C-jun N-terminal Kinase-mediated Signaling Is Essential for Staphylococcus Aureus-induced U937
肿瘤细胞信号转导通路

肿瘤细胞的信号转导通路信号传导通路是将胞外刺激由细胞表面传入细胞内,启动了胞浆中的信号转导通路,通过多种途径将信号传递到胞核内,促进或抑制特定靶基因的表达。
一、MAPK信号通路MAPK信号通路介导细胞外信号到细胞内反应。
丝裂原活化蛋白激酶(mitogen activated protein kinase,MAPK)主要位于细胞浆,很多生长因子所激活,活化后既可以磷酸化胞浆内的靶蛋白,也能进入细胞核作用于对应的转录因子,调节靶基因的表达。
调节着细胞的生长、分化、分裂、死亡各个阶段的生理活动以及细胞间功能同步化过程,并在细胞恶变和肿瘤侵袭转移过程中起重要作用,阻断MAPK途径是肿瘤侵袭转移的治疗新方向。
MAPK信号转导通路是需要经过多级激酶的级联反应,其中包括3个关键的激酶,即MAPK激酶激酶(MKKK)→MAPK激酶(MKK)→MAPK。
(一)MKKK:包括Raf、Mos、Tpl、SPAK、MUK、MLK和MEKK等,其中Raf又分为A-Raf、B-Raf、Raf-1等亚型;MKKK是一个Ser/Thr蛋白激酶,被MAPKKKK、小G蛋白家族成员Ras、Rho激活后可Ser/Thr磷酸化激活下游激酶MKK。
MKK识别下游MAPK分子中的TXY序列(“Thr-X-Tyr”模序,为MAPK第Ⅷ区存在的三肽序列Thr-Glu-Tyr、Thr-Pro-Tyr或Thr-Gly-Tyr),将该序列中的Thr和Tyr分别磷酸化后激活MAPK。
注:TXY序列是MKK活化JNK的双磷酸化位点,MKK4和MKK7通过磷酸化TXY 序列的第183位苏氨酸残基(Thr183)和第185位酪氨酸残基(Tyr185)激活JNK1。
(二)MKK:包括MEK1-MEK7,主要是MEK1/2;(三)MAPK:MAPK是一类丝氨酸/苏氨酸激酶,是MAPK途径的核心,它至少由4种同功酶组成,包括:细胞外信号调节激酶(Extracellular signal Regulated Kinases,ERK1/2)、C-Jun 氨基末端激酶(JNK)/应激激活蛋白激酶(Stress-activated protein kinase,SAPK)、p38(p38MAPK)、ERK5/BMK1(big MAP kinase1)等MAPK亚族,并根据此将MAPK 信号传导通路分为4条途径。
TNF信号通路图 生物帮

TNF信号通路图日期:2012-04-16 来源:未知标签:肿瘤坏死因子信号TNF acts several通路摘要: TNF信号通路图天隆科技NP968自动核酸提取仪,产品试用进行中!佛山泰尔健生物细胞培养器材诚征代理TNF acts on several different signaling pathways through two cell surface receptors, TNFR1 and TNFR2 (See TNFR1 and TNFR2 Signaling Pathways) to regulate apoptotic pathways, NF-kB activation of inflammation, and activate stress-activated protein kinases (SAPKs). Interaction of TNFR1 with TRADD leads to activation of NF-kB and apoptosis pathways, while interaction with TRAF2 has GENE rally been thought to be involved in stress kinase and NF-kB activation but is not required for TNF to induce apoptosis. Activation of NF-kB is mediated by TRAF2 through the NIK kinase and also by RIP but the observation that TNF activates NF-kB in mice lacking TRAF2 indicates that TRAF-2 does not play an essential role in this process. Stress-activated protein kinases, also calledJNKs, are a family of map kinases activated by cellular stress and inflammatory signals. Binding of TNF to the TNFR1 receptor activates the germinal center kinase (GCK) through the TNF adaptor Traf2, activating the map kinase MEKK1. Both GCK and MEKK1 interact with Traf2, and GCK is required for MEKK1 activation by TNF, but GCK kinase activity does not appear to be required for MEKK1 activation. Instead, GCK activates MEKK1 by causing MEKK1 oligo merization and autophosphorylation. Tank increases the affinity of Traf2 for GCK to increase Map kinase activation by TNF. Once activated, MEKK1 stands at the top of a map kinase pathways leading to transcriptional regulation, including JNK phosphorylation of c-Jun to stimulate transcriptional activation by AP-1, a heterodimer of c-jun and fos or ATF proteins. The activation of the p38 Map kinase also contributes to AP-1 activation leading to the transcriptional activation of many stress and growth related genes. RIP has been suggested as a component of the p38 pathway in addition to playing a role in nf-kb activation. MEKK1 knockout mice support the role of MEKK1 in JNK activation in some cells but did not support MEKK1 dependent activation of NF-kB. Alternative redundant mechanisms may obscure the role of MEKK1 in NF-kB mechanisms. TNF activation of stress kinase pathways and downstream transcription factors may help to modulate the apoptotic pathways also activated by TNF.Contributor:REFERENCES: Chadee DN, Yuasa T, Kyriakis JM. Direct activation ofmitogen-activated protein kinase kinase kinase MEKK1 by the Ste20p homologue GCK and the adapter protein TRAF2. Mol Cell Biol 2002 Feb;22(3):737-49 Chin AI, Shu J, Shan Shi C, Yao Z, Kehrl JH, Cheng G. TANK potentiates tumor necrosis factor receptor-associated factor-mediated c-Jun N-terminal kinase/stress-activated protein kinase activation through the germinal center kinase pathway. Mol Cell Biol 1999Oct;19(10):6665-72 Kim JW, Joe CO, Choi EJ. Role of receptor-interacting protein in tumor necrosis factor-alpha -dependent MEKK1 activation. J Biol Chem 2001 Jul20;276(29):27064-70 Natoli G, Costanzo A, Ianni A, Templeton DJ, Woodgett JR, Balsano C, Levrero M. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. science 1997 Jan 10;275(5297):200-3 Song HY, Regnier CH, Kirschning CJ, Goeddel DV, Rothe M. Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-kappaB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc Natl Acad Sci U S A 1997 Sep2;94(18):9792-6 Xia Y, Makris C, Su B, Li E, Yang J, Nemerow GR, Karin M. MEK kinase 1 is critically required for c-Jun N-terminal kinase activation by proinflammatory stimuli and growth factor-induced cell migration. Proc Natl Acad Sci U S A 2000 May9;97(10):5243-8 Yeh WC, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de laPompa JL, Ferrick D, Hum B, Iscove N, Ohashi P, Rothe M, Goeddel DV, Mak TW. Early lethality, function al NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity 1997 Nov;7(5):715-25 Yuasa T, Ohno S, Kehrl JH, Kyriakis JM. Tumor necrosis factor signaling to stress-activated protein kinase (SAPK)/Jun NH2-terminal kinase (JNK) and p38. Germinal center kinase couples TRAF2 to mitogen-activated protein kinase/ERK kinase kinase 1 and SAPK while receptor interacting protein associates with a mitogen-activated protein kinase kinase kinase upstream of MKK6 and p38. J Biol Chem 1998 Aug 28;273(35):22681-92 Yujiri T, Ware M, Widmann C, Oyer R, Russell D, Chan E, Zaitsu Y, Clarke P, Tyler K, Oka Y, Fanger GR, Henson P, Johnson GL. MEK kinase 1 gene disruption alters cell migration and c-JunNH2-terminal kinase regulation but does not cause a measurable defect in NF- B activation Proc Natl Acad Sci U S A 2000 Jun 20;97(13):7272-7作者:xilu 点击:1230次。
c-Jun_N-terminal_kinase抑制剂_激动剂_MCE

JNKc-Jun N-terminal kinaseprotein kinase family, and are responsive to stress stimuli, such ascytokines,ultraviolet irradiation, heat shock, and osmotic shock. JNKsplay a role in T cell differentiation and the cellular apoptosis pathway.Activation occurs through a dual phosphorylation of threonine (Thr)and tyrosine (Tyr) residues within a Thr-Pro-Tyr motif located inkinase subdomain VIII. Activation is carried out by two MAP kinases,MKK4 and MKK7 and JNK can be inactivated by Ser/Thr and Tyrprotein phosphatases. Downstream molecules that are activated byJNK include c-Jun, ATF2, ELK1, SMAD4, p53 and HSF1. JNKs canassociate with scaffold proteins JNK interacting proteins as well as their upstream kinases JNKK1 and JNKK2 following their activation. JNK activity regulates several important cellularfunctions including cell growth, differentiation, survival and apoptosis.JNK Inhibitors & ModulatorsAS601245 is an inhibitor of the c-Jun NH2-terminal kinase (JNK)(hJNK1: IC50=150nM, hJNK2: IC50=220nM and hJNK3: IC50=70 nM),CC-401 is a second generation ATP-competitive anthrapyrazolone c-Jun N terminal kinase (JNK) inhibitor with potential antineoplastic anthrapyrazolone c-Jun N terminal kinase (JNK) inhibitor with JNK1/JNK2/JNK3(IC50=61/7/6 nM) inhibitor and is currently under clinical development for fibrotic and infammatory indications.DB07268 is a potent and selective JNK1 inhibitor with an IC50 value JNK-IN-7 is a relatively selective JNKs inhibitor(IC50= 1.54/1.99/0.75for JNK1/2/3); also bound to IRAK1, PIK3C3, PIP5K3 and PIP4K2C.Email: sales@Cat. No.: HY-11010Cat. No.: HY-13022A(CC 401 hydrochloride; CC401 hydrochlorCat. No.: HY-13022Cat. No.: HY-15495Cat. No.: HY-P0069Cat. No.: HY-15737Cat. No.: HY-100233Cat. No.: HY-15617SR-3306 is a brain penetrant small molecule JNK inhibitor from the TCS JNK 5a(JNK Inhibitor IX) is a selective inhibitor of JNK2 and JNK3(pIC50 values are 6.7, 6.5, <5.0 and <4.8 for JNK3, JNK2, JNK1and p38(alpha) respectively); displays no significant activity at a range of other protein kinases including EGFR, ErbB2, cdk2,Cat. No.: HY-12829Cat. No.: HY-15881。
医学国际会议学术交流壁报模板
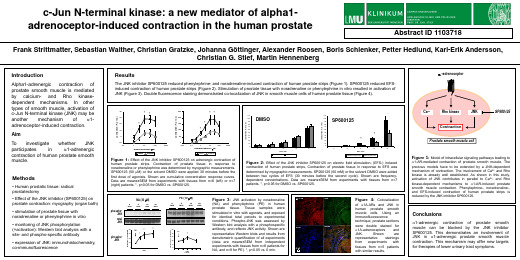
phosphoJNK
stimulated in vitro with agonists, and exposed for identical total periods to experimental
total JNK
conditions. Phospho-JNK was assessed by
phospho-JNK (% of unstimulated) phospho-JNK (% of unstimulated)
Aim
To investigate whether JNK participates in a1-adrenergic contraction of human prostate smooth muscle.
Methods
• Human prostatic tissue: radical prostatectomy
Figure 2: Effect of the JNK inhibitor SP600125 on electric field stimulation- (EFS-) induced
contraction of human prostate strips. Contraction of prostate tissue in response to EFS was determined by myographic measurements. SP200125 (50 mM) or the solvent DMSO were added between two cycles of EFS (30 minutes before the second cycle). Shown are frequencydependent concentrations. Data are means±SEM from experiments with tissues from n=7 patients. *, p<0.05 for DMSO vs. SP600125.
β-Catenin的磷酸化修饰与其生物学意义

