CCX140_DataSheet_MedChemExpress
CXCR4和MMP-13在非小细胞肺癌中的表达

胞 中分离 出来 的 , MMP 激 活 过 程 中起 关键 作用 ,I激 活 在 s I 丁 其 他 亚 型 的 MMP ,在肿 瘤 侵 袭 和 转 移 中具 有 重 要 作 j s l 千
C C 4是 趋 化 因 子 C C 亚 家 族 中 的 一 员 . S F X R X 是 D 一1的
CHI A MODE OCT N RN D OR 中 国现 代 医 生 4 7
收集 我 院 2 0 0 8年 1月 ~ 0 0年 1 非小 细 胞肺 癌 手 术 21 0月
后 的蜡 块 组 织 。纳 入 患者 均 符合 WH O关 于肺 肿 瘤 的诊 断标
准 。排除标准: ①术前行放 、 化疗 的患者 ; ②临床资料不完全
S 一1和 C R DF XC 4构象 的变化 ,进 而启 动 G蛋 白耦联 的信 传 导过程 1 。 8 , 本实 验应 用 免疫 组织 化 学 技术 , 检测 非 小细 胞肺 癌及 常肺 组 织 中 C R XC 4和 MMP 1 一 3的表达 ,结 果显 示 非 小细 胞
肺 癌 中 C C 4及 MMP 1 阳 性 表 达 部 位 均 在 细 胞 质 中 . X R 一3
【 中图分 类 号】R 3 . 7 42
[ 献标 识 码】B 文
[ 章 编 号】1 7 — 7 12 1 )3 0 4 — 2 文 6 3 9 0 (0 2 0 — 0 7 0
Ex r si n n t i n fc n e o p e so s a d i sg i a c f CXCR4 a d M M P- 3 i o s a lc l s i n 1 n n n- m l el
浸 润 和转 移 是非 小 细 胞 肺癌 预 后不 良的 重要 原 因[ 近 1 1 。 来 有 研 究 显 示非 小 细 胞 肺 癌 的 发 生 发 展 涉 及 多种 基 因 和 蛋
CCX140_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jun.-08-2017Print Date:Jun.-08-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :CCX140Catalog No. :HY-101713CAS No. :1100318-47-51.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:CCX–140; CCX 140Formula:C20H13ClF3N5O3SMolecular Weight:495.86CAS No. :1100318-47-54. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Light yellow to yellow (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
多西他赛美国USP40
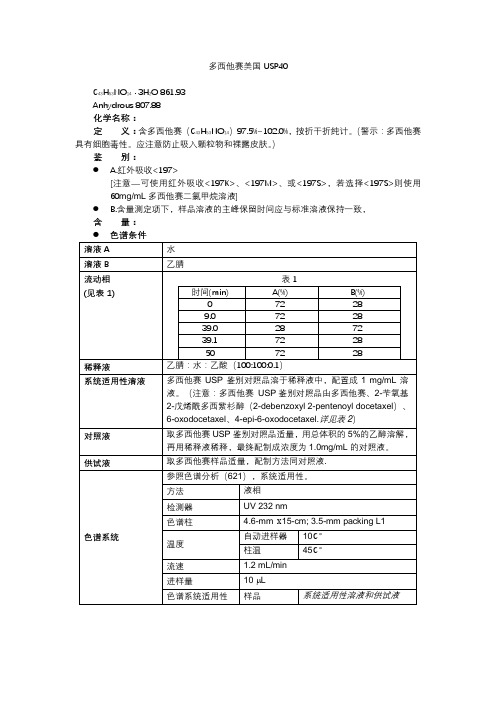
多西他赛美国USP40C43H53NO14· 3H2O 861.93Anhydrous 807.88化学名称:定义:含多西他赛(C43H53NO14)97.5%~102.0%,按折干折纯计。
(警示:多西他赛具有细胞毒性。
应注意防止吸入颗粒物和裸露皮肤。
)鉴别:● A.红外吸收<197>[注意—可使用红外吸收<197K>、<197M>、或<197S>,若选择<197S>则使用60mg/mL多西他赛二氯甲烷溶液]● B.含量测定项下,样品溶液的主峰保留时间应与标准溶液保持一致,含量:杂质:●炽灼残渣(281):不多于0.1%●有关物质,色谱条件1(注意:根据合成路线提供两种色谱条件。
如果特殊杂质为10-deacetyl baccatin and 2-debenzoxyl docetaxel则推荐色谱条件1.如果特殊杂质为O-BOC N-pyruvyl docetaxel则推荐色谱条件2。
)有关物质,色谱条件2特性试验:●微生物限度检测<61>及特殊微生物检测<62>:总需氧微生物限度不超过102cfu/g。
总的霉菌和酵母计数不超过10 cfu/g。
●细菌内毒素<85>:每mg含USP内毒素不超过0.4单位●水分,方法<921>:5.0%-7.0%。
如果是无水物形式则不超过1.5%●比旋度,旋光率<781S>样品溶液:10mg/mL的甲醇溶液可接受标准:-39° to -41° (t = 20°),按折干折纯计。
如果是无水物形式:-35° to -45° (t = 20°),按主要成分原型计其他:●标签:标签应注明,是否无水物形式。
如果有关物质测试用的不是色谱条件1,标签上应该说明该物质的测试方法。
(Where it is an anhydrous form, the label so indicates. If a test for Organic Impurities other than Procedure 1 is used,the labeling states the test with which the article complies)包装和储存:密封、避光、室温下保存。
全球压敏胶带需求增加

全球压敏胶带需求增加
范景阳
【期刊名称】《《造纸信息》》
【年(卷),期】2007(000)011
【摘要】据Freedonia集团的一项新研究显示,预期到2010年,全球压敏胶带(PSA)市场将以年均4.7%的速度增加,最终超过300亿m^2。
【总页数】1页(P51)
【作者】范景阳
【作者单位】
【正文语种】中文
【中图分类】F731
【相关文献】
1.未来全球煤炭需求预计增加中国仍是贸易\"变数\" [J],
2.2019/2020年度预计全球棉花库存增加而需求不确定 [J], 李鹏程
3.中国化工部门最终需求对全球经济体增加值的拉动效应 [J], 齐颖
4.“双草”价格高位等多因素助推百草枯全球需求增加 [J],
5.随着可持续包装需求的增加,全球未漂白硫酸盐针叶木浆市场不断增长,潜力巨大[J],
因版权原因,仅展示原文概要,查看原文内容请购买。
GeXP简介

•Alignment
•Call scores
•Heterozygote Detection
2013/11/12
6
GeXP荧光系统
•GeXP更适合检测突变/杂合子: •波长越长,干扰越少 ,背景噪音低;
•650nm •laser •750nm •laser
•无10%的cut off把噪音,不会把10%以上杂合子去掉;
•NO Interference •from biological materials
7
个体化用药检测
KIT-Exon9
PDGFRA-exon12
EGFR突变检测
肿瘤药物对应相关基因的检测
药物名称 易瑞沙/特罗凯类 检测基因
EGFR-Exon18 突变 EGFR-Exon19 突变 EGFR-Exon21 突变 EGFR-Exon20突变 C-KIT-Exon9 突变 C-KIT-Exon11 突变 C-KIT-Exon13 突变 C-KIT-Exon17 突变 PDGFRα-Exon12 PDGFRα-Exon18 CYP2D6*10 多态性 XRCC1-Exon6 多态性 XRCC1-Exon10 多态性 ERCC1-codon118 多态性 MRP2-Exon10 多态性 BRCA1-Exon2 (女)多态性 BRCA1-Exon20 (女)多态性 XPD基因多态性 UGT1A1 *6 多态性 UGT1A1*28 多态性 DPYD*2A 多态性
伊马替尼 他莫昔芬
铂类
伊立替康 氟脲嘧啶类
HBV分型、耐药突变检测
2、片段分析
• 只需要研究长度,不需要知道具体序列 • 分别率为1bp
片段分析应用
STR/SSR
融合基因,可变剪切体
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
北京春达科技有限公司专营进口体外诊断试剂工业原料,透析产品,纯化填料,标准品

北京春达科技有限公司专营进⼝体外诊断试剂⼯业原料,透析产品,纯化填料,标准品SCLPP209碱性磷酸酶3.1.3.1Alkaline phosphatase from Calf intestine-Activity: >30000 U/mlGlycerol solution-Mw: 100,000-Store at -20 °CSCMAD211苹果酸脱氢酶1.1.1.37Malate dehydrogenase from Microorganism-Activity: >40 U/mgYellowish amorphous powder-Mw: 140,000-Store at -20 °CSCMDH18C苹果酸脱氢酶1.1.1.37Malate dehydrogenase from Pig heart-Activity: >1250 U/mgprot.White amorphous powder-Mw:140,000-Store at -20 °CSCMDL100苹果酸脱氢酶1.1.1.37Malate dehydrogenase from Microorganism-Activity: >60 U/mgWhite amorphous powder-Mw: 80,000-Store at -20 °CSCMUT11C变旋酶5.1.3.3Mutarotase from Porcine kidney-Activity: >1500 U/mgWhite amorphous powder-Mw: 40,000-Store at -20 °CSCMUT12C变旋酶5.1.3.3Mutarotase from Porcine kidney-Activity: >1000 U/mlAmmonium sulfate suspension-Mw: 40,000-Store at 2-8 °CSCNAL301唾液酸醛缩酶4.1.3.3N-Acetylneuraminic acid aldolase from MicroorganismActivity: >15 U/mg-Yellowish amorphous powderMw: 98,000-Store at -20 °CSCPCO301原⼉茶酸3,4双加氧酶1.13.11.3Protocatechuate 3,4-dioxygenase from Pseudomonas sp.Activity: >3 U/mg-Light brown amorphous powderMw: 700,000-Store at -20 °CSCPEO131过氧化物酶1.11.1.7Peroxidase from Horseradish, Grade IActivity: >250 U/mg-Reddish-brown amorphous powderMw: 40,000-Store at -20 °CSCPEO301过氧化物酶1.11.1.7Peroxidase from Horseradish, Grade I-Activity: >250 U/mgReddish-brown amorphous powder-Mw: 40,000-Store at -20°CSCPEO302过氧化物酶1.11.1.7Peroxidase from Horseradish, Grade III-Activity: >110 U/mgReddish-brown amorphous powder-Mw: 40,000-Store at -20°CSCPHO12C磷脂酶D Phospholipase D from Streptomyces chromofuscus3.1.4.4Activity: >40 U/mg-Brown amorphous powderMw: 57,000-Store at -20 °CSCPNP301嘌呤核苷磷酸化酶2.4.2.1Purine-nucleoside phosphorylase from MicroorganismActivity: >15 U/mg-White amorphous powderMw: 120,000-Store at -20 °CSCPPC301磷酸烯醇式丙酮酸羧化酶4.1.1.31Phosphoenolpyruvate carboxylase from Corn leavesActivity: >5 U/mg-White amorphous powderMw: 390,000-Store at -20 °CSCPSP101脯氨酸特定的肽链内切酶3.4.21.26Proline specific endopeptidase from Flavobacterium sp.Activity: >5 U/mg-White amorphous powder-Mw: 78,000-Store at -20 °CSCPYK302L丙酮酸激酶2.7.1.40Pyruvate kinase from Rabbit muscle-Activity:>2000 U/mlwhite ammonium sulphate suspension-Mw:237000-Store at 2-8℃SCPYO311丙酮酸氧化酶1.2.3.3Pyruvate oxidase from Microorganism-Activity: >1.5 U/mgYellowish amorphous powder-Mw: 260,000-Store at -20 °CSCSAO341肌氨酸氧化酶1.5.3.1Sarcosine oxidase from Microorganism-Activity: >8 U/mgYellowish amorphous powder-Mw: 65,000-Store at -20 °CSCSAO351肌氨酸氧化酶1.5.3.1Sarcosine oxidase from Microorganism-Activity: >8 U/mgYellowish amorphous powder-Mw: 43,000-Store at -20 °CSCSOD302超氧化物歧化酶1.15.1.1Superoxide dismutase from Bovine erythrocyte-Activity: >3000U/mgBluish-green amorphous powder-Mw: 32,000-Store at -20 °CSCUAO201尿酸酶1.7.3.3Uricase from Candida sp.