Crolibulin_SDS_MedChemExpress
CIL56-SDS-MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jan.-07-2019Print Date:Jan.-07-20191. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :CIL56Catalog No. :HY-112063CAS No. :300802-28-21.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4), H302Acute aquatic toxicity (Category 1), H400Chronic aquatic toxicity (Category 1), H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word No data availableHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effectsPrecautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor ⁄ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents ⁄ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:C23H27N3O5S2Molecular Weight:489.61CAS No. :300802-28-24. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077 Class: 9 Packing group: III EMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutantIATAUN number: 3077 Class: 9 Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis)reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:Acute Health Hazard16. OTHER INFORMATIONCopyright 2019 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
安进公司研发的PCSK9抑制剂Repatha喜获欧盟批准

安进公司研发的PCSK9抑制剂Repatha喜获欧盟批准佚名
【期刊名称】《临床合理用药杂志》
【年(卷),期】2015(8)23
【摘要】生物技术巨头安进(Amgen)近日在监管方面收获重大里程碑,该公司研发的PCSK9抑制剂Repatha(evolocumab)喜获欧盟批准,标志着全球首个新一代PCSK9抑制剂类降脂药成功诞生!PCSK9抑制剂是一类单抗药物,靶标是一种名为前蛋白转化酶枯草溶菌素9(PCSK9)的蛋白,该蛋白会增加低密度脂蛋白胆固醇(LDL-C)的生成率,而LDL-C可阻塞血管,被公认为心血管疾病(CVD)的主要风险因子,也是心脏病的罪魁祸首。
【总页数】1页(P87-87)
【正文语种】中文
【中图分类】R972.4
【相关文献】
1.诺华公司第二代酪氨酸激酶抑制剂尼洛替尼在欧盟获得批准
2.欧盟批准安进公司denosumab上市
3.欧盟率先批准赛诺菲-安特公司减肥药利莫那班上市
4.欧盟批准安进公司panitumumab用于转移性结直肠癌治疗
5.FDA批准安进公司的新药Amjevita上市
因版权原因,仅展示原文概要,查看原文内容请购买。
华中科技大学学术期刊分类目录(T-D)_最新权威版
24.755 23.917 23.654 23.565 23.333 23.194 22.929 22.864 22.864 22.864 22.490 22.345 22.333 21.757 21.543 21.543 20.833 20.761 20.614 19.966 19.795 19.547 19.352 18.571 18.571 18.038 17.983 17.983 17.949 17.689 17.689 17.689 17.436 17.313 17.215 16.417 16.238 16.179 16.008 16.008 15.766 15.575 15.518 15.389 15.389 15.389 15.333 15.333 15.280 15.280 15.265 15.253 15.251 15.202
ONCOLOGY CLINICAL NEUROLOGY PLANT SCIENCES BIOCHEMICAL RESEARCH METHODS ASTRONOMY & ASTROPHYSICS MATERIALS SCIENCE, MULTIDISCIPLINARY PHYSICS, MULTIDISCIPLINARY BIOCHEMISTRY & MOLECULAR BIOLOGY CELL BIOLOGY MEDICINE, RESEARCH & EXPERIMENTAL MICROBIOLOGY PHARMACOLOGY & PHARMACY PHYSICS, PARTICLES & FIELDS CHEMISTRY, MULTIDISCIPLINARY PHARMACOLOGY & PHARMACY TOXICOLOGY CHEMISTRY, MULTIDISCIPLINARY CELL BIOLOGY NEUROSCIENCES INFECTIOUS DISEASES IMMUNOLOGY PHYSIOLOGY PHYSICS, MULTIDISCIPLINARY BEHAVIORAL SCIENCES NEUROSCIENCES ONCOLOGY CELL BIOLOGY DEVELOPMENTAL BIOLOGY ECOLOGY CHEMISTRY, MULTIDISCIPLINARY MAபைடு நூலகம்ERIALS SCIENCE, MULTIDISCIPLINARY NANOSCIENCE & NANOTECHNOLOGY GENETICS & HEREDITY MICROBIOLOGY MEDICINE, GENERAL & INTERNAL MICROBIOLOGY ASTRONOMY & ASTROPHYSICS MATERIALS SCIENCE, MULTIDISCIPLINARY BEHAVIORAL SCIENCES NEUROSCIENCES NEUROSCIENCES PSYCHOLOGY CLINICAL NEUROLOGY ECOLOGY EVOLUTIONARY BIOLOGY GENETICS & HEREDITY CHEMISTRY, PHYSICAL PHYSICS, CONDENSED MATTER BIOCHEMISTRY & MOLECULAR BIOLOGY CELL BIOLOGY PSYCHOLOGY MEDICINE, GENERAL & INTERNAL NEUROSCIENCES CARDIAC & CARDIOVASCULAR SYSTEMS
Gelucire-14-44-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Nov.-23-2018Print Date:Nov.-23-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Gelucire 14/44Catalog No. :HY-Y1892CAS No. :121548-04-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:N/AMolecular Weight:N/ACAS No. :121548-04-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Pure form-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Oil)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
marked manuscript

Quality evaluation of Flos Lonicerae through a simultaneous determination of seven saponins by HPLC with ELSDXing-Yun Chai1, Song-Lin Li2, Ping Li1*1Key Laboratory of Modern Chinese Medicines and Department of Pharmacognosy, China Pharmaceutical University, Nanjing, 210009, People’s Republic of China2Institute of Nanjing Military Command for Drug Control, Nanjing, 210002, People’s Republic of China*Corresponding author: Ping LiKey Laboratory of Modern Chinese Medicines and Department of Pharmacognosy, China Pharmaceutical University, Nanjing 210009, People’s Republic of China.E-mail address: lipingli@Tel.: +86-25-8324-2299; 8539-1244; 135********Fax: +86-25-8532-2747AbstractA new HPLC coupled with evaporative light scattering detection (ELSD) method has been developed for the simultaneous quantitative determination of seven major saponins, namely macranthoidinB (1), macranthoidin