β-Catenin的磷酸化修饰与其生物学意义刘奕君,吴军舟,于宇,张宏权(北京大学基础医学院,北京100191)5 10 15 20 25 30 35 摘要:β-Catenin 在细胞的社会性中承担了两个很关键的角色:其一是参与cadherin 介导的细胞间的粘附作用;其二是参与Wnt 介导的细胞间信号传递,调控基因转录。
β-catenin的翻译后修饰是其功能的一种重要的调控机制。
其中β-catenin 的磷酸化修饰可以调节β-catenin 自身的稳定性,并且改变其在细胞内的定位,从而实现对其自身功能的调控。
异常的β-catenin 磷酸化修饰将导致β-catenin 生物学功能失常,可能与癌症、肥胖、糖尿病等多种疾病的发生有密切联系。
关于β-catenin 磷酸化修饰在不同生物学过程中的作用和意义已经成为当今研究的热点。
关键词:分子生物学;β-catenin;磷酸化修饰;蛋白质稳定性;转录调控中图分类号:R34Phosphorylation of β-catenin and its biological function LIU Yijun, WU Junzhou, YU Yu, ZHANG Hongquan(School of Basic Medical Sciences, Peking University, Beijing 100191)Abstract:β-Catenin plays a major role in the regulation of cell adhesion and gene transcription.β-catenin is not only as a component of cell-cell adhesion complexes, but also as a key effector of canonical Wnt signalling. Post-translational modification is an important regulation mechanism ofβ-catenin. Phosphorylated state of β-catenin is significant for enhancing its own stability and inducing cellular translocation. Abnormal phosphorylation of β-catenin shows strong correlations with cancer, obesity and diabetes. It has attracted much attention to investigate the roles and significances of β-catenin phosphorylation in different biological process.Key words:Molecular biology; β-catenin; phosphorylation; protein stability; transcription regualtion0引言Wnt/β-catenin 通路作为生物进化过程中高度保守的一条信号通路,参与调控细胞增殖、存活以及命运决定,在胚胎发育中有着很重要的作用,其异常激活也和许多疾病,如恶性肿瘤的发生发展密切相关。
液CD33的检测对阿尔茨海默病的诊断作用

应蛋白(CRP)、同型半胱氨酸(HCY)及载脂蛋白E(ApoE) 表达异常‘4制,本研究首次比较了不同病程的AD患者和健
万方数据
・1386-
主垦医厘苤查!Q!!生!月筮!!鲞筮!期』业婴堂堂£!i!!兰旦!Y!i!i翌:!!P堡!尘笪!Q!i:y丛:!!:№:里 表1受试者的一般特征
CD33+。
达与炎症因子
相关性分析 以AD患者的 CD33阳性表达 率与血清 CRP、HCY和 ApoE水平进行
图l外蒯血单核细胞CD33表达
相关性分析。
结果为:血清ApoE与CD33阳性表达率呈负相关(r=・ 一0.674,P<0.05);血清CRP与CD33阳性表达率呈正相 关(r=0.233,P<0.05);血清HCY与CD33阳性表达率无 相关(P>0.05)。
选25例健康体检者为对照组,测定血液中CRP、HCY、ApoE和CD33的表达。结果重度AD患者、轻度AD患者、对照组CRP
水平分别为(13.78±2.71)ms/L、(5.14 4-0.88)mg/L、(1.24 4-0.41)mg/L;HCY水平分别为(27.9 4-6.6)pJ】nol/L、(17.4
2.4
CRP、HCY、ApoE的检测空腹12 h以上,抽取肘静脉
血2 IIll,分离血清一20℃保存。采用全自动生化分析仪(日 立7180,日本)测定CRP、HCY、ApoE水平。
1.3
CD33+细胞检测空腹12 h以上,抽取肘静脉血2“,
CD33表
EDTA抗凝后分离血浆单核细胞P,配制成浓度为1×106/ml 的细胞悬液。细胞经刺激培养后,加入固定剂(eBioscience, 美国)100“l室温避光反应20 min,PBS缓冲液洗涤,加入破 膜剂(eBioscience,美国)100¨l室温避光破膜20 min,再PBS 洗涤。将细胞平均分为对照管和测定管,对照管中加入 IgGl-PE单抗(赛默飞,美国)20山,测定管加入CD45FITC/ CD33PE单抗(赛默飞,美国)20“l,室温避光反应15 min后 PBS洗涤。流式细胞仪(Beckman Coulter,美国)检测
信号转导通路与疾病

激活Gi AC活性下降
cAMP
PKA对基因表达的调控作用 cAMP应答元件结合蛋白
cAMP应答元件
PKA进入细胞核后使CREB特定的Ser/Thr磷酸化,形成 同源二聚体,结合DNA,激活转录。
(二) 磷脂与Ca2+-蛋白激酶通路
1. IP3、Ca2+—钙调蛋白激酶途径
α1肾上腺素能受体 内皮素受体 血管紧张素Ⅱ受体等
➢ 基本信号过程 胞外刺激 → MAPKKKs→ MEK4和MEK7, JIP1 协同→激活JNK 1/2/3→靶蛋白、转录因子磷酸化 → 细胞效应。 ➢ MAPKKK:确认有MEKKs(MEKK1、2、3、4), 混合连接激酶(MLK2、3),调亡信号调节激酶 (ASKs)和TGF-β激活的蛋白激酶(TAKs)。Rac, cdc42, APC65等 ➢ MAPKK:MEK4和MEK7。为双功能特异激酶, 致JNK第8域“TPY”双磷酸化。
说明P38功能多样,主要调节炎症反应、细胞增殖、分化、 死亡和凋亡、免疫反应、肿瘤发生等。
第三条,JNK- MAPK、化学因 素引起的细胞外环境变化以及致炎细胞因子等。参与多 种系统的促凋亡作用。 细胞外刺激:细胞因子(TNF、EGF、IL-1等)、生长因子、 应激(如渗透压、氧化损伤、还原剂、电离辐射和热休克 等)等多种因素经不同的信号过程激活。
4.第4条信号通路:ERK5/BMK1 (big mitogenactivated protein kinase 1)通路,可被肿瘤坏死因子 ( TNF)、过氧化氢(H2O2)、胞外高渗等刺激激活, 可能参与炎性反应调控 。
ERK5/BMK1 (big mitogen-activated protein kinase)
局部NO对动脉平滑肌的调节
乳腺癌转移相关机制

并对其进行预后分析,更有利于临床预测和监测乳腺
癌的复发和转移。
2.2
癌细胞对缺氧环境的适应能力以及诱导上皮.间质转
化逆转而增强乳腺癌细胞的脑转移能力’2 2|。 4结语 乳腺癌转移相关机制涉及多个方面,包括上皮. 问质转化、细胞外基质溶解、循环肿瘤细胞和播散性
之后未出现复发和转移的迹象,前者肿块的uPA水
平高,而后者则较低,研究者对这两组乳腺癌患者的 肿瘤组织细胞进行了蛋白质组学分析,发现甘油三磷 酸脱氢酶和脂肪酸结核蛋白4在出现复发、转移乳腺 癌组中表达下调,而另外有9种则表达上调。另外有 研究表明,转凝蛋白可以通过干扰细胞外调节蛋白激
酶的激活和AP一1信号途径来抑制MMPO的表达,从 而抑制肿瘤细胞转移,而MMP-9(溶解细胞外基质) 与转移有着密切的关系一J。这一研究发现了与乳腺
在恶劣环境(低氧、化疗)下生存,并具有一定的肿瘤 干细胞分子表型,提示这些蛋白质有可能成为检测循 环肿瘤细胞和播散性肿瘤细胞的分子标志物,从而估
测肿瘤患者的预后一11+。有研究表明,循环肿瘤细胞 和播散性肿瘤细胞对于乳腺癌而言是一个独立性的 预后因素¨2J 3I。在人体血液中检测循环肿瘤细胞,
抑制癌细胞增殖、集落形成、转移和侵袭等多种生物
基金项目:湖南省科技计划(2014FJ6090) 作者单位:410013长沙,湖南省肿瘤医院暨中南大学湘雅医学院 附属肿瘤医院病理科 通信作者:曾亮,Email:zlxx03@qq.con
株与人体存在较大的差异,因此这一理论的发展还有
待进一步体内研究探索。
1.2细胞外基质 细胞外基质是由上皮细胞和间质细胞共同分泌
[12]Rack
B,Schindlbeck
c-Jun N-terminal kinases

