GSK461364_929095-18-1_DataSheet_MedChemExpress
18q_缺失综合征产前诊断方法

第 44卷第4期2023 年7月Vol.44 No.4July 2023中山大学学报(医学科学版)JOURNAL OF SUN YAT⁃SEN UNIVERSITY(MEDICAL SCIENCES)18q缺失综合征产前诊断方法马莉1,李蕾2,吴红1,刘永明1,杨新1(1. 青岛大学附属烟台毓璜顶医院检验中心,山东烟台 264000; 2. 青岛大学附属烟台毓璜顶医院产科,山东烟台 264000)摘要:【目的】 探讨18q缺失综合征的产前诊断方法,提高对无创产前筛查(NIPT)技术在18q缺失综合征产前诊断中应用价值的认识。
【方法】 本研究通过对孕妇进行血清学筛查、超声影像学检查、羊水核型分析及亲本外周血染色体核型分析等传统检查手段以及NIPT检查、染色体微阵列芯片检测(CMA)、流产组织基因组拷贝数变异测序(CNV-Seq)检测等分子生物学技术来诊断18q缺失综合征,并根据检查结果进行遗传咨询。
【结果】 该病例NIPT结果提示18号染色体24 Mb片段缺失,经过羊水核型分析及CMA检测证实,该片段包含BCL2在内的17个基因的缺失变异,均与18q缺失综合征相关。
结合超声影像学检查,确诊为18q缺失综合征。
结合亲本外周血染色体核型分析结果,该变异为新发突变。
【结论】 介入性产前诊断技术是诊断18q缺失综合征的重要标准。
NIPT技术作为中孕期的一项重要筛查,可以在超声影像学未见异常的情况下,早期提示染色体片段缺失的可能性,降低时间及经济成本。
关键词:18q缺失综合征;无创产前筛查;遗传咨询中图分类号:R72 文献标志码:A 文章编号:1672-3554(2023)04-0677-07DOI:10.13471/ki.j.sun.yat-sen.univ(med.sci).20230421.001Prenatal Diagnosis of 18q Deletion SyndromeMA Li1, LI Lei2, WU Hong1, LIU Yong-ming1, YANG Xin1(1. Center for Laboratory Diagnosis, Yantai Yuhuangding Hospital Affiliated to Medical College of Qingdao University,Yantai 264000, China; 2. Obstetrics Department, Yantai Yuhuangding Hospital Affiliated to Medical College of QingdaoUniversity, Yantai 264000, China)Correspondence to: YANG Xin; E-mail:******************Abstract:【Objective】 To explore the prenatal diagnostic methods of 18q deletion syndrome and improve understand⁃ing on the value of non-invasive prenatal testing (NIPT) in prenatal diagnosis of 18q deletion syndrome.【Methods】 18q de⁃letion syndrome was detected by conventional methods such as serological screening,ultrasonic imaging examination,chromosome karyotype analyses of both amniotic fluid cells and parental peripheral blood, and molecular biological tech⁃niques including NIPT, chromosomal microarray analysis (CMA) and copy number variation sequencing (CNV-Seq). Ge⁃netic counseling was conducted based on these examination results.【Results】 NIPT identified a 24 MB deletion on the chro⁃mosome 18 which contained 17 genes including BCL2 by karyotype analysis of amniotic fluid cells and CMA. Further ultra⁃sonic imaging examination confirmed the diagnosis of 18q deletion syndrome and karyotype analysis of parental peripheral blood revealed a de novo deletion mutation.【Conclusions】 Interventional prenatal diagnosis is an integral standard for the diagnosis of 18q deletion syndrome. NIPT, as an important screening test in middle pregnancy, can indicate the early pos⁃sible chromosome segment deletion and reduce the time and economic cost when no abnormality is found in ultrasonic imaging.Key words:18q deletion syndrome; non-invasive prenatal testing (NIPT); genetic counseling[J SUN Yat⁃sen Univ(Med Sci),2023,44(4):677-683]·临床研究·收稿日期:2022-12-21基金项目:烟台科技计划项目(2019YD004)作者简介:马莉,研究方向:分子生物学诊断,E-mail:***************;杨新,通信作者,副主任技师,E-mail:******************第44卷中山大学学报(医学科学版)18q缺失综合征是一种由18号染色体长臂节段缺失引起的疾病,由De Grouchy于1964年首次报道[1]。
中西医结合护理对胰腺炎急性反应期的护理效果分析

中西医结合护理对胰腺炎急性反应期的护理效果分析陈丽娇① 【摘要】 目的:探讨中西医结合护理对胰腺炎急性反应期的护理效果。
方法:选择2021年6月—2022年6月于漳州市中医院接受治疗的80例胰腺炎急性反应期患者作为本次研究对象,按照随机数表法将其分为试验组和对照组,各40例。
对照组给予常规护理,试验组在对照组的基础上给予中西医结合护理。
比较两组的临床症状恢复时间、血清炎症因子[白细胞介素-6(IL-6)、白细胞介素-15(IL-15)、肿瘤坏死因子-α(TNF-α)]水平、肠道屏障功能[血清淀粉酶(AMS)、D-乳酸和二胺氧化酶(DAO)]及并发症发生率。
结果:试验组临床症状恢复时间显著早于对照组,差异有统计学意义(P<0.05)。
干预后,试验组IL-6、IL-15、TNF-α水平均显著低于对照组,差异有统计学意义(P<0.05)。
干预后,试验组AMS、D-乳酸、DAO水平均显著低于对照组,差异有统计学意义(P<0.05)。
试验组并发症发生率显著低于对照组,差异有统计学意义(P<0.05)。
结论:中西医结合护理应用于胰腺炎急性反应期患者能促进患者临床症状恢复、降低炎症反应,加强肠道屏障功能,降低并发症发生率。
【关键词】 中西医结合护理 胰腺炎 急性反应期 肠道屏障功能 doi:10.14033/ki.cfmr.2023.25.026 文献标识码 B 文章编号 1674-6805(2023)25-0105-05 Nursing Effect of Integrated Traditional Chinese and Western Medicine Nursing on Acute Response Period of Pancreatitis/CHEN Lijiao. //Chinese and Foreign Medical Research, 2023, 21(25): 105-109 [Abstract] Objective: To explore the nursing effect of integrated traditional Chinese and western medicine nursing on acute response period of pancreatitis. Method: A total of 80 acute response period of pancreatitis patients who received treatment at Zhangzhou Traditional Chinese Medicine Hospital from June 2021 to June 2022 were selected as the subjects of this study. They were randomly divided into experimental group and control group using a random number table method, with 40 patients in each group. The control group received routine nursing, while the experimental group received integrated traditional Chinese and western medicine nursing on the basis of the control group. The clinical symptom recovery time, serum inflammatory factors [interleukin-6 (IL-6), interleukin-15 (IL-15), tumor necrosis factor-α (TNF-α)] levels, intestinal barrier function [serum amylase (AMS), D-lactate, and diamine oxidase (DAO)] and incidence of complications between the two groups were compared. Result: The recovery time of clinical symptoms in the experimental group were significantly earlier than those in the control group, the differences were statistically significant (P<0.05). After intervention, the levels of IL-6, IL-15, TNF-α in the experimental groups were significantly lower than those in the control group, the differences were statistically significant (P<0.05). After intervention, the levels of AMS, D-lactate, and DAO in the experimental group were significantly lower than those in the control group, the differences were statistically significant (P<0.05). The incidence of complications in the experimental group was significantly lower than that in the control group, the differences were statistically significant (P<0.05). Conclusion: The application of integrated traditional Chinese and western medicine nursing in the acute response period of pancreatitis patients can accelerate the recovery of clinical symptoms, reduce inflammatory reactions, strengthen intestinal barrier function, and reduce the incidence of complications. [Key words] Integrated traditional Chinese and western medicine nursing Pancreatitis Acute response period Intestinal barrier function First-author's address: Zhangzhou Traditional Chinese Medicine Hospital, Zhangzhou 363000, China 胰腺炎是患者在酗酒、暴饮暴食、胆道系统疾病等诱因下发生胰腺组织微循环障碍的多发性消化系统疾病,临床主要表现为急性腹痛、高热、恶心呕吐等[1-2]。
GSK-3β STAR ELISA Kit 说明书

Instruction Manual ForGSK-3β STAR ELISA KitCatalog # 17- 471Sufficient reagents for 96 assays per kitContentsPageI. TEST PRINCIPLE 1 II. BACKGROUND2 III. ASSAY SENSITIVITY, DETECTION LIMITS and SPECIES REACTIVITY 2 IV. STORAGE OF KIT COMPONENTS2 V. KIT COMPONENTS, PRECAUTIONS AND TECHNICAL NOTES3 VI. PREPARATION OF SAMPLE4 VII. REAGENT PREPARATION5 VIII. ASSAY PROTOCOL6 IX. CALCULATION OF RESULTS 7FOR RESEARCH USE ONLY.NOT RECOMMENDED OR INTENDED FOR DIAGNOSIS OF DISEASE IN HUMANS.DO NOT USE IN HUMANS.USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209Australia +61 3 9839 2000 This page left blank intentionally.I. TEST PRINCIPLEThe UPSTATE® colorimetric STAR (Signal Transduction Assay Reaction) ELISA kit is a solid phase sandwich enzyme linked immunosorbent assay that provides a fast, sensitive method to detect specific levels of signaling targets in whole cell extracts. The GSK-3β plate is coated with a specific mouse monoclonal GSK-3β capture antibody on the microwells of the 96-well clear plate. Sample lysate or the standard included in the kit is incubated in the microwells allowing GSK-3βantigen to be captured in the plate wells. The plate is then washed to remove any non-bound unspecific material. The wells are then incubated with a specific rabbit anti-GSK-3β antibody to detect the captured GSK-3β on the plate well. The unbound detection antibody is washed away followed by incubation with an HRP-conjugated anti-rabbit antibody. This allows for a sensitive enzymatic detection of the sample. After the addition of TMB substrate and stop solution the absorbance is measured at 450 nm using a plate reader.The entire assay takes less than 5 hours to complete with minimal hands-on time. Many of the reagents are supplied in ready-to use formulations for ease of use. The kit also includes a standard that is run as both a positive control and to develop a standard line of detection.II. GSK-3β BACKGROUNDGlycogen Synthase Kinase-3 differs from most serine/threonine kinases in that it is active in the absence of the action of signaling pathways. When it becomes phosphorylated, the protein becomes inactive. There are two isoforms that exist, GSK-3α and GSK-3β. The function of GSK-3 is to phosphorylate Glycogen Synthase and thereby inactivate it. Insulin action stimulates the PI3 Kinase pathway that results in the activation of Akt. Akt phosphorylates GSK-3, thereby inactivating it. Glycogen Synthase, GSK-3’s downstream substrate, is then rapidly dephosphorylated and activated. Other GSK-3 substrates include Jun (on inhibitory sites), and eIF2B. Detection of GSK-3’s phosphorylation status of the Akt site (Ser21) on GSK-3 is suitable for surrogate assays of the activation state of the pathway.III. ASSAY SENSITIVITY, DETECTION LIMITS and SPECIES REACTIVITYng/mLSensitivity: 0.4Range of Detection: 0.4 to 25 ng/mLSpecies Reactivity Human, mouse and ratNOTE: This data is presented for reference use only and should not be used to interpret actual assay results. A standard curve must be generated for each assay.IV. STORAGE OF KIT COMPONENTSMaintain the unopened kit at 2-8°C until expiration date.V. KIT COMPONENTS1. GSK-3β Capture Plate: (Part No. 17-471A) One pre-coated 96-stripwell immunoplate sealed in a foil pouch.2. Anti-GSK-3β detection antibody: (Part No. 17-471B) One bottle (6 mL) of anti-GSK-3β detection antibody containingsodium azide, ready to use.3. ELISA Diluent: (Part No. 17-471C) One bottle (25 mL) of ELISA Diluent sodium azide, ready to use.4. 25X ELISA Wash Buffer: (Part No. 17-471D) One bottle (50 mL) of 25X ELISA Wash Buffer.5. Anti-Rabbit IgG HRP conjugate: (Part No. 17-471E) One vial (125 µL) of 100X anti-rabbit HRP conjugate containingthimerosol.6. HRP Diluent: (Part No. 17-471F) One bottle (25 mL) of HRP diluent containing thimerosol.7. TMB Solution: (Part No. 17-471G) One bottle (25 mL) of stabilized tetramethylbenzidine (TMB), ready to use.8. Stop Solution: (Part No. 17-471H) One bottle (25 mL) of stop solution, ready to use.9. GSK-3β Standard: (Part No. 17-471I) Two vials of GSK-3β standard, lyophilized.10. Plate Covers: Two plate covers.Materials Not Supplied1.Multi-channel or repeating pipettes2.Plate shaker (optional)3.Pipettors & tips capable of accurately measuring 1-1000 μL4. Graduated serological pipettes5.96-well microtiter Plate Reader with 450 nm filter6.Graphing software for plotting data or graph paper for manual plotting of data7.Microfuge tubes for standard and sample dilutions8. Mechanical vortex9. 1 liter container10.Distilled or deionized waterPrecautions•The instructions provided have been designed to optimize the kit's performance. Deviation from the instructions may result in suboptimal performance of the kit and the failure to produce accurate data.• Caustic Material: Stop Solution. Caution: Eye, hand, face, and clothing protection should be worn when handling this material.•Safety Warnings and Precautions: This kit is designed for research use only and not recommended for internal use in humans or animals. All chemicals should be considered potentially hazardous and principles of good laboratory practice should be followed.•The Detection Antibody and Elisa Diluent contain sodium azide. Sodium azide may react with copper and lead plumbing to form highly explosive metal azides. Upon disposal, flush with large amounts of water to prevent azide build-up. Avoid contact with skin.•The Anti-Rabbit IgG HRP Conjugate and HRP Diluent contain thimerosal. Thimerosoa is highly toxic by inhalation, contact with skin or if swallowed. Thimerosal is a possible mutagen and should be handled accordingly. Technical Notes•All kit reagents should be at room temperature (20°C to 25°C) prior to use.•Do not use reagents beyond the expiration date of the kit.•Do not mix or interchange reagent from various kit lots.•Manual Plate Washing: Vigorous washing and complete removal of all liquid by aspiration at the end of each washing step is very important to obtain low background values. Gentle agitation during the wash steps or a 2-3 minute soak may reduce background values.•The desiccant enclosed in the 96-well capture plate pouch will keep the plate stable when stored at 2° to 8°C should the plate loose its seal during shipping.VI. PREPARATION OF SAMPLE1. Culture cells stimulating GSK-3β activation as desired.2. Remove culture media and wash cells twice ice-cold with 1X TBS (Tris Buffered Saline) or PBS (Phosphate BufferedSaline). Discard supernatant3. Add 5-10 mL of cold 1X RIPA containing 0.1% SDS and protease inhibitors per 150 mm tissue culture plate.Note: 10 mL of 1X RIPA containing 0.1% SDS can be prepared by adding 10 µL of 1 µg/µL Leupeptin, 10 µL of 1 µg/µL Aprotinin, 10 µL of 1 µg/µL Pepstatin, 100 µL of 100mM PMSF, 100 µL 10% SDS, 1 mL 10X RIPA (Cat. No. 20-188) to 8.77 mL of distilled or deionized water.4. Scrape cells from plate with a rubber policeman.5. Transfer cells in RIPA buffer to a microcentrifuge tube and incubate on ice for 15 minutes.6. Vortex tube for 10 seconds or sonicate briefly for 10 seconds.7. Clarify lysate by centrifugation at 12,000 rpm for 10 minutes at 4ºC in a microcentrifuge prior to use.8. Cell extract containing SDS must be diluted to 0.01% SDS using ELISA Diluent prior to use.9. Collect the supernatant and calculate protein concentration using a Bradford Assay or by densitometry.10. It is suggested that the cell lysate be used immediately following preparation. However, samples can be frozen andstored at -80º C for later use. Frozen samples should be used within 6 months if storing at -80º C. Avoid repeated freeze thaws.Further information of lysate preparation protocols can be obtained at Cell Lysate Extracts-General Protocols.VII. REAGENT PREPARATION1. 1X Wash BufferWarm the 25X ELISA Wash Buffer to room temperature and mix to ensure that any precipitated salts have re-dissolved. For 500 mL of Wash Buffer, combine 20 mL of 25X ELISA Wash Buffer and 480 mL distilled or deionized water. Stir to homogeneity. Wash Buffer can be stored for up to 4 weeks at 2-8°C. Discard the Wash Buffer if it becomes turbid or if a precipitate develops.2. Anti-Rabbit IgG HRP ConjugateDilute the anti-Rabbit IgG HRP Conjugate 100-fold with HRP Diluent immediately before use. Prepare 1 mL for each strip used.3. StandardNote : When opening lyophilized Standard, remove rubber stopper gently as the lyophilizate may have becomedislodged during shipping.Reconstitute the standard with the volume of ELISA Diluent specified on the vial label to give a concentration of 25 ng/mL. Gently swirl the vial and allow the vial to sit for 10 minutes to ensure the material is completely reconstituted. The standard should be reconstituted immediately before the assay. This stock material (tube #1) is then used to generate a standard curve. A suggested 2-fold dilution scheme is as follows:a) Label 7 test tubes #2-7 and “0 dose”. Add 150 μL of the ELISA Diluent to tubes #2-7 and “0 dose”.b) Add 150 μL of the stock Standard solution [25 ng/mL] to tube #2 and vortex. This is Standard tube #2 with aconcentration of 12.5 ng/mL.c) Standards #3-7 are then prepared by performing a 2-fold serial dilution of the preceding standard. Refer to Fig. 1.For example, to make Standard #3, remove 150 μL of Standard #2 and add it to tube #3 and vortex and so on. Do not add any Standard to the "0 Dose" Standard tube.Figure 1: Recommended 2-fold Serial Dilution of StandardNote: The Standard curve can set up with a different serial dilution scheme by making appropriate adjustments to the dilution pattern.25 12.5 6.25 3.13 1.560.780.39 ng/mL150 μL 150 μL 150 μL 150 μL 150 μL150 μLVIII. ASSAY PROTOCOL1. Prepare the reagents as described in the Reagent Preparation section.2. Place the desired number of strips in the strip well plate holder. (Re-bag the extra strips and return unused stripsto refrigerator for future use.)