A (2), dipsacoside B (3), hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester (4), macranthoside B (5), macranthoside A (6), and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside (7)in Flos Lonicerae, a commonly used traditional Chinese medicine (TCM) herb.Simultaneous separation of these seven saponins was achieved on a C18 analytical column with a mixed mobile phase consisting of acetonitrile(A)-water(B)(29:71 v/v) acidified with 0.5% acetic acid. The elution was operated from keeping 29%A for 10min, then gradually to 54%B from 10 to 25 min on linear gradient, and then keep isocratic elution with 54%B from 25 to 30min.The drift tube temperature of ELSD was set at 106℃, and with the nitrogen flow-rate of 2.6 l/min. All calibration curves showed good linear regression (r2 0.9922) within test ranges. This method showed good reproducibility for the quantification of these seven saponins in Flos Lonicerae with intra- and inter-day variations of less than 3.0% and 6.0% respectively. The validated method was successfully applied to quantify seven saponins in five sources of Flos Lonicerae, which provides a new basis of overall assessment on quality of Flos Lonicerae.Keywords: HPLC-ELSD; Flos Lonicerae; Saponins; Quantification1. IntroductionFlos Lonicerae (Jinyinhua in Chinese), the dried buds of several species of the genus Lonicera (Caprifoliaceae), is a commonly used traditional Chinese medicine (TCM) herb. It has been used for centuries in TCM practice for the treatment of sores, carbuncles, furuncles, swelling and affections caused by exopathogenic wind-heat or epidemic febrile diseases at the early stage [1]. Though four species of Lonicera are documented as the sources of Flos Lonicerae in China Pharmacopeia (2000 edition), i.e. L. japonica, L. hypoglauca,L. daystyla and L. confusa, other species such as L. similes and L. macranthoides have also been used on the same purpose in some local areas in China [2]. So it is an important issue to comprehensively evaluate the different sources of Flos Lonicerae, so as to ensure the clinical efficacy of this Chinese herbal drug.Chemical and pharmacological investigations on Flos Lonicerae resulted in discovering several kinds of bioactive components, i.e. chlorogenic acid and its analogues, flavonoids, iridoid glucosides and triterpenoid saponins [3]. Previously, chlorogenic acid has been used as the chemical marker for the quality evaluation of Flos Lonicerae,owing to its antipyretic and antibiotic property as well as its high content in the herb. But this compound is not a characteristic component of Flos Lonicerae, as it has also been used as the chemical marker for other Chinese herbal drugs such as Flos Chrysanthemi and so on[4-5]. Moreover, chlorogenic acid alone could not be responsible for the overall pharmacological activities of Flos Lonicerae[6].On the other hand, many studies revealed that triterpenoidal saponins of Flos Lonicerae possess protection effects on hepatic injury caused by Acetaminophen, Cd, and CCl4, and conspicuous depressant effects on swelling of ear croton oil [7-11]. Therefore, saponins should also be considered as one of the markers for quality control of Flos Lonicerae. Consequently, determinations of all types of components such as chlorogenic acid, flavonoids, iridoid glucosides and triterpenoidal saponins in Flos Lonicerae could be a better strategy for the comprehensive quality evaluation of Flos Lonicerae.Recently an HPLC-ELSD method has been established in our laboratory for qualitative and quantitative determination of iridoid glucosides in Flos Lonicerae [12]. But no method was reported for the determination of triterpenoidal saponins in Flos Lonicera. As a series studies on the comprehensive evaluation of Flos Lonicera, we report here, for the first time, the development of an HPLC-ELSD method for simultaneous determination of seven triterpenoidal saponins in the Chinese herbal drug Flos Lonicerae, i.e.macranthoidin B (1), macranthoidin A (2), dipsacoside B (3), hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester (4), macranthoside B (5), macranthoside A (6), and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside (7) (Fig. 1).2. Experimental2.1. Samples, chemicals and reagentsFive samples of Lonicera species,L. japonica from Mi county, HeNan province (LJ1999-07), L. hypoglauca from Jiujang county, JiangXi province (LH2001-06), L. similes from Fei county, ShanDong province (LS2001-07), L. confuse from Xupu county, HuNan province (LC2001-07), and L. macranthoides from Longhu