-Activity: >4 U/mg-White amorphous powder-Mw: 120,000-Store at -20 °CSCUAO211尿酸酶1.7.3.3Uricase from Bacillus sp.-Activity:>1.5 U/mg-White amorphous powder-Mw: 150,000-Store at -20 °CSCURH10S脲酶3.5.1.5Urease from Jack bean-Activity: >220 U/mg-White amorphous powder-Mw: 480,000-Store at -20 °CSCURH16C脲素酶GUrease G from Jack bean-Activity: >150 U/mgWhite amorphous powder-Mw: 480,000-Store at -20 °C3.5.1.5SCURH201脲素酶Urease from Jack bean-Activity: >100 U/mgWhite amorphous powder-Mw: 480,000-Store at -20 °C3.5.1.5SCXTO212黄嘌呤氧化酶Xanthine oxidase from Microorganism-Activity: >10 U/mgReddish-brown amorphous powder-Mw: 160,000-Store at -20°C1.1.3.222.诊断与研究⽤的辅酶CODES PRODUCTS CAS #SCADP01C 腺苷5'-⼆磷酸单钾盐⼆⽔合物ADP-K.2H2O-Adenosine 5’-diphosphate monopotasium saltdihydrate-C10H14N5O10P2K.2H2O-Mw:501.30-Colorless crystals-Purity:>95 %72696-48-1SCADP22C 腺苷5’-⼆磷酸⼆钠盐ADP-Na2-Adenosine 5’-diphosphate disodium salt-C10H13N5O10P2Na2-Mw:471.20-White powder-Purity:>98 %16178-48-6SCAMP02C 腺苷-5’-磷酸AMP-Adenosine 5’-monophosphoric acid-C10H14N5O7P.H2O-Mw:365.20-White powder-Purity:>98 %18422-05-4SCAMP05C 腺苷5’-磷酸AMP-Na2-Adenosine 5’-monophosphate disodium salt -C10H12N5O7PNa2-Mw:391.18-White powder-Purity:>95%18422-05-4SCATP03C 腺苷5’-三磷酸⼆钠盐3⽔合物ATP-Na2.3H2O-Adenosine 5’-triphosphate disodium salttrihydrate -C10H14N5Na2O13P3.3H2O -Mw:605.19-White powder-Purity:>96 %987-65-5SCBNA207C β-烟酰胺-腺嘌呤⼆核苷酸NAD-β-Nicotinamide-adenine dinucleotide-C21H27N7O14P2-Mw:663.4-White powder-Purity:>98 %53-84-9SCBND210C β-烟酰胺腺嘌呤⼆核苷磷酸,还原型四钠盐NADPH-Na4-β-Nicotinamide-adenine dinucleotide phosphate, reducedtetrasodium salt-C21H26N7O17P3.Na4-Mw:833.40-White powder-Purity:>93 %2646-71-1SCBNH208C β-烟酰胺腺嘌呤⼆核苷酸,还原⼆钠盐NADH-Na2-β-Nicotinamide-adenine dinucleotide, reduceddisodium salt -C21H27N7P2O14Na2-Mw:709.40-White to yellowish powder-Purity:>93 %606-68-8β-烟酰胺腺嘌呤⼆核苷酸磷酸⼆钠盐24292-60-2SCBNP209C β-烟酰胺腺嘌呤⼆核苷酸磷酸⼆钠盐NADP-Na2-β-Nicotinamide-adenine dinucleotide phosphatedisodium salt-C21H26N7O17P3Na2-Mw:787.40-Yellowishpowder-Purity:>97 %24292-60-2SCCOA11C 辅酶A三锂盐Coenzyme A trilithium salt-C21H33Li3N16O7P3S-Mw:785.40-White powder-Purity:>85 %18439-24-2SCDAD631C ⼆(腺苷-5’-)五磷酸三锂盐Ap5A-Li3-P1,P5 –Di(adenosine -5’-)pentaphosphate trilithiumsalt-C20H26N10O22P5Li3-Mw:934.20-Yellowish powder-Purity:>95 %75522-97-3SCFAD11C 黄素腺嘌呤⼆核苷酸⼆钠盐FAD- Na2-Flavine-adenine dinucleotide disodium salt-C27H31N9O15P2Na2-Mw:829.60-Orange powder-Purity:>93 %146-14-5SCNAL24C N-⼄酰-L-半胱氨酸N-Acetyl-L-cysteine-C5H9NO3S-Mw:163.20-White powder-Purity:>98 %616-91-1SASNAD 硫代辅酶IThio-NAD-C21H27N7O13SP2-Purity:≥ 90%4090-29-3SASNADP 硫代辅酶IIThio-NADP-C21H27N7O16P3S•Na- Purity:≥ 90%19254-05-8SAAldNAD 3-吡啶⼄醛腺嘌呤⼆核苷酸3-Pyridinealdehyde adenine dinucleotide C21H27N6O14P21986-7-7SAAC1023-⼄酰基吡啶腺嘌呤⼆核苷酸磷酸钠(Ac-NADP) APADP-C22H28N6O17P3•Na -Purity:≥ 80%102029-67-4SANAAD 烟酸腺嘌呤⼆核苷酸Nicotinic Acid Adenine Dinucleotide- C21H26N6O15P2104809-30-5SADeNAD 脱氨基NADDeamino Nicotinamide Adenine Dinucleotide-C21H26N6O15P2104809-38-3SAGGNAFB γ-L-⾕氨酰基-4-硝基苯胺Gamma-L-glutamyl-4-nitroanilide,-C11H13N3O5-Purity:≥ 96%7300-59-6SACGGN L-γ-⾕氨酸-(3-甲酸-4硝基苯胺)铵盐L-Glutamic Acid Gamma- (3-Carboxy-4-Nitroanilide),Ammonium Salt-C12H12N3O7P•NH4-Purity:≥ 96%63699-78-5SAPEPCHA 磷酸烯醇丙酮酸单环⼰胺盐PEP-C3H4O6P•C6H13N-Purity :>95%10526-80-4SAPEPK 磷酸烯醇式丙酮酸单钾盐PEP-C3H4O6P•K-Purity :>95%4265-07-03.诊断与研究⽤的底物CODES PRODUCTS CAS #SCAKE05C α–酮戊⼆酸⼆钠盐⼆⽔合物α–Ketoglutaric acid, disodium salt dihydrate-C5H4O5Na2.2H2O-Mw:226.10 -White powder-Purity:>98%305-72-6α–酮戊⼆游离酸328-50-7SCAKE115C α–酮戊⼆游离酸α–Ketoglutaric free acid-C5H4O5-Mw:146.10 -White powder-Purity:>99%328-50-7SCBTC06S-丁酰硫胆碱碘S-Butyrylthiocholine Iodide-C9H20NOSI-Mw:317.23 -White powder-Purity:>97%1866-16-6SCCNP005Gal-G2-α-CNP-C28H51O18Cl-Mw:659.98 -White powder-Purity:>90 %157381-11-8SCCRP59C 磷酸肌酸⼆钠盐四⽔合物Phosphocreatine disodium salt tetrahydrate-C4H8N3O5PNa2.4H2O-Mw:327.15 -White powder-Purity:>97 %19333-65-4SCGGC106C ⽢氨酰⽢氨酸Glycylglycine-C4H8N2O3-Mw:132.12-White powder-Purity:>98 %556-50-3SCGLT100L-⾕氨酸L-Glutamic acid-C5H9N2O4- Mw:147.13-White powder-purity:>99%617-65-2SCGPS15C 葡萄糖-6-磷酸⼆钠盐Glucose-6-phosphate disodium salt-C6H11O9PNa2-Mw:304.20-White powder-Purity:>95 %3671-99-6SCLAC171C DL-乳酸锂盐DL-Lactic acid Lithium salt-C3H5O3Li-Mw:96.01-White powder-Purity:>95%16891-53-5SCLAL29C L-丙氨酸游离酸L-Alanine free acid-C3H7NO2-Mw:89.09-White powder-Purity:>99%56-41-7SCLAP41C L-天门冬氨酸L-Aspartic acid-C4H7NO4-Mw:133.10-White powder-Purity:>99 %56-84-8SCLAP42C L-天门冬氨酸,钠盐⼀⽔合物L-Aspartic acid, sodium salt monohydrate-C4H6NO4Na.H2O-Mw:173.10-White powder-Purity:>98 %323194-76-9SCLAP43C L-天门冬氨酸,镁⼆⽔合物L-Aspartic acid, magnesium salt dihydrate-C8H12N2O8Mg.2H2O-MW:324.50-White powder-Purity:>98 %2068-80-6SCLGC244C L-γ-⾕氨酰-3-羧基-4-硝基苯胺Glupa C-L-γ-Glutamyl-3-carboxy-4-nitranilide-C12H12N3O7NH4-Mw:328.30-Yellow powder-Purity:>99 %63699-78-5SCNAY138C 萘磷酸单钠盐Naphtyl phosphate, monosodium salt-C10H8NaO4P.H2O-Mw:264.15-White powder-Purity:>98%81012-89-7SCPG701C 亚⼄基降-4-硝基苯基-β-D-麦芽庚糖苷pNP-G7-Ethylidene-4-nitrophenyl-D-maltoheptaoside-C50H77NO38-Mw:1300.10-Yellowish powder-Purity:>90%96597-16-9对硝基苯基磷酸酯,⼆钠盐六⽔合物4264-83-9SCPNP264C 对硝基苯基磷酸酯,⼆钠盐六⽔合物PNPP-p-Nitrophenylphosphate, disodium salt hexahydrate-C6H4NO6PNa2.6H2O-Mw :371.10-White to yellow powder-Purity:>98%4264-83-9SCPNS265C p-硝基苯基磷酸酯,⼆tris盐PNPP diTris-p-Nitrophenylphosphate, ditris salt-C14H28N3O12P-Mw:461.40-White powder68189-42-4SCPYN100丙酮酸钠Sodium pyruvate-C3H3NaO3-Mw:110-White powder-Purity:>99%113-24-64.缓冲液Buffers for diagnostic & researchPRODUCTS CAS # N-(2-⼄酰胺基)-2-氨基⼄磺酸ACES-N-(2-Acetamido)-2-aminoethanesulfonic acid7365-82-4N-(2-⼄酰胺基)亚氨基⼆⼄酸ADA-(N-(2-Acetamido)iminodiacetic acid)26239-55-4 2-氨基-2-甲基-1-丙醇AMT -2-amino-2-methyl-1-propanol124-68-5 N,N-双(2-羟⼄基)-2-氨基⼄磺酸BES-N,N-Bis-(2-Hydroxyethyl)-2-Aminoethanesulfonic acid10191-18-1 N,N-双-(2-羟基⼄基)⽢氨酸Bicine-N,N-Bis-(2-Hydroxyethyl)glycine150-25-4 N-环⼰基-3-氨基丙磺酸CAPS-N-Cyclohexyl-3-aminopropanesulfonicacid1135-40-6N-环⼰基-2-羟基-3-氨基丙磺酸CAPSO -N-Cyclohexyl-2-hydroxy-3-aminopropanesulfonic acid73463-39-5。