μL of the Standards 1 through 7 or the samples to wells. Add 50 μL of the zero dose to the control503. Addeitherwells. It is recommended that standards and samples be run in duplicate.Note: Do not add standard or sample lysate to wells reserved for TMB blanks.Note: A standard curve must be run at each setting.μL of the detection antibody to each well. Seal the plate and incubate at room temperature for 3 hours at 4. Add50room temperature (on shaker if possible).5. IMPORTANT WASH STEP:Gently remove the plate sealer and wash the plate at least 4 times. A thorough washing of the plate is extremely important to reduce background. We recommend using a multi-channel pipette to fill each well with 250 μL of diluted Wash Buffer. Fluid removal from the wells is best accomplished by inverting the plate over a sink and flicking the fluid out of the wells and then blotting the plate on clean paper towels.Using the multichannel pipet add 250 μL of Wash Buffer to each well; flick and blot the plate. Repeat this procedure for a total of 4 times.For users of automatic plate washers: It is important to ensure that the wash apparatus is properly maintained and operating correctly. Tubing and tips can easily become clogged, leading to incomplete washing and inadequate aspiration of wells. The result may be poor precision and an unsuitable standard curve. For best results, we recommend at least 4 wash cycles.μL of a 1:100 dilution of the anti-Rabbit IgG HRP Conjugate to each well. Cover the plate and incubate 6. Add100at room temperature for 45 minutes (on shaker with mild agitation if possible).7. Wash as described in Step 5. Remove all fluid from the wells and blot the wells dry.μL of the TMB Solution to each well. Incubate at room temperature in the dark for 10 to 45 minutes, 8. Add100monitor the color development. Stop the reaction by adding 100 μL of Stop Solution to each well. Immediately read the plate at 450 nm. Plate should be read within 1 hour of adding the stop solution.9. The plate reader may be blanked against a TMB blank prepared by adding 100 μL of stop solution to 100 μL ofthe TMB solution.CAUTION:Bubbles in the wells will cause inaccurate readings. Ensure that all bubbles are removed prior to taking the absorbance reading.NOTE:For very low starting protein levels, samples can be placed at 37o C during the final incubation to obtain greater sensitivity.IX. CALCULATION OF RESULTSFigure 2. Typical GSK-3β Standard CurveμL of progressive 2 fold dilutions of the GSK-3β standard was run as described in the assay100instructions.NOTE: This data is presented for reference use only and should not be used to interpret actual assay results. A standardcurve must be generated for each assay.WarrantyMillipore Corporation (“Millipore”) warrants its products will meet their applicable published specifications when used in accordance with their applicable instructions for a period of one year from shipment of the products. MILLIPORE MAKES NO OTHER WARRANTY, EXPRESSED OR IMPLIED. THERE IS NO WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. The warranty provided herein and the data, specifications and descriptions of Millipore products appearing in Millipore’s published catalogues and product literature may not be altered except by express written agreement signed by an officer of Millipore. Representations, oral or written, which are inconsistent with this warranty or such publications are not authorized and if given, should not be relied upon.In the event of a breach of the foregoing warranty, Millipore’s sole obligation shall be to repair or replace, at its option, the applicable product or part thereof, provided the customer notifies Millipore promptly of any such breach. If after exercising reasonable efforts, Millipore is unable to repair or replace the product or part, then Millipore shall refund to the Company all monies paid for such applicable Product. MILLIPORE SHALL NOT BE LIABLE FOR CONSEQUENTIAL, INCIDENTAL, SPECIAL OR ANY OTHER DAMAGES RESULTING FROM ECONOMIC LOSS OR PROPERTY DAMAGE SUSTAINED BY ANY COMPANY CUSTOMER FROM THE USE OF ITS PRODUCTS.(c)2008 - 2009: Millipore Corporation. All rights reserved. No part of these works may be reproduced in any form without permission in writing.This page left blank intentionallyCat. No 17-471March 2009Revision B17-471MAN。
滑石粉说明书(英文)

63256-200-04 PRESCRIBING INFORMATION NDCSTERILE TALC POWDERFDA FINAL VERSIONFor Intrapleural Administration OnlyDESCRIPTIONSterile Talc Powder is a sclerosing agent intended for intrapleural administration supplied in a single use 100 mL brown glass bottle, sealed with a gray, 20 mm stopper and covered with a flip-off seal. Each bottle contains a minimum of 5.0 g of Talc USP (Ultra 2000 Talc), either white oroff-white to light gray, asbestos-free and brucite-free grade of talc of controlled particle size. The composition of the talc is ≥ 95% talc as hydrated magnesium silicate. The empirical formula oftalc is Mg3 Si4 010 (OH)2 with a molecular weight of 379.3. Associated naturally occurring minerals include chlorite (hydrated aluminum and magnesium silicate.), dolomite (calcium and magnesium carbonate), calcite (calcium carbonate) and quartz. Talc is practically insoluble in water and in dilute solutions of acids and alkali hydroxides. The finished product has been sterilized by gamma irradiation.CLINICAL PHARMACOLOGYMechanism of ActionThe therapeutic action of talc instilled into the pleural cavity is believed to result from inductionof an inflammatory reaction. This reaction promotes adherence of the visceral and parietal pleura, obliterating the pleural space and preventing reaccumulation of pleural fluid.The extent of systemic absorption of talc after intrapleural administration has not been adequately studied. Systemic exposure could be affected by the integrity of the pleural surface,and therefore could be increased if talc is administered immediately following lung resection or biopsy.CLINICAL STUDIESThe data demonstrating safety and efficacy of talc slurry administered via chest tube for the treatment of patients with malignant pleural effusions are from the published medical literature. The following prospective, randomized studies were designed to evaluate the risk of recurrenceof malignant pleural effusions in patient with a variety of solid tumors. The studies comparedtalc slurry, instilled into the pleural cavity via chest tube, versus a concurrent control. In all studies, after maximal drainage of the pleural effusion, the investigator administered talc slurryvia the chest tube. Chest films documented response (defined as lack of recurrence of fluid for a period of time). Studies differed on the timing of the efficacy assessment. Zimmer et al. did notspecify the time required evaluations. Ong et al. specified the assessment at one month. Sorensen et al. specified the assessment at 3-4 months. The remaining studies assessed response at the completion of the follow-up period.Randomized Controlled Trials Using Talc Slurry as a Sclerosing AgentREFERENCE TREATMENT RESPONSE RATE EVALUABLE PTS*p value*RESPONSERATE ALL PTS*p value*Sorensen et al. Eur J Respir Dis. 1984: 65(2):131-5 Talc Slurry 10g /250ml NS vs. Chest tube drainage alone100% (9/9) vs. 58% (7/12) p=0.04 64% (9/14)vs.41% (7/17)p=0.29Noppen et al . Acta Clin Belg 1997; 52(4):258-62 Talc Slurry 5g/50-ml NS vs. Bleomycin 1mg/kg/50ml NS79% (11/14) vs. 75% (9/12) p=1.00 79% (11/14)vs.75% (9/12)p=1.00Zimmer PW et al. Chest 1997; 112(2):430-434 Talc Slurry 5g/50 ml NS c vs. Bleomycin 60U/50 ml NS c90% (17/19b ) vs. 79% (11/14 b )p=0.63 Not Given Ong KC et al. Respirology 2000;5;99-103 Talc Slurry 5g/150ml NS d vs. Bleomycin 1U/kg/150 ml NS d89% (16/18) vs. 70% (14/20) p=0.24 64% (16/25) vs.56% (14/25)p=0.77 Yim AP et al. Ann Thorax Surg 1996; 62:1655-8 Talc Slurry 5g/50ml NS, lidocaine 2% 10 ml vs. Talc Insufflation 5g powder 90%(26/29) vs. 96% (27/28) p=0.61 90% (26/29) vs.96% (27/28)p=0.61* Two-sided p-value based on Fisher's exact test a Patients were evaluable if chest x-rays were done to assess response per protocol. The Sorensen study excluded patients if incomplete lung re-expansion was noted post drainage. b Data per procedure (33 procedures in 29 evaluable patients, 3 patients with bilateral effusions). c Plus lidocaine 1%, 20 ml. d Plus lidocaine 1%, 10 ml.In single-arm studies of malignant pleural effusions from the published literature, variously defined "success" rates using talc slurry pleurodesis ranged from 75% to 100%. INDICATIONS AND USAGESterile Talc Powder, administered intrapleurally via chest tube, is indicated as a sclerosing agent to decrease the recurrence of malignant pleural effusions in symptomatic patients. CONTRAINDICATIONSNone knownWARNINGSNonePRECAUTIONS1. Future procedures: The possibility of the future diagnostic and therapeutic procedures involving the hemithorax to be treated must be considered prior to administering Sterile Talc Powder. Sclerosis of the pleural space may preclude subsequent diagnostic procedures of the pleura on the treated side. Talc sclerosis may complicate or preclude future ipsilateral lung resective surgery, including pneumonectomy for transplantation purposes.e in potentially curable disease: Talc has no known antineoplastic activity and should not be used alone for potentially curable malignancies where systemic therapy would be more appropriate, e.g., a malignant effusion secondary to a potentially curable lymphoma.3. Pulmonary complications: Acute Pneumonitis and Acute Respiratory Distress Syndrome (ARDS) have been reported in association with intrapleural talc administration. Three of the case reports of ARDS have occurred after treatment with a relatively large talc dose (10 g) administered via intrapleural chest tube instillation. One patient died one month post treatment and two patients recovered without further sequelae.DRUG INTERACTIONSIt is not known whether the effectiveness of a second sclerosing agent after prior talc pleurodesis would be diminished by the absorptive properties of talc.Carcinogenesis, Mutagenesis, Impairment of Fertility: Studies on the carcinogenicity of talc have been performed using non-standard designs which prevent firm conclusions on its carcinogenicity. With single intraperitoneal administration to mice at 20 mg and observation for at least 6 months or 4 weekly doses administered intraperitoneally at 25 mg/dose to rats with observation for at least 84 weeks, tumor incidence was not increased. In these studies the talcand its asbestos content were not characterized.Genotoxicity was tested in cultures of rat pleural mesothelial cells (RPMC) as unscheduled DNA synthesis (UDS) and sister chromatid exchanges (SCEs).None of the talc samples (which were asbestos-free) induced enhancement of UDS or SCEs in treated cultures. No information is available on impairment of fertility in animals by talc.Pregnancy: Pregnancy Category B. An oral administration study has been performed in the rabbit at 900 mg/kg. Approximately 5 fold higher than a human dose on mg/m2 basis, and has revealed no evidence of teratogenicity due to talc. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should not be used during pregnancy unless the benefit outweighs the risk.Pediatric Use: The safety and efficacy of Sterile Talc Powder in pediatric patients have not been established.Geriatric use: The estimated mean and median ages of patients treated with talc slurry from clinical studies (single-arm or randomized) were 60 and 62 years, respectively. No analyses to specifically evaluate the safety and efficacy in the geriatric population have been reported.ADVERSE REACTIONSIntrathoracic administration of talc slurry has been described in medical literature reports involving more than 2000 patients. Patients with malignant pleural effusions were treated with talc via poudrage or slurry. In general, with respect to reported adverse experiences, it is difficult to distinguish the effects of talc from the effects of the procedure(s) associated with its administration. The most often reported adverse experiences to intrapleurally-administered talc were fever and pain.Infection: Complications reported include empyema.Respiratory: Complications reported include hypoxemia, dyspnea, unilateral pulmonary edema, pneumonia, ARDS, brochopleural fistula, hemoptysis and pulmonary emboli. Cardiovascular: Complications reported included tachycardia, myocardial infarction, hypotension, hypovolemia, and asystolic arrestDelivery Procedure: Adverse reactions due to the delivery procedure and the chest tube may include: pain,infection at the site of thoracostomy or thoracoscopy, localized bleeding, and subcutaneous emphysema.Chronic Toxicity: Since patients in clinical studies had a limited life expectancy, data on chronic toxicity are limitedOVERDOSAGENo definite relationship between dose and toxicity has been established. Excessive talc may be partially removed with saline lavage.DOSAGE AND ADMINISTRATIONSterile Talc Powder should be administered after adequate drainage of the effusion. The success of the pleurodesis appears to be related to the completeness of the drainage of the pleural fluid, as well as the full re-expansion of the lung, both of which will promote symphysis of the pleural surfaces.The recommended dose is 5 g, dissolved in 50 - 100 ml Sodium Chloride Injection, USP. Although the optimal dose for effective pleurodesis is unknown, 5 g was the dose most frequently reported in the published literature.Talc PreparationPrepare the talc slurry using aseptic technique in an appropriate laminar flow hood. Remove talc container from packaging. Remove protective flip-off seal.Each brown bottle contains 5 g of Sterilized Talc Powder. To dispense the contents:ing a 16 gauge needle attached to a 60-ml LuerLok syringe, measure and draw up 50 ml ofSodium Chloride Injection, USP. Vent the talc bottle using a needle. Slowing inject the 50 ml of Sodium Chloride Injection, USP into the bottle. For doses more than 5 g, repeat this procedure with a second bottle.2.Swirl the bottle(s) to disperse the talc powder and continue swirling to avoid settling of thetalc in the slurry. Each bottle will contain 5 g Sterile Talc Powder dispersed in 50 ml of Sodium Chloride Injection, USP.3.Divide the content of each bottle into two 60 ml irrigation syringes by withdrawing 25 ml ofthe slurry into each syringe with continuous swirling.QS each syringe with Sodium Chloride Injection, USP to a total volume of 50 ml in each syringe. Draw air into each syringe to the60 ml mark to serve as a headspace for mixing prior to administration.4. When appropriately labeled, each syringe contains 2.5 g of Sterile Talc in 50 ml of SodiumChloride Injection, USP with an air headspace of 10 ml. Once the slurry has been made, use within 12 hours or discard and prepare fresh slurry. Label the syringes appropriately noting the expiration date and time, with the statement “For Pleurodesis Only – NOT FOR IV ADMINISTRATION,” the identity of the patient intended to receive this material and acautionary statement to SHAKE WELL before use.5. Prior to administration, completely and continuously agitate the syringes to evenlyredisperse the talc and avoid settlement. Immediately prior to administration, vent the 10ml air headspace from each syringe.6. Attach the adapter and place a syringe tip on the adapter. Maintain continuous agitationof the syringes.NOTICE: Shake well before installation. Each 25 ml of prepared slurry in the syringe contains 1.25 g of talc. NOT FOR IV ADMINISTRATION.AdministrationAdminister the talc slurry through the chest tube by gently applying pressure to syringe plunger and empty the contents of the syringe into the chest cavity. After application, discard the empty syringe according to general hospital procedures. After the talc slurry has been administered through the chest tube into the pleural cavity, the chest tube may be flushed with 10- 25 ml sodium chloride solution to ensure that the complete dose of talc is delivered.Following introduction of the talc slurry, the chest drainage tube is clamped, and the patient is asked to move, at 20 to 30 minute intervals, from supine to alternating decubitus positions, so that over a period of about 2 hours the talc is distributed within the chest cavity. Recent evidence suggests that this step may not be necessary.At the end of this period, the chest drainage tube is unclamped, and the excess saline is removed by the routine continual external suction on the tube.HOW SUPPLIEDNDC 63256-200-04 Sterile Talc Powder is supplied in a 100 ml brown glass bottle containing 5 g of talc.The sterile bottle is closed with a gray stopper and covered with a flip-off seal. Storage: Store at Room Temperature (18-25°C). Protect against sunlight.DISTRIBUTED BY: Bryan Corporation. Woburn, MA 01801Version: Original September 2003。