county, HuNan province (LM2000-06) respectively, were collected in China. All samples were authenticated by Dr. Ping Li, professor of department of Pharmacognosy, China Pharmaceutical University, Nanjing, China. The voucher specimens were deposited in the department of Pharmacognosy, China Pharmaceutical University, Nanjing, China. Seven saponin reference compounds: macranthoidin B (1), macranthoidin A (2), dipsacoside B (3), hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester (4), macranthoside B (5), macranthoside A (6), and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside (7) were isolated previously from the dried buds of L. confusa by repeated silica gel, sephadex LH-20 and Rp-18 silica gel column chromatography, their structures were elucidated by comparison of their spectral data (UV, IR, MS, 1H- NMR and 13C-NMR) with references [13-15]. The purity of these saponins were determined to be more than 98% by normalization of the peak areas detected by HPLC with ELSD, and showed very stable in methanol solution.HPLC-grade acetonitrile from Merck (Darmstadt, Germany), the deionized water from Robust (Guangzhou, China), were purchased. The other solvents, purchased from Nanjing Chemical Factory (Nanjing, China) were of analytical grade.2.2. Apparatus and chromatographic conditionsAglient1100 series HPLC apparatus was used. Chromatography was carried out on an Aglient Zorbax SB-C18 column(250 4.6mm, 5.0µm)at a column temperature of 25℃.A Rheodyne 7125i sampling valve (Cotati, USA) equipped with a sample loop of 20µl was used for sample injection. The analog signal from Alltech ELSD 2000 (Alltech, Deerfield, IL, USA)was transmitted to a HP Chemstation for processing through an Agilent 35900E (Agilent Technologies, USA).The optimum resolution was obtained by using a linear gradient elution. The mobile phase was composed of acetonitrile(A) and water(B) which acidified with 0.5% acetic acid. The elution was operated from keeping 29%A for 10min, then gradually to 54%B from 10 to 25 min in linear gradient, and back to the isocratic elution of 54%B from 25 to 30 min.The drift tube temperature for ELSD was set at 106℃and the nitrogen flow-rate was of 2.6 l/min. The chromatographic peaks were identified by comparing their retention time with that of each reference compound tried under the same chromatographic conditions with a series of mobile phases. In addition, spiking samples with the reference compounds further confirmed the identities of the peaks.2.3. Calibration curvesMethanol stock solutions containing seven analytes were prepared and diluted to appropriate concentration for the construction of calibration curves. Six concentrationof the seven analytes’ solution were injected in triplicate, and then the calibration curves were constructed by plotting the peak areas versus the concentration of each analyte. The results were demonstrated in Table1.2.4. Limits of detection and quantificationMethanol stock solution containing seven reference compounds were diluted to a series of appropriate concentrations with methanol, and an aliquot of the diluted solutions were injected into HPLC for analysis.The limits of detection (LOD) and quantification (LOQ) under the present chromatographic conditions were determined at a signal-to-noise ratio (S/N) of 3 and 10, respectively. LOD and LOQ for each compound were shown in Table1.2.5. Precision and accuracyIntra- and inter-day variations were chosen to determine the precision of the developed assay. Approximate 2.0g of the pulverized samples of L. macranthoides were weighted, extracted and analyzed as described in 2.6 Sample preparation section. For intra-day variability test, the samples were analyzed in triplicate for three times within one day, while for inter-day variability test, the samples were examined in triplicate for consecutive three days. Variations were expressed by the relative standard deviations. The results were given in Table 2.Recovery test was used to evaluate the accuracy of this method. Accurate amounts of seven saponins were added to approximate 1.0g of L. macranthoides,and then extracted and analyzed as described in 2.6 Sample preparation section. The average recoveries were counted by the formula: recovery (%) = (amount found –original amount)/ amount spiked ×100%, and RSD (%) = (SD/mean) ×100%. The results were given in Table 3.2.6. Sample preparationSamples of Flos Lonicerae were dried at 50℃until constant weight. Approximate 2.0g of the pulverized samples, accurately weighed, was extracted with 60% ethanol in a flask