Fenoterol_hydrobromide_DataSheet_MedChemExpress
LUNA SENSATION 藻类杀菌剂数据安全表说明书

LUNA SENSATION®1/11Version 4.0 / USA Revision Date: 06/30/2020102000012886 Print Date: 06/30/2020SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKINGProduct identifierTrade nameLUNA SENSATION®Product code (UVP)84469882SDS Number102000012886EPA Registration No. 264-1090Relevant identified uses of the substance or mixture and uses advised againstUseFungicideRestrictions on useSee product label for restrictions.Information on supplierSupplier Bayer CropScience LP 800 North Lindbergh Blvd. St. Louis, MO 63167 USAResponsible DepartmentEmail: ************************Emergency telephone no.Emergency Telephone Number (24hr/ 7 days)1-800-334-7577Product Information Telephone Number 1-866-99BAYER (1-866-992-2937)SECTION 2: HAZARDS IDENTIFICATIONClassification in accordance with regulation HCS 29CFR §1910.1200 Acute toxicity(Oral): Category 4Reproductive toxicity: Effects on or via lactationLabelling in accordance with regulation HCS 29CFR §1910.1200Signal word : WarningHazard statementsHarmful if swallowed.May cause harm to breast-fed children.LUNA SENSATION®2/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020 Precautionary statementsWash thoroughly after handling.Do not eat, drink or smoke when using this product.Obtain special instructions before use.Do not breathe mist.Avoid contact during pregnancy/ while nursing.IF SWALLOWED: Call a POISON CENTER/doctor/physician if you feel unwell.Rinse mouth.IF exposed or concerned: Get medical advice/ attention.Dispose of contents/container in accordance with local regulation.Hazards Not Otherwise Classified (HNOC)No physical hazards not otherwise classified.No health hazards not otherwise classified.SECTION 3: COMPOSITION/INFORMATION ON INGREDIENTSHazardous Component Name CAS-No.Concentration % by weight Fluopyram 658066-35-4 21.4 Trifloxystrobin 141517-21-7 21.4 SECTION 4: FIRST AID MEASURESDescription of first aid measuresGeneral advice When possible, have the product container or label with you whencalling a poison control center or doctor or going for treatment.Inhalation Move to fresh air. If person is not breathing, call 911 or an ambulance,then give artificial respiration, preferably mouth-to-mouth if possible.Call a physician or poison control center immediately.Skin contact Take off contaminated clothing and shoes immediately.Wash offimmediately with plenty of water for at least 15 minutes.Call aphysician or poison control center immediately.Eye contact Hold eye open and rinse slowly and gently with water for 15-20minutes.Remove contact lenses, if present, after the first 5 minutes,then continue rinsing eye.Call a physician or poison control centerimmediately.Ingestion Call a physician or poison control center immediately.Rinse out mouthand give water in small sips to drink.DO NOT induce vomiting unlessdirected to do so by a physician or poison control center.Never giveanything by mouth to an unconscious person.Do not leave victimunattended.Most important symptoms and effects, both acute and delayedSymptoms To date no symptoms are known.Indication of any immediate medical attention and special treatment neededLUNA SENSATION®3/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020 Treatment Appropriate supportive and symptomatic treatment as indicated by thepatient's condition is recommended.SECTION 5: FIREFIGHTING MEASURESExtinguishing mediaSuitable Water spray, Carbon dioxide (CO2), Alcohol-resistant foam, Sand Unsuitable High volume water jetSpecial hazards arising from the substance or mixture In the event of fire the following may be released:, Hydrogen chloride (HCl), Hydrogen cyanide (hydrocyanic acid), Hydrogen fluoride, Carbon monoxide (CO), Carbon dioxide (CO2), Nitrogen oxides (NOx)Advice for firefightersSpecial protective equipment for firefighters Firefighters should wear NIOSH approved self-contained breathing apparatus and full protective clothing.Further information Keep out of smoke. Fight fire from upwind position. Cool closedcontainers exposed to fire with water spray. Do not allow run-off fromfire fighting to enter drains or water courses.Flash point> 100 °CAuto-ignition temperature 380 °C / 716 °FLower explosion limit No data availableUpper explosion limit No data availableExplosivity Not explosive92/69/EEC, A.14 / OECD 113SECTION 6: ACCIDENTAL RELEASE MEASURESPersonal precautions, protective equipment and emergency proceduresPrecautions Keep unauthorized people away. Isolate hazard area. Avoid contactwith spilled product or contaminated surfaces.Methods and materials for containment and cleaning upMethods for cleaning up Soak up with inert absorbent material (e.g. sand, silica gel, acidbinder, universal binder, sawdust). Clean contaminated floors andobjects thoroughly, observing environmental regulations. Collect andtransfer the product into a properly labelled and tightly closedcontainer.Additional advice Use personal protective equipment. If the product is accidentallyspilled, do not allow to enter soil, waterways or waste water canal.LUNA SENSATION®4/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020 Reference to other sections Information regarding safe handling, see section 7.Information regarding personal protective equipment, see section 8.Information regarding waste disposal, see section 13.SECTION 7: HANDLING AND STORAGEPrecautions for safe handlingAdvice on safe handling Use only in area provided with appropriate exhaust ventilation. Handleand open container in a manner as to prevent spillage.Hygiene measures Wash hands thoroughly with soap and water after handling and beforeeating, drinking, chewing gum, using tobacco, using the toilet orapplying cosmetics.Remove Personal Protective Equipment (PPE) immediately afterhandling this product. Before removing gloves clean them with soap andwater. Remove soiled clothing immediately and clean thoroughly beforeusing again. Wash thoroughly with soap and water after handling. Conditions for safe storage, including any incompatibilitiesRequirements for storage areas and containers Store in a cool, dry place and in such a manner as to prevent cross contamination with other crop protection products, fertilizers, food, and feed. Store in original container and out of the reach of children, preferably in a locked storage area. Protect from freezing. Keep away from direct sunlight.SECTION 8: EXPOSURE CONTROLS/PERSONAL PROTECTIONControl parameters*OES BCS: Internal Bayer AG, Crop Science Division "Occupational Exposure Standard"Exposure controlsPersonal protective equipmentIn normal use and handling conditions please refer to the label and/or leaflet. In all other cases the following recommendations would apply.Respiratory protection When respirators are required, select NIOSH approved equipmentbased on actual or potential airborne concentrations and inaccordance with the appropriate regulatory standards and/or industryrecommendations.Hand protection Chemical resistant nitrile rubber glovesEye protection Safety glasses with side-shieldsLUNA SENSATION®5/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020 Skin and body protection Wear long-sleeved shirt and long pants and shoes plus socks. General protective measures Follow manufacturer's instructions for cleaning/maintaining PPE. Ifno such instructions for washables, use detergent and warm/tepidwater.Keep and wash PPE separately from other laundry.SECTION 9. PHYSICAL AND CHEMICAL PROPERTIESAppearance white to beigePhysical State suspensionOdor characteristicOdour Threshold No data availablepH 5.0 - 8.0 (100 %) (23 °C)Viscosity, kinematic No data availableVapor Pressure No data availableVapor Density (Air = 1)No data availableDensity ca. 1.17 g/cm³ (20 °C)Evaporation rate No data availableBoiling Point No data availableMelting / Freezing Point No data availableWater solubility suspensiveMinimum Ignition Energy Not applicableDecompositionStable under normal conditions.temperatureSelf-accelaratingNo data availabledecomposition temperature(SADT)Partition coefficient: n-Not applicableoctanol/waterViscosity240 - 350 mPa.s (20 °C) Velocity gradient 20 /sFlammability No data availableOxidizing properties No oxidizing propertiesFlash point> 100 °CAuto-ignition temperature 380 °C / 716 °FLower explosion limit No data availableUpper explosion limit No data availableLUNA SENSATION®6/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020 Explosivity Not explosive92/69/EEC, A.14 / OECD 113Particle size No data availableOther information Further safety related physical-chemical data are not known.SECTION 10: STABILITY AND REACTIVITYReactivityThermal decomposition Stable under normal conditions.Chemical stability Stable under recommended storage conditions.Possibility of hazardous reactions No hazardous reactions when stored and handled according to prescribed instructions.Conditions to avoid Extremes of temperature and direct sunlight.Incompatible materials No incompatible materials known.Hazardous decompositionproductsNo decomposition products expected under normal conditions of use. SECTION 11: TOXICOLOGICAL INFORMATIONExposure routes Skin Absorption, Ingestion, Inhalation, Eye contactImmediate EffectsSkin Harmful if absorbed through skin.Ingestion Harmful if swallowed.Inhalation Harmful if inhaled.Information on toxicological effectsAcute oral toxicity LD50 (female Rat) 2,000 mg/kgAcute inhalation toxicity LC50 (Rat) > 1.7 mg/lExposure time: 4 hDetermined in the form of liquid aerosol.Highest attainable concentration.No deathsAcute dermal toxicity LD50 (Rat) > 2,000 mg/kgSkin corrosion/irritation No skin irritation (Rabbit)Serious eye damage/eyeirritationNo eye irritation (Rabbit)LUNA SENSATION®7/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020Respiratory or skin sensitisation Skin: Non-sensitizing. (Mouse)OECD Test Guideline 429, local lymph node assay (LLNA)Assessment STOT Specific target organ toxicity – single exposureFluopyram: Based on available data, the classification criteria are not met.Trifloxystrobin: Based on available data, the classification criteria are not met.Assessment STOT Specific target organ toxicity – repeated exposureFluopyram did not cause specific target organ toxicity in experimental animal studies.Trifloxystrobin did not cause specific target organ toxicity in experimental animal studies.Assessment mutagenicityFluopyram was not mutagenic or genotoxic in a battery of in vitro and in vivo tests.Trifloxystrobin was not mutagenic or genotoxic in a battery of in vitro and in vivo tests.Assessment carcinogenicityFluopyram caused at high dose levels an increased incidence of tumours in rats in the followingorgan(s): Liver.Fluopyram caused at high dose levels an increased incidence of tumours in mice in the followingorgan(s): Thyroid.The tumours seen with Fluopyram were caused through a non-genotoxic mechanism, which is not relevant at low doses. The mechanism that triggers these tumours is not relevant to humans. Trifloxystrobin was not carcinogenic in lifetime feeding studies in rats and mice.ACGIHNone.NTPNone.IARCNone.OSHANone.Assessment toxicity to reproductionFluopyram caused reproduction toxicity in a two-generation study in rats only at dose levels also toxic to the parent animals. The reproduction toxicity seen with Fluopyram is related to parental toxicity. Trifloxystrobin caused reduced body weight development in offspring during lactation only at doses also producing systemic toxicity in adult rats.Assessment developmental toxicityFluopyram caused developmental toxicity only at dose levels toxic to the dams. The developmental effects seen with Fluopyram are related to maternal toxicity.Trifloxystrobin caused developmental toxicity only at dose levels toxic to the dams. The developmental effects seen with Trifloxystrobin are related to maternal toxicity.Aspiration hazardBased on available data, the classification criteria are not met.LUNA SENSATION®8/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020 Further informationOnly acute toxicity studies have been performed on the formulated product.The non-acute information pertains to the active ingredient(s).SECTION 12: ECOLOGICAL INFORMATIONToxicity to fish LC50 (Oncorhynchus mykiss (rainbow trout)) 0.091 mg/lExposure time: 96 hToxicity to aquatic invertebratesEC50 (Daphnia magna (Water flea)) 0.086 mg/lExposure time: 48 hLC50 (Mysidopsis bahia (mysid shrimp)) 0.00862 mg/l Exposure time: 96 hThe value mentioned relates to the active ingredient trifloxystrobin.Toxicity to aquatic plants IC50 (Raphidocelis subcapitata (freshwater green alga)) 0.292 mg/lGrowth rate; Exposure time: 72 hEC10 (Desmodesmus subspicatus (green algae)) 0.0025 mg/lGrowth rate; Exposure time: 72 hThe value mentioned relates to the active ingredient trifloxystrobin. Biodegradability Fluopyram:Not rapidly biodegradableTrifloxystrobin:Not rapidly biodegradableKoc Fluopyram: Koc: 279Trifloxystrobin: Koc: 2377Bioaccumulation Fluopyram: Bioconcentration factor (BCF) 18Does not bioaccumulate.Trifloxystrobin: Bioconcentration factor (BCF) 431Does not bioaccumulate.Mobility in soil Fluopyram: Moderately mobile in soilsTrifloxystrobin: Slightly mobile in soilsAdditional ecologicalinformationNo other effects to be mentioned.Environmental precautions Do not apply directly to water, to areas where surface water is presentor to intertidal areas below the mean high water mark.Drift and runoff from treated areas may be hazardous to aquaticorganisms in adjacent sites.Do not apply when weather conditions favor runoff or drift.Do not allow product to enter streams, sewers or other waterways.Do not contaminate surface or ground water by cleaning equipment ordisposal of wastes, including equipment wash water.Apply this product as specified on the label.LUNA SENSATION®9/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020 SECTION 13: DISPOSAL CONSIDERATIONSWaste treatment methodsProduct Dispose in accordance with all local, state/provincial and federalregulations.Pesticide, spray mixture or rinse water that cannot be used according tolabel instructions may be disposed of on site or at an approved wastedisposal facility.Follow advice on product label and/or leaflet.Contaminated packaging Do not re-use empty containers.Triple rinse containers.Completely empty container into application equipment, then dispose ofempty container in a sanitary landfill, by incineration or by otherprocedures approved by state/provincial and local authorities.If burned, stay out of smoke.Follow advice on product label and/or leaflet.RCRA Information Characterization and proper disposal of this material as a special orhazardous waste is dependent upon Federal, State and local laws andare the user's responsibility. RCRA classification may apply.SECTION 14: TRANSPORT INFORMATION49CFR Not dangerous goods / not hazardous materialIMDGUN number 3082Class 9Packaging group IIIMarine pollutant YESProper shipping name ENVIRONMENTALLY HAZARDOUS SUBSTANCE, LIQUID,N.O.S.(TRIFLOXYSTROBIN SOLUTION)IATAUN number 3082Class 9Packaging group IIIEnvironm. Hazardous Mark YESProper shipping name ENVIRONMENTALLY HAZARDOUS SUBSTANCE, LIQUID,N.O.S.(TRIFLOXYSTROBIN SOLUTION )This transportation information is not intended to convey all specific regulatory information relating to this product. It does not address regulatory variations due to package size or special transportation requirements.LUNA SENSATION®10/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020 Freight Classification: INSECTICIDES OR FUNGICIDES, N.O.I., OTHER THANPOISONSECTION 15: REGULATORY INFORMATIONEPA Registration No.264-1090US Federal RegulationsTSCA listWater 7732-18-51,2-Propanediol 57-55-6Polyethylene-polypropylene copolymer 9003-11-6US. Toxic Substances Control Act (TSCA) Section 12(b) Export Notification (40 CFR 707, Subpt D) No export notification needs to be made.SARA Title III - Section 302 - Notification and InformationNot applicable.SARA Title III - Section 313 - Toxic Chemical Release ReportingNone.US States Regulatory ReportingCA Prop65This product does not contain any substances known to the State of California to cause cancer.This product does not contain any substances known to the State of California to causereproductive harm.US State Right-To-Know Ingredients1,2-Propanediol 57-55-6 MN, RINone.EPA/FIFRA Information:This chemical is a pesticide product registered by the Environmental Protection Agency and is subject to certain labeling requirements under federal pesticide law. These requirements differ from the classification criteria and hazard information required for safety data sheets, and for workplace labels of non-pesticide chemicals. Following is the hazard information required on the pesticide label:Signal word:Caution!Hazard statements:Harmful if swallowed, inhaled or absorbed through the skin.Avoid contact with skin, eyes and clothing.Avoid inhalation of vapour or mist.SAFETY DATA SHEETLUNA SENSATION®11/11 Version 4.0/USA Revision Date: 06/30/2020 102000012886Print Date: 06/30/2020 SECTION 16: OTHER INFORMATIONAbbreviations and acronyms49CFR Code of Federal Regulations, Title 49ACGIH US. ACGIH Threshold Limit ValuesATE Acute toxicity estimateCAS-Nr. Chemical Abstracts Service numberCERCLA Comprehensive Environmental Response, Compensation, and Liability Act EINECS European inventory of existing commercial substancesELINCS European list of notified chemical substancesIARC International Agency for Research on CancerIATA International Air Transport AssociationIMDG International Maritime Dangerous GoodsN.O.S. Not otherwise specifiedNTP US. National Toxicology Program (NTP) Report on CarcinogensOECD Organization for Economic Co-operation and DevelopmentTDG Transportation of Dangerous GoodsTWA Time weighted averageUN United NationsWHO World health organisationNFPA 704 (National Fire Protection Association):Health - 2 Flammability - 1 Instability - 0 Others - noneHMIS (Hazardous Materials Identification System, based on the Third Edition Ratings Guide) Health - 2 Flammability - 1 Physical Hazard - 0 PPE -0 = minimal hazard, 1 = slight hazard, 2 = moderate hazard, 3 = severe hazard, 4 = extreme hazard Reason for Revision: The following sections have been revised: Section 2: Hazards Identification. Section 3: Composition / Information on Ingredients. Section 11: Toxicological Information. Section 12. Ecological information. Reviewed and updated for general editorial purposes.Revision Date: 06/30/2020This information is provided in good faith but without express or implied warranty. The customer assumes all responsibility for safety and use not in accordance with label instructions. The product names are registered trademarks of Bayer.。
High-powerlithiumbatteriesfromfunctionalizedcarbon-nanotubeelectrodes