人白细胞介素18IL18试剂盒使用方法
人白细胞介素-18(IL-18)试剂盒使用方法检测范围:96T5 ng/L -180 ng/L使用目的:本试剂盒用于测定人血清、血浆及相关液体样本中人白细胞介素-18(IL-18)含量。
实验原理本试剂盒应用双抗体夹心法测定标本中人白细胞介素-18(IL-18)水平。
用纯化的人白细胞介素-18(IL-18)抗体包被微孔板,制成固相抗体,往包被单抗的微孔中依次加入白细胞介素-18(IL-18),再与HRP标记的白细胞介素-18(IL-18)抗体结合,形成抗体-抗原-酶标抗体复合物,经过彻底洗涤后加底物TMB显色。
TMB在HRP酶的催化下转化成蓝色,并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的白细胞介素-18(IL-18)呈正相关。
用酶标仪在450nm波长下测定吸光度(OD值),通过标准曲线计算样品中人白细胞介素-18(IL-18)浓度。
试剂盒组成1.标本采集后尽早进行提取,提取按相关文献进行,提取后应尽快进行实验。
若不能马上进行试验,可将标本放于-20℃保存,但应避免反复冻融2.不能检测含NaN3的样品,因NaN3抑制辣根过氧化物酶的(HRP)活性。
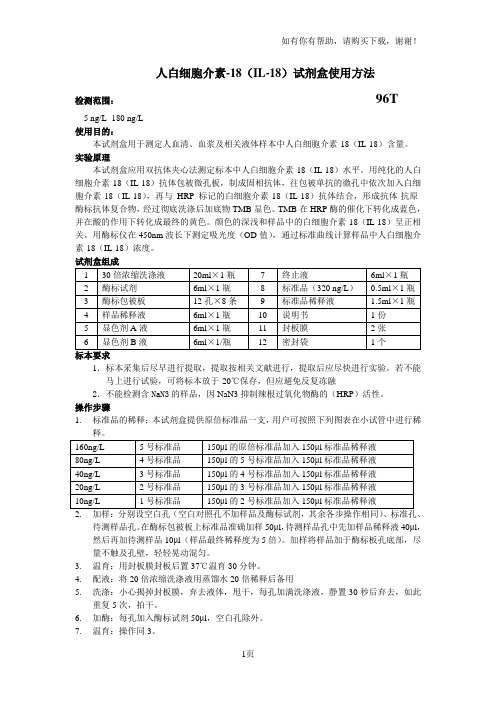
操作步骤1.标准品的稀释:本试剂盒提供原倍标准品一支,用户可按照下列图表在小试管中进行稀2.加样:分别设空白孔(空白对照孔不加样品及酶标试剂,其余各步操作相同)、标准孔、待测样品孔。
在酶标包被板上标准品准确加样50μl,待测样品孔中先加样品稀释液40μl,然后再加待测样品10μl(样品最终稀释度为5倍)。
加样将样品加于酶标板孔底部,尽量不触及孔壁,轻轻晃动混匀。
3.温育:用封板膜封板后置37℃温育30分钟。
4.配液:将20倍浓缩洗涤液用蒸馏水20倍稀释后备用5.洗涤:小心揭掉封板膜,弃去液体,甩干,每孔加满洗涤液,静置30秒后弃去,如此重复5次,拍干。
6.加酶:每孔加入酶标试剂50μl,空白孔除外。
7.温育:操作同3。
8.洗涤:操作同5。
9.显色:每孔先加入显色剂A50μl,再加入显色剂B50μl,轻轻震荡混匀,37℃避光显色15分钟.10.终止:每孔加终止液50μl,终止反应(此时蓝色立转黄色)。
GSK461364_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:GSK461364 is a potent Polo–like kinase 1 (PLK1) inhibitor with a K i value of 2.2 nM.IC50 & Target: Ki: 2.2 nM (PLK1)In Vitro: GSK461364 inhibits cancer cell line proliferation from multiple origins with minimal toxicity in nondividing human cells [1].RNA silencing of WT p53 increases the antiproliferative activity of GSK461364. As many cancer therapies tend to be more effective in p53 WT patients, the higher sensitivity of p53–deficient tumors toward GSK461364 can potentially offer an opportunity to treattumors that are refractory to other chemotherapies as well as early line therapy for these genotypes. GSK461364 is a thiophene amide that inhibits purified Plk1 enzyme in vitro with a K i of 2 nM and has >100–fold selectivity for Plk1 compared with Plk2 and Plk3.GSK461364 is a potent inhibitor of cell proliferation causing 50% growth inhibition (GI 50) below 100 nM in most of the cell lines tested with limited toxicity against human nonproliferating cells. Inhibition of cell cycle progression is concentration dependent with initial delay at G2 phase at high GSK461364 concentrations and arrest at M phase at lower concentrations. Currently,GSK461364 is in a dose–escalation first–time–in–human trial. Cell lines with mutations in the TP53 gene tended to be more sensitive to GSK461364, and that inhibiting the p53 response by RNA silencing confers increased sensitivity in some p53 wild–type (WT) cells.Furthermore, these more sensitive cell lines also have increased levels of chromosome instability, a characteristic associated with TP53 mutations [2]. In preclinical testing, GSK461364 shows antiproliferative activity against multiple (>120) tumor cell lines and potently inhibits the proliferation of greater than 83% and 91% of these cell lines, with IC 50 values lower than 50 and 100 nM,respectively [3].In Vivo: Cell culture growth inhibition by GSK461364 can be cytostatic or cytotoxic but leads to tumor regression in xenograft tumor models under proper dose scheduling. GSK461364 shows clear antitumor activity in human tumor xenograft models [1]. GSK461364shows a dose–dependent mitotic arrest in mouse xenografts, which correlates with effects on tumor growth [2]. Intraperitonealadministration of GSK461364 causes regression or tumor growth delay in different xenograft models, including Colo205 xenografts.Suppression of Plk1 in vivo by using GSK461364 results in mitotic arrest with aberrant mitotic figures consisting of monopolar or collapsed mitotic spindles [3].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]Kinase reactions are performed in a final assay volume of 10 μL using the Z'–Lyte Assay kit (Ser/Thr peptide 16).Briefly, reactions contained 50 mM HEPES (pH 7.5), 10 mM MgCl 2, 1 mM EGTA, 1 mM DTT, 0.01% Brij 35, 0.01 mg/mL casein, 200 μM ATP, 200 μM Polo Box peptide (NH2–MAGPMQS[pT]PLNGAKK–OH), and 6 nM recombinant Plk1 (H6–tev–PLK 1–603). Plk1 ispreincubated for 60 min in the presence or absence of 0 to 1,000 nM GSK461364A. Reactions are then initiated by the addition of 2μM peptide. After 15 min at 23°C, reactions are quenched and processed according the Z'–Lyte protocol and read on an EnVision plate reader. Raw fluorescence values are converted to concentration of product formed using substrate and product standards. IC 50values are determined using a two–parameter fit (Hill coefficient and IC 50) using GraFit software. Because the potency of inhibitionProduct Name:GSK461364Cat. No.:HY-50877CAS No.:929095-18-1Molecular Formula:C 27H 28F 3N 5O 2S Molecular Weight:543.60Target:Polo–like Kinase (PLK)Pathway:Cell Cycle/DNA Damage Solubility:10 mM in DMSOfor GSK461364A is observed to vary as a function of the ATP concentration in a manner consistent with an ATP–competitive modeof inhibition, an upper limit for the K i*app for GSK461364A is determined by applying the Cheng–Prusoff relationship for a competitive inhibitor (ATP K m*app=16 μM) to the IC50 value obtained with 60 min preincubation of GSK461364A.Cell Assay: GSK461364 is dissolved in DMSO.[1]Cell lines grow in the recommended media at 37°C, 5% CO2 in a humidified incubator. Cells are plated in triplicate 96–well microtiter plates at 1,000 cells per well in culture media. GSK461364A dissolved in DMSO or negative control (0.1% DMSO) are added the following day, and one plate is harvested with 50 μL of CellTiter–Glo for a time 0 (T=0) measurement.Animal Administration: GSK461364 is formulated in vehicle [4% DMA/Cremaphore (50:50), pH 5.6].[1]Cells are implanted in Nude mice and grown as tumor xenografts. Dosing began when tumors achieve appr 100 mm3. GSK461364A or the vehicle [4%DMA/Cremaphore (50:50), pH 5.6] is given i.p. to mice every 2 d (q2dx6, q2dx12) or every 4 d (q4dx3) at nominal dose levelsof 25, 50, and 100 mg/kg/dose. Results are reported as median tumor volume for n=7 to 8 mice. Paclitaxel (30 mg/kg i.v.;q4dx3) is used as a positive control for comparison. Tumors are measured thrice a week with Vernier calipers, and tumorvolume is calculated from two–dimensional measurements using an equation approximating the volume of a prolateellipsoid [tumor volume mm3=(length × width2) × 0.5]. The maximum tolerated dose is defined as the highest dose that produces >20% mortality or >20% weight loss (appr 4 g). Antitumor activity is defined as tumor growth delay (TGD), partial regression (PR), or complete regression (CR). TGD represents the time differential between the treated and control tumors to reach a predetermined tumor volume of 1,000 mm3. PR is defined as a decrease in an individual tumor volume to one–half the initial starting volume for at least 1 wk (three consecutive measurements). CR is defined as a decrease in an individual tumor volume to <13 mm3 for at least 1 wk. References:[1]. Gilmartin AG, et al. Distinct concentration–dependent effects of the polo–like kinase 1–specific inhibitor GSK461364A, including differential effect on apoptosis. Cancer Res. 2009 Sep 1;69(17):6969–77.[2]. Degenhardt Y, et al. Sensitivity of cancer cells to Plk1 inhibitor GSK461364A is associated with loss of p53 function and chromosome instability. Mol Cancer Ther. 2010 Jul;9(7):2079–89.[3]. Olmos D, et al. Phase I study of GSK461364, a specific and competitive Polo–like kinase 1 inhibitor, in patients with advanced solid malignancies. Clin Cancer Res. 2011 May 15;17(10):3420–30.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
美国联合保健公司产品说明书:测试生成产品

UnitedHealthcare PharmacyClinical Pharmacy ProgramsProgram Number 2023 P 2018-16Program Prior Authorization/Medical Necessity – TestosteroneMedication Androderm, Androgel*, Fortesta*, Jatenzo*, Natesto*, Kyzatrex*,Testim, testosterone topical solution (generic Axiron)*, testosteronetransdermal gel (generic Testim)*, Tlando*, Vogelxo*, Xyosted*P&T Approval Date 2/2014, 4/2014, 5/2014, 7/2014, 10/2014, 10/2015, 5/2016, 6/2017,6/2018, 2/2019, 6/2019, 7/2020, 8/2021, 9/2022, 1/2023Effective Date 4/1/2023;Oxford only: 4/1/20231.Background:Testosterone products are approved by the Food and Drug Administration (FDA) for testosterone replacement therapy in males with primary hypogonadism (congenital or acquired) orhypogonadotropic hypogonadism (congenital or acquired). Primary hypogonadism originatesfrom a deficiency or disorder in the testicles. Secondary hypogonadism indicates a problem in the hypothalamus or the pituitary gland. Testosterone use has been strongly linked to improvements in muscle mass, bone density, and libido.The purpose of this program is to provide coverage for androgens and anabolic steroid therapy for the treatment of conditions for which they have shown to be effective and are within the scope of the plan’s pharmacy benefit. Coverage for the enhancement of athletic performance or bodybuilding will not be provided.a3.Additional Clinical Rules:•Notwithstanding Coverage Criteria, UnitedHealthcare may approve initial and re-authorization based solely on previous claim/medication history, diagnosis codes (ICD-10)and/or claim logic. Use of automated approval and re-approval processes varies by programand/or therapeutic class.•Supply limits may be in place.•* May be excluded from coverage•+ Coverage for patient population may be dependent upon benefit design4.References:1.AACE Hypogonadism Task Force. American Association of Clinical EndocrinologistsMedical Guidelines for Clinical Practice for the Evaluation and Treatment ofHypogonadism in Adult Male Patients – 2002 Update. Endocr Pract. 2002; 8(No. 6): 439-456.2.The World Professional Association for Transgender Health (WPATH), Standards of Carefor the Health of Transsexual, Transgender, and Gender Nonconforming People, 7thVersion.3.Cook, David M, et al. "American Association of Clinical Endocrinologists medicalguidelines for clinical practice for growth hormone use in growth hormone-deficient adultsand transition patients - 2009 update: executive summary of recommendations." Endocrinepractice 15.6 (2009):580-586.4.Gibney, James, et al. "Growth hormone and testosterone interact positively to enhanceprotein and energy metabolism in hypopituitary men." American journal of physiology:endocrinology and metabolism 289.2 (2005):E266-E2715.Bhasin, S, et al. "Testosterone replacement and resistance exercise in HIV-infected menwith weight loss and low testosterone levels." JAMA. 2000. 283.(6) 763-770.6.Isidori, Andrea M, et al. Effects of testosterone on sexual function in men: results of ameta-analysis. Clinical endocrinology. 2005 63(4):381-394.7.Kenny, A M, et al. Effects of transdermal testosterone on bone and muscle in older menwith low bioavailable testosterone levels. The journals of gerontology. 2001. 56(5) M266-M272.8.Tracz, Michal J, et al. Testosterone use in men and its effects on bone health. A systematicreview and meta-analysis of randomized placebo-controlled trials. The Journal of clinicalendocrinology and metabolism. 2006. 91(6):2011-2016.9.Bolona, Enrique R, et al. Testosterone use in men with sexual dysfunction: a systematicreview and meta-analysis of randomized placebo-controlled trials. Mayo Clinicproceedings.2007. 82(1):20-28.10.Androderm [package insert]. Madison, NJ: Allergan, Inc; May 2020.11.Androgel [package insert]. North Chicago, IL: AbbVie Inc; May 2020.12.Fortesta [package insert]. Malvern, PA: Endo Pharmaceuticals Inc; January 2022.13.Testim [package insert]. Malvern, PA: Endo Pharmaceuticals Inc; August 2021.14.Natesto [package insert]. Mississauga, ON: Acerus Pharmaceuticals Corporation;December 2021.15.Vogelxo [package insert]. Maple Grove, MN: Upsher-Smith Laboratories, LLC; April2020.16.Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline.J Clin Endocrinol Metab 2017; 102:3869.17.The Endocrine Society. Testosterone therapy in Adult Men with Androgen DeficiencySyndromes. J Clin Endocrinol Metab, May 2018, 103(5):1–30.18.Mulhall JP, et al. Evaluation and Management of Testosterone Deficiency: AUA Guideline.American Urological Association Education and Research, Inc 2018.19.Xyosted [package insert]. Ewing, NJ: Antares Pharma, Inc; November 2019.20.Jatenzo [package insert]. Northbrook, IL: Clarus Therapeutics, Inc; March 2019.21.Tlando [package insert]. Ewing, NJ: Antares Pharma, Inc; March 2022.22.Kyzatrex [package insert]. Raleigh, NC: Marius Pharmaceuticals LLC; September 2022.Program Prior Authorization/Medical Necessity - TestosteroneChange ControlDate Change2/2014 Create Prior Authorization Criteria4/2014 Revised Reauthorization Criteria; formatting corrections, referencesupdated.5/2014 Revised the initial authorization criteria to include subsections for themale population and the female to male transsexual population, updatedto include language from the gender identity disorder/ gender dysphoriatreatment medical coverage determination guideline, referencesupdated7/2014 Added Natesto and Vogelxo to criteria. Changed coverage criteria fromspecific product names to topical testosterone products.10/2014 Modified criteria for total testosterone to consider reference range of thelaboratory. Added criteria for when Free Testosterone level may beutilized. Added criteria for conditions that do not require testosteronelevels. Extended initial authorization period for patients already ontherapy.12/2014 Testosterone free level units corrected.10/2015 Clarified initial authorization periods. Clarified that levels forreauthorization should be within the past 6 months for patients new totestosterone and within the past 12 months for continuing users.Updated references.5/2016 Removed age requirement from female to male transsexual coveragerequirements. Updated gender identity disorder to gender dysphoria.6/2017 Updated criteria for Gender Dysphoria. Updated reauthorizationcriteria to clarify that new to therapy refers to use of less than one yearand continuing therapy refers to use of one year or longer.6/2018 Updated required testosterone level to less than 300 ng/dL based on2018 American Urological Society treatment guidelines.2/2019 Program name change from Topical Androgens to Testosterone.Xyosted added to program.6/2019 Jatenzo added to program.7/2020 Updated initial authorization to 6 months for both new and existingusers. Added state mandate language. Updated references.8/2021 Annual review. Updated references. Removed Striant as it is no longeron the market.9/2022 Tlando added to program. Removed brand Axiron from program sinceit is no longer available. Updated to note generic Testim is typicallyexcluded. Updated references.1/2023 Kyzatrex added to program. Increased initial authorization to 12months and changed reauthorization to require a lab value within thepast 12 months.。
