crc-15_02_85-LABORATORY SOLVENTS AND OTHER LIQUID REAGENTS
CRC化学和物理手册1

CRC Handbook of Chemistry and Physics90th EditionCD-ROM Version 2010Editor-in-ChiefDavid R. LideFormer Director, Standard Reference DataNational Institute of Standards and TechnologyAssociate EditorW. M. “Mickey” HaynesScientist EmeritusNational Institute of Standards and TechnologyEditorial Advisory BoardGrace BaysingerSwain Chemistry and Chemical Engineering Library Stanford University Henry V. Kehiaian ITODYS University of Paris VIILev I. BergerCalifornia Institute of Electronics and Materials Science Kozo Kuchitsu Department of Chemistry Josai UniversityMichael FrenkelNational Institute of Standards and Technology Dana L. RothMillikan LibraryCalifornia Institute of TechnologyRobert N. GoldbergNational Institute of Standards and Technology, retired Daniel Zwillinger Mathematics Department Rensselaer Polytechnic InstituteFOREwORD BY ThE PuBLIShERPublishing the 90th edition of this landmark reference is a true milestone in the history of CRC Press. Since its first publication in 1913 – as a 116-page pocket-sized book priced at $2 – the CRC Handbook of Chemistry and Physics has developed into a 2800 page tome that no longer fits anyone’s pocket but still finds a place on every scientist’s bookshelf.Certainly, the tremendous advances in science and technology over the past 96 years have fuelled the increase in the Handbook’s contents, but the immense task of data selection, compilation, and organization has been expertly performed by a succession of Editors, Advisory Board members, and Contributors. These people have played a significant role in shaping the Handbook that we see today, and it is to them that I wish to pay tribute in this Foreword. Covering such large subject areas, the Editors have always relied on a team of subject experts from around the world to contrib-ute articles and tables. A cursory glance over the names credited through the years provides an interesting historical roll call of re-nowned chemists and physicists who have given their time and scientific expertise to the Handbook. These contributors include leaders such as Nobel Laureate Glenn T. Seaborg, space science pioneer James Van Allen, and C. S. “Speed” Marvel, considered the father of synthetic polymer chemistry.Originally conceived by the Ohio-based Chemical Rubber Company as an incentive to encourage sales of their laboratory supplies, the Handbook started life as a small booklet of useful mathematical formulae and laboratory data. By 1913, it had grown to 116 pages and was published in its own right as the Handbook of Chemistry and Physics. The Editor was William R. Veazey, an Associate Professor of Chemistry at the (then) Case School of Applied Science. Who could have predicted that this pocket book was to become so well known that its users came to refer to it as the ‘Rubber Bible’ or, simply, the ‘CRC’? To paraphrase a review of the 88th edition –“if you can’t find a copy in your lab, that’s because someone in the next lab has stolen it.”Veazey’s successor was Charles D. Hodgman, his Assistant Editor for the first edition and an Associate Professor of Physics at Case. Hodgman went on to hold the position of Editor from 1915 to 1963, overseeing 42 editions of the Handbook. Under his Editorship the Handbook grew to over 3000 pages and the cov-erage expanded to include x-ray crystallography, nuclear physics, synthetic polymers, and other fields that did not exist when his first edition appeared.Following Hodgman’s retirement Robert Weast took over the Editorship and published the 45th edition in 1964. Noticeably big-ger with an 8” by 10” page size, the Handbook continued to expand in both scope and magnitude over the next few years. In 1972, The Chemical Rubber Company first published it under the CRC Press imprint, and in the late 1970’s sold off its laboratory supply busi-ness, moved to new headquarters in Florida, and began building its book publishing business.David R. Lide became the Handbook’s fourth Editor in 1989, and took the opportunity to radically overhaul the organization and content to reflect the needs of the modern user. He added, merged, and deleted tables, and during the period of his editorship, up-dated 100 percent of the content. Staying within the confines of a single volume has always meant difficult decisions on which tables to include – often at the expense of others –but with the advent of electronic media, the Handbook is now available electronically and space constraints are less of a problem. Modern production techniques and the move to a larger page size have given the cur-rent Handbook a cleaner and more user-friendly look. Publication of the 90th edition marks David Lide’s final edition as Editor-in-Chief, and the publisher wishes to take this opportunity to thank him for his tremendous expertise and enthusiasm that has helped make the Handbook so indispensable to today’s scientists. Starting with the 91st edition, the Handbook editorship transfers to W.M. “Mickey” Haynes, Editor-in-Chief of the International Journal of Thermophysics, Scientist Emeritus at the National Institute of Standards and Technology (NIST), and former Chief of the NIST Physical and Chemical Properties Division. We look for-ward to a new era in the Handbook’s long and illustrious history.Fiona MacdonaldPublisher, CRC PressBoca Raton, FloridaMarch 2009PREFACE TO 90th EDITIONThe 90th Edition of the CRC Handbook of Chemistry and Physics marks a milestone for this reference work, which first appeared in 1913. For almost a century the Handbook has been updated annually, except for a few wartime years, and has served several generations of R&D professionals, engineers, and students. Its aim has always been to provide broad coverage of all types of physical science data commonly encountered by scientists and engineers, with as much depth as can be accommodated in a one-volume format. The data contained in the Handbook have been carefully selected by experts in each field; quality control is a high priority and the sources are documented. The annual updates make it possible to add new and improved data in a timely fashion, and references to more detailed data sources have helped to establish the Handbook as the first place to look for physical and chemical data.This edition also marks the retirement of the current Editor-in-Chief after 20 years in that post. The reception to the changes I have made in the book is very gratifying, and I greatly appreciate the suggestions that have come from the Editorial Board, the contributors, and many users. The new Editor will be W. M. “Mickey” Haynes, who has had long experience in providing physical and chemical data through the National Institute of Standards and Technology and through his service as Editor of the International Journal of Thermophysics. I am confident that he will continue the tradition of excellence the Handbook has achieved.Many new tables and updates are included in the 90th Edition, especially in the following areas:Fluid properties (Sec. 6) - new data over a wider temperature and pressure range for -Water (including D2O) and steam-Air-Refrigerants and other important industrial fluidsBiochemistry (Sec. 7) – new tables on- Enzyme catalyzed reactions- Structure and functions of common drugs- Chemical constituents of human bloodAnalytical chemistry (Sec. 8) – new and expanded tables on-Proton NMR shifts for solvents and other fluids-Mass spectral peaks-Nuclear moments and other data for NMR spectroscopy-Aqueous solubility of organic compoundsAstronomy and geophysics (Sec. 14) – new data on-Properties of the planets and their satellites-Major world earthquakes, 850 AD to 2008-Interstellar moleculesOther new and expanded tables-International recommendations for the expression of uncertainty of measurements-Description of the new IUPAC chemical identifier (InChI)-Nobel prize winners in physics and chemistry-Threshold limits for airborne contaminantsIn addition to offering the full text of the print edition in searchable pdf format, this CD-ROM Version 2010 presents the major tables of numerical data in the form of interactive tables that can be sorted, filtered, and combined in various ways. Substances in these tables can be retrieved bysearching on name, formula, CAS Registry Number, or chemical structure, and such a search can be combined with a request for a desired property. Thus one can request a specific property of a specific substance (for example, viscosity of benzene) and receive a customized table with exactly that information. In addition, the CD-ROM version includes a section with pdf files of many older tables that have been removed from the print edition to make space for new material.Suggestions on new topics for the Handbook and notification of errors are always appreciated. Input from users plays a key role in keeping the book up to date. Address all comments to Editor-in-Chief, CRC Handbook of Chemistry and Physics, Taylor & Francis Group, 6000 Broken Sound Parkway NW, Suite 300, Boca Raton, FL 33487.The Handbook of Chemistry and Physics is dependent on the efforts of many contributors throughout the world. The list of current contributors follows this Preface. The assistance and support of Dr. Fiona Macdonald, Chemical and Biological Sciences Publisher for CRC Press/Taylor & Francis Books, is greatly appreciated. Finally, I want to thank Mimi Williams, Pam Morrell, Glen Butler, James Yanchak, and Theresa Delforn for their outstanding work in production of the book and the software team at Hampden Data Services for producing the CD-ROM version.David R. Lide2009 AprilThe 90th Edition of the Handbook of Chemistry and Physics is dedicated to my wife,Bettijoyce Breen Lide, and to the members of my familyDavid Alston Lide, Vanessa Lide Whitcomb and David Whitcomb, James Lide and Deborah Horowitz, Quentin Lide and Suzanne Romero, Neil and Lizzie Molino, and Van Molinoand to my grandchildrenDavid A. Lide, Jr., Mary Lide, Grace Lide, David A. Whitcomb, Kate Whitcomb, andZoë LideHow To Cite this ReferenceThe recommended form of citation is: David R. Lide, ed., CRC Handbook of Chemistry and Physics, 90th Edition (CD-ROM Version 2010), CRC Press/Taylor and Francis, Boca Raton, FL. If a specific table is cited, use the format: "Physical Constants of Organic Compounds", in CRC Handbook of Chemistry and Physics, 90th Edition (CD-ROM Version 2010), David R. Lide, ed., CRC Press/Taylor and Francis, Boca Raton, FL.This work contains information obtained from authentic and highly regarded sources. Reprinted material is quoted with permission, and sources are indicated. A wide variety of references are listed. Best efforts have been made to select and verify the data on the basis of sound scientific judgment, but the author and the publisher cannot accept responsibility for the validity of all materials or for the consequences of their use.© Copyright Taylor and Francis Group LLC 2010CuRREnT COnTRIBuTORSRobert A. AlbertyDepartment of Chemistry Massachusetts Institute of Technology Cambridge, Massachusetts 02139Lev I. BergerCalifornia Institute of Electronics and Materials Science2115 Flame Tree WayHemet, California 92545A. K. CovingtonDepartment of Chemistry University of NewcastleNewcastle upon Tyne NE1 7RU EnglandJ. R. FuhrAtomic Physics DivisionNational Institute of Standards and TechnologyGaithersburg, Maryland 20899J. GmehlingUniversität OldenburgFakultät V, Technische ChemieD-26111 Oldenburg, Germany Robert N. Goldberg Biotechnology DivisionNational Institute of Standards and TechnologyGaithersburg, Maryland 20899Allan H. Harvey Thermophysical Properties Division National Institute of Standards and TechnologyBoulder, Colorado 80305Steven R. HellerChemical and Biochemical Reference Data DivisionNational Institute of Standards and TechnologyGaithersburg, Maryland 20899 Norman E. HoldenNational Nuclear Data Center Brookhaven National Laboratory Upton, New York 11973Henry V. Kehiaian7, Allee de la Caravelle94430 Chennevieres sur Marne FranceCarolyn A. KohCenter for Hydrate Research Colorado School of Mines1600 Illinois St.Golden, Colorado 80401Willem H. KoppenolDept CHABLab. f. Anorg. Chemie, HCI H211Wolfgang-Pauli-Strasse 10ETH HönggerbergCH-8093 Zürich, SwitzerlandEric W. LemmonThermophysical Properties DivisionNational Institute of Standards andTechnologyBoulder, Colorado 80305Frank J. Lovas8616 Melwood Rd.Bethesda, Maryland 20817Yu-Ran LuoSchool of Chemistry and Material ScienceUniversity of Science and Technology ofChinaHefei 230026, ChinaWilliam C. MartinAtomic Physics DivisionNational Institute of Standards andTechnologyGaithersburg, Maryland 20899Alan D. McNaught8 Cavendish AvenueCambridge CB1 7USEnglandThomas M. MillerAir Force Research Laboratory/VSBP29 Randolph Rd.Hanscom AFB, Massachusetts 01731-3010N. Moazzen-AhmadiDepartment of Physics and AstronomyUniversity of Calgary2500 University Drive NWCalgary, Alberta T2N 1N4, CanadaPeter J. MohrPhysics LaboratoryNational Institute of Standards andTechnologyGaithersburg, Maryland 20899I. OzierDepartment of Physics and AstronomyUniversity of British Columbia6224 Agricultural RoadVancouver, British Columbia V6T 1Z1,CanadaCedric J. PowellSurface and Microanalysis ScienceDivisionNational Institute of Standards andTechnologyGaithersburg, Maryland 20899Joseph ReaderAtomic Physics DivisionNational Institute of Standards andTechnologyGaithersburg, Maryland 20899E. Dendy SloanCenter for Hydrate ResearchColorado School of Mines1600 Illinois St.Golden, Colorado 80401Lewis E. SnyderAstronomy DepartmentUniversity of IllinoisUrbana, Illinois 61801Barry N. TaylorPhysics LaboratoryNational Institute of Standards andTechnologyGaithersburg, Maryland 20899Petr VanýsekDepartment of ChemistryNorthern Illinois UniversityDeKalb, Illinois 60115Wolfgang L. WieseAtomic Physics DivisionNational Institute of Standards andTechnologyGaithersburg, Maryland 20899Christian WohlfarthMartin Luther UniversityInstitute of Physical ChemistryMühlpforte 106108 Halle (Saale), GermanyDaniel ZwillingerMathematics DepartmentRensselaer Polytechnic InstituteTroy, New York 12180Piotr ZylaParticle Data GroupLawrence Berkeley LaboratoryBerkeley, California 94720。
