62691-EiCentro
哈希水质分析手册总磷27426-45
测试准备工作
仪器型号
DR 5000 DR 2800 DR 2700 DR/2500 DR/2400
表1 仪器详细说明
遮光罩型号
— LZV 646 LZV 646
— —
适配器型号
— — — — 5945700
测试开始前:
针对型号为DR 2800 和DR 2700的仪器:测试前请在适配器模块上方安装上遮光罩。 为了测试结果更加准确,每一批新的试剂都应该测定试剂空白值。试剂空白的测定同样按照测试步骤进行,只是把样品换成了去
数量/每次测量 1 1 1 1
1
1
推荐使用的标准样品
标准样品名称及描述
饮用水无机混合指标标准溶液,用于F-、NO3、PO4、SO4 磷Voluette® 安瓿瓶标准溶液,浓度为50 mg/L PO4 3-,10 mL 磷标准溶液,浓度为1 mg/L PO4 3磷标准溶液,浓度为3 mg/L PO4 3-
50 /pkg
1000 /pkg
12 /pkg 100 /pkg
0.06 mg/L PO4 3-
0.06 mg/L PO4 3-
方法解释
分析测试前,有机和浓缩的无机形式的磷酸盐(偏、焦或多磷酸盐)必须转化为可以反应的正磷酸盐。用酸和加热的样品预处理可 以提供浓缩的无机形式的磷酸盐发生水解作用的条件。有机磷酸盐在酸液和过硫酸盐溶液中加热后可以转化为正磷酸盐。
正磷酸盐与钼酸盐在酸性介质中发生反应,生成一种混合的磷酸盐/钼酸盐配合物。抗坏血酸还原此含钼配合物使溶液呈深蓝色。 测试结果是在波长为880 nm的可见光下读取的。
4. 按“OK(好)”键确认标样浓度、样品体积和加标体积的默认值。按“EDIT(编辑程序)”键可以修改这些默认值。当这些值确认好
CHEMLOK 5150 产品安全数据表说明书

USA SAFETY DATA SHEET3000000027601. CHEMICAL PRODUCT AND COMPANY IDENTIFICATIONProduct name: CHEMLOK 5150 Product Use/Class:AdhesiveLORD Corporation 111 LORD DriveCary, NC 27511-7923 USATelephone: 814 868-3180Non-Transportation Emergency: 814 763-2345 Chemtrec 24 Hr Transportation Emergency No.800 424-9300 (Outside Continental U.S. 703 527-3887)EFFECTIVE DATE: 08/07/20172. HAZARDS IDENTIFICATIONGHS CLASSIFICATION:Flammable liquids Category 2 Acute toxicity Oral Category 4Acute toxicity Inhalation - Vapours Category 3Acute toxicity Inhalation - Dust and Mist Category 2 Serious eye damage/eye irritation Category 2A Reproductive toxicity Category 1ASpecific target organ systemic toxicity (single exposure) Category 1 Central nervous system, retina, Systemic toxicity, EyesSpecific target organ systemic toxicity (single exposure) Category 3Specific target organ systemic toxicity (repeated exposure) Category 1 Liver, Central nervous system, Eyes, retina Specific target organ systemic toxicity (repeated exposure) Category 2 Nervous system Hazardous to the aquatic environment - acute hazard Category 3 Hazardous to the aquatic environment - chronic hazard Category 3GHS LABEL ELEMENTS: Symbol(s)Signal WordD ANGERHazard StatementsHighly flammable liquid and vapor. Harmful if swallowed. Fatal if inhaled.Causes serious eye irritation.May damage fertility or the unborn child.Causes damage to organs.(Central nervous system, retina, Systemic toxicity, Eyes) May cause respiratory irritation. May cause drowsiness or dizziness.Causes damage to organs through prolonged or repeated exposure.(Liver, Central nervous system, Eyes, retina) May cause damage to organs through prolonged or repeated exposure.(Nervous system) Harmful to aquatic life.Harmful to aquatic life with long lasting effects.Precautionary StatementsPreventionKeep away from heat/sparks/open flames/hot surfaces. - No smoking.Ground/Bond container and receiving equipment.Use explosion-proof electrical/ventilating/lighting equipment.Use only non-sparking tools.Take precautionary measures against static discharge.Obtain special instructions before use.Do not handle until all safety precautions have been read and understood.Wear protective gloves/protective clothing/eye protection/face protection.Use personal protective equipment as required.Wear respiratory protection.Do not breathe dust/fume/gas/mist/vapors/spray.Wash thoroughly after handling.Do not eat, drink or smoke when using this product.Use only outdoors or in a well-ventilated area.Avoid release to the environment.ResponseIn case of fire: refer to section 5 of SDS for extinguishing media.Immediately call a POISON CENTER or doctor/physician.Specific treatment is urgent (see supplemental first aid instructions on this label).IF INHALED: Remove to fresh air and keep at rest in a position comfortable for breathing.IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower.IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do.Continue rinsing.Rinse mouth.StorageStore in a well-ventilated place. Keep cool.Store in a well-ventilated place. Keep container tightly closed.Store locked up.Disposal:Dispose of contents/container in accordance with waste/disposal laws and regulations of your country or particular locality.Other Hazards:This product contains component(s) which have the following warnings; however based on the GHS classification criteria of your country or locale, the product mixture may be outside the respective category(s).Acute: Vapor harmful; may affect the brain or nervous system causing dizziness, headache or nausea. May cause central nervous system depression characterized by the following progressive steps: headache, dizziness, staggering gait, confusion, unconsciousness or coma. Possible irritation of the respiratory system can occur causing a variety of symptoms such as dryness of the throat, tightness of the chest, and shortness of breath. The silane material in this product can hydrolyze to form ethanol. Ethanol can cause moderate eye irritation, moderate skin irritation; and can be absorbed through the skin causing headache, nausea, and general discomfort. Ethanol is toxic by inhalation. If swallowed, the silane material in this product can hydrolyze in the stomach to form ethanol. Refer to the ethanol warnings on this (M)SDS. Contains methanol; may be harmful or fatal if swallowed; ingestion of methanol may cause blindness or permanent eye damage. Cannot be made non-poisonous. Dermal absorption possible.May cause mild skin irritation.Chronic: May cause kidney damage. May affect the gastrointestinal system. This material contains methanol.Ingestion of methanol may cause permanent eye damage or blindness. ACGIH considers Ethyl alcohol to be an A3 carcinogen (confirmed animal carcinogen with unknown relevance in humans).3. COMPOSITION/INFORMATION ON INGREDIENTSChemical Name CAS Number RangeMethanol67-56-185 - 90%Ethyl alcohol64-17-5 1 - 5%Organic phosphonium chloride salt PROPRIETARY 1 - 5%Any "PROPRIETARY" component(s) in the above table is considered trade secret, thus the specific chemical and its exact concentration is being withheld.4. FIRST AID MEASURESFIRST AID - EYE CONTACT: Flush eyes immediately with large amount of water for at least 15 minutes holding eyelids open while flushing. Get prompt medical attention.FIRST AID - SKIN CONTACT: Flush contaminated skin with large amounts of water while removing contaminated clothing. Wash affected skin areas with soap and water. Get medical attention if symptoms occur.FIRST AID - INHALATION: Move person to fresh air. Restore and support continued breathing. If breathing is difficult, give oxygen. Get immediate medical attention.FIRST AID - INGESTION: If swallowed, do not induce vomiting. Call a physician or poison control center immediately for further instructions. Never give anything by mouth if victim is rapidly losing consciousness, unconscious or convulsing.5. FIRE-FIGHTING MEASURESSUITABLE EXTINGUISHING MEDIA: Carbon Dioxide, Dry Chemical, Foam, Water FogUNSUITABLE EXTINGUISHING MEDIA: Not determined for this product.SPECIFIC HAZARDS POSSIBLY ARISING FROM THE CHEMICAL: Flammable liquid and vapor. Keep containers tightly closed. Isolate from heat, electrical equipment, sparks, open flame, and other sources of ignition. Closed containers may rupture when exposed to extreme heat. Use water spray to keep fire exposed containers cool. During a fire, irritating and/or toxic gases and particulate may be generated by thermal decomposition or combustion.SPECIAL PROTECTIVE EQUIPMENT AND PRECAUTIONS FOR FIRE-FIGHTERS: Wear full firefighting protective clothing, including self-contained breathing apparatus (SCBA). Water spray may be ineffective. If water is used, fog nozzles are preferable.6. ACCIDENTAL RELEASE MEASURESPERSONAL PRECAUTIONS, PROTECTIVE EQUIPMENT, AND EMERGENCY PROCEDURES: Remove all sources of ignition (flame, hot surfaces, and electrical, static or frictional sparks). Avoid breathing vapors. Use self-contained breathing equipment. Avoid contact.ENVIRONMENTAL PRECAUTIONS: Do not contaminate bodies of water, waterways, or ditches, with chemical or used container.METHODS AND MATERIALS FOR CONTAINMENT AND CLEANUP: Keep non-essential personnel a safe distance away from the spill area. Notify appropriate authorities if necessary. Contain and remove with inert absorbent material and non-sparking tools. Avoid contact. Before attempting cleanup, refer to hazard caution information in other sections of the SDS form.7. HANDLING AND STORAGEHANDLING: Keep closure tight and container upright to prevent leakage. Ground and bond containers when transferring material. Because empty containers may retain product residue and flammable vapors, keep away from heat, sparks and flame; do not cut, puncture or weld on or near the empty container. Do not smoke where this product is used or stored. Avoid breathing of vapor or spray mists. Use with adequate ventilation. Avoid skin and eye contact. Wash thoroughly after handling. Do not handle until all safety precautions have been read and understood. Empty containers should not be re-used. Cannot be made non-poisonous.STORAGE: Do not store or use near heat, sparks, or open flame. Store drum out of sun and away from heat. Store only in well-ventilated areas. Do not puncture, drag, or slide container. Keep container closed when not in use. Refer to OSHA29CFR Part 1910.106 "Flammable and Combustible Liquids" for specific storage requirements.INCOMPATIBILITY: Strong acids, bases, and strong oxidizers.8. EXPOSURE CONTROLS/PERSONAL PROTECTION COMPONENT EXPOSURE LIMITChemical Name ACGIH TLV-TWA ACGIH TLV-STELOSHA PEL-TWAOSHA PEL-CEILINGSkinMethanol200 ppm250 ppm260 mg/m3200 ppmN.E.SEthyl alcohol N.E.1,000 ppm1,900 mg/m31,000 ppmN.E.N.A.Organic phosphoniumchloride saltN.E.N.E.N.E. N.E.N.A.N.A. - Not Applicable, N.E. - Not Established, S - Skin DesignationEngineering controls: Sufficient ventilation in pattern and volume should be provided in order to maintain air contaminant levels below recommended exposure limits. Caution: Solvent vapors are heavier than air and collect in lower levels of the work area. Sufficient ventilation (using explosion-proof equipment) should be provided to prevent flammable vapor/air mixtures from accumulating.PERSONAL PROTECTION MEASURES/EQUIPMENT:RESPIRATORY PROTECTION: Use a NIOSH approved chemical/mechanical filter respirator designed toremove a combination of particulates and organic vapor if occupational limits are exceeded. For emergencysituations, confined space use, or other conditions where exposure limits may be greatly exceeded, use an approved air-supplied respirator. For respirator use observe OSHA regulations (29CFR 1910.134) or use in accordance with applicable laws and regulations of your country or particular locality.SKIN PROTECTION: Use neoprene, nitrile, or rubber gloves to prevent skin contact.EYE PROTECTION: Use safety eyewear including safety glasses with side shields and chemical goggles where splashing may occur.OTHER PROTECTIVE EQUIPMENT: Use disposable or impervious clothing if work clothing contamination is likely. Remove and wash contaminated clothing before reuse.HYGIENIC PRACTICES: Wash hands before eating, smoking, or using toilet facility. Do not smoke in anychemical handling or storage area. Food or beverages should not be consumed anywhere this product is handled or stored. Wash thoroughly after handling.9. PHYSICAL AND CHEMICAL PROPERTIESTypical values, not to be used for specification purposes.ODOR: Alcohol VAPOR PRESSURE: N.D.APPEARANCE: Clear VAPOR DENSITY: Heavier than Air PHYSICAL STATE: Liquid LOWER EXPLOSIVE LIMIT: 4.3 %(V)FLASH POINT: 43 °F, 6 °C SetaflashClosed CupUPPER EXPLOSIVE LIMIT: 36.5 %(V)BOILING RANGE: 65 - 100 °C EVAPORATION RATE: Faster than n-butyl-acetate.AUTOIGNITION TEMPERATURE:N.D.DENSITY: 0.82 g/cm3 - 6.85 lb/gal DECOMPOSITION TEMPERATURE:N.D. VISCOSITY, DYNAMIC: N.D.ODOR THRESHOLD: N.D.VISCOSITY, KINEMATIC: N.D.SOLUBILITY IN H2O: Insoluble VOLATILE BY WEIGHT: 94.65 %pH: N.A.VOLATILE BY VOLUME: 96.15 %FREEZE POINT: N.D. VOC CALCULATED: 6.33 lb/gal, 758 g/l COEFFICIENT OF WATER/OILDISTRIBUTION:N.D.LEGEND: N.A. - Not Applicable, N.E. - Not Established, N.D. - Not Determined10. STABILITY AND REACTIVITYHAZARDOUS POLYMERIZATION: Hazardous polymerization will not occur under normal conditions.STABILITY: Product is stable under normal storage conditions.CONDITIONS TO AVOID: High temperatures. Sources of ignition.INCOMPATIBILITY: Strong acids, bases, and strong oxidizers.HAZARDOUS DECOMPOSITION PRODUCTS: Oxides of nitrogen, Carbon monoxide, carbon dioxide, Oxides of silicon11. TOXICOLOGICAL INFORMATIONEXPOSURE PATH: Refer to section 2 of this SDS.SYMPTOMS:Refer to section 2 of this SDS.TOXICITY MEASURES:Chemical Name LD50/LC50Methanol Oral LD50: Rat6,200 mg/kgDermal LD50: Rabbit15,800 mg/kgInhalation LC50: Rat22500 ppm/8 hEthyl alcohol Oral LD50: Rat7,060 mg/kgInhalation LC50: Rat124.7 mg/l/4 hOrganic phosphonium chloride salt Oral LD50: Rat43 mg/kgGHS LC50 (vapour): Acute toxicity point estimate3 mg/l GHS LC50 (dustand mist): Acute toxicity point estimate0.05 mg/lGerm cell mutagenicity: No classification proposedCarcinogenicity: No classification proposedReproductive toxicity: Category 1A - May damage fertility or the unborn child.Components contributing to classification: Methanol.12. ECOLOGICAL INFORMATIONECOTOXICITY:Chemical Name EcotoxicityMethanol Fish: Pimephales promelas28,200 mg/l96 h flow-throughPimephales promelas> 100 mg/l96 h StaticOncorhynchus mykiss19,500 - 20,700 mg/l96 h flow-throughLepomis macrochirus13,500 - 17,600 mg/l96 h flow-throughEthyl alcohol Fish: Pimephales promelas> 100 mg/l96 h StaticPimephales promelas13,400 - 15,100 mg/l96 h flow-throughInvertebrates: Daphnia magna9,268 - 14,221 mg/l48 hDaphnia magna2 mg/l48 h StaticOrganic phosphonium chloride salt N.D.PERSISTENCE AND DEGRADABILITY:Not determined for this product.BIOACCUMULATIVE: Not determined for this product.MOBILITY IN SOIL: Not determined for this product.OTHER ADVERSE EFFECTS: Not determined for this product.13. DISPOSAL CONSIDERATIONSDISPOSAL METHOD: Disposal should be done in accordance with Federal (40CFR Part 261), state and local environmental control regulations. If waste is determined to be hazardous, use licensed hazardous waste transporter and disposal facility.14. TRANSPORT INFORMATIONUS DOT RoadDOT Proper Shipping Name: AdhesivesDOT Hazard Class: 3SECONDARY HAZARD: NoneDOT UN/NA Number: 1133Packing Group: IIEmergency Response Guide Number: 128IATA CargoPROPER SHIPPING NAME: AdhesivesDOT Hazard Class: 3HAZARD CLASS: NoneUN-NUMBER: 1133PACKING GROUP: IIEMS: 3LIMDGPROPER SHIPPING NAME: AdhesivesDOT Hazard Class: 3HAZARD CLASS: NoneUN-NUMBER: 1133PACKING GROUP: IIEMS: F-EThe listed transportation classification applies to non-bulk shipments. It does not address regulatory variations due to changes in package size, mode of shipment or other regulatory descriptors. For the most accurate shipping information, refer to your transportation/compliance department.15. REGULATORY INFORMATIONU.S. FEDERAL REGULATIONS: AS FOLLOWS:SARA SECTION 313This product contains the following substances subject to the reporting requirements of Section 313 of Title III of the Superfund Amendment and Reauthorization Act of 1986 and 40 CFR part 372.:Chemical Name CAS Number Weight % Less ThanMethanol67-56-190.0%TOXIC SUBSTANCES CONTROL ACT:INVENTORY STATUSThe chemical substances in this product are on the TSCA Section 8 Inventory.EXPORT NOTIFICATIONThis product contains the following chemical substances subject to the reporting requirements of TSCA 12(B) if exported from the United States:None16. OTHER INFORMATIONUnder HazCom 2012 it is optional to continue using the HMIS rating system. It is important to ensure employees have been trained to recognize the different numeric ratings associated with the HazCom 2012 and HMIS schemes.HMIS RATINGS - HEALTH: 2* FLAMMABILITY: 3 PHYSICAL HAZARD: 1* - Indicates a chronic hazard; see Section 2Revision: Section 2Effective Date: 08/07/2017DISCLAIMERThe information contained herein is, to the best of our knowledge and belief, accurate. However, since the conditions of handling and use are beyond our control, we make no guarantee of results, and assume no liability for damages incurred by use of this material. It is the responsibility of the user to comply with all applicable federal, state and local laws and regulations.。
Perkadox 26 商品数据单(Dimyristyl peroxydicarbonate)说明书

