微包纳
栓塞型新型微包纳药物控释载体的制备及性能

刘源 岗等 : 塞型新型微 包纳 药物控释载体 的制备及性 能 栓
苦. 但是 目 前药物缓释微囊/ 球常常面临着药物突释效应 、 调节药物溶出速率手段单一等问题.同时, 由于不同药物之间的理化性质差异 , 在药物载体 中混合使用时可能面临着药物交叉污染的问题.发展 环境友好型的微包纳体系可解决以上问题 , 具有实用性强及广 阔的应用前景.聚精氨酸是人工合成的 可生物降解 的氨基酸类聚合物 , 具有优 良的理化性能.其降解产物精氨酸是人体的半必需氨基酸 , 具 有营养作用和药理功效 , 富精氨酸环境可抑制肿瘤 的生长及转移 , 而且聚精氨酸比其它阳离子型高聚 物更容易穿越细胞. 本课题组 首次报道 了聚精氨酸作为膜材料制备微胶囊 , 该体系有望成为一 种具有介入治疗效果 的药物缓/ 控释载体. 几丁聚糖是天然多糖 中唯一的碱性多糖 , 其来源丰富、 生物 相容性好 , 具有一定 的生物降解性 , 有增强药物吸收 、 促进 伤 口愈合及抑制肿瘤细胞的作用 , 是一种 应用前景广阔的医药载体¨ .海藻酸钠是天然可生物降解高分子聚合物 , 是一种聚阴离子电解质, 其
Vo . 2 13
21 0 1年 1 1月
高 等 学 校 化 学 学 报
CHEMI ALJ C OURNAL OF CHI NES E UNI VERSTI I ES
No 1 .1
2 7 ~2 8 54 5 0
栓 塞 型 新 型微 包 纳药 物 控 释 载体 的制 备及 性 能
单一蛋 白类药物和负载两种药物的缓释性 能并 进行 了动力学模 型拟合 .结果表明 , i e-ep s 型能够较 Rt r p a 模 g P 好地模拟该溶胀控释系统的药物释放过程 , 与实验 结果 比较吻合 .同时也 证 明了该 新型载体 体系具 有无突 释、 释放速率减缓及顺序释放 的功能 , 为新 型药物载体体 系的研究提供 了新 的思路 .
微纳米气泡微纳米气泡研究与应用

微纳米气泡微纳米气泡研究与应用下载提示:该文档是本店铺精心编制而成的,希望大家下载后,能够帮助大家解决实际问题。
文档下载后可定制修改,请根据实际需要进行调整和使用,谢谢!本店铺为大家提供各种类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by this editor. I hope that after you download it, it can help you solve practical problems. The document can be customized and modified after downloading, please adjust and use it according to actual needs, thank you! In addition, this shop provides you with various types of practical materials, such as educational essays, diary appreciation, sentence excerpts, ancient poems, classic articles, topic composition, work summary, word parsing, copy excerpts, other materials and so on, want to know different data formats and writing methods, please pay attention!微纳米气泡研究与应用微纳米气泡是指尺度在微米到纳米范围内的气泡颗粒。
药剂学--微型包囊ppt课件

•混合 •微囊化
A药微囊化+B药微囊化+…+附加剂
• 注意:囊心物与囊材的比例要适当,囊心物过少,
•
则生成无囊心物的空囊。
PPT文档演模板
药剂学课件--微型包囊课件
二、囊材(coating material)
用于包裹囊心物所需的材料称为囊材。 对囊材的一般要求:
①性质稳定;②有适宜的释药速度;③无毒、无刺激; ④能与药物配伍,不影响药物的药理作用及含量测定; ⑤有一定强度和可塑性,能完全包裹囊芯物; ⑥具有符合要求的粘度、渗透性、亲水性、溶解性等。
PPT文档演模板
药剂学课件--微型包囊课件
2.单凝聚法的工艺流程
•?
• 加量为总体 积的3倍
•胶联 剂
•凝聚 剂
PPT文档演模板
药剂学课件--微型包囊课件
单凝聚法: 3、成囊条件
•透明区
•⑴凝聚系统的组成 • • 用三元相图来寻找成囊 系统产生凝聚的组成范围。
• 明胶10% • 硫酸钠10%
• 水80%
PPT文档演模板
药剂学课件--微型包囊课件
•3、合成高分子囊材
(1) 生物不降解囊材 ①不受pH值影响:聚酰胺、硅橡胶等。 ②在一定pH条件下溶解: 聚丙烯酸树脂、聚乙烯醇(PVA)
(2)可生物降解囊材 聚碳酸酯、聚氨基酸、 聚乳酸(PLA)、 聚羟基乙酸(PGA)、 聚乳酸聚羟基乙酸共聚物(PLGA) 特点:无毒、成膜性好、化学稳定性高,可用于注射。
PPT文档演模板
药剂学课件--微型包囊课件
2、半合成高分子材料
⑴羧甲基纤维素钠:常与明胶配合作复合囊材, 配比 CMC-Na(1-5g/L):明胶(30g/L) = 2 :1
【国家自然科学基金】_药物控释系统_基金支持热词逐年推荐_【万方软件创新助手】_20140730

科研热词 推荐指数 纳米粒 2 逐层组装技术 1 药物释放模型 1 药物递送 1 药物控释系统 1 药物控制释放 1 药物/基因传递系统 1 肽类自组装体 1 聚乳酸 1 生物启发型自组装 1 环境响应型 1 牛血清白蛋白 1 海藻酸钙微球 1 水凝胶 1 智能微囊 1 数学模型 1 控制释放 1 急毒性水平 1 微载体 1 微胶囊 1 微凝胶 1 异烟肼 1 叶酸受体 1 右旋糖酐-磁性层状复合氢氧化物-氟尿嘧啶给药系统 1 受体介导 1 双乳化-凝胶法 1 单分散 1 功能材料 1 刺激响应性 1 利福平 1 体外释药 1 仿病毒衣壳 1 两亲聚合物 1 三级超分子组装 1
2010年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28
科研热词 推荐指数 靶向 1 药物控释系统及其载体材料 1 药物传输 1 胶束 1 肝素 1 聚氰基丙烯酸二乙酯 1 罂粟碱 1 缓释制剂 1 缓释 1 缓控释系统 1 纳米胶束 1 紫杉醇 1 溶菌酶 1 正交设计 1 星形聚己内酯 1 明胶 1 控制释放载体材料 1 控制释放 1 成纤维细胞生长因子 1 微球 1 壳聚糖 1 叶酸 1 反向温敏 1 单甲氧基聚乙二醇(mpeg) 1 凝胶给药系统 1 介孔二氧化硅纳米粒子 1 乳腺癌动物模型 1 β -胡萝卜素 1
2013年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32