ACD LABS Fate and Purge Impurity Tracking and Carr

ACD/LABS [ADVANCED CHEMISTRY DEVELOPMENT, INC.]Tracking Fate and Purge ofImpurities and CalculatingCarryoverJoe DiMartinoJesse HarrisSanjivanjit K. BahlIntroduction to Fate and Purge and CarryoverThe purpose of process development in pharmaceutical research is to select and optimize a synthetic route to produce the active pharmaceutical ingredient (API) by the safest, cheapest, fastest, and cleanest pathway. This method should also follow both Good Laboratory Practice (GLP) and Quality by Design (QbD) principles. As with any synthetic process, impurities are generated. Regulatory authorities such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) require that impurities are tracked and identified above a certain threshold. Genotoxic and mutagenic impurities must be reported at any level (as stated in the ICH Q7 guideline1).Route scouting data in process development is typically stored in electronic notebooks. Associated analytical information may be accessible as PDF images stored within an experiment record. Unfortunately, analytical data is not dynamically linked with the process route's individual stage(s). It is unsearchable and inaccessible.Effective API development data management and impurity tracking are necessary to develop an optimal control strategy. To successfully track the fate and purge of impurities, many scientists gather LC/MS and LC area percent values for impurity entities using Excel® spreadsheets. While spreadsheets are adequate for handlingand managing numerical data, they are a weak tool for relating chemical structures with the analytical spectra and chromatograms used to identify and characterize them. For example, Excel cannot map chemical routes, search for compounds based on molecular structure, or process analytical data.2Here, we discuss Luminata®—software designed to help project teams map synthetic routes, track impurities, and access analytical data for process development in a systematized and searchable manner. Luminata enables effective inter- and intra-departmental collaboration and automatically calculates carryover values directly from LC/MS and LC data. In this document, we describe two workflows that are often tedious and time-consuming without Luminata—process optimization and carryover calculations.Convenient Management of Process RoutesLuminata facilitates the import of the whole process route associated with a given dataset, including each synthetic stage. The resulting process map enables clear visualization of the impurities at each route phase and a straightforward comparison of molecular composition across reaction steps.Beyond incorporating good manufacturing practice (GMP) into drug substance production, Luminata also allows users to evaluate in-process samples, filtrates, or other entities to assist with synthesis optimization. Figure 1 illustrates an example route optimization of sulfabenzamide, where in-process samples from the reaction, filtrate liquors from product isolation, and the final isolated product are documented.Figure 1. Optimized synthesis of Sulfabenzamide (green, Stage 2) mapped in Luminata. All steps in the reaction are tracked with starting materials (blue), intermediates and products (green), and stage-specific impurities indicated (orange).Sulfabenzamide is an antibacterial substance that is synthesized through a two-step reaction. Within Luminata, this two-step reaction can be documented with all the stages involved. In Stage 1, the process chemist activates the carboxylic acid with carbonyldiimidazole (CDI) to form the imidazolide.3 The chemist then checks how far the activation has progressed toward completion from Stage 1 via a quench conversion to the methyl ester (Figure 1, Stage 1b—Activation). The next substrate is added (Figure 1, Stage 1c), and reaction completion is tested (Figure 1, Stage Reaction Complete). At this point, all known or unknown impurities within the reaction can be separated. Finally, the analyst proceeds through process work-up (Figure 1, Stage 2—Filtrate) and then purification of the compound (Figure 1, Stage 2—Isolated).For all these individual stages, corresponding HPLC data can be associated with each step. Thus, the process map is a powerful tool for comparing stages, denoting the proportion of each impurity rejected at each stage. The software helps conveniently record and share information about the removal and carryover of impuritiesthroughout the process.Each set of reactions also forms an interactive record. Within a record, analysts can examine the impacts of different conditions, such as temperature or solvents, on process optimization. For example, the analyst can assess whether altering a given reaction will generate more impurities at any/each stage. Most process chemists currently use an electronic notebook (ELN) to store this chemical and analytical information, where a massive amount of valuable data is hidden in largely unsearchable PDF documents.Calculating CarryoverIn addition to storing development information in one place, Luminata can link chemical information about impurity fate with all the relevant analytical data. This enables dynamic calculation of carryover. Once the connection of impurities between each stage has been defined by the user (by creating arrows to indicate a conversion or carryover), the corresponding carryover value is automatically calculated from the associated LC/MS data, as illustrated in Figure 2.Figure 2. Creating an arrow indicating conversion of an impurity in the Luminata process map leads to automatic population of the corresponding detection limit (DL) and quantitation limit (QL) in the impurity carryover table.In addition to calculating the carryover at each stage, Luminata also automatically calculates the cumulative carryover value for the entire reaction (Figure 3).Figure 3. As the reaction pathway is defined in the Process Map in Luminata, the Carryover of Impurities Table populates dynamically where ‘DL’ and ‘QL’ represent detection limit and quantitation limit respectively.Carryover is calculated using ‘Area %’ values for two consecutive stages:Carryover=(Area%stage(x)Area%stage(x−1)) x 100%Cumulative carryover is calculated using the carryover calculated for each individual step in the route, for example:Cumulative Carryover=(Carryover stage1→2100)(Carryover stage2→3100)(Carryover stage3→4100) x 100Detection and quantitation limits (DL and QL, respectively) can be edited at each stage. The software relies on user-defined DL and QL values to calculate carryover. Values falling below these limits are denoted with a ‘<’ to indicate the imprecise nature of the calculated result—a practice widely used in industry.4In addition to calculating cumulative carryover amounts for the fates of each impurity, the software enables the comparison of different batches within a complete record set. One use for this functionality is “spike and purge” experiments, where an impurity is spiked into test batches in varying amounts (i.e., 1%, 2%, 3%, 4%, or 5%) to determine if it is purged at the same final stage. Luminata allows users to compare all these different spiked records and create one cumulative carryover table (Figure 4).Figure 4. Cumulative carryover table of two records with differing spiked impurityamounts in Luminata.Carryover calculations for other impurities within the same record can also be determined by selecting the impurity of interest (Figure 5).Figure 5. Selection of an impurity in Luminata allows the carryover value to be calculated automatically from the associated LC/MS data in the Carryover of Impurities table.ConclusionLuminata supports effective workflow optimization for process chemists. This enables informed decision-making by automatically calculating quantitative carryover values for process-related impurities using associated analytical data.References1.ICH, Q7 Good Manufacturing Practice Guide for Active PharmaceuticalIngredients (2016). Link2.Moser, A., Waked, A.E., DiMartino, J. (2021). Consolidating and ManagingData for Drug Development within a Pharmaceutical Laboratory: Comparingthe Mapping and Reporting Tools from Software Applications. OPRD, 25(10),2177-2187. Link3.Montalbetti, C.A.G.N.; Falque, V. (2005). Amide bond formation andpeptide coupling.Tetrahedron, 61, 10827- 10852. Link4.Armbruster, D.A.; Pry, T. (2008). Limit of Blank, Limit of Detection andLimit of Quantitation.Clin. Biochem. Rev., 29(Suppl 1), S49–S52. Link。
【医疗药品管理】美国FDA原料药生产质量管理规范(中英文)

DIRECTION OF GMP (GOOD MANUFACTURING PRACTICE )OFRAW MATERIALS BY FDA美国FDA原料药生产质量管理规范(中英文)Table of Contents 目录1. INTRODUCTION 简介1.1 Objective 目的1.2 Regulatory Applicability法规的适用性1.3 Scope 范围2. QUALITY MANAGEMENT .质量管理2.1 Principles 总则2.2 Responsibilities of the Quality Unit(s) 质量部门的责任2.3 Responsibility for Production Activities 生产作业的职责2.4 Internal Audits (Self Inspection) 内部审计(自检)2.5 Product Quality Review 产品质量审核3. PERSONNEL 人员3.1 Personnel Qualifications 人员的资质3.2 Personnel Hygiene 人员卫生3.3 Consultants 顾问4. BUILDINGS AND FACILITIES 建筑和设施4.1 Design and Construction 设计和结构4.2 Utilities 公用设施4.3 Water 水4.4 Containment 限制4.5 Lighting 照明4.6 Sewage and Refuse 排污和垃圾4.7 Sanitation and Maintenance 卫生和保养5. PROCESS EQUIPMENT 工艺设备5.1 Design and Construction 设计和结构5.2 Equipment Maintenance and Cleaning 设备保养和清洁5.3 Calibration. 校验5.4 Computerized Systems 计算机控制系统6. DOCUMENTATION AND RECORDS 文件和记录6.1 Documentation System and Specifications 文件系统和质量标准6.2 Equipment cleaning and Use Record 设备的清洁和使用记录6.3 Records of Raw Materials, Intermediates, API Labeling and Packaging Materials 原料、中间体、原料药的标签和包装材料的记录6.4 Master Production Instructions (Master Production and Control Records)生产工艺规程(主生产和控制记录)6.5 Batch Production Records (Batch Production and Control Records)批生产记录(批生产和控制记录)6.6 Laboratory Control Records 实验室控制记录6.7 Batch Production Record Review 批生产记录审核7. MATERIALS MANAGEMENT 物料管理7.1 General Controls 控制通则7.2 Receipt and Quarantine 接收和待验7.3 Sampling and Testing of Incoming Production Materials 进厂物料的取样与测试7.4 Storage 储存7.5 Re-evaluation 复验8. PRODUCTION AND IN-PROCESS CONTROLS 生产和过程控制8.1 Production Operations 生产操作8.2 Time Limits 时限8.3 In-process Sampling and Controls 工序取样和控制8.4 Blending Batches of Intermediates or APIs 中间体或原料药的混批8.5 Contamination Control 污染控制9. PACKAGING AND IDENTIFICATION LABELING OF APIs AND INTERMEDIATES原料药和中间体的包装和贴签9.1 General 总则9.2 Packaging Materials 包装材料9.3 Label Issuance and Control 标签发放与控制9.4 Packaging and Labeling Operations 包装和贴签操作10. STORAGE AND DISTRIBUTION.储存和分发10.1 Warehousing Procedures 入库程序10.2 Distribution Procedures 分发程序11. LABORATORY CONTROLS 实验室控制11.1 General Controls 控制通则11.2 Testing of Intermediates and APIs 中间体和原料药的测试11.3 Validation of Analytical Procedures 分析方法的验证11.4 Certificates of Analysis分析报告单11.5 Stability Monitoring of APIs 原料药的稳定性监测11.6 Expiry and Retest Dating 有效期和复验期11.7 Reserve/Retention Samples 留样12. VALIDATION .验证12.1 Validation Policy 验证方针12.2 Validation Documentation 验证文件12.3 Qualification 确认12.4 Approaches to Process Validation 工艺验证的方法12.5 Process Validation Program 工艺验证的程序12.6 Periodic Review of Validated Systems 验证系统的定期审核12.7 Cleaning Validation 清洗验证12.8 Validation of Analytical Methods 分析方法的验证13. CHANGE CONTROL 变更的控制14. REJECTION AND RE-USE OF MATERIALS.拒收和物料的再利用14.1 Rejection 拒收14.2 Reprocessing 返工14.3 Reworking 重新加工14.4 Recovery of Materials and Solvents 物料与溶剂的回收14.5 Returns 退货15. COMPLAINTS AND RECALLS 投诉与召回16. CONTRACT MANUFACTURERS (INCLUDING LABORATORIES)协议生产商(包括实验室)17. AGENTS, BROKERS, TRADERS, DISTRIBUTORS, REPACKERS, AND RELABELLERS 代理商、经纪人、贸易商、经销商、重新包装者和重新贴签者17.1 Applicability 适用性17.2 Traceability of Distributed APIs and Intermediates已分发的原料药和中间体的可追溯性17.3 Quality Management 质量管理17.4 Repackaging, Relabeling, and Holding of APIs and Intermediates原料药和中间体的重新包装、重新贴签和待检17.5 Stability 稳定性17.6 Transfer of Information 信息的传达17.7 Handling of Complaints and Recalls 投诉和召回的处理17.8 Handling of Returns 退货的处理18. Specific Guidance for APIs Manufactured by Cell Culture/Fermentation用细胞繁殖/发酵生产的原料药的特殊指南18.1 General 总则18.2 Cell Bank Maintenance and Record Keeping 细胞库的维护和记录的保存18.3 Cell Culture/Fermentation 细胞繁殖/发酵18.4 Harvesting, Isolation and Purification 收取、分离和精制18.5 Viral Removal/Inactivation steps 病毒的去除/灭活步骤19. APIs for Use in Clinical Trials 用于临床研究的原料药19.1 General 总则19.2 Quality 质量19.3 Equipment and Facilities设备和设施19.4 Control of Raw Materials 原料的控制19.5 Production 生产19.6 Validation 验证19.7 Changes 变更19.8 Laboratory Controls 实验室控制19.9 Documentation 文件20. Glossary 术语1. INTRODUCTION 1. 简介1.1 Objective 1.1目的This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the quality and purity characteristics that they purport, or are represented, to possess.本文件旨在为在合适的质量管理体系下制造活性药用成分(以下称原料药)提供有关优良药品生产管理规范(GMP)提供指南。
CGMP文件 化学药品和溶剂的接收,保管和处理的标准操作程序SOP
1.0目的Purpose:制定QC用于分析的化学药品和溶剂的接收,保管和处理程序。
Lay down a procedure for receipt, handling and maintenance of chemicals and solvents used for analysis in Quality Control department.2.0范围 Scope:本程序适用于QC化学药品和溶剂的接收,保管和处理,同时也适用于实验室工作的安全。
This procedure is applicable for receipt, maintenance and handling of all chemicals andsolvents used in Quality Control department, and also applies to the safety in laboratoryduring working.3.0定义 Definitions: 无 Nil4.0责任 Responsibility:所有QC人员有责任按照本程序的规定操作。
All Quality Control personnel are responsible for performing the activity as per the procedure.5.0程序 Procedure:5.1通则 General Instructions:5.1.1所有的化学药品只能从经授权的生产商和经销商那获得。
All chemicals should be procured only from authorized manufacturer and dealer.5.1.2在需要的时候,要使用合适的安全防护用具,比如:面具,手套,安全护目镜,安全鞋。
Use appropriate safety appliances like mask, hand gloves, safety goggles andsafety shoes wherever necessary.5.1.3在处理易燃性的溶剂时,要确保旁边有灭火器。
盐酸多西环素ChP2015标准检测的紧邻主成分后杂质的制备及鉴定

·6 9 5·
盐酸多西环素 ChP 2015 标准检测的紧邻主成分后杂质的制备及鉴定
杨帆 1,张娅 2,3,张锦琳 2,杨志明 4,殷学智 4,赵述强 5,袁耀佐 2 *,张玫 2
(1. 南京理工大学医院,南京 210094;2. 江苏省食品药品监督检验研究院,南京 210008;3. 中国药科大学药物化学教研室,南京 210009;4. 常州制药厂有限公司,常州 213018;5. 中国医药城公共平台服务中心,泰州 225300)