Product Data SheetPerkadox 26Dimyristyl peroxydicarbonatePerkadox® 26 is an initiator for (co)polymerization of (meth)acrylates.CAS number53220-22-7EINECS/ELINCS No.258-436-4TSCA statuslisted on inventoryMolecular weight514.8SpecificationsAppearance White flakesAssay≥96.0 %Inorganic + organic hydrolysable chloride≤ 100 mg/kgCharacteristicsBulk density480 kg/m³Melting point42 °CApplicationsPerkadox® 26 can be used for the market segments: polymer production, thermoset composites and acrylics production with their different applications/functions. For more information please check our website and/or contact us.Thermal stabilityOrganic peroxides are thermally unstable substances, which may undergo self-accelerating decomposition. The lowest temperature at which self-accelerating decomposition of a substance in the original packaging may occur is the Self-Accelerating Decomposition Temperature (SADT). The SADT is determined on the basis of the Heat Accumulation Storage Test.SADT35°C (95°C)Emergency temperature (Tₑ)25°C (77°F)Control temperature (Tc)20°C (68°F)Method The Heat Accumulation Storage Test is a recognized test method for the determinationof the SADT of organic peroxides (see Recommendations on the Transport ofDangerous Goods, Manual of Tests and Criteria - United Nations, New York andGeneva).StorageDue to the relatively unstable nature of organic peroxides a loss of quality can be detected over a period of time. To minimize the loss of quality, Nouryon recommends a maximum storage temperatureTs max.15°C (59°F)Note When stored under these recommended storage conditions, Perkadox® 26 will remainwithin the Nouryon specifications for a period of at least 3 months after delivery.Packaging and transportThe standard packaging is a cardboard box for 25 kg or 4 x 5 kg peroxide. Both packaging and transport meet the international regulations. For the availability of other packed quantities contact your Nouryon representative. Perkadox® 26 is classified as Organic peroxide type D; solid, temperature controlled, Division 5. 2; UN 3116.Safety and handlingKeep containers tightly closed. Store and handle Perkadox® 26 in a dry well-ventilated place away from sources of heat or ignition and direct sunlight. Never weigh out in the storage room. Avoid contact with reducing agents (e. g. amines), acids, alkalis and heavy metal compounds (e. g. accelerators, driers and metal soaps). Dissolving Perkadox® 26 in reactive monomer is not recommended. Please refer to the Safety Data Sheet (SDS) for further information on the safe storage, use and handling of Perkadox® 26. This information should be thoroughly reviewed prior to acceptance of this product. The SDS is available at /sds-search.Major decomposition productsCarbon dioxide, TetradecanolAll information concerning this product and/or suggestions for handling and use contained herein are offered in good faith and are believed to be reliable. Nouryon, however, makes no warranty as to accuracy and/or sufficiency of such information and/or suggestions, as to the product's merchantability or fitness for any particular purpose, or that any suggested use will not infringe any patent. Nouryon does not accept any liability whatsoever arising out of the use of or reliance on this information, or out of the use or the performance of the product. Nothing contained herein shall be construed as granting or extending any license under any patent.Customer must determine for himself, by preliminary tests or otherwise, the suitability of this product for his purposes. The information contained herein supersedes all previously issued information on the subject matter covered. The customer may forward, distribute, and/or photocopy this document only if unaltered and complete, including all of its headers and footers, and should refrain from any unauthorized use. Don’t copy this document to a website.Perkadox®, Trigonox and Laurox are registered trademarks of Nouryon Functional Chemicals B.V. or affiliates in one or more territories.Contact UsPolymer Specialties Americas************************Polymer Specialties Europe, Middle East, India and Africa*************************Polymer Specialties Asia Pacific************************2023-4-25© 2023Acrylics production Perkadox 26。
化妆品安全技术规范2015版

化妆品安全技术规范(报送稿)目录正文 (1)1 范围 (1)2术语和释义 (1)3 化妆品安全通用要求 (2)附录一、化妆品禁用组分 (4)表1 化妆品禁用组分(1)(2) (4)表2 化妆品禁用植(动)物组分(1)(2)(3) (95)附录二、化妆品限用组分 (102)表3 限用防腐剂(1) (102)表4 限用防晒剂(1) (109)表5 限用着色剂(1) (113)表6 限用染发剂(1)(3) (4) (136)表7 其他限用组分 (144)附录三、化妆品检测和评价方法 (157)一、理化检验方法 (157)(一)总则 (157)(二)禁用组分 (160)第1节 4-氨基偶氮苯和联苯胺 (160)第2节 4-氨基联苯及其盐 (164)第3节 8-甲氧基补骨脂素等4种物质 (168)第4节α-氯甲苯 (172)第5节氨基己酸 (176)第6节斑蝥素 (179)第7节苯并[а]芘 (181)第8节丙烯酰胺 (184)第9节补骨脂素等4种物质 (188)第10节氮芥 (191)第11节二噁烷 (193)第12节镉 (196)第13节汞 (199)第14节环氧乙烷和甲基环氧乙烷 (204)第15节甲醇 (208)第16节马来酸二乙酯 (211)第17节米诺地尔 (215)第18节铅 (219)第19节氢醌、苯酚 (222)第20节砷 (228)第21节石棉 (234)第22节维甲酸和异维甲酸 (241)第23节维生素D2和维生素D3 (246)(三)限用组分 (249)第1节 6-甲基香豆素 (249)第2节α-羟基酸 (256)第3节二硫化硒 (262)第4节过氧化氢 (265)第5节间苯二酚 (268)第6节可溶性锌盐 (270)第7节奎宁 (272)第8节硼酸和硼酸盐 (274)第9节羟基喹啉 (277)第10节巯基乙酸 (279)第11节水杨酸 (285)第12节酮麝香 (288)第13节游离氢氧化物 (290)第14节总硒 (292)(四)防腐剂 (295)第1节苯甲醇 (295)第2节苯甲酸及其钠盐 (302)第3节苯氧异丙醇 (309)第4节苯扎氯铵 (312)第5节苄索氯铵、劳拉氯铵和西他氯铵 (314)第6节甲醛 (316)第7节甲基氯异噻唑啉酮等12种物质 (322)第8节氯苯甘醚 (325)第9节三氯卡班 (327)第10节山梨酸和脱氢乙酸 (329)(五)防晒剂 (331)第1节苯基苯并咪唑磺酸等15种物质 (331)第2节二苯酮-2 (337)第3节二氧化钛 (340)第4节二乙氨羟苯甲酰基苯甲酸己酯 (342)第5节二乙基己基丁酰胺基三嗪酮 (344)第6节亚苄基樟脑磺酸 (346)第7节氧化锌 (349)(六)着色剂 (351)第1节酸性黄36等5种物质 (351)第2节酸性紫43等7种物质 (357)第3节着色剂CI 16185等10种物质 (360)(七)染发剂 (366)第1节对苯二胺等8种物质 (366)第2节对苯二胺等32种物质 (369)(八)去屑剂 (374)第1节水杨酸等5种物质 (374)(九)抗感染药物 (377)第1节氟康唑等9种物质 (377)第2节盐酸美满霉素等7种物质 (382)第3节依诺沙星等10种物质 (384)(十)激素 (388)第1节雌三醇等7种物质 (388)第2节氢化可的松等7种物质 (397)(十一)有机溶剂 (401)第1节二氯甲烷等15种物质 (401)(十二)其他 (406)第1节二甘醇 (406)第2节化妆品抗UVA能力仪器测定法 (410)第3节邻苯二甲酸二甲酯等10种物质 (412)第4节邻苯二甲酸二丁酯等8种物质 (417)第5节钕等15种元素 (422)第6节 pH值 (425)第7节乙醇胺等5种物质 (427)二、微生物检验方法 (433)(一)总则 (433)(二)菌落总数 (435)(三)耐热大肠菌群 (438)(四)铜绿假单胞菌 (441)(五)金黄色葡萄球菌 (444)(六)霉菌和酵母菌 (447)三、毒理学试验方法 (449)(一)总则 (449)(二)急性经口毒性试验 (450)(三)急性经皮毒性试验 (452)(四)皮肤刺激性/腐蚀性试验 (454)(五)急性眼刺激性/腐蚀性试验 (457)(六)皮肤变态反应试验 (460)(七)皮肤光毒性试验 (464)(八)鼠伤寒沙门氏菌/回复突变试验 (467)(九)体外哺乳动物细胞染色体畸变试验 (474)(十)体外哺乳动物细胞基因突变试验 (477)(十一)哺乳动物骨髓细胞染色体畸变试验 (480)(十二)体内哺乳动物细胞微核试验 (483)(十三)睾丸生殖细胞染色体畸变试验 (486)(十四)亚慢性经口毒性试验 (489)(十五)亚慢性经皮毒性试验 (492)(十六)致畸试验 (495)(十七)慢性毒性/致癌性结合试验 (498)四、人体安全性检验方法 (503)(一)总则 (503)(二)人体皮肤斑贴试验 (504)(三)人体试用试验安全性评价 (507)五、人体功效评价检验方法 (509)(一)总则 (509)(二)防晒化妆品防晒指数(SPF值)测定方法 (510)(三)防晒化妆品防水性能测试方法 (517)(四)防晒化妆品长波紫外线防护指数(PFA值)测定方法 (519)正文1 范围本规范规定了化妆品的安全技术要求,包括通用要求、禁限用组分要求以及检验评价方法等。
Agilent 8890 5977C Series gas chromatograph mass s

Agilent 5977C GC/MSD SystemThe Agilent 8890/5977C Series gas chromatograph/mass selective detector (GC/MSD) builds on a tradition of leadership in GC and MS technology, with the world’s most competitive performance and productivity features.Agilent GC/MSD system featuresAgilent 5977C GC/MSD — the most sensitive and robust MSD provides:–Four EI source options including the revolutionary high-efficiency source (HES), which offers the industry’s lowest instrument detection limit (IDL) and bestcarrier gas applications.signal-to-noise ratio (S/N) and a HydroInert source for H2– A heated monolithic quartz gold quadrupole (heatable up to 200 °C) for rapid elimination of contamination to keep the analyzer clean.– A second-generation triple-axis detector (TAD) for eliminating neutral noise.–Scan speeds up to 20,000 u/sec (extractor ion source and HES).–An optional oil-free IDP-3 roughing pump: a cleaner, quieter, and greener alternative (for use with turbo molecular pump systems).10-Year value promiseSupport is guaranteed for 10 years from the date of purchase, or Agilent will provide credit for the residual value of the system toward a model upgrade.Installation checkout specifications Agilent verifies GC/MSD system performance at the customer site.IDL is a statistically based metricthat more accurately confirms system performance than an S/N measurement. Test specificationsare based on splitless injection intoan Agilent J&W HP-5ms Ultra Inert30 m × 0.25 mm, 0.25 μm column for helium and a 20 m × 0.18 mm, 0.18 μm column for HydroInert with hydrogen. IDL analyses use lab helium (hydrogen for HydroInert) with GC gas filters installed. See more about the IDL test at /Library/ technicaloverviews/Public/5990-8341EN.pdf* IDL was statistically derived at 99% confidence level from the area precision of eight sequential splitless injections of OFN (octafluoronaphthalene). Demonstration of IDL specifications require a compatible system configuration, including a liquid autosampler with a 5 μL syringe.–HES IDL was measured using 10 fg injection, 1 µL injection.–Other IDLs were measured using 100 fg, 1 µL injection.–A 30 m column was used for helium IDL checkout; a 20 m column was used for hydrogenIDL checkout.–Helium carrier gas for Installation Specifications of the HES, Extractor, and Stainless steel sources; hydrogen carrier gas for Installation Specification of the HydroInert source only.–Reference IDL specifications from the above table will be confirmed only when purchased as an additional service with a compatible new system (GC and MS) installation.Signal-to-noise (S/N) specificationsa S/N checkout is performed only if there is no compatible autosampler (which is required for IDL checkout). Helium carrier gas, manual injection using a 30 m × 0.25 mm,0.25 µm column and in scan mode. Hydrogen carrier gas, manual injection using 20 m × 0.18 mm, 0.18 µm column and in scan mode. When the autosampler (ALS) is present, these specifications are a reference of the performance. Reference S/N specifications from the above table will not be confirmed at installation or introduction for ALS equipped systems.b Standard scanning from 50 to 300 u at nominal 272.0 u ion.c 1 μL injection of 100 pg/μL benzophenone (BZP) standard, 80 to 230 u scan at nominal 183 u ion, using methane reagent gas.d 2 μL injection of 100 fg/μL OFN standard scanning from 50 to 300 u at nominal 272 u ion, using methane reagent gas.2a Only applicable with optional Accurate Mass software package. Scan mode only. Not verified during installation.b As scan rate increases, sensitivity will decrease, and resolution may degrade.c A high flow rate into a fixed ion source will cause a loss in sensitivity.d The heated quadrupole mass filter should not require maintenance, but if maintenance is required, it should be performed by an Agilent service engineer.34aInlet temperature should be cool enough to touch when performing maintenance.bA micro ion gauge is shipped standard for the CI system, and is available optionally for EI systems.DE67854286This information is subject to change without notice.© Agilent Technologies, Inc. 2022Printed in the USA, May 26, 20225994-4846EN。
挥发性风味成分保留指数LRI-20M