High-power lithium batteries from functionalized carbon-nanotube electrodesSeung Woo Lee 1†,Naoaki Yabuuchi 2†,Betar M.Gallant 2,Shuo Chen 2,Byeong-Su Kim 1,Paula T.Hammond 1and Yang Shao-Horn 2,3*Energy storage devices that can deliver high powers have many applications,including hybrid vehicles and renewable energy.Much research has focused on increasing the power output of lithium batteries by reducing lithium-ion diffusion distances,but outputs remain far below those of electrochemical capacitors and below the levels required for many applications.Here,we report an alternative approach based on the redox reactions of functional groups on the surfaces of carbon yer-by-layer techniques are used to assemble an electrode that consists of additive-free,densely packed and functionalized multiwalled carbon nanotubes.The electrode,which is several micrometres thick,can store lithium up to a reversible gravimetric capacity of ∼200mA h g 21electrode while also delivering 100kW kg electrode 21of power and providing lifetimes in excess of thousands of cycles,both of which are comparable to electrochemical capacitor electrodes.A device using the nanotube electrode as the positive electrode and lithium titanium oxide as a negative electrode had a gravimetric energy ∼5times higher than conventional electrochemical capacitors and power delivery ∼10times higher than conventional lithium-ion batteries.Amajor challenge in the field of electrical energy storage is to bridge the performance gap between batteries and electroche-mical capacitors by developing materials that can combine the advantages of both devices.Batteries exhibit high energy as a result of Faradaic reactions in the bulk of active particles,but are rate-limited.Electrochemical capacitors 1–3can deliver high power at the cost of low energy storage by making use of surface ion adsorption (referred to as double-layer capacitance)and surface redox reactions (referred to as pseudo-capacitance).Lithium rechargeable batteries ( 150W h kg cell 21and 1kW kg cell 21)therefore have higher gravimetric energy but lower power capability than electrochemical capacitors ( 5W h kg cell 21and 10kW kg cell 21)1.Energy and power versatility are crucial for hybrid applications 2.For example,although conventional batteries have been used in light vehicles,hybrid platforms for heavy vehicles and machineries demand delivery of much higher currents,so higher energy and comparable power capability relative to electrochemical capacitors are needed to meet this demand 1,2.Considerable research efforts have been focused on increasing the power characteristics of lithium rechargeable batteries by redu-cing the dimensions of lithium storage materials down to the nano-metre scale 4–12,which would reduce the lithium diffusion time that accompanies the Faradaic reactions of active particles.However,nanostructured lithium storage electrodes 5,13still have a lower power capability than electrochemical capacitor electrodes.On the other hand,researchers have shown that the gravimetric energy of electrochemical capacitors can be increased by using electrode materials with enhanced gravimetric capacitances (gravimetric charge storage per volt),which can be achieved through the use of carbon subnanometre pores for ion adsorption 1,14or by taking advantage of the pseudocapacitance of nanostructured transition metal oxides 15–17.The high cost of ruthenium-based oxides is prohibitive for many applications,and the cycling instability of manganese-based oxides 17–19remains a major technical challenge.A promising approach is to use the Faradaic reactions of surface functional groups on nanostructured carbon electrodes,which can store more energy than the double-layer capacitance on convention-al capacitor electrodes 1and also provide high power capability.Here,we report the use of an entirely different class of electrodes for lithium storage,which are based on functionalized multiwalled carbon nanotubes (MWNTs)that include stable pseudo-capacitive functional groups,and are assembled using the layer-by-layer (LBL)technique 20.These additive-free LBL-MWNT electrodes exhibit high gravimetric energy (200W h kg electrode 21)delivered at an exceptionally high power of 100kW kg electrode 21in Li /LBL-MWNT cells when normalized to the single-electrode weight,with no loss observed after completing thousands of cycles.LBL-MWNT electrodes show significantly higher gravimetric energy not only over electrochemical capacitor electrodes,but also over high-power lithium battery electrodes,with gravimetric powers greater than 10kW kg electrode 21.In addition,cells (analogous to asymmetric electrochemical capacitors)consisting of LBL-MWNTs and a lithiated Li 4Ti 5O 12(LTO)negative electrode have comparable gravimetric energy to LTO /LiNi 0.5Mn 1.5O 4cells 21at low gravimetric power,but can also deliver much higher energy at higher power.Gravimetric energy and power at the cell level may be estimated by dividing these values based on LBL-MWNT weight by a factor of 5(ref.22).Furthermore,we show that the Faradaic reactions between the lithium ions and the surface functional groups on the MWNTs are responsible for the high gravimetric energy found in Li /LBL-MWNT and LTO /LBL-MWNT cells.Physical characteristics of LBL-MWNT electrodesStable dispersions of negatively and positively charged MWNTs were obtained by functionalization of the exterior surfaces with car-boxylic-acid (MWNT–COOH)and amine-containing (MWNT–NH 2)groups,respectively 23.Uniform MWNT electrodes on glass1Department of Chemical Engineering,Massachusetts Institute of T echnology,Cambridge,Massachusetts 02139,USA,2Department of Mechanical Engineering,Massachusetts Institute of T echnology,Cambridge,Massachusetts 02139,USA,3Department of Materials Science and Engineering,Massachusetts Institute of T echnology,Cambridge,Massachusetts 02139,USA;†These authors contributed equally to this work.*e-mail:shaohorn@coated with indium tin oxide (ITO)(Fig.1a)were assembled by alternate adsorption of charged MWNTs 23.Electrode thickness increases linearly with the number of positively and negatively charged layer pairs (bilayers),as shown in Fig.1b.The transparency of the MWNT films decreased linearly with increasing thickness up to 0.3m m (Fig.1b).The films were then heat-treated sequentially at 1508C in vacuum for 12h,and at 3008C in H 2for 2h to increase film mechanical stability and electrical conductivity bined profilometry and quartz crystal microbalance measurements gave an electrode density of 0.83g cm 23(Supplementary Fig.S1)follow-ing the heat treatments,which is one of the highest densities reported for carbon nanotube electrodes 24,25.Cross-sectional scan-ning electron microscope (SEM)images showed that the individual MWNTs were randomly distributed throughout the film thickness,and the MWNT films were uniform and conformal on the substrate (Fig.1c).Moreover,the LBL-MWNT electrodes had an inter-connected network of individual MWNTs (Fig.1c,inset)with well-distributed pores of 20nm,as revealed by transmission electron microscopy (TEM)imaging of an LBL-MWNT electrode slice (Fig.1d).Role of surface functional groups on LBL-MWNTelectrodesX-ray photoemission spectroscopy (XPS)analysis of the LBL-MWNT electrodes after heat treatment revealed that significant amounts of oxygen-containing and nitrogen-containing surface functional groups remained on the nanotube surface.The atomic composition of a representative LBL-MWNT electrode was found to be 85.7%carbon,10.6%oxygen and 3.7%nitrogen (C 0.86O 0.11N 0.04;Supplementary Table S1).The presence of two dis-tinct peaks (531.7+0.1eV and 533.4+0.1eV)in the O 1s spectrum (Supplementary Fig.S2a)could be attributed to oxygen atoms inthe carbonyl groups 26,27and various other oxygen groups (Supplementary Fig.S2c)bound to the edges 26,28of the graphene sheets forming the MWNT sidewalls.High-resolution TEM images of functionalized MWNTs revealed that acid treatments roughened the exterior walls of the MWNTs (Supplementary Fig.S3),exposing carbon atoms on the edge sites.Edge carbon atoms are known to bind with oxygenated species more strongly than carbon atoms in the basal plane 29,which is in good agreement with the XPS finding that more oxygenated species are detected on LBL-MWNT electro-des than on pristine MWNTs.In addition,the chemical environment of the nitrogen atoms in the LBL-MWNT electrodes was mostly in the form of amide groups (Supplementary Fig.S2b),as indicated by the N 1s peak centred at 400.1eV (ref.30).Two small additional peaks in the N 1s spectrum suggest that some nitrogen atoms are pyr-idinic N-6(ref.30)(398.5+0.1eV)and tied in oxidized nitrogen-containing functional groups (402.3eV)30.These surface functional groups can undergo Faradaic reactions,as indicated by the potential-dependent gravimetric capacitance obtained from cyclic voltammetry measurements (Fig.2a).The typical gravi-metric capacitance of LBL-MWNT electrodes in the voltage range 3–4.25V versus Li (with a comparable voltage scale of 0to 1.2V versus standard hydrogen electrode (SHE))is 125F g 21,which is comparable to the values reported for functionalized MWNTs 23,31and porous carbon materials 32,33in aqueous solutions.Reducing the lower potential limit from 3.0to 1.5V led to a significant increase in the gravimetric capacitance from 125to 250F g 21measured at 4.0and 2.5V versus Li.Recent studies have shown that carbonyl (C ¼O)groups can be reduced by Li þand reversibly oxidized in the voltage range from 3.5to 1.5V versus Li in aromatic carbonyl derivative organic materials such as poly(2,5-dihydroxy-1,4-benzoqui-none-3,6-methylene)34and Li 2C 6O 6(ref.35).It is thereforepostulated0.00.51.01.52.02.53.03.5acbdT h i c k n e s s (µm )Number of bilayersFigure 1|Physical characteristics of LBL-MWNT electrodes.a ,Digital image of representative MWNT electrodes on ITO-coated glass slides.The number on each image indicates the number of bilayers (n )in (MWNT–NH 2/MWNT–COOH)n .b ,Thickness of the LBL-MWNT electrodes as a function of the number of bilayers.A linear relationship is apparent for LBL-MWNT electrodes with thicknesses from 20nm to 3m m.Error bars show the standard deviation of the thickness,computed from three samples for each thickness.T ransmittance measured at 550nm as a function of the number of bilayers is shown in the inset.Error bars show the standard deviation of transmittance computed from three measurements.c ,SEM cross-sectional image of an LBL-MWNT electrode on an ITO-coated glass slide after heat treatments.A higher-magnification image is shown in the inset,revealing that MWNT s are entangled in the direction perpendicular to the electrode surface.d ,TEM image of an LBL-MWNT electrode slice,showing pore sizes of the order of 20nm.DOI:10.1038/NNANO.2010.116that the doubled gravimetric capacitance obtained from lowering the lower voltage limit of Li /LBL-MWNT cells (with open-circuit voltages of 3.2V)from 3.0to 1.5V versus Li can be attributed to the Faradaic reactions of surface oxygen on LBL-MWNTs,such as C ¼O LBL-MWNT þLi þþe 2↔C 2OLi LBL-MWNT ,which can become accessible at vol-tages lower than 3V versus Li.The role of surface functional groups in providing high capaci-tances in LBL-MWNTs was further confirmed by comparing the specific capacitance of LBL-MWNTs before and after exposure to 4%H 2and 96%Ar by volume at 5008C for 10h.The gravimetric current and capacitance values of the LBL-MWNTelectrode decreased considerably (by 40%)after this heat treatment,as shown in Fig.2b.XPS analysis showed that this high-temperature heat treatment decreased the amount of surface oxygen and nitrogen functional groups on the MWNTs.The intensities of the distinct C 1s peaks (assigned to carbon atoms in C 2N (ref.36)or C 2O (ref.37)centred at 285.9+0.1eV,carbonyl C ¼O (refs 27,37)groups at 286.7+0.1eV,and amide N 2C ¼O or carboxylic COOR groups at 288.4+0.1eV (ref.36))were greatly reduced (by 70%)relative to those of sp 2(284.5eV)and sp 3(285.2eV)hybridized carbon 37follow-ing heat treatment,as shown in Fig.2c.This experiment therefore pro-vides further evidence that the redox of surface oxygen-containing functional groups with lithium ions is responsible for the large gravi-metric capacitances of LBL-MWNT electrodes in organic electrolytes.The contribution of double-layer capacitance to LBL-MWNT capacitances is relatively small compared to that of Faradaic reactions,and can be estimated by comparing cyclic voltammograms of compo-site electrodes (80wt%MWNTs and 20wt%binder)that include pristine MWNTs with those containing functionalized MWNTs(see Supplementary Information).Composite electrodes with MWNT–COOH and MWNT–NH 2show much higher gravimetric capacitances (factor of 2)than pristine MWNTs (Fig.2d;Supplementary Fig.S4),indicating the dominant contribution from the surface functional groups.In addition,we show that surface func-tional groups on MWNTs are better used in LBL-MWNT electrodes than in composite electrodes.The capacitance normalized to MWNT weight for LBL-MWNT electrodes is 2.5times higher than that of con-ventional composite electrodes (Fig.2d).Considering that the MWNT–COOH and MWNT–NH 2in the composite electrodes have similar ratios of carbon and oxygen atomic percentages as the LBL electrodes (Supplementary Table S1),this result suggests that the binder-free porous network structure of LBL-MWNT electrodes allows better utilization of the surface functional groups than compo-site electrodes with binder,which can block the redox of the surface functional groups.Moreover,the volumetric capacitances of LBL-MWNT electrodes are even greater (by a factor of 5)than those of composite MWNT electrodes because of their higher elec-trode density (0.83g cm 23for LBL-MWNTs versus 0.45g cm 23for composite electrodes).Lithium storage characteristics of LBL-MWNT electrodesThe specific and volumetric capacitances of LBL-MWNT electrodes are greater than those of conventional composite electrodes based on carbon 32,38in organic electrolytes.Because LBL-MWNT electrodes have no additives,the gravimetric capacitance normalized to electrode weight is identical to that normalized to MWNT weight.It is interesting to point out that the gravimetric capacitances of these LBL-MWNT electrodes are comparable to those of12345−1.0−0.50.00.51.0acbdI (A g M W N T −1)I (A g M W N T −1)I (A g M W N T −1)E (V versus Li)−1,000−50005001,0001.48 ± 0.14 µm at 1 mV s −10.31 ± 0.01 µm at 1 mV s −10.31 ± 0.01 µm at 1 mV s −1PVdF-MWNT (pristine)PVdF-MWNT (−NH 2)PVdF-MWNT (−COOH)3.0 −4.25 VBefore 500 °C H 2 treatmentBefore 500 °C H 2 treatment After 500 °C H 2 treatmentAfter 500 °C H 2 treatment4.2 V4.5 V4.5 V4.2 V d Q /d E (F g MWNT −1)d Q /d E (F g MWNT −1)d Q /d E (F g MWNT −1)12345−0.6−0.30.00.30.6−600−300300600E (V versus Li)292290288286284282C 1s LBL-MWNTC−NN−C C−O sp 2−Csp 3−CI n t e n s i t y (a .u .)Binding energy (eV)12345−1.0−0.50.00.51.0−1,000−5005001,000E (V versus Li)−−O−−O COOR C Figure 2|Potential-dependent electrochemical behaviour of LBL-MWNT and functionalized MWNT composite electrodes measured in two-electrode lithium cells.a ,Cyclic voltammogram data for an LBL-MWNT electrode obtained with different upper-and lower-potential limits.Reducing the lower-potential limit from 3to 1.5V versus Li resulted in increased current and gravimetric capacitance.b ,Cyclic voltammogram data for an LBL-MWNT electrode before and after 5008C H 2-treatment in 4%H 2and 96%Ar by volume for 10h.c ,XPS C 1s spectra of an LBL-MWNT electrode before and after this additional heat treatment,which is seen to remove a considerable amount of surface oxygen and nitrogen functional groups from the MWNT surface.d ,Cyclic voltammogram data for an LBL-MWNT electrode and composite electrodes of pristine MWNT,MWNT–COOH and MWNT–NH 2,with the LBL-MWNT electrode having higher current and capacitance normalized to the MWNT weight than the composite electrodes.The composite electrodesconsisted of 20wt%PVdF and 80wt%posite MWNT electrodes were prepared from slurry casting and dried at 1008C for 12h under vacuum.The thickness of the LBL-MWNT electrode was 0.3m m,and the thicknesses of the pristine MWNT,MWNT–COOH and MWNT–NH 2composite electrodes were 40,50and 30m m,respectively.The density of the composite electrodes was 0.45g cm 23.DOI:10.1038/NNANO.2010.116nanostructured composite electrodes with manganese-based oxides ( 40wt%oxides),even though higher capacitances normalized toactive material mass only (for example,up to 600F g MnO221;ref.39)are typically reported.Moreover,taking into account the LBL-MWNT electrode density of 0.83g cm 23,we obtain a volumetric capacitance of 180F cm 23for LBL-MWNT electrodes,which is higher than that of nanostructured carbon ( 50F cm 23)in organic electrolytes 32and nanostructured MnO 2electrodes( 150F cm 23)in aqueous electrolytes 16,40.Very few studies have reported the volumetric capacitance of entire electrodes,and we note that this is,to our knowledge,the highest value reported.Storing energy on the surfaces of MWNTs enables LBL-MWNT electrodes to have a high rate capability.For a given thickness,the current at 3V was found to increase linearly with scan rate from cyclic voltammetry,indicating a surface-redox limited process (Fig.3a,including inset),which is in good agreement with the proposed mechanism of redox of functional groups on MWNT surfaces.LBL-MWNT electrodes were also examined by means of galvanostatic measurements,allowing direct comparison with the performance of high-power battery materials.The gravi-metric capacity of 0.3-m m electrodes was found to be 200mA h g 21at low rates such as 0.4A g 21,which is in good agreement with the estimated capacity of LBL-MWNT electrodes based on the proposed Faradaic reaction between Li and surface oxygen (C 0.86O 0.11N 0.04).Half of the gravimetric capacity (100mA h g 21)was retained at exceptionally high discharge rates of 180A g 21(corresponding to full discharge in less than 2s),as shown in Fig.3b.Moreover,the capacity (stored charge)of LBL-MWNT electrodes increases linearly with electrode thickness (Fig.3c,inset),and a high power capability is maintained with elec-trode thickness increasing to 3m m (Supplementary Fig.S5).The specific energy and power of LBL-MWNTelectrodes with thick-nesses up to 3.0m m,in the 1.5–4.5V range,are shown in Fig.4a (for volumetric energy and power data see Supplementary Fig.S7).Although the powercapabilityof LBL-MWNTelectrodes reduces some-what with increasing thickness,electrodes of 3.0m m can still deliver a very high gravimetric energy of 200W h kg electrode 21at a large gravimetric power of 100kW kg electrode 21,based on single-electrode weight alone.At low powers,their gravimetric energy ( 500W h kg electrode 21)approaches that of LiFePO 4and LiCoO 2(refs 5,35,41;Supplementary Fig.S6).At high powers (greater than 10kW kg electrode 21),LBL-MWNT electrodes show higher gravimetric energy than carbon-nanotube-based electrodes for electrochemical capacitors ( 70W h kg electrode 21;ref.25),thin-film batteries 22,nano-structured lithium battery materials 5,13and high-power lithium battery materials 4,42(Supplementary Fig.S6).As conventional composite electrodes are much thicker than the 3.0-m m LBL-MWNT elec-trodes (.10times),where ion transport in the electrodes can limit power capability,future studies are needed to examine how the power and energy performance of LBL-MWNT electrodes changes with thicknesses up to tens and hundreds of micrometres.LBL-MWNT electrodes can be tested over 1,000cycles without any observable capacity loss,as shown in Fig.4b.Further cycling of a 1.5-m m LBL-MWNT electrode revealed no capacity loss up to 2,500cycles,even after the cell was left open circuit for 30days (Supplementary Fig.S8).The voltage profiles in the first and 1,000th cycles to 4.5V are virtually unchanged (Fig.4c and Supplementary Fig.S8).TEM and XPS analysis of cycled electro-des (1,000cycles to 4.5V,followed by an additional 1,000cycles to 4.7V versus Li)provided further evidence for this cycling stab-ility,with no distinctive change being noted in the surface atomic structure and surface functional groups after cycling (Supplementary Fig.S9).The stability of the functional groups on these MWNTs is remarkable when compared to the consider-able losses of carbonyl derivative molecules within 50to 100cycles reported recently 34,35,43.We hypothesize that the cycling stability of the LBL-MWNT electrodes can be linked to the strong chemical covalent bonding of the surface functional groups on the MWNTs,in contrast to the gradual separation occurring between the active carbonyl groups and carbon addi-tives in composite electrodes during cycling.Because a lithium negative electrode is not practical for real applications,we investigated the use of LBL-MWNT electrodes with a lithiated Li 4Ti 5O 12(LTO)composite electrode (see−1.0−0.50.00.51.0a bE (V versus Li)I (m A )249 A g −1183 A g −137 A g −14.5 V(0.31±0.01 µm)550 A g −12 A g −10.4 A g −16012345E (V v e r s u s L i )E (V versus Li)70140210Q (mA h g −1)Q (µA h)12345−80−404080I (µA )c Figure 3|Electrochemical characteristics of LBL-MWNT electrodes in two-electrode lithium cells with 1M LiPF 6in a mixture of ethylene carbonate and dimethyl carbonate (volume ratio 3:7).a ,Cyclicvoltammogram data for a 0.3-m m LBL-MWNT electrode over a range of scan rates.The current at 3V versus scan rate is shown in the inset.b ,Charge and discharge profiles of an electrode of 0.3m m obtained over a wide range of gravimetric current densities between 1.5and 4.5V versus Li.Before each charge and discharge measurement for the data in Fig.3b,cells were held at 1.5and 4.5V for 30min,respectively.c ,Cyclic voltammogram data forelectrodes with different thicknesses collected at a scanning rate of 1mV s 21in the voltage range 1.5–4.5V versus Li.The integrated charge increases linearly with electrode thickness,as shown in the inset.DOI:10.1038/NNANO.2010.116Supplementary Information).Although the electrode gravimetric energy and power in LTO /LBL-MWNT cells is reduced due to the lower cell voltage (Fig.4d),the rate capability,gravimetric capacity and capacity retention are comparable to cells with a Li negative electrode (Supplementary Fig.S10).Interestingly,although LTO /LBL-MWNT cells show comparable gravimetric energy to LTO /LiNi 0.5Mn 1.5O 4cells at low power,they exhibit significantly higher gravimetric energy at powers greater than10kW kg electrode 21(ref.21;Fig.4d and Supplementary Fig.S11).Using a conservative assumption that the mass of the battery is five times greater than that of the LBL-MWNT 22,which is higher than the 2.5(ref.4)typically used for conventional lithium rechargeable batteries due to reduced electrode thicknesses (such as 3m m)demonstrated in this study,LTO /LBL-MWNT storage devices are expected to deliver 30W h kg cell 21at 5kW kg cell 21.This value is significantly higher than that of current electrochemical capacitors with a gravimetric energy of 5W h kg cell 21at 1kW kg cell 21(refs 1,32).Finally,we show that LBL-MWNT electrodes can also be used in symmetrical LBL-MWNT /LBL-MWNT cells (cell voltage in the range 0–3V).As there is no net Faradaic reaction of surface oxygen-containing functional groups on MWNTs,they exhibit specific capacitances ( 95F g 21)comparable to those (702120F g 21)in electrochemical capacitors reported previously 44,but considerably lower than that of Li /LBL-MWNT cells.Symmetric cells therefore deliver gravimetric energy comparable toconventional electrochemical capacitors 1,32,but lower than Li /LBL-MWNT and LTO /LBL-MWNT cells (Fig.4d).ConclusionsIn summary,LBL-MWNT electrodes,which are conformal,densely packed and additive-free,can exhibit gravimetric energies up to 200W h kg electrode 21at a gravimetric power of 100kW kg electrode 21,where the gravimetric energy and power at the cell level can be estimated by dividing these values by a factor of 5.The energy stored in the LBL-MWNT electrodes can be con-trolled by the electrode thickness (Fig.3c,inset)and upper voltage limit (Supplementary Fig.S12).Redox of surface oxygen-containing functional groups on LBL-MWNT electrodes by lithium ions in organic electrolytes,which can be accessed reversibly at high power,are predominantly responsible for the observed high energy and power capabilities of LBL-MWNT electrodes,as can be seen in the high-resolution TEM (HRTEM)image of the LBL-MWNT elec-trode in Fig.5.We have demonstrated energy and power capabilities of LBL-MWNT electrodes with thicknesses of a few micrometres that will open up new opportunities in the development of high-performance electrical energy storage for microsystems,and flexible,thin-film devices 22.Further research will seek to validate the reported energy and power capabilities of MWNT electrodes with thicknesses of the order of tens and hundreds of micrometres,and minimize energy loss during charge and discharge (charging voltages of LBL-MWNT107a b cd1061054.5 V (1.48 ± 0.14 µm) at 250 mA g −1104103102G r a v i m e t r i c p o w e r (W k g −1)107106105104103102G r a v i m e t r i c p o w e r (W k g −1)Gravimetric energy (W h kg −1)101102103Gravimetric energy (W h kg −1)Q (m A h g −1)Cycle number01st cycle1,000th cycle 102030012345050100150200Q (mA h g −1)E (V v e r s u s L i )Q (µA h)Figure 4|Gravimetric energy and power densities,and cycle life of LBL-MWNT electrodes obtained from measurements of two-electrode cells.a ,Ragone plot for Li /LBL-MWNT cells with different thicknesses ( 0.3–3.0m m).The corresponding loading density of LBL-MWNT electrodes ranges from 0.025–0.25mg cm 22.Only the LBL-MWNT weight was considered in the gravimetric energy and power density calculations.b ,Gravimetric capacities of Li /LBL-MWNT cells as a function of cycle number,measured at a current density of 0.25A g 21once every 100cycles,after voltage holds at the end of charging and discharging for 30min.Within each 100cycles,these cells were cycled at an accelerated rate of 2.5A g 21.c ,Voltage profiles of a 1.5-m m electrode in the first and 1,000th cycles,for which negligible changes were noted.d ,Ragone plot for Li /LBL-MWNT (black squares),L TO /LBL-MWNT (green circles),L TO /LiNi 0.5Mn 1.5O 4(grey circles)and LBL-MWNT /LBL-MWNT (orange triangles)cells with 4.5V versus Li as the upper-potential limit.The thickness of the LBL-MWNT electrode was 0.3m m for asymmetric Li /LBL-MWNT and L TO /LBL-MWNT,and 0.4m m for symmetric LBL-MWNT /LBL-MWNT.Gravimetric energy and maximum power densities were reduced for the L TO /LBL-MWNT cells subjected to the same testing conditions due to a lower cell voltage.DOI:10.1038/NNANO.2010.116electrodes are higher than those on discharge,even at low rates).Large-scale thicker electrodes of tens of micrometres can be produced using a recently developed sprayed LBL system 45that uses an auto-mated process to reduce assembly time dramatically (about 70times faster than the conventional dipping of LBL systems used in this study).Modification of the surface functional groups on carbon 46,47may allow the tuning of redox potentials and increase efficiency by reducing the voltage difference during charge and discharge.MethodsMaterials.MWNTs prepared by chemical vapour deposition were purchased from NANOLAB (95%purity;outer diameter,15+5nm).Carboxylated MWNTs (MWNT–COOH)and amine-functionalized MWNTs (MWNT–NH 2)wereprepared and assembled onto ITO-coated glass slides (procedures are described in detail elsewhere 23).Cross-sectional scanning electron microscope (SEM)images of MWNT electrodes after heat treatments were obtained using a JEOL 6320SEM operated at 5kV.Fabrication of layer-by-layer MWNT electrodes.MWNT–COOH and MWNT–NH 2powder samples were sonicated for several hours in Milli-Q water (18M V cm)to form a uniform dispersion,and this was followed by dialysis using Milli-Q water for several days,resulting in a stable dispersion of functionalized MWNTs in solution (0.5mg ml 21).pH values of the solutions were adjusted to pH 2.5(MWNT–NH 2)and pH 3.5(MWNT–COOH),respectively,and the solutions were sonicated for 1h just before LBL assembly.All-MWNT electrodes were assembled with a modified Carl Zeiss DS50programmable slide stainer.Details of LBLassembly of MWNT electrodes can be found elsewhere 23.Assembled LBL-MWNT electrodes were dried in air,and these films were then heat-treated sequentially at 1508C in vacuum for 12h,and at 3008C in H 2for 2h to increase mechanical stability.X-ray photoelectron spectroscopy (XPS).A Kratos Axis Ultra XPS instrument (Kratos Analytical)with a monochromatized Al K a X-ray source was used to analyse the surface chemistry of functionalized MWNTs and LBL-MWNTelectrodes.The take-off angle relative to the sample substrate was 908.Curve fitting of the photoemission spectra was performed following a Shirley-type background subtraction.An asymmetric C 1s peak from sp 2hybridized carbons centred at 284.5eV was generated for raw ing this asymmetric peak as a reference,all other peaks were fitted by the Gaussian–Lorentzian function.The experimental uncertainty of the XPS binding energy was +0.1eV.The relative sensitivity factors used to scale the peaks of C 1s ,O 1s and N 1s were 0.278,0.780and 0.477,respectively.Electrochemical measurements.Electrochemical measurements were conducted using a two-electrode electrochemical cell (Tomcell)consisting of an LBL-MWNT electrode on ITO-coated glass,two sheets of microporous membrane (Celgard 2500,Celgard)and lithium foil as the counter-electrode.LBL-MWNT electrode areas of 100or 50mm 2were used for electrochemical measurements with Li foil andlithiated LTO negative electrodes.The weights of the LBL-MWNT electrodes were determined from the area and the mass area density (see SupplementaryInformation).The loading density of the LBL-MWNT electrodes ranged from 0.025to 0.25mg cm 22.A piece of aluminium foil (25m m thick and with an areaof 1mm ×7mm in contact with the LBL-MWNT electrode)was attached to one edge and used as a current collector.The electrolyte solution was 1M LiPF 6dissolved in a mixture of ethylene carbonate (EC)and dimethyl carbonate (DMC)with a 3:7volume ratio (3.5ppm H 2O impurity,Kishida Chem.).The separators were wetted by a minimum amount of electrolyte to reduce the background current.Cyclic voltammetry and galvanostatic measurements of the lithium cells were performed using a Solartron 4170at room temperature.Received 26March 2010;accepted 13May 2010;published online 20June 2010References1.Simon,P.&Gogotsi,Y.Materials for electrochemical capacitors.Nature Mater.7,845–854(2008).ler,J.R.&Simon,P.Materials science—electrochemical capacitors forenergy management.Science 321,651–652(2008).3.Amatucci,G.G.,Badway,F.,Du Pasquier,A.&Zheng,T.An asymmetric hybridnonaqueous energy storage cell.J.Electrochem.Soc.148,A930–A939(2001).4.Kang,B.&Ceder,G.Battery materials for ultrafast charging and discharging.Nature 458,190–193(2009).5.Lee,Y.J.et al .Fabricating genetically engineered high-power lithium-ionbatteries using multiple virus genes.Science 324,1051–1055(2009).6.Nazar,L.F.et al .Nanostructured materials for energy storage.Int.J.Inorg.Mater.3,191–200(2001).7.Arico,A.S.et al .Nanostructured materials for advanced energy conversion andstorage devices.Nature Mater.4,366–377(2005).8.Poizot,P.et al .Nano-sized transition-metaloxides as negative-electrodematerials for lithium-ion batteries.Nature 407,496–499(2000).9.Sides,C.R.et al .Nanoscale materials for lithium-ion batteries.MRS Bull.27,604–607(2002).10.Bruce,P.G.,Scrosati,B.&Tarascon,J.M.Nanomaterials for rechargeablelithium batteries.Angew.Chem.Int.Ed.47,2930–2946(2008).11.Tarascon,J.M.&Armand,M.Issues and challenges facing rechargeable lithiumbatteries.Nature 414,359–367(2001).12.Armand,M.&Tarascon,J.M.Building better batteries.Nature 451,652–657(2008).13.Wu,X.L.et al .LiFePO 4nanoparticles embedded in a nanoporous carbonmatrix:superior cathode material for electrochemical energy-storage devices.Adv.Mater.21,2710–2714(2009).14.Chmiola,J.et al .Anomalous increase in carbon capacitance at pore sizes lessthan 1nanometer.Science 313,1760–1763(2006).15.Hu,C.C.,Chen,W.C.&Chang,K.H.How to achieve maximum utilization ofhydrous ruthenium oxide for supercapacitors.J.Electrochem.Soc.151,A281–A290(2004).16.Fischer,A.E.et al .Incorporation of homogeneous,nanoscale MnO 2withinultraporous carbon structures via self-limiting electroless deposition:implications for electrochemical capacitors.Nano Lett.7,281–286(2007).17.Reddy,A.L.M.,Shaijumon,M.M.,Gowda,S.R.&Ajayan,P.M.CoaxialMnO 2/carbon nanotube array electrodes for high-performance lithium batteries.Nano Lett.9,1002–1006(2009).18.Kim,D.K.et al .Spinel LiMn 2O 4nanorods as lithium ion battery cathodes.NanoLett.8,3948–3952(2008).19.Be´langer,D.,Brousse,T.&Long,J.W.Manganese oxides:battery materials make the leap to electrochemical capacitors.Electrochem.Soc.Interf.17,49–52(2008).20.Decher,G.Fuzzy nanoassemblies:toward layered polymeric multicomposites.Science 277,1232–1237(1997).21.Xiang,H.F.et al .Effect of capacity matchup in the LiNi 0.5Mn 1.5O 4/Li 4Ti 5O 12cells.J.Power Sources 183,355–360(2008).22.Dudney,J.N.Thin film micro-batteries.Electrochem.Soc.Interf.17,44–48(2008).23.Lee,Seung Woo et al .Layer-by-layer assembly of all carbon nanotube ultrathinfilms for electrochemical applications.J.Am.Chem.Soc.131,671–679(2009).24.Niu,C.M.et al .High power electrochemical capacitors based on carbonnanotube electrodes.Appl.Phys.Lett.70,1480–1482(1997).25.Futaba,D.N.et al .Shape-engineerable and highly densely packed single-walledcarbon nanotubes and their application as super-capacitor electrodes.Nature Mater.5,987–994(2006).26.Zielke,U.,Huttinger,K.J.&Hoffman,W.P.Surface-oxidized carbon fibers:I.Surface structure and chemistry.Carbon 34,983–998(1996).27.Kozlowski,C.&Sherwood,P.M.A.X-ray photoelectron-spectroscopic studiesof carbon-fibre surfaces.Part 5.The effect of pH on surface oxidation.J.Chem.Soc.Farad.Trans.I 81,2745–2756(1985).28.Frackowiak,E.et al .Electrochemical storage of lithium multiwalled carbonnanotubes.Carbon 37,61–69(1999).29.Zhu,X.Y.,Lee,S.M.,Lee,Y.H.&Frauenheim,T.Adsorption and desorption ofan O 2molecule on carbon nanotubes.Phys.Rev.Lett.85,2757–2760(2000).30.Burg,P.et al .The characterization of nitrogen-enriched activated carbons by IR,XPS and LSER methods.Carbon 40,1521–1531(2002).Surface functional groups:Faradaic RXN centresOOLi Li Li+OO Figure 5|Schematic of the energy storage mechanism of LBL-MWNT electrodes.Faradaic reactions between surface oxygen functional species (orange arrows)and Li schematically illustrated on an HRTEM image of the LBL-MWNT electrodes.Intact graphite layers inside the MWNT s (white arrows)are indicated as electron conduction channels.DOI:10.1038/NNANO.2010.116。
雷德诺水溶氧化氢抗溅液-精品(乳胶)安全数据表说明书