mitogen-activated protein kinase 8IdentifiersSymbol MAPK8Alt.symbols JNK1, PRKM8Entrez5599 ( /entrez/query.fcgi?db=gene&cmd=retrieve&dopt=default&list_uids=5599&rn=1)HUGO 6881 ( /data/hgnc_data.php?hgnc_id=6881)OMIM 601158 ( /601158)RefSeq NM_002750 (/cgi-bin/hgTracks?Submit=Submit&position=NM_002750&rn=1)UniProt P45983 (/uniprot/P45983)Other dataLocusChr. 10 q11.2 ( /search?index=geneMap&search=10q11.2)mitogen-activated protein kinase 9Identifiersc-Jun N-terminal kinasesFrom Wikipedia, the free encyclopediaMain article: Mitogen-activated protein kinase c-Jun N-terminal kinases (JNKs), were originally identified as kinases that bind and phosphorylate c-Jun on Ser-63 and Ser-73within its transcriptional activation domain.They belong to the mitogen-activated protein kinase family, and are responsive to stress stimuli, such as cytokines, ultravioletirradiation, heat shock, and osmotic shock.They also play a role in T cell differentiation and the cellular apoptosis pathway. Activation occurs through a dual phosphorylation of threonine (Thr) and tyrosine (Tyr) residues within a Thr-Pro-Tyr motif located in kinase subdomain VIII. Activation is carried out by two MAP kinases, MKK4 and MKK7 and JNK can be inactivated by Ser/Thr and Tyr protein phosphatases.[1] It has been suggested that this signaling pathway contributes toinflammatory responses in mammals and insects.[citation needed ]Contents1 Isoforms2 Function3 References4 External linksIsoformsThe c-Jun N-terminal kinases consist of ten isoforms derived from three genes: JNK1(four isoforms), JNK2 (four isoforms) and JNK3 (two isoforms).[2] Each gene isexpressed as either 46 kDa or 55 kDa protein kinases, depending upon how the 3' coding region of the corresponding mRNA isprocessed. There have been no functional differences documented between the 46 kDa and the 55 kDa isoform, however, a second form of alternative splicing occurs withinUn Re gi st er edSymbol MAPK9Alt.symbols JNK2, PRKM9Entrez5601 ( /entrez/query.fcgi?db=gene&cmd=retrieve&dopt=default&list_uids=5601&rn=1)HUGO 6886 ( /data/hgnc_data.php?hgnc_id=6886)OMIM602896 ( /602896)RefSeq NM_002752 (/cgi-bin/hgTracks?Submit=Submit&position=NM_002752&rn=1)UniProt P45984 (/uniprot/P45984)Other dataLocusChr. 5 q35 ( /search?index=geneMap&search=5q35)mitogen-activated protein kinase 10IdentifiersSymbol MAPK10Alt.symbols JNK3, PRKM10Entrez 5602 ( /entrez/query.fcgi?db=gene&cmd=retrieve&dopt=default&list_uids=5602&rn=1)HUGO 6872 ( /data/hgnc_data.php?hgnc_id=6872)OMIM602897 ( /602897)RefSeq NM_002753 (/cgi-bin/hgTracks?Submit=Submit&position=NM_002753&rn=1)UniProt P53779 (/uniprot/P53779)Other datatranscripts of JNK1 and JNK2, yielding JNK1-α, JNK2-α and JNK1-β and JNK2-β.Differences in interactions with protein substrates arise because of the mutually exclusive utilization of two exons within the kinase domain.[1]c-Jun N-terminal kinase isoforms have the following tissue distribution:JNK1 and JNK2 are found in all cells and tissues.[3]JNK3 is found mainly in the brain, but is also found in the heart and the testes.[3]FunctionInflammatory signals, changes in levels of reactive oxygen species, ultraviolet radiation,protein synthesis inhibitors, and a variety of stress stimuli can activate JNK. One way this activation may occur is through disruption of the conformation of sensitive protein phosphatase enzymes; specificphosphatases normally inhibit the activity of JNK itself and the activity of proteins linked to JNK activation.[4]JNKs can associate with scaffold proteins JNK interacting proteins as well as their upstream kinases JNKK1 and JNKK2following their activation.JNK, by phosphorylation, modifies the activity of numerous proteins that reside at the mitochondria or act in the nucleus.Downstream molecules that are activated by JNK include c-Jun, ATF2, ELK1, SMAD4, p53and HSF1. The downstream molecules that are inhibited by JNK activation include NFAT4, NFATC1 and STAT3. By activating and inhibiting other small molecules in this way, JNK activity regulates several important cellular functions including cell growth,differentiation, survival and apoptosis.JNK1 is involved in apoptosis,neurodegeneration, cell differentiation andproliferation, inflammatory conditions and cytokine production mediated by AP-1 (activationUn Re gi st er edprotein 1) such as RANTES, IL-8 and GM-CSF.[5]Recently, JNK1 has been found to regulate Jun protein turnover by phosphorylation and activation of the ubiquitin ligase Itch.References^ a b Ip YT, Davis RJ (April 1998). "Signal transduction by the c-Jun N-terminal kinase (JNK)--from inflammation to development". Curr. Opin. Cell Biol. 10 (2): 205–19.doi:10.1016/S0955-0674(98)80143-9 (/10.1016%2FS0955-0674%2898%2980143-9) . PMID 9561845 (// /pubmed/9561845) .1.^ Waetzig V, Herdegen T (2005). "Context-specific inhibition of JNKs: overcoming the dilemma of protection and damage". Br. J. Pharmacol 26 (9): 455–61. doi:10.1016/j.tips.2005.07.006(/10.1016%2Fj.tips.2005.07.006) . PMID 16054242 (// /pubmed/16054242) .2.^ a b Bode AM, Dong Z (August 2007). "The Functional Contrariety of JNK"(///pmc/articles/PMC2832829/) . Mol. Carcinog. 46 (8): 591–8.doi:10.1002/mc.20348 (/10.1002%2Fmc.20348) . PMC 2832829(///pmc/articles/PMC2832829) . PMID 17538955 (// /pubmed/17538955) . ///pmc/articles/PMC2832829/. "The protein products of jnk1 and jnk2 are believed to be expressed in every cell and tissue type, whereas the JNK3protein is found primarily in brain and to a lesser extent in heart and testis"3.^ Vlahopoulos S, Zoumpourlis VC (August 2004). "JNK: a key modulator of intracellular signaling". Biochemistry Mosc. 69 (8): 844–54. doi:10.1023/B:BIRY .0000040215.02460.45(/10.1023%2FB%3ABIRY .0000040215.02460.45) . PMID 15377263(///pubmed/15377263) .4.^ Oltmanns U, Issa R, Sukkar MB, John M, Chung KF (July 2003). "Role of c-jun N-terminal kinase in the induced release of GM-CSF , RANTES and IL-8 from human airway smoothmuscle cells" (///pmc/articles/PMC1573939/) . Br. J. Pharmacol. 139 (6):1228–34. doi:10.1038/sj.bjp.0705345 (/10.1038%2Fsj.bjp.0705345) .PMC 1573939 (///pmc/articles/PMC1573939) . PMID 12871843(///pubmed/12871843) . ///pmc/articles /PMC1573939/.5.External linksJNK+Mitogen-Activated+Protein+Kinases (/cgi/mesh/2011/MB_cgi?mode=&term=JNK+Mitogen-Activated+Protein+Kinases) at the US National Library of Medicine Medical Subject Headings (MeSH)Getting a Handle on Cellular JNK (/?p=1026) (from Beaker Blog)MAP Kinase Resource (http://www.mapkinases.eu)Retrieved from "/w/index.php?title=C-Jun_N-terminal_kinases&oldid=541721429"Categories: Genes on chromosome 10Genes on chromosome 5Cell signaling Signal transduction EC 2.7.11This page was last modified on 2 March 2013 at 13:46.T ext is available under the Creative Commons Attribution-ShareAlike License;Un Re gi st er edadditional terms may apply. See T erms of Use for details.Wikipedia® is a registered trademark of the Wikimedia Foundation, Inc., a non-profit organization.deretsigeRnU。
Simplex

Individually addressable manual fire alarm stations for releasing applications with:∙Power and data supplied via IDNet or MAPNET II addressable communications using a single wire pair∙Operation that complies with ADA requirements∙Visible LED indicator that flashes duringcommunications and is on steady when the station hasbeen activated∙Pull lever that protrudes when alarmed∙Break-rod supplied (use is optional)∙Dual action push and pull operation∙Label kit provides for six varieties of releasing applications (ordered separately)Compatible with the following Simplex® Releasing System control panels equipped with either IDNet or MAPNET II communications:∙Model Series 4100ES, 4010ES, and 4010∙Installed 4100, 4120, and 4020 systemsCompact construction:∙Electronics module enclosure minimizes dust infiltration ∙Allows mounting in standard electrical boxes∙Screw terminals for wiring connectionsTamper resistant reset key lock∙Locks are keyed the same as Simplex fire alarm cabinets Multiple mounting options:∙Surface or semi-flush with standard boxes or matching Simplex boxes∙Flush mount adapter kit∙Adapters are available for retrofitting to commonly available existing boxesUL listed to Standard 38These 4099 series addressable manual stations combinethe familiar Simplex housing with a compact communication module providing easy installation for releasing applications. The integral individual addressable module (IAM) monitors status and communicates changes to the connected control panel via MAPNET II or IDNet communications wiring.A blank area on the front of the station allows the selection of a label to match the specific releasing application (label kit is ordered separately). (Refer to data sheet S4099-0005 for standard Simplex addressable manual stations.)* This product has been approved by the California State Fire Marshal (CSFM) pursuant to Section 13144.1 of the California Health and Safety Code. See CSFM Listing 7150-0026:224 for allowable values and/or conditions concerning material presented in this document. Additional listings may be applicable; contact your local Simplex product supplier for the latest status. Listings and approvals under Simplex Time Recorder Co. are the property of Tyco Safety Products Westminster.4099-9015 Addressable Manual Station for Releasing Applications (with Manual Release label from4099-9802 Label Kit)Label Kit4099-9802Activation requires that a spring loaded interference plate (marked PUSH) be pushed back to access the station pull lever with a firm downward pull that activates the alarm switch. Completing the action breaks an internal plastic break-rod (visible below the pull lever, use is optional). The use of a break-rod can be a deterrent to vandalism without interfering with the minimum pull requirements needed for easy activation. The pull lever latches into the alarm position and remains extended out of the housing to provide a visible indication.Station reset requires the use of a key to reset the manual station lever and deactivate the alarm switch. (If the break-rod is used, it must be replaced.)Station testing is performed by physical activation of the pull lever. Electrical testing can be also performed by unlocking the station housing to activate the alarm switch.Releasing System PeripheralsUL, ULC, CSFM Listed;IDNet or MAPNET II Communicating Devices; FM Approved *Addressable Manual Stations for Releasing ApplicationsAddressable Manual StationsModelDescription4099-9015 Double action, Push operation, Addressable manual station; red housing with white letters and white pulllever; requires label kit 4099-98024099-9802Label kit, white lettering on red background; select the label required for the specific releasing application; types include: Clean Agent, Extinguishing, Carbon Dioxide, Foam System, Sprinkler, and ManualAccessoriesModelDescriptionReference2975-9178 Surface mount steel box, redRefer to page 3 for dimensions 2975-9022 Cast aluminum surface mount box, red2099-9813 Semi-flush trim plate for double gang switch box, red Typically for retrofit, refer to page 4 2099-9814 Surface trim plate for Wiremold box V5744-2, red 2099-9819 Flush mount adapter kit, black Refer to page 4 for details2099-9820Flush mount adapter kit, beige2099-9804 Replacement break-rodPower and Communications IDNet or MAPNET II communications, 1 address per station, up to 2500 ft (762 m) from fire alarm control panel, up to 10,000 ft (3048 m) total wiring distance (including T-Taps)Address Means Dipswitch, 8 positionWire ConnectionsScrew terminal for in/out wiring, for 18 to 14 AWG wire (0.82 mm 2 to 2.08 mm 2)UL Listed Temperature Range 32° to 120° F (0° to 49° C) intended for indoor operation Humidity Range Up to 93% RH at 100° F (38° F) Housing Color Red with white raised letteringMaterialHousing and pull lever are Lexan polycarbonate or equal Pull Lever ColorWhite with red raised letteringHousing Dimensions 5” H x 3 ¾” W x 1” D (127 mm x 95 mm x 25 mm) Installation Instructions579-11354" (102 mm) square box, 2-1/8" (54 mm) minimum 4" Square Box MountSemi-Flush Mount Side ViewSingle Gang Box MountSingle gang box, 2-1/2" deepPreferred Mounting. For surface mounting of theseaddressable manual stations, the preferred electrical boxes are shown in the illustration to the right.Additional MountingReference. Refer to page 4 for Wiremold box mounting compatibility.2975-9178 Box5-3/16" H x 4" W x 2-3/16" D (132 mm x 102 mm x 56 mm)Knockouts located top and bottom2975-9022 Cast Box 5" H x 3-7/8" W x 2-3/16" D (127 mm x 98 mm x 56 mm)4099-9015 Addressable Manual StationFor retrofit and new installations, additional compatible mounting boxes and the required adapter plates are shown in the illustration to the right.Front ViewFlush mount adapter kit Side ViewTyco Fire Protection Products • Westminster, MA • 01441-0001 • USAS4099-0006 11/2014TYCO, SIMPLEX, and the product names listed in this material are marks and/or registered marks. Unauthorized use is strictly prohibited. Lexan is a trademark of the General Electric Co. Wiremold is a trademark of the Wiremold Company.。
贝伐珠单抗注射液辅助规范化疗治疗非小细胞肺癌的临床效果

