的影响 [J], 王征;方芳;方云祥;邹节明因版权原因,仅展示原文概要,查看原文内容请购买。
芬戈莫德对肾缺血再灌注损伤模型小鼠的肾保护作用及其机制研究

芬戈莫德对肾缺血再灌注损伤模型小鼠的肾保护作用及其机制研究目的:研究芬戈莫德对肾缺血再灌注损伤(RIRI)模型小鼠的肾保护作用及其机制。
方法:将60只小鼠随机分为假手术组、模型组、芬戈莫德组(1 mg/kg)和芬戈莫德+wortmannin组[芬戈莫德1 mg/kg+磷脂酰肌醇3-激酶(PI3K)特异性阻滞药wortmannin 1.4 mg/kg],每组15只。
除假手术组外,其余3组小鼠均建立RIRI模型,术前24 h一次性经尾静脉注射相应的药物。
再灌注24 h后收集每组小鼠血清,使用全自动生化分析仪测量各组小鼠血清中血肌酐(Scr)和尿素氮(BUN)水平;光镜下观察肾组织病理变化;Western blot法检测肾组织中细胞间黏附分子1(ICAM-1)、单核细胞趋化蛋白1(MCP-1)、磷酸化蛋白激酶B(p-Akt)蛋白的表达。
结果:与假手术组比较,模型组小鼠血清中Scr和BUN 水平明显升高(P<0.01);肾组织出现病理性改变,肾小管上皮细胞坏死,炎性细胞浸润;肾组织中ICAM-1和MCP-1蛋白表达水平明显升高(P<0.01),p-Akt 蛋白表达水平轻微升高(P>0.05)。
与模型组比较,芬戈莫德组小鼠除肾组织中p-Akt蛋白表达水平明显升高(P<0.01)外,其余指标均明显改善(P<0.01)。
与芬戈莫德组比较,芬戈莫德+wortmannin组小鼠的上述指标变化均逆转(P<0.05或P<0.01)。
结论:芬戈莫德能减轻RIRI模型小鼠的肾损伤,其机制可能与激活PI3K/Akt信号通路有关。
ABSTRACT OBJECTIVE:To study the protective effect of fingolimod on renal ischemia reperfusion injury (RIRI)model mice and its mechanism. METHODS:A total of 60 mice were randomly divided into sham operation group,model group,fingolimod group (1 mg/kg)and fingolimod+wortmannin group [fingolimod 1 mg/kg+phosphatidylinositol 3-kinase (PI3K)specific blocker wortmannin 1.4 mg/kg],with 15 mice in each group. Except for sham operation group,RIRI model was induced in other 3 groups,and those model mice were given relevant medicine via caudal vein at once 24 h before surgery. Serum of mice were collected in each group after 24 h perfusion. Serum levels of Scr and BUN were measured by automatic biochemical analyzer. The pathological changes of renal tissue were observed under light microscope. The protein expression of intercellular cell adhesion molecule-1 (ICAM-1),monocyte chemoattractant protein-1(MCP-1)and phosphorylated protein kinase B (p-Akt)in renal tissue were measured by Western blot assay. RESULTS:Compared with sham operation group,the serum levels of Scr and BUN in model group were increased significantly (P<0.01). Pathological changes were found in the kidney,and RIRI led to widespread renal tubular epithelial cell injury,apoptosis and inflammatory cells infiltration. The protein expression of ICAM-1 and MCP-1 in renal tissue were increased significantly (P<0.01),the protein expression of p-Akt was increased slightly (P>0.05). Compared with mo- del group,other indexes of fingolimod group were improved significantly (P<0.01)except that the protein expression of p-Akt in renal tissue was increased significantly (P<0.01). Compared with fingolimod group,above indexes of fingolimod+wortmannin group were reversed (P<0.05 or P<0.01). CONCLUSIONS:Fingolimod can obviously ameliorate renal injury induced byRIRI in mice,the mechanism of which may be associated with the activation of PI3K/Akt signaling pathway.KEYWORDS Fingolimod;Renal ischemia reperfusion injury;Mice;Phosphatidylinositol 3-kinase;Phosphorylated protein kinase B肾缺血再灌注损伤(RIRI)是临床常见的病理过程,如处理不及时可能使多个脏器受累。