科研热词 控释 靶向 降解 释放 超声敏感材料 药物递送系统 肿瘤 聚乙二醇 综论 组织工程 类水滑石 盐酸阿霉素 界面材料 炎性反应 温敏凝胶 氧化还原 智能材料 微载体给药系统 微粒 形貌规则 响应性开关 可控释放 口服 双硒 原位给药系统 功能化 体外释放 低强度聚焦超声 介孔材料 中药 两亲性 plm
【国家自然科学基金】_缓释效应_基金支持热词逐年推荐_【万方软件创新助手】_20140803
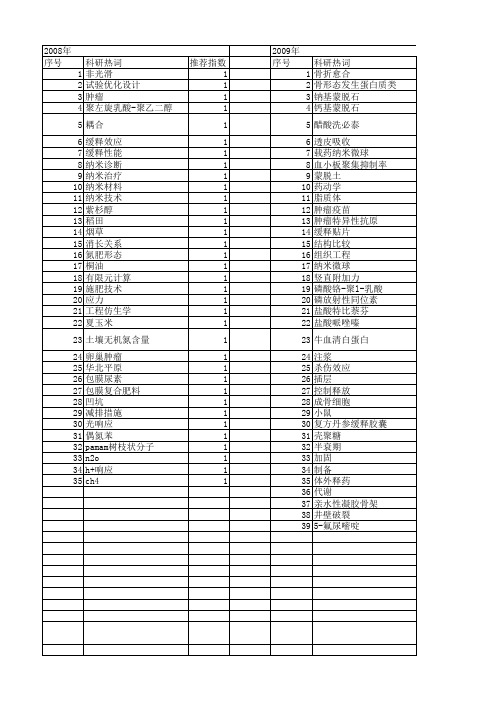
推荐指数 2 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
体外释放度 人骨形态发生蛋白2 乳酸-羟基乙酸共聚物 中药 中空微球 olsen-p h22肝癌小鼠 epr效应 5-氟尿嘧啶
1 1 1 1 1 1 1 1 1
2013年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45
2008年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35
科研热词 推荐指数 非光滑 1 试验优化设计 1 肿瘤 1 聚左旋乳酸-聚乙二醇750 1 耦合 1 缓释效应 1 缓释性能 1 纳米诊断 1 纳米治疗 1 纳米材料 1 纳米技术 1 紫杉醇 1 稻田 1 烟草 1 消长关系 1 氮肥形态 1 桐油 1 有限元计算 1 施肥技术 1 应力 1 工程仿生学 1 夏玉米 1 土壤无机氮含量 1 卵巢肿瘤 1 华北平原 1 包膜尿素 1 包膜复合肥料 1 凹坑 1 减排措施 1 光响应 1 偶氮苯 1 pamam树枝状分子 1 n2o 1 h+响应 1 ch4 1
科研热词 推荐指数 软琼脂克隆形成 1 裸鼠成瘤性 1 裸小鼠 1 药物缓释 1 药物控释系统及其载体材料 1 膨润土/丙烯酸 1 胶质瘤 1 肝细胞癌 1 聚氰基丙烯酸二乙酯 1 羟基磷灰石颗粒 1 缓释机制 1 缓释微球 1 缓释制剂 1 缓释作用 1 缓释 1 给药系统 1 细胞因子缓释微粒 1 纳米胶束 1 纳米微粒 1 紫龙金 1 紫杉醇 1 皮下移植瘤 1 疫苗 1 生物材料 1 活性 1 沉淀聚合 1 氮素缓释效应 1 替莫唑胺 1 成骨细胞 1 巴塞尔新资本协议 1 左旋甲基多巴 1 分子印迹微球 1 免疫治疗 1 保水保肥剂 1 人乳腺癌细胞系mcf-7 1 亲周期效应 1 乳酸羟基乙酸共聚物 1 乳腺癌动物模型 1 丹酚酸b 1 三氧化二砷 1 shg-44胶质瘤细胞 1 p-erk1/2 1
【国家自然科学基金】_药物控释体系_基金支持热词逐年推荐_【万方软件创新助手】_20140802
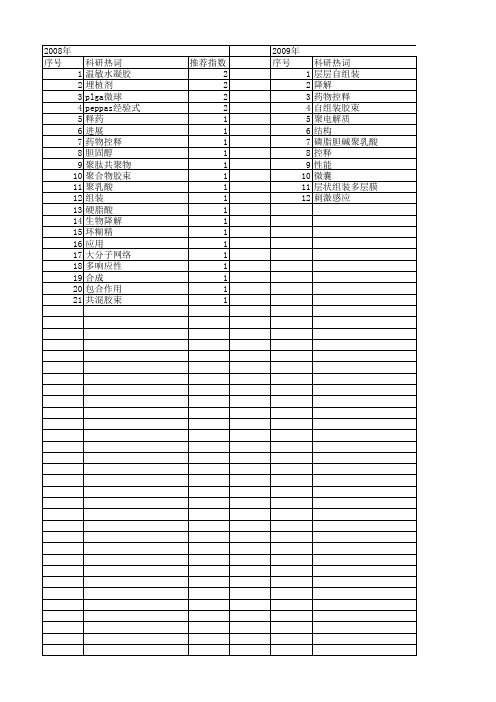
推荐指数 2 2 2 2 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
2009年 序号 1 2 3 4 5 6 7 8 9 10 11 12
科研热词 层层碱聚乳酸 控释 性能 微囊 层状组装多层膜 刺激感应
推荐指数 2 1 1 1 1 1 1 1 1 1 1 1
2011年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30
2011年 科研热词 药物释放 纳米杂化物 层状双金属氢氧化物 顺序释放 静电纺丝 阿霉素 苯丁酸氮芥 聚精氨酸 聚合物药物 缓释 紫杉醇 稀土催化剂 离子交换法 生物降解能力 温敏水凝胶 海藻酸钠 水杨酸 机制 替加氟 无规共聚 控释 控制释放 微球 微包纳药物释放体系 壳聚糖 叶酸 几丁聚糖 主动靶向 ε -己内酯 pcl-peg-pcl共聚物 推荐指数 2 2 2 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
2010年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22
科研热词 推荐指数 高度支化聚合物 1 阿司匹林 1 超支化聚合物 1 胶原海绵 1 缓释 1 细胞聚集体 1 纳米杂化物 1 碱性成纤维细胞生长因子 1 生物材料与药物控释 1 环糊精高分子 1 环糊精 1 热可逆 1 树枝状聚合物 1 来曲唑 1 微胶囊 1 层状双金属氢氧化物 1 凝胶化 1 人肝癌细胞株 1 二次组装法 1 三维培养 1 plga微球 1 diels-alder反应 1
推荐指数 3 2 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
MicroRNAs在恶性肿瘤分子诊断和预后预测中的应用

刘 巾男 任洁钏
应斌武
要 】 微 小 R A( co NA, R A) 近 年 来 发 现 的 一 类 长 度 为 1 ~2 个 核 苷 酸 的非 编 码 小 分 N mi R r miN 是 8 6
子R NA, 它在转 录后水平 调控 基因的表达 , 表达情况 与机体众多生理病理状态密切相 关。 目前发现 , 其 恶性 肿瘤组织和血液循环 中存在不 同于正 常机体 的特征性 miNA表达谱 , 过测定这些 miNA的表达变化可 R 通 R 能可 以成为恶性肿瘤早期诊 断和预后预测 的重要 手段 。本文 总结了 miNA在恶性肿瘤性疾 病分子诊 断领 R 域的研究进展 , 为疾病分子诊断学研究及 临床实践提供参 考。
[B T A ] Mi o N ( R s, n w mi f ma o cdn NAs a po i tl 1- 6 A S R CT c R As r miNA )ak o n f l o ln n o igR a y s l (p rxmae 2 y 8
n c oie n hh, a e enso nt rglt g nt x rs o i a s t nln iio r se gr ul t s nl g t h v e h w euae e ece pes nva rnl i a ih t no sn e e d i e ) b o i i t ao b i me
p y i lg c l o ah l g c l c aa t rsis h so o ia r p t o o ia h r ce it .Re e t su i s h v e e ld t a RNAs h ie s p c f c c n t d e a e r v ae h tmi ,t e d s a e s e i c i e p e so fwh c a e f u d i h i s e o l o ic l t n o a c r , r mie t a e a mp c n x r s i n o ih c n b o n n t e t u r b o d c r u ai fc n e s p o s o h v n i a to s o lb r t r d cn se r i g o t n r g o tcma k r l n n m o s Ou ri l u ma i e e a o ao me ii e a a l d a n si a d p o n si r e si mai a tt y y c n g u r . ra t e s m c rz d t h r c n r g e so n t e r s a c ed o i e e tp o r s in o h e e r h f l f m RNA n t s o ito t a c r d a n ss a d p o n si i a d i a s ca i n wi c n e ig o i n r g o t s h c e au to r e r v d aa f r v l ai n i o d r op o i e d t o l c lrd a n ss n l i a r c ie . n t mo e u a i g o i d ci c l a t s a n p c