r eceptor-3 activity and lymphangiogenesis[J].Cancer Res,2008;68 (12):4754-62.
王 慧 芳 等 贝伐 珠 单 抗 注 射 液 辅 助 规 范 化 疗 治疗 非小 细 胞肺 癌 的 临 床 效 果 第 21期
2 Cao Y.Opinion:emerging mechanisms of tumor lymphangi0gensis and lymphatic met ̄tasis[J].Nat Rev Cancel,2005;5(9):735-43.
[关键词 ] 贝伐珠单抗注射液 (安维汀 );化疗 ;非小 细胞 肺癌
[中图分类号] R73 [文献标识 码] A (文章编号】 lo05-9202(2018)21-5193-03;doi:10.3969/j.issn.1005-9202.2018.21.030
4757-72. 10 Tammela T,Zarkada G,W allgard E,et a1.Blocking VEGFR-3 supples—
ses angiogenic sprouting and vascular network formation(J].Nature, 2008;454(7204):656-60.
8 Parsons JT,Slack-Davis J,Tilghman R ,et a1.Foca l adhesion kina s e:tar-
碧云天生物技术SMT (iNOS抑制剂) 产品说明书

碧云天生物技术/Beyotime Biotechnology订货热线:400-168-3301或800-8283301订货e-mail:******************技术咨询:*****************网址:碧云天网站微信公众号SMT (iNOS抑制剂)产品编号产品名称包装S0008 SMT (iNOS抑制剂) 100mg产品简介:SMT,即S-Methylisothiourea Sulfate,也称2-Methyl-2-thiopseudourea, Sulfate,或S-Methyl-ITU,是iNOS (inducible nitric oxide synthase)高度选择性抑制剂。
对于体外培养巨噬细胞诱导产生的iNOS,EC50=6µM;对于血管平滑肌细胞被诱导产生的iNOS,EC50=2µM。
SMT为白色结晶,分子量278.4,分子式为(C2H6N2S)2·H2SO4,纯度大于99%。
溶解于水;用1M盐酸可以配制成25mg/ml的无色透明溶液。
包装清单:产品编号产品名称包装S0008 SMT (iNOS抑制剂) 100mg—说明书1份保存条件:室温保存,两年有效。
注意事项:如果配制成水溶液,分装后-20ºC保存,半年有效。
本产品仅限于专业人员的科学研究用,不得用于临床诊断或治疗,不得用于食品或药品,不得存放于普通住宅内。
为了您的安全和健康,请穿实验服并戴一次性手套操作。
使用说明:SMT的工作浓度通常为0.1-1mM。
其最佳工作浓度需根据具体的实验,自行摸索。
可以先分别尝试0.1、0.3和1mM这三个浓度。
使用本产品的文献:1.Zhang F, Liao L, Ju Y, Song A, Liu Y. Neurochemical plasticity of nitricoxide synthase isoforms in neurogenic detrusor overactivityafter spinal cord injury. Neurochem Res. 2011 Oct;36(10):1903-9.2.Li W, Ren G, Huang Y, Su J, Han Y, Li J, Chen X, Cao K, Chen Q, ShouP, Zhang L, Yuan ZR, Roberts AI, Shi S, Le AD, Shi Y. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ.2012 Sep;19(9):1505-13.3.Xu J, Jin DQ, Zhao P, Song X, Sun Z, Guo Y, Zhang L. Sesquiterpenesinhibiting NO production from Celastrus orbiculatus. Fitoterapia.2012 Dec;83(8):1302-5.4.Mao YF, Zhang YL, Yu QH, Jiang YH, Wang XW, Yao Y, Huang JL.Chronic restraint stress aggravated arthritic joint swell of rats through regulating nitric oxide production. Nitric Oxide. 2012 Oct 15;27(3):137-42.5.Jiang Q, Zhou Z, Wang L, Shi X, Wang J, Yue F, Yi Q, Yang C, Song L.The immunomodulation of inducible nitric oxide in scallop Chlamys farreri. Fish Shellfish Immunol. 2013 Jan;34(1):100-8.6.Yan K, Zhang R, Chen L, Chen F, Liu Y, Peng L, Sun H, Huang W, SunC, Lv B, Li F, Cai Y, Tang Y, Zou Y, Du M, Qin L, Zhang H, Jiang X.Nitric oxide-mediated immunosuppressive effect of human amniotic membrane-derived mesenchymal stem cells on the viability and migration of microglia. Brain Res. 2014 Nov 24;1590:1-9.7.Sun Z, Jiang Q, Wang L, Zhou Z, Wang M, Yi Q, Song L. Thecomparative proteomics analysis revealed the modulation of induciblenitric oxide on the immune response of scallop Chlamys farreri. Fish Shellfish Immunol. 2014 Oct;40(2):584-94.8.Li Y, Ma C, Shi X, Wen Z, Li D, Sun M, Ding H. Effect of nitric oxidesynthase on multiple drug resistance is related to Wnt signaling in non-small cell lung cancer. Oncol Rep. 2014 Oct;32(4):1703-8.9.Wu C, Zhao W, Zhang X, Chen X. Neocryptotanshinone inhibitslipopolysaccharide-induced inflammation in RAW264.7 macrophages by suppression of NF-κB and iNOS signaling pathways. Acta Pharm Sin B.2015 Jul;5(4):323-9.10.Han Y, Jiang Q, Gao H, Fan J, Wang Z, Zhong F, Zheng Y, Gong Z,Wang C. The anti-apoptotic effect of polypeptide from Chlamys farreri (PCF) in UVB-exposed HaCaT cells involves inhibition of iNOS and TGF-β1. Cell Biochem Biophys. 2015 Mar;71(2):1105-15.11.Su Z, Ye J, Qin Z, Ding X. Protective effects of madecassoside againstDoxorubicin induced nephrotoxicity in vivo and in vitro. Sci Rep. 2015 Dec 14;5:18314.12.Wu B, Geng S, Bi Y, Liu H, Hu Y, Li X, Zhang Y, Zhou X, Zheng G, HeB, Wang B. Herpes Simplex Virus 1 Suppresses the Function of Lung Dendritic Cells via Caveolin-1. Clin Vaccine Immunol. 2015 Aug;22(8):883-95.13.Li S, Chen S, Yang W, Liao L, Li S, Li J, Zheng Y, Zhu D. Allicin relaxesisolated mesenteric arteries through activation of PKA-KATP channel in rat. J Recept Signal Transduct Res. 2017 Feb;37(1):17-24.Version 2017.03.08。
Lassen iQ GPS模块说明书

