CX-5461_1138549-36-6_MSDS_MedChemExpress
MTS法检测细胞增殖活性

Promega Corporation ·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA1.Description (1)2.Product Components and Storage Conditions (4)3.Protocols (5)A.General Protocol (5)B.Example of a Protocol for Bioassay of IL-6 Using B9 Cells (5)4.General Considerations (6)A.Background Absorbance.....................................................................................6B.Optional Wavelengths to Record Data.............................................................7C.Lymphocyte Assays.............................................................................................8D.Reagent Optimization..........................................................................................8E.Cell Number Optimization (8)5.References (9)6.Related Products (9)1.DescriptionThe CellTiter 96®AQ ueous One Solution Cell Proliferation Assay (a)is a colorimetric method for determining the number of viable cells in proliferation or cytotoxicity assays. The CellTiter 96®AQ ueous One Solution Reagent contains a novel tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS (a)] and an electron coupling reagent (phenazine ethosulfate; PES). PES has enhanced chemical stability, which allows it to be combined with MTS to form a stable solution. This convenient “One Solution” format is an improvement over the first version of the CellTiter 96®AQ ueous Assay, where phenazine methosulfate (PMS) is used as the electron coupling reagent, and the PMS Solution and MTS Solution are supplied separately.The MTS tetrazolium compound (Owen’s reagent) is bioreduced by cells into a colored formazan product that is soluble in tissue culture medium (Figure 1, 1).This conversion is presumably accomplished by NADPH or NADH produced by dehydrogenase enzymes in metabolically active cells (2). Assays are performed by adding a small amount of the CellTiter 96®AQ ueous One Solution Reagent directly to culture wells, incubating for 1–4 hours and then recording the absorbance at 490nm with a 96-well plate reader (3,4).CellTiter 96®AQ ueous One Solution Cell Proliferation AssayAll technical literature is available on the Internet at: /tbs Please visit the web site to verify that you are using the most current version of this Technical Bulletin. Please contact Promega Technical Services if you have questions on useofthissystem.E-mail:********************.1.Description (continued)The quantity of formazan product as measured by the absorbance at 490nm is directly proportional to the number of living cells in culture (Figure 2). Because the MTS formazan product is soluble in tissue culture medium, the CellTiter 96®AQ ueous One Solution Assay requires fewer steps than procedures that usetetrazolium compounds such as MTT or INT (5,6). The formazan product of MTT reduction is a crystalline precipitate that requires an additional step in the procedure to dissolve the crystals before recording absorbance readings at570nm (7).If you currently use a [3H]thymidine incorporation assay, addition of theCellTiter 96®AQ ueous One Solution Reagent can be substituted for the pulse of[3H]thymidine at the time point in the assay when the pulse of radioactivethymidine is usually added. Bioassay data comparing [3H]thymidineincorporation to the MTS-based CellTiter 96®AQ ueous Assay and the original MTT-based CellTiter 96®Assay demonstrate that tetrazolium reagents can be substituted for [3H]thymidine incorporation (4,7).Advantages of the CellTiter 96®AQ ueous One Solution Assay include:•Easy-to-Use:Add the CellTiter 96®AQ ueous One Solution Reagent to cells,incubate and read absorbance.•Convenient:Supplied as a single solution, filter-sterilized and ready for adding to assay plates.•Fast:Perform the assay in a 96-well plate with no washing or cell harvesting.Also eliminates solubilization steps normally required for MTT assays.•Non-Radioactive:Requires no scintillation cocktail or radioactive wastedisposal.Promega Corporation ·2800 Woods Hollow Road ·Madison, WI 53711-5399 USAFigure 1. Structures of MTS tetrazolium and its formazan product.N N N NSO 3OCH 2COOHMTS +SN CH 3CH 3N N N N H SO 3OCH 2COOH FormazanS NCH 3CH 31605M B 09_6A•Flexible:Plates can be read and returned to incubator for further color development.•Safe:Requires no volatile organic solvent to solubilize theformazanproduct (unlike MTT).Promega Corporation ·2800 Woods Hollow Road ·Madison, WI 53711-5399 USAFigure 2. Effect of cell number on absorbance at 490nm measured using theCellTiter 96®AQ ueous One Solution Assay. Various numbers of B9 hybridoma cells were added to the wells of a 96-well plate in RPMI containing 50μM 2-mercaptoethanol and supplemented with 5% FBS and 2ng/ml IL-6. The medium was allowed toequilibrate for 1 hour, then 20μl/well of CellTiter 96®AQ ueous One Solution Reagent was added. After 1 hour at 37°C in a humidified, 5% CO 2atmosphere, the absorbance at 490nm was recorded using an ELISA plate reader. Each point represents the mean ± SD of 4 replicates. The correlation coefficient of the line was 0.993, indicating alinear response between cell number and absorbance at 490nm. The backgroundabsorbance shown at zero cells/well was not subtracted from these data.2 × 1044 × 1046 × 1048 × 1041 × 1051.2 × 1051.4 × 1051.6 × 105A b s o r b a n c e (490n m )Cells/Well1606M A 10_9A2.Product Components and Storage ConditionsProduct Size Cat.# CellTiter 96®AQ ueous One SolutionCell Proliferation Assay200 assays G3582 For Laboratory Use. Includes:•4ml CellTiter 96®AQ ueous One Solution ReagentProduct Size Cat.# CellTiter 96®AQ ueous One SolutionCell Proliferation Assay1,000 assays G3580 For Laboratory Use. Includes:•20ml CellTiter 96®AQ ueous One Solution ReagentProduct Size Cat.# CellTiter 96®AQ ueous One SolutionCell Proliferation Assay5,000 assays G3581 For Laboratory Use. Includes:•100ml CellTiter 96®AQ ueous One Solution ReagentStorage Conditions:For long-term storage, store the CellTiter 96®AQ ueous One Solution Reagent at –20°C, protected from light. See the expiration date on the ProductInformation Label. For frequent use, solutions may be stored at 4°C, protected fromlight, for up to 6 weeks.Note:The performance of CellTiter 96®AQ ueous One Solution Reagent after 10 freeze-thaw cycles was demonstrated to be equal to that of freshly prepared solution.Safety:To the best of our knowledge, the chemical, physical and toxicologicalproperties of this product have not been thoroughly investigated; therefore, werecommend the use of gloves, lab coats and eye protection when working with these orany chemicals.Light-Sensitivity:The CellTiter 96®AQ ueous One Solution Reagent is light-sensitive andis supplied in an amber container. Discoloration may occur if solutions are exposed tolight outside of the container for several hours. This discoloration may cause slightlyhigher background 490nm absorbance readings, but it should not affect theperformance of the CellTiter 96®AQ ueous One Solution Assay.Promega Corporation·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA3.ProtocolsMaterials to Be Supplied by the User•96-well plates suitable for tissue culture•repeating pipettes, digital pipettes or multichannel pipettes•96-well plate reader3.A.General Protocol1.Thaw the CellTiter 96®AQ ueous One Solution Reagent. It should takeapproximately 90 minutes at room temperature, or 10minutes in a waterbath at 37°C, to completely thaw the 20ml size.2.Pipet 20μl of CellTiter 96®AQ ueous One Solution Reagent into each well ofthe 96-well assay plate containing the samples in 100μl of culture medium.Note:We recommend repeating pipettes, digital pipettes or multichannelpipettes for convenient delivery of uniform volumes of CellTiter 96®AQ ueous One Solution Reagent to the 96-well plate.3.Incubate the plate at 37°C for 1–4 hours in a humidified, 5% CO2atmosphere.Note:To measure the amount of soluble formazan produced by cellularreduction of MTS, proceed immediately to Step 4. Alternatively, to measurethe absorbance later, add 25μl of 10% SDS to each well to stop the reaction.Store SDS-treated plates protected from light in a humidified chamber atroom temperature for up to 18 hours. Proceed to Step 4.4.Record the absorbance at 490nm using a 96-well plate reader.3.B.Example of a Protocol for Bioassay of IL-6 Using B9 Cells1.Maintain stock cultures of B9 cells in RPMI 1640 medium containing5%FBS, 50μM 2-mercaptoethanol (2-ME) supplemented with 5ng/mlhuman recombinant IL-6 . Subculture the stock cultures of cells to2×104cells/ml, and refeed with human recombinant IL-6 every 3 days orwhen a density of 2 × 105 cells/ml is reached.Note:B9 cells used for the bioassay should be from stock cultures 2 daysafter the last subculture (feeding with IL-6).2.Add 50μl/well of IL-6 samples or standards to be measured, diluted inRPMI 1640 medium containing 5% FBS and 50μM 2-ME. Start the titrationof the IL-6 standard at 4ng/ml in column 12, and perform serial twofolddilutions across the plate to column 2 (to 4pg/ml). (After the cellsuspension is added in Step 5 below, the final concentration of the titratedstandard will be 2ng/ml in column 12 to 2pg/ml in column 2.) Usecolumn1 for the negative control: RPMI 1640 medium (and supplements)without IL-6. Equilibrate the plate at 37°C in a humidified, 5% CO2atmosphere while harvesting the cells for assay.Promega Corporation·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA3.B.Example of a Protocol for Bioassay of IL-6 Using B9 Cells (continued)3.Wash the B9 cells twice in RPMI 1640 containing 5% FBS and 50μM 2-MEby centrifugation at 300 × g for 5 minutes.4.Determine cell number and viability (by trypan blue exclusion), andresuspend the cells to a final concentration of 1 × 105 cells/ml in RPMI 1640supplemented with 5% FBS and 50μM 2-ME.5.Dispense 50μl of the cell suspension (5,000 cells) into all wells of the plateprepared in Step 2. The total volume in each well should be 100μl.6.Incubate the plate at 37°C for 48–72 hours in a humidified, 5% CO2atmosphere.7.Add 20μl per well of CellTiter 96®AQ ueous One Solution Reagent.8.Incubate the plate at 37°C for 1–4 hours in a humidified, 5% CO2atmosphere.Note:To measure the amount of soluble formazan produced by cellularreduction of MTS, proceed immediately to Step 9. Alternatively, to measurethe absorbance at a later time, add 25μl of 10% SDS to each well to stop thereaction. Store SDS-treated plates protected from light in a humidifiedchamber at room temperature for up to 18 hours. Proceed to Step9.9.Record the absorbance at 490nm using a 96-well plate reader.10.Plot the corrected absorbance at 490nm (Y axis) versus concentration ofgrowth factor (X axis). Determine the X-axis value corresponding to one-halfthe difference between the maximum (plateau) and minimum (no growthfactor control) absorbance values; this is the ED50value (ED50= theconcentration of growth factor necessary to give one-half the maximumresponse.)4.General Considerations4.A.Background AbsorbanceA small amount of spontaneous 490nm absorbance occurs in culture mediumincubated with CellTiter 96®AQ ueous One Solution Reagent. The type of culturemedium used, type of serum, pH and length of exposure to light are variablesthat may contribute to the background 490nm absorbance. Backgroundabsorbance