Entropy and Free Energy - Arapahoe High School熵与自由能-阿拉帕霍高中
碳酸锂在水中的溶解度和超溶解度的测定及热力学分析

化工进展CHEMICAL INDUSTRY AND ENGINEERING PROGRESS 2016年第35卷第8期·2350·碳酸锂在水中的溶解度和超溶解度的测定及热力学分析宋昌斌1,李润超2(1青海盐湖镁业有限公司,青海格尔木 816099;2北京四中高三(5)班,北京 100088)摘要:固体溶质在溶剂中的溶解度和超溶解度数值决定了结晶介稳区的宽度,而溶质结晶分离过程又是在介稳区中进行操作,因此固体溶质的溶解度和超溶解度在工业结晶中是很重要的基础数据。
本文以碳酸锂为溶质,在标准压力条件和283.15~318.15K温度条件下,用重量分析法测定其在水中的溶解度;用激光动态法测定其在一定温度条件下在水中的超溶解度,从而得到碳酸锂在水溶液中的介稳区;结果显示,碳酸锂在水中的溶解度和超溶解度均随温度的升高而减小,介稳区宽度随温度的升高而变窄;其溶解度数据用Van’t Hoff方程和修正的Apelblat方程进行了热力学关联计算,结果表明,两种热力学模型对碳酸锂在水中溶解度的关联效果都很好,其中Van’t Hoff方程和修正的Apelblat方程的计算值与实验值的平均相对偏差分别为0.54%和0.20%。
通过溶解热力学计算,得到碳酸锂在水中的溶解焓∆H d、熔解熵ΔS d和溶液标准吉布斯自由能变∆G d,结果表明该溶解过程为放热熵减小的非自发过程,并且溶解熵变对溶解过程的影响较大。
关键词:碳酸锂;水;溶解度;超溶解度;介稳区;热力学计算中图分类号:TQ 013.1 文献标志码:A 文章编号:1000–6613(2016)08–2350–05DOI:10.16085/j.issn.1000-6613.2016.08.07Measurement and thermodynamic analysis of the solubility andsupersolubility of lithium carbonate in waterSONG Changbin1,LI Runchao2(1Qinghai Salt Lake Magnesium Industry Co.,Ltd.,Geermu 816099,Qinghai,China;2Beijing No.4 Middle School,High Senior Three Class 5,Beijing 100088,China)Abstract:The solubility and supersolubility determines the width of the metastable state and the crystallization process is operated in the metastable zone. Therefore,the solubility and supersolubility are an important basic data in industrial crystallization process. In this study,lithium carbonate was used as solute and the solubility of lithium carbonate in water was measured at (283.15 to 318.15) K and atmospheric pressure by using a gravimetric method. The supersolubility was measured by using a laser dynamic method. It was obviously showed that the solubility and supersolubility of lithium carbonate in water and the width of the metastable zone decreased with increasing temperature. The solubility data was correlated by Van’t Hoff equation and modified Apelblat equation. The results indicated that the solubility of calculated values was in good agreement with the experimental values.The average relative deviation of the Van’t Hoff equation and the modified Apelblat equation were0.54% and 0.20%,respectively. The changes of enthalpy(ΔH d),entropy(ΔS d)and Gibbs free energy(ΔG d)of the dissolving process were obtained by the thermodynamic calculation. The dissolving process was a non-spontaneous process of exothermic and Entropy. The entropy change was the main influencing factor in the dissolution process.152****************。
诺贝尔化学奖获得者 艾伦 黑格尔 演讲稿

