MCB-613_COA_19559_MedChemExpress
BLU-554_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :BLU-554Catalog No. :HY-100492CAS No. :1707289-21-11.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:C24H24Cl2N4O4Molecular Weight:503.38CAS No. :1707289-21-14. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to light yellow (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
CAS号254964-60-8_Tasquinimod_MedBio相关资料
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11931
Cyclophosphamide
Cyclophosphamide
50-18-0
10mM (in 1mL DMSO)
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12034
Mupirocin
包装
纯度
MedBio
MED11950
Resminostat hydrochloride
Resminostat hydrochloride
1187075-34-8
100mg
≥98%
品牌
CAS
包装
纯度
MedBio
MED12017
Raltitrexed
Raltitrexed
112887-68-0
10mM (in 1mL DMSO)
≥98%
体内研究
当通过管饲法或饮用水给予成年雄性小鼠(即C57B1 / 6J或无胸腺裸鼠)0.1-30mg / kg(即0.2-74μmoles/ kg)时,Tasquinimod的生物利用度和口服吸收是极好的。Tasquinimod的效力表示为抑制癌症生长50%的Tasquinimod每日口服剂量范围为0.1-1.0 mg / kg / d(即0.24-2.40μmoles/ kg /天),相对于一系列(n> 5)免疫缺陷小鼠中的人前列腺癌异种移植物。通过饮用水以5mg / kg /天的慢性剂量服用的Tasquinimod在免疫活性同系小鼠中产生> 80%的TRAMP-C2小鼠前列腺癌生长抑制(p <0.05)[2]。携带皮下LNCaP肿瘤的裸鼠用Tasquinimod治疗3周。在接种后第7天开始以1mg / kg /天和10mg / kg /天暴露于Tasquinimod。与接种后28天的未处理对照组相比,1 mg / kg /天和10 mg / kg /天的肿瘤重量均有统计学显着的剂量依赖性降低(p <0.001),说明Tasquinimod的抗肿瘤作用[3]。
26291346_基于UPLC-MS

Abstract: A UPLC-MS/MS method was established to quantitatively determine the content of alliin in animal plasma to study whether alliin and alliin in garlic enteric preparations can react to produce the active ingredient allicin in the in vivo environment. Methods Reversed-phase C18 column (Waters ICQUITY UPLC BEH, 100 × 2.1 mm, 1.7μm), column temperature: 40 ℃, flow rate: 0.15 mL/min, injection volume: 2μl, Mobile phase: 0.1% formic acid (A)-acetonitrile (B), gradient elution; mass spectrometry ionization: ESI+, determination of allicin in rat plasma . Results The results of two parallel experiments of garlic enteric preparation and enzymatic garlic powder showed that in the garlic enteric preparation with allinase, the plasma concentration of alliin in the blood of rats was significantly lower. Conclusion A UPLC-MS/MS method for the quantitative determination of alliin in animal plasma has been established. Alliin and alliin in garlic enteric-coated preparations can react in vivo.Key words: Garlic enteric preparation; garbonine; UPLC-MS-MS基于UPLC-MS/MS大蒜肠溶制剂中蒜氨酸、蒜酶体内反应情况研究杨亮1,胡小霞4 ,宋百灵4,关明3,李新霞2*(1.新疆警察学院 新疆 乌鲁木齐 8300112.新疆医科大学药学院 新疆 乌鲁木齐 8300113.新疆师范大学化学化工学院 新疆 乌鲁木齐 8300544.新疆医科大学中心实验室 新疆 乌鲁木齐 830011)Study on the Reaction of Garlic and Uterine in the UPLC-MS / MS of Garlic SausolYANG Liang 1,HU Xiaoxia 4 ,SONG Bailing 4,GUAN Ming 3,LI Xinxia 2*(1. Xinjiang Police College, Urumqi 830054, Xinjiang China2.Chemistry and Chemical Engineering of Xinjiang Normal University College, Urumqi 830054, Xinjiang China3.School of Pharmacy, Xinjiang Medical University, Urumqi 830011, Xinjiang China4.Central Laboratory of Xinjiang Medical University, Urumqi 830011, Xinjiang China )摘要:目的 建立定量测定动物血浆中蒜氨酸含量的UPLC-MS/MS 方法,研究大蒜肠溶制剂中蒜氨酸、蒜酶能否在体内环境下反应生成活性成分大蒜辣素。
