Articaine hydrochloride_23964-57-0_DataSheet_MedChemExpress
钛酸异丙酯产品安全技术说明书(阿拉丁)

GHS02:易燃物; GHS06:急毒性物质钛酸异丙酯Titanium(IV) isopropoxide99.99%CAS No. 546-68-9EC-编号208-909-64急救措施4.1必要的急救措施描述一般的建议请教医生。
向到现场的医生出示此安全技术说明书。
如果吸入如果吸入,请将患者移到新鲜空气处。
如呼吸停止,进行人工呼吸。
请教医生。
在皮肤接触的情况下用肥皂和大量的水冲洗。
请教医生。
在眼睛接触的情况下用大量水彻底冲洗至少15分钟并请教医生。
如果误服禁止催吐。
切勿给失去知觉者喂食任何东西。
用水漱口。
请教医生。
4.2最重要的症状和影响,急性的和滞后的无数据资料4.3及时的医疗处理和所需的特殊处理的说明和指示无数据资料5消防措施5.1灭火介质火灾特征无数据资料灭火方法及灭火剂干粉 干砂不要用水喷射。
5.2源于此物质或混合物的特别的危害无数据资料5.3救火人员的预防如有必要,佩戴自给式呼吸器进行消防作业。
5.4进一步的信息喷水冷却未打开的容器。
6泄露应急处理6.1人员的预防,防护设备和紧急处理程序使用个人防护装备。
避免吸入蒸气、气雾或气体。
保证充分的通风。
消除所有火源。
注意蒸气积累达到可爆炸的浓度,蒸气可蓄积在地面低洼处。
6.2环境预防措施如能确保安全,可采取措施防止进一步的泄漏或溢出。
不要让产品进入下水道。
6.3抑制和清除溢出物的方法和材料围堵溢出物,用非可燃性材料(如砂子、泥土、硅藻土、蛭石)吸收溢出物,将其收集到容器中,根据当地的或国家的规定处理6.4参考其他部分丢弃处理请参阅第13节。
7安全操作与储存7.1安全操作的注意事项避免接触皮肤和眼睛。
避免吸入蒸气或雾滴。
火舌回闪有可能穿过相当长的距离。
容器遇火可能会爆炸切勿靠近火源。
-严禁烟火。
采取措施防止静电积聚。
7.2安全储存的条件,包括任何不兼容性在氩气下操作,避免潮湿。
储存于氩气中 使容器保持密闭,储存在干燥通风处。
阿替卡因(Articaine)杂质
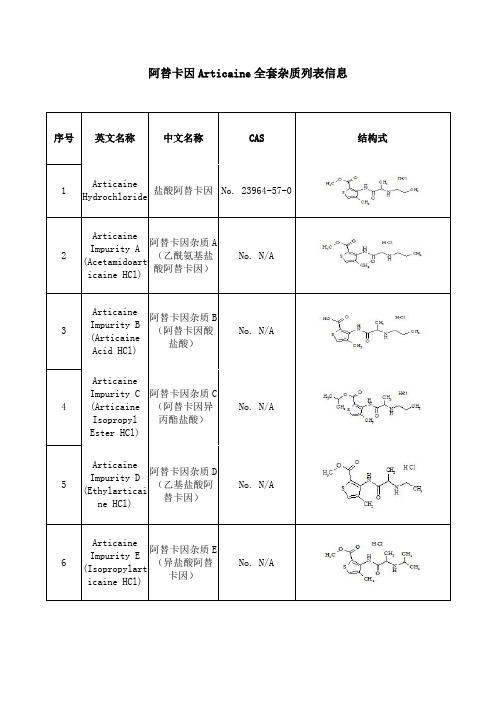
阿替卡因Articaine全套杂质列表信息序号英文名称中文名称CAS 结构式1ArticaineHydrochloride盐酸阿替卡因No. 23964-57-02ArticaineImpurity A(Acetamidoarticaine HCl)阿替卡因杂质A(乙酰氨基盐酸阿替卡因)No. N/A3ArticaineImpurity B(ArticaineAcid HCl)阿替卡因杂质B(阿替卡因酸盐酸)No. N/A4ArticaineImpurity C(ArticaineIsopropylEster HCl)阿替卡因杂质C(阿替卡因异丙酯盐酸)No. N/A5ArticaineImpurity D(Ethylarticaine HCl)阿替卡因杂质D(乙基盐酸阿替卡因)No. N/A6ArticaineImpurity E(Isopropylarticaine HCl)阿替卡因杂质E(异盐酸阿替卡因)No. N/A7ArticaineImpurity F(ArticaineAcidPropionamideHCl)阿替卡因杂质F(阿替卡因酸丙酰胺盐酸)No. N/A8ArticaineImpurity G(Butylarticaine HCl)阿替卡因杂质G(丁基盐酸阿替卡因)No. 23964-59-29ArticaineImpurity H(Dipropylarticaine HCl)阿替卡因杂质H(二丙盐酸阿替卡因)No. N/A10ArticaineImpurity I(Methyl3-Amino-4-Methylthiophene-2-Carboxylate)阿替卡因杂质I(3-氨基-4-甲基噻吩-2-羧酸甲酯No. 85006-31-111ArticaineImpurity J(Methyl3-[[(2RS)-2-Bromopropanoyl]amino]-4-Methylthiophene-2-Carboxylate)阿替卡因杂质J(下甲基3 -[[(2RS)-2-溴丙基]氨基] -4-甲基噻吩-2-羧酸甲酯)No. N/A12 2-ThiopheneAcetylChloride2-噻吩乙酰氯化物No.39098-97-0。
盐酸纳美芬(征求意见稿)

盐酸纳美芬(征求意见稿)Yansuan NameifenNalmefene Hydrochloride. H Cl . H 2ONHOCH2O HOC 21H 25NO 3·HCl·H 2O 375.90 本品为17-环丙甲基-4,5α-环氧-6-亚甲基吗啡喃-3,14-二醇盐酸盐一水合物。
按无水、无溶剂物计算,含C 21H 25NO 3·HCl 应为98.0%~102.0%。
【性状】 本品为白色至类白色结晶性粉末;无臭;有引湿性或略有引湿性。
本品在水或甲醇中易溶,在乙醇中溶解,在丙酮中极微溶解。
比旋度 取本品,精密称定,加水溶解并定量稀释制成每1ml 中约含10mg 的溶液,依法测定(中国药典2010年版二部附录Ⅵ E ),比旋度为-165º至-175º。
【鉴别】 (1)在含量测定项下记录的色谱图中,供试品溶液中主峰的保留时间应与对照品溶液主峰的保留时间一致。
(2)本品的红外光吸收图谱应与对照品的图谱一致(中国药典2010年版二部附录ⅣC )。
(3)本品的水溶液显氯化物的鉴别反应(中国药典2010年版二部附录Ⅲ)。
【检查】 酸度 取本品0.1g ,加水10ml 使溶解,依法测定(中国药典2010年版二部附录Ⅵ H ),pH 值应为5.0~6.5。
溶液的澄清度与颜色 取本品适量,加水制成每1ml 中约含20mg 的溶液,溶液应澄清无色;如显浑浊,与1号浊度标准液(中国药典2010年版二部附录Ⅸ B )比较,不得更浓;如显色,与黄色1号标准比色液(中国药典2010年版二部附录Ⅸ A 第一法)比较,不得更深。
有关物质 取本品适量,精密称定,加流动相溶解并定量稀释制成每1ml 中约含1mg 的溶液,作为供试品溶液;另精密称取盐酸纳曲酮(杂质Ⅰ)对照品适量,加流动相溶解并定量稀释制成每1ml 中约含杂质Ⅰ 1mg 的溶液,作为对照品溶液;精密量取供试品溶液1ml 和对照品溶液2ml ,置同一100ml 量瓶中,用流动相稀释至刻度,摇匀,精密量取5ml ,置50ml 量瓶中,用流动相稀释至刻度,摇匀,作为对照溶液。
洋甘菊提取液MSDS英文版

1. IDENTIFICATION OF THE SUBSTANCE/TREPARATION AND THE COMPANY/UNDERTAKING3.HAZARDS IDENTIFICATION4. FIRST AID MEASURESMATERIAL SAFETY DATA SHEETProduct name:Supplier:Tel:EMERGENCY OVERVIEW: May cause skin irritation and/or dermatitisPrinciple routes of exposure: Inhalation: Ingestion: Skin contact: Eye contact:SkinMay cause irritation of respiratory tract May be harmful if swallowed May cause allergic skin reaction Avoid contact with eyesStatements of hazard MAY CAUSE ALLERGIC SKIN REACTION.Statements of Spill of Leak Label Eliminate all ignition sources. Absorb and/or contain spill with inert materials (e.g., sand, vermiculite). Then place in appropriate container. For large spills, use water spray to disperse vapors, flush spill area. Prevent runoff from entering waterways or sewers.General advice:POSITION/INFORMATION ON INGREDIENTSInhalation:Skin contact:Ingestion:Eye contact:Protection of first – aiders:Medical conditions aggravated by exposure: In the case of accident or if you fell unwell, seek medical advice immediately (show the label where possible).Move to fresh air, call a physician immediately.Rinse immediately with plenty of water and seek medical adviceDo not induce vomiting without medical advice.In the case of contact with eyes, rinse immediately with plenty of water and seek medical advice.No information availableNone knownSuitable extinguishing media:Specific hazards:Special protective equipment for firefighters:Flash point:Autoignition temperature:NFPA rating Use dry chemical, CO2, water spray or “alcohol” foam Burning produces irritant fumes.As in any fire, wear self-contained breathing apparatus pressure-demand, MSHA/NIOSH (approved or equivalent) and full protective gearNot determinedNot determinedNFPA Health: 1 NFPA