Afobazole_173352-21-1_DataSheet_MedChemExpress
Healthy Blue SC会员手册说明书
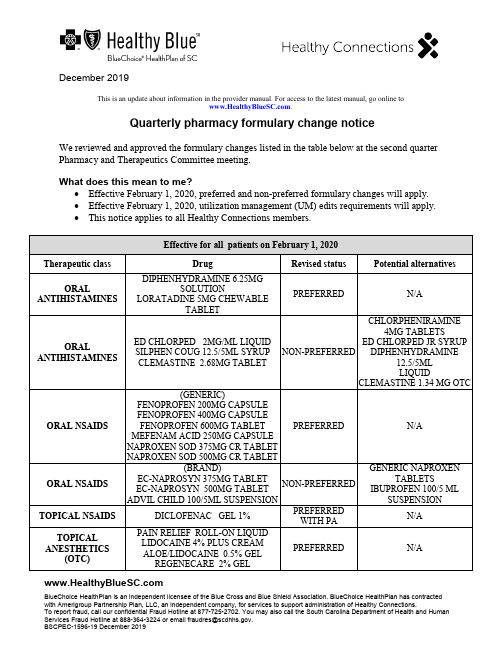
December 2019BlueChoice HealthPlan is an independent licensee of the Blue Cross and Blue Shield Association. BlueChoice HealthPlan has contracted with Amerigroup Partnership Plan, LLC, an independent company, for services to support administration of Healthy Connections.To report fraud, call our confidential Fraud Hotline at 877-725-2702. You may also call the South Carolina Department of Health and Human ************************************************************.BSCPEC-1596-19 December 2019This is an update about information in the provider manual. For access to the latest manual, go online to .Quarterly pharmacy formulary change noticeWe reviewed and approved the formulary changes listed in the table below at the second quarter Pharmacy and Therapeutics Committee meeting.What does this mean to me?• Effective February 1, 2020, preferred and non-preferred formulary changes will apply. • Effective February 1, 2020, utilization management (UM) edits requirements will apply. • This notice applies to all Healthy Connections members.Effective for all patients on February 1, 2020Therapeutic class DrugRevised status Potential alternativesORALANTIHISTAMINESDIPHENHYDRAMINE 6.25MGSOLUTIONLORATADINE 5MG CHEWABLETABLETPREFERREDN/AORALANTIHISTAMINESED CHLORPED 2MG/ML LIQUID SILPHEN COUG 12.5/5ML SYRUP CLEMASTINE 2.68MG TABLET NON-PREFERRED CHLORPHENIRAMINE4MG TABLETSED CHLORPED JR SYRUPDIPHENHYDRAMINE12.5/5MLLIQUIDCLEMASTINE 1.34 MG OTCORAL NSAIDS(GENERIC)FENOPROFEN 200MG CAPSULE FENOPROFEN 400MG CAPSULE FENOPROFEN 600MG TABLET MEFENAM ACID 250MG CAPSULE NAPROXEN SOD 375MG CR TABLET NAPROXEN SOD 500MG CR TABLETPREFERRED N/A ORAL NSAIDS(BRAND) EC-NAPROSYN 375MG TABLET EC-NAPROSYN 500MG TABLET ADVIL CHILD 100/5ML SUSPENSION NON-PREFERRED GENERIC NAPROXENTABLETSIBUPROFEN 100/5 ML SUSPENSIONTOPICAL NSAIDSDICLOFENAC GEL 1% PREFERREDWITH PAN/A TOPICALANESTHETICS(OTC)PAIN RELIEF ROLL-ON LIQUIDLIDOCAINE 4% PLUS CREAMALOE/LIDOCAINE 0.5% GELREGENECARE 2% GELPREFERRED N/ALIDODOSE 3% GELREGENECARE SPRAYALOCANE 4% GELAFTERBURN 2.5% GELXOLIDO 2% CREAM BURN RELIEF 0.5% AEROSAL ASPERCREME 4% SPRAYLIDOCAINE 3% CREAMLIDOCAINE 4% CREAMLIDOCAINE 5% CREAMAFTERSUN 0.5% GELLIDOCAINE 4% PADTOPICAL ANESTHETICS(RX)LIDOCAINE 3% CREAMLIDOCAINE 5% OINTMENT NON-PREFERREDOTC LIDOCAINEPRODUCTSRX LIDOCAINE5% PATCH(PA REQUIRED)MISCELLANEOUS ANTICONVULSANTSPREGABALIN 25MG CAPSULEPREGABALIN 50MG CAPSULEPREGABALIN 75MG CAPSULEPREGABALIN 100MG CAPSULEPREGABALIN 150MG CAPSULEPREGABALIN 200MG CAPSULEPREGABALIN 225MG CAPSULEPREGABALIN 300MG CAPSULEPREGABALIN SOL 20MG/MLPREFERREDWITH NO PRIORAUTHORIZATION(PA)N/AATOPICDERMATITIS PIMECROLIMUS 1% CREAMPREFERREDWITH STEPTHERAPY (ST)N/AFIBRATESFENOFIBRATE 130MG CAPSULEFENOFIBRATE 145MG TABLETFENOFIBRIC 35MG TABLETFENOFIBRIC 105MG TABLETFENOFIBRIC 135MG DR CAPSULENON-PREFERREDWITH STFENOFIBRATE134MG, 160MG, 200MG,43MG, 48MG,54 MG,67 MGFENOFIBRIC ACID 45 MGALCOHOL SWABS (MANUFACTURERS) GLOBAL DIABETICRITE AID NON-PREFERREDMANUFACTURERSBD DIABETESDYNAREXHEALTH MARTULTIMEDALCOHOL SWABS (MANUFACTURERS) BD DIABETESDYNAREXHEALTH MARTULTIMEDPREFERRED N/AIRON SUPPLEMENTS (GENERIC OTC)IRON 45MG TABLETSLOW-RELEASE FE 45MG TABLETHEMAX TABLETGENTLE IRON 28MG CAPSULEHIGH POTENCY FE 27MG TABLETNU-IRON 150 150MG CAPSULEABATRON AF TABLETSLOW IRON 50MG TABLETPREFERRED N/AFERGON 27MG TABLETIRON SUPPLEMENTS(BRAND OTC)FOLITAB 500 TABLET IRON 28MG TABLETFERROUS GLUC 324MG TABLETEZFE 200MG CAPSULEFERROUS GLUC TAB 324MGFERROUS SULF 324MG EC TABLETFERRETTS 325MG TABLETFERREX 150MG CAPSULEFERREX 28 MIS FERREX 150 PLUS CAPSULE FERREX 150 FORTE PL CAPSULECHEWABLE IRONPEDIATRIC IRON CHEWABLEFERROUS SUL 220/5ML LIQUIDFERROUS SULF 300/5ML SYRUPFEOSOL 200MG TABLETSLOW RELEASE FE 143MG CRTABLETNON- PREFERRED OTC GENERIC IRONSUPPLEMENTSRX PRODUCTS:HEMATOGEN FA CAPSULEHEMETAB TABLETMULTIGEN TABLETMULTIGEN PLS TABLETMULTIGEN FOLICTABLETFERRAPLUS 90 TABLETTARON FORTE CAPSULEFOLIVANE-F CAPSULEFOLIVANE-PLS CAPSULECENTRATEX CAPSULEIRON SUPPLEMENTS(PRESCRIPTIONSTRENGTH)IFEREX 150 FORTE CAPSULE HEMATOGEN CAPSULE HEMATOGEN FORTE CAPSULE TRICON CAPSULE MYFERON 150 FORTE CAPSULE FERROCITE PLUS TABLET FEROCON CAPSULE PUREVIT DUA FE PLUS CAPSULE HEMATINIC PL VIT/MIN TABLET HEMATINIC/FA TABLET POLY-IRON 150 FORT CAPSULE CORVITA 150 TABLET TRIGELS-F FORTE CAPSULE TL ICON CAPSULE SE-TAN PLUS CAPSULE NON- PREFERRED OTC GENERIC IRON SUPPLEMENTSRX PRODUCTS:HEMATOGEN FA CAPSULE HEMETAB TABLET MULTIGEN TABLET MULTIGEN PLS TABLET MULTIGEN FOLIC TABLET FERRAPLUS 90 TABLETTARON FORTE CAPSULE FOLIVANE-F CAPSULEFOLIVANE-PLS CAPSULE CENTRATEX CAPSULEUM edits — effective for all members no later than February 1, 2020 No changes in preferred/non-preferred status revision or addition to UM edit onlyANDROGENS*JATENZO CAPSULE ADD ST WITH QUANTITY LIMITS (QL)58 MG AND 198 MG QL: 4 PER DAY 237 MG QL: 2 PER DAY ANTICONVULSANTSNAYZILAM SPRAY 5MG ADD PA WITH QLQL: 50 MG PER 30 DAYS ANTICONVULSANTSOXTELLAR XR 150 MGOXTELLAR XR 600 MGREVISED QL LIMIT:150 MG: 3 TABLETS PER DAY 600 MG: 4 TABLETS PER DAYANTINEOPLASTICAGENTSPIQRAY 200 MG TABLETSPIQRAY 250 MG TABLETSPIQRAY 300 MG TABLETSADD PA WITH QL QL: 1 CARTON PER 28 DAYS ANTINEOPLASTICAGENTSXPOVIO PAK 60MGXPOVIO PAK 80MGXPOVIO PAK 100MGADD QL 1 CARTON PER 28 DAYSANTINEOPLASTICAGENTSNUBEQA 300MG TABLET ADD QL 4 TABLETS PER DAY ANTINEOPLASTICAGENTS TURALIO CAP 200MG ADD QL 4 TABLETS PER DAY ANTINEOPLASTICAGENTS PIQRAY 200MG TAB DOSE PIQRAY 300MG TAB DOSE PIQRAY 250MG TAB DOSE REVISE QL1 CARTON PER 28 DAYS CHOLESTEROLAGENTS EZALLOR SPRINKLE 5 MG CAP EZALLOR SPRINKLE 10 MG CAP EZALLOR SPRINKLE 20 MG CAP EZALLOR SPRINKLE 40 MG CAP ADD PA AND QLQL: 1 TABLET PER DAY COPD AGENTS DUAKLIR 400/12 INHALER ADD ST AND QLQL: 1 INHALER PER 30 DAYSCYSTIC FIBROSISAGENTSKALYDECO PAK 25MG ADD QL2 PACKETS PER DAYCYSTIC FIBROSISAGENTSORKAMBI GRANULES ADD QL2 PACKETS PER DAY HIVDOVATO TABLET EDURANT 25 MG TABLET DELSTRIGO TABLET COMPLERA TABLET ODEFSEY TABLET JULUCA TABLET ADD PA FOR NEW STARTS AND ADD QLQL: 1 PER DAY HIVINTELENCE TABLET ADD PA FOR NEW STARTS AND ADD QLQL:200 MG- 2 TABLETS PER DAY 400 MG- 4 TABLETS PER DAY 25 MG – 16 TABLETS PER DAYHIVATRIPLA TABLET BIKTARVY TABLET CIMDUO TABLET DESCOVY TABLETEMTRIVA 200 MG CAPSULE EPIVIR 300 MG TABLET EPZICOM TABLET EVOTAZ TABLET GENVOYA TABLET PIFELTRO 100 MG TABLET PREZCOBIX TABLET PREZISTA 800 MG TABLET REYATAZ 300 MG CAPSULESTRIBILD TABLET SUSTIVA 600 MG TABLETSYMFI TABLET SYMFI LO TABLET SYMTUZA TABLET TRIUMEQ TABLET TRUVADA TABLET TYBOST 150 MG TABLET VIDEX EC 400 MG CAPSULE VIDEX EC 250 MG CAPSULE VIRAMUNE XR 400 MG TABLETADD QL 1 PER DAYTEMIXYS TABLETHIVREYATAZ 200 MG CAPSULE REYATAZ 150 MG CAPSULE VIDEX EC 200 MG CAPSULE ZERIT 40 MG CAPSULE ZERIT 30 MG CAPSULE COMBIVIR TABLET DUTREBIS TABLET EPIVIR 150 MG TABLET ISENTRESS HD 600 MG TABLET PREZISTA 600 MG TABLET RETROVIR 300 MG TABLET SELZENTRY 75 MG TABLET TIVICAY 10 MG, 25 MG AND 50 MGTABLETTRIZIVIR TABLETVIRAMUNE 200 MG TABLET ZIAGEN 300 MG TABLET ADD QL 2 PER DAYHIV ISENTRESS 100 MG GRANULE PACKET FOR SUSPENSION ADD QL2 PACKETS PER DAYHIVVIDEX EC 125 MG CAPSULE VIRAMUNE XR 100MG TABLET ADD QL 3 PER DAYHIVAPTIVUS 250 MG CAPSULE INVIRASE 500 MG TABLET ISENTRESS 400 MG TABLET KALETRA 200 MG-50 MG TABLETLEXIVA 700 MG TABLET SELZENTRY 300 MG TABLET SELZENTRY 150 MG TABLET SUSTIVA 200 MG CAPSULE VIRACEPT 625 MG TABLET ZERIT 20 MG CAPSULE ZERIT 15 MG CAPSULEADD QL 4 PER DAYHIVREYATAZ 50 MG POWDER FORSUSPENSIONADD QL5 PACKETS PER DAYHIVCRIXIVAN 400 MG CAPSULE PREZISTA 150 MG TABLET RESCRIPTOR 200 MG TABLET RETROVIR 100 MG CAPSULE ISENTRESS 100 MG CHEWABLEADD QL 6 PER DAY HIV SELZENTRY 25 MG TABLET ADD QL 8 PER DAY HIV TROGARZO 150MG/ML VIAL ADD QL8 VIALS PER 28 DAYSHIVINVIRASE 200 MG CAPSULE KALETRA 100 MG-25 MG TABLETPREZISTA 75 MG TABLET VIRACEPT 250 MG TABLET ADD QL 10 PER DAY HIVCRIXIVAN 200 MG CAPSULE NORVIR 100 MG TABLET NORVIR 100 MG CAPSULEADD QL 12 PER DAYNORVIR 100 MG ORAL POWDERPACKETRESCRIPTOR 100 MG TABLET SUSTIVA 50 MG CAPSULEHIV APTIVUS 100 MG/ML SOLUTION ADD QL 13 ML PER DAYHIV PREZISTA 100 MG/ML SUSPENSION ADD QL 14 ML PER DAY HIV KALETRA 400 MG-100 MG/5 MLORAL SOLUTIONNORVIR 80 MG/ML ORAL SOLUTION ADD QL 16 ML PER DAY HIV ISENTRESS 25 MG CHEWABLE ADD QL24 TABLETS PER DAYHIV EMTRIVA 10 MG/ML SOLUTION ADD QL 29 ML PER DAYHIVEPIVIR 10 MG/ML ORAL SOLUTION ZIAGEN 20 MG/ML SOLUTION ADD QL 32 ML PER DAY HIVVIDEX 4 GM PEDIATRIC ORALSOLUTIONVIDEX 2 GM PEDIATRIC ORALSOLUTIONVIRAMUNE 50 MG/5 MLSUSPENSION ADD QL 40 ML PER DAY HIV VIRACEPT 50 MG/G POWDERADD QL 53 GM PER DAYHIV FUZEON 90 MG VIAL ADD QL60 VIALS PER 30 DAYSHIV LEXIVA 50 MG/ML SUSPENSION ADD QL 60 ML PER DAYHIV SELZENTRY 20 MG/ML ORALSOLUTION ADD QL 62 ML PER DAYHIV RETROVIR 10 MG/ML SYRUP ADD QL 64 ML PER DAYHIVZERIT 1 MG/ML SOLUTION ADD QL 80 ML PER DAY IRRITABLE BOWEL SYNDROME (IBS)AGENTSZELNORM 6MG TABLET ADD PA AND QL QL 2 TABLETS PER DAY LAMBERT-EATON MYASTHENIC SYNDROME AGENTSRUZURGI 10MG TABLET ADD PA AND QL QL 10 TABLETS PER DAYNARCOTIC ANTAGONISTS SUBLOCADE 100/0.5 INJECTION SUBLOCADE 300/1.5 INJECTION REMOVE PANARCOTIC ANTAGONISTS VIVITROL 380MG INEJCTION REMOVE PA AND ADD QL QL 1 VIAL PER 28 DAYSNARCOTIC ANTAGONISTS ZUBSOLV 2.9-0.71 SUB REVISE QL QL 5 PER DAY ORAL DIABETICAGENTS*QTERNMET XR TABLETADD ST AND QLQL:5 MG/5 MG/1000 MG, 10 MG/5 MG/1000 MG:1 TABLET PER DAY2.5 MG/2.5 MG/1000 MG, 5 MG/2.5 MG/10000MG: 2 TABLETS PER DAYORAL DIABETICAGENTS QTERN 5-5MG TABLET ADD QL1 TABLET 28 DAYSINJECTABLE DIABETIC AGENTSOZEMPIC 2/1.5ML INJECTION ADD QL 1 PER 28 DAYSPRENATAL VITAMINS DUET DHADUET DHA BALANCEDNESTABS ABC NESTABS DHA OBTREX DHA SELECT-OB+DHATHERANATAL COMPLETEVITAFOL FE+ VITAFOL-OB+DHABAL-CARE DHA ESSENTIAL ADD QL 2 PER DAYPRENATAL VITAMINS CITRANATAL B-CALMADD QL 3 PER DAYTOPICAL ANTIPRURITICS DOXEPIN HCL 5% CREAM,ZONALON 5% CREAM, PRUDOXIN5% CREAM ADD PA AND QLQL 1 TUBE PER FILL; 1 FILL PER 3 MONTHSTOPICAL ANESTHETIC COMBINATIONSLIDOCAINE/PRILOCAINE CREAMREVISE QL30 GM PER 30 DAYS* Clinical edits will be put in place as these new drugs to come market.What action do I need to take?Please review these changes and work with your Healthy Connections members to transition them to formulary alternatives. If you determine preferred formulary alternatives are notclinically appropriate for specific members, you will need to obtain prior authorization (PA) to continue coverage beyond the applicable effective date.What if I need assistance?We recognize the unique aspects of member cases. If your Healthy Connections member cannot be converted to a formulary alternative for medical reasons, call our Pharmacy department at 866-902-1689 and follow the voice prompts for pharmacy PA.You can find the Preferred Drug List on our website at > Providers > Pharmacy Information. If you need assistance with any other item, contact the Customer Care Center at 866-757-8286.。
仿制药参比制剂目录(第七批)
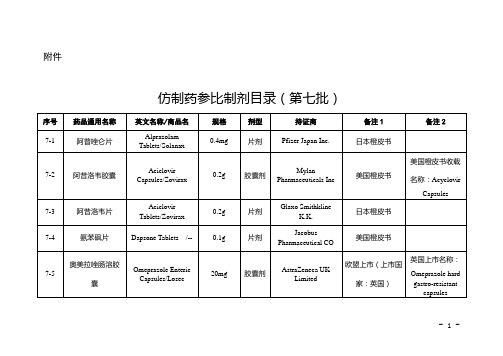
片剂(肠溶片)
AstraZeneca K.K.