【国家自然科学基金】_劳动卫生与职业病_基金支持热词逐年推荐_【万方软件创新助手】_20140731
107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 124 125 126 127 128
乙醛 中华蜜蜂 丙烯醛 三叉神经节 一氧化碳 t细胞受体γ t淋巴细胞 topsis sh-sy5y细胞 sh-sy5y rna干扰 rapd pcb153 pbde-47 mirna jnk1 hmsh2基因 h(+)转运atp合酶 dna损伤 dna加合物 dna修复基因 bak基因
2009年 序号 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52
科研热词 推荐指数 转录因子ap-1 4 基因表达 4 上皮细胞 4 细胞凋亡 3 烃类,芳香 3 铝 2 辐射 2 苯 2 细胞周期蛋白d1 2 细胞周期 2 石英 2 有丝分裂素激活蛋白激酶类 2 巨噬细胞,肺泡 2 信号传递 2 二氧化硅 2 pc12细胞 2 骨骼肌 1 非小细胞肺癌 1 需求评估 1 队列研究 1 钨 1 钙通道阻滞药 1 钙通道 1 采矿 1 邻苯二甲酸丁苄酯 1 遗传载体 1 遗传易感性 1 转染 1 转录因子 1 超氧化物歧化酶 1 质量评价 1 质粒 1 记忆 1 表皮 1 蛋白质p53 1 苯并芘 1 苯并(a)芘 1 芘类 1 色谱法,高压液相 1 致癌物,环境 1 脂质过氧化作用 1 胰岛素样生长因子ii 1 肺肿瘤 1 肺功能 1 职业卫生服务 1 细菌蛋白质类 1 细胞遗传毒性 1 细胞恶性转化 1 细胞周期蛋白质依赖激酶类 1 纤溶酶原激活物抑制物1 1 粉尘 1 神经行为 1
CAS号413611-93-5_10074-G5_MedBio_物理性质

1、产品物理参数:
常用名
10074-G5
英文名
10074-G5
CAS号
413611-93-5
分子量
332.313
密度
1.4±0.1 g/cm3
沸点
538.6±60.0 °C at 760 mmHg
分子式
C18H12N4O3
熔点
无资料
闪点
279.5±32.9 °C
2、技术资料:
体外研究
10074-G5抑制Daudi Burkitt淋巴瘤细胞的生长并破坏c-Myc / Max二聚化。针对Daudi和HL-60细胞的IC50值分别为15.6和13.5μM[1]。10074-G5在区域Arg363-Ile381中结合Myc肽Myc353-437,Kd值为2.8μM。10074-G5结合在由诱导螺旋结构域(Leu370-Arg378)的N末端的扭结(Asp379-Ile381)产生的空腔中[3]。
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11457
CDK inhibitor II
CDK inhibitor II
1269815-17-9
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11591
(S)-CCG-1423
(S)-CCG-1423
None
体内研究
静脉注射20 mg / kg小鼠的血浆半衰期为10074-G5,为37分钟,血药浓度峰值为58μM,比肿瘤峰值浓度高10倍[1]。
3、同类产品列表:
MCB-613_LCMS_19559_MedChemExpress
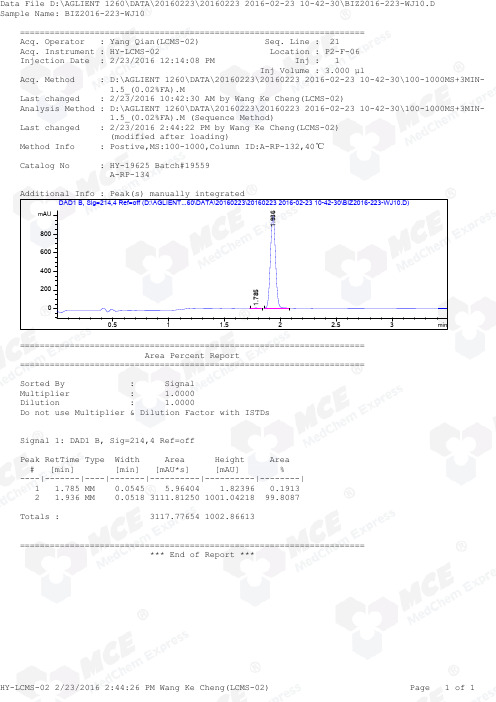
=====================================================================Acq. Operator : Yang Qian(LCMS-02) Seq. Line : 21Acq. Instrument : HY-LCMS-02 Location : P2-F-06Injection Date : 2/23/2016 12:14:08 PM Inj : 1Inj Volume : 3.000 µlAcq. Method : D:\AGLIENT 1260\DATA\20160223\20160223 2016-02-23 10-42-30\100-1000MS+3MIN- 1.5_(0.02%FA).MLast changed : 2/23/2016 10:42:30 AM by Wang Ke Cheng(LCMS-02)Analysis Method : D:\AGLIENT 1260\DATA\20160223\20160223 2016-02-23 10-42-30\100-1000MS+3MIN- 1.5_(0.02%FA).M (Sequence Method)Last changed : 2/23/2016 2:44:22 PM by Wang Ke Cheng(LCMS-02) (modified after loading)Method Info : Postive,MS:100-1000,Column ID:A-RP-132,40℃Catalog No : HY-19625 Batch#19559 A-RP-134Additional Info : Peak(s) manually integratedmin0.511.52 2.53mAU 0200400600800 DAD1 B, Sig=214,4 Ref=off (D:\AGLIENT...60\DATA\20160223\20160223 2016-02-23 10-42-30\BIZ2016-223-WJ10.D)1.7851.936===================================================================== Area Percent Report =====================================================================Sorted By : Signal Multiplier : 1.0000Dilution : 1.0000Do not use Multiplier & Dilution Factor with ISTDsSignal 1: DAD1 B, Sig=214,4 Ref=offPeak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------| 1 1.785 MM 0.0545 5.96404 1.82396 0.1913 2 1.936 MM 0.0518 3111.81250 1001.04218 99.8087Totals : 3117.77654 1002.86613===================================================================== *** End