【国家自然科学基金】_缓释胶囊_基金支持热词逐年推荐_【万方软件创新助手】_20140801
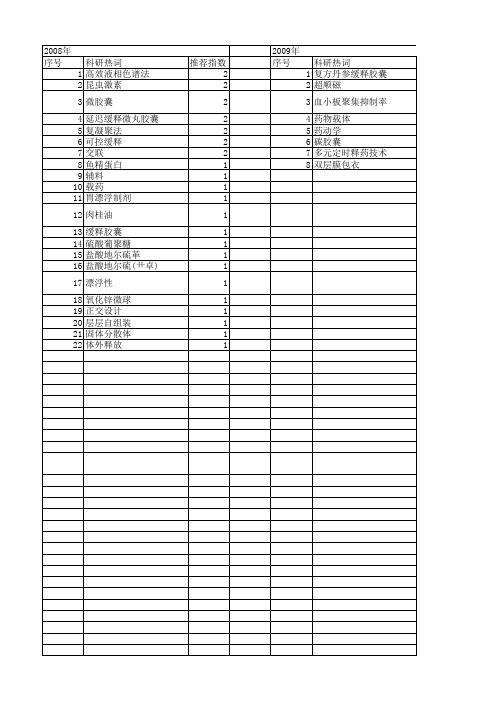
2010年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27
科研热词 推荐指数 药代动力学 2 缓释 2 高效液相色谱-紫外检测器法 1 高效液相色谱 1 高效液相 1 血药浓度 1 药动学 1 苦参碱 1 色谱法 1 聚脲微胶囊 1 聚丙烯酸叔丁酯 1 缓释胶囊 1 缓释制剂 1 细乳液聚合法 1 纳米胶囊 1 生物黏附型缓释胶囊 1 牛血清白蛋白 1 海藻酸钠 1 毒死蜱 1 毒力评价 1 投药, 局部 1 微胶囊 1 巯甲丙脯酸 1 大鼠 1 卡托普利 1 几丁聚糖 1 兔耳草醛 1
2013年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32
科研热词 推荐指数 缓释 2 粒径 2 微胶囊 2 鲁米诺 1 高效液相串联质谱 1 非索非那定 1 载药量 1 表征方法 1 药物缓释 1 药动学 1 胸主动脉 1 聚丙烯酸酯 1 缓释香精 1 牛血清白蛋白 1 流变性 1 流动注射 1 氨基脲类 1 明胶 1 慢性骨盆疼痛综合征 1 微观结构 1 微包纳 1 少腹逐瘀汤 1 夹层动脉瘤 1 壳聚糖 1 壁厚 1 哈乐(盐酸坦索罗辛) 1 化学发光 1 包埋率 1 动物模型 1 伪麻黄碱 1 γ -球蛋白 1 (r)5-(2-氨基丙基)-2-甲氧基苯磺酰胺 1
推荐指数 2 2 2 2 2 2 2 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
2009年 序号 1 2 3 4 5 6 7 8
科研热词 复方丹参缓释胶囊 超顺磁 血小板聚集抑制率 药物载体 药动学 碳胶囊 多元定时释药技术 双层膜包衣
【国家自然科学基金】_高压静电_基金支持热词逐年推荐_【万方软件创新助手】_20140802
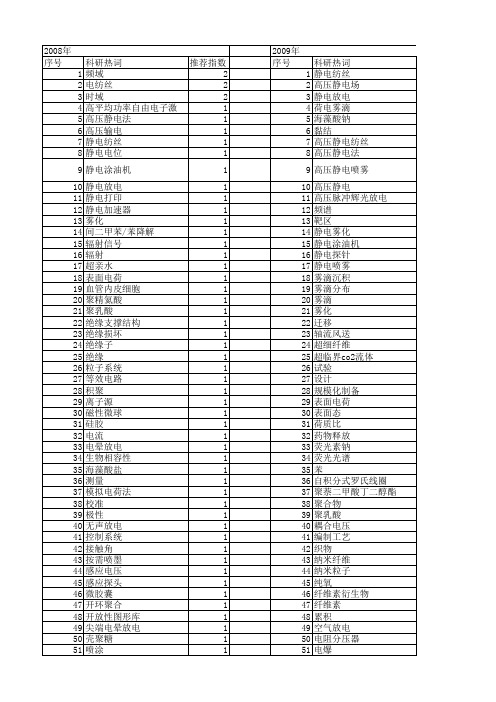
1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78
时域补偿 施药量 放电特性 接触角 微载体 微混合器 强度 弯管当量长度系数 干式电极 小波变换 射流 姜黄素 多聚磷酸钠 外水含量 壳聚糖 喷嘴 同杆双回线 压损 动态补偿 动态校准 分解层数 分布电容电流 冷冻干燥 人体-金属模型 rbf神经网络 apa微囊
推荐指数 2 2 2 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
2011年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52
科研热词 静电纺丝 高压静电场 静电放电 荷电雾滴 海藻酸钠 黏结 高压静电纺丝 高压静电法 高压静电喷雾 高压静电 高压脉冲辉光放电 频谱 靶区 静电雾化 静电涂油机 静电探针 静电喷雾 雾滴沉积 雾滴分布 雾滴 雾化 迁移 轴流风送 超细纤维 超临界co2流体 试验 设计 规模化制备 表面电荷 表面态 荷质比 药物释放 荧光素钠 荧光光谱 苯 自积分式罗氏线圈 聚萘二甲酸丁二醇酯(pbn) 聚合物 聚乳酸 耦合电压 编制工艺 织物 纳米纤维 纳米粒子 纯氧 纤维素衍生物 纤维素 累积 空气放电 电阻分压器 电爆 电晕荷电
【国家自然科学基金】_聚精氨酸_基金支持热词逐年推荐_【万方软件创新助手】_20140802
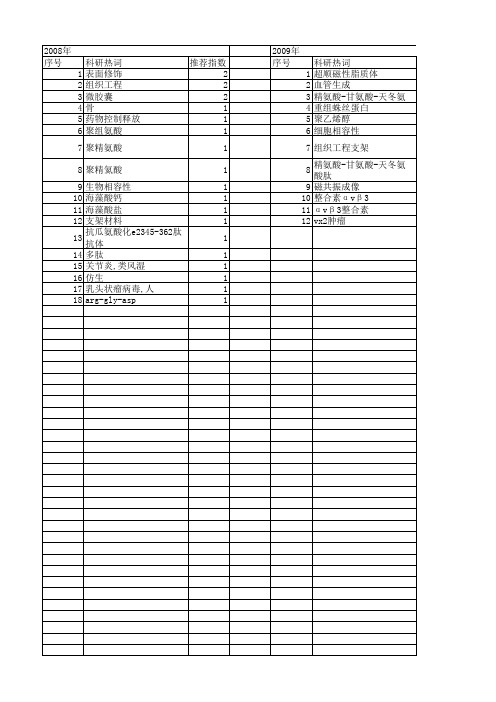
科研热词 推荐指数 表面修饰 2 组织工程 2 微胶囊 2 骨 1 药物控制释放 1 聚组氨酸 1 聚精氨酸 1 聚精氦酸 1 生物相容性 1 海藻酸钙 1 海藻酸盐 1 支架材料 1 抗瓜氨酸化e2345-362肽抗体 1 多肽 1 关节炎,类风湿 1 仿生 1 乳头状瘤病毒,人 1 arg-gly-asp 1
2010年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22
科研热词 靶向治疗 锝,整合素α vβ 3 鉴定 透膜小肽 蛹期 蛋白质转导结构域 蛋白质组 肽类,环 聚集抑制 神经胶质瘤 王浆高产蜜蜂 放射性核素显像 帕金森症 嵌合基因 小鼠,裸 寡聚精氨酸 双向电泳 原种意大利蜜蜂 冠菌素 伯克霍尔德菌 α -synuclein 16srdna
推荐指数 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
2011年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22
Байду номын сангаас
2011年 科研热词 推荐指数 顺序释放 1 色谱分离 1 肿瘤 1 聚精氨酸 1 聚乙二醇 1 聚(n-异丙基丙烯酰胺) 1 精氨酸-甘氨酸-天冬氨酸肽 1 磁共振成像 1 温度敏感聚合物 1 添加剂 1 海藻酸钠 1 微包纳药物释放体系 1 四羟丙基乙二胺 1 化学镀厚铜 1 分子显像 1 分子成像 1 几丁聚糖 1 rrl多肽 1 l-精氨酸 1 k_4fe(cn)_6 1 fe3o4纳米颗粒 1 131i标记 1
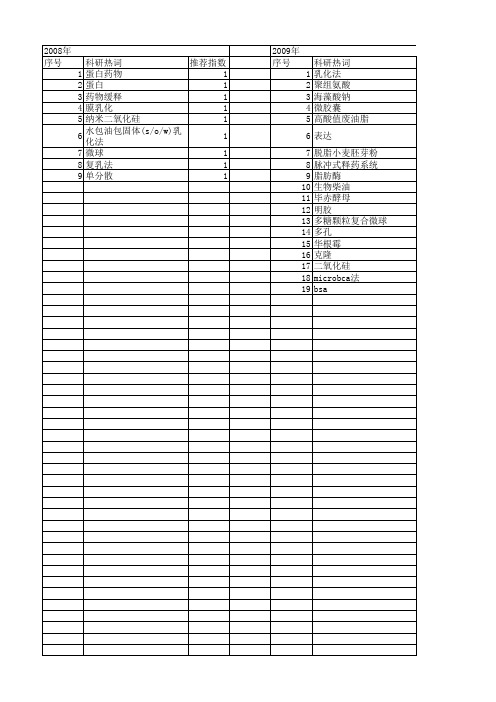
【国家自然科学基金】_乳化法_基金支持热词逐年推荐_【万方软件创新助手】_20140801
推荐指数 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
2014年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14
2014年 科研热词 超声检查 超声 脂肪酶 纳米技术 筛选 离心 湿法磷酸 液态氟碳 正交设计 微气泡 分离 优化 乳状液 1,3-甘油二酯 推荐指数 1 1 1 1 1 1 1 1 1 1 1 1 1 1
