Tyrphostin A9_10537-47-0_DataSheet_MedChemExpress
碧云天细胞自噬染色检测试剂盒(MDC法)说明书

细胞自噬染色检测试剂盒(MDC 法)产品简介:碧云天生产的细胞自噬染色检测试剂盒(MDC 法),即Autophagy Staining Assay Kit with MDC ,是一种使用丹酰尸胺,也称单丹磺酰尸胺、丹酰尸胺或丹酰戊二胺(monodansylcadaverine, MDC)作为荧光探针快速便捷地检测细胞自噬的试剂盒。
自噬(autophagy)是一种在进化上高度保守的通过溶酶体吞噬并降解部分自身组分的细胞内分解代谢途径。
自噬与多种生理功能有关,在饥饿等环境条件下,细胞通过自噬降解多余或异常的细胞内组分,为细胞的生存提供能量及原材料,促进生物体的生长发育、细胞分化及对环境变化产生应答。
自噬异常与多种病理过程如肿瘤、神经退行性疾病、代谢疾病、病原体感染等都有密切关系。
由于细胞自噬在生理和病理过程中都有重要作用,自噬已经成为细胞生物学领域的一个研究热点。
MDC 是细胞自噬检测最常用的荧光探针之一。
MDC 可以通过离子捕获(ion trapping)和与膜脂的特异性结合,从而特异性标记自噬体(autophagosome),也称autophagic vacuole ,因而常用于细胞自噬的检测。
MDC 是一种嗜酸性荧光探针,很多酸性膜性结构也会被MDC 染色,因此MDC 染色时正常的细胞也会有一定的染色背景。
本产品的染色原理决定了本产品只能用于培养的细胞或者组织的细胞自噬荧光染色检测,不能用于冻存的或固定的细胞、组织或者组织切片的染色检测。
使用本产品染色后可以通过荧光显微镜拍照观察,也可以通过荧光酶标仪或流式细胞仪进行荧光检测。
荧光显微镜观察时可以使用紫外区激发光激发,发出绿色荧光。
荧光酶标仪或流式细胞仪推荐的激发波长为335nm (330-360nm 均可),发射波长为512nm (510-540nm 均可)。
本产品用于细胞自噬染色的效果参考图1。
图1. 细胞自噬染色检测试剂盒(MDC 法)的染色效果图。
游离睾酮(Free—Testosterone)测定试剂盒(化学发光法)产品技术要求新产业
2.性能指标
2.1外观和性状
2.1.1试剂盒各组分应齐全、完整、液体无渗漏;包装标签应清晰,准确、牢固。
2.1.2试剂盒内组分(磁性微球溶液除外) 应为清澈透明的液体,无沉淀、无悬浮物、无絮状物。
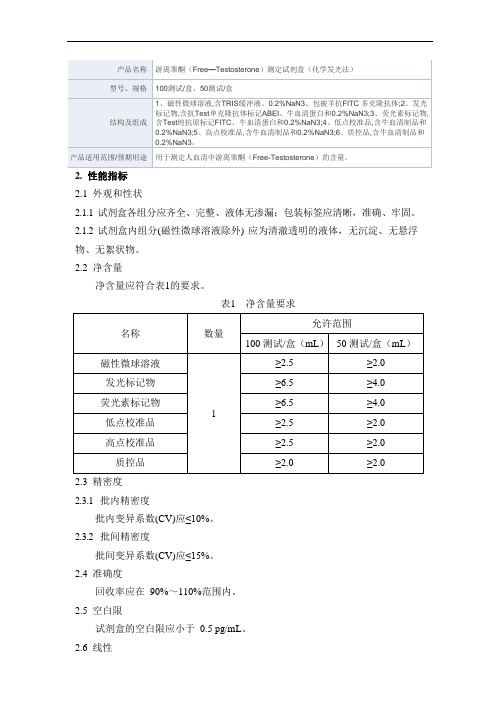
2.2净含量
净含量应符合表1的要求。
表1 净含量要求
2.3精密度
2.3.1批内精密度
批内变异系数(CV)应≤10%。
2.3.2批间精密度
批间变异系数(CV)应≤15%。
2.4准确度
回收率应在90%~110%范围内。
2.5空白限
试剂盒的空白限应小于0.5 pg/mL。
2.6线性
在(2.0-150.0)pg/mL 浓度范围内,线性相关性系数(r)应大于0.9800。
2.7质控品准确度
测量结果在质控范围(可接受区间)内。
2.8质控品均一性
瓶间重复性CV%应≤10%。
1 / 1。
hss-p-5.75.09 - hyaluronic acid derivatives说明书

5.75.09Section:Prescription DrugsEffective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject:Hyaluronic Acid DerivativesPage:1 of 7Last Review Date:March 13, 2020Hyaluronic Acid DerivativesDescriptionDurolane, Euflexxa, GelSyn-3, GenVisc 850, Hyalgan , SodiumHyaluronate, Supartz , Synojoynt*, Triluron, TriVisc, Visco-3 (sodium hyaluronate)Gel-ONE , Hymovis, Monovisc, Orthovisc (hyaluronan)Synvisc, Synvisc-One (hylan G-F 20)Bolded medications are the preferred products*These medications are included in this policy but are not available in the market as of yetBackgroundOsteoarthritis of the knee is a disease in which the elastoviscous property of the synovial fluid in the knee joint becomes diminished, resulting in less protection and shock absorption. Durolane, Euflexxa, Gel-One, GelSyn-3, GenVisc 850, Hyalgan, Hymovis, Monovisc, Orthovisc, Sodium Hyaluronate, Synvisc, Synvisc-One, Supartz, Synojoynt, Triluron, TriVisc, Visco-3 are hyaluronan derivatives that are injected into the knee joints to increase the elastoviscous properties of arthritic joint fluid and slow its outflow from the joint . The goal of therapy is torestore the viscoelasticity in the affected joints, thereby decreasing pain, improving mobility, and restoring the natural protective functions (1).The American College of Rheumatology (ACR) updated its guidelines for the treatment of osteoarthritis (OA) of the knee in 2012. In mild symptomatic OA, treatment may be limited toFederal Employee Program® 1310 G Street, N.W.Washington, D.C. 20005 202.942.1000Fax 202.942.1125Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 2 of 7patient education, physical and occupational therapy and other non-pharmacologic modalities. Nonpharmacologic modalities strongly recommended for the management of knee OA were aerobic, aquatic, and/or resistance exercises as well as weight loss for overweight patients. Nonpharmacologic modalities conditionally recommended for knee OA included medial wedge insoles for valgus knee OA, subtalar strapped lateral insoles for varus knee OA, medially directed patellar taping, manual therapy, walking aids, thermal agents, tai chi, self-management programs, and psychosocial interventions. Pharmacologic modalities conditionally recommended for the initial management of patients with knee OA included acetaminophen, oral and topical NSAIDs, tramadol, and intraarticular corticosteroid injections (1).Regulatory StatusFDA-approved indication: Hyaluronic acid derivatives are indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative non-pharmacologic therapy, simple analgesics (e.g., acetaminophen), NSAIDs, tramadol, or intra-articular steroid injections (2-18).The hyaluronic acid derivatives are contraindicated for use in patients with known hypersensitivity to hyaluronan (sodium hyaluronate) preparations. Orthovisc lists hypersensitivity to gram positive bacterial proteins as an additional contraindication (4). Caution should be exercised when Gel-One, Hyalgan, Visco-3, Synvisc, Synvisc-One, Supartz, and Triluron are administered to patients with allergies to avian proteins, feathers, and egg products (3-8, 18).Hyaluronic acid derivatives are contraindicated to treat patients with knee