P280 P264
Wear protective gloves, protective clothing,eye protection,face protection. Wash face, hands and any exposed skin thoroughly after handling.
P210 Keep away from heat, sparks, open flames, hot surfaces - No smoking.
111-42-2
ETHYLENE OXIDE
75-21-8
*The exact percentage (concentration) of composition has been withheld as a trade secret.
Weight %* 10-20 1-10 <1
<0.0001
March 23, 2019
Section 3: Composition and Information on Ingredients
SUBSTANCES:
Chemical Name
CAS-No
DIMETHYLETHER
115-10-6
NONYLPHENOXYPOLYETHOXYETHANOL
127087-87-0
DIETHANOLAMINE
Radnor Water Based Anti-Spatter - Premium (Aerosol)
Anti-Spatter Prevents Spatter Build Up in Welding Operations 64000111 042R Radnor 259 North Radnor - Chester Road - Suite 100 Radnor, PA, 19087-5283 866-734-3438 March 23 Regulated Hazardous / Regulated
Fenoterol_hydrobromide_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Fenoterol (hydrobromide)Catalog No. :HY-B0976ACAS No. :1944-12-31.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:Fenoterol bromide; Th⁻1165a; Phenoterol hydrobromideFormula:C17H22BrNO4Molecular Weight:384.26CAS No. :1944-12-34. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
伪罗特水溶性抗渗剂技术数据表说明书