仑伐替尼在肝细胞癌中的耐药机制和对策研究进展

收稿日期:2023G08G21基金项目:国家自然科学基金项目(82060435)作者简介:徐永康(1996 ),男,博士研究生,主要从事肝癌的临床和基础研究.通信作者:吴建兵,主任医师,E Gm a i l :h h g w jb @163.c o m .仑伐替尼在肝细胞癌中的耐药机制和对策研究进展徐永康,吴建兵(南昌大学第二附属医院肿瘤科,南昌330006)摘要:仑伐替尼是晚期肝癌有效的一线治疗药物.然而,许多患者在仑伐替尼治疗后出现疾病进展,耐药问题在治疗实践中普遍存在,临床急需逆转耐药策略.众多研究表明表观遗传学㊁细胞转运过程㊁肿瘤微环境㊁肿瘤干性以及细胞自噬㊁铁死亡等与仑伐替尼的耐药机制密切相关.文章对肝癌仑伐替尼耐药的机制研究进展进行归纳,并初步探讨耐药后临床治疗策略,旨在为改善仑伐替尼耐药患者预后提供理论基础.关键词:肝细胞癌;仑伐替尼;耐药性;临床策略中图分类号:R 735.7㊀㊀㊀文献标志码:A㊀㊀㊀文章编号:2095G4727(2024)02-0081-07D O I :10.13764/j.c n k i .n c d m.2024.02.014R e s i s t a n c e o fL e n v a t i n i b a n d I t sU s e i n t h eT r e a t m e n to fH e pa t o c e l l u l a rC a r c i n o m a :aR e v i e w X UY o n g Gk a n g ,W UJ i a n Gb i n g(D e p a r t m e n t o f O n c o l o g y ,t h eS e c o n dA f f i l i a t e d H o s p i t a l o f N a n c h a n gU n i v e r s i t y ,N a n c h a n g 330006,C h i n a )A B S T R A C T :L e n v a t i n i b i s a n e f f e c t i v e f i r s t Gl i n e t h e r a p e u t i c a g e n t f o r a d v a n c e d h e pa t o c e l l u l a r c a r Gc i n o m a .H o w e v e r ,m a n y p a t i e n t s e x p e r i e n c e d i s e a s e p r o g r e s s i o n a f t e r l e n v a t i n ib t r e a t m e n t ,a n d t h e p r o b l e mo f d r u g r e s i s t a nc e i s p r e v a l e n t i n t h e r a p e u t i c p r a c t i c e ,u r g e n t l y r e q u i r i n g s t r a t e gi e s t o r e Gv e r s ed r u g r e s i s t a n c e .N u m e r o u ss t u d i e sh a v es h o w nt h a te p i g e n e t i c s ,c e l l t r a n s po r t p r o c e s s e s ,t u m o rm i c r o e n v i r o n m e n t ,t u m o r d r y n e s s ,a sw e l l a s a u t o p h a g y a n d f e r r o p t o s i s ,a r e c l o s e l y re l a t e d t o t h e r e s i s t a n c e m e c h a n i s m of l e n v a t i n i b .T h i sa r t i c l es u mm a r i z e s t h er e s e a r c h p r o gr e s so nt h e m e c h a n i s mo f l e n v a t i n i b r e s i s t a n c e i nh e p a t o c e l l u l a r c a r c i n o m a ,a n d p r e l i m i n a r i l y e x p l o r e s c l i n i c a l t r e a t m e n t s t r a t e g i e s t o p r o v i d e a t h e o r e t i c a l b a s i s f o r i m p r o v i n g t h e p r o g n o s i s o f l e n v a t i n i b Gr e s i s t Ga n t p a t i e n t s .K E Y W O R D S :h e p a t o c e l l u l a r c a r c i n o m a ;l e n v a t i n i b ;d r u g r e s i s t a n c e ;c l i n i c a l s t r a t e g y㊀㊀据国际癌症研究机构的估算,到2040年,全球新发肝癌病例预计将达到140万例,相较于2020年将增长55%;而全球肝癌导致的死亡人数也预计将达到130万人,较2020年增加56.4%[1].肝细胞癌(H C C )是最常见的肝癌类型,约占肝癌病例的90%[2].当前,全球和中国的肝癌防治形势十分严峻,因此提高肝癌的诊断和治疗水平对于我国的肝癌患者尤为重要.根据R E F L E C T 研究显示,与索拉非尼相比,仑伐替尼在总生存期(O S )㊁客观缓解率(O R R )(24.1%比9.2%)和无进展生存期(P F S )方面表现出非劣效(P F S7.4个月比3.7个月)[3].在晚期肝癌的系统治疗中,我国广泛使用仑伐替尼,并且取得了显著的疗效.然而,随着治疗时间的延长,18南昌大学学报(医学版)2024年第64卷第2期㊀J o u r n a l o fN a n c h a n g U n i v e r s i t y(M e d i c a l S c i e n c e s )2024,V o l .64N o .2肿瘤耐药问题不可避免,导致患者疾病进展.因此,探索仑伐替尼耐药的机制以及新的耐药途径显得尤为迫切.另外,目前临床上对于仑伐替尼耐药患者的治疗方案尚无统一标准.本文对仑伐替尼治疗H C C耐药机制以及耐药后的临床研究进展进行综述,以期为临床提高参考.1㊀表观遗传与仑伐替尼耐药㊀㊀表观遗传是一种与核苷酸序列变化无关但能够改变生物表型的重要机制.已有大量证据显示,异常的表观遗传调控可能导致肿瘤耐药的发生[4].因此,对于仑伐替尼耐药与表观遗传机制之间的关系进行深入阐述,将为未来逆转耐药策略提供理论基础.1.1㊀R N A修饰R N A修饰是表观遗传学调控中的重要组成部分,包括甲基化修饰㊁羟甲基化修饰㊁乙酰化修饰和磷酸化修饰等常见类型,近年来发现这些修饰在仑伐替尼耐药形成过程中扮演着重要角色.在仑伐替尼耐药细胞中,m6A修饰相关蛋白M E T T L3表达显著上调,进一步机制研究[5]发现,M E T T L3通过m6A修饰调节E G F R的m R N A翻译,针对M E TGT L3的特异性抑制剂S T M2457可以提高细胞对仑伐替尼的敏感性.此外,有研究[6]还发现在耐药细胞中m7G甲基转移酶复合物的关键成分M E T T L1和WD R4上调,通过介导t R N A m7G修饰和调控T R I M28表达等途径促进了对仑伐替尼的耐药性[7].另外,N A T10的上调可以通过调节H S P90A A1m R N A a c4C的修饰水平维持H S P90A A1的稳定性,促进了内质网应激H C C的转移和仑伐替尼耐药作用[8].此外,Y R D C也在调节K R A S蛋白质翻译方面发挥作用,其低t6A修饰水平的t R N A降低了K R A S的翻译,从而介导肝癌细胞对仑伐替尼的耐药[9].当前,R N A修饰蛋白成为了治疗肿瘤耐药的新靶标,针对M E T T L3的小分子抑制剂已经应用于临床研究,为提升仑伐替尼治疗反应性提供了新的选择.1.2㊀非编码R N A非编码R N A是基因表达调控的关键因素之一.微小R N A s(m i R N A s)㊁长链非编码R N A s(l nGc R N A s)和环状R N A s(c i r c R N A s)通过影响转录㊁转录后修饰和翻译等生物学过程,对癌症的发生发展和肿瘤耐药发挥着双重作用[10].1.2.1㊀m i c r o R N A在仑伐替尼耐药的人肝癌细胞株H u h7和S MM CG7721中,CGM e t呈现过度表达和活化的现象.有研究[11]表明,m i RG128G3p通过抑制cGM e t表达,调节介导凋亡途径的A k t和调节细胞周期进程的E R K参与了仑伐替尼的耐药机制.此外,在仑伐替尼耐药的肝癌细胞中,A K R1C1和m i c r o R N A 464表达水平显著上调.数据库分析提示A K R1C1和m i c r o R N A464可能存在靶向关系,而m i c r o RGN A464被认为是早期诊断仑伐替尼耐药性的一个潜在生物标志物[12].1.2.2㊀l n c R N A在仑伐替尼耐药的H C C细胞中,L n c R N A MT1J P(MT1J P)呈现上调的情况,导致了MT1J P 和抗凋亡蛋白B C L2L2的过度表达,从而降低了H C C细胞对仑伐替尼的敏感性[13].MT1J P通过吸附m i RG24G3p来靶向B C L2L2,进而促进了仑伐替尼的耐药性.此外,有研究[14]报道了l n c X I S T通过激活H C C细胞中的E Z H2GN O D2GE R K轴来促进仑伐替尼的耐药性.S O N G等[15]观察到在仑伐替尼耐药的细胞和组织中P I N K1的高表达,这有助于维持线粒体的结构和功能,并促进抗氧化应激反应,进一步的机制研究发现F G D5GA S1/m i RG5590G3p/P I N K1轴可以促进肝癌细胞对仑伐替尼的耐药性.机制研究表明L I N C01607/m i RG892b/P62促进了线粒体自噬,降低了R O S水平,从而导致肿瘤的耐药性.最后,敲除L I N C01607联合仑伐替尼能够有效地克服类器官模型中仑伐替尼的耐药性[16].1.2.3㊀c i r c R N A通过对28例接受仑伐替尼治疗后出现耐药的患者进行外周血检测,发现c i r c M E D27水平显著上升.相关研究[17]表明,c i r c M E D27作为m i RG655G3p的竞争内源性R N A发挥着重要作用,其上调促使U S P28的表达增加,在肝癌的进展和仑伐替尼耐药过程中发挥关键作用.此外,L I U等[18]首次发现c i r c K C N N2通过m i RG520cG3p/M B D2轴抑制了H C C的复发.潜在的机制可能在于c i r c K C N N2和仑伐替尼都能够抑制F G F R4的表达,从而加强对仑伐替尼的抗肿瘤效果.H A O等[19]研究发现C i rGc P A K1通过H i p p o信号通路促进了Y A P的核转运,从而促进了H C C的进展.同时,该研究发现在仑伐替尼耐药的L M3GL R和H e pG3BGL R H C C细胞中,C i r c P A K1的表达上调.外泌体中高表达的C i r c P A K1从耐药细胞转运到敏感细胞,诱导细胞对仑伐替尼的耐药性.非编码R N A作为治疗靶点在改善肿瘤耐药方面显示出极具竞争力和前景.然而,在药物的稳定性和传递效率方面仍然存在挑战.28南昌大学学报(医学版)2024年4月,第64卷第2期2㊀信号通路与仑伐替尼耐药㊀㊀作为一种多靶点酪氨酸激酶抑制剂,仑伐替尼主要作用于血管内皮生长因子(V E G F R)1G3㊁成纤维细胞生长因子受体(F G F R)1G4等靶点,从而发挥抗肿瘤的作用.近期的研究逐渐揭示,仑伐替尼治疗导致V E G F R和F G F R的抑制,进而引发E G F R/ S T A T3㊁R A S/R A F/M E K/E R K㊁P I3K/A K T等相关信号通路的反馈激活.这些信号通路的激活被认为是引发肿瘤对靶向治疗产生耐药的关键机制.因此,通过针对H C C内部相关信号通路的交叉对话进行靶向阻断,成为解决耐药问题的一种有效策略.2.1㊀E G F R介导的信号通路J I N等[20]通过C R I S P RGC a s9基因筛选,确定了E G F R作为仑伐替尼的合成致死靶点.研究机制显示,仑伐替尼治疗抑制F G F R,进而导致E G F RGP A K2GE R K5信号轴的反馈激活.另一方面,HU 等[21]的研究表明,A B C B1在E G F R激活下以脂质筏依赖的方式被激活,从而显著增强了仑伐替尼的细胞排出作用.这种激活通过激活E G F R并刺激E G F RGS T A T3GA B C B1轴,导致对仑伐替尼产生耐药性.考虑到E G F R通路在肝癌靶向治疗中的关键作用,L I M等[22]利用c t D N A分析了肝癌患者在治疗前和治疗进展时E G F R途径的遗传变化.他们发现,在仑伐替尼治疗进展期间,患者显示出E GGF R的拷贝数增加(4.8拷贝G7拷贝)和E R B B2的激活突变(S310Y).这些E G F R/E R B B2的遗传改变可能是导致仑伐替尼耐药性的遗传机制之一.2.2㊀R A S/M E K/E R K信号通路耐药细胞中V E G F R2的表达以及其下游R A S/M E K/E R K信号轴显著上调,E T SG1被确认为介导V E G F R2相关的仑伐替尼耐药的原因.此外,槐定碱被发现能够降低E T SG1的表达,从而抑制耐药H C C细胞中V E G F R2及其下游R A S/ M E K/E R K轴的表达[23].L U等[24]发现N F1和D U S P9在H C C中是仑伐替尼耐药的关键驱动因素,研究者通过R N A i敲除和C R I S P R/C a s9敲除模型进一步阐明了N F1激活P I3K/A K T和MA P K/E R K信号通路的机制.另外,D U S P9激活MA P K/E R K信号通路,导致F O X O3失活降解,最终诱导耐药发生.在仑伐替尼耐药细胞中,发现3种血管生成细胞因子(V E G F㊁P D G FGA A㊁A n g)显著上调,加速了肿瘤血管生成,增加了获得性耐药的发生,同时观察到了MA P K/M E K/E R K信号通路的激活和E MT标记物的上调[25].2.3㊀其他信号通路HO U等[26]发现,I T G B8通过H S P90介导的A K T的稳定以及A K T信号的增强来调节仑伐替尼的耐药性.通过使用A K T抑制剂MKG2206或H S P90抑制剂17GA A G,可以使耐药细胞重新对仑伐替尼治疗产生敏感性.此外,另一项研究[27]指出,T HO C2可能通过W n t/βGc a t e n i n通路参与调控肝癌对仑伐替尼的耐药性.肿瘤细胞异常信号通路的激活在获得性耐药中是常见的机制,针对这些关键靶点进行有效干预是防治耐药的有效策略.例如,已证实E G F R抑制剂㊁cGM E T抑制剂以及M E K抑制剂在一定程度上可以逆转耐药现象,有望成为临床上的新对策.3㊀肿瘤微环境与仑伐替尼耐药㊀㊀肿瘤微环境构成了一个极为复杂的生态系统,由各种细胞㊁非细胞成分和分泌产物组成,它们之间的相互作用形成了复杂的保护和修复机制,从而导致肿瘤产生耐药性.因此,深入探索新的针对微环境的靶向策略和药物,以改善耐药性并抑制肿瘤的恶性增殖显得尤为重要.作为一种抗血管生成药物,长期使用仑伐替尼可能会导致肿瘤细胞缺氧,部分细胞因此产生耐药性.在缺氧条件下,H I FG1α诱导P L C/P R F/5细胞中纤维连接蛋白的生成并削弱了仑伐替尼的作用,进而导致耐药性的产生.进一步的研究[28]表明,联合抑制纤维连接蛋白和MA P K通路可能是一种有效的治疗策略.肿瘤相关成纤维细胞(C A F)长期处于激活状态,形成致密的纤维间质包绕瘤块,通过分泌可溶性因子和直接细胞间接触等途径,对耐药性的形成起着潜在作用.研究人员发现,C A F和H C C细胞的共培养显著降低了H C C细胞对索拉非尼/仑伐替尼的体内外反应性.C A F分泌的S P P1通过P K Cα信号通路激活R A F/MA P K和P I3K/A K T/m T O R 通路,并通过E MT增强了肝癌患者对酪氨酸激酶抑制剂的抵抗.因此,治疗前血浆S P P1水平可能成为预测治疗反应的潜在生物标志物[29].4㊀细胞自噬与仑伐替尼耐药㊀㊀自噬是一种细胞死亡编程,与肿瘤的恶性演变和耐药性密切相关,过去的研究[30G31]已经表明在肝癌索拉非尼耐药中自噬发挥着关键作用,近期对仑伐替尼耐药机制的探索中也发现了类似的现象. P A N等[32]证实L A P T M5的上调会导致细胞自噬水平上升,从而降低H C C对仑伐替尼的敏感性,而38徐永康等:仑伐替尼在肝细胞癌中的耐药机制和对策研究进展通过L A P T M5的沉默或者使用H C Q来阻断内在的自噬潮,可以与仑伐替尼协同作用来抑制H C C 的生长.此外,HO A I R M1在仑伐替尼耐药细胞系中被发现是一个独立的耐药因子,与仑伐替尼治疗H C C的疗效显著相关.敲低HO T A I R M1可以增加H u h7GR和H e p G2GR细胞中m i RG34a的水平,并抑制B e c l i nG1的表达.因此,HO T A I R M1可能通过下调m i RG34a和上调B e c l i nG1来诱导自噬的激活,从而导致H C C对仑伐替尼的耐药性[33].5㊀铁死亡与仑伐替尼耐药㊀㊀铁死亡是一种铁依赖性的细胞死亡方式,诸多证据表明此过程与肿瘤治疗耐药相关联,调节铁死亡过程可能有效改善耐药性.I S E D A等[34]分析了仑伐替尼对H C C细胞的细胞毒性,并证实了仑伐替尼通过抑制F G F R4,抑制x C T表达并诱导脂质R O S积累的方式诱导H C C细胞发生铁死亡.此外,活化的N r f2抑制了仑伐替尼诱导的铁死亡,因此抑制N r f2的活化有望提高H C C细胞对仑伐替尼的敏感性,从而改善肝癌的耐药性.另一项研究[35]发现,K E A P1是驱动索拉非尼耐药的关键基因,K E A P1失活导致了K E A P1/N r f2途径的失活,通过上调N r f2下游基因和降低R O S水平,导致人类H C C细胞对索拉非尼的耐药性增加.同时,该研究还发现K E A P1的破坏抑制了仑伐替尼诱导的细胞活力下降,同时降低了对药物反应产生R O S的能力,这表明K E A P1也可能是仑伐替尼的易感基因之一.6㊀其他机制6.1㊀肿瘤细胞干性肿瘤干细胞(C S C)是一种功能细胞状态,具有自我复制和多谱系分化能力,在肿瘤药物抵抗中发挥关键作用,其耐药机制主要涉及药物转运子高表达㊁强D N A修复能力和募集保护性微环境等方面[36].WA N G等[37]研究发现F Z D10在肝C S C中高度表达,从机制上看,M E T T L3介导F Z D10m RGN A的m6A修饰,而m6A读取器Y T H D F2随后与F Z D10m R N A上的m6A位点结合,保持该m R N A 的稳定性.F Z D10通过WN T/βGc a t e n i n和H i p p o 途径增强肝脏C S C的特性,并且F Z D10/βGc a t e n i n/ cGJ u n/M E K/E R K轴促进肝癌细胞对仑伐替尼产生耐药性.采用腺相关病毒靶向F Z D10或βGc a t e n i n 抑制剂治疗仑伐替尼耐药H C C可恢复其抗肿瘤反应.另一项研究[38]表明,T M4S F1通过上调MY H9来调节N O T C H通路,进而促进肝癌中干细胞干性和仑伐替尼耐药性,且C D73通过上调S O X9的表达和增强其蛋白稳定性,在维持C S C性状方面发挥关键作用.C D73的过度表达使H C C 细胞对仑伐替尼产生显著的耐药性,同时也在维持肝癌索拉非尼和卡博替尼的耐药性中发挥作用.因此,将C D73作为靶点可能是根除C S C和逆转肝癌患者对T K I耐药性的一种有希望的策略[39].6.2㊀糖酵解H C C在缺氧和营养缺乏的环境中,通过适应性机制 W a r b u r g效应 ,优先依靠糖酵解产生能量,相关的转运蛋白㊁关键限速酶和代谢产物能够通过多种机制促使肿瘤进展和耐药.因此,探索糖酵解调控的耐药机制对于癌症治疗具有重要意义.A CGY P1在糖酵解中扮演直接调节的角色,并通过A CGY P1/H S P90/MY C/L D H A轴驱动了仑伐替尼耐药性和H C C的进展,靶向A C Y P1可以与仑伐替尼协同作用,更有效地治疗H C C[40].另外,果糖G2,6G二磷酸酶3(P F K F B3)是糖酵解的有效刺激剂, P F K F B3的表达上调可以导致H C C细胞对仑伐替尼的耐药.联合使用P F K F B3抑制剂可以在仑伐替尼耐药后抑制P F K F B3及MM P s的表达,从而逆转H C C细胞对仑伐替尼的耐药性[41].7㊀逆转耐药的机制策略和临床对策7.1㊀机制策略7.1.1㊀E G F R抑制剂J I N等[20]在体外和体内实验中,利用E G F R抑制剂吉非替尼和仑伐替尼的联合作用于表达E G F R 的肝癌细胞系㊁异种肝癌细胞系㊁免疫活性小鼠模型以及病人来源H C C异种移植肿瘤,均观察到显著的抗增殖效果.进一步的临床试验(N C T04642547)结果显示,对于12例经过仑伐替尼治疗后肿瘤仍然进展的E G F R高表达肝癌患者,采用仑伐替尼和吉非替尼联合治疗后,4例部分缓解,4例病情稳定,显示出良好的应用前景.此外, S U N等[42]的研究表明,E G F R抑制剂吉非替尼与仑伐替尼联合应用能够延缓仑伐替尼耐药细胞的增殖并诱导其凋亡,其机制可能是通过抑制E G F R介导的M E K/E R K和P I3K/A K T通路激活.在体内实验中,仑伐替尼与依拉昔达或吉非替尼联合给药抑制了肿瘤生长和血管生成.HU等[21]发现另一种E G F R抑制剂厄洛替尼可以抑制A B C B1,从而减少仑伐替尼的胞吐,在体外和体内实验中均表现出对H C C的抑制效果.7.1.2㊀M E K抑制剂L U等[24]发现一种小分子M E K途径抑制剂曲48南昌大学学报(医学版)2024年4月,第64卷第2期美替尼可逆转H C C细胞中N F1和D U S P9丢失引起的耐药性,即使在小鼠中敲除N F1,曲美替尼仍然能有效阻止H C C的增殖.此外,HU A N G等[43]发现M E K抑制剂S e l u m e t i n i b能够消除D U S P4缺乏引起的仑伐替尼耐药性,表明M E K磷酸化和D U S P4抑制依赖性E R K激活是其耐药性产生的关键.7.1.3㊀其他有研究[44]表明,双硫仑联合铜离子能够增加仑伐替尼对仑伐替尼耐药肝癌细胞H u h7的敏感性,其机制可能与抑制P I3K/A k t通路及促进c a s p a s eG9蛋白表达有关.H AMA Y A等[45]发现化疗也是改善耐药的有效方式,顺铂能够抑制仑伐替尼耐药细胞的增殖,并诱导G2/M细胞周期阻滞.此外,顺铂通过A T M/A T RGC h k1/C h k2信号通路触发D N A损伤反应.另外,Z H A O等[23]发现槐定碱可以进一步提高仑伐替尼治疗仑伐替尼耐药H C C的敏感性.7.2㊀仑伐替尼耐药临床对策仑伐替尼已被证明是晚期H C C患者的一线治疗药物,在临床实践中得到了广泛应用.然而,一旦患者出现对仑伐替尼的耐药,目前尚缺乏标准有效的二线治疗方案.当患者出现仑伐替尼耐药时,通常是各临床中心根据自身实际经验选择治疗方式.7.2.1㊀分子靶向治疗抗血管生成药物作为治疗肝癌的重要药物,在仑伐替尼耐药后仍然是临床实践中的有效手段.根据当前的指南推荐,索拉非尼㊁瑞戈非尼㊁雷莫芦单抗等均是仑伐替尼治疗耐药患者的备用方案.T OMO N A R I等[46]首次报道了13名在仑伐替尼进展后接受索拉非尼治疗的患者.根据m R EGC I S T标准,O R R和D C R分别为15.3%(2/13)和69.2%(9/13).而根据R E C I S T标准,O R R和D C R分别为0%(0/13)和69.2%(9/13),中位P F S 为4.1个月.一项系统评价[47]分析了4054名患者的20项研究,发现进展后生存期(P P S)与总生存期(O S)之间存在更强的相关性(r=0.869,P<0.001).该评价同时探讨了P P S在仑伐替尼耐药患者中的作用,25例接受二线索拉非尼治疗的患者的O R R和D C R分别为12%(3/25)和52%(13/25),P F S为5.7个月(95%C I:0.8~10.6个月).另外,一项来自韩国的研究[48]提示,续贯索拉非尼是一种潜在的治疗选择.该研究发现,索拉非尼治疗的患者的中位O S明显长于接受纳武利尤单抗治疗的患者的中位O S(8.7v s3.0个月;P=0.046).索拉非尼治疗与较低的死亡率相关(H R=0.194;95%C I:0.053~0.708;P=0.013).另外,7例H C C患者在仑伐替尼失败后接受雷莫芦单抗作为二线或三线治疗,D C R为28.6%(2/7例),中位P F S为41d,具有抑制先前接受仑伐替尼治疗的患者H C C进展以及在治疗期间维持肝功能的潜力[49].由于肝癌患者发病机制的复杂性,导致药物疗效存在差异性,因此,目前临床对于药物耐药评价以及换药指征把控存在一定差异.一些临床工作者认为,仑伐替尼的高缓解率和低毒性仍会使患者受益.一项通过倾向性匹配来自11家不同医疗机构的临床数据的研究[50]发现,相较于其他治疗(包括最佳支持治疗㊁索拉非尼㊁瑞戈非尼㊁雷莫芦单抗),继续仑伐替尼治疗的患者可以获得更好的O S(10.8/19.6v s 5.8/11.2个月,P<0.001).7.2.2㊀免疫联合靶向治疗多项免疫联合靶向治疗的优异疗效已经深刻改变了肝癌的系统治疗格局,这些相互联合的治疗方案能够增强治疗效果,其在晚期二线治疗中的应用也在逐渐增加.Z O U等[51]进行了一项回顾性调查,评估了标准剂量的仑伐替尼与P DG1抑制剂的联合治疗对于仑伐替尼治疗进展患者的有效性和安全性,他们发现O R R和D C R分别为23.9%(11/46)和71.7%(33/46),而中位P F S和O S分别为6.9个月和14.5个月.最常见的治疗相关不良事件包括厌食症(43.5%)㊁甲状腺功能减退症(43.5%)和高血压(36.9%),而3/4级不良事件的发生率为34.8%(16/46).另一项研究[52]则比较了在仑伐替尼治疗失败患者中,P DG1联合仑伐替尼和瑞戈非尼治疗的效果,结果显示联合组的中位P F S和D C R 较单药组有所提高(P F S8.7v s4.2个月,P=0.018;D C R82.7%v s53.3%,P=0.01).然而,联合组的O S并未显示出显著的获益(15.3v sN E个月,P=0.5),且O R R也未显著高于瑞戈非尼组(27.6%v s13.3%,P=0.49).此外,瑞戈非尼组和联合组的3/4级治疗相关不良反应发生率分别为26.7%和10.3%,最常见的不良反应包括丙氨酸氨基转移酶升高㊁疼痛和总胆红素升高.然而,来自广州医科大学附属第二医院朱教授团队[53]和笔者团队的研究[54]显示,瑞戈非尼联合P DG1抑制剂相较于瑞戈非尼单药治疗在索拉非尼及仑伐替尼一线治疗失败后具有更高的O R R㊁更长的P F S和更好的O S.不过,纳入一线仑伐替尼治疗失败的患者比例较低可能是导致这些研究结果不一致的原因之一.目前,文献报道索拉非尼或免疫联合靶向药58徐永康等:仑伐替尼在肝细胞癌中的耐药机制和对策研究进展物可能存在一定的获益,但缺乏充分的临床数据支持.仑伐替尼耐药后的治疗方案仍处于探索阶段,但包括C h i C T R2200062854㊁C h i C T R2000036664㊁N C T05718882㊁N C T04642547等临床试验正在进行中.期待未来会有更多的大型临床研究集中于仑伐替尼耐药患者,以及探索免疫治疗联合仑伐替尼耐药后的治疗方案选择.参考文献:[1]㊀R UM G A Y H,A R N O L D M,F E R L A YJ,e t a l.G l o b a l b u r d e n o f p r i m a r y l i v e rc a n c e r i n2020a n d p r e d i c t i o n s t o2040[J].JH e p a t o l,2022,77(6):1598G1606.[2]V O G E LA,M E Y E R T,S A P I S O C H I N G,e t a l.H e p a t o c e l l u l a rc a r c i n o m a[J].L a n c e t,2022,400(10360):1345G1362.[3]K U D O M,F I N N R S,Q I N S K,e ta l.L e n v a t i n i bv e r s u ss o rGa f e n ib i n f i r s tGl i n e t r e a t m e n t o f p a t i e n t sw i t hu n r e s ec t a b l e h e pGa t o c e l l u l a r c a r c i n o m a:a r a n d o m i s e d p h a s e3n o nGi n f e r i o r i t y t r iGa l[J].L a n c e t,2018,391(10126):1163G1173.[4]WA N G N,MA T,Y U B.T a r g e t i n g e p i g e n e t i c r e g u l a t o r s t ooGv e r c o m e d r u g r e s i s t a n c e i n c a n c e r s[J].S i g n a l T r a n s d u c t T a r g e t T h e r,2023,8(1):69.[5]WA N G L N,Y A N G Q X,Z HO U Q Y,e t a l.M E T T L3Gm6AGE GF RGa x i s d r i v e s l e n v a t i n i br e s i s t a n c e i nh e p a t o c e l l u l a r c a r c iGn o m a[J].C a n c e rL e t t,2023,559:216122.[6]HU A N G M L,L O N GJT,Y A OZ J,e t a l.M E T T L1GM e d i a t e d m7G t R N A m o d i f i c a t i o n p r o m o t e s L e n v a t i n i b r e s i s t a n c ei nh e p a t o c e l l u l a r c a r c i n o m a[J].C a n c e rR e s,2023,83(1):89G102.[7]HA N W Y,WA N GJ,Z H A OJ,e t a l.WD R4/T R I M28i s an oGv e lm o l e c u l a r t a r g e t l i n k e dt o l e n v a t i n i br e s i s t a n c e t h a th e l p s r e t a i n t h e s t e mc h a r a c t e r i s t i c s i nh e p a t o c e l l u l a r c a r c i n o m a s[J].C a n c e rL e t t,2023,568:216259.[8]P A NZP,B A O Y W,HU M Y,e t a l.R o l e o fN A T10Gm e d i a t e da c4CGm o d i f i e dH S P90A A1R N Aa c e t y l a t i o n i nE Rs t r e s sGm eGd i a te d m e t a s t a s i sa n d L e n v a t i n i br e s i s t a n c ei nh e p a t o c e l l u l a rc a r c i n o m a[J].C e l lD e a t hD i s c o v,2023,9(1):56.[9]G U OJ,Z HUP,Y EZ,e t a l.Y R D C m ed i a te s t h e r e s i s t a n c eof L e n v a t i n i b i nh e p a t o c a r c i n o m ac e l l sv i am o d u l a t i ng th e t r a n sGl a ti o no fK R A S[J].F r o n tP h a r m a c o l,2021,12:744578.[10]㊀WO N GC M,T S A N GFH C,N GIOL.N o nGc o d i n g R N A s i nh e p a t o c e l l u l a rc a r c i n o m a:m o l e c u l a r f u n c t i o n sa n d p a t h o l o g iGc a l i m p l i c a t i o n s[J].N a tR e vG a s t r o e n t e r o lH e p a t o l,2018,15(3):137G151.[11]X UX,J I A N G WJ,H A NP,e t a l.M i c r o R N AG128G3p m e d i a t e s L e n v a t i n i br e s i s t a n c e o f h e p a t o c e l l u l a rc a r c i n o m a c e l l s b yd o w n re g u l a t i n g cGM e t[J].J H e p a t o c e l lC a r c i n o m a,2022,9:113G126.