for 4h. The ethanol was evaporated to dryness with a rotary evaporator. Residue was dissolved in water, followed by defatting with 60ml of petroleum ether for 2 times, and then the water solution was evaporated, residue was dissolved with methanol into a 25ml flask. One ml of the methanol solution was drawn and transferred to a 5ml flask, diluted to the mark with methanol. The resultant solution was at last filtrated through a 0.45µm syringe filter (Type Millex-HA, Millipore, USA) and 20µl of the filtrate was injected to HPLC system. The contents of the analytes were determined from the corresponding calibration curves.3. Results and discussionsThe temperature of drift tube and the gas flow-rate are two most important adjustable parameters for ELSD, they play a prominent role to an analyte response. In ourprevious work [12], the temperature of drift tube was optimized at 90°C for the determination of iridoids. As the polarity of saponins are higher than that of iridoids, more water was used in the mobile phase for the separation of saponins, therefore the temperature for saponins determination was optimized systematically from 95°C to 110°C, the flow-rate from 2.2 to 3.0 l/min. Dipsacoside B was selected as the testing saponin for optimizing ELSD conditions, as it was contained in all samples. Eventually, the drift tube temperature of 106℃and a gas flow of 2.6 l/min were optimized to detect the analytes. And these two exact experimental parameters should be strictly controlled in the analytical procedure [16].All calibration curves showed good linear regression (r2 0.9922) within test ranges. Validation studies of this method proved that this assay has good reproducibility. As shown in Table 2, the overall intra- and inter-day variations are less than 6% for all seven analytes. As demonstrated in Table 3, the developed analytical method has good accuracy with the overall recovery of high than 96% for the analytes concerned. The limit of detection (S/N=3) and the limit of quantification (S/N=10) are less than 0.26μg and 0.88μg respectively (Table1), indicating that this HPLC-ELSD method is precise, accurate and se nsitive enough for the quantitative evaluation of major non- chromaphoric saponins in Flos Lonicerae.It has been reported that there are two major types of saponins in Flos Lonicerae, i.e. saponins with hederagenin as aglycone and saponins with oleanolic acid as the aglycone [17]. But hederagenin type saponins of the herb were reported to have distinct activities of liver protection and anti-inflammatory [7-11]. So we adoptedseven hederagenin type saponins as representative markers to establish a quality control method.The newly established HPLC-ELSD method was applied to analyze seven analytes in five plant sources of Flos Lonicerae, i.e. L. japonica,L. hypoglauca,L. confusa,L. similes and L. macranthoides(Table 4). It was found that there were remarkable differences of seven saponins contents between different plant sources of Flos Lonicerae. All seven saponins analyzed could be detected in L. confusa and L. hypoglauca, while only dipsacoside B was detected in L. japonica. Among all seven saponins interested, only dipsacoside B was found in all five plant species of Flos Lonicerae analyzed, and this compound was determined as the major saponin with content of 53.7 mg/g in L. hypoglauca. On the other hand, macranthoidin B was found to be the major saponin with the content higher than 41.0mg/g in L. macranthoides,L. confusa, and L. similis, while the contents of other analytes were much lower.In our previous study [12], overall HPLC profiles of iridoid glucosides was used to qualitatively and quantitatively distinguish different origins of Flos Lonicerae. As shown in Fig.2, the chromatogram profiles of L. confusa, L. japonica and L. similes seem to be similar, resulting in the difficulty of clarifying the origins of Flos Lonicerae solely by HPLC profiles of saponins, in addition to the clear difference of the HPLC profiles of saponins from L. macranthoides and L. hypoglauca.Therefore, in addition to the conventional morphological and histological identification methods, the contents and the HPLC profiles of saponins and iridoids could also be used as accessory chemical evidence toclarify the botanical origin and comprehensive quality evaluation of Flos Lonicerae.4. ConclusionsThis is the first report on validation of an analytical method for qualification and quantification of saponins in Flos Lonicerae. This newly established HPLC-ELSD method can be used to simultaneously quantify seven saponins, i.e. macranthoidin B, macranthoidin A, dipsacoside B, hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester, macranthoside B, macranthoside A, and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside in Flos Lonicerae. Together with the HPLC profiles of iridoids, the HPLC-ELSD profiles of saponins could also be used as an accessory chemical evidence to clarify the botanical origin and comprehensive quality evaluation of Flos Lonicerae.AcknowledgementsThis project is financially supported by Fund for Distinguished Chinese Young Scholars of the National Science Foundation of China (30325046) and the National High Tech Program(2003AA2Z2010).