日本橙皮书
7-8
奥美拉唑肠溶片
Omeprazole Enteric-coated Tablets/Omepral
10mg
片剂(肠溶片)
AstraZeneca K.K.
日本橙皮书
7-9
苯巴比妥片
Phenobarbital Tablets/
Luminaletten
7-20
茶碱缓释片
Theophylline Sustained-release Tablets/THEOPHYLLINE
0.1g(按C7H8N4O2计)
片剂(缓释片)
PLIVA INC
美国橙皮书
美国橙皮书收载名称:Theophylline Extended-release Tablets
7-21
地红霉素肠溶片
0.1g
片剂
DESITIN Arzneimittel GmbH
欧盟上市(上市国家:德国;产地:德国)
7-12
苯妥英钠片
Phenytoin Sodium Tablets
50mg
片剂
Aurobindo Pharma - Milpharm Ltd.
欧盟上市(上市国家:英国;产地:英国)
7-13
苯妥英钠片
Phenytoin Sodium Tablets
0.5mg
片剂
HOFFMANN LA ROCHE INC
美国橙皮书
7-47
马来酸氯苯那敏片
Chlorphenamine Maleate Tablets/Chlor-Tripolon
4mg
片剂
BAYER INC CONSUMER CARE
TALON Superflow Metal Affinity Resin说明书

Safety Data SheetRevision Date 2022-12-26Revision Number 91. IdentificationProduct identifier Product NameTALON Superflow Metal Affinity ResinOther means of identification Product Code635670 UN number or ID number UN3082SynonymsNo information availableRecommended use of the chemical and restrictions on use Identified uses No information available Restrictions on useNo information availableDetails of the supplier of the safety data sheetEmergency telephone number Emergency telephoneIn case of emergency, call PERS (Professional Emergency Resource Services) 1-800-633-8253 (US) or 801-629-0667 (international).2. Hazard(s) identificationProduct Classification Data Acute toxicity - Oral Category 2 CarcinogenicityCategory 1AHazards not otherwise classified (HNOC) Not applicable Label elements Supplier USA:Takara Bio USA, Inc. 2560 Orchard Parkway San Jose, CA 95131, USAPhone: 800.662.2566/888.251.6618 Web: DangerHazard statements Fatal if swallowed May cause cancerAppearance Pink slurry Physical state Paste / Gel Liquid Odor Alcohol Precautionary Statements - PreventionObtain special instructions before useDo not handle until all safety precautions have been read and understoodWear protective gloves/protective clothing/eye protection/face protectionWash face, hands and any exposed skin thoroughly after handlingDo not eat, drink or smoke when using this productPrecautionary Statements - ResponseIF exposed or concerned: Get medical advice/attentionSpecific treatment (see supplemental first aid instructions on this label)IF SWALLOWED: Immediately call a POISON CENTER or doctorRinse mouthPrecautionary Statements - StorageStore locked upPrecautionary Statements - DisposalDispose of contents/container to an approved waste disposal plantUnknown acute toxicity50.941 % of the mixture consists of ingredient(s) of unknown acute oral toxicityOther informationHarmful to aquatic life with long lasting effects. Harmful to aquatic life.3. Composition/information on ingredientsSubstanceNot applicable.MixtureChemical name CAS No Weight-% Trade secret Ethanol 64-17-5 10 - 20 *4. First-aid measuresDescription of first aid measuresGeneral advice Immediate medical attention is required. Show this safety data sheet to the doctor inattendance. IF exposed or concerned: Get medical advice/attention.Inhalation Remove to fresh air.Eye contact Rinse thoroughly with plenty of water for at least 15 minutes, lifting lower and upper eyelids.Consult a physician.Skin contact Wash skin with soap and water.Ingestion Get immediate medical advice/attention. Do NOT induce vomiting. Clean mouth with waterand drink afterwards plenty of water. Never give anything by mouth to an unconsciousperson.Most important symptoms and effects, both acute and delayedSymptoms No information available.Indication of any immediate medical attention and special treatment neededNote to physicians Treat symptomatically.5. Fire-fighting measuresSuitable Extinguishing Media Use extinguishing measures that are appropriate to local circumstances and thesurrounding environment.Large Fire CAUTION: Use of water spray when fighting fire may be inefficient.Unsuitable extinguishing media Do not scatter spilled material with high pressure water streams.Specific hazards arising from thechemicalNo information available.Explosion DataSensitivity to mechanical impact None.Sensitivity to static discharge None.Special protective equipment for fire-fighters Firefighters should wear self-contained breathing apparatus and full firefighting turnout gear. Use personal protection equipment.6. Accidental release measuresPersonal precautions, protective equipment and emergency proceduresPersonal precautions Ensure adequate ventilation.Other information Refer to protective measures listed in Sections 7 and 8.Methods and material for containment and cleaning upMethods for containment Prevent further leakage or spillage if safe to do so.Methods for cleaning up Pick up and transfer to properly labeled containers.7. Handling and storagePrecautions for safe handlingAdvice on safe handling Handle in accordance with good industrial hygiene and safety practice. Avoid contact withskin, eyes or clothing.Conditions for safe storage, including any incompatibilitiesStorage Conditions Store locked up. Keep containers tightly closed in a dry, cool and well-ventilated place.Keep out of the reach of children.8. Exposure controls/personal protectionControl parametersExposure LimitsChemical name ACGIH TLV OSHA PEL NIOSHEthanol 64-17-5 STEL: 1000 ppm TWA: 1000 ppmTWA: 1900 mg/m3(vacated) TWA: 1000 ppm(vacated) TWA: 1900 mg/m3IDLH: 3300 ppmTWA: 1000 ppmTWA: 1900 mg/m3Appropriate engineering controlsEngineering controls ShowersEyewash stationsVentilation systems.Individual protection measures, such as personal protective equipmentEye/face protection No special protective equipment required.Hand protection Wear suitable gloves.Skin and body protection Wear suitable protective clothing.Respiratory protection No protective equipment is needed under normal use conditions. If exposure limits areexceeded or irritation is experienced, ventilation and evacuation may be required. General hygiene considerations Do not eat, drink or smoke when using this product. Wash hands before breaks andimmediately after handling the product.9. Physical and chemical propertiesInformation on basic physical and chemical propertiesPhysical state Paste / Gel LiquidAppearance Pink slurryColor No information availableOdor AlcoholOdor Threshold No information availableProperty Values Remarks • MethodpH No data available None knownMelting point / freezing point No data available None knownBoiling point/boiling range (°C) No data available None knownFlash point No data available Open cupEvaporation Rate No data available None knownFlammability (solid, gas) No data available None knownFlammability Limit in Air None knownUpper flammability limit: No data availableLower flammability limit: No data availableVapor pressure No data available None knownVapor density No data available None knownRelative density No data available None knownWater solubility No data available None knownSolubility in other solvents No data available None knownPartition coefficient No data available None knownAutoignition temperature 363 °C / 685.4 °F None knownDecomposition temperature None knownKinematic viscosity No data available None knownDynamic Viscosity No data available None knownOther informationExplosive properties No information availableOxidizing properties No information availableSoftening point No information availableMolecular weight No information availableVOC content No information availableLiquid Density No information availableBulk Density No information available10. Stability and reactivityReactivity No information available.Chemical stability Stable under normal conditions.Possibility of hazardous reactions None under normal processing.Conditions to Avoid None known based on information supplied.Incompatible materials None known based on information supplied.Hazardous decomposition products None known based on information supplied.11. Toxicological informationInformation on likely routes of exposureProduct InformationInhalation Specific test data for the substance or mixture is not available.Eye contact Specific test data for the substance or mixture is not available.Skin contact Specific test data for the substance or mixture is not available.Ingestion Specific test data for the substance or mixture is not available. Fatal if swallowed. (based oncomponents).Symptoms related to the physical, chemical and toxicological characteristicsSymptoms No information available.Acute toxicityNumerical measures of toxicityThe following values are calculated based on chapter 3.1 of the GHS documentATEmix (oral) 9.81 mg/kgATEmix (inhalation-dust/mist) 573.50 mg/lUnknown acute toxicity50.941 % of the mixture consists of ingredient(s) of unknown acute oral toxicityComponent InformationChemical name Oral LD50 Dermal LD50 Inhalation LC50 Ethanol = 7060 mg/kg ( Rat ) - = 116.9 mg/L ( Rat ) 4 h64-17-5 = 133.8 mg/L ( Rat ) 4 hDelayed and immediate effects as well as chronic effects from short and long-term exposureSkin corrosion/irritation No information available.Serious eye damage/eye irritation No information available.Respiratory or skin sensitization No information available.Germ cell mutagenicity No information available.Carcinogenicity Contains a known or suspected carcinogen. Classification based on data available foringredients. May cause cancer.The table below indicates whether each agency has listed any ingredient as a carcinogen.Chemical name ACGIH IARC NTP OSHA Ethanol64-17-5A3 Group 1 Known XLegendACGIH (American Conference of Governmental Industrial Hygienists)A3 - Animal CarcinogenIARC (International Agency for Research on Cancer)Group 1 - Carcinogenic to HumansNTP (National Toxicology Program)Known - Known CarcinogenOSHA (Occupational Safety and Health Administration of the US Department of Labor)X - PresentReproductive toxicity No information available.STOT - single exposure No information available.STOT - repeated exposure No information available.Target organ effects Liver, Respiratory system, Eyes, Skin, Central nervous system, Blood, Reproductivesystem.Aspiration hazard No information available.Other adverse effects No information available.Interactive effects No information available.12. Ecological informationEcotoxicity Harmful to aquatic life with long lasting effects.Chemical name Algae/aquatic plants Fish Toxicity tomicroorganismsCrustaceaEthanol 64-17-5 - LC50: 12.0 - 16.0mL/L(96h, Oncorhynchus- LC50: 9268 - 14221mg/L(48h, Daphnia magna)mykiss)LC50: >100mg/L (96h, Pimephales promelas) LC50: 13400 - 15100mg/L (96h, Pimephalespromelas) EC50: =2mg/L (48h, Daphnia magna)Persistence and degradability No information available.Bioaccumulation There is no data for this product.Component InformationChemical name Partition coefficientEthanol64-17-5-0.35Other adverse effects No information available.13. Disposal considerationsWaste treatment methodsWaste from residues/unused products Dispose of in accordance with local regulations. Dispose of waste in accordance with environmental legislation.Contaminated packaging Do not reuse empty containers.California Hazardous Waste Status This product contains one or more substances that are listed with the State of California asa hazardous waste.14. Transport informationDOTUN number or ID number UN3082Proper shipping name Environmentally hazardous substance, liquid, n.o.s.Transport hazard class(es) 9Packing group IIISpecial Provisions 8, 146, 173, 335, 441, IB3, T4, TP1, TP29DOT Marine Pollutant NPDescription UN3082, Environmentally hazardous substance, liquid, n.o.s., 9, IIIEmergency Response GuideNumber171TDGUN number or ID number UN3082UN proper shipping name Environmentally hazardous substance, liquid, n.o.s.Transport hazard class(es) 9Packing group IIISpecial Provisions 16, 99Description UN3082, Environmentally hazardous substance, liquid, n.o.s. (Ethanol), 9, IIIMEXUN number or ID number UN3082UN proper shipping name Environmentally hazardous substance, liquid, n.o.s.Transport hazard class(es) 9Packing group IIITechnical Name EthanolDescription UN3082, Environmentally hazardous substance, liquid, n.o.s. (Ethanol), 9, IIISpecial Provisions 274, 331, 335ICAO (air)UN number or ID number UN3082UN proper shipping name Environmentally hazardous substance, liquid, n.o.s.Transport hazard class(es) 9Packing group IIIDescription UN3082, Environmentally hazardous substance, liquid, n.o.s. (Ethanol), 9, III Special Provisions A97, A158, A197, A215IATAUN number or ID number UN3082UN proper shipping name Environmentally hazardous substance, liquid, n.o.s.Transport hazard class(es) 9Packing group IIITechnical Name EthanolDescription UN3082, Environmentally hazardous substance, liquid, n.o.s. (Ethanol), 9, III Special Provisions A97, A158, A197ERG Code 9LUN number or ID number UN3082UN proper shipping name Environmentally hazardous substance, liquid, n.o.s.Transport hazard class(es) 9Packing group IIIEmS-No F-A, S-FSpecial Provisions 274, 335, 969Marine pollutant PDescription UN3082, Environmentally hazardous substance, liquid, n.o.s., 9, III, Marine Pollutant RIDUN number or ID number UN3082UN proper shipping name Environmentally hazardous substance, liquid, n.o.s.Transport hazard class(es) 9Packing group IIIClassification code M6Special Provisions 274, 335, 375, 601Description UN3082, Environmentally hazardous substance, liquid, n.o.s. (Ethanol), 9, IIIADRUN number or ID number UN3082UN proper shipping name Environmentally hazardous substance, liquid, n.o.s.Transport hazard class(es) 9Packing group IIIClassification code M6Tunnel restriction code (-)Special Provisions 274, 335, 601, 375Description UN3082, Environmentally hazardous substance, liquid, n.o.s. (Ethanol), 9, III, (-) ADNNotes Could not find a Marine Pollutant Name.UN number or ID number UN3082UN proper shipping name Environmentally hazardous substance, liquid, n.o.s.Transport hazard class(es) 9Packing group IIIClassification code M6Special Provisions 274, 335, 375, 601Description UN3082, Environmentally hazardous substance, liquid, n.o.s. (Ethanol, Cobalt), 9, III Equipment Requirements PP15. Regulatory informationInternational InventoriesTSCA -.