VX-745_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:VX–745 is a potent and selective inhibitor of p38α, and possesses anti–inflammatory activity.In Vitro: VX–745 exhibits PBMC IL–1β and TNFα IC 50 values of 45 and 51 nM, respectively. VX–745 is also effective in whole blood,blocking IL–1β and TNFα release with IC 50 values of 150 and 180 nM, respectively. VX–745 shows a promising selectivity profile,with 20–fold selectivity for p38α over p38β (K i =220 nM)[1]. VX–745 solutions in DMSO/DMEM inhibits the IL–6 production with IC 50of 15±9 nM [2]. VX–745 (5.0 nM) displays potent activity and 1000–fold selectivity over closely related kinases, including ERK1,JNK1–3 and MK2. VX–745 (10 nM–50 μM) increasingly inhibits the anisomycin–induced activity of p38α[3]. VX–745 (0.06 μM–20 μM)inhibits IL–6 and VEGF secretion in BMSCs. VX–745 can inhibit cytokine (TNF–α, IL–6, VEGF)–induced paracrine MM cell growth,survival, and drug resistance in the BM microenvironment. VX–745 induces modest growth inhibition of MM.1S, RPMI8226, and U266 cell lines in a dose–dependent fashion, with inhibitory concentration of 50% (IC 50) of 10 μM [4].In Vivo: VX–745 (2.5, 5, and 10 mg/kg) improves the inflammatory scores in mice by 27%, 31%, and 44%, respectively [1]. VX–745(1.06 mg/kg) significantly decreases the inflammation score from 2.07±0.29 for the control group to 1.42±0.06[2].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]VX–745 inhibits p38α and p38β. The IC 50 for the inhibition of these two p38 homologs are obtained by aspectrophotometric coupled–enzyme assay. A fixed concentration of enzyme (15 nM of p38α or p38β) is incubated with various concentrations of VX–745 in DMSO for 10 min. at 30°C in 0.1 M HEPES buffer, pH 7.5, containing 10% glycerol, 10 mM MgCl 2, 2.5mM phosphoenolpyruvate, 200 μM NADH, 150 μg/mL pyruvate kinase, 50 μg/mL lactate dehydrogenase, and 200 μM EGF receptor peptide. The reaction is initiated with 100 μM and 70 μM ATP for p38α and p38β assays, respectively. The decrease of absorbance at 340 nm is monitored to follow the rate of the reaction. IC 50 is evaluated from the rate data as a function of the inhibitor concentration.Cell Assay: VX–745 is dissolved in DMSO.[2]For these experiments, cells are plated at a density of 60,000 cells/well in 96–well plates.Each condition is tested in triplicate or more. The following day, all particle suspensions, active substances or control solutions are freshly prepared, distributed and incubated for 24 h at 37°C. Then, supernatants are carefully discarded. To perform the MTT test, 50μL of 0.1% MTT solution is added to each well for 3 h. Each well is then incubated for 1 h with 200 μL of dimethyl sulfoxide.Absorbance is measured at 595 nm. Reported results are expressed as the means±SD.Animal Administration: VX–745 is prepared in 100% propylene glycol.[1]Type II collagen–induced arthritis is established in male DBA/1mice with a minor modification. 10–Week old male DBA/1 mice are immunized by two intradermal injections within a 3 week interval using 100 μL of an emulsion consisting of a 1:1 (v/v) mixture of chick type II collagen (200 μg in 10 mM acetic acid) and complete Freund's adjuvant. Following the booster immunization, the mice are left untreated for 2–3 weeks, and are randomized into five treatment groups after they exhibit focal carpal (wrist) swelling (level 2 arthritic severity score) in both front paws. The five treatment groups are: 1: water control, 10 mL/kg, p.o., bid, (n=14); 2: 100% propylene glycol (PG) vehicle control, 10 mL/kg, p.o., bid, (n=8); 3:VX–745 in PG, at 10 mg/kg, p.o., bid, (n=7); 4: VX–745 in PG, at 5 mg/kg, p.o., bid, (n=10); and 5: VX–745 in PG, at 2.5 mg/kg, p.o., bid,Product Name:VX–745Cat. No.:HY-10328CAS No.:209410-46-8Molecular Formula:C 19H 9Cl 2F 2N 3OS Molecular Weight:436.26Target:p38 MAPK Pathway:MAPK/ERK Pathway Solubility:DMSO: 13.08 mg/mL (Need ultrasonic)(n=11). Arthritic symptoms are scored every other day using a level 1 to level 5 scoring system. Paw inflammation begins with erythema at the wrist (level 1), progressing to focal swelling of the wrist (level 2), to complete swelling of the wrist (level 3), to complete swelling of wrist and palm (level 4), and finally to complete swelling of wrist, palm and fingers (level 5). The sums of the scores from both front paw scores are used for plotting disease progression curves. Mice are sacrificed on day 20 and paws are removed, sectioned sagitally, stained with hemotoxylin & eosin, and scored for inflammation. Histologically, wrist joint inflammation begins with an infiltration of the synovium into the joint space (level 1), progressing to joint cartilage erosion (level 2), to joint cartilage and bone erosion (level 3), and finally to erosion of cartilage and bone accompanied by pannus formation (level 4). References:[1]. Duffy JP, et al. The Discovery of VX–745: A Novel and Selective p38α Kinase Inhibitor. ACS Med Chem Lett. 2011 Jul 28;2(10):758–63.[2]. Pradal J, et al. Intra–articular bioactivity of a p38 MAPK inhibitor and development of an extended–release system. Eur J Pharm Biopharm. 2015 Jun; 93:110–7.[3]. Bagley MC, et al. Rapid synthesis of VX–745: p38 MAP kinase inhibition in Werner syndrome cells. Bioorg Med Chem Lett. 2007 Sep 15;17(18):5107–10. Epub 2007 Jul 13.[4]. Hideshima T, et al. Targeting p38 MAPK inhibits multiple myeloma cell growth in the bone marrow milieu. Blood, 2003, 101(2), 703–705.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