姜黄素对肾缺血再灌注损伤的保护作用机制的研究进展

姜黄素对肾缺血再灌注损伤的保护作用机制的研究进展周小园;容松;田梅【摘要】姜黄素是一种从姜黄中提取的黄色酸性酚类物质,广泛应用于食品工业中.肾脏作为内分泌器官,同时也是高灌注器官,对缺血和再灌注尤其敏感.当肾脏出现缺血再灌注时,在缺血再灌注的后期会产生大量的活性氧族,使肾脏处于高度氧化应激状态,并引发一系列有害的细胞反应,导致炎症、细胞凋亡和急性肾衰竭,甚至引起其他器官的损害.姜黄素可以通过上调APPL1的表达、抑制Akt磷酸化途径、抑制活化的INOS/NO/CGMP/PKG信号通路、减轻氧化应激反应、抑制炎症细胞浸润,上调HO-1、抑制NF-κB活性、减少血管活性物质的产生等减少肾缺血再灌注所致的肾损伤.因此姜黄素可作为治疗肾缺血再灌注的一种新的治疗方法.【期刊名称】《中国医药导报》【年(卷),期】2019(016)006【总页数】5页(P20-23,27)【关键词】姜黄素;肾缺血再灌注损伤;氧化应激;炎性因子【作者】周小园;容松;田梅【作者单位】遵义医学院附属医院肾病风湿科,贵州遵义563000;汉诺威莱布尼兹大学,德国汉诺威30167;遵义医学院附属医院肾病风湿科,贵州遵义563000【正文语种】中文【中图分类】R285.5缺血再灌注损伤(ischemia reperfusion injury,IRI)是指经历缺血的器官或组织在恢复供血和供氧后,器官或组织损伤反而加重,甚至出现损伤不可逆的现象,通常由炎症级联反应引发,包括活性氧(reactive oxygen species,ROS)、活性氮(reactive nitrogen species,RNS),细胞因子、趋化因子、白细胞活化等[1]。
肾缺血再灌注损伤(renal ischemia reperfusion injury,RIRI)是一个非常复杂的病理过程,其主要通过线粒体损伤、炎症、凋亡、氧化应激等途径造成肾脏损伤[2]。
姜黄素是从姜黄中提取的一种色素,姜黄主要分布在印度、中国和东南亚等热带和亚热带地区,广泛用于食物色素,其人体安全性好,且具有抗炎、抗氧化、抗纤维化、抗凝、抗肿瘤、降血脂等活性,研究[3-10]表明姜黄素对RIRI具有保护作用,本文就姜黄素对于RIRI的保护作用机制的研究进展予以综述。
大鼠急性肾脏缺血再灌注后处理动物模型的建立

大鼠急性肾脏缺血再灌注后处理动物模型的建立【摘要】目的:建立肾脏缺血后处理动物模型. 方法:将40只SD大鼠随机分为对照组、缺血再灌注组(IR组)和3个缺血后处理组(IPO1,IPO2,IPO3组),分别制作动物模型. IR组在同时夹闭双侧肾动脉45 min后恢复血供,IPO1,IPO2和IPO3组在肾缺血45 min后分别采用不同的后处理方法,其中IPO3组采用反复10次再灌注20 s-缺血20 s的后处理方法. 恢复血供24 h后留取各组大鼠静脉血标本及肾组织,检测肾功能指标,光镜下观察肾组织形态学变化并对肾小管损伤程度进行评分. 结果:IPO3组血尿素氮为(27.9±3.2) mmol/L,血肌酐为(232±49)μmol/L,肾小管损伤程度评分为382±48. 和IR组相比,IPO3组的血尿素氮、血肌酐及肾小管损伤程度评分均明显降低(P<0.01),肾组织损伤明显减轻,其余两个缺血后处理组与IR组相比差异无统计学意义(P>0.05). 结论:在急性肾脏缺血后,采用IPO3组的方法可以减轻急性肾脏缺血再灌注损伤,从而成功建立大鼠急性肾脏IRI的后处理动物模型.【关键词】缺血后处理0引言急性肾脏缺血再灌注损伤(ischemia reperfusion injury,IRI)是一种常见的临床病理生理过程,多见于低血容量性休克、急性肾动脉阻断以及肾脏移植等情况,可以造成急性肾衰竭或使移植肾功能延迟恢复. 因此,如何有效防止因急性肾脏IRI而导致的各种急慢性肾脏疾病是一个亟待解决的问题. 2000年,陶凌等[1]在国内首先证实了缺血后处理(ischemic postconditioning,IPO)对急性心肌缺血再灌注兔心脏具有保护作用,此后,更多研究者[2-4]把研究对象拓宽到不同物种和不同器官,建立了IPO动物模型,并对IPO保护作用机制进行了探讨. 但是,肾脏IPO是否同样具有对急性肾脏IRI的保护作用尚不清楚. 我们在大鼠急性肾脏IRI模型基础上,探讨建立肾脏IPO大鼠模型的方法.1材料和方法1.1材料健康雌性SD大鼠40只,体质量(236±10)g(第四军医大学实验动物中心),动物许可证号SCXK(军2002��005),标准饲料喂养,正常饮水,普通光照. 血尿素氮、血肌酐试剂盒(化学法)(南京建成生物工程研究所);721分光光度计(上海精密科学仪器公司);光学显微镜(日本Olympus公司).1.2方法1.2.1动物分组与模型制作将SD大鼠随机分为5组,每组8只. ①缺血再灌注组(IR组):参考Basile等[5]的方法进行双侧肾脏缺血再灌注,建立缺血再灌注引起的急性肾衰竭的动物模型. 