is typically 0.2–0.3 absorbance units after 4 hours of culture.Background absorbance may result from chemical interference of certaincompounds with tetrazolium reduction reactions. Strong reducing substances,including ascorbic acid, or sulfhydryl-containing compounds, such asglutathione, coenzyme A and dithiothreitol, can reduce tetrazolium saltsnonenzymatically and lead to increased background absorbance values.Culture medium at elevated pH or extended exposure to direct light also maycause an accelerated spontaneous reduction of tetrazolium salts and result inincreased background absorbance values. If phenol red containing medium is Promega Corporation·2800 Woods Hollow Road ·Madison, WI 53711-5399 USAused, an immediate change in color may indicate a shift in pH caused by thetest compounds. Specific chemical interference of test compounds can beconfirmed by measuring absorbance values from control wells containingmedium without cells at various concentrations of test compound.Background 490nm absorbance may be corrected as follows: Prepare atriplicate set of control wells (without cells) containing the same volumes ofculture medium and CellTiter 96®AQ ueous One Solution Reagent as in theexperimental wells. Subtract the average 490nm absorbance from the “no cell”control wells from all other absorbance values to yield corrected absorbances.4.B.Optional Wavelengths to Record DataFigure 3 shows an absorbance spectrum of the formazan product resultingfrom reduction of MTS. We recommend recording data at the absorbance peakof 490nm; however, if your 96-well plate reader does not have a 490nm filter,data can be recorded at wavelengths of 450–540nm. Absorbance may berecorded at other wavelengths if necessary, but loss in sensitivity will result. Areference wavelength of 630–700nm may be used to subtract backgroundcontributed by excess cell debris, fingerprints and other nonspecific absorbance.Promega Corporation ·2800 Woods Hollow Road ·Madison, WI 53711-5399 USAFigure 3. Absorbance spectrum of MTS/formazan. The absorbance spectrum of the formazan product resulting from reduction of the MTS tetrazolium compoundshows an absorbance maximum at 490nm. The negative absorbance values (382nm)correspond to the disappearance of MTS as it is converted into formazan.A b s o r b a n c e Wavelength (nm)3.002.502.001.501.000.500.00-0.503004005006007002284M A 07_8A4.C.Lymphocyte AssaysLymphocytes may produce less formazan than other cell types (8). To achievesignificant absorbance changes with lymphocytes, increase the number ofcells to approximately 2.5–10 × 104cells/well and incubate the plate withCellTiter 96®AQ ueous One Solution Reagent for the entire 4-hour period.4.D.Reagent OptimizationThe concentrations of tetrazolium and electron transfer reagents have beenoptimized for general use with a wide variety of cell lines cultured in 96-wellplates containing 100μl of medium. If different volumes of culture medium areused, adjust the volume to maintain a ratio of 20μl CellTiter 96®AQ ueous OneSolution Reagent per 100μl culture medium. This reagent:medium ratio resultsin a final concentration of 317μg/ml MTS in the assay wells. Minor variationsin the optimum concentrations of tetrazolium and electron transfer reagentsoccur with different cell lines; however, assay sensitivity is seldomcompromised using the formulation in the CellTiter 96®AQ ueous One SolutionReagent. If reagent optimization is critical to your assay procedure, werecommend using the CellTiter 96®AQ ueous Non-Radioactive Cell ProliferationAssay (Cat.# G5421, G5430, G5440) or the CellTiter 96®AQ ueous MTS ReagentPowder products (Cat.# G1111, G1112) that supply the chemicals separately.4.E.Cell Number OptimizationCell proliferation assays require cells to grow over a period of time. Therefore,choose an initial number of cells per well that produces an assay signal nearthe low end of the linear range of the assay. This helps to ensure that the signalmeasured at the end of the assay will not exceed the linear range of the assay.This cell number can be determined by performing a cell titration as shown inFigure 2.Different cell types have different levels of metabolic activity. Factors that affectthe metabolic activity of cells may affect the relationship between cell numberand absorbance. Anchorage-dependent cells that undergo contact inhibitionmay show a change in metabolic activity per cell at high densities, resulting ina nonlinear relationship between cell number and absorbance. Factors thataffect the cytoplasmic volume or physiology of the cells will affect metabolicactivity.For most tumor cells, hybridomas and fibroblast cell lines, 5,000 cells per wellis recommended to initiate proliferation studies, although fewer than 1,000 cellscan usually be detected. The known exception to this is blood lymphocytes,which generally require 25,000–250,000 cells per well to obtain a sufficientabsorbance reading.Promega Corporation·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA5.References1.Barltrop, J.A. et al.(1991) 5-(3-carboxymethoxyphenyl)-2-(4,5-dimenthylthiazoly)-3-(4-sulfophenyl)tetrazolium, inner salt (MTS) and related analogs of3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT) reducing to purplewater-soluble formazans as cell-viability indicators. Bioorg. Med. Chem. Lett.1, 611-4.2.Berridge, M.V. and Tan, A.S. (1993) Characterization of the cellular reduction of3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellularlocalization, substrate dependence, and involvement of mitochondrial electrontransport in MTT reduction. Arch. Biochem. Biophys.303, 474–82.3.Cory, A.H. et al.(1991) Use of an aqueous soluble tetrazolium/formazan assay for cellgrowth assays in culture. Cancer Commun.3, 207–12.4.Riss, T.L. and Moravec, R.A. (1992) Comparison of MTT, XTT, and a noveltetrazolium compound for MTS for in vitro proliferation and chemosensitivity assays.Mol. Biol. Cell (Suppl.)3, 184a.5.Mosmann, T. (1983) Rapid colorimetric assay for cellular growth and survival:Application to proliferation and cytotoxicity assays. J. Immunol. Methods65, 55–63.6.Bernabei, P.A. et al.(1989) In vitro chemosensitivity testing of leukemic cells:Development of a semiautomated colorimetric assay. Hematol. Oncol.7, 243–53.7.CellTiter 96®Non-Radioactive Cell Proliferation Assay Technical Bulletin#TB112,Promega Corporation.8.Chen, C.-H., Campbell, P.A. and Newman, L.S. (1990) MTT colorimetric assay detectsmitogen responses of spleen but not blood lymphocytes. Int. Arch. Allergy Appl.Immunol.93, 249–556.Related ProductsMTS/MTT-Based Cell Viability Assay SystemsProduct Size Cat.# CellTiter 96®AQ ueous Non-RadioactiveCell Proliferation Assay1,000 assays G54215,000 assays G543050,000 assays G5440 CellTiter 96®AQ ueous MTS Reagent Powder*250mg G11121g G1111 CellTiter 96®Non-RadioactiveCell Proliferation Assay1,000 assays G40005,000 assays G4100For Laboratory Use. *PMS is not supplied with MTS Reagent Powder and must beobtained separately.Promega Corporation·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA6.Related Products (continued)Luminescent-Based Cell Viability Assay SystemProduct Size Cat.# CellTiter-Glo®Luminescent Cell Viability Assay10ml G757010 × 10ml G7571100ml G757210 × 100ml G7573Resazurin-Based Cell Viability Assay SystemProduct Size Cat.# CellTiter-Blue®Cell Viability Assay20ml G8080100ml G808110 × 100ml G8082Fluorescent-Based Cell Viability AssayProduct Size Cat.# CellTiter-Fluor™ Cell Viability Assay10ml G60805 × 10ml G60812 × 50ml G6082For Laboratory Use.Cytotoxicity Assay Systems (LDH)Product Size Cat.# CytoTox-ONE™ HomogeneousMembrane Integrity Assay200–800 assays G78901,000–4,000 assays G7891 CytoTox 96®Non-Radioactive Cytotoxicity Assay*1,000 assays G1780 CytoTox-Glo™ Cytotoxicity Assay*10ml G92905 × 10ml G92912 × 50ml G9292*For Laboratory Use.Promega Corporation·2800 Woods Hollow Road ·Madison, WI 53711-5399 USAApoptosis Assay SystemsProduct Size Cat.#Apo-ONE®Homogeneous Caspase-3/7 Assay1ml G779210ml G7790100ml G7791 Caspase-Glo®2 Assay*10ml G094050ml G0941 Caspase-Glo®6 Assay*10ml G097050ml G0971 Caspase-Glo®3/7 Assay* 2.5ml G809010ml G8091100ml G8092 Caspase-Glo®8 Assay* 2.5ml G820010ml G8201100ml G8202 Caspase-Glo®9 Assay* 2.5ml G821010ml G8211100ml G8212CaspACE™ Assay System, Colorimetric*50 assays G7351100 assays G7220 DeadEnd™ Fluorometric TUNEL System60 reactions G3250 DeadEnd™ Colorimetric TUNEL System40 reactions G713020 reactions G7360*For Laboratory Use.Apoptosis ReagentsProduct Size Cat.# CaspACE™ FITC-VAD-FMK In Situ Marker50μl G7461125μl G7462Anti-ACTIVE®Caspase-3 pAb50μl G7481Anti-Cytochrome c mAb100μg G7421Anti-pS473Akt pAb40μl G7441Anti-PARP p85 Fragment pAb50μl G7341 Caspase Inhibitor Z-VAD-FMK125μl G723250μl G7231 Caspase Inhibitor, Ac-DEVD-CHO100μl G5961 Promega Corporation·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA6.Related Products (continued)Viability and Cytotoxicity AssayProductSize Cat.#MultiTox-Fluor Multiplex Cytotoxicity Assay 10ml G92005 × 10ml G9201(live/dead cell protease activity determination) 2 × 50ml G9202CytoTox-Fluor™ Cytotoxicity Assay 10ml G92605 × 10ml G9261(dead cell protease activity determination) 2 × 50ml G9262MultiTox-Glo Multiplex Cytotoxicity Assay 10ml G92705 × 10ml G9271(live/dead cell protease activity determination) 2 × 50ml G9272For Laboratory Use.Promega Corporation ·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA(a)The MTS tetrazolium compound is the subject of U.S. Pat. No. 5,185,450 assigned to the University of South Florida and islicensed exclusively to Promega Corporation.© 1996, 1998, 1999, 2004, 2005, 2007 Promega Corporation. All Rights Reserved.Anti-ACTIVE, Apo-ONE, Caspase-Glo, CellTiter 96, CellTiter-Blue, CellTiter-Glo and CytoTox 96 are registered trademarks of Promega Corporation. CaspACE, CellTiter-Fluor, CytoTox-Fluor, CytoTox-Glo, CytoTox-ONE and DeadEnd are trademarks of Promega Corporation.Products may be covered by pending or issued patents or may have certain limitations. Please visit our Web site for more information.NEN is a registered trademark of NEN Life Science Products, Inc.All prices and specifications are subject to change without prior notice.Product claims are subject to change. Please contact Promega Technical Services or access the Promega online catalog for the most up-to-date information on Promega products.。
碧云天生物技术 Beyotime Biotechnology Anisomycin (JNK激活剂)
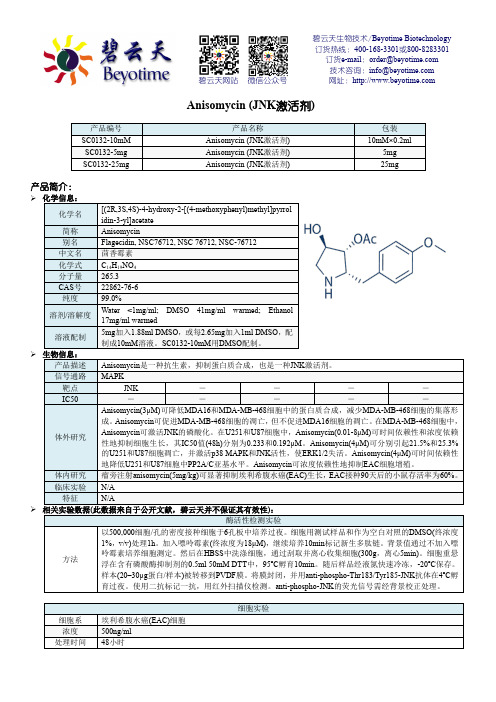
碧云天生物技术/Beyotime Biotechnology订货热线:400-168-3301或800-8283301订货e-mail:******************技术咨询:*****************网址:碧云天网站微信公众号Anisomycin (JNK激活剂)产品编号产品名称包装SC0132-10mM Anisomycin (JNK激活剂) 10mM×0.2mlSC0132-5mg Anisomycin (JNK激活剂) 5mgSC0132-25mg Anisomycin (JNK激活剂) 25mg产品简介:化学信息:化学名[(2R,3S,4S)-4-hydroxy-2-[(4-methoxyphenyl)methyl]pyrrol idin-3-yl]acetate简称Anisomycin别名Flagecidin, NSC76712, NSC 76712, NSC-76712 中文名茴香霉素化学式C14H19NO4分子量265.3CAS号22862-76-6纯度99.0%溶剂/溶解度Water <1mg/ml; DMSO 41mg/ml warmed; Ethanol 17mg/ml warmed溶液配制5mg加入1.88ml DMSO,或每2.65mg加入1ml DMSO,配制成10mM溶液。
SC0132-10mM用DMSO配制。
生物信息:产品描述Anisomycin是一种抗生素,抑制蛋白质合成,也是一种JNK激活剂。
信号通路MAPK靶点JNK ----IC50 -----体外研究Anisomycin(3μM)可降低MDA16和MDA-MB-468细胞中的蛋白质合成,减少MDA-MB-468细胞的集落形成。
Anisomycin可促进MDA-MB-468细胞的凋亡,但不促进MDA16细胞的凋亡。
在MDA-MB-468细胞中,Anisomycin可激活JNK的磷酸化。
FDA批准的放射性药物都有哪些?