The data on this sheet represent typical values. Since application variables are a major factor in product performance, this information should serve only as a general guide. Valspar assumes no obligation or liability for use of this information. UNLESS VALSPAR AGREES OTHERWISE IN WRITING, VALSPAR MAKES NO WARRANTIES, EXPRESS OR IMPLIED, AND DISCLAIMS ALL IMPLIED WARRANTIES INCLUDINGWARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR USE OR FREEDOM FROM PATENT INFRINGEMENT. VALSPAR WILL NOT BE LIABLE FOR ANY SPECIAL, INCIDENTAL OR CONSEQUENTIAL DAMAGES. Your only remedy for any defect in this product is the replacement of the defective product, or a refund of its purchase price, at our option.Version 2: Feb 2011 (supersedes all previous revisions) (Continued on reverse)Waterborne RetarderTECHNICAL DATA SHEETPRODUCT NUMBERYXT0700COMPANION PRODUCTSZenith™ Coatings, stains, and glaze basesDESCRIPTION/USESZenith™ Waterborne Retarder is designed to increase the open time of Zenith™ coatings, stain, and glaze bases where needed. This may be necessary in conditions of high temperature, excessive airmovement, or when applying coatings to very large pieces where longer open time is needed. This product is not designed for thinning purposes and will not affect viscosity at recommended amounts. Zenith™Waterborne Retarder is designed for professional use only. For Wood Substrates Only.PRODUCT ADVANTAGES• HAP’s Free• Formaldehyde Free • Very low odor •Water cleanupAPPLICATION FEATURES• Easy mixing.•Extends the open time of Zenith™ products.PRECAUTIONSThese products are recommended for professional application and are designed for interior use only. Always pre-test the system on your substrate and under your line conditions to verify suitability to the application and to avoid potential need for costly refinishing. Valspar Wood Finishes products aredesigned to protect and enhance the natural beauty of wood, but cannot eliminate natural discoloration or deterioration of wood as it ages. Additional notes:Do not mix with other finishing systems or deviate from these finishing recommendations. Valspar will not be held liable for finish failures resulting from the mixing of products or deviations from finishing recommendations.PHYSICAL PROPERTIES (objective specifications)Viscosity: N/A Weight Solids: 0 Volume Solids: 0%Weight/Gallon: 8.66 lbs/gal¹ Theoretical Coverage: N/A Flash Point: 999ºF Closed CupAir Quality Information: VOC: 8.66 lbs/gal of Product AIM VOC : 1037.7g/l VOC Ratio: N/A VHAP: 0.0 lb VHAP/lb solid-HAP’s Free Photochemically Reactive: NoDry Times (78°F, 50%RH): Air Dry N/AForce DryN/AShelf life: 2 years from the manufacturing datePot Life: N/AApplication Equipment:Use only equipment with plastic, stainless steel, or Teflon coated valves and parts.Recommended tip sizes : N/ANote: All information provided is typical (as formulated) and will not represent exact values for every product. For specific Air Quality Data for each product, VOC reports are available upon request.FINISHING RECOMMENDATIONSGeneral: Surface must be clean and dust free with moisture content of 6-8% prior to finishing. Remove all dirt, dust, wax and wood marks. Proper sanding and preparation of the wood is critical to achieving consistent results. New Wood: Finish sand surface (150-180 grit) and remove all sanding dust.Painted or Varnished Wood: Remove all paint or varnish then follow new wood instructions.Before using, mix product by hand, or if using mechanical agitation such as an air mixer or drill, mix at slow to moderate speed until there is no material on the bottom of the container.The Zenith™ product line is packaged ready to use. However, certain conditions of high temperature, excessive air movement, or size of the piece to be sprayed may require a longer open time than normal. Such conditions may warrant the use of Zenith™ Waterborne Retarder. Amounts of addition are specific to each product. Please refer to the following table to maintain regulatory compliance:Zenith™ clear sealer and topcoatsLKF700X series, PKF720X series, PKF750X series, PKS7200: Add 1-5 oz (1-4%) max per gal. Max VOC after recommended addition is 275 g/l or 2.3 lbs per gallon.Zenith™ basecoats and white topcoatsLKW7100, LKN7200, LKW710X series, PKW730X series: Add 1-4 oz (1-3%) max per gal. Max VOC after recommended addition is 275 g/l or 2.3 lbs per gallon.Zenith™ stains and glazesLWS075X series: Add 1-2.5 oz (1-2%) max per gal. Max VOC after recommended addition is 250 g/l or 2.08 lbs per gallon.LWS4750: Add 1-2oz (1-1.5%) max per gal. Max VOC after recommended addition is 250 g/l or 2.08 lbs per gallon. LWS4725: Add 1-2oz (1-1.5%) max per gal. Max VOC after recommended addition is 250 g/l or 2.08 lbs per gallon. LWS1000: Add 4-7oz (3-5%) max per gal. Max VOC after recommended addition is 120 g/per liter of Material.For Zenith products/series not listed-No retarder is recommended.Note: Adding Zenith™ Waterborne Retarder will increase the dry time needed before re-coating. The amount ofextra time needed will depend on ambient conditions and air movement. Make sure products are thoroughly dry before re-coating.Caution: Exceeding recommended amounts may cause severely extended dry times and cause a soft film in the early curing stages.Clean equipment with warm water. If coating has dried, Acetone may be required to remove dried film. Keep container closed when not in use to avoid skinning. Do not transfer contents to other containers for storage or disposal. In case of spillage, absorb with inert material such as sand or kitty litter. Dispose of empty cans or unused portion in accordance with local state and federal regulations.Store in a cool, dry place. DO NOT FREEZE! Product should be stored in temperatures between 50°-110°F. Close all containers after use. Do not store near heat or sparks. Spills should be cleaned up with non-sparking tools. See the product MSDS for complete safety information.Always pre-test the system on your substrate and under your line conditions to verify suitability to the application and avoid potential need for costly refinishing. All dry times listed are as tested under ideal indoor environmental conditions of 78°F (26°C) with relative humidity not exceeding 50%. These products are recommended for use under temperature conditions of 60-100°F (16-38°C) and when relative humidity is below 50% during application and drying time. Low temperatures, poor air circulation or high humidity will extend dry times. Valspar strongly recommends against use of these products if temperatures of air, material, or surface to be coated are below 60°F (16°C) or below the dew point. Abnormal conditions of temperature or humidity may adversely affect product performance. Please contact your authorized Valspar Wood Finishes distributor for additional product use recommendations and finishing guidance.MSDS AND CPDS SHEETS AVAILABLE UPON REQUEST。
tetracycline hydrochloride结构式名

tetracycline hydrochloride结构式名Tetracycline Hydrochloride: A Breakthrough Antibiotic that Revolutionized MedicineIntroduction:Tetracycline hydrochloride is a potent antibiotic that has played a significant role in the treatment of various infectious diseases since its discovery in the mid-20th century. In this article, we will explore the structural features of tetracycline hydrochloride and delve into its mechanism of action, clinical uses, and potential side effects. Join us on this journey as we uncover the impact this compound has had on the field of medicine.Structural Features and Composition:Tetracycline hydrochloride, also known by its chemical formula C22H24N2O8·HCl, is a semisynthetic derivative of a natural antibiotic produced by Streptomyces bacteria. The compound consists of four fused rings, referred to as rings A, B, C, and D, which are responsible for its antibacterial activity. The presence of various functional groups, such as dimethylamine, hydroxyl, and carbonyl moieties, confers different properties to tetracycline hydrochloride.Mechanism of Action:Tetracycline hydrochloride exerts its antimicrobial effect by inhibiting bacterial protein synthesis. It does so by binding reversibly to the 30S ribosomal subunit in susceptible bacterial cells, preventing the attachment of aminoacyl-tRNA to the messenger RNA-ribosome complex. Additionally, it interferes with the proofreading mechanism of the ribosome, leading to the incorporation of incorrect amino acids into the growing protein chain. This disruption of protein synthesis effectively inhibits bacterial growth and replication.Clinical Uses:Tetracycline hydrochloride has been widely employed in the treatment of various infectious diseases, including respiratory tract infections, urinary tract infections, sexually transmitted infections, and skin and soft tissue infections. Its broad-spectrum activity against both gram-positive and gram-negative bacteria has made it a valuable tool in combating bacterial infections, particularly in settings where other antibiotics are ineffective or contraindicated.Emerging Applications:Beyond its traditional uses, tetracycline hydrochloride has shown promise in a range of non-infectious conditions. Research suggests its potential in treating variousinflammatory disorders, such as rheumatoid arthritis and ocular inflammatory diseases. Additionally, studies have explored its potential role in the treatment of certain cancers, as it possesses anti-tumor properties. However, further research is necessary to fully understand and harness its therapeutic potential in these novel areas.Side Effects and Precautions:While tetracycline hydrochloride has proven to be an invaluable treatment option, it is not without its side effects. Common adverse reactions include gastrointestinal disturbances, such as nausea, vomiting, and diarrhea. Photosensitivity reactions can also occur, necessitating caution when exposed to sunlight or ultraviolet radiation. Long-term use of tetracycline hydrochloride can result in discoloration of teeth and bones, making it unsuitable for use in pregnant women and children under the age of eight.Conclusion:Tetracycline hydrochloride has emerged as a game-changer in the field of antibiotics, revolutionizing the treatment of infectious diseases. Its unique structural features, mechanism of action, and broad-spectrum activity have made it a cornerstone in the fight against bacterial infections.Moreover, ongoing research suggests potential applications in inflammatory disorders and cancer treatment. While side effects must be considered, the benefits of tetracycline hydrochloride far outweigh the risks when used judiciously. Continued exploration of this remarkable compound promises to uncover new therapeutic avenues and improve the health outcomes of countless patients worldwide.。
铁铬液流电池电解液优化研究一中国科学技术大学博士论文

p h m a t e r i a l s , w h i c
i sex
e c t e d t o b e c o m e a c o s t - e f fe c t i v e e n e r g y s t o r a g e e q u i p m e n t .
H C ow e v
^ 对 〇 3 + /(
电 +
反
应动 力学行 为和 析氢反应 的影响 ,
分别研究 了在
和 °
2 5 C
件 下 °
65 C 条
,
电 解 液
中
子 离 2 +
Pb
、
Bi3+离子浓度 变化在玻碳 电极上发 生 的 负 极反应 的影 响 。
结 果 表
明,
升 高温度有助于
离 子 C 3 + r
的
电化 学反应 和析氢反应
摘
要摘 要 Fra bibliotek液 流 电 池 因 其 具 有 高 安 全 性 、 循 环 寿 命 长 、 设 计 灵 活 等 突 出 优 势 , 是 业 界 公
认 最 适 于 建 造 大 规 模 储 能 系 统 的 一 种 储 能 组 件 , 并 在 商 业 化 应 用 中 取 得 了 重 大 进
展 。 其 中 , 铁 铬 液 流 电 池 , 作 为 最 早 出 现 的 一 种 液 流 电 池 技 术 , 采 用 价 格 低 廉 的
l
m
a
t
e
r
i
a
l
fo
r
e
n
e
r
g
y
s
t
o
r
a
ge
o
f
fl
戈尔质子交换膜型号

戈尔质子交换膜是一种用于燃料电池的重要组件,具有良好的电导率和化学稳定性。
在选择戈尔质子交换膜型号时,需要根据应用场景、功率需求、成本等因素进行综合考虑。
以下是一些有关戈尔质子交换膜型号的介绍:首先,戈尔提供多种质子交换膜型号,以满足不同客户的需求。
其中,Gel-Filer Pro 是戈尔常用的燃料电池用质子交换膜之一,具有优异的电导率、化学稳定性和耐久性。
该膜适用于各种燃料电池应用,如轿车、巴士、混合动力车、固定电源等。
此外,戈尔还提供用于固体氧化物燃料电池(SOFC)的Gel-Filer SOFC专用膜,该膜具有更高的离子电导率和渗透性,可提高发电效率。
在选择戈尔质子交换膜型号时,需要考虑以下几个因素:1. 应用场景:不同应用场景对质子交换膜的性能要求不同。
例如,在混合动力车和轿车等小型车辆中,通常使用Gel-Filer Pro膜,而在大型燃料电池系统中,可能需要考虑更高的功率和效率。
2. 功率需求:质子交换膜的功率容量取决于其厚度和渗透性。
在选择戈尔质子交换膜型号时,需要考虑设备的功率需求,以便选择最适合的型号。
3. 成本:在选择戈尔质子交换膜型号时,还需要考虑其成本因素。
不同型号的质子交换膜的成本和制造成本不同,因此在考虑性价比的情况下,选择适合的质子交换膜非常重要。
基于以上因素的综合考虑,在某些情况下,Gel-Filer XPE膜可能是戈尔质子交换膜的一种合适选择。
Gel-Filer XPE膜是一种高性能的燃料电池用质子交换膜,具有优异的电导率、化学稳定性和耐久性。
它适用于各种燃料电池应用,包括小型车辆、固定电源和移动电源等。
此外,Gel-Filer XPE膜还具有更高的离子电导率和渗透性,可以提高发电效率。
总之,在选择戈尔质子交换膜型号时,需要根据具体应用场景、功率需求和成本等因素进行综合考虑。
通过了解戈尔提供的多种质子交换膜型号及其性能特点,可以更好地选择适合的质子交换膜,从而为燃料电池系统的性能和可靠性提供有力保障。
欧洲药典7.5版

INDEX
To aid users the index includes a reference to the supplement in which the latest version of a text can be found. For example : Amikacin sulfate...............................................7.5-4579 means the monograph Amikacin sulfate can be found on page 4579 of Supplement 7.5. Note that where no reference to a supplement is made, the text can be found in the principal volume.
English index ........................................................................ 4707
Latin index ................................................................................. 4739
EUROPEAN PHARMACOPபைடு நூலகம்EIA 7.5
Index
Numerics 1. General notices ................................................................... 7.5-4453 2.1.1. Droppers...................
大环多胺