joint infections, infections or skin diseases in the area of the injection site (2-17).A treatment cycle for most of the hyaluronan derivatives typically involves multiple weekly injections. Euflexxa, GelSyn-3, Sodium Hyaluronate, Synvisc, Triluron, TriVisc, and Visco-3 are given for a total of three injections. Orthovisc is given for three or four injections. GenVisc 850, Supartz and Hyalgan are given for a total of three or five injections. Durolane, Gel-One, Synojoynt, and Synvisc-One differ from the other hyaluronan derivatives in that it only requires one injection. Repeat courses of hyaluronan derivatives may be administered if symptoms return (2-18).Upon the basis of high quality supporting evidence, the American Academy of Orthopedic Surgeons cannot recommend using hyaluronic acid for patients with symptomatic osteoarthritis of the knee (19).Related policiesSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 3 of 7Hyaluronate PowderPolicyThis policy statement applies to clinical review performed for pre-service (Prior Approval, Precertification, Advanced Benefit Determination, etc.) and/or post-service claims.Hyaluronic acid derivatives may be considered medically necessary for the treatment of osteoarthritis of the knee and if the conditions indicated below are met.Hyaluronic acid derivatives may be considered investigational for all other indications.Prior-Approval RequirementsAge18 years or older (22 or older for Synvisc, Synvisc-One, and TriVisc)DiagnosisPatient must have the following:Osteoarthritis of the kneeAND ALL of the following:1. Inadequate response to TWO or more of the following conservative non-pharmacologic therapy:a. Cardiovascular (aerobic) activity, such as: walking, biking, stationarybike, aquatic exerciseb. Resistance exercisec. Weight reduction (for persons who are overweight)d. Participation in self-management programse. Wear of medially directed patellar tapingf. Wear of wedged insolesg. Thermal agentsh. Walking aidsi. Physical therapyj. Occupational therapy2. Inadequate response, intolerance, or contraindication to TWO or more of thefollowing:Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 4 of 7a. Acetaminophenb. Oral NSAIDsc. Topical NSAIDs3. Inadequate response, intolerance, or contraindication to intra-articularsteroid injections in which efficacy lasted less than 8 weeks4. Radiologic confirmation of Kellgren-Lawrence Scale score of grade 2 orgreater5. NO dual therapy with another hyaluronic acid injectable6. Non-preferred medications only: Patient MUST have tried at least TWO ofthe preferred products unless the patient has a valid medical exception (e.g.inadequate treatment response, intolerance, contraindication)Prior – Approval Renewal RequirementsAge18 years or older (22 or older for Synvisc, Synvisc-One, and TriVisc)DiagnosisPatient must have the following:Osteoarthritis of the kneeAND ALL of the following:1. Documentation of improvement in pain with previous course of treatment2. At least 12 months has elapsed since last injection of the prior treatmentcycle3. Documentation of reduction of dosing of NSAIDs or other analgesicsduring the 12 month period following the last injection of the prior treatmentcycle4. NO dual therapy with another hyaluronic acid injectable5. Non-preferred medications only: Patient MUST have tried at least TWOof the preferred products unless the patient has a valid medical exception(e.g. inadequate treatment response, intolerance, contraindication) Policy GuidelinesPre - PA AllowanceNoneSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 5 of 7Prior - Approval LimitsDuration12 monthsQuantity One course of therapy for each kneePrior – Approval Renewal LimitsSame as aboveRationaleSummaryOsteoarthritis of the knee is a disease in which the elastoviscous property of the synovial fluid in the knee joint becomes diminished, resulting in less protection and shock absorption. Durolane, Euflexxa, Gel-One, GelSyn-3, GenVisc 850, Hyalgan, Hymovis, Monovisc, Orthovisc, Sodium Hyaluronate, Synvisc, Synvisc-One, Supartz, Synojoynt, Triluron, TriVisc, Visco-3 are hyaluronan derivatives that are injected into the knee joints to increase the elastoviscous properties of arthritic joint fluid and slow its outflow from the joint. The goal of therapy is to restore the viscoelasticity in the affected joints, thereby decreasing pain, improving mobility, and restoring the natural protective functions (1-18).Prior approval is required to ensure the safe, clinically appropriate and cost effective use of the hyaluronic acid derivatives while maintaining optimal therapeutic outcomes.References1. American