Lab1 report-A0093605
质粒图谱查询方法

3.google scholar: / 有些质粒是经过改造的,所以通过上述方法不能查询到相应信息。这时,可以在google scholar中输入质粒名称,可以直观地看哪些学者在何文章中使用了该质粒,从而可了解到质粒的来源;或者籍此向作者咨询或索取。 4.尝试从各大生物公司,例如invitrogen网站查询. 5. 这个网站收录了大量图谱: http://www.embl-hamburg.de/~geerlof/webPP/vectordb/bact_vectors/table.html
Pe
te
rX u
file:///D|/中科院/Selective Serotonin Transporter/质粒信息/质粒图谱查询方法.txt
file:///D|/中科院/Selective Serotonin Transporter/质粒信息/质粒图谱查询方法.txt(第 2/6 页)[2011/8/4 18:39:52]
By
0099--pGE-1—Stratagene--RNAi载体 0100--pSUPER.p53—OligoEngine--RNAi载体 0101--palter-ex1--promega 0102--pACYCDuet-1--NOVAGEN 0103--pEX lox(+) Vector—NOVAGEN--原核表达 0104--质粒名称:pBACgus-8 Transfer Plasmid—NOVAGEN--CHUANSUO 0105--pSCREEN?-1b(+) Vector Map—novagen--筛选 0106--PGEX-2T--BD Co--pDsRed2--Clontech 0107--pbgal-Basic—Clontech--mammalian reporter vector 0108—pBI—Clontech--express two genes of interest from a bidirectional tet-responsive promoter 0109--质粒名称:pbgal-Control—Clontech--mammalian reporter vector 0110-- pGEX-5X-1--原核表达 0111--pBI-EGFP—Clontech--pBI-EGFP-- coexpress 0112--pBI-G—Clontech--pBI-G--express b-galactosidase 0113--pBI-GL—Clontech--pBI-GL --express luciferase and b-galactosidase 0114--pCMS-EGFP—Clontech--mammalian expression vector 0115--pd2EYFP-1—Clontech--启动子测定 0116--质粒名称--pd2EYFP-N1—Clontech--融合表达 0117--pd4EGFP-Bid—Clontech--融合表达 Bid 0118--pDNR-CMV—Clontech--pDNR-CMV 0119--pDNR-EGFP Vector—Clontech 0120--pDNR-LacZ –Clontech 0121--pECFP-Endo—Clontech--真核表达0122--pECFP-ER—Clontech--真核表达0123--pEGFP-Actin—Clontech--真核表达0124--pGAD GH--Clontech--酵母表达 0125--pGADT7-Rec –Clontech--酵母表达 0126--pGADT7-RecAB—Clontech--酵母表达 0127--pGADT7-Rec2—Clontech--酵母表达 0128--pGBKT7—Clontech--酵母表达 0129--pHAT 10/11/12—Clontech 0130--pHAT20—Clontech 0131—pHygEGFP—Clontech 0132—pLacZi—Clontech 0133—pM—Clontech--pM is used to generate a fusion of the GAL4 DNA-BD 0134--pPKCa-EGFP—Clontech 0135--pPKCb-EGFP—Clontec 0136--pSIREN-DNR Vector—Clontech--RNAi 0137--pSIREN-DNR-DsRed-Express Vector—Clontech--RNAi 0138--pSIREN-RetroQ—Clontech--RNAi 0139--pIRES-EYFP—Clontech--RNAi 0140--pSRE-Luc—Clontech--RNAi 0141--pTK-neo—novagen--原核表达 0142--pZsGreen Vector—Clontech--pZsGreen is a pUC19-derived prokaryotic expression vector 0143--pTandem-1—novagen--原核表达 0144--pZsGreen1-C1Vector—Clontech----真核表达 0145--质粒名称:M13mp18—novagen--原核表达 0146--pZsGreen1-DR Vector—Clontech--真核表达 0147--PZsGreen1-N1 Vector—Clontech --真核表达 0148--T7Select415-1b—novagen----真核表达 0149--pZsYellow Vector—Clontech --真核表达 0150—pTimer—Clontech --真核表达 0151--pTA-Luc—Clontech --真核表达 0152--pTAL-Luc—Clontech --真核表达 0153--pTA-SEAP—Clontech --真核表达 0154--pTAL-SEAP—Clontech --真核表达 0155--pTet-On—Clontech --真核表达 0156--pTet-Off—Clontech --真核表达 0157--pTet-ATF—Clontech --真核表达 0158--pTet-CREB—Clontech --真核表达
IGCS19-0405 : 产品说明书

abnormal vaginal bleeding requiring intervention had no statis-tical difference between VP and WVP patients group (p=0.3074)as other complications as well(table1).Median of related days of vaginal bleeding after the procedure were 7.4days(SD8.75)in VP group and7.34days(SD8.52)in WVP group,with no statistical difference(p=0.912). Conclusions Insert a vaginal pack or not,after LEEP,do not affect the number of postoperative gynecologic intervention due to vaginal bleeding or the amount of postoperative bleed-ing days.Previous pregnancies,hormonal status,cytology or LEEP specimen characteristics did not affect the disclosure. We also could not find any risk factor associated to abnormal bleeding.Based on that,the use of vaginal pack can be omit-ted with no further complications.IGCS19-0405382LATERALLY EXTENDED ENDOPELVIC RESECTION(LEER) AND NEOVAGINE,PATIENT WITH RECTALADENOCARCINOMA AND RECURRENCE IN CERVIX,VAGINA AND PELVIC WALL:A PURPOSE OF A CASE1J Torres*,2J Saenz,3O Suescun,3M Medina,4L Trujillo.1Especialista en entrenamiento–Universidad Militar Nueva Granada–Instituto Nacional de Cancerologia,Department of Gynecologic Oncology,Bogota D.C.,Colombia;2Especialista en entrenamiento–Universidad Militar Nueva Granada–Instituto Nacional de Cancerologia,Department of Gynecologic Oncology,Bogota D.C,Colombia;3Instituto Nacional de Cancerologia, Department of Gynecologic Oncology,Bogota D.C,Colombia;4Instituto Nacional de Cancerologia,Department of Gynecologic Oncology,Bogota D.C.,Colombia10.1136/ijgc-2019-IGCS.382Objectives Exenteration is used to treat cancers of the lower and middle female genital tract in the irradiated pelvis. Höckel described laterally extended endopelvic resection (LEER)as an approach in which the resection line extends to the pelvic side wall.Methods A49-year-old patient diagnosed with rectal adenocar-cinoma10years ago,managed with chemotherapy plus radio-therapy.T umor relapse at3years,management with low abdominoperineal resection and definitive colostomy.Second relapse4years later,compromising the posterior aspect of the coccyx and right side of the pelvis with irresecability criteria, management was decided with chemotherapy with capecita-bine,oxaliplatin and bevacizumab.New relapse at2years in the cervix,vagina and pelvic wall.Images without distance disease,type LEER management with extension of pelvic floor margins and resection of muscle pubococcygeus and right lat-eral iliococcygeus with neovagina(Singapore flap)and non-continent urinary derivation with bilateral cutaneous ureteros-tomy,achieving adequate lateral margin with curative intent. During follow-up with favorable evolution.Results LEER combines at least two procedures:total mesorec-tal excision,total mesometrial resection or total mesovesical resection.It may even require resection of the pelvic wall, internal obturator muscle,pubococcygeus,iliococcygeus,coccy-geus or internal iliac vessels.In combination with neovagina, it would offer better results in non-gynecological cancer relapses.Conclusions LEER with neovagina can be offered as a new therapy to a selected subset of patients with relapse in adja-cent gynecological organs with good oncological,functional and aesthetic results.Symptom Management–Supportive Cancer CareIGCS19-0706383PHOTOBIOMODULATION AND MANUAL LYMPHDRAINAGE FOR NIPPLE NECROSIS TREATMENT INBREAST CANCER:A CASE REPORT1J Baiocchi,2L Campanholi,3G Baiocchi*.1Oncofisio,Physical Therapy,Sao Paulo,Brazil;2CESCAGE,Physical Therapy,Ponta Grossa,Brazil;3AC Camargo Cancer Center, Gynecologic Oncology,Sao Paulo,Brazil10.1136/ijgc-2019-IGCS.383Objectives Recently,breast reconstruction after mastectomywith nipple preservation became an option of breast cancer surgery.Despite its efficacy and aesthetic superiority,the nip-ple preservation is associated with several complications in the postoperative period.The photobiomodulation therapy,for-merly known as low-intensity laser therapy,demonstrated tis-sue promotion repair by cellular repair biostimulation, angiogenesis and anti-inflammatory effects.These characteris-tics suggest a potential role for repair of chronic wounds andmay be applicable in necrosis treatment.Our aim was toreport the effects of the physiotherapeutic intervention through photobiomodulation therapy in a patient with nipple necrosis after risk reducing mastectomy.Methods We report a case of a breast cancer surgery with nip-ple necrosis treated with low-level laser therapy.The patientwas a36-year-old women who developed skin nipple necrosisin the right breast after bilateral reconstructive mastectomy.She had6sessions of low-level laser therapy.Results A female subject developed a nipple necrosis of morethan40%on the right breast after mastectomy and recon-struction.She was referred to Physical Therapy(PT)and thePT sessions were composed by manual lymph drainage,man-ual therapy for de AWS,exercises of strength and flexibility, followed by LLLT with laser660nm,2joules per point atevery1cm.Therapy was implemented for12times in total,from May2016to June2016.A re-evaluation was performed monthly from July13,2016to November2017.After18 months of follow-up,the sustained effects of LLLT were found.Conclusions Low-level laser therapy is effective for the skin cicatrization after nipple necrosis.IGCS19-0446384CONTRACEPTION AND FERTILITY COUNSELING INPATIENTS RECEIVING CHEMOTHERAPY1A Elnaggar*,2A Calfee,1LB Daily,2T Hasley,1T Tillmanns.1West Cancer Center and Research Institute,Gynecologic Oncology,Memphis,USA;2University of Tennessee Health Science Center,Obstetrics and Gynecology,Mempis,USA10.1136/ijgc-2019-IGCS.384Objectives Cancer care advances allow more patients to pursue fertility.Unfortunately,treatments may have detrimental effectson fertility and fetus should pregnancy occur.This study examines physician documentation and patient perceptions of fertility and contraception counseling. on December 24, 2023 by guest. Protected by copyright./ Int J Gynecol Cancer: first published as 10.1136/ijgc-2019-IGCS.384 on 18 September 2019. Downloaded fromMethods IRB approval obtained for a cross-sectional study of men and women,ages18–50,with newly diagnosed malig-nancy between May2017and2018.Prior sterilization,secon-dary or synchronous cancer,or prior chemotherapy were exclusionary.Consented patients received a survey regarding perception on receipt and quality of,counseling.Demographic, sexual,and social information was obtained.Differences were evaluated using chi-square tests.Results Fifty-three of179patients identified participated. Majority were women(75v25%).Patients were more likely to have perceived counseling for contraception and fertility than documented.The majority perceived counseling as suffi-cient regarding contraception and fertility.Men were more likely than women to be perceive counsel-ing regarding fertility(85v43%,p=0.010).However,both felt fertility counseling to be sufficient with similar rates of documentation.Caucasians were more likely to perceive receipt of fertility counseling(68v29%)and to perceive it to be sufficient(70v40%),then African Americans,with the same rate of documentation(35%).Conclusions Significant discrepancies in perception counsel-ing regarding contraception and fertility were seen.Gen-der and race were important factors for the perception of fertility counseling,while only race was a factor to qual-ity of perceived counseling.These differences occurred despite equal rates of physician documentation,across all groups.IGCS19-0430385WHO ARE YOU CALLING OLD?PRACTICE PATTERNS AND MANAGEMENT OF NONAGENARIANS PRESENTINGTO A GYNECOLOGIC ONCOLOGIST FOR INITIALCONSULTATIONE Ryan*,B Margolis,B Pothuri.New York University Langone Health,Obstetrics and Gynecology,New York,USA10.1136/ijgc-2019-IGCS.385Objectives T o describe the practice patterns and treatment of nonagenarians who initiated care with a gynecologic oncologist.Methods Retrospective chart review of women aged90or older who presented to a gynecologic oncologist between10/ 09and12/18at an urban academic medical center.Descrip-tive statistics utilized for variables of interest.Results We identified34nonagenarians(median age92,range 90–98):10(29%)had benign disease,8(24%)pre-malignancy or suspected malignancy,and16(47%)malignancy.Of these, 79%had age and/or functional status discussed in the care plan.Of the8with suspected malignancy,5declined further workup.The cancer distribution revealed5(31%)vulvar,5 (31%)uterine,4(25%)ovarian,1(6%)vaginal and1(6%) cervical bined,37%had stage I disease;6% stage3;6%stage4;13%recurrent;and25%unstaged.All received treatment plans:7(47%)with palliative intent and8 (53%)with curative intent.In the curative group,7under-went surgery(1adjuvant chemotherapy)and1chemotherapy/radiation.In the palliative group,4underwent radiation,1 chemotherapy and2declined/unknown.Overall,13(87%) completed the proposed treatment.T reatment-related complica-tions included1superficial skin infection and1thirty-day readmission.Conclusions Nonagenarians often presented with vulvar or endometrial cancer and87%successfully completed treatmentwith minimal adverse effects or toxicity.Age and/or functionalstatus were considered in the care plan for79%of women,but it did not preclude treatments that had the potential to preserve meaningful quality of life and/or cure patients oftheir disease.IGCS19-0646386RISK FACTORS COMPREHENSIVE GERIATRICASSESSMENT FOR EARLY DEATH IN ELDERLY PATIENTSWITH GYNECOLOGICAL CANCER.A PROSPECTIVECOHORT STUDY1J Sales*,2C Azevedo,2C santos,3L sales,4M Bezerra,5G Bezerra,4Z cavalcanti,6MJ Mello.1IMIP,Geriatric Oncology,Recife,Brazil;2IMIP,Oncology,Recife,Brazil;3FPS,Medical Course,Recife,Brazil;4IMIP,geriatric,Recife,Brazil;5HMV,oncology,caruaru,Brazil;6IMIP,post graduation,Recife,Brazil10.1136/ijgc-2019-IGCS.386Objectives T o determine risk factors for early death identifiedthe Comprehensive Geriatric Assessment(CGA)in elderly patients with gynecological cancer(EPGC).Methods Prospective cohort study.Participants with a recent diagnosis of cancer were from eight community hospitals andone cancer center in Northeast Brazil and were recruited dur-ing their first medical appointment at the outpatient oncologic clinic.A basal CGA was done before the treatment decision (ADL,Charlson Comorbidity Index-CCI,Karnofsky Perform-ance status–KPS,GDS15,IPAQ,MMSE,MNA,MNA-SF,PS,PPS,Polipharmacy,TUG).During the follow up of12 months,information about the treatments performed,the tar-geted interventions and early death was collected.Overall sur-vival was estimated using the Kaplan–Meier method,and survival curves were compared using the Log rank test for cat-egorical variables.A multivariate Cox proportional hazardsmodel was used.Results From2015–2017,84EPGC,mean age69,6±7,9;range60–96),were enrolled,25%were metastatic disease.tumor site:40,4%cervical uterine,36,9%endometrial,20,2%ovary and2,3vulva.Nine(10.7%)ECP died in less than12 months of follow-up.In our multivariate model,controlled byage,site of cancer and cancer stage,the remaining significantrisk factors were malnutrition/nonutrition determined byMNA-SF(HR3.70,95%CI1.81–5.99,p<0.001),Katz index(HR 3.60,CI 1.56–3.81,p<0.001)CCI>2(HR2,74,CI1.0.74–10.20,p=0.013)and Polipharmacy(HR2.65,CI0.71–9.81,p<0.001).Conclusions The CGA at admission identified risk factors (Nutritional risk,polypharmacy,functionality for Katz indexand comorbidity index)for premature death in EPGC.They can help to plan a personalized care. on December 24, 2023 by guest. Protected by copyright./ Int J Gynecol Cancer: first published as 10.1136/ijgc-2019-IGCS.384 on 18 September 2019. Downloaded from。
试剂验证
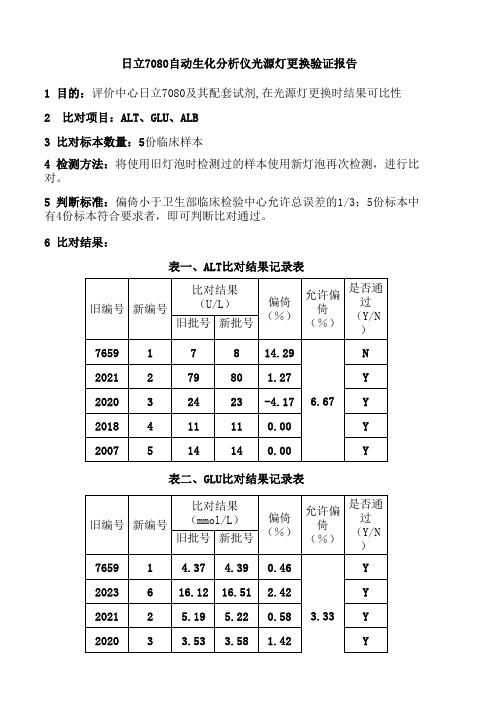
日立7080自动生化分析仪光源灯更换验证报告
1 目的:评价中心日立7080及其配套试剂,在光源灯更换时结果可比性
2 比对项目:ALT、GLU、ALB
3 比对标本数量:5份临床样本
4 检测方法:将使用旧灯泡时检测过的样本使用新灯泡再次检测,进行比对。
5 判断标准:偏倚小于卫生部临床检验中心允许总误差的1/3;5份标本中有4份标本符合要求者,即可判断比对通过。
6 比对结果:
表一、ALT比对结果记录表
表二、GLU比对结果记录表
审核者: 批准者:
2012年6月6日2012年6月6日2012年6月6日
操作者:7 比对结论:光源灯更换时做室内比对,GLU和ALB 5份标本均符合判断标准,ALT的比对中,有4份标本符合判断标准,即比对通过
表三、ALB比对结果记录表。
洗涤加强剂-Spray'N Wash 亮白与Resolve力说明书