Key Featuresand Benefits• Ultra-low power: 86 mW• Trimble quality at low cost• Aided GPS through TSIP for faster acquisition• Dual sensitivity modes with automatic switching• 12-channel simultaneous operation • Supports NMEA 0183, TSIP, TAIP and DGPS Lassen iQ GPS ModuleLow-power, high-quality GPS solution for your mobile productsT rimble’s Lassen® iQ module isone smart buy. It adds powerful,12-channel GPS functionalityto your mobile product in apostage-stamp-sized footprintwith ultra-low power consump-tion and extreme reliability—allat a very economical price.Designed for portable handheld,battery-powered applicationssuch as cell phones, pagers,PDAs, digital cameras, and manyothers, the module is also idealfor standard GPS applicationssuch as tracking.The 12-channel Lassen iQmodule is fully compatible withT rimble’s popular Lassen SQmodule. Using T rimble’s break-through, patented FirstGPS®architecture, the module deliverscomplete position, velocity andtime (PVT) solutions for use inthe host application.Powerful PerformanceThe Lassen iQ module fea-tures two GPS signal sensitivitymodes: Standard and Enhanced.With Enhanced mode enabled,the module automaticallyswitches to higher sensitivitywhen satellite signals are weak.The module also supports TSIPdownload of critical startupinformation for fast acquisition.This aided GPS (A-GPS) startupprovides hot start performancefor each power-up.The Lassen iQ module is the onlystamp-sized GPS product thatsupports the four most popu-lar protocols: DGPS (RTCM),TSIP(T rimble Standard InterfaceProtocol), TAIP (T rimble ASCIIInterface Protocol) and NMEA 0183.The Lassen iQ module combinesT rimble performance and qual-ity with low cost. With an MTBF(mean time between failures) fi gureof 60 years, it is one of the most reli-able GPS receivers on the market.HardwareA metal shield encloses themodule for protection and easeof handling. The package hasa small form factor, (approxi-mately 26 mm x 26 mm,including the shield). It typi-cally requires less than 90 mWof power at 3.3 VDC.The highly integrated moduleis a miniature board containingT rimble GPS hardware corebased on our Colossus® RFASIC and IO-TS digital signalprocessor (DSP), a 32-bit RISCCPU and fl ash memory.AntennasThe Lassen iQ module is com-patible with active, 3.3-VDCantennas. Three such antennasare available from T rimble andare recommended for use accord-ing to your application; see thereverse side for antenna details.The module provides both anten-na open and short detection plusantenna short protection.Starter KitThe Lassen iQ Starter Kit pro-vides everything you need toget started integrating state-of-the-art GPS capability into yourapplication.Lassen iQ GPS receiver with metal shieldLassen iQ GPS ModuleLow-power, high-quality GPS solution for your mobile productsVibration0.008 g 2/Hz 5 Hz to 20 Hz 0.05 g 2/Hz 20 Hz to 100 Hz–3 dB/octave 100 Hz to 900 HzOperating Humidity5% to 95% R.H. non-condensing, at +60° CEnclosureMetal enclosure with solder mounting tabs Dimensions26 mm W x 26 mm L x 6 mm H(1.02” W x 1.02” L x 0.24” H)Weight6.5 grams (0.2 ounce) including shieldnGothDEMI 7ptModuleLassen iQ module, in metal enclosure with soldermounting tabs Starter Kit Includes Lassen iQ module mounted on interface motherboard in a durable metal enclosure, AC/DC power converter, compact magnetic-mount GPS antenna, ultra-compact embedded antenna, serial interface cable, cigarette lighter adapter, TSIP , NMEA, and TAIP protocols, software toolkit and manual on CD-ROMAntenna Transition Cable, MCXRF cable for connecting antennas with MCX connector to on-module H.FL-RF connector. Cable length: 10 cmAntenna Transition Cable, SMARF cable for connecting antennas with SMA connector to on-module H.FL-RF connector.Cable length: 12.9 cm.Ultra-Compact Embedded Antenna3.3V active miniature unpackaged antennaCable length: 8 cmDim: 22 mm W x 21 mm L x 8 mm H (0.866” x 0.827” x 0.315”)Connector: HFL; mates directly to on-module RF connectorCompact Unpackaged Antenna3V active micropatch unpackaged antenna Cable length: 11 cmDim: 34.6 mm W x 29 mm L x 9 mm H (1.362” x 1.141” x 0.354”)Connector: MCX; mates through the optional RF transition cable to on-module RF connectorCompact Magnetic-Mount Antenna, MCX or SMA3V active micropatch antenna with magnetic mount Cable length: 5 mDim: 42 mm W x 50.5 mm L x 13.8 mm H (1.65” x 1.99” x 0.55”)Connectors: MCX or SMA, mates through the optional RF trasition cable to the module RF connectorSpecifi cations subject to change without notice.© C o p y r i g h t 2004, T r i m b l e N a v i g a t i o n L i m i t e d . A l l r i g h t s r e s e r v e d . T h e G l o b e a n d T r i a n g l e , T r i m b l e , C o l o s s u s , F i r s t G P S , a n d L a s s e n a r e t r a d e m a r k s o f T r i m b l e N a v i g a t i o n L i m i t e d r e g i s t e r e d i n t h e U n i t e d S t a t e s P a t e n t a n d T r a d e m a r k O f fi c e . A l l o t h e r t r a d e m a r k s a r e t h e p r o p e r t y o f t h e i r r e s p e c t i v e o w n e r s . T I D 13442 (9/04)• 12-channel simultaneous operation• Ultra-low power consumption: less than 90 mW (27 mA) @ 3.3 V • Dual sensitivity modes with automatic switching • Aided GPS through TSIP• Antenna open and short circuit detection and protection • Compact size: 26 mm W x 26 mm L x 6 mm H• Supports NMEA 0183, TSIP , TAIP , DGPS protocols • Trimble quality at low costGeneralL1 (1575.42 MHz) frequency, C/A code, 12-channel,continuous tracking receiverUpdate Rate TSIP @ 1 Hz; NMEA @ 1 HZ; TAIP @ 1 Hz Accuracy Horizontal: <5 meters (50%), <8 meters (90%) Altitude: <10 meters (50%), <16 meters (90%) Velocity: 0.06 m/sec PPS (static): ±50 nanosecondsAcquisition (Autonomous Operation in Standard Sensitivity Mode) Reacquisition: <2 sec. (90%) Hot Start: <10 sec (50%), <13 sec (90%) Warm Start: <38 sec (50%), <42 sec (90%) Cold Start: <50 sec (50%), <84 sec (90%)Cold start requires no initialization. Warm start implies last position, time and almanac are saved by backup power. Hot start implies ephemeris also saved.Operational (COCOM) LimitsAltitude: 18,000 mVelocity: 515 m/sEither limit may be exceeded, but not bothConnectorsI/O:8-pin (2x4) 2 mm male header, micro terminal strip ASP 69533-01 RF: Low-profi le coaxial connectorH.FL-R-SMT (10), 50 Ohm Serial Port 2 serial ports (transmit/receive)PPS3.3 V CMOS-compatible TTL-level pulse, once per secondProtocolsTSIP , TAIP , NMEA 0183 v3.0, RTCM SC-104 NMEA MessagesGGA, VTG, GLL, ZDA, GSA, GSV and RMC Messages selectable by TSIP commandSelection stored in fl ash memory- BFranGothDEMI 7ptPrime Power+3.0 VDC to 3.6 VDC (3.3 V typ.) Power ConsumptionLess than 90 mW (27 mA) @ 3.3 VBackup Power +2.5 VDC to +3.6 VDC (3.0V typ.)Ripple Noise Max 60 mV, peak to peak from 1 Hz to 1 MHz Antenna Fault Protection Open and short circuit detection and protectionOperating Temperature –40° C to +85° C Storage Temperature–55° C to +105° CT rimble Navigation Limited is not responsible for the operation or failure of operation ofGPS satellites or the availability of GPS satellite signals.Trimble Navigation Limited Corporate Headquarters 645 North Mary Avenue Sunnyvale, CA Trimble Navigation Europe Ltd, UKPhone: 44 1256-760-150Trimble Export Ltd, Korea Phone: 82-2-5555-361***********************Trimble Navigation Ltd, ChinaPhone: 86-21-6391-7814/iQ。
p53信号通路