CCX140-DataSheet-MedChemExpress
CCX140UAER and ACR values during the entire 8-wk dosing regimen[1]. In DIO mice, the CCR2 antagonist completely blocks the recruitment of inflammatory macrophages to visceral adipose tissue. The mice exhibit reduced hyperglycemia and insulinemia, improved insulin sensitivity, increased circulating adiponectin levels, decreased pancreatic islet size and increased islet number. It also reduces urine output, glucose excretion, hepatic glycogen and triglyceride content and glucose 6-phosphatase levels[2].Animal Administration [1]Mice: CCX140-B is formulated as a solution in 1% hydroxypropyl methylcellulose. Male uninephrectomized hCCR2 KI mice are rendered diabetic with the high-fat diet and dosed with 100 mg/kg CCX140-B, but for 8 wk of dosing. Eight-week-old male hCCR2 KI Lepr db/db mice are similarly dosed with 100 mg/kg CCX140-B for 6 wk[1].MCE has not independently confirmed the accuracy of these methods. They are for reference only.REFERENCES[1]. Sullivan T, et al. CCR2 antagonist CCX140-B provides renal and glycemic benefits in diabetic transgenic human CCR2 knockin mice. Am J Physiol Renal Physiol. 2013 Nov 1;305(9):F1288-97.[2]. Sullivan TJ, et al. Experimental evidence for the use of CCR2 antagonists in the treatment of type 2 diabetes. Metabolism. 2013 Nov;62(11):1623-32.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Mc-MMAD_1401963-15-2_MSDS_MedChemExpress

Version 2.1 Revision Date: 07/08/2013Print Date: 01/14/2014MSDS1 Composition7 Accident Release MeasureProduct Name:Mc-MMADChemical Name:PROCEDURE(S) OF PERSONAL PRECAUTION(S)-Wear respirator, chemical safety goggles, rubber boots, and heavyrubber gloves.METHODS FOR CLEANING UP-Sweep up, place in a bag and hold for waste disposal. Avoid raising dust. Ventilate area andwash spill site after material pickup is complete.L-Valinamide, N-[6-(2,5-dihydro-2,5-dioxo-1H-pyrrol-1-yl)-1-oxohexyl]-N-methyl-L-valyl-N-[(1S,2R)-2-methoxy-4-[(2S)-2-[(1R,2R)-1-methoxy-2-methyl-3-oxo-3-[[(1S)-2-phenyl-1-(2-CAS No.:1401963-15-28 Accident Release MeasureAppearance:White to off-white(solid)Formula:C51H77N7O9S 9 Toxicological InformationSolubility:To the best of our knowledge, the chemical, physical, andtoxicological properties have not been thoroughly investigated.No data available.p p p p thiazolyl)ethyl]amino]propyl]-1-pyrrolidinyl]-1-[(1S)-1-methylpropyl]-4-oxobutyl]-N-methyl-25°C: DMSO2 Handling and Storage10 Regulary Information3 Stability and Reactivity11Disposal ConsiderationsCLASSIFICATION- Substance not yet fully tested.SAFETY PHASES- 26-36 (In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Wear suitable protective clothing.) 36/37/38 (Irritating to eyes,respiratory system and skin.)STABILITY- Stable under normal handling conditions.HANDLING- Do not breathe dust. Avoid contact with eyes,skin,and clothing.Avoid prolonged or repeated exposure.STORAGE- Please store the product under the recommended conditions in the Certificate of Analysis.11 Disposal Considerations 4 Hazards Identification12 Transport Information5First Aid RID/ADR- Non-hazardous for road transport. IMDG- Non-hazardous for sea transport.IATA - Non-hazardous for air transport.As specific country, federal, state and local environmentalregulations vary and change frequently we suggest you contact a local, authorized waste disposal contractor for adequate disposal.Special indication of hazards to humans and the environment.Irritating to eyes, respiratory system and skin.MATERIALS TO AVOID- Strong oxidizing agents.REACTIVITY- May emit toxic gasses like Carbon monoxide,Carbon dioxide, Nitrogen oxides upon thermal decomposition.5 First Aid13 Other InformationThe above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. Medchemexpress LLC shall not be held liable for any damage resulting from h dli f t t ith th b d tINHALATION- If inhaled, remove to fresh air. If not breathing give, artificial respiration. If breathing is difficult, give oxygen.SKIN CONTACT- In case of contact, immediately wash skin withsoap and copious amounts of water.EYE CONTACT- In case of contact, immediately flush eyes withcopious amounts of water for at least 15 minutes.INGESTION- If swallowed, wash out mouth with water provided person is conscious. Call a physician.6 Fire Fighting Measureshandling or from contact with the above product.EXTINGUISHING MEDIA Water spray- Carbon dioxide, dry chemical powder, or appropriate foam.SPECIAL RISKS Specific Hazard(s)- Emits toxic fumes under fire conditions. SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS Wear self-contained breathing apparatus and protective clothing Caution: Not fully tested. For research purposes onlyMedchemexpress LLCto prevent contact with skin and eyes.18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
多西他赛杂质

