The microcell mediated transfer of human chromosome 8 into highly metastatic rat liver cancer cell
TAA诱导肝性脑病小鼠中巨噬细胞极化及自噬的改变

TAA诱导肝性脑病小鼠中巨噬细胞极化及自噬的改变摘要:肝性脑病(HE)是肝功能不全导致的神经精神症状,巨噬细胞在HE发病中起重要作用。
本研究旨在探讨TAA(四乙酰氨基)诱导的HE小鼠中巨噬细胞极化及自噬的改变,以及与HE的相关性。
结果显示,TAA诱导HE小鼠中巨噬细胞表型转变为M1型,转录组分析证实了该现象。
此外,巨噬细胞中自噬相关蛋白LC3B-II和Beclin 1水平升高,说明TAA诱导HE小鼠中存在自噬过程的改变。
因此,我们推断TAA诱导的HE小鼠,巨噬细胞M1型极化及自噬的改变可能参与了HE的发病过程。
该研究可能为深入了解HE的病理机制提供参考。
关键词:肝性脑病、TAA、巨噬细胞极化、自噬正文:引言肝性脑病(HE)是由肝功能不全导致的神经精神症状,表现为认知障碍、运动协调障碍、昏迷等,严重影响患者生活质量。
许多因素可导致HE的发生和发展,如肝性脑病引起的炎症反应、肝脏中毒物的堆积和神经递质代谢紊乱等。
近年来,研究表明巨噬细胞在HE的病理机制中起重要作用。
巨噬细胞分为M1型和M2型,前者可释放大量炎症因子,导致神经系统炎症反应。
另一方面,自噬是一种在HE的发生和发展过程中可能参与的细胞自我修复过程。
自噬过程中的关键蛋白包括LC3B、Beclin 1等。
TAA(四乙酰氨基)是一种化学诱导肝脏损伤的药物。
早期研究发现TAA对肝脏有毒性作用,能够引起Necrosis和肝纤维化。
而随着研究的深入,发现TAA可引起HE的发生。
目前,关于TAA诱导HE小鼠中巨噬细胞及自噬活性的改变还未有详细报道。
因此,我们开展了本研究,旨在探究TAA诱导HE小鼠中巨噬细胞极化及自噬的改变,以期为HE的病理机制提供更深入的了解。
材料与方法动物模型:采用C57BL/6J雄性小鼠,体重20-25g。
主要试剂:TAA(四乙酰氨基)。
分组:将小鼠随机分组,分为对照组和TAA治疗组。
HE评估:巨噬细胞在HE的发病中起重要作用,本研究采用Morris水迷宫和旋转棒测试评估HE小鼠的认知和运动协调能力。
丁酸盐在炎症性肠病中的免疫调节机制研究进展

doi :10.3969/j.issn.1002-7386.2024.09.026·综述与讲座·丁酸盐在炎症性肠病中的免疫调节机制研究进展崔馨月 石璠 郑丽红 王海强项目来源:国家中医药管理局第五批全国中医临床优秀人才研修项目(国中医药人教函[2022]1号);黑龙江中医药大学“优秀青年骨干教师”计划(编号:150********)作者单位:150040 哈尔滨市,黑龙江中医药大学(崔馨月、石璠);黑龙江中医药大学附属第四医院消化内科(郑丽红);黑龙江中医药大学附属第一医院消化二科(王海强)通信作者:王海强 E⁃mail:haiqiang915@ 【摘要】 炎症性肠病(IBD )是一组与肠道慢性炎症相关的异质性疾病,丁酸盐是肠道微生物群产生的关键代谢产物,能够调节免疫细胞的发育和功能,调节免疫功能并防止过度免疫反应,从而延缓IBD 的临床进展。
本文就丁酸盐在调节免疫功能方面改善IBD 作用机制的研究进展进行综述,旨在为IBD 的临床治疗提供新的选择。
【关键词】 炎症性肠病;丁酸盐;G 蛋白耦联受体;Th17细胞;Treg 细胞【中图分类号】 R 321.54 【文献标识码】 A 【文章编号】 1002-7386(2024)09-1397-06Research progress on the immunomodulatory mechanism of butyrate in inflammatory bowel diseases CUI Xinyue ∗,SHI Fan ∗,ZHENG Lihong ,et al.∗Heilongjiang University of Chinese Medicine ,Heilongjiang ,Harbin 150040,China【Abstract 】 Inflammatory bowel diseases (IBDs )are a group of heterogeneous diseases associated with chronic inflammation of the gut.Butyrate is a key metabolite produced by the gut microbiota that regulates the development and function of immune cells ,modulates immune function and prevents excessive immune responses ,thereby delaying the clinical progression of IBDs.This paper reviews the progress of research on the mechanism of butyrate in modulating immune function to improve IBD ,aiming to provide new options for the clinical treatment of IBD.【Key words 】 inflammatory bowel disease ;butyrate ;G⁃protein coupled receptor ;Th17cell ;Treg cell 炎症性肠病(inflammatory bowel disease,IBD)是一组与肠道慢性炎症相关的异质性疾病[1],常表现为腹痛、腹泻、血便、体重减轻等,在过去的10年里在全球范围内变得越来越普遍[2]。
上海交通大学基础医学院高小玲课题组发表纳米递药系统脑内命运及其调控机制研究的新成果

上海交通大学基础医学院高小玲课题组发表纳米递药系统脑内命运及其调控机制研究的新成果佚名【期刊名称】《上海交通大学学报(医学版)》【年(卷),期】2024(44)1【摘要】2023年12月29日,上海交通大学基础医学院药理学与化学生物学系高小玲教授联合上海中医药大学陈红专教授和加拿大多伦多大学郑岗教授,在国际权威杂志《自然·纳米技术》(Nature Nanotechnology)正式发表了题为Intracerebral fate of organic and inorganic nanoparticles is dependent on microglial extracellular vesicle function的研究论文。
该杂志同期配发Intracerebral fate of engineered nanoparticles新闻和评述。
该研究通过阐明不同纳米递药系统脑清除差异的原因,揭示小胶质细胞的细胞外囊泡(EVs)对于脑内纳米粒的清除至关重要;发现通过激活ERK1/2通路.【总页数】1页(P97-97)【正文语种】中文【中图分类】G64【相关文献】1.狄文课题组在纳米靶向治疗卵巢研究方面取得新进展上海交通大学医学院附属仁济医院研究人员构建新型纳米载药递送系统2.上海交通大学基础医学院高小玲课题组发表多功能仿生纳米结构靶向神经血管单元改善阿尔茨海默病认知障碍的新成果3.上海交通大学医学院附属瑞金医院蒙国宇课题组发表生物被膜形成机制研究成果4.上海交通大学医学院高小玲课题组提出“纳米刹车”新策略,阻断线粒体功能障碍级联反应并改善阿尔茨海默病认知功能障碍5.上海交通大学基础医学院方超课题组报道基于巨噬细胞的肿瘤靶向递药新技术因版权原因,仅展示原文概要,查看原文内容请购买。
第八章染色体工程ppt课件