p53 SignalingRT² Profiler™ PCR Arrayp53 Signaling Pathway PCR ArrayCellular Senescence PCR ArrayDNA Damage Signaling Pathway PCR ArrayCell Cycle PCR ArraySureSilencing RNAip53 Signaling Pathway Gene RNAiCellular Senescence Gene RNAiDNA Damage Signaling Pathway Gene RNAiCell Cycle Gene RNAiCignal™ Reporter Assaysp53 Pathway Reporter Assay KitE2F Reporter Assay KitEGR1 Reporter Kitp53 is a tumour suppressor protein that regulates the expression of a wide variety of genes involved in Apoptosis, Growth arrest, Inhibition of cell cycle progression, Differentiation and accelerated DNA repair or Senescence in response to Genotoxic or Cellular Stress. As a transcription factor, p53 is compos ed of an N-terminal Activation Domain, a central specific DNA Binding Domain, and a C-terminal Tetramerization Domain, followed by a Regulatory Domain rich in basic Amino acids. Having a short half-life, p53 is normally maintained at low levels in unstress ed mammalian cells by continuous ubiquitylation and subsequentdegradation by the 26S Proteasome. Nonphosphorylated p53 is ubiquitylated by the MDM2 (Mouse Double Minute-2) ubiquitin ligase. MDM2 binding inactivates p53 by two mechanisms. First, MDM2 binds to the transactivation domain of p53, precluding interaction with the transcriptional machinery. Second, this binding mediates the covalent attachment of ubiquitin to p53. Ubiquitylated p53 is then degraded by the Proteasome. Thus MDM2 acts as a major reg ulator of the tumor suppressor p53 by targeting its destruction. When the cell is confronted with stress like DNA damage, Hypoxia, Cytokines, Metabolic changes, Vi ral infection, or Oncogenes, however, p53 ubiquitylation is suppressed and p53 accumulates in the nucleus, where it is activated and stabilized by undergoing multiple covalent modifications including Phosphorylation and Acetylation (Ref.1 & 2).Phosphorylation of p53 mostly occurs in the N-terminal activation domain at the Ser6, Ser9, Ser15, Thr18, Ser20, Ser33, Ser37, Ser46, Thr55, and Thr81 residues, with some phosphorylation occurring in the C-terminal linker and basic regions at Ser315, Ser371, Ser376, Ser378, and Ser392. Phosphorylation on most of these sites is induced by DNA damage, with som e, such as Thr55 and Ser376, being repressed upon genotoxic stress. p53 phosphorylation is mediated by several cellular kinases including Chks (Checkpoint Kinases), CSNK1-Delta (Casein Kinase-1-Delta), CSNK2 (Casein Kinase-2), PKA (Protein Kinase A), CDK7 (Cyclin-Dependent Kinase-7), DNA-PK (DNA-Activated- Protein Kinase), HIPK2 (Homeodomain-Interacting Protein Kinase-2), CAK (CDK-Activating Kinase), p38 and JNK (Jun NH2-terminal kinase). Notably, phosphorylation at Ser15 by ATM (Ataxia Telangiectasia Mutated Gene)/ATR (Ataxia-Telangiectasia and Rad3 Related), either directly or through Chk1 (Cell Cycle Checkpoint Kinase-1)/Chk2 (Cell Cycle Checkpoint Kinase-2), or at Ser20 by Chk1/Chk2 has been shown to alleviate the inhibition or degradation of p53, leading to p53 stabilization and activation. The phosphorylation-induced p53 stabilization and activation are mediated through multiple mechanisms and may vary according to the cellular context or microenvironment. HIF-1Alpha (Hypoxia-Inducible Factor-1-Alpha) has been implicated to be involved in p53 stabilization, the precise mechanism by which HIF-1Alpha regulates p53-mediated function remains unknown. Recently, the interaction between p53 and HIF-1Alpha was reported to evoke HIF-1Alpha degradation. Members of the PIAS (Protein Inhibitor of Activated STAT) protein family have also been found to interact with p53. PIAS1 and PIAS-Gamma function as SUMO (Small Ubiquitin Related Modifier-1) ligases for p53. Moreover, the RING finger domain of PIAS1 binds to the C-terminus of the tumor suppressor p53 and catalyzes its sumoylation, a modification which represses p53 activity on a reporter plasmid containing consensus p53 DNA binding sites. PML (Promyelocytic Leukemia) also activates p53 by recruiting it to multiprotein complexes termed PML-nuclear bodies. PML is a tumor suppressor protein and the major component of multiprotein nuclear complexes that have been variably termed Kremer bodies, ND10, PODs (for PML Oncogenic Domains), and PML-NBs (PML-Nuclear Bodies). PML binds directly with p53 and recruits it to PML-NBs. Recruitment to PML-NBs activate p53 by bringing it in close proximity with CBP (CREB-Binding Protein) /p300. BRCA1 (Breast Cancer-1 Gene) and p53 can also physically associate, both in vitro and in vivo and function in a common pathway of tumor suppression. The ability of BRCA1 to biochemically modulate p53 function suggests that this may be afundamental role of BRCA1 in tumor suppression (Ref.3, 4 & 5).Another important modification of p53 is acetylation. p53 is specifically acetylated at Lys370, Lys372, Lys373, Lys381, and Lys382 by p300/CBP and at Lys320 by PCAF (p300/CBP-associated factor). Acetylation has been shown to augment p53 DNA binding, and to stimulate p53-mediated transactivation of its downstream target genes through the recruitment of coactivators. Acetylation may also regulate the stability of p53 by inhibiting its ubiquitination by MDM2. In vivo, acetylation at Lys320, Lys373, and Lys382 is induced by many genotoxic agents, including UV-radiation, IR (Ionizing Radiation), hypoxia, oxidative stress, and even depletion of ribonucleotide pools. p53 can also be deacetylated by HDAC1 (Histone Deacetylase-1) and SIRT1. Human SIRT1 is an enzyme that deacetylates the p53 tumor suppressor protein and has been suggested to modulate p53-dependent functions including DNA damage-induced cell death. p53 deacetylation has been suggested to down-regulate the activation of genes such as Bax and p21WAF1. Phosphorylation and acetylation are interdependent. In deed, phosphorylation at the p53N-terminus has been shown to enhance its interaction with acetylase p300/CBP and to potentiate p53 acetylation. Activated p53 functions effectively as a transcription factor and induces transcription of several genes. The D NA targets of p53 are consensussequences consisting of a 10-base pair repeat of 5'-PuPuPu-C(A/T)(T/A)GPyPyPy-3' (where Pu is a purine and Py is a pyrimidine). It also can bind to a palindromic site having a four or five-base pair inverted repeat of a similar sequence. Complete p53 is inactive for specific DNA binding unless activated by covalent and noncovalent modifications of the basic C-terminal domain. After p53 is activated it can be involved in cell-cycle inhibition, apoptosis, genetic repair, and inhibition of blood-vesselformation (Ref.5, 6 & 7).Cell cycle inhibition takes place when there is a block in cell-cycle division. p53 does this by stimulating the expression of p21 WAF1/CIP1 (Cyclin Dependent Kinase Inhibitor-p21). This protein is an inhibitor of CDKs (Cyclin-Dependent Kinases) that regulate the cell cycle via perturbation of their partner cyclin. Cyclins are involved to ensure successful transitions from S phase to G1. Since p21 WAF1/CIP1 inhibits CDKs it results in inhibition of both G1-to-S and G2-to-mitosis transitions by causing hypophosphorylation of Rb (Retinoblastoma) and preventing the release of E2F. Additionally p53 can stimulate 14-3-3, a protein that sequesters Cyclin B1-CDK1 complexes out of the nucleus. This results in a G2 block. Activated p53 may also initiate apoptosis and stop cell proliferation. p53 stimulates a wide network of signals that act through two major apoptotic pathways: Extrinsic Pathways and Intrinsic Pathways. The extrinsic pathway involves engagement of pa rticular `death' receptors that belong to the TNFR (Tumor Necrosis Factor Receptor) family and, through the formation of the DISC(Death-Inducing-Signaling-Complex), leads to a cascade of activation of Caspases, including Caspase8 and Caspase3, which in turn induce apoptosis. Most common death receptors involved in extrinsic apoptosis Fas, DR5 (Death Receptor-5) and PERP. The intrinsic apoptotic pathway is dominated by the Bcl2 (B-Cell CLL/Lymphoma-2) family of proteins, which governs the release of CytoC (Cytochrome-C) from the mitochondria. The Bcl2 family comprises anti-apoptotic (pro-survival) and pro-apoptotic members. The Bcl2 family is divisible into three classes: pro-survival proteins, whose members are most structurally similar to Bcl2, such as BclXL; pro-apoptotic proteins, BAX (Bcl2 Associated-X Protein) and BAK (Bcl2 Antagonist Killer-1), which are structurally similar to Bcl2 and BclXL and antagonize their pro-survival functions; and the pro-apoptotic `BH3-only' proteins. Intriguingly, a key subset of the Bcl2 family genes are p53 targets, including BAX, Noxa, PUMA (p53-Upregulated Modulator of Apoptosis) and the most recently identified, BID (BH3 Interacting Domain Death Agonist). p53 may also inhibit Bcl2 that is an inhibitor of apoptosis. p53 may also have a role in maintaining genetic stability by 'nucleotide-excision' repair of DNA, chromosomal recombination and chromosome segmentation. GADD45 (Growth Arrest- and DNA Damage-Inducible Gene-45) is a multifunctional protein that is regulated by p53 and that may play a role in DNA repair and cell cycle checkpoints. p53 can playa role in the inhibition of blood-vessel formation. In order for tumours to reach a large size, they must initiate the growth of nutrient-bringing blood vessels in their vicinity, the process of angiogenesis. p53 stimulates the production of genes that prevent this process from happening. p53 activates the expression of the Tsp1 (Thrombospondin-1), an anti-angiogenic factor, along with other angiogenesis inhibitor BAI1 (Brain-specific Angiogenesis Inhibitor-1) (Ref. 8, 9 & 10).In addition, p53 regulates MDM2 function in a negative feedback loop, because the MDM2 gene is a target for p53. Therefore, activation of p53 eventually leads to its own inactivation by switching on a pathway that leads to its destruction. MDM2 is subject to further regulation by direct binding of the ARF (Active Response Factor) protein, which prevents MDM2-mediated p53 proteolysis. PTEN (Phosphatase and Tensin Homolog), on the other hand inhibits MDM2-mediated p53 degradation. p53 can transcriptionally activate PTEN, which may further inhibit Akt activity. Therefore, inhibition of Akt by the inhibitors may trigger a positive feedback with perhaps additional anti-tumor effects. The c-Fos proto-oncogene is also a target for transactivation by the p53 tumor suppressor. Mutations in p53 are associated with genomic instability and increased susceptibility to cancer. It is the most frequently mutated protein in all cancer with an estimated 60% of all cancers having mutated forms that affect its growth suppressing activities. However some common tumours have a higher incidence such that 90% of cervical and 70% of colorectal are found to have p53 mutations. The p53 protein can be inactivated in several ways, in cluding inherited mutations that result in a higher incidence of certain familial cancers such as Li-Fraumeni syndrome. Certain DNA tumour viruses, such as the human adenovirus and the papilomavirus, bind to and inactivate the protein. Functional p53 is th ought to provide a protective effectagainst tumorigenesis (Ref.2, 11 & 12).。
JNK信号通路与细胞凋亡_傅应亚

重庆医科大学学报2014年第39卷第3期(JournalofChongqingMedicalUniversity2014.Vol.39No.3)1c-Jun 氨基末端激酶(c-Jun N-terminal kinases ,JNK )信号通路简介丝裂原活化蛋白激酶(mitogen activated-protein kinase ,MAPK )是重要的细胞内信号传导系统,负责将各种信号从细胞外传递到细胞核内部,调节着多种重要的生理过程,包括新陈代谢、存活、细胞分裂、凋亡等[1-2]。
在哺乳动物细胞中,已确定出3条MAPK 信号传导通路,它们分别是:①应激活化蛋白激酶/c-Jun 氨基末端激酶(SAPK/JNKs 1、2、3);②p38分裂原活化蛋白激酶(p38mitogen-activated protein kinase ,p38MAPK );③细胞外信号调节蛋白激酶(extracellular signal-regulated protein kinases ,ERKs )1、2、3、4、5[3]。
一般来说,SAPK/JNK 主要被各种物理、化学、生物因素激活,而ERK1/2的激活主要是各种生长因子的作用。
JNK 是MAPK 家族主要成分之一,主要参与细胞存活、肿瘤形成、生长、分化和细胞死亡等生理过程。
在哺乳动物细胞中已发现3种编码jnk 基因,包括jnk1、jnk2和jnk3,其相应的编码产物为JNK1、JNK2和JNK3。
据文献报道,JNK1和JNK2在组织中广泛表达,而JNK3只在脑、心脏和睾丸组织中表达[4]。
2JNK 信号通路的组成及活化机制2.1JNK 信号通路与细胞外活化因素JNK 信号通路可被细胞因子如肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)、白介素1、表皮生长因子、某些G蛋白偶联受体、应激(如电离辐射、渗透压、热休克和氧化损伤)等多种细胞外刺激因素激活,参与细胞增殖与分化、细胞形态维持、细胞骨架构建、细胞凋亡和细胞恶变等多种生物学反应[5]。
细胞信号通路大全.pdf