Flammability: 1 NFPA Reactivity: 0Personal precautions: Environmental precautions: Methods for cleaning up: Use personal protective equipment.Prevent product from entering drains.Sweep up and shovel into suitable containers for disposalStorage:7. HANDLING AND STORAGE5.FIRE-FIGHTING MEASURES6. ACCIDENTAL RELEASE MEASURESRoom temperature Handling:Safe handling advice: Incompatible products:Use only in area provided with appropriate exhaust ventilation.Wear personal protective equipment.Oxidising and spontaneously flammable productsEngineering measures: Respiratory protection: Skin and body protection:Eye protection: Hand protection: Hygiene measures:Ensure adequate ventilation.Breathing apparatus only if aerosol or dust is formed. Usual safety precautions while handling the product will provide adequate protection against this potential effect. Safety glasses with side-shieldsPVC or other plastic material glovesHandle in accordance with good industrial hygiene and safety practice.Melting point/range: Boiling point/range: Density: Vapor pressure: Evaporation rate: Vapor density: Solubility (in water): Flash point:Autoignition temperature:No Data available at this time. No Data available at this time. No data available No data available No data available No data available No data available Not determined Not determinedStability: Stable under recommended storage conditions. Polymerization: None under normal processing.Hazardous decomposition products: Thermal decomposition can lead to release of irritating gases and vapours such as carbon oxides.Materials to avoid: Strong oxidising agents.10. STABILITY AND REACTIVITY9. PHYSICAL AND CHEMICAL PROPERTIES8. EXPOSURE CONTROLS/PERSONAL PROTECTION11. TOXICOLOGICAL INFORMATIONConditions to avoid: Exposure to air or moisture over prolonged periods.Product information Acute toxicityChronic toxicity:Local effects: Chronic exposure may cause nausea and vomiting, higher exposure causes unconsciousness.Symptoms of overexposure may be headache, dizziness, tiredness, nausea and vomiting.Specific effects:May include moderate to severe erythema (redness) and moderate edema (raised skin), nausea, vomiting,headache.Primary irritation: Carcingenic effects: Mutagenic effects: Reproductive toxicity:No data is available on the product itself. No data is available on the product itself. No data is available on the product itself. No data is available on the product itself.Mobility:Bioaccumulation: Ecotoxicity effects: Aquatic toxicity:No data available No data available No data availableMay cause long-term adverse effects in the aquatic environment.12. ECOLOGICAL INFORMATION13. DISPOSAL CONSIDERATIONSWaste from residues/unused products:Contaminated packaging:Waste disposal must be in accordance with appropriate Federal, State and local regulations. This product, if unaltered by use, may be disposed of treatment at a permitted facility or as advised by your local hazardous waste regulatory authority. Residue from fires extinguished with this material may be hazardous.Do not re-use empty containers.UN/Id No:Not regulated14. TRANSPORT INFFORMATIONDOTProper shipping name: Not regulatedTGD(Canada)WHMIS hazard class: Non - controlledIMDG/IMOIMDG – Hazard Classifications Not ApplicableIMO – labels:15. REGULATORY INFOTMATION International Inventories16. OTHER INFORMATIONPrepared by: Health & SafetyDisclaimer: The information and recommendations contained herein are based upon tests believed to be reliable.However, XABC does not guarantee the accuracy or completeness NOR SHALL ANY OF THIS INFORMATION CONSTITUTE A WARRANTY, WHETHER EXPRESSED OR IMPLIED, AS TO THE SAFETY OF THE GOOD, THE MERCHANTABILITY OF THE GOODS, OR THE FITNESS OF THE FITNESS OF THE GOODS FOR A PARTICULAR PURPOSE. Adjustment to conform to actual conditions of usage maybe required. XABC assumes no responsibility for results obtained or for incidental or consequential damages, including lost profits arising from the use of these data. No warranty against infringement of any patent, copyright or trademark is made or implied.End of safety data sheet。
粉状美斯特(蛋氨酸羟基类似物)仅用于为奶牛提供蛋氨酸

2. 产品组成及成分信息
成 分 含量% CAS- No. EINECS-No. 有害杂质
载体
2-羟基-4-甲硫基 丁酸异丙酯
57296-04-5
—
二氧化硅 无
CAS-Nr[7631-86-9]
3. 危害识别
理化危害:供应的物质中无有害影响 健康危害:供应的物质中无有害影响 环境危害:在正常使用时无已知的环境危害
皮肤接触: 对兔子皮肤无刺激 吸 入: 无影响 慢性毒性:致癌影响:无致癌影响 致突变影响:AMES 试验测定无致突变影响;Eukaryote 试验测 定无致突变影响 生殖毒性:无影响 过敏性:无已知过敏反应 12. 生态信息 迁移性:产品可能转移的途径:水 生物积累性:无潜在的生物积累性 13. 善后处理 废弃物处理: 请勿倾注于排水管。在遵从 1990 年环境保护法规的要求下可焚烧处理(请 参照 IPR5/1) 污染物包装处理: 清空污染物; 使用热水和合适的清洗剂去除脂肪; 在遵从环保部门 1990 法规要求下可焚烧处理(请参照 IPR5/1); 请注意按区域地方相关污法规作出染物处理。