“Plastic”Solar Cells:Self-Assembly of Bulk Heterojunction Nanomaterials by SpontaneousPhase SeparationJEFFREY PEET,ALAN J.HEEGER,*AND GUILLERMO C.BAZAN*Center for Polymers and Organic Solids,University of California at SantaBarbara,Santa Barbara,California93106RECEIVED ON FEBRUARY27,2009A s the global demand for low-cost renewable energy sources intensifies,interest in new routes for converting solar energyto electricity is rapidly increasing.Although photovoltaic cells have been commercially available for more than50years, only0.1%of the total electricity generated in the United States comes directly from sunlight.The earliest commercial solar technology remains the basis for the most prevalent devices in current use,namely,highly-ordered crystalline,inorganic solar cells,commonly referred to as silicon cells.Another class of solar cells that has recently inspired significant academic and industrial excitement is the bulk het-erojunction(BHJ)“plastic”solar cell.Research by a rapidly growing community of scientists across the globe is gen-erating a steady stream of new insights into the fundamental physics,the materials design and synthesis,thefilm processing and morphology,and the device science and architecture of BHJ technology.Future progress in the fab-rication of high-performance BHJ cells will depend on our ability to combine aspects of synthetic and physical chem-istry,condensed matter physics,and materials science.In this Account,we use a combination of characterization tools to tie together recent advances in BHJ morphology char-acterization,device photophysics,and thin-film solution processing,illustrating how to identify the limiting factors in solar cell performance.We also highlight how new processing methods,which control both the BHJ phase separation and the internal order of the components,can be implemented to increase the power conversion efficiency(PCE).The failure of many innovative materials to achieve high performance in BHJ solar cell devices has been blamed on“poor morphology”without significant characterization of either the structure of the phase-separated morphology or the nature of the charge carrier recombination.We demonstrate how properly controlling the“nanomorphology”,which is critically dependent on minute experimental details at every step,from synthesis to device construction,provides a clear path to>10% PCE BHJ cells,which can be fabricated at a fraction of the cost of conventional solar cells.IntroductionEnough energy arrives on the earth’s surface every hour to power the entire human race for a year,yet despite the fact that solar photovol-taics have been commercially available for more than half a century,they account for less than a tenth of one percent of our electricity genera-tion.Photovoltaics based on conjugatedmate-1700ACCOUNTS OF CHEMICAL RESEARCH1700-1708November2009Vol.42,No.11Published on the Web07/01//acr10.1021/ar900065j CCC:$71.50©2009American Chemical Societyrials solution-processed onto flexible plastic substrates represent a potential platform for continuous,large-scale printing of thin-film photovoltaics.1,2Rapid development of the technology has led to growing interest in polymer-based solar cells in academic and industrial laboratories and has been the subject of multiple recent reviews.2-7These devices are promising in terms of low-cost power generation and simplicity of fabrication,but are remark-ably complex in terms of device physics and in the size of the parameter space for materials selection and device fab-rication.8The most common polymer solar cell structure is the bulk heterojunction (BHJ)device in which a polymeric electron donor and a fullerene-based electron acceptor are mixed in solution and cast into a thin film that is sandwiched between two electrodes.Due to the inherent symmetry of the BHJ active layer,the anode and cathode of the device must be defined by the nature of the electrodes.9The first reported BHJ solar cell was published in 1995and comprised a film of a poly(phenylene vinylene)(PPV)derivative blended with phenyl C 61-butyric acid methyl ester (PC 61BM),as shown in Figure 1A.9Since that publication,the device architecture has changed only slightly,but improved understanding of the device oper-ation,loss mechanisms,and limitations has enabled signifi-cant improvements in materials,processing,and charac-terization.These advances have been accompanied by progress in large area device fabrication via low-cost coating methods such as inkjet printing,doctor blade coating,slot-die coating,screen printing and rotogravure printing onto flex-ible plastic substrates.1,10In addition,recent measurements of device operational lifetime and environmental stability indi-cate that the BHJ devices,even at the current level of devel-opment,will be viable for years of continuous use.11,12Unlike highly ordered crystalline inorganic solar cells,where photoexcitation yields a pair of free carriers,photons incident on the active layer of a polymer BHJ solar cell cre-ate excitons,that is,electron -hole pairs bound by their Cou-lomb attraction.6In most pristine semiconducting polymers cast from solution,these excitons can diffuse less than 10-20nm before decaying to the ground state.13Subpicosecond photoinduced electron transfer from the polymer to the fullerene with nearly 100%quantum efficiency enables the formation of mobile carriers.9Following exciton dissociation,however,there is evidence that the charges remain weakly bound at the interface before either recombining or separat-ing into free carriers.14-16The weakly bound carriers at the interface are referred to as being in a charge transfer (CT)state.Once formed,the holes are free to drift and diffuse within the polymer domains and the electrons are free to move within the fullerene domains.If the domain isisolatedFIGURE 1.(A)Illustration of BHJ solar cell structure and components from the original 1995publication.Reproduced with permission from ref 9.Copyright 1995American Association for the Advancement of Science.(B)Schematic of the processes that must occur within the BHJ active layer for effective charge collection.Reproduced with permission from ref 5.Copyright 2008Wiley-VCH Verlag GmbH &Co.KGaA.“Plastic”Solar Cells Peet et al.Vol.42,No.11November 20091700-1708ACCOUNTS OF CHEMICAL RESEARCH1701from the appropriate electrode or the carrier is too far from the electrode,it will eventually recombine at a polymer/fullerene interface.Thus,for the mobile carriers to contribute to the pho-tocurrent,the domain in which they form must be in contact with the appropriate electrode.Because of this necessity for fully bicontinuous interpenetrating networks comprising the donor and acceptor materials and the weak intermolecular forces between the molecular partners,these devices are acutely sensitive to processing conditions.10A schematic of the device operation is illustrated in Figure 1B.4Solar power conversion efficiency (PCE)is dictated by the product of three electrical parameters:the device short cir-cuit current (I sc ),the open circuit voltage (V oc ),and the fill fac-tor (FF).The I sc ,or the device photocurrent at zero bias,is the product of the number of photons absorbed and the efficiency of free charge carrier generation and collection.The FF is,in part,a function of the efficiency of charge migration to the electrodes.The V oc of the BHJ cell relates to the difference between the highest occupied molecular orbital (HOMO)of the electron donor and the lowest unoccupied molecular orbital (LUMO)of the electron acceptor.17Note that the V oc is usu-ally reduced from the value predicted by subtracting the elec-tron donor HOMO from the electron acceptor LUMO by approximately 0.3V,presumably because of the energies associated with the molecular distortions involved in carrier formation.18,19As presented in Figure 2,it is possible to calculate the the-oretical efficiency for an electron donor blended with PC 61BM based on the donor absorption and LUMO level and reason-able values for the device external quantum efficiency (EQE)and fill factor (65%each).19The plot indicates that single layer devices in excess of 10%should be possible,given the cor-rect molecular design and processing optimization.19More-over,it has been demonstrated that solution-processable tandem cells are possible and can potentially enable an addi-tional 50%increase in efficiency by harvesting a broader frac-tion of the solar spectrum and by taking advantage of the increased voltage available for wide band gap materials.20,21Characterization of BHJ Solar CellsPoly(3-hexylthiophene)(P3HT)is the most studied polymer in BHJ solar cell applications.7Important qualities include good solubility in a variety of organic solvents,a high field effect hole mobility (>10-2cm 2/(V s))and a tendency to crystallize into ordered domains.22The kinetics of the BHJ ordering are such that the degree of crystallization and polymer/fullerene phase separation can be tailored by altering the film casting conditions.The film morphology can then be further opti-mized through thermal annealing,which leads to better order within the P3HT and demixing of the blend.23,24Other tech-niques,such as slow drying,solvent annealing,the use of pro-cessing additives,and aggregation in solution have also led to increased performance by allowing a degree of control over the polymer packing and polymer/fullerene phase separation during the film formation process.25-27The relative ease with which P3HT/fullerene films can be optimized has resulted in a significant body of work on the relationships between pro-cessing,morphology,and performance.The degree of phase separation is a critical parameter since large domains will prevent efficient charge separation and small domains will lead to a poorly connected network result-ing in increased charge carrier recombination.Directly prob-ing the phase separation between the polymer and the fullerene is difficult for highly optimized BHJ solar cell sys-tems,however,because the domain sizes are on the order of tens of nanometers.28Furthermore,most studies attempting to image the phase separation concentrate on the surface of the film and investigations by X-ray photoelectron spectros-copy (XPS)and ellipsometry indicate vertical concentration gradients.29,30Recent studies have utilized a focused ion beam to “cut”cross sections through the BHJ film for characterization via atomic force microscopy (AFM)and transmission electron microscopy (TEM).31,32Figure 3contains a cross-sectional TEM image of a P3HT/PC 61BM blend film and a contrast-enhanced image of the BHJ morphology.32Both cross-sectional AFM and TEM capture the continuous pathways of donor and acceptor domains.By control of the polymer/fullerene phase separation,charge transport through the BHJ films can be optimized.OneFIGURE 2.Contour plot containing the theoretical solar cell PCE (contour lines)versus the donor polymer band gap and LUMO level for a BHJ device using PC 61BM.Reproduced with permission from ref 19.Copyright 2006Wiley-VCH Verlag GmbH &Co.KGaA.“Plastic”Solar Cells Peet et al.1702ACCOUNTS OF CHEMICAL RESEARCH1700-1708November 2009Vol.42,No.11of the most common charge transport characterization meth-ods is the measurement of field effect mobility from thin film transistor (TFT)transconductance characteristics.33Recent work using bottom contact TFTs with aluminum electrodes indicates that BHJ solar cell active layers can be used to create ambi-polar TFTs.34Fabrication of ambipolar transistors with vari-ous polymer/fullerene ratios to equalize the electron and hole mobilities yields a component ratio which correlates with the optimum ratio for solar cell device performance.35It is logi-cal that equalizing charge transport though the BHJ film would increase performance by reducing space charge build up,but only recently has this been clearly demonstrated.35The use of ambipolar field effect transistors (FETs)to measure carrier mobilities in BHJ blends with different polymer/fullerene ratios can thus be used to compare different BHJ systems and to optimize the performance of new polymer/fullerene combi-nations.Additional details on carrier generation and recombina-tion in BHJ films can be explored by transient photoinduced absorption spectroscopy (TA).TA is a pump -probe technique where an ultrafast laser pulse is incident on the film and the photoinduced change in optical absorption is measured at var-ious wavelengths as a function of time.Since different excited states within the film (excitons,mobile carriers after charge separation,etc.)have specific absorption profiles,their rela-tive populations can be probed as a function of time follow-ing photoexcitation.There has been uncertainty in the literature as to why solar cell PCEs have been highly variable for materials expected to yield high-performance devices based on optical absorption,mobility measurements,and component energy levels.Orig-inally,it was thought that ultrafast exciton dissociation imme-diately yielded a free pair of mobile carriers.36TA data indicate that,for a variety of polymers,a charge transfer (CT)state or bound radical pair is formed at the interface and a sig-nificant percentage of dissociated excitons recombine from the CT state in BHJ films rather than by subsequent mobile car-rier recombination.14-16Analysis of transient absorption data from P3HT/PC 61BM BHJ films indicates that thermal annealing not only improves the carrier lifetime and mobility through improved morphol-ogy of the interpenetrating network but also increases the fraction of mobile carriers that emerge from the CT state.14,37One possible explanation for this phenomenon is that the higher lying HOMO level in ordered polymer domains rela-tive to the disordered polymer/fullerene interface serves as a driving force for hole migration away from the interface.14Several other mechanisms for the decrease in geminate recombination with increased molecular order are possible;one alternate explanation is that ordered polymer chains con-tain fewer kinks and thus intersystem crossing from the CT state to a low-energy triplet state would be less probable.38,39Combining the time-resolved evolution of excitations using TA with mobility measurements,morphology data,optical char-acterization,and device performance analysis enables the emergence of fundamental structure/property relationships in new materials systems.Optimizing the Performance of NewMaterials:The Example of a Low Band Gap PolymerDespite the dominance of P3HT in the literature,new mate-rials are needed to achieve the required improvements in effi-ciency.P3HT lacks the broad absorption profile to collect a large fraction of the solar spectrum;moreover,the high lying LUMO of P3HT is more than 500mV higher than it needs to be for electron transfer to the fullerene.19An example of a new material that has recently been introduced as a candi-date for higher performance solar cells is poly[(4,4-bis(2-eth-ylhexyl)-cyclopenta-[2,1-b ;3,4-b ′]dithiophene)-2,6-diyl-alt -2,1,3-benzothiadiazole-4,7-diyl],PCPDTBT (structure shown in Figure 4A).40PCPDTBT is a “push -pull”copolymer,which uses alternating electron-withdrawing and electron-donat-ing components to increase the double bond character between the units and thereby stabilize the quinoidal form of the polymer and decrease the band gap.3,41This class of polymers is also susceptible to “tuning”of the HOMO and LUMO values since the HOMO value is largely derived from the electron-donating monomer and the LUMO is largely derived from the electron-withdrawing monomer.3,40It should be noted that while the donor and acceptor mono-mers predominantly affect the HOMO and LUMO,respec-tively,mixing of the orbitals as well as chain planarityandFIGURE 3.Cross-sectional TEM of a BHJ solar cell with a contrast-enhanced image to highlight the continuous domains of P3HT and PC 61BM.Reproduced with permission from ref 32.Copyright 2009American Chemical Society.“Plastic”Solar Cells Peet et al.Vol.42,No.11November 20091700-1708ACCOUNTS OF CHEMICAL RESEARCH1703packing can alter the frontier orbitals from what might be predicted based on the monomers alone.PCPDTBT has approximately the same HOMO energy value as P3HT,and thus the same open circuit voltage is observed in BHJ solar cells.Because of the lower lying LUMO,PCPDTBT harvests light and generates photocurrent between 300and 900nm (compared with 300to 650nm for P3HT).42While still not the ideal LUMO based on the theoretical value required for charge separation,PCPDTBT has a significantly greater potential for high-efficiency solar cells than does P3HT.42Measurements of the hole mobility for PCPDTBT indicate that it has a surprisingly high mobility (>10-3cm 2/(V s))given that diffraction measurements indicate a poorly ordered morphology.42,43Initial device results indicated that the I sc obtained from the cells was low,implying poor collection of photogenerated carriers.Despite being a seemingly “better”material for BHJ solar cells,the highest performance cells that could be fabricated exhibited power conversion efficiencies below 4%compared with 5%for P3HT.This limit persisted despite attempts toward optimization via molecular weight,polymer/fullerene ratio,casting solvent,thermal annealing,and device architecture.In response to the need to optimize the PCE of PCPDTBT devices,the use of processing additives was explored.43This approach was suggested by the significant improvements in the photoconductivity of P3HT-based BHJ blends processed with such additives.44Since the use of mixed solvents offers a fundamentally different route to polymer ordering and mor-phology control,the possibility existed that the use of pro-cessing additives might work with PCPDTBT where thermal annealing had failed.The increased versatility of the addi-tives proved effective at optimizing the chain packing and phase separation in PCPDTBT solar cells.43The role of the processing additives was investigated by monitoring the UV and visible absorption spectra of nascent films as the solvents evaporated;because the aromatic organic solvents and the additives have absorption in the UV,the evaporation of each can be monitored with time.45,46This experiment indicated that the additives function by slowing the evaporation of solvent from the nascent film and,perhaps more importantly,by changing the solvent quality during film formation from being dominated by the primary solvent (which must be a good solvent for both the polymer and the fullerene),to being dominated by the additive (which should be a relatively poor solvent for the polymer and a good sol-vent for the fullerene).46,47This shift in solvent quality can be used to optimize the order within the polymer domains and the degree of polymer/fullerene phase separation.46,47More critically,unlike other forms of processing optimization,addi-tives can serve to form unique polymer structures and con-formations that are not formed when only a good solvent is present,as has been shown to be the case for both PCPDTBT and poly(9,9-dioctylfluorene).45,46Additionally,despite their extremely low vapor pressures,no detectable additive remains in the film after drying under vacuum.35,43The effect of using processing additives on BHJ films of PCPDTBT with PC 71BM is immediately apparent via a red shift in the absorption maximum from 760to 800nm,as pre-sented in Figure 4A.43The additive used for processing the PCPDTBT solar cells was octane dithiol;this additive is a suf-ficiently good solvent for the fullerene and a poor solvent for the polymer that BHJ films dipped in additive arecompletelyFIGURE 4.(A)Optical absorption spectra for PCPDTBT/PC 71BM blends cast from pristine chlorobenzene (black)and cast from chlorobenzene containing 2%by volume of various alkane dithiols (colored).The PCPDTBT structure is shown inset where the R represents an ethylhexyl group.(B)EQE specra of solar cells composed of P3HT/PC 61BM before (dotted red line)and after (solid red line)thermal annealing andPCPDTBT/PC 71BM cast from chlorobenzene (dotted green line)and cast from chlorobenzene containing octanedithiol (solid green line).The AM 1.5G solar spectrum is shown in black for reference.Reproduced with permission from ref 43.Copyright 2007Nature Publishing Group.“Plastic”Solar Cells Peet et al.1704ACCOUNTS OF CHEMICAL RESEARCH1700-1708November 2009Vol.42,No.11depleted of fullerene and only a porous PCPDTBT film remains.47The solubility of the fullerene in the additive is crit-ical to prevent macroscale crystallization of the fullerene.46,47When devices were fabricated in which the 800nm absorp-tion peak was maximized,the solar cell current output nearly doubled.The EQE vs wavelength curve,shown in Figure 4B compared with a P3HT/PC 61BM solar cell and the solar spec-trum,indicates that the current output of the device improves across all wavelengths,not only in the region near the absorp-tion edge where the spectral features are better-defined and increased in magnitude by the additive processing.This increase in EQE implies an increase in the ability of the cell to generate and collect carriers independent of where the absorption event takes place.New questions arose when the effects of the additives on PCPDTBT were investigated.Measurements of the carrier mobility of the BHJ films indicated that,despite the increase in the 800nm absorption peak and improvements in the solar cell performance,the hole mobility did not increase.43Prelim-inary diffraction experiments also indicated that the film was amorphous when processed with or without additives.These results represent a departure from the results observed with P3HT,where additive processing increases long-range order within the polymer domains and increases carrier mobility.44Investigations into the effects of additive processing on the morphology of PCPDTBT BHJ blends by cross-sectional AFM indicate that the use of additives increases the scale of the phase separation as well as altering the polymer supramo-lecular structure.47,48Some increases were observed in the electron mobility in bipolar FETs leading to slightly more bal-anced charge transport in films cast using the processing addi-tives,but the changes were not sufficient to explain the increase in collected charge carriers.35,49Detailed analysis of the transient absorption profile pro-vided additional insight into the carrier formation and recom-bination dynamics in the PCPDTBT-based BHJ films.50The TA decay profiles indicated a significant decrease in carrier losses from the CT state for films processed with additives.50,51Despite little observable long-range order and no change in hole mobility,films that contained the 800nm peak much more efficiently generated mobile carriers from the CT state.Studies into the nature of the 800nm peak revealed that it can only be formed when the number-average molecular weight of the polymer is greater than approximately 20000.46If low molecular weight material is used in solar cells processed with additives,the effect of the additives on both absorption and device performance is negligible.Thus,the nanostructure associated with the 800nm peak is neces-sary for achieving high performance.This fact explains both why the additive is necessary for high performance to be achieved and why high molecular weights are used in all high-performance PCPDTBT devices,but does not explain in what way the change in absorption is connected with the increase in performance.It remains unclear to what extent the absorption at 800nm for PCPDTBT arises from a traditional “aggregate”,with excitons delocalized across multiple poly-mer chains,or an effect similar to that observed in the -phase of poly(9,9-dioctylfluorene),in which chain planarization in a poor solvent leads to increased intrachain exciton delocaliza-tion.52The effect of additives in decreasing geminate recom-bination in BHJ films has been observed by multiple groups and additional work is needed to elucidate the nature of the PCPDTBT absorption band at 800nm.51,53New Materials SystemsWhile the smaller band gap of PCPDTBT yields better light har-vesting relative to P3HT,there is opportunity for significant improvement in PCE by increasing V oc and EQE.19Toward this goal,new materials systems are being designed to optimize the polymer and fullerene energy levels in order to increase device photovoltage.One new class of donor materials has been recently introduced that replaces the fused bithiophene in PCPDTBT with a carbazole derivative resulting the polymer poly[N -9′-heptadecanyl-2,7-carbazole-alt -5,5′-(4′,7′-di-2-thie-nyl-2′,1′,3′-benzothiadiazole],PCDTBT (structure shown in Fig-ure 5A).54-56The low-lying HOMO of the carbazole results in a 50%increase in operating voltage compared with P3HT or PCPDTBT.54-56The combination of the larger V oc and a high quantum efficiency results in 6%PCE under AM1.5irradia-tion even though the band gap is approximately the same as P3HT.57It is important in this context that carbazole-based copolymers from this class can be synthesized with signifi-cantly lower band gaps through the use of stronger acceptor units in the push -pull copolymer.Another strategy for increasing photovoltage and thus the device performance is modification of the fullerene.9,58While only small changes to the fullerene orbital energy levels can be realized via chemical modification of the fullerene,ithasFIGURE 5.(A)Molecular structure of PCDTBT where R is an n -octyl chain.(B)Structure of a trimetallic nitride endohedral fullerene recently used to increase the V oc of a P3HT-based BHJ solar cell to more than 800mV.“Plastic”Solar Cells Peet et al.Vol.42,No.11November 20091700-1708ACCOUNTS OF CHEMICAL RESEARCH1705been shown that the use of trimetallic nitride endohedral fullerenes can enable significant offsets in the fullerene LUMO levels.59One such material,using trilutetium nitride incarcer-ated in an80carbon fulleroid(structure shown in Figure5B), has been modified with a phenyl butyric acid hexyl ester solu-bilizing group.When this material is blended with P3HT,one observes an increase in the V oc of nearly300mV with no sig-nificant loss in I sc.The potential of these fullerenes to enable performance increases across multiple established polymer systems is promising and future studies will determine the extent to which they can be effectively combined with low band gap materials.Summary and ConclusionThe synthesis and optimization of new materials for BHJ solar cells will lead to significantly higher performance levels and the discovery of materials that maximize open circuit volt-ages will lead to increasingly efficient tandem solar cell devices,which can potentially enable power conversion effi-ciencies in excess of15%.60It has also been shown that results from spin-casting can be readily transferred to more scalablefilm casting methods at least in the case of P3HT/ PC61BM devices.1,10Moreover,by expansion of the selection of high-performance materials,characterization tools,and opti-mization techniques,the probability will increase that materi-als can be discovered that are capable of achieving high performance and long operational lifetimes when cast via large area continuous coating methods.Since thefirst report of solution-processed photovoltaics based on blends of donor and acceptor molecules,significant progress has been made in the fundamental photophysics that underlies the devices,in the chemical synthesis of the com-ponents,in thefilm processing,and in the characterization tools that are necessary for the optimization of increasingly high-performance devices.Because the details of the materi-als synthesis and purification can so significantly affect the car-rier dynamics and device performance,as can the details of thefilm processing and device fabrication,it is essential that all researchers involved in the fabrication and characteriza-tion of organic solar cells effectively communicate experimen-tal details if the fundamental processing/structure/property relationships for these devices are to be elucidated and used to improve devices.A clear path exists to greater than10% PCE for devices that may be fabricated at a fraction of the cost of conventional solar cells and the synthetic,photophysical, andfilm casting tools are in place to achieve that goal.BIOGRAPHICAL INFORMATIONJeffrey Peet is a doctoral candidate in Materials at the Univer-sity of California at Santa Barbara(UCSB).He was raised in Chapel Hill,North Carolina,and received B.S.degrees in Materials Engi-neering and Textile Chemistry from North Carolina State Univer-sity in2004.He received a National Defense Science and Engineering Graduate Fellowship in2005and a Materials Research Society Graduate Student Award in2008for his work on enhancing performance in polymer solar cells using solvent additives.His current research interests include understanding the dynamics of active layer formation in solution-processed organic electronic devices and the fabrication of high-performance poly-mer photovoltaics.Alan J.Heeger serves as a Professor of Physics and Professor of Materials at the University of California,Santa Barbara,and also heads a research group at the University’s Center for Polymers and Organic Solids.He was awarded the Nobel Prize in Chemis-try(2000)for his pioneering research in and the cofounding of thefield of semiconducting and metallic polymers.His research efforts continue to focus on the science and technology of semi-conducting and metallic polymers with emphasis on“plastic”solar cells.Heeger cofounded(with Howard Berke)Konarka Techonolo-gies in2001;he continues to serve as Chief Scientist.Konarka is commercializing low-cost plastic solar cells fabricated from bulk heterojunction materials fabricated via roll-to-roll manufactur-ing.Other current interests include studies of biospecific sensors for DNA and proteins.Guillermo C.Bazan is a professor in the Departments of Mate-rials and Chemistry&Biochemistry and is Co-Director of the Cen-ter for Polymers and Organic Solids at the University of California, Santa Barbara.He was born in Mendoza,Argentina,and was raised in Argentina,Belgium,and Canada.He obtained his B.Sc. in1986(Summa Cum Laude)from the University of Ottawa.His Ph.D.thesis was done with Professor Richard R.Schrock at MIT. After a postdoctoral appointment at Caltech with Professor John Bercaw,he began his independent career in1992at the Univer-sity of Rochester.He moved to UCSB in1998.His research inter-ests concern the design,synthesis,photophysics,bulk properties, and applications of organic molecules with delocalized electronic structures and the design of homogeneous transition-metal cat-alysts for the controlled polymerization of olefins. FOOTNOTES*To whom correspondence should be addressed.E-mail addresses:bazan@ ;ajhe1@.REFERENCES1Brabec,C.J.;Durrant,J.R.Solution-processed organic solar cells.MRS Bull.2008, 33,670–675.2Dennler,G.;Scharber,M.C.;Brabec,C.J.Polymer-fullerene bulk-heterojunction solar cells.Adv.Mater.2009,21,1323–1338.3Kroon,R.;Lenes,M.;Hummelen,J.C.;Blom,P.W.M.;de Boer,B.Small Bandgap Polymers for Organic Solar Cells.Polym.Rev.2008,48,531–582.4Bundgaard,E.;Krebs,F.C.Low band gap polymers for organic photovoltaics.Sol.Energy Mater.Sol.Cells2007,91,945–985.5Thompson,B.C.;Frechet,anic photovoltaics-Polymer-fullerene composite solar cells.Angew.Chem.,Int.Ed.2008,47,58–77.“Plastic”Solar Cells Peet et al.1706ACCOUNTS OF CHEMICAL RESEARCH1700-1708November2009Vol.42,No.11。
自由能