[1]Ministry of Public Health of the People’s Republic of China, Pharmacopoeia ofthe People’s Republic of China, V ol.1, 2000, p. 177.[2]W. Shi, R.B. Shi, Y.R. Lu, Chin. Pharm. J., 34(1999) 724.[3]J.B. Xing, P. Li, D.L. Wen, Chin. Med. Mater., 26(2001) 457.[4]Y.Q. Zhang, L.C. Xu, L.P. Wang, J. Chin. Med. Mater., 21(1996) 204.[5] D. Zhang, Z.W. Li, Y. Jiang, J. Pharm. Anal., 16(1996) 83.[6]T.Z. Wang, Y.M. Li, Huaxiyaoxue Zazhi, 15(2000) 292.[7]J.ZH. Shi, G.T. Liu. Acta Pharm. Sin., 30(1995) 311.[8]Y. P. Liu, J. Liu, X.SH. Jia, et al. Acta Pharmacol. Sin., 13 (1992) 209.[9]Y. P. Liu, J. Liu, X.SH. Jia, et al. Acta Pharmacol. Sin., 13 (1992) 213.[10]J.ZH. Shi, L. Wan, X.F. Chen.ZhongYao YaoLi Yu LinChuang, 6 (1990) 33.[11]J. Liu, L. Xia, X.F. Chen. Acta Pharmacol. Sin., 9 (1988) 395[12]H.J. Li, P. Li, W.C. Ye, J. Chromatogr. A 1008(2003) 167-72.[13]Q. Mao, D. Cao, X.SH. Jia. Acta Pharm. Sin., 28(1993) 273.[14]H. Kizu, S. Hirabayashi, M. Suzuki, et al. Chem. Pharm. Bull., 33(1985) 3473.[15]S. Saito, S. Sumita, N. Tamura, et al. Chem Pharm Bull., 38(1990) 411.[16]Alltech ELSD 2000 Operating Manual, Alltech, 2001, p. 16. In Chinese.[17]J.B. Xing, P. Li, Chin. Med. Mater., 22(1999) 366.Fig. 1 Chemical structures of seven saponins from Lonicera confusa macranthoidin B (1), macranthoidin A (2), dipsacoside B (3), hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester (4), macranthoside B (5), macranthoside A (6), and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside (7)Fig. 2Representative HPLC chromatograms of mixed standards and methanol extracts of Flos Lonicerae.Column: Agilent Zorbax SB-C18 column(250 4.6mm, 5.0µm), temperature of 25℃; Detector: ELSD, drift tube temperature 106℃, nitrogen flow-rate 2.6 l/min.A: Mixed standards, B: L. confusa, C: L. japonica, D: L. macranthoides, E: L. hypoglauca, F: L. similes.Table 1 Calibration curves for seven saponinsAnalytes Calibration curve ar2Test range(μg)LOD(μg)LOQ(μg)1 y=6711.9x-377.6 0.9940 0.56–22.01 0.26 0.882 y=7812.6x-411.9 0.9922 0.54–21.63 0.26 0.843 y=6798.5x-299.0 0.9958 0.46–18.42 0.22 0.724 y=12805x-487.9 0.9961 0.38–15.66 0.10 0.345 y=4143.8x-88.62 0.9989 0.42–16.82 0.18 0.246 y=3946.8x-94.4 0.9977 0.40–16.02 0.16 0.207 y=4287.8x-95.2 0.9982 0.42–16.46 0.12 0.22a y: Peak area; x: concentration (mg/ml)Table 2 Reproducibility of the assayAnalyteIntra-day variability Inter-day variability Content (mg/g) Mean RSD (%) Content (mg/g) Mean RSD (%)1 46.1646.2846.2246.22 0.1346.2245.3647.4226.33 2.232 5.385.385.165.31 2.405.285.345.045.22 3.043 4.374.304.184.28 2.244.284.464.024.255.204 nd1)-- -- nd -- --5 1.761.801.821.79 1.701.801.681.841.77 4.706 1.281.241.221.252.451.241.341.201.26 5.727 tr2)-- -- tr -- -- 1): not detected; 2): trace. RSD (%) = (SD/Mean) ×100%Table 3 Recovery of the seven analytesAnalyteOriginal(mg) Spiked(mg)Found(mg)Recovery(%)Mean(%)RSD(%)1 23.0823.1423.1119.7122.8628.1042.7346.1351.0199.7100.699.399.8 0.722.692.672.582.082.913.164.735.515.7698.197.6100.698.8 1.632.172.152.091.732.182.623.884.404.6598.8103.297.799.9 2.94nd1)1.011.050.980.981.101.0297.0104.8104.1102.0 4.250.880.900.910.700.871.081.561.752.0197.197.7101.898.9 2.660.640.620.610.450.610.751.081.211.3397.796.796.096.8 0.97tr2)1.021.101.081.031.111.07100.9102.799.1100.9 1.81): not detected; 2): trace.a Recovery (%) = (Amount found –Original amount)/ Amount spiked ×100%, RSD (%) = (SD/Mean) ×100%Table 4 Contents of seven saponins in Lonicera spp.Content (mg/g)1 2 3 4 5 6 7 L. confusa45.65±0.32 5.13±0.08 4.45±0.11tr1) 2.04±0.04tr 1.81±0.03 L. japonica nd2)nd 3.44±0.09nd nd nd nd L. macranthoides46.22±0.06 5.31±0.13 4.28±0.10 tr 1.79±0.03 1.25±0.03 tr L. hypoglauca11.17±0.07 nq3)53.78±1.18nd 1.72±0.02 2.23±0.06 2.52±0.04 L. similes41.22±0.25 4.57±0.07 3.79±0.09nd 1.75±0.02tr nd 1): trace; 2): not detected.. 3) not quantified owing to the suspicious purity of the peak.。
USP微生物限度检查 中文