College of Rheumatology, Subcommittee on Osteoarthritis Guidelines.Recommendations for the medical management of osteoarthritis of the hip and knee:2012 update. Arthritis Care & Research 2012; 64(4):465-474.2. Euflexxa [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc.; July 2016.3. Hyalgan [package insert]. Parsippany, NJ: Fidia Pharma USA Inc.; May 2014.4. Orthovisc [package insert]. Woburn, MA: Anika Therapeutics; June 2005.5. Supartz [package insert]. Durham, NC: Bioventus LLC; April 2015.6. Synvisc [package insert]. Ridgefield, NJ: Genzyme Corp.; December 2014.7. Synvisc-One [package insert]. Ridgefield, NJ: Genzyme Corp.; September 2014;8. Gel-One [package insert]. Warsaw, IN: Zimmer Inc.; May 2011.9. Monovisc [package insert]. Bedford, MA: Anika Therapeutics; December 2013.10. Hymovis [package insert]. Parsippany, NJ: O Fidia Pharma USA Inc.; October 2015.Section: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 6 of 711. GenVisc 850 [package insert]. Doylestown, PA: OrthogenRx Inc.; January 2015.12. GelSyn-3 [package insert]. Durham, NC: Bioventus LLC; January 2016.13. Durolane [package insert]. Durham, NC: Bioventus LLC; November 2017.14. Visco-3 [package insert]. Warsaw, IN: Zimmer, Inc.; May 2017.15. Sodium Hyaluronate [package insert]. North Wales, PA: Teva Pharmaceuticals USA,Inc.; March 2019.16. Synojoynt [package insert]. North Wales, PA: Teva Pharmaceuticals USA, Inc.;September 2019.17. TriVisc [package insert]. Doylestown, PA: OrthogenRx, Inc.; September 2018.18. Triluron [package insert]. Florham Park, NJ: Fidia Pharma USA Inc.; March 2019.19. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee.Evidence-based guideline 2nd edition. May 2013.Policy HistoryDate Action ReasonJanuary 2012 Added minimum age - only approved for adultsDecember 2012 Annual editorial review and reference updateDecember 2013 Annual editorial review and reference updateMarch 2014 Annual editorial reviewAddition of examples of non-pharmacological agents and agents of priorfailure medications.April 2014 Line-Addition of Monovisc to PAMarch 2015 Annual criteria review and reference updateMarch 2016 Change from one tried and failed to two tried and failed non-pharmacologic and pharmacologic therapies and addition of the tried and failed of intra-articular steroid and radiologic confirmation of Kellgren-Lawrence Scalescore of grade 2 or greaterAddition of HymovisPolicy # change from 5.11.04 to 5.75.09May 2016 Addition of GelSyn-3 and GenVisc 850December 2016 Annual editorial review and reference updateAdded: no dual therapy with another hyaluronic acid injectableMarch 2017 Bolded preferred products in the title pageJuly 2017 GelSyn-3 has been changed to preferredSeptember 2017 Annual reviewDecember 2017 Addition of Durolane and Visco-3March 2018 Annual editorial reviewRemoval of Tramadol from the T/F listSeptember 2019 Annual review and reference update. Addition of Sodium Hyaluronate,Synojoynt, and TriViscSection: Prescription Drugs Effective Date: April 1, 2020 Subsection: Neuromuscular Drugs Original Policy Date: June 9, 2011 Subject: Hyaluronic Acid Derivatives Page: 7 of 7December 2019 Annual review. Addition of requirement to trial preferred products January 2020 Addition of TriluronMarch 2020 Annual reviewKeywordsThis policy was approved by the FEP® Pharmacy and Medical Policy Committee on March 13, 2020 and is effective on April 1, 2020.。
磷钨酸负染色液(2%,pH7.0)
版本:A4 修改日期:2023.12.18 磷钨酸负染色液(2%,pH7.0)产品简介:负染色又称阴性染色,是由Hall 发现的相对于普通染色(即正染色)而言的染色技术,其原理在于利用重金属盐包绕低电子密度的样品,增强样本四周的电子密度,造成细微结构之间的"质量-厚度”差异,增强散射吸收反差,使样品在黑暗的背景上呈现明亮的结构,负染色液有磷钨酸、钼酸铵、印度墨汁等,其中最常用的是1~3%磷钨酸。
Leagene 磷钨酸负染色液(2%,pH7.0)适用于显示大分子、细菌、病毒、原生动物、噬菌体、细胞器、核酸大分子、蛋白质晶体及其他大分子材料等,染色后的样品图像呈现透明的亮光,而背景图像呈黑色。
该试剂仅用于科研领域,不适用于临床诊断或其他用途。
产品组成:自备材料:1、 离心机2、 载网3、 显微镜操作步骤(仅供参考):(一)滴染法1、样品低速离心(2000g ,10min)或采用其他方法浓缩样品,制成悬浮液并且使其达到一定浓度和纯度。
2、将样品悬浮液直接滴于带有支持膜的载网上。
3、用滤纸条从液滴边缘吸去多余液体,稍干燥。
4、 滴加负染色液。
5、吸去多余染色液,自然干燥,进行显微镜观察。
(二)漂浮法1、样品低速离心(2000g ,10min)或采用其他方法浓缩样品,制成悬浮液并且使其达到一定浓度和纯度。
2、将带有支持膜的载网置于样品液滴上漂浮以沾取样品。
编号 名称 DZ0035 Storage 磷钨酸负染色液(2%,pH7.0) 100ml RT 避光 使用说明书 1份3、载网置于负染色液上漂浮1~2min。
4、吸去多余染色液,自然干燥,进行显微镜观察。
染色结果:样品透明的亮光背景黑色注意事项:1、目的样本尽量新鲜。
2、样品应为均匀的悬浮液,其纯度和浓度应适宜,否则无法与染色剂之间产生特异和清晰的结合反应。
3、为了您的安全和健康,请穿实验服并戴一次性手套操作。
4、试剂开封后请尽快使用,以防影响后续实验效果。
异泽兰黄素说明书

【产品名称】:异泽兰黄素
【中文别名】:2-(3,4-二甲氧基苯基)-5,7-二羟基-6-甲氧基苯并吡喃-4-酮
【英文名称】:Eupatilin
【CAS编号】:22368-21-4
【分子结构】:
【贮存条件】:4℃冷藏、密封、避光
【熔点】:234~236℃
【外观】:黄色针晶
【纯度】:HPLC≥98%
【用途】:用于含量测定/鉴定/药理实验等
【溶解性】:溶于DMSO、热甲醇,氯仿甲醇混合溶剂,不溶于石油醚等溶剂
【分子式】:C18H16O7
【分子量】:344.32
【有效期】:2年
【鉴别方法】:MS,NMR
【推荐测定方法】:HPLC
【中国药典测定方法】:液相
【注意事项】:防止阳光直射
【包装情况】:20mg, 50mg, 100mg, 1g,10g,100g,1kg,50kg..