材料安全数据表
粉状美斯特Ⓡ (蛋氨酸羟基类似物) 仅用于为奶牛提供蛋氨酸
饲料添加剂 HMBi C.A.S 号:57296-04-5 二氧化硅 C.A.S 号:7631-86-9 保证值:含 2-羟基-4-甲硫基丁酸异丙酯不低于 57%
1. 化学产品及公司名称
产品信息:产品名称粉状美斯特 TM
产品使用:推荐作为动物饲料使用
16. 其他信息
此材料安全数据表严格按照 2001/59/EEC,2001/58/EEC,1999/45/EEC 标准 执行
分子量:192.28g 化学结构:HMBi(2-羟基-4-甲硫基丁酸异丙酯)
海藻糖酶(Trehalase,THL)试剂盒说明书

货号:MS2606 规格:100管/48样海藻糖酶(Trehalase,THL)试剂盒说明书微量法正式测定前务必取2-3个预期差异较大的样本做预测定测定意义:THL(EC 3.2.1.28)广泛存在于动物、植物、微生物和培养细胞中。
海藻糖酶主要功能在于生物体分解海藻糖生成葡萄糖而直接用于能量供应。
测定原理:采用3,5-二硝基水杨酸法测定THL催化海藻糖产生的还原糖的含量。
还原糖与3,5-二硝基水杨酸共热生成棕红色的氨基化合物,在一定范围内还原糖的量和反应液的颜色深度成正比,由此判断THL活性的高低。
自备实验用品及仪器:可见分光光度计/酶标仪、水浴锅、可调式移液器、微量石英比色皿/96孔板、研钵、冰和蒸馏水。
试剂的组成和配制:提取液:液体100mL×1瓶,4℃保存;试剂一:液体7mL×1瓶,4℃保存;试剂二:粉剂×1支,4℃保存,用时加入1mL试剂一,充分溶解待用;用不完的试剂4℃保存;试剂三:液体10mL×1瓶,4℃保存;试剂四:液体10mL×1瓶,常温避光保存;样品测定的准备:1、细菌或细胞的处理:收集细菌或细胞到离心管内,离心后弃上清;按照细菌或细胞数量(104个):提取液体积(mL)为500~1000:1的比例(建议500万细菌或细胞加入1mL提取液),超声波破碎细菌或细胞(冰浴,功率20%或200W,超声3S,间隔10S,重复30次);8000g,4℃离心10min,取上清,置冰上待测。
2、组织的处理:按照组织质量(g):提取液体积(mL)为1:5~10的比例(建议称取约0.1g组织,加入1mL提取液),冰浴中匀浆。
8000g ,4℃离心10min,取上清,置冰上待测。
3、血清(浆)的处理:按照血清(浆)体积(mL):提取液体积(mL)为1:5~10的比例(建议取0.1mL血清(浆)加入1mL提取液),冰浴中匀浆。
8000g ,4℃离心10min,取上清,置冰上待测。
甜菊素质量标准及检验操作规程
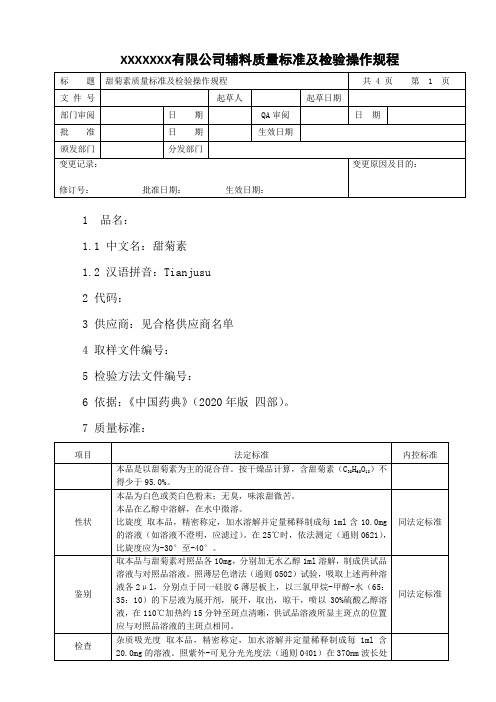
XXXXXXX 有限公司辅料质量标准及检验操作规程1 品名:1.1 中文名:甜菊素1.2 汉语拼音:Tianjusu2 代码:3 供应商:见合格供应商名单4 取样文件编号:5 检验方法文件编号:6 依据:《中国药典》(2020年版 四部)。
7 质量标准:8 检验操作规程:8.1 试药与试剂:水、硫酸乙醇、乙醇、甲醇、三氯甲烷、甜菊糖对照品等。
8.2 仪器与用具:电子天平、烘箱、马福炉等。
8.3 性状:8.3.1 本品为白色或类白色粉末。
8.3.2 本品为白色或类白色粉末;无臭,味浓甜微苦。
8.3.3 比旋度取本品,精密称定,加水溶解并定量稀释制成每1ml含10.0mg 的溶液(如溶液不澄明,应滤过)。
在25℃时,依法测定(附录23),比旋度应为-30°至-40°。
8.4 鉴别:取本品与甜菊素对照品各10mg,分别加无水乙醇1ml溶解,制成供试品溶液与对照品溶液。
照薄层色谱法(附录7)试验,吸取上述两种溶液各2μl,分别点于同一硅胶G薄层板上,以三氯甲烷-甲醇-水(65:35:10)的下层液为展开剂,展开,取出,晾干,喷以30%硫酸乙醇溶液,在110℃加热约15分钟至斑点清晰,供试品溶液所显主斑点的位置应与对照品溶液的主斑点相同。
8.5 检查:8.5.1 杂质吸光度取本品,精密称定,加水溶解并定量稀释制成每1ml含20.0mg的溶液。
照紫外-可见分光光度法(附录5)在370nm波长处测定,吸光度不得大于0.10。
8.5.2酸度取本品0.50g,加中性乙醇(对酚酞指示液显中性)20ml,振摇使溶解,加酚酞指示液1滴,用氢氧化钠滴定液(0.1mol/L)滴定至红色出现,并在10秒钟内不褪,消耗氢氧化钠滴定液(0.1mol/L)不得过0.5ml。
8.5.3 干燥失重取本品,在105℃干燥至恒重,减失重量不得过5.0%(附录14)。
8.5.4 炽灼残渣取本品1.0g,依法检查(附录16),遗留残渣不得过0.1%。
藤茶提取物 二氢杨梅素

藤茶提取物二氢杨梅素【产品名称】二氢杨梅素Dihydromyricetin DMY【别名】双氢杨梅树皮素、福建茶素、白蔽素、二氢杨梅黄酮、蛇葡萄素【化学名称】 3 ,5,7,3’,4’,5’- 六羟基-2,3- 双氢黄酮醇【CAS 号】27200-12--0【分子式】C15H12O8【分子量】320【溶解度】25 ℃水中溶解度为4%,热水中溶解度更大;易溶于乙醇及丙酮,极微溶于醋酸乙酯。
【其他性质】黄酮类化合物,气特殊纯白色针状结晶,微溶于水,呈酸性(pH4~5),在酸性条件下稳定。
【用途】主要作为医药原料药。
【来源】藤茶属葡萄科,蛇葡萄属,学名为显齿蛇葡萄(Ampelopsis grossedentata)百部提取物Stemona Extract 5:1,10:1 TLC 八角提取物Anise Extract 5:1,10:1 TLC 八角莲提取物Dysosma versipellis Extract 5:1,10:1 TLC 菝葜提取物Sarsaparilla Extract 5:1,10:1 TLC 百里香提取物Thyme Extract 5:1,10:1 TLC 白术提取物Atractylodes Rhizome Extract 5:1,10:1 TLC 白芸豆提取物White Kidney Bean Extract 5:1,10:1 TLC 布枯叶提取物Buchu Leaf Extract 5:1,10:1 TLC 薄荷提取物Mint Extract 5:1,10:1 TLC 草莓提取物Strawberry Extract 5:1,10:1 TLC 陈皮提取物Tangerine Peel Extract 5:1,10:1 TLC 苍术提取物Atractylodes Rhizome Extract 5:1,10:1 TLC 柴胡提取物Bupleurum Extract 5:1,10:1 TLC 大枣提取物Jujube Extract 5:1,10:1 TLC 党参提取物Codonopsis Pilosula Extract 5:1,10:1 TLC 丹参提取物Radix Salviae Miltiorrhizae Extract 5:1,10:1 TLC 大豆提取物Soybean Extract 5:1,10:1 TLC 椴树花提取物Linden Extract 5:1,10:1 TLC 番泻叶提取物Senna Extract 5:1,10:1 TLC 茯苓提取物Poria Extract 5:1,10:1 TLC 防风草提取物Wind Grass Extract 5:1,10:1 TLC 甘草提取物Licorice Extract 5:1,10:1 TLC 桂皮提取物Cinnamon Extract 5:1,10:1 TLC 桂圆提取物Longan Extract 5:1,10:1 TLC 狗脊提取物Rhizome Extract 5:1,10:1 TLC 黑胡椒提取物Black Pepper Extract 5:1,10:1 TLC 黄芪提取物Astragalus Extract 5:1,10:1 TLC 红景天提取物Rhodiola Extract 5:1,10:1 TLC 厚朴提取物Magnolia Bark Extract 5:1,10:1 TLC 红花提取物Safflower Extracts 5:1,10:1 TLC【药理作用】此类物质具有清除自由基、抗氧化、抗血栓、抗肿瘤、消炎等多种奇特功效;而二氢杨梅素是较为特殊的一种黄酮类化合物,除具有黄酮类化合物的一般特性外,还具有解除醇中毒、预防酒精肝、脂肪肝、抑制肝细胞恶化、降低肝癌的发病率等作用。
阿司咪唑

合成方法
合成方法
化合物(I)和碘甲烷在乙醇中回流8h,环合得到化合物(Ⅱ)。再水解脱去酯基,得到化合物(Ⅲ)。用对甲氧 基苯乙基溴进行N-烷基化,得化合物(Ⅳ)。再用对氟苄基溴烷基化,得阿司咪唑。
1. 1-[(4-氟苯基)甲基]-苯并咪唑-2-(3H)-酮的制备
在反应瓶中加入2-羟基苯并咪唑5.0g(37.3mmol)和NaH 1.6g(53mmol)(NaH含量大约为80%,浸入矿物油中) 的DMF 100ml的悬浮液.加毕.在60ºC.(最好有N2保护)搅拌反应1h.再加入4-氟苄基氯(FBC)5.4g(37mmol),加热 ( 6 0 ºC ) 搅 拌 反 应 5 . 5 h . 冷 却 至 室 温 后 加 入 冰 水 7 0 0 m l , 用 二 氯 甲 烷 ( 5 0 0 m l × 2 ) 提 取 . 有 机 层 用 食 盐 水 洗 . 无 水 N a 2 S O 4 干燥.过滤.滤液减压浓缩.剩余物用石油醚析晶.得1-[(4-氟苯基)甲基]-苯并咪唑-2-(3H)-酮固体8.0g,为无色 结 晶 m p 1 7 8 ~ 1 7 9 ºC , 收 率 8 8 % .
治疗措施
阿司咪唑中毒的治疗要点为: 1.大量摄入者予洗胃,后灌服活性炭和导泻。 2.对心肌抑制和Q-T间期延长者予5%碳酸氢钠250ml静注可能有效。 3.对症、支持治疗。
专家点评
专家点评
阿司咪阿司咪唑自1983年上市以来,在许多国家得到了广泛应用。国外研究显示阿司咪唑治疗荨麻疹的总有 效率为74%。国内的一项多中心双盲安慰剂对照试验表明阿司咪唑对急性荨麻疹的总有效率为82.9%,对慢性荨麻 疹的总有效率为86.0%,均显著高于安慰剂,主要不良反应为嗜睡、倦怠、口干等,连续用药3个月的患者中,半 数有食欲及体重增加。阿司咪唑的心脏毒性虽然发生率较低,但由于后果严重,已限制了它的应用。阿司咪唑为 强效和长效的H1受体拮抗剂,无中枢镇静和抗毒蕈碱样作用。代谢产物去甲阿司咪唑仍有抗胆胺作用。长期服用 可增进食欲和增加体重,服用过量可引起心脏Q-T间期延长和室性心律失常。适用于各种原因引起过敏性疾病。
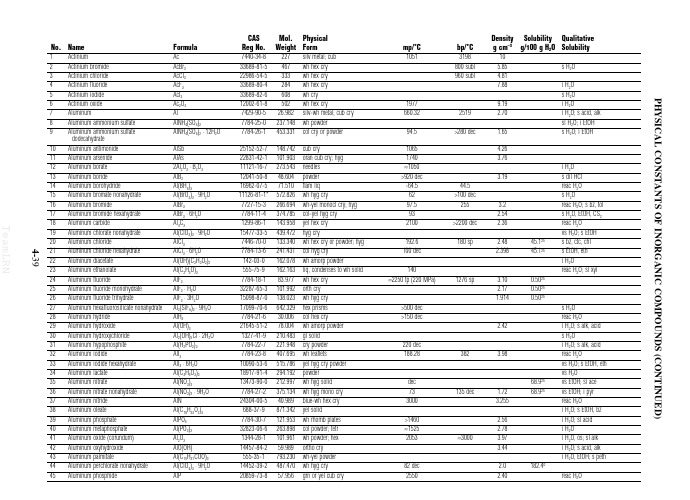
无机物熔点
>500 dec >150 dec 2.42 220 dec 188.28
382