将大鼠用30g/L戊巴比妥腹腔注射麻醉,去毛、固定,沿上腹部正中线逐层切开皮肤、腹直肌及腹膜,先后在结肠脾区及结肠肝区位置暴露左、右肾,游离左、右肾蒂,用玻璃分针小心分离出左、右肾动脉,然后用血管夹同时将双侧肾动脉夹闭,45 min后同时松开双侧血管夹,恢复双侧肾脏血供. ②缺血后处理1组(IPO1组):各缺血后处理组的手术方法基本同IR组. 在缺血45 min后用血管夹同时给予双侧肾脏反复5次的恢复血供3 min-阻断血供3 min处理(即缺血后处理),再恢复血供. ③缺血后处理2组(IPO2组):在缺血45 min后用血管夹同时给予双侧肾脏反复5次的恢复血供20 s-阻断血供20 s处理,再恢复血供. ④缺血后处理3组(IPO3组):在缺血45 min后用血管夹同时给予双侧肾脏反复10次的恢复血供20 s-阻断血供20 s处理,再恢复血供. ⑤对照组(S组):手术方法基本同IR组,但仅分离出大鼠双侧肾动脉,不进行夹闭.1.2.2标本留取在末次恢复肾脏血供24 h后将大鼠用30 g/L戊巴比妥腹腔注射麻醉,固定,分离下腔静脉,用注射器采血2~3 mL,用于血液生化指标检查;然后用冰生理盐水经肾动脉对肾脏进行灌注直至肾脏发白,取下双肾,留取部分组织做光镜检查.1.2.3指标观察①肾功能:采用化学比色法检测血尿素氮及血肌酐. ②肾脏组织形态学:部分肾组织用100 mL/L甲醛固定,常规脱水、包埋、切片后,行HE和PAS染色,光镜下观察形态,参考Paller等[6]的方法进行评分,每只大鼠肾组织随机选择10个无重叠视野(×200),每个视野下随机选择10处肾小管,共按100个肾小管计分,分数越高表示肾小管损伤程度越严重.统计学处理:所得数据采用x±s表示,采用完全随机设计的单因素方差分析(ANOVA),多组之间的两两比较采用LSD��t检验,应用SPSS 13.0软件进行统计学处理,以P<0.05为差异有统计学意义.2结果2.1血尿素氮及肌酐与S组相比, IR, IPO1, IPO2, IPO3组的血尿素氮及血肌酐均显著增高(P<0.01). 与IR组相比,IPO1组的血尿素氮和血肌酐略有增高、IPO2组略有降低,但均无统计学意义(P>0.05),IPO3组的血尿素氮和血肌酐均明显降低,差异有统计学意义(P<0.01,表1).表1各组SD大鼠血尿素氮、血肌酐及肾小管损伤程度评分的比较(略)2.2肾脏组织形态学的变化2.2.1肾小管损伤程度的评分与S组相比,IR,IPO1,IPO2,IPO3组的肾小管损伤程度差异有统计学意义(P<0.01). 与IR组相比,IPO1组的肾小管损伤程度更重(P<0.05),IPO2组的肾小管损伤程度略轻,但无统计学意义(P>0.05),IPO3组的肾小管损伤程度明显减轻(P<0.01,表1).2.2.2肾脏组织形态学改变在光镜下S组大鼠肾组织结构正常,肾小管、肾间质未见明显病理改变(图1A). 经过再灌注24 h后,IR组大鼠肾组织中可见间质水肿,肾小管上皮细胞刷状缘消失,大量上皮细胞脱落、坏死,基底膜裸露,管腔明显扩张,管腔内可见大量管型(图1B). IPO3组大鼠肾组织中可见间质轻度水肿,肾小管上皮细胞扁平,部分刷状缘脱落消失,部分管腔扩张,可见少量管型,相对IR组病理改变明显减轻(图1C).图1各组SD大鼠肾脏形态学改变PAS ×200略3讨论1986年,Murry等[7]发现在急性缺血再灌注出现之前对心脏进行反复多次的短暂缺血再灌注(即缺血预处理)可以减轻心脏的IRI,此后,Islam等[8]证实了预处理对肾脏急性IRI同样有保护作用. 然而在临床中,如果一旦发生IRI,则预处理难以实施,因此,研究在缺血后对再灌注进行处理更具有实用价值. 2000年,我国学者[1]首先证实了IPO对急性心肌IRI具有保护作用,此后,Zhao等[2]和Kin等[9]的研究分别证明了IPO可以减轻犬和大鼠心脏IRI. 不仅在心脏,随后的一些研究针对肝脏[3]、脑[4]等组织进行IPO,证实对其相应器官的IRI也可产生保护作用,并对保护作用机制进行了探讨. 但是,以往的国内外众多研究并未涉及到肾脏器官,而且针对大鼠心脏等器官的研究中,单次后处理的时间和后处理的循环次数也不尽相同[10],因此,在开展针对肾脏IPO作用效果及作用机制的研究之前,首先需要建立成功的大鼠肾脏IPO模型.在国外许多大鼠缺血再灌注的急性肾衰模型研究[5, 11]中,都将肾脏缺血时间限定在45 min,我们在本研究中同样将缺血时间限定在45 min,通过预实验成功复制了大鼠急性肾脏IRI模型. Yang等[12]通过对研究发现,如果在开始再灌注10 min后再进行后处理将丧失对IRI的保护作用,更多的研究[9,13]发现,如果在开始再灌注1 min之后再进行后处理,同样会使其对心肌IRI的保护作用丧失. 最近,Tang等[10]的研究显示,不同的后处理循环次数和心肌梗死面积有关,单次后处理时间过长、后处理循环次数过少都有可能限制其心肌保护作用的发挥. 因此,我们在成功复制了大鼠急性肾脏IRI模型的基础上,重点针对单次后处理的时间和后处理的循环次数进行了研究,以期成功建立大鼠急性肾脏IRI的后处理动物模型.我们在实验中对大鼠肾脏缺血后采用了3种不同的后处理方式,从功能学和形态学角度对后处理的效果进行了分析,以便选择合适的肾脏缺血后处理动物模型. 结果显示,相对于IR组,采用反复5次恢复血供3 min-阻断血供3 min的后处理(IPO1组)并不能减轻急性肾脏IRI,反而可能加重损伤;采用反复5次恢复血供20 s-阻断血供20 s的后处理(IPO2组)能使肾功能指标改善,但无统计学意义. 而采用反复10次恢复血供20 s-阻断血供20 s的后处理组(IPO3组)和IR组相比,肾功能指标明显改善,肾组织中未见肾小管上皮细胞脱落和基底膜裸露,间质水肿、刷状缘消失、管腔扩张、管型等改变也较IR组轻,对肾小管损伤程度评分的分析同样支持上述结果.