【提取来源】:菊科植物艾叶Artemisiae argyi
菊科植物艾 Artemisia argyi Levl. et Vant. 的干燥叶。
多年生草本,高45~120厘米。
茎直立,圆形,质硬,基部木质化,被灰白色软毛,从中部以上分枝。
单叶,互生;茎下部的叶在开花时即枯萎;中部叶具短柄,叶片卵状椭圆形,羽状深裂,裂片椭圆状披针形,边缘具粗锯齿,上面暗绿色,稀被白色软毛,并密布腺点,下面灰绿色,密被灰白色绒毛;近茎顶端的叶无柄,叶片有时全缘完全不分裂,披针形或线状披针形。
Medlife,58-97-9,尿苷单磷酸,技术规格说明书(SDS)

58-97-9
分子式:
C9H13N2O9P
分子量:
324.18
详细描述:
创赛优选提供58-97-9,尿苷單磷酸,UMP,Medlife,上海现货。
Medlife,致力于提供高品质、高性价比小分子化合物的产品。
Medlife小分子化合物大量库存,提供超过2万种的抑制剂、激动剂、拮抗剂等产品,是药物及疾病研究的重要原料供应商。
沸点
700.1±70.0 °C at 760 mmHg
熔点
202 ºC (decomp)
分子式
C9H13N2O9P
分子量
324.181ห้องสมุดไป่ตู้
闪点
377.2±35.7 °C
精确质量
324.035858
PSA
181.12
LogP
-1.8
外观性状
solid
蒸汽压
0.0±5.0 mmHg at 25°C
折射率
1.754
英文名称:
Uridine-5'-monophosphate
英文别名:
5'-Uridylic acid;5'-UMP;UMP;Uridine 5′-monophosphate;Uridine 5'-Monophosphate;IMP;U 5'-P;Uridine 5`-monophosphate;Uridine-5'-phosphate;uridinemonophosphate;uridinephosphate;Uridylate;Uridylic acid;U 5′-P;Uridine 5'-phosphoric acid;Uridine monophosphate;uridine-5'-monophosphate;Uridine phosphate;Uridine 5'-phosphate;UMP (nucleic acid);Uridine 5'-(dihydrogen phosphate);5'Uridylic acid;E2OU15WN0N;2,4(1H,3H)-pyrimidinedione, 1-(5-O-phosphono-beta-D-ribofuranosyl)-;Polyuridylic acids;{[(2R,3S,4R,5R)-5-(2,4-dioxo-1,2,3
金转停Genistein金雀异黄素可抑制人乳腺癌细胞的生长

金转停Genistein金雀异黄素可抑制人乳腺癌细胞的生长作者:G 彼得森,S 巴恩斯摘要:已经检查了金雀异黄素对人乳腺癌细胞系MDA-468(雌激素受体阴性)以及MCF-7 和MCF-7-D-40(雌激素受体阳性)生长的影响。
金雀异黄素是每种细胞系生长的有效抑制剂(IC50 值为6.5 至12.0 μg/ml),而biochanin A 和大豆苷元是较弱的生长抑制剂(IC50 值为20 至34 μg/ml)。
金雀异黄素β-葡萄糖苷,genistin和daidzin,对生长几乎没有影响(IC50值大于100μg/ml)。
金雀异黄素不需要雌激素受体的存在来抑制肿瘤细胞生长(MDA-468 与MCF-7 细胞)。
此外,金雀异黄素和生物素A的作用不会因多重耐药基因产物(MCF-7-D40 与MCF-7 细胞)的过表达而减弱。
收起关键词:精胺色氨酸抗氧化剂电子喷射自由基年份:1991Genistein inhibition of the growth of human breast cancer cells: independence from estrogen receptors and the multi-drug resistance gene作者:G Peterson,S Barnes摘要:The effect of isoflavones on the growth of the human breast carcinoma cell lines, MDA-468 (estrogen receptor negative), and MCF-7 and MCF-7-D-40 (estrogen receptor positive), has been examined.Genistein is a potent inhibitor of the growth of each cell line (IC50 values from 6.5 to 12.0 micrograms/ml), whereas biochanin A and daidzein are weaker growth inhibitors (IC50 values from 20 to 34 micrograms/ml). The isoflavone beta-glucosides, genistin and daidzin, have little effect on growth (IC50 values greater than 100 micrograms/ml). The presence of the estrogen receptor is not required for the isoflavones to inhibit tumor cell growth (MDA-468 vs MCF-7 cells). In addition, the effects of genistein and biochanin A are not attenuated by overexpression of the multi-drug resistance gene product (MCF-7-D40 vs MCF-7 cells).关键词:SPERMINE TRYPTOPHAN ANTIOXIDANT ELECTRON EJECTION FREE RADICALS年份:1991。
α-羟丁酸脱氢酶测定试剂盒(α-酮丁酸底物法)产品技术要求shouyi

α-羟丁酸脱氢酶测定试剂盒(α-酮丁酸底物法)适用范围:本试剂用于体外定量测定人血清中α-羟丁酸脱氢酶的活性。
1.1产品规格试剂1:3×80ml,试剂2:3×20ml;试剂1:4×60ml,试剂2:4×15ml;试剂1:6×40ml,试剂2:3×20ml;试剂1:2×80ml,试剂2:2×20ml;试剂1:1×80ml,试剂2:1×20ml;试剂1:1×60ml,试剂2:1×15ml 1.2产品组成成分试剂1:Tris缓冲液50mmol/L,酮丁酸3.3mmol/L。
试剂2:NADH 0.18mmol/L。