[12]G A O C,C HA N G L,X U T X,e t a l.A K R1C1o v e r e x p r e s s i o n l e a d s t oL e n v a t i n i b r e s i s t a n c e i nh e p a t o c e l l u l a r c a r c i n o m a[J].JG a s t r o i n t e s tO n c o l,2023,14(3):1412G1433.[13]Y U T,Y U JJ,L U L,e ta l.M T1J PGm e d i a t e d m i RG24G3p/B C L2L2a x i s p r o m o t e s L e n v a t i n i b r e s i s t a n c e i nh e p a t o c e l l u l a rc a r c i n o m a c e l l sb y i n h i b i t i n g a p o p t o s i s[J].C e l lO n c o l(D o rGd r),2021,44(4):821G834.[14]D U A N A Q,L I H,Y U W L,e ta l.L o n g n o n c o d i n g R N A X I S T p r o m o t e s r e s i s t a n c e t oL e n v a t i n i b i nh e p a t o c e l l u l a r c a rGc i n o m a c e l l sv i ae p i g e n e t i c i n h i b i t i o no fN O D2[J].JO n c o l,2022,2022:4537343.[15]S O N G M F,MA L Y,S H E N C,e t a l.F G D5GA S1/m i RG5590G3p/P I N K1i n d u c e s L e n v a t i n i b r e s i s t a n c ei n h e p a t o c e l l u l a rc a r c i n o m a[J].C e l l S i g n a l,2023,111:110828.[16]张宇鑫.L I N C01607通过P62依赖的线粒体自噬以及P62/ N r f2依赖的抗氧化途径促进肝癌仑伐替尼耐药[D].武汉:华中科技大学,2022.[17]Z HA N G P F,S U N H X,W E N P H,e ta l.c i r c R N A c i r cGM E D27a c t s a sa p r o g n o s t i c f a c t o ra n d m e d i a t o r t o p r o m o t eL e n v a t i n i b r e s i s t a n c eo fh e p a t o c e l l u l a r c a r c i n o m a[J].M o lTGh e rN u c l e i cA c i d s,2021,27:293G303.[18]L I U D H,L I U W B,C H E N X,e t a l.c i r c K C N N2s u p p r e s s e s t h er e c u r r e n c eo fh e p a t o c e l l u l a rc a r c i n o m aa t l e a s t p a r t i a l l yv i a r e g u l a t i n g m i RG520cG3p/m e t h y lGD N AGb i n d i n g d o m a i np r o t e i n2a x i s[J].C l i nT r a n s lM e d,2022,12(1):e662.[19]H A O X P,Z H A N G Y,S H IX L,e ta l.C i r c P A K1p r o m o t e s t h e p r o g r e s s i o no f h e p a t o c e l l u l a r c a r c i n o m a v i am o d u l a t i o no fY A Pn u c l e u sl o c a l i z a t i o nb y i n t e r a c t i n g w i t h14G3G3ζ[J].JE x p C l i nC a n c e rR e s,2022,41(1):281.[20]J I N HJ,S H IYP,L V Y Y,e t a l.E G F Ra c t i v a t i o n l i m i t s t h e r e s p o n s eo f l i v e rc a n c e r t oL e n v a t i n i b[J].N a t u r e,2021,595(7869):730G734.[21]HU BY,Z O U T T,Q I N W,e t a l.I n h i b i t i o no fE G F Ro v e rGc o m e s a c q u i r e dL e n v a t i n i br e s i s t a n c ed r i ve nb y S T A T3GA BGC B1s i g n a l i n g i n h e p a t o c e l l u l a rc a r c i n o m a[J].C a n c e r R e s,2022,82(20):3845G3857.[22]L I M M,F R A N S E SJ W,I M P E R I A L R,e t a l.E G F R/E R B B2a m p l i f i c a t i o n sa n da l t e r a t i o n sa s s o c i a t e d w i t h r e s i s t a n c et oL e n v a t i n i b i nh e p a t o c e l l u l a r c a r c i n o m a[J].G a s t r o e n t e r o l o g y,2023,164(6):1006G1008.e3.[23]Z H A O Z W,Z H A N G D K,WU FZ,e ta l.S o p h o r i d i n es u pGp r e s s e sL e n v a t i n i bGr e s i s t a n th e p a t o c e l l u l a r c a r c i n o m a g r o w t hb y i n h i b i t i n g R A S/M E K/E R Ka x i sv i ad ec r e a s i n g V E G F R2e x p r e s s i o n[J].J C e l lM o lM e d,2021,25(1):549G560.[24]L U Y G,S H E N H M,HU A N G W J,e ta l.G e n o m eGs c a l eC R I S P RGC a s9k n o c k o u t s c r e e n i n g i nh e p a t o c e l l u l a r c a r c i n o m aw i t hL e n v a t i n i b r e s i s t a n c e[J].C e l lD e a t hD i s c o v,2021,7(1):359.[25]A OJ J,C H I B A T,S H I B A T AS,e t a l.A c q u i s i t i o no fm e s e nGc h y m a lGl i k e p h e n o t y p e s a n do v e r p r od u c t i o no f a n g i o ge n i cf a cGt o r s i nL e n v a t i n i bGr e s i s t a n t h e p a t o c e l l u l a r c a r c i n o m a c e l l s[J].B i o c h e m B i o p h y sR e sC o mm u n,2021,549:171G178.[26]H O U W,B R ID GE M A N B,M A L N A S S Y G,e t a l.I n t e g r i ns u bGu n i t b e t a8c o n t r i b u t e s t oL e n v a t i n i b r e s i s t a n c e i nH C C[J].H e p aGt o l C o mm u n,2022,6(7):1786G1802.[27]陈家诚,刘路政,陈良,等.肝癌细胞仑伐替尼耐药的基因筛选及其通路研究[J].肝胆胰外科杂志,2022,34(3):157G163.[28]T A K A HA S H I M,O K A D A K,O U C H R,e ta l.F i b r o n e c t i n68南昌大学学报(医学版)2024年4月,第64卷第2期p l a y s am a j o r r o l e i nh y p o x i aGi n d u c e dL e n v a t i n i b r e s i s t a n c e i nh e p a t o c e l l u l a rc a r c i n o m a P L C/P R F/5c e l l s[J].P h a r m a z i e,2021,76(12):594G601.[29]E U NJW,Y O O NJH,A H N H R,e t a l.C a n c e rGa s s o c i a t e d f iGb r o b l a s tGd e r i v e d s ec r e t ed p h o s p h o p r o te i n1c o n t r i b u t e s t o r eGs i s t a n c eo fh e p a t o c e l l u l a r c a r c i n o m a t os o r a f e n i ba n dL e n v aGt i n i b[J].C a n c e rC o mm u n(L o n d),2023,43(4):455G479.[30]L I NZY,N I U Y,WA N A,e t a l.R N A m6A m e t h y l a t i o n r e gGu l a t e s s o r a f e n i br e s i s t a n c ei nl i v e rc a n c e rt h r o u g h F O X O3Gm e d i a t e d a u t o p h a g y[J].E M B OJ,2020,39(12):e103181.[31]X U W P,L I UJP,F E N GJF,e t a l.m i RG541p o t e n t i a t e s t h e r e s p o n s e o f h u m a n h e p a t o c e l l u l a r c a r c i n o m a t o s o r a f e n i bt r e a t m e n t b y i n h i b i t i n g a u t o p h a g y[J].G u t,2020,69(7):1309G1321.[32]P A N J M,Z HA N G M,D O N G L Q,e ta l.G e n o m eGs c a l eC R I S P Rs c r e e n i d e n t i f i e sL A P TM5d r i v i n g L e n v a t i n i b r e s i s tGa n c e i nh e p a t o c e l l u l a r c a r c i n o m a[J].A u t o p h a g y,2023,19(4):1184G1198.[33]G U DY,T O N G M,WA N GJ,e t a l.O v e r e x p r e s s i o no f t h e l nGc R N A H O T A I R M1p r o m o t e s L e n v a t i n i b r e s i s t a n c e b yd o w nGr e g u l a t i n g m i RG34a a n d a c t i v a t i n g a u t o p h a g y i nh e p a t o c e l l u l a rc a r c i n o m a[J].D i s c o vO n c o l,2023,14(1):66.[34]I S E D A N,I T O HS,T O S H I D AK,e t a l.F e r r o p t o s i s i s i n d u c e db y L e n v a t i n i b t h r o u g h f i b r o b l a s t g r o w t h f ac t o r r e c e p t o rG4i nGh i b i t i o n i nh e p a t o c e l l u l a r c a r c i n o m a[J].C a n c e rS c i,2022,113(7):2272G2287.[35]Z H E N G A,C H E V A L I E R N,C A L D E R O N I M,e t a l.C R I S P R/C a s9g e n o m eGw i d e s c r e e n i n g i d e n t i f i e sK E A P1a sas o r a f e n i b,L e n v a t i n i b,a n d r e g o r a f e n i b s e n s i t i v i t yg e n e i nh e pGa t o c e l l u l a r c a r c i n o m a[J].O n c o t a r g e t,2019,10(66):7058G7070.[36]G A R C I AGMA Y E AY,M I RC,MA S S O NF,e t a l.I n s i g h t s i n t o n e w m e c h a n i s m s a n dm o d e l s o f c a n c e r s t e mc e l lm u l t i d r u g r eGs i s t a n c e[J].S e m i nC a n c e rB i o l,2020,60:166G180.[37]WA N GJH,Y U H M,D O N G W,e t a l.N6GM e t h y l a d e n o s i n eGm e d i a t e du pGr e g u l a t i o no fF Z D10r e g u l a t e s l i v e r c a n c e r s t e mc e l l s p r o p e r t i e sa n dL e n v a t i n i br e s i s t a n c e t h r o u g h WN T/βGc a t e n i na n dh i p p os i g n a l i n gp a t h w a y s[J].G a s t r o e n t e r o l o g y,2023,164(6):990G1005.[38]Y A N G SB,Z HO U Z H,L E IJ,e ta l.T M4S F1u p r e g u l a t e s MY H9t oa c t i v a t et h e N O T C H p a t h w a y t o p r o m o t ec a n c e rs t e m n e s s a n dL e n v a t i n i br e s i s t a n c e i n H C C[J].B i o lD i r e c t,2023,18(1):18.[39]MA X L,HU B,T A N G W G,e ta l.C D73s u s t a i n e dc a n c e rGs t e mGc e l l t r a i t sb yp r o m o t i n g S O X9e x p r e s s i o na n ds t a b i l i t yi nh e p a t o c e l l u l a rc a r c i n o m a[J].J H e m a t o l O n c o l,2020,13(1):11.[40]WA N GS,Z HO ULY,J IN,e t a l.T a r g e t i n g A C Y P1Gm e d i a t e dg l y c o l y s i s r e v e r s e sL e n v a t i n i b r e s i s t a n c e a n d r e s t r i c t s h e p a t oGc e l l u l a r c a r c i n o m a p r o g r e s s i o n[J].D r u g R e s i s tU pd a t,2023,69:100976.[41]李秋婷.P F K F B3促进肝癌增殖侵袭的机制及其对仑伐替尼耐药性的影响[D].武汉:华中科技大学,2021.[42]S U N D W,L I U J,WA N G Y F,e ta l.C oGa d m i n i s t r a t i o no f M D R1a n dB C R Po rE G F R/P I3Ki n h i b i t o r so v e r c o m e sL e nGv a t i n i b r e s i s t a n c e i nh e p a t o c e l l u l a rc a r c i n o m a[J].F r o n tO nGc o l,2022,12:944537.[43]HU A N G S Z,MA Z Y,Z H O U Q,e ta l.G e n o m eGw i d eC R I S P R/C a s9l i b r a r y s c r e e n i n g i d e n t i f i e dt h a tD U S P4d e f iGc i e n c y i nd u ce sL e n v a t i n i b r e s i s t a n c e i nh e p a t o c e l l u l a r c a r c i n oGm a[J].I n t JB i o l S c i,2022,18(11):4357G4371.[44]董博文,李子一,李伟东,等.双硫仑联合铜离子对乐伐替尼耐药肝癌细胞H u h7增殖及凋亡的影响[J].中国普外基础与临床杂志,2020,27(2):168G172.[45]HAMA Y AS,F U J I HA R AS,I WAMA H,e t a l.C h a r a c t e r i z aGt i o no f c i s p l a t i ne f f e c t s i nL e n v a t i n i bGr e s i s t a n th e p a t o c e l l u l a rc a r c i n o m a c e l l s[J].A n t i c a n c e rR e s,2022,42(3):1263G1275.[46]T OMO N A R IT,S A T O Y,T A N A K A H,e ta l.S o r a f e n i ba s s e c o n dGl i n e t r e a t m e n t o p t i o n a f t e r f a i l u r e o f L e n v a t i n i b i n p aGt i e n t sw i t h u n r e s e c t a b l e h e p a t o c e l l u l a rc a r c i n o m a[J].J G HO p e n,2020,4(6):1135G1139.[47]T A J I R IK,T O K I M I T S U Y,K AWA IK,e t a l.I m p a c t o f p o s tGp r o g r e s s i o ns u r v i v a l o no u t c o m e s o fL e n v a t i n i b t r e a t m e n t f o ru n r e s e c t a b l eh e p a t o c e l l u l a rc a r c i n o m a:a s y s t e m a t i cr e v i e wa n d r e t r o s p e c t i v ec o h o r ts t u d y[J].A n t i c a n c e rR e s,2022,42(12):6007G6018.[48]K I M Y,L E EJ S,L E EH W,e t a l.S o r a f e n i b v e r s u s n i v o l u m a ba f t e rL e n v a t i n i bt r e a t m e n t f a i l u r e i n p a t i e n t sw i t ha d v a n c e dh e p a t o c e l l u l a rc a r c i n o m a[J].E u rJ G a s t r o e n t e r o l H e p a t o l,2023,35(2):191G197.[49]K A S U Y A K,K AWAMU R A Y,K O B A Y A S H IM,e t a l.E f f iGc a c y a nd s a fe t y of r a m u c i r u m a b i n p a t i e n t sw i t hu n r e s e c t a b l eh e p a t o c e l l u l a rc a r c i n o m a w i t h p r o g r e s s i o n a f t e rt r e a t m e n tw i t hL e n v a t i n i b[J].I n t e r n M e d,2021,60(3):345G351.[50]H I R A O K A A,K UMA D A T,T A D A T,e ta l.W h a tc a nb ed o ne t os o l v e t h eu n m e t c l i n i c a l n e e dof h e p a t o c e l l u l a r c a r c iGn o m a p a t i e n t s f o l l o w i n g L e n v a t i n i b f a i l u r e?[J].L i v e rC a n cGe r,2021,10(2):115G125.[51]Z O UJX,HU A N GPX,G E N L,e t a l.A n t iGP DG1a n t i b o d i e s p l u s l e n v a t i n i bi n p a t i e n t s w i t h u n r e s e c t a b l eh e p a t o c e l l u l a rc a r c i n o m aw h o p r o g r e s s e do nL e n v a t i n i b:ar e t r o s p e c t i v ec oGh o r t s t u d y o fr e a lGw o r l d p a t i e n t s[J].J G a s t r o i n t e s t O n c o l,2022,13(4):1898G1906.[52]G U A NRG,M E I J,L I SH,e t a l.C o m p a r a t i v e e f f i c a c y o f P DG1i n h i b i t o r s p l u sL e n v a t i n i ba n dr e g o r a f e n i ba f t e rL e n v a t i n i bf a i l u r ef o ra d v a n c e d h e p a t o c e l l u l a rc a r c i n o m a:ar e a lGw o r l ds t u d y[J].H e p a t o l I n t,2023,17(3):765G769.[53]HU A N G JJ,G U O Y J,HU A N G W S,e ta l.R e g o r a f e n i bc o m b i n ed w i t h P DG1b l o c k a d ei mm u n o t he r a p y v e r s u sr e g oGr a f e n i ba ss e c o n dGl i n et r e a t m e n t f o ra d v a n c e dh e p a t o c e l l u l a rc a r c i n o m a:am u l t i c e n t e r r e t r o s p e c t i v e s t ud y[J].JHe p a t o c e l lC a r c i n o m a,2022,9:157G170.[54]B A R B I E R I I,K O U Z A R I D E S T.R o l eo fR N A m o d i f i c a t i o n si n c a n c e r[J].N a tR e vC a n c e r,2020,20(6):303G322.(责任编辑:李松旻)78徐永康等:仑伐替尼在肝细胞癌中的耐药机制和对策研究进展。
恩格列净联合西格列汀治疗老年2_型糖尿病患者的临床疗效分析

·药物与临床·糖尿病新世界 2023年3月DOI:10.16658/ki.1672-4062.2023.05.059恩格列净联合西格列汀治疗老年2型糖尿病患者的临床疗效分析臧道军,龚红燕江苏省常州市德安医院老年内科,江苏常州213000[摘要]目的探讨老年2型糖尿病患者使用恩格列净+西格列汀治疗的临床效果。
方法选取2020年1月—2021年12月常州市德安医院接诊的100例老年2型糖尿病患者作为研究对象,根据不同用药方式分为对照组与研究组,各50例,对照组接受西格列汀治疗,研究组接受恩格列净+西格列汀治疗,就两组患者血糖指标、炎性指标、胱抑素C(Cys-C)、血尿素氮(BUN)、血同型半胱氨酸(Hcy)指标进行比较。
结果治疗前两组血糖指标相比,差异无统计学意义(P>0.05),治疗后,研究组HbA1c、FPG及2 hPG明显低于对照组,差异有统计学意义(P<0.05);治疗前两组炎性指标比较,差异无统计学意义(P>0.05),治疗后,研究组IL-4、IL-6及TNF-α明显低于对照组,差异有统计学意义(P<0.05);治疗前两组Cys-C、BUN及Hcy相比,差异无统计学意义(P>0.05),治疗后,研究组患者Cys-C、BUN及Hcy明显低于对照组,差异有统计学意义(P<0.05)。
结论对于老年2型糖尿病患者开展恩格列净+西格列汀治疗能有效改善血糖指标,降低Hcy,提升肾功能,治疗效果显著。
[关键词] 老年人群;恩格列净;西格列汀;2型糖尿病[中图分类号] R4 [文献标识码] A [文章编号] 1672-4062(2023)03(a)-0059-04Clinical Efficacy Analysis of Empagliflozin Combined with Sitagliptin in the Treatment of Elderly Patients with Type 2 Diabetes MellitusZANG Daojun, GONG HongyanDepartment of Geriatric Medicine, Changzhou De'an Hospital, Changzhou, Jiangsu Province, 213000 China[Abstract] Objective To investigate the clinical effect of treatment with empagliflozin + sitagliptin in elderly patients with type 2 diabetes mellitus.Methods A total of 100 elderly patients with type 2 diabetes mellitus admitted to Chang⁃zhou De'an Hospital from January 2020 to December 2021 were selected as study subjects. The cases were divided into control group and study group according to different medication administration, fifty cases in each. The control group received sitagliptin treatment and the study group received empagliflozin + sitagliptin treatment. The blood glu⁃cose index, inflammatory index, cystatin C (Cys-C), blood urea nitrogen (BUN), and blood homocysteine (Hcy) index were compared between the two groups.Results There was no statistically significant difference in blood glucose in⁃dexes between the two groups before treatment (P>0.05). After treatment, HbA1c, FPG and 2 hPG of the study group were significantly lower than those in the control group, the difference was statistically significant (P<0.05). There was no statistically significant difference in inflammatory indexes between the two groups before treatment (P>0.05). After treatment, IL-4, IL-6 and TNF-α in the study group were significantly lower than those in the control group, the dif⁃ference was statistically significant (P<0.05). There was no statistically significant difference in the Cys-C, BUN and Hcy between the two groups before treatment (P>0.05). After treatment, the Cys-C, BUN and Hcy of the study group were significantly lower than those in the control group, the difference was statistically significant (P<0.05).Conclusion For elderly patients with type 2 diabetes mellitus, treatment with empagliflozin + sitagliptin can effec⁃tively improve blood glucose index, reduce Hcy and enhance renal function, with significant therapeutic effects.[作者简介]臧道军(1974-),男,本科,副主任医师,研究方向为老年内科。
Rat IgG ELISA Kit, Fluorescent (产品型号ab229388)说明书
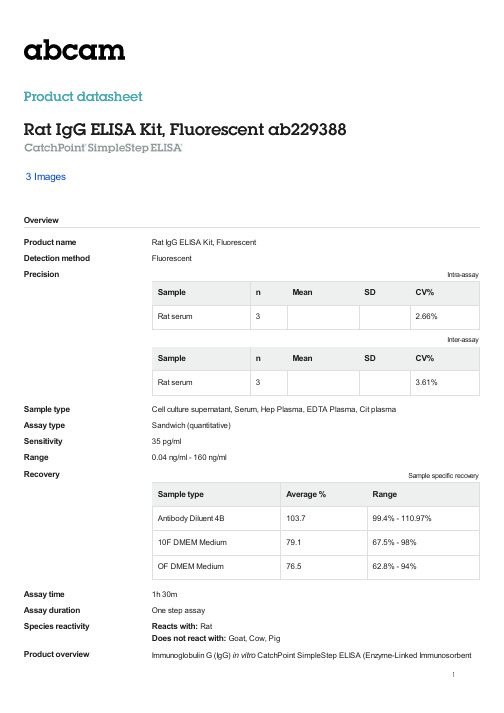