of Report ***=====================================================================Acq. Operator : Yang Qian(LCMS-02) Seq. Line : 21Acq. Instrument : HY-LCMS-02 Location : P2-F-06Injection Date : 2/23/2016 12:14:08 PM Inj : 1Inj Volume : 3.000 µlAcq. Method : D:\AGLIENT 1260\DATA\20160223\20160223 2016-02-23 10-42-30\100-1000MS+3MIN- 1.5_(0.02%FA).MLast changed : 2/23/2016 10:42:30 AM by Wang Ke Cheng(LCMS-02)Analysis Method : D:\AGLIENT 1260\DATA\20160223\20160223 2016-02-23 10-42-30\100-1000MS+3MIN- 1.5_(0.02%FA).M (Sequence Method)Last changed : 2/23/2016 2:38:56 PM by Wang Ke Cheng(LCMS-02) (modified after loading)Method Info : Postive,MS:100-1000,Column ID:A-RP-132,40℃Catalog No : HY-19625 Batch#19559 A-RP-134Additional Info : Peak(s) manually integratedmin0.511.522.53100000200000300000400000500000600000700000 MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20160223\20160223 2016-02-23 10-42-30\BIZ2016-223-WJ10.D) ES-API, Pos, Sca1.938MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts.Reportable Ion Abundance: > 10%.Retention Mol. Weight Time (MS) MS Area or Ion1.938 5038909 306.20 I 305.20 I 153.20 Im/z10020030040050060020406080100*MSD1 SPC, time=1.908:1.981 of D:\AGLIENT 1260\DATA\20160223\20160223 2016-02-23 10-42-30\BIZ2016-223-WJ10.D ES-API,Max: 318797306.2305.2153.2*** End of Report ***。
液相色谱-串联质谱测定小麦粉中11种真菌毒素

液相色谱-串联质谱测定小麦粉中11种真菌毒素李磊;李海畅;周贻兵;林野【摘要】建立小麦粉中11种真菌毒素的超高效液相色谱-串联质谱检测方法.样品用乙腈-水溶液提取,经多功能净化柱PriboFast 100进行净化,采用XTerra MSC18色谱柱进行分离,以20 mmol/L乙酸铵溶液和乙腈梯度洗脱,利用超高效液相色谱-串联质谱进行分析.11种真菌毒素在测定浓度范围内线性关系良好,各组分相关系数R2 >0.998,加标回收率范围72.4 %~90.7%,方法精密度范围6.2 %~11.8%,测定各组分真菌毒素定量限范围1.2μg/kg~2.0μg/kg.该方法前处理过程简洁、灵敏度高、重现性好,适用于小麦粉中11种真菌毒素的测定.【期刊名称】《食品研究与开发》【年(卷),期】2016(037)003【总页数】5页(P156-160)【关键词】小麦粉;真菌毒素;超高效液相色谱-串联质谱【作者】李磊;李海畅;周贻兵;林野【作者单位】贵州省疾病预防控制中心,贵州贵阳550004;贵阳市疾病预防控制中心,贵州贵阳550002;贵州省疾病预防控制中心,贵州贵阳550004;贵州省疾病预防控制中心,贵州贵阳550004【正文语种】中文真菌毒素是霉菌在污染食品后所产生的有毒代谢物,是各种粮食及农副产品的主要污染物之一,据联合国粮农组织(FAO)报道世界上约有25 %的农作物受到真菌毒素的污染[1]。
真菌毒素具有致癌及极强的细胞毒性,极易威胁到人体的健康[2-3]。
我国是小麦制品的消耗大国,小麦运输储存过程中也极易受到真菌毒素的污染,目前已有报道在小麦制品中检出了黄曲霉毒素、呕吐毒素等真菌毒素[4]。
目前对于真菌毒素的检测主要方法有酶联免疫法[5],气相色谱法[6],高效液相色谱法[7-8]和液相色谱-串联质谱法[9-10]。
酶联免疫法虽然设备要求简单,但是准确度上无法保证,大批量检测时容易出现假阳性结果;气相色谱法由于对分析物质要求具有低沸点且热稳定性好,所以在多组分真菌毒素的分析上应用受到了限制;高效液相色谱法稳定性较好,但是单一通过保留时间定性很难应用于多组分的真菌毒素的测定;高效液相色谱-串联质谱法能够克服上诉方法的缺点,具有高分离度、高准确度的特点。
AMG319_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:AMG319 is a potent and selective PI3Kδ inhibitor with IC50 of 18 nM, also inhibits PI3Kγ with IC50 of 850 nM.IC50: 18 nM (PI3Kδ), 850 nM (PI3Kγ), 2.7μM (PI3Kβ), 33 μM (PI3Kα)Target: PI3KIn vitro: AMG319 is a compound with an IC50 of 16 nM in a human whole blood assay (HWB), excellent selectivity over a large panel of protein kinases, and a high level of in vivo efficacy as measured by two rodent disease models of inflammation. AMG319 inhibits anti–IgM/CD40L–induced B cell proliferation with IC50 of 8.6 nM and reduces pAkt level with IC50 of 1.5 nM. AMG319 also inhibits anti–IgD–induce CD–69 expression in HWB.