多西他赛杂质——孟成科技(上海)有限公司名称信息结构式多西他赛杂质Docetaxel Impurity分子式/Molecular Formula :C41H55NO14分子量/Molecular Weight :785.87多西他赛杂质Docetaxel Impurity 分子式/Molecular Formula :C43H51NO14分子量/Molecular Weight :805.86CAS#:167074-97-7多西他赛杂质Docetaxel Impurity 分子式/Molecular Formula :C43H53NO14分子量/Molecular Weight :807.88CAS#:153381-68-1多西他赛杂质Docetaxel Impurity 分子式/Molecular Formula :C43H51NO14分子量/Molecular Weight :805.86CAS#:162784-72-7多西他赛杂质Docetaxel Impurity 分子式/Molecular Formula :C29H36O10分子量/Molecular Weight :544.59CAS#:32981-86-5多西他赛杂质Docetaxel Impurity 分子式/Molecular Formula :C45H55NO15分子量/Molecular Weight :849.92CAS#:125354-16-7多西他赛杂质Docetaxel Impurity 分子式/Molecular Formula :C41H49NO14分子量/Molecular Weight :779.84多西他赛杂质Docetaxel Impurity 分子式/Molecular Formula :C29H36O10分子量/Molecular Weight :544.60多西他赛杂质Docetaxel Impurity 分子式/Molecular Formula :C11H15NO3分子量/Molecular Weight :209.25CAS#:126150-57-0多西他赛杂质Docetaxel Impurity 分子式/Molecular Formula :C16H15NO3分子量/Molecular Weight :269.30CAS#:146924-94-9多西他赛杂质Docetaxel Impurity 分子式/Molecular Formula :C16H15NO3分子量/Molecular Weight :269.30CAS#:146924-91-6多西他赛杂质Docetaxel Impurity 分子式/Molecular Formula :C16H15NO3分子量/Molecular Weight :269.30CAS#:194235-04-6多西他赛杂质Docetaxel Impurity 分子式/Molecular Formula :C16H15NO3分子量/Molecular Weight :269.30CAS#:194235-02-4多西他赛杂质Docetaxel Impurity 分子式/Molecular Formula :C29H34O10分子量/Molecular Weight :542.58CAS#:92950-45-3多西他赛杂质Docetaxel Impurity 分子式/Molecular Formula :C43H55NO15分子量/Molecular Weight :825.91多西他赛杂质Docetaxel Impurity 分子式/Molecular Formula :C43H51NO14分子量/Molecular Weight :805.87。
OXOID药敏纸片中英文对照
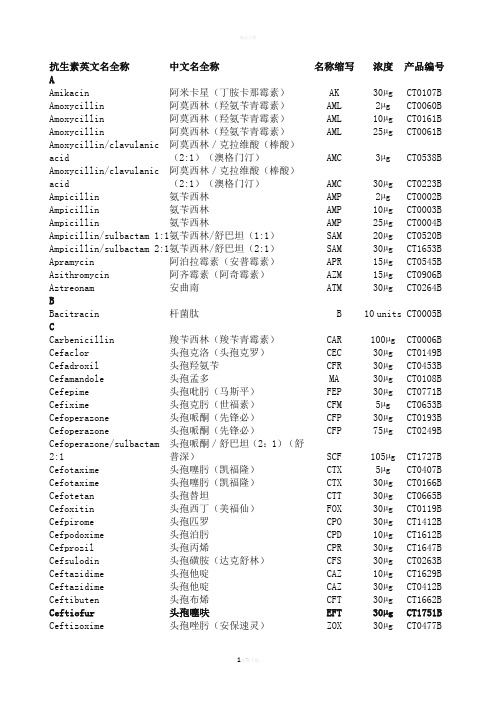
AAmikacin 阿米卡星(丁胺卡那霉素)AK 30µg CT0107B Amoxycillin 阿莫西林(羟氨苄青霉素)AML 2µg CT0060B Amoxycillin 阿莫西林(羟氨苄青霉素)AML 10µg CT0161B Amoxycillin 阿莫西林(羟氨苄青霉素)AML 25µg CT0061BAmoxycillin/clavulanic acid 阿莫西林/克拉维酸(棒酸)(2:1)(澳格门汀)AMC 3µg CT0538BAmoxycillin/clavulanic acid 阿莫西林/克拉维酸(棒酸)(2:1)(澳格门汀)AMC 30µg CT0223BAmpicillin 氨苄西林AMP 2µg CT0002B Ampicillin 氨苄西林AMP 10µg CT0003B Ampicillin 氨苄西林AMP 25µg CT0004B Ampicillin/sulbactam 1:1 氨苄西林/舒巴坦(1:1)SAM 20µg CT0520B Ampicillin/sulbactam 2:1 氨苄西林/舒巴坦(2:1)SAM 30µg CT1653B Apramycin 阿泊拉霉素(安普霉素)APR 15µg CT0545B Azithromycin 阿齐霉素(阿奇霉素)AZM 15µg CT0906B Aztreonam 安曲南ATM 30µg CT0264B BBacitracin 杆菌肽 B 10 units CT0005B CCarbenicillin 羧苄西林(羧苄青霉素)CAR 100µg CT0006B Cefaclor 头孢克洛(头孢克罗)CEC 30µg CT0149B Cefadroxil 头孢羟氨苄CFR 30µg CT0453B Cefamandole 头孢孟多MA 30µg CT0108B Cefepime 头孢吡肟(马斯平)FEP 30µg CT0771B Cefixime 头孢克肟(世福素)CFM 5µg CT0653B Cefoperazone 头孢哌酮(先锋必)CFP 30µg CT0193B Cefoperazone 头孢哌酮(先锋必)CFP 75µg CT0249BCefoperazone/sulbactam 2:1 头孢哌酮/舒巴坦(2:1)(舒普深)SCF 105µg CT1727BCefotaxime 头孢噻肟(凯福隆)CTX 5µg CT0407B Cefotaxime 头孢噻肟(凯福隆)CTX 30µg CT0166B Cefotetan 头孢替坦CTT 30µg CT0665B Cefoxitin 头孢西丁(美福仙)FOX 30µg CT0119B Cefpirome 头孢匹罗CPO 30µg CT1412B Cefpodoxime 头孢泊肟CPD 10µg CT1612B Cefprozil 头孢丙烯CPR 30µg CT1647B Cefsulodin 头孢磺胺(达克舒林)CFS 30µg CT0263B Ceftazidime 头孢他啶CAZ 10µg CT1629B Ceftazidime 头孢他啶CAZ 30µg CT0412B Ceftibuten 头孢布烯CFT 30µg CT1662B Ceftiofur 头孢噻呋EFT 30µg CT1751B Ceftizoxime 头孢唑肟(安保速灵)ZOX 30µg CT0477BCeftriaxone 头孢曲松(头孢三嗪)CRO 30µg CT0417B Cefuroxime sodium 头孢呋新钠CXM 5µg CT0406B Cefuroxime sodium 头孢呋新钠CXM 30µg CT0127B Cephalexin 头孢氨苄(头孢力新,先锋IV)CL 30µg CT0007B Cephalothin 头孢噻吩(头孢菌素,先锋I )KF 30µg CT0010B Cephazolin 头孢唑啉(先锋V)KZ 30µg CT0011B Cephradine 头孢拉定(先锋VI)CE 30µg CT0063B Chloramphenicol 氯霉素 C 10µg CT0012B Chloramphenicol 氯霉素 C 30µg CT0013B Chloramphenicol 氯霉素 C 50µg CT0014B Cinoxacin 西诺沙星CIN 100µg CT0162B Ciprofloxacin 环丙沙星(悉复欢)CIP 1µg CT0623B Ciprofloxacin 环丙沙星(悉复欢)CIP 5µg CT0425B Ciprofloxacin 环丙沙星(悉复欢)CIP 10µg CT1615B Clarithromycin 克拉霉素CLR 2µg CT1599B Clarithromycin 克拉霉素CLR 5µg CT1623B Clarithromycin 克拉霉素CLR 15µg CT0693BClindamycin 克林霉素(氯林可霉素,氯洁霉素)DA 2µg CT0064BClindamycin 克林霉素(氯林可霉素,氯洁霉素)DA 10µg CT0015BCloxacillin 氯唑西林(邻氯青霉素)OB 5µg CT0016B Colistin sulphate 多粘菌素E(硫酸粘杆菌素)CT 10µg CT0017B Colistin sulphate 多粘菌素E(硫酸粘杆菌素)CT 25µg CT0065B Colistin sulphate 多粘菌素E(硫酸粘杆菌素)CT 50µg CT0664B Compound sulphonamides 磺胺复合物S3 300µg CT0059B 抗生素英文名全称中文名全称名称缩写浓度产品编号DDoxycycline 强力霉素DO 30µg CT0018B EEnrofloxacin 恩诺沙星ENR 5µg CT0639B Ertapenem 厄他培南ETP 10µg CT1761B Erythromycin 红霉素 E 5µg CT0066B Erythromycin 红霉素 E 10µg CT0019B Erythromycin 红霉素 E 15µg CT0020B Erythromycin 红霉素 E 30µg CT0021B抗生素英文名全称中文名全称名称缩写浓度产品编号FFlorfenicol 氟苯尼考FFC 30µg CT1754BFluconazole 氟康唑FCA 25µg CT1806B Flumequine 氟甲喹UB 30µg CT0666B Fosfomycin 磷霉素FOS 50µg CT0183B 抗生素英文名全称中文名全称名称缩写浓度产品编号Framycetin 新霉素B FY 100µg CT0071B Fusidic acid 褐霉素(夫西地酸)FD 5µg CT0493B Fusidic acid 褐霉素(夫西地酸)FD 10µg CT0023B Fusidic acid 褐霉素(夫西地酸)FD 50µg CT1617B GGentamicin 庆大霉素CN 10µg CT0024B Gentamicin 庆大霉素CN 30µg CT0072B Gentamicin 庆大霉素CN 120µg CT0794B Gentamicin 庆大霉素CN 200µg CT0695B IImipenem 亚胺培南(配能)IPM 10µg CT0455B KKanamycin 卡那霉素K 5µg CT0025B Kanamycin 卡那霉素K 30µg CT0026B LLatamoxef 拉氧头孢MOX 30µg CT0302B Levofloxacin 左氧氟沙星(可乐必妥)LEV 1µg CT1586B Levofloxacin 左氧氟沙星(可乐必妥)LEV 5µg CT1587B Lincomycin 林可霉素(洁霉素)MY 2µg CT0027B Lincomycin 林可霉素(洁霉素)MY 10µg CT0123B Lincomycin 林可霉素(洁霉素)MY 15µg CT0028BLincomycin/neomycin 林可霉素(洁霉素)/新霉素LN 75µg CT1757BLincomycin/spectinomycin 林可霉素/壮观霉素LS 109µg CT1758B Linezolid 利奈唑胺LZD 10µg CT1649B Linezolid 利奈唑胺LZD 30µg CT1650B Lomefloxacin 洛美沙星LOM 10µg CT1661B MMecillinam 美西林MEL 10µg CT0096B Mecillinam 美西林MEL 25µg CT0091B Meropenem 美罗培南(美平)MEM 10µg CT0774B Metronidazole 甲硝唑(灭滴灵)MTZ 5µg CT0067B Metronidazole 甲硝唑(灭滴灵)MTZ 50µg CT0466B Mezlocillin 美洛西林MEZ 30µg CT0174B Mezlocillin 美洛西林MEZ 75µg CT0192BMinocycline 米诺环素(二甲胺四环素)MH 30µg CT0030BMoxalactam 拉氧头孢MOX 30µg CT0302B Moxifloxacin 莫西沙星MXF 1µg CT1683B Moxifloxacin 莫西沙星MXF 5µg CT1633BMupirocin 莫匹罗星MUP 5µg CT0522B Mupirocin 莫匹罗星MUP 20µg CT1826B Mupirocin 莫匹罗星MUP 200µg CT0523B NNalidixic acid 萘啶酸NA 30µg CT0031B 抗生素英文名全称中文名全称名称缩写浓度产品编号Neomycin 新霉素N 30µg CT0033BNetilmicin 奈替米星(乙基西梭霉素)NET 10µg CT0424BNetilmicin 奈替米星(乙基西梭霉素)NET 30µg CT0225BNitrofurantoin 呋喃妥因(呋喃妥英) F 50µg CT0069B Nitrofurantoin 呋喃妥因(呋喃妥英) F 100µg CT0034B Nitrofurantoin 呋喃妥因(呋喃妥英) F 200µg CT0035B Nitrofurantoin 呋喃妥因(呋喃妥英) F 300µg CT0036B Norfloxacin 诺氟沙星(氟哌酸)NOR 2µg CT0687B Norfloxacin 诺氟沙星(氟哌酸)NOR 5µg CT0668B Norfloxacin 诺氟沙星(氟哌酸)NOR 10µg CT0434B Novobiocin 新生霉素NV 5µg CT0037B Novobiocin 新生霉素NV 30µg CT0038B