Product Safety Data Sheet1. PRODUCT AND COMPANY IDENTIFICATION Product NameSpray 'N Wash Bright & White with Resolve powerProduct Identifier Laundry additive liquid for in-wash applications TDS formula code0167353 (R&D Code: Mira Lab-Book 290-54) Validation Date September 10th, 2008Distributor Reckitt Benckiser North America Inc.Morris Centre IV, 399 Interface Parkway (P.O. 225) Parsippany, N.J. 07054-0225Emergency Contact 1-800-228-4722Transport Emergency 1-800 424 9300 (North America) ; 703-527-3887 (outside North America)2. COMPOSITION IngredientsCAS N. Concentration RangeExposure LimitHydrogen Peroxide 7722-84-1 2% - 4% None Alcohol Ethoxylated C12-C16 7EO 68551-12-2 4% - 6% None62338-81761 (44oz) ; 62338-81762 (75 oz)August 13th, 2009Alcohol Ethoxylated C12-C16 3EO 68551-12-2 1% - 3% NoneSodium Alkylbenzen sulphonate 85117-50-6 2% - 4% None3. HAZARDS IDENTIFICATIONEMERGENCY OVERVIEW:WARNING. EYE AND SKIN IRRITANT. MAY BE HARMFUL IF SWALLOWEDKEEP OUT OF REACH OF CHILDREN.DO NOT get in eyes, on skin or ingest. May be severely irritating to eyes. wash hands after use. For sensitive skin the use of gloves is recommendedContains Hydrogen peroxide and surfactants4. FIRST- AID MEASURESFirst Aid - Eye Contact In case of eye contact, hold eyes open and IMMEDIATELY, rinse thoroughly with plenty of waterRemove any contact lenses and continue rinsing for at least 15 minutes. I f irritation persists get medical attentionFirst Aid - Skin Contact I n keeping good hygienic practices, wash exposed areas thoroughly with soap and water. I f any irritation occurs get medical attentionFirst Aid - Ingestion If swallowed, rinse mouth and drink a glass of water. DO NOT induce vomiting. Call a physician or poison control centreFirst Aid - Inhalation The product is not volatile. In case of accident, move the person to fresh air5. FIRE- FIGHTING MEASURESFlammability Not flammableFire/Explosion Hazard None knownFire fighting media/Instructions Not a fire hazard. Use exthinguishing media for surrounding materialsSpecial equipment for Fire Fighters As in any fire, wear self-contained breathing apparatus, pressure-demand, MSHA/NI OSH (approved or equivalent) and full protecting gearFlash Point (°C) > 93.3°C (based on available information on raw materilas)Autoignition Temperature No information available6. ACCIDENTAL RELEASE MEASURESPersonal precautions Avoid contact with skin and eyes.People working on cleaning-up accidental spillages should be using appropriate gloves and eye/body protection Spillages Small splillages: Cordon-off the area. Soak-up spill with adsorben material and dispose off as appropriate. Rinse surface residue and wipe dry to avoid splippery conditionsLarger spillages: Cordon-off the area. Large spill should be collected and disposed according to local regulations7. HANDLING AND STORAGEHandling Careful handling of chemicals is required. WARNING: EYE AND SKIN IRRITANT. MAY BE HARMFUL IFSWALLOWED. Avoid contact with eyes and skin.Do not ingestStorage Conditions Store the closed packages cool and under dry conditions. Do not freeze. Store in original container in a secure area, inaccessible to children and petsKEEP OUT OF THE REACH OF CHILDREN8. EXPOSURE CONTROL AND PERSONAL PROTECTIONEngineering Control Ensure adequate ventilation, especially in confined areasEye protection Wear safety goggles if the exposure is likely to be prolonged (emergency responders should wear appropriate body protection)Skin protection Wear safety gloves if the exposure is likely to be prolonged (emergency responders should wear appropriate body protection)Respiratory protection In case of airborne dusting wear dust mask (emergency responders should wear appropriate body protection)Body protection Wear suitable protective clothes if the use is likely to be prolonged (emergency responders should wear appropriate body protection).Hand protection Protective gloves (emergency responders should wear appropriate body protection).9. PHYSICAL AND CHEMICAL PROPERTIESAppearance Clear liquidColour AmberOdour perfumed (fruity scent)pH (as is)3,4 - 4,4Solubility in water (kg/m3)high solubilityViscosity (cPs, 25°C)< 100 (Brookfield RV, spindle 1, 60 rpm)Specific gravity (g/mL)1,0179 - 1,027910. STABILITY AND REACTIVITYChemical Stability The product is chemically stable under normal conditions. Store away from direct sun-light and from any source of heatConditions to Avoid T > 50°C, product contaminationHazardous decomposition products Carbon oxide and unknown organic compoundsMaterials to Avoid Hypochlorite based bleaches, metal ions11. TOXICOLOGICAL INFORMATIONEyes contact May be severely Irritating to eyes (based on raw material data)Skin contact Skin Irritant (based on raw material data).Ingestion May be Harmful by ingestion (Oral LD50 > 5000mg/kg, based on raw material data)Sensitization Not expected to be a sensitizer (based on raw material information)Chronic Effects No information availableCarcirogenic Effects Not listed as carcirogenic by OSHA, NTP, or IARCMutagenic Effects No information availableReproductive toxicity No information availableTarget Organ Effects No information availableInformation is based on data on the components and the toxicology of similar products12. ECOLOGICAL INFORMATIONIncorporated surfactants are ultimately aerobically biodegradable according to OECD301 methodsMobility Not Applicable Bioaccumulation Not ApplicableEcotoxicityNot Applicable Aquatic toxicity The liquid contains hydrogen peroxide which decomposes in oxygen and water with no adverse effect For surfactants with EC 50 < 1mg/l no adverse long-term effects to the aquatic environment are foreseen,due to their biodegradability and non-bioacculable properties. the product may exhibit acute effects in case of massive disscharge in watercourses.13. DISPOSAL CONSIDERATIONSProduct Disposal Disposal should be done in accordance with local/state or federal legislation Do not release in ground or on riverContainer DisposalCleaned to recycling, landfill or incinerator 14. TRANSPORT INFORMATIONGeneral Remark: Not classified as hazardous for transport.Dot Classification: Not a DOT regulated material (United States)TDG Classification: Not a TDG regulated material (Canada)UN NumberNot applicableProper Shipping Name Not applicable Packaging Group Not applicable Maritime TransportationNot applicable15. REGULATORY INFORMATION FEDERAL and STATE REGULATIONSARA Title III, Section 313 Toxic Chemical Notification Release Reporting: NonePROPOSITION 65 This product contains the following ingredients which require a warning under theSafe Drinking Water & Toxic Enforcement Act: NoneTSCA Listing Raw materials used in this formula are resistered and compliant with TSCA inventory16. OTHER INFORMATIONHMIS (USA) Health Hazard: 2 Fire Hazard: 0 Reactivity: 1Personal Protection: B NFPA Aerosol Level: Not applicable PSDS first issue September 10th, 2008PSDS last revision November 6th, 2008Reason for revisionUPC Code AdditionAugust 13th, 2009September 10th, 2008The information given in this data sheet is believed to be true and correct. This quality is not intended for wuality assurance purposedFinal determination of suitability of any material is the sole responsibility of the user. All materials may present unknown hazards and should be used with cautionsAlthough certain hazards are described herein, we cannot guarantee that these are the only hazards that exist。
岛津labsolution报告模板

岛津labsolution报告模板1.背景岛津labsolution是一家专注于实验室仪器销售和技术支持的企业,该企业为客户提供仪器的销售、售后服务和维修等一站式服务。
对于岛津labsolution的客户来说,及时且详细的报告是评估实验结果的重要因素。
本文旨在介绍岛津labsolution报告模板的使用方法,以帮助客户快速准确地生成报告。
2.模板说明岛津labsolution报告模板是由专业的化学分析师、工程师和客户服务代表组成的团队共同开发设计的。
该模板基于国际标准及使用经验制定,通过对客户的真实需求和岛津labsolution的实际情况进行分析,将常用的报告要素整理成了易于理解且操作简便的形式。
该模板可适用于多种实验仪器和实验场景,并具有以下特点:•格式清晰,简洁明了。
•报告内容涵盖全面,包括实验方法、实验结果和实验结论。
•报告中可以插入自定义的分析结果和图表(非必要因素)。
•模板支持Markdown文本输入格式,方便客户编辑。
•该模板可免费下载使用。
3.模板使用方法3.1 下载模板客户可以通过以下步骤下载岛津labsolution报告模板:1.打开岛津labsolution官网。
2.导航至“下载中心”页面。
3.在该页面中找到“报告模板”选项,并点击下载链接。
3.2 填写报告安装模板后,客户可以按照以下步骤完成报告:1.打开模板并输入实验名称和实验人员信息。
2.编写实验方法,输入实验步骤和实验条件等信息。
该部分需要客户根据实验实际情况进行填写。
3.输入实验结果,包括实验数据和实验图表等信息(非必要因素)。
客户可以根据自己实验的特点自定义需要包含在报告中的结果内容。
4.撰写实验结论,总结实验结果和结论。
该部分需要客户进行仔细思考和分析,以给出准确有效的评价。
3.3 导出报告完成报告后,客户可以通过以下步骤导出报告:1.点击“导出”按钮。
2.选择报告的导出格式,包括PDF、HTML和Word等。
迈瑞试剂日立上机参数

校准方法 Calib Type Linear Linear Linear Linear Linear Linear Linear Linear Linear Linear Linear Linear Linear Linear Linear Linear Linear Linear Linear Linear Linear Logit-Log(5p) Logit-Log(5p) Logit-Log(4p) Linear Linear
1浓度 0 0 0 0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0 0.00 0 0 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0 0 0
2浓度 # # # # # # # # # # # # # # # # # # # # #
# #
CK CKMB Ca Mg P α -AMY PA IgA IgG IgM C3 C4 CRP hs-CRP ADA
50 50
50 75 100 100 50 100 75 50 150 90 90 40 40 50 40 50 150 60 30 60 40
18800 11700 32000 32000 32000 32000 32000 32000 32000 32000 32000 32000 32000 32000 32000 32000 32000 0 32000 32000 32000 0 5000 32000 32000 32000 32000 32000 32000
2-2 2-2 2-2 2-2 2-2 2-2 6-3 6-3 6-3 6-3 6-3 6-3 6-3 5-3 2-2 2-2 2-2 6-3 2-2 2-2 2-2 2-2 2-2 2-2 6-3 2-2 2-2 2-2 2-2
UN38.3 Test Report(1)