[1]In vivo: In female Lewis rats, AMG319 (3 mg/kg, p.o.) inhibits the KLH–induced inflammatory response by 88%. In the transgenic (IgMm) mice, AMG319 (, p.o.) inhibits in vivo pAKT with IC50 of 1.9 nM.[1]PROTOCOL (Extracted from published papers and Only for reference)Enzyme assay [1]A PI3K Alphascreen assay is used to measure the activity of a panel of four phosphoinositide 3–kinases: PI3Kα, PI3Kβ, PI3Kγ, and PI3Kδ. Enzyme reaction buffer is prepared using sterile water and 50 mM Tris–HCl, pH 7, 14 mM MgCl2, 2 mM sodium cholate, and 100 mM NaCl. 2 mM DTT is added fresh on the day of the experiment. The Alphascreen buffer is made using sterile water and 10 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.10% Tween 20, and 30 mM EDTA. Then 1 mM DTT is added fresh on the day of the experiment.Compound source plates used for this assay are 384–well Greiner clear polypropylene plates containing test compounds at 5 mM and diluted 1:2 over 22 concentrations. Columns 23 and 24 contained only DMSO, as these wells comprised the positive and negativecontrols, respectively. Source plates are replicated by transferring 0.5 μL per well into 384–well Optiplates. Each PI3K isoform is diluted in enzyme reaction buffer to 2× working stocks. PI3Kα is diluted to 1.6 nM, PI3Kβ is diluted to 0.8 nM, PI3Kγ is diluted to 15 nM, and PI3Kδ is diluted to 1.6 nM. PI(4,5)P2 is diluted to 10 μM, and ATP was diluted to 20 μM. This 2× stock is used in the assays for PI3Kαand PI3Kβ. For assay of PI3Kγ and PI3Kδ, PI(4,5)P2 is diluted to 10 μM and ATP was diluted to 8 μM to prepare a similar 2× working stock. Alphascreen reaction solutions are made using beads from the anti–GST Alphascreen kit. Two 4× working stocks of the Alphascreen reagents are made in Alphascreen reaction buffer. In one stock, biotinylated–IP4 is diluted to 40 nM andstreptavadin–donor beads are diluted to 80 μg/mL. In the second stock, PIP3–binding protein is diluted to 40 nM andanti–GST–acceptor beads were diluted to 80 μg/mL. As a negative control, a reference inhibitor at a concentration Ki (40 μM) isincluded in column 24 as a negative (100% inhibition) control. Using a 384–well Multidrop, 10 μL/well of 2× enzyme stock is added to columns 1–24 of the assay plates for each isoform. An amount of 10 μL/well of the appropriate substrate 2× stock (containing 20 μM ATP for the PI3Kα and –β assays and containing 8 μM ATP for the PI3Kγ and –δ assays) is then added to columns 1–24 of all plates.Plates are then incubated at room temperature for 20 min. In the dark, 10 μL/well of the donor bead solution is added to columns 1–24 of the plates to quench the enzyme reaction. The plates are incubated at room temperature for 30 min. Still in the dark, 10Product Name:AMG319Cat. No.:HY-12948CAS No.:1608125-21-8Molecular Formula:C 21H 16FN 7Molecular Weight:385.40Target:PI3K Pathway:PI3K/Akt/mTOR Solubility:10 mM in DMSOμL/well of the acceptor bead solution is added to columns 1–24 of the plates. The plates are then incubated in the dark for 1.5 h. The plates are read on an Envision