2.单倍体育种的优越性
(1)可以克服杂种分离的困难,缩短杂交育种 时间,一般只需一年就可迅速获得自交系,两年 可获得纯系。
(2)大大提高选育效率。
(3)能克服远缘杂交的不孕性,创造出新物 种。
3.人工诱导单倍体的方法 远缘杂交 标记花粉的利用 延迟授粉 核置换 射线照射 染色体有选择地消失 未授粉子房培养和花粉培养(最有效)
微细胞介导的基因转移法
微细胞是指含有一条或几条染色体,外有一薄 层细胞质和一个完移法
染色体介导转移法是指分离得到相应的染色体后转入受体 细胞的一种技术。染色体介导技术一般包括首先诱发细胞 同步分裂,继而用秋水仙胺阻断细胞分裂于中期,再破碎 细胞,通过离心收集大量的中期染色体,然后通过适当的 分部或进一步的分类,即可转移到受体细胞中去。
蛋白质电泳
系列生化分析:如在关东系银鲫中,三倍体红血球的丙
酮酸激酶的含量含著高于二倍体。
形态学检查
直接法
染色体计数:是鉴定多倍性的一个准确的直接方法。
DNA含量测定:流式细胞仪
(三)多倍体育种的重要性
(1)多倍体育种的优势: 植物: ① 对不利环境的适应有明显优势 ② 花大、果大,营养改善 ③ 果树可延长储存期 多倍体植物的性状比原来的二倍体气孔、花、果
二、中期染色体的转移
中期染色体转移:分离到染色体后需要考虑如何将 染色体导入受体细胞。早期实验的基因转移频率很 低,仅-10 -7 - 10 -6,近年来技术上采用磷酸钙沉淀 染色体,再用二甲基亚砜处理受体细胞,或采用卵 磷脂与胆固醇制成脂质体包埋染色体,将染色体转 入受体细胞,可是频率上升至10 -5 - 10 - 4水平。
酵母人工染色体的构建
是基于大肠杆菌的 F 质粒构建 的,高通量低拷贝的质粒载体。 每个环状 DNA 分子中携带一个 抗生素抗性标记,一个来源于大 肠杆菌 F 因子(致育因子)的 严谨型控制的复制子 oriS ( Shizuya et al. 1992 ),一 个易于 DNA 复制的由 ATP 驱动 的解旋酶( (RepE) 以及三 个确保低拷贝质粒精确分配至子 代细胞的基因座 ( parA , parB , 和 parC ) 。
噬菌体展示技术筛选脑靶向功能肽及其修饰纳米粒的脑内递药研究

噬菌体展示技术筛选脑靶向功能肽及其修饰纳米粒的脑内递药研究一、概述在生物医学领域中,脑靶向递药系统一直是研究的热点和难点。
由于血脑屏障的存在,许多药物难以有效进入大脑,从而限制了其在中枢神经系统疾病治疗中的应用。
开发新型的脑靶向递药技术,对于提高药物在脑部的浓度和疗效,降低副作用具有重要意义。
噬菌体展示技术以其独特的优势在药物研发和生物医学领域得到广泛应用。
该技术通过将外源蛋白或多肽的DNA序列插入到噬菌体外壳蛋白结构基因的适当位置,使外源基因随外壳蛋白的表达而表达,同时外源蛋白随噬菌体的重新组装而展示到噬菌体表面。
利用噬菌体展示技术,我们可以筛选到与特定靶标具有高亲和力的多肽或蛋白,为药物研发和疾病治疗提供新的候选分子。
本研究旨在利用噬菌体展示技术筛选具有脑靶向功能的多肽,并将其修饰到纳米粒表面,构建新型的脑靶向递药系统。
通过优化筛选条件和方式,我们成功获得了多个具有脑靶向功能的多肽序列,并通过实验验证了其脑靶向性。
我们还将这些多肽以共价连接的方式修饰到聚乙二醇聚乳酸羟基乙酸共聚物(PEGPLGA)纳米粒表面,以提高药物的稳定性和脑部递送效率。
本研究不仅为脑靶向递药系统的开发提供了新的思路和方法,还为中枢神经系统疾病的治疗提供了新的候选药物和递送策略。
通过进一步的研究和优化,我们相信这种新型的脑靶向递药系统将在未来为更多的患者带来福音。
1. 介绍脑靶向药物递送的重要性与挑战脑靶向药物递送是神经科学领域的一个关键研究方向,对于治疗脑部疾病具有重要意义。
由于血脑屏障的存在,许多药物难以有效穿透并进入脑组织,这使得脑内疾病的治疗面临着巨大的挑战。
开发高效的脑靶向药物递送系统成为当前研究的热点和难点。
脑靶向药物递送的重要性主要体现在以下几个方面:对于脑部疾病如阿尔茨海默病、帕金森病、脑肿瘤等,有效的药物递送能够显著提高治疗效果,改善患者的生存质量。
脑靶向递送系统能够实现药物的精准定位,减少对其他组织器官的副作用。
这种纳米颗粒将小分子药物高效送到肿瘤微环境的巨噬细胞中疗效实现大飞跃

厉害了,这种纳米颗粒!“翻山越岭”将小分子药物高效送到肿瘤微环境的巨噬细胞中,疗效实现大飞跃订阅号APExBIO肿瘤相关巨噬细胞(TAMs,Tumour-associated macrophages)是指浸润在肿瘤组织中的巨噬细胞,是肿瘤微环境中数量最多的免疫细胞。
近年来TAMs引起了研究者们的很多关注,因为它们自身的独特性在肿瘤转移和治疗抗性中起关键作用。
TAMs具有很高的可塑性并且具有相反的表型,受肿瘤微环境细胞因子的影响,可以分化为M1型(杀伤肿瘤)或M2型(促进肿瘤生长)。
巨噬细胞向M1型和M2型分化的过程被称之为极化。
在大多数肿瘤中,促进肿瘤生长的M2表型占上风。
很多研究者选择用一些小分子药物来消除M2型TAMs,这些药物在动物模型中可以延缓肿瘤生长,并在临床中进行了评估。
但是TAMs的耗竭并不能带来持久的抗癌反应。
很多研究者利用这两种表型的可逆性和可调节性,试图将M2表型转化为能杀伤肿瘤的M1表型。
通过利用小分子药物驱动M2型转化为M1型。
然而,这些小分子的临床免疫治疗仍然面临几项挑战:(1)不确定哪些小分子可以有效地影响M2→M1的表型转换;(2)在体内优先将小分子递送至TAMs的能力不足;(3)小分子无法发挥最大效力。
M2型TAMs主要表达甘露糖受体C1样蛋白1(MRC1)和精氨酸酶-1(ARG1)。
相反,M1表型细胞的特征是表达一氧化氮合酶(NOS2)和白细胞介素12(IL12)。
M2型表达MRC1和ARG1,M1型表达NOS2和IL12。
来自美国马萨诸塞州综合医院(也称麻省总医院)的研究团队对一组包含38个与巨噬细胞极化相关的代表性小分子药物进行了筛选。
这些药物作用于巨噬细胞集落刺激因子1受体(CSF1R)、酪氨酸激酶或Toll样受体(TLR),均表现M1型的活化。
通过对比发现TLR7 / 8的激动剂表现出最强的极化效应,这些激动剂中的R848 (resiquimod,双重TLR 7 / 8激动剂)具有最强的效应。
慢病毒介导骨形态发生蛋白2和血管内皮生长因子165双基因转染促进骨髓间充质干细胞向成骨细胞分化

慢病毒介导骨形态发生蛋白2和血管内皮生长因子165双基因转染促进骨髓间充质干细胞向成骨细胞分化周桢杰;李强;李诗鹏;陶旋;马跃刚【摘要】背景:骨形态发生蛋白2(bone morphogenetic protein 2,BMP-2)和血管内皮生长因子165(vascular endothelial growth factor 165,VEGF-165)均为骨修复过程中必不可少的细胞因子,利用慢病毒转染干细胞研究双因子作用下细胞的生物学改变具有重要意义.目的:探讨慢病毒介导人BMP-2和人VEGF-165双基因转染(2次转染)骨髓间充质干细胞的可行性以及转染后细胞的诱导成骨性能.方法:将骨髓间充质干细胞分为4组:①未转染组;②空载组:通过2次相同感染复数的空载慢病毒转染细胞;③BMP-2组:转染了单个目的基因的细胞(Lv-BMP-2/GFP);④BMP-2/VEGF-165组:通过2次转染后携带双目的基因的细胞(Lv-BMP-2/GFP,Lv-VEGH-165/RFP).转染后不同时间点Western Blot检测目的蛋白表达,MTT法检测各组细胞增殖活性,转染后14 d检测各组细胞碱性磷酸酶活性.结果与结论:①空载组、BMP-2组、BMP-2/VEGF-165组在荧光显微镜下均可见相应的绿色及红色荧光蛋白表达;②转染后3 d,BMP-2组、BMP-2/VEGF-165组均有相应目的蛋白高表达;转染后7,14,21 d,目的蛋白检测无明显变化(P>0.05);③BMP-2/VEGF-165组增殖稍快于BMP-2组(P<0.05),BMP-2组明显快于未转染组、空载组(P<0.05);④BMP-2组、BMP-2/VEGF-165组碱性磷酸酶活力明显高于未转染组、空载组;⑤结果表明,慢病毒介导的hBMP-2和hVEGF-165双基因经2次转染成功转入兔骨髓间充质干细胞内,并长期稳定表达,该双因子的表达能通过刺激细胞碱性磷酸酶生成促使细胞更好的向成骨细胞分化.【期刊名称】《中国组织工程研究》【年(卷),期】2018(022)025【总页数】6页(P3950-3955)【关键词】骨髓间充质干细胞;骨形态发生蛋白2;血管内皮生长因子165;慢病毒;基因转染;细胞增殖;碱性磷酸酶;干细胞;国家自然科学基金【作者】周桢杰;李强;李诗鹏;陶旋;马跃刚【作者单位】桂林医学院附属医院,广西壮族自治区桂林市 541001;桂林医学院附属医院,广西壮族自治区桂林市 541001;桂林医学院附属医院,广西壮族自治区桂林市 541001;桂林医学院附属医院,广西壮族自治区桂林市 541001;桂林医学院附属医院,广西壮族自治区桂林市 541001【正文语种】中文【中图分类】R394.2文章快速阅读:文题释义:慢病毒载体:是以人类免疫缺陷Ⅰ型病毒为基础发展起来的基因治疗载体,其对分裂细胞和非分裂细胞均具有较强的感染能力。
细胞质中存在信号纳米架构,可按需打开