右式上面的横线代表对所有非平衡态过程的平均。Jarzynski恒等式 假设初始态为平衡态,终态则不必是。 物理文献中常称其为自由能,并以F表示。也有文献使用A表示
Thank
You!!!
,其中是化学势。一个重要的推论是。也就是说每 个粒子的平均吉布斯自由能等于化学势
吉布斯自由能 在标准状况下,存在一个一般规律:
物理意义
“任何一个封闭系统都尽量使自由能最小”
因此,根据这个自然界的基本趋势,如果对于一个潜在反应,距离 势 这个最小值进行定量测量,当热力学的计算显示吉布斯自由能ΔG的 变化是负值的时候。本质上,这表明了那样一个反应更容易发生并 且将释放能量。释放的能量等于这个化学反应所能够做的最大的功。 相反,如果ΔG为正值,能量必须通过做功的方式进入反应系统使得 此反应能够进行。 吉布斯自由能的物理含义是在等温等压过程中,除体积变化所做的 功以外,从系统所能获得的最大功。换句话说,在等温等压过程中, 除体积变化所做的功以外,系统对外界所做的功只能等于或者小于 吉布斯自由能的减小。数学表示是: 不足
吉布斯自由能 吉布斯自由能(Gibbs free energy),或称吉布斯函数 (Gibbs function)、自由焓(Free Enthalpy)是热力学中描 述等温、等压过程的一个重要参量,常用G表示,它的定义是:
,其中U是系统的内能,T是温度,S是熵,p是压强,V是体积, H是焓。 吉布斯自由能的微分形式是:
Ndg+gdN=SdT+Vdp+μdN
,现在假想保证原来物体属性的情况下,切掉体系的一小部分 dN。这时dT,dp,dg,这些强度量的变化为零。所以必然有
μ=g=G/N
亥姆霍兹自由能
定义
ቤተ መጻሕፍቲ ባይዱ
2022-2023学年福建省福州市鼓楼区格致中学高二(下)期中英语试卷