FDA批准的放射性药物都有哪些?展开全文中华医学会核医学分会放射性药物学组整理1、药物名称:Carbon-11 choline(11C-胆碱)生产商:Mayo Clinic 商品名:—用途:前列腺癌复发诊断2、药物名称:Carbon-14 urea(14C-尿素)生产商:Kimberly-Clark 商品名:PYtest用途:胃中幽门螺杆菌感染诊断3、药物名称:Fluorine-18 florbetaben(18F-AV1)生产商:Piramal Imaging 商品名:Neuraceq™用途:阿尔茨海默(AD)患者和痴呆患者评价4、药物名称:Fluorine-18florbetapir(18F-AV45)生产商:Eli Lilly 商品名:Amyvid™用途:阿尔茨海默症诊断与治疗5、药物名称:Fluorine-18sodium fluoride(18F-氟化钠)生产商:Various 商品名:—用途:成骨能力的骨显像剂6、药物名称:Fluorine-18fludeoxyglucose(18F-FDG)生产商:Various 商品名:—用途:肿瘤、癫痫病灶糖代谢异常检测7、药物名称:Fluorine-18flutemetamol(18F-PIB)生产商:GE Healthcare 商品名:Vizamyl用途:阿尔茨海默(AD)患者和痴呆患者评价8、药物名称:Gallium-67 citrate(67Ga-柠檬酸)生产商:Lantheus MedicalImaging、Mallinckrodt商品名:—用途:霍奇金病、淋巴瘤、支气管癌以及一些急性炎症病变诊断9、药物名称:Indium-111capromab pendetide(111In-卡罗单抗喷地肽)生产商:AytuPharmaceuticals 商品名:ProstaScint®用途:前列腺癌患者、前列腺癌术后高度怀疑转移患者的检测10、药物名称:Indium-111 chloride(111In-氯化铟)生产商:GE Healthcare、Mallinckrodt 商品名:Indiclor™用途:用于放射性标记11、药物名称:Indium-111 pentetate(111In-DTPA)生产商:GE Healthcare 商品名:—用途:放射性核素脑池造影12、药物名称:Indium-111oxyquinoline(111In-羟基喹啉)生产商:GE Healthcare 商品名:—用途:用于自体白细胞标记,炎症及感染的诊断13、药物名称:Indium-111pentetreotide(111In-奥曲肽)生产商:Mallinckrodt 商品名:Octreoscan™用途:原发性和转移性内神经分泌肿瘤生长抑素受体定位14、药物名称:Iodine I-123iobenguane(123I-MIBG)生产商:GE Healthcare 商品名:AdreView™用途:原发或转移性嗜铬细胞瘤或神经母细胞瘤的辅助诊断15、药物名称:Iodine I-123 ioflupane(123I-氟潘)生产商:GE Healthcare 商品名:DaTscan™用途:对疑似帕金森症患者的评估16、药物名称:Iodine I-123sodium iodide capsules(123I-碘化钠胶囊)生产商:Cardinal Health、Mallinckrodt 商品名:—用途:甲状腺功能及形态学评价17、药物名称:Iodine I-125 humanserum albumin(125I-人血清白蛋白)生产商:IsoTex Diagnostics 商品名:Jeanatope用途:全血及血浆容量测定18、药物名称:Iodine I-125iothalamate(125I-酞酸盐)生产商:IsoT ex Diagnostics商品名:Glofil-125用途:肾小球滤过率的评价19、药物名称:Iodine I-131 humanserum albumin(131I-人血清白蛋白)生产商:IsoTex Diagnostics 商品名:Megatope用途:全血及血浆量、心脏输出、心脏及肺血容量、蛋白质周转研究、脑肿瘤定位等20、药物名称:Iodine I-131sodium iodide(131I-碘化钠)生产商:DRAXIMAGE、Mallinckrodt 商品名:HICON™用途:甲状腺疾病的诊断与治疗21、药物名称:MolybdenumMo-99 generator(钼锝发生器)生产商:GE Healthcare、Lantheus MedicalImaging、Mallinckrodt商品名:DRYTEC™、T echnelite、UltraTechneKow®DTE用途:放射性药物的制备22、药物名称:Nitrogen-13 ammonia(13N-氨水)生产商:Various 商品名:—用途:心肌灌注评价冠状动脉疾病23、药物名称:Radium-223 dichloride(223Ra-二氯化镭)生产商:Bayer HealthCarePharmaceuticalsInc. 商品名:Xofigo®用途:去势性前列腺癌治疗24、药物名称:Rubidium-82 chloride(82Ru-氯化铷)生产商:Bracco Diagnostics 商品名:Cardiogen-82®用途:心肌灌注显像剂25、药物名称:Samarium-153lexidronam(153Sm-EDTMP)生产商:Lantheus MedicalImaging 商品名:Quadramet®用途:减轻骨转移患者的疼痛26、药物名称:Strontium-89 chloride(89Sr-氯化锶)生产商:GE Healthcare商品名:MetastronTM用途:减轻骨转移患者的疼痛27、药物名称:T echnetium-99mbicisate(99mT c-ECD)生产商:Lantheus MedicalImaging 商品名:Neurolite®用途:脑卒中患者卒中的诊断与治疗28、药物名称:Technetium-99mdisofenin(99mTc-地索芬宁)生产商:Pharmalucence 商品名:Hepatolite®用途:急性胆囊炎诊断29、药物名称:Technetium-99mexametazine(99mTc-HMPAO)生产商:GE Healthcare 商品名:C eretec™用途:脑卒中患者血脑灌注、白细胞标记显像用用于腹腔感染及肠道炎症定位30、药物名称:T echnetium-99mmacroaggregatedalbumin (99mT c-MAA)生产商:DRAXIMAGE 商品名:—用途:肺灌注评价、腹静脉分流畅通性评价31、药物名称:Technetium-99mmebrofenin(99mT c-甲溴苯宁)生产商:Bracco Diagnostics、Pharmalucence 商品名:Choletec®用途:肝胆显像剂32、药物名称:Technetium-99mmedronate(99mTc-MDP)生产商:DRAXIMAGE、GE Healthcare、Pharmalucence 商品名:MDP-25、MDP Multidose用途:骨显像剂33、药物名称:Technetium-99mmertiatide(99mTc-MAG3)生产商:Mallinckrodt 商品名:TechnescanMAG3TM用途:肾动态显像34、药物名称:Technetium-99moxidronate(99mT c-HDP)生产商:Mallinckrodt 商品名:Tec hnescan™HDP用途:骨显像剂35、药物名称:Technetium-99mpentetate(99mT c-DTPA)生产商:DRAXIMAGE 商品名:—用途:脑显像、肾显像36、药物名称:T echnetium-99mpyrophosphate(99mTc-PYP)生产商:Mallinckrodt、Pharmalucence 商品名:Technescan™、PYP™用途:骨显像、心脏显像剂、血池显像剂37、药物名称:Technetium-99m redblood cells(99mT c-红细胞)生产商:Mallinckrodt 商品名:UltraTag™用途:血池造影、消化道出血定位38、药物名称:T echnetium-99msestamibi(99mTc-MIBI)生产商:Cardinal Health、DRAXIMAGE、Lantheus MedicalImaging、Mallinckrodt、Pharmalucence商品名:Cardiolite®用途:心肌灌注,用于检测缺血、评价心机功能,乳腺成像39、药物名称:Technetium-99msodium pertechnetate (99mT c-高锝酸钠)生产商:GE Healthcare、Lantheus MedicalImaging、Mallinckrodt商品名:—用途:脑显像、甲状腺显像、胎盘定位、膀胱显像等40、药物名称:Technetium-99msuccimer(99mT c-DMSA)生产商:GE Healthcare 商品名:—用途:肾显像41、药物名称:Technetium-99msulfur colloid(99mT c-硫胶体)生产商:Pharmalucence 商品名:—用途:肝、脾、骨髓显像等42、药物名称:Technetium-99mtetrofosmin(99mTc-替曲膦)生产商:GE Healthcare 商品名:MyoviewTM用途:心肌灌注剂43、药物名称:Technetium-99mtilmanocept(99mTc-替马诺噻)生产商:NavideaBiopharmaceuticals,Inc. 商品名:Lymphoseek®用途:淋巴结定位44、药物名称:Thallium-201chloride(201Tl-氯化铊)生产商:GE Healthcare、Lantheus MedicalImaging、Mallinckrodt商品名:—用途:心肌灌注显像45、药物名称:Xenon-133 gas(133Xe气体)生产商:Lantheus MedicalImaging 商品名:—用途:肺功能评估与肺显像、脑血流评估46、药物名称:Yttrium-90chloride(90Y-氯化钇)生产商:MDS Nordion、Eckert&ZieglerNuclitec商品名:—用途:放射性标记47、药物名称:Yttrium-90ibritumomab tiuxetan(90Y-替伊莫单抗)生产商:SpectrumPharmaceuticals商品名:Zevalin®用途:非霍奇金氏淋巴瘤治疗截止至2015年8月1日。
艾德KRAS试剂盒说明书