USP微生物限度检查中文61)微生物限度检测(MICROBIAL LIMIT TESTS)此章提供方法来检测可能存在的好氧微生物其他制药过程中可能出现的微生物的数量,包括原材料和成品中的。
如果经过验证确认可以得到相同或更好的检测结论,也允许采用自动化的检测方法。
在样品检测过程中须进行无菌操作。
若无特别说明,则“培养(incubate)”一词指在30—35℃的培养箱内培养24至48小时;“生长(growth)”一词用于专门的判定,说明“存在和可能存在活的微生物”。
准备实验 (Preparatory Testing)本章涉及实验结果的有效性取决于:提供的被检测样品本身在实验条件下,被充分证明不会抑制可能存在的微生物的生长。
因此,在准备样品时,需要正规的实验操作和符合要求的实验条件,接种稀释样品到含有以下(微生物)培养物的培养基:金黄色(奥里斯)葡萄球菌(Staphylococcus aureus),大肠埃希氏菌(Escherichia coli), 铜绿假单胞菌(Pseudomonas aeruginosa), 和沙门氏菌(Salmonella)。
方法如下:将用肉汤培养基培养24小时后的(微生物)不小于10-3稀释的微生物培养物,加1 ml(微生物)培养液到磷酸(盐)缓冲液(pH 7.2),液体大豆酪蛋白消化物培养基(Fluid Soybean-Casein Digest Medium),或者液体乳糖培养基(Fluid Lactose Medium)。
相应培养基培养失败则需要采取以下方法更改检测程序:(1)增加稀释液体积,检测样品加入量仍维持不变;或者(2)中和一定数量的干扰因子;或者(3)结合(1)、(2)得出适当条件,使接种物得以生长。
以下是一些物质的成分和浓度,该物质及浓度可用于加入培养基、阻止物质发挥抑菌作用:大豆卵磷脂(soy lecithin, 0.5%)或者聚山梨醇酯20(polysorbate 20, 4.0%)。
硫辛酸注射液联合胰激肽原酶肠溶片对DPN_的临床疗效及生存质量的影响

DOI:10.16658/ki.1672-4062.2024.01.174硫辛酸注射液联合胰激肽原酶肠溶片对DPN的临床疗效及生存质量的影响王莉,朱海峰濉溪县中医医院内分泌科,安徽淮北235100[摘要]目的探讨硫辛酸注射液联合胰激肽原酶肠溶片对2型糖尿病周围神经病变(Diabetic Peripheral Neu⁃ropathy, DPN)患者的临床疗效、生存质量及安全性的影响。
方法选取2021年2月—2022年4月濉溪县中医医院60名DPN患者作为研究对象。
通过随机数表法分为两组,每组30例。
对照组采用常规治疗,观察组在对照组基础上加用硫辛酸注射液和胰激肽原酶肠溶片治疗。
比较两组患者的神经病变评分、神经电生理指标、生存质量评分、安全性指标和不良反应发生率。
结果治疗后,观察组神经病变评分(6.2±0.9)分低于对照组(7.6±1.1)分,差异有统计学意义(t=5.438,P<0.05);观察组神经电生理指标、生存质量评分、安全性指标均优于对照组,差异有统计学意义(P均<0.05);两组患者不良反应发生率比较,差异无统计学意义(P>0.05)。
结论硫辛酸注射液联合胰激肽原酶肠溶片对DPN患者有良好的临床疗效,能够改善神经功能、改善神经电生理指标、提高生存质量,且安全性高。
[关键词] 硫辛酸注射液;胰激肽原酶肠溶片;2型糖尿病周围神经病变;临床疗效[中图分类号] R587.2 [文献标识码] A [文章编号] 1672-4062(2024)01(a)-0174-05Effect of Lipoic Acid Injection Combined with Pancreatic Kininogenase Enteric-coated Tablets on Clinical Efficacy and Quality of Survival in DPN WANG Li, ZHU HaifengDepartment of Endocrinology, Suixi County Hospital of Traditional Chinese Medicine, Huaibei, Anhui Province, 235100 China[Abstract] Objective To investigate the effects of lipoic acid injection combined with pancreatic kininogenase enteric-coated tablets on the clinical efficacy, quality of survival and safety of patients with type 2 diabetic peripheral neuropathy (DPN). Methods 60 DPN patients admitted to Suixi County Hospital of Traditional Chinese Medicine from February 2021 to April 2022 were selected as the study objects. They were divided into two groups with 30 cases in each group by random number table method. The control group received conventional treatment, and the observation group was treated with lipoic acid injection and pancreatic kininogenase enteric-coated tablets on the basis of control group. Neuropathy score, neuroelectrophysiological index, quality of life score, safety index and incidence of adverse reactions were compared between the two groups. Results After treatment, the neuropathy score of observation group (6.2±0.9) points was lower than that of control group (7.6±1.1) points, and the difference was statistically significant (t= 5.438, P<0.05). Neuroelectrophysiological indexes, quality of survival scores and safety indexes of the observation group were better than those of the control group, and the differences were statistically significant (all P<0.05). There was no significant difference in the incidence of adverse reactions between the two groups (P>0.05). Conclusion Li⁃[作者简介]王莉(1982-),女,本科,主治医生,研究方向为糖尿病周围神经病变。
国际上著名的从事药剂学研究的专家

Intra Oral Delivery (口腔内传递)直接由口腔黏膜吸收,瞬间进入血液循环,有效成分不流失。
Universities, Departments,FacultiesResearchersButler University College of Pharmacy and Health Sciences Health Sciences USA Associate Professor Nandita G. DasMain focus on her research facilities are about peformulation, biopharmaceutics, drug targeting, anticancer drug delivery.Purdue University School of Pharmacy and Pharmacal Sciences Department of Industrial and Physical Pharmacy (IPPH) USA Professor Kinam ParkControlled Drug Delivery, Glucose-Sensitive Hydrogels for Self-Regulated Insulin Delivery, Superporous Hydrogel Composites, Oral Vaccination using Hydrogel Microparticles, Fractal Analysis of Pharmaceutical Solid Materials.St. John's University School of Pharmacy and Allied Health ProfessionsUSA Professor Parshotam L. MadanControlled and targeted drug delivery systems; Bio-erodible polymers as drug delivery systemsThe University of Iowa College of Dentistry Department of Oral Pathology, Radiology, and Medicine USA Professor Christopher A. Squierpermeability of skin, and oral mucosa to exogenous substances, including alcohol and tobacco, and drug deliveryThe University of Iowa College of Pharmacy Department of Pharmaceutics USA Associate Professor Maureen D. DonovanMucosal drug delivery especially via the nasal, gastrointestinal