2022-2023学年福建省福州市鼓楼区格致中学高二(下)期中英语试卷第一部分听力1.What did the speakers do last weekend?A.They went climbing.B.They played football.C.They watched a match.2.When was the meeting scheduled?A.At 6:00.B.At 6:30.C.At 7:00.3.What is the man dissatisfied with about the store?A.The clothes.B.The service.C.The price.4.What does the woman think of the show?A.Boring.B.Interesting.cational.5.What are the speakers talking about?A.The sea.B.The weekend plan.C.The weather.第二节6.(1)What day is it today?A.Saturday.B.Friday.C.Monday.(2)Why is the woman worried?A.She has no time to prepare.B.Nobody can lead the dance group.C.There aren't enough people in the group.7.(1)Where are the speakers?A.In a store.B.In a street.C.In a cafe.(2)What problem does the man have?A.He forgot to send an email.B.He has to make a phone call.C.He left his wallet in the office.(3)What does the woman suggest the man do?A.Speak with her manager.B.Visit another location.C.Eat in the cafe.8.(1)What might the man be?A.A customer service representative.B.A travel agent.C.A repairman.(2)What will the woman do next?A.Call his son.B.Give some details.C.Sign a form.(3)What do we know about the woman?A.Her son lives abroad now.B.She will go to visit her son soon.C.She should make payment from this month.9.(1)What did the man do yesterday?A.He started a new job.B.He registered for a class.C.He did some shopping online.(2)What does the man want to buy?A.Posters.B.Paintings.C.Brushes.(3)What will the woman do next?A.Go to a store.B.Ask about a sale.C.Email the man.10.(1)How much is the ticket to the Ocean Park?A. £ 2.00.B. £ 6.50.C. £ 8.50.(2)What is unavailable during the trip?A.Lunch.B.Water.C.Fruit.(3)What will the listeners do at 2:30 next Tuesday?A.Listen to a talk.B.Do some worksheets.C.Watch the sharks being fed.(4)What is the topic of the talk for next Tuesday?A.The ocean.B.Sharks.C.Penguins.第二部分阅读11.STEMInnovative.Eye-opening.Inspiring.Science,Technology,Engineering,and Math constantly reshape the communities we livein.Our STEM tours challenge students to develop imaginative solutions to global problems through critical thinking and creative expression.Health Sciences in Great BritainPaging the next generation of doctors,nurses,and health professionals - this tour through England and Scotland is for you.Trace the innovations that shaped Britain's modern healthcare landscape,and participate in hands-on workshops to discover how real-life medical careers operate.Robotics,Engineering,and the Future of Cities in JapanHome to countless organizations specializing in robotics and engineering,Japan is at the forefront of innovation in sustainability and human mobility.From high-speed superconducting magnetic (磁悬浮)trains to ASIMO the robot,see how the future of cities is closer than you think in Japan.You can take a guided tour of Tokyo with a STEM expert before visiting other Japanese cities.Agriculture in IrelandGet your hands dirty as you experience the farm-to-table journey - an important part of life in Ireland - by visiting a variety of local farms around the country.Learn about organic and sustainable farming practices,the impact of climate change on traditional food production,and how each agricultural product requires specialized care.Reef (礁)Regeneration and Conservation in AustraliaAs the world's largest living organism,the Great Barrier Reef is the foundation of Australia's coastal ecosystems.And Townsville,which will serve as your base of operations for this tour,is a world-famous center for scientific reef research and conservation.Conduct a field survey with koalas and snorkel (潜水)alongside the Reef.(1)Which best suits those expecting a career in medicine?A.The tour in Great Britain.B.The tour in Japan.C.The tour in Ireland.D.The tour in Australia.(2)What does the tour in Ireland provide?A.Healthcare workshops.B.Robot programing classes.C.Labor experience in the fields.D.A field survey with sea lives.(3)Who is the text aimed at?A.Artists.B.Adventurers.C.Volunteers.D.Students.12.As a young girl growing up in France,Sarah Toumi dreamed of becoming a leader who could make the world a better place.Her passion to help others was awakened when,from the age of nine,she accompanied her Tunisian father to his birthplace in the east of the country during holidays.There she organized homework clubs and activities for children.Toumi witnessed first-hand the destructive effect of desertification."Within 10 years rich farmers became worse off,and in 10 years from now they will be poor.I wanted to stop the Sahara Desert in its tracks."A decrease in average rainfall and an increase in the severity of droughts(干旱)have led to an estimated 75 per cent of Tunisia's agricultural lands being threatened by desertification.Toumi recognized that farming practices needed to change.She is confident that small land areas can bring large returns if farmers are able to adapt by planting sustainable crops,using new technologies for water treatment and focusing on natural products and fertilisers(肥料)rather than chemicals.In 2012,Toumi consolidated her dream to fight the desert.She moved to Tunisia,and set up a programme named Acacias for All to put her sustainable farming philosophy into action."I want to show young people in rural areas that they can create opportunities where they are.Nobody is better able to understand the impact of desertification and climate change than somebody who is living with no access to water."By September 2016,more than 130,000 acacia trees had been planted on 20 pilot farms,with farmers recording a 60 per cent survival rate.Toumi estimates that some 3 million acacia trees are needed to protect Tunisia's farmland.She expects to plant 1 million trees by 2018.In the next couple of years,Toumi hopes to extend the programme to Algeria and Morocco.(1)How did Toumi's holiday trips to Tunisia influence her?A.They made her decide to leave the country.B.They helped her better understand her father.C.They fired her enthusiasm for helping others.D.They destroyed her dream of being a teacher.(2)What is the main cause of the desertification of Tunisia's farmland?A.Low rainfall.B.Soil pollution.C.Cold weather.D.Forest damage.(3)Why did Toumi set up Acacias for All in Tunisia?A.To create job opportunities for young people.B.To help the children obtain a basic education.C.To persuade the farmers not to use fertilizers.D.To facilitate the protection of their farmland.(4)Which of the following can be the best title for the text?A.Saving Water in Tunisia.B.Planting Trees of Native Species.C.Holding back the Sahara.D.Fighting Poverty in North Africa.13.If humans pump enough carbon dioxide (CO2)into the atmosphere,the stratocumulus clouds(层积云)could disappear,and the earth's temperature could climb sharply to heights not predicted in current climate models.It would burn the planet.That's the conclusion of a paper published in the journal Nature Geoscience and described in detail by Natalie Wolchover for Quanta Magazine.As Wolchover explained,clouds have long been one of the great uncertainties of climate models.Computer models that easily capture the complexity and detail of most climate systems just aren't powerful enough to predict worldwide changes in cloud behavior.But clouds are important.They reflect sunlight away from the earth's surface.And stratocumulus clouds are those white blankets you might have seen as you looked out the window of arm airplane,rolling out below you and hiding the ground Researchers suspect that certain sudden,past jumps in temperature may have been caused by changes to clouds like these.For the new research,scientists modeled just a small patch of sky using a supercomputer.They found that if carbon dioxide levels reach about 1,200 parts per million(ppm)in the atmosphere,stratocumulus clouds break up.That's a very high carbon dioxide concentration Right now,levels have climbed past 410 ppm--a dangerous change from 280 ppm before the Industrial Revolution.But humans put more and more CO2 into the atmosphere every year.If current trends continue,the earth could reach 1,200 ppm within 100 to 150 years.This could happen if our society doesn't follow through on any of its commitments to reduce emissions(排放),Wolchover reported.And even if it does,the result would be another 8 degrees Celsius of heat added to the global average,on top of the dangerous changes already underway due to greenhouse gases.That's an enormous change,and it goes beyond predictions of worldwide ice melt and catastrophic sea-level rise,And,once the stratocumulus clouds are gone,Wolchover reported,they likely wouldn't reappear until atmospheric carbon dioxide levels dropped below where they are currently.There's still some uncertainty in the data.The 1,200 ppm figure could change as scientists look into the issue further.(1)What can we learn from paragraph 2?A.Most climate systems are not complex.B.Cloud behavior is uncertain and hard to predictC.Temperature changes affect the stratocumulus cloudsD.The stratocumulus clouds protect planes from sunlight.(2)How did the scientists study clouds in the new research?A.By measuring the sea levelB.By experimenting in a natural stateC.By comparing climate modelsD.By computer modeling and analyzing(3)What does"it"refer to in paragraph 4?A.The atmosphere.B.The earth.C.Our society.D.The result(4)Which of the following statements would Wolchover most probably agree with?A.The effects of CO2 emissions have beer fully assessedB.The stratocumulus clouds won't return if they are goneC.The breakup of stratocumulus clouds could result in catastrophesD.Once CO2 level reaches 1,200 ppm,stratocumulus clouds will go extinct第二节14.Weighing yourself regularly is a wonderful way to stay aware of any significant weight changes.(1)______As for me,weighing myself every day caused me to shift my focus from being generally healthy and physically active,to focusing only on the scale.The original training program is beneficial for me to develop muscle.(2)______ However,thinking only of lowering the number on the scale,I changed my training program.I found that weighing myself daily did not provide an accurate description of the hard work and progress I was making in the gym.It takes about three weeks to a month to notice significant changes in your weight due to changing your training program.The most immediate changes will be observed inskill level,strength and inches lost.(3)______ Instead,I switched to a bimonthly weighing schedule.Weighing every other month allows me to observe and account for any significant weight changes. (4)______ I use my bimonthly weigh-in results to get information about my nutrition as well.If my training intensity remains the same,but I'm constantly hungry and dropping weight,this is a sign that I need to increase my daily caloric intake.(5)______ I'm experiencing increased passion for working out since I no longer carry the burden of a disappointing morning weigh-in.I've also experienced greater success in achieving my specific fitness goals,because I'm training according to those goals,not the numbers on a scale.Rather than obsessing over the scale,turn your focus to how you look,feel,how your clothes fit and your overall energy level.A.That was bad to my overall fitness goals.B.Muscles rely on more than just activity to grow.C.I had gained weight in the form of muscle mass before.D.For these reasons,I stopped weighing myself every day.E.That tells me whether I need to adjust my training program.F.The decision to stop weighing myself every day has done a lot of wonders.G.However,when done too often,this habit can sometimes hurt more than it helps.第三部分语言运用15.One day,Marga asked if I would join her in hiking to Base Camp at the foot of Mount Qomolangma to celebrate her 60th birthday.I (1)______ the opportunity.We met our guide Achut Pandey in Kathmandu.He was a man of joy and (2)______ ,who had been to Base Camp over 100 times.When we left Namche Bazaar,we took the Three High Passes route,which is less-traveled,longer and more (3)______ .The temperatures ranged from the teens to below 0°F.We were nearly (4)______ .When we were done with dinner,we (5)______ ourselves to our sleeping bags.While we (6)______ were able to down a couple of eggs and a piece of toast,the higher we went,the less we (7)______ .Eventually,it was a lot of (8)______ to just eat a bowl of rice.The altitude (9)______ us in other ways too.As our appetites slowed,so did our (10)______ .Renjo La Pass,the first pass,was supposed to take eight hours.It took 12.Achut's faith in us was never (11)______ .He greeted us each day with a (12)______ "good morning." Before we (13)______ Base Camp,he carried our packs and skillfully negotiated our moods and capabilities.We trusted him as he (14)______ us,confident and strong,up and down icy,(15)______ and sometimes narrow paths.Thanks to Achut,I made it,at 65.(1)A.turned down B.jumped at C.looked for D.gave up(2)A.wealth B.honesty C.kindness D.taste(3)A.preferable B.difficult C.crowded D.deserted(4)A.mad B.starved C.desperate D.frozen(5)A.withdrew B.exposed C.helped D.resigned(6)A.gradually B.ultimately C.initially D.obviously(7)A.expected B.rested C.suffered D.ate(8)A.effort B.fun C.fortune D.pressure(9)A.supported B.humbled C.confused D.directed(10) A.growth B.speech C.breathing D.pace(11) A.restored B.tested C.shaken D.rewarded(12) A.cheerful B.unwilling C.calm D.meaningless(13) A.reached B.left C.guarded D.established(14) A.admired B.praised C.guided D.contacted(15) A.straight B.muddy C.smooth D.uneven第二节课本语法填空16.The first act of the Red Cross was to organise an international conference (the Geneva Convention)(1)______ countries adopted guidelines and measures for protecting(2)______ wounded and medical workers during and after battles.In the years since then,the Red Cross (3)______ (be)active in just about every war,helping to care for both soldiers and civilians.While the Red Cross is (4)______ (most)tasked with helping people in times of war,it also responds to natural disasters and gives aid to people who have to leave their homes.All around the world,the Red Cross continues to help civilians(5)______ (catch)in war,providing medical (6)______ (assist),feeding the needy,and offering many other types of aid.(7)______ (help )meet the need for aid,most countries have National Red Cross and Red Crescent Societies.Not only(8)______ these societies provide support to Red Cross efforts in other countries,but they also help prepare their own communities for disasters,provide medical services,and teach first-aid and life-saving techniques.The Florence Cathedral first decided to have the statue (9)______ (make)in 1464 to stand on its roof,along with statues of other(10)______ (hero)from the Bible.(11)______ (consequence),they purchased a block of white marble more than 5 metres (12)______ length.Over the years,two different sculptors attempted to work on the statue.However,both gave up,complaining that the marble was of poor quality.(13)______ (bad)yet,it appeared that the last sculptor had ruined the marble by(14)______ (cut)a huge hole in it for the gap between David's legs.The hole had (15)______ odd shape,and left very little material on either side to work with.Everyone thought it would be impossible to carve the statue's legs properly,and that they would be too thin- -unable to hold the weight of the rest of the statue.第四部分根据句意选择适当的短语,并用正确形式填空(有两个多余项)17.(1)Having said she wasn't hungry,she then ______ order a three-course meal.(2)A red rose,rather than yellow roses,______ the traditional romantic gift given to your love on Valentine's Day.(3)The zoo goes broke,and Pi's father puts his family and a few valuable animals on a ship ______ Canada.(4)His career has been ______ for some years now.(5)Yesterday,few players turned up because most ______.(6)Having learned that I was in trouble,he came to my aid ______.(7)My grandparents usually ______ the atmosphere of peace and calm in the country.(8)It is important to enrich the soil ______ planting.(9)Jiuqu Stream ______ others due to its rich waterfall based on the forest coverage in Wuyi Mountains.(10)We have already formed a strong working party ______ a number of our former presidents.第五部分书面表达18.假定你是李华,你的美国网友Tom来信说他的家人都很喜欢吃中餐,向你请教如何在中餐馆选择健康的中餐。
TiVNbTa难熔高熵合金的吸放氢动力学