细胞质中存在信号纳米架构,可按需打开细胞同时处理多个信号的原因找到了!来源:科技日报2022-03-18 09:18科技日报柏林3月16日电(记者李山)近日,德国分子医学研究中心(MDC)的研究人员发现,纳米结构域构成独立的信号单元是单个细胞可同时处理成百上千个信号的原因。
研究人员认为,该研究成果将开辟一个全新的细胞生物学研究领域。
相关研究成果发表在最近一期《细胞》杂志上。
活细胞暴露于各种刺激之中。
无数信使物质停靠在它们的表面并触发细胞内的信号级联。
这是细胞表面受体接受外界信号做出应答并将信号逐级在细胞内传递、放大和增强的过程。
其中,G蛋白偶联受体(GPCR)属于“第一信使”,负责将细胞外刺激传递给特定的细胞功能。
尽管有许多不同的GPCR,但所有这些GPCR仅向少数“第二信使”发出信号,例如环磷酸腺苷(cAMP)。
现在,德国亥姆霍兹协会马克斯·德尔布吕克分子医学中心的安德烈亚斯·博克教授领导的研究团队发现,单个GPCR通过与受体相关的独立cAMP纳米结构域(RAIN)发出信号,这些结构域构成自给自足的独立细胞信号单元,不受来自其他受体和细胞区室的cAMP的影响。
研究人员使用荧光显微镜观察分离的单细胞,研究来自两个不同受体的cAMP信号如何在一个细胞中平行出现并进行处理。
其中一种受体对胰岛素分泌很重要,另一种则影响心肺功能。
科学家们发现,激活的受体直接形成一个半径在30到60纳米的微小区域。
博克教授将这个纳米空间比喻成弹出式工厂,当“命令”到来时,它会出现在细胞膜上并立即开始工作。
“当一个这样的纳米空间被填满时,cAMP会溢出到下一个,触发信号级联到细胞内部。
”纳米结构域的发现使得细胞中信号通道的复杂性增加很多倍。
受体产生的信号开始只会影响附近的酶。
细胞中的其他区域不会受影响。
这允许非常精确地打开和关闭信号路径。
研究人员认为,细胞质中存在信号纳米架构,可按需打开。
这样的凝胶状结构会阻止cAMP从微小空间中扩散出来。
染色体工程

PPT文档演模板
染色体工程
基于ARS、CEN、TEL是线性染色体稳定的功能序列,人们 利用这些序列构建载体, 重组后的DNA以线性状态存在, 这样 不仅稳定,而且大大提高了插入外源基因的能力, 并且可以像天 然染色体一样在寄主细胞中稳定复制和遗传,称为人工染色 体。如利用从酵母染色体上分离的ARS、CEN、TEL序列构 建载体,然后用这种载体构建的染色体即称为酵母人工染色 体。
n 蛋白质电泳: n 生化分析:如在关东系银鲫中,三倍体红血球的丙酮酸激酶的含量含
著高于二倍体。 n 染色体计数:是鉴定多倍性的一个准确的直接方法。 n DNA含量测定:流式细胞仪。
PPT文档演模板
染色体工程
n 染色体直接计数法准确、直接,但费时; n 红细胞体积测量法省时、简单,在生产现场就能进行
染色体工程
PPT文档演模板
2020/11/19
染色体工程
PPT文档演模板
染色体工程
染色体变异
n 染色体结构变异
① 染色体易位:一个染色体上某一区段与另一非同源染色体上的区段发 生互换。
② 染色体缺失:染色体的某一区段及其带有的基因一起丢失 ③ 染色体倒位:染色体上某一区段连同它带有的基因顺序发生180度倒
PPT文档演模板
染色体工程
第一节 人工诱导多倍体
n 多倍体(polyploid)这个名词是由Winkler(1916年)首 先使用的,它是指每个体细胞中含有三个或更多染色体组的 个体而言。染色体组成多倍性现象在高等植物中相当多,但 动物界的多倍体现象却少得多。自从60年代发现一种美洲角 蛙是一个确定的四倍体动物以来,学者们陆续在低等脊椎动 物中发现许多多倍体动物,包括鱼类、两栖类和爬行类。
PPT文档演模板
欧洲药典7.5版

INDEX
To aid users the index includes a reference to the supplement in which the latest version of a text can be found. For example : Amikacin sulfate...............................................7.5-4579 means the monograph Amikacin sulfate can be found on page 4579 of Supplement 7.5. Note that where no reference to a supplement is made, the text can be found in the principal volume.
English index ........................................................................ 4707
Latin index ................................................................................. 4739
EUROPEAN PHARMACOPபைடு நூலகம்EIA 7.5
Index
Numerics 1. General notices ................................................................... 7.5-4453 2.1.1. Droppers...................
生物专业英语复习资料(第三版词汇)

名词解释:发酵(fermentation):通过微生物或动植物细胞生长培养和化学变化大量产生和积累专门的代谢产物的过程。
生物固定化(immobilized):将具有一定生理更能的生物细胞,如微生物细胞、植物细胞、动物细胞等,用一定方法将其固定,作为固体催化剂加以利用的一门技术,固定化细胞与固定化酶技术共同组成了现代化的生物催化技术固体培养(solid culture):微生物生长在潮湿不溶于水的基质发酵,在固体发酵过程中几乎不含自由水。
腺病毒(adenovirus):一类DNA病毒主要引起呼吸系统急性感染,经改造的的腺病毒基因组可作为基因载体用于转染,也可用于基因治疗,潜在危险性较小。
在作为基因载体转染的过程中外源基因并整合到宿主靶细胞基因组中,并且表达一定时间自然降解。
佐剂(adjuvant):能非特异性的增强机体对抗原免疫应答的物质,其本身无抗原性,但与抗原结合后可以促进机体产生抗体,或延长抗体产生的时间,还能产生炎症反应,使抗体在组织局部聚集。
胰岛素(insulin):胰腺中的胰岛β细胞分泌的一种蛋白质激素含51个氨基酸其生物学作用包括参与糖代谢、脂代谢、蛋白质代谢的调节。
单克隆抗体(monoclonal antibody):抗体主要由B细胞合成,每个B细胞有合成一种抗体的遗传基因,动物脾脏有上百万种B细胞系,含遗传基因不同的B细胞合成不同的抗体,当机体受到抗原刺激时,抗原分子上的许多决定簇分别激活各个具有不同基因的B细胞,被激活的B细胞分裂增殖形成该细胞的子代细胞,有许多个被激活B细胞的分裂增殖,形成该细胞的子代细胞,有许多个被激活的B细胞的分裂增殖形成多克隆,并合成多种抗体,若能选出一个制造一种专一抗体的细胞进行培养,就可得到由单细胞经分裂增殖而形成的细胞群即单克隆、单克隆细胞将合成一种决定簇的抗体称为Ig。
单克隆抗体技术:要制备单克隆抗体须首先获得能合成转移抗体的单克隆B细胞。
但这种B 细胞不能在体外生长,实验发现骨髓瘤细胞可在体外生长繁殖,应用细胞杂交技术使骨髓瘤细胞与免疫系统的淋巴细胞融合,得到杂交骨髓瘤细胞,这种细胞既具有B细胞合成专一抗体的特性,又具有骨髓瘤细胞能在体外培养增值永存的特性。
肿瘤的自我促进——外泌体miR-934诱导巨噬细胞M2极化促进CRC肝转移