Nystatin 制霉菌素NS 100units CT0073B OOfloxacin 氧氟沙星(泰利必妥)OFX 5µg CT0446B Oleandomycin 竹桃霉素OL 15µg CT0039B Oxacillin 苯唑西林OX 1µg CT0159B Oxacillin 苯唑西林OX 5µg CT0040B Oxolinic acid 奥索利酸(恶喹酸)OA 2µg CT0181BOxytetracycline 土霉素(氧四环素,地霉素)OT 30µg CT0041BPPefloxacin 培氟沙星(甲氟哌酸)PEF 5µg CT0661B Penicillin G 青霉素G P 1unit CT0152B Penicillin G 青霉素G P 1.5unit CT0042B Penicillin G 青霉素G P 2units CT0088B Penicillin G 青霉素G P 5units CT0124B Penicllin G 青霉素G P 10units CT0043B Penicillin/novobiocin 青霉素/新生霉素PNV 40 CT1755B Pipemidic acid 吡哌酸PIP 20µg CT0180BPiperacillin 哌拉西林(氧哌嗪青霉素)PRL 30µg CT1619BPiperacillin 哌拉西林(氧哌嗪青霉素)PRL 75µg CT0261BPiperacillin 哌拉西林(氧哌嗪青霉素)PRL 100µg CT0199BPiperacillin/tazobactam 哌拉西林/他唑巴坦(特治星)TZP 36µg CT1616B Piperacillin/tazobactam 哌拉西林/他唑巴坦(特治星)TZP 40µg CT1628B Piperacillin/tazobactam 哌拉西林/他唑巴坦(特治星)TZP 85µg CT0720B Piperacillin/tazobactam 哌拉西林/他唑巴坦(特治星) TZP 110µg CT0725B Pirlimycin 吡利霉素 PIR 2µg CT1668B Polymyxin B多粘菌素BPB300units CT0044BQQuinupristin/dalfopristin 喹奴普汀/达福普汀 QD 15µg CT1644BR抗生素英文名全称 中文名全称 名称缩写 浓度 产品编号 Rifampicin 利福平 RD 2µg CT0078B Rifampicin 利福平 RD 5µg CT0207B Rifampicin 利福平 RD 30µg CT0104BSSpectinomycin 大观霉素(壮观霉素) SH 10µg CT0046B Spectinomycin 大观霉素(壮观霉素) SH 25µg CT0411B Spectinomycin 大观霉素(壮观霉素) SH 100µg CT0823B Spiramycin 螺旋霉素 SP 100µg CT0232B Streptomycin 链霉素 S 10µg CT0047B Streptomycin链霉素 S 25µg CT0048B Sulbactam/ampicillin 1 : 1舒巴坦/氨苄西林 SAM 20µg CT0520BSulbactam/ampicillin 1 : 2 舒巴坦/氨苄西林(优立新) SAM 30µg CT1653B Sulphafurazole 磺胺异恶唑 SF300µg CT0075B Sulphamethoxazole 磺胺甲基异恶唑(新诺明) RL25µg CT0051B Sulphamethoxazole 磺胺甲基异恶唑(新诺明) RL 100µg CT0074B Sulphamethoxazole/trimethoprim 19 : 1 磺胺甲基异恶唑(新诺明)/甲氧苄氨嘧啶 SXT 25µg CT0052B Sulphonamides compound 磺胺复合物 S3 300µg CT0059BT Teicoplanin 替考拉宁(壁霉素) TEC 30µg CT0647B Telithromycin 泰利霉素 TEL 15µg CT1714B Tetracycline 四环素 TE 10µg CT0053B Tetracycline 四环素 TE 30µg CT0054B Ticarcillin 替卡西林(羧噻吩青霉TIC75µgCT0167B素)Ticarcillin/clavulanic acid 7.5 : 1 替卡西林/克拉维酸(7.5:1) TIM 85µg CT0449B Tigecycline 替加环素 TGC 15µg CT1841B Tilmicosin 替米考星 TIL 15µg CT1756B Tobramycin 妥布霉素(托普霉素) TOB 10µg CT0056B Tobramycin 妥布霉素(托普霉素) TOB 30µg CT1618B Trimethoprim 甲氧苄氨嘧啶 W 1.25µg CT0057B Trimethoprim 甲氧苄氨嘧啶 W 2.5µg CT0070B Trimethoprim 甲氧苄氨嘧啶 W 5µg CT0076B Trimethoprim/sulphamethoxazole 1:19 甲氧苄氨嘧啶/磺胺甲基异恶唑(复方新诺明) SXT 25µg CT0052B V Vancomycin 万古霉素(稳可信) VA 5µg CT0188B Vancomycin 万古霉素(稳可信) VA 30µg CT0058B Voriconazole 优立康唑 VOR 1µg CT1807B 注释: CLSI: Clinical and Laboratory Standards Institute 美国临床实验室标准化研究所DIN: Deutsches Institut f ür Normung 德国标准化学会 BSAC: British Society for Antimicrobial Chemotherapy 英国抗生素化疗协会SRGA: Swedish Reference Group for Antibiotics 瑞典抗生素委员会 SFM: Soci ét é Fran çaise de Microbiologie 法国微生物学会注:红色为兽禽用抗生素,绿色为农作物用抗生素,其余为人用抗生素。
Thermo Scientific HERACELL VIOS 160i 250i CR CO2培养

50163133版本 B 6 2021Thermo ScientificHERA CELL VIOS 160i CR/HERA CELL VIOS 250i CRCO 2 培养箱使用说明书©2020 Thermo Fisher Scientific Inc. 版权所有。
商标Heracell Vios CR™, Steri-Run™, Steri-Cycle CR™, iCan™, THRIVE™ 和 Cell Locker™ 是Thermo Scientific的注册商标。
Thermo Scientific是Thermo Fisher Scientific, Inc.拥有的品牌。
本使用说明书中提到的所有其他商标均属于其各自的制造商所有。
Thermo Electron LED GmbHRobert-Bosch-Straße 1D - 63505 Langenselbold德国Thermo Electron LED GmbH有限公司是下述公司的成员公司:Thermo Fisher Scientific Inc.168 3rd AvenueWaltham, MA 02451美国Thermo Fisher Scientific Inc.为购买其产品的用户提供此手册作为操作指南。
本文档享有版权保护。
未经Thermo Fisher Scientific Inc.公司书面许可,不得复制本文档的部分或全部内容。
我们保留对本使用说明书随时进行修改的权力,恕不另行通知。
该文件中所有的技术信息仅用于参考的目的。
系统的配置及技术参数以该文件为准,并取代用户所有之前获得的信息。
Thermo Fisher Scientific Inc.不承诺该文件是完全完整的、准确的或毫无错误的,我们也不对由此文件导致的错误、疏漏、损坏或损失负责,即使该文件的信息被合适的遵照执行。
此文件不是Thermo Fisher Scientific公司与购方合同的一部分。
F-STOP 对照图片,病毒免疫咖啡草坛说明书

F -S T O P ™ F o r P i c t u r e -P e r f e c t , D i s e a s e -F r e e T u r f g r a s s 12770• Provides Systemic Prevention And Control Of Turfgrass Diseases• Prevents Over 15 Major Lawn Diseases • For Use On All Types Of Home Lawns • One Application Protects For Up To 4 WeeksKEEP OUT OF REACH OF CHILDRENCAUTIONBUYER ASSUMES ALL RISKS OF USE, STORAGE OR HANDLING OF THIS MATERIALNOT IN STRICT ACCORDANCE WITH DIRECTIONS GIVEN HEREWITH.NET WEIGHT 10 LBS. (4.53 KG)ACTIVE INGREDIENT:myclobutanil: a-butyl-a-(4-chlorophenyl)-1-H-1,2,4 triazole-propanenitrile: ...............0.39%OTHER INGREDIENTS: .............................................................................................99.61%TOTAL .....................................................................................................................100.00%This product contains 0.195 lb.. of myclobutanil per 50 lb. bag.C o v e r s U p T o 2,500 S q . F t .F-STOP ™ For Picture-Perfect, Disease-Free TurfgrassF -S T O P ™F o r P i c t u r e -P e r f e c t , D i s e a s e -F r e e T u r f g r a s sF-STOP ™For Picture-Perfect, Disease-Free TurfgrassPRECAUTIONARY STATEMENTSHAZARDS TO HUMANS AND DOMESTIC ANIMALSCauses Moderate Eye Irritation.Avoid contact with eyes or clothing. Wash thoroughly with soap and water after handling and before eating, drinking, chewing gum, using tobacco, or using the toilet.Notice: Read the entire label. Use only according to label directions. Before using this product, read “Warranty Disclaimer,” “Inherent Risks of Use,” and “Limitation of Remedies” at end of Directions for Use. If terms are unacceptable, return at once unopened.In case of emergency endangering health or the environment involving this product,call 1-800-992-5994Agricultural Chemical: Do not ship or store with food, feeds, drugs, or clothing.FIRST AIDIf in eyes: Hold eye open and rinse slowly and gently with water for 15-20 minutes. Remove contact lenses, if present, after the first 5 minutes, then continue rinsing eye. Call a Poison Control Center or doctor for treatment advice. Have the product container or label with you when calling