综上所述,在急性肾脏缺血后及时采取反复10次的短暂(恢复血供20 s-阻断血供20 s)后处理,可以减轻急性肾脏IRI,从而成功建立大鼠急性肾脏IRI的后处理动物模型,为进一步研究IPO对大鼠肾脏的保护作用机制奠定了基础.参考文献[1]陶凌,李源,高峰,等. 缺血后处理对急性心肌缺血再灌注兔心脏的保护作用[J]. 第四军医大学学报, 2000, 21(6): S116-S118.[2] Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning[J]. Am J Physiol Heart Circ Physiol, 2003, 285(2):H579-H588.[3] Sun K, Liu ZS, Sun Q. Role of mitochondria in cell apoptosis during hepatic ischemia��reperfusion injury and protective effect of ischemic postconditioning[J]. World J Gastroenterol, 2004, 10(13): 1934-1938.[4] Danielisova V, Nemethova M, Gottlieb M, et al. The Changes in Endogenous Antioxidant Enzyme Activity After Postconditioning[J]. Cell Mol Neurobiol, 2006, 26(7��8):1179-1189.[5] Basile DP, Donohoe D, Cao X, et al. Resistance to ischemic acute renal failure in the Brown Norway rat: a new model to study cytoprotection[J]. Kidney Int, 2004, 65(6):2201-2211.[6] Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat[J]. J Clin Invest, 1984,74(4): 1156-1164.[7] Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium[J]. Circulation, 1986, 74(5):1124-1136.[8] Islam CF, Mathie RT, Dinneen MD, et al.Ischaemia��reperfusion injury in the rat kidney: the effect of preconditioning[J]. Br J Urol, 1997, 79(6):842-847.[9] Kin H, Zhao ZQ, Sun HY, et al. Postconditioning attenuates myocardial ischemia��reperfusion injury by inhibiting events in the early minutes of reperfusion[J]. Cardiovasc Res, 2004, 62(1):74-85.[10] Tang XL, Sato H, Tiwari S, et al. Cardioprotection by postconditioning in conscious rats is limited to coronary occlusions <45 minutes[J]. Am J Physiol Heart Circ Physiol, 2006, 291(5): H2308-H2317.[11] Spandou E, Tsouchnikas I, Karkavelas G, et al. Erythropoietin attenuates renal injury in experimental acute renal failure ischaemic/reperfusion model[J]. Nephrol Dial Transplant,2006, 21(2): 330-336.[12] Yang XM, Proctor JB, Cui L, et al. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways[J]. J Am Coll Cardiol, 2004,44(5):1103-1110.[13] Philipp S, Downey JM, Cohen MV. Postconditioning must be initiated in less than 1 minute following reperfusion and is dependent on adenosine receptors and PI3��kinase[J]. Circulation, 2004,110(Suppl III):804.。
新型肾脏冷缺血再灌注损伤动物模型的稳定性研究

有关 , 手术组和对照组病理学检查符合急性肾衰竭改变 。结 论
新型 肾脏冷缺 血再灌 注损伤模 型操作更 为
简单 , 稳定性强 , 利于被普 通研究人员所掌握 , 成功率高 , 是一种研究 肾脏冷缺血再灌注损伤 的较好模型。 【 关键词 】 再灌注损伤 ; 模 型 , 动物
Sa it f e dlo in yc l ce a rp rui nu y W I tblyo n w mo e fkd e od i h mi e efs n ijr E i a s o ,A n ,H i —iL CI Mi S IBn y,I g g