2012年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17
科研热词 高温 降滤失剂 钻井液 试验环道 表观粘度 膜乳化 聚乳酸 羟基磷灰石 纳米复合材料 稳定性 稠油 测试 微粒给药系统 复合微球 共表面活性剂 乳状液 乳剂
推荐指数 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
2008年 序号 1 2 3 4 5 6 7 8 9
科研热词 推荐指数 蛋白药物 1 蛋白 1 药物缓释 1 膜乳化 1 纳米二氧化硅 1 水包油包固体(s/o/w)乳化法 1 微球 1 复乳法 1 单分散 1
2009年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19
2011年 科研热词 马钱子碱 顺序释放 靶向效率 非乳化法 过氧化镁 药物控制释放 肝细胞癌 聚精氨酸 纳米载体 纳米给药系统 纳米微粒 纳米复合乳液 甘草次酸 海藻酸钠 海藻酸盐 水基钻井液 氨水 成膜效率 成膜剂 微包纳药物释放体系 天然高分子 双氧水 几丁聚糖 推荐指数 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1
2013年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25
微型包裹技术

第三节微型包囊技术一、概述微型包囊技术(microencapsulation)简称微囊化,系利用天然的或合成的高分子材料(称为囊材)作为囊膜壁壳(membrane wall),将固态药物或液态药物(称为囊心物)包裹而成药库型微型胶囊,简称微囊(microcapsule)。
微球(microsphere)纳米囊(nanocapsule)和纳米球(nanosphere)。
(一) 药物微囊化的应用特点(1) 掩盖药物的不良气味及口味;(2) 提高药物的稳定性;(3) 防止药物在胃内失活或减少对胃的刺激性:(4) 使液态药物固态化便于应用与贮存;(5) 减少复方药物的配伍变化;(6) 控制药物释放速率;(7) 使药物浓集于靶区;(二) 药物微囊化的进展第一阶段开始主要应用于掩盖药物的不良气味,提高药物的稳定性等方面,微囊粒径一般为5~l000μm。
第二阶段微囊粒径减小到l~250 μm,主要应用于控制药物释放。
这种粒径的载药微粒,毫微粒通过非胃肠道给药时,被器官或组织吸收能显著延长药效、降低毒性,提出高活性和生物利用度。
第三阶段主要是靶向给药的纳米囊,粒径为1~ 1000nm。
将微粒或毫微粒引导到体内特定部位,再被吸收而发挥药效。
二、囊心物与囊材(一) 囊心物(core material)(1)可以是固体,也可以是液体。
(2)通常将主药与附加剂混匀后微囊化,亦可先将主药单独微囊化,再加入附加剂。
(3)若有多种主药,可将其混匀再微囊化,或分别微囊化后再混合,这取决于设计要求、药物、囊材和附加剂的性质及工艺条件等(4)囊心物与囊材的比例要适当,如囊心物过少,将生成无囊心物的空囊。
(5)囊心物也可形成单核或多核的微囊。
(二) 囊材囊材(coating material)的一般要求是:①性质稳定;②有适宜的释药速率;③无毒、无刺激性;④能与药物配伍,不影响药物的药理作用及含量测定;⑤有一定的强度、弹性及可塑性,能完全包封囊心物;⑥具有符合要求的粘度、渗透性、亲水性、溶解性等特性。
微纳气泡技术

微纳气泡技术微纳气泡技术是近年来快速发展的一种新型技术,它具有高效、环保、节能等优点,在水处理、材料科学、医药等领域得到了广泛的应用。
本文将综述微纳气泡技术的研究进展和应用前景。
一、微纳气泡技术的原理及形成机制微纳气泡是指气泡直径小于1000μm,甚至小于10μm的气泡。
与传统气泡不同,微纳气泡围绕着几个特征:1. 直径小微纳气泡的直径相比传统气泡小很多,最小的只有几个纳米,这意味着微纳气泡内部的压力要比传统气泡高很多。
2. 寿命长传统气泡的存活时间短,一般只有几分钟,而微纳气泡的存活时间可以达到几个小时、甚至几天。
3. 高压局部在微纳气泡内部,由于气泡的小直径和高浓度的气体,局部气压很高,在水中形成的白色气体云就是微纳气泡团。
微纳气泡的形成主要有两个机制:一个是生物源性的机制,另一个是非生物源性的机制。
生物源性的机制指的是微纳气泡通过有机物的分解和厌氧微生物的代谢而产生,这种机制形成的微纳气泡寿命较短。
二、微纳气泡技术在水处理中的应用1. 水污染控制微纳气泡技术可以用来清除水中的微粒、悬浮物、有机物等污染物,尤其是可以对难以去除的油污进行有效处理。
研究表明,微纳气泡结合化学药剂可以去除水中的重金属等污染物。
微纳气泡也可以用来分离水中的不同离子种类,提高水的纯度。
2. 水资源开发微纳气泡技术可以用于水的深度处理和回用,可以将含有大量有机物质和病菌的废水处理成优质的再生水,并能有效降低生活污水的COD和BOD值,减少对环境的污染。
微纳气泡也可以用来增加水体溶氧量,提高水中生物的生存环境。
3. 水处理设备微纳气泡技术可以应用于水处理设备的清洗和消毒,可以清除管道和设备表面的菌斑、泡沫、水垢等污垢,保护设备的使用寿命。
微纳气泡可以用来增强水处理设备的处理效率,提高流量和处理速度。
微纳气泡技术在材料科学领域也具有广泛的应用前景。
1. 纳米材料制备与改性微纳气泡可以应用在制备纳米材料和改性纳米材料中,利用微纳气泡通过高压和高温的条件下使得材料发生一些物理和化学变化,可以改善纳米材料的性能和稳定性。
循环microRNA

多,但是芯片检测方法的重现性和准确性比
较差,因此只用于疾病相关循环miRNA的初
.
15
循环miRNA研究、研发体系
Solexa技术初筛患者血清中表达上升的一组miRNA qRT-PCR技术对初筛miRNA进行复筛 qRT-PCR技术对复筛结果进行验证 评价筛选出的miRNA临床诊断价值
.
16
循环miRNA展望
循环miRNA与肿瘤
有学者发现口腔黏膜鳞状细胞患者血浆中 miR-31含量较健康人显著增高,手术切除肿 瘤2周后其水平明显下降。 多种miRNA在转移型前列腺癌患者血清中含 量非常高;其中miR-375和miR-141可作为肿瘤 风险高低的生物标志分子。 Hu等发现血清中miR-486、miR-30d、miR-1和 miR-499可以作为NSCLC患者生存率的预测因 子。 用伊马替尼治疗慢性粒. 细胞白血病患者2周后13
《循环microRNA的研究现状与展望》 孙士 鹏,李金明等
.
19
谢谢!
.
20
资料可以编辑修改使用 学习愉快!
课件仅供参考哦, 实际情况要实际分析哈!
感谢您的观看
.
5
microvesicles
eraly endosomes late endosomes
shedding vesicle
exosome
cell
.
6
exosomes
.
7
循环miRNA 耐受降解机制的假说
RNA可能与DNA退火,使得它们既耐受 DNase降解又能耐受RNase降解
RNA可能被包入脂质或脂蛋白复合物内而 受到保护
循环microRNA的发展 和前景
.
