加拿大HnG医学技术有限公司
苯并[a]芘对生殖系统的毒性作用及其机制研究进展
![苯并[a]芘对生殖系统的毒性作用及其机制研究进展](https://img.taocdn.com/s3/m/9f44d8af760bf78a6529647d27284b73f24236e5.png)
生态毒理学报Asian Journal of Ecotoxicology第19卷第2期2024年4月V ol.19,No.2Apr.2024㊀㊀基金项目:国家自然科学基金项目(31960154);中央引导地方科技发展资金项目(2023ZY0004);内蒙古自治区 草原英才 工程青年创新创业人才项目(Q2022085);内蒙古自治区高等学校科学研究项目(NJZZ23017);内蒙古自治区自然科学基金项目(2023QN03047);内蒙古医科大学面上项目(YKD2022MS033)㊀㊀第一作者:王惠增(1997 ),女,硕士研究生,研究方向为生殖毒理㊁分子诊断,E -mail:******************* ㊀㊀*通信作者(Corresponding author ),E -mail:*********************.cnDOI:10.7524/AJE.1673-5897.20230413002王惠增,刘秉春,陈红,等.苯并[a]芘对生殖系统的毒性作用及其机制研究进展[J].生态毒理学报,2024,19(2):165-183Wang H Z,Liu B C,Chen H,et al.Research progress on toxic effects of benzo(a)pyrene on reproductive system and its mechanism [J].Asian Journal of Ecotoxicology,2024,19(2):165-183(in Chinese)苯并[a ]芘对生殖系统的毒性作用及其机制研究进展王惠增1,刘秉春2,陈红1,徐沛欣1,郭鑫1,袁建龙1,*1.内蒙古医科大学附属医院检验科,呼和浩特0100502.内蒙古医科大学附属医院干细胞实验室/内蒙古自治区肿瘤细胞基因检测应用与研究工程实验室,呼和浩特010050收稿日期:2023-04-13㊀㊀录用日期:2023-11-02摘要:苯并[a]芘(benzo(a)pyrene,BaP)作为多环芳烃(polycyclic aromatic hydrocarbons,PAHs)的成员,是最早发现也是最具有代表性的环境污染物,通过空气㊁食物㊁水源等途径进入人体,引起细胞氧化应激损伤㊁DNA 损伤和基因异常表达导致细胞死亡㊂研究表明雄性与雌性动物经BaP 染毒后,其生殖器官㊁生殖细胞甚至激素水平均会受到影响,进而影响受精卵形成和胚胎发育,造成不良妊娠结局㊂因此,近年来BaP 的生殖毒性受到广泛关注,其作用机制包括改变胞内活性氧水平㊁诱导细胞DNA 损伤以及调控生殖发育相关基因㊁类固醇合成相关基因和促凋亡基因影响生殖发育㊂BaP 作为环境毒物,不仅可以影响生态环境的稳定性,还可以影响生物的生殖发育,损害生态环境中的物种多样性,从长远来看,BaP 的不良影响不但会威胁到陆地与海洋生物种群的稳定,还会破坏陆地和海洋生态系统的功能㊂本文将从生殖健康㊁配子与合子形成以及胚胎发育的角度,详细阐述BaP 染毒对生殖系统的毒性作用与机制,为预防BaP 引起的生殖危害㊁减少不良妊娠结局提供理论依据,旨在为BaP 的环境毒性行为和对生物的毒性研究提供有效借鉴,为合理预防和缓解因接触BaP 等环境毒物而带来的健康影响提供参考㊂关键词:苯并[a]芘(BaP);生殖细胞;生殖毒性;生殖器官;激素;细胞毒性文章编号:1673-5897(2024)2-165-19㊀㊀中图分类号:X171.5㊀㊀文献标识码:AResearch Progress on Toxic Effects of Benzo (a )pyrene on Reproductive System and Its MechanismWang Huizeng 1,Liu Bingchun 2,Chen Hong 1,Xu Peixin 1,Guo Xin 1,Yuan Jianlong 1,*1.Department of Laboratory Medicine,The Affiliated Hospital of Inner Mongolia Medical University,Hohhot 010050,China2.Stem Cell Research Center,The Affiliated Hospital of Inner Mongolia Medical University/Inner Mongolia Autonomous Region Tumor Cell Gene Detection Application and Research Engineering Laboratory,Hohhot 010050,ChinaReceived 13April 2023㊀㊀accepted 2November 2023Abstract :Benzo(a)pyrene (BaP),as a member of the polycyclic aromatic hydrocarbons (PAHs),is the earliest dis -covered and most representative environmental pollutant.It enters the human body through the air,food,and water,causing cellular oxidative stress damage,DNA damage,and abnormal gene expression,leading to cell death.Studies have shown that when male and female animals are exposed to BaP,their reproductive organs,cells,and hormone166㊀生态毒理学报第19卷levels are affected,which in turn will affect the formation of fertilized eggs and embryonic development,resulting in adverse pregnancy outcomes.Hence,the reproductive toxicity of BaP has received more attention in recent years.Its mechanism of action on reproductive development includes alteration of intracellular reactive oxygen species levels,induction of cellular DNA damage,and modulation of genetic changes related to reproductive development,steroid synthesis and pro-apoptosis.BaP,as an environmental toxicant,could influence the stability of the ecological environment,the reproductive development of organisms and destroy the diversity of species in the ecosystems.In this review,we will detailly elaborate on the toxic effects and mechanisms of BaP on the reproductive system,and provide a theoretical evidence for prevention reproductive harm caused by BaP and the reduction of adverse pregnancy outcomes,with the aim to providing an effective reference for the study of BaP s toxicity to the environment and organisms,and for the rational prevention and mitigation of the health effects of exposure to BaP or other environmental toxins.Keywords:benzo(a)pyrene;germ cell;reproductive toxicity;genital organ;hormone;cytotoxicity㊀㊀PAHs是由2个或2个以上的稠环芳烃组成的有机化合物[1],由于其化学性质稳定且具有疏水性[2],因此多环芳烃可以在环境中稳定存在,是常见的环境污染物,广泛存在于油炸烧烤食物㊁香烟烟雾[3]㊁汽车尾气[4]㊁煤炭燃烧[5]等中㊂人类可以通过空气㊁饮用水㊁食物等不同方式暴露于多环芳烃[6]㊂此外,多环芳烃的亲脂性有利于它们在水生生物的脂肪中积累[7],并随着食物链进入人体,对人类健康产生威胁㊂BaP是多环芳烃中最具有代表性也是毒性最大的致癌物[8],可以诱发肺癌[9]㊁乳腺癌[10]等癌症,危害人类健康㊂BaP广泛存在于人类生活环境中,2019年公布的美国毒物和疾病登记机构物质优先清单中,BaP被列为第8名,在污染的空气[11]㊁土壤[12]㊁水源[13]㊁食物[14]中均可以检测到BaP㊂近年来,越来越多研究表明BaP与胚胎畸形[15]和不良妊娠[16]有着密切的联系㊂在妊娠早期暴露于BaP会导致小鼠胎儿畸形率增高[17]㊂此外,一项病例对照研究表明,接触BaP与早孕流产之间存在联系,妊娠女性发生流产的风险与血中BaP-DNA加合物的浓度成正比,这进一步说明BaP除了致癌性也具有生殖毒性㊂目前对于BaP的研究多聚焦于其诱发癌症[18-19]尤其是肺癌[20]这一方面,虽有研究表明BaP 具有生殖毒性,其生殖毒性机理尚未研究透彻㊂本综述的目的是总结BaP生殖毒性相关文章,讨论BaP导致生殖毒性的潜在分子机制㊂1㊀BaP在生殖方面的主要致毒途径(The main toxic pathway of BaP in reproduction)近些年研究发现,BaP发挥其致毒作用主要有3种途径:(1)通过氧化应激影响细胞正常代谢;(2)BaP可以与DNA形成加合物,进而导致DNA损伤;(3)BaP可以通过调控基因表达,发挥其毒性作用㊂BaP致毒途径是多种机制相辅相成㊂由于生殖对繁育后代具有重要意义,因此研究BaP的生殖毒性已成为科学家们的研究重点,下文将重点总结BaP的生殖毒性机制㊂1.1㊀氧化应激(Oxidative stress)BaP进入细胞后,通过AHR途径诱导细胞发生氧化应激反应,其主要过程为:BaP刺激细胞质中的一种转录因子 芳香族化合物受体(aryl hydrocar-bon receptor,AHR)[21],使其转入到细胞核后,再与芳香族化合物受体核转运蛋白(aryl hydrocarbon recep-tor nuclear transporter,ARNT)结合形成异二聚体[22],结合在下游靶基因上,激活细胞色素P450目标基因的异常表达,包括细胞色素P4501A1(cytochrome P450family1subfamily A member1,CYP1A1)㊁细胞色素P4501A1(cytochrome P450family1subfamily A member2,CYP1A2)㊁细胞色素P4501B1(cyto-chrome P450family1subfamily B member1, CYP1B1)[21,23],进而引起细胞产生大量活性氧(reac-tive oxygen species,ROS),使机体发生氧化应激反应,如果体内的活性氧产生过多,超出了细胞的清除能力,会影响细胞的正常代谢甚至会破坏细胞结构㊂低㊁高剂量的BaP均可导致小鼠卵母细胞功能障碍,降低精卵结合与融合率,这与线粒体ROS水平增加和卵膜脂质过氧化密切相关[24]㊂Zhang等[25]发现BaP可以削弱雌鼠的繁殖能力,通过增加雌鼠卵母细胞中ROS,扰乱纺锤体组装,染色体配对,阻滞卵母细胞减数分裂过程㊂BaP诱导的氧化应激不仅仅通过产生ROS这一条途径,还可以通过降低过氧第2期王惠增等:苯并[a]芘对生殖系统的毒性作用及其机制研究进展167㊀化氢酶(catalase,CAT)㊁抗坏血酸过氧化物酶(ascor-bate peroxidase,AP)㊁谷胱甘肽过氧化物酶(glutathione peroxidase,GPX)㊁超氧化物歧化酶(superoxide dis-mutase,SOD)㊁谷胱甘肽还原酶(glutathione reductase, GR)等抗氧化酶的活性[26-27]以及促进炎症细胞因子表达[28]导致氧化应激的发生,最终引起细胞功能受损㊂1.2㊀BPDE引起DNA损伤(BPDE induces DNA damage)BaP进入体内经过一系列氧化代谢反应,生成二羟环氧苯并[a]芘(BaP-7,8-dihydrodiol-9,10-epoxide, BPDE),进而发挥其毒性,Penning[29]认为生成BPDE 的主要途径是在细胞色素P450酶的催化下,BaP末端的苯环上发生单加氧化反应,生成BaP-7,8-环氧化物(BaP-7,8epoxide),在环氧化物水解酶作用下转化为BaP-7,8-二氢二醇(BaP-7,8diol),该过程循环往复最终形成致癌物 BPDE[30-32]㊂BPDE可以与DNA共价结合形成加合物,造成DNA损伤㊂Shiizaki等[33]提出一个关于BaP-DNA加合物成因的假设,即CYP1A1是BaP被激活形成BPDE反应中的关键酶,这与Bukowska等[32]提出的观点一致㊂Einaudi等[34]通过建立BaP染毒的雌性小鼠模型,发现BaP可以导致卵母细胞与卵丘细胞DNA损伤,并且他们认为导致DNA断裂的主要原因是由于细胞中的修复机制对BPDE-DNA加合物切除和修复导致的㊂Zhan等[35]研究表明BaP形成的DNA加合物可以干扰DNA复制,进一步引起胚胎的DNA损伤,影响胚胎的发育㊂Zhan等[35]进一步研究发现DNA加合物与ROS共同造成基因组严重损伤,还可以引起卵裂球的端粒功能障碍,最终引起胚胎的异常㊂Miao等[36]发现BaP会引起猪卵母细胞纺锤体组装缺陷进一步引起减数分裂停滞,而导致这一结果的原因可能是DNA加合物引起的㊂Zhang 等[25]将小鼠卵母细胞暴露于BaP后,发现纺锤体的组装㊁染色体的排列和着丝点-微管附着均被破坏,这可能与DNA加合物的形成有关联,与Miao的设想一致㊂1.3㊀基因表达调控(Regulation of gene expression)基因表达调控是生物学研究的重要内容之一,在细胞分化发育的不同时期,基因表达的种类和强度各不相同,共同决定着细胞的形态与功能;细胞为了适应环境变化改变自身的基因表达有利于生存,因而基因表达调控十分重要㊂海洋污染问题日趋严重,BaP具有水生生物生殖毒性,是造成海洋污染的重要原因之一,受到广泛关注㊂有研究发现BaP生殖毒性的潜在分子机制是通过调控相关基因表达㊂数字基因表达技术表明BaP对雄性栉孔扇贝睾丸中的生殖基因有影响,其中热休克蛋白90㊁细胞色素P4503A㊁凋亡抑制蛋白3个基因的改变会引起睾丸组织损伤,此外BaP与性激素合成和睾丸发育相关基因有密切联系[37]㊂Albornoz-Abud等[38]研究表明苯并芘可以通过调控GH/IGF轴发挥其生殖毒性,急性暴露于BaP会导致尼罗罗非鱼睾丸中内分泌相关基因:胰岛素样生长因子1(insulin-likegrowth factor1,IGF1)和生长激素受体基因1(growth hormone receptor1,GHR1)基因表达降低,并造成发育问题㊂BaP通过基因调控引起的生殖毒性不仅仅在海洋生物中体现,陆地生物也同样受这一机制调控㊂BaP通过影响父本基因,最终影响胚胎发育㊂用BaP染毒的雄性小鼠进行体外受精后,发现在8-细胞期和囊胚期存在基因表达异常,包括调控细胞周期以及DNA修复的基因[39]㊂妊娠黄体可以分泌雌孕激素,在生殖系统中发挥重要作用,黄体的发育与血管内皮生成因子有着密切联系[40]㊂苯并芘可以使血管内皮生成因子相关基因,如血管生成素-1(an-giopoietin-1,Ang-1)㊁血管内皮细胞生长因子受体(vascular endothelial growth factor,VEGFR)㊁内皮细胞TEK酪氨酸激酶表达下调,并增加抗血管生成因子血小板反应蛋白(recombinant thrombospondin1, THBS1)的表达,还影响了对黄体血管系统建立至关重要的基因Notch1㊁DLL4㊁Jag1和Hay2的表达,破坏了黄体血管网络系统的形成,最终影响了妊娠过程中黄体的内分泌功能[41]㊂综上所述,在3种BaP发挥致毒作用的机制中(图1),BaP诱导生殖发育相关基因表达异常或提高促凋亡基因表达起主导作用,也是目前研究较为透彻的机制(图2),下面将从雄性生殖㊁雌性生殖以及胚胎发育3个角度详述BaP的毒性机制㊂2㊀BaP的雄性生殖毒性(Male reproductive toxici-ty of BaP)2.1㊀BaP对雄性激素的毒性(Toxicity of BaP to an-drogens)BaP作为内分泌干扰物主要影响睾酮水平[42],睾酮主要是由睾丸间质细胞合成分泌的,其主要成分为类固醇㊂BaP可以降低睾酮的转化率[43]和(或)睾酮的浓度[44]㊂有研究表明睾丸巨噬细胞分泌的白介素1β(interleukin-1β,IL-1β)和肿瘤坏死因子α168㊀生态毒理学报第19卷(tumor necrosis factor α,TNF α)通过抑制类固醇生成急性调节蛋白(steroidogenic acute regulatory protein,STAR)表达进一步抑制间质细胞合成睾酮[45]㊂Zheng 等[46]发现BaP 通过增加IL -1β的表达,显著抑制雄性大鼠睾酮的产生,他们还发现BaP 可以改变睾丸巨噬细胞亚群,激活ED2+睾丸巨噬细胞并促进了IL -1β的产生,最终抑制雄性大鼠睾酮合成㊂此外,3β-羟基类固醇脱氢酶(3β-hydroxysteroid dehy -drogenase,3β-HSD)与细胞色素P450胆固醇侧链裂解酶(cholesterol side -chain lyase P450scc,P450scc)在间质细胞合成睾酮中起着重要作用[47],其表达改变时会影响睾酮水平;STAR 表达的下调也可以导致睾酮合成减少[48-49]㊂雄性大鼠用BaP 灌胃90d 后,检测到BaP 下调间质细胞中的STAR ㊁3β-HSD 以及细胞色素P45017A1(cytochrome P450family 17subfamily A member 1,CYP17A1)表达,并上调P450scc 表达,进而降低大鼠睾丸间质细胞生成睾酮的能力[50]㊂Sheweita 等[51]发现BaP 降低类固醇合成酶CYP17A1和17β-羟基类固醇脱氢酶(17β-hydroxysteroid dehydrogenase,17β-HSD)蛋白表达,使大鼠血浆睾酮浓度降低㊂Banerjee 等[52]进一步验证了BaP 通过抑制类固醇生成蛋白表达,如细胞色素P450ⅡA1(cytochrome P450family Ⅱsubfamily A member 1,CYP ⅡA1)㊁STAR ㊁3β-HSD ㊁17β-HSD ,进一步降低血清睾酮水平,2021年Daoud 等[53]再一次证实了上述观点㊂Yang 等[54]发现BaP 也可以通过影响3β-HSD ㊁CYP17和17β-HSD 表达进一步扰乱雄性栉孔扇贝的激素水平㊂Booc 等[55]研究发现BaP 可降低雄性底鳉的睾酮水平,与其他动物不同的是BaP 并非通过调控类固醇相关基因表达造成这一结果,而是可能通过精原细胞包囊大小进而影响睾酮水平㊂综上所述,BaP 主要通过改变类固醇生成相关基因与酶的表达,抑制睾酮的生成,对雄性的生殖发育产生不利影响㊂epoxide(BPDE)图1㊀BaP 致毒途径机制注:AHR 表示芳香族化合物受体,ARNT 表示芳香族化合物受体核转运蛋白,HSP90表示热休克蛋白90,CYP450表示细胞色素P450,CYP17A1表示细胞色素P45017A1,STAR 表示类固醇生成急性调节蛋白,3β-HSD 表示3β-羟基类固醇脱氢酶,17β-HSD 表示17β-羟基类固醇脱氢酶,Caspase -3表示半胱氨酸蛋白酶-3,Caspase -9表示半胱氨酸蛋白酶-9,Bax 表示Bcl -2相关X 蛋白㊂Fig.1㊀Mechanism of BaP toxicity pathwayNote:AHR represents aryl hydrocarbon receptor,ARNT represents aryl hydrocarbon receptor nuclear transporter,HSP90represents heat shock protein 90,CYP450represents cytochrome P450family,CYP17A1represents cytochrome P450family 17subfamily A member 1,STAR represents steroidogenic acute regulatory protein,3β-HSD represents 3β-hydroxysteroid dehydrogenase,17β-HSD represents 17β-hydroxysteroid dehydrogenase,and Bax represents Bcl -2associated X protein.