smp i h o lxt f mo e , p l r n t u in a d p o l o r s a c . e h s T e e p r n s i l y t e c mp e i o d l a p y mo e i si t n e p e t e e r h M t o f y t o d h x e me t wa i
中华 临 床 医 师杂 志 ( 电子 版 )02年 3月第 6卷 第 5期 21
C i JCi c n ( lc oi d i )Mac , 1 , 0. , o5 h l ii s Eet ncE io 。 rh12 2 V 16 N . n n a r tn 0
t O1
・
论 著 ・
手术组和对照组相 比 , 本模型 大鼠术后各 时间段肌酐 、 尿
素氮和 N .B差异有统计 学意义。两组术后第 6h ld 3d Fe 、 、 肌酐值 比较 , P<00 ; . 5 术后 1d尿素氮 比较 , P< 00 ; .5 术后 1d 3dN —B比较 , 0 0 。可能与省略血管缝合 步骤 , 而减少 大 鼠热缺血 再灌 注损 伤时 间 、 Fe P< .5 从
脑缺血再灌注后局部组织凋亡发生微环境生化因子变化及外源性EPO的影响

脑缺血再灌注后局部组织凋亡发生微环境生化因子变化及外源性EPO的影响罗晓明;程新富;钱晟;张志强;秦晓勇【期刊名称】《四川医学》【年(卷),期】2007(28)3【摘要】目的观察大鼠脑缺血再灌注后局部组织凋亡发生微环境生化因子的变化及外源性促红细胞生成素(EPO)的影响.方法采用血管阻断法制作大鼠单侧(左)脑缺血再灌注模型.将54只SD大鼠随机分为正常组6只、缺血再灌注组(对照组)24只和EPO治疗组(EPO组)24只,观察时相点为缺血后6,12,24,48h,观察脑组织含水量变化,苏木精-伊红染色病理观察及细胞计数,TUNEL法检测细胞凋亡变化,检测脑组织LPO、MDA、谷氨酸(glutamate,GLU)和γ氨基丁酸(gamm-aminobutyric,GABA)含量.结果 EPO组病理变化较对照组轻,脑组织含水量较对照组显著减少,EPO组缺血24h及48h平均脑组织细胞数显著多于对照组、TUNEL 阳性细胞数量显著少于对照组,EPO组脑组织LPO、MDA水平和GLU含量显著下降,GABA含量较对照组显著上升.结论 EPO能有效减轻大鼠脑缺血再灌注后损伤,LPO、MDA、GLU和GABA含量变化参与了其保护作用.【总页数】3页(P249-251)【作者】罗晓明;程新富;钱晟;张志强;秦晓勇【作者单位】中国人民解放军第161中心医院神经外科,湖北,武汉,430010;中国人民解放军第161中心医院神经外科,湖北,武汉,430010;中国人民解放军第161中心医院神经外科,湖北,武汉,430010;中国人民解放军第161中心医院神经外科,湖北,武汉,430010;中国人民解放军第161中心医院神经外科,湖北,武汉,430010【正文语种】中文【中图分类】R651【相关文献】1.外源性拟载脂蛋白E肽对脑血管痉挛后血管内皮生长因子介导的脑动脉内皮细胞凋亡的影响 [J], 王凯杰;李冉;刘江;高俊玲;田艳霞;崔建忠2.脑缺血再灌注后脑梗塞周围区生长相关蛋白-GAP43表达的变化及外源性神经生长因子的影响 [J], 郭宗君;丰岩清;郭云良3.青蒿素对大鼠脑缺血再灌注损伤后凋亡相关因子及炎症介质表达的影响 [J], 袁红刚4.局部组织透析对电损伤后局部微环境电解质的影响 [J], 潘贰;汪道新;范锟铻;黄江鸿;张力勇;李伟萍;朱志祥5.丹红注射液对脑缺血再灌注后凋亡诱导因子(AIF)表达及神经元凋亡的影响[J], 罗德云因版权原因,仅展示原文概要,查看原文内容请购买。
肾缺血再灌注损伤的病理基础及中医药物治疗的研究进展

肾缺血再灌注损伤的病理基础及中医药物治疗的研究进展作者:程丰李伟王光策来源:《云南中医中药杂志》2022年第02期摘要:肾移植术目前已经成为治疗终末期肾病的最有效手段,然而术后发生的肾缺血再灌注损伤会大大影响肾脏的长期存活状态并降低患者生存率,成为目前限制肾移植的一大难题。
肾移植术后的微循环障碍是一个复杂的过程,涉及到多个方面、多个因素、多个途径,很难用某一单独因素解釋,中药运用其整体观辨证论治的思想,在整体调节方面优于传统西药治疗,但是其在防治缓解肾脏缺血再灌注损伤的作用机制仍待深入研究。
现就中医药缓解治疗肾移植术后的缺血再灌注损伤问题结合近些年国内外相关研究做一综述。
关键词:肾移植;缺血再灌注损伤;中医药防治中图分类号:R692 文献标志码:A 文章编号:1007-2349(2022)02-0086-05缺血再灌注(ischemia and reperfusion,I/R)损伤常发生于微血管区,造成微循环障碍,指的是由于各种原因引起的组织器官缺血和血液灌注恢复后所导致的损伤。
组织器官发生缺血时可发生冷缺血和热缺血两个过程,但这两个过程造成的损伤并没有较大的区别,最重要的是再灌注的过程会加重组织器官的损伤。
肾脏是临床上常见的易受缺血再灌注损伤的器官之一,常发生于肾脏移植、急性缺血性肾衰竭、肾肿瘤切除术等[1]。
1 缺血再灌注损伤的机制缺血再灌注损伤涉及多种病理过程,主要包括能量代谢、氧化应激、内质网应激、自噬等。
1.1 氧化应激再灌注后,细胞的自由基形成与细胞防御自由基能力的平衡失调所诱发的氧化应激损伤是I/R损伤的主要原因之一。
缺血再灌注后细胞内活性氧(ROS)的产生有多种途径,包括线粒体电子传递链途径、NADPH氧化酶(NOXs)、黄嘌呤氧化酶途径途径、未耦连的一氧化氮合酶(NOS)途径等[2]。
1.1.1 线粒体电子传递链线粒体不仅是机体内细胞能量的代谢中心而且还是细胞内自由基产生的重要基地之一[3]。
肾缺血再灌注通过促凋亡途径加重糖尿病小鼠肾损伤