【产品名称】通用名称:人类KRAS基因7种突变检测试剂盒(荧光PCR法)英文名称:Human KRAS Gene 7 Mutations Fluorescence Polymerase ChainReaction (PCR) Diagnostic Kit【包装规格】12测试/盒【预期用途】KRAS基因是人体肿瘤中常见的致癌基因。
该基因的突变常见于多种恶性肿瘤,在肺癌患者中的突变率为15~30%,在结直肠癌患者中的突变率为20~50%。
导致KRAS处于激活状态的突变主要位于第12和13密码子上。
KRAS基因突变一般会使肺癌患者对EGFR酪氨酸激酶抑制剂产生耐药,使结直肠癌患者对抗EGFR抗体类药物产生耐药。
但是,2010年10月的最新研究发现第13密码子上的Gly13Asp(G13D)突变亦对抗EGFR抗体类药物有治疗反应性(参见:De Roock. W. JAMA. 2010;304(16):1812-1820)。
因此,KRAS基因突变检测能提高肿瘤临床治疗的针对性,降低治疗费用,节省宝贵的治疗时间。
大部分肿瘤的突变都是体细胞突变,突变细胞往往与野生型细胞混杂在一起,因此所提取的DNA常带有大量野生型DNA,所以对体细胞突变检测需要较高的特异性,而目前广泛使用的直接测序法检测能力有限,不能完全满足临床需要。
本试剂盒用于检测人类KRAS基因的12和13密码子上7种热点体细胞突变(见表1),试剂盒以DNA为检测样本,提供突变状态的定性评估。
辅助临床医生筛选出可受益于肿瘤靶向药物的大肠癌等癌症患者。
该产品用于组织中提取DNA的KRAS基因7种突变的检测,为临床医生对大肠癌或肺癌患者选择肿瘤靶向药物治疗提供参考。
表1 人类KRAS基因的12和13密码子上7种热点体细胞突变突变名称氨基酸变化碱基变化Cosmic ID 公司命名Gly12Asp 甘氨酸到天门冬氨酸GGT>GAT 521 12-2-A Gly12Ala 甘氨酸到丙氨酸GGT>GCT 522 12-2-C Gly12Val 甘氨酸到缬氨酸GGT>GTT 520 12-2-T Gly12Ser 甘氨酸到丝氨酸GGT>AGT 517 12-1-A Gly12Arg 甘氨酸到精氨酸GGT>CGT 518 12-1-C Gly12Cys 甘氨酸到胱氨酸GGT>TGT 516 12-1-T Gly13Asp甘氨酸到天门冬氨酸GGC>GAC 53213-2-A【检测原理】本试剂盒基于实时PCR平台结合了特异引物和双环探针两种技术,检测DNA样品中含有的突变基因。
抗体公司

赛信通(上海)生物试剂有限公司
上海市浦东南路1101号远东大厦514室,200120 info@cst www.cst 2158356288 公司总部: 美国
Established in Beverly, MA in 1999, Cell Signaling Technology (CST) is a privatelyowned company with over 400 employees worldwide. We are dedicated to providing innovative research tools that are used to help define mechanisms underlying cell function and disease. Since its inception, CST has become the world leader in the production of the highest quality activationstate and total protein antibodies utilized to expand knowledge of cell signaling pathways. Our mission is to deliver the world's highest quality research tools that accelerate progress in biological research and personalized medicine. 总引用数为4670,来自于1966篇文章。最常引用的试剂包括: Akt, ERK2, ERK1, p38, Akt1。
AbD Serotec (BioRad)
超敏C_反应蛋白、血脂指标检验结果对糖尿病新发患者的评估研究