Product datasheetRat IgG ELISA Kit, Fluorescent ab2293883 ImagesOverviewProduct name Rat IgG ELISA Kit, FluorescentDetection method FluorescentPrecision Intra-assaySample n Mean SD CV%Rat serum3 2.66%Inter-assaySample n Mean SD CV%Rat serum3 3.61%Sample type Cell culture supernatant, Serum, Hep Plasma, EDTA Plasma, Cit plasmaAssay type Sandwich (quantitative)Sensitivity35 pg/mlRange0.04 ng/ml - 160 ng/mlRecovery Sample specific recov erySample type Average %RangeAntibody Diluent 4B103.799.4% - 110.97%10F DMEM Medium79.167.5% - 98%OF DMEM Medium76.562.8% - 94%Assay time1h 30mAssay duration One step assaySpecies reactivity Reacts with: RatDoes not react with: Goat, Cow, PigProduct overview Immunoglobulin G (IgG) in vitro CatchPoint SimpleStep ELISA (Enzyme-Linked ImmunosorbentAssay) kit is designed for the quantitative measurement of Immunoglobulin G (IgG) protein in ratserum, plasma, and cell culture supernatant.This CatchPoint SimpleStep ELISA kit has been optimized for Molecular Devices MicroplateReaders. Click here for a list of recommended Microplate Readers.If using a Molecular Devices’ plate reader supported by SoftMax® Pro software, a preconfiguredprotocol for these CatchPoint SimpleStep ELISA Kits is available with all the protocol andanalysis settings at .The CatchPoint SimpleStep ELISA employs an affinity tag labeled capture antibody and areporter conjugated detector antibody which immunocapture the sample analyte in solution. Thisentire complex (capture antibody/analyte/detector antibody) is in turn immobilized viaimmunoaffinity of an anti-tag antibody coating the well. To perform the assay, samples orstandards are added to the wells, followed by the antibody mix. After incubation, the wells arewashed to remove unbound material. CatchPoint HRP Development Solution containing theStoplight Red Substrate is added. During incubation, the substrate is catalyzed by HRPgenerating a fluorescent product. Signal is generated proportionally to the amount of boundanalyte and the intensity is measured in a fluorescence plater reader at 530/570/590 nmExcitation/Cutoff/Emission.Notes There are four classes of immunoglobulins in rat: IgA, IgE, IgM, and IgG. IgG is the most abundantimmunoglobulin and is equally distributed in blood and tissue. In rat, the IgG class is furtherdivided into four subclasses: IgG1, IgG2a, IgG2b, and IgG2c. The general immunoglobulinstructure is composed of four polypeptide chains, two heavy and two light chains linked togetherand to each other by disulfide bonds, creating a tetrameric quaternary structure. The resultingtetramer creates two identical halves which together form a Y like structure. While the amino-terminal portions that exhibits highly variable amino-acid composition are involved in antigenbinding, the C terminal constant parts are involved in complement binding, placental passage andbinding to cell membrane.IgG is involved in response to a foreign antigen and the presence of IgG usually signifies a matureantibody response. IgG has a molecular weight of about 150 kDa, can bind to many pathogensand also plays an important role in antibody dependent cell-mediated cytotoxicity. Typically ratserum and plasma samples contain about 7 to 10 mg/ml of IgG.Platform Pre-coated microplate (12 x 8 well strips)PropertiesStorage instructions Store at +4°C. Please refer to protocols.Components 1 x 96 tests100X Stoplight Red Substrate 1 x 120µl10X Rat IgG Capture Antibody 1 x 600µl10X Rat IgG Detector Antibody 1 x 600µl10X Wash Buffer PT (ab206977) 1 x 20ml500X Hydrogen Peroxide (H2O2, 3%) 1 x 50µlCellular localization SecretedOther - Rat IgG ELISA Kit, Fluorescent (ab229388)SimpleStep ELISA technology allows the formation of the antibody-antigen complex in one single step, reducing assay time to 90 minutes. Add samples or standards and antibody mix to wells all at once, incubate, wash, and add your final substrate. See protocol for a detailed step-by-step guide.Example of rat Immunoglobulin G (IgG) standard curv e in Sample Diluent NS.The Immunoglobulin G (IgG) standard curve was prepared as described in Section 10. Background-subtracted data values (mean +/- SD) are graphed.Antibody Diluent 4B 1 x 6ml Plate Seals 1 unitRat IgG Lyophilized Purified Protein 2 vials Sample Diluent NS (ab193972) 1 x 50ml SimpleStep Pre-Coated Black 96-Well Microplate 1 unit Stoplight Red Substrate Buffer 1 x 12ml Components 1 x 96 tests ImagesInterpolated rat IgG value are graphed.Example of rat serum IgG lev el in Sample DiluentNS.Please note: A ll products are "FOR RESEA RCH USE ONLY. NOT FOR USE IN DIA GNOSTIC PROCEDURES"Our Abpromise to you: Quality guaranteed and expert technical supportReplacement or refund for products not performing as stated on the datasheetValid for 12 months from date of deliveryResponse to your inquiry within 24 hoursWe provide support in Chinese, English, French, German, Japanese and SpanishExtensive multi-media technical resources to help youWe investigate all quality concerns to ensure our products perform to the highest standardsIf the product does not perform as described on this datasheet, we will offer a refund or replacement. For full details of the Abpromise, please visit https:///abpromise or contact our technical team.Terms and conditionsGuarantee only valid for products bought direct from Abcam or one of our authorized distributors。
GLP Bio产品说明书:Nystatin (Fungicidin) (GC10090)

Product Data Sheet Product Name:Nystatin (Fungicidin)Cat. No.:GC10090Chemical PropertiesCas No.1400-61-9化学名(4E,6E,8E,10E,14E,16E,18S,19R,20R,21S,35S)-3-[(2S,3S,4S,5S,6R)-4-amino-3,5-dihydroxy-6-methyloxan-2-yl]oxy-19,25,27,29,32,33,35,37-octahydroxy-18,20,21-trimethyl-23-oxo-22,39-dioxabicyclo[33.3.1]nonatriaconta-4,6,8,10,14,16-hexaene-38-carboxylic acidCanonical SMILES CC1C=CC=CCCC=CC=CC=CC=CC(CC2C(C(CC(O2)(CC(C(CCC(CC(CC(CC(=O)OC(C(C1O)C)C)O)O)O)O )O)O)O)C(=O)O)OC3C(C(C(C(O3)C)O)N)O分子式C47H75NO17分子量926.09溶解度≥ 30.45 mg/mL in DMSO储存条件-20°C, sealed storage, away from moisture and light,unstable in solution, ready to use.General tips For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months.Shipping Condition Evaluation sample solution : ship with blue ice All other available size: ship with RT , or blue ice upon request.StructureProtocolCell experiment [1]:Cell lines Oral Candida species and human buccal epithelial cellsPreparation method The solubility of this compound in DMSO is > 30.5 mg/mL. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below - 20 °C for several months.Reacting condition 1 hrApplications The minimal inhibitory concentrations (μg/mL) of Nystatin for C. albicans, C. tropicalis, C. krusei, C. parapsilosis, C. glabrata and C. guilliermondii in RPMI broth were 0.78 ~ 1.56, 1.56 ~ 3.12, 3.12, 1.56 ~ 3.12, 0.78 ~ 1.56 and 0.39 ~ 0.78, respectively. Compared with the control group, Nystatin significantly reduced adhesion of 6 Candida species to buccal epithelial cells. However, the adhesion of C. albicans isolates was least affected by Nystatin treatment, which was significantly different from that of the non-albicans species.Animal experiment [2]:Animal models Aspergillus-infected, neutropenic mice Dosage form2, 4, 6 and 8 mg/kg/day; i.v.Product Data SheetApplications At a dose as low as 2 mg/kg/day, Liposomal Nystatin significantly protected neutropenic mice from Aspergillus-induced death compared to either the no-treatment, the saline or the empty-liposome group. Liposomal Nystatin-treated mice showed no evidence of Aspergillusinfection either at day 5 in all of the treatment groups or at day 52 in the 8 mg/kg/dayliposomal-Nystatin treatment group.Other notesPlease test the solubility of all compounds indoor, and the actual solubility may slightly differwith the theoretical value. This is caused by an experimental system error and it is normal.References:[1]. Ellepola AN, Panagoda GJ, Samaranayake LP. Adhesion of oral Candida species to human buccal epithelial cells following brief exposure to nystatin. Oral Microbiol Immunol. 1999 Dec;14(6):358-63.[2]. Wallace TL, Paetznick V, Cossum PA, Lopez-Berestein G, Rex JH, Anaissie E. Activity of liposomal nystatin against disseminated Aspergillus fumigatus infection in neutropenic mice. Antimicrob Agents Chemother. 1997Oct;41(10):2238-43.BackgroundNystatin (Fungicidin) is a polyene antifungal antibiotic [1].Antifungal antibiotic is a pharmaceutical fungicide used to treat and prevent mycoses.Nystatin is a polyene antifungal antibiotic that is effective against yeast and mycoplasma [1]. In liquid media,Nystatin inhibited C. albicans at concentrations of 5-20 U/ml[2].In a 200 clinical isolates, which comprised of 113 Candida albicans, 54 Candida glabrata, 11 Candida parapsilosis, 11Candida tropicalis and 11 Candida krusei. Nystatin exhibited MIC90 value of 4 mg/L against C. albicans isolates and all non-albicans Candida species tested. The results confirmed C. Albicans was most frequently susceptible andNystatin could be used to treat vulvovaginal candidiasis caused by non-albicans Candida species. Nystatin would be an important choice for women affected by non-albicans Candida species which present higher resistance to the imidazole-based treatments [3].制霉菌素(Fungicidin )是一种多烯类抗真菌抗生素[1]。
孕晚期B族链球菌阴道直肠定植产妇分娩后新生儿的早期干预效果分析

论著China &Foreign Medical Treatment 中外医疗孕晚期B 族链球菌阴道直肠定植产妇分娩后新生儿的早期干预效果分析李小艳,杨晓明徐州仁慈医院产科,江苏徐州 221000[摘要] 目的 探讨孕晚期B 族链球菌(group B streptococcus, GBS )阴道直肠定植产妇分娩后新生儿早期干预效果分析。
方法 随机选取2021年4月—2023年4月徐州仁慈医院妇产科孕龄>32周的100例GBS 筛检阳性孕妇分娩后的新生儿,以随机数表法分为研究组与对照组,研究组新生儿娩出后立即予以阿莫西林颗粒口服,对照组未进行早期干预。
比较两组血细胞数、CRP 和PCT 水平,以及新生儿GBS 感染情况。
结果 出生当日,两组PCT 水平比较,差异有统计学意义(P <0.05);两组血细胞数、CRP 水平比较,差异无统计学意义(P >0.05)。
出生次日和第7日,研究组血细胞数、CRP 和PCT 水平低于对照组,差异有统计学意义(P <0.05)。
共检出20例GBS 感染,研究组早发性GBS 疾病2例,无迟发性GBS 疾病,对照组早发性GBS 疾病11例,迟发性GBS 疾病7例。
研究组GBS 感染发生率低于对照组,差异有统计学意义(P <0.05)。
结论 适当的早期干预可显著降低新生儿GBS 感染。
[关键词] B 族链球菌;感染;干预;效果分析[中图分类号] R722 [文献标识码] A [文章编号] 1674-0742(2023)07(a)-0037-04Analysis of Early Intervention Effect of Neonates in Women with Group B Streptococcus Vaginorectal Colonization in Late Pregnancy after DeliveryLI Xiaoyan, YANG XiaomingDepartment of Obstetrics, Xuzhou Renci Hospital, Xuzhou, Jiangsu Province, 221000 China[Abstract] Objective To explore the effectiveness of early intervention for newborns after vaginal rectal colonization of group B streptococcus (GBS) in late pregnancy. Methods From April 2021 to April 2023, 100 newborns of GBS screening positive pregnant women with gestational age>32 weeks in the obstetrics and gynecology department of Xu⁃zhou Renci Hospital were randomly selected and divided into the study group and the control group by random num⁃ber table. The study group newborns were given Amoxicillin granules orally immediately after delivery, while the con⁃trol group did not receive early intervention. Compared the blood cell count, CRP and PCT levels, as well as the inci⁃dence of neonatal GBS infection between the two groups. Results On the day of birth, there was a statistically signifi⁃cant difference in PCT levels between the two groups (P <0.05); there was no statistically significant difference inblood cell count and CRP levels between the two groups (P >0.05). On the day after birth and the 7th day, the blood cell count, CRP, and PCT levels in the study group were lower than those in the control group, and the difference was statistically significant (P <0.05). A total of 20 cases of GBS infection were detected, with 2 cases of early onset GBS disease and no late onset GBS disease in the study group. There were 11 cases of early onset GBS disease and 7 casesof late onset GBS disease in the control group. The incidence of GBS infection in the study group was lower than that in the control group, and the difference was statistically significant (P <0.05). Conclusion Appropriate early interven⁃tion can significantly reduce GBS infection in newborns.DOI :10.16662/ki.1674-0742.2023.19.037[基金项目] 徐州仁慈医院集团科技创新课题非限制类技术(XZRCKY-KT-202204007)。
USP色谱柱编号对应表
钙型磺化交联苯乙烯-二乙烯基苯共聚物,强阳离子交换柱
L20
二羟基丙烷基化学键合多孔硅胶微ห้องสมุดไป่ตู้固定相(Diol)
二醇基柱
L21
刚性苯乙烯-二乙烯基苯共聚物微球填料柱
L22
带有磺酸基团的多孔苯乙烯阳离子交换柱
L23
带有季胺基团的聚甲基丙烯酸甲酯或聚丙烯酸酯多孔离子交换柱
L24
表面含有大量羟基的半刚性聚乙烯醇亲水凝胶柱
L29
氧化铝,反相键合,含碳量低,氧化铝基聚丁二稀小球,5μm,孔径80Å
L30
全多孔硅胶键合乙基硅烷固定相
L31
季胺基改性孔径2000Å的交联苯乙烯和二乙烯基苯(55%)强阴离子交换树脂
L32
L-脯氨酸铜配合物共价键合于不规则形硅胶微粒的配位体的交换手性色谱填料
L33
能够分离分子量4000~40000范围蛋白质分子的球形硅胶固定相, pH稳定性好
美国药典(USP)规定的色谱柱编号对应表
USP编号
填料/说明
简称
L1
十八烷基键合多孔硅胶或无机氧化物微粒固定相
ODS柱
L2
30~50μm表面多孔薄壳型键合十八烷基固定相
C18柱
L3
多孔硅胶微粒,即一般的硅胶柱
L4
30~50μm表面多孔薄壳型硅胶柱
L5
30~50μm表面多孔薄壳型氧化铝柱
L6
30~50μm实心微球表面包覆磺化碳氟聚合物,强阳离子交换柱
L34
铅型磺化交联苯乙烯-二乙烯基苯共聚物强阳离子交换树脂,9mm球形
L35
锆稳定的硅胶微球键合二醇基亲水分子单层固定相,孔径150Å
L36
5mm胺丙基硅胶键合L-苯基氨基乙酸-3,5二硝基苯甲酰
SensiMix

Shipping: On Dry/Blue Ice Catalog Numbers Batch No.: See vial Concentration: See vial QT605-05: 500 x 50 μL reactions: 10 x 1.25 mL Storage and Stability:SensiMix SYBR ®Hi --20 °C upon receipt. Excessive freeze/thawing is not recommended. Expiry:When stored under the recommended conditions and handled correctly, full activity of the kit is retained until the expiry date on the outer box label.Quality Control:SensiMix SYBR ®Hi -ROX Kit and its components are extensively tested for activity,processivity, efficiency, heat activation, sensitivity, absence of nuclease contamination andabsence of nucleic acid contamination prior to release.Safety Precautions:Please refer to the material safety data sheet for further information.Notes:For research or further manufacturing use only. Trademarks:SensiMix, SensiFAST (Bioline Reagents Ltd), SYBR (Molecular Probes), ROX, LightCycler (Roche), StepOne (ABI), RotorGene (Qiagen), LightCycler (Roche).Kit componentsStore at –20 °CThe SensiMix SYBR ® Hi -ROX Kit has been optimized for use in SYBR ® Green -based qPCR on the real -time instruments listed in the following compatibility table, each of these instruments having the capacity to analyze the qPCR data with the passive reference signal either on or off. The kit is also compatible with several instruments that do not require the use of ROX, such as the BMS Mic, Qiagen Rotor -Gene ™ 6000, Bio -Rad CFX96 or Roche LightCycler ® 480.DescriptionThe SensiMix ™ SYBR ® Hi -ROX Kit is a high -performance reagent designed for superior sensitivity and specificity on various real -time instruments. The SensiMix SYBR ® Hi -ROX Kit employs a hot -start DNA polymerase, for high PCR specificity and sensitivity. SensiMix SYBR ® Hi -ROX is inactivated and possesses no polymerase activity during the reaction set -up, preventing non -specific amplification including primer -dimer formation.For ease -of -use and added convenience, SensiMix SYBR ® Hi -ROX is provided as a 2x master mix containing all the components necessary for real -time PCR (qPCR), including the SYBR ® Green I dye, dNTPs, stabilizers and ROX for optional use. As a ready -to -use premix, only primers and template need to be added. Kit compatibilityPrimers: the sequence and concentration of primer as well as the amplicon length can be critical for specific amplification, yield and overall efficiency of any qPCR. We strongly recommend taking the following into consideration when designing and running your PCR reaction:• use primer -design software, such as Primer3 or visual OMP TM (/primer3/ and DNA Software, Inc ; / respectively). Primers should have a melting temperature (Tm) of approximately 60 °C• optimal amplicon length should be 50-150 bp• a final primer concentration of 250 nM is suitable for most PCR conditions, however to determine the optimal concentration we recommend a primer titration in the range of 0.1–1 μM• use equimolar primer concentrations• when amplifying from cDNA use gene -specific primers. If possible use intron -spanning primers to avoid amplification from genomic DNATemplate: it is important that the DNA template is suitable for use in PCR in terms of purity and concentration. Also, the template needs to be devoid of any contaminating PCR inhibitors (e.g. EDTA). The recommended amount of template for PCR is dependent upon the type of DNA used. The following should be considered when using genomic DNA and cDNA templates:• Genomic DNA: use up to 1 μg of complex (e.g. eukaryotic) genomic DNA in a single PCR. We recommend using the ISOLATE Genomic II DNA Mini Kit (BIO -52066) for high yield and purity from both prokaryotic and eukaryotic sources• cDNA: the optimal amount of cDNA to use in a single PCR is dependent upon the copy number of the target gene. We suggest using 100 ng cDNA per reaction, however it may be necessary to vary this amount. To perform a two -step RT -PCR, we recommend using the SensiFAST cDNA Synthesis Kit (BIO -65053) for reverse transcription of the purified RNA. For high yield and purity of RNA, use the ISOLATE II RNA Mini Kit (BIO -52072)General considerationsTo help prevent any carry -over