multimode plate reader using a 680 nm excitation filter and a 520–620 nm emission filter.Cell assay [1]B cells were purified from human peripheral blood mononuclear cells (PBMCs) by negative selection. Approximately 3 × 104 purified B cells per well were seeded into a 96– well plate. AMG319 was dissolved in DMSO at a concentration of 10 mM, and a 10–point, 3–fold serial dilution of AMG319 was carried out in DMSO. Then 0.5 μL of AMG319 was added to each well in duplicates so that the final DMSO concentration was 0.25% and the highest AMG319 concentration was 10 μM. After preincubating for 30 min, B cells were treated with 2 μg/mL of anti–human IgM antibody plus 300 ng/mL human CD40L or 5 ng/mL human IL–4 plus 200 ng/mL of CD40L as a counterscreen to evaluate the off–target effects. The plates were incubated at 37°C and 5% CO2 for 72 h, then pulsed with 0.5 μCi per well 3H thymidine for 18 h, and B cells were collected to count the incorporation of 3H thymidine.Animal administration [1]Female Lewis rats (N = 8/dose group) were dosed po with AMG319 or vehicle (2% HPMC, 1% Pluronic F68, 10% Captisol, pH 2.0) once a day for 10 days at various doses. Two hours after the first dosing, 200 μL of PBS containing 60 μg of KLH was administered to each rat intravenously. Ten days after the KLH priming, rats were euthanized and blood was taken by cardiac punReferences:[1]. Cushing TD, et al. Discovery and in vivo evaluation of (S)–N–(1–(7–fluoro–2–(pyridin–2–yl)quinolin–3–yl)ethyl)–9H–purin–6–amine (AMG319) and related PI3Kδ inhibitors for inflammation and autoimmune disease. J Med Chem. 2015 Jan 8;58(1):480–511.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Expression, Purification and Crystallization of

Expression, Purification and Crystallization of the Mycobacterium Tuberculosis HSP16.3 Molecular Chaperone Background of Mycobacterium Tuberculosis HSP16.3HSP16.3, a 16.3 kDa protein from Mycobacterium Tuberculosis, was originally identified as a prominent antigen (Kingston et al., 1987). During the stationary phase, HSP16.3 is maximally expressed and becomes a main protein of the latent phase (Yuan et al., 1996). Previous studies showed that HSP16.3 can make the cell structure stable and prevent stationary Mycobacterium Tuberculosis from autolysing (Cunningham et al., 1998). In previous studies, HSP16.3 was found as one of theα-crystallin-related small heat shock proteins (sHSP) with molecular chaperone activity. Experiments in vitro revealed that HSP16.3 can suppress the thermal aggregation of citrate synthase at 39.5˚C, without consumption of A TP (Chang et al., 1996).Now the Mycobacterium Tuberculosis HSP16.3 gene was cloned to the plasmid pSTE-HSP16.3, and transformed to E.Coli. BL21(DE3) strain.Material and MethodExpressionThings to have ready before Starting.-Plate or glycerol culture-Sterile LB 25ml in a 50mL shaker flasker, 250ml in a 500mL shaker flasker, all together autoclaved, antibiotic added afterword.