1 PPAR信号通路:过氧化物酶体增殖物激活受体( PPARs) 是与维甲酸、类固醇和甲状腺激素受体相关的配体激活转录因子超家族核激素受体成员。
它们作为脂肪传感器调节脂肪代谢酶的转录。
PPARs由PPARα、PPARβ和PPARγ 3种亚型组成。
PPARα主要在脂肪酸代谢水平高的组织,如:肝、棕色脂肪、心、肾和骨骼肌表达。
他通过调控靶基因的表达而调节机体许多生理功能包括能量代谢、生长发育等。
另外,他还通过调节脂质代谢的生物感受器而调节细胞生长、分化与凋亡。
PPARa同时也是一种磷酸化蛋白,他受多种磷酸化酶的调节包括丝裂原激活蛋白激酶( ERK-和p38.M APK) ,蛋白激酶A和C( PKA,PKC) ,AM PK和糖原合成酶一3( G SK3) 等调控。
调控PPARa生长信号的酶报道有M APK、PKA和G SK3。
PPARβ广泛表达于各种组织,而PPAR γ主要局限表达在血和棕色脂肪,其他组织如骨骼肌和心肌有少量表达。
PPAR-γ在诸如炎症、动脉粥样硬化、胰岛素抵抗和糖代谢调节,以及肿瘤和肥胖等方面均有着举足轻重的作用,而其众多生物学效应则是通过启动或参与的复杂信号通路予以实现。
鉴于目前人们对PPAR—γ信号通路尚不甚清,PPARs 通常是通过与9-cis维甲酸受体( RXR)结合实现其转录活性的。
2 MAPK信号通路:mapk简介:丝裂原激活蛋白激酶(mitogen—activated protein kinase,MAPK)是广泛存在于动植物细胞中的一类丝氨酸/苏氨酸蛋白激酶。
作用主要是将细胞外刺激信号转导至细胞及其核内,并引起细胞的生物化学反应(增殖、分化、凋亡、应激等)。
:包括ERK1、MAPKs家族的亚族 :ERKs(extracellular signal regulated kinase)ERK2。
生长因子、细胞因子或激素激活此通路,介导细胞增殖、分化。
JNKs(c-Jun N-terminal kinase)包括JNK1、JNK2、JNK3。
c-Jun氨基末端蛋白激酶(JNK)与临床

c-Jun氨基末端蛋白激酶(JNK)与临床【摘要】c-Jun氨基末端蛋白激酶(c-Jun N-terminal protein kainse,JNK)家族是丝裂原活化蛋白激酶(motigen-activated protein kinases, MAPKS)超家族成员之一,分子量为46和54KD的应激蛋白激酶。
JNK可被多种因素如细胞因子、生长因子、应激等通过三级磷酸化级联反应而激活,激活的JNK信号通路在临床上对细胞分化、细胞凋亡、炎症反应、应激反应、缺血再灌注损伤、纤维化、肿瘤、毒性反应、高氧损伤等生理病理过程起着至关重要的调节作用。
【关键词】JNK;MAPK;应激蛋白激酶;JNK信号通路【中图分类号】R977.4 【文献标识码】B 【文章编号】1003-5028(2015)8-0574-03【Abstract】c-Jun N-terminal protein kainse(JNK) is one of motigen-activated protein kinases(MAPKS),which is an activated protein kinase which has 46 and 54KD Molecular weight. JNK can be activated by various factors such as cytokine , growth factor , stress etc. through three-level phosphorylation’s caspase cascade. Activated-JNK’s signal path takes the crucial adjective accommodation in the clinical physiology’s and pathology’s courses about celldifferentiation ,apoptosis, inflammatory response, ischemia reperfusion injury ,fibrosis, tumour , toxic reaction ,hyperoxic injury and so on.【keywords】JNK MAPK activated protein kinase JNK’s signal path1 JNK的一般概述c-Jun氨基末端蛋白激酶(c-Jun N-terminal protein kainse,JNK)家族是JM Kyriakis 等人于1990年发现的分子量为46和54KD的应激蛋白激酶[1],是丝裂原活化蛋白激酶(motigen-activated protein kinase, MAPK)超家族成员之一,进化上保守的丝氨酸/苏氨酸蛋白激酶。
shRNA靶向沉默CNTN1表达抑制乳腺癌细胞株MDA-MB-468增殖及克隆形成能力