肿瘤的自我促进——外泌体miR-934诱导巨噬细胞M2极化促进CRC肝转移越来越多的证据表明肿瘤相关巨噬细胞(TAMs)和外泌体在转移前niche(生态位)形成中发挥重要作用。
TAMs是转移前生态位形成和结直肠癌肝转移(CRLM)的基础。
然而,肿瘤来源的外泌体miRNAs 与TAMs相互作用的分子机制仍然很大程度上未知。
本研究发现肿瘤来源外泌体miR-934可以诱导巨噬细胞M2极化,进而促进CRC的肝转移。
该文于2020年11月发表在《Journal of Hematology and Oncology》IF:11.059期刊上。
技术路线主要实验结果1、miR-934表达升高与CRLM进展及预后不良呈正相关为了揭示参与CRLM的miRNA,作者基于TCGA数据库比较了CRC的1期肿瘤和4期肿瘤的miRNA表达谱,发现miR-934是在4期肿瘤中高表达差异表达最显著的miRNA(Fig. 1a,b)。
此外,作者将110个CRC组织按有无肝转移分为两组,发现肝转移组与未转移组相比,组织和血清miR-934表达上调(Fig.1c, d)。
接下来,为了研究miR-934在CRLM进展中的作用,使用ISH比较了miR-934在包含308个CRC样本的组织芯片(TMA)中的表达,与正常粘膜组织相比,CRC组织中miR-934的表达明显上调;miR-934表达增加与T分期、M分期、晚期AJCC分期和肿瘤复发呈正相关,尤其是在肝转移的病例中(Fig. 1e)。
以上数据提示,miR-934异常高表达与结直肠癌肿瘤进展及肝转移显著相关。
生存分析显示,与miR-934表达较低的患者相比,miR-934表达较高的患者总生存期(OS)和无病生存期(DFS)较低(Fig. 1f, g)。
因此,在CRLM患者中,miR-934高表达与CRLM 进展和预后不良呈正相关。
图1 miR-934表达升高与CRLM进展及预后不良呈正相关2、miR-934存在于CRC细胞衍生的外泌体中miR-934在HCT-8和LoVo的CM中的表达显著高于其它细胞(Fig. 2a)。
血浆外泌体mir-29c在血管性认知障碍患者中的表达及意义

1668
chondrocytes in vitro〔 J〕 . J Nutrꎬ2005ꎻ135(9) :2096 ̄102
25 Lu CꎬLi YꎬHu Sꎬet al. Scoparone prevents IL ̄1β ̄induced inflam ̄
( Warsz) ꎬ2014ꎻ62(5) :395 ̄403
〔2019 ̄07 ̄15 修回〕
( 编辑 王一涵)
gene expression profiling analysis 〔 J 〕 . PLoS Oneꎬ 2014ꎻ 9 ( 2 ) :
血浆外泌体 miR ̄29c 在血管性认知障碍患者中的表达及意义
31 Wojdasiewicz Pꎬ Poniatowski LAꎬ Kotela Aꎬ et al. The chemokine
CX3CL1 ( fractalkine) and its receptor CX3CR1: occurrence and
potential role in osteoarthritis 〔 J 〕 . Arch Immunol Ther Exp
30 Huo LWꎬYe YLꎬWang GWꎬet al. Fractalkine ( CX3CL1 ) : a bio ̄
marker reflecting symptomatic severity in patients with knee osteoar ̄
thritis〔 J〕 . J Investig Medꎬ2015ꎻ63(4) :626 ̄31
全部痴呆人群的 36% ꎬVCI 包括从轻度认知功能受
突触形成、细胞增殖、分化和凋亡过程中的非编码单
GuidelinesforHumaneEndpoints