APPLICATION FOR LOW VOLTAGE DIRECTIVEOn Behalf ofShenzhen Xuxing Telecom Technology Co.,Ltd.Li-ion BatteryModel: A1, X8Prepared For : Shenzhen Xuxing Telecom Technology Co.,Ltd.14I,Block A, Huaqiang Plaza, No.1019Huaqiang North Road, Futian District,Shenzhen,ChinaPrepared By : Most Technology Service Co., Ltd.No. 5, 2nd Langshan Road, North District, Hi-techIndustrial Park, Nanshan, Shenzhen,Guangdong, ChinaTEL : 0755 – 86170306FAX : 0755 – 86170310Date of receipt of test item : January 13, 2011Report Reference Number : SZSTS110103EU______ST/SG/AC.10/11Rev.5 Section 38.3Clause Requirement- Test Result - Remark Verdict38.3 Lithium metal and lithium ion batteries P 38.3.1 Purpose PThis section presents the procedures to be followedfor the classification of Lithium metal and lithium ioncells and batteries.P 38.3.2 Scope P 38.3.2.1 Lithium metal and lithium ion cells and batteries whichdiffer from a tested type by:Pa) For primary cells and batteries, a change of morethan 0.1 g or 20% by mass, whichever is greater, tothe cathode, to the anode, or to the electrolyte.Nb) For rechargeable cells and batteries, a change inwatt-hours of more than 20% or an increase in voltageof more than 20%.Pc) A change that would materially affect the testresults. Shall be considered a new type and shall besubjected to the required test.P38.3.2.2 I For the purposes of classification, the followingdefinitions apply:P38.3.3 When a cell or battery type is to be tested underthis sub-section, the number and condition ofcells and batteries of each type to be tested are asfollows: Tests 1 to 5 must beconducted in sequence onthe same battery,Pa) When testing primary cells and batteries undertests 1 to 5, the following shall be tested:N Ten cells in undischarged states, N Ten cells in fully discharged states, N Four small batteries in undischarged states, N Four small batteries in fully discharged states, N Four large batteries in undischarged states N Four large batteries in fully discharged states N b) when testing rechargeable cells and batteries undertests 1 to 5 the following shall be tested:P Ten cells at first cycle, in fully charged states, N Four small batteries at first cycle, in fully chargedstates.P Four small batteries 50 cycle ending in fully chargedstates.P Two large batteries at first cycle, in fully chargedstates. N Two large batteries 25 cycle ending in fully chargedstates.NClause Requirement- TestResult - RemarkVerdict______c) When testing primary and rechargeable cells under test 6(Impact), the following shall be tested in the quantity indicated:PFor primary cells, five cells in undischarged states and five cells in fully discharged statesPFor component cells of primary batteries, Five cells in undischarged states and five cells in fully discharged states.NFor rechargeable cells, five cells at first cycle at 50% of the design rated capacity,N For components cells of rechargeable batteries, five cells at first cycle at 50% of the design rated capacity.PFor prismatic cells, ten test cells are required instead of the five described above, so that the procedure can be carried out on five cells along the longitudinal axes and, separately, five cells along the other axes. In every case, the test cell is only subjected to one impacPd) When testing rechargeable batteries under test 7(Overcharge), the following shall be tested in the quantity indicated:PFour small batteries at first cycle, in fully charged states.P Four small batteries after 50 cycles ending in fully charged states.P Two large batteries at first cycle, in fully charged states,N Two large batteries after 25 cycles ending in fully charged states.Ne) When testing primary and rechargeable cells under test 8(Forced Discharge), the following shall be tested in the quantity indicated:The requirement is not applicable to test batteries. N Ten primary cells in fully discharged states N Ten rechargeable cells, at first cycle in fully discharged statesN Ten rechargeable cells after 50 cycles ending in fullydischarged statesNf) when testing a battery assembly in which the aggregate lithium content of all anodes, when fully charged, is not more than 500g, or in the case of a lithium ion battery, with a watt-hour rating of not more than 6200 Watt-hoursNClause Requirement- Test Result - Remark Verdict______38.3.4 ProcedurePTest 1 to 5 must be conducted in sequence on the same cell or battery.P Test 6 and 8 should be conducted using not otherwisetested cells or batteriesPTest 7 may be conducted using undamaged batteriespreviously used in tests 1 to 5 for purposes of testing on cycled batteriesP38.3.4.1 Test 1: Altitude Simulation P 38.3.4.1.1 Purpose P This test simulates air transport under low-pressureconditions.-- 38.3.4.1.2 Test procedureP stored at a pressure 11.6 kPa -- ambient temperature (20 ± 5).℃ 24℃ -- Stored times( ≥ 6 hours) 8 hours. -- 38.3.4.1.3 RequirementCells and batteries meet this requirement if there is nomass loss, no leakage, no venting, no disassembly, no rupture and no fire and if the open circuit voltage of each test cell or battery after testing is not less than 90% of its voltage immediately prior to this procedure. The requirement relating to voltage is not applicable to test cells and batteries at fully discharged statesNo mass loss, no leakage, no venting, no disassembly, no rupture and no fire. Battery after testing is not less than 90% of its voltageimmediately prior to this procedure.PMass M of Test Battery (g)OCV (V) Group No.M1 (beforet he test) M2 (after the test)MassLoss limit(0.1%)OCV1 (beforethe test) OCV2 (after the test) OCV (≥90%)01 21.223g 21.223g0.00%3.861 3.861 100.0% 02 21.178g 21.178g 0.00%3.867 3.867 100.0%03 21.338g 21.338g 0.00%3.866 3.866 100.0% Group A (at first cycle, infully charged states) 04 21.385g 21.385g 0.00% 3.862 3.862 100.0% 05 21.142g 21.142g 0.00%3.865 3.865 100.0% 06 21.465g 21.465g 0.00%3.854 3.854 100.0% 07 21.276g 21.276g 0.00%3.860 3.860 100.0% Group B (after fiftycycles ending in fullycharged states)08 21.328g 21.328g 0.00%3.8673.867100.0%Remark1.Mass loss (%)=(M1-M2)/M1*100% (Where M 1 is the mass before the test and M 2 is the mass after the test)2.When mass loss does not exceed the value in Table: Mass loss limit, it shall be considered as "no mass loss".3.The OCV of each test cell after testing is not less than 90% of its voltage immediately prior to this procedure.4. Ambient temperature: 24℃Conclusion:Clause Requirement- TestResult - RemarkVerdict______Li-ion Battery had passed altitude simulation test.38.3.4.2 Test 2: Thermal Test P 38.3.4.2.1 Purpose P This test assesses cell and battery seal integrity andinternal electrical connections. The test is conducted using rapid and extreme temperature changes.P38.3.4.2.2 Test procedurePTest temperature and stored hours 1) 75℃, ≥6h 2) -40℃, ≥6h-- The maximum time intervalBetween test temperature extremes is 30 minutes. --Test timesrepeated 10 times -- After which all test cells and batteries are to be storedfor 24 hours at ambient temperature (20±5℃)24℃ -- For large cells and batteries the duration of exposure tothe test temperature extremes should be at least 12 hours.Small batteryN 38.3.4.2.3 RequirementCells and batteries meet this requirement if there is nomass loss, no leakage, no venting, no disassembly, no rupture and no fire and if the open circuit voltage of each test cell or battery after testing is not less than 90% of its voltage immediately prior to this procedure. The requirement relating to voltage is not applicable to test cells and batteries at fully discharged statesNo mass loss, no leakage, no venting, no disassembly, no rupture and no fire. Battery after testing is not less than 90% of its voltageimmediately prior to this procedure.PMass M of Test Battery (g)OCV (V) Group No. M1 (beforet he test) M2(afterthe test)MassLosslimit(0.1%)OCV1 (beforethe test) OCV2 (after the test) OCV (≥90%) 01 21.223g 21.223g 0.00%3.861 3.835 99.33%02 21.178g 21.178g 0.00%3.867 3.854 99.66% 03 21.338g 21.338g 0.00% 3.866 3.845 99.46% Group A (at first cycle, in fully charged states)04 21.385g 21.385g 0.00% 3.862 3.836 99.33% 05 21.142g 21.142g 0.00%3.865 3.842 99.40% 06 21.465g 21.465g 0.00%3.854 3.836 99.53% 07 21.276g 21.276g 0.00%3.860 3.837 99.40% Group B (after fiftycycles ending in fullycharged states)08 21.328g 21.328g 0.00%3.8673.85399.64%Remark1.Mass loss (%)=(M1-M2)/M1*100% (Where M 1 is the mass before the test and M 2 is the mass after the test)2.When mass loss does not exceed the value in Table: Mass loss limit, it shall be considered as "no mass loss".3.The OCV of each test cell after testing is not less than 90% of its voltage immediately prior to this procedure.4. Ambient temperature: 24℃Conclusion:Clause Requirement- TestResult - RemarkVerdict______Li-ion Battery had passed thermal test.38.3.4.3 Test 3: Vibration P 38.3.4.3.1 PurposePThis test simulates vibration during transport..P 38.3.4.3.2 Test procedure PCells and batteries are firmly secured to the platform of the vibration machine without distorting the cells insuch a manner as to faithfully transmit the vibration.-- The vibration shall be a sinusoidal waveform with a logarithmicP Duration 15min -- Frequency range 7Hz... ..200Hz.....7Hz -- Amplitude 0.8mm -- This cycle shall be repeated 12 times for a total of 3 hours for each of three mutually perpendicular mounting positions of the cell.-- 38.3.4.3.3 Requirement PCells and batteries meet this requirement if there is no mass loss, no leakage, no venting, no disassembly, no rupture and no fire and if the open circuit voltage of each test cell or battery after testing is not less than 90% of its voltage immediately prior to this procedure. The requirement relating to voltage is not applicable to test cells and batteries at fully discharged states No mass loss, no leakage,no venting, no disassembly, no rupture and no fire. PMass M of Test Battery (g) OCV (V)GroupNo. M1 (beforet he test) M2(afterthe test)MassLosslimit(0.1%)OCV1 (beforethe test) OCV2 (after the test) OCV (≥90%) 01 21.223g 21.223g 0.00% 3.835 3.835 100.0% 02 21.178g 21.178g 0.00% 3.854 3.854 100.0% 03 21.338g 21.338g 0.00% 3.845 3.845 100.0% Group A (at first cycle, in fully charged states) 04 21.385g 21.385g 0.00% 3.836 3.836 100.0% 05 21.142g 21.142g 0.00% 3.842 3.842 100.0% 06 21.465g 21.465g 0.00% 3.836 3.836 100.0% 07 21.276g 21.276g 0.00% 3.837 3.837 100.0% Group B (after fifty cycles ending in fully charged states)08 21.328g 21.328g 0.00%3.8533.853100.0%Remark1.Mass loss (%)=(M1-M2)/M1*100% (Where M 1 is the mass before the test and M 2 is the mass after the test)2.When mass loss does not exceed the value in Table: Mass loss limit, it shall be considered as "no mass loss".3.The OCV of each test cell after testing is not less than 90% of its voltage immediately prior to this procedure.4. Ambient temperature: 24℃Conclusion:Li-ion Battery had passed vibration test.Clause Requirement- TestResult - RemarkVerdict______38.3.4.4 Test 4: Shock P 38.3.4.4.1 PurposePThis test simulates vibration during transport..P 38.3.4.4.2 Test procedurePTest cells and batteries shall be secured to the testing machine by means of a rigid mount which will support all mounting surfaces of each test battery.This is small batteries. -- a half-sine shock of peak acceleration 150 g P Pulse duration 6ms -- the positive direction followed three times shocks --Each cell or battery shall be subjected to three shocks in the positive direction followed by three shocks in the negative direction of three mutually perpendicular mounting positions of the cell or battery for a total of18 shocks.--38.3.4.4.3 Requirement PCells and batteries meet this requirement if there is no mass loss, no leakage, no venting, no disassembly, no rupture and no fire and if the open circuit voltage of each test cell or battery after testing is not less than 90% of its voltage immediately prior to this procedure. The requirement relating to voltage is not applicable to test cells and batteries at fully discharged states No mass loss, no leakage,no venting, no disassembly, no rupture and no fire. PMass M of Test Battery (g) OCV (V)GroupNo. M1 (beforet he test) M2(afterthe test)MassLosslimit(0.1%)OCV1 (beforethe test) OCV2 (after the test) OCV (≥90%) 01 21.223g 21.223g 0.00% 3.835 3.835 100.0% 02 21.178g 21.178g 0.00% 3.854 3.854 100.0% 03 21.338g 21.338g 0.00% 3.845 3.845 100.0% Group A (at first cycle, in fully charged states) 04 21.385g 21.385g 0.00% 3.836 3.836 100.0% 05 21.142g 21.142g 0.00% 3.842 3.842 100.0% 06 21.465g 21.465g 0.00% 3.836 3.836 100.0% 07 21.276g 21.276g 0.00% 3.837 3.837 100.0% Group B (after fifty cycles ending in fully charged states)08 21.328g 21.328g 0.00%3.8533.853100.0%Remark1.Mass loss (%)=(M1-M2)/M1*100% (Where M 1 is the mass before the test and M 2 is the mass after the test)2.When mass loss does not exceed the value in Table: Mass loss limit, it shall be considered as "no mass loss".3.The OCV of each test cell after testing is not less than 90% of its voltage immediately prior to this procedure.4. Ambient temperature: 24℃Conclusion:Li-ion Battery had passed shock test.Clause Requirement- TestResult - RemarkVerdict______38.3.4.5 Test 5: External Short Circuit P 38.3.4.5.1 PurposePThis test simulates an external short circuit. P 38.3.4.5.2 Test procedurePThe cell or battery to be tested shall be temperature stabilized so that its external case temperature reaches 55℃--Short circuit condition with a total External resistance of less than 0.1ohm--The cell or battery must be observed for a further six hours for the test to be concluded.--This short circuit condition is continued for at least one hour after the cell or battery external case temperature has returned to 55℃-- 38.3.4.5.3 RequirementPCells and batteries meet this requirement if theirexternal temperature does not exceed 170℃ and there is no disassembly, no rupture and no fire within six hours of this test. Battery externaltemperature does notexceed 170℃, and there is no disassembly, no fire and no rupture within six hours of this testPGroupNo.External Highest Temperature(℃)Criteria Result0155.6℃ P 02 55.5℃ P 03 55.7℃ P Group A (at first cycle, in fully charged states)04 55.9℃ P 05 55.6℃ P 06 55.7℃ P 07 55.8℃ P Group B (after fifty cycles ending in fully charged states) 0855.9℃Battery external temperature does not exceed 170℃, and there is no disassembly, no fire and no rupture within six hours of this testPAmbient temperature: 23℃Conclusion:Li-ion Battery had passed external short circuit test.Clause Requirement- TestResult - RemarkVerdict______38.3.4.6 Test 6: ImpactThe test sample Component cell of chargeable batteries. P 38.3.4.6.1 PurposePThis test simulates an impact. P 38.3.4.6.2 Test procedureP - Dropped height 61±2.5cm, -- - mass9.1Kg -- - diameter bar 15.8mm --- Impact position:Prismatic cell is to be impacted with its longitudinal axis parallel to the flat surface and perpendicular to the longitudinal axis of the 15.8 mm diameter curved surface lying across the centre of the test sample, Prismatic cell is also to be rotated 90 degrees around its longitudinal axis so that both the wide and narrow sides will be subjected to the impact.--A coin or button cell is to be impacted with the flat surface of the sample parallel to the flat surface and the 15.8 mm diameter curved surface lying across its centre.38.3.4.6.3 RequirementPCells and batteries meet this requirement if theirexternal temperature does not exceed 170℃ andthere is no disassembly, no rupture and no fire within six hours of this test.Battery external temperature does not exceed 170℃, and there isno disassembly, no fire and no rupture within six hours of this testPGroup No. ExternalHighestTemperature(℃)Criteria Result0178.2℃ P 02 76.3℃ P 03 79.4℃ P 04 80.6℃ P Group A (at first cycle, in fully charged states)05 78.5℃ P 0648.3℃ P 07 47.6℃ P 08 50.3℃ P 09 48.6℃ P Group B (after fifty cycles ending in fullycharged states) 1047.9℃Battery external temperature does not exceed 170℃, and there is no disassembly, no fire and no rupture within six hours of this testPAmbient temperature: 23℃Clause Requirement- TestResult - RemarkVerdict______Conclusion:Li-ion Battery had passed Impact test.38.3.4.7 Test 7: OverchargeP 38.3.4.7.1 PurposePThis test evaluates the ability of a rechargeable battery to withstand an overcharge condition.P 38.3.4.7.2 Test procedurePThe charge current2×900=1800mA, Twice the manufacturer'srecommended maximum continuous charge current--The minimum voltage of the test:--a) The minimum voltage of the test (Themanufacturer ’s recommended charge voltage is not more than 18V).2×4.2=8.4V, the lesser of two times the maximum charge voltage of the battery or 22V,-- Ambient temperature. 24℃ -- The duration of the test.24 hours 38.3.4.7.3 RequirementPRechargeable batteries meet this requirement if there is no disassembly and no fire within seven days of the testThere is no disassemblyand no fire within seven days of the test.PGroupNo. CriteriaResult 01P 02 P 03 P Group A (at first cycle, in fully charged states)04 P 05 P 06 P 07 P Group B (after fifty cycles ending in fully charged states) 08There is no disassembly and no fire within seven days of the test.P Ambient temperature: 24℃Conclusion:Li-ion Battery had passed overcharge test.Clause Requirement- TestResult - RemarkVerdict______38.3.4.8 Test 8: Forced discharge N 38.3.4.8.1 PurposeNThis test evaluates the ability of a primary or a rechargeable cell to withstand a forced discharge condition.-- 38.3.4.8.2 Test procedureNEach cell shall be forced discharged at ambient temperature by connecting it in series with a 12 V DC, power supply at an initial current equal to the maximum discharge current specified by the manufacturer.NThe specified discharge current is to be obtained by connecting a resistive load of the appropriate size and rating in series with the test cell, Each cell shall be forced discharged for a time interval (in hours) equal to its rated capacity divided by the initial test current (in ampere)N38.3.4.8.3 RequirementNPrimary or rechargeable cells meet this requirement if there is no disassembly and no fire within seven days of the test.NPHOTOGRAPHS OF EUT。
SureSelect Target Enrichment RNA Reagent Kit - HSQ