* 通信作者 Tel:(025)86631609;E-mail:yyzyz7256@ 第一作者 Tel:(025)84315786;E-mail:79214129@
Chinese Journal of Pharmaceutical Analysis
摘要 目的:制备盐酸多西环素中国药典 2015 年版(ChP 2015)标准检测的紧邻主峰后杂质,并鉴定其结 构。方法:以盐酸多西环素成盐用母液为对象,采用室温条件下半制备液相色谱分离纯化杂质;色谱条件: 采用 Synergi C18(50 mm×250 mm,10 μm)色谱柱,以 0.1% 三氟乙酸水溶液(A)-0.1% 三氟乙酸乙腈溶液 (B)为流动相,线性梯度洗脱(A-B):0.0 min(85∶15)→ 40.0 min(60∶40)→ 40.5 min(85∶15)→50.0 min (85∶15),流速为 80 mL·min-1;254 nm 检测收集目标组分,旋转蒸发去除有机溶剂,冷冻干燥制得目标杂 质;采用盐酸多西环素 ChP 2015 标准中有关物质检查的高效液相色谱法确证制得物质为目标杂质并采用 归一化方法测定其纯度;采用光谱综合分析等手段确证其结构。结果:制得目标杂质纯度为 98.7%,结构确 证为 2- 乙酰 -2- 脱氨甲酰多西环素(即 EP 8.0 中的盐酸多西环素杂质 F),在有关物质测定用供试品溶液 中添加该杂质后,多西环素后相邻峰面积显著增加。结论:盐酸多西环素 ChP 2015 标准检测的紧邻主峰后 杂质经制备色谱分离并成功鉴定,该结论被 ChP 2015 采用。 关键词:四环素类抗生素;多西环素;杂质 F;乙酰 - 脱氨甲酰多西环素;杂质制备;结构确证
The Barton reaction using a microreactor and black light. Continuous-flow synthesis of a key steroid
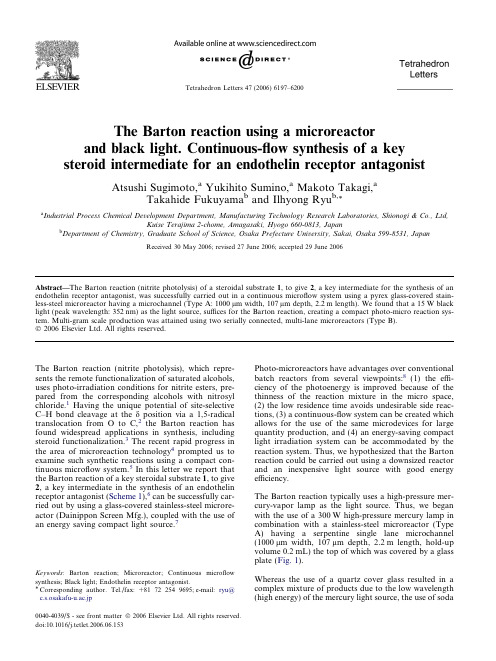
The Barton reaction using a microreactorand black light.Continuous-flow synthesis of a key steroid intermediate for an endothelin receptor antagonistAtsushi Sugimoto,a Yukihito Sumino,a Makoto Takagi,aTakahide Fukuyama b and Ilhyong Ryu b,*aIndustrial Process Chemical Development Department,Manufacturing Technology Research Laboratories,Shionogi &Co.,Ltd,Kuise Terajima 2-chome,Amagasaki,Hyogo 660-0813,JapanbDepartment of Chemistry,Graduate School of Science,Osaka Prefecture University,Sakai,Osaka 599-8531,JapanReceived 30May 2006;revised 27June 2006;accepted 29June 2006Abstract—The Barton reaction (nitrite photolysis)of a steroidal substrate 1,to give 2,a key intermediate for the synthesis of an endothelin receptor antagonist,was successfully carried out in a continuous microflow system using a pyrex glass-covered stain-less-steel microreactor having a microchannel (Type A:1000l m width,107l m depth,2.2m length).We found that a 15W black light (peak wavelength:352nm)as the light source,suffices for the Barton reaction,creating a compact photo-micro reaction sys-tem.Multi-gram scale production was attained using two serially connected,multi-lane microreactors (Type B).Ó2006Elsevier Ltd.All rights reserved.The Barton reaction (nitrite photolysis),which repre-sents the remote functionalization of saturated alcohols,uses photo-irradiation conditions for nitrite esters,pre-pared from the corresponding alcohols with nitrosyl chloride.1Having the unique potential of site-selective C–H bond cleavage at the d position via a 1,5-radical translocation from O to C,2the Barton reaction has found widespread applications in synthesis,including steroid functionalization.3The recent rapid progress in the area of microreaction technology 4prompted us to examine such synthetic reactions using a compact con-tinuous microflow system.5In this letter we report that the Barton reaction of a key steroidal substrate 1,to give 2,a key intermediate in the synthesis of an endothelin receptor antagonist (Scheme 1),6can be successfully car-ried out by using a glass-covered stainless-steel microre-actor (Dainippon Screen Mfg.),coupled with the use of an energy saving compact light source.7Photo-microreactors have advantages over conventional batch reactors from several viewpoints:8(1)the effi-ciency of the photoenergy is improved because of the thinness of the reaction mixture in the micro space,(2)the low residence time avoids undesirable side reac-tions,(3)a continuous-flow system can be created which allows for the use of the same microdevices for large quantity production,and (4)an energy-saving compact light irradiation system can be accommodated by the reaction system.Thus,we hypothesized that the Barton reaction could be carried out using a downsized reactor and an inexpensive light source with good energy efficiency.The Barton reaction typically uses a high-pressure mer-cury-vapor lamp as the light source.Thus,we began with the use of a 300W high-pressure mercury lamp in combination with a stainless-steel microreactor (Type A)having a serpentine single lane microchannel (1000l m width,107l m depth,2.2m length,hold-up volume 0.2mL)the top of which was covered by a glass plate (Fig.1).Whereas the use of a quartz cover glass resulted in a complex mixture of products due to the low wavelength (high energy)of the mercury light source,the use of soda0040-4039/$-see front matter Ó2006Elsevier Ltd.All rights reserved.doi:10.1016/j.tetlet.2006.06.153Keywords :Barton reaction;Microreactor;Continuous microflow synthesis;Black light;Endothelin receptor antagonist.*Corresponding author.Tel./fax:+81722549695;e-mail:ryu@c.s.osakafu-u.ac.jpTetrahedron Letters 47(2006)6197–6200lime glass as a top cover gave good yields of the rear-ranged ing the microreactor with a soda lime glass top cover,we examined the optimal distance between the microreactor and the lamp,while the resi-dence time wasfixed at6min(Fig.2).When the reac-tion was carried out using a distance of7.5cm from the reactor,the oxime2was obtained in59%yield. The yield of oxime2became low at distances greater than7.5cm,but a closer distance,such as5cm,gave an inferior yield of2(33%)presumably due to the excess thermal energy evolving from the light source.In a sep-arate experiment,we confirmed a tendency for tempera-tures greater than50°C to cause extensive degradation of the product,and indeed a closer distance,such as 5cm,caused heat evolution.A high-pressure mercury-vapor lamp(300W)radiates short wavelength light that is not necessary for this reac-tion,and which can cause power loss of the light and the undesirable evolution of heat.It occurred to us that a black light,which has a maxim peak wavelength at 352nm,might be suitable for the Barton reaction.The results are summarized in Table1.In the case of a black light,the Pyrex top glass gave better results than soda lime glass(entries3and4).Probably the use of Pyrex glass has the advantage of better transparency at the wavelength used over soda lime glass,since the shorter wavelengths produced by a black light are weak.Since the power of the black light(15W)is considerably weaker than that of mercury lamp(300W),we adjusted the residence time so as to compensate for this defi-ciency.Gratifyingly,we found that the extension of the residence time to12min resulted in a71%HPLC yield of the desired oxime(entry5).This is worthy of note,since the energy efficiency of the black light is 10times superior to that of the mercury lamp based on the calculated values of yields per Watt hour(entries 2,4,and5).Figure1.Photos of two microreactors(Type A and Type B)used for this study.Type A(channel size:1000l m width,107l m depth,2.2m length,hold-up volume0.2mL).Type B(channel size:1000l m width, 500l m depth,0.5m length,16lanes,hold-up volume4mL).6198 A.Sugimoto et al./Tetrahedron Letters47(2006)6197–6200Although toluene and acetone are good solvents for the Barton reaction,steroidal substrate 1has limited solu-bility in these solvents,which does not permit high throughput production.To investigate this further,we screened solvents and,as a result,found that the solubil-ity of 1in DMF is nearly four times higher than that in acetone.Thus,using a 36mM DMF solution of 1,we carried out a continuous microflow reaction using two serially connected microreactors (Type B)(1000l m width,500l m depth,1m total length,16lanes,total hold-up volume 8mL)and eight 20W black light lamps.As a result,after continuous operation for 20h,we ob-tained 3.1g of the desired product 2(60%isolated yield)(Scheme 2).9In summary,we demonstrated herein that the Barton reaction (nitrite photolysis)of a steroidal substrate 1can be successfully carried out using a stainless-steel microreactor covered by Pyrex glass,a low power blacklight as the light source,and DMF as the solvent.Thus,a gram scale synthesis of oxime product 2was attained in a continuous-flow reaction.AcknowledgmentsThe microreactors were offered by Dainippon Screen Mfg.Co.,Ltd.We thank Hitoshi Haibara and Akiko Murata for helpful discussion and for help with photo-irradiation experiments.T.F.thanks NEDO for finan-cial support.References and notes1.(a)Barton,D.H.R.Pure Appl.Chem.1968,16,1;(b)Studer,A.Chem.Eur.J.2001,7,1159;(c)Grossi,L.Chem.Eur.J.2005,11,5419.2.Majetich,G.;Wheless,K.Tetrahedron 1995,51,7095.Table 1.Energy efficiency of the microflow Barton reaction a21h νpyridine (0.2equiv)Entry Light source/reactor top Flow rate (mL/h)Residence time (min)Yield of 2b (%)W h Yield/W h 1300W Hg lamp/pyrex glass 2.0621300.702300W Hg lamp/lime soda glass 2.065630 1.89315W black light/lime soda glass 2.0615 1.510.3415W black light/pyrex glass 2.0629 1.519.3515W black light/pyrex glass1.01271323.7aMicroreactor Type A,[1]:9mM in acetone,pyridine 0.2equiv.Distance between the lamp and the microreactor surface:7.5cm (Hg),3.0cm (black light).bHPLC yield.A.Sugimoto et al./Tetrahedron Letters 47(2006)6197–620061993.Suginome,H.In CRC Handbook of Organic Photochemis-try and Photobiology,2nd ed.;Horspool,W.M.,Lenci,F., Eds.;CRC Press:Boca Raton,2004;p102-1.4.(a)Ehrfeld,W.;Hessel,V.;Lo¨we,H.Microreactors:NewTechnology for Modern Chemistry;Wiley-VCH:Weinheim, 2000;(b)Ja¨hnisch,K.;Hessel,V.;Lo¨we,H.;Baerns,M.Angew.Chem.,Int.Ed.2004,43,406;(c)Pennemann,H.;Watts,P.;Haswell,S.J.;Hessel,V.;Lo¨we,.Process Res.Dev.2004,8,422;Also see a review on continuousflow reactions:(d)Jas,G.;Kirshning,A.Chem.Eur.J.2003,9, 5708.5.For our recent work on catalytic reactions using microre-actors,see:(a)Fukuyama,T.;Shinmen,M.;Nishitani,S.;Sato,M.;Ryu,.Lett.2002,4,1691;(b)Liu,S.;Fukuyama,T.;Sato,M.;Ryu,.Process Res.Dev.2004,8,477;(c)Rahman,M.T.;Fukuyama,T.;Kamata, N.;Sato,M.;Ryu,mun.2006,2236.6.Konoike,T.;Takahashi,K.;Araki,Y.;Horibe,.Chem.1997,62,960.7.For[2+2]cycloaddition using a photo-microreactor,see:Fukuyama,T.;Hino,Y.;Kamata,N.;Ryu,I.Chem.Lett.2004,33,1430.8.For photoreactions using microreactors,see:(a)Lu,H.;Schmidt,M.A.;Jensen,b Chip2001,1,22;(b) Ueno,K.;Kitagawa,F.;Kitamura,b Chip2002,2, 231;(c)Ehrich,H.;Linke,D.;Morgenschweis,K.;Baerns, M.;Ja¨hnisch,L.Chimia2002,56,647;(d)Wootton,R.C.R.;Fortt,R.;de Mello,.Process Res.Dev.2002, 6,187;(e)Maeda,H.;Mukae,H.;Mizuno,K.Chem.Lett.2005,34,66;(f)Matsushita,Y.;Kumada,S.;Wakaba-yashi,K.;Sakeda,K.;Ichimura,T.Chem.Lett.2006,35, 410.9.The continuous-flow reaction was performed by irradi-ating a solution of nitrite1(5.4g,10.8mmol)in DMF (300mL)containing a small amount of pyridine(0.2mol equiv of1)with two microreactors(Type B)and eight20W black lights through a Pyrex glass cover (flow rate:15mL/h,resident time:32min,reaction time: 20h).Water(600mL)was added to the photoreaction mixture and the resulting slurry was collected by filtration and washed with water(100mL)to give a white solid.The solid was purified by silica gel column chromatography to give oxime2in60%isolated yield(3.1g).6200 A.Sugimoto et al./Tetrahedron Letters47(2006)6197–6200。
对羧基苯甲醛_对甲基苯甲酸_苯甲酸_对苯二_省略_间苯二甲酸在N_N_二甲基甲酰
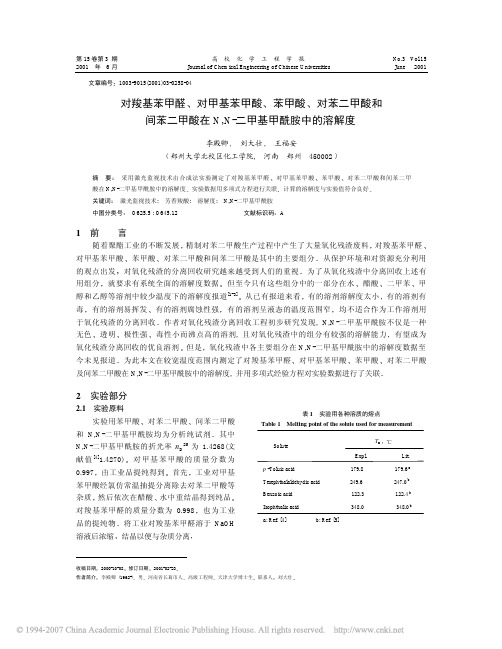
T, K
313.45 316.55 319.35 322.55 327.55 330.85 334.05 336.35 338.95
295.65 298.75 303.05 307.55 311.05 315.55 320.45
294.75 298.75 301.05 302.15 306.35 310.05 312.15 316.55 319.35 323.35 325.65 329.05
第 15 卷第 3 期 2001 年 6 月
高校化学工程学报 Journal of Chemical Engineering of Chinese Universities
文章编号 1003-9015(2001)03-0258-04
No.3 Vol.15 June 2001
对羧基苯甲醛 对甲基苯甲酸 苯甲酸 对苯二甲酸和 间苯二甲酸在 N,N-二甲基甲酰胺中的溶解度
李殿卿 刘大壮 王福安 郑州大学北校区化工学院, 河南 郑州 450002
摘 要: 采用激光监视技术由合成法实验测定了对羧基苯甲醛 对甲基苯甲酸 苯甲酸 对苯二甲酸和间苯二甲
核磁常见溶剂峰

NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities Hugo E.Gottlieb,*Vadim Kotlyar,andAbraham Nudelman*Department of Chemistry,Bar-Ilan University,Ramat-Gan52900,IsraelReceived June27,1997In the course of the routine use of NMR as an aid for organic chemistry,a day-to-day problem is the identifica-tion of signals deriving from common contaminants (water,solvents,stabilizers,oils)in less-than-analyti-cally-pure samples.This data may be available in the literature,but the time involved in searching for it may be considerable.Another issue is the concentration dependence of chemical shifts(especially1H);results obtained two or three decades ago usually refer to much more concentrated samples,and run at lower magnetic fields,than today’s practice.We therefore decided to collect1H and13C chemical shifts of what are,in our experience,the most popular “extra peaks”in a variety of commonly used NMR solvents,in the hope that this will be of assistance to the practicing chemist.Experimental SectionNMR spectra were taken in a Bruker DPX-300instrument (300.1and75.5MHz for1H and13C,respectively).Unless otherwise indicated,all were run at room temperature(24(1°C).For the experiments in the last section of this paper,probe temperatures were measured with a calibrated Eurotherm840/T digital thermometer,connected to a thermocouple which was introduced into an NMR tube filled with mineral oil to ap-proximately the same level as a typical sample.At each temperature,the D2O samples were left to equilibrate for at least 10min before the data were collected.In order to avoid having to obtain hundreds of spectra,we prepared seven stock solutions containing approximately equal amounts of several of our entries,chosen in such a way as to prevent intermolecular interactions and possible ambiguities in assignment.Solution1:acetone,tert-butyl methyl ether,di-methylformamide,ethanol,toluene.Solution2:benzene,di-methyl sulfoxide,ethyl acetate,methanol.Solution3:acetic acid,chloroform,diethyl ether,2-propanol,tetrahydrofuran. Solution4:acetonitrile,dichloromethane,dioxane,n-hexane, HMPA.Solution5:1,2-dichloroethane,ethyl methyl ketone, n-pentane,pyridine.Solution6:tert-butyl alcohol,BHT,cyclo-hexane,1,2-dimethoxyethane,nitromethane,silicone grease, triethylamine.Solution7:diglyme,dimethylacetamide,ethyl-ene glycol,“grease”(engine oil).For D2O.Solution1:acetone, tert-butyl methyl ether,dimethylformamide,ethanol,2-propanol. Solution2:dimethyl sulfoxide,ethyl acetate,ethylene glycol, methanol.Solution3:acetonitrile,diglyme,dioxane,HMPA, pyridine.Solution4:1,2-dimethoxyethane,dimethylacetamide, ethyl methyl ketone,triethylamine.Solution5:acetic acid,tert-butyl alcohol,diethyl ether,tetrahydrofuran.In D2O and CD3OD