3.98
dec 73 3000 >1460 ≈1525 2053
135 dec
1.72 3.255 2.56 2.78 3.97 3.44 2.0 2.40
68.925 68.925
≈3000
Hale Waihona Puke 82 dec 2550182.40
No. Name
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 Actinium Actinium bromide Actinium chloride Actinium fluoride Actinium iodide Actinium oxide Aluminum Aluminum ammonium sulfate Aluminum ammonium sulfate dodecahydrate Aluminum antimonide Aluminum arsenide Aluminum borate Aluminum boride Aluminum borohydride Aluminum bromate nonahydrate Aluminum bromide Aluminum bromide hexahydrate Aluminum carbide Aluminum chlorate nonahydrate Aluminum chloride Aluminum chloride hexahydrate Aluminum diacetate Aluminum ethanolate Aluminum fluoride Aluminum fluoride monohydrate Aluminum fluoride trihydrate Aluminum hexafluorosilicate nonahydrate Aluminum hydride Aluminum hydroxide Aluminum hydroxychloride Aluminum hypophosphite Aluminum iodide Aluminum iodide hexahydrate Aluminum lactate Aluminum nitrate Aluminum nitrate nonahydrate Aluminum nitride Aluminum oleate Aluminum phosphate Aluminum metaphosphate Aluminum oxide (corundum) Aluminum oxyhydroxide Aluminum palmitate Aluminum perchlorate nonahydrate Aluminum phosphide
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
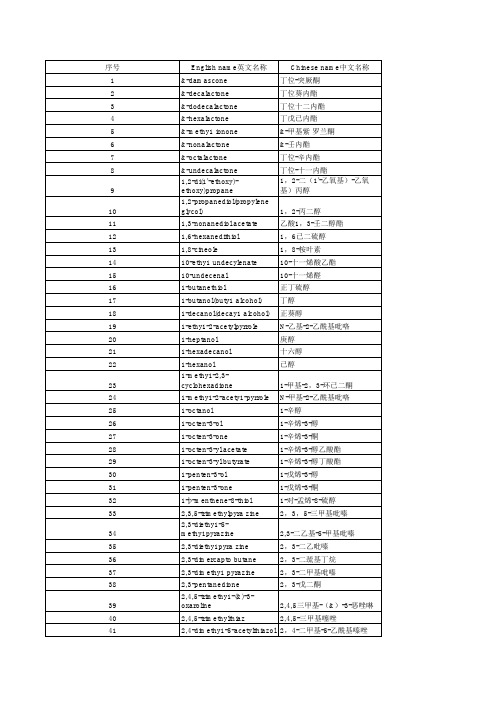
香精香料中英文
118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152
3(mehty1thio)propionaldehyd e 3-甲硫基丙醛 3-(methy1thio)butanal 3-(methy1thio)propanol 3,4-dimethoxy benzaldehyde 3,4-hexanedione 3,5-dimethy1-1,2,4trithiolane 3,5-dimethy1-1,2cyclopenta-dione 3-甲硫基丁醛 3-甲硫基丙醇 3,4二甲氧基苯甲醛 3,4-已二酮 3,5-二甲基-1,2,4-三硫代 环戊烷 3,5-二甲基-1,2-环戊二酮
3-acety1-2,5-dimethyl furan 3-乙酰基-2,5-二甲基呋喃 3-acety1-2,5-dimethyl thiophene 3-乙酰基-2,5-二甲基噻酚 3-butylideneph thalide 3-ethy1-2-hycroxy-2-cyclopenten-1-one 3-heptanone 3-hexenoic acid 3-hexeny1 isovalerate 3-hexeny1-2-methy1 butyrate 3-hydroxy-2-butanone 3-mercapto-2-butanone 3-mercapto-2-pentanone 3-methy1- pentanoic acid 3-methy1-2-penty1-2cyclopenten-1-one 3-methy1thio-1-hexanol 3-octy1 acetate 3-pheny1propionalde hyde 4-(2-fury1)-3-buten-2-one 4-(3,4-methy1enedioxypheny1)-2-butanone 4-(methy1thio)-4-methy1-2pentanone 4-(þ-hydroxypheny1)-2 butanone 4-(þ-methoxypheny1)-2butan-one 4,5-dihydro-3(2H)thiophenon 4,5-dimethy1 thiazole 4,5-dimethy1-2-isobuty1-3thiazoline 4,5-dimethy1-3-hydroxy-2,5dihydrofuran-2-one 4-carvomenthenol 4-ethy1 guaiacol 4-heptenal 3-丁叉苯酞 3-乙基-2-羟基-2-环戊烯-1-酮 3-庚酮 3-已烯酸 异戊酸-3-已烯酯 2-甲基-丁酸-3-已烯酯 3-羟基-2-丁酮 3-巯基-丁-2-酮 3-巯基-戊-2-酮 酐酪酸(3-甲基戊酸) 3-甲基-2-戊基-2-环戊烯-1-酮 3-硫基-1-已醇 乙酸3-辛酯 3-苯基丙醛 4-(2-呋喃基)-3-丁烯-2-酮 胡椒基丙酮 4-甲硫基-4-甲基-2-戊酮 复盆子酮(悬钩子酮) 4-对甲氧基苯基-2-丁酮 4,5-二氢-3-(2H)噻酚酮(四 氢噻酚-3-酮) 4,5-二甲基噻唑 4,5-二甲基-2-异丁基-3-嗪唑 啉 4,5-二甲基-3-羟基-2,5-二 氢呋喃-2-酮 香芹薄荷醇(4-葛缕薄茶脑) 4-乙基愈创木酚 4-庚烯醛
Articaine HCl_牙科局部麻醉剂_23964-57-0_Apexbio

>13.4mg/mL in DMSO
Store at -20°C
For obtaining a higher solubility , please warm the tube at 37°C and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months.
Evaluation sample solution : ship with blue ice All other available size: ship with RT , or blue ice upon request
生物活性
靶点 : 信号通路: 产品描述:
Articaine is a dental local anesthetic which contains an additional ester group that is metabolized by estearases in blood and tissue. 参考文献:
特别声明
产品仅用于研究, 不针对患者销售,望谅解。 每个产品具体的储存和使用信息显示在产品说明书中。ApexBio 产品在推荐的条件下是稳定 的。产品会根据不同的推荐温度进行运输。许多产品短期运输是稳定的,运输温度不同于长 期储存的温度。我们确保我们的产品是在保持试剂质量的条件下运输的。收到产品后,按照 产品说明书上的要求进行储存。
产品说明书
化学性质
产品名: Articaine HCl 修订日期: 6/30/2016
三氟乙酸脱苄基O-debenzylation_of_ortho-substituted_phenols_with_trifluoroacetic_acid

Mild,efficient and rapid O-debenzylation of ortho -substituted phenols with trifluoroacetic acidSteven Fletcher *,Patrick T.Gunning *Department of Chemistry,University of Toronto,Mississauga,ON L5L 1C6,Canadaa r t i c l e i n f o Article history:Received 21May 2008Revised 2June 2008Accepted 4June 2008Available online 10June 2008a b s t r a c tThe mild and efficient deblocking of aryl benzyl ethers with TFA is reported.Cleavage was fastest with ortho -electron-withdrawing groups on the phenolic ring,which we have attributed to a proton chelation effect,furnishing the deprotected phenols in excellent yields.The corresponding para -methoxybenzyl,allyl and iso -propyl ethers were also cleanly removed under these conditions.In addition,the selective aryl benzyl ether debenzylation in the presence of benzyl ester,Cbz carbamate and Boc carbamate func-tionalities was also observed.Crown Copyright Ó2008Published by Elsevier Ltd.All rights reserved.Phosphotyrosines feature in the design of inhibitors of several protein targets,including protein tyrosine phosphatase 1B (PTP1B).1However,these moieties suffer from