DOI:10.16658/ki.1672-4062.2023.12.045超敏C反应蛋白、血脂指标检验结果对糖尿病新发患者的评估研究赵海举湖北省枣阳市中医医院医学检验科,湖北枣阳441200[摘要]目的研究超敏C反应蛋白(hs-CRP)、血脂指标检验结果用于评估糖尿病(diabetic mellitus, DM)新发患者的临床价值。
方法选取2022年1月—2023年1月湖北省枣阳市中医医院医学检验科收入的健康体检人员64名作为对照组,同期收入的糖尿病新发患者64例作为研究组,两组均进行血糖、超敏C反应蛋白(hs-CRP)与血脂指标检测,对比分析两组血糖指标、超敏C反应蛋白及血脂指标水平。
结果研究组空腹血糖(FPG)、餐后2 h血糖(2 hPG)以及糖化血红蛋白(HbA1c)水平均较对照组高,差异有统计学意义(P<0.05);研究组血清hs-CRP、低密度脂蛋白胆固醇(LDL-C)、三酰甘油(TG)以及总胆固醇(TC)水平高于对照组,高密度脂蛋白(HDL-C)低于对照组,差异有统计学意义(P<0.05)。
结论在糖尿病新发患者的疾病诊断中,除血糖常规检测外,超敏C反应蛋白、血脂指标检验能够及时评估患者机体内部状况,为其疾病诊断与治疗提供更多参考依据,临床应用价值较高。
[关键词] 超敏C反应蛋白;血脂指标;检验;糖尿病;新发患者;诊断评估[中图分类号] R587.1 [文献标识码] A [文章编号] 1672-4062(2023)06(b)-0045-04Evaluation Study of Test Results of High-Sensitivity C-Reactive Protein and Blood Fat Index in Patients with New-onset DiabetesZHAO HaijuDepartment of Medical Laboratory Science, Zaoyang Hospital of Traditional Chinese Medicine, Zaoyang, Hubei Prov‐ince, 441200 China[Abstract] Objective To study the clinical value of the test results of high-sensitivity C-reactive protein (hs-CRP) and blood fat index in evaluating patients with new-onset diabetic mellitus (DM). Methods From January 2022 to January 2023, 64 health examiners from the medical laboratory department of Zaoyang Hospital of Traditional Chinese Medicine in Hubei Province were selected as the control group, and 64 new diabetes patients from the same period were selected as the study group. Both groups were tested for blood glucose, hypersensitive C-reactive protein (hs CRP), and blood lipid indicators, and the levels of blood glucose, hs-CRP, and blood lipid indicators were compared and analyzed between the two groups. Results The levels of fasting plasma glucose (FPG), 2 h postprandial plasma glucose (2 hPG) and glycated hemoglobin (HbA1c) in the study group were significantly higher than those in the con‐trol group, the difference was statistically significant (P<0.05). The levels of hs CRP, low-density lipoprotein cholester‐oled (LDL-C), triacylglycerol (TG) and total cholesterol (TC) in the study group were higher than those in the control group, while high-density lipoprotein cholesteroled (HDL-C) was lower than that in the control group, the difference was statistically significant (P<0.05). Conclusion In the disease diagnosis of patients with new-onset diabetes, in ad‐dition to the routine blood glucose detection, the tests of high-sensitivity C-reactive protein and blood fat index can timely evaluate the internal inflammatory status of patients, providing more reference for the disease diagnosis and treatment. Moreover, its clinical application value is high.[Key words] High-sensitivity C-reactive protein; Blood fat index; Test; Diabetes; New-onset patients; Diagnostic [作者简介]赵海举(1976-),女,本科,副主任技师,研究方向为医学检验。
(2-氨基乙基)三甲基氯化铵盐酸盐 氢谱

(2-氨基乙基)三甲基氯化铵盐酸盐(Trimethylammonium methylchloride hydrochloride)是一种常用的有机化合物,具有广泛的应用价值。
其氢谱是对其结构和性质进行研究的重要手段之一。
本文将对该化合物的氢谱进行分析,并就其结构特点和应用进行探讨。
一、(2-氨基乙基)三甲基氯化铵盐酸盐的氢谱分析(2-氨基乙基)三甲基氯化铵盐酸盐是一种季铵盐,其分子结构如下图所示:[图表]根据其结构及成分,可以对其进行氢谱分析。
在氢谱图谱中,可以观察到不同化学位点上的质子信号和相对应的化学位移。
通过仪器检测和数据处理,可以得到该化合物的氢谱峰图谱和一系列参数,如化学位移、峰面积、耦合常数等。
通过对这些数据的分析,可以揭示该化合物内部的结构特点。
二、(2-氨基乙基)三甲基氯化铵盐酸盐的结构特点通过对氢谱数据的分析,可以得到(2-氨基乙基)三甲基氯化铵盐酸盐的结构特点。
其中,可以从化学位移得知不同质子的化学环境和相对位置,从峰面积得知不同质子的相对含量,从耦合常数得知不同质子之间的相互作用。
这些数据可以揭示该化合物内部化学键的性质和构型,为进一步研究提供了重要的参考依据。
三、(2-氨基乙基)三甲基氯化铵盐酸盐的应用(2-氨基乙基)三甲基氯化铵盐酸盐作为一种有机化合物,在化工和生物领域具有广泛的应用价值。
在化工领域,它可作为表面活性剂、阻垢剂等添加剂使用。
在生物领域,它可作为生物催化剂或有机合成试剂使用。
由于其在氢谱中的特殊性质,也可作为理化或分析化学实验的研究对象,为学术研究提供了重要的实验数据。
四、结论(2-氨基乙基)三甲基氯化铵盐酸盐作为一种有机化合物,具有重要的结构特点和广泛的应用价值。
通过对其氢谱的分析,可以揭示其内部的化学键性质和构型特点,为其在化工和生物领域的应用提供了重要的参考依据。
对其氢谱的研究具有重要的理论和实际意义,值得进一步深入研究和应用。
(2-氨基乙基)三甲基氯化铵盐酸盐的氢谱分析是对其结构和性质进行研究的重要手段,通过对其进行氢谱分析,可以揭示其结构特点和应用价值。
不同酶消化法提取猪原代肝细胞的效果比较

532024.4·试验研究0 引言猪圆环病毒(PCV )是Circoviridae 科Circovirus 属的一种无囊膜的单链环状DNA 病毒。
在已知的4个血清型中,PCV2为猪易感的致病性病毒[1]。
PCV2感染会诱导宿主免疫抑制引起猪圆环病毒病(PCVD ),包括断奶仔猪多系统衰竭综合征、新生仔猪先天性脑震颤、皮炎与肾病综合征、猪呼吸道病综合征、母猪繁殖障碍等,给全世界养猪业带来较大的经济损失,是世界各国的兽医与养猪业者公认的造成重大影响的猪传染病[2]。
PCV2的感染在猪生长发育的不同阶段有不同的组织嗜性。
但无论是胎儿阶段还是出生后,肝细胞都是PCV2感染和复制的靶细胞。
因此,PCV2也被视为一种能够诱导猪肝炎的病毒[3]。
且PCV2诱导的肝细胞凋亡在PCV2引发的相关病变和疾病的发病机制中具有关键性作用[4]。
因此,方便、快捷地获取大量有活性的猪肝细胞对于研究PCVD 的致病机制具有重大意义。
目前获取肝细胞常用的方法主要包括机械分离细胞法、非酶分离细胞法、离体酶消化法和酶灌流法等[5]。
因此,本试验采用简便、经济、无需特殊设备、仅需部分肝组织的离体酶消化法,比较不同酶消化分离猪原代肝细胞的效果,为一般实验室提取分离大量有活性的猪肝细胞提供参考。
1 材料与方法1.1 材料1.1.1 主要试剂新鲜猪肝组织,Hank's 平衡盐溶液(HBSS ),磷酸盐缓冲液(无菌PBS ),4%多聚甲醛(PFA ),收稿日期:2024-01-27基金项目:国家自然科学基金项目:复杂器官与组织在脾脏内的功能性再生(32230056)作者简介:周徐倩(1999-),女,汉族,浙江温州人,硕士在读,研究方向:组织工程与再生医学。
*通信作者简介:董磊(1978-),男,汉族,安徽阜阳人,博士,教授,研究方向:组织工程与再生医学、生物材料。
周徐倩,董磊.不同酶消化法提取猪原代肝细胞的效果比较[J].现代畜牧科技,2024,107(4):53-55. doi :10.19369/ki.2095-9737.2024.04.014. ZHOU Xuqian ,DONG Lei .Comparison of the Effect of Different Enzyme Digestion Methods on Extraction of Porcine Primary Hepatocytes[J].Modern Animal Husbandry Science & Technology ,2024,107(4):53-55.不同酶消化法提取猪原代肝细胞的效果比较周徐倩,董磊*(南京大学,江苏 南京 210023)摘要:猪肝细胞是猪圆环病毒的靶细胞,简单快速地提取猪原代肝细胞对于研究猪圆环病毒病的致病机制具有重要意义。
全反式维A酸对肺腺癌H1299细胞放射敏感性研究