- antibiotic and sterile water- TipsPrepare the LB and autoclave:Fomula of the LB medium for 1 Liter:Bacto Tryptone (BT) 10 gBacto Y east Extract (BYE) 10 gNaCl 10gThe LB medium, dd H2O and the tips all together autoclaved at 121 ˚C for 20 minutes.Method:1 Innoculate 25 ml LB Medium ( containing 100 ug) and grow culture overnight(37˚C, 200rpm).2 Next morning inoculate 250 ml prewarmed LB Medium ( containing 100 ug) with the 25 ml overnight culture and grow at 37 ˚C, 200rpm, HSP16.3 was overexpressed in soluble form intracellularly without IPTG induction.3 Incubate the Culture for 10 hours before havesting the cell at 4000 g for 20 minutes.4 Resuspend the cell pellet in 30 ml Butter A and freeze the Sample in -80˚C refigerator.PurificationDE52 Ion-Exchange columnThings to have ready before Starting.-Butter A: 50 mM Imidazole pH 6.5 (1 liter)-Butter B: 50 mM Imidazole pH 6.5 , 300mM NaClall together Fitrate with 0.2 um membrane.- DE52 medium , column ,Gradient maker, UV-monitor and Fractioner- TipsMethod:1 Thaw the cell pellet and vortex .2 Add 0.4ml 100 mM PMSF and sonicate (400kw, 4s-6s 50 cycle* 5 )3 Centrifuge 15000 rpm, 30 minutes to pellet debris4 Transfer supernatant to a 50 ml conicale tube and discard the pellet.5 The supernatant dilute to 50 ml with Buffer A and then load to DE52 ion-exchange columns (20ml), which was pre-equibrated with 100ml Buffer A. And then wash the unbound proteins with 100 ml Buffer A.6 Elute the protein with a linear gradient : 200ml buffer A plus 200ml buffer B, 2ml/min, 6ml each fraction.7 Run 15% SDS-PAGE to determine the HSP16.3 peak.Desalting by dialysis1 Preparation of the dialysis tubeCut the tube in a suitable length (20-30 cm)Boil the tube in solution containing 10 mM NaHCO3 for a few minutes.Boil the tube in solution containing 10 mM EDTA for a few minutes.Rasin the tube with de-ion water2 Pool the HSP16.3 peak and dialysis the Sample against 1000ml Buffer A for more than 6hours.Q-Separose (HP) Ion-Exchange Column1 load the sample to Q-Separose (HP) Ion-Exchange column (20ml), which was pre-equibrated with 100ml Buffer A. And then wash the unbound proteins with 100 ml Buffer A.2 Elute the protein with a linear gradient : 200ml buffer A plus 200ml buffer B, 2ml/min, 6ml each fraction.3 Run 15% SDS-PAGE to determine the purity of the HSP16.3 peak.Gel filtration ColumnThe HSP peak was a final volumn 0.3ml and then run though a Superdex75 (HR, 10/30mm) gel filtration column in 150mM NaCl and 5mM Imdazole, pH6.5. Crystallization1 The purified HSP16.3 was solvent-exchanged to water and concentrated to 20mg/ml before crystallization trails (Bradford). All the crystallization trials were carried out using the hanging-drop vapor-diffusion method at 291K: drops consisted of2 microlitres of HSP16.3 protein solution plus 2 microlitres of the precipitant. The drops were equilibrated against 0.2 ml precipitant at room temperature. The crystallization conditions were investigated with a PEG4000 Kit.Result and discussionThe purity of the final HSP16.3 was over 95% by SDS-PAGE. The crystallization trials of HSP16.3 yielded Cubic crystals with a size of 0.8*0.8*0.6mm in a few days.20040060080010001200mAUBuffer Tris-HCL pH 8.5 Precipitant PEG 4000 MethodV apor Diffusion Temperature 293 K Size0.8*0.8*0.6mmReferencesChang Z., Primm, T.P., Jakana J., Lee H. I., Serysheva I., Chiu W., Gilber H. F., Quiocho F. A., (1996) J Biol Chem 271:7218-7223Cunningham A. F., Spreadbury C. L., (1998) J. Bacteriol. 