2.1 外观试剂1、2均为无色透明溶液。
2.2 装量液体试剂的净含量应不少于标示值。
2.3 试剂空白2.3.1试剂空白吸光度A≥1.0(光径1.0cm,波长340nm)。
2.3.2 试剂空白吸光度变化率ΔA/5min≤0.01。
2.4分析灵敏度测定294U/L被测物,吸光度变化率在0.0732/min~0.1032/min范围内。
2.5 准确度采用比对试验,相关系数r≥0.975,相对偏差≤10%。
2.6 精密度2.6.1 重复性变异系数CV≤5%。
2.6.2 批间差批间相对极差≤6%。
2.7 线性区间a)(0,1000]U/L(37℃)。
在规定的线性区间内,测定值与样本浓度值的相关系数(r)应不低于0.990。
b)(0,100]U/L区间内,线性绝对偏差应不超过±10U/L;(100,1000]U/L 区间内,线性相对偏差应不超过±10%。
2.8稳定性原装试剂2~8℃避光保存,有效期12个月,有效期满后两个月内测定结果应符合2.3、2.4、2.5、2.6.1、2.7的要求。
苯丙氨酸解氨酶活性检测试剂盒说明书 BC0215
苯丙氨酸解氨酶(PAL微量法注意:本产品试剂有所变动,请注意并严格按照该说明书操作。
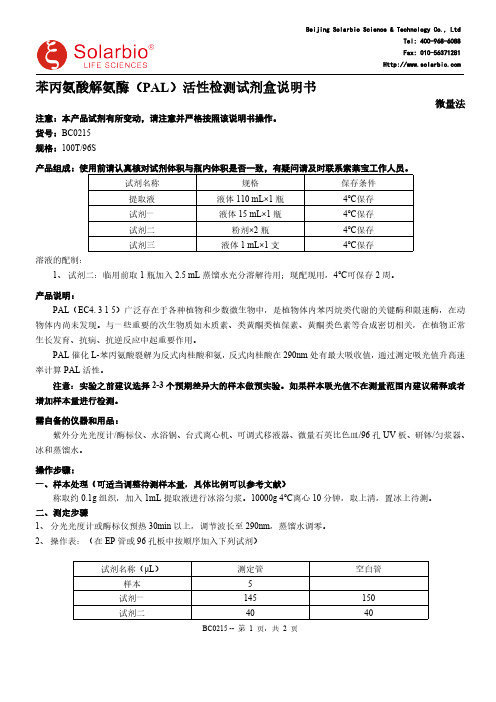
货号:BC0215规格:100T/96S产品组成:使用前请认真核对试剂体积与瓶内体积是否一致,有疑问请及时联系索莱宝工作人员。
试剂名称规格保存条件提取液液体110 mL×1瓶4℃保存试剂一液体15 mL×1瓶4℃保存试剂二粉剂×2瓶4℃保存试剂三液体1 mL×1支4℃保存溶液的配制:1、试剂二:临用前取1瓶加入2.5 mL蒸馏水充分溶解待用;现配现用,4℃可保存2周。
产品说明:PAL(EC4. 3 1 5)广泛存在于各种植物和少数微生物中,是植物体内苯丙烷类代谢的关键酶和限速酶,在动物体内尚未发现。
与一些重要的次生物质如木质素、类黄酮类植保素、黄酮类色素等合成密切相关,在植物正常生长发育、抗病、抗逆反应中起重要作用。
PAL催化L-苯丙氨酸裂解为反式肉桂酸和氨,反式肉桂酸在290nm处有最大吸收值,通过测定吸光值升高速率计算PAL活性。
注意:实验之前建议选择2-3个预期差异大的样本做预实验。
如果样本吸光值不在测量范围内建议稀释或者增加样本量进行检测。
需自备的仪器和用品:紫外分光光度计/酶标仪、水浴锅、台式离心机、可调式移液器、微量石英比色皿/96孔UV板、研钵/匀浆器、冰和蒸馏水。
操作步骤:一、样本处理(可适当调整待测样本量,具体比例可以参考文献)称取约0.1g组织,加入1mL提取液进行冰浴匀浆。
10000g 4℃离心10分钟,取上清,置冰上待测。
二、测定步骤1、分光光度计或酶标仪预热30min以上,调节波长至290nm,蒸馏水调零。
2、操作表:(在EP管或96孔板中按顺序加入下列试剂)试剂名称(μL)测定管空白管样本5试剂一145150试剂二4040BC0215 -- 第1 页,共2 页混匀,30℃准确反应30min试剂三1010混匀,静置10min后,290nm处记录测定管吸光值A1和空白管吸光值A2,ΔA=A1-A2。
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
小分子抑制剂、激动剂、拮抗剂--JAKSTAT信号通路

JAK/STAT
JAK/STAT(Janus激酶/信号转导子和转录激活子)信号通路将来自细胞外的化学信号传递给细胞核,导致与免疫、增殖、分化、凋亡和肿瘤发生等相关基因的DNA转录和表达。
此信号通路是众多细胞因子信号转导的共同途径,其活性在炎性疾病和血液恶性肿瘤等疾病治疗研究中具有重要意义。
JAK-STAT信号级联由三个主要成分组成:由酪氨酸激酶相关受体、酪氨酸激酶JAK和转录因子STAT三个成分组成。
JAK/STAT通路转导过程
细胞因子,如干扰素、白细胞介素和生长因子等配体,与细胞表面受体结合,引起受体分子二聚化。
与受体偶联的JAK 相互接近并通过交互的酪氨酸磷酸化而被激活。
活化后的JAK使受体的酪氨酸磷酸化,为具有SH2结构域的STAT创建结合位点。
STAT结合至受体后,在JAK作用下,STAT酪氨酸705(Tyr 705)被磷酸化。
STAT就会从受体上脱离,形成同/异二聚体。
STAT二聚体进入细胞核后,会结合特定的调节序列以激活或抑制靶基因的转录。
JAK/STAT信号通路图
按靶点分类:。
Tyrphostin_AG_879_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:TyrphostinAG879 is a tyrosine kinase inhibitor that inhibits TrKA phosphorylation, but not TrKB and TrKC.[1] also a ErbB2 kinase inhibitor, has at least 500–fold higher selectivity to ErbB2 (IC50 = 1 μmol/L) than EGFR (IC50 >500 μmol/L).target: TrKA [1], ErbB2 [2].IC 50: ErbB2 1 μmol/L [2].In vitro: TyrphostinAG879 significantly inhibit the A–type potassium currents in the cultured hippocampus neurons.[2]TyrphostinAG879 can reduce gephyrin puncta in GABAergic neurons and PSD–95 puncta in glutamatergic neurons where ErbB4expression was low. [3]In vivo: Treatment with TyrphostinAG879 in immunodepressed mice graft with leiomyosarcoma or promyelocytic leukemia cells result in dramatic reductions in tumor sizes. [1]References:[1]. Rende M et al. Role of nerve growth factor and its receptors in non–nervous cancer growth: efficacy of a tyrosine kinase inhibitor (AG879) andneutralizing antibodies antityrosine kinase receptor A and antinerve growth factor: an in–vitro and in–vivo study. Anticancer Drugs. 2006 Sep;17(8):929–41.