*Contact supplier for details. One or more substances in this product are either not listed on the US TSCA inventory, listed on the confidential US TSCA inventory or are otherwise exempted from inventory listing requirementsDSL/NDSL -.EINECS/ELINCS -.ENCS -.IECSC -.KECL -.PICCS -.AICS -.Legend:TSCA - United States Toxic Substances Control Act Section 8(b) InventoryDSL/NDSL - Canadian Domestic Substances List/Non-Domestic Substances ListEINECS/ELINCS - European Inventory of Existing Chemical Substances/European List of Notified Chemical SubstancesENCS - Japan Existing and New Chemical SubstancesIECSC - China Inventory of Existing Chemical SubstancesKECL - Korean Existing and Evaluated Chemical SubstancesPICCS - Philippines Inventory of Chemicals and Chemical SubstancesAICS - Australian Inventory of Chemical SubstancesUS Federal RegulationsSARA 313Section 313 of Title III of the Superfund Amendments and Reauthorization Act of 1986 (SARA). This product does not contain any chemicals which are subject to the reporting requirements of the Act and Title 40 of the Code of Federal Regulations, Part 372. SARA 311/312 Hazard CategoriesShould this product meet EPCRA 311/312 Tier reporting criteria at 40 CFR 370, refer to Section 2 of this SDS for appropriate classifications.CWA (Clean Water Act)This product does not contain any substances regulated as pollutants pursuant to the Clean Water Act (40 CFR 122.21 and 40 CFR 122.42).CERCLAThis material, as supplied, does not contain any substances regulated as hazardous substances under the Comprehensive Environmental Response Compensation and Liability Act (CERCLA) (40 CFR 302) or the Superfund Amendments and Reauthorization Act (SARA) (40 CFR 355). There may be specific reporting requirements at the local, regional, or state level pertaining to releases of this material.US State RegulationsCalifornia Proposition 65This product contains the following Proposition 65 chemicals:.Chemical name California Proposition 65Ethanol - 64-17-5 CarcinogenDevelopmentalCobalt - 7440-48-4 CarcinogenU.S. State Right-to-Know RegulationsChemical name New Jersey Massachusetts Pennsylvania EthanolX X X 64-17-5X X X Cobalt7440-48-4U.S. EPA Label InformationEPA Pesticide Registration Number Not applicable16. Other informationNFPA Health hazards 3 Flammability 1 Instability 0 Special hazards - HMIS Health hazards * 3 Flammability 1 Physical hazards 0 Personal protection X Chronic Hazard Star Legend * = Chronic Health HazardKey or legend to abbreviations and acronyms used in the safety data sheetLegend Section 8: EXPOSURE CONTROLS/PERSONAL PROTECTIONTWA Time weighted average STEL Short term exposure limitCeiling Maximum limit value * Skin designationKey literature references and sources for data used to compile the SDSAgency for Toxic Substances and Disease Registry (ATSDR)U.S. Environmental Protection Agency ChemView DatabaseEuropean Food Safety Authority (EFSA)EPA (Environmental Protection Agency)Acute Exposure Guideline Level(s) (AEGL(s))U.S. Environmental Protection Agency Federal Insecticide, Fungicide, and Rodenticide ActU.S. Environmental Protection Agency High Production Volume ChemicalsFood Research JournalHazardous Substance DatabaseInternational Uniform Chemical Information Database (IUCLID)Japan GHS ClassificationAustralia National Industrial Chemicals Notification and Assessment Scheme (NICNAS)NIOSH (National Institute for Occupational Safety and Health)National Library of Medicine's ChemID Plus (NLM CIP)National Library of Medicine’s PubMed database (NLM PUBMED)National Toxicology Program (NTP)New Zealand's Chemical Classification and Information Database (CCID)Organization for Economic Co-operation and Development Environment, Health, and Safety PublicationsOrganization for Economic Co-operation and Development High Production Volume Chemicals ProgramOrganization for Economic Co-operation and Development Screening Information Data SetWorld Health OrganizationRevision Date 2022-12-26Revision Note No information available.DisclaimerThe information provided in this Safety Data Sheet is correct to the best of our knowledge, information and belief at the date of its publication. The information given is designed only as a guidance for safe handling, use, processing, storage, transportation, disposal and release and is not to be considered a warranty or quality specification. The information relates only to the specific material designated and may not be valid for such material used in combination with any other materials or in any process, unless specified in the text.End of Safety Data Sheet。
CFDA发布的仿制药参比制剂目录汇总

Amoxicillin for Suspension/Clamoxyl Amoxicillin Capsules/Amoxil Amoxicillin Capsules/Amoxil Amoxicillin Chewable Tablets/Amoxicillin Amoxicillin Chewable Tablets/Amoxicillin Amoxicillin Chewable Tablets/Amoxicillin Amoxicillin Chewable Tablets/Amoxicillin Amoxicillin and Clavulanate Potassium for Suspension/Augmentin Amoxicillin and Clavulanate Potassium for Suspension/Augmentin
1-19 1-20 1-21 3-16
8-8 8-219 8-9 7-1 4-33 3-27 3-26 4-31 4-32 7-97 6-35 3-13 3-13 4-20 4-20 4-19 4-19 3-14 3-15 10-1 10-271 3-18 3-19 4-26
8-10 8-11 8-12 8-13 8-14
8-17
Aminobutyric Acid Tablets/GAMMALON Amlodipine Besylate and Atorvastatin Calcium Tablets/-Amlodipine Besylate and Atorvastatin Calcium Tablets/-Amlodipine Besylate and Atorvastatin Calcium Tablets/-Olanzapine Orally Disintegrating Tablets/ Zyprexa Olanzapine Orally Disintegrating Tablets/ Zyprexa Olanzapine Orally Disintegrating Tablets/ Zyprexa Olanzapine Orally Disintegrating Tablets/ Zyprexa Olanzapine Tablets/ Zyprexa Olanzapine Tablets/ Zyprexa Olanzapine Tablets/ Zyprexa Oxcarbazepine Tablets/Trileptal Oxcarbazepine Tablets/Trileptal Oxcarbazepine Tablets/Trileptal Orlistat Capsules/Alli Orlistat Capsules/Xenical
常用多肽缩合试剂
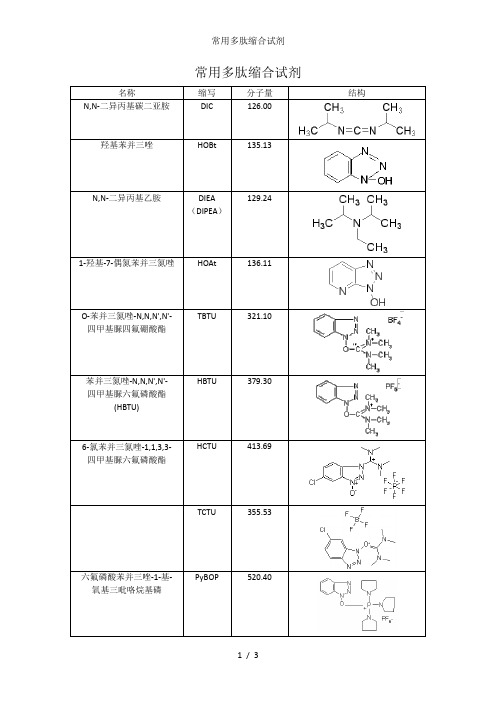
名称缩写分子量结构N,N-二异丙基碳二亚胺DIC 126.00羟基苯并三唑HOBt 135.13129.24N,N-二异丙基乙胺DIEA(DIPEA)1-羟基-7-偶氮苯并三氮唑HOAt 136.11TBTU 321.10O-苯并三氮唑-N,N,N',N'-四甲基脲四氟硼酸酯HBTU 379.30苯并三氮唑-N,N,N',N'-四甲基脲六氟磷酸酯(HBTU)HCTU 413.696-氯苯并三氮唑-1,1,3,3-四甲基脲六氟磷酸酯TCTU 355.53PyBOP 520.40六氟磷酸苯并三唑-1-基-氧基三吡咯烷基磷名称缩写分子量结构PyAOP 521.38(3H-1,2,3-三唑并[4,5-b]吡啶-3-氧基)三-1-吡咯烷基鏻六氟磷酸盐DCC 206.33N,N'-二环己基碳二亚胺4-二甲氨基吡啶DMAP 122.17DBU 152.241,8-二氮杂双环[5.4.0]十一碳-7-烯1,1’-羰基二咪唑CDI 162.15HATU 380.232-(7-偶氮苯并三氮唑)-N,N,N',N'-四甲基脲六氟磷酸酯HOOBt 163.103-羟基-1,2,3-苯并三嗪-4(3H)-酮Cl-HOBt 169.576-氯-1-羟基苯并三氮唑EDC.HCl 191.71-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐TATU 322.1O-(7-氮杂苯并三氮唑)-N,N,N',N'-四甲基脲四氟硼酸盐名称缩写分子量结构O-(1,2-二氢-2-氧-吡啶基)--1,1,3,3-四甲基脲四氟硼酸盐TPTU 297.10O-(N-琥珀酰亚胺基)-N NN'N'-四甲基四氟硼酸脲TSTU 301.10三吡咯烷基溴化鏻六氟磷酸盐PyBrOP 466.20N,N,N',N'-四甲基氯甲脒六氟磷酸盐TCFH 280.583 -二乙氧基磷酰基-1,2,3-苯唑4(3H)-酮DEPBT 299.23O-[(乙氧基羰基)氰基甲胺]-N,N,N',N'-四甲基硫尿四氟硼酸TOTU 328.1苯并三氮唑-1-基氧基三(二甲基氨基)磷鎓六氟磷酸盐BOP(卡特缩合剂)442.50N,N,N',N'-四甲基-O-(3,4-二氢-4-氧代-1,2,3-苯并三嗪-3-基)脲四氟硼酸盐TDBTU 349.09。
美国联合保健公司产品说明书:测试生成产品

UnitedHealthcare PharmacyClinical Pharmacy ProgramsProgram Number 2023 P 2018-16Program Prior Authorization/Medical Necessity – TestosteroneMedication Androderm, Androgel*, Fortesta*, Jatenzo*, Natesto*, Kyzatrex*,Testim, testosterone topical solution (generic Axiron)*, testosteronetransdermal gel (generic Testim)*, Tlando*, Vogelxo*, Xyosted*P&T Approval Date 2/2014, 4/2014, 5/2014, 7/2014, 10/2014, 10/2015, 5/2016, 6/2017,6/2018, 2/2019, 6/2019, 7/2020, 8/2021, 9/2022, 1/2023Effective Date 4/1/2023;Oxford only: 4/1/20231.Background:Testosterone products are approved by the Food and Drug Administration (FDA) for testosterone replacement therapy in males with primary hypogonadism (congenital or acquired) orhypogonadotropic hypogonadism (congenital or acquired). Primary hypogonadism originatesfrom a deficiency or disorder in the testicles. Secondary hypogonadism indicates a problem in the hypothalamus or the pituitary gland. Testosterone use has been strongly linked to improvements in muscle mass, bone density, and libido.The purpose of this program is to provide coverage for androgens and anabolic steroid therapy for the treatment of conditions for which they have shown to be effective and are within the scope of the plan’s pharmacy benefit. Coverage for the enhancement of athletic performance or bodybuilding will not be provided.a3.Additional Clinical Rules:•Notwithstanding Coverage Criteria, UnitedHealthcare may approve initial and re-authorization based solely on previous claim/medication history, diagnosis codes (ICD-10)and/or claim logic. Use of automated approval and re-approval processes varies by programand/or therapeutic class.•Supply limits may be in place.•* May be excluded from coverage•+ Coverage for patient population may be dependent upon benefit design4.References:1.AACE Hypogonadism Task Force. American Association of Clinical EndocrinologistsMedical Guidelines for Clinical Practice for the Evaluation and Treatment ofHypogonadism in Adult Male Patients – 2002 Update. Endocr Pract. 2002; 8(No. 6): 439-456.2.The World Professional Association for Transgender Health (WPATH), Standards of Carefor the Health of Transsexual, Transgender, and Gender Nonconforming People, 7thVersion.3.Cook, David M, et al. "American Association of Clinical Endocrinologists medicalguidelines for clinical practice for growth hormone use in growth hormone-deficient adultsand transition patients - 2009 update: executive summary of recommendations." Endocrinepractice 15.6 (2009):580-586.4.Gibney, James, et al. "Growth hormone and testosterone interact positively to enhanceprotein and energy metabolism in hypopituitary men." American journal of physiology:endocrinology and metabolism 289.2 (2005):E266-E2715.Bhasin, S, et al. "Testosterone replacement and resistance exercise in HIV-infected menwith weight loss and low testosterone levels." JAMA. 2000. 283.(6) 763-770.6.Isidori, Andrea M, et al. Effects of testosterone on sexual function in men: results of ameta-analysis. Clinical endocrinology. 2005 63(4):381-394.7.Kenny, A M, et al. Effects of transdermal testosterone on bone and muscle in older menwith low bioavailable testosterone levels. The journals of gerontology. 2001. 56(5) M266-M272.8.Tracz, Michal J, et al. Testosterone use in men and its effects on bone health. A systematicreview and meta-analysis of randomized placebo-controlled trials. The Journal of clinicalendocrinology and metabolism. 2006. 91(6):2011-2016.9.Bolona, Enrique R, et al. Testosterone use in men with sexual dysfunction: a systematicreview and meta-analysis of randomized placebo-controlled trials. Mayo Clinicproceedings.2007. 82(1):20-28.10.Androderm [package insert]. Madison, NJ: Allergan, Inc; May 2020.11.Androgel [package insert]. North Chicago, IL: AbbVie Inc; May 2020.12.Fortesta [package insert]. Malvern, PA: Endo Pharmaceuticals Inc; January 2022.13.Testim [package insert]. Malvern, PA: Endo Pharmaceuticals Inc; August 2021.14.Natesto [package insert]. Mississauga, ON: Acerus Pharmaceuticals Corporation;December 2021.15.Vogelxo [package insert]. Maple Grove, MN: Upsher-Smith Laboratories, LLC; April2020.16.Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline.J Clin Endocrinol Metab 2017; 102:3869.17.The Endocrine Society. Testosterone therapy in Adult Men with Androgen DeficiencySyndromes. J Clin Endocrinol Metab, May 2018, 103(5):1–30.18.Mulhall JP, et al. Evaluation and Management of Testosterone Deficiency: AUA Guideline.American Urological Association Education and Research, Inc 2018.19.Xyosted [package insert]. Ewing, NJ: Antares Pharma, Inc; November 2019.20.Jatenzo [package insert]. Northbrook, IL: Clarus Therapeutics, Inc; March 2019.21.Tlando [package insert]. Ewing, NJ: Antares Pharma, Inc; March 2022.22.Kyzatrex [package insert]. Raleigh, NC: Marius Pharmaceuticals LLC; September 2022.Program Prior Authorization/Medical Necessity - TestosteroneChange ControlDate Change2/2014 Create Prior Authorization Criteria4/2014 Revised Reauthorization Criteria; formatting corrections, referencesupdated.5/2014 Revised the initial authorization criteria to include subsections for themale population and the female to male transsexual population, updatedto include language from the gender identity disorder/ gender dysphoriatreatment medical coverage determination guideline, referencesupdated7/2014 Added Natesto and Vogelxo to criteria. Changed coverage criteria fromspecific product names to topical testosterone products.10/2014 Modified criteria for total testosterone to consider reference range of thelaboratory. Added criteria for when Free Testosterone level may beutilized. Added criteria for conditions that do not require testosteronelevels. Extended initial authorization period for patients already ontherapy.12/2014 Testosterone free level units corrected.10/2015 Clarified initial authorization periods. Clarified that levels forreauthorization should be within the past 6 months for patients new totestosterone and within the past 12 months for continuing users.Updated references.5/2016 Removed age requirement from female to male transsexual coveragerequirements. Updated gender identity disorder to gender dysphoria.6/2017 Updated criteria for Gender Dysphoria. Updated reauthorizationcriteria to clarify that new to therapy refers to use of less than one yearand continuing therapy refers to use of one year or longer.6/2018 Updated required testosterone level to less than 300 ng/dL based on2018 American Urological Society treatment guidelines.2/2019 Program name change from Topical Androgens to Testosterone.Xyosted added to program.6/2019 Jatenzo added to program.7/2020 Updated initial authorization to 6 months for both new and existingusers. Added state mandate language. Updated references.8/2021 Annual review. Updated references. Removed Striant as it is no longeron the market.9/2022 Tlando added to program. Removed brand Axiron from program sinceit is no longer available. Updated to note generic Testim is typicallyexcluded. Updated references.1/2023 Kyzatrex added to program. Increased initial authorization to 12months and changed reauthorization to require a lab value within thepast 12 months.。
迪雷尔(Drexel)乙醛三氯尿唑(Chlorpyrifos)15G农业昆虫毒(Agricultur

SAFETY DATA SHEETDREXEL CHLORPYRIFOS 15G AGRICULTURAL INSECTICIDEProduct Name: Drexel Chlorpyrifos 15G Agricultural InsecticideEPA Reg No.: 19713-505CAS NO: 2921-88-2Formula: C9H11ClNO3PSCompany: Drexel Chemical Company1700 Channel AvenueMemphis, TN 38106Synonyms: O,O-Diethyl O-3, 5, 6-trichloropyridin-2-yl phosphorothioateIdentifiers:EINECS: 220-864-4RTECS: TF6300000DOT information: See Section 14 for Transportation InformationEmergency Telephone Number:CHEMTREC Drexel Chemical Co.Tel: 1-800-424-9300 901-774-4370This product is an EPA FIFRA registered pesticide. Some of the classifications on this SDS are not the same as the FIFRA label. Certain sections of this SDS are superseded by federal law governed by EPA for a registered pesticide. Please see Section 15. REGULATORY INFORMATION for explanation.GHS classification:Health hazards: Acute toxicity – inhalation Category 2Skin corrosion/irritation Category 2Eye damage/ irritation Category 2BSpecific target organ toxicity –(single exposure) Category 1Specific target organ toxicity –(repeated exposure) Category 2Aquatic acute toxicity Category 1GHS label elements:Signal Word: DANGERHazard Statements: Toxic if swallowed.Fatal if inhaled.Causes skin irritation.Causes eye irritation.Causes damage to nervous system.May cause damage to nervous system through prolonged or repeated exposure.Very toxic to aquatic life.Precautionary Statements:Prevention: Prevention Wash thoroughly after handling.Do not breathe dust/fume/gas/mist/vapors/spray.Wear protective gloves/protective clothing. Examples of preferred glove barriermaterials include: Neoprene, Nitrile/butadiene rubber (“nitrile” or “NBR”) orPolyvinyl chloride (“PVC” or “vinyl”).Wear respiratory protection. Use a respirator with either an organic vapor-removing cartridge with a pre-filter approved for pesticides (MSHA/NIOSHapproval number prefix TC-23C) or a canister approved for pesticides(MSHA/NIOSH approval number prefix TC-14G).Do not eat, drink or smoke when using this product.Use only outdoors or in well-ventilated area.In case if inadequate ventilation wear respiratory protection.Avoid release to the environment from other than intended use.Response: If swallowed: Immediately call a poison control center/doctor. Rinse mouth. SeeSection 4: Note to Physician.If on skin: Wash with plenty of water. Call a poison center/doctor if you feelunwell. If skin irritation occurs; Get medical advice/attention. Take offcontaminated clothing and wash it before reuse.If inhaled: Remove person to fresh air and keep comfortable for breathing.Immediately call a poison center/doctor if person stops breathing.If in eyes: Rinse cautiously with water for several minutes. Remove contactlenses, if easy to do. Continue rinsing. If eye irritation persists: Get medicaladvice/attention.If exposed: Call poison center/doctor. Specific treatment see Note toPhysician, Section 4.Get medical attention if you feel unwell.Collect spillage.Storage: Store locked up. Store in well-ventilated place.Keep cool.Keep container tightly closed.Disposal: Disposal of contents/container must be in accordance with your local or arearegulations.Components CAS No.: % By Wt.: OSHA PEL: ACGIH TLV:Active Ingredient:Chlorpyrifos 2921-88-2 15.0% N/Av 0.2 mg/m3Inert Ingredients: N/A 85.0% N/A N/AHave the product container or label with you when calling a poison control center or doctor.Eye Contact: Hold eye open and rinse slowly and gently with water for 15 to 20 minutes. Remove contact lenses, if present, after the first 5 minutes, then continue rinsing eyes for at least 10 minutes. Obtain medical attention without delay, preferably from an ophthalmologist.If Swallowed: Call a poison control center or doctor immediately for treatment advice. Rinse out mouth then have person sip a glass of water if able to swallow. Do not induce vomiting unless told to do so by the poison control center or doctor. Do not give anything by mouth to an unconscious person. Have product label with you when calling a poison control center or doctor.Skin Contact: Immediately flush skin with water while removing contaminated clothing and shoes. Get medical attention if symptoms occur. Wash clothing before reuse. Destroy contaminated leather items such as shoes, belts, and watchbands.If Inhaled: Move person to fresh air. If person is not breathing, call 911 or an ambulance, then give artificial respiration, preferably mouth-to-mouth, if possible. Call a poison control center or doctor for further treatment advice.Note to Physician: This product contains an organophosphate that inhibits cholinesterase. Treat symptomatically. If exposed, plasma and red blood cell cholinesterase tests may indicate significance of exposure (baseline data are useful). Atropine, only by injection, is the preferable antidote. Oximes, such as 2-PAM/protopam, may be therapeutic if used early; however, use only in conjunction with atropine. In case of severe, acute poisoning, use antidote immediately after establishing an open airway and respiration. Contains petroleum distillate. Do not induce vomiting since vomiting may cause aspiration pneumonia.Fire Hazards: Thermal decomposition during a fire can produce fumes and irritating gases.Flammability classification (OSHA 29 CFR 1910.1200): N/AFlash point: NoneLower flammable limit (% by volume): N/AvUpper flammable limit (% by volume): N/AvFire Fighting Procedures: Keep people away. Isolate fire and deny unnecessary entry. Evacuate the area and fight the fire from upwind at a safe distance to avoid hazardous vapors or decomposition products. Dike and collect fire-extinguishing water to prevent environmental damage and excessive waste runoff.Firefighting media: Use foam, dry chemical, carbon dioxide, or water fog when fighting fires involving this product. Do not use water jet, as this may spread burning material. Minimize the use of water to avoid environmental contamination. Contain all runoff.Special Protective Equipment for Firefighters: Wear positive-pressure self-contained breathing apparatus (SCBA) and protective firefighting clothing (includes firefighting helmet, coat, trousers, boots, and gloves). Use full face shield and operate in positive pressure mode. Avoid contact with this material during firefighting operations. If contact is likely, change to full chemical resistant firefighting clothing with self-contained breathing apparatus. If this is not available, wear full chemical resistant clothing with self-contained breathing apparatus and fight fire from a remote location. For protective equipment in post-fire or non-fire clean-up situations, refer to the relevant sections.Hazardous Combustion Products: Hydrogen chloride, ethyl sulfide, diethyl sulfide, nitrogen oxides, carbon oxides, irritating fumes and smoke.NFPA: Health: Flammability: Reactivity:2 1 0(Rating: 4-Extreme, 3-High, 2-Moderate, 1-Slight, 0-Insignificant)Steps to be taken if Material is Released or Spilled:Sweep as much material as possible, keeping dust to a minimum and place in an approved chemical waste container. Wash the spill area with water containing a strong detergent, absorb with earth, sand or absorbent material and sweep up and place in approved chemical waste container. For large spills contact Drexel Chemical Co. See Section 13, Disposal Considerations, for additional information.Personal Precautions:Isolate area. Keep unnecessary and unprotected personnel from entering the area. Refer to Section 7, Handling for additional precautionary measures. Ventilate area of leak or spill. Use appropriate safety equipment. For additional information, refer to Section 8, Exposure Controls and Personal Protection.Environmental Precautions: Prevent from entering into soil, ditches, sewers, waterways and/or groundwater. See Section 12, Ecological Information.KEEP OUT OF REACH OF CHILDRENGeneral Handling: Avoid contact with eyes, skin, and clothing. Wash thoroughly after handling. Do not swallow. Avoid breathing dust. Use with adequate ventilation. Wear chemical protective equipment when handling. Keep away from heat, sparks and flame. See Section 8, Exposure Controls and Personal Protection.Storage: Store in a cool, dry, ventilated and secure area designated specifically for pesticides and away from heat sources. Keep in original containers and keep containers closed when not in use. Do not store in excessive heat. Do not store near children, food, foodstuffs, drugs or potable water supplies.Exposure Limits: TLV Chlorpyrifos 0.2 mg/m3Personal Protection:Eye/Face Protection: Wear safety glasses with side shields or chemical splash goggles to prevent dust from entering the eyes. If using a full face shield, always use safety glasses or goggles along with the face shield to ensure adequate protection of the eyes.Skin Protection: Wear long-sleeved shirt, long pants and shoes plus socks. Safety shower should be located in immediate work area. Remove contaminated clothing immediately after handling this product, wash skin area with soap and water, and launder clothing before reuse or dispose of properly. Items which cannot be decontaminated, such as shoes, belts and watchbands, should be removed and disposed of properly.Hand protection: Use gloves chemically resistant to this material. Examples of preferred glove barrier materials include: Neoprene, Nitrile/butadiene rubber (“nitrile” or “NBR”) or Polyvinyl chloride (“PVC” or “vinyl”).Respiratory Protection: Not normally required. Respiratory protection should be worn when there is a potential to exceed the exposure limit requirements or guidelines. When handling in enclosed areas, when large quantities of dusts are generated or prolonged exposure is possible in excess of the TLV, use a respirator with either an organic vapor-removing cartridge with a prefilter approved for pesticides (MSHA/NIOSH approval number prefix TC-23C) or a canister approved for pesticides (MSHA/NIOSH approval number prefix TC-14G).Ingestion: Avoid ingestion of even very small amounts; do not consume or store food or tobacco in the work area; wash hands and face before smoking or eating.Engineering Controls:Ventilation: When handling this product proper ventilation is required to maintain exposure below the TLV.Ventilate all transport vehicles prior to unloading. Facilities storing or utilizing this material should be equipped with and eyewash facility and safety shower.Physical State: GranuleColor: BrownOdor: MildFlash Point: NoneVapor Pressure (mmHg): N/ABoiling Point: N/AVapor Density (air = 1): N/ABulk Density (H2O = 1): 46.43 lbs./ft3Freezing Point: N/ASolubility in water: InsolublepH: N/AViscosity: N/A% Volatiles: >3%Stability/Instability: Avoid heating above 60°C (100°F). Chlorpyrifos undergoes exothermic decomposition at approximately 130°C (266°F), which can lead to higher temperatures and violent decomposition if generated heat is not removed.Conditions to Avoid: Keep this product away from excessive heat and moisture.Incompatible Materials: Avoid contact with strong alkalies, amines and oxidizers.Hazardous Polymerization: Will not occurThermal Decomposition: Decomposition products can include but are not limited to: Hydrogen chloride, ethyl sulfide and nitrogen oxides.Data presented for technical Chlorpyrifos:Acute ToxicityIngestion:∙Oral LD50, (rat): 223 mg/kgDermal:∙Dermal LD50, (rabbit): >5,000 mg/kgInhalation:∙LC50, (rat): > 0.2 mg/lEye Irritation (rabbit):∙Slight irritation resolved within 24 hoursSkin Irritation (rabbit):∙Mild irritation resolved within 7 daysSensitization Skin:∙Non-sensitizer (Guinea Pig)Carcinogenicity:∙Not likely to be carcinogenic in humansTeratogenicity, mutagenicity, and other reproductive effects: None knownData presented for the active ingredient Chlorpyrifos:ENVIRONMENTAL FATE:∙Very highly toxic to fish, highly toxic to daphnia, moderately to highly toxic to birds and serious hazard to Honeybees.Persistence and Degradability:∙Moderately persistent in soils degrading slowly through aerobic and anaerobic metabolism. Absorbs strongly to soil particles and is not readily soluble in water.Aquatic Toxicity:∙Rainbow Trout: 96 hour LC50: (0.007 – 0.051 mg/L)∙Bluegill Sunfish: 96 hour LC50: (0.002 – 0.010 mg/L)∙Daphnia magna: 48 hour EC50: (1.7 μg/L)Bees:∙LD50: (oral) 360 ng/bee; (contact) 70 ng/beeBird Toxicity:∙Mallard Duck: 8-day LC50: 180 ppm∙Bobwhite Quail: 8-day LC50: 423 ppmIf wastes and/or containers cannot be disposed of according to the product label directions, disposal of this material must be in accordance with your local or area regulatory authorities. This information presented below only applies to the material as supplied. The identification based on characteristic(s) or listing may not apply if the material has been used or otherwise contaminated. It is the responsibility of the waste generator to determine the toxicity and physical properties of the material generated to determine the proper waste identification and disposal methods in compliance with applicable regulations. If the material as supplied becomes a waste, follow all applicable regional, national and local laws.DOT: UN-3077, Environmentally hazardous substances, solid, n.o.s., (Chlorpyrifos), 9, PG III, Marine Pollutant, RQ 1 lb.IMDG: UN-3077, Environmentally hazardous substances, solid, n.o.s., (Chlorpyrifos), 9, PG III, Marine Pollutant, RQ 1 lb.IATA: UN-3077, Environmentally hazardous substances, solid, n.o.s., (Chlorpyrifos), 9, PG III, Marine Pollutant, RQ 1 lb.Freight description: Agricultural Insecticide, solid, n.o.s.ERG Guide No.: 171This information is not intended to convey all specific regulatory or operational requirements/information relating to this product. Additional transportation system information can be obtained through an authorized sales or customer service representative. It is the responsibility of the transporting organization to follow all applicable laws, regulations and rules relating to the transportation of the material.OSHA Hazard Communication Standard:∙This product is a “Hazardous Chemical” as defined by the OSHA Hazard Communication Standard, 29 CFR 1910.1200.∙EPA FIFRA INFORMATION:This chemical is a pesticide product registered by the United States Environmental Protection Agency and is subject to certain labeling requirements under federal pesticide law. These requirements differ from the classification criteria and hazard information required for safety data sheets (SDS), and for workplace labels of non-pesticide chemical. The hazard information required on the pesticide label is listed out below.The pesticide label also includes other important information, including directions for use.∙EPA/CERCLA Reportable Quantity: Chlorpyrifos RQ = 1 lb.SARA/TITLE III:∙Section 302. Extremely Hazardous Substance Notification: This material is not known to contain any Extremely Hazardous Substances.∙Section 311/312. Hazard Categories:Fire HazardImmediate health hazardChronic health hazard∙Section 313. Toxic Chemical(s): This material is not known to contain any Toxic Chemical constituents.∙RCRA Waste Code: Not applicableCalifornia Proposition 65 (Safe Drinking Water and Toxic Enforcement Act of 1986):∙This product is not listed.Toxic Substances Control Act (TSCA):∙All components of this product are on the TSCA Inventory or are exempt from TSCA Inventory requirements under40 CFR 720.30Drexel Chemical Company recommends that each customer or recipient of this SDS to study it carefully and consult appropriate expertise, as necessary or appropriate, to become aware of and understand the data contained in this SDS and any hazards associated with the product. The information herein is provided in good faith and believed to be accurate as of the effective date shown below. However, no warranty, express or implied, is given. Regulatory requirements are subject to change and may differ between various locations. It is the buyer’s/user’s responsibility to ensure that his activities comply with all federal, state, provincial or local laws. The information presented here pertains only to the product as shipped. Since conditions for use of the product are not under the control of the manufacturer, it is the buyer’s/user’s duty to determine the conditions necessary for the safe use of this product. Due to the proliferation of sources for information such as manufacturer-specific SDSs, we are not and cannot be responsible for SDSs obtained from any source other than ourselves. If you have obtained an SDS from another source or if you are not sure that the SDS you have is current, please contact us for the most current version.Date Revised: June 22, 2016Supersedes: September 8, 2015。
肝病专业英语词汇

3α-羟类固醇脱氢酶(Y' 蛋白) γ -谷氨酰转移酶 γ-氨基丁酸 甲胎蛋白 人兽共患病 异种肝移植 脂肪性纤维瘤,黄色瘤 黄嘌呤氧化酶 黄斑瘤 全球移植中心名录 窗口期 肝豆状核变性 肥达反应 外斐反应 韦克斯勒成人智力测验 呕吐 视觉诱发电位 病毒学应答 病毒复制 病毒性肝炎 静脉-静脉转流 VOD 肝小静脉闭塞病 静脉-动脉转流 血管活性肽 静脉曲张 胆管消失综合征 疫苗 熊去氧胆酸 尿胆素原 尿胆素 二磷酸尿苷异构酶 尿素生成 鸟氨酸循环,尿素循环 上消化道出血 粗纤维调节素 肝未分化肉瘤 充盈不足学说 非结合高胆红素血症 游离胆红素,非结合胆红素 超声(波)检查法
短潜伏期肝炎 移动性浊音 腹水白蛋白浓度梯度 血清肝炎 血清诊断 血清胆红素 血清白蛋白 血清学应答 乙肝血清学检查 血清转换 败血症相关胆汁淤积 正链,有义链 镇静剂 次级胆酸 海蓝组织细胞增多症 硬化疗法 硬化性胆管炎 日本血吸虫病 湄公血吸虫 曼氏血吸虫 日本血吸虫 间插血吸虫 埃及血吸虫 血吸虫 瘢痕形成期 粗面内质网 滚环机制(环状DNA复制的机理) RNA干扰 核酶 核糖体 胆固醇逆向转运
伤寒 Ⅳ型胶原 Ⅲ型前胶原 甲肝和乙肝疫苗 抑癌基因 肿瘤坏死因子 肝结核 滋养体 甘油三酯 颠换效应 经颈静脉肝内门腔分流 转换 输血传染的病毒 输血性肝炎 转化生长因子
transcatheter arterial chemoembolization 肝动脉化疗栓塞 trans-activation toxic hepatitis total cholesterin total bilirubin tomor necrosis factor tocopherol TNF-related apoptosis inducing ligand tissue inhibitor of metalloproteinase thymosin Thymopolypeptides for Injection thromboxane the core promoter element tentative diagnosis tension of muscle tenderness Telbivudine taurocholic acid taurochenodeoxycholate acid systemic inflammatory response syndrome syncytial giant-cell hepatitis sustained virus response sustained response 反式激活 中毒性肝炎 总胆固醇 总胆红素 肿瘤坏死因子 生育酚,维生素E 肿瘤坏死因子相关凋亡诱导配体 基质金属蛋白酶组织抑制物 胸腺肽 胸腺肽 血栓素 核心启动子元件,启动子核心元件 暂时的(假定的)诊断,试验性诊断 肌张力 压痛 LdT 牛(磺)胆酸 牛磺鹅(去氧)胆酸盐,牛磺鹅(脱氧)胆酸盐 全身炎症反应综合症 融合巨细胞性肝炎 持续病毒应答 持久应答
艾氟康唑(英文说明书)

__________________ ______________HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use JUBLIA safely and effectively. See full prescribing information for JUBLIA. JUBLIA® (efinaconazole) topical solution, 10%For topical useInitial U.S. Approval: 2014INDICATIONS AND USAGEJUBLIA is an azole antifungal indicated for the topical treatment of onychomycosis of the toenails due to Trichophyton rubrum and Trichophyton mentagrophytes. (1)_______________DOSAGE AND ADMINISTRATION• Apply JUBLIA to affected toenails once daily for 48 weeks using the integrated flow-through brush applicator. (2)• When applying JUBLIA, ensure the toenail, the toenail folds, toenail bed, hyponychium, and the undersurface of the toenail plate, are completely covered. (2)• For topical use only. (2) • Not for oral, ophthalmic, or intravaginal use. (2)DOSAGE FORMS AND STRENGTHSSolution: 10%. (3)___________________ CONTRAINDICATIONS ___________________ None. (4)___________________ ADVERSE REACTIONS ___________________ The most common adverse reactions (incidence >1%) were ingrown toenails, application site dermatitis, application site vesicles, and application site pain.(6.1)To report SUSPECTED ADVERSE REACTIONS, contact Valeant Pharmaceuticals North America LLC at 1-800-321-4576 or FDA at 1-800FDA-1088 or /medwatch.See 17 for PATIENT COUNSELING INFORMATION and FDA-Approved Patient LabelingRevised: 06/2014FULL PRESCRIBING INFORMATION: CONTENTS*12 CLINICAL PHARMACOLOGY 1 INDICATIONS AND USAGE 12.1 Mechanism of Action2 3 DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHS12.2 Pharmacodynamics12.3 Pharmacokinetics4 6 CONTRAINDICATIONSADVERSE REACTIONS 1312.4 MicrobiologyNONCLINICAL TOXICOLOGY7 6.1 Clinical Trials ExperienceDRUG INTERACTIONS 1413.1 Carcinogenesis, Mutagenesis, Impairment of FertilityCLINICAL STUDIES8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy8.3 Nursing Mothers8.4 Pediatric Use8.5 Geriatric Use 1617*SeareHOW SUPPLIED/STORAGE AND HANDLINGPATIENT COUNSELING INFORMATIONctions or subsections omitted from the full prescribing information not listed.11 DESCRIPTION 1FULL PRESCRIBING INFORMATION1 INDICATIONS AND USAGEJUBLIA (efinaconazole) topical solution, 10% is an azole antifungal indicated for the topical treatment of onychomycosis of the toenail(s) due to Trichophyton rubrum and Trichophyton mentagrophytes.2 DOSAGE AND ADMINISTRATIONApply JUBLIA to affected toenails once daily for 48 weeks, using the integrated flow-through brush applicator. When applying JUBLIA, ensure the toenail, the toenail folds, toenail bed, hyponychium, and the undersurface of the toenail plate, are completely covered.JUBLIA is for topical use only and not for oral, ophthalmic, or intravaginal use.3 DOSAGE FORMS AND STRENGTHSJUBLIA (efinaconazole) topical solution, 10% contains 100 mg of efinaconazole in each gram of clear, colorless to pale yellow solution.4 CONTRAINDICATIONSNone.6 ADVERSE REACTIONS6.1 Clinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.In two clinical trials, 1227 subjects were treated with JUBLIA, 1161 for at least 24 weeks and 780 for 48 weeks. Adverse reactions reported within 48 weeks of treatment and in at least 1% of subjects treated with JUBLIA and those reported in subjects treated with the vehicle are presented in Table 1.Table 1: Adverse Reactions Reported by at Least 1% of Subjects Treated for up to 48 WeeksAdverse Event, n (%) JUBLIAN = 1227 Vehicle N = 413Ingrown toenail 28 (2.3%) 3 (0.7%) Application site dermatitis 27 (2.2%) 1 (0.2%) Application site vesicles 20 (1.6%) 0 (0.0%) Application site pain 13 (1.1%) 1 (0.2%)7 DRUG INTERACTIONSIn vitro studies have shown that JUBLIA, at therapeutic concentrations, neither inhibits nor induces cytochrome P450 (CYP450) enzymes.8 USE IN SPECIFIC POPULATIONS8.1 PregnancyPregnancy Category CThere are no adequate and well-controlled studies with JUBLIA in pregnant women. JUBLIA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.Systemic embryofetal development studies were conducted in rats and rabbits. Subcutaneous doses of 2, 10 and 50 mg/kg/day efinaconazole were administered during the period of organogenesis (gestational days 6-16) to pregnant female rats. In the presence of maternal toxicity, embryofetal toxicity (increased embryofetal deaths, decreased number of live fetuses, and placental effects) was noted at 50 mg/kg/day [559 times the Maximum Recommended Human Dose (MRHD) based on Area Under the Curve (AUC) comparisons]. No embryofetal toxicity was noted at 10 mg/kg/day (112 times the MRHD based on AUC comparisons). No malformations were observed at 50 mg/kg/day (559 times the MRHD based on AUC comparisons).Subcutaneous doses of 1, 5, and 10 mg/kg/day efinaconazole were administered during the period of organogenesis (gestational days 6-19) to pregnant female rabbits. In the presence of maternal toxicity, there was no embryofetal toxicity or malformations at 10 mg/kg/day (154 times the MRHD based on AUC comparisons).In a pre-and post-natal development study in rats, subcutaneous doses of 1, 5 and 25 mg/kg/day efinaconazole were administered from the beginning of organogenesis (gestation day 6) through the end of lactation (lactation day 20). In the presence of maternal toxicity, embryofetal toxicity (increased prenatal pup mortality, reduced live litter sizes and increased postnatal pup mortality) was noted at 25 mg/kg/day. No embryofetal toxicity was noted at 5 mg/kg/day (17 times the MRHD based on AUC comparisons). No effects on postnatal development were noted at25 mg/kg/day (89 times the MRHD based on AUC comparisons).8.3 Nursing