1
微纳米气泡 微纳米泡沫

微纳米气泡微纳米泡沫
微纳米气泡和微纳米泡沫是指在微观尺度下的气泡和泡沫。
在
微观尺度下,气泡和泡沫的特性和行为可能会有所不同于宏观尺度
下的气泡和泡沫。
首先,微纳米气泡和泡沫通常具有非常小的尺寸,通常在微米或纳米级别,因此具有较大的比表面积和较高的表面能。
这使得它们在液体中的分布和行为与宏观尺度下的气泡和泡沫有所
不同。
微纳米气泡和泡沫在许多领域具有重要的应用价值。
例如,在
水处理领域,微纳米气泡被用于提高水的氧化性能,改善水的清洁
效果。
在生物医学领域,微纳米气泡被应用于药物输送系统和影像
学技术,以提高药物的生物利用度和影像学的分辨率。
此外,微纳
米泡沫还被用于食品加工、化妆品和个人护理产品等领域。
此外,微纳米气泡和泡沫的形成和稳定机制也是研究的热点之一。
由于其表面能较高,微纳米气泡和泡沫的稳定性较差,因此研
究人员致力于开发新的方法来稳定微纳米气泡和泡沫,以便更好地
应用于各个领域。
总的来说,微纳米气泡和泡沫作为一种新型的材料,在科学研
究和工程应用中具有广阔的前景,其特殊的性质和潜在的应用价值值得我们进一步深入研究和探索。
制药工程中的药物微纳技术研究

制药工程中的药物微纳技术研究随着科技的不断进步,制药工程中的药物微纳技术研究日益受到重视。
药物微纳技术是指利用微纳米尺度的技术手段研究制药工程中的药物,其应用广泛,包括药物的制备、表征、控释等方面。
本文将重点介绍药物微纳技术在制药工程中的应用与研究进展。
1. 药物微纳技术的背景介绍药物微纳技术是制药工程中的一项重要研究内容,其目的是利用微纳米材料的特殊性质改进药物的性能和疗效。
药物微纳技术已经成为当今制药工程领域的热点研究方向,并取得了显著的进展。
2. 药物微纳技术的应用领域药物微纳技术在制药工程中具有广泛的应用领域。
首先,药物微纳技术可以用于药物的制备,通过纳米材料载体、纳米粒子等方式,改善药物的溶解性、稳定性和生物利用度。
其次,药物微纳技术可以用于药物的表征,通过扫描电子显微镜、透射电子显微镜等技术手段,观察和分析药物的微观结构和形态。
此外,药物微纳技术还可应用于药物的控释,通过纳米载体的特殊结构和性能,实现药物在体内的缓慢释放,提高药效持久性和降低药物副作用。
3. 药物微纳技术的研究进展随着药物微纳技术的不断发展,研究者们在相关领域取得了许多重要的研究成果。
例如,利用纳米材料作为药物的载体,可以提高药物的溶解度和稳定性,延长药物的血药浓度时间,从而增强药物的疗效。
此外,利用纳米技术改良药物控释系统,可以实现药物的准确释放和定向输送,提高药物在人体内的靶向性和治疗效果。
这些研究成果为制药工程中的药物微纳技术应用提供了重要的理论基础和实践指导。
4. 药物微纳技术的挑战和前景虽然药物微纳技术在制药工程中取得了许多重要的进展,但也面临着一些挑战。
首先,药物微纳技术的研究需要多学科的结合,包括化学、物理、生物等学科的交叉与合作。
其次,药物微纳技术的安全性与可控性是研究的重要问题,需要进行深入的研究和探索。
未来,药物微纳技术在制药工程中的应用前景十分广阔,有望为新药研发和制备提供更多可能性。
综上所述,药物微纳技术在制药工程中具有重要的应用和研究价值。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Controlled release of protein drugs from newly developed amphiphilic polymer-based microparticles composed of nanoparticlesYoshinori Kakizawa,Reiji Nishio,Taisuke Hirano,Yoichiro Koshi,Mio Nukiwa,Masakazu Koiwa,Junji Michizoe,Nobuo Ida ⁎New Frontiers Research Laboratories,Toray Industries,Inc.,6-10-1Tebiro,Kamakura,Kanagawa 248-8555,Japana b s t r a c ta r t i c l e i n f o Article history:Received 15June 2009Accepted 26September 2009Available online 3October 2009Keywords:Microparticle NanoparticleSustained releaseHuman growth hormone Protein drugA novel formulation of biodegradable microparticles was developed for the sustained release of peptide and protein drugs.The microparticles were formed by the aggregation of protein nanoparticles through water-in-oil (W/O)emulsion-lyophilization and subsequent solid-in-oil-in-water (S/O/W)emulsion –solvent evaporation.Amphiphilic copolymers were used as an emulsi fier in the W/O emulsion and matrix of the microparticles.Among the various copolymers investigated,poly(lactide-co-glycolide)-grafted dextran (Dex-g-PLGA)was chosen as the best candidate on the basis of the encapsulation ef ficiency and in vitro release pro file,the near zero-order release without a signi ficant initial burst,of human growth hormone (hGH).The release rate of hGH was controllable by changing the composition of Dex-g-PLGA.The in vivo release studies using normal mice revealed that the plasma concentration of hGH was maintained for 1week without a signi ficant initial burst.The enhancement of biological activity of hGH by sustained release was con firmed by measuring the IGF-1concentration and body weight of hypophysectomized mice.These results suggest the high potential of the newly developed microparticles for the sustained release of biopharmaceuticals.©2009Elsevier B.V.All rights reserved.1.IntroductionSustained-release formulations of peptide and protein drugs (biopharmaceuticals)have attracted considerable interest because the short half-life and instability of biopharmaceuticals in plasma necessitate frequent administration by injection.Many microparticle formulations of those drugs are under development in order to reduce the dosing frequency,thereby increasing patient compliance.How-ever,despite the advances in this technology,there are only a few products that have received regulatory approval.Several problems such as the initial burst release,dif ficulty in controlling the release period and denaturation of entrapped proteins have prevented the realization of commercially viable products [1–4].The release behavior and denaturation of proteins depend on the environment that the protein encounters during the particle forma-tion and the internal structure of the microparticles.Numerous studies have been conducted to control these factors.The formation processes utilize various emulsions including water-in-oil-in-water [5],water-in-oil-in-oil [6],solid-in-oil-in-oil-in-water [7],solid-in-oil-in-oil [8]and solid-in-oil-in-water (S/O/W)[9–17].In particular,the S/O/W emulsion methods are effective for the stable encapsulation of proteins in the microparticles.Solidi fied protein microparticles in theS/O/W emulsion may suppress the contact between the protein and water/oil interface,which is one of the major causes of denaturation.However,a severe initial burst is often observed in the case of the solid phase prepared by conventional methods.The objective of this study is to develop a novel formulation of biodegradable microparticles for protein and peptide drugs through the S/O/W emulsion.The solid phase of S/O/W emulsion was prepared by lyophilization of the water-in-oil (W/O)emulsion using amphi-philic copolymers as an emulsi fier.In this process,we aimed to suppress the contact between the protein and water/oil interface using the amphiphilic copolymers aligned at the interface.The obtained solid was dispersed in an organic solvent in the form of nanoparticles.The agglomerated forms of the nanoparticles were then produced by S/O/W emulsion –solvent evaporation method to form protein-loaded microparticles.In this paper,we describe the characteristics of the microparticles and in vitro release behavior of an encapsulated protein drug,human growth hormone (hGH).The in vivo release was studied using a normal mouse and the biological activity of the released hGH was investigated using hypophysectomized mice.2.Materials and methods 2.1.MaterialsRecombinant hGH,methanol,chloroform,t -butanol and dimethyl-carbonate were purchased from Wako Pure Chemical (Osaka).DextranJournal of Controlled Release 142(2010)8–13⁎Corresponding author.Tel.:+81467328974;fax:+81467328363.E-mail address:Nobuo_Ida@nts.toray.co.jp (N.Ida).0168-3659/$–see front matter ©2009Elsevier B.V.All rights reserved.doi:10.1016/j.jconrel.2009.09.024Contents lists available at ScienceDirectJournal of Controlled Releasej ou r n a l h o m e pa g e :ww w.e l s ev i e r.c o m/l o c a t e /j c o n re l(molecular weight(MW)15,000–20,000)was purchased from Nacalai Tesque(Kyoto).DL-Lactide and hexamethyldisilazane were purchased from Tokyo Chemical(Tokyo).Glycolide was purchased from Poly-sciences,Inc.(Warrington,PA).Potassium t-butoxide(t BuOK),acetic acid and tetrahydrofuran(THF)were purchased from Kanto Chemical (Tokyo).Trifluoroacetic acid(TFA)was purchased from Kishida Chemical(Osaka).Pluronic F68was purchased from MP Biomedicals (Morgan Irvine,CA).Fluorecein-labeled dextran(MW40000,FD40) was purchased from Sigma Chemical Co.(St.Louis,MO).Anti-human growth hormone(hGH)monoclonal antibody,biotinylated anti-hGH polyclonal antibody and an ELISA kit for hGH quantitation were purchased from R&D Systems Inc.(Minneapolis,MN).Anti-mouse insulin-like growth factor1(IGF-1)monoclonal antibody and biotinylated anti-mouse IGF-1polyclonal antibody were purchased from R&D Systems Inc.