184:801-808Kingston A. E., Salgame P. R., Mitchison N.A., Colston M. J. (1987) Infect. Immun 55,3149-3154Yuan Y., Crane D. D., Barry C. E. III (1996) J Bacteriol178: 4484-4492。
乙酰胆碱酯酶抑制剂

上海应用技术学院研究生课程《高等天然产物化学》试卷2014 / 2015 学年第1 学期课程代码:NX0702013论文题目:乙酰胆碱酯酶抑制剂的研究进展姓名:芮银146061414康满满146061409专业:制药工程学院:化工学院乙酰胆碱酯酶抑制剂的研究进展芮银,陈祎桐,康满满摘要:本文阐述了乙酰胆碱酯酶抑制剂(AChEI)的研究进展,介绍了用于药物治疗的乙酰胆碱酯酶抑制剂的各种来源如植物、微生物等,及其抑制乙酰胆碱的活性物质。
在此基础上,总结了几种现代分析技术,对AChEIs进行筛选,大大加快AD药物资源的开发利用进程。
这些方法主要有基于比色法的Ellman's法及相关的改进方法、薄层显色法、荧光显色法、电喷雾质谱法等。
但是,到目前为止,现代分析技术在AD药物资源中的应用还处在起步阶段。
关键词:乙酰胆碱酯酶抑制剂,筛选方法,薄层显色法,荧光显色法The progress of acetylcholinesteraseinhibitorsRui Yin, Chen Yitong, Kang ManmanAbstract:In this artical, the research elaborates progress of acetylcholinesterase inhibitors (AChEI), and introduces a variety of sources for drug treatment acetylcholinesterase inhibitors such as plants, microorganisms, and its active ingredients. On this basis, the review summarizes several modern analytic techniques such as Ellman's method which based on the colorimetric method, TLC chromogenic method, fluorescent color method, Electrospray ionization mass spectrometry and so on. However, at present, the application of modern analytic techniques in AD drug resources is still in infancy.Key word: Acetylcholinesterase inhibitors, Screening Methods, TLC chromogenic method, Fluorescent color method目录摘要.................................................................................................错误!未定义书签。
致病疫霉菌效应蛋白Pi05440毒性功能验证及寄主候选靶标筛选

核农学报2024,38(3):0434~0442Journal of Nuclear Agricultural Sciences致病疫霉菌效应蛋白Pi05440毒性功能验证及寄主候选靶标筛选张蝶陈胜男赵迪王荟洁王洪洋 *刘晶 *(云南师范大学,云南省马铃薯生物学重点实验室,云南昆明650500)摘要:致病疫霉菌(Phytophthora infestans)侵染时会分泌大量效应蛋白进入寄主细胞,通过操纵寄主靶标抑制植物免疫反应。
为研究致病疫霉菌效应蛋白Pi05440的功能,本研究重点分析了Pi05440的蛋白特征、毒性功能及鉴定其寄主靶标;利用生物信息学数据库,预测Pi05440的保守结构域和信号肽。
构建pRI101-GFP-Pi05440表达载体用于Pi05440的亚细胞定位和毒性功能分析;同时,利用酵母双杂交技术对Pi05440的寄主靶标蛋白进行了筛选和鉴定。
结果表明,Pi05440基因全长969 bp,编码322个氨基酸。
结构预测结果显示Pi05440含有3个典型的KAZAL功能域。
亚细胞定位结果表明Pi05440定位在质膜和细胞间隙。
在本氏烟中瞬时表达该基因显著促进致病疫霉菌扩展。
通过酵母双杂交筛选,初步鉴定到3个Pi05440的候选靶标蛋白,分别为马铃薯过氧化氢酶12、马铃薯几丁质酶以及马铃薯MYB-like A蛋白。
本研究为探究致病疫霉菌效应蛋白Pi05440及其靶标蛋白如何调控植物免疫提供了重要线索。
关键词:致病疫霉菌;效应蛋白;亚细胞定位;酵母双杂交;靶标蛋白DOI:10.11869/j.issn.1000‑8551.2024.03.0434马铃薯(Solanum tuberosum)富含碳水化合物、维生素、矿物质和抗氧化物质等营养成分,对人类健康和可持续农业发展至关重要[1]。
然而,马铃薯生产过程中会面临许多病害的威胁,其中最为严重的是由致病疫霉菌(Phytophthora infestans)引起的晚疫病。
211232354_华蟾酥毒基通过调控circNFIX

华蟾酥毒基通过调控circNFIX/miR -577通路抑制乳腺癌MDA -MB -231 细胞的增殖、迁移和侵袭何孙香 吴中伟① 刘梅芬 (九江市中医医院肿瘤科,九江 339000)中图分类号 R282.74 R737.9 文献标志码 A 文章编号 1000-484X (2023)04-0770-07[摘要] 目的:研究华蟾酥毒基(CnBu )对乳腺癌细胞MDA -MB -231恶性生物学行为的影响,并探讨其机制是否与调控环状RNA 核因子IX (circNFIX )和miR -577表达相关。