第 1 期第 101-107 页材料工程Vol.52Jan. 2024Journal of Materials EngineeringNo.1pp.101-107第 52 卷2024 年 1 月TiVNbTa难熔高熵合金的吸放氢动力学Hydrogen absorption-desorption kinetics ofTiVNbTa refractory high-entropy alloy龙雁1,2,张李敬1,2,杨继荣1,2,王芬1,2*(1 广东省金属新材料制备与成形重点实验室,广州 510640;2 华南理工大学机械与汽车工程学院,广州 510640)LONG Yan1,2,ZHANG Lijing1,2,YANG Jirong1,2,WANG Fen1,2*(1 Guangdong Provincial Key Laboratory for Processing and Forming ofAdvanced Metallic Materials,Guangzhou 510640,China;2 School ofMechanical and Automotive Engineering,South China University ofTechnology,Guangzhou 510640,China)摘要:通过真空电磁感应悬浮熔炼技术制备TiVNbTa难熔高熵合金试样,采用多通道储氢性能测试仪测试合金的吸放氢性能,并研究该合金的吸(放)氢行为及其动力学机制。
结果表明:单相BCC结构的TiVNbTa难熔高熵合金吸氢后生成TiH1.971,Nb0.696V0.304H和Nb0.498V0.502H2 3种氢化物新相。
氢化高熵合金粉末在 519 ,593 K和640 K 分别发生氢化物的分解反应,放氢后恢复单相BCC结构,因此TiVNbTa合金的吸氢反应属于可逆反应。
该合金在423~723 K温度区间具有较高的吸(放)氢速率,其吸(放)氢动力学模型分别符合Johnson-Mehl-Avrami (JMA)方程和二级速率方程,吸(放)氢的表观活化能E a分别为-21.87 J/mol和8.67 J/mol。
开启片剂完整性的窗户(中英文对照)
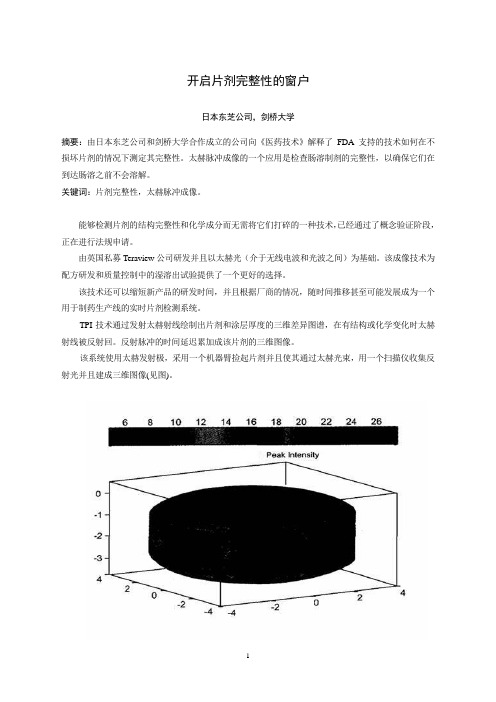
开启片剂完整性的窗户日本东芝公司,剑桥大学摘要:由日本东芝公司和剑桥大学合作成立的公司向《医药技术》解释了FDA支持的技术如何在不损坏片剂的情况下测定其完整性。
太赫脉冲成像的一个应用是检查肠溶制剂的完整性,以确保它们在到达肠溶之前不会溶解。
关键词:片剂完整性,太赫脉冲成像。
能够检测片剂的结构完整性和化学成分而无需将它们打碎的一种技术,已经通过了概念验证阶段,正在进行法规申请。
由英国私募Teraview公司研发并且以太赫光(介于无线电波和光波之间)为基础。
该成像技术为配方研发和质量控制中的湿溶出试验提供了一个更好的选择。
该技术还可以缩短新产品的研发时间,并且根据厂商的情况,随时间推移甚至可能发展成为一个用于制药生产线的实时片剂检测系统。
TPI技术通过发射太赫射线绘制出片剂和涂层厚度的三维差异图谱,在有结构或化学变化时太赫射线被反射回。
反射脉冲的时间延迟累加成该片剂的三维图像。
该系统使用太赫发射极,采用一个机器臂捡起片剂并且使其通过太赫光束,用一个扫描仪收集反射光并且建成三维图像(见图)。
技术研发太赫技术发源于二十世纪九十年代中期13本东芝公司位于英国的东芝欧洲研究中心,该中心与剑桥大学的物理学系有着密切的联系。
日本东芝公司当时正在研究新一代的半导体,研究的副产品是发现了这些半导体实际上是太赫光非常好的发射源和检测器。
二十世纪九十年代后期,日本东芝公司授权研究小组寻求该技术可能的应用,包括成像和化学传感光谱学,并与葛兰素史克和辉瑞以及其它公司建立了关系,以探讨其在制药业的应用。
虽然早期的结果表明该技术有前景,但日本东芝公司却不愿深入研究下去,原因是此应用与日本东芝公司在消费电子行业的任何业务兴趣都没有交叉。
这一决定的结果是研究中心的首席执行官DonArnone和剑桥桥大学物理学系的教授Michael Pepper先生于2001年成立了Teraview公司一作为研究中心的子公司。
TPI imaga 2000是第一个商品化太赫成像系统,该系统经优化用于成品片剂及其核心完整性和性能的无破坏检测。
自由能