(Minneapolis,MN).DL-lactide was purified by recrystallization and sublimation before use.Glycolide was purified by recrystallization.2.2.Synthesis of poly(lactide-co-glycolide)-grafted dextran(Dex-g-PLGA) graft copolymersDex-g-PLGA(LA:GA=50:50)was synthesized by graft polymer-ization of DL-lactide and glycolide on trimethylsilyl dextran(TMS-Dex)with t BuOK as an initiator,using the method reported by Ohya et al.with modification[18].Dextran was silylated using hexam-ethyldisilazane to obtain TMS-Dex with a silylation yield of82–92%. TMS-Dex and t BuOK were dissolved in anhydrous ctide and glycolide monomers dissolved in anhydrous THF were added to TMS-Dex solution by canula.The polymerization reaction was carried out for5min at room temperature under argon atmosphere.The reaction was quenched by adding acetic acid.The products were purified by chloroform/methanol precipitation.The deprotection of the OH group of TMS-Dex-g-PLGA was carried out by treatment with TFA in chloroform to yield Dex-g-PLGA.The molecular weight and graft number of the PLGA segment of Dex-g-PLGA was determined by 1H-NMR spectroscopy.GPC measurement was performed using a Tosoh GPC-8020system(eluent:DMF,column:TSK-GELα-5000×2, detector:refractive index)to check the purity of Dex-g-PLGA and to determine Mw/Mn.2.3.Microparticle preparationA typical microparticle preparation is as follows:An amphiphilic copolymer(5mg)was dissolved in100μL of dimethylcarbonate(DMC) containing20%(v/v)t-butanol.A protein solution(1or10mg/mL for in vitro or in vivo study,respectively,50μL)was added to the copolymer solution.After mixing using a vortex mixer,the obtained W/O emulsion was lyophilized.The lyophilized powder was suspended in200μL of DMC to yield S/O suspension(25mg(polymer)/mL).This S/O suspen-sion was added slowly to10%(w/v)aqueous Pluronic F68solution (2mL)and vortexed for30s or mixed using a stirrer at500,1000and 1600rpm for5min to form the S/O/W emulsion.The organic solvent was removed under reduced pressure.In the size control experiment, O/W ratios of the S/O/W emulsion were varied in the range from0.025 to1,keeping the polymer content and volume of water phase constant (2mL).The resulting microparticles were lyophilized and stored at −20°C.2.4.Particle characterizationThe morphologies of the nano-and microparticles were investi-gated by the scanning electron microscopy(SEM,Hitachi-S4800, Hitachi Ltd.,Japan).The particles suspended in water were centri-fuged at130×g for10min and the supernatant was removed.The precipitated particles were resuspended in water.SEM samples were prepared by placing a droplet of this suspension on a silicon wafer.The samples were dried in a vacuum desiccator and sputter-coated with platinum.SEM images were obtained at1.0–5.0kV.The sizes of nano-and microparticles were determined using SEM, the dynamic light scattering technique(DLS)with ELSZ(Otsuka Electronics Co.Ltd.,Japan)and the laser diffraction technique(LD) with Microtrac FRA(Nikkiso Co.Ltd.,Japan).DLS was employed for the particles with diameters smaller than1μm and LD was employed for the particles with diameters larger than1μm.The samples for DLS and LD were prepared by suspending dry particles(5mg)in water (15ml).In the SEM analysis,the size of microparticles were determined by measuring30to120particles in the SEM images and presented as mean±SD.2.5.Confocal laser scanning microscopyThe microparticles loaded withfluorecein-labeled dextran(MW 40000)as a model compound were suspended in phosphate-buffered saline(PBS)and placed in a glass-bottom96-well plate(BD Falcon). The microparticles werefixed with highly viscous polysaccharide solution.Confocal images were acquired using an Olympus laser scanning microscope(FV1000)equipped with a100×oil-immersion objective.The excitation wavelength was488nm(argon laser).2.6.Drug loading efficiencyThe encapsulation efficiency(EE)of hGH was determined as follows:the lyophilized microparticles were suspended in PBS (pH7.4)containing0.1%bovine serum albumin,0.1%Pluronic F-68 and sodium azide.The suspension was centrifuged at18,800×g for 10min and the supernatant was carefully removed.This procedure was performed thrice.The protein concentrations of the supernatants were determined by sandwich ELISA using monoclonal anti-hGH as a capture antibody and biotinylated polyclonal anti-hGH as a detection antibody.EE was determined by subtracting the amount of hGH in the supernatants from the feed amount.2.7.In vitro release studiesThe microparticles were washed thrice then suspended in PBS. The suspensions were incubated at37°C with continuous rotation at 2rpm.At different time points,an aliquot was centrifuged at 18,800×g for10min and the hGH concentration of the supernatant was determined by ELISA.2.8.In vivo release studiesThe in vivo release of hGH from the microparticles of Dex-g-PLGA (MW(Dex)17500,MW(PLGA)1822,number of graft chains38, Mw/Mn=1.20)was assessed using male BALB/c mice(10weeks old). The animals were immunosuppressed by intraperitoneal treatment with tacrolimus(Astellas Pharma)[13].The microparticles were washed thrice then suspended in PBS.The suspensions or free hGH were injected subcutaneously at a dose of28mg(hGH)/kg using 29-gauge needles.Blood samples were collected via the tail vein.After centrifugation,the heparinized plasma was collected and analyzed for hGH using the ELISA kit(R&D Systems).2.9.Biological activity of released hGHThe biological activity of released hGH was assessed using hypophysectomized male ICR mice(7weeks old).The microparticles of Dex-g-PLGA(MW(Dex)17500,MW(PLGA)1822,number of graft chains38,Mw/Mn=1.20)were washed thrice then suspended in PBS.The suspensions or free hGH were injected subcutaneously at a dose of28mg(hGH)/kg using29-gauge needles.The animals were weighed and blood samples were collected via the tail vein for9Y.Kakizawa et al./Journal of Controlled Release142(2010)8–132weeks.After centrifugation of the blood samples,the plasma was collected and analyzed for IGF-1by sandwich ELISA using monoclonal anti-mouse IGF-1as a capture antibody and biotinylated polyclonal anti-mouse IGF-1as a detection antibody.All the animal experiments were conducted in accordance with the Guidelines for Animal Experiments,Research&Development Divi-sion,Toray Industries,Inc.3.Results and discussion3.1.Microparticle preparationFig.1shows the fabrication of the microparticles entrapping protein or peptide drugs.In thefirst step,a protein aqueous solution is added to an organic solvent containing an amphiphilic copolymer as an emulsifier to form a water-in-oil(W/O)emulsion.The W/O emulsion is then quickly cooled using liquid nitrogen and lyophilized to remove water and organic solvent.The aim of this process is to obtain protein,which is present in the water phase of the W/O emulsion,as nanoparticles surrounded by the amphiphilic copoly-mers.Then,an organic solvent is added to the lyophilized powder to obtain a solid-in-oil(S/O)suspension.The S/O suspension is added to water containing Pluronic F68as an emulsifier to form a solid-in-oil-in-water(S/O/W)emulsion.Finally,the organic phase is evaporated to form microparticles with protein dispersed in the biodegradable polymer matrix.There are several studies in which the S/O/W emulsion–solvent evaporation method is used to prepare protein-loaded microparticles[9–17].In these studies,the solid phases of the protein typically have diameters in the range of2to10μm.In contrast, the size of the solid phase in our process depends on the size of the W/O emulsion formed in thefirst step.By properly choosing the condition of the emulsion formation,we can obtain the solid phase containing protein as nanoparticles.Amphiphilic copolymers play a critical role as a matrix of the microparticles and the stabilizer of W/O emulsion.According to Bancroft's rule,an oil-soluble surfactant is suitable for stabilization of a W/O emulsion[19].We synthesized more thanfifty different