第2期王惠增等:苯并[a]芘对生殖系统的毒性作用及其机制研究进展169㊀图2㊀BaP通过基因调控引起生殖毒性注:GnRH2表示促性腺激素释放激素,GnRH3表示促性腺激素释放激素,IL-1β表示白介素1β,CYP17A1表示细胞色素P45017A1,STAR表示类固醇生成急性调节蛋白,3β-HSD表示3β-羟基类固醇脱氢酶,17β-HSD表示17β-羟基类固醇脱氢酶,CYP1A1代表细胞色素P4501A1, P450scc代表细胞色素P450胆固醇侧链裂解酶,Adcy-PKA代表上游腺苷环化酶-蛋白激酶,Caspase-3表示半胱氨酸蛋白酶-3,Caspase-9表示半胱氨酸蛋白酶-9,Bax表示Bcl-2相关X蛋白,Hsp90aB1代表90kDa热休克蛋白aB1,VTG代表卵黄蛋白原,CD34代表分化簇34, AMH代表抗缪勒管激素,CCND2代表细胞周期蛋白D2,FOXO1代表叉头框蛋白O1,HoxA10代表同源盒基因,BMP2代表骨形态发生蛋白-2,IBA1代表离子钙结合衔接分子1,SNCA代表重组人α-突触核蛋白,CYP19a代表细胞色素P450家族19亚家族a㊂Fig.2㊀BaP causes reproductive toxicity through gene regulationNote:GnRH2represents gonadotropin-releasing hormone2,GnRH3represents gonadotropin-releasing hormone3,IL-1βrepresents interleukin-1β, CYP17A1represents cytochrome P450family17subfamily A member1,STAR represents steroidogenic acute regulatory protein,3β-HSD represents3β-hydroxysteroid dehydrogenase,17β-HSD represents17β-hydroxysteroid dehydrogenase,CYP1A1represents cytochrome P450family1subfamily A member1,P450scc represents cholesterol side-chain lyase P450scc,Adcy-PKA represents adenylate cyclase-protein kinase,Bax represents Bcl-2associated X protein,Hsp90aB1represents recombinant heat shock protein90kDa alpha B1, VTG represents vitellogenin,CD34represents cluster designation34,AMH represents anti-Müllerian hormone, CCND2represents cyclin-D2,FOXO1represents forkhead box O1,HoxA10represents homeobox A10,BMP2represents bone morphogenetic protein-2,IBA1represents ionized calcium-binding adapter molecule1,SNCA representsrecombinant human alpha-synuclein,CYP19a represents cytochrome P450family19subfamily a.2.2㊀BaP对精子的毒性(Toxicity of BaP to sperm) 2.2.1㊀BaP减少精子生成(BaP reduces spermato-genesis)哺乳动物雄性生殖器官主要有睾丸㊁附睾㊁输精管等,其中睾丸的主要作用是生成精子和产生雄性激素,BaP主要通过损害睾丸进一步影响精子生成㊂BaP通过氧化应激或基因调控介导睾丸细胞凋亡,影响睾丸功能受损,减少精子数量㊂Banerjee等[52]证实BaP激活P38蛋白激酶(P38mitogen activated protein kinase,P38MAPK)通路来增加睾丸细胞内ROS,并降低细胞中的抗氧化酶活性[56],使睾丸细胞氧化应激损伤,减少精子的生成㊂Sheweita等[51]研究发现BaP通过降低睾丸组织中抗氧化酶CAT㊁SOD㊁GPX的活性,增加ROS水平,导致睾丸细胞线粒体膜破裂,进而引起睾丸组织凋亡㊂BaP还可通过AHR途径降低睾丸中CAT㊁SOD活性,升高H2O2含量,诱导睾丸细胞氧化应激,影响睾丸功能[57]㊂上述均为BaP对小鼠的生殖毒性,Tian等[58]发现BaP通过可引起雄性栉孔扇贝精巢氧化应激损伤,进一步减少精子生成㊂此外,BaP可以通过基因调控诱导睾丸细胞凋亡,提高睾丸细胞内的凋亡蛋白半胱氨酸蛋白酶-3(Caspase-3)和半胱氨酸蛋白170㊀生态毒理学报第19卷酶-9(Caspase-9)表达;促进细胞色素C转位到细胞质,启动线粒体凋亡途径,导致睾丸细胞凋亡,进一步导致精子生成减少[52,59]㊂BaP不仅通过影响睾丸功能减少精子生成,而且可以直接影响精子生成过程㊂Verhofstad等[60]的研究表明在精子发育各个阶段均可以检测到BPDE 导致的精子DNA损伤,这也是精子数量减少的原因之一㊂BaP可以导致雄鼠精子功能缺陷以及生育能力下降,并且Mohamed等[61]的实验证明了BaP的生殖毒性具有遗传性,但毒性随着子代数增加逐渐减弱㊂BaP可以减少精母细胞和次级精母细胞进入中晚期粗线期,阻止减数分裂过程的完成,导致精子生成减少[62]㊂此外,BaP诱导的氧化应激会降低精原细胞的存活率,并且通过下调基质金属蛋白酶(matrix metalloproteinase,MMP)水平以及上调促凋亡因子Caspase-3和Caspase-9表达促进精原细胞凋亡[63]㊂BaP作为广泛存在于生态系统中的环境污染物,不仅使陆地雄性动物精子生成异常,还影响水生生态系统中的雄性动物的精子生成㊂BaP可以通过基因调控扰乱雄性栉孔扇贝的精子发生相关基因:细胞周期蛋白D2(cyclin-D2,CCND2),联会复合体3㊁核呼吸因子1和水通道蛋白9,进一步减少精子生成[54]㊂斑马鱼胚胎暴露于BaP后,其睾丸中生殖细胞特异基因的启动子发生甲基化上调,进一步下调相关基因表达,最终抑制精子生成,影响雄性斑马鱼的生殖能力[64]㊂2.2.2㊀BaP降低精子活力(BaP reduces sperm motility)BaP可以损害睾丸和附睾的内分泌功能,从而导致储存的精子活力下降[65-67]㊂睾丸的质量和大小与精子的数量和活力成正比[68],雄性小鼠用BaP连续灌胃60d,检测到小鼠的睾丸质量明显降低,精子的活力也随之降低[69]㊂小鼠暴露于BaP后,其睾丸支持细胞和间质细胞均凋亡,进而影响精子发生过程,最终导致精子活力减弱[69]㊂畸形精子的活力及存活率显著低于正常精子,BaP暴露会导致精子形态异常,畸形精子大幅增加,主要异常表现为无尾㊁双头㊁中段弯曲[57]㊂Xu等[59]验证了BaP可导致精子活动力降低,精子头㊁尾部畸形率以及总畸形率均显著升高㊂最新研究表明BaP改变睾丸激素水平引起雄性交配强度减弱,降低精子质量,引起畸形精子增多[70-71]㊂有研究表明精子短端粒可能是导致男性不育的原因之一[72],Ling等[73]研究发现BaP可以使精子端粒变短,且与剂量成反比㊂3㊀BaP的雌性生殖毒性(Female reproductive toxicity of BaP)3.1㊀BaP对雌性激素的毒性(Toxicity of BaP to es-trogen)BaP作为一种常见的环境污染物,是海洋环境污染原因之一,影响水生动物的繁殖㊂雌孕激素对雌性发育有不可或缺的作用,而BaP作为内分泌干扰物可以降低水生动物血浆中的孕酮㊁雌激素和催乳素浓度[74]㊂为进一步探究其发生机制,Tian等[58]用不同浓度的BaP处理雌性栉孔扇贝,发现BaP可以导致类固醇合成相关酶(3β-HSD㊁CYP17㊁17β-HSD)表达下降,并呈剂量依赖性;高浓度的BaP还可抑制AHR㊁ARNT㊁CYP1A1以及17β-雌二醇-雌激素受体转录,2种机制相辅相成,共同抑制雌孕激素的生成㊂BaP通过干扰激素膜受体降低三疣梭子蟹的雌二醇(estradiol,E2)浓度[75]㊂斑马鱼胚胎暴露于BaP会导致成年雌鱼卵巢中E2水平下降,其机制为雌鱼脑中促性腺激素释放激素(gonadotropin-releasing hormone,GnRH)基因中的GnRH3的甲基化水平显著升高,并下调GnRH3mRNA表达,从而影响E2的产生[76]㊂与斑马鱼报道相反的是BaP可促进雌性海马GnRH2和GnRH3mRNA的表达,并导致血浆中E2水平显著下降[77]㊂2种相反结果可能与BaP的浓度㊁作用时间以及实验对象不同有关㊂Yang等[78]发现BaP抑制雌性栉孔扇贝的上游腺苷环化酶-蛋白激酶(adenylate cyclase-protein ki-nase,Adcy-PKA)信号通路,下调促性腺激素受体转录水平,如促卵泡激素受体(follicle-stimulating hor-mone receptor,FSHR)和黄体生成素/绒毛膜促性腺激素受体(luteinizing hormone/choriogonadotropin re-ceptor,LHCGR),导致类固醇生成酶(3β-HSD㊁CYP17㊁17β-HSD)表达减少,最终引起抗雌激素效应㊂Kennedy和Smyth[79]发现雌鲑鱼体内E2的减少并非是通过常规的BaP作用于类固醇机制,而是通过其他内分泌干扰机制来对抗雌激素的方式改变了血浆E2的浓度,这种机制有待进一步研究㊂综上所述,BaP主要通过基因表达调控这一途径降低雌性体内E2和孕酮水平,进而影响雌性的生殖发育㊂3.2㊀BaP对卵巢的毒性(Toxicity of BaP to ovary)卵巢是雌性生殖发育中最重要的生殖器官,具有排卵和内分泌功能,对维持雌性激素水平至关重要,暴露于BaP会扰乱卵巢的结构与功能,进一步第2期王惠增等:苯并[a]芘对生殖系统的毒性作用及其机制研究进展171㊀影响生育㊁妊娠㊂高剂量的BaP可以导致卵巢细胞退化并出现管状结构,而这些组织学变化属于癌前病变[80]㊂Rahmani等[81]发现BaP通过氧化应激导致卵巢表面上皮内陷㊁细胞堆积㊁管状结构形成,卵巢间质出现间质水肿㊁出血等病理学改变,并且BaP 诱导卵巢中Caspase-3表达升高,影响卵巢的生理功能,与睾丸相比,BaP对卵巢的危害更严重,这是因为在BaP处理后,胎儿卵巢中促细胞凋亡蛋白Bcl-2相关X蛋白(Bcl-2associated X protein,Bax)表达增加,并激活下游Caspase-3和Caspase-9,导致卵巢细胞凋亡[82]㊂卵黄蛋白原(vitellogenin,VTG)和CCND2是雌激素介导的卵巢发育相关基因[83],BaP可以下调VTG和CCND2表达,造成雌性栉孔扇贝卵巢受损,组织学检查发现,BaP可引起卵巢发育延迟和卵母细胞退化,并且卵巢的病变情况随着染毒时间和染毒剂量的增加而严重[78]㊂研究发现BaP可以抑制脂联素受体1(adiponectin receptor protein1,AdipoR1)和脂联素受体2(adiponectin receptor protein2,AdipoR2)表达,进而影响卵巢功能[84]㊂最新研究表明,BaP及其代谢产物BPDE可抑制妊娠小鼠卵巢中腺嘌呤核苷酸转运体1(adenine nucleotide translocator1,ANT1)的表达,进一步研究发现ANT1的过表达可以修复BPDE引起的有丝分裂缺陷,恢复卵巢功能[85]㊂3.3㊀BaP对雌性生殖细胞的毒性(Toxicity of BaP to female germ cells)3.3.1㊀BaP影响卵泡发生和发育(BaP affects folli-cular genesis and development)卵泡发育是女性正常的生理过程,卵泡的发育情况直接关系到后代繁殖㊂卵泡作为卵巢的功能单位,支持卵母细胞的发育和成熟[86]㊂卵泡的生长发育过程相当复杂,原始卵泡经历初级卵泡㊁窦前㊁窦卵泡才能发育为成熟卵泡[87-88]㊂有报道称BaP作为卵毒物质,可以破坏原始卵泡[89],或者使原始卵泡迅速枯竭[90],BaP还可以通过香烟烟雾进入卵泡液中,对卵泡发育产生不利影响[91]㊂Sobinoff等[24]研究了BaP卵毒性的机制,连续用BaP处理雌性小鼠7d会导致卵巢中的原始卵泡显著减少,卵泡闭锁,其具体机制为BaP通过干扰AHR发育信号破坏卵泡形成㊂有报道称BaP不仅可以减少或耗尽原始卵泡和初级卵泡的数量[89-90],还可以抑制卵泡生长发育,即BaP处理过的卵泡均发育不到窦前阶段[92]㊂Sadeu和Foster[93]将小鼠卵泡暴露于不同浓度的BaP,发现卵泡存活率均降低,其中高浓度的BaP会抑制窦卵泡发育,使卵泡停滞于窦前卵泡阶段,窦卵泡比例显著减少㊂抗缪勒管激素(anti-Müllerian hormone,AMH)浓度增加与卵泡发育停滞有关[94-95]㊂有研究表明BaP可以通过减少AMH生成,促进卵泡募集到卵泡池中,最终加快卵泡枯竭的速度[96]㊂Sadeu和Foster[93]进一步探索了BaP诱导卵泡发育异常的关键分子途径,发现BaP暴露通过激活窦前㊁窦卵泡和成熟卵泡中AHR信号通路,进一步促进促凋亡因子Bax激活,此外,BaP暴露还会导致90kDa热休克蛋白aB1(recombinant heat shock protein90kDa alpha B1,Hsp90aB1)基因表达上调,导致卵泡生长延迟和存活率下降㊂卵泡生长和卵泡发育在雌性哺乳动物生殖中有着重要地位,BaP不但可以通过基因表达调控导致卵泡生长发育异常,还可能通过氧化应激影响卵泡发育㊂3.3.2㊀BaP影响卵母细胞功能(BaP affects oocyte function)BaP可使卵母细胞线粒体内ROS水平升高,导致精-卵结合和融合障碍,影响动物的繁殖[24,36]㊂BaP可导致卵母细胞和卵丘细胞DNA断裂,细胞功能障碍,影响卵母细胞进一步发育,精卵融合失败[34],这也是BaP生殖毒性机制之一㊂卵母细胞的减数分裂在卵母细胞成熟与成功受精中起着重要作用[97],BaP诱导卵母细胞减数分裂异常,卵母细胞功能障碍,不利于动物繁殖㊂BaP可以阻滞猪卵母细胞减数分裂,使部分卵母细胞停滞在MⅡ期,进一步检测发现BaP通过降低乙酰化α-微管蛋白,导致微管不稳定,损害纺锤体组装,从而干扰卵母细胞减数分裂过程[36]㊂Sui等[98]通过将雌鼠暴露于BaP检测其对子代的影响,验证了BaP对卵母细胞的遗传毒性:生发泡破裂(germinal vesicle breakdown, GVBD)是卵母细胞成熟的关键事件,母体暴露BaP 会降低子代GVBD率[99];并且BaP会扰乱子代卵母细胞的纺锤体组装和染色体配对,使卵母细胞减数分裂停滞;最后,雌鼠暴露于BaP可导致子代卵母细胞基因组高甲基化,损害卵母细胞的发育能力㊂综上所述,母系BaP暴露损害了子代卵母细胞的进一步发育,这与上文Miao等[36]研究结果相一致㊂4㊀BaP对胎儿或胚胎的生殖毒性(Reproductive toxicity of BaP to the fetus or embryo)4.1㊀BaP的胚胎发育毒性(Embryonic developmen-tal toxicity of BaP)早有研究表明,吸烟损害身体健康,还对孕妇以。
国内外独立医学实验室
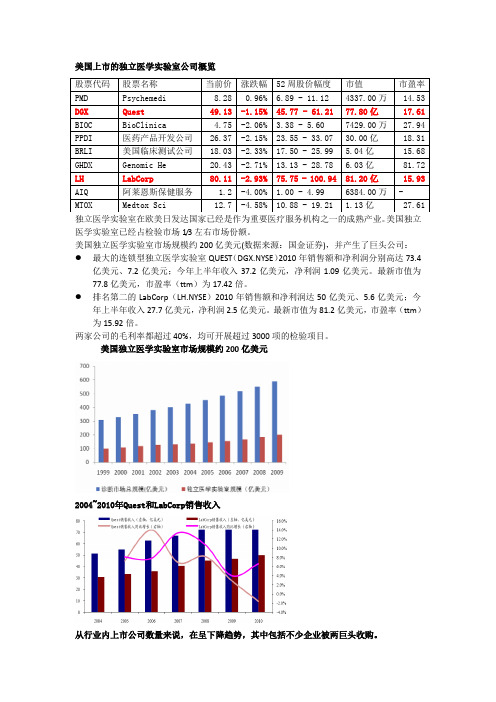
美国上市的独立医学实验室公司概览独立医学实验室在欧美日发达国家已经是作为重要医疗服务机构之一的成熟产业。
美国独立医学实验室已经占检验市场1/3左右市场份额。
美国独立医学实验室市场规模约200亿美元(数据来源:国金证券),并产生了巨头公司:●最大的连锁型独立医学实验室QUEST(DGX.NYSE)2010年销售额和净利润分别高达73.4亿美元、7.2亿美元;今年上半年收入37.2亿美元,净利润1.09亿美元。
最新市值为77.8亿美元,市盈率(ttm)为17.42倍。
●排名第二的LabCorp(LH.NYSE)2010年销售额和净利润达50亿美元、5.6亿美元;今年上半年收入27.7亿美元,净利润2.5亿美元。
最新市值为81.2亿美元,市盈率(ttm)为15.92倍。
两家公司的毛利率都超过40%,均可开展超过3000项的检验项目。
美国独立医学实验室市场规模约200亿美元2004~2010年Quest和LabCorp销售收入从行业内上市公司数量来说,在呈下降趋势,其中包括不少企业被两巨头收购。
独立医学实验室在中国:独立医学实验室在国内还处于起步阶段,市场规模仅有10 亿元,仅占国内医学诊断市场规模的2-3%。
虽然我国公立大医院占主导的特殊性,但相比较于国外30%多的市场份额,国内独立医学实验室还有很大的发展空间。
目前国内已有超过100家独立医学实验室,主要集中在沿海发达地区。
市场份额领先的包括广州金域、杭州艾迪康、杭州迪安、广州达安高新。
整体竞争格局出现“全国仍然分散、区域初步集中”的格局。
广州金域全国来看份额最大,但在长三角迪安诊断份额最大,其次是杭州艾迪康。
国内主要的医学诊断外包企业各地的小规模连锁或单体独立医学实验室较多,而规模最大的独立医学实验室,也只能开展1,000 多项诊断项目(未来新项目的引进也将带来新的利润增长点)。
各地区发展也很不平衡,相比成熟市场的业务种类和地域覆盖,我国第三方医学诊断行业还有较大的增长空间,前景被广泛看好。
(Annex19)totheEUGMPGuide-EuropeanCommission

EUROPEAN COMMISSIONENTERPRISE DIRECTORATE-GENERALSingle market : management & legislation for consumer goodsPharmaceuticals : regulatory framework and market authorisationsBrussels, 23 June 2004Ad Hoc GMP Inspections Services GroupGood Manufacturing PracticeProposed Addition (Annex 19) to the EU GMP GuideTitle:Reference Samples and Retention SamplesAgreed by ad hoc GMP inspectors services group April 2004 Released for public consultation 15 July 2004 Deadline for comments 15 January 2005 Final draft adopted by ad hoc GMP inspectors services groupAdopted by Pharmaceutical CommitteeDate for coming into operationNote:The new annex to the EU GMP Guide provides guidance on the taking and holding of refer-ence samples of starting materials, packaging materials and finished products as well as for retention samples of finished products. The annex provides definitions of the terms "refer-ence sample" and "retention sample", which are often incorrectly considered as synonyms. The guidance is wide ranging in scope and includes the case of multiple manufacturing sites, the position with respect to importers and what should happen when a manufacturing site ceases to operate. Updated guidance is also given on the size of reference samples and a con-sequential amendment will therefore be necessary to Chapter 6 section 14 of the GMP Guide to maintain consistency.PROPOSED ANNEX TO EC GUIDE TO GOOD MANUFACTURING PRACTICE REFERENCE SAMPLES AND RETENTION SAMPLES1. Scope1.1 This Annex to the Guide to Good Manufacturing Practice for Medicinal Products (“the Guide”) gives guidance on the taking and holding of reference samples of starting materials, packag-ing materials or finished products and retention samples of finished products.1.2 The guidance may also be applied to investigational medicinal products, subject to any differ-ence mentioned in Commission Directive 2003/94/EC and any more specific guidance in Annex 13 to the Guide.1.3 This annex also includes guidance on the taking of retention samples for parallel imported / distributed medicinal products.2. Principle2.1 Samples are retained to fulfil two purposes; firstly to provide a sample for analytical testing and secondly to provide a specimen of the fully finished product. Samples may therefore fall into two categories:Reference sample: a sample of a batch of starting material, packaging material or finished product which is stored for the purpose of being analysed should the need arise during the shelf life of the batch concerned. Where stability permits, reference samples from important intermediate stages of manufacture should also be kept. Examples include tablet cores and different stages of coating proc-esses.Retention sample: a sample of a fully packaged unit from a batch of finished product. It is stored for identification purposes. For example, presentation, packaging, labelling, summary of product charac-teristics / patient information leaflet, batch number, expiry date) should the need arise during the shelf life of the batch concerned.For finished products, in many instances the reference and retention samples will be presented identi-cally, i.e. as fully packaged units. In such circumstances, reference and retention samples may be re-garded as interchangeable.2.2 It is necessary for the manufacturer / importer / site of batch release, as appropriate, to keep reference and/or retention samples from each batch of finished product and, for the manufacturer to keep a reference sample from each delivery of a batch of starting material (subject to certain excep-tions – see3.2 below). Each packaging site should keep reference samples of each batch of primary and printed packaging materials.2.3 The reference and/or retention samples serve as a record of the batch of finished product or starting material and can be assessed in the event of, for example, a dosage form quality complaint, a query relating to compliance with the marketing authorisation, a labelling/packaging query, a pharma-covigilance report or