[2]. Zhou Y et al. Blockade of EGFR and ErbB2 by the novel dual EGFR and ErbB2 tyrosine kinase inhibitor GW572016 sensitizes human colon carcinoma GEO cells to apoptosis. Cancer Res. 2006 Jan 1;66(1):404–11.[3]. Ting AK et al. Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J Neurosci. 2011 Jan 5;31(1):15–25.Product Name:Tyrphostin AG 879Cat. No.:HY-20878CAS No.:148741-30-4Molecular Formula:C 18H 24N 2OS Molecular Weight:316.46Target:EGFR; EGFR; Trk Receptor Pathway:JAK/STAT Signaling; Protein Tyrosine Kinase/RTK; Protein Tyrosine Kinase/RTK Solubility:DMSO: ≥ 30 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
细胞程序性坏死诱导试剂盒(TSZ法)说明书
细胞程序性坏死诱导试剂盒(TSZ 法)产品编号 产品名称包装 C1058S 细胞程序性坏死诱导试剂盒(TSZ 法) 100次 C1058M细胞程序性坏死诱导试剂盒(TSZ 法)500次产品简介:细胞程序性坏死诱导试剂盒(TSZ 法) (Necroptosis Inducer Kit with TSZ)是由TNF-α、SM-164和Z-V AD-FMK (简称TSZ)按一定的比例混合而成,可以非常有效地诱导细胞程序性坏死。
本产品可以非常有效地诱导L-929、HT-29等细胞的程序性坏死。
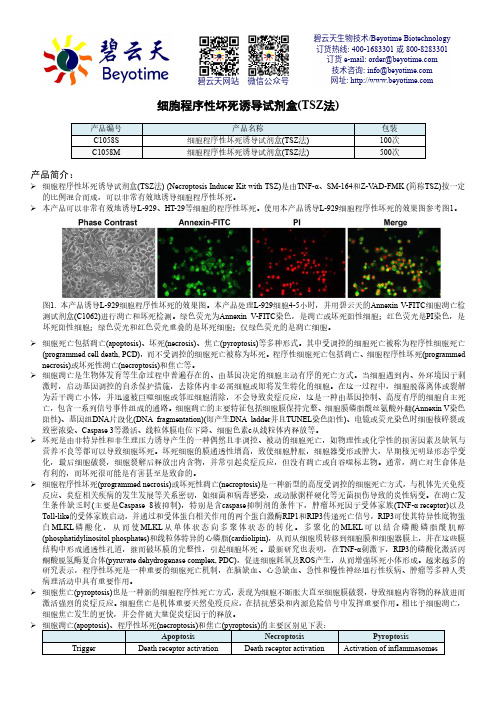
使用本产品诱导L-929细胞程序性坏死的效果图参考图1。
图1. 本产品诱导L-929细胞程序性坏死的效果图。
本产品处理L-929细胞4-5小时,并用碧云天的Annexin V-FITC 细胞凋亡检测试剂盒(C1062)进行凋亡和坏死检测。
绿色荧光为Annexin V-FITC 染色,是凋亡或坏死阳性细胞;红色荧光是PI 染色,是坏死阳性细胞;绿色荧光和红色荧光重叠的是坏死细胞;仅绿色荧光的是凋亡细胞。
细胞死亡包括凋亡(apoptosis)、坏死(necrosis)、焦亡(pyroptosis)等多种形式。
其中受调控的细胞死亡被称为程序性细胞死亡(programmed cell death, PCD),而不受调控的细胞死亡被称为坏死。
程序性细胞死亡包括凋亡、细胞程序性坏死(programmed necrosis)或坏死性凋亡(necroptosis)和焦亡等。
细胞凋亡是生物体发育等生命过程中普遍存在的、由基因决定的细胞主动有序的死亡方式。
当细胞遇到内、外环境因子刺激时,启动基因调控的自杀保护措施,去除体内非必需细胞或即将发生特化的细胞。
在这一过程中,细胞脱落离体或裂解为若干凋亡小体,并迅速被巨噬细胞或邻近细胞清除,不会导致炎症反应,这是一种由基因控制、高度有序的细胞自主死亡,包含一系列信号事件组成的通路。
细胞凋亡的主要特征包括细胞膜保持完整、细胞膜磷脂酰丝氨酸外翻(Annexin V 染色阳性)、基因组DNA 片段化(DNA fragmentation)(即产生DNA ladder 并且TUNEL 染色阳性)、电镜或荧光染色时细胞核碎裂或致密浓染、Caspase 3等激活、线粒体膜电位下降、细胞色素c 从线粒体内释放等。
Trypsin Antigen Retrieval Solution (ab970) 使用说明书

Product nameTrypsin Antigen Retrieval Solution Tested applicationsSuitable for: IHC-P General notes Trypsin-based antigen retrieval solution for Protease-Induced Epitope Retrieval (PIER) (ab970).Contains colored pH indicator for easy pH confirmation.Protocol Notes: 1) Deparaffinize tissues and hydrate to water. If necessary perform a hydrogenperoxide block, wash in water, and rinse in PBS or TBS. The tissue section is ready for trypsindigestion. 2) Combine 1 part trypsin concentrate with 1 part buffer (working solution stable for 5working days at 2-8ºC). Incubate section for 5-10 minutes at 37°C or 15 minutes at roomtemperature. Then, rinse well in PBS or TBS buffer and proceed with immunostaining procedure.This product utilises a color-coded pH indicator (rose) for easy identification. The end-user canvisually inspect the solution and see that the mixture or buffer is at the proper pH. If the mixedsolution is purple, the pH is too high for optimum digestion. If the buffer or trypsin solution turnsorange or yellow, the pH is too low for optimum digestion.Trypsin is a commonly used digestive enzyme. In formalin-fixed, paraffin-embedded tissues,certain antibody protocols required enzyme pretreatment for proper immunohistochemicalstaining.Other products