自由能free energy其他名称:亥姆霍兹函数(Helmholtz function)定义1:热力系工质的一种状态参数,等于内能减去绝对温度与熵之积。
应用学科:电力(一级学科);通论(二级学科)定义2:在恒温、恒压条件下,体系中可用于做功的能量。
符号为“G”。
应用学科:细胞生物学(一级学科);细胞生理(二级学科)以上内容由全国科学技术名词审定委员会审定公布求助编辑百科名片自由能free energy :在热力学当中,自由能指的是在某一个热力学过程中,系统减少的内能中可以转化为对外作功的部分,它衡量的是:在一个特定的热力学过程中,系统可对外输出的“有用能量”。
可分为亥姆霍兹自由能和吉布斯自由能目录生物反应中的自由能自由能的分类自由能做功化学反应中的自由能生物反应中的自由能呼吸链中电子对传递时自由能的变化自由能free energy 在物理化学中,按照亥姆霍兹的定容自由能F与吉布斯的定压自由能G的定义,G=A+PV (p为压力,V为体积)。
在生物的反应中,因为△(PV)可以忽略不计,所以两者是相同的。
只有这样,A的变化△A=△U-T△S 才成为主要讨论的问题(U、T、S分别是该系统的内能、绝对温度、熵)。
△A给出了生物反应中释放出来可用于做功的能量上限。
其变化量(一般用△G*表示)在生物学上使用时必须注意下列事项:(1)水的活度,可随意设为1.0进行计算:(2)因[H+]=1M并不符合实际情况,一般认为[H+]=10-7M(pH7),为了区别其符号写成△G0′;(3)例如反应,因各种成分并非标准浓度(1M),把实际浓度代入下式后其值△G′就有问题了;(4)在共轭反应中,要注意各种成分反应的变化量之和;(5)把△G0改为用平衡常数[1](Keq)表示,往往是很有用的。
例如在25℃下△G0=-RT1nKeq=-1363log10Keq自由能的分类亥姆霍兹自由能设体系从温度为T环的热源吸取热量δQ,根据第二定律的基本公亥姆霍兹dS - δQ/T环≥0;代入第一定律的公式δQ=dU十δW,得δW≤-(dU-T环ds)若体系的最初与最后温度和环境的温度相等,即T1=T2=T环,则δW≤- d (U-Ts) (.2.27)令F===U-TS (中间横线上为def) (2.28) ,F称为亥姆霍兹自由能(Helmholz free energy),亦称亥姆霍兹函数,又称为功函(work function),它显然是体系的状态函数。
初论自由能源(五)磁能,永动,能量,发明物理

初论自由能源(五)惯性推进和电力生成系统,称之为“可持续传输系统”的装置。
该装置基于他们的惯性推进生成轮,用的是他们称为“3维Coraxial 复合感应”(CHI,简称纪氏感应)的技术。
这里“Coraxial”指的是他们的“径-轴复合技术”,既径向磁体脉冲系统提供驱动,而使用轴向磁体/线圈系统来实现能源提取。
允许参观者骑2千瓦的试验车。
以1牛顿米在每分500转上的旋转输入,一个单独的惯性推进产生轮可以产生1千瓦的轴向原动力。
紀大任还说他的电机的150瓦输出实际上产生的机械输出在180到200瓦之间。
当用瓦特计、速度计、扭矩计和示波器测量时,而一个1500瓦输出产生1800瓦到2000瓦之间的机械输出(96伏,20安培)。
当引擎运行并提供它的输出时,陶基和碳基超级电容器用于返回一些输出功率给输入,以保持机器持续运行而无需能量供应。
当用这个装置驱动一辆汽车时,通过无级变速器提供机械驱动力矩。
此时,它表示“径向轴向相加”复合感应,简称“CHI”。
他们也称这为“3维径轴复合感应”系统。
他们陈述电磁铁的3维安排,以一个单一的转子和两个分离的定子,使得轮子既有径向磁通推进,也有轴向磁通产生。
当径向电传动旋转时,机械能由于轮子的惯性和地面动力传输机构使车辆的轮子在旋转时发电。
另一个应用是,装置的电输出以水下等离子弧从水中产生氢氧混合气体:该电机/发电机不寻常之处在于,使用了脉冲转子驱动在转子圆周上安装的磁体,而同时从安装在转子侧面的线圈/磁体上拾取电能,如图所示:基本的驱动器/发电机组可以被复制在一个轴上,以生成更大的能量,仍然没有增加驱动器和发电机之间的摩擦损失:很少看到这种技术使用,因为它可能很难避免不同磁场之间的相互作用。
然而,紀大任完全成功地一直在这样做着,而且这种配置成为系统的整体组成部分时,在驱动电机和发电机之间没有输电损耗。
该系统具有可驱动自供电式空调机组的能力,而一台5千瓦的概念证明原型机如下图所示:此设备也能驱动电力照明,而以5千瓦输出的自供电输出,可以为大多数家庭提供需要的电力。
First-principles study of the structural, vibrational, phonon and thermodynamic

1. Introduction Ultra-high temperature ceramics (UHTCs) with melting temperatures in excess of 3000 K are usually composed by the refractory borides, carbides and nitrides of early transition metals [1–7]. Among the UHTCs, transition metal carbides (TMC) such as TiC, ZrC and HfC are metallic compounds with unique physical and chemical properties including an extremely high melting point and hardness, chemical stability, corrosion resistance combined with metallic electrical and thermal conductivities [5–10]. These features give transition metal carbides the capability to withstand high temperatures in oxidizing environments, making them candidates for applications in the atmosphere of extreme thermal and chemical environments [6,7]. The structural, vibrational, phonon and thermodynamic properties of IVb group transition metal carbides have been investigated experimentally [10–17] and theoretically [13,18–28] in the earlier reports. In the 1970s, the phonon dispersion relations of TiC, ZrC and HfC were measured using inelastic neutron scattering by Pintschovius et al. [10] and Smith et al. [15–17]. Lattice dynamics calculation and the phonon dispersion relations of transition metal carbides such as ZrC and HfC were reported using a phenomenological ‘‘double-shell’’ model theory [18] where long-range interatomic interactions were taken into account in order to get a
Entropy and Free Energy

ENTROPY, S.
Copyright (c) 1999 by Harcourt Brace & Company All rights reserved
Reaction of K with water
Product-Favored Reactions
In general, productfavored reactions are exothermic.
Fe2O3(s) + 2 Al(s) ---> 2 Fe(s) + Al2O3(s)
DH = - 848 kJ
Copyright (c) 1999 by Harcourt Brace & Company All rights reserved
Paper burns — a product-favored reaction. Also kinetically favored once reaction is begun.
Copyright (c) 1999 by Harcourt Brace & Company All rights reserved
Thermodynamics
• Is the state of a chemical system such that a rearrangement of its atoms and molecules would decrease the energy of the system?
• If yes, system is favored to react — a
Copyright (c) 1999 by Harcourt Brace & Company All rights reserved
2060碳达峰碳中和

2060碳达峰碳中和英文回答:Achieving Carbon Peak by 2060 and Carbon Neutrality: China's Path to Net-Zero Emissions.In 2020, China made a historic commitment to reach carbon neutrality by 2060 and peak carbon emissions by 2030. This ambitious goal underscores China's recognition of the urgency of climate change and its determination totransition to a low-carbon, sustainable economy.Achieving carbon peak and carbon neutrality requires a comprehensive and multifaceted strategy that encompassesall sectors of the economy. Key elements of China's approach include:Energy Transformation: Shifting away from coal-fired power plants to renewable energy sources, such as solar, wind, and nuclear.Industrial Innovation: Promoting energy-efficient technologies, circular economy principles, and low-carbon production processes in industries such as steel, cement, and chemicals.Transportation Electrification: Accelerating the adoption of electric vehicles, improving public transportation, and developing smart city transportation systems.Carbon Capture and Storage (CCS): Developing and deploying CCS technologies to capture and store carbon dioxide emissions from industrial and power plants.Forestry and Land Management: Protecting and restoring forests, which serve as carbon sinks, and promoting sustainable land-use practices.China is already making significant progress towards achieving its carbon goals. In 2023, the share of non-fossil energy in total energy consumption reached 18%, andrenewable energy capacity exceeded 1.2 billion kilowatts. The government is also implementing policies to reduce energy intensity, promote energy efficiency, and control carbon emissions.The path to carbon peak and carbon neutrality is not without challenges. China's heavy reliance on coal, itsvast industrial sector, and its rapidly growing population all present significant obstacles. However, China is committed to overcoming these challenges and has demonstrated a strong track record of ambitious policy implementation.Achieving carbon peak and carbon neutrality will bring numerous benefits to China, including:Improved Air and Water Quality: Reducing carbon emissions will significantly reduce air and water pollution, leading to improved public health and environmental quality.Enhanced Energy Security: Transitioning to renewable energy sources will reduce China's dependence on importedfuels and enhance its energy security.Economic Competitiveness: Investing in low-carbon technologies will create new industries, stimulate economic growth, and enhance China's global competitiveness.Climate Change Mitigation: China's carbon peak and carbon neutrality targets will play a vital role in mitigating global greenhouse gas emissions and averting the worst impacts of climate change.中文回答:2060年碳达峰碳中和目标,中国实现净零排放的道路。
Entropy and Free Energy - Senay's Classes熵与自由能- Senay类