SureSelect Target Enrichment RNA Reagent Kit - HSQ *************(24小时)化学品安全技术说明书GHS化学品标识应急咨询电话(带值班时间)::供应商/ 制造商:安捷伦科技(上海)有限公司中国(上海)外高桥自由贸易试验区英伦路412号(邮编:200131)电话号码: 800-820-3278传真号码: 0086 (21) 5048 2818SureSelect Target Enrichment RNA Reagent Kit - HSQ化学品的推荐用途和限制用途5190-4396 / 5190-4404 / 5190-44125190-4397 / 5190-4405 / 5190-4413SureSelect Hyb 45190-4398 / 5190-4406 / 5190-4414SureSelect Binding Buffer 5190-4399 / 5190-4407 / 5190-4415SureSelect Wash Buffer 15190-4400 / 5190-4408 / 5190-4416SureSelect Wash Buffer 25190-4401 / 5190-4409 / 5190-4417SureSelect Elution Buffer 5190-4402 / 5190-4410 / 5190-4418SureSelect Neutralization Buffer 5190-4403 / 5190-4411 / 5190-4419SureSelect RNase Block 5190-4383 / 5190-4386SureSelect Hyb 35190-4382 / 5190-4385SureSelect Block 25190-4381 / 5190-4384SureSelect Indexing Block 15190-4427 / 5190-4428SureSelect Indexing Block 35190-4445 / 5190-4446SureSelect ILM Indexing Post Capture Forward PCR Primer5190-4441 / 5190-4442SureSelect ILM Index Pre Capture PCR Reverse Primer5190-4443 / 5190-4444SureSelect Adaptor Oligo Mix 5190-4932 / 5190-3619SureSelect Primer 5190-4933 / 5190-3620PCR Primer Index 1-16各种各样的*部件号:物质用途:0.4 ml(毫升) - 12 ml(毫升)0.096 ml(毫升) - 1.25 ml(毫升)SureSelect Hyb 40.208 ml(毫升) - 6.25 ml(毫升)SureSelect Binding Buffer 13.2 ml(毫升) - 400 ml(毫升)SureSelect Wash Buffer 18 ml(毫升) - 240 ml(毫升)SureSelect Wash Buffer 224 ml(毫升) - 720 ml(毫升)SureSelect Elution Buffer0.96 ml(毫升) - 29 ml(毫升)SureSelect Neutralization Buffer 0.96 ml(毫升) - 29 ml(毫升)SureSelect RNase Block 0.016 ml(毫升) - 0.096 ml(毫升)SureSelect Hyb 30.16 ml(毫升) - 0.96 ml(毫升)SureSelect Block 20.045 ml(毫升) - 0.24 ml(毫升)SureSelect Indexing Block 10.045 ml(毫升) - 0.24 ml(毫升)SureSelect Indexing Block 30.012 ml(毫升) - 0.058 ml(毫升)SureSelect ILM Indexing Post Capture Forward PCR Primer0.045 ml(毫升) - 0.27 ml(毫升)SureSelect ILM Index Pre Capture PCR Reverse Primer0.045 ml(毫升) - 0.27 ml(毫升)SureSelect Adaptor Oligo Mix 1.2 ml(毫升) (16 反应)SureSelect Primer0.3 ml(毫升) (16 反应)PCR Primer Index 1-160.0125 ml(毫升) - 0.025 ml(毫升)部件号(化学品试剂盒):G9601A, G9601B, G9601C 注解 *:* PCR Primer Index 1-16: 5190-3021, 5190-3037, 5190-3022, 5190-3038, 5190-3023,5190-3039, 5190-3024, 5190-3040, 5190-3025, 5190-3041, 5190-3026, 5190-3042,5190-3027, 5190-3043, 5190-3028, 5190-3044, 5190-3029, 5190-3045, 5190-3030,5190-3046, 5190-3031, 5190-3047, 5190-3032, 5190-3048, 5190-4423, 5190-4468,5190-4424, 5190-4469, 5190-4425, 5190-4470, 5190-4426, 5190-4471安全技术说明书根据 GB/ T 16483-2008 和 GB/ T 17519-2013物质或混合物的分类根据 GB13690-2009 和 GB30000-2013紧急情况概述SureSelect Hyb 1液体。
一种同时检测工业己烷中正己烷和苯等12种化合物的方法

随着科技的不断进步,工业己烷在化工、食品和日化品等领域的应用量日益增加。
由于己烷可通过呼吸、皮肤接触进入人体并蓄积,长期接触甚至会导致慢性中毒和死亡,存在于工业己烷中的苯对人体的血液、神经、生殖系统具有较强危害,所以最新修订的标准GB/T 17602—2018《工业己烷》[1]对正己烷和苯的含量都做了明确要求。
但其规定的检测方法[2-4]专属性差,干扰多,且不能实现同时测定正己烷和苯。
为了填补这一空白,实验小组以丙酮为内标物,建立了一种GC-MS法[5]同时测定工业己烷中正戊烷、2,2-二甲基丁烷、环戊烷、2,3-二甲基丁烷、2,4-二甲基戊烷、2,2,3-三甲基丁烷、环己烷、2-甲基戊烷、正己烷、3-甲基戊烷、甲基环戊烷11种常见物质和苯的检测方法,为监控工业己烷的质量提供了技术保障。
1 实验部分1.1 主要仪器及试剂Agilent 7890B-5977A型气质联用仪,Agilent 7683B自动进样器;色谱柱,Agilent HP-PONA 柱,50m×0.20mm×0.5um;Sartorius CP225D十万分之一天平;SGE微量注射器,量程分别为0~25 μL、0~100μL、0~250μL、0~500μL、0~1000μL。
正戊烷,含量≥99.3%,Dr.Ehrenstorfer;2,2-二甲基丁烷,含量≥99%,SIGMA;环戊烷,含量≥99 %,SIGMA;2,3-二甲基丁烷,含量≥99%,Alfa Aesar;2-甲基戊烷,含量≥99%,SIGMA;3-甲基戊烷,含量≥99%,SIGMA;正己烷,含量≥99%,SIGMA;甲基环戊烷,含量≥99%,SIGMA;2,4-二甲基戊烷,含量≥99%,梯希爱;2,2,3-三甲基丁烷,含量≥99%,ACROS;苯,含量≥99%,SIGMA;环己烷,含量≥99%,SIGMA;丙酮,色谱纯,SIGMA;异辛烷,含量≥99.5 %,天津科密欧。
M190MWW3 Push mura

M190MWW3 R0 Push mura analysis report
2009-4-16
Prepared by: Jungle wei Date: 4/15/2009
IVO Confidential
1
Problem description
Customer: INNOLUX Project: VA1918 & VA1928 End-buyer: Viewsonic Issue name: Push mura
2009-4-16
IVO Confidential
4
Hale Waihona Puke Summary1, At 4/1 pm, FAE、Viewsonic & Innolux had hold a conference call, the meeting minutes is IVO should shipping new sample to Innolux for PP test, the abnormal panel will be send back to IVO. --- The new sample had arrive at 4/7
external force
2009-4-16
IVO Confidential
3
IVO Push mura judge method
Lab-T, Inc. T300 User Manual