a stability query.3. Duration of Storage3.1 Reference and retention samples from each batch of finished product should be retained for at least one year after the expiry date. The reference sample should be contained in its finished primary packaging or in packaging composed of the same material as the primary container in which the prod-uct is marketed (for veterinary medicinal products other than immunologicals, see also Annex 4, para-graphs 8 & 9).3.2 Unless a longer period is required under the law of the Member State of manufacture, samples of starting materials (other than solvents, gases or water used in the manufacturing process) shall be retained for at least two years after the release of product. That period may be shortened if the period of stability of the material, as indicated in the relevant specification, is shorter.4. Size of Reference and Retention Samples4.1 The reference sample should be of sufficient size to permit the carrying out, on two occasions, of the full analytical controls on the batch in accordance with the Marketing Authorisation File which has been assessed and approved by the relevant Competent Authority / Authorities. Any proposed exception to this should be justified to, and agreed with, the relevant competent authority.4.2 Where applicable, national requirements relating to the size of reference samples and, if nec-essary, retention samples, should be followed.4.3 Reference samples should be representative of the batch of starting material or finished prod-uct from which they are taken. Samples should include the most stressed part of a process (e.g. begin-ning or end of a process). Where a batch is packaged in two, or more, distinct packaging operations, at least one retention sample should be taken from each individual packaging operation. Any pro-posed exception to this should be justified to, and agreed with, the relevant competent authority.4.4 It should be ensured that all necessary analytical materials and equipment are still available, or are readily obtainable, in order to carry out all tests given in the specification until one year after ex-piry of the last batch manufactured. This applies also to analytical reference materials used in tests which have been superseded.5. StorageConditions5.1 Storage of reference/retention samples of finished products and reference samples of starting materials should be in accordance with the current version of the Note for Guidance on Declaration of Storage Conditions for Medicinal Products and Active Substances.5.2 Storage conditions should be in accordance with the marketing authorisation (e.g. refriger-ated storage where relevant).Agreements6. Written6.1 Where the marketing authorisation holder is not the same legal entity as the site(s) responsible for batch release within the EEA, the responsibility for taking and storage of reference/retention sam-ples should be defined in a written agreement between the two parties in accordance with Chapter 7 of the EC Guide to Good Manufacturing Practice. This applies also where any manufacturing or batch release activity is carried out at a site other than that with overall responsibility for the batch on the EEA market and the arrangements between each different site for the taking and keeping of reference and retention samples should be defined in a written agreement.6.2 The Qualified Person who releases a batch for sale should ensure that all relevant reference and retention samples are accessible at all reasonable times. Where necessary, the arrangements for such access should be defined in a written agreement.6.3 Where more than one site is involved in the manufacture of a finished product, the availability of written agreements is key to controlling the taking and location of reference and retention samples.7. Reference Samples – General Points7.1 Reference samples are for the purpose of analysis and, therefore, should be conveniently available to a laboratory with validated methodology. For starting materials and packaging materials, used for medicinal products manufactured within the EEA, these are the original site of manufacture and the site(s) of packaging, respectively. For finished products manufactured within the EEA, this is the original site of manufacture.7.2 For finished products manufactured by a third-country manufacturer and where an operational Mutual Recognition Agreement (MRA) is in place, the reference samples may be taken and stored at the third country site of manufacture. This should be covered in a written agreement (as referred to in section 6. above) between the importer/site of batch release and the third country manufacturer.7.3 For finished products manufactured by a third country manufacturer where no MRA is in place, reference samples should be taken and stored at a licensed manufacturer located within the EEA. These samples should be taken in accordance with written agreement(s) between all of the par-ties concerned. The samples should, preferably, be stored at the location where testing on importation has been performed.8. Retention Samples – General Points8.1 A retention sample should represent a batch of finished products as distributed in the EEA and may need to be examined in order to confirm non-technical attributes for compliance with the market-ing authorisation or EU legislation. Therefore, retention samples should in all cases be located within the EEA. These should preferably be stored at the site where the Qualified Person (QP) certifying the finished product batch is located.8.2 In accordance with 8.1 above, where an operational MRA is in place and reference samples are retained at a third country manufacturer (section 7.2 above), separate retention samples should be kept within the EEA.8.3 Retention samples should be stored at the premises of an authorised manufacturer in order to permit ready access by the Competent Authority.8.4 Where more than one manufacturing site within the EEA is involved in the manufacture im-portation/packaging/testing/batch release, as appropriate of a product, the responsibility for taking and storage of retention samples should be defined in a written agreement(s) between the parties con-cerned.9. Reference and Retention Samples for Parallel Imported/Parallel Distributed Products. 9.1 Where the packs are not opened, only the packaging material used needs to be retained, as there is no, or little, risk of product mix up.9.2 Where the packs are opened, for example, to replace the carton or patient information leaflet, then one retention sample, per packaging operation, containing the product should be taken, as there is a risk of product mix-up during the assembly process. It is important to be able to identify quickly who is responsible in the event of a mix-up (original manufacturer or parallel import assembler) as it would affect the extent of any resulting recall.10. Reference and Retention Samples in the Case of Closedown of a Manufacturer10.1 Where a manufacturer closes down and the manufacturing authorisation is surrendered, re-voked, or ceases to exist, it is probable that many unexpired batches of medicinal products manufac-tured by that manufacturer remain on the market. In order for those batches to remain on the market, the manufacturer should make detailed arrangements for transfer of reference and retention samples (and relevant GMP documentation) to an authorised storage site. The manufacturer should satisfy the Competent Authority that the arrangements for storage are satisfactory and that the samples can, if necessary, be readily accessed.10.2 If the manufacturer is not in a position to make the necessary arrangements this may be dele-gated to another manufacturer. The Marketing Authorisation holder (MAH) is responsible for such delegation and for the provision of all necessary information to the Competent Authority. In addition, the MAH should, in relation to the suitability of the proposed arrangements for storage of reference and retention samples, consult with the competent authority of each Member State in which any unex-pired batch has been placed on the market.10.3 These requirements apply also in the event of the closedown of a third country site of manu-facture. In such instances, the importer has a particular responsibility to ensure that satisfactory ar-rangements are put in place and that the competent authority/authorities is/are consulted.--------------------------------------------------------------------------------------------------------------- Consequential amendment to Chapter 6 section 14 of EU GMP Guide6.14 Reference samples from each batch of finished products should be retained till one yearafter the expiry date. Finished products should usually be kept in their final packaging and stored un-der the recommended conditions. Samples of starting materials (other than solvents, gases and water) should be retained for at least two years (1) after the release of the product if their stability allows. This period may be shortened if their stability, as mentioned in the relevant specification, is shorter. Refer-ence samples of materials and products should be of a size sufficient to permit the carrying out, on two occasions, of the full analytical controls on the batch in accordance with the Marketing Authorisation.(1) In Federal Republic of Germany, France, Belgium and Greece, samples of starting materials should be retained for as long as the corresponding finished product.。
猪繁殖与呼吸综合征GP5蛋白和N蛋白在大肠杆菌中的优化表达和纯化

( 上海 市农 业科 学 院 畜 牧 兽 医 研 究 所 , 海 2 1 0 ) 上 0 16
摘
要 : 用 不 同 的培 养 基 、 导 温 度 、 导 时 间 、P G 诱 导 剂 浓 度 以 及 诱 导 菌 浓 度 等 大 肠 杆 菌 表 达 条 件 , 采 诱 诱 IT
ZH A N G hu ln Z H A N G a h C n-i g, W n— ua, LI Chun h — ua, I N G ng y ng, JA Fe - i
ZHOU n — ig HE Xi h n , HU Yo gjn S Wa —u , Zo gqn , — o g Z z n — ,U ng o ZOU Yo g u n
HI — 融 合 蛋 白 。S — AGE电 泳 分 析 表 明 , 化 的 蛋 白纯 度 大 于 9 % 。 SN DS P 纯 5
关 键 词 : 繁 殖 与 呼 吸 综 合 征 ; 合 蛋 白 ; 达 条 件 ; 达 产 物 ; 化 ; 化 猪 融 表 表 优 纯
中 图 分 类 号 :8 8 2 ¥5 .8 文献标识码 : A
pu iy. rt
Ke r : y wo ds PR RSV ; Fuson pr t i Expr s i n c ndii n; x e son pr uc ; ptm i a i i o e n; e so o to E pr s i od t O i z ton; Pur— i fc i iaton
w e e sud e y c ng ng s c . o ie r t i d b ha i u h E c l xpr s i n c e so ond tonsa ulu e m e u ,ndu to em pe a u e ii sc t r di m i c in t r t r an i e, d tm and PTG i uc c c nt a i n.T h r s ls how e t t e pr s e f son I nd er on e r to e e u t s d hat he x e s d u i pr ei s ot n
IVD行业国外原料主要供应商