nitromethane was run separately,as the protons exchanged with deuterium in presence of triethylamine.ResultsProton Spectra(Table1).A sample of0.6mL of the solvent,containing1µL of TMS,1was first run on its own.From this spectrum we determined the chemical shifts of the solvent residual peak2and the water peak. It should be noted that the latter is quite temperature-dependent(vide infra).Also,any potential hydrogen-bond acceptor will tend to shift the water signal down-field;this is particularly true for nonpolar solvents.In contrast,in e.g.DMSO the water is already strongly hydrogen-bonded to the solvent,and solutes have only a negligible effect on its chemical shift.This is also true for D2O;the chemical shift of the residual HDO is very temperature-dependent(vide infra)but,maybe counter-intuitively,remarkably solute(and pH)independent. We then added3µL of one of our stock solutions to the NMR tube.The chemical shifts were read and are presented in Table 1.Except where indicated,the coupling constants,and therefore the peak shapes,are essentially solvent-independent and are presented only once.For D2O as a solvent,the accepted reference peak(δ)0)is the methyl signal of the sodium salt of3-(trimeth-ylsilyl)propanesulfonic acid;one crystal of this was added to each NMR tube.This material has several disadvan-tages,however:it is not volatile,so it cannot be readily eliminated if the sample has to be recovered.In addition, unless one purchases it in the relatively expensive deuterated form,it adds three more signals to the spectrum(methylenes1,2,and3appear at2.91,1.76, and0.63ppm,respectively).We suggest that the re-sidual HDO peak be used as a secondary reference;we find that if the effects of temperature are taken into account(vide infra),this is very reproducible.For D2O, we used a different set of stock solutions,since many of the less polar substrates are not significantly water-soluble(see Table1).We also ran sodium acetate and sodium formate(chemical shifts: 1.90and8.44ppm, respectively).Carbon Spectra(Table2).To each tube,50µL of the stock solution and3µL of TMS1were added.The solvent chemical shifts3were obtained from the spectra containing the solutes,and the ranges of chemical shifts(1)For recommendations on the publication of NMR data,see: IUPAC Commission on Molecular Structure and Spectroscopy.Pure Appl.Chem.1972,29,627;1976,45,217.(2)I.e.,the signal of the proton for the isotopomer with one less deuterium than the perdeuterated material,e.g.,C H Cl3in CDCl3or C6D5H in C6D6.Except for CHCl3,the splitting due to J HD is typically observed(to a good approximation,it is1/6.5of the value of the corresponding J HH).For CHD2groups(deuterated acetone,DMSO, acetonitrile),this signal is a1:2:3:2:1quintet with a splitting of ca.2 Hz.(3)In contrast to what was said in note2,in the13C spectra the solvent signal is due to the perdeuterated isotopomer,and the one-bond couplings to deuterium are always observable(ca.20-30Hz). Figure1.Chemical shift of H DO as a function of tempera-ture..Chem.1997,62,7512-7515S0022-3263(97)01176-6CCC:$14.00©1997American Chemical Societyshow their degree of variability.Occasionally,in order to distinguish between peaks whose assignment was ambiguous,a further1-2µL of a specific substrate were added and the spectra run again.Table1.1H NMR Dataproton mult CDCl3(CD3)2CO(CD3)2SO C6D6CD3CN CD3OD D2O solvent residual peak7.26 2.05 2.507.16 1.94 3.31 4.79 H2O s 1.56 2.84a 3.33a0.40 2.13 4.87acetic acid CH3s 2.10 1.96 1.91 1.55 1.96 1.99 2.08 acetone CH3s 2.17 2.09 2.09 1.55 2.08 2.15 2.22 acetonitrile CH3s 2.10 2.05 2.07 1.55 1.96 2.03 2.06 benzene CH s7.367.367.377.157.377.33tert-butyl alcohol CH3s 1.28 1.18 1.11 1.05 1.16 1.40 1.24 OH c s 4.19 1.55 2.18tert-butyl methyl ether CCH3s 1.19 1.13 1.11 1.07 1.14 1.15 1.21 OCH3s 3.22 3.13 3.08 3.04 3.13 3.20 3.22 BHT b ArH s 6.98 6.96 6.877.05 6.97 6.92OH c s 5.01 6.65 4.79 5.20ArCH3s 2.27 2.22 2.18 2.24 2.22 2.21ArC(CH3)3s 1.43 1.41 1.36 1.38 1.39 1.40chloroform CH s7.268.028.32 6.157.587.90 cyclohexane CH2s 1.43 1.43 1.40 1.40 1.44 1.451,2-dichloroethane CH2s 3.73 3.87 3.90 2.90 3.81 3.78 dichloromethane CH2s 5.30 5.63 5.76 4.27 5.44 5.49diethyl ether CH3t,7 1.21 1.11 1.09 1.11 1.12 1.18 1.17 CH2q,7 3.48 3.41 3.38 3.26 3.42 3.49 3.56 diglyme CH2m 3.65 3.56 3.51 3.46 3.53 3.61 3.67 CH2m 3.57 3.47 3.38 3.34 3.45 3.58 3.61OCH3s 3.39 3.28 3.24 3.11 3.29 3.35 3.37 1,2-dimethoxyethane CH3s 3.40 3.28 3.24 3.12 3.28 3.35 3.37 CH2s 3.55 3.46 3.43 3.33 3.45 3.52 3.60 dimethylacetamide CH3CO s 2.09 1.97 1.96 1.60 1.97 2.07 2.08 NCH3s 3.02 3.00 2.94 2.57 2.96 3.31 3.06NCH3s 2.94 2.83 2.78 2.05 2.83 2.92 2.90 dimethylformamide CH s8.027.967.957.637.927.977.92 CH3s 2.96 2.94 2.89 2.36 2.89 2.99 3.01CH3s 2.88 2.78 2.73 1.86 2.77 2.86 2.85 dimethyl sulfoxide CH3s 2.62 2.52 2.54 1.68 2.50 2.65 2.71 dioxane CH2s 3.71 3.59 3.57 3.35 3.60 3.66 3.75 ethanol CH3t,7 1.25 1.12 1.060.96 1.12 1.19 1.17 CH2q,7d 3.72 3.57 3.44 3.34 3.54 3.60 3.65OH s c,d 1.32 3.39 4.63 2.47ethyl acetate CH3CO s 2.05 1.97 1.99 1.65 1.97 2.01 2.07C H2CH3q,7 4.12 4.05 4.03 3.89 4.06 4.09 4.14CH2C H3t,7 1.26 1.20 1.170.92 1.20 1.24 1.24 ethyl methyl ketone CH3CO s 2.14 2.07 2.07 1.58 2.06 2.12 2.19C H2CH3q,7 2.46 2.45 2.43 1.81 2.43 2.50 3.18CH2C H3t,7 1.060.960.910.850.96 1.01 1.26 ethylene glycol CH s e 3.76 3.28 3.34 3.41 3.51 3.59 3.65“grease”f CH3m0.860.870.920.860.88CH2br s 1.26 1.29 1.36 1.27 1.29n-hexane CH3t0.880.880.860.890.890.90CH2m 1.26 1.28 1.25 1.24 1.28 1.29HMPA g CH3d,9.5 2.65 2.59 2.53 2.40 2.57 2.64 2.61 methanol CH3s h 3.49 3.31 3.16 3.07 3.28 3.34 3.34 OH s c,h 1.09 3.12 4.01 2.16nitromethane CH3s 4.33 4.43 4.42 2.94 4.31 4.34 4.40 n-pentane CH3t,70.880.880.860.870.890.90CH2m 1.27 1.27 1.27 1.23 1.29 1.292-propanol CH3d,6 1.22 1.10 1.040.95 1.09 1.50 1.17 CH sep,6 4.04 3.90 3.78 3.67 3.87 3.92 4.02 pyridine CH(2)m8.628.588.588.538.578.538.52 CH(3)m7.297.357.39 6.667.337.447.45CH(4)m7.687.767.79 6.987.737.857.87 silicone grease i CH3s0.070.130.290.080.10 tetrahydrofuran CH2m 1.85 1.79 1.76 1.40 1.80 1.87 1.88 CH2O m 3.76 3.63 3.60 3.57 3.64 3.71 3.74 toluene CH3s 2.36 2.32 2.30 2.11 2.33 2.32CH(o/p)m7.177.1-7.27.187.027.1-7.37.16CH(m)m7.257.1-7.27.257.137.1-7.37.16 triethylamine CH3t,7 1.030.960.930.960.96 1.050.99 CH2q,7 2.53 2.45 2.43 2.40 2.45 2.58 2.57a In these solvents the intermolecular rate of exchange is slow enough that a peak due to HDO is usually also observed;it appears at2.81and3.30ppm in acetone and DMSO,respectively.In the former solvent,it is often seen as a1:1:1triplet,with2J H,D)1Hz. b2,6-Dimethyl-4-tert-butylphenol.c The signals from exchangeable protons were not always identified.d In some cases(see note a),the coupling interaction between the CH2and the OH protons may be observed(J)5Hz).e In CD3CN,the OH proton was seen as a multiplet atδ2.69,and extra coupling was also apparent on the methylene peak.f Long-chain,linear aliphatic hydrocarbons.Their solubility in DMSO was too low to give visible peaks.g Hexamethylphosphoramide.h In some cases(see notes a,d),the coupling interaction between the CH3and the OH protons may be observed(J)5.5Hz).i Poly(dimethylsiloxane).Its solubility in DMSO was too low to give visible peaks.Notes .Chem.,Vol.62,No.21,19977513.Chem.,Vol.62,No.21,1997NotesTable2.13C NMR Data aCDCl3(CD3)2CO(CD3)2SO C6D6CD3CN CD3OD D2O solvent signals77.16(0.0629.84(0.0139.52(0.06128.06(0.02 1.32(0.0249.00(0.01206.26(0.13118.26(0.02acetic acid CO175.99172.31171.93175.82173.21175.11177.21 CH320.8120.5120.9520.3720.7320.5621.03 acetone CO207.07205.87206.31204.43207.43209.67215.94 CH330.9230.6030.5630.1430.9130.6730.89 acetonitrile CN116.43117.60117.91116.02118.26118.06119.68 CH3 1.89 1.12 1.030.20 1.790.85 1.47 benzene CH128.37129.15128.30128.62129.32129.34tert-butyl alcohol C69.1568.1366.8868.1968.7469.4070.36 CH331.2530.7230.3830.4730.6830.9130.29 tert-butyl methyl ether OCH349.4549.3548.7049.1949.5249.6649.37 C72.8772.8172.0472.4073.1774.3275.62C C H326.9927.2426.7927.0927.2827.2226.60 BHT C(1)151.55152.51151.47152.05152.42152.85C(2)135.87138.19139.12136.08138.13139.09CH(3)125.55129.05127.97128.52129.61129.49C(4)128.27126.03124.85125.83126.38126.11CH3Ar21.2021.3120.9721.4021.2321.38C H3C30.3331.6131.2531.3431.5031.15C34.2535.0034.3334.3535.0535.36chloroform CH77.3679.1979.1677.7979.1779.44cyclohexane CH226.9427.5126.3327.2327.6327.961,2-dichloroethane CH243.5045.2545.0243.5945.5445.11 dichloromethane CH253.5254.9554.8453.4655.3254.78diethyl ether CH315.2015.7815.1215.4615.6315.4614.77 CH265.9166.1262.0565.9466.3266.8866.42 diglyme CH359.0158.7757.9858.6658.9059.0658.67 CH270.5171.0369.5470.8770.9971.3370.05CH271.9072.6371.2572.3572.6372.9271.63 1,2-dimethoxyethane CH359.0858.4558.0158.6858.8959.0658.67 CH271.8472.4717.0772.2172.4772.7271.49 dimethylacetamide CH321.5321.5121.2921.1621.7621.3221.09 CO171.07170.61169.54169.95171.31173.32174.57NCH335.2834.8937.3834.6735.1735.5035.03NCH338.1337.9234.4237.0338.2638.4338.76 dimethylformamide CH162.62162.79162.29162.13163.31164.73165.53 CH336.5036.1535.7335.2536.5736.8937.54CH331.4531.0330.7330.7231.3231.6132.03 dimethyl sulfoxide CH340.7641.2340.4540.0341.3140.4539.39 dioxane CH267.1467.6066.3667.1667.7268.1167.19 ethanol CH318.4118.8918.5118.7218.8018.4017.47 CH258.2857.7256.0757.8657.9658.2658.05 ethyl acetate C H3CO21.0420.8320.6820.5621.1620.8821.15 CO171.36170.96170.31170.44171.68172.89175.26CH260.4960.5659.7460.2160.9861.5062.32CH314.1914.5014.4014.1914.5414.4913.92 ethyl methyl ketone C H3CO29.4929.3029.2628.5629.6029.3929.49 CO209.56208.30208.72206.55209.88212.16218.43C H2CH336.8936.7535.8336.3637.0937.3437.27CH2C H37.868.037.617.918.148.097.87 ethylene glycol CH263.7964.2662.7664.3464.2264.3063.17“grease”CH229.7630.7329.2030.2130.8631.29n-hexane CH314.1414.3413.8814.3214.4314.45CH2(2)22.7023.2822.0523.0423.4023.68CH2(3)31.6432.3030.9531.9632.3632.73HMPA b CH336.8737.0436.4236.8837.1037.0036.46 methanol CH350.4149.7748.5949.9749.9049.8649.50c nitromethane CH362.5063.2163.2861.1663.6663.0863.22 n-pentane CH314.0814.2913.2814.2514.3714.39CH2(2)22.3822.9821.7022.7223.0823.38CH2(3)34.1634.8333.4834.4534.8935.302-propanol CH325.1425.6725.4325.1825.5525.2724.38 CH64.5063.8564.9264.2364.3064.7164.88 pyridine CH(2)149.90150.67149.58150.27150.76150.07149.18 CH(3)123.75124.57123.84123.58127.76125.53125.12CH(4)135.96136.56136.05135.28136.89138.35138.27 silicone grease CH3 1.04 1.40 1.38 2.10 tetrahydrofuran CH225.6226.1525.1425.7226.2726.4825.67 CH2O67.9768.0767.0367.8068.3368.8368.68 toluene CH321.4621.4620.9921.1021.5021.50C(i)137.89138.48137.35137.91138.90138.85CH(o)129.07129.76128.88129.33129.94129.91CH(m)128.26129.03128.18128.56129.23129.20CH(p)125.33126.12125.29125.68126.28126.29triethylamine CH311.6112.4911.7412.3512.3811.099.07 CH246.2547.0745.7446.7747.1046.9647.19a See footnotes for Table1.b2J PC)3Hz.c Reference material;see text.For D2O solutions there is no accepted reference for carbon chemical shifts.We suggest the addition of a drop of methanol,and the position of its signal to be defined as49.50ppm;on this basis,the entries in Table2were recorded.The chemical shifts thus obtained are,on the whole,very similar to those for the other solvents. Alternatively,we suggest the use of dioxane when the methanol peak is expected to fall in a crowded area of the spectrum.We also report the chemical shifts of sodium formate(171.67ppm),sodium acetate(182.02and 23.97ppm),sodium carbonate(168.88ppm),sodium bicarbonate(161.08ppm),and sodium3-(trimethylsilyl)-propanesulfonate[54.90,19.66,15.56(methylenes1,2, and3,respectively),and-2.04ppm(methyls)],in D2O. Temperature Dependence of HDO Chemical Shifts.We recorded the1H spectrum of a sample of D2O, containing a crystal of sodium3-(trimethylsilyl)propane-sulfonate as reference,as a function of temperature.The data are shown in Figure1.The solid line connecting the experimental points corresponds to the equation which reproduces the measured values to better than1 ppb.For the0-50o C range,the simplergives values correct to10ppb.For both equations,T is the temperature in°C.Acknowledgment.Generous support for this work by the Minerva Foundation and the Otto Mayerhoff Center for the Study of Drug-Receptor Interactions at Bar-Ilan University is gratefully acknowledged.JO971176Vδ)5.060-0.0122T+(2.11×10-5)T2(1)δ)5.051-0.0111T(2)Notes .Chem.,Vol.62,No.21,19977515。
防腐蚀涂料(Anticorrosive coating)