REPORT No.:TRRFCC16-0004FCC ID : 2ACZXT300T User ManualCopyright ⓒ 2016. Lab-T, Inc.4. Press and release the CH UP key to5.enter the automatic code search mode.The remote will transmit the TV power code every 1.5 seconds and the LED will blink at the same time.off, the remote has found a candidate group device code.the code and to exit the search mode.Time Warner Cable Enterprises LLC.assumes no responsibility for errors or omissions that may appear in this guide. We reserve the right to change this guide at any time without notice.Time Warner Cable Enterprises and the Time Warner Cable logo aretrademarks or registered trademarks of Time Warner Cable Enterprises in the U.S. and other countries.Third-party trademarks mentioned are the property of their respective owners.The use of the word partner does not Time Warner Cable Enterprises and any other company.2015 Time Warner Cable Enterprises LLC. All rights reserved.Last Updated: December 2015Printed in ChinaDisclaimerAll TV Setup CodesMost Common TV Codes1. Universal Power:2. TV:The universal power button simply sends the power code for all mapped with the DTA.4. MENU:application if available.5. GUIDE:The Guide button will launch the onscreen Guide UI.6. EXIT:The Exit button will dismiss all on screen UI.7. INFO:or a channel banner if available devices.code to any TV that is programmed.states are out of sync.3. PROG:Press and hold PROG with another button on the remote to RF4CE pair This is intended to allow the user to correct when the TV and DTA power 8. Directional Pad:Up, Down, Right, Left and OK keys 11.Volume:Increases or decreases the volume of the currently programmed TV.9. Last:Quick access to the most recently watched channels.10.Mute:on the currently programmed TV.12.Channel::This button toggles on/off the closed 15.INPUT:on the currently programmed TV.13.Number Keys:Understanding Your Remote Control KeysThe unified DTA Remote controls your Cisco, Arris and Technicolor DTA and the Volume, Mute, Power,Input on many connected TVs. Use the instructions in this guide to set up and use your unified DTA remote.WelcomeUse two AAA batteries.Check the diagram inside the battery compartment to ensure the batteries are inserted in the correct direction.BatteriesThis device complies with Part 15 of the FCC Rules.Operation is subject to the following two conditions:(1) This device may not cause harmful interference, and(2) This device must accept any interference received, including interference that may cause undesired operation.NOTE:tested and found to comply with the limits for a Class B digital FCC Rules.FCC Notice- Insert batteries correctly.There may be a risk of explosion if the batteries are incorrectly inserted.- Do not attempt to recharge ‘disposable’ or ‘non-reusable’batteries.- Please follow instructions provided for charging ‘rechargeable’ batteries.- Replace batteries with the same or equivalent type that we recommend.- Do not expose batteries to excessive heat (such as sunlight of fire).- Do not expose batteries to temperatures above 100(212).The batteries may containperchlorate, a known hazardous substance, so special handling and disposal of this product might be necessary. For more informaion about perchlorate and best management practices for perchlorate-containingsubstance, see /hazardouswaste/perchlorateThese limits are designed to provide reasonable protection against harmful interference in a residential installation.This equipment generates, uses and can radiate radio frequency energy and if not installed and used in accordance with the instructions, may cause harmful interference to radio communications.However, there is no guarantee that interference will not occur in a particular installation.If this equipment does cause harmful interference to radio or television reception, which can be determined by turning the equipment off and on, the user is encouraged to try to correct the interference by one or more of the following measures:- Reorient or relocate the receiving antenna.-the equipment and receiver.- Connect the equipment into an outlet on a circuit different from that to which the receiver is connected.- Consult the dealer or anexperienced radio/TV technician for help.The changes or modifications not expressly approved by the party responsible for compliance could void the user's authority to operate the equipment.CAUTION:Exposure to RadioFrequency Radiation.Antenna shall be mounted in such a manner to minimize the potential for human contact during normal operation.The antenna should not be contacted during operation exceeding the FCC radio frequency exposure limit.Battery SafetyThe batteries may contain substances that could be harmful to the environment.Recycle or dispose of batteries in accordance with the battery manufacturer’s instructions and local/national disposal and recycling regulations.Battery DisposalThis product may contain disposable batteries. Heed the following warning and follow the Battery Safety and Battery Disposal instructions below.BatteriesHandling Disposable Automatic Code Search begins with the through all device in the brand list.If the device code for your TV brand can’t be found in the list, please try to search the device code database using the following steps:1. Turn on your TV.3. Release the PROG key and make sure the RED LED stays on.Automatic Code SearchThis remote control is initiallyprogrammed for the Cisco DTA. Use to use the remote to control Arris,Technicolor DTA and your TV.Important Information about Your RemoteYour remote control can be paired with 1. Turn on the target (DTA) device.2. Press the PROG key for three seconds or until the RED LED turns on and then press "INFO" key.3. The LED on the remote control blinks mode.4. The DTA will prompt the user, via a message on the TV to enter a unique three (3) digit number.5. If the three digits entered are correct,the target (DTA) will prompt the user that pairing is successful.the DTA, allowing you to place the DTA out of the line of sight(behind your TV, for example)RF Pairing between Remote Control and DTA6. If the three digits entered are incorrect,the target (DTA) will prompt the user that pairing fails and to try again until the correct code is entered.Long Key Press Limit1.2.If you want to restore all settings to a default status or unpair the RCU,follow the steps below:Factory Reset(including RF unpairing)Technicolor, and Cisco box.Default state: the remote is initially Follow the steps below to program the remote to match DTA vendor:1. Check the brand of DTA box you have3. If your DTA box is Cisco, press the [101] keyIf your DTA box is Arris, press the [102] keyIf your DTA box is Technicolor, press the [103] keyassigned to control DTA VOLUME and MUTE.Your remote control can be used to control three DTAs which are Arris,Programming the remote to match DTA vendor1. Press and hold the PROG key for three seconds or until the RED LED turns on.2. Press [VOL+] key.If you want to lock Volume and Mute to your TV, follow the steps below:Locking Volume/Mute Controls to your TVProgram the remote control for TV following these steps:1. Turn on your TV.5. Find the four (4) digit device code for your TV brand.keys in order.code will be retained.If the device code is invalid, the REDWhile in programming mode, if a key is not pressed within 15 seconds, the remote exits the programming mode and doesn't make any changes.Program the Remote to Control Your TVWarning: There is danger of explosion if the battery is mishandled or incorrectly replaced. Replace only with the same type of battery.Do not crush, puncture,dispose of in fire, short the external contacts, or expose to water or other liquids.Dispose of the battery in accordance with localregulations and instructions from your service provider.User Manual for TWC, Unified DTA Remote Control1. Press and hold the PROG key for three seconds or until the RED LED turns on.If you want to lock Volume and Mute to your DTA, follow the steps below:Locking Volume/Mute Controls to your DTA2. Press [VOL ] key.Caution: This remote control should be kept out of reach of the children under the age of 6.124691114571012831315。
Phyton 35 产品安全数据表说明书

PHYTON CORPORATIONSAFETY DATA SHEETProduct Identity: Phyton 35Recommended use: Restrictions on Use: Bactericide and FungicideUse only as directed on the product label.Supplier: Phyton CorporationP.O. Box 385370Minneapolis, MN 55438Telephone: +1 (952) 378-1157, 800-356-8733Emergency Phone:For Chemical EmergencySpill, Leak, Fire, or AccidentCall CHEMTREC Day or NightWithin USA and Canada: 1-800-424-9300Outside USA and Canada: +1 703-527-3887 (collect calls accepted)GHS Classification:GHS Label Elements:DANGER!Statements of Hazard ResponseCauses serious eye irritation. Very toxic to aquatic life with long lasting effects.PreventionWash hands thoroughly after handling.Avoid release to the environment.Wear eye protection.DisposalDispose of contents and container in accordance withlocal, regional, and national regulations.IF IN EYES: Rinse cautiously with water for several minutes.Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice or attention. Collect spillage.Eye: First check victim for contact lenses and remove if present. Flush victim's eyes with large quantities of water for at least 15 minutes, holding the eyelids apart. Get medical attention if irritation persists.Skin: Remove contaminated clothing. Wash skin thoroughly with soap and water. If irritation or symptoms develop, get medical attention. Launder clothing before re-use. (Discard contaminated shoes)Ingestion: Do not induce vomiting unless directed by a medical professional. If conscious, rinse mouth with water. Never give anything by mouth to an unconscious or convulsing person. Get medical attention if symptoms occur or if the victim feels unwell.Inhalation: Immediately remove victim to fresh air. Get medical attention if symptoms occur and persist.Most important Symptoms: Causes eye irritation. May Cause skin irritation. Ingestion may cause headache, dizziness, nausea, vomiting, irregular heartbeat, respiratory difficulty, metallic taste, gastrointestinal bleeding, liver and kidney failure.Indication of immediate medical attention/special treatment: Immediate medical attention is not generally required.Suitable (and Unsuitable) Extinguishing Media: Use water spray, carbon dioxide, foam or dry chemical. Specific hazards arising from the chemical: This product is not classified as flammable but may burn under fire conditions. Combustion may produce ammonia, copper fumes and oxides of carbon, sulfur and nitrogen. Special Protective Equipment and Precautions for Fire-Fighters: Firefighters should wear positive pressure self-contained breathing apparatus and full protective clothing. Cool fire exposed containers with water spray. Do not allow run-off from fire fighting to enter drains or water courses.Personal Precautions, Protective Equipment, and Emergency Procedures: Evacuate spill area and keep unprotected personnel away. Do not breathe vapors or mists.Avoid contact with the eyes, skin and clothing. Wear appropriate protective clothing.Methods and Materials for Containment and Cleaning Up: Contain and collect using inert absorbent materials and place in appropriate containers for disposal. Do not flush to sewer! Report releases as required by local, state and federal authorities. Contact your local pesticide, environmental control agency, or hazardous waste representative for advice.Precautions for Safe Handling: Avoid contact with the eyes, skin and clothing. Avoid breathing vapors or mists. Wear appropriate protective clothing and equipment. Use only with adequate ventilation. Wash thoroughly with soap and water after handling. Keep containers closed when not in use.Do not cut, drill, grind or weld on or near containers, even empty containers. Do not reuse containers. Empty containers retain product residues which can be hazardous. Follow all SDS precautions when handling empty containers.Conditions for Safe Storage, Including Any Incompatibilities: Do not contaminate water, food or feed by storage or disposal. Do not freeze or store below 45°F. Store in original container. Store away from oxidizers and other incompatible materials.Engineering Controls: Use with adequate ventilation to maintain exposures below the occupational exposure limits.Personal Protective Equipment: Refer to the product label for additional requirements for pesticide use. Respiratory Protection: In operations where exposure levels are excessive, an approved respirator withdust/mist cartridges or supplied air respirator should be used. Respirator selection and use should be based on contaminant type, form and concentration. Follow applicable regulations and good Industrial Hygiene practice. Skin Protection: Wear impervious gloves.Eye Protection: Chemical safety goggles should be worn.Other: Impervious coveralls, apron and boots is recommended to prevent skin contact and contamination of personal clothing. An eye wash should be available in the immediate work area.Appearance and Odor: Opaque brown-green liquid.Reactivity: Not normally reactiveChemical Stability: Stable under normal storage and handling conditions.Possibility of Hazardous Reactions: Reaction with oxidizers may generate heat.Conditions to Avoid: Avoid heat, flames and sources of ignition.Incompatible Materials: Avoid oxidizing agents.Hazardous Decomposition Products: Thermal decomposition yields ammonia and oxides of nitrogen, carbon, copper and sulfur.HEALTH HAZARDS:Eye: Contact may cause irritation with redness, tearing and stinging Moderate irritant in rabbit study.Skin: May causes mild skin irritation. Repeated or prolonged contact may cause defatting of the skin and dermatitis. Slight irritant in rabbits. Low toxicity in rabbits.Ingestion: Ingestion may cause headache, dizziness, nausea, vomiting, diarrhea, irregular heartbeat, respiratory difficulty, metallic taste, gastrointestinal bleeding, liver and kidney failure.Inhalation: Inhalation of vapors or mists may cause mucous membrane and respiratory irritation and effects similar to ingestion.Chronic: None known.Sensitization: Negative in the guinea pig Buehler test.Carcinogenicity: None of the components are listed as a carcinogen or suspected carcinogen by IARC, NTP, ACGIH, OSHA or the EU Substance Directive.Germ Cell Mutagenicity: Components are not germ cell mutagens.Reproductive Toxicity: Components are not reproductive toxins.Numerical Measures of Toxicity:Copper (II) Sulfate: Oral rat LD50 481 mg/kgProduct Test Data: Oral rat LD50 >5000 mg/kg; Dermal rabbit LD50 >5000 mg/kg; Inhalation rat LD50 >2.06 mg/L (no mortalities at highest attainable dose)Ecotoxicity:Copper (II) Sulfate – LC50 Cyprinus carpio – 810 ug/L/96 hr; LC50 freshwater trout – 190-210 ug/L/96 hr; This product is classified as highly toxic to the aquatic environment with long-term effects. Releases to the environment should be avoided.Persistence and Degradability: Not expected to be readily biodegradable.Bioaccumulative Potential: No data available.Mobility in Soil: No data available.Other Adverse Effects: No data availablePesticide wastes are acutely toxic. Improper disposal of excess pesticide, spray mixture, or rinsate is a violation of federal law. If these wastes cannot be disposed of by use according to label instructions, contact your State Pesticide or Environmental Control Agency, or the Hazardous Waste representative at the nearest EPA Regional Office for guidance. Open dumping is prohibited.CONTAINER DISPOSAL: Nonrefillable container. Do not reuse or refill this container. Triple rinse container (orequivalent) promptly after emptying. Triple rinse as follows: Empty the remaining contents into application equipment or a mix tank and drain for 10 seconds after the flow begins to drip. Fill the container 1/4 full with water and recap. Shake for 10 seconds. Pour rinsate into application equipment or a mix tank or store rinsate for later use or disposal. Drain for 10 seconds after the flow begins to drip. Repeat this procedure two more times. Then offer for recycling or reconditioning, or puncture and dispose of in a sanitary landfill, or, if allowed by State and local authorities, by burning. If burned, stay out of smoke.DOT Hazardous Materials Description: This product is not regulated for transport in containers less than 46 lbs. For containers with more than 46 lbs, the following shipping information applies:Proper Shipping Name: Environmentally hazardous substance, liquid, n.o.s. (Copper (II) Sulfate), RQUN Number: UN3082Hazard Class/Packing Group: 9, IIILabels Required: Class 9, Marine PollutantIMDG Shipping Name: Environmentally hazardous substance, liquid, n.o.s. (Copper (II) Sulfate)IMDG Hazard Class: 9, IIIUN Number: UN3082IMDG Hazard Labels Required: Class 9, Marine PollutantIATA Shipping Name: Environmentally hazardous substance, liquid, n.o.s. (Copper (II) Sulfate)IATA Hazard Class: 9, PG IIIUN Number: UN3082IATA Hazard Labels Required: Class 9, EHS MarkEPA FIFRA INFORMATIONThis chemical is a pesticide product registered by the United States Environmental Protection Agency and is subject to certain labeling requirements under federal pesticide law. These requirements differ from the classification criteria and hazard information required for safety data sheets (SDS), and for workplace labels of non-pesticide chemicals. The hazard information required on the pesticide label is reproduced below. The pesticide label also includes other important information, including directions for use.WARNING: Causes substantial but temporary eye injury. Harmful if swallowed or absorbed through the skin. Do not get into eyes or on clothing. Avoid contact with skin.CERCLA 103 Reportable Quantity: This product has a Reportable Quantity (RQ) of 46 lbs. (based on the RQ for Copper (II) Sulfate of 10 lbs present at 21.27%). Releases above the RQ must be reported to the National Response Center. Many states have more stringent release reporting requirements. Report spills required under federal, state and local regulations.Hazard Category for Section311/312: Acute HealthSection313Toxic Chemicals: This product contains the following chemicals subject to SARA Title III Section 313 Reporting requirements:Copper (II) Sulfate 7758-98-7 21.27%Section302 Extremely Hazardous Substances (TPQ): NoneSDS Date of Preparation: June 1, 2015NOTICEThis above information is believed to be correct but does not propose to be all inclusive and shall be used only as a guide. Phyton Corporation shall not be held liable for any damage resulting from handling or from contact with the above product. This information relates only to the product designated herein and does not relate to its use in combination with any other material or process.。
Incucyte