.aaltobioreagents.ie .aaltoscientific..aetltd..biocell..npods.ru.diarect..endocrinetech..scipac..eastcoastbio..haemtech. .immunovision..mainebiotechnology. .operon.es.equitech-bio..quadfive..promeddx..seracare..chemogen..modiquest..seramon..midlandbio..capricornproducts. .instruchemie.nl.sheffield-products. .biogenes.de.biocheckinc..biospacific..bioprocessinginc..fitzgerald-fii..microbix..inventdiagnostica.de .biomarket.fi.calbioreagents..xema-medica..scrippslabs..silverlakeresearch..ssi.dk.virostat-inc..virusys..oycus..accessbiologicals. .anshlabs..arlingtonscientific..auditmicro..brt-us..cardinalbiologicals. .diasource.be.diazyme..dsitaly..icllab..immunoreagents. .magsphere.丹麦提供诊断试剂盒和抗体、抗原和血清,有特色的产品是 CE认证的NGAL诊断试剂盒,MBL试剂盒重症监护和止血,临床化学仪器,试剂盒日本提供诊断试剂盒产品的公司,特色产品是低密度LDL 和胱抑素C 试剂盒。
产品涉及质控品,转染病,糖尿病,肿瘤,生殖,甲状腺等试剂盒Acris 是一家德国的著名抗体公司,提供近 3 万种各种优质抗体、蛋白及抗体纯化试剂盒,产品X 围涉及免疫学、细胞生物学、细胞神经信号传导、蛋 白组学、肿瘤生物学等。
中极公司企业介绍

公司介绍GHDE BIOENGINEERIG2006年,"中极科技”作为高胜药业兽药销售部正式组建,是公司药业重点打造的一个集药品科研、开发、销售为一体的新型高科股份制企业,公司成立伊始便与“江西农大、南京农大、华北农大、江西生物科技学院”等多家专业院校及实验室合作,重点致力于新产品的研发工作,在治疗病毒性疾病的中兽药研发方面居国际领先地位。
拥有多项发明专利、三个高新技术产品(千目毒抗、高热疫舒、中极康),并荣获多项国际国内金奖。
“中极科技”作为江西省重点中兽药产品走出去企业,公司产品“高热疫舒、中极康、千目毒抗、痢扶康”等四个产品已出口至俄罗斯、西班牙、越南、南非等9个国家,不仅为企业创造了巨大经济效益,更为我国特色中草药文化的对外发展做出了一定的贡献!2007年,公司又采取与美国jonat.han博士、俄罗斯OOO《ArpoColo3》、OOO 《Betmapkt》、加拿大CANADIANSWINEEXPORTERSASSCIATION、西班牙GROUP BAT ALLe等多家跨国公司及台湾恩能国际集团合作的形式,在国际领先技术与产品上进行沟通与交流,并将国际上先进的高效化设备引入公司(千目粉碎机),为中国的畜牧事业及经济做出自己的贡献。
2008年,公司着手组建国内销售团队,成立伊始,公司一直秉承“以科研、技术为依托,走合作、共赢之路,创国际品牌”为战略思想,坚持“自强不息、厚德载物”的经营理念;致力于“打造中国中兽药千目粉散剂第一品牌”的行业使命,始终坚持“绿色中药,让养殖回归自然健康状态”的服务理念,务实创新,自我超越。
中极科技,专业与猪群保健的发展思路必将受到同行业尊重,受到经销商和养殖户的信赖!“中极科技兽药”愿与各界同仁一道,竭尽全力、坚持不懈,为中国养殖业的健康成长,为中国农业经济的发展贡献自己的全部力量。
ADC药物研发现状

Title Originator Highest Dev Status 111In-capromab pendetide Cytogen Corp Launched111In-imciromab pentetate Janssen Biotech Inc Launched131I-chTNT-1/B Peregrine Pharmaceuticals Inc Launched131I-metuximab Fourth Military Medical University PLA Launchedbrentuximab vedotin Seattle Genetics Inc Launchedgemtuzumab Wyeth Research Launchedibritumomab tiuxetan IDEC Pharmaceuticals Corp Launchedtrastuzumab emtansine Genentech Inc LaunchedATL-101, ATLAB Cornell University Phase 3 Clinical inotuzumab ozogamicin Wyeth Research Phase 3 Clinical oportuzumab monatox (intratumoral, head and neck cancer), Viventia University of Zurich Phase 3 ClinicalRIGS CC49Navidea Biopharmaceuticals Inc Phase 3 ClinicalABT-414Abbott Laboratories Phase 2 ClinicalCDX-1401Celldex Therapeutics Inc (pre-merger)Phase 2 Clinical glembatumumab vedotin CuraGen Corp Phase 2 ClinicalLMB-2National Cancer Institute Phase 2 Clinical lorvotuzumab mertansine ImmunoGen Inc Phase 2 Clinical moxetumomab pasudotox National Cancer Institute Phase 2 Clinical oportuzumab monatox (intravesicular, bladder cancer), Viventia University of Zurich Phase 2 ClinicalPSMA-ADC Cytogen Corp Phase 2 ClinicalRG-7593Genentech Inc Phase 2 ClinicalRG-7596Genentech Inc Phase 2 ClinicalSAR-3419ImmunoGen Inc Phase 2 Clinical212-Pb-TCMC-trastuzumab National Cancer Institute Phase 1 ClinicalActimab-A PDL BioPharma Inc Phase 1 ClinicalAGS-16M8F Agensys Inc Phase 1 Clinicalanti-CD3/anti-CD20 bispecific antibody-armed activated T-cells (non-Hodgkin's lymphoma), Wayne State University/Barbara Ann KarmanosCancer Institute Barbara Ann Karmanos Cancer Institute Phase 1 ClinicalASG-5ME Agensys Inc Phase 1 ClinicalBAY-79-4620MorphoSys AG Phase 1 Clinical citatuzumab bogatox Viventia Biotech Inc Phase 1 Clinical doxorubicin-loaded anti-EGFR immunoliposomes (solid tumors), UniversityHospital Basel University Hospital of Basel Phase 1 ClinicalHuM195/rGel (intravenous infusion, AML/CML/meylodisplastic syndrome),Targa Therapeutics Memorial Sloan-Kettering Cancer Center Phase 1 ClinicalIMGN-529ImmunoGen Inc Phase 1 ClinicalIMGN-853ImmunoGen Inc Phase 1 ClinicalIMMU-132Immunomedics Inc Phase 1 Clinical labetuzumab-SN-38Immunomedics Inc Phase 1 ClinicalNHS-IL-12National Cancer Institute Phase 1 ClinicalRG-7450Genentech Inc Phase 1 ClinicalRG-7458Genentech Inc Phase 1 Clinical RG-7598Genentech Inc Phase 1 Clinical RG-7599Genentech Inc Phase 1 Clinical RG-7600Genentech Inc Phase 1 Clinical RG-7636Genentech Inc Phase 1 Clinical SAR-566658ImmunoGen Inc Phase 1 Clinical T-Guard University Medical Center St Radboud Phase 1 Clinical vorsetuzumab mafodotin Seattle Genetics Inc Phase 1 Clinical 131I-catuximab (colorectal cancer), Pacific Meinuoke Fourth Military Medical University PLA Discovery177Lu-tetraxetan-tetulomab (non-Hodgkin's lymphoma), Nordic Nanovector Nordic Nanovector AS Discovery227Th-epratuzumab (hematological cancer), Algeta Algeta ASA Discovery227Th-rituximab (cancer), Algeta Algeta ASA Discovery227Th-trastuzumab (cancer), Algeta Algeta ASA Discovery4s3-0014s3 Bioscience Inc Discovery4s3-0024s3 Bioscience Inc Discovery64Cu-NOTA-ALT-836Altor BioScience Corp DiscoveryAA-A225Actinium Pharmaceuticals Inc Discovery AbGn-107AbGenomics Corp Discovery Actimab-B Fred Hutchinson Cancer Research Center Discovery Actimab-C Actinium Pharmaceuticals Inc Discovery Actimab-P Actinium Pharmaceuticals Inc Discovery adalimumab + anti-Ang2 Zybody (rheumatoid arthritis/inflammatory boweldisease), Zyngenia Zyngenia Inc DiscoveryAGS-15E ADC Agensys Inc DiscoveryAGT-160ArmaGen Technologies Inc DiscoveryAGT-185ArmaGen Technologies Inc DiscoveryAGT-190ArmaGen Technologies Inc Discovery amanitin-trastuzumab conjugate (cancer), Heidelberg Pharma Heidelberg Pharma Holding Ltd Discoveryanti-CD133-immunotoxin conjugates (photochemical internalization, cancer),PCI Biotech PCI Biotech Holding ASA Discoveryanti-ET8R-MC-vc-PAB-MMAE Genentech Inc Discoveryanti-NaPi3b antibody-drug conjugate (cancer), Genentech/Roche Genentech Inc Discovery antibody drug conjugates (cancer), Sanofi Sanofi Discovery antibody-drug conjugates (cancer), ADC Therapeutics ADC Therapeutics Sarl Discovery antibody-drug conjugates (cancer), Seattle Genetics/Oxford BioTherapeutics Seattle Genetics Inc Discovery antibody-drug conjugates (TAP, cancer), Lilly Eli Lilly & Co Discovery antibody-IFN lambda conjugates (cancer), Immunomedics Immunomedics Inc Discovery anticancer therapy (TAP technology), Amgen Amgen Inc DiscoveryAPH-0912Aphios Corp Discovery Aurixin BioIntegrator DiscoveryAZ-05Allozyne Inc Discovery BIOO-1BIOO Therapeutics DiscoveryBIOO-2BIOO Therapeutics Discovery BIOO-3BIOO Therapeutics Discovery BIOO-4BIOO Therapeutics Discovery BIOO-5BIOO Therapeutics Discovery BIOO-6BIOO Therapeutics Discovery BIOO-7BIOO Therapeutics Discovery botulinum toxin B inhibitor (injectable, heteropolymer mAbs, botulism),Immunome Immunome Inc Discovery BT-2111biOasis Technologies Inc Discovery C2-2b-2b Immunomedics Inc Discovery CDX-014CuraGen Corp Discovery chiHEA-125-Ama Heidelberg Pharma Holding Ltd Discovery CK-22-(20)-(20)Immunomedics Inc Discovery complement factor H-derived short consensus repeat-antibody constructs(infection), LysoVac University of Innsbruck Discovery Cymac-001Cytoguide ApS Discovery CYP-Ab Cytune Pharma Discovery D2C7-based immunotoxins (glioma), Duke University Duke University Discovery EGFR modulators (antibody conjugates, PIT, cancer), Aspyrian Aspyrian Therapeutics Inc Discovery engineered cysteine drug conjugates mAbs (cancer), Seattle Genetics Seattle Genetics Inc Discovery epratuzumab-SN-38Immunomedics Inc Discovery ETBs (cancer), Molecular Templates/ Imclone Molecular Templates Inc Discovery Fluorescent-labeled bevacizumab (imaging, ocular disease), Mivenion mivenion Gmbh Discovery gemcitabine + paclitaxel (prodrug, nanomAb, cancer), ImmunePharmaceuticals Immune Pharmaceuticals Corp Discovery Herceptin:Endostatin-P125A University of Miami Discovery hLL1-CL2A-SN-38Immunomedics Inc Discovery hPAM4-CL2A-SN-38Immunomedics Inc Discovery human monoclonal antibody-toxin conjugates (myocardial infarction), Celdara Celdara Medical LLC Discovery HuMax-TF-ADC Genmab A/S Discovery IFNalpha-fused mAbs (HBV infection), Roche Roche Holding AG Discovery IL-13 receptor alpha 2 inhibitors (iv, cancer), Pfizer Pfizer Inc Discovery IMGN-289ImmunoGen Inc Discovery intracellular antibodies (Intraphilin, inflammatory diseases/infectiousdiseases/ophthalmic diseases), Permeon Biologics Permeon Biologics Inc Discovery mapp-66Mapp Biopharmaceutical Inc Discovery MB-2003Mapp Biopharmaceutical Inc Discovery monoclonal antibody-drug conjugates, Chirogenix/ImmunoGen/Celltrion Chirogenix Co Ltd Discovery MP-Ter-ADC MediaPharma Srl Discovery N01-OX2Intellect Neurosciences Inc Discovery PC-91ProCell Therapeutics Inc Discovery ProstaLite PhotoBiotics Ltd Discoveryrecombinant mAb-biocide fusion proteins (oral/Directed Biocide,cryptosporidium infection), ioGenetics ioGenetics Inc Discovery SGN-CD33A Seattle Genetics Inc Discovery SGN-LIV1A Seattle Genetics Inc Discovery SL-101Stemline Therapeutics Inc Discovery SYD-983Synthon Biopharmaceuticals Discovery T01-OX2Intellect Neurosciences Inc Discovery TBL-0306L Transgene Biotek Ltd Discovery TBL-0306M Transgene Biotek Ltd Discovery TBL-0805E Transgene Biotek Ltd Discovery thio-trastuzumab-mpeo-DM1Genentech Inc Discovery trastuzumab-PNU-159682 antibody-drug conjugate (cancer), Genentech Genentech Inc Discovery veltuzumab-IFN alpha 2b conjugate (cancer), IBC/Immunomedics IBC Pharmaceuticals Inc Discovery BIIB-015Biogen Inc Suspended Pretarget technology (gastrointestinal adenocarcinoma), NeoRx Poniard Pharmaceuticals Inc Suspended125I-AnnA1 IgG Sidney Kimmel Cancer Center No Development Reported131I-CC49-SCA Enzon Labs Inc No Development Reported177Lu-capromab pendetide Cytogen Corp No Development Reported90Y-capromab pendetide Cytogen Corp No Development Reported99mTC-BERH2Medac GmbH No Development Reportedanti-CD133-vcMMAF Seattle Genetics Inc No Development Reportedanti-CD22 antibody drug-conjugates, Medarex/BMS Medarex Inc No Development Reportedanti-PSMA antibody-drug conjugates (cancer), Medarex Medarex Inc No Development Reportedantibody-drug conjugates (solid tumors), Daiichi Sankyo Seattle Genetics Inc No Development ReportedAVE-9633ImmunoGen Inc No Development Reportedbectumomab Immunomedics Inc No Development ReportedCA125/MUC16-targeting antibody-drug conjugate (ovarian cancer),Genentech Genentech Inc No Development Reportedcathepsin B-sensitive prodrugs, BMS Bristol-Myers Squibb Pharmaceutical ResearchInstituteNo DevelopmentReportedCC49 humanized radioimmunoconjugates, National Cancer Institute,University of Alabama at Birmingham National Cancer Institute No Development ReportedCD4-BFFI Roche Holding AG No Development ReportedCHB-111ViRexx Medical Corp No Development ReportedCHT-25University College London No Development ReportedCMD-193Wyeth No Development ReportedCNTO-95 immunoconjugates (cancer), Centocor