Hunter College Animal FacilityGuidelines for Humane EndpointsIntroductionExperimental studies may involve procedures that cause clinical symptoms or morbidity in the animals. The Animal Care and Use Committee (ACUC) must consider the selection of the most appropriateendpoint(s). This requires careful consideration of the scientific requirements of the study, the expected and possible adverse effects the research animals may experience (pain, distress, illness, etc.), the most likely time course and progression of those adverse effects, and the earliest most predictive indicators of present or impending adverse effects. The effective use of endpoints requires that properly qualified individuals perform both general and study-specific observations of the research animals at appropriate time points. Studies should be designed to minimize pain and/or distress. If pain or distress isunavoidable, then a scientific justification and the humane endpoints for removing animals from the study or for their euthanasia must be approved by the ACUC prior to the study. Such endpoints are preferable to death or moribundity since they minimize pain and distress. Efforts must be made to minimize pain and distress experienced by animals used in research.MorbidityAnimal Study Proposals that include morbidity as an endpoint or that include animal procedures that have the potential to cause adverse sequelae should address the following:1. Criteria that establish when the endpoint has been reached.a. There are several examples in the literature that might be considered, including:1) Evaluation of five aspects of an animal's condition as described by Morton and Griffiths 6. These are body weight, physical appearance, measurable clinical signs,unprovoked behavior and response to external stimuli.2) Clinical observations used in cancer research and toxicological studies as described by Montgomery 5. Parameters include changes in general appearance, skin and hair,eyes, nose, mouth and head, respiration, urine, feces and locomotion (Table 1). 3) Body condition scoring as described by Ullman-Culler and Foltz 10.b. The clinical signs, depending on severity and duration, that may constitute an endpointinclude, but are not limited to: Rapid weight loss.• Diarrhea, if debilitating.• Progressive dermatitis.• Rough hair coat, hunched posture, lethargy or persistent recumbency.• Coughing, labored breathing, nasal discharge.• Jaundice and/or anemia.• Neurological signs.• Bleeding from any orifice.• Self-induced trauma.• Any condition interfering with eating or drinking (e.g. difficulty with ambulation).• Excessive or prolonged hyperthermia or hypothermia.c. Additional signs in neoplasia studies that may constitute an endpoint include, but are notlimited to: 1) A tumor burden greater than 10% body weight. In an adult mouse, a tumor may not exceed 20 mm in any one dimension; in an adult rat, a tumor may not exceed 40 mm in any one dimension. Formulas for calculating tumor size can be found in the literature (see tumor sizereferences). 2) Tumors that ulcerate, become necrotic or infected.d. Any animal found unexpectedly to be moribund, cachectic, or unable to obtain food or water.2. A plan for monitoring the animals both before and after a change in any of the above aspects, providing care if appropriate, and increasing the level of monitoring. Monitoring or clinical care on weekends and holidays may require involvement of the investigative staff to supplement that provided by the animal care and veterinary staff.3. Identification of personnel responsible for evaluation, record keeping, notification of the investigator and/or veterinarian and persons responsible for euthanasia. Checklists or score sheets may be helpful in ensuring appropriate observations are made, consistently interpreted, and properly documented.Death or MoribundityWhile it is preferable to use the earliest endpoints compatible with the scientific requirements of each study, there are studies that require moribundity or mortality as an endpoint. The moribund condition is defined as a clinically irreversible condition leading inevitably to death. Commonly used signs of moribundity include, but are not limited to: a) lack of responsiveness to manual stimulation; b) immobility; and/or c) an inability to eat or drink. In these studies, animals are permitted to die or become moribund, as a result of experimental procedures. In some cases, pain relieving measures are not used because such measures may compromise the experimental integrity of the study. Examples of research proposals that may have death or moribundity as an endpoint include: infectious disease studies, drug and toxicity studies, and cancer research. The following guidelines are suggested to assist the Animal Care and Use Committees in reviewing proposals with death or moribundity as endpoints.Animal Study Proposals utilizing death or moribundity as an endpoint should contain the following information:1. The scientific rationale for death or moribundity as an endpoint, including:a. What alternatives were considered, why morbidity as an endpoint cannot be used, and howalternatives will be used whenever possible.b. Why measures to relieve pain and/or distress cannot be utilized.c. Number of animals to be used and why this is the minimal number of animals required.d. Whether animals will be euthanized when moribund and if not, what information is to be gainedin the interval between moribundity and death.2. A plan for the following animal care and monitoring procedures:a. Animals involved in experiments that may lead to moribundity or death will be monitored dailyby personnel experienced in recognizing signs of morbidity(illness, injury, or abnormal behavior) for at least the following: abnormal posture, rough haircoat, head tucked into abdomen, exudate around eyes and/ or nose, skin lesions, or abnormalbreathing, difficulty with ambulation, decreased food or water intake, or self mutilation.b. The frequency of observation will be increased when animals exhibit the above or other signsof morbidity. Monitoring on weekends and holidays may require involvement of the investigative staff to supplement that provided by the animal care and veterinary staff. Designated personnel, including a veterinarian, should be notified as soon as animals show signs of disease. Anassessment of the animals' condition should be made as soon as possible and a plan of action established.c. Consideration will be given to moving animals to individual cages when their conditiondeteriorates to the point that injury from other animals is likely. Dead animals must be promptly removed.d. Written records will be kept of monitoring.General endpoint references:1 Alternatives to Animal Testing on the Web (2004), Humane Endpoints Database. (/humane-endpoints.htm ) Johns Hopkins Center for Alternatives to Animal Testing. Baltimore.2 Canadian Council on Animal Care (1998), Guidelines on: Choosing an appropriate endpoint in experiments using animals for research, teaching and testing. Ottawa, Canada.3 Hendriksen CFM and Morton DB, ed. (1998), Humane Endpoints in Animal Experiments for Biomedical Research. Proceedings of the International Conference, 22-25 November 1998, Zeist, The Netherlands. Laboratory Animals Ltd, by Royal Society of Medicine Press Limited, London, England.4 Institute for Laboratory Animal Research Journal (2000), Humane Endpoints for Animals Used in Biomedical Research and Testing. 41: No. 25 Montgomery CA (1990), Oncological and toxicological research: Alleviation and control of pain and distress in laboratory animals. Cancer Bulletin 42:230-237.6 Morton DB and Griffiths PHM (1985), Guidelines on the recognition of pain, distress anddiscomfort in experimental animals and an hypothesis for assessment. Veterinary Record 116:431-43. 7 OECD Guidance Document on the Recognition, Assessment, and Use of Clinical Signs as Humane Endpoints for Experimental Animals Used in Safety Evaluation (2000)/olis/2000doc.nsf/4f7adc214b91a685c12569fa005d0ee7/c125692700623b74c12569bb005aa3d5/$FILE/00087372.pdf8 Stokes WS (1999), Humane Endpoints in Animal Experiments for Laboratory Animals Used in Toxicity Testing Proceedings of the 3rdWorld Congress on Alternatives and Animal use in the Life Sciences, 31 August - 2 September 1999, Bologna, Italy.9 Toth (1997), The moribund state as an experimental endpoint. Contemp Top Lab Anim Sc 36:44-48.10 Ullman-Culleré MH and Foltz CJ (1999), Body condition scoring: a rapid and accurate method for assessing health status of mice. Lab Anim Sc 49:319-323.11 United Kingdom Co-ordinating Committee on Cancer Research (1997), UKCCCR Guidelines for the Welfare of Animals in Experimental Neoplasia, 2nd ed. London, England.12 Netherlands Centre Alternatives to Animal Use http://www.vet.uu.nl/nca/documents/humane_endpointsTumor size references:1 Bullard DE, Schold SC Jr, Bigner SH, Bigner DD (1981), Growth and chemotherapeutic response in athymic mice of tumors arising from human glioma-derived cell lines. J Neuropath Exp Neurol 40:410-427.2 Hamm (1995), Proposed institutional animal care and use committee guidelines for death as an endpoint in rodent studies. Contemp Top Lab Anim Sc 34:69-71.3 Sung C, Dedrick RL, Hall WA, Johnson PA, Youle RJ (1993), The spatial distribution ofimmunotoxins in solid tumors: assessment by quantitative autoradiography. Cancer Research 53: 2092-2099.4 Tomayko MM and Reynolds CP (1989), Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol 24:148-154.5 Welch DR, Chen P, Miele ME, McGary CT, Bower JM, Stanbridge EJ, Weissman BE (1994), Microcell-mediated transfer of chromosome6 into metastatic human C8161 melanoma cells suppresses metastasis but does not inhibit tumorigenicity. Oncogene 9: 255-262.Table 1. Selected Clinical Observations Used in Cancer Research and Toxicological Studies Parameter What to look forGeneral Appearance Dehydration, decreased body weight, missing anatomy, abnormal posture, hypothermia, fractured appendage, swelling, tissue masses, prolapse, paraphimosisSkin and fur Discoloration, urine stain, pallor, redness, cyanosis, icterus, wound, sore,abscess, ulcer, alopecia, ruffled furEyes Exophthalmos, microphthalmia, ptosis, reddened eye, lacrimation, discharge OpacityNose, Mouth, andHeadHead tilted, nasal discharge, malocclusion, salivationRespiration Sneezing, dyspnea, tachypnea, ralesUrine Discoloration, blood in urine, polyuria, anuriaFeces Discoloration, blood in the feces, softness/diarrheaLocomotor Hyperactivity, hyperactivity, coma, ataxia, circling, muscle, tremors, Montgomery, C.A. Jr. (1990), Cancer Bulletin 42:230-237 and appeared in AWIC Newsletter, Spring 1995 6:4。
科学家用皮肤治疗糖尿病