Odorant MW CAS No. Structure Odorpentane 72.1 109-66-0 alkanepropanal58.09 123-38-6solvent, pungenthexane 86.1 110-54-3 alkanetrimethylamine59.11 75-50-3fishmethanethiol48.10 74-93-1sulfur, gasoline, garlicheptane 100.1 142-82-5 alkaneethanal44.0 75-07-0pungent, etherdimethyl sulfide62.0 75-18-3cabbage, sulfur, gasolineoctane 114.1 111-65-9 alkane2-methylpropanal 72.1 78-84-2pungent, malt, greenbutanal72.1 123-72-8pungent, greenethyl formate74.0 109-94-4pungentdiethyl acetal118.17 105-57-7fruit, creamnonane 128.2 111-84-2 alkaneethyl acetate88.1 141-78-6pineapple3-methylbutanal 86.1 590-86-3malt2-methylbutanal 86.1 96-17-3cocoa, almondmethyl methylpropanoate102.1 547-63-7flowerethanol46.0 64-17-5sweetpentanal86.1 110-62-3almond, malt, pungentmethyl ethyl ketone72.11 78-93-3etherethyl propionate102.1 105-37-3fruitethyl isobutyrate116.16 97-62-1sweet, rubbermethyl ethyl sulfide76.16 624-89-5sulfur, garlicmethylbutenol86.1 115-18-4herb2,3-butanedione 86.0 431-03-8butterpentenone84.1 1629-58-9fish, pungent2-pentanone86.1 107-87-9ether, fruit3-pentanone86.1 96-22-0ethermethyl butanoate102.1 623-42-7ether, fruit, sweetthiophene84.14 110-02-1garlicdecane 142.2 124-18-5 alkane2-methylpentan-3-one 100.1 565-69-5mintpyrrolidine71.22 123-75-1alkalinemethyl 2-methylbutanoate 116.1 868-57-5apple2-methylpropyl acetate 116.16 110-19-0fruit, apple, bananaα-thujene136.23 2867-05-2wood, green, herbmethyl 3-methylbutanoate 116.1 556-24-1applebutanol74.1 78-92-2winemercaptoacetaldehyde76.11 4124-63-4cabbageethyl butyrate116.1 105-54-4appleα-pinene136.1 80-56-8pine, turpentinemethyl thiocyanate73.11 556-64-9sulfurpropanol60.1 71-23-8alcohol, pungenttoluene92.1 108-88-3paint(S)-(+)-ethyl-2-methylbutanoate 130.18 10307-61-6apple(E)-2-butenal 70.09 123-73-9flowerethyl methylbutyrate130.1 7452-79-1apple2,3-pentadione100.1 600-14-6cream, butterhexanone130.19 108-64-5ether, grapealpha,γ-dimethylallyl alcohol86.13 1569-50-2green, vinylethyl 3-methylbutanoate130.19 108-64-5fruitdimethyl disulfide94.0 624-92-0onion, cabbage, putridbutyl acetate116.1 123-86-4pearcamphene136.1 79-92-5camphor2-methylthiophene98.0 554-14-3sulfurhexanal100.1 66-25-1grass, tallow, fatpentanoic acid102.1 109-52-4sweatmethylbutenthiol102.20 5287-45-6amine, smokeisobutanol74.1 78-83-1wine, solvent, bitterundecane 156.2 1120-21-4 alkanemethyl-2-butenal84.12 1115-11-3green, fruithexenone98.1 1629-60-3cooked vegetable, metal3-pentanol96.5 584-02-1fruitfucoserratene108.18 33580-05-1green, plastic2-methyl-1-butanol88.50 1565-80-6malt(Z)-2-penten-1-ol86.13 1576-95-0green, plastic, rubberepoxylinalool170.25 14049-11-7flowermethylpentanol102.6 590-36-3pungent2-methylbutyl acetate130.18 624-41-9fruitβ-pinene136.1 127-91-3pine, resin, turpentineisoamyl acetate130.1 123-92-2banana2-pentanol88.1 6032-29-7greensabinene136.24 3387-41-5pepper, turpentine, woodmethyl-2-butenol86.1 556-82-1herb(E)-2-pentenal 84.1 1576-87-0strawberry, fruit, tomatoethyl pentanoate 130.1 539-82-2yeast, fruit4-methyl-3-penten-2-one98.14 141-79-7sweet, chemical2-hexanone100.1 591-78-6ether1-butanol74.1 71-36-3medicine, fruitmyrcene136.24 123-35-3balsamic, must, spice(Z)-3-hexenal 98.1 6789-80-6leaf, greenδ-3-carene136.1 13466-78-9lemon, resinthiophane88.17 110-01-0cabbagem-xylene106.17 108-38-3plastic3-hydroxy-2-pentanone 102.5 3142-66-3herb, truffle3-mercapto-2-butanone 104.17 40789-98-8onionpentenol86.1 616-25-1butter, pungentpropyl propanoate116.1 106-36-5pineapple8-nonen-2-one 140.22 5009-32-5bakedα-phellandrene136.1 99-83-2turpentine, mint, spice2-hydroxy-3-pentanone 102.5 5704-20-1truffle, earth, nutazine,pyridine 79.10 110-86-1rancid2-heptanone114.1 110-43-0soapheptanal114.1 111-71-7fat, citrus, rancid2-methylpyrazine 94.1 109-08-0popcornlimonene136.1 138-86-3lemon, orangeα-terpinene136.1 99-86-5lemon3-ethoxy-1-propanol 104.1 111-35-3fruitethyl isohexanoate144.21 25415-67-2fruito-xylene106.17 95-47-6geranium1,4-cineole154.25 470-67-7spicemethyl hexanoate130.1 106-70-7fruit, fresh, sweet1-(methylthio)ethanethiol108.23 31331-53-0thiamin(E)-2-hexenal98.14 85761-70-2green, leaf2-propyl butanoate 130.1 638-11-9pungent, fruit2,3-dehydro-1,8-cineole152.23 92760-25-3mint, lemon2-hydroxymethylfuran 98.0 98-00-0burntdodecane 170.2 112-40-3 alkane(+)-limonene,136.24 5989-27-5citrus, mint2-hexenal98.14 505-57-7fat, rancid3-methyl-1-butanol88.1 123-51-3whiskey, malt, burnt2-methyl-1-butanol88.15 137-32-6wine, onionβ-phellandrene136.1 555-10-2mint, terpentine1,8-cineole154.25 470-82-6mint, sweet3-methyl-2-butanone 86.1 563-80-4camphor(E)-2-hexenal 98.1 6728-26-3apple, greenethyl hexanoate144.1 123-66-0apple peel, fruit1-hepten-3-one 112.2 2918-13-0metal4-methoxy-2-methyl-2-butanethiol 134.24 94087-83-9cat, black currant3-heptanol116.20 589-82-2herb(Z)-ocimene 136.1 27400-71-1herb2,4-dimethylthiazole 113.2 541-58-2rubber, mold(Z)-2-hexenol 100.6 928-94-9leaf, green, wine, fruitpropyl butyrate130.1 105-66-8pineapple, solvent(Z)-4-heptenal 112.1 6728-31-0biscuit, cream(Z)-6-decenal 154.25 105683-99-6tallow, green2-methylpyridine 93.13 109-06-8sweatallyl isothiocyanate, allyspol99.16 57-06-7sulfur, pungent, garlic3-mercapto-2-pentanone 118.2 67633-97-0sulfur, onion, meatγ-terpinene136.1 99-85-4gasoline, turpentinedimethyl pyrazine108.14 5910-89-4nut, peanut butter, cocoa, meat2-pentylfuran138.21 3777-69-3green bean, butterstyrene104.15 100-42-5balsamic, gasolinemethyl-2-(methylthio)acetate120.17 16630-66-3cooked potato, roasted nut(E)-β-ocimene136.1 3779-61-1sweet, herb(E)-2-heptenal 112.1 18829-55-5soap, fat, almond3-octanone128.1 106-68-3herb, butter, resin(Z)-ocimene,136.23 3338-55-4citrus, herb, flower2-mercapto-3-pentanone 118.2 17042-24-9roasted meatbornyl methyl ether168.28 10395-54-7earth, mustdimethyl pyrazine108.14 123-32-0cocoa, roasted nut, roast beef, medicine1-pentanol88.1 71-41-0balsamic2,4-hexadienal96.1 80466-34-8green4-methylhexanol116.20 818-49-5sweatp-cymene134.22 99-87-6solvent, gasoline, citrus1-cyclohexen-3-one96.13 930-68-7pesticideethyl mercaptopropionate134.2 19788-49-9sulfurhexyl acetate144.21 142-92-7fruit, herbdimethyl sulfone90.13 67-71-0sulfur, burnthexanethiol118.1 111-31-9sulfur2-heptanol116.1 543-49-7mushroomdimethylthiazole113.18 3581-91-7roast, smokedimethylfuranthiol128.2 55764-23-3meatoctanal128.0 124-13-0fat, soap, lemon, green(Z)-1,5-octadien-3-one 124.1 65767-22-8geranium, metalmethyldihydrofuranthiol116.18 26486-13-5meatδ-terpinene136.1 586-62-9pine, plastic2-octanone128.1 111-13-7soap, gasoline(Z)-3-octen-2-one 126.20 51193-77-2nut2-ethylpyridine107.15 100-71-0grass3-hydroxy-2-butanone 88.1 513-86-0butter, cream5-methyl-(E)-2-hepten-4-one 126.20 102322-83-8hazelnut, nut2-pentylpyridine149.24 2294-76-0fat(+)-α-phellandrene136.24 2243-33-6dillmethyl-p-xylene120.19 95-63-6plasticbutyl isothiocyanate115.20 592-82-5sulfur, pungent, greenperillen150.22 539-52-6wood2-hepten-1-al112.17 2463-63-0greentridecane 184.2 629-50-5 alkane2-methyl-3-furanthiol 114.2 28588-74-1meatdimethyl pyrazine108.14 108-50-9roasted nut, cocoa, roast beef1-octen-3-one 126.1 4312-99-6mushroom, metal(E)-2-methyl-3-tetrahydrofuranthiol 118.20 26548-78-7onion3-thienyl mercaptan 116.21 7774-73-4cooked meat4-methylthio-2-butanone118.2 34047-39-7fruit2-acetyl-1-pyrroline 111.14 85213-22-5nut, roast(Z)-2-octenal 126.1 20664-46-4green leaf, walnut(E)-2-penten-1-ol86.13 1576-96-1mushroom(Z)-3-hexenyl acetate 142.1 3681-71-8green, banana2-octanol130.1 123-96-6mushroom, fat2-acetyl-1,4,5,6-tetrahydropyridine125.17 25343-57-1caramel6-methyl-5-hepten-2-one 126.1 110-93-0pepper, mushroom, rubber1,5-octadien-3-ol126.20 83861-74-9earth, herb(-)-cis-rose oxide154.25 3033-23-6sweet, rose(+)-cis-rose oxide154.25 876-17-5green, flower(E)-1,5-octadien-3-one 260.40 359794-78-8earth, mustethylmethyl pyrazine122.1 13360-64-0fruit, sweet(E)-2-octenal 126.1 2548-87-0green, nut, fat(Z)-3-nonenal 142.9 31823-43-5cucumber2-methyl-dithiacyclopentane 120.0 5616-51-3sulfur2-methyl anisole122.16 578-58-5warm, flower, walnut(E)-3-nonenal 140.22 109351-28-2fatβ-carene136.23 554-60-9orange peel2-ethyl pyrazine108.14 13925-00-3peanut butter, woodethyl lactate118.1 97-64-3fruit1-hexanol102.1 111-27-3resin, flower, green2-propionylpyrrole123.15 1073-26-3roast, popcorn2,3,4,5-tetrahydroanisole112.17 931-57-7herb, spice5-(methylthio)-valeronitrile129.22 59121-25-4broccoli, cabbage3-mercapto-4-methyl-2-pentanone 132.22 75832-79-0black curranttrimethylthiazole127.0 13623-11-5earth(E,Z)-2,4-heptadienal 110.16 4313-02-4fried(E)-rose oxide154.25 5258-11-7flowerp-menthatriene134.1 18368-95-1turpentine(E)-2-hexen-1-ol 100.1 928-95-0green, leaf, walnutdimethyl trisulfide126.0 3658-80-8sulfur, fish, cabbagemethylethylpyrazine122.17 33504-66-4sweatnonanal142.1 124-19-6fat, citrus, green1-nitro-2-phenylethane 151.16 6125-24-2flower, spicebenzylmethyl ether122.1 538-86-3metal(3E)-3-hexen-1-ol 100.16 928-97-2moss, freshethyl 4-hydroxybutanoate 132.16 999-10-0caramel3-octanol130.23 589-98-0moss, nut, mushroomisopropyl hexanoate158.1 2311-46-8fresh3-octen-2-one126.20 1669-44-9nut, crushed bug2-nonanone142.1 821-55-6hot milk, soap, greenmethyl octanoate158.1 111-11-5orange2-formylthiophene112.0 98-03-3sulfur(Z)-3-hexen-1-ol 100.1 928-96-1grass2-isobutyl thiazole141.24 18640-74-9tomato leaf, green4-mercapto-4-methyl-2-pentanone132.22 19872-52-7box treebutyl methylbutyrate158.24 15706-73-7fruit, cocoa2-acetylpyrrole109.13 1072-83-9nut, walnut, bread1-octen-3-ol 128.1 3391-86-4mushroomtrimethyl-pyrazine122.1 14667-55-1roast, potato, must2-methyl-3-ethylpyrazine 122.1 15707-23-0roast2-methyl-3-thiophenethiol 130.23 2527-76-6medicine5-isopropyl-2-methylpyrazine136.19 13925-05-8sweattetradecane 198.2 629-59-4 alkane(E,E)-2,4-heptadienal 110.1 4313-03-5nut, fat2,3-dimethyl-6-ethylpyrazine 136.1 15707-34-3burnt, popcorn2,6-dimethyl-5-heptenal 140.1 106-72-9fruit, green, melonα-ocimene136.23 502-99-8fruit, wet cloth1-nonen-3-one 140.22 24415-26-7pungent, mushroom2-propionyl-1-pyrroline 125.17 133447-37-7roast2-octenal126.20 2363-89-5greenartemisia ketone152.23 546-49-6green, herb2-methoxy-3,6-dimethylpyrazine138.17 19846-22-1earthα-p-dimethylstyrene132.20 1195-32-0citrus, pine(Z)-linalool oxide170.25 5989-33-3flowercis-sabinene hydrate154.25 15537-55-0balsamiclinalool oxide170.25 1365-19-1flower, woodisopropylmethoxypyrazine152.1 25773-40-4pea, earthlimonene oxide152.1 1195-92-2fruitthenylthiol130.23 6258-63-5sulfurethyl cyclohexanoate156.22 3289-28-9fruitfufuryl mercaptan114.0 98-02-2coffee, roastp, a -dimethylstyrol132.20 27576-03-0gasolineartemisia alcohol154.25 29887-38-5herb3,5-octadien-2-one 124.18 38284-27-4fruit, fat, mushroom2,5-dimethyl-3-ethylpyrazine136.1 13360-65-1potato, roast5-ethyl-2,4-dimethylthiazole 141.1 38205-61-7earthdurene134.22 95-93-2rancid, sweetethyl octanoate172.1 106-32-1fruit, fat4-methyl-3-thiazoline 101.2 52558-99-3garlicdihydromyrcenol156.27 18479-58-8tart lime, citrus, cologne2-pentylthiophene154.27 4861-58-9sweet, fruitpentyl butanoate158.1 540-18-1banana(Z,Z)-3,6-nonadienal 138.9 21944-83-2fat, soappropyl hexanoate158.24 626-77-7fruitethyl heptylate158.24 106-30-9fruit2-ethyl-3,5-dimethylpyrazine 136.20 13925-07-0potatodihydrolinalool156.27 18479-49-7wood, citrus, camphor(E)-linalool oxide170.25 34995-77-2floweracetic acid60.0 64-19-7sourfurfural96.0 98-01-1bread, almond, sweetdimethylethyl pyrazine136.20 13925-07-0roastmethional104.0 3268-49-3cooked potatotrans-sabinene hydrate154.25 17699-16-0wood, balsamicα-cubebene204.35 17699-14-8herb, wax(methylbutenyl)-methylfuran150.22 15186-51-3mintmethyl cyclohexanecarboxylate142.20 4630-82-4fruit, esterheptanol116.1 111-70-6chemical, green(Z)-limonene oxide152.23 13837-75-7fresh, citrus4-ethyl-6-hepten-3-one140.22 131671-56-2fish(E)-p-mentha-2,8-dien-1-ol152.23 7212-40-0fresh, mint2-pinen-5-ol 152.23 168564-54-3must, dust6(10)-dihydromyrcenol156.27 18479-58-8tart lime, citrus, colognementhone154.25 89-80-5fresh, greenphenol94.11 108-95-2phenolnerol oxide152.23 1786-08-9oil, flower(E)-limonene oxide152.23 4959-35-7fresh, citrus4-mercapto-4-methyl-2-pentanol134.24 31539-84-1flower, lemondecanal156.2 112-31-2soap, orange peel, tallow2-ethyl-1-hexanol 130.2 104-76-7rose, greenpentyl methylbutyrate172.27 68039-26-9applecitronellal154.25 106-23-0fatα-copaene204.34 3856-25-5wood, spice(+)-(E)-limonene oxide152.23 6909-30-4greenacetylfuran110.0 1192-62-7balsamicmenthone154.25 10458-14-7mintcamphor152.1 76-22-2camphorbenzaldehyde106.0 100-52-7almond, burnt sugaroctyl acetate172.1 2051-50-5fruitbutylmethoxypyrazine166.22 24168-70-5carrot, earthpentadecane 212.3 629-62-9 alkane2-nonenal140.22 2463-53-8papernonanol144.25 143-08-8fat, greenmethyldihydrothiophenone116.18 13679-85-1cabbage, onion, mustisoborneol154.25 124-76-5must, camphordiethylmethylpyrazine150.22 18138-05-1baked4-pentenyl isothiocyanate127.21 18060-79-2mustard, horseradishisobutylmethoxypyrazine166.1 24683-00-9earth, spice, green pepper(Z)-2-nonenal 140.22 60784-31-8orris, fat, cucumberbenzyl acetate150.1 140-11-4fresh, boiled vegetablecis-isocitral153.0 72203-97-5greenmethylcyclopentapyrazine132.16 65128-99-6roastcamphene hydrate154.25 465-31-6camphor2-methyl-3-(methyldithio) furan 160.26 65505-17-1thiamin, meat3-mercapto-3-methylbutyl formate 148.22 50746-10-6cat, roastpropanoic acid74.08 79-09-4pungent, rancid, soyethyl 3-hydroxybutanoate132.1 5405-41-4marshmallow1-octen-3-hydroperoxide 144.8 72755-76-1metal, mushroom(epoxymethylbutyl)-methylfuran166.22 92356-06-4green, earth, citrus(Z)-3-hexenyl butanoate170.25 16491-36-4wine, green5-methyl-2-furfurylthiol 128.19 59303-05-8sulfur, roast(E)-2-nonenal140.22 18829-56-6cucumber, fat, greendill ether151.1 74410-10-9dill(E)-3,7-dimethyl-3,6-octadienal 152.24 72203-98-6greenhexyl butanoate172.1 2639-63-6apple peeldiethylmethyl pyrazine150.1 18138-04-0potato, meat, roast2-nonanol144.25 628-99-9cucumberlinalool154.25 78-70-6flower, lavenderp-2-menthen-1-ol154.25 619-62-5herb8-p-menthen-2-ol 154.25 619-01-2mint, spice4-acetyl-1-methyl-1-cyclohexene 138.1 70286-20-3spice(Z)-3-hexenyl-2-methylbutanoate184.28 53398-85-9herb, sweetbutyl hexanoate172.1 626-82-4fruit(Z)-dihydrocarvone152.23 3792-53-8herb, warm2-undecanone170.2 112-12-9orange, fresh, green(E,Z)-2,4-nonadienal138.21 21661-99-4geranium, pungent(Z)-4-decenal154.25 21662-09-9green, mustcarveol152.23 99-48-9fresh, spearmint, carawayβ-cubebene204.35 13744-15-5citrus, fruit2,4-nonadienal138.1 6750-03-4watermelonethyl octenoate171.0 2351-90-8must, oil, fruit, pungent(E)-dihydrocarvone152.23 5948-04-9warm, herb1-octanol130.1 111-87-5chemical, metal, burntethenyl-dimethylpyrazine205.2 157615-33-3earthα-fenchyl alcohol154.25 14575-74-7camphor(E)-6-decenal154.25 147159-48-6cucumber5-methylfurfural110.0 620-02-0almond, caramel, burnt sugardihydroterpinyl acetate306.40 58985-18-5pine, citrusisodihydrocarveol154.25 18675-35-9wood, spiceisobutyric acid88.1 79-31-2rancid, butter, cheesemethyl geranate182.26 1189-09-9flower, green, fruitlinalyl acetate196.29 115-95-7sweet, fruit2-methyl-2-(methyldithio)propanal150.3 67952-60-7smoke, fatlinalyl formate182.26 115-99-1citrus, corianderisocaryophyllene204.36 118-65-0woodmethyl nonanoate172.1 1731-84-6coconutepoxy-p-menthene152.23 13955-48-1mint, dillfenchyl alcohol154.25 1632-73-1camphor2,6-nonadienal138.21 557-48-2cucumber, wax, greendiethyl malonate160.1 105-53-3applesyntexan, dimethyl sulfoxide 78.13 67-68-5garlicdimethylmethoxyfuranone142.15 4077-47-8caramel, sweet, mildewdimethyl tetrasulfide157.9 5756-24-1cabbage, sulfur2,3-butanediol90.1 513-85-9fruit, onion2-acetylpyridine121.1 1122-62-9popcornisopulegyl acetate196.26 89-49-6mint, leafβ-bourbonene5208-59-3herbnonyl acetate186.29 143-13-5sweet, fruitethenyl-ethylmethylpyrazine148.21 181589-32-2earthmethylisoborneol168.28 2371-42-8earth, mustepoxy-2-octenal140.18 134452-45-2metalhexyl methylbutyrate186.2 10032-15-2strawberry(R)-linden ether150.2 125811-37-2mintbenzothiazole135.0 95-16-9gasoline, rubber3-terpinen-1-ol154.25 586-82-3must2-decenal154.25 3913-81-3tallow1-terpinen-4-ol154.25 562-74-3turpentine, nutmeg, mustphenyl cyanide103.12 100-47-0rancidmethyl decanoate186.2 110-42-9winepiperitone152.23 89-81-6mint, freshβ-caryophyllene204.35 87-44-5wood, spiceβ-elemene204.35 33880-83-0herb, wax, freshmyrtenal150.1 23727-16-4spiceisobornyl formate182.26 1200-67-5green, earth, camphordihydromethylcyclopentapyrazine134.18 23747-48-0roast, nutchavicol134.18 501-92-8medicine, phenolβ-cyclocitral152.1 432-25-7mintp-menthenethiol170.32 71159-90-5grapefruitmethyl benzoate136.1 93-58-3prune, lettuce, herb, sweetethyl phenylacetate164.20 101-97-3fruit, sweethydrocinnamic alcohol136.19 122-97-4cinnamon, anise, fruitaromadendrene204.35 489-39-4woodhexadecane 226.3 544-76-3 alkane(Z)-2-decenal154.25 2497-25-8tallowcitral152.1 5392-40-5lemonacetylpyrazine122.0 22047-25-2roast2,4-octadienal124.1 30361-28-5green, seaweed, cucumberp-anisyl alcohol138.17 105-13-5flowersulfurol143.0 137-00-8sulfurcitronellyl acetate198.2 150-84-5rose, dust(E)-octenol128.1 18409-17-1soap, plasticbornyl formate188.26 7492-41-3greenbutyl octanoate200.2 589-75-3fruit2-decenal154.25 3913-71-1orangebutyric acid88.1 107-92-6rancid, cheese, sweat2-acetyl-3,4,5,6-tetrahydropyridine125.17 27300-27-2caramelphenylethylthiol138.23 4410-99-5rubberhotrienol152.1 20053-88-7hyacinth2-acetylthiazole127.0 24295-03-2roast, nut, sulfurbenzoic acid122.0 65-85-0urinephenylethanal120.1 122-78-1hawthorne, honey, sweetl-menthol156.27 2216-51-5peppermintsafrole162.19 94-59-7spice, sweet, warm(E)-cinnamaldehyde132.16 14371-10-9cinnamon, paintpinocarveol152.23 4955-29-7flower2,4-decadienal152.1 65909-91-3seaweedethyl decanoate200.1 110-38-3grape(-)-γ-elemene204.35 29873-99-2green, wood, oilmethyl quinoxaline144.17 7251-61-8roast, nut, fruit(+)-carvone150.1 2244-16-8carawayalloaromadendrene204.35 25246-27-9woodacetophenone120.1 98-86-2must, flower, almondβ-terpineol154.25 138-87-4mustneoisomenthol156.27 491-02-1mentholγ-butyrolactone86.0 96-48-0caramel, sweetethyl benzoate150.1 93-89-0camomile, flower, celery, fruit(Z)-β-Farnesene204.35 28973-97-9citrus, greensafranal150.22 116-26-7herb, sweet(Z)-piperitol154.25 491-04-3herbpiperitol154.25 16721-39-4herb3-mercapto-3-methyl-1-butanol 118.2 34300-94-2meat brothestragole148.20 140-67-0licorice, aniseethyl undecanoate214.35 627-90-7cognac, coconutβ-farnesene204.35 18794-84-8wood, citrus, sweet2-isopentyl-3,6-dimethyl pyrazine 178.28 18433-98-2fruitmethylbutyric acid102.1 116-53-0cheese, sweatα-humulene204.35 6753-98-6woodisovaleric acid102.1 503-74-2sweat, acid, rancidmethyl-(methyldithio)furan160.26 65505-17-1cooked meat, thiaminneral152.1 106-26-3lemondihydrocarvyl acetate196.26 20777-49-5mint, camphor, medicine4-hexanolide114.14 695-06-7coumarin, sweet1,3-p-menthadien-7-al150.22 1197-15-5fat, spiceisobornyl propionate210.32 2756-56-1fruit, turpentineborneol154.25 507-70-0camphorlavandulol154.25 498-16-8herbethyl-3-hydroxyhexanoate 160.1 2305-25-1freshdimethoxytoluene152.19 131092-10-9hummuslinalyl butyrate224.34 78-36-4pear, sweetp-anisaldehyde136.1 123-11-5mint, sweet4-oxodecanal170.25 43160-78-7fat4-methylthiazole99.16 693-95-8roasted meatγ-muurolene204.35 30021-74-0herb, wood, spicebenzyl butanoate178.1 103-37-7plumδ-muurolene204.35 120021-96-7oilp-methoxystyrene134.18 637-69-4sweetheptyl 2-methylbutyrate200.32 50862-12-9appleα-terpineol154.25 98-55-5oil, anise, mintδ-elemene204.35 20307-84-0wooddiethyl succinate174.20 123-25-1wine, fruitethylfuranone112.1 2313-01-1spiceepoxy-2-nonenal154.21 128386-31-2metalbutyl benzoate178.1 136-60-7balsamicdecyl acetate 200.32 112-17-4orange, oillinalyl isovalerate238.37 1118-27-0sweet, apple, citrus3-(acetylthio)-2-methylfuran156.20 55764-25-5roasted meatmethylnonanedione170.25 113486-29-6straw, fruitterpinyl acetate196.29 80-26-2waxheptadecane 240.3 629-78-7 alkanecitronellyl isobutyrate226.36 97-89-2fruit, rosegermacrene D204.35 23986-74-5wood, spice2,4-nonadienal138.1 5910-87-2fat, wax, green(E,Z)-2,4-decadienal 152.1 25152-83-4fried, fat(E,E)-2,4-decadienal 152.1 25152-84-5fried, wax, fatβ-selinene204.2 17066-67-0herbgeranyl acetate196.29 105-87-3rose2-undecenal168.2 2463-77-6sweetα-muurolene204.35 10208-80-7wood(-)-β-bisabolene204.2 495-61-4balsamicgeranial152.1 141-27-5lemon, mintnaphthalene128.1 91-20-3tarundecanol172.2 112-42-5mandarinDL-carvone150.22 99-49-0mint, basil, fennellauric aldehyde184.2 112-54-9lily, fat, citrusmethionol106.2 505-10-2sweet, potatoα-farnesene204.35 502-61-4wood, sweetvalencene204.35 4630-07-3green, oilhexyl hexanoate200.2 6378-65-0apple peel, peach(Z)-3-hexenyl hexanoate198.30 31501-11-8fruit, prune(E,Z)-3,6-nonadien-1-ol140.22 56805-23-3fish3-mercaptohexyl-acetate176.28 136954-20-6box treestyrene glycol138.2 25779-13-9sweetdehydro-ar-ionene172.27 30364-38-6licoricebicyclogermacrene204.35 24703-35-3green, woodneryl acetate196.29 141-12-8fruitmethyl salicylate152.0 119-36-8peppermintethylfuranone112.1 2407-43-4spicep-mentha-dien-hydroperoxide168.23 32495-14-0turpentineδ-cadinene204.2 483-76-1thyme, medicine, woodcis-linalool pyran oxide170.25 14009-71-3citrus, green(-)-carvone150.22 6485-40-1mintγ-cadinene204.2 39029-41-9woodethylbenzaldehyde134.1 4748-78-1sweet2,6-nonadienol140.22 7786-44-9cucumbermethyl eugenol178.23 93-15-2clove, spicecumin aldehyde148.1 122-03-2acid, sharp4-acetyltoluene134.1 122-00-9bitter almondcarvyl acetate194.27 97-42-7green, spearmint(E)-2-undecenal168.28 53448-07-0soap, fat, greenp-menthadienhydroperoxide168.23 77026-83-6turpentineα-gurjunene204.35 489-40-7wood, balsamicbornyl butyrate146.86 13109-70-1herb, woodcitronellol156.27 106-22-9rosedecanol158.2 112-30-1fatperillaldehyde150.1 18031-40-8fatlinalyl valerate238.37 10471-96-2citrus, lavenderacetylthiazoline129.2 29926-41-8roast, popcornnerol154.1 106-25-2sweetγ-heptalactone128.2 105-21-5nut, fat, fruitgeosmin182.30 19700-21-1beet, earthnonadienol140.1 28069-72-9cucumberα-curcumene202.34 644-30-4herbbornyl isovalerate238.37 76-50-6herb, earth, green(E)-α-bergamotene204.35 13474-59-4wood, warm, teasesquiphellandrene204.35 73744-93-1sweet, fruit, herbisogeraniol154.1 5944-20-7roseβ-sesquiphellandrene204.35 20307-83-9woodethyl salicylate166.1 118-61-6wintergreen, mintacetylthiophene126.0 88-15-3sulfurS-(2-furfuryl)-ethanethioate156.20 13678-68-7roastformylmethyl thiophene126.0 13679-70-4sulfurcitronellyl butyrate226.36 141-16-2fruit, sweet, rosecadinadiene204.35 29837-12-5spice, fruiterucin161.29 4430-36-8cabbagemethyl laurate214.2 111-82-0fat, coconutoctadecane 254.3 593-45-3 alkaneα-guaiene204.35 3691-12-1wood, balsamicγ-selinene204.2 515-17-3wood(E)-β-damascone192.30 23726-91-2applep-menthadienhydroperoxide168.23 94268-57-2turpentinehexyl octanoate228.4 1117-55-1herb, green, oil2-dodecenal182.2 20407-84-5green, fat, sweetα-ionone192.2 127-41-3wood, violetisogeraniol154.25 16750-94-0roseβ-damascenone190.1 23726-93-4apple. rose, honeybutyl decanoate228.2 30673-36-0whiskeyperilla aldehyde150.22 2111-75-3spicep-menthadienhydroperoxide168.23 77026-84-7turpentinefurfurylmethyldisulphide160.26 57500-00-2smoketridecanal198.34 10486-19-8flower, sweet, mustasaricin192.21 18607-93-7spice, peppercaproic acid,hexanoic acid 116.1 142-62-1sweatβ-phenethyl acetate164.20 103-45-7rose, honey, tobaccoβ-guaiene204.35 88-84-6wood, spiceethyl-(E,Z)-2,4-decadienoate196.29 3025-30-7pearp-coumaric acid164.16 501-98-4balsamicp-menth-1-en-9-yl acetate196.29 28839-13-6fruit, herbp-cymenol150.22 1197-01-9citrus, must(E)-carveol152.1 1197-07-5caraway, solventhydroxydimethylcyclopentenone126.7 21835-00-7caramelgeranyl acetone194.2 3796-70-1magnolia, greenisopiperitone152.23 58615-39-7sweet, fruitethyl laurate228.2 106-33-2leafα-zingiberene204.35 495-60-3spice, fresh, sharp(Z)-carveol152.23 1197-06-4carawaygeraniol154.1 106-24-1rose, geraniumguaiacol124.14 90-05-1smoke, sweet, medicineelemicin208.25 487-11-6spice, flowerraspberry ketone164.1 5471-51-2raspberry12-methyltridecanal212.37 75853-49-5cooked meat, tallow, fat, meat broth,sweatgermacrene B204.35 15423-57-1wood, earth, spicebenzyl alcohol108.1 100-51-6sweet, flowerberteroin175.32 4430-42-6cabbagegeranyl butyrate224.34 106-29-6fruit, rose, apple3-mercaptohexanol134.24 51755-83-0sulfurcitronellyl valerate240.39 7540-53-6warm, honey, herb, roseγ-octalactone142.1 104-50-7coconut(Z)-oak-lactone156.22 55013-32-6spicegeranyl isovalerate238.37 109-20-6fruit, rose, applenonadecane 268.3 629-92-5 alkanep-menth-1-en-9-ol154.25 18479-68-0fruit, herbcaryophyllene alcohol56747-96-7moss, earth, spiceethyl dihydrocinnamate178.23 2021-28-5flowerβ-ionone192.3 79-77-6seaweed, violet, flower, raspberryisopropyl benzoate164.20 939-48-0sweet, fruit5-octanolide142.20 698-76-0peach2-phenylethyl alcohol122.16 60-12-8honey, spice, rose, lilaccalamenene202.34 483-77-2herb, spicemyristicin192.21 607-91-0spice, warm, balsamic(E)-oak lactone156.22 39638-67-0coconut, flowerguaiol222.37 489-86-1wood, balsamictetradecyl aldehyde212.37 124-25-4flower, waxdehydrocarveol150.22 28982-60-7oil, herb(E)-isoelemicin208.25 5273-85-8spice, flowerdill apiol222.24 484-31-1wood, spicetridecanol200.2 112-70-9mustmaltol140.14 118-71-8caramelepoxy-β-ionone208.30 23267-57-4fruit, sweet, woodgeranyl valerate238.37 10402-47-8rose, fruitcaryophyllene oxide220.36 1139-30-6herb, sweet, spice(E)-2-hexenoic acid114.14 13419-69-7must, fatlauryl alcohol186.2 112-53-8fat, waxmethyl jasmonate224.14 1211-29-6jasmine(E)-Whiskey lactone156.22 80041-01-6flower, lactone(Z)-whiskey lactone156.22 80041-00-5coconut(-)-cubenol222.37 21284-22-0spice, herb, green teamethyl tetradecanoate242.2 124-10-7orrisbulnesol222.37 22451-73-6spiceeicosane 282.3 112-95-8 alkaneβ-caryophyllene alcohol222.37 472-97-9earth, mossacetyloxy-dimethylfuranone170.16 4166-20-5caramelmethyl epijasmonate225.3 95722-42-2jasminecadina-1,4-dien-3-ol220.35 114791-16-1wood, spicemethylene bis(methyl sulfide)108.23 1618-26-4garlic, sulfurnerolidol222.2 40716-66-3wood, flower, wax(Z)-nerolidol222.37 3790-78-1waxmethyl dihydroepijasmonate212.2 39647-11-5jasmineβ-bisabolol222.37 15352-77-9sweet, herbhumulene oxide220.35 19888-33-6herbo-cresol108.1 95-48-7phenolepoxy-2-decenal168.23 134454-31-2metal, greenbutyl laurate256.4 106-18-3oillevomenol222.37 23089-26-1herb4-ethylguaiacol152.1 2785-89-9spice, cloveacetylpyrrolizine149.19 55041-85-5medicinepantolactone130.14 599-04-2cotton candyzingiberenol222.37 58334-55-7metal2,6-dimethylnaphthalene156.22 581-42-0grass(E)-2-dodecen-1-ol184.32 69064-37-5oil7-heptadecene239.7 54290-12-9alkaneγ-nonalactone156.1 104-61-0coconut, peachethyl tetradecanoate256.2 124-06-1etherfuraneol™112.1 3658-77-3caramelβ-farnesol222.37 58181-76-3flower, oilcinnamic acid148.1 140-10-3honeymethyl cinnamate162.1 1754-62-7strawberrydiethyl malate190.19 7554-12-3brown sugar, sweetmethyl cinnamate162.1 103-26-4strawberrycuminic alcohol150.22 536-60-7wood, herbpentadecanal226.2 2765-11-9freshp-cresol108.1 106-44-5medicine, phenol, smokebenzyl benzoate212.25 120-51-4balsamic, oil, herb。
超高效液相色谱-串联质谱法测定化妆品中15种N-亚硝胺化合物