Janssen Biotech Inc No Development Reportedconjugated PEI/anti-CD133 mAb plasmid-based gene therapy (brain tumor),Discovery genomics Discovery Genomics Inc No Development ReportedcT84.66City of Hope No Development Reporteddelta 9-cadherin targeting antibody (gastric cancer), Actinium Helmholtz Zentrum München No Development Reporteddiphtheria toxin, RCT Research Corporation Technologies No Development Reporteddoxorubicin-C225 conjugate (STEALTH), SEQUUS SEQUUS Pharmaceuticals Inc No Development ReportedDTPA-BrE-3University of Colorado System No Development ReportedDXL-625InNexus Biotechnology Inc No Development ReportedG3.519-PAP-S Tanox Inc No Development ReportedhuHMFG1-caspase Antisoma plc No Development ReportedIMGN-007ImmunoGen Inc No Development ReportedIMGN-009ImmunoGen Inc No Development Reportedimmunoconjugate (cancer MN), Bayer Bayer AG No Development ReportedImmuRAID-AFP-99mTc, Immunomedics Immunomedics Inc No Development ReportedIMTOX 22-97A University of Texas Southwestern MedicalCenterNo DevelopmentReportedKSB-201KS Biomedix Holdings plc No Development ReportedLA22-radioimmunoconjugates (cancer), Welson/Peking University Welson Pharmaceuticals Inc No Development Reportedlabetuzumab Immunomedics Inc No Development ReportedLMB-1, NIH National Institutes of Health No Development ReportedLu-177-trastuzumab Tarbiat Modares University No Development ReportedLymphoScan Immunomedics Inc No Development ReportedMDX-11Medarex Inc No Development ReportedMDX-1203Medarex Inc No Development ReportedMDX-1206Medarex Inc No Development Reportedmonoclonal-porphyrins, Quadra Logic QLT Inc No Development Reportedmonoclonals, Quest Quest Biotechnology Inc No Development ReportedNogo receptor modulators, Biogen Idec Yale University No Development ReportedONS-1210Oncobiologics Inc No Development ReportedOP-06 program (cancer), Onco-Pharmakon Onco-Pharmakon Inc No Development Reportedpaclitaxel analogs and immunoconjugates (cancer), Bioxel Bioxel Pharma Inc No Development ReportedPE38-conjugated anti-CD30 immunotoxin, NCI National Cancer Institute No Development Reportedprostate-specific MAb, NIH National Institutes of Health No Development ReportedR-1549The UK Imperial Cancer Research Fund No Development Reportedradiolabeled Tx3.833Beth Israel Deaconess Medical Center No Development Reportedscu-PA-59D8Bristol-Myers Squibb Co No Development ReportedSGN-17/19Seattle Genetics Inc No Development Reportedtaxane-monoclonal antibody conjugates, ImClone ImClone Systems Inc No Development Reportedtranscobalamin (vitamin B12) receptor-targeting mAbTCR23-saporinconjugate (cancer), Kyto Kyto Biopharma Inc No Development Reportedtrastuzumab-autophilic peptide conjugate (breast cancer), InNexusBiotechnology InNexus Biotechnology Inc No Development Reportedtrastuzumab-MC-vc-PAB-MMAF Genentech Inc No Development ReportedTRP-targeted antibody conjugate (Yttrium 90/MX-DTPA), SomantaPharmaceuticals Immunodex Inc No Development Reportedtucotuzumab celmoleukin EMD Lexigen Research Center Corp No Development ReportedVB4-011Viventia Biotech Inc No Development ReportedVB6-011Viventia Biotech Inc No Development ReportedVB6-050Viventia Biotech Inc No Development ReportedXomaZyme-791XOMA Corp No Development Reportednofetumomab Poniard Pharmaceuticals Inc Withdrawn 131I-81C6Duke University Discontinued 131I-ImmuRAIT-HCG, Immunomedics Immunomedics Inc Discontinued B-B4-DC1ImmunoGen Inc Discontinued CC49 radioimmunoconjugates, University of Alabama at Birmingham University of Alabama at Birmingham Discontinued CD5 monoclonals/RIPs, Italfarmaco Italfarmaco SpA Discontinued CVX-045CovX Pharmaceuticals Inc Discontinued CVX-060CovX Pharmaceuticals Inc Discontinued CVX-241CovX Pharmaceuticals Inc Discontinued CVX-343CovX Pharmaceuticals Inc Discontinued doxorubicin-BR96 conjugate, BMS Bristol-Myers Squibb Co Discontinued doxorubicin-CEA conjugate, Immunomedics Immunomedics Inc Discontinued doxorubicin-LL2 conjugate, Immunomedics Immunomedics Inc Discontinued FAP5-DM1Boehringer Ingelheim Corp Discontinued FGFR4-CovX-Body CovX Pharmaceuticals Inc Discontinued HuM-195-Bi-213PDL BioPharma Inc Discontinued human placental growth factor 1-CVX-2000 monoclonal antibody conjugatedtherapeutic (CovX-body, cancer), CovX CovX Pharmaceuticals Inc Discontinued huN901-CC-1065ImmunoGen Inc Discontinued huN901-DC1ImmunoGen Inc Discontinued ImmuRAID-HCG-99mTc, Immunomedics Immunomedics Inc Discontinued ImmuRAIT-CEA-rhenium-188, Immunomedics Immunomedics Inc Discontinued MDX-214Medarex Inc Discontinued MEDI-547MedImmune LLC Discontinued MLN-2704Cornell University Discontinued Oncolym Peregrine Pharmaceuticals Inc Discontinued Oncolysin B Dana-Farber Cancer Institute Inc DiscontinuedOncolysin CD6Dana-Farber Cancer Institute Inc Discontinued Oncolysin M Dana-Farber Cancer Institute Inc Discontinued Oncolysin S Dana-Farber Cancer Institute Inc Discontinued Oncopurge Poniard Pharmaceuticals Inc Discontinued rhenium-188-LL2, Immunomedics Immunomedics Inc Discontinued SMART ABL-364Novartis AG Discontinued targeted ranpirnase conjugates (cancer), Alfacell Tamir Biotechnology Inc DiscontinuedHighest Dev Status。
药理学实验教学采用PBL教学模式的分析

参考 文献 :
『 M c aln M .N o l L .Lvn so G.T e f c v n s l l pr d a be M iigtn h e e f e es i
ofP o l m — bae Le r ng c r b e — sd ani ompa e t ta i on lta hng i r d o r d f a e c i n
u drrd a sci r[. e d . 0 4 3 ( n egaut py hay1 M dE u 2 0 . 8 8 8 9— 8 7 e t ] ): 5 6. 『 盛瑞 . 明 华.药理 学 实验 教 学 中 开展 创 新 科 研 性 实验 U. 2 1 耿 】
实验 室研 究 与探 索 . 0 9 2 ( ) 1 - 8 20 . 5 8 :6 1
索性实验 的开展打下坚实的基础 。 2激发学生的学 习兴趣和 主动性 . 高等教育 要以学生为本 , 面向学生 , 努力促进 学生 的个性发 展和全 面发展 。教师是学生学习的指 导者 与学生发展 的促进者。 教师的作用主要体现在指导学生 的有效学 习 ,而不再是单纯地 向学生灌 输知识 。学生应学会学 习, 学会思维 。 P L教学方法改变可以往填鸭式被动学习状况 , B 让学生更多 地参 与药理实验教学活动 , 使他们在 主动积极 的心理状态下获取 知识信息 , 激发学 习的兴趣和学习主动性。P L教学弥补了部分 B 学生学习 自控性差的弊端 , 调动了学 生学 习的主动性和索取知识 的兴趣。学生在问题的引导下展开课堂讨论 , 教师可需事先制定 讨论提纲 , 在讨论提纲 的指导下进行 , 由学生个别发 言或 中心 可 发言 , 学生也可提问或答辩 , 最后 由老师总结 。 学生通过阐明 自己 观点 , 听取 同学的意见和教师总结 , 阔了视野 , 开 解决 了听课或 自 学过程 中的疑难 问题 , 自学意识增强 , 自学能力提高 , 理论知识得 到强化和掌握 , 逻辑推理 、 学思维得 到锻炼 , 科 提高了学生 的整体
慢性移植肺功能障碍的诊断与治疗现状

第12卷 第5期2021年9月Vol. 12 No.5Sep. 2021器官移植Organ Transplantation·专家论坛·【摘要】 慢性移植肺功能障碍(CLAD )是影响肺移植受者远期生存的最大阻碍,代表了一系列术后移植肺功能显著且持续恶化的复杂临床表现。
由于缺乏有效的早期诊断和预防策略,一半以上的肺移植受者在5年内会出现CLAD ,且这一比例将在10年内提高到75%。
目前没有任何药物可以完全阻止或逆转CLAD 的进展。
近年来,随着2019年国际心肺移植学会(ISHLT )更新了CLAD 的定义、诊断和治疗,国际上对CLAD 的认识有了较大提高。
本文将对CLAD 的综合诊断方式和潜在治疗策略进行详细总结,为CLAD 发生、发展的早期监测和管理提供理论参考和见解。
【关键词】 肺移植;慢性移植肺功能障碍;闭塞性细支气管炎综合征;限制性移植物综合征;供者来源性细胞游离DNA ;阿奇霉素;孟鲁司特;吡非尼酮【中图分类号】 R617,R563 【文献标志码】A 【文章编号】1674-7445(2021)05-0004-08慢性移植肺功能障碍的诊断与治疗现状黄桁 田东【Abstract 】 Chronic lung allograft dysfunction (CLAD) is the largest obstacle to the long-term survival of lung transplant recipients, which represents a series of complicated clinical manifestations of significant and persistent deterioration of lung allograft function after surgery. Due to lack of effective strategies for early diagnosis and prevention, over half of lung transplant recipients will develop CLAD within postoperative 5 years, which is likely to increase to 75% within postoperative 10 years. At present, no drug can be administered to completely prevent or reverse the progression of CLAD. In recent years, since the definition, diagnosis and treatment of CLAD have been updated by International Society of Heart and Lung Transplantation (ISHLT) in 2019, the understanding of CLAD has been significantly deepened within the international community. In this article, comprehensive diagnostic methods and potential treatment strategies of CLAD were explicitly illustrated, aiming to provide theoretical reference and insights for early monitoring and management of the incidence and progression of CLAD.【Key words 】 Lung transplantation; Chronic lung allograft dysfunction; Bronchiolitis obliterans syndrome; Restrictive allograft syndrome; Donor-derived cell-free DNA; Azithromycin; Montelukast; PirfenidoneCurrent status of diagnosis and treatment of chronic lung allograft dysfunction Huang Heng, Tian Dong. Heart and Lung Transplant Research Laboratory, Affiliated Hospital of North Sichuan Medical College, Academician (Expert) Workstation, Affiliated Hospital of North Sichuan Medical College, Medical Imaging Key Laboratory of Sichuan Province, North Sichuan Medical College, Nanchong 637000, China DOI: 10.3969/j.issn.1674-7445.2021.05.004基金项目:医学影像四川省重点实验室开放课题(MIKLSP202007);南充市杰出青年科技人才专项(20SXJCQN0002);川北医学院附属医院博士科研启动基金(2021BK01);川北医学院附属医院科研发展计划项目(2021ZK003)作者单位:637000 四川南充,川北医学院附属医院心肺移植研究室 川北医学院附属医院院士(专家)工作站 川北医学院医学影像四川省重点实验室作者简介:黄桁,男,1997年生,硕士研究生,研究方向为肺移植基础与临床研究,Email :通信作者:田东,Email :·526·第12卷器官移植慢性移植肺功能障碍(chronic lung allograft dysfunction ,CLAD )是肺移植术后限制受者远期结果的最重要因素,约一半的受者在术后5年内可能罹患CLAD ,这直接导致了肺移植受者5年总体生存率仅为54% [1-2]。
WADiana全自动配血系统进行献血者Rh(D)阴性确认试验方法的建立

R () hD 阴性确 认试 验是献 血者 R 血 型鉴定 中的重 要试 h 验。通 过该试 验可 以检 出经 IM型 抗 一 g D试 剂鉴 定为 阴性 ,
公 司、加 拿 大 D mn n 司、 法 国 Daat 司、 英 国 o ii 公 o i s公 g
成都
6 0 4 10 1
幼临床医学杂志 ( 电子版 ) 0 5 (:2 — 2 , ,2 0 ,81 14 1 6 ) HF I U作为近 年来发 展起 来的 日渐成 熟 的局部 非手术 治 2 】 聚焦多束超 声对猴胎 定位损伤及再 疗肿瘤的新技术 ,越 来越被 人们认 同和接受。其治疗子宫肌 【 王智彪,伍 烽,王芷龙 ,等 .
Miioe 司 ( 别 以 D 、D 、D 、D 表 示 ) g lp r 公 l 分 1 2 3 4 ,IG抗 一 D
但是用 多个批次或 厂家 的 IM+g g IG型或 IG型抗 一 g D试 剂进 行 间接 抗 球蛋 白试 验鉴 定 出现部 分或 全部 结果 阳性 的献 血 者,这部分 献血者 即为 R () 异型,这种血液 临床应作为 hD 变
是 19 90年开始应 用于 红细胞 血型 血清学 临床检验 的,在经
典抗人球蛋 白试验基础上 发展 起来的新型检测方法 ,在 国外 柱凝胶技术 的优势 ,结合 WA in 自动配 血系统的功能 和 D aa全
特点,建立 了全 自动微 柱凝胶 技术进 行献 血者 R () hD 阴性确 认试 验的方法,现报告如—
【 摘要 】 目的 : 建立一种运用全 自动微柱凝胶 技术进行献 血者 R () hD 阴性确认试验 的方法。 方法 : 采用西 班牙 Gil公 司 WA i a 自 r s l Da 全 n
新型HMG—CoAG还原酶抑制剂赛伐他汀钠

《 中国 医药情报 3 0 2年 第 8卷 第 2期 20 认定为违法 所得 , 我个人 认为 , 二 种观点 是曲 第 解 原意 , 对违 法违 规者 难以起 到惩戒 作用 ; 我主 张第一 种 观点 , 为 只有 将全 部 违法 收 入予 以 因
水平 可 降低 3 , 3 而其 它 他 汀类 药 物 的剂量 要
高 5 ~ 1 0 才可 能 达 到相似 的 功效 。 由于本 0 0倍 品 对 血 清 T 和 HD c 的 作 用 ( 别 为 降 低 G I 一 分
1 ~ 1 和 升 高 5 ~ 9 ) 应 并 不 突 出 。 0 7 相
( 江 医药 包装 材 料 质 量 检 测 站 , 州 1浙 . 杭
2 杭 州华 东医药集团有 限公 司, 州市 . 杭
摘要
30 1 ) 1 0 1
综 述 了赛伐他汀 钠药教学 、 动学、缶 药 I 床应用 及不 良反应 。该 品作 为一种 新型 、 I 合垒成 的高教 HMG—
G A 还 原 酶 抑 制 剂 . 内 活 性 为 洛伐 他 汀 的 I 0 。 最 大 优点 是 微 克 级 剂 量 起 作 用 , o 体 1倍 其 而其 它 同 类 药 物 均 以 毫 克 级 剂量 起 作 用 。预 测本 品 的 年销 售 额 很 快将 达 6亿 美 元 . 望 成 为 市 场 前 景极 佳 的 “ 磅 炸 弹 ” 可 重 。
故在 临床 上特 别适用 于那些 单 以胆 固醇升 高 为
特 征 的 Ⅱ型 病 人 。
通 常 本 品使 用 1周 后 起 效 , 4周时 达 到最
大 疗 效 2 药 动 学 B ’
本 晶 口 服 易 吸 收 , 康 自愿 者 口 服 本 品 健
一种达玛烷苷元复合物及其应用[发明专利]
![一种达玛烷苷元复合物及其应用[发明专利]](https://img.taocdn.com/s3/m/7cabdafc6bd97f192379e98f.png)
专利名称:一种达玛烷苷元复合物及其应用
专利类型:发明专利
发明人:黄冬,刘新民,王天山,祁东风,杨艳艳,许秋霞申请号:CN201010620245.6
申请日:20101223
公开号:CN102125571A
公开日:
20110720
专利内容由知识产权出版社提供
摘要:本发明涉及一种达玛烷苷元复合物及其应用。
具体地,是利用加拿大天马药业集团独有的专利技术,采用亚洲人参茎叶总皂苷通过碱水解制备而成的达玛烷苷元复合物单独或与其他药用许可的辅料配合后制成口服和注射剂型,用于各种化疗、放疗所致骨髓抑制的预防和治疗方面的用途。