Randomness decreases
Entropy decreases (DS < 0)
(b) Forming sucrose crystals from a supersaturated solution
• If there is no net change in the total number of gas molecules, then DS0 may be positive or negative BUT DS0 will be a small number.
What is the sign of the entropy change for the following
nonspontaneous
7
• All these are spontaneous processes.
A spontaneous process, Has a definite direction in which it occurs Occurs without any ongoing outside intervention.
Ssolid < Sliquid << Sgas
H2O (s)
H2O (l)
DS > 0
19
Guidelines for Determining if DS is + or -.
S (gases) > S (liquids) > S (solids)
So (J/K•mol) H2O(liq) 69.91 H2O(gas) 188.8
10
Enthalpy is a factor in whether a reaction is
spontaneous; but is not the only factor.
Entropy and Free Energy熵与自由能

Higher T means : • more randomness • larger S
11
Entropy of Ionic Substances
• Ionic Solids : Entropy depends on extent of motion of ions. This depends on the strength of coulombic attraction.
3. The entropy (S) of a pure, perfectly crystalline compound at T = 0 K is ZERO. (no disorder) ST=0 = 0 (perfect xll)
18
2nd Law of Thermodynamics
A reaction is spontaneous (productfavored) if S for the universe is positive.
19
Calculating S for a Reaction
So = So (products) - So (reactants)
Consider 2 H2(g) + O2(g) ---> 2 H2O(l) So = 2 So (H2O) - [2 So (H2) + So (O2)] So = 2 mol (69.9 J/K•mol) -
6
Directionality of Reactions
How probable is it that reactant molecules will react?
PROBABILITY suggests that a product-favored reaction will result in the dispersal of energy
Entropy and Free Energy - Senay's Classes熵与自由能- Se

N2 (g) + 3 H2 (g) -> 2 NH3 (g)
4 moles gas
2 moles gas
- DS
How does the entropy of a system 30
change for each of the following
processes?
(a) Condensing water vapor
15
Entropy, S
❖The disorder is expressed by a thermodynamic quantity called ENTROPY (S) (however, entropy is not “disorder”).
❖The more disordered or random a system, the larger its entropy.
Enthalpy is a factor in whether a reaction is
spontaneous; but is not the only factor.
Spontaneous reactions
H2O (s)
H2O (l) DH0 = +6.01 kJ
NH4NO3 (s) H2O NH4+(aq) + NO3- (aq) DH0 = +25 kJ
reaction? 2Zn (s) + O2 (g)
2ZnO (s)
The total number of gas molecules goes down, DS is
negative.
18.3
Guidelines for Determining if DS is + or -.29
Entropy and Free Energy - Chemistry - Louisiana Te

What is 1st Law of Thermodynamics
Eenergy is conserved in the Universe
All forms of energy are inter-convertible and conserved
Energy is neither created nor destroyed.
Other reactions:
SO2(g) ------> S(s) + O2(g) ; DH = 297kJ H2SO4(l)------> H2(g) + S(s) + 2O2(g); DH = H2(g) +1/2O2(g) -----> H2O(g); DH = -242 kJ
814 kJ
= [-2754.5 - 2287.2] - [-198.8] = -5041.7 + 198.8 = -4842.9 kJ = -4843 kJ
Why is DHof of elements is zero?
DHof, Heat formations are for compounds Note: DHof of elements is zero
What is Hess's Law of Summation of Heat?
To heat of reaction for new reactions.
Two methods?
1st method: new DH is calculated by adding DHs of other reactions.
Thermodynamics
Will the rearrangement of a system decrease its energy?
Entropy and Free Energy熵与自由能-70页精品文档
Non-exothermic spontaneous reactions But many spontaneous reactions or
processes are endothermic . . . NH4NO3(s) + heat NH4+ (aq) + NO3- (aq)
Hsol = +25.7 kJ/mol or have H = 0 . . .Leabharlann 188.8 69.9 47.9
S (gases) >> S (liquids) > S (solids)
10
Entropy and Temperature
The entropy of a substance increases with temperature.
Molecular motions different temps.
3. The entropy (S) of a pure, perfectly crystalline compound at T = 0 K is ZERO. (no disorder) ST=0 = 0 (perfect xll)
18
2nd Law of Thermodynamics
A reaction is spontaneous (productfavored) if S for the universe is positive.
So - with UNITS of J.K-1.mol-1
• The larger the value of So, the greater the degree of disorder or randomness
e.g. So (in J K-1 mol-1) : Br2 (liq) = 152.2 Br2 (gas) = 245.5
RHU 2 项目简介海水发电厂

RHU海水发电厂拯救地球卓越科技1、0项目描述1、1引言能源需求的日益增长压力,由温室气体排放造成的环境恶化与燃料价格上升就是催动使用各种有效的可再生能源的主要驱动力。
可再生技术被认为就是清洁能源,优先使用,减少对环境影响,产生最少的二次废物,而且基于当今与将来经济社会需要这些能源就是可持续的。
在世界经济进步与工业化过程中,各种形式能源都起到了更加重要的作用。
世界人口增长与原材料需求的耦合式上升已经增加了能源使用率。
过去50-100年能源使用特性的快速增长不能无限期持续,因为地球上有限的能源会枯竭的。
因此,需要去开发可再生能源来满足当前环境下的能源需求。
全世界都在积极追求可持续发展。
马来西亚政府已经制定了未来30 年发展纲要与战略,来获得国家的政治目标,减轻安全,能源效率与环境的影响以便满足增长的能源需求。
马来政府现今关注于发展有效的可再生能源政策上,以便减少化石燃料的依赖与减轻气候改变的效应。
当今,马来的可再生能源项目已经慢慢地成型了,然而马来政府从政策上正齐心协力积极地推动发展使用可再生生源。
据马来政府统计,2012年年底总装机发电容量就是29、1 千兆瓦,多数位于马来西亚半岛。
为满足国家计划用电需求,政府预计在2015与2020年间将再增加6千兆瓦的装机容量。
在今后10年,政府致力于满足增长的电力需求,采用更均衡的发电装机组合,使用煤,可再生能源与小部分的天然气。
马来政府政策减少电力消耗,也进行用电价格改革,更能反应出市场价值,促使用电方采取保护措施。
所有的发达发展国家都面临着以持续方法满足人类日益需求的挑战。
关键问题包括化石燃料消耗,全球变暖,水缺乏,生物多样性缺失与人类健康影响,由于全球人口的膨胀,所有这些问题都复合在一起。
为解决这些复合的与内部相关的问题,社会的所有阶层都需要革新的解决方案。
个体与团体,小的商业与全球合作,政府机构与非政府组织,每一个选民在创造可持续的世界上都起着关键作用。
Soilless_culture