hydrolytic lability to cellular phosphatases and poor cell penetration due to the asso-ciated dianionic charge.1To address these issues,salicylic acid derivatives (and closely-related analogues)have become popular mimetics of phosphotyrosine in small molecule inhibitors.1–5Turk-son et al.have recently reported on NSC74859(1),a potent,sali-cylic acid-based inhibitor of the oncogenic protein Stat3.6As part of our structure–activity relationship (SAR)studies on NSC74859(1),we sought to debenzylate both the phenol ether and benzoate ester in 2without reducing the aryl-bromide bond,a common undesired side reaction that occurs with hydrogen gas and Pd/C catalyst.7O -Benzyl-protected phenols are known to undergo debenzyla-tion with trifluoroacetic acid (TFA)8by an initial protonation of the weakly basic phenol oxygen,although additives such as strongorganic acids (e.g.,trifluoromethanesulfonic acid 9)or a large excess of nucleophilic scavenger (e.g.,thioanisole,which accelerates the reaction by a ‘push–pull’mechanism 10)are typically required.Re-cent work by Ploypradith et al.describes the mild deprotection of aromatic ethers with sub-stoichiometric para -toluenesulfonic acid on solid support.11In a special case,O -benzyl-protected ortho -nitrophenol was cleaved rapidly (<5min)with neat TFA,12which we considered was due to the ability of the substrate to chelate a proton since the structurally-similar ortho -hydroxybenzoates (salicylates)are well-known to chelate copper ions and iron ions.We reasoned that 2(and indeed 3)may similarly undergo acceler-ated debenzylation with TFA.In fact,as shown in Scheme 1,treat-ment of 2(or 3)with a 1:1mixture of TFA/toluene led to rapid debenzylation (5min for 2;1h for 3)in 91%yield for 2(or 85%yield for 3).In this Letter,we will explore the structural require-ments of the phenol component that increase the lability of the O -benzyl phenol ether bond in the presence of TFA.In addition,0040-4039/$-see front matter Crown Copyright Ó2008Published by Elsevier Ltd.All rights reserved.*Tel.:+19058285354;fax:+19058285425(P.T.G.).E-mail addresses:steven.fletcher@utoronto.ca (S.Fletcher),patrick.gunning@utoronto.ca (P.T.Gunning).Tetrahedron Letters 49(2008)4817–4819Contents lists available at ScienceDirectTetrahedron Lettersj o ur na l h om e pa ge :w w w.e ls e v ie r.c o m/lo c at e/t et l e twe will explore the selectivity of this mild debenzylation tech-nique with respect to other aromatic ethers and examine the sta-bility of other benzyl-based protecting groups to these reaction conditions.A series of 12O -benzyl-protected phenols was prepared by standard procedures in near quantitative yields.Each of these ethers was then deprotected with a 1:1mixture of TFA/toluene;our observations are summarized in Table 1.In certain cases,O ?C benzyl migration (Friedel–Crafts reaction)by-products (610%)were occasionally inseparable from the product by silica gel flash column chromatography.Thus,several benzyl cation cap-tors were investigated for their abilities to improve yields and puri-ties of the debenzylation reactions.Three to ten equivalents of p -cresol,anisole and triethylsilane were employed,but these exerted little effects on reducing by-product formation.Conversely,we dis-covered that including the more nucleophilic scavenger thioanisole as an additive to the co-solvent toluene typically,after silica gel flash column chromatography,furnished products in P 95%puri-ties (and higher yields),as judged by 1H NMR.Nevertheless,we envisaged any Friedel–Crafts impurities would be more readily separable on slightly more complex aryl benzyl ethers,as we ob-served with the substrates shown in Scheme 1and Tables 3and 4(>99%purities (1H NMR)in each case).Whilst likely leading to even higher yields and purities,large excesses of thioanisole (50equiv)are also known to accelerate TFA-mediated debenzyla-tion.10However,in our hands just 3equiv of thioanisole had little effect on the rate of debenzylation,allowing us to attribute the deprotection rates solely to the structure of the phenol.Electron-rich phenols are good scavengers of benzyl cations,13and since preliminary experiments with electron-rich phenols generated complex mixtures of Friedel–Crafts by-products under these deb-enzylation conditions,we chose to investigate only electron-poor phenols in this study.O -Benzyl-protected phenols with p -ortho -electron-withdraw-ing groups (6a ,6b ,6d ,6f )were swiftly (several in less than 3h cf.24h for unsubstituted phenol 6l )and cleanly debenzylated,with less than 5%of the undesired C-benzylated phenol