第42 卷第 11 期2023 年11 月Vol.42 No.111469~1478分析测试学报FENXI CESHI XUEBAO(Journal of Instrumental Analysis)超高效液相色谱-串联质谱法测定化妆品中15种N-亚硝胺化合物汪毅1,梁文耀1,何国山1,陈张好2,周智明2,吴谦1,席绍峰1,谭建华1*(1.广州质量监督检测研究院,国家化妆品质量检验检测中心(广州),广东广州511447;2.广东省药品检验所,广东广州510663)摘要:采用超高效液相色谱-串联质谱(UPLC-MS/MS)建立了化妆品中15种痕量N-亚硝胺化合物的分析方法。
水剂样品以水或乙腈分组超声提取,膏霜乳液样品采用亚铁氰化钾-乙酸锌溶液沉淀大分子或者饱和氯化钠-乙腈盐析分组处理后,以Agilent Poroshell 120 SB-Aq(100 mm×3.0 mm,2.7 μm)色谱柱分离,经大气压化学电离源(APCI)电离,多反应监测模式检测,以同位素内标法定量。
结果表明,15种N-亚硝胺化合物在相应质量浓度范围内线性关系良好(r2>0.995),检出限和定量下限分别为5~15 ng/g和15~45 ng/g。
水、乳、膏霜3种化妆品基质在25、50、100 ng/g加标水平下的平均回收率为88.0%~111%,相对标准偏差(RSD,n=6)为1.4%~9.8%。
该方法用于市售化妆品检测,发现13批次样品检出N-亚硝基二乙醇胺(NDELA),其中1批次超限量值。
方法的专属性强,灵敏度高,精密度好,解决了N-亚硝胺化合物稳定性差、易被干扰等问题,适用于化妆品中15种N-亚硝胺化合物的痕量测定。
关键词:N-亚硝胺化合物;化妆品;超高效液相色谱-串联质谱法(UPLC-MS/MS);大气压化学电离源中图分类号:O657.63;O623.732文献标识码:A 文章编号:1004-4957(2023)11-1469-10 Determination of Fifteen N-nitrosamine Compounds in Cosmetics by Ultra Performance Liquid Chromatography-TandemMass SpectrometryWANG Yi1,LIANG Wen-yao1,HE Guo-shan1,CHEN Zhang-hao2,ZHOU Zhi-ming2,WU Qian1,XI Shao-feng1,TAN Jian-hua1*(1.Guangzhou Quality Supervision and Testing Institute,National Quality Supervision and Testing Center for Cosmetics(Guangzhou),Guangzhou 511447,China;2.Guangdong Institute for Drug Control,Guangzhou 510663)Abstract:An ultra performance liquid chromatography-tandem mass spectrometric(UPLC-MS/MS)method was established for detecting 15 trace N-nitrosamine compounds in cosmetics. The final estab⁃lished method involved ultrasonic extraction of cosmetics using water or acetonitrile for different com⁃pounds. The samples were treated with potassium ferrocyanide-zinc acetate solution for precipitating macromolecules or saturated sodium chloride-acetonitrile for salting out.An Agilent Poroshell 120 SB-Aq(100 mm × 3.0 mm,2.7 μm) chromatography column was used for separation,followed by atmospheric pressure chemical ionization(APCI) source and multiple reaction monitoring mode detec⁃tion in the isotope internal standard method for quantification. The result showed good linearity(r2> 0.995) for the 15 N-nitrosamine compounds in their respective concentration ranges,with detection and quantitation limits of 5-15 ng/g and 15-45 ng/g,respectively.The average recoveries for the three cosmetic matrices(aqueous,emulsion,cream) at spiked levels of 25,50,100 ng/g were be⁃tween 88.0% and 111%,with relative standard deviations(RSD,n=6) of 1.4%-9.8%. The method was applied to the detection of commercial cosmetics and N-nitrosodiethanolamine(NDELA) was de⁃tected in 13 batches,with one batch exceeding the limit. The strong specificity,high sensitivity,and good precision made the method could solve the problems of poor stability and easy interference ofdoi:10.19969/j.fxcsxb.23051602收稿日期:2023-05-16;修回日期:2023-06-10基金项目:广东省药品监督管理局化妆品风险评估重点实验室专项(2021ZDZ03);广东省市场监督管理局科技项目(2022CZ06)∗通讯作者:谭建华,博士,正高级工程师,研究方向:色谱-质谱检测技术研究,E-mail:tanjianhua0734@第 42 卷分析测试学报N-nitrosamine compounds,and was suitable for the trace determination of 15 N-nitrosamine com⁃pounds in cosmetics.Key words:N-nitrosamine compounds;cosmetics;ultra performance liquid chromatography-tan⁃dem mass spectrometry(UPLC-MS/MS);atmospheric pressure chemical ionization(APCI) sourceN-亚硝胺化合物是一类具有N-亚硝基结构的化合物,因取代基的不同,形成了种类繁多的同系物,目前已发现超过300种[1]。
瑞卢戈利杂质清单
中文名称英文名称CAS规格用途结构式
瑞卢戈利Relugolix(TAK-385)737789-87-6
10mg
25mg
50mg
100mg
更大规格请咨询
项目报批
纯度高于95%
扬信医药代理各品种杂质对照品:舒更葡糖钠杂质,达托霉素杂质,依维莫司杂质,他克莫司杂质,阿奇霉素杂质,克拉维酸钾杂质,红霉素杂质,克拉霉素杂质,林可霉素杂质,罗红霉素杂质,克林霉素杂质,恩曲他滨杂质,艾地那非杂质,瑞卢戈利杂质,艾氟康唑杂质等;并提供COA、NMR、HPLC、MS等结构确证图谱。
专注各种杂质对照品 代理中检所/EP/BP/USP/LGC/TRC/TLC/MC/SIGMA/BACHEM/STD等品牌
瑞卢戈利杂质种类整理列表。
J. Label. Compd. Radiopharm. 2004, 47, 821–835.