for IHCOther enzymes for antigen retrieval include: Proteinase K Antigen Retrieval Solution ab64220 and Pepsin Solution ab64201.Find more kits and reagents for antigen retrieval, blocking, signal amplification, visualization,counterstaining, and mounting in the IHC kits and reagents guide .FormLiquid Storage instructionsStore at +4°C.Storage bufferPreservative: 0.05% Sodium azide Constituents: PBS, Carrier protein Reagent notes Trypsin is a commonly used digestive enzyme. In formalin-fixed, paraffin-embedded tissues,certain antibody protocols required enzyme pretreatment for proper immunohistochemicalstaining.Product datasheetTrypsin Antigen Retrieval Solution ab9701 Abreviews 24 ReferencesOverview PropertiesThe Abpromise guarantee ApplicationsOur Abpromise guarantee covers the use of ab970 in the following tested applications.The application notes include recommended starting dilutions; optimal dilutions/concentrations should be determined by the end user.ApplicationAbreviews Notes IHC-P Use at an assay dependent dilution. Ratio for 25ml concentratedtrypsin and 25 ml buffer is 1:1 - 1:3.Please note: A ll products are "FOR RESEA RCH USE ONLY . NOT FOR USE IN DIA GNOSTIC PROCEDURES"Our Abpromise to you: Quality guaranteed and expert technical support Replacement or refund for products not performing as stated on the datasheet Valid for 12 months from date of delivery Response to your inquiry within 24 hours We provide support in Chinese, English, French, German, Japanese and Spanish Extensive multi-media technical resources to help youWe investigate all quality concerns to ensure our products perform to the highest standardsIf the product does not perform as described on this datasheet, we will offer a refund or replacement. For full details of the Abpromise,please visit https:///abpromise or contact our technical team.Terms and conditions Guarantee only valid for products bought direct from Abcam or one of our authorized distributors。
还原型谷胱甘肽检测试剂盒(DTNB速率比色法)
还原型谷胱甘肽(GSH)检测试剂盒(DTNB 速率比色法)简介:谷胱甘肽(glutathione ,GSH)存在于几乎身体的每一个细胞,参与细胞许多功能活动,是一种氧自由基消除剂。
谷胱甘肽是研究活性氧和自由基的重要指标,亦是机体氧化物牵累的重要指标。
还原型谷胱甘肽(GSH)是一种含γ-酰胺键和巯基的三肽,由谷氨酸、半胱氨酸及甘氨酸组成,能可逆的转变为氧化型谷胱甘肽(GSSG),其存在形式会随着细胞内代谢的情况而发生相互转变。
还原型谷胱甘肽(GSH)检测试剂盒(DTNB 速率比色法)(Glutathione Assay Kit)是一种简单易行的检测还原型谷胱甘肽的试剂盒,其检测原理是待测样品中的还原型谷胱甘肽(GSH)与发色底物DTNB 反应,产生稳定黄色的TNB 和GSSG ,获得样品的GSH 含量。
该试剂盒可用于检测血浆、血清、组织、细胞等样品中还原型谷胱甘肽(GSH)含量。
该试剂盒仅用于科研领域,不宜用于临床诊断或其他用途。
产品组成:操作步骤(仅供参考):1、 配制GSH 标准储存液:取10mg 还原型谷胱甘肽(GSH)标准加入3.25ml ddH 2O ,溶解并混匀,即为还原型GSH 标准储存液(10mM)。
一部分立即使用,其余适当分装后-20℃保存。
2、 配制GSH 检测工作液:按下表配制GSH 检测工作液。
1个样品 10个样品 50个样品 GSH assay buffer 1.6ml 16ml 80ml DTNB 储存液10μl100μl500μl3、 配制标准品:注意:由于GSH 标准在蛋白沉淀工作液中不太稳定,用蛋白沉淀工作液配制的GSH 溶液必须新鲜配制后使用,不可冻存后再使用。
4、 配制NADPH 工作液:在本试剂盒提供的10mg NADPH 中加入1ml ddH 2O ,溶解并编号 名称TO1037 100T Storage试剂(A): 还原型谷胱甘肽(GSH)标准 10mg 4℃ 避光 试剂(B): GSH assay buffer 250ml RT 试剂(E): NADPH 10mg -20℃ 避光 试剂(F): DMSO 1.5ml RT 避光 试剂(G): ddH 2O 50mlRT 避光 使用说明书1份混匀,即为NADPH储存液(10mg/ml)。
气相色谱法测定非布司他原料药中的基因毒性杂质盐酸羟胺