肾缺血再灌注通过促凋亡途径加重糖尿病小鼠肾损伤韩庆玲;郑德义;李伟人;王志伟;杜娇;王毅【摘要】目的通过检测凋亡相关蛋白在糖尿病小鼠肾缺血再灌注(I/R)损伤中的表达变化,探讨肾I/R加重糖尿病肾损伤的可能机制.方法 28 只健康清洁级雄性C57BL/6J小鼠,随机分为4组:正常血糖假手术组(Sham组)(n=6),糖尿病假手术组( DM+Sham组) ( n=6),正常血糖肾缺血组(I/R组)(n=8),糖尿病肾缺血组(DM+I/R 组)(n =8).通过连续5 d腹腔注射链脲佐菌素(55 mg/kg)的方法制备1型糖尿病模型,4 周后,通过开腹用无创微动脉夹夹闭双侧肾动静脉30 min,然后松开动脉夹再灌注12 h建立肾缺血再灌注损伤小鼠模型.常规生化检测血肌酐、尿素氮反应肾功能情况. Western blot 方法检测肾组织中 Bcl-2、Bax、Caspase-3蛋白的表达变化.结果糖尿病小鼠空腹血糖明显高于正常血糖小鼠(P<0.05),而糖尿病小鼠空腹体重明显低于正常血糖小鼠( P<0.05). I/R组和DM+Sham组小鼠血清肌酐、尿素氮高于Sham组(P<0.05),DM+I/R组小鼠血清肌酐、尿素氮高于DM+Sham 组和I/R组( P<0.05).与Sham组比较, Bax和Caspase-3 蛋白表达在I/R组和DM+Sham组小鼠肾组织中升高( P<0.05),Bcl-2 蛋白表达降低(P<0.05);与DM+Sham和I/R组比较,DM+I/R组小鼠肾组织中Bax和Caspase-3蛋白表达明显升高( P<0.05),Bcl-2蛋白表达明显降低(P<0.05).结论肾缺血再灌注损伤导致糖尿病小鼠肾组织促凋亡蛋白 Bax 和Caspase-3表达升高,而抗凋亡蛋白Bcl-2 降低,肾缺血再灌注通过促凋亡途径加重糖尿病肾损伤.【期刊名称】《安徽医科大学学报》【年(卷),期】2018(053)012【总页数】4页(P1834-1837)【关键词】凋亡;糖尿病;缺血再灌注;肾损伤【作者】韩庆玲;郑德义;李伟人;王志伟;杜娇;王毅【作者单位】贵州医科大学临床医学院,贵阳 550004;贵州省人民医院烧伤整形科,贵阳 550002;贵州医科大学附属医院烧伤整形科,贵阳 550004;贵州医科大学临床医学院,贵阳 550004;贵州省人民医院烧伤整形科,贵阳 550002;贵州省人民医院烧伤整形科,贵阳 550002【正文语种】中文【中图分类】R692糖尿病(diabetes mellitus,DM)是一组以高血糖为特征的代谢性疾病,糖尿病时长期存在的高血糖,导致各种组织损伤,特别是肾、心脏、血管和神经等多器官组织的慢性损害及功能障碍[1]。
移植肾缺血-再灌注损伤的防治研究进展 谢彪

移植肾缺血-再灌注损伤的防治研究进展谢彪【摘要】肾移植是终末期肾病的有效治疗手段之一,缺血-再灌注损伤(IRI)是影响移植肾早期肾功能恢复和长期存活的重要因素。
本文从供体保存及IRI的防治等几个方面,针对该病理发生发展的过程,对移植肾IRI防治方法的研究进展作一综述。
【关键词】缺血-再灌注损伤;肾移植;抗氧化剂;抗炎症药物;研究进展缺血-再灌注损伤(IRI)是指组织或器官在缺血一段时间后,当重新恢复血供时,不仅不能使组织器官的功能恢复,反而加重了组织、器官的功能障碍和结构损伤。
肾脏为血流高灌注器官,对缺血缺氧等损伤的反应极为敏感,在DCD供肾肾移植手术中,供体经历血流动力学紊乱、热缺血、冷缺血和血流再灌注等一系列过程,移植肾不可避免的发生IRI。
移植肾IRI可引起移植肾功能延迟恢复(DGF)、移植肾原发性无功能(primary non-function,PNF)、急性排斥反应甚至导致移植肾丢失,是影响移植肾早期肾功能恢复和长期存活的重要因素[1]。
因此,对移植肾IRI的防治也成为近年肾移植领域研究的热点,本文将就移植肾IRI防治方法的研究进展展开综述。
1.缺血预处理(ischemic-preconditioning,IPC)是指预先给予一定次数的、短暂的、非致死性的、重复性的缺血,诱导供体产生内源性保护机制,使移植物“耐受”随后的缺血期。
在此期间,对脑死亡大鼠给予胸腺球蛋白(rATS)可减少促炎细胞因子的表达和减轻肾损伤[2]。
补充Klotho蛋白(一种具有多效性功能的跨膜蛋白),可以减轻IRI并且可以抑制纤维化[3]。
最近对动物肾移植模型的系统回顾已证实缺血预处理能有效降低IRI[4]。
但该项结论还有待进一步证实。
2.保存方法2.1静态低温保存法目前普遍采用低温机器灌注法。
相对于静态低温保存,机器灌注法可降低DGF发生率、提高移植成功率,为供肾提供更好的保护作用,这在DCD供体中尤为显著[5],机器灌注的优势在于可以为供肾在冷保存时提供流动的液体、持续的营养供给以及带走有毒代谢产物。
缺血后处理对大鼠肢体缺血再灌注后肾细胞凋亡的抑制作用

缺血后处理对大鼠肢体缺血再灌注后肾细胞凋亡的抑制作用赵利军;李开济;吴静;门秀丽【期刊名称】《吉林大学学报(医学版)》【年(卷),期】2017(043)004【摘要】目的:观察缺血后处理对大鼠肢体缺血再灌注(LIR)后肾组织细胞凋亡的影响,探讨其可能机制.方法: 30只SD大鼠随机分为对照组、缺血再灌注组(IR组)和缺血后处理加再灌注组(I-postC组),每组10只.建立大鼠肢体缺血再灌注(LIR)模型,即橡皮带环绕大鼠双后肢根部阻断血流4 h再恢复血流灌注4 h.对照组大鼠仅松弛环绕橡皮带不阻断血流,I-postC组大鼠则在再灌注前附加反复3次缺血5 min-再灌注5 min操作,即缺血后处理.全自动生化分析仪测各组大鼠血浆肌酐(Cr)、尿素氮(BUN)和C反应蛋白(CRP)水平;免疫组织化学法检测大鼠肾组织凋亡相关蛋白Bcl-2和Bax的表达,采用自动图像分析系统统计其定量结果并计算Bcl-2/Bax比值;TUNEL染色后在激光共聚焦显微镜下观察肾组织细胞凋亡情况,电镜下观察肾组织超微结构.