amphiphilic copolymers with sufficient oil solubility using this rule as a guideline and examined the particle formation capability,encapsu-lation efficiency and in vitro release behavior of a protein drug using hGH as a model(data not shown).On the basis of the screening assay results,we chose Dex-g-PLGA as the candidate polymer and further investigated the characteristics of microparticles composed of this polymer.3.2.Morphology and internal structure of microparticles of Dex-g-PLGAFig.2A shows the SEM image of the particles in the S/O suspension that was formed by the addition of the organic solvent to the lyophilized powder of the W/O emulsion.The morphologies of the microparticles obtained by solvent evaporation of the S/O/W emulsion are shown in Fig.2B.The microparticles were spherical and the surface was smooth,suggesting that the free polymer that existed in the organic phase of the S/O/W suspension forms the polymer matrix in which the nanoparticles are dispersed.The distribution of the encapsulated molecules in the microparticles was examined by confocal laser scanning microscopy usingfluorescein-labeled dextran(FD40)as a model compound(Fig.2C and D). Punctuate distribution offluorescence of FD40was observed in the microparticles,which is consistent with the proposed nanoparticle-aggregated structure of the microparticles.3.3.Size control of microparticlesThe size of the biodegradable microparticles has a profound impact on the release rate of protein[20,21].In addition,particle size is important with respect to the phagocytosis of particles by macro-phages and the needle diameter used for administration.To control the size of the microparticles,we investigated various parameters of the microparticle fabrication.Among the parameters studied,the stirring speed and ratio of the volume of the organic phase to that of the water phase(O/W ratio)for the formation of S/O/W emulsion have a considerable effect on the size of the particles.We changed the stirring speed during the formation of the S/O/W emulsion from500 to1600rpm and measured the diameter of the particles observed by SEM(Fig.3).As the stirring speed was decreased,the mean diameter of the microparticles increased from24±12to59±12μm.As for the O/W ratio,the increase in the ratio resulted in a decrease in the diameter,which was determined by SEM,DLS and LD(Figs.3and4). In these experiments,the concentration of the solid phase ofS/O Fig.1.Scheme of preparation of protein-loadedmicroparticles.Fig.2.Scanning electron micrographs of(A)nanoparticles in the S/O suspension and(B)microparticles of Dex-g-PLGA.(C)Confocal microscopy image and(D)differentialinterference micrograph of Dex-g-PLGA microparticles containing a model compound(FD40).10Y.Kakizawa et al./Journal of Controlled Release142(2010)8–13suspension varies with the O/W ratio.The diameter of the resulting particles is presumably affected by the emulsion size and concentra-tion of the S/O suspension.The diameter reached the minimum of 270±120nm at the O/W ratio of 0.25.The particles above this O/W ratio are likely to correspond to the single nanoparticle present in the S/O suspension.It is noteworthy that the range of diameters of the particles depends on the properties of the organic phase.Similar results were obtained for ethyl acetate used as the organic phase of the S/O/W emulsion.On the other hand,the diameters were in the range from 1.1±0.1to 1.2±0.2μm when chloroform was used to form the S/O/W emulsion in the range from 0.025to 0.25in O/W ratio (data not shown).Taken together,we can control the diameter of theparticles by over two orders of magnitude by properly choosing the stirring speed,O/W ratio and type of organic phase.3.4.Effects of polymer compositions on in vitro release of hGH The in vitro release of hGH from the microparticles was investigated with particular focus on the molecular weight of Dex-g-PLGA.Table 1shows the parameters of Dex-g-PLGA polymers and microparticles used for the in vitro release study.The EE of hGH was more than 94%and the diameter of all the microparticles was approximately 5μm.Fig.5shows the release pro file from the microparticles composed of Dex-g-PLGA with three different compositions.In these experi-ments,the molecular weight (MW)of dextran segments is fixed at 17500and those of the PLGA segments were varied from 2790to 4330.Because the graft numbers of the polymers are almost constant,the ratio of total MW of PLGA segments to the MW of dextran is proportional to the MW of PLGA.The microparticles composed of Dex-g-PLGA with low-molecular-weight PLGA segments released hGH at a high release rate.The time periods at which 50%of hGH was released from microparticles composed of polymer A,B and C were 7,11and 20days,respectively.It is reported that the release rate is higher for the particles composed of block copolymers with low hydrophobic segment content [22,23].As for the microparticles composed of Dex-PLGA with low PLGA content,the water molecules would diffuse inside the particles more easily because of the more hydrophilic interior.3.5.In vivo release of hGH from subcutaneously injected microparticles Fig.6shows the change in the plasma hGH concentration after subcutaneous injection to normal mice.The concentration of hGH was determined by ELISA.In the case of free drug,theplasmaFig. 3.Scanning electron micrographs of microparticles formed under various conditions.O/W ratio of the formation of S/O/W emulsion is 0.1and stirring speed during the formation of the S/O/W emulsion is (A)500,(B)1000and (C)1600rpm.The S/O/W emulsion was formed using a vortex mixer and O/W ratio is (D)0.1(E)0.175and (F)0.5.Fig.4.Microparticle size as a function of O/W ratio of the S/O/W emulsion determined by (▲)SEM,(□)DLS and (○)LD.Table 1Characteristics of Dex-g-PLGA used in in vitro release study.Polymer MW(Dex)MW(PLGA)a Number of graft chains a TotalMW b Mw/Mn c EE/%dA 17,50027902792,830 1.1794.2±0.5B 17,50030002798,500 1.1595.7±0.1C17,500433033160,390 1.1797.5±0.1a Determined by 1H-NMR.b Calculated by the equation:MW(dextran)+MW(PLGA)×number of graft chains.c Determined by GPC.dMean ±SD (n =3).Fig.5.In vitro release pro files of hGH from Dex-g-PLGA microparticles composed of various molecular weights of PLGA segments in PBS at 37°C.(▲)polymer A,(●)polymer B and (■)polymer C.Characteristics of the polymers are described in Table 1.Values are the mean and standard deviation of three experiments.11Y.Kakizawa et al./Journal of Controlled Release 142(2010)8–13concentration increased rapidly and decreased to the control level within 3days.On the other hand,the rapid increase after adminis-tration was not observed and the constant plasma level was maintained for a week in the case of the microparticles.Fourteen days after the microparticle administration,hGH was no longer detectable in the plasma;this may be due to the production of anti-hGH antibody,even though we used an immunosuppressive reagent in this experiment.The antibody production is attributed to the fact that human growth hormone is a foreign protein for a mouse [24].These results demonstrate the potential of our microparticles to overcome the problems associated with commercially available hGH-loaded microparticles,such as the initial burst and relatively short release period.3.6.In vivo study of biological activity of released proteinTo con firm the biological activity of the released hGH,the plasma concentration of insulin-like growth factor-1(IGF-1),a cytokine induced by the growth hormone activity,and change in body weight,also a marker of growth hormone action,were measured using