DNA contamination we recommend that separate areas be maintained for PCR set -up, PCR amplification and any post -PCR gel analysis. It is essential that any amplified PCR product should not be opened in the PCR set -up area.Website:/sensimixemail:****************************PI -50231 V11__________________________________________________________________________________________________________________________LICENSING INFORMATION2) Purchase of this product conveys a licence from Life Technologies to use this SYBR ® containing reagent in an end -user RUO assay. Parties wishing to incorporate this SYBR ® containing reagent into a downstream kit, should contact Life Technologies for SYBR ® Licencing information.Website:/sensimixemail:****************************Associated ProductsBioline Reagents Ltd UNITED KINGDOMTel: +44 (0)20 8830 5300 Fax: +44 (0)20 8452 2822Meridian Life Science Inc. USATel: +1 901 382 8716 Fax: +1 901.382.0027Bioline GmbH GERMANYTel: +49(0)3371 60222 00 Fax: +49(0)3371 60222 01Bioline (Aust) Pty. Ltd AUSTRALIATel: +61 (0)2 9209 4180 Fax: +61 (0)2 9209 4763Technical SupportIf the troubleshooting guide does not solve the difficulty you are experiencing, please contact Technical Support with details of reaction setup, cycling conditions and relevant data. Email: ********************************MgCl 2: The MgCl 2 concentration in the 1x reaction mix is 3 mM. In the majority of qPCR conditions this is optimal for both the reverse transcriptase and the hot -start DNA polymerase. If necessary, we suggest titrating the MgCl 2 to a maximum of 5mM.PCR controls: It is important to detect the presence of contaminating DNA that may affect the reliability of the data. Always include a no template control (NTC), replacing the template with PCR -grade water. When performing a two -step RT -qPCR, set -up a no RT control as the NTC for the PCR.ProcedureReaction mix composition: Prepare a PCR master mix. The volumes given below are based on a standard 50 μL final reaction mix and can be scaled accordingly.Optional ROX: The SensiMix ™SYBR Hi -ROX Kit is premixed with ROX (5-carboxy -X -rhodamine, succinymidyl ester), so that where necessary, ROX fluorescence can be optionally detected on certain real -time instruments. If your real -time instrument has the capability of using ROX and you wish to use this option, then this option must be selected by the user in the software.Troubleshooting GuideSuggested thermal cycling conditionsThe PCR conditions described below are suitable for the SensiMix ™ SYBR ® Hi -ROX Kit for the majority of amplicons and real -time PCR instruments. However, the cycling conditions can be varied to suit customer or machine -specific protocols. The critical step of the PCR is the 10 minute initial activation at 95 °C. The detection channel on the real -time instrument should be set to (SYBR ®) Green or FAM.*Non -variable parameterOptional analysis:After the reaction has reached completion refer to the instrument instructions for the option of melt -profile analysis.Website:/sensimixemail:****************************Website:/sensimixemail:****************************Troubleshooting Guide (Continued)。
3种含铋剂四联方案根除幽门螺杆菌的临床疗效研究

3种含铋剂四联方案根除幽门螺杆菌的临床疗效研究潘萍,杨芸,张立江苏大学附属武进医院药学部,江苏常州213002[摘要]目的比较3种含铋剂四联疗法治疗幽门螺杆菌的临床治疗效果,为选择理想的初次根治方案提供依据。
方法回顾性分析2022年1—9月江苏大学附属武进医院收治的96例幽门螺杆菌阳性患者的临床资料,根据不同治疗方案分为3组,A组(35例):阿莫西林胶囊+克拉霉素缓释片+雷贝拉唑肠溶胶囊+枸橼酸铋钾胶囊;B组(32例):阿莫西林胶囊+左氧氟沙星片+雷贝拉唑肠溶胶囊+枸橼酸铋钾胶囊;C组(29例):克拉霉素缓释片+左氧氟沙星片+雷贝拉唑肠溶胶囊+枸橼酸铋钾胶囊。
比较各组方案的临床疗效和不良反应。
结果A、B、C 组幽门螺杆菌根除率分别为85.71%、81.25%、75.86%,差异无统计学意义(χ2= 1.010,P=0.609)。
3组方案药物不良反应发生率分别为5.71%、6.25%、10.34%,差异无统计学意义(χ2=0.694,P=0.789)。
结论阿莫西林联合克拉霉素方案Hp根除率相对较高、安全性较好,可作为初次根治治疗的首选方案;克拉霉素联合左氧氟沙星方案Hp根除率低,不宜选用。
[关键词]幽门螺杆菌;四联疗法;铋剂;疗效[中图分类号]R4 [文献标识码]A [文章编号]2096-1782(2023)09(b)-0183-04Clinical Efficacy Study of Three Bismuth-containing Quadruple Regimens for the Eradication of Helicobacter pyloriPAN Ping, YANG Yun, ZHANG LiDepartment of Pharmacy, Wujin Hospital Affiliated to Jiangsu University, Changzhou, Jiangsu Province, 213002 China [Abstract] Objective To compare the clinical therapeutic effects of three bismuth-containing quadruple regimens for the treatment of Helicobacter pylori, and to provide a basis for selecting an ideal initial eradication program. Methods The clinical data of 96 Helicobacter pylori positive patients admitted to Wujin Hospital Affiliated to Jiangsu Univer⁃sity from January to September 2022 were retrospectively analyzed and divided into 3 groups according to different treatment plans. Group A (35 cases): Amoxicillin capsule+clarithromycin sustained-release tablet+rabeprazole enteric-coated capsule+bismuth potassium citrate capsule; Group B (32 cases): amoxicillin capsule+Levofloxacin tab⁃let+Rabeprazole enteric-coated capsule+bismuth potassium citrate capsule; Group C (29 cases):clarithromycin sustained-release tablet+levofloxacin tablet+rabeprazole enteric capsule+bismuth potassium citrate capsule. The clini⁃cal efficacy and adverse reactions of each group were compared. Results The eradication rates of H. pylori in groups A, B and C were 85.71%, 81.25% and 75.86%, respectively, and the difference was not statistically significant (χ2= 1.010, P=0.609). The incidence of adverse drug reactions in the three groups was 5.71%, 6.25% and 10.34%, respec⁃tively, and the difference was not statistically significant (χ2=0.694, P=0.789). Conclusion Amoxicillin combined with clarithromycin has higher eradication rate and good safety of Hp, which can be used as the first choice of radical treat⁃ment. Clarithromycin combined with levofloxacin has a low eradication rate of Hp and should not be used.[Key words] Helicobacter pylori; Quadruple therapy; Bismuth; Efficacy幽门螺杆菌(helicobacter pylori, Hp)是一种螺旋状的革兰氏阴性菌,进入人体后可寄生在口腔、胃和十二指肠等部位[1]。
老年脓毒症患者血清氨基末端脑钠肽前体升高的影响因素分析

论著DOI:10.16662/ki.1674-0742.2023.17.001老年脓毒症患者血清氨基末端脑钠肽前体升高的影响因素分析李程锦,石松菁,陈湘平,陈凤朱福建医科大学教学医院福建省老年医院重症医学科,福建福州350000[摘要]目的探讨影响老年脓毒症患者NT-proBNP升高的影响因素。
方法采用回顾性研究分析2017年10月—2019年12月福建省老年医院收治的老年脓毒症患者114例的临床资料。
根据患者确诊入院时的血清NT-proBNP水平是否升高,将其分为观察组(NT-proBNP升高)60例和对照组(NT-proBNP正常)54例。
收集患者的人口学特征以及既往病史、BMI、是否有脓毒性休克及SOFA评分、APACHE Ⅱ评分等。
入院时采集患者外周静脉血,送检血清肌酐、NT-proBNP、心肌损伤标志物、炎症指标、心肌抑制因子、心肌自身免疫抗体等指标。
以单因素和多因素分析老年脓毒症患者NT-proBNP的影响因素。
结果与对照组比较,观察组的脓毒性休克占比、SOFA评分、APACHEⅡ评分、CK-MB、cTnI、PCT、CRP、IL-1β、TNF-α、β1-AAB、M2-AA显著较高,Ccr水平更低,差异有统计学意义(P<0.05)。
Logistic多因素回归分析结果表明老年脓毒症患者NT-proBNP水平的独立影响因素为PCT、IL-1β、BMI、Ccr(P<0.05)。
结论老年脓毒症患者NT-proBNP的升高与BMI、PCT、IL-1β、Ccr指标相关,上述指标是老年脓毒症患者NT-proBNP升高的影响因素,但这些发现需要样本量更大的前瞻性研究进一步验证。
[关键词]脓毒症;氨基末端脑钠肽前体;炎症因子;心肌损伤标志物;心肌自身免疫抗体[中图分类号]R5 [文献标识码]A [文章编号]1674-0742(2023)06(b)-0001-07Analysis of Factors Affecting Elevated Serum Amino-terminal Brain Natri⁃uretic Peptide Precursors in Elderly Patients with SepsisLI Chengjin, SHI Songjing, CHEN Xiangping, CHEN FengzhuDepartment of Critical Care Medicine, Fujian Provincial Geriatric Hospital, Fujian Medical University Teaching Hos⁃pital, Fuzhou, Fujian Province, 350000 China[Abstract] Objective To investigate the influencing factors affecting the elevation of NT-proBNP in elderly patients with sepsis. Methods A retrospective study was used to analyze the clinical data of 114 cases of geriatric sepsis pa⁃tients admitted to Fujian Provincial Geriatric Hospital from October 2017 to December 2019. The patients were di⁃vided into 60 cases in the observation group (elevated NT-proBNP) and 54 cases in the control group (normal NT-proBNP) according to whether their serum NT-proBNP levels were elevated at the time of confirmed admission. The patients' demographic characteristics as well as past medical history, BMI, presence of septic shock and SOFA score and APACHE Ⅱ score were collected. Peripheral venous blood was collected from patients on admission and sent for serum creatinine, NT-proBNP, myocardial injury markers, inflammatory indexes, myocardial inhibitory factors, and myocardial autoimmune antibodies. Analyzed the influencing factors of NT proBNP levels in elderly sepsis patients us⁃ing single and multiple factors. Results Compared with the control group, septic shock percentage, SOFA score, APACHEⅡ score, CK-MB, cTnI, PCT, CRP, IL-1β, TNF-α, β1-AAB, and M2-AA in the observation group were significantly higher, Ccr levels was lower, the difference was statistically significant (P<0.05). Logistic multi-factor re⁃[基金项目]福建省卫生计生科研人才培养项目青年科研课题(2017/2/19)。
AAMI标准

Association for the Advancement of Medical Instrumentation协会中文名称:AAMI;美国医疗器械促进协会1 AAMI 7198-1998 (R 2004) CARDIOVASCULAR IMPLANTS - TUBUAL VASCULAR PROSTHESES-FDA RECOGNIZED2 AAMI 10993-1-2003 Biological evaluation of medical devices - Part 1: Evaluation and testing-FDA RECOGNIZED3 AAMI 10993-2-2006 Biological evaluation of medical devices - Part 2: Animal welfare requirements4 AAMI 10993-3-2003 Biological Evaluation of Medical Devices, Part 3: Tests for Genotoxicity, Carcinogenicity and Reproductive Toxicity5 AAMI 10993-4-2002 Biological evaluation of medical devices - Part 4: Selection of tests for interaction with blood-Incorporates Amendment 1: 20066 AAMI 10993-5-1999 BIOLOGICAL EVALUATION OF MEDICAL DEVICES, PART 5: TEST FOR IN VITRO CYTOTOXICITY-FDA RECOGNIZED7 AAMI 10993-6-1995 (R 2001) BIOLOGICAL EVALUATION OF MEDICAL DEVICES, PART 6: TESTS FOR LOCAL EFFECTS AFTER IMPLANTATION-FDA RECOGNIZED8 AAMI 10993-7-1995 (R 2001) Biological Evaluation of Medical Devices, Part 7: Ethylene Oxide Sterilization Residuals-Only FDA RECOGNIZED if used in conjunction to AAMI TIR19: 1988; Replaces AAMI ST29: 1988 and AAMI ST30: 1989 [Use: AAMI TIR19 AMD 1]9 AAMI 10993-9-1999 (R 2005) Biological evaluation of medical devices - Part 9: for identification and quantification of potential degradation products10 AAMI 10993-11-2006 Biological evaluation of medical devices - Part 11: Tests for systemic toxicity11 AAMI 10993-12-2002 Biological evaluation of medical devices - Part 12: Sample preparation and reference materials-FDA RECOGNIZED12 AAMI 10993-13-1999 (R 2004) Biological evaluation of medical devices, Part 13: Identification and quantification of degradation products from polymeric devices.13 AAMI 10993-14-2001 (R 2006) BIOLOGICAL EVALUATION OF MEDICAL DEVICES, PART 14: IDENTIFICATION AND QUANTIFICATION OF DEGRADATION PRODUCT FROM CERAMICS14 AAMI 10993-15-2000 (R 2006) Biological evaluation of medical devices?Part 15: Identification and quantification of degradation products from metals and alloys15 AAMI 10993-16-1997 (R 2003) BIOLOGICAL EVALUATION OF MEDICAL DEVICES - PART 16: TOXICOKINETIC STUDY DESIGN FOR DEGRADATION PRODUCTS AND LEACHABLES FROM MEDICAL DEVICES16 AAMI 10993-17-2002 BIOLOGICAL EVALUATION OF MEDICAL DEVICES, PART17: ESTABLISHMENT OF ALLOWABLE LIMITS FOR LEACHABLE SUBSTANCES17 AAMI 11135 ERTA-2005 Medical devices ?Validation and routine control of ethylene oxide sterilization-Erratum issued: 20 January 1995 (print copy only) and Erratum issued: 6 April 2005 (PDF copy only)18 AAMI 11135-1994 Medical devices - Validation and routine control of ethylene oxide sterilization-FDA RECGOGNIZED; esignations: AAMI OPEO, AAMI ST27, and AAMI TIR1 [Refer to: AAMI TIR14, AAMI TIR15, AAMI TIR16, AAMI TIR20, AAMI TIR28]19 AAMI 11137-1-2006 Sterilization of health care products - Radiation - Part 1: Requirements for the development, validation and routine control of a sterilization process for medical devices-Also replaces AAMI ST31, AAMI ST32, AAMI TIR520 AAMI 11137-3-2006 Sterilization of health care products - Radiation - Part 3: Guidance on dosimetric aspects-Also replaces AAMI ST31, AAMI ST32, AAMI TIR521 AAMI 11138-1-2006 Sterilization of health care products - Biological indicators - Part 1: General requirements [Refer to: AAMI 18472] 22 AAMI 11138-2-2006 Sterilization of health care products - Biological indicators - Part 2: Biological indicators for ethylene oxide sterilization processes [Refer to: AAMI 18472]23 AAMI 11138-3-2006 Sterilization of health care products - Biological indicators - Part 3: Biological indicators for moist heat sterilization processes [Refer to: AAMI 18472]24 AAMI 11138-4-2006 Sterilization of health care products - Biological indicators - Part 4: Biological indicators for dry heat sterilization processes [Refer to: AAMI 18472]25 AAMI 11138-5-2006 Sterilization of health care products - Biological indicators - Part 5: Biological indicators for low-temperature steam and aldehyde sterilization processes [Refer to: AAMI 18472]26 AAMI 11607-2-2006 Packaging for terminally sterilized medical devices - Part 2: Validation requirements for ing, sealing and assembly processes27 AAMI 11737-2-1998 Sterilization of Medical Devices - Microbiological Methods - Part 2: Tests of Sterility Per ed in the Validation of a Sterilization Process-FDA RECOGNIZED28 AAMI 13485-2003 Medical devices - Quality management systems - Requirements for regulatory purposes29 AAMI 14155-1-2003 Clinical investigation of medical devices for human subjects - Part 1: General requirements30 AAMI 14155-2-2003 Clinical investigation of medical devices for human subjects?Part 2: Clinical investigation plans31 AAMI 14160-1998 Sterilization of Single-Use Medical Devices Incorporating Materials of Animal Origin - Validation and Routine Control of Sterilization by Liquid Chemical Sterilants-FDA RECOGNIZED32 AAMI 14161-2000 Sterilization of health care products?Biological indicators?Guidance for the selection, use, and interpretation of results-FDA RECOGNIZED33 AAMI 14937-2000 Sterilization of health care products?General requirements for characterization of a sterilizing agent and the development, validation, and routine control of a sterilization process for medical devices-FDA RECOGNIZED34 AAMI 15223-2000 Medical Devices - Symbols to be used with medical device labels, labeling and in ation to be supplied.