JOURNAL OF LABELLED COMPOUNDS AND RADIOPHARMACEUTICALSJ Label Compd Radiopharm2004;47:821–835.Published online in Wiley InterScience().DOI:10.1002/jlcr.870Research ArticleIsotope labeled‘HEA Moiety’in the synthesisof labeled HIV-protease inhibitors–Part1I.Victor Ekhato1,*,Yuan Liao1,2and Mihaela Plesescu1,31Bristol-Myers Squibb Company,Pharmaceutical Research Institute,5Research Parkway,Wallingford,CT06492,USA2Pfizer Global Research and Development,Ann Arbor,MI48105,USA3Millennium Pharmaceuticals,Cambridge,MA023139,USASummary[(S)-10-((N-tert-Butyloxycarbonyl)amino)-2S-[2H5]phenyl-ethyl]oxirane11,made from [2H5]-bromobenzene,was transformed into the HIV-protease inhibitors[2H5]-DPH 153893and[2H5]-DPH140662.Both compounds are members of the hydroxyethy-lamine class of protease inhibitors(HIV-PIs).The method of synthesis is applicable to members of this class and the HEE group of HIV-PIs.Copyright#2004John Wiley &Sons,Ltd.Key Words:protease inhibitor;anti-HIV;AIDS;[2H5]-HEA isostereIntroductionDPH153893and DPH140662,like amprenavir1and saquinavir,2are members of the hydroxyethylamine(HEA)isostere class of HIV protease inhibitors(HIV-PIs).3The two compounds are highly potent HIV-aspartyl protease inhibitors with an average IC90of4–8nM against wild-type viruses. Both were selected for pre-clinical evaluation as potential new generation anti-HIV drugs.4Aspects of the studies involved LC-MS analysis and the stable isotope-labeled forms of DPH153893and DPH140662were needed as internal standards.We wanted to make the penta-deuterated DPH153893and DPH 140662molecules in which the[2H5]is situated at the core as the[phenyl ring-2H5]-HEA.We preferred to make the penta-deuterated analog based on the calculation that the molecular weight is sufficiently increased,such that overlap with other ions due to isotopic mass distribution is avoided.*Correspondence to:I.V.Ekhato,Bristol-Myers Squibb Company,Pharmaceutical Research Institute, 5Research Parkway,Wallingford,CT06492,USA.E-mail:ihoezo.ekhato@Copyright#2004John Wiley&Sons,Ltd.Received15July2003Since both compounds possess the HEA core structure,we planned to prepare DPH153893and DPH140662from a common labeled precursor bearing the elements of the[phenyl ring-2H5]-HEA moiety.We believe that the phenyl group of the HEA sub-unit is metabolically stable,since metabolites have not been reported.5Similarly,these metabolites are absent from our internal study6of DPH153893or DPH140662.As a result,we were encouraged to explore the current synthesis with the prospect that a protease inhibitor containing the[phenyl ring-2H5]-HEA moiety might,in addition to our initial reason for synthesis,be suitable for in vivo investigation.By routine reactions we made the required[2H5]-intermediate from[2H5]-bromobenzene and transformed this compound into[2H5]-DPH153893and[2H5]-DPH 140662.Results and discussionIn the synthetic literature on anti-HIV protease inhibitors,unlabeled(1S,2S)-(1-oxiranyl-2-[2H5]phenylethyl)-carbamic acid tert-butyl ester11is extensively used as an intermediate3,7for synthesis of aspartyl protease inhibitors.We considered11to be an attractive key intermediate from which to make[2H5]-protease pound11,Figure1,has the epoxide functionality from which to build[2H5]-DPH153893and[2H5]-DPH140662.In addition,11 provides the[phenyl ring-2H5]-HEA isostere core of both inhibitors.Unlike previous work,7the present approach does not draw on any chiral pools of reagents,such as optically pure amino acids or derivatives,to construct11. Therefore,constructing the[2H5]-labeled carbon skeleton from[2H5]-bromo-benzene1with the desired1S,2S-configuration in11was a challenging task. To address this point,we implemented a facially selective syn-aldol condensation reaction with S-4-benzyl-(3-[2H5]phenylpropionyl)-oxazolidin-2-one5.[2H5]-Bromobenzene was used to prepare compound5,and theSYNTHESIS OF LABELED HIV-PROTEASE INHIBITORS823 sequence of routine reactions that was employed is shown in Scheme1.Thus, following a lithium-halogen exchange reaction with[2H5]-bromobenzene1, DMF was added and acid workup provided the aldehyde,2.A Wittig-type homologation protocol8with compound2furnished3-[2H5]phenylacrylic acid ethyl ester,3.The carboxylic acid made by the hydrolysis of3was activated as the mixed pivaloyl anhydride,and reacted with(S)-(-)-4-benzyl-N-lithio-2-oxazolidinone,according to a literature procedure,9to form(S)-4-benzyl-3-(3-[2H5]phenylacryloyl)-oxazolidin-2-one,pound4was saturated by subjecting it to Pd/C catalyzed hydrogenation to furnish(S)-4-benzyl-3-(3-[2H5]phenylpropionyl)-oxazolidin-2-one,5.2222Et4Scheme1.Conversion of[2H5]-bromobenzene into the acyl oxazolidinone5to enable the asymmetric synthesis of11To complete the required carbon skeleton and simultaneously achieve the desired(S,S)-configuration,compound5was condensed with(benzyloxy) acetaldehyde,10as shown in Scheme2.The combination of dibutylboron triflate and diisopropylethylamine reagent resulted in the highly diastereofa-cially selective syn-aldol condensation reaction yielding6.In the manner described in the literature,11lithium peroxide was used to cleave the amide bond in compound6to obtain(2S,3S)-[(2-[2H5]benzyl-4-(benzyloxy)-3-hydroxybutyric acid,7,in84%pound7was refluxed in a solution of toluene containing diphenylphosphoryl azide and triethylamine to produce 8,an oxazolidinone.Formation of compound8is explained by the intra-molecular cyclization reaction of the intermediate isocyanate with the participation of the neighboring hydroxy group,as described in the literature.10The deprotection of8and the formation of(1S,2S)-1-[N-tert-butyloxycarbonyl)amino]-1-[25H5]benzyl-3-(benzyloxy)propan-2-ol,9,wasachieved in a one-pot pound5was subsequently hydrogenated under Pd/C catalysis to yield10(see Scheme2).The diol grouping in10was converted to the epoxide ring by a modified literature protocol12to give11(Scheme3).Specifically,compound10was treated with1-(bromocarbonyl)-1-methylethyl acetate in ethanol-free chloro-form at08C,converting it to an intermediate bromohydrin.The bromohydrin was cyclized by treatment with a solution of sodium methoxide in THF to provide(1S,2S)-(1-oxiranyl-2-[2H5]phenyl-ethyl)-carbamic acid tert-butyl ester,11,in73%yield.From intermediate11we proceeded to make[2H5]-DPH153893and[2H5]-DPH140662.At the second step in the reaction sequence isobutylamine wasreacted with11in hot isopropyl alcohol to pound12is the lastintermediate common to both synthetic targets,i.e.the reaction step after 12is the point of divergence of the reaction sequences to DPH 140662and DPH 153893.In order to make [2H 5]-DPH 140662,compound 12was treated with 4-nitro-benzenesulfonyl chloride in the presence of potassium carbonate as acid scavenger.After removal of the Boc group,13was obtained as the methanesulfonic acid salt in 80%overall yield over the two steps.By a standard coupling method with EDC.HCl,4-methylmorpholine and HOBt 13in DMF,compound 13and Boc-l -tert -leucine formed the Boc protected 14.We found that the stepwise sequence of coupling Boc-l -tert -leucine to make the Boc protected 14,the removal of Boc to yield 14and the coupling reaction of compound 14with IS066(IS066was generously supplied bythe 11into 14via compound 12,a commonintermediate to [2H 6]-DPH 153893and [H 5]-DPH 140662SYNTHESIS OF LABELED HIV-PROTEASE INHIBITORS 825Department of Chemical Process,DuPont Pharmaceuticals Company,Wilmington,DE)to give 15,as shown in Scheme 4,was the best sequence for making [2H 5]-DPH 140662.An alternative single coupling reaction to DPH 140662from 13was complicated by intra-molecular cyclization of the dipeptide reagent under our reaction conditions.Chromatographic purifica-tion was required to obtain 15,and then only in poor yield.The single coupling approach was therefore abandoned.A standard coupling protocol in DMF containing EDC.HCl,HOBt and 4-methylmorpholine as acid scavenger converted 14to compound 15.After Pd/C catalyzed hydrogenation of 15to the 4-aminobenzenesulfonamide,the Boc group was removed with TFA-Et 3SiH and the reaction mixture was neutralized with NaHCO 3to provide [2H 5]-DPH 140662in 61%yield.By substituting 3-nitrobenzenesulfonyl chloride for 4-nitrobenzenesulfonyl chloride in Scheme 3,and following a sequence of reactions analogous to Schemes 3and 4,we accomplished the synthesis of [2H 5]-DPH 153893in comparable yield.ConclusionTwo HIV protease inhibitors were specifically labeled with [2H 5]at the HEA sub-unit by transforming [2H 5]-bromobenzene into a common intermediate for these agents.The intermediate was made using established reactionsthat 14into [2H 5]-DPH 140662I.V.EKHATO ET AL.826included an asymmetric reaction step.The intermediate was used to create the[phenyl ring-2H 5]labeled HEA core structure of DPH 140662and DPH 153893.Very high deuterium incorporation was achieved by the sequence we have described and the synthetic approach may be applicable,with some modifications,to other HIV-protease inhibitors.ExperimentalAll reactions were carried out under an atmosphere of argon unless otherwise specified.Solvents were commercial grade and used without purification or drying.Column chromatography was carried out on Merck Kiesegel 60(230m)silica gel.Flash chromatographic separations were on a Biotage Flash System using a pre-packed silica gel cartridge.TLC visualization reagents included (10%iodine plus 10%AcOH)in 40%aqueous KI.1H NMR spectra were recorded at 300,400or 500MHz.Chemical shifts are given in ppm relative to tetramethylsilane (TMS).Only the important IR absorptions (cm À1)and the molecular ion and/or base peaks in MS are given.HPLC columns used include Zorbax Rx C 18,Discovery Amide C 16,and Waters Symmetry C 18columns.4S-Benzyl-3-(3-[2H 5]phenyl-propionyl)-oxazolidin-2-one,%5To a stirred solution of [2H 5]-phenylacrylic acid (10.1g,65.07mmol,see Scheme 1for preparation)and 4-methylmorpholine (7.15ml,65.07mmol)in dry THF (120ml)maintained at À788C with a dry ice-acetone bath was added trimethylacetyl chloride (8.01ml,65.07mmol).After 30min the cold bath was replaced with an ice-water bath and the reaction was stirred for another 45min.The precipitate was removed by filtration,and the filtrate was transferred to a solution of N -lithio-oxazolidinone that was pre-generated as follows.(S)-(-)-4-Benzyl-2-oxazolidinone (11.53g,65.07mmol)in dry THF (120ml)was stirred under argon atmosphere at À788C while BuLi (1.6M solution in hexanes,40.6ml,65.07mmol)was added dropwise.The solution was stirred at À788C for 1.0h and the above filtrate,a solution of mixed anhydride in THF,was added in one portion.The reaction was quenched after 1h with saturated NH 4Cl solution (100ml)and the organic phase was separated.The aqueous portion was further extracted with ethyl acetate (3Â200ml),the combined organic extract was dried and evaporated to dryness to give (S)-4-benzyl-3-(3-[2H 5]phenylacryloyl)-oxazolidin-2-onepound 4was dissolved in ethyl acetate (150ml)and a suspension of 10%Pd/C (1.2g)in ethyl acetate (20ml)was added.After the reaction mixture was degassed,it was stirred under a hydrogen atmosphere for 3h and filtered through a pad of Celite to remove the spent catalyst.The filtrate was evaporated to a product which crystallized from EtOAc-hexane to give 5(17.10g,84%).1H NMR (400MHz)d 2.74(dd,1H),3.03(m,2H),3.28(m,SYNTHESIS OF LABELED HIV-PROTEASE INHIBITORS 8273H),4.16(m,2H),4.65(m,1H),7.17(d,2H)and 7.32(m,3H).13C NMR (75MHz)d 30.30,37.12,37.94,55.17,66.26,127.4,128.0,128.2,129.0,129.5,140.4,153.4and 172.5.IR n max (CHCl 3)2276,1785,1699,1490,1478,1390,1372,and 1202cm À1.4S-Benzyl-3-[(2S-[2H 5]benzyl-4-(benzyloxy)-3S-hydroxy-butyryl)]-oxazoli-din-2-one,%6To a stirred solution 4S-benzyl-3-(3-[2H 5]phenylpropionyl)-oxazolidin-2-one 5(23.68g,75.3mmol)in CH 2Cl 2(140ml)at 08C was added dibutylboron triflate (1.0M solution in dichloromethane,98.0ml,98mmol)followed by diisopro-pylethylamine (19.67ml,112.97mmol).After stirring the mixture for 1h at room temperature it was cooled to À788C and a solution of (benzyloxy)ace-taldehyde (20g,133.17mmol)in CH 2Cl 2(30ml)was added.The reaction mixture was stirred for 30min at À788C and warmed to room temperature for 2h.A phosphate buffer of pH 7.3(40ml)was added and while the reaction was cooled to 08C,methanol (140ml)was added followed by a mixture of (2:1)MeOH and 30%H 2O 2(180ml).The resulting mixture was stirred for 1h,concentrated to a small volume and extracted with EtOAc (2Â200ml).The organic portions were combined and washed with saturated NaHCO 3(200ml),brine (200ml)and dried over anhydrous magnesium sulfate.Evaporation of the solvent gave the crude product which was purified by chromatography on silica gel.The column was eluted with 10–25%ethyl in hexane to give 6(25g,71.46%).1H NMR (500MHz)d 2.10(dd,Hz,1H),2.64(brs,1H),2.83(dd,1H),3.08(q,Hz,1H),3.17(q,1H),3.59(m,2H),3.78(tr,1H),3.86(q,1H),4.18(q,1H),4.42(m,1H),4.50(s,2H),4.64(m,1H),6.93(dd,2H),7.20–7.343(m,10H).IR n max (CHCl 3)2274,1778,1694,1496,1386,1210,and 1104cm À1.S-2-[2H 5]Benzyl-4-(benzyloxy)-3S-hydroxy-butyric acid,%7To a stirred solution of 4S-benzyl-3-[(2S-[2H 5]benzyl-4-(benzyloxy)-3S-hydro-xy-butyryl)]-oxazolidin-2-one 6(23.1g,49.72mmol)in 50%aqueous THF (105ml)at 08C were added 30%H 2O 2(25ml,220mmol)and LiOH.H 2O (4.25g,101.2mmol)in succession.The reaction mixture was stirred in an ice bath and monitored by TLC until acyloxazolidinone was consumed in a calculated 6h.Saturated sodium metabisulfite (100ml)was added and the mixture was extracted with EtOAc (3Â200ml).After acidification of the aqueous portion to pH 2with dilute HCl,it was extracted with EtOAC (2Â120ml).The organic portions were combined,dried over anhydrous Na 2SO 4,filtered and evaporated to an oil.TLC on silica gel (ether:pet.ether:acetic acid,60/40/1v/v/v)showed fast running desired product and slow running oxazolidinone.The mixture was applied to a column of silica gel and I.V.EKHATO ET AL.828eluted with (ether:pet.ether:acetic acid 60/40/1,v/v/v/)to afford crystalline (CH 2Cl 2-hexane)7(12.98g,84%).1H NMR (500MHz)d 2.95(m,1H),3.10(2H),3.49(dd,1H),3.59(dd,1H),4.03(m,1H),4.54(s,2H),7.31–7.36(m,5H).13C NMR (75MHz)d 33.67,50.09,70.38,71.64,73.57,127.9,128.0,128.2,128.3,128.6,137.6,138.6,and 177.9.IR n max (CHCl 3)3254,2275,1728,1454and 1169cm À1.S-4-[2H 5]Benzyl-5S-((benzyloxymethyl)-oxazolidin-2-one,%8A mixture of S-(2-[2H 5]benzyl-4-(benzyloxy)-3S-hydroxy-butyric acid 7(11.0g,36.0mmol),diphenylphosphoryl azide (9.84ml,45.66mmol)and triethylamine (6.68ml,47.92mmol)in toluene (78ml)was refluxed for 4h and cooled to room temperature.Ethyl acetate (250ml)was added and the solution was washed with saturated NaHCO 3(100ml),dried and evaporated to give a residue that was chromatographed on silica gel.The fast running impurities were first eluted with 25%EtOAc in hexane,and 35%EtOAc in hexane was used to elute the desired product 8(11.7g,98%).1H NMR (300MHz)d 2.75(dd,1H),2.92(dd,1H),3.797(d,2H),4.12(m,1H),5.62(s,2H),5.8(m,2H)and 7.40(m,5H).(S)-1-[N-tert-Butyloxycarbonyl)amino]-1-[2H 5]benzyl-3-(benzyloxy)propan-2S-ol,%9S-4-[2H 5]Benzyl-5S-(benzyloxymethyl)-oxazolidin-2-one 8(11.7g,38.74mmol)in 50%aqueous ethanol (40ml)containing KOH (9.0g,160.4mmol)was heated at 708C for 3h and then cooled to room temperature.The solution was adjusted to pH 7with 1.0M HCl and concentrated under reduced pressure.Di-tert -butyl dicarbonate (16.99g,77.84mmol)in CH 2Cl 2(60ml)was added at 08C and the reaction was stirred for 2h at room temperature.Saturated NH 4Cl solution (40ml)was added.The mixture was extracted with CH 2Cl 2(2Â50ml),the combined organic portions were dried and evaporated to yield a solid residue.The crude product was applied to a column of silica gel and the excess di-tert -dibutyl dicarbonate was eluted with 10%EtOAc in hexane.Further elution with 35%EtOAc in hexane gave a solid which crystallized from hexane to afford 9(15.2g,94%).1H NMR (300MHz)d 1.35(s,9H),2.62(brs),2.88(d,2H),3.56(m,2H),3.73(brs,1H),3.93(brs,1H),4.51(s,2H),4.72(d,1H),7.33(m,C 6H 5).13C NMR (75MHz)d 28.34,36.36,54.20,71.72,71.82,73.68,79.53,125.9(tr)127.8,127.9,128.1,128.5,129.1(tr),137.8,137.9and 155.9.IR n max (CHCl 3)1685,2273,1526,and 1172cm À1.S-1-[N-tert-Butyloxycarbonyl)amino]-1-[2H 5]benzyl-propan-2S,3-diol,10To a solution of S-1-[N -tert -butyloxycarbonyl)amino]-1-[2H 5]benzyl-3-(benzy-loxy)-propan-2S-ol 9(14.0g,37.18mmol)in EtOAc (140ml)was added a SYNTHESIS OF LABELED HIV-PROTEASE INHIBITORS 829suspension of 5%Pd/C (1.40g)in ethyl acetate (10ml).The stirred reaction mixture was purged to remove air and stirred under hydrogen atmosphere for 18h.The spent catalyst was filtered offand the filtrate was concentrated to a white solid that crystallized from hexane-ethyl acetate to give 10(10.48g,%100%).1H NMR (500MHz)d 1.37(s,9H),1.59(brs,1H),2.75(d,1H),2.91(dd,1H),3.09(dd,1H),3.33(brs,2H),3.65(m,2H),3.82(m,1H),4.53(d,1H).13C NMR (75MHz)d 28.30,36.55,52.59,63.06,73.27,80.41,126.2(tr),128.2(tr),129.0(tr),137.3,and 157.1.IR n max (CHCl 3)3356,2273,1685,1525,1445,1250,and 1171cm À1.[(S)-10-((N-tert-Butyloxycarbonyl)amino)-2S-[2H 5]phenyl-ethyl]oxirane,111-Bromocarbonyl-1-methylethyl acetate (2.0ml,13.63mmol)was added to a stirred suspension of (S)-1-[N -tert -butyloxycarbonyl)amino]-1-[2H 5]benzyl-propan-2S,3-diol 10(3.0g,10.48mmol)in dry,ethanol free,chloroform (30ml)maintained at 08C in an ice water bath.After 3h at room temperature the reaction was quenched by the addition of saturated NaHCO 3solution (20ml)and extracted with EtOAc (3Â30ml).The organic extract was washed with brine (30ml),dried over anhydrous Na 2SO 4and evaporated to give a solid.The solid was stirred as a solution in dry THF (40ml),cooled to 08C in an ice bath and NaOCH 3(0.92g,17.03mmol)was added.After stirring the reaction mixture at room temperature for 4h,saturated ammonium chloride solution (30ml)was added and the mixture extracted with EtOAc (3Â30ml).The organic extract was dried over anhydrous Na 2SO 4,filtered,and the filtrate was evaporated to a small volume.The material was applied to a column of silica gel and the compound was eluted with 22%EtOAc in hexane to give a white solid 11(2.08g,73%).1H NMR (300MHz)d 1.75(d,J =7.0Hz,3H),2.45(s,3H),4.97(q,J =7.0Hz,1H),7.10–7.27(m,3H),7.32–7.43(m,2H),7.51(d,J =8.2Hz,2H)and 7.75(d,J =7.3Hz,1H).13C NMR (75MHz)d 28.34,37.65,41.61,46.81,53.3,79.71,126.2(tr),128.1(tr),129.1(tr),136.7and 155.3.IR n max (CHCl 3)2275,1679,1523and 1168cm À1.3S-[N-(tert-Butyloxycarbonyl)amino-1-(2-methylpropyl)amino-4-[2H 5]phe-nylbutan-2R-ol,12To a solution of 2S-[10-(S)-((N -tert -butyloxycarbonyl)amino)-2-[2H 5]phenyl-ethyl]-oxirane 11(2.06g,7.20mmol)in isopropanol (6ml)was added isobutylamine (6ml,111.5mmol)and heated at 868C for 4h under argon atmosphere.The solvent was evaporated after cooling to room temperature.The white solid obtained was crystallized from ether-petroleum ether to give 12(2.32g,%100%).1H NMR (500MHz)d 0.89(dd,6H),1.35(s,9H),1.70(m,1H),2.39(d,3H),2.68(d,2H),2.86(dd,1H),2.98(dd,1H),3.44(m,1H),3.79(brs,1H),4.67(d,1H).IR n max (CHCl 3)3365,1648,1521,and 1173cm À1.I.V.EKHATO ET AL.830N-(3S-Amino-2R-hydroxy-4-[2H5]phenyl-butyl)-N-isobutyl-4-nitro-benzene-sulfonamide,13A solution of K2CO3(430mg,3.11mmol)in water(3ml)was added to a solution of3S-[N-(tert-butyloxycarbonyl)amino-1-(2-methylpropyl)amino-4-[2H5]phenylbutan-2R-ol12(575.19mg,1.68mmol)in isopropyl acetate(3ml). The mixture was stirred at568C to attain solution.4-Nitro-benzenesulfonyl chloride(360mg,1.62mmol)in isopropyl acetate(3ml)was added and stirring continued for30min after the addition was completed.A solid product separated upon cooling the reaction to room temperature,and it was collected byfiltration.This material(Boc protected amine)(885.8mg,1.681mmol)was stirred in isopropyl acetate(18ml)and the temperature was brought to858C under argon atmosphere.Methanesulfonic acid(130.9m l, 2.0mmol)in isopropyl acetate(5ml)was added slowly and the reaction mixture was stirred for another30min.Upon cooling to room temperature a crystalline product separated and it was collected byfiltration to afford13(722.6mg, 80%).1H NMR(300MHz)(DMSO-d6)d0.89(dd,6H),1.80(m,1H),2.36(s, 3H),2.85(m,2H),3.10(m,3H),3.49(dd,1H),3.95(m,1H),5.58(d,1H),7.80 (brs,2H),8.15(d,1H),8.40(d,1H).2-Amino-N-{1-[2H5]benzyl-2-hydroxy-3-[isobutyl-(4-nitro-benzenesulfonyl)-amino]-propyl-3,3-dimethylbutyramide,14N-tert-Butyloxycarbonyl tert-butylglycine(Boc-l-tert-leucine)(360.80mg, 1.56mmol),1-hydroxybenzotriazole hydrate(210.8mg,1.56mmol)in anhy-drous DMF(10ml)was treated with4-methylmorpholine(343m l,3.12mmol) followed by1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (298.8mg,1.56mmol).Compound13(680mg,1.302mmol)was added and the reaction mixture was stirred under argon atmosphere overnight.The reaction mixture was concentrated to a small volume,partitioned between ethyl acetate(20ml)and water(15ml),and the aqueous portion was separated.The aqueous portion was further extracted with ethyl acetate (3Â20ml)and the combined organic portions were washed sequentially with saturated NH4Cl solution(20ml),saturated K2CO3solution(20ml),brine (20ml)and dried.Thefiltrate was evaporated to a residue(843mg),and then dissolved in isopropyl acetate(10ml).While the stirred solution was maintained at858C,methanesulfonic acid(102m l,1.85mmol)in isopropyl acetate(3ml)was added dropwise.The reaction mixture was cooled to room temperature after30min,and concentrated to a small volume.Upon the addition of ether,a crystalline solid precipitated,and the material was collected byfiltration to afford14(708mg,84%).HPLC analysis on a Zorbax Rx C18column4.6Â250mm at aflow rate of1.0ml/min under isocratic conditions using40%water in acetonitrile containing0.05%TFA gave aretention time of3.3min.1H NMR(300MHz)(DMSO-d6)d0.75(dd,6H), 0.96(s,9H),1.89(m,1H),2.28(s,3H),2.65(dd,1H),2.82(m,1H),2.96(dd, 1H),3.08(dd,1H),3.40(m,2H),3.65(m,1H),3.85(brs),4.10(m,1H),7.85 (brs,2H),8.10(d,1H),8.38(d,1H).IR n max(CHCl3)2276,1691,1530,1352, 1350,and1150cmÀ1.[{1-{1-[2H5]Benzyl-2-hydroxy-3-[isobutyl-{4-nitro-benzenesulfonyl)-amino]-propylcarbamoyl}-2,2-dimethyl-propylcarbamoyl}-methyl](3-fluoro-benzyl)-carbamic acid tert-butyl ester,15To a solution of N-[(tert-butyloxycarbonyl)-(3-fluorobenzyl)]glycine(IS066) (373.95mg,1.32mmol),1-hydroxybenzotriazole hydrate(HOBT),178.38mg,1.32mmol)in anhydrous DMF(5ml)was added4-methylmorpholine(290m l,2.64mmol)followed by EDC.HCl(252.56mg, 1.32mmol).While stirring under an argon atmosphere,14(700mg,1.1mmol)was added.After stirring overnight,the mixture was concentrated under high vacuum to a small volume and partitioned between EtOAc(20ml)and water(15ml).The organic phase was separated and the aqueous portion was further extracted with ethyl acetate(2Â20ml).The combined organic portions were washed with water (20ml),saturated NH4Cl solution(20ml),saturated K2CO3(20ml),brine (20ml)and dried over magnesium sulfate.The solvent was evaporated to givea foamy solid15(880mg,91%).HPLC analysis on a Zorbax Rx C18column4.6Â250mm at aflow rate of1.0ml/min under isocratic conditions using40% water in acetonitrile containing0.05%TFA gave a retention time of 11.12min.N-{3-[(4-Amino-benezenesulfonyl)-isobutyl-amino]-1-[2H5]benzyl-2-hydroxy-propyl}-2-[2-(3-fluorobenzylamino)-acetylamino]-3,3-dimethyl-butyramide, [2H5]-DPH140662To a solution of15(700mg,0.869mmol)in methanol(25ml)was added a suspension of10%Pd/C(224mg)in methanol(8ml).The reaction mixture was stirred at room temperature in an atmosphere of hydrogen for3h and thenfiltered.The solvent was evaporated to yield a foamy solid.Trifluor-oacetic acid(2.616ml)and triethylsilane(Et3SiH)(0.4ml)were added successively to a solution of the solid in methylene chloride(18ml).The mixture was stirred at room temperature and the reaction was monitored by TLC(3%MeOH in dichloromethane).After2h the reaction mixture was evaporated to a residue and,EtOAc(20ml),water(15ml),and solid NaHCO3 (1.3g)were added.The mixture was stirred for20min and the organic portion was separated.The aqueous portion was further extracted with ethyl acetate (2Â20ml)and the combined organic extract was dried over MgSO4. The product was purified by chromatography on silica gel column eluted with8%EtOH in toluene to give an oil.Trituration of the oil with ether gave[2H5]-DPH140662(385mg,61%;D598.9%,D4, 1.1%).[a]20D–15.648 (c=0.41%in MeOH).The product was analyzed by HPLC on a Discovery Amide C16Supelco250Â4.6mm,5m m column.The solvent combination of A (10mmol ammonium formate)and B(acetonitrile)was used under gradient conditions of20–30%B in0–5min,30–50%B in5–18min and20–30%B in 18–20min at aflow rate of ml/min.The product peak was detected by UV at a wavelength of254nm and gave>98%chemical purity at a retention time of 14.13min.IR n max(CHCl3)1314,1285,and1143cmÀ1.1H NMR(300MHz, d6-DMSO)d0.85(m,15H),1.82(m,1H),2.52(dd,1H),2.68(m,1H),2.85(m, 2H),2.95(dd,1H),3.05(s),3.22(dd,1H),3.30(s,3H),3.50(s),3.60(m,1H), 3.89(m,1H),4.18(d,1H),4.89(d,1H),5.98(brs,2H),6.60(d,2H),7.12(m, 3H),7.42(m,3H),7.78(d,1H),7.98(d,1H).HRMS(TOF MS ES+;M+1); calcd.675.3762,found675.3766.ESI full ms gave z at675(M+1,100%)and 397(5%),372(15%)and354(2%).N-{1-[2H5]Benzyl-2-hydroxy-3-[isobutyl-(3-nitro-benzenesulfonyl)-amino]-propyl}-2-[2-(3-fluorobenzylamino)-acetylamino]-3,3-dimethyl-butyramide, [2H5]-DPH153893N-[(tert-butyloxycarbonyl)-(3-fluorobenzyl)]glycine(356.0mg, 1.256mmol) was reacted with2-amino-N-{1-[2H5]benzyl-2-hydroxy-3-[isobutyl-(3-nitro-benzenesulfonyl–amino]-propyl-3,3-dimethyl-butyramide methanesulfonic acid salt17(799mg, 1.256mmol)to make[(1{1-[2H5]benzyl-2-hydroxy-3-[isobutyl-(3-nitro-benzenesulfonyl)-amino]-propyl-carbamoyl}-2,2-dimethyl-propylcarbamoyl)-methyl]–(3-fluorobenzylcarbamic acid tert-butyl ester (902mg,89%)as described under experiment15.Sequential hydrogenolysis and boc deprotection with methanesulfonic acid in isopropyl acetate gave N-{1-[2H5]benzyl-2-hydroxy-3-[isobutyl-(3-nitro-benzenesulfonyl)-amino]pro-pyl}-2-[2-(3-fluorobenzylamino)-acetylamino]-3,3-dimethyl-butyramide[2H5]-DPH153893(947mg, 1.09mmol,98%;D5,98.9%,D4, 1.1%)as the methanesulfonic acid salt.[a]20D–17.848(c=0.43%in MeOH).HPLC analysis on Discovery Amide C16Supelco250Â4.6mm,5m m column with elution solvent combination of A(10mmol ammonium formate)and B(acetonitrile), under the gradient conditions of20–30%B in0–5min,30–50%B in5–18min and20–30%B in18–20min and aflow rate of1.0ml/min.The product peak was detected by UV at a wavelength of254nm,and gave>99.6%chemical purity at a retention time of10.7min.HRMS(TOF MS ES+;M+1);calcd. 675.3762,found675.3765.1H NMR(300MHz,d6-DMSO)d0.85(dd,and s, overlapped,15H),1.92(m,1H),2.50(dd,1H overlapped with DMSO),2.80 (m,2H),2.90(m,2H),3.03(s),3.50(s),3.60(m,1H),3.95(m,1H),4.20(d, 1H),4.90(d,1H),5.51(brs),6.70(d,1H),6.80(d,1H),6.90(s,1H),7.10(m, 3H),7.30(m,1H),6.65(d,1H),7.90(d,1H).ESI full MS gave z675(M+1, 100%),397(2%),372(18%),and354(5%).AcknowledgementsThe authors are grateful to Dr Gerald Miwa and Shimoga Prakash (Millennium Pharmaceuticals)for their support.IVE gratefully acknowledges D.R.Schroeder and S.Huang(DAS,BMS Wallingford,CT)for NMR support;Julie Nielsen(DAS,BMS,Wallingford,CT)for the determination of Optical Rotations of Protease Inhibitors and Richard E.Gedamke(ARD, BMS,Lawrenceville,NJ)for the determination of at%D.References1.(a)Kim EE,Baker CT,Dwyer MD,Murcko MA,Rao BG,Tung RD,NaviaMA.J Am Chem Soc1995;117:1181–1182.(b)Tung RD,Murcko MA,Bhissetti GR.Vertex Pharmaceuticals Inc.,1994;WO94/05639.2.(a)Roberts NA,Martin JA,Kinchington D,Broadhurst AV,Craig JC,DuncanIB,Gaplin SA,Handa BA,Kay J,Kroehn A,Lambert RW,Merret JH, Mills JS,Parkes KEB,Redshaw S,Ritchie AJ,Taylor DL,Thomas GJ, Machin PJ.Science1990;248:358.(b)Skinner C,Sedwick A,Bragman K, Pinching AJ,Weber J.Abstracts at the9th International Conference on AIDS,Berlin,1993.(c)Parkes KEB,Bushnell DJ,Crackett PH, Dunsdon SJ,Freeman AC,Gunn MP,Hopkins RA,Lambert RW,Martin JA, Merrett JH,Redshaw S,Spurden WC,Thomas GJ.J Org Chem1994;59: 3656–3664.3.(a)Rich DH,Green J,Toth MV,Marshall GR,Kent SBH.J Med Chem1990;33:1285–1287.(b)Rich DH,Sun CQ,Vara Prasad JVN,Pathiasseril A, Toth MV,Marshall GR,Clara M,Mueller RA,Houseman KJ.J Med Chem 1991;34:1222–1225.(c)Beaulieu PL,Wernic D.J Org Chem1996;61: 3635–3645.4.Kaltenbach III RF,Trainor D,Getman G,Harris S,Diamond M,Saye JJ,Erickson-Viitanen S.Antimicrobial Agents Chemother2001;45:3021–3028.5.Treluyer JM,Bowers G,Cazali N,Sonnier M,Rey E,Pons G,Cresteil T.DrugMetab Dispos2003;31:275–281.6.Solon EG,Yang TD.Dept of Drug Metabolism and Pharmacokinetics,DupontPharmaceutical Company,Newark,DE19714;Personal Communication.7.Albeck A,Persky R.Tetrahedron1994;50:6333–6346.(b)Thottahthil JK,Polniaszek RP.Synlett2000;6:902–904.(c)Rotella DP.Tetrahedron Lett1995;35:5453–5456.(d)Beaulieu PL,Wernic D,Duceppe J-S,Guindon Y.Tetrahedron Lett1995;36:3317–3319.(e)Beier C,Schaumann E,Adiwidjaja G.Synlett1998;1:42–44.8.(a)Lombardo L,Taylor RJK.Synthesis1978;2:131–132.(b)Coutrot P,SnoussiM,Savignac P.Synthesis1978;2:133–134.(c)Monache GD,Di Giovanni MC, Maggio F,Misiti D,Zappia G.Synthesis1995;9:1155–1158.9.Dobarro A,Velasco D.Tetrahedron1996;52:13525–13530.10.(a)Ghosh AK,Hussain KA,Fidanze S.J Org Chem1997;62:6080–6082.(b)Ghosh AK,Fidanze S.J Org Chem1998;63:6146–6152.。
(完整版)介电常数
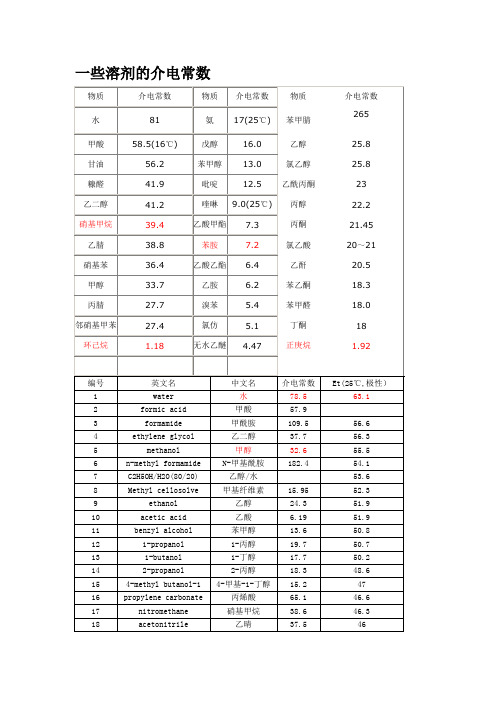
一些溶剂的介电常数介电常数(Dielectric constants)表1列出常见气体在20℃,101 325 Pa条件下的介电常识(ε)。
数据中的有效数字表示测试精度,其中Ar,H2,He,N2,O2,CO2等被推荐为参比数据,其精度为百万分之一或更高。
1 气体的介电常数(Dielectric constants of gases)表1 气体的介电常数Table 1 Dielectric constants of gases2 饱和水蒸气的介电常数(Dielectric constants of saturated water vapor)表2给出不同温度下的液态水成平衡的水蒸气的介电常数。
表2 饱和水蒸气的介电常数Table 2 Dielectric constants of saturated water vapor3 液体的介电常数(Dielectric constants of liquid)表3给出常见液体在指定温度下的介电常数(ε),测试压力为101325Pa。
加*表示测试压力为液体的饱和蒸气压(该温度下其饱和蒸气压大于101325Pa)。
表3 液体的介电常数Table3 Dielectric constants of liquid3 2.58 He 氦-269 1.408I2 碘118 11.1 NH3 氨-77 25 N2氮-195 1.433 N2H4 肼20 52.9 N2O 一氧化二氮0 1.61114.9 CH2Br2 二溴甲烷10 7.77 CH2Cl2二氯甲烷20 9.08 CH2I2二碘甲烷25 5.32 CH2O2甲酸16 58.5 CH3Br 溴甲烷0 9.82 CH3Cl 氯甲烷-20 *12.6 CH3I 碘甲烷20 7.00242C2H4Cl21,1-二氯乙烷18 10.0 C2H4Cl21,2-二氯乙烷25 10.37 C2H4O 乙醛20 21.1 C2H4O 环氧乙烷-1 13.9 C2H4O2乙酸20 6.15 C2H4O2甲酸甲酯20 8.5 C2H5Br 溴乙烷20 9.39 C2H5CI 氯乙烷0 12.2536C3H6O 丙醛17 18.5 C3H6O2 甲酸乙酯25 7.16 C3H6O2乙酸甲脂25 6.68 C3H6O2丙酸40 3.30 C3H6O3 乳酸18 22 C3H7Br 1-溴丙烷25 8.09 C3H7Br 2-溴丙烷25 9.46 C3H7Cl 1-氯丙烷20 7.7 C3H7I 1-碘丙烷20 7.00410C4H10O 2-丁醇25 15.8 C4H10O 2-甲基-2-丙醇30 10.9 C4H10O2-甲基-1-丙醇25 17.7 C4H10O 二乙基醚20 4.335 C4H10O2 1,4丁二醇30 30.2 C4H11S 1-丁硫醇25 4.95 C4H11S2过硫化二乙基25 5.72 C4H11N 丁胺21 5.3 C4H11N 异丁胺21 4.4 C4H11N 二乙基胺22 3.6 C5feO5五羰基铁20 2.60511C5H11Cl 1-氯戊烷11 6.6 C5H11Cl 1-氯-3-甲基丁烷20 6.05 C5H11I 1-碘戊烷20 5.81 C5H11N 哌啶22 5.8 C5H12戊烷20 1.844 C5H12异戊烷20 1.843 C5H12O 1-戊醇25 13.9 C5H12O 2-甲基-2-丁醇25 5.82 C5H12O 3-甲基-1-丁醇25 14.7 C5H12S 1-戊硫醇25 4.55 C5H13N 戊胺22 4.5 C6H4ClNO2o-氯硝基苯50 37.7610C6H10O 环己酮20 18.6 C6H10O 异亚丙基丙酮0 15.6 C6H10O 乙酰乙酸乙酯22 15.7 C6H10O 丙酸酐16 18.3 C6H10O 草酸二乙酯21 8.1 C6H11Cl 氯代环己烷25 7.6 C6H12环己烷25 2.016 C6H12甲基环戊烷20 1.985 C6H12乙基环丁烷20 1.965 C6H12O 环己醇25 15.0 C6H12O 2-己酮14 14.6 C6H12O 4-甲基-2-丁酮20 13.1 C6H12O 3,3-二甲基-2-丁酮14 13.1614C6H15N 二丙基胺21 2.9 C6H18Osi2六甲基二硅氧烷20 2.17 C7H5ClO 苯甲酰氯20 23 C7H6N 苄腈25 25.20 C7H6Cl22,4-二氯甲苯20 6.9 C7H6O 苯甲醛20 17.8 C7H7O 水杨醛30 17.1 C7H7Br p-溴甲苯58 5.49 C7H7Cl o-氯甲苯20 4.45 C7H7Cl m-氯甲苯20 5.55 C7H7Cl o-氯甲苯20 6.08 C7H7Cl 苄基氯13 7.0 C7H7F o-氟甲苯30 4.22 C7H7F m-氟甲苯30 5.42714C7H141-庚烯20 2.05 C7H14O 2-庚酮20 11.95 C7H14O2乙酸戊酯20 4.75 C7H14O2乙酸异戊酯30 4.63 C7H14O2戊酸乙酯18 4.71 C7H14O2丁酸乙酯20 4.3 C7H14O2庚酸71 2.59 C7H15Br 1-溴庚烷25 5.33 C7H15Cl 1-氯庚烷22 5.48 C7H16庚烷20 1.924 C7H162-甲基已烷20 1.919 C7H163-甲基已烷20 1.927 C7H162,2-二甲基戊烷20 1.912 C7H162,3-二甲基戊烷20 1.939 C7H162,4-二甲基戊烷20 1.914811C8H11N N-乙基苯胺20 5.76 C8H14O3丁酸酐20 12.9 C8H16O2辛酸20 2.45 C8H16O2丁酸异丁酯20 4.1 C8H16O2丙酸异戊酯20 4.2 C8H17Br 1-溴辛烷25 5.00 C8H17Cl 1-氯辛烷25 5.05 C8H18辛烷20 1.948 C8H182,2,3-三甲基戊烷20 1.96 C8H182,2,4-三甲基戊烷20 1.940 C8H18O 1-辛醇20 10.34 C8H18O 2-辛醇20 8.20 C8H18O 4-甲基-3-庚醇20 5.25 C8H18O 5-甲基-3-庚醇20 6.13 C8H18O 二丁基醚25 3.06 C8H19N 二异丁基胺22 2.7107C10H8萘85 2.54 C10H10O4邻苯二甲酸二甲酯24 8.5 C10H121,2,3,4-四氢化萘20 2.757 C10H14异丁基苯17 2.35 C10H14叔丁基苯20 2.38 C10H14p-甲基异丙基苯20 2.243 C10H18顺十氢化萘20 2.197 C10H18反十氢化萘20 2.172 C10H20O 薄荷醇42 3.95 C10H22癸烷20 1.991 C10H22O 1-癸烷20 1.983 C11H101-甲基萘20 2.71 C11H24十一烷20 2.005 C12H10联苯75 2.53 C12H10N2O 氧化偶氮苯40 5.1 C12H10O 二苯醚30 3.65 C12H11N 二苯胺53 3.34 固体的介电常数(Dielectric constants of solid)表4给出常见无机固体的介电数(e),对于各向异性的材料则给出几个独立的介电常数。
Fisher 262K过滤器安装和维护说明书