防腐蚀涂料(Anticorrosive coating)防腐蚀涂料(Anticorrosive coating)This paper consists of 316935929 contributionsPdf documents may experience poor browsing on the WAP side. It is recommended that you first select TXT, or download the source file to the local view.-.Page.20 / 46220.72) P0I2, one, 0815 (80.9; ICH1109B)Sperm CsttogrsascaAcid) 3% (two - 9 equivalent) of the acid component, 00 (alcohol, heat, flexibility, and electrical insulation). Coated conductors (coated with an amide insulating coating) and coated with an enamelled wire with the composition. For example, useTwo a two amino benzene methane benzene two formic acid two methyl glycol phenyl anhydride of three B a partial (ethyl) isocyanurate copolymer composition coated copper wire, bake 2 hydroxyl ISO roast, the breakdown voltage of enameled wire in the 70g load is l.k, 017VThe conductive coating contains a silicone base material and a conductive polymer, and is prepared in the organic (- ethyl) - 2 hydroxy urea ester. The composition has a good dispersion in solvent resistance. The workpiece has a conductive (or antistatic) coating on the base material (e.g., plastics, rubber, glass, metal, ceramics, and paper). For example, WD12N- 060 (ethyl silicate solution) methanol mixture, a composition, with its 08 water and coated with H1W (E) K3FPT film, drying, the conductive coating mist.% 03, 11 x 1 "E surface resistance, pencil hardness 2. L0Q /.HM (conductive polymer polyaniline doped with methanol dispersion) N - one of BK45 polymers containing long chain amino acid polyester amide salt containing 4.T and TLY0 (and), 4The softening resistance is 45o. 2C21019 inorganic high temperature antistatic coating: Cl1705 [0070N065, 0AChina's patent for invention patent / China: North coatings industry research and Design Institute (Wang 21014) an insulating coating for overhead high voltage wires and its preparation 0071Zhiqiang etc.) 21..0 111 (91;.0003.20902948020.13) a6ICC9l0P0D / 0 method: C1189 [country to apply for a patent for invention: China: N07148A PLA University of Science and Technology Engineering / China Force Engineering Institute (Harvest etc.) 21. gold.0 0O.1.011911 (000.8:ICC9I7I72, 210010.21.12) P0D / 80221010 preparation of solvent-free long lasting antistaticcoating: C110071N070lA China to apply for a patent for invention: North China: 6L / 3 coatings industry research and Design Institute (Xi fachen etc.) 21..0 01l1..0003.209029461, one, 6(1) I01 / 2910PCD600.3; c923;Anticorrosive coating21011 rare earth modified barium zirconate titanate nano powder as anti corrosion 21015 conductive filler and aqueous coating of metal and plastic adhesion 00710071Electrostatic coating and its preparation method: C1185lA @ China invention special agent and its preparation: W20 48, N0711Es009l490 international patent application date: Japan / apply for public benefit. China: Harbin Institute of Technology (21.7.00. / Hao Sue) 021.211176232.40) ICC916 / 2000. (010.1:P0D30030)DCCroainSiaaiJn) 20.20.5Ioprto (hrkm, u, etc..091. one, 8 pages. One, 3, one.)J20 / 65820.52) ICC9110P0812 (80.6; P0D / 635)The finishing agent containing a () A core-shell type resin particle, with each particle (A) shell (by carboxyl orcarboxylic acid ester; / T and 2000 N0 ~ 550m? Mlgo / K alicyclic structure prepared polyurethane) (nuclear layer (and) containing basic nitrogen atoms theElectrical insulating coating21012 polyamide polyimide coating and insulated wire: 0071In the case of ethyl acetate, 07:0 olefin polymer is better than AA / 1 ~ 03 ~B (9) and with epoxy group (Group); alcohol / water dry our silicon O (B) with dry mouth) or silicon B}A compound of alkyl groups. A water-based coating containing Z6400 and a core / shell treeJ2111l Japan Patent: Japan: Smtmlcrc fat particles (by butyl acrylate, P0O3 / uiooEeti containing 0 methyl two dimethylamino ethyl methacrylate and methylWnen.ohd, Kno.21.21.1.20 / methyl acrylate was prepared by the 14 nuclear and two methanol cyclohexane, adipic acid, jtcIc (siaeg) 0O0.2 1Y - 08, 130020..8ICC9I907 (0802) P0D / 8977 (A) of the title coatings containing (0O ~ O1B) diisocyanate and.3.3 containing benzene partial equivalentFor example: 1 = 13, 44 D three benzene anhydride copolymer coating. 1, MI bias22 dimethylol propionic acid, neopentyl glycol, ethylenehydrogenation of MI prepared by D and a nuclear shell, 27, light transmittance of 9% TFH / 3:7 shell solution containing the particles, 34)Good in character, solvent resistance and hot water resistance.Three acid anhydride and 12 -, 4 - amino acids,The equivalence ratio of AB is > 1, and the resultant film has good adhesion to metal and plastic, and has the advantages of corrosion resistance, heat resistance, and / orThe insulated wires covered with copper wires have good adhesion, flexibility, heat resistance and impact resistance.21013 dielectric resin composition and enameled wire: J20 - 80071P092685: Japan open Japanese Patent: Htcihmclo, t.ao2 / iaheia.Ld (T, CCS21016 iron base anticorrosive coating water diluted resin composition: 0071J21 758: P038, Japan Japan Patent O / CioaCeiahydhmclC.t.ad, Ai) 21.21.2 page.20 / 0o, Ld (eakoM.0O0.8 - 0 - 8205820.80 05 (80) P2F / ICC3I041;(Sih) 20.21.8, page.20178120.52) EII one, 91, one, C., one, 0819 (080.7:3)ICC87 / 8P0L90The resin composition containing (A = 1) diluted water (B), more than 1 kinds of coupling agents,(C = 1) from aqueous wax, resin modifier, curing agent, waterborne emulsion,In the composition, a long chain amino amide salt of 00 to 1.10 portions of polymeric acid polyester is added to a 10 polyester and 0 imide resins (1, 5) (from 6% to 5Compositions of levelling agents, heterocyclic compounds, organic acids, lanolin derivatives, rosin derivatives, sulfonates and oils, and (D) equilibrium water. For example, the JSG34I11CotaasnS...SThe SC board is soaked in ammonia containing poly ~ (uefe7) three ethanol titanate (PCSprlxl0, IAfter soaking, anticorrosive coating has good adhesion. 21010 has good adhesion, abrasion resistance, alkali resistance, salt resistance and aging 0072 fat rot coating compositions containing phthalate coupling agent of K3R99.72 page (090.0ICC9130 34220.81) P0D / 83Coupling agent) composition of glycidyl methacrylate (and resin modifier)3, and 10C drying 1, the test film thickness of 1 ~ 50sOC0Sm, salt spray test has good corrosion resistance. 21017 anticorrosive coating composition with good ethanol resistance and 0071Corrosion protection coating structure: W21 - 138 international patent application, m0002021..5 one, 6 pages,.J20 / 17420.82) IC002.202, one P0825 (080.1:P2)C9] 700D6 / 6The coating composition comprises acrylic resin 1% to 4%72, and water-based alkyd resin02% to 1, aluminium tripolyphosphate I2%%.5%? 5 ~ 2, zinc phosphate 12%%? ~ 2, 5 ~ 7, 5 iron oxide red iron oxide yellow pigment 1%4 5 ~ 7, 0 ~ 1%,%Ben: S0aHqD] mrC.Ld (ioht, ihai. resin, l%3, fluorocarbon resin 1.5 ~ 1%hwihoye).. T.nsiaMcik) K 1 ~ 1%02%, titanate coupling agent 2matter.sliceNoPolyester composition, the"One..."...OneComposition containing unsaturated polyester () "(" containing 7oIl%:b by B (010) ~ fIf%0 / hAnd () 5%0%c to the main chain and C B containing 0~10 with O side chains of ethylene glycol of multielement 1And water 5%-70%21011 preparation of low shrinkage polyethylene vinyl ester heavy duty anticorrosive coating 0072Composition of alcohols] B and (polymerizable unsaturated monomers). A paint containing peroxide method: CII701A Chinese Chinese. To apply for a patent for invention: North N65 O1 / RF10 and compound, a composition containing 4%5ms (C6 ~ n5%5s ~ party coatings industry research and Design Institute (Li Qingcai) 21.63.01000.0 2090One.A polyester obtained from glycol, 15 amyl glycol and maleic anhydride, 1 of which is formedFor OI%.2, thickness varies to 22. .240820.13) ICC913i012 (091.0:P0D / 96).21012 synthesis of modified waterborne epoxy resin and its anticorrosion coating 0072Aqueous solution and method of 21018 metal surface anticorrosive coating with the European patent application: P0071E21141 / CeiceWreKuh8, in 5 De Dehmsheklte058620..1:ICC91037 (0800): P0D / 08OneChina: Harbin Institute of Technology (Huang Yudong)21.71.2100.000.4 01 P0F8 6. (0. / 1295821.32);ICC82313006021013 two-component epoxy phenolic resin with rust primer and preparation process thereof: 0072 (Guo Fang Zhu) 21? 63? 20802014i20 22).000 (081? 0 - 13 - 4 - 7;Environmental safety anticorrosive coatings for metals, 5I content, C] 160 [5N070i9A] [Chinese patent applications] [Guo Fangzhu, China]Surface modified silica, organic solvents and other additives. IA, a kind of > NJPH is 45. containing 2gL / surface modified silica sol and 02gHr. .Children F and water ICC9111ZP0D6 / 4The composition is coated with a pre treated aluminum plate, a steel plate, and a galvanized sheet surface.:Oh, oh, oh, oh, ohSexual rot... 06 anti C10N11710I.....21015 organic acid resistant coatings developed L / 0072 J Guo Jinyan, paintThe combination of the coating J2100 patent this Ai two ~: 0O76 P3 / Ah SVKsihmclCroainGt, Tii, etc.) 0..AeCeiasoprto (ooej.210040.12 - L pages, 2O / 33. 8O? 7Icc9l?. One 0B27742. (9L) P0D, 82The composition contains I0 partial vinylidene chloride copolymer emulsion (8%0 from 0 to9%5.%% vinyl vinylidene chloride and / or vinyl chloride, 05 ~ 5 with carboxylIndustry.One21, 4 () 1, 30005.3 - 3M 'n3%i% to l other vinyl monomer emulsion polymerization) 00 ~ 321016 two-component waterborne acrylic primer design, M / 5, and Chen.50072 JournalAlkane coupling agent (in latex solids). For example, the pigment dispersion and C China, industrial silicon coatedmaterial ".One2) 00, 4 () 9205. - 6, one, 5Acid butyl acrylate vinyl vinylidene chloride copolymer latex helium (H 4P, by the regulation of ammonia. (05), with latex solids) B03 chloro%KH73 (- propyl trimethoxy silane) sodium nitrite and film-forming agent, a mixed coating obtained. 21017 a polyether polyurethane marine heavy anticorrosive coating and its preparation method: 0072 C1162 N070I3A China to apply for a patent for invention: / Chinese.With the coating of polished steel, drying, and then coated with Plto (Li Yue) 2l..0 ~ 158 (081.6:oylnF LiYue.0003.2O0l90820.12) 68380 acrylic latex base) 3 (acrylic finish), to test piece, get ICC9150P0D187 in water,CoatngsiS...S21018, an underwater painting heavy-duty coating and its preparation method: 0072C1180 [N07155A China patent for invention] in China / ShenyangThe journal / fouling paint Li Shucai and "paint industry.21, 4 (521017)] - 0005.1I containing base polymer and antifouling coatings containing the polymer group 0073 complexes: W21 - 8l4 international patent application, English: LAN.Az00O14 / Ko bearingN B LC I G N E N T O ..我需要它我N E O T、N、Si RW O T R I N (N I是LBV RA D E F科腐蚀控制工程技术中心等(今人等)2 1. 7王。
沃士达罗利克高功率厚膜片电阻器说明书

CRCW06032K20FKEAHPCRCW-HP e3Vishay DraloricPulse Proof, High Power Thick Film Chip ResistorsLINKS TO ADDITIONAL RESOURCESThe pulse proof, high power thick film chip resistors series is the perfect choice for most fields of power measurement electronics where reliability, stability, high power rating and excellent pulse load performance are of major concern. Typical applications include battery management systems in automotive appliances.FEATURES•Excellent pulse load capability •Enhanced power rating•Double side printed resistor element •AEC-Q200 qualified•Material categorization: for definitions of compliance please see /doc?99912APPLICATIONS•Automotive •Industrial •Commercial •High powerNotes(1)Please refer to APPLICATION INFORMATION below(2)CRCW0402-HP resistors feature a single side printed resistive layer only, except jumpers (3)Specified power rating requires a thermal resistance of R th≤ 110 K/W APPLICATION INFORMATIONWhen the resistor dissipates power, a temperature rise above the ambient temperature occurs, dependent on the thermal resistance of the assembled resistor together with the printed circuit board. The rated dissipation applies only if the permitted film temperature is not exceeded.These resistors do not feature a limited lifetime when operated within the permissible limits. However, resistance value drift increasing over operating time may result in exceeding a limit acceptable to the specific application, thereby establishing a functional lifetime.3D 3D3D ModelsTECHNICAL SPECIFICATIONSDESCRIPTIONCRCW0402-HP e3CRCW0603-HP e3CRCW0805-HP e3CRCW1206-HP e3CRCW1210-HP e3CRCW1218-HP e3CRCW2010-HP e3CRCW2512-HP e3Imperial size 04020603080512061210121820102512Metric size code RR1005M RR1608M RR2012MRR3216M RR3225M RR3246M RR5025M RR6332M Resistance range 1 Ω to 1 M Ω; jumper (0 Ω)Resistance tolerance ± 5 %; ± 1 %; ± 0.5 %Temperature coefficient ± 200 ppm/K; ± 100 ppm/K Rated dissipation, P 70 (1)0.2 W (2)0.33 W 0.5 W 0.75 W (3)0.75 W 1.5 W 1.0 W 1.5 W Operating voltage, U max. AC RMS /DC 50 V 75 V 150 V 200 V 200 V 200 V 400 V 500 V Permissible film temperature, ϑF max. (1)155 °C Operating temperature range-55 °C to +155 °C Max. resistance change at P 70 for resistance range, |ΔR /R | after:1000 h ≤ 2.0 %8000 h ≤ 4.0 %Permissible voltage against ambient (insulation):1 min, U ins 75 V 100 V 200 V 300 V 300 V 300 V 300 V 300 VFailure rate: FIT observed ≤ 0.1 x 10-9/hCRCW-HP e3Vishay DraloricNote•The temperature coefficient of resistance (TCR) is not specified for 0 Ω jumpersTEMPERATURE COEFFICIENT AND RESISTANCE RANGETYPE / SIZE TCR TOLERANCERESISTANCE E-SERIES CRCW0402-HP e3± 200 ppm/K ± 5 % 1 Ω to 1 M ΩE24± 100 ppm/K ± 1 % 1 Ω to 1 M ΩE24; E96± 0.5 %Jumper, I max. = 3 A ≤ 10 m Ω0 Ω-CRCW0603-HP e3± 200 ppm/K± 5 % 1 Ω to 1 M ΩE24± 100 ppm/K ± 1 % 1 Ω to 1 M ΩE24; E96± 0.5 %Jumper, I max. = 5 A ≤ 8 m Ω0 Ω-CRCW0805-HP e3± 200 ppm/K± 5 % 1 Ω to 1 M ΩE24± 100 ppm/K ± 1 % 1 Ω to 1 M ΩE24; E96± 0.5 %Jumper, I max. = 6 A ≤ 5 m Ω0 Ω-CRCW1206-HP e3± 200 ppm/K± 5 % 1 Ω to 1 M ΩE24± 100 ppm/K ± 1 % 1 Ω to 1 M ΩE24; E96± 0.5 %Jumper, I max. = 10 A≤ 5 m Ω0 Ω-CRCW1210-HP e3± 200 ppm/K± 5 % 1 Ω to 1 M ΩE24± 100 ppm/K ± 1 % 1 Ω to 1 M ΩE24; E96± 0.5 %Jumper, I max. = 12 A≤ 4 m Ω0 Ω-CRCW1218-HP e3± 200 ppm/K± 5 % 1 Ω to 1 M ΩE24± 100 ppm/K ± 1 % 1 Ω to 1 M ΩE24; E96± 0.5 %Jumper, I max. = 20 A≤ 4 m Ω0 Ω-CRCW2010-HP e3± 200 ppm/K± 5 % 1 Ω to 1 M ΩE24± 100 ppm/K ± 1 % 1 Ω to 1 M ΩE24; E96± 0.5 %Jumper, I max. = 12 A≤ 5 m Ω0 Ω-CRCW2512-HP e3± 200 ppm/K± 5 % 1 Ω to 1 M ΩE24± 100 ppm/K ± 1 % 1 Ω to 1 M ΩE24; E96± 0.5 %Jumper, I max. = 16 A≤ 5 m Ω0 Ω-PACKAGINGTYPE / SIZE CODE QUANTITY PACKAGING STYLEWIDTHPITCH PACKAGING DIMENSIONS CRCW0402-HP e3ED = ET710 000Paper tape acc. to IEC 60286-3, type 1a8 mm2 mmØ 180 mm / 7"EE = EF450 000Ø 330 mm / 13"CRCW0603-HP e3EI = ET25000 2 mmØ 180 mm / 7"ED = ET310 000Ø 180 mm / 7"EL = ET420 000Ø 285 mm / 11.25"EE = ET820 000Ø 330 mm / 13"EA = ET15000 4 mmØ 180 mm / 7"EB = ET510 000Ø 285 mm / 11.25"EC = ET620 000Ø 330 mm / 13"CRCW0805-HP e3EA = ET15000 4 mmØ 180 mm / 7"EB = ET510 000Ø 285 mm / 11.25"EC = ET620 000Ø 330 mm / 13"CRCW1206-HP e3EA = ET15000 4 mmØ 180 mm / 7"EB = ET510 000Ø 285 mm / 11.25"EC = ET620 000Ø 330 mm / 13"CRCW1210-HP e3EA = ET15000 4 mm Ø 180 mm / 7"EB = ET510 000Ø 285 mm / 11.25"EC = ET620 000Ø 330 mm / 13"CRCW1218-HP e3EK = ET94000Blister tape acc. to IEC 60286-3, type 2a12 mm4 mm Ø 180 mm / 7"CRCW2010-HP e3EF = E024000 4 mm Ø 180 mm / 7"EJ = E0816 000 4 mm Ø 330 mm / 13"CRCW2512-HP e3EG = E6720008 mm Ø 180 mm / 7"EH = E8240004 mmCRCW-HP e3 Vishay DraloricPART NUMBER AND PRODUCT DESCRIPTIONPart Number: CRCW0603562RFKEAHPPart Number: CRCW06030000Z0EAHPTYPE / SIZE VALUE TOLERANCE TCR PACKAGING SPECIALCRCW0402 CRCW0603 CRCW0805 CRCW1206 CRCW1210 CRCW1218 CRCW2010 CRCW2512R = decimalK = thousandM = million0000 = jumperD = ± 0.5 %F = ± 1 %J = ± 5 %Z = jumperK = ± 100 ppm/KN = ± 200 ppm/K0 = jumperEAEBECEDEEEFEGEIEJEHEKELUp to 2 digitsHP = pulse proof,high powerProduct Description: CRCW0603-HP 100 562R 1 % ET1 e3Product Description: CRCW0603-HP 0R0 ET1 e3CRCW0603-HP100562R 1 %ET1e3 TYPE / SIZE TCR RESISTANCE VALUE TOLERANCE PACKAGING LEADCRCW0402-HP CRCW0603-HP CRCW0805-HP CRCW1206-HP CRCW1210-HP CRCW1218-HP CRCW2010-HP CRCW2512-HP ± 100 ppm/K± 200 ppm/K10R = 10 Ω562R = 562 Ω10K = 10 kΩ1M = 1 MΩ0R0 = jumper± 0.5 %± 1 %± 5 %ET1, ET2,ET3, ET4,ET5, ET6,ET7, ET8,ET9, EF4,E02, E08,E67, E82e3 = pure tinterminationfinishC R C W0603562R F K E A H PCRCW-HP e3 Vishay DraloricDESCRIPTIONProduction is strictly controlled and follows an extensive set of instructions established for reproducibility. A cermet film layer and a glass-over are deposited on both sides of a high grade (Al2O3) ceramic substrate with its prepared inner contacts on both sides. A special laser is used to achieve the target value by smoothly fine trimming the resistive layer without damaging the ceramics. The resistor elements are covered by a protective coating designed for electrical, mechanical and climatic protection. The terminations receive a final pure tin on nickel plating.The result of the determined production is verified by an extensive testing procedure on 100 % of the individual chip resistors. Only accepted products are laid directly into the tape in accordance with IEC 60286-3 Type 1a and Type 2a (1).ASSEMBLYThe resistors are suitable for processing on automatic SMD assembly systems. They are suitable for automatic soldering wave, reflow or vapor phase as shown in IEC 61760-1 (1). The encapsulation is resistant to all cleaning solvents commonly used in the electronics industry, including alcohols, esters and aqueous solutions. The suitability of conformal coatings, potting compounds and their processes, if applied, shall be qualified by appropriate means to ensure the long-term stability of the whole system.The resistors are RoHS-compliant, the pure tin plating provides compatibility with lead (Pb)-free and lead-containing soldering processes. Solderability is specified for 2 years after production or requalification. The permitted storage time is 20 years. The immunity of the plating against tin whisker growth has been proven under extensive testing.MATERIALSVishay acknowledges the following systems for the regulation of hazardous substances:•IEC 62474, Material Declaration for Products of and for the Electrotechnical Industry, with the list of declarable substances given therein (2)•The Global Automotive Declarable Substance List (GADSL) (3)•The REACH regulation (1907/2006/EC) and the related list of substances with very high concern (SVHC) (4) for its supply chain The products do not contain any of the banned substances as per IEC 62474, GADSL, or the SVHC list, see /how/leadfree.Hence the products fully comply with the following directives:•2000/53/EC End-of-Life Vehicle Directive (ELV) and Annex II (ELV II)•2011/65/EU Restriction of the Use of Hazardous Substances Directive (RoHS) with amendment 2015/863/EU•2012/19/EU Waste Electrical and Electronic Equipment Directive (WEEE)Vishay pursues the elimination of conflict minerals from its supply chain, see the Conflict Minerals Policy at /doc?49037.APPROVALSThe resistors are qualified according to AEC-Q200.Where applicable, the resistors are tested in accordance with EN 140401-802 which refers to EN 60115-1, EN 60115-8 and the variety of environmental test procedures of the IEC 60068 (1) series.RELATED PRODUCTSF or more information about products with superior surge and pulse performance please refer to datasheet:D/CRCW-IF e3, Pulse Proof Thick Film Chip Resistors /doc?20024.For thick film resistors with standard requirements for power rating, please refer to datasheet:D/CRCW e3, Standard Thick Film Chip/doc?20035.For anti-surge products and high power rating, please refer to datasheet:RCS e3, Anti-Surge High Power Thick Film Chip Resistors /doc?20065.Notes(1)The quoted IEC standards are also released as EN standards with the same number and identical contents(2)The IEC 62474 list of declarable substances is maintained in a dedicated database, which is available at http://std.iec.ch/iec62474(3)The Global Automotive Declarable Substance List (GADSL) is maintained by the American Chemistry Council and available at (4)The SVHC list is maintained by the European Chemical Agency (ECHA) and available at http://echa.europa.eu/candidate-list-tableCRCW-HP e3Vishay DraloricFUNCTIONAL PERFORMANCESingle PulseMaximum pulse load, single pulse; applicable if → 0 and n < 1000 and Û ≤ Ûmax.;for permissible resistance change equivalent to 8000 h operationContinuous PulseMaximum pulse load, continuous pulses; applicable if ≤ P (ϑamb ) and Û ≤ Ûmax.;for permissible resistance change equivalent to 8000 h operation0.010.1110100100010 0000.0000010.000010.00010.0010.010.1110100^P m a x .-P u l s e L o a d (W )t i -Pulse Duration (s)P 0.010.1110100100010 0000.0000010.000010.00010.0010.010.1110100^P m a x .-P u l s e L o a d (W )t i -Pulse Duration (s)PCRCW-HP e3Vishay DraloricPulse VoltageMaximum pulse voltage, single and continuous pulses; applicable if ;for permissible resistance change equivalent to 8000 h operationDerating2004006008001000120014001600180020000.0000010.000010.00010.0010.010.1110^U m a x .-P u l s e V o l t a g e (V )t i -Pulse Duration (s)P ˆP ˆmax .≤00.20.40.60.81.01.21.41.61.8-70-60-50-40-30-20-10102030405060708090100110120130140150160P -P o w e r D i s s i p a t i o n (W )ϑamb -Ambient Temperature (°C)CRCW-HP e3Vishay DraloricTESTS AND REQUIREMENTSAll executed tests are carried out in accordance with the following specifications:EN 60115-1, generic specificationEN 60115-8 (successor of EN 140400), sectional specificationEN 140401-802, detail specification IEC 60068-2-xx, test methodsThe parameters stated in the Test Procedures and Requirements table are based on the required tests and permitted limits of EN 140401-802. The table presents only the most important tests, for the full test schedule refer to the documents listed above. However, some additional tests and a number of improvements against those minimum requirements have been included.The testing also covers most of the requirements specified by EIA/IS-703 and JIS-C-5201-1.The tests are carried out under standard atmospheric conditions in accordance with IEC 60068-1, 4.3, whereupon the following values are applied:Temperature: 15 °C to 35 °C Relative humidity: 25 % to 75 %Air pressure: 86 kPa to 106 kPa (860 mbar to 1060 mbar).A climatic category LCT / UCT / 56 is applied, defined by the lower category temperature (LCT), the upper category temperature (UCT), and the duration of exposure in the damp heat, steady state test (56 days).The components are mounted for testing on boards in accordance with EN 60115-8, 2.4.2 unless otherwise specified.TEST PROCEDURES AND REQUIREMENTSEN 60115-1 CLAUSE IEC60068-2 (1)TESTMETHODTESTPROCEDUREREQUIREMENTSPERMISSIBLE CHANGE (ΔR )Stability for product types:STABILITY CLASS 2 OR BETTERCRCW-HP e31 Ω to 1 M Ω4.5-Resistance -± 0.5 %; ± 1 %; ± 5 %4.8-Temperature coefficient(20 / -55 / 20) °C and (20 / 155 / 20) °C ± 100 ppm/K; ± 200 ppm/K4.25.1-Endurance at 70 °CU = or U = U max.;whichever is the less severe;1.5 h on; 0.5 h off70 °C; 1000 h ± (2 % R + 0.1 Ω)70 °C; 8000 h± (4 % R + 0.1 Ω)4.25.3-Endurance at upper category temperature 155 °C, 1000 h ± (2 % R + 0.1 Ω)4.2478 (Cab)Damp heat,steady state (40 ± 2) °C; 56 days;(93 ± 3) % RH;± (1 % R + 0.05 Ω)4.3767 (Cy)Damp heat,steady state,accelerated (85 ± 2) °C; (85 ± 5) % RH;U = ≤ 100 V;1000 h± (2 % R + 0.1 Ω)4.23-Climatic sequence:-± (2 % R + 0.1 Ω)4.23.2 2 (Bb)dry heat 125 °C; 16 h 4.23.330 (Db)damp heat, cyclic55 °C; 24 h; ≥ 90 % RH;1 cycle4.23.4 1 (Ab)cold -55 °C; 2 h4.23.513 (M)low air pressure 8.5 kPa; 2 h; (25 ± 10) °C4.23.630 (Db)damp heat, cyclic55 °C; 24 h;≥ 90 % RH; 5 cycles4.23.7-DC load U = ≤ U max.; 1 min-1 (Aa)Cold-55 °C; 2 h± (0.5 % R + 0.05 Ω)P 70 x R 0.1 x P 85 x R P 70 x RCRCW-HP e3Vishay DraloricNote(1)The quoted IEC standards are also released as EN standards with the same number and identical contents4.1914 (Na)Rapid change of temperature 30 min at -55 °C and 30 min at 125 °C;1000 cycles ± (1 % R + 0.05 Ω)no visible damage 4.13-Short time overloadU = 2.5 x ≤ 2 x U max.;whichever is the less severe;5 s ± (2 % R + 0.05 Ω)4.27-Single pulse high voltage overload Severity no. 4:U = 10 x orU = 2 x U max.;whichever is the less severe;10 pulses 10 μs/700 μs ± (1 % R + 0.05 Ω)no visible damage4.39-Periodic electricoverload U = orU = 2 x U max.;whichever is the less severe;0.1 s on; 2.5 s off;1000 cycles ± (1 % R + 0.05 Ω)no visible damage4.38-Electrostatic discharge(human body model)IEC 61340-3-1 (1);3 pos. + 3 neg. discharges;ESD voltage acc. to the size ± (1 % R + 0.05 Ω)4.22 6 (Fc)VibrationEndurance by sweeping;10 Hz to 2000 Hz;no resonance;amplitude ≤ 1.5 mm or≤ 200 m/s 2;7.5 h ± (0.5 % R + 0.05 Ω)no visible damage4.1758 (Td)SolderabilitySolder bath method;Sn60Pb40non-activated flux;(235 ± 5) °C;(2 ± 0.2) s Good tinning (≥ 95 % covered)no visible damageSolder bath method;Sn96.5Ag3Cu0.5non-activated flux;(245 ± 5) °C;(3 ± 0.3) s 4.1858 (Td)Resistance to soldering heat Solder bath method(260 ± 5) °C;(10 ± 1) s ± (0.5 % R + 0.05 Ω)4.2945 (XA)Component solventresistanceIsopropyl alcohol;+50 °C; method 2No visible damage4.3221 (Uu 3)Shear (adhesion)CRCW0402-HP and CRCW0603-HP: 9 N No visible damageCRCW0805-HP to CRCW2512-HP: 45 N4.3321 (Uu 1)Substrate bending Depth 2 mm;3 times ± (0.25 % R + 0.05 Ω)no visible damage, no open circuit in bent position4.7-Voltage proof U = 1.4 x U ins ; 60 s No flashover or breakdown 4.35-Flammability,needle flame testIEC 60695-11-5 (1);10 sNo burning after 30 sTEST PROCEDURES AND REQUIREMENTSEN 60115-1 CLAUSE IEC60068-2 (1)TESTMETHODTESTPROCEDUREREQUIREMENTSPERMISSIBLE CHANGE (ΔR )Stability for product types:STABILITY CLASS 2 OR BETTERCRCW-HP e31 Ω to 1 M ΩP 70 x R P 70 x R 15 x P 70 x RCRCW-HP e3Vishay DraloricDIMENSIONSSOLDER PAD DIMENSIONSNotes•The given solder pad dimensions reflect the considerations for board design and assembly as outlined e.g in standards IEC 61188-5-x (1) or in publication IPC-7351.Still, the given solder pad dimensions will be found adequate for most general applications(1)The quoted IEC standards are also released as EN standards with the same number and identical contentsDIMENSIONS AND MASSTYPE / SIZE L (mm)W (mm)H (mm)T1(mm)T2(mm)MASS (mg)CRCW0402-HP e3 1.0 ± 0.050.5 ± 0.050.3 ± 0.100.25 ± 0.100.2 ± 0.100.65CRCW0603-HP e3 1.6 ± 0.100.85 ± 0.100.45 ± 0.100.3 ± 0.200.3 ± 0.202CRCW0805-HP e3 2.0 ± 0.15 1.25 ± 0.150.5 ± 0.100.4 ± 0.200.35 ± 0.20 5.5CRCW1206-HP e3 3.1 ± 0.20 1.6 ± 0.150.5 ± 0.150.5 ± 0.200.45 ± 0.2010CRCW1210-HP e3 3.2 ± 0.20 2.5 ± 0.200.6 ± 0.100.45 ± 0.200.4 ± 0.2018CRCW1218-HP e3 3.1 ± 0.20 4.6 ± 0.200.6 ± 0.100.45 ± 0.200.4 ± 0.2031CRCW2010-HP e3 5.0 ± 0.15 2.5 ± 0.150.6 ± 0.100.6 ± 0.200.6 ± 0.2025.5CRCW2512-HP e36.3 ± 0.203.15 ± 0.150.6 ± 0.100.6 ± 0.200.6 ± 0.2042RECOMMENDED SOLDER PAD DIMENSIONSTYPE / SIZE WAVE SOLDERINGREFLOW SOLDERING G (mm)Y (mm)X (mm)Z (mm)G (mm)Y (mm)X(mm)Z (mm)CRCW0402-HP e3----0.450.60.6 1.65CRCW0603-HP e30.65 1.10 1.25 2.850.750.75 1.00 2.25CRCW0805-HP e30.90 1.30 1.60 3.50 1.000.95 1.45 2.90CRCW1206-HP e3 1.40 1.40 1.95 4.20 1.50 1.05 1.8 3.60CRCW1210-HP e3 1.80 1.452.95 4.70 1.70 1.10 2.803.90CRCW1218-HP e3 1.60 1.50 5.104.60 1.70 1.10 4.90 3.90CRCW2010-HP e3 3.60 1.65 2.85 6.90 3.70 1.20 2.70 6.10CRCW2512-HP e34.901.603.508.105.001.253.357.50Legal Disclaimer Notice VishayDisclaimerALL PRODUCT, PRODUCT SPECIFICATIONS AND DATA ARE SUBJECT TO CHANGE WITHOUT NOTICE TO IMPROV E RELIABILITY, FUNCTION OR DESIGN OR OTHERWISE.V ishay Intertechnology, Inc., its affiliates, agents, and employees, and all persons acting on its or their behalf (collectively,“Vishay”), disclaim any and all liability for any errors, inaccuracies or incompleteness contained in any datasheet or in any other disclosure relating to any product.Vishay makes no warranty, representation or guarantee regarding the suitability of the products for any particular purpose or the continuing production of any product. To the maximum extent permitted by applicable law, Vishay disclaims (i) any and all liability arising out of the application or use of any product, (ii) any and all liability, including without limitation special, consequential or incidental damages, and (iii) any and all implied warranties, including warranties of fitness for particular purpose, non-infringement and merchantability.Statements regarding the suitability of products for certain types of applications are based on Vishay's knowledge of typical requirements that are often placed on Vishay products in generic applications. Such statements are not binding statements about the suitability of products for a particular application. It is the customer's responsibility to validate that a particular product with the properties described in the product specification is suitable for use in a particular application. Parameters provided in datasheets and / or specifications may vary in different applications and performance may vary over time. All operating parameters, including typical parameters, must be validated for each customer application by the customer's technical experts. Product specifications do not expand or otherwise modify Vishay's terms and conditions of purchase, including but not limited to the warranty expressed therein.Hyperlinks included in this datasheet may direct users to third-party websites. These links are provided as a convenience and for informational purposes only. Inclusion of these hyperlinks does not constitute an endorsement or an approval by Vishay of any of the products, services or opinions of the corporation, organization or individual associated with the third-party website. Vishay disclaims any and all liability and bears no responsibility for the accuracy, legality or content of the third-party website or for that of subsequent links.Except as expressly indicated in writing, Vishay products are not designed for use in medical, life-saving, or life-sustaining applications or for any other application in which the failure of the Vishay product could result in personal injury or death. Customers using or selling Vishay products not expressly indicated for use in such applications do so at their own risk. Please contact authorized Vishay personnel to obtain written terms and conditions regarding products designed for such applications. No license, express or implied, by estoppel or otherwise, to any intellectual property rights is granted by this document or by any conduct of Vishay. Product names and markings noted herein may be trademarks of their respective owners.© 2022 VISHAY INTERTECHNOLOGY, INC. ALL RIGHTS RESERVEDRevision: 01-Jan-20221Document Number: 91000CRCW06032K20FKEAHP。