[文章编号]1000-2200(2021)03-0281-05-基础医学-全反式维A酸对肺腺癌H1299细胞放射敏感性研究孙谦,宣爱丽,汪庚明,周咏春,徐洪波,何泽来,周燕,周育夫,江浩[摘要]目的:探讨全反式维A酸(ATRA)对肺腺癌细胞株H1299放射敏感性影响及其分子机制。
方法:MTT法检测ATRA对H1299细胞存活率的影响;平板克隆形成实验检测H1299细胞的放射敏感性;流式细胞术检测细胞周期;Western blotting检测survivin与NF-k B的蛋白表达情况。
结果:不同浓度ATRA对H1299细胞均有抑制作用,浓度为10滋mo^L时最佳(P< 0.05)。
相对单独ATRA处理,10滋mo^LATRA联合不同剂量的射线照射后,细胞生长抑制率明显增加(P<0.01)。
ATRA作用和射线照射后的细胞凋亡增多(P<0.01),ATRA联合射线照射作用后的细胞总凋亡率明显高于单纯射线照射(P<0.01)。
与对照组、放射组、ATRA组相比,ATRA+放射组G0/G1期比例明显增加(P<0.01)。
与放射组相比,ATRA+放射组的细胞存活分数值降低,ATRA可以增加肺腺癌H1299细胞放射敏感性,增敏比为1.406。
Western blotting结果显示,ATRA+放射组细胞survivin、NF-KB蛋白表达明显降低(P<0.01)。
结论:ATRA对肺腺癌H1299细胞具有放射增敏作用,其机制可能与ATRA直接抑制H1299细胞增殖、促进H1299细胞凋亡,下调survivin及NF-k B蛋白表达有关。
[关键词]肺肿瘤;全反式维A酸;放射敏感性[中图法分类号]R734.2;R811.5[文献标志码]A DOI:10.13898/ki.issn.1000-2200.2021.03.001Effect of all-trans retinoic acid on radiosensitivity of lung adenocarcinoma H1299cells SUN Qian,XUAN Ai-li,WANG Geng-ming,ZHOU Yong-chun,XU Hong-bo,HE Ze-lai,ZHOU Yan,ZHOU Yu-fu,JIANG Hao (Department of Radiation Oncology,The First Affiliated Hospital of Bengbu Medical College,Bengbu Anhui233004,China)[Abstract]Objective:To investigate the effects of all-trans retinoic acid(ATRA)on the radiosensitivity of lung adenocarcinoma cell line H1299,and explore its possible molecular mechanisms.Methods:MTT assay was used to determine the effects of ATRA on the survival rate of H1299cells,the radiosensitivity of H1299cells was detected using plate cloning formation experiment,the H1299cell cycle was detected using flow cytometry and the protein expression levels of survivin and NF-k B in the cells were detected using Western blotting.Results:The H1299cells could be inhibited by ATRA at different concentrations, and the best concentration of which was10滋moL/L(P<0.05).Compared with the cells treated with ATRA alone,10滋moL/L ATRA combined with different doses of radiation,the inhibition rate of cell growth significantly increased(P<0.01),the number of cell apoptosiss increased(P<0.01),and the total apoptosis rate of ATRA combined with radiation was significantly higher than that of ATRA alone(P<0.01).Compared with the control group,radiation group,and ATRA group,the proportion of G0/G1phase in ATRA combined with radiation group significantly increased(P<0.01).Compared with the radiation group,the cell survival fraction in ATRA combined with radiation group decreased,and ATRA could increase the radiosensitivity of lung adenocarcinoma H1299cells with a sensitization ratio of1.406.The results of Western blotting showed that the expression levels of survivin and NF-k B in H1299cells significantly decreased in ATRA combined with radiation group(P<0.01).Conclusions:ATRA can increase the radiosensitivity of lung adenocarcinoma H1299cells, and the mechanism may be related to ATRA directly inhibiting the proliferation of H1299cells,promoting the apoptosis of H1299cells and down-regulating the survivin protein and NF-k B protein expression.[Key words]lung neoplasms;all-trans retinoic acid;radiosensitivity2015年世界卫生组织统计,癌症是91个国家70岁前人群的主要死亡原因。
重组贻贝粘蛋白的表征及功效评价

生物技术进展 2023 年 第 13 卷 第 4 期 596 ~ 603Current Biotechnology ISSN 2095‑2341研究论文Articles重组贻贝粘蛋白的表征及功效评价李敏 , 魏文培 , 乔莎 , 郝东 , 周浩 , 赵硕文 , 张立峰 , 侯增淼 *西安德诺海思医疗科技有限公司,西安 710000摘要:为了推进重组贻贝粘蛋白在医疗、化妆品领域的应用,对大肠杆菌规模化发酵及纯化生产获得的重组贻贝粘蛋白进行了表征及功效评价。
经Edman 降解法、基质辅助激光解吸电离飞行时间质谱、PITC 法、非还原型SDS -聚丙烯酰胺凝胶电泳法、凝胶法、改良的Arnow 法对重组贻贝粘蛋白进行氨基酸N 端测序、相对分子量分析、氨基酸组成分析、蛋白纯度分析、内毒素含量测定、多巴含量测定;通过细胞迁移、斑马鱼尾鳍修复效果对重组贻贝粘蛋白进行功效评价。
结果显示,获得的重组贻贝粘蛋白与理论的一级结构一致,蛋白纯度达95%以上,内毒素<10 EU ·mg -1,多巴含量大于5%;重组贻贝粘蛋白浓度为60 μg ·mL -1时能够显著促进细胞增殖的活性(P <0.01);斑马鱼尾鳍面积样品组与模型对照组相比极显著增加(P <0.001)。
研究结果表明,重组贻贝粘蛋白具有显著的促细胞迁移和修复愈合的功效,具备作为生物医学材料的潜质。
关键词:贻贝粘蛋白;基因重组;生物材料;表征;功效评价DOI :10.19586/j.20952341.2023.0021 中图分类号:S985.3+1 文献标志码:ACharacterization and Efficacy Evaluation of Recombinant Mussel Adhesive ProteinLI Min , WEI Wenpei , QIAO Sha , HAO Dong , ZHOU Hao , ZHAO Shuowen , ZHANG Lifeng ,HOU Zengmiao *Xi'an DeNovo Hith Medical Technology Co., Ltd , Xi'an 710000, ChinaAbstract :In order to promote the application of recombinant mussel adhesive protein in the medical and cosmetics field , the recombi⁃nant mussel adhesive protein obtained from scale fermentation and purification of Escherichia coli was characterized and its efficacy was evaluated. Amino acid N -terminal sequencing , relative molecular weight analysis , amino acid composition analysis , protein purityanalysis , endotoxin content , dihydroxyphenylalanine (DOPA ) content of recombinant mussel adhesive protein were determined by the following methods : Edman degradation , matrix -assisted laser desorption ionization time -of -flight mass spectrometry (MALDI -TOF -MS ), phenyl -isothiocyanate (PITC ), nonreductive SDS -polyacrylamide gel electrophoresis (SDS -PAGE ), gel method , modified Ar⁃now. The efficacy of recombinant mussel adhesive protein was evaluated by cell migration and repairing effect of zebrafish tail fin. Re⁃sults showed that the obtained recombinant mussel adhesive protein was confirmed to be consistent with the theoretical primary structure , protein purity of more than 95%, endotoxin <10 EU ·mg -1, DOPA content above 5%. When the recombinant mussel adhesive protein concentration was 60 μg ·mL -1, the effect of promoting cell proliferation was the most obvious , and it had very significant activity (P <0.01). The caudal fin area of zebrafish in sample group was significantly increased compared with model control group (P <0.001). The results indicated that recombinant mussel adhesive protein can promote cell migration and repair healing and has the potential to be used as biomedical materials.Key words :mussel adhesive protein ; gene recombination ; biological materials ; representation ; efficacy evaluation贻贝粘蛋白(mussel adhesive protein , MAP )也称作贻贝足丝蛋白(mussel foot protein ,Mfps ),收稿日期:2023⁃02⁃24; 接受日期:2023⁃03⁃31联系方式:李敏 E -mail:*******************;*通信作者 侯增淼 E -mail:***********************.cn李敏,等:重组贻贝粘蛋白的表征及功效评价是海洋贝类——紫贻贝(Mytilus galloprovincalis)、厚壳贻贝(Mytilus coruscus)、翡翠贻贝(Perna viri⁃dis)等分泌的一种特殊的蛋白质,贻贝中含有多种贻贝粘蛋白,包括贻贝粘蛋白(Mfp 1~6)、前胶原蛋白(precollagens)和基质蛋白(matrix proteins)等[1]。
HPLC_MS分析薏苡仁油中的甘油三酯成分

HP LC 2MS 分析薏苡仁油中的甘油三酯成分向智敏3,祝 明,陈碧莲,陈 勇(浙江省药品检验所,浙江杭州310004)[摘要] 目的:对薏苡仁油中的甘油三酯成分进行定性分析研究。
方法:采用高效液相色谱2大气压化学电离2质谱法(HP LC 2APCI 2MS )。
实验条件:正离子检测模式,喷雾电压3000V ,毛细管温度250℃,APCI 源温度400℃,corona 电流4μA 。
鞘气(高纯液氮)压力35K Pa 。
质谱扫描范围为300~900amu ,扫描时间1s ,Q1宽度为017。
液相色谱的固定相为Z orbax Extend C 18柱(416mm ×250mm ,5μm )。
流动相为二氯甲烷2乙腈(35∶65),流速1m L ・min -1。
柱温25℃。
结果:共鉴定出12种甘油三酯。
结论:此测定结果可作为薏苡仁注射液指纹图谱中组分的定性依据。
[关键词] 薏苡仁油;甘油三酯;液相色谱-质谱法[中图分类号]R 284.1 [文献标识码]A [文章编号]100125302(2005)1821436203[收稿日期] 2004204205[通讯作者] 3向智敏,T el :(0571)86459425,E 2mail :xiangzm @sina 1com 薏苡仁是禾本科薏苡仁属植物薏苡Coix lacry 2ma 2jobi L 1var 1ma 2yuen (R oman 1)Stapf 的种仁,民间药用已有几千年的历史。
由薏苡仁提取的薏苡仁油经药效学实验证实具有抑杀癌细胞和调整机体免疫功能的作用,其静脉制剂康莱特注射液已用于临床,对肝癌等均有较好疗效。
康莱特注射液的活性成分薏苡仁油的主要成分为甘油三酯。
为了制订薏苡仁油及其制剂的指纹图谱,必须研究其甘油三酯的组成。
目前对薏苡仁油脂化学成分研究较少,文献[1,2]采用T LC 研究其中的甘油三酯和用G C 2MS 研究甘油三酯的脂肪酸成分。
血清生物标志物在缺血缺氧性脑病新生儿中的研究进展