by-prod-ucts.In contrast,meta -and para -electron-withdrawing groups slo-wed down the debenzylation (e.g.,entries 6g and 6h ),relative to the control compound 6l ,which itself could only be obtained in moderate purity by this method.The r -withdrawing (and p -donating)bromophenols 6i –k were insufficiently deactivated to benzyl cation scavenging and were contaminated with several by-products.Importantly,n -butyl benzyl ether 8was unaffected by TFA under the reaction conditions,indicating this procedure is selective for aryl benzyl ethers.In addition,the results in Table 1suggest that this procedure is suitable only for phenols substituted with p -electron-withdrawing groups.Since the debenzylation mechanism with TFA proceeds via an initial protonation of the phenol ether oxygen,the more available the ether oxygen lone pairs are,the faster the reaction will be.Hence,the slower reaction times for the phenols bearing meta -and para -electron-withdrawing groups make sense,although this is not true for the ortho -functionalized aryl benzyl ethers.As hypothesized for the bis-benzyl salicylate derivative 2earlier,we considered these ortho -substituted phenols were capable of chelat-ing the acidic hydrogen atom from TFA which therein facilitated the acid-mediated debenzylation via a six-membered cyclic inter-mediate,as proposed in Scheme 2.A similar chelation intermediate has been put forward by Baldwin and Haraldsson to account for the Lewis acid MgBr 2-mediated debenzylation of aromatic benzyl ethers ortho to an aldehyde group.14Accordingly,to test this hypothesis we expanded this series of ortho -substituted aryl benzyl ethers,and the results from their deb-enzylation reactions with TFA are summarized in Table 2.These substrates have been listed in order of increasing approximateTable 1TFA-mediated debenzylation of O -benzyl-protected phenols aTFAtolueneOBnROHR67Substrate RTime (h)b Yield c (%)6a o -CO 2Me,m d -NHAc 5min 936b o -CO 2Me 5min 946c p -CO 2Me 36e 63(85f )6d o -CO 2Bn 5min 936e p -CO 2Bn 36e 58(79f )6f o -NO 23976g m -NO 236e 75(98f )6h p -NO 236e 66(98f )6i o -Br 16—g 6j m -Br 30—g 6k p -Br 36—g 6lH 24—gn -BuOBn (8)—24No reactionaThe reaction was carried out with 6(0.5mmol)in a 1:1mixture of TFA/toluene (5ml)at rt,with 3equiv of thioanisole.bTime taken for all starting material to be consumed.cIsolated yield after silica gel flash column chromatography.dmeta to phenol oxygen AND para to ester.eReaction was slow and incomplete after 3days.fYield based on recovered starting material.gComplex mixture of products.Table 2TFA-mediated debenzylation of O -benzyl-protected,ortho -substituted phenols aTFA tolueneOBnOH67RRSubstrate R p K aH b Time c (h)Yield d (%)Relative rate 6m CO 2NH 2À2248316n CHO À7 3.594e 6.96o CO 2H À8191246b CO 2Me À8.55min 942886d CO 2Bn À8.55min 932886p CN À10>4851(95f )—6f NO 2À1239786i Br —16—g 1.56lH—24—g1aThe reaction was carried out with 6(0.5mmol)in a 1:1mixture of TFA/toluene (5ml)at rt,with 3equiv of thioanisole.bApproximate p K aH of conjugate acid of R group.15cTime taken for all starting material to be consumed.dIsolated yield after silica gel flash column chromatography.eIncluding thioanisole in the deprotection of 6n led to further by-products,thus no scavenger was used and compound 7n could be obtained in only 90%purity.fYield based on recovered starting material.gComplex mixture of products.4818S.Fletcher,P.T.Gunning /Tetrahedron Letters 49(2008)4817–4819acidity of the conjugate acid (decreasing p K aH )of the ortho -elec-tron-withdrawing substituent.15There appears to be an optimal p K aH of around À8.5,that is exhibited by carboxylic esters,which lead to the fastest rate of debenzylation with TFA.In an approxi-mate bell-shaped distribution of reaction rate versus ortho -substi-tuent p K aH —that was interrupted only by ortho -cyanophenol 6p —protonatable groups with p K aH ’s <À8.5or >À8.5were less effective at accelerating the TFA-mediated debenzylation.These data concur with our chelation hypothesis:groups that are too ba-sic bind more strongly to the TFA proton making it less available for sharing with,and ultimately releasing to,the phenol ether oxygen;groups that are weakly basic do not bind the TFA proton as well,leading to reduced chelation and hence less rate enhancement.The anomalous result for ortho -cyanophenol 6p was anticipated since this compound was selected as a negative control.Phenol 6p is geometrically incapable of chelating a proton,because the lin-ear,sp -hybridized nitrile functionality directs its basic nitrogen atom (p K aH %À10)away from