New1H-Pyrazole-Containing Polyamine Receptors Able ToComplex L-Glutamate in Water at Physiological pH ValuesCarlos Miranda,†Francisco Escartı´,‡Laurent Lamarque,†Marı´a J.R.Yunta,§Pilar Navarro,*,†Enrique Garcı´a-Espan˜a,*,‡and M.Luisa Jimeno†Contribution from the Instituto de Quı´mica Me´dica,Centro de Quı´mica Orga´nica Manuel Lora Tamayo,CSIC,C/Juan de la Cier V a3,28006Madrid,Spain,Departamento de Quı´mica Inorga´nica,Facultad de Quı´mica,Uni V ersidad de Valencia,c/Doctor Moliner50, 46100Burjassot(Valencia),Spain,and Departamento de Quı´mica Orga´nica,Facultad deQuı´mica,Uni V ersidad Complutense de Madrid,A V plutense s/n,28040Madrid,SpainReceived April16,2003;E-mail:enrique.garcia-es@uv.esAbstract:The interaction of the pyrazole-containing macrocyclic receptors3,6,9,12,13,16,19,22,25,26-decaazatricyclo-[22.2.1.111,14]-octacosa-1(27),11,14(28),24-tetraene1[L1],13,26-dibenzyl-3,6,9,12,13,16,-19,22,25,26-decaazatricyclo-[22.2.1.111,14]-octacosa-1(27),11,14(28),24-tetraene2[L2],3,9,12,13,16,22,-25,26-octaazatricyclo-[22.2.1.111,14]-octacosa-1(27),11,14(28),24-tetraene3[L3],6,19-dibenzyl-3,6,9,12,13,-16,19,22,25,26-decaazatricyclo-[22.2.1.111,14]-octacosa-1(27),11,14(28),24-tetraene4[L4],6,19-diphenethyl-3,6,9,12,13,16,19,22,25,26-decaazatricyclo-[22.2.1.111,14]-octacosa-1(27),11,14(28),24-tetraene5[L5],and 6,19-dioctyl-3,6,9,12,13,16,19,22,25,26-decaazatricyclo-[22.2.1.111,14]-octacosa-1(27),11,14(28),24-tetra-ene6[L6]with L-glutamate in aqueous solution has been studied by potentiometric techniques.The synthesis of receptors3-6[L3-L6]is described for the first time.The potentiometric results show that4[L4]containing benzyl groups in the central nitrogens of the polyamine side chains is the receptor displaying the larger interaction at pH7.4(K eff)2.04×104).The presence of phenethyl5[L5]or octyl groups6[L6]instead of benzyl groups4[L4]in the central nitrogens of the chains produces a drastic decrease in the stability[K eff )3.51×102(5),K eff)3.64×102(6)].The studies show the relevance of the central polyaminic nitrogen in the interaction with glutamate.1[L1]and2[L2]with secondary nitrogens in this position present significantly larger interactions than3[L3],which lacks an amino group in the center of the chains.The NMR and modeling studies suggest the important contribution of hydrogen bonding andπ-cation interaction to adduct formation.IntroductionThe search for the L-glutamate receptor field has been andcontinues to be in a state of almost explosive development.1 L-Glutamate(Glu)is thought to be the predominant excitatory transmitter in the central nervous system(CNS)acting at a rangeof excitatory amino acid receptors.It is well-known that it playsa vital role mediating a great part of the synaptic transmission.2However,there is an increasing amount of experimentalevidence that metabolic defects and glutamatergic abnormalitiescan exacerbate or induce glutamate-mediated excitotoxic damageand consequently neurological disorders.3,4Overactivation ofionotropic(NMDA,AMPA,and Kainate)receptors(iGluRs)by Glu yields an excessive Ca2+influx that produces irreversible loss of neurons of specific areas of the brain.5There is much evidence that these processes induce,at least in part,neuro-degenerative illnesses such as Parkinson,Alzheimer,Huntington, AIDS,dementia,and amyotrophic lateral sclerosis(ALS).6In particular,ALS is one of the neurodegenerative disorders for which there is more evidence that excitotoxicity due to an increase in Glu concentration may contribute to the pathology of the disease.7Memantine,a drug able to antagonize the pathological effects of sustained,but relatively small,increases in extracellular glutamate concentration,has been recently received for the treatment of Alzheimer disease.8However,there is not an effective treatment for ALS.Therefore,the preparation of adequately functionalized synthetic receptors for L-glutamate seems to be an important target in finding new routes for controlling abnormal excitatory processes.However,effective recognition in water of aminocarboxylic acids is not an easy task due to its zwitterionic character at physiological pH values and to the strong competition that it finds in its own solvent.9†Centro de Quı´mica Orga´nica Manuel Lora Tamayo.‡Universidad de Valencia.§Universidad Complutense de Madrid.(1)Jane,D.E.In Medicinal Chemistry into the Millenium;Campbell,M.M.,Blagbrough,I.S.,Eds.;Royal Society of Chemistry:Cambridge,2001;pp67-84.(2)(a)Standaert,D.G.;Young,A.B.In The Pharmacological Basis ofTherapeutics;Hardman,J.G.,Goodman Gilman,A.,Limbird,L.E.,Eds.;McGraw-Hill:New York,1996;Chapter22,p503.(b)Fletcher,E.J.;Loge,D.In An Introduction to Neurotransmission in Health and Disease;Riederer,P.,Kopp,N.,Pearson,J.,Eds.;Oxford University Press:New York,1990;Chapter7,p79.(3)Michaelis,E.K.Prog.Neurobiol.1998,54,369-415.(4)Olney,J.W.Science1969,164,719-721.(5)Green,J.G.;Greenamyre,J.T.Prog.Neurobiol.1996,48,613-63.(6)Bra¨un-Osborne,H.;Egebjerg,J.;Nielsen,E.O.;Madsen,U.;Krogsgaard-Larsen,P.J.Med.Chem.2000,43,2609-2645and references therein.(7)(a)Shaw,P.J.;Ince,P.G.J.Neurol.1997,244(Suppl2),S3-S14.(b)Plaitakis,A.;Fesdjian,C.O.;Shashidharan,S Drugs1996,5,437-456.(8)Frantz,A.;Smith,A.Nat.Re V.Drug Dico V ery2003,2,9.Published on Web12/30/200310.1021/ja035671m CCC:$27.50©2004American Chemical Society J.AM.CHEM.SOC.2004,126,823-8339823There are many types of receptors able to interact with carboxylic acids and amino acids in organic solvents,10-13yielding selective complexation in some instances.However,the number of reported receptors of glutamate in aqueous solution is very scarce.In this sense,one of the few reports concerns an optical sensor based on a Zn(II)complex of a 2,2′:6′,2′′-terpyridine derivative in which L -aspartate and L -glutamate were efficiently bound as axial ligands (K s )104-105M -1)in 50/50water/methanol mixtures.14Among the receptors employed for carboxylic acid recogni-tion,the polyamine macrocycles I -IV in Chart 1are of particular relevance to this work.In a seminal paper,Lehn et al.15showed that saturated polyamines I and II could exert chain-length discrimination between different R ,ω-dicarboxylic acids as a function of the number of methylene groups between the two triamine units of the receptor.Such compounds were also able to interact with a glutamic acid derivative which has the ammonium group protected with an acyl moiety.15,16Compounds III and IV reported by Gotor and Lehn interact in their protonated forms in aqueous solution with protected N -acetyl-L -glutamate and N -acetyl-D -glutamate,showing a higher stability for the interaction with the D -isomer.17In both reports,the interaction with protected N -acetyl-L -glutamate at physiological pH yields constants of ca.3logarithmic units.Recently,we have shown that 1H -pyrazole-containing mac-rocycles present desirable properties for the binding of dopam-ine.18These polyaza macrocycles,apart from having a highpositive charge at neutral pH values,can form hydrogen bonds not only through the ammonium or amine groups but also through the pyrazole nitrogens that can behave as hydrogen bond donors or acceptors.In fact,Elguero et al.19have recently shown the ability of the pyrazole rings to form hydrogen bonds with carboxylic and carboxylate functions.These features can be used to recognize the functionalities of glutamic acid,the carboxylic and/or carboxylate functions and the ammonium group.Apart from this,the introduction of aromatic donor groups appropriately arranged within the macrocyclic framework or appended to it through arms of adequate length may contribute to the recognition event through π-cation interactions with the ammonium group of L -glutamate.π-Cation interactions are a key feature in many enzymatic centers,a classical example being acetylcholine esterase.20The role of such an interaction in abiotic systems was very well illustrated several years ago in a seminal work carried out by Dougherty and Stauffer.21Since then,many other examples have been reported both in biotic and in abiotic systems.22Taking into account all of these considerations,here we report on the ability of receptors 1[L 1]-6[L 6](Chart 2)to interact with L -glutamic acid.These receptors display structures which differ from one another in only one feature,which helps to obtain clear-cut relations between structure and interaction(9)Rebek,J.,Jr.;Askew,B.;Nemeth,D.;Parris,K.J.Am.Chem.Soc.1987,109,2432-2434.(10)Seel,C.;de Mendoza,J.In Comprehensi V e Supramolecular Chemistry ;Vogtle,F.,Ed.;Elsevier Science:New York,1996;Vol.2,p 519.(11)(a)Sessler,J.L.;Sanson,P.I.;Andrievesky,A.;Kral,V.In SupramolecularChemistry of Anions ;Bianchi,A.,Bowman-James,K.,Garcı´a-Espan ˜a,E.,Eds.;John Wiley &Sons:New York,1997;Chapter 10,pp 369-375.(b)Sessler,J.L.;Andrievsky,A.;Kra ´l,V.;Lynch,V.J.Am.Chem.Soc.1997,119,9385-9392.(12)Fitzmaurice,R.J.;Kyne,G.M.;Douheret,D.;Kilburn,J.D.J.Chem.Soc.,Perkin Trans.12002,7,841-864and references therein.(13)Rossi,S.;Kyne,G.M.;Turner,D.L.;Wells,N.J.;Kilburn,J.D.Angew.Chem.,Int.Ed.2002,41,4233-4236.(14)Aı¨t-Haddou,H.;Wiskur,S.L.;Lynch,V.M.;Anslyn,E.V.J.Am.Chem.Soc.2001,123,11296-11297.(15)Hosseini,M.W.;Lehn,J.-M.J.Am.Chem.Soc.1982,104,3525-3527.(16)(a)Hosseini,M.W.;Lehn,J.-M.Hel V .Chim.Acta 1986,69,587-603.(b)Heyer,D.;Lehn,J.-M.Tetrahedron Lett.1986,27,5869-5872.(17)(a)Alfonso,I.;Dietrich,B.;Rebolledo,F.;Gotor,V.;Lehn,J.-M.Hel V .Chim.Acta 2001,84,280-295.(b)Alfonso,I.;Rebolledo,F.;Gotor,V.Chem.-Eur.J.2000,6,3331-3338.(18)Lamarque,L.;Navarro,P.;Miranda,C.;Ara ´n,V.J.;Ochoa,C.;Escartı´,F.;Garcı´a-Espan ˜a,E.;Latorre,J.;Luis,S.V.;Miravet,J.F.J.Am.Chem.Soc .2001,123,10560-10570.(19)Foces-Foces,C.;Echevarria,A.;Jagerovic,N.;Alkorta,I.;Elguero,J.;Langer,U.;Klein,O.;Minguet-Bonvehı´,H.-H.J.Am.Chem.Soc.2001,123,7898-7906.(20)Sussman,J.L.;Harel,M.;Frolow,F.;Oefner,C.;Goldman,A.;Toker,L.;Silman,I.Science 1991,253,872-879.(21)Dougherty,D.A.;Stauffer,D.A.Science 1990,250,1558-1560.(22)(a)Sutcliffe,M.J.;Smeeton,A.H.;Wo,Z.G.;Oswald,R.E.FaradayDiscuss.1998,111,259-272.(b)Kearney,P.C.;Mizoue,L.S.;Kumpf,R.A.;Forman,J.E.;McCurdy,A.;Dougherty,D.A.J.Am.Chem.Soc.1993,115,9907-9919.(c)Bra ¨uner-Osborne,H.;Egebjerg,J.;Nielsen,E.;Madsen,U.;Krogsgaard-Larsen,P.J.Med.Chem.2000,43,2609-2645.(d)Zacharias,N.;Dougherty,D.A.Trends Pharmacol.Sci.2002,23,281-287.(e)Hu,J.;Barbour,L.J.;Gokel,G.W.J.Am.Chem.Soc.2002,124,10940-10941.Chart 1.Some Receptors Employed for Dicarboxylic Acid and N -AcetylglutamateRecognitionChart 2.New 1H -Pyrazole-Containing Polyamine Receptors Able To Complex L -Glutamate inWaterA R T I C L E SMiranda et al.824J.AM.CHEM.SOC.9VOL.126,NO.3,2004strengths.1[L1]and2[L2]differ in the N-benzylation of the pyrazole moiety,and1[L1]and3[L3]differ in the presence in the center of the polyamine side chains of an amino group or of a methylene group.The receptors4[L4]and5[L5]present the central nitrogens of the chain N-functionalized with benzyl or phenethyl groups,and6[L6]has large hydrophobic octyl groups.Results and DiscussionSynthesis of3-6.Macrocycles3-6have been obtained following the procedure previously reported for the preparation of1and2.23The method includes a first dipodal(2+2) condensation of the1H-pyrazol-3,5-dicarbaldehyde7with the corresponding R,ω-diamine,followed by hydrogenation of the resulting Schiff base imine bonds.In the case of receptor3,the Schiff base formed by condensation with1,5-pentanediamine is a stable solid(8,mp208-210°C)which precipitated in68% yield from the reaction mixture.Further reduction with NaBH4 in absolute ethanol gave the expected tetraazamacrocycle3, which after crystallization from toluene was isolated as a pure compound(mp184-186°C).In the cases of receptors4-6, the precursor R,ω-diamines(11a-11c)(Scheme1B)were obtained,by using a procedure previously described for11a.24 This procedure is based on the previous protection of the primary amino groups of1,5-diamino-3-azapentane by treatment with phthalic anhydride,followed by alkylation of the secondary amino group of1,5-diphthalimido-3-azapentane9with benzyl, phenethyl,or octyl bromide.Finally,the phthalimido groups of the N-alkyl substituted intermediates10a-10c were removed by treatment with hydrazine to afford the desired amines11a-11c,which were obtained in moderate yield(54-63%).In contrast with the behavior previously observed in the synthesis of3,in the(2+2)dipodal condensations of7with 3-benzyl-,3-phenethyl-,and3-octyl-substituted3-aza-1,5-pentanediamine11a,11b,and11c,respectively,there was not precipitation of the expected Schiff bases(Scheme1A). Consequently,the reaction mixtures were directly reduced in situ with NaBH4to obtain the desired hexaamines4-6,which after being carefully purified by chromatography afforded purecolorless oils in51%,63%,and31%yield,respectively.The structures of all of these new cyclic polyamines have been established from the analytical and spectroscopic data(MS(ES+), 1H and13C NMR)of both the free ligands3-6and their corresponding hydrochloride salts[3‚4HCl,4‚6HCl,5‚6HCl, and6‚6HCl],which were obtained as stable solids following the same procedure previously reported18for1‚6HCl and2‚6HCl.As usually occurs for3,5-disubstituted1H-pyrazole deriva-tives,either the free ligands3-6or their hydrochlorides show very simple1H and13C NMR spectra,in which signals indicate that,because of the prototropic equilibrium of the pyrazole ring, all of these compounds present average4-fold symmetry on the NMR scale.The quaternary C3and C5carbons appear together,and the pairs of methylene carbons C6,C7,and C8are magnetically equivalent(see Experimental Section).In the13C NMR spectra registered in CDCl3solution, significant differences can be observed between ligand3,without an amino group in the center of the side chain,and the N-substituted ligands4-6.In3,the C3,5signal appears as a broad singlet.However,in4-6,it almost disappears within the baseline of the spectra,and the methylene carbon atoms C6and C8experience a significant broadening.Additionally,a remark-able line-broadening is also observed in the C1′carbon signals belonging to the phenethyl and octyl groups of L5and L6, respectively.All of these data suggest that as the N-substituents located in the middle of the side chains of4-6are larger,the dynamic exchange rate of the pyrazole prototropic equilibrium is gradually lower,probably due to a relation between proto-tropic and conformational equilibria.Acid-Base Behavior.To follow the complexation of L-glutamate(hereafter abbreviated as Glu2-)and its protonated forms(HGlu-,H2Glu,and H3Glu+)by the receptors L1-L6, the acid-base behavior of L-glutamate has to be revisited under the experimental conditions of this work,298K and0.15mol dm-3.The protonation constants obtained,included in the first column of Table1,agree with the literature25and show that the zwitterionic HGlu-species is the only species present in aqueous solution at physiological pH values(Scheme2and Figure S1of Supporting Information).Therefore,receptors for(23)Ara´n,V.J.;Kumar,M.;Molina,J.;Lamarque,L.;Navarro,P.;Garcı´a-Espan˜a,E.;Ramı´rez,J.A.;Luis,S.V.;Escuder,.Chem.1999, 64,6137-6146.(24)(a)Yuen Ng,C.;Motekaitis,R.J.;Martell,A.E.Inorg.Chem.1979,18,2982-2986.(b)Anelli,P.L.;Lunazzi,L.;Montanari,F.;Quici,.Chem.1984,49,4197-4203.Scheme1.Synthesis of the Pyrazole-Containing MacrocyclicReceptorsNew1H-Pyrazole-Containing Polyamine Receptors A R T I C L E SJ.AM.CHEM.SOC.9VOL.126,NO.3,2004825glutamate recognition able to address both the negative charges of the carboxylate groups and the positive charge of ammonium are highly relevant.The protonation constants of L 3-L 6are included in Table 1,together with those we have previously reported for receptors L 1and L 2.23A comparison of the constants of L 4-L 6with those of the nonfunctionalized receptor L 1shows a reduced basicity of the receptors L 4-L 6with tertiary nitrogens at the middle of the polyamine bridges.Such a reduction in basicity prevented the potentiometric detection of the last protonation for these ligands in aqueous solution.A similar reduction in basicity was previously reported for the macrocycle with the N -benzylated pyrazole spacers (L 2).23These diminished basicities are related to the lower probability of the tertiary nitrogens for stabilizing the positive charges through hydrogen bond formation either with adjacent nonprotonated amino groups of the molecule or with water molecules.Also,the increase in the hydrophobicity of these molecules will contribute to their lower basicity.The stepwise basicity constants are relatively high for the first four protonation steps,which is attributable to the fact that these protons can bind to the nitrogen atoms adjacent to the pyrazole groups leaving the central nitrogen free,the electrostatic repulsions between them being therefore of little significance.The remaining protonation steps will occur in the central nitrogen atom,which will produce an important increase in the electrostatic repulsion in the molecule and therefore a reduction in basicity.As stated above,the tertiary nitrogen atoms present in L 4-L 6will also contribute to this diminished basicity.To analyze the interaction with glutamic acid,it is important to know the protonation degree of the ligands at physiological pH values.In Table 2,we have calculated the percentages ofthe different protonated species existing in solution at pH 7.4for receptors L 1-L 6.As can be seen,except for the receptor with the pentamethylenic chains L 3in which the tetraprotonated species prevails,all of the other systems show that the di-and triprotonated species prevail,although to different extents.Interaction with Glutamate.The stepwise constants for the interaction of the receptors L 1-L 6with glutamate are shown in Table 3,and selected distribution diagrams are plotted in Figure 1A -C.All of the studied receptors interact with glutamate forming adduct species with protonation degrees (j )which vary between 8and 0depending on the system (see Table 3).The stepwise constants have been derived from the overall association constants (L +Glu 2-+j H +)H j LGlu (j -2)+,log j )provided by the fitting of the pH-metric titration curves.This takes into account the basicities of the receptors and glutamate (vide supra)and the pH range in which a given species prevails in solution.In this respect,except below pH ca.4and above pH 9,HGlu -can be chosen as the protonated form of glutamate involved in the formation of the different adducts.Below pH 4,the participation of H 2Glu in the equilibria has also to be considered (entries 9and 10in Table 3).For instance,the formation of the H 6LGlu 4+species can proceed through the equilibria HGlu -+H 5L 5+)H 6LGlu 4+(entry 8,Table 3),and H 2Glu +H 4L 4+)H 6LGlu 4(entry 9Table 3),with percentages of participation that depend on pH.One of the effects of the interaction is to render somewhat more basic the receptor,and somewhat more acidic glutamic acid,facilitating the attraction between op-positely charged partners.A first inspection of Table 3and of the diagrams A,B,and C in Figure 1shows that the interaction strengths differ markedly from one system to another depending on the structural features of the receptors involved.L 4is the receptor that presents the highest capacity for interacting with glutamate throughout all of the pH range explored.It must also be remarked that there are not clear-cut trends in the values of the stepwise constants as a function of the protonation degree of the receptors.This suggests that charge -charge attractions do not play the most(25)(a)Martell,E.;Smith,R.M.Critical Stability Constants ;Plenum:NewYork,1975.(b)Motekaitis,R.J.NIST Critically Selected Stability Constants of Metal Complexes Database ;NIST Standard Reference Database,version 4,1997.Table 1.Protonation Constants of Glutamic Acid and Receptors L 1-L 6Determined in NaCl 0.15mol dm -3at 298.1KreactionGluL 1aL 2aL 3bL 4L 5L 6L +H )L H c 9.574(2)d 9.74(2)8.90(3)9.56(1)9.25(3)9.49(4)9.34(5)L H +H )L H 2 4.165(3)8.86(2)8.27(2)8.939(7)8.38(3)8.11(5)8.13(5)L H 2+H )L H 3 2.18(2)7.96(2) 6.62(3)8.02(1) 6.89(5)7.17(6)7.46(7)L H 3+H )L H 4 6.83(2) 5.85(4)7.63(1) 6.32(5) 6.35(6) 5.97(8)L H 4+H )L H 5 4.57(3) 3.37(4) 2.72(8) 2.84(9) 3.23(9)L H 5+H )L H 6 3.18(3) 2.27(6)∑log K H n L41.135.334.233.634.034.1aTaken from ref 23.b These data were previously cited in a short communication (ref 26).c Charges omitted for clarity.d Values in parentheses are the standard deviations in the last significant figure.Scheme 2.L -Glutamate Acid -BaseBehaviorTable 2.Percentages of the Different Protonated Species at pH 7.4H 1L aH 2LH 3LH 4LL 11186417L 21077130L 3083458L 4083458L 51154323L 6842482aCharges omitted for clarity.A R T I C L E SMiranda et al.826J.AM.CHEM.SOC.9VOL.126,NO.3,2004outstanding role and that other forces contribute very importantly to these processes.26However,in systems such as these,which present overlapping equilibria,it is convenient to use conditional constants because they provide a clearer picture of the selectivity trends.27These constants are defined as the quotient between the overall amounts of complexed species and those of free receptor and substrate at a given pH[eq1].In Figure2are presented the logarithms of the effective constants versus pH for all of the studied systems.Receptors L1and L2with a nonfunctionalized secondary amino group in the side chains display opposite trend from all other receptors. While the stability of the L1and L2adducts tends to increase with pH,the other ligands show a decreasing interaction. Additionally,L1and L2present a close interaction over the entire pH range under study.The tetraaminic macrocycle L3is a better(26)Escartı´,F.;Miranda,C.;Lamarque,L.;Latorre,J.;Garcı´a-Espan˜a,E.;Kumar,M.;Ara´n,V.J.;Navarro,mun.2002,9,936-937.(27)(a)Bianchi,A.;Garcı´a-Espan˜a,c.1999,12,1725-1732.(b)Aguilar,J.A.;Celda,B.;Garcı´a-Espan˜a,E.;Luis,S.V.;Martı´nez,M.;Ramı´rez,J.A.;Soriano,C.;Tejero,B.J.Chem.Soc.,Perkin Trans.22000, 7,1323-1328.Table3.Stability Constants for the Interaction of L1-L6with the Different Protonated Forms of Glutamate(Glu) entry reaction a L1L2L3L4L5L6 1Glu+L)Glu L 3.30(2)b 4.11(1)2HGlu+L)HGlu L 3.65(2) 4.11(1) 3.68(2) 3.38(4) 3Glu+H L)HGlu L 3.89(2) 4.48(1) 3.96(2) 3.57(4) 4HGlu+H L)H2Glu L 3.49(2) 3.89(1) 2.37(4) 3.71(2)5HGlu+H2L)H3Glu L 3.44(2) 3.73(1) 2.34(3) 4.14(2) 2.46(4) 2.61(7) 6HGlu+H3L)H4Glu L 3.33(2) 3.56(2) 2.66(3) 4.65(2) 2.74(3) 2.55(7) 7HGlu+H4L)H5Glu L 3.02(2) 3.26(2) 2.58(3) 4.77(2) 2.87(3) 2.91(5) 8HGlu+H5L)H6Glu L 3.11(3) 3.54(2) 6.76(3) 4.96(3) 4.47(3) 9H2Glu+H4L)H6Glu L 2.54(3) 3.05(2) 3.88(2) 5.35(3) 3.66(4) 3.56(3) 10H2Glu+H5L)H7Glu L 2.61(6) 2.73(4) 5.51(3) 3.57(4) 3.22(8) 11H3Glu+H4L)H7Glu L 4.82(2) 4.12(9)a Charges omitted for clarity.b Values in parentheses are standard deviations in the last significantfigure.Figure1.Distribution diagrams for the systems(A)L1-glutamic acid, (B)L4-glutamic acid,and(C)L5-glutamicacid.Figure2.Representation of the variation of K cond(M-1)for the interaction of glutamic acid with(A)L1and L3,(B)L2,L4,L5,and L6.Initial concentrations of glutamate and receptors are10-3mol dm-3.Kcond)∑[(H i L)‚(H j Glu)]/{∑[H i L]∑[H j Glu]}(1)New1H-Pyrazole-Containing Polyamine Receptors A R T I C L E SJ.AM.CHEM.SOC.9VOL.126,NO.3,2004827receptor at acidic pH,but its interaction markedly decreases on raising the pH.These results strongly suggest the implication of the central nitrogens of the lateral polyamine chains in the stabilization of the adducts.Among the N-functionalized receptors,L4presents the largest interaction with glutamate.Interestingly enough,L5,which differs from L4only in having a phenethyl group instead of a benzyl one,presents much lower stability of its adducts.Since the basicity and thereby the protonation states that L4and L5 present with pH are very close,the reason for the larger stability of the L4adducts could reside on a better spatial disposition for formingπ-cation interactions with the ammonium group of the amino acid.In addition,as already pointed out,L4presents the highest affinity for glutamic acid in a wide pH range,being overcome only by L1and L2at pH values over9.This observation again supports the contribution ofπ-cation inter-actions in the system L4-glutamic because at these pH values the ammonium functionality will start to deprotonate(see Scheme2and Figure1B).Table4gathers the percentages of the species existing in equilibria at pH7.4together with the values of the conditional constant at this pH.In correspondence with Figure1A,1C and Figure S2(Supporting Information),it can be seen that for L1, L2,L5,and L6the prevailing species are[H2L‚HGlu]+and[H3L‚HGlu]2+(protonation degrees3and4,respectively),while for L3the main species are[H3L‚HGlu]+and[H4L‚HGlu]2+ (protonation degrees4and5,respectively).The most effective receptor at this pH would be L4which joins hydrogen bonding, charge-charge,andπ-cation contributions for the stabilization of the adducts.To check the selectivity of this receptor,we have also studied its interaction with L-aspartate,which is a competitor of L-glutamate in the biologic receptors.The conditional constant at pH7.4has a value of3.1logarithmic units for the system Asp-L4.Therefore,the selectivity of L4 for glutamate over aspartate(K cond(L4-glu)/K cond(L4-asp))will be of ca.15.It is interesting to remark that the affinity of L4 for zwiterionic L-glutamate at pH7.4is even larger than that displayed by receptors III and IV(Chart1)with the protected dianion N-acetyl-L-glutamate lacking the zwitterionic charac-teristics.Applying eq1and the stability constants reported in ref17,conditional constants at pH7.4of 3.24and 2.96 logarithmic units can be derived for the systems III-L-Glu and IV-L-Glu,respectively.Molecular Modeling Studies.Molecular mechanics-based methods involving docking studies have been used to study the binding orientations and affinities for the complexation of glutamate by L1-L6receptors.The quality of a computer simulation depends on two factors:accuracy of the force field that describes intra-and intermolecular interactions,and an adequate sampling of the conformational and configuration space of the system.28The additive AMBER force field is appropriate for describing the complexation processes of our compounds,as it is one of the best methods29in reproducing H-bonding and stacking stabiliza-tion energies.The experimental data show that at pH7.4,L1-L6exist in different protonation states.So,a theoretical study of the protonation of these ligands was done,including all of the species shown in5%or more abundance in the potentiometric measurements(Table4).In each case,the more favored positions of protons were calculated for mono-,di-,tri-,and tetraprotonated species.Molecular dynamics studies were performed to find the minimum energy conformations with simulated solvent effects.Molecular modeling studies were carried out using the AMBER30method implemented in the Hyperchem6.0pack-age,31modified by the inclusion of appropriate parameters. Where available,the parameters came from analogous ones used in the literature.32All others were developed following Koll-man33and Hopfinger34procedures.The equilibrium bond length and angle values came from experimental values of reasonable reference compounds.All of the compounds were constructed using standard geometry and standard bond lengths.To develop suitable parameters for NH‚‚‚N hydrogen bonding,ab initio calculations at the STO-3G level35were used to calculate atomic charges compatible with the AMBER force field charges,as they gave excellent results,and,at the same time,this method allows the study of aryl-amine interactions.In all cases,full geometry optimizations with the Polak-Ribiere algorithm were carried out,with no restraints.Ions are separated far away and well solvated in water due to the fact that water has a high dielectric constant and hydrogen bond network.Consequently,there is no need to use counteri-ons36in the modelization studies.In the absence of explicit solvent molecules,a distance-dependent dielectric factor quali-tatively simulates the presence of water,as it takes into account the fact that the intermolecular electrostatic interactions should vanish more rapidly with distance than in the gas phase.The same results can be obtained using a constant dielectric factor greater than1.We have chosen to use a distance-dependent dielectric constant( )4R ij)as this was the method used by Weiner et al.37to develop the AMBER force field.Table8 shows the theoretical differences in protonation energy(∆E p) of mono-,bi-,and triprotonated hexaamine ligands,for the (28)Urban,J.J.;Cronin,C.W.;Roberts,R.R.;Famini,G.R.J.Am.Chem.Soc.1997,119,12292-12299.(29)Hobza,P.;Kabelac,M.;Sponer,J.;Mejzlik,P.;Vondrasek,put.Chem.1997,18,1136-1150.(30)Cornell,W.D.;Cieplak,P.;Bayly,C.I.;Gould,I.R.;Merz,K.M.,Jr.;Ferguson,D.M.;Spelmeyer,D.C.;Fox,T.;Caldwell,J.W.;Kollman,P.A.J.Am.Chem.Soc.1995,117,5179-5197.(31)Hyperchem6.0(Hypercube Inc.).(32)(a)Fox,T.;Scanlan,T.S.;Kollman,P.A.J.Am.Chem.Soc.1997,119,11571-11577.(b)Grootenhuis,P.D.;Kollman,P.A.J.Am.Chem.Soc.1989,111,2152-2158.(c)Moyna,G.;Hernandez,G.;Williams,H.J.;Nachman,R.J.;Scott,put.Sci.1997,37,951-956.(d)Boden,C.D.J.;Patenden,put.-Aided Mol.Des.1999, 13,153-166.(33)/amber.(34)Hopfinger,A.J.;Pearlstein,put.Chem.1984,5,486-499.(35)Glennon,T.M.;Zheng,Y.-J.;Le Grand,S.M.;Shutzberg,B.A.;Merz,K.M.,put.Chem.1994,15,1019-1040.(36)Wang,J.;Kollman,P.A.J.Am.Chem.Soc.1998,120,11106-11114.Table4.Percentages of the Different Protonated Adducts[HGlu‚H j L](j-1)+,Overall Percentages of Complexation,andConditional Constants(K Cond)at pH7.4for the Interaction ofGlutamate(HGlu-)with Receptors L1-L6at Physiological pH[H n L‚HGlu]an)1n)2n)3n)4∑{[H n L‚HGlu]}K cond(M-1)L13272353 2.44×103L2947763 4.12×103L31101324 3.99×102L423737581 2.04×104L51010222 3.51×102L6121224 3.64×102a Charges omitted for clarity.A R T I C L E S Miranda et al. 828J.AM.CHEM.SOC.9VOL.126,NO.3,2004。
氢氧化物50%安全数据表说明书