申请人:天马药业(集团)股份有限公司,大连泓亿北美人参制品有限公司
地址:加拿大哥伦比亚省
国籍:CA
代理机构:上海新天专利代理有限公司
更多信息请下载全文后查看。
叠氮高铁血红蛋白(hin3)法

叠氮高铁血红蛋白(hin3)法叠氮高铁血红蛋白(Hin3)法是一种用于检测血红蛋白缺陷的方法。
血红蛋白是一种在红细胞中负责运输氧气的蛋白质,而血红蛋白缺陷可能导致一些遗传性疾病,如镰状细胞贫血。
Hin3法是通过检测血红蛋白分子中的DNA序列来确定是否存在缺陷。
它是利用化学试剂叠氮高铁(DAB)与血红蛋白结合,并生成可见的深紫色沉淀物的反应。
如果血红蛋白分子中存在缺陷,那么该反应会产生不同的结果,通常表现为颜色变浅或者无法产生沉淀物。
Hin3法主要用于筛查和诊断一些血红蛋白缺陷性疾病,例如镰状细胞贫血、地中海贫血等。
通过这种方法,可以快速且准确地检测出血红蛋白缺陷,从而指导医生进行相应的治疗和管理。
高纯度植物源重组人血清白蛋白的制备方法及其应用

高纯度植物源重组人血清白蛋白的制备方法及其应用高纯度植物源重组人血清白蛋白(HSA)的制备方法及其应用植物源重组人血清白蛋白(HSA)是一种重要的生物医药制剂,在临床和科研中有着广泛的应用。
传统上,HSA通常是通过提取人类血液中的白蛋白获得,但这种方法存在血液供应紧张和传染病传播的风险。
近年来,利用转基因植物制备高纯度植物源重组HSA的方法得到了广泛关注。
高纯度植物源重组HSA的制备方法主要包括基因克隆、转基因植物的构建与转化、植物体内表达和HSA的提取纯化等步骤。
首先,对人类血浆中的HSA基因进行克隆,获取HSA基因的DNA序列。
然后,将HSA基因与适当的启动子、终止子和调控元件进行连接构建成适用于植物表达的表达载体。
接下来,将表达载体通过农杆菌介导的方法导入到目标植物中,使目标植物细胞中含有HSA基因。
在经过一系列的培养和筛选后,选出转基因植物株系并进行扩繁。
在大规模的转基因植物培养过程中,需要对植株进行适当的生长条件控制,以提高HSA的表达水平。
常见的培养方法如液体培养和固体培养等,可以根据需求选择合适的培养方式。
当目标植物达到一定生长期时,可以进行HSA的提取和纯化。
常用的提取方法包括机械破碎和细胞溶解等,用以破坏植物细胞结构,释放HSA。
然后,可通过凝胶过滤层析、离子交换层析、亲和层析和透析等技术手段对提取液进行纯化,以去除杂质,并得到高纯度的植物源重组HSA。
高纯度植物源重组HSA在医药领域具有广泛的应用价值。
首先,HSA可以作为生物医药制剂的原料药使用,如制备血浆代用品,用于治疗休克、肝功能不全等疾病。
其次,HSA可以用于制备口服给药的载体,如微球、纳米颗粒等,用于提高药物的生物利用度和稳定性。
此外,HSA还可以作为药物传递系统的载体,如携带药物靶向癌细胞。
与传统的人血浆源HSA相比,高纯度植物源重组HSA具有以下优势:一是植物不具备传染病的风险,可以避免传染病的传播;二是植物源HSA可以实现大规模生产,提高制备效率和降低制备成本;三是植物源HSA可以通过基因工程技术进行定制化的改良,以满足特定的临床和科研需求。
加拿大HnG医学技术有限公司

加拿大HnG医学技术有
限公司
公司简介
加拿大HnG医学技术有限公司总部位于加拿大安大略省,从2007年起,陆续在宁波保税区、杭州和南京设立了分公司,在新加坡建立了办事处。
公司专业从事心脑血管介入类医疗器械的加工工艺研发,为亚洲广大的医疗器械生产厂商提供医疗器械的原料、配件和生产设备。
公司依托在该领域的先进技术和丰富经验,以及与该领域最领先的多家美国公司的战略合作伙伴关系,自成立起发展迅速,已为国内及国外知名医疗器械生产商提供了产品和服务。
公司设有技术部、市场部、销售部、行政部、财务部等多个职能部门。
在中国工作地点有宁波保税区、杭州滨江区、杭州余杭区和南京建邺区四处。
业务范围
我们是17家美国公司在亚洲的战略合作伙伴;
我们的产品主要是介入类医疗器械的生产设备、原材料和配件;
我们提供技术服务和售后服务;
我们是经授权的Vante的亚太售后服务中心。
产品简介
一、设备
导管挤出机
导管成型机
导丝磨床
绕簧机
缠簧机
二、原材料
医用级高分子材料
医用涂层材料
镍钛材料
金属丝/管/芯轴
三、配件
管材
精密激光雕刻
硅胶制品
医用小配件
导丝
微型弹簧
微型冲压件
精密机加工
企业文化
我们的使命:全球最先进的植入介入医疗器械制造解决方案 我们的愿景:领导行业转型升级
我们的价值观:公平的价格,最好的产品。
IPCS International Programme on Chemical Safety

IPCSInternational Programme on Chemical SafetyREPORT OF THE IPCS NINTH FINAL REVIEW BOARDMEETING ON CONCISE INTERNATIONAL CHEMICALASSESSMENT DOCUMENTS (CICADs)Ottawa, Ontario, Canada: 29 October -1 November 2001Programme international sur la Sécurite des Substances ChimiquesInternal Technical Report Rapport Technique InterneUnited Nations Environment ProgrammeProgramme des Nations Unies pour l'Environnement International Labour Organization Bureau International du Travail World Health Organization Organisation m,ondiale de la SantéUNITED NATIONS ENVIRONMENT PROGRAMME IPCS/CICAD/01.26 INTERNATIONAL LABOUR ORGANIZATION English only WORLD HEALTH ORGANIZATION Distribution:Limited REPORT OF THE IPCS NINTH FINAL REVIEW BOARDMEETING ON CONCISE INTERNATIONAL CHEMICALASSESSMENT DOCUMENTS (CICADs)Ottawa, Ontario, Canada: 29 October -1 November 2001The issue of this document does not constitute formal publication. It should not be reviewed, abstracted, or quoted without the written permission of the Coordinator, Programme for the Promotion of Chemical Safety, WHO, Geneva, Switzerland.CONTENTS INTRODUCTION (1)BACKGROUND (1)DOCUMENT EVALUATION (1)Acrolein (1)Bromoethane (1)4-Chloroaniline (1)Polychlorinated biphenyls (Human health aspects) (2)Diethyl phthalate (2)Carbon disulphide (2)Silver (Environmental aspects) (2)Ethylene glycol (Human health aspects) (2)OTHER BUSINESS210th and subsequent Final Review Board meetingss (2)General issues for consideration by the Steering Group (3)APPENDIX I LIST OF PARTICIPANTS (4)APPENDIX II AGENDA (8)APPENDIX III TERMS OF REFERENCE (9)APPENDIX IV FINAL REVIEW BOARD COMMENTSON DRAFT CICADS (10)Acrolein (10)Bromoethane (12)4-Chloroaniline (13)Polychlorinated biphenyls (Human health aspects) (15)Diethyl phthalate (18)Carbon disulphide (21)Silver (Environmental aspects) (22)Ethylene glycol (Human health aspects) (23)ACKNOWLEDGEMENTS (25)INTRODUCTIONDr Aitio, on behalf of the Programme for the Promotion of Chemical Safety, World Health Organization, and Ms Meek, on behalf of Health Canada, opened the meeting and welcomed the participants. Dr Aitio expressed thanks for the support of Health Canada in making available the facilities for the meeting.The meeting opened with the election of officers: Dr De Rosa was elected as Chair and Dr Dobson as Vice-Chair (Chair for polychlorinated biphenyls CICAD) and Dr Kielhorn and Mr Howe as Rapporteurs. Following a brief introduction of each participant (List of participants, Appendix I), the agenda was adopted as proposed (Appendix II).None of the members of the 9th Final Review Board (FRB) declared any conflict of interests. BACKGROUNDDr A. Aitio outlined the Terms of Reference for the meeting (Appendix III), and described the international peer-review process for the production of CICADs. He clarified the roles of Members and Observers, namely that Members are responsible for taking the formal decisions on the CICADs, whereas Observers are restricted to commenting on the factual content of the documents. Members were reminded that they are selected to serve on the FRB for their individual scientific expertise and not, in any way, as representatives of their goverments.DOCUMENT EVALUATIONThe FRB systematically reviewed responses of the authors to each comment submitted during the peer-review phase. Areas where additional changes were recommended are noted in Appendix IV. All other comments were considered to have been adequately addressed by the authors. The tables of peer-review comments are to be held by the Secretariat and made available, upon request.AcroleinThe CICAD on Acrolein was approved by the Final Review Board as an international assessment and recommended for publication subject to the requested changes being made as noted in Appendix IV.BromoethaneThe CICAD on Bromoethane was approved by the Final Review Board as an international assessment and recommended for publication subject to the requested changes being made as noted in Appendix IV.4-ChloroanilineThe CICAD on 4-Chloroaniline was approved by the Final Review Board as an international assessment and recommended for publication subject to the requested changes being made as noted in Appendix IV and pending peer review and approval of the revisions in the evaluation section by a consultative group.Polychlorinated biphenyls (Human health aspects)The CICAD on Polychlorinated biphenyls(Human health aspects) was approved by the Final Review Board as an international assessment and recommended for publication subject to the requested changes being made as noted in Appendix IV and pending the approval of authors' responses to the late comments by the Chair and Discussion leaders.Diethyl phthalateThe CICAD on Diethyl phthalate was approved by the Final Review Board as an international assessment and recommended for publication subject to the requested changes being made as noted in Appendix IV and pending peer review and approval of the revisions in the Section on the evaluation of human health effects by a consultative group and of the probabilistic environmental risk characterisation (to be formulated by the author) by another consultative group.Carbon disulphideThe CICAD on Carbon disulphide was approved by the Final Review Board as an international assessment and recommended for publication subject to the requested changes being made as noted in Appendix IV.Silver (Environmental aspects)The CICAD on Silver was approved by the Final Review Board as an international assessment and recommended for publication subject to the requested changes being made as noted in Appendix IV.Ethylene glycol (Human health aspects)The CICAD on Ethylene glycol (Human health aspects) was approved by the Final Review Board as an international assessment and recommended for publication subject to the requested changes being made as noted in Appendix IV and pending the verification of the reliability of the essential non-public source material used as well as approval of authors' responses to late comments by the Chair and the Discussion leader.OTHER BUSINESS10th and subsequent Final Review Board meetingssThe 10th FRB is planned to take place in August-September 2002. The draft CICADs proposed for consideration at the 10th FRB are: Arsine, Asphalt, 1,1-Dichloroethylene, Ethylene oxide, Hydrogen cyanide and cyanides, Hydrogen sulphide, Thiourea, and 1,2,3-Trichloropropane. The closing date for the receipt of first draft of CICADs is the end of January 2002.Other draft CICADs proposed for consideration at future FRB meetings are: Creosote; 2-Diethylaminoethanol; Crotonaldehyde; Glyoxal; 2-Mercapto benzothiazole.General issues for consideration by the Steering Group•Citation of unfinished documents, for example, EU documents.•Dealing with papers with different information content and importance vis-a-vis risk characterization published beyond the cut-off date for the CICAD literature searches.•Identifying conflicts of interest of peer reviewers and informing the FRB of such conficts•International evaluations included in Section 12.•Informing the peer reviewers and CICAD readers of the availability of the supporting document•Process and format of the response of authors to peer review comments•The level of detail on a study or end-point specifically in relation to inclusion in CICADs vs reference to the source document.