Managing the Root Zonein Soilless CultureAuthor: Eyal Ronen Haifa Chemicals Chief AgronomistIn solid growing media, there are five important parameters that should be monitored around the root zone to optimize plant growth and yields. EYAL RONEN offers some guidelines on how to manage these parameters to prevent major crop problems, and explains the importance of measuring fertiliser solution at both the dripper and drainage points.General terminologySoilless culture, commonly referred to as ‘hydroponics’, is a cultivation technique by which plants are grown detached from the soil. Plants are cultivated in containers filled with several possible growing media. If these media are solid, the method is called ‘soilless culture’. If no medium is present and the plant roots are bathed in circulated nutrient solution, the method is called ‘hydroponics. If no medium is present and plant roots get their nutrients by frequent spraying or misting, the method is called‘aeroponics’. The method we are concerned with in this article uses solid growing media.Figure 1. Different soilless cultivation methods.Soilless culture characteristicsThe limited volume of medium and water availability generally causes rapid changes in the status of water and nutrients. Changes in the medium solution, such as electrical conductivity (EC), pH and nutrients level, should be monitored for the efficient use of water and nutrients. Failures in the careful supervision of fertilisation and/or the accuracy of irrigation are likely to result in severe plant damage and reduced yields. Hydroponics, however, offers several major advantages in the management of both plant nutrition and plant protection, if the right tools are applied and careful management is carried out.There are five important parameters that should be monitored by the grower, employing simple devices and methods. However, the common perception of some of these parameters is not always correct and this can result in some major problems.There are two reference points of special importance used to determine the status of the medium. These are the ‘drip-line point’ (fertigation input) and the ‘drainage’ point (output), based on the understanding that the drainage point best reflects the condition in the active root zone. By monitoring these two points, it is possible to see what changes are occurring in the medium after fertigation. Changes in the medium environmentWater functions as a source of some nutrients, and as a delivery vehicle to transport nutrients into the plant via the xylem vascular system. When plants are exposed to low relative humidity, they lose water by transpiration through their stomata. Water also evaporates from the medium. However, transpiration and evaporation can lead to a salt build-up in the medium if proper management practices are ignored. Although some salts are absorbed by the plant, there is a sharp increase in the concentration and a build-up of some undesirable salts.When growing in soil, root volume and soil space are large enough that salt accumulation does not interfere with plant growth as quickly. But in soilless culture there is no space to buffer this saltbuild-up, and immediate action is needed to purge the medium and lower the concentration of these dangerous components by washing them away. To avoid this problem, the common practice is to supply extra water at every irrigation cycle to ensure sufficient drainage - irrigation water should pass through most of the medium volume and leach away high salt concentrations at the drainage point. Theoretically, a 10% increase in water volume during daily irrigation cycles should be sufficient, but practically, an extra 30-50% of water is used.When plants are supplied with mineral fertilisers, although some are consumed and some are lost by leaching, the medium solution electrical conductivity is increasing compared to the drip-line point. The accumulation is mainly of nitrate and chloride.It is important to identify the reason for any EC elevation to avoid taking wrong corrective action. For example, chloride is a micronutrient and is required by plants in very small amounts. However, excess chloride will not be absorbed and will easily accumulate in the medium. Because chloride is highly soluble, it will almost always be present in the solution and affect the EC.Nutrient consumption can be roughly estimated by checking the changes in the nitrate (NO3-) content of the nutrient solution. Compared to the drip-line point, it will go up and down, generally reflecting changes in plant consumption. Another form of nitrogen that can tell us about the status of the growing medium is nitrite (NO2-) concentration. In case of over-irrigation, water accumulation in the medium leads to waterlogging, and to decreased availability of oxygen in the medium. This change uncouples the chemical transformation of ammonium to nitrate causing nitrite to appear and accumulate in the medium. The nitrite anion is toxic to plant roots and will eventually lead to plant death.Another important parameter is the pH of the solution flowing through the medium, which can affect the availability of microelements and phosphate to plants. One of the advantages of soilless culture is the ability to control pH in the medium solution. This is achieved by adding acid to the irrigation water to change the ratio between NH4+ and NO3- , which are the only two forms of nitrogen allowed in this cultivation method. It is a common phenomenon that while passing through the root system, the pH will drop slightly due to root respiration and lack of buffer capacity in the soilless medium.It is possible to check these other parameters in the growing medium as well, but this requires relatively sophisticated lab equipment. It should be stressed that EC and pH should be monitored over time, where the trends are more important than the absolute values which are not always accurate. Although the day-to-day measurements of EC and pH are not the most accurate for predicting changes in the crop, relatively inexpensive meters are adequate for quick field evaluations.Table 1. Most important parameters, and how to manage them.Parameter Factorsaffected Units Recommendedrelationshipbetween valueat drainagepoint and at thedripperAction to be taken if undesirable valuesshow at the drainage pointpH Availability ofphosphorusand ofmicronutrients pH units Lower by oneunit, and at therange of 5.5 –6.0Add more acid to the irrigation waterand/or increase ammonium share out oftotal nitrogen.E.C Salinity andwateravailability Deci-siemens/meter orMillisiemens/cmUp to 20%higher.Rinse the medium with a lot of water (2-3fold of the regular volume), enriched witha small amount of 50 ppm nitrogen, if thedrainage EC is 20% above the optimum.Cl-Irrigationvolume ppm Up to 50 ppmhigher.Same action as the above if value atdrainage is 50 ppm or more higher thanat dripper.NO3-Fertilizationstatus ppm Can be higheror lowerIncrease or decrease fertilizationconcentration according to change.NO2-Irrigationintervals or rate ppm Should not bepresent, andmax of 10 ppm.Rinse the medium if >10 ppm is detected.Increase intervals between irrigations andincrease acid application rate.Table 1 is a compilation of common management guidelines used in soilless cultivation. Crop management is generally achieved by comparing EC and pH values between the drip-line point and drainage point; but is the latter the optimal point to compare?In many cases, the values obtained at the drainage point do not reflect the real situation in the active root zone and may mislead the grower about the status of the growing medium. To obtain a more accurate picture, we need to be sure that the irrigation water is passing through all possible pore spaces in the growing medium.Although irrigation water passes horizontally and vertically through the growing medium, several factors can influence which way it moves. A low density of emitters and a high flow capacity, and a growing medium with large aggregate particles and square ‘packing’, will dictate a more vertical movement of the irrigation water. Conversely, a high density of emitters (two lines) and low flow capacity (0.2 l/h), and a medium with small aggregates and triangular shape, will dictate a more horizontal flow of water, thus ensuring a better flush of the medium and a drainage that will be more representative of the status of the growing medium (see Fig. 2)..Big aggregates Small aggregatesLow emitters density High emitters densityLow flow capacity High flow capacityFigure 2. Water movement through the medium in different conditions.Generally, the water found in the drainage does not characterise the real situation near the active root zone. Most of the water in the drainage is collected beneath the dripper from a narrow, sausage-like basin; where water movement is faster than in its periphery. Undesirable salts like chloride show in this water at a lower concentration than can be found in the active root zone, which has a much lower‘hydraulic conductance’. While drainage chloride may show an acceptable situation, a careful inspection of the medium around the active root zone (which is difficult to perform), may show an undesirable concentration of salts, expressed by a high EC.One of the functions of water supplied to soilless culture, other than fulfilling the need of transporting nutrients in plants, is to maintain a low level of salts in the medium and to prevent a possible build-up of salts. Commonsense tells us material cannot disappear from the system. Therefore, although water evaporates from the system, salts do not; hence they will always remain behind. Every anion and cation not consumed by the plant will accumulate in the medium.If the salt is not outside in the drainage it is inside the medium!Growers should aim to allow for an EC value at the drainage point as high as possible and notjust higher by 20% more of the irrigation water at the drip-line point. Also, as high as possible chloride and not only 50 ppm at the drainage, as compared to the irrigation water at the dripper. If the values are only higher by 20% in E.C and 50 ppm in chloride the grower should be worried, since it means the drainage was not effective enough and in this equation the salt is still hiding in the medium. The drainage is ineffective in removing excessive salts with most left in the medium.Growers should check the status of the growing medium at the active root zone. There are two ways to do this. One can dig into some of the medium, squeeze it by hand over a filter paper, and analyse the solution for the relevant parameters. A better method uses a fixed soil-solution extractorthat can extract the medium solution from any location selected by the grower as being a characteristic point to determine the real environment that the plant roots are surrounded by (see Fig. 3).Figure 3. Soil solution extrector inside a pot.Above all, the grower should bear in mind that both methods described for checking the drainage solution and the medium itself are only an approximation. However, because the grower needs some indication of what is going on in the medium, this method of medium evaluation will suffice as long as the EC is not higher than 20% compared to the drip-line EC, and chloride does not exceeds 50ppm over the drip line point, referring to the drainage analysis over there parameters should be higher as possible in comparison to the drip line point.One must also remember that these guidelines are not crop-specific. A better way is to balance the EC specifically for each crop according to its threshold level, above which plant productivity will decrease (see Table 2). The idea is to always keep the EC below this threshold, and when it does exceed this level to take some corrective action and flush the medium with extra water. It’s always better to make frequent small adjustments to the irrigation and fertiliser regime, than to make extreme changes when it is already too late. As a cautionary note, the action of flushing the medium should only be taken when EC levels exceed the threshold and not as a routine, because it will upset the sensitive balance the plants live in.Table 2. Crop salinity sensitivity, threshold and yield decrease (Maas and Hoffman, 1993).Crop Salinity threshold expressedin ds/m Percentage of yield decrease above the salinity threshold % per every ds/mLettuce 1.313 Pepper 1.514 Cucumber 2.513 Tomato 2.59.9Some growers might consider this procedure as labour intensive and rather than checking the ECat the drainage point, they wash the medium heavily from time to time instead. Although this may work,it is the wrong management practice. The methods described in this article will ensure plants are grownin the right zone, beneath the threshold of reduced dry-matter production (see Fig. 4 and Fig 5).TimeFigure 4. Characteristic EC pattern in non-controlled soilless cultivation, with rinsing once in a while. The grey part of the triangle is over the threshold, where productivity decreases.E.C levelTimeFigure 5. Characteristic EC pattern in a controlled soilless cultivation, with rinsing that maintains optimum level with no production losses.Further investigation of ECWhen checking EC of the medium, it is also important to breakdown this general term into its major components. Checking the EC itself will reflect that the concentration of electrolytes is high. At the working range in soilless cultivation, it is important to know whether this EC is due to nitrate nitrogen or chloride. If the majority is from nitrate, it means that the grower has over-fertilised and fertiliser concentration should be lowered. If the majority is from chloride, it means that the grower is not irrigating with a proper volume of water; water consumption is exceeding supply and since chloride only comes from water, it is accumulating very fast. In this case, the appropriate action is to increase the irrigation volume. In both cases, the high EC can be diminished by extra washing, if needed.To sum up, one needs to understand that soilless cultivation is a flexible growing method that lets the grower have full control over the growing environment, including the active root zone. However, plants and yields will suffer if the grower doesn’t have a good understanding of EC and the specific limitations of the crop, the growing medium, and irrigation water. “About the authorEyal Ronen is the Chief Agronomist in charge of market and product development worldwide for Haifa Chemicals. His role includes field research, attending seminars and providing technical support to Haifa agronomists and companies in the distribution channel throughout the world.Email: eyalr@。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
How to use the free energy to determine whether a reaction will be spontaneous or not
ΔG0 = ΔG0 (products) - ΔG0 (reactants) Table 19.4 on page 563
Gibbs Free Energy
If the ΔH (enthalpy) and the ΔS (entropy) are known, the (free energy) can be found by:
Entropy and Free Energy
19.4
After reading Section 19.4, you should know:
How to calculate the amount of free energy of a reaction
How to use the free energy to determine whether a reaction will be spontaneous or not
Entropy – a quantitative measure of th( Joule / Kelvin* mol) ΔS0 = ΔS0 (products) - ΔS0 (reactants) Table 19.2 on page 558
Values in the book
Free Energy (ΔG0) values – pg 563 Enthalpy (ΔH0) values – pg 316 Entropy (Δ S0) values – pg 558
After reading Section 19.4, you should know:
happen ▪ ΔH0 = positive ▪ Δ S0 = negative
** A system may be spontaneous under one set of conditions and nonspontaneous under another (example – if the temperature changes)
Practice
Using Table 19.2, determine the standard entropy change(ΔS0 ) for the following reaction.
2NO(g) + O2(g) 2NO2(g)
ΔS0 = ΔS0 (products) - ΔS0 (reactants)
ΔG0 = ΔH -T ΔS0
T = temp in Kelvin Must convert units!!!
▪ ΔG0 (kJ / mol) ▪ ΔH0 (kJ / mol) ▪ Δ S0 ( Joule / Kelvin*mol)
Spontaneous or not??
Spontaneous reactions release free energy
ΔS0 = -145.2 J/K
*** Remember to multiply the ΔS0 by 2 since you have 2 mol of NO and NO2
Gibbs Free Energy – the energy available to do
work
Symbol = ΔG0 (kJ / mol)
▪ ΔG0 is negative ▪ The system loses free energy ▪ ΔH0 = negative ▪ Δ S0 = positive
Spontaneous or not??
Nonspontaneous reactions absorb free energy
▪ ΔG0 is positive ▪ The system needs the free energy to make the reaction