血清生物标志物在缺血缺氧性脑病新生儿中的研究进展兰雪,崔艳芳,陈国萍,于嘉,肇颖新(哈尔滨医科大学附属第一医院新生儿科,黑龙江哈尔滨150001)摘要:治疗性低温疗法作为低氧缺血性脑病(hypoxic ischemic encephalopathy,HIE)治疗标准的广泛引入,给临床医生带来了越来越大的压力,要求他们对发生的低氧损伤(hypoxic injury,H I)的程度和随之而来的脑病的严重程度做出早期和准确的评估。
然而,目前还没有任何一种基于血液的标志物足以检测HI或预测预后。
许多炎症蛋白、神经元特异性蛋白和MicroRNA表达可预测HIE病情变化,这些变化在出生的几小时至几天内迅速演变。
将临床数据与生化检测结果相结合是目前改善新生儿HIE的检测和预测结局的最可能途径。
本文总结了目前对HIE血清生物标志物的研究,显示了其预测HIE预后的潜力。
关键词:血清生物标记物;新生儿;缺血缺氧性脑病;研究进展中图分类号:R7222文献标识码:AResearch Progresses of Serum Biomarkers in Neonateswith Hypoxic Ischemic EncephalopathyLAN Xue,CUI Yanfang,CHEN Guoping,YU Jia,ZHAO Yingxin (Department of Neonatology,The First Affiliated Hospital of Harbin Medical University,Harbin150001,China) Abstract:The widespread introduction of therapeutic hypothermia as a standard of care for hypoxic ischemic encephalopathy(HIE)has created an increasing pressure on clinicians to make an early and accurate asse s ment ofthe degree of hypoxicinjury(HI)that occurs and the severity ofthe accompanyingencephalopathy.However,none of the blood-based markersisyetgoodenoughto accuratelydetectsignificantHIorpredictoutcomes.HIEisa s ociatedwith manypredictablechanges in inflammatory proteins,neuron-specific proteins,and MicroRNA expressions that evolve rapidly withinhoursto daysafterbirth.Thecombination of clinical data and biochemicaltestresultsis currentlythe mostpromising approachtoimprovethe detection and prediction of neonatal HIE outcomes.This paper summarizes the current research on SERUM biomarkers of HIE and shows its potentialtopredictHIEresults.Key words:;Serum biomarkers;Newborn;Hypoxic ischemic encephalopathy;Progress在新生儿低氧缺血性脑病(hypoxic ischemic encephalopathy,HIE)的管理中,最大的难点是对于HIE的预测、检测和分级,分级的结果会影响治疗干预的方式。
芯片电泳

[9]Deanne KT,Simon KW.John BC,et a1.Perinatal risk factors altering regional brain structure in the preterm infant[J].Brain,2007,130
(3):667—77.
apolipopm・rein E3 transgenie
Pratt
injury[J].Best
Clin
Obstet Gynaecol,2007.21
brain
[14]u BM.Stmcturc and remodeling of people’s brain[J].Zhongguo Dangdai Erke Zazhi(Chin J Conte Pediatr)2002,2(S):25—7. [15]Maegele M,Lippert—Gruener M,Ester—Bode T,et a1.Muhimodal eal-=
在一般厚度不超过5咖、面积为数平方厘米的平板芯片上,
是将传统毛细管电泳技术(CE)与微电机加工技术(MSMS) 相结合的一种分离分析技术,它以刻蚀在芯片上的纤细管道 为分离通道,以高压直流电场为驱动力,是毛细管电泳技术的 重大技术延伸,它不但继承了传统毛细管电泳技术中纳升水 平检测及可分离分析大分子样品的优势,还发挥了微系统体 积小、检测效率高、时间短、耐用性好、成本低廉、可以在一块 微芯片上实现多路并行检测等优势旧J,不仅是减小尺寸,而 且大大提高了分析效能。已用于DNAp J、氨基酸H J、蛋白 质"J、细胞‘60等的检测和分析,在食品安全、新药筛选、环境 检测、司法鉴定、临床疾病诊断等领域逐步得到应用。 1芯片电泳原理与方法 微流控芯片电泳主要以电渗流作为蛋白区带的驱动力。 玻璃或石英芯片在中性或碱性pH下,其表面带负电荷,液流 中与其相邻的部分形成沿通道壁的带正电荷的界面。在通道 两端施加高电压,带正电荷的界面在电场作用下产生迁移,继 而带动通道内界面包裹的液流产生电渗流,使液体向负极移
C反应蛋白、乳酸脱氢酶和β2-微球蛋白

DOI:10.19368/ki.2096-1782.2022.20.017C反应蛋白、乳酸脱氢酶和β-微球蛋白检测对2急性白血病患者治疗预后评估中的价值丁晨江苏省人民医院浦口分院检验科,江苏南京211800[摘要]目的探讨C反应蛋白(C-reactiveprotein,CRP)、乳酸脱氢酶(lactate dehydrogenase,LDH)和β2-微球蛋白(beta-2-microglobulin,β2-MG)检测对急性白血病患者治疗预后评估中的价值。
方法选取2018年8月—2021年8月江苏省人民医院浦口分院收治的76例急性白血病患者,设定为观察组,并选取同时期健康体检者76名,设定为对照组。
比较两组CRP、LDH和β2-MG水平及预后情况。
结果观察组患者CRP(76.13±5.06)mg/L、LDH(586.74±13.58)U/L、β2-MG(3.81±0.25)mg/L水平明显高于对照组,差异有统计学意义(t= 126.793、202.249、87.016,P<0.05);观察组ANLL患者血清CRP、LDH、β2-MG水平高于ALL患者,差异有统计学意义(P<0.05);相比较初治组,缓解组CRP(2.89±0.11)mg/L、LDH(158.54±11.69)U/L、β2-MG(1.29±0.11)mg/L 明显降低,差异有统计学意义(t=116.611、198.872、75.247,P<0.05);与未缓解组(74.85±5.65)mg/L、(579.75±12.55)U/L、(3.68±0.23)mg/L相比较,缓解组CRP、LDH、β2-MG水平显著降低,差异有统计学意义(t=106.140、109.396、55.236,P<0.05);未缓解组血清CRP、LDH、β2-MG水平与初治组相比较,差异无统计学意义(P> 0.05);观察组患者治疗后,65例缓解,11例病情未缓解,患者均无严重不良反应。
UPLC-MS

UPLC-MS/MS法同时快速测定保健食品中10种降压类非法添加化学药王晓峰,许琨琨,卢文斌,吴芳海,蔡振世*,林晓明(泉州市食品药品检验所,福建泉州 362000)摘 要:目的:建立同时测定保健食品中10种降压类非法添加化学药的UPLC-MS/MS快速检测方法。
方法:液相采用ACQUITY UPLC®BEH C18(2.1 mm×100 mm,1.7 μm)柱,以超纯水(含0.1%甲酸)-乙腈(0.1%甲酸)为流动相,进行梯度洗脱,进样量2 μL,流速0.3 mL·min-1,柱温30 ℃。
质谱采用ESI,正、负离子多反应监测模式,进行定性分析和定量分析。
结果:盐酸普萘洛尔、酒石酸美托洛尔、艾司洛尔、比索洛尔、坎地沙坦、厄贝沙坦、替米沙坦、氯沙坦、缬沙坦和吲达帕胺的分离度良好,线性范围内相关性均较好,R2均大于0.996 3;平均回收率为93%~118%;RSD为1.4%~4.9%(n=6);方法检出限为0.10~5.00 μg·kg-1;方法定量限为0.30~15.00 μg·kg-1;单次分析仅需12 min。
结论:该方法操作便捷、检测时间短、灵敏度高、结果准确,适用于保健食品中10种降压类非法添加化学药的快速筛查及定量检测。
关键词:UPLC-MS/MS;保健食品;降压类药物Simultaneous and Rapid Determination of 10 illegally Added Antihypertensive Chemicals in Health Food by UPLC-MS/MS WANG Xiaofeng, XU Kunkun, LU Wenbin, WU Fanghai, CAI Zhenshi*, LIN Xiaoming(Quanzhou Institute for Food And Drug Control, Quanzhou 362000, China) Abstract: Objective: To establish a UPLC-MS/MS rapid detection method for simultaneously determining 10 illegally added chemical antihypertensive drugs in health foods. Method: ACQUITY UPLC®BEH C18( 2.1 mm×100 mm, 1.7 μm) column was used in the liquid phase with ultrapure water (containing 0.1% formic acid)-acetonitrile (0.1% formic acid) as the mobile phase for gradient elution, the injection volume was 2 μL, flow rate was 0.3 mL·m in-1, and column temperature was 30 ℃. The mass spectrometry was performed by ESI, positive/negative ion multiple reaction monitoring mode for qualitative and quantitative analysis. Result: The separation degree of propranolol, metoprolol, amlodipine, bisoprolol, candesartan, irbesartan, telmisartan, losartan, valsartan, and indapamide was good, and the linear range correlations were good, all R2 values were greater than 0.996 3. The average recovery rate was 93%~118%. RSD was 1.4%~4.9% (n=6). The LOD was between 0.10~5.00 μg·kg-1, and the LOQ was between 0.30~15.00 μg·kg-1. A single analysis only requires 12 min. Conclusion: The method is simple to operate, has short detection time, high sensitivity, and accurate results, and it is suitable for the rapid screening and quantitative detection of 10 illegally added chemical antihypertensive drugs in health foods.Keywords: UPLC-MS/MS; health food; antihypertensive drugs随着近年来我国经济的迅速发展,人们的生活质量得到了很大提高,同时老年人口不断增加,导致出现越来越多的高血压患者,市场上也出现了越来越多的辅助降压类保健食品。
CX-5461_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :CX-5461Catalog No. :HY-13323CAS No. :1138549-36-61.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:CX 5461; CX5461Formula:C27H27N7O2SMolecular Weight:513.61CAS No. :1138549-36-64. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to yellow (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
CX-5461_1138549-36-6_DataSheet_MedChemExpress