方法:将MDA -MB -231细胞分为对照(Con )组、CnBu -低剂量(L )组、 CnBu -中剂量(M )组、CnBu -高剂量(H )组、si -NC 组、si -circNFIX 组、pcDNA 组、pcDNA -circNFIX 组、CnBu+pcDNA 组、CnBu+pcDNA -circNFIX 组。
MTT 、Transwell 实验分析细胞增殖、迁移和侵袭能力,Western blot 分析Ki -67、基质金属蛋白酶2(MMP -2)和MMP -9蛋白表达。
RT -qPCR 分析circNFIX 、miR -577表达量。
双荧光素酶报告实验和RT -qPCR 验证circNFIX 和miR -577的靶向调控关系。
结果:CnBu 以剂量依赖方式下调Ki -67、MMP -2、MMP -9蛋白和circNFIX 表达(P <0.05),上调miR -577表达(P <0.05),减弱MDA -MB -231细胞的增殖、迁移和侵袭能力(P <0.05)。
miR -577与circNFIX 序列直接结合,干扰或过表达circNFIX 可显著提高或降低miR -577表达水平(P <0.05)。
干扰circNFIX 表达显著下调Ki -67、MMP -2、MMP -9蛋白表达(P <0.05),减弱MDA -MB -231细胞的增殖、迁移和侵袭能力(P <0.05)。
百草枯所致小鼠肺纤维化时肺组织酸性鞘磷脂酶表达上调

百草枯所致小鼠肺纤维化时肺组织酸性鞘磷脂酶表达上调李燕飞;王会;胡长平【期刊名称】《中国临床药理学与治疗学》【年(卷),期】2016(21)7【摘要】目的:观察百草枯所致C57BL/6小鼠肺纤维化时肺组织酸性鞘磷脂酶(acid sphingomyelinase,ASM)的表达变化,初步探讨ASM在百草枯所致肺纤维化发生发展中的潜在病理意义。
方法:将20只C57BL/6雄性小鼠随机分为对照组和百草枯组,百草枯组动物腹腔注射20 mg/kg百草枯,对照组动物注射等体积生理盐水,单次注射百草枯21 d后麻醉动物进行支气管肺泡灌洗,取支气管肺泡灌洗液(bronchial alveolar lavage fluid,BALF)检测灌洗液中细胞总数、总蛋白浓度,ELISA法检测BALF中IL-6和TNF-α水平;取肺组织,HE染色和Masson染色观察组织形态学变化和胶原沉积,TUNEL染色和Caspase-3活性检测细胞凋亡情况,DHE染色检测活性氧(reactive oxtgen species,ROS)水平,Real time PCR、免疫组织化学、Western Blot检测肺组织中ASM mRNA和蛋白表达。
结果:用百草枯处理后,C57BL/6小鼠肺组织肺泡排列紊乱、部分肺泡出现塌陷、肺泡间隔增厚、炎性细胞浸润,肺组织中出现大量胶原沉积。
与对照组相比,百草枯组小鼠BALF中细胞总数增多(P<0.01),总蛋白浓度增加(P<0.01),IL-6和TNF-α水平升高(P<0.05);肺组织中TUNEL染色阳性细胞数增多(P<0.01),Caspase-3活性增加(P<0.05),ROS水平升高,ASM mRNA和蛋白表达上调(P<0.05)。
结论:ASM可能参与百草枯所致肺纤维化的病理生理过程,其具体作用机制有待进一步研究证实。
【总页数】6页(P765-770)【关键词】酸性鞘磷脂酶;百草枯;肺纤维化【作者】李燕飞;王会;胡长平【作者单位】中南大学药学院药理学系【正文语种】中文【中图分类】R563【相关文献】1.卡托普利及氯沙坦对百草枯中毒大鼠肺纤维化和肺组织PlGF表达的影响 [J], 郑敏辉;戴木森;林立芳2.百草枯中毒致大鼠肺组织损伤时氨溴索对肺组织Bcl-2/Bax的表达及细胞凋亡的影响 [J], 马玉腾;刘建辉;田英平;石汉文;郭宪立;高恒波;苏建玲;霍淑花;胡军利;姚冬奇;王荣英;陈慧3.MMP7蛋白在百草枯致肺纤维化大鼠肺组织中的表达及其意义 [J], 杜妍;张罡;肖莉4.百草枯中毒大鼠肺组织中KL-6黏糖蛋白和羟脯胺酸的表达及肺纤维化的变化[J], 张引;刘阳;何静春;刘亮亮;刘斌;赵希洋5.百草枯所致肺损伤大鼠肺组织凋亡情况及凋亡相关基因表达情况 [J], 武梓萌;罗毅沣;张剑锋因版权原因,仅展示原文概要,查看原文内容请购买。
表阿霉素-羧甲基葡聚糖磁性纳米颗粒的制备及其协同恒定外磁场体内外对人膀胱癌细胞增殖和凋亡的影响研究

表阿霉素-羧甲基葡聚糖磁性纳米颗粒的制备及其协同恒定外磁场体内外对人膀胱癌细胞增殖和凋亡的影响研究何灵生;邓少珍;刘百川;王健富【期刊名称】《黑龙江医学》【年(卷),期】2016(040)003【摘要】目的研究表阿霉素-羧甲基葡聚糖磁性纳米颗粒(EPI-CDMN)的制备方法,并分析外磁场协同EPI-CDMN对人体膀胱癌细胞(BIU-87)株的体内外增殖、凋亡的影响作用.方法本研究采用氧化还原法制备EPI-CDMN并检测其化学性质,分成对照组、磁场组、EPI-CDMN组、磁场+EPI-CDMN组,观察各组细胞凋亡等指标的差异.结果磁场组、EPI-CDMN组、磁场+EPI-CDMN组的BIU-87细胞生长抑制率、死亡率、凋亡率均显著高于对照组(P<0.05);磁场+EPI-CDMN组的BIU-87细胞生长抑制率、死亡率、凋亡率均显著高于磁场组、EPI-CDMN组(P<0.05);磁场组、EPI-CDMN组、磁场+EPI-CDMN组裸鼠移植瘤的瘤体平均重量、瘤体平均体积均显著低于对照组(P<0.05),磁场组、EPI-CDMN组、磁场+EPI-CDMN组裸鼠移植瘤的瘤体BIU-87细胞凋亡指数显著高于对照组(P<0.05);磁场+EPI-CDMN组裸鼠移植瘤的瘤体平均重量、瘤体平均体积均显著低于磁场组、EPI-CDMN组(P<0.05),磁场+EPI-CDMN组裸鼠移植瘤的瘤体BIU-87细胞凋亡指数显著高于磁场组、EPI-CDMN组(P<0.05).结论外磁场协同EPI-CDMN对人体BIU-87细胞的杀伤作用显著的增强,为膀胱癌的磁导向靶向治疗提供了实验基础.【总页数】4页(P200-203)【作者】何灵生;邓少珍;刘百川;王健富【作者单位】东莞市凤岗医院,广东东莞523690;东莞市凤岗医院,广东东莞523690;广东省第二人民医院,广东广州510317;广东省第二人民医院,广东广州510317【正文语种】中文【中图分类】R737.14【相关文献】1.恒定磁场协同阿霉素诱导肝癌细胞凋亡的研究 [J], 夏克勤2.表阿霉素-羧甲基葡聚糖磁性纳米颗粒协同外磁场对人膀胱癌BIU-87细胞增殖和凋亡的影响 [J], 曹正国;周四维;刘继红;孙凯;叶章群3.外磁场协同卟啉-葡聚糖磁性纳米微粒的光动力学对人膀胱癌细胞的杀伤作用 [J], 罗道升;米其武;孟祥军;高勇;戴宇平;邓春华4.去甲氧基姜黄素对人膀胱癌T24细胞增殖及凋亡的影响研究 [J], 倪晓辰;陈砚凝;武新慧;张爱莉;赵志红5.miR-186介导的PTTG1下调对膀胱癌细胞增殖、凋亡、侵袭和迁移的影响研究[J], 余洋; 刘斌波; 熊飞因版权原因,仅展示原文概要,查看原文内容请购买。