•Process to deal with important changes in the CICAD caused by comments received in the international peer review, such as changes in critical end-points, key studies, approaches in hazard characterization, setting uncertainty factors, major changes in exposure scenarios for the sample risk characterization•Coordination in time of FRB and Steering Group meetings; processes to get Steering Group advice on a very short notice•Use of data from developing countries (including how to include data; a more formalized system for retrieving information)APPENDIX I MembersDr Rajendra Chhabra, National Institute of Environmental Health Sciences, National Institutes of Health, PO Box 12233, Research Triangle Park, North Carolina 27709, USA, tel: 1-919-541 3386, fax: 1-919-541 4704, e-mail: cchabrar@(teleconference participation).Dr Tapan Chakrabarti, Environmental Biotechnology Division, National Environmental Engineering, Research Institute, Nehru Marg, Nagpur 440 020, India, tel: 91 0712 226026; fax: 91 712 226026/222725; e-mail: twmneeri@.inMr Richard Cary, Health and Safety Executive, Industrial Chemicals Unit HD D3, Room 202, Magdalen House, Trinity Road, Bootle, Merseyside, L20 3QZ, UK, tel: 44-151-951 4820, fax: +44-151-951 3308, e-mail: richard.cary@Dr Bing-Heng Chen, Department of Environmental Health, School of Public Health, Fudan University (Former Shanghai Medical University), 138 Yixueyuan Road, Shanghai 200032, China, tel + fax: 86 21 64163061, e-mail: bhchen@, bhchen98@Dr Christopher De Rosa, Agency for Toxic Substances and Disease Registry (ATSDR) Centers for Disease Control, 1600 Clifton road, N.E. Atlanta, Georgia 30333, USA, tel: 1 404 498 0160 / 0744, fax: 1 404 498 0094, e-mail: cyd0@Dr Stuart Dobson, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, PE28 2LS, UK, tel: 44-1487-772 494 (direct) 44-1487-772 400 (Switchboard), fax: 44-1487-773 467, e-mail: SD@Dr Obaid Faroon, Agency for Toxic Substances and Disease Registry (ATSDR), Division of Toxicology, 1600 Clifton Road, NE, Mail Stop E29, Atlanta, Georgia 30333, USA, tel: 1 404 498 0729, fax: 1 404 498 0092, e-mail: oxs0@Dr Herman Gibb, National Center for Environmental Assessment, US Environmental Protection Agency (8601D), Ariel Rios Building, 1200 Pennsylvania Avenue, NW, Washington, DC 20460, USA, tel: 1-202-564 3334, fax: 1-202 565 0059, e-mail: Gibb.Herman@Ms Rose Gomes,Existing Substances Division, Healthy Environments and Consumer Safety Branch, Health Canada, AL 0802B1, Tunney's Pasture, Ottawa, Ontario K1A OL2, Canada, tel: 1 613 957 0166, fax: 1 613 946 9673, e-mail: rose_gomes@hc-sc.gc.caDr Mary Gulumian, Toxicology & Biochemistry Research, National Centre for Occupational Health, P.O, Box 4788, Johannesburg 2000, South Africa, tel: 27 11 7205734; fax: 27 11 7206608; e-mail: gulumm@.zaDr Rolf F. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, FG-82, BGVV, Thielallee 88-92, 14195 Berlin, Germany, tel: 49 30 8412 3931, fax: 49 30-8412 3003, e-mail: ExChem@bgvv.deDr Akihiko Hirose, Division of Risk Assessment, National Institute of Health Sciences, 1-18-1 Kamiyoga, Setagaya-ku, Tokyo 158-8501 Japan, tel: 81 3-3700-1429, fax: 81-3-3707-6950, e-mail hirose@nihs.go.jpMr Paul Howe, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, PE28 2LS, UK, tel: 44 1487 772 499, fax: 44 1487 773 467, e-mail: pho@Dr Janet Kielhorn, Fraunhofer Institute for Toxicology and Aerosol Research, Nikolai-Fuchs-Strasse 1, D-30625 Hannover, Germany, tel: 49 511 5350 329, fax: 49 511 5350 335 email: kielhorn@ita.fhg.deDr Se-Hoon Lee, Department of Preventive Medicine, College of Medicine, The Catholic University of Korea, 505 Banpodong, Seochoku, Seoul 137-701, Korea, tel: 82 2 590 1236, fax 82 2 532 3820, e-mail: ashlee@cmc.cuk.ac.krMs Bette Meek, Existing Substances Division, Room 145, Environmental Health Centre, Healthy Environments and Consumer Safety Branch, Health Canada, AL 0801C2, Tunney's Pasture, Ottawa, Ontario K1A OL2, Canada tel: 1 613 957 3129, fax: 1 613 954 2486, e-mail: bette_meek@hc-sc.gc.caDr José Antonio Menezes Filho, Faculty of Pharmacy, Federal University of Bahia, Av. Barao Geremoabo, Campus Ondina, Salvador, Bahia, Brazil, tel: 55 71 399 6325 or 6311, fax: 55 71 399 6427, e-mail: antomen@ufba.brDr Ryszard Rolecki, Nofer Institute of Occupational Medicine, 8, Sw Teresy Str., 90-950 Lodz, Poland, tel: 48 42 6314842, fax: 48 42 6314572; 48 42 6568331, e-mail: rrol@imp.lodz.plDr Jun Sekizawa, Division of Chem-Bio Informatics, National Institute of Health Sciences, 1-18-1 Kamiyoga, Setagaya-ku, Tokyo 158-8591, Japan, tel: 813 3700 9548, fax: 813 5717 7180, e-mail: sekizawa@nihs.go.jpDr Salah A. Soliman, Department of Pesticide Chemistry, Faculty of Agriculture, Alexandria University, El-Shatby, Alexandria 21545, Egypt, tel: 203 592 0067 or 203 544 8403, fax: 203 592 2780 or 203-592-0067, e-mail: samsoliman@Dr Marie Haring Sweeney, Document Development Branch, Education and Information Division, NIOSH Mailstop C32, 4676 Columbia Parkway, Cincinnati, OH 45226, USA, tel: 1-513-533-8339, fax: 1-513-533-8230, e-mail: mhs2@Dr. Jan Temmink, Wageningen University; Dept. Agrotechnology & Food Sciences; Sub-dept., Toxicology, P.O. Box 8000, NL-6700 EA Wageningen, The Netherlands, tel. +31-317-48 26 56, fax +31-317.48 49 31, e-mail: hans.temmink@algemeen.tox.wag-ur.nlMs. Deborah Willcocks, National Industrial Chemicals Notification and Assessment Scheme (NICNAS), GPO Box 58, Sydney NSW 2001, Australia, tel: 61 2 8577 8890, fax: 61 2 8577 8888, e-mail: deborah.willcocks@.auRepresentative of the European UnionDr. Koula Ziegler-Skylakakis, European Commission, DG Employment and Social Affairs, EUFO 3263, Rue Alcide de Gasperi, 2920 Luxembourg, tel: (352)4301 34424, fax: (352) 4301 34259, e-mail: kyriakoula.Ziegler-Skylakakis@cec.eu.intSecretariatDr Antero Aitio, International Programme on Chemical Safety, World Health Organization, 20 Avenue Appia, 1211 Geneva 22, Switzerland, tel: 41 22 791 3592, fax: 41 22 791 4848,e-mail: aitioa@wh.chMr Teruyoshi Ehara, International Programme on Chemical Safety, World Health Organization, 20 Avenue Appia, 1211 Geneva 22, Switzerland, tel: 41 22 791 4334, fax: 41 22 791 4848, e-mail: eharat@who.chDr Philip Jenkins, International Programme on Chemical Safety, World Health Organization, 20 Avenue Appia, 1211 Geneva 22, Switzerland, tel: 41 22 791 3572, fax: 41 22 791 4848, e-mail: jenkinsp@who.chObserversDr Raymond M.David, Eastman Kodak Company, 1100 Ridgeway Avenue, Rochester, NY 14652-6255, USA, tel: 1-716 588 4763, fax: 1-716 722 7561Dr Robert J. Golden, ToxLogic LC, 9808 Clagett Farm Drive, Potomac, MD10854, USA, tel: 301 309 1947, fax: 301 424 2728, email: rgolden124@Mr Joseph W. Gorsuch, Eastman Kodak Company, 1100 Ridgeway Avenue, Rochester, NY 14652-6255, USA, tel: 1-716 588 2140, fax: 1-716 722 3173,Mr Bill Gulledge, American Chemistry Council, 1300 Wilson Avenue, Arlington, VA 22209, USAMr Stephen B.Hamilton, General Electric Company, 3135 Easton Turnpike, Fairfield, CT 06431, USA, tel: 1-203 373 3316, fax: 1-203 373 2650,email: stephen.hamilton@Dr Jay B.Silkworth, GE Corporate Research & Development, P O Box 8, Schenectady, NY 12301-0008, USA, tel: 1-518 387 5895, fax: 1-518 387 7611,email: silkworth@Dr William M.Snellings, Union Carbide Corporation, 39 Old Ridgebury Road, Danbury, CT 06817-0001, USA, tel: 1-203 794 3588, fax: 1-203 794 5275 email:snellib@Dr E.Watson, American Chemistry Council, 1300 Wilson Boulevard, Arlington, VA 22209, USA, tel:1-716 588 4763, fax: 1-703 741 6091,e-mail: Elizabeth_Watson@APPENDIX II Agenda NINTH FINAL REVIEW BOARD ON CONCISE INTERNATIONAL CHEMICALASSESSMENT DOCUMENTSOttawa, Ontario, Canada: 29 October-1 November 2001AGENDA1.Opening of the meeting, election of officers and adoption of the agenda2.Introduction to the Terms of Reference for Final Review Board members3.Draft CICAD on Acrolein4.Draft CICAD on Bromoethane5.Draft CICAD on Chloroaniline6.Draft CICAD on PCB (Human health aspects)7.Draft CICAD on Diethyl phthalate8.Draft CICAD on Carbon disulphide9.Draft CICAD on Silver (Environmental aspects)10.Draft CICAD on Ethylene glycol (Human health aspects)11.Future CICADs12.Any other business13.Closure of the meeting= = =APPENDIX III Terms of Reference NINTH FINAL REVIEW BOARD ON CONCISE INTERNATIONAL CHEMICAL ASSESSMENT DOCUMENTSOttawa, Ontario, Canada: 29th October-1st November 2001TERMS OF REFERENCE FOR A FINAL REVIEW BOARDThe Final Review Board is responsible for the following functions:•ensuring that each CICAD has been subjected to an appropriate and thorough peer review;•verifying that peer reviewers’ comments have been addressed appropriately;•providing guidance to authors on how to resolve any remaining issues if, in the opinion of the Board, all comments of the reviewers have not been adequately addressed;•approving CICADs as international assessments.The Final Review Board conducts most of its business at meetings, but also by correspondence between meetings. It is guided in its work by the IPCS Programme Advisory Committee, and functions in collaboration with the newly-formed IPCS Steering Group on Risk Assessment.APPENDIX IV FINAL REVIEW BOARD COMMENTS ON DRAFT CICADSAcroleinDiscussion of the draft CICAD on Acrylonitrile was led by Mr. Richard CaryDiscussion leader notesAcrolein is a highly reactive molecule and does not seem to persist very long in vivo or in the environment. Aquatic organisms appear to be more sensitive to acrolein than terrestrial organisms. However, the information available to the authors indicates that that the likely exposure levels do not result in a risk of adverse effects. There are no adverse effects anticipated for other environmental factors (e.g. ozone depletion, climate change or the formation of photochemical smog). In terms of human health, there are no immediate concerns of carcinogenicity and genotoxicity from the available data on acrolein, although these data are of limited quality. Hence, the main adverse health effects observed relate to upper respiratory tract irritation and these are the effects that form the basis of the guidance values that are derived.Specific commentsSection 1Summary section to be changed to reflect changes in main text.Section 1, para 5Sentence on epidemiological data to be expanded to a paragraph of its own toindicate that available human data on systemic effects are very limited and did not enter into overall conclusions. A reference be made to effects on the respiratorytract and eye.No need to include information on anti-cancer drug (reviewer 10 commentunaddressed in table of comments)Section 2, para 2In response to the reviewer’s comment; it would be possible to resolve the reasons for the considerable ranges on the physicochemical data. However, this would be time-consuming and is not relevant to determining risk for this compound. The current ranges can be left as they are.Section 4.3Dr Kielhorn to provide production levels data.Section 5.2Anaerobic and aerobic appear to be reversed since intuitively aerobic degradation should be greater - to be checked by author.Section 7If there is no information on oral and dermal absorption, and the magnitude of binding, then it was suggested that it should be stated that there are no data. Section 8.1Insert specific reference to source document for detail on signs of acute toxicity. Section 8.2Add that limited data indicate that acrolein may be a skin sensitizer.The EU document cannot be cited as the source since it is not yet complete.Citation of individual studies together with a footnote on the existence of the EUdocument.The poor quality of the studies should be indicated.Add sentence on effects on ciliary beat in the upper respiratory tract (reviewer 7 –comment on extra study from the