科学家用皮肤治疗糖尿病
佚名
【期刊名称】《微循环学杂志》
【年(卷),期】2017(27)3
【摘要】在一个概念验证实验中,美国芝加哥大学研究人员使用CRISPR基因编辑技术改良了小鼠皮肤植片的干细胞生长,以分泌一种血糖调节的激素。
当研究人员将这些转基因皮肤移植到糖尿病小鼠身上后,这些皮肤能在4个月里调节小鼠的血糖水平,并逆转了胰岛素耐受性以及与高膳食饮食有关的体重增加。
【总页数】1页(P46-46)
【关键词】糖尿病小鼠;皮肤治疗;家用;科学;胰岛素耐受性;研究人员;血糖调节;芝加哥大学
【正文语种】中文
【中图分类】R587.1
【相关文献】
1.德科学家用光疗法治疗皮肤癌 [J], 方留民
2.科学家用转基因皮肤治疗糖尿病 [J],
3.我国科学家用干细胞移植治疗法治疗糖尿病足获成功 [J],
4.美科学家用皮肤造血管可用于糖尿病透析治疗 [J],
5.科学家用基因改造益生菌治疗糖尿病 [J],
因版权原因,仅展示原文概要,查看原文内容请购买。
关于国自然热点—外泌体“套路”的那些事
关于国自然热点—外泌体“套路”的那些事最近一段时间正是国自然标书撰写进入最后的冲刺阶段,相信很多科研工作者们都已经拟好内容,只差润色了。
但是不知道这两年的国自然一线明星外泌体,大家的内容里都靠上了没?如果想靠但还没有靠上的,需要抓紧时间了啊,还剩下最后的补实验基础的时间。
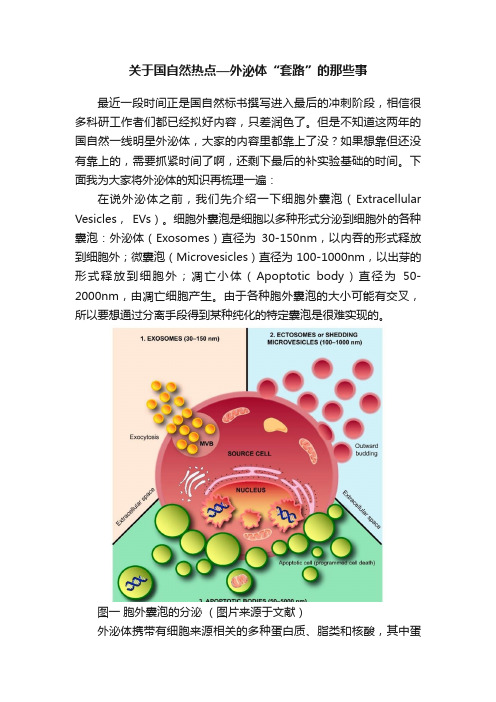
下面我为大家将外泌体的知识再梳理一遍:在说外泌体之前,我们先介绍一下细胞外囊泡(Extracellular Vesicles, EVs)。
细胞外囊泡是细胞以多种形式分泌到细胞外的各种囊泡:外泌体(Exosomes)直径为30-150nm,以内吞的形式释放到细胞外;微囊泡(Microvesicles)直径为100-1000nm,以出芽的形式释放到细胞外;凋亡小体(Apoptotic body)直径为50-2000nm,由凋亡细胞产生。
由于各种胞外囊泡的大小可能有交叉,所以要想通过分离手段得到某种纯化的特定囊泡是很难实现的。
图一胞外囊泡的分泌(图片来源于文献)外泌体携带有细胞来源相关的多种蛋白质、脂类和核酸,其中蛋白质包括跨膜蛋白、信号转导蛋白、细胞支架蛋白、酶等,现在很多文献会选择其中的几种膜蛋白和膜内蛋白作为外泌体的鉴定标志物,核酸包括DNA、mRNA、miRNA、lncRNA、circRNA等,这些组分参与了细胞的各种代谢以及生理活动的变化。
图二外泌体组分分布(图片来源于网络)为什么要研究外泌体?外泌体是细胞与细胞间通讯中起关键作用的胞外基质成分,广泛参与细胞间物质运输与信息传递,调控细胞生理活动;同时,外泌体具有抗原提呈、免疫逃逸等免疫调节作用和诱导正常细胞转化,促进肿瘤发生及转移的作用;此外,外泌体还可以作为“天然的纳米粒子”来进行药物递送,装载的药物类型包括小分子化学药物、蛋白质和肽、核酸药物等。
外泌体相关数据库有哪些?•exoRBase()数据库收集和描述人类血液外泌体中所有长的RNA,提供了注释、表达水平和可能的来源组织。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
P.O.Box 2345, Beijing 100023,China World J Gastroenterol 2003;9(3):449-453 Fax: +86-10-85381893 World Journal of Gastroenterology E-mail: wjg@ Copyright © 2003 by The WJG Press ISSN 1007-9327• LIVER CANCER •The microcell mediated transfer of human chromosome 8 into highly metastatic rat liver cancer cell line C5FHu Liu, Sheng-Long Ye, Jiong Yang, Zhao-You Tang, Yin-Kun Liu, Lun-Xiu Qin, Shuang-Jian Qiu, Rui-Xia SunHu Liu, Sheng-Long Ye, Jiong Yang, Zhao-You Tang, Yin-Kun Liu, Lun-Xiu Qin, Shuang-Jian Qiu, Rui-Xia Sun, Liver Cancer Institute, Zhong Shan Hospital, Fudan University, Shanghai 200032, ChinaSupported by The State Key Basic Research Program, No.G1998051200 and National Scientific Foundation of China, No. 30271459 Correspondence to: Sheng-Long Ye, Professor, Liver Cancer Institute, Zhong Shan Hospital, Fudan University, Shanghai 200032, China. slye@Telephone: +86-21-64041990-2150 Fax: +86-21-64037181 Received: 2002-10-05 Accepted: 2002-11-04AbstractAIM: Our previous research on the surgical samples of primary liver cancer with CGH showed that the loss of human chromosome 8p had correlation with the metastatic phenotype of liver cancer. In order to seek the functional evidence that there could be a metastatsis suppressor gene (s) for liver cancer on human chromosome 8, we tried to transfer normal human chromosome 8 into rat liver cancer cell line C5F, which had high metastatic potential to lung.METHODS: Human chromosome 8 randomly marked with neo gene was introduced into C5F cell line by MMCT and positive microcell hybrids were screened by double selections of G418 and HAT. Single cell isolation cloning was applied to clone microcell hybrids. Finally, STS-PCR and WCP-FISH were used to confirm the introduction.RESULTS: Microcell hybrids resistant to HAT and G418 were obtained and 15 clones were obtained by single-cell isolation cloning. STS-PCR and WCP-FISH proved that human chromosome 8 had been successfully introduced into rat liver cancer cell line C5F. STS-PCR detected a random loss in the chromosome introduced and WCP-FISH found a consistent recombination of the introduced human chromosome with the rat chromosome.CONCLUSION: The successful introduction of human chromosome 8 into highly metastatic rat liver cancer cell line builds the basis for seeking functional evidence of a metastasis suppressor gene for liver cancer harboring on human chromosome 8 and its subsequent cloning.Liu H, Ye SL, Yang J, Tang ZY, Liu YK, Qin LX, Qiu SJ, Sun RX. The microcell mediated transfer of human chromosome 8 into highly metastatic rat liver cancer cell line C5F.World J Gastroenterol 2003; 9(3): 449-453/1007-9327/9/449.htmINTRODUCTIONWith the practice of diagnosis of primary liver cancer at early stage, surgical resection of small hepatocellular carcinoma (HCC) and other improvements in medical diagnosis, imaging, nonsurgical therapies, etc, important progresses have been made toward the control of liver cancer. For example, surgical resection of small HCC has resulted in a marked increase in 5-year survival rate from 20-30 % to 40-60 %. In our institute, the 5-year survival rate of 963 patients with small HCC (450 ISSN 1007-9327 CN 14-1219/ R World J Gastroenterol March 15, 2003 Volume 9 Number 3Vision TM Chromosome Analysis System (Cytovision TM Image Analysis Workstations, USA) was the product of Applied Imagining, USA.MMCT [8-10]Micronuleation of A9(neo8) A9(neo8) cells were seeded in six straight-neck T25 flasks respectively. Colcemid was added to the culture to the final concentration of 0.05 µg·mL-1 when the cells reached confluence of 80 %. The cells weresubsequently incubated at 37. Flasks were sealed with parafilm, and then put into a R12A3 rotor (fixed-angle 28°) to centrifuge at 7 500×g at 36for 20 min and thereafter resuspended with 2 mL of DMEM with 100 µg·mL-1 PHA-P.Microcell fusion & drug selection of microcell hybrids The recipient cell line C5F was trypsinized to make single cell suspension and counted on a hematocytometer for its cell density. C5F cells equivalent to 1/10 of microcells were taken and washed twice with PBS to get rid of remnant serum and thereby collected by centrifugation and resuspended with the PHA-P solution containing the microcells. The mixture was incubated at 37for 48 h and replaced with selective media of 1×HAT and 800 µg·mL-1 G418. The selective medium was refreshed twice per week.Single cell Isolation cloning[11]Viable cells in selective media of G418 and HAT were trypsinized to make single cell suspension. Cell density was examined on a hematocytometer. Fifty to one hundred cells were added into a P100 culture plate with serum free DMEM. Single cells were picked up with a P20 pipette under microscope in laminar flow. It was assured that there was only one cell in view under a low-power objective to exclude the possibility of aspirating more than one cell each time. The opening of tip was pointed by the side of the selected cell and 5 µL media was aspirated each time. The single cell could be seen disappear into the tip under the low magnification microscope. Single cells were added to 96-well cell culture plate containing 0.1 mL of selective media (DMEM supplemented with 20 %FBS containing 1×HAT and 800µg·mL-1 G418) in each well. After about 10 days, clones that are actively proliferating were picked up and transferred to a 24-well plate. After about 7 days, cells were transferred again to T25 flask. When cells reached large quantity, they were freezed down in liquid nitrogen to keep in stock.Genomic DNA extraction from cell lines[12]About 1×106 cultured cells were harvested by trypsinization and centrifugation, into which 0.5 mL of cell lysis buffer (100 mmol·L-1 NaCl , 1 mmol·L-1 EDTA, 0.5 %SDS, 50 mmol·L-1 Tris-HCl, pH 8.0) and 10 µL of Proteinase K (2 g×L-1) wereadded. The mixture was incubated at 37. STS-PCR[13,14]A human STS, D8S277, which located at 8p23.3-p22, was randomly chosen to confirm the result of MMCT. Primers were designed according to the