结果:与对照组比较,IR组和I-postC组大鼠血浆Cr、BUN和CRP水平均明显增高(P<0.01);与IR组比较,I-postC组大鼠血浆Cr、BUN和CRP水平均明显降低(P<0.01).与对照组比较,IR组和I-postC组大鼠肾组织中Bax和Bcl-2表达水平明显升高(P<0.05或P<0.01), Bcl-2/Bax比值降低 (P<0.05);与IR组比较,I-postC组大鼠肾组织中Bax表达水平降低(P<0.05),Bcl-2表达水平升高(P<0.01),Bcl-2/Bax比值升高 (P<0.05).激光共聚焦显微镜下观察,与对照组比较,IR组大鼠肾组织中凋亡细胞明显增多;与IR组比较,I-postC组大鼠肾组织中凋亡细胞明显减少.透射电镜下观察,对照组大鼠肾组织细胞结构清晰完整;IR组大鼠肾组织中肾近曲小管上皮细胞核固缩,溶酶体和致密颗粒沉积增多,线粒体数目减少,部分线粒体嵴断裂或模糊,肾小球足突细胞突起不规则、融合,有空泡现象,粗面内质网扩张;与IR组比较,I-postC组大鼠肾组织中肾小管上皮细胞及肾小球损伤有一定程度改善.结论:LIR可诱发大鼠肾组织细胞凋亡,缺血后处理可抑制肢体缺血再灌注后的肾细胞凋亡,对改善大鼠肾功能有一定作用.%Objective:To observe the inhibitory effect of ischemic postconditioning (I-postC) on the apoptosis of renal cells after limb ischemia reperfusion(LIR) in the rats, and to investigate the possible mechanisms. Methods:Thirty SD rats were randomly divided control group, ischemia-reperfusion group(IR group) and I-postC group(n=10).4 h ischemia and 4 h reperfusion with the rubber band in two hind limbs of the rats were performed to establish the models.In control group, the rubber band around the limb was loose and the blood flow was not blocked.As for I-postC group, before perfusion, 5 min ischemia and 5 min reperfusion were performed in the rats and repeated 3 times named I-post C.The levels of blood creatinine (Cr), blood urea nitrogen(BUN) and C-reactive protein (CRP) in plasma of the rats in various groups were measured by automatic biochemistry analyzer.The expressions of Bcl-2 protein and Bax protein in renal were detected by immunohistochemical method,and its quantitative results were observed with automatic image analysis system and the ratio of Bcl-2/Bax was calculated.The apoptotic cells in kidney tissue were determined by terminal-deoxynucleotidy1 transferase-mediated d-UTP nick end labeling (TUNEL) under laser scanning confocal microscope(LSCM).The ultrastructures of kidney tissue were observed under electron microscope.Results:Compared with control group, the levels of Cr,BUN andCRP in plasma of the rats in IR group and I-postC group wereincreased(P<0.05 or P<0.01);compared with IR group, the levels of Cr, BUN and CRP in plasma of the rats in I-postC group weredecreased(P<0.01).Compared with control group,the expression levels Bax and Bcl-2 in kidney tissue of the rats in IR group and I-postC group were significantly increased (P<0.05 or P<0.01),and the ratios of Bcl-2/Bax were reduced(P<0.05);compared with IR group,the expression level of Bax in kidney tissue of the rats in I-postC group was decreased (P<0.05),the expression level of Bcl-2 was increased(P<0.01),and the ratio of Bcl-2/Bax was increased(P<0.05).Under laser confocal microscope,the number of apoptotic cells in kidney tissue of the rats in IR group was increased compared with control group;the number of apoptotic cells in I-postC group was decreased compared with IR group.Under transmission electron microscope,the changes in IR group were found as follows: renal proximal convoluted tubule epithelial cell nucleus vacuoles,increased lysosome and dense particle deposition, some mitochondria crest fracture orfuzzy;irregular and fusion, glomerular podocyteprotuberance,mitochondrial cristae fracture and reducetion with vacuoles, rough endoplasmic reticulum expansion;the damage levels of renal tubular epithelial cells and glomerulus in I-postC group were improved compared with IR group.Conclusion: Limb ischemia reperfusion can induce the apoptosis of renal cells, I-postC can inhibit the apoptosis of renal cells,and it would be helpful to improve the kidney function.【总页数】5页(P725-728,前插2)【作者】赵利军;李开济;吴静;门秀丽【作者单位】华北理工大学基础医学院病理生理学系,河北唐山 063000;华北理工大学基础医学院病理生理学系,河北唐山 063000;华北理工大学基础医学院病理生理学系,河北唐山 063000;华北理工大学基础医学院病理生理学系,河北唐山063000【正文语种】中文【中图分类】R363【相关文献】1.丙泊酚后处理对大鼠肝缺血再灌注损伤时肾细胞凋亡以及Bcl-2,Bax的影响 [J], 刘臻;曹定睿2.肢体缺血预处理和BQ123药物后处理对大鼠肢体缺血再灌注后肝损伤的影响[J], 赵林静;王永玲;杨保胜;张金盈3.肢体缺血后处理对局灶性脑缺血再灌注大鼠Caspase-3和细胞凋亡的影响 [J], 韦家俊;李浩;廖小明;吴岚;王耀辉;刘开祥4.依托咪酯后处理对大鼠肝缺血再灌注肾细胞凋亡及Bcl-2和Bax表达的影响 [J], 武莉;曹定睿;刘臻;李敏5.肢体缺血后处理对大鼠脑缺血再灌注后血脑屏障的保护作用 [J], 阳昀;周茜;廖小明;刘开祥;李浩因版权原因,仅展示原文概要,查看原文内容请购买。
分析强力霉素对移植肺缺血-再灌注损伤中细胞凋亡的探究

分析强力霉素对移植肺缺血-再灌注损伤中细胞凋亡的探究
日期: 08月24日
【摘要 目的 探索外源性基质金属蛋白酶(matrix metalloproteinases, MMPs)抑制剂强力霉素在移植肺缺血-再灌注损伤中细胞凋亡的影响。 方法 建立大鼠自体左肺肺缺血再灌注模型,16只SD受体大鼠随机分为两组摘要:缺血再灌注组和强力霉素组,用免疫组化方法观察移植肺Bcl-2、Bax和Caspase-3表达情况,并取肺组织平滑肌细胞进行培养,采用荧光技术测定肺组织细胞凋亡。结果 肺缺血再灌注后,和对照组相比,强力霉素组移植肺Bax和Caspase-3表达明显降低(P%26lt;0.01), Bcl-2 的表达无明显变化(P%26gt;0.05),但Bcl-2/Bax比例升高;荧光图片显示,强力霉素组的细胞凋亡明显较对照组减少。结论 强力霉素可能通过下调Bax、Caspase-3表达,提高Bcl-2/Bax比例而抑制肺缺血再灌注损伤的细胞凋亡,并通过降低基底膜的降解,减轻肺水肿,达到肺保护功能。
1.3 统计学分析 所有数据均以均数±标准差(x±s)表示,两组间比较采用t检验。统计学处理由SPSS11.0统计软件完成。P%26lt;0.05为有统计学意义。
2 结果
2.1 Bcl-2、Bax、Caspase-3蛋白水平变化 和对照组相比,强力霉素组Bax和Caspase-3表达明显降低(P%26lt;0.01),Bcl-2表达无明显增高(P>0.05)(表1)。
表1 各组Bcl-2、Bax和Caspase-3的表达(%) (x±s,n=8)
注摘要:vs Con*P%26lt;0.01
2.2 细胞核形态的Hoechst 33258荧光染色 Hoechst 33258 是和 DNA 特异结合的蓝色荧光染料经荧光显微镜观察, 经强力霉素对照组的细胞核呈现均匀的红色荧光,其细胞核边界规则整洁,染色质均匀分布(图 1),对照组的PMVECs 细胞核则出现不均匀亮度的蓝色荧光, 细胞核先后呈现凋亡早期(波纹状)、中期(核染色质凝聚, 边缘化)和晚期(核裂解, 产生凋亡小体)的典型变化,其凋亡的细胞主要是肺泡上皮细胞及内皮细胞(图2)。