MothersIt is not known whether efinaconazole is excreted in human milk. After repeated subcutaneous administration, efinaconazole was detected in milk of nursing rats. Because many drugs are excreted in human milk, caution should be exercised when JUBLIA is administered to nursing women.8.4 Pediatric UseSafety and effectiveness of JUBLIA in pediatric subjects have not been established.8.5 Geriatric UseOf the total number of subjects in clinical trials of JUBLIA, 11.3% were 65 and over, while none were 75 and over. No overall differences in safety and effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and the younger subjects, but greater sensitivity of some older individuals cannot be ruled out.11 DESCRIPTIONJUBLIA (efinaconazole) topical solution, 10% is a clear colorless to pale yellow solution for topical use. Each gram of JUBLIA contains 100 mg of efinaconazole. Efinaconazole is an azole antifungal with a chemical name of ((2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylenepiperidin-1yl)-1-(1H-1,2,4-triazol-1-yl) butan-2-ol). The structural formula for efinaconazole is represented below:Molecular Formula: C18H22F2N4O Molecular Weight: 348.39JUBLIA contains the following inactive ingredients: alcohol, anhydrous citric acid, butylated hydroxytoluene, C12-15 alkyl lactate, cyclomethicone, diisopropyl adipate, disodium edetate, and purified water.12 CLINICAL PHARMACOLOGY12.1 Mechanism of ActionJUBLIA topical solution is an azole antifungal [see Clinical Pharmacology (12.4)].12.2 PharmacodynamicsThe pharmacodynamics of JUBLIA is unknown.12.3 PharmacokineticsSystemic absorption of efinaconazole in 18 adult subjects with severe onychomycosis was determined after application of JUBLIA once daily for 28 days to patients 10 toenails and 0.5 cm adjacent skin. The concentration of efinaconazole in plasma was determined at multiple time points over the course of 24-hour periods on days 1, 14, and 28. Efinaconazole mean ± SD plasma C max on Day 28 was 0.67 ± 0.37 ng/mL and the mean ± SD AUC was 12.15 ± 6.91ng*h/mL. The plasma concentration versus time profile at steady state was generally flat over a 24-hour dosing interval. In a separate study of healthy volunteers, the plasma half-life of efinaconazole following daily applications when applied to all 10 toenails for 7 days was 29.9 hours.Drug InteractionsJUBLIA is considered a non-inhibitor of the CYP450 enzyme family. In in vitro studies using human liver microsomes, efinaconazole did not inhibit CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2PE1 and CYP3A4 enzyme activities at expected clinical systemicconcentrations. In vitro studies in human primary hepatocytes showed that efinaconazole did not induce CYP1A2 or CYP3A4 activities.12.4 MicrobiologyMechanism of ActionEfinaconazole is an azole antifungal. Efinaconazole inhibits fungal lanosterol 14α-demethylase involved in the biosynthesis of ergosterol, a constituent of fungal cell membranes.Activity In Vitro and In VivoEfinaconazole has been shown to be active against isolates of the following microorganisms, both in vitro and in clinical infections. Efinaconazole exhibits in vitro minimum inhibitory concentrations (MICs) of 0.06 μg/mL or less against most (≥90%) isolates of the following microorganisms:Trichophyton rubrumTrichophyton mentagrophytesMechanism of ResistanceEfinaconazole drug resistance development was studied in vitro against T. mentagrophytes, T. rubrum and C. albicans. Serial passage of fungal cultures in the presence of sub-growth inhibitory concentrations of efinaconazole increased the MIC by up to 4-fold. The clinical significance of these in vitro results is unknown.13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of FertilityA 2-year dermal carcinogenicity study in mice was conducted with daily topical administration of 3%, 10% and 30% efinaconazole solution. Severe irritation was noted at the treatment site in all dose groups, which was attributed to the vehicle and confounded the interpretation of skin effects by efinaconazole. The high dose group was terminated at week 34 due to severe skin reactions. No drug-related neoplasms were noted at doses up to 10% efinaconazole solution (248 times the MRHD based on AUC comparisons).Efinaconazole revealed no evidence of mutagenic or clastogenic potential based on the results of two in vitro genotoxicity tests (Ames assay and Chinese hamster lung cell chromosome aberration assay) and one in vivo genotoxicity test (mouse peripheral reticulocyte micronucleus assay).No effects on fertility were observed in male and female rats that were administered subcutaneous doses up to 25 mg/kg/day efinaconzole (279 times the MRHD based on AUC comparisons) prior to and during early pregnancy. Efinaconazole delayed the estrous cycle in females at 25 mg/kg/day but not at 5 mg/kg/day (56 times MRHD based on AUC comparisons).14 CLINICAL STUDIESThe safety and efficacy of once daily use of JUBLIA for the treatment of onychomycosis of the toenail were assessed in two 52-week prospective, multi-center, randomized, double-blind clinical trials in patients 18 years and older (18 to 70 years of age) with 20% to 50% clinical involvement of the target toenail, without dermatophytomas or lunula (matrix) involvement. The trials compared 48-weeks of treatment with JUBLIA to the vehicle solution. The Complete Cure rate was assessed at Week 52 (4-weeks after completion of therapy). Complete cure was defined as 0% involvement of the target toenail (no clinical evidence of onychomycosis of the target toenail) in addition to Mycologic Cure, defined as both negative fungal culture and negative KOH. Table 2 lists the efficacy results for trials 1 and 2.Table 2: Efficacy EndpointsTrial 1 Trial 2JUBLIA Vehicle JUBLIA VehicleN = 656 N = 214 N = 580 N = 201Complete Cure a11717.8%73.3%8815.2%115.5%Complete or Almost Complete Cure b17326.4%157.0%13623.4%157.5%Mycologic Cure c 362 36 310 3455.2% 16.8% 53.4% 16.9%a Complete cure defined as 0% clinical involvement of the target toenail plus negative KOH and negative culture.b Complete or almost complete cure defined as ≤5% affected target toenail area involved and negative KOH and culture.c Mycologic cure defined as negative KOH and negative culture.16 HOW SUPPLIED/STORAGE AND HANDLINGJUBLIA (efinaconazole) topical solution, 10% is a clear, colorless to pale yellow solution supplied in a white plastic bottle with an integrated flow-through brush applicator as follows:• 4 mL (NDC 0187-5400-04)•8 mL (NDC 0187-5400-08)Storage and Handling Conditions:Store at 20°C -25°C (68°F -77°F); excursions permitted to 15°C -30°C (59°F -86°F) [see USP Controlled Room Temperature].• Solution is flammable; keep away from heat or flame• Protect from freezing• Keep out of the reach of children• Keep bottle tightly closed• Store in upright position17 PATIENT COUNSELING INFORMATIONSee FDA-Approved Patient Labeling (Patient Information)• JUBLIA is for external use only and is not for ophthalmic, oral, or intravaginal use. It is for use on toenails and immediately adjacent skin only.• Apply JUBLIA once daily to clean dry toenails. Wait for at least 10 minutes after showering, bathing, or washing before applying.• Use JUBLIA only on the affected toenails, as directed by your healthcare provider.• Inform a health care professional if the area of application shows signs of persistent irritation (for example, redness, itching, swelling).• Avoid pedicures, the use of nail polish, and cosmetic nail products while using JUBLIA.• Flammable, avoid use near heat or open flame.Manufactured for: Valeant Pharmaceuticals North America LLC, Bridgewater, NJ 08807 USA Manufactured by: Kaken Pharmaceutical Co. Ltd, Shizuoka, JapanProduct of JapanU.S. Patents 8,039,494; 7,214,5069391900 Issued: 06/2014PATIENT INFORMATIONJUBLIA (joo-blee-uh)(efinaconazole) topical solution, 10%Important information: JUBLIA is for use on toenails and surrounding skin only. Do not use JUBLIA in your mouth, eyes, or vagina.What is JUBLIA?JUBLIA is a prescription medicine used to treat fungal infections of the toenails.It is not known if JUBLIA is safe and effective in children.What should I tell my healthcare provider before using JUBLIA?Before you use JUBLIA, tell your healthcare provider about all your medical conditions, including if you:•are pregnant or plan to become pregnant. It is not known if JUBLIA can harm your unborn baby.•are breastfeeding or plan to breastfeed. It is not known if JUBLIA passes into your breast milk. Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.How should I use JUBLIA?See the “Instructions for Use” at the end of this Patient Information leaflet for detailed information about the right way to use JUBLIA.•Use JUBLIA exactly as your healthcare provider tells you to use it. Apply JUBLIA to your affected toenails 1 time each day. Wait for at least 10 minutes after showering, bathing, or washing before applying JUBLIA. JUBLIA is used for 48 weeks.What should I avoid while using JUBLIA?•JUBLIA is flammable. Avoid heat and flame while applying JUBLIA to your toenail.•Avoid pedicures, use of nail polish, or cosmetic nail products, while using JUBLIA.What are the possible side effects of JUBLIA?JUBLIA may cause irritation at the treated site. The most common side effects include: ingrown toenail, redness, itching, swelling, burning or stinging, blisters, and pain. Tell your healthcare provider if you have any side effects that bother you or that does not go away.These are not all the possible side effects of JUBLIA.Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1800-FDA-1088.How should I store JUBLIA?•Store JUBLIA at room temperature, between 68°F to 77°F (20°C to 25°C). Do not freeze JUBLIA.•Keep the bottle tightly closed and store in an upright position.•JUBLIA is flammable. Keep away from heat and flame.Keep JUBLIA and all medicines out of the reach of children.General information about the safe and effective use of JUBLIAMedicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about JUBLIA that is written for health professionals. Do not use JUBLIA for a condition for which it was not prescribed. Do not give JUBLIA to other people, even if they have the same condition you have. It may harm them.What are the ingredients in JUBLIA?Active ingredients: efinaconazoleInactive ingredients: alcohol, anhydrous citric acid, butylated hydroxytoluene, C12-15 alkyl lactate, cyclomethicone, diisopropyl adipate, disodium edetate, and purified water. Manufactured for: Valeant Pharmaceuticals North America LLC, Bridgewater, NJ 08807 Manufactured by: Kaken Pharmaceutical Co. Ltd, Shizuoka, Japan. Product of JapanFor more information, call 1-800-321-4576.This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 06/2014Instructions for UseJUBLIA® (joo-blee-uh)(efinaconazole) topical solution, 10%Important information: JUBLIA is for use on toenails and surrounding skin only. Do not use JUBLIA in your mouth, eyes or vagina.Read the Instructions for Use that comes with JUBLIA before you start using it. Talk to your healthcare provider if you have any questions.How to apply JUBLIA:Your toenails should be clean and dry before you apply JUBLIA.Step 1: Before you apply JUBLIA to your affected toenail, remove the cap from the JUBLIA bottle (See Figure A).Step 2: Hold the bottle directly over the affected toenail and gently squeeze the bottle to apply one drop of JUBLIA onto the toenail (See Figure B).Step 3: For the big toenail, also apply a second drop to the end of the toenail (See FigureC).Step 4: Use the brush attached to the bottle to gently spread JUBLIA around the entire toenail including: the cuticle, folds of the skin next to the sides of the toenail, andunderneath the nail (See Figure D). Do not squeeze the bottle while spreadingJUBLIA with the brush.Step 5: Repeat Steps 2 to 4 to apply JUBLIA to each affected toenail.Step 6: Let JUBLIA dry completely.Step 7: After applying JUBLIA to your affected toenails, place the cap on the bottle and screw it on tightly.Step 8: Wash your hands with soap and water after applying JUBLIA.This Patient Information and Instructions for Use has been approved by the U.S. Food and Drug Administration.Manufactured for: Valeant Pharmaceuticals North America LLC, Bridgewater, NJ 08807 USA Manufactured by: Kaken Pharmaceutical Co. Ltd, Shizuoka, Japan.Product of JapanIssued: 06/2014。
艾微停产品介绍

Avitene®
艾微停网剂
• 优势
– 艾微停网剂有各种尺寸可根据需要止血的范围选择 – 比艾微停粉剂更容易操作 – 在平坦的出血表面使用方便 – 可以裁剪出适合的形状适合不同创面 – 可以用于包裹血管极大程度方便了血管外科手术 – 用冲洗或吸引可清除多余止血剂
• 减少吸收热、结痂和粘连的形成
多余的止血剂
Avitene®
腹腔镜专用艾微停网剂
• 优势
– 专为腹腔镜手术设计的艾微停有5mm和10mm推送器适应 不同尺寸的腹腔镜穿刺器
– 唯一的腹腔镜手术专用止血剂 – 钝性的头端设计不会损伤组织可以安全在腹腔镜下压迫止血 – 预装的网剂含更多止血剂 – 用冲洗或吸引可清除多余止血剂
• 减少吸收热、结痂和粘连的形成
泰绫
再生氧化纤维素S-99 可溶性止血纱布
北京
可即邦
国产 科劳德 创必复
大清生物纸
倍菱
牛源(筋腱)
胶原蛋白海绵
牛源(跟腱)
胶原蛋白海绵
猪源(猪皮及跟腱) 胶原蛋白海绵
透明质酸钠
可吸收止血膜
牛腓肠肌肌腱
胶原蛋白海绵
无锡 北京 辽宁 北京 北京
重点型号 价格区间
注册证
1690 2400 446 380-1400