the phenol oxygen.As predicted,there was no rate enhancement for the TFA-mediated debenzyla-tion of 6p relative to phenol 6l .In fact,6p was only slowly deben-zylated,at a rate that was comparable with the m -nitro and p -nitro derivatives 6g and 6h ,respectively.We next wanted to investigate the selectivity for the deprotec-tion of the benzyl group over other phenol protecting groups.Accordingly,the benzyl group in salicylate derivative 9a was varied with para -methoxybenzyl (PMB;9b ),methyl (9c ),allyl (9d )and iso -propyl (i -Pr;9e ).These substrates were then debenzylated with a 1:1mixture of TFA/toluene;our findings are reported in Table 3.Any impurities this time were minor and readily separable from the products,eliminating the need for the additive thioanisole.The relative rates at which these protecting groups were removed was para -methoxybenzyl >benzyl >allyl >iso -propyl )methyl,which reflects the stability of the carbocations.These data suggest that in salicylates such as 9,the benzyl phenol protecting group (R =Bn)can be removed with TFA in the presence of the corres-ponding allyl,iso -propyl and methyl ethers.Finally,we explored the selectivity of this mild debenzylation technique over other benzyl-based protecting groups,as shown in Table 4.As the results demonstrate,it was possible to deblock the O -benzyl ether in the presence of a benzyl ester (6d )and in the presence of a benzyl carbamate (11b ),thereby increasing the orthogonality of O -benzyl phenol ethers of salicylate derivatives.Interestingly,it was even possible to cleave the benzyl group in 11c with TFA in the presence of an N -Boc-protected aniline.In summary,we have presented the mild,efficient and rapid deblocking of ortho -substituted aryl benzyl ethers with TFA.Deb-enzylation was fastest when the ortho group was a carboxylic ester,which we have attributed to a proton chelation effect.Other ortho groups that accelerated the TFA-mediated debenzylation included carboxylic acid,aldehyde and nitro.In addition,we have shown that in such ortho -functionalized phenols,benzyl could be removed in the presence of the corresponding iso -propyl,allyl and methyl ethers.Moreover,the benzyl ether could be selectively cleaved in the presence of benzyl ester,Cbz carbamate and Boc carbamate functionalities.AcknowledgementsThe authors gratefully acknowledge financial support for this work from the Canadian Foundation of Innovation and the Univer-sity of Toronto (Connaught Foundation).References and notes1.Zhang,S.;Zhang,Z.-Y.Drug Discov.Today 2007,12,373–381.2.(a)Pei,Z.;Li,X.;Liu,G.;Abad-Zapatero,C.;Lubben,T.;Zhang,T.;Ballaron,S.J.;Hutchins,C.W.;Trevillyana,J.M.;Jirouseka,M.R.Bioorg.Med.Chem.Lett.2003,13,3129–3132;(b)Xin,Z.;Liu,G.;Abad-Zapatero,C.;Pei,Z.;Szczepankiewicz,B.G.;Li,X.;Zhang,T.;Hutchins,C.W.;Hajduk,P.J.;Ballaron,S.J.;Stashko,M.A.;Lubben,T.H.;Trevillyana,J.M.;Jirouseka,M.R.Bioorg.Med.Chem.Lett.2003,13,3947–3950.3.Tautz,L.;Bruckner,S.;Sareth,S.;Alonso,A.;Bogetz,J.;Bottini,N.;Pellecchia,M.;Mustelin,T.J.Biol.Chem.2005,280,9400–9408.4.Shrestha,S.;Bhattarai,B.R.;Chang,K.J.;Leea,K.-H.;Choa,H.Bioorg.Med.Chem.Lett.2007,17,2760–2764.5.Liljebris,C.;Larsen,S.D.;Ogg,D.;Palazuk,B.J.;Bleasdale,J.E.J.Med.Chem.2002,45,1785–1798.6.Siddiquee,K.;Zhang,S.;Guida,W.C.;Blaskovich,M.A.;Greedy,B.;Lawrence,H.R.;Yip,M.L.R.;Jove,R.;Laughlin,M.M.;Lawrence,N.J.;Sebti,S.M.;Turkson,J.Proc.Natl.Acad.Sci.U.S.A.2007,104,7391–7396.7.Pandey,P.N.;Purkayastha,M.L.Synthesis 1982,876–878.8.(a)Greene,T.W.;Wuts,P.G.M.Protective Groups in Organic Synthesis ,3rd ed.;John Wiley &Sons:New York,1999;(b)Kocienski,P.J.Protecting Groups ,3rd ed.;Georg Thieme:Stuttgart,Germany,2003.9.Kiso,Y.;Isawa,H.;Kitagawa,K.;Akita,T.Chem.Pharm.Bull.1978,26,2562–2564.10.Kiso,Y.;Ukawa,K.;Nakamura,S.;Ito,K.;Akita,T.Chem.Pharm.Bull.1980,28,673–676.11.Ploypradith,P.;Cheryklin,P.;Niyomtham,N.;Bertoni,D.R.;Ruchirawat,.Lett.2007,9,2637–2640.12.Marsh,J.P.,Jr.;Goodman,.Chem.1965,30,2491–2492.13.(a)Eberle,A.N.J.Chem.Soc.,Perkin Trans.11986,361–367;(b)Bodanszky,M.;Tolle,J.C.;Deshmane,S.S.;Bodanszky,A.Int.J.Pept.Protein Res.1978,12,57–68.14.Haraldsson,G.G.;Baldwin,J.E.Tetrahedron 1997,53,215–224.15.