SAFETY DATA SHEETHYDROGEN PEROXIDE, 50%1. PRODUCT AND COMPANY IDENTIFICATIONProduct InformationProduct name: HYDROGEN PEROXIDE 50% (ALL GRADES)Synonyms: H2O2 50%Molecular formula: H2O2Chemical family: peroxidesMolecular weight: 34.01 g/molProduct use: Bleaching agent, Oxidizing agent, Cosmetics, Water treatmentDetails of the supplier of the safety data sheetCompany Compass Remediation Chemicals2028 East Ben White Blvd#240-1974Austin, TX 78741Telephone (866) 221-9167Emergency telephone numberEmergency Phone #: CHEMTREC 1-800-424-93002. HAZARDS IDENTIFICATIONEmergency OverviewColor: colorlessPhysical state: liquidOdor: pungent*Classification of the substance or mixture:Oxidizing liquids, Category 2, H272Oral: Acute toxicity, Category 3,H301 Skin corrosion, Category 1C,H314 Serious eye damage, Category1, H318Specific target organ toxicity - single exposure, Category3, H335 Chronic aquatic toxicity, Category 3, H412*For the full text of the H-Statements mentioned in this Section, see Section 16GHS-LabellingHazard pictograms:Signal word: DangerSAFETY DATA SHEET – HYDROGEN PEROXIDE, 50%Hazard Statements:H272 : May intensify fire; oxidiser.H301 : Toxic if swallowed.H314 : Causes severe skin burns and eye damage.H335 : May cause respiratory irritation.H412 : Harmful to aquatic life with long lasting effects.Prevention:P210 : Keep away from heat.P220 : Keep/Store away from clothing/ combustiblematerials.P221 : Take any precaution to avoid mixing withcombustibles.P261 : Avoid breathing gas/mist/vapours/spray.P264 : Wash skin thoroughly after handling.P270 : Do not eat, drink or smoke when using thisproduct.P271 : Use only outdoors or in a well-ventilated area.P273 : Avoid release to the environment.P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection.Response:P301 + P310 : IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P301 + P330 + P331 : IF SWALLOWED: Rinse mouth. Do NOT inducevomiting.P303 + P361 + P353 : IF ON SKIN (or hair): Remove/ Take off immediately all contaminatedclothing. Rinse skin with water/ shower.P304 + P340 : IF INHALED: Remove victim to fresh air and keep at rest in a position comfortablefor breathing. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes.Remove contact lenses, if present and easy to do. Continue rinsing.P310 : Immediately call a POISON CENTER or doctor/physician. P363 : Wash contaminated clothing beforereuse.P370 + P378 : In case of fire: Use dry sand, dry chemical or alcohol-resistant foam for extinction.Storage:P403 + P233 : Store in a well-ventilated place. Keep container tightlyclosed. P405 : Store locked up.Disposal:P501 : Dispose of contents/ container to an approved waste disposal plant.Supplemental information:Potential Health Effects:If swallowed:May cause: gastrointestinal symptoms, ulceration, burns, accumulation of fluid in the lungs whichmay be delayed for several hours, (severity of effects depends on extent of exposure).SAFETY DATA SHEET – HYDROGEN PEROXIDE, 50%3. COMPOSITION/INFORMATION ON INGREDIENTSChemical Name CAS-No.Wt/Wt GHS Classification**HYDROGEN PEROXIDE 7722-84-150 %H271, H301, H332, H335,H314, H318, H412 Water7732-18-550 %Not classified**For the full text of the H-Statements mentioned in this Section, see Section 16.4. FIRST AID MEASURESInhalation:If inhaled, remove victim to fresh air. If not breathing, give artificial respiration. If breathing isdifficult, give oxygen. Get medical attention immediately.Skin:In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical attention immediately. Remove contaminated clothing and shoes. Wash clothing before reuse. Destroy contaminated shoes.Eyes:In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical attention immediately.Ingestion:If swallowed, DO NOT induce vomiting unless directed to do so by medical personnel. Call a Poison Control Center. If victim is fully conscious, give a cupful of water. Never give anything by mouth to an unconscious person. Rinse mouth.Notes to physician:Exposure to material may cause delayed lung injury resulting in pulmonary edema and pneumonitis.Exposed individuals should be monitored for 72 hours after exposure for the onset of delayedrespiratory symptoms.5. FIREFIGHTING MEASURESExtinguishing media (suitable):water spray, water fogProtective equipment:Fire fighters and others who may be exposed to products of combustion should wear full fire fighting turn out gear (full Bunker Gear) and self-contained breathing apparatus (pressure demand / NIOSH approved or equivalent).Further firefighting advice:Oxidizing materialIn case of major fire and large quantities: Evacuate area. Fight fire remotely due to the risk of explosion.SAFETY DATA SHEET – HYDROGEN PEROXIDE, 50%Decomposition will release oxygen, which will intensify a fire.Cool closed containers exposed to fire with water spray.Closed containers of this material may explode when subjected to heat from surrounding fire. Do not allow run-off from fire-fighting to enter drains or water courses.Fire-fighting equipment should be thoroughly decontaminated after use.Fire and explosion hazards:Solutions above 65% are especially hazardous as they do not contain enough water to remove the heat of decomposition by evaporation.Explosive when mixed with combustible material. Avoid breathing fumes from fire exposed material.6. ACCIDENTAL RELEASE MEASURESIn case of spill or leak:Prevent further leakage or spillage if you can do so without risk. Evacuate area of all unnecessarypersonnel. Ventilate the area. Eliminate all ignition sources. Avoid generation of vapors. Avoidcontact with cellulose, paper, sawdust or similar substances. Risk of self-ignition or promotion offires. Combustible materials exposed to hydrogen peroxide should be rinsed immediately with large amounts of water to ensure that all the hydrogen peroxide is removed. Contain and collect spillagewith non-combustible absorbent material such as clean sand, earth, diatomaceous earth or non-acidic clay and place into suitable properly labeled containers for prompt disposal. Avoid dispersal of spilled material and runoff and contact with soil, waterways, drains and sewers. Consult a regulatoryspecialist to determine appropriate state or local reporting requirements, for assistance in wastecharacterization and/or hazardous waste disposal and other requirements listed in pertinentenvironmental permits.7. HANDLING AND STORAGEHandlingGeneral information on handling:Do not taste or swallow.Do not get in eyes, on skin, or on clothing. Avoid breathing vapor or mist.Keep from contact with clothing and other combustible materials. Keep away from heat, sparks and flames.Use only with adequate ventilation. Wash thoroughly after handling.Wear fire/ flame resistant/ retardant clothing. Prevent product contamination.Keep only in the original container. Store in tightly closed container.DO NOT CUT, DRILL, GRIND, OR WELD ON OR NEAR THIS CONTAINER.Emptied container retains vapor and product residue.Observe all labeled safeguards until container is cleaned, reconditioned or destroyed. Avoid contamination.StorageGeneral information on storage conditions:Store in tightly closed container. Store in cool, dry, well ventilated area away from sources of ignition such as flame, sparks and static electricity. Store out of direct sunlight in a cool well-ventilated place. Store in original container.Store away from combustibles and incompatible materials. Refer to National Fire Protection Association(NFPA) 430, Code for the Storage of Solid and Liquid Oxidizers.Storage incompatibility – General:Store separate from acids, alkalies, reducing agents, and combustibles.Store separate from: Metallic oxides Organic materialsSAFETY DATA SHEET – HYDROGEN PEROXIDE, 50%8. EXPOSURE CONTROLS/PERSONAL PROTECTIONAirborne Exposure Guidelines:HYDROGEN PEROXIDE (7722-84-1)US. ACGIH Threshold Limit ValuesTime weighted average 1 ppmUS. OSHA Table Z-1 Limits for Air Contaminants (29 CFR1910.1000) PEL: 1 ppm (1.4 mg/m3)Only those components with exposure limits are printed in this section. Limits with skin contactdesignation above have skin contact effect. Air sampling alone is insufficient to accurately quantitate exposure. Measures to prevent significant cutaneous absorption may be required. Limits with asensitizer designation above mean that exposure to this material may cause allergic reactions.Engineering controls:Investigate engineering techniques to reduce exposures below airborne exposure limits or tootherwise reduce exposures. Provide ventilation if necessary to minimize exposures or to controlexposure levels to below airborne exposure limits (if applicable see above).If practical, use localmechanical exhaust ventilation at sources of air contamination such as open process equipment.Consult ACGIH ventilation manual or NFPA Standard 91 for design of exhaust systems.Respiratory protection:Avoid breathing vapor or mist. Where airborne exposure is likely or airborne exposure limits areexceeded (if applicable, see above), use NIOSH approved respiratory protection equipmentappropriate to the material and/or its components. Full facepiece equipment is recommendedand, if used, replaces need for face shield and/or chemical goggles. Consult respiratormanufacturer to determine appropriate type equipment for a given application. Observerespirator use limitations specified by NIOSH or the manufacturer. For emergency and otherconditions where there may be a potential for significant exposure or where exposure limitmay be significantly exceeded, use an approved full face positive-pressure, self-containedbreathing apparatus or positive-pressure airline with auxiliary self-contained air supply.Respiratory protection programs must comply with 29 CFR § 1910.134.Skin protection:Wear appropriate chemical resistant protective clothing and chemical resistant gloves to preventskin contact. When handling this material, gloves of the following type(s) should be worn:NeoprenePolyvinylchlorideImpervious butyl rubber glovesWear a face shield, chemical goggles and chemical resistant clothing such as an approvedsplash protective suit made of SBR Rubber, PVC, Gore-Tex or a HAZMAT Splash Protective Suit(Level A, B, or C) when splashing may occur (such as connecting/disconnecting, mechanicalfirst break). For foot protection, wear boots made of NBR, PVC, polyurethane, or neoprene.Overboots made of Latex or PVC, as well as firefighter boots or specialized HAZMAT boots arealso permitted. DO NOT wear any form of boot or overboots made of nylon or nylon blends. DONOT use cotton, wool or leather, as these materials react RAPIDLY with higher concentrationsSAFETY DATA SHEET – HYDROGEN PEROXIDE, 50%of hydrogen peroxide. Rinse immediately if skin is contaminated. Remove contaminated clothing and shoes immediately. Thoroughly rinse the outside of gloves and protective clothing with water prior to removal. Completely submerge hydrogen peroxide contaminated clothing or othermaterials in water prior to drying. Residual hydrogen peroxide, if allowed to dry on materialssuch as paper, fabrics, cotton, leather, wood or other combustibles can cause the material toignite and result in a fire. Clean protective equipment before reuse. Provide a safety shower at any location where skin contact can occur. Wash thoroughly after handling.Eye protection:Where there is potential for eye contact, wear a face shield, chemical goggles, and have eyeflushing equipment immediately available.9. PHYSICAL AND CHEMICAL PROPERTIESColor: colorlessPhysical state:liquidOdor:pungentOdor threshold:No data availableFlash point None.Auto-ignition temperature:Not applicableLower flammable limit (LFL):Not applicableUpper flammable limit (UFL):Not applicablepH: No data availableDensity: 1.196 g/cm3 (68 °F (20 °C))Vapor pressure:18 mmHg (68 °F (20 °C))Relative vapor density: 1.0Vapor density:not determinedBoiling point/boiling range: 237 °F (114 °C)Freezing point:-62 °F (-52 °C)Evaporation rate:No data availableSolubility in water:completely soluble% Volatiles:100 %Molecular weight: 34.01 g/molOil/water partition coefficient: No data availableThermal decomposition No data availableFlammability: See GHS Classification in Section 210. STABILITY AND REACTIVITYStability:This material is chemically stable under normal and anticipated storage, handling and processing conditions.Materials to avoid:MetalsOrganic materialsReducing agentsMetallic oxidesDustsCombustible materials (e.g., wood, sawdust)Alkaline materialsConditions/hazards to avoid:Material decomposes with the potential to produce a rupture of unvented closed containers.SAFETY DATA SHEET – HYDROGEN PEROXIDE, 50%Hazardous decomposition products:This material decomposes if contaminated, causing fire and possible explosions. Oxygen can beliberated at temperatures above ambient.11. TOXICOLOGICAL INFORMATIONData for HYDROGEN PEROXIDE 50% (ALL GRADES)Acute toxicityOral:Toxic if swallowed. (Rat) LD50 = 225 - 1,200 mg/kg. (50 %) (as aqueous solution)Dermal:Practically nontoxic. (Rat) LD50 = 9,200 mg/kg. (70 %) (as aqueous solution)Inhalation:No deaths occurred. (Rat) 4 h LC0 > 0.17 mg/l. (50 %) (saturated vapor)Specific target organ toxicity - single exposure:May cause respiratory irritation.Skin Irritation:Causes severe skin burns. (Rabbit) (1 h) (50 %) (aqueous solution)Eye Irritation:Causes serious eye damage. (Rabbit) (70 %) (aqueous solution)12. ECOLOGICAL INFORMATIONChemical Fate and PathwayData on this material and/or a similar material are summarized below.EcotoxicologyData on this material and/or a similar material are summarized below.13. DISPOSAL CONSIDERATIONSWaste disposal:Dilution with water is the preferred method of disposal. Dispose of in accordance with federal, state and local regulations. Consult a regulatory specialist to determine appropriate state or local reporting requirements, for assistance in waste characterization and/or hazardous waste disposal and otherrequirements listed in pertinent environmental permits. Note: Chemical additions to, processing of, or otherwise altering this material may make this waste management information incomplete, inaccurate, or otherwise inappropriate. Furthermore, state and local waste disposal requirements may be more restrictive or otherwise different from federal laws and regulations.Take appropriate measures to prevent release to the environment.14. TRANSPORT INFORMATIONUS Department of Transportation (DOT)UN Number : 2014Proper shipping name : Hydrogen peroxide, aqueous solutionsClass : 5.1Subsidiary hazard class : (8)Packaging group : IIMarine pollutant : noSAFETY DATA SHEET – HYDROGEN PEROXIDE, 50%International Maritime Dangerous Goods Code (IMDG)UN Number : 2014Proper shipping name : HYDROGEN PEROXIDE, AQUEOUS SOLUTIONClass : 5.1Subsidiary hazard class : (8)Packaging group : IIMarine pollutant : no15. REGULATORY INFORMATIONChemical Inventory StatusEU. EINECS EINECS Conforms toUS. Toxic Substances Control Act TSCA The components of this product are all onthe TSCA Inventory.Australia. Industrial Chemical AICS Conforms to(Notification and Assessment) ActCanada. Canadian Environmental DSL All components of this product are on the Protection Act (CEPA). Domestic Canadian DSL.Substances List (DSL)Japan. Kashin-Hou Law List ENCS (JP) Does not conformKorea. Existing Chemicals Inventory (KECI) KECI (KR) Conforms toPhilippines. The Toxic Substances PICCS (PH) Does not conformand Hazardous and Nuclear Waste Control ActChina. Inventory of Existing Chemical IECSC (CN) Does not conformSubstancesUnited States – Federal RegulationsSARA Title III – Section 302 Extremely Hazardous Chemicals:Chemical Name CAS-No. SARA Reportable SARA ThresholdQuantities Planning QuantityHYDROGEN PEROXIDE 7722-84-1 1000 lbs 1000 lbsSARA Title III - Section 311/312 Hazard Categories:Acute Health Hazard, Fire Hazard, Reactivity HazardSARA Title III – Section 313 Toxic Chemicals:This material does not contain any chemical components with known CAS numbers thatexceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) -Reportable Quantity (RQ):The components in this product are either not CERCLA regulated, regulated but present innegligible concentrations, or regulated with no assigned reportable quantity.SAFETY DATA SHEET – HYDROGEN PEROXIDE, 50%United States – State RegulationsNew Jersey Right to KnowChemical Name CAS-No.Water 7732-18-5HYDROGEN PEROXIDE 7722-84-1New Jersey Right to Know – Special Health Hazard Substance(s)Chemical Name CAS-No.HYDROGEN PEROXIDE 7722-84-1Pennsylvania Right to KnowChemical Name CAS-No.Water 7732-18-5HYDROGEN PEROXIDE 7722-84-1Pennsylvania Right to Know – Environmentally Hazardous Substance(s)Chemical Name CAS-No.HYDROGEN PEROXIDE 7722-84-1California Prop. 65This product does not contain any chemicals known to the State of California to causecancer, birth defects, or any other reproductive defects.16. OTHER INFORMATIONFull text of H-Statements referred to under sections 2 and 3.H272 May intensify fire;oxidiser.H301 Toxic ifswallowed.H314 Causes severe skin burns and eyedamage.H318 Causes serious eye damage.H332 Harmful if inhaled.H335 May cause respiratory irritation.H412 Harmful to aquatic life with long lasting effects.DisclaimerThe information contained herein is accurate to the best of our knowledge. However, data, safetystandards and government regulations are subject to change and, therefore, holders and users shouldsatisfy themselves that they are aware of all current data and regulations relevant to their particular use of product. COMPASS REMEDIATION CHEMICALS DISCLAIMS ALL LIABILITY FOR RELIANCE ON THECOMPLETENESS OR ACCURACY OR THE INFORMATION INCLUDED HEREIN. COMPASS REMEDIATIONCHEMICALS MAKES NO WARRANTY, EITHER EXPRESS OR IMPLIED, INCLUDING, BUT NOT LIMITED TO, ANY WARRANTIES OF MERCHANTIABILITY OR FITNESS FOR PARTICULAR USE OR PURPOSE OF THEPRODUCT DESCRIBED HEREIN. All conditions relating to storage, handling, and use of the product arebeyond the control of Compass Remediation Chemicals and shall be the sole responsibility of the holder or user of the product.。
iron hydroxide化学式