Filtro Fisher™ 262KIntroducciónAlcance del manualEste manual ofrece instrucciones para la instalación y el mantenimiento de los filtros 262K así como información sobre piezas de repuesto.No instalar, utilizar ni dar mantenimiento a un filtro Fisher 262K sin contar con una formación sólida en instalación, utilización y mantenimiento de válvulas, actuadores y accesorios. Para evitar lesiones o daños materiales, es importante leer atentamente, entender y seguir el contenido completo de este manual, incluidas todas sus precauciones y advertencias. Ante cualquier pregunta sobre estas instrucciones, consultar a la oficina de ventas de Emerson antes de continuar.Descripción del productoEl filtro 262K está diseñado para eliminar la suciedad, las cascarillas de óxido y demás cuerpos sólidos que deja el suministro de aire en los actuadores e instrumentos.El filtro 262K está disponible con un cuerpo de hierro fundido (tipo B) o de acero inoxidable (tipo SSB) y conexiones NPT atornilladas. Consultar la tabla 1 para conocer las capacidades térmicas.El filtro tiene un elemento filtrante de celulosa impregnado con resina que es capaz de capturar partículas pequeñas de hasta40 micrones.Figura 1. Filtro Fisher 262K típico W7910Figura 2. Detalles de la estructuraW0225-1ELEMENTO FILTRANTETAPÓN DE TUBERÍALa temperatura máxima superficial depende de lascondiciones operativasGas: T6...T21. No deben sobrepasarse los límites de presión y temperatura indicados en este manual de instrucciones ni ninguna otra norma o código pertinentes.2. Las presiones superiores a este valor pueden ocasionar fallos o fugas de los componentes que contienen presión.EspecificacionesLas especificaciones del filtro 262K se indican en la tabla 1.ADVERTENCIASi personal no cualificado lleva a cabo los procedimientos de instalación, operación y mantenimiento puede resultar peligroso usar este equipo. Como consecuencia, se pueden producir lesiones personales o daños en el equipo. Confiar la instalación, operación y mantenimiento de este filtro a personal cualificado.Si se produce una fuga en el sistema, el gas que escape puede acumularse y ocasionar un incendio o una explosión. Si surgieran problemas, llamar inmediatamente a personal técnico cualificado.InstalaciónADVERTENCIASi el filtro se instala en condiciones que sobrepasen sus capacidades o las especificaciones de la tubería o de las conexiones de tuberías adyacentes, puede escapar el gas o explotar las piezas que contienen presión y producirse lesiones personales, daños del equipo o fugas. Para evitar estos riesgos, instalar el filtro solo si:D Las condiciones de aplicación corresponden con las capacidades de la unidad.D Las condiciones de aplicación cumplen con los códigos, reglamentos o normas pertinentes.D Además, el deterioro físico del filtro puede dar lugar a su ruptura y ocasionar lesiones personales y daños materialescomo consecuencia de escapes de gas. Para evitar estas lesiones o daños, instalar la unidad en un emplazamiento seguro.D Confirmar con el ingeniero de proceso o de seguridad si se deben tomar medidas adicionales para protegerse contra elfluido del proceso.2D Si se está realizando la instalación en una aplicación existente, consultar también la ADVERTENCIA que se encuentra alprincipio de la sección Mantenimiento de este manual de instrucciones.Mediante buenas técnicas de conexión de tuberías, instalar el filtro en el conducto de manera que la dirección del caudal coincida con la flecha marcada en el cuerpo. Como se indica en la figura 2, cuando el filtro 262K se monta en un conducto horizontal, el elemento filtrante debe apuntar hacia abajo. Cuando se monta en un conducto vertical, el elemento filtrante debe también apuntar hacia abajo, de manera que el caudal sea de arriba hacia abajo.Figura 3. Filtro Fisher 262K19B0172-AMantenimientoLas piezas de los filtros están sujetas al desgaste normal y deben examinarse regularmente y reemplazarse cuando sea necesario. La frecuencia de la inspección y del reemplazo depende de la exigencia de las condiciones de la aplicación y de los códigos y reglamentos gubernamentales pertinentes.ADVERTENCIAEvitar las lesiones personales o daños materiales que puedan producirse como consecuencia de una repentina liberación de presión o de un fluido de proceso no controlado. Antes de desmontar, descargar con cuidado toda la presión. Usar unmanómetro para controlar la presión mientras se descarga.Confirmar con el ingeniero de proceso o de seguridad si se deben tomar medidas adicionales para protegerse contra el fluido del proceso.3Pedido de piezasSiempre debe mencionarse el tipo de filtro (hierro fundido, tipo B; acero inoxidable, tipo SSB) cuando se contacte con la oficina de ventas de Emerson con respecto a este filtro.Cuando se pida el juego de repuesto, especificar el número de pieza de 11 caracteres que indica la siguiente lista de piezas.Usar solo piezas de repuesto originales de Fisher. En las válvulas Fisher nunca deben usarse, bajo ninguna circunstancia,componentes que no sean suministrados por Emerson, ya que podrían anular la garantía, perjudicar el funcionamiento de las válvulas y poner en riesgo la seguridad del personal y dañar el equipo.Lista de piezasNotaConsultar a la oficina de ventas de Emerson para obtener información sobre el pedido de piezas.Filtro 262K (figura 3)Clave Descripción Número de pieza1Body2Drain Plug 3Filter Element 4Cartridge Gasket 5Cap Gasket 6Pipe Plug *Repair Kit19B0173X012*Repuestos recomendadosEmerson Automation Solutions Marshalltown, Iowa 50158 USA Sorocaba, 18087 Brazil Cernay, 68700 FranceDubai, United Arab Emirates Singapore 128461 SingaporeEl contenido de esta publicación se presenta con fines informativos solamente y, aunque se han realizado todos los esfuerzos posibles para asegurar su exactitud, no debe tomarse como garantías, expresas o implícitas, que acogen los productos o los servicios descritos en esta publicación o su uso o aplicación.Todas las ventas se rigen por nuestros términos y condiciones, que están disponibles si se solicitan. Nos reservamos el derecho de modificar o mejorar los diseños o especificaciones de los productos en cualquier momento sin previo aviso.Emerson, Emerson Automation Solutions y sus entidades afiliadas no se hacen responsables de la selección, el uso o el mantenimiento de ningún responsabilidad de la selección, del uso y del mantenimiento correctos de cualquier producto corresponde exclusivamente al comprador y al usuario final.Fisher es una marca de una de las compañías de la unidad comercial Emerson Automation Solutions de Emerson Electric Co. Emerson Automation Solutions,Emerson y el logotipo de Emerson son marcas comerciales y marcas de servicio de Emerson Electric Co. Todas las demás marcas son propiedad de sus respectivos dueños.。
Perkadox 16产品数据表说明书