published sequences on NCBI UniSTS database (/genome/sts/). Forward primer: CCAGGTGAGTTTATCAATTCCTGAG; reverse primer: TGAGAGGT CTGAGT GAC ATCCG. PCR product size: 148-180 (bp). PCR reaction solution (10 mmol·L-1 Tris, 50 mmol·L-1 KCl, 2 mmol·L-1 MgCl2, 0.001 % Gelatin, 200 mmol·L-1 dNTPs, 0.5 mmol·L-1 primers and 2U of Taq polymerase) was added into 50 ng of DNA template for each sample respectively. PCR program was run as: 9440 s 604 min, 1 cycle; keep at 4and trypsinized subsequently to make single cell suspension. Cells were pelleted and then exposed to 5 mL of hypotonic solution (0.075 M KCl) by incubating for 10 to 15 min at 37±1- 50±1~50incubator for 16 h. Coverslips were removed from slides, which were immediately immersed in 0.4×SSC/ 3 mL·L-1 NP-40prewarmed to 73observed among dead cells in selective media containing G418 and HAT. Figure 1. The viable cells had good refraction and round shape while the dead cells had poor refraction and morphologic shrinkage.Figure 1 Double selection of microcell hybrids in HAT and G418 (10×25 magnification).Fifteen microcell hybrid clones were obtained by single cell isolation cloning, which were named neo8/C5F-1~15 respectively. It took about 4 weeks for the progress of enlargement culture serially from 96- to 24-well cell culture plate and finally to T25 flask. Feeder cells are proven unnecessary for the single cell isolation cloning of C5F microcell hybrids. Figure 2 showed the microcell hyrid clone obtained in 96-well plate containing selective media of HAT (1×) and G418 (800 µg·mL-1) one week after single cell isolation.Figure 2 Single-cell isolation cloning in 96-well cell culture plate (10×25 magnification).STS-PCRThe chromosome donor cell line A9 (neo8) was taken as positive control and the recipient cell line C5F as negative control. DNA extracted from A9 (neo8), C5F and microcell hybrids was used as template. PCR was performed with the primers for D8S277, which is a unique STS located on human chromosome 8p23.3-p22. PCR products, when checked on 2 % agarose gel elctrophoresis, were found of the same length with those reported in UniSTS database of NCBI. Figure 3 showed that neo8/C5F-3 had the deletion of D8S277.WCP-FISHWCP-FISH was performed using whole chromosome painting DNA probe for human chromosome 8 (WCP 8 probe SpectrumGreen TM, Vysis) to confirm its presence. Figure 4 (a and b) showed that the human chromosome 8 had been successfully introduced into rat liver cancer cell line. Meanwhile, the recombination of human chromosome 8 with rat chromosome could be observed clearly by comparing the probe with its DAPI counterstain image. This recombination was found to be consistent in different metaphase cells.Figure 3 STS-PCR Amplification of D8S277. 1: chromosome donor cell line A9(neo8); 2: recipient cell line C5F; 3-7: microcell hybrids neo8/C5F-1-5; M: 100bp ladders.Figure 4a WCP-FISH analysis of neo8/C5F microcell hybrids.Figure 4b WCP-FISH of neo8/C5F microcell hybrids DAPI counterstain.DISCUSSIONMetastasis is a major problem puzzling both specialists of cancer biology and of clinical oncology. Thousands of cancer patients die of it each year while clinicians can do little to deal with it. The main reason is that the molecular mechanisms of metastasis have not been totally clarified yet. Researchers fascinated by metastasis hope that the discovery of metastasis suppressor genes could shed light on the solution of this problem, while hitherto few of them have been discovered[17,18].Metastasis suppressor genes suppress the formation of spontaneous, macroscopic metastases without affecting the growth rate of the primary cancer. Until presently, only five genes, nm23, KAI1, KISS1, MKK4 and BrMS1, have been proved to meet this criteria[17,18]. Despite the first metastasis suppressor gene nm23 was discovered by subtractive hybridization, most of encoding regions of metastasis suppressor genes have been discovered with the methodology of MMCT[18], which is thought to be the methodology particularly suitable for the functional localization of metastasis suppressor genes[19]. MMCT is established on somatic cellLiu H et al. Transfer of human chromosome 8 into rat liver cancer cell line C5F451 1 2 3 4 5 6 7 M500400300200hybrid. Before MMCT was introduced, researchers had found that somatic cell fusion of metastatic cell line with normal cells could change its metastatic phenotype[20]. Microcells that contain only one single human chromosome are obtained by th e m icro nuc lea tion an d e nucleation of hum an monochromosome donor cells, which include only one intact human chromosome labeled with selective markers such as the neo gene. The target monochromosomes are thereby introduced into recipient cells through the fusion of microcells with recipient cells and the cloning screening of microcell hybrids based on drug resistance conferred. Due to unknown reasons, the introduced chromosome is unstable and random loss or recombination can occur, which result in a series of microcell hybrid clones with different regions of the target chromosome. In this study, the human chromosome 8 introduced into C5F was found to not only have random loss by STS-PCR but also have recombined with rat chromosome by FISH. Those clones in all are called human monochromosome somatic cell panel[21]. In order to judge which region on the target chromosome harbors the gene of deserved function, spontaneous metastasis assay will be performed with these microcell hybrids. Based on the comparison of changes of metastatic phenotype and different chromosome regions each microcell hybrid contains, the target gene can be narrowed down to specific chromosome region.Most of current researches for the discovery of metastasis suppressor genes focused on the functional localization of metastasis suppressor genes for prostate cancer, melanoma and breast cancer[19, 22-31]. However, there hasn’t been any report of such work on liver cancer. Our previous study on the surgical samples of primary liver cancer through comparative genomic hybridization (CGH) showed that the loss of the short arm of human chromosome 8 had correlation with metastasis, which suggested that there could be metastasis suppressor gene(s) on chromosome 8p[6]. This discovery made our present research work imperative. In this study, the highly metastatic rat liver cancer cell line C5F, whose incidence of subcutaneous tumorigenecity is 100 % and that of lung metastasis is 89 %, was chosen as the recipient. The genetic background of rat facilitated STS-PCR screening of the regions remained on the chromosome introduced, as STS is a unique genetic marker different between species. One STS on human chromosome 8p23.3-22, D8S277, was chosen to confirm the introduction of human chromosome with the genomic DNA of microcell hybrids as templates. The results indicated that the microcell hybrids contained the STS specific for human chromosome 8, which could be taken as a proof for the successful chromosome transfer. Meanwhile some clones had deletions of STS, which showed that random losses of regions on human chromosome had occurred. This phenomenon built the basis for construction of human monochromosome panel. The target chromosome was randomly labeled with the G418 resistance gene, neo, which facilitated the selection of positive microcell hybrid clones. In the mean time, the