化学实验室英语单词

化学实验室英语单词The Essentials of a Chemistry Laboratory.Chemistry, a branch of science dealing with the composition, structure, properties, and reactions of matter, is an inherently experimental discipline. The chemistry laboratory, therefore, plays a pivotal role in the advancement of this field. It is not just a place where reactions are carried out; it's an environment where scientists explore, discover, and innovate. Let's delveinto the essential elements of a chemistry laboratory.1. Laboratory Bench.The laboratory bench, often made of durable materials like stainless steel or laminated wood, is the heart of the laboratory. It provides a stable platform for performing experiments and supports various equipment and glassware. The benches are usually equipped with built-in storage compartments for reagents, glassware, and other laboratorytools.2. Glassware and Apparatus.Glassware is an integral part of any chemistry laboratory. From beakers and flasks to pipettes and burettes, glassware is used for containing, measuring, and manipulating chemicals. These precision-made glass instruments are crucial for accurate measurements and safe handling of chemicals.3. Equipment and Instruments.A chemistry laboratory is equipped with a range of instruments for various purposes. These include balances for weighing chemicals, spectrometers for analyzing the composition of matter, and centrifuges for separating mixtures. These instruments enable scientists to carry out precise measurements and analyses.4. Chemical Storage.Proper storage of chemicals is crucial in a chemistry laboratory. Cabinets and shelves are used to store reagents, solvents, and other chemicals. These storage areas are designed to ensure the safety of the chemicals, prevent cross-contamination, and maintain the integrity of the reagents.5. Safety Equipment.Safety is paramount in a chemistry laboratory. Labs are equipped with safety equipment such as fire extinguishers, eye washes, and first aid kits. In addition, laboratory workers must wear protective gear, including lab coats, gloves, and safety goggles, to minimize the risk of accidents and injuries.6. Ventilation System.Chemistry experiments often involve the handling of volatile chemicals and the generation of fumes. A well-designed ventilation system is essential to ensure the removal of these harmful gases and maintain a safe workingenvironment.7. Laboratory Furniture.In addition to the benches, a chemistry laboratory also requires other furniture such as stools, chairs, and cabinets. These furniture items not only enhance the functionality of the lab but also contribute to the overall comfort of the lab workers.8. Cleanliness and Organization.A well-maintained chemistry laboratory is essential for accurate results and safe working conditions. Labs should be regularly cleaned and organized to prevent contamination and ensure the integrity of experiments.In conclusion, a chemistry laboratory is a complex ecosystem that requires meticulous planning and execution. It is not just a collection of equipment and glassware;it's a dynamic workspace where scientists can explore, innovate, and advance the field of chemistry. With theright tools, equipment, and safety measures, a chemistry laboratory can be a thriving ground for scientific discovery and progress.。
100种有机溶剂的极性和其他物理常数列表

100种有机溶剂的极性和其他物理常数列表pp.408-411 AppendixTable A-1. Compilation of hundred important organic solvents together with their physical constants, arranged by decreasing E No. (1) (2) (3) (4) (5) (6) (7) (8) (9) Solvent Name Water Formamide 1,2-Ethanediol Methanol N-Methylformamide Diethylene glycol TriethyIene glycol 2-Methoxyethanol Tetraethylene glycol Name in Chinese 水甲酰胺乙二醇甲醇甲基甲酰胺双乙二醇三乙二醇2-甲氧基乙醇四乙二醇甲基乙酰胺乙醇氨基乙醇乙酸丙醇苄醇丁醇戊醇异戊醇异丁醇异丙醇2-丁醇环己醇碳酸丙二酯2-戊醇硝基甲烷3-戊醇乙腈二甲亚砜苯胺环丁砜乙酸酐二甲基甲酰胺ET (h) 1 0.799 0.79 0.762 0.722 0.713 0.704 0.667 0.664 0.657 0.654 0.651 0.648 0.617 0.608 0.602 0.568 0.565 0.552 0.546 0.506 0.5 0.491 0.488 0.481 0.463 0.46 0.444 0.42 0.41 0.407 0.404 bp (° C) 100 210.5 197.5 64.5 180-185 245.7 288 124.6 327.3 206.7 78.3 170.95 117.9 97.15 205.45 117.7 138 130.5 107.9 82.2 99.5 161.1 241.7 119 101.2 115.3 81.6 189 184.4 287.3 (dec.) 140 153(10) N-Methylacetamide (11) Ethanol (12) 2-Aminoethanol (13) Acetic acid (14) 1-Propanol (15) Benzyl alcohol (16) 1-Butanol (17) 1-Pentanol (18) 3-Methyl-1-butanol, Isoamyl alcohol (19) 2-Methyl-1-propanol, Isobutyl alcohol (20) 2-Propanolm) (21) 2-Butanol (22) Cyclohexanol (23) Propylene carbonate (24) 2-Pentanol (25) Nitromethane (26) 3-Pentanol (27) Acetonitrile (28) Dimethylsulfoxide (29) Aniline (30) SulfoIane (31) Acetic anhydride (32) N,N-Dimethyl-formamidepp.408-411 AppendixTable A-1. Compilation of hundred important organic solvents together with their physical constants, arranged by decreasing E(33) N,N-Dimethyl-acetamide (34) Propanenitrile (35) 2-Methyl-2-propanol, t-Butanol (36) 1.3-Dimethyl-imidazolidin-2-one, DMEU (37) 1-Methylpyrrolidin-2-one (38) Acetone (39) 1.3-Dimethyl-2-oxo-hexahydropyrimidine, DMPU (40) 1,2-Diaminoethane (41) Cyanobenzene (42) 1,2-Dichloroethane (43) 2-Butanone (44) Nitrobenzene (45) 2-Methyl-2-butanol, t-Pentyl alcohol (46) 2-Pentanone (47) Tetramethylurea (48) Morpholine (49) Hexamethylphosphoric acid triamide, HMPT (50) 3-Methyl-2-butanone (51) Dichloromethane“ (52)Acetophenone (53) Pyridine (54) Methyl acetate (55) Cyclohexanone (56) 4-Methyl-2-pentanone (57) 1,1-Dichloroethane (58) Quinoline (59) 3-Pentanone (60) Chloroform (61) 3,3-Dimethyl-2-butanone (62) Triethylene glycol dimethyl ether (63) 2,4-Dimethyl-3-pentanone (64) Diethylene glycol dimethyl ether (65) 1,2-Dimethoxyethane (66) Ethyl acetate (67) 1,2-Dichlorobenzene (68) 2,6-Dimethyl-4-heptanone (69) Diethylene glycol diethyl ether二甲基乙酰胺丙腈叔丁醇二甲基乙撑脲甲基砒咯烷酮丙酮二甲基亚丙基脲乙二胺腈基苯1,2-二氯乙烷丁酮, 甲乙酮硝基苯叔戊醇2-戊酮四甲基脲吗啉六甲基磷酰胺异戊酮二氯甲烷苯乙酮吡啶乙酸甲酯环己酮4-甲基-2-戊酮1,1-二氯乙烷喹啉3-戊酮氯仿3,3-二甲基-2-丁酮三乙二醇二甲基醚2,4-二甲基-3-戊酮双乙二醇二甲基醚乙二醇二甲基醚乙酸乙酯邻二氯苯2,6-二甲基-4-庚酮双乙二醇二乙基醚0.401 0.401 0.389 0.364 0.355 0.355 0.352 0.349 0.333 0.327 0.327 0.324 0.321 0.321 0.318 0.318 0.315 0.315 0.309 0.306 0.302 0.287 0.281 0.269 0.269 0.269 0.265 0.259 0.256 0.253 0.247 0.244 0.231 0.228 0.225 0.225 0.21166.1 97.35 82.3 225.5 202 56.1 230 116.9 191.1 83.5 79.6210.8 102 102.3 175.2 128.9 233 94.2 39.6 202 115.25 56.9 155.65 117.4 573 237.1 102 61.2 106.3 216 125.25 159.8 (dec.) 84.5 77.1 180.5 168.2 188.9(70) Tetrahydrofuran (71) Methoxybenzene (72) Diethyl carbonate (73) Fluorobenzene (74) 1,1-Dichloroethene (75) Chlorobenzene (76) Bromobenzene (77) Ethoxybenzene (78) lodobenzene (79) 1,1,1-Trichloroethane (80) 1,4-Dioxane (81) Trichloroethene (82) t-Butyl methyl ether (83) Piperidine (84) Diethylamine (85) Diphenyl ether (86) Diethylether (87) Benzene (88) Di-n-propyl ether (89) Toluene (90) 1,4-Dimethylbenzene (91) Di-n-butyl ether (92) Carbon disulfide (93) Tetrachloromethane (94) Triethylamine (95) Tri-n-butylamine (96) cis-Decahydro-naphthaline (97) n-Heptane (98) n-Hexane (99) n-Pentane (100) Cyclohexane四氢呋喃苯甲醚碳酸二乙酯氟苯1,1-二氯乙烯氯苯溴苯苯乙醚碘苯1,1,1-三氯乙烷二f烷三氯乙烯叔丁基甲醚哌啶二乙基胺二苯醚乙醚苯二丙醚甲苯对二甲苯二丁醚二硫化碳四氯甲烷三乙基胺三丁基胺顺十氢萘正庚烷正己烷正戊烷环己烷0.207 0.198 0.194 194 0.194 0.188 0.182 0.182 0.17 0.17 0.164 0.16 0.148 0.148 0.145 0.142 0.117 0.111 0.102 0.099 0.0740.071 0.065 0.052 0.043 0.043 0.015 0.012 0.009 0.009 0.00666 153.6 126.8 84.7 31.6 131.7 155.9 169.8 188.3 74.1 101.3 87.2 55.2 106.2 55.55 258.1 344 801 90.1 110.6 138.4 140.3 46.276.6 88.9 214 195.8 98.4 68.7 36.1 80.7a The physical constants were taken from the following references: (1) R. C. Weast and M. J. Astle: CRC Handbook of Data o c Melting point. d Boiling point at 1013 mbar. e Relative permittivity (dielectric constant) for the pure liquid at 25° C unless followed by another temperature in parentheses.b C. Reichardt and E. Harbusch-G° rnert. Liebigs Ann. Chem 1983,721; C. Laurence, P. Nicolet. M. Lucon, and C. Reichardt, Ba The physical constants were taken from the following references: (1) R. C. Weast and M. J. Astle: CRC Hac Melting point.d Boiling point at 1013 mbar.e Relative permittivity (dielectric constant) for the pure liquid at 25°C unless followed by another temperature in parentheses.b C. Reichardt and E. Harbusch-G°rnert. Liebigs Ann. Chem 1983,721; C. Laurence, P. Nicolet. M. Lucon, and C. Reichardt, Bf Dipole moment in Coulombmeter (10^(-30) C m). measured in benzene, tetrachloromethane, 1,4-dioxane. Oh Normalised ET-values, derived from the transition energy at 25 °C of the long-wavelength absorption of a sI Y. Marcus and S. Glikberg, Pure Appl. Chem. 57. 855 (1985) (Methanol).l Y. Marcus, Pure Appl. Chem. 57, 860 (1985) (Ethanol)m Y. Marcus, Pure Appl. Chem. 58, 1411 (1986) (1-Propanol, 2-Propanol, 1-Butanol).n J. F. Coetzee and T.-H. Chang, Pure Appl. Chem. 58, 1541 (1986) (Nitromethane).p M. Breant, Bull. Soc. Chim. Fr. 197/,725 (I-Methylpyrrolidin-2-one).q J. F. Coetzee and T.-H. Chang, Pure Appl. Chem. 58, 1535 (1986) (Acetone).r C. Agarni, Bull. Soc. Chim. Fr. /968, 1205 (1,2-Dimethoxyethane).s J. F. Coetzee and T.-H. Chang, Pure Appl. Chem. 57, 633 (1985) (THF, 1,4-Dioxane).g Refractive index at the average D-line of sodium (***** cm-1) at 20°C unless followed by another temperature in parenthesek J. F. Coetzee (ed.): Recommended Methods for Purification of Solvents and Tests for Impurities (Acetonitrile, Sulfolane. Proo B. J. Barker, J. Rosenfarb, and J. A.Caruso, Angew. Chem. 91, 560(1979); Angew. Chem., Int. Ed. Engl. 18, 503 (1979) (Dt K. M. Kadish and J. E. Anderson, Pure Appl. Chem. 59, 703 (1987) (Benzonitrile, Dichloromethane. 1,1-Dichloroethane, 1,2-Dnstants, arranged by decreasing ET-values as empirical parameter of solvent polarity. mp (° C) 0 2.55 -12.6 -97.7 -3.8 -7.8 -4.3 -85.1 -6.2 30.6 -114.5 10.5 16.7 -126.2 -15.3 -88.6 -78.2 -117.2 -108 -88 -114.7 25.15 -54.5 -28.55 -75 -43.8 18.5 -6 28.45 -73.1 -60.4 nD20 (g) 1.333 1.4475 1.4318 1.3284 1.4319 1.4475 1.4558 1.4021 1.4577 1.4253(35° ) 1.3614 ***** 1.3719 1.3856 1.5404 1.3993 1.41 1.4072 1.3959 1.3772 1.3971 1.4648(25° ) 1.4215 1.4064 1.3819 1.4104 1.3441 1.4793 1.5863 1.4816(30° ) 1.3904 1.4305 Er(e) 78.3 111.0(20° ) 37.7 32.66 182.4 31.69(20° ) 23.69(20° ) 16.93 19.7 191.3(32° ) 24.55 37.72 6.17(20° ) 20.45 13.1(20° ) 17.51 13.9 15.19 17.93 19.92 16.56 15 64.92 13.71 35.94 13.35 35.94 46.45 6.71(30° ) 43.3(30° )20.7(19° ) 36.71 u (f) 5.9 11.2 7.7 5.7 12.9 7.7 10.0 6.8 10.8 14.2 5.8 7.6 5.6 5.5 5.5 5.8 5.7 6.1 6.0 5.5 5.5 6.2 16.5 5.5 11.9 5.5 11.8 13.5 5.0 16 9.4 10.8anged by decreasing ET-values as empirical parameter of solvent polarity.-20 -92.8 25.6 8.2 -24.4 -94.7 -20 11.3 -12.75 -35.7 -86.7 5.8 -8.8 -76.9 -1.2 -4.8 7.2 -92 -94.9 19.6 -41.55 -98.05 -32.1 -84.7 -97 -14.85 -39 -63.5 -49.8 -45 -69 -64 -69 -83.55 -17 -46 -44.31.4384 1.3658 1.3877 1.4707(25° ) 1.47 1.3587 1.4881(25° ) 1.4568 1.5282 1.4448 1.3788 1.5562 1.405 1.3908 1.4493(25° ) 1.4542 ***** 1.388 1.4242 1.5342 1.5102 1.3614 1.451 1.3958 1.4164 1.6273 1.3923 1.4459 1.3952 1.4224 1.3999 1.4078 1.3796 1.3724 1.5515 1.4122 1.411537.78 28.86(20° ) 12.47 37.6 32.2 20.56 36.12 12.9 25.2 10.37 18.51(20° ) 34.78 5.78 15.38(20° ) 23.6 7.42 296 15.87(30° ) 8.93 17.39 12.91 6.68 16.10(20° ) 13.11(20° ) 10.0(18° ) 8.95 17.00(20° )4.81(20° ) 13.1(14.5° ) 7.5 17.2(20° )5.8 7.26.02 9.93 9.91(20° )5.712.4 11.7 5.5 13.6 13.6 9.0 14.1 6.3 13.4 6.1 9.2 133.0 5.7 90.0 11.7 5.2 18.5 9.2 5.2 9.8 7.9 5.7 10.3 2.7 6.1 7.3 9.4 3.8 9.3 9.1 6.6 5.7 6.1 7.1 8.9-108.4 -37.5 -43 -42.2 -122.6 -45.6 -30.8 -29.5 -31.35 -30.4 11.8 -86.4 -108.6 -10.5 -49.8 26.9 -116.3 5.5 -123.2 -95 13.3 -95.2 -111.6 -22.8 -114.7 -70 -43 -90.6 -95.3 -129.7 6.71.4072 1.517 1.3837 1.4684(15° ) 1.4247 1.5248 1.55681.5074 1.62 1.438 1.4224 1.4773 1.369 1.4525 1.3846 1.5763(30° ) 1.3524 1.5011 1.3805 1.4969 1.4958 1.3992 1.6275 14,602 1.401 1.4291 1.481 1.3876 1.3749 1.3575 1.42627.58 4.33 2.82(20° ) 5.42 4.82(20° ) 5.62 5.4 4.22(20° ) 4.49(20° ) 7.25(20° ) 2.21 3.42(16° ) 4.5(20° ) 5.8(20° ) 3.78 3.69(20° ) 4.2 2.27 3.39(26° ) 2.38 2.27(20° ) 3.08(20° ) 2.64(20° ) 2.23 2.42(20° ) 2.20(20° )1.92(20° ) 1.88 1.84(20° )2.02(20° )5.8 4.2 3.0 4.9 4.3 5.4 5.2 4.5 4.7 5.7 1.5 2.7 4.1 4.0 4.0 3.9 3.8 0.0 4.4 1.0 0.0 3.9 0.0 0.0 2.9 2.6 0.0 0.0 0.0 0.0 0.0J. Astle: CRC Handbook of Data on Organic Compounds. Vol.I and II, CRC Press, Boca Raton/Florida 1985; (2) R C. Weast (ed.): Handbook oolet. M. Lucon, and C. Reichardt, Bull. Soc Chim Fr 1987,125: ibid. 1987, 1001; cf. also Table 7-3 in Chapter 7.her temperature in parentheses.J. Astle: CRC Handbook of Data on Organic Compounds. Vol.I and II, CRC Press, Boca Raton/Florida 1985; (2) R C. Weast (ed.): Handbook oolet. M. Lucon, and C. Reichardt, Bull. Soc Chim Fr 1987,125: ibid. 1987, 1001; cf. also Table 7-3 in Chapter 7.her temperature in parentheses.e, 1,4-dioxane. Or n-hexane at 20-30°C.1 Debye=3.336x10-30 Cm.of a standard pyridinium-N-phenoxide betaine dye, ET(30); cf. Eqs. (7-27) and (7-29) in Section 7.4tonitrile, Sulfolane. Propylene carbonate, Dimethyl sulfoxide, N,N-Dimethylformamide, Hexamethylphosphoric triamanother temperature in parentheses.m., Int. Ed. Engl. 18, 503 (1979) (DMEU, DMPU, Tetramethylurea).methane. 1,1-Dichloroethane, 1,2-Dichloroethane).。
含CuSBA-15催化剂的合成、表征及DeNPAC反应性能评价

e h n e e cin atvt fCu / B —5 o NP o l e a tiue o t e b—u cin l y o cd n n a cd ra t ciiy o A1S A— r De AC c ud b trb td t h i n t ai f a i o 1 f f o t a d meal o f A1S tlcino i Cu / BA— 5 l.