Product Information Presentation, Storage and StabilityThe Incucyte® Fabfluor-pH Antibody Labeling Reagents for antibody internalization are supplied as lyophilized solids in sufficient quantity to label 50 μg of test antibody, when used at the suggested molar ratio (1:3 of test antibody to labeling Fab). The lyophilized solid can be stored at 2-8° C for one year. Once re-hydrated, any unused reagent should be aliquoted and stored at -80° C for up to one year. Avoid repeated freeze-thaw cycles.Incucyte® Fabfluor-pH Antibody Labeling ReagentsFor Antibody Internalization AssaysAntibody Labeling Reagent Rehydrated: -80° C *Excitation and Emission maxima were determined at a pH of 4.5.Fabfluor_quick_guideBackgroundIncucyte ® Fabfluor-pH Antibody Labeling Reagents are designed for quick, easy labeling of Fc-containing test antibodies with a Fab fragment-conjugated pH-sensitive fluorophore. The pH-sensitive dye based system exploits the acidic environment of the lysosomes to quantify in-ternalization of the labeled antibody. As Fabfluor labeled antibodies reside in the neutral extracellular solution (pH 7.4), they interact with cell surface specific antigens and are internalized. Once in the lysosomes, they enter an acidic environment (pH 4.5–5.5) and a substantial in-crease in fluorescence is observed. In the absence of ex-pression of the specific antigen, no internalization occurs and the fluorescence intensity of the labeled antibodies remains low. With the Incucyte ® integrated analysis soft-ware, background fluorescence is minimized. These reagents have been validated for use with a number of different antibodies in a range of cell types. The Incucyte ® Live-Cell Analysis System enables real-time, kinetic eval -uation of antibody internalization.Recommended UseWe recommend that the Incucyte ® Fabfluor-pH Antibody Labeling Reagents are prepared at a stock concentration of 0.5 mg/mL by the addition of 100 μL of sterile water and triturated (centrifuge if solution not clear). The reagent may then be diluted directly into the labeling mixture with test antibody. Do NOT sonicate the solution.Additional InformationThe Fab antibody was purified from antisera by a combination of papain digestion and immunoaffinity chromatography using antigens coupled to agarose beads. Fc fragments and whole IgG molecules have been removed.Human Red (Cat. No. 4722) or Human Orange (Cat. No. 4812)—Based on immunoelectrophoresis and/ or ELISA, the antibody reacts with the Fc portion of human IgG heavy chain but not the Fab portion of human IgG. No antibody was detected against human IgM, IgA or against non-immunoglobulin serum proteins. The anti-body may cross-react with other immunoglobulins from other species.Mouse IgG1 (Cat. No. 4723), IgG2a (Cat. No. 4750) or IgG2b (Cat. No. 4751)—Based on antigen-binding assay and/or ELISA, the antibody reacts with the Fc portion of mouse IgG, IgG2a or IgG2b, respectively, but not the Fab portion of mouse immunoglobulins. No antibody was detected against mouse IgM or against non–immunoglobulin serum proteins. The antibody may cross-react with other mouse IgG subclasses or with immunoglobulins from other species.Rat (Cat. No. 4737)—Based on immunoelectrophoresis and/or ELISA, the antibody reacts with the Fc portion of rat IgG heavy chain but not the Fab portion of rat IgG. No antibody was detected against rat IgM, IgA or against non-immunoglobulin serum proteins. The antibody may cross-react with other immunoglobulins from other species.A.B.C.D.R e d O b j e c t A r e a (x 105 μm 2 p e r w e l l )Time (hours)A U C x 106 (0–12 h )log [α–CD71] (g/mL)Example DataFigure 1: Concentration-dependent increase in antibody internalization of Incucyte ® Fabfluor labeled-α-CD71 in HT1080 cells. α-CD71 and mouse IgG1 isotype control were labeled with Incucyte ® Mouse IgG1 Fabfluor-pH Red Antibody Labeling Reagent. HT1080 cells were treated with either Fabfluor-α-CD71 or Fabfluor-IgG1 (4 μg/mL); HD phase and red fluorescence images were captured every 30 minutes over 12 hours using a 10X magnification. (A) Images of cells treated with Fabfluor-α-CD71 display red fluorescence in the cytoplasm (images shown at 6 h). (B) Cells treated with labeled isotype control display no cellular fluorescence. (C) Time-course of Fabfluor-α-CD71 internalization with increasing concentrations of Fabfluor-α-CD71 (progressively darker symbols). Internalization has been quantified as the red object area for each time-point. (D) Concentration response curve to Fabfluor-α-CD71. Area under the curve (AUC) values have been determined from the time-course shown in panel C (0-12 hours) and are presented as the mean ± SEM, n=3 wells.CD71-FabfluorIgG-FabfluorProtocols and ProceduresMaterialsIncucyte® Fabfluor-pH Antibody Labeling ReagentTest antibody of interest containing human, mouse, or rat IgG Fc region (at known concentration)Target cells of interestTarget cell growth mediaSterile distilled water96-well flat bottom microplate (e.g. Corning Cat. No. 3595) for imaging96-well round black round bottom ULA plate (e.g. Corning Cat. No. 45913799) or amber microtube (e.g. Cole Parmer Cat. No. MCT-150-X, autoclaved) for conjugation step0.01% Poly-L-Ornithine (PLO) solution (e.g. Sigma Cat. No. P4957), optional for non-adherent cells Recommended control antibodiesIt is strongly recommended that a positive and negative control is run alongside test antibodies and cell lines. For example, CD71, which is a mouse anti-human antibody, is recommended as a positive control for the mouse Fab.Anti-CD71, clone MEM-189, IgG1 e.g. Sigma Cat. No. SAB4700520-100UGAnti-CD71, clone CYG4, IgG2a e.g. BioLegend Cat. No. 334102Isotype controls, depending on isotype being studied—Mouse IgG1, e.g. BioLegend Cat. No. 400124, Mouse IgG2a e.g. BioLegend Cat. No. 401501Preparation of Incucyte® Antibody Internalization Assay 1. Seed target cells of interest1.1 Harvest cells of interest and determine cell concentra-tion (e.g. trypan blue + hemocytometer).1.2 Prepare cell seeding stock in target cell growth mediawith a cell density to achieve 40–50% confluence be-fore the addition of labeled antibodies. The suggested starting range is 5,000–30,000 cells/well, although the seeding density will need to be optimized for each cell type.Note: For non-adherent cell types, a well coating may be required to maintain even cell distribution in the well. For a 96-well flat bottom plate, we recommend coating with 50 μL of either 0.01% Poly-L-Or-nithine (PLO) solution or 5 μg/mL fibronectin diluted in 0.1% BSA.Coat plates for 1 hour at ambient temperature, remove solution from wells and then allow the plates to dry for 30-60 minutes prior to cell addition.1.3 Using a multi-channel pipette, seed cells (50 µL perwell) into a 96-well flat bottom microplate. Lightly tapplate side to ensure even liquid distribution in well. Toensure uniform distribution of cells in each well, allowthe covered plate sit on a level surface undisturbed at room temperature in the tissue culture hood for 30minutes. After cells are settled, place the plate insidethe Incucyte® Live-Cell Analysis System to monitor cell confluence.Note: Depending on cell type, plates can be used in assay once cells have adhered to plastic and achieved normal cell morphology e.g.2-3 hours for HT1080 or 1-2 hours for non-adherent cell types. Some cell types may require overnight incubation.2. Label Test Antibody2.1 Rehydrate the Incucyte® Fabfluor-pH Antibody Label-ing Reagent with 100 µL sterile water to result in a final concentration of 0.5 mg/mL. Triturate to mix (centrifuge if solution is not clear).Note: The reagent is light sensitive and should be protected fromlight. Rehydrated reagent can be aliquoted into amber or foilwrapped tubes and stored at -80° C for up to 1 year (avoid freezing and thawing).2.2 Mix test antibody with rehydrated Incucyte® Fabfluor–pH Antibody Labeling Reagent and target cell growth media in a black round bottom microplate or ambertube to protect from light (50 µL/well).a. Add test antibody and Incucyte® Fabfluor–pH Anti-body Labeling Reagent at 2X the final concentration.We suggest optimizing the assay by starting with afinal concentration of 4 µg/mL of test antibody or theFabfluor-pH Antibody Labeling Reagent (i.e. 2Xworking concentration = 8 µg/mL).Note: A 1:3 molar ratio of test antibody to Incucyte® Fabfluor-pHAntibody Labeling Reagent is recommended. The labeling re-agent is a third of the size of a standard antibody (50 and 150KDa, respectively). Therefore, labeling equal quantities will pro-duce a 1:3 molar ratio of test antibody to labeling Fab.b. Make sufficient volume of 2X labeling solution for50 µL/well for each sample. Triturate to mix.c. Incubate at 37° C for 15 minutes protected from light.Note: If performing a range of concentrations of test antibody,e.g. concentration response-curve, it is recommended to createthe dilution series post the conjugation step to ensure consistentmolar ratio. We strongly recommend the use of both a negativeand positive control antibody in the same plate.3. Add labeled antibody to cells3.1 Remove cell plate from incubator.3.2 Using a multi-channel pipette, add 50 µL of 2X labeledantibody and control solutions to designated wells.Remove any bubbles and immediately place plate in the Incucyte® Live-Cell Analysis System and start scanning.Note: To reduce the risk of condensation formation on the lid priorto first image acquisition, maintain all reagents at 37° C prior toplate addition.4. Acquire images and analyze4.1 In the Incucyte® Software, schedule to image every15-30 minutes, depending on the speed of the specific antibody internalization.a Scan on schedule, standard. If the Incucyte® Cell-by-Cell Analysis Software Module (Cat. No. 9600-0031)is available, adherent cell-by-cell or non-adherentcell-by-cell scan types can be selected.b Channel selection: select “phase” and “red” or“phase” and "orange” (depending on reagent used).c Objective: 10X or 20X depending on cell types used,generally 10X is recommended for adherent cells,and 20X for non-adherent or smaller cells.NOTE: The optional Incucyte® Cell-by-Cell Analysis SoftwareModule enables the classification of cells into sub-populationsbased on properties including fluorescence intensity, size andshape. For further details on this analysis module and its appli-cation, please see: /cell-by-cell.4.2 To generate the metrics, user must create an AnalysisDefinition suited to the cell type, assay conditions andmagnification selected.4.3 Select images from a well containing a positiveinternalization signal and an isotype control well(negative signal) at a time point where internalizationis visible.4.4 In the Analysis Definition:Basic Analyzer:a. Set up the mask for the phase confluence measurewith fluorescence channel turned off.b. Once the phase mask is determined, turn the fluores-cence channel on: Exclude background fluorescencefrom the mask using the background subtractionfeature. The feature “Top-Hat” will subtract localbackground from brightly fluorescent objects withina given radius; this is a useful tool for analyzing ob-jects which change in fluorescence intensity overtime.i The radius chosen should reflect the size of thefluorescent object but contain enough backgroundto reliably estimate background fluorescence inthe image; 20-30 μm is often a useful startingpoint.ii The threshold chosen will ensure that objectsbelow a fluorescence threshold will not bemasked.iii Choose a threshold in which red or orange objectsare masked in the positive response image but lownumbers in the isotype control, negative responsewell. For a very sensitive measurement, for example,if interested in early responses, we suggest athreshold of 0.2.NOTE: The Adaptive feature can be used for analysis but maynot be as sensitive and may miss early responses. If interestedin rate of response, Top-Hat may be preferable.Cell-by-Cell (if available):a. Create a Cell-by-Cell mask following the softwaremanual.b. There is no need to separate phase and fluorescencemasks. The default setting of Top-Hat No Mask forthe fluorescence channel will enable backgroundsubtraction without generation of a mask. Ensurethat the Top-Hat radius is set to a value higher thanthe radius of the larger clusters to avoid excess back-ground subtraction.c. The threshold of fluorescence can be determined inCell-by-Cell Classification.Specifications subject to change without notice.© 2020. All rights reserved. Incucyte, Essen BioScience, and all names of Essen BioScience prod -ucts are registered trademarks and the property of Essen BioScience unless otherwise specified. Essen BioScience is a Sartorius Company. Publication No.: 8000-0728-A00Version 1 | 2020 | 04Sales and Service ContactsFor further contacts, visit Essen BioScience, A Sartorius Company /incucyte Sartorius Lab Instruments GmbH & Co. KGOtto-Brenner-Strasse 20 37079 Goettingen, Germany Phone +49 551 308 0North AmericaEssen BioScience Inc. 300 West Morgan Road Ann Arbor, Michigan, 48108USATelephone +1 734 769 1600E-Mail:***************************EuropeEssen BioScience Ltd.Units 2 & 3 The Quadrant Newark CloseRoyston Hertfordshire SG8 5HLUnited KingdomTelephone +44 (0) 1763 227400E-Mail:***************************APACEssen BioScience K.K.4th floor Daiwa Shinagawa North Bldg.1-8-11 Kita-Shinagawa Shinagawa-ku, Tokyo 140-0001 JapanTelephone: +81 3 6478 5202E-Mail:*************************5. Analysis GuidelinesAs the labeled antibody is internalized into the acidic environment of the lysosome, the area of fluorescence intensity inside the cells increases.This can be reported in two ways:Ways to Report Basic AnalyzerCell-by-Cell Analysis* To correct for cell proliferation, it is advisable to normalize the fluorescence area to the total cell area using User Defined Metrics.For Research Use Only. Not For Therapeutic or Diagnostic Use.LicensesFor non-commercial research use only. Not for therapeutic or in vivo applications. Other license needs contact Essen BioS cience.Fabfluor-pH Red Antibody Labeling Reagent: This product or portions thereof is manufactured under license from Carnegie Mellon University and U.S. patent numbers 7615646 and 8044203 and related patents. This product is licensed for sale only for research. It is not licensed for any other use. There is no implied license hereunder for any commercial use.Fabfluor-pH Orange Antibody Labeling Reagent: This product or portions thereof is manufactured under a license from Tokyo University and is covered by issued patents EP2098529B1, JP5636080B2, US8258171, and US9784732 and related patent applications. This product and related products are trademarks of Goryo Chemical. Any application of above mentioned technology for commercial purpose requires a separate li -cense from: Goryo Chemical, EAREE Bldg., SF Kita 8 Nishi 18-35-100, Chuo-Ku, Sapporo, 060-0008 Japan.SupportA complete suite of cell health applications is available to fit your experimental needs. Find more information at /incucyte Foradditionalproductortechnicalinformation,************************************************************/incucyte。
Agilent Biodiesel Analyzers