a Poison Control Center or doctor, or going for treatment. You may also contact 1-800-992-5994 for emergency treatment information.ENVIRONMENTAL HAZARDSThis pesticide is toxic to fish. To protect the environment, do not allow pesticide to enter or run off into storm drains, drainage ditches, gutters or surface waters. Applying this product in calm weather when rain is not predicted for the next 24 hours will help ensure that wind or rain does not blow or wash pesticide off the treatment area. Sweeping any product that lands on a driveway, sidewalk, or street, back onto the treated area of the lawn or garden will help to prevent runoff to water bodies or drainage systems.DIRECTIONS FOR USEIt is a violation of Federal law to use this product in a manner inconsistent with its labeling. Read all directions carefully before applying this product.Not for use on turfgrass being grown for sale or other commercial use as sod, or for commercial seed productions, or for research purposes.STORAGE AND DISPOSALDo not contaminate water, food or feed by storage and disposal.PESTICIDE STORAGE: Keep away from fire and sparks. Store in a cool, dry, well-ventilated area. CONTAINER HANDLING: Nonrefillable container. Do not reuse or refill this container.If empty: Place in trash or offer for recycling if available.If partly filled: Call your local solid waste agency for disposal instructions. Never place unused product down any indoor or outdoor drain.HOW THIS PRODUCT WORKSferti•lome® F-STOP™is a systemic, protectant and curative fungicide for the control of listed diseases in established home lawns and ornamental turfgrass. Optimum disease control is achieved when this product is applied to established turfgrass in a regularly scheduled preventative program. Use this product in conjunction with turf management practices that promote good plant health and optimum disease control. The key to selecting a fungicide is the proper diagnosis of the organism causing the disease. Diagnostic kits, extension experts, or other identification methods should be used when developing disease control strategies.HOW TO APPLYApply ferti•lome® F-STOP™ uniformly over the turfgrass area using a properly calibrated drop or rotary-type spreader designed to apply granules. Before each application, calibrate the spreader according to the equipment manufacturer’s directions. Check frequently to make sure equipment is operating properly and distributing product uniformly. A more uniform application may be achieved by spreading half of the required amount of product over the area and then applying the remaining half in swaths at a right angle to the first. Avoid skips and excessive overlaps during application. Avoid the use of spreaders that apply this product in narrow rows or concentrated bands.Wash hands with soap and water promptly after use.Do not allow people or pets to contact treated area until after product dust has settled into the turfgrass, or if watered in, after the turfgrass surface in the treated area has dried.WHEN TO APPLYReduce the interval between applications of this product when conditions are favorable for disease development. Unless otherwise specified, when disease pressure is high or when used as a curative, use higher rates of ferti•lome® F-STOP™ and shorter application interval. Under light to moderate disease pressure apply this product at the low use rate and/or longer application intervals. To avoid pick-up, lightly irrigate treated areas soon after application. On short cut bentgrass (1/2 inch or less) when temperatures are above 80°F, apply only to dry foliage.HOW MUCH TO APPLYOptimum disease control is achieved when ferti•lome® F-STOP™ is applied in a preventative disease control program at a rate of 4 lb per 1,000 sq. ft. See the following table for specific application rates for various diseases. Under any circumstances, do not apply more than 46 lb of this product per1,000 sq. ft. per year.SPREADER GUIDEONE BAG WILL COVER UP TO 2,500 SQUARE FEETSPREADER SPREADER SETTINGS Scotts®/Republic Accugreen (Drop) 4 1/4Scotts®/Republic Speedy Green (Broadcast) 3 3/4 ferti•lome® /EarthWay Ev-N-Spred (Broadcast)14TERMS AND CONDITION OF USEIf terms of the following Warranty Disclaimer, Inherent Risks of Use, and Limitation of Remedies are not acceptable, return unopened package at once to the Seller for a full refund of purchase price paid. Otherwise, use by the Buyer or any other user constitutes acceptance of the terms under Warranty Disclaimer, Inherent Risks of Use, and Limitations of Remedies.WARRANTY DISCLAIMERSeller warrants that this product conforms to the chemical description of the label and is reasonably fit for the purposes stated on the label when used in strict accordance with the directions, subjectto the Inherent Risks set forth below. Seller MAKES NO OTHER EXPRESS OR IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE OR ANY OTHER EXPRESS OR IMPLIED WARRANTY.INHERENT RISKS OF USEIt is impossible to eliminate all risks associated with use of this product. Plant injury, lack of performance, or other unintended consequences may result because of such factors as use of the product contrary to label instructions (including conditions noted on the label, such as unfavorable temperature, soil conditions, etc.), abnormal conditions (such as excessive rainfall, drought, tornadoes, hurricanes), presence of other materials, the manner of application, or other factors, allof which are beyond the control of the Seller. To the extent allowed by law, all such risks shall be assumed by the Buyer.LIMITATION OF REMEDIESTo the extent consistent with applicable law, the exclusive remedy for losses or damages resulting from this product (including claims