BUA document).Section 8.3Deleted LOAELs to be retained or a description of the lowest effect concentrations to be added to the text.Section 8.3.1, para 6Number of animals to be added for the Cassee et al. (1996) study where it currently states these are not available – text in Chapter 11 (11.1.3.1 para 17) indicates numbers are available.Section 8.3.1 para 11Copy response statement on systemic effects from table of comments (reviewer 10) to section 8.3.1.Section 8.5Extra material on genotoxicity to be provided by Dr Chakrabarti to update to 1998.Drosophila test not to be included in this section – leave as is.Section 8.8, para 33Replace DNA adducts by the more general term DNA damage. (see also 11.1.1.2)Section 9Add to Ott study: include confidence limits - if not possible then add p-value. Section11.1.2,para 14Check that first sentence is consistent with the main text (Sect 8.5)Section 11.1.3.1, para 19Currently, the median figure has been used rather than the lower 95% confidence limit (note in bullet point 1 after equation). Indicate the value derived if the lower 95% limit was used instead either here in the text, in a footnote or an appendix.Figure 2Interchange the names of S-(2-carboxyethyl)-mercapturic acid and S-(3-hydroxypropyl)mercapturic acidTable of comments – list of reviewersReplace C. Eichler with R. Hertel; correct Dr Ziegler-Skylakakis' affiliation; add Dr Ziegler-Skylakakis among reviewers (comments received on early document drafts)BromoethaneDiscussion of the draft CICAD on Bromoethane was led by Dr Jun Sekizawa Comments have been responded to in an appropriate manner.Specific commentsSection 2,para 1Dimensionless Henry’s Law discrepancy between Sec 2 and Sec 5. Check.Section 2,para 2Check conversion factorsSec 4.2Add production data (Jun Sekizawa to provide)Sec 5 para 3Check solubilitySec 6.2.1 para 5EASE Model: Introduce model e.g. This model is used to measure..... together with source where it can be obtained. Give input data either as footnote or appendix. Give reference to EU Technical Guidance Document.Sec 7 Para 1The authors are to consider Paper from Thier et al., which Jun Sekizawa will provide (response to Reviewer 7)Sec 7 p. 9.para 2Line 6 change bromine to bromideSec 8.1.1Paras 2 and3Check conversions mg/L - ppm.Sec 8.2. Para9Delete paraSec 8.4 paras 15 & 17Recheck incidence figures. Add information about historical tumour incidence data from tabulated comments page 9 Sec 8.4 para 17 reviewer 10.Paras 19 & 20Change order of the paragraphs 19 & 20Section 11.1.4Weight of evidence for carcinogenicity. Put together information from Section 11. Para. 6,11 and 12 (Is bromoethane a genotoxic carcinogen or not? Bromoethane is a direct-acting alkylating agent but induces tumours not at the site of entry but rather at distant sites, such as the uterus) into new para 21. Delete old para 21------Table of comments & responses: P.4 Sec 6.2 Reviewer 9. Change in response: Not usual to add occupational exposure limits in a CICAD.4-ChloroanilineDiscussion of the draft CICAD on 4-chloroaniline was led by Dr Raj Chhabra in a teleconferenceThe FRB considered the document to be well written and that the authors had made considerable effort to deal with reviewers comments.The critical end-point and key study have been changed following proposals received in the peer review. While the FRB endorsed these changes, it considered it essential that the revised evaluation of health effects be reviewed by peers, and suggests the consultative group approach.Specific commentsSection 1Changes in the text (especially in section 11) to be reflected in the executivesummary.Section 1, para2Include MAK document as source document.Section 1, para17To be changed to reflect the wording in section 11.2Section 4.2Mr Howe to provide US EPA release data..Section 4.3,para 5Delete ‘can affect human health’ in last sentence.Section 5.1,para 1Comma missing in sentence, which makes the meaning ambiguous.Section 5.2,para 4Terminology (half-time vs. half-life) to be verified.Section 5.2, 5.3Not degradation – adsorption. Dr Dobson to aid with making the conclusionclearer.Section 6.1, para 3Analytical methods – explain where limits of detection refer to analytical equipment and where to method. (current text gives the impression that some reported concentrations are below the limit of detection).Sections 6.2.1,para 12 & 6.2.2,para 15Calculations need to be checked.Section 6.2.2,para 19Add ‘because products may contain PCA as a residual’.Section 8.1.1, para 2Uncertainties – possible contribution of dermal adsorption of vapour to body burden. Author to check paper (Russian)Section 8.4,para 10Replace NOEL with NOAEC(L)Section 8.5.2, para 17Add author’s response to reviewer 10 to section 8.9 (Mode of Action Section).Section 8.6.2Dr Chhabra to provide further data on MN from NTP to author. Section 8.6.2,para 23bParagraph to be deleted.Section 8.7,para 24bDelete paragraph.Section 8.9, para 28Second sentence to be reworded to indicate that there is one positive in vivo study on MN, but this was only positive at a dose level in the range of the LD50.Section 9, paras1 & 3Replace resorption with adsorption.Section 9, para 2Dr Gibb to check the studies in this paragraph with view on the interpretation of causal link between exposure to chloroaniline and methaemoglobinaemia in humans.Section 9, para4Replace ‘traced to’ with ‘reported to be associated with’.Section 9, paras 4b & 4c Paragraphs to be revised by Drs Sweeney and Gibb to verify the causality and exposure-response relationships reported.Section 9, para4cLast sentence of paragraph to be deleted.Section 10.1 &Table 10.1Common names of organisms to be provided by Mr Howe.Section 11Ms Meek to aid author with reorganizing hazard identification, exposure-response assessment and criteria for setting guidance values:Reorganize order and remove subheadingsDelete first paragraph (summary).Emphasize the methaemoglobin formation (critical endpoint).Remove more detailed descriptions of the data throughout.Animal data in paragraphs 4 and 5 to be moved before the human data.Delete LD50 values.Cross-reference between animal and human data.Section 11.1.1,para 7Paragraph needs to be revised subsequent to the NTP study being reviewed. Section 11.1.2Animal data should be used to set the guidance value with human data being used to bound the animal data.Section 11.1.2 para 8g Delete second sentence and emphasise that the human data is based on a sensitive population.Section 11.1.2 para 9Delete paragraph.Section 11.1.3,para 19Delete paragraph.Section 11.1.4Add uncertainties regarding human exposure data (possibility of dermal exposure) to this subsection. Note the weaknesses of the human studydesign)Section 11.1.4,para 22bParagraph to be deleted.Section 11.2Add scattergramSection 12Delete second paragraph (one line).Figure 7.3For the 4-aminophenol replace Cl with OHPolychlorinated biphenyls (Human health aspects)Discussion of the section 9 of the draft CICAD on Polychlorinated biphenyls (Human health aspects) was led by Dr MH Sweeney, and of the other sections, by Ms ME MeekGeneralThe draft has vastly improved since presentation at 8th Meeting. It is more explicit and inclusive. It should be made sure that the executive summary accurately reflects the body of the text and the evaluation section. It should be clearly stated what is the object of evaluation and what is our explicit approach. For different exposure scenarios there are different approaches, notably because of different isomer composition, e.g., in the environment the profile of the mixture changes. This CICAD concentrates on situations where the mixtures approach is valid. Other approaches should not be described in detail but should be noted with an indication of the special circumstances where these approaches might be more appropriate (with reference where possible).The Secretariat had received additional comments on the document from a stakeholder organisation, mostly on details in the section on human health effects. Because of the late arrival of these comments, it was not possible for the author or the FRB to consider these comments by the time of the meeting. Furthermore, the health evaluation section of the document is mainly based on experimental studies, rather than on epidemiological findings. Thus the FRB considered it sufficient that the author considers these new comments after the meeting, and revises the document where needed. The adequacy of the author's response to these comments will be verified by the discussion leaders, and the Chair.Specific commentsAdd list of abbreviations and acronyms used.Section 1Changes in the text to be reflected in the Section 1.Section 1 para 1Remove description of history of the document (Executive summary, para 1) to the Appendix.Section 1 para 2(which will become para 3 in the final Section 1, see below). Put into historical context the sentence on risk management.Section 1Paragraph 14 should be subsumed in a new para 2 in the Executive summary, which identifies the PCB mixtures that are discussed in this CICAD (commercialPCB mixtures and mixtures generated from them, e.g. in the environment andfood chain), and makes explicit the hazard characterization approach chosen asthe basis in this document. The other approaches should be briefly mentionedwith a reference (and thus deleted from Section 10, para 17-18).It should be stated that there is much more data in the source document.It should be stated that this document does not deal with heating-inducedcontaminants of PCB, but describes, however, briefly the studies on Yusho andYucheng patients. It should also be made clear that, in many instances of bothepidemiological and experimental studies, the exposure has been to a mixturenot only of PCBs with varying and unknown composition, but also to PCDD/PCDF, and even other POPs.Ongoing work at WHO should be mentioned, perhaps as a foot-note.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
加拿大HnG医学技术有
限公司
公司简介
加拿大HnG医学技术有限公司总部位于加拿大安大略省,从2007年起,陆续在宁波保税区、杭州和南京设立了分公司,在新加坡建立了办事处。
公司专业从事心脑血管介入类医疗器械的加工工艺研发,为亚洲广大的医疗器械生产厂商提供医疗器械的原料、配件和生产设备。
公司依托在该领域的先进技术和丰富经验,以及与该领域最领先的多家美国公司的战略合作伙伴关系,自成立起发展迅速,已为国内及国外知名医疗器械生产商提供了产品和服务。
公司设有技术部、市场部、销售部、行政部、财务部等多个职能部门。
在中国工作地点有宁波保税区、杭州滨江区、杭州余杭区和南京建邺区四处。
业务范围
我们是17家美国公司在亚洲的战略合作伙伴;
我们的产品主要是介入类医疗器械的生产设备、原材料和配件;
我们提供技术服务和售后服务;
我们是经授权的Vante的亚太售后服务中心。
产品简介
一、设备
导管挤出机
导管成型机
导丝磨床
绕簧机
缠簧机
二、原材料
医用级高分子材料
医用涂层材料
镍钛材料
金属丝/管/芯轴
三、配件
管材
精密激光雕刻
硅胶制品
医用小配件
导丝
微型弹簧
微型冲压件
精密机加工
企业文化
我们的使命:全球最先进的植入介入医疗器械制造解决方案 我们的愿景:领导行业转型升级
我们的价值观:公平的价格,最好的产品。