• 压迫止血 • 机械止血-缝合、止血夹、门钉 • 电凝 • 氩气刀、超声刀 • 骨蜡 • 止血剂
– 主动止血剂:主动参与人体凝血机制,参与血凝块形成 – 被动止血剂:不参与人体凝血机制
止血剂的适用科室
--包括神外及泌尿外科的全手术科室
常用止血剂的科室
心胸外科
普外等其他外科
16%
14%
2%
矫形外科
国家药监局关于发布仿制药参比制剂目录(第三十九批)的通告

国家药监局关于发布仿制药参比制剂目录(第三十九
批)的通告
文章属性
•【制定机关】国家药品监督管理局
•【公布日期】2021.03.01
•【文号】国家药品监督管理局通告2021年第20号
•【施行日期】2021.03.01
•【效力等级】部门规范性文件
•【时效性】现行有效
•【主题分类】药政管理
正文
国家药品监督管理局通告
2021年第20号
国家药监局关于发布仿制药参比制剂目录(第三十九批)的
通告
经国家药品监督管理局仿制药质量和疗效一致性评价专家委员会审核确定,现发布仿制药参比制剂目录(第三十九批)。
特此通告。
附件:仿制药参比制剂目录(第三十九批)
国家药监局
2021年3月1日附件
仿制药参比制剂目录(第三十九批)。
药用辅料中英文对照

药用辅料中英文对照1 阿拉伯胶(Acacia)2 乙酰舒泛钾(Acesulfame Potassium)3 冰醋酸(Acetic Acid,Glacial)4 乙酰枸橼酸三丁酯(AcetyltributylCitrate)5 乙酰枸橼酸三乙酯(AcetyltriethylCitrate)6 人血白蛋白(Albumin)7 乙醇(Ethanol)8 海藻酸(Alginic Acid)9 脂肪族聚酯(Aliphatic Polyesters)10 阿力糖(Alitame)11 杏仁油(Almond Oil)12 维生素E(Alpha Tocopherol)13 氨溶液(Ammonia Solution)14 维生素C(Ascorbic Acid)15 棕榈酸维生素C酯(Ascorbyl Palmitate)16 阿司帕坦(Aspartame)17 绿坡缕石(Attapulgite)18 皂土(Bentonite)19 苯扎氯铵(Benzalkonium Chloride)20 苄索氯铵(Benzethonium Chloride)21 苯甲酸(Benzoic Acid)22 苯甲醇(Benzyl Alcohol)23 苯甲酸苄酯(Benzyl Benzoate)24 溴硝丙二醇(Bronopol)25 丁羟茴醚(Butylated Hydroxyanisole)26 丁羟甲苯(Butylated Hydroxytoluene)27 羟苯丁酯(Butylparaben)28 碳酸钙(Calcium Carbonate)29 无水磷酸氢钙(Calcium Phosphate,Dibasic Anhydrous)30 磷酸氢钙二水合物(Calcium Phosphate,Dibasic Dihydrate)31 磷酸钙(Calcium Phosphate,Tribasic)32 硬脂酸钙(Calcium Stearate)33 硫酸钙(Calcium Sulfate)34 低芥酸菜籽油(Canola Oil)35 卡波姆(Carbomer)36 二氧化碳(Carbon Dioxide)37 羧甲纤维素钙(Carboxymethylcellulose Calcium)38 羧甲纤维素钠(Carboxymethylcellulose Sodium)39 角叉菜胶(Carrageenan)40 蓖麻油(Castor Oil)41 氢化蓖麻油(Castor Oil,Hydro-genated)42 微晶纤维素(Cellulose,Microcrystalline)43 粉状纤维素(Cellulose,Powdered)44 微粉硅胶微晶纤维素(Cellulose, Silicified Microcrystalline)45 醋酸纤维素(Cellulose Acetate)46 纤维醋法酯(Cellulose Acetate Phthalate)47 角豆胶(Ceratonia)48 十八十六醇(Cetostearyl Alcohol)49 西曲溴铵(Cetrimide)50 十六醇(Cetyl Alcohol)51 壳聚糖(Chitosan)52 氯己定(Chlorhexidine)53 三氯叔丁醇(Chlorobutanol)54 氯甲酚(Chlorocresol)55 一氯二氟乙烷(Chlorodifluoroe-thane)56 氟里昂(Chlorofluorocabons)57 对氯间二甲酚(Chloroxylenol)58 胆固醇(Cholesterol)59 枸橼酸(Citric Acid Monohydrate)60 胶态二氧化硅(微粉硅胶)(Colloidal Silicon Dioxide)61 着色剂(Coloring Agents)62 玉米油(Corn Oil)63 棉籽油(Cottonseed Oil)64 甲酚(Cresol)65 交联羧甲纤维素钠(Croscarmellose Sodium)66 交联聚维酮(Crospovidone)67 环糊精(Cyclodextrins)68 环甲基硅酮(Cyclomethicone)69 苯甲地那铵(Denatonium Benzoate)70 葡萄糖结合剂(Dextrates)71 糊精(Dextrin)72 葡萄糖(Dextrose)73 邻苯二甲酸二丁酯(Dibutyl Phthalate)74 癸二酸二丁酯(Dibutyl Sebacate)75 二乙醇胺(Diethanolamine)76 邻苯二甲酸二乙酯(Diethyl Phthalate)77 二氟乙烷(Difluoroethane)78 二甲硅油(Dimethicone)79 二甲醚(Dimethyl Ether)80 邻苯二甲酸二甲酯(Dimethyl Phthalate)81 二甲亚砜(Dimethyl Sulfoxide)82 多库酯钠(Docusate Sodium)83 依地酸(乙二胺四乙酸)(Edetic Acid)84 乙酸乙酯(Ethyl Acetate)85 乙基麦芽酚(Ethyl Maltol)86 油酸乙酯(Ethyl Oleate)87 乙基香草醛(Ethyl Vanillin)88 乙基纤维素(Ethylcellulose)89 硬脂酸棕榈酸乙二醇酯(Ethylene Glycol Palmitostearate)90 羟苯乙酯(Ethylparaben)91 果糖(Fructose)92 富马酸(Fumaric Acid)93 明胶(Gelatin)94 液体葡萄糖(Glucose,Liquid)95 甘油(Glycerin)96 山萮酸甘油酯(Glyceryl Behenate)97 单油酸甘油酯(Glyceryl Monooleate)98 单硬脂酸甘油酯(Glyceryl Monostearate)99 硬脂酸棕榈酸甘油酯(Glyceryl Palmitostearate)100 四氢呋喃聚乙二醇醚(Glycofurol)101 瓜耳胶(Guar Gum)102 七氟丙烷(HFC)(Heptafluoro-propane)103 海克西定(Hexetidine)104 烷烃类(HC) (Hydrocarbons)105 盐酸(Hydrochloric Acid)106 羟乙纤维素(Hydroxyethyl Cellulose)107 羟乙甲纤维素(Hydroxyethylmethyl Cellulose)108 羟丙纤维素(Hydroxypropyl Cellulose)109 低取代羟丙纤维素(Hydroxypropyl Cellulose,Low-substituted) 110 羟丙甲纤维素(Hypromellose)111 羟丙甲纤维素酞酸酯(Hypromellose Phthalate)112 咪唑烷脲(Imidurea)113 异丙醇(Isopropyl Alcohol)114 肉豆蔻酸异丙酯(Isopropyl Myristate)115 棕榈酸异丙酯(Isopropyl Palmitate)116 白陶土(Kaolin)117 乳酸(Lactic Acid)118 拉克替醇(Lactitol)119 乳糖(Lactose)120 羊毛脂(Lanolin)121 含水羊毛脂(Lanolin,Hydrous)122 羊毛醇(Lanolin Alcohols)123 卵磷脂(Lecithin)124 硅酸镁铝(Magnesium Aluminum Silicate)125 碳酸镁(Magnesium Carbonate)126 氧化镁(Magnesium Oxide)127 硅酸镁(Magnesium Silicate)128 硬脂酸镁(Magnesium Stearate)129 三硅酸镁(Magnesium Trisilicate)130 苹果酸(Malic Acid)131 麦芽糖醇(Maltitol)132 麦芽糖醇溶液(Maltitol Solution)133 麦芽糖糊精(Maltodextrin)134 麦芽酚(Maltol)135 麦芽糖(Maltose)136 甘露醇(Mannitol)137 中链脂肪酸甘油三酯(Medium-chain Triglycerides)138 葡甲胺(Meglumine)139 薄荷脑(Menthol)140 甲基纤维素(Methylcellulose)141 羟苯甲酯(Methylparaben)142 液体石蜡(Mineral Oil)143 轻质液体石蜡(Mineral Oil,Light)144 液体石蜡羊毛醇(Mineral Oil and Lanolin Alcohols)145 单乙醇胺(Monoethanolamine)146 谷氨酸一钠(Monosodium Glutamate)147 硫代甘油(Monothioglycerol)148 氮(Nitrogen)149 一氧化二氮(Nitrous Oxide)150 油酸(Oleic Acid)151 橄榄油(Olive Oil)152 石蜡(Paraffin)153 花生油(Peanut Oil)154 凡士林(Petrolatum)155 凡士林羊毛醇(Petrolatum and Lanolin Alcohols)156 苯酚(Phenol)157 苯氧乙醇(Phenoxyethanol)158 苯乙醇(Phenylethyl Alcohol)159 醋酸苯汞(Phenylmercuric Acetate)160 硼酸苯汞(Phenylmercuric Borate)161 硝酸苯汞(Phenylmercuric Nitrate)162 磷酸(Phosphoric Acid)163 波拉克林钾(Polacrilin Potassium)164 泊洛沙姆(Poloxamer)165 葡聚糖(Polydextrose)166 聚乙二醇(Polyethylene Glycol)167 聚氧乙烯(Polyethylene Oxide)168 聚(甲基)丙烯酸树脂(Polymethacr-ylates)169 聚氧乙烯烷基醚(Polyoxyethylene Alkyl Ethers)170 聚氧乙烯蓖麻油衍生物(Polyoxyeth-ylene Castor Oil Derivatives) 171 聚山梨酯(Polyoxyethylene Sorbitan Fatty Acid Esters)172 硬脂酸聚氧乙烯酯(Polyoxyethylene Stearates)173 聚醋酸乙烯酞酸酯(Polyvinyl Acetate Phthalate)174 聚乙烯醇(Polyvinyl Alcohol)175 苯甲酸钾(Potassium Benzoate)176 碳酸氢钾(Potassium Bicarbonate)177 氯化钾(Potassium Chloride)178 枸橼酸钾(Potassium Citrate)179 氢氧化钾(Potassium Hydroxide)180 焦亚硫酸钾(Potassium Metabisulfite)181 山梨酸钾(Potassium Sorbate)182 聚维酮(Povidone)183 丙酸(Propionic Acid)184 没食子酸丙酯(Propyl Gallate)185 碳酸丙烯酯(Propylene Carbonate)186 丙二醇(Propylene Glycol)187 海藻酸丙二醇酯(Propylene Glycol Alginate)188 羟苯丙酯(Propylparaben)189 糖精(Saccharin)190 糖精钠(Saccharin Sodium)191 芝麻油(Sesame Oil)192 虫胶(Shellac)193 二氧化硅二甲硅油(Simethicone)194 海藻酸钠(Sodium Alginate)195 抗坏血酸钠(Sodium Ascorbate)196 苯甲酸钠(Sodium Benzoate)197 碳酸氢钠(Sodium Bicarbonate)198 氯化钠(Sodium Chloride)199 枸橼酸钠二水合物(Sodium Citrate Dihydrate)200 环拉酸钠(Sodium Cyclamate)201 氢氧化钠(Sodium Hydroxide)202 月桂硫酸钠(十二烷基硫酸钠)(Sodium Lauryl Sulfate)203 焦亚硫酸钠(偏亚硫酸钠)(Sodium Metabisulfite)204 磷酸氢二钠(Sodium Phosphate,Dibasic)205 磷酸二氢钠(Sodium Phosphate ,Monobasic)206 丙酸钠(Sodium Propionate)207 羧甲淀粉钠(Sodium Starch Glycolate)208 硬脂富马酸钠(Sodium Stearyl Fumarate)209 山梨酸(Sorbic Acid)210 山梨坦酯Sorbitan Esters(Sorbitan Fatty Acid Esters) 211 山梨醇(Sorbitol)212 大豆油(Soybean Oil)213 淀粉(Starch)214 预胶化淀粉(Starch,Pregelatinized)215 灭菌玉米淀粉(Starch,Sterilizable Maize)216 硬脂酸(Stearic Acid)217 硬脂醇(Stearyl Alcohol)218 羟糖氯(Sucralose)219 蔗糖(Sucrose)220 可压性蔗糖(Sugar,Compressible)221 蔗糖粉(Sugar,Confectioner’s)222 蔗糖球形颗粒(Sugar Spheres)223 硫酸(Sulfuric Acid)224 葵花籽油(Sunflower Oil)225 氢化植物油(硬脂)栓剂基质(Sup-pository Bases,Hard Fat) 226 滑石粉(Talc)227 酒石酸(Tartaric Acid)228 四氟乙烷(HFC)(Tetrafluoroe-thane)229 硫柳汞(Thimerosal)230 二氧化钛(Titanium Dioxide)231 西黄蓍胶(Tragacanth)232 海藻糖(Trehalose)233 三醋汀(Triacetin)234 枸橼酸三丁酯(Tributyl Citrate)235 三乙醇胺(Triethanolamine)236 枸橼酸三乙酯(Triethyl Citrate)237 香草醛(Vanillin)238 氢化植物油(Vegetable Oil,Hydrogenated)239 纯化水(Water)240 阴离子乳化蜡(Wax,Anionic Emulsifying)241 巴西棕榈蜡(Wax,Carnauba)242 十六醇酯蜡(Wax,Cetyl Esters)243 微晶蜡(Wax,Microcrystalline)244 非离子乳化蜡(聚西托醇乳化蜡)(Wax,Nonionic Emulsifying) 245 白蜡(Wax,White)246 黄蜡(Wax,Yellow)247 黄原酸胶(Xanthan Gum)248 木糖醇(Xylitol)796249 玉米朊(玉米蛋白)(Zein)250 硬脂酸锌(Zinc Stearate)。
世界卫生组织儿童标准处方集

WHO Model Formulary for ChildrenBased on the Second Model List of Essential Medicines for Children 2009世界卫生组织儿童标准处方集基于2009年儿童基本用药的第二个标准目录WHO Library Cataloguing-in-Publication Data:WHO model formulary for children 2010.Based on the second model list of essential medicines for children 2009.1.Essential drugs.2.Formularies.3.Pharmaceutical preparations.4.Child.5.Drug utilization. I.World Health Organization.ISBN 978 92 4 159932 0 (NLM classification: QV 55)世界卫生组织实验室出版数据目录:世界卫生组织儿童标准处方集基于2009年儿童基本用药的第二个标准处方集1.基本药物 2.处方一览表 3.药品制备 4儿童 5.药物ISBN 978 92 4 159932 0 (美国国立医学图书馆分类:QV55)World Health Organization 2010All rights reserved. Publications of the World Health Organization can be obtained fromWHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: ******************). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the aboveaddress(fax:+41227914806;e-mail:*******************).世界卫生组织2010版权所有。
Crafters Choice Cocamidopropyl Betaine产品说明书

Safety Data SheetCrafters Choice™ Cocamidopropyl BetaineDate of Issue: 03/30/2016Version: 1.0EN (English US) 1/71.1. Product IdentifierProduct Form: MixtureProduct Name: Crafters Choice™ Cocamidopropyl BetaineCAS No: 61789-40-0Synonyms: CAPB 1.2.Intended Use of the Product Use of the substance/mixture: Personal Care products1.3. Name, Address, and Telephone of the Responsible Party CompanyCrafter’s Choice Brands, LLC 7820 E. Pleasant Valley Road Independence, Ohio 44131 Phone: 1-800-908-7028 1.4. Emergency Telephone NumberEmergency Number(800) 255-3924 ChemTel Domestic USA, Canada, Puerto Rico, and USVl + (813) 248-0585 International SECTION 2: HAZARDS IDENTIFICATION2.1. Classification of the Substance or MixtureGHS-US classificationSkin Irrit. 2 H315Eye Irrit. 2AH319Aquatic Acute 1 H400Full text of hazard classes and H-statements : see section 162.2. Label ElementsGHS-US LabelingHazard Pictograms (GHS-US) : GHS07GHS09Signal Word (GHS-US): Warning Hazard Statements (GHS-US): H315 - Causes skin irritation H319 - Causes serious eye irritation H400 - Very toxic to aquatic life Precautionary Statements (GHS-US) : P264 - Wash exposed areas. thoroughly after handling.P273 - Avoid release to the environment.P280 - Wear protective gloves, protective clothing, and eye protection.P302+P352 - If on skin: Wash with plenty of water.P305+P351+P338 - If in eyes: Rinse cautiously with water for several minutes.Remove contact lenses, if present and easy to do. Continue rinsing.P321 - Specific treatment (see section 4 on this SDS).P332+P313 - If skin irritation occurs: Get medical advice/attention.P337+P313 - If eye irritation persists: Get medical advice/attention.P362+P364 - Take off contaminated clothing and wash it before reuse.P391 - Collect spillage.P501 - Dispose of contents/container in accordance with local, regional, national,and international regulations.2.3. Other HazardsOther Hazards Not Contributing to the Classification: Exposure may aggravate pre-existing eye, skin, or respiratory conditions.2.4. Unknown Acute Toxicity (GHS-US)No data available3.1. SubstanceNot applicable3.2. MixtureName Product Identifier % GHS-US classification Water (CAS No) 7732-18-5 69 - 71 Not classified1-Propanaminium, 3-amino-N-(carboxymethyl)-N,N-dimethyl-, N-coco acyl derivatives, hydroxides, inner salts (CAS No) 61789-40-0 29 - 31 Skin Irrit. 2, H315Eye Irrit. 2A, H319Aquatic Acute 1, H400Full text of H-phrases: see section 164.1. Description of First Aid MeasuresFirst-aid Measures General: Never give anything by mouth to an unconscious person. If you feel unwell, seek medical advice (show the label where possible).First-aid Measures After Inhalation: When symptoms occur: go into open air and ventilate suspected area. Obtain medical attention if breathing difficulty persists.First-aid Measures After Skin Contact: Remove contaminated clothing. Drench affected area with water for at least 15 minutes. Obtain medical attention if irritation develops or persists.First-aid Measures After Eye Contact: Rinse cautiously with water for at least 15 minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Obtain medical attention.First-aid Measures After Ingestion: Rinse mouth. Do NOT induce vomiting. Obtain medical attention.4.2. Most Important Symptoms and Effects, Both Acute and DelayedSymptoms/Injuries: Causes serious eye irritation. Causes skin irritation.Symptoms/Injuries After Inhalation: Not expected to present a significant inhalation hazard under anticipated conditions of normal use. Prolonged exposure may cause irritation.Symptoms/Injuries After Skin Contact: Redness, pain, swelling, itching, burning, dryness, and dermatitis.Symptoms/Injuries After Eye Contact: Contact causes severe irritation with redness and swelling of the conjunctiva. Symptoms/Injuries After Ingestion: If a large quantity has been ingested: May cause nausea, vomiting, and diarrhea. Ingestion may cause adverse effects.4.3. Indication of Any Immediate Medical Attention and Special Treatment NeededIf medical advice is needed, have product container or label at hand. If exposed or concerned, get medical advice and attention.5.1. Extinguishing MediaSuitable Extinguishing Media: Alcohol foam, dry chemical, carbon dioxide, water spray, fog. Use extinguishing media appropriate for surrounding fire.Unsuitable Extinguishing Media: Do not use a heavy water stream. Use of heavy stream of water may spread fire.5.2. Special Hazards Arising From the Substance or MixtureFire Hazard: Not considered flammable but may burn at high temperatures.Explosion Hazard: Product is not explosive.Reactivity: Stable at ambient temperature and under normal conditions of use. Hazardous reactions will not occur under normal conditions.5.3. Advice for FirefightersPrecautionary Measures Fire: Exercise caution when fighting any chemical fire.Firefighting Instructions: Exercise caution when fighting any chemical fire. Use water spray or