(a)Ionization Constants of Organic Acids in Solution ;Serjeant,E.P.,Dempsey,B.,Eds.IUPAC Chemical Data Series No.23;Pergamon Press:Oxford,UK,1979;(b)see also:/labs/evans/pdf/evans_pKa_table.pdf .Table 3TFA-mediated deprotection of O-blocked phenol ether derivatives of methyl 4-acetamidosalicylate aTFAtolueneNHAcNHAcORO OMeOH OMeO 910Substrate R Time b (h)Yield c (%)9a Bn 5min 919b PMB 2min 909c Me 480d 9d Allyl 20919ei -Pr3692aThe reaction was carried out with 9(0.5mmol)in a 1:1mixture of TFA/toluene (5ml)at rt.bTime taken for all starting material to be consumed.cIsolated yield after silica gel flash column chromatography.dOnly starting material remained after 48h,at which point the reaction was aborted.Table 4Selectivity investigation into the TFA-mediated debenzylation of aryl benzyl ethers aTFA tolueneOBnOH2Bn2Bn1112RRSubstrate R Yield b (%)6d c H 9311a NHAc 9211b NHCbz 9311c dNHBoc54aThe reaction was carried out with 11(0.5mmol)in a 1:1mixture of TFA/toluene (5ml)at rt for 5min,then all solvents were evaporated.bIsolated yield after silica gel flash column chromatography.cFor compound 6d ,3equiv of thioanisole were also used.dAfter 5min,the reaction mixture was diluted with CH 2Cl 2and then immedi-ately neutralized with 1M NaOH.The organic layer was then separated and evaporated.S.Fletcher,P.T.Gunning /Tetrahedron Letters 49(2008)4817–48194819。
盐酸特比萘芬

非常罕见:已报告的有血液系统疾病如中性粒细胞减少症,粒细胞缺乏症或血小板减少症
非常罕见,已报告的有脱发,尽管病因成分过敏者禁用
【用法用量】根据感染的严重程度和适应症调整疗程。
口服,成人每次0.25克,每日一次,疗程如下:皮肤感染的疗程:手足癣[趾(指)间型和跖型]:2—6周;体癣、股癣:2—4周:皮肤患珠菌病:2—4周。在真菌学治愈几周,才可见到皮肤外观完全正常以及感染症状消失。
成人:每次1片(0.25g)每天一次
3.相互作用研究证明,特比萘芬几乎不影响经由细胞色素P450酶系代谢的药物(如环孢素、D860或口服避孕药)的清除。然而,使用口服避孕药的妇女应慎用本品,因为极少数人可能月经失调。此外,肝药酶诱导药(如利福平等)可加速特比萘芬的血浆清除,肝药酶抑制药(如西米替丁等)则可抑制其清除,故如果需要用以上药物,则需将特比萘芬的剂量作适当调整。
青少年,体重>40kg(通常年龄>12岁):每次1片(0.25g)每天一次
儿童,体重20-40kg(通常年龄5-12岁):每次半片(0.125g)每天一次
儿童,体重<20kg 通常年龄<5岁:关于此组病人,从对照试验中获得的资料非常有限,所以药物只有在没有其它可选择的治疗方法以及潜在的治疗疗效益大于可能的危险情况才可使用。
注意事项:
1.肝或肾功能不全(肌酐清除率<50ml/分,血清肌酐>300u mol/升)者,特比萘芬剂量应减少50%(见“不良反应”)。
2.妊娠与哺乳动物研究显示,本品对胎儿和生育力无不良影响。由于本品用于孕妇的经验极有限,因此,应权衡利弊,原则上孕妇不应使用。特比萘芬可经乳汁排泄,故接受特比萘芬口服治疗的母亲不应哺乳。
海藻糖含量检测试剂盒说明书

海藻糖含量检测试剂盒说明书微量法注意:本产品试剂有所变动,请注意并严格按照该说明书操作。
货号:BC0335规格:100T/96S产品组成:使用前请认真核对试剂体积与瓶内体积是否一致,有疑问请及时联系索莱宝工作人员。
试剂名称规格保存条件提取液液体100 mL×1瓶2-8℃保存试剂一粉剂×2瓶2-8℃保存标准品粉剂×1支2-8℃保存溶液的配制:1、标准品:10 mg 海藻糖。
临用前加1 mL蒸馏水,溶液浓度为10 mg/mL,2-8℃保存两周;2、工作液的配制:临用前取1瓶试剂一加入3.5 mL蒸馏水后,缓慢加入14 mL浓硫酸,不断搅拌,充分溶解,待用。
用不完的试剂2-8℃保存一周。
产品说明:海藻糖存在于大量有机体中,包括细菌、藻类、酵母、植物、昆虫和其他无脊椎动物。
由于海藻糖具有独特的不同于其他碳水化合物的生物学特性,能在干旱、高温、脱水、冷冻、高渗透压及毒性物质等恶劣环境下保护生物体细胞蛋白质、脂肪、糖类、核酸等组分不受损害。
测定方法采用蒽酮比色法。
具有灵敏度高﹑简便快捷﹑适用于微量样本的测定等优点。
但是蒽酮比色法也存在一定缺陷,如果样本中含有可溶性糖,则会影响测定。
本试剂盒建议用于除海藻糖外不含其他可溶性糖样本的测定。
Trehalose+ H2SO4Furfural Derivatives(620nm)技术指标:最低检出限:0.0055 mg/mL线性范围:0.00625-0.4 mg/mL注意:实验之前建议选择2-3个预期差异大的样本做预实验。
如果样本吸光值不在测量范围内建议稀释或者增加样本量进行检测。
需自备的仪器和用品:可见分光光度计/酶标仪、水浴锅、台式离心机,可调式移液器、超声破碎仪、微量玻璃比色皿/96孔板、研钵/匀浆器、浓硫酸和蒸馏水。
操作步骤:一、样本处理(可适当调整待测样本量,具体比例可以参考文献)1、细菌或细胞处理:收集细菌或细胞到离心管内,离心后弃上清;按照每500万细菌或细胞加入1mL提取液,超声波破碎细菌或细胞(功率200w,超声3秒,间隔10秒,重复30次),室温静置45min,振荡3~5次,冷却后,8000g,常温离心10min,取上清。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Product Name:
Articaine hydrochloride CAS No.:
23964-57-0Cat. No.:
HY-B0516
Product Data Sheet
MWt:
320.84Formula:
C13H21ClN2O3S Purity :>98%
DMSO 64 mg/mL; Water 64 mg/mL Solubility:Mechanisms:
Biological Activity:
A ti i H d hl id i d t l l l th ti
Pathways:Others; Target:Others SO 6g/;ate 6g/
Articaine Hydrochloride is a dental local anesthetic.
Target: Others Articaine Hydrochloride is a dental local anesthetic. Articaine: VAS (Visual Analogue Scale) scores (from 0 to 10 cm) by patients 4 to < 13 years of age are 0.5 for simple procedures and 1.1 for complex procedures, and average investigator scores are 0.4 and 0.6 for simple and complex procedures, respectively. No serious adverse events related to the articaine occurres, the only adverse event considered related to articaine is accidental lip injury in one patient [1]. Articaine results in success rate of 64.5% in electronic pulp testing in healthy adult volunteers injected with 4%articaine.Articaine infiltration produces significantly more episodes of no response to maximum References:
[1]. Malamed, S.F., S. Gagnon, and D. Leblanc, A comparison between articaine HCl and lidocaine
HCl in pediatric dental patients. Pediatr Dent, 2000. 22(4): p. 307-11.[2]. Kanaa, M.D., et al., Articaine and lidocaine mandibular buccal infiltration anesthesia: a
4% articaine. Articaine infiltration produces significantly more episodes of no response to maximum stimulation in first molars than lidocaine. Mandibular buccal infiltration is more effective with 4%articaine with epinephrine...
prospective randomized double-blind cross-over study. J Endod, 2006. 32(4): p. 296-8.[3]. Robertson, D., et al., The anesthetic efficacy of articaine in buccal infiltration of mandibular
posterior teeth. J Am Dent Assoc, 2007. 138(8): p. 1104-12.Caution: Not fully tested. For research purposes only
Medchemexpress LLC
18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