Solutions for biodiesel –amazing precision thatanyone can achieve Agilent Biodiesel Analyzers•Ready-to-go solutions that ensure precision•Industry-leading knowledge built into eachanalyzer•Unrivaled, highest quality hardware andsoftware, expertly configuredBiodiesel is a motor or heating fuel produced from vegetable oils oranimal fats. The high cost and limited availability of crude oil make biodiesel and other renewable fuels a viable way to replace, supplement or extend traditional petroleum fuels.A process called transesterification produces biodiesel. The vegetable oil is reacted with methanol in the presence of a catalyst. This produces a mixture of fatty acid methyl esters (FAME) and glycerin. Once the glycerin and any other contaminants are removed, the remaining FAME mixture is pure biodiesel.Depending on the oil source, a typical biodiesel contains FAME mixtures that have both saturated and unsaturated carbon chains from C8 to C24.Responding to a fast-growing renewable fuel sourceAgilent Technologies has developed a comprehensive set of four biodiesel analyzers all compliant with ASTM and CEN standards.Method compliance made simple by Agilent’s superior precision!Measuring Glycerin and Glycerides in Pure Biodiesel B100 – EN 14105/ASTM D6584Analysis of soy biodiesel using ASTM D6584/E14105 methods. Inset shows improved peak shape using a retention gap and Microfluidics connector.Repeatability for Soybean Oil Biodiesel. Exceeds EN14105 specifications.Free glycerin 0.00160.00010.00010.00010.0001Monoglycerides 0.03800.00500.00700.00700.0000Diglycerides 0.03600.00800.00800.01400.0000Triglycerides 0.05880.00770.00450.00950.0040Total glycerin0.01740.00340.00350.00470.0002• Retention gap significantly improves peak shape for better accuracy, reproducibility as well as extend column life• Agilent’s “Ultimate” union joins retention gap to column for high temperature, leak free, inert, and easy to use connections.• Uses a standard autosampler syringe instead of special narrow bore syringe – improving reliability and precision• Exceeds ASTM and CEN specifications for calibration and precision.This method ensures the key FAME compounds are in the biodiesel in the correct proportion. Once again, the Agilent Solution for this simple method exceeds the precision requirements of the method.A complex sample matrix makes this analysis difficult. However, it is possible to separate the individual FAME compounds from the petroleum hydrocarbons using two-dimensional GC. Existing methods for analyzing only B2 blends requires a complicated and costly sample preparation before GC analysis. Agilent has developed a new 2-D GC method that uses a “Microfluidics” Deans Switch to easily and reproducibly perform this analysis. This method can be used for commercial blended biodiesel fuels from B1 to B25 with no sample preparation.A key ingredient in all of these analyzers:Agilent innovation.Improved Analysis of Commercial Biodiesel Blends Using Two Dimensional GCBreak through for analyzing biodiesel components in commercial blended fuel – An alternative to EN 14331 method2-D GC Analysis of B20 biodiesel sample using a new Agilent method employing Microfluidics Deans Switch. Exceeds EN 14331 precision specifications.C16:00.500.03C18:10.600.06C18:30.400.02CompliantwithASTM and CENstandardsReady-to-go Solutions that Ensure PrecisionTo learn more; visit/chemOr contact your Agilent Representative or Agilent authorized distributorInformation, descriptions, and specifications in this publication are subject to change without notice.© Agilent Technologies, Inc. 2006Printed in Germany, November 1, 2006 Publication Number 5989-5945ENAll of the Agilent Biodiesel Solutions come equipped with columns and consumables so analysts can quickly and easily start using their systems to produce results for this fast growing industry. Biodiesel will be even more important in the future. Now is the time to learn more about theseexcellent solutions for measuring the quality of biodiesel fuels. So take a moment to investigate these applications and their Agilent Solutions.Biodiesel solutions from Agilent:• Determination of free and total Glycerin andGlycerides: EN14105/ASTM D6584• FAME Determination of Ester and Linoleic Acid Methyl Ester Content: EN14103• Determination of Trace Methanol in Biodiesel:EN14110• Muicrofludic dean switch for biodiesel blends analysis: Breakthrough alternative to EN 14331• Simulated Distillation of biodiesel: ASTM D2887• Elemental contamination in biodiesel by ICP-MS: EN 14107; EN 14108; EN 14109,and EN14538。
lab on a chip

lab on a chipLab on a Chip: A Revolution in Laboratory TestingIntroductionIn recent years, the field of laboratory testing has witnessed a significant revolution with the advent of microfluidic technology. Lab on a Chip, also known as micro total analysis systems (µTAS), has emerged as a promising solution to miniaturize and automate laboratory processes onto a microscale platform. This revolutionary technology has the potential to transform various fields, including medical diagnostics, environmental monitoring, and drug discovery. In this document, we will explore the concept of Lab on a Chip and its applications, as well as the advantages and challenges associated with this technology.What is Lab on a Chip?Lab on a Chip refers to the integration of multiple laboratory functions onto a single microchip. It utilizes microfluidics, the science and technology of manipulating and controlling fluids on a microscale, to perform precise and accurateanalysis. A typical Lab on a Chip device consists of a network of microchannels and chambers where fluids can be transported, mixed, separated, and analyzed. The small size and scalability of Lab on a Chip devices enable high-throughput analysis and allow for the integration of various laboratory processes that were previously conducted in separate, larger-scale systems.Applications of Lab on a Chip1. Medical Diagnostics:One of the most significant applications of Lab on a Chip technology is in the field of medical diagnostics. Lab on a Chip devices can perform a wide range of diagnostic tests, including blood tests, DNA analysis, and immunoassays, with minimal sample volume and quick turnaround time. These devices offer the potential for point-of-care diagnostics, providing faster results and reducing the need for laboratory infrastructure. Lab on a Chip technology can revolutionize healthcare delivery, especially in resource-limited settings, by enabling rapid and cost-effective diagnosis of various diseases.2. Environmental Monitoring:Lab on a Chip devices can also be used for environmental monitoring. They can be designed to analyze water quality, detect pathogens in food, and monitor air pollution. These devices offer real-time monitoring capabilities and can provide accurate measurements of various environmental parameters. Lab on a Chip technology can enhance our ability to detect and respond to environmental hazards promptly, thereby contributing to the protection of public health and the environment.3. Drug Discovery:In the field of drug discovery, Lab on a Chip technology has the potential to accelerate the development of new drugs. These devices can be used to screen thousands of compounds simultaneously, enabling high-throughput screening of potential drug candidates. Lab on a Chip devices can also mimic the complexity of human organs on a microscale, allowing researchers to study drug effects in a more physiologically relevant environment. This technology can improve the efficiency and success rate of drug discovery, ultimately leading to the development of safer and more effective therapies.Advantages of Lab on a Chip1. Miniaturization:The miniaturization of laboratory processes onto a microchip offers several advantages. Lab on a Chip devices require smaller sample volumes, reducing the need for expensive reagents. The small size also enables faster reaction times, leading to shorter analysis time. Additionally, the portability of Lab on a Chip devices allows for on-site testing and remote diagnostics, saving time and resources.2. Automation:Lab on a Chip technology enables the automation of laboratory processes, reducing human error and increasing efficiency. The integration of multiple functions onto a single chip eliminates the need for complex, time-consuming manual operations. This automation enables rapid, parallel analysis and eliminates the need for specialized laboratory personnel for routine testing.Challenges and Future DirectionsWhile Lab on a Chip technology holds great promise, several challenges must be overcome for its widespread implementation. One of the primary challenges is the fabrication and mass production of microfluidic devices. The complex design and fabrication processes require specialized equipment and expertise, making large-scale production costly and time-consuming. Standardization of fabrication processes and the development of low-cost manufacturing techniques are crucial for the widespread adoption of Lab on a Chip technology.Another challenge is the integration of various analytical techniques onto a single chip. Each laboratory process requires specific conditions and sensors, and compatibility issues can arise when multiple processes are combined. The development of universal interfaces and standard protocols can facilitate the integration of different techniques, ensuring compatibility and reliability.ConclusionLab on a Chip technology has the potential to revolutionize laboratory testing in various domains. Its miniaturization and automation capabilities offer numerous advantages, from faster and cheaper diagnostics to improved drug discovery. However, significant challenges need to be addressed for thewidespread adoption of Lab on a Chip devices. With ongoing research and development, it is hopeful that these challenges will be overcome, paving the way for a future where laboratory testing is more accessible, efficient, and precise.。
Trypsin Antigen Retrieval Solution (ab970) 使用说明书

Product nameTrypsin Antigen Retrieval Solution Tested applicationsSuitable for: IHC-P General notes Trypsin-based antigen retrieval solution for Protease-Induced Epitope Retrieval (PIER) (ab970).Contains colored pH indicator for easy pH confirmation.Protocol Notes: 1) Deparaffinize tissues and hydrate to water. If necessary perform a hydrogenperoxide block, wash in water, and rinse in PBS or TBS. The tissue section is ready for trypsindigestion. 2) Combine 1 part trypsin concentrate with 1 part buffer (working solution stable for 5working days at 2-8ºC). Incubate section for 5-10 minutes at 37°C or 15 minutes at roomtemperature. Then, rinse well in PBS or TBS buffer and proceed with immunostaining procedure.This product utilises a color-coded pH indicator (rose) for easy identification. The end-user canvisually inspect the solution and see that the mixture or buffer is at the proper pH. If the mixedsolution is purple, the pH is too high for optimum digestion. If the buffer or trypsin solution turnsorange or yellow, the pH is too low for optimum digestion.Trypsin is a commonly used digestive enzyme. In formalin-fixed, paraffin-embedded tissues,certain antibody protocols required enzyme pretreatment for proper immunohistochemicalstaining.Other products for IHCOther enzymes for antigen retrieval include: Proteinase K Antigen Retrieval Solution ab64220 and Pepsin Solution ab64201.Find more kits and reagents for antigen retrieval, blocking, signal amplification, visualization,counterstaining, and mounting in the IHC kits and reagents guide .FormLiquid Storage instructionsStore at +4°C.Storage bufferPreservative: 0.05% Sodium azide Constituents: PBS, Carrier protein Reagent notes Trypsin is a commonly used digestive enzyme. In formalin-fixed, paraffin-embedded tissues,certain antibody protocols required enzyme pretreatment for proper immunohistochemicalstaining.Product datasheetTrypsin Antigen Retrieval Solution ab9701 Abreviews 24 ReferencesOverview PropertiesThe Abpromise guarantee ApplicationsOur Abpromise guarantee covers the use of ab970 in the following tested applications.The application notes include recommended starting dilutions; optimal dilutions/concentrations should be determined by the end user.ApplicationAbreviews Notes IHC-P Use at an assay dependent dilution. Ratio for 25ml concentratedtrypsin and 25 ml buffer is 1:1 - 1:3.Please note: A ll products are "FOR RESEA RCH USE ONLY . NOT FOR USE IN DIA GNOSTIC PROCEDURES"Our Abpromise to you: Quality guaranteed and expert technical support Replacement or refund for products not performing as stated on the datasheet Valid for 12 months from date of delivery Response to your inquiry within 24 hours We provide support in Chinese, English, French, German, Japanese and Spanish Extensive multi-media technical resources to help youWe investigate all quality concerns to ensure our products perform to the highest standardsIf the product does not perform as described on this datasheet, we will offer a refund or replacement. For full details of the Abpromise,please visit https:///abpromise or contact our technical team.Terms and conditions Guarantee only valid for products bought direct from Abcam or one of our authorized distributors。
北京海岸鸿蒙标准物质粒子不合格

北京海岸鸿蒙标准物质粒子不合格下载提示:该文档是本店铺精心编制而成的,希望大家下载后,能够帮助大家解决实际问题。
文档下载后可定制修改,请根据实际需要进行调整和使用,谢谢!本店铺为大家提供各种类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by this editor. I hope that after you download it, it can help you solve practical problems. The document can be customized and modified after downloading, please adjust and use it according to actual needs, thank you! In addition, this shop provides you with various types of practical materials, such as educational essays, diary appreciation, sentence excerpts, ancient poems, classic articles, topic composition, work summary, word parsing, copy excerpts, other materials and so on, want to know different data formats and writing methods, please pay attention!近日,北京市质量监督局对北京海岸鸿蒙标准物质进行抽检,并发现部分产品的粒子不合格。
云康达安核酸报告英文

云康达安核酸报告英文Cloud Health and Safety Nucleic Acid ReportDate: [Insert Date]Patient Information:- Name: [Insert Patient's Full Name]- Gender: [Insert Gender]- Age: [Insert Age]- Identification Number: [Insert Identification Number]- Contact Number: [Insert Contact Number]- Address: [Insert Address]Test Information:- Test Date: [Insert Test Date]- Test Method: Nucleic Acid Testing (NAT)- Test Result: NegativeExplanation of Test Result:Our laboratory conducted a Nucleic Acid Testing (NAT) on [Insert Patient's Full Name] to detect the presence of SARS-CoV-2, the virus responsible for COVID-19. We are pleased to inform you that the test result for [Insert Patient's Full Name] is negative.A negative test result indicates that no evidence of the SARS-CoV-2 virus was detected in the sample provided by [Insert Patient's Full Name] at the time of testing. It is important to note that anegative test result does not guarantee that [Insert Patient's Full Name] is free from the virus, as the accuracy of the test can be influenced by various factors, such as the timing of the test, quality of the sample, and the sensitivity of the testing method.Recommendations:- [Insert Patient's Full Name] is advised to continue practicing good hygiene, including frequent hand washing with soap and water for at least 20 seconds, or using alcohol-based hand sanitizers if soap and water are not available.- Maintaining social distancing is strongly advised. It is recommended to keep a minimum distance of 1 meter (3 feet) from others, especially individuals who are coughing, sneezing, or showing other symptoms of respiratory illness.- Wearing a mask in public places is highly recommended, especially when it is not possible to maintain social distancing.- [Insert Patient's Full Name] should monitor their health closely for any symptoms related to COVID-19, such as fever, cough, sore throat, shortness of breath, fatigue, body aches, loss of taste or smell, and gastrointestinal symptoms. If any of these symptoms occur, it is important to seek medical advice and follow the local health authority's guidelines.- Regularly disinfecting frequently-touched surfaces, such as doorknobs, light switches, and mobile phones, is recommended to minimize the risk of transmission.Please note that the information provided in this report is based on the test result at the time of testing and is subject to change. It is essential for [Insert Patient's Full Name] to remain vigilant andfollow the latest guidance from health authorities to protect themselves and the community from COVID-19.If you have any further questions or concerns, please do not hesitate to contact us at [Insert Contact Information]. Wishing you good health and safety.Yours sincerely,[Insert Name][Insert Position][Insert Laboratory/Organization Name]。
LAB.MICRO.300 微生物实验室服务指南说明书