iron hydroxide化学式
铁羟化合物是一种化学式为Fe(OH)2的化合物。
它由铁离子和羟基离子组成,是一种常见的金属羟化物之一。
铁羟化合物具有一些特殊的性质和用途。
首先,它是一种弱碱性物质,可以与酸反应生成盐和水。
例如,当铁羟化合物与盐酸反应时,会生成氯化铁和水的反应。
这种反应可以用化学方程式表示为:
Fe(OH)2 + 2HCl → FeCl2 + 2H2O
铁羟化合物还具有一定的溶解度。
在水中,铁羟化合物会发生部分溶解,生成铁离子和羟基离子。
这使得铁羟化合物在一些化学反应和实验中具有重要的应用。
例如,铁羟化合物可以用作析出铁离子的试剂,可以通过加入氢氧化钠溶液来沉淀出铁羟化合物。
铁羟化合物还具有一定的吸湿性。
它可以吸收周围的水分,形成水合物。
这种性质使得铁羟化合物在一些湿度控制的领域,如干燥剂的制备和使用中发挥重要作用。
铁羟化合物在工业上也有广泛的应用。
它可以用作催化剂的载体,用于催化剂的制备和催化反应的进行。
此外,铁羟化合物还可以用于废水处理和环境修复中,通过吸附重金属离子和有机污染物来净化水体和土壤。
铁羟化合物还具有一些其他的特点。
例如,它是一种具有磁性的物
质,可以在外加磁场下表现出磁性行为。
这种特性使得铁羟化合物在磁学研究和应用中具有潜在的用途。
铁羟化合物是一种重要的化学物质,具有弱碱性、溶解性、吸湿性和磁性等特点。
它在酸碱中和、水处理、催化剂制备、环境修复和磁学研究等领域都有广泛的应用。
铁羟化合物的研究和应用对于促进化学科学的发展和解决环境问题具有重要意义。
硝酸铁9水合物英文缩写