Product Data SheetPerkadox 16Di(4-tert-butylcyclohexyl) peroxydicarbonatePerkadox® 16 is applied as an initiator for the suspension and mass polymerization of vinyl chloride in the temperature range between 40°C and 65°C. Perkadox® 16 can be used alone or in combination with other peroxides, such as 1,1,3,3-Tetramethylbutyl peroxyneodecanoate (Trigonox 423), Cumyl peroxyneodecanoate (Trigonox 99) or Dilauroyl peroxide (Laurox), to increase reactor efficiency.CAS number15520-11-3EINECS/ELINCS No.239-557-1TSCA statuslisted on inventoryMolecular weight398.5SpecificationsAppearance White powderAssay94.0-97.0 %Inorganic + organic hydrolysable chloride≤ 4000 mg/kgCharacteristicsBulk density, 20 °C450-480 kg/m³Density, 20 °C 1.13 g/cm³ApplicationsPerkadox® 16 can be used for the market segments: polymer production, thermoset composites and acrylics with their different applications/functions. For more information please check our website and/or contact us.Half-life dataThe reactivity of an organic peroxide is usually given by its half-life (t½) at various temperatures. For Perkadox® 16 in chlorobenzene:0.1 hr82°C (180°F)1 hr64°C (147°F)10 hr48°C (118°F)Formula 1kd = A·e-Ea/RTFormula 2t½ = (ln2)/kdEa126.39 kJ/moleA7.44E+15 s-1R8.3142 J/mole·KT(273.15+°C) KThermal stabilityOrganic peroxides are thermally unstable substances, which may undergo self-accelerating decomposition. The lowest temperature at which self-accelerating decomposition of a substance in the original packaging may occur is the Self-Accelerating Decomposition Temperature (SADT). The SADT is determined on the basis of the Heat Accumulation Storage Test.SADT40°CEmergency temperature (Tₑ)35°CControl temperature (Tc)30°CMethod The Heat Accumulation Storage Test is a recognized test method for thedetermination of the SADT of organic peroxides (see Recommendations on theTransport of Dangerous Goods, Manual of Tests and Criteria – United Nations,New York and Geneva).StorageDue to the relatively unstable nature of organic peroxides a loss of quality can be detected over a period of time. To minimize the loss of quality, Nouryon recommends a maximum storage temperature (Ts max. ) for each organic peroxide product.Ts max.20°C (please see note below)Note When stored under the recommended storage conditions, Perkadox® 16 willremain within the Nouryon specifications for a period of at least 3 months afterdelivery. The Ts max of 20°C is not to be interpretated as ambient or roomtemperature as this differs per region and season. Perkadox® 16 has a high qualitycomposition and to hold that it should be stored at below 20°C. At temperaturesabove 20°C the decomposition of Perkadox® 16 progresses fast which leads tosignificant loss of quality. If you have questions about this, please contact yourlocal Nouryon account manager for advice.Packaging and transportIn North America Perkadox® 16 is packed in non-returnable cartons containing 25 polyethylene bags of 1 lb net weightor 5 polyethylene bags of 5 lb net weight. In other regions the standard packaging is a cardboard box for 20 kg peroxide. Both packaging and transport meet the international regulations. For the availability of other packed quantities contact your Nouryon representative. Perkadox® 16 is classified as Organic peroxide type C; solid, temperature controlled; Division 5. 2; UN 3114.Safety and handlingKeep containers tightly closed. Store and handle Perkadox® 16 in a dry well-ventilated place away from sources of heat or ignition and direct sunlight. Never weigh out in the storage room. Avoid contact with reducing agents (e. g. amines), acids, alkalis and heavy metal compounds (e. g. accelerators, driers and metal soaps). Please refer to the Safety Data Sheet (SDS) for further information on the safe storage, use and handling of Perkadox® 16. This information should be thoroughly reviewed prior to acceptance of this product. The SDS is available at /sds-search.Major decomposition products Carbon dioxide, 4-tert-Butyl-cyclohexanolAll information concerning this product and/or suggestions for handling and use contained herein are offered in good faith and are believed to be reliable.Nouryon, however, makes no warranty as to accuracy and/or sufficiency of such information and/or suggestions, as to the product's merchantability or fitness for any particular purpose, or that any suggested use will not infringe any patent. Nouryon does not accept any liability whatsoever arising out of the use of or reliance on this information, or out of the use or the performance of the product. Nothing contained herein shall be construed as granting or extending any license under any patent. Customer must determine for himself, by preliminary tests or otherwise, the suitability of this product for his purposes.The information contained herein supersedes all previously issued information on the subject matter covered. The customer may forward, distribute, and/or photocopy this document only if unaltered and complete, including all of its headers and footers, and should refrain from any unauthorized use. Don’t copythis document to a website.Perkadox® and Trigonox are registered trademarks of Nouryon Functional Chemicals B.V. or affiliates in one or more territories.Contact UsPolymer Specialties Americas************************Polymer Specialties Europe, Middle East, India and Africa*************************Polymer Specialties Asia Pacific************************2023-1-10© 2023Polymer production Perkadox 16。
UN编号查询