2 Hu a a c an t o h m ia o . n n Ji n h g Pe r c e c l C
,
Lt , Yu y n 1 0 2 Ch n d. e a g 4 4 1 , i a)
Ab ta t sr c :Cu S O/ BA一 5 1 ,Cu / B 1 n OAIS A1S A- 5a dCu / BA一 5we es n h s e ywe— rg aina dine c a g 1 r y t ei db ti e n t n o —x h n e z mp o
Cu / B 1 h we h e tc tlt e fr n eb iig t ehg etc n eso fp r iewi h o s A1S A一5 s o dt eb s aayi p ro ma c y gvn h ih s o v rin o y i n t te lwe t c d h
wa n r d cd t BA一 5 a d A1S sito u e o S 1 n / BA一 5 s p o t y we—mp e n t n a d in e c a g t o 1 u p rs b ti rg ai n o —x h n emeh d.rs e t ey o e p ci l. v
Ke r s y i i eo i a i n;N0 ;S y wo d :p rd n x d t o BA一 5 iu c in l cd c i nc st s 1 ;b f n t a i i o i i o a — e
一种核酸免提取试剂及其使用方法[发明专利]
![一种核酸免提取试剂及其使用方法[发明专利]](https://img.taocdn.com/s3/m/a5c37af459f5f61fb7360b4c2e3f5727a5e9240c.png)
(19)中华人民共和国国家知识产权局(12)发明专利申请(10)申请公布号 (43)申请公布日 (21)申请号 202011191934.X(22)申请日 2020.10.30(71)申请人 山东省滨州畜牧兽医研究院地址 256600 山东省滨州市国家级经济技术开发区黄河二路169号绿都生物工程高科技园(72)发明人 张松林 刘鸿艳 肖跃强 刘磊 马永彪 公鑫婧 沈志强 (74)专利代理机构 北京中索知识产权代理有限公司 11640代理人 周国勇(51)Int.Cl.C12N 15/10(2006.01)(54)发明名称一种核酸免提取试剂及其使用方法(57)摘要本发明适用于微生物技术领域,提供了一种核酸免提取试剂,包括如下组分:Chelex ‑100、Triton X ‑100、Tris ‑Cl、NaCl和洋地黄皂苷;各组分中,Chelex ‑100、Triton X ‑100和洋地黄皂苷的质量浓度分别为0.8~1.2%、0.3~0.5%和1.3~1.7%,Tris ‑Cl和NaCl的摩尔浓度分别为45~55mmol/L和80~120mmol/L;本发明还提供了核酸免提取试剂的使用方法。
借此,本发明温度适应范围广,能够在较差的实验环境中进行样品处理和核酸提取,耗时短,效率高,使用便捷且生产成本低,具有较高的市场推广价值。
权利要求书1页 说明书5页 附图2页CN 112322613 A 2021.02.05C N 112322613A1.一种核酸免提取试剂,其特征在于,包括如下组分:Chelex -100、Triton X -100、Tris -Cl、NaCl和洋地黄皂苷;各组分中,所述Chelex -100、Triton X -100和洋地黄皂苷的质量浓度分别为0.8~1.2%、0.3~0.5%和1.3~1.7%,所述Tris -Cl和NaCl的摩尔浓度分别为45~55mmol/L和80~120mmol/L。
欧盟发布《起始物料生产审计指南》-2018

欧盟发布《起始物料生产审计指南》-2018近日,欧盟APIC发布了《APIC GUIDE FOR AUDITING REGISTERED STARTING MATERIAL MANUFACTURERS(APIC指南:已注册起始物料生产商审计指南)》-2018,该指南旨在为起始物料生产商及其用户企业提供以下指导:•Consider key areas of focus for such audits•审计关注的关键领域•Consider the application of risk management principles in their audit process•审计过程中风险管理原则的应用•Consider key parameters of a quality management system needed for the•manufacture of such materials该物料生产商所需要具备的质量管理体系的关键要素该指南适用于人用药品API起始物料的生产,也同样适用于兽用药品API起始物料的生产。
该指南内容包括如下:1. PREAMBLE序2. INTRODUCTION简介2.1 Objective目的2.2 Regulatory Applicability监管适用性2.3 Scope范围3. QUALITY MANAGEMENT质量管理3.1 Principles原则3.2 Internal Audits (Self Inspection)内部审计(自检)4. PERSONNEL人员4.1 Personnel Qualification人员资质4.2 Personnel Hygiene人员卫生5. BUILDINGS AND FACILITIES厂房和设施5.1 Design and Construction设计和构造5.2 Water水5.3 Containment控制5.4 Lighting照明5.5 Sanitation and Maintenance卫生和维护6. PROCESS EQUIPMENT工艺设备6.1 Design and Construction设计和构造6.2 Equipment Maintenance and Cleaning设备维护和清洁6.3 Calibration校准6.4 Computerized Systems计算机化系统7. DOCUMENTATION AND RECORDS文件和记录7.1 Documentation System and Specifications文件系统和规范7.2 Master Production Instructions (Master Production and Control Records)主生产文件(主生产和控制记录)7.3 Batch Production Records (Batch Production and Control Records)批生产记录(批生产和控制记录)7.4 Laboratory Control Records实验室控制记录7.5 RSM Batch Documentation Review and Batch Release起始物料生产商文件审核和批放行8. MATERIALS MANAGEMENT物料管理8.1 General Controls一般控制8.2 Receipt and Quarantine收货和验货8.3 Storage储存9. PRODUCTION AND IN-PROCESS CONTROLS生产和中间控制9.1 Production Operations生产操作9.2 In-process Sampling and Controls生产过程取样和控制9.3 Contamination Control污染控制10. PACKAGING AND IDENTIFICATION LABELLING OF RSM 起始物料的包装和识别标签10.1 Packaging Materials包装材料10.2 Packaging and Labelling Operations包装和贴签操作11. STORAGE AND DISTRIBUTION储存和运输11.1 Warehousing Procedures仓储规程11.2 Distribution Procedures发运规程12. LABORATORY CONTROLS实验室控制12.1 General Controls一般控制12.2 Testing of RSM起始物料的检验12.3 Certificates of Analysis检验报告书12.4 Expiry and Retest Dating有效期和复验期12.5 Reserve/Retention Samples留样13. VALIDATION验证14. CHANGE CONTROL变更控制15. REPROCESSING OR REWORK OF MATERIALS返工和重新加工15.1 General一般原则15.2 Recovery of Materials and Solvents物料和溶剂回收15.3 Returns退货16. COMPLAINTS投诉17. CONTRACT MANUFACTURERS (INCLUDING LABORATORIES)合同制造(包括实验室)18. SPECIFIC GUIDANCE FOR RSM MANUFACTURED BY FERMENTATION通过发酵生产的起始物料的特殊指南18.1 General一般原则18.2 Fermentation发酵18.3 Harvesting, Isolation and Purification收取、分离和精制19. GLOSSARY术语。
用于核酸提取样本容积最优化的等频率运动控制

引用格式:蒋新, 姚佳, 李东书, 等. 用于核酸提取样本容积最优化的等频率运动控制[J]. 中国测试,2023, 49(11): 157-165. JIANG Xin, YAO Jia, LI Dongshu, et al. Equal-frequency motion control for volume optimization ofnucleic acid extraction samples[J]. China Measurement & Test, 2023, 49(11): 157-165. DOI: 10.11857/j.issn.1674-5124.2023040071用于核酸提取样本容积最优化的等频率运动控制蒋 新1, 姚 佳2,3, 李东书2, 王天一3,李金泽2, 李莹雪2, 周连群1,2(1. 徐州医科大学,江苏 徐州 221000; 2. 中国科学院苏州生物医学工程技术研究所 中国科学院生物医学检验技术重点实验室,江苏 苏州 215163; 3. 苏州国科芯感医疗科技有限公司,江苏 苏州 215163)摘 要: 为实现核酸提取振荡运动下样本容积最优化,将等频率运动控制算法应用于核酸提取过程中,通过提高核酸提取运动过程的平稳性,使振荡运动下核酸样本容积得到有效提升。
基于搭建的STM32高精度控制平台,通过等频率离散化的方式控制步进电机使用Sigmoid 曲线实现加减速过程,实现步进电机的平滑、精确控制。
提出的等频率离散加减速运动控制算法可解决变速过程中速度变化不平稳问题。
对比等时间采样的实验表明,相同实验参数下,采用等频率离散的方式比等时间离散能更快达到目标频率。
在精度对比上,采用等频率离散的方式在定位精度上比等时间离散法提高39.47%。
在核酸提取应用中,等频率离散法比等时间离散法可有效提高核酸样本容积20.62 μL 。
与传统Sigmoid 函数模型实现S 型曲线加减速控制算法相比,等频率离散法可提升运动定位精度使抽提过程更加顺滑,有效提高了核酸提取振荡运动下的样本容积。
实验室规章制度

实验室规章制度英文回答:As a researcher, it is imperative to adhere to laboratory regulations and safety protocols to ensure the well-being of myself, my colleagues, and the integrity of our research. These regulations are not merely a set of rules but rather a framework that guides our daily operations and helps us maintain a safe and productive work environment.One of the fundamental regulations in our laboratory is the requirement to wear appropriate personal protective equipment (PPE). This includes lab coats, safety glasses, and gloves when handling hazardous materials. By donning PPE, I am not only protecting myself from potential hazards but also preventing the contamination of samples and equipment. For instance, in our cell culture experiments, sterile gloves are essential to avoid introducing bacteria or viruses into the cell culture, which could compromisethe results of our research.Another critical aspect of laboratory safety is the proper handling and disposal of chemicals. Our laboratory has a comprehensive chemical inventory system that tracks the usage and storage of all chemicals. Before using any chemical, I thoroughly read the safety data sheet (SDS) to understand its potential hazards and appropriate handling procedures. This knowledge empowers me to take necessary precautions to minimize the risk of accidents or exposure. For example, when working with flammable solvents, I ensure that the work area is well-ventilated and that there are no open flames nearby.Regular maintenance and calibration of laboratory equipment are equally important. Malfunctioning equipment can lead to inaccurate results or even accidents. As a responsible researcher, I am obligated to inspect and maintain the equipment I use regularly. This includes checking the calibration of pipettes, microscopes, and other instruments according to the manufacturer's specifications. By ensuring that our equipment is operatingoptimally, I can have confidence in the accuracy and reliability of our experimental data.Waste management is another area where compliance with laboratory regulations is crucial. Our laboratory has designated areas for the disposal of different types of waste, including chemical, biological, and radioactive waste. I strictly adhere to the waste disposal procedures to prevent environmental contamination and protect the health of others. For instance, chemical waste is disposed of in designated containers and labeled appropriately before being sent for proper disposal by a licensed waste disposal company.In addition to these specific regulations, our laboratory also emphasizes the importance of good laboratory practices (GLPs). GLPs are a set of guidelines that ensure the quality and integrity of our research data. These practices include maintaining accurate records, following established protocols, and documenting all experimental procedures and observations. By adhering to GLPs, I am not only ensuring the reproducibility of ourresults but also demonstrating the credibility andreliability of our research findings.By adhering to laboratory regulations and GLPs, I am not only fulfilling my ethical and professional responsibilities but also contributing to a positive and productive research environment. These regulations provide me with the knowledge and tools necessary to work safely and efficiently, ultimately enabling me to make meaningful contributions to the advancement of science and technology.中文回答:作为一名研究人员,我必须遵守实验室规章制度和安全规程,以确保我自己、我的同事和我们研究的完整性。
溶剂的选择

第四章溶剂选择I.介绍选择适当的溶剂可以提高反应速率,提高反应的可重复性和操作的便利性,并且能够确保目标产物的质量和产率。
另外,从减少浪费以及溶剂的有效回收和重复使用上来说,溶剂的选择也是相当重要的。
以上这些都对合成产品的生产效率和生产成本有着直接的影响。
在研发的早期阶段,以任何方式提供的原料都是至关重要的,而溶剂的正确选择是为了能够保证在规定时间内,在难度最小化的条件下得到目标产物。
对于溶剂的分类,溶剂化,以及重要的物理参数的简单讨论如下,更多细节请参阅Reichardt关于溶剂的文章[1]。
I.A. 溶解性和主要溶剂的性质溶剂的许多性质取决于其官能团。
溶剂的类别包括• 质子性溶剂,或氢键供体的溶剂(HBD,路易斯酸),例如,H2O,NH3,CH3OH和AcOH;• 氢键受体的溶剂(HBA,路易斯碱),比如,H2O,Et3N,EtOAc,THF,NMP(N-甲基吡咯烷酮)和丙酮;• 极性非质子溶剂,或称为“非羟基溶剂”,例如,DMSO和DMF;• 氯代烷烃和氟代烷烃溶剂;• 饱和烃类和不饱和烃类溶剂[1]。
当溶质被分散在溶剂中,它们就叫做被溶解。
溶解,即每一个被分散的分子或者离子的周围都被溶剂分子紧密约束着。
被分散在水中的分子或者离子叫做被水合。
溶解可以是一个吸热的过程,也可以是放热的过程;类似的,结晶也可以是吸热或者放热的过程。
(实验室中的结晶过程的放热现象可能不易被注意到,但是在放大生产时,结晶过程中反应釜的温度升高1-2℃是很普遍的)。
溶剂化的程度随着离子电荷的增大和离子半径的减小而增大。
不同溶剂的离子溶剂化值(包围一个溶质分子的溶剂分子数)不同。
例如,Li+在环丁砜中的离子溶剂化值为1.4,在甲醇中上升至7,在乙腈中为9,在水中达到21[1]。
溶剂化程度能够影响反应性。
在相转移催化剂下产生裸露的阴离子只能最低限度的在有机溶剂中溶剂化,其反应性比高溶剂化的离子要大得多。
极性非羟基溶剂,例如DMSO,能够将阳离子溶剂化,但C-H键不能被极化成为明显的溶剂化阴离子。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Data on the temperature dependence of viscosity, dielectric constant, and vapor pressure can be found in the pertinent tables in this Handbook.
REFERENCES
1.491
2.022 2.05 2.392
11.9 0.049 16.2 38.4 36.2
14.1 3.14 33.1
2.061 1.840
0.090 0.472
FP/ °C -39 39 49 -20 74 6 77
4 -26 50 0 21 -29 67 80 70 52
1.621 1.741
ቤተ መጻሕፍቲ ባይዱ
tm/°C -123.37 16.64 -74.1 -94.7 -19 -43.82 20.5 -96 -112.8 -87.7 12.5 -83.48 -129 -88.2 25.5 12.4 -6.02 -37.13 4 8.3 -16 -57.1 5.49 -23.8 <0 -27 -30 14.5 -14.93 -13.99 -0.4
/ g mL–1
0.783418 1.044625 1.08220 0.784525 0.93219 0.785720 1.028120 1.662516 1.105120 0.84020 1.051120 0.800725 0.854020 0.75820 0.93420 0.982426 1.021720 0.994020 2.34 3.10 2.150 1.040125 0.876520 1.020515 0.964025 1.020220 1.05820 1.347015 1.077520 1.009315 1.212020
LABORATORY SOLVENTS AND OTHER LIQUID REAGENTS
This table summarizes the properties of 575 liquids that are commonly used in the laboratory as solvents or chemical reagents. The properties tabulated are:
2.068
0.169 63 12.7 -11
113
96
1.572 1.602
0.11 0.084 72
Fl. Lim. 4-60% 4-20% 2.7-10.3% 3-13% 2.2-12% 3-16%
IT/ °C
175 463 316 465 688 524 570
390
2.8-31% 220
2.4-8%
438
3-17%
481
3-18%
378
2-22%
374
1.3-11% 615 475
192
1-8%
498
TLV/ ppm 10 5 500 40 10
2 2 0.5
2
0.5
0.5
15-16
LABORATORY SOLVENTS AND OTHER LIQUID REAGENTS (continued)
/D 2.750 1.70 ≈2.8 2.88
3.92 3.02
2.72 3.1
3.87 1.60 1.2
1.13 1.38
1.59 3.0 0 3.5
1.23 4.18
cp/
vp/
J g–1K–1 kPa
2.020 2.053 1.648 2.175
120 2.07 0.680 30.8
2.229 1.703
/ mPa s 1.056 0.843 0.306 0.369 1.681 0.368
1.218
3.85 1.056
0.604
1.267
⑀ 21.0 6.20 22.45 21.01
36.64 17.44
15.8
33.0 19.7
7.06 4.30 3.222
17.85 2.2825 17.87
12.31 4.705 28.90 4.26 25.9 23.0
Mol. Form.
C2H4O C2H4O2 C4H6O3 C3H6O C4H7NO C2H3N C8H8O C2H3BrO C2H3ClO C3H4O C3H4O2 C3H3N C3H6O C3H7N C4H11NO C3H9NO C6H7N C7H8O Cl5Sb F5Sb AsCl3 C7H6O C6H6 C8H7N C8H11N C8H10O C7H8S C6H5ClO2S C6H6S C7H5N C7H5ClO
Name
Benzyl acetate Benzyl alcohol Benzylamine 2,2’-Bioxirane Bis(2-aminoethyl)amine N,N’-Bis(2-aminoethyl)-1,2-
ethanediamine Bis(2-chloroethyl) ether Bis(chloromethyl) ether Bis(2-ethylhexyl) phthalate Bis(2-hydroxyethyl) sulfide Boron tribromide Boron trichloride Bromine Bromobenzene 1-Bromobutane 2-Bromobutane, (±)Bromochloromethane Bromodichloromethane Bromoethane Bromoethene 2-Bromo-2-methylpropane 1-Bromopentane 1-Bromopropane 2-Bromopropane 3-Bromopropene 2-Bromotoluene Bromotrichloromethane Butanal 1,3-Butanediol 1,4-Butanediol 2,3-Butanediol 2,3-Butanedione Butanenitrile 1-Butanethiol 2-Butanethiol Butanoic acid Butanoic anhydride 1-Butanol 2-Butanol 2-Butanone trans-2-Butenal cis-2-Butenoic acid 2-Butoxyethanol Butyl acetate sec-Butyl acetate Butyl acrylate Butylamine sec-Butylamine tert-Butylamine Butylbenzene tert-Butylbenzene Butyl benzoate tert-Butyl ethyl ether tert-Butyl hydroperoxide 1-tert-Butyl-4-methylbenzene Butyl vinyl ether ␥-Butyrolactone Carbon disulfide Chloroacetaldehyde Chloroacetone Chloroacetyl chloride 2-Chloroaniline 3-Chloroaniline Chlorobenzene 2-Chloro-1,3-butadiene
Name
Acetaldehyde Acetic acid Acetic anhydride Acetone Acetone cyanohydrin Acetonitrile Acetophenone Acetyl bromide Acetyl chloride Acrolein Acrylic acid Acrylonitrile Allyl alcohol Allylamine 2-Amino-2-methyl-1-propanol 3-Amino-1-propanol Aniline Anisole Antimony(V) chloride Antimony(V) fluoride Arsenic(III) chloride Benzaldehyde Benzene Benzeneacetonitrile Benzeneethanamine Benzeneethanol Benzenemethanethiol Benzenesulfonyl chloride Benzenethiol Benzonitrile Benzoyl chloride
1. Lide, D. R., Handbook of Organic Solvents, CRC Press, Boca Raton, FL, 1994. 2. Lide, D. R., and Kehiaian, H. V., Handbook of Thermophysical and Thermochemical Data, CRC Press, Boca Raton, FL, 1994. 3. Riddick, J. A., Bunger, W. B., and Sakano, T. K., Organic Solvents, Fourth Edition, John Wiley & Sons, New York, 1986. 4. Fire Protection Guide to Hazardous Materials, 11th Edition, National Fire Protection Association, Quincy, MA, 1994. 5. Urben, P. G., Ed., Bretherick’s Handbook of Reactive Chemical Hazards, 5th Edition, Butterworth-Heinemann, Oxford, 1995
Mol. Form.
C9H10O2 C7H8O C7H9N C4H6O2 C4H13N3 C6H18N4
Mr : tm: tb : ρ: η: ε: µ: cp: vp: FP: Fl.Lim: IT TLV