气相色谱法测定非布司他原料药中的基因毒性杂质盐酸羟胺李彬;李丽婉
【期刊名称】《天津药学》
【年(卷),期】2024(36)2
【摘要】目的:建立了气相色谱衍生化法测定非布司他中潜在基因毒性杂质盐酸羟胺的含量。
方法:用环己酮将盐酸羟胺衍生为环己酮肟,通过气相色谱进行测定。
色谱柱为安捷伦DB-624色谱柱(30 m×0.53 mm,3μm);检测器为氢火焰离子化检测器(FID),检测器的温度260℃;进样口温度为230℃;高纯氮气为载气,恒流模式,流速为1.5 ml/min;色谱柱的升温程序为:起始温度55℃,维持2 min,以20℃/min的升温速率升温至95℃,维持2 min,再以40℃/min的升温速率升温至250℃,维持20 min。
进样量为1μl;分流比为5:1。
结果:盐酸羟胺在0.2247~2.696μg/ml范围内,浓度与峰面积线性关系良好(r=0.9997);平均回收率为99.24%~106.59%之间,RSD 为2.29%。
结论:本方法简单,准确度高,重现性好,可用于非布司他原料药中盐酸羟胺的质量控制。
【总页数】4页(P18-21)
【作者】李彬;李丽婉
【作者单位】开封市中医院;上海药明康德新药开发有限公司
【正文语种】中文
【中图分类】R927.1
【相关文献】
1.气相色谱法测定沙格列汀原料药中基因毒性杂质残留量
2.盐酸兰地洛尔原料药中基因毒性杂质的定量分析方法研究
3.气相色谱法测定盐酸苯海索中潜在基因毒性杂质
4.高效液相法测定5-氟-1H-吲哚-2,3-二酮中基因毒性杂质盐酸羟胺的含量
5.盐酸倍他司汀中基因毒性杂质甲醛的测定
因版权原因,仅展示原文概要,查看原文内容请购买。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Product Name:
Tyrphostin A9CAS No.:
10537-47-0Cat No :HY-15511Product Data Sheet
Cat. No.:
HY 15511MWt:
282.38Formula:
C18H22N2O Purity :>98%
DMSO
Ethanol Solubility:Mechanisms:
Biological Activity:
Pathways:Protein Tyrosine Kinase/RTK; Target:VEGFR DMSO Ethanol
Tyrphostin A9(AG 17), a tyrosine kinase inhibitor, is a potent inducer of mitochondrial fission.Tyrphostin A9 emerged as the most potent and selective of 51 tyrosine kinase inhibitors tested
against the TNF-induced respiratory burst.
IC50 value:
Target: Multi tyrosine kinase Tyrphostin A9 inhibited TNF-induced tyrosine phosphorylation of pyk2 without blocking the cells'bactericidal activity. Tyrphostin A9 is a PDGF receptor tyrosine kinase inhibitor (IC50 = 500 nM).Recent findings suggest that signaling via PDGF receptor tyrosine kinases is not necessary for the shift of the smooth muscle cells from a contractile to a synthetic phenotype On the other hand these References:
[1]. Park SJ, Park YJ, Shin JH, et al. A receptor tyrosine kinase inhibitor, Tyrphostin A9 induces cancer cell death through Drp1 dependent mitochondria fragmentation. Biochem Biophys Res
Commun. 2011 May 13;408(3):465-70.[2]Ri h d F L Ch i t h B F th All M S l t l Fib ti F t
shift of the smooth muscle cells from a contractile to a synthetic phenotype. On the other hand these enzymes apparently carry out important functions in the control of intracellular membrane traffic and cell division....
[2]. Richard F. Loeser, Christopher B. Forsyth, Allen M. Samarel et al. Fibronectin Fragment Activation of Proline-rich Tyrosine Kinase PYK2 Mediates Integrin Signals Regulating Collagenase-3Expression by Human Chondrocytes through a Protein Kinase C-dependent Pathway. The Journal
of Biological Chemistry, 2003, 278, 24577-24585.[3]. Michele Fuortes, Maxine Melchior, Hyunsil Han, et al. Role of the tyrosine kinase pyk2 in the
integrin-dependent activation of human neutrophils by TNF. J Clin Invest. 1999;104(3):327-335.[4]. Johan Thyberg . Tyrphostin A9 and wortmannin perturb the Golgi complex and block
proliferation of vascular smooth...
Caution: Not fully tested. For research purposes only
Medchemexpress LLC
18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