详情
供应商
93-82-3
硬脂酰胺DEA;
硬脂酰胺DEA;
Octadecanamide,N,N-bis(2-hydroxyethyl)-;
AlkamideDS-280;
Amisol SDE;
Clindrol 868;
Cyclomide SD;
Diethanolamine stearic acidamide;
C12H15NO2
详情
供应商
124-26-5
硬脂酰胺;
十八酰胺
stearamide;
Stearamide(6CI,7CI,8CI);
AP 1;
Adogen 42;
Advawax 290;
Alflow S 10;
Amide AP;
Amide S;
Amide S (binder);
Amide T;
Armid 18;
Mercury,[3-(a-carboxy-o-anisamido)-2-hydroxypropyl]hydroxy-(8CI);
Acetic acid, [2-[[(2-hydroxypropyl)amino]carbonyl]phenoxy]-, mercurycomplex;
Mercuderamid;
Armoslip 18LF;
Atmer SA 1750;
Crodamide S;
Crodamide SR;
Diamid AP 1;
EBF;
Fatty Amide S;
Fatty Amide T;
G 270;
Hidorin F792;
Hidorin M 7;
Hymicron G 270;
CleanGredients 产品指南:更安全的选择成分说明书

Second generation bio-based water soluble polymer which is more sustainably sourced and readily biodegradable.
• Anti-redeposition, anti-encrustation and anti-film forming • Best-in-class readily biodegradable water soluble polymer • Dispersant and anti-scalent with improved calcium, iron and
• Available as aqueous solution or powder
Unique, high-purity hydrotroping acrylic/styrene copolymer.
• Hydrotroping of surfactants into heavy duty liquids • Particulate and oily soil dispersion/suspension • Protective corrosion inhibition • Hypochlorite stable • Calcium binding
• Hard surface cleaning • Vehicle cleaning • Water based alkaline cleaners • Microemulsions • All Purpose Cleaners (APC)
• All Purpose Cleaners (APC) • Hydrotroping • Acidic and alkaline cleaners
化学品英文名称

Filling material A AC 45 UBI / FR 310 5000 Acetic acid (5% at 20°C) Acetic acid (5% at 50°C) Acetic acid (at 40°C) Acetic acid (at 50°C) Acetic acid anhydride Acetoacetate, ethylAcetoacetate, methylAcetone Acetulan Acetyl chloride Acricid 40 EC Acricid liquid Acryl amide Acrylat 38092, solvent base Acrylic acid Acrylic Polymere RDZ 1263 Acrylic Polymere RDZ 2738 Acrylic Polymere RDZ 2771 Acrylic Polymere RDZ 958 Acrylic Polymere RZ 20810 Acrylic Polymere RZ 21376 Acrylic Polymere RZ 421 Activated carbon Activoll EFL Addipast 350 WD black Additol XK 391 Additol XK 406 Adhesion Promoter AMS 70 After Shave Afugan Aircraft fuel Aircraft fuel, Jet A1 Akypo RO 90 VG Akypo RO 90 VG Aldehyde C12 Aldehyde C8 Aldurol VUP 21 Aldurol VUP 51 Alkydal R 35 W Allylic heptylate
罗地亚产品目录

HLB:8/10.5/11.4/12.1/13.3/13.7
浊点:48-50/64-66/69-72/73-75/57-59/75-77
FentacareF08
无溶剂C9-C11烷基乙氧基化物
RhodafacRP-710
有机磷酸酯阴离子表面活性剂
微浊液体
245KG/桶
低泡,良好清洗、润湿、乳化性能;高电流忍受能力,可用于电解除油、化学除油除蜡和PCB除油整孔,也可以和低泡非离子复配,用于喷淋。
RhodocalTS-60/MX
磷酸酯类阴离子表面活性剂
粘稠液体
200KG/桶
含氨基的阴离子表面活性剂,低泡,高电流负荷稳定,常与油酸类的表活复配用于电解除油粉
产品名称
化学成分
外观
产品特点及应用
MirapolWT
阳离子聚合物
淡黄色液体
200KG/桶
无氰碱性镀锌的走位剂、整平剂,能明显缩短电镀时间,改善镀层的均匀性和深镀能力,节省锌的用量,同时提高镀层的耐腐蚀能力。
PH:7.5-8.5;添加量:1.5g/L;消耗量:40-70g/KAh
沉降剂:主用于磨削液中吸附金属屑沉降
黄色液体
200KG/桶
用于水基清洗配方表面活性剂混合物,低泡、耐酸碱,对于清除油脂类的有机油污非常有效,经常可以取代溶剂,降低成本,适用于高压和低压清洗设备。
Antarox L61/62/64
EO/PO嵌段共聚物
透明液体
204.12KG/桶
泡沫控制能力优异,适用范围广。可替代陶氏TergitolL、巴斯夫Pluronic PE、亨斯曼Surfonic POA-L系列。
弗罗里硅固相萃取柱测定废水中的64种半挥发性有机物

弗罗里硅固相萃取柱测定废水中的 64种半挥发性有机物摘要:本文对固相萃取法测定废水样品中的SVOCs进行了探讨。
废水样品先用滤纸过滤,再通过3μm滤膜过滤。
取1L过滤后的水样,经过硅酸镁固相萃取小柱处理后上机测试。
同时做64种SVOCs的检出限测试,并对废水中SVOCs加标进行了回收率和相对标准偏差测试,结果表明加标回收率在65.2-124.3%,相对标准偏差在0.94%~9.78%之间;64种半挥发性有机物检出限分别在0.20μg/L~0.60μg/L之间。
关键词:废水;半挥发性有机物;固相萃取;加标回收率;相对标准偏差;检出限1、引言半挥发性有机物(SVOCs),是指沸点一般在170~350℃之间、蒸汽压在13.3~10.5Pa的有机物,包括多环芳烃、氯苯类、硝基苯类、有机农药、亚硝基胺类、苯胺类和苯酚类等化合物。
随着经济的发展,目前SVOCs对水环境的污染日益严重。
国家相关部门对水中SVOCs提出了明确的质量要求。
目前水中SVOCs的测定大多数采用EPA 8270E:2018标准中的液液萃取气质联用法,此方法检测有机溶剂用量大,易产生乳化现象,且破乳操作较难、重现性差,整个前处理过程用时较长。
本文尝试采用弗罗里硅固相萃取小柱对废水中的SVOCs进行富集、浓缩,采用气质联用法测定64种SVOCs取得满意效果。
使样品前处理过程大幅缩短,有利于大规模样品测试。
2、仪器和设备(1)气相色谱质谱联用仪:美国安捷伦7890b/5977b;毛细色谱柱:HP-5MS (30m×0.32mm×0.25μm),安捷伦;(2)全自动氮吹浓缩仪: LABTECH ,Multivap-10型;(3)固相萃取装置:LABTECH,W-SPE24 ;(4)固相萃取小柱:弗罗里硅柱;(5)隔膜真空泵1.标准品1)64种SVOCs混和标准溶液:1000mg/L;1.内标溶液:4000mg/L;2.替代物标准溶液:2000mg/l;3.十氟三苯基膦(DFTPP):ρ=50 mg/L;4、化学试剂耗材1)甲醇 (CH3OH):HPLC 级2)乙酸乙酯:HPLC级3)超纯水4)二氯甲烷:HPLC级5、样品前处理5.1样品准备将取好的废水样品,先用滤纸过滤掉泥沙等杂质,再用3μm滤膜过滤。
有机溶剂极性表

有机溶剂极性表极性与非极性是针对分子说的。
首先化学共价键分为极性键与非极性键.非极性键就是共用电子对没有偏移,出现在单质中比如O2;极性键就是共用电子对有偏移比如HCl。
而当偏移的非常厉害之后,看上去一边完全失电子另一边得到了电子,就会变成离子键了,如NaCl再说极性分子与与非极性分子。
由于极性键的出现,所以就使某些分子出现了电极性,但是并不是说所有有极性键的分子都是极性分子。
比如CH4,虽然含有4个极性的C-H键,但是因为其空间上成对称的正四面体结构,所以键的极性相消,整个分子没有极性对与H2O,虽然与CO2有相同类型的分子式,也同样有极性共价键,但二者分子的极性却不同。
CO2是空间对称的直线型,所以分子是非极性分子,H2O是折线型,不对称,所以是极性分子,作为溶剂称为极性溶剂非极性溶剂由非极性分子组成,是指分子中各原子的化学键的合力为零,如CCl4分子为正四面体,四个C—Cl键的合力为零,CCl4分子无极性,CCl4就是非极性溶剂非极性溶剂"脂肪油(fattyoils) ”液状石蜡(1iquidparaffin) ”醋酸乙酯(ethyloleate) ”肉豆蔻酸异丙酯(isopropylmyristate)非极性溶剂是由非性分子溶液组成的溶剂,非极性分子多由共价键构成,无或电子活性很小。
最简单准确的说就是偶极矩为零的溶剂。
知道什么是偶极矩吗?非极性溶剂是由非性分子溶液组成的溶剂,非极性分子多由共价键构成,无或电子活性很小。
偶极矩小的溶剂溶剂英文名称solvent又称溶媒。
能溶解气体、固体、液体而成为均匀混合物的一种液体。
习惯上把气体和固体叫溶质,液体叫溶剂.对于两种液体所组成的溶液,通常把含量较多的组分叫溶剂,少者叫溶质.分为无机溶剂和有机溶剂两大类。
水是应用最广泛的无机溶剂,酒精、汽油、氯仿及丙酮等是常用的有机溶剂.溶剂是一种可以溶化固体,液体或气体溶质的液体,继而成为溶液.在日常生活中最普遍的溶剂是水。