hypophysectomized mice injected with hGH microparticles [25,26].Fig.7shows the change in the IGF-1concentration.Following free drug administration,the IGF-1level became highest (61and 75ng/mL)1day after the administration and decreased to the base level after 4days.On the other hand,following microparticle administration,the IGF-1concentration reached 108and 167ng/mL after 4days and remained high for about 2weeks.As for the body weight change,the increments at 2weeks after administration were 9%and 20%for free drug and microparticles,respectively.These results demonstrate that the hGH released from the microparticles maintained its biological activity and,because of the sustained release,the hGH encapsulated in the microparticle showed better effects than free drug.4.ConclusionsA new formulation of protein-loaded microparticles was developed through S/O/W emulsion using amphiphilic copolymers as a matrix.The near zero-order release without a signi ficant initial burst was achieved using Dex-g-PLGA.In addition,it was possible to control the size of the microparticles from 270nm to 59μm by changing the parameters including the O/W ratio of the S/O/W emulsion and the stirring speed.The release rate of hGH is dependent on the composition of Dex-g-PLGA.The in vivo release studies using normal mice revealed that the plasma concentration of hGH was maintained for 1week without a signi ficant initial burst.The biological activity of hGH and the enhancement ofactivity by sustained release were con firmed.These results suggest the high potential of the newly developed microparticles for the sustained release of biopharmaceuticals.AcknowledgementsThe authors express their great appreciation to Drs.Yuichi Ohya,Masahiro Goto and Noriho Kamiya for helpful discussion.This research was financially supported by the Japan Science and Technology Agency (JST).References[1]R.T.Bartus,M.A.Tracy,D.F.Emerich,S.E.Zale,Sustained delivery of proteins fornovel therapeutic products,Science 281(1998)1161–1162.[2]S.D.Putney,P.A.Burke,Improving protein therapeutics with sustained-releaseformulations,Nat.Biotechnol.16(1998)153–157.[3]J.L.Cleland,O.L.Johnson,S.Putney,A.J.S.Jones,Recombinant human growthhormone poly(lactic-co-glycolic acid)microsphere formulation development,Adv.Drug Deliv.Rev.28(1997)71–84.[4]F.Wu,T.Jin,Polymer-based sustained-release dosage forms for protein drugs,challenges,and recent advances,AAPS PharmSciTech 9(4)(2009)1218–1229.[5]J.L.Cleland,E.Duenas,A.Daugherty,M.Marian,J.Yang,M.Wilson,A.C.Celniker,A.Shahzamani,V.Quarmby,H.Chu,V.Mukku,A.Mac,M.Roussakis,N.Gillette,B.Boyd,D.Yeung,D.Brooks,Y.F.Maa,C.Hsu,A.J.S.Jones,Recombinant human growth hormone poly(lactic-co-glycolic acid)(PLGA)microspheres provide a long lasting effect,J.Control.Release 49(1997)193–205.[6]M.K.Yeh,A.G.A.Coombes,P.G.Jenkins,S.S.Davis,A novel emulsi fication-solventextraction technique for production of protein loaded biodegradable micropar-ticles for vaccine and drug delivery,J.Control.Release 33(1995)437–445.Fig.6.hGH concentrations in plasma of normal mice treated with (○)free hGH and (●)hGH entrapped in Dex-g-PLGA microparticles.Values are the mean and standard deviation of threemice.Fig.7.(A)IGF-1concentrations in plasma and (B)body weight of hypophysectomized mice treated with (Δ)saline,(□)free hGH and (●)hGH entrapped in Dex-g-PLGA microparticles.Values of two independent experiments at each point are presented.12Y.Kakizawa et al./Journal of Controlled Release 142(2010)8–13[7]W.Yuan,F.Wui,M.Guo,T.Jin,Development of protein delivery microspheresystem by a novel S/O/O/W multi-emulsion,Eur.J.Pharm.Sci.36(2009)212–218.[8]W.Jiang,S.P.Schwendeman,Stabilization and controlled release of bovine serumalbumin encapsulated in poly(D,L-lactide)and poly(ethylene glycol)microsphere blends,Pharm.Res.18(6)(2001)878–885.[9]J.L.Cleland, A.J.S.Jones,Stable formulations of recombinant human growthhormone and interferon-γfor microencapsulation in biodegradable microspheres, Pharm.Res.13(10)(1996)1464–1475.[10]O.L.Johnson,W.Jaworowicz,J.L.Cleland,L.Bailey,M.Charnis,E.Duenas,C.Wu,D.Shepard,S.Magil,st, A.J.S.Jones,S.D.Putney,The stabilization and encapsulation of human growth hormone into biodegradable microspheres, Pharm.Res.14(6)(1997)730–735.[11]M.Iwata,T.Tanaka,Y.Nakamura,J.W.McGinity,Selection of the solvent systemfor the preparation of poly(D,L-lactic-co-glycolic acid)microspheres containing tumor necrosis factor-alpha(TNF-α),Int.J.Pharm.160(1998)145–156.[12]T.Morita,Y.Sakamura,Y.Horikiri,T.Suzuki,H.Yoshino,Protein encapsulationinto biodegradable microspheres by a novel S/O/W emulsion method using poly (ethylene glycol)as a protein micronization adjuvant,J.Control.Release69 (2000)435–444.[13]S.Takada,Y.Yamagata,M.Misaki,K.Taira,T.Kurokawa,Sustained release ofhuman growth hormone from microcapsules prepared by a solvent evaporation technique,J.Control.Release88(2003)229–242.[14]I.J.Castellanos,K.Griebenow,Improvedα-chymotrypsin stability upon encapsu-lation in PLGA microspheres by solvent replacement,Pharm.Res.20(11)(2003) 1873–1880.[15]I.J.Castellanos,R.Crespo,K.Griebenow,Poly(ethylene glycol)as stabilizer andemulsifying agent:a novel stabilization approach preventing aggregation and inactivation of proteins upon encapsulation in biodegradable polyester micro-spheres,J.Control.Release88(2003)135–145.[16]J.W.Wang,K.M.Chua,C.H.Wang,Stabilization and encapsulation of humanimmunoglobulin G into biodegradable microspheres,J.Colloid Interface Sci.271 (2004)92–101.[17]garce,E.Garcion,N.Faisant,O.Thomas,P.Kanaujia,P.Menei,J.P.Benoit,Development and characterization of interleukin-18-loaded biodegradable micro-spheres,Int.J.Pharm.314(2006)179–188.[18]T.Ouchi,T.Saito,T.Kontani,Y.Ohya,Encapsulation and/or release behavior ofbovine serum albumin within and from polylactide-grafted dextran microspheres, Macromol.Biosci.4(2004)458–463.[19]J.Raynaud,B.Choquenet,E.Marie,E.Dellacherie,C.Nouvel,J.-L.Six,A.Durand,Emulsifying properties of biodegradable polylactide-grafted dextran copolymers, Biomacromolecules9(2008)1014–1021.[20]C.Berkland,M.King,A.Cox,K.Kim,D.W.Pack,Precise control of PLG microspheresize provides enhanced control of drug release rate,J.Control.Release82(2002) 137–147.[21]J.Panyam,M.M.Dali,S.K.Sahoo,W.Ma,S.S.Chakravarthi,G.L.Amidon,R.J.Levy,V.Labhasetwar,Polymer degradation and in vitro release of a model protein from poly(D,L-lactide-co-glycolide)nano-and microparticles,J.Control.Release92 (2003)173–187.[22]J.M.Bezemer,R.Radersma,D.W.Grijpma,P.J.Dijkstra,C.A.van Blitterswijk,J.Feijen,Microspheres for protein delivery prepared from amphiphilic multiblock copolymers2.Modulation of release rate,J.Control.Release67(2000)249–260.[23]J.M.Bezemer,R.Radersma,D.W.Grijpma,P.J.Dijstra,J.Feijen,C.A.van Blitterswijk,Zero-order release of lysozyme from poly(ethylene glycol)/poly(butylene terephthalate)matrices,J.Control.Release64(2000)179–192.[24]K.D.F.Vlugt-Wensink,R.de Vrueh,M.G.Gresnigt,C.M.Hoogerbrugge,S.C.vanBuul-Offers,L.G.J.de Leede,L.G.W.Sterkman,D.J.A.Crommelin,W.E.Hennik,R.Verrijk,Preclinical and clinical in vitro and in vivo correlation of an hGH dextran microsphere formulation,Pharm.Res.24(12)(2007)2239–2248.[25]L.S.Mathews,G.Norstedt,R.D.Palmiter,Regulation of insulin-like growth factor Igene expression by growth hormone,Proc.Natl.Acad.Sci.U.S.A.83(1986) 9343–9347.[26]M.Søndergaard,F.Dagnæs-Hansen,A.Flyvbjerg,T.G.Jense,Normalization ofgrowth in hypophysectomized mice using hydrodynamic transfer of the human growth hormone gene,Am.J.Physiol.:Endocrinol.Metab.285(2003)427–432.13Y.Kakizawa et al./Journal of Controlled Release142(2010)8–13。