and vaginal epithelia; and mechanisms of drug absorption and disposition.The University of Texas at San Antonio College of Engineering Department of Biomedical Engineering USA Professor Jeffrey Y. ThompsonDental restorative materials and implantsThe University of Utah Pharmaceutics & Pharmaceutical Chemistry USA Professor John W. MaugerDr. Maugner is mainly focused on dissolution testing and coating technology of orally administered drug products with bitter taste about which he is one of the inventors of a filed patent.University of Kentucky College of Pharmacy Pharmaceutical Sciences USA Professor Peter CrooksDr. Crooks is internationally known for his research work in drug discovery, delivery, and development, which includes drug design and synthesis, pharmacophore development, drug biotransformation studies, prodrug design, and medicinal plant natural product research. His research also focuses on preclinical drug development, including drug metabolism and pharmacokinetics in animal models, dosage form development, and drug delivery assessment using both conventional and non-conventional routes, and preformulation/formulation studies.Associate Professor Russell MumperDr. Mumper's main research areas are thin-films and mucoadhesive gels for (trans)mucosal delivery of drugs, microbicides, and mucosal vaccines, and nanotemplate engineering of nano-based detection devices and cell-specific nanoparticles for tumor and brain targeting, gene therapy and vaccines.West Virginia University School of Pharmacy Department of Basic Pharmaceutical Sciences USA Associate Professor Paula Jo Meyer StoutDr. Stout's research areas are composed of dispersed pharmaceutical systems, sterile product formulation DDS for dental diseases and coating of sustained release formulations.Monash University Victorian College of Pharmacy Department of Pharmaceutics Australia Professor Barrie C. FinninTransdermal Drug Delivery. Physicochemical Characterisation of Drug Candidates. Topical Drug Delivery. Drug uptake by the buccal mucosaProfessor Barry L. ReedTransdermal Drug Delivery. Topical Drug Delivery. Formulation of Dental Pharmaceuticals.University of Gent Faculty of Pharmaceutical Sciences Department of Pharmaceutics Belgium Professor Chris Vervaet-Extrusion/spheronisation - Bioadhesion - Controlled release based on hot stage extrusion technology - Freeze-drying - Tabletting and - GranulationPh.D. Els AdriaensMucosal drug delivery (Vaginal and ocular) Nasal BioadhesionUniversity of Gent Faculty of Pharmaceutical SciencesLaboratory of Pharmaceutical Technology Belgium Professor Jean Paul Remonbioadhesive carriers, mucosal delivery, Ocular bioerodible minitablets, Compaction of enteric-coated pellets; matrix-in-cylinder system for sustained drug delivery; formulation of solid dosage forms; In-line monitoring of a pharmaceutical blending process using FT-Raman spectroscopy; hot-melt extruded mini-matricesDanish University of Pharmaceutical Sciences Department of Pharmaceutics Denmark Associate Professor Jette JacobsenLow soluble drugs ?in vitro lymphatic absorption Drug delivery to the oral cavity ?in vitro models (cell culture, diffusion chamber) for permeatbility and toxicity of drugs, in vivo human perfusion model, different formulation approaces, e.g. iontophoresis.。
Blonanserin_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :BlonanserinCatalog No. :HY-13575CAS No. :132810-10-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:AD⁻5423; AD5423; AD 5423Formula:C23H30FN3Molecular Weight:367.50CAS No. :132810-10-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
C反应蛋白、乳酸脱氢酶和β2-微球蛋白

DOI:10.19368/ki.2096-1782.2022.20.017C反应蛋白、乳酸脱氢酶和β-微球蛋白检测对2急性白血病患者治疗预后评估中的价值丁晨江苏省人民医院浦口分院检验科,江苏南京211800[摘要]目的探讨C反应蛋白(C-reactiveprotein,CRP)、乳酸脱氢酶(lactate dehydrogenase,LDH)和β2-微球蛋白(beta-2-microglobulin,β2-MG)检测对急性白血病患者治疗预后评估中的价值。
方法选取2018年8月—2021年8月江苏省人民医院浦口分院收治的76例急性白血病患者,设定为观察组,并选取同时期健康体检者76名,设定为对照组。
比较两组CRP、LDH和β2-MG水平及预后情况。
结果观察组患者CRP(76.13±5.06)mg/L、LDH(586.74±13.58)U/L、β2-MG(3.81±0.25)mg/L水平明显高于对照组,差异有统计学意义(t= 126.793、202.249、87.016,P<0.05);观察组ANLL患者血清CRP、LDH、β2-MG水平高于ALL患者,差异有统计学意义(P<0.05);相比较初治组,缓解组CRP(2.89±0.11)mg/L、LDH(158.54±11.69)U/L、β2-MG(1.29±0.11)mg/L 明显降低,差异有统计学意义(t=116.611、198.872、75.247,P<0.05);与未缓解组(74.85±5.65)mg/L、(579.75±12.55)U/L、(3.68±0.23)mg/L相比较,缓解组CRP、LDH、β2-MG水平显著降低,差异有统计学意义(t=106.140、109.396、55.236,P<0.05);未缓解组血清CRP、LDH、β2-MG水平与初治组相比较,差异无统计学意义(P> 0.05);观察组患者治疗后,65例缓解,11例病情未缓解,患者均无严重不良反应。
上海百斯杰生物工程有限公司介绍企业发展分析报告