-FDA RECOGNIZED; Includes Amendment 1: 11/5/2001 and Amendment 2: 4/23/200435 AAMI 15225-2000 (R 2006) NOMENCLATURE - SPECIFICATION FOA A NOMENCLATURE SYSTEM FOR MEDICAL DEVICES FOR THE PURPOSE OF REGULATORY DATA EXCHANGE-Includes Amendment 1: 200436 AAMI 15674-2001 Cardiovascular implants and artificial organs —Hard shell cardiotomy/venous reservoir systems (with/without filter) and soft venous reservoir bags37 AAMI 15675-2001 Cardiovascular implants and artificial organs?Cardiopulmonary bypass systems?Arterial blood line filters38 AAMI 15882-2003 Sterilization of health care products - Chemical indicators - Guidance for selection, use, and interpretation of results-FDA RECOGNIZED39 AAMI 17665-1-2006 Sterilization of health care products - Moist heat - Part 1 Requirements for the development, validation and routine control of a sterilization process for medical devices- er Designation: AAMI ST2540 AAMI 25539-1 AMD 1-2005 Cardiovascular implants - Endovascular devices - Part 1: Endovascular prostheses, Amendment 1: Test methods 41 AAMI 25539-1-2003 Cardiovascular implants?Endovascular devices?Part 1: Endovascular prostheses42 AAMI 60601-2-21-2000 Medical electrical equipment, Part 2: Particular requirements for the safety of infant radiant warmers-FDA RECOGNIZED; er Designation: AAMI II52; Incorporates Amendment 1: 2000 43 AAMI 62304-2006 Medical device software - Software life cycle processes [Refer to: AAMI TIR32]44 AAMI BE78-2002 BIOLOGICAL EVALUATION OF MEDICAL DEVICES, PART 10: TEST FOR IRRITATION AND DELAYED TYPE HYPERSENSITIVITY-In place of AAMI 10993-10; FDA RECOGNIZED45 AAMI BE83-2006 Biological evaluation of medical devices - Part 18: Chemical characterization of materials46 AAMI BF7-1989 (R 2002) Blood Transfusion Micro-Filters-FDA RECOGNIZED47 AAMI BF64-2002 LEUKOCYTE REDUCTION FILTERS48 AAMI 7199-1996 (R 2002) Cardiovascular implants and artificial organs - Blood-ga* **changers (oxygenators)49 AAMI BP22-1994 (R 2006) Blood Pressure Transducers-FDA RECOGNIZED; Incorporates Errata: 08/05/200450 AAMI 5840-2005 Cardiovascular implants - Cardiac valve prostheses-FDA RECOGNIZED51 AAMI DF80-2003 Medical electrical equipment, Part 2: Particular requirements for the safety of cardiac defibrillators [including automated external defibrillators]-Also repalces AAMI TIR252 AAMI EC11-1991 (R 2001) Diagnostic electrocardiographicdevices-FDA RECOGNIZED53 AAMI EC12-2000 (R 2005) Disposable ECG Electrodes-FDA RECOGNIZED54 AAMI EC13 ERTA-2002 Cardiac Monitors, Heart Rate Meters and Alarms55 AAMI EC13-2002 Cardiac Monitors, Heart Rate Meters and Alarms56 AAMI EC38-1998 Ambulatory Electrocardiographs-FDA RECOGNIZED57 AAMI EC53 ERTA-2001 ECG cables and leadwires-Includes Erratum issued: 31 May 199858 AAMI EC53-1995 (R 2001) ECG cables and leadwires59 AAMI EC57-1998 (R 2003) Testing and reporting per ance results of cardiac rhythm and ST segment measurement algorithms-Includes Errata: 4/14/2004; Revision of ECAR: 1987; FDA-Recognized60 AAMI EC71-2001 Standard Communications Protocol - Computer Assisted Electrocardiography-Proposed equivalency to IEC60601-2-53/Ed.1: Harmonized61 AAMI EQ56-1999 (R 2004) Recommended practices for a medical equipment management program62 AAMI ES60601-1-2005 Medical electrical equipment, Part 1: General requirements for basic safety and essential per ance63 AAMI HE48-1993 Human Factors Engineering Guidelines and Preferred Practices for the Design of Medical Devices64 AAMI HE74-2001 Human Factors Design Process for MedicalDevices-FDA RECOGNIZED65 AAMI HFMD-1996 An Introduction to Human Factors in Medical Devices66 AAMI ID26 ERTA-2006 Medical electrical equipment, Part 2: Particular requirements for the safety of infusion pumps and controllers 67 AAMI ID54-1996 (R 2005) Enteral Feeding Set Adapters and Connectors-FDA RECOGNIZED68 AAMI NS4-1986 (R 2002) Transcutaneous Electrical Nerve Stimulators69 AAMI NS14-1995 (R 2002) Implantable spinal cord stimulators70 AAMI NS15-1995 (R 2002) Implantable peripheral nerve stimulators71 AAMI NS28-1988 (R 2006) Intracranial Pressure Monitoring Devices-FDA RECOGNIZED; Incorporates Errata: 06/200172 AAMI PAC49-1993 (R 2000) Pacemaker Emergency Intervention System73 AAMI PB70-2003 LIQUID BARRIER PER ANCE AND CLASSIFICATION OF PROTECTIVE APPAREL AND DRAPES INTENDED FOR USE IN HEALTH CAREFACILITIES-FDA RECOGNIZED74 AAMI PC69-2000 ACTIVE IMPLANTABLE MEDICAL DEVICES ELECTROMAGNETIC COMPATIBILITY EMC TEST PROTOCOLS FOR IMPLANTABLE CARDIAC PACEMAKERS AND IMPLANTABLE CARDIOVERTER DEFIBRILLATORS75 AAMI RD5-2003 Hemodialysis Systems76 AAMI RD16 AMD 1-2002 (R 2005) Hemodialyzers-FDA RECOGNIZED77 AAMI RD16-1996 (R 2005) Hemodialyzers [Refer to: IEC 60601-2-16]78 AAMI RD17-1994 (R 2005) Hemodialyzer Blood Tubing-Includes Amendment 1: 200279 AAMI RD47-2002 Reuse of Hemodialyzers-Incorporates Amendment 1: 7/2003 and Errata: 07/200380 AAMI RD52-2004 Dialysate for hemodialysis81 AAMI RD62-2006 WATER TREATMENT EQUIPMENT FOR HEMODIALYSIS APPLICATIONS82 AAMI SP10-2006 Manual, electronic, or automated sphygmomanometers-FDA REGOGNIZED; Incorporates Amendment 1: 2003; Amendment 2: 200683 AAMI ST8-2001 Hospital Steam Sterilizers-FDA RECOGNIZED84 AAMI ST24-1999 (R 2005) Automatic, General-Purpose Ethylene Oxide Sterilizers and Ethylene Oxide Sterilant Sources Intended for Use in Health Care Facilities-FDA RECONIZED85 AAMI ST40-2004 Table-top dry heat (heated air) sterilization and sterility assurance in health care facilities-FDA RECOGNIZED86 AAMI ST41-1999 (R 2005) Ethylene Oxide Sterilization in Health Care Facilities: Safety and Effectiveness-FDA RECOGNIZED87 AAMI ST50-2004 Dry Heat (Heated Air) Sterilizers-FDA RECOGNIZED88 AAMI ST55-2003 Table-Top Steam Sterilizers-FDA RECOGNIZED89 AAMI ST63-2002 Sterilization of health care products—Requirements for the development, validation, and routine control of an industrial sterilization process for medical devices—Dry heat-FDA RECOGNIZED90 AAMI ST65-2000 PROCESSING OF REUSABLE SURGICAL TEXTILES FOR USE IN HEALTH CARE FACILITIES91 AAMI ST66-1999 STERILIZATION OF HEALTHCARE PRODUCTS - CHEMICAL INDICATORS - PART 2 : CLASS 2 INDICATORS FOR AIR REMOVAL TEST SHEETS AND PACKS-FDA RECOGNIZED [Refer to: AAMI 18472]92 AAMI ST67-2003 Sterilization of health care products—Requirements for products labeled “STERILE?FDA RECOGNIZED93 AAMI ST77-2006 Containment devices for reusable medical device sterilization94 AAMI ST81-2004 Sterilization of medical devices - In ation to be provided by the manufacturer for the processing of resterilizable medical devices-FDA RECOGNIZED95 AAMI TIR4-1989 Apnea Monitoring by Means of Thoracic ImpedancePneumography96 AAMI TIR11-2005 Selection and use of protective apparel and surgical drapes in health care facilities97 AAMI TIR12-2004 Designing, Testing and Labeling Reusable Medical Devices for Reprocessing in Health Care Facilities: A Guide for Device Manufacturers-2nd Edition98 AAMI TIR13-1997 Principles of Industrial Moist HeatSterilization-(Updated material from ANSI/AAMI ST25:1987 )99 AAMI TIR14-1997 Contract Sterilization for EthyleneOxide-Updated material from AAMI ST27; Cited by FDA as "relevant guidance" for ANSI/AAMI/ISO 11135: 1994; Includes Amendment 1: 2004 [Refer to: AAMI 11135 ERTA]100 AAMI TIR15-1997 Ethylene Oxide Sterilization Equipment, Process Considerations and Pertinent Calculations-Updated material from AAMI ST27; Cited by FDA as "relevant guidance" for ANSI/AAMI/ISO 11135: 1994 [Refer to: AAMI 11135 ERTA]101 AAMI TIR16-2000 PROCESS DEVELOPMENT AND PER ANCE QUALIFICATION FOR ETHYLENE OXIDE STERILIZATION - MICROBIOLOGICAL ASPECTS-Updated material from AAMI ST27; Cited by FDA as "relevant guidance" forANSI/AAMI/ISO 11135: 1994 [Refer to: AAMI 11135 ERTA]102 AAMI TIR17-1997 Radiation Sterilization MaterialQualification-Cited by FDA as "relevant guidance" for ANSI/AAMI/ISO 11137: 1994; Replaces AAMI ST32 [Refer to: AAMI 11137]103 AAMI TIR18-1997 Guidance on Electromagnetic Compatibility of Medical Devices for Clinical/Biomedical Engineers - Part 1: Radiated Radio-Frequency Electromagnetic Energy [Replaced by: IEC 61326-2-6] 104 AAMI TIR19 AMD 1-1999 Guidance for ANSI/AAMI/ISO 10993-7:1995, Biological evaluation of medical deices—Part 7: Ethylene oxide sterilization residuals Amendment105 AAMI TIR19-1998 Guidance for ANSI/AAMI/ISO 10993-7:1995, Biological Evaluation of Medical Devices - Part 7: Ethylene Oxide Sterilization Residuals-Replaces AAMI ST29 and AAMI ST30; Cited as relevant guidance to FDA-recognized standard ANSI/AAMI/ISO 10993-7 [Refer to: AAMI 10993-7]106 AAMI TIR20-2001 PARAMETRIC RELEASE FOR ETHYLENE OXIDE STERILIZATION-Cited by FDA as "relevant guidance" for ANSI/AAMI/ISO 11135: 1994 [Refer to: AAMI 11135 ERTA]107 AAMI TIR21-1998 Systems Used to Forecast Remaining Pacemaker Battery Service Life108 AAMI TIR22-1998 Guidance for ANSI/AAMI/ISO 11607, Packaging for Terminally Sterilized Medical Devices-AMD 1: 2001109 AAMI TIR23-1999 Signal Averaging110 AAMI TIR24-1999 Acquisition and use of physiologic wave databases for testing of medical devices111 AAMI TIR26-2000 Ventricular assist and heart replacement systems 112 AAMI TIR28-2001 PRODUCT ADOPTION AND PROCESS EQUIVALENCY FOR ETHYLENE OXIDE STERILIZATION [Refer to: ISO 11135, AAMI 11135 ERTA] 113 AAMI TIR29-2002 GUIDE FOR PROCESS CONTROL IN RADIATION STERILIZATION-Cited by FDA as "relevant guidance" for ANSI/AAMI/ISO 11137: 1994 [Refer to: AAMI 11137]114 AAMI TIR30-2003 A Compendium of processes, materials, test methods, and acceptance criteria for cleaning reusable medical devices 115 AAMI TIR31-2003 PROCESS CHALLENGE DEVICES/TEST PACKS FOR USE IN HEALTH CARD FACILITIES116 AAMI TIR32-2004 Medical device software risk management [Refer to: AAMI 14971, AAMI 62304]117 AAMI TIR35-2006 Sterilization of health care products - Radiation sterilization - Alternative sampling plans for verification dose experiments and sterilization dose audits- er designation: AAMI ST31, AAMI ST32, and AAMI TIR5118 AAMI TIR10993-19-2006 Biological evaluation of medical devices - Part 19: Physico-chemical, morphological and topographical characterization of materials119 AAMI TIR10993-20-2006 Biological evaluation of medical devices - Part 20: Principles and methods for immunotoxicology testing of medical devices120 AAMI TIR11139-2006 Sterilization of health careproducts ?Vocabulary121 AAMI TIR14969-2004 Medical devices—Quality management systems?Guidance on the application of ISO 13485:2003122 AAMI TIR15844-1998 Sterlization of Health Care Products - Radiation Sterlization - Selection of a Sterilization Dose for a Single Production Batch-Edition 1123 AAMI TIR16142-2005 Guidance on the selection of standards in support of recognized essential principles of safety and per ance of medical devices-2006 printing124 AAMI TIR19218-2006 Medical devices - Coding structure for adverse event type and cause125 AAMI TIR60878-2003 Graphical symbols for electrical equipment in medical practice126 AAMI TIR62296-2003 Considerations of unaddressed safety aspects in the Second Edition of IEC 60601-1 and proposals for new requirements [Refer to: IEC 60601-1]127 AAMI TIR62348-2006 Mapping between the clauses of the third edition of IEC 60601-1 and the 1988 edition as amended。
GSK461364_Cell Cycle_PLK_CAS号929095-18-1说明书_AbMole中国
分子量543.6溶解性(25°C)DMSO 10 mg/mL分子式C H F N O S Water <1 mg/mLCAS号929095-18-1Ethanol 30 mg/mL储存条件3年 -20°C 粉末状生物活性GSK461364抑制纯化的Plk1,K为2 nM,比作用于Plk2/3选择性高1000倍。
GSK461364作用于非细胞分裂细胞,从多起点抑制癌细胞系增殖。
野生型p53的RNA沉默提高GSK461364的抗增殖活性。
许多癌症疗法对p53野生型患者更有效, p53缺陷肿瘤对GSK461364的高度敏感性可提供治疗肿瘤的机会。
实验操作来自于公开的文献,仅供参考细胞实验细胞系MDA-MB-231, A549, and PC3 cell line方法Cell lines were obtained from American Type Culture Collection and grown in the recommended media at 37°C, 5% CO2 in a humidified incubator. Cells were plated in triplicate 96-well microtiter plates at 1,000 cells per well in culture media. GSK461364Adissolved in DMSO or negative control (0.1% DMSO) were added the following day, and one plate was harvested with 50 μL ofCellTiter-Glo (Promega) for a time 0 (T = 0) measurement. Remaining duplicate cell plates were typically incubated for 72 h. Cells werethen lysed with 50 μL CellTiter-Glo, and chemiluminescent signal was read on the Wallac EnVision 2100 plate reader. All data werenormalized to signal at time of compound addition (T = 0). Curves were analyzed using the XLfit (IDBS Ltd.) curve-fitting tool forMicrosoft Excel to determine the gI50 (concentration of 50% growth inhibition relative to T = 0 and Ymax values), the gI-maximum(concentration giving maximum growth inhibition), and the Ymin (bottom of the four-parameter curve at gI-maximum).浓度0~10 μM处理时间72 h动物实验动物模型mice bearing Colo205 tumor xenograft model配制4% DMA/Cremaphore (50:50), pH 5.6剂量every 2 d (q2dx6, q2dx12) or every 4 d (q4dx3) at nominal dose levels of 25, 50, and 100 mg/kg/dose给药处理i.p.不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)小鼠大鼠兔豚鼠仓鼠狗重量 (kg)0.020.15 1.80.40.0810体表面积 (m)0.0070.0250.150.050.020.5K系数36128520动物 A (mg/kg) = 动物 B (mg/kg) ×动物 B的K系数动物 A的K系数例如,依据体表面积折算法,将白藜芦醇用于小鼠的剂量22.4 mg/kg 换算成大鼠的剂量,需要将22.4 mg/kg 乘以小鼠的K系数(3),再除以大鼠的K系数(6),得到白藜芦醇用于大鼠的等效剂量为11.2 mg/kg。
GSK461364_929095-18-1_DataSheet_MedChemExpress
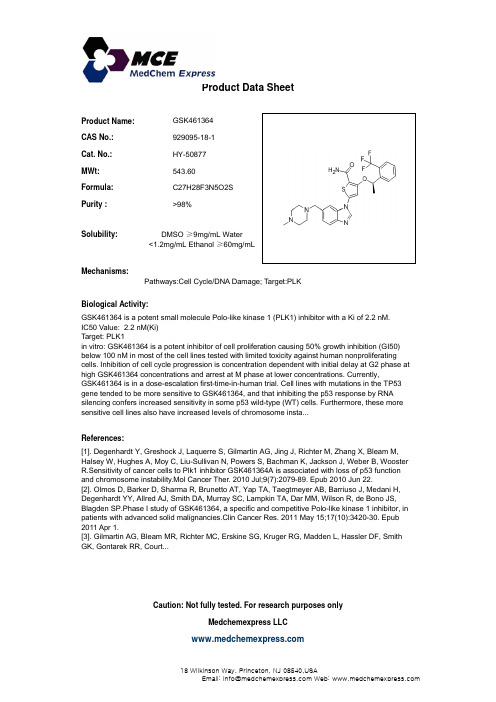
Product Name:GSK461364CAS No.:929095-18-1Cat. No.:HY-50877Product Data SheetMWt:543.60Formula:C27H28F3N5O2S Purity :>98%Solubility:DMSO ≥9mg/mL Water y Mechanisms:Biological Activity:GSK461364is a potent small molecule Polo like kinase 1(PLK1)inhibitor with a Ki of 22nMPathways:Cell Cycle/DNA Damage; Target:PLK g<1.2mg/mL Ethanol ≥60mg/mLGSK461364 is a potent small molecule Polo-like kinase 1 (PLK1) inhibitor with a Ki of 2.2 nM.IC50 Value: 2.2 nM(Ki)Target: PLK1in vitro: GSK461364 is a potent inhibitor of cell proliferation causing 50% growth inhibition (GI50)below 100 nM in most of the cell lines tested with limited toxicity against human nonproliferating cells. Inhibition of cell cycle progression is concentration dependent with initial delay at G2 phase at high GSK461364 concentrations and arrest at M phase at lower concentrations. Currently,GSK461364 is in a dose-escalation first-time-in-human trial. Cell lines with mutations in the TP53gene tended to be more sensitive to GSK461364, and that inhibiting the p53 response by RNA References:[1]. Degenhardt Y, Greshock J, Laquerre S, Gilmartin AG, Jing J, Richter M, Zhang X, Bleam M,Halsey W, Hughes A, Moy C, Liu-Sullivan N, Powers S, Bachman K, Jackson J, Weber B, Wooster R.Sensitivity of cancer cells to Plk1 inhibitor GSK461364A is associated with loss of p53 function g ,g p p ysilencing confers increased sensitivity in some p53 wild-type (WT) cells. Furthermore, these more sensitive cell lines also have increased levels of chromosome insta...and chromosome instability.Mol Cancer Ther. 2010 Jul;9(7):2079-89. Epub 2010 Jun 22.[2]. Olmos D, Barker D, Sharma R, Brunetto AT, Yap TA, Taegtmeyer AB, Barriuso J, Medani H,Degenhardt YY, Allred AJ, Smith DA, Murray SC, Lampkin TA, Dar MM, Wilson R, de Bono JS,Blagden SP.Phase I study of GSK461364, a specific and competitive Polo-like kinase 1 inhibitor, in patients with advanced solid malignancies.Clin Cancer Res. 2011 May 15;17(10):3420-30. Epub2011 Apr 1.[3]. Gilmartin AG, Bleam MR, Richter MC, Erskine SG, Kruger RG, Madden L, Hassler DF, Smith GK, Gontarek RR, Court...Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Product Name:
GSK461364CAS No.:
929095-18-1Cat. No.:
HY-50877
Product Data Sheet
MWt:
543.60Formula:
C27H28F3N5O2S Purity :>98%
Solubility:DMSO ≥
9mg/mL Water y Mechanisms:
Biological Activity:
GSK461364is a potent small molecule Polo like kinase 1(PLK1)inhibitor with a Ki of 22nM
Pathways:Cell Cycle/DNA Damage; Target:PLK g
<1.2mg/mL Ethanol ≥60mg/mL
GSK461364 is a potent small molecule Polo-like kinase 1 (PLK1) inhibitor with a Ki of 2.2 nM.
IC50 Value: 2.2 nM(Ki)
Target: PLK1in vitro: GSK461364 is a potent inhibitor of cell proliferation causing 50% growth inhibition (GI50)below 100 nM in most of the cell lines tested with limited toxicity against human nonproliferating cells. Inhibition of cell cycle progression is concentration dependent with initial delay at G2 phase at high GSK461364 concentrations and arrest at M phase at lower concentrations. Currently,
GSK461364 is in a dose-escalation first-time-in-human trial. Cell lines with mutations in the TP53gene tended to be more sensitive to GSK461364, and that inhibiting the p53 response by RNA References:
[1]. Degenhardt Y, Greshock J, Laquerre S, Gilmartin AG, Jing J, Richter M, Zhang X, Bleam M,Halsey W, Hughes A, Moy C, Liu-Sullivan N, Powers S, Bachman K, Jackson J, Weber B, Wooster R.Sensitivity of cancer cells to Plk1 inhibitor GSK461364A is associated with loss of p53 function g ,g p p y
silencing confers increased sensitivity in some p53 wild-type (WT) cells. Furthermore, these more sensitive cell lines also have increased levels of chromosome insta...
and chromosome instability.Mol Cancer Ther. 2010 Jul;9(7):2079-89. Epub 2010 Jun 22.[2]. Olmos D, Barker D, Sharma R, Brunetto AT, Yap TA, Taegtmeyer AB, Barriuso J, Medani H,Degenhardt YY, Allred AJ, Smith DA, Murray SC, Lampkin TA, Dar MM, Wilson R, de Bono JS,Blagden SP.Phase I study of GSK461364, a specific and competitive Polo-like kinase 1 inhibitor, in patients with advanced solid malignancies.Clin Cancer Res. 2011 May 15;17(10):3420-30. Epub
2011 Apr 1.[3]. Gilmartin AG, Bleam MR, Richter MC, Erskine SG, Kruger RG, Madden L, Hassler DF, Smith GK, Gontarek RR, Court...
Caution: Not fully tested. For research purposes only
Medchemexpress LLC
18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