based on contract, negligence, strict liability, or other legal theories), shall be limited to, at Seller’s election, one of the following:1. Refund of purchase price paid by Buyer or user for product bought, or2. Replacement of amount of product used.To the extent allowed by law, Seller shall not be liable for the losses of damages resulting from the handling or use of this product unless Seller is promptly notified of such loss or damage in writing. In no case shall Seller be liable for consequential or incidental damages or losses.The terms of the Warranty Disclaimer and Inherent Risks of Use above and this Limitation of Remedies cannot be varied by any written or verbal statements or agreements. No employee or sales agent of Seller or the Seller is authorized to vary or exceed the terms of the Warranty Disclaimer or this Limitation of Remedies in any manner.Disease ferti•lome® F-STOP™(lb/1,000 sq ft)ApplicationInterval/Timing(Days)Directions RestrictionsAnthracnose Red Thread Septoria Leaf Spot 414 - 21Apply when conditions are favorable for disease development.Do not apply more than 46pounds of product per 1,000sq. ft. per year.For Nassau and Suffolkcounties in New York State,do not apply more than 11.5lb of this product per 1,000sq ft per year.Brown Patch14Begin applications when conditions are favorable for disease development andb efore disease symptoms are apparentCopper SpotZonate Leaf SpotApply when conditions are favorable for disease development.Crown Rot Leaf Spot Melting-Out Apply when conditions are favorable for disease development. For crown rot, water in with 3 to 4 gallons of water per 1,000 sq. ft. to increase penetration to crown a nd roots.Dollar Spot14 - 28Apply when conditions are favorable for disease development. Make no morethan 3 consecutive applications for Dollar Spot control before rotating to aregistered fungicide with a diiferent mode of actionFusarium Blight14 - 21Apply when conditions are favorable for disease development.Fusarium Patch(Pink Snow Mold)21 - 28Apply when conditions are favorable for disease development.Gray Leafspot14Apply prior to snow cover.Leaf Spot Apply in the fall after turfgrass enters dormancy and/or in the spring prior to theinitiation of growth.Necrotic Ring Spot Spring: 28Make applications on a preventative basis in early to mid-spring.Necrotic Ring Spot Fall: 28Make 2 applications beginning in August before the turfgrass goes dormant.Powdery MildewRusts14 - 28Apply when conditions are favorable for disease development.Summer Patch Begin applications in the spring when conditions are favorable for disease de-velopment. Make 2 to 4 applications depending on recommendations from localTurfgrass Extension Experts.Water in with at least 3 to 4 gallons of water per 1,000 sq. ft. to increase penetra-tion to crown and roots.Manufactured by:230 FM 87 • BONHAM, TEXAS 75418EPA Reg. No. 62719-461-7401 EPA Est. No. 7401-TX-01Visit Us At: Product Questions? 855-270-477612770-0515-TP。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries
Data Sheet
BIOLOGICAL ACTIVITY:
CCX140 is a potent CCR2 antagonist.
In Vitro: CCX140–B potently inhibits CCL2–induced chemotaxis of purified human blood monocytes with IC 50 values of 8 nM in buffer and 200 nM in the presence of 100% human serum. CCX140–B also inhibits CCL2–induced Ca 2+ mobilization in monocytes with an IC 50 value of 3 nM. CCX140–B inhibits the binding of 125I–CCL2 to monocytes with an IC 50 value of 17 nM. CCX140–B has a K d value of 2.3 nM toward hCCR2. CCX140–B also inhibits monocyte chemotaxis mediated by the other CCR2 ligands: CCL8/MCP–2,CCL7/MCP–3, and CCL13/MCP–4[1].
In Vivo: Treatment of hCCR2 KI mice with CCX140–B causes a dose–dependent reduction in the number of peritoneal leukocytes after thioglycollate challenge: CCX140–B strongly blocks leukocyte infiltration at 30 mg/kg, partially blocks leukocyte infiltration at 10 mg/kg, and fails to block leukocyte infiltration at 3 mg/kg. In DIO hCCR2 KI mice, treatment with 100 mg/kg CCX140–B blocks
the progressive increase in UAER and ACR. CCX140–B maintains lower UAER and ACR values during the entire 8–wk dosing regimen [1].In DIO mice, the CCR2 antagonist completely blocks the recruitment of inflammatory macrophages to visceral adipose tissue. The mice exhibit reduced hyperglycemia and insulinemia, improved insulin sensitivity, increased circulating adiponectin levels,
decreased pancreatic islet size and increased islet number. It also reduces urine output, glucose excretion, hepatic glycogen and triglyceride content and glucose 6–phosphatase levels [2].
PROTOCOL (Extracted from published papers and Only for reference)
Animal Administration:[1]Mouse: CCX140–B is formulated as a solution in 1% hydroxypropyl methylcellulose. Male
uninephrectomized hCCR2 KI mice are rendered diabetic with the high–fat diet and dosed with 100 mg/kg CCX140–B, but for 8 wk of dosing. Eight–week–old male hCCR2 KI Lepr db/db mice are similarly dosed with 100 mg/kg CCX140–B for 6 wk [1].
References:
[1]. Sullivan T, et al. CCR2 antagonist CCX140–B provides renal and glycemic benefits in diabetic transgenic human CCR2 knockin mice. Am J Physiol Renal Physiol. 2013 Nov 1;305(9):F1288–97.
[2]. Sullivan TJ, et al. Experimental evidence for the use of CCR2 antagonists in the treatment of type 2 diabetes. Metabolism. 2013 Nov;62(11):1623–32.
Product Name:
CCX140Cat. No.:
HY-101713CAS No.:
1100318-47-5Molecular Formula:
C 20H 13ClF 3N 5O 3S Molecular Weight:
495.86Target:
CCR; CCR Pathway:
Immunology/Inflammation; GPCR/G Protein Solubility:
10 mM in DMSO
Caution: Product has not been fully validated for medical applications. For research use only.
Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