fog for cooling exposed containers.Protection During Firefighting: Do not enter fire area without proper protective equipment, including respiratory protection. Other Information: Do not allow run-off from fire fighting to enter drains or water courses.6.1. Personal Precautions, Protective Equipment and Emergency ProceduresGeneral Measures: Avoid breathing (vapor, mist, spray). Avoid all contact with skin, eyes, or clothing.6.1.1. For Non-emergency PersonnelProtective Equipment: Use appropriate personal protection equipment (PPE).Emergency Procedures: Evacuate unnecessary personnel.6.1.2. For Emergency RespondersProtective Equipment: Equip cleanup crew with proper protection.Emergency Procedures: Ventilate area. Upon arrival at the scene, a first responder is expected to recognize the presence of dangerous goods, protect oneself and the public, secure the area, and call for the assistance of trained personnel as soon as conditions permit.6.2. Environmental PrecautionsPrevent entry to sewers and public waters. Avoid release to the environment. Collect spillage.6.3. Methods and Material for Containment and Cleaning UpFor Containment: Absorb and/or contain spill with inert material, then place in suitable container. Contain any spills with dikes or absorbents to prevent migration and entry into sewers or streams.Methods for Cleaning Up: Clean up spills immediately and dispose of waste safely. Transfer spilled material to a suitable container for disposal. Contact competent authorities after a spill.6.4. Reference to Other SectionsSee heading 8, Exposure Controls and Personal Protection. See Section 13, Disposal Considerations.7.1. Precautions for Safe HandlingPrecautions for Safe Handling: Wash hands and other exposed areas with mild soap and water before eating, drinking or smoking and when leaving work. Avoid breathing vapors, mist, spray. Avoid contact with skin, eyes and clothing.Hygiene Measures: Do not eat, drink or smoke when using this product. Handle in accordance with good industrial hygiene and safety procedures.7.2. Conditions for Safe Storage, Including Any IncompatibilitiesTechnical Measures: Comply with applicable regulations.Storage Conditions: Keep container closed when not in use. Store in a dry, cool place. Keep/Store away from direct sunlight, extremely high or low temperatures and incompatible materials.Incompatible Products: Strong acids, strong bases, strong oxidizers.Incompatible Materials: Sources of ignition. Direct sunlight.7.3. Specific End Use(s)Personal Care products8.1. Control ParametersFor substances listed in section 3 that are not listed here, there are no established exposure limits from the manufacturer, supplier, importer, or the appropriate advisory agency including: ACGIH (TLV), AIHA (WEEL), NIOSH (REL), or OSHA (PEL).8.2. Exposure ControlsAppropriate Engineering Controls : Emergency eye wash fountains and safety showers should be available in theimmediate vicinity of any potential exposure. Ensure adequate ventilation,especially in confined areas. Ensure all national/local regulations are observed. Personal Protective Equipment : Gloves. Safety glasses. Protective clothing. Protective goggles.Hand Protection : Wear protective gloves.Eye Protection : Chemical safety goggles.Skin and Body Protection : Wear suitable protective clothing.Respiratory Protection : In case of inadequate ventilation wear respiratory protection. If exposure limits areexceeded or irritation is experienced, approved respiratory protection should beworn.Other Information: When using, do not eat, drink or smoke.9.1. Information on Basic Physical and Chemical PropertiesPhysical State : LiquidAppearance : Colorless to pale yellowOdor : Slight fattyOdor Threshold : No data availablepH : 4 - 6Relative Evaporation Rate (butylacetate=1) : No data availableMelting Point : < -10 °C (14 °F)Freezing Point : No data availableBoiling Point : > 100 °C (212 °F)Flash Point : > 100 °C (212 °F)Auto-ignition Temperature : No data availableDecomposition Temperature : No data availableFlammability (solid, gas) : No data availableVapor Pressure : No data availableRelative Vapor Density at 20 °C : No data availableRelative Density : No data availableSpecific Gravity : 1.045-1.065 @ 20°CSolubility : Water: SolublePartition Coefficient: N-Octanol/Water : 4.2 Log KowViscosity : < 100 cP 30°C (Brookfield, #1, 50 rpm)Explosive Properties : No data availableOxidizing Properties : No data availableExplosive Limits : Not applicable9.2. Other Information No additional information available10.1 Reactivity: Stable at ambient temperature and under normal conditions of use. Hazardous reactions will not occur under normal conditions.10.2 Chemical Stability: Product is stable.10.3 Possibility of Hazardous Reactions: Hazardous polymerization will not occur.10.4 Conditions to Avoid: Direct sunlight, extremely high or low temperatures, and incompatible materials.10.5 Incompatible Materials: Strong acids, strong bases, strong oxidizers.10.6 Hazardous Decomposition Products: Under fire conditions this material may produce hazardous carbon dioxide (CO2), carbon monoxide (CO), various low molecular weight hydrocarbons, and smoke.11.1. Information On Toxicological EffectsAcute Toxicity : Not classifiedCocoamidopropyl Betaine (61789-40-0)1-Propanaminium, 3-amino-N-(carboxymethyl)-N,N-dimethyl-, N-coco acyl derivatives, hydroxides, inner salts (61789-40-0) LD50 Oral Rat 4900 mg/kgSkin Corrosion/Irritation: Causes skin irritation.pH: 4 - 6Serious Eye Damage/Irritation: Causes serious eye irritation.pH: 4 - 6Respiratory or Skin Sensitization: Not classifiedGerm Cell Mutagenicity: Not classifiedCarcinogenicity: Not classifiedCocoamidopropyl Betaine (61789-40-0)Reproductive Toxicity: Not classifiedSpecific Target Organ Toxicity (Single Exposure): Not classifiedCocoamidopropyl Betaine (61789-40-0)Specific Target Organ Toxicity (Repeated Exposure): Not classifiedAspiration Hazard: Not classifiedPotential Adverse Human Health Effects and Symptoms: Based on available data, the classification criteria are not met. Symptoms/Injuries After Inhalation: Not expected to present a significant inhalation hazard under anticipated conditions of normal use. Prolonged exposure may cause irritation.Symptoms/Injuries After Skin Contact: Redness, pain, swelling, itching, burning, dryness, and dermatitis.Symptoms/Injuries After Eye Contact: Contact causes severe irritation with redness and swelling of the conjunctiva. Symptoms/Injuries After Ingestion: If a large quantity has been ingested: May cause nausea, vomiting, and diarrhea. Ingestion may cause adverse effects.12.1. ToxicityEcology - General : Very toxic to aquatic life.Cocoamidopropyl Betaine (61789-40-0)1-Propanaminium, 3-amino-N-(carboxymethyl)-N,N-dimethyl-, N-coco acyl derivatives, hydroxides, inner salts (61789-40-0) LC50 Fish 1 1 (1 - 10) mg/l (Exposure time: 96 h - Species: Brachydanio rerio)EC50 Daphnia 1 6.5 mg/l (Exposure time: 48 h - Species: Daphnia magna)EC50 Other Aquatic Organisms 1 1 (1 - 10) mg/l (Exposure time: 72 h - Species: Desmodesmus subspicatus)LC 50 Fish 2 2 mg/l (Exposure time: 96 h - Species: Brachydanio rerio [semi-static])12.2. Persistence and DegradabilityCocoamidopropyl Betaine (61789-40-0)Persistence and Degradability Not established.12.3. Bioaccumulative PotentialCocoamidopropyl Betaine (61789-40-0)Bioaccumulative Potential Not established.12.4. Mobility in Soil1-Propanaminium, 3-amino-N-(carboxymethyl)-N,N-dimethyl-, N-coco acyl derivatives, hydroxides, inner salts (61789-40-0) Log Koc 2.812.5. Other Adverse EffectsOther Information : Avoid release to the environment.13.1. Waste treatment methodsWaste Disposal Recommendations: Dispose of contents/container in accordance with local, regional, national, and international regulations.Additional Information: Container may remain hazardous when empty. Continue to observe all precautions.Ecology – Waste Materials: Avoid release to the environment. This material is hazardous to the aquatic environment. Keep out of sewers and waterways.In Accordance With ICAO/IATA/IMDG/DOT14.1. UN NumberUN-No.(DOT) : 3082DOT NA no. UN308214.2. UN Proper Shipping NameProper Shipping Name (DOT) : Environmentally hazardous substances, liquid, n.o.s.Class (DOT) : 9 - Class 9 - Miscellaneous hazardous material 49 CFR 173.140Hazard Labels (DOT) : 9 - Class 9 (Miscellaneous dangerous materials)DOT Symbols : G - Identifies PSN requiring a technical namePacking Group (DOT) : III - Minor DangerDOT Special Provisions (49 CFR 172.102) : 8 - A hazardous substance that is not a hazardous waste may be shipped under theshipping description “Other regulated substances, liquid or solid, n.o.s.”, asappropriate. In addition, for solid materials, special provision B54 applies146 - This description may be used for a material that poses a hazard to theenvironment but does not meet the definition for a hazardous waste or ahazardous substance, as defined in 171.8 of this subchapter, or any hazard class asdefined in Part 173 of this subchapter, if it is designated as environmentallyhazardous by the Competent Authority of the country of origin, transit ordestination173 - An appropriate generic entry may be used for this material335 - Mixtures of solids that are not subject to this subchapter andenvironmentally hazardous liquids or solids may be classified as “Environmentallyhazardous substances, solid, n.o.s,” UN3077 and may be transported under thisentry, provided there is no free liquid visible at the time the material is loaded orat the time the packaging or transport unit is closed. Each transport unit must beleak-proof when used as bulk packagingIB3 - Authorized IBCs: Metal (31A, 31B and 31N); Rigid plastics (31H1 and 31H2);Composite (31HZ1 and 31HA2, 31HB2, 31HN2, 31HD2 and 31HH2). AdditionalRequirement: Only liquids with a vapor pressure less than or equal to 110 kPa at50 C (1.1 bar at 122 F), or 130 kPa at 55 C (1.3 bar at 131 F) are authorized, exceptfor UN2672 (also see Special Provision IP8 in Table 2 for UN2672)T4 - 2.65 178.274(d)(2) Normal............. 178.275(d)(3)TP1 - The maximum degree of filling must not exceed the degree of fillingdetermined by the following: Degree of filling = 97 / (1 + a (tr - tf)) Where: tr is themaximum mean bulk temperature during transport, and tf is the temperature indegrees celsius of the liquid during fillingTP29 - A portable tank having a minimum test pressure of 1.5 bar (150.0 kPa) maybe used provided the calculated test pressure is 1.5 bar or less based on theMAWP of the hazardous materials, as defined in 178.275 of this subchapter, wherethe test pressure is 1.5 times the MAWP: 155DOT Packaging Exceptions (49 CFR173.xxx): 203DOT Packaging Non Bulk (49 CFR173.xxx)DOT Packaging Bulk (49 CFR 173.xxx) : 24114.3. Additional Information: 171Emergency Response Guide (ERG)NumberOther information : No supplementary information available.Transport by SeaDOT Vessel Stowage Location : A - The material may be stowed ‘‘on deck’’ or ‘‘under deck’’ on a cargo vessel andon a passenger vesselEmS-No. (1) : F-AEmS-No. (2) : S-FAir Transport: No limitDOT Quantity Limitations PassengerAircraft/Rail (49 CFR 173.27)DOT Quantity Limitations Cargo Aircraft: No limitOnly (49 CFR 175.75)15.1 US Federal RegulationsCocoamidopropyl Betaine (61789-40-0)SARA Section 311/312 Hazard Classes Immediate (acute) health hazard1-Propanaminium, 3-amino-N-(carboxymethyl)-N,N-dimethyl-, N-coco acyl derivatives, hydroxides, inner salts (61789-40-0) Listed on the United States TSCA (Toxic Substances Control Act) inventoryWater (7732-18-5)Listed on the United States TSCA (Toxic Substances Control Act) inventory15.2 US State Regulations Neither this product nor its chemical components appear on any US state lists.Other Information : This document has been prepared in accordance with the SDSrequirements of the OSHA Hazard Communication Standard 29 CFR1910.1200.GHS Full Text Phrases:Aquatic Acute 1 Hazardous to the aquatic environment - Acute Hazard Category 1 Eye Irrit. 2A Serious eye damage/eye irritation Category 2ASkin Irrit. 2 Skin corrosion/irritation Category 2H315 Causes skin irritationH319 Causes serious eye irritationH400 Very toxic to aquatic lifeThe data herein are based on our current knowledge and believed to be reliable. Crafter's Choice Brands, LLC, provides this information without any representation or warranty, expressed or implied, regarding its accuracy or correctness.Users must make their own determination that handling, storage, use and disposal of the product in the anticipated manner is safe and appropriate. Because these actions of the user are out of our control, and may be beyond our knowledge, we do not assume responsibility and expressly disclaim liability for loss, damage, expense or any other claim arising out of or in any way connected with the handling, storage, use or disposal of the product or container.。