biomarkerfortheproliferationandchemoresistanceofcolorectalcancerviamiR-203a-3p-mediatedWnt/β-Cateninsigna lingpathway[J].CellPhysiolBiochem,2018,46(3):1275-1285.[17] JIANGN,JIANGX,CHENZ,etal.MiR-203a-3psuppressescellproliferationandmetastasisthroughinhibitingLASP1innaso pharyngealcarcinoma[J].JExpClinCancerRes,2017,36(1):138.[18] JINQC,GODWINAK,BELLACOSAA,etal.AKT2,aputativeoncogeneencodingamemberofasubfamilyofprotein-serine/threoninekinases,isamplifiedinhumanovariancarcinomas[J].ProcNatlAcadSciUSA,1992,89(19):9267-9271.[19] JINQC,RUGGERIB,KLEINWM,etal.AmplificationofAKT2inhumanpancreaticcancercellsandinhibitionofAKT2expres sionandtumorigenicitybyantisenseRNA[J].ProcNatlAcadSciUSA,1996,93(8):3636-3641.[20] ZHUY,ZHOUJ,JIY,etal.ElevatedexpressionofAKT2correlateswithdiseaseseverityandpoorprognosisinhumanosteosarco ma[J].MolMedRep,2014,10(2):737-742.(编校:蔺癑)shRNA靶向沉默CNTN1表达抑制乳腺癌细胞株MDA-MB-468增殖及克隆形成能力贺 赛1,耿 洁2,杨晓民1,侯艳妮1,范拥国1,赵 静1,张静远1,陈 楠1shRNAsilencingCNTN1geneexpressioninhibitsproliferationandcloneformationofbreastcancerMDA-MB-468cellsHESai1,GENGJie2,YANGXiaomin1,HOUYanni1,FANYongguo1,ZHAOJing1,ZHANGJingyuan1,CHENNan11DepartmentofBreastOncology,ShaanxiProvincialCancerHospital,ShaanxiXi'an710061,China;2DepartmentofCardiovascularInternalMedicine,theSecondAffiliatedHospitalofXi'anJiaotongUniversity,ShaanxiXi'an710004,China.【Abstract】 Objective:ToinvestigatetheeffectofshorthairpinRNA(shRNA)targetedsilencingofCNTN1geneonproliferationandcloneformationofbreastcancerMDA-MB-468cells.Methods:Real-timequantitativePCR(RT-PCR)andWesternblotwereusedtodetectthemRNAandproteinlevelsofCNTN1inMCF7-ADR,MDA-MB-468、MCF7andHs578Tcells.MDA-MB-468cellswererandomlytransfectedwiththesuccessfullyconstruc tedshRNAvectorfragmentstargetingCNTN1gene(silencinggroup)ornonsenseshRNAfragments(controlgroup)viaLipofectamineTM2000.CNTN1mRNAandproteinexpressionwasdetectedafter48htransfectionbyRT-PCRandWesternblot.TheproliferationandcloneformationabilitiesofcellsweredetectedbyMTT,cloneformationassayandflowcytometry,respectively.Results:TherelativeexpressionlevelofCNTN1mRNAandproteininMDA-MB-468cellshadthehighestexpressionsignificantly(P<0.05).MDA-MB-468cellsintransfectiongroupunderwentG1phaseblock.Thecapabilitiesofproliferation,cloneformationofMDA-MB-468cellsintransfectiongroupde creasedsignificantlycomparedwiththenon-transfectiongroupandmock-vehiclegroup(P<0.05).Conclusion:CNTN1geneishighlyexpressedinbreastcancerMDA-MB-468cells,anditssilencingexpressionmayinhibitpro liferationandcloneformation.【Keywords】breastcancer,MDA-MB-468,CNTN1,proliferation,cloneformationModernOncology2021,29(04):0564-0568【摘要】 目的:探讨短发夹RNA(shRNA)靶向沉默CNTN1基因表达对乳腺癌MDA-MB-468细胞增殖及【收稿日期】 2020-02-05【修回日期】 2020-02-13【基金项目】 陕西省自然科学基础研究计划(编号:2020JM-680)【作者单位】 1陕西省肿瘤医院乳腺病院,陕西 西安 7100612西安交通大学第二附属医院心内科,陕西 西安 710004【作者简介】 贺赛(1987-),男,陕西延安人,主治医师,主要从事乳腺肿瘤的基础与临床研究。
JNK-pathway
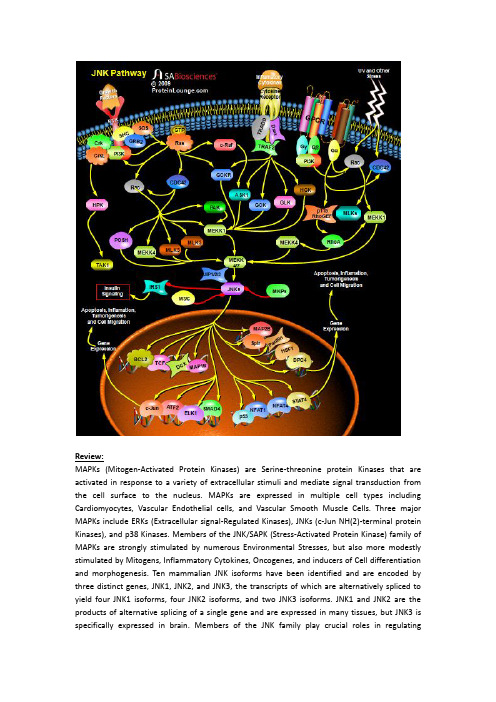
Review:MAPKs (Mitogen-Activated Protein Kinases) are Serine-threonine protein Kinases that are activated in response to a variety of extracellular stimuli and mediate signal transduction from the cell surface to the nucleus. MAPKs are expressed in multiple cell types including Cardiomyocytes, Vascular Endothelial cells, and Vascular Smooth Muscle Cells. Three major MAPKs include ERKs (Extracellular signal-Regulated Kinases), JNKs (c-Jun NH(2)-terminal protein Kinases), and p38 Kinases. Members of the JNK/SAPK (Stress-Activated Protein Kinase) family of MAPKs are strongly stimulated by numerous Environmental Stresses, but also more modestly stimulated by Mitogens, Inflammatory Cytokines, Oncogenes, and inducers of Cell differentiation and morphogenesis. Ten mammalian JNK isoforms have been identified and are encoded by three distinct genes, JNK1, JNK2, and JNK3, the transcripts of which are alternatively spliced to yield four JNK1 isoforms, four JNK2 isoforms, and two JNK3 isoforms. JNK1 and JNK2 are the products of alternative splicing of a single gene and are expressed in many tissues, but JNK3 is specifically expressed in brain. Members of the JNK family play crucial roles in regulatingresponses to various Stresses, and in Neural Development, Inflammation, and Apoptosis. JNK activation is much more complex than that of ERK1/ERK2 owing to inputs by a greater number of MAPKKKs (Mitogen-Activated Protein Kinase Kinase Kinases) (at least 13, including MEKK1 (MAP/ERK Kinase-Kinase-1)-MEKK4 (MAP/ERK Kinase-Kinase-4), ASK (Apoptosis Signal-regulating Kinase) and MLKs (Mixed-Lineage Kinases), which are activated by upstream Rho-family GTPases). These activate JNK MAPKKs MEK4 (MAPK/ERK Kinase-4) and MEK7 (MAPK/ERK Kinase-7), which further activate JNKs. The JNK MAPK modules are regulated by a number of different scaffold proteins, including JIP1 (JNK Interacting Protein-1), JIP2 (JNK Interacting Protein-2), JIP3 (JNK Interacting Protein-3), JIP4 (JNK Interacting Protein-4), Beta-Arrestin-2, Filamin and CrkII. The scaffold proteins presumably target the MAPK modules to different sites in the cell and play roles in kinase activation and/or substrate selection (Ref.1 & 2).Stress or Genotoxic agents are the most powerful inducers of JNK. Different forms of stress have been shown to mediate JNK activation via various cellular pathways. JNK activation in response to UV irradiation is mediated by upstream signaling components, including Rac (Ras-Related C3 Botulinum Toxin Substrate), CDC42 (Cell Division Cycle-42), PAK (p21/CDC42/Rac1-Activated Kinase), ASK1 (Apoptosis Signal-regulating Kinase-1), MLK, MEKK1, SEK1 (SAPK/ERK Kinase-1)/MKK4, MKK7 and p21Ras, in concert with nuclear DNA lesions. Besides Stress, JNKs can also be activated via GPCRs (G-Protein Coupled Receptors), RTKs (Receptor Tyrosine Kinases) and Cytokine Receptors. How GPCRs activate the JNKs is still an unanswered question. Free Beta-Gamma dimers and GN-Alpha12 and GN-Alpha13 proteins are able to activate JNK in a Rac1-CDC42 or p115RhoGEF and RhoA-dependent manner. However, the nature of the GEFs (Guanine nucleotide Exchange Factors) that connect Beta-Gamma and GN-Alpha12/ GN-Alpha13 to Rac1 and CDC2 is still unclear. Interestingly, GN-Alpha12 can also activate JNK by activating the MEKK (MEK kinase). The activation of JNK by Cytokine receptors appears to be mediated by the TRAF (TNF Receptor-Associated Factor) group of Adaptor proteins. Activation of the TNF receptor leads to recruitment of TRAF2 (TNF Receptor-Associated Factor-2), which is required for JNK activation. These adaptor proteins (TRADD (Tumor Necrosis Factor Receptor-1-Associated Death Domain Protein), RIP (Receptor-Interacting Protein) and Daxx) have been reported to bind MEKK1 and ASK1. TRAF2 activates MAPK4Ks like GCK (Germinal Center Kinase), GCKR (GCK-Related Kinase), GLK (GCK-Like Kinase) and HGK (HPK/GCK-like Kinase), which further activates JNKs via MEKK1 and MKK4/7 respectively. ASK1 also interacts with TRAF2 and activates JNK via MKK4/7 (Ref. 3, 4 & 5).Growth Factors also activate JNKs. Although the Signaling cascade from Growth Factor Receptors to ERKs is relatively well understood, the pathway leading to JNK activation is more obscure. Activation of JNK by EGF (Epidermal Growth Factor) or NGF (Nerve Growth Factor) is dependent on H-Ras activation. Growth Factors and Growth Factor Receptors stimulate Ras by recruiting SOS (Son of Sevenless), GRB2 (Growth Factor Receptor-Bound Protein-2) and SHC to the membrane. PI3K (Phosphatidylinositde-3-Kinase) also activate Ras. Ras activates two protein kinases, Raf1 and MEKK (MEK (MAPK, or ERK, kinase) Kinase). Raf1 contributes directly to ERK activation but not to JNK activation, whereas MEKK participated in JNK activation but caused ERK activation only after overexpression. Recently, Raf1 is found to interact with the proapoptotic, stress-activated protein kinase ASK1 in vitro and in vivo. This interaction allows Raf1 to act independently of the MEK–ERK pathway to activate JNK pathway (Ref.6 & 7). The Rho family GTPases, CDC42 (Cell Division Cycle-42) and Rac also initiate a cascade leading to JNK/SAPK,presumably by binding and activating the protein kinase PAK (p21-Activated Kinases), a kinase that phosphorylates and promotes activation of MEKK1. Rac/CDC42 are also involved in JNK activation via a pathway consisting of a sequential cascade MLKs and MKK4/7 (MAP Kinase Kinase-4/7. MLK2 (Mixed-Lineage Kinase-2) and MLK3 (Mixed-Lineage Kinase-3) interact with the activated (GTP-bound) forms of Rac and CDC42, with a slight preference for Rac. Besides MLKs, MEKK1/4 and Posh (Plenty of SH3) are also activated by Rac/CDC42 to activate MKK4/7 and thus JNKs. Adaptor proteins such as Crk (v-Crk Avian Sarcoma Virus Ct10 Oncogene Homolog) and CrkL (v-Crk Avian Sarcoma Virus Ct10 Oncogene Homolog-Like) also leads to activation of JNKs in response to RTK. HPK1 (Hematopoietic Progenitor Kinase-1) associates with Crk and CrkL through binding to the SH3 (Src-Homology Domain-3) of these proteins. Furthermore, association of HPK1 with these proteins increases HPK1's kinase activity. HPK1 then act as upstream of MEKK1 and TAK1 (Transforming Growth Factor-Beta-activated Kinase-1) in the JNK kinase cascade. JNKs are negatively regulated by MKP (MAP Kinase Phosphatase) (Ref.2, 8 & 9).The activated JNK/SAPKs translocate to the nucleus where they phosphorylate transcription factors such as c-Jun, Elk1, DPC4 (Deleted In Pancreatic Carcinoma 4)/ SMAD4 (Sma and MAD (Mothers Against Decapentaplegic) Related Protein-4), p53, ATF2 (Activating T ranscription Factor-2), NFAT4 (Nuclear Factor of Activated T-Cell-4) and NFAT1 (Nuclear Factor of Activated T-Cell-1). JNK1 directly phosphorylates Bcl2 (B-Cell CLL/Lymphoma-2) in vitro, co-localizes and collaborates with Bcl2 to mediate prolonged cell survival. JNK cascade also activates TCF (Ternary Complex Factor) protein. JNK also phosphorylate HSF1 (Heat Shock Factor-1) and JNK-mediated phosphorylation of HSF1 selectively stabilize the HSF1 protein and confers protection to cells under conditions of severe stress. DCX is also a substrate of JNK and interacts with both JNK and JIP. MAPs (Microtubule-Associated Proteins), both MAP1B and MAP2B are also found to be the substrates of JNK. Ser-727 phosphorylation of STAT3 (Signal Transducer and Activator of Transcription-3) can also be induced by JNK. JNK also regulates Insulin signaling by negatively regulating IRS1 (Insulin Receptor Substrate-1). JNK is generally thought to be involved in inflammation, proliferation and Apoptosis. Accordingly, its substrates are transcription factors and Anti-apoptotic proteins. However, JNK also phosphorylates Serine 178 on Paxillin and regulate cell migration. Despite extensive progress in the understanding of the JNK MAP kinase pathway, the mechanisms by which the pathway contributes to the many cellular programs where JNKs are activated are poorly defined. The JIP1 proteins have been proposed to act as molecular scaffolds that organize the JNK signal transduction pathway in response to specific stimuli. The JNK stress pathway is thought to be important in many pathological conditions including the progression of some neurodegenerative diseases su ch as Huntington’s and also in cancer. This pathway therefore offers potential targets for therapeutic intervention. The identification of critical components of this signaling pathway, such as JIP1, offers new routes to understand how this pathway is regulated and potential ways of manipulating it to combat disease (Ref.10, 11 & 12).References:1. Himes SR, Sester DP, Ravasi T, Cronau SL, Sasmono T, Hume DA.The JNK are important for development and survival of macrophages.J Immunol. 2006 Feb 15;176(4):2219-28.PubMed ID: 164559782. Moulin N, Widmann C.Islet-brain (IB)/JNK-interacting proteins (JIPs): future targets for the treatment of neurodegenerative diseases?Curr Neurovasc Res. 2004 Apr;1(2):111-27.PubMed ID: 161851883. Zhou JY, Liu Y, Wu GS.The role of mitogen-activated protein kinase phosphatase-1 in oxidative damage-induced cell death.Cancer Res. 2006 May 1;66(9):4888-94.PubMed ID: 166514454. Yang L, Mao L, Chen H, Catavsan M, Kozinn J, Arora A, Liu X, Wang JQ.A signaling mechanism from G alpha q-protein-coupled metabotropic glutamate receptors to gene expression: role of the c-Jun N-terminal kinase pathway.J Neurosci. 2006 Jan 18;26(3):971-80.PubMed ID: 164213175. Yang Q, Kim YS, Lin Y, Lewis J, Neckers L, Liu ZG.Tumour necrosis factor receptor 1 mediates endoplasmic reticulum stress-induced activation of the MAP kinase JNK.EMBO Rep. 2006 May 5;PubMed ID: 166800936. Kraus S, Benard O, Naor Z, Seger R.c-Src is activated by the epidermal growth factor receptor in a pathway that mediates JNK and ERK activation by gonadotropin-releasing hormone in COS7 cells.J Biol Chem. 2003 Aug 29;278(35):32618-30.PubMed ID: 127503727. Matsukawa J, Matsuzawa A, T akeda K, Ichijo H.The ASK1-MAP kinase cascades in mammalian stress response.J Biochem (Tokyo). 2004 Sep;136(3):261-5.PubMed ID: 155988808. Yamauchi J, Miyamoto Y, Kokubu H, Nishii H, Okamoto M, Sugawara Y, Hirasawa A, Tsujimoto G, Itoh H.Endothelin suppresses cell migration via the JNK signaling pathway in a manner depen dent upon Src kinase, Rac1, and Cdc42.FEBS Lett. 2002 Sep 11;527(1-3):284-8.PubMed ID: 122206759. Zhou JY, Liu Y, Wu GS.The role of mitogen-activated protein kinase phosphatase-1 in oxidative damage-induced cell death.Cancer Res. 2006 May 1;66(9):4888-94.PubMed ID: 1665144510. Baan B, van Dam H, van der Zon GC, Maassen JA, Ouwens DM.The role of JNK, p38 and ERK MAP-kinases in insulin-induced Thr69 and Thr71-phosphorylation of transcription factor ATF2.Mol Endocrinol. 2006 Apr 6;PubMed ID: 1660107111. Sprowles A, Robinson D, Wu YM, Kung HJ, Wisdom R.c-Jun controls the efficiency of MAP kinase signaling by transcriptional repression of MAP kinase phosphatases.Exp Cell Res. 2005 Aug 15;308(2):459-68.PubMed ID: 1595021712. Heasley LE, Han SY.JNK Regulation of Oncogenesis.Mol Cells. 2006 Apr 30;21(2):167-73.PubMed ID: 16682809。