chromosome donor cell line A9 is hypoxanthine-guanine phosphoribosyl transferase (HGPRT) deficient. Therefore A9 can’t survive in the media with HAT. Double selections of G418 and HAT were applied in this study to exclude the fake clones from either chromosome donor cell line or recipient cell line. Based on the acquisition of drug resistance, we can reasonably infer that the target chromosome has been transferred into C5F. The results of FISH provide the most direct evidence for the successful chromosome transfer. Recombination between human chromosome and rat chromosome was also observed in different metaphase cells, which showed that the recombination had been stabilized. The introduction of human chromosome can cause transient genetic instability, but with the selection the genetic change will become stable, which is the foundation for further analysis.All in all, the human chromosome 8 has been successfully transferred into the highly metastatic rat liver cancer cell line C5F, which builds solid basis for future researches on the discovery of metastasis suppressor genes for liver cancer. ACKNOWLEDGEMENTSWe are much grateful to Dr. Kumiko Ogawa (First Department of Pathology, Nagoaya City University, Japan) for his generous offer of C5F cell line, Prof. Hong-Xuan Lin (Plant Physiology and Ecology Insitute, Chinese Academy of Science, Shanghai, China) for his warmhearted provision and assistance in the usage of Hitachi high speed centrifuge and Dr. M.Z.Zdzienicka and Wouter Wiegant (Leiden Univerity Medical College, Netherland) for kindly providing us the detailed protocol of MMCT.REFERENCES1Tang ZY. Hepatocellular carcinoma- cause, treatment and metastasis. World J Gastroenterol 2001; 7: 445-4542Tang ZY. Hepatocellular carcinoma. J Gastroenterol Hepatol 2000;15 (Suppl): G1-G73Tang ZY, Yu YQ, Zhou XD, Ma ZC, Wu ZQ. Progress and pros-pects in hepatocellular carcinoma surgery. Ann Chir 1998; 52: 558-5634Qin LX, Tang ZY. The prognostic significance of clinical and pathological features in hepatocellular carcinoma. World J Gastroenterol 2002; 8: 193-1995Qin LX, Tang ZY. The prognostic molecular markers in hepato-cellular carcinoma. World J Gastroenterol 2002; 8: 385-3926Qin LX, Tang ZY, Sham JS, Ma ZC, Ye SL, Zhou XD, Wu ZQ, Trent JM, Guan XY. The association of chromosome 8p deletion and tumor metastasis in human hepatocellular carcinoma. Can-cer Res1999; 59: 5662-56657Ogawa K, Nakanishi H, Takeshita F, Futakuchi M, Asamoto M, Imaida K, Tatematsu M, Shirai T. Establishment of rat hepato-cellular carcinoma cell lines with differing metastatic potential in nude mice. Int J Cancer 2001; 91: 797-8028Fournier RE. A general high-efficiency procedure for produc-tion of microcell hybrids. Proc Natl Acad Sci U S A 1981; 78: 6349-63539McNeill CA, Brown RL. Genetic manipulation by means of microcell-mediated transfer of normal human chromosomes into recipient mouse cells. Proc Natl Acad Sci U S A 1980; 77: 5394-539810Kraakman-van der Zwet M,Overkamp WJ, Jaspers NG, Natarajan AT, Lohman PH, Zdzienicka MZ. Complementation of chromosomal aberrations in AT/NBS hybrids: inadequacy of RDS as an endpoint in complementation studies with immortal NBS cells. Mutation Res 2001; 485: 177-18511Cowell JK. Single-cell Isolation Cloning. Human Chromosome Principles and techniques, Verma RS and Babu A eds. 2nded.McGraw-Hill Inc USA 1995: 37-3812Sambrook J, Russell DW. Molecular cloning: A laboratory manual. 3rded. New York: Cold Spring Harbor Laboratory Press 2000: 542-54513Schuler GD. Electronic PCR: bridging the gap between genome mapping and genome sequencing. Trends Biotechnol 1998; 16: 456-45914Nelson DL. Applications of polymerase chain reaction methods in genome mapping. Curr Opin Genet Dev 1991; 1: 62-6815Verma RS,Babu A. Human Chromosome Principles and techniques. 2ned. Mc Graw-Hill Inc USA 1995: 12-1316Jenkins RB, Le Beau MM, Kraker WJ, Borell TJ, Stalboerger PG, Davis EM, Penland L, Fernald A, Espinosa R 3rd, Schaid DJ. Fluo-rescence in situ hybridization: a sensitive method for trisomy 8 detection in bone marrow specimens. Blood 1992; 79: 3307-3315 17Welch DR, Rinker-Schaeffer CW. What defines a useful marker of metastasis in human cancer? J Natl Cancer Inst 1999; 91: 1351-1353 18Yoshida BA, Sokoloff MM, Welch DR, Rinker-Schaeffer CW. Me-tastasis-suppressor genes: a review and perspective on an emerg-ing field. J Natl Cancer Inst2000; 92: 1717-1730452 ISSN 1007-9327 CN 14-1219/ R World J Gastroenterol March 15, 2003 Volume 9 Number 319Mashimo T, Watabe M, Cuthbert AP, Newbold RF, Rinker-Schaeffer CW, Helfer E, Watabe K. Human chromosome 16 sup-presses metastasis but not tumorigenesis in rat prostatic tumor cells. Cancer Res 1998; 58: 4572-457620Ramshaw IA, Carlsen S, Wang HC, Badenoch-Jones P. The use of cell fusion to analyze factors involved in tumor cell metastasis.Int J Cancer 1983; 32: 471-47821Overhauser J. Somatic Cell Hybrid Mapping Panels: Resources for Mapping Disease Genes. In: Human Genome Methods, Adolph KW ed. CRC Press LLC USA 1998: 258-26422Dong JT, Lamb PW, Rinker-Schaeffer CW, Vukanovic J, Ichikawa T, Issacs JT,Barrett JC. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science 1995; 268: 884-88623Chekmareva MA, Hollowell CM, Smith RC, Davis EM, LeBeau MM, Rinker-Schaeffer CW. Localization of prostate cancer me-tastasis suppressor activity on human chromosome 17. Prostate 1997; 33: 271-28024Nihei N, Kouprina N, Larionov V, Oshima J, Martin GM, Ichikawa T, Barrett JC. Functional evidence for a metastasis sup-pressor gene for rat prostate cancer within a 60-kilobase region on human chromosome 8p21-p12. Cancer Res 2002; 62: 367-370 25Ichikawa T, Ichikawa Y, Dong J, Hawkins AL, Griffin CA, Isaacs WB, OshimuraM, Barrett JC, Isaacs JT. Localization of metasta-sis suppressor gene(s) for prostatic cancer to the short arm of human chromosome 11. Cancer Res 1992; 52: 3486-349026Yoshida BA, Dubauskas Z, Chekmareva MA, Christiano TR, Stadler WM, Rinker-Schaeffer CW. Mitogen-activated protein kinase kinase 4/stress-activated protein/Erk kinase 1 (MKK4/ SEK1), a prostate cancer metastasis suppressor gene encoded by human chromosome 17. Cancer Res 1999; 59: 5483-548727Miele ME, Jewett MD, Goldberg SF, Hyatt DL, Morelli C, Gualandi F, Rimessi P, Hicks DJ, Weissman BE, Barbanti-Brodano G, Welch DR. A human melanoma metastasis-suppressor locus maps to 6q16.3-q23. Int J Cancer 2000; 86: 524-52828Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma me-tastasis-suppressor gene. J Natl Cancer Inst 1996; 88: 1731-1737 29Mashimo T, Goodarzi G, Watabe M, Cuthbert AP, Newbold RF, Pai SK, Hirota S, Hosobe S, Miura K, Bandyopadhyay S, Gross SC, Watabe K. Localization of a novel tumor metastasis suppres-sor region on the short arm of human chromosome 2. Genes Chro-mosomes Cancer 2000; 28: 285-29330Goodarzi G, Mashimo T, Watabe M, Cuthbert AP, Newbold RF, Pai SK, Hirota S, Hosobe S, Miura K, Bandyopadhyay S, Gross SC, Balaji KC, Watabe K. Identification of tumor metastasis sup-pressor region on the short arm of human chromosome 20. Genes Chromosomes Cancer 2001; 32: 33-4231Seraj MJ, Samant RS, Verderame MF, Welch DR. Functional evi-dence for a novel human breast carcinoma metastasis suppressor, BRMS1, encoded at chromosome 11q13. Cancer Res 2000; 60: 2764-2769Edited by Zhang JLiu H et al. Transfer of human chromosome 8 into rat liver cancer cell line C5F453。