Product Name:CX-5461CAS No.:1138549-36-6Product Data SheetCat. No.:HY-13323MWt:513.61Formula:C27H27N7O2S Purity :>98%Solubility:Mechanisms:Biological Activity:Pathways:Cell Cycle/DNA Damage; Target:DNA/RNA Synthesis DMSO <1.2mg/mL Water<1.2mg/mL Ethanol <1.2mg/mLg y CX-5461 is a first-in-class non-genotoxic small molecule targeted inhibitor of RNA polymerase I (Pol I) that activates the p53 pathway without causing DNA damage. CX-5461 selectively inhibits rRNA synthesis by Pol I in the nucleolus, but does not inhibit mRNA synthesis by RNA Polymerase II (Pol II) and does not inhibit DNA replication or protein synthesis. Inhibition of Pol I results in nucleolar stress and release of ribosomal proteins (RP) from the nucleolus. The RP bind to Mdm2 and liberate p53 to orchestrate apoptosis in cancer cells. CX-5461 demonstrates a favorable preclinical profile,potently and selectively kills cancer cells, demonstrates robust in vivo efficacy in multiple models,and has demonstrated oral bioavailability in multiple species. cGMP manufacture has been References:[1]. Drygin D et al. Targeting RNA polymerase I with an oral small molecule CX-5461 inhibitsribosomal RNA synthesis and solid tumor growth. Cancer Res. 2011 Feb 15;71(4):1418-30.[2]. Bywater MJ, Poortinga G, Sanij E, Hein N, Peck A, Cullinane C, Wall M, Cluse L, Drygin D,Anderes K, Huser N, Proffitt C, Bliesath J, Haddach M, Schwaebe MK, Ryckman DM, Rice WG,y p pcompleted and the molecule is being advanced to the clinic and will be developed for the trea...ySchmitt C, Lowe SW, Johnstone RW, Pearson RB, McArthur GA, Hannan RD.Inhibition of RNA Polymerase I as a Therapeutic Strategy to Promote Cancer-Specific Activation ofp53.Cancer Cell. 2012 Jul 10;22(1):51-65.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
化学发光法对丙肝抗体筛查阳性的结果分析

CHINESE COMMUNITY DOCTORS 中国社区医师2021年第37卷第11期化学发光法对丙肝抗体筛查阳性的结果分析李亦君许兴101100北京中医药大学东直门医院通州院区医学检验科,北京丙型肝炎是由丙型肝炎病毒(HCV)引起的一种常见传染病。
据统计,丙肝感染率约为3%,每年约有3.5万人感染[1]。
HCV 在肝细胞中的定植和复制会逐渐破坏肝脏结构,损害肝脏功能。
丙型肝炎主要分为病毒性肝炎、急性病毒性肝炎和慢性病毒性肝炎以及肝硬化。
急性病毒性肝炎患者多有无力、恶心、疲软、尿黄等症状;慢性病毒性肝炎主要表现为疲劳和食欲不振,HCV-RNA 持续表达为阳性[2]。
在过去20~30年,感染丙肝病毒的人群中,有10%~20%的人发展为肝硬化[3]。
丙肝具有高度传染性,目前还没有疫苗接种或特殊治疗方法。
早期诊断和干预是防止疾病恶化的重要手段。
化学发光法具有较高的敏感性和特异性,窗口期短,应用广泛[4]。
化学发光法分析利用免疫反应和化学发光的结合来检测大量的物质,具有很高的灵敏度[5]。
本研究选取在我院拟行有创检查前,进行感染性疾病初筛的8773名患者进行化学发光法的丙肝抗体初筛,对其中阳性患者同时进行HCV-RNA 的检测,通过整理数据,研究两种方法在丙型肝炎初筛和诊疗中的作用。
现报告如下。
资料与方法选取2019年6月-2020年5月拟行有创检查前进行感染性疾病筛查的患者8773例,男5968例,女6808例;年龄4~94岁,平均(48.73±4.58)岁。
展开临床研究,应用化学发光法初筛丙肝抗体,血清标本均符合规定检测标准,排除其余患病可能。
方法:按照操作规范从患者肘部采静脉血3mL,置于一次性分离胶采血管内,以3000r/min 的速度离心10min,分离血清待检。
化学发光法检测丙肝抗体:应用日本希森美康全自动化学发光仪及配套封闭试剂进行检测。
根据说明书,发光强度值1.0为临界值,>1.0为丙肝抗体阳性,<1.0为丙肝抗体阴性。
iTRAQ

百泰派克生物科技
iTRAQ
iTRAQ(isobaric tags for relative and absolute quantitation)即同位素标记相对和绝对定量技术,是由AB SCIEX公司研发的一种基于标签的蛋白质定量技术。
iTRAQ技术通过同位素标记来实现蛋白质定量研究,其基本原理为:将水解得到的蛋白质多肽N末端或赖氨酸侧链基团用同位素试剂标签标记,再将该混合物进行高精度串联质谱分析,根据信号峰强度等质谱信息实现对样品蛋白的定量和定性分析。
iTRAQ含有8种不同的试剂标签,可同时对多达8个样品进行鉴别和定量;该技术在体外对样品蛋白进行标记,检测范围广,精确度高,理论上可用于对所有物种的胞内、胞外蛋白质进行定量分析。
百泰派克生物科技采用Thermo公司最新推出的Obitrap Fusion Lumos质谱仪结合Nano-LC,提供iTRAQ蛋白定量分析服务技术包裹,您只需要将您的实验目的告诉我们并将您的蛋白寄给我们,我们会负责项目后续所有事宜,包括蛋白酶切、肽段标价、肽段分离、质谱分析、质谱原始数据分析、生物信息学分析。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
MSDS
1 Composition
7 Accident Release Measure
Product Name:CX-5461
Chemical Name:
PROCEDURE(S) OF PERSONAL PRECAUTION(S)-Wear respirator, chemical safety goggles, rubber boots, and heavy
rubber gloves.METHODS FOR CLEANING UP-Sweep up, place in a bag and hold for waste disposal. Avoid raising dust. Ventilate area and
wash spill site after material pickup is complete.
5H-Benzothiazolo[3,2-a][1,8]naphthyridine-6-carboxamide, 2-(hexahydro-4-methyl-1H-1,4-diazepin-1-yl)-N-[(5-methyl-2-pyrazinyl)methyl]-5-oxo-
CAS No.:1138549-36-68 Accident Release Measure
Appearance: White to off-white (solid)
Formula:C27H27N7O2S
9 Toxicological Information
Solubility:
To the best of our knowledge, the chemical, physical, and
toxicological properties have not been thoroughly investigated.
No data available.
p p p p DMSO <1.2mg/mL Water <1.2mg/mL
Ethanol <1.2mg/mL
2 Handling and Storage
10 Regulary Information
3 Stability and Reactivity
11Disposal Considerations
CLASSIFICATION- Substance not yet fully tested.SAFETY PHASES- 26-36 (In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Wear suitable protective clothing.) 36/37/38 (Irritating to eyes,respiratory system and skin.)
STABILITY- Stable under normal handling conditions.HANDLING- Do not breathe dust. Avoid contact with eyes,
skin,and clothing.Avoid prolonged or repeated exposure.STORAGE- Store in a properly sealed container store at -20℃,shelflife is 2 years.
11 Disposal Considerations 4 Hazards Identification
12 Transport Information
5First Aid RID/ADR- Non-hazardous for road transport. IMDG- Non-hazardous for sea transport.IATA - Non-hazardous for air transport.
As specific country, federal, state and local environmental
regulations vary and change frequently we suggest you contact a local, authorized waste disposal contractor for adequate disposal.
Special indication of hazards to humans and the environment.Irritating to eyes, respiratory system and skin.
MATERIALS TO AVOID- Strong oxidizing agents.REACTIVITY- May emit toxic gasses like Carbon monoxide,Carbon dioxide, Nitrogen oxides upon thermal decomposition.
5 First Aid
13 Other Information
The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. Medchemexpress LLC shall not be held liable for any damage resulting from h dli f t t ith th b d t
INHALATION- If inhaled, remove to fresh air. If not breathing give, artificial respiration. If breathing is difficult, give oxygen.SKIN CONTACT- In case of contact, immediately wash skin with
soap and copious amounts of water.EYE CONTACT- In case of contact, immediately flush eyes with
copious amounts of water for at least 15 minutes.INGESTION- If swallowed, wash out mouth with water provided person is conscious. Call a physician.
6 Fire Fighting Measures
handling or from contact with the above product.
EXTINGUISHING MEDIA Water spray- Carbon dioxide, dry chemical powder, or appropriate foam.
SPECIAL RISKS Specific Hazard(s)- Emits toxic fumes under fire conditions. SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS Wear self-contained breathing apparatus and protective clothing Caution: Not fully tested. For research purposes only
Medchemexpress LLC
to prevent contact with skin and eyes.
18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