硝酸铁9水合物英文缩写The Abbreviation of Iron (III) Nitrate NonahydrateIron (III) nitrate nonahydrate, commonly known as ferric nitrate nonahydrate or iron (III) nitrate enneahydrate, is a chemical compound with the chemical formula Fe(NO3)3·9H2O. This complex salt is used in various industries, such as in the production of pigments, inorganic salts, and catalysts. The abbreviation for iron (III) nitrate nonahydrate isFe(NO3)3·9H2O. In this article, we will explore the properties and applications of this compound.Properties of Iron (III) Nitrate NonahydrateIron (III) nitrate nonahydrate exists as green crystals or powder. It is highly soluble in water, alcohol, and acetone. The compound has a molecular weight of 404.0 g/mol. Iron (III) nitrate nonahydrate is an oxidizing agent and reacts with reducing agents, which makes it important in a variety of chemical reactions. The compound also has the ability to form coordination compounds with various ligands due to the presence of a transition metal ion.Applications of Iron (III) Nitrate NonahydrateThe versatility of iron (III) nitrate nonahydrate allows for its application in different industries.1. Pigment Production:Iron (III) nitrate nonahydrate is commonly used in the production of pigments. It serves as a source of iron ions, which can provide various colorsto the pigments. By controlling the concentration and reaction conditions, different shades and intensities of color can be obtained. The pigment produced using iron (III) nitrate nonahydrate is widely used in the paint, ink, and textile industries.2. Inorganic Salt Synthesis:Iron (III) nitrate nonahydrate is a precursor in the synthesis of various inorganic salts. By reacting it with different metal or non-metal ions, a wide range of iron-containing compounds can be obtained. For example, when reacting with sodium hydroxide (NaOH), iron (III) hydroxide (Fe(OH)3) can be obtained. These inorganic salts find applications in water treatment, agriculture, and as catalysts in chemical reactions.3. Oxidation Reactions:Due to its oxidizing properties, iron (III) nitrate nonahydrate is utilized in several oxidation reactions. It can act as a catalyst or reactant in organic synthesis processes. For instance, it can be employed in the synthesis of aldehydes and ketones from primary and secondary alcohols. The presence of the iron (III) ion aids in the oxidation of the alcohol to the desired product.4. Analytical Chemistry:Iron (III) nitrate nonahydrate is also used in analytical chemistry as a reagent. In qualitative analysis, it is used for the detection of phenols and phosphates. Additionally, iron (III) nitrate nonahydrate can be utilized as a standard solution in complexometric titrations to determine various substances, such as EDTA (ethylene diamine tetraacetic acid), which forms a stable complex with the iron ion.5. Chemical Education:Iron (III) nitrate nonahydrate is utilized in educational laboratories for conducting experiments and demonstrations. Its colorful properties make it an interesting compound to study. It is often used in crystal growing experiments to demonstrate the formation of solid crystals and the crystallization process.In conclusion, the abbreviation for iron (III) nitrate nonahydrate isFe(NO3)3·9H2O. This compound has various applications in industries such as pigment production, inorganic salt synthesis, oxidation reactions, analytical chemistry, and chemical education. Its properties as an oxidizing agent and source of iron ions make it valuable in numerous chemical processes.。
Poly-D-lysine Hydrobromide 产品说明书
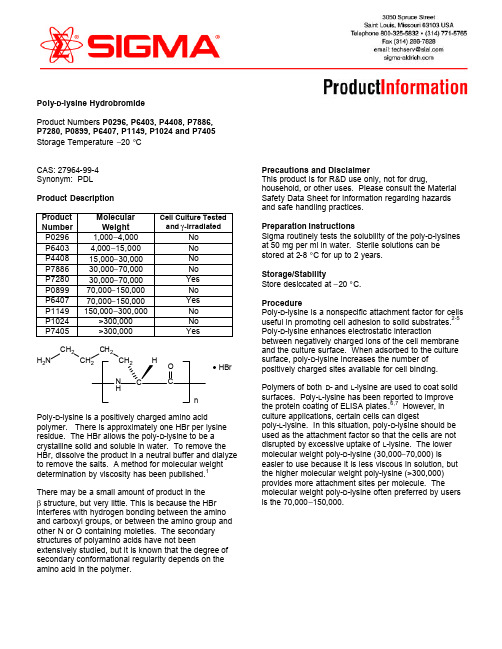
Poly-D -lysine HydrobromideProduct Numbers P0296, P6403, P4408, P7886, P7280, P0899, P6407, P1149, P1024 and P7405 Storage Temperature −20 °CCAS:27964-99-4Synonym: PDLProduct DescriptionProduct Number MolecularWeightCell Culture Tested and γ-irradiated P02961,000−4,000 No P6403 4,000−15,000 No P4408 15,000−30,000 No P7886 30,000−70,000 No P7280 30,000−70,000 Yes P0899 70,000−150,000 No P6407 70,000−150,000 Yes P1149 150,000−300,000 No P1024 >300,000 No P7405 >300,000 YesH 2NCH 2CH 2CH 2CH 2N HO nHBrPoly-D -lysine is a positively charged amino acidpolymer. There is approximately one HBr per lysine residue. The HBr allows the poly-D -lysine to be a crystalline solid and soluble in water. To remove the HBr, dissolve the product in a neutral buffer and dialyze to remove the salts. A method for molecular weightdetermination by viscosity has been published.1There may be a small amount of product in the β structure, but very little. This is because the HBr interferes with hydrogen bonding between the amino and carboxyl groups, or between the amino group and other N or O containing moieties. The secondary structures of polyamino acids have not beenextensively studied, but it is known that the degree of secondary conformational regularity depends on the amino acid in the polymer.Precautions and DisclaimerThis product is for R&D use only, not for drug,household, or other uses. Please consult the Material Safety Data Sheet for information regarding hazards and safe handling practices.Preparation InstructionsSigma routinely tests the solubility of the poly-D -lysines at 50 mg per ml in water. Sterile solutions can be stored at 2-8 °C for up to 2 years.Storage/StabilityStore desiccated at −20 °C.ProcedurePoly-D -lysine is a nonspecific attachment factor for cellsuseful in promoting cell adhesion to solid substrates.2-5Poly-D -lysine enhances electrostatic interactionbetween negatively charged ions of the cell membrane and the culture surface. When adsorbed to the culture surface, poly-D -lysine increases the number of positively charged sites available for cell binding.Polymers of both D - and L -lysine are used to coat solid surfaces. Poly-L -lysine has been reported to improvethe protein coating of ELISA plates.6,7However, in culture applications, certain cells can digestpoly-L -lysine. In this situation, poly-D -lysine should be used as the attachment factor so that the cells are not disrupted by excessive uptake of L -lysine. The lower molecular weight poly-D -lysine (30,000−70,000) iseasier to use because it is less viscous in solution, but the higher molecular weight poly-lysine (>300,000) provides more attachment sites per molecule. The molecular weight poly-D -lysine often preferred by users is the 70,000−150,000.Cell Culture:When using poly-D-lysine as an attachment factor, the optimal conditions must be determined for each cell line and application. In general, the following steps can be used.1. Add 50 ml of sterile tissue culture grade water to5 mg of poly-D-lysine.2. Aseptically coat culture surface with 0.5−1.0 ml ofsolution per 25 cm2. Rock gently to ensure evencoating of the culture surface.3. After 5 minutes, remove solution by aspiration andthoroughly rinse surface with sterile tissue culturegrade water.4. Allow to dry at least two hours before introducingcells and medium.If glassware or slides must be sterilized after coating with poly-lysine, γ-irradiation is recommended insteadof autoclaving.If uneven coating occurs, glass slides may be pretreated with 1 mM magnesium acetate for2−3 hours and then rinsed well before coating. Alternatively, they may be acid washed (hydrochloric acid or sulfuric acid). This treatment should allow for an even coating with the poly-D-lysine solution. Histology:In general, a 0.1% (w/v) poly-D-lysine solution is recommended as a dip for histology slide preparation. After a five-minute exposure, drain slides and dry at room temperature overnight or in an oven (~60 °C) for ~1 hour. Store the solution in plastic bottles in the refrigerator and limit use to four times.Related ProductsPoly-L-lysine Solution (suitable for histochemical techniques)• Poly-L-lysine Solution, 0.1% (w/v) in water (preservative added), Prod. No. P8920References1. Yaron, A., and Berger, A., The effect of urea andguanidine on the helix content of poly-N5-(3-hydroxypropyl)-L-glutamine in aqueous solventsystems. Biochim. Biophys. Acta, 69, 397 (1963).2. Jacobson, B.S., and Branton, D. Plasmamembrane: rapid isolation and exposure of thecytoplasmic surface by use of positively chargedbeads. Science, 195, 302, (1977).3. Leifer, D., et al., Monoclonal antibody to Thy-1enhances regeneration of processes by rat retinalganglion cells in culture. Science, 224, 303 (1984).4. Cannela, M., and Ross, R. Influence of substratumon the retrograde response of the rat superiorcervical ganglion in vitro. Exp. Neurology, 95, 652(1987).5. Needham, L., et al., Endothelial functionalresponses and increased vascular permeabilityinduced by polycations. Lab. Invest.59(4), 538-548 (1988).6. Banerjee, D.S., et al., An ELISA method forquantitation of tubulin using poly-l-lysine coatedmicrotiter plates. Indian J. Exp. Bio., 27, 972-976(1989).7. Stinitz, M. Quantitation of the blocking effect ofTween 20 and bovine serum albumin in ELISAmicrowells. Anal. Biochem., 282, 232-238 (2000).CMH,ARO,TES,KTA 09/05-1Cell Culture Poly-Lysine Selection GuideSigma offers both Poly-D-Lysine and Poly-L-Lysine in several molecular weight ranges. Poly-Lysine enhances electrostatic interaction between negatively charged ions of the cell membrane and positively charged surface ions of attachment factors on the culture surface. When adsorbed to the culture surface, it increases the number of positively charged sites available for cell binding.Product Molecular Target Cells Concentration Number Product Weight Source Storage For Attachment For Use Refs. P7280 POLY-D-LYSINE MW 30,000−70,000 synthetic −20 °C; store Attachment of a variety Use 0.5 ml of 3,4,5 HYDROBROMIDE solubilized of cell types a 0.10 mg/mlP6407 Lyophilized,MW 70,000−150,000 product at solution to Sterilized −20 °C coat 25 cm2P7405 by γ-irradiation MW >300,000P9155 POLY-L-LYSINE MW 30,000−70,000HYDROBROMIDEP6282 Lyophilized,MW 70,000−150,000SterilizedP5899 by γ-irradiation MW >300,000P4707 POLY-L-LYSINE MW 70,000−150,0000.01% SolutionP4832 Sterile MW 150,000−300,000Please refer to the catalog for additional extracellular matrices/attachment factors.Sigma brand products are sold through Sigma-Aldrich, Inc.Sigma-Aldrich, Inc. warrants that its products conform to the information contained in this and other Sigma-Aldrich publications.Purchaser must determine the suitability of the product(s) for their particular use. Additional terms and conditions may apply.Please see reverse side of the invoice or packing slip.。
叔丁基过氧化氢(tbhp)-连二亚硫酸钠(sh)氧化还原体系

叔丁基过氧化氢(tbhp)-连二亚硫酸钠(sh)氧化还原体系下载提示:该文档是本店铺精心编制而成的,希望大家下载后,能够帮助大家解决实际问题。
文档下载后可定制修改,请根据实际需要进行调整和使用,谢谢!本店铺为大家提供各种类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by this editor. I hope that after you download it, it can help you solve practical problems. The document can be customized and modified after downloading, please adjust and use it according to actual needs, thank you! In addition, this shop provides you with various types of practical materials, such as educational essays, diary appreciation, sentence excerpts, ancient poems, classic articles, topic composition, work summary, word parsing, copy excerpts, other materials and so on, want to know different data formats and writing methods, please pay attention!叔丁基过氧化氢(TBHP)连二亚硫酸钠(SH)氧化还原体系1. 引言在有机合成领域中,氧化还原反应是一种常见而重要的反应类型。
铁铬液流电池析氢氧化

铁铬液流电池析氢氧化
铁铬液流电池是一种利用铁和铬溶液进行电化学反应的设备,通过该反应析出氢氧化物。
这种电池的工作原理是利用铁和铬的电化学特性,以及液流电池的特殊构造。
铁铬液流电池中,铁和铬溶液分别充当阳极和阴极。
当电流通过电解质溶液时,铁在阳极处氧化,释放出电子,并溶解成铁离子。
而铬在阴极处还原,接受电子,并从溶液中析出。
这一反应过程可以表示为:
阴极:Cr3+ + 3e- → Cr
阳极:Fe → Fe2+ + 2e-
由于电流的通过和溶液的循环流动,铁铬液流电池可以持续进行反应,从而持续地析出氢氧化物。
这种电池的优势在于可以提供连续的电流输出,并且可以根据需要调整电流的大小。
铁铬液流电池析氢氧化的过程是一个重要的能量转化过程。
氢氧化物是一种重要的化学品,广泛应用于工业生产、环境保护和能源储存等领域。
通过铁铬液流电池析氢氧化,可以实现能源的高效转换和可持续利用。
然而,铁铬液流电池析氢氧化的过程也存在一些挑战和问题。
比如,电池的效率和稳定性需要进一步提高,电解质的选择和优化也是一个重要的研究方向。
此外,电池的制造和操作也需要更多的技术支
持和改进。
铁铬液流电池是一种重要的能量转化设备,可以通过电化学反应析出氢氧化物。
这种电池在能源转换和储存方面具有潜在的应用前景,但也面临一些挑战和问题。
通过持续的研究和创新,相信铁铬液流电池在未来会发挥更大的作用,并为人类社会的可持续发展做出贡献。
氢氧化二茂铁

氢氧化二茂铁氢氧化二茂铁是一种由铁离子和茂基离子组成的化合物,具有多种特殊的性质和应用。
本文将从不同角度介绍氢氧化二茂铁,并探讨其在科学研究和工业领域中的重要性。
一、氢氧化二茂铁的基本性质氢氧化二茂铁化学式为[(η5-C5H5)2Fe]OH,是一种具有橙红色晶体结构的固体。
它在常温下稳定,不易溶于水,但可溶于有机溶剂。
氢氧化二茂铁具有良好的热稳定性,在高温下不易分解。
同时,它还具有较高的磁性,可被应用于磁性材料的研究和制备。
1. 催化剂:氢氧化二茂铁作为一种重要的过渡金属化合物,具有优良的催化性能。
它在有机合成反应中广泛应用,可用于催化羰基化合物的加成反应、烯烃的环化反应等。
在有机合成领域中,氢氧化二茂铁作为催化剂具有高效、环境友好等特点,受到了广泛关注。
2. 电池材料:氢氧化二茂铁具有优异的电化学性能,可以作为电池正极材料进行利用。
其中,锂离子电池是目前最为常见的应用之一。
氢氧化二茂铁作为锂离子电池正极材料,具有较高的比容量和循环稳定性,可用于电动汽车、移动通信设备等的电源系统。
3. 生物医学:氢氧化二茂铁在生物医学领域中也有一定的应用。
它可以作为抗肿瘤药物,通过抑制肿瘤细胞的生长和分裂,达到治疗肿瘤的效果。
同时,氢氧化二茂铁还可以用于磁共振成像(MRI)等医学诊断技术中,通过其磁性性质对人体组织进行成像。
三、氢氧化二茂铁的研究进展近年来,氢氧化二茂铁的研究得到了广泛关注,相关的科研论文和专利也层出不穷。
研究者们通过改变氢氧化二茂铁的结构和组成,探索其在新能源、环境保护和生物医学等领域的更多应用。
同时,还有一些研究关注氢氧化二茂铁的制备方法和性质的研究,以提高其在实际应用中的效果和性能。
氢氧化二茂铁作为一种重要的过渡金属化合物,在催化剂、电池材料和生物医学等领域具有广泛的应用前景。
通过不断的研究和开发,相信氢氧化二茂铁将为人类社会的发展做出更大的贡献。