Enterprise Development专业品质权威Analysis Report企业发展分析报告上海百斯杰生物工程有限公司免责声明:本报告通过对该企业公开数据进行分析生成,并不完全代表我方对该企业的意见,如有错误请及时联系;本报告出于对企业发展研究目的产生,仅供参考,在任何情况下,使用本报告所引起的一切后果,我方不承担任何责任:本报告不得用于一切商业用途,如需引用或合作,请与我方联系:上海百斯杰生物工程有限公司1企业发展分析结果1.1 企业发展指数得分企业发展指数得分上海百斯杰生物工程有限公司综合得分说明:企业发展指数根据企业规模、企业创新、企业风险、企业活力四个维度对企业发展情况进行评价。
该企业的综合评价得分需要您得到该公司授权后,我们将协助您分析给出。
1.2 企业画像类别内容行业科技推广和应用服务业-技术推广服务资质增值税一般纳税人产品服务人体干细胞、基因诊断与治疗技术开发和应用1.3 发展历程2工商2.1工商信息2.2工商变更2.3股东结构2.4主要人员2.5分支机构2.6对外投资2.7企业年报2.8股权出质2.9动产抵押2.10司法协助2.11清算2.12注销3投融资3.1融资历史3.2投资事件3.3核心团队3.4企业业务4企业信用4.1企业信用4.2行政许可-工商局4.3行政处罚-信用中国4.4行政处罚-工商局4.5税务评级4.6税务处罚4.7经营异常4.8经营异常-工商局4.9采购不良行为4.10产品抽查4.11产品抽查-工商局4.12欠税公告4.13环保处罚4.14被执行人5司法文书5.1法律诉讼(当事人)5.2法律诉讼(相关人)5.3开庭公告5.4被执行人5.5法院公告5.6破产暂无破产数据6企业资质6.1资质许可6.2人员资质6.3产品许可6.4特殊许可7知识产权7.1商标7.2专利7.3软件著作权7.4作品著作权7.5网站备案7.6应用APP7.7微信公众号8招标中标8.1政府招标8.2政府中标8.3央企招标8.4央企中标9标准9.1国家标准9.2行业标准9.3团体标准9.4地方标准10成果奖励10.1国家奖励10.2省部奖励10.3社会奖励10.4科技成果11土地11.1大块土地出让11.2出让公告11.3土地抵押11.4地块公示11.5大企业购地11.6土地出租11.7土地结果11.8土地转让12基金12.1国家自然基金12.2国家自然基金成果12.3国家社科基金13招聘13.1招聘信息感谢阅读:感谢您耐心地阅读这份企业调查分析报告。
【JACS】双磷酸类脂-递送mRNA至骨微环境
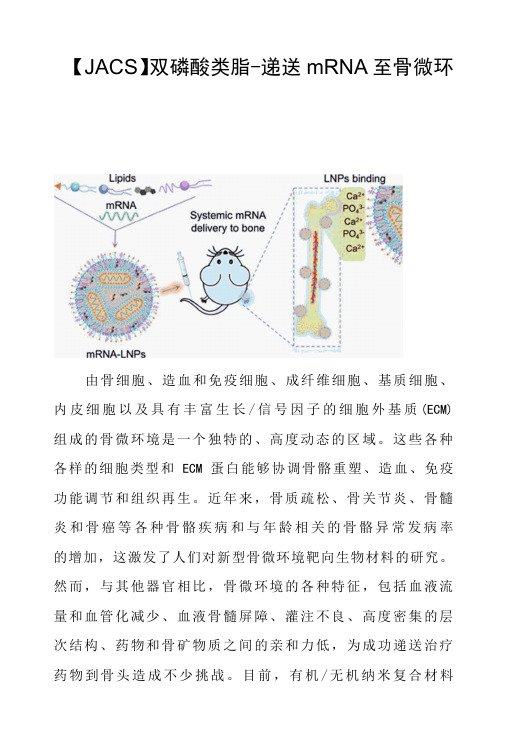
【JACS】双磷酸类脂-递送mRNA至骨微环由骨细胞、造血和免疫细胞、成纤维细胞、基质细胞、内皮细胞以及具有丰富生长/信号因子的细胞外基质(ECM) 组成的骨微环境是一个独特的、高度动态的区域。
这些各种各样的细胞类型和ECM蛋白能够协调骨骼重塑、造血、免疫功能调节和组织再生。
近年来,骨质疏松、骨关节炎、骨髓炎和骨癌等各种骨骼疾病和与年龄相关的骨骼异常发病率的增加,这激发了人们对新型骨微环境靶向生物材料的研究。
然而,与其他器官相比,骨微环境的各种特征,包括血液流量和血管化减少、血液骨髓屏障、灌注不良、高度密集的层次结构、药物和骨矿物质之间的亲和力低,为成功递送治疗药物到骨头造成不少挑战。
目前,有机/无机纳米复合材料和水凝胶已被开发用于靶向和局部输送治疗药物至骨微环境,如抗生素、生长因子、抗炎分子、抗癌制剂和激素等。
然而,这些材料的传递效率较低,需要高剂量才能在病变部位达到理想的治疗剂量和长期的生物利用度,但也增加了严重副作用的发生率。
这些局限性表明需要有效和安全的治疗方法来将药物靶向输送到骨微环境。
一类对骨传递特别有吸引力的治疗方法是核酸疗法,它通过传递外源性核酸来调控靶向部位的治疗性基因表达,以治疗疾病。
目前,临床上领先的RNA治疗非病毒载体是脂质纳米颗粒(LNPs),它是一种由电离脂质、胆固醇、磷脂和聚乙二醇(PEG)脂质组成的四组分配方,已经用于Alnylam制药公司的siRNA治疗ONPATTRO,以及辉瑞∕BioNTech和Moderna公司生产的COVID-19 mRNA疫苗。
最近,一些靶向谷微环境的核酸递送材料被报道。
例如,LNPs封装的siRNA 被递送到骨髓细胞,以沉默炎性单核细胞和内皮细胞中的基因,选择性地抑制这些细胞及其后代的迁移。
尽管取得了一些进展,但被动扩散LNPs将mRNA特异地递送到骨微环境仍然极具挑战性性。
因此,开发一种靶向LNP技术,将mRNA 传递到骨中,这是一个亟待解决的问题。
P0332 抗酒石酸酸性磷酸酶检测试剂盒

抗酒石酸酸性磷酸酶检测试剂盒产品简介:碧云天生产的抗酒石酸酸性磷酸酶检测试剂盒(Tartrate Resistant Acid Phosphatase Assay Kit)是一种用于快速、便捷地检测细胞或组织的裂解液或匀浆液、血浆、血清、尿液等样品中内源性的抗酒石酸酸性磷酸酯酶活性的试剂盒。
酸性磷酸酶(Acid Phosphatase),也称酸性磷酸酯酶,在酸性条件下可以催化磷酸酯键的水解。
酸性磷酸酶是一个蛋白家族,哺乳动物中其分子量从18kD到100kD不等。
酸性磷酸酶主要分为两类,一类为抗酒石酸酸性磷酸酶(tartrate resistant acid phosphatase, TRAP),一类为抗氟离子酸性磷酸酶。
抗酒石酸酸性磷酸酶是一种糖基化的含金属蛋白酶,在破骨细胞(osteoclast)和破软骨细胞(chondroclast)中高表达。
在活化的巨噬细胞和神经元中也有表达。
抗酒石酸酸性磷酸酶在细胞信号转导、细胞增殖和分化等方面起重要作用,也和活性氧产生和铁离子转运等有关。
抗酒石酸酸性磷酸酶可以被破骨细胞释放到血液中,几乎被认为是是机体破骨活性的唯一血液指标。
在一些疾病状态下,如白血病性网状内皮组织增生症(leukaemic reticuloendotheliosis)、戈谢病(Gaucher’s disease)、艾滋病导致的脑病(HIV-induced encephalopathy)、破骨细胞瘤(osteoclastoma)、骨质疏松(osteoporosis)和代谢性骨疾病(metabolic bone disease)有关。
TRAP敲除小鼠有轻微的骨骼石化症,TRAP转基因小鼠有轻微的骨质疏松。
本试剂盒可以检测细胞或组织的裂解液或匀浆液、血浆、血清、尿液或纯化的酶样品等中的抗酒石酸酸性磷酸酶活性。
Para-nitrophenyl phosphate (pNPP)是一种常用的磷酸酶显色底物,在酸性条件下,可在酸性磷酸酶作用下生成para-nitrophenol。
我院生化药物和酶诊断试剂的研制

我院生化药物和酶诊断试剂的研制
杜平中
【期刊名称】《中国医药工业杂志》
【年(卷),期】1997(28)3
【摘要】概述近20年我院在药用酶(胶原酶,链激酶,尿激酶,弹性酶,溶菌酶)、工业用酶(青霉素酰化酶,头孢菌素酰化酶,乙内酰脲酶)、诊断用酶(胆固醇,甘油三酯,肌酸激酶,乳酸脱氢酶。
【总页数】5页(P103-107)
【关键词】生化药物;酶诊断试剂;药用酶
【作者】杜平中
【作者单位】上海医药工业研究院
【正文语种】中文
【中图分类】TQ464.8
【相关文献】
1.艰难梭菌A毒素单克隆抗体酶标诊断试剂盒的研制 [J], 常学荣;傅思武
2.肺炎衣原体抗体酶免疫诊断试剂盒的研制及应用 [J], 薛晓英;何素华;陈锡枚;刘
士德
3.3-磷酸甘油醛脱氢酶(GAPD)诊断试剂盒的研制及对心梗病人诊断价值的研究 [J], 李彬先;张玉民
4.单胺氧化酶(MAO)诊断试剂盒的研制及对肝硬化病人诊断价值的研究 [J], 董丽刚
5.烟草环斑病毒、马铃薯X病毒碱性磷酸酶酶联诊断试剂盒的研制简报 [J], 李明福;魏梅生;朱水芳;李桂芬;陈燕芳;黄文胜;相宁;李尉民;施宗伟
因版权原因,仅展示原文概要,查看原文内容请购买。
USP微生物限度检查 中文

USP微生物限度检查中文61)微生物限度检测(MICROBIAL LIMIT TESTS)此章提供方法来检测可能存在的好氧微生物其他制药过程中可能出现的微生物的数量,包括原材料和成品中的。
如果经过验证确认可以得到相同或更好的检测结论,也允许采用自动化的检测方法。
在样品检测过程中须进行无菌操作。
若无特别说明,则“培养(incubate)”一词指在30—35℃的培养箱内培养24至48小时;“生长(growth)”一词用于专门的判定,说明“存在和可能存在活的微生物”。
准备实验 (Preparatory Testing)本章涉及实验结果的有效性取决于:提供的被检测样品本身在实验条件下,被充分证明不会抑制可能存在的微生物的生长。
因此,在准备样品时,需要正规的实验操作和符合要求的实验条件,接种稀释样品到含有以下(微生物)培养物的培养基:金黄色(奥里斯)葡萄球菌(Staphylococcus aureus),大肠埃希氏菌(Escherichia coli), 铜绿假单胞菌(Pseudomonas aeruginosa), 和沙门氏菌(Salmonella)。
方法如下:将用肉汤培养基培养24小时后的(微生物)不小于10-3稀释的微生物培养物,加1 ml(微生物)培养液到磷酸(盐)缓冲液(pH 7.2),液体大豆酪蛋白消化物培养基(Fluid Soybean-Casein Digest Medium),或者液体乳糖培养基(Fluid Lactose Medium)。
相应培养基培养失败则需要采取以下方法更改检测程序:(1)增加稀释液体积,检测样品加入量仍维持不变;或者(2)中和一定数量的干扰因子;或者(3)结合(1)、(2)得出适当条件,使接种物得以生长。
以下是一些物质的成分和浓度,该物质及浓度可用于加入培养基、阻止物质发挥抑菌作用:大豆卵磷脂(soy lecithin, 0.5%)或者聚山梨醇酯20(polysorbate 20, 4.0%)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jan.-23-2018Print Date:Jan.-23-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :CrolibulinCatalog No. :HY-13603CAS No. :1000852-17-41.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:EPC2407Formula:C18H17BrN4O3Molecular Weight:417.26CAS No. :1000852-17-44. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Light yellow to yellow (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