Laboratory ServicesLAB.MICRO.300Anaerobic and Aerobic Collection Guide Copy of version1.1(approved and current)Last Approval orPeriodic Review Completed12/29/2017 Next Periodic ReviewNeeded On or Before12/29/2019 Effective Date8/31/2017Controlled Copy ID62016Location Lab Test Catalog Organization Memorial Health SystemComments for version1.0(last major revision)Initial versionComments for version1.1(this revision)Added tissue;condensed photos.Approval and Periodic Review SignaturesType Description Date Version Performed By Notes Periodic review Designated Reviewer12/29/2017 1.1Amery RayApproval Lab Director3/26/2016 1.0Cory DunnApproval Department Medical Director3/24/2016 1.0Nathan JohnstonApproval Manager Approval3/21/2016 1.0Patricia DilleyApproval Supervisor Approval3/18/2016 1.0Amery RayApproval QA check3/18/2016 1.0Tianna McCormickVersion HistoryVersion Status Type Date Added Date Effective Date Retired 1.1Approved and Current Minor revision8/31/20178/31/2017Indefinite1.0Retired Initial version3/14/20163/26/20168/31/2017Anaerobic and Aerobic Culture CollectionPURPOSEThis process describes how to collect specimens for Anaerobic or Aerobic Culture PROCESSFor Anaerobic CultureTwo (2) swabs are requested/collected. Use a sterile container for fluid specimens or tissue specimens. Do not submit fluid specimens or tissue specimens in swabs. Requests for anaerobic culture will always have aerobic culture and gram stain performed.1.Aerobic Culture and Gram Stain2.Anaerobic CultureSuitable sources for Anaerobic Culture*:∙Aspirate (by needle and syringe)∙Bartholin’s gland/cyst and Boils, Skin ∙Bone Marrow/Blood∙Bronchoscopy, Protected Brushing ∙Culdocentesis (aspirate)∙Fallopian Tube (aspirate/biopsy)∙IUD (for Actinomyces species)∙Pilonidal Cysts∙Placenta via C-section delivery ∙Sinus aspirate∙Surgical wounds and tissues (aspirate, tissue, swab)∙Transtracheal aspirate ∙Urine, suprapubic aspirateFor Aerobic ONLY CultureOne (1) swab is requested/collected. This does not include Gram Stain. Submit two (2) swabs if Gram Stain is desired.*Murray, Patrick R., *Manual Clinical Microbiology, ASM, Current Ed, Washington, D.C. 2015, ASM PressMicrobiology Lab is available 24/7 for questions: 719-365-5686Approved and current. Effective starting 8/31/2017. LAB.MICRO.300 (version 1.1) Anaerobic and Aerobic Collection GuideControlled copy ID 62016. Printed on 1/3/2018 10:34 AM (MST). Page 1 of 1。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Lab 1SHI ZHEN WEN A0093605BACHELOR OF TECHNOLOGYLAB1AA. Problem ObjectiveDesign a program to convert currency from Singapore dollar to other countries dollars, able to input amount and should be able to see all the other currency equivalents for buying and selling rate. Show as below: (a) From US to SGD & vice-versa (b) From JAP to SGD & vice-versa (c) From RMB to SGD & vice-versa (a) From RM to SGD & vice-versa (b) From EUR to SGD & vice-versa (c) From GBP to SGD & vice-versaB. Currency conversion listingThis program to prompt user to convert amount of SGD Dollar and other dollars .it will convert it to a currency selected and following conversion functions: 1. For Singapore dollar covert to foreign dollars (SGD): (a) From SGD to US (b) From SGD to JAP (c) From SGD to RMB (d) From SGD to RM (e) From SGD to EUR (f) From SGD to GBP2. For foreign dollars covert to Singapore dollar (SGD): (a) From US to SGD (b) From JAP to SGD (c) From RMB to SGD (d) From RM to SGD (e) From EUR to SGD (f) From GBP to SGDC. Flow Chart:Program StartDisplay 6 foreign currency exchange ratesAsk user to key in amount need to convertInput Greater than zeroNOYESSelect the type user want to convertCalculate SGD to US or US to SGDCalculate SGD to JAP or JAP to SGDCalculate SGD to RMB or RMB to SGDCalculate SGD to RM or RM to SGDCalculate SGD to EUR or EUR to SGDCalculate SGD to GBP or GBP to SGDDisplay foreign currency exchange amount based on the input SGD amountD. Learning point1. Use the (#define) function to create the constants. 2. Use the (\t) to make text alignment nicely. 3. Use output function printf() & input function scanf(), getch(). 4. Use basic function for avoid unnecessary repetition code. 5. Use while loop function for input value checking. 6. Use switch function for multiple selection.E. Program Code #include <stdio.h> // program header for standard nput & output #include<stdlib.h> // program header for standard library #include<conio.h> #define SGD_TO_US_RATE 0.795 // constant rate for sell out US dollar #define SGD_TO_JAP_RATE 62.819 // constant rate for we sell out Japaness yen #define SGD_TO_RMB_RATE 5.0041 // constant rate for we sell out chinese yuan #define SGD_TO_RM_RATE 2.419 // constant rate for we sell out malaysian dollar #define SGD_TO_EUR_RATE 0.605 // constant rate for we sell out EURO dollar #define SGD_TO_GBP_RATE 0.502 // constant rate for we sell out British pound#define US_TO_SGD_RATE 1.258 dollar #define JAP_TO_SGD_RATE 0.0159 Japaness yen #define RMB_TO_SGD_RATE 0.19984 chinese yuan #define RM_TO_SGD_RATE 0.4134 malaysian dollar #define EUR_TO_SGD_RATE 1.653 dollar // constant rate for we buy in EURO // constant rate for we buy in // constant rate for we buy in // constant rate for we buy in // constant rate for we buy in US#define GBP_TO_SGD_RATE 1.992 pound void ShortLine(); void LongLine(); void main () { float amount; int convert_type;// constant rate for we buy in British// declare function for short line //declare function for long line //main fuction header//declaration variable/* DISPLAY rate for BUY IN & SELL OUT in mantissa component) */ printf("\n\t Currency Rate for Singapore Dollar\n\n"); printf("\tRate of Buy in\t\tRate of sell out \n"); LongLine(); printf("*\t%6f\t*\t%6f\t*\n",SGD_TO_US_RATE, US_TO_SGD_RATE); LongLine(); printf("*\t%6f\t*\t%6f\t*\n",SGD_TO_JAP_RATE, JAP_TO_SGD_RATE); LongLine(); printf("*\t%6f\t*\t%6f\t*\n",SGD_TO_RMB_RATE, RMB_TO_SGD_RATE); LongLine(); printf("*\t%6f\t*\t%6f\t*\n",SGD_TO_RM_RATE, RM_TO_SGD_RATE); LongLine(); printf("*\t%6f\t*\t%6f\t*\n",SGD_TO_EUR_RATE, EUR_TO_SGD_RATE); LongLine(); printf("*\t%6f\t*\t%6f\t*\n",SGD_TO_GBP_RATE, GBP_TO_SGD_RATE); LongLine();/* DISPLAY rate for BUY IN & SELL OUT in exponential mode */ printf("\n\n\tRate of Buy in\t\tRate of sell out \n"); LongLine(); printf("*\t%e\t*\t%e\t*\n",SGD_TO_US_RATE, US_TO_SGD_RATE); LongLine(); printf("*\t%e\t*\t%e\t*\n",SGD_TO_JAP_RATE, JAP_TO_SGD_RATE); LongLine(); printf("*\t%e\t*\t%e\t*\n",SGD_TO_RMB_RATE, RMB_TO_SGD_RATE); LongLine(); printf("*\t%e\t*\t%e\t*\n",SGD_TO_RM_RATE, RM_TO_SGD_RATE); LongLine(); printf("*\t%e\t*\t%e\t*\n",SGD_TO_EUR_RATE, EUR_TO_SGD_RATE); LongLine(); printf("*\t%e\t*\t%e\t*\n",SGD_TO_GBP_RATE, GBP_TO_SGD_RATE);LongLine(); //input data for convert printf("\nHow much amount would you need to convert?\n"); scanf("%f",&amount); while(amount<=0) { printf("Invalid number key in.Try again.\n"); printf("How much amount would you need to convert?\n"); scanf("%f",&amount); } //end while //ask user want to buy or sell printf("\nWould you like to convert your money to?(1-2):\n"); printf("Press [1] for BUY Foreign currency\n"); printf("Press [2] for SELL Foreign currency\n"); scanf("%d",&convert_type); switch(convert_type) { case 1: //out put for buy in convert printf("\n\tForeign Money YOU Get\n" ); ShortLine(); printf("*\t%6f\tUSD\t*\n",SGD_TO_US_RATE*amount ); ShortLine(); printf("*\t%6f\tJAP\t*\n",SGD_TO_JAP_RATE*amount); ShortLine(); printf("*\t%6f\tRMB\t*\n",SGD_TO_RMB_RATE*amount); ShortLine(); printf("*\t%6f\tRM\t*\n",SGD_TO_RM_RATE*amount); ShortLine(); printf("*\t%6f\tEUR\t*\n",SGD_TO_EUR_RATE*amount); ShortLine(); printf("*\t%6f\tGBP\t*\n",SGD_TO_GBP_RATE*amount); ShortLine();break; case 2: //out put for sell out convertprintf("\n\tTotal SGD YOU Get\n" ); ShortLine(); printf("*\t%6f\tSGD\t*\n",US_TO_SGD_RATE*amount ); ShortLine(); printf("*\t%6f\tSGD\t*\n",JAP_TO_SGD_RATE*amount); ShortLine(); printf("*\t%f\tSGD\t*\n",RMB_TO_SGD_RATE*amount); ShortLine(); printf("*\t%6f\tSGD\t*\n",RM_TO_SGD_RATE*amount); ShortLine(); printf("*\t%6f\tSGD\t*\n",EUR_TO_SGD_RATE*amount); ShortLine(); printf("*\t%f\tSGD\t*\n",GBP_TO_SGD_RATE*amount); ShortLine(); break; default: printf("Sorry, this system do not support the convertion\n"); break;} /* say goodbye and exit */ printf("\nThank you for using this Currency Converter!\nGoodbye!"); printf("\tSHI ZHENWEN A0093605!\n"); getch(); exit(0); } void ShortLine() { printf("*********************************\n"); } //function definition for line void LongLine() { printf("*************************************************\n"); } //function body for short lineF. OutputConstant rate display for buy in or sell out. Tow different mode has be showed.Input amount to be valided & select buy in, Print output.Input amount & select sell out. Prin output.LAB1BA. Problem Objective1. Design a program to get the value of F for the user input parameter values. 2. The project requires the program to display error message if the user inputs values out of the given range, an error message should be displayed. 3. The values of the expressions G(r) and Q(r) must also be printed along with the value of F. 4. Print messages to the user indicating if G(r) is greater or less or equal to Q(r) value. 5. Q(r) cannot generate a real value, then print an error message and use Q(r) = 1 and determine the result. Display following:C. Learning points1.How to use if…else structure for program decision making.2.For if condition checking relational operator has different tomathematical operator.e while loop for user input checking for given condition.e mathematical function for A B & square root(A+B).5.Understanding of math operator precedence.D. Program Code#include<stdio.h>#include<stdlib.h>#include<conio.h>#include<math.h>void main(void){//varable declarationint r;float K,alpha,beta,W,Z,delta,Q_r,C,G_r,L,F;//input for K value, if K out of rang, ask rekey in.printf(" Please key in K value rang is (0.1_10) ");scanf(" %f",&K);while((K<0.1)||(K>10)){printf(" Invalid number key in.\n");printf(" Please key in K value rang is (0.1-10): ");scanf(" %f",&K);}printf(" Check K value is %f.\n\n",K);//input for ala value, if alpha out of rang, ask rekey in.printf(" Please key in alpha value range is(0-1): ");scanf(" %f",&alpha);while((alpha<0)||(alpha>1)){printf(" Invalid number key in.\n");printf(" Please key in alpha value range is(0-1): ");scanf(" %f",&alpha);}printf(" Check alpha value is %f.\n\n",alpha);//input for belt value, if beta out of rang, ask rekey in.printf(" Please key in beta value range is(0-1): ");scanf(" %f",&beta);while((beta<0)||(beta>1)){printf(" Invalid number key in.\n");printf(" Please key in beta value range is(0-1): ");scanf(" %f",&beta);}printf(" Check beta value is %f.\n\n",beta);//input for delta value, if delta out of rang, ask rekey in. printf(" Please key in delta value range is(0-1): ");scanf(" %f",&delta);while((delta<0)||(delta>1)){printf(" Invalid number key in.\n");printf(" Please key in delta value range is(0-1): ");scanf(" %f",&delta);}printf(" Check delta value is %f.\n\n",delta);//input for W value, if W out of rang, ask rekey in.printf(" Please key in W value range (1-5):");scanf(" %f",&W);while((W<1)||(W>5)){printf(" Invalid number key in.\n");printf(" Please key in W value range (1-5): ");scanf(" %f",&W);}printf(" Check W value is %f.\n\n",W);//input for Z value, if Z out of rang, ask rekey in.printf(" Please key in Z value range is(1-10): ");scanf(" %f",&Z);while((Z<1)||(Z>10)){printf(" Invalid number key in.\n");printf(" Please key in Z value range is (1-10): ");scanf(" %f",&Z);}printf(" Check Z value is %f.\n\n",Z);//input for r value, if r out of rang, ask rekey in.printf(" Please key in r value of interger:");scanf(" %d",&r);//calculation forC=pow(K,2)- beta*delta;printf("\n C value is %f \n",C);L= delta*pow(Z,r);printf("\n L value is %f \n",L);//condition check for Q_r to be real number or not if ((L==0)||(C<0)||(beta==0)){printf("\n Q_r can't generate a real value");Q_r=1;}else{Q_r= beta*pow(W,r)*(1+sqrt(C))/L;}//calculation for G_rG_r = (beta*pow(W,r)+L)/(pow((W/Z),r)+ 1);//comparation for G_r & Q_rif (G_r>Q_r)printf("\n G_r value is greater Q_r value\n");else if (G_r<Q_r)printf("\n G_r value is less Q_r value\n");elseprintf("\n G_r value is equal to Q_r value\n");//calculation for FF = ( G_r*(1+ pow(K,3))*alpha/Q_r);//print out outputprintf("\n Q_r value is %.5f \n",Q_r);printf("\n G_r value is %.5f .\n",G_r);printf("\n F value is %.5f.\n",F);printf("\n Goodbye!\tSHI ZHENWEN A0093605!\n");getch();exit(0);} //end mainE. OutputInput data from keyboard, any invalid data would be display error message. Print out G_r, Q_r & F. G_r is greater than Q_r.Input the data for Q_r can not get the real value & set Q_r=1, print output.。
